Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02304495 2007-06-26
- 1
NON-THROMBOGENIC STENT JACKET
~..
BACKGROUND OF THE INVENTION
This invention relates to the field of expandable intraluminal support
devices such as stents and the like. Typically, stents are expandable, tubular
metallic devices that are positioned within a patient's vasculature or other
body
lumen and expanded in order to support a vessel or body lumen at a desired
intraluminal location to allow the flow of blood or other body fluids
therethrough.
Often, the stents are formed from a deformable metal and delivered to the
desired intraluminal location by mounting the stent onto an expandable
portion,
e.g. a balloon, on the distal extremity of a delivery catheter. By advancing
the
catheter through the body lumen, the stent may be delivered to a desired
position and expanded therein by expanding the expandable member, e.g. the
balloon to an expanded configuration, seating it within the artery or other
body
lumen. Other implementations make use of a self-expanding stent formed from
a suitable material such as pseudoelastic material that is delivered in a
constricted condition and when released spontaneously expands to an enlarged
configuration. 'In other embodiments, a stent made of shape memory alloy (e.g.
NiTi alloy) may be inserted into the body lumen in a martensitic phase and
transformed to an austenite phase which has an expanded memory when raised
to a temperature above the transformation temperature, usually less than 45 0
C.
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
2
Stents are often used in conjunction with an intravascular treatment for
obstructive coronary artery disease. For example, ablation, atherectomy,
balloon
dilation, laser treatment or other procedures are among the methods used to
widen a stenotic region of a patient's vasculature. However, restenosis occurs
in large percentage of percutaneous transiuminal coronary angioplasty (PTCA)
patients and rates can be even higher with other procedures. The prior art has
employed a number of mechanical and pharmacological strategies to reduce the
restenosis rate, but none have been particularly effective. Accordingly,
stents
have been proposed to maintain the patency of a treated vessel and prevent
restenosis. Using stents, restenosis rates have fallen to less than 20%.
Restenosis is thought to be a natural healing reaction provoked by injury
from the intravascular procedure. The healing process frequently causes
thrombosis and may lead to intimal hyperplasia that occludes the vessel.
Although helpful in reducing restenosis, stents do not represent a complete
solution. The framework of the stent may still allow migration and
proliferation
of the smooth muscle cells, while the stent itself can be thrombogenic. To
address these problems, stents have been covered with DACRON, PTFE and
autologous vein and the stent surface has been seeded with endothelial cells
or
otherwise treated. Each of these solutions suffer from certain drawbacks, such
as not being 'biocompatible, lacking sufficient mechanical strength, having a
surface that is difficult to prepare, lack of ready availability and being
thrombogenic. Antithrombotic drug regimens, in which anticoagulants and
thrombolytic agents are administered during and after deployment of the stent,
have also been employed to reduce the risk of thrombosis.
Thus, there remains a need for a stent capable of minimizing restenosis
while having a consistency similar to the native artery, a non-thrombogenic
surface and sufficient mechanical strength as well as being biocompatible and
readily available. This invention satisfies these and other needs.
SUMMARY OF THE INVENTION
The invention is directed to a stent assembly suitable for maintaining the
patency of a bodily lumen, generally comprising an expandable stent and an
expandable, biocompatible, non-thrombogenic jacket such as heterologous tissue
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
3
disposed about the exterior of the expandable stent. Preferably, the
heterologous tissue is selected from the group consisting of bovine
pericardium,
porcine pericardium, aortic leaflet and other suitable heterologous tissue.
The
stent may be an expandable, tubular framework and may be a conventional self
expanding or balloon expandable stent. The jacket is disposed about either or
both of the outer and inner surfaces of the stent. In a preferred embodiment,
the jacket is generally cylindrical for corresponding to the tubular framework
or
the stent.
This invention is also directed to a method for maintaining the patency of
a bodily lumen generally comprising providing a delivery catheter having an
expandable member on the distal extremity thereof, mounting the stent
assembly, including a tubular stent with a jacket of biocompatible, non-
thrombogenic expandable material such as heterologous tissue disposed about at
least part of the stent, on the expandable member on the distal extremity of
the
delivery catheter. The catheter is advanced through the body lumen within the
patient until the distal extremity of the catheter having the stent assembly
is
positioned at a desired location therein. The stent assembly is expanded by
expanding the expandable member onto which the stent assembly is mounted to
anchor the stent assembly within the body lumen. Once the stent assembly is
effectively positioned within the body lumen, the expanded expandable member
may be contracted, e.g. by deflating the balloon, and then the delivery
catheter
may be withdrawn.
A presently preferred embodiment of the invention is directed to a stent
assembly suitable for expansion within a body lumen and delivery of a
therapeutic or diagnostic agent therein, generally comprising an expandable
stent
and an expandable, biocompatible, non-thrombogenic jacket such as
heterologous tissue, which contains the therapeutic or diagnostic agent and
which is disposed about the expandable stent. The jacket releasably contains
at
least one therapeutic or diagnostic agent.
A wide variety of therapeutic or diagnostic agents for a variety of
indications can be used, including angiogenesis agents and antithrombotic
agents. The term "antithrombotic agents" is meant to include various agents
for
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
4
reducing the risk of thrombosis, including anticoagulants such as heparin,
thrombolytic agents such as urokinase, streptokinase, tissue plasminogen
activator (ACTILYSE), monoclonal antibodies such as abciximab (REOPRO),
fibrinolytic agents, and the like. Angiogenesis agents that stimulate the
growth
of neo-vessels include agents such as basic Fibroblast Growth Factor (bFGF)
and
Vascular Endothelial Growth Factor (VEGF).
In a presently preferred embodiment, the jacket is impregnated with a
liquid containing the therapeutic or diagnostic agent. For example, a jacket
formed from heterologous tissue which is submerged in a solution of the
therapeutic agent will absorb the solution. A variety of suitable methods of
applying the agent to the jacket may be used, including using
electrodeposition,
heat and pressure. Thereafter, the stent assembly can be positioned at a
desired
site within the patient's body lumen, where the jacket will release the
therapeutic agent. The jacket on the stent assembly may be impregnated just
before use, or alternatively, stored in the therapeutic or diagnostic agent so
that
the stent assembly is preimpregnated.
The invention is also directed to a method for delivery of a therapeutic or
diagnostic agent within a body lumen. The stent assembly including a tubular
stent with a jacket of biocompatible, non-thrombogenic expandable material,
such as heterologous tissue, containing a therapeutic or diagnostic agent is
positioned within the body lumen as outlined above. With the stent assembly
positioned at a desired location, the therapeutic or diagnostic agent is
released
from the jacket into the body lumen and thereby delivered at and around the
location of the stent assembly within the body lumen.
The expanded jacket of biocompatible, non-thrombogenic expandable
material such as heterologous tissue should extend over a substantial portion,
preferably all, of the stenotic region in which it is disposed in order
to minimize the restenosis.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
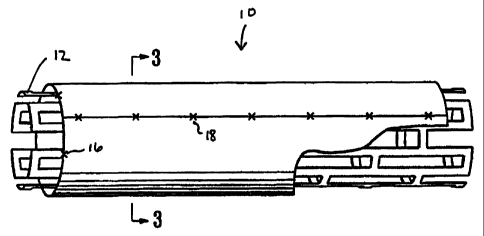
Figure 1 is a perspective view, partially broken away, of a stent assembly
of the invention showing a tubular, expandable stent with an expandable
biocompatible non-thrombogenic cylindrical exterior jacket.
Figure 2 is an elevational view, partially in section, of a delivery catheter
5 having a jacketed stent mounted on an inflatable balloon on the distal
extremity
of the catheter.
Figure 3 is a transverse cross sectional view of the stent assembly shown
in Figure 1, taken along lines 3-3.
Figure 4 is a perspective view, partially in section, of one embodiment of
the stent assembly, shown in the expanded configuration, having a
biocompatible non-thrombogenic jacket covering the length of the expandable
stent.
Figure 5 is a transverse cross sectional view of one embodiment of the
stent assembly prior to being expanded, illustrating the biocompatible non-
thrombogenic jacket in a S-shaped folded configuration.
Figure 6 is a transverse cross sectional view of another embodiment of
the stent assembly prior to being expanded, illustrating the biocompatible non-
thrombogenic jacket in a U-shaped folded configuration.
Figure 7 is a perspective view, partially broken away, of one embodiment
of the stent assembly having a biocompatible non-thrombogenic jacket
comprising an overlapping ribbon.
Figure 8 is transverse cross sectional view of one embodiment of the
stent assembly prior to being expanded, having a biocompatible non-
thrombogenic jacket in an overlapping wrapped configuration.
DETAILED DESCRIPTION OF THE INVENTION
In the embodiment of the invention shown in Fig. 1, stent assembly 10
comprises a tubular, expandable metallic framework forming the stent 12 with
an exterior jacket 14 of heterologous tissue. In the embodiment illustrated in
Figure 1, metallic stent 12 extends about 1 mm beyond each end of jacket 14 to
prevent prolapse of the tissue into the lumen of the stent when it is
expanded.
Jacket 14 may be secured to metallic framework 12 by any suitable means. For
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
6
example, four radially spaced sutures 16 may be placed at each end of jacket
14.
In a presently preferred embodiment of the stent assembly illustrated in
Fig. 1, the jacket 14 contains a therapeutic or diagnostic agent, as shown in
Fig.
3, illustrating a transverse cross section of the stent assembly shown in Fig.
1,
taken along lines, 3-3.
Exterior jacket 14 preferably comprises bovine pericardium, a material
shown to resist suture line bleeding, require no pre-clotting, support
endothelialization and have an excellent host-tissue response. Further, bovine
pericardial tissue has an elasticity of up to about 30% which allows the
tissue
cylinder to conform to both the unexpanded and expanded configurations of the
stent 12 with out adding a great deal of bulk which increases the profile on
the
balloon. Other heterologous tissue suitable in the practice of the invention
includes porcine pericardium, aortic leaflet, veins and arteries, and others.
Useful heterologous tissue is relatively impervious and impenetrable so as to
prevent tissue build up and the migration of smooth muscle cells through the
stent framework. A particularly preferred bovine pericardium has cross-linked
collagen and is available from Bio Vascular. Bovine pericardium tissue is
available in a thickness ranging from about 0.25 mm to about 0.75 mm, with an
average of about 0.45 mm.
In a presently preferred embodiment of the invention, the biocompatible
non-thrombogenic jacket 14 has a thickness of less than about 0.25 mm, and
preferably has a thickness of about 0.05 mm to about 0.20 mm, and most
preferably about 0.1 mm to about 0.15 mm. However, biocompatible non-
thrombogenic jackets having a thickness of up to about 0.75 mm may be used.
In the embodiment of the invention in which a thin biocompatible non-
thrombogenic jacket having a thickness of less than about 0.25 mm is used, the
heterologous tissue used to form the jacket is typically thinned before being
assembled with the stent. The tissue may be thinned by a variety of suitable
methods including peeling, shaving or otherwise removing a thin layer of the
tissue. In a presently preferred embodiment, the thin jacket comprises the
serous pericardium, which is the smooth, inner layer of the pericardium, which
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
7
has been separated from at least a part of the outer layer of the pericardium.
Similarly, where other forms of heterologous tissue are used, such as veins or
arteries, the venous or arterial walls may be thinned to the presently
preferred
thickness of about 0.05 mm to about 0.20 mm. As a result of being thinned,
the jacket may have reduced elasticity, so that the thin jacket is preferably
provided on the unexpanded stent in a folded or overlapping wrapped
configuration which provides sufficient material to cover the larger
circumference of the expanded stent, as will be discussed in greater detail
below.
The biocompatible non-thrombogenic jacket 14 preferably has a length
configured to cover the length of the expanded stent, as illustrated in Fig.
4,
showing an expanded stent 12 with a jacket 14 extending the length of the
stent, with a length equal to the stent length. However, the jacket may have a
length that is not equal to the length of the stent. For example, the jacket
may
have a length less than the stent length, as illustrated in Fig. 1, preferably
not
more than about 10%-20% less than the length of the stent. However, the
jacket may cover an even smaller percentage of the length of the stent, as for
example, when the stent assembly is used in a Transjugular Intrahepatic Portal
Shunt (TIPS) application, where the jacket length is about 50% less than the
length of the stent. Alternatively, the jacket may have a length greater than
the
length of the stent, preferably not more than about 5% greater than the stent
length. The jacket preferably has a circumference about equal to the
circumference of the expanded stent, configured to fit on an inner or outer
surface of the expanded stent. The jacket preferably fits on the expanded
stent
so that the jacket conforms to the expanded stent without flaps of excess
material.
Metallic stent 12 may comprise any suitable conventional stent. For
example, Micro Stent II and GFX stents available from Arterial Vascular
Engineering, and Multi-Link, available from Guidant, have proven useful. Other
stents that may be used in the practice of this invention include the Palmaz-
Shatz stent from Johnson and Johnson, the Gianturco stent from Cook
incorporated and other commercially available stents. Conventional balloon
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
8
expandable stents are preferred, but, as previously mentioned, self-expanding
stents, such as those formed from shape memory materials, are also suitable.
The stent assembly is formed by covering a surface of the unexpanded
stent with the heterologous tissue forming the jacket 14. In one embodiment,
the heterologous tissue is mounted onto the unexpanded stent in the form of a
cylinder of tissue. The cylinder of heterologous tissue forming the jacket 14,
may be formed by cutting a rectangle of tissue having a length about 2 mm
shorter than the stent on which it is to be mounted and a width about equal to
the circumference of the expanded stent. The two sides corresponding to the
length of the stent then may be secured together, such as by sewing with 6-0,
7-0, 8-0 or 10-0 polypropylene sutures. Other means for securing the sides of
the stent cover together include mechanical means such as staples, adhesive or
chemical bonding and the like. It may be desirable to support the tissue while
manipulating it. For example, a 9 French introducer dilator may be used to
support a 3 mm diameter cylinder, an 11 French dilator for a 3.5 mm cylinder
and a 12 French dilator for a 4 mm cylinder. The cylinder of tissue having a
circumference about equal to the circumference of the expanded stent may be
provided on the unexpanded stent in a folded or wrapped configuration. In one
embodiment, the tissue on the unexpanded stent forms wings 30 on either side
of the stent which are folded about stent, reducing the profile of the
assembly,
and unfolding upon expansion of the stent. In the embodiment illustrated in
Fig.
5, the wings are folded in the same direction in an S-shaped configuration. In
another embodiment, illustrated in Fig. 6, the wings of the cylinder of tissue
on
the unexpanded stent are folded about stent in opposite directions in a U-
shaped
configuration. However, the cylinder of tissue may be placed about the
unexpanded stent in a variety of suitable configurations, as for example,
where
the wings of the cylinder of tissue are collapsed toward the stent, such as in
an
accordion type configuration (not shown). It would be apparent to one of skill
in
the art that the heterologous tissue forming the jacket could be folded about
the
unexpanded stent as outlined above whether or not the tissue had been formed
into a cylinder of tissue before mounting onto the unexpanded stent.
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
9
In another embodiment, the heterologous tissue is wrapped around the
unexpanded stent, so that sufficient tissue to cover the expanded stent is
provided. In one embodiment, illustrated in Fig. 7, a ribbon of tissue is
spirally
wrapped around the unexpanded stent down a length thereof. The adjacent
turns of the ribbon of tissue overlap, so that the ribbon unwraps as the stent
expands to provide the jacket 14 configured to cover the expanded stent and
having a circumference about equal to the circumference of the expanded stent.
Preferably, the ribbon of tissue is wrapped along the entire length of the
stent.
In another embodiment, a rectangle of tissue having a width about equal to the
circumference of the expanded stent on.which it is to be mounted is repeatably
wrapped around the outer circumference of the unexpanded stent, so that
multiple layers of tissue are present on at least a part of the unexpanded
stent,
as shown in Fig. 8, illustrating a transverse cross section of an unexpanded
stent with a wrapped jacket thereon. Preferably, one end of the tissue is
fixed
to the stent, and the tissue is then tightly wrapped around the stent. Upon
expansion of the stent, the tissue unwraps to provide the jacket 14 having a
circumference about equal to the circumference of the expanded stent.
Preferably the length of the tissue is about equal to the length of the stent.
The tissue can be caused to remain in the folded or wrapped
configurations until the stent is expanded by pressing the fluid out of the
folded
or wrapped tissue. Additionally, securing members such as surgical tape, ties,
or breakable bands may be provided to releasably hold the tissue in the folded
or
wrapped configurations.
Depending upon the jacket material, the tissue may be kept wet at all
times during manipulation or it may be dry until advanced into the patient's
blood stream. Additionally, radio-opaque markers, such as rings of gold or
platinum, may be added to the outer layer of the tissue so that the integrity
of
the cylinder may be assured before deployment within the body lumen. The
cylinder of heterologous tissue configured to be mounted onto a stent and the
jacket 14 formed by the cylinder of tissue or the unwrapped or unfolded tissue
generally has a length, for coronary applications, of about 4 to greater than
about 80 mm, typically about 5 to about 80 mm, preferably about 10 to about
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
50 mm, and a diameter of about 1.5 to about 35 mm, typically about 2 to about
6 mm, preferably about 2.5 to about 5 mm. The actual length and diameter of
the cylinder of heterologous tissue may vary, and will depend on the nature of
the vessel in which the stent assembly is implanted. For example, for.
peripheral
5 vessel applications, such as an aortic abdominal aneurysm, a larger cylinder
of
heterologous tissue having a length of about 5 mm to about 200 mm and a
diameter of about 2 mm to about 60 mm would be used.
The jacketed stent assembly 10 is inserted into the body lumen in the
following fashion. A guidewire 20 is backloaded into a delivery catheter 22
10 having the jacketed stent assembly 10 mounted over an inflatable balloon 24
on
the distal extremity of the delivery catheter (as schematically shown in Fig.
2) or
on a self expanding stent delivery system (not shown). The catheter 22 and
guidewire 20 are percutaneously introduced by means of a conventional
Seldinger technique and a 5-9 or 10 French guiding catheter (not shown) into
the patient's arterial system. Larger guiding catheters, for example up to
about
Fr, may be used depending on the application. The guidewire 20 is advanced
out delivery catheter 22 through the vasculature under fluoroscopic imaging
until
it crosses a stenotic region. Then the catheter 22 is advanced over the
guidewire 20 until the stent assembly 10 is positioned at the desired location
20 within the stenotic region. Then, the balloon 24 is inflated or the
securing
mechanism of the self expanding stent is released to expand the stent 12 and
cylindrical jacket 14, seating the assembly 10 within the vessel. The balloon
24
is then deflated and the catheter 22 is removed, leaving the expanded stent
assembly 10 in place.
25 Although primarily described with respect to preventing restenosis in
angioplasty patients, the covered stents of this invention may be used in a
number of coronary artery, peripheral artery and non-vascular applications.
For
example, coronary artery applications include use in ectatic arteries and
ectatic
arteries containing an obstructive lesion, aneurismatic arteries, saphenous
vein
grafts and native arteries, coronary perforation, coronary fistula, and ostial
coronary lesions. Peripheral artery applications include aortic abdominal
aneurysm and other aneurismatic peripheral arteries, transjugular intrahepatic
CA 02304495 2000-03-23
WO 99/15105 PCT/IB98/01459
- 11
portal shunt, percutaneous transluminal angioplasty, fistula closing and neuro
interventions (such as aneurysms and arterial-venous malformations), small
vessel intraluminal grafting, and ostial renal artery lesions. Finally, the
covered
stents of this invention may be used in urological, gastroenterological,
respiratory, neurological, and other non-vascular applications. For example,
urological field applications include urethral stenting for stenosis due to
tumors,
fibrous tissue and perforation. Gastroenterological field applications include
fistula closing, reconstruction such as esophagus reconstruction, and
esophageal
bleeding. Respiratory field applications include tracheal and bronchial
obstructions, and neurological field applications include carotid angioplasty.
A general description of the device of the present invention as well as a
preferred embodiment of the present invention has been set forth above. One
skilled in the art will recognize and be able to practice many changes in many
aspects of the device described above, including variations that fall within
the
teachings of this invention. For example, the assembly may include a second
expandable stent, so that the heterologous tissue layer is between two
coaxially
disposed stents. Additionally, the jacket may cover the entire stent or only a
portion thereof. Additionally, the stent assembly may be used in branched body
lumens, and positioned to block one or more of the branch lumens. The spirit
and scope of the invention should be limited only as set forth in the claims
which follow.