Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~5~~~1
P-669 -1-
COATED ARTICLE
TECHNICAL FIELD
The present invention relates to coating
compositions for protecting titanium or titanium
alloys, the coated article, and the method of coating
the article. More specifically, the present
invention relates to coating compositions which are
ideally suited for application to parts, such as
aircraft and turbine engine parts, made from titanium
and titanium alloys that are exposed to oils and
hydraulic fluids at high temperatures.
BACKGROUND ART
The use of titanium alloys in aerospace
construction is of significant interest. Titanium
alloys have superior strength-to-weight ratios.
Densities range between 4.4 and 4.85 gm/cm3 and yield
strengths from 172 MPa to 1,880 MPa for different
titanium alloys. This combination of high strength
and low density results in exceptionally favorable
strength-to-weight ratios. These ratios are superior
to almost all other metals in the range of
P-669 -2-
temperatures reached in the compressors of aircraft
gas turbines making the use of titanium very
desirable for weight saving.
In addition to high strength-to-weight
ratios, titanium alloys possess excellent erosion
resistance, high heat transfer efficiency and good
corrosion resistance in most environments. Like
stainless steel, in the presence of air titanium
alloys form a tightly adherent oxide scale that is
self healing and severely reduces material loss due
to erosion/corrosion.
In airframe/turbine engine applications, it
is desirable to replace ferrous alloys including
stainless steels, and some nickel based alloys with
titanium alloys to save weight. Presently, titanium
alloys account for 7% of the weight of airframe
structures for commercial aircraft and 20-25~ of
weight in such structures used for military
applications. Uses include bulkheads, air ducting,
fairings, keels, and fuselage panels. In addition,
casings and larger structures made by electron beam
welding plates of various titanium alloys can be
substituted for ferrous materials.
One obstacle to greater utilization of
titanium within airframes is its reactivity with hot
oils and hydraulic fluids. In aircraft, excess heat,
20~~941
P-669 -3-
age or catalytic reaction can cause lubricants and
hydraulic fluids to decompose to acidic materials
which quickly attack titanium structures. One major
manufacturer of commercial aircraft has noted that
phosphate ester based hydraulic fluid has little
effect on most metals up to about 115°C (240°F), but
that titanium alloys can be severely etched, pitted,
and embrittled when exposed to such a fluid at
temperatures above 132°C (270°F). This corrosion
occurs on all of the titanium alloys that could be
used in airframe construction. Similar effects are
seen on corrosion-resistant steel, often known as
CRES, at temperatures above 204°C (400°F).
Temperatures greater than 132°C can be generated in a
variety of airframe locations - for example, in
braking systems and in/near engines and support
pylons.
Of the hydraulic fluids and oils most
commonly used, phosphate ester type synthetic
hydraulic fluid exhibits the most rapid attack on
airframe materials. This fluid is used in aircraft
because it is fire resistant, e.g., it has a high
autoignition temperature and shows little tendency to
propagate a flame. In addition to attacking titanium
(and steels), it strips most finishes from metals and
attacks other organic polymer structures. This
CA 02055941 2001-06-13
68086-469
-4-
phosphate ester hydraulic fluid typically contains
dibutyl phenyl phosphate: and tributyl phosphate.
Typical hydraulic fluids. of this type are Skydrol
TM
500B and Skydrol LD manufactured by Monsanto Chemical
~~ Company.
When this fluid drips on titanium alloys
that are operating at a temperature greater than
132°C (270°F), black decomposition products
accumulate which contain acidic products such as acid
phosphates which rapidly attack the titanium
structure.
In the past, use of titanium structures in
any area exposed to such, hydraulic fluid was
forbidden since there was no known way of controlling
the attack of the hydraulic fluid on the hot
component. The temperature of exposure can be as
high as 260'C (500'F) and can be produced by cool
hydraulic fluid dripping' on a hot component or hot
hydraulic fluid dripping on a cool component.
20~ Because the attack produces embrittlement and
pitting, protection must be total since embrittlement
and pitting both lead to cracks and catastrophic
failure.
Applicant has been quite active in
developing and patenting various coating and bonding
compositions which are highly suitable for coating
~05~~41
P-669 -5-
various surfaces, particularly metal, to impart
protective or other characteristics thereto or which
may be used as compositions for bonding two surfaces
together. Examples of these compositions are
5 disclosed in the U.S. Patents 3,248,251 to Allen,
issued April 26, 1966, 4,537,632 issued August 27,
1985, and 4,606,967 issued August 19, 1986, both to
Mosser, and U.S. Patents 4,617,056, issued October
15, 1986, 4,659,613, issued April 21, 1987, and
10 4,724,172, issued February 9, 1988, all to Mosser and
McMordie. All of the aforementioned patents are
assigned to the assignee of the present invention.
However, none of the aforementioned patents or other
prior art known to applicant addresses the problem
15 inherent in coatings of titanium structures which are
exposed to hydraulic fluids at extremely high
temperatures. Further, neither applicant nor others
skilled in the art have previously been able to
provide coatings for controlling the attack of the
20 hydraulic fluid on hot components made from titanium
or titanium alloys.
Applicant herein provides a coating, a
method of coating an article, and a coated article,
all related to titanium and titanium alloys as
CA 02055941 2001-06-13
68086-469
-6-
substrates, which now allows such coated substrates to be used
in what was previously forbidden i.n environments.
SUMMARY OF THE INVENTION
In accordance with the present invention, there is
c~ provided a coated article consisting essentially of a substrate
selected from the group consisting of titanium and a titanium
alloy; an outer coating consisting essentially of polyphenylene
sulfide resin for prot:ect.ing said substrate from chemical
degradation by acid decomposition and maintaining stability
during exposure to temperatures of 400° to 500°F; an adhesion
primer layer disposed between said substrate and said outer
coating, saicL adhesion. primer layer including an inorganic
binder phase containing a reactive metal powder for increasing
adhesion to :>aid titanium and titanium alloys and thermoplastic
resin particles for ir~c:reasing adhesion to said outer coating,
said thermoplastic resin particles consisting essentially of
polyphenylene sulfide resin; and a sublayer between said
substrate ancL said pri.mc=r layer, said sublayer selected from
the group consisting es;~entially of aluminum, aluminum alloy
2C and aluminum ceramic.
The present invention further provides a method of
making a coated article including the steps of: forming a
substrate from titanium or titanium alloys, and adhering an
outer coating of thermoplastic resin capable of protecting the
substrate against chemical attack and temperature of at least
as high as 2E~0°C by appl.yi:ng an adhesion primer layer over the
substrate, the primer 1<~yer including an inorganic binder phase
increasing acLhesion to the substrate and thermoplastic resin in
particulate form increa;~ing adhesion to the outer coating, and
3C further including the s1=ep of increasing the adhesion of the
adhesive binder layer for the outer coating by admixing a
pigment with the adhesive binder layer.
CA 02055941 2001-06-13
68086-469
F:rGURES IN THE DRAWINGS
Other advantages of the present invention will be
readily apprE~ciated as t:he same becomes better understood by
reference to the following detailed description when considered
in connection with the accompanying drawings wherein:
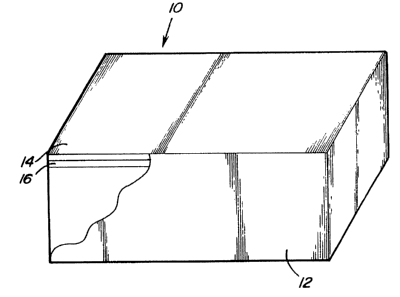
Figure 1 is a perspective view partially broken away
of a substrate coated in accordance with the present invention;
and
Figure 2 is a perspective view partially broken away
1C of a second embodiment.c~f the present invention.
DETAILED DESCRIPTION OF THE INVENTION
A coated article made in accordance with the present
invention is generally ;shown at 10 in Figure 1. The article
includes a substrate consisting essentially of titanium or
titanium allc>y.
~05~9~1
P-669 -8-
Examples of typical titanium alloys are described in
AMS Specification 4911 (6% aluminum, 4% vanadium),
AMS Specification 4919 (6% aluminum, 2% tin, 4%
zironium, 2% molybdenum). Alloys are described
further in U.S. Military Specification MIL-T-9046.
The substrate can be in the form of an airframe
component, such as a pylon, that is used for securing
a jet engine to an aircraft wing or the like. As
discussed above, these pylons are commonly exposed to
hot hydraulic fluid dripping on a cool substrate or
alternatively, the exposure can be to cool fluid
dripping on a high temperature substrate.
Although titanium and titanium alloys are
preferred substrates in these applications because of
their relatively lighter weight compared to ferrous
alloys, the present invention can be suitable for use
on other related corrosion resistant metals, such as
stainless steel.
The coated article 10 includes an outer
coating 14 of thermoplastic resin for protecting the
substrate from chemical degradation and maintaining
stability during exposure to temperatures over 260°C
(500°F). The thermoplastic resin can be selected
from the group including, but not limited to,
fluorinated ethylene/proplyene copolymer (FEP),
polytetra fluoroethylene (PTFE), polyfluoroalkoxy
P-669 . _g_
resins (PFA), polyvinylidene fluoride (PVF2),
trifluorochloro-ethylene, and polyphenylene sulfide
(PPS).
These resins are useful because they share
a series of properties. The resins are all generally
considered to be resistant to most chemicals. The
resins further are all useful at elevated
temperatures, for example, greater than 120°C
(250°F). Some of these resins are stable during
prolonged exposure to temperatures over 260°C
(500°F). Additionally, the resins are all
thermoplastic. Accordingly, the resins all melt and
flow thereby forming films having significant
cohesive strength. The resins set forth above are
not attacked by hydraulic fluids or the decomposition
products of hydraulic fluids.
Prior to the development of the present
invention, it has been demonstrated that although the
coatings listed above and coatings similar thereto
and films containing the above listed thermoplastic
polymers can be applied to substrates like titanium,
they suffer from poor adhesion. In addition, in
areas where such films have imperfections, such as
pin holes, there is ready access to the substrate.
At these pin holes, rapid localized attack can be
initiated.
P-669 -10-
The present invention addresses the
aforementioned problems by providing an adhesion
primer layer 16 disposed between the substrate 12 and
the outer coating 14. The adhesion primer layer 16
5 includes an inorganic binder phase for increasing
adhesion to the titanium and titanium alloys and
related metals. The adhesion primer layer 16 further
includes thermoplastic resin in particulate form for
increasing adhesion to the outer coating 14. In
10 other words, applicant has found that adhesion of the
protective thermoplastic films forming the outer
layer 14 is greatly improved if the film is applied
on the primer layer 16 which contains an inorganic
binder phase and the high temperature thermoplastic
15 resin in particulate form.
Although applicant has utilized similar
coatings in other environments, applicant has
discovered, as claimed herein, that such coatings are
useful in combination with substrates made from
20 titanium, titanium alloys, and related metals for the
protection of these metals in the aggressive
environment set forth above. Moreover, applicant has
discovered these coating compositions to include an
oil and hydraulic fluid decomposition product
25 protection component for protecting the titanium,
.\
2~J~~~~
P-669 -11-
titanium alloy, and related metals from the high
temperature acids produced by the decomposition of
oils and hydraulic fluids.
Generally, the primer layer 16 includes a
cured binder or matrix phase prepared by thermally
curing a primer slurry consisting essentially of an
aqueous solution of a combination of inorganic
compounds from the group consisting of phosphoric
acid, chromic acid, molybdic acid and metal salts of
these acids. Preferred solutions contain phosphate
anion and chromate (or dichromate) and/or molybdate
anions. Such coating composition binders are
disclosed in the U.S. Patents 3,248,251 to Allen,
3,869,293 to Brumbaugh, and 4,537,632 to Mosser.
The primer layer can include certain
pigments for further increasing the adhesion of the
outer coating 14 thereto. Specifically, pulverulent
aluminum is beneficial in such primer layers in that
the pulverulent aluminum bonds tenaciously to all of .
the thermoplastic resins listed above. Additionally,
aluminum metal does~not oxidize during the cure cycle
required for the resin in the outer coating 14 and
further improves adhesion. An additional benefit of
the pigment is that if the outer coating 14 has pin
holes or other imperfections, the aluminum powder in
the primer layer 16 can react with the acid
P-669 -12-
phosphates or other acidic compounds produced by
decomposing hydraulic or other fluid thereby
neutralizing the effects of the acid and preventing
corrosion.
Preferably, the aluminum powder consists
essentially of atomized aluminum spheroids. Such
particles are disclosed in the U.S. Patent 4,537,632
to Mosser, assigned to the assignee of the present
invention.
Although aluminum powder is the preferred
pigment, other insoluble inorganic and metallic
particles can be incorporated into the binder. Among
these inorganic particles are chromium and zinc.
Furthermore, other additives, pigments can be
included in the film.
As shown in Figure 1, the primer layer 16
can be applied directly over the surface of the
substrate 12. Alternatively, as shown in Figure 2
wherein primed numerals are used to indicate like
structure between the several embodiments, the primer
layer 16 can be applied over a sublayer 18 containing
aluminum or aluminum alloys. This sublayer can be in
the form of aluminum powder thermally sprayed using
powder plasma, arc wire or similar techniques.
Alternatively, the aluminum layer can be applied by
physical vapor deposition, specifically ion vapor
P-669 -13-
deposition. The sublayer 18 may also consist of
aluminum/ceramic coatings such as those described in
the U.S. Patents 3,248,251 to Allen, 4,537,632 and
4,606,967 to Mosser, and 4,617,056, 4,659,613 and
4,724,172 to Mosser and McMordie. These coatings are
of particular significance because of their
exceptionally high bond strength, high temperature
stability, and ease of application to a wide variety
of structures.
The aluminum sublayer insures that if the
barrier polymer outer coating 14 or primer layer 16
fails in service, the substrate 12 will still be
protected from the hydraulic fluid by a layer 18
capable of reacting with the fluid and neutralizing
its effects. Although it is possible that this layer
may add undesired weight to the final coated product,
the ambient condition of the coated article may be
. such that the benefit of the additional layer
providing protection to the substrate 12 will out-
weight the cost of the additional weight.
As stated above, the primer layer 16
includes a high temperature thermoplastic resin in
particulate form and a bonding agent. Optimally,
other pigments, particularly metal powders, can be
added to this layer. The bonding agent may be a
metal powder and the metal powder/thermoplastic resin
P-669 -14-
may be applied by thermal spray techniques. Although
different compositions are described in the U.S.
Patent 3,723,165 to Longo et al, the Longo patent
does disclose that such composite materials can be
5 thermally sprayed onto a substrate surface. In
addition to this thermal spray technique, the resin
or resin/metal pigment mixtures can be compounded in
inorganic binders such as acid phosphates,
chromate/phosphates, or inorganic silicates, as
disclosed in the above cited Allen patent.
With regard to the thermoplastic resin in
particulate form, the size of the resin particles can
vary from colloidal to about 150 microns (100 mesh)
with typical sizes being between 5 microns to 100
15 microns. The primer layer composition is designed
such that after application to the substrate surface
and thermal cure, if required, the resin particles
are present on the surface of the primer layer. This
is critical because these particles are useful in
bonding the outer coating film 14 to the primer layer
16.
Thermal cure of the inorganic primers is
usually carried out at temperatures between 232°C
(450°F) and 371°C (700°F) to cure the inorganic
25 binder portion of the primer layer 16 and to sinter
the particulate resin portion.
20~~~~~.
P-669 -15-
Preferably, the outer coating 14 includes
the same or similar resin as the primer resin 16.
For example, a primer layer may utilize polytetra
fluoroethylene (PTFE) as a pigment. A subsequent
barrier film forming the outer coating 14 may contain
fluorinated ethylene proplyene (FEP) or
polyfluoroalkoxy resin (PFA) provided that the top
coat film forming the outer coating 14 is heated at
least to 360°C (680°F) to partially melt the
particles of PTFE resin in the primer as well as the
resin in the outer coating l4. In this manner, the
resins are bonded thereby bonding together the two
layers.
The outer ccating 14 can be prepared as a
slurry of solvent carriers and finely divided resin
or it may be preformed film. Solvent carriers used
will vary with the resin selected. PPS resin is
usually applied from a water dispersion. Thin film
FEP and PTFE coatings are also applied via a water
borne slurry. PVF2 resin normally is combined with a
mixture of solvents and diluents to provide viscosity
control after the resin has melted. Additionally,
both the outer coating 14 and primer layer 16 may
include other components, such as surfactants, to
P-669 -16-
improve the fluid characteristics of the coating to
be compatible and form desirable films over
particular substrates.
An alternative method of applying the outer
5 coating 14 is by the coalescence of electrostatically
deposited resin powder to form a film during
subsequent thermal treatment. The film itself would
then be applied to the primer layer 16 and thermally
cured therewith to bond the two layers together.
EXPERIMENTAL EXAMPLES
In general, evaluation of the coating
systems used and made in accordance with the present
invention to meet end user requirements is done by
heating a horizontal titanium alloy panel to a
suitable test temperature, as high as 260°C (500°).
Then, hydraulic fluid is dripped onto the panel at a
slow constant rate. Decomposition occurs and a black
char-like substance remains. Every 24 hours, the
specimen is cooled and the char scraped off with a
blunt wood scraper. After clean up, the test is
continued until four 24 hour cycles have been
completed. Finally, the coating is removed entirely
25 and any weight changes noted in addition to any
erosion, pits, or other indications of attack.
20~~9~1
P-669 -17-
Quantitative evaluation may include measurement of
yield strength changes or fatigue testing may be
undertaken to insure that there have been no
undesirable effects on the substrate.
Although other test methods are possible,
the one described above simulates a typical airframe
application where a leaking hydraulic line drips
fluid on a hot airframe component.
E%AMPLE I
A primer was prepared by manufacturing the
following binder A (Taken from U.S. Patent to Allen .
discussed above, example 7).
Binder A
Deionized Water 800 milliliters
Phosphoric Acid (85%) 220 milliliters
Chromic Acid 92 grams
Magnesium Oxide 152 grams
2~55~~~~
P-669 -18-
Primer
Binder A 1750 milliliters
Deionized Water 1750 milliliters
Aluminum Pawder, air 1225 grams
atomized, 5.5 um ave.
particle size
Polyphenylene Sulfide 1435 grams
resin powder (Ryton V-1
grade) (Phillips Petroleum Co.)
Surfactant, Triton X-100 10 grams
(Rohm and Haas Co.)
The primer was mixed and screened through a
100 mesh (150 hum) screen. The primer was spray
applied to a titanium alloy panel that had been
blasted with 100 mesh (150 jzm) aluminum oxide. The
primer was cured at 343°C (650°F) for 30 minutes,
then cooled.
A polyphenylene sulfide topcoat was
prepared as follows:
205~~~1
P-669 -19-
Deiani2ed water 1900 milliliters
Glycerol 1667 milliliters
Surfactant, Triton X-100 266 milliliters
(Rohm & Haas Co.)
Polyphenylene Sulfide 1333 grams
Resin (Ryton V-1 Grade)
Titanium Dioxide 320 grams
rutile pigment grade
The ingredients were added in order,
stirred, and then ball milled for 24 hours. This ,
topcoat was spray applied over the cured primer layer
and then cured at 371°C (700°F) for 30 minutes. A
second coat was applied and cured in the same way.
Total coating thickness was 90~um (0.0035 inches).
Primed and topcoated panels titanium allay
panels were exposed to the dripping hydraulic fluid
(Skydrol) test for 96 hours at 232°C (450°F) and
exhibited no weight change or evidence of pitting.
Bare titanium alloy panels were severely eroded by
the same exposure.
2~~~~~~1
P-669 -20-
EXAMPhE II
Sheet titanium alloy panels (AMS 4911) were
solvent degreased, grit blasted with 100 mesh (150
5 dam) aluminum oxide, then coated with an aluminum
coating described in U.S. Patent 4,724,172 example 7,
to Mosser and McMcrdie, dried at 80°C (176°F) for 30
minutes, then cured at 343°C (650°F) for 30 minutes.
After cooling to ambient temperature, a second coat
10 was applied, dried, and cured. A total of 125 pm
(0.0049 inches) average coating thickness was
applied.
This aluminum/ceramic layer was primed and
topcoated with the coating primer and topcoat
15 described in Example I. When tested per the
procedure described in Example I, it showed corrosion
resistance at least equivalent to that of the primed
and topcoated panels described in Example I.
P-669 -21-
EXAMPLE III
A primer was prepared as follows:
Binder A (Example I) 1000 milliliters
Polytetrafluoroethylene
dispersion, 60% solids 1000 milliliters
(DuPont T-30)
Polytetrafluoroethylene 220 grams ,
powder, average particle size
3-4 hum (Hostaflon TFVP 9202
(Farbwerke Hoechst AG)
Deionized Water 400 milliliters
This primer was thoroughly mixed and spray
applied to alumina blasted (90-120 mesh) C.P.
Titanium panels. It was dried and cured at 371°C
(700°F) for 40 minutes to cure the binder and fuse
the PTFE resin particles. It was topcaated with a
proprietary fluorinated ethylene propylene resin
dispersion, DuPont 856-204 then cured at 385°C
(725°F) for 10 minutes. Additional coats of 856-204
were applied and cured at 329°C (625°F). This
coating showed excellent stability in the Skydrol
drip test.
2~~~~~:~
P-669 -22-
The PTFE primer described in Example III
was applied to blasted C.P. Titanium panels. A
fluorinated ethylene propylene (FEP) polymer film,
200 um (0.008 inches) thick was bonded to this primed
surface by heating the panel and film under pressure
to 400°C (752°F) for 15 minutes. The bonded film
showed superior corrosion resistance when immersed in
hot synthetic oil at 250°C (482°F).
EXAMPLE D
Grit blasted titanium panels were plasma
sprayed with an aluminum silicon alloy powder (12~
silicon ) having a particle size between 10 um and
120 um. A thickness of 150 pm (+ 25 Vim) was applied.
A primer was applied over the plasma
coating. The primer composition is as follows:
Primer
Binder A (Example I) 945 milliliters
Chromic Acid 278.5 grams
Zinc Oxide 25.6 grams
Magnesum Carbonate 97.5 grams
20~~~4i
P-669 -23-
Deionized water 2840 milliliters
Polytetrafluoroethylene 3500 milliliters
dispersion, 60% solids
(DuPont T-30j
The slurry was spray applied over the
plasma applied coating and allowed to air dry. It
was cured at 371°C (700°F) for 30 minutes.
A polytetrafluoroethylene (PTFEj thin film
water borne topcoat was spray applied over the cured
primer. A DuPont 850 - 204 coating was used. Two
coating layers were applied and cured at 385°C
(725°F). The coating system showed superior adhesion
and good resistance to hot hydraulic fluid.
The above examples demonstrate the ability
of the present invention to provide a coated article
with means for protecting titanium or titanium alloy
substrate from acids produced by decomposition
products of oils and hydraulic fluids at temperatures
up to 260°C (500°). The examples demonstrate the
ability of either embodiment illustrated in Figures 1
and 2 to provide corrosion protection in a severe
chemical and heat environment.
~~~~~~1
P-669 -24-
The invention has been described in an
illustrative manner, and it is to be understood that
the terminology which has been used is intended to
be in the nature of words of description rather than
of limitation.
Obviously, many modifications and
variations of the present invention are possible in
light of the above teachings. It is, therefore, to
be understood that within the scope of the appended
claims wherein reference numerals are merely for
convenience and are not to be in any way limiting,
the inven~.ion may be practiced otherwise than as
specifically described.