Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
Cysteine Protease Inhibitors
Field of the invention.
This invention relates to inhibitors of cysteine proteases, especially those
of the papain
superfamily. The invention provides novel compounds useful in the prophylaxis
or
treatment of disorders stemming from misbalance of physiological proteases
such as
cathepsin F or S, or pathogenic proteases such as malarial falcipain.
Description of the related art.
The papain superfamily of cysteine proteases are widely distributed in diverse
species
including mammals, invertebrates, protozoa, plants and bacteria. A number of
mammalian cathepsin enzymes, including cathepsins B, F, H, K, L, N and S, have
been
ascribed to this superfamily, and inappropriate regulation of their activity
has been
implicated in a number of metabolic disorders including arthritis, muscular
dystrophy,
inflammation, glomerulonephritis and tumour invasion. Pathogenic cathepsin
like
enzymes include the bacterial gingipains, the malarial falcipains I, II, III
et seq and
cysteine proteases from Pneumocystis carinii, Trypanosoma cruzei and brucei,
Crithidia fusiculata, Schistosoma spp.
In WO 97/40066, the use of inhibitors against Cathepsin S is described. The
inhibition
of this enzyme is suggested to prevent or treat disease caused by protease
activity.
Cathepsin S is a highly active cysteine protease belonging to the papain
superfamily.
Its primary structure is 57%, 41 % and 45% homologous with that of the human
cathepsin L and H and plant cysteine proteases papain respectively, although
only 31%
homologous with Cathepsin B. It is found mainly in lymph nodes, spleen, and
macrophages and this limited occurrence suggests the potential involvement of
this
enzyme in the pathogenesis of degenerative disease. Moreover, it has been
found that
destruction of Ii by proteolysis is required for MHC class II molecules to
bind antigenic
peptides, and for transport of the resulting complex to the cell surface.
Furthermore, it
has been found that Cathepsin S is essential in B cells for effective Ii
proteolysis
necessary to render class II molecules competent for binding peptides.
Therefore, the
inhibition of this enzyme may be useful in modulating class II-restricting
immune
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
response (WO 97/40066). Other disorders in which cathepsin S is implicated are
chronic obstructive pulmonary disease and endometriosis.
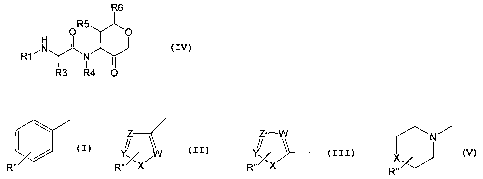
WO 98/50533 describes the use of compounds according to the formula (I).
R3 ~ O
R~~ N
IRs
C ~Rs
R~ O
X
(I)
It is suggested the compounds of this formula, are useful as inhibitors to
proteases, in
particular the papain superfamily; specifically those of the Cathepsin family;
and
particularly Cathepsin K. The ketone bearing ring structure in these compounds
has a
tendency to spontaneously racemise, limiting their clinical utility. Other SKB
applications describing ketone cathepsin K inhibitors include WO 98/46582,
W099/
64399, WO00/29408, WO00/38687 and WO00/49011. However none of these
applications disclose an a- ring substituent adjacent to the linkage to the
peptidomimetic chain.
Shenai et al., J. Biol. Chem. 275 37 29000-29010 describe the isolation of a
major
cysteine protease, denoted falcipain 2 from trophozoites of Plasmodium
falciparium.
The enzyme appears inter alia to hydrolyse erythrocyte haemoglobin in acidic
food
vacuoles. This publication also describes the isolation of the corresponding
gene using
an N-terminus tag, which is autocatalytically removed during folding.
SmithKline Beecham's WO 99/53039 describes the cysteine protease inhibitory
activity of a diverse range of peptidomimetics on a trophozoite preparation
from
Plasmodium falciparium. No guidance is provided as to which cysteine protease
is
being inhibited. Although most of the peptidomimetics are linear structures,
one
compound (R,S)-3-[N-(3-benzyloxybenzoyl)-L-leucinylamino]tetrahydrofuran-4-one
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
3
belongs to the furanones of formula I depicted above. As would be expected of
such
chirally unstable structures, the ketone bearing ring is racemic.
Summary of the invention
A first aspect of the invention provides a compound according to
formula IV
R6
R5
O ~O
H
R1~N N
I
R3 R4 O
where
Rl is R'-C(=Q)- or R'-S(=O)2-
R' is
N~
// // W
/ . ~ . ~ X.
R~~~ R~~~X R~~~X R,
X, = O, S, NH,
W, Y, Z = CH, N;
R" = single or multiple ring substitution combinations taken from:
H, C1-7-alkyl, C3-6-cycloalkyl, OH, SH, amine, halogen;
R3 = C1-7-alkyl, C2-C7 alkenyl, C2-C7 alkenyl, C3-7-cycloalkyl, Ar, Ar-C1-
7alkyl;
R4 = H, C1-7-alkyl, C3-7-cycloalkyl; C2-7alkenyl, Ar, Ar-C1-C7-alkyl;
RS = Cl-7-alkyl; hydroxy- or halo-substituted C1-C7alkyl, halogen, Ar-Cl-7-
alkyl,
CO-3-alkyl-CONR3R4 or R'°;
R'°
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
n n n )n ~ n
N A 02S
Rv~N~Rv; N /
Rv/ ~Rvi
N+
Rvii
Rviii
where n = 1-3, m = 1-3;
R°, R°' = H, C 1-7-alkyl;
A = N, CH;
B = N, O, S, CH;
Rv" = absent when B = O"S; or R°" = H, C1-7-alkyl when B = N, CH;
R°"' = O, C 1-7-alkyl;
R6 =H, C1-7-alkyl, Ar-C1-7-alkyl, C1-3-alkyl-S02-R"', C1-3-alkyl-C(O)-NHR"' or
CHZXAr;
R"' is C1-7-alkyl, Ar-C1-7-alkyl or C3-6-cycloalkyl.
'C1-7-alkyl' as applied herein is meant to include straight and branched chain
aliphatic
carbon chains such as methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-
butyl,
pentyl, isopentyl, hexyl, heptyl and any simple isomers thereof. Additionally,
any C1-
7-alkyl may optionally be substituted by one or two halogens and/or a
heteroatom S, O,
NH. If the heteroatom is located at a chain terminus then it is appropriately
substituted
with one or 2 hydrogen atoms, for example as hydroxymethyl. An S heteroatom
may
be oxidised to the sulphone, especially in the case of R3 C1-7 alkyl or ArCl-
7alkyl.
'C1-3-alkyl' as applied herein includes methyl, ethyl, propyl, isopropyl,
cyclopropyl,
any of which may be optionally substituted as described in the paragraph
above.
'Amine' includes NH2, NHC1-3-alkyl or N(C1-3-alkyl)2.
'Halogen' as applied herein is meant to include F, Cl, Br, I, particularly
chloro and
preferably fluoro.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
'C3-6-cycloalkyf (or C3-C7 cycloalkyl) as applied herein is meant to include
any
variation of 'C1-7-alkyl' which additionally contains a C3-6 (or C3-7)
carbocyclic ring
such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl. Alternatively the C3-
6 or C3-
7 cyclopropyl may be spiro bound to the adjacent carbon without an intervening
C1-C7
alkyl.
'Ar- C 1-7-alkyl' as applied herein is meant to include a phenyl, pyrazolyl,
pyridyl,
imidazolyl, oxazolyl, isoxazolyl, thiazinolyl, isothiazinolyl, thiazolyl,
oxadiazolyl,
1,2,3-triazolyl, 1,2,4-triazolyl, furanyl or thienyl aromatic ring (Ar)
attached through a
'C1-7-alkyl' (defined above) to the dihydro-(3H)-furanone ring system or in
the case of
R2, R3 or R4 linked directly to the molecule backbone. Optionally, the
aromatic ring
Ar may be substituted with halogen, C1-3-alkyl, OH, OC1-3-alkyl, SH, SC1-3-
alkyl,
amine and the like.
'C1-3-alkyl-CONR"', R'°' as applied herein is meant to include straight
or branched
carbon chain substituted with a 1°, 2° or 3° carboxamide
wherein R"', R'° includes H
and Me.
'C 1-3-alkyl-S02-R"', as applied herein is meant to include straight or
branched carbon
chain substituted with a sulphone wherein R"' includes 'C 1-7-alkyl', 'Ar- C 1-
7-alkyl,
'C3-6-cycloalkyf.
'C1-3-alkyl-C(O)-NHR'X, as applied herein is meant to include straight or
branched
carbon chain substituted with a secondary carboxamide wherein R"' includes 'C1-
7-
alkyl, 'Ar- Cl-7-alkyl', 'C3-6-cycloalkyf.
If a chiral centre is present, all isomeric forms are intended to be covered.
Both (R) and
(S) stereochemistries at the position corresponding to the pyranone 5-position
(ie RS
adjacent the linkage to the peptidomimetic chain) are encompassed by the
invention
with (S) being preferred in some cases, for instance with cathepsin S
inhibitors. Other
cysteine proteases appeax to favour the R stereoisomer at this position, such
as
cathepsin K and falcipain, but can accept the S.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
The compounds of the invention are cysteine protease inhibitors, notably
against
cathepsins or cathepsin-like proteases of the papain superfamily. Ideally the
compound
displays selective inhibition of a single protease in the complex mixture of
proteolytic
enzymes characterising the physiological environment, for example a greater
than 10
fold selectivity, preferably greater than 100. Most preferably inhibitory
specificity is
exhibited over other members of the same enzyme class or family, such as the
Cathepsin family, which have a high degree of homology, as incorrect
regulation of
proteolytic activity can lead to unwanted pathological conditions such as
hypertension,
blood clotting or worse. This is especially desirable for disorders such as
autoimmune
disorders where administration of the drug is likely to be protracted.
However, compounds can be useful notwithstanding that they exhibit a degree of
promiscuity in relation to inhibition of physiological proteases. For example
the
physiological functions of many cathepsins are redundant, that is inhibition
of a
particular cysteine protease can be compensated by the presence or
upregulation of
other non-inhibited proteases or alternative metabolic routes. Alternatively,
treatments
of short duration can result only in transient toxicity or other side effects.
The cross-specificity of cysteine proteases for a given putative inhibitor (ie
the
selectivity if the inhibitor) is readily ascertained with conventional enzyme
and cell
culture assays, for instance as depicted in the examples in relation to
cathepsins S, K
and L.
A further aspect of the invention comprises a method employing the compounds
of
formula IV for the treatment of diseases wherein cathepsin S is a factor, ie
diseases or
conditions alleviated or modified by inhibition of cathepsin S, preferably
without
substantial concomitant inhibition of other members of the papain superfamily.
Examples of such diseases or conditions include those enumerated in WO
97/40066,
such as autoimmune diseases, allergies, multiple sclerosis, rheumatoid
arthritis and the
like. The invention further provides the use of the compounds of formula IV in
therapy
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
and in the manufacture of a medicament for the treatment of diseases or
conditions
alleviated or moderated by inhibition of cathepsin S.
In one preferred embodiment, cathepsin S inhibitors have
Rl = R'C(O)
Where R' _
p Me p
~N~ j /
R4 and R6 = H;
R3 = n-butyl, t-butyl, 3-(2,2-dimethylpropyl), 4-(2-methylbutyl), 4-(3,3-
dimethylbutyl), 4-(3,3-dimethyl-2-methylbutyl), 4-(3-methyl-2-methylbutyl), 5-
(2-methyl-3-methylpentyl, cyclohexylmethyl, cyclopentylmethyl);
RS = CH3, C2H5, CH20H, CH2Ar, CH2CONH2, (CH2)2CONH2,
N N
CH3
or permutations thereof.
A favoured group of cathepsin S inhibitors comprises compounds otherwise as
defined
in the immediately preceding paragraph, wherein RS is methyl, ethyl, propyl or
hydroxymethyl. A further group of cathepsin inhibitors comprises compounds as
defined in the paragraph above, but wherein R' as phenyl bears multiple
substitutions,
such as C1-C7alkyl, hydroxy, halo and the like, typically at the 3 and 4
positions.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
Additional preferred definitions for R3 in formula IV include sulphone
substituted C1-
7 alkyl and especially sulphone substituted ArCl-7alkyl, such as
benzenesulphonylmethyl, phenylsulphonylmethyl and phenylethylsulphonylmethyl.
These R3 groups are conveniently combined wth the preferred variables in the
preceding two paragraphs.
A further aspect of the invention provides methods for the treatment or
prophylaxis of
a parasitic infection, such as a protozoal or bacterial infection, comprising
the
administration of a compound of formula IV, to a mammal in need thereof. A
still
further aspect provides a method for the control of protozoal parasites
comprising the
administration of a compound of formula IV, to an invertebrate vector and/or
to a locus
prone to infestation of such a vector.
Conveniently the protozoal or bacterial parasite is a Plasmodium, Leishmania,
Schistosoma, Giardia, Entamoeba, Trypansoma, Crithidia, Pneumocystis or
Porphyromonas species.
Suitably, the treatment or prophylaxis of Plasmodium falciparium comprises
inhibition
of a falcipain II enzyme.
Preferred R3 groups for parasite treatment and prophylaxis include 2-
methylpropen-1-
y1, isobutyl and benzyl, especially the enantiomers defining the side chain of
L-leucine
or L-phenylalanine.
The compounds of the invention can form salts which form an additional aspect
of the
invention. Appropriate pharmaceutically acceptable salts of the compounds of
Formula
IV include salts of organic acids, especially carboxylic acids, including but
not limited
to acetate, trifluoroacetate, lactate, gluconate, citrate, tartrate, maleate,
malate,
pantothenate, isethionate, adipate, alginate, aspartate, benzoate, butyrate,
digluconate,
cyclopentanate, glucoheptanate, glycerophosphate, oxalate, heptanoate,
hexanoate,
fumarate, nicotinate, palmoate, pectinate, 3-phenylpropionate, picrate,
pivalate,
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
proprionate, tartrate, lactobionate, pivolate, camphorate, undecanoate and
succinate,
organic sulphonic acids such as methanesulphonate, ethanesulphonate,
2-hydroxyethane sulphonate, camphorsulphonate, 2-napthalenesulphonate,
benzenesulphonate, p-chlorobenzenesulphonate and p-toluenesulphonate; and
inorganic acids such as hydrochloride, hydrobromide, hydroiodide, sulphate,
bisulphate, hemisulphate, thiocyanate, persulphate, phosphoric and sulphonic
acids.
The compounds of formula IV may in some cases be isolated as the hydrate.
It will be appreciated that the invention extends to prodrugs, solvates,
complexes and
other forms releasing a compound of formula IV in vivo.
While it is possible for the active agent to be administered alone, it is
preferable to
present it as part of a pharmaceutical formulation. Such a formulation will
comprise
the above defined active agent together with one or more acceptable
carriers/excipients
and optionally other therapeutic ingredients. The carriers) must be acceptable
in the
sense of being compatible with the other ingredients of the formulation and
not
deleterious to the recipient.
The formulations include those suitable for rectal, nasal, topical (including
buccal and
sublingual), vaginal or parenteral (including subcutaneous, intramuscular,
intravenous
and intradermal) administration, but preferably the formulation is an orally
administered formulation. The formulations may conveniently be presented in
unit
dosage form, e.g. tablets and sustained release capsules, and may be prepared
by any
methods well known in the art of pharmacy.
Such methods include the step of bringing into association the above defined
active
agent with the carrier. In general, the formulations are prepared by uniformly
and
intimately bringing into association the active agent with liquid carriers or
finely
divided solid carriers or both, and then if necessary shaping the product. The
invention
extends to methods for preparing a pharmaceutical composition comprising
bringing a
compound of Formula IV or its pharmaceutically acceptable salt in conjunction
or
association with a pharmaceutically acceptable carrier or vehicle. If the
manufacture of
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
pharmaceutical formulations involves intimate mixing of pharmaceutical
excipients
and the active ingredient in salt form, then it is often preferred to use
excipients which
are non-basic in nature, i.e. either acidic or neutral.
Formulations for oral administration in the present invention may be presented
as
discrete units such as capsules, cachets or tablets each containing a
predetermined
amount of the active agent; as a powder or granules; as a solution or a
suspension of
the active agent in an aqueous liquid or a non-aqueous liquid; or as an oil-in-
water
liquid emulsion or a water in oil liquid emulsion and as a bolus etc.
With regard to compositions for oral administration (e.g. tablets and
capsules), the term
suitable carrier includes vehicles such as common excipients e.g. binding
agents, for
example syrup, acacia, gelatin, sorbitol, tragacanth, polyvinylpyrrolidone
(Povidone),
methylcellulose, ethylcellulose, sodium carboxymethylcellulose,
hydroxypropylmethylcellulose, sucrose and starch; fillers and carriers, for
example
corn starch, gelatin, lactose, sucrose, microcrystalline cellulose, kaolin,
mannitol,
dicalcium phosphate, sodium chloride and alginic acid; and lubricants such as
magnesium stearate, sodium stearate and other metallic stearates, glycerol
stearate
stearic acid, silicone fluid, talc waxes, oils and colloidal silica.
Flavouring agents such
as peppermint, oil of wintergreen, cherry flavouring or the like can also be
used. It may
be desirable to add a colouring agent to make the dosage form readily
identifiable.
Tablets may also be coated by methods well known in the art.
A tablet may be made by compression or moulding, optionally with one or more
accessory ingredients. Compressed tablets may be prepared by compressing in a
suitable machine.the active agent in a free flowing form such as a powder or
granules,
optionally mixed with a binder, lubricant, inert diluent, preservative,
surface-active or
dispersing agent. Moulded tablets may be made by moulding in a suitable
machine a
mixture of the powdered compound moistened with an inert liquid diluent. The
tablets
may be optionally be coated or scored and may be formulated so as to provide
slow or
controlled release of the active agent.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
11
Other formulations suitable for oral administration include lozenges
comprising the
active agent in a flavoured base, usually sucrose and acacia or tragacanth;
pastilles
comprising the active agent in an inert base such as gelatin and glycerin, or
sucrose and
acacia; and mouthwashes comprising the active agent in a suitable liquid
carrier.
The appropriate dosage for the compounds or formulations of the invention will
depend upon the indication and the patient and is readily determined by
conventional
animal trials. Dosages providing intracellular (for inhibition of
physiological proteases
of the papain superamily) concentrations of the order 0.01-100 uM, more
preferably
.O1-10 uM, such as 0.1-SuM are typically desirable and achievable. Ex vivo or
topical
administration against parasites will typically involve higher concentrations.
The term ~T-protecting group~or ~T-protected~and the like as used herein
refers to
those groups intended to protect the N-terminus of an amino acid or peptide or
to
protect an amino group against undesirable reactions during synthetic
procedures.
Commonly used N-protecting groups are disclosed in Crreene, q'rotective Groups
in
Organic Synthesis~(John Wiley & Sons, New York, 1981), which is hereby
incorporated by reference. N-protecting groups include acyl groups such as
formyl,
acetyl, propionyl, pivaloyl, t-butylacetyl, 2-chloroacetyl, 2-bromoacetyl,
trifluoracetyl,
trichloroacetyl, phthalyl, o-nitrophenoxyacetyl, a-chlorobutyryl, benzoyl, 4-
chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl, and the like; sulfonyl groups
such as
benzenesulfonyl, p-toluenesulfonyl, and the like, carbamate forming groups
such as
benzyloxycarbonyl, p-chlorobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl,
p-nitrobenzyloxycarbonyl, 2-nitrobenzyloxycarbonyl, p-bromobenzyloxycarbonyl,
3,4-dimethoxybenzyloxycarbonyl, 4-methoxybenzyloxycarbonyl,
2-vitro-4,5-dimethoxybenzyloxycarbonyl, 3,4,5-trimethoxybenzyloxycarbonyl,
1-(p-biphenylyl)-1-methylethoxycarbonyl, a,a-dimethyl-3,5-
dimethoxybenzyloxycarbonyl, benzhydryloxycarbonyl, t-butoxycarbonyl,
diisopropylmethoxycarbonyl, isopropyloxycarbonyl, ethoxycarbonyl,
methoxycarbonyl, allyloxycarbonyl, 2,2,2-trichloroethoxycarbonyl,
phenoxycarbonyl,
4-nitrophenoxycarbonyl, fluorenyl-9-methoxycarbonyl, cyclopentyloxycarbonyl,
adamantyloxycarbonyl, cyclohexyloxycarbonyl, phenylthiocarbonyl, and the like;
alkyl
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
12
gropus such as benzyl, triphenylmethyl, benzyloxymethyl and the like; and
silyl groups
such as trimethylsilyl and the like. Favoured N-protecting groups include
formyl,
acetyl, allyl, Fmoc, benzoyl, pivaloyl, t-butylacetyl, phenylsulfonyl, benzyl,
t-butoxycarbonyl (Boc) and benzyloxycarbonyl (Cbz).
Hydroxy and/or carboxy protecting groups are also extensively reviewed in
Greene ibid
and include ethers such as methyl, substituted methyl ethers such as
methoxymethyl,
methylthiomethyl, benzyloxymethyl, t-butoxymethyl, 2-methoxyethoxymethyl and
the
like, silyl ethers such as trimethylsilyl (TMS), t-butyldimethylsilyl (TBDMS)
tribenzylsilyl, triphenylsilyl, t-butyldiphenylsilyl (TBDPS), triisopropyl
silyl and the
like, substituted ethyl ethers such as '1-ethoxymethyl, 1-methyl-1-
methoxyethyl, t-butyl,
allyl, benzyl, p-methoxybenzyl, dipehenylmethyl, triphenylmethyl and the like,
aralkyl
groups such as trityl, and pixyl (9-hydroxy-9-phenylxanthene derivatives,
especially
the chloride). Ester hydroxy protecting groups include esters such as formate,
benzylformate, chloroacetate, methoxyacetate, phenoxyacetate, pivaloate,
adamantoate,
mesitoate, benzoate and the like. Carbonate hydroxy protecting groups include
methyl
vinyl, allyl, cinnamyl, benzyl and the like.
Compounds are synthesised by a combination of chemistries, performed either in
solution or on the solid phase.
The RS substituent confers many beneficial qualities to molecules of general
formula
IV including improvements in potency and offers the potential to append
inhibitor
molecules with a basic functionality to improve solubility and pharmacokinetic
properties. Additionally, molecules of formula IV where RS is alkyl or other
substituent and not simply hydrogen show good chiral stability at the pyranone
alpha
carbon (unless the context otherwise requires referred to as ring position 4
or C4
herein). By chirally stable is meant that the compounds of the invention exist
as a
predominant stereoisomer rather than an equal mixture of stereoisomers
differing in
stereochemistry at C4. Preferably the compounds of the invention are greater
than 90%
diastereomically pure after a protracted time period..
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
13
Note particularly the presence of the RS substituent in the compounds of the
invention
in comparison with the absence of any substituent in the same position in
formula (I)
according to WO 98/50533, WO 98/46582, W099/64399, WO00/29408, WO00/38687
and WO00/49011.
Many active inhibitors contain commercially available amino acid residues such
as L-
leucine, L-norleucine etc . Alternatively, active inhibitors contain new and
novel
hydrophobic amino acids, which are prepared following the chemistry detailed
in
scheme 7. The synthesis detailed in Scheme 7 was adapted from Dexter, C. S.
and
Jackson, R. F. W. Chem.Commuu. 75-76, 1998, and allows ready access to
analogues
embraced by R3 in formula IV. The side chains of some of the novel, multiply
branched alpha-amino acid building blocks exemplified herein can be thought of
as
hybrids of the properties of combinations of other amino acid side chains,
such as those
of norleucine and t-butylalanine. This synthesis methodology is also described
in
Medivir UK's copending PCT application no PCT/GBO1/02162 claiming priority
from
GB00025386.4 entitled Branched Amino Acids filed in the UK patent office on 17
October 2000, the contents of which are specifically incorporated herein.
Access to sulphonyl bearing C1-C7alkyl or ArCl-C7alkyl R3 groups, for instance
arylalkylCO-2sulphonylinethyl functionalities can come from the suitably
protected
amino acid cysteine. Mitsunobu coupling of the cysteinyl thiol with aryl
alcohols such
as phenol yield the protected amino acid containing,the phenylthiomethyl R3
sidechain
that is readily oxidised using m-chloroperbenzoic acid to provide the R3
sidechain
phenylsulphonylinethyl. The benzylsulphonylmethyl and phenethylsulphonylmethyl
R3 sidechain containing amino acids can be prepared by nucleophilic
substitution of
the cysteinyl thiol with benzyl bromide and phenethyl bromide respectively.
Oxidation
of the resulting sulphides with m-chloroperbenzoic acid provides the suitably
protected
amino acids with the benzylsulphonylinethyl and phenethylsulphonylmethyl R3
sidechain.
The pyranone or N-protected aminopyranone building blocks (synthesis
exemplified in
Schemes 1-SA) are utilised in a solid phase synthesis of inhibitor molecules
(typically
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
14
5-25mg product) detailed in Scheme 8. Alternatively, for larger scale
syntheses, full
preparation of inhibitors can be achieved by solution phase chemistry.
Compounds of the invention can be accessed as illustrated below with reference
to
Schemes 1-4.
Scheme 1
OH OTBDPS
HO HO
a b c,d
Cbz~N OCHZCH3 Cbz~N OCHZCH3 --~ Cbz~N OCHZCH3
H H H
O O O
1_ 2 3
OTBDPS OTBDPS OTBDPS
~O ~O ' ~O
a
Cbz~N OH ~ Cbz~N O 'BU f . Cbz.N
~N
H H ~ H
p O O O
4 5 6
~O O Hp O HO O
Cbz h Cbz~ I Cbz~N j,k
H H . ---~ H
O O H3C0 OCH3
7 8
O _O
O m N n,o
Cbz~ --. Boc N
N HzN ~ H3C0 OCH3
HH3C0 OCH3 HzCO OCH3
14
- 11 12 -
0 w0 P O O
N R ~N~N
O = HH3C0 OCH3 IO H O
13 ~ 14
a) Os04, NMM; b) TBDPSC1, imidazole, DMFlCH2C12; c) allyl bromide, TBAF,
Bu2Sn0; d) LiOH in THF/H20; e) 'BuOCOCI, NMM; f) diazomethane in EtaO; g)
LiCI (10e~ in 80 % acetic acid; h) (Ph3P)4Pd, CHC13, AcOH, NMM; i) (Me0)3CH, p-
toluenesulphonic acid, MeOH; j) TsCI, pyridine; k) Me2CuCNLi2; I) 10 % Pd on
carbon, H2; m) Boc-Leu-Opfp, HOBt, NMM, DMF; n) 4M HCl in dioxane; o) Rl
capping group eg benzoic acid, HBTLJ, HOBt, NMM, DMF; p) TFA, NaHC03
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
Compounds of the general formula IV, are prepared by methods shown in Scheme
1.
Treatment of the known Cbz-ethyl ester 1-Scheme-1 with osmium tetroxide and 4-
methylmorpholine provides the diol 2-Scheme-1. Protection of the primary
alcohol
may be effected with test-butyldiphenylsilylchloride and imidazole to provide
3-
Scheme-1. Protection of the secondary alcohol 3-Scheme-1 may be achieved with
allyl
bromide and subsequent base hydrolysis of the ethyl ester provides 4-Scheme-1.
Activation of the acid 4-Scheme-1 may be achieved with isobutyl chloroformate
and 4-
methylmorpholine to provide 5-Scheme-1. Subsequent treatment of 5-Scheme-1
with
diazomethane provides the diazoketone 6-Scheme-1. Cyclization of diazoketone 6-
Scheme-1 can be effected by lithium chloride/aqueous acetic acid to give the 3-
pyranone 7-Scheme-1. The allyl protection may be removed from 7-Scheme-1, by
treatment with palladium(0) and acid, to provide alcohol 8-Scheme-1. Fetal
formation
from ketone 8-Scheme-1 may be effected by treatment with trimethylorthoformate
and
p-toluenesulphonic acid to provide 9-Scheme-1. Conversion of the alcohol 9-
Scheme-1
to the methyl derivative 10-Scheme 1 can be achieved utilising methods that
are known
in the art, such as tosylation with tosylchloride and pyridine, with
subsequent reaction
with the higher order cuprate prepared from methyl lithium. Removal of the Cbz
protecting group from 10-Scheme 1 may be achieved with 10% Pd on carbon in the
presence of hydrogen to provide 11-Scheme-1. The amine 11-Scheme-1 can be
coupled with a carboxylic acid by methods that are known in the art, such as
coupling
with a pentafluorophenol derivative in the presence of HOBT and NMM, to
provide
the amide 12-Scheme-1. The tef~t-butoxycarbonyl group may be removed by
treatment
with an acid, such as hydrogen chloride in dioxane and the amine salt
subsequently
coupled with a carboxylic acid by methods that are known in the art, such as
coupling
with an acid in the presence of HBTU and HOBT, to provide the amide 13-Scheme-
1.
Removal of the ketal functionality from 13-Scheme-1 may be achieved with
trifluoroacetic acid in the presence of sodium hydrogen carbonate to provide
14-
Scheme-1.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
16
Building blocks toward compounds of general formula IV are additionally
conveniently prepared by Schemes 2-4:
Scheme 2
°H OAc OAc
'o 'OH a O OAc b O c
OH
OH OAc OAc OAc
1_ 2 3
OH
OH O O
o d ~ a °
f
O O
~H O
OH
6
O
O O j
g h ~
< O ° ~ ~ \\~~H
g
7 _
O k O I
O 1
~ N\\' 3~ n
n \\y~Bn
OH
11 12
O m O
BOCH\\y~~ ~ BOCH\\vM/'~
13 14
a) pyridine, acetic anhydride; b) triethylsilane, trimethylsilyl triflate; c)
sodium
methoxide, methanol; d) cyclohexanone diethylacetal; e) Swern oxidation; f)
PPh3CHCH3, THF; g) H2, palladium on carbon, sodium bicarbonate; h) 80%
aqueous acetic acid; i) sodium hydride, benzyl bromide; j) mesyl chloride,
pyridine; k) sodium azide, DMF; I) H2, palladium on carbon, di-(tert
butyloxy)carbonyl; m) Dess-Martin periodinane
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
17
Lyxose 1-scheme-2 can be peracetylated to give 2-Scheme-2 with acetic
anhydride in pyridine at room temperature overnight. Reduction at the
anomeric centre to afford 3-Scheme-2 may be achieved using triethylsilane in
the presence of trimethylsilyl triflate. Hydrolysis of the triacetate 3-Scheme-
2
affords 4-Scheme-2 whereupon the vicinal diol can be protected as the
cyclohexanone acetal 5-Scheme-2. Swern oxidation of the unprotected
alcohol functionality gives 6-Scheme-2, a key intermediate for the
introduction
of the required C5 pyranone substitution. Ethyl substitution is achieved here
by treatment with ethyl triphenylphosphonium bromide with potassium tert
butoxide in THF at 0°C to produce 7-Scheme-2. Hydrogenation of 7-Scheme-
2 in ethyl acetate with sodium bicarbonate gives the ethyl derivative 8-
Scheme-2 with the stereochemistry shown. Deprotection of the
cyclohexanone acetal 8-Scheme-2 can be achieved with aqueous acetic acid
overnight to afford the diol 9-Scheme-2. Selective benzylation of the
equatorial hydroxyl group gives 10-Scheme-2, which can then be mesylated
using mesyl chloride in pyridine at 50°C to produce 11-Scheme-2. Azide
displacement of mesylate anion using sodium azide in DMF at 80°C
affords
12-Scheme-14, from which the pyranol 13-Scheme-2 can be obtained by
hydrogenolysis in the presence of BOC-anhydride. Oxidation to the pyranone
14-Scheme-2 is achieved using the Dess-Martin periodinane.
In Scheme 2, the C5 substitution is introduced using Wittig chemistry followed
by hydrogenation, and hence compound 6-Scheme-2 could be converted to
the C5 ethyl derivative 8-Scheme-2. Alternative C5 substitution can be
achieved using this route. For example, alternative Wittig or Horner-Emmons
chemistry will lead to different alkyl substituents. In an analogous manner,
the
C5 hydroxymethyl group can be prepared and this itself can be further
derivatised to other groups such as halogen, amino and other basic groups
and sulfhydryl.
A general methodology starting from L-lyxose has been established for the
preparation of various 5-substituted 4-amino 3-hydroxy pyranols with all four
possible combinations of configuration at position 4 and 5 i.e. 4S,5S; 4S,5R;
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
18
4R,5S and 4R,5R. This methodology is exemplified in Scheme 2A. The
pyranols can then N-extended and capped as described herein and
subsequently oxidised to the keto compounds, for example by Dess-Martin
periodination.
OH OR OR
4 5 O O O
3 2 OH ~ OR
OH OH1 OR OR OR OR
L-Lyxose 1 R=acyl 2 R=acyl
3 R=H
Scheme 2A
R2 OH
O O~ O O
ORS ORS E ~ OR OR
OR
6 Rl=ketal 5 R=ketal 4 R=ketal
7 R2=alkyl
R4 R4
\ O\
R3 O R R2~
---~ I OBn
ORz ORS R1
8 R1,R2=ketal, R3=alkyl, R4=H 13 Rl=H, R2 N3, R3=alkyl, R4=H
9 Rl=R2=H, R3=alkyl, R4=H 14 Rl=H, R2 N3, R3=H, R4=alkyl
Rl=R2=H, R3=H, R4=allcyl
11 Rl=Bn, R2=H, R3=alkyl, R4=H 15 Rl=N3, R2=H, R3=alkyl, R4=H
12 Rl=Bn, R2=H, R3=H, R4=allcyl 16 Rl=N3, R2=H, R3=H, R4=alkyl
4S, 5S: Rl=H, R2=NHBoc, R3=alkyl, R4 H R4
4S, 5R: Rl=H, R2=NHBoc, R3=H, R4=alkyl
4R, 5S: Rl=NHBoc, R2=H, R3=alkyl, R4=H R3 O
4R, SR: Rl=NHBoc, R2=H, R3=H, R4=alkyl R~
OH
R1
L-lyxose can be acylated with a suitable acylating agent such as acid
anhydride, acyl
halide in an organic solvent like pyridine or other mixed organic solvents, to
give the
peracylated compound 1-scheme-2A. This compound can then be subjected to
anomeric reduction with a trialkyl silane together with a Lewis acid such as
triethyl
silane and trimethylsilyl trifluormethanesulphonate. Transforming the compound
into
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
19
the corresponding halo-, sulpho- or thiocarbo-glycoside followed by a radical
reduction, using known methodology, can also bring about the anomeric
reduction.
Deacylation under basic condition provides the triol 3-scheme-2A, which can be
selectively protected on the 2,3-hydroxylgroups forming a ketal 4-scheme 2A by
using
standard protecting group methodology. Oxidation of the 4-OH group into the
keto
function 5-scheme-2A can be performed with the Swern procedure, Dess-Martin or
any
other suitable oxidation method. 'Various 4-substituted alkenes 6-scheme-2A
can be
achieved by using appropriate Wittig reagents for example
triphenylalkylphosphonium
halide or triphenylalkylarylphosphonium halide together with a base. Catalytic
hydrogenation of the Wittig product in the presence of a buffer provides
predominantly
compound 8-scheme-2A. Alternatively, the compound with the other configuration
at
this position 10-scheme-2A can be obtained by removal of the ketal protecting
group
prior to the hydrogenation. The alkene compound can also be subjected to
hydroboration, which will introduce a hydroxyl group, suitable for further
modifications.
Another possibility to achieve the 4-alkyl compounds is to transform the 4-OH
group
into a leaving group for example a sulphonate followed by displacement by a
cuprous
or Grignard reagent of the desired alkylgroup.
The ketal protecting group can be removed under acidic conditions such as 1 M
HCl/THF 1:1 at room temperature or heating to 80 °C in aqueous acetic
acid which
will give the diol 8-scheme-2A. Selective protection of the 2-OH group with an
alkylating agent such as benzyl halide or any other similar reagent in the
presence of a
base can give exclusively or predominantly the 2-O-protected compound 11,12-
scheme-2A. The 3-OH can be converted to a suitable leaving group such as a
sulphonate, which subsequently can be displaced by an azide 13,14-scheme-2A.
Alternatively, a Mitsunobu reaction can be used to produce the azide-
substituted
compound. Hydrogenation of the azide-compound in the presence of a
carbamoylating
agent like di-tent-butyl dicarbonate provides the desired 1,5-anhydro-3-[(te~t-
butoxycarbonyl)amino]-3,4-dideoxy-4-ethyl-D-xylitol and 1,5-anhydro-3-[(tert-
butoxycarbonyl)amino]-2,3-dideoxy-2-ethyl-L-arabinitol.
The series of compounds with the other configuration at carbon 3 can be
prepared by
inversion of the configuration of the 3-OH in compound 11,12-scheme-2A by
methods
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
that are known in the art, followed by the above procedure i. e. putting on a
leaving
group and azide displacement. They can also be prepared by the following
sequence.
Oxidation of the 3-OH into a ketone, using the oxidation reagents previously
described, transformation of the ketone into an oxime, utilising reagents such
as
benzyloxyamine halide and finally reduction of the oxime into the amino
function.
This will provide a mixture of the compounds with the two different
configurations,
which can be separated using known methodology. Boc-protection of the amino
group
and reductive removal of the benzyl protecting group provides the compounds
with the
remaining two configurations 4R,SS and 4R,SR.
Scheme 3
//~~~' COaBn
BnO~C
INHZ
1
COZtBu O b OH C OtBu
HO NHZ ~ ZHN ~ZHN N~OMe ~ZHN N~OMe
O
O 4
f
~OtBu a ~otBU ~OtBU
9 t
ZHN H ~ ZHN OH ~ ZHN Ou0 Bu
O O O CIO
6 7 8
wOtBu I w0
ZHN ~N2 ~ ZHN
IOI O
9 10
a) TFA; b) Me3Al, HC1.HNMe(OMe), DCM; c) CC13(NH)OtBu, BF3.Et20, DCM,
cyclohexane; d) LAH in Et20; e) tBuOH, 2-methyl-2-butene, NaC102, NaH2P04,
H20;
f) tBuOK, Et20, H20; g) 'BuOCOCI, NMM, THF; h) diazomethane in Et20; i) LiCI
(l0eq) in 80 % acetic acid.
Compounds of the general formula IV are alternatively prepared by methods
shown in
Scheme 3. Alcohol 2-Scheme-3 can be prepared following the literature
procedure
reported by J. E. Baldwin et al. (Tetrahedron, 1995, 51 (43), 11581). Removal
of the
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
21
ester functionality from 2-Scheme-3 can be achieved with trifluoroacetic acid
to
provide the lactone 3-Scheme-3. Lactone 3-Scheme-3 can be ring opened by
MeONHMe in the presence of Me3A1 to provide the alcohol 4-Scheme-3. The tey~t-
butoxycarbonyl group may be introduced onto alcohol 4-Scheme-3 to provide 5-
Scheme-3. The Weinreb amide 5-Scheme-3 can then be treated with lithium
aluminum
hydride to provide the aldehyde 6-Scheme-3. Oxidation of the aldehyde 6-Scheme-
3
can be effected by sodium chlorite to provide the acid 7-Scheme-3.
Alternatively, the
Weinreb amide 5-Scheme-3 can then be treated with potassium-test-butoxide to
provide the acid 7-Scheme-3. Activation of the acid 7-Scheme-3 with isobutyl
chloroformate and 4-methylmorpholine provides 8-Scheme-3. Subsequent treatment
of
8-Scheme-3 with diazomethane provides the diazoketone 9-Scheme-3. Cyclization
of
diazoketone 9-Scheme-3 can be effected by lithium chloride/aqueous acetic acid
to
give the dihydro-3(2H)-:furanone 10-Scheme-3.
Scheme 4
a b c d
O ---~ O --. O _ O --.
HO TfO~ N3~ pTsO-NH3+
O O 0 0
1_ 2 3
~OH f ~OTBDMS
0 -
Boc NH BocHN OH BocHN OH -'
O O O
6 7
~OTBDMS h ~OTBDMS t
O OtBu~
BocHN ~ BocHN ~NZ BocHN
0 O O O
9_ 10
a) (CF3S02)20, pyridine, DCM; b) (nBu)4NN3, toluene; c) H2, 10% Pd/C, pTsOH,
MeOH; d) BocaO, NEt3, THF; e) 1M LiOH, THF; f) TBDMSCI, NEt3, cat DMAP,
DCM; g) 'BuOCOCI, NMM, THF; h) diazomethane in Et20; i) LiCI (l0eq) in 80
acetic acid.
Compounds of the general formula (IV) can be prepared analogously to the model
compound depicted in scheme 4.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
22
Pantolactone 1-Scheme-4 is commercially available and is first converted to
the triflate
2-Scheme-4. The triflate 2-Scheme-4 may be displaced with tetrabutylammonium
azide to provide the corresponding azide 3-Scheme-4. Azide 3-Scheme-4 may be
reduced to provide the amine salt 4-Scheme-4. Protection of the amine salt 4-
Scheme-4
provides 5-Scheme-4. Ring opening of the lactone 5-Scheme-4 with lithium
hydroxide
provides the acid 6-Scheme-4. Protection of the primary alcohol 6-Scheme-4
with
tetrabutyldimethylsilyl chloride in the presence of base provides acid 7-
Scheme-4.
Activation of the acid 7-Scheme-4 with isobutyl chloroformate and 4-
methylmorpholine provides 8-Scheme-4. Subsequent treatment of 8-Scheme-4 with
diazomethane provides the diazoketone 9-Scheme-4. Cyclization of diazoketone 9-
Scheme-4 can be effected by lithium chloride/aqueous acetic acid to give the
model
dihydro-3(2H)-pyranone 10-Scheme-4. Corresponding ring closure can be perfomed
on mono-RS variants of the invention.
Although schemes 1-4 have been illustrated by reference to particular Rl-R4
values, it
will be appreciated that the methodology is more generally applicable to
precursors
- bearing the other claimed values in these positions, where necessary in
conjunction
with conventional protection of functionalities on Rl-R4. Similarly other
values for RS
and R6 can be accessed analogously.
Unless otherwise specified, where a chiral centre is present in a molecule but
not
assigned, both R and S isomers are intended. Preferably the stereochemistry at
R3 is
that of the corresponding L-amino acid. Most preferably the stereochemistry at
RS is S,
especially when the adjacent linkage from the ring to the amine of the
backbone, (ie
C4) is also S. Alternatively the compounds of the invention are R,R at the
latter stereo
centres.
Currently preferred compounds of the present invention include, but are not
limited to,
the following examples:
Furan-3-carboxylic acid (1~-[3,3-dimethyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-
_
ylcarbamoyl)-butyl]-amide
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
23
Furan-3-carboxylic acid (1~-[2-cyclohexyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-
ylcarbamoyl)-ethyl]-amide
(1~-N [3,3-Dimethyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-
benzamide
(1ST-N [2-Cyclohexyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(l~-N [3,3-Dimethyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-4-
hydroxy-3-methyl-benzamide
(1ST-N [2-Cyclohexyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
4-
hydroxy-3-methyl-benzamide
Furan-3-carboxylic acid (1~-[2-cyclopentyl-1-(3-methyl-5-oxo-tetrahydro-pyran-
4-
ylcarbamoyl)-ethyl]-amide
(1~-N [2-Cyclopentyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(1ST-N [2-Cyclopentyl-1-(3-methyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
4-
hydroxy-3-methyl-benzamide
Furan-3-carboxylic acid (1~-[3,3-dimethyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-
ylcarbamoyl)-butyl]-amide
Furan-3-carboxylic acid (1S)-[2-cyclohexyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-
ylcarbamoyl)-ethyl]-amide
(1~-N [3,3-Dimethyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-
benzamide
(1ST-N [2-Cyclohexyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(1ST-N [3,3-Dimethyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-4-
hydroxy-3-methyl-benzamide
(1ST-N [2-Cyclohexyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-4-
hydroxy-3-methyl-benzamide
Furan-3-carboxylic acid (1ST-[2-cyclopentyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-
4-
ylcarbamoyl)-ethyl]-amide
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
24
(1ST-N [2-Cyclopentyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(1~-N [2-Cyclopentyl-1-(3-ethyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-4-
hydroxy-3-methyl-benzamide
Furan-3-carboxylic acid (1ST-[3,3-dimethyl-1-(3-propyl-5-oxo-tetrahydro-pyran-
4-
ylcarbamoyl)-butyl]-amide
Furan-3-carboxylic acid (1ST-[2-cyclohexyl-1-(3-propyl-5-oxo-tetrahydro-pyran-
4-
ylcarbamoyl)-ethyl]-amide
(l~-N [3,3-Dimethyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-
benzamide
(1ST-N [2-Cyclohexyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(1ST-N [3,3-Dimethyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-butyl]-
4-
hydroxy-3-methyl-benzamide
(1ST-N [2-Cyclohexyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
4-
hydroxy-3-methyl-benzamide .
Furan-3-carboxylic acid (1S7-[2-cyclopentyl-1-(3-propyl-5-oxo-tetrahydro-pyran-
4-
ylcarbamoyl)-ethyl]-amide
(l~-N [2-Cyclapentyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
benzamide
(1S7-N [2-Cyclopentyl-1-(3-propyl-5-oxo-tetrahydro-pyran-4-ylcarbamoyl)-ethyl]-
4-
hydroxy-3-methyl-benzamide,
particularly the respective 3R,4R enantiomers,
most preferably the respective 3S, 4S enantiomers;
and pharmaceutically acceptable salts thereof
Detailed disclosure of the embodiments
Experimental Section
Solution Phase Chemistry
Example 1.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
Following general chemistry schemes 5 and SA
(a) General method for the synthesis of N-Boc protected 4-aminopyranol,
exemplified
by 1,5-anhydro-3-[(tert-butoxycarbonyl)amino]-3,4-dideoxy-4-ethyl-D-xylitol
4S, SS
OH OAc OAc
0 O O
O H --~ OAc
OH OH OAc OAc OAc OAc
L-Lyxose 1 2
H3C O~ O OH
.~ O O
O O EO O E-O O
5 4
3
O O O
i
O O OH OH ~ OH O
6
8
N\\r
O O
BocHN
OH 9
(4S, SS)
Scheme SA
1,2,3,4-Tetra-O-acetyl-L-lyzopyroanose (1).
L-Lyxopyroanose (25.0 g, 166 mmol) was dissolved in pyridine (150 ml) and
cooled
on an icebath, acetic anhydride (75 ml) was added and the solution was stirred
at room
temperature. After 2 hours tlc (pentane:ethyl acetate 1:1) indicated complete
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
26
conversion of the starting material into a higher migrating spot. The solution
was
concentrated and co-evaporated three times with toluene which gave a pale
yellow
syrup.
NMR data 400 MHz (CDC13): 1H, 8 2.06 (s, 3H), 2.08 (s, 3H), 2.14 (s, 3H), 2.16
(s,
3H), 3.71 (dd, 1 H), 4.01 (dd, J=5.0, 11.7 Hz, 1 H), 5.17-5.26 (m, 2H), 5.3 7
(dd, J=3.5,
8.8 Hz, 1 H), 6.0 (d, J=3 .2 Hz, 1 H).
13C, 8 20.9, 20.9, 21.0, 21.0, 62.2, 66.7, 68.4, 68.4, 90.8, 168.8, 169.9,
170.0, 170.1.
2,3,4-Tri-O-acetyl-1,5-anhydro-L-arabinitol (2). a
Trimethylsilyl trifluormethanesulphonate (60 ml, 333 mmol) was added to a
solution
of crude 1,2,3,4-tetra-O-acetyl-L-lyxopyroanose constituting the yield from
the step
above in acetonitrile (200 ml), the solution was cooled on an ice bath and
triethylsilane
(80 ml, 500 mmol) was added dropwise. The solution was stirred at room
temperature
and reaction was monitored by GC. When the reaction was complete (after 3
hours),
the solution was neutralised with sodium hydrogen carbonate (s), diluted with
dichloromethane and washed with water. The organic phase was dried with
magnesium
sulphate, filtered and concentrated. The obtained oil was purified by silica
gel flash
column chromatography (pentane:ethyl acetate 5:1, 4:1, 3:1) which gave 32 g,
74
(from free lyxose) of the reduced compound.
NMR data 400 MHz (CDCl3): 1H, 8 2.06 (s, 3H), 2.07 (s, 3H), 2.11 (s, 3H), 3.36-
3.41
(m, 1H), 3.64 (dd, J=2.4, 12.2 Hz, 1H), 3.87 (m, 1H), 4.03 (m, 1H), 5.10-5.15
(m, 2H),
5.28-5.31 (m, 1H).
1,5-Anhydro-3,4-O-cyclohegylidene-L-arabinitol (3).
A solution of 1-deoxy-2,3,4-tri-O-acetyl-L-lyxopyroanose (20.8 g, 80 mmol) in
methanol (125 ml) was treated with a catalytic amount of 1M methanolic sodium
methoxide. After stirring for 1 hour at room temperature tlc (ethyl
acetate:methanol
3:1) indicated complete conversion into a lower migrating spot. The solution
was
neutralised with Dowex H+, filtered and concentrated, which gave a colourless
oil.
The oil was suspended in dichloromethane (70 ml) and cyclohexanone diethyl
ketal
(41 g, 240 mmol) was added followed byp-toluenesulphonic acid until acidic pH.
After a few minutes the suspension became a clear solution, which was stirred
at room
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
27
temperature. After 18 hours, when tlc (pentane:ethyl acetate 1:2) indicated
complete
conversion into a higher migrating spot, the solution was neutralised with
triethyl
amine, concentrated and the residue was purified by silica gel flash column
chromatography (toluene:ethyl acetate 3:2, 1:1) which gave 9.6 g, 56% of the
title
compound as white crystals.
NMR data 400 MHz (CDC13): 1H, 8 1.38-1.43 (m, 2H), 1.56-1.75 (m, 8H), 2.43 (d,
J=4.9 Hz, 1H), 3.28 (m, 1H), 3.75 (dd, J=3.9, I2.7 Hz, 1H), 3.82-3.94 (m, 3H),
4.05 (t,
J=5 .4 Hz, 1 H), 4.22 (m, 1 H).
13C, 8 23.9, 24.3, 25.2, 35.7, 38.3, 67.8, 68.7, 69.1, 71.9, 77.5, 110.5.
1,5-Anhydro-3,4-O-cyclohegylidene-L-ribulose (4).
A solution of dimethyl sulphoxide (2.65 ml, 37.3 mmol) in dichloromethane (30
ml)
was added dropwise at -60 °C under nitrogen to a stirred solution of
oxalyl chloride
(1.79 ml, 20.5 mmol) in dichloromethane (30 ml) during a period of 15 min. To
this
solution a solution of 2,3-O-cyclohexylidene-1-deoxy-L-lyxopyroanose (4 g,
18.7
mmol) in dichloromethane (20 ml) was added dropwise during a period of 5 min.
A
white suspension was obtained and additional dichloromethane was added twice
(10+30 ml). The temperature was allowed to rise to -25 °C when the
suspension
became a colourless solution. The temperature was again lowered to -45
°C and a
solution of triethyl amine (12.9 ml, 93.3 mmol) in dichloromethane (20 ml) was
added.
After 10 min, when tlc (toluene:ethyl acetate 1:1) indicated complete
conversion of the
alcohol into the ketone, the reaction mixture was poured into water (100 ml),
the water
layer was extracted once with dichloromethane (50 ml), the combined organic
phases
were dried with sodium sulphate, filtered and concentrated. Flash column
chromatography on silica gel (eluent pentane:diethyl ether 1:1) of the residue
gave a
colourless solid 3.4 g, 86%.
The oxidation was also performed by the Dess-Martin procedure:
A suspension of 2,3-O-cyclohexylidene-1-deoxy-L-lyxopyroanose (0.5 g, 2.33
mmol)
and Dess-Martin periodinane (1.39 g, 3.29 mmol) in dichloromethane (5 ml) was
stirred for 10 min then "wet dichloromethane~(46 ~,1 water in 10 ml
dichloromethane)
was added dropwise during 15 min. After 1h tlc (toluene:ethyl acetate 1:1)
indicated
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
28
complete conversion of the starting material into a higher migrating spot. The
reaction
mixture was diluted with diethyl ether (100 ml) and washed with an aqueous
solution
of sodium hydrogen carbonate/sodium thiosulphate 1:1 (50 ml), dried with
sodium
sulphate, filtered and concentrated. Purification of the residue by flash
column
chromatography on silica gel (eluent pentane:diethyl ether 1:1) gave the title
compound, 0:42 g, 84%, as a crystalline solid.
NMR data 400 MHz (CDC13): 1H, 8 I.39-I.43 (m, 2H), 1.56-1.72 (m, 8H), 3.92-
4.07
(m, 3H), 4.18-4.23 (m, 1 H), 4.45 (d, J=6.8 Hz, 1 H), 4.64-4.67 (m, 1 H).
13C, 8 23.9, 24.1, 25.1, 35.3, 36.8, 68.5, 74.1, 75. l, 76.3, 112.4, 205Ø
1,5-Anhydro-4-deo~y-4-ethylidene-2,3-O-cyclohegylidene-D-erythro-pentitol (5).
Potassium-t-butoxide (3.41 g, 30.4 mmol) was added in one portion to a stirred
suspension of ethyltriphenylphosphonium bromide (11.9 g, 32.0 mmol) in THF (60
ml)
at -10 °C under nitrogen. The obtained orange-red mixture was allowed
to reach room
temperature, then cooled again to -10 °C and a solution of 1,5-anhydro-
3,4-O-
cyclohexylidene-L-ribulose (3.4 g, 16.0 mmol) in THF (40 ml) was added
dropwise.
The mixture was allowed to attain room temperature. Twenty minutes after the
final
addition, when tlc (toluene:ethyl acetate 1:1) indicated complete conversion
of the
starting material into a higher migrating spot, the reaction mixture was
partitioned
between diethyl ether (400 ml) and water (200 ml). The organic layer was
washed with
water (1x200 ml) and brine (1x200 ml), dried with sodium sulphate, filtered
and
concentrated to a 10 ml residue. The residue was purified by flash column
chromatography on silica gel (eluent pentane:ethyl acetate 95:5, 9:1) and
appropriate
fractions were carefully concentrated (bath temperature 25 °C) to 10 g
that was used
directly in the next step.
1,5-Anhydro-4-deogy-4-ethyl-2,3-O-cyclohegylidene-D-ribitol (6).
The above solution was diluted with ethyl acetate (30 ml), Pd/C ( 10%, 0.2 g)
was
added and the mixture was hydrogenated at atmospheric pressure. Additional
Pd/C was
added (0.16 g + 0.20 g) after 40 and 90 minutes. After 100 minutes tlc
indicated almost
complete consumption of the starting material. The reaction mixture was
filtered
through celite, concentrated into a liquid (5 ml) and purified by flash column
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
29
chromatography on silica gel (eluent pentane:ethyl acetate 95:5, 9:1).
Appropriate
fractions were concentrated to 2.08 g and this solution was used directly in
the next
step.
NMR data 400 MHz (CDC13): 1H, 8 0.98 (t, 3H), 1.31-1.74 (m, 12H), 1.82-1.92
(m,
1H), 3.18-3.26 (m, 2H), 3.64-3.68 (m, 1H), 3.84 (dd, J=6.4, 11.4 Hz, 1H), 4.08-
4.14
(m, 1H), 4.27-4.29 (m, 1H).
13C, 8 11.3, 20.9, 24.0, 24.3, 25.3, 35.7, 38.3, 38.7, 67.7, 68.3, 70.8, 72.6,
109.5.
1,5-Anhydro-4-deogy-4-ethyl-D-ribitol (7).
The above 1,5-anhydro-4-deoxy-4-ethyl-2,3-O-cyclohexylidene-D-ribitol was
dissolved in aqueous acetic acid (80 %, 25 ml) and the solution was stirred at
70 °C.
After 18 hours, when tlc (pentane:ethyl acetate 9:1 and l :l) indicated almost
complete
consumption of the starting material (,~5% left), the solution was
concentrated.
Purification of the residue by flash column chromatography on silica gel
(eluent
pentane:ethyl acetate 1:1, 2:3) gave 0.91 g 39 % (from the keto compound) of a
colourless solid.
NMR data 400 MHz (CDC13): 1H, 8 0.94 (t, 3H), 1.24-1.42 (m, 2H), 1.58-1.67 (m,
1 H), 3 .3 5 (t, 1 H), 3 .43 (t, 1 H), 3 .56 (dd, 1 H), 3 .67-3 .71 (m, 2H).
13C, 8 11.4, 20.1, 42.1, 66.1, 66.3, 68.3, 68.7.
1,5-Anhydro-2-O-benzyl-4-deogy-4-ethyl-D-ribitol (8).
Sodium hydride (60%, 0.27 g, 6.84 mmol) was added in one portion, at room
temperature, under nitrogen, to a stirred solution of 1,5-anhydro-4-deoxy-4-
ethyl-D-
ribitol (0.5 g, 3.42 mmol) in dimethylformamide (7 ml). After 30 minutes
benzyl
bromide (0.53 ml, 4.45 mmol) was added dropwise during 30 minutes. After 20
minutes, when tlc (petroleum ether:ethyl acetate 4:1) indicated complete
conversion of
the diol, methanol (1 ml) was added and the mixtuxe was stirred for 20
minutes. The
reaction mixture was diluted with ethyl acetate (100 ml), washed with water
(3x50 ml),
dried with sodium sulphate, filtered and concentrated. Purification of residue
by flash
column chromatography on silica gel (eluent pentane:ethyl acetate 9:1, 4:1)
gave 0.52
g, 64% of a colourless solid.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
NMR data 400 MHz (CDC13): 1H, 8 0.94 (t, 3H), 1.25-1.36 (m, 1H), 1.37-1.48 (m,
1H), 1.54-1.62 (m, 1H), 2.14 (s, 1H), 3.40 (t, 1H), 3.51-3.56 (m, 3H), 3.72-
3.79 (m,
1H), 4.13 (s, 1H), 4.58 (d, J=11.7 Hz, 1H), 4.63 (d, J=11.7 Hz, 1H), 7.29-7.38
(m, SH).
13C, 8 11.5, 20.1, 42.0, 64.1, 66.5, 66.6, 71.1, 75.6, 127.9, 128.2, 128.8,
138.1.
1,5-Anhydro-3-azido-2-O-benzyl-3,4-dideogy-4-ethyl-D-xylitol (9).
Methanesulphonyl chloride (0.34 g, 2.96 mmol) was added to a stirred solution
of 1,5-
anhydro-2-O-benzyl-4-deoxy-4-ethyl-D-ribitol (0.28 g, 1.18 mmol) in pyridine
(5 ml).
The reaction mixture was warmed to 50 °C and stirred for one hour.
Dichloromethane
(100 ml) was added and the reaction mixture was washed successively with 1M
aqueous sulphuric acid (2x50 ml), 1M aqueous sodium hydrogen carbonate, dried
with
sodium sulphate, filtered and concentrated. The residue was dissolved in
dimethylformamide (10 ml) and sodium azide (0.3I g, 4.74 mmol) was added. The
obtained mixture was stirred at 80 °C over night, diluted with ethyl
acetate (100 ml),
washed with water (3x50 ml), dried with sodium sulphate, f ltered and
concentrated.
Purification of residue by flash column chromatography on silica gel (eluent
toluene:ethyl acetate 95:5) gave 0.25 g, 81% of a colourless oil.
NMR data 400 MHz (CDCl3): 1H, b 0,90 (t, 3H), 1.12-1.24 (m, 1H), 1.44-1.54 (m,
1 H), 1.69-1.79 (m, 1 H), 3 .01 (t, 1 H), 3 .08-3.16 (m, 2H), 3 .44-3.50 (m, 1
H), 3.92 (dd,
J=4.9, I I .7 Hz, I H), 4.04 (ddd, J=1.0, 4.9, 11.2 Hz, 1 H), 4.62 (d, J=11.7
Hz, 1 H), 4.71
(d, J=11.2 Hz, 1H) 7.29-7.37 (m, SH).
13C, 8 11.3, 22.0, 42.4, 68.5, 69.2, 70.9, 73.1, 78.2, 128.2, 128.2, 128.7,
138Ø
1,5-Anhydro-3-[(tent-butogycarbonyl)amino]-3,4-dideoxy-4-ethyl-D-gylitol (10).
Pd/C (10%, 30 mg) was added to solution of 1,5-anhydro-3-azido-2-O-benzyl-3,4-
dideoxy-4-ethyl-D-xylitol (88 mg, 0.34 mmol) and di-tef°t-butyl
Bicarbonate (77 mg,
0.35 mmol) in ethyl acetate (4 ml) and the mixture was stirred under hydrogen.
After
18 hours, when tlc (pentane: ethyl acetate 9:1, ninhydride) indicated complete
consumption of the starting material, the mixture was filtered through celite
and
concentrated. The residue was purified by flash column chromatography on
silica gel
(eluent toluene:ethyl acetate 4:1) which gave a colourless solid that still
contained a
benzyl group according to lHnrnr. The solid was dissolved in ethyl
acetate:ethanol 1:1
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
31
and hydrogenated over Pd/C (10% 20 mg). After 1 hour, when tlc (toluene:ethyl
acetate 1:1, ninhydride) indicated complete conversion of the starting
material into a
lower migrating spot, the mixture was filtered through celite and
concentrated.
Purification of residue by flash column chromatography on silica gel (eluent
toluene:ethyl acetate 1:1, 2:3) gave 59 mg, 71% ofthe desired monool as a
colourless
solid.
NMR data 400 MHz (CDCl3): 1H, b 0.90 (t, 3H), 1.12-I.24 (m, IH), 1.42-1.52 (m,
10H), 1.59-1.70 (m, 1H), 3.05-3.16 (m, 2H), 3.26-3.30 (m, 1H), 3.43-3.48 (m,
2H),
3.96-4.05 (m, 2H).
13C, 8 11.5, 21.2, 28.5, 42.4, 59.2, 71.4, 71.8, 72.7.
Alternative method for the preparation of 5-methyl pyranones as building
blocks and intermediates towards 5-functionalised pyranones
2-Benzyloxycarbonylamino-4-hydroxy-3-methyl-butyric acid tent-butyl ester
coZtBu
HO' Y 'NHZ
2-Benzyloxycarbonylamino-4-hydroxy-3-methyl-butyric acid tent butyl ester was
prepared following procedures reported by J.E. Baldwin et al (Tetrahedron
1995, 51 (42), 11581 ).
(4-Methyl-2-oxo-tetrahydro-furan-3-yl)-carbamic acid benzyl ester
0
ZHN
O
2-Benzyloxycarbonylamino-4-hydroxy-3-methyl-butyric acid tent butyl ester
(1.00g, 3 mmol) was dissolved in TFA (30 mL). This solution was stirred for 45
minutes and then concentrated in vacuo. The residual TFA was removed
azeotropically with toluene. This residue was purified by flash column
chromatography to yield the title compound as a crystalline solid (750mg,
80%), MS (ES+) 250 (M+H).
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
32
[3-Hydroxy-1-(methoxy-methyl-carbamoyl)-2-methyl-propyl]-
carbamic acid benzyl ester 4
'OH
N~
ZHN home
O
The lactone ring of (4-methyl-2-oxo-tetrahydro-furan-3-yl)-carbamic acid
benzyl ester can be opened using N,O-dimethylhydroxylamine hydrochloride in
the presence of Me3Al to give the title compound.
[3-tart Butoxy-1-(methoxy-methyl-carbamoyl)-2-methyl-propyl]-carbamic acid
benzyl ester 5
N~
ZHN home
O
The primary alcohol of [3-hydroxy-1-(methoxy-methyl-carbamoyl)-2-methyl-
propyl]-carbamic acid benzyl ester can be protected using tart-butyl-2,2,2-
trichloroacetimidate and boron trifluoride etherate to give the title
compound.
(3-tart Butoxy-1-formyl-2-methyl-propyl)-carbamic acid benzyl ester 6
~o~Bu
H
ZHN
O
The Weinreb amide function of [3-tent-butoxy-1-(methoxy-methyl-carbamoyl)-
2-methyl-propyl]-carbamic acid benzyl ester can be reduced using lithium
aluminium hydride in ether to provide the title compound.
2-Benzyloxycarbonylamino-4-tart-butoxy-3-methyl-butyric acid 7
OtBu
OH
ZHN
O
(3-fiert butoxy-1-formyl-2-methyl-propyl)-carbamic acid benzyl ester in tart
butyl
alcohol in the presence of 2-methyl-2-butane can be oxidised using a solution
of sodium chlorite and monobasic sodium phosphate in water to give the title
compound.
[3-tent Butoxy-1-(2-diazo-acetyl)-2-methyl-propyl]-carbamic acid benzyl ester
9
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
33
~otBu
ZHN ~ ~ NZ
O
Activation of 2-benzyloxycarbonylamino-4-tent butoxy-3-methyl-butyric acid
with isobutyl chloroformate and 4-methylmorpholine, and subsequent
treatment of the activated acid with diazomethane allows for the preparation
of
the title compound.
(3-Methyl-5-oxo-tetrahydro-pyran-4-yl)-carbamic acid benzyl ester 10
-o
ZHN
O
Cyclisation of tert butoxy-1-(2-diazo-acetyl)-2-methyl-propyl]-carbamic acid
benzyl ester using lithium chloride in aqueous acetic acid gives the title
compound. The CBz protecting group is readily replaced with Boc or Fmoc etc
by conventional protecting group manipulation.
Pyranone or pyranol building blocks are N-terminal extended and capped as
shown in Scheme 5A:
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
34
R6
R5 _ 1. 4.0M HCI dioxan
O 2. Boc-L-AA-Ofp. HOBt, DMF, NMM
Boc ~
N
H R6
O
R5
~O
H
N
Boc~ =
' R3 O
1. 4M HCI dioxan
2. R'COOPfpIHOBT 1. 4M HCI dioxan
DMF, NMM 2. R'SOZCI Et3NH cat DMAP
R6 R6
OR5 O OR5 O
R~~N H
N
R' ~S~ N H
O R3 H O O O R3 O
Scheme SA - N terminal e$tension & capping
(b) General method for N-terminal extension, exemplified by L-butylalanine
The N-Boc-protected 4-aminopyranone from step a) is treated with a solution of
4.0M
HCl in dioxan (25mL) at room temperature for lhr. The solvents are removed in
vacuo
and the residue azeotroped with 2 x toluene to give the hydrochloride salt.
Boc-L-tef°t-
butylalanine pentafluorophenyl ester ( l.OSeq) and 1-hydroxybenzotriazole
hydrate (,
1.OSeq) are dissolved in DMF (20mL) and after Smins added to the above salt.
The
solution is then treated with N-methylmorpholine (, 1.1 eq) and left at room
temperature for 2hrs. The solvents are removed in vacuo and the crude product
purified
by flash chromatography over silica gel (50g) eluting with EtOAc / heptane
(1:3, v/v),
then EtOAc / heptane (1:2, v/v). Fractions are pooled and reduced in vacuo to
give the
title intermediate.
(c) General method for addition of capping group, exemplified by furylcarbonyl
The N-extended aminopyranone from step b) is treated with a solution of 4.0M
HCl in
dioxan (25mL) at room temperature for lhr. The solvents were removed in vacuo
and
the residue azeotroped with 2 x toluene to give the hydrochloride salt..
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
Furan-3-carboxypentafluorophenyl ester (I.OSeq) and I-hydroxybenzotriazole
hydrate
(l.OSeq) are dissolved in DMF (lSmL) and after Smins added to the above salt.
The
solution is then treated with N-methylmorpholine (l.leq) and left at room
temperature
for 2 hr. The solvents are removed ih vacuo and the crude product purified by
flash
chromatography over silica gel (50g) eluting with EtOAc / heptane (3:2, vlv).
Fractions
are pooled and reduced in vacuo to give the title compound.
Example 2.
(a) General method for addition of sulphonyl capping group
The N-extended 4-aminopyranone from example 1 ~ step b) is treated with a
solution of
4.0M HCl in dioxan (SmL) at room temperature for lhr. The solvents were
removed i~
vacuo and the residue azeotroped with 2 x toluene to give the hydrochloride
salt.
Hydrochloride salt was dissolved in dry DCM (2mL) and furan-3-
sulphonylchloride
added followed by diisopropylethylamine (3 eq) and catalytic N,N-
dimethylaminopyridine (2mg). After 2 hr at room temperature, the solution is
diluted
with DCM (lSmL) and washed successively with O.1N HCl (25mL), water (2 x 25mL)
and brine (25mL), then dried over sodium sulphate. The solvent is removed in
vacuo
and the crude product purified by flash chromatography over silica gel (15g)
eluting
with EtOAc / heptane (l :l, v/v). Fractions are pooled and reduced ih vacuo to
give the
title intermediate, lyophilised from 0.1 %aq TFA / acetonitrile.
Preparation of Building Block-Linker Constructs
General method for the synthesis of Pyranone - Linker Constructs (following
Scheme
6).
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
36
R6
R5 O
~O
OH
Fmoc ~N H H
HzN
H O ,N"N
O
R6
construct linker O
Fmoc
OH
H
N~.;,,,
R6 attach to solid phase
R5 O
-O
Fmoc ~ / gear
H NON N H
N terminal extension
Addition of capping group
R6
R5
O ~O
H
R1~N H
R3 N- linker-gear
R6 TFA/H20
R5
O ~O
H
R1~N N
H
R3 O
Scheme 6 - Solid phase synthesis of compounds of formula IV
N-Fmoc-4-amino-5-ethylpyranone(l.Oeq) is dissolved in a mixture of ethanol /
water
(7:1 v/v, lOmL per mmole compound) containing sodium acetate trihydrate
(l.Seq). 4-
[[(hydrazinocarbonyl)amino]methyl]cyclohexanecarboxylic acid trifluoro acetate
(mw
329.3, 1.Oeq) (see Murphy, A. M., et al, J. Am. Chem. Soc, 114, 3156-3157,
1992) is
added and the mixture heated under reflux for 2hrs. The mixture is then
cooled, poured
into dichloromethane (100mL per mmole compound) and water (100mL) added. The
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
37
organic layer is separated, backwashed with saturated brine (100mL). The
organic layer
is dried (Na2S04), filtered and evaporated i~ vacuo to yield the title
construct, which
may be used without further purification
Chemistry Towards P2 Hybrid Aminoacids
The general chemistry depicted in Scheme 7 will shortly be published in full
in the
academic literature, by its inventors CS Dexter and RFW Jackson at the
University of
Newcastle, England.
~Znl
+ ~ Br
BocHN COZMe
~~~~,~NHBoc + / NHBoc
(76) COZMe (75) C02Me
~~~i~~NHBoc NHBoc
', + ~ Y
COZMe COZMe
(81 ) (77)
i'1
NHFmoc + NHFmoc
COZH C02H
(84) (80)
Scheme 7 . Novel P2 hybrids by the CuCN catalysed cross coupling of Zn
activated [3-
iodoalanine with allyl bromides
(a) General Procedure for the zinc coupling reactions
(b) Zinc activation
Zinc dust (150mg, 2.3mmol, 3.Oeq, Aldrich) was weighed into a 25mL round
bottom
flask with a side arm and fitted with a three way tap. The zinc powder was
heated with
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
38
a heat gun under vacuum and the flask was flushed with nitrogen and evacuated
and
flushed a further three times. With the flask filled with nitrogen, dry DMF
(1mL) was
added. Trimethylsilylchloride (301, 0.23mmo1, 0.3eq) was added and the zinc
slurry
was vigorously stirred for a further 30mins.
(c) Zinc insertion; N-(tent-Butoxycarbonyl)-3-iodozinc-L-alanine methyl ester
(61)
N-(tent-Butoxycarbonyl)-3-iodo-L-alanine methyl ester (247mg, 0.75mmo1, l.Oeq)
dissolved in dry DMF (O.SmL) was added dropwise, via cannula, to the activated
zinc
slurry at 0°C prepared as described above. The reaction mixture was
then allowed to
warm up to room temperature and stirred for lhr to give the organozinc
reagent.
(d) CuBr.SMe2 preparation
Whilst the zinc insertion reaction was in progress, CuBr.SMe2 (20mg, O.lmmol,
0.13eq) was weighed into a 25m1 round bottom flask fitted with a three way tap
and
dried "gently~lwith a heat gun under vacuum until CuBr.SMe2 changed appearance
from a brown powder to give a light green powder. Dry DMF (O.SmL) was then
added
followed by addition of the electrophile (either 1-bromo-2-methylbut-2-ene,
toluene-4-
sulfonic acid-(E)-2-methyl-but-2-enyl ester or 1-bromo-2,3-dimethylbut-2-ene)
(l.Ommol, l.3eq). The reaction mixture was then cooled to -15°C.
(e) Coupling Reaction
Stirring of the organozinc reagent solution was stopped to allow the zinc
powder to
settle and the supernatant was carefully removed via cannula (care taken to
avoid
transferring too much zinc powder) and added dropwise to the solution of
electrophile
and copper catalyst. The cooling bath was removed and the solution was stirred
at
room temperature overnight. Ethyl acetate (20mL) was added and stirring was
continued for a further l5mins. The reaction mixture was transferred to a
separating
funnel and a fiuther aliquot of EtOAc (30mL) was added. The organic phase was
washed successively with 1M Na2S203 (20mL), water (2 x 20mL), brine (40mL),
dried
over sodium sulphate and filtered. The solvent was removed in vacuo and the
crude
product purified by flash chromatography on silica gel as described.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
39
(f) Hydrogenation of alkene
The alkene (l.Ommol) was dissolved in ethanol (lOmL), 10% palladium on carbon
(80mg) added and hydrogen introduced. Once the reaction had been deemed to
have
reached completion, the hydrogen was removed, the reaction filtered through
Celite
and the catalyst washed with ethanol (30mL). The combined organic filtrate was
concentrated in vacuo and the alkane used directly in the subsequent reaction.
(g) Saponification of methyl ester
The methyl ester (l.Ommo1) was dissolved in THF (6mL) and whilst stirring, a
solution
of LiOH (l.2mmol, l.2eq) in water (6mL) was added dropwise. Once the reaction
was
deemed to have reached completion, the THF was removed in vacuo and diethyl
ether
(1 OmL) added to the residue. The reaction mixture vas then acidified with
1.0M HCl
until pH =3. The organic phase was then removed and the aqueous layer
extracted with
diethyl ether (2 x l OmL). The combined organic extracts were dried over
magnesium
sulphate, filtered and the solvent removed i~c vacuo to give the carboxylic
acid used
directly in the subsequent reaction.
(h) Removal of N-Boc protecting group
The N-Boc protected material (l.Ommol) was dissolved in DCM (2mL) and cooled
to
0°C. Trifluoroacetic acid (2mL) was added dropwise and when the
reaction was
deemed to have reached completion, the solvents were removed in vacuo to yield
the
amine used directly in the subsequent reaction. Alternatively, the N-Boc
protected
material (l.Ommol) was cooled to 0°C and 4M HCl in dioxane (SmL) added
dropwise
and when the reaction was deemed to have reached completion, the solvents were
removed in vacuo to yield the amine used directly in the subsequent reaction.
(i) Fmoc protection of amine
The amine (l.Ommol) in 1,4-dioxane (2mL) was cooled to 0°C and 10%
sodium
carbonate (2.2mmol, 2.2eq, 2mL) added. The biphasic reaction mixture was
stirred
vigorously and Fmoc-Cl (l.lmmol, l.leq) added. Once the reaction was deemed to
have reached completion, diethyl ether (lOmL) added and the reaction mixture
acidified to pH = 3 with 1M HCI. The organic phase was removed and the aqueous
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
layer extracted with diethyl ether (2 x l OmL). The combined organic extracts
were
dried over sodium sulphate, filtered, the solvent removed in vacuo and the
residue
purified by flash chromatography over silica gel.
Example Synthesis 1
Preparation of 2S-2-(9H fluoren-9-ylmethoxycarbonylamino)-4,4-dimethylhexanoic
acid (68)
The following scheme explains how optically pure (S)-2-test-
Butoxycarbonylamino-
4,4-dimethyl-hex-5-enoic acid methyl ester (62) was prepared and isolated.
IZn~NHBoc Br CuBr.SMe2 ' ~ / NHBoc
+ ~NHBoc+ ~~f\~'
COzMe ~ DMF COZMe
(61) CO~Me (62)
~mCPBA
NHBoc
~NHBoc ~ O~NHBoc + ~~~ ~~
nBuLi COZMe
Co2Me (63) (64) CO~Me (62)
Separated by column chromatography
(a) 2S-2-test-Butoxycarbonylamino-4, 4-dimethyl-hex-5-enoic acid methyl ester
(62),
2S-2-te~°t-butoxycarbonylamino-4-(2S-3,3-dimethyl-oxiranyl)-butyric
acid methyl
ester (63) and 2S-2-tef~t-butoxycarbonylamino-4-(2R-3,3-dimethyl-oxiranyl)-
butyric acid methyl ester (64)
Following the general procedure for zinc coupling reactions, 1-bromo-3-
methylbut-2-
ene (I 15~,L, I .Ommol) was coupled to compound (61) (247mg, 0.75mmol) in the
presence of CuBr.SMe2 (20mg, O.lmmol) to give a residue which was purified by
flash
column chromatography over silica gel eluting with EtOAc / 40:60 petroleum
ether
(1:9, v/v). Fractions were pooled and reduced i~c vacuo to give a mixture of
regioisomers (2:1 formal SN2' vs SN2), inseparable by column chromatography,
as a
colourless oil, yield 190mg, 93%.
To a mixture of regioisomers (190mg, 0.7mmo1) in chloroform (3mL) was added
dropwise over Smins, 3-chloroperbenzoic acid (156mg, 85% pure, 0.8mmol, l.le~
in
chloroform (2mL). The reaction mixture was stirred at room temperature for a
further
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
41
2hr. The reaction mixture was then washed successively with 1M Na2S205 (SmL),
saturated sodium bicarbonate solution (SmL) and brine (lOmL). The organic
phase was
dried over sodium sulfate, filtered, the solvent removed i~t vacuo and the
residue was
purified by flash chromatography over silica gel eluting with EtOAc / 40:60
petroleum
ether (2:8, v/v). Three products were obtained; compound (62) was eluted first
and
further elution afforded an inseparable mixture of compound (63) and compound
(64).
Fractions of the initial component were pooled and reduced in vacuo to give 2S-
2-tef°t-
butoxycarbonylamino-4,4-dimethyl-hex-5-enoic acid methyl ester (62) as a-clear
oil,
yield 93mg, 49%. Electrospray-MS m/z 272 (MH+). Analytical HPLC Rt = 21.45mins
(95%), HRMS CloHm04N~requires M, 215.1158, found: M+-C4H8 215.1152 (b - 2.8
ppm); IR (cap. film)/crri 1 3369 (s), 3084 (m), 2965 (s), 1748 (s), 1715 (s),
1517 (s),
1167 (s), 1007 (s), 914 (s)
~H (500 MHz; CDC13) 1.06 (6H, s, CH2=CHC(CH )2), 1.42 (9H, s, C(CH )3) 1.55
(1H,
dd, J 14, 9, CH2=CHC(CH3)aCH A), 1.82 (1H, dd, J 14, 3, CHZ=CHC(CH3)2CH B),
3.69 (3H, s, OCH3), 4.30 (1H, m, NHCHC02CH3), 4.83 (1H, br d, J 7, NH), 4.97
(2H,
m, CH =CH) and 5.78 (1H, dd, .Itr~s 17.5, J°;S 11, CHa=CH)
8c (125 MHz; CDC13) 26.93 (CH2=CHC(CH3)2), 28.34 (C(CH3)3), 36.33
(~H2=CHC(CH3)2CH2), 45.06 (CH2=CHC(CH3)2), 51.25 (NHCHCOaCH3), 52.15
(OCH3), 79.77 (C(CH3)3), 111.39 (CH2=CH), 146.87 (CH2=CH), 154.97 (NHC02But)
and 174.04 (C02CH3).
(b) 2S-2-text-Butoxycarbonylamino-4,4-dimethyl-hexanoic acid methyl ester (65)
Following the general procedure for alkene hydrogenation, compound (62) (93mg,
0.3mmo1) yielded compound (65) as a colourless oil, yield 90mg, 96% and used
directly in the subsequent reaction. Electrospray-MS m/z 274 (MH+). Analytical
HPLC
Rt = 22.SSmins (100%).
(c) 2S-2-tent-Butoxycarbonylamino-4,4-dimethyl-hexanoic acid (66)
Following the general procedure for methyl ester saponification, compound (65)
(90mg, 0.3mmo1) gave compound (66) as crystals, yield 79mg, 92% and used
directly
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
42
in the subsequent reaction. Electrospray-MS m/z 260 (MHO). Analytical HPLC Rt
=
20.90mins (100%).
(d) 2S-2-Amino-4,4-dimethyl-hexanoic acid trifluoroacetic acid salt (6'n
Following the general procedure of N-Boc removal using TFA, compound (66)
(79mg,
0.3mmol) gave compound (6'n as a solid, yield 80mg, 96% and used directly in
the
subsequent reaction. Electrospray-MS m/z 274 (MH+).
(e) 2S-2-(9H Fluoren-9-ylmethoxycarbonylamino)-4,4-dimethyl-hexanoic acid (68)
Following the general procedure for Fmoc protection of an amine, compound (6'n
(80mg, 0.3mmol) gave on purification by flash chromatography over silica gel
eluting
with CHC13 / CH30H (100:0 to 96:4, v/v) 2S-2-(9H fluoren-9-
ylmethoxycarbonylamino)-4,4-dimethyl-hexanoic acid (68) as a solid, yield
60mg,
S4%. Electrospray-MS m/z 382(MH+). Analytical HPLC Rt = 23.63mins (100%); [a
]Dl' -18.4 (c 0.25 in EtOH)
8H (SOOMHz, CDC13) 0.88 (3H, t, J7, CH3CH2), 0.95 (6H, s, CH3CH2C(CH )a), 1.31
(2H, m, CH3CHz), 1.46 (1H, dd, J 14.5, 10, CH3CH2C(CH3)2CH A), 1.85 (1H, br d,
J
14.5, CH3CH2C(CH3)2CH B), 4.21_(1H, t, J 6.5, CH-Fmoc), 4.41 (3H, m, NHCHCO2H
and CH20), 5.02 ( 1 H, br d, J 8, NH-Fmoc), 7.29 (2H, m, H-2' and H-7' ), 7.3
8 (2H, m,
H-3' and H-6'), 7.58 (2H, m, H-1' and H-8') and 7.74 (2H, d, J 7, H-4' and H-
S').
Example Synthesis 2
Preparation of 2S,4RS-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-4,S-dimethyl-
hexanoic acid (74)
Optically pure 2S,4S-2-tef~t-Butoxycarbonylamino-4,S-dimethyl-hex-S-enoic acid
methyl ester (69) and 2S,4R-2-tef°t-Butoxy-carbonylamino-4,S-dimethyl-
hex-S-enoic
acid methyl ester (70) were obtained directly after zinc coupling reaction by
flash
chromatography.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
43
IZn~NHBoc + OTs CuBr.SMe~ NHBoc + NHBoc
CO~Me ~ DMF
C02Me - CO~Me
(61) (69) (70)
(a) 2S,4S-2-te~°t-Butoxycarbonylamino-4,5-dimethyl-hex-5-enoic acid
methyl ester
(69) and 2S,4R-2-tent-butoxy-carbonylamino-4,5-dimethyl-hex-5-enoic acid
methyl ester (70)
Following the general procedure for zinc coupling reactions, toluene-4-
sulfonic acid
(E)-2-methyl-but-2-enyl ester (1.45mL, l.Ommol) was coupled to compound (61)
(247mg, 0.75mmol) in the presence of CuBr.SMe2 (20mg, O.lOmmol) to give a
residue
which was purified by flash chromatography over silica gel eluting with EtOAc
/ 40:60
petroleum ether (1:9, v/v) to give two diastereoisomers. Analytical HPLC Rt =
22.49mins (60%) and Rt = 22.52mins (40%). Fractions of the first eluted
component
were pooled to give one of the diastereoisomers obtained as a colourless oil,
yield
36mg, 18%. Next a mixture of the diastereomers as a colourless oil, yield
75mg, 37%
was obtained. Pure fractions containing the later eluted component were pooled
to give
the other diastereoisomer as a colourless oil, yield l9mg, 9%. (The
stereochemistry at
the 4 position was not investigated). Spectral data obtained for the fast
running
diastereomer: Electrospray-MS m/z 272 (MH+); [oc]Dao +12.3 (c 1.06 in CHC13);
IR
(cap. film)/cni 1 3382 (s), 3070 (m), 2966 (s), 1746 (s), 1716 (s), 1616 (w),
1507 (s),
886 (m)
8H (500 MHz, CDC13) 1.06 (3H, d, J 7, CH CH), 1.45 (9H, s, C(CH )3), 1.58 (1H,
m,
CH3CH), 1.68 (3H, s, CH C=CH2), 1.85 (1H, m, CH ACH), 1.97 (1H, m, CH BCH),
3.73 (3H, s, OCH3), 4.29 (1H, m, NHCHC02CH3), 4.72 (1H, s, CH A=CH), 4.95 (1H,
d, J1.5, CH B=CH) and 5.04 (1H, d, J7, NH)
8~ (125 MHz, CDCl3) 18.61 (CH3C=CH2), 21.64 (CH3CH), 28.32 (C(CH3)3), 30.79
(CH3CHCH2), 38.06 (CHZCHNH), 52.00 (NHCHC02CH3), 52.22 (OCH3), 79.53
(C(CH3)3), 110.19 (CH2=C(CH3)), 144.62 (CH2=C(CH3)), 155.18 (OCONH) and
173.30 (COZCH3).
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
44
Spectral data obtained for the slow running diastereoismer: Electrospray-MS
m/z 272
(~; ~a~Dao +16.0 (c 0.60 in CHCl3); IR (cap. film)/cni 13369 (s), 3073 (m),
2969
(s), 1747 (s), 1717 (s), 1617 (w), 1517 (s), 893 (m)
8H (500 MHz, CDCl3) 1.04 (3H, d, J 7, CH CH), 1.44 (9H, s, C(CH3)3), 1.55 (1H,
m,
CH3CH)~ 1.67 (3H, s, CH C=CH2), 1.91 (1H, m, CH ACH), 2.37 (1H, m, CH BCH),
3.73 (3H, s, OCH3), 4.26 (1H, m, NHCHC02CH3), 4.75 (1H, d, J1.5, CH A=CH),
4.79
(1H, d, J1.5, CH B=CH) and 5.46 (1H, d, J6.1, NH)
8~ (125 MHz, CDC13) 18.51 (CH3C=CH2), 20.14 (CH3CH), 28.31 (C(CH3)3), 30.55
(CH3CHCH2), 37.64 (CH2CHNH), 52.17 (NHCHC02CH3), 52.22 (OCH3), 79.74
(C(CH3)3), 111.27 (CH2=C(CH3)), 147.94 (CH2=C(CH3)), 155.36 (OCONH) and
173.83 (CO2CH3).
These diastereoisomers were not separated routinely and used as a mixture in
subsequent reactions.
(b) 2S,4RS-2-test-Butoxycarbonylamino-4,5-dimethyl-hexanoic acid methyl ester
(71)
Following the general procedure for alkene hydrogenation, compounds (69) and
compound (70) (130mg, 0.48mmo1) yielded a mixture of two diastereoisomers (71)
which were not separated, obtained as a colourless oil, yield 128mg, 98%.
Analytical
HPLC Rt 22.49mins, electrospray-MS m/z 274 (MH+).
(c) 2S,4RS-2-test-Butoxycarbonylamino-4,5-dimethyl-hexanoic acid (72)
Following the general procedure for methyl ester saponification, compounds
(71)
(128mg, 0.47mmo1) gave a inseparable mixture of compounds (72) as a colourless
oil,
yield 106mg, 87%. Electrospray-MS m/z 260 (MH+). Analytical HPLC Rt =
20.65mins
(100%).
(d) 2S,4RS-2-Amino-4,5-dimethyl-hexanoic acid trifluoroacetic acid salt (73)
Following the general procedure of N-Boc removal using TFA, compounds (72)
(106mg, 0.41mmo1) gave an inseparable mixture of compounds (73) as a solid,
yield
107mg, 96% and used directly in the subsequent reaction. Electrospray-MS m/z
160
(MH~.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
(e) 2S,4RS-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-4,5-dimethyl-hexanoic acid
(74)
Following the general procedure for Fmoc protection of an amine, compounds
(73)
(107mg, 0.39mmo1) gave on purification by flash chromatography over silica gel
eluting with CHC13 / CH30H (100:0 to 95:5, v/v) 2S,4RS-2-(9H-fluoren-9-
ylinethoxycarbonylamino)-4,5-dimethyl-hexanoic acid (74) as a solid, yield
60mg,
40% as a mixture of two diastereoisomers. Analytical HPLC Rt = 23.83mins (40%)
and Rt = 24.06mins (60%). First eluted diastereomer: Electrospray-MS m/z 382
(MH+). Later eluted diastereomer: Electrospray-MS m/z 382 (MH~.
Example Synthesis 3
Preparation of 2S,SRS-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-5,6-dimethyl-
heptanoic acid (80) and 2S-2-(9H-Fluoren-9-ylinethoxycarbonylamino)-4,4,5-
trimethyl-hexanoic acid (84)
(S)-2-tent-butyloxycarbonylamino-5,6-dimethyl-hept-5-enoic methyl ester (75)
and (S)-
2-test-butyloxycarbonylamino-4,4,5-trimethyl-hex-5-enoic methyl ester (76)
were
obtained directly after zinc coupling reaction by flash chromatography.
IZn~NHBoc + Br CuBr.SMe~ NHBoc + NHBoc
C02Me ~ DMF
CO~Me C02Me
(61) (75) (76)
(a) 2S-2-test-Butyloxycarbonylamino-5,6-dirriethyl-hept-5-enoic methyl ester
(75)
and 2S-2-text-butyloxycarbonylamino-4,4,5-trimethyl-hex-5-enoic methyl ester
(76)
Following the general procedure for zinc coupling reactions, 1-bromo-2,3-
dimethylbut-
2-ene (163mg, l.Ommol) was coupled to compound (61) (247mg, 0.75mmol) in
presence of CuBr.SMea (20mg, 0.1 Ommol) to give a residue which on
purification by
flash chromatography over silica gel eluting with EtOAc/ 40:60 petroleum ether
(1:9)
gave two regioisomers. The first eluted component compound (75) as a
colourless oil,
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
46
yield 60mg, 28% and the second eluted component was compound (76) as a
colourless
oil, yield S l mg, 24%.
Spectral data obtained for compound (75); Electrospray-MS m/z 285 (MH+).
Analytical HPLC Rt = 22.85mins (100%); HRMS C15H2~N04 requires M, 285.1940,
found: M+ 285.1954 (b - 4.9 ppm); [a]Daa +26.1 (c 1.01 in CH2C12); elemental
analysis
C15H2~N04 (req) %C 63.1, %H 9.5, %N 4.9, (fnd) %C 62.4, %H 9.6, %N 5.3; IR
(cap.
film)/crri 13366 (s), 3154 (m), 2978 (s), 1744 (s), 1718 (s), 1506 (s), 1366
(s), 1164 (s)
8H (500 MHz, CDCl3) 1.45 (9H, s, C(CH3)3), 1.62 (9H, m, (CH )2=C(CH3)), 1.87
(1H,
m, CH ACH2CH), 2.03 (1H, m, CH BCH2CH), 2.09 (1H, dd, J6, 10.5, CHZCH ACH),
2.12 (1H, dd, J6.5, 10.5, CH2CH BCH), 3.74 (3H, s, OCH3), 4.29 (1H, m,
NHCHC02CH3) and 5.02 (1H, d, J7, NH)
S~ (125 MHz, CDC13) 18.19 ((CH3)2C=C(CH3)), 20.00 ((CH3)z~;SC=C(CH3)), 20.61
((CHs)2transC=C(CH3)), 28.33 (C(CH3)3), 30.07 (CHZCH2CH), 30.92 (CH2CH2CH),
52.20 (NHCHC02CH3), 53.47 (OCH3), 80.00 (C(CH3)3), 95.90 ((CH3)2C=C(CH3)),
96.49 ((CH3)2C=C(CH3), 155.33 (OCONH) and 173.42 (COZCH3).
Spectral data obtained for compound (76); Electrospray-MS m/z 285 (MH+).
Analytical HPLC Rt = 22.91mins (100%); HRMS ClIHnNOa. requires M229.1314,
found: M+-C4H$ 229.1309 (8 - 2.2 ppm); [a]Da3 +4.8 (c 1.01 in CH2Clz);
elemental
analysis C15H2~N04 (req) %C 63.1, %H 9.5, %N 4.9, (fnd) %C 62.5, %H 9.5, %N;
IR (cap. film)/crri 13368 (s), 3091 (m), 2934 (s), 1748 (s), 1717 (s), 1516
(s)
8H (500 MHz, CDC13) 1.10 (3H, s, (CH3)aaC), 1.12 (3H, s, (CH3)2BC), 1.43 (9H,
s,
C(CH3)3), 1.60 (1H, m, CH ACH), 1.74 (3H, s, CH3C=CH2), 1.92 (1H, dd, J 14.5,
4,
CH BCH), 3.70 (3H, s, OCH3), 4.24 (1H, m, NHCHCOZCH3), 4.79 (1H, s,
CH A=C(CH3)), 4.82 (1H, s, CH B=C(CH3)) and 4.83 (1H, br d, Jl 1, NH)
8C (125 MHz, CDC13) 19.38 (CH3), 27.19 (CH3), 27.61 (CH3), 28.34 (C(CH3)3),
38.50
(CH2CH), 38.95 ((CH3)aC), 51.34 (NHCHC02CH3), 52.13 (OCH3), 79.71 (C(CH3)3),
110.95 (CH2=C(CH3)), 150.62 (CH2=C(CH3)), 155.00 (OCONH) and 174.24
(CO~CH3). .
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
47
(b) 2S,SRS-2-test-Butoxycarbonylamino-5,6-dimethyl-heptanoic acid methyl ester
(Tn
Following the general procedure for alkene hydrogenation, 2S-2-
tee°t-
butyloxycarbonylamino-5,6-dimethyl-hept-5-enoic methyl ester (75) (60mg,
0.21mmo1) yielded compound (77) as a colourless oil, yield 54mg, 89%.
Electrospray-
MS m/z 288 (MH+). Analytical HPLC Rt = 24.06mins (100%).
(c) 2S,SRS-2-test-Butoxycarbonylamino-5,6-dimethyl-heptanoic acid (78)
Following the general procedure for methyl ester saponification, compounds
(77)
(54mg, 0.19mmo1) gave compounds (78) as a colourless oil, yield 54mg, 100%.
Electrospray-MS m/z 274 (MH+). Analytical HPLC Rt = 21.44mins (100%).
(d) 2S,SRS-2-Amino-5,6-dimethyl-heptanoic acid hydrochloride salt (79)
Following the general procedure of N-Boc removal using 4M HCl in dioxane,
compounds (78) (54mg, 0.20mmo1) gave compounds (79) as a solid, yield 40mg,
97%.
Electrospray-MS m/z 174 (MH+).
(e) 2S,SRS-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-5,6-dimethyl-heptanoic acid
(80)
Following the general procedure for Fmoc protection of an amine, compounds
(79)
(40mg, 0. I 9mmo1) gave on purification by flash chromatography over silica
gel eluting
with CHC13 / CH30H (100:0 to 95:5, v/v) 2S,SRS-2-(9H-fluoren-9-
ylmethoxycarbonylamino)-5,6-dimethyl-heptanoic acid (80) as a solid, yield
27mg,
36%. Electrospray-MS m/z 395 (MH+). Analytical HPLC Rt = 24.52mins (100%),
HRMS C~4Ha904NNa requires M418.1994, found: MNa , 418.1993. (b - 0.38 ppm)
8H (500 MHz; CDCl3) 0.73 (6H, m, (CH3)2CH), 0.82 (3H, d, J6.5,
(CH3)2CHCH(CH )), 1.23 (1H, m, (CH3)2CHCH(CH3)CH A), 1.39 (1H, m,
(CH3)zCHCH(CH3)CH B), 1.55 (2H, rn, (CH3)2CHCH(CH3) and
(CH3)2CHCH(CH3)CHZCH A), 1.63 (1H, m, (CH3)ZCHCH(CH3)), 1.90 (1H, m,
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
48
(CH3)2CHCH(CH3)CH2C~B), 4.18 (1H, t, J6.5, CH-Fmoc), 4.40 (3H, m,
NHCHCOZH and CHaO), 5.30 (1H, br d, J 8, NH-Fmoc), 7.27 (2H, m, H-2' and H-
7'),
7.37 (2H, m, H-3' and H-6'), 7.56(2H, m, H-1' and H-8') and 7.75 (2H, d, J7, H-
4'
and H-5')
8~ (125 MHz; CDC13) 14.91 (CH3)aCHCH(CH3)), 17.49 and 17.73 ((CH3)anCH),
19.93 and 20.05 ((CH3)2BCH), 28.08 ((CH3)2CH), 29.26 and 29.44
((CH3)2CHCH(CH3)CH2CH2), 30.04 and 30.17 ((CH3)2CHCH(CH3)CHaCH2), 31.38
and 31.68 ((CH3)aCHCH(CH3)), 37.89 and 38.07 (NHCHC02H), 46.88 (CH-1'), 66.84
(CH20), 119.72 (CH-5' and CH-10'), 124.80 (CH-4' and CH-11'), 126.81 (CH-6'
and
CH-9'), 127.46 (CH-3' and CH-12'), 141.05 ( C-7' and C-8'), 143.47 (C-2' and C-
13')
and 155.89 (OCONH). The quaternary signal for the carboxylic acid was not
observed.
(f) 2S-2-text-Butoxycarbonylamino-4,4,5-trimethyl-hexanoic acid methyl ester
(81)
Following the general procedure for alkene hydrogenation, 2S-2-te~t-
butyloxycarbonylamino-4,4,5-trimethyl-hex-5-enoic methyl ester (76) (5lmg,
0.18mmo1) yielded compound (81) as a colourless oil, yield 46mg, 90%.
Electrospray-
MS m/z 288 (MH+). Analytical HPLC Rt = 22.91mins (100%).
(g) 2S-2-test-Butoxycarbonylamino-4,4,5-trimethyl-hexanoic acid (82)
Following the general procedure for methyl ester saponification, compound (81)
(46mg, 0.16mmo1) gave compound (82) as a colourless oil, yield 44mg, 100%.
Electrospray-MS m/z 274 (MH+).
(h) 2S-2-Amino-4,4,5-trimethyl-hexanoic acid hydrochloride salt (83)
Following the general procedure of N-Boc removal using 4M HCl in dioxane,
compound (82) (44mg, 0.16mmo1) gave compound (83) as a solid, yield 33mg, 99%.
Electrospray-MS m/z 174 (MH+).
(i) 2S-2-(9H-Fluoren-9-ylmethoxycarbonylamino)-4,4,5-trimethyl-hexanoic acid
(84)
Following the general procedure for Fmoc protection of an amine, compound (83)
(33mg, 0.16mmo1) gave on purification by flash chromatography over silica gel
eluting
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
49
with CHC13 / CH30H (100:0 to 95:5, v/v) 2S-2-(9H-fluoren-9-
ylinethoxycarbonylamino)-4,4,5-trimethyl-hexanoic acid (84) as a solid, yield
20mg,
32%. Electrospray-MS m/z 396 (MH+). Analytical HPLC Rt = 24.28mins (100%),
HRMS C24Ha904NNa requires M418.1994, found: MNa , 418.1993. (8 - 0.38 ppm)
8H (500 MHz; CDC13) 0.93 (9H, m, (CH )2CHC(CH3)2A), 0.98 (3H, s,
(CH3)2CHC(CH )2B), 1.48 (1H, dd, J 14, 10, (CH3)2CHC(CH3)2CH A), 1.57 (1H, m,
(CH3)2CH), 1.91 (1H, d, J 14, (CH3)ZCHC(CH3)2CH B), 4.21 (1H, t, J 6.5, CH-
Fmoc),
4.40 (3H, m, NHCHC02H and CH20), 5.10 (1H, br d, J7.5, NH-Fmoc), 7.27 (2H, m,
H-2' and H-7'), 7.36 (2H, m, H-3' and H-6'), 7.57 (2H, m, H-1' and H-8') and
7.74
(2H, d, J 7, H-4' and H-5')
8~ (125 MHz; CDCl3) 17.01 ((CH3)2ACH), 17.16 ((CH3)aBCH), 23.69
((CH3)2CHC(CH3)2A), 24.27 ((CH3)2CHC(CH3)2B), 35.27 ((CH3)2CHC(CH3)Z), 35.73
((CH3)2CH), 41.88 ((CH3)2CHC(CH3)2CH2), 46.93 (CH-1'), 54.20 (NHCHC02H),
66.79 (CH2O), 119.70 (CH-5' and CH-10'), 124.78 (CH-4' and CH-11'), 126.79 (CH-
6' and CH-9'), 127.44 (CH-3' and CH-12'), 141.05 ( C-7' and C-8'), 143.61 (C-
2' and
C-13') and 155.68 (OCONH). The quaternary signal for the carboxylic acid was
not
observed.
General Solid Phase procedures
Molecules are assembled using pyranone building blocks and novel protected
aminoacids described earlier, by solid phase procedures on Chiron multipins
following
the protocols detailed below.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
R6
R5
-O
Fmoc ~
N
H
O
construct link
OH
NON
O
R6 /~ attach to solid phase
O
Fmoc ~ A gear
N
H
N terminal extension
Addition of capping group
R6
R5
O ~O
H
R1~N H
R3 N- linker-gear
R6 TFA/H~O
R5
O ~O
H
R1~N N
H
R3 O
Preparation of Crown Assembly
The compounds are synthesised in parallel fashion using the appropriately
loaded
Fmoc-Building block-linker-DA/MDA derivatised macrocrowns (see above) loaded
at
approximately 3.5 - 9.1 .moles per crown. Prior to synthesis each crown is
connected
to its respective stem and slotted into the 8 x I2 stem holder. Coupling of
the amino
acids employs standard Fmoc amino acid chemistry as described in ' Solid Phase
Peptide Synthesis', E. Atherton and R.C. Sheppard, IRL Press Ltd, Oxford, LTK,
1989.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
51
Removal of Na-Fmoc Protection
A 250 mL solvent resistant bath is charged with 200 mL of a 20% piperidine/DMF
solution. The multipin assembly is added and deprotection allowed to proceed
for 30
minutes. The assembly is then removed and excess solvent removed by brief
shaking.
The assembly is then washed consecutively with (200 mL each), DMF (5 minutes)
and
MeOH (5 minutes, 2 minutes, 2 minutes) and left to air dry for 15 minutes.
Quantitative W Measurement of Fmoc Chromophore Release
A 1 cm path length UV cell is charged with 1.2 mL of a 20% piperidine/DMF
solution
and used to zero the absorbance of the LTV spectrometer at a wavelength of
290nm. A
UV standard is then prepared consisting of 5.0 mg Fmoc-Asp(OBut)-Pepsyn KA
(0.08
mmol/g) in 3.2 mL of a 20% piperidine/DMF solution. This standard gives Abs29o
=
0.55-0.65 (at room temperature). An aliquot of the multipin deprotection
solution is
then diluted as appropriate to give a theoretical Absz9o = 0.6, and this value
compared
with the actual experimentally measured absorbance showing the efficiency of
previous
coupling reaction.
Standard Coupling Of Amino Acid Residues
Coupling reactions are performed by charging the appropriate wells of a
polypropylene
96 well plate with the pattern of activated solutions required during a
particular round
of coupling. Macrocrown standard couplings were performed in DMF (500 ~,l).
Coupling of an Amino-acid Residue To Appropriate Well
Whilst the multipin assembly is drying, the appropriate Na Fmoc amino acid pfp
esters
(10 equivalents calculated from the loading of each crown) and HOBt (10
equivalents)
required for the particular round of coupling are accurately weighed into
suitable
containers. Alternatively, the appropriate Na Fmoc amino acids (10 equivalents
calculated from the loading of each crown), desired coupling agent e.g. HBTU
(9.9
equivalents calculated from the loading of each crown) and activation e.g.
HOBt (9.9
equivalents calculated from the loading of each crown), NMM (19.9 equivalents
calculated from the loading of each crown) are accurately weighed into
suitable
containers.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
52
The protected and activated Fmoc amino acid derivatives are then dissolved in
DMF
(500 ~l for each macrocrown e.g. for 20 macrocrowns, 20 x 10 eq. x 7 ,moles of
derivative would be dissolved in 10 mL DMF). The appropriate derivatives are
then
dispensed to the appropriate wells ready for commencement of the 'coupling
cycle'. As
a standard, coupling reactions are allowed to proceed for 6 hours. The coupled
assembly was then washed as detailed below.
Washine Following Coupling
If a 20% piperidine/DMF deprotection is to immediately follow the coupling
cycle,
then the multipin assembly is briefly shaken to remove excess solvent washed
consecutively with (200 mL each), MeOH (5 minutes) and DMF (5 minutes) and de-
protected. If the multipin assembly is to be stored or reacted further, then a
full
washing cycle consisting brief shaking then consecutive washes with (200 mL
each),
DMF (5 minutes) and MeOH (5 minutes, 2 minutes, 2 minutes) is performed.
Addition of Capping Group
Whilst the multipin assembly is drying, the appropriate acid capping group (10
equivalents calculated from the loading of each crown), desired coupling agent
e.g.
HBTU (9.9 equivalents calculated from the loading of each crown) and
activation e.g.
HOBt (9.9 equivalents calculated from the loading of each crown), NMM (19.9
equivalents calculated from the loading of each crown) are accurately weighed
into
suitable containers. The acid derivatives / coupling agents are then dissolved
in DMF
(500 ~,1 for each macrocrown e.g. for 20 macrocrowns, 20 x 10 eq. of
derivative would
be dissolved in 10 mL DMF) and left to activate for 5 minutes. The appropriate
derivatives are then dispensed to the appropriate wells ready for commencement
of the
'capping cycle'. As a standard, capping reactions are allowed to proceed for
18 hours
overnight. The capped assembly was then washed as detailed above.
Acidolytic Mediated Cleavage of Molecule-Pin AssemblX
Acid mediated cleavage protocols are strictly performed in a fume hood. A
polystyrene
96 well plate (1 mL/well) is labelled and weighed to the nearest mg.
Appropriate wells
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
53
are then charged with a trifluoroacetic acid/water (95:5, v/v, 600 ~.1)
cleavage solution,
in a pattern corresponding to that of the multipin assembly to be cleaved.
The multipin assembly is added, the entire construct covered in tin foil and
left for 2
hours. The multipin assembly in then added to another polystyrene 96 well
plate (1
mL/well) containing trifluoroacetic acid/water (95:5, v/v, 600 ~,1) (as above)
for 5
minutes.
Work up of Cleaved Molecules
The primary polystyrene cleavage plate (2 hour cleavage) and the secondary
polystyrene plate (5 minute wash) are then placed in the GeneVac evaporator
and the
solvents removed (minimum drying rate) for 90 minutes. The contents of the
secondary
polystyrene plate are transferred to their corresponding wells on the primary
plate using
an acetonitrile/water (50: 50 v/v/v) solution (3 x 150 ~,l) and the spent
secondary plate
discarded. Aliqouts (5-20~,L) are taken for analysis. The plate was covered in
tin foil,
pin-pricked over wells containing compounds, placed into the freezer for lhr,
then
lyophilised.
Analysis and Purification of Molecules
The (5-20~.L) aliquots are analysed by analytical HPLC and electrospray-MS. In
virtually all cases, crude parities are >90% by HPLC with the desired m/z.
Sample
were purified by semi-preparative reverse phase HPLC, using Vydac
C4.Appropriate
fractions are combined and lyophilised in tared l OmL glass vials, then re-
weighed.
Molecules were prepared on a 15-90~,mole scale, yielding 2.0-26.Omg of
purified
products. The purity of each product was confirmed by analytical HPLC at >95%
(215nm LTV detection) and gave the appropriate [MH]~ by electrospray mass
spectrometry analysis.
Loadin , of Macrocrowns With Constructs
General method for the loading of multipins with Pyranone - Linker Constructs
Amino functionalised DA/MDA macrocrowns (ex Chiron Mimotopes, Australia,
9.1 .mole loading) or amino functionalised HEMA gears (ex Chiron Mimotopes,
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
54
Australia, 1.3 mole loading) are used for all loadings and subsequent solid
phase
syntheses.
Pyranone - Linker Construct (3 eq compared to total surface functionalisation
of crowns
/ gears) is carboxyl activated with 2-(1H-benzotriazole-1-yl)-1,1,3,3-
tetramethyluronium
hexafluorophosphate (3eq), 1-hydroxybenzotriazole (3eq) and N-methylmorpholine
(6eq) in dimethylformamide (SmL) for Smins. This mixture is added to the
crowns /
gears, additional DMF added to cover the reaction surface and the mixture left
overnight.
Standard washing and Fmoc deprotection readings (see procedures above)
typically
indicates virtually quantitative loading.
K; determinations for cathepsins S, L, and K
Cathepsin S (Mammalian, marine and rat)
General
Assays were performed in 100 mM sodium phosphate, 100 mM NaCI, pH 6.5 (buffer)
in white 384 well plates (Corning Costar). Eight inhibitors (were assayed per
plate.
Inhibitor dilutions
Inhibitor dilutions were performed on a 96 well V-bottomed polypropylene plate
(Corning Costar). 100 ~,1 of buffer was placed in wells 2-5 and 7-12 of rows
A, B, C
and D. Sufficient of each inhibitor at 10 mM in DMSO was placed into wells Al-
D1
and A6-D6 to give the desired final concentration when the volume in the well
was
made up to 200 ~,1 with buffer. Column 1 was made up to 200 ~.1 with buffer,
mixed by
aspirating and dispensing 100 ~,1 in the well, and 100 ~,1 transferred to
column 2. The
pipette tips were changed and the mixing and transferral repeated to column 5.
This
process was repeated for columns 6-10.
Substrate dilutions
Substrate dilutions were performed on a 96 deep well polypropylene plate
(Beckman
Coulter). 280 ~,l of buffer was placed in wells B-H of columns 1 and 2. 70 ~,1
of 10 mM
boc-Val-Leu-Lys-AMC was placed in A1 and A2. 2 ~ 250 ~1 of buffer was added to
wells Al and A2, mixed by aspirating and dispensing 280 ~,1 in the well, and
280 ~.l
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
transferred to row B. The pipette tips were changed and the process repeated
down the
plate to row H.
Assay
Column 1 of the substrate dilution plate was distributed at 10 w1 per well
into alternate
rows beginning at row A. Column 2 was distributed to alternate rows beginning
at row
B. Row A of the inhibitor dilution plate was distributed at 10 ~,1 per well to
alternate
rows and columns starting at A1. Row B was distributed to alternate rows and
columns starting at A2. Row C was distributed to alternate rows and columns
starting
at B 1 and row D was distributed to alternate rows and columns starting at B2.
The
assay was started by the addition of 30 ~,1 per well of 20 nM cathepsin S in
buffer
containing 10 mM 2-mercaptoethanol.
Data were saved as text files and imported into Excel. The initial rates were
determined by linear regression and then fitted to the competitive inhibition
equation
using Sigmal'lot.
Cathepsins L and K
Assays were performed essentially as above. For cathepsin L, the buffer used
was 100
mM sodium acetate, 1 ~mM EDTA, pH 5.5 and the substrate was D-Val-Leu-Lys-AMC
with a highest concentration of 100 ~,M. For cathepsin K, the buffer used was
100 mM
MES/Tris, 1 mM EDTA, pH 7.0 and the substrate was D-Ala-Leu-Lys-AMC with a
highest concentration of 250 ~M.
Determination of cathepsin K proteol is catalytic activity
Convenient assays for cathepsin K arere carried out using human recombinant
enzyme, as described above. Standard assay conditions for the determination of
kinetic
constants used a fluorogenic peptide substrate,.typically H-D-Ala-Leu-Lys-AMC,
and
were determined in either 100 mM Mes/Tris, pH 7.0 containing 1 mM EDTA and 10
mM 2-mercaptoethanol or 100 mM Na acetate, pH 5.5 containing 5 mM EDTA and 20
mM cysteine. The enzyme concentration used was 5 nM. The stock substrate
solution
was prepared at 10 mM in DMSO. Screens were carried out at a fixed substrate
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
56
concentration of 60 p.M and detailed kinetic studies with doubling dilutions
of
substrate from 2S0 ~M. The total DMSO concentration in the assay was kept
below
3%. All assays were conducted at ambient temperature. Product fluorescence
(excitation at 390 nm, emission at 460 nm) was monitored with a Labsystems
Fluoroskan Ascent fluorescent plate reader. Product progress curves were
generated
over 1 S minutes following generation of AMC product.
Inhibition Studies
Potential inhibitors are screened using the above assay with variable
concentrations of
test compound. Reactions were initiated by addition of enzyme to buffered
solutions
of substrate and inhibitor. I~; values were calculated according to equation 1
TES
yo -
KM 1+ 1 +S (1)
Kl
where yo is the velocity of the reaction, his the maximal velocity, S' is the
concentration of substrate with Michaelis constant of KM, and I is the
concentration of
inhibitor.
Determination of falcipain 2 proteol is catalytic activity
Generation of Falcipain 2
Cloning
The deoxyoligonucleotide primers:
(SEQ ID NO.: 1) S'CGCGGATCCGCCACCATGGAATTAAACAGATTTGCCGAT-
3' and (SEQ ID N0.:2)
S'CGCGTCGACTTAATGATGATGATGATGATGTTCAATTAATGGAATGAATG
CATCAGT-3' were designed based on sequences deposited at the Sanger Centre,
Cambridge, UI~ (http://www.san~er.ac.uk/Projects/P falciparum/blast
server.shtml).
These primers were designed to amplify a portion of the cDNA sequence of the
cysteinyl proteinase now known as Falcipain 2 and to include relevant terminal
cloning
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
57
enzymes sites and a carboxy-terminal hexahistidine coding sequence immediately
upstream of the stop codon.
Polymerase chain reaction was performed with the above primers and Plasmodium
falciparum phage library DNA as a template using the following conditions;
94°C for 2
minutes then 35 cycles of 94°C for 10 seconds, 50°C for 1
minute, and 60°C for 2
minutes, this was followed by a 60°C 5 minute incubation. The 880bp PCR
arnplicon
was purified and phosphorylated using T4 polynucleotide kinase. This DNA was
then
ligated into EcoRV cleaved, dephosphorylated Bluescript II cloning vector and
transformed into DIIS alpha E.coli. The DNA sequence of the plasmid inserts in
isolated recombinant E. coli clones were determined using an Amersham Megabace
sequencing instrument. To create an authentic ORF a three-way ligation was
conducted
bringing together the N-terminus of truncated falcipain-2 (NcoI/NdeI), the C-
terminus
of falcipain-2 (NdeI/BamHI) and the vector pQE-60 (NcoI/BamHI).
Nucleotide Sequence of TF2.10 (SEQ ID NO.: 3):
CCATGGAATTAAACAGATTTGCCGATTTAACTTATCATGAATTTAAAAACA
AATATCTTAGTTTAAGATCTTCAAAACCATTAAAGAATTCTAAATATTTATT
AGATCAAATGAATTATGAAGAAGTTATAAAAA.A.ATATAGAGGAGAAGAAA
ATTTCGATCATGCAGCTTACGACTGGAGATTACACAGTGGTGTAACACCTG
TAAAGGATCAAAAAAATTGTGGATCTTGCTGGGCCTTTAGTAGTATAGGTT
CCGTAGAATCACAATATGCTATCAGAAAAAATAAATTAATAACCTTAAGTG
AACAAGAATTAGTAGATTGTTCATTTAAAAATTATGGTTGTAATGGAGGTC
TCATTAATAATGCCTTTGAGGATATGATTGAACTTGGAGGTATATGTCCAG
ATGGTGATTATCCATATGTGAGTGATGCTCCAAATTTATGTAACATAGATA
GATGTACTGAAAAATATGGAATCAAAAATTATTTATCCGTACCAGATAATA
AATTAAAAGAAGCACTTAGATTCTTGGGACCTATTAGTATTAGTGTAGCCG
TATCAGATGATTTTGCTTTTTACAAAGAAGGTATTTTCGATGGAGAATGTG
GTGATGAATTAAATCATGCCGTTATGCTTGTAGGTTTTGGTATGAAAGAAA
TTGTTAATCCATTAACCAAGAAAGGAGAAAA.ACATTATTATTATATAATTA
AGAACTCATGGGGACAACAATGGGGAGAAAGAGGTTTCATAAATATTGAA
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
58
ACAGATGAATCAGGATTAATGAGAAA.ATGTGGATTAGGTACTGATGCATTC
ATTCCATTAATTGAACATCATCATCATCATCATTAAGTCGACGCGATCGAA
TTCCTGCAGCCCGGGGATCC
Coding for the Protein Sequence (SEQ ID NO.: 4):
MELNRFADLTYHEFKNKYLSLRSSKPLKNSKYLLDQMNYEEVIKKYRGEENFD
HAAYDWRLHSGVTPVKDQKNCGSCWAFSSIGSVESQYAIRKNKLITLSEQELV
DCSFKNYGCNGGLINNAFEDMIELGGICPDGDYPYVSDAPNLCNIDRCTEKYGI
KNYLSVPDNKLKEALRFLGPISISVAVSDDFAFYKEGIFDGECGDELNHAVMLV
GFGMKEIVNPLTKKGEKHYYYIIKNS W GQQ W GERGFINIETDES GLMRKCGLG
TDAFIPLIEHHHHHH.
The TF2.10 insert was excised from the pQE-60 vector using the restriction
enzymes
NcoI and BaxnHI, ligated into NcoI/BamHI cut expression vector pET-11D and
transformed into DHS alpha E. coli. The presence of a recombinant expression
plasmid
(pET-TF2.10) in an isolated E. coli colony was confirmed by restriction enzyme
digest
of plasmid DNA. BL21 (DE3) E. coli were transformed with pET-TF2.10 and used
for
expression of the recombinant cysteinyl proteinase.
Protein Expression
pET-TF2.10-Transformed BL21(DE3) E.coli (BLTF2.10) were grown up overnight at
200 rpm, 37°C in Luria broth containing 100 ~,g/ml ampicillin. Fresh
medium was
then inoculated and grown to an OD6oonm of 0.8 before protein expression was
induced
using 1 mM IPTG. Induction was performed for 3 hours at 200 rpm, 37°C
then the
bacterial cells harvested by centrifugation and stored at -80°C until
protein purification
performed.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
59
Protein Purification and Refolding
An E coli cell pellet equivalent to 250m1 culture was lysed by resuspension in
solubilisation buffer (6M guanidine hydrochloride, 20mM Tris-HCI, 250mM NaCI,
20mM imidazole, pH8.0) for 30 minutes at room temperature. After
centrifugation at
120008 for 10 minutes at 4°C the cleared lysate was applied to 1 ml
nickel-NTA
agarose, and agitated for 1 hour at room temperature.
Protein Refolding Method 1
The protein bound to nickel-NTA was batch washed with 6M guanidine
hydrochloride,
20mM Tris-HCI, pH 8.0, 250mM NaCI then 8M urea, Tris-HCI, pH 8.0, SOOmM NaCI
then 8M urea, Tris-HCI, pH 8.0 including 30 mM imidazole and protein elution
performed using 8M urea, Tris-HCI, pH 8.0 with 1 M imidazole. The eluted
protein
was then diluted 100 fold in refolding buffer (100mM Tris-HCI, 1mM EDTA, 20%
glycerol, 250mM L-arginine, 1mM reduced glutathione, O.ImM oxidised
glutatione,
pH8.0) and left stirring overnight at 4°C. The protein could then be
concentrated either
by filter centrifugation or repurification using a nickel-agarose column
(after dialysis to
remove the EDTA).
Protein Refolding Method 2
The protein bound to nickel-NTA was batch washed with 8M urea, Tris-HCI, SOOmM
NaCI, pH 8.0 then 8M urea, Tris-HCI, pH 8.0 including 20 mM imidazole, then 2M
urea, Tris-HCI, pH 8Ø The protein was then refolded on the column by the
addition
of 100mM Tris-HCI, pH8.0, 250mM L-arginine, 1mM reduced glutathione, O.lmM
oxidised glutatione with incubation at 4°C and protein elution
performed using,
100mM Tris-HCI, pH 8.0 with 0.5 M imidazole.
Immediately active (mature) proteinase was obtained using protein refolding
method 1
and concentrating the dilute refolded enzyme by filter centrifugation.This
method,
however, did result in a large degree of enzyme loss due to autoproteolysis.
Both
concentrating the protein refolded using method 1 by nickel column
purification and
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
using refolding method 2 resulted in greater recovery of the enzyme in its
stable
inactive pro-form. The pro-form could also be used to generate mature active
falcipain
2, after incubation at 37°C.
Convenient assays for falcipain 2 are carried out using the above recombinant
enzyme.
Alternatively, Sijali et al. Prot Exp Purif 22 128-134 2001 describes a
usefixl assay.
Standard assay conditions for the determination of kinetic constants used a
fluorogenic
peptide substrate, typically Boc-Val-Leu-Lys-AMC, and were determined in
either
100 mM Mes/Tris/acetate, pH 7.0 containing 1 M NaCI and 10 mM 2-
mercaptoethanol
or 100 mM Na phosphate, pH 5.5 containing 1 M NaCI and 10 mM 2-
mercaptoethanol. The enzyme concentration used was 2 nM. The stock substrate
solution was prepared at 10 mM in DMSO. Screens were carried out at a fixed
substrate concentration of 80 ~,M and detailed kinetic studies with doubling
dilutions
of substrate from 250 ~,M. The total DMSO concentration in the assay was kept
below
3%. All assays were conducted at ambient temperature. Product fluorescence
(excitation at 390 nm, emission at 460 nm) was monitored with a Labsystems
Fluoroskan Ascent fluorescent plate reader. Product progress curves were
generated
over 15 minutes following generation of AMC product.
Inhibition Studies
Potential inhibitors were screened using the above assay with variable
concentrations
of test compound. Reactions were initiated by addition of enzyme to buffered
solutions of substrate and inhibitor. I~; values were calculated according to
equation 1
vs
v° -
KM 1+ I +S (1)
K;
where v0 is the velocity of the reaction, Tr is the maximal velocity, S is the
concentration of substrate with Michaelis constant of K,yl, and 1 is the
concentration of
inhibitor.
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
1/3
SEQUENCE LISTING
<11.0> Medivir UK Ltd
Genzyme Corporation
<120> Cysteine Protease Inhibitors
<130> 1718-195FPC ,
<150> US 60/252,840
<151> 2001-11-17
<160> 4
<170> PatentIn version 3.0
<210> 1
<211> 39
<212> DNA
<213> artificial sequence
<220>
<223> Primer for cDNA of cysteinyl proteinase (Falcipain 2)
<400> 1
cgcggatccg ccaccatgga attaaacaga tttgccgat 39
<210> 2
<211> 57
<212> DNA
<213> artificial sequence
<220>
<223> primer for cDNA of cysteinyl proteinase (Falcipain 2)
<400> 2
cgcgtcgact taatgatgat gatgatgatg ttcaattaat ggaatgaatg catcagt 57
<210> 3
<211> 886
<212> DNA
<213> artificial sequence
<220>
<223> PCR product from amplification using primers for the cDNA sequenc
a of cysteinyl proteinase (Falcipain 2)
<220>
<221> CDS
<222> (3)..(848)
<400> 3
cc atg gaa tta aac aga ttt gcc gat tta act tat cat gaa ttt aaa 47
Met Glu Leu Asn Arg Phe Ala Asp Leu Thr Tyr His Glu Phe Lys
1 5 10 Z5
aac aaa tat ctt agt tta aga tct tca aaa cca tta aag aat tct aaa 95
Asn Lys Tyr Leu Ser Leu Arg Ser Ser Lys Pro Leu Lys Asn Ser Lys
20 25 30
tat tta tta gat caa atg aat tat gaa gaa gtt ata "aaa aaa tat aga 143
Tyr Leu Leu Asp Gln Met Asn Tyr Glu Glu Val Ile Lys Lys Tyr Arg
35 40 45
gga gaa gaa aat ttc gat cat gca get tac gac tgg aga tta cac agt 191
Gly Glu Glu Asn Phe Asp His Ala Ala Tyr Asp Trp Atg Leu His Ser
50 55 60
ggt gta aca cct gta aag gat caa aaa aat tgt gga tct tgc tgg gcc 239
Gly Val Thr Pro Val Lys Asp Gln Lys Asn Cys Gly Ser Cys Trp Ala
65 70 . 75
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
2/3
ttt agt agt ata ggt tcc gta gaa tca caa tat get atc aga aaa aat 287
Phe Ser Ser Ile Gly Ser Val Glu Ser Gln Tyr Ala Ile Axg Lys Asn
80 85 90 95
aaa tta ata acc tta agt gaa caa gaa tta gta gat tgt tca ttt aaa 335
Lys Leu Ile Thr Leu Ser Glu Gln Glu Leu Val Asp Cys Ser Phe Lys
100 105 110
aat tat ggt tgt aat gga ggt ctc att aat aat gcc ttt gag gat atg 383
Asn Tyr Gly Cys Asn Gly Gly Leu Ile Asn Asn Ala Phe Glu Asp Met
115 120 125
att gaa ctt gga ggt ata tgt cca gat ggt gat tat cca tat gtg agt 431
Ile Glu Leu Gly Gly Ile Cys Pro Asp Gly Asp Tyr Pro Tyr Val Ser
130 135 140
gat get cca aat tta tgt aac ata gat aga tgt act gaa aaa tat gga 479
Asp Ala Pro Asn Leu Cys Asn Ile Asp Arg Cys Thr Glu Lys Tyr Gly
145 150 155
atc aaa aat tat tta tcc gta cca gat aat aaa tta aaa gaa gca ctt 527
Ile Lys Asn Tyr Leu Ser Val Pro Asp Asn Lys Leu Lys Glu Ala Leu
160 165 170 175
aga ttc ttg gga cct att agt att agt gta gcc gta tca gat gat ttt 575
Arg Phe Leu Gly Pro Ile Ser Ile Ser Val Ala Val Ser Asp Asp Phe
180 185 190
get ttt tac aaa gaa ggt att ttc gat gga gaa tgt ggt gat gaa tta 623
Ala Phe Tyr Lys G1u Gly Ile Phe Asp Gly Glu Cys Gly Asp Glu Leu
195 200 205
aat cat gcc gtt atg ctt gta ggt ttt ggt atg aaa gaa att gtt aat 671
Asn His Ala Val Met Leu Val Gly Phe Gly Met Lys Glu Ile Val Asn
210 215 220
cca tta acc aag aaa gga gaa aaa cat tat tat tat ata att aag aac 719
Pro Leu Thr Lys Lys Gly Glu Lys His Tyr Tyr Tyr Ile Ile Lys Asn
225 230 235
tca tgg gga caa caa tgg gga gaa aga ggt ttc ata aat att gaa aca 767
Ser Trp Gly Gln Gln Trp.Gly Glu Arg Gly Phe Ile Asn Ile Glu Thr
240 245 250 255
gat gaa tca gga tta atg aga aaa tgt gga tta ggt act gat gca ttc 815
Asp Glu Ser Gly Leu Met Arg Lys Cys Gly Leu Gly Thr Asp Ala Phe
260 265 270
att cca tta att gaa cat cat cat cat cat cat taagtcgacg cgatcgaatt 868
Ile Pro Leu Ile Glu His His His His His His
275 280
cctgcagccc ggggatcc 886
<210> 4
<211> 282
<212> PRT
<213> artificial
<400> 4
Met Glu Leu Asn Arg Phe Ala Asp Leu Thr Tyr His Glu Phe Lys Asn
1 5 10 15
Lys Tyr Leu Ser Leu Arg Ser Ser Lys Pro Leu Lys Asn Ser Lys Tyr
20 25 30
Leu Leu Asp Gln Met Asn Tyr Glu Glu Val Ile Lys Lys Tyr Arg Gly
35 40 45
CA 02428863 2003-05-14
WO 02/40462 PCT/USO1/46486
3/3
Glu Glu Asn Phe Asp His Ala Ala Tyr Asp Trp Arg Leu His Ser Gly
50 55 60
Val Thr Pro Val Lys Asp Gln Lys Asn Cys Gly Ser Cys Trp Ala Phe
65 70 75 80
Ser Ser Ile Gly Ser Val Glu Ser Gln Tyr Ala Ile Arg Lys Asn Lys
85 90 95
Leu Ile Thr Leu Ser Glu Gln Glu Leu Val Asp Cys Ser Phe Lys Asn
100 105 110
Tyr Gly Cys Asn Gly Gly Leu Ile Asn Asn Ala Phe Glu Asp Met Ile
115 120 125
Glu Leu Gly Gly Ile Cys Pro Asp Gly Asp Tyr Pro Tyr Val Ser Asp
130 135 140
Ala Pro Asn Leu Cys Asn Ile Asp Arg Cys Thr Glu Lys Tyr Gly Ile
145 150 155 160
Lys Asn Tyr Leu Ser Val Pro Asp Asn Lys Leu Lys Glu Ala Leu Arg
165 170 175
Phe Leu Gly Pro Ile Ser Ile Ser Val Ala Val Ser Asp Asp Phe Ala
180 185 190
Phe Tyr Lys Glu Gly Ile Phe Asp Gly Glu Cys Gly Asp Glu Leu Asn
19S 200' 205
His Ala Val Met Leu Val Gly Phe Gly Met Lys Glu Ile Val Asn Pro
210 215 220
Leu Thr Lys Lys Gly Glu Lys His Tyr Tyr Tyr Ile Ile Lys Asn Ser
225 230 235 240
Trp Gly Gln Gln Trp Gly Glu Arg Gly Phe Ile Asn Ile Glu Thr Asp
245 250 255
Glu Ser Gly Leu Met Arg Lys Cys Gly Leu Gly Thr Asp Ala Phe Ile
260 265 270
Pro Leu Ile Glu His His His His His His
275 280