Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
A TRANSPARENT COMPOSITION CONTAINING
POLYCARBONATE AND A COPOLYMER OF METHYLMETHACRYLATE
FIELD OF THE INVENTION
The present invention is directed to a thermoplastic molding
composition and more particularly to a transparent blend that contains
polycarbonate resin and atactic copolymer of PMMA.
SUMMARY OF THE INVENTION
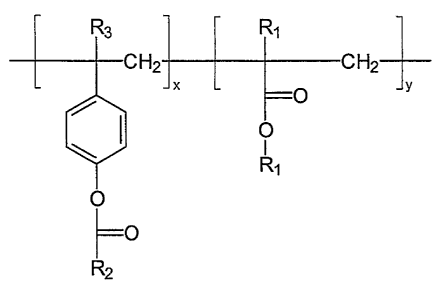
A thermoplastic molding composition is disclosed containing
polycarbonate resin and a copolymer of methyl methacrylate conforming
structurally to
O
R2
wherein x and y are integers calculated to result in a content of PMMA
in the copolymer in the range of 80 to 90 mole %, and where R~
denotes -CH3, R2 denotes -C6H5 and R3 is a C~-C2-alkyl group.
The composition is useful in preparing transparent articles,
including films.
BACKGROUND OF THE INVENTION
Polycarbonate resins are characterized by dimensional stability at
relatively high temperatures, excellent resistance to impact, stiffness and
transparency. These properties make polycarbonate the material of choice
for a variety of applications including glazing containers and medical
devices. A notable drawback characterizing this resin has been its
susceptibility to scratching. Polymethylmethacrylate ("PMMA"), known for
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-2-
its clarity and scratch resistance is noted for its shortcomings in terms of
dimensional stability, low impact strength and relatively poor thermal
stability. While some blends of polycarbonates and PMMA reflecting a
more attractive profile of properties are known, it is also known that a wide
range of blends with typical PMMA are immiscible and opaque. It has been
suggested that free radical polymerized PMMA does not form a single
thermo-dynamically miscible transparent blend but does demonstrate
mechanical compatibility with polycarbonate. While this compatibility is
taken to mean that the phases exhibit good adhesion one to the other, the
resulting blend is not transparent.
Literature relative to mixtures of polycarbonate and PMMA include
U.S. Patent 4,319,003 that disclosed opaque blends. Also noted in this
connection are JP 7216063 and EP 297285. Ways of overcoming the
drawbacks associated with the immiscibility of PMMA and polycarbonate
have been proposed. These include the addition of copolymer additives
(DE 2264268); PMMA/acrylamide copolymers (DE 3632946 and PMMA-
ester copolymers containing carboxylic groups (U.S. Patent 4,906,696).
Further, transparent blends of PMMA and polycarbonate are said to have
been prepared, in accordance with DE 3,833,218, by melting the two
components in the presence of supercritical gas. U.S. Patents 4,743,654
and 4,745,029 disclosed producing solutions of the two polymers in
organic solvents and evaporating the solvents as means to prepare
transparent material. Transparent films of polycarbonate and polymethyl-
methacrylate produced by special solvent-removal methods, however,
tend to become opaque at temperatures above 145°C due to the
immiscibility of these polymers. Mixtures of polycarbonate and
stereoregular polymethyl methacrylate in which at least 60% of the
monomer units are in the syndiotactic configuration have been disclosed in
EP 573,109. U.S. Patent 5,284,916 is noted for disclosing a blend of
polycarbonate and a block copolymer containing a polyaromatic(alkyl)-
methacrylate block.
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-3-
DETAILED DESCRIPTION OF THE INVENTION
The present invention concerns a thermoplastic molding
composition containing polycarbonate and a specifically structured
copolymer of methyl methacrylate (herein "COPMA"). The composition,
containing about 5 to 20, preferably 7 to 15 percent of a suitable COPMA,
the percent being relative to the total weight of polycarbonate and COPMA
is characterized by its transparency. The inventive transparent blend may
be processed thermoplastically in accordance with conventional
procedures and using conventional means.
Aromatic polycarbonates within the scope of the present invention
are homopolycarbonates, copolycarbonates, branched polycarbonates
and mixtures thereof. The polycarbonates generally have a weight
average molecular weight of 10,000 to 200,000, preferably 20,000 to
80,000 and their melt flow rate, per ASTM D-1238 at 300°C, is about 1
to
about 65 g!10 min., preferably about 2 to 15 g/10 min. They may be
prepared, for example, by the known diphasic interface process from a
carbonic acid derivative such as phosgene and dihydroxy compounds by
polycondensation (see German Offenlegungsschriften 2,063,050;
2,063,052; 1,570,703; 2,211,956; 2,211,957 and 2,248,817; French Patent
1,561,518; and the monograph H. Schnell, "Chemistry and Physics of
Polycarbonates", Interscience Publishers, New York, New York, 1964 (all
incorporated herein by reference).
In the present context, dihydroxy compounds suitable for the
preparation of the polycarbonates of the invention conform to the structural
formulae (1 ) or (2).
H
(
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-4-
(2)
wherein
A denotes an alkylene group with 1 to 8 carbon atoms, an alkylidene group
with 2 to 8 carbon atoms, a cycloalkylene group with 5 to 15 carbon atoms,
a cycloalkylidene group with 5 to 15 carbon atoms, a carbonyl group, an
oxygen atom, a sulfur atom, -SO- or -S02- or a radical conforming to
CH3
CH3
C ~ CH3
CH3
a and g both denote the number 0 to 1; Z denotes F, CI, Br or C~-C4-alkyl
and if several Z radicals are substituents in one aryl radical, they may be
identical or different from one another; d denotes an integer of from 0 to 4;
and f denotes an integer of from 0 to 3.
Among the dihydroxy compounds useful in the practice of the
invention are hydroquinone, resorcinol, bis-(hydroxyphenyl)-alkanes, bis-
(hydroxyphenyl)-ethers, bis-(hydroxyphenyl)-ketones, bis-(hydroxyphenyl)-
sulfoxides, bis-(hydroxyphenyl)-sulfides, bis-(hydroxyphenyl)-sulfones, and
a,a-bis-(hydroxyphenyl)-diisopropylbenzenes, as well as their nuclear-
alkylated compounds. These and further suitable aromatic dihydroxy
compounds are described, for example, in U.S. Patents 3,028,356;
2,999,835; 3,148,172; 2,991,273; 3,271,367; and 2,999,846, all
incorporated herein by reference.
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-5-
Further examples of suitable bisphenols are 2,2-bis-(4-hydroxy-
phenyl)-propane (bisphenol A), 2,4-bis-(4-hydroxyphenyl)-2-methyl-
butane, 1,1-bis-(4-hydroxyphenyl)-cyclohexane, a,a'-bis-(4-hydroxy-
phenyl)-p-diisopropylbenzene, 2,2-bis-(3-methyl-4-hydroxyphenyl)-
propane, 2,2-bis-(3-chloro-4-hydroxyphenyl)-propane, bis-(3,5-dimethyl-4-
hydroxyphenyl)-methane, 2,2-bis-(3,5-dimethyl-4-hydroxyphenyl)-propane,
bis-(3,5-dimethyl-4-hydroxyphenyl)-sulfide, bis-(3,5-dimethyl-4-hydroxy-
phenyl)-sulfoxide, bis-(3,5-dimethyl-4-hydroxyphenyl)-sulfone, dihydroxy-
benzophenone, 2,4-bis-(3,5-dimethyl-4-hydroxyphenyl)-cyclohexane, a,a'-
bis-(3,5-dimethyl-4-hydroxyphenyl)-p-diisopropylbenzene, 2,2,4-trimethyl
cyclohexyl 1,1-diphenol and 4,4'-sulfonyl diphenol.
Examples of particularly preferred aromatic bisphenols are 2,2,-bis-
(4-hydroxyphenyl)-propane, 2,2-bis-(3,5-dimethyl-4-hydroxyphenyl)-
propane, 2,2,4-trimethyl cyclohexyl 1,1-diphenol and 1,1-bis-(4-
hydroxyphenyl)-cyclohexane.
The most preferred bisphenol is 2,2-bis-(4-hydroxyphenyl)-propane
(bisphenol A).
The polycarbonates of the invention may entail in their structure
units derived from one or more of the suitable bisphenols.
The copolymer of methyl methacrylate of the present invention
(herein COPMA), conforms structurally to
H2 H2
y
30
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-6-
wherein x and y are integers calculated to result in a content of PMMA
in the copolymer in the range of about 80 to 90 mole %, R~ denotes
-CH3, R2 denotes -C6H5 and R3 is hydrogen or a C~-C2-alkyl group.
The preparation of the inventive COPMA may be carried out by free
radical polymerization of methyl methacrylate in the presence of an
appropriate vinyl-benzoate comonomer.
Suitable benzoate monomer may be synthesized from a-methyl-
para-hydroxystyrene with benzoyl chloride in the presence of an amine
base (such as triethylamine). In the course of the work leading to the
present invention, 4-phenol ispropenylbenzoate was thus prepared.
ci
0
CH2 I ~ CH2
i
\
OH O
a-Methyl-p-hydroxystyrene O
I\
4-Phenol, p-isopropenylbenzoate
The invention is further illustrated but is not intended to be
limited by the following examples in which all parts and percentages
are by weight unless otherwise specified.
EXAMPLES
Methylmethacrylate and toluene were mixed with the 4-phenol
p-isopropenylbenzoate prepared in accordance with the procedure
above. The amount of 4-phenol p-isopropenylbenzoate is calculated to
yield COPMA having 80 to 95 mol % of PMMA. Polymerization was
initiated with about 0.5% of azobisisobutyronitrile, a free radical
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-7-
initiator. The polymerizations were allowed to proceed at 25 to 60°C
with stirring until such time as a viscous solution was obtained.
2. The resulting COPMA is isolated, such as by precipitation in
methanol, and dried in a vacuum oven at 80°C. Characterized by
spectroscopy and measurements of glass transition temperatures, the
resulting COPMA had glass transition temperatures (shown below) that
are higher than that of PMMA (=125°C).
MMA Tg (C)
content
(%)
95 132
90 130
80 135
3. Blends in accordance with the invention were prepared and their
properties were determined. The polycarbonate component of all the
exemplified blends was a homopolycarbonate based on bisphenol A
(Makrolon 2608, a product of Bayer Corporation). In preparing the
blends, a low shear melt kneader was used, operating at 280°C for 20
to 25 minutes. Additional inventive blends were prepared in a 16 mm
twin-screw extruder which has conveying and mixing sections along
the extruder screws at 250 to 285°C. No catalysts or other additives
are necessary for preparing the transparent materials. The blends
contained 90 wt.% polycarbonate and 10 wt% COPMA (the COPMA
containing 90 mole % MMA). These were injection molded
conventionally to make test specimens for determination of optical
properties (see results below in Table 2).
4. A comparative example containing 90 wt % polycarbonate and
10 wt % PMMA were prepared by the same procedures and optical
properties (total light transmission -TLT- and haze) of specimens thus
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
_$_
molded were determined (in accordance with ASTM D 1003). These
are reported below (see Table 2).
Table 2
TLT Haze
(%) (%)
Inventive blend 79 7.24
Comparative blend 19.5 99.3
Clearly, the composition in accordance with the invention exhibit far
better optical properties than does a corresponding, largely similar
composition.
5. Corresponding blends containing the indicated amounts of
polycarbonate resin and copolymers having molecular structures
similar to COPMA were prepared as described below. The structures
of the various copolymers and the relevant properties of the resulting
compositions are noted below.
(a) A copolymer conforming structurally to
MMA >
Y
wherein MMA denotes units derived from methylmethacrylate comonomer
and
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
_g_
wherein x amounted to 10 mole % relative to the molar amount of the
copolymer was blended with polycarbonate. The resulting blend (10
percent by weight (wt.%) of copolymer and 90 wt% of polycarbonate) was
opaque.
(b) A copolymer conforming structurally to
MMA
Y
wherein x amounted to 10 mole % relative to the molar amount of the
copolyemer was blended with polycarbonate. The resulting blend (10 wt.%
of copolymer and 90 wt% of polycarbonate) was opaque.
(c) A copolymer conforming structurally to
C H3
MMA
o ~ y
0
w
I
wherein x amounted to 10 mole % relative to the molar amount of the
copolymer was blended with polycarbonate. The resulting blend (10 wt.%
of copolymer and 90 wt% of polycarbonate) was opaque.
(d) A copolymer conforming structurally to
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-10-
MMA
' Y
/.
OH
where x amounted to 50 mole % relative to the molar amount of the
copolymer , a product of Aldrich proved to be immiscible with
polycarbonate.
(e) A copolymer conforming structurally to
M MA
Y
0
~o
C H3
(i) where x amounted to 10 mole%: a blend of 10 wt
copolymer and 90 wt % polycarbonate was opaque.
(ii) where x amounted to 20 mole%: a blend of 10 wt
copolymer and 90 wt % polycarbonate was opaque.
(iii) where x amounted to 10 mole%: a blend of 5 wt
copolymer and 95 wt % polycarbonate was opaque.
6. The copolymer of the invention - COPMA conforming to
Y
CA 02418707 2003-02-06
WO 02/14427 PCT/USO1/24549
-11 -
where x amounted to 10 mole% a blend containing 90 wt
polycarbonate resin was transparent
(ii) where x amounted to 10 mole% a blend containing 93 wt
polycarbonate resin was transparent
(iii) where x amounted to 10 mole% a blend containing 70 wt
polycarbonate resin was opaque.
(iv) where x amounted to 20 mole% a blend containing 90 wt
polycarbonate resin was transparent.
(v) where x amounted to more than 20 mole%, a blend
containing 90 wt.% polycarbonate was opaque.
Although the invention has been described in detail in the
foregoing for the purpose of illustration, it is to be understood that such
detail is solely for that purpose and that variations can be made therein
by those skilled in the art without departing from the spirit and scope of
the invention except as it may be limited by the claims.