Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
5 ~ æ ~
HOECHST ARTIENGESELLSCHAFT HOE 90/F 37~ Dr.Sl/pe
Description
Agistatins, novel meta~olites from Fusarium 8p., a
process for their pr~paration, and their use
It has been found that Fusaxium strains can produce novel
metabolites, the agi~ta~ins, which have pharmacological
and theref ore therapeutic activity and can be employed
particularly advantageou~ly ~8 cholesterol biosynthesi~
inhibitors having corresponding pha~m~cological utility.
The invention therefore relates to:
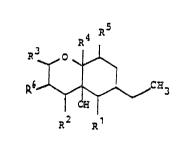
1. A process for the preparation of the com~ounds of
the formula I
~5
~ I
in which
is hydrogen or hydroxyl r
R2 is hydroxyl or an oxo group,
R3 is methoxy or hydroxyl or, together with Rl, forms an
eth~r bridge, and
R4 is hydrogen or hydxoxyl,
R5 is hydrogen or, together with R4, is an additional
bond, and
R5 is hydrogen or, together with R3, i an additional
bond ,
which comprises culturing Fusarium DSM 5983 in a
nutrient medium until the compounds of the formula I
are accumulated in the culture.
~7~ ~
2. A use of the compound of the formula I a~ choles-
terol bio~ynthesis inhibitors, and pharmacological
utilization thereof.
The following text will describe the invention in detail,
in particular in its preferred embodLmen~s. The ~nvention
is furthermore determined by the con~ents of the patent
claLms.
The compounds according to the invention ar~ preferably
produced with ~usarium sp. DSM 5983. The strain was
isolated from a soil sample from Hawaii and deposi~ed on
June 6, 1990, under the abovementioned number, at the
Deutsche Sammlung von Nikroorganismen ~German Collection
of Microorganisms] in compliance with the provi~ions of
the Budapest Convention.
The strain Fusarium sp. DSM 5983 has a white aerial
mycelium and white conidia. It forms ~he macro- and
microconidia and chlamydospores which are characteristic
of Fusaria.
In a nutrient solution which contains a carbon source and
a nitrogen sour~e as well as the con~entional inorganic
salts, Fusarium sp., preferably DSM 5983, produce~ the
compounds of the formula I. Instead of strain DSM 5983,
it is, of course, also possible to 4mploy the mutant~ and
variants thereof, a~ long a~ they synthesize this ~om-
pound. Such mutants can be produced in a manner known per
se using physical means, for e~ample irr~diation, such as
with ultraviolet rays or ~-ray~, or chemi-~al mutagens,
for example ethyl methanesulfonate (E~S), 2-hydroxy
methoxybenzophenone ~OB~ or N-methyl-N~-nitro-N-nitroso-
guanidine (MNNG).
Preferred car~on ~ources which are 5uitable for the
aerobic fermentation are carbohydrate and ~ugar alcohol~
2~7~ ~
~ 3 --
which can be assimilated, such as glucose, lactose or
D-mannitol, and carbohydrate-containing natural product~,
such as malt extract. The following are suitable as
nitrogen-containing nutrients: amino acids, peptides and
proteins, and their degradation produc~s such as peptones
or tryptones, furthermore meat extracts, ground seeds,
for example of maize, wheat, beans, soybeans or of the
cotton plant, distillation residue~ of alcohol produc-
tion, meat meals or yeast extracts, but also ammonium
salts and nitrates. Inorganic ~alt6 which the nutrient
solution may contain are, for example, chlorides, carbo-
nates, sulfates or phosphates of the alkali metals or of
the alkaline earth metals, iron, zinc, cobalt and
manganese.
~he compounds of the formula I are particularly readily
formed in a nutrient solution which contains approxi-
mately 0.2 to 5 ~, preferably 1 to 4 %, of malt extract
and 0.02 to 0.5 %, preferably 0.1 to 0.4 ~, of yeast
extract, as well as 0.2 to 5.0 %, preferably 0.5 ~o 2 %,
of glucose and 0.01 to 0.1 ~, preferably 0.04 to 0.04 to
0.06 %, of (NH4)2HPO4, in each case based on the weight of
the entire nutrient solution. Culturing is carried out
under aerobic conditions, that i~ to say, for example,
submerse conditions with shaking or stirring in shaker
~5 flasks or fermenters, if necessary while passing in air
or oxygen. Culturing can be carried out in a temperature
range from approximately 15 to 30C, preferably at
approximately 20 to 30C, in particular at 23 to 28C.
The pH range should be between 1 and 7, advantageously
between 2.5 and 4.5. In general, the microorganism is
cultured under these conditions over a period of 24 to
300 hours, preferably 3Ç to 140 hours.
It is advantageous to carry out the culture in several
steps, i.e. one or more precultures are first e~tablished
in a liquid nutrient medium, and the actual production
medium, the main culture, is then inoculated with these,
for example in a ratio by volume of 1:10. The preculture
r,~
-- 4
is obtained, for example, by inoculating a nutrient
solution with a sporulating mycelium and allowing it to
grow for approximately 36 to 120 hours, preferably from
48 to 72 hours. The sporulating mycelium can be obtained
for e~ample by allowing the strain to grow for apprvxi-
mately 3 to 40 days, preferably 4 to 10 days, on a solid
or liquid medium, for example yeast/malt agar or potato/
dextrose agar.
The course of the fermentation can be monitored with the
aid of the pH of the culture or of ~he volume of myce-
lium, and also by chromatographic methods, for example
thin-layer chromatography or high pressure liquid chroma-
tography, or by assays of the biological activity. The
compounds of the formula I are contained both in the
mycelium and in the culture filtrate.
~he compounds mentioned are isolated from the culture
medium by known methods, taking into account the chemi-
cal, physical and biological properties of the products.
For an assay of the metabolite concentration in the
culture medium or in the individual isolation steps, it
is possible to use thin-layer chromatography, for example
on silica ~el using butanol/glacial acetic acid/water or
ethyl acetate/methanol/water mixtures as the eluent. In
the case of separation by means of thin-layer chromato-
graphy, detection can be effected, for example, bystaining reagents such as anis~ldehyde, or by bioassays,
for example as a cholesterol biosynthesis inhibitor, the
amount of substance formed advantageously being compared
with a calibration solution.
To isolate the compounds I, culture liquor and mycelium
are first extracted with non-polar organic solvents, for
example n-hexane, petroleum ether or halogenated hydro-
carbons such as, for example, chloroform etc., so as to
remove the non-polar impurities. This is followed by
extraction with a more polar organic solvent, for example
lower alcohols, acetone and/or ethyl acetate, and also
- 5 _
mixtures of these solvents.
The isolation of the pure compounds of the formula I is
carried out on sui~able materials, preferably, for
example, on silica gel, aluminum oxide, ion exchangers or
adsorber resins, followed by elution with organic, polar
solvents or solvent mixtures, fo.r example alkyl acetates,
mixtures of alkyl acetates with a lower alkanol, chloro-
form or methylene chloride, or mixtures of these 801vent8
with lower alkanols, if appropriate also with water, or
a pH or salt gradient which is ~uitable for ion-exchanger
resins, for example sodium chloride or tris(hydroxy-
methyl)aminomethane HCl (tris buffer), and combination of
the biologically active fractions.
In the solid state and in solutions in the pH range
between 1 and 9, and particularly 3 and 7, the compounds
of the formula I are stable and can therefore be
incorporated in pharmaceutical preparations.
The compounds according to the invention can be used as
lipid regulators, in particular for inhibiting the
biosynthesis of cholesterol.
The compounds of the formula I can be used for the
prophylaxis and treatment of diseases which are based on
an increased cholesterol level, in particular of coronary
heart diseases, arteriosclerosis and sLmilar diseases.
The use also relates to pharmaceutical preparations of
the compounds of the formula I.
In the preparation of pharmaceuticals, it is also pos-
sible to use pharrnaceutically acceptable additives, such
as diluents and/or excipients, in addition to the active
substance. These are underskood as meaning physiologic-
ally acceptable substances which, after having been mixed
with the active substance, bring the latter into a form
which is suitable for administration.
-- 6 --
Examples of suitable solid or li~uid pharmaceutical
preparation forms are tablets, coated tablets, powders,
capsules, suppositories, syrups, emulsions, suspensions,
drops or solutions for injection, as well as preparations
with a protracted release of active substance. Examples
of excipients or diluents which are frequently used are
various sugars or types of starch, cellulose derivatives,
magnesium carbonate, gelatin, animal and vegetable oilR,
polyethylene glycols, water or other suitable solvents,
and also buffers which contain water and which can be
made isotonic by adding glucose or salts.
In addition, other additives which can be used in the
pharmaceutical preparations according to the invention
are, if appropriate, surface-active agents, colorants and
flavorings, stabilizers and al~o preservatives. It is
also possible to use pharmacologically acceptable poly-
meric excipients, for example polyphenylpyrrolidone, or
other pharmaceutically acceptable additives, for example
cyclodextrin or polysaccharides. In particular, the
compounds can also be co~bined with additives which bind
bile acid, in particular non-toxic/ basic anion exchan-
gers which cannot be absorbed in the gastrointestinal
tract.
The preparations can be administered orally, rectally or
parenterally. The preparations can preferably be prepared
in dosage units; examples of suitable dosage units are,
in particular, tablets, capsules or suppositories. Each
dosage unit, in particular for oral administration, can
contain up to 1000 mg, but preferably 10 to 100 mg, of
~0 the active ingredient. However, it is also possihle to
use dosage units which are above or below this amount and
which, if required, are to be divided or multiplied prior
to administration.
If appropriate, the dosage units for oral administration
can be microencapsulated so as to slow down release, or
extend it over a longer period, for example by coating
or embedding the particula~e active substance in suitable
polymers, waxes or the like.
Parenteral administration can be effected using liquid
dosage forms, such as sterile solutions and suspensions
which are intended for in~ramuscular or subcutan~ous
injection. Dosage forms of this type are prepared by
dissolving, or suspending, a measured amount of active
substance in a suitable physiologically acceptable
diluent, for example an aqueous or oily medium, and
sterilizing the solution, or ~he suspension, if
appropriate with the concomitan~ use of suitable
stabilizers, emulsifiers and/or preservatives and/or
antioxidants.
The preferred administration form, in particular with
regard to long-term therapy, i8 the oral adminis~ration
form, which facilitates the preven~ion and therapy of the
abovementioned diseases substantially.
The pharmaceutical preparations are prepared by generally
customary processes. The dosage schedule may depend on
the type, age, weight, sex and medical condition of the
patient or person at risk.
The examples which follow will describe the invention in
greater detail. Percentages are by weight, unless other-
wise defined.
Examples:
1. a) Preparation of a spore suspension of the
producer strain:
100 ml of nutrient solution (60 g of malt extract, 2 g of
yeast extract, 10 g of glucose, 0.5 g of (NH4)2HPO4 in 1 1
of tap water, pH before sterilization 6.0) in a ~terile
500 ml Erlenmeyer flask are inoculated with strain DSM
5983 and the culture is incubated on a rotary shaker for
-- 8 --
72 hours at 25C and 140 rpm. In a sterile 500 ml
Erlenmeyer flask, 20 ml of culture liquid are
subsequently uniformly distributed in the nutrient mQdium
(potato infusion 4.0 g/l [infusion of 200 g of potatoes],
20.0 g/l D-glucose, pH before sterilization 5.6~, to
which 15 g of agar/l had additionally been add2d for
solidification, and the mixture is decanted. The cultures
are incubated for 10 to 14 days at 25C. The spores of
one flask which have been formed after this time are
rinsed with 500 ml of demineralizad water containing one
drop o~ a commercially available non ionic surfactant
(for example Triton0 X 100, manufactured by Serva), and
either reused immediately or stored in 50 % glycerol at
-22C-
5 b) Production of a culture, or precul~ure, of theproducer strain in an Erlenmeyer flask
A sterile 500 ml Erlenmeyer flask containing
100 ml of the nutrient solution described under a) is
inoculated with a culture which has been yrown on agar
slants or with 0.2 ml of spore suspension, and the
mixture is incubated on a rotary shaker at 140 rpm and
25C. Maximum production of the compounds of the formula
I is attained after approximately 120 hours. To inoculate
10 and 100 l fermenters, it is sufficient to use a
72 hour old submerse culture (inoculum approximately 5 %)
from the same nutrient ~olution.
2. Preparation of the compounds of the formula I:
A 10 l fermenter is operated under the following condi-
tions:
0 Nutrient medium: 20 g/l of malt extract
10 g/l of glucose
2 g/l of yeast extract
0,5 g/l of (NH")2HPO4
pH 6.0 (before sterilization)5 Incubation time: 120 houra
2~
Incubation
temperature: 25C
5tirring speed: 200 rpm
Aeration: 2 1 of air/min.
Foam formation can be suppressed by repeatedly adding a
few drops of ethanolic polyol solution. Maximum produc-
tion takes place after approximately 96 hours. The pH
during harvesting is approximately 3.5. The yields ar~
approximately 80 mg/l of the compounds of the formula I.
3. Isolation of the compounds of the formula I:
When the fermentation of DSM 5983 has ended, the culture
liquor is filtered with the addition of approximately 2 %
of filter aid (for example Celite~. Working-up can be
effected following the diagrams below:
lo ~ ~ ~ 7 ~ ;3~
Working-up/i~olation
Diagram 1: Culture supexnatant
_
Culture
_
Filtration ~-~ Culture supernatant
Adsorption on Amberlite XAD-16
(20-25% by volume of the amount applied)
_ ~
Washing with water (50 % by volume)
Elution with 80 % MeOH
/
Crude product
. I ,
ND
silica gel
CHCl3/MeOli ( 50 : 1 )
Sephadex LH-20
in MeOH
Silica gel
CHCl3/NeOH/n-hexane
(20 : 1 : 5)
Compounds of the formula I
Working-up/isolation
Diagram 1: Mycelium
-r3
~L
NycelLum I
Ext lction with
acetone
~3
I ~
Chromatography on silica gel
CHCl3/MeOH (50 : 1)
/
Sephadex LH-20
in MeOH
Silica gel
CHCl3/MeOH/n-hexane
(20 : 1 : 5)
Compounds of the formula I
7 ~
- 12 -
4. Bioassay as cholesterol biosynthesis inhibitors:
a) In-vitro determination:
HEP-G2 cell monolayers in lipoprotein-free nutrient
medium are preincubated for one hour with appropriate
concentrations of the test substances of the formula I.
The l4C-tagged biosynthesis precursor [14CJsodium acetate
is added, and incubation is then continued for 3 hours.
After this r some of the cells are subjected to alkaline
hydrolysis after previously adding an internal ~tandard
of 3H cholesterol. The lipids of the hydrolyzed cells are
extracted with a chloroform/me~hanol mixture. Support
cholesterol is added, and this lipid mixture is separat~d
by preparative thin-layer chromatography, the cholesterol
band is stained and then isolated, and the amount of
14C-cholesterol formed from the 14C-precursor is determined
by scintigraphy. In an aliquot of the cells, cell protein
was determined, so that the amount of 14C-cholesterol
formed from l4C-precursor per mg of cell protein can be
calculated per cell unit. To compare the inhibitory
action of an added test preparation, the control is used,
so that the inhibition of cholesterol biosynthesis at a
particular molar concentration of the test preparation in
~he medium can be indicated directly. In aliquots of the
cell culture, the integrity of the cell culture and the
absence of damage to cells caused by action of prepara-
tions is assessed morphologically (light microscopy) and
measured biochemically by determining the secretion of
lactate dehydrogenase into the incubation medium.
Lovostatin was used as standard preparation. The extent
of cholesterol biosynthesis inhibition by the compounds
of the formula I where R = H is, at a concentration of
10-8 mol/l, 41 %.
~he inhibition of chole~terol biosynthesis at 10-7 ~ol/l
lovostatin is 74 %, and at 10-8 mol/l 48 %.
- 13 - 2~$~
b) In-vivo determination:
Inhibition of hepatic cholesterol biosynthesis effect
the reduction of serum lipids, as can be demonstrated in
chronic tests on male rats. The tes~ preparations in
polyethylene glycol 400 were administered daily in the
morning to groups of male rat~ of the strain HOE: WISKf
(SPF 71) of starting weight of approximat01y 240 g, by
gavage, the control group in question only receiving the
vehicle. 24 hours after the last administration and after
24 hours~ fasting, blood samples were taken, and the
lipoproteins in the serum obtained from a pooled group of
rats were separated by means of the preparative ultra-
centrifuge technique. In ~his process, the following
density limi~s were used for the separation of VLDL, LDL
and HDL:
VLDL 1. 006
LDL 1.04
HDL 1.21
To determine the cholesterol and the triglycerides,
fully-enzymatic Boehringer/Mannheim methods were used,
and to determine the protein, Lowry and coworkers' method
was 1l sed.
The values measured for the compounds of the formula I in
comparison with clofibrate are listed below:
o
h
Cl~ U~
~ ~ o
tq ~ ,1
O ~t` o
h ~~ _
a) ;>l l
~ ~Ct~ o~
_I
~:1
~D CO
~ l
o~ o~
~:
S~ ~ r~ ~
a
~ ~ l
~n
~1 ~
~ o a D ~
o ~ ~ ~ U~
l l
a) ~
~n ~o o
o ~~ O
$ a ~ ,,
0 h
O ~ ~ .
~ r~
- 15 -
5.a) Characterization of the compound of the formula I
o~ ~
Agistatin A ~figure) was isolated as an amorphou6 solid
and is solu~le in CH2Clz, CHC13, ether, ethyl acetate,
acetone, ethanol and isopropanol. Agistatin ~ i8
insoluble in n pentane, n-hexane and cyclohexane.
Melting point: 73C
Optical rotation: [~ 2U = _ 36.2 (c = 0.9; methanol)
Thin-layer chromatography:
Silica gel 60, F254: chloroform/methanol (9:1
vsv) Rf 0.70
n-Butanol/acetic acid/water (top phase) (4:1:5,
v:v:v):Rf 0.75
Staining behavior: orcinol: brown; anisaldehyde/sulfuric
acid: brown.
EI MS: m/e = 242 (8 %, M~, high re~olution: 242.1154,
C12H1~Os), 224 (10 %, M-~2O), 210 (10~), 182 (32%) 168
t46%)~ 140 (24%3, 13g (~4~)~ 111 (64%~, ~5 (9
Molecular mass: 242-27 (C12Hl8O5)
IR (KBr): 34~0, 2960, 2920, 1730, 1880 cml
UV (MeOH): A = 203 nm ~ - 3250;224l1~00),261(1000)nm
+ HCL:~ = 203(~ = 4450);224~1750),271(900~nm
~NaOH:A = 211~ = 4700,271(1600,303(1450)nm
H NMR (200 MHz, CDCl3):
= 0~98(t,3H,J = 7v0 Hz,1 1'H3~ 32-1,84~m,2~,1~H2),1.85~m,1H, 6-H);
1,94-2.26(m,2H,7-H2);2944(d,lH,J6~H,6-3~5 Hz,~-OH); ~B2~dd,1H, J3~3b-
14,5 Hz, J3~,2= 2,5 Hz,3-Ha);2,76(s,1H,4a-OH);3,32(dd,1H,J3~ 14.5 Hz,
J3b2= 4 Hz,3-Hb);3.42(s,3H,g-H3);4,09(dd,1H,J~,~.~ s 3,5 andJ = 3,5 Hz,5-
H);5.13(dd,1H, J2,3b= 4 HZ~J2~a~= 2~5 Hz,2-H);5~61(dd,lH,J = 2~-andS.O Hz,
8-H).
.
.
~ ~ rj ~ d,
-- 16 --
13c NMR ( 50 MHz, CDCl3):
~ = 11,6 (q, G11); 24.3 (t, C-10~; 2~,1 (t, e-7); 34~(d,C-8);43~8(t,C-3);5~o~(q~
G9);71.5(d, G5),73~6(s,C-4a);100.U(~,C-2~ .3~,C-~);146~1(s,C-8a);
205.5(s,C-4).
Elemental analysis: calculatedO C 59.53 H 7.43
found: C 59.56 H 7.38
b) Characterization of the compound of ~he formula I:
1 ~o
HO ~ C~3
OH
Agistatin B (figure):
Empirical formula: C11H18O4
Molecular mass: 214.26
Consistency: colorless, cry~talline
Soluble in: MeOH, EtOH
Insoluble in: n-pentane, n-hexane
Melting point: l6lC
IR (KBr): 3440, 3400, 2940, ll~0, 1085, 930 cm
Elemental analy~is: found calculated
C 61.46 61.66
H 8.39 8.47
Optical rotations [~]DO = +38.2; (c = l.0)
Mass spectrum: m/z = 214 (22 %, Mt, high resolution:
2l4.l205, C1lH1ôO4)
196(4 %, M-18),186(4 %, M-28),170(6 %, M-44),167
(14 %, M-47),142(66 %, M-72),113(66 ~, M-101),
95(99 %, M-119)
3C NMR (50.3 MHz, CD30D):
,9 (q, C-11); 22,2 5t, C-10); 26.1 (t, C-7); 2~.~ (t, C:-8); 37,~; ~d, 1::-6);
40,8(t, G2);67,4(S, G4); 6908 (d, G3); 71.5 (d, G~)i 74.5 (d, C-9); 91,2 (d, C-l)
- 17 -
H NMR (500 MHz, CDCl3):
= 0"94 (t, 3H, Jt1,10= 7.~ Hz, 1 l-H~); 1.3~1,80 (m, 7H, 4tl, 7~Ha~ 8-H2, 10-H2);
1~97 (ddd, 1H, J2-.2b = 14 Hz, J2~,3 = 3.5 Hz, J2nl = 2 Hz, 2-Ha); 2.09 ~hr s, 1H, OH~;
2~14 ~br s, 1 H, OH); 2.55 (ddd, 1H, `12h,2. = 14 Hz, J2b,3 Z 10 Hz, J2b,1 = 2 Hz, 2-Hb);
3,81 (m, 1H, 9-H); 3.99 ~dd, lH, J3,2b - 10 Hz, J3,2~, = 3 ~z, 3-~1); 4,19 (br s, 1~1, 5
H); 4.96 (dd, 1H, J=2and 2 Hz, 1-H).
c) Characterization of the compound of +~he formula I
3 ~ CH3
C)H
OH
Agistatin D (figure):
Empirical formula: C11H14O4
Molecular mass: 2l0.23
Consistency: white powder
Soluble in: CH2Clz, CHCl3, ether, EtO~c, acetone,
EtOH, isopropanol
Insoluble in: n-pentane, n-hexane, cyclohe~ane
Melting point: 93C
UV~MeOH)nm l~): 201(31743,227(1132),289~6942)nm
(MeOH/HCI) (~): 202 (5234), 225 (2339), 288 (7128) nrn
(MeOH/NaOH) (~): 215 (8037), 274 (4770), 305 (4937) nm
IR (I<Br): 33~0 br, 2950, 29~0, 2860, 1~80, 1B5:), 1620, 1600,
1585, 1~10, 1280, 100~ cm
Optical rotation
(MeOH): [~]DO = ~252-4; (c = 0.55
Mass spectrum: m/z - 210 ~84 %, M+)
High resolution: calculated for C1lH14O4 2l0.0892
found 210.08937
- 18 ~ 7~
132(44 %,M-18),163~8~ %, M-47),13~100 %, M-71),
137(80 %, M-73),126~75 %, M-84~,71~87 %, M-139),
55(76 %, M-155)
3C NMR (50 MHz, d6-acetone):
9(q~C-11);25~1(t,C-9);26~7(t,C-7);35~8(d,C-~);70~4(d,C-5~;
72~1~s~-4a);105.5(d,C-3);117~4(~C-8);1~0,2~s~c-8a);15904(d~c-2);
192.1(s,C-4)
H NMR (200 MHz, CDC13)
~ = 0~98(~,3H,J= 7~0 Hz,10-H3);1~36-1~72~m,2~,9-H2);1~5~m,1H, 6-H);
2~04 2.38(m,2H,7-H2); ~50(s,1H,5-OH);2.82(s,1H,4a-OH);425(s,1H,5-H);
5,54(d,1H,J2= 6,0 Hz,3H);6.00(dd,1H,J= 2.5 and5,0 Hz,8-H);7~45(d,1H,
J3= 6~0 Hz,2H)
d) Characteri~ation of ~he compound of the formula I
OH
, ~ ~ CH3
3 OH .5 l
H
Agistatin E (figure):
Empirical formula: CllH15O5
Molecular mas~: 228.24
Consistency: colorless, c~ystalline
Soluble in: MeOH, EtOH, acetone, CHCl3
Insoluble in: n-pentane, n-hexane, n-heptane
Melting point: 124C
UV (MeOH/NaOH) 214 (2929), 266 (14798), 304 (3794) nm
nm (~):
IR (KBr): 3430, 2970, 2940, 1745, 1460, 1435, 1350, 1270 cm
Optical rotation
(MeOH): [~D = ~107-7; (c = 0.44)
Mass spectrum: m/z = 228 (4 %, M~)
- 19 2~7~
~igh resolution: calculated ~or CllHl605 228 09972
found 228 . 09972
210 (4 %, M-18), 181 (17 %, M-47), 164 (14 ~6, M-~4),
140 (54 %, M-88), 139 (52 %, M-~9), 111 (62 %, M-l 17),
83 (79 %, M-145), 55 171 %, M-173), 43 (100 96, M-185)
3C NMR (50.3 MHz, CDCl3) s
o' = 11.6 (q, C-11); 22,2 (t, C-7); 24.2 (t, C-10); 32~,4 (t, C-8); 35,4 (d, C-6);
44,6 (t, C-2~; 74~8 (d, C-5); 75,5 Is, C-4); 92,3 (d, C-1); 9802 (s, C-9~; 206,4 (s, C-3)
1H NMR ( 2 O O MHZ , CDC13 ):
~5 = 0,92 (t, 3H, J = 7~,0 HZ, 1 1 -H3); 1,40 (m, 2H, 1 0-H2); 1,~0 (m, 1 H, 6-HJ; 1.65 (m,
2H, 7-H2); 1.75/1098 (m, 2H, 8-H2), 2~70 Is, 11i, 9-OH); 2~8312,.90 (dd, ~H, JA,~ 19.0
HZ~ JA1= ~IB1 = 2.0 HZ, 2-HAand 2-H~ ,74 (s, 1H, 4-OH); 3.94 (s, 1H, 5-H); 5.48(dd, 1 H, J1 2-HA = J1 2.HB3 = 2"0 Hz, 1-H)