Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02240393 1998-06-23
SPECIFICATION
METHOD FOR PRODUCING TEMPERATURE
TOLERANT PLANTS
FIELD OF THE INVENTION
The present invention relates to a method for
producing plants with novel properties, more specifically,
a method for producing temperature-tolerant plants which
are highly resistant to environmental stress.
PRIOR ART
Many organisms adapt themselves to severe
environmental stress by synthesizing and accumulating a
specific compound called "compatible solute" in their
cytoplasm to protect themselves against such stress.
Environmental stress to which organisms have been shown to
adapt themselves by such a mechanism include salts (Imhoff
et al., FEMS Microbiol. Rev. 39:57-66, 1986; Mackay et
al., J. Gen. Microbiol. 130:2177-2191, 1984; Rhodes and
Hanson, Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:357-
384, 1993), dehydration (Yancy et al., Science 217:1214-
1222, 1982) and low temperatures (Ko et al., J. Bacteriol.
176:426-431, 1994).
Among those compatible solutes, glycine betaine
(hereinafter referred to as betaine) is widely distributed
in higher plants (Robinson and Jones, Aust. J. Plant
Physiol. 13:659-668, 1986), bacteria (Csonka, Microbiol.
Rev. 53:121-147, 1989) and animals (Garcia-Perez and Burg,
Physiol. Rev. 71:1081-1115, 1991; Lever et al., Biochim.
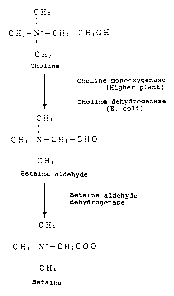
Biophys. Acta. 1200:259-264, 1994). As shown in Fig. 1,
- 1 -
CA 02240393 1998-06-23
betaine is a bipolar compound having a positive charge and
a negative charge in its molecules (Rhodes and Hanson,
Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:357-384,
1993). A long discussion regarding the physiological
functions of betaine has suggested that betaine may
protect cells by maintaining an osmotic balance with the
environment (Robinson and Jones, Aust. J. Plant Physiol.
13:659-668, 1986) and that betaine may stabilize higher-
order structures of proteins (Bernard et al., Acad. Sci.
111, 307:99-104, 1988; Papageorgiou and Murata, Phtosynth.
Res. 44:243-252, 1995). However, betaine is not
exclusively synthesized in cells under salt stress or
dehydration stress. Thus, it could not be concluded that
betaine has a direct effect on the protection of cells
against such stress.
In Escherichia coli and spinach (Spinacia oleracea),
betaine is biosynthesized from choline via two steps of
oxidation as shown in Fig. 1. On the other hand, choline
oxidase obtained from the gram-positive soil bacterium
Arthrobacter globiformis can oxidize choline to betaine in
one-step oxidation reaction (Ikuta, S. et al., J. Biochem.
82:1741-1749, 1977).
In an attempt to study a direct effect of betaine,
we isolated the codA gene encoding a novel choline oxidase
which catalyzes oxidation from choline to betaine
(Japanese Society of Plant Physiologist, Annual meeting of
1994, the 34th Symposium held March 28-30, 1994) and
integrated it into cells of the cyanobacterium strain
- 2 -
CA 02240393 1998-06-23
Synechococcus PCC7942 and brassicaceous plants, thus
succeeded in obtaining salt-tolerant and/or osmotolerant
plants (Japanese Patent Application No. 106819/95). This
confirmed that betaine functions to protect organisms
against salt stress.
However, no report has shown that betaine confers
temperature tolerance on plants or bacteria.
It is an object of the present invention to produce
plants that are tolerant to environmental changes such as
high temperatures or low temperatures by gene
recombination techniques.
DISCLOSURE OF THE INVENTION
As a result of careful study to solve the above
problems, we succeeded in obtaining temperature-tolerant
plants by integrating and expressing a gene encoding
choline oxidase in cyanobacteria, brassicaceous plants and
gramineous plants.
Accordingly, the present invention provides a method
for producing temperature-tolerant plants, which comprises
transforming a plant with a recombinant vector carrying a
gene encoding choline oxidase.
The present invention also provides temperature-
tolerant plants produced by said method or progenies
thereof having the same properties.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1: Schematic representation showing the
oxidation process from choline to betaine.
FIG. 2A: Schematic representation showing constructs
- 3 -
CA 02240393 1998-06-23
used for transformation of Synechococcus PCC7942. PAM
refers to Synechococcus PCC7942 transformed with pAM1044,
and PAMCOD refers to Synechococcus PCC7942 transformed
with pAM1044 carrying the codA gene. Dashed arrows
indicate primers used for PCR. Triangles represent the
conII promoter. Arrows indicate the orientation of genes.
FIG. 2B: SDS-PAGE (photograph of electrophoresis)
showing the complete replacement of chromosomes by the
spectinomycin-resistant gene and codA gene in DNA of
Synechococcus PCC7942. Lane a: ~,-HindIII/~x174-HaeIII
fragment; Lane b: the wild-type strain of Synechococcus
PCC7942; Lane c: the strain PAM; Lanes d and e: the strain
PAMCOD (Lanes b, c and d show the results of PCR with
primers 1 and 2, and lane a shows the results of PCR with
primers 1 and 3).
FIG. 3: Western blot analysis (photograph of
electrophoresis) showing the expression of choline oxidase
in the Synechococcus PCC7942 strains PAM and PAMCOD. Lane
a: protein extracts from the strain PAMCOD; Lane b:
protein extracts from the strain PAM; Lane c: purified
choline oxidase.
FIG. 4: Results of growth of Synechococcus PCC7942
strains grown at various temperatures for 10 days on an
agar plate in BG 11 medium supplemented with 1 mM choline
chloride (photographs showing morphology of organisms). 1
and 4: PAM strain; 2 and 3: PAMCOD strain.
FIG. 5: Growth of the Synechococcus strains PCC7942
PAM (~) and PAMCOD (~) in BG11 medium supplemented with 1
- 4 -
CA 02240393 1998-06-23
mM choline chloride under illumination. A: Growth at
42°C; B: Growth at 20°C.
FIG. 6: Photosynthetic oxygen release level from the
Synechococcus strains PCC7942 PAM (~) and PAMCOD
grown at low temperatures in the presence of 1 mM NaHC03
(A) or in the presence of 1,4-benzoquinone and 1 mM
K3Fe(CN)6 (B) .
FIG. 7: Schematic representation showing the
restriction enzyme map of the codA gene.
FIG. 8: Schematic representation showing the
structure of the binary vector plasmid pGAH/codA used for
transformation of Arabidopsis.
FIG. 9: Western blot analysis (photograph of
electrophoresis) of choline oxidase in soluble fractions
of the wild-type and transformed plants of Arabidopsis.
Lane 1: choline oxidase derived from commercially
available Arthrobacter globiformis (Sigma Chemical Co.,
St. Louis, MO, USA); Lane 2: soluble fraction of the wild-
type plant; Lane 3: soluble fraction of a transformed plant.
FIG. 10: Influence of low temperature on
photochemical system II in leaves of the wild-type and
transformed plants of Arabidopsis. (~): wild-type plant;
): transformed plant.
FIG. 11: Inhibition of photosynthesis at low
temperature (A) and recovery from inhibition of
photosynthesis at low temperature (B) in leaves of the
wild-type and transformed plants of Arabidopsis. (~):
wild-type plant; (~): transformed plant.
- 5 -
CA 02240393 1998-06-23
FIG. 12: Results of freeze resistance tests on
leaves of the wild-type and transformed plants of
Arabidopsis. (~): wild-type plant; (~): transformed plant.
FIG. 13: Influence of low temperature at water
absorption stage on germination of seeds of the wild-type
and transformed plants of Arabidopsis (photographs showing
morphology of the organisms). A: seeds germinated without
low-temperature treatment at water absorption stage; B:
seeds germinated after low-temperature treatment at water
absorption stage. In both of A and B, W on the left shows
the results from seeds of the wild-type plant and T on the
right shows the results from seeds of the transformed plant.
FIG. 14: Structures of two chimeric codA genes used
for transformation of rice, i.e. 35SINTPcodA and
35SINcodA.
FIG. 15: NMR charts representing betaine
accumulation in rice plants of the wild-type strain, a
transformant (A) which does not express the codA gene, and a
transformant (B) which expresses the codA gene. In the
figure, GB and Ch represent peaks corresponding to betaine
and choline, respectively.
FIG. 16: Effect of the introduction of the codA gene
into Synechococcus PCC7942 on electron transport mediated
by photochemical system II in terms of relative values vs.
maximum activation assumed as 100. (~): PAM cells under
illumination; (~): PAMCOD cells under illumination;
PAM cells in the dark; (~): PAMCOD cells in the dark.
FIG. 17: Recovery of electron transport mediated by
- 6 -
CA 02240393 1998-06-23
photochemical system II from photoinhibition at low
temperature when the codA gene was introduced into
Synechococcus PCC7942. (0): PAM cells under
illumination; (~): PAMCOD cells under illumination.
FIG. 18: Effect of low-temperature treatment under
dark conditions on photosynthetic oxygen release when the
codA gene was introduced into Synechococcus PCC7942.
(0): PAM cells under illumination; (~): PAMCOD cells
under illumination.
FIG. 19: Effect of low-temperature treatment on
lipid phase transition when the codA gene was introduced
into Synechococcus PCC7942. (0): PAM cells under
illumination; (~): PAMCOD cells under illumination.
FIG. 20: Electrophoretogram showing changes of
protein in the soluble fractions and membrane fractions when
the codA gene was introduced into Synechococcus PCC7942.
Lane 1: choline oxidase; 2: protoplasmic membrane of PAM
cells; 3: protoplasmic membrane of PAMCOD cells; 4:
thylakoid membrane of PAM cells; 5: thylakoid membrane of
PAMCOD cells; 6: soluble fraction of PAM cells; 7: soluble
fraction of PAMCOD cells. An arrow indicates choline
oxidase.
THE BEST EMBODIMENTS OF THE INVENTION
As used herein, temperature tolerance means an
ability of a transformed plant to grow at higher or lower
temperatures than the temperatures which normally allow
non-transformed plants to grow.
The gene encoding choline oxidase used in the
CA 02240393 1998-06-23
present invention is a gene which encodes a protein
capable of converting choline into betaine in a one-step.
reaction and which may be derived from gram-positive soil
bacteria of the genus Arthrobacter. For example, it may
be preferably derived from Arthrobacter globiformis and
Arthrobacter pascens, especially Arthrobacter globiformis.
We cloned the codA gene encoding choline oxidase
from Arthrobacter globiformis and determined its nucleotide
sequence. The codA gene contains an open reading frame of
1641 bp, which encodes 547 amino acids. The nucleotide
sequence and amino acid sequence of the codA gene are shown
as SEQ ID NO: 1 in Sequence Listing.
Such a gene encoding choline oxidase can be
integrated into appropriate vectors to transform a plant.
Then, an appropriate promoter or a sequence involved in
the expression of a character can be introduced into these
vectors to express the gene in the plant.
The gene encoding choline oxidase may include not
only the nucleotide sequence encoding the amino acid
sequence of SEQ ID NO: 1 in Sequence Listing but also
nucleotide sequences in which one or more amino acids are
added to, deleted from or substituted for said amino acid
sequence provided that they encode a protein having choline
oxidase activity.
The method of the present invention can confer
temperature tolerance on a wide variety of plants ranging
from cyanobacteria to higher plants. Cyanobacteria are
widely used as model organisms of higher plants because
_ g _
CA 02240393 1998-06-23
they have basically the same photosynthetic mechanism as
that of higher plants and they can be readily transformed
to give results in a short time. Some easy-to-transform
cyanobacteria readily take up extracellular DNA into their
cells to cause efficient recombination. Such
cyanobacteria include Synechococcus PCC7942, Synechococcus
PCC6301 (ATCC 27144) and Synechocystis PCC6803 (ATCC
27184) (Protein, Nucleic Acid, Enzyme, Vol. 35, No. 14,
pp. 2542-2551, 1991; Crit. Rev. Microbiol. Vol. 13, No.
1, pp. 111-132, 1985).
Higher plants include dicotyledons and
monocotyledons. In the examples described below, highly
temperature-tolerant plants could be obtained from a
brassicaceous plant as a dicotyledon, but it is not
limitative and other families and genera of dicotyledons
may be used. The method of the present invention may also
be applicable to monocotyledons. It was found that a
monocotyledonous plant rice, which originally lacks
betaine-synthesizing ability, gained this ability after
transformation by the method of the present invention.
The vector into which the choline oxidase-coding
gene can be integrated and the method for transformation
and selection of transformed plants can be appropriately
chosen dependent on the nature of the plant to be
transformed, including plant cells.
For example, a plasmid such as pUC303 can be used
for cyanobacteria. Then, transformants having desired
properties can be selected by an antibiotic-resistant gene
- 9 -
CA 02240393 1998-06-23
inserted into the plasmid. According to the present
invention, plants showing tolerance to both of high and low
temperatures were successfully obtained by transforming the
cyanobacterium Synechococcus PCC7942 with the codA gene
encoding choline oxidase derived from Arthrobacter
globiformis.
When the Synechococcus PCC7942 transformed with the
codA gene was cultivated in BG11 medium supplemented with
choline chloride, the transformed Synechococcus was found
to take up exogenously supplied choline to convert it into
betaine and accumulate betaine up to a level of about 80
mM. However, such accumulation was not observed with a
control group of Synechococcus strain lacking the codA
gene.
When the Synechococcus strain transformed with the
codA gene and a control group of non-transformed strain
were cultivated in BG11 medium supplemented with choline
chloride at 42°C to examine their reaction to high
temperatures, the transformed Synechococcus stopped
growing for a day and then began to grow again. The non-
transformed control group did not grow at all at 42°C.
When they were cultivated at 20°C to examine their reaction
to low temperatures, the transformed strain grew slowly for
4 days but then began to grow rapidly. However, the non-
transformed control group still grew very slowly even after
4 days. A growth test using a solid medium also showed that
the transformed strain grew well at both high and low
temperatures as compared with the non-transformed strain.
- 10 -
CA 02240393 1998-06-23
These results revealed that the Synechococcus strain
transformed with the codA gene grows significantly better
than the non-transformed strain both at high and low
temperatures.
It has been pointed out that betaine not only acts
as an osmoprotectant but also plays an essential role in
the protection of a photosynthetic mechanism in
photoautotrophic organisms (Murata et al., FEBS Lett.
296:187-189, 1992). Thus, the Synechococcus strain
transformed according to the present invention and the
non-transformed strain were cultivated in the dark at
various low temperatures to examine temperature tolerance
of photosynthesis. As a result, a great difference was
observed between the transformed strain and non-
transformed strain in photosynthetic oxygen release level
and inactivation of electron transport mediated by
photochemical system II in cells at low temperatures.
Namely, photosynthetic oxygen release level of the
transformed strain was more tolerant to low temperatures
than that of the non-transformed strain. Electron
transport activity mediated by photochemical system II in
cells of the transformed strain was also more tolerant to
low temperatures than that of the non-transformed strain,
i.e. the activity of the non-transformed strain was
lowered to 50~ of the original level at 5°C while the
activity of the transformed strain remained at almost the
original level at 5°C and began to decrease below 5°C.
It has previously been known that the optimal
- 11 -
CA 02240393 1998-06-23
temperature for growth of Synechococcus PCC7942 ranges
from 30 to 38°C. Thus, it is deduced that the non-
transformed strain was inhibited from cell growth by
denaturation of the structures of some proteins at such a
high temperature as 42°C. However, the transformed strain
carrying the codA gene could grow probably because about
80 mM betaine accumulated in cells prevented denaturation
of proteins. At 20°C, the non-transformed strain could
not grow, but the transformed strain in which the codA
gene had been integrated could grow. This may also result
from accumulation of betaine. Further, betaine
accumulation in cytoplasm is thought to enhance the
tolerance of cyanobacterial cells to low temperatures in
the dark. However, the present invention is not
restricted to such an action mechanism.
These results mean that excellent tolerance to both
high and low temperatures has been conferred on the
Synechococcus transformed with the gene encoding choline
oxidase according to the method of the present invention.
Dicotyledons may be transformed by gene transfer
techniques using protoplasts or a part of tissue. In case
of the gene transfer using tissue fragments, the Ti
plasmid from Agrobacterium may be used. Tissue fragments
of a callused plant may be infected with Agrobacterium into
which the choline oxidase-coding gene has been integrated,
selected by resistance to an antibiotic such as kanamycin,
and then differentiated in shoots to give a transformed
plant.
- 12 -
CA 02240393 1998-06-23
In the present invention, a highly temperature-
tolerant plant could be obtained by transforming the
brassicaceous plant Arabidopsis thaliana with the choline
oxidase-coding gene as follows.
A binary vector plasmid pGAH-codA carrying the codA
gene was prepared and integrated into Agrobacterium
tumefaciens EHA101 bearing the Ti plasmid. Hypocotyl
calli of Arabidopsis were infected with the resultant
Agrobacterium EHA101 (pGAH/codA) incorporating the codA
gene, then shoots were formed and selected by kanamycin
and hygromycin resistance to induce roots and to form
seeds. The plants obtained from the resultant
heterozygous T2 seeds were self-fertilized to give
homozygous T3 individuals, which were sown to form a
transformed plant.
Thus obtained transformed plant showed that choline
oxidase had been transported to chloroplasts. When
choline and betaine levels in leaf were measured, only
choline was observed in the wild-type plant while both of
choline and betaine were observed in the transformed plant,
suggesting that betaine is accumulated in plants by transfer
of the codA gene. The transformed plant showed a remarkable
temperature tolerance as compared with the wild type.
The monocotyledonous plant rice (Oryza sativa L. cv.
Nippon bare) can be transformed with, for example, two
chimeric codA genes prepared on the plasmid pUC119, which
are localized in cytosol or plastid after translation
under transcriptional control of the cauliflower mosaic
- 13 -
CA 02240393 1998-06-23
under transcriptional control of the cauliflower mosaic
virus 35S promoter. Both of the chimeric genes include a
rice-derived intron in the 5' non-translated sequence to
enhance expression level.
A transformed rice can be produced by the following
procedure. Namely, a transformed plant can be obtained by
introducing said chimeric codA genes into suspension culture
cells from scutellum calli of rice seeds together with a
selection marker hygromycin-resistant gene by a particle gun
device, then selecting the transformed calli based on
antibiotic resistance, and redifferentiating them into a
plant.
Although the wild-type rice lacks betaine-
synthesizing ability, the rice transformed by the method
of the present invention gained betaine-synthesizing
ability. The transformed rice expressing the codA gene grew
equally to the non-transformed plant without showing any
apparent abnormality under both of geoponic and hydroponic
conditions. This may conclude that hydrogen peroxide formed
as a by-product of betaine synthesis was efficiently
detoxified in cells. In view of the relation between
acquisition of betaine-synthesizing ability and temperature
tolerance in cyanobacteria and dicotyledons, the transformed
rice obtained by the method of the present invention can be
expected to also have gained temperature tolerance. This is
the first case in which rice has gained betaine-
synthesizing ability through genetic engineering
techniques.
- 14 -
CA 02240393 1998-06-23
When recovery of photochemical system II of
transformed and non-transformed plants after placed in
dark conditions was compared in experiments using
Synechococcus, the transformed plant recovered more
rapidly, indicating that the presence of betaine
accelerated recovery of photochemical system II. It was
also shown that photosynthesis of the transformed plant is
more tolerant to low temperatures than that of the non-
transformed plant. In view that any great difference was
not found in membrane lipid and protein between the
transformed plant and non-transformed plant, the
protection of photochemical system II observed in the
transformed plant at low temperatures seemed to be an
effect of betaine.
The scope of the present invention covers not only
temperature-tolerant plants produced as described above or
progenies thereof having the same properties, but also
plant cells (for example, callus cultured cells) and plant
portions (for example, flowers, seeds, fruits, tubers,
etc.) obtained therefrom as well as progenies thereof.
According to the present invention, temperature-
tolerant,transformed plants that are highly resistant to
environmental stresses can be obtained. The method of the
present invention can be used to produce plants that can
grow under high-temperature or low-temperature conditions
under which plants normally can not grow. The range of
plants on which can be conferred temperature tolerance by
the method of the present invention is very wide, from
- 15 -
CA 02240393 1998-06-23
photosynthetic bacteria to higher plants. Especially, the
present invention is industrially very useful, because it
is the first case in which stable transformed plants were
obtained from monocotyledons including most main crops and
confirmed for their betaine synthesis.
The method for producing temperature-tolerant plants
according to the present invention is very useful, because
it can be used to produce plants that can tolerate both
high and low temperatures. The following examples
illustrate the present invention more in detail, but are
not construed as limiting the scope thereof.
EXAMPLES
Example 1: Transformation of the cyanobacterium
Synechococcus PCC7942 with the codA gene
(1) Cloning of the codA gene
The choline oxidase gene was isolated from
Arthrobacter globiformis by the method described in the
Abstracts of Oral Reports published in the 34th symposium
of the annual meeting of the Japanese Society of Plant
Physiologists, 1994. In brief, 1) choline oxidase is
fragmented with cyanogen bromide, 2) the N-terminal amino
acid sequence of an appropriate fragment is determined, 3)
appropriate portions are selected from said amino acid
partial sequence to synthesize oligonucleotides
corresponding thereto, 4) a partial sequence of the
choline oxidase gene is amplified by PCR (Polymerase Chain
Reaction) using these oligonucleotides as primers, 5) the
amplified partial sequence of the choline oxidase gene is
- 16 -
CA 02240393 1998-06-23
used as a probe to screen the genomic DNA library of
Arthrobacter globiformis.
Thus obtained positive clones were subcloned into
the plasmid pBluescript (SK+) (Stratagene) to isolate
positive clones, which were subjected to Southern blot
analysis. A 3.6 kbp XbaI-XhoI fragment which hybridized
to said probe was subcloned into pBluescript and mapped with
restriction enzymes. The nucleotide sequence of the region
spanning from the first SalI-site to XhoI-site (about 2.5
kbp) was determined.
The results showed that the choline oxidase gene
contains an open reading frame of 1641 by which encodes a
polypeptide of 547 amino acid residues. The amino acid
sequence and the nucleotide sequence of the choline
oxidase-coding gene are shown as SEQ ID NO: 1 in Sequence
Listing.
(2) Transformation of Synechococcus PCC7942 with the codA
gene
The plasmid pBluescript carrying the codA gene was
digested with the restriction enzymes BstEII (at position
-40 from the translation origin) and SmaI (downstream of
the stop codon). The BstE II-cohesive end was filled with
a Klenow fragment (Takara, Tokyo, Japan). The blunt-ended
fragment containing the coding region of the codA gene and
a putative ribosome binding site was inserted into the
SmaI site of the plasmid pAM1044. The correct orientation
of the gene, which seems to be expressed under control of
the conII promoter of pAM1044, was confirmed by
- 17 -
CA 02240393 1998-06-23
restriction enzyme analysis. The conII promoter is a
consensus sequence of E. coli promoters, containing the
nucleotide sequences TTGGACA (-35) and TATAAT (-10).
The plasmid pAM1044 and the plasmid containing the
codA gene were used to transform Synechococcus PCC7942 by
the method of Elhai et al. The resultant transformant was
designated as the strain PAMCOD. As a control,
Synechococcus PCC7942 was transformed with pAM1044 alone
and designated as the strain PAM.
Selection of transformants was performed on BG11
agar plates containing 30 ~,g/ml of spectinomycin. After
several passages of a single colony to fresh BG11 plates
containing spectinomycin, the complete insertion of the
spectinomycin-resistant gene and the codA gene into all
the copies of chromosomes was confirmed by PCR (Polymerase
Chain Reaction) using the primers shown in Fig. 2A. The
complete insertion of the spectinomycin-resistant gene and
the codA gene into Synechococcus chromosomes was confirmed
by PCR using a combination of primers 1 and 2.
Cultivation of the strains PAMCOD and PAM was
performed in BG 11 medium (Stanier et al., Bacteriol. Rev.
35:171-205, 1971) supplemented with 1 mM choline chloride
(Kitayama Chemical) with aeration at 1~ COZ at 30°C under
illumination with an incandescent lamp of 70 ~,E m-Zs-1.
Logarithically growing cells were used in all the
experiments described below. Photosynthetic activity was
measured at a cell density adjusted to a chlorophyll
concentration of 5-10 ~g/ml.
- 18 -
CA 02240393 1998-06-23
Example 2: Confirmation of the gene inserted into
transformants
DNAs from the strains of wild-type, PAM and PAMCOD
of Synechococcus PCC7942 were used as templates for PCR,
and the amplified products were analyzed by SDS-PAGE. The
results are shown in Fig. 2B.
PCR of DNA from the wild-type strain produced an
amplified product of about 400 by (Fig. 2B, lane b). PCR
using DNA from the strain PAM as a template produced a
band of about 2.4 kb, indicating that pAM1044 had been
inserted into chromosomes. The band of about 400 by as
observed in the wild-type strain does not exist,
indicating that native chromosomes had been completely
replaced by mutant chromosomes in the strain PAM.
When DNA from PAMCOD was used as a template, the
band corresponding to wild-type chromosomes was not
observed (Fig. 2B, lane c). However, the predicted band
of about 4.1 kb was not amplified, either, probably due to
the large size of the insert and the high GC content in
the codA sequence. Therefore, primer 3 corresponding to
the coding region of the codA gene (Fig. 2A) was used in
combination with primer 1. The predicted band of about
2.6 kb was amplified (Fig. 2B, lane d), indicating that
the codA gene exists in chromosomes of the strain PAMCOD.
Example 3: Expression of the codA gene in the
Synechococcus strain PAMCOD
The expression of the codA gene in the strain PAMCOD
obtained in Example 1 was examined by Western blot
- 19 -
CA 02240393 1998-06-23
analysis using a polyclonal antiserum to purified choline
oxidase. The results are shown in Fig. 3. Signals were
detected at 60 kDa in protein extracts from the strain
PAMCOD (lane a) and purified choline oxidase (lane c).
This signal was not detected in protein extracts from the
strain PAM (lane b). This result confirmed that the codA
gene had been expressed in Synechococcus PCC7942 under
control of the conII promoter.
Example 4: Analysis of betaine concentration in cells
Transformed cells were grown in one liter of BG11
medium supplemented with 5 mM choline chloride. Salt
stress was given by adding NaCl at various concentrations.
The harvested cells were treated with 1M HzS04 at 25°C for
hours and betaine was recovered from the mixture by the
15 periodate precipitation technique (Wall, J.S. et al.,
Analyt. Chem. 32:870-874, 1960). Betaine periodate was
dissolved in 1 ml of methanol-d4 (Wako) containing 2 mM 2-
methyl-2-propanol (Wako) as an internal standard. This
solution was measured for 1H NMR spectra in an NMR tube
20 using a Bruker AMX 360 Wb. Betaine was quantified by
comparing the integrated peaks with a standard curve.
Betaine concentration in cells of the strain PAMCOD
was determined on the basis of cell volumes estimated from
the electron micrograph of negatively stained cells. The
cytoplasm of a single cell had a cylindrical shape of 2.14
N,m in length and 0.82 Eun in diameter and the cell volume
was estimated to be approximately 1.13 E.im3.
As a result, betaine concentration in cells of the
- 20 -
CA 02240393 1998-06-23
strain PAMCOD was calculated at about 80mM. However, no
trace of betaine could be detected in the strain PAM lacking
the codA gene.
Example 5: Growth under high- and low-temperatures
stresses
(1) Growth tests in a solid medium
The Synechococcus strains PAM and PAMCOD were
transferred to an agar plate in BG11 medium supplemented
with 1 mM choline chloride and observed for growth at
40°C, 42°C and 44°C. The results are shown in Fig. 4A.
Both
strains almost equally grew at 40°C. Neither strain grew at
44°C. At 42°C, the strain PAM grew very slowly while the
strain PAMCOD grew very well.
Growth at low temperatLres
The Synechococcus strains PAM and PAMCOD were
transferred to an agar plate in BG11 medium supplemented
with 1 mM choline chloride and observed for growth at
22°C, 20°C and 18°C. The results are shown in Fig. 4B.
Both
strains almost equally grew at 22°C. At 20°C, the strain
PAMCOD grew more rapidly than the strain. Neither strain
grew well at 18°C .
(2) Growth tests in a liquid medium
Growth at h-ugh temperatures
Cells of the Synechococcus strains PAM and PAMCOD
preliminarily grown in BG11 medium supplemented with 1 mM
choline chloride at 30°C were transferred to 42°C and
assessed for growth by monitoring the turbidity at a
- 21 -
CA 02240393 1998-06-23
wavelength of 730 nm. The results are shown in Fig. 5A.
Cells of the strain PAMCOD stopped growing on the first
day, but then resumed to grow. However, cells of the
strain PAM did not grew at all.
Growth at low temperatures
Cells of the Synechococcus strains PAM and PAMCOD
preliminarily grown in BG11 medium supplemented with 1 mM
choline chloride at 30°C were transferred to 20°C and
assessed for growth by monitoring the turbidity at a
wavelength of 730 nm. The results are shown in Fig. 5B.
Cells of the strain PAMCOD grew slowly for 4 days, but
then began to rapidly grow. However, cells of the strain
PAM still grew slowly after 4 days.
Example 6: Photosynthetic activity under low-temperature
stress
Inactivation of photosynthetic oxygen release
induced by low-temperature stress was examined. Cells of
the strains PAM and PAMCOD preliminarily grown at 30°C
were grown in the dark at various temperatures. After
then, photosynthetic oxygen release activity was measured
at 30°C in the presence of 1 mM NaHC03 or in the presence of
1,4-benzoquinone and 1 mM K3Fe(CN)6 using a Clark-type
oxygen electrode.
photosynthetic activii~y at low temperatures
Cells were grown in the dark at various temperatures
from 0 to 20°C for 1 hour, then at 30°C for 5 minutes .
After growth, photosynthetic oxygen release activity was
measured in the manner described above. Cells of the
- 22 -
CA 02240393 1998-06-23
strains PAM and PAMCOD showed absolute activities of
photosynthetic oxygen release of 387 ~ 23 and 379 t 19
,mole Oz / mg chlorophyll / hour, respectively, in the
presence of CO2, and the absolute activities of
photosynthetic oxygen release of 802 ~ 36 and 740 ~ 82
mole OZ / mg chlorophyll / hour, respectively, in the
presence of 1,4-benzoquinone and K3Fe(CN)6.
The results are shown in Fig. 6 (A: in the presence
of NaHC03; B: in the presence of 1,4-benzoquinone and
K3Fe(CN)6). As shown in Fig. 6A, the photosynthetic oxygen
release activity of the strain PAMCOD was more tolerant to
low temperatures than that of the strain PAM. As shown in
Fig. 6B, the electron transport activity mediated by
photochemical system II of the strain PAMCOD was also more
tolerant to low temperatures than that of the strain PAM,
i.e. the activity of the strain PAM decreased to 50~ of
the initial level at 5°C while the activity of the strain
PAMCOD remained almost at the initial level at 5°C and
began to decrease below 5°C.
Example 7: Preparation of a binary vector plasmid carrying
the codA gene
A rbcS (ribulose 1,5-bisphosphate carboxylase small
subunit) transit signal XbaI-NdeI fragment (about 200 bp)
from tobacco (Nicotiana sylvestris) was amplified by PCR
using 5'CTGTCTAGATGTAATTAACAATGGCT3' and
5'CCACATATGCATGCATTGCACTCT3' as primers, and XbaI and NdeI
sites were introduced.
Then, an N-terminal-BamHI fragment (about 100 bp) of
- 23 -
CA 02240393 1998-06-23
the codA gene was amplified by PCR using
5'AACCATATGCACATCGACAACATC3' and 5'GCTCCATCCAGCGGTCCAGC3'
as primers, then an NdeI site was introduced. A BamHI-
SmaI fragment (about 1.6 kbp) of the codA gene was
prepared by restriction enzymes. Finally, an SmaI-C-
terminal fragment (about 80 bp) of the codA gene was
amplified by PCR using 5'GAAACAGTCCTGCTTCCACAC3' and
5'GCGAGCTCTGCCTACACCGCCAT3' as primers, and an SacI site
was introduced.
The GUS ((3-glucuronidase) gene in the binary vector
plasmid pBI221 was replaced by these fragments.
The restriction enzyme map of the codA gene is shown
in Fig. 7.
A HindIII-EcoRI fragment containing the cauliflower
mosaic virus 35S promoter and the NOS (nopalin synthase)
terminator was introduced into the binary vector plasmid
pGAH to prepare a plasmid pGAH/codA (Fig. 8). This
plasmid contains kanamycin- and hygromycin-resistant
genes.
Example 8: Introduction of the binary vector plasmid into
Agrobacterium
The Agrobacterium tumefaciens EHA 101 bearing the Ti
plasmid was mixed with the binary vector plasmid pGAH/codA
obtained in Example 7, then frozen and melted, and screened
on LB plates containing tetracycline and kanamycin. The
resultant agrobacterium in which the codA gene had been
integrated was designated as EHA101 (pGAH/codA).
Example 9: Transformation of Arabidopsis
- 24 -
CA 02240393 1998-06-23
The Arabidopsis thaliana strain WS was germinated to
prepare a hypocotyl segment. This hypocotyl was callused
in B5 medium (ICN Biochemicals) (pH 5.7) containing 0.05
mg/1 of kinetin (Wako) and 0.5 mg/1 of 2,4-D (Wako) to
form hypocotyl calli.
Then, the calli were infected with the codA-
containing Agrobacterium EHA101(pGAH/codA) prepared in
Example 8 and cocultivated. After removal of Agrobacterium
by B5 medium containing 250 mg/1 of vancomycin, 500 mg/1 of
carbenicillin and 200 mg/1 of Claforan, the cultures were
transferred to a differentiation medium containing kanamycin
and hygromycin (B5 medium containing 25 mg/1 of kanamycin
and 15 mg/1 of hygromycin) to form shoots. Thus, kanamycin-
and hygromycin-resistant shoots were selected to induce
roots and to form seeds. The resultant T2 seeds are
heterozygous individuals in which only one of the
chromosomes has been transformed.
Then, the plants obtained from the T2 seeds were
self-fertilized and selected by kanamycin and hygromycin
to give homozygous T3 seeds.
The plants of the wild-type and transformant strains
were used for experiments, after grown in a medium (pH
5.2) containing 0.1~ HYPONEX (Hyponex Corporation,
Marysville, OH, USA) at 22°C for 30 days on water or soil
consisting of vermiculite and perlite with illumination of
75 ~,mol m-Zs-1 for 16 hours in a day and in the dark for the
remaining 8 hours unless otherwise indicated.
Example 10: Immunological study of the expressed choline
- 25 -
CA 02240393 1998-06-23
oxidase
An antibody to choline oxidase was prepared
according to the method described in literature by the
inventors of the present invention (Deshniumu, P. et al.,
Plant Mol. Biol. 29:897-907, 1995).
Leaves from 20-day old plants of the wild-type and
transformant strains of Arabidopsis thaliana were ground
in a microcentrifuge at 0°C and the homogenates were
centrifuged at 10,000 x g for 10 minutes to prepare
soluble fractions. Soluble proteins of the supernatant
were separated by SDS-PAGE and transferred to a nylon
membrane (Immobilon PVDF; Millipore, Bedford, MA, USA).
The membrane was incubated with the above antibody to
choline oxidase and detected with a system consisting of a
biotinylated secondary antibody, avidin and biotinylated
horse radish peroxidase (ABC Kit; Vectastain, Burlingane,
CA, USA).
The results of Western blot analysis are shown in
Fig. 9. The presence of an immunoresponsive protein of 64
kDa corresponding to choline oxidase was identified. A
small amount of a protein of 70 kDa corresponding to a
precursor of choline oxidase and the rbcS transit peptide
were also observed. These results show that the codA gene
was correctly integrated and expressed in chromosomes and
that the expressed precursor was processed into a manure
protein.
Then, localization of the expressed choline oxidase
in plants was detected with the above antibody to choline
- 26 -
CA 02240393 1998-06-23
oxidase by a method described in literature (Mustardy, L.
et al., Plant Physiol. 94:334-340, 1990). A small piece
of young leaf from a plant was fixed with 1~
glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2)
for one hour. After rinsed with the same buffer, the
sample was dehydrated with ethanol and placed in Lowicryl
K4M resin (TAAB Laboratories Equipment Ltd., Berkshire,
U.K.). Immuno-gold labeling was done by a method
described in literature (Mustardy et al., supra).
As a result, the expressed choline oxidase was
observed to be localized in stroma of chloroplasts,
indicating that choline oxidase had been transported to
chloroplasts.
Example 11: Determination of betaine and chlorophyll
levels in transformed plants
Betaine content in leaf of plants was calculated by
measuring NMR spectra of a quaternary ammonium compound
(Wall, J. et al., Analyt. Chem. 32:870-874, 1960). 5 g of
leaf of the wild-type and transformed plants were powdered
in liquid nitrogen by a ceramic motor. This powder was
suspended in 25 ml of 1.0 M HZS04 and incubated at 25°C for 2
hours. After unsoluble matters were removed, the
supernatant was recovered by centrifugation at 1000 x g for
10 minutes. The supernatant was incubated with 10 ml of a
KI-IZ solution at 0°C for 2 hours. Betaine and choline
modified with periodide were recovered by centrifugation at
1000 x g for 30 minutes and dissolved in 0.5 ml of CD40H
(Wako) containing 0.5 mM 2-methyl-2- propanol (Wako) as an
- 27 -
CA 02240393 1998-06-23
internal standard to measure 1H NMR spectra. Two main peaks
corresponding to betaine and choline were observed, and the
integrated betaine peaks were used for determination of the
concentration.
Chlorophyll content in leaf was measured by the
following procedure. Leaf (1 g) was powdered in liquid
nitrogen by a ceramic motor. The powder was suspended in
ml of acetone . water (4:1, v/v). After incubation for
30 minutes, unsoluble matters were removed and the
10 supernatant was subjected to spectrophotometry (Arnon,
D.I. Plant Physiol. 24:1-15, 1949).
As a result, both of betaine and choline were
observed in the transformed plant, while only choline was
observed in the wild-type plant. Betaine content was 1.0
E.imol/g fresh leaf . Chlorophyll content was 0 . 3 ~umol/g
fresh leaf .
Example 12: Tolerance of transformed Arabidopsis to low-
temperature stress
A test was performed to determine whether or not the
introduction of the codA gene and accumulation of betaine
confer tolerance on low-temperatures stress.
The wild-type and transformed plants were incubated at
5°C for 7 days under continuous illumination of 250 Eunol
m-Zs-1. No significant difference was observed with the naked
eye between the wild-type and transformed plants. When
these plants were incubated at 22°C for further 2 days,
leaves of the wild-type plant began to droop and whiten.
However, the transformed plant was not apparently affected
- 28 -
CA 02240393 1998-06-23
at all by this treatment.
Example 13: Inactivation of photochemical system II
activity at low temperatures
Influences of low-temperature stress on
photochemical system II activity of leaf of the transformed
plant were assessed by monitoring fluorescence of
chlorophyll. Activity of photochemical system II was
measured as a ratio of variable chlorophyll fluorescence to
maximum chlorophyll fluorescence (Fv/Fm) by using a pulse
intensity-modulated fluorometer (PAM- 2000; Walts,
Effeltrich, Germany) (Annu. Rev. Plant Physiol. Plant Mol.
Biol. 42:313-349, 1991).
The results are shown in Fig. 10. After incubation
at 5°C for 7 days under continuous illumination of 250
~unol m-Zs-1, photosynthetic system II activity decreased in
both of the wild-type and transformed plants. Decline was
sharp on the first day and then slowed down in both of the
wild-type and transformed plants. However, the transformed
plant showed much slower inactivation than the wild-type
plant at every instant. After incubation for 5 days, the
wild-type plant almost completely lost activity, but the
transformed plant kept about 30$ of the original level of
activity. However, no significant difference was observed
in photosynthetic system II activity between them at 10 to
15°C.
Example 14: Inhibition of photosynthesis by low
temperatures and its recovery, as well as freeze
resistance
- 29 -
CA 02240393 1998-06-23
(1) Tests on inhibition of photosynthesis by low
temperatures and recovery from the inhibition of
photosynthesis
The extent of inhibition of photosynthesis was
measured at a temperature as low as 1°C. The results are
shown in Fig. 11A. Leaves of the transformed plant were
more tolerant to inhibition of photosynthesis by low
temperatures than leaves of the wild-type plant. Namely,
leaves of the wild-type plant lost about 75~ of
photosynthetic system II activity after 2.5 hours, while
it took 3.5 hours or more until leaves of the transformed
plant were inactivated to the same extent.
Fig. 11B shows results of recovery test from
photosynthesis inhibition by low temperatures. After the
above low-temperature test, leaves were incubated at 17°C
with 70 E,Lmol m-Zs-1. Leaves of both of the wild-type and
transformed plants showed recovery from photosynthesis
inhibition by low temperatures. However, the transformed
plant showed a higher extent of recovery than the wild-
type plant. After incubation for 4 hours, leaves of the
wild-type plant recovered 25 to 50~ of the original
activity. However, leaves of the transformed plant showed
recovery of 25 to 75~.
(2) Freeze resistance tests on leaves
Leaves of the wild-type and transformed plants of
Arabidopsis were torn and immersed in water at a
temperature decreasing at a rate of 3°C/min. When the
temperature was lowered to -3°C, a needle cooled by liquid
- 30 -
CA 02240393 1998-06-23
nitrogen was applied to leaves to freeze them. Then,
leaves were cooled at a rate of 1°C/hour to various
measuring temperature ranging from -2°C to -12°C. The leaves
were removed and allowed to stand overnight at 4°C so that
they melted. On the following day, the temperature was
returned to room temperature and photochemical system II
activity was measured. The results are shown in Fig. 12.
At any temperature, the transformed plant showed higher
activity than the wild-type plant.
Example 15: Influences of low-temperature treatment at the
water-absorption stage on germination of seeds
Seeds of the wild-type plant and T3 seeds of the
transformed plant were maintained in ice water (about 0°C)
for 2 hours and sterilized, then germinated on MS
(Murashige-Skoog) medium containing 2~ sucrose and 0.5~
gellan gum. Seeds were germinated at 22°C for 20 days
with light for 16 hours and in the dark for 8 hours each
day.
The results are shown in Fig. 13. As apparent from
the figure, both of the wild-type and transformed plants
germinated when their seeds were not subjected to cooling
treatment at the water-absorption stage (Fig. 13A). When
the seeds were subjected to cooling treatment at the
water-absorption stage, the wild-type plant did not
germinate, but the transformed plant germinated and grew
equally to untreated seeds (Fig. 13B).
Example 16: Preparation of a chimeric codA gene used for
transformation of rice
- 31 -
CA 02240393 1998-06-23
Two chimeric codA genes (designated as 35SINcodA and
35SINTPcodA, respectively) which are localized in cytosol
or plastid after translation of the choline oxidase gene
(codA) derived from Arthrobacter globiformis under
transcriptional control of the cauliflower mosaic virus
35S promoter were prepared on the plasmid pUC119 by the
procedure described in Example 6 (see Fig. 14).
Considering that the presence of an intron is required for
high expression of a gene in rice (for example, see
Tanaka, A. et al., Nucleic Acids Res. 18:6767-6770, 1990),
an intron in the 5' non-translated sequence of the
superoxide dismutase gene of rice (SodCc2: Sakamoto, A. et
al., FEBS Lett. 358:62-66, 1995) was introduced into both
chimeric genes. Further, a DNA sequence derived from the
rbcS transit peptide (Coruzz, G. et al., EMBO J 3:1671-1679,
1984) from pea was added to 35SINTPcodA, in order to
transfer the codA protein to chloroplasts.
Example 17: Transformation of rice
Each of the two chimeric codA genes prepared in
Example 16 was introduced into suspension culture cells
from scutellum calli of rice seeds together with the
selection marker hygromycin-resistant gene by a particle
gun device. The transformed calli were selected based on
the antibiotic resistance and redifferentiated into
plants. Polymerase Chain Reaction (PCR) was run on the
transformed calli or transformed/redifferentiated
individuals showing hygromycin resistance, to assess
integration and transcription of the codA gene into the
- 32 -
CA 02240393 1998-06-23
nuclear genome by Northern blot technique and select 80 to
100 or more transformants for each codA gene.
Example 18: Analysis of expression of the codA gene in
transformed rice
The transformants obtained in Example 17 were
screened by Western blot technique to obtain the transformed
rice (the present generation) expressing the codA gene at
the protein level, finally including 6 individuals carrying
the gene localized in plastid and 10 individuals carrying
the gene localized in cytosol.
Rice lacks endogenous choline oxidase activity, but
soluble fractions prepared from leaves or roots of the
transformants showed choline oxidase activity. Contrary
to expectation, all the individuals of the plastid-type
transformants were found to express a lower amount of
choline oxidase protein than the cytosol-type, despite of
the same expression promoter used.
When the expression of the codA gene was further
examined by Northern blot technique, any significant
difference was not found in the amount of both genes
expressed at the transcription level. When processing of
the intron was examined by reverse transcriptive PCR, a
plurality of splicing variants containing different 3'-
acceptor sites which may not bring about normal
translation into protein were detected from the mRNA
transcribed from the plastid-type gene. This suggested
that the low level protein expression by the plant
transformed with the plastid-type gene might be due to
- 33 -
CA 02240393 1998-06-23
abnormal processing of the mRNA precursor. This
phenomenon seems to be related to the fact that the
sequence encoding the transit peptide used for plastid-
targeting of choline oxidase was derived from a
dicotyledon (pea rbcS gene). Therefore, it may be readily
expected that the expression of the codA in rice
chloroplasts would be more efficient and the resultant
transformed rice would be more tolerant to temperature
stress if the sequence encoding the transit peptide was
derived from a monocotyledon such as rice rbcS:
Example 19: Betaine biosynthesis in transformed rice
Betaine accumulating in tissues of transformants
expressing choline oxidase was detected by proton NMR.
Fig. 15 shows the results of the NMR of the wild-type
strain, a transformant which does not express the codA
gene (Fig. 15A) and a transformant which expresses the
codA gene (Fig. 15B).
The transformant which expresses choline oxidase
biosynthesized betaine and the accumulating amount of
betaine showed a positive correlation with the amount of
choline oxidase detected by Western blot technique. The
accumulating amount of betaine was greater in leaves than
in roots and reached 4 ~,mol/g fresh leaf in individuals
highly expressing the codA gene. This is the first case
in which rice gained betaine-synthesizing ability through
a genetic engineering technique.
Example 20: Inactivation of photosynthesis under
illumination at low temperatures
- 34 -
CA 02240393 1998-06-23
In order to examine whether transformed plants gained
temperature tolerance by the presence of betaine or other
causes, the following tests were performed using the
transformant prepared by transforming the Synechococcus
PCC7942 with a plasmid containing the codA gene and PAM
prepared by transforming it with pAM1044 alone in Example
1.
PAM and PAMCOD cells preliminarily grown at 30°C in
the presence of 1 mM choline chloride were grown at 20°C
under illumination or dark conditions. Under dark
conditions, activity of photochemical system II was
maintained. However, activity of photochemical system II
of PAM cells decreased to 35~ of the original level after
cultivation with 500 ~,Eiri Zs-1 for 120 minutes (Fig. 16A) .
Activity of photochemical system II of PAMCOD cells also
decreased but to a lesser extent than PAM cells (Fig.
16A). This revealed that photochemical system II of
PAMCOD cells is more tolerant to photoinhibition than that
of PAM cells.
Photoinhibition of photochemical system II is caused
by competition between light-induced inactivation of D1
protein and recovery of photochemical system II by uptake
of D1 protein newly synthesized (Aro, E.-M. et al.,
Biochim. Biophys. Acta 1019:269-275, 1990; Aro, E.-M. et
al., Biochim. Biophys. Acta 1143:113-134, 1993). In order
to examine whether the tolerance of PAMCOD cells to
photostress at low temperatures results from suppression
of inactivation of D1 protein or promotion of D1 protein
- 35 -
CA 02240393 1998-06-23
synthesis, photoinhibition was induced in the presence of
a protein synthesis inhibitor, linomycin (400 mg/ml).
Under dark conditions, linomycin had no influence on
photochemical system II activity of PAM and PAMCOD cells.
Under illumination, photochemical system II complexes were
inactivated at the same speed in cells of both
transformants (Fig. 16B). This result shows that the
improvement of tolerance of PAMCOD cells to photostress at
low temperatures does not result from suppression of
inactivation of D1 protein. The presence of betaine
seemed to promote recovery of photochemical system II.
Example 21: Recovery from photoinhibition
PAM and PAMCOD cells were tested for recovery of
photochemical system II from photoinhibition by measuring
oxygen release activity. Cells were exposed to light of
3500 ~.Em2s-lto inhibit photochemical system II complexes
to 15~ of the original level. Then, cells were grown at
20°C or 30°C under illumination of 70 ~Em-Zs-1. The results
are shown in Fig. 17.
At 20°C, PAM cells showed only slight recovery of
photochemical system II complexes from photoinhibition.
However, PAMCOD cells recovered to 60~ of the original
level after 2 hours (Fig. 17A). At 30°C, cells of both
strains completely recovered activity of photochemical
system II after 2 hours. However, PAMCOD cells recovered
much faster than than PAM cells (Fig. 17B).
Example 22: Inactivation of photosynthesis under dark
conditions at low temperatures
- 36 -
CA 02240393 1998-06-23
Tolerances of PAM and PAMCOD cells to low-
temperature stress were compared under dark conditions at
low temperatures. Effects of various low-temperature
treatments on inactivation of net photosynthesis and
electron transport mediated by photochemical system II in
both cells are shown in Fig. 18. Oxygen release activity
by photosynthesis of PAMCOD cells was more tolerant to low
temperatures than that of PAM cells (Fig. 18A). Similar
results were obtained for electron transport mediated by
photochemical system II. The activity of PAM cells
decreased to 50~ of the original level at 5°C, while the
activity of PAMCOD cells remained almost at the same level
as that of the control at 5°C and decreased below 5°C (Fig.
18B). These results show that photosynthesis of PAMCOD
cells is more tolerant to low temperatures than that of
PAM cells.
Example 23: Phase transition of protoplasmic membranes
Cyanobacterial cells exposed to low temperatures
have been reported to lessen growth or photosynthetic
activity because of change of the lipid phase of
protoplasmic membranes from liquid crystal state into
phase separation state (Murata, N., J. Bioenerg. Biomembr.
21:61-75, 1989). A test was performed to examine whether
or not the improvement of low-temperature tolerance of
PAMCOD cells is related to the change of the lipid phase
of membranes .
Transition of the lipid phase of protoplasmic
membranes can be tested by agglutination of zeaxanthin by
- 37 -
CA 02240393 1998-06-23
monitoring change of absorbance in cells of Synechococcus
PCC7942 and PCC6301 (previously called as Anacystis
nidulans) at 388 nm (Brand, J.J., Plant Physiol. 59:970-
973, 1977; Gombos, Z. et al., Plant Physiol. 80:415-419,
1986; Murata N., J. Bioenerg. Biomembr. 21:61-75, 1989;
Ono, T. et al., Plant Physiol. 67:176-181; 1981; Wada, H.
et al., Nature 347:200-203, 1990; Yamamoto, H.Y. et al.,
Biochim. Biophys. Acata 507:119-127, 1978). Fig. 19 shows
the results from PAM cells and PAMCOD cells tested by a
similar method. Fig. 19 shows that phase transition of
membrane lipid of PAM cells appears at 10°C and terminates
at 2°C, with the intermediate temperature being 6°C. On
the other hand, transition of membrane lipid of PAMCOD
cells starts at 5°C. This means that lipid transition of
protoplamic membranes of PAMCOD cells occurs at a
temperature 5°C lower than PAM cells.
Example 24: Change of membrane lipid and protein
Transition temperature of membrane lipid of
cyanobacteria has been known to depend on the extent of
unsaturation of fatty acid and the nature of lipid
(Murata, N., J. Bioenerg. Biomembr. 21:61-75, 1989).
Thus, membrane lipid of PAMCOD cells was tested. Tables 1
and 2 show components of the fatty acid and glycerolipid
in protoplasmic membranes and thylakoid membranes of PAM
and PAMCOD cells. Table 1 shows lipid composition in
cells grown at 30°C in the presence of 1 mM choline
chloride. Table 2 shows glycerolipid composition in cells
grown at 30°C in the presence of 1 mM choline chloride.
- 38 -
CA 02240393 1998-06-23
Table 1
Fatty acid
14:0 14:1 16:0 16:1 18:0 18:1(9) 18:1(11)
(mole
Protoplasmic
membrane
PAM 1 1 54 36 3 2 2
PAMCOD 2 2 53 38 2 2 2
Thylakoid
membrane
PAM 1 2 52 40 2 2 2
PAMCOD 1 2 50 40 2 2 2
Abbreviation: 14:0: myristic acid
14:1: ~9-myristic acid
16:0: palmitic acid
16:1: 09-palmitic acid
18:0: stearic acid
18:1(9): 09-stearic acid
18:1(11): 011-stearic acid
All the double bonds are in cis-form.
- 39 -
CA 02240393 1998-06-23
Table 2
Tylakoid membrane Protoplasmic membrane
Lipid class PAM PAMCOD PAM PAMCOD
(mole ~) (mole
MGDG 54 53 56 55
DGDG 22 23 19 19
SQDG 14 14 15 15
PG 10 10 10 11
Abbreviation: MGDG: monogalactosyl diacylglycerol
DGDG: digalactosyl diacylglycerol
SQDG: sulfoquinovosyl diacylglycerol
PG: phosphatidylglycerol
As is apparent from Tables 1 and 2, no significant
difference was observed between both.
Fig. 20 shows electrophoretic patterns of proteins
of membrane and soluble fractions of PAM and PAMCOD cells.
A slight difference was observed in membrane fractions.
Namely, PAMCOD cells showed an increase in amount of the
protein of 14 kda and a decrease in amount of the protein
of 16 kda. No other difference was found in soluble
fractions except that PAMCOD cells showed a band
corresponding to choline oxidase.
Thus, there was found no significant difference in
membrane lipid or protein between PAM and PAMCOD cells,
indicating that the protection of photochemical system II
- 40 -
CA 02240393 1998-06-23
as seen in PAMCOD cells at low temperatures is an effect of
betaine.
- 41 -
CA 02240393 1998-06-23
SEQUENCE LISTING
(1} GENERAL INFORMATION
(i) APPLICANT:
(A) NAME: SUNTORY LIMITED
(B} STREET: 14-64-602 Fubuki-cho
(C) CITY: Okazaki-shi
(D) STATE: Aichi
(E) COUNTRY: JAPAN
(F) POSTAL CODE (ZIP): 444-0817
(ii) TITLE OF THE INVENTION: Method for producing temperature-
tolerant plants
(iii) NUMBER OF SEQUENCES: 1
(iv} CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Swabey Ogilvy Renault
{B) STREET: 1981 McGill College, suite 1600
(C) CITY: Montreal
(D) STATE: Quebec
(E) COUNTRY: Canada
(F) ZIP: H3A 2Y3
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Diskette
(B) COMPUTER: IBM Compatible
(C) OPERATING SYSTEM: DOS
(D) SOFTWARE: Word 6.0 for Windows
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B} FILING DATE: 27-DEC-1996
(C) CLASSIFICATION:
(vii} PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: PCT/JP96/03873
(B) FILING DATE: 27-DEC-1996
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: JP 343354/1995
(B) FILING DATE: 28-DEC-1995
(vii) PRIOR APPLICATION DATA:
(A} APPLICATION NUMBER: JP 97534/1996
(B) FILING DATE: 27-MAR-1996
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: CaT$, France
(B) REGISTRATION NUMBER: 4166
(C) REFERENCE/DOCKET NUMBER: 4734-183 FC/ntb
{ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (514) 845-7126
(B) TELEFAX: (514) 288-8389
(C) TELEX:
- 42 -
CA 02240393 1998-06-23
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2400 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(ix) FEATURE:
(A) NAME/KEY: mat peptide
(B) LOCATION: 361..2002
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:1:
GGGAATATCC GTCGTCGTAG ACGAGCCCTT CGGCCCGTGT AAAGGTGGAG ACCTTCCACA 60
CCGAGGACGA GGCCGTCGCG ACCGCCAACG ACACCAACTA CGGGCTGTCC GGCGCGGTCC 120
TGGACCCAGG ACGCCGGCAA GACGCAGCGC GTGGCCGGCC GGCTGCGACA CGGCACCGTC 180
TGGATCAACG ACTTCCACCC CTACCTCCCA CAGACCGAGT GGGGCGGCTT CGGCCAGTCC 240
GGCGTCGGCC GCGAACTCGG CCCGACCGGC CTGGCCGAGT ACCAGGAGGC CAAGCACATC 300
TACCAGAACA CCAGCCCGCA GGTCACCGGC TGGTTCGCTG ACCACGGCAA GGAGAACTAG 360
ATGCAC GAC GAG CTG GAG TTCGACTAC 408
ATC AAC AAC AGC
ATC GAC
AGG
MetHis Asp IleGlu LeuSer ArgGlu PheAspTyr
Ile Asn Asn Asp
1 5 10 15
ATCGTC GGC GGGTCC GGGGCC GTCGCC GCCCGGCTG 455
GTC GGC GCC GCC
IleVal Gly GlySer GlyAla ValAla AlaArgLeu
Val Gly Rla Ala
20 25 30
AGCGAG CCC GTGAGC GCGCTG GAGGCC GGCCCGGAT 504
GAT GCA GTG GTG
SerGlu Pro ValSer AlaLeu GluAla GlyProAsp
Asp Ala Val Val
35 40 45
GACCGC GTG GAGGTG CAGCTG CGCTGG ATGGAGCTG 552
GGC CCC CTG GRC
AspArg Val GluVal GlnLeu ArgTrp MetGluLeu
Gly Pro Leu Asp
50 55 60
CTGGAA GGC GACTGG TACCCG GAGCCG CAGGAGAAC 600
TCG TAC GAC ATC
LeuGlu Gly AspTrp TyrPro GluPro GlnGluAsn
Ser Tyr Asp Ile
65 70 75 80
GGCAAC TTC CGCCAT CGTGCC GTCATG GGCGGCTGC 648
TCC ATG GCC AAG
- 43 -
CA 02240393 1998-06-23
Gly Asn Ser Phe Met Arg His Ala Arg Ala Lys Val Met Gly Gly Cys
85 90 95
TCC AGC CAC AAC TCC TGC ATC GCC TTC TGG GCC CCG CGC GAG GAC CTG 696
Ser Ser His Asn Ser Cys Ile A1a Phe Trp A1a Pro Arg Glu Asp Leu
100 105 110
GAC GAG TGG GAG GCC AAG TAC GGC GCC ACC GGC TGG AAC GCC GAG GCG 744
Asp Glu Trp Glu Ala Lys Tyr Gly Ala Thr Gly Trp Asn Ala Glu Ala
115 120 125
GCC TGG CCG CTG TAC AAG CGG CTG GAA ACC AAC GAG GAC GCG GGC CCG 792
Ala Trp Pro Leu Tyr Lys Arg Leu Glu Thr Asn Glu Asp Ala Gly Pro
130 135 140
GAC GCG CCG CAC CAC GGG GAC TCC GGC CCC GTG CAC CTG ATG AAC GTG 840
Asp Ala Pro His His Gly Asp Ser Gly Pro Val His Leu Met Asn Val
145 150 155 160
CCC CCG AAG GAC CCG ACC GGC GTC GCG CTC CTG GAC GCC TGC GAG CAG 888
Pro Pro Lys Asp Pro Thr Gly Val A1a Leu Leu Asp Ala Cys Glu Gln
165 170 175
GCC GGC ATC CCG CGC GCG AAG TTC AAC ACC GGC ACC ACC GTG GTC AAC 936
Ala Gly Ile Pro Arg Ala Lys Phe Asn Thr Gly Thr Thr Val Val Asn
180 185 190
GGC GCC AAC TTC TTC CAG ATC AAC CGG CGC GCG GAC GGC ACC CGC TCC 984
Gly Ala Asn Phe Phe Gln Ile Asn Arg Arg Ala Asp Gly Thr Arg Ser
195 200 205
TCC AGC TCG GTC TCC TAC ATC CAC CCG ATC GTC GAG CRG GAG AAC TTC 1032
Ser Ser Ser Val Ser Tyr Ile His Pro Ile Val Glu Gln Glu Asn Phe
210 215 220
ACC CTG CTA RCC GGC CTG CGC GCC CGC CAG CTG GTG TTC GAC GCG GAC 1080
Thr Leu Leu Thr Gly Leu Arg Ala Arg Gln Leu Val Phe Asp Ala Asp
225 230 235 240
RGG CGC TGC ACC GGC GTC GAC ATC GTG GAC TCC GCC TTC GGC CGC ACC 1128
Arg Arg Cys Thr Gly Val Asp Ile Val Asp Ser Ala Phe Gly Arg Thr
245 250 255
CAT CGG CTG ACG GCG CGC AAT GAA GTC GTG CTC TCC ACC GGC GCG ATC 1176
His Arg Leu Thr Ala Arg Asn Glu Val Val Leu Ser Thr Gly Ala Ile
260 265 270
GAT ACG CCG AAG CTG TTG ATG CTC TCC GGA ATC GGC CCC GCC GCC CAC 1224
Asp Thr Pro Lys Leu Leu Met Leu Ser Gly Ile Gly Pro Ala Ala His
275 280 285
CTC GCC GAG CAC GGC ATC GAG GTC CTT GGT GGA CTC CCC CGG CGT GGG 1272
-44-
CA 02240393 1998-06-23
Leu Ala Glu His Gly Ile Glu Val Leu Gly Gly Leu Gly
Pro Arg Arg
290 295 300
CGA GCA CCT GCA GGA CCA CCC GGR AGG CGT GGT GCA CAA 1320
GTT CGA GGC
Arg Ala Pro Ala Gly Pro Pro Gly Arg Arg Gly Ala Gln
Val Arg Gly
305 310 315 320
GCA GCC CAT GGT CGC CGA GTC CAC GCA GTG GTG GGA CTT 1368
GAT CGG CAT
Ala Ala His Gly Arg Arg Val His Ala Val Val Gly Leu
Asp Arg His
325 330 335
CAC CCC CAC CGA GGA CGG CCT GGA CCG CCC CGA CCT CTA 1416
GAT GAT GCA
His Pro His Arg Gly Arg Pro Gly Pro Pro Arg Pro Leu
Asp Asp Ala
340 345 350
CGG CTC CGT GCC GTT CGA CAT GAA CAC CCT GCG GCA CAC 1464
CGG CTA CCC
Arg Leu Arg Ala Val Arg His Glu His Pro Ala A1a His
Arg Leu Pro
355 360 365
CAC GGA GAA CGG GCT TCA GCC TCA CCC CGA ACG TCA GCT 1512
CGC ACG CCC
His Gly Glu Arg Ala Ser Ala Ser Pro Arg Thr Ser Ala
Arg Thr Pro
370 375 380
CCC GCG GCA CTG TCC GGC TGC GCA GCC GCG ACT TCC CCA 1560
GCG ATA AGC
Pro Ala Rla Leu Ser Gly Cys A1a Ala A1a Thr Ser Pro
Ala Ile Ser
385 390 395 400
TGG TCG ACC CGC GCT ACT TCA CCG ACC CAG AAG GGC CGC 1608
CAT GAC RTG
Trp Ser Thr Arg Ala Thr Ser Pro Thr Gln Lys Gly Arg
His Asp Met
405 410 415
GTC ATG GTC GCC GGC ATC CGC AAG GCC CGC GAA ATC CCC 1656
GCC GCC CAG
Val Met Val Ala Gly Ile Arg Lys Ala Arg Glu Ile Pro
Ala Ala Gln
420 425 430
GCC ATG GCG GAA TGG ACC GGC CGC GAG CTC TCC CCC GCG 1704
GGC GTC GAG
Ala Met Ala Glu Trp Thr Gly Arg Glu Leu Ser Pro Ala
Gly Val Glu
435 440 445
CAG RCC GAC GAG GAG CTG CAG GAC TAC ATC CGC AAG ACC 1752
ACG CAC AAC
Gln Thr Asp Glu Glu Leu Gln Asp Tyr Ile Arg Lys Thr
Thr His Asn
450 455 460
GTC TAC CAC CCC GTG GGC ACC GTG CGC ATG GGC GCG GAG 1800
GTC GAG GAC
Val Tyr His Pro Val Gly Thr Val Arg Met Gly Ala Glu
Val Glu Asp
465 470 475 480
ATG TCC CCG CTC GAC CCC GAG CTG CGG GTC AAG GGC CTG 1848
GTC ACC GGT
Met Ser Pro Leu Asp Pro Glu Leu Arg Val Lys Gly Leu
Val Thr Gly
485 490 495
CGC GTC GGC GAC GCC TCG GTC ATG CCC GAG CAC GTG CCC 1896
ACC GTC AAC
- 45 -
CA 02240393 1998-06-23
Arg Val Gly Asp Ala Ser Val Met Pro Glu His Val Thr Val Asn Pro
500 505 510
AAC ATC ACC GTC ATG ATG ATC GGC GAG CGC TGC GCG GAC CTT ATC CGC 1944
Asn Ile Thr Val Met Met Ile Gly Glu Arg Cys Ala Asp Leu Ile Arg
515 520 525
TCC GCC CGC GCC GGT GAA ACA ACG ACG GCG GAC GCC GAG CTG AGC GCG 1992
Ser Ala Arg Ala Gly Glu Thr Thr Thr Ala Asp Ala Glu Leu Ser Ala
530 535 540
GCC CTC GCC TAAGCGGGAG CGGCCAGCCG CGGGGCCTGT CCGGAACCAC CTGGCGGGCC 2051
Ala Leu Ala
545 547
CCGCATGGGG CCGGACACAA TGCCGGTAAC TAAGGGTGCG GAAGCAGTCC TGCTTCCACA 2111
CCCGCGTTTT GCACGCCCGG GCCGGCAACT GGCCCGGCCG GCTAAGCCGA AGGTCTTCCG 2171
GGGGCGGGCC GGATCGCTGC GGGCAGTCCG TCGGCCAGCC GCTGCAGCGT GCCGGCGGTA 2231
ATGGCGGTGT AGGCAGGGAT CGCGTCGGGG TAGATGTACT CGTTGCGGGC GTGCGCGCCG 2291
TCGCCCACCG CGCCCAGGCC GCACAGGACC GGGATGCCGA GGGCGGAGAC GAAGTTGGCG 2351
TCGCTGCCCC CGCCCACCGA GGCGGTTTCC AGCTCCCGGC CCTGCTCCA 2400
-46-