Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-1-
PROTEIN PRODUCTION METHODS AND
MODIFIED CELLS FOR USE THEREIN
Background of the Invention
This invention relates the field of cell biology. More particularly, this
invention
relates to protein production by eukaryotic cells.
Proteins produced by eukaryotic cells can have significant therapeutic value.
Such proteins may be naturally produced by the eukaryotic cell, or the
eukaryotic cell
may be manipulated by recombinant molecular biology techniques to produce a
heterologous protein. Non-limiting examples of proteins produced, either
naturally or
by artifice, include erythropoietin, insulin, and factor IX.
In eukaryotic cell culture, production of protein from a cultured cell is a
function of the specific activity (i. e., the amount of protein produced per
cell) and the
total viable cell mass over the course of a bioreactor run (ICA). However, the
production of protein by cultured cells is limited by cell death in the
bioreactor. There
are two forms of cell death, necrosis and apoptosis. Necrosis is a form of
cell death
that is typically due to a traumatic injury or insult to the cell. Shear
forces and foaming
are probable causes of necrosis in the bioreactor. Apoptosis, also known as
programmed cell death, is a form of cell death where, through a variety of
signaling
pathways, the cell self destructs. Examples of apoptosis stimuli include
growth factor
withdrawal, the limitation of various nutrients and exposure to toxins. Recent
literature
on the subject of cell death in the bioreactor supports the notion of
apoptosis as a major
contributor (Moors A et al., "Apoptosis in CHO cell batch cultures:
examination by
flow cytometry," Cytotechhology 17:1-11, 1995; Goswami J et al., "Apoptosis in
Batch
Cultures of Chinese Hamster Ovary Cells," Biotech. & Bioeng. 62:632-640,
1999).
There is a need to identify methods for prolonging cell lifespan as a means
for
enhancing the cell's production of a protein, regardless of whether that
protein is one
naturally produced by the cell, or whether that protein is a heterologous
protein to the
cell.
Summary of the Invention
The invention provides methods for prolonging cell lifespan as a means for
enhancing the cell's production of a protein, regardless of whether that
protein is one
naturally produced by the cell, or whether that protein is a heterologous
protein to the
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-2-
cell.
Accordingly, in one aspect, the invention provides a method for increasing
production of a protein by a cell, comprising increasing expression of an anti-
apoptosis
gene in the cell. In certain embodiments, the cell does not express a
heterologous
cyclin-dependent kinase inhibitor. In particular embodiments, the cell is a
human cell,
a murine cell, a hamster cell, an insect cell, or an amphibian cell.
In another aspect, the invention provides a method for increasing the
production
of a heterologous protein by a cell, comprising increasing expression of an
anti-
apoptosis gene in the cell, wherein the cell does not express a heterologous
cyclin-
dependent kinase inhibitor.
In a further aspect, the invention provides a method for increasing production
of
a protein by a cell, comprising increasing expression of a Bcl-xL gene in the
cell,
wherein the cell does not express a heterologous cyclin-dependent kinase
inhibitor.
In particular embodiments, the cell is a human cell, a murine cell, a hamster
cell, an insect cell, or an amphibian cell.
In another aspect, the invention provides a method for increasing the
production
of a heterologous protein by a cell, comprising increasing expression of a Bcl-
xL gene in
the cell, wherein the cell does not express a heterologous cyclin-dependent
kinase
inhibitor.
In a further aspect, the invention provides a cell comprising increased
expression of an anti-apoptosis gene and does not express a heterologous
cyclin-
dependent kinase inhibitor, wherein the cell produced an increased amount of a
protein
as compared to a cell that does not comprise increased expression of the anti-
apoptosis
gene.
In a further aspect, the invention provides a cell comprising increased
expression of a Bcl-xL gene and does not express a heterologous cyclin-
dependent
kinase inhibitor, wherein the cell produced an increased amount of a protein
as
compared to a cell that does not comprise increased expression of the Bcl-xL
gene.
In another aspect, the invention provides a cell comprising increased
expression
of an anti-apoptosis gene and a gene encoding a protein of interest, and does
not
express a heterologous cyclin-dependent kinase inhibitor, wherein the cell
produced an
increased amount of a protein of interest as compared to a cell that does not
comprise
increased expression of the anti-apoptosis gene.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-3-
In a further aspect, the invention provides a cell comprising increased
expression of a Bcl xL gene and a gene encoding a protein of interest, and
does not
express a heterologous cyclin-dependent kinase inhibitor, wherein the cell
produced an
increased amount of a protein of interest as compared to a cell that does not
comprise
increased expression of the Bcl xL gene.
The invention includes a cell comprising an increased amount of Bcl-xL
protein,
where the cell does not express a heterologous cyclin-dependent kinase
inhibitor. The
cell can be a mammalian, rodent, insect, or amphibian cell, such as a human,
murine, or
hamster cell (e.g., a Chinese hamster ovary cell). In addition, the cell can
be adapted
for growth in suspension or for growth in a medium free of serum (e.g., fetal
bovine
serum). The medium used for culturing the cell, whether free of serum or not,
can
further contain butyrate (e.g., sodium butyrate) to increase protein yields.
The Bcl-xL protein can be expressed from an expression vector introduced into
the cell or made to overexpress the endogenous Bcl xL gene of the cell, e.g.,
by
inducing the endogenous promoter of the gene. The Bcl-xL protein can be of a
species
different than that of the cell. For example, as shown below, the human Bcl-xL
protein
can be expressed in Chinese hamster ovary cells to obtain the cells and
methods of the
invention.
The cells of the invention, as described immediately above, are especially
useful
for robust production of proteins, either already produced by the cell or
exogenously
produced by introducing of an expression vector encoding the protein (e.g., a
secreted
protein). Where the cells of the invention are used to express a cloned
monoclonal
antibody, the cell can contain one vector that expresses both the heavy and
light chain
or two vectors, each expressing a heavy or light chain.
Accordingly, the invention further includes a method of producing a
polypeptide by culturing a cell of the invention and purifying the polypeptide
from the
cell culture.
Any publications or other documents cited in this disclosure is hereby
incorporated by reference.
Brief Description of the Drawings
Fig. 1 A is a schematic representation of the Bcl-xL -neo plasmid, a non-
limiting
vector of the invention. The expression of Bcl-xL in this vector is driven by
the CMV
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-4-
immediate-early promoter and the neomycin gene provides the selection marker
(for
resistance in the presence of G418).
Figs. 2A and 2B are schematic representations of growth curves showing viable
cell density (VCD) over time (Fig. 2A) and percentage viabilities (%
viability) over
time (Fig. 2B) for five out of ten non-limiting Bcl-xL transfected Chinese
Hamster
Ovary (CHO) DG44 cells and two controls (i.e., the untransfected DG44 host and
the
DG44 transfected with empty vector).
Figs. 3A and 3B are schematic representations of growth curves showing DG44/
Bcl-xL clone #3, a non-limiting clone of the invention, and two controls
(i.e., the
untransfected DG44 CHO host and the DG44 CHO cells transfected with empty
vector)
cultured in the absence of 6418 as measured by viable cell density (VCD) over
time
shown (Fig. 3A) and percentage viability (% viability) (Fig. 3B).
Fig. 4 is a bar graph showing caspase-3 activity, as measured daily for twelve
days, in a non-limiting Bcl-xL transfected cells of the invention, DG44/ Bcl-
xL #3
(black bars), DG44 CHO cells transfected with empty vector (medium gray bars),
and
untransfected DG44 CHO cells (light gray bars).
Fig. 5 is a representation of a Western blotting analysis probing cell lysates
of
the following non-limiting cells of the invention: DG44/ Bcl-xL #3 cells (left
lane),
DG44/ Bcl-xL #8 cells (middle lane), and DG44 CHO cells transfected with empty
vector (right lane) with a murine monoclonal antibody that specifically binds
to human
Bcl-xL protein.
Figs. 6A and 6B are schematic representations of growth curves showing viable
cell density (VCD) over time (Fig. 6A) and percentage viabilities (%
viability) over
time (Fig. 6B) for the following non-limiting cells of the invention: DG44/
Bcl-xL #3
(black circles), DG44/ Bcl-xL #8 (blue triangles), and the untransfected DG44
host
(open circles).
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-5-
Fig. 7A is a schematic representation of the Bcl xL -zeo plasmid, a non-
limiting
vector of the invention. The expression of Bcl-xL in this vector is driven by
the CN1V
immediate-early promoter and the zeocin gene provides the selection marker
(for
resistance in the presence of zeocin).
Fig. 7B is a schematic representation of a flow cytometry histogram showing
expression of AQC2 by parent 1 OOAB-37 cells (grey [green] line) and the pool
of Bcl-
xL transfected 100AB-37 cells (bold black [blue] line) as determined by
staining with
an antibody that specifically binds to AQC2 . The control (black line) was
DG44 host
cells stained with the same anti-AQC2 antibody
Figs. 8A and 8B are schematic representations of growth curves showing viable
cell density (VCD) over time (Fig. 8A) and percentage viabilities (%
viability) over
time (Fig. 8B) for the following non-limiting cells of the invention: 100AB-
37/ Bcl-xL
isolate #11 (purple diamonds), 100AB-37/ Bcl-xL isolate #21 (black triangles),
100AB-
37/ Bcl-xL isolate #25 (red circles), and 100AB-37 parent (blue circles).
Fig. 9 is a line graph showing the AQC2 titer from for the following non-
limiting cells of the invention: 100AB-37/ Bcl-xL isolate #11 (purple
diamonds),
100AB-37/ Bcl-xL isolate #21 (black triangles), 100AB-37/ Bcl-xL isolate #25
(red
circles), and 100AB-37 parent (blue circles). The 100AB-37/ Bcl-xL isolates
demonstrated significantly higher titers and up to 80% increase in throughput
cultured
in spinner flasks.
Figs. l0A and 10B are schematic representations of growth curves showing
viable cell density (VCD) over time (Fig. l0A) and percentage viabilities (%
viability)
over time (Fig. 10B) for the following non-limiting cells of the invention: 1
OOAB-37
parent run 1 (open blue diamonds), 100AB-37 parent run 2 (open red squares),
100AB-
37/ Bcl-xL isolate #21, run 1 (black triangles), and 100AB-37/ Bcl-xL isolate
#21, run 2
(red squares) in 2 liter model bioreactors. The results were consistent with
previous
results obtained in smaller scale spinner cultures.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-6-
Fig. 11 is a line graph showing the AQC2 titer from the following non-limiting
cells of the invention: 100AB-37 parent run 1 (open triangles), 100AB-37
parent run 2
(open squares), 100AB-37/ Bcl-xL isolate #21, run 1 (black triangles), and
100AB-37/
Bcl-xL isolate #21, run 2 (red squares) in 2 liter model bioreactors.
Figs. 12A and 12B are schematic representations of growth curves showing
viable cell density (VCD) over time (Fig. 12A) and percentage viabilities (%
viability)
over time (Fig. 12B) for the following non-limiting cells of the invention:
100AB-
37/21.15 Bcl-xL (green squares) and 37.32 OBcI-xL (open diamonds) cultured in
spinners in chemically defined growth media (CDM).
Fig. 13 is a line graph showing the AQC2 titer from the following non-limiting
cells of the invention: 100AB-37/21.15 Bcl-xL (green squares; lead Bcl-xL-
expressing
subclone) and 37.32 OBcl-xL (open diamonds; lead subclone of parent) cultured
in
spinners in chemically defined growth media (CDM).
Fig. 14 is a bar graph showing caspase-3 activity, as measured daily for
twelve
days, in the following non-limiting cells of the invention: 21.15 Bcl-xL (red
bars; lead
Bcl-xL-expressing subclone) and 37.32 ~Bcl-xL (gray bars; lead subclone of
parent).
Fig. 15 is a bar graph showing the amount of AQC2 secretion by the following
non-limiting cells of the invention: 100AB-37 parent cells, 100AB-37.320Bc1-xL
cells
(lead subclone of parent), 100AB-37-21 Bcl-xL cells, and 100AB-37-21.15 Bcl-xL
cells
(lead Bcl-xL expressing subclone) in the absence (white bars) or presence
(black bars)
of 2 mM sodium butyrate in shaker flasks.
Fig. 16 is a bar graph of percent viability for the following non-limiting
cells of
the invention: 100AB-37 parent cells, 100AB-37.32~Bc1-xL cells (lead subclone
of
parent), 100AB-37.21 Bcl-xL cells, and 100AB-37-21.15 Bcl-xL cells (lead Bcl-
xL
expressing subclone) in the absence (white bars) or presence (red bars) of 2
mM
sodium butyrate in shaker flasks.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
_7_
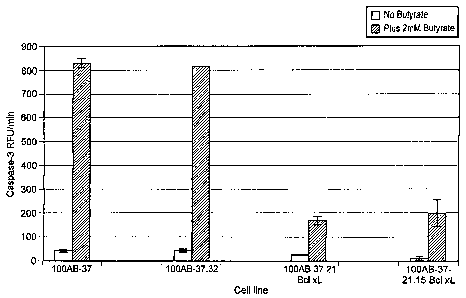
Fig. 17 is a bar graph showing caspase-3 activity in the following non-
limiting
cells of the invention: 100AB-37 parent cells, 100AB-37.32~Bc1-xL cells (lead
subclone of parent), 100AB-37-21 Bcl-xL cells, and 100AB-37-21.15 Bcl-xL cells
(lead
Bcl-xL expressing subclone) in the absence (blue bars) or presence (red bars)
of 2 mM
sodium butyrate in shaker flasks.
Detailed Descriution of the Preferred Embodiments
The patent and scientific literature referred to herein establishes knowledge
that
is available to those with skill in the art. The issued U.S. patents, allowed
applications,
published foreign applications, and references, including GenBank database
sequences,
that are cited herein are hereby incorporated by reference to the same extent
as if each
was specifically and individually indicated to be incorporated by reference.
The present invention stems from the inventors' unexpected discovery that
when an anti-apoptosis gene (e.g., Bcl-xL) is expressed in a cell, that cell
produces more
protein. Surprisingly, cells co-expressing the anti-apoptosis gene and a
second protein
(e.g., a heterologous protein) do not show an increase in the number of viable
cells.
The invention allows for methods to increase protein production by a cell,
both ih vitro
(i.e., in tissue culture) and in vivo.
Many different genes are involved in the induction and prevention of cell
death,
including apoptosis and necrosis. Two genes, Bcl-2 and Bcl-xL have been
identified as
having anti-apoptosis activities. For example, Fussenegger et al. (Nature
Biotechnology 16(5):468-72,1998) describes engineered Chinese Hamster Ovary
cells
that were engineered to inducibly express three different proteins, Bcl-xL,
p27 (a
cyclin-dependent kinase inhibitor), and SEAP (secreted alkaline phosphatase).
Upon
induction of expression of these proteins, the cells were held in the Gl phase
by p27,
which allowed an increase in SEAP production. Mastrangelo A.J. et al.
(Biotech.
Bioeng. 67(5):544-554, 2000) describe induction of cell death of BHK and
Chinese
Hamster Ovary (CHO) cells by infection with recombinant alphavirus vectors
engineered to express IL-12 protein. The lifespans of these infected cells was
prolonged by the overexpression of Bcl-2 or Bcl-xL in these cells, thereby
allowing the
cells to produce more IL-12. Indeed, overexpression of Bcl-2 or Bcl-xL in BHK
and
CHO cells was able to prolong the cells' lifespans after other, non-alphavirus
infection
induced cell death stimuli, including extended periods of glucose deprivation,
serum
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
_g_
withdrawal, and treatment with ammonium chloride (Mastrangelo A.J. et al.,
Biotech.
Bioeyzg. 67(5):555-564, 2000).
Thus, as used in accordance with the invention, by "anti-apoptosis gene" is
meant the gene encoding the Bcl-2 protein or the gene encoding the Bcl-xL
protein (or
other nucleic acid (e.g., cDNA or mRNA) encoding Bcl-2 protein or Bcl-xL
protein,
respectively), regardless of what species the genes are from. For example, the
Bcl-xL
gene may be from a human (GenBank Accession No. 223115 or L20121; Boise et
al.,
Cell 74(4): 597-608,1993). Other non-limiting Bcl xL anti-apoptosis genes of
the
invention include the feline Bcl-xL gene (GenBank Accession No. AB080951); the
bovine Bcl xL gene (GenBank Accession No. AF245489); the canine Bcl xL gene
(GenBank Accession No. AB073983); the Xenopus laevis Bcl xL gene (GenBank
Accession No. NP 494134); the porcine Bcl xL gene (GenBank Accession Nos.
AF216205 or AJ001203); the murine Bcl xL genes (GenBank Accession No. U51278,
Yang et al., Immunity 7(5):629-639, 1997; GenBank Accession No. X83574; and
GenBank Accession No L35049); and the rat Bcl-xL gene (GenBank Accession No.
U34963; Tilly et al., Eudocrihology 136(1): 232-241, 1995).
Similarly, the Bcl-2 gene may be from a human (GenBank Accession No.
M14745; Cleary et al., Cell 47(1): 19-28, 1986). Other non-limiting anti-
apoptosis Bcl-
2 genes of the invention include the rat Bcl-2 gene (GenBank Accession No.
U34964;
Tilly et al., Endocrinology 136(1): 232-241, 1995); the bovine Bcl-2 gene
(GenBank
Accession No. U92434); the chicken Bcl-2 gene (GenBank Accession No. Zl 1961;
Cazals-Hatem et al., Biochim. Biophys. Acta 1132(1): 109-113, 1992); and
murine Bcl-
2 gene (GenBanlc Accession Nos. NM 009741, M16506, and L31532; Negrini, Cell
49(4): 455-463, 1987).
In the field of biopharmaceuticals, there are several benefits for delaying
the
death phase of cultured cells. One such benefit is the opportunity to harvest
the product
while cell viability is still high thereby reducing the exposure of the
product to debris
and degradative enzymes produced by cell lysis. Other benefits include
reduction in
expensive bioreactor runs as a consequence of higher titers, better
performance in scale
up, simplified down stream processes, and improved cost effectiveness.
In addition, the invention allows the generation of cell lines that may be
robust
in either chemically defined medium (CDM) or PFM protein free medium (PFM),
which, although useful for purifying proteins produced by the cells, are
disfavored
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-9-
since cells grown in protein free media or chemically defined media are highly
susceptible to apoptosis. Use of a cell line that is more robust in such media
would be
highly favorable as this would have an impact on the cost of media, thus
eliminating the
more expensive (and regulation strict) media components in current
formulations.
Accordingly, in one aspect, the invention provides a method for increasing
production of a protein by a cell, comprising increasing expression of an anti-
apoptosis
gene in the cell. In some embodiments, wherein the cell does not express a
heterologous cyclin-dependent kinase inhibitor. In particular embodiments, the
cell is a
human cell, a murine cell, a hamster cell, an insect cell, or an amphibian
cell.
In another aspect, the invention provides a method for increasing the
production
of a heterologous protein by a cell, comprising increasing expression of an
anti-
apoptosis gene in the cell. In some embodiments, wherein the cell does not
express a
heterologous cyclin-dependent kinase inhibitor.
Note that as used herein, the term "cell" encompasses all eukaryotic cells
including, without limitation, cells from mammals (e.g., human or mouse),
insect,
amphibian (e.g., ~ehopus laevis), and birds. Non-limiting examples of cells
for use in
the invention include CHO cells, NSO cells, BHK cells, NIH-3G3 cells, HEK-293
cells, COS cells, CV1 cells, HeLa cells, Jurkat cells, Raji cells, Daudi
cells, S~ cells,
and A549 cells (all of which are commercially available from the American Type
Culture Collection (ATCC), Manassas, VA).
As used herein, by "increasing the expression" is meant that the expression
level of an anti-apoptosis gene in a cell is increased as compared to the
expression level
in the starting cell. For example, where the cell in which the expression of
an anti-
apoptosis gene does not express any protein encoded by the anti-apoptosis gene
(e.g.,
see the parent CHO DG44 cells described below), any expression of a protein
encoded
by the anti-apoptosis gene is increasing the expression of that anti-apoptosis
gene.
Where, however, the parent cell naturally expresses some level of protein
encoded by
the anti-apoptosis gene, "increasing the expression" of the apoptosis gene
results in an
increased level of protein as compared to the level expressed by the parent
cell.
As used herein, by "expressing" or "expression" is meant that the anti-
apoptosis
gene is transcribed and/or translated in the cell to produce a protein. For
example,
where the human Bcl-xL gene is expressed in a murine cell, that murine cell
produces
human Bcl-xL protein. Of course, it will be understood that when a cell, in
accordance
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-10-
with the invention, is induced to increase production of a protein by
increasing the
expression of an anti-apoptosis gene in that cell, the protein the cell is
increasing
production of is not encoded by the anti-apoptosis gene. For example, if the
native Bcl-
xL gene is expressed in a marine cell, then, in accordance with the invention,
the marine
cell that expresses an increased level of its native marine Bcl-xL protein
also increases
production of a non-Bcl-xL protein.
Thus, in accordance with the invention, where a cell of the invention
increases
production of a protein of interest, that protein can be any protein except
for the protein
encoded by the anti-apoptosis gene expressed in that cell. The protein can be
a secreted
protein, a transmembrane protein, or an intracellular protein. Thus, non-
limiting
examples of proteins include antibodies, hormones (e.g., follicle-stimulating
hormone),
insulin, nuclear proteins, ribosomal proteins, erythropoietin, cytokines
(e.g.,
interleukin-2 or (3-interferon), and blood factors (e.g., Factor IX). The
protein can be a
native protein to the cell, or can be a heterologous protein to the cell.
As used herein, by the term, "native protein" is meant a protein encoded by a
nucleic acid molecule that naturally occurs in the cell. Thus, if the cell is
a human cell,
a human protein is one that is native to that cell.
As used herein, by the term, "heterologous protein" is meant a protein that is
not
encoded by a nucleic acid molecule that naturally occurs in the cell. For
example, if the
cell is a marine cell, a humanized marine antibody is one that is heterologous
to that
cell. One non-limiting example of a heterologous protein of the invention is
the AQC2
antibody, which specifically binds to the cell surface protein, VLA-1 (e.g.,
human
VLA-1). The AQC2 antibody preferably comprises the same heavy and/or light
chain
sequences as the antibody produced by one of the following hybridoma cell
lines, all of
which have been deposited with the American Type Culture Collection (Manassas,
Virginia, USA) in accordance with the Budapest Treaty: mAQC2 (ATCC Accession
No. PTA3273, deposited April 18, 2001); hAQC2 (ATCC Accession No. PTA3275,
deposited April 18, 2001); haAQC2 (ATCC Accession No. PTA3356, deposited May
4, 2001); and hsAQC2 (ATCC Accession No. PTA3274, deposited April 18, 2001).
These antibodies are described in PCT Publication No. W002/083854.
Note that the invention encompasses the increased production of an antibody by
a cell, including a hybridoma cell. For example, if a hybridoma cell comprises
a
nucleic acid molecule encoding a particular monoclonal antibody, improved
production
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-11-
of that monoclonal antibody by that cell can be achieved, in accordance with
the
invention, by expression of an anti-apoptosis gene in that hybridoma cell.
In a non-limiting example, a human B cell is identified as containing nucleic
acid that encodes a particular antibody. This B cell is immortalized according
to
standard methods known in the art (e.g., infection with Epstein Barr virus).
Next, to
improve the production of the antibody by the now-immortalized B cell, an anti-
apoptosis gene can be expressed in the B cell. For example, an expression
plasmid
encoding an anti-apoptosis gene can be introduced into the cell. Such plasmids
are
described in the Examples below. Alternatively, the protein encoded by the B
cell's
own anti-apoptosis gene can be upregulated, such that the native protein is
expressed in
the cell, thereby resulting in increased production of the antibody.
In accordance with the invention, the cell expressing an anti-apoptosis gene
does not express a heterologous cyclin-dependent kinase inhibitor (i.e., a
cyclin-
dependent kinase inhibitor encoded by a nucleic acid molecule that does not
naturally
occur in the cell). In certain embodiments, the heterologous cylin-dependent
kinase
inhibitor is p27. In certain embodiments, the heterologous cylin-dependent
kinase
inhibitor is p21.
In accordance with the invention, two non-limiting different approaches can be
taken for increasing production of a protein. In one approach, a new cell line
is
generated which has increased expression of an anti-apoptosis gene. As
described
below, one such non-limiting cell line, Chinese Hamster Ovary cells, was
generated.
The anti-apoptosis human gene, Bcl-xL, was expressed in these cells,
generating a stable
Bcl-xL expressing cell line.
In another non-limiting approach to generate such a cell, the anti-apoptosis
gene
can also be turned on in a cell that has the gene, but does not express a
protein encoded
by the gene. For example, a human cell comprises a human Bcl-xL gene, but that
does
not express human Bcl-xL, can be induced to express human Bcl-xL. Such a human
cell
is included within the scope of the invention.
Once such an anti-apoptosis gene expressing cell line is established, it is
ready
to produce increased amounts of a protein, regardless of whether that protein
is
heterologous to the cell or native to the cell. For example, the Bcl-xL
expressing CHO
cell may be used to produce increased amounts of a hamster protein.
Alternatively, a
nucleic acid molecule encoding a heterologous protein (e.g., encoding human (3-
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-12-
interferon) may be introduced (e.g., by transfection, infection, or
transformation) into
the Bcl-xL expressing CHO cell, where more heterologous protein is produced by
the
Bcl-xL expressing CHO cell as compared to a CHO cell not expressing Bcl-xL.
Accordingly, in a further aspect, the invention provides a cell comprising
increased expression of an anti-apoptosis gene and that does not express a
heterologous
cyclin dependent kinase inhibitor, wherein the cell produces an increased
amount of a
protein as compared to a cell that does comprise increased expression the anti-
apoptosis
gene.
In a second approach of the invention, expression of an anti-apoptosis gene is
increased in a cell already producing a protein of interest. In one non-
limiting example
of the invention, a murine Bcl xL gene may be expressed in a human (3-islet
cell (i. e.,
that already produces human insulin), such that the cell produces more
insulin. In
another non-limiting example, an anti-apoptosis gene may be expressed in a
cell
already expressing a heterologous protein (e.g., a CHO cell expressing human
(3-
interferon), such that the cell having increased expression of an anti-
apoptosis gene
produces more heterologous protein than the cell that does not have an
increased
expression of the anti-apoptosis gene.
Thus, in a further aspect, the invention provides a cell comprising increased
expression of an anti-apoptosis gene and a gene encoding a protein of
interest, and does
not express a heterologous cyclin-dependent kinase inhibitor, wherein the cell
produced
an increased amount of a protein of interest as compared to a cell that does
not
comprise increased expression of the anti-apoptosis gene.
The following examples are intended to further illustrate certain preferred
embodiments of the invention and are not limiting in nature. Those skilled in
the art
will recognize, or be able to ascertain, using no more than routine
experimentation,
numerous equivalents to the specific substances and procedures described
herein.
Example I
Generation of an Enhanced CHO Cell Host
To generate a CHO host with improved growth characteristics, which may be
potentially used for improved expression of heterologous proteins, Bcl-xL was
expressed in CHO cells.
To do this, the Bcl xL gene was isolated by using oligonucleotides designed to
anneal to the 5' and 3' ends of the open reading frame (ORF) based on the
sequence of
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-13-
Bcl-xL provided in Boise L.H. et al., Cell 74: 597-608: 1993 (also see GenBank
Accession No. 223115). The sequences of the oligonucleotides used are as
follows:
5' PCRprimer 5'-GCCCTCGAGATGTCTCAGAGCAACCGG-3' (SEQ ID NO: 1),
where the italicized sequence is an added linker region with an ~hoI site;
and 3'PCR primer 5'-GCCTCTAGATCATTTCCGACTGAAGAGTG -3'(SEQ ID
NO: 2), where the italicized sequence is an added linker region with an XbbaI
site
The Bcl xL gene was generated using the polymerase chain reaction (PCR; using
PfuTurbo DNA polymerase, Cat# 600250, commercially available from Stratagene)
from Human Brain, whole Marathon-Ready cDNA (Clontech Laboratories, Palo
Alto, CA). The expression vector, expression vector pcDNA3.1 (+) (commercially
available form Promega, Madison, WI), was digested with XhoI and XbaI, and the
Bcl-
xL PCR fragment was ligated into the linearized vector. This resulted in the
plasmid
pBcl-xL-neo schematically depicted in Fig. 1A.
The pBcl-xL-neo plasmid was used to transfect CHO-DG44 host cells using
electroporation according to standard techniques (see, e.g., Ausubel et al.,
Current
Protocols in Molecular Biology, John Wiley & Sons Inc., New York City, NY,
1993).
Note that CHO cells are commercially available from the ATCC. CHO-DG44 cells
as
described in Urlaub, et al., Cell 33:405-412, 1983. As a control, the empty
pcDNA3.1 (+) vector was also transfected into CHO cells.
After electroporation, the cells were grown for forty-eight hours in 6418-free
media, and then selected in the presence of 400 ug/ml 6418 (neomycin). The
living,
adherent cells were selected while the dead, non-adherent cells were removed
when the
media was changed. After approximately two weeks, stable isolates were
selected.
Example II
Improved Growth of the Enhanced CHO Cell Host
The cell death kinetics of these Bcl-xL transfected cells described in Example
I
were next compared the to the original unmodified host cells.
To do this, ten DG44/ Bcl-xL out of fifty isolates, the CHO cell transfected
with
empty vector were cultured in serum free media supplemented with 6418
alongside the
untransfected host CHO cell cultured typically in the absence of 6418. The
cells were
cultured for ten days, and were counted daily. For a fed batch mode, cultures
were fed
every other day with one fiftieth volume of a solution of substrates, without
removal of
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-14-
culture suspension. As shown in Fig. 2A (viable cell density) and Fig. 2B
(percentage
viability), both controls (i.e., the untransfected DG44 host and the DG44
transfected
with empty vector) exhibited maximum VCD on day 4 at approximately 2.6 x
106/ml.
In comparison, DG44/ Bcl-xL clones #2, 3, 8, and 9 reached peak VCD on day 5
ranging between 3.0 x 106/ml to 3.9 x 106/ml, and clone #5 reached a peak of
3.1 x
106/ml on day 6. Percentage viabilities for clones #2, 3, and 5 remained high
on day 6
at 95, 96, and 87% respectively, whereas viabilities for DG44 (i.e.,
untransfected host)
and DG44 vector alone (i.e., host cells transfected with empty vector)
controls had
fallen to 59 and 54% respectively. By day 8, viabilities for clones #2, 3, and
5 were at
76, 79, and 81% respectively, whereas viabilities for DG44/neo (i.e., host
cells
transfected with empty vector) and DG44 controls (i. e., untransfected host)
were at 46
and 14% respectively.
The integral cell area (ICA) is defined as the area under a growth profile
curve
representing the total number of live cells during the course of a culture
run. An
estimate of the ICA (based on the viable cell density data) on day 8 indicated
that
isolate #2, 3, 5, and 8 had an increased ICA of 38, 51, 52, and 51%,
respectively, over
the vector control (i. e., host cells transfected with empty vector).
Stability of the
previously observed enhanced viability over control cultures for isolates #2,
3, 5, and 8
was repeated and consistent to at least ten passages, (see data for isolate #8
in Figs. 5
and 6). On average the increase in ICA of DG44/ Bcl-xL cells over the vector
only
control was 40 to 75%, and was 30 to 100% over the untransfected DG44 host
control.
Both an increased peak cell density and prolonged cell viability contributed
towards the
enhanced ICA.
Next, the cells were cultured in the absence of 6418 to determine if the
presence or absence of 6418 had any effect on the growth of the Bcl-xL
transfected
cells. To do this, DG44/ Bcl-xL isolates and control transfected with empty
vector
alongside the untransfected control, were cultured in the absence of 6418.
Again,
growth curves representing viable cell density (VCD) over time and percentage
viability (% viability) over time were monitored.
As shown in Fig. 3A, DG44/ Bcl-xL clone #3 (one exemplary DG44/ Bcl-xL
isolate) maintained both a higher and prolonged peak cell density of 4 x 106
cells/ml up
to day 8. Moreover, percent viability was at 90% on day 10 compared to 25% in
the
vector control (Fig. 3B), and the ICA (approximately 30 x 106 cells/ml for a
11-12 day
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-15-
run) was up to three fold higher than the DG44 host. This characteristic was
stable up
to at least 7 passages for isolates #2, 3, and 5, but not for #8 (see below).
Example III
Further Characterization of the Enhanced CHO Cell Host
One way to detect and quantify apoptosis is by measurement of caspase-3
proteolytic activity in sample lysates. Caspase-3 is one caspase that plays a
critical role
in the execution of apoptosis by proteolytic disassembly of cells. Given the
known
ability of Bcl-xL to inhibit apoptosis, the CHO cells transfected with Bcl-xL
were next
tested for caspase activity using a caspase-3 assay.
Caspase proteins cleave proteins after aspartic acid. It is known that the 3
or 4
amino acids prior to aspartic acid confer specificity. This allows the use of
four amino
acid labeled peptides to be used as substrates for caspases. For the caspase
assay, the
peptide substrate used had the amino acid sequence DEVD, with the D (i. e.,
the aspartic
acid residues) labeled with a fluorimetric marker AMC (cat #P-411, BIOMOL
Research Labs, Inc., Plymouth Meeting, PA). The marker fluoresces once
cleavage has
occurred. Thus, without cleavage, little or no signal was observed.
Caspase-3 proteolytic activity was determined from lysates of CHO cells
cultured as described in Example II daily for twelve days. Fluorescence of AMC
from
samples compared to samples treated with non-cleavable analogue DEVD-CHO
(BIOMOL cat#P-410), allowed determination of the increase in caspase-3
activity.
DG44/ Bcl-xL #3 (a non-limiting Bcl-xL-transfected CHO cell generated
according to Examples I and II) showed a delayed onset of peak caspase-3
proteolytic
activity as compared to empty vector control cells (i.e., DG44 CHO cells
transfected
with empty vector) and DG44 CHO control cells (i. e., untransfected cells). As
shown
in Fig. 4, onset of peak caspase-3 proteolytic activity in DG44/ Bcl-xL #3
occurred on
day 1 l, with minimal activity exhibited on other days. In contrast the DG44
host alone
exhibited over two fold higher peak activity as early as day 5, while the
DG44/vector
alone control showed close to peak activity starting on day 8 (see Fig. 4).
These results
demonstrated that genetic manipulation of the host cell line with an anti-
apoptosis gene
leads to prolonged cell viability.
Next, Western blotting analysis was performed to determine whether or not Bcl-
xL was in fact expressed in DG44/ Bcl-xL #3. The presence of Bcl-xL was also
assessed
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-16-
in DG44/ Bcl-xL #8, a 6418 resistant DG44 CHO clone generated at the same time
as
DG44/ Bcl-xL #3. To do this, Western blotting was performed according to
standard
methods (see, e.g., Ausubel et al., supYa). Briefly, cells lysates were
resolved by SDS-
Page, and transferred to nitrocellulose or PVDF membranes. The membranes were
blotted using a mouse monoclonal antibody that specifically binds to human Bcl-
xL
(Clone 2H12, commercially available from Oncogene Research Sciences, San
Diego,
CA).
As shown in Fig. 5, left lane, Bcl-xL was clearly expressed in DG44/ Bcl-xL
#3.
However, no Bcl-xL was expressed by either DG44/ Bcl-xL #8 (middle lane, Fig.
5) or
DG44 CHO cells transfected with empty vector (right lane, Fig. 5). The results
were
the same from isolates grown either in the presence or absence of 6418
selection.
Indeed, when DG44/ Bcl-xL #8 was grown in the absence of 6418 for selection,
it showed no enhanced survival as compared to untransfected DG44 CHO control
cells. To do this, DG44/ Bcl-xL #8 was released from 6418 selection and
evaluated
against DG44/ Bcl-xL #3 and the DG44 host control. Growth curves representing
viable cell densities (VCD) over time and the percentage viabilities (%
viability) were
assessed as described in Example I. Although DG44/ Bcl-xL #8 was 6418
resistant
with an improved ICA of approximately 50%, when the same cells were cultured
in the
absence of selection, the increase in ICA was not significant (see Fig. 6A).
Moreover,
the percent viability over time was not improved and in fact fared worse than
the
control (see Fig. 6B).
These results demonstrate a correlation between the undetectable Bcl-xL
expression and lack of enhancement in ICA in DG44/ Bcl-xL #8, as compared to
the
other isolates such as DG44/ Bcl-xL #3 in which expression was clearly
detected. The
results also demonstrate that Bcl-xL expression is linked to enhanced growth
and
viability. The observed increase in ICA for DG44/ Bcl-xL #8 under selective
conditions would indicate that the process of 6418 enrichment generates cells
expressing the neomycin gene (but not always the Bcl-xL gene) with additional
robust
growth for survival in the presence of 6418.
Example IV
Generation of a Bcl-xn Transfected Cell Line Secreting a Heterolo~ous Protein
The above Examples established the feasibility of generating a more robust
CHO host with prolonged cell viability through delay of cell death by
expressing the
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
_1'/_
anti-apoptosis gene, Bcl-xL, in the cells. To further expand the application
of Bcl-xL,
the next goal was to transfect an established CHO-DG44 cell line expressing a
heterologous protein with the Bcl-xL gene and examine the Bcl-xL transfected
cells for
an increased production of the heterologous protein arising from expected
prolonged
viability.
To do this, a second construct was generated as described above in Example I,
but with the zeocin resistance gene. Briefly, the Bcl-xL PCR fragment (see
Example I)
was cloned into the XhoI and XbaI sites of expression vector pcDNA3.1 /Zeo (+)
(Promega, Madison, WI), where expression is driven by the CMV immediate-early
promoter and the zeocin gene provides selection marker, to yield final plasmid
pBcl-xL-
zeo. A schematic representation of this plasmid is depicted in Fig. 7A.
The pBcl-xL-zeo plasmid was used to transfect (by electroporation) the cell
line
100AB-37, which is a DG44 CHO cell previously transfected with a nucleic acid
molecule encoding the monoclonal antibody, AQC2. The 100AB-37 parent secretes
the AQC2 monoclonal antibody with a specific productivity (s.p.) of 10 pg cell
-1 day 1.
The 100AB-37 cells transfected with the Bcl-xL-zeo plasmid were cultured in
the presence of 600 ug/ml zeocin. Next, the pool of transfectants cultured in
the
absence of fetal bovine serum (FBS) and in the presence of selective zeocin
was
analysed for AQC2 secretion. Flow cytometry analysis was performed using a
conjugated antibody against AQC2. As shown in Fig. 7B, AQC2 secreted from Bcl-
xL
transfectants (histogram in bold black) was compared to that of the
untransfected parent
(grey) and control (black). The results shown in Fig. 7B demonstrated that the
ability
to express and secrete AQC2 was not suppressed by the presence of Bcl-xL.
Titer
analysis by ELISA of conditioned media sampled from the cells above, confirm
the
flow cytometric data. In fact productivity was higher for Bcl-xL-transfected
cells, at 150
p,g/ml for the 100AB-37/ Bcl-xL pool (2.24 x 106/ml cells) compared to 105
p.g/ml for
the non-modified parent line (2.1 x106 cells/ml).
Next, individual isolates of Bcl-xL transfected 100AB-37 cells were generated,
screened for secretion of AQC2, and ranked according to AQC2 titer (i.e., the
amount
of AQC2 antibody secreted by an isolate). Eight 100AB-37/ Bcl-xL isolates
expressing
the highest titer were released from zeocin selection and cultured further for
stability
before examination for growth and titer (see below) in spinner flasks. As
described in
Example I, growth curves and % viabilities were monitored as parameters of
cell death
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-18-
kinetics, compared to the parent control, over a period of 14 days in a fed
batch mode
(as described above). As shown in Fig. 8A, on day 6 the % viability of the
parent line
was at 95%, which steadily decreased to 62% on day 13, whereas the top three
Bcl-xL
isolates (#11, 21, and 25) maintained high % cell viability ranging from 84%
to 96%.
Interestingly, the sustained higher % viabilities did not result in a
significant increase in
ICA over the unmodified parent since the overall cell density was not
increased (Fig.
8B). Only Bcl-xL clone #25 showed a moderate 20% increase in ICA (see Fig.
8B).
To assess whether productivity was improved since viability was sustained, the
titer of the secreted AQC2 was assayed by protein A-HPLC binding on the eight
1 OOAB-37/ Bcl-xL isolates evaluated as described above. As many as five out
of the
eight isolates examined had improved titer ranging from 306 to 434 ~.g/ml (see
below)
compared to the parent control of 236 ~g/ml on day 12 of culture. As shown in
Fig. 9,
protein A titer data from clones 11, 21, and 25 shown previously to maintain
higher
viabilities (see Figs. 8A and 8B) indicated significant enhancement in
productivity.
Protein A titer data on day 14 was as shown below in Table I.
Table I
Cell Line Day 14 % increase % increaseSpecific
AQC2 in in viabilityactivity
(ug/ml) throughput pg/cell/day
100AB-37/ Bcl-xL 368 28 84 14
#11
100AB-37/ Bcl-xL 522 91 95 19
#21
100AB-37/ Bcl-xL 441 53 90 12
#25
100AB-37 parent 289 63 9
(Note that throughput is g/L/day (i. e., the total titer divided by the number
of days
needed to get to that titer plus two additional days (for bioreactor
turnaround time).
Thus, percent increase in titer is equivalent to percent increase in
throughput).
As Table I demonstrates, 100AB-37/ Bcl-xL isolates 1 l, 21, and 25 (%
viability
above 84%) produced titers of 368, 441, and 522 ug/ml respectively compared to
288
ug/ml from the parent (% viability 62%). The increase in throughput (i.e.,
titer) of
clones ranged from 28% for isolate #11 to as high as 81 % for top isolate #21.
The
specific productivity was also enhanced in the 100AB-37/ Bcl-xL isolates (12
to 14 pg
cell-lday-1 compared to 9 pg cell-lday 1).
To assess the validity of the previous results demonstrating the marked
increase
in titer, the evaluation was repeated under the controlled environment of 2 L
scale
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-ly-
bioreactors. Bioreactors of this size are typically used to model 200 L
manufacture-
scale bioreactors. The most desirable 100AB-371 Bcl-xL isolate, #21, was run
in
duplicate reactors along side the 100AB-37 parent. Growth curves and %
viabilities
were monitored as parameters of cell death kinetics compared to the parent
control over
a period of 13 days in cltures run in fed-batch mode. Bcl-xL containing cells
were still
high in % viabilities at 84 to 89% on day 13, whereas the parent cell line
viability had
already decreased to 60 and 66% on day 13 (Fig. l OB). As predicted from
previous
spinner data (see Figs. 8A and 8B), the sustained higher % viabilities did not
result in a
significant increase in ICA over the unmodified parent since the overall cell
density
was not increased (FIG. l0A).
Moreover, consistent with small scale spinner data (see Fig. 9), 100AB-37.21/
Bcl-xL isolate #21 also produced significantly higher titers and up to 60%
increase in
throughput grown in 2L bioreactors, as determined by protein A binding (Fig.
11 ).
Protein A titer data on day 14 was as shown below in Table II.
Table II
Cell Line Day 14 % increase % increaseSpecific activity
in
AQC2 throughput in viabilitypg/cell/day
(ug/ml)
100AB-37.21/ Bcl-xL597 62 89 21
isolate #21, run
1
100AB-37.21/ Bcl-xL585 59 84 23
isolate #21. run
2
100AB-37 parent, 365 66 12
runl
100AB-37 parent, 371 60 11
runl
Protein A results indicated that 100AB-37.21/ Bcl-xL isolate #21 yielded 591
mg/L on
day 13 as opposed to only 368 mg/L for the parent line, representing 60%
increase in
titer and throughput (Fig. 11 and Table II). Significant increase in titer was
clearly
evident starting on day 7 and 9 and continued throughout the run (see Fig.
11). As
previously observed, the ICA from day 0 to 13 was not significantly different;
in
addition the specific activity was doubled from 11.5 to 22 pg cell-1 day 1
(Table II).
Furthermore Westerns (non-reduced and reduced SDS-PAGE analysis) and
carbohydrate analysis of the AQC2 mAB product from these bioreactor runs
indicated
no change in product quality.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-20-
Example V
Further Characterization of the Bcl-xT. Transfected Cell Line
Secreting a Heterolo~ous Protein
Since 100AB-37.21/ Bcl-xL isolate, #21 was not verified as being clonal, both
this isolate along with OBcI-xL 100AB-37 were further subcloried, with the
~Bcl-xL
100AB-37 isolate being a subclone of the untransfected parent 100AB-37 cell
line.
This was done because the comparison of the most desirable subclone from each
cell
line would provide a more strict comparison between Bcl-xL and OBcI-xL cell
lines. To
do this, an equivalent number of subclones was screened for each cell line.
Specific
activity was determined for the selected subclones generated and ranked
according to
superior growth and ability to produce high titers (data not shown). The lead
subclone
of Bcl-xL isolate #21, namely 21.15, was then evaluated and compared to lead
subclone
37.32 of the umnodified parent 100AB-37 in spinners vessels. The growth media
selected for the evaluation was a chemically defined growth media (CDM) that
had few
animal derived components. CDM is highly desirable for large scale
manufacturing
due to the elimination of undefined components, reduction in raw material
variability,
reduction in complexity of downstream processes and elimination of potential
contaminants of animal origin (Jayme and Smith, Cytotechfzology 33: 27-36,
2000).
Moreover, the CDM environment with markedly reduced protein content is more
likely
to predispose cells to apoptosis (Moore A. et al., Cytotechrcology 17:1-11,
1995 and
Zhangi et al., Biotech Bioehg. 64:108-119, 1999). Thus the presence of Bcl-xL
expression may provide a cell line that maintains robustness even under such
media
conditions.
Growth curves and percent viabilities were monitored as parameters of cell
death kinetics compared to the parent control over a period of 14 days in
cultures run in
fed-batch mode (Fig. 12A). However, as shown in Fig. 12B, 21.15 Bcl-xL cells
sustained high viability well, at above 90% throughout the length of the
culture to day
14, whereas cell death occurred in 37.32 ~Bcl-xL subclone on day 9 (72%
viability) and
dropped considerably to 43% by day 14. These results demonstrated that Bcl-xL
overexpression delays cell death under media conditions in which cells are
particularly
susceptible to apoptosis. As expected with previous observations, there was no
significant difference in ICA.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-21-
Next, to assess the cells' productivity, the titer of the secreted AQC2 was
assayed by protein A-HPLC binding. As shown in Fig. 13, lead subclone 100AB-
37/21.15 Bcl-xL produced dramatically higher titers and up to 89% increase in
throughput (i.e., titer) compared to lead subclone 37.32 OBcI-xL, even when
cultured in
chemically defined growth media (CDM).
Protein A titer data on day 14 was as shown below in Table III.
Table III
Cell Line Day 14 AQC2% increase % increase Specific
in in
(ug/ml) throughput viability activity
pg/cell/day
21.15 Bcl-xL667 89 93 22
37.32 OBcI-xL353 43 12
As Table III demonstrates, Protein A results of samples taken from cultures
described in Figs.l2A and 12B indicated that Bcl-xL 21.15 yielded 667 ~.glml
on day
14 as opposed to only 341 ~g/ml for the lead subclone of the parent, clone
37.32. This
represented as much as 89% increase in throughput when 21.15 cells were still
high in
percent viability at 93%, compared to 37.32 cells which were low at 43% (Fig.
13 and
Table III). Fuuthermore the specific productivity was almost doubled from 12
to 22 pg
cell-lday 1 (see Table III). Thus, lead subclone 100AB-37/21.15 Bcl-xL
produced
dramatically higher titers and up to 89% increase in throughput compared to
lead
subclone 37.32 ~ Bcl-xL, even when cultured in chemically defined growth media
(CDM).
To address whether over-expression of Bcl-xL was responsible for the observed
enhancement in titer, assays including the detection and quantitation of Bcl-
xL
expression and caspase-3 activity were conducted. Both flow cytometric and
Western
analysis demonstrated an increase in Bcl-xL expression from day 3 to a
constant level
on day 5 in 100AB-37121.15 Bcl-xL cells (data not shown). Next, the caspase
activity
in the 100AB-37/21.15 Bcl-xL and 37.32 OBcI-xL was assessed. A caspase-3 assay
on
the cells cultured as described in Fig. 13 (caspase-3 assay performed as
described
above).
The results showed caspase-3 was dramatically suppressed to control levels
throughout the production run of clone 21.15 Bcl-xL, whereas activity in 37.32
increased almost 10 fold of that of 21.15 on day 14 (see Fig. 14). That the
Bcl-xL
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-22-
expressing 21.15 Bcl-xL cells exhibited minimal caspase-3 activity throughout
the
production run in CDM, demonstrated active suppression of apoptosis. The data
clearly indicated significant delay in apoptosis in a cell line that
overexpresses Bcl-xL.
Example VI
Increased Productivity and Viability of Bcl-xr. Transfected Cell Line
Secreting a
Heterologous Protein Cultured in Media Containing Sodium Butyrate
Sodium butyrate (NaBu) is commonly used as an attempt to enhance specific
heterologous protein expression by augmenting transcription (Chang et al.,
Free Rad.
Es. 30:85-91, 1999; Palermo et al., J. Biotech. 19:35-47, 1991; and Laubach,
V.E. et
al., Biochem. Biophy. Res. Comnzun. 218:802-807, 1996). However, a serious
drawback to the high concentrations of NaBu required for increased expression,
is the
negative competing effect of rapid induction of apoptosis known to occur in
CHO cells
(Chang et al., Ff°ee Rad Es 30:85-91, 1999; and Kim and Lee, Biotech.
Bioeyzg. 71:184-
193, 2000-2001). For this reason NaBu is not universally effective in
enhancing titer in
all cell lines.
To further analyze the ability of Bcl-xL to protect cells from premature
apoptosis, cells were evaluated up to 4 days with and without 2mM NaBu in
shaker
flasks cultured in CDM suspension culture. As shown in Fig. 15, additive Na Bu
did
indeed increase AQG2 productivity of both parent 100AB-37 and the #21 Bcl-xL
expressing isolate, as well as the respective subclones 37.320Bc1-xL, and
21.15.
Moreover, under the conditions described above by day 4, although NaBu
increases titer in the parent and 37.320Bc1-xL subclone, the percent viability
predictably dropped significantly to 41 % and 46% respectively (see Fig. 16).
In
contrast, % viability for #21 Bcl-xL remained high at above 80% viability in
Bcl-xL
21.15 cells remained unchanged at 96% (see Fig. 16). These percent viability
results
demonstrate active suppression of NaBu-induced apoptosis by expression of Bcl-
xL in
#21 Bcl-xL isolate and subclone 21.15 Bcl-xL.
At day 3 of culture (i. e., in shaker flasks in CDM suspension culture),
caspase 3
activity, a marker for apoptosis, was measured in the cells. As shown in Fig.
17, a
significant delay in apoptosis was observed in cell lines overexpresing Bcl-
xL. Caspase
activity in #21 and 21.15 Bcl-xL cells was not completely suppressed in the
presence of
2 mM butyrate, but was significantly diminished compared to 100AB-37 and 37.32
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
-Z3-
~Bcl-xL, which exhibited 4 fold greater activity (Fig. 17). These results show
a
positive correlation between Bcl-xL expression and an active delay of
apoptosis
chemically induced by NaBu.
EQUIVALENTS
Those skilled in the art will recognize, or be able to ascertain, using no
more
than routine experimentation, numerous equivalents to the specific embodiments
described specifically herein. Such equivalents are intended to be encompassed
in the
scope of the following claims.
CA 02491212 2004-12-24
WO 2004/003151 PCT/US2003/020207
A167SEQLIST.TXT
SEQUENCE LISTING
<110> BIOGEN, INC.
CHIANG, Gisela
slsK, William
<120> Protein Production Methods and Modified
cells for use Therein
<130> A167 PCT
<150> 60/391,738
<151> 2002-06-26
<150> 60/440,498
<151> 2003-01-16
<160> 2
<170> FastsEQ for Windows Version 4.0
<210> 1
<211> 27
<212> DNA
<213> Artificial sequence
<220>
<223> synthesized Primer
<400> 1
gccctcgaga tgtctcagag caaccgg 27
<210> 2
<211> 29
<212> DNA
<213> Artificial sequence
<220>
<223> Synthesized Primer
<400> 2
gcctctagat catttccgac tgaagagtg 29
Page 1