Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98116290
CONJUGATES TARGETED TO THE INTERLEDKIN-2 RECEPTOR
Background of the Invention
This invention relates to delivery of chemical
agents to cells. More particularly, this invention
relates to compositions and methods for intracellular
delivery of chemical agents to a specific cell type,
i.e. cells bearing the interleukin-2 (IL-2) receptor.
Toxins that target cell surface receptors or
antigens on tumor cells have attracted considerable
attention for treatment of cancer. Era., I. Pastan &
D. FitzGerald, Recombinant Toxins for Cancer
Treatment, 254 Science 1173 (1991); Anderson et al.,
U.S. Patent Nos. 5,169,933 and 5,135,736; Thorpe et
al., U.S. Patent No. 5,165,923: Jansen et al., U.S.
Patent No. 4,906,469; Frankel, U.S. Patent No.
4,962,188; Uhr et al., U.S. Patent No. 4,792,447;
Masuho et al., U.S. Patent Nos. 4,450,154 and
4,350,626. These agents include a cell-targeting
moiety, such as a growth factor or an antigen-binding
protein, linked to a plant or bacterial toxin. They
kill cells by mechanisms different from conventional
chemotherapy, thus potentially reducing or eliminating
cross resistance to conventional chemotherapeutic
agents.
Copending U.S. Patent Application Serial No.
08/305,770, filed September 13, 1994, describes
compositions and methods for specific intracellular
delivery of a chemical agent into a CR2-receptor-
bearing cell, e.g. B lymphocytes. The compositions
comprise a CR2-receptor-binding and endocytosis-
inducing ligand (CBEL) coupled to the chemical agent.
The CBEL binds to the CR2 receptor on the surface of B
lymphocytes and elicits endocytosis of the composition
such that the composition is transported to lysosomes.
Optionally, the composition can include a spacer,
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
2
which can be either biodegradable (in the lysosome) or
non-biodegradable, for coupling the CBEL to the
chemical agent. Chemical agents can include
cytotoxins, transforming nucleic acids, gene
regulators, labels, antigens, drugs, and the like.
The composition can further comprise a carrier such as
another water soluble polymer, liposome, or
particulate.
Copending U.S. Patent Applications Serial No.
08/616,693, filed March 15, 1996, and Serial No.
08/857,009, filed May 15, 1997, describe compositions
and methods for specific intracellular delivery of a
chemical agent into T lymphocytes. The compositions
are represented by the formula [L-S]a-C-[S-A]b wherein
L is a ligand configured for binding to a receptor on
a T lymphocyte and stimulating receptor-mediated
endocytosis of the composition, A is a chemical agent,
S is a spacer moiety, C is a water soluble polymer
having functional groups compatible with forming
covalent bonds with the ligand, chemical agent, and
spacer, and a and b are positive integers. These
compositions are also designed to be transported to
lysosomes. Preferred water soluble polymers include
polyethylene glycol) and a copolymer of N-(2-
hydroxypropyl)methacrylamide (HPMA). Preferred
chemical agents include cytotoxins, transforming
nucleic acids, gene regulators, labels, antigens,
drugs, and the like. The composition can further
comprise a carrier such as other water soluble
polymers, liposomes, or particulates.
It would also be advantageous to develop
additional compositions that are specifically targeted
to other receptors on T lymphocytes. For example,
targeting of T lymphocytes would enable therapeutic
applications for T-cell-associated diseases and tissue
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
3
graft rejection. Such T-cell-associated diseases
include arthritis, T-cell lymphoma, skin cancers,
psoriasis, and diseases resulting from HIV infection.
In view of the foregoing, it will be appreciated
that compositions for intracellular delivery of
chemical agents to T cells and methods of use thereof
would be significant advancements in the art.
0~lects end summary of the Invention
It is an object of the present invention to
provide compositions for intracellular delivery of
selected chemical agents to a specific cell type, i.e.
IL-2-receptor-bearing cells.
It is also an object of the invention to provide
methods of making and methods of using compositions
for intracellular delivery of selected chemical agents
to IL-2-receptor-bearing cells.
It is another object of the invention to provide
compositions and methods for delivering selected
chemical agents to IL-2-receptor-bearing cells using
water soluble polymers that are inexpensive, FDA-
approved, and resistant to development of an antibody
response.
It is yet another object of the invention to
provide compositions and methods of use thereof for
intracellular delivery of selected chemical agents to
activated T cells.
These and other objects are achieved by providing
a composition for intracellular delivery of a chemical
agent into an IL-2-receptor bearing cell, the
composition comprising (a) a water-soluble,
biocompatible polymer, (b) the chemical agent
covalently, releasably coupled to the polymer, and (c)
at least two copies of a ligand comprising an IL-2-
CA 02299368 2000-02-04
WO 99/07324 PCTlUS98/16290
4
receptor-binding peptide covalently coupled to the
polymer.
In a preferred embodiment of the invention, the
composition has a formula selected from the group
consisting of P- [Ta-L-Sb-A] ~ and [A-Sb] d-P- [Te-L] ~,
wherein L is the ligand; A is the chemical agent: S
and T are spacers, wherein at least S is
biodegradable; P is a water soluble polymer having
functional groups compatible with forming covalent
bonds with the ligand; a and b are integers of 0 or 1~
c is an integer of at least 2; and d is an integer of
at least 1.
Preferably, P is a polyalkylene oxide. Preferred
polyalkylene oxides are selected from the group
consisting of alpha-substituted polyalkylene oxide
derivatives, polyethylene glycol (PEG} homopolymers,
polypropylene glycol homopolymers, alkyl-capped
polyethylene oxides, bis-polyethylene oxides,
copolymers of poly(alkylene oxides), and block
copolymers of poly(alkylene oxides} or activated
derivatives thereof. Preferably, the polyalkylene
oxide has a molecular weight of about 200 to about
50,000. More preferably, the polyalkylene oxide has a
molecular weight of about 2,000 to about 20,000. Most
preferably, the polyalkylene oxide has a molecular
weight of about 5,000. Especially preferred
polyalkylene oxides are polyethylene glycol and
polyethylene oxide.
The IL-2-receptor-binding peptide is preferably a
member selected from the group consisting of SEQ ID
NO:l and biologically functional equivalents thereof.
More preferably, the IL-2-receptor-binding peptide is
a member selected from the group consisting of SEQ ID
N0:1 through SEQ ID NO:11.
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98l16290
The chemical agent is preferably selected from
the group consisting of cytotoxins, transforming
nucleic acids, gene regulators, labels, antigens, and
drugs.
5 Preferably, the spacer comprises a peptide. A
preferred peptide spacer comprises Gly-Phe-Leu-Gly
( SEQ I D NO : 21 ) .
In one preferred embodiment, the composition
further comprises a carrier selected from the group
consisting of other water soluble polymers, liposomes,
and particulates. Preferably, such water soluble
polymers are selected from the group consisting of
dextran, inulin, poly(L-lysine) with modified epsilon
amino groups, poly(L-glutamic acid), and N-substituted
methacrylamide-containing polymers.
A method of delivering a chemical agent in vitro
into an TL-2-receptor-bearing cell in a heterogeneous
population of cells, comprises the steps of:
(a) providing a composition comprising (i) a
water-soluble, biocompatible polymer, (ii) the
chemical agent covalently, releasably coupled to the
polymer, and (iii) at least two copies of a ligand
comprising an IL-2-receptor-binding peptide covalently
coupled to the polymer; and
(b) contacting the population of cells with an
effective amount of the composition under conditions
wherein the ligand binds to an IL-2 receptor on the
IL-2-receptor-bearing cell and elicits endocytosis of
the composition.
A method of delivering a chemical agent
intracellularly into an IL-2-receptor-bearing cell in
a warm-blooded animal, comprising the steps of:
(a) providing a composition comprising (i) a
water-soluble, biocompatible polymer, (ii) the
chemical agent covalently, releasably coupled to the
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
6
polymer, and {iii) at least two copies of a ligand
comprising an IL-2-receptor-binding peptide covalently
coupled to the polymer; and
(b) systemically administering to the warm-
s blooded animal an effective amount of the composition
under conditions wherein the ligand contacts and binds
to an IL-2 receptor on the IL-2-receptor-bearing cell
and elicits endocytosis of the composition.
Brief Description of ,the Drawing
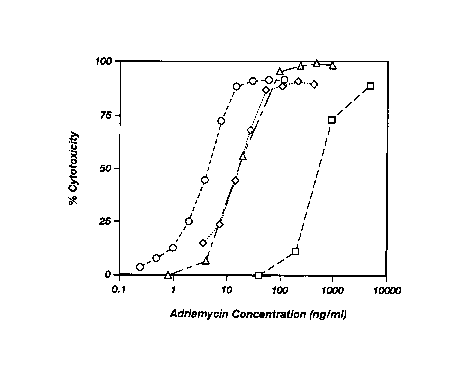
FIG. 1 shows the in vitro cytotoxic activity of a
composition according to the present invention and
control compositions against human HSB-2 T cells:
PEG-GFLG-ADR (SEQ ID N0:21); (n) PEG-TT13-ADR {SEQ ID
N0:13); (0) PEG-TT7-ADR (SEQ ID N0:14): and
unconjugated adriamycin.
Detailed Description of the Invention
Before the present compositions and methods for
targeted delivery to IL-2-receptor-bearing cells are
disclosed and described, it is to be understood that
this invention is not limited to the particular
embodiments, process steps, and materials disclosed
herein as such embodiments, process steps, and
materials may vary somewhat. It is also to be
understood that the terminology used herein is used
for the purpose of describing particular embodiments
only and is not intended to be limiting since the
scope of the present invention will be limited only by
the appended claims and equivalents thereof.
It must be noted that, as used in this
specification and the appended claims, the singular
forms "a," "an," and "the" include plural referents
unless the context clearly dictates otherwise. Thus,
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
7
for example, reference to a composition containing "a
ligand" includes reference to two or more ligands,
reference to "a chemical agent" includes reference to
one or more of such chemical agents that may be the
same or different chemical agents, and reference to "a
spacer" includes reference to two or more spacers.
In describing and claiming the present invention,
the following terminology will be used in accordance
with the definitions set out below.
As used herein, "peptide" means peptides of any
length and includes proteins. The terms "polypeptide"
and "oligopeptide" are used herein without any
particular intended size limitation, unless a
particular size is otherwise stated.
As used herein, "TL-2-receptor-binding peptide"
means a peptide configured for binding to an IL-2
receptor and stimulating internalization thereof by
receptor-mediated endocytosis. According to the
present invention, ligands comprising such IL-2-
receptor-binding peptides are coupled to various
functional molecules so that upon endocytosis of the
ligands the various functional molecules coupled
thereto are also internalized by the cells.
Preferred IL-2-receptor-binding peptides include
the peptide having the amino acid sequence identified
as SEQ ID NO:l and biologically functional equivalents
thereof. Such functional equivalents retain
functionality in binding the IL-2 receptor and
eliciting receptor-mediated endocytosis although they
may be truncations, deletion variants, or substitution
variants of SEQ ID N0:1 or include additional amino
acid residues attached thereto. It is also preferred
that the IL-2-receptor-binding peptides have a size of
about 6-20 amino acid residues, more preferably about
CA 02299368 2000-02-04
WO 99/07324 PCT/US98116290
8
6-12 amino acid residues, and most preferably about 6-
8 amino acid residues.
As mentioned above, changes may be made in the
structure of the IL-2 receptor-binding peptide while
maintaining the desirable receptor-binding
characteristics. For example, certain amino acid
residues may be substituted for other amino acid
residues in a protein structure without appreciable
loss of interactive binding capacity with structures
such as, for example, antigen-binding regions of
antibodies or binding sites of ligands such as an IL-2
receptor-binding peptide. Since it is the interactive
capacity and nature of a protein that defines that
protein's biological functional activity, certain
amino acid sequence substitutions can be made in a
protein sequence and nevertheless obtain a protein
with like properties. It is thus contemplated that
various changes may be made in the sequence of an IL-2
receptor-binding peptide without appreciable loss of
its biological utility or activity.
It is also well understood by the skilled artisan
that inherent in the definition of a biologically
functional equivalent protein or peptide is the
concept that there is a limit to the number of changes
that may be made within a defined portion of the
molecule and still result in a molecule with an
acceptable level of equivalent biological activity.
It is also well understood that where certain residues
are shown to be particularly important to the
biological or structural properties of a protein or
peptide, e.g. residues in active sites, such residues
may not generally be exchanged.
Amino acid substitutions are generally based on
the relative similarity of the amino acid side-chains
relative to, for example, their hydrophobicity,
CA 02299368 2000-02-04
WO 99107324 PCT/US98/16290
9
hydrophilicity, charge, size, and the like. An
analysis of the size, shape, and type of the amino
acid side-chains reveals, for example, that arginine,
lysine, and histidine are all positively charged
residues; that alanine, glycine, and serine are all a
similar size; and that phenylalanine, tryptophan, and
tyrosine all have a generally similar shape.
Therefore, based upon these considerations, the
following conservative substitution groups or
biologically functional equivalents have been
defined: (a) Cys; (b) Phe, Trp, Tyr; (c) Gln, Glu, Asn,
Asp; (d) His, Lys, Arg; (e) Ala, Gly, Pro, Ser, Thr;
and (f) Met, Ile, Leu, Val. M. Dayhoff et al., Atlas
of Protein Sequence and Structure (Nat'1 Biomed. Res.
Found., Washington, D.C., 1978), hereby incorporated
by reference.
To effect more quantitative changes, the
hydropathic index of amino acids may be considered.
Each amino acid has been assigned a hydropathic index
on the basis of its hydrophobicity and charge
characteristics, which are as follows: isoleucine
(+4.5); valine (+4.2); leucine (+3.8); phenylalanine
(+2.8); cysteine (+2.5); methionine (+1.9): alanine
(+1.8); glycine (-0.4); threonine (-0.7); serine
(-0.8); tryptophan (-0.9); tyrosine (-1.3); proline
(-1.6); histidine (-3.2); glutamate (-3.5); glutamine
(-3.5); aspartate (-3.5); asparagine (-3.5); lysine
(-3.9); and arginine (-4.5).
The importance of the hydropathic amino acid
index in conferring interactive biological function on
a protein is generally understood in the art. J. Kyte
& R. Doolittle, A simple method for displaying the
hydropathic character of a protein, 157 J. Mol. Biol.
105-132 (1982), inccrporated herein by reference. It
is known that certain amino acids may be substituted
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98/16290
for other amino acids having a similar hydropathic
index or score and still retain a similar biological
activity. In making changes based on the hydropathic
index, the substitution of amino acids whose
5 hydropathic indices are within ~ 2 is preferred,
within ~ 1 is particularly preferred, and within ~ 0.5
is even more particularly preferred.
It is also understood that an amino acid can be
substituted for another having a similar
10 hydrophilicity value and still obtain a biologically
equivalent protein. As detailed in U.S. Patent No.
4,554,101, the following hydrophilicity values have
been assigned to amino acid residues: arginine (+3.0);
lysine (+3.0); aspartate (+3.0); glutamate (+3.0 ~ 1);
serine (+0.3); asparagine (+0.2): glutamine (+0.2);
glycine (0): threonine (-0.4); proline (-0.5 t 1);
alanine (-0.5); histidine (-0.5); cysteine (-1.0);
methionine (-1.3): valine (-1.5); leucine (-1.8);
isoleucine (-1.8); tyrosine (-2.3); phenylalanine
(-2.5): tryptophan (-3.4).
In making changes based upon similar
hydrophilicity values, the substitution of amino acids
whose hydrophilicity values are within ~ 2 is
preferred, within ~ 1 is particularly preferred, and
within ~ 0.5 is even more particularly preferred.
A hexapeptide believed to be a part of IL-2 that
binds to the IL-2 receptor has been identified (SEQ ID
N0:1), D.A. Weigent et al., 139 Biochem. Biophys. Res.
Commun. 367-74 (1986). Moreover, regions of homology
between this IL-2 hexapeptide and env proteins of
immunosuppressive retroviruses have been discovered.
D.A. Weigent et al., supra; W.E. Reiher III et al., 83
Proc. Nat'1 Acad. Sci. USA 9188-92 (1986). Thus,
amino acid substitutions in these regions of homology
as compared to the IL-2 hexapeptide are also
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
11
considered to be biologically functional equivalents.
Therefore, illustrative biologically functional
equivalents of SEQ ID N0:1 include the following: SEQ
ID N0:2; SEQ ID N0:3; SEQ ID N0:4; SEQ ID N0:5; and
SEQ ID N0:6. Other illustrative biologically
functional equivalents have also been discovered,
including: SEQ ID N0:7; SEQ ID N0:8; SEQ ID N0:9; SEQ
ID NO:10; and SEQ ID N0:11. Additional biologically
functional equivalents can be discovered by a person
of ordinary skill in the art according to the guidance
and principles disclosed herein without undue
experimentation.
As used herein, "macromolecule" means a
composition comprising a water soluble polymer with a
ligand and a chemical agent bound thereto. Preferably
the polymer is a polyalkylene oxide and the ligand is
an oligopeptide. The chemical agent can be from many
different classes of molecules, as explained in more
detail herein.
As used herein, "prodrug" means a chemical agent
that is chemically modified to overcome a biological
barrier. When a chemical agent is converted into its
prodrug form, its biological activity is eliminated or
substantially reduced, but the biological barrier that
inhibited its effectiveness is no longer problematic.
The chemical group that is attached to the chemical
agent to form the prodrug, i.e. the "pro-moiety", is
removed from the prodrug by enzymatic or nonenzymatic
means to release the active form of the chemical
agent. egg A. Albert, Ohemical Asgects of Selective
Toxicity, 182 Nature 421 (1958). The instant
compositions are prodrugs because the chemical agent
that has the selected effect when internalized in Ih-
2-receptor-bearing cells is modified with a ligand,
water soluble polymer, and, optionally, spacers such
CA 02299368 2000-02-04
WO 99107324 PCT/US98/16290
12
that the composition is delivered into the IL-2-
receptor-bearing cells, thus penetrating the cell
membrane thereof. The biological effect of the
chemical agent is greatly reduced or eliminated until
the composition is delivered intracellularly and the
chemical agent is released from the remainder of the
composition by biodegradation of the spacer.
As used herein, "chemical agent" means and
includes any substance that has a selected effect when
internalized into an IL-2-receptor-bearing cell.
Certain chemical agents have a physiological effect,
such as a cytotoxic effect or an effect on gene
regulation, when internalized into the cell. A
"transforming nucleic acid" (RNA or DNA), when
internalized into a cell, can be replicated and/or
expressed within the cell. Other nucleic acids can
interact with regulatory sequences or regulatory
factors within the cell to influence gene expression
within the cell in a selected manner. A detectable
label delivered intracellularly can permit
identification of cells that have internalized the
compositions of the present invention by detection of
the label. Drugs or pharmacologically active
compounds can be used to ameliorate pathogenic effects
or other types of disorders. Particularly useful
chemical agents include polypeptides, and some such
chemical agents are active fragments of biologically
active proteins, or are specific antigenic fragments
(e. g., epitopes) of antigenic proteins. Thus, chemical
agents include cytotoxins, gene regulators,
transforming nucleic acids, labels, antigens, drugs,
and the like.
As used herein, "drug" or "pharmacologically
active agent" means any chemical material or compound
suitable for intracellular administration in a IL-2
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
13
receptor bearing cell, e.g. an activated T lymphocyte,
that stimulates a desired biological or
pharmacological effect in such cell.
As used herein, "carrier" means water soluble
polymers, particulates, or liposomes to which a
composition according to the instant invention can be
coupled. Such carriers increase the molecular size of
the compositions and may provide added selectivity
and/or stability. Such selectivity arises because
carrier-containing compositions are too large to enter
cells by passive diffusion, and thus are limited to
entering cells through receptor-mediated endocytosis.
The potential for use of such carriers for targeted
drug delivery has been established. See, e.g., J.
Kopecek, 5 Biomaterials 19 (1984). E. Schacht et al.,
Polysaccharides as Drug Carriers, in Controlled-
Release Technology 188 (P. I. Lee & W.R. Good, eds.,
1987); F. Hudecz et al., Carrier desicrn~ Cvtotoxicity
and Immunogenicity of $yn~hetic Branched Polypeptides
wig h Polv(L-lysine) Backbone, 19 J. Controlled Release
231 (1992); Z. Brich et al., Preparation and
Characterization of a Water Soluble Dextran
Im~unoconj,ugate of Doxorubicin and the Monoclonal
Antibocy (ABL364), 19 J. Controlled Release 245
(1992). Thus, illustrative water soluble polymers
include dextran, inulin, poly(L-lysine) with modified
epsilon-amino groups, poly(L-glutamic acid), N-
substituted methacrylamide-containing synthetic
polymers and copolymers, and the like.
As used herein, "effective amount" is an amount
sufficient to produce a selected effect. For example,
a selected effect of a composition containing a
cytotoxin as the chemical agent could be to kill a
selected proportion of IL-2-receptor-bearing cells,
e.g. activated T cells, within a selected time period.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98116290
14
An effective amount of the composition would be the
amount that achieves this selected result, and such an
amount could be determined as a matter of routine by a
person skilled in the art.
The compositions of the present invention provide
intracellular delivery of a chemical agent capable of
eliciting a selected effect when delivered
intracellularly into an IL-2-receptor-bearing cell.
Illustrative embodiments of the composition have a
formula selected from the group consisting of
P- [T$-L-Sb-A] ~ and [A-Sb] d-P- [Ta-L] ~, wherein L is a
ligand configured for binding to an IL-2 receptor on
the IL-2-receptor-bearing cell and stimulating
receptor-mediated endocytosis of the composition; A is
the chemical agent; S and T are spacers, wherein at
least S is biodegradable: P is a water soluble polymer
having functional groups compatible with forming
covalent bonds with the ligand; a and b are integers
of 0 or 1; c is an integer of at least 2; and d is an
integer of at least 1 Preferably, c is an integer of 2
to about 1000. The spacers are preferably
biodegradable such that the chemical agent is detached
from the composition by hydrolysis and/or enzymatic
cleavage inside IL-2-receptor-bearing cells, e.g. T
cells, especially in lysosomes. Once detached, the
chemical agent can exert its functional effect in the
cell. Illustrative of such spacers is the peptide
Gly-Phe-Leu-Gly (SEQ ID N0:21). Equivalent peptide
spacers are well known in the art. The chemical agent
is selected from the group consisting of cytotoxins,
transforming nucleic acids, gene regulators, labels,
antigens, drugs, and the like. The water soluble
polymer (represented by P in the formula above) is
preferably a poly(alkylene oxide). Within this group
of substances are alpha-substituted polyalkylene oxide
CA 02299368 2000-02-04
WO 99107324 PCT/US98/16290
derivatives, such as methoxypolyethylene glycols or
other suitable alkyl-substituted derivatives, such as
those containing C1-C4 alkyl groups. It is preferred
that the polymer be a monomethyl-substituted PEG
5 homopolymer. Other poly(alkylene oxides) are also
useful, including other polyethylene glycol (PEG)
homopolymers, polypropylene glycol homopolymers, other
alkyl-capped polyethylene oxides, bis-polyethylene
oxides, copolymers of poly(alkylene oxides), and block
10 copolymers of poly(alkylene oxides) or activated
derivatives thereof. In those aspects of the
invention where PEG-based polymers are used, it is
preferred that they have molecular weights of from
about 200 to about 50,000. Molecular weights of about
15 2,000 to about 20,000 are preferred, and molecular
weights of about 5,000 are particularly preferred.
PEG is preferred because it is inexpensive, approved
by the FDA for administration to humans, and is
resistant to eliciting an antibody response.
Polyethylene oxide) (PEO) is another preferred water
soluble polymer represented by P. The coupling of a
ligand to a chemical agent can be, without limitation,
by covalent bond, electrostatic interaction,
hydrophobic interaction, physical encapsulation, and
the like. The compositions of the present invention
can further comprise a carrier selected from the group
consisting of other water soluble polymers, liposomes,
and particulates. Such water soluble polymers for use
as carriers are selected from the group consisting of
dextran, inulin, poly(L-lysine) (PLL) with modified
epsilon amino groups, poly(L-glutamic acid) (PGA), N-
substituted methacrylamide-containing polymers and
copolymers, and the like. A preferred water soluble
polymer is a copolymer of N-(2-
hydroxypropyl)methacrylamide (HPMA).
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98/16290
16
Thus, according to the invention, the composition
provides means for preferential binding to an IL-2
receptor, such as on activated T cells, thus
triggering internalization of the composition by
endocytosis. The chemical agent provides means for
achieving a selected effect in the IL-2-receptor
bearing cells. Accordingly, for example, chemical
agents comprise cytotoxins, including radionuclides,
for selective killing or disabling of cells; nucleic
acids for genetically transforming or regulating gene
expression in cells; drugs or other pharmacologically
active agents for achieving a selected therapeutic
effect: labels, including fluorescent, radioactive,
and magnetic labels, for permitting detection of cells
that have taken up the compositions: and the like.
IL-2 is a lymphocyte growth factor produced by T
cells that is essential for a normal immune response.
Binding of IL-2 to the IL-2 receptor precedes
internalization by receptor-mediated endocytosis. The
human IL-2 gene has been sequenced, T. Taniguchi et
al., 302 Nature 305-10 (1983), hereby incorporated by
reference, as has the gene for the human IL-2
receptor, W.J. Leonard et al., 311 Nature 626-31
(1984); T. Nikaido et al., 311 Nature 631-35 (1984);
D. Cosman et al., 312 Nature 768-71 (1984). The IL-2
receptor is a heterotrimeric glycoprotein complex on
the cell membrane with a 55 kDa a subunit, a 75 kDa ~i
subunit, and a 64 kDa y subunit. The only normal
human tissues expressing the a and (3 subunits are
activated T cells, B cells, LGL cells, and monocytes
and some liver Kupffer cells, macrophages, and skin
Langerhans' cells. A.E. Frankel et al., 11 Leukemia
22-30 (1997). A variety of hematologic neoplasms may
show high affinity IL-2 receptor expression including
hairy cell leukemia, adult T cell leukemia, and a
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98116290
17
fraction of cutaneous T cell lymphomas and B cell
chronic lymphocytic leukemias. Recombinant toxins
targeted to the IL-2 receptor have been described
wherein the ligand is IL-2. A.E. Frankel et al.,
supra; U.S. Patent No. 4,675,382; J. vanderSpek et
al., 268 J. Biol. Chem. 120?7-82 (1993): I. Pastan &
D. FitzGerald, supra.
In some embodiments of the present invention, the
compositions are constructed by chemically conjugating
the ligand and chemical agent to the water soluble
polymer. "Chemically conjugating" the ligand and the
chemical agent to the water soluble polymer, as that
term is used herein, means covalently bonding the
ligand and chemical agent to each other, preferably by
way of a spacer moiety, and conjugating the resulting
ligand/agent conjugate to the water soluble polymer.
In particular embodiments, a spacer moiety is used to
form a linkage between the ligand and the chemical
agent.
Peptide portions of the compositions of the
present invention can be produced in a genetically
engineered organism, such as E. coli, as a "fusion
protein." That is, a hybrid gene containing a
sequence of nucleotides encoding a ligand, spacer, or
peptide chemical agent can be constructed by
recombinant DNA technology. This hybrid gene can be
inserted into an organism such that the "fusion
protein" encoded by the hybrid gene is expressed. The
fusion protein can then be purified by standard
methods, including affinity chromatography. Peptides
containing a ligand, spacer, or peptide chemical agent
can also be constructed by chemical synthesis. Short
peptide ligands are generally preferred, both because
short peptides can be manipulated more readily and
because the presence of additional amino acids
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98/16290
18
residues, and particularly of substantial numbers of
additional amino acids residues, may interfere with
the function of the peptide ligand in stimulating
internalization of the chemical agent by endocytosis.
Compositions according to the present invention
preferably also further include a protease digestion
site, preferably in the spacer moiety, situated such
that once the composition is within the cell, such as
in a lysosome, the chemical agent can be separated
from the remainder of the composition by proteolysis
of the digestion site. Such a protease susceptible
spacer can be added regardless of whether the peptide
portions of the composition are synthesized chemically
or as expression peptides in a genetically engineered
organism. In the latter case, nucleotides encoding
the protease susceptible spacer can be inserted into
the hybrid gene encoding the ligand and or a peptide
chemical agent by techniques well known in the art.
In one illustrative embodiment, the protease-
susceptible spacer is designed to be cleaved by
proteolysis in the lysosome of the target cell. The
composition that is internalized by endocytosis is
packaged in an endocytic vesicle, which is transported
to a lysosome. Once in the lysosome, the protease-
susceptible spacer is cleaved, and the chemical agent
is then available to be transported to the cytoplasm.
Another aspect of the present invention features
a method for specifically effecting a desired activity
in IZ-2-receptor-bearing cells, e.g. activated T
lymphocytes, contained in a heterogeneous population
of cells, by the step of contacting the population of
cells with a composition, prepared according to the
present invention, that directs such activity
intracellularly. The compositions of the invention
are selectively bound to IL-2-receptor-bearing T cells
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
19
in the mixed population, whereupon endocytosis of the
composition into such activated T cells is stimulated,
and the chemical agent effects its activity within
such T cells.
This application employs, except where otherwise
indicated, standard techniques for manipulation of
peptides and for manipulation of nucleic acids for
expression of peptides. Techniques for conjugation of
oligopeptides and oligonucleotides are known in the
art, and are described for example in T. Zhu et al., 3
Antisense Res. Dev. 265 (1993); T. Zhu et al., 89
Proc. Nat'1 Acad. Sci. USA 7934 (1992); P. Rigaudy et
al., 49 Cancer Res. 1836 (1989), which are hereby
incorporated by reference.
As is noted above, the invention features
peptides, employed as ligands, spacers, and/or
chemical agents. The peptides according to the
invention can be made by any of a variety of
techniques, including organic synthesis and
recombinant DNA methods. Techniques for chemical
synthesis of peptides are described, for example, in
B. Merrifield et al., 21 Biochemistry 5020 (1982):
Houghten, 82 Proc. Nat'1 Acad. Sci. USA 5131 (1985);
M. Bodanszky & A. Bodanszky, The Practice of Peptide
Synthesis (Springer-Verlag 2d ed., 1994), incorporated
herein by reference. Techniques for chemical
conjugation of peptides with other molecules are known
in the art.
A fusion protein according to the invention can
be made by expression in a suitable host cell of a
nucleic acid containing an oligonucleotide encoding a
ligand and/or spacer and/or chemical agent. Such
techniques for producing recombinant fusion proteins
are well-known in the art, and are described generally
in, e.g., J. Sambrook et al., Molecular Cloning: A
CA 02299368 2000-02-04
WO 99/07324 PCTIUS98/16290
Laboratory Manual (2d ed., 1989), the pertinent parts
of which are hereby incorporated herein by reference.
Reagents useful in applying such techniques, such as
restriction endonucleases and the like, are widely
5 known in the art and commercially available from any
of several vendors.
Construction of compositions according to the
present invention will now be described, with
particular reference to examples in which a peptide
10 ligand coupled to a biodegradable spacer (SEQ ID
N0:21) and a cytotoxic chemical agent, adriamycin, are
coupled to PEG.
Example 1
15 NHZ-Gly-Leu-OH (Sigma Chemical Co. St. Louis, M0;
2.74 g, 14.6 mmol) was dissolved in 30 ml of
phosphate-buffered saline (PBS) containing 0.5 M NaCl.
The reaction mixture was stirred and 5 g of solid
activated PEG {succinimidyl ester of 5 pendent
20 polyethylene glycol propionic acid: p-5-SPA-5000
Shearwater Polymers, Inc., Huntsville, AL) was added
to the solution. The reaction was continued for 4.5
hours at pH 7-8. The reaction mixture was then
extracted four times with 500 ml each of
dichloromethane EA-C. S., HPLC grade, Sigma or Aldrich,
Milwaukee, WI). An emulsion was formed during
extraction and was partially broken by adding NaCl to
the emulsion. The organic layers were pooled and
dried over MgS04 overnight. The solution was then
filtered, and the filtrate was concentrated by
evaporating the dichloromethane with a rotary
evaporator at about 35°C using a water pump. The
final volume of solution was reduced to about 15 ml.
The solution was added to ether {750 ml; anhydrous,
Fisher Scientific), and the product {PEG-Gly-Leu-OH)
CA 02299368 2000-02-04
WO 99/07324 PCTlUS98116290
21
was precipitated. The precipitates were filtered,
washed with ether, and dried in air.
PEG-Gly-Leu-OH (3.5 g, 3.4 mmol) and p-
nitrophenol were dissolved in 50 ml of tetrahydrofuran
(anhydrous, Aldrich) and 10 ml of ethylacetate
(Aldrich). The solution was cooled in an ice bath,
and dicyclohexylcarbodiimide (DCC, Sigma; 2.4 g, 11.5
mmol) was added to the reaction mixture in four
aliquots. The reaction solution was stirred for 30
minutes in the ice bath. The temperature of the
reaction solution was then raised to room temperature,
and the reaction was then continued for another 91
hours. The reaction solution was then filtered
through filter paper, and the filtrate was
concentrated by evaporating the solvent with a rotary
evaporator using a water pump. The clear concentrated
solution (30 ml) was added to ether (750 ml). The
precipitate was filtered, washed in ether, and dried
in air. An aliquot of the product was dissolved in
0.1 N NaOH, and the concentration of the liberated p-
nitrophenol was estimated by spectrophotometry at 400
nm using a molar extinction coefficient of E = 1.8 x
104 1/mol-cm. The product (PEG-Gly-Leu-ONp) was
determined to have an ONp content of 375 ~cmol/g.
The product (PEG-Gly-Leu-ONp; 430.88 mg, Onp
content 161.2 ~cmol) was dissolved in 5 ml anhydrous
dimethylformamide (DMF), and a peptide (Gln-His-Leu-
Phe-Leu-Gly, SEQ ID N0:12) was added to the solution.
About 150 E.cl of triethylamine diluted 1:2 with DMF was
added to the reaction mixture four times in an
interval of 15 minutes, and the solution was stirred
for 17 hours at room temperature. The reaction
solution was added to cold ether (400 ml), and the
conjugate precipitates (PEG-TT13-OHM SEQ ID N0:13)
were filtered, washed with 400 ml ether, and dried.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
22
Adriamycin (63.97 mg, 111.5 E.cmol, Sigma) and PEG-
TT13-OH (450.26 mg, 111.5 ~cmol) were dissolved in 5 ml
DMF and DCC solid (59.3 mg) was added to the solution.
The reaction was carried out for 22 hours and then
filtered through Whatman No. 4 paper. The precipitate
on the filter paper was dried under vacuum and then
dissolved in PBS buffer and dialyzed against PBS
overnight. After a change of buffer, the solution was
dialyzed for 2 additional hours. Adriamycin content
was determined by spectrophotometry at 490 nm. The
resulting conjugate had the structure PEG-Gly-Leu-Gln-
His-Leu-Phe-Leu-Gly-Adriamycin (hereinafter, "PEG-
TT13-ADR;" SEQ ID N0:13).
Example 2
In this example, a control composition having the
structure PEG-Gly-Phe-Leu-Gly-ADR {hereinafter, "PEG-
GFLG-ADR;" SEQ ID N0:21) was prepared according to the
procedure of Example 1.
Example 3
In this example, a composition having the
structure PEG-Gly-Leu-Glu-Arg-Ile-Leu-Leu-Gly-Phe-Leu-
Gly-Adriamycin (hereinafter, "PEG-TT7-ADR;" SEQ ID
N0:14) was prepared according to the procedure of
Example 1.
Example 4
In this example, a composition having the
structure PEG-Gly-Leu-GlutBt-His-Ile-Leu-Leu-Gly-Phe-
Leu-Gly-Adriamycin (SEQ ID N0:15), where tBt is a
tart-butyl group coupled to the COOH side-chain of the
glutamic acid residue, was prepared according to the
procedure of Example 1. The tart-butyl derivative of
glutamic acid was purchased commercially (Bachem, King
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
23
of Prussia, PA) and was incorporated into the
oligopeptide during peptide synthesis. The tert-
butyl group blocks the COOH group to prevent reaction
of the COON side-chain of the glutamic acid residue
with an NHZ group of adriamycin.
Example 5
In this example, a composition having the
structure PEG-Gly-Leu-Gln-His-Ile-Leu-Leu-Gly-Phe-Leu-
Gly-Adriamycin (SEQ ID N0:16) was prepared according
to the procedure of Example 1.
Example 6
In this example, a composition having the
structure PEG-Gly-Leu-Asp-His-Ile-Phe-Leu-Gly-Phe-Leu-
Gly-Adriamycin (SEQ ID NO:I7} is prepared according to
the procedure of Example 1.
Example 7
In this example, a composition having the
structure PEG-Gly-Leu-Asn-His-Ile-Phe-Leu-Gly-Phe-Leu-
Gly-Adriamycin (SEQ ID N0:18) is prepared according to
the procedure of Example 1.
Example 8
In this example, a composition having the
structure PEG-Thr-Gly-Leu-Gln-His-Ile-Leu-Leu-Gly-Phe-
Leu-Gly-Adriamycin (SEQ ID N0:19} is prepared
according to the procedure of Example 1.
Example 9
In this example, a composition having the
structure PEG-Ser-Leu-Gln-His-Ile-Leu-Leu-Gly-Phe-Leu-
Gly-Adriamycin (SEQ ID N0:20) is prepared according to
the procedure of Example 1.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
24
Example 10
The in vitro effects of PEG-TT13-ADR prepared
according to the procedure of Example 1, PEG-GFLG-ADR
prepared according to the procedure of Example 2, PEG-
TT7-ADR prepared according to the procedure of Example
3, and unconjugated adriamycin were tested on human
HSB-2 T cells (ATCC No. CCL 120.1) as follows.
Triplicate samples of 1 x 105 cells each were mixed
with different concentrations of the purified
compositions in 0.1 ml of culture medium (RPMI 1640,
10% fetal calf serum) in the wells of a 96-well
microtiter plate (Falcon Microtest 111), and incubated
for 48 hours at 37°C in a humidified, 5~ COZ
atmosphere. Thereafter, cell viability was assessed
by a colorimetric method using the tetrazolium
compound MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-
carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-
tetrazolium, inner salt) and an electron coupling
reagent, PMS (phenazine methosulfate). A.J. Cory et
al., 3 Cancer Commun. 207 (1991); T.L. Riss & R.A.
Moravec, 3 Mol. Biol. Cell. 184a (Supp.; 1992); T.M.
Buttke et al., 157 J. Immunol. Methods 233 (1993),
hereby incorporated by reference. MTS is bioreduced
by living cells into a soluble formazan product. The
absorbance of the formazan at 490 nm can be measured
directly from 96 well assay plates without additional
processing. The quantity of formazan product as
measured by the absorbance at 490 nm is directly
proportional to the number of living cells in culture.
Reagents for the MTS assay were obtained from Promega
Corp. (Madison, Wisconsin). According to this method,
20 ul of MTS/PMS solution (Promega No. G-5421) was
added to each well of the assay plate. The plate was
then further incubated at 37°C in a humidified, 5~ COZ
atmosphere for 4 hours. The absorbance of each well
CA 02299368 2000-02-04
WO 99!07324 PCT/US98/16290
was then measured at 490 nm with an EL311 Microplate
Autoreader (Bio-Tek Instruments). The mean absorbance
for each treatment was then calculated, and the
percent cytotoxicity was determined using the formula:
A
cytotoxity = (1-AS) x 100
c
5 wherein AS represents the mean absorbance for each
treatment and AC represents mean absorbance of the
control treatment, i.e. cells not exposea to a
conjugate.
FIG. 1 shows that PEG-TT7-ADR (0) and PEG-TT13-
10 ADR (v) kill such HSB-2 T cells at concentrations
about 100-fold lower than that required for PEG-GFLG-
ADR (0) to effect similar levels of cytotoxicity. The
cytotoxicities of PEG-TT7-ADR and PEG-TT13-ADR were
substantially identical. These results show that the
15 presence of a ligand specific for binding to the IL-2
receptor and inducing receptor-mediated endocytosis
results in much greater cytotoxicity that a PEG- and
adriamycin-containing conjugate lacking such ligand.
Thus, a conjugate bearing an IL-2-receptor specific
20 ligand is internalized with much greater efficiency
that similar conjugates lacking such a ligand. The
unconjugated adriamycin control rapidly diffuses into
the cells and kills them. As expected, cytotoxicities
from PEG-TT7-ADR and PEG-TT13-ADR require higher
25 concentrations of adriamycin than unconjugated
adriamycin due to the requirement that PEG-TT7-ADR and
PEG-TT13-ADR be internalized by endocytosis.
The compositions according to the present
invention can be employed for targeted delivery of a
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
26
chemical agent to IL-2-receptor-bearing cells, e.g.
activated T cells, generally by contacting the cells
with the composition under conditions in which binding
of the ligand to a receptor stimulates endocytosis of
the composition into the cells. The chemical agent
then acts on or within the targeted cell into which
the composition is internalized, and the desired
effect of the active agent can be confined to those
cells having the receptor.
For example, a composition according to the
invention can be employed as an effective antitumor
agent in vivo for killing activated T cells.
Preferably, the composition is administered to the
subject by systemic administration, typically by
subcutaneous, intramuscular, or intravenous injection,
or intraperitoneal administration, which are methods
well known in the art. Injectables for such use can
be prepared in conventional forms, either as a liquid
solution or suspension or in a solid form suitable for
preparation as a solution or suspension in a liquid
prior to injection, or as an emulsion. Suitable
excipients include, for example, water, saline,
dextrose, glycerol, ethanol, and the like; and if
desired, minor amounts of auxiliary substances such as
wetting or emulsifying agents, buffers, and the like
may be added. Effective amounts of such compositions
can be determined by those skilled in the art without
undue experimentation according to the guidelines
provided herein.
The composition can be contacted with the cells
in vitro or in vivo. The T cells constitute a
subpopulation of a mixed population of cell types; the
ligand according to the invention can provide for
endocytosis of the conjugate into T cells and possibly
CA 02299368 2000-02-04
WO 99/07324 PCT/US98l16290
27
into a small proportion of other cells having a
closely related receptor.
The chemical agent can have any of a variety of
desired effects in the targeted cells. As mentioned
above, in some particularly useful embodiments the
chemical agent is effective on a cell only when, or
principally when, the agent is internalized into the
cell.
Example 11
In Vivo Targeted Delivery to T cells
Compositions according to the present invention
can be administered to a warm-blooded animal for
targeted delivery to IZ-2-receptor-bearing cells, e.g.
activated T cells. Particularly, the composition
provides for receptor-mediated internalization of the
composition into the targeted cells.
About 1 x 106 CCRF-CEM human T-cell leukemia cells
in 500 ul of PBS were injected intraperitoneally into
male CB 17 SCID (HSD)mice, and the cells were allowed
to colonize the mice for 24 hours. The human T-cell
leukemia cells were found to preferentially colonize
the spleen and liver. The mice were divided into 6
groups of 6 animals each: Group A was treated with 100
,ug of conjugate; Group B were treated with 75 E.cg of
conjugate; Group C was treated with 50 ~cg of
conjugate; Group D was treated with 25 ,ug of
conjugate; Group E was treated with 10 ~cg of
conjugate; and Group F was not treated with any
conjugate. After 24 hours, the mice were injected
intraperitoneally with 10-100 ug of PEG-TT13-ADR
(calculated on the mass of ADR in the conjugate) or,
in the case of Group F, were not treated. After an
CA 02299368 2000-02-04
WO 99/0732A PCTIUS98/16290
28
additional 24 and 48 hours, Groups B, C, D, and E were
injected again.
Based on previous experiments, it is expected
that by 60 days after injection, all of the animals in
Group F will die from uncontrolled growth of the T-
cell leukemia cells, whereas all of the animals in
Groups A-E, i.e. injected with PEG-TT13-ADR, will be
alive. These results will show that a composition
according to the present invention, wherein the
chemical agent is a cytotoxin, preferentially is
internalized by IL-2-receptor-bearing cells, i.e. T
cells, and such T cells are killed by the cytotoxin.
Example 12
In this example, mice are injected with CCRF-CEM
human T-cell leukemia cells and with either PEG-TT13-
ADR or PEG-GFLG-ADR according to the procedure of
Example 11 to determine whether the liver and spleen
of such animals contain human cells. The spleen and
liver are harvested upon death of the animals or at
120 days post-inoculation, whichever occurs earlier.
PCR assay of genomic DNA prepared from these organs is
used to determine the presence or absence of human
cells therein.
Genomic DNA is prepared from mouse spleen and
liver according to methods that are generally well
known in the art. Set, e.a., J. Sambrook et al.,
Molecular Cloning: A Laboratory Manual (2d ed., 1989):
T. Maniatis et al., Molecular Cloning: A Laboratory
Manual (1982); F. Ausubel et al., Current Protocols in
Molecular Biology (1987). An illustrative method for
preparation of genomic DNA will now be briefly
described. The excised spleen and liver are disrupted
and the resulting cells are washed in PBS. The cells
are then resuspended in a buffer containing 100 mM
CA 02299368 2000-02-04
WO 99/07324 PCT1LJS98116290
29
NaCl, 10 mM Tris-HC1, pH 8.0, 25 mM EDTA, 0.5~ SDS,
and 0.1 mg/ml proteinase K, and incubated overnight at
37°C. The resulting lysate is then twice extracted
with phenol/chloroform/isoamylalcohol. The DNA in the
aqueous phase is then precipitated with ethanol,
washed, dried, and resuspended in a buffer containing
mM Tris~HCl, pH 8.0, 1 mM EDTA.
PCR is well known in the art for determining the
presence of selected sequences in genomic DNA samples.
10 The following references illustrate PCR methodology:
PCR Technology: Principles and Applications for DNA
Amplification (H. Erlich ed., Stockton Press, New
York, 1989): PCR Protocols: A Guide to Methods and
Applications (Innis et al. eds, Academic Press, San
Diego, Calif., 1990); U.S. Patent Nos. 4,683,195;
4,683,202; 4,800,159 4,965,188. Briefly, PCR
reactions are carried out in glass capillary tubes in
10 ul volumes containing 1.25 mM of each of the four
deoxynucleotide triphosphates, 0.72 units of Thermus
aquaticus (Taq) DNA polymerase, 35-70 pmol of each
primer (20-23 nucleotides in length), 2 ~ccg genomic
DNA, and a reaction buffer containing 50 mM Tris~HCl,
pH 8.3, 3 mM MgCl2, 20 mM KC1, and 0.5 mg/ml of bovine
serum albumin. Amplification is routinely carried out
by 60 cycles of PCR. Variations from these
parameters, such as the amount of DNA and number of
cycles of amplification can be determined empirically
by a person of ordinary skill in the art without undue
experimentation.
The reaction mixtures are sealed in capillary
tubes and then the capillaries are placed in a Model
1605 Air Thermocycler (Idaho Technology, Idaho Falls,
Idaho). Parameters of annealing temperature,
elongation time, and number of cycles are selected.
Increasing the annealing temperature increases the
CA 02299368 2000-02-04
WO 99/07324 PCT/US98116290
specificity of PCR amplification reactions and
decreases the amounts of nonspecific products.
Annealing temperature can be estimated from thermal
melting temperature according to the formula:
5 Tm = 4°C(no. of G and C residues in primer) + 2°C(no.
of A and T residues in primer). A person of ordinary
skill in the art can optimize the annealing
temperature according to known principles. The
elongation time depends on the size of product to be
10 amplified. As a rule of thumb, about 4 seconds is
sufficient for products of about 100-150 bp, about 8
seconds is sufficient for products of about 200-300
bp, and about 20 seconds is sufficient for products
larger than about 500 bp. Increasing elongation times
15 may result in amplification of nonspecific products.
After amplification, the reaction mixture is
removed from the capillary, mixed with an equal volume
of stop solution (95~ formamide, 20 mM EDTA, 0.05
bromphenol blue, 0.058 xylene cyanol FF), and either
20 stored frozen or immediately heated at 95°C for 5
minutes and subjected to agarose gel electrophoresis.
The fractionated products are then detected by
ethidium bromide staining.
An illustrative method of determining the
25 relative amounts of human and mouse cells in spleen
and liver tissues involves comparison of amplified
products from reactions with mouse (3-actin and human
~i-actin specific primers. Illustrative mouse a-actin
primers are as follows:
GTAACAATGC CATGTTCAAT (SEQ ID N0:22)
CTCCATCGTG GGCCGCTCTA G (SEQ ID N0:23}
Illustrative human primers areas follows:
~i-actin
CTTAGTTGCG TTACACCCTT TC (SEQ ID N0:24)
GGGCCATTCT CCTTAGAGAG AAG (SEQ ID N0:25)
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
31
Based on previous experiments, the results of
this experiment are expected to show that all of the
mice treated with the control PEG-GFLG-ADR composition
exhibit the presence of both human and mouse DNA by
PCR analysis with specific (3-actin primers. Further,
mice treated with the PEG-TT13-ADR composition that
die within 120 days of administration of the human T
cell leukemia cells also exhibit the presence of human
DNA. However, mice treated with the PEG-TT13-ADR
composition that live for 120 days after
administration of the human T cell leukemia cells do
not exhibit the presence of human DNA. These results
demonstrate that a ligand and cytotoxin-containing
composition according to the present invention
selectively kills T cells in animals to which it is
administered.
Example 13
A method of treating T cell lymphoma in a human
comprises (a) providing a composition according to the
present invention including a ligand, such as the
ligand (SEQ ID N0:1) or a biologically functional
equivalent thereof, and a cytotoxin, such as
adriamycin, both of which are coupled to water soluble
polymer, such as PEG, by means of a spacer (Gly-Phe-
Leu-Gly: SEQ ID N0:21) and (b) systemically
administering an effective amount of the composition
to an individual. Such composition can be made, for
example, as shown above in Example 1. An effective
amount of the composition is systemically administered
to the individual such that the composition enters the
bloodstream and contacts T cells. The composition
binds to an IL-2 receptor on the T cells and
stimulates internalization of the composition by
endocytosis. The biodegradable spacer is digested by
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
32
intracellular proteases, releasing the adriamycin.
The adriamycin then kills the cell by intercalating
with DNA in the cell. This procedure reduces the
number of malignant T cells in the body of the
individual, thereby having a positive effect in
treatment of the disease.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
1
SEQUENCE LISTING
<110> Prakash, Ramesh K.
<120> CONJUGATES TARGETED TO THE INTERLEUKIN-2
RECEPTOR
<130> T5181.PCT
<140>
<141> 1998-08-05
<150> US 08/914,042
<151> 1997-08-05
<160> 25
<170> WordPerfect 8.0
<210> 1
<211> 6
<212> PRT
<213> Homo sapiens
<400> 1
Leu Glu His Leu Leu Leu
1 5
<210> 2
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Found in HTLV-III, this sequence exhibits
similarity to a sequence found in human IL-
2.
<400> 2
Leu Glu Arg Ile Leu Leu
1 5
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
2
<210> 3
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 3
Leu Glu Arg Leu Leu Leu
1 5
<210> 4
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<900> 4
Leu Asp Leu Leu Phe Trp
1 5
<210> 5
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 5
Leu Asp Leu Leu Phe Leu
CA 02299368 2000-02-04
WO 99107324 PCT/US98116290
3
1 5
<210> 6
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 6
Leu Asp Ile Leu Phe Leu
1 5
<210> 7
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 7
Leu Gln His Leu Phe Leu
1 5
<210> 8
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 8
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
4
Leu Glu His Ile Leu Leu
1 5
<210> 9
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 9
Leu Gln His Ile Leu Leu
1 5
<210> 10
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
<400> 10
Leu Asp His Ile Phe Leu
1 5
<210> 11
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion
of
human IL-2.
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
<400> 11
Leu Asn His Ile Phe Leu
1 5
5
<210> 12
<211> 6
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion of
human IL-2.
<400> 12
Gln His Leu Phe Leu Gly
1 5
<210> 13
<211> 8
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion of
human IL-2.
<400> 13
Gly Leu Gln His Leu Phe Leu Gly
1 5
<210> 14
<211> 11
<212> PRT
<213> Artificial Sequence
<220>
CA 02299368 2000-02-04
WO 99107324 PCTIUS98/1b290
6
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 14
Gly Leu Glu Arg Ile Leu Leu Gly Phe
Leu Gly
1 5 10
<210> 15
<211> I1
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 15
Gly Leu Glu His Ile Leu Leu Gly Phe Gly
Leu
1 5 10
<210> 16
<211> 11
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 16
Gly Leu Gln His Ile Leu Leu Gly Phe Gly
Leu
1 5 10
<210> 17
<211> 11
<212> PRT
<213> Artificial Sequence
CA 02299368 2000-02-04
WO 99/07324 PCT/US98116290
7
<220>
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 17
Gly Leu Asp His Ile Phe Leu Gly Phe
Leu Gly
1 5 10
<210> 18
<211> 11
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 18
Gly Leu Asn His Ile Phe Leu Gly Phe Gly
Leu
1 5 IO
<210> 19
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to portion
a of
human IL-2.
<400> 19
Thr Gly Leu Gln His Ile Leu Leu Gly Leu Gly
Phe
1 5 10
<210> 20
<211> 11
<212> PRT
CA 02299368 2000-02-04
WO 99/07324 PCT/US9$/16290
8
<213> Artificial Sequence
<220>
<223> Exhibits sequence similarity to a portion of
human IL-2.
<400> 20
Ser Leu Gln His Ile Leu Leu Gly Phe Leu Gly
1 5 10
<210> 21
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Biodegradable spacer sequence.
<400> 21
Gly Phe Leu Gly
1
<210> 22
<211> 20
<212> DNA
<2I3> Mus musculus
<400> 22
gtaacaatgc
catgttcaat
20
<210> 23
<211> 21
<212> DNA
<213> Mus musculus
<400> 23
ctccatcgtg
ggccgctcta
g 21
CA 02299368 2000-02-04
WO 99/07324 PCT/US98/16290
9
<210> 24
<211> 22
<212> DNA
<213> Homo sapiens
<400> 24
cttagttgcg
ttacaccctt
tc 22
<210> 25
<211> 23
<212> DNA
<213> Homo Sapiens
<400> 25
gggccattct ccttagagag aag 23