Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02454854 2003-12-30
3
TITLE OF THE INVENTION
METHOD OF RECOVERING SULFUR FROM LEACHING RESIDUE
UNDER ATOMOSPHERIC PRESSURE
BACKGROUND OF THE INVENTION
1. Field of the invention
The present invention relates to a method of
recovering sulfur from a residue that is generated by
leaching sulphide minerals (e. g. copper concentrates)
under atmospheric pressure.
2. Description of the Related Art
In earlier times, the pyrometallurgical process
had been predominantly used as a copper refining
process. However, recently, an SX-EW process, which is
one of the hydrometallurgical processes, has become
remarkably popular as the copper refining process to
extract copper typically from cooper oxide ores or
secondary copper ores, and the process has achieved
about 200 of the world copper production.
When producing copper metal at copper mine site,
they prefer the hydrometallurgical process to the
pyrometallurgical process because of its lower
production cost and an easier environmental protection
action. This is the reason why the h.ydrometallurgical
process has made progress in producing copper. However,
the hydrometallurgical process has not yet reached a
technical level to process main coppE:r mineral such as
primary copper mineral and chalcopyrite. Several
researches have been carried out to achieve a technical
breakthrough of processing the primary copper mineral
and the chalcopyrite.
One of the hydrometallurgical copper refining
processes, such as an Intec copper process using a
chloride salt solution, is a promising method of
refining the primary copper material, since the method
can facilitate a leaching operation and reduce
electricity consumption of univalent copper
- 1 -
CA 02454854 2003-12-30
electrolysis by half. Therefore, there is a need of
efficiently recovering sulfur from a chlaride leaching
residue generated by the hydrometallurgical copper
refining process.
For example, a leaching process used in the Intec
copper process leaches the chalcopyrite from Cu(2+) and
BrCl2(-) contained in a solution aftE=r electrolysis,
and produces a leaching liquid containing Cu(+) and a
leaching residue in the form of Fe00H and elemental
sulfur. Therefore, there is also a need of separating
sulfur and iron from the leaching residue in an
industrially and economically viable manner.
Another known the hydrometallurgical copper
refining process has acid leaching where the copper
concentrate is leached using Fe(3+) at room temperature
and under atmospheric pressure. However, this acid
leaching also has a problem of recovering sulfur
contained in the leaching residue.
Conventional methods of separating and recovering
sulfur from the residue can be classified into two
categories: (1) a first method having the steps of
extracting sulfur using a solvent and separating sulfur
from iron, and (2) a second method including
pressurization and screening (flotation).
The first method that uses the solvent is not
preferable to recover sulfur, because the solvent is
costly and not easy to handle in an industrially simple
manner. The second method that includes the
pressurization is not feasible because of a high cost
of the pressurization. Consequently, both methods are
not industrially and economically viable.
Japanese Patent Publication No. 6-43619, "PROCESS
FOR THE LEACHING OF SULPHIDES CONTAINING ZINC AND IRON",
discloses a leaching process based on a. combination of
leaching under pressure and leaching under atmospheric
pressure. It is to be noted that the leaching process
of the cited patent necessitates the leaching under
- 2 -
CA 02454854 2003-12-30
2 -
pressure applied.
It is known that, in case of leaching of sulphide
minerals (e. g. copper concentrates) under pressure,
sulfur that remains in a residue is easily recovered
from the residue by flotation. Thus, the leaching
process of the cited patent involves the leaching under
pressure. At the time of the leaching under pressure,
the sulfur contained in the residue may easily be
recovered by the flotation. A pressurization process
causes sulfur to reach its melting point. Once the
sulfur has fused, the sulfur can re-crystallize in the
form of a certain size of particles and rather suitable
to float in the liquid.
On the contrary, since the sulfur contained in
the residue after leaching under atmospheric pressure
remains in the form of fine particles, it is not easy
to selectively separate the sulfur by the flotation.
SUMMARY OF THE INVENTION
It is an object of the present invention to
provide a method of economically and industrially
recovering sulfur from a leaching residue produced by
leaching sulphide minerals (e. g. copper concentrates)
under atmospheric pressure, in which. the above
disadvantages are eliminated.
The inventor of the present invention has studied
various methods of recovering sulfur from a residue and
has reached the following invention.
(1) The object of the present invention is achieved
by a method of recovering sulfur from a leaching
residue produced by leaching sulphide minerals (e. g.
copper concentrates) under atmospheric pressure, the
method comprising the steps of:
pretreating the leaching residue with an acid
solution; and
selectively recovering sulfur from the leaching
residue by a flotation process.
- 3 -
CA 02454854 2003-12-30
a
(2) According to an embodiment of the present
invention, the acid solution is a sulfuric acid
solution.
(3) According to an embodiment of the present
invention, the step of pretreating the leaching residue
with an acid solution comprises the step of:
pretreating the leaching residue with a treatment
solution of sulfuric acid having a pH value equal to or
less than 1.5, at a pulp density of 10 to 50 mass
percent, for a processing time of 20 to 60 minutes, and
at a processing temperature of 30 to 90 °C.
(4) According to an embodiment of the present
invention, the step of selectively recovering sulfur
from the leaching residue by a flotation process
comprises the step of:
applying the Rougher flotation to the pretreated
residue at a pulp density of 10 to 35 mass percent, in
a treatment solution having a pH value equal to or less
than 2.2, and at a processing temperature of 20 to
90 °C.
(5) According to a further embodiment of the present
invention, the step of selectively recovering sulfur
from the leaching residue by a flotation process
further comprises the step of:
applying the cleaner flotation to a product
produced by the floatation process.
(6) According to a still further embodiment of the
present invention, the step of selectively recovering
sulfur from the leaching residue by a flotation process
further comprises the step of:
if a product containing sulfur produced by the
cleaner flotation includes sulfides which have not
leached away, applying a gravity separation process to
the product so as to selectively removing the sulfides
from the product.
- 4 -
CA 02454854 2003-12-30
BRIEF DESCRIPTION OF THE DRAWINGS
Other objects, features and advantages of the
present invention will become more apparent from the
following detailed description when read in conjunction
with the accompanying drawings, in which
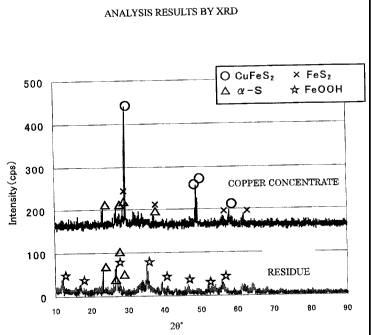
Fig. 1 shows results of an x-ray diffraction
analysis (XRD) according to the present invention;
Fig. 2 shows a particle size distribution of feed
(Cu concentrate) and residue from a processing ore
according to the present invention;
Fig. 3 plots a sulfur recovery percentage in
relation to a warming time during a pretreatment
according to the present invention;
Fig. 4 plots a sulfur recovery percentage in
relation to a pH during the pretreatment according to
the present invention;
Fig. 5 plots a sulfur recovery percentage in
relation to a warming temperature during the
pretreatment according to the present invention;
Fig. 6 plots a sulfur recovery percentage in
relation to a pH during rougher flotation;
Fig. 7 plots a sulfur recovery percentage in
relation to sulfur grade at each process of the present
invention; and
Fig. 8 shows a processing flow according to one
embodiment of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention will be described in detail,
by way of an example, with reference to the
accompanying drawings. It should be noted that the
present invention is not limited to the specifically
disclosed embodiments.
A processing object of the present invention,
that is to say a leaching residue, is composed of, for
example, 10 to 35 masso Fe (iron), 1.5 to 35 mass°s S
(sulfur) and 0.7 to 1.2 masso Cu (copper).
- 5 -
CA 02454854 2003-12-30
If the residue is a chloride leaching residue,
then Fe is leached out of chalcopyrite and exists in
the form of Fe00H, and most of S exists in the form of
elemental sulfur, as can be seen from the results of an
x-ray diffraction analysis shown in Fig. 1. The
residue contains some ores which have not yet leached
away.
Sulfur essentially has a native hydrophobic
property, whereas Fe00H is very hydrophilic. Therefore,
if particles of sulfur and Fe00H are physically
separated from each other, it is appreciated that
sulfur and Fe00H c:an be separated from each other by
flotation utilizing a difference between hydrophilic
and hydrophobic aspects.
With reference to Fig. 2, however, the particle
size of the residue is much smaller than that of copper
concentrates prior to a leaching process, and has a
very small 50o passing size of between 2 and 4 microns.
The floatation process is not ~~apable of
separating fine particles which are smaller than an
applicable particle size limit, because such fine
particles will not attach to an air bubble. The
particle size limit is said to be between 20 and 30
microns for ordinary ores and 7 microns for MacArthur
River mineral ores which probably has the smallest
grain size limit.
For the reasons stated above, though the
flotation process can be performed at attractive costs,
it is a very difficult approach to reduction in
practice in technical point of view.
According to one aspect of the present invention,
the residue is previously pretreated with an acid
solution. Preferably, the acid solut=ion is a sulfuric
acid solution, as described later.
Preferably, a ratio of the leaching residue to
the sulfuric acid solution is such that a pulp density
is equal to 10 to 35 masso. This is because industrial
- 6 -
CA 02454854 2003-12-30
production efficiency is low at the pulp density below
masso and the pretreatment process becomes difficult
to carry out above the pulp density of 35 masso as the
solution will not be sufficiently stirred.
5 The time of a pretreatment process is preferably
set to be between 20 and 60 minutes, as shown in Fig. 3.
Otherwise, a final sulfur recovery percentage will not
go beyond 90 masso, because the pretreatment with the
sulfuric acid solution is insufficient at the
10 processing time below 20 minutes and the industrial
production efficiency becomes inferior at the
processing time over 60 minutes.
More preferably, the processing time is set to be
between 30 and 60 minutes. This is because the sulfur
recovery percentage during a first flotation process
(hereinafter referred to as '°rougher flotation")
amounts to 95 mass% or more.
Properties of several acid treatment solutions,
such as sulfuric acid, hydrochloric acid and nitric
acid, used in the pretreatment process are shown in
Table I. Under a 'treating condition where an identical
pH value is used, the sulfur recovery percentages for
the sulfuric acid, hydrochloric acid and nitric acid
are 96.0 mass%, 44.8 masso and 58.5 masso, respectively.
An addition of the hydrochloric acid or the nitric acid
improves the sulfur recovery percentage in comparison
with the case where no acid is added. Preferably, the
sulfuric acid solution is used as th~~ acid solution in
the pretreatment process in terms of its sulfur
recovery percentage. Furthermore, the sulfuric acid is
more cost effective and easier to handle in later
processes than the hydrochloric acid and the nitric
acid.
Of course, the sulfur recovery percentage may be
improved with increasing acid concentration of the
hydrochloric acid or the nitric acid.
It can be seen from the property of the solution
CA 02454854 2003-12-30
after the pretreatment process that the solution
produced using the sulfuric acid has a higher iron
concentration than using the hydrochloric acid or the
nitric acid. From this point of view, we can consider
that Fe00H covering a surface of sulfur particle elutes
by a reaction represented by the following formula (1)
and is removed from the surface of the sulfur particle,
so that an essential floating characteristic of sulfur
is revealed and improved.
2Fe00H+3H2S0~--jFe2 (S04) 3+4H20 (1)
Table I
Type Kinds Grade Recovery
o o (masso)
(masso)
of of S Fe Cu Wt S Fe Cu
Acid Ores
No F 27.9 30.3 1.1 100.0 100.0 100.0 100.0
Acid C 47.2 30.4 1.1 22.<? 37.7 22.3 22.7
T 22.3 30.2 1.1 77.8 62.3 77.7 77.3
H2S04 F 30.0 29.0 1.0 100.0 100.0 100.0 100.0
C 52.4 21.6 1.5 55.0 96.0 41.0 83.0
T 2.7 38.0 0.4 45.0 4.0 59.0 17.0
HN03 F 29.9 29.2 0.9 100.0 100.0 100.0 100.0
C 53.3 26.4 1.3 32.8 58.5 29.7 47.9
T 18.5 30.5 0.7 67.2 41.5 70.3 52.1
HCl F 29.9 29.9 0.9 100.() 100.0 100.0 100.0
C 60.2 23.9 1.5 22.2 44.8 17.8 34.1
T 21.2 31. 0.8 77.8 55.2 82.2 65.
~ 6 1 I 9
"F" indicates feed (residue).
"C" indicates concentrate.
"T" indicates tailing.
Preferably, the sulfuric acid treatment solution
for the pretreatment is adjusted such that its pH value
is equal to or less than 1.5, as shown in Fig. 4. This
is because the sulfur recovery percentage reaches 90
masso or more during rougher flotation. More
_ g _
CA 02454854 2003-12-30
preferably, the pH value of the sulfuric acid treatment
solution is adjusted to be less than 1.0, because the
sulfur recovery percentage reaches 95 masso or more
during rougher flotation.
The processing temperature during the
pretreatment is preferably between 30 and 90 °C,
because the sulfur recovery percentage reaches 90 masso
or more during rougher flotation. More preferably, the
processing temperature during the pretreatment is
between 40 and 90 °C, because the sulfur recovery
percentage reaches 95 masso or more during rougher
flotation at a processing temperature higher than 40 °C.
After the pretreatment process, the pulp density
during rougher flotation is preferably between 10 and
35 masso. This is because the rougher flotation is not
industrially efficient at the pulp density below 10
masso and the viscosity of the solution is too high to
facilitate the rougher flotation at the pulp density
above 35 masso.
The processing time for the pretreatment with the
sulfuric acid solution is preferably between 5 and 75
minutes. This is because the sulfur recovery
percentage will not reach 90 masso due to the
insufficient pretreatment with the sulfuric acid
solution at the processing time below 5 minutes, and
the industrial efficiency is inferior at the processing
time above 75 minutes.
More preferably, the processing time is between
and 75 minutes, because the sulfur recovery
30 percentage reaches 95 masso or more at the processing
time above 30 minutes during rougher flotation.
Preferably, the sulfuric acid -treatment solution
for rougher flotation is adjusted such that its pH
value is equal to or less than 2.2, as shown in Fig. 6.
This is because the sulfur recovery percentage reaches
90 masso or more during rougher flotation. More
preferably, the pH value of the sulfuric acid treatment
_ o _
CA 02454854 2003-12-30
solution is adjusted to be between 1.5 and 2.1, because
the sulfur recovery percentage reaches 95 mass% or more
during rougher flotation.
The processing temperature during rougher
flotation is preferably between 20 and 90 °C, because
the sulfur recovery percentage reaches 90 mass% or more
during rougher flotation. More preferably, the
processing temperature during coarse screening is
between 40 and 90 °C, because the sulfur recovery
percentage reaches 95 masss or more during coarse
screening at the processing temperature above 40 °C.
It is noted that the processing temperature above 90
C will not be effective to further improve the sulfur
recovery percentage.
It is obvious that a frother, such as Methyl
Isobutyl Carbinol (MIBC), of 40 to 80 g/t (g/ton} is
added to the residue in dried form.
In the rougher flotation, a floatation machine,
such as a column flotation machine, may be used.
After the rougher flotation, the cleaner
flotation may be performed. Preferably, the cleaner
flotation repeats cleaner flotation two or more times.
This is because grade of the recovered sulfur reaches
about 80 masss or more, as shown in Fig. 7.
Since forming property of the :Eorther added to
the dry residue in the rougher flotation is maintained
during cleaner flotation, there is no need of further
adding the forther to the residue. l.n stead of adding
the agent, air bubbles are kept in a floating position
by applying air blowing of 250 to 700 1/min/m2 to the
residue and cleaner flotation is-app.lied to the residue
in normal industrial water.
The column floatation machine may also be used as
the flotation machine for cleaner flotation.
Grade of a product containing sulfur generated by
cleaner flotation is as follow:
FIRST FINE CLEANER FLOTATION: S grade is 65 to 69
- 10 -
CA 02454854 2003-12-30
masso, Fe grade is 13.0 to 17.0 masso, and Cu grade is
between 1.7 to 2.1 masso; and
SECOND FINE CLEANER FLOTATION: S grade is 77 to
81 masso, Fe grade is 3.0 to 7.0 masso, and Cu grade is
1.6 to 1.9 masso. It can be seen that S grade at the
second stage is higher than that of the first stage.
If the Fe grade and/or the Cu grade of the
product containing sulfur are high, gravity flotation,
or gravity separation may be performed in order to
recover sulfides from the product, as the sulfides have
not yet leached away. Since the specific gravity of
sulfides is about 5 and the specific gravity of sulfur
is about 2, the sulfides are subject to the gravity
flotation in water using a plate inclined at an angle
of about 0.5 to 1.0 degrees and by applying a vibration
having the number of vibrations of about 230 to 280 rpm
and vibration width of 13 to 25 mm.
Alternatively, the gravity flotation may be
performed by a gravity flotation machine based on
centrifugal separation.
After this gravity flotation, on one hand, the
grade of a resultant sulfur concentrate is such that S
grade is 78.0 to 82.0 masso, Fe grade is 3.0 to 3.8
masso, and Cu grade is 1.0 to 1.4 masso. On the other
hand, the grade of the sulfides which have not yet
leached away is such that S grade is 40.0 to 48.0 masso,
Fe grade is 34.0 to 38.0 masso, and Cu grade is 13.0 to
17.0 masso. It can be seen that sulf=ur grade of the
sulfur concentrate is increased and Cu grade and Fe
grade are decreased.
As will be apparent from the above mentioned
description, the present invention provides a method of
recovering sulfur from a leaching residue produced by
leaching sulphide minerals (e. g. copper concentrates)
simply under atmospheric pressure.
EXAMPLE
- 11 -
CA 02454854 2003-12-30
Referring to Figs. 7 and 8, an embodiment of the
present invention will be explained, by way of an
example, in conjunction with Table II representing
processing objects and processing results for each step
of a method of recovering sulfur from a. leaching
residue according to the embodiment of the present
invention.
A processing object, that is to say, a chloride
leaching residue of a copper concentrate contains S, Fe
and Cu such that S grade is 30.0 masso, Fe grade is
29.0 masso, and Cu grade is 1.0 masso, as shown in
Table II.
Table II
Object Grade (masso) Recovery
o (masso)
S Fe Cu Wt S Fe Cu
(1) 30.0 29.0 1.0 100.0 100.0 100.0 100.0
(2) 54.0 22.0 1.6 53.0 95.4 40.2 84.8
(3) 67.0 15.0 1.9 42.0 93.8 21.7 79.8
(4) 79.0 5.0 1.8 33.8 89.0 5.8 60.8
(5) 80.6 3.4 1.2 32.3 86.8 3.8 38.4
(6) 44.5 38.6 15.0 1.5 2.2 2.0 22.4
Object (1) indicates a residue or a processing
object.
Object (2) indicates a concentrate produced by
rougher flotation.
Object (3) indicates a product produced by the
first stage of cleaner flotation.
Object (4) indicates a product produced by the
second stage of cleaner flotation.
Object (5) indicates a resultant sulfur
concentrate.
Object (6) indicates sulfides which have not
leached away.
The processing object was pretreated with a
sulfuric acid solution under treatment conditions:
- 12 -
CA 02454854 2003-12-30
pH=1.0, a pulp density of 30 masso, a treatment liquid
temperature of 80 °C, and a processing time of 60
minutes.
Then, the object (1) was subject to rougher
flotation under rougher flotation conditions: pH=1.0,
pulp density of 3G masso, treatment liquid temperature
of 20 °C, processing time (flotation time) of 10
minutes.
Methyl Isobutyl Carbinol (MIBC) was used as a
frother. 60g/t of MIBC was added to the dry residue.
This rougher flotation was carried out in a column
flotation machine.
The resultant object (2) after rougher flotation
was the rougher flotation concentrate such that S grade
was 54 masso, Fe grade was 22.0 masso, and Cu grade was
1.6 masso, as shown in the object (2) of Table II and
Fig. 7.
It can be seen that this rougher flotation
increased S grade of the object (2) to about twice that
of the object (1).
Subsequently, the cleaner flotation was applied
to the object (2), and thus producing the object (3),
as shown in Fig. 8.
During fine screening, since a:ir bubbles
generated by the previous rougher flotation were flowed
into a solution, industrial water was used as the
solution and air blowing of 318 1/mi:n/m2 (i.e. 0.1
1/min/3.14cm2) was introduced from a lower part of a
flotation machine in order to move the air bubbles
upwards.
The solution was kept at a roorn temperature of
20 °C.
A column flotation machine was used as the
flotation machine.
The fine screening process included two stages of
cleaner flotation. After the first stage, the
resultant object (3) was composed as follows: S grade
- 13 -
CA 02454854 2003-12-30
of 67.0 mass%, Fe grade of 15.0 masso, and Cu grade of
1.9 masso, as shown in Table II and Fig. 7. Sulfur
grade of the object (3) was more increased than that of
the object (2) produced by rougher flotation.
After the second stage of the cleaner flotation
process, the resultant object (4) was composed as
follows: S grade of 79.0 masso, Fe grade of 5.0 masso,
and Cu grade of 1.8 masso, as shown in Table II and Fig.
7. Sulfur grade of the object (4) was more increased
than that of the object (3) produced by rougher
flotation and iron grade of the object (4) was lower
than that of the object (3).
'In addition, gravity separation using the shaking
table inclined at an angle of about 0.5 degrees was
applied to a product containing sulfur, i.e. the object
(4) generated by the second cleaner flotation stage,
with vibration of 250 rpm and vibration width of 20 mm
in water.
In this manner, sulfides that have not leached
away and a sulfur concentrate were produced, as shown
in objects (5) and (6) of Table II. On one hand, the
sulfides contained Cu such that Cu grade is 15 mass%.
On the other hand, the sulfur concentrate contained S
such that S grade much increased to 80.6 masso and F
grade and Cu grade decreased, and thus resulted in the
desirable sulfur concentrate.
As apparent from steps (1) to (4) of Fig. 7,
sulfur grade increased as the step proceeded. It is
noted that numbers (1) to (4) of Fig. 7 correspond to
those of Fig. 8. The object numbers (1) to (6) in
Table II also correspond to the numbers in Fig. 8.
Treatment conditions for each of the steps should
be determined so that desired sulfur recovery
percentage and sulfur grade can be achieved.
In the above mentioned example, the method of
recovering sulfur :From a leaching re:~idue according to
one embodiment of the present invention includes a
- 14 -
CA 02454854 2003-12-30
cleaner flotation having two stages and an additional
gravity separation process. However, the present
invention is not limited to the specifically disclosed
embodiments, and variations and modifications may be
made without departing from the scope of the present
invention mentioned before.
According to an embodiment of the present
invention, since a leaching residue that has been
leached out of sulphide minerals (e. g. copper
concentrates) is pretreated with an acid solution,
high-grade sulfur can be recovered from a chloride
leaching residue using a simple flotation process.
According to an embodiment of -the present
invention, since a sulfuric acid solution is used as
the acid solution, sulfur grade is further improved.
According to a further embodiment of the present
invention, since a flotation process includes rougher
flotation and cleaner flotation to separate sulfur from
the residue, a prcduct containing sulfur with a high
sulfur concentration can be recovered from the residue.
According to a still further embodiment of the
present invention, since the method further comprises a
gravity separation process after the flotation process,
sulfides that have not leached away can be efficiently
removed from the product and more high-grade sulfur can
be achieved.
- 15 -