Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-1-
METHODS AND COMPOSITIONS FOR ASSAYING ANALYTES
Related Applications
This application is related to U.S. application Serial No. 09/347,878, filed
July 6, 1999,
entitled "METHODS AND COMPOSITIONS FOR ASSAYING ANALYTES" and U.S.
application Serial No. 09/457,205, filed December 6, 1999, entitled "METHODS
AND
COMPOSITIONS FOR ASSAYING ANALYTES." The subject matter of the above U.S.
applications is incorporated in its entirety.
Field of the Invention
The present invention relates to compositions and methods for assaying
analytes,
preferably, small molecule analytes. More particularly, assay methods that
employ, in place of
antibodies, modified enzymes that retain binding affinity or have enhanced
binding affinity, but
that have attenuated catalytic activity, are provided. The modified enzymes
and fusion proteins
containing the modified enzymes are also provided.
Background of the Invention
Methods for assaying analytes have wide applications. Many analytes including
small
molecule analytes are essential components and/or participants of biological
systems and
processes. Methods for assaying these analytes can be used in monitoring the
biological
systems/processes, or prognosis or diagnosis of diseases or disorders caused
by deficiencies
and/or imbalances of the analytes. For instance, homocysteine (Hcy), a
thiolated amino acid;
folic acid, an organic acid; and cholesterol, a lipid are all important
prognostic and diagnostic
markers for a wide range of cardiovascular diseases. Vitamins are important
prognostic and
diagnostic markers for various vitamin deficient diseases or disorders.
Glucose, a
monosaccharide, is a diagnostic marker for numerous glycemic conditions such
as diabetic
mellitus. Ethanol, an alcohol, is important in monitoring liquor consumption
and potential liver
damage. Bile acids or bile salts are important prognostic and diagnostic
markers for certain
cancers such as colon cancer. Monitoring uric acid is important because
abnormally high
concentration of uric acid is the diagnostic marker and cause of hyperuricemia
leading to gout,
which is very painful and can cause damage to the kidney. In addition to these
prognostic and
diagnostic uses, methods for assaying analytes have applications in other
agricultural, industrial
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-2-
or environmental protection processes where determining the presence, location
and amount of
the analytes is critical.
Assays for Homocysteine .
Homocysteine (Hcy) is a thiol-containing amino acid formed from methionine
during S-
adenosylmethionine-dependent transmethylation reactions. Intracellular Hcy is
remethylated to
methionine, or is irreversibly catabolized in a series of reactions to form
cysteine. Intracellular
Hcy is exported into extracellular fluids such as blood and urine, and
circulates mostly in
oxidized form, and mainly bound to plasma protein (Refsum, et al., Annu. Rev.
Medicine,
49:31-62 (1998)). The amount of Hcy in plasma and urine reflects the balance
between Hcy
production and utilization. This balance may be perturbed by clinical states
characterized by
genetic disorders of enzymes involved in Hcy transsulfuration and
remethylation (e.g.,
cystathionine 13-synthase and NS°1°-methylenetetrahydrofolate
reductase or dietary deficiency of
vitamins (e.g., vitamin B6, B12 and folate) involved in Hcy metabolism (Baual,
et al., Cleveland
Clinic Journal of Medicine, 64:543-549 (1997)). In addition, plasma Hcy levels
may also be
perturbed by some medications such as anti-folate drugs (e.g., methotrexate)
used for
treatments of cancer or arthritis (Foody, et al., Clinician Reviews, 8:203-210
(1998)).
Severe cases of homocysteinemia are caused by homozygous defects in genes
encoding
for enzymes involved in Hcy metabolisms. In such cases, a defect in an enzyme
involved in
either Hcy remethylation or transsulfuxation leads to as much as 50-fold
elevations of Hcy in
the blood and urine. The classic form of such a disorder, congenital
homocysteinemia
(Hcyemia), is caused by homozygous defects in the gene encoding cystathionine
13-synthase
(CBS). These individuals suffer from thromboembolic complications at an early
age, which
result in stroke, myocardial infarction, renovascular hypertension,
intermittent claudication,
mesenteric ischemic, and pulmonary embolism. Such patients may also exhibit
mental
retardation and other abnormalities resembling ectopia lentis and skeletal
deformities (Perry T.,
Homocysteine: Selected aspects in Nyham W.L. ed Heritable disorders of amino
acid
metabolism. New York, John Wiley ~ Sons, pp. 419-451 (1974)). It is also known
that
elevated Hcy levels in pregnant women is related to birth defects of children
with neurotube
closures (Scott, et al., "The etiology of neural tube defects" in Graham, L,
Refsum, H.,
Rosenberg, LH., and Ureland P.M ed. "Homocysteine metabolism: from basic
science to
clinical medicine" Kluwer Academic Publishers, Boston, pp. 133-136 (1995)).
Thus, the
diagnostic utility of Hcy determinations has been well documented in these
clinical conditions.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-3-
It has been demonstrated that even mild or moderately elevated levels of Hcy
also
increase the risk of atherosclerosis of the coronary, cerebral and peripheral
arteries and
cardiovascular disease (Boushey, et al., JAMA, 274:1049-1057 (1995)). The
prevalence of
Hcyemia was shown to be 42%, 28%, and 30% among patients with cerebral
vascular disease,
S peripheral vascular disease and cardiovascular disease, respectively
(Moghadasian, et al., Arch.
Intern. Med., 157:2299-2307 (1997)). A meta-analysis of 27 clinical studies
calculated that
each increase of 5 ~tM in Hcy level increases the risk for coronary artery
disease by 60% in
men and by 80% in women, which is equivalent to an increase of 20 mg/dl-1 (0.5
mmol/dl-1) in
plasma cholesterol, suggesting that Hcy, as a risk factor, is as strong as
cholesterol in the
general population. Results from these clinical studies concluded that
hyperhomocysteinemia
is an emerging new independent risk factor for cardiovascular disease, and may
be accountable
for half of all cardiovascular patients who do not have any of the established
cardiovascular
risk factors (e.g., hypertension, hypercholesterolemia, cigarette smoking,
diabetes mellitus,
marked obesity and physical inactivity).
Mild homocysteinemia is mainly caused by heterozygosity of enzyme defects. A
common polymorphism in the gene for methylenetetrahydrofolate reductase
appears to
influence the sensitivity of homocysteine levels to folic acid deficiency
(Boers, et al., J. Inher.
Metab. Dis., 20:301-306 (1997)). Moreover, plasma homocysteine levels are also
significantly
increased in heart and renal transplant patients (LJeland, et al., J. Lab.
Clin. Med , 114:473-501
(1989)), Alzheimer patients(Jacobsen, et al., Clin. Chem., 44:2238-2239
(1998)), as well as in
patients of non-insulin-dependent diabetes mellitus (Ducloux, et al., Nephrol.
Dial.
Transplantl, 13:2890-2893 (1998)). The accumulating evidence linking elevated
homocysteine
with cardiovascular disease has prompted the initiation of double-blind,
randomized and
placebo controlled multicenter clinical trials to demonstrate the efficacy of
lowering plasma
Hcy in preventing or halting the progress of vascular disease (Diaz-Arrastia,
et al., Arch.
Neurol., 55:1407-1408 (1998)). Determination of plasma homocysteine levels
should be a
common clinical practice.
As a risk factor for cardiovascular disease, the determination of total plasma
Hcy levels
(reduced, oxidized and protein-bound) has been recommended in clinical setting
(Hornberger,
et al., American J. ofPublic Health, 88:61-67 (1998)). Since 1982, several
methods for
determining total plasma Hcy have been described (Mansoor, et al., Anal.
BioChem., 200:218-
229 (1992); Steir, et al., Arch. Intern. Med., 158:1301-1306 (1998); Ueland,
et al., Clin. Chem.,
39:1764-1779 ()1993); and Ueland, et al., "Plasma homocysteine and
cardiovascular disease"
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-4-
in Francis, R.B.Jr.eds. Atherosclerotic Cardiovascular Disease, Hemostasis,
and Endothelial
Function. New York, Marcel Dokker, pp. 183-236 (1992); see, also, Ueland, et
al., "Plasma
homocysteine and cardiovascular disease" in Francis, R.B.Jr.eds.
Atherosclerotic
Cardiovascular Disease, Hemostasis, and Endothelial Function. New York, Marcel
Dokker,
pp. 183-236 (1992)). The assay of total Hcy in plasma or serum is complicated
by the fact that
70% of plasma Hcy is protein-bound and 20-30% exists as free symmetric or
mostly
asymmetric mixed disulfides. Free reduced Hcy exists in only trace amounts
(Stehouwer, et al.,
Kidney International, 55308-314 (1999)).
Most of the methods require sophisticated chromatographic techniques such as
HPLC,
capillary gas chromatography, or mass spectrometry (GClMS) to directly or
indirectly (e.g.,
enzymatic conversion of Hcy to SAH (S-adenosylhomocysteine) by SAH hydrolase
followed
by HPLC or TLC separation) measure Hcy. Radioenzymatic conversion of Hcy to
radiolabeled
SAH by SAH hydrolase prior to TLC separation has also been used. A feature
common to
these methods includes the following four steps: ( 1 ) reduction of oxidized
Hcy to reduced Hcy;
(2) precolumn derivitization or enzymic conversion to SAH; (3) chromatographic
separation;
and (4) detection of the Hcy derivative or SAH. In these assays,
chromatographic separation,
which is often time-consuming and cumbersome to perform, is a common key step
of these
methods. More particularly, these methods require highly specialized and
sophisticated
equipment and well-trained analytic specialists. The use of such equipment is
generally not
well-accepted in routine clinical laboratory practice.
Immunoassays for Hcy that use a monoclonal antibody against SAH (Araki, et
al., J.
Chromatog., 422:43-52 (1987) are also known. These assays are based upon
conversion of
Hcy to SAH, which is then detected by a monoclonal antibody. Monoclonal
antibody against
albumin-bound Hcy has been developed for determination of albumin-bound Hcy
(Stabler, et
al., J. Clin. Invest., 81:466-474 (1988)), which is the major fraction of
total plasma Hcy. Other
immunological protocols are also available (see, e.g., U.S. Patent No.
5,885,767 and U.S.
Patent No. 5,631,127) Though immunoassays avoid a time-consuming
chromatographic
separation step and are amenable to automation, production of monoclonal
antibody is
expensive, somewhat unpredictable, and often requires secondary or even
tertiary antibodies
for detection.
Hence, in general, methods for assaying analytes suffer from several
deficiencies. First,
for many analytes, specific binding partners are not readily available and
this lack of specific
binding partner often compromises the specificity of the assay method.
Although such
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-5-
deficiencies may be overcome by generating antibodies for macromolecule
analytes, generating
antibodies, especially monoclonal antibodies with the desired specificity and
uniformity, is
often time consuming and expensive. In addition, for many small molecule
analytes, the option
of generating antibodies is often not available because small molecules are
poor antigens.
Generation of antibodies against small molecules usually requires conjugation
of the small
molecules to macromolecules, and this often makes the antibody screening more
tedious and
laborious. Second, many methods for assaying analytes, especially small
molecule analytes,
involve chemical derivations and chromatographic separation which can be time
consuming.
Third, many such assay methods use sophisticated and expensive analytical
equipment such as
HPLC's and GC/MS. Hence there is a need for rapid simpler assays that address
these
deficiencies.
It is an object herein to provide assays for detecting analytes. It is also an
object herein
to provide such an assay for quantifying and/or detecting homocysteine in body
fluids and body
tissues.
SUMMARY OF THE INVENTION
Assays, particularly assays that are based on immunoassay formats, but that
employ
mutant analyte-binding enzymes that, substantially retain binding affinity or
have enhanced
binding affinity for desired analytes or an immediate analyte enzymatic
conversion products
but have attenuated catalytic activity, are provided. In place of antibodies,
these assays use
modified enzymes that retain binding affinity or have enhanced binding
affinity, but have
attenuated catalytic activity. These methods are designated substrate trapping
methods; and the
modified enzymes, are designated as "substrate trapping enzymes." The
substrate trapping
enzymes (also designated pseudoantibodies) and methods for preparing them are
also provided.
These substrate trapping enzymes are intended to replace antibodies,
monoclonal, polyclonal or
any mixture thereof, in reactions, methods, assays and processes in which an
antibody
(polyclonal, monoclonal or specific binding fragment thereof) is a reactant.
They can also act
as competitive inhibitors with analytes for binding to entities such as
receptors and other anti-
ligands and other analytes. Hence, they can be used in competitive binding
assays in place of,
for example, receptor agonists or modulators of receptor activity, and for
assays that monitor
drugs.
Any process or method, particularly immunoassays or assays in which an
antibody aids
in detection of a target analyte, can be modified as described herein, by
substituting a substrate
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-6-
trapping enzyme for the antibody used in the process or method. The substrate
trapping
enzymes can be prepared by any method known to those of skill in the art by
which the
catalytic activity of an enzyme is substantially attenuated or eliminated,
without affecting or
without substantially reducing the binding affinity of the resulting modified
enzyme for an
analyte. Other methods in which antibodies may be substituted by mutant
analyte binding
enzymes, include but are not limited to, affinity purification methods, and
methods in which the
mutant enzymes replace neutralizing antibodies.
The methods are particularly useful for detecting analytes indicative of
metabolic
conditions, inborn errors of metabolism, such as hypothyroidism, galactosemia,
phenylketonuria (PKU), and maple syrup urine disease; disease markers, such as
glucose
levels, cholesterol levels, Hcy levels and other such parameters in body fluid
and tissue samples
from mammals, including humans. The methods also include methods for detecting
contaminants in food, for testing foods to quantitate certain nutrients, for
screening blood. The
assays readily can be automated. In addition, the assays can be adapted for
use in point of care
systems and in home test kits. For example, blood test point of care systems
can be adapted for
measuring homocysteine levels using the mutant enzymes provided herein. Home
test kits may
also be adapted for use with the methods and mutant enzymes provided herein.
Accordingly, methods in which an antibody is a reactant, wherein the
improvement is
replacement of the antibody with a substrate trapping enzyme, as defined
herein, are provided.
The methods may also rely on competitive binding of the modified enzyme for a
target analyte.
In another embodiment, a method is provided for assaying an analyte,
preferably a
small molecule analyte, in a sample by: a) contacting the sample with a mutant
analyte-binding
enzyme, the mutant enzyme substantially retains its binding affinity or has
enhanced binding
affinity for the analyte or an immediate analyte enzymatic conversion product
but has
attenuated catalytic activity; and b) detecting binding between the analyte or
the immediate
analyte enzymatic conversion product and the mutant analyte-binding enzyme.
The small molecule analyte to be assayed can be any analyte, including organic
and
inorganic molecules. Typically the small molecule to be assayed has a
molecular weight that is
about or less than 10,000 daltons. Preferably, the small molecule has a
molecular weight that is
about or less than 5,000 daltons.
Inorganic molecules include, but are not limited to, an inorganic ion such as
a sodium,
a potassium, a magnesium, a calcium, a chlorine, an iron, a copper, a zinc, a
manganese, a
cobalt, an iodine, a molybdenum, a vanadium, a nickel, a chromium, a fluorine,
a silicon, a tin,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
a boron or an arsenic ion. Organic molecules include, but are not limited to,
an amino acid, a
peptide, typically containing less than about 10 amino acids, a nucleoside, a
nucleotide, an
oligonucleotide, typically containing less than about 10 nucleotides, a
vitamin, a
monosaccharide, an oligosaccharide containing less than 10 monosaccharides or
a lipid.
The amino acids, include, but are not limited to, D- or L-amino-acids,
including the
building blocks of naturally-occurring peptides and proteins including Ala
(A), Arg (R), Asn
(N), Asp (D), Cys (C), Gln (Q), Glu (E), Gly (G), His (H), Ile (I), Leu (L),
Lys (K), Met (M),
Phe (F), Pro (P) Ser (S), Thr (T), Trp (W), Tyr (Y) and Val (V).
Nucleosides, include, but are not limited to, adenosine, guanosine, cytidine,
thymidine
and uridine. Nucleotides include, but are not limited to, AMP, GMP, CMP, UMP,
ADP, GDP,
CDP, UDP, ATP, GTP, CTP, UTP, dAMP, dGMP, dCMP, dTMP, dADP, dGDP, dCDP,
dTDP, dATP, dGTP, dCTP and dTTP.
Vitamins, include, but are not limited to, water-soluble vitamins such as
thiamine,
riboflavin, nicotinic acid, pantothenic acid, pyridoxine, biotin, folate,
vitamin B1z and ascorbic
acid, fat-soluble vitamins such as vitamin A, vitamin D, vitamin E, and
vitamin K.
Monosaccharides, include but are not limited to, D- or L-monosaccharides and
whether
aldoses or ketoses. Monosaccharides include, but are not limited to, triose,
such as
glyceraldehyde, tetroses such as erythrose and threose, pentoses such as
ribose, arabinose,
xylose, lyxose and ribulose, hexoses such as allose, altrose, glucose,
mannose, gulose, idose,
galactose, talose and fructose and heptose such as sedoheptulose.
Lipids, include, but are not limited to, triacylglycerols such as tristearin,
tripalmitin and
triolein, waxes, phosphoglycerides such as phosphatidylethanolamine,
phosphatidylcholine,
phosphatidylserine, phosphatidylinositol and cardiolipin, sphingolipids such
as sphingomyelin,
cerebrosides and gangliosides, sterols such as cholesterol and stigmasterol
and sterol fatty acid
esters. The fatty acids can be saturated fatty acids such as lauric acid,
myristic acid, palmitic
acid, stearic acid, arachidic acid and lignoceric acid, or can be unsaturated
fatty acids such as
palmitoleic acid, oleic acid, linoleic acid, linolenic acid and arachidonic
acid.
In an exemplary embodiment, mutant S-adenosylhomocysteine (SAH) hydrolases,
substantially retaining binding affinity or having enhanced binding affinity
for homocysteine
(Hcy) or SAH but having attenuated catalytic activity, are provided. Also
provided are
methods, combinations, kits and articles of manufacture for assaying analytes,
preferably small
molecule analytes such as inorganic ions, amino acids (e.g., homocysteine),
peptides,
nucleosides, nucleotides, oligonucleotides, vitamins, monosaccharides (e.g.,
glucose),
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
_g_
oligosaccharides, lipids (e.g., cholesterol), organic acids (e.g., folate
species, bile acids and uric
acids).
In another embodiment, provided herein are purified mutant SAH hydrolases, the
mutant SAH hydrolases substantially retain their binding affinity or have
enhanced binding
affinity for homocysteine (Hcy) or SAH but have attenuated catalytic activity.
Examples of such mutant SAH hydrolases include those in which the attenuated
catalytic activity is caused by mutations) in the mutant SAH hydrolase's
binding site for
NAD+, or mutations) in the mutant SAH hydrolase's catalytic site or a
combination thereof;
those that have attenuated 5'-hydrolytic activity but substantially retain the
3'-oxidative activity;
those that irreversibly bind SAH; those that have a Km for SAH that is about
or less than 10.0
~M; those that have a Kcat for SAH that is about or less than 0.1 S-~; those
that have one or
more insertion, deletion, or point mutation(s); those that are derived from
the sequence of
amino acids set forth in SEQ ID No. 1 or encoded by the sequence of
nucleotides set forth in
SEQ ID No. 2 and have one or, preferably at least two or more mutations
selected from Phe
302 to Ser (F302S), Lys 186 to Ala (K186A), His 301 to Asp (H301D), His 353 to
Ser
(H353S), Arg 343 to Ala (R343A), Asp 190 to Ala (D190A), Phe 82 to Ala (F82A),
Thr 157 to
Leu (T157L), Cys 195 to Asp (C195D), Asn 181 to Asp (N181D), and deletion of
Tyr 432
( O 432); or those that are derived from the sequence of amino acids set forth
in SEQ ID No. 1
or encoded by the sequence of nucleotides set forth in SEQ ID No. 2 and have a
combination of
Arg 431 to Ala (R431 A) and Lys 426 to Arg (K426R) mutations; or any that
hybridize under
conditions of low, more preferably moderate, most preferably high, stringency
along their full-
length and have a Km at least about 10%, more preferably at least about 50% of
the Km of the
wildtype enzyme for the analyte or substrate, but having substantially
attenuated catalytic
activity to the coding portion of the sequence of nucleotides set forth in SEQ
ID No. 1 or
encoding the sequence of amino acids set forth in SEQ ID No. 2.
Isolated nucleic acid fragments encoding the above-described mutant SAH
hydrolases,
preferably in the form of plasmid or expression vectors, are also provided.
Recombinant host
cells, especially recombinant bacterial cells, yeast cells, fungal cells,
plant cells, insect cells and
animal cells, containing the plasmids or vectors are further provided. Methods
for producing
the mutant SAH hydrolases using the recombinant host cells are further
provided.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-9-
Assays for homoc~steine and metabolically related analytes
Assays for homocysteine, which as noted above, is a risk factor for
cardiovascular
disease and other diseases, are provided herein.
Homocysteine
In these embodiments, the small molecule to be assayed is homocysteine (Hcy)
and the
mutant analyte-binding enzymes are mutant Hcy-binding enzymes that
substantially retain their
binding affinity or that have enhanced binding affinity for Hcy or an
immediate Hcy enzymatic
conversion product but have attenuated catalytic activity.
Mutant Hcy-binding enzymes that can be used in the assay include those in
which the
attenuated catalytic activity is caused by mutation in the mutant enzyme's
binding site for its
co-enzyme or for a non-Hcy substrate, or mutation in the mutant enzyme's
catalytic site or a
combination thereof.
In another embodiment, the mutant enzyme is a mutant cystathionine 13-synthase
and the
attenuated catalytic activity is caused by mutation in the mutant
cystathionine 13-synthase's
catalytic site, its binding site for pyridoxal 5'-phosphate or L-serine, or a
combination thereof.
In another embodiment, the mutant enzyme is a mutant methionine synthase and
the
attenuated catalytic activity is caused by mutation in the mutant methionine
synthase's catalytic
site, its binding site for vitamin B12 or 5-methyltetrahydrofolate (5-CH3-
THF), or a combination
thereof. More preferably, the mutant methionine synthase is an E. coli.
methionine synthase,
the mutant methionine synthase has one or more of the following mutations:
His759G1y,
Asp757G1u, Asp757Asn, and Ser810A1a.
In another embodiment, the mutant enzyme is a mutant methioninase and the
attenuated
catalytic activity is caused by mutation in the mutant methionine synthase's
catalytic site, its
binding site for a compound with the formulae of R'SH, in which R'SH is a
substituted thiol,
where R is preferably alkyl, preferably lower alkyl (1 to 6 carbons,
preferably 1 to 3 carbons, in
a straight or branched chain), heteroaryl, where the heteroatom is O, S or N,
or aryl, which is
substituted, such as with alkyl, preferably lower alkyl, or hal, or
unsubstituted, preferably aryl
or heteroaryl with one ring or two to three fused rings, preferably with about
4 to 7 members in
each ring, or combinations of any of the above.
In a preferred embodiment, the mutant enzyme is a mutant SAH hydrolase, where
the
mutant SAH hydrolase substantially retains its binding affinity or has
enhanced binding affinity
for Hcy or SAH but has attenuated catalytic activity. Examples of such mutant
SAH
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 10-
hydrolases that can be used in the assay include those in which the attenuated
catalytic activity
is caused by mutations) in the mutant SAH hydrolase's binding site for NAD+,
or mutations)
in the mutant SAH hydrolase's catalytic site or a combination thereof; those
that have
attenuated 5'-hydrolytic activity but substantially retains its 3'-oxidative
activity; those that
irreversibly bind SAH; those that have a Km for SAH that is about or less than
10.0 ~.M; those
that have a Kcat for SAH that is about or less than 0.1 S-1; those that have
one or more
insertion, deletion, or point mutation(s); those that are derived from the
sequence of amino
acids set forth in SEQ ID No. 1 or encoded by the sequence of nucleotides set
forth in SEQ ID
No. 2 but have one or more of the following mutations: Phe 302 to Ser (F302S),
Lys 186 to Ala
(K186A), His 301 to Asp (H301D), His 353 to Ser (H353S), Arg 343 to Ala
(R343A), Asp 190
to Ala (D190A), Phe 82 to Ala (F82A), Thr 157 to Leu (T157L), Cys 195 to Asp
(C195D),
Asn 181 to Asp (N181D), and deletion of Tyr 432 (0432); or those that are
derived from a
sequence of amino acids set forth in SEQ ID No. 1 or encoded by the sequence
of nucleotides
set forth in SEQ ID No. 2 and have a combination of Arg 431 to Ala (R431A) and
Lys 426 to
Arg (K426R) mutations or any that hybridize under conditions of low, more
preferably
moderate, most preferably high, stringency 'along their full-length and have a
Km at least about
10%, more preferably at least about 50% of the Km of the wildtype enzyme for
the analyte or
substrate, but having substantially attenuated catalytic activity.
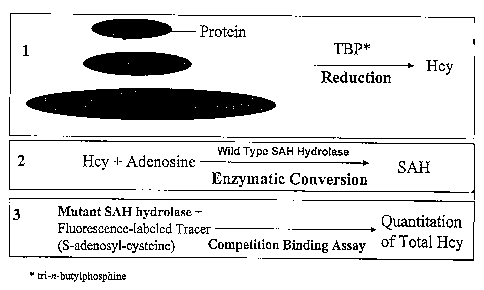
In one embodiment that uses a mutant SAH hydrolase, oxidized Hcy in the sample
is
converted into reduced Hcy prior to the contact between the sample and the
mutant SAH
hydrolase. The oxidized Hcy in the sample is converted into reduced Hcy by a
reducing agent,
such as, but not limited to, tri-n-butylphosphine (TBP),13-ME, DTT,
dithioerythritol,
thioglycolic acid, glutathione, tris (2-carboxyethyl)phosphine, sodium
cyanoborohydride,
NaBH4, KBH4 and free metals.
In another embodiment that uses a mutant SAH hydrolase, prior to the contact
between
the sample and the mutant SAH hydrolase, the Hcy in the sample is converted
into SAH. More
preferably, the Hcy in the sample is converted into SAH by a wild-type SAH
hydrolase. Also
more preferably, the SAH in the sample is contacted with the mutant SAH
hydrolas° in the
presence of a SAH hydrolase catalysis inhibitor, such as, but are not limited
to, neplanocin A or
thimersal.
In another embodiment that uses a mutant SAH hydrolase, prior to the contact
between
the SAH and the mutant SAH hydrolase, free adenosine is removed or degraded.
More
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-11-
preferably, free adenosine is degraded by combined effect of adenosine
deaminase, purine
nucleoside phosphorylase and xanthine oxidase.
In another embodiment that uses a mutant SAH hydrolase, the SAH is contacted
with
the mutant SAH hydrolase in the presence of a labeled SAH or a derivative or
an analog
thereof, whereby the amount of the labeled SAH bound to the mutant SAH
hydrolase inversely
relates to amount of the SAH in the sample. More preferably, the labeled SAH
derivative or
analog is a fluorescence labeled adenosyl-cysteine.
In another embodiment that uses a mutant SAH hydrolase, the mutant SAH
hydrolase is
labeled mutant SAH hydrolase. More preferably, the mutant SAH hydrolase is
labeled by
fluorescence.
In still another embodiment, the mutant enzyme is a mutant betaine-
homocysteine
methyltransferase and the attenuated catalytic activity is caused by mutation
in the mutant
betaine-homocysteine methyltransferase's binding site for betaine, its
catalytic site, or a
combination thereof.
In another embodiment, the Hcy assay is performed in combination with assays
for
other analytes associated with cardiovascular disease and/or regulation of Hcy
levels, such as
assays for cholesterol and/or folic acid.
Folate
In another embodiment, the mutant enzyme is a mutant methionine synthase. In
this
embodiment, the folate species can be a 5,-methyl-tetrahydrofolate, the mutant
folate-species-
binding enzyme is a mutant methionine synthase, and the attenuated catalytic
activity of the
mutant methionine synthase is caused by mutation in its catalytic site, its
binding site for
vitamin B~2, Hcy, or a combination thereof.
In another embodiment, the folate species is tetrahydrofolate, the mutant
folate-species-
binding enzyme is a mutant tetrahydrofolate methyltransferase, and the
attenuated catalytic
activity of the mutant tetrahydrofolate methyltransferase is caused by
mutation in its catalytic
site, its binding site for serine, or a combination thereof.
In still another embodiment, the folate species is 5, 10,-methylene
tetrahydrofolate, the
mutant folate-species-binding enzyme is a mutant methylenetetrahydrofolate
reductase, and the
attenuated catalytic activity of the methylenetetrahydrofolate reductase is
caused by mutation in
its catalytic site.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-12-
In yet another embodiment, the folate species is 5, 10,-methylene
tetrahydrofolate, the
mutant folate-species-binding enzyme is a mutant folypolyglutamate synthase,
and the
attenuated catalytic activity of the folypolyglutamate synthase is caused by
mutation in its
catalytic site, its binding site for ATP, L-glutamate, Mg2+, or a combination
thereof.
In yet another preferred embodiment, the folate species is dihydrofolate, the
mutant
folate-species-binding enzyme is a mutant dihydrofolate reductase, and the
attenuated catalytic
activity of the mutant dihydrofolate reductase is caused by mutation in its
catalytic site, its
binding site for NADH/NADPH, or a combination thereof. More preferably, the
mutant
dihydrofolate reductase is a Lactobacillus casei dihydrofolate reductase
having the Arg43Ala
or Trp2lHis mutation (Basran, et al., Protein Eng., 10 7 :815-26 91997)).
In yet another embodiment, the folate species is 5, 10,-methylene
tetrahydrofolate (5,
10-methylene-FH4), the mutant folate-species-binding enzyme is a mutant
thymidylate
synthase, and the attenuated catalytic activity of the mutant thymidylate
synthase is caused by
mutation in its catalytic site, its binding site for dUMP, or a combination
thereof. More
preferably, the mutant thymidylate synthase is a human thymidylate synthase
having a mutation
selected from Tyr6His, G1u214Ser, Ser216A1a, Ser216Leu, Asn229A1a and His199X,
where X
is any amino acid that is not His (Schiffer, et al., Biochemistry, 34 50
:16279-87 (1995);
Steadman, et al., Biochemistry, 37:7089-7095 (1998); Williams, et al.,
Biochemistry,
37 20 :7096-102 (1998); Finer-Moore, et al., J. Mol. Biol., 276 1 :113-29
(1998); and Finer-
Moore, et al., Biochemistry, 35(16):5125-36 (1996)). Also more preferably, the
mutant
thymidylate synthase is an E coli thymidylate synthase having an Arg126G1u
mutation (Strop,
et al., Protein Sci., 6(12):2504-11 (1997)) or a Lactobacillus casei
thymidylate synthase having
a V316Am mutation (Carreras, et al., Biochemistry, 31(26):6038-44 (1992)).
Cholesterol
In another embodiment, the analyte is cholesterol and the mutant analyte-
binding
enzyme is a mutant cholesterol-binding enzyme, where the mutant enzyme
substantially retains
its binding affinity or has enhanced binding affinity for cholesterol but has
attenuated catalytic
activity. In a preferred embodiment, the mutant cholesterol-binding enzyme is
a mutant
cholesterol esterase, and the attenuated catalytic activity of the mutant
cholesterol esterase is
caused by mutation in its catalytic site, its binding site for H20 or a
combination thereof. More
preferably, the cholesterol esterase is a pancreatic cholesterol esterase
having a Ser194Thr or
Ser194A1a mutation (DiPersio, et al., J. Biol. Chem., 265 28 :16801-6 (1990)).
In another
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-13-
preferred embodiment, the mutant cholesterol-binding enzyme is a mutant
cholesterol oxidase,
and the attenuated catalytic activity of the mutant cholesterol oxidase is
caused by mutation in
its catalytic site, its binding site for 02 or a combination thereof. More
preferably, the
cholesterol oxidase is a Brevibacterium sterolicum cholesterol oxidase having
a His447Asn or
His447G1n mutation (Yue, et al., Biochemistry, 38(14):4277-86 (1999)).
Bile acid (salt)
In still another specific embodiment, the small molecule analyte is a bile
acid (salt) and
the mutant analyte-binding enzyme is a mutant bile-acid (salt)-binding enzyme,
the mutant
enzyme substantially retains its binding affinity or has enhanced binding
affinity for the bile
acid (salt) but has attenuated catalytic activity. Preferably, the mutant bile-
acid (salt)-binding
enzyme is a mutant 3-a-hydroxy steroid dehydrogenase, and the attenuated
catalytic activity of
the mutant 3-a-hydroxy steroid dehydrogenase is caused by mutation in its
catalytic site, its
binding site for NAD+ or a combination thereof.
Assays for disorders associated with glucose metabolism
In yet another specific embodiment, the small molecule analyte is glucose and
the
mutant analyte-binding enzyme is a mutant glucose-binding enzyme, the mutant
enzyme
substantially retains its binding affinity or has enhanced binding affinity
for glucose but has
attenuated catalytic activity. Preferably, the mutant glucose-binding enzyme
is a Clostridium
thermosulfurogenes glucose isomerase having a mutation selected from His 1 O 1
Phe,
His 101 Glu, His 101 Gln, His 101 Asp and His 101 Asn (Lee, et al., J. Biol.
Chem., 265(31 ):19082-
90 (1990)). Also preferably, the mutant glucose-binding enzyme is a mutant
hexokinase or
glucokinase, and the attenuated catalytic activity of the mutant hexokinase or
glucokinase is
caused by mutation in its catalytic site, its binding site for ATP or Mg2+, or
a combination
thereof. Further preferably, the mutant glucose-binding enzyme is a mutant
glucose oxidase,
and the attenuated catalytic activity of the mutant glucose oxidase is caused
by mutation in its
catalytic site, its binding site for H20 or 02, or a combination thereof. Any
disorders associated
with glucose metabolism may be monitored or assessed.
Ethanol
In yet another specific embodiment, the small molecule analyte is ethanol and
the
mutant analyte-binding enzyme is a mutant ethanol-binding enzyme, the mutant
enzyme
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 14-
substantially retains its binding affinity or has enhanced binding affinity
for ethanol but has
attenuated catalytic activity. Preferably, the mutant ethanol-binding enzyme
is a mutant
alcohol dehydrogenase, and the attenuated catalytic activity of the mutant
alcohol
dehydrogenase is caused by mutation in its catalytic site, its binding site
for NAD+ or Zn2+, or a
combination thereof. More preferably, the mutant alcohol dehydrogenase is a
human liver
alcohol dehydrogenase having a His5lGln mutation (Ehrig, et al., Biochemistry,
30 4 :1062-8
(1991)). Also more preferably, the mutant alcohol dehydrogenase is a horse
liver alcohol
dehydrogenase having a Phe93Trp or Va1203A1a mutation (Bahnson, et al., Proc.
Natl. Acad.
Sci., 94 24 :12797-802 (1997); Colby, et al., Biochemistry, 37(26):9295-304
(1998)).
Assays for disorders, such as gout, associated with uric acid metabolism
In another exemplary embodiment, the small molecule analyte is uric acid and
the
mutant analyte-binding enzyme is a mutant uric-acid-binding enzyme, the mutant
enzyme
substantially retains its binding affinity or has enhanced binding affinity
for uric acid but has
attenuated catalytic activity. Preferably, the mutant uric-acid-binding enzyme
is a mutant orate
oxidase, and the attenuated catalytic activity of the mutant orate oxidase is
caused by mutation
in its catalytic site, its binding site for 02, H20, or copper ion, or a
combination thereof. More
preferably, the mutant orate oxidase is a rat orate oxidase having a mutation
selected from
H127Y, H129Y and F131S (Chu, et al., Ann. N. Y. Acad. Sci., 804:781-6 (1996)).
In all embodiments, the sample being assayed typically is a body fluid or
tissue,
including, but not limited to blood, urine, cerebral spinal fluid, synovial
fluid, amniotic fluid,
and tissue samples, such as biopsied tissues. Preferably, the body fluid is
blood or urine. More
preferably, the blood sample is further separated into a plasma or sera
fraction.
Further provided herein are combinations that include: a) a mutant analyte-
binding
enzyme, the mutant enzyme substantially retains its binding affinity or has
enhanced binding
affinity for the analyte or an immediate analyte enzymatic conversion product
but has
attenuated catalytic activity; and b) reagents and or other means for
detecting binding between
the analyte or the immediate analyte enzymatic conversion product with the
mutant analyte-
binding enzyme. Preferably, binding between the analyte or the immediate
analyte enzymatic
conversion product with the mutant analyte-binding enzyme is detected using a
labeled analyte,
a labeled immediate analyte enzymatic conversion product, or a derivative or
an analog thereof,
or a labeled mutant analyte-binding enzyme. Also preferably, the combination
where the
analyte is Hcy further also includes reagents for detecting cholesterol and/or
folic acid.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-15-
Kits and articles of manufacture that include the above combinations and
optional
instructions for performing the assay of interest are provided. Articles of
manufacture that
contain the mutant enzymes with a label indicating the assay in which the
enzyme is used, and
also packaging material that contains the enzyme.
Other diagnostic and prognostic assays
Other assays include, but are not limited to, diagnostic and prognostic assays
in which
markers, especially small molecule markers associated with various diseases,
defects,
conditions or drugs are monitored. Exemplary small molecule analytes and
mutant enzymes
include, but are not limited to, any of the following in which:
the small molecule analyte is creatinine and the mutant analyte-binding enzyme
is a
mutant creatinine amidohydrolase,
the small molecule analyte is serotonin and the mutant analyte-binding enzyme
is a
mutant serotonin N-acetyltransferase,
the small molecule analyte is hyaluronic acid and the mutant analyte-binding
enzyme is
a mutant hyaluronidase,
the small molecule analyte is catecholamine and the mutant analyte-binding
enzyme is a
mutant catechol O-methyltransferase,
the small molecule analyte is homovanillic acid and the mutant analyte-binding
enzyme
is a mutant monoamine oxidase,
the small molecule analyte is vanilylmandelic acid and the mutant analyte-
binding
enzyme is a mutant dopamine 13-hydroxylase,
the small molecule analyte is cyclosporin A and the mutant analyte-binding
enzyme is a
mutant calcineurine or cyclophilin,
the small molecule analyte is mycophenoric acid and the mutant analyte-binding
enzyme is a mutant inosine monophosphate dehydrogenase,
the small molecule analyte is leflunomide and the mutant analyte-binding
enzyme is a
mutant dihydroorotate dehydrogenase,
the small molecule analyte is N-acetylprocainamide and the mutant analyte-
binding
enzyme is a mutant procainamide N-acetyltransferase,
the small molecule analyte is selected from the group consisting of
fluvastatin,
lovastatin, provastatin, simvastatin and atorvastatin and the mutant analyte-
binding enzyme is a
mutant hydroxymethylglutaryl-CoA reductase.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-16-
Coniu~ates, preparation and uses thereof
Also provided herein are conjugates of the mutant analyte binding enzymes and
an
additional portion, referred to herein as a facilitating agent, linked
directly or indirectly via a
linker to the analyte binding protein. The facilitating agent is linked
directly or indirectly,
typically covalently or via ionic interactions, and is selected to facilitate,
for example: i) affinity
isolation or purification of the conjugate, such as a tag that specifically
binds to an immobilized
receptor; ii) immobilization, such as attachment of the conjugate to a
surface; or iii) detection
of the conjugate, such as a linker.
Hence the conjugates provided herein contain the following components: (mutant
analyte binding enzyme)", (L)q, and (facilitating agent)", in which at least
one mutant analyte
binding enzyme is linked directly or via one or more linkers (L) to at least
one facilitating
agent. L refers to a linker. Any suitable association among the elements of
the conjugate is
contemplated as long as the resulting conjugate substantially retains binding
affinity or has
enhanced binding affinity for desired analytes or immediate analyte enzymatic
conversion
products but has attenuated catalytic activity, and the facilitating agent
retains the desired
activity.
The variables n and m are integers of 1 or greater and q is 0 or any integer.
The
variables n, q and m are selected such that the resulting conjugate interacts
with the targeted
receptor and a targeted agent is internalized by a cell to which it has been
targeted. Typically n
is between 1 and 3; q is 0 or more, depending upon the number of linked
moieties and/or
functions of the linker, q is generally 1 to 4; m is 1 or more, generally 1 or
2. When more than
one facilitating agent and/or mutant analyte binding enzyme is/are present in
a conjugate the
each agent may be the same or different and each mutant analyte binding enzyme
may be the
same or different.
The conjugates can be produced by any means, including, by chemical
conjugation
methods and, where both moieties are proteinaceous, as fusion proteins. The
conjugates can
include a fusion protein portion and a chemically linked portion or any
combination thereof.
Any agent, such as a protein or peptide fragment or other moiety that
facilitates:
i) affinity isolation or purification of the fusion protein; ii) attachment of
the fusion protein to a
surface; or iii) detection of the fusion protein, is contemplated for use in
the conjugate. In one
exemplary embodiment, the facilitating agent is a protein binding moiety, such
as an epitope
tag or an IgG binding protein, a nucleotide binding protein such as a DNA or
RNA binding
protein, a lipid binding protein, a polysaccharide binding protein, or a metal
binding protein. In
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
17-
another exemplary embodiment, the facilitating agent is derived from an
enzyme, a transport
protein, a nutrient or storage protein, a contractile or motile protein, a
structural protein, a
defense protein, a regulatory protein, or a fluorescent protein.
Also provided herein are isolated nucleic acid molecules that contain a
sequence of
nucleotides encoding the fusion protein. Plasmids containing the molecules,
and cells
containing the plasmids are also provided. Methods for producing the fusion
proteins by
culturing the cells containing the plasmids under conditions whereby the
fusion protein is
expressed by the cell, and recovering the expressed fusion protein are
provided.
Further provided herein are methods for assaying an analyte in a sample using
the
conjugates. In practicing these methods, the conjugate is contacted with the
sample, and
interaction between the analyte or an immediate analyte enzymatic conversion
product and the
conjugate is detected. The presence or amount of the analyte in the sample is
then assessed.
Prior to the contact between the sample and the conjugate, the conjugate could
be isolated or
purified through affinity binding between the facilitating agent and an
affinity binding moiety.
In addition, prior to the contact between the sample and the conjugate, the
conjugate can be
linked, directly or indirectly, to a surface preferably through affinity
binding between the
facilitating agent and an affinity binding moiety on the surface, thereby
readily permitting solid
phase assays to be performed.
Particular compositions, combinations, kits and articles of manufacture for
assaying
analytes, preferably small molecule analytes, and methods are described in the
sections and
subsections that follow.
H~i h throughput protocols
The methods and compositions provided herein may be adapted for use in high
throughput protocols. In particular, solid supports with a plurality of linked
mutant analyte
binding enzymes and/or conjugate provided herein may used to screen a
plurality of samples.
Each of the linked enzymes or conjugates may be the same or different from
each other.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 depicts Hcy assay using wild type and mutant SAH hydrolase.
Fig. 2 depicts total plasma Hcy assay procedure with wild type and mutant SAH
hydrolase.
Fig. 3 depicts design and synthesis of fluorescence labeled tracer.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-18-
Fig. 4 depicts selection of mutant SAH hydrolase that lacks catalytic activity
but retains
substrate binding affinity.
DETAILED DESCRIPTION OF THE INVENTION
A. DEFINITIONS
B. METHODS FOR ASSAYING ANALYTES
1. Analytes
2. Mutant analyte-binding enzymes ("substrate trapping enzymes")
a. Nucleic acids encoding analyte-binding enzymes
b. Selecting and producing mutant analyte-binding enzymes
3. Sample collection
C. METHODS FOR ASSAYING HOMOCYSTEINE
1. Homocysteine metabolism
2. Mutant Hcy-binding enzymes
1 S a. Nucleic acids encoding Hcy-binding enzymes
b. Selecting and producing Hcy-binding enzymes
c. Mutant SAH hydrolase and nucleic acids encoding the mutant sah
hydrolase
3. Hcy assays using mutant SAH hydrolase
D. METHODS FOR ASSAYING FOLATE SPECIES
E. METHODS FOR ASSAYING CHOLESTEROL
Cholesterol-binding enzymes
F. HCY ASSAYS IN CONJUNCTION WITH CHOLESTEROL AND/OR FOLIC ACID
1. Cholesterol assay
2. Folic acid assay
G. METHODS FOR ASSAYING BILE ACID AND BILE SALTS
H. METHODS FOR ASSAYING GLUCOSE
I. METHODS FOR ASSAYING ETHANOL
J. METHODS FOR ASSAYING URIC ACID
K. OTHER PROGNOSTIC AND DIAGNOSTIC ASSAYS AND ASSAYS FOR
MONITORING THERAPEUTIC INTERVENTION
1. Diagnostic and prognostic assays
2. Drug assays
L. COMBINATIONS, KITS AND ARTICLES OF MANUFACTURE
M. PREPARATION OF CONJUGATES
1. Conjugation
a. Fusion proteins
b. Chemical conjugation
1. Heterobifunctional cross-linking reagents
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 19-
2. Exemplary Linkers
a. Acid cleavable, photocleavable and heat sensitive linkers
b. Other linkers for chemical conjugation
c. Peptide linkers
N. Selection of and preparation of facilitating agents
1. Selection of facilitating agents
a. Protein binding moieties
1) Interaction trap/two-hybrid system
2) Phage-based expression cloning
3) Detection of protein-protein interactions
b. Epitope tags
c. IgG binding proteins
1) pEZZ 18 Protein A gene fusion vector
Expression
Sequencing
Cloning
Hosts)
Selectable markers)
Amplification
2) pRIT2T Protein A gene fusion vector
Induction
Expression
Hosts)
Selectable markers)
3) The IgG Sepharose 6 fast flow system
d. ~-galactosidase fusion proteins
Expression
Host(s):
Selectable markers)
e. Nucleic acid binding moieties
1) DNA binding proteins
2) RNA binding proteins
3) Preparation of nucleic acid binding proteins
Preparation of nuclear and cytoplasmic extracts
4) Assays for identifying nucleic acid binding proteins
a. Mobility shift DNA-binding assay
b. Basic mobility shift assay procedure
c. Competition mobility shift assay
d. Antibody supershift assay
e. Methylation and uracil interference assay
1) Methylation interference assays
2) Uracil interference assay
3) DNase I footprint analysis
4) Screening a 71gt11 expression library with
recognition-site DNA
5) Rapid separation of protein-bound DNA from free
DNA
f. Lipid binding moieties
g. Polysaccharide binding moieties
h. Metal binding moieties
i. Other facilitating agents
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-20-
1) Peroxidase
2) urease
3) Alkaline phosphatase
4) Luciferase
5) Glutathione S-transferase
6) Defense proteins
7) Fluorescent moieties
2. Selection of Mutant analyte-binding enzymes
3. Nucleic acids, plasmids and cells
4. Immobilization and supports or substrates therefor
EXAMPLES
A. DEFINITIONS
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as is commonly understood by one of ordinary skill in the art to which
this invention
belongs. All patents, applications, published applications and other
publications and sequences
from GenBank and other data bases referred to herein are incorporated by
reference in their
entirety.
As used herein, "analyte" refers to a molecule that can specifically bind to
an enzyme,
either as a co-enzyme, a co-factor or a substrate.
As used herein, "enzyme" refers to a protein specialized to catalyze or
promote a
specific metabolic reaction. Generally, enzymes are catalysts, but for
purposes herein, such
"enzymes" include those that would be modified during a reaction. Since the
enzymes are
modified to eliminate or substantially eliminate catalytic activity, they will
not be so-modified
during a reaction.
As used herein, "analyte-binding enzyme" refers to an enzyme that uses the
analyte as
its co-enzyme, co-factor, or as a substrate. For instance, "Hcy-binding
enzyme" refers to an
enzyme that uses Hcy as its co-enzyme, co-factor, or its sole or one of its
substrates. Examples
of Hcy-binding enzymes include SAH hydrolase, cystathionine f3-synthase,
methionine
synthase, betaine-homocysteine methyltransferase and methioninase. It is
intended that
analyte-binding enzymes include those conservative amino acid substitutions
that do not
substantially alter its activity. Suitable conservative substitutions of amino
acids are known to
those of skill in this art and may be made generally without altering the
biological activity of
the resulting molecule. Those of skill in this art recognize that, in general,
single amino acid
substitutions in non-essential regions of a polypeptide do not substantially
alter biological
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-21 -
activity (see, e.g., Watson, et al., Molecular Biology of the Gene, 4th
Edition, 1987, The
Bejacmin/Cummings Pub. Co., p. 224).
Such substitutions are preferably made in accordance with those set forth in
TABLE 1
as follows:
TABLE 1
Original residue Conservative substitution
Ala (A) Gly; Ser
Arg (R) Lys
Asn (N) Gln; His
Cys (C) Ser
Gln (Q) Asn
Glu (E) Asp
Gly (G) Ala; Pro
His (H) Asn; Gln
Ile (I) Leu; Val
Leu (L) Ile; Val
Lys (K) Arg; Gln; Glu
Met (M) Leu; Tyr; Ile
Phe (F) Met; Leu; Tyr
Ser (S) Thr
Thr (T) Ser
Trp (W) Tyr
Tyr (Y) Trp; Phe
Val (V) Ile; Leu
Other substitutions are also
permissible and may be determined
empirically or in accord with
known conservative substitutions.
As used herein, the "amino acids," which occur in the various amino acid
sequences
appearing herein, are identified according to their well-known, three-letter
or one-letter
abbreviations. The nucleotides, which occur in the various DNA fragments, are
designated
with the standard single-letter designations used routinely in the art.
As used herein, "a mutant analyte-binding enzyme" (used interchangeably with
"modified enzyme" and "substrate trapping enzyme" that substantially retains
its binding
affinity or has enhanced binding affinity for the analyte or an immediate
analyte enzymatic
conversion product" refers to a mutant form of an analyte-binding enzyme that
retains
sufficient binding affinity for the analyte to be detected in the process or
method, particularly
assay, of interest. Typically this is at least about 10%, preferably at least
about 50% binding
affinity for the analyte or an immediate analyte enzymatic conversion product,
compared to its
wildtype counterpart. Preferably, such mutant analyte-binding enzyme retains
60%, 70%, 80%,
90%, 100% binding affinity for the analyte or an immediate analyte enzymatic
conversion
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-22-
product compared to its wildtype counterpart, or has a higher binding affinity
than its wildtype
counterpart. Such mutant analyte-binding enzyme is herein referred to as a
"substrate trapping
enzyme", i.e., a molecule that specifically binds to a selected analyte or
target molecule, but
does not catalyze conversion thereof.
As used herein, "immediate analyte enzymatic conversion product" refers to a
product
derived from the analyte by catalysis of a single analyte-binding enzyme. For
example, the
"immediate Hcy enzymatic conversion product" of SAH hydrolase is SAH. The
"immediate
Hcy enzymatic conversion product" of cystathionine 13-synthase is
cystathionine. The
"immediate Hcy enzymatic conversion product" of methionine synthase and
betaine-
homocysteine methyltransferase is methionine.
As used herein, a conjugate refers to the compounds provided herein that
include one or
more mutant analyte-binding enzymes and one or more facilitating agents. These
conjugates
include those produced by recombinant means as fusion proteins, those produced
by chemical
means, such as by chemical coupling, through, for example, coupling to
sulfhydryl groups, and
those produced by any other method whereby at least one mutant analyte-binding
enzyme is
linked, directly or indirectly via linkers) to a facilitating agent.
As used herein, a facilitating agent, is any moiety, such as a protein or
effective portion
thereof, that promotes or facilitates, for example, preferably:
i) affinity isolation or purification of the conjugate;
ii) attachment of the conjugate to a surface; or
iii) detection of the conjugate or complexes containing the conjugate.
As used herein the term "assessing" is intended to include quantitative and
qualitative
determination in the sense of obtaining an absolute value for the amount or
concentration of the
analyte, e.g., a homocysteine co-substrate, present in the sample, and also of
obtaining an
index, ratio, percentage, visual or other value indicative of the level of
analyte in the sample.
Assessment may be direct or indirect and the chemical species actually
detected need not of
course be the analyte itself but may for example be a derivative thereof or
some further
substance.
As used herein, "attenuated catalytic activity" refers to a mutant analyte-
binding
enzyme that retains sufficiently reduced catalytic activity to be useful as a
"pseudo-antibody,"
i. e., a molecule used in place of an antibody in immunoassay formats. The
precise reduction in
catalytic activity for use in the assays can be empirically determined for
each assay. Typically,
the enzyme will retain less than about 50% of one of its catalytic activities
or less than 50% of
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 23 -
its overall catalytic activities compared to its wildtype counterpart.
Preferably, a mutant
analyte-binding enzyme retains less than 40%, 30%, 20%, 10%, 1 %, 0.1 %, or
0.01 % of one of
its catalytic activities or its overall catalytic activities compared to its
wildtype counterpart.
More preferably, a mutant analyte-binding enzyme lacks detectable level of one
of its catalytic
activities or its overall catalytic activities compared to its wildtype
counterpart. In instances in
which catalytic activity is retained and/or a further reduction thereof is
desired, the contacting
step can be effected in the presence of a catalysis inhibitor. Such
inhibitors, include, but are
not limited to, heavy metals, chelators or other agents that bind to a co-
factor required for
catalysis, but not for binding, and other such agents.
As used herein, "macromolecule" refers to a molecule that, without attaching
to another
molecule, is capable of generating an antibody that specifically binds to the
macromolecule.
As used herein, "small molecule" refers to a molecule that, without forming
homo-
aggregates or without attaching to a macromolecule or adjuvant, is incapable
of generating an
antibody that specifically binds to the small molecule. Preferably, the small
molecule has a
molecular weight that is about or less than 10,000 daltons. More preferably,
the small molecule
has a molecular weight that is about or less than 5,000 dalton.
As used herein, "inorganic molecule" refers to a molecule that does not
contain
hydrocarbon group(s).
As used herein, "organic molecule" refers to a molecule that contains
hydrocarbon
group(s).
As used herein, "vitamin" refers to a trace organic substance required in
certain
biological species. Most vitamins function as components of certain coenzymes.
As used herein, "biomolecule" refers to an organic compound normally present
as an
essential component of living organisms.
As used herein, "lipid" refers to water-insoluble, oily or greasy organic
substances that
are extractable from cells and tissues by nonpolar solvents, such as
chloroform or ether.
As used herein, "homocysteine" (Hcy) refers to a compound with the following
molecular formula: HSCHZCHZCH(NH2)COOH. Biologically, Hcy is produced by
demethylation of methionine and is an intermediate in the biosynthesis of
cysteine from
methionine. The term "Hcy" encompasses free Hcy (in the reduced form) and
conjugated Hcy
(in the oxidized form). Hcy can conjugate with proteins, peptides, itself or
other thiols through
disulfide bond.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-24-
As used herein, "SAH hydrolase" refers to an ubiquitous eukaryotic enzyme,
which is
also found in some prokaryotes, which catalyzes hydrolysis of SAH to Ado and
Hcy. SAH
hydrolase also catalyzes the formation of SAH from Ado and Hcy. The co-enzyme
of SAH
hydrolase is NAD+/NADH. SAH hydrolase has several catalytic activities. In the
hydrolytic
direction, the first step involves oxidation of the 3'-hydroxyl group of SAH
(3'-oxidative
activity) by enzyme-bound NAD+ (E-NAD+), followed by 13-elimination of L-Hcy
to give 3'-
keto-4',5'-didehydro-5'-deoxy-Ado. Michael addition of water to the 5'-
position to this tightly
bound intermediate (5'-hydrolytic activity) affords 3'-keto-Ado, which is then
reduced by
enzyme-bound NADH (E-NADH) to Ado (3'-reduction activity). It is intended to
encompass
SAH hydrolase with conservative amino acid substitutions that do not
substantially alter its
activity.
As used herein, "SAH hydrolase catalysis inhibitor" refers to an agent that
inhibits one
or all of SAH hydrolase catalytic activities, e.g., 3'-oxidative activity, 5'-
hydrolytic activity, or
3'-reduction activity, while not affecting SAH hydrolase's binding affinity
for Hcy and/or SAH.
As used herein, "cystathionine 13-synthase" refers to an enzyme that
irreversibly
catalyzes the formation of cystathionine from Hcy and serine. The co-enzyme of
cystathionine
13-synthase is pyridoxal S'-phosphate. It is intended to encompass
cystathionine 13-synthase with
conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "methionine synthase" refers to an enzyme that irreversibly
catalyzes
the formation of methionine from Hcy and 5-methyltetrahydrofolate (5-CH3-THF).
The co-
enzyme of cystathionine 13-synthase is vitamin B~2. It is intended to
encompass methionine
synthase with conservative amino acid substitutions that do not substantially
alter its activity.
As used herein, "betaine-homocysteine methyltransferase" refers to an enzyme
that
irreversibly catalyzes the formation of methionine and dimethyl-glycine from
Hcy and betaine.
It is intended to encompass betaine-homocysteine methyltransferase with
conservative amino
acid substitutions that do not substantially alter its activity.
As used herein, "methioninase" refers to an enzyme that catalyzes a,13- and a,
I-'-eliminations from S-substituted amino acids and also catalyzes a variety
of 13- and
h-exchange reactions, according to the following equations:
RSCH2CH(NHZ)COOH+R'SH in
equilibrium with R'SCH2CH(NH2)COOH+RSH (13-exchange) and RSCHZCHZCH(NHZ)COOH
+ R'SH in equilibrium with R'SCH2CH2CH(NH2)COOH + RSH (r-exchange), where R'SH
represents an alkanethiol or a substituted thiol (Ito, et al., J. Biochem.,
(Tokyo) 80~6~:1327-34
(1976)). In particular, R and R' independently are selected preferably from
alkyl, aryl, alkynyl,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 25 -
cycloalkly, heteroaryl, alkenyl, amino acids, proteins and other suitable
moieties or mixtures
thereof. R and R' typically contain less than about SO atoms, are substituted
or unsubstituted,
the carbon chains can be straight or branched or cyclized, heteroatoms include
S, N, O; the aryl
and heteroaryl or other cyclic groups can include one ring or two or more
fused rings, each ring
preferably containing from 3 to 7, more preferably 4 to 6, members.
As used herein, "adenosine deaminase" refers to an enzyme that catalyzes the
deamination of adenosine to form inosine. It is intended to encompass
adenosine deaminase
with conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "purine nucleoside phosphorylase" refers to an enzyme that
catalyzes
the formation of hypoxanthine and D-ribose from inosine and water. It is
intended to
encompass purine nucleoside phosphorylase with conservative amino acid
substitutions that do
not substantially alter its activity.
As used herein, "xanthine oxidase" refers to an enzyme that catalyzes the
conversion of
hypoxanthine to uric acid via xanthine. It is intended to encompass xanthine
oxidase with
conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "folate species" refers to folate or folic acid, which is
chemically N-[4-
[[2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzxoyl]-L-glutamic
acid, or a
derivative thereof. Examples of folate derivatives include, but are not
limited to,
dihydrofolate, tetrahydrofolate, 5,-methyl-tetrahydrofolate and 5,10-methylene
tetrahydrofolate.
As used herein, "tetrahydrofolate methyltransferase" refers to an enzyme that
catalyzes
the formation of 5,10-methylene tetrahydrofolate and glycine from
tetrahydrofolate and serine.
It is intended to encompass tetrahydrofolate methyltransferase with
conservative amino acid
substitutions that do not substantially alter its activity.
As used herein, "methylenetetrahydrofolate reductase" refers to an enzyme that
catalyzes the formation of 5,-methyl-tetrahydrofolate from S,10-methylene
tetrahydrofolate. It
is intended to encompass methylenetetrahydrofolate reductase with conservative
amino acid
substitutions that do not substantially alter its activity.
As used herein, "folypolyglutamate synthase" refers to an enzyme that
catalyzes the
formation of 5,10-methylenetetrahydrofolate-diglutamate derivative, ADP and Pi
from 5,10-
methylenetetrahydrofolate, L-glutamate and ATP. The cofactor of
folypolyglutamate synthase
is Mg2+. It is intended to encompass folypolyglutamate synthase with
conservative amino acid
substitutions that do not substantially alter its activity.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-26-
As used herein, "dihydrofolate reductase" refers to an enzyme that catalyzes
the
formation of tetrahydrofolate and NADP+ from dihydrofolate, NADPH and H+. It
is intended
to encompass dihydrofolate reductase with conservative amino acid
substitutions that do not
substantially alter its activity.
As used herein, "thymidylate synthase" refers to an enzyme that catalyzes the
formation
of dihydrofolate and dTMP from 5,10-methylenetetrahydrofolate and dUMP. It is
intended to
encompass thymidylate synthase with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "cholesterol esterase" refers to an enzyme that catalyzes the
formation
of cholesterol and fatty acids from cholesterolester and H20. It is intended
to encompass
cholesterol esterase with conservative amino acid substitutions that do not
substantially alter its
activity.
As used herein, "cholesterol oxidase" refers to an enzyme that catalyzes the
formation
of cholesterol-4-en-3-one and H202 from cholesterol and 02. It is intended to
encompass
cholesterol oxidase with conservative amino acid substitutions that do not
substantially alter its
activity.
As used herein, "calcineurin (also called phosphoprotein phosphatase 2B or
PP2B)"
refers to a Ca2+/calmodulin-dependent protein phosphatase that is an element
of many
intracellular signaling pathways including T cell activation. In T cells,
calcineurin participates
in regulation of IL-2 expression following T cell stimulation. Nuclear factor
of activated T
cells (NFATp) has been shown to be a substrate for calcineurin phosphatase
activity. Following
T cell stimulation, calcineurin-mediated NFATp dephosphorylation allows
translocation of
NFATp from the cytoplasm to the nucleus where NFATP interacts with Fos and Jun
to induce
expression of the IL-2 gene. It is intended to encompass calcineurin with
conservative amino
acid substitutions that do not substantially alter its activity.
As used herein, "catechol O-methyltransferase (COMT)" refers to an enzyme that
catalyzes the transfer of the methyl group of S-adenosyl-L-methionine (AdoMet)
to one of the
hydroxyl groups of a catechol substrate in the presence of Mgz+. The
physiological substrates
of COMT include dopa, catecholamines (e.g., dopamine, noradrenaline,
adrenaline), their
hydroxylated metabolites, catechol estrogens and ascorbic acid. COMT is mainly
a cellular
enzyme. In vertebrates, the COMT protein appears mostly in soluble form and a
minor fraction
is in a membrane-bound form. It is intended to encompass catechol O-
methyltransferase with
conservative amino acid substitutions that do not substantially alter its
activity.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-27-
As used herein, "creatinine amidohydrolase" refers to an enzyme that catalyses
the
following reaction:
Creatinine + HZO <---> Creatine.
It is intended to encompass creatinine amidohydrolase with conservative amino
acid
substitutions that do not substantially alter its activity.
As used herein, "cyclophilin" refers to an enzyme that: 1 ) has cis-trans
peptidyl-prolyl
isomerase (PPIase) activity; 2) binds drug cyclosporin A (CsA); and 3)
inhibits calcineurin in
the presence of CsA. It is intended to encompass cyclophilin with conservative
amino acid
substitutions that do not substantially alter its activity.
As used herein, "dihydroorotate dehydrogenase" refers to an enzyme that
catalyzes the
conversion of L-dihydroorotate to orotate in the presence of OZ. It is
intended to encompass
dihydroorotate dehydrogenase with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "dopamine-13-hydroxylase" refers to an enzyme that
hydroxylates
dopamine to norepinephrine in the presence of oxygen and ascorbic acid. It is
intended to
encompass dopamine-13-hydroxylase with conservative amino acid substitutions
that do not
substantially alter its activity.
As used herein, "hyaluronidase" refers to the class of enzymes that act on the
disaccharide unit of D-glucuronic acid and N-acetyl-D-glucosamine. Such
enzymes mediate
the hydrolysis of polymers of repeating disaccharides comprising D-glucuronic
acid and N-
acetyl-D-glucosamine. One example of such polymer is hyaluronic acid.
Hyaluronidase
catalyzes the release of reducing groups of N-acetylglucosamine from
hyaluronic acid. It is
intended to encompass hyaluronidase with conservative amino acid substitutions
that do not
substantially alter its activity.
As used herein, "hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase)"
refers
to an enzyme that catalyzes the conversion of 3-hydroxy-3-methylglutaryl-CoA
to mevalonate
in a reaction requiring NADPH as the co-enzyme. It is intended to encompass
HMG-CoA
reductase with conser~~ative amino acid substitutions that do not
substantially alter its activity.
As used herein, "hydroxysteroid dehydrogenase" refers to a family of enzymes
which
play a pivotal role in the regulation of steroid hormone action. These enzymes
catalyze the
interconversion of secondary alcohols to ketones in a positional and
stereospecific manner on
the steroid nucleus and side chain. They require nicotinamide dinucleotide
(phosphate) NADP+
as cofactor. For example, 3a-hydroxysteroid dehydrogenase catalyzes the
reduction
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-28-
Sa-dihydrotestosterone to Sa-androstan-3a,1713-diol. It is intended to
encompass
hydroxysteroid dehydrogenase with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "inosine-5'-monophosphate dehydrogenase (IMPDH)" refers to an
enzyme that is involved in the de novo synthesis of guanosine nucleotides.
IMPDH catalyzes
the NAD+-dependent oxidation of inosine-5'-monophosphate (IMP) to xanthosine-
5'-
monophosphate (XMP). IMPDH is ubiquitous in eukaryotes, bacteria and protozoa.
Regardless of species, the enzyme follows an ordered Bi--Bi reaction sequence
of substrate and
cofactor binding and product release. First, IMP binds to IMPDH. This is
followed by the
binding of the cofactor NAD+. The reduced cofactor, NADH, is then released
from the
complex, followed by the product, XMP. It is intended to encompass IMPDH with
conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "monoamine oxidase" refers to an enzyme that catalyzes the
oxidative
deamination of a wide variety of dietary amines and neurotransmitters such as
dopamine,
norepinephrine, and serotonin. It is an integral protein of the outer
mitochondrial membrane
and is present in all types of cells. Two isoenzymic forms (Types A and B)
have been
identified. It is intended to encompass monoamine oxidase with conservative
amino acid
substitutions that do not substantially alter its activity.
As used herein, "procainamide N-acetyltransferase" refers to an enzyme that
catalyzes
the transfer of the acetyl moiety of acetyl CoA to an acceptor amine such as
procainamide. It is
intended to encompass serotonin N-acetyltransferase with conservative amino
acid
substitutions that do not substantially alter its activity.
As used herein, "serotonin N-acetyltransferase (AANAT)" refers to an enzyme
that
catalyzes the conversion of serotonin to N-acetylserotonin in a reaction
requiring acetyl
coenzyme A (AcCoA). It is intended to encompass serotonin N-acetyltransferase
with
conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "bile acid" refers to acidic sterols synthesized from
cholesterol in the
liver. Following synthesis, the bile acids are secreted into bile and enter
the lumen of the small
intestine, where they facilitate absorption of fat-soluble vitamins and
cholesterol. In humans,
the most abundant bile acid is cholic acid.
As used herein, "bile salt" refers to salt of bile acid. The major human bile
salts are
sodium glycocholate and sodium taurocholate.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-29-
As used herein, "3-a-hydroxy steroid dehydrogenase" refers to an enzyme that
catalyzes
the 3-oxo-bile-acid, H+ and NADH from 3-a-hydroxy-bile-acid and NAD+. It is
intended to
encompass 3-a-hydroxy steroid dehydrogenase with conservative amino acid
substitutions that
do not substantially alter its activity.
As used herein, "glucose isomerase" refers to an enzyme that catalyzes the
reversible
conversion between D-glucose and D-fructose. It is intended to encompass
glucose isomerase
with conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "hexokinase or glucokinase" refers to an enzyme that catalyzes
the
formation of D-glucose 6-phosphate and ADP from a-D-glucose and ATP. The
cofactor of
hexokinase or glucokinase is Mg2+. It is intended to encompass hexokinase or
glucokinase with
conservative amino acid substitutions that do not substantially alter its
activity.
As used herein, "glucose oxidase" refers to an enzyme that catalyzes the
formation of
gluconic acid and H202 from glucose, HZO and O2. It is intended to encompass
glucose
oxidase with conservative amino acid substitutions that do not substantially
alter its activity.
As used herein, "alcohol dehydrogenase" refers to an enzyme that catalyzes the
formation of acetaldehyde, NADH and H+ from ethanol and NAD+. The cofactor of
alcohol
dehydrogenase is Zn2+. It is intended to encompass alcohol dehydrogenase with
conservative
amino acid substitutions that do not substantially alter its activity.
As used herein, "orate oxidase or uricase" refers to an enzyme that catalyzes
the
formation of allantoin and C02 from uric acid, 02 and H20. The cofactor of
orate oxidase or
uricase is copper. It is intended to encompass orate oxidase or uricase with
conservative amino
acid substitutions that do not substantially alter its activity.
As used herein, "serum" refers to the fluid portion of the blood obtained
after removal
of the fibrin clot and blood cells, distinguished from the plasma in
circulating blood.
As used herein, "plasma" refers to the fluid, noncellular portion of the
blood,
distinguished from the serum obtained after coagulation.
As used herein, "substantially pure" means sufficiently homogeneous to appear
free of
readily detectable impurities as determined by standard methods of analysis,
such as thin layer
chromatography (TLC), gel electrophoresis and high performance liquid
chromatography
(HPLC), used by those of skill in the art to assess such purity, or
sufFciently pure such that
further purification would not detectably alter the physical and chemical
properties, such as
enzymatic and biological activities, of the substance. Methods for
purification of the
compounds to produce substantially chemically pure compounds are known to
those of skill in
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-30-
the art. A substantially chemically pure compound may, however, be a mixture
of
stereoisomers or isomers. In such instances, further purification might
increase the specific
activity of the compound.
As used herein, "biological activity" refers to the in vivo activities of a
compound or
physiological responses that result upon in vivo administration of a compound,
composition or
other mixture. Biological activity, thus, encompasses therapeutic effects and
pharmaceutical
activity of such compounds, compositions and mixtures. Biological activities
may be observed
in vitro systems designed to test or use such activities. Thus, for purposes
herein the biological
activity of a luciferase is its oxygenase activity whereby, upon oxidation of
a substrate, light is
produced.
As used herein, a "receptor" refers to a molecule that has an affinity for a
given ligand.
Receptors may be naturally-occurring or synthetic molecules. Receptors may
also be referred
to in the art as anti-ligands. As used herein, the receptor and anti-ligand
are interchangeable.
Receptors can be used in their unaltered state or as aggregates with other
species. Receptors
may be attached, covalently or noncovalently, or in physical contact with, to
a binding member,
either directly or indirectly via a specific binding substance or linker.
Examples of receptors,
include, but are not limited to: antibodies, cell membrane receptors surface
receptors and
internalizing receptors, monoclonal antibodies and antisera reactive with
specific antigenic
determinants [such as on viruses, cells, or other materials], drugs,
polynucleotides, nucleic
acids, peptides, cofactors, lectins, sugars, polysaccharides, cells, cellular
membranes, and
organelles.
Examples of receptors and applications using such receptors, include but are
not
restricted to:
a) enzymes: specific transport proteins or enzymes essential to survival of
microorganisms, which could serve as targets for antibiotic [ligand]
selection;
b) antibodies: identification of a ligand-binding site on the antibody
molecule that
combines with the epitope of an antigen of interest may be investigated;
determination of a
sequence that mimics an antigenic epitope may lead to the development of
vaccines of which
the immunogen is based on one or more of such sequences or lead to the
development of
related diagnostic agents or compounds useful in therapeutic treatments such
as for auto-
immune diseases
c) nucleic acids: identification of ligand, such as protein or RNA, binding
sites;
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-31 -
d) catalytic polypeptides: polymers, preferably polypeptides, that are capable
of
promoting a chemical reaction involving the conversion of one or more
reactants to one or
more products; such polypeptides generally include a binding site specific for
at least one
reactant or reaction intermediate and an active functionality proximate to the
binding site, in
which the functionality is capable of chemically modifying the bound reactant
[see, e.g., U.S.
Patent No. 5,215,899];
e) hormone receptors: determination of the ligands that bind with high
affinity to a
receptor is useful in the development of hormone replacement therapies; for
example,
identification of ligands that bind to such receptors may lead to the
development of drugs to
control blood pressure; and
f) opiate receptors: determination of ligands that bind to the opiate
receptors in the brain
is useful in the development of less-addictive replacements for morphine and
related drugs.
As used herein, "antibody" includes antibody fragments, such as Fab fragments,
which
are composed of a light chain and the variable region of a heavy chain.
As used herein, "humanized antibodies" refer to antibodies that are modified
to include
"human" sequences of amino acids so that administration to a human will not
provoke an
immune response. Methods for preparation of such antibodies are known. For
example, the
hybridoma that expresses the monoclonal antibody is altered by recombinant DNA
techniques
to express an antibody in which the amino acid composition of the non-variable
regions is
based on human antibodies. Computer programs have been designed to identify
such regions.
As used herein, "production by recombinant means" refers to production methods
that
use recombinant nucleic acid methods that rely on well known methods of
molecular biology
for expressing proteins encoded by cloned nucleic acids.
As used herein, "substantially identical" to a product means sufficiently
similar so that
the property of interest is sufficiently unchanged so that the substantially
identical product can
be used in place of the product.
As used herein, "equivalent," when referring to two sequences of nucleic acids
means
that the two sequences in question encode the same sequence of amino acids or
equivalent
proteins. It also encompasses those that hybridize under conditions of
moderate, preferably
high stringency, whereby the encoded protein retains desired properties.
As used herein, when "equivalent" is used in referring to two proteins or
peptides, it
means that the two proteins or peptides have substantially the same amino acid
sequence with
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-32-
only conservative amino acid substitutions [see, e.g., Table 1, above] that do
not substantially
alter the activity or function of the protein or peptide.
When "equivalent" refers to a property, the property does not need to be
present to the
same extent [e.g., two peptides can exhibit different rates of the same type
of enzymatic
activity], but the activities are preferably substantially the same.
"Complementary," when
referring to two nucleic acid molecules, means that the two sequences of
nucleotides are
capable of hybridizing, preferably with less than 25%, more preferably with
less than 15%,
even more preferably with less than 5%, most preferably with no mismatches
between opposed
nucleotides. Preferably the two molecules will hybridize under conditions of
high stringency.
As used herein: "stringency of hybridization" in determining percentage
mismatch is as
follows:
1) high stringency: 0.1 x SSPE, 0.1% SDS, 65°C;
2) medium stringency: 0.2 x SSPE, 0.1% SDS, 50°C (also referred to as
moderate
stringency); and
3) low stringency: 1.0 x SSPE, 0.1% SDS, 50°C.
It is understood that equivalent stringencies may be achieved using
alternative buffers, salts and
temperatures.
The term "substantially" identical or homologous or similar varies with the
context as
understood by those skilled in the relevant art and generally means at least
70%, preferably
means at least 80%, more preferably at least 90%, and most preferably at least
95% identity.
As used herein, a "composition" refers to a any mixture of two or more
products or
compounds. It may be a solution, a suspension, liquid, powder, a paste,
aqueous, non-aqueous
or any combination thereof.
As used herein, a "combination" refers to any association between two or among
more
items.
As used herein, "fluid" refers to any composition that can flow. Fluids thus
encompass
compositions that are in the form of semi-solids, pastes, solutions, aqueous
mixtures, gels,
lotions, creams and other such compositions.
As used herein, "vector (or plasmid)" refers to discrete elements that are
used to
introduce heterologous DNA into cells for either expression or replication
thereof. Selection
and use of such vehicles are well known within the skill of the artisan. An
expression vector
includes vectors capable of expressing DNA's that are operatively linked with
regulatory
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-33-
sequences, such as promoter regions, that are capable of effecting expression
of such DNA
fragments. Thus, an expression vector refers to a recombinant DNA or RNA
construct, such as
a plasmid, a phage, recombinant virus or other vector that, upon introduction
into an
appropriate host cell, results in expression of the cloned DNA. Appropriate
expression vectors
are well known to those of skill in the art and include those that are
replicable in eukaryotic
cells and/or prokaryotic cells and those that remain episomal or those which
integrate into the
host cell genome.
As used herein, "a promoter region or promoter element" refers to a segment of
DNA or
RNA that controls transcription of the DNA or RNA to which it is operatively
linked. The
promoter region includes specific sequences that are sufficient for RNA
polymerise
recognition, binding and transcription initiation. This portion of the
promoter region is referred
to as the promoter. In addition, the promoter region includes sequences that
modulate this
recognition, binding and transcription initiation activity of RNA polymerise.
These sequences
may be cis acting or may be responsive to traps acting factors. Promoters,
depending upon the
1 S nature of the regulation, may be constitutive or regulated. Exemplary
promoters contemplated
for use in prokaryotes include the bacteriophage T7 and T3 promoters, and the
like.
As used herein, "operatively linked or operationally associated" refers to the
functional
relationship of DNA with regulatory and effector sequences of nucleotides,
such as promoters,
enhancers, transcriptional and translational stop sites, and other signal
sequences. For example,
operative linkage of DNA to a promoter refers to the physical and functional
relationship
between the DNA and the promoter such that the transcription of such DNA is
initiated from
the promoter by an RNA polymerise that specifically recognizes, binds to and
transcribes the
DNA. In order to optimize expression and/or in vitro transcription, it may be
necessary to
remove, add or alter 5' untranslated portions of the clones to eliminate
extra, potential
inappropriate alternative translation initiation (i.e., start) codons or other
sequences that may
interfere with or reduce expression, either at the level of transcription or
translation.
Alternatively, consensus ribosome binding sites (see, e.g., Kozak, J. Biol.
Chem., 266:19867-
19870 (1991)) can be inserted immediately 5' of the start codon and may
enhance expression.
The desirability of (or need for) such modification may be empirically
determined.
As used herein, "sample" refers to anything which may contain an analyte for
which an
analyte assay is desired. The sample may be a biological sample, such as a
biological fluid or a
biological tissue. Examples of biological fluids include urine, blood, plasma,
serum, saliva,
semen, stool, sputum, cerebral spinal fluid, tears, mucus, amniotic fluid or
the like. Biological
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-34-
tissues are aggregates of cells, usually of a particular kind together with
their intercellular
substance that form one of the structural materials of a human, animal, plant,
bacterial, fungal
or viral structure, including connective, epithelium, muscle and nerve
tissues. Examples of
biological tissues also include organs, tumors, lymph nodes, arteries and
individual cell(s).
As used herein, the abbreviations for any protective groups, amino acids and
other
compounds, are, unless indicated otherwise, in accord with their common usage,
recognized
abbreviations, or the IUPAC-IUB Commission on Biochemical Nomenclature (see,
(1972)
Biochem. 11:1726).
As used herein, "protein binding sequence" refers to a protein or peptide
sequence that
is capable of specific binding to other protein or peptide sequences
generally, to a set of protein
or peptide sequences or to a particular protein or peptide sequence.
As used herein, "epitope tag" refers to a short stretch of amino acid residues
corresponding to an epitope to facilitate subsequent biochemical and
immunological analysis of
the "epitope tagged" protein or peptide. "Epitope tagging" is achieved by
appending the
sequence of the "epitope tag" to the protein-encoding sequence in an
appropriate expression
vector. "Epitope tagged" proteins can be affinity purified using highly
specific antibodies
raised against the tags.
As used herein, "Protein A or Protein G" refers to proteins that can bind to
Fc region of
most IgG isotypes. Protein A or Protein G are typically found in the cell wall
of some strains
of staphylococci. It is intended to encompass Protein A or Protein G with
conservative amino
acid substitutions that do not substantially alter its activity.
As used herein, "nucleotide binding sequence" refers to a protein or peptide
sequence
that is capable of specific binding to nucleotide sequences generally, to a
set of nucleotide
sequences or to a particular nucleotide sequence.
As used herein, "A-form DNA" refers to a DNA structure wherein the presence of
the 2'
hydroxyl group prevents adoption of the B-form. The A-form DNA structure is
very close to
the conformation of double-stranded RNA. Hybrid duplexes with one strand of
DNA and one
strand of RNA also lie in the A-form.
As used herein, "B-form DNA" refers to a DNA structure that follows the Watson
and
Crick model and represents the general structure of DNA. The DNA in living
cells exist in the
B-form.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-35-
As used herein, "Z-form DNA" refers to a DNA structure that follows the left-
handed
helix. The Z-form double helix occurs in polymers that have a sequence of
alternating purines
and pyrimidines.
As used herein, "replication" refers to a process of DNA-dependent DNA
synthesis
wherein the DNA molecule is duplicated to give identical copies.
As used herein, "transcription" refers to a process of DNA-dependent RNA
synthesis.
As used herein, "DNA repair" refers to a process wherein the sites of
mutations in DNA
are recognized by special nuclease that excise the damaged region from DNA;
and then further
enzymes synthesize a replacement sequence so that the original DNA sequence is
preserved.
As used herein, "recombination" refers to a reaction between homologous
sequences of
DNA. The critical feature is that the enzymes responsible for recombination
can use any pair
of homologous sequences as substrates, although some types of sequences may be
favored over
others. Recombination allows favorable or unfavorable mutations to be
separated and tested as
individual units in new assortments.
As used herein, "DNA structure maintenance" refers to DNA sequences, through
binding to proteins, that maintain the DNA molecule in particular structures
such as
chromatids, chromatins or chromosomes.
As used herein, "DNA polymerase" refers to an enzyme that synthesizes DNA
using a
DNA as the template. It is intended to encompass DNA polymerise with
conservative amino
acid substitutions that do not substantially alter its activity.
As used herein, "DNA-dependent RNA polymerise" or "transcriptase" refers to an
enzyme that synthesizes RNA using a DNA as the template. It is intended to
encompass DNA-
dependent RNA polymerise with conservative amino acid substitutions that do
not
substantially alter its activity.
As used herein, "DNAase" refers to an enzyme that attacks bonds in DNA. It is
intended to encompass DNAase with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "DNA ligase" refers to an enzyme that catalyses the formation
of a
phosphodiester bond to link two adjacent bases separated by a nick in one
strand of double
helix of DNA. It is intended to encompass DNA ligase with conservative amino
acid
substitutions that do not substantially alter its activity.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-36-
As used herein, "DNA topoisomerase" refers to an enzyme that can change the
linking
number of DNA. It is intended to encompass DNA topoisomerase with conservative
amino
acid substitutions that do not substantially alter its activity.
As used herein, "DNA transposase" refers to an enzyme that is involved in
insertion of a
transposon at a new site. It is intended to encompass DNA transposase with
conservative
amino acid substitutions that do not substantially alter its activity.
As used herein, "Transposon" refers to a DNA sequence that is able to
replicate and
insert one copy at a new location in the genome.
As used herein, "DNA kinase" refers to an enzyme that phosphorylates DNA. It
is
intended to encompass DNA kinase with conservative amino acid substitutions
that do not
substantially alter its activity.
As used herein, "restriction enzyme" refers to an enzyme that recognizes
specific short
sequences of DNA and cleaves the duplex at the recognition site or other site.
It is intended to
encompass a restriction enzyme with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "rRNA" or "ribosomal RNA" refers to the RNA components of the
ribosome, a compact ribonucleoprotein particle that assembles amino acids into
proteins.
As used herein, "mRNA" or "messenger RNA" refers to the RNA molecule that
bears
the same sequence of the DNA coding strand and is used as the template in
protein synthesis.
As used herein, "tRNA" or "transfer RNA" refers to the RNA molecule that
carries
amino acids to the ribosome for protein synthesis.
As used herein, "reverse transcription" refers to the RNA-dependent DNA
synthesis.
As used herein, "RNA splicing" refers to the removal of introns and joining of
exons in
RNA so that introns are spliced out and exons are spliced together.
As used herein, "RNA-dependent DNA polymerase" or "reverse transcriptase"
refers to
an enzyme that synthesizes DNA using a RNA as the template. It is intended to
encompass a
RNA-dependent DNA polymerase with conservative amino acid substitutions that
do not
substantially alter its activity.
As used herein, "RNA-dependent RNA polymerase" refers to an enzyme that
synthesizes RNA using a RNA as the template. It is intended to encompass a RNA-
dependent
RNA polymerase with conservative amino acid substitutions that do not
substantially alter its
activity.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-37-
As used herein, "RNA ligase" refers to an enzyme that catalyses the formation
of a
phosphodiester bond to link two adjacent bases separated by a nick in one
strand of RNA. It is
intended to encompass a RNA ligase with conservative amino acid substitutions
that do not
substantially alter its activity.
As used herein, "RNA maturase" refers to an enzyme that catalyses the removal
of
intron in the RNA splicing. It is intended to encompass a RNA maturase with
conservative
amino acid substitutions that do not substantially alter its activity.
As used herein, "lipid binding sequence" refers to a protein or peptide
sequence that is
capable of specific binding to lipids generally, to a set of lipids or to a
particular lipid.
As used herein, "C2 motif' refers to a protein domain that has the similar
binding
affinity as the C2 domain of approximately 130 residues in length originally
identified in the
Ca2+-dependent isoforms of protein kinase C (Nalefski and Falke, Protein Sci.,
5:2375-90
(1996)). Single and multiple copies of C2 domains have been identified in a
number of
eukaryotic signaling proteins that interact with cellular membranes and
mediate a broad array
of critical intracellular processes, including membrane trafficking, the
generation of lipid-
second messengers, activation of GTPases, and the control of protein
phosphorylation. As a
group, C2 domains display the remarkable property of binding a variety of
different ligands and
substrates, including Ca2+, phospholipids, inositol polyphosphates, and
intracellular proteins.
C2 domain exists in two topologies: the fold of the original synaptotagmin C2A
domain as
"topology I," while that of the phosphoinositide-specific phospholipase C-bl
domain as
"topology IL" Each of these structures forms an eight-stranded anti-parallel
(3-sandwich
including a pair of four-stranded 13-sheets, with a slight difference in their
13-strand connection.
As used herein, "amphipathic a-helix motif' refers to an a helix with opposing
polar
and nonpolar faces oriented along its long axis (Segrest, et al., Adv. Protein
Chem., 45:303-69
(1994)).
As used herein, "polysaccharide binding sequence" refers to a protein or
peptide
sequence that is capable of specific binding to polysaccharides generally, to
a set of
polysaccharides or to a particular polysaccharide.
As used herein, "metal binding sequence" refers to a protein or peptide
sequence that is
capable of specific binding to metal ions generally, to a set of metal ions or
to a particular metal
ion.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-38-
As used herein, "transport protein" refers to a protein that carries specific
molecules or
ions from one organ to another. Non-limiting examples of transport proteins
include
hemoglobin, serum albumin, myoglobin and 131-lipoprotein.
As used herein, "nutrient or storage protein" refers to a protein that is used
by the cell as
the nutrient source or storage form for such nutrient. Non-limiting examples
of nutrient or
storage proteins include gliadin, ovalbumin, casein, and ferritin.
As used herein, "contractile or motile protein" refers to a protein that
endows cells and
organisms with the ability to contract, to change shape, or to move about. Non-
limiting
examples of contractile or motile proteins include actin, myosin, tubulin and
dynein.
As used herein, "structural protein" refers to a protein that serves as
supporting
filaments, cables, or sheets to give biological structures strength or
protection. Non-limiting
examples of structural proteins include keratin, fibroin, collagen, elastin
and proteoglycans.
As used herein, "defense protein" refers to a protein that defends organisms
against
invasion by other species or protect them from injury. Non-limiting examples
of defense
proteins include antibodies, fibrinogen, thrombin, botulinus toxin, diphtheria
toxin, snake
venoms and ricin.
As used herein, "regulatory protein" refers to a protein.that helps regulate
cellular or
physiological activity. Non-limiting examples of regulatory proteins include
insulin, growth
hormones, corticotropin and repressors.
As used herein, "luminescence" refers to the detectable EM radiation,
generally, UV, IR
or visible EM radiation that is produced when the excited product of an
exergic chemical
process reverts to its ground state with the emission of light.
Chemiluminescence is
luminescence that results from a chemical reaction. Bioluminescence is
chemiluminescence
that results from a chemical reaction using biological molecules or synthetic
versions or
analogs thereof as substrates and/or enzymes.
As used herein, "bioluminescence," which is a type of chemiluminescence,
refers to the
emission of light by biological molecules, particularly proteins. The
essential condition for
bioluminescence is molecular oxygen, either bound or free in the presence of
an oxygenase, a
luciferase, which acts on a substrate, a luciferin. Bioluminescence is
generated by an enzyme
or other protein (luciferase) that is an oxygenase that acts on a substrate
luciferin (a
bioluminescence substrate) in the presence of molecular oxygen and transforms
the substrate to
an excited state, which upon return to a lower energy level releases the
energy in the form of
light.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-39-
As used herein, the substrates and enzymes for producing bioluminescence are
generically referred to as luciferin and luciferase, respectively. When
reference is made to a
particular species thereof, for clarity, each generic term is used with the
name of the organism
from which it derives, for example, bacterial luciferin or firefly luciferase.
As used herein, "luciferase" refers to oxygenases that catalyze a light
emitting reaction.
For instance, bacterial luciferases catalyze the oxidation of flavin
mononucleotide [FMN] and
aliphatic aldehydes, which reaction produces light. Another class of
luciferases, found among
marine arthropods, catalyzes the oxidation of Cypridina [Vargula] luciferin,
and another class
of luciferases catalyzes the oxidation of Coleoptera luciferin.
Thus, luciferase refers to an enzyme or photoprotein that catalyzes a
bioluminescent
reaction [a reaction that produces bioluminescence]. The luciferases, such as
firefly and
Renilla luciferases, that are enzymes which act catalytically and are
unchanged during the
bioluminescence generating reaction. The luciferase photoproteins, such as the
aequorin
photoprotein to which luciferin is non-covalently bound, are changed, such as
by release of the
luciferin, during bioluminescence generating reaction. The luciferase is a
protein that occurs
naturally in an organism or a variant or mutant thereof, such as a variant
produced by
mutagenesis that has one or more properties, such as thermal stability, that
differ from the
naturally-occurnng protein. Luciferases and modified mutant or variant forms
thereof are well
known. For purposes herein, reference to luciferase refers to either the
photoproteins or
luciferases.
As used herein, "peroxidase" refers to an enzyme that catalyses a host of
reactions in
which hydrogen peroxide is a specific oxidizing agent and a wide range of
substrates act as
electron donors. It is intended to encompass a peroxidase with conservative
amino acid
substitutions that do not substantially alter its activity. Peroxidases are
widely distributed in
nature and are produced by a wide variety of plant species. The chief
commercially available
peroxidase is horseradish peroxidase.
As used herein, "urease" refers to an enzyme that catalyses decomposition of
urea to
form ammonia and carbon dioxide. It is intended to encompass an unease with
conservative
amino acid substitutions that do not substantially alter its activity. Unease
is widely found in
plants, animals and microorganisms.
As used herein, "alkaline phosphatases" refers to a family of functionally
related
enzymes named after the tissues in which they predominately appear. Alkaline
phosphatases
carry out hydrolase/transferase reactions on phosphate-containing substrates
at a high pH
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-40-
optimum. It is intended to encompass alkaline phosphatases with conservative
amino acid
substitutions that do not substantially alter its activity.
As used herein, "glutathione S-transferase" refers to a ubiquitous family of
enzymes
with dual substrate specificities that perform important biochemical functions
of xenobiotic
biotransformation and detoxification, drug metabolism, and protection of
tissues against
peroxidative damage. The basic reaction catalyzed by glutathione S-transferase
is the
conjugation of an electrophile with reduced glutathione (GSH) and results in
either activation
or deactivation/detoxification of the chemical. It is intended to encompass a
glutathione S-
transferase with conservative amino acid substitutions that do not
substantially alter its activity.
As used herein, high-throughput screening (HTS) refers to processes that test
a large
number of samples, such as samples of diverse chemical structures against
disease targets to
identify "hits" (see, e.g., Broach, et al., High throughput screening for drug
discovery, Nature,
384:14-16 (1996); Janzen, et al., High throughput screening as a discovery
tool in the
pharmaceutical industry, Lab Robotics Automation: 8261-265 (1996); Fernandes,
P.B., Letter
from the society president, J. Biomol. Screening, 2:1 (1997); Burbaum, et al.,
New technologies
for high-throughput screening, Curr. Opin. Chem. Biol., 1:72-78 (1997)]. HTS
operations are
highly automated and computerized to handle sample preparation, assay
procedures and the
subsequent processing of large volumes of data.
As used herein, "disease or disorder" refers to a pathological condition in an
organism
resulting from, e.g., infection or genetic defect, and characterized by
identifiable symptoms.
As used herein, "infection" refers to invasion of the body of a multi-cellular
organism
with organisms that have the potential to cause disease.
As used herein, "infectious organism" refers to an organism that is capable to
cause
infection of a mufti-cellular organism. Most infectious organisms are
microorganisms such as
viruses, bacteria and fungi.
For clarity of disclosure, and not by way of limitation, the detailed
description is
divided into the subsections that follow.
B. METHODS FOR ASSAYING ANALYTES
Provided herein are methods for assaying an analyte in a sample. Any assays
that
employ an antibody as a reagent can be modified as described herein by
replacing the antibody
with an enzyme that has been modified such that it retains the ability to bind
to an analyte of
interest but has substantially reduced catalytic activity (i.e., a substrate
trapping enzyme).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-41 -
Assays provided herein include the steps of: a) contacting a sample with a
mutant or
modified enzyme that binds to the analyte of interest; and b) detecting
binding between the
analyte or the immediate analyte enzymatic conversion product with the mutant
analyte-
binding enzyme. The mutant or modified enzyme substantially retains the
binding affinity has
enhanced binding affinity of the wildtype or unmodified enzyme for the analyte
or an
immediate analyte enzymatic conversion product but has attenuated catalytic
activity.
1. Analytes
Any analyte that can specifically bind to an enzyme, either as a co-enzyme, a
co-factor
or a substrate can be assayed by the presently claimed methods. Analytes can
be any
molecules, including biological macromolecules and small molecules, ligands,
anti-ligands and '
other species. Preferably, the analyte to be assayed is a small molecule. In
one embodiment,
the small molecule analyte to be assayed is an inorganic molecule. Preferably,
the inorganic
molecule is an inorganic ion such as a sodium, a potassium, a magnesium, a
calcium, a
chlorine, an iron, a copper, a zinc, a manganese, a cobalt, an iodine, a
molybdenum, a
vanadium, a nickel, a chromium, a fluorine, a silicon, a tin, a boron or an
arsenic ion.
In another specific embodiment, the small molecule analyte is an organic
molecule.
Preferably, the organic molecule to be assayed is an amino acid, a peptide
containing less than
10 amino acids, a nucleoside, a nucleotide, an oligonucleotide containing less
than 10
nucleotides, a vitamin, a monosaccharide, an oligosaccharide containing less
than 10
monosaccharides or a lipid.
Any amino acids can be assayed by the presently claimed methods. For example,
a D-
and a L-amino-acid can be assayed. In addition, any building blocks of
naturally occurring
peptides and proteins including Ala (A), Arg (R), Asn (N), Asp (D), Cys (C),
Gln (Q), Glu (E),
Gly (G), His (H), Ile (I), Leu (L), Lys (K), Met (M), Phe (F), Pro (P) Ser
(S), Thr (T), Trp (V~,
Tyr (Y) and Val (V) can be assayed. Further, any derivatives of the naturally
occurring amino
acids, e.g., Hcy as a derivative of Cys, can be assayed.
Any nucleosides can be assayed by the presently claimed methods. Examples of
such
nucleosides include adenosine, guanosine, cytidine, thymidine and uridine.
Any nucleotides can be assayed by the presently claimed methods. Examples of
such
nucleotides include AMP, GMP, CMP, UMP, ADP, GDP, CDP, UDP, ATP, GTP, CTP,
UTP,
dAMP, dGMP, dCMP, dTMP, dADP, dGDP, dCDP, dTDP, dATP, dGTP, dCTP and dTTP. In
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-42-
addition, any oligonucleotides containing less than 10 such nucleotides or
other nucleotides can
be assayed.
Any vitamins can be assayed by the presently claimed methods. For example,
water-
soluble vitamins such as thiamine, riboflavin, nicotinic acid, pantothenic
acid, pyridoxine,
biotin, folate, vitamin B12 and ascorbic acid can be assayed. Similarly, fat-
soluble vitamins
such as vitamin A, vitamin D, vitamin E, and vitamin K can be assayed.
Any monosaccharides, whether D- or L-monosaccharides and whether aldoses or
ketoses, can be assayed by the presently claimed methods. Examples of
monosaccharides
include triose such as glyceraldehyde, tetroses such as erythrose and threose,
pentoses such as
ribose, arabinose, xylose, lyxose and ribulose, hexoses such as allose,
altrose, glucose,
mannose, gulose, idose, galactose, talose and fructose and heptose such as
sedoheptulose.
Any lipids can be assayed by the presently claimed methods. Examples of lipids
include triacylglycerols such as tristearin, tripalmitin and triolein, waxes,
phosphoglycerides
such as phosphatidylethanolamine, phosphatidylcholine, phosphatidylserine,
phosphatidylinositol and cardiolipin, sphingolipids such as sphingomyelin,
cerebrosides and
gangliosides, sterols such as cholesterol and stigmasterol and sterol fatty
acid esters. The fatty
acids can be saturated fatty acids such as lauric acid, myristic acid,
palmitic acid, stearic acid,
arachidic acid and lignoceric acid, or can be unsaturated fatty acids such as
palmitoleic acid,
oleic acid, linoleic acid, linolenic acid and arachidonic acid.
In still another specific embodiment, the small molecule to be assayed has a
molecular
weight that is about or less than 10,000 daltons. More preferably, the small
molecule has a
molecular weight that is about or less than 5,000 daltons.
Examples of specific analytes that can be assayed by the presently claimed
methods
include, but are not limited to, Hcy, folate species, cholesterol, glucose,
ethanol and uric acid.
2. Mutant analyte-binding enzymes ("substrate trapping enzymes")
Any mutant analyte-binding enzyme that substantially retains its binding
affinity or has
enhanced binding affinity for the analyte or an immediate analyte enzymatic
conversion
product but has attenuated catalytic activity can be used in the assay. For
example, if Hcy is
the analyte to be assayed, mutant Hcy-binding enzymes such as mutant
cystathionine 13-
synthase, mutant methionine synthase, mutant betaine-homocysteine
methyltransferase, mutant
methioninase and mutant SAH hydrolase can be used.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 43 -
Mutant enzymes having the desired specificity can be prepared using routine
mutagenesis methods. Residues to mutate can be identified by systematically
mutating
residues to different residues, and identifying those that have the desired
reduction in catalytic
activity and retention of binding activity for a particular substrate.
Alternatively or
additionally, mutations may be based upon predicted or known 3-D structures of
enzymes,
including predicted affects of various mutations (see, e.g., Turner, et al.
(1998) Nature
Structural Biol. 5:369-376; Ault-Richie, et al. (1994) J. Biol. Chem.
269:31472-31478; Yuan,
et al. (1996) J. Biol. Chem. 271:28009-28016; Williams, et al. (1998)
Biochemistry 37:7096;
Steadman, et al. (1998) Biochemistry 37:7089-7095; Finer-Moore, et al. (1998)
J. Mol. Biol.
276:113-129; Strop, et al. (1997) Protein Sci. 6:2504-2511; Finer-Moore, et
al. (1996)
Biochemistry 35:5125-5136; Schiffer, et al. (1995) Biochemistry 34:16279-
16287; Costi, et al.
(1996) Biochemistry 35:3944-3949; Graves, et al. (1992) Biochemistry 31:15-21;
Cameras, et
al. (1992) Biochemistry 31:6038-6044). Such predictions can be made by those
of skill in the
art of computational chemistry. Hence, for any selected enzyme, the mutations
need to
inactivate catalytic activity but retain binding activity can be determined
empirically.
a. Nucleic acids encoding analyte-binding enzymes
Nucleic acids encoding analyte-binding enzymes can be obtained by methods
known in
the art. Known nucleic acid sequences of analyte-binding enzymes can be used
in isolating
nucleic acids encoding analyte-binding enzymes from natural or other sources.
Alternatively,
complete or partial nucleic acids encoding analyte-binding enzymes can be
obtained by
chemical synthesis according to the known sequences or obtained from
commercial or other
sources.
Eukaryotic cells and prokaryotic cells can serve as a nucleic acid source for
the isolation
of nucleic acids encoding analyte-binding enzymes. The DNA can be obtained by
standard
procedures known in the art from cloned DNA (e.g., a DNA "library"), chemical
synthesis,
cDNA cloning, or by the cloning of genomic DNA, or fragments thereof, purified
from the
desired cell (see, for example, Sambrook, et al., 1989, Molecular Cloning, A
Laboratory
Manual, 2d Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New
York; Glover,
D.M. (ed.), 1985, DNA Cloning: A Practical Approach, MRL Press, Ltd., Oxford,
U.K. Vol. I,
IL). Clones derived from genomic DNA can contain regulatory and intron DNA
regions in
addition to coding regions; clones derived from cDNA or RNA contain only exon
sequences.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-44-
Whatever the source, the gene is generally molecularly cloned into a suitable
vector for
propagation of the gene.
In the molecular cloning of the gene from cDNA, cDNA can be generated from
totally
cellular RNA or mRNA by methods that are known in the art. The gene can also
be obtained
from genomic DNA, where DNA fragments are generated (e. g., using restriction
enzymes or by
mechanical shearing), some of which will encode the desired gene. The linear
DNA fragments
can then be separated according to size by standard techniques, including but
not limited to,
agarose and polyacrylamide gel electrophoresis and column chromatography.
Once the DNA fragments are generated, identification of the specific DNA
fragment
containing all or a portion of the analyte-binding enzymes gene can be
accomplished in a
number of ways.
A preferred method for isolating an analyte-binding enzyme gene is by the
polymerase
chain reaction (PCR), which can be used to amplify the desired analyte-binding
enzyme
sequence in a genomic or cDNA library or from genomic DNA or cDNA that has not
been
incorporated into a library. Oligonucleotide primers which hybridize to the
analyte-binding
enzyme sequences can be used as primers in PCR.
Additionally, a portion of the analyte-binding enzyme (of any species) gene or
its
specific RNA, or a fragment thereof, can be purified (or an oligonucleotide
synthesized) and
labeled, the generated DNA fragments may be screened by nucleic acid
hybridization to the
labeled probe (Benton, W. and Davis, R., 1977, Science 196:180; Grunstein, M.
And Hogness,
D., 1975, Proc. Natl. Acad. Sci. U.S.A. 72:3961). Those DNA fragments with
substantial
homology to the probe will hybridize. The analyte-binding enzyme nucleic acids
can be also
identified and isolated by expression cloning using, for example, anti-analyte-
binding enzyme
antibodies for selection.
Alternatives to obtaining the analyte-binding enzyme DNA by cloning or
amplification
include, but are not limited to, chemically synthesizing the gene sequence
itself from the known
analyte-binding enzyme nucleotide sequence or making cDNA to the mRNA which
encodes
the analyte-binding enzyme. Any suitable method known to those of skill in the
art may be
employed.
Once a clone has been obtained, its identity can be confirmed by nucleic acid
sequencing (by methods known in the art) and comparison to known analyte-
binding enzyme
sequences. DNA sequence analysis can be performed by techniques known in the
art,
including but not limited to, the method of Maxam and Gilbert (1980, Meth.
Enrymol. 65:499-
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 45 -
560), the Sanger dideoxy method (Sanger, F., et al., 1977, Proc. Natl. Acad.
Sci. US.A.
74:5463), the use of T7 DNA polymerase (Tabor and Richardson, U.S. Patent No.
4,795,699),
use of an automated DNA sequenator (e.g., Applied Biosystems, Foster City,
CA).
Nucleic acids which are hybridizable to an analyte-binding enzyme nucleic
acid, or to a
nucleic acid encoding an analyte-binding enzyme derivative can be isolated, by
nucleic acid
hybridization under conditions of low, high, or medium stringency (Shilo and
Weinberg, 1981,
Proc. Natl. Acad. Sci. USA 78:6789-6792).
b. Selecting and producing mutant analyte-binding enzymes
Once nucleic acids encoding the analyte-binding enzymes are obtained, these
nucleic
acids can be mutagenized and screened and/or selected for analyte-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for the analyte or an
immediate analyte enzymatic conversion product but have attenuated catalytic
activity.
Insertion, deletion, or point mutations) can be introduced into nucleic acids
encoding the
analyte-binding enzymes. Techniques for mutagenesis known in the art can be
used, including,
but not limited to, in vitro site-directed mutagenesis (Hutchinson, et al.,
1978, J. Biol. Chem
253:6551), use of TAB~ linkers (Pharmacia), mutation-containing PCR primers,
etc.
Mutagenesis can be followed by phenotypic testing of the altered gene product.
Site-directed mutagenesis protocols can take advantage of vectors that provide
single
stranded as well as double stranded DNA, as needed. Generally, the mutagenesis
protocol with
such vectors is as follows. A mutagenic primer, i.e., a primer complementary
to the sequence
to be changed, but including one or a small number of altered, added, or
deleted bases, is
synthesized. The primer is extended in vitro by a DNA polymerase and, after
some additional
manipulations, the now double-stranded DNA is transfected into bacterial
cells. Next, by a
variety of methods, the desired mutated DNA is identified, and the desired
protein is purified
from clones containing the mutated sequence. For longer sequences, additional
cloning steps
are often required because long inserts (longer than 2 kilobases) are unstable
in those vectors.
Protocols are known to one skilled in the art and kits for site-directed
mutagenesis are widely
available from biotechnology supply companies, for example from Amersham Life
Science,
Inc. (Arlington Heights, IL) and Stratagene Cloning Systems (La Jolla, CA).
Information regarding the structural-functional relationship of the analyte-
binding
enzymes can be used in the mutagenesis and selection of analyte-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for the analyte or an
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-46-
immediate analyte enzymatic conversion product but have attenuated catalytic
activity. For
example, mutants can be made in the enzyme's binding site for its co-enzyme,
co-factor, a non-
analyte substrate, or in the mutant enzyme's catalytic site, or a combination
thereof.
Once a mutant analyte-binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for the
analyte or an
immediate analyte enzymatic conversion product but has attenuated catalytic
activity, is
identified, such mutant analyte-binding enzyme can be produced by any methods
known in the
art including recombinant expression, chemical synthesis or a combination
thereof. Preferably,
the mutant analyte-binding enzyme is obtained by recombinant expression.
For recombinant expression, the mutant analyte-binding enzyme gene or portion
thereof
is inserted into an appropriate cloning vector for expression in a particular
host cell. A large
number of vector-host systems known in the art may be used. Possible vectors
include, but are
not limited to, plasmids or modified viruses, but the vector system must be
compatible with the
host cells used. Such vectors include, but are not limited to, bacteriophages
such as lambda
derivatives, or plasmids such as pBR322 or pUC plasmid derivatives or the
Bluescript vector
(Stratagene). The insertion into a cloning vector can, for example, be
accomplished by ligating
the DNA fragment into a cloning vector which has complementary cohesive
termini. If,
however, the complementary restriction sites used to fragment the DNA are not
present in the
cloning vector, the ends of the DNA molecules can be enzymatically modified.
Alternatively, a
desired site can be produced by ligating sequences of nucleotides (linkers)
onto the DNA
termini; these ligated linkers can include specific oligonucleotides encoding
restriction
endonuclease recognition sequences. Recombinant molecules can be introduced
into host cells
via transformation, transfection, infection, electroporation, etc., so that
many copies of the gene
sequence are generated.
In an alternative method, the desired gene can be identified and isolated
after insertion
into a suitable cloning vector in a "shot gun" approach. Enrichment for the
desired gene, for
example, by size fractionation, can be done before insertion into the cloning
vector.
In specific embodiments, transformation of host cells with recombinant DNA
molecules
that incorporate the isolated mutant analyte-binding enzyme gene, cDNA, or
synthesized DNA
sequence enables generation of multiple copies of the gene. Thus, the gene can
be obtained in
large quantities by growing transformants, isolating the recombinant DNA
molecules from the
transformants and, when necessary, retrieving the inserted gene from the
isolated recombinant
DNA.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-47-
The nucleotide sequence coding for a mutant analyte-binding enzyme or a
functionally
active analog or fragment or other derivative thereof, can be inserted into an
appropriate
expression vector, e.g., a vector which contains the necessary elements for
the transcription and
translation of the inserted protein-coding sequence. The necessary
transcriptional and
translational signals can also be supplied by the native mutant analyte-
binding enzyme gene
and/or its flanking regions. A variety of host-vector systems can be utilized
to express the
protein-coding sequence. These systems include but are not limited to
mammalian cell systems
infected with virus (e.g., vaccinia virus, adenovirus, etc.); insect cell
systems infected with
virus (e.g., baculovirus); microorganisms such as yeast containing yeast
vectors, or bacteria
transformed with bacteriophage, DNA, plasmid DNA, or cosmid DNA. The
expression
elements of vectors vary in their strengths and specificities. Depending on
the host-vector
system utilized, suitable transcription and translation elements can be used.
The methods previously described for the insertion of DNA fragments into a
vector can
be used to construct expression vectors containing a chimeric gene containing
appropriate
1 S transcriptional/translational control signals and the protein coding
sequences. These methods
can include in vitro recombinant DNA and synthetic techniques and in vivo
recombinants
(genetic recombination). Expression of a nucleic acid sequence encoding a
mutant analyte-
binding enzyme or peptide fragment can be regulated by a second nucleic acid
sequence so that
the mutant analyte-binding enzyme or peptide is expressed in a host
transformed with the
recombinant DNA molecule. For example, expression of a mutant analyte-binding
enzyme can
be controlled by a promoter/enhancer element as is known in the art. Promoters
which can be
used to control a mutant analyte-binding enzyme expression include, but are
not limited to, the
SV40 early promoter region (Bernoist and Chambon, 1981, Nature 290:304-310),
the promoter
contained in the 3' long terminal repeat of Rous sarcoma virus (Yamamoto, et
al., 1980, Cell
22:787-797), the herpes thymidine kinase promoter (Wagner, et al., 1981, Proc.
Natl. Acad.
Sci. U.SA. 78:1441-1445), the regulatory sequences of the metallothionein gene
(Brinster, et
al., 1982, Nature 296:39-42); prokaryotic expression vectors such as the (3-
lactamase promoter
(Villa-Kamaroff, et al., 1978, Proc. Natl. Acad. Sci. U.S.A. 75:3727-3731), or
the tac promoter
(DeBoer, et al., 1983, Proc. Natl. Acad. Sci. U.S.A. 80:21-25); see also
"Useful proteins from
recombinant bacteria" in Scientific American, 1980, 242:74-94; promoter
elements from yeast
or other fungi such as the Gal 4 promoter, the ADC (alcohol dehydrogenase)
promoter, PGK
(phosphoglycerol kinase) promoter, alkaline phosphatase promoter, and certain
animal
transcriptional control regions.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-48-
For example, a vector can be used that contains a promoter operably linked to
a nucleic
acid encoding a mutant analyte-binding enzyme, one or more origins of
replication, and,
optionally, one or more selectable markers (e.g., an antibiotic resistance
gene).
In a specific embodiment, an expression construct is made by subcloning a
mutant
analyte-binding enzyme coding sequence into the EcoRI restriction site of each
of the three
pGEX vectors (Glutathione S-Transferase expression vectors; see, e.g., Smith
and Johnson,
1988, Gene 7:31-40). This allows for the expression of a mutant analyte-
binding enzyme
product from the subclone in the correct reading frame.
Expression vectors containing a mutant analyte-binding enzyme gene inserts can
be
identified by three general approaches: (a) nucleic acid hybridization, (b)
presence or absence
of "marker" gene functions, and (c) expression of inserted sequences. In the
first approach, the
presence of a mutant analyte-binding enzyme gene inserted in an expression
vector can be
detected by nucleic acid hybridization using probes containing sequences that
are homologous
to an inserted mutant analyte-binding enzyme gene. In the second approach, the
recombinant
vector/host system can be identified and selected based upon the presence or
absence of certain
"marker" gene functions (e.g., thymidine kinase activity, resistance to
antibiotics,
transformation phenotype, occlusion body formation in baculovirus, etc.)
caused by the
insertion of a mutant analyte-binding enzyme gene in the vector. For example,
if the mutant
analyte-binding enzyme gene is inserted within the marker gene sequence of the
vector,
recombinants containing the mutant analyte-Binding enzyme insert can be
identified by the
absence of the marker gene function. In the third approach, recombinant
expression vectors
can be identified by assaying the mutant analyte-binding enzyme product
expressed by the
recombinant. Such assays can be based, for example, on the physical or
functional properties
of the mutant analyte-binding enzyme in in vitro assay systems, e.g., binding
with anti-mutant
analyte-binding enzyme antibody.
Once a particular recombinant DNA molecule is identified and isolated, several
methods known in the art can be used to propagate it. Once a suitable host
system and growth
conditions are established, recombinant expression vectors can be propagated
and prepared in
quantity. As previously explained, the expression vectors which can be used
include, but are
not limited to, the following vectors or their derivatives: human or animal
viruses such as
vaccinia virus or adenovirus; insect viruses such as baculovirus; yeast
vectors; bacteriophage
vectors (e.g., lambda), and plasmid and cosmid DNA vectors, to name but a few.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-49-
In addition, a host cell strain can be chosen which modulates the expression
of the
inserted sequences, or modifies and processes the gene product in the specific
fashion desired.
Expression from certain promoters can be elevated in the presence of certain
inducers; thus,
expression of the genetically engineered mutant analyte-binding enzyme can be
controlled.
Furthermore, different host cells have characteristic and specific mechanisms
for the
translational and post-translational processing and modification (e.g.,
glycosylation,
phosphorylation) of proteins. Appropriate cell lines or host systems can be
chosen to ensure
the desired modification and processing of the foreign protein expressed. For
example,
expression in a bacterial system can be used to produce an unglycosylated core
protein product.
Expression in yeast will produce a glycosylated product. Expression in
appropriate animal
cells can be used to ensure "native" glycosylation of a heterologous protein.
Furthermore,
different vector/host expression systems can effect processing reactions to
different extent.
3. Sample collection
Any sample can be assayed for an analyte using the above-described methods. In
one
embodiment, the sample being assayed is a biological sample from a mammal,
particularly a
human, such as a biological fluid or a biological tissue. Biological fluids,
include, but are not
limited to, are urine, blood, plasma, serum, saliva, semen, stool, sputum,
hair and other
keratinous samples, cerebral spinal fluid, tears, mucus and amniotic fluid.
Biological tissues
contemplated include, but are not limited to, aggregates of cells, usually of
a particular kind
together with their intercellular substance that form one of the structural
materials of a human,
animal, plant, bacterial, fungal or viral structure, including connective,
epithelium, muscle and
nerve tissues, organs, tumors, lymph nodes, arteries and individual cell(s).
In one specific
embodiment, the body fluid to be assayed is urine. In another specific
embodiment, the body
fluid to be assayed is blood. Preferably, the blood sample is further
separated into a plasma or
sera fraction.
Serum or plasma can be recovered from the collected blood by any methods known
in
the art. In one specific embodiment, the serum or plasma is recovered from the
collected blood
by centrifugation. Preferably, the centrifugation is conducted in the presence
of a sealant
having a specific gravity greater than that of the serum or plasma and less
than that of the blood
corpuscles which will form the lower, whereby upon centrifugation, the sealant
forms a
separator between the upper serum or plasma layer and the lower blood
corpuscle layer. The
sealants that can be used in the processes include, but are not limited to,
styrene resin powders
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-50-
(Japanese Patent Publication No. 38841/1973), pellets or plates of a hydrogel
of a crosslinked
polymer of 2-hydroxyethyl methacrylate or acrylamide (U.S. Patent No.
3,647,070), beads of
polystyrene bearing an antithrombus agent or a wetting agent on the surfaces
(U.S. Patent
No. 3,464,890) and a silicone fluid (U.S. Patent Nos. 3,852,194 and
3,780,935). In a preferred
embodiment, the sealant is a polymer of unsubstituted alkyl acrylates and/or
unsubstituted alkyl
methacrylates, the alkyl moiety having not more than 18 carbon atoms, the
polymer material
having a specific gravity of about 1.03 to 1.08 and a viscosity of about 5,000
to 1,000,000 cps
at a shearing speed of about 1 second-1 when measured at about 25°C
(U.S. Patent
No. 4,140,631 ).
In another specific embodiment, the serum or plasma is recovered from the
collected
blood by filtration. Preferably, the blood is filtered through a layer of
glass fibers with an
average diameter of about 0.2 to 5 p, and a density of about 0.1 to 0.5
g./cm3, the total volume
of the plasma or serum to be separated being at most about 50% of the
absorption volume of
the glass fiber layer; and collecting the run-through from the glass fiber
layer which is plasma
or serum (U.S. Patent No. 4,477,575). Also preferably, the blood is filtered
through a layer of
glass fibers having an average diameter 0.5 to 2.5 p, impregnated with a
polyacrylic ester
derivative and polyethylene glycol (U.S. Patent No. 5,364,533). More
preferably, the
polyacrylic ester derivative is poly(butyl acrylate), poly(methyl acrylate) or
poly(ethyl
acrylate), and (a) poly(butyl acrylate), (b) poly(methyl acrylate) or
poly(ethyl acrylate) and
(c) polyethylene glycol are used in admixture at a ratio of (10-12):(1-4):(1-
4).
In still another specific embodiment, the serum or plasma is recovered from
the
collected blood by treating the blood with a coagulant containing a lignan
skeleton having
oxygen-containing side chains or rings (U.S. Patent No. 4,803,153).
Preferably, the coagulant
contains a lignan skeleton having oxygen-containing side chains or rings,
e.g., d-sesamin, 1-
sesamin, paulownin, d-asarinin, l-asarinin, 2a-paulownin, 6a-paulownin,
pinoresinol, d-
eudesmin, l-pinoresinol 13-D-glucoside,1-pinoresinol, l-pinoresinol monomethyl
ether 13-D-
glucoside, epimagnolin, lirioresinol-B, syringaresinol (dl), lirioresinonB-
dimethyl ether,
phillyrin, magnolin, lirioresinol-A, 2a, 6a-d-sesamin, d-diaeudesmin,
lirioresinol-C dimethyl
ether (ddiayangambin) and sesamolin. More preferably, the coagulant is used in
an amount
ranging from about 0.01 to 50 g per 1 1 of the blood.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-51 -
C. METHODS FOR ASSAYING HOMOCYSTEINE
Also provided herein is a method for assaying Hcy in a sample. The method
includes at
least the steps of: a) contacting the sample with a mutant Hcy-binding enzyme,
the mutant
enzyme substantially retains its binding affinity or has enhanced binding
affinity for Hcy or an
immediate Hcy enzymatic conversion product but has attenuated catalytic
activity; and b)
detecting binding between the Hcy or the immediate Hcy enzymatic conversion
product with
the mutant Hcy-binding enzyme.
1. Homocysteine metabolism
Homocysteine is an intermediary amino acid produced when methionine is
metabolized
to cysteine. There are two routes by which homocysteine produced in the body
is rapidly
metabolized: (1) condensation with serine to form cystathione or (2)
conversion to methionine.
As discussed above, homocysteine levels in biological samples are of clinical
significance. Homocysteine plays a role sulflnydryl amino acid metabolism; its
accumulation
may be indicative of various disorders occurring in these pathways, including
in particular
inborn errors of metabolism. Thus, for example homocystinuria (an abnormal
build-up of
homocysteine in the urine) is a disorder of amino acid metabolism resulting
from deficiencies
in the enzymes cystathione ~i-synthetase or methyltetrahydrofolic acid
methyltransferase, which
catalyses the methylation of homocysteine to methionine.
In the second pathway, which is illustrated as follows:
4
FHz dTMP
NADPH + H NADP~ 5
Met dUMP
_ FHa 2 ' 5,10,-METHYLENE-1
B,2 I eS r C,ly
3
Hcy 5,-METHYL-FH,
where: 1 is methylene synthase; 2 is tetrahydrofolate (FH4) methyltransferase;
3 is
methylenetetrahydrofolate reductase; 4 is dihydrofolate reductase; 5 is
thymidylate synthase;
FH4 is tetrahydrofolate and FHZ is dihydrofolate, homocysteine levels are
related, among other
things, to folate levels and also vitamin B12 levels. The various enzymes in
these pathways
may be assessed and correlated with homocysteine levels.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-52-
Sulfliydryl amino acid metabolism is closely linked to that of folic acid and
vitamin Biz
(cobalamin), which act as substrates or co-factors in the various
transformations involved.
Homocysteine accumulation can be an indicator of malfunction of cobalamin or
folate
dependent enzymes, or other disorders or diseases related to cobalamin or
folate metabolism.
Homocysteine metabolism also may be affected by anti-folate drugs, such as
methotrexate, administered to treat disorders, such as cancer and asthma,
since homocysteine
conversion to methionine relies on a reaction requiring S-methyl
tetrahydrofolate as the methyl
donor. Monitoring of homocysteine has therefore also been proposed in the
management of
malignant disease treatment with anti-folate drugs. More recently, elevated
levels of
homocysteine in the blood have been correlated with the development of
atherosclerosis (see
Clarke, et al., New Eng. J. Med. 324:1149-1155 (1991)) and even moderate
homocysteinemia
is a risk factor for cardiac and vascular diseases. Measurement of plasma or
blood levels of
homocysteine is thus also of importance in the diagnosis and treatment of
vascular disease.
2. Mutant Hcy-binding enzymes
Any mutant Hcy-binding enzymes that substantially retain their binding
affinity or have
enhanced binding affinity for Hcy or an immediate Hcy enzymatic conversion
product but have
attenuated catalytic activity can be used in the Hcy assay. Examples of such
mutant Hcy-
binding enzyme include mutant cystathionine 13-synthase, mutant methionine
synthase, mutant
betaine-homocysteine methyltransferase, mutant methioninase and mutant SAH
hydrolase.
a. Nucleic acids encoding Hcy-binding enzymes
Nucleic acids encoding Hcy-binding enzymes can be obtained by methods known in
the
art. Additional nucleic acid molecules encoding such enzymes are known and the
molecules or
sequences thereof are publicly available. If the molecules are available they
can be used;
alternatively the known sequences can be used to obtain clones from selected
or desired
sources. For example, the nucleic acid sequences of Hcy-binding enzymes, such
as
cystathionine 13-synthase, methionine synthase, betaine-homocysteine
methyltransferase,
methioninase and SAH hydrolase, can be used in isolating nucleic acids
encoding Hcy-binding
enzymes from natural sources. Alternatively, nucleic acids encoding Hcy-
binding enzymes can
be obtained by chemical synthesis according to the known sequences.
In one embodiment, the nucleic acid molecules containing sequences of
nucleotides
with the following GenBank accession Nos. can be used in obtaining nucleic
acid encoding
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-53-
SAH hydrolase: AF129871 (Gossypium hirsutum); AQ003753 (Cryptosporidium
parvum);
AF105295 (Alexandrium fundyense); AA955402 (Rattus norvegicus); AA900229
(Rattus
norvegicus); AA874914 (Rattus norvegicus); AA695679 (Drosophila melanogaster
ovary);
AA803942 (Drosophila melanogaster ovary; AI187655 (Manduca sexta male
antennae);
S U40872 (Trichomonas vaginalis); AJ007835 (Xenopus Laevis); AF080546
(Anopheles
gambiae); AI069796 (T. cruzi epimastigote); 297059 (Arabidopsis thaliana);
AF059581
(Arabidopsis thaliana); U82761 (Homo Sapiens); AA754430 (Oryza sativa); D49804
(Nicotiana tabacum); D45204 (Nicotiana tabacum); X95636 (D. melanogaster);
T18277
(endosperm Zea mays); 875259 (Mouse brain); 226881 (C. roseus); X12523 (D.
discoideum);
X64391 (Streptomyces fradiae); W21772 (Maize Leaf); AH003443 (Rattus
norvegicus);
U 14963 (Rattus norvegicus); U 14962 (Rattus norvegicus); U 14961 (Rattus
norvegicus);
U 14960 (Rattus norvegicus); U 14959 (Rattus norvegicus); U 14937 (Rattus
norvegicus);
U14988 (Rattus norvegicus); U14987 (Rattus norvegicus); U14986 (Rattus
norvegicus);
U14985 (Rattus norvegicus); U14984 (Rattus norvegicus); U14983 (Rattus
norvegicus);
U14982 (Rattus norvegicus); U14981 (Rattus norvegicus); U14980 (Rattus
norvegicus);
U14979 (Rattus norvegicus); U14978 (Rattus norvegicus); U14977 (Rattus
norvegicus);
U14976 (Rattus norvegicus); U14975 (Rattus norvegicus); L32836 (Mus musculus);
L35559
(Xenopus laevis); 219779 (Human foetal Adrenals tissue); L23836 (Rhodobacter
capsulatus);
M15185 (Rat); L11872 (Triticum aestivum); M19937 (Slime mold (D. discoideum);
M80630
(Rhodobacter capsulatus). Preferably, the nucleic acid molecules containing
nucleotide
sequences with the GenBank accession Nos. M61831-61832 can be used in
obtaining nucleic
acid encoding SAH hydrolase (SEQ ID No. 1; see also Coulter-Karis and
Hershfield, Ann.
Hum. Genet., 53:169-175 (1989)). Also preferably, the nucleic acid molecule
containing the
sequence of nucleotides or encoding the amino acids set forth in SEQ ID No. 3
can be used (see
also U.S. Patent No. 5,854,023).
In another specific embodiment, the nucleic acid molecules containing
sequences of
nucleotides with the following GenBank accession Nos. can be used in obtaining
nucleic acid
encoding methionine synthase: AI547373 (Mesembryanthemum crystallinum);
AI507856
(COBALAM1NE-INDEPENDENT METHION1NE SYNTHASE); AI496185
(COBALAM1NE-INDEPENDENT METHIONINE SYNTHASE); AI496016
(COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI495904
(COBALAM1NE-INDEPENDENT METHIONINE SYNTHASE); AI495702; AI495399;
AI461276 (COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI460827
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-54-
(COBALAMINE-INDEPENDENT METHION1NE SYNTHASE); AI460549; AI443293;
AI443243 (COBALAMINE-INDEPENDENT METHION1NE SYNTHASE); AI443242
(COBALAMINE-INDEPENDENT METHION1NE SYNTHASE); AI442736
(COBALAMINE-INDEPENDENT METHION1NE SYNTHASE); AI442546; AI442173
(COBALAMINE-INDEPENDENT METHION1NE SYNTHASE); AI442136
(COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI441314; AI440982;
AI438053; AI416939 (COBALAMINE-INDEPENDENT METHIONINE SYNTHASE);
AI416601; AI391967 (Conidial Neurospora crassa); AF034214 (Rattus norvegicus);
U77388
(Chlamydomonas moewusii); AF093539 (Zea mays); U97200 (Arabidopsis thaliana);
U36197
(Chlamydomonas reinhardtii); AF025794 (Homo sapiens); AJ222785 (Hordeum
vulgare);
249150 (C. blumei kinetoplast met gene); AB004651 (Hyphomicrobium methylovorum
gene);
AA661438 (Maize Leaf); AA661023 (Medicago truncatula); AA660965 (Medicago
truncatula); AA660880 (Medicago truncatula); AA660780 (Medicago truncatula);
AA660708
(Medicago truncatula); AA660643 (Medicago truncatula); AA660558 (Medicago
truncatula);
AA660475 (Medicago truncatula); AA660444 (Medicago truncatula); AA660382
(Medicago
truncatula); AA660310 (Medicago truncatula); AA660241 (Medicago truncatula);
U75743
(Human); AA389835 (Arabidopsis thaliana); U84889 (Mesembryanthemum
crystallinum);
U73338 (Human); AA054818 (Maize Leaf); AA030695 (Maize Leaf); X83499 (C.
roseus);
U15099 (Saccharomyces cerevisiae (MET6)); J02804 (E. coli speED operon speE
and speD
genes); M87625 (Escherichia coli); J04975 (E. coli). Preferably, the nucleic
acid molecules
containing sequences of nucleotides with GenBank accession Nos. U75743 (SEQ ID
No. 4)
and U73338 (SEQ ID No. 6) can be used to obtain nucleic acid encoding
methionine synthase.
In still another specific embodiment, nucleic acid molecules containing
sequences of
nucleotides with the following GenBank accession Nos. can be used in obtaining
nucleic acid
encoding cystathionine 13-synthase: AI584826 (Zebrafish L19501); AI566920
(Homo sapiens);
AI558544 (Zebrafish); AI529762 (Sugano mouse liver); AI528420 (Sugano mouse
liver);
AI494445 (Homo Sapiens); AI500425 (Homo Sapiens); AI421007 (Homo Sapiens);
AI369768
(Homo Sapiens); AI368618 (Homo Sapiens); AI312384 (Homo Sapiens); AI266220
(Homo
Sapiens); AI307196 (Homo Sapiens); 885449 (Homo sapiens); 884640 (Homo
Sapiens);
AI371928 (Homo Sapiens); AI281692 (Homo Sapiens); AI198353 (Homo sapiens);
AI222601
(Homo Sapiens); AI188666 (Snares placenta); AI088293 (Snares Homo Sapiens);
AI039450
(Homo Sapiens); AA995138 (Homo Sapiens); AI053744 (Homo Sapiens); AA921824
(Homo
Sapiens); AA876324 (Homo Sapiens); AA218777 (neuronal precursor Homo Sapiens);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-55-
AA243110 (neuronal precursor Homo Sapiens); AA232188 (neuronal precursor Homo
Sapiens); AA227066 (neuronal precursor Homo sapiens); AA180443 (HeLa cell Homo
Sapiens); AA179769 (HeLa cell Homo Sapiens); AA620410 (lung carcinoma Homo
sapiens);
AA173243 (neuroepithelium Homo Sapiens); AA173133 (neuroepithelium Homo
Sapiens);
AA811740 (Homo Sapiens); AA659341 (Homo Sapiens); AA729802 (Homo Sapiens);
AA063294 (corneal stroma); AA063180 (corneal stroma); AA701200 (fetal liver
spleen);
AA699637 (fetal liver spleen); AA652920 (Homo Sapiens); AA430416 (ovary
tumor);
AA430367 (ovary tumor); AA642534 (Homo Sapiens); AA618538 (Homo sapiens);
AA548257 (Homo Sapiens); AA554953 (Homo Sapiens); AA548561 (Homo Sapiens);
AA136426 (lung carcinoma); AA136339 (lung carcinoma); AA057714 (corneal
stroma);
AA260332 (mouse NML Mus musculus); AA239916 (mouse NML Mus musculus);
AA239480 (mouse NML Mus musculus); AA096780 (mouse lung); AA105071 (mouse
kidney); N76209 (fetal liver spleen); N54505 (fetal liver spleen); AA171542
(neuroepithelium); AA171511 (neuroepithelium); 578267 (human, homocystinuria
patient 12,
skin fibroblasts); AA057541 (corneal stroma); N50670 (multiple sclerosis);
N29067
(melanocyte); T28038 (Human Brain Homo Sapiens); H11280 (infant brain); 878956
(placenta); 838394 (infant brain); 835233 (placenta); T91706 (lung); T70457
(liver); T69322
(liver); T69248 (liver); L00972 (Human). Preferably, a nucleic acid molecule
containing
sequences of nucleotides set forth in SEQ ID No. 8 can be used in obtaining
nucleic acid
encoding cystathionine 13-synthase (see also U.S. Patent No. 5,523,225).
In yet another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding betaine
homocysteine
S-methyltransferase: AI629131 (Zebrafish); AI601766 (Zebrafish); NM001713
(Homo
sapiens); AH007531 (Homo sapiens); AF118378 (Homo Sapiens); AF118377 (Homo
sapiens);
AF118376 (Homo Sapiens); AF118375 (Homo Sapiens); AF118374 (Homo Sapiens);
AF118373 (Homo Sapiens); AF118372 (Homo Sapiens); AF118371 (Homo Sapiens);
AI550844
(mouse lung); AI529920 (mouse liver); AI529834 (mouse liver); AI529135 (mouse
liver);
AI527147 (mouse liver); AI527097 (mouse liver); AI497458 (Zebrafish); AI497232
(Zebrafish); AI496988 (Zebrafish); AI496904 (Zebrafish); AI496821 (Zebrafish);
AI496747
(Zebrafish); AI471640 (Homo sapiens); AA901407 (Rattus norvegicus); AI390284
(mouse);
AI244216 (Homo Sapiens); AI316045 (mouse liver); AI303938 (mouse liver);
AI303911
(mouse liver); AI303222 (mouse liver); AI287146 (mouse liver); AI287008 (mouse
liver);
AI286878 (mouse liver); AI266927 (mouse liver); AI256283 (mouse liver);
AI227233 (mouse
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-56-
liver); AI227053 (mouse liver); U50929 (Human); U53421 (Sus scrofa); AI132261
(mouse
liver); AI132254 (mouse liver); AI118276 (mouse liver); AI116416 (mouse
liver); AI115840
(mouse kidney); AI115838 (mouse kidney); AI048111 (mouse liver); AI043140
(mouse liver);
AA989805 (mouse kidney); AA986591 (mouse kidney); AA986590 (mouse kidney);
AA985983 (mouse liver); AA755243 (mouse diaphragm); AF038870 (Rattus
norvegicus);
AA693837 (fetal liver); U96133 ((Rattus norvegicus). Preferably, the
nucleotide sequences
with the GenBank accession No. AH007531 can be used in obtaining nucleic acid
encoding
betaine homocysteine S-methyltransferase (SEQ ID No. 10; see also Garrow, J.
Biol. Chem.,
271 37 :22831-8 (1996)).
In yet another specific embodiment, the nucleotide sequences described in U.S.
Patent
No. 5,891,704 (SEQ ID No. 11) and the nucleotide sequences with the GenBank
Accession
No. L43133 (SEQ ID No. 13) (Hori, et al., Cancer Res., 56(9):2116-22 (1996))
can be used in
obtaining nucleic acid encoding methioninase.
b. Selecting and producing Hcy-binding enzymes
Once nucleic acids encoding Hcy-binding enzymes are obtained, these nucleic
acids can
be mutagenized and screened and/or selected for Hcy-binding enzymes that
substantially retain
their binding affinity or have enhanced binding affinity for Hcy or an
immediate Hcy
enzymatic conversion product but have attenuated catalytic activity.
Insertion, deletion, or
point mutations) can be introduced into nucleic acids encoding Hcy-binding
enzymes
according to methods known to those of skill in the art, and, particularly,
those described in
Section C2. herein.
Information regarding the structural-functional relationship of the Hcy-
binding enzymes
can be used in the mutagenesis and selection of Hcy-binding enzymes that
substantially retain
their binding affinity or have enhanced binding affinity for Hcy or an
immediate Hcy
enzymatic conversion product but have attenuated catalytic activity. For
example, mutants can
be made in the enzyme's binding site for its co-enzyme, co-factor, a non-Hcy
substrate, or in
the mutant enzyme's catalytic site, or a combination thereof.
In one specific embodiment, wherein cystathionine 13-synthase is mutagenized,
mutants
can be made in cystathionine 13-synthase's binding site for pyridoxal 5'-
phosphate or L-serine,
or a combination thereof (Kim, et al., Proc. Nat. Acad. Sci., 7:4821-4825
(1974)). For
example, Lys 119 of human cystathionine 13-synthase can be deleted or mutated,
preferably to a
non-charged or acidic amino acid residue (Kery, et al., Biochemistry,
3,~9~:2716-24 ( 1999)).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-57-
In another specific embodiment, wherein methionine synthase is mutagenized,
mutants
can be made in methionine synthase's binding site for vitamin B12 or S-
methyltetrahydrofolate
(5-CH3-THF), or a combination thereof. For example, Asp946, G1u1097, Argl 134,
A1a1136,
Gly 113 8, Tyrl 139 and Tyr 1189 of human methionine synthase can be deleted
or mutated,
preferably to a different type of amino acid residue, i.e., Asp and Glu are
changed to non-
charged or basic residue, Arg is changed to non-charged or acidic residue, Ala
and Gly are
changed to charged residue or non-charged residue with larger sidechain, and
Tyr is charged to
residue without an aromatic sidechain (Dixon, et al., Structure, 411):1263-75
(1996)).
Preferably, E. coli. methionine synthase with amino acid sequence set forth in
SEQ ID No. 3,
containing His759G1y, Asp757G1u, Asp757Asn, or Ser810A1a is used in the Hcy
assay
(Amaratunga, et al., Biochemistry, 35 7 :2453-63 (1996))
In still another embodiment, wherein SAH hydrolase is mutagenized, mutants can
be
made in SAH hydrolase's binding site for NAD+, or mutations) in the mutant SAH
hydrolase's
catalytic site, e.g., the 5'-hydrolytic catalytic site, or a combination
thereof.
In yet another embodiment, wherein betaine-homocysteine methyltransferase is
mutagenized, mutants can be made in betaine-homocysteine methyltransferase's
binding site for
Zn+ or betaine. For example, Cys299 and Cys300 of human betaine-homocysteine
methyltransferase can be deleted or mutated, preferably to amino acid residue
without -SH
sidechain, e.g., Serine (Millian and Garrow, Arch. Biochem. Biophys., 356 1
:93-8 (1998)).
In yet another specific embodiment, wherein methioninase is mutagenized,
mutants can
be made in methioninase's binding site for R'SH which represents an
alkanethiol or a
substituted thiol (Ito, et al., J. Biochem., (Tokyo) 80~6~:1327-34 (1976)).
Once a mutant Hcy-binding enzyme with desired properties, i.e., substantially
retaining
its binding affinity or having enhanced binding affinity for Hcy or an
immediate Hcy enzymatic
conversion product but has attenuated catalytic activity, is identified, such
mutant Hcy-binding
enzyme can be produced by any methods known in the art including recombinant
expression,
chemical synthesis or a combination thereof as described in Section B.
Preferably, the mutant
Hcy-binding enzyme is obtained by recombinant expression.
c. Mutant SAH hydrolase and nucleic acids encoding the mutant SAH
hydrolase
SAH hydrolase from mammalian sources are homotetramer of approximate molecular
weight of 180-190 KD. The enzyme contains 4 molecules of tightly-bound NAD+ as
a co-
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-58-
enzyme. The catalytic mechanism of the enzyme in the hydrolytic direction
includes two
consecutive reactions, i. e., the 3'-oxidation of the substrate to 3'-keto in
concomitant with the
reduction of the enzyme-bound NAD+ to NADH, and followed by the 5'-hydrolysis
to release
the reaction products Hcy and Ado (Refsum, et al., Clin. Chem., 31:624-628
(1985)). The C-
terminal regions of all known SAH hydrolase are extremely conserved and
contain essential
amino acid residues to the enzyme catalysis. The crystal structure of human
SAH hydrolase in
complex with a substrate analog inhibitor was recently determined. This x-ray
structure of
SAH hydrolase indicates that at least twenty amino acid residues are directly
or indirectly
interacting with the substrate analog inhibitor and co-enzyme NAD+. Mutations
of those amino
acid residues that are involved directly or indirectly in the substrate
binding and catalysis can
readily be made by site-directed mutagenesis, and the sequence of the
resulting mutant enzyme
can be confirmed by comparing the mutant SAH hydrolase DNA sequence with the
sequence
of the wild type enzyme to ensure no other mutations are introduced to the
specific mutant
enzyme.
Provided herein is a substantially purified mutant SAH hydrolase that
substantially
retains its binding affinity or has enhanced binding affinity for homocysteine
(Hcy) or SAH but
has attenuated catalytic activity.
In one specific embodiment, the attenuated catalytic activity of the mutant
SAH
hydrolase is caused by mutations) in the mutant SAH hydrolase's binding site
for NAD+, or
mutations) in the mutant SAH hydrolase's catalytic site or a combination
thereof.
In another specific embodiment, the mutant SAH hydrolase has attenuated 5'-
hydrolytic
activity but substantially retains its 3'-oxidative activity.
In still another specific embodiment, the mutant SAH hydrolase irreversibly
binds SAH.
In yet another specific embodiment, the mutant SAH hydrolase has a Km for SAH
that
is about or less than 10.0 pM. Preferably, the mutant SAH hydrolase has a Km
for SAH that is
about 1.0 p,M or less than 1.0 pM.
In yet another specific embodiment, the mutant SAH hydrolase has a Kcat for
SAH that
is about or less than 0.1 S-1.
In yet another specific embodiment, the mutant SAH hydrolase has one or more
insertion, deletion, or point mutation(s). Preferably, the mutant SAH
hydrolase is derived from
the sequence of amino acids set forth in SEQ ID No. 1 or encoded by the
sequence of
nucleotides set forth in SEQ ID No. 2 but has one or more of the following
mutations: Phe 302
to Ser (F302S), Lys 186 to Ala (K186A), His 301 to Asp (H301D), His 353 to Ser
(H353S),
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-59-
Arg 343 to Ala (R343A), Asp 190 to Ala (D190A), Phe 82 to Ala (F82A), Thr 157
to Leu
(T157L), Cys 195 to Asp (C195D), Asn 181 to Asp (N181D), and deletion of Tyr
432 (0432).
Also more preferably, the mutant SAH hydrolase is a derived sequence of amino
acids set forth
in SEQ ID No. 1 or encoded by the sequence of nucleotides set forth in SEQ ID
No. 2 and has a
combination of Arg 431 to Ala (R431 A) and Lys 426 to Arg (K426R) mutations.
The nucleic
acid molecules contemplated also include those that have conservative amino
acid changes, and
include those that hybridize along their full length to the coding portion of
the sequence of
nucleotides set forth in SEQ ID No. 2, under medium stringency, or preferably
high stringency,
such that the encoded protein retains ability to bind to the selected analyte
without substantial
conversion of the analyte.
Also provided herein is an isolated nucleic acid fragment, either DNA or RNA,
that
includes a sequence of nucleotides encoding a mutant S-adenosylhomocysteine
(SAH)
hydrolase, the mutant SAH hydrolase substantially retains its binding affinity
or has enhanced
binding affinity for homocysteine (Hcy) or SAH but has attenuated catalytic
activity.
In one specific embodiment, the isolated nucleic acid fragment encodes a
mutant SAH
hydrolase wherein the attenuated catalytic activity is caused by mutations) in
the mutant SAH
hydrolase's binding site for NAD+, or mutations) in the mutant SAH hydrolase's
catalytic site
or a combination thereof.
In another specific embodiment, the isolated nucleic acid fragment encodes a
mutant
SAH hydrolase wherein the mutant SAH hydrolase has attenuated 5'-hydrolytic
activity but
substantially retains its 3'-oxidative activity.
In still another specific embodiment, the isolated nucleic acid fragment
encodes a
mutant SAH hydrolase wherein the mutant SAH hydrolase irreversibly binds SAH.
In yet another specific embodiment, the isolated nucleic acid fragment encodes
a mutant
SAH hydrolase wherein the mutant SAH hydrolase has a Km for SAH that is about
or less than
10.0 ~,M. Preferably, the isolated nucleic acid fragment encodes a mutant SAH
hydrolase
wherein the mutant SAH hydrolase has a Km for SAH that is about 1.0 ~,M or
less than 1.0 ~M.
In yet another specific embodiment, ~he isolated nucleic acid fragment encodes
a mutant
SAH hydrolase wherein the mutant SAH hydrolase has a Kcat for SAH that is
about or less
than 0.1 S-~.
In yet another specific embodiment, the isolated nucleic acid fragment encodes
a mutant
SAH hydrolase wherein the mutant SAH hydrolase has one or more insertion,
deletion, or point
mutation(s). Preferably, the isolated nucleic acid fragment encodes a mutant
SAH hydrolase
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-60-
wherein the mutant SAH hydrolase is derived from a sequence of nucleotides set
forth in SEQ
ID No. 1 and has one or more mutation selected from Phe 302 to Ser (F302S),
Lys 186 to Ala
(K186A), His 301 to Asp (H301D), His 353 to Ser (H353S), Arg 343 to Ala
(R343A), Asp 190
to Ala (D190A), Phe 82 to Ala (F82A), Thr 157 to Leu (T157L), Cys 195 to Asp
(C195D),
Asn 181 to Asp (N181D), and deletion of Tyr 432 (0432). Also more preferably,
the isolated
nucleic acid fragment encodes a mutant SAH hydrolase wherein the mutant SAH
hydrolase is
derived from a sequence of nucleotides set forth in SEQ ID No. 1 and has a
combination of
Arg 431 to Ala (R431 A) and Lys 426 to Arg (K426R) mutations.
Further provided is a plasmid, including the nucleic acid fragment encoding
the above
mutant SAH hydrolases. Preferably, the plasmid is an expression vector
including a sequence
of nucleotides encoding: a) a promoter region; and b) a mutant S-
adenosylhomocysteine (SAH)
hydrolase, the mutant SAH hydrolase substantially retains its binding affinity
or has enhanced
binding affinity for homocysteine (Hcy) or SAH but has attenuated catalytic
activity. The
sequence of nucleotides encoding the mutant SAH hydrolase is operatively
linked to the
promoter, whereby the mutant SAH hydrolase is expressed. More preferably, the
plasmid also
includes a selectable marker.
Further provided is a recombinant host cell containing the above plasmid. The
recombinant host cell can be any suitable host cell, including, but not
limited to, a bacterial cell,
a yeast cell, a fungal cell, a plant cell, an insect cell or an animal cell.
Also provided are methods for producing a mutant SAH hydrolase. The
recombinant
host cells can be grown or cultured under conditions whereby the mutant SAH
hydrolase is
expressed by the cell. The expressed mutant SAH hydrolase can then be isolated
or recovered.
Additional mutant SAH hydrolase that substantially retains its binding
affinity or has
enhanced binding affinity for homocysteine (Hcy) or SAH, but has attenuated
catalytic activity
can be produced according to the procedures known to the those of skill in the
art, including
procedures exemplified herein (see, e.g., Section B). The above-described
mutant SAH
hydrolases and additional mutant SAH hydrolase that substantially retain
binding affinity or
have enhanced binding affinity for homocysteine (Hcy) or SAH but have
attenuated catalytic
activity can be used for assaying Hcy in a sample.
3. Hcy assays using mutant SAH hydrolase
In one specific embodiment, the mutant Hcy-binding enzyme used in the Hcy
assay is a
mutant SAH hydrolase, the mutant SAH hydrolase substantially retains its
binding affinity or
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-61 -
has enhanced binding affinity for homocysteine (Hcy) or SAH but has attenuated
catalytic
activity. This assay, described in detail in the EXAMPLES, is depicted in
Figure I . In this
Figure, the homocysteine-containing analyte is reduced to produce Hcy, which,
is quantified or
detected by binding it to a mutant (substrate trapping) SAH hydrolase; the Hcy
is then
converted to SAH by reaction with adenosine in the presence of wild type SAH
hydrolase. As
exemplified in the Figure, instead of using a monoclonal antibody to effect
quantitation (see,
e.g., U.S. Patent No. 5,885,767 and U.S. Patent No. 5,631,127). Quantitation
is effected using
a fluorescence-labeled tracer S-adenosylcysteine in a competition binding
format in which the
mutant SAH is used to trap the substrate. Any suitable quantitation assay with
any suitable
label can be used in the substrate trapping method. Figure 2 depicts an
exemplary assay
performed in a 96 well format; and figure 3 exemplifies preparation of
labeling of adenosyl-
cysteine with a fluorescent moiety.
In one preferred embodiment, the attenuated catalytic activity in the mutant
SAH
hydrolase is caused by mutations) in the mutant SAH hydrolase's binding site
for NAD+, or
mutations) in the mutant SAH hydrolase's catalytic site or a combination
thereof.
In another preferred embodiment, the mutant SAH hydrolase has attenuated 5'-
hydrolytic activity but substantially retains its 3'-oxidative activity.
In another preferred embodiment, the mutant SAH hydrolase irreversibly binds
SAH.
In still another preferred embodiment, the mutant SAH hydrolase has a Km for
SAH
that is about or less than 10.0 ~,M. More preferably, the mutant SAH hydrolase
has a Km for
SAH that is about 1.0 p.M or less than 1.0 ~M.
In yet another preferred embodiment, the mutant SAH hydrolase has a Kcat for
SAH
that is about or less than 0.1 S-~.
In yet another preferred embodiment, the mutant SAH hydrolase has one or more
insertion, deletion, or point mutation(s). More preferably, the mutant SAH
hydrolase is derived
from the sequence of amino acids set forth in SEQ ID No. 1 or encoded by the
sequence of
nucleotides set forth in SEQ ID No. 2 and has one or more of the following
mutations: Phe 302
to Ser (F302S), Lys 186 to Ala (K186A), His 301 to Asp (H301D), His 353 to Ser
(H353S),
Arg 343 to Ala (R343A), Asp 190 to Ala (D190A), Phe 82 to Ala (F82A), Thr 157
to Leu
(T157L), Cys 195 to Asp (C195D), Asn 181 to Asp (N181D), and deletion of Tyr
432 (0432).
Also more preferably, the mutant SAH hydrolase is derived from a sequence of
amino acids set
forth in SEQ ID No. 2 and has a combination of Arg 431 to Ala (R431 A) and Lys
426 to Arg
(K426R) mutations.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-62-
In yet another preferred embodiment, prior to the contact between the sample
and the
mutant SAH hydrolase, oxidized Hcy in the sample is converted into reduced
Hcy. More
preferably, the oxidized Hcy in the sample is converted into reduced Hcy by a
reducing agent
such as tri-n-butylphosphine (TBP),13-ME, DTT, dithioerythritol, thioglycolic
acid,
glutathione, tris(2-carbxyethyl)phosphine, sodium cyanoborohydride, NaBH4,
KBH4 and free
metals. .
In yet another preferred embodiment, prior to the contact between the sample
and the
mutant SAH hydrolase, the Hcy in the sample is converted into SAH. More
preferably, the
Hcy in the sample is converted into SAH by a wild-type SAH hydrolase. Also
more preferably,
the SAH is contacted with the mutant SAH hydrolase in the presence of a SAH
hydrolase
catalysis inhibitor such as neplanocin A or thimersol.
In yet another preferred embodiment, prior to the contact between the SAH and
the
mutant SAH hydrolase, free adenosine is removed or degraded. More preferably,
the free
adenosine is degraded by combined effect of adenosine deaminase, purine
nucleoside
phosphorylase and xanthine oxidase.
Any adenosine deaminase can be used. Preferably, the adenosine deaminase
encoded
by the nucleic acids having the following GenBank accession Nos. can be used:
AF051275
(Caenorhabditis elegans); AI573492 (mouse mammary gland); AI462267 (mouse
mammary
gland); AI429519 (mouse embryo); AI429513 (mouse embryo); AI326688 (Mus
musculus);
AI324114 (mouse placenta); AI322477 (mouse placenta); AI152550 (mouse uterus);
U76422
(Human, SEQ ID No. 15; see also Lai, et al., Mol. Cell. Biol., 17 5 :2413-24
(1997)); U76421
(Human); U76420 (Human); AI120695 (mouse mammary gland); AI049175 (Mus
musculus);
U73107 (Mus musculus); AF052506 (Mus musculus); AA871919 (Barstead bowel, Mus
musculus); AA871917 (Barstead bowel, Mus musculus); AA871865 (Barstead bowel);
AA871752 (Barstead bowel); AA871702 (Barstead bowel); AA871324 (Barstead
bowel);
AA871189 (Barstead bowel); AA869711 (Mus musculus); AA869187 (Mus musculus);
AA869184 (Mus musculus); AA869176 (Mus musculus); AA869120 (Mus musculus);
U75503
(Homo sapiens); AA646698 (mouse mammary gland); AA646681 (mouse mammary
gland);
AA427106 (mouse mammary gland); D50624 (Streptomyces virginiae); AA389303
(mouse
embryo); AA389067 (mouse embryo); U88065 (Xenopus laevis); AA124740 (Mus
musculus);
U74586 (Rattus norvegicus); AA036487 (mouse placenta); AA035873 (mouse
placenta);
AA030290 (mouse placenta); AA023505 (mouse placenta); AA023331 (mouse
placenta);
AA111514 (mouse embryo); AA111327 (mouse embryo); AA110493 (mouse embryo);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-63-
U73185 (Mus musculus); AA107590 (mouse embryo); AA102891 (mouse embryo);
AA097525 (mouse embryo); AA096642 (mouse embryo); AA087094 (mouse embryo);
AA060462 (mouse); U10439 (Human); M13792 (Human); U18942 (Rattus norvegicus);
K02567 (Human); M10319 (Mouse); M59033 (E. coli adenosine). Preferably, the
adenosine
deaminase encoded by the nucleic acids having the following GenBank accession
No. can be
used: U76422 (Human, SEQ ID No. 15; see also Lai, et al., Mol. Cell. Biol.,
17~5~:2413-24
( 1997)).
Any purine nucleoside phosphorylase can be used. Preferably, the purine
nucleoside
phosphorylase encoded by the nucleic acids having the following GenBank
accession Nos. can
be used: U88529 (E.coli); U24438 (E.coli, SEQ ID No. 17; see also Cornell and
R.iscoe,
Biochim. Biophys. Acta, 1396 1 :8-14 (1998)); U83703 (H. pylori); and M30469
(E coli).
Any xanthine oxidase can be used. Preferably, the xanthine oxidase encoded by
the
nucleic acids having the following GenBank accession Nos. can be used:
AF080548
(Sinorhizobium meliloti); and U39487 (Human, SEQ ID No. 19; see also Saksela
and Raivio,
Biochem. J., 315 1 :235-9 (1996)).
In yet another preferred embodiment, the sample containing or suspected of
containing
SAH is contacted with the mutant SAH hydrolase in the presence of a labeled
SAH or a
derivative or an analog thereof, whereby the amount of the labeled SAH bound
to the mutant
SAH hydrolase inversely relates to amount of the SAH in the sample. The SAH,
or the
derivative or analog thereof, can be labeled by methods known in the art,
e.g., to become
radioactive, enzymatic, fluorescent, luminescent (including chemo- or bio-
luminescent)
labeled. More preferably, the labeled SAH derivative or analog is a
fluorescence labeled
adenosyl-cysteine.
In yet another preferred embodiment, the sample containing or suspected of
containing
SAH is contacted with a labeled mutant SAH hydrolase. The mutant SAH hydrolase
can be
labeled by methods known in the art, e.g., to become radioactive, enzymatic,
fluorescent,
luminescent (including chemo- or bio-luminescent) labeled. More preferably,
the mutant SAH
hydrolase is fluorescently labeled. For example, a mutant SAH hydrolase
derived from an
SAH hydrolase having sequence of amino acids encoded by the sequence of
nucleotides set
forth in SEQ ID No. 2 is used and the mutant SAH hydrolase is fluorescently
labeled at residue
Cys421.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-64-
D. METHODS FOR ASSAYING FOLATE SPECIES
Further provided herein is a method for assaying a folate species in a sample.
This
method includes at least the steps of: a) contacting the sample with a mutant
folate-species-
binding enzyme, which substantially retains its binding affinity or has
enhanced binding
affinity for the folate species but has attenuated catalytic activity; and b)
detecting binding
between the folate species with the mutant folate-species-binding enzyme.
Any mutant folate-species-binding enzymes that substantially retain their
binding
affinity or have enhanced binding affinity for the folate species but have
attenuated catalytic
activity can be used in the folate species assay. Examples of such mutant
folate-species-
binding enzymes include mutant methionine synthase, tetrahydrofolate
methyltransferase,
methylenetetrahydrofolate reductase, folypolyglutamate synthase, dihydrofolate
reductase and
thymidylate synthase.
Nucleic acids encoding folate-species-binding enzymes can be obtained by
methods
known in the art. Where the molecules are available or the sequence known,
they can be
obtained from publicly available sources. Known nucleic acid sequences of
folate-species-
binding enzymes, such as methionine synthase, tetrahydrofolate
methyltransferase,
methylenetetrahydrofolate reductase, folypolyglutamate synthase, dihydrofolate
reductase and
thymidylate synthase, can be used in isolating nucleic acids encoding folate-
species-binding
enzymes from natural sources. Alternatively, nucleic acids encoding folate-
species-binding
enzymes can be obtained by chemical synthesis according to the known
sequences.
In specific embodiment, the nucleotide sequences with the following GenBank
accession Nos. can be used in obtaining nucleic acid encoding methionine
synthase: AI547373
(Mesembryanthemum crystallinum); AI507856 (COBALAMINE-INDEPENDENT
METHIONINE SYNTHASE); AI496185 (COBALAM1NE-INDEPENDENT METHIONINE
SYNTHASE); AI496016 (COBALAMINE-INDEPENDENT METHIONINE SYNTHASE);
AI495904 (COBALAM1NE-INDEPENDENT METHIONINE SYNTHASE); AI495702;
AI495399; AI461276 (COBALAMINE-INDEPENDENT METHIONINE SYNTHASE);
AI460827 (COBALAMINE-INDEPENDENT ME'THIONINE SYNTHASE); AI460549;
AI443293; AI443243 (COBALAM1NE-INDEPENDENT METHIONINE SYNTHASE);
AI443242 (COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI442736
(COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI442546; AI442173
(COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI442136
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-65-
(COBALAMINE-INDEPENDENT METHIONINE SYNTHASE); AI441314; AI440982;
AI438053; AI416939 (COBALAMINE-INDEPENDENT METHION1NE SYNTHASE);
AI416601; AI391967 (Conidial Neurospora crassa); AF034214 (Rattus norvegicus);
U77388
(Chlamydomonas moewusii); AF093539 (Zea mays); U97200 (Arabidopsis thaliana);
U36197
(Chlamydomonas reinhardtii); AF025794 (Homo sapiens); AJ222785 (Hordeum
vulgare);
249150 (C. blumei kinetoplast met gene); AB004651 (Hyphomicrobium methylovorum
gene);
AA661438 (Maize Leaf); AA661023 (Medicago truncatula); AA660965 (Medicago
truncatula); AA660880 (Medicago truncatula); AA660780 (Medicago truncatula);
AA660708
(Medicago truncatula); AA660643 (Medicago truncatula); AA660558 (Medicago
truncatula);
AA660475 (Medicago truncatula); AA660444 (Medicago truncatula); AA660382
(Medicago
truncatula); AA660310 (Medicago truncatula); AA660241 (Medicago truncatula);
U75743
(Human); AA389835 (Arabidopsis thaliana); U84889 (Mesembryanthemum
crystallinum);
U73338 (Human); AA054818 (Maize Leaf); AA030695 (Maize Leaf); X83499 (C.
roseus);
U15099 (Saccharomyces cerevisiae (MET6)); J02804 (E coli speED operon speE and
speD
genes); M87625 (Escherichia coli); J04975 (E. coli). Preferably, the
nucleotide sequences with
the GenBank accession Nos. U75743 (SEQ ID No. 4) and U73338 (SEQ ID No. 6) can
be
used in obtaining nucleic acid encoding methionine synthase.
In another embodiment, the nucleotide sequences with the following GenBank
accession Nos. can be used in obtaining nucleic acid encoding tetrahydrofolate
methyltransferase: 299115 (SEQ ID No. 21; see also Kunst, et al., Nature, 390
6657 :249-56
( 1997)).
In still another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding
methylenetetrahydrofolate reductase: AJ237672 (Homo Sapiens); AH007491 (Mus
musculus);
AF105998 (Mus musculus); AF105997 (Mus musculus); AF105996 (Mus musculus);
AF105995 (Mus musculus); AF105994 (Mus musculus); AF105993 (Mus musculus);
AF105992 (Mus musculus); AF105991 (Mus musculus); AF105990 (Mus musculus);
AF105989 (Mus musculus); AF105988 (Mus musculus); AF102543 (Zymomonas
mobilis);
AH007464 (Homo sapiens complete CDs); AF105987 (Homo Sapiens); AF105986 (Homo
sapiens); AF105985 (Homo sapiens); AF105984 (Homo Sapiens); AF105983 (Homo
sapiens);
AF105982 (Homo Sapiens); AF105981 (Homo sapiens); AF105980 (Homo Sapiens);
AF105979 (Homo Sapiens); AF105978 (Homo sapiens); AF105977 (Homo sapiens);
AI327505
(mouse); U74302 (Erwinia carotovora); AA660667 (Medicago truncatula); W11807
(mouse);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-66-
AA368389 (Placenta I Homo Sapiens); AA363389 (Ovary I Homo sapiens); U57049
(Rattus
norvegicus); X07689 (X. typhimurium); and U09806 (Human). Preferably, the
nucleotide
sequences with the GenBank accession No. AH007464 can be used in obtaining
nucleic acid
encoding methylenetetrahydrofolate reductase (SEQ ID No. 23; see also Goyette,
et al., Mamm.
Genome., x:652-6 (1998)).
In yet another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding
folypolyglutamate
synthase: AL031852 (S. pombe); and M32445 (E. coli). Preferably, the
nucleotide sequences
with the GenBank accession No. M32445 can be used in obtaining nucleic acid
encoding
folypolyglutamate synthase (SEQ ID No. 25; see also Bognar, et al., J. Biol.
Chem.,
262 25 :12337-43 (1987)).
In yet another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding
dihydrofolate
reductase: AF083501 (Macaca mulatta rhadinovirus); AF028812 (Enterococcus
faecalis);
U83347 (Kaposi's sarcoma-associated herpesvirus); U41366 (Cryptosporidium
pamum);
U03885 (Paramecium tetraurelia); AF006616 (Mycobacterium avium); U71365
(Kaposi's
sarcoma-associated herpes-like virus fragment I); AF055727 (Streptococcus
pneumoniae strain
R6); AF055726 (Streptococcus pneumoniae strain AP183); AF055725 (Streptococcus
pneumoniae strain AP13); AF055724 (Streptococcus pneumoniae strain AP173);
AF055723
(Streptococcus pneumoniae strain AP92); AF055722 (Streptococcus pneumoniae
strain AP71);
AF055721 (Streptococcus pneumoniae strain AP188); AF055720 (Streptococcus
pneumoniae
strain AP48); AF077008 (Salmonella typhimurium plasmid pIE1142); AF073488 (Zea
mays);
M12742 (Coliphage T4); U84588 (Candida albicans); U12275 (Plasmodium berghei
ANKA);
U12338 (Pseudomonas aeruginosa); M18578 (S. cerevisiae); J03772 (Plasmodium
falciparum);
L22484 (Trypanosoma cruzi); U09476 (Synthetic construct Tn7 (dhfr) gene);
U31119
(Escherichia coli plasmid pDG0100); L08489 (Toxoplasma gondii); M69220 (E.
coli plasmid
pDG0100); L17041 (Synthetic construct); U40997 (Listeria monocytogenes);
U20781
(Trypanosoma brucei); J01609 (E coli); U43152 (Listeria monocytogenes); U36276
(Escherichia); U09273 (Shigella sonnei); M55264 (Herpesvirus saimiri); M20407
(Synthetic
mini type II); J05088 (H. volcanii); U10186 (Escherichia coli); M28071
(Herpesvirus saimiri);
U12338 (Pseudomonas aeruginosa plasmid R1033); M18578 (S. cerevisiae); J03772
(Plasmodium falciparum (clone HB3)); L22484 (Trypanosoma cruzi); U09476
(Synthetic
construct); U31119 (Escherichia coli plasmid pDG0100); L08489 (Toxoplasma
gondii);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-67-
M69220 (E. coli plasmid pDG0100); L17041 (Synthetic construct); U40997
(Listeria
monocytogenes); U20781 (Trypanosome brucei); J01609 (E. coli); U43152
(Listeria
monocytogenes); U36276 (Escherichia coli); U09273 (Shigella sonnei); M55264
(Herpesvirus
saimiri); M20407 (Synthetic mini type II); J05088 (H. volcanii); U10186
(Escherichia coli);
M28071 (Herpesvirus saimiri); M19237 (Herpesvirus saimiri); L26316 (Mus
musculus);
L15311 (Cricetulus sp.); M37124 (Chinese hamster); M19869 (Chinese hamster);
M26668
(Saccharomyces cerevisiae); M26496 (Pneumocystis carinii); M26495 (P.
carinii); L08594
(Arabidopsis thaliana); L08593 (Arabidopsis thaliana); K01804 (Bacteriophage
T4); M22852
(C. fasciculata); M30834 (P. chabaudi); J04643 (P. falciparum); J03028 (P.
falciparum);
M22159 (P. falciparum); M14330 (L. tropica); M12734 (Leishmania); K02118
(Plasmid R67
from E. coli); J03306 (Plasmid pAZI type III); M10922 (Lactobacillus casei);
M26022
(Enterobacter aerogenes); M84522 (Escherichia coli); M26023 (Citrobacter
freundii); and
U06861 (Drosophila melanogaster). Preferably, the nucleotide sequences with
the GenBank
accession No. M37124 can be used in obtaining nucleic acid encoding
dihydrofolate reductase
(SEQ ID No. 27; see also Dicker, et al., J. Biol. Chem., 265 14 :8317-21
(1990)).
In yet another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding
thymidylate synthase:
AF083501 (Macaca mulatta rhadinovirus, thymidylate synthase); AF059506 (chilo
iridescent
virus); AI531067 (Drosophila melanogaster Schneider L2 cell); AI515689 (LD
Drosophila
melanogaster embryo; AI514354 (Drosophila melanogaster embryo; AB023402 (Oryza
sativa
thyA); AI406263 (Drosophila melanogaster head; AI390061 (Drosophila
melanogaster head;
AF099673 (Caenorhabditis elegans); AF099672 (Ascaris suum); AI297939
(Drosophila
melanogaster larval-early pupal); AI293665 (Drosophila melanogaster larval-
early pupal);
AI136006 (Drosophila melanogaster head); AI258021 (Drosophila melanogaster
larval-early
pupal); D00596 (Homo Sapiens); AF029302 (Rhesus monkey rhadinovirus); U83348
(Kaposi's
sarcoma-associated herpesvirus); U69259 (Synthetic Plasmodium falciparum);
U12256
(Filobasidiella neoformans); U41366 (Cryptosporidium parvum); U03885
(Paramecium
tetraurelia); U86637 (Neisseria gonorrhoeae); U71365 (Kaposi's sarcoma-
associated herpes-
like virus); AF073994 (Drosophila melanogaster); AF073488 (Zee mays); M12742
(Coliphage
T4); U12275 (Plasmodium berghei ANKA); J03772 (Plasmodium falcipanlm (clone
HB3);
L22484 (Trypanosome cruzi); L08489 (Toxoplasma gondii); L12138 (Rattus);
U20781
(Trypanosome brucei); M29019 (Synthetic Lactobacillus); L31962 (Bacteriophage
beta-22);
M13190 (Herpesvirus saimiri); M14080 (Herpesvirus saimiri); M22036
(Herpesvirus ateles);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-68-
M13019 (Mouse); M30774 (Mouse); J04230 (C.albicans); L08594 (Arabidopsis
thaliana);
L08593 (Arabidopsis thaliana); K01804 (Bacteriophage T4); M30834 (P.chabaudi);
J04643 (P.
falciparum); J03028 (P.falciparum); M14330 (L.tropica); M12734 (Leishmania);
M19653
(L.casei (thyA)); and M33770 (L.lactis (thyA)). Preferably, the nucleotide
sequences with the
GenBank accession No. D00596 can be used in obtaining nucleic acid encoding
thymidylate
synthase (SEQ ID No. 29; see also Kaneda, et al., J. Biol. Chem., 265 33
:20277-84 (1990)).
Once nucleic acids encoding folate-species-binding enzymes are obtained, these
nucleic
acids can be mutagenized and screened and/or selected for folate-species-
binding enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for the folate
species but have attenuated catalytic activity. Insertion, deletion, or point
mutations) can be
introduced into nucleic acids encoding folate-species-binding enzymes
according to the
methods described in Section B.
Information regarding the structural-functional relationship of the folate-
species-
binding enzymes can be used in the mutagenesis and selection of the folate-
species-binding
enzymes that substantially retain their binding affinity or have enhanced
binding affinity for the
folate species but have attenuated catalytic activity. For example, mutants
can be made in the
enzyme's binding site for its co-enzyme, co-factor, a non-folate-species
substrate, or in the
mutant enzyme's catalytic site, or a combination thereof.
In one specific embodiment, the folate species is 5,-methyl-tetrahydrofolate,
the mutant
folate-species-binding enzyme is a mutant methionine synthase, and the
attenuated catalytic
activity of the mutant methionine synthase is caused by mutation in its
catalytic site, its binding
site for vitamin B ~ 2, Hcy, or a combination thereof.
In another specific embodiment, the folate species is tetrahydrofolate, the
mutant folate-
species-binding enzyme is a mutant tetrahydrofolate methyltransferase, and the
attenuated
catalytic activity of the mutant tetrahydrofolate methyltransferase is caused
by mutation in its
catalytic site, its binding site for serine, or a combination thereof.
In still another specific embodiment, the folate species is 5, 10,-methylene
tetrahydrofolate, the mutant folate-species-binding enzyme is a mutant
methylenetetrahydrofolate reductase, and the attenuated catalytic activity of
the
methylenetetrahydrofolate reductase is caused by mutation in its catalytic
site.
In yet another specific embodiment, the folate species is S, 10,-methylene
tetrahydrofolate, the mutant folate-species-binding enzyme is a mutant
folypolyglutamate
synthase, and the attenuated catalytic activity of the folypolyglutamate
synthase is caused by
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-69-
mutation in its catalytic site, its binding site for ATP, L-glutamate, Mg2+, a
combination
thereof.
In yet another specific embodiment, the folate species is dihydrofolate, the
mutant
folate-species-binding enzyme is a mutant dihydrofolate reductase, and the
attenuated catalytic
activity of the mutant dihydrofolate reductase is caused by mutation in its
catalytic site, its
binding site for NADPH, or a combination thereof. Preferably, the mutant
dihydrofolate
reductase is a Lactobacillus casei dihydrofolate reductase having an Arg43Ala
or Trp2lHis
mutation (Basran, et al., Protein Eng., 10 7 :815-26 91997)).
In yet another specific embodiment, the folate species is 5, 10,-methylene
tetrahydrofolate, the mutant folate-species-binding enzyme is a mutant
thymidylate synthase,
and the attenuated catalytic activity of the mutant thymidylate synthase is
caused by mutation
in its catalytic site, its binding site for dUMP, or a combination thereof.
Preferably, the mutant
thymidylate synthase is a human thymidylate synthase having a mutation
selected from
Tyr6His, G1u214Ser, Ser216A1a, Ser216Leu, Asn229A1a and His199X, X being any
amino
acid that is not His (Schiffer, et al., Biochemistry, 34 50 :16279-87 (1995);
Steadman, et al.,
Biochemistry, 37:7089-7095 (1998); Williams, et al., Biochemistry, 37(20):7096-
102 (1998);
Finer-Moore, et al., J. Mol. Biol., 276 1 :113-29 (1998); and Finer-Moore, et
al., Biochemistry,
35 16 :5125-36 (1996)). Also more preferably, the mutant thymidylate synthase
is an E: coli
thymidylate synthase having an Arg126G1u mutation (Strop, et al., Protein
Sci., 6(12):2504-11
(1997)) or a Lactobacillus casei thymidylate synthase having a V316Am mutation
(Cameras, et
al., Biochemistry, 31 26 :6038-44 (1992)).
Once a mutant folate-species-binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for the
folate species but has
attenuated catalytic activity, is identified, such mutant folate-species-
binding enzyme can be
produced by any methods known in the art including recombinant expression,
chemical
synthesis or a combination thereof as described in Section B. Preferably, the
mutant folate-
species-binding enzyme is obtained by recombinant expression.
E. METHODS FOR ASSAYING CHOLESTEROL
Further provided herein is a method for assaying cholesterol in a sample. This
method
includes at least the steps of: a) contacting the sample with a mutant
cholesterol-binding
enzyme, the mutant enzyme substantially retains its binding affinity or has
enhanced binding
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-70-
affinity for cholesterol but has attenuated catalytic activity; and b)
detecting binding between
cholesterol with the mutant cholesterol-binding enzyme.
Any mutant cholesterol-binding enzymes that substantially retain their binding
affinity
or have enhanced binding affinity for cholesterol but have attenuated
catalytic activity can be
used in the cholesterol assay. Examples of such mutant cholesterol-binding
enzymes include
mutant cholesterol esterase and cholesterol oxidase.
Cholesterol-binding enzymes
Nucleic acids encoding cholesterol-binding enzymes can be obtained by methods
known in the art or obtained from public or commercial sources. Known nucleic
acid
sequences of cholesterol-binding enzymes, such as cholesterol esterase and
cholesterol oxidase,
can be used in isolating nucleic acids encoding cholesterol-binding enzymes
from natural
sources. Alternatively, nucleic acids encoding cholesterol-binding enzymes can
be obtained by
chemical synthesis according to the known sequences.
In one embodiment, the nucleotide sequences with the following GenBank
accession
Nos. can be used in obtaining nucleic acid encoding cholesterol esterase:
AI558069 (Mouse
mammary gland); AI465062 (Mouse mammary gland); AA793597 (Mouse diaphragm);
AA762311 (Mouse mammary gland); AA759540 (Mouse mammary gland); AA672047
(Mouse mammary gland); AA571290 (Mouse diaphragm); AA537778 (Mouse diaphragm);
AA265434 (Mouse); M69157 (Rat pancreatic); U33169 (Mus musculus); L46791
(Rattus
norvegicus); M85201 (Human). Preferably, the nucleotide sequences with the
GenBank
accession Nos. M85201 (SEQ ID No. 31), nucleotide sequences described in U.S.
Patent
No. 5,624,836 (bovine pancreatic cholesterol esterase; SEQ ID No. 33) can be
used in
obtaining nucleic acid encoding cholesterol esterase.
In another specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used in obtaining nucleic acid encoding cholesterol
oxidase: E07692;
E07691; E03850 (Brevibacterium sterolicum); E03828; E03827; D00712 (B.
sterolicum choB
gene); U13981 (Streptomyces A19249 choM gene); and M31939 (Streptomyces A19249
chop
gene). Preferably, the nucleotide sequences with the GenBank accession No.
U13981 (SEQ ID
No. 35; see also Corbin, et al., Appl. Environ. Microbiol., 6012):4239-44
(1994)) and the
nucleotide sequence described in U.S. Patent No. 5,665,560 (SEQ ID No. 37) can
be used in
obtaining nucleic acid encoding cholesterol oxidase.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-71-
Once nucleic acids encoding cholesterol-binding enzymes are obtained, these
nucleic
acids can be mutagenized and screened and/or selected for cholesterol-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for cholesterol but
have attenuated catalytic activity. Insertion, deletion, or point mutations)
can be introduced
S into nucleic acids encoding cholesterol-species-binding enzymes according to
the methods
described in Section B.
Information regarding the structural-functional relationship of the
cholesterol-binding
enzymes can be used in the mutagenesis and selection of the cholesterol-
binding enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for cholesterol but
have attenuated catalytic activity. For example, mutants can be made in the
enzyme's binding
site for its co-enzyme, co-factor, a non-cholesterol substrate, or in the
mutant enzyme's
catalytic site, or a combination thereof.
In one specific embodiment, the mutant cholesterol-binding enzyme is a mutant
cholesterol esterase, and the attenuated catalytic activity of the mutant
cholesterol esterase is
caused by mutation in its catalytic site, its binding site for HZO or a
combination thereof.
Preferably, the cholesterol esterase is a pancreatic cholesterol esterase
having a Ser194Thr or
Ser194A1a mutation (DiPersio, et al., J. Biol. Chem., 265 28 :16801-6 (1990)).
In another specific embodiment, the mutant cholesterol-binding enzyme is a
mutant
cholesterol oxidase, and the attenuated catalytic activity of the mutant
cholesterol oxidase is
caused by mutation in its catalytic site, its binding site for 02 or a
combination thereof.
Preferably, the cholesterol oxidase is a Brevibacterium sterolicum cholesterol
oxidase having a
His447Asn or His447G1n mutation (Yue, et al., Biochemistry, 38(14):4277-86
(1999)).
Once a mutant cholesterol-binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for the
cholesterol but has
attenuated catalytic activity, is identified, such mutant cholesterol-binding
enzyme can be
produced by any methods known in the art including recombinant expression,
chemical
synthesis or a combination thereof as described in Section B. Preferably, the
mutant
cholesterol-binding enzyme is obtained by recombinant expression.
F. HCY ASSAYS IN CONJUNCTION WITH CHOLESTEROL AND/OR FOLIC
ACID ASSAY
The Hcy assays described in Section C can be conducted in conjunction with a
cholesterol and/or a folic acid assay.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-72-
1. Cholesterol assay
Cholesterol assay can be conducted according to any methods known in the art.
For
example, the Hcy assays described in Section C can be conducted in conjunction
with
cholesterol assays described in Section E. In addition, the Hcy assays can be
conducted in
conjunction with cholesterol assays described in U.S. Patent Nos. 4,161,425,
4,164,448,
4,188,188, 4,211,531, 5,034,332, 5,047,327, 5,217,873 and 5,593,894.
U.S. Patent No. 4,161,425 describes cholesterol assay enzymatic reagents for
rate
determination of cholesterol in a sample to be assayed. The reagents contain
cholesterol
oxidase, and a buffering agent in ari amount to produce a solution having a pH
of between
about 5.5 and about 8. The reagent acts by neutralizing substantially all
oxygen consumption
inhibiting effects of inhibiting agents present in the sample to be assayed,
such as an
alkyldimethylbenzylammonium salt in an amount sufficient to neutralize
substantially all
oxygen consumption inhibiting effects of inhibiting agents present in the
sample to be assayed.
U.S. Patent No. 4,161,425 also describes methods for determining the
cholesterol concentration
in a cholesterol containing sample by: (a) oxidizing the cholesterol present
in the sample in an
oxygen saturated aqueous solution by means of a cholesterol assay enzymatic
reagent;
(b) generating a first electrical signal related to the oxygen concentration;
(c) differentiating the
first electrical signal to produce an output signal proportional to the
instantaneous time rate of
change of oxygen concentration; and (d) measuring the output signal to
determine the
cholesterol concentration. In this method substantially all oxygen consumption
inhibiting
effects of inhibiting agents in the sample to be assayed is neutralized by
including in the
cholesterol assay enzymatic reagent a cationic surfactant in an amount
sufficient to neutralize
substantially all oxygen consumption inhibiting effects of inhibiting agents
present in the
sample to be assayed, preferably, from about 0.01 to about 0.4 percent by
weight of the reagent
of a cationic surfactant. The enzymatic agent is cholesterol oxidase and a
buffering agent in an
amount to produce a solution having a pH of between 5.5 and about 8; in the
presence of a
sensor which serves to monitor a property or characteristic of oxygen in the
solution related to
the oxygen concentration thereof;
U.S. Patent No. 4,164,448 describes diagnostic agents in solid form for the
detection
and determination of cholesterol and cholesterol esters in body fluids. The
agents include a
solid carrier having impregnated or embedded therein cholesterol oxidase, a
system for the
detection of hydrogen peroxide, buffer and from 2 to 30%, based on the total
solid diagnostic
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 73 -
agent of at least one surface-active compound with lipophilic and hydrophilic
properties. U.S.
Patent No. 4,164,448 also describes processes for the activation of
analytically pure, detergent-
free, storage-stable cholesterol oxidase, recovered from a micro-organism by
extraction with a
surfactant, for the analytic determination of cholesterol. The processes
include removing all
traces of the surfactant from the cholesterol oxidase to produce a surfactant-
free cholesterol
oxidase and then adding to an aqueous solution of the surfactant-free
cholesterol oxidase
between 0.005% to 0.1% by weight, based on the weight of the aqueous
cholesterol oxidase
solution, of at least one surface-active compound with lipophilic and
hydrophilic properties
before use of the cholesterol oxidase.
U.S. Patent No. 4,188,188 describes compositions for use in a HDL cholesterol
separation. The compositions contain heparin, a divalent cation salt having
the formula: CX2,
where C is selected from Group IIA metals and manganese and X is a halogen,
and an inert
filler that includes a polysaccharide, a terminal interlocking linear glucose
polymer and a
vinylpyrrolidone polymer. This patent also describes high density lipoprotein
cholesterol
assays utilizing heparin/MnCl2 precipitation. In these assays the serum sample
to be assayed is
added to a reagent composition as described above. The resulting supernatant
is assayed for
cholesterol.
U.S. Patent No. 4,211,531 describes methods of determining cholesterol in a
biological
sample. The methods include a precipitation step for precipitating protein in
the sample, a
color forming step for forming in the resulting supernatant a color
proportional to the
concentration of at least one form of cholesterol in the sample, and a step of
determining the
depth of color formed. The precipitation step is carried out by means of a
reagent that contains
colorimetric amounts of propionic acid and ferric ion. U.S. Patent No.
4,211,531 also describes
methods of determining cholesterol in a biological sample using a color
forming step in which
a reaction mixture including at least a fraction of the serum and a color
forming reagent is
formed. The depth of color formed is related to the amount of at least one
form of cholesterol
in the reaction mixture. In these assays, the reaction mixture contains a
colorimetric amount of
sulfuric acid and propionic acid. U.S. Patent No. 4,211,531 also describes
methods of
determining cholesterol in a sample of human serum, by first precipitating
protein in the sample
by means of a protein precipitation reagent that contains colorimetric amounts
of propionic acid
and fernc ion to produce a generally protein-free supernatant. Color is then
developed in a
reaction mixture containing the supernatant and a cholesterol color reagent,
which contains
colorimetric amounts of propionic acid and sulfuric acid. The depth of color
formed is related
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-74-
to the amount of cholesterol in the sample. U.S. Patent No. 4,211,531 also
provides reagent
kits for determination of total cholesterol, which include a first container
containing a
colorimetric amount of ferric chloride and propionic acid and a second
container containing a
reagent that contains colorimetric amount of propionic acid and sulfuric acid.
U.S. Patent No. 5,034,332 describes assays for the presence of HDL cholesterol
in a
blood plasma sample. This method includes the steps of: mixing the sample with
a
proteinaceous material that is also present in protein H of boar vesicle
seminal plasma so as to
cause a precipitation of HDL cholesterol bound to the proteinaceous material;
and measuring
either the amount of cholesterol in a supernatant formed by the mixing step,
or the amount of
precipitant formed in the mixing step.
U.S. Patent No. 5,217,873 describes stable cholesterol assay compositions that
contain:
(a) at least one acidic compound selected from a bile acid and a salt of a
bile acid, the total of
the acid compound being present in an amount of up to about S mM; (b) a
nonionic surfactant
present in a concentration of from about 0.15 to about 1.5 percent by volume;
(c) a buffer in a
concentration of from 0 to about v (d) cholesterol oxidase in a concentration
of at least about
0.02 KIU/1; (e) cholesterol esterase present in a concentration of at least
about 0.07 KIU/l; and
(f) a chromogen system for determination of hydrogen peroxide, the cholesterol
assay solution
having a pH of from about 5.5 to about 7.5 and a completion time of less than
10 minutes at
37°C. U.S. Patent No. 5,217,873 also describes stable total cholesterol
chromogen assay
compositions containing an aqueous solution have a pH of from about 6.5 to
about 7.5 and
(a) phenol in a concentration of from about 8 to about 35 mM; (b) a metal salt
of cholic acid
present in a concentration of from about 0.2 to about 5 mM; (c) a nonionic
surfactant present in
a concentration of from about 0.2 to about 1.5 percent volume by volume; (d) a
phosphate
buffer present in a concentration of from about 0.5 to about 30 mM and
sufficient to maintain a
pH of from about 6 to about 7.5; (e) 4-aminoantipyrine in a concentration up
to about 0.3 mM;
(f) cholesterol esterase present in a concentration of at least about 0.07
KIU/l; (g) cholesterol
oxidase present in a concentration of at least about 0.02 KIU/1; and (h)
peroxidase, the amount
of peroxidase and 4-aminoantipyrine being sufficient to enable quantitative
determination of
the amount of hydrogen peroxide formed from oxidation of cholesterol within 10
minutes at
37°C. U.S. Patent No. 5,217,873 further describes stable total
cholesterol chromogen assay
compositions containing an aqueous solution of: a) phenol in a concentration
of about 17 mM;
b) 2,4dichlorophenol present in a concentration of about 0.5 mM; c) a metal
salt of cholic acid
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-75-
present in a concentration of up to about 5 mM; d) polyethylene glycol p-
isooctylphenyl ether
present in a concentration of from about 0.2 to about 0.6 percent volume by
volume; e)
KHZP04 present in a concentration of about 12.5 mM; f) peroxidase present in a
concentration
of about 30 KIU/l; g) cholesterol oxidase present in a concentration of at
least about 0.05
KIU/1; h) microbial cholesterol esterase present in a concentration of at
least about 0.1 KIU/1;
and i) 4-aminoantipyrene present in concentration of about 0.3 mM, the stable
total cholesterol
chromogen assay composition having a pH of from about 6.0 to about 7.5.
U.S. Patent No. 5,593,894 describes methods for forming a
spectrophotometrically
active product of cholesterol, such as HDL-C, LDL-C and VLDL-C. The method
includes
contacting cholesterol with an acyl compound and a perchlorate effective to
form a
spectrophotometrically active product of the cholesterol, the perchlorate
selected from zinc
perchlorate, barium perchlorate and perchloric acid. U.S. Patent No. 5,593,894
also describes
methods for determining the amount of cholesterol present in a test sample by
contacting a test
sample in which cholesterol is present with an acyl compound and a perchlorate
effective to
form a spectrophotometrically active product with the cholesterol, the
perchlorate selected from
zinc perchlorate, barium perchlorate and perchloric acid, and evaluating the
spectrophotometric
activity to determine the amount of the cholesterol present in the sample.
U.S. Patent No. 5,047,327 describes stable cholesterol assay compositions.
These
compositions contain a polyhydroxy compound free aqueous solution of: (a) at
least one acidic
compound selected from a bile acid and a salt of a bile acid, the total of the
acidic compound
being present in a positive amount of up to about 5 mM; (b) a nonionic
surfactant present in a
concentration of from about 0.15 to about 1.5 percent volume by volume; (c) a
buffer in a
concentration of from 0 to about 65 mM; (d) cholesterol oxidase in a
concentration of at least
about 0.02 KIU/l, (e) microbial cholesterol esterase in a concentration of at
least about 0.07
KIU/l; and (~ a chromogen system for determination of hydrogen peroxide; the
cholesterol
assay solution having a pH of from about 5.5 to about 8.5 a stability of at
least 3 days at 41 °C
and an assay completion time within 10 minutes at 37°C. U.S. Patent No.
5,047,327 also
describes stable total cholesterol chromagen assay compositions. These
compositions are
aqueous solutions having a pH of from about 6.5 to about 7.5 and (a) phenol in
a concentration
of from about 8 to about 35 mM; (b) sodium cholate present in a concentration
of from about
0.2 to about 5 mM; (c) a nonionic surfactant present in a concentration of
from about 0.15 to
about 1.5 percent volume by volume; (d) a buffer present in a concentration of
from 0.5 to
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-76-
about 65 mM; (e) 4-aminoantipyrine; (f) microbial cholesterol esterase present
in a
concentration of at least about 0.07 KIU/l; (g) cholesterol oxidase present in
a concentration of
at least about 0.02 KIU/1; and (h) peroxidase, the amount of peridase and 4-
aminoantipyrine
being sufficient to enable quantitative determination of the amount of
hydrogen peroxide
formed from oxidation of cholesterol within 10 minutes at 37° C., the
assay composition
having a stability of at least 3 days at 41 °C.
2. Folic acid assay
Folic acid assay can be conducted according to any methods known in the art.
For
example, the Hcy assays described in Section C can be conducted in conjunction
with folic acid
assays described in Section D. In addition, the Hcy assays can be conducted in
conjunction
with cholesterol assays described in U.S. Patent Nos. 4,276,280, 4,336,185,
4,337,339,
5,374,560 and 5,800,979.
U.5. Patent No. 4,276,280 describes derivatives of folic acid wherein the a-
carboxyl
group of the glutamyl moiety is substituted with a radical which is capable of
being
radioiodinated, such as, substituted and unsubstituted tyrosyl and histidyl.
The radioiodinated
derivatives can be employed as tracers for the assay of folates.
U.5. Patent No. 4,336,185 describes protein conjugates and iodinated
conjugates of
folic acid and its salts, esters and amides which retain the ability to
competitively bind on a
binding protein, such as folic acid binding globulin or on an antibody which
is specific to folic
acid. The compounds are useful in analysis of body fluids such as blood serum,
blood plasma,
urine and the like, to assay for the presence of folic acid by competitive
protein binding assay
(CPSA) or by radioimmunoassay (RIA) procedures.
U.S. Patent No. 4,337,339 describes that folic acid derivatives, such as
radiolabeled
pteroyltyrosine, are conveniently synthesized from either pteroic acid or by
the direct
condensation of 6-formylpterin with p-aminobenzoyltyrosine methyl ester. The
radioiodinated
derivatives are particularly useful in competitive protein binding and
radioimmuno-assays of
folate compounds.
U.5. Patent No. 5,374,560 describes methods for detecting a deficiency of
cobalamin or
folic acid in warm-blooded animals, by: assaying a body fluid for an elevated
level of
cystathionine; and correlating an elevated level of cystathionine in the body
fluid with a
likelihood of a deficiency of cobalamin or folic acid. U.5. Patent No.
5,374,560 also describes
methods for detecting a deficiency of cobalamin in warm-blooded animals, by:
assaying a body
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
_77_
fluid for an elevated level of 2-methylcitric acid I or 2-methylcitric acid II
or; and correlating
an elevated level of 2-methylcitric acid I or 2-methylcitric acid II or in the
body fluid with a
likelihood of a deficiency of cobalamin. U.S. Patent No. 5,374,560 further
describes methods
for detecting a deficiency of cobalamin or folic acid in warm-blooded animals
and for
distinguishing between a deficiency of cobalamin and a deficiency of folic
acid, by: assaying a
first body fluid from the warm-blooded animal for an elevated level of
cystathionine;
correlating an elevated level of cystathionine in the body fluid with a
likelihood of a deficiency
of cobalamin or folic acid; assaying a second body fluid from the warm-blooded
animal having
an elevated level of cystathionine in the first body fluid correlating with a
likelihood of a
deficiency of cobalamin or folic acid, for an elevated level of 2-methylcitric
acid I or 2-
methylcitric acid II or; and correlating an elevated level of 2-methylcitric
acid I or 2-
methylcitric acid II or in the second body fluid with a likelihood of a
deficiency of cobalamin
but a likelihood of a deficiency of folic acid. U.S. Patent No. 5,374,560
further describes
methods for detecting a deficiency of cobalamin or folic acid in warm-blooded
animals, by:
assaying a first body fluid for an elevated level of cystathionine; assaying a
second body fluid
for an elevated level of homocysteine; and correlating an elevated level of
cystathionine and
homocysteine with a likelihood of a deficiency of cobalamin or folic acid.
U.S. Patent No. 5,800,979 describes methods for determination of concentration
in a
body fluid of at least one member of an endogenous folate co-enzyme pool
selected from: (1)
pool I containing tetrahydrofolate, dihydrofolate and 5,10-
methylenetetrahydrofolate; (2) pool
II containing 5-methyltetrahydrofolate; and (3) pool III containing 3-
formyltetrahydrofolate,
10-formyltetrahydrofolate, 5,10-methyleneyltetrahydrofolate, and 5-
formiminotetrahydrofolate.
The method includes the steps of: (a) combining a known amount of at least one
internal
standard folate co-enzyme which is a non-radioactively-labeled stable isotope
of a member of
the selected folate co-enzyme pool with the body fluid, wherein the internal
standard folate
coenzyme is recovered from harvested bacterial cells grown on a medium
containing non-
radioactively-labeled stable isotope paraaminobenzoic acid; (b) at least
partially purifying the
endogenous and internal standard folate coenzymes from other components in the
body fluid in
a partial purification step; (c) quantitating the endogenous folate co-enzymes
in the purified
body fluid of step (b) by gas chromatography/mass spectrometry analysis; and
(d) determining
the concentration of the selected endogenous folate coenzyme pool by
correcting the
concentrations of endogenous folate coenzymes quantitated in step (c) for
endogenous losses as
reflected by losses in the known amount of internal standard folate co-enzyme
of step (a).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-78-
G. METHODS FOR ASSAYING BILE ACID AND BILE SALTS
Further provided herein is a method for assaying bile acids or bile salts in a
sample by:
a) contacting the sample with a mutant bile-acid-binding enzyme or bile-salt-
binding enzyme,
the mutant enzyme substantially retains its binding affinity or has enhanced
binding affinity for
the bile acid or bile salt but has attenuated catalytic activity; and b)
detecting binding between
the bile acid or bile salt with the mutant bile-acid-binding enzyme or bile-
salt-binding enzyme.
Any mutant bile-acid-binding enzyme or bile-salt-binding enzyme that
substantially
retain their binding affinity or have enhanced binding affinity for the bile
acid or bile salt but
have attenuated catalytic activity can be used in the bile acid or bile salt
assay. Example of
such mutant bile-acid-binding enzyme or bile-salt-binding enzyme includes 3-a-
hydroxy
steroid dehydrogenase.
Nucleic acids encoding bile-acid-binding enzymes or bile-salt-binding enzymes
can be
obtained by methods known in the art. Known nucleic acid sequences of bile-
acid-binding
enzyme or bile-salt-binding enzyme, such as 3-a-hydroxy steroid dehydrogenase,
can be used
in isolating nucleic acids encoding bile-acid-binding enzymes or bile-salt-
binding enzymes
from natural sources. Alternatively, nucleic acids encoding bile-acid-binding
enzymes or bile-
salt-binding enzymes can be obtained by chemical synthesis according to the
known sequences.
In one specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used in obtaining nucleic acid encoding 3-a-hydroxy
steroid
dehydrogenase: AA866404 (Rattus norvegicus); AA866403 (Rattus norvegicus);
U34072 (Mus
musculus); AF064635 (Mus musculus putative steroid); AB009304 (Anas
platyrhynchos);
D17310 (Rat); U32426 (Molluscum contagiosum virus); L23428 (Comamonas
testosteroni);
M67467 (Macaca fuscata); M27137 (Human). Preferably, the nucleotide sequences
with the
GenBank accession No. M27137 (SEQ ID No. 39; see also The, et al., Mol.
Endocrinol.,
x:1310-2 (1989)) can be used in obtaining nucleic acid encoding 3-a-hydroxy
steroid
dehydrogenase.
Once nucleic acids encoding bile-acid-binding enzymes or bile-salt-binding
enzymes
are obtained, these nucleic acids can be mutagenized and screened and/or
selected for bile-acid-
binding enzymes or bile-salt-binding enzymes that substantially retain their
binding affinity or
have enhanced binding affinity for bile acids or bile salts but have
attenuated catalytic activity.
Insertion, deletion, or point mutations) can be introduced into nucleic acids
encoding bile-acid-
binding enzymes or bile-salt-binding enzymes according to the methods
described in Section B.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-79-
Information regarding the structural-functional relationship of the bile-acid-
binding
enzymes or bile-salt-binding enzymes can be used in the mutagenesis and
selection of the bile-
acid-binding enzymes or bile-salt-binding enzymes that substantially retain
their binding
affinity or have enhanced binding affinity for bile acids or bile salts but
have attenuated
catalytic activity. For example, mutants can be made in the enzyme's binding
site for its co-
enzyme or for a non-bile-acid or non-bile-salt substrate, or in the mutant
enzyme's catalytic site,
or a combination thereof.
In one specific embodiment, the mutant bile-acid-binding enzyme is a mutant 3-
a-
hydroxy steroid dehydrogenase, and the attenuated catalytic activity of the
mutant 3-a-hydroxy
steroid dehydrogenase is caused by mutation in its catalytic site, its binding
site for NAD+ or a
combination thereof.
Once a mutant bile-acid-binding enzyme or bile-salt-binding enzyme with
desired
properties, i.e., substantially retaining its binding affinity or having
enhanced binding affinity
for the bile acid or bile salt but having attenuated catalytic activity, is
identified, such mutant
bile-acid-binding enzyme or bile-salt-binding enzyme can be produced by any
methods known
in the art including recombinant expression, chemical synthesis or a
combination thereof as
described in Section B. Preferably, the mutant bile-acid-binding enzyme or
bile-salt-binding
enzyme is obtained by recombinant expression.
H. METHODS FOR ASSAYING GLUCOSE
Further provided herein is a method for assaying glucose in a sample. This
method
includes at least the steps of: a) contacting the sample with a mutant glucose-
binding enzyme,
the mutant enzyme substantially retains its binding affinity or has enhanced
binding affinity for
glucose but has attenuated catalytic activity; and b) detecting binding
between glucose with the
mutant glucose-binding enzyme.
Any mutant glucose-binding enzymes that substantially retain their binding
affinity or
have enhanced binding affinity for glucose but have attenuated catalytic
activity can be used in
the glucose assay. Examples of such mutant glucose-binding enzyme include
mutant glucose
isomerase, glucokinase, hexokinase and glucose oxidase.
Nucleic acids encoding glucose-binding enzymes can be obtained by methods
known in
the art. Known nucleic acid sequences of glucose-binding enzymes, such as
glucose isomerase,
glucokinase, hexokinase and glucose oxidase, can be used in isolating nucleic
acids encoding
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-80-
glucose-binding enzymes from natural sources. Alternatively, nucleic acids
encoding glucose-
binding enzymes can be obtained by chemical synthesis according to the known
sequences.
In one specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used in obtaining nucleic acid encoding glucose
isomerase: AF065160
(Toxoplasma gondii); AF050755 (Giardia intestinalis (GPI2)); AF050754,(Giardia
intestinalis
(GPI1)); AI117811 (mouse mammary gland); AA636682 (mouse myotubes); AA611494
(mouse irradiated colon); AA529061 (mouse irradiated colon); AA522284 (mouse
embryonic
region); AA472600 (mouse mammary gland); L27675 (Drosophila yakuba isofemale
line 4);
D13777 (Synechocystis Sp.); AA265107 (mouse pooled organs); AA162075 (mouse
skin);
AA139952 (mouse heart); AAl 17013 (mouse embryonic region); W36773 (mouse);
W16112
(mouse); AA03546 (mouse embryo); W77098 (mouse embryo); W61997 (mouse embryo);
W53620 (mouse embryo); U17225 (Zea mays); L27685 (Drosophila yakuba isofemale
line 1);
L27684 (Drosophila yakuba isofemale line 13); L27683 (Drosophila yakuba
isofemale line 12);
L27682 (Drosophila yakuba isofemale line 11 ); L27681 (Drosophila yakuba
isofemale line 10);
L27680 (Drosophila yakuba isofemale line 9); L27679 (Drosophila yakuba
isofemale line 8);
L27678 (Drosophila yakuba isofemale line 7); L27677 (Drosophila yakuba
isofemale line 6);
L27676 (Drosophila yakuba isofemale line 5); L27555 (Drosophila melanogaster
isochromosomal line); L27554 (Drosophila melanogaster isochromosomal line);
L27553
(Drosophila melanogaster isochromosomal line); L27674 (Drosophila yakuba
isofemale line 3);
and L27673 (Drosophila yakuba isofemale). Preferably, the nucleotide sequences
with the
GenBank accession No. U17225 (SEQ ID No. 41; see also Lal and Sachs, et al.,
Plant Physiol.,
108 3 :1295-6 (1995)) can be used in obtaining nucleic acid encoding glucose
isomerase.
In another specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used in obtaining nucleic acid encoding glucokinase:
AI386017 (Mouse
testis); AI325384 (Mouse embryo); AI323376 (Mouse embryo); AI255715 (Mouse
liver mlia);
AI196901 (Mouse liver); AI194797 (Mouse liver); AI194643 (Mouse liver); U44834
(Mycobacterium tuberculosis); U21919 (Brucella abortus); L41631 (Mus
musculus); AI035808
(Mouse kidney); AI035659 (Mouse liver); AA882226 (Mouse lung); AH045826 (Homo
Sapiens pancreatic beta cell specific glucokinase (GCK) and major liver
specific glucokinase
(GCK) genes); AF041022 (Homo Sapiens glucokinase); M69051 (Human liver
glucokinase
(ATP:D-hexose 6-phosphotransferase); AA109998 (Mouse testis); AA014441 (Mouse
embryo); L38990 (Mus musculus); U22490 (Escherichia coli); M24077
(Saccharomyces
cerevisiae); M90299 (Human); M88011 (Human pancreatic beta-cell); M25807
(Rat); J04218
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-81-
(Rat); M60615 (Z.mobilis). Preferably, the nucleotide sequences with the
GenBank accession
No. M90299 (SEQ ID No. 43; see also Koranyi, et al., Diabetes, 41 7 :807-11
(1992)) can be
used in obtaining nucleic acid encoding glucokinase.
In still another specific embodiment, the nucleotide sequences with the
following
GenBank accession Nos. can be used in obtaining nucleic acid encoding glucose
oxidase:
AF012277 (Penicillium amagasakiense); U56240 (Talaromyces flavus); X16061
(Aspergillus
niger gox gene); X56443 (A.niger god gene); J05242 (A.niger); AF012277
(Penicillium .
amagasakiense); U56240 (Talaromyces flavus); X16061 (Aspergillus niger gox
gene); X56443
(A.niger god gene); J05242 (A.niger glucose). Preferably, the nucleotide
sequences with the
GenBank accession No. J05242 (SEQ ID No. 45; see also Frederick, et al., J.
Biol. Chem.,
265 7 :3793-802 (1990)) and the nucleotide sequences described in U.S. Patent
No. 5,879,921
(SEQ ID No. 47) can be used in obtaining nucleic acid encoding glucose
oxidase.
Once nucleic acids encoding glucose-binding enzymes are obtained, these
nucleic acids
can be mutagenized and screened and/or selected for glucose-binding enzymes
that
substantially retain their binding affinity or have enhanced binding affinity
for glucose but have
attenuated catalytic activity. Insertion, deletion, or point mutations) can be
introduced into
nucleic acids encoding glucose-binding enzymes according to the methods
described in Section
B.
Information regarding the structural-functional relationship of the glucose-
binding
enzymes can be used in the mutagenesis and selection of the glucose-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for glucose but have
attenuated catalytic activity. For example, mutants can be made in the
enzyme's binding site
for its co-enzyme, co-factor, non-glucose substrate, or in the mutant enzyme's
catalytic site, or a
combination thereof.
In one specific embodiment, the mutant glucose-binding enzyme is a Clostridium
thermosulfurogenes glucose isomerase having a mutation selected from
His101Phe,
His101G1u, HislOlGln, His101Asp and His101Asn (Lee, et al., J. Biol. Chem.,
265(31):19082-
90 (1990)). In another specific embodiment, the mutant glucose-binding enzyme
is a mutant
hexokinase or glucokinase, and the attenuated catalytic activity of the mutant
hexokinase or
glucokinase is caused by mutation in its catalytic site, its binding site for
ATP or Mg2+, or a
combination thereof. In still another specific embodiment, the mutant glucose-
binding enzyme
is a mutant glucose kinase, and the attenuated catalytic activity of the
mutant glucose kinase is
caused by mutation in its catalytic site, its binding site for HZO or 02, or a
combination thereof.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-82-
Once a mutant glucose-binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for glucose
but has attenuated
catalytic activity, is identified, such mutant glucose-binding enzyme can be
produced by any
methods known in the art including recombinant expression, chemical synthesis
or a
combination thereof as described in Section B. Preferably, the mutant glucose-
binding enzyme
is obtained by recombinant expression.
I. METHODS FOR ASSAYING ETHANOL
Further provided herein is a method for assaying ethanol in a sample. This
method
includes at least the steps of: a) contacting the sample with a mutant ethanol-
binding enzyme,
the mutant enzyme substantially retains its binding affinity or has enhanced
binding affinity for
ethanol but has attenuated catalytic activity; and b) detecting binding
between ethanol with the
mutant ethanol-binding enzyme.
Any mutant ethanol-binding enzymes that substantially retain their binding
affinity or
have enhanced binding affinity for ethanol but have attenuated catalytic
activity can be used in
1 S the ethanol assay. Examples of such mutant ethanol-binding enzyme include
alcohol
dehydrogenase.
Nucleic acids encoding ethanol-binding enzymes can be obtained by methods
known in
the art. Known nucleic acid sequences of ethanol-binding enzymes, such as
alcohol
dehydrogenase, can be used in isolating nucleic acids encoding ethanol-binding
enzymes from
natural sources. Alternatively, nucleic acids encoding ethanol-binding enzymes
can be
obtained by chemical synthesis according to the known sequences.
In one specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used for producing mutant nucleic acid molecules
encoding alcohol
dehydrogenase: AI194923 (mouse liver); U16293 (Human class IV); U73514 (Human
short-
chain); U09623 (Human); M30471 (Human class III); 221104 (Human adult Testis
tissue);
L33179 (Human class IV sigma-1); M24317 (Human class I); M29872 (Human);
M81118
(Human); M21692 (Human class I); M12963 (Human class I); M68895 (Human);
U07821
(Human); AF044127 (Homo sapiens peroxisomal short-chain); M12272 (Homo
Sapiens);
D00137 (Homo Sapiens); L47166 (Homo sapiens); M12271 (Homo Sapiens class I);
221104
(Human adult Testis tissue). In addition, nucleic acid molecules, such as
those provided in the
following U.S. Patents can be used in obtaining and producing mutant nucleic
acid encoding
alcohol dehydrogenase:
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-83-
U.S. Patent No. alcohol dehydrogenase
5,908,924 thermoanaerobacter ethanolicus 39E secondary-alcohol
dehydrogenase
5,855,881 Mammalian alcohol dehydrogenase
5,385,833 Pseudomonas sp. ATCC No. 49794 alcohol dehydrogenase
5,344,777 membrane-bound alcohol dehydrogenase complex
5,342,767 Lactobacillus kefir alcohol dehydrogenase
5,225,339
5,162,516 alcohol dehydrogenase II gene from Zymomonas
mobilis
Nucleic acid molecules that include the sequences of sequences with the
GenBank
accession Nos. U73514 (SEQ ID No. 49), U09623 (SEQ ID No. 51; see also
Kedishvili, et al.,
J. Biol. Chem., 270 8 :3625-30 (1995)), M30471 (SEQ ID No. 53; see also
Sharma, et al.,
Biochem. Biophys. Res. Commun., 164(2):631-7 (1989)) and M24317 (SEQ ID No.
55; see also
Xu, et al., Genomics, 23,):209-14 (1988); Ikuta, et al., Proc. Natl. Acad.
Sci., 82:2703-7
(1985)) can be used in obtaining nucleic acid encoding alcohol dehydrogenase.
Once nucleic acids encoding ethanol-binding enzymes are obtained, these
nucleic acids
can be mutagenized and screened and/or selected for ethanol-binding enzymes
that
substantially retain their binding affinity or have enhanced binding affinity
for ethanol but have
attenuated catalytic activity. Insertion, deletion, or point mutations) can be
introduced into
nucleic acids encoding ethanol-binding enzymes according to the methods
described in Section
B.
Information regarding the structural-functional relationship of the ethanol-
binding
enzymes can be used in the mutagenesis and selection of the ethanol-binding
enzymes that
substantially retain their binding amity or have enhanced binding affinity for
ethanol but have
attenuated catalytic activity. For example, mutants can be made in the
enzyme's binding site
for its co-enzyme, co-factor, non-ethanol substrate, or in the mutant enzyme's
catalytic site, or a
combination thereof.
In one specific embodiment, the mutant ethanol-binding enzyme is a mutant
alcohol
dehydrogenase and the attenuated catalytic activity of the mutant alcohol
dehydrogenase is
caused by mutation in its catalytic site, its binding site for NAD+ or Zn2+,
or a combination
thereof. Preferably, the mutant alcohol dehydrogenase is a human liver alcohol
dehydrogenase
having a Hiss 1 Gln mutation (Ehrig, et al., Biochemistry, 30 4 :1062-8 ( 1991
)). Also
preferably, the mutant alcohol dehydrogenase is a horse liver alcohol
dehydrogenase having a
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-84-
Phe93Trp or Va1203A1a mutation (Bahnson, et al., Proc. Natl. Acad. Sci.,
94(24):12797-802
(1997); Colby, et al., Biochemistry, 37 26 :9295-304 (1998)).
Once a mutant ethanol-binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for ethanol
but having
attenuated catalytic activity, is identified, such mutant ethanol-binding
enzyme can be produced
by any methods known in the art including recombinant expression, chemical
synthesis or a
combination thereof as described in Section B. Preferably, the mutant ethanol-
binding enzyme
is obtained by recombinant expression.
J. METHODS FOR ASSAYING URIC ACID
Further provided herein is a method for assaying uric acid in a sample. This
method
includes at least the steps of: a) contacting the sample with a mutant uric-
acid-binding enzyme,
the mutant enzyme substantially retains its binding affinity or has enhanced
binding affinity for
uric acid but has attenuated catalytic activity; and b) detecting binding
between uric acid with
the mutant uric-acid-binding enzyme.
Any mutant uric-acid-binding enzymes that substantially retain their binding
affinity or
have enhanced binding affinity for uric acid but have attenuated catalytic
activity can be used
in the uric acid assay. Examples of such mutant uric acid-binding enzyme
include orate
oxidase or uricase.
Nucleic acids encoding uric-acid-binding enzymes can be obtained by methods
known
in the art. Known nucleic acid sequences of uric-acid-binding enzymes, such as
orate oxidase
or uricase, can be used in isolating nucleic acids encoding uric-acid-binding
enzymes from
natural sources. Alternatively, nucleic acids encoding uric-acid-binding
enzymes can be
obtained by chemical synthesis according to the known sequences.
In one specific embodiment, the nucleotide sequences with the following
GenBank
accession Nos. can be used in obtaining nucleic acid encoding orate oxidase or
uricase:
AB028150 (Medicago sativa); AB028149 (Medicago sativa); E13225 (Arthrobacter
globiformis); U72663 (Phaseolus vulgaris); D86930; D86929; D32043 (Candida
utilis):
D49974 (Bacillus sp.); M10594 (Soybean nodulin-35 (N-35)); M24396 (Rat);
M27695
(Mouse); M27694 (Baboon); and M27697 (Pig). In addition, the nucleotide
sequences
described in the following U.S. Patent Nos. can be used in obtaining nucleic
acid encoding
orate oxidase or uricase: 5,541,098 (SEQ ID No. 57) and 5,728,562 (SEQ ID No.
59).
Preferably, the nucleotide sequences with the GenBank accession No. M27694
(SEQ ID
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-85-
No. 61; see also Wu, et al., Proc. Natl. Acad. Sci., 86(2:9412-6 (1989)) can
be used in
obtaining nucleic acid encoding orate oxidase or uricase.
Once nucleic acids encoding uric-acid-binding enzymes are obtained, these
nucleic
acids can be mutagenized and screened and/or selected for uric-acid-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for uric acid but
have attenuated catalytic activity. Insertion, deletion, or point mutations)
can be introduced
into nucleic acids encoding uric-acid-binding enzymes according to the methods
described in
Section B.
Information regarding to structural-functional relationship of the uric-acid-
binding
enzymes can be used in the mutagenesis and selection of the uric-acid-binding
enzymes that
substantially retain their binding affinity or have enhanced binding affinity
for uric acid but
have attenuated catalytic activity. For example, mutants can be made in the
enzyme's binding
site for its co-enzyme, co-factor, non-uric-acid substrate, or in the mutant
enzyme's catalytic
site, or a combination thereof.
In one specific embodiment, the mutant uric-acid-binding enzyme is a mutant
orate
oxidase or uricase, and the attenuated catalytic activity of the mutant orate
oxidase or uricase is
caused by mutation in its catalytic site, its binding site for 02, H20, or
copper ion, or a
combination thereof. Preferably, the mutant orate oxidase is a rat orate
oxidase having a
mutation selected from H 127Y, H 129Y and F 131 S (Chu, et al., Ann. N. Y.
Acad. Sci. , 804:781-6
( 1996)).
Once a mutant uric acid binding enzyme with desired properties, i.e.,
substantially
retaining its binding affinity or having enhanced binding affinity for uric
acid but having
attenuated catalytic activity, is identified, such mutant uric-acid-binding
enzyme can be
produced by any methods known in the art including recombinant expression,
chemical
synthesis or a combination thereof as described in Section B. Preferably, the
mutant uric-acid-
binding enzyme is obtained by recombinant expression.
K. OTHER PROGNOSTIC AND DIAGNOSTIC ASSAYS AND ASSAYS FOR
MONITORING THERAPEUTIC lINTERVENTION
1. Diagnostic and prognostic assays
Small molecule markers associated with various diseases, defects or conditions
can be
monitored for diagnostics and prognostics. The presence, absence or
quantitation of any
diagnostic and prognostic small molecule markers can be monitored, and a
diagnostic or
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-86-
prognostic determination can be made based on the assay results. The following
Table 2
illustrates exemplary markers for certain diseases or conditions and mutant
enzymes to be used
in the substrate trapping assay methods.
Table 2
Metabolites Enzymes Diseases
Bile acid 3a-hydroxysteroid biliary cirrhosis
dehydrogenase
Uric acid Uricase gout, leukemia
Creatinine creatinine amidohydrolaserenal disfunction
Serotonin serotonin N- neuron disease
acetyltransferase
Hyaluronic acid hyaluronidase rheumatoid arthritis
Catecholamine catechol O- neuroblastoma
methyltransferase
Homovanillic acid monoamine oxidase neuroblastoma
Vanilylmandelic aciddopamine beta- neuroblastoma
hydroxylase
In a specific embodiment, the small molecule analyte to be assayed is bile
acid and the
mutant analyte-binding enzyme is a mutant 3a-hydroxysteroid dehydrogenase, the
mutant 3a-
hydroxysteroid dehydrogenase substantially retains its binding affinity or has
enhanced binding
affinity for bile acid but has attenuated catalytic activity. Preferably, the
attenuated catalytic
activity is caused by mutation in the mutant 3a-hydroxysteroid dehydrogenase's
catalytic site,
its binding site for NADP+, or a combination thereof.
In another specific embodiment, the small molecule analyte to be assayed is
creatinine
and the mutant analyte-binding enzyme is a mutant creatinine amidohydrolase,
the mutant
creatinine amidohydrolase substantially retains its binding affinity or has
enhanced binding
affinity for creatinine but has attenuated catalytic activity. Preferably, the
attenuated catalytic
activity is caused by mutation in the mutant creatinine amidohydrolase's
catalytic site, its
binding site for H20, or a combination thereof.
In still another specific embodiment, the small molecule analyte to be assayed
is
serotonin and the mutant analyte-binding enzyme is a mutant serotonin N-
acetyltransferase, the
mutant serotonin N-acetyltransferase substantially retains its binding
affinity or has enhanced
binding affinity for serotonin but has attenuated catalytic activity.
Preferably, the attenuated
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
_87_
catalytic activity is caused by mutation in the mutant serotonin N-
acetyltransferase's catalytic
site, its binding site for AcCoA, or a combination thereof.
In yet another specific embodiment, the small molecule analyte to be assayed
is
hyaluronic acid and the mutant analyte-binding enzyme is a mutant
hyaluronidase, the mutant
hyaluronidase substantially retains its binding affinity or has enhanced
binding affinity for
hyaluronic acid but has attenuated catalytic activity. Preferably, the
attenuated catalytic
activity is caused by mutation in the mutant hyaluronidase's catalytic site,
its binding site for
H20, or a combination thereof.
In yet another specific embodiment, the small molecule analyte to be assayed
is
catecholamine and the mutant analyte-binding enzyme is a mutant catechol O-
methyltransferase, the mutant catechol O-methyltransferase substantially
retains its binding
affinity or has enhanced binding affinity for catecholamine but has attenuated
catalytic activity.
Preferably, the attenuated catalytic activity is caused by mutation in the
mutant catechol O-
methyltransferase's catalytic site, its binding site for AdoMet or Mg2+, or a
combination thereof.
In yet another specific embodiment, the small molecule analyte to be assayed
is
homovanillic acid and the mutant analyte-binding enzyme is a mutant monoamine
oxidase, the
mutant monoamine oxidase substantially retains its binding affinity or has
enhanced binding
affinity for homovanillic acid but has attenuated catalytic activity.
Preferably, the attenuated
catalytic activity is caused by mutation in the mutant monoamine oxidase's
catalytic site, its
binding site for 02, or a combination thereof.
In yet another specific embodiment, the small molecule analyte to be assayed
is
vanilylmandelic acid and the mutant analyte-binding enzyme is a mutant
dopamine 13-
hydroxylase, the mutant dopamine 13-hydroxylase substantially retains its
binding affinity or has
enhanced binding affinity for vanilylmandelic acid but has attenuated
catalytic activity.
Preferably, the attenuated catalytic activity is caused by mutation in the
mutant dopamine 13-
hydroxylase's catalytic site, its binding site for Oz or ascorbic acid, or a
combination thereof.
More preferably, the mutant dopamine 13-hydroxylase is a mammalian enzyme,
such as
the bovine dopamine beta-hydroxylase having one or more mutations that
correspond to
mutations of the bovine enzyme at Tyr477, His249 or Arg503 (Robertson, et al.,
J. Biol.
Chem., 265:1029-1035 (1990); and Farrington, et al., J. Biol. Chem.,
265(2):1036-40 (1990));
or a dopamine 13-hydroxylase having one or more mutations at its copper
binding site
(Blackburn, et al., Biochemistry, 27(16):6001-8 (1988); or a dopamine J3-
hydroxylase having
one or more mutations within a region of the enzyme corresponding to the
sequence (SEQ ID
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
_88_
No. 154): Ala-Pro-Asp-Val-Leu-Ile-Pro-Gly-Gln-Gln-Thr-Thr-Tyc-Trp-Cys-Tyr-Val-
Thr-Glu-
Leu-Pro-Asp-Gly-Phe-Pro-Arg, where Tyc is an amino acid residue with the in-
chain mass of a
cresol-Tyr adduct (106 + 163 Da) (see, e.g., DeWolf, et al., Biochemistry,
27(26):9093-101
(1988) of the bovine enzyme.
2. Drug assays
The substrate trapping methods provided herein can also be used for monitoring
presence, absence, quantitation, kinetics and metabolism of therapeutic or
preventive agents,
especially small molecule agents. Assays for any drug or therapeutic agent for
which an
enzyme that binds to the drug or agent can be identified are contemplated
herein. The
following Table 3 illustrates exemplary therapeutic or preventive agents that
can be assayed
and the target enzymes for modifying for use in the assays provided herein.
Table 3
Drug Enzyme Therapeutic target
Cyclosporin A calcineurine/cyclophilinimmunosuppressant
Mycophenoric acid inosine monophosphateimmunosuppressant
dehydrogenase
Leflunomide dihydroorotate immunosuppressant
dehydrogenase
N-acetylprocainamideprocainamide N- cardiac arrhythmias
acetyltransferase
Fluvastatin HMG-CoA reductase hypercholesterolemia
Lovastatin HMG-CoA reductase hypercholesterolemia
Provastatin HMG-CoA reductase hypercholesterolemia
Simvastatin HMG-CoA reductase hypercholesterolemia
Atorvastain HMG-CoA reductase hypercholesterolemia
Finasteride S2-reductase benign prostate hyperplasia
Thus, if the small molecule analyte to be assayed is cyclosporin A, the mutant
analyte-
binding enzyme is a mutant calcineurine or cyclophilin, that has been designed
to substantially
retain its binding affinity or have enhanced binding affinity for cyclosporin
A but have
attenuated catalytic activity. Preferably, the attenuated catalytic activity
is achieved by a
mutation in calcineurine's catalytic site, its binding site for Ca2+ and/or
calmodulin, or a
combination thereof. More preferably, mutant calcineurine to be used is the
bovine brain
calcineurin containing mutations in its Fe3+-Zn2+ binding site (see, Yu, et
al., Biochemistry,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-89-
36 35 :10727-34 (1997)); and the mutant cyclophilin to be used is the human
cyclophilin A
having one or more of the following mutations: W121A, H54Q, RSSA, F60A, Q111A,
F113A,
and H126Q (Liu, et al., Biochemistry, 30:2306-2310 (1991); and Zydowsky, et
al., Protein Sci.,
x:1092-9 (1992)).
S In another specific embodiment, the small molecule analyte to be assayed is
mycophenoric acid and the mutant analyte-binding enzyme is a mutant inosine
monophosphate
dehydrogenase (IMPDH), the mutant inosine monophosphate dehydrogenase
substantially
retains its binding affinity or has enhanced binding affinity for mycophenoric
acid but has
attenuated catalytic activity. Preferably, the attenuated catalytic activity
is caused by mutation
in the mutant inosine monophosphate dehydrogenase's catalytic site, its
binding site for NAD+,
or a combination thereof. More preferably, the mutant IMPDH is the human type
II isoform of
IMPDH having mutations) at Cys 331 (Colby, et al., Proc. Natl. Acad. Sci.,
9:3531-6
(1999)).
In still another specific embodiment, the small molecule analyte to be assayed
is
leflunomide and the mutant analyte-binding enzyme is a mutant dihydroorotate
dehydrogenase,
the mutant dihydroorotate dehydrogenase substantially retains its binding
affinity or has
enhanced binding affinity for leflunomide but has attenuated catalytic
activity. Preferably, the
attenuated catalytic activity is caused by mutation in the mutant
dihydroorotate
dehydrogenase's catalytic site, its binding site for 02, or a combination
thereof. More
preferably, the mutant dihydroorotate dehydrogenase is the Lactococcus lactis
dihydroorotate
dehydrogenase having one or more of the mutations: C130S, C130A, K43A, K43E,
N132A and
K164A (Bjornberg, et al., Biochemistry, 36 51 :16197-205 (1997); or the E.coli
dihydroorotate
dehydrogenase having the S175C mutation (Bjornberg, et al., Biochemistry, 38
10 :2899-908
(1999)); or the Lactococcus lactis dihydroorotate dehydrogenase having one or
more mutations
at the following locations: Asn 67, Asn 127, Asn 132, Asn 193, Lys 43, Ser
194, Met 69,
Gly 70 and Leu 71 (Rowland, et al., Protein Sci., x:1269-79 (1998)).
In yet another specific embodiment, the small molecule analyte to be assayed
is N-
acetylprocainamide and the mutant anal5rte-binding enzyme is a mutant
procainamide N-
acetyltransferase, the mutant procainamide N-acetyltransferase substantially
retains its binding
affinity or has enhanced binding affinity for N-acetylprocainamide but has
attenuated catalytic
activity. Preferably, the attenuated catalytic activity is caused by mutation
in the mutant
procainamide N-acetyltransferase's catalytic site, its binding site for acetyl
CoA, or a
combination thereof.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-90-
In yet another specific embodiment, the small molecule analyte to be assayed
is selected
from fluvastatin, lovastatin, provastatin, simvastatin and atorvastatin and
the mutant analyte-
binding enzyme is a mutant HMG-CoA reductase (hydroxymethylglutaryl-CoA
reductase), the
mutant HMG-CoA reductase substantially retains its binding affinity or has
enhanced binding
affinity for N-fluvastatin, lovastatin, provastatin, simvastatin or
atorvastatin, but has attenuated
catalytic activity. Preferably, the attenuated catalytic activity is caused by
mutation in the
mutant HMG-CoA reductase's catalytic site, its binding site for NADPH, or a
combination
thereof. More preferably, the mutant HMG-CoA reductase is the Pseudomonas
mevalonii
HMG-CoA reductase having one or more of the following mutations: K267A, K267H,
K267R,
or having one or more mutations at His 381 (Bochar, et al., Biochemistry, 38
28 :8879-83
(1999); Tabernero, et al., Proc. Natl. Acad Sci., 96 13 :7167-71 (1999); or
the Syrian hamster
HMG-CoA reductase having one or more of the following mutations: E558D, E558Q,
D766N
and phosphorylated Ser 871 (Omkumar and Rodwell, J. Biol. Chem., 269 24 :16862-
6 (1994);
and Frimpong and Rodwell, J. Biol. Chem., 269 2 :1217-21 (1994)).
L. COMBINATIONS, KITS AND ARTICLES OF MANUFACTURE
Combinations and kits containing such combination are provided. The
combination
includes: a) a mutant analyte-binding enzyme, the mutant enzyme substantially
retains its
binding affinity or has enhanced binding affinity for the analyte or an
immediate analyte
enzymatic conversion product but has attenuated catalytic activity; and b)
reagents and/or other
means for detecting binding between the analyte or the immediate analyte
enzymatic
conversion product and the mutant analyte-binding enzyme. Preferably, the
analyte to be
assayed is Hcy. Also preferably, binding between the Hcy or the immediate Hcy
enzymatic
conversion product and the mutant Hcy-binding enzyme is detected using a
labelled Hcy, a
labelled immediate Hcy enzymatic conversion product, a labelled mutant Hcy-
binding enzyme,
or a derivative or an analog thereof. More preferably, wherein the analyte to
be assayed is Hcy,
the combination also includes reagents for detecting cholesterol and/or folic
acid. The kit can
also include instructions for assaying an analyte in a sample using the mutant
analyte binding
enzymes.
The packages discussed herein in relation to diagnostic systems are those
customarily
utilized in diagnostic systems. Such packages include glass and plastic, such
as polyethylene,
polypropylene and polycarbonate, bottles and vials, plastic and plastic-foil
laminated
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-91 -
envelopes and the like. The packages may also include containers appropriate
for use in auto
analyzers. The packages typically include instructions for performing the
assays.
In still another embodiment, an article of manufacture is provided. The
article includes:
a) packaging material; b) a mutant analyte-binding enzyme, the mutant enzyme
substantially
retains its binding affinity or has enhanced binding affinity for the analyte
or an immediate
analyte enzymatic conversion product but has attenuated catalytic activity;
and c) a label
indicating that the mutant analyte-binding enzyme and the reagents are for use
in assaying the
analyte in a sample. The article of manufacture may also include reagents for
detecting binding
between the analyte or the immediate analyte enzymatic conversion product and
the mutant
analyte-binding enzyme.
M. PREPARATION OF CONJUGATES
Conjugates, such as fusion proteins and chemical conjugates, of the mutant
analyte-
binding enzyme with a protein or peptide fragment (or plurality thereof) that
functions, for
example, to facilitate affinity isolation or purification of the mutant
enzyme, attachment of the
mutant enzyme to a surface, or detection of the mutant enzyme are provided.
The conjugates
can be produced by chemical conjugation, such as via thiol linkages, but are
preferably
produced by recombinant means as fusion proteins. In the fusion protein, the
peptide or
fragment thereof is linked to either the N-terminus or C-terminus of the
mutant enzyme. In
chemical conjugates the peptide or fragment thereof may be linked anywhere
that conjugation
can be effected, and there may be a plurality of such peptides or fragments
linked to a single
mutant enzyme or to a plurality thereof.
1. Conjugation
Conjugation can be effected by any method known to those of skill in the art.
As
described below, conjugation can be effected by chemical means, through
covalent, ionic or
any other suitable linkage.
a. Fusion proteins
Fusion proteins are provided herein. A fusion protein contains: a) one or a
plurality of
mutant analyte-binding enzymes and b) at least one protein or peptide fragment
that facilitates,
for example: i) affinity isolation or purification of the fusion protein; ii)
attachment of the
fusion protein to a surface; or iii) detection of the fusion protein, or any
combination thereof.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-92-
The facilitating agent is selected to perform the desired purpose, such as (i)
- (iii), and is
linked a mutant analyte-binding enzyme such that the resulting conjugate
retains the analyte
binding property and also pocesses the property(ies) of the facilitating
agent. For example, the
facilitating agent can be a protein or peptide fragment, such as a protein
binding peptide,
including but not limited to an epitope tag or an IgG binding protein, a
nucleotide binding
protein, such as a DNA or RNA binding protein, a lipid binding protein, a
polysaccharide
binding protein, and a metal binding protein or fragments thereof that possess
the requisite
desired facilitating activity.
Such facilitating agents can be designed, screened or selected according to
the methods
known in the art. The screening or selection process begins, for example, with
nucleic acid
encoding a particular protein or peptide to be used in the fusion protein, and
screened or
selected for its specific binding partner. Alternatively, the screening or
selection process can
start with a specific molecule that can be used in the subsequent
isolation/purification,
attachment or detection, and screen or select for a particular protein or
peptide sequence to be
used in the fusion protein that can specifically bind to the pre-selected
molecule.
The conventional technique of random screening of natural products can be used
in
screening and selecting a protein or peptide sequence and its specific binding
partner. In
addition, numerous strategies can be used for preparing proteins having new
binding
specificities. These new approaches generally involve the synthetic production
of large
numbers of random molecules followed by some selection procedure to identify
the molecule
of interest. For example, epitope libraries have been developed using random
polypeptides
displayed on the surface of filamentous phage particles. The library is made
by synthesizing a
repertoire of random oligonucleotides to generate all combinations, ,followed
by their insertion
into a phage vector. Each of the sequences is separately cloned and expressed
in phage, and the
relevant expressed peptide can be selected by finding those phage that bind to
the particular
target. The phages recovered in this way can be amplified and the selection
repeated. The
sequence of the peptide is decoded by sequencing the DNA (See, e.g., Cwirla,
et al., Proc.
Natl. Acad. Sci., USA, 87:6378-6382 (1990); Scott, et al., Science, 249:386-
390 (1990); and
Devlin, et al., Science, 249:404-406 (1990).
Another approach involves large arrays of peptides that are synthesized in
parallel and
screened with acceptor molecules labelled with fluorescent or other reporter
groups. The
sequence of any effective peptide can be decoded from its address in the array
(See, e.g.,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-93-
Geysen, et al., Proc. Natl. Acad. Sci., USA, 81:3998-4002 (1984); Maeji, et
al., J. Immunol.
Met., 146:83-90 (1992); and Fodor, et al., Science, 251:767-775 (1991).
Combinatorial approaches can also be employed. For example, in one exemplary
approach, combinatorial libraries of peptides are synthesized on resin beads
such that each resin
bead contains about 20 pmoles of the same peptide. The beads are screened with
labeled
acceptor molecules and those with bound acceptor are searched for by visual
inspection,
physically removed, and the peptide identified by direct sequence analysis
(Lam, et al., Nature,
354:82-84 (1991)). Another useful combinatory method for identification of
peptides of
desired activity is that of Houghten, et al. (see, e.g." Nature, 354:84-86
(1991)). For
hexapeptides of the 20 natural amino acids, 400 separate libraries are
synthesized, each with the
first two amino acids fixed and the remaining four positions occupied by all
possible
combinations. An assay, based on competition for binding or other activity, is
then used to find
the library with an active peptide. Twenty new libraries are then synthesized
and assayed to
determine the effective amino acid in the third position, and the process is
reiterated in this
fashion until the active hexapeptide is defined.
b. Chemical conjugation
To effect chemical conjugation herein, the targeting agent is linked via one
or more
selected linkers or directly to the targeted agent. Chemical conjugation must
be used if the
targeted agent is other than a peptide or protein, such a nucleic acid or a
non-peptide drug.
Any means known to those of skill in the art for chemically conjugating
selected moieties may
be used.
1. Heterobifunctional cross-linking reagents
Numerous heterobifunctional cross-linking reagents that are used to form
covalent
bonds between amino groups and thiol groups and to introduce thiol groups into
proteins, are
known to those of skill in this art (see, e.g., the PIERCE CATALOG,
ImmunoTechnology
Catalog & Handbook, 1992-1993, which describes the preparation of and use of
such reagents
and provides a commercial source for such reagents; see, also, e.g., Cumber,
et al. (1992)
Bioconjugate Chem. 3':397-401; Thorpe, et al. (1987) Cancer Res. 47:5924-5931;
Gordon, et
al. (1987) Proc. Natl. Acad Sci. 84:308-312; Walden, et al. (1986) J. Mol.
Cell Immunol.
2:191-197; Carlsson, et al. (1978) Biochem. J. 173: 723-737; Mahan, et al.
(1987) Anal.
Biochem. 162:163-170; Wawryznaczak, et al. (1992) Br. J. Cancer 66:361-366;
Fattom, et al.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-94-
(1992) Infection & Immun. 60:584-589). These reagents may be used to form
covalent bonds
between the the mutant analyte binding enzyme and the facilitating agent.
These reagents
include, but are not limited to: N-succinimidyl-3-(2-pyridyldithio)propionate
(SPDP; disulfide
linker); sulfosuccinimidyl 6-[3-(2-pyridyldithio)propionamido]hexanoate (sulfo-
LC-SPDP);
succinimidyloxycarbonyl-a-methyl benzyl thiosulfate (SMBT, hindered disulfate
linker);
succinimidyl 6-[3-(2-pyridyldithio) propionamido]hexanoate (LC-SPDP);
sulfosuccinimidyl 4-
(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC); succinimidyl 3-(2-
pyridyldithio)butyrate (SPDB; hindered disulfide bond linker);
sulfosuccinimidyl 2-(7-azido-4-
methylcoumarin-3-acetamide) ethyl-1,3'-dithiopropionate (SAED); sulfo-
succinimidyl 7-azido-
4-methylcoumarin-3-acetate (SAMOA); sulfosuccinimidyl 6-[alpha-methyl-alpha-(2-
pyridyldithio)toluamido]hexanoate (sulfo-LC-SMPT); 1,4-di-[3'-(2'-
pyridyldithio)propionamido]butane (DPDPB); 4-succinimidyloxycarbonyl-a-methyl-
a-(2-
pyridylthio)toluene (SMPT, hindered disulfate linker); sulfosuccinimidyl6[a-
methyl-a-(2-
pyridyldithio)toluamido]hexanoate (sulfo-LC-SMPT); m-maleimidobenzoyl-N-
hydroxysuccinimide ester (MBS); m-maleimidobenzoyl-N-hydroxysulfosuccinimide
ester
(sulfo-MBS); N-succinimidyl(4-iodoacetyl)aminobenzoate (SIAB; thioether
linker);
sulfosuccinimidyl(4-iodoacetyl)amino benzoate (sulfo-SIAB); succinimidyl4(p-
maleimidophenyl)butyrate (SMPB); sulfosuccinimidyl4-(p-
maleimidophenyl)butyrate (sulfo-
SMPB); azidobenzoyl hydrazide (ABH).
Other heterobifunctional cleavable cross-linkers include, N-succinimidyl (4-
iodoacetyl)-aminobenzoate; sulfosuccinimydil (4-iodoacetyl)-aminobenzoate; 4-
succinimidyl-
oxycarbonyl-a-(2-pyridyldithio)toluene; sulfosuccinimidyl-6- [a-methyl-a-
(pyridyldithiol)-
toluamido] hexanoate; N-succinimidyl-3-(-2-pyridyldithio) - proprionate;
succinimidyl 6[3(-(-
2-pyridyldithio)-proprionamido] hexanoate; sulfosuccinimidyl 6[3(-(-2-
pyridyldithio)-
propionamido] hexanoate; 3-(2-pyridyldithio)-propionyl hydrazide, Ellman's
reagent,
dichlorotriazinic acid, S-(2-thiopyridyl)-L-cysteine. Further exemplary
bifunctional linking
compounds are disclosed in U.S. Patent Nos. 5,349,066. 5,618,528, 4,569,789,
4,952,394, and
5,137,877.
2. Exemplary Linkers
Any linker known to those of skill in the art for preparation of conjugates
may be used
herein. These linkers are typically used in the preparation of chemical
conjugates; peptide
linkers may be incorporated into fusion proteins.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-95-
Linkers can be any moiety suitable to associate the mutant analyte binding
enzyme and
the facilitating agent. Such linkers and linkages include, but are not limited
to, peptidic
linkages, amino acid and peptide linkages, typically containing between one
and about 60
amino acids, more generally between about 10 and 30 amino acids,cheW ical
linkers, such as
heterobifunctional cleavable cross-linkers, including but are not limited to,
N-succinimidyl (4-
iodoacetyl)-aminobenzoate, sulfosuccinimydil (4-iodoacetyl)-aminobenzoate, 4-
succinimidyl-
oxycarbonyl-a- (2-pyridyldithio)toluene, sulfosuccinimidyl-6- [a-methyl-a-
(pyridyldithiol)-
toluamido] hexanoate, N-succinimidyl-3-(-2-pyridyldithio) - proprionate,
succinimidyl 6[3(-(-
2-pyridyldithio)-proprionamido] hexanoate, sulfosuccinimidyl 6[3(-(-2-
pyridyldithio)-
propionamido] hexanoate, 3-(2-pyridyldithio)-propionyl hydrazide, Ellman's
reagent,
dichlorotriazinic acid, and S-(2-thiopyridyl)-L-cysteine. Other linkers
include, but are not
limited to peptides and other moieties that reduce stearic hindrance between
the mutant analyte
binding enzyme and the facilitating agent, intracellular enzyme substrates,
linkers that increase
the flexibility of the conjugate, linkers that increase the solubility of the
conjugate, linkers that
increase the serum stability of the conjugate, photocleavable linkers and acid
cleavable linkers.
Other exemplary linkers and linkages that are suitable for chemically linked
conjugates
include, but are not limited to, disulfide bonds, thioether bonds, hindered
disulfide bonds, and
covalent bonds between free reactive groups, such as amine and thiol groups.
These bonds are
produced using heterobifunctional reagents to produce reactive thiol groups on
one or both of
the polypeptides and then reacting the thiol groups on one polypeptide with
reactive thiol
groups or amine groups to which reactive maleimido groups or thiol groups can
be attached on
the other. Other linkers include, acid cleavable linkers, such as
bismaleimideothoxy propane,
acid labile-transferrin conjugates and adipic acid diihydrazide, that would be
cleaved in more
acidic intracellular compartments; cross linkers that are cleaved upon
exposure to UV or visible
light and linkers, such as the various domains, such as CH1, CH2, and CH3,
from the constant
region of human IgGI (see, Batra, et al. (1993) Molecular Immunol. 30:379-
386). In some
embodiments, several linkers may be included in order to take advantage of
desired properties
of each linker.
Chemical linkers and peptide linkers may be inserted by covalently coupling
the linker
to the mutant analyte binding enzyme and the facilitating agent. The
heterobifunctional agents,
described below, may be used to effect such covalent coupling. Peptide linkers
may also be
linked by expressing DNA encoding the linker and TA, linker and targeted
agent, or linker,
targeted agent and TA as a fusion protein. Flexible linkers and linkers that
increase solubility
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-96-
of the conjugates are contemplated for use, either alone or with other linkers
are also
contemplated herein.
a. Acid cleavable, photocleavable and heat sensitive
linkers
Acid cleavable linkers, photocleavable and heat sensitive linkers may also be
used,
particularly where it may be necessary to cleave the targeted agent to permit
it to be more
readily accessible to reaction. Acid cleavable linkers include, but are not
limited to,
bismaleimideothoxy propane; and adipic acid dihydrazide linkers (see, e.g.,
Fattom, et al.
(1992) Infection & Immun. 60:584-589) and acid labile transferrin conjugates
that contain a
sufficient portion of transferrin to permit entry into the intracellular
transferrin cycling pathway
(see, e.g., Welhoner, et al. (1991) J. Biol. Chem. 266:4309-4314).
Photocleavable linkers are linkers that are cleaved upon exposure to light
(see, e.g.,
Goldmacher, et al. (1992) Bioconj. Chem. 3:104-107, which linkers are herein
incorporated by
reference), thereby releasing the targeted agent upon exposure to light.
Photocleavable linkers
that are cleaved upon exposure to light are known (see, e.g., Hazum, et al.
(1981) in Pept.,
Proc. Eur. Pept. Symp., 16th, Brunfeldt, K (Ed), pp. 105-110, which describes
the use of a
nitrobenzyl group as a photocleavable protective group for cysteine; Yen, et
al. (1989)
Makromol. Chem 190:69-82, which describes water soluble photocleavable
copolymers,
including hydroxypropylmethacrylamide copolymer, glycine copolymer,
fluorescein copolymer
and methylrhodamine copolymer; Goldmacher, et al. (1992) Bioconj. Chem. 3:104-
107, which
describes a cross-linker and reagent that undergoes photolytic degradation
upon exposure to
near UV light (350 nm); and Senter, et al. (1985) Photochem. Photobiol 42:231-
237, which
describes nitrobenzyloxycarbonyl chloride cross linking reagents that produce
photocleavable
linkages), thereby releasing the targeted agent upon exposure to light. Such
linkers would have
particular use in treating dermatological or ophthalmic conditions that can be
exposed to light
using fiber optics. After administration of the conjugate, the eye or skin or
other body part can
be exposed to light, resulting in release of the targeted moiety from the
conjugate. Such
photocleavable linkers are useful in connection with diagnostic protocols in
which it is
desirable to remove the targeting agent to permit rapid clearance from the
body of the animal.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-97-
b. Other linkers for chemical conjugation
Other linkers, include trityl linkers, particularly, derivatized trityl groups
to generate a
genus of conjugates that provide for release of therapeutic agents at various
degrees of acidity
or alkalinity. The flexibility thus afforded by the ability to preselect the
pH range at which the
therapeutic agent will be released allows selection of a linker based on the
known physiological
differences between tissues in need of delivery of a therapeutic agent (see,
e.g., U.S. Patent
No. 5,612,474). For example, the acidity of tumor tissues appears to be lower
than that of
normal tissues.
c. Peptide linkers
The linker moieties can be peptides. Petide linkers can be employed in fusion
proteins
and also in chemically linked conjugates. The peptide typically a has from
about 2 to about 60
amino acid residues, for example from about 5 to about 40, or from about 10 to
about 30 amino
acid residues. The length selected will depend upon factors, such as the use
for which the
linker is included.
The proteinaceous ligand binds with specificity to a receptors) on one or more
of the
target cells) and is taken up by the target cell(s). In order to facilitate
passage of the chimeric
ligand-toxin into the target cell, it is presently preferred that the size of
the chimeric ligand-
toxin be no larger than can be taken up by the target cell of interest.
Generally, the size of the
chimeric ligand-toxin will depend upon its composition. In the case where the
chimeric ligand
toxin contains a chemical linker and a chemical toxin (i.e., rather than
proteinaceous one), the
size of the ligand toxin is generally smaller than when the chimeric ligand-
toxin is a fusion
protein. Peptidic linkers can conveniently be encoded by nucleic acid and
incorporated in
fusion proteins upon expression in a host cell, such as E. coli.
Peptide linkers are advantageous when the facilitating agent is proteinaceous.
For
example, the linker moiety can be a flexible spacer amino acid sequence, such
as those known
in single-chain antibody research. Examples of such known linker moieties
include, but are not
limited to, peptides, such as (GlymSer)" and (SermGly)~, in which n is 1 to 6,
preferably 1 to 4,
more preferably 2 to 4, and m is 1 to 6, preferably 1 to 4, more preferably 2
to 4, enzyme
cleavable linkers and others.
Additional linking moieties are described, for example, in Huston, et al.,
Proc. Natl.
Acad. Sci. U.S.A. 85:5879-5883, 1988; Whitlow, M., et al., Protein Engineering
6:989-995,
1993; Newton, et al., Biochemistry 35:545-553, 1996; A. J. Cumber, et al.,
Bioconj. Chem.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-98-
3:397-401, 1992; Ladurner, et al., J. Mol. Biol. 273:330-337, 1997; and U.S.
Patent.
No. 4,894,443. In some embodiments, several linkers may be included in order
to take
advantage of desired properties of each linker.
N. PREPARATION OF CONJUGATES
Conjugates with linked targeted agents can be prepared either by chemical
conjugation,
recombinant DNA technology, or combinations of recombinant expression and
chemical
conjugation. The mutant analyte binding enzyme and the facilitating agent may
be linked in
any orientation and more than one targeting agent and/or targeted agent may be
present in a
conjugate.
1. Selection of facilitating agents
Any agent that facilitates detection, immobilization, or purification of the
conjugate is
contemplated for use herein. For chemical conjugates any moiety that has such
properties is
contemplated; for fusion proteins, the facilitating agent is a protein,
peptide or fragment thereof
that is sufficient to effect the facilitating activity.
a. Protein binding moieties
The conjugate contains a protein binding moiety, particularly a protein
binding protein,
peptide or effective fragment thereof. Its specific binding partner can be
proteins or peptides
generally, a set of proteins or peptides or mixtures thereof, or a particular
protein or peptide.
Any protein-protein interaction pair known to those of skill in the art is
contemplated. For
example, the protein-protein interaction pair can be enzyme/protein or peptide
substrate,
antibody/protein or peptide antigen, receptor/protein or peptide ligand, etc.
Any protein-protein
interaction pair can be designed, screened or selected according to the
methods known in the art
(See generally, Current Protocols in Molecular Biology (1998) ~ 20, John Wiley
& Sons, Inc.).
Examples of such methods for identifying protein-protein interactions include
the interaction
trap/two-hybrid system and the phage-based expression cloning.
1) Interaction trap/two-hybrid system
Interacting proteins can be identified by a selection or screen in which
proteins that
specifically interact with a target protein of interest are isolated from a
library. One particular
approach to detect interacting proteins is the two-hybrid system or
interaction trap (See
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-99-
generally, Current Protocols in Molecular Biology (1998) ~ 20.1.-20.2., John
Wiley & Sons,
Inc.), which uses yeast as a "test tube" and transcriptional activation of a
reporter system to
identify associating proteins.
In the two-hybrid system, a yeast vector such as the plasmid pEG202 or a
related vector
can be used to express the probe or "bait" protein as a fusion to the
heterologous DNA-binding
protein LexA. Many proteins, including transcription factors, kinases, and
phosphatases, can
be used as bait proteins. The major requirements for the bait protein are that
it should not be
actively excluded from the yeast nucleus, and it should not possess an
intrinsic ability to
strongly activate transcription. The plasmid expressing the LexA-fused bait
protein can be
used to transform yeast possessing a dual reporter system responsive to
transcriptional
activation through the LexA operator.
In one such example, the yeast strain EGY48 containing the reporter plasmid
pSHl8-34
can be used. In this case, binding sites for LexA are located upstream of two
reporter genes. In
the EGY48 strain, the upstream activating sequences of the chromosomal LELJ2
gene, which is
required in the biosynthetic pathway for leucine (Leu), are replaced with LexA
operators (DNA
binding sites). PSH18-34 contains a LexA operator-lacZ fusion gene. These two
reporters
allow selection for transcriptional activation by permitting selection for
viability when cells are
plated on medium lacking Leu, and discrimination based on color when the yeast
is grown on
medium containing Xgal.
The EGY48/PSH18-34 transformed with a bait is first characterized for its
ability to
express protein, growth on medium lacking Leu, and for the level of
transcriptional activation
of lacZ. A number of alternative strains, plasmids, and strategies can be
employed if a bait
proves to have an unacceptably high level of background transcriptional
activation.
In an interactor hunt, the stain EGY48/PSH18-34 containing the bait expression
plasmid
is transformed, preferably along with carrier DNA, with a conditionally
expressed library made
in a suitable vector such as the vector pJG4-5. This library uses the
inducible yeast GAL 1
promoter to express proteins as fusions to an acidic domain ("acid blob") that
functions as a
portable transcriptional activation motif (act) and to other useful moieties.
Expression of
library-encoded proteins is induced by plating transformants on medium
containing galactose
(Gal), so yeast cells containing library proteins that do not interact
specifically with the bait
protein will fail to grow in the absence of Leu. Yeast cells containing
library proteins that
interact with the bait protein will form colonies within 2 to 5 days, and the
colonies will turn
blue when the cells are streaked on medium containing Xgal. The DNA from
interaction trap
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-100-
positive colonies can be analyzed by polymerase chain reaction (PCR) to
streamline screening
and detect redundant clones in cases where many positives are obtained in
screening. The
plasmids can be isolated and characterized by a series of tests to confirm
specificity of the
interaction with the initial bait protein.
An alternative way of conducting an interactor hunt is to mate a strain that
expresses the
bait protein with a strain that has been pretransformed with the library DNA,
and screen the
resulting diploid cells for interactors (Bendixen, et al., Nucl. Acids. Res.,
22:1778-1779 (1994);
and Finley and Brent, Proc. Natl. Sci. U.S.A., 91:12980-12984 (1994)). This
"interaction
mating" approach can be used for any interactor hunt, and is particularly
useful in three special
cases. The first case is when more than one bait will be used to screen a
single library.
Interaction mating allows several interactor hunts with different baits to be
conducted using a
single high-efficiency yeast transformation with library DNA. This can be a
considerable
savings, since the library transformation is one of the most challenging tasks
in an interactor
hunt. The second case is when a constitutively expressed bait interferes with
yeast viability.
For such baits, performing a hunt by interaction mating avoids the difficulty
associated with
achieving a high-efficiency library transformation of a strain expressing a
toxic bait.
Moreover, the actual selection for interactors will be conducted in diploid
yeast, which are
more vigorous than haploid yeast and can better tolerate expression of toxic
proteins. The third
case is when a bait cannot be used in a traditional interactor hunt using
haploid yeast stains
because it activates transcription of even the least sensitive reporters. In
diploids the reporters
are less sensitive to transcription activation than they are in haploids.
Thus, the interaction
mating hunt provides an additional method to reduce background from
transactivating baits.
The interaction trap/two-hybrid system and the identified protein-protein
interaction
pairs have been successfully used (see, e.g., Bartel, et al., Using the two-
hybrid system to
detect protein-protein interactions, In Cellular Interactions in Development:
A Practical
Approach, (D.A. Hartley, ed.) pp. 153-179, Oxford University Press, Oxford
(1993); Bartel, et
al., A protein linkage map of Escherichia coli bacteriophage T7, Nature
Genet., 12:72-77
(1996); Bendi~:en, et al., A yeast mating-selection scheme for detection of
protein-protein
interactions, Nucl. Acids. Res., 22:1778-1779 (1994); Breeden and Nasmyth,
Regulation of the
yeast HO gene., Cold spring Harbor Symp. Quant. Biol, 50:643-650 (1985); Brent
and Ptashne,
A bacterial repressor protein or a yeast transcriptional terminator can block
upstream activation
of a yeast gene, Nature, 312:612-615 (1984); Brent, et al., A eukaryotic
transcriptional
activator bearing the DNA specificity of a prokaryotic repressor, Cell, 43:729-
736 (1985);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 101 -
Chien, et al., The two-hybrid system: A method to identify and clone genes for
proteins that
interact with a protein of interest, Proc. Natl. Acad. Sci. U.S.A., 88:9578-
9582 (1991); Chiu, et
al., RAPT1, a mammalian homolog of yeast Tor, interacts with the
FKBP12/rapamycin
complex, Proc. Nat. Acad. Sci., U.S.A., 91:12574-12578 (1994); Colas, et al.,
Genetic selection
of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2.,
Nature, 380:548-550
(1996); Durfee, et al., The retinoblastoma protein associates with the protein
phosphatase type
1 catalytic subunit, Genes & Dev., 7:555-569 (1993); Estojak, et al.,
Correlation of two-hybrid
affinity data with in vitro measurements, Mol. Cell. Biol., 15:5820-5829
(1995); Fearon, et al.,
Karyoplasmic interaction selection strategy: A general strategy to detect
protein-protein
interaction in mammalian cells, Proc. Nat., Acad. Sci. U.S.A., 89:7958-7962
(1992); Fields and
Song, A novel genetic system to detect protein-protein interaction, Nature,
340:245-246
(1989); Finley and Brent, Interaction mating revels binary and ternary
connections between
Drosophila cell cycle regulators, Proc. Natl. Sci. U.S.A., 91:12980-12984
(1994); Gietz, et al.,
Improved method for high-efficiency transformation of intact yeast cells,
Nucl. Acids. Res.,
20:1425 (1992); Golemis and Brent, Fused protein domains inhibit DNA biding by
LexA, Mol.
Cell Biol., 12:3006-3014 (1992); Gyuris, et al., Cdil, a human G1 and S-phase
protein
phosphatase that associates with Cdkl, Cell, 75:791-803 (1993); Kaiser, et
al., A., Methods in
Yeast Genetics, a Cold Spring Harbor Laboratory Course Manual, pp. 135-136.
Cold Spring
Harbor Laboratory Press, Cold Spring Harbor, N.Y. (1994); Kolonin and Finley,
Jr., Targeting
cyclin-dependent kinases in Drosophila with peptide aptamers, Proc. Natl.
Acad. Sci. U. S.A., In
press (1998); Licitra and Liu, A three-hybrid system for detecting small
ligand-protein receptor
interactions, Proc. Nat. Acad. Sci. U.S.A., 93:12817-12821 (1996); Ma and
Ptashne, A new
class of yeast transcriptional activators, Cell, 51:113-119 (1987); Ma and
Ptashne, Converting
an eukaryotic transcriptional inhibitor into an activator, Cell, 55:443-446
(1988); Osborne, et
al., The yeast tribrid system: Genetic detection of transphosphorylated ITAM-
SH2 interactions,
BiolTechnology, 13:1474-1478 (1995); Ruden, et al., Generating yeast
transcriptional
activators containing no yeast protein sequences, Nature, 350:426-430 (1991);
Samson, et al.,
Gene activation and DNA binding by Drosophila Ubx and abd-A proteins, Cell,
57:1045-1052
(1989); Stagljar, et al., Use of the two-hybrid system and random sonicated
DNA to identify
the interaction domain of a protein, BioTechniques, 21:430-432 (1996);
Vasavada, et al., A
contingent replication assay for the detection of protein-protein interactions
in animal cells,
Proc. Nat. Acad. Sci. U.S.A., 88:10686-10690 (1991); Vojtex, et al., Mammalian
Ras interacts
directly with the serine/threonine kinase Raf, Cell, 74:205-214 (1993);
Watson, et al., Vectors
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 102 -
encoding alternative antibiotic resistance for use in the yeast two-hybrid
system,
BioTechniques, 21:255-259 (1996); West, et al., Saccharomyces cerevisiae GAL10
divergent
promoter region: Location and function of the upstream activator sequence
UASG, Mol. Cell
Biol., 4:2467-2478 (1984); and Yang, et al., Protein-peptide interactions
analyzed with the
yeast two-hybrid system, Nucl. Acids Res., 23:1152-1156 (1995)) and can be
used in the
present system.
2) Phage-based expression cloning
Interaction cloning (also known as expression cloning) is a technique to
identify and
clone genes that encode proteins that interact with a protein of interest, or
"bait" protein.
Phage-based interaction cloning requires a gene encoding the bait protein and
an appropriate
expression library constructed in a bacteriophage expression vector, such as
~gtl 1 (See
generally, Current Protocols in Molecular Biology (1998) ~ 20.3, John Wiley &
Sons, Inc.).
The gene encoding the bait protein is used to produce recombinant fusion
protein in E. coli.
The cDNA is radioactively labeled with 32P. A recognition site for a protein
kinase such as the
cyclic adenosine 3',5'-phosphate (cAMP)--dependent protein kinase (Protein
kinase A; PKA) is
introduced into the recombinant fusion protein to allow its enzymatic
phosphorylation by the
kinase and [h-32P]ATP.
In one example, the procedure involves a fusion protein containing bait
protein and
glutathione-S-transferase (GST) with a PKA site at the junction between them:
The labeled
protein is subsequently used as a probe to screen a ~ bacteriophage-derived
cDNA expression
library, which expresses (3-galactosidase fusion proteins that contain in-
frame gene fusions.
The phages lyse cells, form plaques, and release fusion proteins that are
adsorbed onto
nitrocellulose membrane filters. The filters are blocked with excess
nonspecific protein to
eliminate nonspecific binding and probed with the radiolabeled bait protein.
This procedure
leads directly to the isolation of genes encoding the interacting protein, by
passing the need for
purification and microsequencing or for antibody production.
The phage-based interaction cloning system and the identified protein-protein
interaction pairs have been successfully employed (Blanar, et al., Interaction
cloning:
Identification of a helix-loop-helix zipper protein that interacts with c-Fos,
Science, 256:1014-
1018 (1992); Carr and Scott, Blotting and band-shifting: Techniques for
studying protein-
protein interactions, Trends Biochem. Sci., 17:246-249 (1992); Chapline, et
al., Interaction
cloning of protein kinase C substrates, J. Biol. Chem., 268:6858-6861 (1993);
Hoeffler, et al.,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-103-
Identification of multiple nuclear factors that interact with cyclic AMP
response element-
binding protein and activation transcription factor-2 by protein interactions,
Mol. Endocrinol.,
5:256-266 (1991); Kaelin, et al., Expression cloning of a cDNA encoding a
retinoblastoma-
binding protein with E2F-like properties, Cell, 70:351-364 (1992); Lester, et
al., Cloning and
characterization of a novel A-kinase anchoring protein: AKAP220, association
with testicular
peroxisomes, J. Biol. Chem., 271:9460-9465 (1996); Lowenstein, et al., The SH2
and SH2
domain-containing protein GRB2 links receptor tyrosine kinase to ras
signaling, Cell, 70:431-
442 (1992); Margolis, et al., High-efficiency expression/cloning of epidermal
growth factor-
receptor-binding proteins with src homology 2 domains, Proc. Natl. Acad. Sci.
U.S.A.,
89:8894-8898 (1992); Skolnik, et al., Cloning of P13 kinase-associated p85
utilizing a novel
method of expression/cloning of target proteins for receptor tyrosine kinases,
Cell, 65:83-90
( 1991 ); and Stone, et al., Interaction of a protein phosphatase with an
Arabidopsis serine-
threonine receptor kinase, Science, 266:793-795 (1994)) and can be used in the
present system.
3) Detection of protein-protein interactions
1 S Surface plasmon resonance (SPR) can be used to verify the protein-protein
interactions
identified by other systems such as the interaction trap/two-hybrid system and
the phage-based
expression cloning systems (See generally, Current Protocols in Molecular
Biology (1998) ~
20.4, John Wiley & Sons, Inc.). This is an in vitro technique based on an
optical phenomenon,
called SPR, that can simultaneously detect interactions between unmodified
proteins and
directly measure kinetic parameters of the interaction.
SPR devices are commercially available. The BIAcore instrument (BIAcore) is
presently preferred herein. This instrument contains sensing optics, an
automated sample
delivery system, and a computer for instrument control, data collection, and
data processing.
Experiments are performed on disposable chips. In practice, a ligand protein
is immobilized on
the chip while buffer continuously flows over the surface. The sensing
apparatus monitors
changes in the angle of minimum reflectance from the interface that result
when a target protein
associates with the ligand protein. Molecular interactions can be directly
visualized (on the
computer monitor) in real time as the optical response is plotted against
time. This response is
measured in resonance units (RUs, where 1000 RUs = 1 ng protein/mm2).
The SPR system has been successfully used (see, e.g., BioSupplyNet Source
Book,
BioSupplyNet, Plainview, N.Y., and Cold Spring Harbor Laboratory Press, Cold
Spring
Harbor, N.Y. (1999); Feng, et al., Functional binding between G~3 and the LIM
domain of SteS
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 104 -
is required to activate the MEKK Stel 1, Cur. Biol., 8:267-278 (1998); Field,
et al., Purification
of RAS-responsive adenylyl cyclase complex from Sacchariomyces cerevisiae by
use of an
epitope addition method, Mol. Cell. Biol., 8:2159-2165 (1988); Phizicky and
Fields, Protein-
protein interactions: Methods for detection and analysis, Microbiol. Rev.,
59:94-123 (1995);
Tyers, et al., Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may
be an
upstream activator of Clnl, Cln2, and other cyclins, EMBO J., 11:1773-1784
(1993)) and the
identified protein-protein interaction pairs can be used in the present
system.
b. Epitope tags
The facilitating agent can be any moiety, particularly a protein, peptide or
effective
fragment thereof that is specifically recognized by an antibody. In these
embodiments, the
conjugate contains an epitope tag that is specifically recognized by a set of
antibodies or by a
particular antibody. Any epitope/antibody pair can be used in the present
system (See
generally, Current Protocols in Molecular Biology (1998) 10.15, John Wiley &
Sons, Inc.).
The following Table 4 provides exemplary epitope tags and illustrates certain
properties of
several commonly used epitope tag systems.
Table 4. Exemplary epitope tag systems
EpitopePeptide SEQ AntibodyReference
ID
FLAG AspTyrLysAspAspAspLys 63 4E11 Prickett'
HA TyrProTyrAspVaIPRoAspTyrAla 64 12Ca5 Xiez
HAl CysGlnAspLeuProGlyAsnAspAsnSerThr65 mouse Nagelkerken3
MAb
c-Myc GluGlnLysLeuIleSerGluGluAspLeu66 9E10 Xie2
6-His HisHisHisHisHisHis 67 BAbCO~
AU1 AspThrTyrArgTyrIle 68 BAbCO
EE GluTyrMetProMetGlu 69 anti-EE Tolbert4
T7 AIaSerMetThrGlyGlyGInGInMetGlyArg70 InvitrogenChens
Tsengb
4A6 SerPheProGlnPheLysProGlnGlulle71 4A6 Rudiger~
a LysGlyPheSerTyrPheGlyGluAspLeuMetPro72 anti-PKCsOlaha
B GlnTyrProAlaLeuThr 73 D11, Wang9
F10
gE GlnArgGlnTyrGlyAspValPheLysGlyAsp74 3B3 Grose'
Tyl GluValHisThrAsnGlnAspProLeuAsp75 BB2, Bastin"
TYGS
1. Prickett, et al., BioTechniques, 7:580-584 (1989)
2. Xie, et al., Endocrinology, 139(11):4563-4567 (1998)
3. Nagelkerke, et al., Electrophoresis, 18:2694-2698 (1997)
4. Tolbert and Lameh, J. Neurochem., 70:113-119 (1998)
5. Chen and Katz, BioTechniques, 25~1~:22-24 (1998)
6. Tseng and Verma, Gene, 169:287-288 (1996)
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-105-
7. Rudiger, et al., BioTechniques, 2:96-97 (1997)
8. Olah, et al., Biochem., 221:94-102 (1994)
9. Wang, et al., Gene, 169 1 :53-58 (1996)
10. Grose, U.S. Patent No. 5,710,248
11. Bastin, et al., Mol. Biochem. Parasitology, 77:235-239 (1996)
* Invitrogen, Sigma, Santa Cruz Biotech
For example, in one embodiment, the selected epitope tag is the 6-His tag.
Vectors for
constructing a fusion protein containing the 6-His tag and reagents for
isolating or purifying
such fusion proteins are commercially available. For example, the Poly-His
gene fusion vector
from Invitrogen, Inc. (Carlsbad, CA) includes the following features: 1) high-
level regulated
transcription for the trc promotor; 2) enhanced translation efficiency of
eukaryotic genes in
E.coli; 3) the LacO operator and the Lacl9 repressor gene for transcriptional
regulation in any
E. coli system; N-terminal Xpress epitope for easy detection with an Anti-
Xpress antibody; and
4) enterokinase cleaving site for removal of the fusion tag. The fusion
protein can be purified
by nickel-chelating agarose resin, and the purified fusion protein can be
coated onto a
microtiter plate pre-coated with nickel (e.g., Reacti-Binding meta chelate
polystyrene plates,
Pierce) for diagnostic usage.
In addition, the fusion protein containing the 6-His tag can be isolated or
purified using
the His MicroSpin Purification Module or HisTrap Kit from Amersham Pharmacia
Biotech,
Inc. The His MicroSpin Purification Module provides fifty MicroSpin columns
prepacked with
nickel-charged Chelating Sepharose Fast Flow. The module enables the simple
and rapid
screening of large numbers of small-scale bacterial lysates for the analysis
of putative clones
and optimization of expression and purification conditions. Each column
contains 50 p,1 bed
volume, enough to purify > 100 p,g his-tagged fusion protein, from up to 400
p1 of His-tagged
fusion protein sample, e.g., crude lysate and purification intermediates. The
HisTrap Kit is
designed for rapid, mild affinity purification of histidine-tagged fusion
proteins in a single step.
The high dynamic capacity of HiTrap Chelating enables milligrams of protein to
be purified in
less than 15 minutes at flow rates of up to 240 column volumes per hour. The
high capacity is
maintained after repeated use ensuring cost-effective, reproducible
purifications. The Kit
includes three HiTrap Chelating columns and buffer concentrates to perform F10-
12
purifications with a syringe. The anti-His antibody from Amersham Pharmacia
Biotech, Inc. is
an IgG2 subclass of monoclonal antibody directed against 6 Histidine residues.
The antibody is
unconjugated to offer the flexibility of detection with a secondary antibody
conjugated with
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 106 -
either horseradish peroxidase or alkaline phosphatase. The antibody provides
high sensitivity
with low background.
Further examples of epitope tagging can be found in Kolodziej and Young,
Epitope
tagging and protein surveillance, Methods Enzymol., 194:508-519 (1991).
Methods for
preparing and using other such tags and other such tags similarly can be used
in the methods
and products provided herein.
c. IgG binding proteins
In other embodiments, the conjugate contains an IgG binding protein, which,
for
example provides a means for selective binding of the conjugate. Any IgG
binding protein/IgG
pair can be used in the present system. Protein A and Protein G are suitable
facilitating. Any
Protein A or Protein G can be used in the present system.
For example, the following nucleotide sequences can be used for amplifying and
constructing Protein A or Protein G fusion proteins: E04365 (Primer for
amplifying IgG
binding domain AB of protein A); E04364 (Primer for amplifying IgG binding
domain AB of
protein A); E01756 (DNA sequence encoding subunit which can bind IgG of
protein A like
substance); M74187 (Cloning vector pKP497 (cloning, screening, fusion vector)
encoding an
IgG-binding fusion protein from protein A analogue (ZZ) and beta-Gal'(lacZ)
genes). In
addition, several Protein A gene fusion vectors such as pEZZ 18 and pRIT2T are
commercially
available (Amersham Pharmacia Biotech, Inc.).
1) pEZZ 18 Protein A gene fusion vector
pEZZ 18 Protein A gene fusion vector can be used for rapid expression of
secreted
fusion proteins and their one-step purification using IgG Sepharose 6FF. The
phagemid pEZZ
18 contains the proteins A signal sequence and two synthetic "Z" domains based
on the "B"
IgG binding domain of Protein A (Lowenadler, et al., Gene, 58:87 (1987); and
Nilsson, et al.,
Prot. Engineering, 1:107 (1987)). Proteins are expressed as fusions with the
"ZZ" peptide and
secreted into the aqueous culture medium under the direction of the protein A
signal sequence.
They are easily purified using IgG Sepharose 6FF to which the "ZZ" domain
binds tightly.
Because of its unique folding properties, the 14 kDa "ZZ" peptide has little
effect on folding of
the fusion partner into a native conformation.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 107 -
Expression
Expression is controlled by the lacUV 5 and protein A promotors and is not
inducible.
Elements of the protein A gene provide the ATG and ribosome-binding sites.
Stop
codons must be provided by the insert.
Sequencing
The M13 Universal Sequencing Primer is used for double-stranded and single-
stranded
sequencing. A protocol for production of single-stranded DNA is provided with
the
vector.
Cloning
Inserts containing a stop codon will yield white colonies when grown on media
containing X-gal.
Hosts)
E. coli strains carrying a lac deletion but capable of a-complementation of
lacZ'.
Selectable markers)
Plasmid confers resistance to ampicillin.
Amplification
Amplification, though not necessarily required can be included.
2) pRIT2T Protein A gene fusion vector
The pRIT2T Protein A gene fusion vector (available from Pharmacia) can be used
for
high-level expression of intracellular fusion proteins. pRIT2T, a derivative
of pRIT2 (Nilsson,
et al., EMBO J., 4:1075 (1985)), contains the IgG-binding domains of
staphylococcal protein A
which permits rapid affinity purification of fusion proteins on IgG Sepharose
6 FF. Thermo-
inducible expression of the fusion protein is achieved in a suitable E. coli
host strain which
carries the temperature-sensitive repressor c1857 (N4830-1) (Zabeau and
Stanley, EMBO J.,
1:1217 (1982)).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 108 -
Induction
The APR promoter is induced by shifting the growth temperature from
30°C to 42°C for
90 minutes.
Expression
Genes inserted into the MCS are expressed from the ~ right promoter (PR) as
fusions
with the IgG-binding domains of staphylococcal protein A. A portion of the ~
cro gene,
fused to the IgG-binding domain, supplies the ATG start codon. Since no signal
sequence is provided, the protein remains intracellular. Protein A gene
transcription
and translation termination signals are provided. Fusion protein can be
purified on IgG
Sepharose 6FF (17-0969-O1). The protein A carrier protein is ~30 kDa.
Hosts)
E. coli N4830-1/N99cI+. Supplied with E. coli N4830-1 which contains the
temperature-sensitive c1857 repressor.
Selectable markers)
Plasmid confers resistance to ampicillin.
3) The IgG Sepharose 6 fast flow system
The Protein A and Protein G fusion protein can be isolated or purified by
affinity
binding with IgG, such as the IgG Sepharose 6 Fast Flow System (Amersham
Pharmacia
Biotech, Inc.). The IgG Sepharose 6 Fast Flow System includes IgG coupled to
the highly
cross-linked 6% agarose matrix Sepharose 6 Fast Flow, and is designed for the
rapid
purification of Protein A and Protein A fusion conjugates. The system binds at
least 2 mg
Protein A/ml drained gel with flow possible rates of 300 cm/hr at 1 bar (14.5
psi, 0.1 MPa) in
an XK 50/30 column (Lundstrom, et al., Biotechnology and Bioengineering,
36:1056 (1990)).
d. ~-galatosidase fusion proteins
The pMC 1871 fusion vector (commercially available from Pharmacia, see, also
Shapira, et al., Gene 25:71 (1983); Casadaban, et al., Methods Enzymol.
100:293 (1983)) for
production of enzymatically active ~3-galactosidase hybrid proteins for gene
expression or
functional studies. Vector pMC1871 is derived from pBR322 and contains a
promoterless lacZ
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 109 -
gene, which also lacks a ribosome-binding site and the first eight non-
essential N-terminal
amino acid codons. Its unique Sma I site allows fusions to the N-terminal part
of the (3-
galactosidase gene. Insertion of a gene into the E. coli lacZ gene results in
the production of a
hybrid protein, whose presence can be readily detected by following its (3
galactosidase activity
(Miller, J.H., in Experiments in Molecular Gener. (Cold Spring Harbor, N.Y.)
(1972); Nielsen,
et al., Proc. Natl. Acad. Sci. U.S.A., 80:5198 (1983)). Hybrid proteins can
then be easily
purified by affinity chromatography (Germino, et al., Proc. Natl. Acad. Sci.
U.S.A., 81: 4692
(1984)). Multiple cloning sites flanking the lacZ gene permit its excision as
a BamH I, Sal I, Pst
I or EcoR I gene cassette. If lacZ is excised as an EcoRI cassette, a portion
of its 3'-end will be
deleted. The resulting (3-glactosidase protein (a-donor) will be functional if
the C-terminus of
the (3-galactosidase protein (a-acceptor) is available through intercistronic
complementation.
Expression
Inserts cloned into the unique Sma I site give fusion proteins with the N-
terminal part of
~i-galactosidase. Insert must contain a promoter, ATG and ribosome-binding
site.
Hosts)
E. coli strains carrying a lac deletion.
Selectable markers)
Plasmid confers resistance to 15 ~,g/ml tetracycline.
GenBank Accession Number L08936.
e. Nucleic acid binding moieties
In another embodiment, the conjugate includes a nucleotide binding protein,
peptide or
effective fragment thereof as a facilitating agent. The specific binding
partner can be
nucleotide sequences generally, a set of nucleotide sequences or a particular
nucleotide
sequence. Any protein-nucleotide interaction pair can be used in the present
system. For
example, the protein-nucleotide interaction pair can be protein/DNA or
protein/RNA pairs, or a
combination thereof. Protein-nucleotide interaction pairs can be designed,
screened or selected
according to the methods known in the art (See generally, Current Protocols in
Molecular
Biology (1998) ~ 12, John Wiley & Sons, Inc.). Examples of such methods for
identifying
protein-nucleotide interactions include the gel mobility shift assay,
methylation and uracil
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 110 -
interference assay, DNase I footprint analysis, ~gtl l expression library
screening and rapid
separation of protein-bound DNA from free DNA using nitrocellulose filters.
1) DNA binding proteins
The conjugate can contain a DNA binding protein and its specific binding
partner can
be DNA molecules generally, a set of DNA molecules or a particular sequence of
nucleotides.
Any DNA binding protein can be used in the present system. For example, the
DNA binding
protein can bind to a single-stranded or double-stranded DNA sequence, or to
an A-, B- or Z-
form DNA sequence. The DNA binding sequence can also bind to a DNA sequence
that is
involved in replication, transcription, DNA repair, recombination,
transposition or DNA
structure maintenance. The DNA binding sequence can further be derived from a
DNA
binding enzyme such as a DNA polymerase, a DNA-dependent RNA polymerase, a
DNAase, a
DNA ligase, a DNA topoisomerase, a transposase, a DNA kinase, or a restriction
enzyme.
Any DNA binding sequence/DNA sequence pair can be designed, screened or
selected
according to the methods known in the art including methods described in
Section L.2. above.
The following Table 5 illustrates certain properties of several DNA binding
sequence/DNA sequence pair systems.
Table 5. Exam les of DNA bindin se uence/DNA se uence bindin airs
DNA binding DNA binding DNA sequence Reference
sequence sequence motif (U.S. Patent
No.)
NF-ATp T lymphocyte GCCCAAAGAGGAA 5,656,452
(SEQ ID NO. 76) DNA-binding AATTTGTTTCATAC
protein AG (SEQ ID NO.
77)
Max helix-loop-helixCACGTG 5,693,487
(SEQ ID NO. 78) zipper protein
Chicken Lung Z-DNA 5,726,050
140
Kd Protein
EGR1, EGR2, GLI,Zinc finger GACC, GCAC 5,789,538
Wilm's tumor proteins
gene,
Sp 1, Hunchback,
Kruppel, ADR1
and
BrLA
LIL-Stat proteinStat TTNCNNAGA, 5,821,053
family of TTCCTGAGA
transcription
factors
Egr zinc finger CGCCCCCGC 5,866,325
(SEQ ID NO. 79) protein
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 111 -
DNA binding DNA binding DNA sequence Reference
sequence sequence motif (U.S. Patent
No.)
S1-3 protein zinc finger CATRRWWG 5,905,146
(SEQ
ID NO. 80) protein
2) RNA binding proteins
In another preferred embodiment, the conjugate can contain an RNA binding
protein
and its specific binding partner can be RNA generally, a set of RNA molecules
or a particular
S sequence of ribonucleotides. Any RNA binding protein can be used in the
present system. For
example, the RNA binding protein can bind to a single-stranded or double-
stranded RNA, or to
rRNA, mRNA or tRNA. The RNA binding protein may specifically bind to a RNA
that is
involved in reverse transcription, transcription, RNA editing, RNA splicing,
translation, RNA
stabilization, RNA destabilization, or RNA localization. The RNA binding
protein can be
derived from or be an RNA binding enzyme such as a RNA-dependent DNA
polymerase, a
RNA-dependent RNA polymerase, a RNase, a RNA ligase, a RNA maturase, or a
ribosome.
Other RNA recognition sequence or binding motifs that can be used in the
present
system include the zinc-finger motif, the Y-box, the KH motif, AUUUA, histone,
RNP motif
(U 1 ), arginine-rich motif (ARM or PRE), double-stranded RNA binding motifs
(IRE) and RGG
box (APP) (U.S. Patent Nos. 5,834,184, 5,859,227 and 5,858,675). The RNP motif
is a 90-100
amino acid sequence that is present in one or more copies in proteins that
bind pre mRNA,
mRNA, pre-ribosomal RNA and snRNA. The consensus sequence and the sequences of
several exemplary proteins containing the RNP motif are provided in Burd and
Dreyfuss,
Science, 265:615-621 (1994); Swanson, et al., Trends Biochem. Sci., 13:86
(1988); Bandziulis,
et al., Genes Dev., 3:431 (1989); and Kenan, et al., Trends Biochem. Sci.,
16:214 (1991). The
RNP consensus motif contains two short consensus sequences RNP-1 and RNP-2.
Some RNP
proteins bind specific RNA sequences with high affinities (dissociation
constant in the range of
10'g-10-" M). Such proteins often function in RNA processing reactions. Other
RNP proteins
have less stringent sequence requirements and bind less strongly (dissociation
constant about
10-6-10-~ M) (Bard & Dreyfuss, EMBOJ., 13:1197 (1994)).
A second characteristic RNA binding motif found in viral, phage and ribosomal
proteins is an arginine-rich motif (ARM) of about 10-20 amino acids. RNA
binding proteins
having this motif include the HIV Tat and Rev proteins. Rev binds with high
affinity
disassociation constant (10-9 M) to an RNA sequence termed RRE, which is found
in all HIV
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 112 -
mRNAs (Zapp, et al., Nature, 342:714 (1989); and Dayton, et al., Science,
246:1625 (1989)).
Tat binds to an RNA sequence termed TAR with a dissociation constant of 5X10-9
M
(Churcher, et al., J. Mol. Biol., 230:90 (1993)). For Tat and Rev proteins, a
fragment
containing the arginine-rich motif binds as strongly as the intact protein. In
other RNA binding
proteins with ARM motifs, residues outside the ARM also contribute to binding.
The double-stranded RNA-binding domain (dsRBD) exclusively binds double-
stranded
RNA or RNA-DNA. A dsRBD motif includes a region of approximately 70 amino
acids which
includes basic residues and contains a conserved core sequence with a
predicted a-helical
structure. The dsRBD motif is found in at least 20 known or putative RNA-
binding proteins
from different organisms. There are two types of dsRBDs; Type A, which is
homologous
along its entire length with the defined consensus sequence, and Type B, which
is more highly
conserved at its C terminus than its N terminus. These domains have been
functionally
delineated in specific proteins by deletion analysis and RNA binding assays
(St Johnston, et al.,
Proc. Natl. Acad. Sci., 89:10979-10983 (1992)).
Any RNA binding sequence/RNA sequence pair can be designed, screened or
selected
according to the methods known in the art including the methods described in
Section L.2.
above and the methods, such as those decribed in U.S. Patent Nos. 5,834,184
and 5,859,227,
and in SenGupta, et al., A three-hybrid system to detect RNA-protein
interactions in vivo,
Proc. Nat. Acad. Sci. U.S.A., 93:8496-8501 (1996)).
For example, U.S. Patent No. 5,834,184 describes a method of screening a
plurality of
polypeptides for RNA binding activity. The method includes the steps of: (1)
culturing a
library of procaryotic cells that constitute a library, and (2) detecting
expression of the reporter
gene in a cell from the library, the expression indicating that the cell
comprises a polypeptide
having RNA binding activity. The cells contain at least one vector that
contains a first DNA
segment that encodes a fusion protein of a prokaryotic anti-terminator protein
having anti-
terminator activity linked in-frame to the test polypeptide, which varies
among the cells in the
library, that is operably linked to a second DNA segment. The second DNA
segment contains
a promoter, an RNA recognition sequence foreign to the anti-terminator
protein, a transcription
termination site and a reporter gene. The termination site blocks
transcription of the reporter
gene in the absence of a protein with anti-termination activity and affinity
for the RNA
recognition sequence. If the test polypeptide has specific affinity for the
recognition sequence,
it binds via the polypeptide to the RNA recognition sequence of a transcript
from the second
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-113-
DNA segment thereby inducing transcription of the second DNA segment to
proceed through
the termination site to the reporter gene resulting in expression of the
reporter gene.
U.S. Patent No. 5,859,227 describes methods for identifying possible binding
sites for
RNA binding proteins in nucleic acid molecules, and confirming the identity of
such
prospective binding sites by detection of interaction between the prospective
binding site and
RNA binding proteins. These methods involve identification of possible binding
sites for RNA
binding proteins, by either searching databases for untranslated regions of
gene sequences or
cloning untranslated sequences using a single specific primer and an universal
primer, followed
by confirmation that the untranslated regions in fact interact with RNA
binding proteins using
the RNA/RBP detection assay. Genomic nucleic acid can further be screened for
putative
binding site motifs in the nucleic acid sequences. Information about binding
sites that are
confirmed in the assay then can be used to redefine or redirect the nucleic
acid sequence search
criteria, for example, by establishing or refining a consensus sequence for a
given binding site
motif.
SenGupta, et al., Proc. Nat. Acad. Sci. U.S.A., 93:8496-8501 (1996) describes
a yeast
genetic method to detect and analyze RNA-protein interactions in which the
binding of a
bifunctional RNA to each of two hybrid proteins activates transcription of a
reporter gene in
vivo (see also Wang, et al., Genes & Dev., 10:3028-3040 (1996)). SenGupta, et
al. demonstrate
that this three-hybrid system enables the rapid, phenotypic detection of
specific RNA-protein
interactions. As examples, SenGupta, et al. use the binding of the iron
regulatory protein 1
(IRP 1 ) to the iron response element (IRE), and of HIV trans-activator
protein (Tat) to the HIV
trans-activation response element (TAR) RNA sequence. The three-hybrid assay
relies only on
the physical properties of the RNA and protein, and not on their natural
biological activities; as
a result, it may have broad application in the identification of RNA-binding
proteins and RNAs,
as well as in the detailed analysis of their interactions.
The following Table 6 illustrates certain properties of several RNA binding
sequence/RNA sequence pair systems.
Table 6. Examples of RNA binding sequence/RNA sequence pairs
RNA binding RNA binding RNA sequence Reference
(U.S.
sequence sequence motif Patent No.)
BINDR double-stranded double-stranded 5,858,675
RNA
RNA-binding poly(rI) and poly
(rC)
Protein extract 5' untranslated UTR of Glutl (SEQ5,859,227
from
SH-SYSY cells region (UTR) ID NO. 81); 5'
UTR
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 114 -
of (HMG,CoA Red)
(SEQ ID NO. 82);
S'
UTR of human
C4b-binding a
chain
(SEQ ID NO. 83);
5'
UTR of human CD45
(SEQ ID NO. 84)
3) Preparation of nucleic acid binding proteins
Preparation of nuclear and cytoplasmic extracts
Extracts prepared from the isolated nuclei of cultured cells are functional in
accurate in
vitro transcription and mRNA processing (See generally, Current Protocols in
Molecular
Biology (1998) ~ 12.1., John Wiley & Sons, Inc.). Thus, such extracts can be
used directly for
functional studies and as the starting material for purification of the
proteins involved in these
processes. To prepare nuclear extracts, tissue culture cells are collected,
washed, and
suspended in hypotonic buffer. The swollen cells are homogenized and nuclei
are pelleted.
The cytoplasmic fraction is removed and saved, and nuclei are resuspended in a
low-salt buffer.
Gentle dropwise addition of a high-salt buffer then releases soluble proteins
from the nuclei
(without lysing the nuclei). Following extraction, the nuclei are removed by
centrifugation, the
nuclear extract supernatant is dialyzed into a moderate salt solution, and any
precipitated
protein is removed by centrifugation.
The nuclear and cytoplasmic extraction procedure (see, e.g., Dignam, et al.,
1983, Nucl.
Acids. Res. 11:1475-1489 (Accurate transcription initiation by RNA polymerase
II in a soluble
extract from isolated mammalian nuclei); Dignam, et al., 1983, Methods
Enzymol. 101:582-598
(Eukaryotic gene transcription with purified components); Krainer, et al.,
1984, Cell 36:993-
1005 (Normal and mutant human (3-globin pre-mRNAs are faithfully and
efficiently spliced in
vitro); Lue, et al., 1987, Proc. Natl. Acad. Sci. U.S.A. 84:8839-8843
(Accurate initiation at
RNA polymerase II promoters in extracts from Saccharomyces cerevisiae);
Manley, et al.,
1980, Proc. Natl. Acad. Sci. U.S.A. 77:3855-3859 (DNA-dependent transcription
of adenovirus
genes in a soluble whole-cell extract); Weil, et al., 1979, J. Biol. Chem.
254:6163-6173
(Faithful transcription of eukaryotic genes by RNA polymerase III in systems
reconstituted
with purified DNA templates); and Weil, et al., 1979, Cell 18:469-484
(Selective and accurate
initiation of transcription at the Ad2 major late promotor in a soluble system
dependent on
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 115 -
purified RNA polymerase II and DNA)) and the identified protein-DNA
interaction pairs can
be used in the present system.
4) Assays for identifying nucleic acid binding proteins
a. Mobility shift DNA-binding assay
The DNA-binding assay using nondenaturing polyacrylamide gel electrophoresis
(PAGE) provides a simple, rapid, and extremely sensitive method for detecting
sequence-
specific DNA-binding proteins (See generally, Current Protocols in Molecular
Biology (1998)
~ 12.2., John Wiley & Sons, Inc.). Proteins that bind specifically to an end-
labeled DNA
fragment retard the mobility of the fragment during electrophoresis, resulting
in discrete bands
corresponding to the individual protein-DNA complexes. The assay can be used
to test binding
of purified proteins or of uncharacterized factors found in crude extracts.
This assay also
permits quantitative determination of the affinity, abundance, association
rate constants,
dissociation rate constants, and binding specificity of DNA-binding proteins.
b. Basic mobility shift assay procedure
The basic mobility shift assay procedure includes 4 steps: ( 1 ) preparation
of a
radioactively labeled DNA probe containing a particular protein binding site;
(2) preparation of
a nondenaturing gel; (3) a binding reaction in which a protein mixture is
bound to the DNA
probe; and (4) electrophoresis of protein-DNA complexes through the gel, which
is then dried
and autoradiographed. The mobility of the DNA-bound protein is retarded while
that of the
non-bound protein is not retarded.
c. Competition mobility shift assay
One important aspect of the mobility shift DNA-binding assay is the ease of
assessing
the sequence specificity of protein-DNA interactions using a competition
binding assay. This
is necessary because most protein preparations will contain specific and
nonspecific DNA
binding proteins. For a specific competitor, the same DNA fragment (unlabeled)
as the probe
can be used. The nonspecific competitor can be essentially any fragment with
an unrelated
sequence, but it is useful to roughly match the probe and specific competitor
for size and
configuration of the ends. For example, some proteins bind blunt DNA ends
nonspecifically.
These would not be competed by circular plasmid or a fragment with overhands,
leading to the
false conclusion that the protein-DNA complex represented specific binding.
Perhaps the best
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 116 -
control competitor is a DNA fragment that is identical to the probe fragment
except for a
mutations) in the binding site that is known to disrupt function (and
presumably binding).
d. Antibody supershift assay
Another useful variation of the mobility shift DNA-binding assay is to use
antibodies to
identify proteins present in the protein-DNA complex. Addition of a specific
antibody to a
binding reaction can have one of several effects. If the protein recognized by
the antibody is
not involved in complex formation, addition of the antibody should have no
effect. If the
protein that forms the complex is recognized by the antibody, the antibody can
either block
complex formation, or it can form an antibody-protein-DNA ternary complex and
thereby
specifically result in a further reduction in the mobility of the protein-DNA
complex
(supershift). Results may be different depending upon whether the antibody is
added before or
after the protein binds DNA (particularly if there are epitopes on the DNA-
binding surface of
the protein).
The mobility shift DNA-binding assay has been successfully employed (see,
e.g.,
1 S Carthew, et al., 1985, Cell 43:439-448 (An RNA polymerase II transcription
factor binds to an
upstream element in the adenovirus major late promoter); Chodosh, et al.,
1986, Mol. Cell.
Biol. 6:4723-4733 (A single polypeptide possesses the binding and activities
of the adenovirus
major late transcription factor); Fried, et al., 1981, Nucl. Acids. Res.,
9:6505-6525 (Equilibria
and kinetics of lac repressor-operator interactions by polyacrylamide gel
electrophoresis);
Fried, et al., 1984, J. Mol. Biol. 172:241-262 (Kinetics and mechanism in the
reaction of gene
regulatory proteins with DNA); Fried, et al., 1984, J. Mol. Biol. 172:263-282
(Equilibrium
studies of the cyclic AMP receptor protein-DNA interaction); Garner, et al.,
1981, Nucl. Acids
Res. 9:3047-3060 (A gel electrophoresis method for quantifying the binding of
proteins to
specific DNA regions: Application to components of the Escherichia coli
lactose operon
regulatory system); Hendrickson, et al., 1984, J. Mol. Biol. 174:611-628
(Regulation of the
Escherichia coli L-arabinose operon studied by gel electrophoresis DNA binding
assay);
Kristie, et al., 1986, Proc. Natl. Acad. Sci. U.S.A. 83:3218-3222 (The major
regulatory protein
of herpes simplex virus type 1, is stably and specifically associated with
promoter-regulatory
domains of a genes and/or selected viral genes); Lieberman, et al., 1994,
Genes & Dev. 8:995-
1006 (A mechanism for TAFs in transcriptional activation: Activation domain
enhancement of
TFIID-TFIIA-promoter DNA complex formation); Riggs, et al., 1970, J. Mol.
Biol. 48:67-83
(Lac repressor-operator interactions: I. Equilibrium studies); Singh, et al.,
1986, Nature
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 117 -
319:154-158 (A nuclear factor that binds to a conserved sequence motif in
transcriptional
control elements of immunoglobulin genes); Staudt, et al., 1986, Nature
323:640-643 (A
lymphoid-specific protein binding to the octamer motif of immunoglobulin
genes); Strauss, et
al., 1984, Cell 37:889-901 (A protein binds to a satellite DNA repeat at three
specific sites that
would be brought into mutual proximity by DNA folding in the nucleosome); and
Zinkel, et al.,
1987, Nature 328:178-181 (DNA bend direction by phase-sensitive detection))
and the
identified protein-DNA interaction pairs can be used in the present system.
e. Methylation and uracil interference assay
Interference assays identify specific residues in the DNA binding site that,
when
modified, interfere with binding of the protein (See generally, Current
Protocols in Molecular
Biology (1998) ~ 12.3., John Wiley & Sons, Inc.). These protocols use end-
labeled DNA
probes that are modified at an average of one site per molecule of probe.
These probes are
incubated with the protein of interests, and protein-DNA complexes are
separated from free
probe by the mobility shift assay. A DNA probe that.is modified at a position
that interferes
with binding will not be retarded in this assay; thus, the specific protein-
DNA complex is
depleted for DNA that contains modifications on bases important for binding.
After gel
purification the bound and unbound DNA are specifically cleaved at the
modified residues and
the resulting products analyzed by electrophoresis on polyacrylamide
sequencing gels and
autoradiography. These procedures provide complementary information about the
nucleotides
involved in protein-DNA interactions.
1) Methylation interference assays
In methylation interference, probes are generated by methylating guanines (at
the N-7
position) and adenines (at the N-3 position) with DMS; these methylated bases
are cleaved
specifically by piperidine. Methylation interference identifies guanines and
adenines in the
DNA binding site that, when methylated, interfere with binding of the protein.
The protocol
uses a single end-labeled DNA probe that is methylated at an average of one
site per molecule
of probe. The labeled probe is a substrate for a protein-binding reaction. DNA-
protein
complexes are separated from the free probe by the mobility shift DNA-binding
assay. A DNA
probe that is methylated at a position that interferes with binding will not
be retarded in this
assay. Therefore, the specific DNA-protein complex is depleted for DNA that
contains methyl
groups on purines important for binding. After gel purification, DNA is
cleaved with
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 118 -
piperidine. Finally, these fragments are electrophoresed on polyacrylamide
sequencing gels
and autoradiographed. Guanines and adenines that interfere with binding are
revealed by their
absence in the retarded complex relative to a lane containing piperidine-
cleaved free probe.
This procedure offers a rapid and highly analytical means of characterizing
DNA-protein
interactions.
2) Uracil interference assay
In uracil interference, probes are generated by PCR amplification in the
presence of a
mixture of TTP and dUTP, thereby producing products in which thymine residues
are replaced
by deoxyuracil residues (which contains hydrogen in place of the thymine S-
methyl group).
Uracil bases are specifically cleaved by uracil-N glycosylase to generate
apyrimidinic sites that
are susceptible to piperidine. Uracil interference identifies thymines in a
DNA binding site
that, when modified, interfere with binding of the protein. Probes generated
by PCR
amplification in the presence of TTP and dUTP incorporate deoxyuracil in place
of thymine
residues. PCR products are incubated with the binding protein and resulting
complexes are
separated from unbound DNA. The DNA recovered from the protein-DNA complex is
treated
with uracil-N-glycosylase and piperidine, and the products are then
electrophoresed on a
denaturing polyacrylamide gel.
The methylation and uracil interference assays have been successfully used
(see, e.g.,
Baldwin, et al., 1988, Proc. Natl. Acad. Sci. U.SA. 85:723-727 (Two
transcription factors,
H2TF 1 and NF-kB, interact with a single regulatory sequence in the class I
MHC promoter);
Brunelle, et al., 1987, Proc. Natl. Acad Sci. U.S.A. 84:6673-6676 (Missing
contact probing of
DNA-protein interactions); Goeddel, et al., 1978, Proc. Natl. Acad Sci. U.S.A.
75:3579-3582
(How lac repressor recognizes lac operator); Ivarie, et al., 1987, Nucl. Acids
Res. 15:9975-9983
(Thymine methyls and DNA-protein interactions); Maxam, et al., 1980, Methods
Enzymol
65:499-560 (Sequencing end-labeled DNA with base-specific chemical cleavages);
Pu, et al.,
1992, Nucl. Acids Res. 20:771-775 (Uracil interference, a rapid and general
method for
defining protein-DNA interactions involving the 5-methyl group of thymines:
The GCN4-
DNA complex); Siebenlist, et al., 1980, Proc. Natl. Acad. Sci. U.S.A. 77:122-
126 (Contacts
between E. coli RNA polymerase and an early promoter of phase T7); and
Hendrickson, et al.,
1985, Proc. Natl. Acad Sci. U.S.A. 82:3129-3133 (A dimer of AraC protein
contacts three
adjacent major groove regions at the Ara I DNA site)) and the identified
protein-DNA
interaction pairs can be used in the present system.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 119 -
3) DNase I footprint analysis
Deoxyribonuclease I (DNase I) protection mapping, or footprinting, is a
valuable
technique for locating the specific binding sites of proteins on DNA (See
generally, Current
Protocols in Molecular Biology (1998) ~ 12.4., John Wiley & Sons, Inc.). The
basis of this
assay is that bound protein protects that phosphodiester backbone of DNA from
DNase I
catalyzed hydrolysis. Binding sites are visualized by autoradiography of the
DNA fragments
that result form hydrolysis, following separation by electrophoresis on
denaturing DNA
sequencing gels. Footprinting has been developed further as a quantitative
technique to
determine separate binding curves for each individual protein-binding site on
the DNA. For
each binding site, the total energy of binding is determined directly from
that site's binding
curve. For sites that interact cooperatively, simultaneous numerical analysis
of all the binding
curves can be used to resolve the intrinsic binding and cooperative components
of these
energies.
DNase I footprint analysis has been successfully employed (see, e.g., Ackers,
et al.,
1982, Proc. Natl. Acad Sci. U.SA. 79:1129-1133 (Quantitative model for gene
regulation by
lambda phage repressor); Ackers, et al., 1983, J. Mol. Biol. 170:223-242 (Free
energy coupling
within macromolecules: The chemical work of ligand binding at the individual
sites in
cooperative systems); Brenowitz, et al., 1986, Proc. Natl. Acad. Sci. U.S.A.
83:8462-8466
(Footprint titrations yield valid thermodynamic isotherms.); Brenowitz, et
al., 1986, Meth.
Enzymol. 130:132-181 (Quantitative DNase I footprint titration: A method for
studying protein-
DNA interactions); Dabrowiak, et al., 1989, In Chemistry and Physics of DNA-
Ligand
Interactions (N.R. Kallenback, ed.) Adenine Press. (Quantitative footprinting
analysis of drug-
DNA interactions); Galas, et al., 1978, Nucl. Acids Res. 5:3157-3170 (DNase
footprinting: A
simple method for the detection of protein-DNA binding specificity);
Hertzberg, et al., 1982, J.
Am. Chem. Soc. 104:313-315 (Cleavage of double helical DNA by (methidiumpropyl-
EDTA)
iron (II)); Johnson, et al., 1979, Proc. Natl. Acad. Sci. U.S.A. 76:5061-5065
(Interactions
between DNA-bound repressors govern regulation by the lambda phage repressor);
Johnson, et
al., 1985, Meth. Enzymol. 117:301-342 (Nonlinear least-squares analysis);
Senear, et al., 1986,
Biochemistry 25:7344-7354 (Energetics of cooperative protein-DNA interactions:
Comparison
between quantitative DNase I footprint titration and filter binding); and
Tullius, et al., 1987,
Meth. Enzymol. 155:537-558 (Hydroxyl radical footprinting: A high resolution
method for
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 120 -
mapping protein-DNA contacts), and the identified protein-DNA interaction
pairs can be used
in the present system.
4) Screening a agtll expression library with
recognition-site DNA
A clone encoding a sequence-specific protein can be detected in a Sgt 11
library because
its recombinant protein binds specifically to a radiolabeled recognition-site
DNA (See
generally, Current Protocols in Molecular Biology (1998) ~ 12.7., John Wiley &
Sons, Inc.).
Bacteriophage from a cDNA library constructed in the vector ~gtl 1 are plated
under lytic
growth conditions. After plaques appear, expression of the (3-galactosidase
fusion proteins
encoded by the recombinant phage is induced by placing nitrocellulose filters
impregnated with
IPTG onto the plate. Phage growth is continued and is accompanied by the
immobilization of
proteins, from lysed cells, onto the nitrocellulose filters. The filters are
lifted after this
incubation, blocked with protein, then reacted with a radiolabeled recognition-
site DNA
(containing one or more binding sites for the relevant sequence-specific
protein) in the presence
of an excess of nonspecific competitor DNA. After the binding reaction, the
filters are washed
to remove nonspecifically bound probe and processed for autoradiography.
Potentially positive
clones detected in the primary screen are rescreened after a round of plaque
purification.
Recombinants which screen positively after enrichment and whose detection
specifically
requires the recognition-site probe (non detected with control probes lacking
the recognition
site for the relevant protein) are then isolated by further rounds of plaque
purification.
The ~gtl l expression screening methods have been successfully used (see,
e.g.,
Androphy, et al., 1987, Nature (Loud.) 325:70-73 (Bovine papillomavirus E2
trans-activating
gene product binds to specific sites in papillomavirus DNA); Arndt, et al.,
1986, Proc. Natl.
Acad. Sci. U.S.A. 83:8516-8520 (GCN4 protein, a positive transcription factor
in yeast, binds
general control promoters at 5'TGACTC3' sequences); Chodosh, et al., 1988,
Cell 53:25-35 (A
yeast and a human CCAAT-binding protein have heterologous subunits that are
functionally
interchangeable); Desplan, et al., 1985, Nature (Lond.) 318:630-635 (The
Drosophila
developmental gene, engrailed, encodes a sequence-specific DNA binding
activity); Hoeffler,
et al., 1988, Science 242:1430-1433 (Cyclic AMP-responsive DNA-binding
protein: Structure
based on a cloned placental cDNA); Hsiou-Chi, et al., 1988, Science 242:69-71
(Distinct
cloned class II MHC DNA binding proteins recognize the X box transcription
element);
Ingraham, et al., 1988, Cell 55:519-529 (A tissue-specific transcription
factor containing a
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 121 -
homeo domain specifies a pituitary phenotype); Kadonaga, et al., 1987, Cell
51:1079-1090
(Isolation of cDNA encoding transcription factor Spl an functional analysis of
the DNA
binding domain); Keegan, et al., 1986, Science 231:699-704 (Separation of DNA
binding from
the transcription-activating function of a eukaryotic regulatory protein);
Miyamoto, et al., 1988,
S Cell 54:903-913 (Regulated expression of a gene encoding a nucleic factor,
IRF-1, that
specifically binds to IFN-(3 gene regulatory elements); Murre, et al., 1989,
Cell 56:777-783 (A
new DNA binding and dimerization motif in immunoglobulin enhancer binding,
daughterless,
MyoD and myc proteins); Miiller, et al., 1988, Nature (Loud.) 336:544-551 (A
cloned octamer
transcription factor stimulates transcription from lymphoid specific promoters
in non-B cells);
Rawlins, et al., 1985, Cell 42:859-868 (Sequence-specific DNA binding of the
Epstein-Barr
viral nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance
region); Reith, et
al., 1989, Proc. Natl. Acid. Sci. U.S.A. 86:4200-4204 (Cloning of the major
histocompatibility
complex class II promoter affected in a hereditary defect in class II gene
regulation); Singly et
al., 1988, Cell 52:415-423 (Molecular cloning of an enhancer binding protein:
Isolation by
screening of an expression library with a recognition site); Staudt, et al.,
1988, Science
241:577-580 (Molecular cloning of a lymphoid-specific cDNA encoding a protein
that binds to
the regulatory octamer DNA motif); Sturm, et al., 1988, Genes & Dev. 2:1582-
1599 (The
ubiquitous octamer protein Oct-1 contains a Pou domain with a homeo
subdomain); Vinson, et
al., 1988, Genes cY~ Dev. 2:801-806 (In situ detection of sequence-specific
DNA binding
activity specified by a recombinant bacteriophage); Weinberger, et al., 1985,
Science 228:740-
742 (Identification of human glucocorticoid receptor complementary DNA clones
by epitope
selection); and Young, et al., 1983, Science 222:778-782 (Yeast RNA polymerise
II genes:
Isolation with antibody probes)) and the identified protein-DNA interaction
pairs can be used in
the present system.
5) Rapid separation of protein-bound DNA from
free DNA
This method relies on the ability of nitrocellulose to bind proteins but not
double-
stranded DNA (See generally, Current Protocols in Molecular Biology (1998) ~
12.8., John
Wiley & Sons, Inc.). Use of radioactively labeled double-stranded DNA
fragments allows
quantitation of DNA bound to the protein at various times and under various
conditions,
permitting kinetic and equilibrium studies of DNA-binding interactions.
Purified protein is
mixed with double-stranded DNA in an appropriate buffer to allow interaction.
After
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 122 -
incubation, the mixture is suction filtered through nitrocellulose, allowing
unbound DNA to
pass through the filter while the protein (and any DNA interacting with it) is
retained.
Nitrocellulose filter methods have been successfully used (see, e.g., Barkley,
et al.,
1975, Biochemistry 14:1700-1712 (Interaction of effecting ligands with lac
repressor and
repressor-operator complex); Fried, et al., 1981, Nucl. Acids Res. 9:6505-6525
(Equilibria and
kinetics of lac repressor-operator interactions by polyacrylamide gel
electrophoresis); Hinkle,
et al., 1972, J. Mol. Biol. 70:157-185 (Studies of the binding of Escherichia
coli RNA
polymerise to DNA I. The role of sigma subunit in site selection); Hinkle, et
al., 1972, J. Mol.
Biol. 70:187-195 (Studies of the binding of Escherichia coli RNA polymerise to
DNA'II. The
kinetics of the binding reaction); Hinkle, et al., 1972, J. Mol. Biol. 70:197-
207 (Studies of the
binding of Escherichia coli RNA polymerise to DNA III. Tight binding of RNA
polymerise
holoenzyme to single-strand breaks in T7 DNA); Jones, et al., 1966, J. Mol.
Biol. 22:199-209
(Studies on the binding of RNA polymerise to polynucleotides); Lin, et al.,
1972, J. Mol. Biol.
72:671-690 (Lac repressor binding to non-operator DNA: Detailed studies and a
comparison of
equilibrium and rate competition methods); Lin, et al., 1975, Cell 4:107-111
(The general
affinity of lac repressor for E. coli DNA: Implications for gene regulation in
procaryotes and
eucaryotes); Nirenberg, et al., 1964, Science 145:1399-1407 (RNA codewords and
protein
synthesis: The effect of trinucleotides upon the binding of sRNA to
ribosomes); Ptashne, et al.,
1987, A Genetic Switch: Gene Control and Phage ~ pp. 80-83 and 109-118. Cell
Press,
Cambridge, MA and Blackwell Scientific, Boston, MA; Riggs, et al., 1970, J.
Mol. Biol. 48:67-
83 (Lac repressor=operator interactions: I. Equilibrium studies); Strauss, et
al., 1980,
Biochemistry 19:3496-3504 (Binding of Escherichia coli ribonucleic acid
polymerise
holoenzyme to a bacteriophage T7 promoter-containing fragment: Selectivity
exists over a
wide range of solution conditions); Strauss, et al., 1980, Biochemistry
19:3504-3515 (Binding
of Escherichia coli ribonucleic acid polymerise holoenzyme to a bacteriophage
T7 promoter-
containing fragment: Evaluation of promoter binding constants as a function of
solution
conditions); and Strauss, et al., 1981, Gene 13:75-87 (Variables affecting the
selectivity and
efficiency of retention of DNA fragments by E. coli RNA polymerise in the
nitrocellulose-
filter binding assay)) and the identified protein-DNA interaction pairs can be
used in the
present system.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-123-
f. Lipid binding moieties
The conjugate can also contain a lipid binding protein, peptide or effective
fragment
thereof. Its specific binding partner can be lipids generally, a set of lipids
or a particular lipid.
Any lipid binding moiety, particularly proteins, peptides or effective
fragments thereof can be
S used in the present system. For example, the lipid binding protein can bind
to a triacylglycerol,
a wax, a phosphoglyceride, a sphingolipid, a sterol and a sterol fatty acid
ester. More
preferably, the lipid binding sequence comprises a C2 motif or an amphipathic
a-helix motif.
Any lipid binding sequence/lipid pair can be designed, screened or selected
according to
the methods known in the art (see, e.g., Kane, et al., Anal. Biochem.,
233(2):197-204 (1996);
Arnold, et al., Biochim. Biophys. Acta, 1233(2):198-204 (1995); Miller and
Cistola, Mol. Cell.
Biochem., 123(1-2):29-37 (1993); and Teegarden, et al., Anal. Biochem., 199 2
:293-9 (1991).
For example, Kane, et al., Anal. Biochem., 233(2):197-204 (1996) describes
that the
fluorescent probe 1-anilinonapthalene 8-sulfonic acid (1,8-ANS) has been used
to characterize
a general assay for members of the intracellular lipid-binding protein (iLBP)
multigene family.
The adipocyte lipid-binding protein (ALBP), the keratinocyte lipid-binding
protein (KLBP), the
cellular retinol-binding protein (CRBP), and the cellular retinoic acid-
binding protein I
(CRABPI) have been characterized as to their ligand binding activities using
1,8-ANS. ALBP
and KLBP exhibited the highest affinity probe binding with apparent
dissociation constants
(Kd) of 410 and 530 nM, respectively, while CRBP and CRABPI bound 1,8-ANS with
apparent dissociation constants of 7.7 and 25 microM, respectively. In order
to quantitate the
fatty acid and retinoid binding specificity and affinity of ALBP, KLBP, and
CRBP, a
competition assay was developed to monitor the ability of various lipid
molecules to displace
bound 1,8-ANS from the binding cavity. Oleic acid and arachidonic acid
displaced bound 1,8-
ANS from ALBP, with apparent inhibitor constants (Ki) of 134 nM, while all-
traps-retinoic
acid exhibited a seven-fold lower Ki (870 nM). The short chain fatty acid
octanoic acid and
all-traps-retinol did not displace the fluorophore from ALBP to any measurable
extent. In
comparison, the displacement assay revealed that KLBP bound oleic acid and
arachidonic acid
with high affinity (Ki = 420 and 400 nM, respectively) but bound all-traps-
retinoic acid with a
markedly reduced affinity (Ki = 3.6 microM). Like that for ALBP, neither
octanoic acid nor
all-traps-retinol were bound by KLBP. Displacement of 1,8-ANS from CRBP by all-
trans-
retinal and all-traps-retinoic acid yielded Ki values of 1.7 and 5.3 microM,
respectively. These
results indicate the utility of the assay for characterizing the ligand
binding characteristics of
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 124 -
members of the iLBP family and suggests that this technique may be used to
characterize the
ligand binding properties of other hydrophobic ligand binding proteins.
Arnold, et al., Biochim. Biophys. Acta, 1233(2):198-204 (1995) describes an
assay for
analyzing the specific binding of proteins to lipid ligands contained within
vesicles or micelles.
S This assay, referred to as the electrophoretic migration shift assay, was
developed using a
model system composed of cholera toxin and of its physiological receptor,
monosialoganglioside GM1. Using polyacrylamide gel electrophoresis in non-
denaturing
conditions, the migration of toxin components known to interact with GM1 was
retarded when
GMl was present in either lipid vesicles or micelles. This effect was
specific, as the migration
of proteins not interacting with GM1 was not modified. The localization of
retarded proteins
and of lipids on gels was further determined by autoradiography. The
stoichiometry of binding
between cholera toxin and GM1 was determined, giving a value of five GM1 per
one
pentameric assembly of cholera toxin B-subunits, in agreement with previous
studies. The
general applicability of this assay was further established using streptavidin
and annexin V
together with specific lipid ligands. This assay is fast, simple,
quantitative, and requires only
microgram quantities of protein.
Miller and Cistola, Mol. Cell. Biochem., 123 1-2 :29-37 (1993) teaches that
titration
calorimetry can be used as a method for obtaining binding constants and
thermodynamic
parameters for the cytosolic fatty acid- and lipid-binding proteins. A feature
of this method is
its ability to accurately determine binding constants in a non-perturbing
manner. This is
acheived because the assay does not require separation of bound and free
ligand to obtain
binding parameters. Also, the structure of the lipid-protein complex was not
perturbed, since
native ligands were used rather than non-native analogues. As illustrated for
liver fatty acid-
binding protein, the method distinguished affinity classes whose dissociation
constants differed
by an order of magnitude or less. It also distinguished endothermic from
exothermic binding
reactions, as illustrated for the binding of two closely related bile salts to
ileal lipid-binding
protein. The main limitations of the method are its relatively low sensitivity
and the difficulty
working with highly insoluble ligands, such as cholesterol or saturated long-
chain fatty acids.
The signal-to-noise ratio was improved by manipulating the buffer conditions,
as illustrated for
oleate binding to rat intestinal fatty acid binding protein.
Teegarden, et al., Anal. Biochem., 199(2):293-9 (1991) describes an assay for
measurement of the affinity of serum vitamin D binding protein for 25-
hydroxyvitamin D3,
1,25-dihydroxyvitamin D3, and vitamin D3, using uniform diameter (6.4 microns)
polystyrene
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-125-
beads coated with phosphatidylcholine and vitamin D metabolites as the vitamin
D donor. The
lipid metabolite coated beads have a solid core, and thus all of the vitamin D
metabolites are on
the bead surface from which transfer to protein occurs. After incubating these
beads in neutral
buffer for 3 h, essentially no 3H-labeled vitamin D metabolites desorb from
this surface.
Phosphatidylcholine/vitamin D metabolite-coated beads (1 microM vitamin D
metabolite) were
incubated with varying concentrations of serum vitamin D binding protein under
conditions in
which the bead surfaces were saturated with protein, but most of the protein
was free in
solution. After incubation, beads were rapidly centrifuged without disturbing
the equilibrium
of binding and vitamin D metabolite bound to sDBP in solution was assayed in
the supernatant.
All three vitamin D metabolites became bound to serum vitamin D binding
protein, and after 10
min of incubation the transfer of the metabolites to serum vitamin D binding
protein was time
independent. The transfer followed a Langmuir isotherm, and the Kd for each
metabolite
binding to serum vitamin D binding protein was derived by nonlinear least-
squares fit analysis.
From this analysis the following values for the Kd were obtained: 5.59 x 10-6
M, 25-
hydroxyvitamin D; 9.45 x 10-6 M, 1,25-dihydroxyvitamin D; and 9.17 x 10-5 M,
vitamin D.
The method disclosed herein avoids problems encountered in previous assays and
allows the
precise and convenient determination of binding affinities of vitamin D
metabolites and serum
vitamin D binding protein.
In addition, known protein/lipid binding pairs can be used in the methods and
with the
products provided herein (see, e.g., Hinderliter, et al., Biochim. Biophys.
Acta, 1448(2):227-35
(1998) (C2 motif binds phospholipid in a manner that is modulated by Ca2+ and
confers
membrane-binding ability on a wide variety of proteins, primarily proteins
involved in signal
transduction and membrane trafficking events); Campagna, et al., J. Diary
Sci., 81 12 :3139-48
(1998) (an amphipathic helical lipid-binding motif of a glycosylated
phosphoprotein,
component PP3 in bovine milk); Chae, et al., J. Biol. Chem., 273(40):25659-63
(1998) (The
C2A domain of synaptotagmin I, which binds Ca2+ and anionic phospholipids);
Johnson, et al.,
Biochemistry, 37(26):9509-19 (1998) (the membrane binding domain of
phosphocholine
cy~tidylyltransferase (CT) includes a continuous amphipathic alpha-helix
between residues
approximately 240-295 anionic lipids); Kiyosue, et al., Plant Mol. Biol.,
35:969-72 (1997)
(Ca2+-dependent lipid-binding domains of cytosolic phospholipase A2, protein
kinase C,
Rabphilin-3A, and Synaptotagmin 1 of animals); Welters, et al., Proc. Natl.
Acad. Sci. USA,
91(24):11398-402 (1994) (calcium-dependent lipid-binding domain is near the N
terminus of
phosphatidylinositol (PI) 3-kinase cloned from Arabidopsis thaliana); and
Filoteo, et al., J.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 126 -
Biol. Chem., 267 17 :11800-5 (1992) (Peptide G25:
LysLysAlaVaILysValProLysLysGIuLysSerValLeuGlnGIyLysLeuThrArgLeuAlaValGlnIle
(SEQ ID
No. 85)) representing the putative lipid-binding region (G region) of the
erythrocyte Ca2+
pump interacted with acidic lipids, as shown by the increase in size of
phosphatidylserine
liposomes in its presence)).
g. Polysaccharide binding moieties
The conjugate can include a polysaccharide binding protein, peptide or
effective
fragment thereof. Its specific binding partner can be polysaccharides
generally, a set of
polysaccharides or a particular polysaccharide. Any polysaccharide binding
moiety, such as a
protein, can be used in the present system and include but are not limited to
a polysaccharide
binding sequence that binds to starch, glycogen, cellulose or hyaluronic acid.
Any polysaccharide binding protein/polysaccharide pair can be designed,
screened or
selected according to the methods known in the art including the methods
disclosed in Kuo, et
al., J. Immunol. Methods, 4:35-47 (1981); and Brandt, et al., J. Immunol.,
108(4):913-20
(1972) (a radioactive antigen-binding assay for Neisseria meningitides
polysaccharide
antibody). Kuo, et al., J. Immunol. Methods, 43 1 :35-47 (1981) provides a
polyethylene
glycol (PEG) radioimmunoprecipitation assay for the detection of antibody to
Haemophilus
influenza b capsular polysaccharide, polyribosylribitol phosphate (PRP). The
radioactive
antigen, [3H]PRP, with a high specific activity, was produced by growing the
organism in the
presence of [3H]ribose and was purified by hydroxylapatite and SepharoseTM 4B
column
chromatography. In the assay, PEG (12.5%) was used to separate antibody-bound
[3H]PRP
from free [3H]PRP. The assay covered the range of 0.5 and 20 ng antibody/assay
at a
maximum sensitivity of 0.5 approximately 1.0 ng antibody/assay. With various
dilutions (1-20
ng antibody/assay) of S. Klein reference antiserum, the within-run coefficient
of variation (CV)
of 10 replicates ranged from 3.5 to 8.5%. Average CVs of 8.9% and 11.0% were
obtained in
the between-run and day-to-day reproducibility studies. The binding of [3H]PRP
to S. Klein
reference antiserum was severely inhibited by a minute amount of non-
radioactive PRP;
however, no significant interference was found in the presence of high
concentrations of
polysaccharides from Escherichia coli K100 and Streptococcus pneumoniae
indicating that the
RIA was highly specific for antibody to H. influenza b PRP.
In addition, known protein/polysaccharide binding pairs can be used in the
methods and
with the products provided herein (see, e.g., Yamaguchi, et al., Oral
Microbiol. Immunol.,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 127 -
13:348-54 (1998) (capsule-like serotype-specific polysaccharide antigen
lipopolysaccharide
from Actinobacillus actinomycetemcomitans/human complement-derived opsonins);
Lucas, et
al., J. Immunol., 161 7 :3776-80 (1998) (kappa II-A2 light chain CDR-3
functional residues in
human antibody/Haemophilus influenza type b polysaccharide); Miller, et al.,
Carbohydr. Res.,
309(3):219-26 (1998) (fragments of the Shigella dysenteriae type 1 O-specific
polysaccharide/monoclonal IgM 3707 E9); Prelim, et al., Protein Expr. Purif.,
x:343-6
(1996) (digitonin/hyaluronate synthase); Jiang, et al., Infect. Immun., 6:2537-
40 (1995)
(mannose-binding protein/Klebsiella 03 lipopolysaccharide); Pelkonen, et al.,
J. Bacteriol.,
174 23 :7757-61 (1992) (bacteriophage depolymerase/bacterial polysaccharide);
Morishita, et
al., Biochem. Biophys. Res. Commun., 176(3):949-57 (1991) (Microbial
polysaccharide, HS-
142-1/guanylyl cyclase-containing receptor); Ohtomo, et al., Can. J.
Microbiol., 3:206-10
(1990) (staphylococcal cell surface polysaccharide/human fibrinogen);
Yamagishi, et al., FEBS
Lett., 225 1-2 :109-12 (1987) (heparin or dermatan sulfate/thrombin);
DeAngelis, et al., J. Biol.
Chem., 262(29):13946-52 (1987) (sulfated fucans/bindin, the adhesive protein
from sea urchin
sperm); Volanakis, et al., Mol. Immunol., 20(11):1201-7 (1983) (human C4/C-
reactive protein-
pneumococcal C-polysaccharide complexes); Naruse, et al., J. Biochem. (Tokyo),
9:581-7
(1981) (a polysaccharide from the cortex of sea urchin egg/microtubule-
associated proteins);
Levy, et al., J. Exp. Med., 153(4):883-96 (1981) (agaropectin and
heparin/human IgG proteins);
Hu, et al., Biochemistry, 14(10):2224-30 (1975) (glycogen phosphorylase A/a
series of
semisynthetic, branched saccharides); Fagerstrom, Microbiology, 140(9):2399-
407 (1994)
(raw-starch-binding consensus amino acids in the C-terminal part of
glucoamylase P); Murata,
et al., J. Vet. Med. Sci., 57:419-25 (1995) (C-polysaccharide/C-reactive
protein (CRP));
Reason, et al., Infect. Immun., 67:994-7 (1999) (Antibodies having light (L)
chains encoded
by the kappaII-A2 variable region/Haemophilus influenza type b polysaccharide
(Hib PS)).
h. Metal binding moieties
The conjugate can contain a metal binding moiety, such as a metal binding
protein,
peptide or effective fragment thereof. The specific binding partner can be
metal ions generally,
a set of metal ions or a particular metal ion. Any metal binding moiety is
contemplated. For
example, the metal binding sequence can bind to a sodium, a potassium, a
magnesium, a
calcium, a chlorine, an iron, a copper, a zinc, a manganese, a cobalt, an
iodine, a molybdenum,
a vanadium, a nickel, a chromium, a fluorine, a silicon, a tin, a boron or an
arsenic ion.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 128 -
Any metal binding moiety/metal ion pair can be designed, screened or selected
according to the methods known in the art including the methods disclosed in
U.S. Patent
No. 5,679,548; Kang, et al., Virus Res., 49~2~:147-54 (1997); Dealwis, et al.,
Biochemistry,
34 43 :13967-73 (1995); and Hutchens, et al., .l. Chromatogr., 604(1):125-32
(1992).
U.S. Patent No. 5,679,548 discloses a method for producing a metal binding
site in a
polypeptide capable of binding a preselected metal ion-containing molecule,
the step of
inducing mutagenesis of a complementarity determining region (CDR) of an
immunoglobulin
heavy or light chain gene, wherein said mutagenesis introduces a metal binding
site, by
amplifying the CDR of said gene by a primer extension reaction using a primer
oligonucleotide, said oligonucleotide comprising: a) a 3' terminus and a 5'
terminus comprising;
b) a nucleotide sequence at said 3' terminus complementary to a first
framework region of said
heavy or light chain immunoglobulin gene; c) a nucleotide sequence at said 5'
terminus
complementary to a second framework region of said heavy or light chain
immunoglobulin
gene; and d) a nucleotide sequence between said 3' terminus and 5' terminus
according to the
formula; [NNS]a, wherein N is independently any nucleotide, S is G or C, and a
is from 3 to
about 50, and said 3' and 5' terminal nucleotide sequences having a length of
about 6 to 50
nucleotides, and sequences complementary thereto.
U.S. Patent No. 5,679,548 also describes a method for producing a metal
binding site in
a polypeptide capable of binding a preselected metal ion-containing molecule,
the step of
inducing mutagenesis of a complementarity determining region (CDR) of an
immunoglobulin
heavy or light chain gene by amplifying the CDR of said gene by a primer
extension reaction
using a primer oligonucleotide, said oligonucleotide comprising: a) a 3'
terminus and a 5'
terminus; b) a nucleotide sequence at said 3' terminus complementary to a
first framework
region of said heavy or light chain immunoglobulin gene; c) a nucleotide
sequence at said 5'
terminus complementary to a second framework region of said heavy or light
chain
immunoglobulin gene; and d) a nucleotide sequence between 3' terminus and S'
terminus
according to the formula: -X-[NNK]a X-[NNK]-X, wherein N is independently any
nucleotide,
K is G or T, X is a trinucleotide encoding a native amino acid residue coded
by said
immunoglobulin gene and a is from 3 to about 50, and said 3' and 5' terminal
nucleotide
sequences having a length of about 6 to 50 nucleotides, and sequences
complementary thereto.
Preferably, the immunoglobulin to be mutagenized is a human immunoglobulin,
the CDR is
CDR3, the mutagenizing oligonucleotide has the formula: 5'-
GTGTATTATTGTGCGAGA[NNS]aTGGGGCCAAGGGACCACG-3' (SEQ ID No. 86), and
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 129 -
the preselected metal ion-containing molecule is magnetite, copper(II),
zinc(II), lead(II),
cerium(III), or iron(III).
Kang, et al., Virus Res., 4:147-54 (1997) isolated human papillomavirus (HPV)
type 18 E7 gene by polymerase chain reaction (PCR) amplification from tissues
of Korean
cervical cancer patients and cloned into a plasmid vector, pET-3a, for the
expression of
recombinant E7 protein (rE7) in Escherichia.coli. The rE7 protein was purified
to the
homogeneity and its purity was confirmed by HPLC. The purified protein was
analyzed for the
metal-binding properties by UV spectroscopy and it was shown that two Cd2+ or
Zn2+ ions bind
to one E7 protein by the metal-sulfur ligand formation via two Cys-X-X-Cys
motifs in E7
protein. When the change of intrinsic fluorescence of tryptophan residue was
analyzed for rE7-
Zn complex, the blue shift of emission wavelength and the decrease in maximum
intensity of
emission were observed compared with rE7. These results suggest that Zn2+-
bound rE7 has
undergone conformational change, in which a tryptophan residue located in the
second Cys-X-
X-Cys motif was moved into solvent-inaccessible or hydrophobic environment.
Dealwis, et al., Biochemistry, 34 43 :13967-73 (1995) present the refined
crystal
structures of three different conformational states of the Asp153-->Gly mutant
(D153G) of
alkaline phosphatase (AP), a metalloenzyme from Escherichia coli. The apo
state is induced in
the crystal over a 3 month period by metal depletion of the holoenzyme
crystals. Subsequently,
the metals are reintroduced in the crystalline state in a time-dependent
reversible manner
without physically damaging the crystals. Two structural intermediates of the
holo form based
on data from a 2 week (intermediate I) and a 2 month soak (intermediate II) of
the apo crystals
with Mg2+ and Znz+ have been identified. The three-dimensional crystal
structures of the apo
(R = 18.1%), intermediate I (R = 19.5%), and intermediate II (R = 19.9%) of
the D153G
enzyme have been refined and the corresponding structures analyzed and
compared. Large
conformational changes that extend from the mutant active site to surface
loops, located 20 A
away, are observed in the apo structure with respect to the holo structure.
The structure of
intermediate I shows the recovery of the entire enzyme to an almost native-
like conformation,
with the exception of residues Asp 51 and Asp 369 in the active site and the
surface loop (406
410) which remains partially disordered. In the three-dimensional structure of
intermediate II,
Asp 51 and Asp 369 are essentially in a native-like conformation, but the main
chain of
residues 406-408 within the loop is still not fully ordered. The D153G mutant
protein exhibits
weak, reversible, time dependent metal binding in solution and in the
crystalline state.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 130 -
Hutchens, et al., J. Chromatogr., 604(1):125-32 (1992) prepared synthetic
peptides
representing metal-binding protein surface domains from the human plasma metal
transport
protein known as histidine-rich glycoprotein (HRG) to evaluate biologically
relevant peptide-
metal ion interactions. Three synthetic peptides, representing multiples of a
5-residue repeat
sequence (Gly-His-His-Pro-His) (SEQ ID No. 87) from within the histidine- and
proline-rich
region of the C-terminal domain were prepared. Prior to immobilization, the
synthetic peptides
were evaluated for identity and sample homogeneity by matrix-assisted UV laser
desorption
time-of flight mass spectrometry (LDTOF-MS). Peptides with bound sodium and
potassium
ions were observed; however, these signal intensities were reduced by
immersion of the sample
probe tip in water. Mixtures of the three different synthetic peptides were
also evaluated by
LDTOF-MS after their elution through a special immobilized peptide-metal ion
column
designed to investigate metal ion transfer. It was found that LDTOF-MS to be a
useful new
method to verify the presence of peptide-bound metal ions.
In addition, the protein/metal binding pairs, which are known (see, e.g.,
DiDonato, et
al., Adv. Exp. Med. Biol., 448:165-73 (1999) (copper/copper binding domain
from the Wilson
disease copper transporting ATPase (ATP7B)); Buchko, et al., Biochem Biophis.
Res.
Commun., 254(1):109-13 (1999) (Zn2+/Xenopus laevis nucleotide excision repair
protein XPA);
Lai, et al., Biochemistry, 37 48 :7005-15 (1998) (Zn2+/hdm2 RING finder
domain); Mitterauer,
et al., Biochemistry, 37(46):16183-91 (1998) (The C2 catalytic domain of
adenylyl cyclase
contains the second metal ion (Mn2+) binding site); Hess, et al., Protein
Sci., x:1970-5
(1998) (Zn2+/Human nucleotide excision repair protein XPA); Goedken, et al.,
Proteins,
33:135-43 (1998) (Mg2+ and Mn2+/ribonuclease H domain of Moloney marine
leukemia
virus reverse transcriptase); Chang, et al., Protein Eng., 11(1):41-6 (1998)
(beta-domain of
metallothionein); Champeil, et al., J. Biol. Chem., 273 12 :6619-31 (1998)
(cytosolic portion of
sarcoplasmic reticulum Ca2+-ATPase); Bavoso, et al., Biochem. Biophys. Res.
Commun.,
242(2):385-9 (1998) (zinc forger peptide containing the Cys-X2-Cys-X4-His-X4-
Cys domain
encoded by the Drosophila Fw-element); Gitschier, et al., Nat. Struct. Biol.,
5 1 :47-54 (1998)
(metal-binding domain from the Menkes copper-transporting ATPase); Gadhavi,
FEBS Lett.,
417 1 :145-9 (1997) (Zn2+/ion binding site in the DNA binding domain of the
yeast
transcriptional activator GAL4); Roehm, et al., Biochemistry, 36(33):10240-5
(1997)
(Zn2+/RING finger domain of BRCA1); Dalton, et al., Mol. Cell Biol.,
17~5~:2781-9 (1997)
(metal response element-binding transcription factor 1 DNA binding involves
zinc interaction
with the zinc finger domain); Essen, et al., Biochemistry, 310):2753-62 (1997)
(Ca2+/A
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 131 -
ternary metal binding site in the C2 domain of phosphoinositide-specific
phospholipase C-
deltal); Curtis, et al., EMBO J., 1:834:43 (1997) (Zn2+/CCHC metal-binding
domain in
Nanos); Worthington, et al., Proc. Natl. Acad. Sci. USA, 93(24):13754-9 (1996)
(zinc-binding
domain of Nup475); Mahadevan, et al., Biochemistry, 34~7~:2095-106 (1995)
(Ba2+, Ca2+,
Mg2+, Mnz+, Ni2+, Zn2+/A divalent metal ion binding site in the kinase insert
domain of the
alpha-platelet-derived growth factor receptor); Pan, et al., Biochem. Biophys.
Res. Commun.,
202(1):621-8 (1994) (alpha and beta domains of mammalian metallothionein);
Borden, et al.,
FEBSLett., 335 2 :255-60 (1993) (Cu2+, Zn2+/cysteine/histidine-rich metal
binding domain
from Xenopus nuclear factor XNF7); Chauhan, et al., J. Bacteriol.,
175(22):7222-7 (1993)
(Mg2+/Bradyrhizobium japonicum delta-aminolevulinic acid dehydratase is metal-
binding
domain); Knegtel, et al., Biochem. Biophys. Res. Commun., 192 2 :492-8 (1993)
(Zn2+/metal
coordination in the human retinoic acid receptor-beta DNA binding domain);
Spencer, et al.,
Biochem. J., 290 1 :279-87 (1993) (Co2+, Mgz+, Zn2+/5-aminolaevulinic acid
dehydratase from
Escherichia coli reactive thiols at the metal-binding domain); Mau, et al.,
Protein Sci.,
1:1403-12 (1992) (Zn2+/GAL4 DNA-binding domain); Vaughan, et al., Virology,
189(1):377-84 (1992) (Zn2+/The herpes simplex virus immediate early protein
ICP27 metal
binding domain); Boese, et al., J. Biol. Chem., 266(26):17060-6 (1991)
(Mgz+/Aminolevulinic
acid dehydratase in pea metal-binding domain); Hutchens, et al., J. Biol.
Chem.,
264(29):17206-12 (1989) (Cu2+, Ni2+, Znz+/DNA-binding estrogen receptor);
Stillman, et al.,
Biochem. J., 262 1 :181-8 (1989) (Cd2+ and Zn2+/rabbit liver metallothionein
2); Freedman, et
al., Nature, 334(6182):543-6 (1988) (Cd2+ and Zn2+/metal coordination sites
within the
glucocorticoid receptor DNA binding domain); Stillman, et al., J. Biol. Chem.,
263(13):6128-
33 (1988) (Cd2+ and Zn2+/metallothionein); and Corson, et al., Biochemistry,
2:1817-26
(1986) (Caz+/calcium-binding proteins C-terminal alpha-helix of a helix-loop-
helix metal-
binding domain)) can be used in the present system.
Among the preferred pairs, are the following metal binding sequence/metal ion
pairs
(see, U.S. Patent No. 5,679,548) set forth in the following Table 7.
Table 7. Examplcs of Metal Ion Binding Sequence/Metal Ion Pairs
Metal Ion Metal Ion Binding Sequence SEQ ID NO.
Mg(II) SerArgArgSerArgHisHisProArgMetTrpAsnGlyLeuAspVa1 88
GlyArgPheLysArgValArgAspArgTrpValValIlePheAspPhe 89
GlyValAlaArgSerLysLysMetArgGlyLeuTrpArgLeuAspVa1 90
GlyLeuAlaValArgSerLysArgGlyArgPhePheLeuPheAspVa1 91
Cu(II) GIyArgValHisHisHisSerLeuAspVa1 92
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 132 -
Metal Metal Ion Binding Sequence SEQ ID
Ion NO.
SerTrpLysHisHisAlaHisTrpAspVal 93
GlySerTrpAspHisArgGlyCysAspGly 94
GIyHisHisMetTyrGlyGlyTrpAspHis 95
GIyHisTrpGIyArgHisSerLeuAspThr 96
GlyHisIleLeuHisHisGlnLeuAspLeu 97
SerSerGlnArgLeuMetLeuGlyAspAsn 98
SerHisHisGlyHisHisTyrLeuAsnHis 99
GlyLysLeuMetMetSerTrpCysArgAspThrGluGlyCysAspHis100
GlyAspThrHisArgGlyHisLeuArgHisHisLeuProHisAspTrp1O1
GlyTrpGlyLeuTrpMetLysProPheValTrpArgAlaTrpAspMet102
Zn(II) GIyArgValHisHisHisSerLeuAspVa1 103
SerHisThrHisAlaLeuProLeuAspPhe 104
GlyGlnSerSerGlyGlyAspThrAspAsp 1 OS
GlyGlnTrpThrProArgGIyAspAspPhe 106
GlyArgCysCysProSerSerCysAspGlu 107
GlyProAlaLysHisArgHisArgHisValGlyGlnMetHisAspSer108
Pb(III) GlyAsnLeuArgArgLysThrSerAspIle 109
GlyGluSerAspSerLysArgGluAspGly 110
GIyGlyProSerLeuAlaVaIGIyAspTrp 111
GlyProLeuGlnHisThrTyrProAspTyr 112
GlyTrpLysValThrAlaGluAspSerThrGluGlyLeuPheAspLeu113
GIyThrArgValTrpArgValCysGlnTrpAsnHisGluGluAspGly114
GlyGluTrpTrpCysSerPheAlaMetCysProAlaArgTrpAspPhe115
GlyAspThrIlePheGlyValThrMetGlyTyrTyrAlaMetAspVa1116
Ce(III) GlyGlnVaIMetGlnGluLeuGlyAspAla 117
GIyLeuThrGluGlnGlnLeuGInAspGly 118
GlyTyrSerTyrSerValSerProAspAla 119
GlyArgLeuGlyLeuValMetThrAspGlu 120
SerThrTrpProGlyArgGlnArgLeuGlyGlnAlaLeuSerAspSer121
GlyTyrGIuLeuSerTrpGlyValAspGlnGlnGluTrpTrpAspIle122
GlyProValArgGlyLeuAspGlnSerLysGlyValArgTyrAspAsn123
GlyLeuSerGlnHisIleValSerGIuThrGlnSerSerGIyAspLeu124
GlyLeuGluSerLeuLysValLeuGlyVaIGlnLeuGlyGlyAspLeu125
GlyAsnMetIleLeuGIyGlyProGlyCysTrpSerSerAlaAspIle126
GlyCysTrpAsnValGlnArgLeuValValTyrHisProProAspGly127
GlyPheGluValThrCysSerTrpPheGlyHisTrpGlyArgAspSer128
Fe(III) SerAlaSerMetArgSerAlaIleGlyLeuTrpArgThrMetAspTyr129
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-133-
Metal Ion Metal Ion Binding Sequence SEQ ID
NO.
GIyAspArgGluIlePheHisMetGlnTrpProLeuArgValAspVa1130
SerGlnAsnProGlnGlnValCysGlyValArgCysGlyGlnAspLys131
GlyAsnArgLeuSerSerGlyHisLeuLeuLysGlnGlyGlnAspGly132
GlyGlySerAspTrpGlnIleGIyAlaCysCysArgGluAspAspLeu133
GlyMetVaISerMetMetGlyGlnSerArgProThrGlnCysAspCys134
GlyValIleLysTrpIleArgArgTrpValArgThrAlaArgAspVa1135
GlyTrpPheTrpArgLeuLeuProThrProArgAlaProSerAspVa1136
i. Other facilitating agents
Facilitating agents can be derived from an enzyme, a transport protein, a
nutrient or
storage protein, a contractile or motile protein, a structural protein, a
defense protein, a
regulatory protein, or a fluorescent protein. Exemplary of such other
fragments are those
derived from an enzyme such as a peroxidase, a urease, an alkaline
phosphatase, a luciferase
and a glutathione S-transferase.
1) Peroxidase
Any peroxidase can be used in the present system. More preferably, a
horseradish
peroxidase is used. For example, the horseradish peroxidases with the
following GenBank
accession Nos. can be used: E01651; D90116 (prxC3 gene); D90115 (prxC2 gene);
J05552
(Synthetic isoenzyme C(HRP-C)); S 14268 (neutral); OPRHC (C 1 precursor);
S00627 (C 1 C
precursor); JH0150 (C3 precursor); S00626 (C1B precursor); JH0149 (C2
precursor);
CAA00083 (Armoracia rusticana); and AAA72223 (synthetic horseradish
perioxidase
isoenzyme C (HRP-C)).
2) urease
Any urease can be used in the present system. For example, the ureases with
the
following GenBank accession Nos. can be used: AF085729 (Ureaplasma urealyticum
serovar);
AF056321 (Actinomyces naeslundii); AF095636 fYersinia pesos); AF006062
(Filobasidiella
neoformans var. neoformans (URE1)); U81509 (Coccidioides immitis urease);
AF000579
(Bordetella bronchiseptica); U352248 (Streptococcus salivarius); U33011
(Mycobacterium
tuberculosis); U89957 (Actinobacillus pleuropneumoniae urease operon
(ureABCXEFGD);
D14439 (Thermophilic Bacillus); L40490 (Ureaplasma urealyticum T960 urease);
L40489
(Ureaplasma urealyticum strain 7); U40842 (Yersinia pseudotuberculosis);
M65260 (Canavalia
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 134 -
ensiformis); U29368 (Bacillus pasteurii ure operon); L25079 (Heliobacter
heilmannii unease);
L24101 (Yersinia enterocolitica); M31834 (P.mirabilis unease operon); M36068
(K.aerogenes);
L07039 (Klebsiella pneumoniae); M60398 (H.pylori); L03308 (E.coli unease gene
cluster);
L03307 (E.coli unease gene cluster).
3) Alkaline phosphatase
Any alkaline phosphatase can be used in the present system. For example, the
alkaline
phosphatases encoded by nucleic acids with the following GenBank accession
Nos. can be
used: AB013386 (Bombyx mori s-Alp soluble alkaline phosphatase); AF154110
(Enterococcus
faecalis (phoZ); M13077 (Human placental); AF052227 (Bos taurus intestinal);
AF052226
(Bos taurus intestinal); AF079878 (Thermus sp. (TAP)); AF047381 (Pseudomonas
aeruginosa
(phoA)); U49060 (Bacillus subtilis (phoD)); J03930 (Human intestinal (ALPI));
J03252
(Human alkaline (ALPP)); U19108 (Gallus tissue-nonspecific); M13345 (E coli);
U31569
(Felis catus (alpl)); L36230 (Zymomonas mobilis (phoD)); M19159 (Human
placental heat-
stable (PLAP-1)); M12551 (Human placental (PLAP)); M31008 (Human intestinal);
J04948
IS (Human (ALP-1); J03572 (Rat); M61705 (Mouse intestinal (IAP); M61704 (Mouse
embryonic); M61706 (Mouse (AP) pseudogene); M21134 (S.cerevisiae (rALPase));
L07733
(Cow intestinal (IAP)); M18443 (Bovine); M77507 (Synechococcus sp. atypical);
M33965
(S.marcescens (phoA)); M33966 (E.fergusonii (phoA)); M29670 (E.coli (phoA));
M29669
(E.coli (phoA)); M29668 (E.coli (phoA)); M29667 (E.coli (phoA)); M29666
(E.coli (phoA));
M29665 (E.coli (phoA)); M29664 (E.coli (phoA)); M29663 (E.coli (phoA)); M23549
(Bacillus
subtilis (phoP gene, 3' end and phoR gene); M16775 (B.subtilis phoP); M33634
(B.subtilis
(phoAIII); L27993 (Neurospora crassa); U02550 (Bacillus subtilis (phoA)).
4) Luciferase
Any luciferase can be used in the present system. Numerous luciferases are
available
and have been cloned. For example, the luciferases encoded by nucleic acids
with the following
GenBank accession Nos. can be used: AH007711 (Streptomyces clavuligerus
(cvm5));
AF124929 (cvm5); U43958 (Cloning vector pRcCMV-luc luciferase gene); M90092
(Xenorhabdus luminescens (luxA)); AF093688 (MMTV-luciferase reporter vector
pHH Luc
*SA *PS); AF093687 (MMTV-luciferase reporter vector PHH Luc *SA); AF093686
(MMTV-
luciferase reporter vector pHH Luc); AF093685 (Luciferase reporter vector pXP2
*SA *PS);
AF093684 (Luciferase reporter vector pXP2 *SA); AF093683 (Luciferase reporter
vector
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-135-
pXPI); AF093682 (Luciferase reporter vector pXP2); U40374 (Luciferase reporter
gene shuttle
vector pMH30); AF003893 (Gonyaulax polyedra luciferase); L39928 (Pyrocoelia
miyako
(clone pB-PmL41); L39929 (Hotaria parvula (clone pB-Hp); AF085332 (Gonyaulax
polyedra);
U89490 (Vargula hilgendorfii); AF027129 (Eukaryotic luciferase expression
vector
pCMVtkLUC+); AF027128 (Eukaryotic luciferase expression vector ptkLUC+);
AF027127
(Eukaryotic luciferase expression vector pTATALUC+); AF027126 (Eukaryotic
luciferase
expression vector pLUC+); U31240 (Photuris pennsylvanica); D25416 (Firefly
clone pPFL7);
D25415 (Firefly clone pPFLl9); U84006 (Expression vector pBSII-LUCINT firefly
luciferase
(LUCINT); U55819 (Plasmid pRL765 with transposon Tn5 and luciferase (luxA and
luxB)
genes); U55385 (Plasmid pRL1063a with transposon Tn5 and luciferase (luxA and
luxB)
genes); U51019 (Luciola lateralis); U49182 (Luciola lateralis); U49181
(Luciola lateralis);
M36597 (K. alfredi symbiont); U47298 (Cloning vector pGL-3-Promoter firefly
luciferase
(luc+) gene); U47297 (Cloning vector pGL3-Enhancer firefly luciferase (luc+)
gene); U47296
(Cloning vector pGL3-Control firefly luciferase (luc+) gene); U47295 (Cloning
vector pGL3-
Basic firefly luciferase (luc+) gene); U47123 (Cloning vector pSP-luc+NF,
luciferase cassette
fusion vector); U47122 (Cloning vector pSP-luc+, Luciferase cassette vector);
M10961
(V.harveyi (luxA and luxB); M65067 (Photobacterium phosphoreum (luxA and
luxB); M62917
(Xenorhabdus luminescens (luxA, luxB, luxC, and luxD); M25666
(V.hilgendorfii); M63501
(Renilla reniformis); M15077 (P.pyralis (firefly)); M26194 (Luciola cruciata);
M55977
(X.luminescens (luxA and luxB)); M90093 (Xenorhabdus luminescens (luxA) and
(luxB)
(luxE)); U03687 (Photinus pyralis modified luciferase gene).
5) Glutathione S-transferase
A glutathione S-transferase (GST), more preferably a Schistosoma japonicum
glutathione S-transferase, can be included in the conjugate. GST occurs
naturally as a 26 kDa
protein which can be expressed in E coli with full enzymatic activity.
Conjugates that contain
the full length GST also demonstrate GST enzymatic activity and can undergo
dimerization as
observed in nature (Parker, et al., J. Mol. Biol., 213:221 (1990); Ji, et al.,
Biochemistry,
31:10169 (1992); and Maru, et al., J. Biol. Chem., 271:15353 (1996)). The
crystal structure of
recombinant Schistosoma japonicum GST from pGEX vectors has been determined
(McTigue,
et al., J. Mol. Biol., 246:21 (1995)) and matches that of the native protein.
Conjugates that
contain a GST can be readily purified.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 136 -
For example, fusion proteins are easily purified from bacterial lysates by
affinity
chromatography using Glutathione Sepharose 4B contained in the GST
Purification Modules
(Amersham Pharmacia Biotech, Inc.). Cleavage of the desired protein from GST
is achieved
using a site-specific protease whose recognition sequence is located
immediately upstream
from the multiple cloning site on the pGEX plasmids. Fusion proteins can be
detected using a
colorimetric assay or immunoassay provided in the GST Detection Module, or by
Western
blotting with anti-GST antibody. The system has been used successfully in many
applications
such as molecular immunology (Toye, et al., Infect. Immun., 58:3909 (1990)),
the production of
vaccines (Fikrig, et al., Science, 250:553 (1990); and Johnson, et al.,
Nature, 338:585 (1989))
and studies involving protein-protein (Kaelin, et al., Cell, 64:521 ( 1991 ))
and DNA-protein
(Kaelin, et al., Cell, 65:1073 ( 1991 )) interactions.
Any glutathione S-transferase is contemplated. For example, the glutathione S-
transferase encoded by nucleic acid with the following GenBank accession Nos.
can be used:
[AF112567], Fasciola gigantica; [M77682], Fasciola hepatica; [AB016426], Cavia
porcellus;
[AF144382], Arabidopsis thaliana; [AF133251], Gallus; [AB021655], Issatchenkia
orientalis;
[AF133268], Manduca sexta; [AF125273], Homo Sapiens tissue-type skeletal
muscle;
[AF125271], Homo Sapiens tissue-type pancreas; [AB026292], Sphingomonas
paucimobilis;
[AB026119], Oncorhynchus nerka; [U49179], Bos taurus; [AF106661], Rattus
norvegicus
(GstYb4); [L15387], Gallus class-alpha; [AF051318], Clonorchis sinensis;
[AF101269],
Echinococcus granulosus; [AF077609], Boophilus microplus; [AA956087], Homo
sapiens
microsomal; [AF004358], Aegilops squarrosa; [AF109714], Triticum aestivum;
[U86635],
Rattus norvegicus glutathione; [AF111428], Drosophila melanogaster microsomal;
[AF111426], Drosophila melanogaster microsomal; [AF071163], Anopheles gambiae;
[AF071162], Anopheles gambiae; [AF071161 ], Anopheles gambiae; [AF071160],
Anopheles
gambiae; [D10524], Nicotiana tabacum; [AF062403), Oryza sativa; [U77604], Homo
Sapiens
microsomal (MGST2); [U30897], Human (Plb); [U62589), Human (GSTpIc); [U42463],
Coccomyxa sp. PA; [AF001779], Sphingomonas paucimobilis strain epa505;
[U51165],
Cycloclasticus oligotrophus (XYLK); [AF025887], Homo Sapiens (GSTA4);
[U66342],
Plutella xylostella; [AF051238], Picea mariana (Sb52); [AF051214], Picea
mariana (Sbl8);
[AF079511], Mesembryanthemum crystallinum clone R6-R37; [D10026], Rattus
norvegicus
Yrs-Yrs; [AF048978], Glycine max 2,4-D inducible (GSTa); [AF043105], Homo
Sapiens
(GSTM3); [AF057172], Homo Sapiens (GSTT2P); [U21689], Human; [AH006027], Homo
sapiens (GSTT2); [AF057176], Homo sapiens (GSTT2); [AF050102], Oryza sativa
(GST1);
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 137 -
[AF044411], Schistosoma japonicum; [U87958], Culicoides variipennis (CVGST1);
[AF026977], Homo sapiens microsomal (MGST3); [AF027740], Homo Sapiens
microsomal
(MGST1L1); [AF005928], Echinococcus granulosus; [AF001103], Pseudomonas
(phnC);
[AF010241], Caenorhabditis elegans (CeGST3); [AF010240], Caenorhabditis
elegans
(CeGST2); [AF010239], Caenorhabditis elegans (CeGSTl); [AF002692], Solanum
commersonii (GST1); [L38503], Homo Sapiens (GSTT2); [M97937], E. colilS.
japonicium;
[L29427], Rat GST-P gene; [M14654], Schistosoma japonicum Sj26 antigen;
[AB000884], Sus
scrofa; [D44465], Arabidopsis thaliana; [D17673], Arabidopsis thaliana;
[D17672],
Arabidopsis thaliana; [U78784], Anopheles dirus; [U71213], Human microsomal;
[U70672],
Arabidopsis thaliana; [U24428], Mus musculus; [U43126], Naegleria fowleri;
[X14233],
D.melanogaster (GST); [L32092], Manduca sexta; [L32091], Manduca sexta;
[U30489],
Arabidopsis thaliana; [M24889], Artificial maize; [L05915], Dianthus
caryophyllus; [M15872],
Human; [L23766], Oryctolagus cuniculus; []03679], Solanum tuberosum; [U12472],
Human
(GST phi); [U15654], Mus musculus; [M24485], Homo sapiens (GSTP1); [L28771],
Onchocerca volvulus; [M14777], Human; [M16594], Human; [M21758], Human;
[]03914],
Rat; [K01932], Rat liver; []02810], Rat prostate; [M25891], Rat; [M11719], Rat
liver;
[M28241], Rat; []03752], Rat; [M73483], Mouse (GST Yc); []04696], Mouse (GSTS-
S);
[]04632], Mouse (GST1-1); [M59772], M.auratus; [L20466], Chinese hamster;
[M25627],
Human liver; []03746], Human (SEQ ID No. 137); [M16901], Maize; [M64268],
Dianthus
caryophyllus; [L11601], Arabidopsis thaliana; [L07589], Arabidopsis thaliana;
[M74529],
Oryctolagus cuniculus; [M74528], Oryctolagus cuniculus; [M98271 ], Schistosoma
mansoni 28
kDa; [L23126], Lucilia cuprina; [M95198], Drosophila melanogaster; [L26544],
Methylophilus
sp.; [U14753], Dirofilaria immitus; [U12679], Zea mays; [L02321], Human
(GSTMS);
[L15386], Chicken.
In addition, commercially available Glutathione S-transferase (GST) gene
fusion system
can be used. For example, the Glutathione S-transferase (GST) Gene Fusion
System
(Amersham Pharmacia Biotech, Inc.) can be used. The system from Amersham
Pharmacia
Biotech, Inc. is an integrated system for the expression, purification and
detection of fusion
proteins produced in E coli. The system includes three primary components:
pGEX plasmid
vectors, various options for GST purification and a variety of GST detection
products. A series
of site-specific proteases complements the system. The pGEX plasmids are
designed for
inducible, high-level intracellular expression of genes or gene fragments as
fusions with
Schistosoma japonicum GST (Smith and Johnson, Gene, 67:31 (1988)). All pGEX
Vectors
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 138 -
(GST Gene fusion) offer: 1 ) A tac promoter for chemically inducible, high-
level expression; 2)
an internal lac Ig gene for use in any E. coli host; 3) very mild elution
conditions for release of
fusion proteins form the affinity matrix, thus minimizing effects on
antigenicity and functional
activity; and 4) PreScission, thrombin or factor Xa protease recognition sites
for cleaving the
desired protein from the fusion product.
The GST Detection Module from Amersham Pharmacia Biotech, Inc. can be used for
identification of GST fusion proteins using either a biochemical or
immunological assay. In
the biochemical assay, glutathione and 1-chloro-2-4-dinitrobenzene (CDNB)
serve as substrates
for GST to yield a yellow product detectable at 340 nm (Habig, et al., J.
Biol. Chem., 249:7130
(1974)). An affinity-purified goat anti-GST polyclonal antibody suitable for
Western blots is
used in the immunoassay.
The GST 96-Well Detection Module from Amersham Pharmacia Biotech, Inc.
contains
five microtitre strip plates, horseradish perioxidase (HRP) conjugated anti-
GST antibody and
recombinant GST protein. The wells of each plate are coated with purified anti-
GST antibody
to capture GST fusion proteins and are preblocked to provide a low background.
HRP
conjugated antibody enables sensitive detection of GST proteins.
The anti-GST antibody supplied in the system from Amersham Pharmacia Biotech,
Inc.
is a polyclonal antibody purified from the sera of goats immunized with
purified schistosomal
glutathione S-transferase (GST). Because of its polyclonal nature, it can
recognize more than
one epitope on GST, thereby improving its capacity for recognizing GST fusion
proteins even
if some binding sites are masked due to recombinant protein folding.
Factor Xa can be used for site-specific separation of the GST affinity tag
from proteins
expressed using pGEX X vectors. Factor Xa enables the site-specific cleavage
of fusion
proteins containing an accessible Factor Xa recognition sequence. It can be
used either
following affinity purification or while fusion proteins are bound to
Glutathione Sepharose 4B.
Factor Xa, purified from bovine plasma, is used to digest fusion proteins
prepared from pGEX
vectors containing the recognition sequence for factor Xa (pGEX-3X, pGEX-SX-1,
pGEX-SX-
2 and pGEX-SX-3). It specifically cleaves following the t°trapeptide
Ile-Glu-Gly-Arg (SEQ ID
No. 139) (Nagai and Thragersen, Nature, 309:810 (1984); and Nagai and
Thragersen, Methods
Enrymol., 153:461 (1987)). In the system from Amersham Pharmacia Biotech,
Inc., one unit of
Factor Xa cleaves ~ 90% of 100 ~g of a test GST fusion protein when incubated
in 1 mM
CaCl2, 100 mM NaCI and 50 mM Tris-HCl (pH 8.0) at 22°C for 16
hours.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 139 -
PreScission protease can be used for site-specific separation of the GST
affinity tag
from proteins expressed using pGEX-6P vectors. It enables the low-temperature
cleavage of
fusion proteins containing the PreScission Protease recognition sequence. It
can be used either
following affinity purification or while fusion proteins are bound to
Glutathione Sepharose 4B.
PreScission Protease is a genetically engineered fusion protein containing
human rhinovirus 3C
protease and GST (Walker, et al., BiolTechnology, 12:601 (1994)). This
protease was
specifically designed to facilitate removal of the protease by allowing
simultaneous protease
immobilization and cleavage of GST fusion proteins produced from pGEX-6P
vectors (pGEX-
6P-1, pGEX-6P-2, and pGEX-6P-3). PreScission Protease specifically cleaves
between the Gln
and Gly residues of the recognition sequence of LeuGluValLeuPheGln/GlyPro (SEQ
ID
No. 140) (Cordingley, et al., J. Bio. Chem., 265:9062 (1990)). In the system
from Amersham
Pharmacia Biotech, Inc., one unit of PreScission protease will cleave ~ 90% of
100 ~g of a test
GST-fusion protein in 50 mM Tris-HCI, 150 mM NaCI, 1 mM EDTA, 1 mM DTT, pH 7.0
at
5°C for 16 hours.
Thrombin can be used for site-specific separation of the GST affinity tag from
proteins
expressed using pGEX T vectors. It enables the site-specific cleavage of
fusion proteins
containing an accessible thrombin recognition sequence. It is purified from
bovine plasma;
functionally free of other clotting factors, plasminogen and plasmin. It can
be used either
following affinity purification or while fusion proteins are bound to
Glutathione Sepharose 4B.
Thrombin is used to digest fusion proteins prepared from pGEX vectors
containing the
recognition sequence for thrombin (pGEX-1~T, pGEX-2T, pGEX-2TK, pGEX-4T-1,
pGEX-
4T2 and pGEX-4T-3). In the system from Amersham Pharmacia Biotech, Inc., one
unit of
Thrombin cleaves ~ 90% of 100 ~,g of a test GST fusion protein when incubated
in lx PBS at
22°C for 16 hours.
6) Defense proteins
The conjugates can contain defense protein, such as an antibody. Any antibody,
including polyclonal, monoclonal, single chain or Fab fragments, can b2 used.
7) Fluorescent moieties
The conjugates can contain a fluorescent moiety, such as a green, a blue or a
red
fluorescent protein. Any green, blue or red fluorescent protein can be used in
the present
system. For instance, the green fluorescent proteins encoded by nucleic acids
with the
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 140 -
following GenBank accession Nos. can be used: U47949 (AGP1); U43284; AF007834
(GFPuv); U89686 (Saccharomyces cerevisiae synthetic green fluorescent protein
(cox3::GFPm-3) gene); U89685 (Saccharomyces cerevisiae synthetic green
fluorescent protein
(cox3::GFPm) gene); U87974 (Synthetic construct modified green fluorescent
protein GFPS-
ER (mgfp5-ER)); U87973 (Synthetic construct modified green fluorescent protein
GFPS
(mgfp5)); U87625 (Synthetic construct modified green fluorescent protein GFP-
ER (mfgp4-
ER)); U87624 (Synthetic construct green fluorescent protein (mgfp4) mRNA));
U73901
(Aequorea victoria mutant 3); U50963 (Synthetic); U70495 (soluble-modified
green
fluorescent protein (smGFP)); U57609 (enhanced green fluorescent protein
gene); U57608
(enhanced green fluorescent protein gene); U57607 (enhanced green fluorescent
protein gene);
U57606 (enhanced green fluorescent protein gene); U55763 (enhanced green
fluorescent
protein (egfp); U55762 (enhanced green fluorescent protein (egfp); U55761
(enhanced green
fluorescent protein (egfp); U54830 (Synthetic E. coli Tn3-derived transposon
green fluorescent
protein (GF); U36202; U36201; U19282; U19279; U19277; U19276; U19281; U19280;
U19278; L29345 (Aequorea victoria); M62654 (Aequorea victoria); M62653
(Aequorea
victoria); AAB47853 ((U87625) synthetic construct modified green fluorescent
protein (GFP-
ER)); AAB47852 ((U87624) synthetic construct green fluorescent protein).
Similarly, the blue fluorescent proteins encoded by nucleic acids with the
following
GenBank accession Nos. can be used: U70497 (soluble-modified blue fluorescent
protein
(smBFP); 1 BFP (blue variant of green fluorescent protein); AAB 16959 (soluble-
modified blue
fluorescent protein).
Also similarly, the red fluorescent proteins encoded by nucleic acids with the
following
GenBank accession Nos. can be used: U70496 (soluble-modified red-shifted green
fluorescent
protein (smRSGFP); AAB16958 ((U70496) soluble-modified red-shifted green
fluorescent
protein).
2. Selection of Mutant analyte-binding enzymes
Any mutant analyte-binding enzyme described herein can be used in the
conjugate,
including any described herein. In a preferred embodiment, the mutant analyte-
binding enzyme
is a mutant SAH hydrolase that at least substantially retains its binding
affinity for Hcy or
SAH, but has attenuated catalytic activity. Exemplary mutant SAH hydrolases,
such as those
set forth above can be included in the conjugate.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 141 -
3. Nucleic acids, plasmids and cells
Isolated nucleic acid fragments encoding fusion proteins are provided. The
nucleic acid
fragment that encodes the fusion protein includes: a) nucleic acid encoding a
mutant analyte-
binding enzyme, wherein the mutant enzyme has binding affinity for the analyte
or an
immediate analyte enzymatic conversion product but has attenuated catalytic
activity; and b)
nucleic acid encoding a protein, peptide or effective fragment thereof that
facilitates: i) affinity
isolation or purification of the fusion protein; ii) attachment of the fusion
protein to a surface;
or iii) detection of the fusion protein. Preferably, the nucleic acid is DNA.
Plasmids for replication and vectors for expression that contain the nucleic
acid
fragments are also provided. Cells containing the plasmids and vectors are
also provided. The
cells can be any suitable host including, but are not limited to, bacterial
cells, yeast cells, fungal
cells, plant cells, insect cells and animal cells. The nucleic acids,
plasmids, and cells containing
the plasmids can be prepared according to methods known in the art including
any described
herein.
In a specific embodiment, a method for producing the above fusion proteins is
provided,
which method comprises growing cells containing a plasmid encoding the fusion
protein under
conditions whereby the fusion protein is expressed by the cell, and recovering
the expressed
fusion protein. Methods for expressing and recovering recombinant proteins are
well known in
the art (See generally, Current Protocols in Molecular Biology (1998) ~ 16,
John Wiley &
Sons, Inc.) and such methods can be used for expressing and recovering the
expressed fusion
proteins. Preferably, the recombinant expression and recovery methods
disclosed in Section
B.2. can be used.
The recovered fusion proteins can be isolated or purified by methods known in
the art
such as centrifugation, filtration, chromatograph, electrophoresis,
immunoprecipitation, etc., or
by a combination thereof (See generally, Current Protocols in Molecular
Biology (1998) ~ 10,
John Wiley & Sons, Inc.). Preferably, the recovered fusion protein is isolated
or purified
through affinity binding between the protein or peptide fragment of the fusion
protein and an
affinity binding moiety. As discussed in the above sections regarding the
construction of the
fusion proteins, any affinity binding pairs can be constructed and used in the
isolation or
purification of the fusion proteins. For example, the affinity binding pairs
can be protein
binding sequences/protein, DNA binding sequences/DNA sequences, RNA binding
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 142 -
sequences/RNA sequences, lipid binding sequences/lipid, polysaccharide binding
sequences/polysaccharide, or metal binding sequences/metal.
4. Immobilization and supports or substrates therefor
In certain embodiments, where the facilitating agents are designed for linkage
to
surfaces, recovered, isolated or purified conjugates, such as fusion proteins
can be attached to a
surface of a matrix material. Immobilization may be effected directly or via a
linker. The
conjugates may be immobilized on any suitable support, including, but are not
limited to,
silicon chips, and other supports described herein and known to those of skill
in the art. A
plurality of conjugates, which may contain the same or different or a variety
of mutant analyte
binding enzymes (substrate trapping enzymes) may be attached to a support,
such as an array
(i.e., a pattern of two, typically three or more) of conjugates on the surface
of a silicon chip or
other chip for use in high throughput protocols and formats.
It is also noted that the mutant analyte binding enzymes can be linked
directly to the
surface or via a linker without a facilitating agent linked thereto. Hence
chips containing arrays
of mutant analyte binding enzymes are contemplated.
For example, an isolated or purified fusion protein can be attached to the
surface of a
solid or insoluble support, such as a silicon chip, as the intact fusion
proteins. Alternatively,
the protein or peptide fragment portion can be cleaved off and the mutant
analyte-binding
enzyme be attached to the surface. The fusion protein can be cleaved by any
methods known in
the art such as chemical or enzymatic means. The cleavage means must be
compatible with the
linking sequence between the protein or peptide fragment portion and the
mutant analyte-
binding enzyme so that the cleavage is linker sequence specific and the
cleaved mutant enzyme
is functional, i.e., can be used as a substrate-trapping enzyme. Those skilled
in the art can
readily determine, if necessary, with empirical studies, which cleavage/linker
sequence pair to
be used. Many cleavage/linker sequence pairs are well known in the art. For
example, Factor
Xa can be used for site-specific separation of the GST affinity tag from
proteins expressed
using pGEX X vectors; PreScission protease can be used for site-specific
separation of the GST
affinity tag from proteins expressed using pGEX-6P vectors; and Thrombin can
be used for
site-specific separation of the GST affinity tag from proteins expressed using
pGEX T vectors.
The matrix material substrates contemplated herein are generally insoluble
materials
used to immobilize ligands and other molecules, and are those that are used in
many chemical
syntheses and separations. Such substrates, also called matrices, are used,
for example, in
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-143-
affinity chromatography, in the immobilization of biologically active
materials, and during
chemical syntheses of biomolecules, including proteins, amino acids and other
organic
molecules and polymers. The preparation of and use of matrices is well known
to those of skill
in this art; there are many such materials and preparations thereof known. For
example,
naturally-occurring matrix materials, such as agarose and cellulose, may be
isolated from their
respective sources, and processed according to known protocols, and synthetic
materials may
be prepared in accord with known protocols.
The substrate matrices are typically insoluble materials that are solid,
porous,
deformable, or hard, and have any required structure and geometry, including,
but not limited
to: beads, pellets, disks, capillaries, hollow fibers, needles, solid fibers,
random shapes, thin
films and membranes. Thus, the item may be fabricated from the matrix material
or combined
with it, such as by coating all or part of the surface or impregnating
particles.
Typically, when the matrix is particulate, the particles are at least about 10-
2000 pM,
but may be smaller or larger, depending upon the selected application.
Selection of the
matrices will be governed, at least in part, by their physical and chemical
properties, such as
solubility, functional groups, mechanical stability, surface area swelling
propensity,
hydrophobic or hydrophilic properties and intended use.
If necessary, the support matrix material can be treated to contain an
appropriate
reactive moiety. In some cases, the support matrix material already containing
the reactive
moiety may be obtained commercially. The support matrix material containing
the reactive
moiety may thereby serve as the matrix support upon which molecules are
linked. Materials
containing reactive surface moieties such as amino silane linkages, hydroxyl
linkages or
carboxysilane linkages may be produced by well established surface chemistry
techniques
involving silanization reactions, or the like. Examples of these materials are
those having
surface silicon oxide moieties, covalently linked to gamma-aminopropylsilane,
and other
organic moieties; N-[3-(triethyoxysilyl) propyl]phthelamic acid; and bis-(2-
hydroxyethyl)
aminopropyltriethoxysilane. Exemplary of readily available materials
containing amino group
reactive functionalities, include, but are not limited to, para-
aminophenyltriethyoxysilane.
Silicon or silicon-coated chips and wafers used in high throughput protocols
are among those
preferred.
Also derivatized polystyrenes and other such polymers are well known and
readily
available to those of skill in this art (e.g., the Tentagel~ Resins are
available with a multitude of
functional groups, and are sold by Rapp Polymere, Tubingen, Germany; see, U.S.
Patent
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 144 -
No. 4,908,405 and U.S. Patent No. 5,292,814; see, also Butz, et al., Peptide
Res., 7:20-23
(1994); and Kleine, et al., Immunobiol., 190:53-66 (1994)).
These matrix materials include any material that can act as a support matrix
for
attachment of the molecules of interest. Such materials are known to those of
skill in this art,
and include those that are used as a support matrix. These materials include,
but are not limited
to, inorganics, natural polymers, and synthetic polymers, including, but are
not limited to:
cellulose, cellulose derivatives, acrylic resins, glass, silica gels,
polystyrene, gelatin, polyvinyl
pyrrolidone, co-polymers of vinyl and acrylamide, polystyrene cross-linked
with
divinylbenzene and others (see, Merrifield, Biochemistry, 3:1385-1390 (1964)),
polyacrylamides, latex gels, polystyrene, dextran, polyacrylamides, rubber,
silicon, plastics,
nitrocellulose, celluloses, natural sponges. Of particular interest herein,
are highly porous
glasses (see, e.g., U.S. Patent No. 4,244,721) and others prepared by mixing a
borosilicate,
alcohol and water.
Synthetic matrices include, but are not limited to: acrylamides, dextran-
derivatives and
dextran co-polymers, agarose-polyacrylamide blends, other polymers and co-
polymers with
various functional groups, methacrylate derivatives and co-polymers,
polystyrene and
polystyrene copolymers (see, e.g., Merrifield, Biochemistry, 3:1385-1390
(1964); Berg, et al.,
in Innovation Perspect. Solid Phase Synth. Collect. Pap., Int. Symp., 1st,
Epton, Roger (Ed),
pp. 453-459 (1990); Berg, et al., Pept., Proc. Eur. Pept. Symp., 20th, Jung,
G., et al. (Eds), pp.
196-198 (1989); Berg, et al., J. Am. Chem. Soc., 111:8024-8026 (1989); Kent,
et al., Isr. J.
Chem., 17:243-247 (1979); Kent, et al., J. Org. Chem., 43:2845-2852 (1978);
Mitchell, et al.,
Tetrahedron Lett., 42:3795-3798 (1976); U.S. Patent No. 4,507,230; U.S. Patent
No. 4,006,117; and U.S. Patent No. 5,389,449). Methods for preparation of such
matrices are
well-known to those of skill in this art.
Synthetic matrices include those made from polymers and co-polymers such as
polyvinylalcohols, acrylates and acrylic acids such as polyethylene-co-acrylic
acid,
polyethylene-co-methacrylic acid, polyethylene-co-ethylacrylate, polyethylene-
co-methyl
acrylate, polypropylene~~co-acrylic acid, polypropylene-co-methyl-acrylic
acid, polypropylene-
co-ethylacrylate, polypropylene-co-methyl acrylate, polyethylene-co-vinyl
acetate,
polypropylene-co-vinyl acetate, and those containing acid anhydride groups
such as
polyethylene-co-malefic anhydride, polypropylene-co-malefic anhydride and the
like.
Liposomes have also been used as solid supports for affinity purifications
(Powell, et al.
Biotechnol. Bioeng., 33:173 (1989)).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-145-
For example, U.S. Patent No. 5,403,750, describes the preparation of
polyurethane-
based polymers. U.S. Pat. No. 4,241,537 describes a plant growth medium
containing a
hydrophilic polyurethane gel composition prepared from chain-extended polyols;
random
copolymerization is preferred with up to 50% propylene oxide units so that the
prepolymer will
be a liquid at room temperature. U.S. Pat. No. 3,939,123 describes lightly
crosslinked
polyurethane polymers of isocyanate terminated prepolymers containing
poly(ethyleneoxy)
glycols with up to 35% of a poly(propyleneoxy) glycol or a poly(butyleneoxy)
glycol. In
producing these polymers, an organic polyamine is used as a crosslinking
agent. Other
matrices and preparation thereof are described in U.S. Patent Nos. 4,177,038,
4,175,183,
4,439,585, 4,485,227, 4,569,981, 5,092,992, 5,334,640, 5,328,603.
U.S. Patent No. 4,162,355 describes a polymer suitable for use in affinity
chromatography, which is a polymer of an aminimide and a vinyl compound having
at least
one pendant halo-methyl group. An amine ligand, which affords sites for
binding in affinity
chromatography is coupled to the polymer by reaction with a portion of the
pendant halo-
methyl groups and the remainder of the pendant halo-methyl groups are reacted
with an amine
containing a pendant hydrophilic group. A method of coating a substrate with
this polymer is
also described. An exemplary aminimide is 1,1-dimethyl-1-(2-hydroxyoctyl)amine
methacrylimide and vinyl compound is a chloromethyl styrene.
U.S. Patent No. 4,171,412 describes specific matrices based on hydrophilic
polymeric
gels, preferably of a macroporous character, which carry covalently bonded D-
amino acids or
peptides that contain D-amino acid units. The basic support is prepared by
copolymerization of
hydroxyalkyl esters or hydroxyalkylamides of acrylic and methacrylic acid with
crosslinking
acrylate or methacrylate comonomers are modified by the reaction with
diamines, aminoacids
or dicarboxylic acids and the resulting carboxyterminal or aminoterminal
groups are condensed
with D-analogs of aminoacids or peptides. The peptide containing D-aminoacids
also can be
synthesized stepwise on the surface of the carrier. For example, U.S. Patent
No. 4,178,439
describes a cationic ion exchanger and a method for preparation thereof. U.S.
Patent
No. 4,180,524 describes chemical syntheses on a silica support.
The fusion protein can be attached to the surface of the matrix material by
methods
known in the art. Numerous methods have been developed for the immobilization
of proteins
and other biomolecules onto solid or liquid supports (see, e.g., Mosbach,
Methods in
Enzymology, 44 (1976); Weetall, Immobilized Enzymes, Antigens, Antibodies, and
Peptides,
(1975); Kennedy, et al., Solid Phase Biochemistry, Analytical and Synthetic
Aspects, Scouten,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 146 -
ed., pp. 253-391 (1983); see, generally, Affinity Techniques. Enzyme
Purification: Part B.
Methods in Enzymology, Vol. 34, ed. W. B. Jakoby, M. Wilchek, Acad. Press,
N.Y. (1974); and
Immobilized Biochemicals and Affinity Chromatography, Advances in Experimental
Medicine
and Biology, vol. 42, ed. R. Dunlap, Plenum Press, N.Y. (1974)).
Among the most commonly used methods are absorption and adsorption or covalent
binding to the support, either directly or via a linker, such as the numerous
disulfide linkages,
thioether bonds, hindered disulfide bonds, and covalent bonds between free
reactive groups,
such as amine and thiol groups, known to those of skill in art (see, e.g., the
PIERCE
CATALOG, ImmunoTechnology Catalog & Handbook, 1992-1993, which describes the
preparation of and use of such reagents and provides a commercial source for
such reagents;
Wong, Chemistry of Protein Conjugation and Cross Linking, CRC Press (1993);
see also
DeWitt, et al., Proc. Natl. Acad. Sci. U.S.A., 90:6909 (1993); Zuckermann, et
al., J. Am. Chem.
Soc., 114:10646 (1992); Kurth, et al., J. Am. Chem. Soc., 116:2661 (1994);
Ellman, et al., Proc.
Natl. Acad. Sci. U.S.A., 91:4708 (1994); Sucholeiki, Tetrahedron Lttrs.,
35:7307 (1994); Su-
Sun Wang, J. Org. Chem., 41:3258 (1976); Padwa, et al., J. Org. Chem., 41:3550
(1971); and
Vedejs, et al., J. Org. Chem., 49:575 (1984), which describe photosensitive
linkers).
To effect immobilization, a composition containing the protein or other
biomolecule is
contacted with a support material such as alumina, carbon, an ion-exchange
resin, cellulose,
glass or a ceramic. Fluorocarbon polymers have been used as supports to which
biomolecules
have been attached by adsorption (see, U.S. Patent No. 3,843,443; Published
International PCT
Application WO/86 03840).
A large variety of methods are known for attaching biological molecules,
including
proteins and nucleic acids, molecules to solid supports (see, e.g., U.S.
Patent No. 5451683).
For example, U.S. Pat. No. 4,681,870 describes a method for introducing free
amino or
carboxyl groups onto a silica matrix. These groups may subsequently be
covalently linked to
other groups, such as a protein or other anti-ligand, in the presence of a
carbodiimide.
Alternatively, a silica matrix may be activated by treatment with a cyanogen
halide under
alkaline conditions. The anti-ligand is covalently attached to the surface
upon addition to the
activated surface. Another method involves modification of a polymer surface
through the
successive application of multiple layers of biotin, avidin and extenders
(see, e.g., U.S. Patent
No. 4,282,287). Other methods involve photoactivation in which a polypeptide
chain is
attached to a solid substrate by incorporating a light-sensitive unnatural
amino acid group into
the polypeptide chain and exposing the product to low-energy ultraviolet light
(see, e.g., U.S.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
147 -
Patent No. 4,762,881). Oligonucleotides have also been attached using a
photochemically
active reagent, such as a psoralen compound, and a coupling agent, which
attaches the
photoreagent to the substrate (see, e.g., U.S. Patent No. 4,542,102 and U.S.
Patent
No. 4,562,157). Photoactivation of the photoreagent binds a nucleic acid
molecule to the
substrate to give a surface-bound probe.
Covalent binding of the protein or other biomolecule or organic molecule or
biological
particle to chemically activated solid matrix supports such as glass,
synthetic polymers, and
cross-linked polysaccharides is a more frequently used immobilization
technique. The
molecule or biological particle may be directly linked to the matrix support
or linked via linker,
such as a metal (see, e.g., U.S. Patent No. 4,179,402; and Smith, et al.,
Methods: A Companion
to Methods in Enz., 4:73-78 (1992)). An example of this method is the cyanogen
bromide
activation of polysaccharide supports, such as agarose. The use of
perfluorocarbon polymer-
based supports for enzyme immobilization and affinity chromatography is
described in U.S.
Pat. No. 4,885,250. In this method the biomolecule is first modified by
reaction with a
perfluoroalkylating agent such as perfluorooctylpropylisocyanate described in
U.S. Pat.
No. 4,954,444. Then, the modified protein is adsorbed onto the fluorocarbon
support to effect
immobilization.
The activation and use of matrices are well known and may be effected by any
such
known methods (see, e.g., Hermanson, et al., Immobilized Affinity Ligand
Techniques,
Academic Press, Inc., San Diego (1992)). For example, the coupling of the
amino acids may
be accomplished by techniques familiar to those in the art and provided, for
example, in
Stewart and Young, Solid Phase Synthesis, Second Edition, Pierce Chemical Co.,
Rockford
(1984).
Other suitable methods for linking molecules to solid supports are well known
to those
of skill in this art (see, e.g., U.S. Patent No. 5,416,193). These include
linkers that are suitable
for chemically linking molecules, such as proteins, to supports and include,
but are not limited
to, disulfide bonds, thioether bonds, hindered disulfide bonds, and covalent
bonds between free
reactive groups, such as amine and thiol groups. These bonds can be produced
using
heterobifunctional reagents to produce reactive thiol groups on one or both of
the moieties and
then reacting the thiol groups on one moiety with reactive thiol groups or
amine groups to
which reactive maleimido groups or thiol groups can be attached on the other.
Other linkers include, acid cleavable linkers, such as bismaleimideothoxy
propane, acid
labile-transferrin conjugates and adipic acid diihydrazide, that would be
cleaved in more acidic
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 148 -
intracellular compartments; cross linkers that are cleaved upon exposure to UV
or visible light
and linkers, such as the various domains, such as CH1, CH2, and CH3, from the
constant region
of human IgGI (Batra, et al., Molecular Immunol., 30:379-386 (1993)).
Presently preferred
linkages are direct linkages effected by adsorbing the molecule to the surface
of the matrix.
Other linkages are photocleavable linkages that can be activated by exposure
to light
(see, e.g., Goldmacher, et al., Bioconj. Chem., 3:104-107 (1992)). The
photocleavable linker is
selected such that the cleaving wavelength does not damage linked moieties.
Photocleavable
linkers are linkers that are cleaved upon exposure to light (see, e.g., Hazum,
et al., Pept., Proc.
Eur. Pept. Symp., 16th, Brunfeldt, K (Ed), pp. 105-110 (1981), which describes
the use of a
nitrobenzyl group as a photocleavable protective group for cysteine; Yen, et
al., Makromol.
Chem., 190:69-82 (1989), which describes water soluble photocleavable
copolymers, including
hydroxypropylmethacrylamide copolymer, glycine copolymer, fluorescein
copolymer and
methylrhodamine copolymer; Goldmacher, et al., Bioconj. Chem., 3:104-107
(1992), which
describes a cross-linker and reagent that undergoes photolytic degradation
upon exposure to
near UV light (350 nm); and Senter, et al., Photochem. Photobiol., 42:231-237
(1985), which
describes nitrobenzyloxycarbonyl chloride cross linking reagents that produce
photocleavable
linkages). The selected linker will depend upon the particular application
and, if needed, may
be empirically selected.
In a preferred embodiment, the recovered fusion protein is attached to the
surface
through affinity binding between the protein or peptide fragment of the fusion
protein and an
affinity binding moiety on the surface.
5. Use of the conjugates in assays
In a specific embodiment, a method for assaying an analyte in a sample is
provided,
which method comprises: 1) contacting the sample with a conjugate that
contains: a) at least on
mutant analyte-binding enzyme, and b) a facilitating agent that, for example,
facilitates: i)
affinity isolation or purification of the fusion protein; ii) attachment of
the conjugate to a
surface; or iii) detection of the conjugate; and 2) detecting binding between
the analyte or the
immediate analyte enzymatic conversion product and the conjugate, whereby, for
example, the
presence or amount of the analyte in the sample is assessed.
In some embodiments, the conjugate is a fusion protein, which prior to the
contact
between the sample and the fusion protein, is isolated or purified. More
preferably, the fusion
protein is isolated or purified through affinity binding between the protein
or peptide fragment
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 149 -
of the fusion protein and an affinity binding moiety. Any kind of affinity
interaction can be
used for isolating or purifying the fusion protein. The affinity interactions,
such as those
desribed herein, but not limited to, are protein/protein, protein/nucleotide,
protein/lipid,
protein/polysaccharide, or protein/metal interactions.
In other embodiments, prior to the contact between the sample and the
conjugate, such
as a fusion protein, the conjugate is attached to a surface. More preferably,
the conjugate is
attached to the surface through affinity binding between the facilitating
agent of conjugate and
an affinity binding moiety on the surface. Any kind of affinity interaction
can be used for
attaching the conjugate, including the protein/protein, protein/nucleotide,
protein/lipid,
protein/polysaccharide, or protein/metal interactions.
Any analytes, particular small molecule analytes can be assayed using the
above assay
methods. For example, the analyte to be analyzed is Hcy and the mutant analyte-
binding
enzyme of the fusion protein is a mutant Hcy-binding enzyme.
The following examples are included for illustrative purposes only and are not
intended
to limit the scope of the invention.
EXAMPLE 1
Preparation of mutant SAH hydrolase-encoding nucleic acid
Human placental SAH hydrolase gene (SEQ ID No. 1 ) was subcloned into an
expression vector pKK223-3 (Pharmacia Biotech, Piscataway, New Jersey) at the
EcoR I site.
pKK223-3 contains the strong tac promoter upstream from the multiple cloning
site and the
strong rrnB ribosomal terminator downstream for control of protein expression.
The SAH
hydrolase gene-containing expression vector was transferred into an E coli
strain JM109
(Invitrogen, Carlsbad, CA). Site-directed mutagenesis of SAH hydrolase was
conducted in two
ways: 1) single-strand DNA-based M13 method; and 2) double-strand DNA-based
PCR
method.
Single-strand DNA-based mutagenesis
Single-strand DNA-based mutagenesis was conducted based on the method
described
by Taylor, et al., Nucleic Acids Res., 13:8765-8785 (1985), which exploits the
inability of NciI
to cleave a thio-containing DNA strand. SculptorTM invitro mutagenesis system
RPN1526
(Amersham Life science, UK) was used. The pKK223-3 vector containing the wild
type gene
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 150 -
of SAH hydrolase was prepared using the method of alkaline lysis followed by
plasmid
purification using Promega's DNA purification kit (Wizard plus Minipreps,
Promega, Madison
WI). The purified plasmid was digested with EcoR I (Stratagene, La Jolla, CA)
at 37°C for 2
hours to obtain the EcoR I fragment by agarose gel electrophoresis followed by
DNA
purification using Promega DNA purification kit. The purified EcoR I fragment
was subcloned
into M 13 mp 19 DNA (Pharmacia Biotech, Piscataway, New Jersey) by T4 DNA
ligase
(Pharmacia Biotech Piscataway, New Jersey). The ligation was conducted in One-
phor-All
buffer (10 mM tris-Ac, pH 7.5, 10 mM Mg(Ac)2, 50 mM KAc; Pharmacia LKB
Biotechnology
AB, Uppsala, Sweden) at 4°C overnight. The ligation product was
transferred into TG1 cells
(Stratagene, La Jolla, CA) by incubation of 10 ~1 of the M13 with 90 ~1 of
competent TG 1
cells at 0°C for 30 min. and 42°C for 75 sec. After being
chilled to 0°C for 2 min, 500 ~,1 of
2XYT media was added to the cells and incubated for 10 min. at 37°C.
Two hundred ~1 of
growing nontransformed TG1 cells were mixed with the transformed TG1 cells,
and to which
2.5 ml of soft agarose LB (42°C) was added. The cell mixture was
immediately poured onto
preheated LB agar plates (40°C), and incubated at 37°C
overnight. Phage clones were picked
up for examination of the insertion of SAH hydrolase gene and the orientation
through DNA
sequencing and restriction enzyme analysis. The selected phage clone was used
for preparation
of single strand DNA template.
The M13 phage containing the SAH hydrolase gene were incubated with TG1 cells
in 3
ml of 2xYT media overnight. One drop of the overnight culture was mixed with
growing TG1
cells (in log phase) in 30 ml of 2XYT media. Cells were incubated for 8 hours
with shaking.
After centrifugation, the supernatant was collected for single-strand template
DNA purification.
The purification was conducted according to the manufacture's procedure
provided by
Amersham Life Science.
Design of primers for point mutation
Oligonucleotides (15-30 bases) were synthesized by CruaChem (Sterling, VA).
The
sequence of the oligonucleotides were designed to be complementary to the
sequence in the
region covering both sides of the mutation site. For example, to mutate lys
426 to glu 426, the
oligonucleotides used as primer contained the following sequence:
GGCCCCTTCGAGCCGGATCACTACCGC (SEQ ID No. 141) where GAG codes for glu
instead of original (wild type) AAG which codes for lys.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 151 -
The selection of mutation sites was based on x-ray structure of the substrate
binding site
and coenzyme binding site of human SAH hydrolase (Turner, et al., Nature
Structural Biology,
5:369-376 (1998)). Amino acid residues such as Thr 157, Asp 131, Hys 301, Lys
186,
Asn 191, Glu 156, Asp 190, Phe 362, Phe 302, Asn 181, His 353, Glu 59, Ser 83,
His 55,
Leu 54, Cys 79, His 301, Arg 343, Asp 303, Leu 344, Asn 80, Asn 346, Asp 107
and entire C-
terminal residues can be the mutagenesis targets (see Table 2 for particular
mutations
generated). The coenzyme binding domain contains residues from Tyr193-Asn346.
The oligonucleotides were dissolved in water to a concentration of 5 ng/~,1.
The
oligonucleotide solution was then phosphorylated at the 5'-end using
polynucleotide kinase.
The phosphorylation reaction mixture contained the following materials: 2.5 ~1
of
oligonucleotides (S ng/~1), 3 ~1 of one-phor-all l OX kinase buffer (Pharmacia
Biotech), 21.5 ~1
of water, 2 ~1 of 10 mM ATP, and 1 ~,1 of polynucleotide kinase ( 1
OO,OOOU/ml) (Pharmacia
Biotech). The reaction mixture was incubated at 37°C for 30 min.
followed by heating at 70°C
for 10 min. to inactivate the enzyme.
Table 8. Oligonucleotides used for site-directed mutagenesis of human SAH
hydrolases
Mutant Muta enic oli onucleotide Codon Chan SEQ
a ~
K186A GACTTCGTCACCGCCAGCAAGTTTGGG AAG~GCC 142
F302S AACATTGGACACTCTGACGTGGAGATC TTT~TCT 143
H301D TGTAACATTGGAGACTTTGACGTGGAG CAC~GAC 144
H353S TGTGCCATGGGCTCCCCCAGCTTCGTG CAC~TCC 145
R343A CTGGCCGAGGGTGCGCTGGTCAACCTG CGG~GCG 146
D190A AAGAGCAAGTTTGCCAACCTCTATGGC GAC~GCC 147
F82A AGCTGCAACATCGCCTCCACCCAGGAC TTC~GCC 148
N181D AACCTCTATGGCGACCGGGAGTCCCTC AAT--~GAC 149
R431A CCGGATCACTACGCCTACTGAGAATTC CGC->GCC 150
K426R TGTGATGGCTTCCGCCCGGATCACTAC AAG~CGC 151
C195S AACCTCTATGGCTCCCGGGAGTCCCTC TGC~TCC 152
0432 GATCACTACCGCTGATGAGAATTCGAG ATC~TGA 153
The mutagenized codon is underlined, and the nucleotides changed are in
boldface type.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 152 -
Table 9. Additional oligonucleotides used for site-directed mutagenesis of
human SAH hydrolases
Sequence Seq.
ID Sequence ID F/R
No.
G1u156A1a GGCATCTCTGAGGCGACCACGACTGGG 155 Fo
G1u156A1a CCCAGTCGTGGTCGCCTCAGAGATGCC 156 Re
G1u156As GGCATCTCTGAGGACACCACGACTGGG 157 Fo
G1u156As CCCAGTCGTGGTGTCCTCAGAGATGCC 158 Re
As 131 CTCAACATGATTCTGGACAAGGGGGGCGACCTCACC 159 Fo
L s
As 131 GGTGAGGTCGCCCCCCTTGTCCAGAATCATGTTGAG 160 Re
Lys
As 131Asn CTCAACATGATTCTGGACAACGGGGGCGACCTCACC 161 Fo
As 131Asn GGTGAGGTCGCCCCCGTTGTCCAGAATCATGTTGAG 162 Re
L s186A1a GACTCCGTCACCGCGAGCAAGTTTGAC 163 Fo
Lys186A1a GTCAAACTTGCTCGCGGTGACGGAGTC 164 Re
Lys186As GACTCCGTCACCGACAGCAAGTTTGAC 165 Fo
Lys186As GTCAAACTTGCTGTCGGTGACGGAGTC 166 Re
His55Pro GCTGGCTGCCTGCCCATGACCGTGGAGACG 167 Fo
His55Pro CGTCTCCACGGTCATGGGCAGGCAGCCAGC 168 Re
Arg343A1a CTGCTGGCCGAGGGTGCGCTGGTCAACCTG 169 Fo
Arg343A1a CAGGTTGACCAGCGCACCCTCGGCCAGCAG 170 Re
As 303G1u GTGTGTAACATTGGACACTTTGAGGTGGAGATCGATGTC 171 Fo
As 303G1u GACATCGATCTCCACCTCAAAGTGTCCAATGTTACACAC 172 Re
Phe302I1e GTGTGTAACATTGGACACATTGACGTGGAGATC 173 Fo
Phe302I1e GATCTCCACGTCAATGTGTCCAATGTTACACAC 174 Re
Leu344G1 GCCGAGGGTCGGGGGGTCAACCTGGGTTGTGCC 175 Fo
Leu344G1 GGCACAACCCAGGTTGACCCCCCGACCCTCGGC 176 Re
Phe82Ser CAGTGGTCCAGCTGCAACATCTCCTCCACCCAGGAC 177 Fo
Phe82Ser GTCCTGGGTGGAGGAGATGTTGCAGCTGGACCACTG 178 Re
Thr159Ser GAGGAGAGGACGTCCGGGGTCCACAACCTC 179 Fo
Thr159Ser GAGGTTGTGGACCCCGGACGTCCTCTCCTC 180 Re
Asn346G1y GGTCGGCTGGTCGGCCTGGGTTGTGCC 181 Fo
Asn346G1y GGCACAACCCAGGCCGACCAGCCGACC 182 Re
Asn346As GGTCGGCTGGTCGACCTGGGTTGTGCC 183 Fo
Asn346As GGCACAACCCAGGTCGACCAGCCGACC 184 Re
C s79Ala GTGCAGTGGTCCAGCGCCAACATCTTCTCCACC 185 Ro
C s79Ala GGTGGAGAAGATGTTGGCGCTGGACCACTGCAC 186 Re
C s79Gly GTGCAGTGGTCCAGCGGCAACATCTTCTCCACC 187 Fo
Cys79G1 GGTGGAGAAGATGTTGCCGCTGGACCACTGCAC 188 Re
His301A1a GTGTGTAACATTGGA('~CCTTTGACGTGGAG 189 Fo
His301A1a CTCCACGTCAAAGGCTCCAATGTTACACAC 190 Re
As 303A1a GTGTGTAACATTGGACACTTTGCCGTGGAG 191 Fo
Asp303A1a GACATCGATCTCCACGGCAAAGTGTCCAATGTTACACAC 192 ~
Re
F: forward oligonucleotide
R: backward oligonucleotide.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-153-
The 5'-phosphorylated oligonucleotides DNA was annealed with single-stranded
DNA
(M13 phage containing wild type human SAH hydrolase gene, 1 ~.g/pl) in a ratio
of
oligonucleotide: template of 2:1 in annealing buffer. The annealing reaction
was performed by
incubating the annealing mixture at 70°C for 3 min. followed by 30 min.
at 37°C or followed by
transferring the micro centrifuge tube to a 55°C beaker and then
allowed to cool to room
temperature. To the annealing mixture (17 p1), 19 p,1 of dNTP A (a-S) mix, 1.5
~1 of T7 DNA
polymerase (0.8 units), and 2.5 p,1 of T4 DNA ligase (92.5 units), and 6 ~l of
water were added.
After 10 min. at room temperature and 30 min. at 37°C, the reaction was
stopped by heat
inactivation at 70°C for 15 min. To the reaction mixture was added TS
exonuclease (2000
units) and exonuclease buffer to remove single-strand non-mutant DNA at
37°C for 30 min.
followed by 15 min. of heat inactivation at 70°C. NciI (5 units) was
added to the reaction
mixture to nicking the non-mutant strand by incubating NciI at 37°C for
90 min. The non-
mutant strand was digested by adding 160 units of Exonuclease III and
incubating at 37°C for
30 min. followed by heat inactivation. To repolymerize the gaped DNA, dNTP mix
B and 3.5
units of DNA polymerase I and 2.5 units of T4 DNA ligase were added to the
reaction mixture,
and incubated at 37°C for 1 h.
The M13 plasmid containing the mutated SAH hydrolase gene was then transferred
into
competent TG 1 host cells by heat shock method or an electroporation method.
Ten p1 of the
mutant M13 plasmid was added to 90 p1 of water and mixed with competent TG1
cells in ice
for 40 min. The TG1 cells were shocked by incubation at 42°C for 45
sec. and immediately at
0°C for 5 min. The transferred TG1 cells were allowed to return to room
temperature, and
mixed with 200 p,1 of growing non-transferred TG1 cells (served as lawn
cells). Three ml of
molten Htop agar was added and mixed followed by immediately pouring the cells
onto a L
plate. The plate was incubated in 37°C for overnight. Phage plaques
formed were picked by
sterile tooth pick and swirling in a tube containing 3 ml of 2XYT medium.
After overnight
culture, cells were collected by centrifugation, and the double-strand M13
plasmid from the
cells was purified by using Promega DNA purification kit (Wizard plus
Minipreps).
The supernatant from centrifugation was used to purify single-strand M13 DNA.
The
mutation was confirmed by DNA sequencing of the single-strand M13 DNA using
Sequenase
Version 2.0 (Unites States Biochemical). The double-strand M13 DNA containing
correct
mutation sequence was selected, and digested with EcoR I. The EcoR I fragment
containing
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 154 -
the mutant SAH hydrolase gene was purified by agarose electrophoresis followed
by gene
cleaning using Qlaquick Gel Extraction kit (Qiagen, Valencia, CA). The
purified EcoR I
fragment was subcloned into pKK223-3 expression vector using T4 ligase. Two
~.l of EcoR 1
treated and 5'-dephosphorylated pKK223-3 vector backbone was incubated with 10
~1 of the
purified mutant insert DNA in a backbone to insert ratio of 2:1. The ligation
reaction was
carried out in One-phore-All buffer containing 0.01 M ATP at 16C overnight.
The ligated
vector containing mutant SAH hydrolase gene was transferred into competent E.
Coli JM109
cells by heat shock method. The transformed cells were selected against 100
~,1/ml ampicillin.
Ampicillin-resistant clones were picked and grown in 10 ml of 2xYT medium
containing 35
~,l/ml ampicillin for 2 hours at 37°C and then induced with 1 mM
isopropyl-1-thib-(3-D-
galactopyranoside (IPTG) and grown overnight at 37°C. The cells were
harvested by
centrifugation, and suspended in 0.8 ml of 50 mM Tri-HCI, pH 7.5, containing 2
mM EDTA.
Cells were lysed by rapid freezing and thawing. After centrifugation at 13,500
rpm for 1 hour
at 4°C, the supernatant was collected for SDS-PAGE analysis for over-
expression of SAH
hydrolase mutant protein. A heavy protein band at molecular size of 47,000 Da
indicates the
overexpression of mutant SAH hydrolase protein.
PCR-based mutagenesis method
PCR-based mutagenesis was performed using the ExSite PCR-based Site-Directed
Mutagenesis Kit (Stratagene, La Jolla, CA). The ExSite method uses increased
template
concentration and <10 PCR cycles. The resulting mixture of template DNA, newly
synthesized
DNA and hybrid parental/newly synthesized DNA is treated with Dpn I and Pfu
DNA
polymerase. Dpn I digests the in vivo methylated parental template and hybrid
DNA, and Pfu
DNA polymerase polishes the ends to create a blunt-ended PCR product. The end-
polished
PCR product is then intramolecularly ligated together and transformed into E.
coli cells. The
detailed experimental procedure is described as follows:
To a microcentrifuge tube were added 0.5 pmol of template DNA, 2.5 ~,1 of l Ox
mutagenesis buffers, 1 w1 of 25 mM dNTY mix, 15 pmol of each primer, and ddH20
to a final
volume of 24 ~1. To the reaction mixture was then added 1 ~1 of ExSite DNA
polymerase
blend (5 U/~1). The reaction solution was overlayed with 20 ~1 of mineral oil
and thermal cycle
the DNA using 7012 amplification cycles. The cycling parameters are listed in
Table 10.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-155-
Table 10. Mutagenesis Cycling Parameters
Se ment C cles Tem erature Time
1 1 94C 4 min.
50C 2 min.
72C 2 min.
2 8 94C 1 min.
56C 2 min.
72C 1 min.
72C 5 min.
3 72C 5 min.
Following amplification, the reaction tube was placed on ice for 2 min. to
cool the
reaction to <37°C. To the reaction tube were added 1 ~1 of the Dpn I
restriction enzyme (10
S U/~,1) and 0.5 p1 of cloned Pfu DNA polymerase (2.5 U/pl) followed by
incubation at 37°C for
30 min. The reaction was stopped by heating at 72°C for 30 min. For
ligating the product, to
the reaction tube were added 100 ~1 of ddH20, 10 ~1 of l Ox mutagenesis
buffer, and 5 ~1 of 10
mM rATP. Transfer 10 p1 of the above reaction mixture to a new micocentrifuge
tube and add
1 g1 of T4 DNA ligase (4 U/~.1). The ligation was incubated at 37°C for
1 hour. 2 ~,1 of the
ligated DNA was added to 80 g1 of Epicurian Coli XL1-Blue supercompetent cells
on ice and
incubated for 30 min. followed by 45 seconds at 42defendant and 2 min. on ice.
The
transformed cells were immediately plated on LB-ampicillin agar plates which
had been spread
with 20 ~,1 of 10% X-gal prepared in DMF and 20 ~1 of 100 M IPTG in H20. The
plate was
incubated overnight at 37°C. The blue colonies were selected as
colonies containing the
mutagenized plasmid. The selected colonies were further confirmed by DNA
sequencing.
Protein overexpression and substrate trapping screening were performed as
described above.
Double-strand pKK223-3 containing human SAH hydrolase (wild type) was purified
from 50 ml of E. coli JM109 culture using Promega DNA purification kit (Wizard
plus
Minipreps). The purified plasmid was annealed with PCR primers containing the
desired
mutation sequence.
Deletion and insertion mutations were also performed according to the
manufacture's
protocol using ExSite PCR-based Site-directed Mutagenesis Kit. Double
mutations or
combinations of mutation and deletion or insertion were carried out using
mutated or deleted
DNA as template for secondary mutation or deletion using either M13-based
mutagenesis or
PCR-based mutagenesis methods.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 156 -
Identification of substrate trapping SAH hydrolase
The cell-free extracts from colonies that inducibly overexpressed mutant SAH
hydrolase proteins were chromatographed on a monoQ column (HR5/5) equipped
with FPLC
system. Proteins were eluted with a linear gradient of NaCI from 0 to 1 M in
10 mM sodium
phosphate buffer, pH 7.0 over 35 min. The major protein peak that eluted at
the same or close
retention time as that of the wild type SAH hydrolase was collected. An
aliquot collected
mutant SAH hydrolase (1-10 ~,g) was incubated with [3H]SAH (10 mCi/mmole, 200
~,M) and
30 pM of 5, 5'-dithiobis (2-nitrobenzoic acid) (DTNB) at room temperature for
5-30 min.
The reaction solution was filtered through a membrane of molecular weight cut-
off at
30,000 by centrifugation. The filtrate was measured at 412 nm for Hcy
formation (enzyme
activity) and the [3H] radioactivity on the membrane was measured by
scintillation counting
after membrane washing with 1 ml of 50 mM phosphate buffer, pH 7Ø
The mutant hydrolases that show high radioactivity on the membrane and low
O.D. at
412 nm of the filtrate relative to the wild type enzyme were selected as
candidates for further
characterization including determination of Km or Kd and binding energy (0G).
Mutant SAH
hydrolases with Km value lower than 10 p,M toward SAH and kcat value lower
than 0.1 per
second were overexpressed in larger quantity (1-2 L of E coli culture) and the
enzyme proteins
were purified to homogenous as judged by single band on SDA-PAGE.
FXAMPT.F 2
Lame scale overexpression and purification of wild type and mutant forms of
SAH hydrolases
Purification
The cell-free extract of IPTG-induced E. Coli JM109 (containing SAH hydrolase
gene
in pKK223-3 vector) culture was mixed with powder DEAF-cellulose (Sigma, St.
Louis, MO)
equilibrated with 0.1 M sodium phosphate buffer, pH 7.2 containing 1 mM EDTA
(buffer A).
The cell-free extract and DEAC-cellulose mixture was placed in a funnel and
filtrated under
vacuum. After washing with 3 volumes of buffer A, the filtrate was
precipitated by solid
ammonium sulfate (30-60%). The precipitated protein was collected by
centrifugation at
13000 rpm, and resuspended in 50 mM sodium phosphate buffer, pH 7.2,
containing 1 mM
EDTA. The protein was chromatographed through a Sephacryl S-300 size exclusion
column
(2.5X100 cm) (Pharmacial Biotech, Piscataway, New Jersey) followed by a DEAE-
Sepharose
ion exchange column (2.5X30 cm) eluted by a linear NaCI gradient. The major
protein peak
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 157 -
from DEAE-Sepharose was examined by SDS-PAGE. In most of the times, this
purification
procedure gave a single protein band on SDS-PAGE. Sometime, minor bands were
observed
on SDS-PAGE. In this case, rechromatography on DEAE-Sepharose column was
performed to
obtain pure protein. SAH hydrolase activity or [3H]SAH binding affinity was
also measured to
confirm the protein peak.
Storage of the purified SAH hydrolase
The purified wild type and mutant SAH hydrolases were dialyzed against 5 mM
sodium
phosphate buffer, pH 7.0 for 6 hours at 4°C. The protein was then
frozen in liquid nitrogen and
lyophilized under vacuum. The lyophilized protein was stored at -70~~. The
protein was stable
for at least 2 years. The purified protein can also be stored in liquid
containing 20% of glycerol
at -20°C. For wild type enzyme, addition of 5 mole excess of adenosine
(Ado) to the 20%
glycerol solution stabilizes the enzyme activity even better.
Assays for enzyme activity
The assay of SAH hydrolase activity in the hydrolytic direction was performed
as
described in Yuan, et al., J. Biol. Chem., 271:28008-28016, 1996). The assay
measures the
hydrolysis of SAH into Ado and Hcy. The reaction product Hcy was derivatized
by thiol
specific reagent DTNB for colometric determination at 412 nm. The assay for
SAH hydrolase
in the synthetic direction was measured by the formation of SAH from substrate
Ado and Hcy
using HPLC (see, Yuan, et al., J. Biol. Chem., 268:17030-17037 (1993). One
unit of the
enzyme activity was defined as the amount of enzyme that can hydrolyze or
synthesize 1 p,
mole of SAH/min/mg.
Assay for binding affinity (Kd)
For mutant enzyme that completely lacks activity, the binding constant (Kd)
values
were determined by an equilibrium dialysis technique using [3H] SAH and
Spectrum 5-cell
Equilibrium Dialyzer) (Spectrum, Houston, Texas). The membrane disc used had
molecular
cut-off of 25,000.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 158 -
EXAMPLE 3
Preparation of reagents
Preparation of fluorophore-labeled Ado and SAH analogs
Method 1
Ado-5'-carboxylic acid (Sigma, St. Louis, MO) was derivatized with 9-
(hydroxylmethyl)anthracene (HMA) (Fluka, Buchs, Switzerland). To 10 mg of Ado-
5'-
carboxylic acid dissolved in 100 ml of chloroform (10 min sonication) was
added 50 mg 1-
hydroxybenzotriazole (HOBT) (Janssen Chimica, Beerse, Belgium). After
evaporation to
dryness under nitrogen, 300 mg of N-ethyl-N'-(3-
dimethylaminopropyl)carbodiimide
hydrochloride in 300 ml chloroform and 5 ml of triethylamine were added. The
resulting
solution was kept at 0°C for 30 min. To the above reaction mixture was
added 200 mg HMA in
100 ml of chloroform. The mixture was allowed to stand at room temperature for
10 min. and
then evaporated to dryness under a stream of nitrogen. The residue obtained
was dissolved in
10 ml of HPLC mobile phase (methanol-water mixture, 90:10, w/w). One ml of the
above
solution was injected into a semi-preparative column (Econosphere, C18, 7x300
mm, Alltech,
Dearfield, IL). The column was eluted with an isocratic method. The flow rate
was 2 ml/min.
The peaks were monitored at UV260 nm and fluorescence at Ex-365nm, Em-415nm.
The
peaks with UV and fluorescence absorbance were collected as HMA-labeled Ado-5'-
ester.
Method 2
Ado-5'caroboxylic acid and 4-bromomethyl-7-methoxycoumarin (Br-Mmc) (Sigma,
St.
Louis, MO) were dissolved in ethyl acetate in a molar ratio of 1:3. The
reaction volume was 25
ml. After addition of 2 g of finely powdered K2C03 the solution was refluxed
for 1 hour using
a ml-reluxer. After cooling, the reaction solution was injected into a C18
column
(Econosphere, C18, 7x300 mm, Alltech, Deerfield, IL) for HPLC separation. The
elution was
monitored by UV (260 nm) and fluorescence (Em 328nm and Ex390nm). The elution
was
performed in a linear gradient of methanol:water from 20 to 100% over 30 min.
The flow rate
was 2 ml/min.
Method 3
This method is depicted in Figure 3. Adenosyl-L-cysteine (Ado-Cys) and 4-
Bromomethyl-7-methoxycoumarin (Br-Mmc) were dissolved in ethyl acetate in a
molar ration
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 159 -
of 1:3. The final volume was 25 ml (ca, 1 mg Ado-Cys). After addition of 200
mg of finely
powdered KZC03, the solution was refluxed for 1 hour using a ml-refluxer at
80°C. After
cooling, the reaction solution was injected into a C18 column (Econosphere,
C18, 7x300 mm,
Alltech, Dearfield, IL) for separation using HPLC. The fluorescently labeled
Ado-Cys was
eluted by a linear gradient of methanol; water from 20 to 100% in 30 min. The
flow rate was 2
ml/min.
Method 4
Ado-Cys was dissolved in carbonate buffer, pH 9.0 in 1 mM concentration.
Fluorescein
isotiocyanate (FITC) (PcPierce, Rockford, IL) was dissolved in DMSO in 5 mM
concentration,
and diluted to 1 mM with carbonate buffer, pH 9Ø Equal volumes of Ado-Cys
and FITC in
carbonate buffer were mixed and incubated in room temperature for 1 hour. The
Ado-Cys-
FITC conjugate was then isolated by HPLC using a C 18 column (Econsphere, C
18, Alltech,
Deerfield, IL). The elution was monitored at UV 260 nm and fluorescence at
Ex484 nm and
Em520 nm. The mobile phases were water and methanol in a linear gradient from
0 to 80% of
methanol in 35 min.
Coating mutant SAH hydrolase on microtiter well (96 well plate)
Mutant SAH hydrolase (F302S) was coated on flat-bottomed 96 well plate (Dynex
Technologies, Chantilly, Virginia). 200 ~,1 of 20 pg/ml of F302S mutant
hydrolase in 50 mM
sodium phosphate buffer, pH 7.6. was added to each well. After incubation at
4°C overnight,
the plate was emptied by inversion. After blocking with 0.5% BSA, the plate
was then washed
three times with 10 mM PBS containing 0.1 NaCI and 0.05% of Tween 20. After
inversion and
tapping, the plate was stored at 4°C before use.
Preparation of standard samples and chemical reagents
1. Construction of a standard Hcy curve
Human albumin (Fraction V powder, Sigrr~a) was dissolved in PBS in a protein
concentration equal to that of human plasma. To 10 ml of the albumin was added
4 ml of 1
tri-n-butylphosphine (TBP). The mixture was incubated at room temperature for
15 min.
followed by gel filtration through a size exclusion column (Sephacryl-S 100, 2
x 90 cm). The
albumin protein concentration was normalized to human plasma concentration
using protein
concentrator (Bio-Rad). The protein concentration was determined by Bradford
reagent (Bio-
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 160 -
Rad). A series of known concentration of L-homocysteine and L-homocystine were
spiked into
the TBP-treated human albumin in a final concentrations ranging from 0 to 50
~M. After
incubation at 37°C for 1 hour, the L-homocysteine spiked albumin and L-
homocystine albumin
were aliquoted in 70 ~1/tube as standard samples, and stored at -20°C
before use.
2. Wild type SAH hydrolase solution
The wild type SAH hydrolase (20 mU/50 ~.1) was dissolved in 50 mM phosphate
buffer,
Ph 7.2, containing 1 mM EDTA, 0.25 mM Ado and 1 mg/ml of BSA.
3. Tri-n-butylphosphine (TBP) solution
Tri-n-butylphoshine (Sigma) was dissolved in dimethylformamide (DMF) to 1
concentration.
4. Fluorophore-labeled Ado-Cys solution
Br-Mmc-labeled Ado-Cys or FITC-labeled Ado-Cys was dissolved in 50 mM
phosphate buffer, pH 7.2, in a concentration of 0.5 mM.
5. SAH hydrolase inhibitor solution
Neplanocin A (natural product), an inhibitor of SAH hydrolase, and a substrate
of
adenosine deaminase, was dissolved in 50 mM phosphate buffer, pH 7.2. The
inhibitor
solution (50 pM) was used in an enzyme to inhibitor ratio of 1:1.5.
6. Multi-enzyme solution
Adenosine (0.2 U/p,l), nucleoside phosphorylase (0.2 U/1) and xanthine oxidase
(0.2
U/~1) were dissolved in 50 mM potassium phosphate buffer, pH 7.2. All the
enzymes were
from Sigma.
7. Washing solution
The plate washing solution contains of 10 mM PBS, pH 7.2, 0.1 M NaCI, and
0.05%
Tween 20.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-161-
EXAMPLE 4
Assays of Hcy using the mutant SAH enzyme
Plasma Hcy assay procedure 1
Step 1. Conversion of Hcy to SAH
To 50 ~.1 of plasma sample in microcentrifuge tube or in uncoated 96-well
plate was
added 20 ~,1 of 1 % TBP and 50 ~1 of the wild type SAH hydrolase solution.
After incubation at
25°C for 15 min, 20 ~,1 of the enzyme inhibitor solution was added to
the reaction mixture, and
incubated for 10 min. to inactivate SAH hydrolase.
Step 2. Removal of remaining Ado and enzyme inhibitor
To the solution in Step 1 was added 30 ~1 of the multi-enzyme solution, and
incubated
for 15 min at room temperature.
Step 3. Trapping the formed SAH onto the mutant SAH hydrolase
150 ~,1 solution in Step 2 was transferred to a microtiter well pre-coated
with mutant
SAH hydrolase. After 30 min. incubation at room temperature, the solution was
emptied by
inversion.
Step 4. Washing
The plate from Step 3 was washed three times with the washing solution
followed by
inversion and tapping.
Step 5. Binding of fluorophore-labeled Ado-Cys to the mutant
enzyme
100 ~1 of the fluorophore-labeled Ado-Cys or fluorophore-labeled Ado-5' ester
was
added to the microtiter well in Step 4. After 20 min. incubation at room
temperature, the plate
was washed three times with the washing solution.
Step 6. Detection of the mutant SAH hydrolase-bound fluorophore-
labeled Ado-Cys
To the microtiter well from Step 5, 200 ~1 of 50 mM phosphate buffer, pH 7.2,
was
added, and the plate was read for fluorescence using a plate reader (Molecular
Devices, fmax).
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 162 -
The plasma Hcy concentration was calculated from the standard curve
constructed under the
same conditions.
Alternative Hcy assay
Alternatively, the Hcy assay can also be performed by pre-coating SAH on
microtiter
well, and using fluorophore-labeled mutant SAH hydrolase for competition
binding assay. The
details are described as follows:
1. pre-coating SAH on microtiter well
SAH was conjugated to polylysine by activating the carboxylic group of SAH
with PC13
at 50°C. The SAH-polylysine conjugate was purified by HPLC, and
dissolved in 0.1 M
carbonate buffer, pH 9.6. 300 ~l of 100 pg/ml SAH-polylysine solution was
added to each
well, and incubated at 37°C for 6 hours. The plate was then washed
three times with washing
solution containing 10 mM PBS, 0.1 M NaCI and 0.05% Tween 20. After inversion
and
tapping, the plate was stored at 4°C before use.
2. Fluorophore-labeled mutant SAH hydrolase
Mutant SAH hydrolase (e.g., F302S) was specifically fluorescence labels on
Cys421, an
non-essential cysteine residue which is located on the surface of the protein
that is not involved
in substrate binding and catalysis. Cys421 residue is readily accessible by
thiol reactive
molecules, and can be modified without effecting the binding affinity of the
enzyme. Thiol
specific reactive probes such as 7-diethylamino-3(4'-maleimidylphenyl)-4-
methylcoumarin
(CPM) can specifically label protein thiols. Mutant SAH hydrolase (F302S) (0.5
mg/ml) in 50
mM phosphate buffer, pH 7.2, was incubated with 2 mM of adenine to protect
other thiols in
the substrate binding site, followed by addition of CPM to final concentration
of 50 ~,M. The
reaction mixture was incubated at room temperature for 30 min. followed by gel
filtration on a
size exclusion column (Sephacryl S-300, 4.Smmx60cm) to remove adenine and
excess CPM.
The CPM-labeled F302S mutant SAH hydrolase (2 mg/ml) was kept in 50 mM
phosphate
buffer containing 20% glycerol at -20°C. The comparison of Km (SAH) and
Kcat (SAH) for
wild type and mutant F302S is shown below in Table 11.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-163-
Table 11. Comparison of kinetic constants between mutant and wild type SAH
hydrolases
Enzyme Km SAH Kcat SAH)
wild type 7.9 ~M 3.8 S-'
F302S 1.0 ~M 0.1 S-'
Plasma Hcy assay procedure 2
Step 1. Conversion of Hcy to SAH
To SO ~1 of plasma sample in microcentrifuge tube or in uncoated 96-well plate
was
added 20 ~,l of 1% TBP and 50 ~,1 of the enzyme inhibitor solution was added
to the reaction
mixture, and incubated for 10 min. to inactivate SAH hydrolase.
Step 2. Removal of remaining Ado and enzyme inhibitor
To the solution in Step 1 was added 30 ~,l of the multi-enzyme solution, and
incubated
for 15 min. at room temperature.
Step 3. Competition binding of SAH to the Mutant SAH hydrolase
One hundred ~,1 of the solution from Step 2 was transferred to a microtiter
well pre-
coated with polylysine-SAH conjugate to which 150 p1 of the fluorophore-
labeled mutant SAH
hydrolase was added. After incubation at room temperature for 30 min., the
plate was inverted
and tapped followed by three times of washing with the washing solution.
Step 4. Detection of the fluorophore-labeled mutant SAH hydrolase bound
to the microtiter well
To the plate from Step 3 was added 200 ~,l of 10 nM PBS, and the plate was
read by a
plate reader (Molecular Devices, fmax) at Ex390 nm and Em460 nm. The plasma
concentration of Hcy was calculated from the standard curve constructed under
the same
conditions with the standard samples.
EXAMPLE 5
Determination of folate contents in serum and er t~ytes
Sample Preparation
Serum folate, which exists primarily as methyltetrahydrofolic acid (Me-THF) is
readily
determined by a Me-THF-trapping enzyme such as mutant forms of thymidylate
synthase,
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
- 164 -
methionine synthase, dihydrofolate reductase, or folylpolyglutamate
synthetase. In contrast,
erythrocyte folate exists as polyglutamate derivatives and have to be treated
with conjugase to
convert folylpolyglutamates to folate before quantitation with mutant folate
trapping enzyme.
Different forms of folates are converted to one form using folate
interconverting enzymes
including dihydrofolate reductase, tetrahydrofolate methyltransferase,
methylenetetrahydrofolate reductase, thymidylate synthase, methionine
synthase. Any one of
these enzymes can be chosen for preparation of a folate trapping enzyme using,
for example
site-directed mutagenesis of nucleic acid that encodes the enzyme.
Preparation of folate trapping enzymes
a. Mutation of thymidylate synthase
Glutamine 214 of human thymidylate synthase is highly conserved in all
thymidylate
synthases and is postulated to interact with nucleotide ligands that bind at
the active site.
Mutation of G1u214 to serine results in attenuated catalytic activity of the
enzyme but retains
substrate binding ability. Residue Asn 229 is involved in formation of
hydrogen bonds to
constrain the orientation of dUMP in binary complexes with dUMP, and in
ternary complexes
with dUMP and cofactor 5,10-methylenetetrahydrofolate. Mutation of Asn 229 to
Ala results
in a 2000-fold decrease in the Kcat of the enzyme with a modest increase in Km
and Kd. In
addition, mutation of His 199 to any other amino acid results reduced
catalytic activity of the
enzyme. The C-terminal residues of thymidylate synthase are involved in the
enzyme catalysis.
Mutation of these residues results in attenuated enzyme activity, but retains
the substrate or
cofactor binding affinity.
b. Mutation of dihydrofolate reductase
Mutation of Arg 43 to Ala or Trp 21 to His results in a folate-trapping
enzyme.
c. Mutation of folylpolyglutamate synthetase
The C-terminal domain (aa's 300-425) of folypolyglutamate synthetase is
involved in
the folate-binding site of the enzyme. Mutation of G1n421 to Ser leads to an
interruption of
hydrophobic interactions in the C-terminal domain and results in decreased
catalytic activity,
but substantially retains substrate-binding ability of the enzyme.
CA 02377665 2001-12-12
WO 01/02600 PCT/US00/18057
-165-
Binding of folate to folate trapping enzyme
Folate in serum is incubated with a folate trapping enzyme, such as Asn 229-
thymidylate synthase, which has been precoated on a 96-well plate. After 30
minutes of
incubation at room temperature, the plate is washed three times with PBS
buffer. Fluorescein-
labeled folate is then added to the plate as competitor tracer. The plate is
incubated for another
30 min at room temperature.
Detection of bound folate
After being washed for three times with PBS buffer, the plate is read, using
an
excitation wavelength Ex of 492 nm and an Em at 515 nm with a fluorescence
plate reader. The
folate content in serum is calculated based on a folate standard curve
prepared and tested under
the same conditions using known concentrations of folate.
Since modifications will be apparent to those of skill in this art, it is
intended that this
invention be limited only by the scope of the appended claims.