Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
1
GENETIC MODIFICATION OF
MALE GERM CELLS FOR GENERATION OF
TRANSGENIC SPECIES & GENETIC THERAPIES
BACKGROUND OF THE INVENTION
Throughout this application various publications are referenced within
parentheses. The
disclosures of these publications in their entireties are hereby incorporated
by reference in this
application in order to more fully describe the state of the art to which this
invention pertains.
1. THE FIELD OF THE INVENTION
This invention relates to the medical arts, particularly to the field of
transgenics and gene
therapy. The invention is particularly directed to in vitro and in vivo
methods for genetically
modifying male germ cells and support cells (i.e., Leydig and Sertoli cells),
which methods incorporate
a method of incorporating exogenous genetic material into the genome of a
vertebrate to
produce transgenic vertebrates and transgenic vertebrate animal lines.
2. DISCUSSION OF THE RELATED ART
The field of transgenics was initially developed to understand the action of a
single gene in
the context of the whole animal and the phenomena of gene activation,
expression, and interaction.
This technology has been used to produce models for various diseases in humans
and other animals.
Transgenic technology is amongst the most powerful tools available for the
study of genetics, and the
understanding of genetic mechanisms and function.
It is also used to study the relationship between genes and diseases. About
5,000 diseases are
caused by a single genetic defect. More commonly, other diseases are the
result of complex
interactions between one or more genes and environmental agents, such as
viruses or carcinogens. The
understanding of such interactions is of prime importance for the development
of therapies, such as
gene therapy and drug therapies, and also treatments such as organ
transplantation. Such treatments
compensate for functional deficiencies and/or may eliminate undesirable
functions expressed in an
organism.
Transgenesis has also been used for the improvement of livestock, and for the
large scale
production of biologically active pharmaceuticals. Historically, transgenic
animals have been
produced almost exclusively by microinjection of the fertilized egg. The
pronuclei of fertilized eggs
are microinjected in vitro with foreign, i.e., xenogeneic or allogeneic DNA or
hybrid DNA molecules.
The microinjected fertilized eggs are then transferred to the genital tract of
a pseudopregnant female.
(E.g., P.J.A. Krimpenfort et al., Transgenic mice depleted in mature T cells
and methods for making
transgenic mice, U.S. Pat. Nos. 5,175,384 and 5,434,340; P.J.A. Krimpenfort et
al., Transgenic mice
depleted in mature lymphocytic cell-type, U.S. Pat. No. 5,591,669).
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
2
This widely used technique requires large numbers of fertilized eggs,
equipment to handle
embryos and the facility to microinject them in vitro. This is partly because
there is a high rate of egg
loss due to lysis during microinjection. Moreover manipulated embryos are less
likely to implant and
survive in utero. These factors contribute to the technique's extremely low
efficiency. Superovulated
mammals (e.g., primates, cows, horses, pigs, and mice) produce only 10-20 or
less eggs per female
animal per cycle, even after hormonal stimulation, and only 1% of
microinjected mouse eggs
(Palmiter, R.D. and Brinster, R.L., Germline transformation of mice, Annu.
Rev. Genet. 20:465-99
[1986]), and 0.1% of cattle, sheep and pig eggs (Wall, R.J., et al., Making
transgenic livestock:
genetic engineering on a large scale, J. Cell Biochem. 49:113-120 [1992])
develop into transgenic
animals. Typically, 300-500 fertilized eggs must be microinjected to produce
perhaps three transgenic
animals. Consequently, generating large animals with these techniques is
prohibitively expensive. For
this reason, mammalian transgenic technology has been confined almost
exclusively to mice due to
their high fecundity. Little has been done to improve the generation of
transgenic animals by the
microinjection of a transgene into fertilized eggs (Gordon, J. and Ruddle,
F.H., Integration and stable
germ line transmission of genes injected into mouse pronuclei, Science
214:1244-1246 [1981]).
While small animals such as mice have proved to be suitable models for certain
diseases, their
value in this respect is limited. Larger transgenic animals would be much more
suitable than mice for
the study of the effects and treatment of most human diseases because of their
greater similarity to
humans in many aspects, and better for studying organ systems or behavior.
Larger mammals are also
more suitable than mice as potential organ donors to humans due to the
comparable size of their
organs. Now that transgenic animals with the potential for human
xenotransplantation are being
developed, more of these larger animals will be required. Transgenic
technology will allow that such
donor animals will be immunocompatible with the human recipient.
In contrast to only 10-20 eggs per female even after treatment with
superovulatory drugs, most
male mammals, including mice and nearly all larger mammals, generally produce
at least about 10g
spermatozoa (male germ cells) in each ejaculate. For this reason alone, male
germ cells will be a better
target for introducing foreign DNA into the germ line, leading to the
generation of transgenic animals
with increased efficiency and after simple, natural mating. Nevertheless,
attempts to generate
transgenic mice using spermatozoa to carry DNA into the egg (Lavitrano, M., et
al., Spernz cells as
vectors for introduction of DNA into eggs: genetic transformation of mice,
Cell 57: 717-723 [ 1989];
WO-A-90/08192), have not been validated (Brinster, R. L., et al., No simple
solution for making
transgenics, Cell 59:239-241 [1989]). Recently, transgenic mice were produced
after the injection
of exogenous DNA together with sperm heads into oocytes ( Perry, A.C., et al.,
Mammalian
transgenesis by intracytoplasmic sperm injection, Science 284:1180-1183 [
1999] ). Following uterine
transfer, 20% of these embryos developed into transgenic offspring.
Genetic information has been transferred to embryos using retroviral vectors
(Jaenisch, R.,
_ . _~___. ___ _. w .. _
.- _ ~_____...._.. .
06-09-201 CA 02368620 2001-11-13 . US()()~3
3
y Germ line integration tmd Mendelian transmission of the exogenous Moloney
leukemia virus,
Proc. Natl. Aced. Sci. USA 73.1260-1264 j1976]), but the animals were mosaics
with di~er~ent gave
iasertivas in di>'Bxent tissues. (Jaenisch, R, Retroviruses weed
embryogerresis: mieroinjection of Moloney
lerrJbemia virus inro midgestation mouse embryos, Cull 19:181-188 [19$0]).
Reveotly, five transgeeuc
calves were produced by iqjection of a paeudotypod replication~icieert vector
based aan the Molooey
marine leukeania virus. The vector wag i~oduocd into the perivitell~e space of
me~phase II oocytea
(Char, A..W., et al., Transgartic cattle Irradrrced by reverse-transcribed
gtne transfer in oorytes, Proc_
Nad_ Aced. Sa. USA 95:14028-14033 [1998.
An a)ternative, net yet fully realized, is the stable trsasfec;tion of male
gene cells is vi~o sad their
br~onsfer to a recipient testis. Transfer of genetically marlmd germ cells to
the testis yielded offspring, but
so far uo transgenic progeny have been produced (Brioater, ~R.L. and Avarbok,
M.R, Germlfne
transmission of donor haplorype following spermatogonial transplantation,
Prac_ Natl_ Aced Sci. USA
91.11303-1130? [1994]).
Lip~naof male germ cells is viv~o vwas acoomplisbed by direct injection of
IiposomeJDNA c~pleaes into rat and mouse testes. ' (Chang, IC: T_ et al.,
Production oJtransgenic rats
and mice by the testis-mediated gene tmnsfer, J_ Reptnd. L~ev: 43(1):29-36
[1999]; Qgagwa, S. et al.,
Method oJpreparing trarugentc aramal, published European Patsm ppplce EP 0867
114 AI). After
the iajecced male rodents were mated with flunales of their species, some
tran:ganic o~priag were
mpo:tedly produced.
Spetmartogeoesis is the process by which a diploid sperrewtogonial ateen cell
provides daug~ ceps
whidi undergo dramatic and distiad eeeoephological changes to become self-
propalhr,g haploid cells (male
gametes) eapablo, whew fielly maou~e, of feetilizmg an ovum.
Primordial germ cells are 5rst seed is the endodermal yolk sac epithelium at
E8 and are thought to
arise from the etnbrymic.octoderm (A. McLarea and M. Buehr, Cell Diff pev
31;185 [1992]; Y Matsvi
er a1, Nature 353:750 [1991_ .'lbey migeate from the yoll~ sac epithelium
through the ]~~ ~,
to the genital ridges and proliserate thmugb,~ mitotic divisive to pthe
testis.
At sexual mat<erity the spenaoatogonium goes through 5 or 6 mitotic divigioe~a
before it erters
meiosis. The. primitive sperenatogonisl stem cells (AO/As) proti~te and form a
populatiaat of
intetaradiate spermatvEoaia types Apr, Aal, Al-4 a$er which they c~erantiate
into type B sperim~togea~is,
3ho type B apertoatogania di>i~,orendiate to form primary socy~ y~ch eretor a
prolonged meiotic
prophase during wdich hoereologous chromosomes pair ~ ,tire. The states of
meiosis that are
morphologically distinguishable are; preteptotene, Ieptatane, zygataue, sad
pachyt~e; ~ secondary
spertreatocytes and the haploid apeematids are Later stages. Spernmtids
uerdergo great morphological
AMENDED SHEET
Fmofanec~oit R.Cen. RW 1
06-09-2001 .. . CA 02368620 2001-11-13 US0013000
3A
changos d~umg aPernatoganesis, such a' ree6aping the nacleus, formation of the
acrosonle and assembly of
' the tail (A.R Bellvo ct al., Recovery, capacitatloa, acrosorrre reaction,
sad fiactiarrati~ of sperm,
Mdliods F.azyre~ol. 225:113-36 [1993]). The spermato~,ytea sad ~ y~
the Seitoli cells tbrouymiqae herai jandiooal atG,cb~o~ w~ the Sertofi cell
membrane. 'Ihe final
ch~ges.in the maturing sp~rn~to~o~ (i.e., apecmatomooa) take plats in the 1
tract of the Female prior
Lo fertilizatian_
lait~lly. ette~ts were made to produce traoagaoic ~imals by adding DNA to
spermatozoa Which
wem than usod to fertilize mo<tse eggs in vitro. 'I7ro fertilized eggs were
then traosFor~ed to
AMENDED SHEET
FmnfanRC~ait fi.San. H:.'~I
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
4
pseudopregnant foster females, and of the pups born, 30% were reported to be
transgenic and express
the transgene. Despite repeated efforts by others, however, this experiment
could not be reproduced
and no transgenic pups were obtained. Indeed, there remains little doubt that
the transgenic animals
reputed to have been obtained by this method were not transgenic at all and
the DNA incorporation
reported was mere experimental artifact. Data collected from laboratories
around the world engaged
in testing this method showed that no transgenics were obtained from a total
of 890 pups generated.
In summary, it is currently possible to produce live transgenic progeny but
the available
methods are costly and extremely inefficient. Spermatogenic transfection in
accordance with this
invention, either in vitro or in vivo, provides a simple, less costly and less
invasive method of
producing transgenic animals and one that is potentially highly effective in
transferring allogeneic as
well as xenogeneic genes into the animal's germ cells.
To facilitate in vitro transfection of male germ cells and implantation into a
testis of a recipient
male vertebrate it is advantageous first to depopulate the testis of the
recipient vertebrate of
untransfected male germ cells before transferring transfected male germ cells
into it.
Depopulation of testis has commonly been done by exposing the whole vertebrate
to gamma
irradiation (X-ray), or localizing irradiation to the testis. (E.g., G. Pinon-
Lataillade et al.,
Erzdocrinological and histological changes induced by continuous low dose
gamma-irradiation of rat
testis, Acta Endocrinol. (Copenh) 109(4):558-62 [ 1985]; G. Pinon-Lataillade
and J. Maas, Continuous
gam»aa-irradiation of rats: dose-rate effect on loss and recovery of
spermatogenesis, Strahlentherapie
161(7):421-26 [1985]; C.R. Hopkinson et al., The effect of local testicular
irradiation on testicular
histology and plasma hormone levels in the male rat, Acta Endocrinol. (Copenh)
87(2):413-23 [ 1978];
G. Pinon-Lataillade et al., Influence of germ cells upon Sertoli cells during
continuous low-dose rate
gamma-irradiation of adult rats, Mol. Cell Endocrinol. 58(1):51-63 [1988]; P.
Kamtchouing et al.,
Effect of continuous low-dose rate gamma-irradiation on rat Sertoli cell
function, Reprod. Nutr. Dev.
28(4B):1009-17 [1988]; C. Pineau et al., Assessment of testicular function
after acute and chronic
irradiation: further evidence for influence of late spermatids on Sertoli cell
function in the adult rat,
Endocrinol. 124(6):2720-28 [1989]; M. Kangasniemi et al., Cellular regulation
of basal and FSH-
stimulated cyclic AMP production in irradiated rat testes, Anat. Rec.
227(1):32-36 [1990]; G. Pinon-
Lataillade et al., Effect of an acute exposure of rat testes to gamma rays on
germ cells and on Sertoli
and Leydig cell functions, Reprod. Nutr. Dev. 31(6):617-29 [1991]).
The mechanism of gamma radiation-induced spermatogonial degeneration is
thought to be
related to the process of apoptosis. (M. Hasegawa et al., Resistance of
differentiating spermatogonia
to radiation-induced apoptosis and loss in p53-defccient mice, Radiat.
Res.149:263-70 [ 1998]).
Another method of depopulating a vertebrate testis is by administering a
composition
containing an alkylating agent, such as busulfan (Myleran). (E.g., F.X. Jiang,
Behaviour of
spermatogonia following recovery from busulfan treatment in the rat, Anat.
Embryol. 198(1):53-61
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
[1998]; L.D. Russell and R.L. Brinster, Ultrastructural observations of
spermatogenesis following
transplantation of rat testis cells into mouse seminiferous tubules, J.
Androl. 17(6):615-27 [ 1996]; N.
Boujrad et al., Evolution of somatic and germ cell populations after busulfan
treatment in utero or
neonatal cryptochidism in the rat, Andrologia 27(4):223-28 [1995]; R.E. Linder
et al., Endpoint of
spermatotoxicity in the rat after short duration exposures to fourteen
reproductive toxicants, Reprod.
Toxicol. 6(6):491-505 [ 1992] ; F. Kasuga and M. Takahashi, The endocrine
function of rat gonads with
reduced number of germ cells following busulfan treatment, Endocrinol. Jpn
33(1):105-15 [1986]).
Cytotoxic alkylating agents, such as busulfan, chlorambucil, cyclophosphamide,
melphalan,
or ethyl ethanesulfonic acid, are frequently used to kill malignant cells in
cancer chemotherapy. (E.g.,
Andersson et al., Parenteral busulfan for treatment of malignant disease, U.S.
Patent Nos. 5,559,148
and 5,430,057; Stratford et al., Stimulation of stem cell growth by the
bryostatins, U.S. Patent No.
5,358,711; Luck et al., Treatment employing vasoconstrictive substances in
combination with cytotoxic
agents for introduction into cellular lesion, U.S. Patent No. 4,978,332).
Treatment of mice with
busulfan (13 mg-40 mg/kg body wt.), was reported to deplete male germs cells
in the testis; both stems
cells and differentiating spermatogonia were killed; doses over 30mg/kg body
weight resulted in
azoospermia for up to 56 days after treatment. (L.R. Bucci and M.L. Meistrich,
Effects of busulfan
on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities and
dominant lethal mutations,
Radiation Research 176:259-68 [1987]).
The present invention addresses the need for spermatogenic genetic
modification, either in
vitro or in vivo, that is highly effective in transferring allogeneic as well
as xenogeneic genes into the
animal's germ cells and in producing transgenic vertebrate animals. The
present technology addresses
the requirements of germ line and stem cell line gene therapies in humans and
other vertebrate species,
including the need for a superior method of depopulating a testis of
untransfected male germ cells.
The present technology is of great value in producing transgenic animals in
large species as well as
for repairing genetic defects that lead to male infertility. Male germ cells
that have stably integrated
the DNA are selectable. These and other benefits and features of the present
invention are described
herein.
CA 02368620 2001-11-13
WO 00/69257 PCT/LIS00/13000
6
SUMMARY OF THE INVENTION
The present invention arose from a desire by the present inventors to improve
on existing
methods for the genetic modification of an animal's germ cells and for
producing transgenic animals.
The pre-existing art methods rely on direct injection of DNA into zygotes
produced in vitro or in vivo,
or by the production of chimeric embryos using embryonal stem cells
incorporated into a recipient
blastocyst. Following this, such treated embryos are transferred to the primed
uterus or oviduct.
These prior methods are extremely slow and costly, rely on several invasive
steps, and only produce
transgenic progeny sporadically and unpredictably.
In their search for a less costly, faster, and more efficient approach for
producing transgenics,
the present inventors devised the present method which relies on the in vivo
or in vitro (ex vivo)
genetic modification of vertebrate male germ cells with a nucleic acid
segment, i.e., a polynucleotide,
encoding a desired trait or product.
The present invention relates to the in vivo and in vitro (ex vivo) genetic
modification, for
example, by transfection or transduction, of vertebrate animal germ cells with
a desired genetic
material. Briefly, the in vivo method involves injection of genetic material
together with a suitable
vector directly into the testicle of the animal. In this method, all or some
of the male germ cells within
the testicle are genetically modified in situ, under effective conditions. The
in vitro method involves
obtaining germ cells from the gonad (i.e., testis) of a suitable donor or from
the animal's own testis,
using a novel isolation or selection method, transfecting or otherwise
genetically altering them in vitro,
and then returning them to the substantially depopulated testis of the donor
or of a different recipient
male vertebrate under suitable conditions where they will spontaneously
repopulate the depopulated
testis. The in vitro method has the advantage that the transfected germ cells
can be screened by
various means before being returned to the testis of the same or a different
suitable recipient male to
ensure that the transgene is incorporated into the genome in a stable state.
Moreover, after screening
and cell sorting only enriched populations of germ cells can be returned. This
approach provides a
greater chance of transgenic progeny after mating.
In particular, the inventive in vivo method of incorporating exogenous genetic
material into
the genome of a vertebrate involves administering to a male vertebrate's
testis(es) a gene delivery
mixture comprising a viral vector, such as, but not limited to, a retroviral
vector, that comprises at least
one polynucleotide defining a gene encoding a desired trait or product and,
optionally, a gene encoding
a genetic selection marker. The genes) are operatively linked to a promoter
sequence (all the
individual genes used are not necessarily linked to a single promoter
sequence), such that a
transcriptional unit is formed, and are administered under conditions
effective to reach at least one of
the spermatozoa, or precursors of spermatozoa, residing in the vertebrate's
testis. The delivery
mixture, including the polynucleotide(s), are administered in amounts and
under conditions effective
such that a polynucleotide encoding a desired trait or product is incorporated
into the genome of at
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
7
least one male germ cell, such as a spermatozoan or precursor cell, so that a
genetically modified male
gamete is produced by the male vertebrate. Then, the male vertebrate is bred,
naturally or with the aid
of artificial reproductive technologies, with a female vertebrate of its
species such that a transgenic
progeny is thereby produced that carries the polynucleotide in its genome.
The invention also includes an in vitro method of incorporating at least one
polynucleotide
encoding a desired trait or product into the genome of a vertebrate. The in
vitro method involves
obtaining from a donor male vertebrate a male germ cell, such as a
spermatozoan cell or a precursor
cell, and genetically modifying the cell in vitro with at least one
polynucleotide encoding a desired
trait or product other than an immortalizing molecule, and a polynucleotide
defining a gene encoding
a genetic selection marker, in the presence of a gene delivery mixture
comprising a viral vector, at
about or below the vertebrate's body temperature and for an effective period
of time such that the
polynucleotide encoding a desired trait or product is incorporated into the
genome of the cell. Then
the genetically modified germ cell is isolated or selected, with the aid of
the genetic selection marker
expressed in the genetically modified cell, and transferred to a testis of a
recipient male vertebrate such
that the cell lodges in a seminiferous tubule of the testis, such that a
genetically modified male gamete
is produced therein. The male vertebrate is bred with a female vertebrate of
its species such that a
transgenic progeny is thereby produced that carries the polynucleotide in its
genome.
This invention also relates to a non-human transgenic male vertebrate produced
in accordance
with either the in vivo or in vitro method of incorporating exogenous genetic
material into the genome
of a vertebrate. Produced in accordance with the in vivo method, the
transgenic vertebrate is the
recipient of the gene delivery mixture. Produced in accordance with the in
vitro method, the
transgenic vertebrate is the recipient of the genetically modified male germ
cell that was transferred
to its testis. The transgenic male vertebrate can be bred with a female of its
species, because it
comprises a native male germ cell carrying in its genome a polynucleotide of
exogenous origin
defining a gene encoding a desired trait or product. But somatic cells in
tissues outside the testis of
the transgenic vertebrate lack the polynucleotide.
This invention also relates to a non-human transgenic vertebrate produced in
accordance with
either the in vivo or in vitro method of incorporating exogenous genetic
material into the genome of
a vertebrate. The non-human transgenic vertebrate is the direct or indirect
progeny of the male
vertebrate that received the gene delivery mixture, in accordance with the in
vivo method.
Alternatively, the non-human transgenic vertebrate is the direct or indirect
progeny of the recipient of
the genetically modified male germ cell that was transferred to its testis, in
accordance with the in vitro
method. Thus, the transgenic progeny is the immediate offspring of the
transgenic male vertebrate,
or is an offspring thereof separated by one or more generations. The
transgenic vertebrate includes
one or more cells carrying in their genome a polynucleotide of exogenous
origin that encodes a desired
trait or product.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
8
Also, the invention includes a transgenic cell derived from the transgenic
progeny. The cell
is a germ cell, such as a spermatozoan (i.e., spermatozoon) or ovum, a
precursor cell of either of these,
or a somatic cell.
The invention also relates to vertebrate semen containing a plurality of the
inventive transgenic
male germ cell.
The invention is also directed to a method of producing a non-human transgenic
vertebrate
animal line comprising native germ cells carrying in their genome at least one
xenogeneic
polynucleotide. The method involves breeding of the transgenic progeny with a
member of the
opposite sex of the same species; and selecting its progeny for the presence
of the polynucleotide.
This technology is applicable to the production of transgenic animals for use
as animal
models, and to the modification of the genome of an animal, including a human,
by addition,
modification, or subtraction of genetic material, often resulting in
phenotypic changes. The present
methods are also applicable to altering the carrier status of an animal,
including a human, where that
individual is carrying a gene for a recessive or dominant gene disorder, or
where the individual is
prone to pass a multigenic disorder to his offspring.
These and other advantages and features of the present invention will be
described more fully
in a detailed description of the preferred embodiments which follows. In
further describing the
invention, the disclosures of related applications U.S. Serial Nos.
09/191,920; 09/292,723; and
09/311,599 are incorporated by reference.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates the process of microinjection of gene delivery mixture
into a mammalian
(mouse) testis. Figure 1(a) shows a preferred site of microinjection into a
vas efferens; in mammals,
the vasa efferentia connect to the lumen of all seminiferous tubules. Figure
1(b) shows a vas efferens
supported by a pipette tip, lmm diameter. Figure 1(c) shows a mouse testis
perfused with
bromophenol blue after being injected in the vas efferens. Figure 1(d) shows
air bubbles in the testis,
confirming satisfactory delivery of viral particles.
Figure 2 shows testicular cells transduced by a pseudotyped lentiviral vector
expressing Green
Fluorescent Protein (GFP) in Zeiss 410 confocal images (wavelength 488 nm; 19
stacked images) of
a cryosection of mouse testis. Figure 2(a) shows a transduced Sertoli cell
expressing GFP. Figure
2(b) shows transduced spermatogonia; GFP expression is visible in the
cytoplasm surrounding large
dark nuclei.
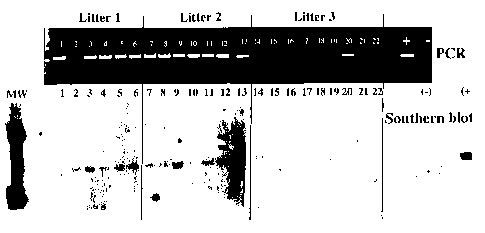
Figure 3 shows a DNA analysis from three consecutive litters of progeny from
one male
treated in accordance with the in vivo method of incorporating exogenous
genetic material into the
genome of a vertebrate. The top panel shows GFP-specific PCR amplification
products separated on
an agarose gel from embryonic DNA of 22 individual progeny. In this run, there
was an absence of
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
9
amplification from fetus No. 2, but other PCR assays confirmed the presence of
the transgenic reporter
gene. The bottom panel shows a Southern blot analysis of the same DNA. The
Southern blot was
probed with a radiolabed GFP DNA fragment.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present method of incorporating exogenous genetic material into the genome
of a
vertebrate relies on at least one of the following strategies. A first method,
an in vivo method of
incorporating exogenous genetic material into the genome of a vertebrate,
delivers a polynucleotide
using known gene delivery systems to male germ cells in situ in the testis of
the male vertebrate (e.g.,
in vivo transfection or transduction), allows the genetically modified germ
cells to differentiate in their
own milieu, and then selects for progeny animals exhibiting the nucleic acid's
integration into its germ
cells (transgenic animals). The thus selected progeny can be mated, or their
sperm utilized for
insemination or in vitro fertilization to produce further generations of
transgenic progeny.
Alternatively, the in vitro method of incorporating exogenous genetic material
into the genome
of a vertebrate involves obtaining male germ cells from the testis of a
suitable donor or from the
animal's own testis, genetically modifying them in vitro, isolating or
selecting genetically modified
germ cells, and then transferring them to the testis under suitable conditions
where they will
spontaneously repopulate it.
By either the in vivo or in vitro route, the inventive method is suitable for
application to a
variety of vertebrate animals, all of which are capable of producing gametes,
i.e. sperm or ova. Thus,
in accordance with the invention novel genetic modifications) and/or
characteristics) can be imparted
to vertebrates, including mammals, such as humans, non-human primates, for
example simians,
marmosets, domestic agricultural animals such as ovines (e.g., sheep), bovines
(e.g., cattle), porcines
(e.g., pigs), equines (e.g., horses), particularly race horses, marine
mammals, feral animals, rodents
such as mice and rats, gerbils, hamsters, rabbits, and the like. Other
vertebrate animals include fowl
such as chickens, turkeys, ducks, ostriches, emus, geese, guinea fowl, doves,
quail, rare and ornamental
birds, and the like. Of particular interest are endangered species of wild
animal, such as rhinoceros,
tigers, cheetahs, species of condor, and the like.
"Gene delivery (or transfection) mixture", in the context of this patent,
means selected genetic
material together with an appropriate vector mixed, for example, with an
effective amount of lipid
transfecting agent, for example, a cationic or polycationic lipid, such as
polybrene. (E.g., Clark et al.,
Polycations and cationic lipids enhance adenovirus transduction and transgene
expression in tumor
cells, Cancer Gene Ther. 6(5):437-46 [ 1999] ). The efficiency of adenoviral-,
retroviral-, or lentiviral-
mediated transduction is enhanced significantly by including polybrene during
the infection. The
amount of each component of the mixture is chosen so that the genetic
modification, e.g., by
transfection or transduction, of a specific species of male germ cell is
optimized. Such optimization
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
requires no more than routine experimentation. The ratio of DNA to lipid is
broad, preferably about
1:1, although other proportions can also be utilized depending on the type of
lipid transfecting agent
used. (E.g., Banerjee, R. et al. [ 1999]; Jaaskelainen, I. et al. , A lipid
carrier with a membrane active
component and a small complex size are required for efficient cellular
delivery of anti-sense
5 phosphorothioate oligonucleotides, Eur. J. Pharm. Sci. 10(3):187-193 [2000];
Sakurai, F. et al., Effect
of DNAlliposome mixing ratio on the physicochemical characteristics, cellular
uptake and
intracellular traff cking of plasmid DNAlcationic liposome complexes and
subsequent gene
expression, J. Controlled Release 66(2-3):255-69 [2000]).
"Genetic material", as used herein, means DNA sequences capable of imparting
novel genetic
10 modification(s), or biologically functional characteristic(s), to the
recipient animal. The novel genetic
modifications) or characteristics) can be encoded by one or more genes or gene
segments defined
by a polynucleotide, or can be caused by removal or mutation of one or more
genes, and can
additionally contain regulatory sequences, such as, but not limited to
enhancers, promoters, or
activator/suppressor binding sites. The transfected genetic material is
preferably functional, that is
it expresses a desired trait by means of a product or by suppressing the
production of another.
Examples of other mechanisms by which a gene's function can be expressed are
genomic imprinting,
i.e. inactivation of one of a pair of genes (alleles) during very early
embryonic development, or
inactivation of genetic material by mutation or deletion of gene sequences, or
by repression of a
dominant negative gene product, among others.
The desired product is any preselected product other than an immortalizing
molecule, such as
SV40 large T or polyoma virus large T antigens. An immortalizing molecule can
transform cells into
"cancer-like" cells. "Immortalization" resulting from the expression of an
immortalizing molecule
can cause a male germ cell to lose many of its important germ cell
characteristics, for instance the
ability to undergo meiosis, which is crucial for the production of normally
functioning male gametes.
(E.g., see, Wolkowicz, M.J., Coonrod, S. M., Reddi, P.P. Millan, J. L.,
Hofmann, M-C, Herr, J.C.,
Refinement of the differentiated phenotype of the spermatogenic cell line GC-
2spd(ts), Biology of
Reproduction 55:923-32 [1996]). Male germ cells genetically modified to
express an immortalizing
molecule are, therefore, not useful for the production of transgenic
vertebrate progeny in accordance
with the present invention.
In addition, novel genetic modifications) can be artificially induced
mutations or variations,
or natural allelic mutations or variations of a gene(s). Mutations or
variations can be induced
artificially by a number of techniques, all of which are well known in the
art, including chemical
treatment, gamma irradiation treatment, ultraviolet radiation treatment,
ultraviolet radiation, the use
of specific chimeric DNA/RNA oligonucleotides (chimeraplasty), and the like.
Chemicals useful for
the induction of mutations or variations include carcinogens such as ethidium
bromide and others
known in the art.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
11
DNA segments of specific sequences can also be constructed to thereby
incorporate any
desired mutation or variant or to disrupt a gene or to alter genomic DNA.
Those skilled in the art will
readily appreciate that the genetic material is inheritable and is, therefore,
present in almost every cell
of future generations of the progeny, including the germ cells. Among novel
characteristics are the
expression of a previously unexpressed trait, augmentation or reduction of an
expressed trait, over
expression or under expression of a trait, ectopic expression, that is
expression of a trait in tissues
where it normally would not be expressed, or the attenuation or elimination of
a previously expressed
trait. Other novel characteristics include the qualitative change of an
expressed trait, for example, to
palliate or alleviate, or otherwise prevent expression of an inheritable
disorder with a multigenic basis.
"Transfecting agent", as utilized herein, means a composition of matter added
to the genetic
material for enhancing the uptake of exogenous DNA segments) into a eukaryotic
cell, preferably
a mammalian cell, and more preferably a mammalian germ cell. The enhancement
is measured relative
to the uptake in the absence of the transfecting agent. Examples of
transfecting agents include
adenovirus-transferrin-polylysine-DNA complexes. These complexes generally
augment the uptake
of DNA into the cell and reduce its breakdown during its passage through the
cytoplasm to the nucleus
of the cell. These complexes can be targeted to the male germ cells using
specific ligands which are
recognized by receptors on the cell surface of the germ cell, such as the c-
kit ligand or modifications
thereof.
Other preferred transfecting agents include lipofectin, lipfectamine, DIMRIE
C, Superfect, and
Effectin (Qiagen), unifectin, maxifectin, DOTMA, DOGS (Transfectam;
dioctadecylamidoglycylspermine), DOPE ( 1,2-dioleoyl-sn-glycero-3-
phosphoethanolamine), DOTAP
(1,2-dioleoyl-3-trimethylammonium propane), DDAB (dimethyl dioctadecylammonium
bromide),
DHDEAB (N,N-di-n-hexadecyl-N,N-dihydroxyethyl ammonium bromide), HDEAB (N-n-
hexadecyl
N,N-dihydroxyethylammonium bromide), polybrene, or poly(ethylenimine) (PEI).
(E.g., Banerjee,
R. et al., Novel series of non-glycerol-based cationic transfection lipids for
use in liposomal gene
delivery, J. Med. Chem. 42(21):4292-99 [1999]; Godbey, W.T. et al., Improved
packing of
poly(ethyleniminelDNA complexes increases transfection e~ciency, Gene Ther.
6(8):1380-88 [ 1999];
Kichler, A et al., Influence of the DNA complexation medium on the
transfection efficiency of
liposperminelDNA particles, Gene Ther. 5(6):855-60 [1998]; Birchaa, J.C. et
al., Physico-chemical
characterisation and transfection efficiency of lipid-based gene delivery
complexes, Int. J. Pharm.
183(2):195-207 [1999]). These non-viral agents have the advantage that they
facilitate stable
integration of xenogeneic DNA sequences into the vertebrate genome, without
size restrictions
commonly associated with virus-derived transfecting agents.
"Virus", as used herein, means any virus, or transfecting fragment thereof,
which can facilitate
the delivery of the genetic material into male germ cells. Examples of viruses
which are suitable for
use herein are adenoviruses, adeno-associated viruses, retroviruses such as
human immune-deficiency
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
12
virus, lentiviruses, mumps virus, and transfecting fragments of any of these
viruses, and other viral
DNA segments that facilitate the uptake of the desired DNA segment by, and
release into, the
cytoplasm of germ cells and mixtures thereof. A preferred viral vector is
Moloney murine leukemia
virus and the retrovirus vector derived from Moloney virus called vesicular-
stomatitis-virus-
glycoprotein (VSV-G)-Moloney murine leukemia virus. A most preferred viral
vector is a
pseudotyped (VSV-G) lentiviral vector derived from the HIV virus (Naldini et
al. [1996]). Also, the
mumps virus is particularly suited because of its affinity for immature sperm
cells including
spermatogonia. All of the above viruses may require modification to render
them non-pathogenic or
less antigenic. Other known vector systems, however, are also useful within
the confines of the
invention.
In the in vivo method of incorporating exogenous genetic material into the
genome of a
vertebrate, administering to a male vertebrate's testis a gene delivery
mixture involves the in vivo
introduction of the gene delivery mixture to the germ cells by direct delivery
into at least one of the
animal's testes, where it is distributed to male germ cells at various stages
of development. The in
vivo method employs injection of the gene delivery mixture, preferably into
the seminiferous tubules,
or into the rete testis, and most preferably into the vas efferens or vasa
efferentia, using, for example,
a micropipette. To ensure a steady infusion of the gene delivery mixture,
under pressures which will
not damage the delicate tubule system in the testis, the injection is made
through the micropipette with
the aid of a picopump delivering a precise measured volume under controlled
amounts of pressure.
The micropipette is made of a suitable material, such as, metal or glass, and
is usually made from glass
tubing which has been drawn to a fine bore at its working tip, e.g. using a
pipette puller. The tip can
be angulated in a convenient manner to facilitate its entry into the
testicular tubule system. Also, the
micropipette can be provided with a beveled working end to allow a better and
less damaging
penetration of the fme tubules at the injection site. This bevel can be
produced by means of a specially
manufactured grinding apparatus. The diameter of the tip of the pipette for
the in vivo method of
injection is typically about 15 to 45 microns, although other sizes can be
used as needed, depending
on the animal's size. The tip of the pipette can be introduced into the rete
testis or the tubule system
of the testicle, with the aid of a binocular microscope with coaxial
illumination, with care taken not
to damage the wall of the tubule opposite the injection point, and keeping
trauma to a minimum. On
average, a magnification of about 25x to 80x is suitable, and bench mounted
micromanipulators are
not severally required as the procedure can be carried out by a skilled
artisan without additional aids.
A small amount of a suitable, non-toxic dye, can be added to the gene delivery
mixture (fluid) to
confirm delivery and dissemination to the seminiferous tubules of the testis.
It can include a dilute
solution of a suitable, non-toxic dye, which can be visualized and tracked
under the microscope.
In this manner, the gene delivery mixture reaches and is brought into intimate
contact with the
male germ cells. Male germ cells include spermatozoa (i.e., male gametes) and
developmental
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
13
precursors thereof. In fetal development, primordial germ cells are thought to
arise from the
embryonic ectoderm, and are first seen in the epithelium of the endodermal
yolk sac at the E8 stage.
From there they migrate through the hindgut endoderm to the genital ridges. In
the sexually mature
male vertebrate animal, there are several types of cells that are precursors
of spermatozoa, and which
can be genetically modified, including the primitive spermatogonial stem
cells, known as AO/As,
which differentiate into type B spermatogonia. The latter further
differentiate to form primary
spermatocytes, and enter a prolonged meiotic prophase during which homologous
chromosomes pair
and recombine. Useful precursor cells at several morphological/developmental
stages are also
distinguishable: preleptotene spermatocytes, leptotene spermatocytes, zygotene
spermatocytes,
pachytene spermatocytes, secondary spermatocytes, and the haploid spermatids.
The latter undergo
further morphological changes during spermatogenesis, including the reshaping
of their nucleus, the
formation of acrosome, and assembly of the tail. The final changes in the
spermatozoan (i.e., male
gamete) take place in the genital tract of the female, prior to fertilization.
The polynucleotide contained
in the gene delivery mixture administered in the in vivo method to the testis
will reach germ cells that
are at any one of the above described stages, and will be taken up
preferentially by those that are at
a relatively more receptive stage.
In the in vitro (ex vivo) method of incorporating exogenous genetic material
into the genome
of a vertebrate, the male germ cells are preferably, but not exclusively,
diploid spermatogonia, which
are exposed to or contacted with the gene delivery mixture. Whether employed
in the in vivo
method or in vitro method, the gene delivery mixture, once in contact with the
male germ cells,
facilitates the uptake and transport of exogenous genetic material into the
appropriate cell location for
integration into the genome and expression. A number of known gene delivery
methods can be used
for the uptake of nucleic acid sequences into the cell. In either the in vivo
or vitro method, the gene
delivery mixture typically comprises the polynucleotide encoding the desired
trait or product, together
with a suitable promoter sequence, and optionally agents which increase the
uptake of or comprise the
polynucleotide sequence, such as liposomes, retroviral vectors, adenoviral
vectors, adenovirus
enhanced gene delivery systems, or combinations thereof. A reporter construct,
including a genetic
selection marker, such as the gene encoding for Green Fluorescent Protein, can
further be added to the
gene delivery mixture. Targeting molecules, such as c-kit ligand, can be added
to the gene delivery
mixture to enhance the transfer of genetic material into the male germ cell.
An immunosuppressing
agent, such as cyclosporin or a corticosteroid can also be added to the gene
delivery mixture as known
in the art.
In the in vitro method of incorporating exogenous genetic material into the
genome of a
vertebrate, the male germ cells are obtained or collected from the donor male
vertebrate, by means
known in the art. The thus obtained germ cells are then exposed to the gene
delivery mixture,
preferably within several hours, or cryopreserved for later use.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
14
In one embodiment of the in vitro method, obtaining the male germ cells from
the donor
vertebrate can be accomplished by transection of the testes. Transection of
the isolated testicular
tissue can be accomplished, for example, by isolation of the vertebrate's
testes, decapsulation and
teasing apart and mincing of the seminiferous tubules. The separated cells can
then be incubated in
an enzyme mixture comprising enzymes known for gently breaking up the tissue
matrix and releasing
undamaged cells such as, for example, pancreatic trypsin, collagenase type I,
pancreatic DNAse type
I, as well as bovine serum albumin and a modified DMEM medium. The cells can
be incubated in the
enzyme mixture for a period of about 5 min to about 30 min, more preferably
about 15 to about 20
min, at a temperature of about 33 °C to about 37 °C, more
preferably about 36 to 37 °C. After washing
the cells free of the enzyme mixture, they can be placed in an incubation
medium such as DMEM, and
the like, and plated on a culture dish for genetic modification by exposure to
the gene delivery mixture.
This transection method is not suitable when the donor and recipient male
vertebrates are intended
to be the same animal, in which case induced a less destructive biopsy method
or induced ejaculation
by means known in the art is preferred.
Any of a number of commercially available gene delivery mixtures can be used,
to which the
polynucleotide encoding a desire trait or product is further admixed. The
final gene delivery mixture
comprising the polynucleotide can then be admixed with the cells and allowed
to interact for a period
of about 2 hrs to about 16 hrs, preferably about 3 to 4 hrs, at a temperature
of about 33 °C to about
37 °C, preferably about 36 ° C to 37 ° C, and more
preferably in a constant and/or controlled atmosphere.
After this period, the cells are preferably placed at a lower temperature of
about 33 °C to about 34°C,
preferably about 30-35°C for a period of about 4 hrs to about 20 hrs,
preferably about 16 to 18 hrs.
Other conditions which do not deviate radically from the ones described can
also be utilized as an
artisan would know.
With respect to either the in vivo or in vitro methods, a most preferred
embodiment employs
a retroviral vector system, which was developed for gene therapy (Naldini, L.,
et al., In vivo gene
delivery and stable transduction of nondividing cells by a lentiviral vector,
Science 272: 263-267
[1996]), which is used to transduce male germ cells in vivo or in vitro. This
gene delivery system
employs retroviral particles generated by a three-plasmid expression system.
In this system a
packaging construct contains the human cytomegalovirus (hCMV) immediate early
promoter, driving
the expression of all viral proteins. The construct's design eliminates the
cis-acting sequences crucial
for viral packaging, reverse transcription and integration of these
transcripts. The second plasmid
encodes a heterologous envelope protein (env), namely the G glycoprotein of
the vesicular stomatitis
virus (VSV-G). The third plasmid, the transducing vector (pHR'), contains cis-
acting sequences of
human immunodeficiency virus (HIV) required for packaging, reverse
transcription and integration,
as well as unique restriction sites for cloning heterologous complementary
DNAs (cDNAs). For
example, a genetic selection marker, such as the enhanced green fluorescent
protein (EGFP), and/or
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
a gene encoding another preselected or desired trait or product is cloned
downstream of the hCMV
promoter in the HR'vector, and is operatively linked so as to form a
transcriptional unit. A VSV-G
pseudotyped retroviral vector system is capable of infecting a wide variety of
cells including cells from
different species and of integrating into the genome. Some retroviruses, i.e.,
lentiviruses, such as HIV,
5 have the ability to infect non-dividing cells. They have a limited capacity
for heterologous DNA
sequences, the size limit for this vector being 7-7.5 kilobases (Verma, LM.
and Somia, N., Gene
Therapy-promises, problems and prospects, Nature 389:239-242 [ 1997]). In vivo
experiments with
lentiviruses show that expression does not shut off like other retroviral
vectors and that in vivo
expression in brain, muscle, liver or pancreatic-islet cells, is sustained at
least for over six months -
10 the longest time tested so far (Verma and Somia [1997]; Anderson, WF.,
Hurnan Gene Therapy,
Nature (Supply. 392:25-30 [1998]).
For the expression of delivered genetic material by transfection,
transduction, or other means
to obtain expression of a desired trait or product, a promoter sequence is
operatively linked to a
polynucleotide sequence encoding the desired trait or product. For purposes of
the present invention,
15 "operatively linked" means that, within a transcriptional unit, the
promoter sequence, is located
upstream (i.e., 5' in relation thereto) from the coding sequence and the
coding sequence, is 3' to the
promoter, or alternatively is in a sequence of genes 3' to the promoter and
expression is coordinately
regulated thereby. Both the promoter and coding sequences are oriented in a 5'
to 3' manner, such that
transcription can take place in vitro in the presence of all essential
enzymes, transcription factors, co-
factors, activators, and reactants, under favorable physical conditions, e.g.,
suitable pH and
temperature. This does not mean that, in any particular cell, conditions will
favor transcription. For
example, transcription from a tissue-specific promoter is generally not
favored in heterologous cell
types from different tissues.
A promoter sequence is chosen that operates in the cell type of interest
and/or under the
physiologic or developmental conditions of interest. Useful promoter sequences
include constitutive
promoters, such as, but not limited to, cytomegalovirus (CMV ) promoter, or
inducible promoters, such
as, but not limited to, the human C-reactive protein (CRP) promoter (e.g.,
Kanzler, S., et al., TGF
betal in liver fibrosis: an inducible transgenic mouse model to study liver
fzbrogenesis, Am. J.
Physiol. 276(4Pt 1 ):61059-68 [ 1999] ), or the insulin-like growth factor
(IGF-I) promoter (e.g., Meton
L, et al., Growth hormone induces insulin-like growth factor-I gene
transcription by synergistic action
of STATS and HNF-1 alpha, FEBS Lett. 444(2-3):155-59 [ 1999] ). Useful
promoters include those that
promote transcription in cells of diverse tissues, such as, but not limited
to, an insulin receptor (IR)
gene promoter (e.g., Tewari, D.S., et al., Characterization of the promoter
region and 3' end of the
human insulin receptor gene, J. Biol. Chem. 264(27):16238-45 [1989]); growth
hormone receptor
(GHR) P2 or P3 promoters (e.g., Jiang, H., et al., Isolation and
characterization of a novel promoter
for the bovine growth hormone receptor gene, J. Biol. Chem. 274(12):7893-900
[1999]); or a leptin
CA 02368620 2001-11-13
WO 00/69257 PCT/LIS00/13000
16
promoter (e.g., Chen, X.L., et al., Analysis of a 762-by proximal leptin
promoter to drive and control
regulation of transgene expression of growth hormone receptor in mice,
Biochem. Biophys. Res.
Commun. 262(1):187-92 [1999]).
Also useful for various applications are tissue-selective (i.e., tissue-
specific) promoters, i.e.,
promoters from which expression occurs preferentially in cells of a particular
kind of tissue, compared
to one or more other types of tissue. Tissue-specific promoters are
particularly useful in applications
directed to gene therapy or to the genetic enhancement of non-human
vertebrates.
For example, a promoter sequence, which is only active in cycling
spermatogonial stem cell
populations can be used for differential expression in male germ cells, for
example, B-Myb or a male
germ cell-specific promoter, such as the c-kit promoter region, c-raf-1
promoter, ATM (ataxia-
telangiectasia) promoter (also active in cerebellar cells and thymocytes),
vasa promoter, cyclin
Alpromoter, RBM (ribosome binding motif) promoter, DAZ (deleted in
azoospermia) promoter,
XRCC-1 promoter, HSP 90 (heat shock gene) promoter, or FRMI (from fragile X
site) promoter.
For hematopoietic tissue-selective expression in hematopoietic precursor
cells, useful
promoters include cyclin Al promoters (e.g., Miiller, C., et al., Cloning of
the cyclin Al genomic
structure and characterization of the promoter region, J. Biol. Chem.
276(16):11220-28 [1999]);
CD34 promoters (e.g., Burn, T.C., etal., Hematopoietic stem cell specific gene
expression, U.S. Patent
No. 5,556,954); a c-kit promoter, or an integrin alphaIIb promoter (e.g.,
Wilcox, D.A., et al., Integrin
alphallb promoter-targeted expression of gene products in rnegakaryocytes
derived from retrovirus-
transduced human hematopoietic cells, Proc. Natl. Acad. Sci. USA 96( 17):9654-
59 [ 1999]).
Cartilage-selective promoters for expression in chondrocytes, for example, an
osteocalcin
(OC) promoter (e.g., Newberry, E.P., et al., The RRM domain of MINT, a novel
Msx2 binding protein,
recognizes and regulates the rat osteocalcin promoter, Biochemistry
38(33):10678-90 [1999]); a
SOX9 promoter, aggrecan gene promoter (AGC1), or collagen oligomeric matrix
protein (COMP)
gene promoter (e.g., Kanai, Y. & Koopman, P., Structural and functional
characterization of the
mouse Sox9 promoter: implications for campomelic dysplasia, Hum. Mol. Genet.
8(4):691-96 [ 1999];
Newton et al., Characterization of human and mouse cartilage oligomeric matrix
protein, Genomics
24:435-39 [1994); or a promoter from a collagen gene, such as, but not limited
to promoters for
COL2A1, COL9A1, or COL10A1. (e.g., Ganguly, A., et al., Targeted insertions of
two exogenous
collagen genes into both alleles of their endogenous loci in cultured human
cells: the insertions are
directed by relatively short fragments containing the promoters and the 5'
ends of the genes, Proc Natl
Acad Sci USA 91(15):7365-9 [1994]; Dharmavaram, R. M., et al., Detection and
characterization of
Spl binding activity in human chondrocytes and its alterations during
chondrocyte dedifferentiation,
J. Biol Chem 272(43):26918-25 [1997]; Zhou, G., et al., Three high mobility
group-like sequences
within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-
specific expression in
vivo, J. Biol Chem 273(24):14989-97 [ 1998]; Seghatoleslami, M. R., et al.,
Differential regulation of
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
17
COL2A1 expression in developing and mature chondrocytes, Matrix Biol 14(9):753-
64 [1995];
Lefebvre, V., et al., An 18-base-pair sequence in the mouse proalphal (II)
collagen gene is sufficient
for expression in cartilage and binds nuclear proteins that are selectively
expressed in chondrocytes,
Mol Cell Biol 16(8):4512-23 [ 1996]; Zhou, G., et al., A 182 by fragment of
the mouse pro alpha 1 (11)
collagen gene is sufficient to direct chondrocyte expression in transgenic
mice, J. Cell Sci, 108(Pt
12):3677-84 [3677-84]; Mukhopadhyay, K., et al., Use of a hew rat
chondrosarcoma cell line to
delineate a 119-base pair chondrocyte-specific erzhancer element and to define
active promoter
segments in the mouse pro-alpha 1 (II) collagen gene, J. Biol Chem
270(46):27711-9 [ 1995]; Vikkula,
M., et al., Structural analysis of the regulatory elements of the tyupe-II
procollagen gene.
Conservation of promoter and first intron sequences between human arid mouse,
Biochem J 285(Pt
1):287-94 [ 1992]; Beier, F., et al., Localization of silencer and enharzcer
elements in the human type
X collagen gene, J Cell Biochem 662(2):210-8 [1997]; Thomas, J. T., Sequence
comparison of three
rnarnmalian type-X collagen promoters and preliminary functional analysis of
the human promoter,
Gene 160(2):291-6 [1995]; Apte, S. S., Characterization of the mouse type X
collagen gene, Matrix
13(2):165-79 [1993]). A cartilage-derived retinoic acid-sensitive protein (CD-
RAP) gene promoter
is also useful for cartilage-selective expression by chondrocytes. (e.g., Xie,
W.F., et al.,
Transactivation of the mouse cartilage derived retinoic acid-sensitive protein
gene by Sox9, J. Bone
Miner. Res. 14(5):757-63 [1999]).
For liver-selective expression in hepatocytes, useful promoter sequences
include, an albumin
gene promoter (e.g., Pastore, L., et al. , Use of a liver-specific promoter
reduces immune response to
the transgene in adenoviral vectors, Hum. Gen. Ther. 10(11):1773-81 [ 1999]);
a CYP7A or CYP7A1
promoter (e.g., Nitta, M., et al., CPF: an orphan nuclear receptor that
regulates liver-specific
expression of the human cholesterol 7alpha-hydroxylase gene, Proc. Natl. Acad.
Sci. USA
96(12):6660-65 [1999]; Chen, J., et al., Hepatocyte nuclear factor 1 binds to
and transactivates the
human but not the rat CYP7A1 promoter, Biochem. Biophys. Res. Commun.
260(3):829-34 [1999]
); a GHR P1 promoter (e.g., Zou, L., et al., Isolation of a liver-specific
promoter for human growth
hormone receptor gene, Endocrinology 138(4):1771-74 [ 1997]; Jiang, H., et al.
[ 1999]; Adams, T.E.,
Differential expression of growth hormone receptor messenger RNA from a second
promoter, Mol.
Cell Endocrinol. 108(1-2):23-33 [1995]); or a thrombin-activatable
fibrinolysis inhibitor (TAFI)
promoter (e.g., Boffa, M.B., et al., Characterization of the gene encoding
human TAFI (thrombin-
activatable fibrinolysis inhibitor; plasma procarboxypeptidase BJ,
Biochemistry 38(20):6547-58
[ 1999] ).
Neuronal specific promoters are also useful, for example, a neurofilament
promoter or a
neural-specific enolase promoter.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
18
Many other tissue specific promoters are useful for tissue specific expression
of a preselected
gene for phenotypic expression of a desired trait or product in the various
tissues or organs of the
vertebrate body.
Other useful promoters are related to the expression of cytokine-inducible
proteins, including
S promoters that regulate the expression of products and modulators of the Jak-
STAT signaling cascade,
for example, a SOCS-3 promoter (C.J. Auernhammer et al., Autoregulation of
pituitary corticotroph
SOCS-3 expression: characterization of the murine SOCS-3 promoter, Proc. Natl.
Acad Sci. USA
96:6964-69 [1999]), a STAT-3 promoter (C. Bousquet & S. Melmed, J. Biol. Chem.
274:10723-30
[1999]), a POMC promoter (C.J. Auernhammer et al. [1998b]), or Spi 2.1
promoter (T.E. Adams et
al. [1995]).
Useful promoters also include exogenously inducible promoters. These are
promoters that can
be "turned on" in response to an exogenously supplied agent or stimulus, which
is generally not an
endogenous metabolite or cytokine. Examples include an antibiotic-inducible
promoter, such as a
tetracycline-inducible promoter; a heat-inducible promoter; a light-inducible
promoter; or a laser-
inducible promoter. (E.g., Halloran, M.C. et al., Laser-induced gene
expression in specific cells of
transgenic zebrafish, Development. 127(9):1953-1960 [2000]; Gerner, E.W. et
al., Heat-inducible
vectors for use in gene therapy, Int. J. Hyperthermia 16(2):171-81 [2000];
Rang, A.. and Will, H.,
The tetracycline-responsive promoter contains functional interferon-inducible
response elements,
Nucleic Acids Res. 28(5):1120-5 [2000]; Hagihara Y. et al., Long-term
functional assessment of
encapsulated cells transfected with Tet-On system, Cell Transplant. 8(4):431-4
[1999]; Huang, C.J.
et al., Expression of green fluorescent protein in oligodendrocytes in a time-
and level-controllable
fashion with a tetracycline-regulated systena, Mol. Med. 5(2):129-37 [1999];
Forster, K. et al.,
Tetracycline-inducible expression systems with reduced basal activity in
mammalian cells, Nucleic
Acids Res. 27(2):708-10 [1999]; Liu, H.S. et al., LaclTet dual-inducible
system functions in
mammalian cell lines, Biotechniques 24(4):624-8, 630-2 [1998]).
Other useful promoters include developmentally or temporally regulated
promoters. Examples
include the myelin PO promoter (P. Thatikunta et al., Reciprocal Id expression
and myelin gene
regulation in Schwann cells, Mol. Cell Neurosci. 14(6):519-28 [1999]), Gabra3
or GABRA3
promoters (W. Mu and D.R. Burt, the mouse GABA(A) receptor alpha3 subunit gene
and promoter,
Brain Res. Mol. Brain Res. 73(1-2):172-80 [1999]), tyrosine hydroxylase
promoter (J.J. Schimmel et
al. , 4.5 kb of the rat tyrosine hydroxylase 5'flanking seguence directs
tissue specific expression during
development and contains consensus sites for multiple transcription factors,
Brain Res. Mol. Brain
Res. 74(1-2):1-14 [1999]), vimentin promoters (A. Benazzouz and P. Duprey, The
vimentin promoter
as a tool to analyze the early events of retinoic acid-induced differentiation
of cultured embryonal
carcinoma cells, Differentiation (65(3):171-80 [1999]), GATA-6 promoters (A.
Brewer et al., The
human and mouse GATA-6 genes utilize two promoters and two initiation codons,
J. Biol. Chem.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
19
274(53):38004-16 [2000]), SHIP1 or SHIP2 promoters (S. Schurmans et al., The
mouse SHIP2
(Inppll )gene: complementary DNA, genonaic structure, promoter analysis, and
gene expression in the
embryo and adult mouse, Genomics 62(2):260-71 [1999]), or hGH-N promoters
(B.M. Shewchuk et
al., Pit-1 binding sites at the somatotrope-specific DNase I hypersensitive
sites I, 11, of the human
growth hormone locus control region are essential for in vivo hGH-Ngene
activation, J. Biol. Chem.
274(50):35725-33 [1999]).
The foregoing examples of useful promoter sequences are by no means an
exhaustive list, but
are merely illustrative of the promoters available to the skilled artisan in
practicing the present
invention.
The in vivo and in vitro methods of incorporating exogenous genetic material
into the genome
of a vertebrate involve incorporating the polynucleotide encoding a desired
trait or product into the
genome of at least one spermatozoan or precursor thereof, so that a
genetically modified male gamete
is produced by the male vertebrate. Thus, the genetically modified germ cells
of the vertebrate
animal, now transgenic, have the non-endogenous (exogenous) genetic material
integrated into their
chromosomes. This is what is often referred to as a "stable transfection" or
"stable integration". This
is applicable to all vertebrate animals, including humans. Animals that are
shown to carry suitably
modified sperm cells then can be either allowed to mate naturally, or
alternatively their spermatozoa
are used for insemination or in vitro fertilization.
Isolating and/or selecting of genetically modified cells, including transgenic
germ cells and
transgenic somatic cells, and of transgenic vertebrates, is by any suitable
means, such as, but not
limited to, physiological and/or morphological phenotypes of interest using
any suitable means, such
as biochemical, enzymatic, immunochemical, histologic, electrophysiologic,
biometric or like methods;
and analysis of cellular nucleic acids, for example the presence or absence of
specific DNAs or RNAs
of interest using conventional molecular biological techniques, including
hybridization analysis,
nucleic acid amplification (such as but not limited to, polymerise chain
reaction [PCR], reverse
transcriptase-mediated polymerise chain reaction [RT-PCR], transcription-
mediated amplification
[TMA], reverse transcriptase-mediated ligase chain reaction [RT-LCR]), and/or
electrophoretic
technologies.
In a preferred embodiment, the gene delivery mixture includes at least one
polynucleotide
comprising a gene encoding a genetic selection marker that is operatively
linked to a promoter
sequence such that a transcriptional unit is formed. The promoter sequence can
be the same or
different from the promoter regulating the expression from the gene encoding
the desired trait or
product. The genetic selection marker, also known as a reporter gene, is, for
example, a gene
encoding an enzyme, such as (3-galactosidase, or encoding a fluorescent
protein, such as Green
Fluorescent Protein (GFP), enhanced Green Fluorescent Protein (EGFP), Yellow
Fluorescent Protein,
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
Blue Fluorescent Protein, a phycobiliprotein, such as phycoerythrin or
phycocyanin, or any other
protein which fluoresces under a suitable wave-length.
Another preferred genetic selection marker or reporter gene, suitable for some
applications
is a gene encoding a protein that can enzymatically lead to the emission of
light from a substrate(s);
5 for purposes of the present invention, such a protein is a "light-emitting"
or luminescent protein. For
example, a light-emitting protein includes proteins such as luciferase or
apoaequorin. Transgenic cells
expressing a fluorescent or luminescent protein encoded by the reporter
construct can be sorted with
the aid of, for example, a flow activated cell sorter (FAGS) set at the
appropriate wavelength(s), or
they can be selected by chemical methods.
10 A preferred method of isolating or selecting male germ cell populations,
comprises obtaining
specific male germ cell populations, such as spermatogonia, from a mixed
population of testicular cells
by extruding the cells from the seminiferous tubules and gentle enzymatic
disaggregation. The
spermatogonia or other male germ cell populations, which are to be genetically
modified, can be
isolated from a mixed cell population by a method including the utilization of
a promoter sequence,
15 which is specifically or selectively active in cycling male germ line stem
cell populations, for
example, B-Myb or a specific promoter, such as the c-kit promoter region, c-
raf-1 promoter, ATM
(ataxia-telangiectasia) promoter, vasa promoter, RBM (ribosome binding motif)
promoter, DAZ
(deleted in azoospermia) promoter, XRCC-1 promoter, HSP 90 (heat shock gene)
promoter, cyclin A1
promoter, or FRMI (from fragile X site) promoter, linked to a reporter
construct, for example, a
20 construct comprising a gene encoding Green Fluorescent Protein (or EGFP),
Yellow Fluorescent
Protein, Blue Fluorescent Protein, a phycobiliprotein, such as phycoerythrin
or phycocyanin, or any
other protein which fluoresces under suitable wave-lengths of light, or
encoding a light-emitting
protein, such as luciferase or apoaequorin. These unique promoter sequences
drive the expression of
the reporter construct only during specific stages of male germ cell
development, as is known to those
skilled in the art. (E.g., Miiller, C., et al., Cloning of the cyclin Al
genomic structure and
characterization of the promoter region, J. Biol. Chem. 276(16):11220-28
[1999]; Schrans-Stassen,
B., H. et al., Differential expression of c-kit in mouse undifferentiated and
differentiating type A
spermatogonia, Endocrinology 140:5894-5900 [1999]). Populations of male germ
cells at specific
developmental stages, thus, are the only cells in the mixed population which
will express the reporter
constructs) and they, thus, can be isolated on this basis. In the case of a
fluorescent reporter construct,
the cells can be sorted with the aid of, for example, a FACS set at the
appropriate wavelength(s), or
they can be selected by chemical methods.
Further with respect to the in vitro method of incorporating exogenous genetic
material into
the genome of a vertebrate, in which male germ cells are obtained from a donor
animal and genetically
modified in vitro to impart a gene encoding a desired trait or product, male
germ cells which exhibit
any evidence that the DNA has been modified in the desired manner are isolated
or selected, and
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
21
transferred to the testis of a suitable recipient animal. After transfer,
further selection can be attempted
after biopsy of one or both of the recipient male's testes, or after
examination of the animal's ejaculate
amplified by the polymerase chain reaction to confirm whether the desired
nucleic acid sequence was
actually incorporated. As described above, the initial gene delivery can have
included a reporter gene,
such as a gene encoding the Green Fluorescent Protein, enhanced Green
Fluorescent Protein (EGFP),
Yellow Fluorescent Protein, Blue Fluorescent Protein, a phycobiliprotein, such
as phycoerythrin or
phycocyanin, or any other protein which fluoresces under light of suitable
wave-lengths, or encoding
a light-emitting protein.
In the in vitro method of incorporating exogenous genetic material into the
genome of a
vertebrate, the genetically modified germ cells, thus isolated or selected,
are preferably transferred to
a testis of a recipient male vertebrate, which can be, but need not be, the
same donor animal. Before
transferring the genetically modified male germ cells to one or more of the
testes of the recipient male
vertebrate, the testes of the recipient animal are preferably depopulated of
native germ cells.
Substantial depopulation of the endogenous male germ cells facilitates the
colonization of the
recipient testis by the genetically modified germ cells from the donor animal.
The depopulation can
be done by any suitable means, including by gamma irradiation, by chemical
treatment, by means of
infectious agents such as viruses, or by autoimmune depletion or by
combinations thereof.
Whichever means of depopulating the testis of endogenous male germ cells is
used, the basic
rigid architecture of the gonad should not be destroyed, nor badly damaged. If
there is disruption of
the fine system of tubule formation, it may be impossible for the exogenous
spermatogonia to
repopulate the testis. Disruption of tubules would also presumably lead to
impaired transport of
testicular sperm and result in infertility. Any controlled testicular injury
of this kind should also be
limited so that the Sertoli cells are not irreversibly damaged, as they are
needed to provide a base for
development of the germ cells during maturation. Moreover they may play a role
in preventing the
host immune defense system from destroying grafted foreign spermatogonia.
But vertebrate testes are most preferably depopulated by a combined treatment
of the
vertebrate with an alkylating agent and gamma irradiation in accordance with
the inventive method
of substantially depopulating a vertebrate testes. The method involves a
treatment with a cytotoxic
alkylating agent, such as, but not limited to, busulfan ( 1,4-butanediol
dimethanesulphonate; Myleran,
Glaxo Wellcome), chlorambucil, cyclophosphamide, melphalan, or ethyl
ethanesulfonic acid,
combined with gamma irradiation, to be administered in either sequence. The
combination of a dose
of an alkylating agent and a dose of gamma radiation yields unexpectedly
superior results in
depopulating the testes of germ cells, compared to either treatment alone. The
dose of the alkylating
agent and the dose of gamma radiation are in an amount sufficient to
substantially depopulate the
testis.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
22
The preferred dose of alkylating agent is about 4 to 10 milligrams per
kilogram of body weight,
and about 6 to 8 milligrams per kilogram of body weight is most preferred. The
alkylating agent can
be administered by any pharmaceutically acceptable delivery system, including
but not limited to,
intraperitoneal, intravenous, or intramuscular injection, intravenous drip,
implant, transdermal or
transmucosal delivery systems.
A recovery period between the administration of alkylating agent and
irradiation is not
essential, and the two treatments are most preferably done within zero to 24
hours of each other.
Preferably, the time between the two treatments should not exceed 2 weeks,
because this yields less
than optimal results for purposes of transferring genetically modified or
heterologous male germ cells
to recipient testes.
The recipient vertebrate is gamma irradiated with a dose of about 200 to 800
Rads, most
preferably about 350 to 450 Rads, directed locally to the testis to be
depopulated. Less than 200 Rad
yields little effect; greater than 800 Rad commonly produces symptoms of
radiation sickness,
particularly in the gastrointestinal tract. Within 3 days to 2 months after
treatment to depopulate the
recipient testis(es) in accordance with the present method, male germ cells
can be transferred thereto
as described herein. Prior to three days, traces of cytotoxic alkylating agent
or endogenous apoptotic
signal molecules may remain in the recipient testis to harm the male germ
cells transferred thereto.
After two months, the endogenous population of male germ cells will typically
begin to restablish
itself, yielding less than optimal results when transfected, genetically
altered, or heterologous male
germ cells are transferred to a recipient testes for breeding purposes.
Thus in the in vitro (ex vivo) method, preferably within three days to two
months after the
final treatment to depopulate the testis(es) of the recipient male vertebrate,
the gene delivery mixture
is administered to the male germ cells of the donor vertebrate, in vitro, in
sufficient amount and under
effective conditions such that one or more of them is genetically modified.
Genetically modified male
germ cells from the donor male vertebrate can then be transferred to the
testis(es) of the recipient male
such that they lodge in a seminiferous tubule of the testis, where they then
mature into genetically
modified gametes.
Transferring the isolated or selected genetically modified germ cells into the
recipient testis
can be accomplished by direct injection using a suitable micropipette. Support
cells, such as Leydig
or Sertoli cells that provide hormonal stimulus to spermatogonial
differentiation, can be transferred
to a recipient testis along with the modified germ cells. These transferred
support cells can be
unmodified, or, alternatively, can themselves have been genetically modified,
together with- or
separately from the germ cells. These transferred support cells can be
autologous or heterologous to
either the donor or recipient testis. A preferred concentration of cells in
the transfer fluid can easily
be established by simple experimentation, but will likely be within the range
of about 1 x 105 - 10 x
lOscells per 10 ~I of fluid. This micropipette can be introduced into the vasa
efferentia, the rete testis
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
23
or the seminiferous tubules, optionally with the aid of a picopump to control
pressure and/or volume,
or this delivery can be done manually. The micropipette employed is in most
respects similar to that
used for the in vivo injection, except that its tip diameter generally will be
about 45 to about 70
microns. The microsurgical method of introduction is similar in all respects
to that used for the in vivo
method described above. A suitable dyestuff or bubbles (less than 1 mm in
diameter) can also be
incorporated into the carrier fluid for easy identification of satisfactory
delivery of the transfected
germ cells to at least one seminiferous tubule of the testis (Figure 1).
With respect to both the in vivo and in vitro methods of incorporating
exogenous genetic
material into the genome of a vertebrate involves, breeding the male
vertebrate with a female
vertebrate of its species means causing the union of male and female gametes
so that fertilization
occurs and a transgenic zygote is formed; a transgenic progeny or offspring is
thereafter produced
during gestation of the developing fetus. A union of male and female gametes
is brought about by
natural mating, i.e., copulation by the male and female vertebrates of the
same species, or by in vitro
or in vivo artificial means. If artificial means are chosen, then
incorporating into the genome a genetic
selection marker that is expressed in male germ cells is particularly useful.
Preferably expression of
the genetic selection marker is regulated from a constitutive or male germ-
cell specific promoter,
operatively linked to the gene encoding the genetic selection marker.
Artificial means include, but are not limited to, artificial insemination, in
vitro fertilization
(IVF) and/or other artificial reproductive technologies, such as
intracytoplasmic sperm injection
(ICSI), subzonal insemination (SUZI), or partial zona dissection (PZD).
However, others, such as
cloning and embryo transfer, cloning and embryo splitting, and the like, can
also be employed.
The thus obtained transgenic vertebrate progeny can, in turn, also be bred,
whether by natural
mating, artificial insemination, or by in vitro fertilization (IVF) and/or
other artificial reproductive
technologies, such as intracytoplasmic sperm injection (ICSI), subzonal
insemination (SUZI), orpartial
zona dissection (PZD), to obtain further generations of transgenic progeny.
Those skilled in the art
will readily appreciate that any desired traits generated as a result of
changes to the genetic material
of any transgenic animal produced by the inventive method are inheritable.
Although the genetic
material was originally inserted solely into the germ cells of a parent
animal, it will ultimately be
present in the germ cells of future progeny and subsequent generations
thereof. In addition, the
genetic material is also present in cells of the progeny other than germ
cells, i.e., somatic cells.
Broadly speaking, a "transgenic" vertebrate is one that has had foreign or
exogenous DNA
permanently introduced into its cells. The exogenous genes which have been
introduced into the
animal's cells are called "transgenes" and are xenogeneic and/or allogeneic
transgenic genetic material,
including biologically functional genetic material. The present invention is
applicable to the
production of transgenic animals containing xenogeneic, i.e., exogenous DNA
from a different species,
either in its native, undisturbed form, or in artificially mutated form. In
other embodiments, the
CA 02368620 2001-11-13
WO 00/69257 PCT/LTS00/13000
24
genetic material is "allogeneic" genetic material, exogenous transgenic
material obtained from a
different strain, race, breed, or individual of the same species, for example,
from an animal having a
"normal" form of a gene, or a desirable allele, variant, or mutation thereof.
Also the gene can be a
hybrid construct consisting of promoter DNA sequences and DNA coding sequences
operatively
linked together. These sequences can be obtained from different species or DNA
sequences from the
same species that are not normally juxtaposed. The DNA construct can also
contain DNA sequences
from prokaryotic organisms, such as bacteria, or from viruses.
The transfected germ cells of the transgenic vertebrate animal preferably have
the
non-endogenous (exogenous) genetic material integrated into their chromosomes.
Those skilled in the
art will readily appreciate that any desired traits generated as a result of
changes to the genetic material
of any transgenic vertebrate produced by this invention are heritable.
Although the genetic material
was originally inserted solely into the germ cells of a parent animal, it will
ultimately be present in the
germ cells of direct progeny and subsequent generations thereof. The genetic
material is also present
in the differentiated cells, i.e. somatic cells, of the progeny.
Included in the invention is a non-human transgenic male vertebrate produced
by the in vivo
or in vitro method of incorporating exogenous genetic material into the genome
of a vertebrate.
Produced in accordance with the in vivo method, the transgenic vertebrate is
the recipient of the gene
delivery mixture. Alternatively, the non-human transgenic male vertebrate is
the recipient of the
genetically modified male germ cell that was transferred to its testis, in
accordance with the in vitro
method. The transgenic male vertebrate can be bred with a female of its
species, because it comprises
a native male germ cell carrying in its genome a polynucleotide of exogenous
origin defining a gene
encoding a desired trait or product. But somatic cells in tissues outside the
testis of the transgenic
vertebrate lack the polynucleotide. Preferably, but not necessarily, the
transgenic male vertebrate will
continue to produce genetically modified gametes for an indefinite period.
However, in some
embodiments the transgenic state is temporary, lasting for at least several
weeks or months, after which
non-modified gametes are again exclusively or predominantly produced by the
animal.
Also included in the invention is a non-human transgenic vertebrate produced
in accordance
with the in vivo or in vitro method of incorporating exogenous genetic
material into the genome of a
vertebrate, wherein the non-human vertebrate is the direct or indirect progeny
of the transgenic male
vertebrate described above. Thus, this transgenic progeny is the immediate
offspring, male or female,
of the transgenic male vertebrate or is an offspring thereof separated by one
or more generations. The
transgenic vertebrate includes one or more cells carrying in their genome a
polynucleotide of
exogenous origin that encodes a desired trait or product.
Also, the invention includes a transgenic cell derived from the transgenic
vertebrate progeny.
The cell is a germ cell, such as a spermatozoan or ovum, a precursor germ cell
of either of these, or
a somatic cell.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
Male germ cells are obtained from a male animal's semen, or spermatozoa,
spermatogonia, or
immature spermatocytes are separated from whole biopsies of testicular tissue
containing the male
germ cells. Alternatively, male germ line stem cells can be isolated from
embryonic tissue. Female
germ cells are obtained by known means, including hormonally induced
"ripening" and harvesting
5 from the oviducts or aspiration by way of the cervix or by way of a
laparoscopic incision.
Somatic cells include stem cells. A stem cell is an undifferentiated mother
cell that is self-
renewable over the life of the organism and is multipotent, i.e., capable of
generating various
committed progenitor cells that can develop into fully mature differentiated
cell lines. (E.g., T. Zigova
and P.R. Sanberg, The rising star of neural stem cell research, Nature
Biotechnol. 16(11):1007-08
10 [1998]). All vertebrate tissues arise from stem cells, including
hematopoietic stem cells, from which
various types of blood cells derive; ectodermal stem cells; neural stem cells,
for example, neural
progenitors from which brain and nerve tissues derive. Somatic cells also
include progenitor cells or
terminally differentiated cells of any kind associated with any tissue or
organ of the vertebrate body.
Somatic cells are obtained by known sampling or biopsy means from any bodily
tissue, organ,
15 or fluid, including but not limited to, blood, heart, kidney, ureter,
bladder, urethra, brain, thyroid,
parotid gland, pancreas, hypothalamus, pituitary gland, submaxillary gland,
sublingual gland, lymph
node, bone, bone marrow, cartilage, lung, mediastinum, breast, uterus, ovary,
testis, prostate, cervix
uteri, endometrium, liver, spleen, adrenal, esophagus, stomach, intestine,
hair root, muscle, nerve,
urine, amniotic fluid, chorionic villus, skin, vascular or oral epithelium, or
spinal fluid. The inventive
20 transgenic cells can be cultured or stored by well known means.
The invention also relates to vertebrate semen containing a plurality of the
inventive transgenic
male germ cell. The inventive vertebrate semen is useful for breeding or other
suitable purposes. The
semen is obtained fromejaculate produced by the inventive transgenic male
vertebrate or its transgenic
male progeny (either immediate progeny or progeny separated by one or more
generations), and
25 methods of inducing ejaculation by a male vertebrate and capturing the
semen are well known. The
semen can be processed, e.g., by washing, and/or stored by means such as are
known in the art. For
example, storage conditions include the use of cryopreservation using
programmed freezing methods
and/or the use of cryoprotectants, for example, dimethyl sulfoxide (DMSO),
glycerol, trehalose, or
propanediol-sucrose, and the use of storage in substances such as liquid
nitrogen.
Such storage techniques are particularly beneficial to young adult humans or
children,
undergoing oncological treatments for such diseases such as leukemia or
Hodgkin's lymphoma. These
treatments frequently irreversibly damage the testicle and, thus, render it
unable to recommence
spermatogenesis after therapy by, for example, irradiation or chemotherapy. In
species other than
humans, the present techniques are valuable for transport of gametes as frozen
germ cells. Such
transport will facilitate the establishment of various valued livestock or
fowl lines, at a remote distance
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
26
from the donor animal. This approach is also applicable to the preservation of
endangered species
across the globe.
Thus, the invention is also includes a method of producing a non-human
transgenic vertebrate
animal line, the individuals of which comprise native germ cells carrying in
their genome at least one
xenogeneic polynucleotide. The transgenic vertebrates bred with other
transgenic or non-transgenic
animals of the same species will produce some transgenic progeny, including
fertile individuals. The
method involves breeding of the fertile transgenic progeny with a member of
the opposite sex of the
same species as described above; and selecting its progeny for the presence of
the polynucleotide.
The inventive method of producing a non-human transgenic vertebrate animal
line is simple and
efficient, and is more easily accomplished in large mammals than in mice
because of the larger size
of the testicular ducts. Far fewer animals are needed to produce transgenic
progeny by genetic
modification of male germ cells, which can be produced continually from
repeated mating without
interruption by pregnancy or parturition. It requires no expensive equipment,
nor the training
necessary for microinjection.
The inventive technology is applicable to the field of gene therapy, since it
permits the
introduction of genetic material encoding and regulating specific genetic
traits. Thus, in the human,
for example, by treating parents it is possible to correct many single gene
disorders which otherwise
might affect their children. It is similarly possible to alter the expression
of fully inheritable disorders
or those disorders having at least a partially inherited basis, which are
caused by interaction of more
than one gene, or those which are more prevalent because of the contribution
of multiple genes. This
technology can also be applied in a similar way to correct disorders in
animals other than human
primates. In some instances, it may be necessary to introduce one or more
"gene(s)" into the germ
cells of the animal to attain a desired therapeutic effect, as in the case
where multiple genes are
involved in the expression or suppression of a defined trait. In the human,
examples of multigenic
disorders include diabetes mellitus caused by deficient production of, or
response to, insulin,
inflammatory bowel disease, certain forms of atheromatous cardiovascular
disease and hypertension,
schizophrenia and some forms of chronic depressive disorders, among others. In
some cases, one gene
can encode an expressible product, whereas another gene encodes a regulatory
function, as is known
in the art. Other examples are those where homologous recombinant methods are
applied to repair
point mutations or deletions in the genome, inactivation of a gene causing
pathogenesis or disease, or
insertion of a gene that is expressed in a dominant negative manner, or
alterations of regulating
elements such as gene promoters, enhancers, the untranslated tail region of a
gene, or regulation of
expansion of repeated sequences of DNA which cause such diseases as
Huntingdon's chorea,
Fragile-X syndrome and the like.
A specific reproductive application of the present invention is to the
treatment of animals,
particularly humans, with disorders of spermatogenesis. Defective
spermatogenesis or spermiogenesis
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
27
frequently has a genetic basis, that is, one or mutations in the genome can
result in failure of
production of normal sperm cells. This can happen at various stages of the
development of germ cells,
and may result in male infertility or sterility. The present invention is
applicable, for example, to the
insertion or incorporation of nucleic acid sequences into a recipient's genome
and, thereby, establish
spermatogenesis in the correction of oligozoospermia or azoospermia in the
treatment of infertility.
Similarly, the present methods are also applicable to males whose subfertility
or sterility is due to a
motility disorder with a genetic basis.
The present invention is additionally applicable to the generation of
transgenic animals
expressing agents which are of therapeutic benefit for use in human and
veterinary medicine or well
being. Examples include the production of pharmaceuticals in domestic cows'
milk, such as factors
which enhance blood clotting for patients with types of haemophilia, or
hormonal agents such as
insulin and other peptide hormones.
The present method is further applicable to the generation of transgenic
animals, for example
pigs, of a suitable anatomical and physiological phenotype for human xenograft
transplantation. The
inventive transgenic technology permits the generation of animals which are
immune-compatible with
a human recipient. Appropriate organs, for example, can be removed from such
animals to allow the
transplantation of, for example, the heart, lung and kidney.
In addition, male germ cells genetically modified in accordance with this
invention can be
obtained from the transgenic animal, and stored under conditions effective for
later use, as is known
in the art.
The invention will now be described in greater detail by reference to the
following
non-limiting examples. The pertinent portions of the contents of all
references, and published patent
applications cited throughout this patent necessary for enablement purposes
are hereby incorporated
by reference.
EXAMPLES
GENETIC MODIFICATION OF MALE GERM CELLS IN VIVO AND IN VITRO
In Vivo Adenovirus-enhanced Transferrin-polylysine-mediated Delivery
of Green Lantern Reporter Gene Deliver~vstem to Testicular Cells
The adenovirus enhanced transferrin-polylysine-mediated gene delivery system
has been
described and patented by Curiel et al. (Curiel D.T.,et al., Adenovirus
enhancement of transferrin-
polylysine-mediated gene delivery, PNAS USA 88: 8850-8854 ( 1991 ). The
delivery of DNA depends
upon endocytosis mediated by the transferrin receptor (Wagner et al.,
Transferrin-polycation
conjugates as carriers for DNA uptake into cells, PNAS (USA) 87: 3410-3414
(1990). In addition
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
28
this method relies on the capacity of adenoviruses to disrupt cell vesicles,
such as endosomes and
release the contents entrapped therein. This system can enhance the gene
delivery to mammalian cells
by as much as 2,000 fold over other methods.
The gene delivery system employed for the in vivo experiments was prepared as
shown in
examples below.
Example 1: Preparation of Transferrin-poly-L-Lysine Complexes
Human transferrin was conjugated to poly (L-lysine) using EDC (1-ethyl-3-(3-
dimethyl
aminopropyl carbodiimide hydrochloride) (Pierce), according to the method of
Gabarek and Gergely
(Gabarek & Gergely, Zero-length cross-linking procedure with the use of active
esters, Analyt.
Biochem 185 : 131 (1990)). In this reaction, EDC reacts with a carboxyl group
of human transferrin
to form an amine-reactive intermediate. The activated protein was allowed to
react with the poly
(L-lysine) moiety for 2 hrs at room temperature, and the reaction was quenched
by adding
hydroxylamine to a final concentration of 10 mM. The conjugate was purified by
gel filtration, and
stored at -20°C.
Example 2: Preparation of DNA for In Vivo Transfection
The Green Lantern-1 vector (Life Technologies, Gibco BRL, Gaithersberg, MD) is
a reporter
construct used for monitoring gene transfection in mammalian cells. It
consists of the gene encoding
the Green Fluorescent Protein (GFP) driven by the cytomegalovirus (CMV )
immediate early promoter.
Downstream of the gene is a SV40 polyadenylation signal. Cells transfected
with Green Lantern-1
fluoresce with a bright green light when illuminated with blue light. The
excitation peak is 490 nm.
Example 3: Preparation of Adenoviral Particles
Adenovirus dI312, a replication-incompetent strain deleted in the Ela region,
was propagated
in the Ela trans-complementing cell line 293 as described by Jones and Shenk
(Jones and Shenk,
PNAS USA ( 1979) 79: 3665-3669). A large scale preparation of the virus was
made using the method
of Mittereder and Trapnell (Mittereder et al., "Evaluation of the
concentration and bioactivity of
adenovirus vectors for gene therapy", J. Urology, 70: 7498-7509 ( 1996)). The
virion concentration was
determined by LTV spectroscopy, 1 absorbance unit being equivalent to 10 viral
particles /ml. The
purified virus was stored at -70°C.
Example 4: Formation of Transferrin-poly-L
Lysine-DNA-Viral Complexes
Six (6) micrograms of transferrin-polylysine complex from Example 1 were mixed
in 7.3 x
10' adenovirus d1312 particles prepared as in Example 3, and then mixed with 5
~g of the Green
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
29
Lantern DNA construct of Example 2, and allowed to stand at room temperature
for 1 hour. About
100 pl of the mixture were drawn up into a micropipette, drawn on a pipette
puller, and slightly bent
on a microforge. The filled micropipette was then attached to a picopump
(Eppendorf), and the DNA
complexes were delivered under continuous pressure, in vivo to mice as
described in Example 6.
Controls were run following the same procedure, but omitting the
transferrin-poly-lysine-DNA-viral complexes from the administered mixture.
Example 5: Comparison of Adenovirus-enhanced Transferrin-polylysine
& Lipofectin Mediated Transfection Efficiency
The conjugated adenovirus particle complexed with DNA were tested on CHO cells
in vitro
prior to in vivo testing. For these experiments a luciferase reporter gene was
used due to the ease of
quantifying luciferase activity. The expression construct consists of a
reporter gene encoding
luciferase, is driven by the CMV promoter (Invitrogen, Carlsbad, CA 92008).
CHO cells were grown
in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum. For
gene transfer
experiments CHO cells were seeded into 6 cm tissue culture plates and grown to
about 50%
confluency (Sx 105 cells). Prior to transfection the medium was aspirated and
replaced with serum free
DMEM. Cells were either transfected with transferrin-polylysine-DNA complexes
or with lipofectin
DNA aggregates. For the transferrin-polylysine mediated DNA transfer, the DNA-
adenovirus
complexes were added to the cells at a concentration of 0.05-3.2 x 104
adenovirus particles per cell.
Plates were returned to the 5% COZ incubator for 1 hour at 37°C. After
1 hour 3 ml of complete media
was added to the wells and the cells were allowed to incubate for 48 hours
before harvesting. The
cells were removed from the plate, counted and then lysed for measurement of
luciferase activity.
For cells transfected by lipofectin, leg of CMV-luciferase DNA was incubated
with 17p1 of
Lipofectin (Life Technologies). The DNA-lipofectin aggregates were added to
the CHO cells and
allowed to incubate at 37°C at 5% COZ for 4 hours. Three milliliters of
complete medium was added
then to the cells and they were allowed to incubate for 48 hours. The cells
were harvested, counted
and lysed for luciferase activity. The luciferase activity was measured by a
luminometer. The results
obtained are shown in Table 1.
The data included in Table 1 below show that the adenovirus-enhanced
transferrin-polylysine
gene delivery system is 1,808 fold more efficient than lipofection for
transfection of CHO cells.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
Table 1: Comparison of Lipofection & Adenovirus Enhanced
Transferrin-polylysine Transfection of CHO Cells
Sample Treatment L a c i f a r a s a
Activity (RLU)
5
1 1 x 10' particles + hug CMV-Luc 486
2 2.5 x 10' particles + hug CMV-Luc 1201
3 5.0 x 10' particles + hug CMV-luc 11119
4 1 x 109 particles + hug CMV-Luc 2003503
10 5 Lipofection 1108
6 Unmanipulated cells 155
Example 6: In Vivo Delivery of DNA to Animal's Germ Cells
via Transferrin-L-lysine-DNA-Viral Complexes
15 The GFP DNA-transferrin-polylysine viral complexes, prepared as described
in Example 4
above, were delivered into the seminiferous tubules of three (3)-week-old
B6D2F1 male mice. The
DNA delivery by transferrin receptor-mediated endocytosis is described by
Schmidt et al. and Wagner
et al. (Schmidt et al., Cell 4: 41-51 (1986); Wagner, E., et al. PNAS (1990),
(USA) 81: 3410-3414
[ 1990]). In addition, this delivery system relies on the capacity of
adenoviruses to disrupt cell vesicles,
20 such as endosomes and release the contents entrapped therein. The
transfection efficiency of this
system is almost 2,000 fold higher than lipofection.
The male mice were anesthetized with 2% Avertin ( 100% Avertin comprises 10 g
2,2,2-
tribromoethanol (Aldrich) and 10 ml t-amyl alcohol (Sigma), and a small
incision made in their skin
and body wall, on the ventral side of the body at the level of the hind leg.
The animal's testis was
25 pulled out through the opening by grasping at the testis fat pad with
forceps, and the vas efferens
tubules exposed and supported by a glass syringe. The GFP DNA-transferrin-
polylysine viral
complexes were injected into a single vasa efferentia using a glass
micropipette attached to a hand held
glass syringe or a pressurized automatic pipettor (Eppendorf), and Trypan blue
added to visualize the
entry of the mixture into the seminiferous tubules. The testes were then
placed back in the body
30 cavity, the body wall was sutured, the skin closed with wound clips, and
the animal allowed to recover
on a warm pad.
Example 7: Detection of DNA and Transcribed Messaee
Nine (9) days after delivery of the genetic material to the animals' testis,
two of the animals
were sacrificed, their testes removed, cut in half, and frozen in liquid
nitrogen. The DNA from one
half of the tissues, and the RNA from the other half of the tissues were
extracted and analyzed.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
31
(a) Detection of DNA
The presence of GFP DNA in the extracts was tested 9 days after administration
of the
transfection mixture using the polymerase chain reaction, and GFP specific
oligonucleotides. GFP
DNA was present in the testes of the animals that had received the DNA
complexes, but was absent
from sham operated animals.
(b) Detection of RNA
The presence of GFP mRNA was assayed in the testes of experimental animals as
follows.
RNA was extracted from injected, and non-injected testes, and the presence of
the GFP messages was
detected using reverse transcriptase PCR (RT PCR) with GFP specific primers.
The GFP message
was present in the injected testes, but not in the control testes. The DNA
detected above by PCR
analysis is episomal GFP DNA. The transfected gene was being transiently
expressed.
Example 8: Expression of Non-endo~ynous DNA
Two males, one having received an injection with the GFP transfection mixture
and a control
to whom only surgery was administered, were sacrificed 4 days after injection,
and their testes excised,
and fixed in 4% paraformaldehyde for 18 hours at 4°C. The fixed testis
was then placed in 30%
sucrose in PBS with 2 mM MgClz for 18 hours at 4°C, embedded in OCT
frozen on dry ice, and
sectioned. When the testes of both animals were examined with a confocal
microscope with
fluorescent light at a wavelength of 488 nM, bright fluorescence was detected
in the tubules of the
GFP-injected mice, but not in the testes of the controls. Many cells within
the seminferous tubules
of the GFP-injected mouse showed bright fluorescence, which evidences that
they were expressing
Fluorescent Green Protein.
Example 9: Generation of Offspring from Normal Mating
GFP transfected males were mated with normal females. The females were allowed
to
complete gestation, and the pups to be born. The pups (F1 offspring or
progeny) were screened for
the presence of the novel genetic material(s).
Example 10: In Vitro Transfection of Testicular Cells
Cells were isolated from the testes of three 10-day-old mice. The testes were
decapsulated and
the seminiferous tubules were teased apart and minced with sterile needles.
The cells were incubated
in enzyme mixture for 20 minutes at 37 °C. The enzyme mixture was made
up of 10 mg bovine serum
albumin (embryo tested), 50 mg bovine pancreatic trypsin type III, Clostridium
collagenase type I, 1
mg bovine pancreatic DNAse type I in 10 mls of modified HTF medium (Irvine
Scientific, Irvine, CA).
The enzymes were obtained from Sigma Company (St. Louis, Missouri 63178).
After digestion, the
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
32
cells were washed twice by centrifugation at 500 x g with HTF medium and
resuspended in 250,u1
HTF medium. The cells were counted, and 0.5 x 106 cells were plated in a 60mm
culture dish in a total
volume of Sml DMEM (Gibco-BRL, Life Technologies, Gaithesburg, MD 20884). A
transfection
mixture was prepared by mixing S~g Green Lantern DNA (Gibco-BRL, Life
Technologies,
Gaithesburg, MD 20884) with 201 Superfect (Qiagen, Santa Clarita, CA 91355)
and 150~c1 DMEM.
The transfection mix was added to the cells and they were allowed to incubate
for 3 hours at 37~C, 5%
COZ The cells were transferred to a 33~C incubator and incubated overnight.
The following morning the cells were assessed for transfection efficiency by
counting the
number of fluorescent cells. In this experiment the transfection efficiency
was 90% (Figure not
shown). The testicular cells transfected with Green Lantern viewed with
Nomaski optics x20 show the
same cells viewed with FITC. Nearly all the cells were fluorescent, which is
confirmation of their
successfultransfection.
Example 11: Preparation of a Cell Suspension from Testicular Tissue for
Cryopreservation
A cell suspension was prepared from mice of different ages as described below.
Group I: 7-10 day olds
Group II: 15-17 day olds
Group III: 24-26 day olds
The mice's testes were dissected, placed in phosphate buffered saline (PBS)
decapsulated, and
the seminiferous tubules were teased apart. Seminiferous tubules from groups I
and II were transferred
to HEPES buffered culture medium (D-MEM) (Gibco-BRL, Life Technologies,
Gaithersberg, MD
20884) containing lmg/ml Bovine serum albumin (BSA) (Sigma, St. Louis, MO
63178) and
Collagenase Type I (Sigma) for the removal of interstitial cells. After a 10
minute incubation at 33~C,
the tubules were lifted into fresh culture medium. This enzymatic digestion
was not carried out on the
testes from group I because of their fragility.
The tubules from group II and III mice or the whole tissue from group I mice
were transferred
to a Petri dish with culture medium and were cut into 0.1-lmm pieces using a
sterile scalpel and
needle. The minced tissue was centrifuged at 500 x g for 5 minutes and the
pellet was resuspended in
lml of enzyme mix. The enzyme mix was made up in D-DMEM with HEPES (Gibco-BRL)
and
consisted of lmg/ml bovine serum albumin (BSA) (Sigma, embryo tested), lmg/ml
collagenase I
(Sigma) and 5 mg/ml bovine pancreatic trypsin (Sigma) and O.lmg/ml
deoxyribonuclease I (DN-EP,
Sigma). The tubules were incubated in enzyme mix for 30 minutes at 33 ~C.
After the incubation, lml
of medium was added to the mix and the cells were centrifuged at 500 x g for 5
min. The cells were
washed twice in medium by centrifugation and resuspension. After the final
wash the cell pellet was
resuspended in 250~c1 of culture medium and counted.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
33
Example 12: Transferring Transfected Male Germ Cells Into Recipient Testis
The cells were injected into the testis via the vasa efferentia using a
micropipette. 3 x 105 cells
in a total volume of SO,uI were used for the injection. The cells were mixed
with Trypan blue prior
to the injection. The recipient mice were anesthetized with 0.017mL/g body wt.
Avertin. An incision
was made across the lower abdominal wall and the testis was gently pulled to
the exterior through the
incision by pulling on the fat pad associated with the testis. The vas
efferens was exposed and
approximately 20 ,uL of cell suspension was injected into the vas efferens
using a glass micropipette
held in a steel micropipette holder (Leitz). The cells were expelled from the
pipette using air pressure
from a 20 mL glass syringe. Prior to the transfer of transfected germ cells to
the recipient animals, the
recipient testes were depopulated of endogenous male germ cells.
Example 13: Depopulating the Recipient Testis of Male Germ Cells.
Comparison of Depopulating Treatments. Eight-week-old C57BL/6J mice were
allowed to acclimatize
for a few days and then were assigned to one of the following three treatment
groups. They received:
(1) 400 Rad gamma irradiation; (2) 4 /.cG/g body weight of busulfan (1,4-
butanediol
dimethanesulphonate; Myleran, Glaxo Wellcome); or (3) a combination treatment
of busulfan (4~g/g
body wt) followed one week later by 400 Rad of gamma irradiation
("busulfan/400 Rad" treatment).
A fourth group of untreated C57BL/6J mice of the same age as the treatment
groups was used as a
control. There were 24 mice in each treatment group, and 3 mice were mice
sacrificed at each of the
following time intervals after treatment: 5 hours, 24 hours, 48 hours, 72
hours, 1 week, 2 weeks, 1
month and 2 months after treatment.
In addition, other C57BL/6J mice receiving the combined busulfan/400 Rad
treatment were
examined histologically at time points up to five months after treatment (the
testes of these other mice
were fixed overnight in 4% paraformaldehyde in PBS, pH 7.4 at 4°C,
dehydrated and embedded in
paraffin before sectioning and H&E staining).
Delivery of an Alkylating Agent to Recipient Vertebrates. The male mice
receiving busulfan received
a dose of 4 ~cg busulfan per g body wt. The busulfan was first dissolved
8mg/mL in 100% dimethyl
sulfoxide (DMSO) then, immediately before injection, was diluted 1:1 in
phosphate buffered saline,
pH 7.4. The mice were injected with the diluted busulfan solution
intraperitoneally.
Irradiation Treatment of Recipient Vertebrates. For the gamma irradiation
treatment, mice were
anesthetized with 0.017 mL/g body wt. of 2.5% Avertin. Gamma irradiation was
specifically directed
to the testis in the following manner. Each mouse was placed in a lead chamber
with only the testis
and lower abdomen exposed through elliptical holes to the irradiating source
('3'Cs Gammacell 40
irradiator [Nordion] ). There were six aligned holes in the floor and roof of
the chamber through which
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
34
the gamma radiation passed unobstructed. After irradiation the animals were
allowed to recover from
the anesthesia on a warm heating pad or water bed until they regained
consciousness.
Histolo~v. At selected time points, mice from each treatment group were
euthanized, and testicular
tissues to be examined were fixed in 10% formalin in PBS, pH 7.4, at
4°C for 24 hours. Small slits
in the testis capsule were made to allow penetration of the fixative. Fixed
samples were washed four
times with PBS, and embedded in paraffin using a Tissue Tek-II tissue
processor (MET). Sections of
8 ~m thickness were cut, stained with haemotoxylin and eosin (H&E), and
mounted with Aquamount
(Lerner Laboratories) on glass slides with coverslips. The sections were
viewed on a Zeiss or Olympic
light microscope with a 40X objective lens (total magnification 400X).
Quantitative Histolo~ic Analysis. Quantitative data were collected from the
testes of two animals for
each of the treatment groups at two months after treatment. (Table 2). For the
control group only one
mouse was used. The seminiferous tubules in a single section were counted
using a SX objective on
a Zeiss light microscope (SOx total magnification). Individual seminiferous
tubules were examined
at 400X total magnification. Seminiferous tubules were considered severely
damaged if hardly any
cells remained in the tubule, and the tubule consisted of a basement membrane
with a single layer of
cells, mostly spermatogonia, lying along the basement membrane. Moderately
damaged tubules were
tubules, in which some of the spermatogenic layers close to the lumen were
partially sloughed off.
Spermatozoan heads were counted in the tubules and averaged over the total
number of tubules
counted.
Results of Histological Analysis. Obvious histological changes were not seen
in the testis until two
weeks after treatment. (Data no shown). After two weeks changes included
severe disruption of
spermatogenesis; all the mature spermatozoa were lost and no spermatids or
spermatocytes were
present. A few Sertoli cell nuclei and spermatogonia were detectable in the
periphery along the
basement membrane. Six weeks after busulfan/400 Rad treatment there was
evidence of the re-
establishment of spermatogenesis. Some spermatids and spermatozoa were seen as
well as a few
spermatocytes.
By about 3 months most of the seminiferous tubules had at least partially
recovered and all
stages of spermatogenesis appear to be represented. (Data not shown).
Spermatogenesis had returned
to normal at this stage by five months after busulfan/400 Rad treatment.
The three treatment groups described above were also compared. The most
dramatic
differences among the groups were seen at two months after treatment. At two
months the mice that
were treated with the combined busulfan/400 Rad gamma irradiation treatment
showed the greatest
number of substantially depopulated seminiferous tubules. Seminiferous tubules
from this group also
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
contained a smaller average number of sperm heads per seminiferous tubule and
the greatest
proportion of severely and moderately damaged seminiferous tubules compared to
the other treatment
groups and the control mice. (Table 2). Treatment of the mice with either 400
Rad gamma irradiation
or busulfan alone also resulted in damage to the spermatogenic process,
including sloughing of cells
5 into the lumen of the tubule, and substantially fewer mature spermatozoan
heads compared to the
controls, but to a significantly lesser extent than exemplified by the
busulfan/400 Rad treatment group.
These results clearly demonstrate that a combination of treatment with an
alkylating agent and
gamma irradiation is a more effective method of depopulating a vertebrate
testis of male germ cells
than either of the two treatments alone.
10 Table 2. Comparison of Various Methods of Depopulating a Vertebrate Testis
of Male Germ Cells.
No. TubulesNo. SeverelyNo. ModeratelyTotal No. Average
Sperm
TreatmentCounted damaged damaged heads No.
sperm
heads/tu
bule
Control 50 0 0 1733 in 50 35
tubules
Busulfan/50 15 (30%) 3 (6%) 460 in 50 9
tubules
400 R
15 Busulfan/57 14 (24%) 8 (14%) 383 in 50 8
tubules
4008
Busulfan70 1 (1%) 5 (7%) 957 in 50 19
tubules
Busulfan69 3 (10%) 2 (3%) 764 in 50 15
tubules
4008 52 2 (4%) 1 (2%) 1005 in 50 20
tubules
20 4008 41 2 (5%) 3 (7%) 827 in 41 20
tubules
Example 14: In vivo Transduction Using a Viral Vector
A retroviral vector was used to transduce (genetically alter or modify) male
germ cells of mice
in vivo. Specifically, a pseudo-typed HIV-derived viral vector (L. Naldini et
al., In vivo gene delivery
and stable transduction of nondividing cells by a Lentiviral vector, Science
272:263-67 [ 1996]), was
25 used, as modified by Carlos Lois to express Green Flourescent Protein (GFP)
instead of the LacZ
reporter gene, under the transcriptional control of the CMV promoter (HR'GFP).
Recipient C57BL/6J mice were treated with busulfan 44 days prior to viral
infection.
C57BL/6J male mice were injected intraperitoneally with 0.1 ml busulfan at a
concentration of 1
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
36
mg/ml. The dose was 4 ~cg busulfan/gm body wt. One pretreated mouse was
anesthetized with
Avertin (0.017m1s/gm body wt.), and a ventral midline incision was made and
the right testis exposed.
The vas efferentia were dissected away from the fat, and ten microlitres of
HIV-derived GFP
vector, HR' GFP, at a titer of 1 x 109 particles/ml were inj ected into the
seminiferous tubules of the right
testis via the vas efferens of a busulfan-treated C57 BL/6J mouse. Injection
was done with a quartz
glass micropipette attached to a Picospritzer II. The Picospritzer was set at
80psi and gave 1 second
bursts upon manual depression of a foot pedal. All the seminiferous tubules of
the testis can be
reached with a single injection as the vas efferens leads to a common chamber,
the rete testis, from
which all the tubules radiate. The left testis was not injected and was used
as a control. Transduction
of the testicular cells within the tubules was widespread.
Twenty one days after infection, the mouse was sacrificed and the testes were
fixed overnight
in 4% paraformaldehyde in PBS, pH 7.4 at 4°C. The testes were washed
three times in PBS and
placed in 20% sucrose overnight at 4°C. The testes were frozen in OCT
and sectioned at 8~m on a
cryostat. The sections were thawed to room temperature immersed in phosphate
saline buffer and
viewed on a Zeiss 310 confocal microscope. The laser was set at a wavelength
of 488 nm.
Green fluorescence was seen in all the seminiferous tubules that were viewed,
although the
intensity was greatest in the tubules at the surface of the testis.
Transduction was seen in the Sertoli
support cells as well as in the spermatogonia along the basement membrane, but
little was seen in the
spermatocytes or spermatids. Very few mature spermatozoa were present due to
the Busulfan
treatment. No fluorescence was seen in the left testes used as control. This
shows that male germ
cells can be transduced by a viral vector and that the transduced gene is
expressed.
Example 15: Optimization Microiniection of Gene Delivery Mixture through the
Vas Efferens.
The method of delivery (Winston, R.M.L., Microsurgical reanastomosis of the
rabbit oviduct and its
functional and pathological sequelae, Brit. J. Obstet. Gynaecol. 82:513 - 522
([1975]) of viral
particles into a single vas efferens, and thence to the seminiferous tubules,
was first optimized in
several mice (Figs la-ld). The mice were treated with busulfan (Myleran: Glaxo
Wellcome) 14 days
before microsurgery to maximize the chance of viral particles gaining access
to spermatogonia, which
lie on the basement lamella of the tubules. Busulfan, an alkylating cytotoxic
agent, depopulates the
testis (Bucci, L. and Meistrich, M., Effects of busulfan on murine
spermatogenesis: cytotoxicity,
sterility, sperm abnormalities, and dominant lethal mutations, Mut. Res.
176:259-268 [1987]). At
the intraperitoneal (IP) dose given (4pg/g body wt.) many of the
spermatocytes, spermatids and
spermatozoa were eliminated from the tubules, but the testis recovered three
to four months afterward
and fertility was restored. This implies that stem cells remain viable and can
repopulate the testis.
Stem cell spermatogonia are known to be resistant to insults, often surviving
when other germ cell
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
37
types are destroyed (Huckins, C. & Oakberg, W.F., Morphological and
quantitative analysis of
spernaatogonia in mouse testes using whole mounted seminiferous tubules. II.
The irradiated testes,
Anat. Rec. 192:529-42 [1978]).
Example 16: Production of Trans~enic Pro e~ny by In Vivo Transduction of Male
Germ Cells
Followed ~ Natural Mating
Microsurgery. After depopulation of the testis as described in Example 15,
viral particles were
delivered to the seminiferous tubules as follows: Mice were anaesthetised with
isofluorane (0.5-2%
in oxygen). Each testis was exposed through a midline abdominal incision.
Using a microsurgical
approach (Winston [1975]; Zeiss microscope at magnification 4 to 50x) the
tissue bundle containing
the vasa efferentia was visualised (Fig 1 a-1 b). Dissection from the
surrounding fat was aided by a
stream of phosphate buffered saline forced through a fine needle. A quartz
glass micropipette was
back-filled with 10 ~L viral particles ( 109pfu/ml) mixed with 1 ,uL polybrene
(80mg/mL). This was
attached to a micropipette (Eppendorf) and the particles introduced into the
vas efferens under 2.2 bar
pressure in pulses of 1.5 seconds, controlled by foot pedal. Earlier trials
using 1 % Bromophenol dye
showed that most seminiferous tubules could be filled (Fig. lc), but during
treatments, no dye was
used and small air bubbles were introduced into the liquid containing viral
particles to confirm
dispersion into the seminiferous tubules (Fig. I d). To preserve fertility,
only single vasa efferentia
were injected, reducing injury to the remaining ducts.
Preparation of the Viral Vector. The plasmid, pHR'-CMVLacZ (L. Naldini et al.
[1996]), was
modified by replacing the BamHI - XhoI fragment containing the LacZ gene with
a fragment
containing the EGFP gene ( 'humanised' GFP, Clontech). For the production of
viral particles
40 ,ug plasmid DNA was used to transfect a 10-cm plate of 293T cells. The 40
~g of plasmid
DNA was made up of 10 ~cg pCMV R9, 201sg of modified pHR' and 10 pg envelope
plasmid.
Vesicular-stomatitis-virus-glycoprotein (VSV-G) pseudotyped vectors were
produced by
contransfection of the vector plasmid with the Moloney murine leukemia virus
(MLV) gag-pol
packaging plasmid pCMV-GAGPOL and the VSV-G plasmid. The supernatant was
harvested
48-60 hours after transfection, subjected to high speed centrifugation,
filtered through 0.45 ~tm
filters and assayed. The transducing viral particles had the MLV restricted
envelope protein,
env, substituted with a broad-spectrum env protein from the vesicular
stomatitis virus.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
38
In Vivo Transduction of Male Germ Cells. Six mice were now treated with viral
particles
containing the transducing vector pHR' (109particles/mL). A single vas
efferens was injected
with a volume of lOpL retroviral concentrate together with 1pL (80mg/mL)
polybrene. After 24
days the mice were sacrificed and the testes removed and fixed for
cryosectioning and histological
examination. Testes were fixed for 48 hours in 4% Paraformaldehyde pH 7.4, and
placed in 20%
sucrose in phosphate saline buffer pH 7.4 at 4°C for 24 hours. They
were embedded in OCT and
stored at-70°C. They were cryosectioned at 8pm and viewed in a Zeiss
410 confocal microscope
(Fig. 2).
Nearly all tubules sectioned contained cells expressing GFP. Expression was
highest in
Sertoli and spermatogonia cells (Fig. 2a -b).
Natural Matings with Females after Transduction of Male Germ Cells. Eleven
C57B 1/6J young
males were then selected to test whether transduced male germ cells could
transmit the
retrovirally integrated transgene to the next generation. Six of these mice
were treated with a
bolus of busulfan (IP; 4pg/gm body wt.) 14 days before in vivo transduction
microsurgery in
accordance with the in vivo method of incorporating exogenous genetic material
into the genome
of a vertebrate, as described above, and three received the same dose only one
week before in
vivo transduction. Two other mice were not pre-treated with busulfan before
the in vivo
transduction operation. Lentiviral particles were introduced into the
seminiferous tubules. After
14 weeks, B6D2F1 females were introduced into cages with the males. Transduced
males
fathered at least two successive litters. Litters were conceived 14, 15, 19
and 20 weeks after
transduction. All the males, except one dying immediately after surgery,
fathered transgenic
offspring. (Table 3).
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
39
Table 3. Production of transgenic offspring per litter fathered by treated
males at various times
after mating.
Mouse Pre-treatment14 weeks15 weeks 19 weeks20 weeks
#
1 Busulfan 1 2/9 (22%)8/10 0/9 (0%)
week (90%)
2 Busulfan 1 - 1/7 (14%)1/7 (14%)2/7 (28%)
week
3 Busulfan 1 4/7 1/8 (12%)4/6 (66%)0/7 (0%)
week (57%)
4 Busulfan 1 7/8 3/7 (43%)1/6 (17%)1/8 (12%)
week (87%)
5 Busulfan 2 5/6 8/9 (89%)- 0/8 (0%)
weeks (83%)
6 Busulfan 2 - 2/8 (25%)8/8 (100%)1/9 (11%)
weeks
7* Busulfan 2 - - - -
weeks
8 Busulfan 2 - 6/6 (100%)- 1/8 (12%)
weeks
9 Busulfan 2 - 8/8 (100%)- 3/10
weeks (30%)
10** none 2/5 5/6 (83%)- -
(40%)
11 none 3/7 7/8 (88%)- 0/6 (0%)
(43%)
*Mouse No. 7 died immediately after surgery;
**Mouse No. 10 died 17 weeks after surgery.
PCR and Southern blot analysis of DNA from embryonic offspring. Embryos at
approximately
embryonic day 12.5, were screened for presence of the transgene by polymerase
chain reaction
(PCR) and Southern blot analysis. For the PCR, GFP specific primers were used
and a
radiolabeled GFP cDNA probe was used for the Southern blot analysis (Fig. 3).
DNA was
purified from embryos using the Gentra purification system. The presence of
the transgene was
ascertained using PCR amplification with the following GFP specific primers:
(A) forward primer: 5'-GGT GAG CAA GGG CGA GGA GCT-3' (SEQ. ID. NO.:1)
(B) reverse primer: 5'-TCG GGC ATG GCG GAC TTG AAG A-3' (SEQ. ID. N0.:2)
The PCR cycling conditions were: denaturing 94°C for 1 minute,
annealing at 60°C for 1 minute
and extension at 72°C for 3 minutes. PCR ran for 35 cycles and yielded
a specific GFP product
470 base pairs in length. Each cycle step can be reduced to one second - "one
second PCR" to .
yield a distinct 470-by PCR amplification product. Southern blot analysis was
also done on the
same embryo DNA extracts. The DNA was cut with BamHI-XhoI, run on a 0.8%
agarose gel and
blotted overnight in 20x SSC onto Hydrobond XL paper. The blots were
hybridised overnight
at 65°C with a 32P-radiolabeled BamHI-XhoI GFP fragment isolated from
the pHR'plasmid. The
blots were washed at 65°C (30 minutes) each in 2x SSC with 0.1 % SDS,
lx SSC with 0.1 % SDS,
0.1 X SSC with 0.1% SDS and exposed to X-ray film.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
PCR and Southern analysis showed that a high percentage of transgenic
offspring were
obtained in litters conceived within 15 weeks. The results are summarized in
Table 3. By 20
weeks the percentage of transgenic progeny had dropped in all of the treatment
groups, implying
that the self-renewing spermatogonia were not transduced, but rather a
population of
5 differentiating spermatogonia. Once the daughter cells from this population
had matured and left
the testis they were not renewed (Huckins, C. & Oakberg, W.F. [1978]). In
Table 3, the ratios
are the number of transgenic offspring out of the total number of embryos in
the litter.
Although pre-treatment with busulfan enhanced the transduction of
spermatogonia, mice
untreated with busulfan also generated transgenic offspring. Male germ cells
take 60 days to
10 differentiate from spermatogonia (Russell, L.D., et al. In: Histological
and Histopathological
evaluation of the testis, Cache River Press [1990]), undergo meiosis and form
spermatozoa.
Since conception was more than 60 days after transduction, it is presumed that
the transgenic
offspring were conceived from differentiated daughter cells of transduced
spermatogonia. EGFP
expression was driven by the CMV promoter and was evident in the testicular
cells of the founder
15 males 24 days after infection. The animals that were infected did not
appear to have toxic side
effects (Verma, LM. and Somia, N. [1997]) with the possible exception of one
dying 17 weeks
after surgery.
The foregoing examples being illustrative but not an exhaustive description of
the
embodiments of the present invention, the following claims are presented.
CA 02368620 2001-11-13
WO 00/69257 PCT/US00/13000
SEQUENCE LISTING
<110> Cedars-Sinai Medical Center;
Imperial College Innovations Ltd.
<120> Genetic Modification of Male Germ Cells
for Generation of Transgenic Species & Genetic Therapies
<130> CEDAR-44904
<140> NOT ASSIGNED
<141> 2000-05-12
<150> US 09/311,599
<151> 1999-05-13
<160> 2
<170> FastSEQ for tnlindows Version 4.0
<210> 1
<211> 21
<212> DNA
<213> Artificial Sequence
<220>
<223> Specific primer for green fluorescent protein
(GFP)
<400> 1
ggtgagcaag ggcgaggagc t 21
<210> 2
<211> 22
<212> DNA
<213> Artificial Sequence
<220>
<223> Specific primer for green fluorescent protein
(GFP)
<400> 2
tcgggcatgg cggacttgaa ga 22
- 1 -