Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02298890 2000-02-17
BACKROUND OF THE INVENTION
Field of the Invention
The present invention relates to an method for preparing manometer-sized
ceramic particles, and
more particularly, it relates to prepare manometer-sized composite ceramic
powders, at a high
production rate.
The manometer-sized metal-oxide powders are known to exhibit unique physical
and mechanical
properties. The novel properties of nano-crystalline materials are the result
of their small residual
pore sizes, limited grain size, phase or domain dimain dimensions, and large
fraction of atoms
residing in interfaces. In a mufti-phase material, limited phase dimensions
could imply a limited
crack propagation path if the brittle phase is surrounded by ductile phase so
the cracks in a brittle
phase would not easily reach a critical crack size. Even with only one
constituent phase, nano-
crytstalline materials may be considered as two-phase materials, composed of
distinct interface and
crystalline phase. The possibilities for reacting, coating, and mixing various
types of nanometer-
sized materials create the potential for fabricating new composites with nano-
sized phases and novel
properties.
Ultra-fine particles with a narrow sized distribution have enormous potential
in ceramic processing.
For example, a green density of 75as been achieved by compaction of nano-
crystalline titanic
prepared by inert gas condensation of metal vapors. Mono-dispersed particles
are known to form a
more uniform green micro-structure, which allows for a better control of the
micro-structure during
densification. In addition, smaller particles can be sintered at much lower
temperatures. Not only
the structure, but also the mechanical, electronic, optical, magnetic and
thermal properties of nano-
crystalline materials are different from those exhibited by their bulk
counterparts. Specifically,
ceramics fabricated from ultra-fine particles are known to possess high
strength and toughness
because of the ultra-small intrinsic defect sizes and the ability for grain
boundaries to undergo a
large plastic deformation. Additionally, ultra-fine grained metals could
exhibit unusually high
strength and hardness. For a review on nano-phase materials please refer to
R.P. Anders, et al.
"Research Opportunities on Clusters and Cluster-Assembled Materials," in
Journal of Materials
Research, Vol.4, 1989, pp.704-736 and A. N. Goldstein,"Handbook of Nanophase
Materials,"
Marcel Dekker, Inc., New York, 1997.
-1-
CA 02298890 2000-02-17
The techniques for the generation of nanometer-sized particles may be divided
into three broad
categories: vacuum, gas-phase, and condensed-phase synthesis. Vacuum synthesis
techniques
include sputtering, laser ablation, and liquid-metal ion sources. Gas-phase
synthesis includes inert
gas condensation, oven sources (for direct evaporation into a gas to produce
an aerosol or smoke of
clusters), laser-induced vaporization, laser pyrolysis, and flame hydrolysis.
Condensed-phase
synthesis includes reduction of metal ions in an acidic aqueous solution,
liquid phase precipitation
of semiconductor clusters, and decomposition-precipitation of ionic materials
for ceramic clusters.
Other methods include high-energy milling, mix-alloy processing, chemical
vapor deposition
(CVD), and sol-gel techniques. All of these techniques have one or more of the
following
problems or shortcomings:
(1) Most of these prior-art techniques suffer from a severe drawback:
extremely low production
rates. It is not unusual to find a production rate of several grams a day.
Vacuum sputtering, for
instance, only produces small amounts of particles at a time. Laser ablation
and laser-assisted
chemical vapor deposition techniques are well-known to be excessively slow
processes. The
high-energy ball milling method, known to be a "quantity" process, is capable
of producing only
several kilograms of nanometer-scaled powders in approximately 100 hours.
These low
production rates, resulting in high product costs, have severely limited the
utility value of nano-
phase materials. There is, therefore, a clear need for a faster, more cost-
effective method for
preparing nanometer-sized powder materials.
(2) Condensed-phase synthesis such as direct reaction of metallic silicon with
nitrogen to produce
silicon nitride powder requires pre-production of metallic silicon of high
purity in finely
powdered form. This reaction tends to produce a silicon nitride powder product
which is
constituted of a broad particle size distribution. Furthermore, this
particular reaction does not
yield a product powder finer than 100 nm (nanometers) except with great
difficulty. Due to the
limited availability of pure metallic silicon in finely powdered form, the use
of an impure
metallic powder necessarily leads to an impure ceramic product. These
shortcomings are true of
essentially all metallic elements, not just silicon.
(3) Some processes require expensive precursor materials to ceramic powders
and could result in
harmful gas that has to be properly disposed o~ For instance, the reaction
scheme of
3SiCl~+4NH3=Si3N4+l2HCl involves the utilization of expensive SiC~ and
produces dangerous
HCl gas. Processes that begin with the high temperature treatment of expensive
precursor
compounds are exemplified by those disclosed in CANADA Pat. CA 2000232 (Oct. 6
, 1989 to
Riedel, Ralf ), CANADA Pat. CA 2031330 (Nov. 30, 1990 to Laubach, Benno),U.S.
Pat.
-2-
CA 02298890 2000-02-17
5,075,090 (Dec. 24,1991 to Lewis, et al.) and U.S. Pat. 4,948,762 (Aug. 14,
1990 to Krumbe,
et al.).
(4) Most of the prior-art processes are capable of producing a particular type
of ceramic powder at a
time, but do not permit the reparation of a uniform mixture of two or more
types of nano-scaled
ceramic powder at a predetermined proportion.
(5) Most of the prior-art processes require heavy and/or expensive equipment
(e.g., a high power
laser source or a plasma generator), resulting in high production costs.In the
precipitation of
ultra fine particles from the vapor phase, when using thermal plasmas or laser
beams as energy
sources, the particle sizes and size distribution cannot be precisely
controlled. Also, the reaction
conditions usually lead to a broad particles size distribution as well as the
appearance of
individual particles having diameters that are multiples of the average
particle size. Processes
that involve hot plasma- or flame-induced gas phase reactions are disclosed in
several
CANADA Pat. CA 2089874 (Aug. 30, 1991 to Marantz, Daniel R.), CANADA Pat. CA
2160367 (May 10, 1994 to Helble, Joseph J.), CANADA Pat. CA 2153048 (Feb. 2 ,
1999 to
Helble, Joseph J.),U.S.patents: e.g., 4,282,195 (Aut.4, 1981 to H.H.Hoekje),
4,689,075 (Aug.25,
1987 to Uda, et al.), 4,889,665 (Dec.26,1989 to Uda, et al.), 5,599,511
(Feb.4, 1997 to Helble, et
al.), and 5,019,686 (May 28, 1991 to Marantz).
The Conventional mechanical attrition and grading processes have the
disadvantages that powders
can only be produced up to a certain fineness and with relatively broad
particle-size distribution. As
a matter of fact, with the currently familiar large-scale process for
manufacturing powders it is
rarely possible, or only possible with considerable difficulty, to produce
powders having average
particle sizes of less than 0.5 (micrometers).It is known that melt
atomization technique has been
employed to produce ultra fine metal powders, but rarely for producing ceramic
powders directly.
This is largely due to the fact that ceramic materials such as oxides and
carbides have much higher
melting temperatures as compared to their metal counterparts and require ultra-
high temperature
melting facilities. Therefore, ultra fine ceramic particles are usually
produced by first preparing
ultra fine base metal particles, which are then converted to the desired
ceramics by a subsequent
step of oxidation, carbonization, nitrogenization, etc.. These multiple-step
processes are tedious and
expensive. In solution or sol-gel type processes, atomization of precursor
solutions to ceramics
requires an additional step of solvent removal. Furthermore, the production
rates of these processes
are relatively low and the final products are expensive.The present invention
is to provide a
technique for producing manometer-sized ceramic powder, and more particularly,
it can prepare
manometer-sized composite ceramic powders at a high production rate.
-3-
CA 02298890 2000-02-17
SUMMARY OF THE INVENTION
The nano-sized ceramic powders can be prepared rapidly using an atomizing-
reaction technique by
which the superheated fluid reactive element is sprayed into a reaction
chamber by means of a
pressured preheated fluid medium through an atomizer, then the reactive
element will occur
chemical reaction with the fluid medium . The reactive element is metal or
alloy mainly, and the
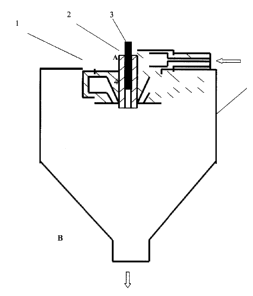
fluid medium is oxygen , nitrogen or chlorine mainly. In figure l, the metal
or alloy melt 3 is
supplied into the reaction chamber through a guiding pipe 2 , the pressurized
reactant fluid medium
is introduced into reaction chamber 4 through a nozzle 1 from inlet A . A
reaction procedures
between the super-heated metal or alloy melt and the fluid medium take place
in the reaction
chamber immediately, and the nano-sized reactant ceramic powder can be
collected in outlet B.
The above-cited superheated temperature is approximately defined to be the
temperature at which
the surface tension of the metal melt is negligibly small or at which the
metal melt is capable of
initiating a substantially spontaneous reaction with a reactant species
contained in the atomizing
medium. The pressurized fluid not only possesses a sufficient kinetic energy
to break up the metal
melt stream into finely divided droplets, but also contains active reactant
species to undergo a
reaction with these fine metal droplets at high temperatures in a
substantially spontaneous
fashion.This process comprises: (a) providing a super-heated melt of a
metallic element or the alloy
into a reaction chamber through an atomizer ; (b) during the melt supplying
procedure, introducing
a pressurized reactant fluid medium element into this atomizer for atomizing
the melt and initiating
a reaction between the metal melt droplets and the reactant fluid medium to
produce nano-sized
reactant ceramic powder. Advantages of the present invention may be summarized
as follows:
1. A wide variety of nano-scaled ceramic particles can be readily produced.
The corresponding
reactants may be selected from the group of hydrogen, oxygen, nitrogen,
carbon, chlorine,
fluorine, and sulfur to form respectively metal hydrides, oxides, nitrides,
carbides, chlorides,
fluorides, and sulfides and combinations thereof. No known prior-art technique
is so versatile in
terms of readily producing so many different types of nano-scaled ceramic
powders.
2. The presently invented process makes use of the concept that a metal melt,
when superheated to
an ultra-high temperature (e.g., reaching 2 to 6 times its melting temperature
in degrees K) has a
negligibly small surface tension so that a melt stream can be easily broken up
into ultra-fine
droplets .So the metal element can contact with the atomizing medium as much
as possible.
-4-
CA 02298890 2000-02-17
More particularly, when the temperature of the melt up to the critical value,
the atoms of the
melt can be easily chemical combined with the atoms of the atomizing medium.
3. The metal melt can be an alloy of two or more elements which are uniformly
dispersed. When
broken up into ultrafme clusters, these elements remain uniformly dispersed
and are capable of
reacting with selected reactant species to form uniformly ceramic powder
particles.
4. The selected super-heat temperatures also fall into the range of
temperatures within which a
spontaneous reaction between a metallic element and a reactant gas such as
oxygen can occur.
The reaction heat released is automatically used to maintain the reacting
medium in a
sufficiently high temperature so that the reaction can be self sustaining
until completion. The
reaction between a metal and certain gas reactant (e.g., oxygen) can rapidly
produce a great
amount of heat energy, which can be used to drive other reactions that occur
concurrently or
subsequently when other reactant elements (e.g., nitrogen) are introduced.
DESCRIPTION OF PREFERRED EMBODIMENTS
Figurel schematically shows an apparatus, in accordance with a preferred
embodiment of the
present invention, for preparing composite nanosized ceramic powders. This
apparatus comprises
four necessary main components. The superheated metal or alloy melt 3 from a
smelter is supplied
into the reaction chamber 4 through a guiding pipe 2 , the pressurized
reactant fluid medium is
introduced into reaction chamber through a nozzle 1 from inlet A . A reaction
procedures between
the super-heated metal or alloy melt and the fluid medium take place in the
reaction chamber
immediately, and the nano-sized reactant ceramic powder can be collected in
outlet B. In present
invention, the metallic element may be selected from the group of some low
melting point elements
containing bismuth(Bi), tin(Sn), lead(Pb), zinc(Zn), Indium(In), cesium(Cs)
and rubidium(Rb) etc;
and the alloy may be the low melting point one in which containing foresaid
low melting point
metals . For example, the Bi-Sn alloy, Pb-Sn alloy, In present inventon the
foresaid metal or alloy
melt should be superheated to reach 3 to 6 times its melting temperature. In
present invention, the
fluid medium may be oxygen, nitrogen or chlorine etc; the reactant will be
metal oxides ,nitrides
and chlorides etc; In present invention , when the superheated melt is an
alloy , the reactant will
be multi-components. For example, if the melt is Sn-Zn alloy and the medium is
oxygen, the
reactant will be Sn02 / Zn0 nanosized composite powder.
-5-