Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02316518 2000-08-31
i .
-1-
Use of EC-Son for treatment of systemic autoimmune diseases
Field of the Inve tn ion
This invention relates to use of the EC-SOD gene yr th8 protein
encoded thereby for preventing or treating systemic autoimmune
diseases.
Ba- groan of th~Tn~ n.ion
In rheumatoid arthritis, the autoantibodies ( igG, IgM and igE;
and IgG in the joint) to the Fc domain of IgG, which are called
rheumatoid factor, increase and form immune complex together with
IgG, and the resulting immune complex sediments in the joint.
Neutrophils react to the sediments, thereby exacerbating the
symptoms . It is believed that some superantigens activate T cells,
which release lymphokines to activate macrophages and synovial
membrane, the synovial Cells release collagenase, proteases and
active oxygens, which cause cytotoxicity in rheumatoid arthritis.
The immune complex occasionally causes complications of
systemic angitis in malignant rheumatoid arthritis. The act~.ve
oxygen, Oz~', is a direct cause of inflammation and cytotoxicity
in rheumatoid arthritis and thus is one of the major therapeutic
targets to be removed. The removal of active oxygens is also a
chief therapeutic target in systemic lupus erythematosus.
Under these circumstances, the possibility to treat
inflammatory diseases by using Cu/Zn- superoxide dismutase (SOD)
and Mn-SOD has been sought. SOD is the enzyme catalyzing the
reaction involved in the superoxide anion radical, Oz~', 2oz~'+2H+
-~Oz+H2Oz. This enzyme protects cells from the toxic effects of
OZ~- and other active oxygens generated from os~'. ,
It was known that the direct, local administration of SOD
protein to the joint is effective for the treatment of arthritis
in horse. The substance, which Huber et al. named Orgotein in
1965, was SOD. In the englobement, the cell membrane of
polymorphonuclear leukocytes produce oZ~' by the action of NADPH
oxidase, and HzOz, Oz~', and others generated by the
disproportionating reaction decrease the viscosity of hyalurvnic
acid to cause pain.
CA 02316518 2000-08-31
r
~z-
When IgG molecules adhere to bacteria, leukocytes easily take
in the bactexia; this phenomenon is called phagocytvs~.s. The
development of rheumatoid arthritis is considered to resemble
phagocytosis.
Cu/Zn-SOD purified from bovine erythrocytes has been used.
Attempts have been made to incorporate liposomes containing
Cu/zn-SOD into cells. The SOD covalently linked tv ~ styrene
maleie acid ester derivative (SMA-SOD) for keeping the blood sop
level is known to accumulate in inflammatory regions and exerts
its effect.
An animal diabetes mellitus model can be prepared by
administering alloxan to an animal. In this animal model, the
development of diabetes mellitus can reportedly be prevented by
pre-administration of SOD to the animal prior to the
administration of alloxan.
A transgenic NOD mouse to which the Cu/zn-SOD gene is
introduced for the removal of intracellular active oxygens is
resistant to the development of alloxan- or streptozotocin-
indueed diabetes.
It has also been shown that the administration of SOD
conjugated with gelatin has the therapeutic effects vn arthritis
induced by collagen (CIA) (x. xakimoto et al. , 1993, Clin. Exp.
Immunol. 94: 241-246). However, SOD used in the report was
Cu/zn-SOD, and effects of extracellular superoxide dismutase
(EC-SOD) were not described there. There is a problem of short
half-life of administered SOD protein or its modified protein.
EC-SOD is a subtype of SOD and belongs to a group of SOD that
is released from the cells. EC-SOD reduces cerebral edema and
ischemic cardiopathy and suppresses neutrophil infiltration and
inflammation. '
EC-SOD anchors to heparan sulfate proteoglycans of glycocalyx
on the endothelial cell. The V-type EC-SOD (EC-SOD V), which
was originally synthesized, can be yielded by fractionation based
on its affinity to heparin (EC-SOD V is induced by heparin
administration).
It is assumed that the EC-SOD concentrations in the blood and
the endothelial cell surface are equilibrated. A decrease in the
CA 02316518 2000-08-31
r ~ ,
- 3 -
amount of EC-SOD bound to the endothelial cells reportedly reduces
the resistance to the oxidative stress (Adachi T, et al., Hiol.
Pharm. sull., 1998, 21(10): 1090-3). Miller et al. reported
that the level of O,~- can be decreased by the adenoviral
6 vector-mediated expression of Cu/2n-SAD and EC-SOD in the artery
(Miller F. J. Jr, et al., (1998) Circ. Res. 82(12): 1298-305).
However, the authors describe that the endothelial cell-dependent
relaxation was not recognized. irlos and EC-SOD are known to be
highly expressed in the macrophages present even in
arteriosclerotic lesions. Luoma et al. suggested the
possibility that arteriosclerosis might be suppressed by
controlling the balance of the expression levels of iN05 and
EC-SOD in the artery (Luvma, J. S., et al., Arterioscler Thromb.
Vasc. Hiol., 1998, 18(2): 157-67). However, its therapeutic
x5 effects vn systemic autoimmune diseases, especially, on
rheumatoid arthritis are not implied.
Oxidized low density lipoprotein (LDL) particles are involved
in the adhesion of leukocytes to the endothelium in initial
arteriosclerosis. Furthermore, it is known that administered
Cu/Zn-SOD and SOD induced by heparin reduce such adhesion (Lehr,
H. A. , et al _ , Arterioscler Thromb. , 1992, 12 ( 7 ) : 824-9 ) . These
facts suggest that the presence of a required amount of EC-SOD
in the blood may not only cause anchoring of the enzyme to the
heparan sulfate on the entire vascular endothelia tv suppress the
leukocyte adhesion to the endothelia but also scavenge the active
oxygens released from neutrophxls.
However, it is so far not clear whether EC-SOD exerts the above
effect on the joint edema already developed. Furthermore, it is
difficult to keep the blood EC-SOD at an enough level because i.ts
molecule weight is low and zt is thus rapidly excreted in the urine.
An attempt was made to prolong ~.ts half-life in blood by chemical
modification. However, steric hindrance may prevent the
modified EC-sOD from anchoring to the endothelium, and
consequently EC-SOD cannot function properly.
Application of gene therapy to the systemic autvimmune
diseases has been studied. Methods of gene therapy include 1)
a method in which a specific gene ligated in a vector is introduced
CA 02316518 2000-08-31
a ~ ~ ,
- 4 -
into cells lacking the gene in the diseased tissues f 2 ) a method
using antisense nucleotides; 3) a method using ribozyme that
cleaves RrlA site-specifically to suppress synthesis of a specific
pxotein; 4 ) the gene knockout method in which a specific gene is
knocked out so as not to be expressed; 5) a method in Which
transcription initiation of a specific gene is regulated to
control its expression, etc. In animal tests, these methods of
gene therapy are being recognized as effective when applied to
the treatment of systemic autoimmune diseases, especially to
arthritis and rheumatoid arthritis. These methods may become
side-effect-free fundamental therapy in future in place of the
currently avairable antirheumatic drugs. However, these methods
are still at the stage of basic research, and their clinical
effectiveness has not yet been proven.
There are three classes of drugs used currently for treating
rheumatism: non-steroidal anti-inflammatory drugs, steroidal
drugs and disease-modifying antirheumatic drugs (DMARD). The
DMARD is most effective but the problems are strong side effects
and the weakening of the therapeutic effect during long-term
administration. Drugs, far which clinical studies are underway
for the future therapeutic use, include, for example, a soluble
TNF a receptor-Fc fusion protein and IL.-1Ra as biological
preparations and monoclonal antibodies including anti-TNF a
monoclonal antibody, anti-IL-6receptor monoclonal antibody, etc.
Specifically, good xesu~.ts have been given in experimental model
animals treated by gene therapy using the vector with the genes
encoding these agents. Examples of such vectors are adenoviral
vectors for the gene of the soluble TNF a receptor-FC fusion
protein, the vIL-10 gene, or the I kappa B gene; retroviral vectors
for the IL-1Ra gene; and HVJ' liposome for the NF - .B decoy gene.
P.H. Goossen et al. expressed a suicide gene for the removal
of synovial cells (P.H. Gvossen et al., "Feasibility of
Adenovirus-Mediated Nonsurgical Synovectomy in Collagen-Induced
Arthritis-Affected Rhesus Monkeys", Human Gene Therapy
10:1139-1149, 1999). The clinical gene therapy of rheumatoid
arthritis has been approved and progressed in the United States
( gene therapy using retroviral vectors with the IL-1Ra gene ) . The
CA 02316518 2000-08-31
a ~ ~ ,
- 5 -
therapeutic purposes of gene therapy of rheumatoid arthritis (RA)
are relief of synovitis and prevention of cartilage and bone
destxuetions. The methods are divided into an in vivo direct
method and an ex vivo indirect method. The direct method requires
high effxc~,ency of gene transfer but a practical vector that meets
this requirement is so far unavailable, and therefore the indirect
method is used for the trials. rn this case, the adhesion
efficiency of the vectors to the cells is a key factor. However,
the safety of the treatment is being examined at present, and the
therapeutic effect remains to be clarified.
Hasunuma et al. examined whether the expression of caspase
3, caspase 8 or FADD and the MAP kinase activity are involved in
the difference in susceptibil ity of synovial cells indigenous to
rheumatoid arthritis(RA) patientsto the Fas-dependent apotosis,
and further examined the possibility of gene therapy using Fas
ligand. Based on the hyperactivity of Fas-dependent apvtvsis in
RA synovial cells and the possibility of close association of this
phenomenon with FAriD molecules, they administered the Fas
ligand-expressing cells to the synovial tissue and confirmed that
intensive apotosis was induced and the proliferating synovial
membrane was removed (T. Hasunuma, et al. , The Japan Society of
Cliriical Biochemistry and Metabolism, vN. voL. 35p. 48,1998).
zhang et al. have proposed a new model for gene therapy by the
transfer of the Fas ligand (Fast) in the treatment of
collagen-induced arthritis known as a model of human rheumatoid
arthritis (J. Clin. Invest., 100:1951, 1.997). In their basic
study on gene therapy, they compared the Fast-adenvvirus
administered mice with the PBS or control adenovirus-vector
administered mice to evaluate the therapeutic effect of the Fash
gene transfer to the model mice affected with the collagen-induced
arthritis. The results showed that the progress of the autoimmune
disease was able to be stopped by inducing apotosis of
inflammatory cells resulted from the expression of the Fast gene
introduced into the inflammatory joint. Moreover, it is reported
that the expression of the introduced Fas7G gene in the
inflammation region xndueed systemic immunotolerance of the
autoreactive cells (N. Suzuki, Clinical Immunology VN VOL. 30,
CA 02316518 2000-08-31
- 6 -
N0.11P, 1544--1549, 1998).
zn addition, basic examinations for establishing gene therapy
targeting synovial cells were reported {K. Nishioka, Uehara
Memorial Life Science Foundation, record of research reports vN
VoL. 9 P.337-339, 1995). ~n this report, a tax gene-introduced
model mice for gene therapy by the transfer of antisense
oligonucleotides targeting HTLV-I-infected synovial cells was
prepared and used to examine the therapeutic effect of the gene
therapy, the development of a program for therapeutic control of
synovial lesion of rheumatoid arthritis by utilizing the apototic
property, and planning for the fundamental treatment by inducing
apotosis by anti-Fas antibody. The effectiveness of the transfer
of naked nNA to the affected part has been reported by a research
group of National Institute of Dental Research (NIDR) which is
a research organization belonging to National Institutes of
Health (NIA) in the united States and a group of Food and Drug
Administration ( FDA) in the United States . They have found that
the directly introduced transforming growth factor,Q (TGF,Q ) gene
reduces the symptoms of rheumatoid arthritis in a mouse model of
rheumatoid arthritis (slanchette, F. et al., (1997) J. C7,in.
Invest. 99(8): 1974-83). They have also confirmed that
subcutaneous injection of the naked TGF,C~DNA is mere effective
than its intra-articular injection, suggesting the possibility
that TGF,Q is expected to relieve inflammation through the cells
localized in the regions other than the joint.
The proliferation of synovial cells is reportedly inhibited
by the expression of cyclin-dependent kinase inhibitor introduced
using adenovirus in the cells (Ren Tariiguchi et al., Nature
Medicine, "Induction of the pl6zNK4a senescence gene as a new
therapeutic strategy for the treatment of rheumatoid arthritis"
Vol. 5, Number 7. p760, 1999 ) . Whalen, J. D et al. (university
of Pittsburgh; GenVec, Inc.) have also reported on use of
adenovirus tar the expression of IL-10 and its homologs (Whalen
J, n., ~'Adenoviral transfer of the viral IL-10 gene
periarticularly tv mouse paws suppresses development of
collagen-induced arthritis in bath injected and uninfected
paws-adeno virus vector used tv transfer Epstein-Harr virus
CA 02316518 2000-08-31
r ~ , ,
- '7 -
interleukin-10 homolog gene in rheumatoid arthritis gene therapy"
J. Immunol. vol. 162, 6, p3625--32, 1999) . However, no report has
been available on the use of the EC-SOD gene for gene therapy of
systemic autoimmune diseases including rheumatoid arthritis.
s
ntiQ.n
An objective of this invention is to provide the EC-SOD gene,
vectors containing the gene, cells expressing the gene, EC-SOD
protein, which are used for preventing and treating systemic
autoimmune diseases, and compositions comprising the gene or the
protein as an active ingredient for pr~aventing and treating
autoimmune diseases.
EC-SOD is a sOD existing extracellularly, whereas Cu/zn-SOn
is localized on the cell membrane and Mn-SOD in the mitochondria.
This information suggests that EC-SOD functioned optimally
outside the cells. The present inventors considered that the
blood Ec-son level could be elevated by administering EC-SOD or
its gene to systemically exert its effect, which made it possible
to treat systemic autoimmune diseases effectively. Furthermore,
l0 the inventors contemplated that the lining cells such as
endothelial cells and synovial cells were able to be protected
from the attack of leukocytes in autoimmune diseases by gene
therapy for maintaining the blood EC-SOD at an adequate le~rel for
a prolonged period of time, utilizing the affinity of EC-SOD for
the endothelium.
The present ~.nventors tr~us introduced the EG-SOD into a
retroviral vector and transfected the fibxoblast cells prepared
from DBA/1 mouse embryos with the vector. Secretion of EC-SOD
protein from the cells was confix~ned. After the cells were
inoculated into DBA/1 mice subcutaneously, type-II collagen was
injected to the joint to induce arthritis. The plasma
concentration of EC-SOD was increased significantly in the mice
to which the cells with the EC-SOD gene was inoculated, and the
incidence and the severity of the arthritis were decreased
markedly in the inoculated group as compaxed with the no
inoculated group. In addition, inoculation of the EC-SOD
gene..introduced cells into the mice that developed collagen-
CA 02316518 2000-08-31
z ~ ,
- g -
induced arthritis suppressed the arthritis. Eistological
indication of arthritis, such as infiltration of monocytes in the
joint cavity, the proliferation of synovial cells, or the
cartilage destruction, were hardly recognizable in the
arthritis-induced mice to which the EC-SOD-introduced cells were
inoculated, demonstrating the arthritis- suppressing effect of
EC-SOD.
Furthermore, the present inventors tested the effect of EC-S
OD gene transduction on mice with colitis. As the result, EC-S
OD gene was shown to have the therapeutic effect on colitis.
The present inventors thus found that autoimmune diseases
including arthritis and others could be prevented and treated
effectively by increasing the EC-SOD protein level by means of
gene therapy using EC-SOD expression vectors, and completed the
present invention.
Specifically, the present invention relates to the prevention
or the treatment of systemic autoimmune diseases using EC-50D gene
or EC-SOD protein, and more specifically to:
( 1 ) a nucleic acid encoding EC-SOD protein used for treating or
preventing systemic autoimmune diseases;
(2) the nucleic acid as described in (1), wherein the systemic
' sutoimmune disease is rheumatoid arthritis ar co~.itis;
( 3 ) a vector containing the nucleic acid as described in { 1 ) or
{z):
(4) the vector as described in (3), wherein the vector is a
retroviral vector ox a sendai virus vector;
(5) a cell expressively carrying the nucleic acid as described
in (1) or (2) as a foreign gene;
( 6 ) the cell as described in ( 5 ) , wherein the cell secretes EC-SOD
protein;
(7) an EC-SOD protein used for treating or preventing systemic
autoimmune diseases;
{e) the protein as describe in {7), wherein the systemic
autoimmune disease is rheumatoid arthritis or colitis.
the present invention also relates to a pharmaceutical
composition for treating or preventing systemic autoimmune
diseases, which comprises a vector containing a DNA encoding
CA 02316518 2000-08-31
' ,
- g
EC-SOD protein or a EC-SOD protein as an active ingz-edien,t.
Furthermore, the present invention relates to a method of
treating or preventing systemic autoimmune diseases, the method
comprising administering a vector containing a DNA encoding
EC-SOD protein to a recipient.
Sri ef De~c_riy~ i on of the Drawin ~s
Figure 1 shows the structures of pRx-ZpN and pRx-ZpN-EC-SOD.
In the promoter, the enhancer sequence in the U3 region within
5'LTR was replaced with CMV IE (CMVIE~LTR). "gag-killed"
indicates that a mutation i.s introduced so that the gag protein
is not expressed. "PGKpro." denotes the PGK promoter.
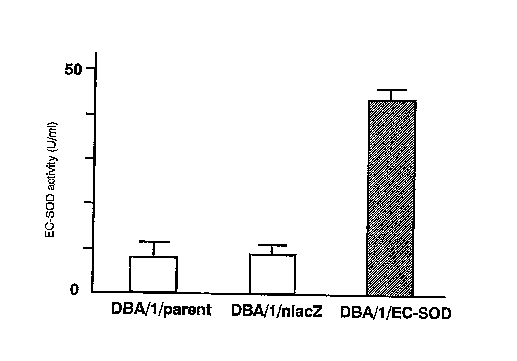
Figure 2 shows the EC-SOD activity in the culture supernatant
of DBA/1 fibroblast cells (DHA/1/EC-SOD) into which the EC-8oD
gene was introduced. "DHA/1/parent" denotes D8A/lfibroblast cell
(parental cell); "DBA/1/nlacz" indicates the mock cell
transfected With an nlacZ expression vector.
Figure 3 shows a time course of the EC-SOD activity in the
serum of DBA/1 mice, measured after the inoculation of
DBA/1/EC-SOD fibroblast cells into the mice.
Figure 4 shows the effect of EC-SOD gene expression on the
development of CIA in mice affected with collagen-induced
arthritis. The abscissa indicates days after the initiation of
induction by collagen, and the ordinate indicates the CIA
incidence.
Figure 5 shows the relationship between the effect of EC
SOD expression on the prevention of CIA and the clinical score
of CIA in mice affected with CIA. The abscissa indicates days
after the initiation of induction by collagen, and the ordinate
indicates the clinical score.
Figure 6 shows the relationship between the effect of EC-
SOD expression on the treatment of CIA and the clinical score of
CIA in mice affected with CIA (the inoculation was carried out
once). The abscissa indicates days after the initiation of
induction by collagen, and the ordinate indicates the clinical
score.
Figure 7 shows the relationship between the effect of EC-
CA 02316518 2000-08-31
- 10 -
SoD expression on the treatment of CIA and the clinical score of
CIA in mice affected with CIA (the inoculation was carried out
three times ) . The abscit3sa indicates days after the initiation
of induction by collagen, and the ordinate indicates the clinical
$ score.
Figure 8 shows maximum disease scores in the group to which
the cells were inoculated three times.
Figure 9 is a photograph of the mouse joint cavity on day 50,
histolvgically showing the post-symptomatic effect of EC-SOD.
Figure 10 shows the EC-SOD activity in the supernatant of
Balb/c fibroblasts transduced EC-SOD gene.
Figure 17, shows the time course of EC-SOD activity in Balb/e
mice serum after inoculation of EC-Sop gene transduced
fibroblasts.
Figure 12 shows the survival rates of EC~SOD gene treated and
non-treated mice with DSS-induced colitis.
Figure 13 shows the body weight loss of EC-SOD gene treated
and non-treated mice with DSS-induced colitis.
Figure 14 shows the body weight loss of EC-SOD gene treated
and non-treated mice with DSS-induced colitis at day 8.
Figure 15 shows the colonic length of EC-SOD gene treated and
non-treated mice with DSS-induced colitis.
Figure 16 shows the colonic blood contents of EC-SOD gene
treated and non-treated mice with DSS-induced colitis.
2.5 Figure 17 is a photograph of the histopathological change of
EC-SOD gene treated and non-treated mice with Dss-induced
colitis.
Figure 18 shows the pathological score of EC-SOD gene treated
and non-treated mice with DSS-induced colitis.
Figure 19 shows the effect of EC-SOD gene transduetion on
production of mouse IL-1,Q in serum and colvnic mueosa of
DSS-induced colitis mice.
Figure 20 shows the effect of EC-SOD gene transduction on
production of mouse TNF a in serum and cvlonic mueosa of DSS-
induced colitis mice.
CA 02316518 2000-08-31
, , - 11 -
D ai l d D .s in ion d h Tnv noon
The present invention relates to a method for preventing or
treating systemic autoimmune diseases using EC-SOD or its gene.
Herein, systemic autoimmune disease" means a disease in Which
the presence of autoantibody is recognized and the lesions caused
by immunological abnormality are found in almost all the organs.
Examples of the diseases include, for example, those listed below
(see Jun-ichi Yada, Medical immunology, Chugaiigaku).
1. Systemic lupus erythematosus (SLE)
The disease exhibits the symptoms of angitis, arthritis and
nephritis. The immune complex of a nuclear antigen and the
antinuclear antibody is considered to cause tissue injury.
2. Rheumatoid arthritis
rn rheumatoid arthritis, the autoantibody against the Fc
domain of IgG is detected and pyogenic diseases associated with
arthritis and carditis, etc. may develop occasionally.
3. Rheumatic myocarditis
Rheumatic myocarditis is believed to be caused by the
cross-reaction of the autoantibodies between streptococcal N
24 protein and cardiac tissue antigens. The antibodies further
react with the autoantigens released as a result of some tissue
injuries, and which causes a vicious cycle. Accordingly, there
is the possibility that the treatment can be performed by
inhibiting the cytotoxicity resulted from the active oxygens and
neutral proteases produced by autoreactive lymphocytes and
neutrophils mediating the tissue injuries.
4. Progressive systemic sclerosis (PSS}
In the disease, the production of the connective tissue
collagenosis fibers develops obstructive lesions of the blood
vessels. Edema, sclerosis and the subseguent atrophy develop
mainly in the skin of the fingers. This disease is characterized
by the presence of the antibody to topoisomerase I (Scl-70 } . The
di 9Pe~9P Wlth h~ni sin v~i ~r:Rr~1 1 P~ i nns i s csl 1 ~d cRFST syndrome,
which is characterized by the presence of anti-centromere
antibody.
5. Dermatomyositis/polymyositis (DM/PM)
The weakened muscular power of the limbs and the skin erythema
CA 02316518 2000-08-31
- 12
are especially noted. swallowing difficulty is also observed.
Among antinuclear antibodies, fro-1 antibody is a characteristic
of this disease. Ku antibody is often positive in cases of
complication with progressive systemic sclerosis.
6. Mixed connective tissue disease (MCTD)
Sausage-like fingers and Raynaud's phenomenon are clinical
manifestations. This disease is defined as the disease with a
partial combination of the symptoms of systemic lupus
erythematosus, progressive systemic sc7.erosis, and
dermatomyositis, and characterized by the presence of anti-
ribonucleoprotein (RNP) antibody and the absence of Sm antibody
among ENA antibodies. SSA antibody is frequently positive in
cases of complication with Sjogren's syndrome.
7. Sjogren's syndrome
The disease is considered to be caused by autoimmune disorders
of the lachrymal gland and the salivary gland. SSA antibody and
SSS antibody are characteristically recognized among antinuclear
antibodies.
8. Polyarteritis
Periarteritis nodosa (pN) is a classical type. The tissue
injury caused by the immune complex and the antibodies reactive
to the arterial wall will develop.
9. Rheumatic fever
Main symptoms are arthritis and carditis that are developed
after streptococcal infection. Chorea, erythema circinatum, and
subcutaneous nodule are occasionally seen. The presence of an
antigen in the cardiac muscle cross-reactive to the anti-
streptococcal M protein antibody as well as that in the cardiac
valve cross-reactive to the antibody against polysaccharide of
streptococcus has been proved by the serum autoantibodies
reactive to the cardiac tissues.
10. Wegener gxanulomatosis
Granulomatous lesions develop in the respiratory organs such
as the nasal cavities, accessory sinus cavities and lungs, and
systemic angitis develops. Among the anti-neutrvphil cytoplasm
antibodies (ANCA), the antibody (C-ANCA) against a proteinase
that is distributed thoroughly in the cytoplasm is frequently
CA 02316518 2000-08-31
- 13 -
detected in the serum. An antibody, P-ANCA, reactive to the
perinuclear myeloperoxidase is observed in the sera from the
patients with necrotizing crescentic glomerulonephritis and
microscopic polyarterxtis.
11. colitis
So far, in spite of a number of researches, cause of colitis,
for example, inflammatory bowel disease, which is also called
Crohn disease and ulcerative colitis, has not yet elucidated among
autoimmune disease. Recently, it is thought that the cause is
not single and that hereditary factors, immune abnormality, and
environmental factors are complexly involved in it. In some cases,
colitis involves food allergy, and therefore, diet therapy is
effective. However, in many cases, colitis requires long
hospitalization and is not completely curedi
The EC-SOD protein used for the prevention or treatment of
autoimmune diseases in this invention may be the natural protein
or can be prepared as a recombinant protein by using gene
engineering technology. The amino acid sequence of human EC-son
protein is set forth in SEQ ID N0: 2. The natural EC-SOD protein
can be purified from the extracts or culture supernatants of
tissues or cells expressing the protein by using known
technologies for protein separation such as affinity
chromatography, ion exchange chromatography, reverse phase
chromatography, gel filtration, and salting-out, etc.
Furthermore, the protein can be prepared by affinity
chromatography using the antibody to the EC-SOD protein. The
antibody to the EC-SOD protein can be prepared by known methods
using EC-SOD protein and its partial peptides as antigens. The
antibody may be a polyclonal antibody or a monoclonal antibody,
The EC-SOD protein can be prepared by the method of Marklund
for example (Marklund, S. L., Proc. Natl. Acad. Sei. t~SA
79 :7634-7638 ( 1982 ) ) . This method produces the protein at a poor
yield (1.3% recovery) and thus the analysis of this protein was
progressing at a sivw pace. Eventually, it has been clarified
that the protein consists of four subunit of 30kDa by Marklund
(ditto), Tibell et al, (Tibell, L. et al. (19$7) Proc. Natl. Acad.
Sci. uSA 84 s 6634-6638 ) , and Stromqvist (Stromqvist, M. ( 1993 ) J.
CA 02316518 2000-08-31
- 1.4 -
Chromatogr 621:139 - 148) . A method has also been developed for
purifying the EC-SOD protein at a high yield ( Oury, T. D . et a1. ,
(1996) Biochem. J. 317:51-57). The protein should be expressed
at a high level in the tissue used as the sources of the protein.
Such tissues include, for example, the b7.ood vessels surrounding
the outer membrane or smooth muscle, trachea, lung, aorta,
umbilical cord, placenta, and others. The recombinant protein
can also be prepared by culturing the cells transformed with the
DNA encoding EC-SOD protein, expressing the EC-SOD protein and
recovering the EC-SOD protein from the culture supernatant or the
cell extracts. An example of the DNA encoding EC-SOD protein is
a DNA segment of from nucleotide positions 124 to 789 of the human
EC-SOD cDNA set forth in SEQ ID N0: 1. When the EC-sOD protein
is secreted outside the cells using mammalian cells or others as
hosts, a DNA segment of from nucleotide positions 70 to 789 (which
contains the signal sequence ) of the human EC-SOD cDNA set forth
in SEQ ID NOs 1 can be used.
The EC-SOD protein of this invention can be used to prepare
a composition for the treatment or the prevention of autoimmune
diseases by mixing the protein with pharmaceutically acceptable
carriers or solvents ( for example, physiological saline, buffer
solutions, stabilizers, preservat~.ves, and suspending agents,
etc.).
The expression vector used for the production of the
recombinant protein may be an appropriate vector selected, as
necessary, from known vectors, depending on the host to be used.
For example, vectors suoh as pUClB, pUCl9, pSPORT1, and
pSPOItT2 (GraCO-sRD) can be used, when E. colj is used as a host.
Vectors for the eukaryotic expression may be PSFV1, pCMV~SPORT
3d ,Q-gal (GrBCO-BRL) , etc. PBS185, pBS246, pBS302, pSFl, yr pSS226
( GIBCO-BRL ) can be used as the vector for the integration of DNA
into chromosomes of host cells that can utilize the Cre/lvxP
system.
A wide variety of vectors can be used as the expression vector
in mammal cells (R. J. Kaufman, 1990, Methods zn Enzymology vol.
185[39], p. 487-511, Academic Press, Ino.). Such mammalian
expression vectors are, for example, pTARGETT" vector (pTARGET
CA 02316518 2000-08-31
- 15 -
T''' Mammalian Expression Vector System; Promega) , pSI (Promega ) ,
pCI (Promega), pCI-neo (Promega), PALTER-MAX (Promega),
pAdVAntage (Promega); tetracycline-responsive vector (pTet-On,
pTet-Off, ptTA2/3/4, pTRE, pTRE-d2EGFP, pBI, pBI-EGFP, pBI-L,
pBI-G, pTK-Hyg; Clvntech), tetracycline-responsive retroviral
vector (pRevTet-On, pRevTet-Off, pRev-TRE, pRetro-on, pRetro-
Off; Clontech); retroviral vector (pLAPSN, phNCX, pLXIN, pLXSN,
ASIA; Clontech); IRES bicistronic vector (pIRESbleo, pIREShyg,
pIRESneo, pIRESpuro ,AIRES-EGPF, AIRES-EYFP; Clvntech);
mammalian selection vector (pHygEGFP, pNeoEGFP, pPUR; Cloritech);
ecdysone-inducible vectors (pIND vectorssuch aspIND,pIND(SP1),
pIND/Hygro, pIND(SP1)/HygrO pIND/V5-His, pIND/V5-His-TOPO, and
pIND/GS; Invitrogen); EpiTag ~' vector (pcDNA3.1/His,
pcDNA3.1/V5-His, pcDN~A3.1/myc-His, pcDNA3.1 (-)/myc-His,
pcDNA4/His, pcDNA4/V5-His, pcDNA4/myc-His, pcDNA6/His,
pcDNA6/V5-His, pcDNA6/myc-His, pEF1/His, pcEF1/V5-His,
pcEFl/myc-His, pEF4/His, pcEF4/V5-His, pcEF4/myc-His, pEFG/His,
pcEF6/V5-His, pcEF6/myc-His, and pvB6/v5-His; Invitrogen);
selectable expression vectors (pcDNA3.1, pcDNA3.1/Zeo,
pcDNA3.1/Hygro, pcDNA4/HisMax, pRc/CMV2, pRc/RSV, pZeoSV2;
Invitrogen); transient expression vectors (pCDMB, pcDNAl.l,
pcDNAl.l/Amp; Znvitrogen); specialized expression vectors
(pcDNA3.1/V5-His/TOPO, pCR3.l, pSecTag2, pSecTag2/Hygro,
pbisplayT°°, pVAXl; Invitrogen); intracellular targeting
expression vectors (vectors of pShooter ~' system such as
pEF/myc/nuc, pCMV/myc/nuc, pEF/myc/mit, pCMV/myc/mit,
pEF/myc/ER, pCMV/myc/ER, pEF/myc/cytv, and pCMV/myc/cyto;
Invitrogen); VP22 expression vector (voyagerT" vectors such as
pVP22/myc-His; Invitrogen); sindbis expression vectors such as
80 pSinHis/pSinRep5 (Invitrogen); EBNA-1-gene containing vectors
(pCEP4, pREP4,pREP7, pREP8,pREP9, pREPlO, pEBVHis; Invitrogen);
vectors of Capture-Tec ~'" system such as pHook T" -1, pHVOk "' -
2, and pHOOk~"-3 (Invitrogen).
In addition, expression vector systems in which yeast is used
as a host are, for example, YEX Yeast Expression Systems
(Clontech) and MATCHMAKER Yeast Expression vectors (Clontech),
etc. When insects ar insect cells are used as hosts, it is
CA 02316518 2000-08-31
- 16 -
possible to use the systems including HacPSIR saculovirus
Expression System, BacPAK Rapid Titer Kit, psacPAK8 & pBacPAK9
Transfer Vectors, pAcUW31 Transfer Vector, and BacPAK6 Viral DNA
(Clontech) as well as Hac-to-Bac~Baculoviral Expression Systems
including pFASTBac T" 1, pFASTBac T" HTa, b, c, and pFASTBac ''~' DvAI.
(GxHCO-HRL Co.).
The DNA encoding EC-SOD protein used in this invention is nvt
particularly liminted as long as the DNA encodes the EC-SOD
protein. The DNA may be any of DNAs encoding EC-SOD including
cDN~s, genome DNAs, and synthetic DNRs. In addition, the DNA also
includes any of nNAS with nucleotide sequences degenerated based
on the codon degeneracy as far as the DNAs encode the protein of
this invention. Human EC-soD cDNA is preferably used. The
nucleotide sequence of the human EC-SOD gene is set forth in the
SEQ ID NO: 1.
EC-SOD cDNA can be screened, for example, by hybridizing a
''P-labeled oligonucleotide probe which is synthesized to contain
a partial sequ~nce of the human EC-SOD gene to a cnNA library
derived from a tissue (the blood vessels surrounding the outer
meiubxane or smooth muscle, trachea, lung, aoxta, umbilical cord,
placenta, and others). EC-SOD cDNA can also be cloned by
polymerise chain reaction using oligonucleotide primers
synthesized based on the EC-SOD gene sequence and, as a template,
cDNA derived from an appropriate tissue (blood vessels
surrounding the outer membrane or smooth muscle, trachea, lung,
aorta, umbilical cord, placenta, and others}, thereby amplifying
the cDNA of interest . The genomic DNA can be screened, fox example,
by hybridizing a '~P-labeled synthetic oligonucleotide probe
containing a partial sequence of the EC-50D gene to a genomic DNA
Xi.bxary. The EC-SOD gene can also be cloned by polymerise chain
reaction using oligonucleotide primers synthesized based on the
EC-SOD gene sequence and genomic DNA as a template to amplify the
desired gene. The EC-SOD gene can also be synthesized, for
example, by chemically synthesizing oligonucleotide pairs with
partial nucleotide sequences of the EC-SOD gene, annealing the
pair of oligonucleotide to each other, and ligating the annealed
DNAs using DNA ligase. The DNA encoding EC-SOD protein can be
CA 02316518 2000-08-31
-- 17 -
used for gene therapy as well as for the production of recombinant
Ec-SOD protein.
The vector, into which the EC-SOD gene is inserted, used fax
gene therapy using the Ec-SOD gene, is not particularly limited
g as long as the vector can express the gene in mammalian cells.
As described in "R. ~. Raufman, 1990, Methods in Enzymology vol.
185[39], p. 487-511, Academic Press, and Iric.", any known gene
transfer methods can be applied to the method in which expression
vectors are transfected to the cells taken from the body and then
the cells are returned to the body, namely, the ex vivo method
(V. S. Patent 5,399,346). For example, the mammalian expression
plasmid vector can be introduced into cells by DEAE-dextran method,
ration liposome method, polycation (polylysine,
polyethyleneimine, ete.) method, and calcium phosphate
precipitation method, etc., when the vector to be introduced is
a non-viral vector. specifically, the mammalian-cell expression
vectors provided by the suppliers, such as Promega, CLONETECH,
and Invitrogen, are designed to have some built-in promoter and
enhancer, and, if required, inducible promoters that control the
expression can be applied to these vectors (Methods in Ezlzymology
vol. 185[39], p. 497). Viral vectors used in this invention
include Sendai virus vector, Sv40 vector, vaccinia virus vector,
Epstein-sarr virus vector, adeno-associated virus vector,
adenovirus vector, retrovirus vector, 7.entivirus vector or other
vectors.
Especially, 5endai virus vector is suitably used in this
invention because it can achieve the high level expression of a
foreign gene in the living body to which the vectro is administered.
The Sendai virus expression vector can be prepared by known
methods (WO97/16539 and W097/16538) . The host cells used far the
reconstitution of the viral vector are not particularly limited
as lung as the viral vector can be reconstituted in the cells.
Por example, culture cells such as CV-I cells and LLCMK2 cells
derived from monkey kidney, and BHIi cells derived from hamster
kidney can be used for the reconstitution of Sendai virus vector.
The infectious virus particles with the envelope can be obtained
by allowing these cells to express the adequate envelope proteins.
CA 02316518 2000-08-31
- 18 -
Furthermore, a large amount of the virivn of Sendai virus can be
obtained by infecting embryonated chicken eggs with the viral
vector obtained from the above host. The method of manufacturing
the virus using chicken eggs has already been developed
("Cutting-edge technologies in neuroscience: protocol III,
molecular and cellular neurvphysiology" Eds., Nakanishi et al.,
(1993), Kouseisya, Osaka, pp. 153-172). Moreover, the
separation and purification of the virions of Sendai virus from
the allantoic fluids can be performed in the usual, manner (Masato
Tashiro, "Experimental Protocols for virus°, general eds., Nagai
and Ishihama, Medical View, pp. 68-73, (1995)). It is also
possible to produce the infectious virus particles in a large
quantity by expressing the desired envelope protein in chicken
eggs.
In the recombinant sendai virus vector, the viral genes may
be modified, for example, to reduce the immunogenicity or to
improve the efficiency of RNA transcription and the replication
efficiency.
In the following, the construction of Sendai virus vector wil
1 be further explained.
Paramyxoviruses generally contain a complex
(ribonucleoprotein; RNP)comprised of RNA and proteins within
their envelope. RNA contained in RNP are single stranded RNA of
the negative strand (minus strand), the paramyxovirus genome. The
complex is formed when NP, P, and L proteins bind to this RNA.
The RNA contained in this RNP becomes the template for the
transcription and replication of the viral genome (Lamb, R.A.,
and D. Kolakofaky, 1996, Paramyxoviridaes The viruses and their
replication. pp.i177~12o4. Yn Fields Virology, 3rd edn. Fields,
B. N., D. M. Kriipe, and p. M. Howley et al. (ed.), Raven Press,
New York, N. Y. ) . The RNP complex replicates autonomously within
cells, to increase copies of genes (RNA contained in the complex) .
Thus, a h~.gh foreign gene expression is facilitated by a vector
having the foreign gene.
zn the case of the Sendai virus (Sendai virus; 5ev), the
genome size of the natural virus is app. 15,000 nucleotides, and
in the negative strand, following the 3' short readex sequence,
six genes encoding NP (nucleocapsid), P (phvsphv), M (matrl.c),
F (fusion), HN (hemagglutinin neuraminidase), and L (large)
CA 02316518 2000-08-31
- 19 -
proteins are lined, with a short 5 ~ trailer region in the other
end. As long as the ability to replicate is maintained, a part
of the genes may be deficient, and the configuration of these genes
may not be the same as the wild type. Since M, HN, and F proteins
are not needed for RNP formation, RNP is constituted by
transcribing this genomic RNA (positive strand or negative
strand) under the presence of NP, P, and L proteins. Infectious
virions are constituted from this RNP. The reconstitution of the
vector could be carried out within LLC-MK2, for example. NP,
P, and L proteins are supplied by transfecting expression vectors
encoded by each gene into cells. Also, each gene may be
incorporated into the chromosomes of host cells. NP, P, and L
genes expressed to form itNP do not need to be completely equivalent
to NP, P, and L genes encoded in the vector genome. Namely, even
if the amino acid sequence of proteins encoded by these genes is
. not the same as the amino acid sequence of proteins encoded by
RNP genome, it forms RNP together with genomic RNA, and as long
as it has the activity to induce gene expression from this RNp,
mutations may be added, or may be substituted by a homologous gene
of another virus. If RNP is formed, NP, p, and L genes will be
expressed from this RNP, RNp will replicate autonomously within
cells, and viral vectors will be produced together with the
envelope protein.
The virus produced is re-infected into cultured cells,
hen-egg, animal ( for example, a mammal such as a mouse ) , and so
on, to amplify or passage the virus . The viral vector could be
amplified by re-transfeeting the RNP formed at the reconstitution
of the virus into host cells such as LLC-MK2. This process
includes the steps of , (a) transfecting the complex comprised
of paramyxovirus-derived negative strandsingle stranded RNA,Np,
P/C, and L protein, and (b) culturing the cells, and recovering
virions from the culture supernatant.
RNP could be transfected into cells by forming a complex
to
together with lipofectamine and polycationic liposome.
Specifically, various transfection reagents may be used. For
example, DOTMA (Boehringer),Superfect (QIAGEN
#301305) ,DOTAP,DOPE,DOSPER(8oehringer #1,811169), and such can
be given. To prevent degradation within the endosome,
chloroquine may also be added ( Calos, M. P . , 1983 , Proc . Natl . Acad.
Sci. uSA 80: 3015).
CA 02316518 2000-08-31
- 20 -
At the time of virus reconstitution, an envelope protein other
than that encoded by genomic RNA may be expressed within cells.
As such proteins, envelope proteins of other viruses, far example,
G protein (VSV-G) of vesicular stomatitis virus {VSV) can be given.
The Paramyxoviridae virus vectors may be vectors comprising
envelope proteins deriving from viruses other than those of
genomic origin, such as the VSV-G protein. Other than viral
envelope proteins, for example, chimera proteins, and such, that
comprise adhesion factors, ligands, receptors, and such, which
are capable of the adhesion onto specific cells, and also
comprising polypeptidesof viral envelope origin in intracellular
regions, may be used. Thereby, vectors targeting specific
tissues could be created. These may be encoded in the virus genome,
or may be supplied by the expression of genes other than the
genome(e.g. expression vectors or genes of host chromosome) at
the time of viral vector reconstitution.
In the viral vector, the viral genes contained in the vector
may be modified genes, in order to reduce immunogenicity, or to
enhance RNA transcription rate and replication rate.
Specifically, the transcription or replication functions could
be enhanced by modifying, for example, at least one of the
replication factors, rip gene, p/C gene and L gene. The HN protein,
which is 'one of the structural proteins, has both the activities
of the erythrocyte agglutinins hemagglutinin and neuraminidase.
For example, if the activity of the former can be weakened, the
stability of the virus within blood could be enhanced, and if the
activity of the latter could be modified, it will be possible to
regulate the ~.nfectivity. The fusion ability of membrane fusion
liposomes could be regulated by modifying the F protein involved
i.n membrane fusion. Furthermore, since the analysis of antigen
presenting epitopes of F proteins and HN proteins that could
become cell surface antigen molecules became possible through the
establishment of the reconstitution system, using this analysis,
it is possible to prepare Sendai viruses with a weak antigen
presentation ability.
The viral vector encodes a foreign gene within its negative
strand single stranded RNA, or comprises a site for inserting a
foreign gene. A desired gene one would like to express within
a target cell could be used as the foreign gene. For example,
for objectives such as gene therapy, a therapeutic gene against
a target disease is inserted into the DNA of said viral vector.
CA 02316518 2000-08-31
- 21 -
The foreign gene could be inserted downstream of each viral gene
(NP,P,M,F,HN, and L gene) (refer Examples). Herein,
"downstream" means, 3' flanking region of the sense strand
encoding proteins . Namely, for a negative strand RNA ( or DNA) ,
downstream of a gene refers to the 5' flanking region of said gene,
and for a positive strand RNA (or DNA) , it ~.s the 3' flanking region
of said gene.
For example, in the wild-type paramyxovirus, the viral genes
are located l.n the order of NP, P, M, F, HN, and L from the 3'
side of the negative genvme, but for the vector, the location may
be any other as well. In order not to disturbed the expression
of genes existing prior and subsequent to the foreign gene, a
suitable E-r-S sequence(transcription end sequence - intervening
sequence - transcription start sequence) or a portion of it is
inserted prior and subsequent to the foreign gene. For example,
when transfecting a foreign gene into the DNA encoding the Sendai
virus genome, it is preferable to insert a sequence with a multiple
of six bases between the genes encoding the viral proteins (J.
Virol., Vol. 67, No. 8, 1993, 4822-4830). The expression of the
inserted foreign gene could be regulated by the type of the
transcription start sequence added to the 5' side (the heady of
the foreign gene. The regulation could also be done by the site
to which the gene is inserted, or by the nucleotide sequence prior
and subsequent to the foreign gene.
In the Sendai virus, the expression amount of the inserted
gene elevates as the position of insertion neaxs the 3' end of
the negative strand RNA. In order tv obtain a high foreign gene
expression, the foreign gene is inserted downstream (namely,
between the 1" and 2°d genes) of the gene that is most upstream
( at the 3 ' s ide of the negative strand ) among the genes encoding
viral proteins. Specifically, in the gene configuration of the
w~.~,d-type genome, the foreign gene is inserted downstream (for
the negative strand, 5' flanking region of the NP gene) of the
NP gene, in other words , between the NP and P genes . Alternatively,
a relatively high expression could also be obtained by inserting
the foreign gene between the 2nd and 3rd genes from the upstream
of the genes encoding viral proteins . In this case, in the gene
configuration of the wild~type genome, the foreign gene is
inserted downstream(for the negative strand, 5'adjacent region
of the P gene) of the P gene, in other words, it is preferable
to insert the foreign gene between the P gene and the M gene.
CA 02316518 2000-08-31
22 -
Conversely, the expression amount of the inserted gene declines
as the position of insertion nears the 5' end of the negative strand
RNA (in the gene configuration upon the genome of the wild-type
virus, as it nears the L gene). In order to suppress the
expression of a foreign gene, the foreign gene is inserteds into
the downstream (namely, between 1" gene from the 5' end of the
negative strand and the trailer sequence; in the wild-type genome,
between the 6t" gene from the 3' side and the trailer sequence)
of the gene encoding the viral protein, which is most downstream
lp (5~ side of the negative chain) of the genes encoding the viral
proteins; or into the upstream (namely, between thg 1" and 2"'
genes from the 5' side; in the wild-type virus genome, between
the 5'y and 6"' genes from the 3' side). Specifically, in the
wild-type genome gene configuration, the foreign gene is inserted
downstream (in the negative strand, 5'flanking region of the L
gene) or into the upstream of the L gene ( in the negative strand,
3' flanking region of the L gene ) , namely, between the L gene and
trailer sequence or between the HN gene and the L gene,
respectively. The expression can also be suppressed by inserting
the foreign gene between the 4'~ and 5t'' genes from the upstream
of the genes encoding viral proteins . In this case, in the wild
type genome gene configuration, it is preferable to insert the
foreign gene downstream of the F gene (in the negative strand,
5' adjacent region of the F gene), in other words, between the
F gene and HN gene. The vector may maintain some other foreign
gene in locations other than those into which the foreign gene
is inserted.
The position into which the foreign gene is inserted, may
be properly adjusted to facilitate a desired expression amount
of said gene, or to maximize the combination of the genes encoding
viral proteins that exist prior and subsequent to the inserted
gene.
To easily insert a foreign gene, a cloning site could be
designed at the position of insertion. Typically, the cloning
site can be a recognition sequence of a restriction enzyme.
Preferably, a restriction enzyme site is designed, which is not
a site that is present in the foreign gene to be inserted. As such
a restriction enzyme, one that has a long recognition sequence,
such as an 8bp recognizing restriction enzyme, is preferable. As
~0 8bp recognizing restriction enzymes, for example, Asc
I(GG.CGCGCG),Fse Z(GGCCGG.CC),NOt I(GC.GGCCGC),Pac
CA 02316518 2000-08-31
- 23 -
I(TTAAT.TAA),Pme I(GTTT.AAAC),Sfi I(GGCCNNNN.NGGCC),Sgf
I(GCGAT.CGC),Srf I(GCCC.GGGC),Sse232 I(CG.CCGGCG),Sse8387
Z(CCTGCA.GG),and Swa I(ATTT.AAAT), and such Can be given, but are
not restricted thereto. The cloning site may be one that
comprises several restriction enzyme recognition sequences, the
so-called mufti-cloning site. Also, it may be a sequence that
is cleaved by an endonuclease other than a restriction enzyme.
It is also possible to envisage inserting a foreign gene by
recombination by making the cloning site be a recognition sequence
of a recombinase. The designing of these sequences within the
DNA encoding the viral genome, could be done by commonly known
mutation induction methods. Furthermore, it is also conceivable
to create a cloning site by segmenting the foreign gene inserting
position beforehand. rf the 5' end of the segmented vector nNA
is de-phosphorylated beforehand, the clone into which the foreign
gene has been inserted could be preferentially generated. Also,
if the 3' end of the fragmented vector DNA is single nucleotide
blunt ended at T, the foreign gene (where the blunt end is A)
amplified by PCR, can be conveniently cloned. When the vector
DNA is a circular DNA as a plasmid, a high ligation efficiency
can be obtained as both ends dp not disengage even when the cloning
site is segmented.
The insertion of a foreign gene into DNA (vector DNA)
encoding the viral genome Could done as follows according to "Kato,
A. et al., 1997, EMBO J. 16: 578-587 and Yu, D. et al., 1997, Genes
Cells 2: 457-466".
First, a DNA sample containing the cDNA nucleotide sequence
of a desired foreign gene is readied. The DNA sample is preferably
one that can be verified to be a single plasmid by electrophoresis
at a concentration of 25ng/.1 or more. 7Che following is a
description of inserting a foreign gene into the DNA encoding the
viral genome using the Notl site. When a NotI site is contained
within the objective cDNA nucleotide sequence, the nucleotide
sequence is modified in a way that the encoded amino acid sequence
36 is not changed, using site-specific mutagenesis, and such method,
and the Not I site is preferably removed beforehand. A desired
gene fragment is amplified and recovered from this sample. Both
ends of the amplified fragment is made into NotI sites, and
furthermore, in order to add a copy of the Sendai virus
transcription end sequence (E), intervening sequence(I) and
transcription start sequence (S) (EIS sequence), forward side
CA 02316518 2000-08-31
24 -
synthetic DNA sequence and reverse side synthetic nNA
sequence(antisense strand) is prepared as a pair of primers
containing Notr restriction enzyme cleaving site sequence,
transcription end sequence (E), intervening sequence(I) and
transcription start sequence ( S ) , and a partial sequence of the
objective gene.
For example, for the forward side synthetic DNA sequence,
two or more arbitrary nucleotides (preferably, four nucleotides
- not containing Notl recognition site derived sequences, such as
la GCG, and GCC, more preferably ACTT) are selected in the 5' side
to ensure cleaving by Notl. Then, a Notl recognition site
gcggccgc is added to the 3' side, and to the 3' side thereof,
further nine arbitrary nucleotides or a number of nucleotides
where multiples of 6 hive been added to nine are attached, and
x5 to the 3' side thereof, an oFtF sequence corresponding to app.25
nucleotides from the initiation codon ATG of the desired CDNA
including the initiation codon ATG, is added.
For the reverse side synthetic DNA sequence, two or more arbitrary
nucleotides (preferably, four nucleotides not containing NotI
20 recognition site-derived sequences, such as GCG, and GCC, more
preferably ACTT) are selected from the 5' side, and to the 3' side
thereof, a Notz recognition site gcggccgc is added, and to the
3' side thereof, an oligo DNA insert fragment is added to control
the length. The length of this vligo DNA ~.s designed so that the
25 suet of CDNA complementary strand nucleotide sequence and the EIS
nucleotide sequence of the Sendai virus genome of Sendai virus
origin described later on, becomes a multiple of six (the so called
"rule of six" ; xolakofski, D. et al. , J, virol. 72 :891-899, 1998 ) .
Furthermore, the complementary strand sequence of the Sendai.
$0 virus S strand, preferably 5'-CTTTCACCCT-3'/SEQ ID N0: 5 ,I
sequence, preferably, 5'-AAG-3', the complementary strand
sequence of the E sequence, preferably 5'-TTTTTCTTACTACGG-3'/SEQ
ID N0:6, is added to the 3' side of the insert fragment, and
furthermore, to the 3' side thereof, a sequence, the length of
35 which was selected so that the last nucleotide of the
complementary strand corresponding to app.25 nucleotides counted
inversely from the end codon of a desired cDNA sequence becomes
G or C, to make the 3' end of reverse side synthetic oligo DNA.
For PCR, the usual method that utilizes, for example, ExTaq
40 polymerise (TaxaRa) can be used. Preferably, PCR is done using
vent polymerise (NEE), and after digesting the amplified
CA 02316518 2000-08-31
- 25 -
objective fragment by NotI, it is inserted into the Notl site of
plasmid vector pBluescript. The nucleotide sequence of the
obtained PCR product is verified using a sequences, and the
plasmid having the correct sequence is selected. The insert
fragment is excised from this plasmid using NotI, and cloned
into the Notl site of a plasmid containing genomic cDNA. It is
also possible to obtain recombinant Sendai virus cDNA by directly
inserting the objective fragment into the Notl site without
mediating the plasmid vector pBluescript.
DNA encoding the virus genome can be produced by:
constructing vector DNA by ligating a suitable transcription
promoter; transcribing this within a test tube or cell=
reconstituting under the presence of L, P, and NP proteins of the
virus; and producing viral vectors containing this RNP. The
reconstitution of viruses from viral vector DNA can be carried
out according to commonly known methods (International
Publication No. 97/16539; International Publication No.
97/16538; Durbin, A.P. et al. , 1997, Virology 235: 323-332; Whelan.
S.P. et al., 1995, Proc. Natl. Acad. Sci. USA 92: 8388-8392;
Schnell. M.J. et al., 1994, EMBO J. 13: 4195-4203; Radecke, F.
et al., 1995, EMHO J. 14: 5773-5784; Lawson, N.D. et al., riroc.
Natl. Acad. Sci. USA 92 : 4477-4481; Garcin, D. et al. , 1995, EMBO
J. 14: 6087-6094; Kato, A. et al., 1996, Genes Cells 1: 569-579;
Baron, M.D. and Barrett, T., 1997, J. virol. 71: 1265-1271;
Bridgen, A. and Elliott, R.M. , 1996, Proc. Natl. Acad. Sci. USA
93: 15400-15404.
To transfect vector DNA into cells, the following methods,
(1) the method of preparing a DNA precipitate capable being
incorporated by cells, ( 2 ) the method of producing a complex that
is suitable for the incorporation by cells, which is also
low-toxic and comprises DNA having a negative charge, (3) the
method of instantly opening a hole sufficient enough for DNA
molecules to pass through using an electric pulse.
Various txansfection reagents could be used for (2).
Examples are, DOTMA (Boehringer),Superfect(QIAGEN
#301305),DOTAP,DaPE,D48PER(Boehxinger #1811169), etc. The
transfection method using calcium phosphate can be given as an
example for (1), and it is known that even though the DNA
incorporated ~.nto cells by this method is taken up by phagocytic
celJ.ules, a sufficient amount enters the nucleus as well (Graham,
F.L. and van Der Eb, J., 1973, Virology 52: 456; Wigler, M. and
CA 02316518 2000-08-31
.- 2 6 -
Silverstein, S . , 1977 , cell 11: 223 ) a Chen and Okayama examined
ways to optimize transfer techniques and report that 1) the best
incubation conditions for the cells and precipitates are 2~-4
GOz ,35'C,15~-24 hr,2) circular DNA has a higher activity than
linear DNA, and 3 ) the optimal precipitate could be obtained when
the DNA concentration of the precipitate mixture is 20-~30.g/ml
(Chen, C. and Okayama, H., 1987, Mol. Cell. Biol. 7: 2745). The
method of(2),is suitable for transient transfection. The method
of transfection by preparing DEAE-dextran (Sigma #D-9885 M.W.
5X105 ) mixture by a desired DNA concentration ratio has been known.
Chloroquine may also be added to enhance efficiency as most of
the complexes are degradated inside endozymes (Calos, M.P. , 1983,
Prvc. Natl. Aead. Sci. USA 80a 3015). Method of (3) is called
eleetroporation and has a high versatility compared to methods
tb or ( i ) ana ( z ) as there is no ceit se.~ectivicy. rne a==iciency
is said to be good under optimal conditions of pulse current
duration, form of pulse, the strength of the electxic field (the
gap between the electrodes, voltage), electric conductivity of
the buffer, DNA concentration, and cell density.
Among the above tt~z~ee categories , the method of ( 2 ) is easy
to handle, and many samples can be examined using a large number
of cells, and therefore, transfection reagents are appropriate
for the present invention. Suitable is Superfect Transfection
Reagent(QIAGEN, Cat No.301305), or DOSPER Liposvmal Transfection
Reagent(Boehringer Mannheim, Cat No. 1811169).
Reconstitution from cDNA is specifically done as follows.
Monkey kidney derived cell line LLC-MK2 cells were cultivated
until 70~-80% cvnfluency ( 1x106 cells )in a 6-well plastic plate
using 10 % bovine fetal serum ( Fc$ ) and antibiotics ( 100 units/ml
penicillin G and 100.g/ml streptomycin) containing minimum
essential medium (MEM). Then T7 polymerase-expressing
recomb~.nant vaccinia virus vTF7-3 (Fuerst, T.R. et al., Proc. Natl.
Acad. Sci. USA 83: 8122-8126,1986,Kato, A. et al., Genes Cells
1 s 569-579, 1996 ) inactivated by a 20 min W irradiation treatment
under the presence 1. g/ml psoralen is infected at 2PFU/cell . The
amount of proralen added and the duration of Uv irradiation can
be suitably adjusted. After a one hour infection, 2~-60.g, more
preferably 3~~5 . g of the above-mentioned recombinant sendai virus
cDNA is transfected by the lipofectivn method using plasmids
( 24-0 . 5 . g of pGEM-N, 12-0 .25 . g of pGEM-P, and 24-0 . 5. g of pGEM-L,
CA 02316518 2000-08-31
- 27 -
more preferably l.g of pGEM~.N,0.5.g of pGEM-P, and l.g of
pGEM-L) (Kato, A. et al . , Genes Cells 1 s 569-579,1996 ) expressing
viral proteins functioning in the trans_needed for the production
of the full-length Sendai virus, together with Superfect(QIAGEN).
The transfected cells are cultured in serum-free MEM containing
desirably 100.g/ml of rifampicin (Sigma) cytosine arabinoside
(AraC), more preferably 40.g/lnl of cytosine arabinoside only,
and the optimal cvncentrati.~n of the reagents are set so as to
maximize the viral recovery rate and to minimize the toxicity
due to the vaccinia virus (Kato, A. et al. , 1996, Genes Cells 1 s
569-579). After culturing about 48 to 72 hr following
transfection, cells are collected, and freeze thawing is repeated
three times to lyze cells, transfected into LLC-rslc2 cells, and
cultured. Alternatively, the culture supernatant is collected,
this is added to the LLC-MK2 cell culture medium, and cultured.
The culture medium is collected after a 3 to 7 day culture.
Alternatively, the collected cells may be co-cultured with other
cells by culturing in layers. The virus titer contained in the
culture supernatant could be determined by measuring
haemagglutination activity (HA). HA could be determined by Nend
point dilution method" (Kato, A. et al., 1996, Genes Cells is
569-579). The obtained viral stock is stored at -80°C.
The recombinant viral vector can be made into a constituent
by diluting suitably using, for example, physiological saline and
PBS, etc. When the recombinant viral vector is proliferated
within hen-eggs, chorioallantoic fluid could also be contained.
A constituent comprising the recombinant viral, vector may also
contain a vehicle such as deionized water, 5% dextrose solution,
and such physiologically acceptable vehicles. Furthermore,
other than these, stabilizers, pesticides, and such may also be
contained.
The host cells used for reconstitution are not restricted
as long as the viral vector is reconstituted. For example, for
the reconstitution of the sendai virus, monkey kidney derived Cv-I
cells and LLC-MK2 cells, hamster kidney derived BHK cells and such
culture cells could be used. sy expressing a suitable envelope
protein in these cells, it is possible obtain infective virivns
comprising that envelope. Also, in order to obtain large amounts
of the Sendai virus, the viral vector obtained from the above
host could be infected into developing hen-eggs to amplify said
CA 02316518 2000-08-31
- 28 -
vector. The method of producing viral vectors using hen-eggs has
already been developed (Leading edge techniques protocol III in
neuroscience research, edited by Nakanishi, et al., KOUSEISFiA,
Osaka, 1993, pp. 153-172). Specifically, for example, the
fertilized eggs are moved to an incubator, and cultured for 9 to
12 days at 37. to 38. to grow the fetus. The Sendai viral vector
is inoculated into the chorio-allantoic membrane cavity, the egg
is incubated a few days to proliferate the viral vector.
Conditions such as the culture duration changes according to the
recombinant Sendai virus used. Then, the chorio-allantoic fluid
containing the virus is collected. The separation and
purification of the Sendai virus vector from the chorio-allantoic
fluid is done according to the usual methods ( "Virus Experiment
Protocols" by Makoto Tashiro, edited by Nagai and ~shihama,
Medical View, pp.68-?3,(7,995)).
The retrovirus vector can also be suitably used as the vector
of this invention, as shown in the example. Various known
retroviral vectors are usable (DNA cloning 4, mammalian systems,
eds., D. M. Glover et al., Chapter 4, p.117, Maruzen).
This invention enables easily supplying EC-SOn protein
locally or systemically in the body using the EC-SOD gene inserted
in the proper vectors. Fvr example, the gene constructs are
administered by a in v~vo or ex vivo method in the gene therapy
. using the vectors of this invention.
The suitable cells used in the ex vivo administration in this
invention are the somatic cells of a recipient without
immunogenicity. The longer the period of temporary in-vitro cell
culture and the growing period after the cells were brought back
in the body are, the more preferable the therapeutic effect can
be expected to be. When hemocytes axe used, the hematopoietic
stem cells are desirable. 7Ct is also possible to use an initial
culture of cells isolated with an appropriate enzyme treatment
from the panrii excised by a surgical operation in the treatment
of rheumatoid arthritis (the pannus is a cell mass comprising
aberrantly propagated synovial cells and fibroblast cells derived
from the connective tissue). When the main part to be treated
is the joint, a higher local concentration of EC-SOD is expected
CA 02316518 2000-08-31
- 29 -
to be given by administering the cells to the joint cavities and
the tissues surrounding the joint using a proper apparatus such
as a syringe (equipped with a needle thicker than 29G) . When the
therapeutic targets spread all over the body, an identical
therapeutic effect is expected to be obtained by administering
the cells at any sites as long as EC-SOD is kept at an effective
level in the blood circulating the body. Similarly,~in the
administration in vivo, the direct administration to the affected
part or the surrounding tissues is preferable when the local
effect is expected, while the administration of naked DNA to the
muscle or others is preferable when the targets are distributed
ovEr the body. The DNA can be administered to the synovial cells
in vivo, for example, as reported in Gene Therapy 2, 424-
428(1995).
The vector of this invention can be used to prepare a
composition for the treatment or the prevention of autoimmune
diseases by mixing the vector with pharmaceutically acceptable
carriers or solvents (for example, physiological saline, buffer
solutions, stabilizers, preservatives, and suspending agents,
etc.). To increase the effect of gene therapy, the vectors can
be embedded in biodegradable gels. For example, a method for
sustainable release of DNA using atelocollagen has been reported
(KOKEN Takyo, Japan) (T. OCHZYA et al., 1999, Nature Medicine,
5, 707-710 ) . rn the present invention, a dosage form similar to
28 that described in the reference can also be utilized for the
continued expression by intramuscular administration of the DNA.
The vector of this invention can be administered to a patient
at a dose of 0.1 to 100 ml of the above-described dosage form with.
the vector concentration of 106 to 101 pfu/ml.
The present invention enables preventing and treating
systemic autoimmune diseases using the EC-SOD gene.
Specifically, it is possible to treat systemic autoimmune
diseases including rheumatoid arthritis effectively by gene
therapy using the EC-SOD gene expression vectors.
The present invention will be described in more detail by
referring to the following examples, but is not to be construed
as being restricted thereto.
CA 02316518 2000-08-31
- 30 -
Preparation of EC-SOD gene transduced fibroblasts (DSA/7./EC-SOD
fibroblast)
A cDNA library was prepared from human placenta. The library
was screened by the plaque hybridization method with an EC-SOD
aDNA fragment of 29obp, as a probe, which was amplified by RT-PCR
from human placental RNA. The sequence of the positive clone was
verified, and the full length of the human EC-SOD cDNA was
subcloned.
PCR amplification was performed using the obtained full
length cDNA of human EC-.SOD as a template and using a sense primer
(5'-RCTctagaC~CTGGCGCT-3', SEQ ID NO: 3)(the lowercase letters
indicate the bases changed from the authentic ones to introduce
a new xbal site; the base triplet underlined indicates the
initiation codon of EC-SOD) and an antisense primer (5'-
attgatcaGC~GGCGGCCTT-3, 'SEQ ID NO: 4)(the lowercase letters
indicate the bases changed fzom the authentic ones to introduce
a new sclY site; the base triplet underlined indicates the
termination codon of EC-SOD).
The amplified segment of the EC-SOD gene was digested with
XbaI and BclI and inserted at a Xbal-BamHI site of the lacZ gene
into pRx-ZpN (Fig. 1 )', which was a retroviral vector originating
from pMFG, to prepare pRx-ZpN-EC-SOD (Fig. 1).
A fetal DBA/1 mouse was euthanatized, cut into pieces, and
treated with collagenase and dispase. The resulting cells were
suspended in a culture medium, seed in a culture dish, and cultured
in D-MEM (Nissui Pharmaceutical) containing J,0% FCS at 37°C in
a 5% CO, atmosphere. A packaging cell, BOSC23 cell was transfected
with pRx-ZpN-EC-SOD obtained above to prepare the recombinant
virus. This v~.rus was the nintroduced into bHA/1 fibroblast cells.
The transductants were selected from these cells using 6418 tv
prepare EC-SOD gene transductant fibroblast cells (DBA/1/nlacZ
fibrvblast). The mock-transductants expressing the nlacZ gene
(DBA/1/nlaczfibroblast)were prepared by introducing the pRx-ZpN
to the fibroblast cells. The introduction of the EC-SOD gene was
verified by RT-PCR.
CA 02316518 2000-08-31
- 31 -
lna2
EC-SOD activity in culture supernatant of DHA/1/EC-SOD
fibroblasts
5 x 10a DBA/1/EC-SOD fibroblasts were cultivated for 5 days,
and the EC-SOD activity in the culture supernatant was measured
by direct spectrophotomeric method according to the method of
Markulund et al.(Murklund, S.L. (1976) J. Biol. Chew. 251s
7504-7507; Murklund, S .L. ( 1985 ) in Handbook of Methods for Oxygen
Radical Research (Greenwald. R.A., ed.), pp.2~49-255, cltC press,
Boca Raton) . A sample was applied tv a Con A-Sepharose column,
the column was washed with PBS, and EC-SOD that was bound to the
column was eluted with 0.5M a -methylmannoside. Catalase (40
u/ml ) was added to 50 mM AMP-HCl ( pA 9 . 5 ) supplemented with 0 . 2
mM DTPA, and 5 mg of x0, was dissol~red therein. The resulting
mixture was mixed with an eluted sample and the EC-SOD activity
in the sample was determined by measuring the absorbance (250 nm)
of superoxide (0~') .
The result showed that the EC-SOD act~:v~.ty in the culture
supernatant of DBA/1/EC-SOD fibroblasts was about four times as
high as that in the control (medium, nlacZ) (Fig. 4).
In vivo EC-SOD activity
2 x 10' DBA/1/EC-SOD fibroblasts were inoculated to a DBA/1
mouse. Blood was collected from the mice on days 1 tv 14, arid
EC-SOD activity in the serum was examined in the same manner as
in Example 2. The EC-SOD activity in the DBA/1/EC-SOD fibroblast
inoculation group was raised approximately 3 times after 4 days,
and approximately 1.5 times after 7 days compared with the no
DHA/1/EC-SOD fibroblast inoculation group (treatment group) and
the DHA/1/EC-SOD nlacZ inoculation group. On day 14,~the EC--
SOD activity in the treatment group became almost the same as that
in the control group (Fig. 3).
Examol g. 4
Preventive arid therapeutic effect of EC-SOD on collagen-induced
CA 02316518 2000-08-31
- 32 -
arthritis (CIA)-induced mouse
Seven to eight-week-old female DHA/1 mice were purchased
from Charles River Laboratories. The mice were kept under the
SPF condit;ons. For induction of collagen-induced arthritis
( CIA) , bovine type I I collagen ( BIIC ) ( collagen research center )
was dissolved in O.1M acetic acid to 2 mg/ml, and the solution
was allowed to stand overnight at 4°C_ It was then emulsified in
the same amount of Freund's complete adjuvant (Difco). On day
0, 100, 1 of the emulsion (100,u g as collagen) was intradermally
injected into the rood of the tale of the mice. On day 21, the
same amount (100,u1, 100,ug as collagen) of BIIC emulsified in
Freund's complete adjuvant was intradermally injected into the
root of the tale of the mice.
2X10' DBA/1/EC-SOD fibroblasts were subcutaneously
in jected into the several points at the back of the CIA prevention
group mice (n=5) on day 14 after immunization. Thereafter, the
same amount of the cells was inoculated into the mice once a week
three times in total (on days 14, 21, 26). On day 29 after
sensitization with BIIC, definite swelling of the limb was
macroscopically observes in the c:lA treatment group mice (n=5)
on day 29, and 2 X 10' DBA/1 /EC-SOD fibroblasts were subcutaneously
inoculated into the several points at the back of these mice. The
same amount of the cells were also inoculated on days 34 and 39.
The severity of arthritis in their paws was scored according
to the following clinical score.
0 : normal
1 : Erythema and mild swelling confined to the ankle joint and
toes
2 : Erythema and mild swelling extending from the ankle to the
midfoot
3:Erythema and severe swelling extending from the ankle to
the metatarsal joints
4:Ankylosing deformation with joint swelling
<Preventive effect of EC-SOD on CIA>
The incidence of CzA in the no Dsall/EC-SOD fibroblast
treatment group was 50.0% on day 35 and 87.5% on day 44, while
CA 02316518 2000-08-31
- 33 -
that in the DBA/1/EC-son fibroblast treatment group was 0% on
day 35 and 16.7% on day 44. Thug, the incidence of CIA was
remarkably suppressed in the treatment group, ~.ndicating that
DsA/1/EC-SOD fibroblasts inoculatipn had a preventive effect on
the onset of CIA (Fig. 4).
The clinical score of the DHA/1/EC-SOD fibroblast treatment
group was far less tha the no treatment group. On day 44, the
clinical score of the treatment group was about 12% of that of
the no treatment group (Fig. 5).
< Therapeutic effect of EC-SOD on CIA
We examined whether or not EC-SOD has post-symptomatic
therapeutic effect on CTA mice. First, reduction of redness of
the joint and of swelling were observed after day 35 in the group
into which DBA/1/EC-Sop fibroblast was inoculated once. These
symptomatic improvements were statistically significant, and 63%
of the suppression was seen with clinical sCOre on day 38 (Fig.
6). However the clinical score of arthritis was also xaised in
the DHA/ 1 /nlacZ f ibr4blast inoculation group and the no treatment
group after day 28.
The preventive effect on arthritis was observed in the group,
into which DsA/1/EC-SOD fibroblasts were inoculated on days 29,
34, and 39, from 3 days after the inoculation. Furthermore, the
clinical score of the treatment group was about 40% on day 36 and
about 35% on day 40 based on the no treatment group (Fig. 7).
2,5 <Maximum disease score>
The maximum clinical score of each foot was examined on day
42 (Fig. 8) . On day 29, most of CIA mice had 1-2 points on clinical
score, and their arthritis had progressed further Without
DHA/1/EC-SOD fibroblast inoculation. The maximum score in the non
treatment group and the DBA/1/nlacz fibroblast group raised up
to 2.71~1.08, 2.7511.22, respectively, however, the DHA/1/EC-
SOD fibroblast inoculation group remained at 1.351.22 (pc0.05).
The rate which feet wEre determined to have incurred serious
illness turned to by more than the clinical scare 3 points was
16 out of 24 feet ( 66.7% ) in the no treatment group andl4 out of
24 feet (58.3%) in the DBA/1/EC-SOD nlacZ inoculation group. on
the other hand, only 3 out of 20 feet ( 15 . 0% ) incurred the serious
CA 02316518 2000-08-31
- 34 -
illness in the DBA/1/EC-SOD fibroblast group.
Histological examination
On day 50 after the sensitization with sriC, the mice was
sacrificed by anesthesia, and articular tissues were stained with
hematoxilin-eosin to observe the presence of infiltration of
inflammatory cells, destruction of joints, and generation of
pannus. As a result, remarkable indication of arthritis
1Q including infiltration of mononuclear cells and growth of
synovial cells in the joint cavity, and the destruction of
cartilage was not observed in the DHA/1/EC-SOD fibroblast
inoculation group compared to the non treatment group and
DBA/1/nlacZ fibxoblast inoculation group. These results
histologically confirm that EC-SOD inoculation suppresses
arthritis (Fig. 9).
Effect of EC-SOD on mice with colitis
salb/c mice, a strain known to be susceptible tv oral dextran
sulfate sodium (DSS), were obtained from Charles River
Laboratories (Wilmington, MA). All mice were female and 6-8
weeks of age at the beginning of the trial.
( ~ ) Pz-epaxation of primary embryonic fibroblasts
On embryonic day 14, Balb/c pregnant mice were dissected,
and the embryos were collected. The embryos were placed in pBS,
and the internal organs and head$ were removed. The remaining
torsos were minced and placed into Zml of 0.25% trypsin for 30min
at 30'C. The torsos were suspended by pipetting, and the reaction
was stopped with incubation medium (DMEMwith 10% fetal calf serum,
100 unit/ml penicillin and 100 mg/ml streptomycin). Then
adherent cells were collected and cultured. The medium was
replaced every 3 days, and all spindle shaped cells were used as
embryonic fibroblasts.
( 2 ) Construction of EC-SOD gene and its trarisduCtiori into
fibrvblasts
CA 02316518 2000-08-31
- 35 -
The r~aroviral vector pRx-ZpN was kindly provided by Dr.
samada (Dept. of Molecular Medicine, Sapporo Medical university).
Total MRNA was prepared from a human placenta, and reverse
transcribed into cDNA as described previously. The cDNA of
EC-SOD was amplified by polymerase chain reaction using
oligonucleotides as the primer set (5'-ACTCZ'AGACATGCTGGCGCT-
3' /SEQ ID N0:7and 5'-ATTGATCAGGCI'CAGGCGGCTT-3'/SEQ ID N0:8).
The 5' primer includes XbaI, and the 3' primer contains the BclI
restriction site for cloning into the 'expression vector. The
construction of EC-SOD cDNA was confirmed by the
dideoxynuclevtide sequencing method. Then human EC-SOD cDNA was
inserted at the lacZ gene site (Xba I and BamHI ) , and pRx-ZpN vector
containing human EC-SOD cDNA(pRx-ZgNhECSOD) wasgenerated. The
pR7C-ZpNIIECSOD was next transfected into the Balb/c fibroblasts
as described earlier, and the transfectants were selected by G-418
(GIBCO, BRL, Rvckville, MD). Transfected Balb/c fibroblasts
with pRx-ZpN were also established to be utilized as a control
transfectant. Staining of the control cells for ,(3ga1 revealed
complete positivity after 6418 selection.
( 3 ) EC-SOD activity
EC-SOD activity was assayed by the direct spectrophotometric
method employing KOz as described by Marklund.
As for mesurement of EC-SOD activity in the cell supertnant,
2x 105 ECSOD-EF cells (DBA/1/EC-SOD fibroblast) were seeded on
a 150 mm dish and 10 ml of medium was added. After 2 days of
cultivation, cell supernatants were collected, and SOD activity
in the supernatant was measured.
on the other hand, as for mesurement of EC-SOD activity in the
serum, ECSOD-EF or mock-EF ( DHA/1/nlacZ fibrvbla6t ~ was
subcutaneously inoculated to Balb/c mice, and their EC-SOD
activities in the serum were monitored
To separate EC-SOD from CuZu and cyanide-resistant SOD activity
(predominantly Mn-SOD), an affinity column of EC-SOD for
concanavalin A-Sepharose was used. axiefly, a sample was
applied to a Con A-Sepharose column (Amersham Pharmacia Biotech,
Buckinghamshire, uK) which had been equilibrated with PBS. The
ConA-Sepharose vvlumn was washed with pHS, and EC-SOD which bound
CA 02316518 2000-08-31
- 36 -
to the column was eluted with 0.5M-methylmannoside. SOD
activity in the elute with aliquot of KOz was detezmined by
measuring the absorbance at 250 nm. One unit of SOD activity
was defined as the amount of protein in the solution necessary
to decrease the reference rate of NBT reduction by 50%.
As the result of the mesurement of EC-SOD activity in the cell
supertnant, the value was shown to be approximately two times
higher than that of parent f ibroblast ox mock..F ( Figure 10 ) . As
the result of the mesurement of EC-SOD activity in the serum, some
apgreciable EC-SOD activity was detected both in parent and mock-F
groups at day 7. indicating endogenous production of mouse EC-
SOD. The activity in the ECSOD-EF group gradually increased for
three days to 1.6 fold the initial activity and gradually
increased to reach 1.8 fold at day 14 (Figure 11).
lu ( 4 ) Induction of colitis
Four, groups of Balb/c mice were used. In three of the groups
5% (w/v) DSS in distilled water Was provided ab Libitum, whereas
the one control group was received distilled water only. In one
colitis group, 2x10' ECSOD-EF were administrated at several sites
on the back subcutaneously on day 0 and 4. The other colitis group
received 2x10' mock-EF or DSS only.
Raplan-Meier survival curve in combined controls vs. ECSOD-EF
treated group. Treatment with ECSOD-EF resulted in
statisticalll~ significant prolongation of survival (p c 0.005)
(Figurel2).
(5) Observation of mice with colitis
Each mouse was weighed daily, and visually inspected for diarrhea
and rectal bleeding. Mice were sacrificed by carbon dioxide
narcosis on day 8. The entire colon was dissected out
longitudinally and blood content assessed using a scale from 0
to 3+ (O: no blood; 1+: blood in up to one third of the colon;
2+: blood in up to two thirds of the colon; 3+; blood in the entire
colon) . The entire colon embedded in paraffin, cut in transverse
sections, and stained with hematoxylin-eosin. Sequential
high-power fields of the colon were evaluated histologically.
Severities were graded on a scale from 0 to 3 and expressed as
the pathological index according to the standard system (0:
CA 02316518 2000-08-31
- 37 -
normal; 1: focal inflammatory cell infiltration including
polymorphonuclear leukocytesl; 2: inflammatory cell
infiltration, gland drop out, and crypt abscess; 3: mucosal
ulceration).
( 6 ) Cytokine assays
Full thickness biopsies from the colon of 3mm diameter were
obtained from the entire colon of each group at day 7. Tissue
specimens were cultured at 37°C in 5% COs, 95% oz for 24 hours.
After 24 hours
the supernatants were harvested for cytokine assays.
The serum of mice and the supernatants of organ cultures were
collected. Interleukin (IL)-1,(3, TNF- a levels were measured
using commercially available ELTSA kits (ENDOGEN).
As a result, the loss of body weight in the ECSOD-EF group was
x5 less pronounced. Balb/c mice administrated ECSOD-EF had a
significantly less decrease in body weight loss (Figure 13 ) . The
decrease was 14% of total body weight and 23% (p < 0.005) in
ECSOD-EF and non-treated group, respectively {Figure 14). The
decrease in colonic length was less pronounced in ECSOD-EF group
(26 %) compared to non-treated (39 %) and mock-EF group (38 %)
(Figure 15). Trace amounts of colonic blood were found in the
ECSOD-EF group (mean 0.5+), whereas significantly more blood was
detected in non-treated and mock-EF group (mean 2.5+ and 2.4+
respectively) (Figure 16).
In the Histopathological evaluation, effect of administrated
EC-50D on the pathological change was observed in the colon of
salb/c mice (Figure~l7). In the non-treated group (s), there
are characteristic features of ulcerative colitis, namely,
mueosal ulceration, inflammatory cell infiltration, gland drop
out. In the ECSOD-EF group (C), no mucosal ulceration or
remarkable gland drop out was seen. The histopathological
severity of colitis was calculated by adding each score for whole
colon. The medianscores are indicated horizontal lines (Figure
18}.
As shown in Figure 19,20, production of both IL- 1,Q and TNF-
rx in the serum and colonic mucosa of non-treated and mock-EF group
was significantly elevated in comparison with normal mice. In ,
CA 02316518 2000-08-31
- 38 -
the EGSOD-EF group, ri,-I ,(3 and TNF- a production was
significantly xeduced.
Exam ,rile 7
Construction and reconstitution of a recombinant Sendai virus
vector comprising EC-SOD gene
Recombinant Sendai virus was constructed according to the
method described in the literatures (Kato, A. et al., EMBO J. 16:
578-598, 1997; Hasan, M.K. et al. s. Gen. Virol. 78: 2813-2820,
1997 ) . First, a 18-by spacer sequence with Notl restriction site
(5'-(G)-CGGCCGGAGATCTTCAGG-3'/SEQ ID N0:9) was inserted into the
proximal locus between the leader sequence and the 5'-end of the
sequence encoding N-protein of cloned Sendai virus genomic cDNA
(pSev(+)) to obtain a plasmid pSeviB+b(+), containing a self-
cleaving ribozyme site from the antigenomic strand of hepatitis
delta virus . In order to introduce EC-SOD gene with a stop codon
TGA (723 bp) into the NotI restriction site of the plasmid
pSeVl8+b(+), primers 5'-
ACTTGCGGCCGCCAAAGTTCAATGCTGGCGCTACTGTGTTCCTG-3'/SEQ ID N0:10
and 5~-
ATCCGCGGCCGCGATGAACTTTCACCCTAAGTTTTTCTTACTACGGTCAGGCGGCCTTGCA
CTCGCTCTCGCGCCGCC-3'/SEQ ID N0:11, Containing Notr sites and new
sets of Sendai virus E and S signal sequence-tags for a exogenous
gene, were synthesized. Using these primers and a template EC-SaD
gene, the desired fragment was amplified by polymerase chain
reaction and inserted into thA rtotz site of the plasmid containing
SeV genomic cDNA. A plasmid comprising template Sendai virus
genome with EC-SOD gene and plasmids encoding N-, P-, and L-
proteins (pGEM-N, pGEM-P, pGEM-L) were complexed with
commercially available active dendrimer molecules (SuperFect
Transfection Reagent; Qiagez~) , and co-trans~ected with vaccinia
virus vT7-3 (Fuerst, T.R. et al. , Pxoc. Natl. Acad. Sci. USA 83:
8122-8126,1986; Kato, A. et al., Genes Cells 1: 569-579, 1996)
into LLCrzK2 cells. Forty hours later, the cells were disrupted
by 3 cycles of freezing and thawing, and injected into the
chrioallantoic cavity of 10-day-old embryonated chicken eggs.
Then, the virus was recovered, and the vaccinia virus was
CA 02316518 2000-08-31
- 39 -
eliminated by passage in eggs. virus titer Was determined by
hemagglutination assay using chicken red blood cells (Kato, A.
et al. , 1996, Genes Cells 1: 569-579 ) , and chvriaallantoic fluid
containing the virus was preserved at -8D .C until just before
use as a composition comprising a recombinant Sendai virus vector
of the present inventian.
CA 02316518 2000-08-31
1/8
SEQUENCE LISTING
GE,~NE~aL INFOReu~TxoN
APPLICANT: DNAVEC Research Inc.
TITLE OF INVENTION: L:$a a!' EC-SOD for Ti~atment of Sys:tonic Autoimnune
Dis~ases
NUMBER OF S~QLT~NCES t 11
CtN'1' APPLICATION DATA:
APPLICATION NUMBERS
FILING DATE:
PRIOR APPLICATION DATAS
APPLICATION NUMBER: JP 11/248032
FILING DATE: 1-SEP-1999
APPLICATION N~ERs cA 2,304,4:3
FILING DATE: 27-RFR-2000
INFORMATION FOR SEQ ID NO: 1:
SEQUENCE CHARACTERISTICS
LENGTH: 1389
TYPE: nucleic acid
TOPOLOGY: 11n~ar
MOLECULE TYPE : Df~lA
ORIGINAL 50URCEs
ORGI~IISMs Homo Sapiens
FLATVRS:
NAME/KEY: CDS
LOCATION: 70..789
FEATURES
NAME/KEY: sig~Cptidd~
L~OCATIONr 70..123
CA 02316518 2000-08-31
2/8
FEATURE:
NAME/IarY: mat~eptid~
LOCATIONS 124..789
SEA DESCRIPTION: SEQ ID NOs is
ctgggtgeag ctetettttc aggagagaea gctctcttgg aggagctgga aaggtgcccg 60
actccagec atg otg gcg cta ctg tgt tcc tgc ctg ctc ctg qca ycc ggt 111
Met Leu Ala Leu Leu cys ser cys Leu Leu hau ala AJ.e Gly
-15 -10 -5
gCC tcg gac gcc tgg acg ggc gag gaa tcg gcg gag ccc aac tct gac 159
Ala Ssr Assp Ala Trp Thr Gly Glu Asp Ser A7.s Glu Pro Asn S~r Asp
-1 1 5 10
tcg gcg gag,tgg atc cga gac atg tac gcc aag gto acg gag atc tgg 207
Ser Ala Glu Trp Ile Arg Asp Met Tyr Ala Lys Val Thr Glu Ile Trp
15 20 25
cag gag gtc atg cag cgg cgg gac gac gac ggc acg ctc cac gco goc 255
Gln Glu Val Met Gln Arg Arg App A~p Asp Gly Thr Lau His Ala Ala
3D 35 40
tgc cag gtg cag ccg tcg gcc acg ctg gac gcc gcg Cag ccc cgg gtg 303
Cye Gln Val Gln Pro :er Ala Thx Leu Asp Ale Ale Gln Pro Arg Val
45 50 55 60
acc ggc gto gtc ctc ttc cgg cag ctt gcg ccc cgc gcc aag ctc gac 351
Thr Gly Val Val Leu Phe Arg Gln Leu Ala Pro Arg Ala Lyc Lmu Asp
65 70 75
gcc ttc ttc gcc ctg gag ggc ttc ccg acc gag ccg aac agc tcc agc 399
Ala Phe Phe Ala Leu Glu G1y Phe Pro Thr Glu Pro Asn Ser Ser Ser
80 85 90
cgc gcc atc cac gtg cac cag ttc ggg gac otg agc cag ggc tgc gag 447
Arg Ala I1~ His Val Hia Gln Pha Gly Asp Leu Ser Gln Gly Cya Glu
CA 02316518 2000-08-31
3/8
95 100 105
tac aco ggg ccc cac tac aac cCg ctg gcc gtg ccg caC ccg cag cac d95
Sar Thr Gly Pro Hie Tyr Aen Pro Leu Ala Val Pro Hl.s Pro Gln His
110 115 120
ceg ggc gac tto ggo eac ttc gcg gtc cgo gao ggc agc ttc tgg agg 543
Pzo Gly Asp Phe Gly Asn Phe Ala Val Arg Aep Cly Ser xeu Tip Arg
125 130 135 190
tac cgc gcc ggc ctg gcc gcc tcg ctc gcg ggc ccg cac tcc atc gtg 591
Tyr Arg Ala Gly Lett Alit Ala Ser Leu Ala Gly Pro His Ser Ile Val
145 150 155
ggc cgg gcc gtg gtc gtc cae get ggc gag gac gac ctg ggc cgc ggc 639
Giy Arg Ala Val Val Val His Ala Gly Glu Asp Aep Leu Gly Arg G1y
160 165 170
ggc eac cag gcc agc gtg gag aac ggg asc gcg ggc cgg cgg ctg gcc 687
Gly Asn Gln Ala Sor Val Glu Asn Gly Asn Ala Gly Arg Arg Leu Ala
175 1B0 1B5
tgc tgc gtg gtg ggc gtg tgc ggg ccc ggg ctc tgg gag cgc cag gcg 735
cya eys Val Val Gly val G~~ Gly 8ro Gly Leu Trp Glu Arg Gln Ala
190 195 200
cgg gag cac tca gag cgc aag aag cgg cgg cgc gag agc gag tgc aag 783
Arg Glu His Ser Glu Arg Lys Lys Arg Arg Arg Glu Ser Glu ~.s Lys
205 210 215 220
gcc gcc tgagcgcggc ccccacccgg cggcggccag ggacccccga ggcccccctc 839
Ala Ala
tgcctttgag cttctcctct gctccaacag acaccttcca ctctgaggta tcnccttcgc 899
ctctgctgaa gtctccccgc agccctctcc acccagaggt ctccctatac cgagacccac 959
CA 02316518 2000-08-31
4/8
catccttcca tcctgaggac cgcccceacc ctcggagccc cccactcagt aggtctgaag 1019
gcctccattt gtaccgeaec accccgctca cgctgacagc ctcctaggct ccctgaggta 1079
cctttccacc cagaccctcc ttccccaccc cateagccct gagactcccg cctttgacct 1139
gacgatcttc ccccttcccg ccttcaggtt cctoctaggc gctcagaggc cgctctgggg 1199
ggttgcctcg agtcccccca cccctcccca cceaccaccg ctcccgcggc aagccagccc 1259
gtgcaacgga agccaggcca actgccccgc gtcttcagct gtttcgcatc caCcgCCacC 1319
ccactgagag ctgctccttt gggggaatgt ttggcaacct ttgtgttaca gattaaaaat 1379
tcagcaattc 1389
IN~a~TxON FoR SEQ ID No: 2:
SEQUENCE CHARACTERISTICS
LENGTH: 240
xX7?Ex amino acid
TOPOLOGY: linear
MCILECULE TYPE: prot6iri
ORIGINAL SOL1I2G'E a
ORGANISM: Homo Sapiens
SEQUENCE DESCRIPTION: SEQ ID NO: 2:
Met Leu Ala Leu Leu Cys Ser Cyg Leu Leu Leu Ala Ala Gly Ala sor
-15 -10 -5
Asp Ala Trp Thr Gly Glu Asp Ser Ala Glu Pro Asn S~r Asp Ser A1a
-1 1 5 10
Glu Trp Ile Arg Asp Met Tyr Ala Ly~ Val Thr Glu Ile Trp Gln Glu
15 20 25 30
Val Met Gln Arg Arg Asp Asp Asp Gly Thr Leu His Ala Ala Cya Gln
CA 02316518 2000-08-31
5/8
35 40 45
Val G1n Pro Gar Ala Thr Leu Asp Als Ala Gln Pro Arg Val Thr Gly
50 55 60
val VaI Leu Phe Arg Gln Leu Ala Pro Arg Ala Lye Isu Asp Ala Phe
65 70 75
Phe Ala zaeu Glu Gly Phe Pro Thr Glu pro Asn Ser S~r Ser Arg Ala
80 85 90
Its His Val His Gln Phe Gly Asp Leu Ser Gla Gly ~s Glu Ser Thr
95 100 105 x10
Gly Pro His Tyr Asn Pro L~su Ala Val Pro His Pxo Gin Flis Pro Gly
115 120 125
Asp Phe Gly Asn Phe Ala Val Arg Asp Gly Ser Leu Trp Arg Tyr Arg
130 135 140
Alw G1y L~u Ala Ala Ser Lsu Ala Gly Pro Hic S~r Ile Vnl Gly Arg
145 150 155
Ala Val Val Val His Ala Gly Glu Aap Asp x.eu Gly Arg Gly Gly Asn
160 165 170
Gln Ala Ser Val Glu Asn Gly Aan Ala Gly Arg Arg Leu Ala Coo Cys
I75 180 185 190
Val Val Gly Val Cys Gly Pro Gly Leu Trp Glu Arg 61n Ala Arg Glu
195 200 205
His Sor Glu Arg Lys Lya Arg Arg Arg Glu Ser Glu Cps Lys Alat Ala
210 215 220
CA 02316518 2000-08-31
6/8 .
IPTFCRMATION FOR 5EQ ID N0: 3:
SEQUENCE CHARACTERISTICS
LENGTH: 20
TYPE: nucl~io acid
TOPOLOGY: linear
MOLECULE TYPE: ether nuclei.a acid (synthetic DNA)
SEQ~1ENCE DESCRIPTION: SEQ ID NO: 3s ,
actctagaca tgctggcgct 20
INFORMATION FOR SEQ Ib NO: 4:
SEQUENCE CHARACTERISTICS
LENGTH: 22
TYPE: nucleic ascid
TOPOLOGY: linear
MOLECULE T7fFE: other nucleic acid (synthetic DNAj
SEQUENCE DESCRIPTION: SEQ ID NOs 4:
attgatcagc tcaggcggcc tt 22
INFORMATION FOR SEQ ID NO: 5:
SEQUENCE CHARACTERISTICS
LENGTH: 10
TYPE: nucleic acid
TOPOhOGY: linear
MOL~Ct7hE TYPES other nucleic acid (synthetic DNA)
SEQUENCE DESCRIPTION: SEQ ID NO: 5:
ctttcaccct 10
INFORMATION FOR SEQ ID NO: 6:
SEQUENCE CHARACTERISTICS
LENGTH: 14
TYPE: nucloie acid
TOPOLOGY: linear
MGLECVLE TYPE: other nucleic acid (synthetic aNA)
SEQttENCL DESCRIPTION: SEQ 2D NO: 6:
tttttettac tacgg 14
CA 02316518 2000-08-31
7/8
INFORMATION FoR 5EQ iD No: 7:
SEQUENCE CHARACTERISTICS
LENGTH: 20
TYPE: nucleic aoid
TOPOLOGY: linear
MOLECULE TYPES other nucleic acid (cynthatie pNA)
SEQUENCE DESCRIPTION: SEQ ID NOs 7s
aetctagaca tgctggcgct 20
INFORMATIDrT FOR SEQ ID NO: 8:
SEQUENCE CHARRCTERZSTICS
LENGTH: 22
TYpEs nucleic acid
TOPOLOGY: linear
MOLECULE TYPE: other nucleic acid (synthetic DNA)
SEQUENCE DESCRIPTION: SEQ ID NO: B:
attgatcagg ctcaqgcggc tt Zy
INFORMATION FOR SEQ ID NO: 9:
$EQUETTCE CHARACTERISTICS
r-F.'NGTH : 18
TYPE: nucleic acid
TOPOLOGY: linear
MOLECULE TYPE: other lnucleic acid (synth~tia DNA)
SEQUENCE pESCRIPTION: SEQ ID NO: 9:
cggccgcaga tcttcacg 18
IDTFORMATION FOR SEQ Ip N0: 10:
SEQUENCE CFI~RACTERISTICS
L~NGTHs 44
TYPE: nucleic acid
TOPOLOGY: linear
MOLECin-F TYPE: other nucleic acid (sy~nthetiC DNA)
SEQUENCE DESCRIPTION: SEQ ID N0: 10:
acttgcggcc gccaaagttc eatgctggcg ctactgtgtt cctg 44
CA 02316518 2000-08-31
a
INFORMATION FOR SEQ ID NO: 11:
SEQUENCE CHARACTERISTICS
LENGTH: 78
TYPE: nucleic said
TOPOLOGY: linear
MOhECULE TYPEf other nucleic acid (synthetic DNA)
SEQUENCE DESCRIPTION: S$Q ID NO: 11s
~tccgcggcc gcgatgaact ttcaccctaa gtttttctta ctt~cggtcag gcggccttge 60
actcgctctc gcgccgcc 78,