Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
1
INTERACTION-ACTIVATED PROTEINS
GOVERNMENT LICENSE RIGHTS
The U. S. government has a paid-up license in this invention and the right in
limited
circumstances to require the patent owner to license others on reasonable
terms as provided
for by the terms of grant No. awarded by
to INTRODUCTION
Technical Field
The present invention is concerned with detecting interactions between
proteins by
expressing them as part of a fusion sequence that also encodes for one
fragment of a
fragment pair that reassembles into a directly detectable protein. The
interaction-dependent
is enzyme association (IdEA) systems of the present invention are exemplified
by the bacterial
(3-lactamases, a large group of structurally-related enzymes which segregate
into several
groups on the basis of structural homologies and substrate specificities.
Background
20 Most physiological processes depend on complex networks of cells
interacting with
one another and their environments, primarily through specific recognition
between
proteins - from the ligand-mediated assembly of multi-protein complexes at the
cell
surface, through the labyrinth of intracellular signal transduction cascades,
to the assembly
of transcription-modulating complexes on the promoters of specific genes.
Thus, for most
25 pathological conditions, protein-protein interactions are instrumental and
provide a wealth
of targets for diagnostic and therapeutic intervention. As a result, new and
improved
methods are in constant demand for (1) identifying natural ligands of key
participants to
study their roles in disease, and (2) developing surrogate ligands for
therapeutic
intervention and diagnosis. A number of methods have been developed over the
years to
3o address each of these goals. The most widely used current methods for
identifying natural
proteins which interact with a protein-of interest generally involve screening
libraries of
expressed cDNAs. A few genes for ligands of proteins-of interest have been
isolated by
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
2
direct screening of cDNA expression libraries on filters for binding to
labeled versions of
the protein-of interest, as in antibody probing (Blackwood and Eisenman,
Science (1991)
251:1211; Defeo-Jones et al., Nature (1991) 352:251). However, a great many
important
protein interactions are not robust enough for the harshness of such methods,
where
conditions of interaction are usually far from native. Also, the false
positive frequencies of
these methods is high, due to the presence of denatured protein in cells which
have been
fixed to make the target proteins accessible to probes.
A major advance in cDNA screening methodology came with the development of
systems in which screenable or selectable cellular phenotypes could be
engineered to
1o depend on desired protein interactions within living cells (Fields and Song
Nature (1989)
340:245; Chien et al., Proc Natl Acad Sci (1991) 88:9578; Zervos et al., Cell
(1993)
72:223; Vojtek et al., Cell (1993) 74:205; and Luban et al., Cell (1993)
73:1067). The
most widely used of these is the yeast "two hybrid" system of Fields and Song
(1989,
supra). This system takes advantage of the "modularity" of many functional
domains in
proteins which allows the linking of functions to be manipulated. This is
particularly true
for transcriptional activators, in which an activation domain which interacts
with the core
transcription complex is "homed" to specific genes by a sequence-specific DNA-
binding
domain. For many transcriptional activators these domains can function
independently,
and in fact are often in separate, interacting subunits. In the yeast two-
hybrid system, the
"bait" protein is expressed as a fusion with a cis-element sequence-specific
DNA-binding
domain, and cDNAs are expressed as fusions with a transactivation domain.
When, and
only when, these two domains are brought together by interaction of a cDNA
product with
the "bait" protein, can the reporter gene be expressed, since its
transcription is dependent
on transactivation from the cis-element. Reporters can be either screenable
(e.g.,
(3-galactosidase for color) or selectable (e.g., HIS3 for growth in the
absence of histidine).
Variations of this system have been successfully employed to identify a number
of
important protein-protein interactions (Chien et al., 1991, supra; Zervos et
al., 1993,
supra; Vojtek et al., 1993, supra; and Luban et al., 1993, supra; Bartel et
al., Nature
Genetics (1996) 2:72; Fromont-Racine et al., Nature Genetics (1997) 3:277; Xu
et al.,
3o Proc Natl Acad Sci (1997) 94:12473). In spite of its success, however, the
original yeast
two-hybrid system has serious drawbacks for the high-throughput applications
required to
accelerate pharmaceutical target discovery from genomics. The fundamental
limitation with
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
3
this system is that many steps are required between the test interaction and
the generation
of a selectable phenotype. Each such step presents an opportunity for non-
specific
interaction to raise the false positive background, and for dissociation to
allow bona fide
interactors to be missed. The false positive problem is exacerbated by the
highly
combinatorial nature of the transcription machinery and the abundance of
protein domains
encoded in cDNA libraries which can interact with one or more components of
the
transcription initiation complex, including transactivator-bound promoter DNA
(Bartel
et al., BioTechniques (1993) 14:920). Another limitation of the original two-
hybrid system
is that it generally cannot accommodate secreted or membrane proteins and
cytoplasmic
proteins must be stable in the yeast nucleus.
Recently the two-hybrid concept has been expanded to include other types of
protein
functionalities for use as protein-protein interaction reporting systems. For
example, in the
Selective Infective Phage (SIP) system a protein which confers infectivity on
filamentous
bacteriophage has been fragmented in such a way that it is functional only
when the
fragments are fused to heterologous interactors (Krebber et al., J Mol Biol
(1997)
268:607). The interaction is then monitored by its ability to allow phage
encoding the
interactors to transfer a selectable phenotype to susceptible cells by
infection. However,
this method also suffers from requiring many low-efficiency steps between the
target
interaction and the expression of the selectable phenotype by the recipient
cell. Also like
the two-hybrid system, the efficiency of this system suffers from the fact
that most natural
protein-protein interactions have affinities in the micromolar range, with
half lifes on the
order of seconds. When the time delay between interaction and signal
generation exceeds
this half life, which it does in these systems, the efficiency of interaction
detection declines
sharply.
More recently still, the two-hybrid concept has been adapted to proteins which
can
confer selectable phenotypes directly from protein-protein interactions, with
few or no
intervening steps between the target interaction and signal generation. For
example,
interactors can be fused to variants of the Green Fluorescent Protein of
Aequorea victoria
(GFP), which are capable of detectable fluorescence resonance energy transfer
(FRET)
when brought into close proximity by the interactors (Cubitt et al., Trends
Biochem (1995)
20:448). Some enzymes which confer selectable or screenable phenotypes on
cells can also
be adapted for two-hybrid type protein-protein interaction detection (Rossi et
al., Proc Natl
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
4
Acad Sci (1997) 94:8405; Pelletier et al., Proc Natl Acad Sci (1998)
95:12141). In this
variation, protein interactors are fused to enzyme fragments, which by
themselves are
inactive. However, when the enzyme fragments are brought together by the
interaction of
the protein domains to which they are fused, the fragments are able to
associate to
reconstitute the selectable activity of the enzyme. This is an example of
interaction-
dependent enzyme activation (IdEA), and it is illustrated in Figure 1. Both
IdEA and GFP
FRET systems present advantages over previous versions of the two-hybrid
concept. For
instance, the selectable signal is produced directly from the desired
interaction, without any
intervening steps which are the main sources of inefficiency in the earlier
systems. Such
l0 improvements in efficiency and background should make these methods more
amenable to
high-throughput applications. However, although both IdEA and GFP FRET systems
in
theory can be set up in both prokaryotic and eukaryotic cells, and either in
the cytoplasm
or in a secretory pathway to allow interactions to be monitored in natural
milieus, they
have not. All IdEA systems reported to date have only utilized cytoplasmic
enzymes and
have only been shown to be operative in that compartment (Rossi et al., 1997,
supra; Pelletier
et al., 1998, supra; Karimova et al., Proc Natl Acad Sci (1998) 95:5752).
Indeed, because of
their design, these reported systems would not be expected to function in the
secretory
pathway or in the bacterial periplasm. Thus, they are not considered useful
for monitoring
the interactions of secreted proteins.
The most widely used current systems for the detection of extra-cellular
protein-
protein interactions, namely viral or cellular display systems, are
essentially in vitro methods
with high stringencies of selection and/or high backgrounds. Thus, they are
not well suited for
high-throughput applications. These systems also usually require the use of a
purified known
heterologous interactor domain or "bait protein'', and are therefore not
suitable for multiplex
applications where neither heterologous interactor domain of a protein binding
pair is known
a priori, i.e., the combinatorial interaction of two protein libraries with
one another for
simultaneous identification of all protein binding pair interactions. One
system which does
not require bait purification for identification of extra-cellular
interactions is the E. coli Dimer
Detection System (EDDS; Small Molecule Therapeutics, Inc., Monmouth Junction,
NJ). Bait
proteins for this system are restricted to type I membrane receptors which
have single
transmembrane domains and require simple dimerization for signaling. The ecto-
domain of
the bait receptor is fused to the transmembrane domain and endo-domain of an
E. coli
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
receptor. When this fusion protein is co-expressed with an expression library
in the bacterial
periplasm, ligands for the receptor can be identified by their ability to
dimerize the receptor
and induce expression of a selectable phenotype. However, this system suffers
from the same
limitation as the yeast two-hybrid and SIP systems, namely, that multiple
steps between
interaction and phenotype cause severe loss of efficiency due to high false
positive and false
negative rates.
It is therefore of interest to develop IdEA systems capable of simultaneous
detection
of multiple interactions between extra-cellular as well as intracellular
proteins in a high
throughput format.
Relevant Literature
USPN 5,585,245 discloses a ubiquitin-based protein sensor complementation
system
where binding of two predetermined proteins of a binding pair is detected as
specific
proteolysis of ubiquitin by ubiquitinases. PCT publication WO 98/44350
discloses a
reporter subunit complementation system employing fusion proteins of (3-
galactosidase
subunits. PC.T publication WO 98/34120 discloses a protein fragment
complementation
system employing dihydrofolate reductase.
SUMMARY
2o Compositions and methods are provided for identifying interactions between
polypeptides using an interaction-dependent protein association system. The
system is
characterized by using fragment pairs comprised of a first and a second member
that
functionally reassemble into a marker protein having a directly detectable
signal, such as a
visible phenotypic change or antibiotic resistance. The fragment
complementation system
of the present invention involves co-expression in a host cell of a first and
a second
oligopeptide, where each is a fusion protein separated by a flexible
polypeptide linker with
a member of the marker protein fragment pair. Binding of the first
oligopeptide to the
second oligopeptide results in the functional reconstitution of the fragment
pair into a
marker protein, and the interacting first and second oligopeptides are
identified by isolating
3o and sequencing plasmids from a host cell that displays a directly
detectable signal indicative
of the marker protein. Functional reconstitution of the fragment pairs into a
marker protein
can be enhanced by including elements such as a cysteine residue or a randomly
encoded
CA 02374476 2001-11-19
WO 00/71702 PCT/US00l07108
6
peptide of from 3-12 amino acids at or near the break-point termini of the
fragment pair
member, or by introducing 1-3 codon changes within the nucleotide sequence
encoding for
a member of a fragment pair. The invention also provides for efficient methods
of fording
functional fragment pairs of a marker protein that involve identifying
functional break-
s points within flexible loops using tertiary or secondary structural
information. The
interaction-dependent protein activation systems of the present invention ford
particular use
in identifying immunoglobulin epitopes, polypeptide sequences that bind to
extracellular
proteins, and inhibitors of phophorylation-regulated signal transducer
proteins. The
systems also find use in allowing single antibiotic selection of cells
transformed to express
1o genes for multiple traits and for targeted and localized activation of
derivitized anti-tumor
prodrugs.
BRIEF DESCRIPTION OF THE DRAWINGS
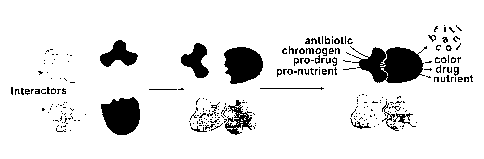
Figure 1. Mechanism for Interaction-dependent Enzyme Activation (IdEA).
Interaction-
15 dependent fragment complementation requires enzyme a and cu, fragments
which can refold
to form active enzyme when and only when they are brought together by an
interaction of
heterologous domains fused to their termini.
Figure 2. Nucleotide coding sequence for the mature form of TEM-1 (3-lactamase
and the
2o encoded amino acid sequence (Sutcliffe, Proc Natl Acad Sci (1978) 75:3737).
From the
sequence for plasmid pBR322 (SYNPBR322), Genbank accession no. J01749. The
break-
points between the a and w fragments at residues Asn52/Ser53, G1u63/G1u64,
G1n99/Asn100, Prol74/Asn175, Glul97/Leul98, Lys215/Va1216, A1a227/G1y228 and
G1y253/Lys254 are indicated.
Figure 3. Three-dimensional structure of mature TEM-1 (3-lactamase. Rendering
of the
x-ray crystal structure of Jelsch et al. (Proteins Struct Funct (1993)
16:364ff), using red
and blue solid ribbons to show a-helix and ~-sheet, respectively. The molecule
is oriented
to emphasize the two-domain structure (a-w and ~c). The active site
nucleophile, Ser70, is
3o shown as a ball-and-stick model.
Figure 4. Three-dimensional representation of interaction-dependent activation
of
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
7
(3-lactamase by fragment complementation. Docking of TEM-1 a 197 and cu 198
fragments
by the interaction of the hetero-dimerizing helixes from the fos and jun
subunits of the
AP-1 transcription activator allows re-folding of the fragments into the
active conformation
of the enzyme (compare with Figure 3).
Figure 5. Structures of some anti-cancer drugs and their cephalosporin
prodrugs.
YW-200 and YW-285 are a DNA-binding tri-indole and its cephalosporin prodrug
(Wang
et al., 1998, US Patent 5,843,937)
1o Figure 6. Vectors and strategy for the expression of heterologous proteins
as fusions to
the a 197 and c~ 198 fragments of TEM-1 (3-lactamase for interaction-dependent
(3-lactamase
activation by fragment complementation. Vector pA01 is a high-copy pUC119-
based
phagemid for expression of w 198 fusions and free ligands from dicistronic
transcripts,
which can be rescued as phage for quantitative introduction into host cells by
high-
.multiplicity infection. Vector pAEl is a low-copy plSA replicon with a strong
promoter
for expression of a 197 fusions at comparable or higher levels than expression
from the
pA01 vector. Trxpeps are 12-mer peptides inserted into the active site of
thioredoxin.
Tripep-trx libraries are random tri-peptides at the N-terminus of thioredoxin
with an
intervening Gly4Ser linker. ScFv, single-chain antibody Fv fragment. LC-CH1,
antibody
2o fragment composed of light chain and first constant region of heavy chain.
VL, antibody
light chain variable region. lac prom, lactose operon promoter. SP, signal
peptide.
(Gly4Ser)3, flexible 15-mer linker. pUC ori, plSA ori, plasmid origins of
replication. fl
ori, filamentous phage origin of replication. cat, chloramphenicol resistance
gene. m.o.i.,
multiplicity of infection. trc prom, fusion promoter from tryptophan and
lactose operons.
2s tt, transcription terminator. kan, kanamycin resistance gene. Vector sizes
in base pairs
(bp) do not include interactors.
Figure 7. TEM-1 (3-lactamase fragment complementation by interaction between
representative single-chain antibody Fv fragment (scFv) and thioredoxin-
scaffolded peptide
3o (Trx). The N-terminal ~3-lactamase fragment, a197 (a), is colored red. The
C-terminal
fragment, w198 (w), is colored blue. TEM-1, thioredoxin, and the scFv were
rendered
from published structures. The peptide and the linkers were drawn in.
WO 00/71702 CA 02374476 2001-11-19 pCT/US00/07108
8
Figure 8. TEM-1 (3-lactamase fragment complementation by interaction between
the CD40
extra-cellular domain (CD40) and a thioredoxin-scaffolded peptide (Trx). The N-
terminal
(3-lactamase fragment, x,197 (a,), is colored red. The C-terminal fragment, cu
198 (w), is
colored blue. TEM-l, thioredoxin, and the scFv were rendered from published
structures.
The peptide and the linkers were drawn in.
Figure 9. Vectors and protocol for construction of a multiplex protein-protein
interaction
library using interaction-dependent (3-lactamase fragment complementation
systems.
1o Expressed sequence (ES), i.e., random-primed cDNA libraries, are subcloned
into
phagemid vectors for expression as fusions to the (3-lactamase a and cu
fragments, via the
flexible linker (Gly4Ser)3. The vectors encode a peptide epitope tag, such as
the 12-residue
Myc tag, at the C-terminus of the ES. When co-expressed with anti-Tag scFv,
such as anti-
myc 9E10, fused to the other fragment, the ES libraries can be selected for ~3-
lactamase
activity driven by the Tag-anti-Tag interaction, which will require stable
expression of the
ES fragment. The resultant libraries, enriched for stable expressors of
autonomously
folding domains (AFD), may then be rescued as phage and co-infected into male
cells for
selection of interacting AFD pairs (Multiplex Interaction Library). The AFD
libraries can
also be co-infected with scFv libraries, antibody light chain variable region
libraries (VL),
or peptide libraries displayed on thioredoxin (trx-peptide) for simultaneous
selection of
binding proteins for each AFD (Multiplex Antibody/Peptide Binder Selection).
See legends
to Figures 6 and 10 for identification of other abbreviations.
Figure 10. Abbreviated output of the PredictProtein Program for prediction of
secondary
structure and solvent exposure for NPTII (Rost and Sander, 1993, 1994). The
top line
shows the amino acid sequence in single letter code. The second and third
lines show
secondary structure prediction. H, helix; E, strand; L, loop. The fourth line
shows a
measure of reliability on a scale from 1 to 10, with 10 being highest. The
fifth line shows
solvent accessibility - e, exposed; b, buried. The bottom line shows a measure
of reliability
3o for solvent accessibility on a scale of 1 to 10, with 10 being highest. Ten
regions of the
sequence predicted to have little secondary structure and to be exposed to
solvent are
indicated by underlining as potential sites for productive fragmentation.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
Figure 11. Expression vectors for production of (3-laca253 and (3-lacc~254
fusion proteins
with scFv. Arrows denote translation start sites. T7 prom, bacteriophage T7
promoter;
SP, pelB signal peptide; scFv is comprised of VH (antibody heavy chain
variable region),
(Gly4Ser)3 (15-mer flexible linker), and VL (antibody light chain variable
region); kan,
kanamycin resistance; His6, hexa-histidine tag for metal ion affinity
purification; laclg,
high-affinity lac operon repressor mutant; fl ori, phage origin of
replication.
BRIEF DESCRIPTION OF THE SPECIFIC EMBODIMENTS
to Methods and compositions are provided for an interaction-dependent protein
activation system useful in detecting an interaction between a first protein
and a second
target protein. The method detects the interaction of a first known or unknown
interactor
domain with a second unknown interactor domain by bringing into close
proximity
members of a fragment pair of a marker protein, such that the parent marker
protein is
15 reassembled to its original functionality, and such that reassembly
requires the prior
interaction of.the heterologous interactor domains. The system is
characterized by N-
terminal and C-terminal fragment members that comprise fragment pairs which
are derived
from, and can functionally reassemble into a marker protein that provides for
a directly
detectable signal that does not involve downstream steps necessary for
recognition. For
20 example, a marker protein of interest for the instant invention functions
of itself to produce
a selectable signal such as a visible phenotypic change or antibiotic
resistance.
The fragment pairs are used in methods that involve the co-expression of a
first and
a second oligopeptide sequence, in which the first oligopepride sequence is a
fusion protein
comprised of in the direction of translation, an N-terminal fragment fused
through a break-
25 point terminus to a flexible polypeptide linker and a first interactor
domain, and the second
oligopeptide sequence is a fusion protein comprised of in the direction of
translation, a
second interactor domain and a flexible polypeptide linker fused through a
break-point
terminus to a C-terminal fragment. The flexible polypeptide linker separates
the fragment
domain from the interactor domain and allows for their independent folding.
The linker is
30 optimally 15 amino acids or 60 A in length ( - 4 A per residue) but may be
as long as 30
amino acids but preferably not more than 20 amino acids in length. It may be
as short as 3
amino acids in length, but more preferably is at least 6 amino acids in
length. To ensure
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
flexibility and to avoid introducing steric hindrance that may interfere with
the independent
folding of the fragment domain and the interactor domain, the linker should be
comprised
of small, preferably neutral residues such as Gly, Ala and Val, but also may
include polar
residues that have heteroatoms such as Ser and Met, and may also contain
charged
5 residues.
The first interactor domain is a known or unknown protein or protein fragment
that
binds directly or indirectly to a second target interactor domain that is an
unknown protein
or protein fragment and either or both the first and second interactor domain
can be a
member of a library. The interactor domain libraries are preferably
constructed from
to cDNA, but may also be constructed from, for example, synthetic DNA, RNA and
genomic
DNA. When combining the first and second oligopeptide sequences, the
reconstitution of
the N-terminal and C-terminal fragments into the marker protein requires the
prior
interaction of the first and second interactor domains. Bound interactor
domains are
identified by expressing a functionally reconstituted marker protein, and then
the nucleotide
sequences encoding for bound interactor domains or the bound interactor
domains
themselves are characterized by methods including electrophoresis, polymerase
chain
reaction (PCR), nucleotide and amino acid sequencing and the like.
Advantages of the present invention over previously disclosed fragment
complementation systems include a reporter protein that provides for a
directly detectable
2o signal upon reassembly, and background levels of 1 in 106 or less.
Additionally, the
invention provides for rationally incorporated enhancement modifications to
the fusion
oligopeptides that increase the functional activity of the reconstituted
protein to wild-type
levels by improving folding and reassembly of the fragments into the parent
protein, while
at the same time maintaining dependence on the interactor domains for
reassembly.
The interaction-dependent enzyme activation system of the subject invention
may be
used to detect in vitro protein interactions, such as in cell lysates, or the
interactions of
intracellular or extracellular proteins of a host cell. For evaluating
interactions between
extracellular proteins, the first and second fusion oligopeptides can be
expressed with a
signal peptide. In bacterial host cells, for example, an N-terminal signal
peptide can
3o provide for translocation of the fusion oligopeptides to the periplasm. The
combined
lengths of the N-terminal fragment and the C-terminal fragment may be
discontinuous with
residues around the break-point deleted, contiguous, or overlapping with
residues around
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
11
the break-point repeated, thereby comprising from 90 % to 110 % of the total
length of the
parent protein. Break-point termini are herein defined as the C-terminus of
the N-terminal
fragment and the N-terminus of the C-terminal fragment.
The subject invention provides for enhancing the performance of the
reassembled
parent protein by introducing at least one of the following modifications,
including: i) a
randomly-encoded peptide of 3-12 amino acids between the break-point terminus
of each
fragment and the flexible polypeptide linker, ii) a randomly-encoded peptide
of 3-12 amino
acids expressed separately as a fusion to the N-terminus of a thioredoxin with
an
intervening flexible linker, iii) a cysteine residue encoded at or within 5
amino acid
1o positions of the break-point and between the break-point terminus of each
fragment and the
flexible polypeptide linker so that a disulfide bond can form between the
members of a
fragment pair, and iv) 1-3 codon changes within a member of a fragment pair
introduced,
for example, by PCR amplification of a nucleotide sequence encoding for a
member of a
fragment pair under error-prone conditions, to enhance the folding stability
of a
functionally reconstituted marker protein.
The invention is also directed to plasmids containing expression cassettes
constructed to express fusion oligopeptides comprised of a fragment domain and
an
interactor domain. The expression cassettes for the N-terminal and C-terminal
fragment
pair members are designed with their components in different sequential
orders. For the
2o C-terminal fragment pair member, the expression cassette will comprise as
operably linked
components in the direction of transcription nucleotide sequences encoding for
(i) a
promoter functional in a host cell, (ii) a polypeptide interactor domain,
(iii) a flexible
polypeptide linker and (iv) a C-terminal fragment of a marker protein that
provides for a
directly selectable phenotype. The expression cassette for the N-terminal
fragment pair
2s member will comprise as operably linked components in the direction of
transcription
nucleotide sequences encoding for (i) a promoter functional in a host cell,
(ii) an
N-terminal fragment of a marker protein that provides for a directly
selectable phenotype.
(iii) a flexible polypeptide linker and (iv) a polypeptide interactor domain.
The invention is
also concerned with host cells that contain plasmids having the sequences of
the above-
3o described expression cassettes.
Appropriate host cells for application of the subject invention include both
eukaryotic cells, such as mammalian, yeast and plant cells, and prokaryotic
cells, such as
WO 00/71702 CA 02374476 2001-11-19 pCT~jS00/07108
12
bacterial cells. A variety of prokaryotic expression systems can be used to
express the
fusion oligopeptides of the subject invention. Expression vectors can be
constructed which
contain a promoter to direct transcription, a ribosome binding site, and a
transcriptional
terminator. Examples of regulatory regions suitable for this purpose in E.
coli are the
promoter and operator region of the E. coli tryptophan biosynthetic pathway as
described
by Yanofsky (1984) J. Bacteriol., 158:1018-1024 and the leftward promoter of
phage
lambda (P~,) as described by Herskowitz and Hagen, (1980) Ann. Rev. Genet.,
14:399-445.
Vectors used for expressing foreign genes in bacterial hosts generally will
contain a
sequence for a promoter which functions in the host cell. Plasmids useful for
transforming
to bacteria include pBR322 (Bolivar, et al, (1977) Gene 2:95-113), the pUC
plasmids
(Messing, (1983) Meth. Enzymol. 101:20-77, Vieira and Messing, (1982) Gene
19:259-
268), pCQV2 (Queen, ibid.), and derivatives thereof. Plasmids may contain both
viral and
bacterial elements. Methods for the recovery of the proteins in biologically
active form are
discussed in U.S. Patent Nos. 4,966,963 and 4,999;422, which are incorporated
herein by
reference. See Sambrook, et al (In Molecular Cloning: A Laboratory Manual,
2°d Ed.,
1989, Cold Spring Harbor Laboratory Press, Cold Spring Harbor) for a
description of
other prokaryotic expression systems.
For expression in eukaryotes, host cells for use in practicing the present
invention
include mammalian, avian, plant, insect, and fungal cells. As an example, for
plants, the
2o choice of a promoter will depend in part upon whether constitutive or
inducible expression
is desired and whether it is desirable to produce the fusion oligopeptides at
a particular
stage of plant development and/or in a particular tissue. Expression can be
targeted to a
particular location within a host plant such as seed, leaves, fruits, flowers,
and roots, by
using specific regulatory sequences, such as those described in USPN
5,463,174, USPN
4,943,674, USPN 5,106,739, USPN 5,175,095, USPN 5,420,034, USPN 5,188,958, and
USPN 5,589,379.
Where the host cell is a yeast cell, transcription and translational regions
functional
in yeast cells are provided, particularly from the host species. The
transcriptional initiation
regulatory regions can be obtained, for example from genes in the glycolytic
pathway, such
3o as alcohol dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase (GPD),
phosphoglucoisomerase, phosphoglycerate kinase, etc. or regulatable genes such
as acid
phosphatase, lactase, metallothionein, glucoamylase, etc. Any one of a number
of
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
13
regulatory sequences can be used in a particular situation, depending upon
whether
constitutive or induced transcription is desired, the particular efficiency of
the promoter in
conjunction with the open-reading frame of interest, the ability to join a
strong promoter
with a control region from a different promoter which allows for inducible
transcription,
ease of construction, and the like. Of particular interest are promoters which
are activated
in the presence of galactose. Galactose-inducible promoters (GAL1, GAL7, and
GAL10)
have been extensively utilized for high level and regulated expression of
protein in yeast
(Lue et al, (1987) Mol. Cell. Biol. 7:3446; Johnston, (1987) Microbiol. Rev.
51:458).
The invention also provides for efficient methods of identifying functional
fragment
1o pairs of a marker protein of interest that involves preparing a
multiplicity of fragment pair
members with break-point termini within a solvent exposed loop or a flexible
loop defined
by tertiary or secondary structure analysis to obtain a fragment pair library.
The fragment
pair members are expressed in a multiplicity of host cells, and the host cells
exhibiting the
directly detectable signal associated with the marker protein of interest are
isolated as
1s indicative of containing fragment pair members that functionally
reconstitute the marker
protein. Plasmids containing expression cassettes coding for the fragment pair
members
are then sequenced to identify functional fragment pairs. To aid in the
identification of
functional fragment pair members of a marker protein of interest, the fragment
pair
members can be expressed as fusion proteins with interactor domains known to
bind to
20 each other, such as the fos and jun transcription factors that associate
through a leucine
zipper interaction. The sequences encoding the hetero-dimerizing helices of
the fos and jun
transcription factors are sufficient to use as effective interactor domain for
this purpose.
The systems and methods of the subject invention find particular use in
identifying
epitopes recognized by immunoglobulin molecules, polypeptide sequences that
bind to
25 extracellular domains of a transmembrane protein, inhibitors of
phosphorylation-regulated
signal transducer proteins, and interaction between oligopeptides of two
different
proteomes. For the identification of epitopes, first and second fusion
oligopeptides
comprised of a fragment domain and an interactor domain are expressed in a
host cell
where the first fusion oligopeptide has an interactor domain comprised of a
randomly
3o encoded peptide inserted into the active site of a thioredoxin protein and
the interactor
domain of the second fusion oligopeptide is comprised of a single-chain
variable region
(scFv) or antibody light chain variable region (VL). A similar strategy is
followed for
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
14
identifying polypeptide sequences that interact with the extracellular domain
of a
transmembrane protein, where the first interactor domain is comprised of a
randomly
encoded peptide inserted into the active site of a thioredoxin protein and the
second
interactor domain is comprised of a transmembrane protein. Identification of
inhibitors of
a phosphorylation-regulated signal transduction protein involves expressing a
first fusion
oligopeptide with a first interactor domain comprised of a phosphorylation-
regulated signal
transduction protein, such as Her-2/neu, and a second fusion oligopeptide with
a second
interactor domain comprised of a scFv or antibody light chain variable region
that only
binds to the unphosphorylated signal transduction protein. Inhibitory
compounds are
l0 identified from host cells that change color in the presence of a
chromogenic (3-lactamase
substrate. For identifying or monitoring polypeptide-polypeptide interactions
between the
members of two different proteomes, members of a first and second cellular
expression
library comprise the first and second interactor domain, respectively, of a
fusion
oligopeptide. The expression library is preferably a cDNA library, but may
also be
constructed from synthetic nucleotides to screen randomly generated
polypeptides. A
library of particular application .for the present invention should represent
all the protein
members of a proteome of interest. Libraries derived from nucleotide sequences
that all
members of a total protein population (i.e. a proteome) of interest may be
isolated from a
host cell such as a prokaryotic or a eukaryotic cell, or also from a viral
host. Viral hosts
2o that encode for oncogenes are of particular interest. Mammalian tumor
cells, immune cells
and endothelial cells also provide proteomes of particular interest for the
subject invention.
The invention also finds use in selecting with a single marker protein the
incorporation of multiple genetic traits in a host cell, where detectable
expression of a
functionally reassembled marker protein is indicative of co-expression of
multiple genes
that encode for individual traits in a host. Finally, the invention provides
therapeutic utility
in a method for specifically activating derivitized prodrugs in the vicinity
of a target organ
in a host, where each member of a marker protein fragment pair is expressed as
a fusion
protein with individual immunoglobulin molecules that recognize neighboring
but non-
overlapping epitopes on a target protein. Binding of both antibodies to the
target protein
3o allows functional reconstitution of the marker protein which then activates
subsequently
administered prodrug only in the vicinity of a target organ.
The invention is exemplified by the antibiotic resistance enzyme, TEM-1 (3-
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
lactamase, although fragment pairs of other enzymes that provide for
antibiotic resistance
are included in the present invention, including: aminoglycoside
phosphotransferases,
particularly neomycin phosphotransferase, chloramphenicol acetyl transferase,
and the
tetracycline resistance protein described by Backman and Boyer (Gene (1983)
26:197).
5 Other proteins that can directly elicit a visible phenotypic change such as
a color change or
fluorescence emission also are applicable to the subject invention. Examples
of such
proteins include (3-galactosidase and green fluorescent protein (GFP) or other
related
fluorescent proteins.
The TEM-1 (3-lactamase of E. coli is the 264 amino acid product of the
ampicillin
to resistance gene of plasmid pBR322 (Sutcliffe, 1978, supra), the nucleotide
sequence of
which is shown in Figure 2 along with the encoded amino acid sequence. TEM-1
is the
archetype member of the homologous Class A (3-lactamases, or penicillinases.
Its three-
dimensional structure is shown in Figure 3 (Jelsch et al., Proteins Struct
Funct (1993)
16:364ffj. The Class A (3-lactamases are comprised..of two domains. One
domain, a-w, is
15 made up of N-terminal and C-terminal sequences, whichform an anti-parallel
two-helix
bundle:packed against a flat 5-stranded (3-sheet. The inner face of the sheet
packs against
the other domain (~,), a seven helix bundle with two extended loops and two
small (3-
structures. An outside strand of the (3-sheet borders the substrate binding
pocket, opposite
the catalytic nucleophile, Ser70, and contributes substrate-binding residues.
The remainder
of the 'active site residues, including Ser70, are contributed by the ~,
domain. The two
domains are connected by two loops: R61-R65 and D214-W229.
The subject invention also provides a method of identifying optimal break-
points in
a parent protein that provides for a directly detectable signal. A search of
the "fragment
space" of TEM-1 (3-lactamase was conducted to identify fragment pairs which
could
complement for activity only when the break-point termini of the fragments
were
genetically fused to hetero-dimerizing helixes from the c fos and c jun
subunits of the AP-1
transcription factor (Karin et al., Curr Opin Cell Biol (1997) 9:240. To do
this, libraries
of all possible N- and C- terminal fragments of the enzyme were generated by
progressive
exonucleolytic digestion of the full coding sequence from both termini.
Fragments of less
than 25 amino acids were considered non-viable. When libraries were
constructed with
compatible vectors, the fragment sequences co-expressed in the same E. coli
cells so that
each cell expressed a single pair of N- and C- terminal fragments and every
possible pair
WO 00/71702 CA 02374476 2001-11-19 pCT/US00/07108
16
may be represented. For example, for a 100 kDa enzyme there are only 106
possible N-
and C-terminal fragment pairs, so an exhaustive search of the fragment space
of most
enzymes could be conducted with libraries of a manageable size. An exposed
loop was
identified by this method between two a-helixes of E. coli TEM-1 (3-lactamase
(approximately Thr195 to A1a202, between helixes 7 and 8) within which the
chain could
be broken to produce fragments which could only complement for activity when
fused to
the fos and jun helixes. Representative fragments with contiguous break point
termini at
G1u197 and Leu198 were designated a197 (N-terminal fragment) and c~198 (C-
terminal
fragment), and subsequently shown to produce selectable activity in the E.
coli periplasm
1o with interactions between a variety of heterologous domains fused to the
break-point
termini, including single-chain antibody Fv fragments (scFv), antibody light
chains (LC),
thioredoxin with 12-mer peptides inserted into the active site (trxpeps), and
the extra-
cellular domain of the B-cell activation antigen CD40 (CD40ED). Activation by
complementation of a 197 and w 198 could also be driven by interaction of the
heterologous
domains with a third polypeptide, such as a receptor. Contiguous break-point
termini of
interest in E. coli TEM-1 ~3-lactamase in addition to E197/L198 include amide-
bond
junctions between amino acid residues N52/S53, E63/E64, Q99/N100, P174/N175,
K215/V216, A227/G228, and G253/K254. The combined lengths of the fragment
pairs
may be discontinuous or overlapping, however, comprising from 90 % to 110 % of
the total
length of the parent protein, and the actual break-point could be within ten
amino acid
residues in either direction from an identified functional contiguous break-
point junction.
The specific activity of the reconstituted enzyme can be enhanced to near wild-
type levels
by the interaction-driven formation of a disulfide at the break-point, which
restores the
integrity of the native polypeptide backbone (see Figure 4).
The (3-lactamase a 197 and ~ 198 fragments cooperatively produce selectable
activity
in the bacterial periplasm in a manner that is strictly dependent on specific
interaction
between heterologous domains fused to the break-point termini of the fragments
is an
example of an enzyme-based molecular interaction sensor that can undergo
secretory
translocation across a plasma membrane into an extra-cellular compartment, and
therefore
3o can reliably detect interactions between and among extra-cellular proteins.
The interaction-dependent enzyme association systems of the present invention
fords
use in many applications in human therapeutics, diagnostics, and prognostics,
as well as in
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
17
high-throughput screening systems for the discovery and validation of
pharmaceutical
targets and drugs.
One particular application is concerned with the localized and controlled
activation
of inactive or weakly active compounds. For example, many useful compounds,
such as
drugs, chromophores, and fluorophores, can be inactivated by conjugation of an
essential
moiety on the compound, such as a hydroxyl or amino group, to a substrate for
enzymatic
hydrolysis, such as an ester, amide, carbamate, phosphate, glycoside, or
glucuronide
(Jungheim and Shepherd, Chem Rev. (1994) 94:1553). Such conjugates can then be
activated by the appropriate hydrolytic enzymes such as esterases,
carboxypeptidases,
1o alkaline phosphatases, glycosidases, glucuronidases, (3-lactamases, and
Penicillin-amidases.
In one particularly versatile system, cephalosporins may be conjugated at the
3' position
via a variety of different leaving groups to a variety of anti-cancer drugs,
such as nitrogen
mustards, methotrexate, anthracyclines, and vinca alkaloids (Svensson et al.,
J Med Chem
(1998) 41:1507; Vrudhula et al., J Med Chem (1995) 38:1380; Jungheim and
Shepherd,
1994, supra; Alexander et al. Tetrahedron Lett (1991) 32:3269;. see also
Figure 5). All of
these are good substrates for broad spectrum (3-lactamases, and most are much
less active
than their parent drugs. As a result, these prodrugs are promising candidates
for use in
Antibody-Directed Enzyme Prodrug Therapy (ADEPT; Bagshawe, Drug Devel Res
(1995)
34:220). In addition to these compounds a vast array of antibiotics (Holbrook
and Lowy,
Cancer Invest (1998) 16:405), as well as a variety of chromagenic and
fluorogenic
substrates have been developed for (3-lactamases (Jones et al., J Clin
Microbiol (1982)
15:677; Jones et al., J Clin Microbiol (1982) 15:954; Zlokarnik et al.,
Science (1998)
279:84), making them one of the most versatile known classes of enzymes.
Nevertheless, the utility of such enzymes would be greatly enhanced if they
could
be engineered so that their catalytic activities could be positively
controlled by allosteric
interaction with ligands of choice. In this way the catalytic power of these
enzymes could
be harnessed to multiple new applications, including (1) rapid, ultra-
sensitive detection of
trace analytes and pathogens in biological specimens or in food, (2) targeted
activation of
therapeutic and diagnostic reagents at specific locations in the body, (3)
rapid enrichment
of expressed sequence libraries for autonomously folding domains (AFDs), (4)
massive
parallel mapping of pair-wise protein-protein interactions within and between
the proteomes
of cells, tissues, and pathogenic organisms, (5) rapid selection of antibody
fragments or
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
18
other binding proteins to whole proteomes, (6) rapid antigen identification
for anti-cell and
anti-tissue antibodies, (7) rapid epitope identification for antibodies, (8)
high-throughput
screens for inhibitors of any protein-protein interaction.
For example, enzymes which could be activated to hydrolyze chromogenic
substrates only upon binding to target analytes could form the basis of assays
for those
analytes of unparalleled sensitivity and convenience. Such assays would be
homogeneous,
requiring no manipulations other than the mixing of two components, namely the
enzyme
and substrate, with a biological specimen, in which the presence of the
analyte is then
quantitatively indicated by the rapid development of color. Current
homogeneous
1o enzymatic assays rely on inhibition of the enzyme by binding of anti-
analyte antibody to the
analyte, or mimic thereof, immobilized on the surface of the enzyme (Coty et
al.,
J Clin Immunoassay (1994) 17:144; Legendre et al., Nature Biotech (1999)
17:67). Free
analyte is estimated by its ability to competitively displace the antibody,
thereby activating
the enzyme. Such enzymes are thus activated competitively, not allosterically.
For assays
employing such enzymes the maximum signal increment occurs at equilibrium with
roughly
Kd concentrations of reagents, so that typically .only. a fraction of analyte
molecules
participates in signal generation, and equilibration is often slow or does not
even reach
completion. However, an enzyme which is activated by direct allosteric
interaction with
analyte, can be used in excess, so that equilibration is rapid and independent
of the analyte
2o concentration, and the analyte can be saturated to produce signal from
every molecule. In
the case of microbial or viral pathogens, where unique surface markers may be
present in
hundreds to thousands of copies per cell or particle, such enzymes, which
would be
activated by binding to the marker, could allow rapid detection of as little
as a single cell
or particle, whereas the sensitivity of equilibrium assays for such analytes
would typically
be much lower.
In another class of applications interaction-activated enzymes could be
adapted for
activation by binding to specific cell surface molecules. This would allow the
enzyme to
become localized and activated at specific sites in the body for target-
restricted activation
of reagents for therapy or imaging. Antibody-Directed Enzyme Prodrug Therapy
3o (ADEPT; Bagshawe, 1995, supra) is a promising chemotherapeutic strategy for
the
treatment of cancer, in which a prodrug-activating enzyme, such as a ~3-
lactamase, is
targeted to the tumor by a tumor-specific antibody to which it is chemically
or genetically
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
19
conjugated. After unbound conjugate has cleared the circulation, an inactive
prodrug, such
as an anthracycline cephalosporin, is administered, which is converted to a
potent tumor-
killing cytotoxin at the site of the tumor by the remaining tumor-bound
enzyme. The main
problem with ADEPT is that the unbound conjugate must clear the circulation
before the
prodrug can be administered in order to minimize systemic toxicity. However,
by the time
the conjugate has cleared the circulation > 90 % of the tumor bound enzyme has
been lost
(Bagshawe, 1995, supra; Springer and Niculescu-Duvaz, Anti-Cancer Drug Design
(1995)
10:361). In spite of this, ADEPT has been able to achieve higher active drug
concentrations in the tumor than any other procedure (Sedlacek et al., 1992 In
1o Contributions to Oncology, Huber H and Queisser V, eds. pp. 208ff Karger,
Basel), and
has shown promise in the clinic (Bagshawe et al., Dis Markers (1991) 9:233;
Springer and
Niculescu-Duvaz, 1995, supra; Martin et al., Cancer Chemother Pharmacol (1997)
40:189). The unbound conjugate problem could be completely obviated by a
prodrug-
activating enzyme which would be active only when bound to the tumor, so that
the
prodrug could be administered simultaneously with the enzyme or at the point
of peak
tumor loading without regard for unbound enzyme which would be inactive.
In the same way, interaction-activated enzymes could be targeted for
activation by
surface markers on the cells of other types of diseased tissues, such as sites
of inflammation
or atherogenesis, or even healthy tissues. The target-localized and activated
enzymes could
2o then be used to activate not just cytotoxins, but other types of
therapeutic agents such as
small molecule agonists or antagonists of biological response modifiers, as
well as imaging
reagents for precise localization of tissue with disease or other phenotype of
interest. For
example, target-activatable enzymes could be used to deliver: (1) immune
stimulants to
tumors, (2) immuno-suppressants to sites of chronic inflammation or to organ
transplants,
(3) antibiotics to specific pathogens, (4) cytotoxins and anti-virals to virus-
infected cells,
(5) hormones and other pleiotropic agents to specific cells and/or tissues, or
(6) neuro-
transmitters and other neuro-modulators to specific nerves or tissues. In
short, interaction-
activated enzymes could be used to deliver to any tissue any small molecule
cytotoxin,
hormone, steroid, prostaglandin, neurotransmitter, or agonist/antagonist of
peptide
3o hormone, cytokine, or chemokine, etc., which could be inactivated by
conjugation to the
appropriate substrate.
In yet another class of applications, interaction-activated enzymes could be
adapted
WO 00/71702 CA 02374476 2001-11-19 pCT~7S00/07108
for efficient simultaneous detection of multitudes of interactions among
proteins within
cells, including expressed sequence libraries, single-chain antibody fragment
(scFv)
libraries, and scaffolded peptide libraries. For example, enzyme-based
interaction traps
could enable the comprehensive mapping of pairwise protein-protein
interactions within and
5 between the proteomes of human cells, tissues, and pathogens for the rapid
identification
and validation of new pharmaceutical targets. They could also be used for
rapid selection
of binding molecules from single-chain antibody fragment (scFv) libraries, or
from
scaffolded peptide libraries for use as reagents in functional genomics
studies, or for
identification of natural ligands and epitopes by homology. Target
interactions identified
1o using interaction-dependent ~3-lactamases could be used immediately to
screen for inhibitors
of the interaction by exploiting the great substrate diversity of these
enzymes to reverse the
polarity of selection. Whereas interaction-dependent activation of (3-
lactamase could be
used to confer selective growth on host cells in the presence of (3-lactam
antibiotics, it
could also be used to confer selective cytotoxicity on the cells in the
presence of ~3-lactam
is pro-antibiotics: The latter substrates would only become cytotoxic upon
hydrolysis of the
(3-lactam moiety by the interaction-activated enzyme, and so could be used to
select
inhibitors of the interaction by their ability to confer selective growth on
host cells.
Finally, enzyme-based interaction sensors could be used for rapid detection of
the
activation or inhibition of key molecular interactions in signal transduction
pathways,
2o enabling high-throughput cellular screens for inhibitors or activators of
those pathways.
For example, screening for agonists or antagonists of receptor tyrosine
kinases usually
requires coupling receptor ligation to a selectable phenotype which results
from de novo
gene expression. Such mufti-step signal generating mechanisms are prone to
high rates of
false positive and false negative selection, like the yeast two-hybrid system,
and are
therefore poorly suited to high-throughput screening. However, interaction-
dependent
(3-lactamases could be set up for activation by phospho-tyrosine sensitive
interactions, so
that a selectable phenotype would be generated just downstream from receptor
ligation.
Interaction between the receptor tyrosine kinase substrate and a binder
peptide could be
designed to be either dependent on, or inhibited by phosphorylation, so that
either receptor
3o agonists or receptor antagonsists could be selected.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
21
General Strategies for Making High-Performance Enzyme
Fragment Complementation Systems
The present invention provides for general strategies for the use of
heterologous
interactors, break-point disulfides. random tri-peptide libraries, and
mutagenesis to obtain
stable enzyme fragments which are capable of forming of catalytically robust
complexes. It
has been suggested that it might be possible to identify such fragment pairs
for any enzyme
simply by conducting thorough searches of all possible fragment pairs for the
enzymes in
question (Ostermeier et al., Proc Natl Acad Sci (1999) 96:3562). In practice,
however, the
success of such endeavors is strongly dependent on the stringency of
selection, that is, how
much functional enzyme must be produced by the expressed fragments to produce
an
efficiently selectable phenotype. An efficiently selectable phenotype is one
in which the
background frequency, or false positive rate, is not appreciably higher than
the frequencies
of the desired fragments in the fragment libraries.
In fact the most useful fragment complementation systems for a given enzyme
are
not necessarily those fragments of wild-type sequence which are most capable
of unassisted
complementation, but rather the most useful fragment complementation systems
comprise
those fragments which, when using the engineering techniques described, can be
made to
meet more specific performance requirements. For example, naturally evolved
proteins are
generally expected to exhibit a roughly inverse correlation between fragment
stability and
2o complex stability. This is due to the energy cost of inter-conversion. The
more stable the
fragments are, the more energy is required to form the complex and vice versa.
As a
result, those fragments capable of producing the highest specific activities
might be missed
or dismissed because fragment instability may prevent them from producing
selectable
levels of activity. To circumvent such pitfalls, libraries of fragment pairs
can be
simultaneously expressed with libraries of random tri-peptides to insure that
every fragment
pair has a chance to perform in the presence of fragment-stabilizing tri-
peptides, thereby
minimizing the dependence of the phenotype on fragment stability. This
strategy is
especially useful if dependence of activation on the interaction of
heterologous domains
fused to the fragments is desired. If constitutive activation is desired, the
fragment
libraries could also be amplified by error-prone PCR to introduce fold-
accelerating
mutations which could mitigate both fragment instability and complex
instability, as was
found for (3-lactamase.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
22
For in vitro applications such as homogeneous assays, biosensors, and target-
activated reagents fragment stability is especially important, but the most
stable fragments
might not be selectable if they cannot produce stable complexes without
assistance, as
would be predicted by the inverse correlation of fragment stability and
complex stability.
Thus, fragment libraries could be expressed in the E. coli periplasm with a
disulfide at the
break-points and heterologous interactors fused to the break-point termini.
These tools
provide mechanisms for docking the fragments, accelerating folding, and
stabilizing the
active complex. As was shown with (3-lactamase, a substantial fraction of
fragment pairs
can be made to produce robust selectable activity in the bacterial periplasm
with such
1o molecular prostheses.
Each of the four tools described for enhancement of functional reconstitution
of the
parent protein of the fragment pairs, i.e., heterologous interaction, break-
point disulfide,
tri-peptide stabilizers, and mutagenesis, can be used alone or in combination
to insure
selection of the best fragments for the desired application, and also to
improve and
optimize the performance of selected fragment pairs for a desired application.
As
demonstrated, each tool enhances performance by a different mechanism; so that
the effects
of multiple tools are generally additive. Heterologous interactors bring and
hold the
- fragments together to facilitate re-folding into the active complex. Break-
point disulfides
can stabilize the active fold by restoring the integrity of the polypeptide
backbone at the
2o break-point. Tethered or free tri-peptides can protect the fragments from
aggregation
without interfering with folding into the active complex. Mutagenesis can
protect the
fragments by accelerating folding into the active complex.
The first step in the development of high-performance enzyme fragment
complementation systems is to construct vectors to express each fragment in
the fragment
pair library. A convenient system for selective fragment library expression
may be derived
from the expression system illustrated in Figure 6. All fragment pairs
regardless of the
intended application can potentially benefit from and would not be impaired by
the docking
function provided by interactors such as the fos and jun helixes fused to the
break-point
termini. Thus, the C-terminal, or w fragment library would be expressed as N-
terminal
3o fusions via a flexible polypeptide linker such as a (Gly4Ser)3 linker to
the fos helix
(Interactor 2 in Figure 6) from the lac promoter in the phagemid vector pA01
(the
upstream cistron could be removed if desired). The amino acid sequence of the
flexible
W~ 00/71702 CA 02374476 2001-11-19 pCT/US00/07108
23
polypeptide linker is not critical, however, it must be of a sufficient length
and flexibility
such that the fragment domain and heterologous interactor domain fold
independently and
unhindered. The N-terminal, or a fragment library would be expressed as C-
terminal
fusions via a flexible polypeptide linker such as a (Gly~Ser)3 linker to the
jun helix
s (Interactor 1 in Figure 6) from the trc promoter in the compatible pAEl
vector. Coding
sequences for signal peptides would be included if translocation to the
periplasm were
desired.
As discussed above, depending on whether the intended applications) were in
vitro
or in vivo, or if in vivo, whether in the cytoplasm or secreted, one or more
of the
to performance-enhancing tools may be incorporated into the expression vectors
to maximize
the probability of selecting the best fragment pair for the intended
application(s). If
periplasmic expression is desired, cysteines should be encoded at the break-
point termini to
allow disulfide formation. If the enzyme contains other cysteines, at least 1
mM and not
more than 5 mM of a reducing agent such as GSH or DTT should be included in
the
15 growth medium to inhibit the formation of mixed disulfides. If fragment
stabilization is
desired to increase the importance of specific activity in selection, a random
or VRK tri-
peptide library may be encoded in frame with each fragment fusion between the
break-point
terminus and the flexible polypeptide linker. If VRK libraries were used for
each fragment
in a 50-fragment pair library, every possible tri-peptide-fragment combination
would be
2o contained in a combined library of < 10g. Alternatively, a single tri-
peptide library could
be used for each fragment pair in trans, as was described above. The tri-
peptide library
would be fused operably in frame via the flexible polypeptide linker to the N-
terminus of
thioredoxin and expressed from the upstream cistron in the pA01 phagemid
vector (see
Figure 6).
25 The second step in the development of high-performance enzyme fragment
complementation systems is to construct an expression library of candidate
enzyme
fragment pairs. Methods for generating libraries of random fragment pairs have
been
described (Ostermeier et al., 1999, supra). However, such libraries are quite
inefficient as
the vast majority of fragment pairs will be dysfunctional. For combinatorial
screening of
3o fragment pair libraries with mutagenic or random tri-peptide libraries,
much more efficient
fragment pair libraries will be necessary. For a variety of reasons it may be
assumed that
the most functional fragment pairs will correspond to scission of the
polypeptide chain in
WU 00/71702 CA 02374476 2001-11-19 pCT/US00/07108
24
exposed regions between elements of secondary structure. Exposed break-points
will be
required for use of tethered heterologous interactors and tri-peptides, and
scission within
secondary structure elements can irreversibly destabilize such elements. If a
3-dimensional
structure is available for the enzyme of interest, or for a homolog, it can be
used to identify
exposed loops as candidate sites for chain scission. Typical globular proteins
will not have
more than 20-25 such sites that are far enough from the ends so that the
larger fragment is
not independently active. This is a manageable number for construction of
coding
sequences for each fragment pair by PCR. Two end-specific primers would be
required,
plus a head-to-head pair of primers for each break-point, which should be
located more or
1o less in the center of the exposed loop. If a 3-d structure is not
available, reliable
algorithms are available on the Internet for computational prediction of
secondary structure
and hydropathy, such as the ProteinPredict program of Rost and Sander (J Mol
Biol (1993)
232:584; Proteins (1994) 19:55; Proteins (1994) 20:216). With such programs,
most of
the exposed loops can be identified as hydrophilic regions between secondary
structure
elements. Again, it would not be excessively burdensome to prepare coding
sequences by
PCR for up to 50 fragment pairs.
If fragment complementation does not need to be dependent on the direct or
ligand-
mediated interaction of heterologous domains fused to the break-point termini,
then fold-
accelerating mutations could also be selected by using error-prone PCR in the
initial
2o amplification of the fragment coding sequences. Under appropriate
conditions of Mg++,
Mn++, and nucleoside triphosphate concentrations, as well as cycle number,
mutagenesis
can be limited to 1-3 unbiased coding changes per molecule (Cadwell and Joyce,
1995, in
PCR Primer-A Laboratory Manual C. Dieffenbach and G. Dveksler, Eds. Cold
Spring
Harbor Press, Cold Spring Harbor, NY, pp. 583-590). Since most mutations would
be
non-phenotypic, this could easily be combined with the other performance-
enhancing tools
without compromising the selectability of optimal fragment-tri-peptide
combinations. Once
the fragment coding sequences have been amplified, gel-purified, and ligated
into the
vectors, the ligation products may be desalted and concentrated to allow
efficient co-
transformation of E. coli cells by high-voltage electroporation. If both the
tri-peptide
libraries and mutagenesis are used it is advisable to collect at least 10g and
preferably at
least 109 transformants to insure comprehensive representation of the full
diversity of the
library. The full library is then plated onto each of a range of non-
permissive conditions,
CA 02374476 2001-11-19
WO 00171702 PCT/US00/07108
the least stringent being that on which the host cells would plate with an
efficiency not
greater than ten times the inverse of the library size. This would insure a
manageable
frequency of true positives among false positives. The maximum selection
stringency
would be that above which nothing is recovered from the library.
5 If fragment complementation is to be dependent on the direct or ligand-
mediated
interaction of heterologous domains fused to the break-point termini, then
mutagenesis
should not be used because folding acceleration usually eliminates the need
for docking
assistance. In this case selected fragment pairs must be counter-screened for
loss of
activity in the absence of the fos jun interaction and activation indexes must
be determined
1o as the ratio of interaction-dependent activity to interaction-independent
activity. For
interaction mapping within or between proteome libraries activation indexes of
the order of
at least 106 are preferred since rare genes are expected to have frequencies
in that range.
For ligand-specific or interaction-specific biosensors lower activation
indexes are usually
acceptable. For example, to detect nanomolar concentrations of a ligand for
which
15 fragment-binder fusion. affinities (Kd) are in the 10 nM range, the
fragment binder fusions
need only to be used at 100 nM concentrations to saturate the ligand. Under
these
conditions ~ 90 % of .the fragment-binder fusions will be unbound. If the
activation index
is > 100, the background will be G 10 % of the signal.
Selected fragment pairs can be optimized for maximum activity and/or maximum
2o activation index. In our experience break-point disulfides produce the
highest specific
activities because they allow the greatest amount of native structure in the
fragment
complex. However, they also may in the background so that activation indexes
are often
lower. To retain the specific activity benefit of the break-point disulfide
and reduce the
background it may be necessary to retard the rate of disulfide formation so
that it would
25 not have sufficient time to occur during the abortive attempts of the
unaided fragments to
fold, but would occur efficiently when folding is catalyzed by the
heterologous interaction.
Two parameters may be adjusted to control the formation of break-point
disulfides. (1)
The proximity of the disulfide-forming cysteines to the break-point may be
adjusted to
place greater orientational stringency on disulfide formation. (2) The
concentration of
3o reducing agent in the medium may be increased to reduce the effective
concentration of
DsbA, the principle disulfide-forming oxidase in the periplasm.
It is possible to use TEM-1 (3-lactamase fragment complementation to select
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
26
fragment pairs of other proteins which do not produce selectable phenotypes in
E. toll for
their ability to form stable complexes because such complexes will usually be
in the native
conformation and should be functionally active. It has been amply demonstrated
that
naturally evolved proteins have unique minimum energy conformations in which
they are
s stable and active (Li et al., Science (1996) 273:666). All other
conformations are unstable.
Thus, if a fragment pair library of a non-phenotypic protein is expressed as
fusions to the
interaction-dependent TEM-1 (3-lactamase fragments, it is expected that only
those
fragment pairs which associate and fold into the native conformation will
provide sufficient
docking function to facilitate selectable ~-lactamase activation. In this
case, the subject
1o fragments serve the purpose of the heterologous interactors in facilitating
complementation
of ~3-lactamase fragments. However, additional modifications could be encoded
into the
fragment/heterologous interactor fusion sequences to enhance functional
reassociation of
the (3-lactamase fragments, including a break-point disulfide, a randomly-
encoded peptide
of from 3-12 amino acids, and mutagenesis of several amino acids within the
fragment
15 domain. All of these tools would specifically impact-only. complementation
of the subject
fragments by stabilizing the fragments, accelerating folding, and/or
stabilizing the active
fragment complex. Selected fragment pairs could then be tested individually
for
reconstitution of enzymatic activity or other function of the parental
protein. In this way
many useful fragment complementation systems could be developed for proteins
which are
2o active in eukaryotic cells, such as kinases or herbicide-resistance
proteins.
The interaction-activated enzyme association systems of the subject invention,
as
exemplified by prokaryotic (3-lactamase, find use in many applications as
summarized
below.
(1) Simplex and multiplex protein-protein interaction mapping. Simplex refers
to the use
25 of single bait proteins to fish natural interactors out of expressed
sequence libraries.
Multiplex refers to the combinatorial pair-wise interaction of two expressed
sequence
libraries for the purpose of simultaneously isolating as many natural
interactions as
possible. Individual interactors can be readily identified by nucleic acid
hybridization.
(2) Interaction-dependent (3-lactamase systems may also be used to enrich
randomly-
3o primed expressed sequence libraries for fragments which encode autonomously-
folding domains (AFD). Interference with folding by the fusion partner is
avoided by
using epitope tags and hetero-dimerizing helixes only at the N- and C-termini
of the
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
27
expressed sequence, respectively. The fragments would have N- and C-terminal
anti-
tag binder and the partner hetero-dimerizing helix. The disulfide switch can
accommodate diverse interaction geometries.
(3) Simplex and multiplex selection of binding molecules such as single chain
antibody
fragments (scFv) and antibody light chain variable regions (VL). Non-immune
human scFv repertoire libraries can be used with TEM-1 (3-lactamase
interaction-
dependent activation systems to isolate scFv to single baits or simultaneously
to
expressed sequence libraries. In the latter case scFv specific for individual
targets
can be readily identified by nucleic acid hybridization.
1o (4) Interface mapping and ligand identification by mimotope homology.
Constrained
peptide libraries displayed on the surface of a carrier or ''scaffold" protein
may be
used with ~3-lactamase interaction-dependent activation systems to isolate
surrogate
ligands for proteins or AFDs of interest. Consensus sequences from panels of
such
surrogate ligands for a given polypeptide may then be used to identify natural
ligands
- . of the polypeptide or interaction surfaces on natural ligands of the
polypeptide. A
common application of interface mapping is epitope mapping for antibodies,
whereby
the specific region to which an antibody binds on the surface of its antigen
is
identified.
(5) Bio-Action Sensors. The efficiencies of most screening systems for signal
2o transduction agonists and antagonists are compromised by the need for
multiple steps
between receptor ligation and selectable phenotype generation, which usually
requires
de novo gene expression. Interaction-activated ~3-lactamases can be tailored
for
activation or inhibition by any component of a target signal transduction
pathway to
allow selection of agonists or antagonists of the pathway in any appropriate
cell type
without the need to wait for gene expression to generate a selectable
phenotype.
(6) Homogeneous Assays. Interaction-dependent complementing fragments can be
fused
to two scFv or other binding molecules which bind non-overlapping epitopes on
target molecules, so that ~3-lactamase activation becomes dependent on binding
to the
target ligand. The use of ligand-dependent /3-lactamases in homogeneous assays
for
3o two-epitope analytes from proteins to pathogens affords unparalleled
sensitivity
because saturation kinetics can be used instead of the equilibrium kinetics
required by
most assays. The binding molecules could also be oligonucleotides which anneal
to
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
28
contiguous sequences in the genome of a target pathogen. Such sequence-
activated
(3-lactamases could also be used for rapid quantitation of specific PCR
products
without the need for gel eletrophoresis.
(7) Target-Activated Enzyme Prodrug Therapy (TAcEPT) and Target-Activated
Enzyme
Imaging (TAcEI). Antibody-directed enzyme prodrug therapy is a promising chemo-
therapeutic strategy in which patients are treated with prodrug-activating
enzymes
such as (3-lactamase conjugated to tumor-targeting antibodies (Bagshawe, 1995,
supra). When unbound antibody-enzyme conjugate has cleared the circulation,
prodrugs can be administered which are preferentially activated at the site of
the
tumor. The efficacy of this therapy is severely limited by the need for
unbound
conjugate to clear the circulation before the prodrug can be administered in
order to
avoid excessive toxicity, during which time most of the bound enzyme is lost
from
the tumor. The use of tumor-activated (3-lactamases allows the prodrug to be
administered at peak tumor loading of the enzyme since the latter is inactive
in the
Is circulation, and can only activate the prodrug when bound to he tumor. The
same
strategy can be used for antibody-directed site-specific activation of
reagents for
imaging of tumors or other tissue pathologies, or for other.therapeutic
indications
such as inflammation or transplant rejection.
2o The following examples are offered by way of illustration of the present
invention,
not limitation.
EXPERIMENTAL
25 EXAMPLE 1
(3-lactamase Activation by Interaction-Mediated Complementation of a197 and
c~198:
Interactions between scFv and trxpeps
This example demonstrates the ability of the system to detect and discriminate
specific interactions between single-chain antibody Fv fragments (scFv) and 12-
amino acid
30 peptides by inserted into the active site of E. coli thioredoxin (trxpeps,
Colas et al., Nature
(1996) 380:548). ScFv are comprised of antibody heavy chain and light chain
variable
regions (VH and VL) tethered into a continuous polypeptide by most commonly a
CA 02374476 2001-11-19
~~.TEr~T ?9 ~~o»n~E~ DOe>~ET rra. ~~.R~.oa2.a~wo~
(GIy~Ser)3 linker encoded between most commonly the C-terminus of VH and the N-
terminus
of VL.
ScFv from a human non-immune antibody repertoire were amplified by PCR using a
consensus primer mix (Marks et al., Eur J Immunol (1991 ) 21:985), and
subcloned into a
pUC 119-based phagemid vector (Sambrook et al., supra) for expression of the
seFv as
fusions to the N-terminus of the c~ 198 fragment with an intervening
(Gly4Ser)3 linker (pA01;
see Figure 6). An N-terminal .signal peptide was provided for translocation to
the bacterial
periplasm. A commercial trxpep library was obtained and amplified by PCR using
primers
specific for the N- and C-termini of E. toll thioredoxin (Genbank accession
no. M54881 ).
This product was subcloned into a plSA replicon (Rose, Nuc Acids Res (1988)
16:355) for
expression as fusions to the C-terminus of the a 197 fragment from the trp-lac
fusion
promoter (pAEl; see Figure 6). Again, an N-terminal signal peptide was
provided for
translocation to the periplasm. Figure 7 illustrates the activation of TEM-1
by
complementation of a 197 and w 198, mediated by interaction between an scFv
and a trxpep.
It was estimated that about 20% of the original scFv library clones produced
soluble,
full-length scFv as judged by immunoblot analysis (Harlow and Lane, (1988)
In~Amibodies:
A Laboratory Manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor)
of
periplasmic~extracts obtained by osmotic shock (Neu and Heppel, J Biol Chem
(1965)
240:3685). Thus, approximately 60 clones had to be screened in this way to
obtain twelve
clones expressing functional scFv. Plasmid~NA representing these twelve clones
of the scFv-w198 construct was co-transformed with DNA representing
approximately 5x106
clones of the a197-trxpep construct into E. toll strains DHSa and TG1
(Sambrook et al.,
1989, supra), and plated onto solid LB medium containing kariamycin and
chloramphenicol
to determine the total number of co-transformants. Aliquots were also plated
onto 25 lzg/ml
ampicillin (amp25). Out of approximately 1x10 total co-transformants, 40
ampicillin-
resistant clones were recovered, 36 of which replated on amp25. A similar
number of co-
transforrriants of a single randomly selected a197-trxpep construct with the
twenty seFv-
c~ 198 constructs produced no colonies on amp25. All twelve scFv were
represented in the 36
arnpicillin-resistant clones with from one to five different trxpeps each.
None of the 12 scFv
cross-reacted with any trxpep originally selected by another scFv, as
determined by
co-transforming each scFv-~ 198 construct with
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
a pool of the a197-trxpep constructs selected by the other scFv. Thus, all 36
selected
clones were bona fide positives, representing unique and specific scFv-trxpep
interactions.
No scFv bound thioredoxin in the absence of its peptide mimotope(s), and no
selected
trxpep bound common determinants on the scFvs. Selections were performed in
the E. coli
5 host strain TG1 without the gratuitous de-repressor of the lac promoter,
isopropyl
thiogalactoside (IPTG), so that transcription was minimal. When transcription
was
increased by the presence of 1 mM IPTG, many more colonies were obtained.
Several of
these were shown to be bona fide interactions which were too weak to confer
selectable
ampicillin resistance at lower levels of expression. Thus, the stringency of
selection can be
1o tuned by adjusting the expression levels of the interactors.
These results have several important implications. First, the false positive
rate was
exceedingly low, much lower than has been reported for other intra-cellular
interaction
sensors such as the yeast two-hybrid system (Bartel et al., 1993, supra;
Bartel et al., 1996,
supra). This property is essential for high-throughput applications. Secondly,
the false
15 negative rate with respect to the scFv was immeasurably low, as trxpeps
were recovered
for all functional scFv, and this too is essential for high-throughput
applications. The fact
that mimotopes were recovered for all scFv enables the system for high-
throughput
multiplex epitope mapping for scFv. Finally, the system is capable of
efficient recovery of
multiple interactions between two diverse populations of proteins
simultaneously.
2o Ultimately, given the high efficiency of the system, i.e., low rates of
false positive and
false negative selection, the throughput of the system should be limited only
by the sizes of
the interacting libraries, and/or the number of co-transformants which can be
handled
conveniently. For example, construction of recombinant protein libraries in
the 109-10'°
range is routinely possible for scFv, trxpeps, or cDNAs (Hoogenboom et al.,
Immurcotech
25 (1998) 4:1). Combinatorial pair-wise interaction trapping for any two such
libraries would
require at least 10'8-102° clones, but with quantitative phagemid
infection methods
(Sambrook et al., 1989, supra) and automated fermentation and plating methods,
such
throughput levels could be realistically achieved.
?~_nR_?r~01 US000710~
CA 02374476 2001-11-19
PATENT 31 ~'fI'~3RNE~' 1?~C)~l;'r NQ. P~~.oU2.OZ~~'U
Example 2
~-lactarnase Activation by Interaction-Mediated Complementation of x197 and
co198:
Interactions between antibody light chain V-regions (VL) and trxpeps
This example demonstrates the ability of the system to work with larger
antibody
S fragments, such as Fab, which are comprised of entire light chains disulfide-
bonded to Fd
fragments which contain VL plus the fast heavy chain constant region. A subset
of Fabs from
a human repertoire library was subcloned for expression as C-terminal w198
fusions from a
dicistronic transcript from the lac promoter in the pA01 vector (see Figure
6). The first
cistron encoded the light chain with a signal peptide for translocation to the
periplasm. The
light chain termination codon was followed by a short spacer sequence and then
a ribosome
binding site approximately 10 by upstream from the start of translation for
the signal peptide
of the Fd fragment, which was followed by ~ 198 with an intervening (Gly4Ser)3
linker. This
construct was then co-expressed with the a197-trxpep library in the pAEl
vector in strains
DHSa and TG1. Spontaneous association of the light chain with the Fd-ca198
fusion protein
in the periplasm was expected to produce a functional Fab fragment_ Binding of
the latter to
the peptide on a a197-trxpep fusion was then expected to facilitate assembly
of the functional
TEM-1 ~i-lactamase in amounts sufficient to confer selectable resistance to
ampicillin on the
host cells.
Many clones were in fact recovered on 25~,g1m1 ampicillin. Some of these
are.listed in
Table 1 befivv. Several were resistant to up to 100 ~,glml and one was
resistant to up to 600
~,g/ml. Unexpectedly, all recovered Fabs were missing the VH region. 'That is,
they
contained the full-length light chain (LC) with only the first heavy chain
constant region
(CH 1) . The reasons for this were as follows . The original Fab library was
constructed by
first inserting tl~e VL repertoire into the vector which already contained the
constant regions
-25 ready for expression. This intermediate construct was capable of
expressing a complex of the
light chain with the first heavy chain constant region fused to ~ 198. Plasmid
DNA was then
purified from this light chain library and used as the recipient for insertion
of the VH
repertoire to complete the Fab library. The resulting library was contaminated
with
approximately 15 %a of clones which contained the intermediate vector. Only
these LC-CN 1
complexes were capable of driving a197-w198 complementation by binding of the
VL
combining site with the peptide on the appropriate trxpep. It is not known why
full-length
Eabs were not selected, however, the larger size
~, = . .
_.li='~i_~__ _. .__ .
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
32
and rigidity of the Fab-trxpep complex ( ~ 67 kDa) may have sterically
inhibited fragment
complementation, whereas the smaller size and flexibility of the LC-CH 1
complex did not.
TABLE 1.
Ampicillin-Resistance of TEM-1 (3-lactamase oc197/~198 Fragment
Complementation Driven by Interaction of Selected Pairs of
Antibody Light Chain-CH1 Complexes and Trxpeps
LC-CH1 Trxuen A
P44-2-2B 1 P44-2-2A 1 + + + + + w
P44-2-3B 1 P44-2-3A 1 + +
P44-1-6B 1 P44-2-6A 1 +
P64-17B 1 P64-17A 1 + +
P65-1-lOBI P65-1-10A1 + + +
P66-3-2B 1 P66-3-2A 1 + +
P66-3-l OB 1 P66-3-l0A 1 +
P66-3-14B 1 P66-3-14A 1 + +
P75-7-7 ? _>+
P75-7-13 ? >_+
P75-7-30 ? >_+
a +, + +, + + +, + + + + + , > 10 % plating efficiency on 25, 50, 100, 600
~g/ml ampicillin
This result shows that light chain V-regions alone, which are only -~ 12 kDa
in size,
could make convenient high-affinity binding molecules for antigen-dependent
activation of
~3-lactamase by fragment complementation. To test this, the VLs from several
of the
selected LC-CH1 were subcloned for expression alone as C-terminal fusions to
w198.
When each was co-expressed with its partner a 197-trxpep, approximately one-
third of the
VL conferred selectable resistance to ampicillin comparable to the parent LC-
CHls.
2o Example 3
~3-lactamase Activation by Interaction-Mediated Complementation of a197 and
w198:
Interactions between CD40 and trxpeps
This example demonstrates the ability of the present system to isolate panels
of
~5_pg_?001 U ~000?108
- CA 02374476 2001-11-19
PATEI'T 33 ATTORNEY )COCKED' NQ. )aARE.042.0I~~'O
trxpeps that bind to a given protein of interest, and which could be used to
map interaction
surfaces on the protein, and which could also assist in the identification of
new ligands by
homology. The extra-cellular domain of the human B-cell activation antigen
CD40 is
known to reliably express in the E. coli periplasm (Noelle et al., Immunol
Today (1992)
13:431; Bajorath and Aruffo, Proteins: Struct, Funct, Genet (1997) 27:59). A T-
cell
surface molecule, CD40 ligand (CD40L), is known to co-activate B-cells by
ligation to
CD40, but there may be other ligands. Therefore, TEM-1 a197/w198 fragment
complementation was used to select a panel of CD40-binding trxpeps. The
sequences of
these peptides would then be examined for homology to the known ligand and
other
potential ligands. The coding sequence for the mature form of the extra-
cellular domain
(CD40ED) was amplified by PCR using primers homologous to the N-terminus of
the
mature protein and to the C-terminus of the -190-residue extra-cellular domain
(Genbank
accession no. X60592). The PCR product was then subcloned into the pA01
phagemid
vector (Figure 6) for expression from the lac promoter as a C-terminal fusion
to the TEM-1
w 198 fragment with an intervening (GlyaSer)3 linker. Expression of the
correct product
was confrmed by PAGE, and the CD40 fusion vector was then rescued as phage and
traiisfected into TG-1 cells bearing the same trxpep library construct as
described above.
Approximately 10' co-transformants were collected by double selection on
kanamycin and
chloramphenicol, and then plated onto 25~cg/ml ampicillin. Activation of TEM-1
~by a
trxpep-Cl?4tJ interaction-mediated complementation of a 197 and w 198 is
depicted in
Figure 8.
Ampicillin-resistant clones encoding thirteen unique trxpeps were recovered.
In all
cases amp resistance was strictly dependent on the presence of CD40ED and the
peptide
portion of the ~rxpep. No activity was seen if CD40ED was replaced with an
irrelevant
protein or if the trxpep was replaced by wild-type thioredoxin. The sequences
of the
selected CD40-binding peptides are shown in Table 2 below along with their
homologies to
each other and to CD40L. The thirteen peptides sort into eight homology
groups: two
groups with three each (1 and 2), one with two (3), and five with one each.
Groups 1 and
2 are defined by homology of three peptides in each group to the same region
of CD40L.
Group 1 is homologous to the region of CD40L from Pro217 to G1y234, and Group
2 is
homologous to the region from GIyl58 to Leul68. Group 3 is defined only by
inter-
peptide homology and has no detectable homology to CD~!OL. Group =1 is
homologous to
.~a-;,;:_ _~ _. ._-
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
34
CD40L from Ser110 to Pro120, and Group 5 is homologous to CD40L from Pro244 to
G1y257. Groups 6-8 have no discernable homologies. However, a number of the
peptides
had striking homology to other human extra-cellular proteins, including CTLA-
2A, a
matrix metalloproteinase, a receptor Tyr phosphatase, vascular endothelial
cell growth
inhibitor (VEGI), transferrin receptor, CD3~, and bone morphogenetic protein
3B (BMP-
3B). These may define an interaction motif or motifs, which have been used
repeatedly for
extra-cellular protein-protein interactions. They may also indicate multiple
interaction sites
on CD40.
Inter-trxpep competition was tested by expressing each of five selected CD40-
binding trxpeps from a second cistron in the pA01 phagemid vector, downstream
from the
CD40 - w 198 fusion. Each of these constructs was then co-expressed with each
of the
same five plus three additional selected a 197-trxpep fusion constructs in
strain TG 1 and
scored for growth on 25 ~,g/ml ampicillin. The results are shown in Table 3
below. The
eight trxpeps sorted into five groups. BW10-1 competes moderately with groups
2 and 3.
p58-12-9A1, BW10-4, and BW10-8 compete strongly with each other and have
similar
competition profiles. They do not compete with group 3, except for BW10-8,
which
competes.slightly with group 3 and BW10-9. All three compete with BW10-1, and
p58-12-
9A1 also competes slightly with BW10-9. p44-4-2A1 and p45-7-2A3 compete
strongly and
have similar competition profiles. They compete with BW10-1 and nothing else
except
2o BW10-8 slightly. BW10-9 competes slightly with BW10-8 and p58-12-9A1. p65-2-
9A1 is
inhibited by nothing.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
PATENT 35
N
fl.
~'b
0
i"'
O
O
n
>r
O
y
v, M r-I y
O I
.~ N ~ ~ '
U FC ~ FC N v
U U a U U
U w p P ~I~~ a - w ~ a '
' ~ m 7 o .~
bn - U W OI N ~ ~l - - C~z calCSI~
a c~C~
w ~ o a ~ ~ ~n ona v~w w w
~ w r~ m f c~c~ ~ ~ z r~ w
I c~
~e fx~ W ~ x OIIOIOI ~ C~ E-nE--~OI U O w
~ ~
c~c~ ~ c~ rxx rxrx ~ x W ~ w ~ of~C
~ I I I I I ~
~ ~ ~''' ~ ~ W a ~ H ~ z ' o
r"
H ~ ~ a ~ w ~ c 0
I 7
A o4~C ~ x ~ c~ x ~ W w w w ~Ix w ~ w ~ o
l
~ ~ I
x C7 P 01 ~ P ~ a ~ ~ ~C~ W ~ !~ x W b
-i ..~
w of ~ a ~ c~ c~~ ~ ~ w x ~ on z ~ ~ ~ o
~ I I
_
U U ~
e~
N UIw U t~ P W ~ W Ua~ W~0.i1W ni0.i~ 3
.~
. I I ~
C7 ~ C~ U HI U U U w U I U U U ~
v' U FC U' ,~~ I I -o b
~1
t~,. I U ~-l ~ N
~I7 I
y ~ N rl N O
tip ~ ..O
4~ N f-i 'D y
TS
O ~ c~"a
r~ G
~ U
_
..~. ~.
.
i. ~
b~ ~ N M V7 -~M M ~ O
~ ~ ~ O
O
O ~ ~ O O ~ c O N N p p ~ O O ~ O~~' U
~1
O ~ ~ N ~ ~ N N ~ ~ l~ ~ ~ N ~ ~ N N
Ca ~
y
3
0
w .
.
o
~
~
U
r.. N M ~t v W D l~Oo~
a.~n
ap
.
o ~
~ O
~
~.~
.n
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
36
o,
'
+
N i i i i i
a1
N + v v + w w
' +
~' ' + +
P~
M
+ ' + +
i
O
O
O
n
U
v v h
+ ' i + -+ i i 23
~
it ~ v O
p-1 CC
GCCr O U
w
R
v
O
~ i i ~ ~ i -~ p w
I ~"'
0. I
o
~ IC
O
00 SC 'B
O
V 0 + + w -j~w + ~ C
+ W ~ ~CS
'O
'
-~-I~ ~j + " ~
~O
G5
L U
d O
~ L
'L7
O
~~ M ~ o
~ >'
' + + y ~ w + w ~ ~ ~ by
~ CC
~ + i ' + ' 00 N .~ O
bD
.~
O r ~ ~
R
u
c
C
t .O
O ..
C
O
r., Q. II ~
+ c
Q ri rr
+ + -H' + + '
N N ~; p CD
w
,., ~-I ~ i.
~' O~ N ~;
es
y
O Op ef ,
-' O 1(~ ,r
~
.-.M '_".~ s a~
pa ~ G. a~
0. G o
v v
V
0oO~ N N C; O~ C
0 0 0 0 ' A N N IlcacC
y -, N M d' ~ y,
V~ Y.
N ' ~ G. CL G1.
2 G G
G
OOOOD Tr
~rwr
~
O O O O
O
CJ CJ L7 .~ N
C7 CJ M ~
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
37
In general, the competition data is consistent with the homology data with the
caveat that simultaneous binding to non-overlapping epitopes is sometimes not
tolerated.
This allows unrelated sequences like p58-12-9A1 and BW10-8 to compete strongly
with
one another and have similar competition profiles. This is probably due to
steric
interference with enzyme reassembly, and may account for the discordance
between
homology and competition data for BW10-1 and p58-12-9A1 in particular. These
two
probably bind near the same CD40 interaction epitope, which may sterically
inhibit
fragment complementation for many (but not all) other trxpeps.
For some applications it will be useful for (3-lactamase activation to be
mediated by
simultaneous binding of both x,197 and cu 198 to non-overlapping epitopes on a
separate
molecule, either a free ligand or cell surface receptor. Two CD40-binding
trxpeps, which
had been identified as non-competing by the competition tests, were used to
test this utility.
One of the two trxpeps was subcloned for expression as the C-terminal w 198
fusion from
the pA01 vector (see Figure 6). The other trxpep was expressed as the a197
fusion from
the pAEl vector as before. Co-expression of these two constructs was used as
the negative
control. To test for CD40-mediated activation, the CD40ED coding sequence
(including
signal peptide) was subcloned into the trxpep-w 198 expression cassette
between the
promoter and the trxpep-w 198 sequence. An additional 20 by containing a
ribosome
binding site was included downstream from the CD40 stop codon to allow
expression of
both CD40 and trxpep-w 198 from the same dicistronic transcript, as was
described above
for the Fab. As shown in Table 4 below, CD40 expression induced resistance to
50 ~.g/ml
ampicillin, whereas without CD40 the cells expressing the control constructs
produced
fewer than 10-6 colonies per cell on 25 ~,g/ml ampicillin. Thus, (3-lactamase
fragment
complementation can be efficiently induced by a tri-molecular protein-protein-
protein
interaction.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
38
Table 4
Ligand activation of TEM-la/w fragment complementation using
non-competing CD40-binding trxpeps and CD40ED.
Molecule#1 Molecule#2 Molecule#3 Amp'
a-p44-4-2 CD40-w - + +
a-p44-4-2 CD40 ~BW10-1-w ++
a-p44-4-2 - BW10-1-w
a. plating efficiencies on 25 ~g/ml ampicillin in colonies per cell. -, < 10-
6;
+, >10%; ++, >25% +++, >50%.
Example 4
~i-lactamase Activation by Interaction-Mediated Complementation of a197 and
w198:
Interaction between a CD40-specific scFv and CD40
Since [3-lactamase activation by a197-w198 fragment complementation could be
driven efficiently by interaction between scFv and trxpeps, it was important
to show that it
could also be driven by interaction between scFv and .a bona fide protein
antigen,
preferably a cell surface receptor. This was especially important because the
ligand-
binding domains for type 1 trans-membrane receptors are N-terminal, therefore
their
expression as C-terminal fusions is preferred. However, the preferred
orientation for scFv
expression is also N-terminal. To allow expression of both scFv and antigen as
C-terminal
fusions, (3-lactamase activation by a tri-molecular interaction was tested,
including the C-
terminal fusion of the scFv with w 198, a C-terminal fusion of CD40 with the
fos helix, and
a C-terminal fusion of a197 with the jun helix. The expression constructs were
analogous
to those used for CD40 ligation of the trxpep-fragment fusions. The CD40-fos
fusion and
the scFvw 198 fusion were expressed from a dicistronic transcript in the pA01
vector, and
a197 jun fusion was expressed from the pAEl vector. The fos jun interaction
has a Kd in
the 10-8M range, so it should quantitatively ligate CD40 with x197, which are
much more
abundant than this in the periplasm. Binding of the scFv to CD40 should then
dock w 198
with the complex to facilitate fragment complementation. As shown in Table 4,
CD40-fos
expression induced resistance to up to 100~g/ml ampicillin, whereas cells
expressing only
the control constructs without CD40-fos again produced fewer than 10-6
colonies per cell
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
39
on 25 ~,g/ml ampicillin. Thus, (3-lactamase fragment complementation can be
efficiently
induced by a tri-molecular interaction of two extra-cellular proteins in
preferred C-terminal
fusions.
Example 5
Disulfide-Enhanced Fragment Complementation
The (3-lactamase activity produced by interaction-dependent complementation of
the
a 197 and w 198 fragments is substantially less than that of the wild-type
enzyme under the
same expression conditions. This loss of activity could be due to a tendency
of the
fragments to aggregate or turnover when they are not folded into the native
conformation,
and it could also reflect a loss of specific activity due to the reduced
ability of the loosely
tethered heterologous interaction to stabilize the native conformation. It was
reasoned that
both folding kinetics and stability could be enhanced by the introduction of a
disulfide at
the break-point, and this could lead to a substantial increase in interaction-
dependent
activity. The expectation was that when the fragments were docked by the
heterologous
interaction, the integrity of the polypeptide backbone would be restored at
some point in
the folding pathway by the formation of a disulfide linkage between cysteines
added at the
break-point, and this would accelerate folding and/or stabilize the active
conformation.
The disulfide would form very rapidly in the highly oxidizing environment of
the bacterial
periplasm. However, if the fragments were unstable until they were docked and
folded,
but once folded the activity was stable, then the break-point disulfide might
have little
effect on activity if it did not form until late in the folding pathway.
Cysteines were added to the sequences of a 197 and w 198, between the break-
point
termini and the linkers leading to the heterologous interactors. With the fos
and jun
helixes as the interactors, quantitative ampicillin resistance ( > 10 %
plating efficiency)
increased from 50 pg/ml to more than 100 ~g/ml, and the plating efficiency on
25 ~g/ml
ampicillin increased at least 2-fold. Thus, disulfide formation must be
accelerating folding
and/or stabilizing the active conformation. However, the disulfide produced
nearly as
much activity without the interactors. This contrasts sharply with the
activity of the
fragments in the absence of either the disulfide or interactors, for which
plating efficiencies
are less than 10-6 on 25 yg/ml ampicillin. This result suggests that the
fragments probably
associate and refold readily on their own at these intra-cellular
concentrations, but that
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
without a heterologous interaction or disulfide at the break-point, either
folding cannot
progress to the active conformation, or the latter is not stable enough to
produce selectable
activity. There must be a finite window of opportunity for disulfide formation
when the
thiols are proximal during unassisted folding. This window should be much
wider during
interaction-assisted folding. Thus, it should be possible to retard disulfide
formation and
thereby make it more dependent on the heterologous interaction.
Disulfide formation was made to be more dependent on the heterologous
interaction
by two modifications. First, disulfide formation could be inhibited by
inclusion of a
reducing agent in the growth medium. Dithiothreitol (DTT) at 10 mM reduced the
plating
efficiency of the disulfide-assisted fragments on 100 pg/ml ampicillin to <
10~ colonies per
cell in the absence of an interaction, whereas with the fos-jun interaction
the activity of the
same fragments was little affected by DTT, so that the activation index was
increased to
> 1000-fold. Secondly, the cysteines were shifted by one residue each away
from the
break-point and into the (3-lactamase sequence, so that they became separated
in the native
fold by an additional -- 8A. This reduced activity to a plating efficiency of
< 10-6 on 50
pg/ml ampicillin without the interaction, whereas with the fos-jun interaction
the plating
efficiency was reduced to --10 % on 50 ~g/ml ampiciliin for an activation
index of > 105.
Thus, a combination of reducing agent and thiol separation may be expected to
increase the
increment of interaction-dependent activation over background even further,
perhaps to
> 106. In any case the 8 A increase in thiol separation alone increased the
activation
increment substantially over that of the fos jun interaction without
disulfide. The
enhancement of interaction-dependent specific activity provided by the
disulfide should
allow weak interactions and/or poor expressors to produce selectable (3-
lactamase activity
with fewer than 10 molecules per cell of the activated enzyme.
The ability of the break-point disulfide to enhance activation of TEM-1 a
197/w 198
fragment complementation, suggests that break-point disulfides might be able
to activate
many enzyme fragment pairs which produce weak or no selectable activity with a
heterologous interaction alone. The heterologous interaction may be essential
for fragment
docking, but since it is tethered with ~ 60A linkers it cannot restore the
tight junction of
the polypeptide backbone at the break-point. However, formation of a disulfide
across the
break-point should restore the integrity of the backbone, and should thereby
help stabilize
the active site of the complex. This idea was tested by screening nine
additional pairs of
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
41
TEM-1 (3-lactamase fragments, corresponding to scission in nine exposed loops
of the
polypeptide chain. The nine fragment pairs were screened for selectable
activity with the
break-point disulfide alone, the fos-jun interaction alone, and with both
together. The
results are summarized in Table 5.
Addition of the break-point disulfide to the fos jun interaction strongly
increased the
activity of seven of the nine fragment pairs, which makes eight out of ten
pairs when
a197/ca198 is included. The ten fragment pairs may be sorted into three
groups. One
group comprises the two negative pairs. The second group comprises three pairs
which
can only be activated by disulfide and fos jun interaction together. In each
case, the
plating efficiency is at least 10% on 25 ~g/ml ampicillin, with an activation
index of at
least 1000. The third group comprises five pairs, all from break-points in the
C-terminal
third of the molecule, which produce modest-to-robust activity with fos jun
alone, but
potent activity with both fos jun and the disulfide together. Most
importantly, four of the
five produce no selectable activity with the disulfide alone, so they have
very large
activation indexes. P174/N175 had the highest activation index, ---10' on 100
~g/ml
ampicillin. G253/K254 had the highest activity with a plating efficiency of >
25 %o on 400
p,g/ml ampicillin. Interestingly, the first fragment pair identified to
exhibit interaction-
dependent activation, a 197/ 198, remains the only pair to produce robust
selectable
activity with the break-point disulfide alone. It is possible that activation
of some pairs is
inhibited by the formation of mixed disulfides between the break-point
cysteines and the
internal cysteines, and it is also possible that such inhibition could be
alleviated with
exogenous reducing agent. However, it is at least as likely that in these
cases unassisted
refolding could not proceed far enough to allow efficient formation of the
break-point
disulfide before aborting.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
42
; +~ o
~
3 x
' n
~ I I I I I I I I I I '
x ~
~ ~3
s. ao
+
w
~
o
. a.
,
-'
o ~ c I I I I I I I I I I ~ ~ i
~~ ~ y -..n U
~ ~ .
~T.I
b
-0
a, ~
~
v~
.~
d d I I I I I a~ ?
,I~, ,~,1n N ~, m U
nO r'3 ~ _ N
U .T'
. . U
~
a. ~ by
O CL x ccs
~
~
N
U
..,I I I I I + '~ + ~' + ~ ~ ~
>, + + ' 3
o
c
~
~r ,
.o .~ .3 o
~
~. o
1n o
3
~,
~ x' o .
p a
e V~ ~
e .
E~ G. ~ ~ '~ o
~
+ .
O N + O ~
O
I I I I I I I I I
~ + ~ O "
O
G~ ,~ Y U
.~
C C
'~
bD U .
s-~.
. ~'
p p p p p H y y
I I
Q N N N N ~ ~ N d'~ ~ 017
~
C~ O ',~'" ~ . ~
.
i
.
_
~
ti. +
N + +' + +'
+ + I + I
v,
C2 ~' ~ '~ '~ +. ~' +.~ p y
N Y s.
O
c.
+.. o
W
N ~ .n w
~ ~~
% ~ ~ ~ ~ ~ -, ...~
"
O 1 ~ N N ~
p a; ~ W U ~ ~ 1 ~ U ~ ,,.,
.
~
~ 3 .
~ _ a
c'1 ~ OO ~ ~ ~ ~~~> ~v
AS ~ M ~ ~ I I M +
~
p G \p O~ ~ ~ t~ Q1 n N tf7~ ~ Q
~ -I-
z w a a ~ ~ w ~ ~ ~ Qo x;
w~
, . ~
U
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
43
The fact that the fragment pairs which produced the highest activities are not
the
same as those with the highest activation indexes and vice versa, indicates
that different
fragment pairs may be optimally suited for different applications. For
example, the
activation index is more important than maximum activity for intra-cellular
interaction
mapping, where natural interactions must be identified against backgrounds of
106 or more
non-interacting pairs. Thus, P174/N175 may be the best fragment pair for intra-
cellular
interaction mapping. On the other hand, maximum activity is more important
than the
activation index for in vitro applications because the activating target
ligands will always be
limiting in such applications. Since for maximum activation the fragments need
only be
1o used in ten-fold excess over their Kds for the ligand, the activation index
need only be 1000
for a signal-to-noise ratio of 100. Thus, G253/K254 may be the best fragment
pair for in
vitro applications such as biosensors or homogeneous assays.
The break-point disulfide overcomes a significant shortcoming of interaction-
dependent enzyme fragment complementation systems. It is essential for high-
throughput
applications that such systems be capable of efficient activation by a wide
range of
heterologous protein-protein interactions. In other words, to.minimize the
false negative
rate, the system must be activatable by any interaction between two proteins
or fragments
within the size range of single, naturally evolved protein domains, i.e.,
between ~-100 and
300 amino acids in length. Globular proteins in this size range have radii in
the range
~-30-SOA. This means that the points of attachment for the linkers could be up
to 100A
apart, and this distance must be spanned by the linkers in order for the break-
points of the
fragments to be able to come together. For this reason, the (Gly4Ser)3 linker
was selected,
which is expected to be fully extended and flexible, and to have a length of ~
60A, thereby
providing a combined length of up to 120A to allow close approach of the break-
point
termini during folding. Nevertheless, it is reasonable to expect the stability
of the active
conformation to be quite sensitive, and generally inversely proportional to
the dimensions
of the heterologous interaction. Thus, for all such systems described to date
it may be
assumed that the longer the linkers, the larger the proportion of possible
interactions that
can accommodate refolding, but the less the interaction can contribute to
stabilization of the
active conformation.
The break-point disulfide overcomes this limitation because, if the linkers
are long
enough, it will form readily during re-folding, and once the break-point
disulfide is formed
WO 00/71702 CA 02374476 2001-11-19 pCT~S00/07108
44
the specific activity of the reconstituted enzyme should be independent of the
dimensions of
the heterologous interaction, and in fact should not even require the
continued integrity of
the interaction. Thus, the break-point disulfide acts as a one-way switch,
with an
activation energy which can be supplied by a broad range of heterologous
interactions,
limited only by the ability of the interactors to fold properly, and by the
length of the
linkers to allow close approach of the break-point cysteines. This has two
important
consequences which allow a larger proportion of natural interactions to
produce selectable
activity. Longer linkers can be used, and interactions which are too weak to
sustain
selectable enzyme activity by themselves should still be able to "throw the
disulfide
to switch" to produce selectable activity.
Example 6
Peptide-Enhanced Fragment Complementation
Another way to enhance interaction-dependent enzyme fragment complementation
is
to introduce short, random peptide sequences at the break-points, and then to
select for
increased activity with a model interaction. Such peptide-dependent
enhancements could
occur by any of several mechanisms. For example, the peptides could stabilize
the active
conformation of the reconstituted enzyme by interacting with each other or
with the enzyme
itself, or the peptides could stabilize one or both of the fragments, thereby
increasing
2o steady-state activity by increasing fragment concentration.
Synthetic oligonucleotides were used to add three randomized residues to each
fragment between the break-point residue and the linker for the heterologous
domain. As
the model interaction, the c-fos helix at the N-terminus of ~ 198 and the c-
jun helix at the
C-terminus of x,197 was used. For each randomized position, a degenerate codon
was
used, which encoded a subset of amino acids which was biased toward charged
residues to
favor charge-charge interactions, which are the strongest. The VRK codon
places c, a, or
g in the first position, a or g in the second position, and t or g in the
third position. The
encoded amino acids are His, Gln, Arg, Asn, Lys, Ser, Asp, Glu, and Gly. For
three
randomized positions in both fragments there are a total of 126 = 3x106
possible codon
3o combinations, and 96 = 5.3x105 possible different amino acid sequences.
Initially, ten
thousand clones of the library were plated onto successively higher
concentrations of
ampicillin until no colonies were recovered. Six clones in the DHSa, strain
were recovered
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
from 800 ~,g/ml ampicillin, and all six showed strict dependence on the fos
jun interaction
for growth. In fact, the jun helix was removed from a 197 in the same starting
104 clones
of the library, and when these clones were plated onto the same concentrations
of
ampicillin, only a few colonies grew on 200 ~,g/ml ampicillin, and no colonies
appeared on
5 higher concentrations. This level of ampicillin resistance is comparable to
that produced
by the fos jun interaction alone.
Unexpectedly, all six selected clones recovered from DHSa had the same a tri-
peptide, Gly-Arg-Glu (GRE), and each had a different cu tri-peptide. When the
cu tri-
peptides were removed, there was no significant reduction in activity,
suggesting that the
1o ability of the GRE sequence to enhance fragment complementation did not
depend on the
presence of the w tri-peptide. Thus, the GRE a tri-peptide produced a profound
enhancement of the interaction-dependent activity, but it cannot substitute
for the
interaction. In fact, without the interaction the GRE tri-peptide does not
seem to increase
the background at all, thus it does not either accelerate refolding or
stabilize the folded
15 complex. The most likely effect of the GRE tri-peptide is to stabilize the
ai97 fragment by
interfering with loss of the fragment by amorphous aggregation. Since the cu
198 fragment
is quite stable, but the a 197 fragment is somewhat less so, the latter is
expected to be
limiting for fragment complementation, and any stabilization of a 197 leading
to an increase
in its concentration would increase the steady state activity of the
interaction-activated
2o enzyme accordingly. Though the GRE tri-peptide could inhibit aggregation of
x197, it
apparently did not interfere' with re-folding of the fragment complex. Since
aggregate
formation proceeds exponentially, it is exquisitely sensitive to small shifts
in the inter-
molecular association rate constants (Dobson, Trends Biochem Sci (1999)
24:329). Thus,
even weak binding of the tethered tri-peptide to the interacting surfaces
could effectively
25 defeat inter-molecular aggregation. As the complementary fragments fold
cooperatively
into the active complex; however, the weakly bound tri-peptide would be
readily stripped
from its binding site by steric strain as the two become separated in the
emerging native
conformation. In this way the general ability of tethered small peptides to
stabilize larger
proteins without interfering with protein folding may be understood.
3o When the same random tri-peptide libraries were screened for fos/jun-
mediated
ampicillin resistance in the TG 1 strain, five clones were recovered on
400~,g/ml ampicillin.
With the fos jun interaction alone TGl cells will not plate above 50 ~,g/ml
ampicillin.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
46
Thus, as before, tri-peptides were selected which substantially increased the
level of
ampicillin resistance produced by the fos jun interaction alone. This time
four different a
tri-peptides were recovered, each with a different w tri-peptide.
Pairs a w
FHT400-lAl, - 1B1 HSE (cat agt gag) REQ (cgg gag cag)
FHT400-2A 1, - 2B 1 NGR (aat ggg cgg) QGN (cag ggt aat)
FHT400-4A 1, - 4B 1 GRE (ggt cgg gag) DGR (gat ggg agg)
FHT400-9A l , - 9B 1 EKR (gag aag cgt) GRR (ggt agg agg)
FHT400-10A2, -IOBl NGR (aat ggg cgg) GNS (ggt aat agt)
GRE was selected again from the a tri-peptide library. NGR was selected twice
from the
a tri-peptide library, with two different cu tri-peptides. In all cases,
activation continued to
be dependent on the fos jun interaction. However, in contrast to the original
GRE tri-
peptide, activity was enhanced in all cases by the presence of the both the a
and w tri-
peptides. Even the activity of the GRE tri-peptide was enhanced by the DGR tri-
peptide on
the ~ fragment. Also, the fragments were interchangeable to some extent.
Different a tri-
peptides could be paired with different w tri-peptides. The fact that enhanced
activity was
2o still fully dependent on the heterologous interaction suggests that the
primary effect of the
peptides was protection of the fragments to which they were attached from
aggregation,
rather than stabilization of the final fragment complex. The latter would be
expected to
confer constitutive activity, independent of the heterologous interaction.
The GRE tri-peptide was also found to stabilize a 197 in traps. When the a 197-
fos
and jun-w 198 fusions were co-expressed in the E. coli periplasm with the GRE
tri-peptide
fused to the N-terminus of thioredoxin via a Gly4Ser linker, the cells plated
with 100
efficiency on 50 ~.g/ml ampicillin, whereas cells expressing the a 197-fos and
jun-~ 198
fusions either alone, without the GRE-trxA fusion, or with a different tri-
peptide-trxA
fusion, plated with only ~ 1 % efficiency on 50 ~cg/ml ampicillin. The GRE-
trxA fusion
3o conferred no resistance to ampicillin in the absence of the interacting
helixes, thus it does
not stabilize the re-folded fragment complex, but rather it must stabilize the
a 197 fragment
since activity is limited by the amount of soluble a197. Since the GRE tri-
peptide had the
same stabilizing effect on a 197 fragment when a different carrier was used,
its activity
must be context independent. Thus, an 18 kDa enzyme fragment could be
stabilized at least
100-fold by a tri-peptide selected from a random sequence library. As with the
tethered
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
47
tri-peptide, the free GRE tri-peptide could inhibit aggregation of a 197
without apparently
interfering with re-folding of the fragment complex. In this case, however,
displacement
of the tri-peptide would have been greatly assisted by the fact that the
effective intra-
molecular concentrations of structural elements relative to one another would
have been
much higher than the tri-peptide concentration. In this way the general
ability of small
peptides to stabilize large proteins in traps without interfering with protein
folding may be
understood. This phenomenon is not widely appreciated, and in fact this may be
the first
demonstration that a functional protein could be deliberately stabilized by
something as
small as a tri-peptide.
to
Example 7
Mutationally-Enhanced Fragment Complementation
The ability of tri-peptides to stabilize (3-lactamase fragments and thereby to
increase
both the interaction-dependent activity and activation index of the TEM-1 a
197/ 198
complex should be of great benefit for in vitro applications of (3-lactamase
fragment
complementation, where utility is most limited by fragment instability. Thus,
it was of
interest to determine if a comparable stabilization of the a 197 fragment
could be achieved
by random mutagenesis and selection. To test this, the a 197 coding sequence
was
mutagenized by error-prone PCR (Cadwell and Joyce, 1995, supra). The PCR
conditions
of Cadwell and Joyce mis-incorporate nucleotides in an unbiased fashion at a
rate of one
mutation every ~ 150 nucleotides. Since the a197 coding sequence is actually
about 520
nucleotides in length, and ~ 75 % of mutations change the encoded amino acids,
less than
three coding changes per molecule should be produced. About 108 clones of the
a197
mutant library were collected and co-expressed as the jun helix fusion with
the fos helix
fusion of wild-type w 198. The mutagenized a 197 jun fusion was expressed from
the pAE 1
vector and the fos-w 198 fusion was expressed from the pA01 phagemid vector
(see Figure
6). When both constructs were co-expressed in strain DHSa colonies were
recovered in
the presence of 600 ~.g/ml ampicillin. Upon sequencing, two of three clones
recovered
(FI600-1 and -3) had the same sequence with two coding mutations, KSSE (aag-
gag) and
3o M182T (atg--~acg). The third clone (FI600-4) also had two coding mutations,
one of
which was shared with the other two (M182T), and the other of which, P62S (ccc-
-~tcc),
was proximal to the other mutation of the other clones.
WO 00/71702 CA 02374476 2001-11-19 pCT~S00/07108
48
Cells expressing either mutant consistently plated at > 30 % efficiency on
100~.g/ml
ampicillin, whereas cells expressing the wild-type a 197 plated at < 10-6
colonies per cell
on 100 ~.g/ml ampicillin, and -- 30 % on 25 ~g/ml ampicillin. However, for
both mutants,
plating efficiencies were just as high or higher in the absence of the
heterologous
interaction, i.e., with the jun helix removed. An exhaustive search for more
mutations did
not turn up any mutants with interaction-dependent activity. Thus, in contrast
to the results
obtained with random tri-peptides, where activation remained interaction-
dependent,
adaptive mutations of a 197 invariably eliminated interaction dependence. This
may be
understood as follows. The tri-peptides stabilized the fragments by reversibly
interfering
1o with aggregation. Reversibility allows them to inhibit aggregation without
interfering with
folding. However, mutations are not reversible in this sense. If aggregation
is caused
primarily by the inter-molecular formation of native folding contacts,
disruption of these by
mutation might be expected to interfere with folding. In fact, it may be
thermodynamically
impossible to stabilize the fragments by mutation without inhibiting the re-
folding process
required to form the active fragment complex. This is because the native folds
of the
fragments have too much exposed hydrophobic surface to be stable. Thus,
mutations can
only stabilize the fragments by stabilizing alternative folds, which minimize
exposed
hydrophobic surface. However, these alternative folds must be unfolded before
the native
folding pathway can proceed to the active complex, and the energy required for
this
2o process may be prohibitive.
Since most aggregation is driven by aggregation-prone intermediates in the
folding
pathway, the rate of aggregation is proportional to the lifetimes of such
species. The effects
of the break-point disulfide described above indicated that the fragments are
capable of
association and initiation of folding in the absence of the heterologous
interaction, but that
the folding process is aborted when the fragments are not held together in
some way, such
as by the heterologous interaction or by the formation of a disulfide at the
break-point. In
the absence of either of these the probability that the fragments will
dissociate before
folding is complete is proportional to the folding rate, which in turn is
proportional to the
lifetimes of the folding intermediates. Thus, if the most likely mechanism for
mutational
3o inhibition of aggregation is to destabilize folding intermediates, this
would also accelerate
folding and thereby reduce the probability that fragment dissociation would
occur before
folding were complete. In this way it may be understood why mutations which
stabilize
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
49
the folded complex are more likely to be selected than mutations which
stabilize the
fragments, and why the former, but not the latter would give rise to
constitutive,
interaction-independent activity.
For the TEM-1 (3-lactamase of E. coli, the type member of the Class A
penicillinases, fragments have been identified which can complement to form
active
enzyme when and only when the "break-point" termini of the fragments are fused
to
proteins or other molecules which interact with each other directly or
preferably through a
second molecule. Furthermore, the subject invention presents new methods
whereby
enzyme fragments capable of interaction-dependent complementation may be
identified and
to modified specifically to confer dependence of their activity on the
interaction of
heterologous domains fused to the break-point termini. Ligand-activated or
interaction-
activated (3-lactamases can be activated in multiple locations, including the
bacterial
periplasm, bacterial cytoplasm, eukaryotic cell cytoplasm, or in vitro. They
are highly
active against a wide variety of substrates, including antibiotics,
chromogens, and
fluorogens, as well as ~3-lactam pro-drugs, pro-antibiotics, and pro-
nutrients, which can
thus be used for both positive and negative viability selection and color
selection. The
utility of (3-lactamase fragment complementation systems has been demonstrated
for
monitoring interactions between and among cell-surface receptors, antibodies,
and random
peptide libraries displayed on the surface of a natural protein.
Example 8
Construction of a Human Peripheral Blood Lymphocyte Proteome Interaction
Library.
The large number of functional interactions among both membrane-bound and
secreted proteins of circulating immune cells include many which are yet to be
discovered.
For example, among the 150 or so CD antigens discovered so far, functions and
ligands
remain unknown for a substantial fraction (Ager et al., in Immunology
Today.lmmune
Receptor Supplement, 2°d Ed. (1997). In addition, the highly
combinatorial mechanisms by
which signalling specificity is generated imply that many signalling proteins
participate in
3o multiple functional interactions, and that even the best known of these
proteins may have
ligands and functions which remain to be discovered. Thus, the functional
interactions of
the extra-cellular proteome of the circulating cells of the immune system
represent a
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
potentially rich reservoir of pharmacological targets which are not readily
accessible by
currently available interaction mapping technologies. This proteome presents a
unique
opportunity to demonstrate the power of interaction-dependent (3-lactamase
fragment
complementation systems for interaction mapping in that, while many important
5 interactions remain to be discovered, many are already known by which the
efficiency of
the system can be gauged.
As discussed above, the activation index is the most important parameter of
the
interaction-dependent fragment complementation system for cleanly
discriminating bona
fide interactions from large pools of non-interacting protein pairs. Thus, for
this
to application one would use the P174/N175 fragment pair of TEM-1 (3-lactamase
(x,174 and
cu 175) because with the break-point disulfide this pair has the largest
activation index,
-10'. It also has a robust specific activity, but this could probably be
improved even
further with some fragment-stabilizing tri-peptides, so one may first wish to
insert the VRK
or NNK tri-peptide library into the expression vectors between the break-point
cysteines
15 and the linkers (see Figure 6), and select for growth on 300-800 ~g/ml
ampicillin. Sa long
as the activation index is not compromised, higher specific activity conferred
by fragment-
stabilizing tri-peptides should allow weaker bona fide interactions in the
expressed
sequence libraries to confer selectable activity. In order to maximize the
quality of the
expressed sequence library, one might wish to subject the full-length cDNA
library first to
2o a normalization protocol to normalize the frequencies of rare and abundant
sequences.
From this normalized cDNA one would then prepare random primed cDNA by PCR,
and
size-select fragments > 200 base-pairs to enrich the library for sequences
which encode
fragments which are at least the size of single protein domains. Finally the
library could
be run through a fold-selection protocol to enrich for coding sequences which
are expressed
25 in the correct reading frame and in register with autonomously-folding
protein domains
(AFD) .
Rough microsomes, which are derived from membranes of rough ER and are
therefore enriched in mRNA for secreted and membrane proteins, may be isolated
from
unfractionated lymphocytes from pooled human blood by sedimentation velocity
in sucrose
3o density gradients (Gaetani et al., Methods in Enzymology (1983) 96:3;
Natzle et al., J Biol
Chem (1986) 261:5575; Kopczynski et al., Proc Natl Acad Sci (1998) 95:9973).
Messenger RNA may then be purified from the rough microsomes using a
commercially
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
51
available kit (e.g., Poly(A) Select, Promega, Inc., Madison, WI). A randomly-
primed
cDNA library is then made from the RNA template and cloned directionally.
First-strand
cDNA is made with AMV reverse transcriptase (RT) and random hexamer primers
(Sambrook et al., 1989, pp. 8.11-8.21). The primers contain a unique 5'
extension with
convenient restriction sites for ligation into the (3-lactamase a and w fusion
expression
vectors. The template is destroyed by the RNAseH activity of AMV RT and the
unused
primers are removed using a spun column. The second strand is then made with
the
Klenow fragment of DNA polymerase I and random hexamer primers containing a
different
unique 5' extension with a different restriction site for insertion into the
expression vectors.
After removal of unused primers, the cDNA is PCR-amplified with primers
corresponding
to only the unique sequence on each original primer (Dieffenbach and Dveksler,
in PCR
Primer. A Laboratory Manual, Cold Spring Harbor Press, cold Spring Harbor, NY,
1995), so that the majority of amplified fragments have the correct
orientation for
expression in E. coli. The product is then normalized by exhaustive
hybridization to a
limiting amount of human genomic DNA immobilized on magnetic beads (Kopczynski
et
al., 1998, supra). Since coding sequences are naturally normalized in.genomic
DNA,
cDNA recovered from the genomic DNA hybrids should be normalized. After a
final
amplification, the PCR product is size selected by centrifugal gel filtration
on Sephacryl S-
400 spun columns for fragments > --200 bp. The cDNA is then digested with
appropriate
2o restriction enzymes and ligated into the interaction-dependent /3-lactamase
a 174 and w 175
fusion expression vectors, which are essentially the same as those shown in
Figure 6,
except for some modifications required for fold selection. The vectors and
protocol for
fold selection and interaction mapping of the cDNA library are illustrated in
Figure 9.
For convenient fold selection, both vectors for expression of the library as a
and cu
fusions are compatible phagemids. In addition, a peptide epitope tag, such as
the well-
known 12-mer derived from the c-myc oncogene (Hoogenboom et al., 1998, supra)
is
encoded at the C-terminus of the cDNA, or expressed sequence (ES) library in
the a-fusion
vector, and at the N-terminus of the ES library in the w-fusion vector. When
co-expressed
with an anti-tag scFv, such as the anti-myc 9E10 scFv (Hoogenboom et al.,
1998, supra)
fused to the other (3-lactamase fragment, each fusion library can be enriched
for clones
which express autonomously folding domains (AFD) in the correct reading frame.
The
principle of the selection is that only fragments which can fold into their
native
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
52
conformations will be stable enough to support selectable levels of (3-
lactamase fragment
complementation driven by the tag-anti-tag interaction.
The normalized cDNA library-vector ligation products are transduced into E.
coli
strain TG-1 by high-voltage electroporation (Dower et al., Nucleic Acids Res
(1988)
16:6127), and plated onto the minimum ampicillin concentration on which non-
interactors
are known to plate with efficiencies of <_10-3 since at least a 100-fold
excess of non-AFD-
encoding fragments is expected in the libraries. For the a 174/w 175 system,
the
recommended ampicillin concentration would be ~ 25 ~g/ml. Since there is not
likely to
be more than 104 secreted or membrane protein genes expressed in PBLs, and the
1o frequencies of expressible AFDs may be in the range of 10-Z per gene, one
should collect at
least 10' clones of each library to insure representation of all expressible
extra-cellular
AFDs.
Once the normalized ES libraries have been enriched for AFD-encoding clones,
the
libraries can be rescued as filamentous phage by high-.multiplicity super-
infection of at least
108 cells of each library with the helper phage M13K07 (Sambrook et al., 1989,
pp. 4.17-
4.19). ..After overnight growth in suspension the library phage are recovered
from the
culture supernatant by precipitation with polyethylene: glycol, and
reconstituted in
phosphate-buffered saline. The library phage stocks may be stored frozen in 15
% glycerol.
Fresh E. coli TG-1 cells may then be co-infected with a high-multiplicity of
each phage
library and plated onto a concentration of ampicillin on which the activation
index of the
system is known to be maximal. For the a 174/c~ 175 system, 100 ~g/ml
ampicillin is
optimal, since the activation index is at least 10' and the fos-jun
interaction-mediated
plating efficiency is at least 50 % . At least 10'4 transforming units of each
fusion library
phage should be used to infect at least 10'z log phase TG-1 cells to insure
that most of the
possible pair-wise combinations of 106 clones of each AFD library are present
in the
doubly infected cell population before selection. After a one-hour adsorption
at 109 cells
per ml, the cells are washed, resuspended in fresh medium, and incubated for
another hour
with gentle shaking to allow the phagemid genes to express. The cells are then
concentrated and plated on 100 large petri dishes (150 mm dia.) containing
solid LB
3o medium containing 1 mM IPTG and 100 ~g/ml ampicillin. A small aliquot is
plated on
chloramphenicol and kanamycin to determine the number of co-transformants.
Since --10'° cells are being seeded onto each plate, it is possible
that the interaction
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
53
frequency might be high enough for the plates to overgrow. This would take at
least 104
clones per plate. In this case, all of the selected clones would have to be
recovered by
scraping and replated at lower densities. If a large number of clones is
recovered, at least
100 should be replated anyway to determine the background frequency due to
ampicillin
escapes. From those that breed true, each candidate interactor should be
recovered and
tested for interaction with an unselected partner. Selected pairs should be
sequenced and
BLAST-searched for homology to known genes (Altschul et al., J Mol Biol (1990)
215:403; Altschul et al., Nucleic Acids Res (1997) 25:3389). A large number of
interactions among secreted and membrane proteins of immune cells are already
known,
such as the B-cell co-activation antigen, CD40 and its T-cell ligand, CD40L,
and the T-cell
activation antigens B7.1 and B7.2 and their ligands CD28 and CTLA4. Labeled
oligonucleotide hybridization probes may be prepared for these known
interactions, and
colony lifts of the entire interaction library may be probed to see what
fraction of expected
interactors are .actually represented in the library. Interaction partner
sequences from
~ positive clones may be recovered, and :homology searched to determine if
known or new
interactors have been identified. Colonies expressing bona fide interactions
may be grown
up and stored indefinitely in 15 % glycerol at -70°C, pending further
characterization or
use for a . g . , drug screening .
2o Example 9
Construction of an Intra-Cellular Signal Transduction Biosensor.
Interaction-dependent (3-lactamase fragment complementation systems can be
adapted for activation or inactivation by virtually any post-translational
modification that
occurs naturally in cells. As a result they may be deployed intra-cellularly
as biosensors to
monitor the activity of any process which is regulated by post-translational
modification. A
major class of such processes is phosphorylation-regulated signal transduction
pathways.
Phosphorylation-regulated intermediates are obligatory components of most
processes by
which cells respond to extra-cellular conditions or messenger molecules by
altering gene
expression. Cellular responses to extra-cellular signals may be fall into
three general
categories, growth, survival, and differentiation. A ubiquitous component of
neoplastic
transformation is the deregulation of growth control signaling, often
accompanied by the
deregulation of survival signalling as well. This often occurs by over-
expression of
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
54
phosphorylation-regulated signal transducers, or by mutational disabling of
phosphorylation-mediated regulation. Thus, most so-called oncogenes are
phosphorylation-
regulated growth signal transducers, which become over-expressed or mutated to
constitutive activity in cancer cells.
The Her-2/neu oncogene is a 185 kDa Type I transmembrane receptor tyrosine
kinase, which is a member of the epidermal growth factor receptor (EGFR)
family. This
growth factor receptor is over-expressed in particularly aggressive
adenocarcinomas of
epithelial origin in a number of tissues, notably breast. When normally
expressed, Her-
2/neu hetero-dimerizes with other EGF-family receptors when they are ligated
by growth
factor. This leads to cross phosphorylation of multiple tyrosines on the
cytoplasmic
domains of the receptors. Phosphorylation of tyrosine 1068 (Tyr1068) on Her-
2/neu leads
via phospho-tyrosine-binding accessory proteins and guanosine nucleotide
exchange factors
to activation of p21'°~, and thence to activation of cell division via
the MAP kinase cascade.
When Her-2/neu is sufficiently over-expressed, the background level of ligand-
independent EGFR hetero-dimerization rises to a level which is in
turn.sufficient to
maintain constitutive mitogenic signaling even in the absence of growth
factor, leading to
the characteristically uncontrolled growth of tumor cells. Thus, there is much
interest in
finding drugs which can block the activation of Her-2/neu, particularly in a
manner which
can prevent constitutive signaling in tumor cells without blocking EGF
signalling in normal
2o cells.
A cell-based biosensor, which produces a readily detectable and quantifiable
signal
when Her-2/neu activation is blocked, would be particularly useful for high-
throughput
screening of chemical libraries for compounds with anti-breast tumor
potential. Such a
biosensor may be set up with a (3-lactamase fragment complementation system as
follows.
The w fragment could be fused via flexible linker to the C-terminus of Her-
2/neu, which is
proximal to the Tyr1068 substrate of the receptor kinase. The a fragment could
then be
fused to a binding protein, such as a scFv or VL, which binds to the Tyr1068
region of the
receptor only when Tyr1068 is unphosphorylated. Since Tyr1068 is mostly
phosphorylated
in Her-2/neu over-expressing cells, especially in the presence of EGF, (3-
lactamase
3o activation would be minimal. However, in the presence of an inhibitor of
Her-2/neu
activation, the proportion of unphosphorylated Tyr1068 would rise, recruiting
the a-
Tyr1068 binder fusion to the receptor where a-c~ complementation would
increase (3-
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
lactamase activity in the cells. In the presence of a fluorogenic ~3-lactamase
substrate,
inhibitors of Her-2/neu activation could be readily identified by increasing
fluorescence in
a matter of minutes, since dephosphorylation of Tyr1068 occurs rapidly upon
inhibition of
the Her-2/neu kinase activity.
5 For intra-cellular biosensors both maximum activity and the activation index
will be
important. However, for all five of the best TEM-1 fragment pairs the
activation index is
expected to depend almost entirely on the difference in the affinity of the
binder for Tyr vs
phospho-Tyr. Thus, the fragment pair with the highest activity, i.e.,
G253/K254 (x,253
and w254), would be preferred, especially since for intra-cellular
applications the break-
1o point disulfide cannot be used. It may be possible to increase the intra-
cellular activity of
a253/c~254, if desired, by selecting one or two fragment stabilizing tri-
peptides, as
described above.
The first step in developing the Her-2/neu inactivation biosensor would be to
obtain
a Tyr1068-binding protein. This could be accomplished by inserting the coding
sequence
15 for the substrate peptide, PVPEYINQS, into the active site of thioredoxin;
between G33
and P34, flanked by short flexible linkers such as PGSGG to minimize
structural
constraints on the peptide, which does not require a rigid structure for
binding to its natural
ligand, the Grb2 SH2 domain. This Tyr1068 trxpep can then be fused via a
(Gly4Ser)3
linker to the N-terminus of w254, and co-expressed in E. coli TG-1 cells with
a scFv
20 library of at least 108 clones, or a VL library of at least 106 clones
fused to the C-terminus
of x,253 via the (Gly4Ser)3 linker. Since the Tyr1068-binder is being selected
for
deployment in the mammalian cell cytoplasm, it might be prudent to perform the
selections
in the E. coli cytoplasm. For this purpose the vectors in Figure 6 could be
used with the
signal peptides removed. Then a chromogenic substrate such as nitrocefm (~,max
= 485
25 nm; s = 17,420 M-' cm'; McManus-Munoz and Crowder, Biochemistry (1999)
38:1547)
would be used to select Tyr1068=binders by color. By plating at least 106-10g
transformants at moderate to high stringency, i.e., on decreasing
concentrations of the
substrate, it should be possible to identify binders with sub-micromolar
affinities since Tyr
is the most common amino acid in high-affinity protein-protein interfaces.
Such affinities
3o will be desirable for maximum discrimination between Tyr and phospho-Tyr.
Selected
Tyr1068-binders must be tested for inhibition by phosphorylation of the Tyr.
This can
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
56
easily be accomplished by expressing the vectors in isogenic cells which over-
express a
broad spectrum Tyrosine kinase (TKXI cells, Stratagene, Inc., La Jolla, CA).
Once a suitable phosphate-sensitive Tyr1068-binder has been identified, the
entire
coding sequence for the x253 - Tyr1068-binder fusion may be subcloned into a
mammalian expression vector, such as the pCMV-Tag vectors (TKXl cells,
Stratagene,
Inc., La Jolla, CA) for expression in mammalian cells from the cytomegalovirus
promoter.
The w254 fragment must be expressed as a fusion to the C-terminus of the Her-
2/neu
cytoplasmic domain, which contains Tyr1068. The coding sequence of the 1210-
residue
EGF receptor (Genbank accession no. X00588; Ullrich et al., Nature (1984)
309:418) may
be used as it is operationally identical to Her-2/neu, and its Tyr1068 will
become
phosphorylated under the same conditions of over-expression and/or growth
factor ligation
in tumor cells. When fused to the C-terminus of EGFR via the (Gly4Ser)3
linker, the 35
residue c~254 (3-lactamase fragments will be only 152 residues away from
Tyr1068. Both
the EGFR-X254 fusion and the a253-Tyr1068-binder fusion may be expressed from
the
~5 same vector from a dicistronic mRNA. This is accomplished by inserting an
roternal
ribosome entry site (IRES; Martinez-Salas, Curr Opin Biotechnol (1999) 10:458)
between
the termination codon of the upstream cistron and the initiation codon of the
downstream
cistron. This will allow both proteins to be made simultaneously from the same
mRNA.
The vector may be introduced into the tumor cell line by cationic liposome-
mediated
20 transfection, using e.g., lipofectamine (Gibco-BRL, Gaithersburg, MD)
according to the
protocol in the product literature. Operation of the biosensor may be tested
in transiently
transfected cells, and if operational, stable transformants may then be
isolated by selection
for long term antibiotic resistance. Multiple free-diffusible chromogenic and
fluorogenic
substrates are available for continuous monitoring of ~-lactamase activity.
Operationally,
25 the c~254 fragment will be anchored to the plasma membrane at the C-
terminus of the
cytoplasmic domain of the receptor near Tyr1068, and the x253 fragment will be
free in
the cytoplasm as the Tyr1068-binder fusion. ATP-analog tyrosine kinase
inhibitors are
available commercially and can be used as positive controls for inhibitor
selection, and to
determine the signal increment from fully-activated to fully-inhibited EGFR.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
57
Example 10
A Fragment Complementation System for Neomycin Phosphotransferase.
Enzyme fragment complementation systems may also be useful for selection for
the
simultaneous incorporation of multiple genetic elements into the same cell or
organism.
s For example, the production of secretory IgA antibodies in plants requires
the introduction
of four different genes into the same plant. For practical reasons this
requires the
introduction of at least two and preferably three different DNA molecules. For
the
production of genetically stable transgenic plants, each DNA molecule must
carry its own
selectable marker. The use of multiple antibiotic selection systems on the
same
transformants is cumbersome and inefficient, as the overall false positive and
false negative
rates tend to scale as the product of the rates for the individual
antibiotics. Thus, two- or
three-piece fragment complementation systems for a single antibiotic offer a
distinct
advantage over multiple antibiotic selection.
For a two fragment system, dependence of activation on the interaction of
IS heterologous domains is not necessary. However, for simultaneous selection
of triple
transgenics, complementation of the enzyme fragment pair must be dependent on
a
heterologous interaction mediated by a free ligand, analogous to the
activation of (3-
lactamase by the tri-molecular interaction of x197 jun, scFv-w198, and CD40-
fos, as
described above. For these applications, the most important parameter is the
maximum
activity of the reconstituted enzyme, which is a function of both the specific
activity and
the efficiency of complementation. The activation index is not relevant
because the each
fragment alone will have essentially no detectable activity, providing a
background of zero.
Thus, to insure recovery of the most competent fragment pairs for intra-
cellular activity,
the fos and jun interactors should be used with tri-peptide libraries between
the break-
points and the (Gly4Ser)3 linkers. The tri-peptide libraries will provide
stabilizers for each
fragment so that the selection will be biased toward the fragments producing
the highest
specific activities. For two-trait selection applications, i.e., bi-molecular
selections, where
a heterologous interaction is not required, specific activity may be increased
further by
mutagenesis and selection for fold accelerating mutations. For three-trait
selection
3o applications, selected fragment pairs will have to be tested for dependence
on the
heterologous interaction. In this case, the activation index will be of some
importance, but
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
58
as with in vitro applications a modest index of 1000 will be more than
adequate for clean
selections.
Neomycin phosphotransferase II (NPTII; Genbank accession no. M77786) is a 267-
amino acid enzyme from E. coli which inactivates aminoglycoside antibiotics
such as
neomycin and kanamycin by phosphorylation from ATP. NPTII is widely used as a
selectable marker for plant and animal cell transformation. Thus, fragment
complementation systems for NPTII would be particularly useful for facile
generation of
multiple-trait plant and animal transgenics. The three-dimensional structure
of NPTII is
not known, and its homology to known structures is too low for reliable
prediction.
1o However, as described above, empirically-derived neural net algorithms are
available
which allow fairly accurate prediction of secondary structure and solvent
exposure for any
protein sequence. The best of these algorithms is the PredictProtein program
of Rost and
Sander (1993, 1994, supra). Application of this program to the protein
sequence of NPTII
produced the result shown in Figure 10. Ten regions of the sequence have been
predicted
to have little secondary structure and to be exposed to solvent, and therefore
to be potential
sites for productive fragmentation. Fragment pairs. corresponding to breakage
in the center
of each of these ten regions, or at two equally-spaced sites in the longer
regions, may be
generated by PCR with appropriate primers, and subcloned into vectors like
those
illustrated in Figure 6 for expression as the fos and jun helix fusions with
intervening
linkers. The vectors would differ from those in Figure 6 in not encoding
signal peptides,
and the pA01 vector would have ampicillin resistance instead of kanamycin
resistance.
Also, the vectors should contain VRK or NNK random tri-peptide-encoding
sequences
between the cloning sites for the enzyme fragments and the (Gly4Ser)3 linkers.
The PCR product for each fragment is restriction digested and ligated into the
appropriate vector, a fragments into the pAEI-type vector and c~ fragments
into the pA01-
type vector. The ligation products are then introduced into TG-1 cells by high-
voltage
electroporation, and plated onto chloramphenicol or ampicillin. At least 10~
transformants
should be collected for each fragment. Also, kanamycin sensitivity should be
determined
for each fragment library, both to prevent false positives and to determine
the minimum
3o quantitatively selective kanamycin concentration. This should be the
concentration on
which single fragment plating efficiencies are < 10-6, since the frequencies
of the fragment-
stabilizing peptides could be this low. Since ---10g co-transformants will be
needed for
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
59
each fragment pair for complete coverage of the tri-peptide libraries,
quantitative phage
infection should be used to combine the two libraries for each fragment pair.
This is
accomplished by rescuing the w-fragment libraries (in the pA01-type phagemid
vector) as
phage using M 13K07 helper phage as described above. For facile quantitative
infection at
least 109 cells bearing each a, fragment library should be inoculated with at
least 10" phage
bearing the corresponding cu fragment library. After one-two hours in
suspension culture
with gentle shaking to allow phage adsorption, penetration, and initiation of
gene
expression, the cells of each fragment pair are centrifuged, washed, and
plated onto ten
150-mm dishes containing solid LB medium with the minimum quantitatively
selective
concentration of kanamycin.
After overnight growth at 37°C, all kanamycin-resistant colonies may be
pooled
and re-plated onto increasing concentrations of kanamycin to identify those
tri-
peptide/fragment pair combinations producing the highest levels of kanamycin
resistance.
As many of the most active clones as necessary should be tested for dependence
of activity
on the fos-jun interaction. This can most easily be accomplished by removing
one of the
helixes by restriction digestion at sites in the gene construct included for
this purpose. The
digestion products are then re-ligated, re-transformed into '/'G-1 cells, and
replated on
kanamycin. As explained above activation indexes of 1000 are more than
adequate, so the
most active pairs with indexes of at least 1000 would be optimal. For tri-
molecular
activation in the cytoplasm, two hetero-dimerizing helix pairs may
conveniently be used,
such as the parallel-binding helixes from fos and jun as described above, and
the anti-
parallel-binding helixes from yeast DNA topoisomerase II (TopII; Berger et al.
, Nature
(1996) 379:225). One of each helix pair would be fused to an NPTII fragment,
and the
other two helixes would be fused to each other, so that the NPTII fragments
would only
come together when the 2-helix fusion was present to form the tri-molecular
complex. For
example, an a,-TopIIN fusion and a fos-c~ fusion could only be brought
together and
activated by a jun-TopIIC fusion. Genes encoding each of the three fusions
could then be
distributed among three different DNA constructs which also encode genes of
interest. In
this way eukaryotic cells could be transformed with a mixture of the three
different
3o constructs and selected for the simultaneous presence of all three genes in
the same cell
simply by selection for growth on a single antibiotic.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
Example 11
Target-Activated Enzyme Prodrug Therapy.
Antibody-directed enzyme prodrug therapy (ADEPT) is a promising anti-cancer
chemotherapeutic strategy which takes advantage of the catalytic power of
enzymes to
5 amplify the cytotoxicity-targeting power of tumor-specific antibodies.
Enzymes are
concentrated at the tumor site when administered as conjugates of tumor-
specific
antibodies. After unbound conjugate has cleared from the circulation, prodrugs
may be
administered which are relatively non-toxic until activated by the tumor-bound
enzyme,
whereupon the cytotoxic product may accumulate at the tumor site to
concentrations which
1o would be unattainable by parenteral administration of the drug without
excessive toxicity.
Enzymes such as (3-lactamase have been chemically or genetically conjugated to
tumor-
targeting antibodies and used with (3-lactam derivatives of anti-tumor drugs
such as
cephalosporin mustards and anthracyclines to achieve promising anti-tumor
effects in
animals. The efficacy of ADEPT is limited, however, by the need for unbound
conjugate to
15 clear the circulation before the prodrug can be administered. By the time
the circulating
conjugate is depleted to the threshold below which systemic activation of the
prodrug
would produce acceptable levels of toxicity, so much of the conjugate has been
lost from
the tumor that efficacy is often seriously compromised.
This problem may be overcome by using an interaction-dependent (3-lactamase
2o fragment complementation system with tumor targeting antibodies. When fused
to single-
chain antibody fragments (scFv) which recognize non-overlapping epitopes on
tumor
markers, the (3-lactamase fragments can localize to the tumor and reconstitute
sufficient (3-
lactamase activity on the tumor cell surface to produce high levels of tumor-
localized
cytotoxicity from [3-lactam prodrugs. The great advantage of such a system is
that prodrug
25 activation cannot occur in the general circulation or anywhere the tumor
marker is not
encountered, so that the prodrug may be administered either simultaneously
with high
doses of the scFv-fragment fusions, or at the point of highest tumor load of
the fragments,
without regard for the circulating levels of the fragments which would be
completely
inactive.
3o As an example, the construction and purification of fusions of interaction-
dependent
(3-lactamase fragments with scFv which bind non-overlapping epitopes on the
human breast
tumor marker Her-2/neu is described. One may then determine the kinetics of
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
61
reconstitution of ~3-lactamase activity on the surface of Her-2/neu -
expressing SKOV3
human ovarian cancer cells. Under conditions of optimum loading, killing of
the cells may
then be assessed for different cephalosporin prodrugs as a function of
concentrations known
to be limiting in vivo. The resulting Tumor-Activated Enzyme Prodrug Therapy
(TAcEPT)
system may then be tested for its ability to ablate SKOV3 and other Her-2/neu-
expressing
human tumors in severe combined immuno-deficient (scid) mice. Once the
efficacy and
safety of the system has been demonstrated in animal models, toxicity and
efficacy trials
may be initiated in human breast cancer subjects.
The requirements for therapeutic use of (3-lactamase fragment complementation
systems are similar to those for in vitro use in general. The most important
parameters are
specific activity and fragment stability, while activation indexes above 1000
confer little
additional efficacy. Thus, the a,253/c~254 would be the recommended fragment
pair for
this application because it has the highest interaction-dependent specific
activity, the
fragments are moderately stable, and its activation index is more than
adequate. However,
the stability of the x,253 fragment could~probably be improved by a custom
fragment-
stabilizing tri-peptide. Thus, before setting up the .tumor-activated system,
one might first
subclone a degenerate sequence encoding the VRK or .NNK tri-peptide library
into the
x,253 expression construct between the break-point cysteine and the linker
(see pAEl in
Figure 6). x,253-stabilizing tri-peptides would then be selected by plating at
least 104
library transformants on increasing ampicillin from 400 to 1000 pg/ml, since
a253/~254
plates quantitatively on 400 p,g/ml even without a stabilizing peptide, and
wild-type TEM-1
(3-lactamase does not plate on more than 1000 yg/ml when expressed under these
conditions.
l la. Expression of TEM-1 ~3-lactamase H25-6253 (x,253) and K254-W288 (c~254)
fragments as fusions to scFv against non-overlappip n~epitopes on the Her-
2/neu human
breast tumor marker.
The tumor activation mechanism for these fragments may employ two scFvs such
as
those described by Schier et al. (Gene (1996) 169:147), which were derived
from a phage
3o display library of a human non-immune repertoire (Marks et al., 1991) by
panning against
a recombinant fragment comprising the extra-cellular domain (ED) of Her-2/neu.
These
two scFv, appear to recognize non-overlapping epitopes, since they do not
compete for
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
62
binding to the Her-2/neuED by ELISA. The affinity of one of these scFv was
improved to
sub-nM Kd in vitro (Schier et al. , 1996, supra), and similar improvements in
the other
could be made using the same methods (Balint and Larrick, Gene (1993)
137:109). The
coding sequences for the scFv may be subcloned into the ~-lactamase a and cu
fusion
production vectors, p~3laca and p~3lacc~, shown in Figure 11. These vectors
are derived
from pET26b (Novagen), and have convenient restriction sites for insertion of
both scFv
and ~3-lactamase fragment sequences. Each fusion protein is inducibly
expressed (IPTG)
from the strong phage T7 promoter under the control of the lac repressor. Each
primary
translation product contains a pelB signal peptide for secretion into the
bacterial periplasm
1o and a C-terminal Hisb tag for one-step purification from osmotic shock
extracts by
immobilized metal ion affinity chromatography (IMAC, Janknecht et al., Proc
Natl Acad
Sci (1991) 88:8972). The yield of each fusion protein can be optimized
primarily by
manipulation of the inducer concentration and the growth temperature.
Each scFv may be expressed as both a and ~ fusions to determine which
arrangements) (1) support the highest binding activity, (2) support the
highest enzymatic
activity, and (3) support the highest yields. Initially, expression may be
optimized by the
.. criterion of silver-stained PAGE. Then fusion proteins should be purified
from osmotic
shock extracts (Neu and Heppel, 1965, supra) by IMAC. The purified fusion
proteins may
be tested for binding to an immobilized recombinant fusion of the Her-2/neu
extra-cellular
2o domain (ED) to a stabilizing immunoglobulin domain (Ig) by ELISA using an
anti-Hisb tag
antibody (Qiagen). The purified fusion proteins may then be tested for
reconstitution of (3-
lactamase activity on immobilized rc- Her-2/neu ED-Ig using a chromogenic
substrate,
nitrocefm (~,max = 485 nm; s = 17, 420 M-' cm' ; McManus-Munoz and Crowder,
1999,
supra). Immobilized BSA may be used as the negative control.
l 1b. Determination of the kinetics of specific ~~3-lactamase activation by
bindingof a-
laca/c~-scFv fusions to immobilized recombinant antigen.
One may determine ~3-lactamase activity quantitatively as a function of
binding of
the fusion proteins to the immobilized antigen. This rate may then be compared
to that
obtainable with intact (3-lactamase fused to the same scFv as an indication of
how much
activity may be localized on a tumor compared to an established vehicle, i.e.,
an antibody-
(3-lactamase conjugate.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
63
First, conditions are established for saturating the antigen with one of the
scFv-(3-lac
fragment fusion proteins. The wells of microtiter plates are coated with
antigen, and
exposed to increasing amounts of the first scFv-fragment fusion until the
ELISA signal
plateaus. At this level, i.e., saturating amounts of the first fusion protein,
increasing
amounts of the second fusion is added. After binding and washing, (3-lactamase
activity is
determined spectrophotometrically after a 30' incubation with excess
nitrocefin. If the
assay is performed in triplicate, V",~ should be a more or less linear
function of the
concentration of the second fusion. As the amount of second fusion is
increased, at some
point V",~ should plateau. The amount of the second fusion bound can be
determined by
ELISA, and a relative specific activity (k~Q,'e') may be computed for the
fragment-
reconstituted (3-lactamase. The KM may be estimated in solution with
saturating antigen and
saturating first fusion and limiting amounts of the second fusion. A range of
nitrocefm
concentrations is added and the initial rates of change of absorbance at 485
nm is measured
as a function of second fusion concentration. The KM is then computed from
standard
regression analysis.
To compare with intact (3-lactamase, a fusion of intact (3-lactamase to the
second
scFv may be prepared. This is then added in increasing amounts to antigen-
coated wells
which had been saturated with the first fusion as had been done before. Again,
V"~ should
be a more or less linear function of the amount of intact (3-lactamase fusion
and should
2o plateau at saturation. At each point, the amount of intact (3-lactamase
fusion bound, as
determined by ELISA, should be comparable to the amount of the second fragment
fusion
bound, and the ratio of V,na,. should reflect the ratio of specific activities
of the intact and
fragment-reconstituted (3-lactamases. For comparison, the KM should be
estimated as
described above for the fragment-reconstituted enzyme. The TEM-1 a253/c~254
fragment
complex is expected to have a maximum activity (k~a,) near that of the intact
enzyme. If the
KM are also comparable, activities on a tumor up to 100-fold higher at the
peak of prodrug
activation than with the conventional antibody-(3-lactamase fusion might be
expected, which
may have 1 % or less of its peak activity left when the unbound fusion has
cleared the
circulation enough to allow prodrug administration.
11c. Determination of killing kinetics of Her-2/neu-expressing SKOV3 ovarian
carcinoma
cells by scFv-mediated (3-laca/w activation of cephalosporin prodru~
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
64
The arrangements) of scFv-(3-lactamase fragment coupling which produces) the
highest specific (3-lactamase activities on immobilized antigen may then be
tested for
activation of (3-lactamase activity in the presence of human tumor cells
expressing the Her-
2/neu antigen. Cell killing may be assayed using any of the three
cephalosporin prodrugs
shown in Figure 5. The fragment-reconstituted activity may again be compared
with the
intact (3-lactamase activity, this time with respect to tumor cell killing.
Such results should
indicate the dose range which may be required to show a significant anti-tumor
effect in
animals, which will be the next step in preclinical evaluation of the tumor-
targeted (3-
lactamase.
1o The SK-OV-3 line of human ovarian adenocarcinoma cells (ATCC) may be seeded
in 6-well tissue culture plates at 3x105 cells per well in Dulbecco's Minimum
Essential
Medium (DMEM) supplemented with 10 % fetal calf serum (FCS), and allowed to
grow to
confluency at 37°C in 10% CO2. The saturability of both Her-2/neu
epitopes on the cells
may be determined with increasing amounts of_ intact (3-lactamase fused to
each scFv, as
determined spectrophotometrically after nitrocefin hydrolysis. The V",~ of the
fragment-
reconstituted enzyme may then be determined on the.cells with saturating
concentrations of
both fusions and nitrocefin. It would be expected to conform to the predicted
activity
based on the maximum intact [3-lactamase activity and the ratio of V",~
observed on the
immobilized recombinant antigen. The sensitivity of the cells to any of the
three prodrugs
shown in Figure 5 may be determined essentially as described by Marais et al.
(Cancer
Research (1996) 56:4735) with and without the intact (3-lactamase-scFv fusions
and the a./c~
fragment-scFv fusions under saturating conditions. The prodrugs are dissolved
in DMSO
and diluted into DMEM/FCS to a range of concentrations immediately prior to
use. One ml
is added to each well and the cells are incubated overnight. The cells are
then washed,
trypsinized, and viability is determined by dye exclusion. Aliquots are then
seeded into
fresh dishes. After four days of growth, cell viability is assessed by
incorporation of [3H]
thymidine as determined by liquid scintillation counting of acid insoluble
material. The
results are expressed as percentage of untreated control cells. Again, the
relative
cytotoxicities of the prodrugs with the (3-lactamase fragment system may be
compared to
3o those of the intact (3-lactamase fusions, particularly at the lower prodrug
concentrations
where second order rate constants (k~Q,IKM) may be important, to give an
indication of the
potential increase in efficacy of TAcEPT over conventional ADEPT in vivo.
CA 02374476 2001-11-19
WO 00/71702 PCT/US00/07108
All publications and patent applications mentioned in this specification are
indicative
of the level of skill of those skilled in the art to which this invention
pertains. All
publications and patent applications are herein incorporated by reference to
the same extent
5 as if each individual publication or patent application was specifically and
individually
indicated to be incorporate by reference.
The invention now having been fully described, it will be apparent to one of
ordinary skill in the art that many changes and modifications can be made
thereto without
departing from the spirit or scope of the appended claims.