Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-1
MODULATION OF I E RECEPTOR CELL SURFACE EXPRESSION
Government Support
This work was funded in part by grant number AI41995 from the National
Institutes
s of Health. Accordingly, the United States Government may have certain rights
to this
invention.
Field of the Invention
The invention relates to methods and related compositions for modulating cell
surface
m expression of the high affinity receptor for immunoglobulin E, the FcsRI
receptor. The
invention also relates to methods and related compositions for the treatment
and/or prevention
of conditions mediated by IgE such as allergic conditions.
Background of the Invention
~s The FcsRI complex is the high affinity cell surface receptor for the Fc
region of
antigen specific immunoglobulin E (IgE) molecules. FcsRI is multimeric and is
a member of
a family of related antigen/Fc receptors which have conserved structural
features and which
exhibit similar functional activities in initiating intracellular signaling
cascades. In humans,
FcsRI controls the activation of mast cells and basophils, and participates in
IgE mediated
ao antigen presentation. Multivalent antigens bind and crosslink IgE molecules
held at the cell
surface by FcsRI. Receptor aggregation induces multiple signaling pathways
that control
diverse effector responses, including secretion of allergic mediators and the
induction of
cytokine gene transcription (such as IL-4, IL-6, TNFa and GM-CSF). FcERI
therefore is
central to the induction and maintenance of an allergic response and
physiologically may
as confer protection in parasitic infections.
It is a conserved feature of antigen receptors that they are multimeric, and
that their
individual subunits perform different functions. In the case of FcsRI, the
receptor is
composed of three distinct polypeptides. The a chain (FcsRIa) binds the Fc
portion of IgE
with high affinity, and the (3 chain (FcERI~3) has four transmembrane domains
between
~o amino- and carboxyl-terminal cytoplasmic tails. A homodimer of two
disulfide linked y
chains (FcsRIy) completes the tetrameric structure. In humans, the tetrameric
structure is
not obligatory, .and an alternate aye trimer is present. In terms of
devolution of function, the
a chain contains two immunoglobulin type domains, D1 and D2, that mediate
binding to IgE.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-2-
The (3 and y chains contain conserved Immunoreceptor Tyrosine-based Activation
Motifs
(ITAM) in their cytoplasmic tails (2, 3). These motifs reportedly are
phosphoacceptors,
through which the receptor subunits interact with signaling proteins.
The events that control mast cell activation via FcsRI are sequential. First,
IgE binds
s via its Fc fragment to the FcERIa, chain. Second, IgE molecules are cross-
linked by
multivalent antigen, causing aggregation of a, chains in the plane of the
plasma membrane.
Third, information concerning productive a chain aggregation is transmitted to
the /3y
signaling subunits, via an unknown mechanism. The resulting initiation of
intracellular
signaling pathways controls downstream events such as allergic mediator
production and
m cytokine gene transcription. These downstream signaling events are
extensively reviewed in
Kinet, J-P., et al, ( 1 ), and Turner, H.K. & Kinet, J-P. (4).
Antigenic crosslinking of the FcsRI initiates a chain of phosphate transfer
events
within the receptor microenvironment. The (3 and y chains of the FcERI contain
ITAMs,
where the tyrosine residues are phosphoacceptor sites for the action of
receptor-associated
Is protein tyrosine kinases (PTKs) (12, 13). Phospho-ITAMs link receptor and
signal
transduction cascades. In the FcsRI context, the (3 and y ITAMs have slightly
different
structures and serve distinct functions. There are two species of FcsRI
associated PTK; the
src family kinase Lyn and the p72 Syk kinase. The former is found associated
with FcsRI(3,
the latter is able to bind (3 and y but has higher affinity for interaction
with y.
2n Several studies report on the immediate events following FcsRI aggregation
(1, 14), in
which: 1) The ~ and y chains act cooperatively. Both in vitro and genetic
reconstitution
studies illustrate this point. Reconstitution of FcsRI deficient mast cell
lines show that
mutation of the two canonical tyrosines in the (3 ITAM abolish activation
dependent
phosphorylation of both the (3 and the y ITAMs, suggesting that the
phosphorylation of the
a former has bearing on the status of the latter (15). Moreover, while Lyri ~-
mast cells exhibit
no (3 or y phosphorylation, Syk-~- cells have intact (3 and y phosphorylation
but still lack
downstream signaling events (16, 17, 18). 2) Lyn binds to,l3 under resting
conditions. An
obvious candidate for the mediation of this interaction is the Lyn SH2 domain,
since (3 is
slightly tyrosine phosphorylated under resting conditions. However, others
have reported that
3n the 'unique' (SH4 containing) domain of Lyn interacts with FcsRI(3 (19). 3)
Active Lyn
phosphorylates the ~3 and y ITAMs. Upon receptor aggregation, Lyn is activated
and its
catalytic activity becomes directed toward the /3 and y ITAMs. Syk is then
recruited to the
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-3- _
receptor y chain via one of the tandem SH2 domains in the kinase. An important
feature is
that Lyn may transphosphorylate ITAM in other receptor complexes. 4) Syk
binding to
FcsRly leads to Lyn-dependent tyrosine phosphorylation and activation of the
kinase. This
step finally potentiates the productive interaction of active Syk with its
many targets. In
s summary, aggregation leads to Lyn-dependent ITAM phosphorylation.
Rodent FcsRI receptor complexes have an obligatory tetrameric a(3y2 structure.
In
humans, both aye and a(3y2 complexes are observed at the cell surface. Rodent
FcERI
receptor complexes are confined to the surface of mast cells and basophils. In
humans,
however, it is now recognized that there is a far wider distribution of FcsRI.
On the mast cell
~n and basophil surface there is a mixture of a(3y2 and aye complexes, while
monocytes,
Langerhans cells, eosinophils and dendritic cells express surface aye.
Finally, in rodent but
not human, there is crosstalk between IgE and IgG mediated cellular
activation. In the rodent,
the IgE Fc region can bind to two classes of low affinity FcyR, activatory and
inhibitory
isotypes. Both of these are expressed on mast cells and so there is a route by
which IgE or
~s IgG immune complexes may regulate mast cell function independently of
FcsRI.
FcsRI receptor complexes are first assembled in the ER. Here, nascent a, (3
and y
chains are thought to interact non-covalently. Only the trafficking of a
chains to the cell
surface has been monitored extensively. In the ER, the a chain reportedly is
core-
glycosylated. During trafficking through the Golgi apparatus, the core, high-
mannose
zn glycosylations of a are replaced by complex sugar, terminal glycosylations.
This difference
can be exploited experimentally since the latter are insensitive to the action
of
Endoglycosidase H. The reason for this two stage glycosylation may be that
both during
biosynthesis and at the cell surface, it is important that a chains do not
aggregate in the
absence of antigen. In vitro, de-glycosylated FcsRIa aggregates spontaneously
without
?s antigen. The glycosylation of potential a-a interaction surfaces prevents
premature
aggregation and permits interaction with the synthetic machinery. Later,
terminal
glycosylation does permit aggregation by a multivalent antigen. Mature FcERIa
glycosylation does not extend to the top surface of the molecule, where the
IgE interaction is
proposed to take place.
3o Summary of the Invention
The invention is based, in part, on the discovery that an FcsRI(3 chain
variant
modulates the expression of an FcERI receptor (the high affinity receptor for
IgE) in cells.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-4-
Accordingly, the invention provides methods and compositions for modulating
cell surface
expression of an FcsRI receptor in a cell (e.g., contained in a tissue or a
subject) which
expresses an FcsRI receptor.
More specifically, the invention is based, in part, on the discovery that
expression of a
s variant of one of the constituent FcsRI receptor chains (e.g., an FcERI(3
chain variant, an
FcsRIa chain variant, or an FcsRIy chain variant) in a cell that expresses a
wild type FcsRI
receptor, results in the abrogation or decrease of FcsRI receptor cell surface
expression. As a
result, activity usually associated with such a receptor and mediation of IgE
signals are also
decreased or abolished. The invention, therefore, is useful whenever it is
desirable to
io modulate such activity, for example, in the treatment of conditions
mediated by IgE. Thus,
the invention also provides methods and related compositions for identifying
pharmacological
agents useful in the treatment of such conditions.
According to one aspect of the invention, a method for inhibiting expression
of an
Fc~RI receptor in a cell, is provided. The method involves contacting a cell
expressing (i.e., a
is cell expressing or capable of expressing), an FcsRI receptor with an
FcERI~i chain variant in
an effective amount to inhibit expression of the FcsRI receptor in the cell.
In some
embodiments, the FcERI(3 chain variant is an isolated nucleic acid molecule
that inhibits
expression of an FcsRI receptor in the cell. In preferred embodiments, the
isolated nucleic
acid molecule comprises the nucleotide sequence of SEQ ID N0:3. In certain
embodiments,
zo the FcsRI(3 chain variant is an isolated peptide molecule that inhibits
expression of an FcERI
receptor in the cell. In preferred embodiments, the isolated peptide molecule
comprises the
nucleotide sequence of SEQ ID N0:4. The contacting can occur in vitro or in
vivo.
According to another aspect of the invention, a method for inhibiting
expression of an
FcsRI receptor in a subject to treat a condition mediated by IgE, is provided.
The method
?s involves administering to a subject in need of such treatment an FcsRI(3
chain variant in an
effective amount to inhibit FcsRI receptor expression in a cell of the
subject. In one
embodiment, the condition mediated by IgE is an allergic condition. In
preferred
embodiments the allergic condition is atopy, rhinitis, conjunctivitis,
anaphylaxis, urticaria, or
angioedema. In a certain embodiment, conditions mediated by IgE are IgE-
dependent late
3n phase reactions. In some embodiments, the FcERI(3 chain variant is an
isolated nucleic acid
molecule that inhibits expression of an FcsRI receptor in the cell. In
preferred embodiments,
the isolated nucleic acid molecule comprises the nucleotide sequence of SEQ ID
N0:3. In
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-5-
certain embodiments, the FcsRI(3 chain variant is an isolated peptide molecule
that inhibits
expression of an FcERI receptor in the cell. In preferred embodiments, the
isolated peptide
molecule comprises the amino acid sequence of SEQ ID N0:4. Any of the
foregoing
embodiments can further comprise co-administering to the subject an anti-
allergic (anti-
s atopic) agent other than an FcsRI~i chain variant.
According to a further aspect of the invention, a method of screening for
FcsRI
receptor expression modulating agents, is also provided. The method involves
(a) contacting
a putative FcsRI receptor expression modulating agent with cell expressing
(i.e., a cell
expressing or capable of expressing) an FcsRI receptor, (b) measuring FcsRI
receptor
m expression by the cell, and (c) determining whether FcsRI receptor
expression by the cell is
altered compared to FcsRI receptor expression by a control cell, wherein 'the
control cell is
contacted with an FcsRI(3 chain variant. In some embodiments, the Fc~RI(3
chain variant is
an endogenous nucleic acid molecule of the cell. In another embodiment the
FcsRI(3 chain
variant is a heterologous nucleic acid molecule of the cell. In a preferred
embodiment, the
~s FcsRI(3 chain variant comprises the nucleotide sequence of SEQ ID N0:3. In
certain
embodiments, measuring FcsRI receptor expression by the cell comprises using
an anti-FcBRI
chain-specific antibody.
According to another aspect of the invention, a method of screening for
FcsRI~3 chain
variant expression modulating agents, is provided. The method involves (a)
contacting a
~n putative FcsRI(3 chain variant expression modulating agent with a test cell
expressing (i.e., a
cell expressing or capable of expressing) an FcsRI(3 chain variant, (b)
measuring FcsRI(3
chain variant expression by the cell, and (c) determining whether FcsRI(3
chain variant
expression by the cell is altered, compared to a control cell expressing an
FcsRI(3 chain
variant in the absence of a putative FcsRI(3 chain variant expression
modulating agent. In
zs certain embodiments the control cell expresses an FcsRI(3 chain variant
identical to the
FcERI(3 chain variant expressed by the test cell. In some embodiments,
measuring FcsRI(3
chain variant expression by the cell comprises using reverse transcription-
polymerase chain
reaction (RT-PCR).
According to yet another aspect of the invention, a method for inhibiting
expression of
3n an FcsRIa chain in a cell, is provided. The method involves contacting a
cell expressing (i.e.,
a cell expressing or capable of expressing), an FcsRIa chain with an FcERI~3
chain variant in
an effective amount to inhibit expression of the FcsRIa chain in the cell. In
some
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-6-
embodiments, the FcsRI(3 chain variant is an isolated nucleic acid molecule
that inhibits
expression of an FcsRIa chain. In a preferred embodiment, the isolated nucleic
acid
molecule comprises the nucleotide sequence of SEQ ID N0:3. The contacting can
occur in
vitro or in vivo.
s According to a further aspect of the invention, a method for determining
whether a
subject has a condition mediated by IgE or a predisposition thereto, is
provided. The method
involves determining FcsRI[3 chain variant expression in a subject suspected
of having a
condition mediated by IgE or a predisposition thereto, and comparing the
FcsRI(3 chain
variant expression to a control. Lower levels of FcsRI~3 chain variant
expression in the
to subject as compared to the control are indicative for the presence of, or a
predisposition to, a
condition mediated by IgE in the subject. In some embodiments, FcsRI(3 chain
variant
expression is mRNA expression. In certain embodiments, FcERI(3 chain variant
expression is
peptide expression.
These and other aspects of the invention, as well as various advantages and
utilities,
rs will be more apparent with reference to the drawings and the detailed
description of the
preferred embodiments.
Brief Description of the drawings
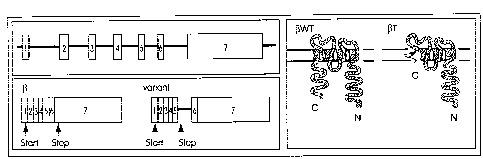
Figure 1. Diagrammatic sketches of: A. The domain structure of WT (wild type)
~n and (3T (variant) FcsRI(3 chains; and B. Predicted topologies of WT and (3T
FcsRI(3 chains.
Figure 2. Graph depicting levels of surface FcsRIa polypeptide chains in U937
cells
stably transfected with a(3Ty, ay, and a(3y FceRI(3 isoforms. Each point
represents a different
clone.
Figure 3. Graph depicting FcsRI receptor cell surface expression in U937 a(3y
(WT)
zs FcsRI stable transfectants, transiently retransfected with either control
or (3T FcsRI(3 cDNA.
Brief Description of the Sequences
SEQ ID NO:I is the wild type (WT) human FcsRI(3 chain cDNA sequence.
SEQ ID N0:2 is the wild type (WT) human FcsRI(3 chain polypeptide sequence
3o encoded by the cDNA sequence set forth in SEQ ID NO: 1.
SEQ ID N0:3 is the variant ((3T or FcsRI(3T) Fc~RI(3 chain cDNA sequence.
SEQ ID N0:4 is the variant ((3T or FcsRI(3T) FcERI(3 chain polypeptide
sequence
encoded by the cDNA sequence set forth in SEQ ID NO: 3.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
_7_
SEQ ID NO:S is a sense PCR primer from exon 2 of FcsRI(3.
SEQ ID N0:6 is an antisense PCR primer from exon 7 of FcsRI[3.
SEQ ID N0:7 is the wild type (WT) human FcsRIa chain cDNA sequence.
SEQ ID N0:8 is the wild type (WT) human FcsRIy chain cDNA sequence.
s SEQ ID N0:9 is the wild type (WT) mouse FcsRI(3 chain cDNA sequence.
Detailed Description of the Invention
The invention is based, in part, on the discovery that a variant form of the
(3 chain
(FcERI(3) of the FcERI receptor, FcsRI(3T (SEQ ID N0:4), inhibits FcsRI
receptor expression
m in a cell expressing (i.e., a cell expressing or capable of expressing), an
FcsRI receptor. More
specifically, we have discovered that the FcsRI(3 chain variant prevents
maturation (i.e., post-
translational modifications) of FcsRI receptor chain FcsRIa, leading to
inhibition of FcsRI
receptor expression in a cell expressing (i.e., a cell expressing or capable
of expressing), an
FcsRI receptor.
~s Accordingly, the invention provides methods and compositions for modulating
cell
surface expression of an FcsRI receptor in a cell (e.g., contained in a tissue
or a subject)
which expresses an FcsRI receptor. For ease of discussion, the phrase "cell
expressing" is
meant to embrace cells already expressing a particular polypeptide, as well as
cells capable of
such expression. More specifically, the invention is based, in part, on the
discovery that
2n expression of a variant of one of the constituent FceRI receptor chains
(e.g., an FcsRI[3 chain
variant, an FcsRIa chain variant, or an FcERIy chain variant ) in a cell that
expresses a wild
type FcERI receptor, results in the abrogation or decrease of FcsRI receptor
cell surface
expression. As a result, activity usually associated with such a receptor and
mediation of IgE
signals are also abolished. The invention, therefore, is useful whenever it is
desirable to
~s modulate such activity, for example, in the treatment of conditions
mediated by IgE. Thus,
the invention also provides methods and related compositions for identifying
pharmacological
agents useful in the treatment of such conditions.
As used herein, "inhibit expression" refers to inhibiting (i.e., reducing to a
detectable
extent) replication, transcription, and/or translation of one or more FcERI
receptor constituent
;o polypeptide genes [i.e., the gene encoding the a chain (FcsRIa), the (3
chain (FcsRI(3), or the
y chain (FcsRIy)], since inhibition of any of these processes results in the
inhibition of
expression of an FcERI receptor constituent polypeptide encoded by its
respective gene. The
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
_g_
term also refers to inhibition of post-translational modifications on the
FcsRI receptor
constituent polypeptide, since inhibition of such modifications will also
prevent proper
expression (i.e., expression as in a wild type cell) of the encoded
polypeptide. The inhibition
of gene expression can be directly determined by detecting a decrease in the
level of mRNA
s for the gene, or the level of protein expression of the gene, using any
suitable means known to
the art, such as nucleic acid hybridization or antibody detection methods,
respectively.
Inhibition of gene expression can also be determined indirectly by detecting a
change in
FcsRI receptor activity as a whole (e.g., histamine and/or cytokine release by
the cell upon
triggering with IgE, etc.), or activity of each of the constituent FcsRI
receptor chains (e.g.,
m phosphorylation, glycosylation, etc.).
An "FcERI~3 chain variant" as used herein, refers to a wild type FcsRI[3 chain
nucleic
acid or polypeptide which contains one or more modifications (as described
below) in its
primary sequence, giving rise to a peptide with functional properties that
differ from those of
the wild type. In certain instances, for example, the variant will have
"dominant negative"
~s peptide properties. In other instances, the variant will have a direct
effect on the expression
of the FcsRIa, and FcsRIy receptor chains (e.g., at the nucleotide and/or
amino acid level -
transcriptionally, translationally, post-translationaly), resulting in
inhibition of FcERI receptor
expression. A "dominant negative" peptide is an inactive variant of a protein,
which, by
interacting with the cellular machinery, displaces an active protein (e.g.,
wild type FcsRI(3
zn chain) from its interaction with the cellular machinery or competes with
the active protein,
thereby reducing the effect of the active protein. For example, a dominant
negative receptor
which binds a ligand but does not transmit a signal in response to binding of
the ligand can
reduce the biological effect of expression of the ligand. Likewise, a dominant
negative
catalytically-inactive kinase which interacts normally with target proteins
but does not
?s phosphorylate the target proteins can reduce phosphorylation of the target
proteins in
response to a cellular signal. Similarly, a dominant negative transcription
factor which binds
to a promoter site in the control region of a gene but does not increase gene
transcription can
reduce the effect of a normal transcription factor by occupying promoter
binding sites without
increasing transcription. The end result of the expression of a dominant
negative polypeptide
3o in a cell is a reduction in function of active proteins. As used herein,
therefore, an FcsRI(3
chain variant refers to a nucleic acid with a nucleotide sequence as set forth
in SEQ ID N0:3,
a polypeptide with an amino acid sequence as set forth in SEQ ID N0:4, and
structurally
related nucleic acids and polypeptides, respectively, which share a common
function with
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-9-
these nucleic acids and polypeptides, namely, the ability to function in a
dominant negative
fashion and inhibit expression of the FcsRI receptor in screening assays such
as those
described herein.
By "structurally related," as used herein, refers to nucleic acids and
polypeptides that
s are homologous and/or allelic to the FcsRI(3 chain variant. In general
homologs and alleles
typically will share at least 40% nucleotide identity and/or at least 50%
amino acid identity to
SEQ ID N0:3 and SEQ ID N0:4, respectively, in some instances will share at
least 50%
nucleotide identity and/or at least 65% amino acid identity and in still other
instances will
share at least 60% nucleotide identity and/or at least 75% amino acid
identity. The homology
m can be calculated using various, publicly available software tools developed
by NCBI
(Bethesda, Maryland). Exemplary tools include the heuristic algorithm of
Altschul SF, et al.,
(J Mol Biol, 1990, 215:403-410), also known as BLAST. Pairwise and ClustalW
alignments
(BLOSUM30 matrix setting) as well as Kyte-Doolittle hydropathic analysis can
be obtained
using public (EMBL, Heidelberg, Germany) and commercial (e.g., the MacVector
sequence
rs analysis software from Oxford Molecular Group/enetics Computer Group,
Madison, WI).
Watson-Crick complements of the foregoing nucleic acids also are embraced by
the
invention.
Modifications which create an FcsRI(3 chain variant are typically made to the
nucleic
acid which encodes the FcsRI(3 chain polypeptide. Other similar methods for
creating and
zn testing variants of a protein according to the invention will be apparent
to one of ordinary
skill in the art. Thus functionally equivalent variants of FceRI(3T chain
polypeptides, i.e.,
variants of FcsRI(3 polypeptides which retain the above-identified function of
the FcsRI(3T
polypeptide, are contemplated by the invention.
One of ordinary skill in the art can screen for a dominant negative variant of
a protein,
~s and using standard mutagenesis techniques to create one or more dominant
negative FcsRI(3
chain variant polypeptides. For example, given the teachings contained herein
of FcERI(3
chain variant nucleic acids and polypeptides, one of ordinary skill in the art
can modify the
FcERI(3 nucleotide (SEQ ID NO:1) and/or peptide sequence (SEQ ID N0:2) by site-
specific
mutagenesis, scanning mutagenesis, partial gene deletion or truncation, and
the like. See,
3o e.g., U.S. Patent No. 5,580,723 and Sambrook et al., Molecular Cloning: A
Laboratory
Manual, Second Edition, Cold Spring Harbor Laboratory Press, 1989. The skilled
artisan
then can test the population of mutagenized polypeptides for their effect in
the inhibition of
FcsRI receptor expression in a cell expressing (i.e., a cell expressing or
capable of
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-10
expressing) an FcsRI receptor. These results can be compared to the results
obtained in a
control experiment, typically an identical experiment performed side-by-side
utilizing an
FcsRI(3 chain variant polypeptide of the invention (e.g., the preferred
FcsRI(3 chain variant
polypeptide, FcsRI[3T, having an amino acid sequence as set forth in SEQ ID
N0:4).
s Methods for detecting FcsRI receptor expression inhibition are described
elsewhere herein.
In another important aspect, the invention provides a method for inhibiting
expression
of an FcsRI receptor in a cell of a subj ect to treat a condition mediated by
IgE. The method
involves administering to a subject in need of such treatment an FcERI(3 chain
variant in an
effective amount to inhibit FcsRI receptor expression in a cell of the
subject. A "subject" as
~o used herein, is a human, non-human primate, cow, horse, pig, sheep, goat,
dog, cat, or rodent.
A "cell" of a subject, as used herein, refers to a cell that expresses, or is
capable of
expressing, an FcsRI receptor, and it includes cells of hematopoietic origin,
and more
specifically mast cells and/or basophils. A "condition mediated by IgE" as
used herein, refers
to Type I hypersensitivity responses (also known as immediate hypersensitivity
reactions) and
~s IgE-dependent "late phase reactions," characterized by an infiltration of
inflammatory cells
that appears days after exposure to an allergen (e.g., as in the inflammatory
reaction present in
the airways of asthmatic patients).
The immune system of humans and animals normally functions to protect its host
from infectious organisms or from cancerous transformation by host cells. In
many instances
~n however, the immune system manifests a response that itself results in
considerable damage
to otherwise healthy cells and organs. Such over-reactivity of immune
responsiveness is
responsible for many serious conditions or diseases including allergies and
autoimmune
diseases. In order to classify the processes by which the immune system
produces cellular
damage, immunologists have divided immune responses into four broad classes
(Type I, II,
zs III and IV) (Roitt, I. M., et al., Immunology, C. V. Mosby, N.Y., 1985, p.
19.1). "Type I
hypersensitivity responses" are also called immediate hypersensitivity
reactions and refer to
those conditions which produce the symptoms classically associated with
"allergies" or the
"allergic syndrome" including atopy [e.g., allergic rhinitis (hay fever),
allergic asthma,
allergic conjunctivitis], urticaria, angioedema, and allergic reactions to
insect stings or foods
30 (anaphylaxis). These allergic conditions are characterized by a rapid
clinical manifestation of
allergic symptoms within minutes after exposure to an antigen (allergen) to
which the subject
has been previously sensitized.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-11-
In order for Type I hypersensitivity to occur, a specialized sequence of
events within
mast cells and basophils must be triggered by immunoglobulin E (IgE)
antibodies that have
been manufactured within the body. In this process IgE directed toward an
antigen (allergen)
must bind to a receptor (FcsRI receptor) on mast cells and basophils which
specifically bind
s to the Fc region of IgE. Mast cells and basophils that have anti-allergen-
IgE bound to them
are considered to be sensitized or "armed" for subsequent exposure to
allergen. Should
allergen be introduced into the local environment of the mast cells or
basophils, the cells are
automatically stimulated or "triggered" to release histamine and other
vasoactive chemicals
(e.g., lipid mediators such as leukotrienes B4 and C4, prostaglandin D2,
platelet-activating
~o factor, cytokines such as IL-4, IL-5, IL-6, TNF-oc, etc.) which produce the
familiar "allergic
symptoms" characteristic of allergic conditions.
The hypersensitivity states characterized by types II, III and IV
hypersensitivity are
very distinct from type I hypersensitivity. For example, allergic inflammation
in type I
hypersensitivity (allergy) begins within minutes after allergen exposure. By
contrast, other
~s hypersensitivity states exhibit inflammation only after hours to days
following reexposure to
the sensitizing agent. Additionally, in type I hypersensitivity, the
sensitizing agent (allergen)
is not a part or component of the host body but a substance found outside of
the host body
that is later introduced into the body by exposure to the environment. Types
II, III and IV
hypersensitivity, by contrast, may have immune responses directed towards
antigens located
ao on cells and molecules that are normal constituents of the body. Such
immune responses
toward normal constituents of the body are termed "autoimmune diseases" and
constitute a
medically class of diseases distinct from conditions mediated by IgE (e.g.,
allergies). A
further distinction is the degree to which cell killing occurs. In type I
hypersensitivity, the
IgE mediated triggering reaction which causes the release of vasoactive
allergic mediators
?s does not result in the death of the releasing mast cell or basophil.
Instead, the "trigger"
reaction is the result of an active secretory process that may recur after a
length of time.
Similarly, the effect of the vasoactive allergic mediators on surrounding
cells is regulatory,
not cytotoxic. Allergic mediators serve to increase the permeability of small
blood vessels
and activate a variety of vasoregulatory and immunoregulatory processes that
do not normally
~o result in cell death. Types II, III and IV hypersensitivity, by contrast,
have as a principal
function cell killing reactions which normally lead to the destruction of
infectious agents or
cancer cells.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-12-
In a further aspect, the invention involves a method of screening for FcsRI
receptor
expression modulating agents. The method involves (a) contacting a putative
FcsRI receptor
expression modulating agent with a cell expressing (i.e., a cell expressing or
capable of
expressing) an FcsRI receptor, (b) measuring FcsRI receptor expression by the
cell, and (c)
s determining whether FcsRI receptor expression by the cell is altered
compared to Fc~RI
receptor expression by a control cell, wherein the control cell is contacted
with an FcsRI(3
chain variant. Additional controls may also include measuring FcsRI receptor
expression by
a cell in the absence of a putative FcsRI receptor expression modulating
agent. As mentioned,
supra, "measuring FcsRI receptor expression by the cell" is accomplished using
a number of
m different methods, most of which are well known by a person of ordinary
skill in the art. For
example, a direct way would be to measure mRNA expression for one of the
constituent
FcsRI receptor chains using Northern blotting, RT-PCR, etc. The human nucleic
acid
sequences encoding each of the three constituent FcsRI receptor chains are
known in the art
and are publicly available through NCBI's GenBank databases (Accession nos.:
X06948 for
~s FcsRIa -SEQ ID N0:7; M89796 for FcERI(3-SEQ ID NO:l; and L03533 for FcsRIy -
SEQ ID
N0:8). Another direct way to measure FcERI receptor expression by the cell is
to use
antibodies specific for one of the constituent FcsRI receptor chains and a
number of
immunocyto- and immunohisto- chemical protocols. Antibodies specific for each
of the
constituent FcERI receptor chains are commercially available and can be
obtained, for
~n example, through Upstate Biotechnology, Lake Placid, NY [rabbit polyclonal
anti-human
FcsRIa peptide (997) cat. 06-725; mouse monoclonal anti-human FcsRIa chain
(clone 3G6)
cat. OS-491; rabbit polyclonal anti-human FcsRI(3 peptide cat. 06-726; rabbit
polyclonal anti-
human FcsRIy peptide (934) cat. 06-727J. In a preferred embodiment, expression
of more
than one chain is measured at the same time. For example, expression of the
FcERIa chain is
~_s measured at the same time as FcsRI(3 chain expression. Another indirect
way to measure
FcsRI receptor expression by the cell is to measure FcERI receptor activity.
For example,
FcsRI receptor activity can be measured by the downstream effects of IgE
binding to the
receptor. Such downstream effects include secretion of chemical compounds by
the cell,
supra, in response to antigen-IgE binding to the receptor.
3o In one embodiment, the FcsRI(3 chain variant is an endogenous nucleic acid
molecule
of the cell. By "endogenous" it is meant that it is naturally present in the
cell genome. In
another embodiment the FcsRI(3 chain variant is a heterologous nucleic acid
molecule of the
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-13
cell. As used herein, "heterologous" or foreign nucleic acid molecule (DNA and
RNA) are
used interchangeably and refer to DNA or RNA that does not occur naturally as
part of the
genome in which it is present or which is found in a location or locations in
the genome that
differ from that in which it occurs in nature. Preferably, it is DNA or RNA
that is not
s endogenous to the cell and has been artificially introduced into the cell.
Examples of
heterologous DNA include, but are not limited to, DNA that encodes an FcsRI(3
chain variant
polypeptide according to the invention, and DNA that encodes RNA or proteins
that mediate
or alter expression of endogenous DNA (for example, that of wild type FcsRI(3
chain) by
affecting transcription, translation, or other regulatable biochemical
processes. The cell that
~o expresses the heterologous DNA, such as DNA encoding an FcsRI(3 chain
variant
polypeptide, may contain DNA encoding the same or different FcERI(3 chain
variant
polypeptide.
Generally, the screening methods of the invention involve assaying for
compounds
which interfere with FcsRI receptor expression activity, and can be detected
by any of the
Is above-identified methods (e.g., downstream effects after the FcsRI receptor
binds its natural
ligand, IgE). Such methods are adaptable to automated, high throughput
screening of
compounds. The target therapeutic indications for agents detected by the
screening methods
are limited only in that the target cellular function be subject to modulation
by alteration of
the formation of a complex comprising an FcsRI receptor, and its natural
ligand, IgE. Target
an indications can include cellular processes mediated by the FcsRI receptor
following its
binding to IgE (e.g., histamine or cytokine release, etc.).
In addition to the FcERI receptor, a screening assay mixture includes a
binding partner
for the receptor, e.g., a naturally occurring ligand (i.e., IgE) that is
capable of binding to the
FcsRI receptor or, alternatively, is comprised of an analog which mimics the
FcsRI receptor
binding properties of the naturally occurring ligand for purposes of the
assay. The screening
assay mixture also comprises a candidate agent (e.g., an agent that modulates
expression of
the FcsRI receptor, and most preferably inhibits expression of the FcsRI
receptor).
Typically, a plurality of assay mixtures are run in parallel with different
agent
concentrations to obtain a different response to the various concentrations.
Typically, one of
3n these concentrations serves as a negative control, i.e., at zero
concentration of agent or at a
concentration of agent below the limits of assay detection. An essential
control according to
the invention is the comparison of the effects of the inhibitory agent on
FceRI receptor
expression with the FcsRI receptor expression in a cell expressing (i.e., a
cell expressing or
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-14-
capable of expressing) an FcsRI(3 chain variant of the invention. Candidate
agents encompass
numerous chemical classes, although typically they are organic compounds.
Preferably, the
candidate agents are small organic compounds, i.e., those having a molecular
weight of more
than 50 yet less than about 2500, preferably less than about 1000 and, more
preferably, less
s than about 500. Candidate agents comprise functional chemical groups
necessary for
structural interactions with polypeptides and/or nucleic acids, and typically
include at least an
amine, carbonyl, hydroxyl or carboxyl group, preferably at least two of the
functional
chemical groups and more preferably at least three of the functional chemical
groups. The
candidate agents can comprise cyclic carbon or heterocyclic structure and/or
aromatic or
m polyaromatic structures substituted with one or more of the above-identified
functional
groups. Candidate agents also can be biomolecules such as peptides,
saccharides, fatty acids,
sterols, isoprenoids, purines, pyrimidines, derivatives or structural analogs
of the above, or
combinations thereof and the like. Where the agent is a nucleic acid, the
agent typically is a
DNA or RNA molecule, although modified nucleic acids as defined herein are
also
~s contemplated.
Candidate agents are obtained from a wide variety of sources including
libraries of
synthetic or natural compounds. For example, numerous means are available for
random and
directed synthesis of a wide variety of organic compounds and biomolecules,
including
expression of randomized oligonucleotides, synthetic organic combinatorial
libraries, phage
~o display libraries of random peptides, and the like. Alternatively,
libraries of natural
compounds in the form of bacterial, fungal, plant and animal extracts are
available or readily
produced. Additionally, natural and synthetically produced libraries and
compounds can be
readily be modified through conventional chemical, physical, and biochemical
means.
Further, known pharmacological agents may be subjected to directed or random
chemical
zs modifications such as acylation, alkylation, esterification, amidification,
etc. to produce
structural analogs of the agents.
A variety of other reagents also can be included in the mixture. These include
reagents such as salts, buffers, neutral proteins (e.g., albumin), detergents,
etc. which may be
used to facilitate optimal protein-protein and/or protein-nucleic acid
binding. Such a reagent
3n may also reduce non-specific or background interactions of the reaction
components. Other
reagents that improve the efficiency of the assay such as protease inhibitors,
nuclease
inhibitors, antimicrobial agents, and the like may also be used.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-15
The mixture of the foregoing assay materials is incubated under conditions
whereby,
but for the presence of the candidate putative inhibitor agent, the FcERI
receptor specifically
binds its natural binding target, a portion thereof or analog thereof. The
order of addition of
components, incubation temperature, time of incubation, and other parameters
of the assay
s may be readily determined. Such experimentation merely involves optimization
of the assay
parameters, not the fundamental composition of the assay. Incubation
temperatures typically
are between 4°C and 40°C. Incubation times preferably are
minimized to facilitate rapid,
high throughput screening, and typically are between 0.1 and 10 hours.
After incubation, the presence or absence of specific binding between the
FcERI
m receptor and one or more binding targets is detected by any convenient
method available to
the user, supra.
The therapeutics of the invention embrace isolated nucleic acids and
polypeptides.
The term "isolated", as used herein in reference to a nucleic acid molecule,
means a nucleic
acid sequence: (i) amplified in vitro by, for example, polymerase chain
reaction (PCR); (ii)
!s synthesized by, for example, chemical synthesis; (iii) recombinantly
produced by cloning; or
(iv) purified, as by cleavage and gel separation. The term "isolated", as used
herein in
reference to a polypeptide (protein), means a polypeptide encoded by an
isolated nucleic acid
sequence, as well as polypeptides synthesized by, for example, chemical
synthetic methods,
and polypeptides separated from biological materials, and then purified using
conventional
~o protein analytical procedures.
The therapeutics of the invention are in effective amounts. The "effective
amount"
will depend upon the mode of administration, the particular condition being
treated and the
desired outcome. It will also depend upon, the stage of the condition, the age
and physical
condition of the subject, the nature of concurrent therapy, if any, and like
factors well known
~s to the medical practitioner. For therapeutic applications, it is that
amount sufficient to
achieve a medically desirable result. In some cases, for example, this is a
decrease in a
subject's immune hypersensitivity to allergens as evidenced by a decrease in
circulating
histamines, cytokines, etc. (i.e., inhibition of allergen effects).
Generally, doses of active compounds of the present invention would be from
about
3n 0.01 mg/kg per day to 1000 mg/kg per day. It is expected that doses ranging
from 50-500
mg/kg will be suitable. A variety of administration routes are available. The
methods of the
invention, generally speaking, may be practiced using any mode of
administration that is
medically acceptable, meaning any mode that produces effective levels of the
active
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877,
-16
compounds without causing clinically unacceptable adverse effects. Such modes
of
administration include oral, rectal, topical, nasal, intradermal, or
parenteral routes. The term
"parenteral" includes subcutaneous, intravenous, intramuscular, or infusion.
Intravenous or
intramuscular routes are not particularly suitable for long-term therapy and
prophylaxis.
s They could, however, be preferred in emergency situations. Oral
administration will be
preferred for prophylactic treatment because of the convenience to the patient
as well as the
dosing schedule. When peptides are used therapeutically, in certain
embodiments a desirable
route of administration is by pulmonary aerosol. Techniques for preparing
aerosol delivery
systems containing peptides are well known to those of skill in the art.
Generally, such
~n systems should utilize components which will not significantly impair the
biological
properties of the peptides, for example, the paratope binding capacity of a
peptide (see, for
example, Sciarra and Cutie, "Aerosols," in Remington's Pharmaceutical
Sciences, 18th
edition, 1990, pp 1694-1712; incorporated by reference). Those of skill in the
art can readily
determine the various parameters and conditions for producing antibody or
peptide aerosols
~s without resort to undue experimentation.
Preferred methods for administering FcsRI(3 variants or agents that induce
FceRI(3
variant expression of the invention also include spliceosome-mediated RNA
trans-splicing as
described by Puttaraju, M., et al (53) and using agents commercially available
from Intronn
LLC (Durham, NC), and ribozymes using methods well known in the art and
reviewed by
~n Woolf, TM (58).
Compositions suitable for oral administration may be presented as discrete
units, such
as capsules, tablets, lozenges, each containing a predetermined amount of the
active agent.
Other compositions include suspensions in aqueous liquids or non-aqueous
liquids such as a
syrup, elixir or an emulsion.
zs Preparations for parenteral administration include sterile aqueous or non-
aqueous
solutions, suspensions, and emulsions. Examples of non-aqueous solvents are
propylene
glycol, polyethylene glycol, vegetable oils such as olive oil, and injectable
organic esters such
as ethyl oleate. Aqueous carriers include water, alcoholic/aqueous solutions,
emulsions or
suspensions, including saline and buffered media. Parenteral vehicles include
sodium
3r~ chloride solution, Ringer's dextrose, dextrose and sodium chloride,
lactated Ringer's or fixed
oils. Intravenous vehicles include fluid and nutrient replenishers,
electrolyte replenishers
(such as those based on Ringer's dextrose), and the like. Preservatives and
other additives
may also be present such as, for example, antimicrobials, anti-oxidants,
chelating agents, and
inert gases and the like. Lower doses will result from other forms of
administration, such as
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-17-
intravenous administration. In the event that a response in a subject is
insufficient at the
initial doses applied, higher doses (or effectively higher doses by a
different, more localized
delivery route) may be employed to the extent that patient tolerance permits.
Multiple doses
per day are contemplated to achieve appropriate systemic levels of compounds.
s The FcsRI(3 chain variant polypeptides or functionally equivalent fragments
thereof
may be combined, optionally, with a pharmaceutically-acceptable carrier. The
term
"pharmaceutically-acceptable carrier" as used herein means one or more
compatible solid or
liquid filler, diluents or encapsulating substances which are suitable for
administration into a
human. The term "carrier" denotes an organic or inorganic ingredient, natural
or synthetic,
~o with which the active ingredient is combined to facilitate the application.
The components of
the pharmaceutical compositions also are capable of being co-mingled with the
molecules of
the present invention, and with each other, in a manner such that there is no
interaction which
would substantially impair the desired pharmaceutical efficacy.
When administered, the pharmaceutical preparations of the invention are
applied in
!s pharmaceutically-acceptable amounts and in pharmaceutically-acceptable
compositions.
Such preparations may routinely contain salt, buffering agents, preservatives,
compatible
carriers, and optionally other therapeutic agents. When used in medicine, the
salts should be
pharmaceutically acceptable, but non-pharmaceutically acceptable salts may
conveniently be
used to prepare pharmaceutically-acceptable salts thereof and are not excluded
from the scope
.n of the invention. Such pharmacologically and pharmaceutically-acceptable
salts include, but
are not limited to, those prepared from the following acids: hydrochloric,
hydrobromic,
sulfuric, nitric, phosphoric, malefic, acetic, salicylic, citric, formic,
malonic, succinic, and the
like. Also, pharmaceutically-acceptable salts can be prepared as alkaline
metal or alkaline
earth salts, such as sodium, potassium or calcium salts.
as FcsRI(3 chain variant polypeptides, or functionally equivalent fragments
thereof,
preferably are produced recombinantly, although such polypeptides may be
isolated from
biological extracts. Recombinantly produced FcsRI(3 chain variant polypeptides
include
chimeric proteins comprising a fusion of a FcsRI(3 chain variant peptide with
another
polypeptide, e.g., a polypeptide capable of providing or enhancing protein-
protein binding,
so sequence specific nucleic acid binding (such as GAL4), enhancing stability
of FcsRI(3 chain
variant polypeptide under assay conditions, or providing a detectable moiety,
such as green
fluorescent protein. A polypeptide fused to a FcERI(3 chain variant
polypeptide or fragment
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-18-
may also provide means of readily detecting the fusion protein, e.g., by
immunological
recognition or by fluorescent labeling.
Various techniques may be employed for introducing FcsRI(3 chain variant
encoding
nucleic acids of the invention into cells, depending on whether the nucleic
acids are
s introduced in vitro or in vivo in a host. Such techniques include
transfection of nucleic acid-
CaP04 precipitates, transfection of nucleic acids associated with DEAE,
transfection with a
retrovirus including the nucleic acid of interest, liposome mediated
transfection, and the like.
For certain uses, it is preferred to target the nucleic acid to particular
cells. In such instances,
a vehicle used for delivering a nucleic acid of the invention into a cell
(e.g., a retrovirus, or
ro other virus; a liposome) can have a targeting molecule attached thereto.
For example, a
molecule such as an antibody specific for a surface membrane protein on the
target cell or a
ligand for a receptor on the target cell can be bound to or incorporated
within the nucleic acid
delivery vehicle. For example, where liposomes are employed to deliver the
nucleic acids of
the invention, proteins which bind to a surface membrane protein associated
with endocytosis
~s may be incorporated into the liposome formulation for targeting and/or to
facilitate uptake.
Such proteins include capsid proteins or fragments thereof tropic for a
particular cell type,
antibodies for proteins which undergo internalization in cycling, proteins
that target
intracellular localization and enhance intracellular half life, and the like.
Polymeric delivery
systems also have been used successfully to deliver nucleic acids into cells,
as is known by
zo those skilled in the art. Such systems even permit oral delivery of nucleic
acids.
Other delivery systems can include time-release, delayed release or sustained
release
delivery systems. Such systems can avoid repeated administrations of the anti-
inflammatory
agent, increasing convenience to the subject and the physician. Many types of
release
delivery systems are available and known to those of ordinary skill in the
art. They include
Zs polymer base systems such as poly(lactide-glycolide), copolyoxalates,
polycaprolactones,
polyesteramides, polyorthoesters, polyhydroxybutyric acid, and polyanhydrides.
Microcapsules of the foregoing polymers containing drugs are described in, for
example, U.S.
Patent 5,075,109. Delivery systems also include non-polymer systems that are:
lipids
including sterols such as cholesterol, cholesterol esters and fatty acids or
neutral fats such as
3o mono- di- and tri-glycerides; hydrogel release systems; sylastic systems;
peptide based
systems; wax coatings; compressed tablets using conventional binders and
excipients;
partially fused implants; and the like. Specific examples include, but are not
limited to: (a)
erosional systems in which the anti-inflammatory agent is contained in a form
within a matrix
such as those described in U.S. Patent Nos. 4,452,775, 4,667,014, 4,748,034
and 5,239,660
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-19
and (b) difusional systems in which an active component permeates at a
controlled rate from a
polymer such as described in U.S. Patent Nos. 3,832,253, and 3,854,480. In
addition, pump-
based hardware delivery systems can be used, some of which are adapted for
implantation.
Use of a long-term sustained release implant may be particularly suitable for
treatment
s of chronic conditions. Long-term release, are used herein, means that the
implant is
constructed and arranged to delivery therapeutic levels of the active
ingredient for at least 30
days, and preferably 60 days. Long-term sustained release implants are well-
known to those
of ordinary skill in the art and include some of the release systems described
above.
In other aspects, the agents of the invention are "co-administered," which
means
~o administered substantially simultaneously, with another anti-allergic agent
other than an
FcsRI(3 chain variant molecule. By substantially simultaneously, it is meant
that an FcsRI(3
chain variant related molecule of the invention is administered to a subject,
as an admixture in
a single composition, or sequentially, close enough in time with the
administration of the
other anti-allergic agent, whereby the two compounds may exert an additive or
even
is synergistic effect, e.g. decreasing or completely eliminating a Type I
hypersensitivity
response.
The invention also embraces determining whether a subject has a condition
mediated
by IgE or a predisposition thereto. The method involves determining whether an
FcsRI(3
chain variant according to the invention is present in a subject, and/or
whether this variant is
?n expressed at different levels in the subject when compared to a control. In
some
embodiments, the method involves determining whether the FcsRI(3 chain variant
is present
and/or expressed at lower levels (amounts) in the subject in comparison to a
control. By
"control" as used herein, refers to FcsRI(3 chain variant expression in a
control subject. A
"control subject" is an apparently healthy subject with no symptoms indicative
of a condition
's mediated by IgE, or predisposition to a condition mediated by IgE (e.g., no
family history of a
condition mediated by IgE ). In a preferred embodiment, the Fc~RI(3 chain
variant is a
polypeptide designated herein as FcsRI(3T having an amino acid sequence as set
forth in SEQ
ID N0:4. Variant polypeptide FcsRI(3T arises from an FcsRI(3 chain mRNA
(having a cDNA
as depicted in SEQ ID N0:3), in which intron 5 (402 nucleotides in length) of
the genomic
3o FcsRI(3 chain gene, is not spliced out and forms part of the mRNA. The
resulting translated
variant polypeptide, FcsRI(3T, is shorter than the wild type FcsRI(3
polypeptide because of an
in frame early termination signal in the the fifth intron and, as a result, is
missing the C-
terminus of the wild type protein (see Figure 1 ).
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-20-
In determining the presence and/or amount of an FcERI(3 chain variant of the
invention, a suitable tissue sample is obtained from the subject suspected of
having a
condition mediated by IgE or a predisposition thereto. Lymph or blood are the
preferred
tissues where samples can be obtained, but other tissues that contain cells of
hematopoietic
s origin (such as mast cells and basophils) can also be used according to the
invention. A
preferred method for determining the presence and/or levels of FcsRI(3 chain
variant
expression according to this aspect of the invention is quantitative RT-PCR
(54-57). Given
the teachings of the present invention and the public availability of the wild
type FcsRI(3
genomic sequence, the skilled artisan can easily select a pair of primers that
spans any of the
m introns of the FcsRI(3 genomic sequence. In certain embodiments, primers
that span intron 5
(e.g., from exons 2 and 7) to perform the detection and/or quantitation of the
variant trancript
are selected. Examples of such primers include those whose sequences are set
forth in SEQ
ID NOs 5 and 6. The assay is performed in parallel with or in reference to a
control assay in
which samples obtained from control subjects, preferably of like tissue, are
used to establish a
~s control amount of the variant. The wild type transcript optionally serves
as an internal control
for the reaction and/or quantitation. In some embodiments, a lower level
(amount) of the
FcsRI(3 chain variant transcript relative to an FceRI(3 chain variant
transcript of a control
subject, is indicative of the subject having a condition mediated by IgE or a
predisposition
thereto.
zo The invention will be more fully understood by reference to the following
examples.
These examples, however, are merely intended to illustrate the embodiments of
the invention
and are not to be construed to limit the scope of the invention.
Examples
zs ExRerimental procedures
Cell Culture
NIH3T3 a(3y2 transfectants were maintained in DMEM (Biofluids, Rockville, MD)
with 10% calf serum (Biofluids, Rockville, MD) and 300 ~g/ml of hygromycin
(Calbiochem,
La Jolla, CA) and 500 ~g/ml of neomycin (Gibco/BRL, Baltimore, MD). All the
other NIH-
30 3T3 transfected cell lines were maintained in the same medium without
neomycin. U937
transfected cell lines were maintained in RPMI-1640 (Biofluids) with 20% fetal
bovine serum
(Biofluids) and 1 mg/ml of neomycin. Mouse Bone Marrow-derived Mast Cells
(BMMC)
were obtained as previously described (42, 43). Human Cord Blood Mast Cells
were
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-21
generated as previously described (44). In brief, mononucleated cells were
isolated from
human umbilical cord blood by centrifugation over Histopaque-1077 (Sigma), and
then were
cultured in Iscove's modified Dulbecco's medium supplemented with 10 % FBS,
100 ng/ml
recombinant human stem cell factor (Biosource), 10 ng/ml recombinant human IL-
6
s (Endogen) and 1 mM prostaglandin E2 (Cayman Chemical, Ann Arbor, MI). The
entire
culture medium was changed 3 times a week during the first 2 weeks, then half
of it was
replaced weekly, and the cells were maintained in culture for at least 10
weeks. Human
basophil-enriched leukocyte suspensions were prepared over a single-step
Percoll gradient
according to an etablished protocol (45).
m Expression Vectors
The human a subunit and mutated forms of rat (3 cDNA ((3YM, tyrosines at
position
218, 224, and 228 with the (3ITAM were changed to phenylalanines) of FcERI
were subcloned
into pCDL-SRa 296. The rat a and y subunits of FcERI were subcloned into
pSHSX, a
pCDL-SRa 296 derivative containing a hygromycin resistance cassette. Human (3
and y
~s subunit, rat ~3 subunit cDNAs and the mutated form of rat y cDNA (yYM,
tyrosines at position
65 and 76 within the y ITAM were changed to phenylalanines) were subcloned
into pBJlneo,
a neomycin derivative of pCDL-SRa 296 (15). The trunctated forms of rat (3
((3NT, the first
57 amino acids of NH2-terminal cytoplasmic domain were deleted; (3CT, the last
38 amino
acids of COON-terminal cytoplasmic domain were deleted) have been described
(40). (3NT
ao and (3CT were subcloned into pCDL-SRa 296. To construct the181L-183L and
2376 (3
variants, the WT (3 cDNA subcloned in the eukaryotic expression vector pBJlneo
was used as
a template for site-directed mutagenesis with the Quick-change kit
(Stratagene) using two
different oligonucleotides containing the sequences from the 181L-183L and
2376 vaxiants.
Confirmation of the mutations was obtained by DNA sequencing. For the spice
variant of
as human FcERI(3 (see RT PCR paragraph below). The /3WT and splice variant
cDNAs were
FLAG-tagged at the N terminus using the FLAG epitope (Kodak, New Haven, CO).
The
flagged cDNAs were subcloned in pBJ 1 neo.
Antibodies
The monoclonal anti-phosphotyrosine antibody (4610) was purchased from Upstate
sn Biotechnology, Inc. (Lake Placid, NY). PE-streptavidin was from PharMingen
(San Diego,
CA). The monoclonal anti-DNP IgE used in culture supernatant form, rabbit anti-
mouse IgE,
anti-rat FcsRI~3N tail monoclonal antibody (JRK), rabbit anti-FcRy subunit
antibody and
monoclonal anti-human-FcsRIa subunit antibody (15-1), were prepared as
described (15).
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-22-
Rabbit anti-porcine syk, and anti-marine lyn were described previously ( 14).
The anti-human
syk antibody 996 was raised against a synthetic peptide representing amino
acids 150-159 of
human syk sequence (15). Monoclonal anti-rat FcsRI(3C tail antibody (NB) was
from Dr. D
Holowka, and chimeric anti-Nip human IgE were from Dr. Z. Eshhar. The
monoclonal FLAG
s antibody covalently attached to agarose beads was from Sigma (Saint Louis,
MO).
Recombinant Vaccinia hirus Construction
Recombinant vaccinia virus expressing marine Lyn A kinase, porcine Syk kinase,
and
virus with the empty pSC-65 vaccinia recombinant plasmid were constructed as
described
( 14).
m Construction of NIH 3T3 and U937 sublines
The generation of a[3y2 cells has been previously described (14). Construction
of
other NIH-3T3 sublines expressing FceRI variants was similar. In brief, cells
were co-
electroporated (270V, 960~F) with appropriate constructs. Resistant clones
were selected,
and screened for surface FcERI expression by flow cytometry with FITC-IgE.
U937 cells
Is were co-transfected by electroporation (260V, 960~F) with human a, (3 and y
constructs or
with a and y constructs only. Cells were grown under 1 mg/ml 6418 selection,
and resistant
clones were selected for FcERIa surface expression by binding with
biotinylated human IgE
and PE-labeled streptavidin. Two clones of each type (a~iy-2 and a(3y-8, ay-1
and ay-10)
were selected for analysis. Transient transfection KU812 cells (5 x 106) were
co-transfected
2n by electroporation (300V, 960 ~F) with 10 ~.g of (3T expression vector and
1 ~g of green
fluorescent protein (GFP; pGreen Lantern, Gibco). Human CD81 subcloned in
PBJlneo was
used as a control vector (Fleming 1997). At different times after the
transfection, FceRI
expression was analysed on GFP positive gated cells.
Infection with Recombinant Vaccinia Viruses
2s Adherent NIH 3T3 cells in one 150cm2 culture flask were infected with the
appropriate virus at 5 pfu/cell in 5 ml DMEM with 2.5% calf serum for 30 min
at 4°C with
gentle rocking, then for 30 min at 37°C. Control virus bearing the
pSC65 vaccinia
recombinant vector was added to single recombinant virus infection in order to
allow valid
comparisons with recombinant virus coinfections.
3n Cell Activation with Antigen and Lysis
Infected NIH-3T3 sublines (3-5 x 106 cells/sample) or U937 sublines (3 x 10'
cells/sample) were harvested in 1 ml medium. Cells were stimulated with 200
ng/ml DNP24-
40-HSA (Sigma, St Louis, MO) in 1 ml for 4 min at room temperature with
rocking. Towards
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-23-
the end of stimulation, cells were pelleted briefly in a microcentrifuge, the
medium was
aspirated and the cell pellet was resuspended in ice cold lysis buffer (0.5%
TritonX-100, 150
mM NaCI, 200 mM boric acid [pH 8.0], 5 mM EDTA, 5 mM sodium floride, 1 mM
sodium
vanadate, 10 ~g/ml pepstatin, 5 ~.g/ml leupeptin, 10 ~g/ml aprotinin) at a
ratio of 3 x 107
s cells/ml of lysis buffer, and kept on ice for 10-15 min. Lysates were spun
for 10 min at
14,000 rpm to remove cell nuclei prior to immunoprecipitation or SDS-PAGE. In
experiments
involving a[3Tyz receptor, cells were lysed in 10 mM CHAPS instead of 0.5 %
TritonX-100.
Immunoprecipitations, In Vitro Kinase Assays, Western Blotting
Immunoprecipitations were performed as previously described (14), except for
the
io cells lysed in 10 mM CHAPS. In this case immunoprecipitates were washed
with lysis buffer
containing 2 mM CHAPS. In vitro kinase assays were performed as described
(15), and
analyzed by SDS-PAGE and autoradiography. Where indicated, immunoprecipitates
were
treated with endo-(3-N-acetylglucosamidase (Endo H) (New England Biolabs) as
previously
described (47). After immunoprecipitation with appropriate antibodies, samples
were
~s resolved on SDS polyacrylamide gel, transferred to PVDF membrane, and
blotted with the
antibodies indicated. Immunoreactive proteins were visualized by using
alkaline phosphatase
coupled second-step reagents and enhanced chemifluorescence (ECF, Amersham).
Fluorescence was quantified using a Storm scanner (Molecular Dynamics). Where
appropriate, cells were treated with proteasome inhibitors (ALLN 250 ~M)
(Calbiochem) or
~n vehicle for 2 hours at 37°C before lysis.
Measurement of Calcium Moblization
U937 transfectant cells were washed and incubated with 2 ~M Fura-2
acetoxymethyl
ester (Moleular Probes, Portland, OR) and 0.08% Pluronic F-127 (Molecular
Probes) in
calcium buffer (135 mM NaCI, 5 mM KCI, 1 mM CaClz, 1 mM MgCl2, 5.6 mM glucose,
10
~s mM HEPES, pH 7.4, 0.1% BSA and 2.5 mM Probenecid [Sigma, St. Louis, MO])
for 1 hr
with gentle rocking at room temperature. Cells were washed once in the same
buffer, and
transferred into the cuvette of a Deltascan spectrofluorometer (Photon
Technology
International Inc., South Brunswick, NJ). Fura-2 loaded cells (5 x 106 cells
in 2 ml calcium
buffer) were exposed to 5 ~g/ml of biotinylated human IgE for 10 min before
the addition of
30 25 ~g/ml streptavidin at at room temperature. Calcium moblization was
monitored and
quantified as previously described (15).
Targeting of FcR/3 Gene
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-24-
The mouse FcR[3 gene was isolated from a 129 ~,-DASH library (49) (a gift from
Dr.
P. Love, NIH, Bethesda, MD). Targeted disruption of the gene was achieved by
replacement
of exons IV-VI (corresponding to by 344-577 of the mouse FcR(3 cDNA sequence -
SEQ ID
N0:9) (GenBank Acc. No. AB033617, and 50) with a Neo cassette. Briefly, a
genomic 4 kb
s fragment encompassing exons I-IV (bp 38-343 of the mouse FcR[3 cDNA
sequence) and a
genomic 1.4 kb fragment encompassing exons VI-VII were amplified by polymerise
chain
reaction using appropriate primers and cloned respectively into the XhoI and
XbaI of pJNS2
vector (42). The construct was linearized by NotI and electroporated into D3
or E14 TG2a
ES cells. ES cells were grown and selected as previously described (48).
Genomic DNA was
m extracted from clonal ES cells, digested with DraI and hybridized by
Southern blot to a 440
by fragment located 16 by downstream of exon VII. The hybridizing bands were
3.4 kb for
the WT and 2.8kb for the disrupted allele. Two out of 109 6418 resistant-
ganciclovir
sensitive clones were found positive for homologous recombination.
Animals
is Chimera, heterozygous (+/-) and homozygous (-/-) animals for the disrupted
FcR(3
allele were generated as previously described (48); FcR(3 -/- animals were on
a Balb/c F2
background. FceRIa -/-animals have been previously described (42), and were on
a Balb/c F6
background. FcR(3 -/- animals were compared to age-matched Fc~RIa -/- for all
relevant
experiments. FcR(3 -/- were also crossed with the previously described
transgenic mice for
2n the human FcERIa gene (hFcsRIaTg) (43). Progeny of the second generation
thus contained
hFcsRIaTg/FcR(3-/- or hFcERIaTg/FcR(3 +/- animals. Littermates were used in
all
appropriate experiments. Human hFcsRIaTg mice used for these crosses were in a
Balb/c
WT background, so that hFcERIaTg/FcR(3 +/- animals were expressing both human
and
marine FcERI on their mast cells. Animals used for the study of hFcERI
cellular distribution
?s were on a FcsRIa -/- background and were thus expressing only hFcERI.
Degranulation
Cellular degranulation was measured by the release of (3-hexoseaminidase as
described before with minor modifications (51). Cells were incubated with 50
ng/ml/106 cells
humanIgE over night or with various doses of anti- FcsRIa (15-1) for 2 hours,
washed twice
3o and stimulated with NIP-BSA or with 10 ~g/ml goat anti-mouse IgG F(ab')2
for 20 min.
Anaphylaxis
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-25-
Anaphylactic reactions were induced and measured as described in Dombrowicz et
al.,
1997 (52), except that human IgE-induced systemic anaphylaxis was achieved by
3 iv
injections of 50 pg anti-NIP hIgE in 200 p1 PBS at 12h intervals followed 4h
later by an iv
challenge with lmg NIP-BSA in 200 ~1 PBS.
s RT PCR
Total RNA was isolated with RNAzoI B (Tel-Test, Inc., Friendswood, TX)
following
manufacturer instructions. Aliquots of 1 ~,g of total RNA were converted to
cDNA by using
poly(dT) 18 primers and transcribed with 100 units of Moloney murine leukemia
virus reverse
transcriptase (Clontech, Palo Altp, CA) at 42°C for 60 min. For PCR,
aliquots of DNA
~o equivalent to 0.1 ~g total RNA were used in each reaction (50 ~1)
containing 50 pmol of each
primer, 200 ~,M of each deoxynucleoside trisphosphate, and 1.25 units of Taq
polymerase
(Fisher Scientific, Pittsburg, PA). (3WT and its splice variant were amplified
using a sense
primer located in exon 2 (5'-GTGCCTGCATTTGAAGTCTTG-3', SEQ ID NO:S) and
antisense primer located in exon 7 (5'-TGGATCCTTGGCTGTGAATC-3', SEQ ID N0:6).
~s The PCR reactions were performed under the following conditions : lmin at
94°C, followed
by 22 to30 cycles of 45 sec at 94°C, 45 sec at 60°C, 1 min at
72°C. PCR products were
visualised on 1.2 % agarose gels stained with ethidium bromide.
Cloning of PCR Products
PCR products were isolated after agarose gel separation, purified with
QIAquick PCR
~n Purification kit (QIAGEN, Valencia, CA) and cloned into a pCRII vector
using TA cloning
(Invitrogen).
RNase Protection Assay (RPA)
Total RNA from untransfected or transfected U937 cells was prepared with
RNAzoI B
(Tel-Test, Inc., Friendswood, TX). Poly A RNA from CBMC was purified using the
zs FastTrack 2.0 kit (Invitrogen, Carlsbad, CA). Antisense radioactive RNA
probes were
generated by in vitro transcription using the Maxiscript kit (Ambion, Austin,
TX). The ~3
probe was designed to overlap the end of exon 5 and the beginning of intron 5.
Probes were
also designed in the oc and y cDNAs and used as controls. RPA was performed
using the
RPAIII kit (Ambion, Austin, TX). Ten ~g total RNA from U937 cells and the mRNA
3n obtained from 30 x 106 CBMC were incubated with 105 cpm freshly prepared
probe, and
probe was then digested in the presence of 0.375 units RNAse A and 15 units
RNAse T1 or
buffer. Protected probe fragments were separated by denaturing polyacrylamide
gel
electrophoresis. Gels were exposed to fluorescent screens that were read on a
Storm 840
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-26-
scanner (Molecular Dynamics, CA), and analyzed using the ImageQuant software
(Molecular
Dynamics, CA).
Pulse-Chase Analysis
45 x 106 actively growing cells were washed twice in cysteine and methionine-
free
s RPMI medium supplemented with 1 mM L-glutamine and 10 mM Hepes. Cells were
then
incubated in the same medium containing 10% dialyzed FBS for 20 min at
37°C. This
medium was then removed and the cells at 5 x 106/m1 were pulsed with 0.2
mCi/ml TRAN
35S-LABEL (ICN) for 10 min at 37°C. An aliquot of 15 x 106 cells for
time 0 was collected,
and the remaining cells were washed and resuspended in complete medium
supplemented
m with cysteine and methionine for the chase times indicated. At each time
point, 1 S x 106 cells
were pelleted by centrifugation, washed twice with ice-cold PBS and
resuspended in 0.5 ml of
ice-cold lysis buffer (0.5 % Triton X-100, 300 mM NaCI, 50 mM Tris, pH 7.5) in
the
presence of protease inhibitors). Samples were then precleared and
immunoprecipitated with
anti-FLAG antibody as above. Proteins were separated on SDS 14% polyacrylamide
gels,
~s and the dried gels were exposed to a Molecular Dynamic phosphor Image
screen for 15 h.
Radiolabeled proteins detected by PhosphorImager were quantitated with Image-
Quant
software (Molecular Dynamics).
Example 1: FcsRl/3 is a signal amplifier
The FcERI(3 molecule is a unique subunit among human antigen receptors. It has
a
~n topology where both tails are cytoplasmic, and contains an atypical ITAM
motif with an extra
tyrosine and decreased spacing relative to the consensus.
We used a fibroblast based reconstitution system to investigate (3 function.
NIH3T3
stably transfected with human FcERI a~3y cDNAs are infected with vaccinia
viruses
separately encoding the Lyn and Syk tyrosine kinases. Surface a levels were
assessed by flow
a cytometry and then antigen stimulation of equivalent numbers of FcsRI
between clones was
performed. When a(3y2 receptors are stimulated, there is substantially
enhanced Syk tyrosine
phosphorylation, Syk kinase activity, and FcsRIy tyrosine phosphorylation
relative to that
achieved by stimulation of aye receptors. These data were subsequently
reproduced precisely
in a hematopoietic cell line system, the U937. The latter cell line also
enabled us to perform a
3n functional assay to assess whether the biochemical amplification observed
translated to a
significant increase in a functional response to antigen stimulation.
We examined FcsRI induced calcium mobilization in U937 transfected with either
ayz
or a(3y2 receptors. Calcium mobilization by FcsRI is biphasic, an early phase
of IP3-
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-27-
dependent calcium release from intracellular stores is followed by a sustained
calcium influx.
We also observed that in two a(3y2 expressing U937 clones, relative to two aye
clones, there
is a remarkable increase in the magnitude of FcERI induced calcium signals.
This translates
the amplification of early signaling events to an enhancement in a functional
response.
s Calcium signals contribute to allergic mediator secretion and controls
induction of several
cytokine genes (e.g. IL-4, IL-6) in mast cells. Therefore, the data suggested
that the presence
or absence of (3 profoundly affected the outcome of FcsRI signaling at the
whole animal level.
We generated a novel humanized FcERIa transgenic mouse to assess the
contribution
of FcERI(3 to in vivo responses. In bone marrow derived mast cells (BMMC)
prepared from
m a~3y2 or aye animals, we found that the biochemical evidence for an
amplifier function of (3
(e.g., enhanced FcsRIy phosphorylation), exactly paralleled that seen in our
in vitro system.
We also found a similar enhancement of calcium mobilization. Most importantly,
we were
able to assess in vivo functional responses in the absence and presence of (3.
We observed
that secretion of allergic mediators in response to antigen stimulation of
BMMC is
~s significantly enhanced in a(3y2 mice relative to aye, an effect also
observed for IL-6
production. Finally, even systemic anaphylactic responses were amplified by
(3.
Example 2: FcsRl~3 is an amplifier of receptor surface expression
Stable cell lines expressing FcsRI isoforms are important tools in our
analyses of
FcERI biology. We generated such lines in both fibroblast and hematopoietic
cell
Zn backgrounds (NIH 3T3 and U937), and analyzed surface FcsRI expression by
flow
cytometry. There is, in all cases, a statistically significant difference
between surface
expression of aye and a(3y2 receptors. The presence of (3 correlates with
increased expression
of receptor at the cell surface in both NIH3T3 and U937 cells. Moreover, these
results were
subsequently reproduced in transient expression systems.
zs These data led us to the intriguing possibility that the presence of (3 may
enhance
FcsRI surface levels. We tested the idea that (3 could inducibly alter FcERI
expression levels.
FcsRI(3 was transiently transfected into KU812 which had been stably
transfected with a~yz
receptors. KU812 are a transformed mast cell line with no endogenous
expression of FcERI at
their surface. We stained the cells for surface a levels and analyzed the
population over a 48h
3n time course. We observed that from 15h after transfection onward, KU812
transfected with
FcsRI(3 have significantly enhanced FcsRI surface expression relative to
controls. Thus,
introduction of FcERI(3 induces expression of FceRI complexes at the cell
surface.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-28-
Without wanting to be bound by a particular hypothesis, we formulated two
hypotheses which, while not mutually exclusive, could explain the apparent
ability of (3 to
enhance FcERI surface expression. First, [3 could be promoting the trafficking
of FcsRI
subunits from their sites of synthesis in the ER to the plasma membrane.
Second, (3 may affect
s the stability of surface and nascent complexes, decreasing their turnover
and causing a
cumulative increase in surface receptor numbers. We then addressed whether
either or both of
these mechanisms were likely to operate.
FcsRI complexes undergo the following maturation process: 1) In the ER,
subunits
associate non-covalently and a is core-glycosylated, and thus sensitive to the
action of
~o Endoglycosidase H (Endo H). 2) Trafficking from ER to Golgi follows; in the
Golgi, terminal
glycosylation occurs which places complex sugars (Endo H resistant) on the a
chain. We can
use SDS-PAGE to look at trafficking of nascent a(3y2/ay2 complexes,
differentiating between
stages on the basis of molecular weight and EndoH sensitivity.
We compared trafficking of FcERIa in the absence and presence of FcsRI(3. We
used
~s stable cell lines expressing either a(3y2 or aye, and immunoprecipitated a
chains from lysates
treated either with vehicle or Endo H. Samples were resolved by SDS-PAGE and
Western
analysis was performed using polyclonal anti-a. Three a species are apparently
visible.
Unglycosylated (early ER) a appears as a single, 30 kDa band; immature a,
which is
glycosylated, runs at 48 kDa, and is sensitive to Endo H. Endo H treatment
leads to a
Zn reduction in intensity of this 48 kDa species and a concomitant increase in
levels of
unglycosylated a. Terminally glycosylated a runs as a diffuse species at
around 66 kDa. In
contrast with the 48 kDa species, mature, complex-sugar glycosylated, a is
resistant to Endo
H. Clear differences in a content are observed between aye and a(3y2 cell
lines. In the
presence of (3 chains there is less of the Endo H sensitive (immature) a
species and an
?s increased amount of mature a chains. Densitometry was performed to
quantitate these
differences and a ratio of mature to immature a chains was calculated. Thus,
the presence of
(3 affects the intracellular trafficking of a chains, apparently accelerating
their progress
towards a mature form.
Example 3: A novel splice variant of ~3, /3I; is an intrinsic downregulator of
FcsRl
3o surface expression
When RT-PCR was performed with the pair of (3 chain specific primers whose
sequences are set forth in SEQ ID NOs 5 and 6, on human cord blood derived
mast cells or
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-29-
peripheral blood basophils, a second (3 transcript is observed, at 1.1 kb,
compared with the
0.7 kb expected transcript. The additional transcript is present in all the
patient samples
analyzed. We hypothesized that this additional mRNA species could correspond
to an
alternate splice form of (3.
s The additional PCR product was purified, subcloned and placed in a mammalian
expression vector. Sequencing revealed that the variant form of (3 was indeed
a product of
alternate splicing. The domain structure of the human FcERI~3 gene is shown in
Figure 1. In
the alternate splice form of (3, intronic sequence from the fifth intron
contributes a novel 16 as
sequence that replaces the normal (3 carboxyl terminus and terminates with a
stop codon from
rn the intron.
The splice variant of (3 would produce a truncated protein, (3T, lacking the C-
terminal
transmembrane and cytoplasmic domains. Since the new 16 as sequence has some
hydrophobic character, it is possible that there is some interaction with the
membrane to form
an imperfect transmembrane domain. Alternatively, (3T may be topologically
distinct from
~s wild-type ((3WT), with the C- and N-termini on opposite sides of the
membrane.
In order for the splice variant to have any important biological function, it
is a
prerequisite that both the transcript and truncated protein are actually
produced. To test
whether this was the case, we first performed RNAse protection assays (RPA) to
test that (3T
was a transcript that could be detected in normal cell and was not a PCR
artifact. RPA can
.o detect as little as 5 fg RNA. We used an antisense probe covering the 3'
end of (3 exon 5 and
the 5' end of (3 intron 5. We performed the RNase Protection Assay in U937
cells
(untransfected, or transfected with either a(3y2 or a(3Ty2), or primary human
cord blood
derived mast cells.
We next assessed whether (3T was translated into a protein. In Western
analysis of
~s CBMC lysates, the (3T signal was present, but extremely hard to visualize.
Transfection of [3T
into U937 caused the appearance of a band that co-migrated with the low
molecular weight
form of (3 seen in the CBMC, suggesting that in fact we were able to see the
(3T protein
species in human mast cells. The difficulty in visualizing ~iT protein could
be attributed to
either 1) (3T being a very low abundance molecule, or 2) (3T being rapidly
turned over, having
3n a short half life and thus very little steady-state accumulation. We
performed 35S-
methionine/cysteine metabolic labeling on U937 cells transfected with either
a[3y2 or a(3Ty2.
FcsRI~3 or (3T were immunoprecipitated from lysates using their FLAG epitope
tags and
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-30-
samples were resolved by SDS-PAGE. After autoradiography, we observed that
wild-type (3
was stable over the 30 min time course. In contrast, we observed that (3T had
an extremely
short half life. Densitometry indicated that (3T had a half life of
approximately 10 min under
the conditions of this experiment.
We observed herein that (3T is an expressed variant protein. It is reasonable
to assume,
therefore, that (3T may form FcsRI complexes. However, due to its variant
structure and
rapid turnover, we might expect that FcsRI formed of a(3Ty would behave
differently than
their wild-type counterparts. We generated novel a(3Ty2-expressing NIH 3T3 and
U937 cell
lines. We then examined the surface FcsRI expression levels in multiple clones
expressing
io either aye, a(3y2 or a(3TyZ receptor isoforms. Cells were stained for
surface a chain levels as
described above. We observed that receptors with the wild-type (3 chain are
significantly more
expressed at the cell surface that the aye isoforms. Remarkably, the presence
of (3T decreases
surface expression of a to levels consistently below those seen with ay
transfectants. This
effect was statistically significant in both the U937 and NIH 3T3 cell lines.
Since (3T is
~s rapidly turned over, (3T can therefore potentially negatively regulate cell
surface expression of
a by causing turnover of nascent receptor complexes.
To confirm this finding, we took a stable cell clone expressing a(3WTy and
retransfected it in transient with either control cDNA, or the (3 variant
cDNA. We then
assessed the level of surface receptor expression as before. Transient
transfection of the (3
.n variant in a(3WTy2 clones resulted in a significant decrease in surface
FcsRI receptor
expression. These results show that the (3T variant acts as a dominant
negative form of (3 for
receptor expression, capable of competing with WT FcERI(3, and of actively
preventing FcsRI
receptor expression.
Time (hours after control DNA (3T cDNA p value
retransfection) pT versus control DNA
8 (n=I) 44 42
16 (n=3) 42.3 t I .80 36.6 t 2.28 p = 0.028
24 (n=4) 39.1 t 2.93 28.8 ~ 3.91 p = 0.005
40 (n=4) 37.1 ~ 2.31 26.8 ~ 3.61 p = 0.005
The results are expressed as mean tSD of MFI. n = number of samples from 3
independent experiments pooled together. Unpaired t tests were used to
calculate p values.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-31-
Example 4: The ~3T Fc~RI~i variant acts by preventing a maturation
We also compared the intracellular trafficking of the a chain in the absence
of ~3, in
the presence of WT FcERI(3, and in the presence of the (3T FcsRI(3 variant.
Intracellular
trafficking of the a chain can be followed quite easily experimentally due to
the fact that a is
s highly glycosylated, and that this glycosylation is modified during
trafficking. In the ER,
FcsRI is glycosylated with high manose type sugars which can be cleaved in
vitro by
treatment with the enzyme Endoglycosidase H (Endo H). During trafficking
through the
Golgi apparatus, these sugars are replaced by complex sugars which are
resistant to the action
of Endo H. These different forms of FcsRIa can be identified based on their
Endo H
m sensitivity, and apparent molecular weight after separation by
polyacrylamide gel
electrophoresis (PAGE). We observed a band around 30 kD that corresponds to
unglycosylated FcsRIa, a band around 46 kD that corresponds to high manose
glycosylated
pre-Golgi FcsRIa, and a smear around 66 kD that corresponds to mature post-
Golgi FccRIa.
These characteristics can be used to interpret other experiments without
performing
Is biosynthetic labeling and EndoH digestion, but with a simple PAGE and
western with an anti-
FcERIa antibody (Ab). Figure 2 shows the results of such an experiment. Three
clones each
of transfectants expressing either aye, a(3WTyz, or a(3Ty2 were lysed,
immunoprecipitated
with an anti- FcsRIa Ab, separated by PAGE, and Western blotted with an anti-
FcsRIa Ab.
Ab binding was revealed by chemifluorescence and each band was quantified. For
each clone
2o the amount of mature post-Golgi FcsRIa was plotted as a function of
immature ER FcERIa.
Comparison of the different types of transfectants shows that for a given
amount of mature
post-Golgi FcsRIa, a(3WTy2 clones have much less immature ER FcsRIa than aye,
and even
less than a(3Ty2. This shows that the presence of the FcERI(3 variant results
in an inefficient
maturation of FcERIa which is more pronounced than in the absence of WT
FcsRI(3,
zs confirming the active role of the (3T FcsRI(3 variant in preventing
receptor expression.
Example 5: The FcsRI/3 variant is degraded by the proteasome
We also tested whether the (3 variant is degraded by the proteasome. We
observed that
when the (3 variant is immunoprecipitated and revealed by Western blotting, it
is barely
detectable in transfectants, even though the amount of (3 variant protein made
by the cell is
3n probably large compared to endogenous proteins since transcription of the
transfected cDNA
is controlled by a strong viral promoter. By contrast, when cells are
incubated before lysis
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-32-
with a proteasome inhibitor (calpain inhibitor I), the (3 variant is easily
detected. We conclude
that at least one degradation pathway utilized by the (3 variant involves the
proteasome.
In summary, we have described 1) the identification of a novel FcsRI(3 splice
variant,
(3T, 2) that (3T is a translated variant protein with the C-terminal
transmembrane/cytoplasmic
s domains of (3 removed and replaced with 16 amino acids derived from intronic
sequence, 3)
that ~iT is unstable, with an apparent half life of approximately 10 min, and
4) that a/3Ty
receptor isoforms are very inefficient at attaining the cell surface, in
comparison with
either a(3y or ay complexes. These data therefore support that (3T is an
expressed negative
regulator of FcsRI trafficking to the cell surface, a function that would form
a stark contrast
m with the positive regulatory function of the wild-type (3 molecule. Although
not wanting to be
bound by a particular hypothesis, we believe that the rapid turnover of (3T
represents its
targeting for degradation.
References
rs 1. Kinet, J.-P. 1999. The high-affinity IgE receptor (FcsRI): from
physiology to pathology.
Annu Rev Immunol 17:931-72.
2. Reth, M. 1989. Antigen receptor tail clue. Nature 338:383-384.
3. Cambier, J., editor. 1995. Conserved Signaling motifs in antigen and Fc
receptors. Vol.
7. Seminars in Immunology. Academic Press.
zn 4. Turner, H.K., Kinet, J.-P. 1999. FcsRI signaling; thresholds and tuning
in the generation
of an allergic response. Nature in press.
5. Keown, M.B., A.J. Henry, R. Ghirlando, B.J. Sutton, and H.J. Gould. 1998.
Thermodynamics of the interaction of human immunoglobulin E with its high-
affinity
receptor FcERI. Biochemistry 37, no. 25:8863-9.
~s 6. Presta, L., R. Shields, L. O'Connell, S. Lahr, J. Porter, C. Gorman, and
P. Jardieu. 1994.
The binding site on human immunoglobulin E for its high affinity receptor. J
Biol Chem
269, no. 42:26368-73
7. Henry, A.J., J.-P. Cook, J.M. McDonnell, G.A. Mackay, J. Shi, B.J. Sutton,
and H.J.
Gould. 1997. Participation of the N-terminal region of Cs3 in the binding of
human IgE
3n to its high-affinity receptor FcsRI. Biochemistry 36, no. 50:15568-78.
8. Robertson, M.W. 1993. Phage and Escherichia coli expression of the human
high
affinity immunoglobulin E receptor alpha-subunit ectodomain. Domain
localization of
the IgE-binding site. JBiol Chem 268, no. 17:12736-43.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-3 3-
9. Letourneur, O., S. Sechi, J. Willette Brown, M.W. Robertson, and J.-P.
Kinet. 1995.
Glycosylation of human truncated Fc epsilon RI alpha chain is necessary for
efficient
folding in the endoplasmic reticulum. JBiol Chem 270, no. 14:8249-56.
10. Garman, S.C., J.-P. Kinet, and T.S. Jardetzky. 1998. Crystal structure of
the human
s high-affinity IgE receptor. Cell 95, no. 7:951-61.
11. Alber, G., L. Miller, C.L. Jelsema, N. Varin-Blank, and H. Metzger. 1991.
Structure
function relationships in mast cell high affinity receptor for IgE. J Biol
Chem
266:22613-22620.
12. Irving, B.A., A.C. Chan, and A. Weiss. 1993. Functional characterization
of a signal
m transducing motif present in the T cell antigen receptor ~ chain. J Exp Med
177, no.
4:1093-1103.
13. Letourneur, F., and R.D. Klausner. 1992. Activation of T cells by a
tyrosine kinase
activation domain in the cytoplasmic tail of CD3 epsilon. Science 255:79-82.
14. Scharenberg, A.M., S. Lin, B. Cuenod, H. Yamamura, and J.-P. Kinet. 1995.
~s Reconstitution of interactions between tyrosine kinases and the high
affinity IgE
receptor which are controlled by receptor clustering. EMBO Journal 14, no.
14:3385-94.
15. Jouvin, M.H., M. Adamcewzewski, R. Numerof, O. Letourneur, A. Valle, and
J.-P.
Kinet. 1994. Differential control of the tyrosine kinases Lyn and Syk by the
two
signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem
269:5918
zo 5925.
16. Nishizumi, H., and T. Yamamoto. 1997. Impaired tyrosine phosphorylation
and Ca2+
mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast
cells. J
Immunol 158, no. 5:2350-5.
17. Costello, P.S., M. Turner, A.E. Walters, C.N. Cunningham, P.H. Bauer, J.
Downward,
zs and V.L. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in
signalling
through the high affinity IgE receptor of mast cells. Oncogene 13, no. 12:2595-
605.
18. Zhang, J., E.H. Berenstein, R.L. Evans, and R.P. Siraganian. 1996.
Transfection of Syk
protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated
degranulation
in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med
184, no.
30 1:71-9.
19. Vonakis, B.M., H. Chen, H. Haleem-Smith, and H. Metzger. 1997. The unique
domain
as the site on Lyn kinase for its constitutive association with the high
affinity receptor
for IgE. JBiol Chem 272, no. 38:24072-80.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-34-
20. Pribluda, V.S., C. Pribluda, and H. Metzger. 1994. Transphosphorylation as
the
mechanism by which the high-affinity receptor for IgE is phosphorylated upon
aggregation. Proceedings of the National Academy of Sciences of the United
States of
America 91, no. 23:11246-50.
s 21. El-Hillal, O., T. Kurosaki, H. Yamamura, J.-P. Kinet, and A.M.
Scharenberg. 1997. syk
kinase activation by a src kinase-initiated activation loop phosphorylation
chain
reaction. Proc Natl Acad Sci U S A 94, no. 5:1919-24.
22. Honda, Z., T. Suzuki, N. Hirose, M. Aihara, T. Shimizu, S. Nada, M. Okada,
C. Ra, Y.
Morita, and K. Ito. 1997. Roles of C-terminal Src kinase in the initiation and
the
io termination of the high affinity IgE receptor-mediated signaling. J Biol
Chem 272, no.
41:25753-60.
23. Sicheri, F., I. Moarefi, and J. Kuriyan. 1997. Crystal structure of the
Src family tyrosine
kinase Hck . Nature 385, no. 6617:602-9.
24. Xu, W., S.C. Harrison, and M.J. Eck. 1997. Three-dimensional structure of
the tyrosine
Is kinase c-Src. Nature 385, no. 6617:595-602.
25. Thomas, M.L. 1999. The regulation of antigen-receptor signaling by protein
tyrosine
phosphatases: a hole in the story. Curr Opin Immunol 11, no. 3:270-6.
26. Moarefi, L, M. LaFevre-Bernt, F. Sicheri, M. Huse, C.H. Lee, J. Kuriyan,
and W.T.
Miller. 1997. Activation of the Src-family tyrosine kinase Hck by SH3 domain
ao displacement. Nature 385, no. 6617:650-3.
27. Takizawa, F., M. Adamczewski, and J.-P. Kinet. 1992. Identification of the
low affinity
receptor for immunoglobulin E on mouse mast cells and macrophages as FcyRII
and
FcyRIII. JExp Med 176, no. 2:469-75.
28. Letourneur, F., S. Hennecke, C. Demolliere, and P. Cosson. 1995. Steric
masking of a
zs dilysine endoplasmic reticulum retention motif during assembly of the human
high
affinity receptor for immunoglobulin E. JCell Biol, no. 129:971-978.
29. Blank, U., C.S. Ra, and J.-P. Kinet. 1991. Characterization of truncated
alpha chain
products from human, rat, and mouse high affinity receptor for immunoglobulin
E. J
Biol Chem 266, no. 4:2639-46.
30 30. Maurer, D., E. Fiebiger, B. Reininger, B. Wolff Winiski, M.H. Jouvin,
O. Kilgus, J.-P.
Kinet, and G. Stingl. 1994. Expression of functional high affinity
immunoglobulin E
receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med 179,
no. 2:745-
50.
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-35-
31. Maurer, D., C. Ebner, B. Reininger, E. Fiebiger, D. Kraft, J.-P. Kinet,
and G. Stingl.
1995. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent
allergen
presentation. Jlmmunol 154, no. 12:6285-90.
32. Tedder, T.F., and P. Engel. 1994. CD20: a regulator of cell-cycle
progression of B
s lymphocytes. Immunol Today 15, no. 9:450-4.
33. Adra, C.N., J.M. Lelias, H. Kobayashi, M. Kaghad, P. Morrison, J.D.
Rowley, and B.
Lim. 1994. Cloning of the cDNA for a hematopoietic cell-specific protein
related to
CD20 and the beta subunit of the high-affinity IgE receptor: evidence for a
family of
proteins with four membrane-spanning regions. Proc Natl Acad Sci U S A 91, no.
~0 21:10178-52.
34. Sandford, A.J., T. Shirakawa, M.F. Moffatt, S.E. Daniels, C. Ra, J.A.
Faux, R.P. Young,
Y. Nakamura, G.M. Lathrop, W.O. Cookson, and et al. 1993. Localisation of
atopy and
beta subunit of high-affinity IgE receptor (Fc epsilon RI) on chromosome 11 q.
Lancet
341, no. 8841:332-334.
is 35. Hizawa, N., L.R. Freidhoff, E. Ehrlich, Y.F. Chiu, D.L. Duffy, C.
Schou, G.M.
Dunston, T.H. Beaty, D.G. Marsh, K.C. Barnes, and S.K. Huang. 1998. Genetic
influences of chromosomes Sq31-q33 and 11q13 on specific IgE responsiveness to
common inhaled allergens among African American families. Collaborative Study
on
the Genetics of Asthma (CSGA). JAllergy Clin Immunol 102, no. 3:449-53.
an 36. Lin, S., C. Cicala, A.M. Scharenberg, and J.-P. Kinet. 1996. The
Fc(epsilon)RIbeta
subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell
activation
signals. Cell 85, no. 7:985-95.
37. Dombrowicz, D., S. Lin, V. Flamand, A.T. Brini, B.H. Koller, and J.-P.
Kinet. 1998.
Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG- mediated
in vivo
zs responses. Immunity 8, no. 4:517-29.
38. Paolini, R., R. Numerof, and J.-P. Kinet. 1992.
Phosphorylation/dephosphorylation of
high-affinity IgE receptors: a mechanism for coupling/uncoupling a large
signaling
complex. Proc Natl Acad Sci U S A 89, no. 22:10733-7.
39. Scharenberg, A.M., O. El-Hillal, D.A. Fruman, L.O. Beitz, Z. Li, S. Lin,
I. Gout, L.C.
3n Cantley, D.J. Rawlings, and J.-P. Kinet. 1998. Phosphatidylinositol-3,4,5-
trisphosphate
(PtdIns-3,4,5-P3)/Tec kinase- dependent calcium signaling pathway: a target
for SHIP-
mediated inhibitory signals. Embo J 17, no. 7:1961-72.
40. Varin-Blank, N., and H. Metzger. 1990. Surface expression of mutated
subunits of the
high affinity mast cell receptor for IgE. JBiol Chem 265, no. 26:15685-94
CA 02385778 2002-03-21
WO 01/21816 _36- PCT/US00/25877.
41. Fields, S., and O. Song. 1989. A novel genetic system to detect protein-
protein
interactions. Nature 340, no. 6230:245-6.
42. Dombrowicz, D., Flamand, V., Brigman, K. K., Koller, B. H., and Kinet, J.
P. 1993.
Abolition of anaphylaxis by targeted disruption of the high affinity
immunoglobulin E
s receptor alpha chain gene. Cell 75:969-976.
43. Dombrowicz, D., Brini, A., Flamand, V., Hicks, E., Snouwaert, J. N.,
Kinet, J.-P., and
Koller, B. H. 1996. Anaphylaxis mediated through a humanized IgE high affinity
receptor. J. Immunol. 157:1645-1651.
44. Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K,
Iikura Y,
~n Awaji T,Tsujimoto G, Yanagida M, Uzumaki H, Takahashi G, Tsuji K, Nakahata
T.
1996. Selective growth of human mast cells induced by Steel factor, IL-6,and
prostaglandin E2 from cord blood mononuclear cells. Jlmmunol 157(1):343-50
45. Metcalfe D Isolation of human basophils. in Current Protocols in
Immunology. edited
by JE Coligan, AM Kruisbeek, DH Margulies, EM Shevach, W Strober. Greene
is Publishing Associates and Wiley-Intersciences, 1991.
46. Fleming TJ, Donnadieu E, Song CH, Laethem FV, Galli SJ, Kinet JP. 1997.
Negative
regulation of Fc epsilon RI-mediated degranulation by CD81. JExp Med 186:1307-
14.
47. Letourneur O, Sechi S, Willette-Brown J, Robertson MW, Kinet JP. 1995.
Glycosylation of human truncated Fc epsilon RI alpha chain is necessary for
efficient
zn folding in the endoplasmic reticulum. JBiol Chem 270(14):8249-56.
48. Koller, B.H., and Smithies, O. 1989. Inactivating the (32-microglobulin
locus in mouse
embryonic stem cells by homologous recombination. Proc. Natl. Acad. Sci. U S A
86:8932-8935.
49. Sambrook, J., Fritsh, E.F., and Maniatis, T. 1989. Molecular cloning. A
laboratory
Zs manual. (Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY, USA.
) pp
2.36-2.37.
50. Ra, C., Jouvin, M.H., and Kinet, J.-P. 1989. Complete structure of the
mouse mast cell
receptor for IgE (FceRI) and surface expression of chimeric receptors (rat-
mouse-
human) on transfected cells. J. Biol. Chem. 264:15323-15327.
3n 51. Hirasawa, N., Scharenberg, A., Yamamura, H., Beaven, M.A., and Kinet,
J.-P. 1995. A
requirement for Syk in the activation of the microtubule-associated protein
kinase/phospholipase A2 pathway by FceRI is not shared by a G protein-coupled
receptor. JBiol Chem 270:10960-10967.
CA 02385778 2002-03-21
WO 01/21816 -37- PCT/US00/25877
52. Dombrowicz, D., Flamand, V., Miyajima, L, Ravetch, J.V., Galli, S.J., and
Kinet, J.-P.
1997. Absence of FcERIa chain results in upregulation of FcyRIII-dependent
mast cell
degranulation and anaphylaxis. Evidence of competition between FcsRI and
FcyRIII for
limiting amounts of FcR(3 and y chains. J. Clin. Invest. 99:915-925.
s 53. Puttaraju, M, Jamison, SF, Mansfield, SG, Garcia-Blanco, MA, Mitchell,
LG. 1999.
Spliceosome-mediated RNA trans-splicing as a tool for gene therapy. Nat
Biotechnol
17:246-52.
54. Gelfand DH, Holland PM, Saiki RK, Watson RM. US Patent 5, 210,015.
55. Lee LG, Connell CR, Bloch W. 1993. Allelic discrimination by nick-
translation PCR
~o with fluorogenic probes. Nucl. Acids Research, 21, 3761-66.
56. Livak KJ, Flood SJA, Marmaro J, Giusti W, Deetz K. 1995. Oligonucleotides
with
fluorescent dyes at opposite ends provide a quenched probe system useful for
detecting
PCR product and nucleic acid hybridization. PCR Methods and Applications, 4,
357-62.
57. Halford, P. 1999. The essential prerequisites for quantitative RT-PCR. Nat
Biotechnol
~s 17:835.
58. Woolf, TM. 1998. Therapeutic repair of mutated nucleic acid sequences. Nat
Biotechnol
16(4):341-4.
All references disclosed herein are incorporated by reference in their
entirety.
so What is claimed is presented below and is followed by an Abstract and a
Sequence
Listing.
We claim:
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-1-
SEQUENCE FISTING
<110> Isis Innovation Limited
Beth Israel Deaconess
Medical Center,
Inc.
Donnadieu, Emmanuel
Jouvin, Marie-Helene
Kinet, Jean-Pierre
Cookson, William
Moffatt, Miriam
Fleur
<120> MODULATION OF OR ELL SURFACE EXPRESSI ON
IgE RECEPT C
<130> I0308/7000W0/ERP/KA
<150> US 60/154,924
<151> 1999-09-21
<160> 9
<170> FastSEQ for WindowsVersion3.0
<210> 1
<211> 3729
<212> DNA
<213> Homo Sapiens
<220>
<221> CDS
<222> (103)...(837)
<400> 1
aacccatttc cagtaagagg aaatccacca
60
aactgcctat agtctcaata
tcagagcatg
taataatatt gttaatgaaa as 114
ctttattcct atg
ggacagctcg gac
aca
gaa
Met
Asp
Thr
Glu
1
agtaat aga gca aat ctt ctcccacag gagccttcc agtgtg 162
agg get
SerAsn Arg Ala Asn Leu LeuProGln GluProSer SerVal
Arg Ala
10 15 20
cctgca gaa gtc ttg gaa tctccccag gaagtatct tcaggc 210
ttt ata
ProAla Glu Val Leu Glu SerProGln GluValSer SerGly
Phe Ile
25 30 35
agacta aag tcg gcc tca ccaccactg catacatgg ctgaca 258
ttg tcc
ArgLeu Lys Ser Ala Ser ProProLeu HisThrTrp LeuThr
Leu Ser
40 45 50
gttttg aaa gag cag gag ctgggggta acacaaatt ctgact 306
aaa ttc
ValLeu Lys Glu Gln Glu LeuGlyVal ThrGlnIle LeuThr
Lys Phe
55 60 65
getatg tgc ctt tgt ttt acagttgtc tgctctgta cttgat 354
ata gga
AlaMet Cys Leu Cys Phe ThrValVal CysSerVal LeuAsp
Ile Gly
70 75 80
atttca att gag gga gac ttttcatca tttaaagca ggttat 402
cac att
IleSer Ile Glu Gly Asp PheSerSer PheLysAla GlyTyr
His Ile
85 90 95 100
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-2-
cca ttc gga gcc ata ttt ttt tct gga atg tca att 450
tgg att tct ttg
Pro Phe Gly Ala Ile Phe Phe Ser Gly Met Ser Ile
Trp Ile Ser Leu
105 110 115
ata tct agg aga aat gca aca tat aga gga ctg gga 498
gaa ctg gtg agc
Ile Ser Arg Arg Asn Ala Thr Tyr Arg Gly Leu Gly
Glu Leu Val Ser
120 125 130
gca aac gcc agc agc ata get ggg gga att atc ctg 546
act gga acg acc
Ala Asn Ala Ser Ser Ile Ala Gly Gly Ile Ile Leu
Thr Gly Thr Thr
135 140 145
atc atc ctg aag aag agc ttg gcc cac atc agt tgc 594
aac tat atc cac
Ile Ile Leu Lys Lys Ser Leu Ala His Ile Ser Cys
Asn Tyr Ile His
150 155 160
cag aaa ttt gag acc aag tgc ttt tcc ttt act gaa 642
ttt atg get tcc
Gln Lys Phe Glu Thr Lys Cys Phe Ser Phe Thr Glu
Phe Met Ala Ser
165 170 175 180
att gta atg atg ctg ttt ctc acc gga ctt agt get 690
gtg att ctg ggt
Ile Val Met Met Leu Phe Leu Thr Gly Leu Ser Ala
Val I1e Leu Gly
185 190 195
gtg tca aca atc tgt gga get ggg ctc aaa aac aag 738
ctc gaa gaa gga
Val Ser Thr Ile Cys Gly Ala Gly Leu Lys Asn Lys
Leu Glu Glu Gly
200 205 210
gtt cca gat cgt gtt tat gaa gaa ata tat get act 786
gag tta aac tca
Val Pro Asp Arg Val Tyr Glu Glu Ile Tyr Ala Thr
Glu Leu Asn Ser
215 220 225
tac agt ttg gaa gac cca ggg gaa cct ccc gat tta 834
gag atg tct att
Tyr Ser Leu Glu Asp Pro Gly Glu Pro Pro Asp Leu
Glu Met Ser Ile
230 235 240
taa gaatcacgtg tccagaacac tctgattcac gcc 887
agccaaggat ccagaag
aaggttttgttaaggggcta ctggaaaaat ttctattctctccacagcctgctggtttta947
cattagatttattcgcctga taagaatatt ttgtttctgctgcttctgtccaccttaata1007
tgctccttctatttgtagat atgatagact cctatttttcttgttttatattatgaccac1067
acacatctctgctggaaagt caacatgtag taagcaagatttaactgtttgattataact1127
gtgcaaatacagaaaaaaag aaggctggct gaaagttgagttaaactttgacagtttgat1187
aatatttggttcttagggtt tttttttttt ttagcattcttaatagttacagttgggcat1247
gatttgtaccatccacccat acccacacag tcacagtcacacacacatatgtattactta1307
cactatatataacttcctat gcaaatattt taccaccagtcaataatacatttttgccaa1367
gacatgaagttttataaaga tctgtataat tgcctgaatcaccagcacattcactgacat1427
gatattatttgcagattgac aagtaggaag tggggaacttttattaagttactcgttgtc1487
tggggaggtaaataggttaa aaacagggaa attataagtgcagagattaacatttcacaa1547
atgtttagtgaaacatttgt gaaaaaagaa gactaaattaagacctgagctgaaataaag1607
tgacgtggaaatggaaataa tggttatatc taaaacatgtagaaaaagagtaactggtag1667
attttgttaacaaattaaag aataaagtta gacaagcaactggttgactaatacattaag1727
cgtttgagtctaagatgaaa ggagaacact ggttatgttgatagaatgataaaaagggtc1787
gggcgcggaggctcacgcct gtaatcccag ccctttgggaggccgaggtgggcagatcac1847
gaagtcagtagtttgagacc agcctggcca acatagtgaaaccccgtctctactaaaaat1907
acaaaaaaaaaattagctgg gtgtggtggc agtcacctgtagtcccagctacttgggagg1967
atgaggcaggagaatcgctt gaacctggga ggcggaggttgcagtgagccgagatcgcac2027
cagtgcactccagccttggt gacaatggga gactccatctcaaaaaaaaaaaaaaaaaaa2087
aaaagataaaaagtcagaaa tctgaaaagt ggaggaagagtacaaatagacctaaattaa2147
gtctcattttttggctttga ttttggggag acaaagggaaatgcagccatagagggcctg2207
atgacatccaatacatgagt tctggtaaag ataaaatttgatacacggtttggtgtcatt2267
ataagagaaatcattattaa atgaagcaag ttaacactctaagagaattattttgagata2327
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-3-
gaagtgaagctaagctaaacttcacatgcctataattggagggaaaaactaaggataaaa2387
tctagcctagaagatacaataattagtcataaacatgcattgtgaaactgtagagagcag2447
gtagcccaaaatagagaaagattagataaagagaaaataagtatccatcagagacagtat2507
ctctaggcttgggcaagagaaaagtccacagtgataagcaactccacctaaggcatgaat2567
atgcggcagagaaaacagcaatagtgaatgaatgcaaaaggtgctgagcaaattccacac2627
atgagtattgtgcatgagtaaatgaataaaacatttgcaaagacctttagagaaagagaa2687
tgggagcatatgtgcgaaataagatagttgattatgaatagaaggtagtgaagaaaagca2747
agctaagaaaaaattctgtttataaaagaaggaaaagatagtttatgtttttagcctaag2807
tataagagtcctacagatggactgaaaaaaatcagtctgagagtattagtcacaattaat2867
gaaataattacattttatgtattgaggatgccaagattaaaaggtgacaggtagatgtta2927
atttccctagattgtgaaagtgatcacgacaatcacacaacaaataattaagtgacttgg2987
tatgctttatttaattgtagggcctgaggttttccattctcatttttctaaaatacaatt3047
ttgtttctccaaatttgacagcagaataaaaaccctaccctttcactgtgtatcatgcta3107
agctgcatctctactcttgatcatctgtaggtattaatcacatcacttccatggcatgga3167
tgttcacatacagactcttaaccctggtttaccaggacctctaggagtggatccaatcta3227
tatctttacagttgtatagtatatgatatctcttttatttcactcaatttatattttcat3287
cattgactacatatttcttatacacaacacacaatttatgaattttttctcaagatcatt3347
ctgagagttgccccaccctacctgccttttatagtacgcccacctcaggcagacacagag3407
cacaatgctggggttctcttcacactatcactgccccaaattgtctttctaaatttcaac3467
ttcaatgtcatcttctccatgaagaccactgaatgaacaccttttcatccagccttaatt3527
tcttgctccataactactctatcccacgatgcagtattgtatcattaattattagtgtgc3587
ttgtgacctccttatgtattctcaattacctgtatttgtgcaataaattggaataatgta3647
acttgatttcttatctgtgtttgtgttggcatgcaagatttaggtacttatcaagataat3707
ggggaattaaggcatcaataas 3729
<210> 2
<211> 244
<212> PRT
<213> Homo sapiens
<400> 2
Met Asp Thr Glu Ser Asn Arg Arg Ala Asn Leu Ala Leu Pro Gln Glu
1 5 10 15
Pro Ser Ser Val Pro Ala Phe Glu Val Leu Glu Ile Ser Pro Gln Glu
20 25 30
Val Ser Ser Gly Arg Leu Leu Lys Ser Ala Ser Ser Pro Pro Leu His
35 40 45
Thr Trp Leu Thr Val Leu Lys Lys Glu Gln Glu Phe Leu Gly Val Thr
50 55 60
Gln Ile Leu Thr Ala Met Ile Cys Leu Cys Phe Gly Thr Val Val Cys
65 70 75 80
Ser Val Leu Asp Ile Ser His Ile Glu Gly Asp Ile Phe Ser Ser Phe
85 90 95
Lys Ala Gly Tyr Pro Phe Trp Gly Ala Ile Phe Phe Ser Ile Ser Gly
100 105 110
Met Leu Ser Ile Ile Ser Glu Arg Arg Asn Ala Thr Tyr Leu Val Arg
115 120 125
Gly Ser Leu Gly Ala Asn Thr Ala Ser Ser Ile Ala Gly Gly Thr Gly
130 135 140
Ile Thr Ile Leu Ile Ile Asn Leu Lys Lys Ser Leu Ala Tyr Ile His
145 150 155 160
Ile His Ser Cys Gln Lys Phe Phe Glu Thr Lys Cys Phe Met Ala Ser
165 170 175
Phe Ser Thr Glu Ile Val Val Met Met Leu Phe Leu Thr Ile Leu Gly
180 185 190
Leu Gly Ser Ala Val Ser Leu Thr Ile Cys Gly Ala Gly Glu Glu Leu
195 200 205
Lys Gly Asn Lys Val Pro Glu Asp Arg Val Tyr Glu Glu Leu Asn Ile
210 215 220
Tyr Ser Ala Thr Tyr Ser Glu Leu Glu Asp Pro Gly Glu Met Ser Pro
225 230 235 240
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-4-
Pro Ile Asp Leu
<210> 3
<211> 690
<212> DNA
<213> Homosap iens
<220>
<221> CDS
<222> (103)...(690)
<221> intron
<222> (640)... (1041)
<223> Intron
5
<400> 3
aacccatttc t cagtaagagg aaatccacca gtctcaata
60
aactgccta tcagagcatg a
taataatatt t acagctcg gttaatgaaa as tg ca 114
ctttattcc gg a gac gaa
a
Met
Asp
Thr
Glu
1
agtaat agagca aatcttget ctcccacag gagccttcc agtgtg 162
agg
SerAsn ArgAla AsnLeuAla LeuProGln GluProSer SerVal
Arg
10 15 20
cctgca gaagtc ttggaaata tctccccag gaagtatct tcaggc 210
ttt
ProAla GluVal LeuGluIle SerProGln GluValSer SerGly
Phe
25 30 35
agacta aagtcg gcctcatcc ccaccactg catacatgg ctgaca 258
ttg
ArgLeu LysSer AlaSerSer ProProLeu HisThrTrp LeuThr
Leu
40 45 50
gttttg aaagag caggagttc ctgggggta acacaaatt ctgact 306
aaa
ValLeu LysGlu GlnGluPhe LeuGlyVal ThrGlnIle LeuThr
Lys
55 60 65
getatg tgcctt tgttttgga acagttgtc tgctctgta cttgat 354
ata
AlaMet CysLeu CysPheGly ThrValVal CysSerVal LeuAsp
Ile
70 75 80
atttca attgag ggagacatt ttttcatca tttaaagca ggttat 402
cac
IleSer IleGlu GlyAspI1e PheSerSer PheLysAla GlyTyr
His
85 90 95 100
ccattc ggagcc atatttttt tctatttct ggaatgttg tcaatt 450
tgg
ProPhe GlyAla IlePhePhe SerIleSer GlyMetLeu SerIle
Trp
105 110 115
atatct aggaga aatgcaaca tatctggtg agaggaagc ctggga 498
gaa
IleSer ArgArg AsnAlaThr TyrLeuVal ArgGlySer LeuGly
Glu
120 125 130
gcaaac gccagc agcataget gggggaacg ggaattacc atcctg 546
act
AlaAsn AlaSer SerIleAla GlyGlyThr GlyIleThr IleLeu
Thr
135 140 145
atcatc ctgaag aagagcttg gcctatatc cacatccac agttgc 594
aac
IleIle LeuLys LysSerLeu AlaTyrIle HisIleHis SerCys
Asn
150 155 160
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-5-
cag aaa ttt ttt gag acc aag tgc ttt atg get tcc ttt tcc act gta 642
Gln Lys Phe Phe Glu Thr Lys Cys Phe Met Ala Ser Phe Ser Thr Val
165 170 175 180
tgt att ttt ttt tgt gtg gga aga cta aga ttc tgg gtc cta atg taa 690
Cys Ile Phe Phe Cys Val Gly Arg Leu Arg Phe Trp Val Leu Met
185 190 195
gtaagaagccctcttctcctgttccatgaacaccatccttttctgtaacttctattacac750
agtatagtggttctgtaagttcacacagcccagggagatgctggctgcccactcccctca810
acccaggcaaattcctcggggttaaagttatctactgcaagtgacgatctctgggttttt870
ctgtgcctgtgtttgtgtgtgtgtgtgtgtgtgtgtgtgtgtgtatgtgtcactttaaaa930
ggactggtcagatggtagggagatgaaaacaggagatgctataagaaaataaacttttgg990
ggcgaataccaatgtgactctttttgtttgtcatttgttgctgttcaataggaaattgta1050
gtgatgatgctgtttctcaccattctgggacttggtagtgctgtgtcactcacaatctgt1110
ggagctggggaagaactcaaaggaaacaaggttccagaggatcgtgtttatgaagaatta1170
aacatatattcagctacttacagtgagttggaagacccaggggaaatgtctcctcccatt1230
gatttataagaatcacgtgtccagaacactctgattcacagccaaggatccagaaggcca1290
aggttttgttaaggggctactggaaaaatttctattctctccacagcctgctggttttac1350
attagatttattcgcctgataagaatattttgtttctgctgcttctgtccaccttaatat1410
gctccttctatttgtagatatgatagactcctatttttcttgttttatattatgaccaca1470
cacatctctgctggaaagtcaacatgtagtaagcaagatttaactgtttgattataactg1530
tgcaaatacagaaaaaaagaaggctggctgaaagttgagttaaactttgacagtttgata1590
atatttggttcttagggttttttttttttttagcattcttaatagttacagttgggcatg1650
atttgtaccatccacccatacccacacagtcacagtcacacacacatatgtattacttac1710
actatatataacttcctatgcaaatattttaccaccagtcaataatacatttttgccaag1770
acatgaagttttataaagatctgtataattgcctgaatcaccagcacattcactgacatg1830
atattatttgcagattgacaagtaggaagtggggaacttttattaagttactcgttgtct1890
ggggaggtaaataggttaaaaacagggaaattataagtgcagagattaacatttcacaaa1950
tgtttagtgaaacatttgtgaaaaaagaagactaaattaagacctgagctgaaataaagt2010
gacgtggaaatggaaataatggttatatctaaaacatgtagaaaaagagtaactggtaga2070
ttttgttaacaaattaaagaataaagttagacaagcaactggttgactaatacattaagc2130
gtttgagtctaagatgaaaggagaacactggttatgttgatagaatgataaaaagggtcg2190
ggcgcggaggctcacgcctgtaatcccagccctttgggaggccgaggtgggcagatcacg2250
aagtcagtagtttgagaccagcctggccaacatagtgaaaccccgtctctactaaaaata2310
caaaaaaaaaattagctgggtgtggtggcagtcacctgtagtcccagctacttgggagga2370
tgaggcaggagaatcgcttgaacctgggaggcggaggttgcagtgagccgagatcgcacc2430
agtgcactccagccttggtgacaatgggagactccatctcaaaaaaaaaaaaaaaaaaaa2490
aaagataaaaagtcagaaatctgaaaagtggaggaagagtacaaatagacctaaattaag2550
tctcattttttggctttgattttggggagacaaagggaaatgcagccatagagggcctga2610
tgacatccaatacatgagttctggtaaagataaaatttgatacacggtttggtgtcatta2670
taagagaaatcattattaaatgaagcaagttaacactctaagagaattattttgagatag2730
aagtgaagctaagctaaacttcacatgcctataattggagggaaaaactaaggataaaat2790
ctagcctagaagatacaataattagtcataaacatgcattgtgaaactgtagagagcagg2850
tagcccaaaatagagaaagattagataaagagaaaataagtatccatcagagacagtatc2910
tctaggcttgggcaagagaaaagtccacagtgataagcaactccacctaaggcatgaata2970
tgcggcagagaaaacagcaatagtgaatgaatgcaaaaggtgctgagcaaattccacaca3030
tgagtattgtgcatgagtaaatgaataaaacatttgcaaagacctttagagaaagagaat3090
gggagcatatgtgcgaaataagatagttgattatgaatagaaggtagtgaagaaaagcaa3150
gctaagaaaaaattctgtttataaaagaaggaaaagatagtttatgtttttagcctaagt3210
ataagagtcctacagatggactgaaaaaaatcagtctgagagtattagtcacaattaatg3270
aaataattacattttatgtattgaggatgccaagattaaaaggtgacaggtagatgttaa3330
tttccctagattgtgaaagtgatcacgacaatcacacaacaaataattaagtgacttggt3390
atgctttatttaattgtagggcctgaggttttccattctcatttttctaaaatacaattt3450
tgtttctccaaatttgacagcagaataaaaaccctaccctttcactgtgtatcatgctaa3510
gctgcatctctactcttgatcatctgtaggtattaatcacatcacttccatggcatggat3570
gttcacatacagactcttaaccctggtttaccaggacctctaggagtggatccaatctat3630
atctttacagttgtatagtatatgatatctcttttatttcactcaatttatattttcatc3690
attgactacatatttcttatacacaacacacaatttatgaattttttctcaagatcattc3750
tgagagttgccccaccctacctgccttttatagtacgcccacctcaggcagacacagagc3810
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
-6-
acaatgctggggttctcttcacactatcactgccccaaattgtctttctaaatttcaact3870
tcaatgtcatcttctccatgaagaccactgaatgaacaccttttcatccagccttaattt3930
cttgctccataactactctatcccacgatgcagtattgtatcattaattattagtgtgct3990
tgtgacctccttatgtattctcaattacctgtatttgtgcaataaattggaataatgtaa4050
cttgatttcttatctgtgtttgtgttggcatgcaagatttaggtacttatcaagataatg4110
gggaattaaggcatcaataaa 4131
<210> 4
<211> 195
<212> PRT
<213> Homo Sapiens
<400> 4
Met Asp Thr Glu Ser Asn Arg Arg Ala Asn Leu Ala Leu Pro Gln Glu
1 5 10 15
Pro Ser Ser Val Pro Ala Phe Glu Val Leu Glu Ile Ser Pro Gln Glu
20 25 30
Val Ser Ser Gly Arg Leu Leu Lys Ser Ala Ser Ser Pro Pro Leu His
35 40 45
Thr Trp Leu Thr Val Leu Lys Lys Glu Gln Glu Phe Leu Gly Val Thr
50 55 60
Gln Ile Leu Thr Ala Met Ile Cys Leu Cys Phe Gly Thr Val Val Cys
65 70 75 80
Ser Val Leu Asp Ile Ser His Ile Glu Gly Asp Ile Phe Ser Ser Phe
85 90 95
Lys Ala Gly Tyr Pro Phe Trp Gly Ala Ile Phe Phe Ser Ile Ser Gly
100 105 110
Met Leu Ser Ile Ile Ser Glu Arg Arg Asn Ala Thr Tyr Leu Val Arg
115 120 125
Gly Ser Leu Gly Ala Asn Thr Ala Ser Ser Ile Ala Gly Gly Thr Gly
130 135 140
Ile Thr Ile Leu Ile Ile Asn Leu Lys Lys Ser Leu Ala Tyr Ile His
145 150 155 160
Ile His Ser Cys Gln Lys Phe Phe Glu Thr Lys Cys Phe Met Ala Ser
165 170 175
Phe Ser Thr Val Cys Ile Phe Phe Cys Val Gly Arg Leu Arg Phe Trp
180 185 190
Val Leu Met
195
<210> 5
<211> 21
<212> DNA
<213> Homo sapiens
<220>
<223> PCR primer
<400> 5
gtgcctgcat ttgaagtctt g 21
<210> 6
<211> 20
<212> DNA
<213> Homo Sapiens
<220>
<223> PCR primer
<400> 6
tggatccttg gctgtgaatc 20
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
_'7_
<210> 7
<211> 1198
<212> DNA
<213> Homo sapiens
<400> 7
tactaagagtctccagcatcctccacctgtctaccaccgagcatgggcctatatttgaag 60
ccttagatctctccagcacagtaagcaccaggagtccatgaagaagatggctcctgccat 120
ggaatcccctactctactgtgtgtagccttactgttcttcgctccagatggcgtgttagc 180
agtccctcagaaacctaaggtctccttgaaccctccatggaatagaatatttaaaggaga 240
gaatgtgactcttacatgtaatgggaacaatttctttgaagtcagttccaccaaatggtt 300
ccacaatggcagcctttcagaagagacaaattcaagtttgaatattgtgaatgccaaatt 360
tgaagacagtggagaatacaaatgtcagcaccaacaagttaatgagagtgaacctgtgta 420
cctggaagtcttcagtgactggctgctccttcaggcctctgctgaggtggtgatggaggg 480
ccagcccctcttcctcaggtgccatggttggaggaactgggatgtgtacaaggtgatcta 540
ttataaggatggtgaagctctcaagtactggtatgagaaccacaacatctccattacaaa 600
tgccacagttgaagacagtggaacctactactgtacgggcaaagtgtggcagctggacta 660
tgagtctgagcccctcaacattactgtaataaaagctccgcgtgagaagtactggctaca 720
attttttatcccattgttggtggtgattctgtttgctgtggacacaggattatttatctc 780
aactcagcagcaggtcacatttctcttgaagattaagagaaccaggaaaggcttcagact 840
tctgaacccacatcctaagccaaaccccaaaaacaactgatataattactcaagaaatat 900
ttgcaacattagtttttttccagcatcagcaattgctactcaattgtcaaacacagcttg 960
caatatacatagaaacgtctgtgctcaaggatttatagaaatgcttcattaaactgagtg 1020
aaactggttaagtggcatgtaatagtaagtgctcaattaacattggttgaataaatgaga 1080
gaatgaatagattcatttattagcatttgtaaaagagatgttcaatttcaataaaataaa 1140
tataaaaccatgtaacagaatgcttctgagtaaaaaaaaaaaaaaaaaaaaaaaaaaa 1198
<210> 8
<211> 2363
<212> DNA
<213> Homo Sapiens
<400>
8
ttggctctctgcaaccactgtctcctgggttcaagcgattctcctgccttagtctcccga60
gtagctgggattacaggcacccaccaccacgcccagctaatttttgtatttttagtagag120
acggggtttcactctgttggccaggctggtcttgaacacctgacctcttgatctgcccac180
ctcagccacccaaagtgctggaattacaagcgtgagtcaccgcacccggccatataagag240
attcttaatctcccatagctctcctgttttattctgtatcctctctgcttactgactgac300
atgtggctttagtctccctgagggtagagattattttctgtgctgggggaggcctaggag360
acagagtttggatatggtttattgatgctccctgtttcctctcttcagacttccatggag420
tcactgattcatcactttaacttgtatactgagggctaccaagttcctccaggagccaca480
tatactgccattgaggctcccaaggtaaggagaggaggggaaggaaaagaccatatgtag540
agtaggtagctaaagatagatctttaacaaatagctcattcatcaatgatatataaaaca600
gaatgaatagaggttttgttggcagagaaaaatactcttcatgttaatacagagtcacac660
ccaaccttcttccttgaacagggagagtttggggtgtacctggtggtctgatggcagcag720
ccgcccttatcgatgcaagatcaaggctcctggttttgcccatctgtaagaatcaatccc780
agtaactataactccaatgaattaaacctgaccttggttgaggtttttatgaactcttct840
ttctcctcccaccttgcaagtcttaactaacattgttgccatctcaatctccctaggctg900
gtttggacaagatgtctaagggacacatgttggcagatgtcgttgccatcataggtacga960
ggcctattgtgtagtagaggtatcctagacaaaggagttcgggacgcccactggggacag1020
aaggagaacacttcctgttcaccataggccatggcatggactcgggtcctcaatcttttg1080
agcacagtaatgggttctggatcttgggtaacaccactttttttgtttgttttgcctcac1140
aacaggaagataagtaacatcacttttttcctccatcctctcacctaggtacccaagata1200
ttgtatttggagaagtagatcggtgagcaggggagcagcgtttgatcccccctgcctatc1260
agcttcttctgtggagcctgttcctcactggaaattggcctctgtgtgtgtgtgtgtgtg1320
tgtgtgtgtgtgtgtgtgtatgttcatgtacacttggctgtcaggctttctgtgcatgta1380
ctaaaaaaggagaaattataataaattagccgtcttcgcgcccctaggcctaaacttctg1440
gtatcttagtgtctcagtatcttagtgtccttcactcggactgtaaacctaagaatgttc1500
attaaccctccattcctgttagattcagtcaggtcttagcaatttttcctgctcgtctcc1560
acccccttctctgactcttgtcctttccacttctctattcccaatttcctctttcgctca1620
CA 02385778 2002-03-21
WO 01/21816 PCT/US00/25877
_g_
gtcctccttgcccaaaccttctcagtgcccacataacttggtaaaccactcaaatcaaga1680
cctggggtaaagttgggagggaaagggctatagtggggtctgagggaatgttgacgggca1740
gtttcacacagataaatctctgaaccagccgggcgcagtgcctcacgcctgtaatcccag1800
cactttgggaggccgaggcggatggatcacctgagatcaggagttcaagaccagcctgat1860
caacatggagaaaccccatctctactaaaaatacaaaattagctgggcgtggtggtgcat1920
gcctgtaatgccagctactcgggaggctgagagaatcgcttgaacctgggaggcggaggt1980
tgcggtgagccgagatggcgccattgcactccagcctgggcaacaaagcaagagtccgtc2040
tcaaaaaaaaaaaaaaaaaaaaaaatctgaaccaggtggagtggaaaatggcagatgtag2100
acagcctttcctgagcgtgagagtctcctcattctgtgggttaggagttggtcattgaag2160
ggctgacgcttaagagcccagatctcccaactcccttagttggccttccgggagccgccc2220
ggtctcttgtgcaggaaggggaaggggccaaagcatgggggaaggcgtggcaggaagagg2280
gggactctgtggtcagggaactgctcgctgagcacagctgcacagtgctggctgtcagaa2340
cggccgatctccagcccaagatg 2363
<210> 9
<211> 708
<212> DNA
<213> Mus musculus
<400> 9
atggacacagaaaataggagcagagcagatcttgctctcccaaatccacaagaatcctcc 60
agtgcacctgacattgaactcttggaagcatctcctgccaaagcagccccaccaaagcag 120
acatggcggacatttttgaagaaagagttggagttcctgggagcaacacaaattctggtt 180
ggtttgatatgcctttgttttggaacaattgtctgctccgtactctatgtttcagacttt 240
gatgaagaagtgcttttactttataaactaggctatccattctggggtgcagtgctgttt 300
gttttgtctggatttttgtcaattatctccgaaagaaaaaacacattgtatctggtgaga 360
ggcagcctgggagcaaacattgtcagtagcatcgctgcagggacggggatcgccatgctg 420
atcctcaatctgaccaataacttcgcttatatgaacaactgcaagaatgtaaccgaagac 480
gacggctgctttgtggcttctttcaccacagaactggtgttgatgatgctgtttctcacc 540
atcctggccttttgcagtgctgtgttgttcactatctataggattggacaagagttagaa 600
agtaaaaaggtcccagatgatcgtctttatgaagaattaaatgtgtattcaccaatttac 66C
agtgagttggaagacaaaggggaaacatcttctccagttgattcataa 708
ttgca