Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
` 2~5~ 1
`_
Conductive coatinqs
In the field of electronics there is a requirement for
electrically conductive, transparent electrodes, for ex-
ample, for LCD displays. Up to the present in most cases
glasses or plastic sheets vapour-deposited with metal
oxides are employed for these applications. Materials
vapour-deposited or sputtered with ITO (indium tin oxide)
have particularly good properties. The surface resistance
of the ITO layers is of the order of magnitude of less than
500 ~
The production of such layers by sputtering under vacuum is
very costly. There is therefore a need for a material which
renders possible the production by simple application
techniques of transparent coatings having good
conductivity.
The production of conductive coatings based on organic
conductive materials is known in principle. Thus for
example coatings made of polypyrrole (EP-A 302 304) or of
polythiophene derivatives (EP-A 440 957) have been
described. These coatings can be produced using simple
coating processes but they are not sufficiently conductive
or transparent for many fields of application.
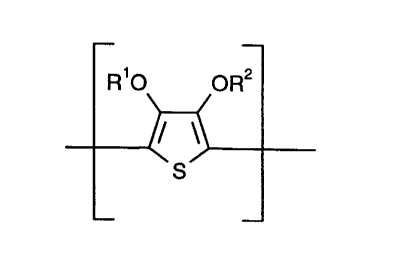
The present invention provides mixtures of
A) neutral polythiophenes of the recurring structural
unit of the formula I,
R O OR
S
Le A 30 329-Eoreiqn Countries - 1 -
2~54~
'_
wherein
Rl and R2 independently of one another represent
hydrogen or a Cl4 alkyl group or together form an
optionally substituted Cl4 alkylene radical,
preferably a methylene radical which is option-
ally substituted by alkyl groups, an ethylene-
1,2 radical optionally substituted by Cll2 alkyl
or phenyl groups, or a cyclohexylene-1,2
radical, and
B) organic compounds containing dihydroxy or poly-hydroxy
and/or carboxyl groups or amide groups or lactam
groups.
These mixtures can be applied in thin layers to the
substrate and by drying and annealing can be converted
into transparent and electrically conducting layers.
Suitable organic compounds cont~; n; ng dihydroxy or
polyhydroxy and/or carboxyl groups or amide groups
correspond to formula (II)
/(H)n
R \ (II)
(COX)m
wherein
n and m independently of one another denote an
integer from 1 to 20, preferably from 2 to 8 and
R denotes a linear, branched or cyclic alkylene
radical having 2 to 20 C atoms or an optionally
substituted arylene radical having 6 to 14 C
atoms or a heterocyclic radical having 4 to 10 C
Le A 30 329-Forei~n Countries - 2 -
i~148544
-
atoms or a sugar radical or sugar alcohol
radical and
X denotes -OH or -NYZ, wherein Y, Z independently
of one another represent hydrogen or alkyl,
preferably hydrogen or Cl-Cl2-alkYl-
Examples of suitable organic compounds containing lactam
groups are N-methylpyrrolidone, pyrrolidone, caprolactam,
N-methylcaprolactam, N-octylpyrrolidone.
Preferred radicals R are derived from the furan structure
or the pyran structure.
Particularly preferred organic compounds corresponding to
formula (II) are:
sugar and sugar derivatives such as sucrose, glucose,
fructose, lactose; sugar alcohols such as sorbitol,
mannitol; furan derivatives such as 2-furancarboxylic acid,
3-furancarboxylic acid; alcohols such as ethylene glycol,
glycerol, di- or triethylene glycol.
In addition to water, other protic solvents can also be
used as solvents for the polythiophene dispersions accord-
ing to the invention, such as for example lower alcoholssuch as methanol, ethanol and isopropanol, as well as mix-
tures of water with lower alcohols and other water-miscible
organic solvents, such as acetone.
The average particle diameters of the particles in the
dispersion can be up to 10 um, preferably up to 3 ,um and
most preferably up to 1 ,um.
The polythiophenes of the recurring structural unit of the
formula (I) are known (cf. EP-A 440,958 and 339,340). The
preparation of the dispersions or solutions according to
Le A 30 329-Eoreiqn Countries - 3 -
21 18~4'1
-
the invention is described in EP-A 440,958 and DE-OS 42 11
459.
The polythiophenes are preferably used in the dispersion or
solution in a cationic form, i.e. in the form in which they
are obtained for example by treating the neutral thiophenes
with oxidising agents. Known oxidising agents, such as
potassium peroxodisulphate are used for the oxidation. As
a result of oxidation the polythiophenes acquire positive
charges which are not shown in the formulae, since the
number thereof and their positions cannot be accurately
determined.
The number of recurring structural units of the formula (I)
is generally >5.
The polythiophene dispersions or solutions according to the
invention contain, based on the sum of polythiophene
cations and polyanions, that is, based on the total solids
content of the solution, from 1 to 100,000% by weight,
preferably 10 to 1,000% by weight, of the compounds of
formula (II) containing hydroxy and carboxyl groups.
Preferably compounds of formula (II) which are soluble in
water are employed.
Organic, polymeric binders and/or organic, low-molecular
cross-linking agents may also be added to the coating
solutions according to the invention. Appropriate binders
are described, for example, in EP-A 564 911.
Epoxysilanes, of the kind described in EP-A 564 911, can be
added to the coating solutions according to the invention,
particularly for the production of adhesive layers on
glass.
The coatings according to the invention can be produced by
known methods, for example, by spraying, application by a
Le A 30 329-Foreiqn Countries - 4 -
~148S4~
-
doctor blade, dipping, application with roller applicator
systems, by printing processes such as gravure printing,
silk screen printing, curtain casting, and can be dried at
room temperature or at temperatures of up to 300C,
preferably up to 200C.
The coatings according to the invention can be annealed in
order to increase electrical conductivity. Annealing can
follow drying at temperatures of below 100C and can be
combined with drying at temperatures of above 100C. This
annealing is carried out at temperatures of from 100C to
400C, preferably at temperatures of up to 250C. The
duration of the annealing is between 0.5 and 3600 seconds,
and preferably 1 ànd 90 seconds.
The thickness of the coatings according to the invention,
depending on the intended use and requirements as to
transparency and conductivity, is from 0.025 to 250 um,
preferably from 0.05 to 10 ~m; the surface resistance is
generally from 0.1 to 2000 D / O, preferably from
1 to 300 D/O.
The coatings according to the invention are used in areas
which require good electrical conductivities, for example,
as electrodes in electroluminescent displays, in LCD
displays, in solid electrolyte capacitors, for the
deposition of metals such as copper, nickel, for example,
in the manufacture of printed circuits, in solar cells, in
electrochromic displays or for the screening of
electromagnetic radiation or for leading away electrical
charges, for example, in picture tubes or as anticorrosive
coatings on metals, for the production of touch screens.
Other areas of application are systems for picture
production, for example, silver halide photography, dry-
plate systems, electrophotography.
Le A 30 329-Forei~n Countries - 5 -
2~485~
_
The conductive layers can optionally be coated with further
layers, for example, W-curing coatings or organic or
inorganic scratch-resistant coatings.
The layers according to the invention can be applied to
organic and inorganic substrates. Examples of suitable
inorganic substrates are glass, oxides or oxidic or non-
oxidic ceramics such as aluminium oxide, silicon nitride.
Examples of suitable organic substrates are sheets or other
mouldings of pure organic polymers, copolymers or mixtures
of, for example, polycarbonate, polystyrene, polyacrylates,
polyesters such as polyethylene terephthalate, polybutylene
terephthalate, polyethylene naphthalate, polyamides,
polyimides, optionally glass-fibre reinforced epoxy resins,
cellulose derivatives such as cellulose triacetate,
polyolefins such as polyethylene, polypropylene.
The invention also relates to electroluminescent systems
which contain the polythiophene dispersions according to
the invention in the form of a transparent conductive layer
or electrode.
The electroluminescent systems according to the invention
consist of an upper and a lower electrode, between which an
electroluminescent layer and optionally other auxiliary
layers, such as for example charge-injecting layers are
inserted, and they are characterised in that they contain
as the electrode a conductive layer consisting of the po-
lythiophene mixtures according to the invention.
The electroluminescent system can contain one or more elec-
trodes, the conductive layers of which contain the
abovementioned polythiophene dispersions. The conductive
layers are preferably transparent.
The conductive layer can be integrated in various positions
in the structure of the electroluminescent systems. The
Le A 30 329-Foreiqn Countries - 6 -
~148544
conductive layer can be applied for example in the form of
a transparent conductive electrode between a transparent
substrate and an electroluminescent layer.
For this purpose the mixtures according to the invention
are applied in the form of a film to a suitable substrate
in the systems according to the invention.
Suitable substrates are transparent substrates such as
glass or plastic films (e.g. polyesters, such as polye-
thylene terephthalate or polyethylene naphthalate, poly-
carbonate, polyacrylate, polysulphone or polyimide film).
The polythiophene mixture according to the invention is
distributed evenly on the substrate by techniques such as
spin-coating, casting, application by a doctor blade, prin-
ting, curtain casting, etc.
After the film has dried the substrate thus coated can be
subjected to a temperature of 150-250C for at least 1 sec,
generally 30 secs. This annealing step increases the con-
ductivity of the layer.
The thickness of the transparent conductive electrode is
5 nm to several ,um, preferably 10 nm to 1500 nm.
An electroluminescent layer is applied to this conductive
transparent electrode in the form of a thin film. The
substances described for example in EP-A 443,861 can be
used as electroluminescent substances.
After the EL layer has dried it is coated with a counter-
electrode. This consists of a conductive substance which
can be transparent. Preferably metals such as Al, Au, Ag
etc. or alloys or oxides thereof are suitable, which are
applied by techniques such as vapour deposition, sputtering
or platinisation.
Le A 30 329-Foreiqn Countries - 7 -
2~ 48S4~
The system according to the invention is brought into con-
tact with the two electrodes by two electrical supply leads
(such as for example metal wires).
When direct voltage in the range of 2 to 100 volt is ap-
plied the systems emit light of a wavelength of 400 to
700 nm. They display photoluminescence in the range from
400 to 700 nm.
The electroluminescent layer can contain one or more elec-
trooptically active substances. It also optionally con-
tains customary additives such as inert binders, charge-
carrier-transporting substances and mixtures of inert bin-
ders and charge-carrier-transporting substances. Charge-
carrier-transporting substances increase the electrolumi-
nescent intensity and reduce the inception voltages.
Suitable inert binders are preferably soluble, transparent
polymers, such as for example polycarbonates, polystyrene
and copolymers of polystyrene such as SAN, polysulphones,
polyacrylates, polyvinylcarbazole, and vinyl acetate and
vinyl alcohol polymers and copolymers, etc.
One or more intermediate layers can be additionally ar-
ranged between the electroluminescent systems and the
electrodes. These intermediate layers - charge-carrier-
transporting substances - are known (for example from Appl.
Phys. Lett. 57 (1990)531) and are defined therein as HTL
(hole transport layer) and ETL (electron transport layer).
The conductive layers can also be applied as transparent
conductive electrodes to form a covering layer on an elec-
troluminescent layer.
Contrary to the arrangement described above, in which the
mixture according to the invention is arranged between an
electroluminescent layer and a transparent substrate, the
Le A 30 329-Foreiqn Countries - 8 -
214~54 1
substance according to the invention can also be used as a
covering electrode.
In this use the electroluminescent substance is arranged on
a conductive or conductively coated substrate, such as for
example metal plates or metal coatings applied by vapour
deposition. The substance according to the invention is
applied to the electroluminescent layer in the manner des-
cribed above.
The advantage of this structure is that it also allows
electroluminescent layers which are exposed to high tempe-
ratures during their production to be provided with a rea-
dily applicable, transparent, conductive electrode.
Example: luminescent plates produced from a luminescent
enamel based on ZnS.
The mixtures according to the invention can also be used as
a charge-transporting intermediate layer in polymeric lumi-
nescent diodes. This intermediate layer increases the
efficiency of the systems.
The mixture according to the invention is applied in the
abovementioned manner in the form of an intermediate layer.
The intermediate layer can be arranged:
- between the transparent conductive electrode and the
electroluminescent polymeric layer,
- between the electroluminescent polymeric layer and the
25covering electrode.
The thickness of the intermediate layer is about 3-200 nm,
and generally between 10 - 100 nm, and most preferably
about 10 nm.
Le A 30 329-Forei~n Countries - 9 -
2148~44
-
ExamPles
A) Preparation of a 3,4-polyethylene dioxythiophene
solution
20 g of free polystyrene sulphonic acid (Mn approx.
40,000), 13.0 g of potassium peroxydisulphate and 50 mg of
iron(III) sulphate are stirred together in 2000 ml of
water. 5.6 g of 3,4-ethylene dioxythiophene is added with
stirring. The solution is stirred for 24 h at room
temperature. Then 100 g of an anion exchanger (commercial
product from Bayer AG, Lewatit MP 62) and 100 g of a cation
exchanger (commercial product from Bayer AG, Lewatit S
100), both moistened by water, are added and stirred for 8
hours.
The ion exchangers are removed by filtration. A solution
having a solids content of approximately 1.2% by weight is
obtained, which is ready for use.
Exam~le 1:
10.0 g of the solution prepared in Example A) together with
10 g of isopropanol are mixed with each of the quantities
of sorbitol and 3-glycidoxypropyltrimethoxysilane (com-
mercial product A 187 Union Carbide) given in the Table.
The mixture is applied to glass plates and dried in air
(approx. 400 mg/m2 dry).
The surface resistance of the dried layers is determined.
The coated glass plates are then placed for 90 seconds on
a hot plate at a temperature of 200C and the surface
resistance is again determined after cooling.
Le A 30 329-Foreign Countries - 10 -
2148~44
-
Table:
A 187 Sorbitol Surface resistance [Q/O]
[g] [g] beforeafter annealing
0.2 _ 3500 3500
0.1 0.2 3400 120
0.2 0.2 3500 180
0.4 0.2 3300 300
0.1 0.6 4000 90
0.2 0.6 3800 105
0.4 0.6 3950 125
It is apparent from the Table that the process according to
the invention results in significantly superior conductive
coatings than does the 3,4-polyethylene dioxythiophene
solution without additives and annealing.
Example 2: An electroluminescent system
B) Preparation of the coating solution
3.0 g of sorbitol are dissolved with stirring in 50 g of
solution A. Then 50 g of isopropanol are added dropwise
with stirring and 0.5 g of glycidoxypropyl trimethoxysilane
(A 187 = a trade product of Union Carbide) is added.
C) Preparation of the 3,4-polyethylenedioxythiophene
electrode
Solution B is applied to a glass slide (20 X 30 mm2). The
substrate is then rotated in a coating centrifuge for 10
seconds at 500 r.p.m. The substrate coated with the film
is placed on a heating plate of a temperature of 180C for
60 seconds, during which the surface resistance is reduced
to 80 Q/O. The layer thickness of the film is 1.3 ~m. The
film is transparent in the visible region of the spectrum.
Le A 30 329-Foreign Countries - 11 -
2~8541
-
The electroluminescent polymer is then applied to this
layer.
D) Application of an electroluminescent layer to the 3,4-
polyethylenedioxythiophene electrode
The electroluminescent material used is MEH-PPV (methoxy-
ethylhexyloxy phenylenevinylene) known from the literature.
A 0.75% solution of the polymer in chloroform is
distributed on the polythiophene-coated substrate of Ex-
ample 2C) for 10 seconds at 2000 r.p.m. using a coating
centrifuge. Al point contacts are then applied to the
polymer film of a thickness of 130 nm by vapour deposition.
E) Use of the flexible polymeric luminescent diodes
When the positive contact of a voltage source is connected
to the PEDT layer and the negative contact with the Al, a
current flows through the electroluminescent polymer. At
the same time electroluminescence occurs. The luminescent
intensity is proportional to the diode current and increa-
ses as the voltage increases.
Le A 30 329-Foreiqn Countries - 12 -