Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02219681 1997-10-28
F-7672
Alkylation Process for DesulfUrization of Gasoline
This invention relates to a process for the upgrading
of hydrocarbon streams. The invention more particularly
relates to a process for upgrading gasoline boiling range
petroleum fractions containing olefins and substantial
S proportions of sulfur impurities.
Heavy petroleum fractions, such as vacuum gas oil, or
even resids such as atmospheric resid, may be catalytically
cracked to lighter and more valuable products, especially
gasoline. The product of catalytic cracking is
conventionally recovered and the products fractionated into
various fractions such as light gases; naphtha, including
light and heavy gasoline; distillate fractions, such as
heating oil and Diesel fuel; lube oil base fractions; and
heavier fractions.
Sulfur in various forms is commonly found in petroleum
and petroleum products either as dissolved free sulfur,
hydrogen sulfide, or as organic compounds, such as
thiophenes, sulfonic acids, mercaptans, alkylsulfates, and
alkyl sulfides. Where a petroleum fraction is being
catalytically cracked and contains sulfur, the products of
catalytic cracking usually contain sulfur impurities which
normally require removal, usually by hydrotreating, in
order to comply with the relevant product specifications.
Such hydrotreating can be done either before or after
catalytic cracking. Because naphtha streams from both
catalytic, e.g.,FCC, and thermal, e.g.,coking, cracking
processes contribute most of the sulfur present in the
gasoline pool, reducing the sulfur content of cracked
naphthas will be important in order to meet liquid
transportation sulfur specifications and emission
standards.
The ease of sulfur removal from petroleum and its
products is dependent upon the type of sulfur-containing
compound. Hydrogen sulfide and mercaptans are relatively
easy to remove whereas aromatic sulfur compounds such as
thiophenes are more difficult to remove. Sulfur impurities
CA 02219681 1997-10-28
F-7672
tend to concentrate in the heavy fraction of the gasoline,
as noted in U.S. Patent No. 3,957,625 (Orkin) which
proposes a method of removing the sulfur by hydrodesulfu-
rization of the heavy fraction of the catalytically cracked
S gasoline so as to retain the octane contribution from the
olefins which are found mainly in the lighter fraction. In
one type of conventional, commercial operation, the heavy
gasoline fraction is treated in this way. As an
alternative, the selectivity for hydrodesulfurization
relative to olefin saturation may be shifted by suitable
catalyst selection, for example, by the use of a magnesium
oxide support instead of the more conventional alumina.
Cracked naphtha, as it comes from the catalytic
cracker and without any further treatments such as
purifying operations, has a relatively high octane number
as a result of the presence of olefinic components. It
also has an excellent volumetric yield. As such, cracked
gasoline is an excellent contributor to the gasoline pool.
It contributes a large quantity of product at a high
blending octane number. In some cases, this fraction may
contribute as much as up to half the gasoline in the
refinery pool. Therefore, it is one of the most desirable
components of the gasoline pool.
Other unsaturated fractions boiling in the gasoline
boiling range, which are produced in some refineries or
petrochemical plants, include pyrolysis gasoline. This is
a fraction which is often produced as a by-product in the
cracking of petroleum fractions to produce light
unsaturates, such as ethylene and propylene. Pyrolysis
gasoline may have a very high octane number but is quite
unstable in the absence of hydrotreating because, in
addition to the desirable olefins boiling in the gasoline
boiling range, it also contains a substantial proportion of
diolefins, which tend to form gums upon storage or
standing.
CA 02219681 1997-10-28
F-7672 ~ ~I/u~ Y ~ 6
IPEA/u~ 18 JUL ~Q
Cracking of naphtha is a highly useful process to
increase the yield of gasoline. However, the cracking
process also affects sulfur containing materials and
results in a reduction in their molecular weight from a
range that is greater than the average molecular weight of
the gasoline boiling range fraction into a range that is
within the molecular weight range of the gasoline fraction.
Much of this gasoline boiling range sulfur is contained in
aromatic compounds and, consequently, needs to removed by
lo hydrotreating. However, hydrotreating of any of the sulfur
containing cracked fractions which boil in the gasoline
boiling range, e.g., FCC, pyrolysis and coker naphtha,
causes a reduction in the olefin content, and consequently
~a reduction in the octane number. Further, as the degree
of desulfurization increases, the octane number of the
normally liquid gasoline boiling range product decreases.
Depending on the conditions of the hydrotreating operation,
some of the hydrogen may also cause some hydrocracking or
aromatic saturation as well as olefin saturation.
Various proposals have been made for removing sulfur
while retaining the more desirable olefins. U.S. 4,049,542
(Gibson), for instance, discloses a process in which a
copper catalyst is used to desulfurize an olefinic
hydrocarbon feed such as catalytically cracked light
naphtha.
Other processes for treating catalytically cracked
gasolines have also been proposed in the past. For
example, U.S. 3,759,821 (Brennan) discloses a process for
upgrading catalytically cracked gasoline by fractionating
it into a heavier and a lighter fraction and treating the
heavier fraction over a ZSM-5 catalyst, after which the
treated fraction is blended back into the lighter fraction.
Another process in which the cracked gasoline is
fractionated prior to treatment is described in U.S.
4,062,762 (Howard) which discloses a process for
desulfurizing naphtha by fractionating the naphtha into
nl~ENt)ED SHEEI
CA 02219681 1997-10-28
v ~ ~ 1 J
F-7672
IPEAQIS 18 JUL "
three fractions each of which is desu'furized by a
different procedure, after which the fractions are
recombined.
In any case, regardless of the mechanism by which it
happens, the decrease in octane which takes place as a
consequence of sulfur removal by hydrotreating creates a
tension between the growing need to produce gasoline fuels
with higher octane number and - because of current
ecological considerations -the need to produce cleaner
burning, less polluting fuels, especially low sulfur fuels
,
to avoid poisoning of catalyst converters which would
adversely affect hydrocarbon emissions.This inherent
tension is yet more marked in the current supply situation
-for low sulfur crudes.
An objective of the present invention is to provide a
process for reducing the sulfur level in naphtha streams
especially the sulfur in naphtha attributable to thiophene
or thiophenic compounds, while minimizing product losses in
volume and octane number.
Sulfur species present in cracked naphthas may be
converted and removed by first passing the naphtha over an
J
acid catalyst to alkylate the thiophenic compounds in the
naphtha using the indigenous olefins and diolefins present
in the naphtha as alkylating agent. Such alkylation
reactions provide alkylated thiophenes that concentrate the
sulfur species in the heavy portion of the naphtha, greatly
reducing the amount of naphtha that needs to be
hydrodesulfurized. Furthermore, because the majority of
the olefins in cracked naphthas remain concentrated in the
light portion of the naphtha which is not subsequently
hydrotreated, the octane and hydrogen consumption penalties
associated with the hydrotreating of only the sulfur-
enriched heavy naphtha are minimized. Similar results can
be achieved through a process according to the invention
with virgin naphthas having a low olefin content by
cofeeding olefin-rich streams.
~MEN~DSHEET
CA 02219681 1997-10-28
F-7672 . ~lJU~ 5 ~
- -IP~WS 18 JUL 'S
More particularly, the invention comprises a process
for upgrading a sulfur-containing feedstream comprising
olefinic gasoline boiling range hydrocarbons rich in
thiophenic sulfur compounds. The process is carried out by
contacting the feedstream with acidic alkylation catalyst
particles under alkylation conditions in an alkylation zone
to provide an effluent stream comprising hydrocarbons
containing alkylated thiophenic sulfur compounds. The
alkylated thiophenic compounds are separated from the
effluent stream by fractional distillation to provide a
heavy naphtha of higher boiling point rich in alkylated
thiophenic compounds and a light naphtha portion. The light
naphtha portion is recovered to provide gasoline boiling
~range hydrocarbons containing a reduced amount of thiophe-
nic sulfur compounds. Optionally, the heavy naphthaportion may be desulfurized using conventional
hydrotreating or other desulfurization processes.
While the process according to the invention
specifically achieves the intended benefit of a lowering of
the sulfur content of the naphtha feedstream, there are
corollary benefits. It is to be expected that A process
according to the invention will also lower the amount of
aromatic nitrogen compounds in the naphtha as well as the
amount of diolefins.
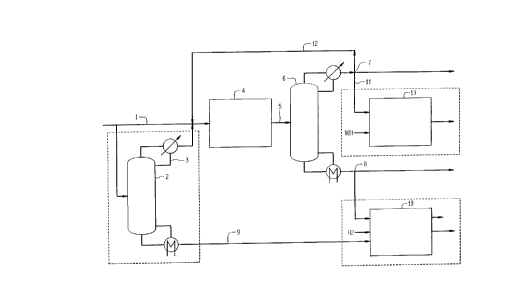
The Figure is a schematic drawing of one embodiment of
a process according to the invention.
Feed
The feed to the process comprises a sulfur-containing
petroleum fraction, generally olefinic, which boils in the
gasoline boiling range. Feeds of this type include
olefinic light naphthas typically having a boiling range of
about C6 to 165~C, full range naphthas typically ha~ing a
boiling range of about Cs to 215-C, heavier naphtha
fractions boiling in the range of about 127-C to 210~C, or
heavy gasoline fractions boiling at, or at least within,
A~EN~I}SHE~
CA 02219681 1997-10-28
F-7672 ~ U ll~v ~ U I 1 ~ ~ U ~
IPEAIU~ 1 8 JUL '97
the range of about 165~C to 260~C, preferably about }65~C
to 210~C. The preferred feed is a light naphtha or full
range naphtha.
While the feedstream to the process preferably
comprises a sulfur-containing olefinic petroleum fraction
which boils in the gasoline boiling range in which
indigenous olefins are used to carry out the alkylation
reaction, it is possible to use an additional or cofeed
olefin feedstream to the process to provide or supplement
' 10 alkylating agents for the process. This optional variation
of the process could be elected depending on conditions
extant in the refinery, including an abundant supply of
light olefins or a sulfur-rich gasoline boiling range
-stream that is not sufficiently rich in indigenous olefins.
The process may be operated with the entire gasoline
fraction obtained from the catalytic cracking step or,
alternatively, with part of it. Because the sulfur tends
to be concentrated in the higher boiling fractions, it is
preferable, particularly when unit capacity is limited, to
separate the higher boiling fractions and process them
~- through the steps of the present process without processing
the lower boiling cut. The cut point between the treated
and untreated fractions may vary according to the sulfur
compounds present but usually, a cut point in the range of
from about lOO-F (38-C) to about 300-F (150-C), more
usually in the range of about 200-F(93-C) to about
300-F(150-C) will be suitable. The exact cut point
selected will depend on the sulfur specification for the
' gasoline product as well as on the type of sulfur compounds
present: lower cut points will typically be necessary for
lower product sulfur specifications. Sulfur which is
present in components boiling below about lSO-F(65-C) is
mostly in the form of mercaptans which may be removed by
extractive type processes such as Merox. Removal of
thiophenic compounds and present in higher boiling
components, e.g., component fractions boiling above about
~N~SH~
CA 02219681 1997-10-28
F-7672 IP~ l~JUe'
180-F(82-C), is carried out according to a process
according to the instant invention.
The sulfur content of these catalytically cracked
fractions will depend on the sulfur content of the feed to
the cracker as well as on the boiling range of the selected
fraction used as the feed in the process. Lighter
fractions, for example, will tend to have lower sulfur
contents than the higher boiling fractions. As a practical
matter, the sulfur content will exceed 50 ppmw and usually
will be in excess of 100 ppmw, and in most cases in excess
of about 500 ppmw. For the fractions which have 95 percent
points over about 380-F(193-C), the sulfur content may
eYcee~ about 1,000 ppmw and may be as high as 4,000 or
~5,000 ppmw or even higher. Since much of the nitrogen
compounds in the feed to a cracker end up as coke, the
nitrogen content of cracked naphtha is not as characteris-
tic of the feed as is the sulfur content and is preferably
not greater than about 20 ppmw although higher nitrogen
levels typically up to about 50 ppmw may ~e found in
certain higher boiling feeds with 95 percent points in
- excess of about 380-F(193-C). The nitrogen level will,
however, usually not be greater than 250 or 300 ppmw. As a
result of the cracking which has preceded the steps of the
present process, the feed to the process according to the
invention will be olefinic, with an olefin content of at
least 3 and more typically in the range of 10 to 20, e.g.
15 - 20, weight percent.
C~t~ly~t
Many heterogeneous acid catalysts containing either
Bronsted acid sites or Lewis acid sites are useful for the
process according to the invention. Typical Lewis acids
include those derived from AlCl3, FeCl3, SbCl3, BF3, ZnCl2,
TiCl4 and P2O5; but particularly, Lewis acids such as
AlCl3/silica, AlClJsilica, BF3/silica, Co/Mo/alumina, Mo/-
alumina, MoS2 are useful for the process according to the
,~ O~ S~
CA 02219681 1997-10-28
F-7672 r~u~ 7 ~
8 ~P~IUS 18 JUL '5
invention. Typical Bronsted acids include HF, H2SO4,
metallosilicates, silica-alumina, sulfonic acid resins, and
the like. Well-known methods of maintaining or recovering
catalyst activity, such as promoter cofeed or hydrogenative
or oxidative regeneration, may also be employed.
The useful catalysts include the crystalline
aluminosilicate zeolites especially the medim pore size
zeolites having a silica:alumina ratio of at least 12, and
constraint index of about 1 to 12. Representative of these
zeolites are ZSM-5, ZSM-ll, ZSM-22, ZSM-23, ZSM-35, MCM-22,
MCM-36, MCM-49, MCM-49 and ZSM-48. The larger pore size
zeolites may also be used as catalysts in the present
process, i.e., those zeolites having a Constraint Index of
no greater than about 2. Representative of these zeolites
are zeolite Beta, TEA mordenite, faujasites, USY and ~SM-
12.
The method by which Constraint Index is determined is
described fully in U.S. Patent No. 4,016,218, to which
reference is made for details of the method.
One group of preferred catalysts for use in the
present invention are the members of the MCM-22 group which
includes MCM-22, MCM-36, MCM-49 and MCM-56. MCM-22 is
described in U. S. patent 4,954,325. MCM-36 is described
in U. S. patent 5,250,277 and MCM-36 (bound) is described
in U. S. patent 5,292,698. MCM-49 is described in U. S.
patent 5,236,575 and MCM-56 is described in U. S. patent
5,362,697.
The Process
The process according to the invention reduces the
sulfur level in naphtha streams while minimizing volume and
octane loss. Olefins, either present in cracked naphthas or
fed to virgin naphtha, are used to convert sulfur species
to higher molecular weight compounds thereby concentrating
the sulfur in the "back-end" of the naphtha. Upon
fractionation, this redistribution of the sulfur in the
A~o~Ds~
CA 02219681 1997-10-28
F-7672 r~/u~ 7 ~ 0 ~
- ~PEWS ~8~L~
naphtha leads to a relatively sulfur-free light naphtha and
a sulfur-rich heavy naphtha which may be desulfurized via
conventional hydrotreating. Conversion of the sulfur in the
heavy fraction of naphtha reduces the amount of naphtha
that must be hydrodesulfurized which, in the case of
cracked naphthas, leads to lower hydrogen consumption and
greater octane-barrels.
The conversion carried out in the process is one of
alkylation of aromatic heterocyclic sulfur compounds, i.e.,
thiophene and related thiophenic compounds, in contact with
acidic alkylation catalyst. Preferably, the process is
carried out on a cracked naphtha feedsteam at temperatures
between lOO-F (38-C) and 700-F (371-C) and pressure between
-atmospheric or autogenous pressure and 7000 kPa. The
preferred t~mrerature is 300-400-F (149-204-C).
Various reactor configurations can be employed to
carry out the alkylation step of the process according to
the invention. These include a down-flow, liquid phase,
fixed bed process; an up-flow, fixed bed, trickle phase
~~- process; an ebulating, fluidized bed process; or a
transport, fluidized bed process. The fixed bed
arrangements are preferred for simplicity of operation.
A preferred implementation of the proposed concept is
shown schematically in the Figure. Cracked naphtha (1),
possibly prefractionated (2) to obtain a light fraction
(3), is fed to a condensation or alkylation reactor (4)
containing acid catalyst where naphtha-range olefins
alkylate sulfur species producing heavier sulfur compounds.
The reactor effluent (S) is distilled (6) to obtain low-
sulfur light naphtha (7) and a heavy naphtha (8) enriched
in sulfur. This high-sulfur heavy naphtha may be combined
with heavy naphtha (9) from the prefractionator and
hydrodesulfurized in reactor (10) using conventional
hydrotreating processes or alternatively sent to the
distillate pool. The low-sulfur light naphtha (7) may be
AMEND~ ~
- - -
' CA 02219681 1997-10-28
I ~IU~ ~ O / 1 ~ C~ U
F-7672 ~
~ . ~P~AIU~
optionally etherified (11) in etherification reactor (13)
or optionally recycled (12) to the sulfur conversion
reactor depending on overall desulfurization targets. The
naphtha splitter may also have utility in meeting Tgo
distillation targets.
A series of experiments was performed to illustrate
the novelty and advantages of the invention. These
experiments are depicted in the following Example 1.
~s~mple 1
Selective condensation of sulfur compounds in cracked
naphthas was scoped over zeolite catalysts ZSM-5, MCM-22,
and USY in batch studies. Feedstocks included both light
(C5 -lOO C, 230 ppmw S) and full-range (c5t, 0.14 wt%S) FCC
naphthas. These batch runs were conducted at 350-F (177~C)
for three hours at autogenous pressure with loadings of 10
grams of light naphtha per gram of catalyst and 11.6 grams
of full-range naphtha per grams of catalyst. Results for
the light FCC are shown in Table 1 and for the full-range
FCC in Table 2.
-~O~û SH~E~
CA 02219681 1997-10-28
F-7672
11
Table 1
Li~ht FCC Naphtha Sulfur Redistribution
Acid Catalyst
Feed ZSM-5 MCM-22 USY
5 Sulfur Distribution~wt% of S
<Thiophene 16.8 0.0 0.0 0.0
Thiophene 44.5 0.0 0.0 8.8
Methylthiophenes 33.2 0.0 0.0 0.0
>Methylthiophenes 5 5 100.0 100.0 91.2
Total 100.0 100.0 100.0 100.0
Composition. wt% of HC
Butenes 1 0 0.7 0.0 0.8
Pentenes 26.8 11.5 2.0 16.3
Hexenes 19.7 11.4 3.2 14.6
C4-C6 P+N+A 27.6 32.8 35.9 34.3
C7+ 24.9 43.7 59 0 34.1
Total 100.0 100.0 100.0 100.0
Table 2
Full-ran~e FCC Naphtha Sulfur Redistribution
Acid Catalyst
Feed ZSM-5 MCM-22 USY
Sulfur Distribution~wt% of S
<Benzothiophene 51.7 22.5 14.9 15.7
Benzothiophene 27.8 24.1 9.0 13.5
>Benzothiophene 20.4 53 4 76 1 70 8
Total 100.0 100.0 100.0 100.0
Composition~ wt% of HC
>430~F (Benzothiophene) 5.2 8.6 10.7 10.1
CA 02219681 1997-10-28
F-7672 12 IPE~S 18~P~
As shown in Table 1, all three catalysts were
extremely effective in converting the sulfur compounds
present in the light FCC naphtha feed to sulfur species
boiling above the methylthiophenes (113-116~C)(235-240-F)
and C,olefins (80-106~C)(177-223-F). This sulfur
conversion was also accompanied by significant olefin
conversion to C7+ products as shown in the detailed
hydrocarbon composition.
All three catalysts were also effective in converting
sulfur species present in full-range FCC naphtha as shown
in Table 2.
~ENOFD