Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
s5794
26/9
- 1 ZC'5~
SPECIFICATION
ALUMINUM-CONTAINING OXIDE, ITS MOLDED ARTICLE,
AND PROCESS FOR PRODUCING ALUMINUN-CONTAINING OXIDE
1 TECHNICAL FIELD
The present invention relates to an aluminum-
cont~ining oxide for use as a machine part, an electronic
part such as an electronic circuit substrate or a cerdip
package, a catalyst, a catalyst support, a sensor, an
adsorbent, and a filler for chromatography, a molded
article obtained by molding the aluminum-contAin;ng
oxide, an article obtained by subjecting the molded
article to heat treatment, and a process for producing
the aluminum-contAi n i ng oxide.
~~C~NICAL BACKGROUND
A conventional molded article of an aluminum-
contAin~ng oxide i8 obtained by Ad~1~g an organic
compound or an inorganic compound as a binder to an
alumina powder and molding the resultant mixture. A
machine part or an electronic part of aluminum-contAining
oxide is produced by further subjecting the molded
article to a sintering treatment at a high temperature of
1,400~C or higher (For example, see the Supplement to
Kogyo Zairyo tIndustrial Materials), Nov. 1987, on a
process for producing an alumina substrate as one example
of molded articles of an aluminum-containing oxide,
issued by Nikkan Kogyo Shinbunsha). As a conventional
- 2 - Z~ 82
1 process for producing an aluminum oxide powder, there is
available a method in which alumina produced by a Bayer
method i8 milled, a method in which aluminum hydroxide
obtAined from an aluminum compound by a co~ecipitation
method is ignited and then milled, a CVD m~thod (gaseous
phase reaction deposition method) in which an alumina
oxide po.ler is synthesized in a gaseous phase from a
- special all i ~< -und having a volatility.
In a conveltional method for producing a molded
article from an aluminum oxide powder, an article
obtAined by A~ i ng an organic compound or an inorganic
compound as a binder to an aluminum oxide powder and
molding the resultant mixture is required to be treated
at a high temperature of 1,400~C or higher as described
above. On the other hand, when an aluminum powder is
molded without a binder, it i8 requirèd to mold it at a
high temperature of l,100~C or higher, or to employ a
means such as a hot-pre~s, etc.
In other col.ven~ional methods for producing
aluminum oY~es, ~uch as the Bayer method, the method of
igniting aluminum hydroxide, etc., it is burdensome to
remove harmful impuritie~, and the finely milling step is
complicated. Furthermore, aluminum oxides obt~ined by
these methods are usually crystalline. A sputtering
method of a CVD method is known to be a method for
obtAining an amorphou~ aluminum oxide. Il~ ve~, the
hAn~l~ng of a raw material or the production process
according to that method is complicated and troublesome.
_ 3 _ 2~5~
1 DISCLOSURE OF THE INV~hllON
With regard to a process for producing a molded
article from an aluminum oxide powder, the present
inventors have made a diligent study for a process for
producing a molded article at a low temperature. As a
result, it has been found that a novel aluminum-
contAining oxide powder having a composition cont~ining a
trace amount of bismuth is effective for producing a
molded article at a low temperature, and that the above
novel powder can be easily produced by rapidly cooling a
melt of an all i n metal to which a trace amount of
bismuth metal has been incorporated to coagulate the melt
and then oxidizing the coagulation product. On the basis
of this fin~ing, the present invention has been
completed. That is, it is an object of the present
invention to provide a substantially amorphous, aluminum-
contAini ng oxide having a composition Al~ 2Ri M1~lM2~2O,
(wherein x is defined by 0.0001 S x S 0.10, Ml i8 at least
one selected from Si, P, B, Sb, Se, Te, Sn, Zn, In, Cr,
Nb, Sc, Y, Sr, Ba, Ca, Na, Li, Mg, Mn, W, Ti, Zr, Hf, Be
and rare earth mstals, M2 is at least one selected from
Fe, Ni, Co, Rh, Ru, Re, Cu and Pb, yl is defined by 0 S
yl S 0.1, y2 is defined by 0 ~ y2 S 0.01, z is defined by
1.2 S z 5 1.5, and x, yl, y2 and z are each an atomic
ratio), a metal composition contAining a trace amount of
bismuth metal and/or aluminum metal, processes for
producing these oxides and metal compositions, and molded
articles obtained from these oxides and metal
- 4 - z~3~
1 compositions or contA;ning these oxides and metal
compositions.
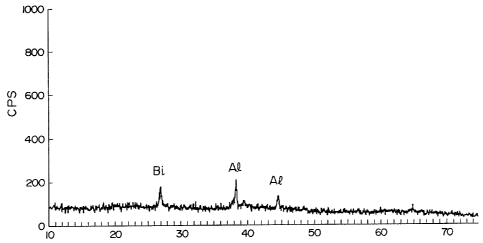
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a scAnning electron microscope
photograph showing the measuremen~ of the particle size
of an oxide powder of the present invention obtained in
Example 1. Fig. 2 is an X-ray diffraction chart showing
existing states of bismuth metal and aluminum metal
contained in the above oxide powder.
MOST PREFERRED EMBODIMENTS FOR W~R~lNG THE lNV llON
The first embodiment of the present invention
relates to a substantially amorphous, aluminum-contAining
oxide having the composition Al~ 2~i Ml,lM2~2Oz (wherein x
i~ defined by 0.0001 ~ x ~ 0.10, Ml is at least one
~elected from Si, P, B, Sb, Se, Te, Sn, Zn, In, Cr, Nb,
Sc, Y, Sr, Ba, Ca, Na, Li, Mg, Mn, W, Ti, Zr, Hf, Be and
rare earth metal~, M2 is at least one selected from Fe,
Ni, Co, Rh, Ru, Re, Cu and Pb, yl is defined by
0 5 yl S 0.1, y2 is defined by 0 ~ y2 S 0.01, z is
defined by 1.2 ~ z ~ 1.5, and x, yl, y2 and z are each an
atomic ratio).
The second embodiment relates to an aluminum-
contAining metal composition contAining an amorphous
oxide of an alloy of an AlBi system and Al or ~i in a
metallic state.
The third embodiment relates to an aluminum-
2~53~2
1 contA;ning metal composition cont~ining an amorphousoxide having the composition Al~ 2BirNlylM2~2O~ (wherein x
is defined by 0.0001 ~ x ~ 0.10, Ml is at least one
selected from Si, P, B, Sb, Se, Te, Sn, Zn, In, Cr, Nb,
Sc, Y, Sr, Ba, Ca, Na, Li, Mg, Mn, W, Ti, Zr, Hf, Be and
rare earth metals, M2 is at least one selected from Fe,
Ni, Co, Rh, Ru, Cu and Pb, yl is defined by 0 ' yl ~ 0.1,
y2 is defined by 0 ~ y2 ~ 0.01, z is defined by 1.2 ~ z <
1.5, and x, yl, y2 and z are each an atomic ratio) and Al
or Bi in a metallic state.
The fourth embodiment relates to the above
amorphous aluminum-cont~ining oxide that is a powder
having an average particle diameter of 0.1 to 100
microns.
The fifth embodiment relates to the above
a1uminum-cont~ning metal composition that is a powder
having an average particle ~ir -ter of 0.1 to 100
microns.
The sixth embodiment relates to a molded
article formed by molding an amorphous aluminum-
contAining oxide or an aluminum-containing metal
composition.
The seventh embodiment relates to a process for
producing the aluminum-cont~ining oxide or the aluminum-
contAining metal composition of any one of the first tofifth embodiments, which comprises rapidly cooling a melt
having a composition Al~ 2Bi~M1~lM2~2 (wherein x is
defined by 0.0001 S x ~ 0.10, Ml is at least one selected
2~'5~8Z
1 from Si, P, B, Sb, Se, Te, Sn, Zn, In, Cr, Nb, Sc, Y, Sr,
Ba, Ca, Na, Li, Mg, Mn, W, Ti, Zr, Hf, Be and rare earth
metals, M2 is at least one selected from Fe, Ni, Co, Rh,
Ru, Re, Cu and Pb, yl is defined by 0 ~ yl ~ 0.1, y2 is
defined by 0 ~ y2 s 0.01, and x, yl and y2 are each an
atomic ratio~ to coagulate the melt, and then oxidizing
the resultant coagulation product.
The eighth invention relates to a crystalline
aluminum-contAining oxide obtained by subjecting the
above amorphous aluminum-contAining oxide or the above
aluminum-contAining metal composition to a heat
treatment.
The ninth embodiment relates to a molded
article of a crystalline aluminum-contAining oxide
obtAine~ by subjecting a molded article of an aluminum-
contA i n i ng oxide to a heat treatment.
When x in the above Al~ 2Bi~Ml~lM2~2 composition
(in which x, yl, y2, Ml and M2 are as defined above) is
less than 0.0001, the molding at a low temperature is
dlfficult. When x exceeds 0.10, the electrical
insulation decreases. The range of x is preferably 0.001
inclusive to 0.005 inclusive, more preferably 0.002
inclusive to 0.02 inclusive. Ml and M2 may be absent, or
may be incorporated as required. When, however, yl
exceeds 0.1 and y2 exceeds 0.01, the electrical
insulation decreases.
Although differing depending upon the oxidation
conditions, in view of low-temperature moldability, the
2(~t;S~ r~
-- 7 --
1 value of z in the above composition is in the range of
1.2 ~ z < 1.5, preferably 1.3 ~ z ' 1.495, more
preferably 0.4 s z s 1.49. However, the value of z is
rigorously determined by correcting the amount of oxygen
adsorbed on and taken into a sample when measured.
As a preferred process for producing the
aluminum oxide powder of the present invention, a melt
having the composition of A~ _,2Ri-Ml~lM2~2 (wherein 0.0001
< x ~ 0.10, each of Ml and M2 is at least one selected
from the above-described elements, 0 ~ yl < 0.1, 0 < y2 <
0.01, atomic ratio) is rapidly cooled for coagulation,
and then oxidized. Raw materials, aluminum, metallic
bismuth, Ml and M2 may contain a small amount of
impurities.
The rapid cooling rate is preferably not less
than 103~C/~econd, more prefe~ably not less than
104~C/second. As a rapid cooling and coagulation method,
there is available a gas atomizing method, a high-
pressure water atomizing method, a method in which a melt
is rapidly cooled by allowing it to collide against a
rotor, a rotational electrode method, a method which is a
combination of the gas atomizing method with the method
in which a melt is rapidly cooled by allowing it to
collide against a rotor. The gas used in the gas
atomizing method i8 preferably selected from gases which
are not reactive with a melt used in the present
invention, such as argon, helium and nitrogen gases, or
gases having low reactivity. The gas may contain a small
Z~5S3~32
-- 8 --
1 amount of oxygen and water.
The gas atomizing method is a rapid cooling and
coagulation method in which a melt mixture of metal
aluminum into which a trace amount of bismuth has been
incorporated (and Ml and M2 have been incorporated as
required) (to hereinafter be referred to as ~present
melt') i5 jetted through a nozzle and atomized with a
high-velocity gas stream to form fine particles. The
low-temperature and high-velocity gas can be obtained by
a method in which a high-pressure gas is adiabatically
pAnde~ or a method in which a liquefied gas is jetted.
The high-pressure water atomizing method is a
ràpid cooling and coagulation method in which high-
pressure water is allowed to collide against a melt
~etted through a nozzle to atomize the melt.
In the method in which a melt is rapidly cooled
by allowing it to collide AgAinRt a rotor, the present
melt flows out through a nozzle on a slit and is fed onto
a rotor having a low-temperature surface to rapidly cool
and coagulate it, whereby a ribbon-shaped thin piece is
obtA~ne~ A preferred rotor used in this method is a
rotor which has the form of a drum, roll, disk, etc., and
which is made of a metal, etc., having good thermal
conductivity. The rotation rate of the rotor in the
position where the present melt collides against it is
preferably 100 to 100,000 m/minute, more preferably 200
to 10,000 m/minute. The surface temperature of the rotor
is preferably not more than 200~C, more preferably not
2~'5S~
1 more than 100~C. In the method which is a c~- ~inAtion of
the gas atomizing method with the method in which a melt
is rapidly cooled by allowing it to collide against a
rotor, the present melt is rapidly cooled by jetting it
through a nozzle to atomize it with a high-velocity gas
stream, and ; -~iAtely allowing the resultant atomized
droplets to collide against a rotor having a low-
temperature surface.
The rapidly cooled and coagulated aluminum-
contAining alloy used in the present invention easily
undergoes oxidation when brought into contact with an
oxygen-contA i n i ng gas. In general, a rigid oxide layer
is formed on an aluminum surface due to oxidation.
Therefore, no oxidation proceeds inside the aluminum.
Surprisingly, however, the aluminum alloy COntAi ni ng a
trace -~unt of bismuth used in the present invention
undergoes oxidation even at a low temperature until
aluminum in~ide the alloy is oxidized, and the alloy
forms a substantially amorphou~ aluminum-contAining
oxide. The oxygen-cont-Aining gas for the oxidation is
~elected from air, oxygen or a gas mixture of oxygen with
an inert gas of argon or nitrogen. Although the oxy~en
concentration of the o~y~en-contAining gas is not
specially limited, air is preferred in view of its ease
of handling. The temperature at which the aluminum alloy
is brought into contact with the oxygen-contA i n ing gas
may be ambient temperature. The oxidation may be
accelerated by heating the alloy as required, or may be
2~538~
1 moderated by cooling it. When an oxygen-contAining gas
which also contains a small amount of water is used, the
rate of forming a powder can be accelerated. According
to the method of rapidly cooling the present melt to
coagulate it, a coagulation product is obtained in the
form of a ribbon, a milled fragment or a powder. The
coagulation product in the form of a ribbon, a milled
fragment, or the like is formed into a powder by only
oxidizing it without mechanically milling it. The
coagulation product may be further finely milled, or the
time for fc_ ing a powder may be decreased by the use of
a ball mill, etc., as required. When the value of x in
the All~ 2Bi~MI~lM2~2 of the present invention is not less
than 0.001, it is easily formed into a powder by
oxidation. When the value of x is not less than 0.002,
it becomes a powder having a large surface area and an
average particle diameter of not more than 100 microns
when it is only allowed to ~tand in air at room
temperature.
The aluminum-cont~ining oxide of the present
invention preferably contains a small amount of bismuth
and/or aluminum in a metallic state, and these metals can
be incorporated if the conditions (temperature, time,
etc.) are properly ~elected. The amounts of both
aluminum and bismuth in a metallic state are preferably
not more than 0.1 and not more than 0.02 in terms of
atomic ratio, respectively, in view of electrical
insulation.
ZC~3S,~.
1 The aluminum-cont~ining oxide of the present
invention is substantially amorphous, and it can be
rendered crystalline by heat treatment at a high
temperature. For example, when heated at 700~C for 8
hours, it becomes a crystalline alumina. When this heat
treatment is carried out in an inert gas, a crystalline
alumina can be produced which contains a small amount of
metallic bismuth and/or metallic aluminum.
In the measurement of the average particle
diameter of the aluminum-cont~ining oxide powder of the
present invention, usually, 100 pieces of the powder
particles are measured with a scAnning electron
microscope, and the average particle diameter is
expressed as an average of the measurement values
obtAined. The average particle diameter of the aluminum-
contA1n1ng oxide powder of the present invention for a
molded article is preferably not more than 100 microns.
When the average particle diameter exceeds 100 microns,
the resultant molded artlcle shows a decrease in
strength. The average particle diameter is preferably
0.1 to 30 microns.
As a method of producing a molded article from
the aluminum-contAining oxide powder of the present
invention, a press molding method, a cast molding method,
a doctor blade method, an extrusion molding method, an
in~ection molding method, etc. can be used. The
al, i - contAining oxide powder may be molded in the
absence of a known binder, such as a resin, etc.
2~ 32
- 12 -
1 However, such a binder may be incorporated as required.
The powder of the present invention can be
press-molded by heating it at a low temperature even
without using any binder. The heating temperature is
preferably between 250~C and 700~C, particularly
preferably between 300~C and 500~C. The pressure for the
press molding is preferably not less than 100 MPa
(megapascal), more preferably not less than 500 MPa. The
so-obtA i ned molded article has a high hardness and high
electrical insulation properties. When the molded
article is further heated at a high temperature of 700~C
or higher, there can be obtAined an article of a
crystalline aluminum-cont~ining oxide which has a higher
strength, a higher hardness, higher electrical insulation
properties, etc.
The aluminum-contA i n i ng oxide powder of the
present invention can be molded at a low temperature
without incorporating any binder. According to the
present invention for producinq the aluminum-contAin~ng
oxide powder, a finely milled powder can be obt~ined by
only oxidation without proceeding with any special
milling step. The aluminum-contAining oxide and the
powder and molded article therefrom, provided by the
pre~ent invention, are useful as or for a machine part,
an electronic part and a raw material for a catalyst.
EXAMPLES
The present invention will be specifically
2('~ ~8~
- 13 -
1 explained hereinafter by reference to the Examples.
Example 1
17.6 Grams of a metaliic aluminum powder (with
a purity of not less than 99.9%, supplied by High Purity
Chemicals Co., Ltd~ and 0.48 g of metallic bismuth (with
a purity of not less than 99.9~, supplied by High Purity
Chemicals Co., Ltd) are fused and mixed in an arc
furnace, filled into a silica tube having a nozzle
(nozzle diameter 5 mm~), and melted by high-frequency
induction heating. The melt was jetted to a metal roll
(made of copper, diameter 200 mm, width 10 mm) rotating
at 3,000 rpm and having an ordinary temperature in an
argon atmosphere at a pressure difference of 0.2 kg/cm2.
The resultant ribbon-shaped thin fragments were allowed
to ~tand in the atmosphere at an ordinary temperature to
give a powder in 3 hours. The powder was observed with a
scAnning electron microscope and an average particle
diameter of 15 microns was measured (Fig. 1).
The contents (Al, Bi and O) of the aluminum-
contA;n1ng oxide of the present invention were determinedwith an ICP and a thermobalance. At first, the aluminum-
contA;ning oxide was weighed before a powder was formed.
A predetermined amount of the oxide was dissolved in
concentrated hydrochloric acid, and the resultant
solution was analyzed with an ICP (high-frequency,
inductively coupled plasma emission analyzer) to
determine the compositional ratio of Al and Bi.
2~S382
- 14 -
1 Furthermore, a powder obtained by allowing the aluminum-
cont~ining oxide to stand in an oxygen-containing gas
(e.g.~ air) was dried under vacuum at 300~C for l hour to
fully l~- -,ve water, and a predetermined amount of the
powder was dissolved in a solvent. The resultant
solution was measured with an ICP, and the amount of
oxygen was calculated from the balance of Al and Bi.
The above-obtained powder had a compositional
ratio of Al0~9965Bio~36Ol~4l (atomic ratio). Although the
measurement of the powder by X-ray diffractometry showed
the presence of trace amounts of crystals of metallic
bismuth (0.0001) and metallic aluminum (0.0589), the
powder was amorphous as a whole (Fig. 2).
Example 2
17.6 Grams of a metallic aluminum powder and
0.41 g of a metallic bismuth powder were mixed, and
rapidly cooled and coagulated in the ~ame manner as in
Example 1. The resultant ribbon-shaped coagulation
product was allowed to stand in the atmosphere for 2
hours to form a powder. The powder had an average
particle diameter of 17 microns. The powder was measured
for a specific surface area by a BET method using
nitrogen. The powder measured 16 m2/g. Although the
X-ray diffraction showed the presence of small amounts of
crystals of metallic bismuth 0.0001 and metallic aluminum
0.033, the powder was amorphous as a whole. The powder
had a composition, determined by ICP, of Al0.997BiO.003O1.4S
- 15 - 2~3~3~
1 (atomic ratio).
Example 3
17.6 Grams of a metallic aluminum powder and
0.27 g of a metallic bismuth powder were mixed, and
rapidly cooled and coagulated in the same manner as in
Example 1. The resultant ribbon-shaped coagulation
product was allowed to stand in the atmosphere overnight
to form a powder. The powder had an average particle
~i~ Ler of 20 microns and a composition of Alo.g9~Bio.oo2ol.4
(atomic ratio). Although the X-ray diffraction showed
the presence of small amounts of crystals of metallic
bismuth 0.0001 and metallic aluminum 0.066, the powder
was amorphous as a whole.
Example 4
11 Grams of a metallic aluminum powder and
0.085 g of a metallic bismuth powder were mixed, and
rapidly cooled and coagulated in the same manner as in
Example 1. The resultant ribbon-shaped coagulation
product was allowed to stand overnight in the atmosphere
at 100~C to give a powder. The powder had an average
particle ~ -Ler of 30 microns and a composition of
Alo.ggsBio.oolol.~9 -
Example 5
11 Grams of a metallic aluminum powder and
0.425 g of a metallic bismuth powder were mixed, and
S~8~
- 16 -
1 rapidly cooled and coagulated in the same manner as in
Example 1. The resultant ribbon-shaped coagulation
product was allowed to stand in the atmosphere to form a
powder. The powder had an average particle diameter of
10 microns and a composition of AlosssBio.oos~l.4s (atomic
ratio). Although the X-ray diffraction showed the
presence of a small amount of a crystal of metallic
bismuth (0.0066), the powder was amorphous as a whole.
Example 6
11 Grams of a metallic aluminum powder and 0.85
g of metallic bismuth were mixed, and rapidly cooled and
coagulated in the same -nner as in Example 1. The
resultant ribbon-shaped coagulation product was allowed
to stand in the atmosphere to form a powder. The powder
had an average particle diameter of 10 microns and a
composition of AloggBio~olol~49 (atomic ratio). Although the
X-ray diffraction showed the presence of a small amount
of a crystal of metallic bismuth (0.0066), the powder was
amorphouR as a whole.
Example 7
11 Grams of a metallic aluminum powder and 0.2
g of a metallic bismuth powder were mixed, and rapidly
cooled and coagulated in the sam0 manner as in Example 1.
The resultant ribbon-shaped coagulation product was
allowed to stand in the atmosphere to form a powder. The
powder had an average particle diameter of 10 microns and
2~5~82
- 17 -
1 a composition of Alo.ss77Bio.00~~l.4s
Example 8
17.6 Grams of a metallic aluminum powder and
4.94 g of a metallic bismuth powder were mixed, and
rapidly cooled and coagulated in the same manner as in
Example 1. The resultant ribbon-shaped coagulation
product was allowed to stand in the atmosphere for 3
hours to form a powder. The powder had an average
particle ~i r -ter of 8 microns and a composition of
Alo~965Bio.o3sol.~8 [atomic ratio). Although the X-ray
diffraction showed the presence of a small amount of a
crystal of metallic bismuth (0.0133), the powder was
amorphous as a whole.
Example 9
17.6 Grams of a metallic aluminum powder and
0.1 g of a metallic bismuth powder were mixed, and
rapidly cooled and coagulated in the same ~nner as in
Example 12. When the resultant ribbon-shaped coagulation
product was allowed to ~tand in the atmosphere overnight,
the ribbon-shaped fragments were partially formed into a
powder. The ribbon-shaped portion and the powder portion
were mixed, and the mixture was measured. The measured
composltion wa8 Alo.sss3Bio.ooo7ol.3s
Example 10
An aluminum~cont~ in i ng oxide powder prepared in
2~S~2
- 18 -
1 the same manner as in Example 1 was dried under vacuum
and charged into a die having a cavity having a diameter
of 5 mm and a length of 50 mm (maraging steel), and the
powder was vacuumed (2 x 10-3 torr) at room temperature
for 30 minutes. Thereafter, the temperature in the die
was increased up to 380~C over 30 minutes, and the
pressure of 900 megapascal was applied for 10 minutes.
The temperature was decreased to room temperature with
va~u, i ng, and the resultant molded article was taken
out. The molded article was a disk having a diameter of
5 mm and a thickness of 1.5 mm. The disk had a Vickers
hardness, measured under a load of 100 g, of 318 kgf/mmZ.
The electric resistance thereof, measured according to
JIS K6911-1979 5.13, was 1.1 x 10l2 Q-cm. The thermal
conductivity thereof was 0.25 cal/cm-s-~C.
Example 11
11 Grams of a metallic aluminum powder and 8.55
g of a metallic bismuth powder were mixed, and rapidly
cooled and coagulated in the same ~nner as in Example 1.
The resultant ribbon-shaped coagulation product was
allowed to stand in the atmosphere to form a powder. The
powder had an average particle diameter of 10 microns and
a cGmposition of Al0.9Bio.lOl.~8 (atomic ratio). Although
the X-ray diffraction ~howed the presence of a small
amount of a crystal of metallic bismuth (0.0133), the
powder was amorphous as a whole.
The powder was molded with the same device as
- 19 - Z~55 ~
1 that described in Example 10 under the same conditions as
those in Example 1. The molded article had a hardness of
340 kgf/mm2. The electric resistance thereof was 5.6 x
10l~ Q-cm.
S Example 12
The powders obtained in Examples 2 to 9 were
molded with the same shaping device as that described in
Example 10 at a temperature of 250 to 400~C under a
pressure of 500 to 1,000 megapascal. Before use,
however, the ribbon-shaped coagulation product obt~ined
in Example 9 was milled with a ball mill until it had an
average particle ~ic -Ler of 10 microns. All of the
resultant molded articles had a hardness of 300 kgf/mm2 or
higher.
Example 13
220 Grams of metal aluminum and 6 g of metallic
bismuth were mixed, and the mixture was charged into a
silica crucible (having a nozzle) ancl melted up to
1,100~C by high-frequency induction heating in a nitrogen
atmosphere. The resultant melt was jetted through the
nozzle under a nitrogen atmosphere for 10 seconds. At
the same time, nitrogen contained in a cylinder (cylinder
pressure 150 atmospheres pressure) was jetted through a
peripheral nozzle at 1.7 NTPm3 against the melt being
jetted. The resultant powder was observed with a
scanning electron microscope to show the form of spheres
5538,~
- 20 -
1 (average particle diameter 35 microns). When the powder
was allowed to stand in the atmosphere for one day at
100~C, there was obtained a fine powder having an average
particle diameter of lO microns. This fine powder was
subjected to X-ray diffractometry to show the presence of
small amounts of crystals of metallic bismuth (0.002) and
metallic aluminum (0.018). However, the fine powder was
amorphous as a whole. The fine powder had a composition
of Al0~9965Bio~0035Ol47 ~atomic ratio).
Example 14
The molded article obtained in Example 8 was
calcined at a temperature of 1,000~C in the atmosphere,
and the calcined article was measured for a Vickers
hardness and an electric resistance according to the
methods de~cribed in Example 8. The hardness and
resistance were 900 kgf/mm2 and 1.5 x 1014 Q-cm,
respectively.
Example 15
Aluminum oxides of Al0994Bio~oo5Mlo~oolo~ were
prepared in the same manner as in Example 1. Si, B, Sn
and Mn were used as Ml. The ribbon-shaped coagulation
products obtained were allowed to stand in the atmosphere
for one day to form powders. The value of Y measured
from 1.45 to 1.48. These powders were molded with the
same shaping device as that described in Example 10 under
the same conditions as those in Example 10. All of the
Z~SS3~
- 21 -
1 resultant molded articles were rigid articles having a
hardness of 300 kgf/mm2 or higher.
Example 16
Aluminum oxides of Alo.994Bio~w~sio~lM2o~l were
jetted in the same manner as in Example l. Fe, Ni and Pb
were used as M2. The resultant ribbon-shaped coagulation
products were allowed to stand in the atmosphere for one
day to form a powder. The powders obtained had a
composition of Al0,994Bio,~KSio~lM2o~lo~ in which z was 1.44
to 1.49. These powders were molded with the same shaping
device as that described in Example 10 under the same
conditions as those in Example 10. All of the resultant
molded articles were rigid articles having a hardness of
300 kgf/mm2 or higher.
COMPARATIVE EXAMPLES
Comparative Example 1
~ commercially available aluminum oxide powder
(a-alumina hzving a diameter of 2 to 3 microns, ~-alumina
having a diameter of 2 to 3 microns, with a purity of
99.9% or higher) was molded with a shaping device shown
in Example 8 at a temperature of 400~C under a pressure
of 1,000 megapascal. The molded article taken out of the
die was easily disintegrable and could not be measured
for a Vickers hardness. The powder was therefore
substantially unmoldable.
- 22
1 Comparative Example 2
Metallic aluminum without metallic bismuth was
rapidly cooled and coagulated in the same -nn~r as in
Example 1 to give ribbon-shaped thin fragments. These
fragments were allowed to stand in the atmosphere at an
ordinary temperature for one day. However, no formation
of a powder took place. The result of the X-ray
diffraction thereof showed that these were in the state
of metallic aluminum.
Even when this sample was heated in the
atmosphere at 500~C for 3 hours, no formation of a powder
took place. X-ray diffraction results showed that the
oxidation of the metallic aluminum hardly took place.
C ~-rative Example 3
A melt of Al~ (in which M was at least any of
Mo, Si, Pb, Zn, Sn and Mn) and in which x was O.OOS
(atomic ratio) was rapidly cooled and coagulated in the
same manner as in Example 1 to give ribbon-shaped thin
fragments. The~e fragments were allowed to stand in the
atmosphere at an ordinary temperature for one day.
However, no formation of a powder took place. X-ray
diffraction results thereof showed that these were in the
state of metallic aluminum, and the formation of a powder
hardly took place.
As is clear from the above, the phenomenon that
aluminum is oxidized at a low temperature to form a
powder is a specific one which take~ place only when
- 23 -
1 bismuth has been incorporated. 2 ~ ~S~~
~U~l~IAL UTILITY
The aluminum-cont~ining powder of the present
invention can be molded at a low temperature, and is
characterized in that said powder can be obt~i~ed by
simple oxidation without proceeding with a special
milling step. The aluminum-cont~ining oxide and the
powder and article therefrom are useful as or for a
machine part, an electronic part and a raw material for a
catalyst, etc.