Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 0221462~ 1997-09-0~
Polyamines cont~; n; nq ester and amide qroups
The present invention relates to a group of novel
polyamine cont~; n; ng an ester group and an amine group, and to
a process for their preparation.
The preparation of a reaction product of a diamine
and two moles of maleic ester is described in DE-A 16 70 812
as an intermediate stage in the preparation of hydatoins. The
use of this aspartic acid derivative (PADE for short) as a
reaction component of a polyisocyanate for preparing a coating
takes place in EP 0 403 921. In EP 0 470 461, the use of the
aspartic acid derivative PADE (+ polyisocyanates) is claimed
as a two-component binder combination in an automative
refinish. Surprisingly, the literature contains no reference
to the use of the aspartic acid derivative for preparing a
two-component (two pack) polyurethane (PU) adhesive. As our
own experiments show, bonds produced using the PADE and a
polyisocyanate exhibit only moderate tensile shear strength.
It has surprisingly been found to be possible to
bring about a considerable increase in the tensile shear
strength if the aspartic acid derivative employed for bo~; ng
contains at least one amide group.
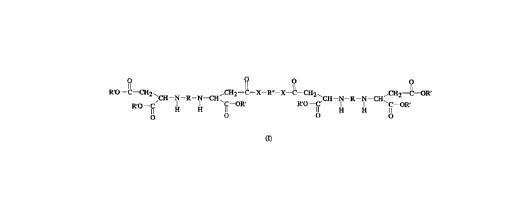
The present invention accordingly provides a
polyamine cont~;n;ng an ester group and an amide group, of the
formula:
23443-622
CA 0221462~ 1997-09-0~
R 1~ R R
R'O--C--CH2~ ,CH2--C--X--R"--X--C--CH2' ,CH2--C--OR'
CH--N--R--N--CH~ ,CH--N--R--N--CH~
R O fi H H- fi fi H~ H fi--OR'
o O O O
where the substituents are defined as follows:
R and R" are each a divalent linear, branched or cyclic
hydrocarbon radical of 4 to 18 carbon atoms, wherein
the cyclic hydrocarbon radicals may be substituted
with one or more C1-C4 alkyl groups,
R' is an alkyl radical of 1 to 14 carbon atoms,
X is NH or NR"', and
R''' is an alkyl radical of 1 to 14 carbon atoms, wherein
R, R' and R" may each have one or more oxygen atoms
in place of a CH2 group.
The novel compound preferably has a basic amine
content of from 1.5 to 4 mmol of NH2/g, an ester group content
of from 3 to 7 mmol/g, more preferably from 4 to 6.5 mmol/g,
an amide group content of from 0.6 to 2 mmol/g, more
preferably from 0.8 to 1.7 mmol/g, and an NH functionality of
from 2.5 to 4, more preferably 3. They may likewise be in
admixture with up to 3 moles of compounds of the formula A
described hereinunder.
The viscosity of the novel compound at 23~C varies
within a wide range, for example, from 1000 to 10 mPa.s.
The novel compound can be processed without
problems, either in solvent-free form or in an inert solvent,
with a polyisocyanate to form a two-pack polyurethane (PU)
-- 2
23443-622
CA 0221462~ 1997-09-0~
coating material. A preferred field of application of the
novel compound is the use as a reaction component for the
preparation of two-pack polyurethane (PU) adhesive.
The present invention additionally provides a
process for preparing the novel compound, which comprises
reacting a product of the reaction of a diprimary diamine and
maleic or fumaric ester (molar ratio 1:2) of the following
formula:
1~l 1~l
R'O--C--CH2, ,CH2--C--OR'
CH--N--R--N--CH (A)
R'O--IC H H 'C--OR'
wherein R and R' are as defined above, with a diamine HX-R"-XH
contA;n;ng two primary or one primary and one secondary, or
two secondary amino groups. The reaction may be carried out
in such a way that from about 2 to 5 mols of the compound (A)
is used per mole of the diamine.
The starting compound (A) employed in the novel
process is known and is described in EP O 403 921.
The starting compound (A) employed in the novel
process may be prepared in a m~nner known per se by reacting a
diprimary diamine H2N-R-NH2 with 2 mol~ of maleic or fumaric
ester as illustrated by the following scheme:
23443-622
CA 0221462~ 1997-09-0~
Il O O
H2N--R--NH2 + 2 HC 'OR' ~ ,CH--N--R--N--CH 2
HC'C'OR R'O--ICl H H ICl--OR'
o O O
(A)
Examples of the diprimary diamine which may be used for
preparing the compound (A) are 1,4-diAm;nohutane,
1,6-diaminohexane, 2-methylpentamethylenediamine,
2,2,4(2,4,4,)-trimethylhexamethylenediamine, isophoronediamine
(IPD); 4,4'-diaminodicyclohexylmethane, 3,3'-dimethyl-4,4'-
diaminodicyclohexylmethane, 2,5-diamino-2,5-dimethylh~Ane,
1,11-diaminoundecane or 1,12-diaminododecane.
Examples of maleic and fumaric esters which may be
used for preparing the starting component (A) are dimethyl,
diethyl, di-n- or -isopropyl, di-n-butyl, di-i-butyl,
di-t-butyl, di-n-pentyl, di-i-pentyl, di-neopentyl, di-hexyl,
di-heptyl, di-octyl and di-2-ethylhexyl maleates or the
correspo~; ng fumaric esters.
The reaction of the starting compound (A) with the
diamine HX-R"-XH is carried out preferably in bulk, but it is
of course also possible to operate in an inert solvent, at
from 50 to 150~C. If a diprimary diamine H2N-R"-NH2 is used,
it is preferably carried out at a temperature of from 80 to
100~C, since at a higher temperature, the formation of a
cyclic imide must be expected. The alcohol R OH which forms
is distilled off from the reaction product during the
reaction. The diamine HX-R"-XH involved may be a diprimary
-- 4
23443-622
CA 0221462~ 1997-09-0~
diamine such as 1,4-diAm;nohutane, 1,6-diaminoh~Ane,
2-methylpentamethylenediamine (MPDA), 2,2,4(2,4,4)-trimethyl-
1,6-diaminohexane (TMD), isophoronediamine (IPD),
4,4'-diaminodicyclohexylmethane, 3,3'-dimethyl-4,4'-
diaminodicyclohexylmethane, 2,5-diamino-2,5-dimethyl h~n~,
l,ll-diaminoundecane or l,12-diaminododecane; a diamine
contA; n; ng one primary amino group and one secondary amino
group such as N-isobutyltrimethylhexamethylenediamine,
N-isobutylisophoronediamine; or a disecondary diamine such as
N,N'-diisobutyltrimethylh~Amethylenediamine and N,N'-diiso-
propyl-IPD.
The diamine having one primary amino group and one
secondary amino group suitable for the reaction with the
starting compound A may be prepared according to a two stage
method: in the 1st stage the diprimary diamine is co~n~ed
with an aldehyde or ketone to form the Schiff base and in the
2nd stage the Schiff base is hydrogenated and separated from
the starting materials, for example, by fractional
distillation. In order to m; n;m; ze the production of by-
product (di-Schiff base), it is necessary to work with a large
excess of diamine; preferably 10 mols of the diamine are
reacted with one mole of the carbonyl compound in a known
mAnner. The diprimary diamines suitable for the co~ncation
to form the Schiff base are, in principle, all
(cyclo)aliphatic diamines, examples of which have already been
listed. As the carbonyl compound to be employed for the
preparation of the Schiff base, all (cyclo)aliphatic aldehydes
and ketones are suitable in principle. Preference, however,
-- 5
23443-622
CA 0221462~ 1997-09-0~
is given to the use of isobutyraldehyde, 2-ethylh~n~l,
methyl ethyl ketone, diisobutyl ketone and cycloh~no~e. The
disecondary diamines may be prepared using a similar method to
that for the diamines just described that had one primary and
one secondary amino group; the only difference is that in this
case the preferred molar ratio of the diprimary diamine to
carbonyl compound is not 10:1 but 1:2.
In some cases it has proven expedient to leave the
mono-alcohol, which is liberated in the course of the novel
process, in the reaction product rather than removing it by
distillation.
The following examples illustrate the invention and
are intended in no way to limit its scope.
Experimental section
1. General preparation procedure for the novel comPounds
a) Preparation of the startinq compound A
The maleic ester is heated at 40~C and the diamine (H2N-
R-NH2) is metered in at a rate such that the temperature
does not rise above 60~C. After the end of the addition
of diamine, heating is continued at 60~C for about 3 h in
order to bring the reaction to completion.
b) Reaction of A with diamines
A is heated with the diamine in the desired molar ratio
at from 50 to 150~C until two moles of amide groups have
formed per mole of diamine employed. This can be readily
monitored by determ;n;ng the amine content of the
reaction product. The reaction temperature is greatly
dependent on the structure of the diamine employed. In
-- 6
23443-622
CA 0221462~ 1997-09-0~
the case of the diprimary diamines, from 80~C to a
m~ lm of 100~C has been found appropriate; in the case
of the disecondary diamines, which are slower to react,
it is necessary to work at from 130 to 150~C. The
alcohol formed during the reaction is distilled off after
the reaction. This is the case when the alcohols
involved are relatively high-boiling; the low-boiling
alcohols liberated are distilled off continuously during
the actual reaction.
Diamine-maleic ester (1:2) adducts employed in the
examples:
A1: 1 mol TMD + 2 mol diethyl maleate
A2: 1 mol hexamethylenediamine + 2 mol dibutyl maleate
A3: 1 mol 2-methylpentamethylenediamine (MPDA) + 2 mol
di-2-ethylhexyl maleate
Table: Composition and properties of some novel compounds
Example Reactants NH2 Viscosity
No. (23~C)
[mmol/g] [mPa.s]
mol of A mol of
diamine
1 2 A3 1 TMD 2.7 41.10
2 3 A3 1 TMD 2.5 7.10
3 4 A1 1 IPD 3.7 5.103
4 2.5 A2 1 MPDA 3.4 32.10
23443-622