Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _5
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME OF
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02521464 2011-06-30
51912-8
iRNA CONJUGATES
TECHNICAL FIELD
The invention relates to RNAi and related methods, e.g., methods of
making and using iRNA agents. It includes methods and compositions for
silencing
genes expressed in the liver, and methods and compositions for directing iRNA
agents to the liver.
BACKGROUND
RNA interference or "RNAi" is a term initially coined by Fire and
co-workers to describe the observation that double-stranded RNA (dsRNA) can
block
gene expression when it is introduced into worms (Fire et al., Nature 391:806-
811,
1998). Short dsRNA directs gene-specific, post-transcriptional silencing in
many
organisms, including vertebrates, and has provided a new tool for studying
gene
function. RNAi may involve mRNA degradation.
1
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Work in this field is typified by comparatively cumbersome approaches to
delivery of
dsRNA to live mammals. E.g., McCaffrey et al. (Nature 418:38-39, 2002)
demonstrated the use
of dsRNA to inhibit the expression of a luciferase reporter gene in mice. The
dsRNAs were
administered by the method of hydrodynamic tail vein injections (in addition,
inhibition
appeared to depend on the injection of greater than 2 mg/kg dsRNA). The
inventors have
discovered, inter alia, that the unwieldy methods typical of some reported
work are not needed
to provide effective amounts of dsRNA to mammals and in particular not needed
to provide
therapeutic amounts of dsRNA to human subjects. The advantages of the current
invention
include practical, uncomplicated methods of administration and therapeutic
applications, e.g., at
1 o dosages of less than 2 mg/kg.
SUMMARY
Aspects of the invention relate to compositions and methods for silencing
genes
expressed in the liver, e.g., to treat disorders of or related to the liver.
An iRNA agent
composition of the invention can be one which has been modified to alter
distribution in favor of
the liver. A composition of the invention includes an iRNA agent, e.g., an
iRNA agent or sRNA
agent described herein.
In one aspect, the invention features a method for reducing apoB-100 levels in
a subject,
e.g., a mammal, such as a human. The method includes administering to a
subject an iRNA agent
which targets apoB-100. The iRNA agent can be one described here, and can be a
dsRNA that is
substantially identical to a region of the apoB-100 gene. The iRNA can be less
than 30
nucleotides in length, e.g., 21-23 nucleotides. Preferably, the iRNA is 21
nucleotides in length.
In one embodiment, the iRNA is 21 nucleotides in length, and the duplex region
of the iRNA is
19 nucleotides. In another embodiment, the iRNA is greater than 30 nucleotides
in length.
In a preferred embodiment, the subject is treated with an iRNA agent which
targets one
of the sequences listed in Tables 9 or 10. Ina preferred embodiment it targets
both sequences of
a palindromic pair provided in Tables 9 or 10. The most preferred targets are
listed in
descending order of preferrability, in other words, the more preferred targets
are listed earlier in
Tables 9 or 10.
2
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the iRNA agent will include regions, or strands,
which are
complementary to a pair in Tables 9 or 10. In a preferred embodiment the iRNA
agent will
include regions complementary to the palindromic pairs of Tables 9 or 10 as a
duplex region.
In a preferred embodiment the duplex region of the iRNA agent will target a
sequence
listed in Tables 9 or 10 but will not be perfectly complementary with the
target sequence, e.g., it
will not be complementary at at least 1 base pair. Preferably it will have no
more than 1, 2, 3, 4,
or 5 bases, in total, or per strand, which do not hybridize with the target
sequence.
The iRNA agent that targets apoB-100 can be administered in an amount
sufficient to
reduce expression of apoB-100 mRNA. In one embodiment, the iRNA agent is
administered in
an amount sufficient to reduce expression of apoB-100 protein (e.g., by at
least 2%, 4%, 6%,
10%, 15%, 20%). Preferably, the iRNA agent does not reduce expression of apoB-
48 mRNA or
protein. This can be effected, e.g., by selection of an iRNA agent which
specifically targets the
nucleotides subject to RNA editing in the apoB-100 transcript.
The iRNA agent that targets apoB-100 can be administered to a subject, wherein
the
subject is suffering from a disorder characterized by elevated or otherwise
unwanted expression
of apoB- 100, elevated or otherwise unwanted levels of cholesterol, and/or
disregulation of lipid
metabolism. The iRNA agent can be administered to an individual at risk for
the disorder to
delay onset of the disorder or a symptom of the disorder. These disorders
include HDL/LDL
cholesterol imbalance; dyslipidemias, e.g., familial combined hyperlipidemia
(FCHL), acquired
hyperlipidemia; hypercholestorolemia; statin-resistant hypercholesterolemia;
coronary artery
disease (CAD) coronary heart disease (CHD) atherosclerosis. In one embodiment,
the iRNA that
targets apoB-100 is administered to a subject suffering from statin-resistant
hypercholesterolemia.
The apoB-100 iRNA agent can be administered in an amount sufficient to reduce
levels
of serum LDL-C and/or HDL-C and/or total cholesterol in a subject. For
example, the iRNA is
administered in an amount sufficient to decrease total cholesterol by at least
0.5%, 1%, 2.5%,
5%, 10% in the subject. In one embodiment, the iRNA agent is administered in
an amount
sufficient to reduce the risk of myocardial infarction the subject.
In a preferred embodiment the iRNA agent is administered repeatedly.
Administration of
3o an iRNA agent can be carried out over a range of time periods. It can be
administered daily,
once every few days, weekly, or monthly. The timing of administration can vary
from patient to
3
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072 WO I
patient, depending on such factors as the severity of a patient's symptoms.
For example, an
effective dose of an iRNA agent can be administered to a patient once a month
for an indefinite
period of time, or until the patient no longer requires therapy. In addition,
sustained release
compositions containing an iRNA agent can be used to maintain a relatively
constant dosage in
the patient's blood.
In one embodiment, the iRNA agent can be targeted to the liver, and apoB
expression
level are decreased in the liver following administration of the apoB iRNA
agent. For example,
the iRNA agent can be complexed with a moiety that targets the liver, e.g., an
antibody or ligand
that binds a receptor on the liver.
The iRNA agent, particularly an iRNA agent that targets apoB, beta-catenin or
glucose-6-
phosphatase RNA, can be targeted to the liver, for example by associating,
e.g., conjugating the
iRNA agent to a lipophilic moiety, e.g., a lipid, cholesterol, oleyl, retinyl,
or cholesteryl residue.
Other lipophilic moieties that can be associated, e.g., conjugated with the
iRNA agent include
cholic acid, adamantane acetic acid, 1-pyrene butyric acid,
dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol, geranyloxyhexyl group, hexadecylglycerol, borneol,
menthol, 1,3-
propanediol, heptadecyl group, palmitic acid, myristic acid,03-
(oleoyl)lithocholic acid, 03-
(oleoyl)cholenic acid, dimethoxytrityl, or phenoxazine. In one embodiment, the
iRNA agent can
be targeted to the liver by associating, e.g., conjugating, the iRNA agent to
a low-density
lipoprotein (LDL), e.g., a lactosylated LDL. In another embodiment, the iRNA
agent can be
targeted to the liver by associating, e.g., conjugating, the iRNA agent to a
polymeric carrier
complex with sugar residues.
In another embodiment, the iRNA agent can be targeted to the liver by
associating, e.g.,
conjugating, the iRNA agent to a liposome complexed with sugar residues. A
targeting agent
that incorporates a sugar, e.g., galactose and/or analogues thereof, is
particularly useful. These
agents target, in particular, the parenchymal cells of the liver (see Table
1). In a preferred
embodiment, the targeting moiety includes more than one galactose moiety,
preferably two or
three. Preferably, the targeting moiety includes 3 galactose moieties, e.g.,
spaced about 15
angstroms from each other. The targeting moiety can be lactose. A lactose is a
glucose coupled
to a galactose. Preferably, the targeting moiety includes three lactoses. The
targeting moiety can
3o also be N-Acetyl-Galactosamine, N-Ac-Glucosamine. A mannose, or mannose-6-
phosphate
targeting moiety can be used for macrophage targeting.
4
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
The targeting agent can be linked directly, e.g., covalently or non
covalently, to the iRNA
agent, or to another delivery or formulation modality, e.g., a liposome. E.g.,
the iRNA agents
with or without a targeting moiety can be incorporated into a delivery
modality, e.g., a liposome,
with or without a targeting moiety.
It is particularly preferred to use an iRNA conjugated to a lipophilic
molecule to
conjugate to an iRNA agent that targets apoB, beta-catenin or glucose-6-
phosphatase iRNA
targeting agent.
In one embodiment, the iRNA agent has been modified, or is associated with a
delivery
agent, e.g., a delivery agent described herein, e.g., a liposome, which has
been modified to alter
lo distribution in favor of the liver. In one embodiment, the modification
mediates association with
a serum albumin (SA), e.g., a human serum albumin (HSA), or a fragment
thereof.
The iRNA agent, particularly an iRNA agent that targets apoB, beta-catenin or
glucose-6-
phosphatase RNA, can be targeted to the liver, for example by associating,
e.g., conjugating the
iRNA agent to an SA molecule, e.g., an HSA molecule, or a fragment thereof. In
one
embodiment, the iRNA agent or composition thereof has an affinity for an SA,
e.g., HSA, which
is sufficiently high such that its levels in the liver are at least 10, 20,
30, 50, or 100% greater in
the presence of SA, e.g., HSA, or is such that addition of exogenous SA will
increase delivery to
the liver. These criteria can be measured, e.g., by testing distribution in a
mouse in the presence
or absence of exogenous mouse or human SA.
The SA, e.g., HSA, targeting agent can be linked directly, e.g., covalently or
non-
covalently, to the iRNA agent, or to another delivery or formulation modality,
e.g., a liposome.
E.g., the iRNA agents with or without a targeting moiety can be incorporated
into a delivery
modality, e.g., a liposome, with or without a targeting moiety.
It is particularly preferred to use an iRNA conjugated to an SA, e.g., an HSA,
molecule
wherein the iRNA agent is an apoB, beta-catenin or glucose-6-phosphatase iRNA
targeting
agent.
In another aspect, the invention features, a method for reducing glucose-6-
phosphatase
levels in a subject, e.g., a mammal, such as a human. The method includes
administering to a
subject an iRNA agent which targets glucose-6-phosphatase. The iRNA agent can
be a dsRNA
that has a sequence that is substantially identical to a sequence of the
glucose-6-phosphatase
gene.
5
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment, the subject is treated with an iRNA agent that
targets one of
the sequences listed in Table 11. In a preferred embodiment it targets both
sequences of a
palindromic pair provided in Table 11. The most preferred targets are listed
in descending order
of preferability, in other words, the more preferred targets are listed
earlier in Table 11.
In a preferred embodiment the iRNA agent will include regions, or strands,
which are
complementary to a pair in Table 11. In a preferred embodiment the iRNA agent
will include
regions complementary to the palindromic pairs of Table 11 as a duplex region.
In a preferred embodiment the duplex region of the iRNA agent will target a
sequence
listed in Table 11 but will not be perfectly complementary with the target
sequence, e.g., it will
lo not be complementary at at least 1 base pair. Preferably it will have no
more than 1, 2, 3, 4, or 5
bases, in total, or per strand, which do not hybridize with the target
sequence
In a preferred embodiment the iRNA agent includes overhangs, e.g., 3' or 5'
overhangs,
preferably one or more 3' overhangs. Overhangs are discussed in detail
elsewhere herein but are
preferably about 2 nucleotides in length. The overhangs can be complementary
to the gene
sequences being targeted or can be other sequence. TT is a preferred overhang
sequence. The
first and second iRNA agent sequences can also be joined, e.g., by additional
bases to form a
hairpin, or by other non-base linkers.
Table 11 refers to sequences from human glucose-6-phosphatase. Table 12 refers
to
sequences from rat glucose-6-phosphatase. The sequences from table 12 can be
used, e.g., in
experiments with rats or cultured rat cells.
In a preferred embodiment iRNA agent can have any architecture, e.g.,
architecture
described herein. E.g., it can be incorporated into an iRNA agent having an
overhang structure,
overall length, hairpin vs. two-strand structure, as described herein. In
addition, monomers other
than naturally occurring ribonucleotides can be used in the selected iRNA
agent.
The iRNA that targets glucose-6-phosphatase can be administered in an amount
sufficient
to reduce expression of glucose-6-phosphatase mRNA.
The iRNA that targets glucose-6-phosphatase can be administered to a subject
to inhibit
hepatic glucose production, for the treatment of glucose-metabolism-related
disorders, such as
diabetes, e.g., type-2-diabetes mellitus. The iRNA agent can be administered
to an individual at
risk for the disorder to delay onset of the disorder or a symptom of the
disorder.
6
CA 02521464 2011-06-30
51912-8
In other embodiments, iRNA agents having sequence similarity to the
following genes can also be used to inhibit hepatic glucose production. These
other
genes include "forkhead homologue in rhabdomyosarcoma (FKHR); glucagon;
glucagon receptor; glycogen phosphorylase; PPAR-Gamma Coactivator (PGC-1);
Fructose-1,6-bisphosphatase; glucose-6-phosphate locator; glucokinase
inhibitory
regulatory protein; and phosphoenolpyruvate carboxykinase (PEPCK).
In one embodiment, the iRNA agent can be targeted to the liver, and
RNA expression levels of the targeted genes are decreased in the liver
following
administration of the iRNA agent.
The iRNA agent can be one described herein, and can be a dsRNA that
has a sequence that is substantially identical to a sequence of a target gene.
The
iRNA can be less than 30 nucleotides in length, e.g., 21-23 nucleotides.
Preferably,
the iRNA is 21 nucleotides in length. In one embodiment, the iRNA is 21
nucleotides
in length, and the duplex region of the iRNA is 19 nucleotides. In another
embodiment, the iRNA is greater than 30 nucleotides in length.
In a particular embodiment, the present invention relates to an iRNA
agent comprising a sense strand and an antisense strand, wherein the sense and
antisense strands are complementary to each other, and wherein the sense
strand
comprises a sequence that is substantially identical to a sequence of any one
of SEQ
ID NO: 5495-5541, and the antisense strand comprises a sequence that is
substantially identical to a sequence of any one of SEQ ID NO: 5542-5588, and
wherein each strand of the iRNA agent is less than 30 nucleotides in length.
In another embodiment, the present invention provides a
pharmaceutical composition comprising the iRNA agent as described herein and a
pharmaceutically acceptable carrier.
In another aspect, the invention features a method for reducing
beta-catenin levels in a subject, e.g., a mammal, such as a human. The method
includes administering to a subject an iRNA agent that targets beta-catenin.
The
7
CA 02521464 2011-06-30
51912-8
RNA agent can be one described herein, and can be a dsRNA that has a sequence
that is substantially identical to a sequence of the beta-catenin gene. The
iRNA can
be less than 30 nucleotides in length, e.g., 21-23 nucleotides. Preferably,
the RNA is
21 nucleotides in length. In one embodiment, the RNA is 21 nucleotides in
length,
and the duplex region of the iRNA is 19 nucleotides. In another embodiment,
the
RNA is greater than 30 nucleotides in length.
In a preferred embodiment, the subject is treated with an RNA agent
which targets one of the sequences listed in Table 13. In a preferred
embodiment it
targets both sequences of a palindromic pair provided in Table 13. The most
preferred targets are listed in descending order of preferrability, in other
words, the
more preferred targets are listed earlier in Table 13.
In a preferred embodiment the iRNA agent will include regions, or
strands, which are complementary to a pair in Table 13. In a preferred
embodiment
the iRNA agent will include regions complementary to the palindromic pairs of
Table 13 as a duplex region.
In a preferred embodiment the duplex region of the iRNA agent will
target a sequence listed in Table 13 but will not be perfectly complementary
with the
target sequence, e.g., it will
7a
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
not be complementary at at least 1 base pair. Preferably it will have no more
than 1, 2, 3, 4, or 5
bases, in total, or per strand, which do not hybridize with the target
sequence
In a preferred embodiment the iRNA agent includes overhangs, e.g., 3' or 5'
overhangs,
preferably one or more 3' overhangs. Overhangs are discussed in detail
elsewhere herein but are
preferably about 2 nucleotides in length. The overhangs can be complementary
to the gene
sequences being targeted or can be other sequence. TT is a preferred overhang
sequence. The
first and second iRNA agent sequences can also be joined, e.g., by additional
bases to form a
hairpin, or by other non-base linkers.
The iRNA agent that targets beta-catenin can be administered in an amount
sufficient to
lo reduce expression of beta-catenin mRNA. In one embodiment, the iRNA agent
is administered
in an amount sufficient to reduce expression of beta-catenin protein (e.g., by
at least 2%, 4%,
6%,10%,15%,20%).
The iRNA agent that targets beta-catenin can be administered to a subject,
wherein the
subject is suffering from a disorder characterized by unwanted cellular
proliferation in the liver
or of liver tissue, e.g., metastatic tissue originating from the liver.
Examples include, a benign
or malignant disorder, e.g., a cancer, e.g., a hepatocellular carcinoma (HCC),
hepatic metastasis,
or hepatoblastoma.
The iRNA agent can be administered to an individual at risk for the disorder
to delay
onset of the disorder or a symptom of the disorder
In a preferred embodiment the iRNA agent is administered repeatedly.
Administration of
an, iRNA agent can be carried out over a range of time periods. It can be
administered daily,
once every few days, weekly, or monthly. The timing of administration can vary
from patient to
patient, depending on such factors as the severity of a patient's symptoms.
For example, an
effective dose of an iRNA agent can be administered to a patient once a month
for an indefinite
period of time, or until the patient no longer requires therapy. In addition,
sustained release
compositions containing an iRNA agent can be used to maintain a relatively
constant dosage in
the patient's blood.
In one embodiment, the iRNA agent can be targeted to the liver, and beta-
catenin
expression level are decreased in the liver following administration of the
beta-catenin iRNA
agent. For example, the iRNA agent can be complexed with a moiety that targets
the liver, e.g.,
an antibody or ligand that binds a receptor on the liver.
8
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In another aspect, the invention provides methods to treat liver disorders,
e.g., disorders
characterized by unwanted cell proliferation, hematological disorders,
disorders characterized by
inflammation disorders, and metabolic or viral diseases or disorders of the
liver. A proliferation
disorder of the liver can be, for example, a benign or malignant disorder,
e.g., a cancer, e.g, a
hepatocellular carcinoma (HCC), hepatic metastasis, or hepatoblastoma. A
hepatic hematology
or inflammation disorder can be a disorder involving clotting factors, a
complement-mediated
inflammation or a fibrosis, for example. Metabolic diseases of the liver can
include
dyslipidemias, and irregularities in glucose regulation. Viral diseases of the
liver can include
hepatitis C or hepatitis B. In one embodiment, a liver disorder is treated by
administering one or
1o more iRNA agents that have a sequence that is substantially identical to a
sequence in a gene
involved in the liver disorder.
In one embodiment an iRNA agent to treat a liver disorder has a sequence which
is
substantially identical to a sequence of the beta-catenin or c-jun gene. In
another embodiment,
such as for the treatment of hepatitis C or hepatitis B, the iRNA agent can
have a sequence that is
substantially identical to a sequence of a gene of the hepatitis C virus or
the hepatitis B virus,
respectively. For example, the iRNA agent can target the 5' core region of
HCV. This region
lies just downstream of the ribosomal toe-print straddling the initiator
methionine.
Alternatively, an iRNA agent of the invention can target any one of the
nonstructural proteins of
HCV: NS3, 4A, 4B, 5A, or 5B. For the treatment of hepatitis B, an iRNA agent
can target the
protein X (HBx) gene, for example.
In a preferred embodiment, the subject is treated with an iRNA agent which
targets one
of the sequences listed in Table 14. In a preferred embodiment it targets both
sequences of a
palindromic pair provided in Table 14. The most preferred targets are listed
in descending order
of preferrability, in other words, the more preferred targets are listed
earlier in Table 14.
In a preferred embodiment the iRNA agent will include regions, or strands,
which are
complementary to a pair in Table 14. In a preferred embodiment the iRNA agent
will include
regions complementary to the palindromic pairs of Table 14 as a duplex region.
In a preferred embodiment the duplex region of the iRNA agent will target a
sequence
listed in Table 14, but will not be perfectly complementary with the target
sequence, e.g., it will
not be complementary at at least I base pair. Preferably it will have no more
than 1, 2, 3, 4, or 5
bases, in total, or per strand, which do not hybridize with the target
sequence
9
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the iRNA agent includes overhangs, e.g., 3' or 5'
overhangs,
preferably one or more 3' overhangs. Overhangs are discussed in detail
elsewhere herein but are
preferably about 2 nucleotides in length. The overhangs can be complementary
to the gene
sequences being targeted or can be other sequence. TT is a preferred overhang
sequence. The
first and second iRNA agent sequences can also be joined, e.g., by additional
bases to form a
hairpin, or by other non-base linkers.
In another aspect, an iRNA agent can be administered to modulate blood
clotting, e.g., to
reduce the tendency to form a blood clot. In a preferred embodiment the iRNA
agent targets
Factor V expression, preferably in the liver. One or more iRNA agents can be
used to target a
1o wild type allele, a mutant allele, e.g., the Leiden Factor V allele, or
both. Such administration
can be used to treat or prevent venous thrombosis, e.g., deep vein thrombosis
or pulmonary
embolism, or another disorder caused by elevated or otherwise unwanted
expression of Factor V,
in, e.g., the liver. In one embodiment the iRNA agent can treat a subject,
e.g., a human who has
Factor V Leiden or other genetic trait associated with an unwanted tendency to
form blood clots.
In a preferred embodiment administration of an iRNA agent which targets Factor
V is
with the administration of a second treatment, e.g, a treatment which reduces
the tendency of the
blood to clot, e.g., the administration of heparin or of a low molecular
weight heparin.
In one embodiment, the iRNA agent that targets Factor V can be used as a
prophylaxis in
patients, e.g., patients with Factor V Leiden, who are placed at risk for a
thrombosis, e.g., those
about to undergo surgery, in particular those about to undergo high-risk
surgical procedures
known to be associated with formation of venous thrombosis, those about to
undergo a
prolonged period of relative inactivity, e.g., on a motor vehicle, train or
airplane flight, e.g., a
flight or other trip lasting more than three or five hours. Such a treatment
can be an adjunct to
the therapeutic use of low molecular weight (LMW) heparin prophylaxis.
In another embodiment, the iRNA agent that targets Factor V can be
administered to
patients with Factor V Leiden to treat deep vein thrombosis (DVT) or pulmonary
embolism (PE).
Such a treatment can be an adjunct to (or can replace) therapeutic uses of
heparin or coumadin.
The treatment can be administered by inhalation or generally by pulmonary
routes.
In a preferred embodiment, an iRNA agent administered to treat a liver
disorder is
3o targeted to the liver. For example, the iRNA agent can be complexed with a
targeting moiety,
e.g., an antibody or ligand that recognizes a liver-specific receptor.
CA 02521464 2011-06-30
51912-8
The invention also includes preparations, including substantially pure or
pharmaceutically acceptable preparations of iRNA agents which silence any of
the genes
discussed herein and in particular for any of apoB-100, glucose-6-phosphatase,
beta-catenin,
factor V, or any of the HVC genes discussed herein.
The methods and compositions of the invention, e.g., the methods and
compositions to
treat diseases and disorders of the liver described herein, can be used with
any of the iRNA
agents described. In addition, the methods and compositions of the invention
can be used for the
treatment of any disease or disorder described herein, and for the treatment
of any subject, ag.,
any animal, any mammal, such as any human.
The methods and compositions of the invention, e.g., the methods and iRNA
compositions to treat liver based diseases described herein, can be used with
any dosage and/or
formulation described herein, as well as with any route of administration
described herein.
A "substantially identical" sequence includes a region of sufficient homology
to the
target gene, and is of sufficient length in terms of nucleotides, that the
iRNA agent, or a fragment
thereof, can mediate down regulation of the target gene. Thus, the iRNA agent
is or includes a
region which is at least partially, and in some embodiments fully,
complementary to a target
RNA transcript. It is not necessary that there be perfect complementarity
between the iRNA
agent and the target, but the correspondence must be sufficient to enable the
iRNA agent, or a
cleavage product thereof, to direct sequence specific silencing, e.g., by RNAi
cleavage of the
target RNA, e.g., mRNA. Complementarity, or degree of homology with the target
strand, is
most critical in the antisense strand. While perfect complementarity,
particularly in the antisense
strand, is often desired some embodiments can include, particularly in the
antisense strand, one
or more but preferably 6, 5, 4, 3, 2, or fewer mismatches (with respect to the
target RNA). The
mismatches, particularly in the antisense strand, are most tolerated in the
terminal regions and if
present are preferably in a terminal region or regions, e.g., within 6, 5, 4,
or 3 nucleotides of the
5' and/or 3' terminus. The sense strand need only be sufficiently
complementary with the
antisense strand to maintain the over all double strand character of the
molecule.
The details of one or more embodiments of the invention are set forth in the
accompanying drawings and the description below. Other features, objects, and
advantages of
the invention will be apparent from this description, and from the claims.
11
CA 02521464 2011-06-30
51912-8
BRIEF DESCRIPTION OF THE DRAWINGS
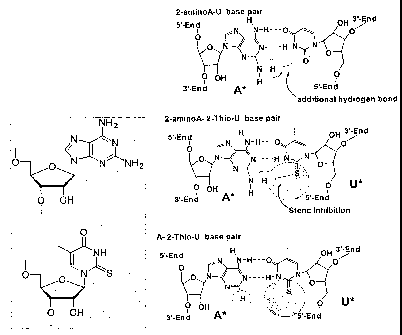
FIG 1 is a structural representation of base pairing in psuedoconiplementary
siRNA2.
FIG. 2 is a schematic representation of dual targeting siRNAs designed to
target the HCV
genome.
FIG 3 is a schematic representation of psuedocomplementary, bifunctional
siRNAs
designed to target the HCV genome.
FIG 4 is a general synthetic scheme for incorporation of RRMS monomers into an
oligonucleotide.
FIG. 5 is a table of representative RRMS carriers. Panel l shows pyrroline-
based
RRMSs; panel 2 shows 3-hydroxyproline-based RRMSs;. panel 3 shows piperidine-
based
RRMSs; panel 4 shows morpholine and piperazine-based RRMSs; and panel 5 shows
decalin-
based RRMSs.. R1 is succinate or phosphoramidate and R2 is H or a conjugate
ligand.
FIG 6A is a graph depicting blood glucose levels in mice treated with
nonspecific Renilla<
RNA or not treated with siRNA. Mice treated with nonspecific Renilla RNA were
injected on
Day 7.
FIG 6B is a graph depicting blood glucose levels in mice treated with siRNA
targeting
glucose 6-phosphatase. Mice treated with siRNA targeting glucose 6-phosphatase
were injected
on Day 7.
FIG 6C is a graph depicting blood glucose levels in mice that were either not
injected
with siRNA, or were injected but the injection failed. Mice that were
injected, were injected on
Day 7.
FIG 7 is a graph depicting average blood glucose levels in four mice treated
with siRNA
targeting glucose 6-phosphatase, and in four mice either treated with
nonspecific Renilla RNA or
not treated with siRNA (triangles). siRNA or Renilla RNA was administered on
day 7 by
hydrodynamic tail vein injection.
FICT. 8A is a graph depicting levels of luciferase mRNA in livers of CMV Luc
mice
(Xanogen) following intervenous injection (iv) of buffer or siRNA into the
tail vein. Each bar
represents data from one mouse. RNA levels were quantified by QuantiGene Assay
" Trade-mark
12
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(Genospectra, Inc.; Fremont, CA)). The Y axis represents chemiluminescence
values in counts
per second (CPS).
FIG. 8B is a graph depicting levels of luciferase mRNA in livers of CMV-Luc
mice
(Xanogen). The values are averaged from the data depicted in FIG. 8A.
FIG 9 is a graph depicting the pharmacokinetics of cholesterol-conjugated and
unconjugated siRNA. The diamonds represent the amount of unconjugated 33P-
labeled siRNA
(ALN-3000) in mouse plasma over time; the squares represent the amount of
cholesterol-
conjugated 33P-labeled siRNA (ALN-3001) in mouse plasma overtime. "L1163" is
equivalent to
ALN3000; "Ll163Chol" is equivalent to ALN-3001.
FIG 10 is a graph indicating the amount of cholesterol-conjugated (dark bars)
and
unconjugated siRNA (light bars) detected in mouse whole liver tissue isolated
over a period of
time following intravenous tail vein injection. The amount of siRNA is
represented as a
percentage of the total dose or 33P-labeled siRNA delivered to the mouse.
"L1163" is equivalent
to ALN3000 (light bars); "L1163Chol" is equivalent to ALN-3001 (dark bars).
FIG. 11 is a graph indicating the amount of cholesterol-conjugated siRNA
detected in
various tissues of two different CMV-Luc mice ("Mouse 69" (light bars) and
"Mouse 63" (dark
bars)). Mice were injected with 50 mg/kg AL-3001 siRNA by intravenous tail
vein injection,
and tissue was harvested 22 hours later. SiRNA was detected by RNAse
protection, and
phosphorimager scanning was used to quantitate the siRNA. The amount of siRNA
is expressed
as ug/g liver tissue.
FIG. 12 is a gel of U/U siRNA (see Table 19) detected in the liver of Balbc
mice at
increasing time points following hydrodynamic (hd) tail vein injection. U/U
siRNA was injected
at a concentration of 4 mg/kg. siRNA was detected by RNAse protection assay.
Lanes labeled
"stand." were loaded with clean siRNA to serve as size and quality standards.
"non" represents
control samples isolated from livers of mice that were not injected with U/U
siRNA. The control
samples were further used in parallel RNAse protection assays.
FIG. 13 is a gel comparing different siRNA species detected in the livers of
Balbc mice at
increasing time points following hydrodynamic (hd) or nonhydrodynamic (iv)
tail vein injection.
U/U siRNA was injected by hd and by iv injection. 3'C/3'C and 3'C/U (see Table
19) were each
injected by iv injection. at a concentration of 4 mg/kg. siRNA was detected by
RNAse
protection assay. Lanes labeled "stand." were loaded with clean siRNA to serve
as size and
13
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
quality standards. "non" represents control samples isolated from livers of
mice that were not
injected with siRNA. The control samples were further used in parallel RNAse
protection
assays.
FIG 14 is a graph depicting the percentage of luciferase activity in liver
extracts of CMV
Luc mice injected with siRNA (ALN-3001). Percentage of luciferase activity was
relative to
activity in CMV-Luc mice injected with PBS, pH 4.7. "Bufferl siRNA1," "Buffer2
siRNA2,"
and "Buffer3 siRNA3" represent the average activity observed in three separate
experiments.
DETAILED DESCRIPTION
Double-stranded (dsRNA) directs the sequence-specific silencing of mRNA
through a
process known as RNA interference (RNAi). The process occurs in a wide variety
of organisms,
including mammals and other vertebrates.
It has been demonstrated that 21-23 nt fragments of dsRNA are sequence-
specific
mediators of RNA silencing, e.g., by causing RNA degradation. While not
wishing to be bound
by theory, it may be that a molecular signal, which may be merely the specific
length of the
fragments, present in these 21-23 nt fragments recruits cellular factors that
mediate RNAi.
Described herein are methods for preparing and administering these 21-23 nt
fragments, and
other iRNAs agents, and their use for specifically inactivating gene function.
The use of iRNAs
agents (or recombinantly produced or chemically synthesized oligonucleotides
of the same or
similar nature) enables the targeting of specific mRNAs for silencing in
mammalian cells. In
addition, longer dsRNA agent fragments can also be used, e.g., as described
below.
Although, in mammalian cells, long dsRNAs can induce the interferon response
which is
frequently deleterious, sRNAs do not trigger the interferon response, at least
not to an extent that
is deleterious to the cell and host. In particular, the length of the iRNA
agent strands in an sRNA
agent can be less than 31, 30, 28, 25, or 23 nt, e.g., sufficiently short to
avoid inducing a
deleterious interferon response. Thus, the administration of a composition of
sRNA agent (e.g.,
formulated as described herein) to a mammalian cell can be used to silence
expression of a target
gene while circumventing the interferon response. Further, use of a discrete
species of iRNA
14
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
agent can be used to selectively target one allele of a target gene, e.g., in
a subject heterozygous
for the allele.
Moreover, in one embodiment, a mammalian cell is treated with an iRNA agent
that
disrupts a component of the interferon response, e.g., double stranded RNA
(dsRNA)-activated
protein kinase PKR. Such a cell can be treated with a second iRNA agent that
includes a
sequence complementary to a target RNA and that has a length that might
otherwise trigger the
interferon response.
In a typical embodiment, the subject is a mammal such as a cow, horse, mouse,
rat, dog,
pig, goat, or a primate. The subject can be a dairy mammal (e.g., a cow, or
goat) or other farmed
1o animal (e.g., a chicken, turkey, sheep, pig, fish, shrimp). In a much
preferred embodiment, the
subject is a human, e.g., a normal individual or an individual that has, is
diagnosed with, or is
predicted to have a disease or disorder.
Further, because iRNA agent mediated silencing persists for several days after
administering the iRNA agent composition, in many instances, it is possible to
administer the
composition with a frequency of less than once per day, or, for some
instances, only once for the
entire therapeutic regimen. For example, treatment of some cancer cells may be
mediated by a
single bolus administration, whereas a chronic viral infection may require
regular administration,
e.g., once per week or once per month.
A number of exemplary routes of delivery are described that can be used to
administer an
iRNA agent to a subject. In addition, the iRNA agent can be formulated
according to an
exemplary method described herein.
Liver Diseases
Exemplary diseases and disorders that can be treated by the methods and
compositions
of the invention are liver-based diseases.
Disorders involving the liver include, but are not limited to, hepatic injury;
jaundice and
cholestasis, such as bilirubin and bile formation; hepatic failure and
cirrhosis, such as cirrhosis,
portal hypertension, including ascites, portosystemic shunts, and
splenomegaly; infectious
disorders, such as viral hepatitis, including hepatitis A-E infection and
infection by other
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
hepatitis viruses, clinicopathologic syndromes, such as the carrier state,
asymptomatic infection,
acute viral hepatitis, chronic viral hepatitis, and fulminant hepatitis;
autoimmune hepatitis; drug-
and toxin-induced liver disease, such as alcoholic liver disease; inborn
errors of metabolism and
pediatric liver disease, such as hemochromatosis, Wilson disease, a1-
antitrypsin deficiency, and
neonatal hepatitis; intrahepatic biliary tract disease, such as secondary
biliary cirrhosis, primary
biliary cirrhosis, primary sclerosing cholangitis, and anomalies of the
biliary tree; circulatory
disorders, such as impaired blood flow into the liver, including hepatic
artery compromise and
portal vein obstruction and thrombosis, impaired blood flow through the liver,
including passive
congestion and centrilobular necrosis and peliosis hepatis, hepatic vein
outflow obstruction,
1o including hepatic vein thrombosis (Budd-Chian syndrome) and veno-occlusive
disease; hepatic
disease associated with pregnancy, such as preeclampsia and eclampsia, acute
fatty liver of
pregnancy, and intrehepatic cholestasis of pregnancy; hepatic complications of
organ or bone
marrow transplantation, such as drug toxicity after bone marrow
transplantation, graft-versus-
host disease and liver rejection, and nonimmunologic damage to liver
allografts; tumors and
tumorous conditions, such as nodular hyperplasias, adenomas, and malignant
tumors, including
primary carcinoma of the liver and metastatic tumors.
An iRNA agent can also be administered to inhibit Factor V expression in the
liver. Two
to five percent of the United States population is heterozygous for an allele
of the Factor V gene
that encodes a single amino acid change at position 1961. These heterozygous
individuals have a
3-8 fold increased risk of venous thrombosis, a risk that is associated with
increased factor V
activity. The increased activity leads to increased thrombin generation from
the prothrombinase
complex. An iRNA agent directed against Factor V can treat or prevent venous
thrombosis or
treat a human who has Factor V Leiden. The iRNA agent that targets Factor V
can be also be
used as a prophylaxis in patients with Factor V Leiden who undergo high-risk
surgical
procedures, and this prophylaxis can be an adjunct to the therapeutic use of
low molecular
weight (LMW) heparin prophylaxis.
An iRNA agent that targets Factor V can also be administered to patients with
Factor V
Leiden to treat deep vein thrombosis (DVT) or pulmonary embolism (PE), and
this treatment can
be an adjunct to therapeutic uses of heparin or coumadin. Any other disorder
caused by elevated
or otherwise unwanted levels of Factor V protein can be treated by
administering an iRNA agent
against Factor V.
16
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
iRNA agents of the invention can be targeted to any gene whose overexpression
is
associated with the liver diseases.
Targeting to the Liver
The iRNA agents of the invention are particularly useful when targeted to the
liver. An
iRNA agent can be targeted to the liver through a composition that includes
the iRNA agent and
a liver-targeting agent. For example, a liver-targeting agent can be a
lipophilic moiety.
Preferred lipophilic moieties include lipid, cholesterols, oleyl, retinyl, or
cholesteryl residues (see
Table 1). Other lipophilic moieties that can function as liver-targeting
agents include cholic acid,
1o adamantane acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol, geranyloxyhexyl group, hexadecylglycerol, borneol,
menthol, 1,3-
propanediol, heptadecyl group, palmitic acid, myristic acid,03-
(oleoyl)lithocholic acid, 03-
(oleoyl)cholenic acid, dimethoxytrityl, or phenoxazine.
An iRNA agent can also be targeted to the liver by association with a low-
density
lipoprotein (LDL), such as lactosylated LDL. Polymeric carriers complexed with
sugar residues
can also function to target iRNA agents to the liver.
A targeting agent that incorporates a sugar, e.g., galactose and/or analogues
thereof, is
particularly useful. These agents target, in particular, the parenchymal cells
of the liver (see
Table 1). For example, a targeting moiety can include more than one or
preferably two or three
galactose moieties, spaced about 15 angstroms from each other. The targeting
moiety can
alternatively be lactose (e.g., three lactose moieties), which is glucose
coupled to a galactose.
The targeting moiety can also be N-Acetyl-Galactosamine, N-Ac-Glucosamine. A
mannose or
mannose-6-phosphate targeting moiety can be used for macrophage targeting.
Conjugation of an iRNA agent with a serum albumin (SA), such as human serum
albumin, can also be used to target the iRNA agent to the liver.
An iRNA agent can be targeted to a particular cell type in the liver by using
specific
targeting agents, which recognize particular receptors in the liver. Exemplary
targeting moieties
and their associated receptors are presented in Table 1.
17
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Table 1 Targeting agents (Ligands) and their associated receptors
Liver Cells Ligand Receptor
1) Parenchymal Cell (PC) Galactose ASGP-R
(Hepatocytes) (Asiologlycoprotein receptor)
Gal NAc ASPG-R
(n-acetyl-galactosamine) Gal NAc Receptor
Lactose
Asialofetuin ASPG-r
2) Sinusoidal Endothelial Hyaluronan Hyaluronan receptor
Cell (SEC)
Procollagen Procollagen receptor
Negatively charged Scavenger receptors
molecules
Mannose Mannose receptors
N-acetyl Glucosamine Scavenger receptors
Immunoglobulins Fc Receptor
LPS CD14 Receptor
Insulin Receptor mediated transcytosis
Transferrin Receptor mediated transcytosis
Albumins Non-specific
Sugar-Albumin conjugates
Mannose-6-phosphate Mannose-6-phosphate receptor
3) Kupffer Cell (KC) Mannose Mannose receptors
Fucose Fucose receptors
Albumins Non-specific
Mannose-albumin
conjugates
iRNA AGENT STRUCTURE
Described herein are isolated iRNA agents, e.g., RNA molecules, (double-
stranded;
single-stranded) that mediate RNAi. The iRNA agents preferably mediate RNAi
with respect to
an endogenous gene of a subject or to a gene of a pathogen.
An "RNA agent" as used herein, is an unmodified RNA, modified RNA, or
nucleoside
lo surrogate, all of which are defined herein (see, e.g., the section below
entitled RNA Agents).
While numerous modified RNAs and nucleoside surrogates are described,
preferred examples
18
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
include those which have greater resistance to nuclease degradation than do
unmodified RNAs.
Preferred examples include those which have a 2' sugar modification, a
modification in a single
strand overhang, preferably a 3' single strand overhang, or, particularly if
single stranded, a 5'
modification which includes one or more phosphate groups or one or more
analogs of a
phosphate group.
An "iRNA agent" as used herein, is an RNA agent which can, or which can be
cleaved
into an RNA agent which can, down regulate the expression of a target gene,
preferably an
endogenous or pathogen target RNA. While not wishing to be bound by theory, an
iRNA agent
may act by one or more of a number of mechanisms, including post-
transcriptional cleavage of a
lo target mRNA sometimes referred to in the art as RNAi, or pre-
transcriptional or pre-translational
mechanisms. An iRNA agent can include a single strand or can include more than
one strands,
e.g., it can be a double stranded iRNA agent. If the iRNA agent is a single
strand it is
particularly preferred that it include a 5' modification which includes one or
more phosphate
groups or one or more analogs of a phosphate group.
The iRNA agent should include a region of sufficient homology to the target
gene, and be
of sufficient length in terms of nucleotides, such that the iRNA agent, or a
fragment thereof, can
mediate down regulation of the target gene. (For ease of exposition the term
nucleotide or
ribonucleotide is sometimes used herein in reference to one or more monomeric
subunits of an
RNA agent. It will be understood herein that the usage of the term
"ribonucleotide" or
"nucleotide", herein can, in the case of a modified RNA or nucleotide
surrogate, also refer to a
modified nucleotide, or surrogate replacement moiety at one or more
positions.) Thus, the iRNA
agent is or includes a region which is at least partially, and in some
embodiments fully,
complementary to the target RNA. It is not necessary that there be perfect
complementarity
between the iRNA agent and the target, but the correspondence must be
sufficient to enable the
iRNA agent, or a cleavage product thereof, to direct sequence specific
silencing, e.g., by RNAi
cleavage of the target RNA, e.g., mRNA.
Complementarity, or degree of homology with the target strand, is most
critical in the
antisense strand. While perfect complementarity, particularly in the antisense
strand, is often
desired some embodiments can include, particularly in the antisense strand,
one or more but
19
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
preferably 6, 5, 4, 3, 2, or fewer mismatches (with respect to the target
RNA). The mismatches,
particularly in the antisense strand, are most tolerated in the terminal
regions and if present are
preferably in a terminal region or regions, e.g., within 6, 5, 4, or 3
nucleotides of the 5' and/or 3'
terminus. The sense strand need only be sufficiently complementary with the
antisense strand to
maintain the over all double strand character of the molecule.
As discussed elsewhere herein, an iRNA agent will often be modified or include
nucleoside surrogates in addition to the RRMS. Single stranded regions of an
iRNA agent will
often be modified or include nucleoside surrogates, e.g., the unpaired region
or regions of a
hairpin structure, e.g., a region which links two complementary regions, can
have modifications
or nucleoside surrogates. Modification to stabilize one or more 3'- or 5'-
terminus of an iRNA
agent, e.g., against exonucleases, or to favor the antisense sRNA agent to
enter into RISC are
also favored. Modifications can include C3 (or C6, C7, C12) amino linkers,
thiol linkers,
carboxyl linkers, non-nucleotidic spacers (C3, C6, C9, C12, abasic,
triethylene glycol,
hexaethylene glycol), special biotin or fluorescein reagents that come as
phosphoramidites and
that have another DMT-protected hydroxyl group, allowing multiple couplings
during RNA
synthesis.
iRNA agents include: molecules that are long enough to trigger the interferon
response
(which can be cleaved by Dicer (Bernstein et al. 2001. Nature, 409:363-366)
and enter a RISC
(RNAi-induced silencing complex)); and, molecules which are sufficiently short
that they do not
trigger the interferon response (which molecules can also be cleaved by Dicer
and/or enter a
RISC), e.g., molecules which are of a size which allows entry into a RISC,
e.g., molecules which
resemble Dicer-cleavage products. Molecules that are short enough that they do
not trigger an
interferon response are termed sRNA agents or shorter iRNA agents herein.
"sRNA agent or
shorter iRNA agent" as used herein, refers to an iRNA agent, e.g., a double
stranded RNA agent
or single strand agent, that is sufficiently short that it does not induce a
deleterious interferon
response in a human cell, e.g., it has a duplexed region of less than 60 but
preferably less than
50, 40, or 30 nucleotide pairs. The sRNA agent, or a cleavage product thereof,
can down
regulate a target gene, e.g., by inducing RNAi with respect to a target RNA,
preferably an
endogenous or pathogen target RNA.
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Each strand of an sRNA agent can be equal to or less than 30, 25, 24, 23, 22,
21, or 20
nucleotides in length. The strand is preferably at least 19 nucleotides in
length. For example,
each strand can be between 21 and 25 nucleotides in length. Preferred sRNA
agents have a
duplex region of 17, 18, 19, 29, 21, 22, 23, 24, or 25 nucleotide pairs, and
one or more
overhangs, preferably one or two 3' overhangs, of 2-3 nucleotides.
In addition to homology to target RNA and the ability to down regulate a
target gene, an
iRNA agent will preferably have one or more of the following properties:
(1) it will be of the Formula 1, 2, 3, or 4 set out in the RNA Agent section
below;
(2) if single stranded it will have a 5' modification which includes one or
more
lo phosphate groups or one or more analogs of a phosphate group;
(3) it will, despite modifications, even to a very large number, or all of the
nucleosides,
have an antisense strand that can present bases (or' modified bases) in the
proper three
dimensional framework so as to be able to form correct base pairing and form a
duplex structure
with a homologous target RNA which is sufficient to allow down regulation of
the target, e.g., by
cleavage of the target RNA;
(4) it will, despite modifications, even to a very large number, or all of the
nucleosides,
still have "RNA-like" properties, i.e., it will possess the overall
structural, chemical and physical
properties of an RNA molecule, even though not exclusively, or even partly, of
ribonucleotide-
based content. For example, an iRNA agent can contain, e.g., a sense and/or an
antisense strand
in which all of the nucleotide sugars contain e.g., 2' fluoro in place of 2'
hydroxyl. This
deoxyribonucleotide-containing agent can still be expected to exhibit RNA-like
properties.
While not wishing to be bound by theory, the electronegative fluorine prefers
an axial
orientation when attached to the C2' position of ribose. This spatial
preference of fluorine can,
in turn, force the sugars to adopt a C3 -endo pucker. This is the same
puckering mode as
observed in RNA molecules and gives rise to the RNA-characteristic A-family-
type helix.
Further, since fluorine is a good hydrogen bond acceptor, it can participate
in the same hydrogen
bonding interactions with water molecules that are known to stabilize RNA
structures.
(Generally, it is preferred that a modified moiety at the 2' sugar position
will be able to enter into
21
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
H-bonding which is more characteristic of the OH moiety of a ribonucleotide
than the H moiety
of a deoxyribonucleotide. A preferred iRNA agent will: exhibit a C3'-endo
pucker in all, or at
least 50, 75,80, 85, 90, or 95 % of its sugars; exhibit a C3'-endo pucker in a
sufficient amount of
its sugars that it can give rise to a the RNA-characteristic A-family-type
helix; will have no more
than 20, 10, 5, 4, 3, 2, orl sugar which is not a C3'-endo pucker structure.
These limitations are
particularly preferably in the antisense strand;
(5) regardless of the nature of the modification, and even though the RNA
agent can
contain deoxynucleotides or modified deoxynucleotides, particularly in
overhang or other single
strand regions, it is preferred that DNA molecules, or any molecule in which
more than 50, 60,
or 70 % of the nucleotides in the molecule, or more than 50, 60, or 70 % of
the nucleotides in a
duplexed region are deoxyribonucleotides, or modified deoxyribonucleotides
which are deoxy at
the 2' position, are excluded from the definition of RNA agent.
A "single strand iRNA agent" as used herein, is an iRNA agent which is made up
of a
single molecule. It may include a duplexed region, formed by intra-strand
pairing, e.g., it may
be, or include, a hairpin or pan-handle structure. Single strand iRNA agents
are preferably
antisense with regard to the target molecule. In preferred embodiments single
strand iRNA
agents are 5' phosphorylated or include a phosphoryl analog at the 5' prime
terminus. 5'-
phosphate modifications include those which are compatible with RISC mediated
gene silencing.
Suitable modifications include: 5'-monophosphate ((HO)2(O)P-O-5'); 5'-
diphosphate
((HO)2(O)P-O-P(HO)(O)-0-5'); 5'-triphosphate ((HO)2(O)P-O-(HO)(O)P-0-P(HO)(O)-
0-5');
5'-guanosine cap (7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-
(HO)(O)P-O-
P(HO)(O)-O-5'); 5'-adenosine cap (Appp), and any modified or unmodified
nucleotide cap
structure (N-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-0-5'); 5'-monothiophosphate
(phosphorothioate; (HO)2(S)P-O-5'); 5'-monodithiophosphate
(phosphorodithioate;
(HO)(HS)(S)P-O-5'), 5'-phosphorothiolate ((HO)2(O)P-S-5'); any additional
combination of
oxygen/sulfur replaced monophosphate, diphosphate and triphosphates (e.g. 5'-
alpha-
thiotriphosphate, 5'-gamma-thiotriphosphate, etc.), 5'-phosphoramidates
((HO)2(O)P-NH-5',
(HO)(NH2)(O)P-O-5'), 5'-alkylphosphonates (R=alkyl=methyl, ethyl, isopropyl,
propyl, etc., e.g.
RP(OH)(O)-0-5'-, (OH)2(O)P-5'-CH2-), 5'-alkyletherphosphonates
22
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(R=alkylether=methoxymethyl (MeOCH2-), ethoxymethyl, etc., e.g. RP(OH)(O)-0-5'-
). (These
modifications can also be used with the antisense strand of a double stranded
iRNA.)
A single strand iRNA agent should be sufficiently long that it can enter the
RISC and
participate in RISC mediated cleavage of a target mRNA. A single strand iRNA
agent is at least
14, and more preferably at least 15, 20, 25, 29, 35, 40, or 50nucleotides in
length. It is preferably
less than 200, 100, or 60 nucleotides in length.
Hairpin iRNA agents will have a duplex region equal to or at least 17, 18, 19,
29, 21, 22,
23, 24, or 25 nucleotide pairs. The duplex region will preferably be equal to
or less than 200,
100, or 50, in length. Preferred ranges for the duplex region are 15-30, 17 to
23, 19 to 23, and 19
I o to 21 nucleotides pairs in length. The hairpin will preferably have a
single strand overhang or
terminal unpaired region, preferably the 3', and preferably of the antisense
side of the hairpin.
Preferred overhangs are 2-3 nucleotides in length.
A "double stranded (ds) iRNA agent" as used herein, is an iRNA agent which
includes
more than one, and preferably two, strands in which interchain hybridization
can form a region
of duplex structure.
The antisense strand of a double stranded iRNA agent should be equal to or at
least, 14,
15, 16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It should be equal
to or less than 200,
100, or 50, nucleotides in length. Preferred ranges are 17 to 25, 19 to 23,
and 19 to21
nucleotides in length.
The sense strand of a double stranded iRNA agent should be equal to or at
least 14, 15,
16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It should be equal to
or less than 200, 100,
or 50, nucleotides in length. Preferred ranges are 17 to 25, 19 to 23, and 19
to21 nucleotides in
length.
The double strand portion of a double stranded iRNA agent should be equal to
or at least,
14, 15, 16 17, 18, 19, 20, 21, 22, 23, 24, 25, 29, 40, or 60 nucleotide pairs
in length. It should be
equal to or less than 200, 100, or 50, nucleotides pairs in length. Preferred
ranges are 15-30, 17
to 23, 19 to 23, and 19 to 21 nucleotides pairs in length.
23
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In many embodiments, the ds iRNA agent is sufficiently large that it can be
cleaved by an
endogenous molecule, e.g., by Dicer, to produce smaller ds iRNA agents, e.g.,
sRNAs agents
It may be desirable to modify one or both of the antisense and sense strands
of a double
strand iRNA agent. In some cases they will have the same modification or the
same class of
modification but in other cases the sense and antisense strand will have
different modifications,
e.g., in some cases it is desirable to modify only the sense strand. It may be
desirable to modify
only the sense strand, e.g., to inactivate it, e.g., the sense strand can be
modified in order to
inactivate the sense strand and prevent formation of an active sRNA/protein or
RISC. This can
be accomplished by a modification which prevents 5'-phosphorylation of the
sense strand, e.g.,
1o by modification with a 5'-O-methyl ribonucleotide (see Nykanen et al.,
(2001) ATP requirements
and small interfering RNA structure in the RNA interference pathway. Cell 107,
309-321.)
Other modifications which prevent phosphorylation can also be used, e.g.,
simply substituting
the 5'-OH by H rather than O-Me. Alternatively, a large bulky group may be
added to the 5'-
phosphate turning it into a phosphodiester linkage, though this may be less
desirable as
phosphodiesterases can cleave such a linkage and release a functional sRNA 5'-
end. Antisense
strand modifications include 5' phosphorylation as well as any of the other 5'
modifications
discussed herein, particularly the 5' modifications discussed above in the
section on single
stranded iRNA molecules.
It is preferred that the sense and antisense strands be chosen such that the
ds iRNA agent
includes a single strand or unpaired region at one or both ends of the
molecule. Thus, a ds iRNA
agent contains sense and antisense strands, preferable paired to contain an
overhang, e.g., one or
two 5' or 3' overhangs but preferably a 3' overhang of 2-3 nucleotides. Most
embodiments
will have a 3' overhang. Preferred sRNA agents will have single-stranded
overhangs, preferably
3' overhangs, of 1 or preferably 2 or 3 nucleotides in length at each end. The
overhangs can be
the result of one strand being longer than the other, or the result of two
strands of the same length
being staggered. 5' ends are preferably phosphorylated.
Preferred lengths for the duplexed region is between 15 and 30, most
preferably 18, 19,
20, 21, 22, and 23 nucleotides in length, e.g., in the sRNA agent range
discussed above. sRNA
agents can resemble in length and structure the natural Dicer processed
products from long
24
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
dsRNAs. Embodiments in which the two strands of the sRNA agent are linked,
e.g.', covalently
linked are also included. Hairpin, or other single strand structures which
provide the required
double stranded region, and preferably a 3' overhang are also within the
invention.
The isolated iRNA agents described herein, including ds iRNA agents and sRNA
agents
can mediate silencing of a target RNA, e.g., mRNA, e.g., a transcript of a
gene that encodes a
protein. For convenience, such mRNA is also referred to herein as mRNA to be
silenced. Such
a gene is also referred to as a target gene. In general, the RNA to be
silenced is an endogenous
gene or a pathogen gene. In addition, RNAs other than mRNA, e.g., tRNAs, and
viral RNAs,
can also be targeted.
As used herein, the phrase "mediates RNAi" refers to the ability to silence,
in a sequence
specific manner, a target RNA. While not wishing to be bound by theory, it is
believed that
silencing uses the RNAi machinery or process and a guide RNA, e.g., an sRNA
agent of 21. to 23
nucleotides.
As used herein, "specifically hybridizable" and "complementary" are terms
which are
used to indicate a sufficient degree of complementarity such that stable and
specific binding
occurs between a compound of the invention and a target RNA molecule. Specific
binding
requires a sufficient degree of complementarity to avoid non-specific binding
of the oligomeric
compound to non-target sequences under conditions in which specific binding is
desired, i.e.,
under physiological conditions in the case of in vivo assays or therapeutic
treatment, or in the
case of in vitro assays, under conditions in which the assays are performed.
The non-target
sequences typically differ by at least 5 nucleotides.
In one embodiment, an iRNA agent is "sufficiently complementary" to a target
RNA,
e.g., a target mRNA, such that the iRNA agent silences production of protein
encoded by the
target mRNA. In another embodiment, the iRNA agent is "exactly complementary"
(excluding
the RRMS containing subunit(s))to a target RNA, e.g., the target RNA and the
iRNA agent
anneal, preferably to form a hybrid made exclusively of Watson-Crick basepairs
in the region of
exact complementarity. A "sufficiently complementary" target RNA can include
an internal
region (e.g., of at least 10 nucleotides) that is exactly complementary to a
target RNA.
Moreover, in some embodiments, the iRNA agent specifically discriminates a
single-nucleotide
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
difference. In this case, the iRNA agent only mediates RNAi if exact
complementary is found in
the region (e.g., within 7 nucleotides of) the single-nucleotide difference.
As used herein, the term "oligonucleotide" refers to a nucleic acid molecule
(RNA or
DNA) preferably of length less than 100, 200, 300, or 400 nucleotides.
RNA agents discussed herein include otherwise unmodified RNA as well as RNA
which
have been modified, e.g., to improve efficacy, and polymers of nucleoside
surrogates.
Unmodified RNA refers to a molecule in which the components of the nucleic
acid, namely
sugars, bases, and phosphate moieties, are the same or essentially the same as
that which occur in
nature, preferably as occur naturally in the human body. The art has referred
to rare or unusual,
1o but naturally occurring, RNAs as modified RNAs, see, e.g., Limbach et al.,
(1994) Summary:
the modified nucleosides of RNA, Nucleic Acids Res. 22: 2183-2196. Such rare
or unusual
RNAs, often termed modified RNAs (apparently because the are typically the
result of a post
transcriptionally modification) are within the term unmodified RNA, as used
herein. Modified
RNA as used herein refers to a molecule in which one or more of the components
of the nucleic
acid, namely sugars, bases, and phosphate moieties, are different from that
which occur in
nature, preferably different from that which occurs in the human body. While
they are referred
to as modified "RNAs," they will of course, because of the modification,
include molecules
which are not RNAs. Nucleoside surrogates are molecules in which the
ribophosphate backbone
is replaced with a non-ribophosphate construct that allows the bases to the
presented in the
correct spatial relationship such that hybridization is substantially similar
to what is seen with a
ribophosphate backbone, e.g., non-charged mimics of the ribophosphate
backbone. Examples of
all of the above are discussed herein.
Much of the discussion below refers to single strand molecules. In many
embodiments of
the invention a double stranded iRNA agent, e.g., a partially double stranded
iRNA agent, is
required or preferred. Thus, it is understood that that double stranded
structures (e.g. where two
separate molecules are contacted to form the double stranded region or where
the double
stranded region is formed by intramolecular pairing (e.g., a hairpin
structure)) made of the single
stranded structures described below are within the invention. Preferred
lengths are described
elsewhere herein.
26
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
As nucleic acids are polymers of subunits or monomers, many of the
modifications
described below occur at a position which is repeated within a nucleic acid,
e.g., a modification
of a base, or a phosphate moiety, or the a non-linking 0 of a phosphate
moiety. In some cases
the modification will occur at all of the subject positions in the nucleic
acid but in many, and
infact in most cases it will not. By way of example, a modification may only
occur at a 3' or 5'
terminal position, may only occur in a terminal regions, e.g. at a position on
a terminal
nucleotide or in the last 2, 3, 4, 5, or 10 nucleotides of a strand. A
modification may occur in a
double strand region, a single strand region, or in both. A modification may
occur only in the
double strand region of an RNA or may only occur in a single strand region of
an RNA. E.g., a
lo phosphorothioate modification at a non-linking 0 position may only occur at
one or both termini,
may only occur in a terminal regions, e.g., at a position on a terminal
nucleotide or in the last 2,
3, 4, 5, or 10 nucleotides of a strand, or may occur in double strand and
single strand regions,
particularly at termini. The 5' end or ends can be phosphorylated.
In some embodiments it is particularly preferred, e.g., to enhance stability,
to include
particular bases in overhangs, or to include modified nucleotides or
nucleotide surrogates, in
single strand overhangs, e.g., in a 5' or 3' overhang, or in both. E.g., it
can be desirable to
include purine nucleotides in overhangs. In some embodiments all or some of
the bases in a 3'
or 5' overhang will be modified, e.g., with a modification described herein.
Modifications can
include, e.g., the use of modifications at the 2' OH group of the ribose
sugar, e.g., the use of
deoxyribonucleotides, e.g., deoxythymidine, instead of ribonucleotides, and
modifications in the
phosphate group, e.g., phosphothioate modifications. Overhangs need not be
homologous with
the target sequence.
27
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Modifications and nucleotide surrogates are discussed below.
II 5'
BASE
O
W\\\\~, ~~~sOH (2' OH)
I
X P Y
-BASE
O
3, H (2' OH)
FORMULA 1
The scaffold presented above in Formula 1 represents a portion of a
ribonucleic acid.
The basic components are the ribose sugar, the base, the terminal phosphates,
and phosphate
intemucleotide linkers. Where the bases are naturally occurring bases, e.g.,
adenine, uracil,
guanine or cytosine, the sugars are the unmodified 2' hydroxyl ribose sugar
(as depicted) and W,
X, Y, and Z are all 0, Formula 1 represents a naturally occurring unmodified
oligoribonucleotide.
Unmodified oligoribonucleotides may be less than optimal in some applications,
e.g.,
unmodified oligoribonucleotides can be prone to degradation by e.g., cellular
nucleases.
Nucleases can hydrolyze nucleic acid phosphodiester bonds. However, chemical
modifications
28
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
to one or more of the above RNA components can confer improved properties,
and, e.g., can
render oligoribonucleotides more stable to nucleases. Umodified
oligoribonucleotides may also
be less than optimal in terms of offering tethering points for attaching
ligands or other moieties
to an iRNA agent.
Modified nucleic acids and nucleotide surrogates can include one or more of-
(i) alteration, e.g., replacement, of one or both of the non-linking (X and Y)
phosphate
oxygens and/or of one or more of the linking (W and Z) phosphate oxygens (When
the phosphate
is in the terminal position, one of the positions W or Z will not link the
phosphate to an
additional element in a naturally occurring ribonucleic acid. However, for
simplicity of
1o terminology, except where otherwise noted, the W position at the 5' end of
a nucleic acid and the
terminal Z position at the 3' end of a nucleic acid, are within the term
"linking phosphate
oxygens" as used herein.);
(ii) alteration, e.g., replacement, of a constituent of the ribose sugar,
e.g., of the 2'
hydroxyl on the ribose sugar, or wholesale replacement of the ribose sugar
with a structure other
than ribose, e.g., as described herein;
(iii) wholesale replacement of the phosphate moiety (bracket I) with
"dephospho" linkers;
(iv) modification or replacement of a naturally occurring base;
(v) replacement or modification of the ribose-phosphate backbone (bracket II);
(vi) modification of the 3' end or 5' end of the RNA, e.g., removal,
modification or
replacement of a terminal phosphate group or conjugation of a moiety, e.g. a
fluorescently
labeled moiety, to either the 3' or 5' end of RNA.
The terms replacement, modification, alteration, and the like, as used in this
context, do
not imply any process limitation, e.g., modification does not mean that one
must start with a
reference or naturally occurring ribonucleic acid and modify it to produce a
modified ribonucleic
acid bur rather modified simply indicates a difference from a naturally
occurring molecule.
29
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
It is understood that the actual electronic structure of some chemical
entities cannot be
adequately represented by only one canonical form (i.e. Lewis structure).
While not wishing to
be bound by theory, the actual structure can instead be some hybrid or
weighted average of two
or more canonical forms, known collectively as resonance forms or structures.
Resonance
structures are not discrete chemical entities and exist only on paper. They
differ from one
another only in the placement or "localization" of the bonding and nonbonding
electrons for a
particular chemical entity. It can be possible for one resonance structure to
contribute to a
greater extent to the hybrid than the others. Thus, the written and graphical
descriptions of the
embodiments of the present invention are made in terms of what the art
recognizes as the
lo predominant resonance form for a particular species. For example, any
phosphoroamidate
(replacement of a nonlinking oxygen with nitrogen) would be represented by X =
0 and Y = N
in the above figure.
Specific modifications are discussed in more detail below.
The Phosphate Group
The phosphate group is a negatively charged species. The charge is distributed
equally
over the two non-linking oxygen atoms (i.e., X and Y in Formula 1 above).
However, the
phosphate group can be modified by replacing one of the oxygens with a
different substituent.
One result of this modification to RNA phosphate backbones can be increased
resistance of the
oligoribonucleotide to nucleolytic breakdown. Thus while not wishing to be
bound by theory, it
can be desirable in some embodiments to introduce alterations which result in
either an
uncharged linker or a charged linker with unsymmetrical charge distribution.
Examples of modified phosphate groups include phosphorothioate,
phosphoroselenates,
borano phosphates, borano phosphate esters, hydrogen phosphonates,
phosphoroamidates, alkyl
or aryl phosphonates and phosphotriesters. Phosphorodithioates have both non-
linking oxygens
replaced by sulfur. Unlike the situation where only one of X or Y is altered,
the phosphorus
center in the phosphorodithioates is achiral which precludes the formation of
oligoribonucleotides diastereomers. Diastereomer formation can result in a
preparation in which
the individual diastereomers exhibit varying resistance to nucleases. Further,
the hybridization
affinity of RNA containing chiral phosphate groups can be lower relative to
the corresponding
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
unmodified RNA species. Thus, while not wishing to be bound by theory,
modifications to both
X and Y which eliminate the chiral center, e.g. phosphorodithioate formation,
may be desirable
in that they cannot produce diastereomer mixtures. Thus, X can be any one of
S, Se, B, C, H, N,
or OR (R is alkyl or aryl). Thus Y can be any one of S, Se, B, C, H, N, or OR
(R is alkyl or
aryl). Replacement of X and/or Y with sulfur is preferred.
The phosphate linker can also be modified by replacement of a linking oxygen
(i.e., W or
Z in Formula 1) with nitrogen (bridged phosphoroamidates), sulfur (bridged
phosphorothioates)
and carbon (bridged methylenephosphonates). The replacement can occur at a
terminal oxygen
(position W (3') or position Z (5'). Replacement of W with carbon or Z with
nitrogen is
1o preferred.
Candidate agents can be evaluated for suitability as described below.
The Sugar Group
A modified RNA can include modification of all or some of the sugar groups of
the
ribonucleic acid. E.g., the 2' hydroxyl group (OH) can be modified or replaced
with a number of
different "oxy" or "deoxy" substituents. While not being bound by theory,
enhanced stability is
expected since the hydroxyl can no longer be deprotonated to form a 2'
alkoxide ion. The 2'
alkoxide can catalyze degradation by intramolecular nucleophilic attack on the
linker phosphorus
atom. Again, while not wishing to be bound by theory, it can be desirable to
some embodiments
to introduce alterations in which alkoxide formation at the 2' position is not
possible.
Examples of "oxy"-2' hydroxyl group modifications include alkoxy or aryloxy
(OR, e.g.,
R = H, alkyl, cycloalkyl, aryl, aralkyl, heteroaryl or sugar);
polyethyleneglycols (PEG),
O(CH2CH2O)õCH2CH2OR; "locked" nucleic acids (LNA) in which the 2' hydroxyl is
connected,
e.g., by a methylene bridge, to the 4' carbon of the same ribose sugar; O-
AMINE (AMINE =
NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino, or
diheteroaryl amino, ethylene diamine, polyamino) and aminoalkoxy, O(CH2)õ
AMINE, (e.g.,
AMINE = NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl
amino, or diheteroaryl amino, ethylene diamine, polyamino). It is noteworthy
that
oligonucleotides containing only the methoxyethyl group (MOE), (OCH2CH2OCH3, a
PEG
31
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
derivative), exhibit nuclease stabilities comparable to those modified with
the robust
phosphorothioate modification.
"Deoxy" modifications include hydrogen (i.e. deoxyribose sugars, which are of
particular
relevance to the overhang portions of partially ds RNA); halo (e.g., fluoro);
amino (e.g. NH2;
alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl
amino, diheteroaryl
amino, or amino acid); NH(CH2CH2NH)õ CH2CH2-AMINE (AMINE = NH2; alkylamino,
dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl amino,or
diheteroaryl amino), -
NHC(O)R (R = alkyl, cycloalkyl, aryl, aralkyl, heteroaryl or sugar), cyano;
mercapto; alkyl-thio-
alkyl; thioalkoxy; and alkyl, cycloalkyl, aryl, alkenyl and alkynyl, which
maybe optionally
lo substituted with e.g., an amino functionality. Preferred substitutents are
2'-methoxyethyl, 2'-
OCH3, 2'-O-allyl, 2'-C- allyl, and 2'-fluoro.
The sugar group can also contain one or more carbons that possess the opposite
stereochemical configuration than that of the corresponding carbon in ribose.
Thus, a modified
RNA can include nucleotides containing e.g., arabinose, as the sugar.
Modified RNAs can also include "abasic" sugars, which lack a nucleobase at C-
1'. These
abasic sugars can also be further contain modifications at one or more of the
constituent sugar
atoms.
To maximize nuclease resistance, the 2' modifications can be used in
combination with
one or more phosphate linker modifications (e.g., phosphorothioate). The so-
called "chimeric"
oligonucleotides are those that contain two or more different modifications.
The modificaton can also entail the wholesale replacement of a ribose
structure with
another entity at one or more sites in the iRNA agent. These modifications are
described in
section entitled Ribose Replacements for RRMSs.
32
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Candidate modifications can be evaluated as described below.
Replacement of the Phosphate Group
The phosphate group can be replaced by non-phosphorus containing connectors
(cf.
Bracket I in Formula 1 above). While not wishing to be bound by theory, it is
believed that since
the charged phosphodiester group is the reaction center in nucleolytic
degradation, its
replacement with neutral structural mimics should impart enhanced nuclease
stability. Again,
while not wishing to be bound by theory, it can be desirable, in some
embodiment, to introduce
alterations in which the charged phosphate group is replaced by a neutral
moiety.
Examples of moieties which can replace the phosphate group include siloxane,
carbonate,
1o carboxymethyl, carbamate, amide, thioether, ethylene oxide linker,
sulfonate, sulfonamide,
thioformacetal, formacetal, oxime, methyleneimino, methylenemethylimino,
methylenehydrazo,
methylenedimethylhydrazo and methyleneoxymethylimino. Preferred replacements
include the
methylenecarbonylamino and methylenemethylimino groups.
Candidate modifications can be evaluated as described below.
Replacement of Ribophosphate Backbone
Oligonucleotide- mimicking scaffolds can also be constructed wherein the
phosphate
linker and ribose sugar are replaced by nuclease resistant nucleoside or
nucleotide surrogates
(see Bracket II of Formula 1 above). While not wishing to be bound by theory,
it is believed that
the absence of a repetitively charged backbone diminishes binding to proteins
that recognize
polyanions (e.g. nucleases). Again, while not wishing to be bound by theory,
it can be desirable
in some embodiment, to introduce alterations in which the bases are tethered
by a neutral
surrogate backbone.
Examples include the mophilino, cyclobutyl, pyrrolidine and peptide nucleic
acid (PNA)
nucleoside surrogates. A preferred surrogate is a PNA surrogate.
Candidate modifications can be evaluated as described below.
33
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Terminal Modifications
The 3' and 5' ends of an oligonucleotide can be modified. Such modifications
can be at
the 3' end, 5' end or both ends of the molecule. They can include modification
or replacement of
an entire terminal phosphate or of one or more of the atoms of the phosphate
group. E.g., the 3'
and 5' ends of an oligonucleotide can be conjugated to other functional
molecular entities such as
labeling moieties, e.g., fluorophores (e.g., pyrene, TAMRA, fluorescein, Cy3
or Cy5 dyes) or
protecting groups (based e.g., on sulfur, silicon, boron or ester). The
functional molecular
entities can be attached to the sugar through a phosphate group and/or a
spacer. The terminal
atom of the spacer can connect to or replace the linking atom of the phosphate
group or the C-3'
I o or C-5' 0, N, S or C group of the sugar. Alternatively, the spacer can
connect to or replace the
terminal atom of a nucleotide surrogate (e.g., PNAs). These spacers or linkers
can include e.g., -
(CH2)n , -(CH2)õN-, -(CH2)õ O-, -(CH2)r,S-, O(CH2CH2O)õ CH2CH2OH (e.g., n = 3
or 6), abasic
sugars, amide, carboxy, amine, oxyamine, oxyimine, thioether, disulfide,
thiourea, sulfonamide,
or morpholino, or biotin and fluorescein reagents. When a spacer/phosphate-
functional
molecular entity-spacer/phosphate array is interposed between two strands of
iRNA agents, this
array can substitute for a hairpin RNA loop in a hairpin-type RNA agent. The
3' end can be an -
OH group. While not wishing to be bound by theory, it is believed that
conjugation of certain
moieties can improve transport, hybridization, and specificity properties.
Again, while not
wishing to be bound by theory, it may be desirable to introduce terminal
alterations that improve
nuclease resistance. Other examples of terminal modifications include dyes,
intercalating agents
(e.g. acridines), cross-linkers (e.g. psoralene, mitomycin C), porphyrins
(TPPC4, texaphyrin,
Sapphyrin), polycyclic aromatic hydrocarbons (e.g., phenazine,
dihydrophenazine), artificial
endonucleases (e.g. EDTA), lipophilic carriers (e.g., cholesterol, cholic
acid, adamantane acetic
acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol, geranyloxyhexyl
group, hexadecylglycerol, bomeol, menthol, 1,3-propanediol, heptadecyl group,
palmitic acid,
myristic acid,03-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid,
dimethoxytrityl, or
phenoxazine)and peptide conjugates (e.g., antennapedia peptide, Tat peptide),
alkylating agents,
phosphate, amino, mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]2, polyamino,
alkyl,
substituted alkyl, radiolabeled markers, enzymes, haptens (e.g. biotin),
transport/absorption
facilitators (e.g., aspirin, vitamin E, folic acid), synthetic ribonucleases
(e.g., imidazole,
34
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
bisimidazole, histamine, imidazole clusters, acridine-imidazole conjugates,
Eu3+ complexes of
tetraazamacrocycles).
Terminal modifications can be added for a number of reasons, including as
discussed
elsewhere herein to modulate activity or to modulate resistance to
degradation. Terminal
modifications useful for modulating activity include modification of the 5'
end with phosphate or
phosphate analogs. E.g., in preferred embodiments iRNA agents, especially
antisense strands,
are 5' phosphorylated or include a phosphoryl analog at the 5' prime terminus.
5'-phosphate
modifications include those which are compatible with RISC mediated gene
silencing. Suitable
modifications include: 5'-monophosphate ((HO)2(O)P-O-5'); 5'-diphosphate
((HO)2(O)P-O-
1o P(HO)(O)-0-5'); 5'-triphosphate ((HO)2(O)P-O-(HO)(O)P-O-P(HO)(O)-0-5'); 5'-
guanosine cap
(7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-0-
5'); 5'-
adenosine cap (Appp), and any modified or unmodified nucleotide cap structure
(N-O-5'-
(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-0-5'); 5'-monothiophosphate (phosphorothioate;
(HO)2(S)P-O-5'); 5'-monodithiophosphate (phosphorodithioate; (HO)(HS)(S)P-O-
5'), 5'-
phosphorothiolate ((HO)2(O)P-S-5'); any additional combination of oxgen/sulfur
replaced
monophosphate, diphosphate and triphosphates (e.g. 5'-alpha-thiotriphosphate,
5'-gamma-
thiotriphosphate, etc.), 5'-phosphoramidates ((HO)2(O)P-NH-5', (HO)(NH2)(O)P-O-
5'), 5'-
alkylphosphonates (R=alkyl=methyl, ethyl, isopropyl, propyl, etc., e.g.
RP(OH)(O)-0-5'-,
(OH)2(O)P-5'-CH2-), 5'-alkyletherphosphonates (R=alkylether=methoxymethyl
(MeOCH2-),
ethoxymethyl, etc., e.g. RP(OH)(O)-0-5'-).
Terminal modifications can also be useful for monitoring distribution, and in
such cases
the preferred groups to be added include fluorophores, e.g., fluorscein or an
Alexa dye, e.g.,
Alexa 488. Terminal modifications can also be useful for enhancing uptake,
useful
modifications for this include cholesterol. Terminal modifications can also be
useful for cross-
linking an RNA agent to another moiety; modifications useful for this include
mitomycin C.
Candidate modifications can be evaluated as described below.
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The Bases
Adenine, guanine, cytosine and uracil are the most common bases found in RNA.
These
bases can be modified or replaced to provide RNA's having improved properties.
E.g., nuclease
resistant oligoribonucleotides can be prepared with these bases or with
synthetic and natural
nucleobases (e.g., inosine, thymine, xanthine, hypoxanthine, nubularine,
isoguanisine, or
tubercidine) and any one of the above modifications. Alternatively,
substituted or modified
analogs of any of the above bases, e.g., "unusual bases" and "universal
bases," can be employed.
Examples include without limitation 2-aminoadenine, 6-methyl and other alkyl
derivatives of
adenine and guanine, 2-propyl and other alkyl derivatives of adenine and
guanine, 5-halouracil
1o and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and
thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-halouracil, 5-(2-aminopropyl)uracil, 5-amino
allyl uracil, 8-halo,
amino, thiol, thioalkyl, hydroxyl and other 8-substituted adenines and
guanines, 5-
trifluoromethyl and other 5-substituted uracils and cytosines, 7-
methylguanine, 5-substituted
pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines,
including 2-
aminopropyladenine, 5-propynyluracil and 5-propynylcytosine, dihydrouracil, 3-
deaza-5-
azacytosine, 2-aminopurine, 5-alkyluracil, 7-alkylguanine, 5-alkyl cytosine,7-
deazaadenine, N6,
N6-dimethyladenine, 2,6-diaminopurine, 5-amino-allyl-uracil, N3-methyluracil,
substituted
1,2,4-triazoles, 2-pyridinone, 5-nitroindole, 3-nitropyrrole, 5-methoxyuracil,
uracil-5-oxyacetic
acid, 5-methoxycarbonylmethyluracil, 5-methyl-2-thiouracil, 5-
methoxycarbonylmethyl-2-
thiouracil, 5-methylaminomethyl-2-thiouracil, 3-(3-amino-
3carboxypropyl)uracil, 3-
methylcytosine, 5-methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-
methyladenine, N6-
isopentyladenine, 2-methylthio-N6-isopentenyladenine, N-methylguanines, or 0-
alkylated bases.
Further purines and pyrimidines include those disclosed in U.S. Pat. No.
3,687,808, those
disclosed in the Concise Encyclopedia Of Polymer Science And Engineering,
pages 858-859,
Kroschwitz, J. I., ed. John Wiley & Sons, 1990, and those disclosed by
Englisch et al.,
Angewandte Chemie, International Edition, 1991, 30, 613.
Generally, base changes are less preferred for promoting stability, but they
can be useful
for other reasons, e.g., some, e.g., 2,6-diaminopurine and 2 amino purine, are
fluorescent.
Modified bases can reduce target specificity. This should be taken into
consideration in the
3o design of iRNA agents.
36
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Candidate modifications can be evaluated as described below.
Evaluation of Candidate RNA's
One can evaluate a candidate RNA agent, e.g., a modified RNA, for a selected
property
by exposing the agent or modified molecule and a control molecule to the
appropriate conditions
and evaluating for the presence of the selected property. For example,
resistance to a degradent
can be evaluated as follows. A candidate modified RNA (and preferably a
control molecule,
usually the unmodified form) can be exposed to degradative conditions, e.g.,
exposed to a milieu,
which includes a degradative agent, e.g., a nuclease. E.g., one can use a
biological sample, e.g.,
one that is similar to a milieu, which might be encountered, in therapeutic
use, e.g., blood or a
1o cellular fraction, e.g., a cell-free homogenate or disrupted cells. The
candidate and control could
then be evaluated for resistance to degradation by any of a number of
approaches. For example,
the candidate and control could be labeled, preferably prior to exposure,
with, e.g., a radioactive
or enzymatic label, or a fluorescent label, such as Cy3 or Cy5. Control and
modified RNA's can
be incubated with the degradative agent, and optionally a control, e.g., an
inactivated, e.g., heat
inactivated, degradative agent. A physical parameter, e.g., size, of the
modified and control
molecules are then determined. They can be determined by a physical method,
e.g., by
polyacrylamide gel electrophoresis or a sizing column, to assess whether the
molecule has
maintained its original length, or assessed functionally. Alternatively,
Northern blot analysis can
be used to assay the length of an unlabeled modified molecule.
A functional assay can also be used to evaluate the candidate agent. A
functional assay
can be applied initially or after an earlier non-functional assay, (e.g.,
assay for resistance to
degradation) to determine if the modification alters the ability of the
molecule to silence gene
expression. For example, a cell, e.g., a mammalian cell, such as a mouse or
human cell, can be
co-transfected with a plasmid expressing a fluorescent protein, e.g., GFP, and
a candidate RNA
agent homologous to the transcript encoding the fluorescent protein (see,
e.g., WO 00/44914).
For example, a modified dsRNA homologous to the GFP mRNA can be assayed for
the ability to
inhibit GFP expression by monitoring for a decrease in cell fluorescence, as
compared to a
control cell, in which the transfection did not include the candidate dsRNA,
e.g., controls with no
agent added and/or controls with a non-modified RNA added. Efficacy of the
candidate agent on
37
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
gene expression can be assessed by comparing cell fluorescence in the presence
of the modified
and unmodified dsRNA agents.
In an alternative functional assay, a candidate dsRNA agent homologous to an
endogenous mouse gene, preferably a maternally expressed gene, such as c-rnos,
can be injected
into an immature mouse oocyte to assess the ability of the agent to inhibit
gene expression in
vivo (see, e.g., WO 01/36646). A phenotype of the oocyte, e.g., the ability to
maintain arrest in
metaphase II, can be monitored as an indicator that the agent is inhibiting
expression. For
example, cleavage of c-rnos mRNA by a dsRNA agent would cause the oocyte to
exit metaphase
arrest and initiate parthenogenetic development (Colledge et al. Nature 370:
65-68, 1994;
Hashimoto et al. Nature, 370:68-71, 1994). The effect of the modified agent on
target RNA
levels can be verified by Northern blot to assay for a decrease in the level
of target mRNA, or by
Western blot to assay for a decrease in the level of target protein, as
compared to a negative
control. Controls can include cells in which with no agent is added and/or
cells in which a non-
modified RNA is added.
References
General References
The oligoribonucleotides and oligoribonucleosides used in accordance with this
invention
may be with solid phase synthesis, see for example "Oligonucleotide synthesis,
a practical
approach", Ed. M. J. Gait, IRL Press, 1984; "Oligonucleotides and Analogues, A
Practical
Approach", Ed. F. Eckstein, IRL Press, 1991 (especially Chapter 1, Modem
machine-aided
methods of oligodeoxyribonucleotide synthesis, Chapter 2, Oligoribonucleotide
synthesis,
Chapter 3, 2'-O--Methyloligoribonucleotide- s: synthesis and applications,
Chapter 4,
Phosphorothioate oligonucleotides, Chapter 5, Synthesis of oligonucleotide
phosphorodithioates,
Chapter 6, Synthesis of oligo-2'-deoxyribonucleoside methylphosphonates, and.
Chapter 7,
Oligodeoxynucleotides containing modified bases. Other particularly useful
synthetic
procedures, reagents, blocking groups and reaction conditions are described in
Martin, P., Helv.
Chinn. Acta, 1995, 78, 486-504; Beaucage, S. L. and Iyer, R. P., Tetrahedron,
1992, 48, 2223-
2311 and Beaucage, S. L. and Iyer, R. P., Tetrahedron, 1993, 49, 6123-6194, or
references
referred to therein.
38
CA 02521464 2011-06-30
51912-8
Modification described in WO 00/44895, W001175164, or W002/44321 can be used
herein.
Phosphate Group References
The preparation of phosphinate oligon'bonucleotides is described in U.S. Pat.
No.
5,508,270. The preparation of alkyl phosphonate oligoribonucleotides is
described in U.S. Pat.
No. 4,469,863. The preparation of phosphoramidite oligoribonucleotides is
described in U.S.
Pat. No. 5,256,775 or U.S. Pat. No. 5,366,878. The preparation of
phosphotriester
oligoribonucleotides is described in U.S. Pat. No. 5,023,243. The preparation
of borano
phosphate oligoribonucleotide is described in U.S. Pat. Nos. 5,130,302 and
5,17,7,198. The
preparation of 3'-Deoxy-3'-amino phosphoramidate oligoribonucleotides is
described in U.S. Pat.
No. 5,476,925. 3'-Deoxy-3'-methylenephosphonate oligonboniicleotides is
described in An, H,
et al. J. Org Chem. 2001, 66, 2789-2801. Preparation of sulfur bridged
nucleotides is described
in Sproat et al. Nucleosides Nucleotides 1988, 7,651 and Crosstick et al.
Tetrahedron Lett. 1989,
30,4693.
Sugar Group References
Modifications to the 2' modifications can be found in Verma, S. et al. Annu.
Rev.
Biochem.1998, 67, 99-134 and all references therein. Specific modifications to
the ribose can be
found in the following references: 2'-fluoro (Kawasaki et. al., J. Merl Chem.,
1993,36,831-
841), 2'-MOE (Martin, P. HeIv. Chim. Acta 1996, 79, 1930-1938), "LNA" (Wengel,
J. Acc
Chem. Res. 1999, 32, 301-310).
Replacement of the Phosphate Croup References
Methylenemethylimino linked oligoribonucleosides, also identified herein as
MMI linked
oligoribonucleosides, methylenedimethylhydrazo linked oligoribonucleosides,
also identified
herein as MDH linked oligoribonucleosides, and methylenecarbonylamino linked
oligonucleosides, also identified herein as amide-3 linked
oligoribonucleosides, and
39
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
methyleneaminocarbonyl linked oligonucleosides, also identified herein as
amide-4 linked
oligoribonucleosides as well as mixed backbone compounds having, as for
instance, alternating
MMI and PO or PS linkages can be prepared as is described in U.S. Pat. Nos.
5,378,825,
5,386,023, 5,489,677 and in published PCT applications PCT/US92/04294 and
PCT/US92/04305 (published as WO 92/20822 WO and 92/20823, respectively).
Formacetal and
thioformacetal linked oligoribonucleosides can be prepared as is described in
U.S. Pat. Nos.
5,264,562 and 5,264,564. Ethylene oxide linked oligoribonucleosides can be
prepared as is
described in U.S. Pat. No. 5,223,618. Siloxane replacements are described in
Cormier,J.F. et al.
Nucleic Acids Res. 1988, 16, 4583. Carbonate replacements are described in
Tittensor, J.R. J.
1o Chem. Soc. C 1971, 1933. Carboxymethyl replacements are described in Edge,
M.D. et al. J
Chem. Soc. Perkin Trans. 1 1972, 1991. Carbamate replacements are described in
Stirchak, E.P.
Nucleic Acids Res. 1989, 17, 6129.
Replacement of the Phosphate-Ribose Backbone References
Cyclobutyl sugar surrogate compounds can be prepared as is described in U.S.
Pat. No.
5,359,044. Pyrrolidine sugar surrogate can be prepared as is described in U.S.
Pat. No.
5,519,134. Morpholino sugar surrogates can be prepared as is described in U.S.
Pat. Nos.
5,142,047 and 5,235,033, and other related patent disclosures. Peptide Nucleic
Acids (PNAs) are
known per se and can be prepared in accordance with any of the various
procedures referred to in
Peptide Nucleic Acids (PNA): Synthesis, Properties and Potential Applications,
Bioorganic &
Medicinal Chemistry, 1996, 4, 5-23. They may also be prepared in accordance
with U.S. Pat. No.
5,539,083.
Terminal Modification References
Terminal modifications are described in Manoharan, M. et al. Antisense and
Nucleic Acid
Drug Development 12, 103-128 (2002) and references therein.
Bases References
N-2 substitued purine nucleoside amidites can be prepared as is described in
U.S. Pat.
No. 5,459,255. 3-Deaza purine nucleoside amidites can be prepared as is
described in U.S. Pat.
No. 5,457,191. 5,6-Substituted pyrimidine nucleoside amidites can be prepared
as is described in
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO1
U.S. Pat. No. 5,614,617. 5-Propynyl pyrimidine nucleoside amidites can be
prepared as is
described in U.S. Pat. No. 5,484,908. Additional references can be disclosed
in the above
section on base modifications.
41
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Preferred iRNA Agents
Preferred RNA agents have the following structure (see Formula 2 below):
Al
RI
O
R7
x
A2 R4
R2
O
R7
i
A3 R5
R3
O
R7
Ate` ''/~
4 R6
FORMULA 2
Referring to Formula 2 above, R1, R2, and R3 are each, independently, H, (i.e.
abasic
nucleotides), adenine, guanine, cytosine and uracil, inosine, thymine,
xanthine, hypoxanthine,
nubularine, tubercidine, isoguanisine, 2-aminoadenine, 6-methyl and other
alkyl derivatives of
adenine and guanine, 2-propyl and other alkyl derivatives of adenine and
guanine, 5-halouracil
and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and
thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-halouracil, 5-(2-aminopropyl)uracil, 5-amino
allyl uracil, 8-halo,
amino, thiol, thioalkyl, hydroxyl and other 8-substituted adenines and
guanines, 5-
42
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
trifluoromethyl and other 5-substituted uracils and cytosines, 7-
methylguanine, 5-substituted
pyrimidines, 6-azapyrimidines and N-2, N-6 and 0-6 substituted purines,
including 2-
aminopropyladenine, 5-propynyluracil and 5-propynylcytosine, dihydrouracil, 3-
deaza-5-
azacytosine, 2-aminopurine, 5-alkyluracil, 7-alkylguanine, 5-alkyl cytosine,7-
deazaadenine, 7-
deazaguanine, N6, N6-dimethyladenine, 2,6-diaminopurine, 5-amino-allyl-uracil,
N3-
methyluracil, substituted 1,2,4-triazoles, 2-pyridinone, 5-nitroindole, 3-
nitropyrrole, 5-
methoxyuracil, uracil-5-oxyacetic acid, 5-methoxycarbonylmethyluracil, 5-
methyl-2-thiouracil,
5-methoxycarbonylmethyl-2-thiouracil, 5-methylaminomethyl-2-thiouracil, 3-(3-
amino-
3carboxypropyl)uracil, 3-methylcytosine, 5-methylcytosine, N4-acetyl cytosine,
2-thiocytosine,
1 o N6-methyladenine, N6-isopentyladenine, 2-methylthio-N6-isopentenyladenine,
N-
methylguanines, or O-alkylated bases.
R4, R5, and R6 are each, independently, ORB, O(CH2CH2O),CH2CH2OR8; O(CH2)r,R9;
O(CH2)õ OR9, H; halo; NH2; NHR8; N(R)2; NH(CH2CH2NH)mCH2CH2NHR9; NHC(O)R8; ;
cyano; mercapto, SR8; alkyl-thio-alkyl; alkyl, aralkyl, cycloalkyl, aryl,
heteroaryl, alkenyl,
alkynyl, each of which may be optionally substituted with halo, hydroxy, oxo,
nitro, haloalkyl,
alkyl, alkaryl, aryl, aralkyl, alkoxy, aryloxy, amino, alkylamino,
dialkylamino, heterocyclyl,
arylamino, diaryl amino, heteroaryl amino, diheteroaryl amino, acylamino,
alkylcarbamoyl,
arylcarbamoyl, aminoalkyl, alkoxycarbonyl, carboxy, hydroxyalkyl,
alkanesulfonyl,
alkanesulfonamido, arenesulfonamido, aralkylsulfonamido, alkylcarbonyl,
acyloxy, cyano, or
ureido; or R4, R5, or R6 together combine with R7 to form an [-O-CH2-]
covalently bound bridge
between the sugar 2' and 4' carbons.
43
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Al is:
W1
I
XI 1-Yl
W1 Z,
X P'1 or
1 ZI Zl
or
X1 P Y1 X
1I Y1 X1 I Y1 I
Z, Z1 Zl
1
; H; OH; OCH3; W'; an abasic nucleotide; or absent;
i
(a preferred Al , especially with regard to anti-sense strands, is chosen from
5'-
monophosphate ((HO)2(O)P-O-5'), 5'-diphosphate ((HO)2(O)P-O-P(HO)(O)-0-5'), 5'-
triphosphate ((HO)2(O)P-O-(HO)(O)P-O-P(HO)(O)-0-5'), 5'-guanosine cap (7-
methylated or
non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-0-5'), 5'-adenosine
cap
(Appp), and any modified or unmodified nucleotide cap structure (N-O-5'-
(HO)(O)P-O-
(HO)(O)P-O-P(HO)(O)-0-5'), 5'-monothiophosphate (phosphorothioate; (HO)2(S)P-O-
5'), 5'-
monodithiophosphate (phosphorodithioate; (HO)(HS)(S)P-O-5'), 5'-
phosphorothiolate
((HO)2(O)P-S-5'); any additional combination of oxgen/sulfur replaced
monophosphate,
diphosphate and triphosphates (e.g. 5'-alpha-thiotriphosphate, 5'-gamma-
thiotriphosphate, etc.),
5'-phosphoramidates ((HO)2(O)P-NH-5', (HO)(NH2)(O)P-O-5'),'5'-
alkylphosphonates
(R=alkyl=methyl, ethyl, isopropyl, propyl, etc., e.g. RP(OH)(O)-0-5'-,
(OH)2(O)P-5'-CH2-), 5'-
44
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
alkyletherphosphonates (R=alkylether=methoxymethyl (MeOCH2-), ethoxymethyl,
etc., e.g.
RP(OH)(O)-0-5'-)).
A2 is:
Z2
X2 i Y2
Z2
A3 is:
z3
x3 i Y3
Z3
and
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
A4 is:
Z1
I
X4 P Y4
II
Z1 Z,
X i Y4 Or X4 P Y4
4
W4 Z1 Z1
Or
x4 I X4 ,P Y4 4 I 4
Z4 Z4 Z4
; H; Z4; an inverted nucleotide; an abasic nucleotide; or absent.
W' is OH, (CH2)õR10, (CH2)õ NHR10, (CH2)õ OR10, (CH2)õ SR10; O(CH2)õR1 ;
O(CH2)õOR10, O(CH2)r,NR10, O(CH2).SR10; O(CH2)õ SS(CH2)õ OR10,
O(CH2)nC(O)OR10,
NH(CH2)õR10; NH(CH2)õ NR10 ;NH(CH2)õOR10, NH(CH2)õSR10; S(CH2)õR10,
S(CH2)õNR10,
S(CH2)õ OR10, S(CH2)õSR10 O(CH2CH2O)mCH2CH2OR10; O(CH2CH2O),,,CH2CH2NHR10
NH(CH2CH2NH)mCH2CH2NHR10; Q-R10, O-Q-R10 N-Q-R10, S-Q-R10 or -0-. W4 is 0,
CH2,
NH, or S.
XI, X2, X3, and X4 are each, independently, 0 or S.
Y1, Y2, Y3, and Y4 are each, independently, OH, 0-, ORB, S, Se, BH3-, H, NHR9,
N(R9)2
alkyl, cycloalkyl, aralkyl, aryl, or heteroaryl, each of which may be
optionally substituted.
Z1, Z2, and Z3 are each independently 0, CH2, NH, or S. Z4 is OH, (CH2)õR10,
(CH2)õNHR10, (CH2)õ OR'O, (CH2)õ SR10; O(CH2) R10; O(CH2)nOR10, O(CH2)õNR10,
46
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
O(CH2)r,SR10, O(CH2)õ SS(CH2)õ OR10, O(CH2)õC(O)OR10; NH(CH2)õ R10; NH(CH2)õ
NR10
;NH(CH2)õ OR10, NH(CH2)õSR10; S(CH2).R10, S(CH2).NR10, S(CH2)õOR10,
S(CH2)õSR1O
O(CH2CH2O)mCH2CH2OR10, O(CH2CH2O),,,CH2CH2NHR10 ,
NH(CH2CH2NH)mCH2CH2NHR10; Q-R10, O-Q-R1 N-Q-R'O, S-Q-RIO.
x is 5-100, chosen to comply with a length for an RNA agent described herein.
R7 is H; or is together combined with R4, R5, or R6 to form an [-O-CH2-]
covalently
bound bridge between the sugar 2' and 4' carbons.
R8 is alkyl, cycloalkyl, aryl, aralkyl, heterocyclyl, heteroaryl, amino acid,
or sugar; R9 is
NH2, alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
1o diheteroaryl amino, or amino acid; and R10 is H; fluorophore (pyrene,
TAMRA, fluorescein, Cy3
or Cy5 dyes); sulfur, silicon, boron or ester protecting group; intercalating
agents (e.g. acridines),
cross-linkers (e.g. psoralene, mitomycin C), porphyrins (TPPC4,texaphyrin,
Sapphyrin),
polycyclic aromatic hydrocarbons (e.g., phenazine, dihydrophenazine),
artificial endonucleases
(e.g. EDTA), lipohilic carriers (cholesterol, cholic acid, adamantane acetic
acid, 1-pyrene butyric
acid, dihydrotestosterone, 1,3-Bis-O(hexadecyl)glycerol, geranyloxyhexyl
group,
hexadecylglycerol, borneol, menthol, 1,3-propanediol, heptadecyl group,
palmitic acid,myristic
acid,03-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid, dimethoxytrityl,
or phenoxazine)and
peptide conjugates (e.g., antennapedia peptide, Tat peptide), alkylating
agents, phosphate, amino,
mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]2, polyamino; alkyl, cycloalkyl,
aryl, aralkyl,
2o heteroaryl; radiolabelled markers, enzymes, haptens (e.g. biotin),
transport/absorption facilitators
(e.g., aspirin, vitamin E, folic acid), synthetic ribonucleases (e.g.,
imidazole, bisimidazole,
histamine, imidazole clusters, acridine-imidazole conjugates, Eu3+ complexes
of
tetraazamacrocycles); or an RNA agent. in is 0-1,000,000, and n is 0-20. Q is
a spacer selected
from the group consisting of abasic sugar, amide, carboxy, oxyamine, oxyimine,
thioether,
disulfide, thiourea, sulfonamide, or morpholino, biotin or fluorescein
reagents.
47
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Preferred RNA agents in which the entire phosphate group has been replaced
have the
following structure (see Formula 3 below):
A1o
Rio
O
Rio
A20 R40
O
R7o
Aso R50
Rso
O
Rio
A40 R60
FORMULA 3
5 Referring to Formula 3, A1 -A40 is L-G-L; A10 and/or A4 may be absent, in
which L is a
linker, wherein one or both L may be present or absent and is selected from
the group consisting
of CH2(CH2)g; N(CH2)g; O(CH2)g; S(CH2)g. G is a functional group selected from
the group
consisting of siloxane, carbonate, carboxymethyl, carbamate, amide, thioether,
ethylene oxide
linker, sulfonate, sulfonamide, thioformacetal, formacetal, oxime,
methyleneimino,
lo methylenemethylimino, methylenehydrazo, methylenedimethylhydrazo and
methyleneoxymethylimino.
48
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R10, R20, and R30 are each, independently, H, (i.e. abasic nucleotides),
adenine, guanine,
cytosine and uracil, inosine, thymine, xanthine, hypoxanthine, nubularine,
tubercidine,
isoguanisine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine
and guanine, 2-
propyl and other alkyl derivatives of adenine and guanine, 5-halouracil and
cytosine, 5-propynyl
uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-
halouracil, 5-(2-aminopropyl)uracil, 5-amino allyl uracil, 8-halo, amino,
thiol, thioalkyl,
hydroxyl and other 8-substituted adenines and guanines, 5-trifluoromethyl and
other 5-
substituted uracils and cytosines, 7-methylguanine, 5-substituted pyrimidines,
6-azapyrimidines
and N-2, N-6 and 0-6 substituted purines, including 2-aminopropyladenine, 5-
propynyluracil
and 5-propynylcytosine, dihydrouracil, 3-deaza-5-azacytosine, 2-aminopurine, 5-
alkyluracil, 7-
alkylguanine, 5-alkyl cytosine,7-deazaadenine, 7-deazaguanine, N6, N6-
dimethyladenine, 2,6-
diaminopurine, 5-amino-allyl-uracil, N3-methyluracil substituted 1,2,4-
triazoles, 2-pyridinone,
5-nitroindole, 3-nitropyrrole, 5-methoxyuracil, uracil-5-oxyacetic acid, 5-
methoxycarbonylmethyluracil, 5-methyl-2-thiouracil, 5-methoxycarbonylmethyl-2-
thiouracil, 5-
methylaminomethyl-2-thiouracil, 3-(3-amino-3carboxypropyl)uracil, 3-
methylcytosine, 5-
methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-methyladenine, N6-
isopentyladenine, 2-
methylthio-N6-isopentenyladenine, N-methylguanines, or O-alkylated bases.
R40, R50, and R60 are each, independently, ORB, O(CH2CH2O)mCH2CH2OR8; O(CH2)õ
R9;
O(CH2)õ OR9, H; halo; NH2; NHR3; N(R8)2; NH(CH2CH2NH),,,CH2CH2R9; NHC(O)R8;;
cyan;
mercapto, SR7; alkyl-thio-alkyl; alkyl, aralkyl, cycloalkyl, aryl, heteroaryl,
alkenyl, alkynyl, each
of which may be optionally substituted with halo, hydroxy, oxo, nitro,
haloalkyl, alkyl, alkaryl,
aryl, aralkyl, alkoxy, aryloxy, amino, alkylamino, dialkylamino, heterocyclyl,
arylamino, diaryl
amino, heteroaryl amino, diheteroaryl amino, acylamino, alkylcarbamoyl,
arylcarbamoyl,
aminoalkyl, alkoxycarbonyl, carboxy, hydroxyalkyl, alkanesulfonyl,
alkanesulfonamido,
arenesulfonamido, aralkylsulfonamido, alkylcarbonyl, acyloxy, cyano, and
ureido groups; or R40,
R50, or R60 together combine with R70 to form an [-O-CH2-] covalently bound
bridge between the
sugar 2' and 4' carbons.
x is 5-100 or chosen to comply with a length for an RNA agent described
herein.
49
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R7 is H; or is together combined with R40, R50, or R60 to form an [-O-CH2-]
covalently
bound bridge between the sugar 2' and 4' carbons.
R8 is alkyl, cycloalkyl, aryl, aralkyl, heterocyclyl, heteroaryl, amino acid,
or sugar; and
R9 is NH2, alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid. m is 0-1,000,000, n is 0-20, and g is 0-2.
Preferred nucleoside surrogates have the following structure (see Formula 4
below):
SLR100-(M-SLR200)X M-SLR3
FORMULA 4
S is a nucleoside surrogate selected from the group consisting of mophilino,
cyclobutyl,
pyrrolidine and peptide nucleic acid. L is a linker and is selected from the
group consisting of
CH2(CH2)g; N(CH2)g; O(CH2)g; S(CH2)g; -C(O)(CH2)n or maybe absent. M is an
amide bond;
sulfonamide; sulfinate; phosphate group; modified phosphate group as described
herein; or may
be absent.
R100, R200, and R300 are each, independently, H (i.e., abasic nucleotides),
adenine,
guanine, cytosine and uracil, inosine, thymine, xanthine, hypoxanthine,
nubularine, tubercidine,
isoguanisine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine
and guanine, 2-
propyl and other alkyl derivatives of adenine and guanine, 5-halouracil and
cytosine, 5-propynyl
uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-
2o halouracil, 5-(2-aminopropyl)uracil, 5-amino allyl uracil, 8-halo, amino,
thiol, thioalkyl,
hydroxyl and other 8-substituted adenines and guanines, 5-trifluoromethyl and
other 5-
substituted uracils and cytosines, 7-methylguanine, 5-substituted pyrimidines,
6-azapyrimidines
and N-2, N-6 and 0-6 substituted purines, including 2-aminopropyladenine, 5-
propynyluracil
and 5-propynylcytosine, dihydrouracil, 3-deaza-5-azacytosine, 2-aminopurine, 5-
alkyluracil, 7-
alkylguanine, 5-alkyl cytosine,7-deazaadenine, 7-deazaguanine, N6, N6-
dimethyladenine, 2,6-
diaminopurine, 5-amino-allyl-uracil, N3-methyluracil substituted 1, 2, 4,-
triazoles, 2-
pyridinones, 5-nitroindole, 3-nitropyrrole, 5-methoxyuracil, uracil-5-
oxyacetic acid, 5-
CA 02521464 2011-06-30
51912-8
methoxycarbonylmethyluracil, 5-methyl-2-thiouracil, 5-methoxycarbonylmethyl-2
thiouracil, 5-
methylaminomethyl-2-thiouracil, 3-(3-amino-3carboxypropyl)uracil, 3-
methylcytosine, 5-
methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-methyladenine, N6-
isopentyladenine, 2-
methylthio-N6-isopentenyladenine, N-methylguanines, or O-alkylated bases.
x is 5-100, or chosen to comply with a length for an RNA agent described
herein; and g is
0-2.
Nuclease resistant monomers
An RNA, e.g., an iRNA agent, can incorporate a nuclease resistant monomer
(NRM),
such as those described herein and those described in copending, co-owned
United States
Provisional Application Serial No. 60/469,612, filed on May 9, 2003, and
International
Application No. PCT/USO4/07070.
In addition, the invention includes iRNA agents having an NRM and another
element
described herein. E.g., the invention includes an iRNA agent described herein,
e.g., a
palindromic iRNA agent, an iRNA agent having a non canonical pairing, an iRNA
agent which
targets a gene described herein, e.g., a gene active in the liver, an iRNA
agent having an-
architecture or structure described herein, an iRNA associated with an
amphipathic delivery
agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
which also incorporates an NRM.
An iRNA agent can include monomers which have been modifed so as to inhibit
degradation, e.g., by nucleases, e.g., endonucleases or exonucleases, found in
the body of a
subject. These monomers are referred to herein as NRMs, or nuclease resistance
promoting
monomers or modifications. In many cases these modifications will modulate
other properties of
the iRNA agent as well, e.g., the ability to interact with a protein, e.g., a
transport protein, e.g.,
serum albumin, or a member of the RISC (RNA-induced Silencing Complex), or the
ability of
the first and second sequences to form a duplex with one another or to form a
duplex with
another sequence, e.g., a target molecule.
51
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
While not wishing to be bound by theory, it is believed that modifications of
the sugar,
base, and/or phosphate backbone in an iRNA agent can enhance endonuclease and
exonuclease
resistance, and can enhance interactions with transporter proteins and one or
more of the
functional components of the RISC complex. Preferred modifications are those
that increase
exonuclease and endonuclease resistance and thus prolong the half-life of the
iRNA agent prior
to interaction with the RISC complex, but at the same time do not render the
iRNA agent
resistant to endonuclease activity in the RISC complex. Again, while not
wishing to be bound by
any theory, it is believed that placement of the modifications at or near the
3' and/or 5' end of
antisense strands can result in iRNA agents that meet the preferred nuclease
resistance criteria
lo delineated above. Again, still while not wishing to be bound by any theory,
it is believed that
placement of the modifications at e.g., the middle of a sense strand can
result in iRNA agents
that are relatively less likely to undergo off-targeting.
Modifications described herein can be incorporated into any double-stranded
RNA and
RNA-like molecule described herein, e.g., an iRNA agent. An iRNA agent may
include a duplex
comprising a hybridized sense and antisense strand, in which the antisense
strand and/or the
sense strand may include one or more of the modifications described herein.
The anti sense
strand may include modifications at the 3' end and/or the 5' end and/or at one
or more positions
that occur 1-6 (e.g., 1-5, 1-4, 1-3, 1-2) nucleotides from either end of the
strand. The sense
strand may include modifications at the 3' end and/or the 5' end and/or at any
one ofthe
intervening positions between the two ends of the strand. The iRNA agent may
also include a
duplex comprising two hybridized antisense strands. The first and/or the
second antisense strand
may include one or more of the modifications described herein. Thus, one
and/or both antisense
strands may include modifications at the 3' end and/or the 5' end and/or at
one or more positions
that occur 1-6 (e.g., 1-5, 1-4, 1-3, 1-2) nucleotides from either end of the
strand. Particular
configurations are discussed below.
Modifications that can be useful for producing iRNA agents that meet the
preferred
nuclease resistance criteria delineated above can include one or more of the
following chemical
and/or stereochemical modifications of the sugar, base, and/or phosphate
backbone:
52
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(i) chiral (Sp) thioates. Thus, preferred NRMs include nucleotide dimers with
an enriched
or pure for a particular chiral form of a modified phosphate group containing
a heteroatom at the
nonbridging position, e.g., Sp or Rp, at the position X, where this is the
position normally
occupied by the oxygen. The atom at X can also be S, Se, Nr2, or Bra. When X
is S, enriched or
chirally pure Sp linkage is preferred. Enriched means at least 70, 80, 90, 95,
or 99% of the
preferred form. Such NRMs are discussed in more detail below;
(ii) attachment of one or more cationic groups to the sugar, base, and/or the
phosphorus
atom of a phosphate or modified phosphate backbone moiety. Thus, preferred
NRMs include
monomers at the terminal position derivatized at a cationic group. As the 5'
end of an antisense
lo sequence should have a terminal -OH or phosphate group this NRM is
preferably not used at the
5' end of an anti-sense sequence. The group should be attached at a position
on the base which
minimizes interference with H bond formation and hybridization, e.g., away
form the face which
interacts with the complementary base on the other strand, e.g, at the 5'
position of a pyrimidine
or a 7-position of a purine. These are discussed in more detail below;
(iii) nonphosphate linkages at the termini. Thus, preferred NRMs include Non-
phosphate
linkages, e.g., a linkage of 4 atoms which confers greater resistance to
cleavage than does a
phosphate bond. Examples include 3' CH2-NCH3-O-CH2-5' and 3' CH2-NH-(O=)-CH2-
5'.;
(iv) 3'-bridging thiophosphates and 5'-bridging thiophosphates. Thus,
preferred NRM's
can included these structures;
(v) L-RNA, 2'-5' linkages, inverted linkages, a-nucleosides. Thus, other
preferred
NRM's include: L nucleosides and dimeric nucleotides derived from L-
nucleosides; 2'-5'
phosphate, non-phosphate and modified phosphate linkages (e.g.,
thiophosphates,
phosphoramidates and boronophosphates); dimers having inverted linkages, e.g.,
3'-3' or 5'-5'
linkages; monomers having an alpha linkage at the 1' site on the sugar, e.g.,
the structures
described herein having an alpha linkage;
(vi) conjugate groups. Thus, preferred NRM's can include e.g., a targeting
moiety or a
conjugated ligand described herein conjugated with the monomer, e.g., through
the sugar, base,
or backbone;
53
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(vi) abasic linkages. Thus, preferred NRM's can include an abasic monomer,
e.g., an
abasic monomer as described herein (e.g., a nucleobaseless monomer); an
aromatic or
heterocyclic or polyheterocyclic aromatic monomer as described herein.; and
(vii) 5'-phosphonates and 5'-phosphate prodrugs. Thus, preferred NRM's include
monomers, preferably at the terminal position, e.g., the 5' position, in which
one or more atoms
of the phosphate group is derivatized with a protecting group, which
protecting group or groups,
are removed as a result of the action of a component in the subject's body,
e.g, a carboxyesterase
or an enzyme present in the subject's body. E.g., a phosphate prodrug in which
a carboxy
esterase cleaves the protected molecule resulting in the production of a
thioate anion which
lo attacks a carbon adjacent to the 0 of a phosphate and resulting in the
production of an
unprotected phosphate.
One or more different NRM modifications can be introduced into an iRNA agent
or into
a sequence of an iRNA agent. An NRM modification can be used more than once in
a sequence
or in an iRNA agent. As some NRM's interfere with hybridization the total
number
incorporated, should be such that acceptable levels of iRNA agent duplex
formation are
maintained.
In some embodiments NRM modifications are introduced into the terminal the
cleavage
site or in the cleavage region of a sequence (a sense strand or sequence)
which does not target a
desired sequence or gene in the subject. This can reduce off-target silencing.
Chiral Sp Thioates
A modification can include the alteration, e.g., replacement, of one or both
of the non-
linking (X and Y) phosphate oxygens and/or of one or more of the linking (W
and Z) phosphate
oxygens. Formula X below depicts a phosphate moiety linking two sugar/sugar
surrogate-base
moieties, SB1 and SB2.
54
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
SB1
w
X P Y
SB2
FORMULA X
In certain embodiments, one of the non-linking phosphate oxygens in the
phosphate
backbone moiety (X and Y) can be replaced by any one of the following: S, Se,
BR3 (R is
hydrogen, alkyl, aryl, etc.), C (i.e., an alkyl group, an aryl group, etc.),
H, NR2 (R is hydrogen,
alkyl, aryl, etc.), or OR (R is alkyl or aryl). The phosphorus atom in an
unmodified phosphate
group is achiral. However, replacement of one of the non-linking oxygens with
one of the above
atoms or groups of atoms renders the phosphorus atom chiral; in other words a
phosphorus atom
1o in a phosphate group modified in this way is a stereogenic center. The
stereogenic phosphorus
atom can possess either the "R" configuration (herein Rp) or the "S"
configuration (herein Sp).
Thus if 60% of a population of stereogenic phosphorus atoms have the Rp
configuration, then the
remaining 40% of the population of stereogenic phosphorus atoms have the Sp
configuration.
In some embodiments, iRNA agents, having phosphate groups in which a phosphate
non-
linking oxygen has been replaced by another atom or group of atoms, may
contain a population
of stereogenic phosphorus atoms in which at least about 50% of these atoms
(e.g., at least about
60% of these atoms, at least about 70% of these atoms, at least about 80% of
these atoms, at least
about 90% of these atoms, at least about 95% of these atoms, at least about
98% of these atoms,
at least about 99% of these atoms) have the Sp configuration. Alternatively,
iRNA agents having
phosphate groups in which a phosphate non-linking oxygen has been replaced by
another atom
or group of atoms may contain a population of stereogenic phosphorus atoms in
which at least
about 50% of these atoms (e.g., at least about 60% of these atoms, at least
about 70% of these
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
atoms, at least about 80% of these atoms, at least about 90% of these atoms,
at least about 95%
of these atoms, at least about 98% of these atoms, at least about 99% of these
atoms) have the Rp
configuration. In other embodiments, the population of stereogenic phosphorus
atoms may have
the Sp configuration and may be substantially free of stereogenic phosphorus
atoms having the
Rp configuration. In still other embodiments, the population of stereogenic
phosphorus atoms
may have the Rp configuration and may be substantially free of stereogenic
phosphorus atoms
having the Sp configuration. As used herein, the phrase "substantially free of
stereogenic
phosphorus atoms having the Rp configuration" means that moieties containing
stereogenic
phosphorus atoms having the Rp configuration cannot be detected by
conventional methods
lo known in the art (chiral HPLC, 1H NMR analysis using chiral shift reagents,
etc.). As used
herein, the phrase "substantially free of stereogenic phosphorus atoms having
the Sp
configuration" means that moieties containing stereogenic phosphorus atoms
having the Sp
configuration cannot be detected by conventional methods known in the art
(chiral HPLC, 1H
NMR analysis using chiral shift reagents, etc.).
In a preferred embodiment, modified iRNA agents contain a phosphorothioate
group, i.e.,
a phosphate groups in which a phosphate non-linking oxygen has been replaced
by a sulfur atom.
In an especially preferred embodiment, the population of phosphorothioate
stereogenic
phosphorus atoms may have the Sp configuration and be substantially free of
stereogenic
phosphorus atoms having the Rp configuration.,
Phosphorothioates may be incorporated into iRNA agents using dimers e.g.,
formulas X-
1 and X-2. The former can be used to introduce phosphorothioate
56
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
DMTO DMTO
O
Z W BASE O BASE
W
R2' XAI R21
S i ~' S P Y
Z I
Z
OZ- BASE O BASE
O 2 O R2
NC I
solid phase reagent 0 P
N(ipr)2
X-1 X-2
at the 3' end of a strand, while the latter can be used to introduce this
modification at the 5' end
or at a position that occurs e.g., 1, 2, 3, 4, 5, or 6 nucleotides from either
end of the strand. In the
above formulas, Y can be 2-cyanoethoxy, W and Z can be 0, R2, can be, e.g., a
substituent that
can impart the C-3 endo configuration to the sugar (e.g., OH, F, OCH3), DMT is
dimethoxytrityl,
and "BASE" can be a natural, unusual, or a universal base.
X-1 and X-2 can be prepared using chiral reagents or directing groups that can
result in
phosphorothioate-containing dimers having a population of stereogenic
phosphorus atoms
1o having essentially only the Rp configuration (i.e., being substantially
free of the Sp configuration)
or only the Sp configuration (i.e., being substantially free of the Rp
configuration). Alternatively,
dimers can be prepared having a population of stereogenic phosphorus atoms in
which about
57
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
50% of the atoms have the Rp configuration and about 50% of the atoms have the
SF
configuration. Dimers having stereogenic phosphorus atoms with the Rp
configuration can be
identified and separated from dimers having stereogenic phosphorus atoms with
the Sp
configuration using e.g., enzymatic degradation and/or conventional
chromatography techniques.
Cationic Groups
Modifications can also include attachment of one or more cationic groups to
the sugar,
base, and/or the phosphorus atom of a phosphate or modified phosphate backbone
moiety. A
cationic group can be attached to any atom capable of substitution on a
natural, unusual or
universal base. A preferred position is one that does not interfere with
hybridization, i.e., does
lo not interfere with the hydrogen bonding interactions needed for base
pairing. A cationic group
can be attached e.g., through the C2' position of a sugar or analogous
position in a cyclic or
acyclic sugar surrogate. Cationic groups can include e.g., protonated amino
groups, derived
from e.g., O-AMINE (AMINE = NH2i alkylamino, dialkylamino, heterocyclyl,
arylamino, diaryl
amino, heteroaryl amino, or diheteroaryl amino, ethylene diamine, polyamino);
aminoalkoxy,
e.g., O(CH2)nAMINE, (e.g., AMINE = NH2; alkylamino, dialkylamino,
heterocyclyl, arylamino,
diaryl amino, heteroaryl amino, or diheteroaryl amino, ethylene diamine,
polyamino); amino
(e.g. NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid); or NH(CH2CH2NH)nCH2CH2-AMINE (AMINE = NH2;
alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl
amino,or
diheteroaryl amino).
Nonphosphate Linkages
Modifications can also include the incorporation of nonphosphate linkages at
the 5'
and/or 3' end of a strand. Examples of nonphosphate linkages which can replace
the phosphate
group include methyl phosphonate, hydroxylamino, siloxane, carbonate,
carboxymethyl,
carbamate, amide, thioether, ethylene oxide linker, sulfonate, sulfonamide,
thioformacetal,
formacetal, oxime, methyleneimino, methylenemethylimino, methylenehydrazo,
methylenedimethylhydrazo and methyleneoxymethylimino. Preferred replacements
include the
methyl phosphonate and hydroxylamino groups.
58
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
3'-bridging thiophosphates and 5 '-bridging thiophosphates; locked-RNA, 2'-5'
likages,
inverted linkages, a-nucleosides; conjugate groups; abasic linkages; and 5'
phosphonates and
5' phosphate prodrugs
Referring to formula X above, modifications can include replacement of one of
the
bridging or linking phosphate oxygens in the phosphate backbone moiety (W and
Z). Unlike the
situation where only one of X or Y is altered, the phosphorus center in the
phosphorodithioates is
achiral which precludes the formation of iRNA agents containing a stereogenic
phosphorus
atom.
Modifications can also include linking two sugars via a phosphate or modified
phosphate
lo group through the 2' position of a first sugar and the 5' position of a
second sugar. Also
contemplated are inverted linkages in which both a first and second sugar are
eached linked
through the respective3' positions. Modified RNA's can also include "abasic"
sugars, which
lack a nucleobase at C-1'. The sugar group can also contain one or more
carbons that possess the
opposite stereochemical configuration than that of the corresponding carbon in
ribose. Thus, a
modified iRNA agent can include nucleotides containing e.g., arabinose, as the
sugar. In another
subset of this modification, the natural, unusual, or universal base may have
the a-configuration.
Modifcations can also include L-RNA.
Modifications can also include 5'-phosphonates, e.g., P(O)(O-)2-X-C5'-sugar
(X= CH2,
CF2, CHF and 5'-phosphate prodrugs, e.g., P(O)[OCH2CH2SC(O)R]2CH2C5'-sugar. In
the
latter case, the prodrug groups may be decomposed via reaction first with
carboxy esterases. The
remaining ethyl thiolate group via intramolecular SN2 displacement can depart
as episulfide to
afford the underivatized phosphate group.
Modification can also include the addition of conjugating groups described
elseqhere
herein, which are prefereably attached to an iRNA agent through any amino
group available for
conjugation.
Nuclease resistant modifications include some which can be placed only at the
terminus
and others which can go at any position. Generally the modifications that can
inhibit
hybridization so it is preferably to use them only in terminal regions, and
preferrable to not use
59
CA 02521464 2011-06-30
51912-8
them at the cleavage site or in the cleavage region of an sequence which
targets a subject
sequence or gene.. The can be used anywhere in a sense sequence, provided that
sufficient
hybridization between the two sequences of the iRNA agent is maintained. In
some
embodiments it is desirabable to put the NRM at the cleavage site or in the
cleavage region of a
sequence which does not target a subject sequence or gene,as it can minimize
off-target
silencing.
In addition, an iRNA agent described herein can have an overhang which does
not form a
duplex structure with the other sequence of the iRNA agent it is an overhang,
but it does
hybridize, either with itself, or with another nucleic acid, other than the
other sequence of the
lo iRNA agent.
. In most cases, the nuclease-resistance promoting modifications will be
distributed
differently depending on whether the sequence will target a sequence in the
subject (often
referred to as an anti-sense sequence) or will not target a sequence in the
subject (often referred
to as a sense sequence). If a sequence is to target a sequence in the subject,
modifications which
interfer with or inhibit endonuclease cleavage should not be inserted in the
region which is
subject to RISC mediated cleavage, e.g., the cleavage site or the cleavage
region (As described
in Elbashir et al., 2001, Genes and Dev. 15: 188, cleavage of
the target occurs about in the middle of a 20 or 21 ntt guide RNA, or about 10
or 11 nucleotides
upstream of the first nucleotide which is complementary to the guide sequence.
As used herein
cleavage site refers to the nucleotide on either side of the cleavage site, on
the target or on the
iRNA agent strand which hybridizes to it. Cleavage region means an nucleotide
with 1, 2, or 3
nucletides of the cleave site, in either direction.)
Such modifications can be introduced into the terminal regions, e.g., at the
terminal
position or with 2, 3, 4, or 5 positions of the terminus, of a sequence which
targets or a sequence
which does not target a sequence in the subject.
An iRNA agent can have a first and a second strand chosen from the following:
a first strand which does not target a sequence and which has an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
a first strand which does not target a sequence and which has an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a first strand which does not target a sequence and which has an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a first strand which does not target a sequence and which has an NRM
modification at the
cleavage site or in the cleavage region;
a first strand which does not target a sequence and which has an NRM
modification at the
cleavage site or in the cleavage region and one or more of an NRM modification
at or within 1,
2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at or within
1, 2, 3, 4, 5 , or 6
positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4, 5 ,
or 6 positions from
both the 3' and the 5' end; and
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end (5' end NRM
modifications are preferentially
not at the terminus but rather at a position 1, 2, 3, 4, 5 , or 6 away from
the 5' terminus of an
antisense strand);
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a second strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
a second strand which targets a sequence and which does not have an NRM
modification
at the cleavage site or in the cleavage region and one or more of an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at
or within 1, 2, 3, 4, 5 ,
or 6 positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4,
5 , or 6 positions
61
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
from both the 3' and the 5' end(5' end NRM modifications are preferentially
not at the terminus
but rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5' terminus of an
antisense strand).
An iRNA agent can also target two sequences and can have a first and second
strand
chosen from:
a first strand which targets a sequence and which has an NRM modification at
or within
1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a first strand which targets a sequence and which has an NRM modification at
or within
1, 2, 3, 4, 5 , or 6 positions from the 5' end (5' end NRM modifications are
preferentially not at
the terminus but rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5'
terminus of an antisense
strand);
a first strand which targets a sequence and which has an NRM modification at
or within
1, 2, 3, 4, 5, or 6 positions from the 3' end and which has a NRM modification
at or within 1, 2,
3, 4, 5 , or 6 positions from the 5' end;
a first strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
a first strand which targets a sequence and which dose not have an NRM
modification at
the cleavage site or in the cleavage region and one or more of an NRM
modification at or within
1, 2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at or
within 1, 2, 3, 4, 5 , or 6
positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4, 5, or
6 positions from
both the 3' and the 5' end(5' end NRM modifications are preferentially not at
the terminus but
rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5' terminus of an
antisense strand) and
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5, or 6 positions from the 5' end (5' end NRM modifications
are preferentially
not at the terminus but rather at a position 1, 2, 3, 4, 5 , or 6 away from
the 5' terminus of an
antisense strand);
62
CA 02521464 2011-06-30
51912-8
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a second strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
a second strand which targets a sequence and which dose not have an NRM
modification
at the cleavage site or in the cleavage region and one or more of an NRM
modification at or
within 1, 2, 3, 4, 5, or 6 positions from the 3' end, a NRM modification at or
within 1, 2, 3, 4, 5,
or 6 positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4,
5, or 6 positions
1o from both the 3' and the 5' end(5' end NRM modifications are preferentially
not at the terminus
but rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5' terminus of an
antisense strand).
Ribose Mimics
An RNA, e.g., an iRNA agent, can incorporate a ribose mimic, such as those
described
herein and those described in copending co-owned United States Provisional
Application Serial
No. 60/454,962, filed on March 13, 2003, and International Application No.
PCT/USO4/07070,
A
In addition, the invention includes iRNA agents having a ribose mimic and
another
element described herein. E.g., the invention includes an iRNA agent described
herein, e.g., a
palindromic iRNA agent, an iRNA agent having a non canonical pairing, an IRNA
agent which
targets a gene described herein, e.g., a gene active in the liver, an iRNA
agent having an
architecture or structure described herein, an iRNA associated with an
amphipathic delivery
agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
which also incorporates a ribose mimic.
. Thus, an aspect of the invention features an iRNA agent that includes a
secondary
hydroxyl group, which can increase efficacy and/or confer nuclease resistance
to the agent.
Nucleases, e. g., cellular nucleases, can hydrolyze nucleic acid
phosphodiester bonds, resulting in
partial or complete degradation. of the nucleic acid. The secondary hydroxy
group confers
63
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
nuclease resistance to an iRNA agent by rendering the iRNA agent less prone to
nuclease
degradation relative to an iRNA which lacks the modification. While not
wishing to be bound
by theory, it is believed that the presence of a secondary hydroxyl group on
the iRNA agent can
act as a structural mimic of a 3' ribose hydroxyl group, thereby causing it to
be less susceptible
to degradation.
The secondary hydroxyl group refers to an "OH" radical that is attached to a
carbon atom
substituted by two other carbons and a hydrogen. The secondary hydroxyl group
that confers
nuclease resistance as described above can be part of any acyclic carbon-
containing group. The
hydroxyl may also be part of any cyclic carbon-containing group, and
preferably one or more of
the following conditions is met (1) there is no ribose moiety between the
hydroxyl group and the
terminal phosphate group or (2) the hydroxyl group is not on a sugar moiety
which is coupled to
a base.. The hydroxyl group is located at least two bonds (e.g., at least
three bonds away, at least
four bonds away, at least five bonds away, at least six bonds away, at least
seven bonds away, at
least eight bonds away, at least nine bonds away, at least ten bonds away,
etc.) from the terminal
phosphate group phosphorus of the iRNA agent. In preferred embodiments, there
are five
intervening bonds between the terminal phosphate group phosphorus and the
secondary hydroxyl
group.
Preferred iRNA agent delivery modules with five intervening bonds between the
terminal
phosphate group phosphorus and the secondary hydroxyl group have the following
structure (see
formula Y below):
AW
I
Y P X
Z
~CH2 R3
` /R4
R, / CH~ )'C"',NHT
n
R2 I I R5
OR7 R6
64
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(Y)
Referring to formula Y, A is an iRNA agent, including any iRNA agent described
herein.
The iRNA agent may be connected directly or indirectly (e.g., through a spacer
or linker) to "W"
of the phosphate group. These spacers or linkers can include e.g., -(CH2)n , -
(CH2)õ N-, -
(CH2)õ O-, -(CH2)õS-, O(CH2CH2O),jCH2CH2OH (e.g., n = 3 or 6), abasic sugars,
amide, carboxy,
amine, oxyamine, oxyimine, thioether, disulfide, thiourea, sulfonamide, or
morpholino, or biotin
and fluorescein reagents.
The iRNA agents can have a terminal phosphate group that is unmodified (e.g.,
W, X, Y,
lo and Z are 0) or modified. In a modified phosphate group, W and Z can be
independently NH, 0,
or S; and X and Y can be independently S, Se, BH3-, C1-C6 alkyl, C6-C10 aryl,
H, 0, O-, alkoxy or
amino (including alkylamino, arylamino, etc.). Preferably, W, X and Z are 0
and Y is S.
R1 and R3 are each, independently, hydrogen; or C1-Cloo alkyl, optionally
substituted with
hydroxyl, amino, halo, phosphate or sulfate and/or may be optionally inserted
with N, 0, S,
alkenyl or alkynyl.
R2 is hydrogen; C1-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, 0, S, alkenyl
or alkynyl; or, when
n is 1, R2 maybe taken together with with R4 or R6 to form a ring of 5-12
atoms.
R4 is hydrogen; Cl-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, 0, S, alkenyl
or alkynyl; or, when
n is 1, R4 may be taken together with with R2 or R5 to form a ring of 5-12
atoms.
R5 is hydrogen, C1-Cloo alkyl optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, 0, S, alkenyl
or alkynyl; or, when
n is 1, R5 maybe taken together with with R4 to form a ring of 5-12 atoms.
R6 is hydrogen, C1-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, 0, S, alkenyl
or alkynyl, or, when
n is 1, R6 maybe taken together with with R2 to form a ring of 6-10 atoms;
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
R7 is hydrogen, C1-Cloo alkyl, or C(O)(CH2)gC(O)NHR9; T is hydrogen or a
functional
group; n and q are each independently 1-100; R8 is C1-C10 alkyl or C6-C10
aryl; and R9 is
hydrogen, C1-C10 alkyl, C6-C10 aryl or a solid support agent.
Preferred embodiments may include one of more of the following subsets of iRNA
agent
delivery modules.
In one subset of RNAi agent delivery modules, A can be connected directly or
indirectly
through a terminal 3' or 5' ribose sugar carbon of the RNA agent.
In another subset of RNAi agent delivery modules, X, W, and Z are 0 and Y is
S.
In still yet another subset of RNAi agent delivery modules, n is 1, and R2 and
R6 are
lo taken together to form a ring containing six atoms and R4 and R5 are taken
together to form a
ring containing six atoms. Preferably, the ring system is a trans-decalin. For
example, the RNAi
agent delivery module of this subset can include a compound of Formula (Y-1):
A`
O O NHT
P
S/ `O
HO
The functional group can be, for example, a targeting group (e.g., a steroid
or a
carbohydrate), a reporter group (e.g., a fluorophore), or a label (an
isotopically labelled moiety).
The targeting group can further include protein binding agents, endothelial
cell targeting groups
(e.g., RGD peptides and mimetics), cancer cell targeting groups (e.g., folate
Vitamin B12,
Biotin), bone cell targeting groups (e.g., bisphosphonates, polyglutamates,
polyaspartates),
multivalent mannose (for e.g., macrophage testing), lactose, galactose, N-
acetyl-galactosamine,
monoclonal antibodies, glycoproteins, lectins, melanotropin, or thyrotropin.
66
CA 02521464 2011-06-30
51912-8
As can be appreciated by the skilled artisan, methods of synthesizing the
compounds of
the formulae herein will be evident to those of ordinary skill in the art.The
synthesized
compounds can be separated from a reaction mixture and further purified by a
method such as
column chromatography, high pressure liquid chromatography, or
recrystallization.
Additionally, the various synthetic steps may be performed in an alternate
sequence or order to
give the desired compounds. Synthetic chemistry transformations and protecting
group
methodologies (protection and deprotection) useful in synthesizing the
compounds described
herein are known in the art and include, for example, those such as described
in R. Larock,
Comprehensive Organic Transformation, VCH Publishers (1989); T.W. Greene and
P.GM.
1o Wuts, Protective Groups in Organic Synthesis, 2d. Ed., John Wiley and Sons
(1991); L. Fieser
and M. Fieser, Fieser and Fieser's Reagents for Organic Synthesis, John Wiley
and Sons (1994);
and L. Paquette, ed., Encyclopedia ofReagents for Organic Synthesis,, John
Wiley and Sons
(1995), and subsequent editions thereof.
Ribose Replacement Monomer Subunits
iRNA agents can be modified in a number of ways which can optimize one or more
characteristics of the iRNA agent. An RNA agent, e.g., an iRNA agent can
include a ribose
replacement monomer subunit (RRMS), such as those described herein and those
described in
one or more of United States Provisional Application Serial No. 60/493,986,
filed on
August 8, 2003; United States Provisional Application Serial No. 60/494,597,
filed on
August 11, 2003; United States Provisional Application Serial No. 60/506,341,
filed on
September 26, 2003; and International Application No. PCTIUSO4/07070,
filed March 8, 2004.
In addition, the invention includes iRNA agents having a RRMS and another
element
described herein. E.g., the invention includes an iRNA agent described herein,
e.g., a
palindromic iRNA agent, an iRNA agent having a non canonical pairing, an iRNA
agent which
targets a gene described herein, e.g., a gene active in the liver, an iRNA
agent having an
67
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
architecture or structure described herein, an iRNA associated with an
amphipathic delivery
agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
which also incorporates a RRMS.
The ribose sugar of one or more ribonucleotide subunits of an iRNA agent can
be
replaced with another moiety, e.g., a non-carbohydrate (preferably cyclic)
carrier. A
ribonucleotide subunit in which the ribose sugar of the subunit has been so
replaced is referred to
herein as a ribose replacement modification subunit (RRMS). A cyclic carrier
may be a
carbocyclic ring system, i.e., all ring atoms are carbon atoms, or a
heterocyclic ring system, i.e.,
1o one or more ring atoms may be'a heteroatom, e.g., nitrogen, oxygen, sulfur.
The cyclic carrier
may be a monocyclic ring system, or may contain two or more rings, e.g. fused
rings. The cyclic
carrier may be a fully saturated ring system, or it may contain one or more
double bonds.
The carriers further include (i) at least two "backbone attachment points" and
(ii) at least
one "tethering attachment point." A "backbone attachment point" as used herein
refers to a
functional group, e.g. a hydroxyl group, or generally, a bond available for,
and that is suitable for
incorporation of the carrier into the backbone, e.g., the phosphate, or
modified phosphate, e.g.,
sulfur containing, backbone, of a ribonucleic acid. A "tethering attachment
point" as used herein
refers to a constituent ring atom of the cyclic carrier, e.g., a carbon atom
or a heteroatom (distinct
from an atom which provides a backbone attachment point), that connects a
selected moiety.
The moiety can be, e.g., a ligand, e.g., a targeting or delivery moiety, or a
moiety which alters a
physical property, e.g., lipophilicity, of an iRNA agent. Optionally, the
selected moiety is
connected by an intervening tether to the cyclic carrier. Thus, it will
include a functional group,
e.g., an amino group, or generally, provide a bond, that is suitable for
incorporation or tethering
of another chemical entity, e.g., a ligand to the constituent ring.
Incorporation of one or more RRMSs described herein into an RNA agent, e.g.,
an iRNA
agent, particularly when tethered to an appropriate entity, can confer one or
more new properties
to the RNA agent and/or alter, enhance or modulate one or more existing
properties in the RNA
molecule. E.g., it can alter one or more of lipophilicity or nuclease
resistance. Incorporation of
one or more RRMSs described herein into an iRNA agent can, particularly when
the RRMS is
68
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
tethered to an appropriate entity, modulate, e.g., increase, binding affinity
of an iRNA agent to a
target mRNA, change the geometry of the duplex form of the iRNA agent, alter
distribution or
target the iRNA agent to a particular part of the body, or modify the
interaction with nucleic acid
binding proteins (e.g., during RISC formation and strand separation).
Accordingly, in one aspect, the invention features, an iRNA agent preferably
comprising
a first strand and a second strand, wherein at least one subunit having a
formula (R-1) is
incorporated into at least one of said strands.
R1 R6
X
R2 R5
R3 Y Z
4
R
(R-1)
Referring to formula (R-1), X is N(CO)R7, NR7 or CH2; Y is NRB, 0, S, CR9R10,
or
absent; and Z is CR11R12 or absent.
Each of R1, R2, R3, R4, R9, and R10 is, independently, H, ORa, ORb, (CH2)nORa,
or
(CH2)nORb, provided that at least one of R1, R2, R3, R4, R9, and R10 is ORa or
ORb and that at
least one of R1, R2, R3, R4, R9, and R10 is (CH2)nORa, or (CH2)nORb (when the
RRMS is terminal,
one of R1, R2, R3, R4, R9, and R10 will include Ra and one will include Rb;
when the RRMS is
internal, two of R1, R2, R3, R4, R9, and R10 will each include an R); further
provided that
preferably ORa may only be present with (CH2)nORb and (CH2)nORa may only be
present with
W.
Each of R5, R6, R11, and R12 is, independently, H, C1-C6 alkyl optionally
substituted with
1-3 R13, or C(O)NHR7; or R5 and R11 together are C3-C8 cycloalkyl optionally
substituted with
R14
69
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R7 is C1-C20 alkyl substituted with NR Rd; R8 is C1-C6 alkyl; R13 is hydroxy,
C1-C4
alkoxy, or halo; and R14 is NRCR7.
Ra is:
A
11
B
C
and
Rb is:
A
P-0 Strand
I
C
Each of A and C is, independently, 0 or S.
BisOH,0-, or
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO I
O 0
Ii OII
O OH
I I
O O-
R' is H or C1-C6 alkyl; Ra is H or a ligand; and n is 1-4.
In a preferred embodiment the ribose is replaced with a pyrroline scaffold,
and X is
N(CO)R7 or NR7, Y is CR9R10, and Z is absent.
In other preferred embodiments the ribose is replaced with a piperidine
scaffold, and X is
N(CO)R7 or NR7, Y is CR9R10, and Z is CR11R12.
In other preferred embodiments the ribose is replaced with a piperazine
scaffold, and X is
N(CO)R7 or NR7, Y is NR8, and Z is CR11R12.
In other preferred embodiments the ribose is replaced with a morpholino
scaffold, and X
is N(CO)R7 or NR7, Y is 0, and Z is CR11R12
In other preferred embodiments the ribose is replaced with a decalin scaffold,
and X
isCH2; Y is CR9R10; and Z is CR11R12; and R5 and R11 together are C6
cycloalkyl.
In other preferred embodiments the ribose is replaced with a decalin/indane
scafold and,
and X is CH2; Y is CR9R10; and Z is CR11R12; and R5 and R11 together are C5
cycloalkyl.
In other preferred embodiments, the ribose is replaced with a hydroxyproline
scaffold.
RRMSs described herein may be incorporated into any double-stranded RNA-like
molecule described herein, e.g., an iRNA agent. An iRNA agent may include a
duplex
comprising a hybridized sense and antisense strand, in which the antisense
strand and/or the
sense strand may include one or more of the RRMSs described herein. An RRMS
can be
introduced at one or more points in one or both strands of a double-stranded
iRNA agent. An
71
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
RRMS can be placed at or near (within 1, 2, or 3 positions) of the 3' or 5'
end of the sense strand
or at near (within 2 or 3 positions of) the 3' end of the antisense strand. In
some embodiments it
is preferred to not have an RRMS at or near (within 1, 2, or 3 positions of)
the 5' end of the
antisense strand. An RRMS can be internal, and will preferably be positioned
in regions not
critical for antisense binding to the target.
In an embodiment, an iRNA agent may have an RRMS at (or within 1, 2, or 3
positions
of) the 3' end of the antisense strand. In an embodiment, an iRNA agent may
have an RRMS at
(or within 1, 2, or 3 positions of) the 3' end of the antisense strand and at
(or within 1, 2, or 3
positions of) the 3' end of the sense strand. In an embodiment, an iRNA agent
may have an
lo RRMS at (or within 1, 2, or 3 positions of) the 3' end of the antisense
strand and an RRMS at the
5' end of the sense strand, in which both ligands are located at the same end
of the iRNA agent.
In certain embodiments, two ligands are tethered, preferably, one on each
strand and are
hydrophobic moieties. While not wishing to be bound by theory, it is believed
that pairing of the
hydrophobic ligands can stabilize the iRNA agent via intermolecular van der
Waals interactions.
In an embodiment, an iRNA agent may have an RRMS at (or within 1, 2, or 3
positions
of) the 3' end of the antisense strand and an RRMS at the 5' end of the sense
strand, in which
both RRMSs may share the same ligand (e.g., cholic acid) via connection of
their individual
tethers to separate positions on the ligand. A ligand shared between two
proximal RRMSs is
referred to herein as a "hairpin ligand."
In other embodiments, an iRNA agent may have an RRMS at the 3' end of the
sense
strand and an RRMS at an internal position of the sense strand. An iRNA agent
may have an
RRMS at an internal position of the sense strand; or may have an RRMS at an
internal position
of the antisense strand; or may have an RRMS at an internal position of the
sense strand and an
RRMS at an internal position of the antisense strand.
In preferred embodiments the iRNA agent includes a first and second sequences,
which
are preferably two separate molecules as opposed to two sequences located on
the same strand,
have sufficient complementarity to each other to hybridize (and thereby form a
duplex region),
72
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
e.g., under physiological conditions, e.g., under physiological conditions but
not in contact with a
helicase or other unwinding enzyme.
It is preferred that the first and second sequences be chosen such that the ds
iRNA agent
includes a single strand or unpaired region at one or both ends of the
molecule. Thus, a ds iRNA
agent contains first and second sequences, preferable paired to contain an
overhang, e.g., one or
two 5' or 3' overhangs but preferably a 3' overhang of 2-3 nucleotides. Most
embodiments
will have a 3' overhang. Preferred sRNA agents will have single-stranded
overhangs, preferably
3' overhangs, of 1 or preferably 2 or 3 nucleotides in length at each end. The
overhangs can be
the result of one strand being longer than the other, or the result of two
strands of the same length
lo being staggered. 5' ends are preferably phosphorylated.
An RNA agent, e.g., an iRNA agent, containing a preferred, but nonlimiting
RRMS is
presented as formula (R-2) in FIG. 4. The carrier includes two "backbone
attachment points"
(hydroxyl groups), a "tethering attachment point," and a ligand, which is
connected indirectly to
the carrier via an intervening tether. The RRMS may be the 5' or 3' terminal
subunit of the RNA
molecule, i.e., one of the two "W" groups may be a hydroxyl group, and the
other "W" group
may be a chain of two or more unmodified or modified ribonucleotides.
Alternatively, the
RRMS may occupy an internal position, and both "W" groups may be one or more
unmodified
or modified ribonucleotides. More than one RRMS may be present in a RNA
molecule, e.g., an
iRNA agent.
The modified RNA molecule of formula (R-2) can be obtained using
oligonucleotide
synthetic methods known in the art. In a preferred embodiment, the modified
RNA molecule of
formula (II) can be prepared by incorporating one or more of the corresponding
RRMS monomer
compounds (RRMS monomers, see, e.g., A, B, and C in FIG. 4) into a growing
sense or
antisense strand, utilizing, e.g., phosphoramidite or H-phosphonate coupling
strategies.
The RRMS monomers generally include two differently functionalized hydroxyl
groups
(OFG1 and OFG2 above), which are linked to the carrier molecule (see A in FIG
4), and a
tethering attachment point. As used herein, the term "functionalized hydroxyl
group" means that
the hydroxyl proton has been replaced by another substituent. As shown in
representative
structures B and C, one hydroxyl group (OFG1) on the carrier is functionalized
with a protecting
73
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
group (PG). The other hydroxyl group (OFG2) can be functionalized with either
(1) a liquid or
solid phase synthesis support reagent (solid circle) directly or indirectly
through a linker, L, as in
B, or (2) a phosphorus-containing moiety, e.g., a phosphoramidite as in C. The
tethering
attachment point may be connected to a hydrogen atom, a tether, or a tethered
ligand at the time
that the monomer is incorporated into the growing sense or antisense strand
(see R in Scheme 1).
Thus, the tethered ligand can be, but need not be attached to the monomer at
the time that the
monomer is incorporated into the growing strand. In certain embodiments, the
tether, the ligand
or the tethered ligand may be linked to a "precursor" RRMS after a "precursor"
RRMS monomer
has been incorporated into the strand.
The (OFGI) protecting group maybe selected as desired, e.g., from T.W. Greene
and
P.G.M. Wuts, Protective Groups in Organic Synthesis, 2d. Ed., John Wiley and
Sons (1991).
The protecting group is preferably stable under amidite synthesis conditions,
storage conditions,
and oligonucleotide synthesis conditions. Hydroxyl groups, -OH, are
nucleophilic groups (i.e.,
Lewis bases), which react through the oxygen with electrophiles (i.e., Lewis
acids). Hydroxyl
groups in which the hydrogen has been replaced with a protecting group, e.g.,
a triarylmethyl
group or a trialkylsilyl group, are essentially unreactive as nucleophiles in
displacement
reactions. Thus, the protected hydroxyl group is useful in preventing e.g.,
homocoupling of
compounds exemplified by structure C during oligonucleotide synthesis. A
preferred protecting
group is the dimethoxytrityl group.
When the OFG2 in B includes a linker, e.g., a long organic linker, connected
to a soluble
or insoluble support reagent, solution or solid phase synthesis techniques can
be employed to
build up a chain of natural and/or modified ribonucleotides once OFGI is
deprotected and free to
react as a nucleophile with another nucleoside or monomer containing an
electrophilic group
(e.g., an amidite group). Alternatively, a natural or modified ribonucleotide
or
oligoribonucleotide chain can be coupled to monomer C via an amidite group or
H-phosphonate
group at OFG2. Subsequent to this operation, OFG1 can be deblocked, and the
restored
nucleophilic hydroxyl group can react with another nucleoside or monomer
containing an
electrophilic group (see FIG. 1). R' can be substituted or unsubstituted alkyl
or alkenyl. In
preferred embodiments, R' is methyl, allyl or 2-cyanoethyl. R" may a C1-Clo
alkyl group,
preferably it is a branched group containing three or more carbons, e.g.,
isopropyl.
74
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
OFG2 in B can be hydroxyl functionalized with a linker, which in turn contains
a liquid
or solid phase synthesis support reagent at the other linker terminus. The
support reagent can be
any support medium that can support the monomers described herein. The monomer
can be
attached to an insoluble support via a linker, L, which allows the monomer
(and the growing
chain) to be solubilized in the solvent in which the support is placed. The
solubilized, yet
immobilized, monomer can react with reagents in the surrounding solvent;
unreacted reagents
and soluble by-products can be readily washed away from the solid support to
which the
monomer or monomer-derived products is attached. Alternatively, the monomer
can be attached
to a soluble support moiety, e.g., polyethylene glycol (PEG) and liquid phase
synthesis
techniques can be used to build up the chain. Linker and support medium
selection is within
skill of the art. Generally the linker may be -C(O)(CH2)gC(O)-, or -
C(O)(CH2)qS-, preferably, it
is oxalyl, succinyl or thioglycolyl. Standard control pore glass solid phase
synthesis supports can
not be used in conjunction with fluoride labile 5' silyl protecting groups
because the glass is
degraded by fluoride with a significant reduction in the amount of full-length
product. Fluoride-
stable polystyrene based supports or PEG are preferred.
Preferred carriers have the general formula (R-3) provided below. (In that
structure
preferred backbone attachment points can be chosen from R1 or R2; R3 or R4; or
R9 and R10 if Y
is CR9R10 (two positions are chosen to give two backbone attachment points,
e.g., R1 and R4, or
R4 and R9. Preferred tethering attachment points include R7; R5 or R6 when X
is CH2. The
carriers are described below as an entity, which can be incorporated into a
strand. Thus, it is
understood that the structures also encompass the situations wherein one (in
the case of a
terminal position) or two (in the case of an internal position) of the
attachment points, e.g., R1 or
R2; R3 or R4; or R9 or R10 (when Y is CR9R10), is connected to the phosphate,
or modified
phosphate, e.g., sulfur containing, backbone. E.g., one of the above-named R
groups can be -
CH2-, wherein one bond is connected to the carrier and one to a backbone atom,
e.g., a linking
oxygen or a central phosphorus atom.)
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R1 R6
R2 R5
R3 4 Y /Z
R
(R-3)
X is N(CO)R7, NR7 or CH2; Y is NR8, 0, S, CR9R10; and Z is CR1'R12 or absent.
Each of R1, R2, R3, R4, R9, and R10 is, independently, H, ORa, or (CH2)"ORb,
provided
that at least two of R1, R2, R3, R4, R9, and R10 are ORa and/or (CH2)0ORb.
Each of R5, R6, R11, and R12 is, independently, a ligand, H, C1-C6 alkyl
optionally
substituted with 1-3 R13, or C(O)NHR7; or R5 and R11 together are C3-C8
cycloalkyl optionally
substituted with R14
R7 is H, a ligand, or C1-C20 alkyl substituted with NR Rd; R8 is H or C1-C6
alkyl; R13 is
hydroxy, C1-C4 alkoxy, or halo; R14 is NRCR7; R15 is C1-C6 alkyl optionally
substituted with
cyano, or C2-C6 alkenyl; R16 is Cl-C10 alkyl; and R17 is a liquid or solid
phase support reagent.
L is -C(O)(CH2)gC(O)-, or -C(O)(CH2)gS-; Ra is CAr3; Rb is P(O)(O-)H,
P(OR15)N(R16)2
or L-R17; Rc is H or C1-C6 alkyl; and Rd is H or a ligand.
Each Ar is, independently, C6-C10 aryl optionally substituted with C1-C4
alkoxy; n is 1-4;
and q is 0-4.
Exemplary carriers include those in which, e.g., X is N(CO)R7 or NR7, Y is
CR9R10, and
Z is absent; or X is N(CO)R7 or NR7, Y is CR9R10, and Z is CR11R12; or X is
N(CO)R7 or NR7, Y
is NR8, and Z is CR11R12; or X is N(CO)R7 or NR7, Y is 0, and Z is CR11R12; or
X is CH2; Y is
CR9R10; Z is CR11R12, and R5 and R11 together form C6 cycloalkyl (H, z = 2),
or the indane ring
76
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
system, e.g., X is CH2; Y is CR9R10; Z is CR11R12, and R5 and R11 together
form C5 cycloalkyl
(H, z= 1).
In certain embodiments, the carrier maybe based on the pyrroline ring system
or the 3-
hydroxyproline ring system, e.g., X is N(CO)R7 or NR7, Y is CR9R10, and Z is
absent (D). OFG1
is preferably attached to a primary carbon, e.g., an exocyclic alkylene
OFG2
14_j_CH2OFG1
/C2
N
LIGAND
D
group, e.g., a methylene group, connected to one of the carbons in the five-
membered ring (-
CH2OFG1 in D). OFG2 is preferably attached directly to one of the carbons in
the five-
membered ring (-OFG2 in D). For the pyrroline-based carriers, -CH2OFG1 may be
attached to C-
2 and OFG2 may be attached to C-3; or -CH2OFG1 may be attached to C-3 and OFG2
maybe
attached to C-4. . In certain embodiments, CH2OFG1 and OFG2 may be geminally
substituted to
one of the above-referenced carbons.For the 3-hydroxyproline-based carriers, '-
CH2OFG1 may be
attached to C-2 and OFG2 may be attached to C-4. The pyrroline- and 3-
hydroxyproline-based
monomers may therefore contain linkages (e.g., carbon-carbon bonds) wherein
bond rotation is
restricted about that particular linkage, e.g. restriction resulting from the
presence of a ring.
Thus, CH2OFG1 and OFG2 may be cis or trans with respect to one another in any
of the pairings
delineated above Accordingly, all cis/trans isomers are expressly included.
The monomers may
also contain one or more asymmetric centers and thus occur as racemates and
racemic mixtures,
single enantiomers, individual diastereomers and diastereomeric mixtures. All
such isomeric
77
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
forms of the monomers are expressly included. The tethering attachment point
is preferably
nitrogen.
In certain embodiments, the carrier may be based on the piperidine ring system
(E), e.g.,
Xis N(CO)R7 or NR7, Y is CR9R10, and Z is CR11R12. OFG1 is preferably
OFG2
C4/
C-(CH2)nOFG'
/C2
N
LIGAND
E
attached to a primary carbon, e.g., an exocyclic alkylene group, e.g., a
methylene group (n=1) or
ethylene group (n=2), connected to one of the carbons in the six-membered ring
[-(CH2)nOFG1 in
E]. OFG2 is preferably attached directly to one of the carbons in the six-
membered ring (-OFG2
in E). -(CH2)nOFG1 and OFG2 maybe disposed in a geminal manner on the ring,
i.e., both
lo groups may be attached to the same carbon, e.g., at C-2, C-3, or C-4.
Alternatively, -
(CH2)nOFG1 and OFG2 may be disposed in a vicinal manner on the ring, i.e.,
both groups may be
attached to adjacent ring carbon atoms, e.g., -(CH2)õOFG1 may be attached to C-
2 and OFG2
may be attached to C-3; -(CH2)nOFG1 may be attached to C-3 and OFG2 may be
attached to C-2;
-(CH2)nOFG1 may be attached to C-3 and OFG2 may be attached to C-4; or -
(CH2)0OFG1 may be
attached to C-4 and OFG2 may be attached to C-3. The piperidine-based monomers
may
therefore contain linkages (e.g., carbon-carbon bonds) wherein bond rotation
is restricted about
that particular linkage, e.g. restriction resulting from the presence of a
ring. Thus, -(CH2)nOFG1
and OFG2 may be cis or trans with respect to one another in any of the
pairings delineated
above. Accordingly, all cis/traps isomers are expressly included. The monomers
may also
78
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
contain one or more asymmetric centers and thus occur as racemates and racemic
mixtures,
single enantiomers, individual diastereomers and diastereomeric mixtures. All
such isomeric
forms of the monomers are expressly included. The tethering attachment point
is preferably
nitrogen.
In certain embodiments, the carrier may be based on the piperazine ring system
(F), e.g.,
X is N(CO)R7 or NR7, Y is NRB, and Z is CR11R12, or the morpholine ring system
(G), e.g., X is
N(CO)R7 or NR7, Y is 0, and Z is CR11R12. OFG' is preferably
R"'
OFG2 OFG2
Nom/ O'/
C3 C3
-CH2OFG1 iCH2OFG1
C2 C2
LIGAND LIGAND
F G
attached to a primary carbon, e.g., an exocyclic alkylene group, e.g., a
methylene group,
connected to one of the carbons in the six-membered ring (-CH2OFG1 in F or G).
OFG2 is
preferably attached directly to one of the carbons in the six-membered rings (-
OFG2 in F or G).
For both F and G, -CH2OFG1 may be attached to C-2 and OFG2 may be attached to
C-3; or vice
versa. In certain embodiments, CH2OFG1 and OFG2 may be geminally substituted
to one of the
above-referenced carbons.The piperazine- and morpholine-based monomers may
therefore
contain linkages (e.g., carbon-carbon bonds) wherein bond rotation is
restricted about that
particular linkage, e.g. restriction resulting from the presence of a ring.
Thus, CH2OFG1 and
OFG2 may be cis or trans with respect to one another in any of the pairings
delineated above.
Accordingly, all cis/traps isomers are expressly included. The monomers may
also contain one
or more asymmetric centers and thus occur as racemates and racemic mixtures,
single
79
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
enantiomers, individual diastereomers and diastereomeric mixtures. All such
isomeric forms of
the monomers are expressly included. R"' can be, e.g., C1-C6 alkyl, preferably
CH3. The
tethering attachment point is preferably nitrogen in both F and G.
In certain embodiments, the carrier maybe based on the decalin ring system,
e.g., X is
CH2; Y is CR9R10; Z is CR11R12, and R5 and R11 together form C6 cycloalkyl (H,
z = 2), or the
indane ring system, e.g., X is CH2; Y is CR9R10; Z is CR11R12, and R5 and R11
together form C5
cycloalkyl (H, z =1). OFG1 is preferably attached to a primary carbon,
OFG2
C7\C/C5/C
z I6 --(CH2)nOFG1
C1, C3
C2
H
e.g., an exocyclic methylene group (n=1) or ethylene group (n=2) connected to
one of C-2, C-3,
1o C-4, or C-5 [-(CH2),,OFG1 in H]. OFG2 is preferably attached directly to
one of C-2, C-3, C-4,
or C-5 (-OFG2 in H). -(CH2)õ OFG1 and OFG2 may be disposed in a geminal manner
on the ring,
i.e., both groups may be attached to the same carbon, e.g., at C-2, C-3, C-4,
or C-5.
Alternatively, -(CH2)õOFG1 and OFG2 may be disposed in a vicinal manner on the
ring, i.e., both
groups maybe attached to adjacent ring carbon atoms, e.g., -(CH2)õ OFG1 maybe
attached to C-2
and OFG2 may be attached to C-3; -(CH2)õ OFG1 may be attached to C-3 and OFG2
may be
attached to C-2; -(CH2)õOFG1 may be attached to C-3 and OFG2 may be attached
to C-4; or -
(CH2)õ OFG1 may be attached to C-4 and OFG2 may be attached to C-3; -(CH2)õ
OFG1 may be
attached to C-4 and OFG2 may be attached to C-5; or -(CH2)õ OFG1 may be
attached to C-5 and
OFG2 may be attached to C-4. The decalin or indane-based monomers may
therefore contain
linkages (e.g., carbon-carbon bonds) wherein bond rotation is restricted about
that particular
linkage, e.g. restriction resulting from the presence of a ring. Thus, -
(CH2)õOFG1 and OFG2 may
be cis or trans with respect to one another in any of the pairings delineated
above. Accordingly,
all cis/traps isomers are expressly included. The monomers may also contain
one or more
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
asymmetric centers and thus occur as racemates and racemic mixtures, single
enantiomers,
individual diastereomers and diastereomeric mixtures. All such isomeric forms
of the monomers
are expressly included. In a preferred embodiment, the substituents at C-1 and
C-6 are trans
with respect to one another. The tethering attachment point is preferably C-6
or C-7.
Other carriers may include those based on 3-hydroxyproline (J). Thus, -
(CH2),,OFG1 and
OFG2 may be cis or trans with respect to one another. Accordingly, all
cis/traps isomers are
expressly included. The monomers may also contain one or more asymmetric
centers
2GFO(CH2) OFG1
N
LIGAND
J
and thus occur as racemates and racemic mixtures, single enantiomers,
individual diastereomers
lo and diastereomeric mixtures. All such isomeric forms of the monomers are
expressly included.
The tethering attachment point is preferably nitrogen.
Representative carriers are shown in FIG. 5.
In certain embodiments, a moiety, e.g., a ligand may be connected indirectly
to the carrier
via the intermediacy of an intervening tether. Tethers are connected to the
carrier at the tethering
attachment point (TAP) and may include any C1-C100 carbon-containing moiety,
(e.g. C1-C75, C1-
C50, C1-C20, C1-C10, C1-C6), preferably having at least one nitrogen atom. In
preferred
embodiments, the nitrogen atom forms part of a terminal amino group on the
tether, which may
serve as a connection point for the ligand. Preferred tethers (underlined)
include TAP_
CHIõ h; TAP-C O CH)nl; or TAP-NR" "(CH,)õ h, in which n is 1-6 and R"" is C1-
C6 alkyl. and Ra is hydrogen or a ligand. In other embodiments, the nitrogen
may form part of a
terminal oxyamino group, e.g., -ONH2, or hydrazino group, -NHNH2. The tether
may optionally
81
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
be substituted, e.g., with hydroxy, alkoxy, perhaloalkyl, and/or optionally
inserted with one or
more additional heteroatoms, e.g., N, 0, or S. Preferred tethered ligands may
include, e.g.,
TAP- CH)õNH(LIGAND),
TAP-C O CHNH(LIGAND), or TAP- " "(CH2)nNH(LIGAND);
TAP- CH2)õONH(LIGAND), TAP-C O CHONH(LIGAND), or
TAP-NR" " (CH?, ONH(LIGAND); TAP- CH2õNHNH, LIGAND ,
TAP-C O CHIõ NHNH LI( GAND), or TAP-NR" "(CHNHNH, LIGAND .
In other embodiments the tether may include an electrophilic moiety,
preferably at the
terminal position of the tether. Preferred electrophilic moieties include,
e.g., an aldehyde, alkyl
1o halide, mesylate, tosylate, nosylate, or brosylate, or an activated
carboxylic acid ester, e.g. an
NHS ester, or a pentafluorophenyl ester. Preferred tethers (underlined)
include TAP_
CH2 CHO; TAP-C O CH -CHO; or TAP-NR ""(CH-CHO, in which n is 1-6 and R.... is
C1-C6 alkyl; or TAP-(CH,JC(O)ONHS; TAP-C(O)(CHõC(O)ONHS; or
TAP-NR" "(CHC(O)ONHS, in which n is 1-6 and R"" is C1-C6 alkyl;
TAP-(CH22 C O OC6F5; TAP-C O CHC(O) OC6F5i or TAP-NR ""(CH,)C O OC6F5, in
which n is 1-6 and R"" is C1-C6 alkyl; or-(CH2)nCH,LG; TAP-C O CH CLG; or TAP-
NR""(CH,CH,LG, in which n is 1-6 and R"" is C1-C6 alkyl (LG can be a leaving
group,
e.g., halide, mesylate, tosylate, nosylate, brosylate). Tethering can be
carried out by coupling a
nucleophilic group of a ligand, e.g., a thiol or amino group with an
electrophilic group on the
tether.
Tethered Entities
A wide variety of entities can be tethered to an iRNA agent, e.g., to the
carrier of an
RRMS. Examples are described below in the context of an RRMS but that is only
preferred,
entities can be coupled at other points to an iRNA agent. Preferred entities
are those which
target to the liver.
82
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Preferred moieties are ligands, which are coupled, preferably covalently,
either directly or
indirectly via an intervening tether, to the RRMS carrier. In preferred
embodiments, the ligand is
attached to the carrier via an intervening tether. As discussed above, the
ligand or tethered
ligand may be present on the RRMS monomer when the RRMS monomer is
incorporated into
the growing strand. In some embodiments, the ligand may be incorporated into a
"precursor"
RRMS after a "precursor" RRMS monomer has been incorporated into the growing
strand. For
example, an RRMS monomer having, e.g., an amino-terminated tether (i.e.,
having no associated
ligand), e.g., TAP-(CH2)nNH2 may be incorporated into a growing sense or
antisense strand. In a
subsequent operation, i.e., after incorporation of the precursor monomer into
the strand, a ligand
1o having an electrophilic group, e.g., a pentafluorophenyl ester or aldehyde
group, can
subsequently be attached to the precursor RRMS by coupling the electrophilic
group of the
ligand with the terminal nucleophilic group of the precursor RRMS tether.
In preferred embodiments, a ligand alters the distribution, targeting or
lifetime of an
iRNA agent into which it is incorporated. In preferred embodiments a ligand
provides an
enhanced affinity for a selected target, e.g, molecule, cell or cell type,
compartment, e.g., a
cellular or organ compartment, tissue, organ or region of the body, as, e.g.,
compared to a species
absent such a ligand. Preferred ligands will not take part in duplex pairing
in a duplexed nucleic
acid.
Preferredligands can improve transport, hybridization, and specificity
properties and may
also improve nuclease resistance of the resultant natural or modified
oligoribonucleotide, or a
polymeric molecule comprising any combination of monomers described herein
and/or natural or
modified ribonucleotides.
Ligands in general can include therapeutic modifiers, e.g., for enhancing
uptake;
diagnostic compounds or reporter groups e.g., for monitoring distribution;
cross-linking agents;
and nuclease-resistance conferring moieties. General examples include lipids,
steroids, vitamins,
sugars, proteins, peptides, polyamines, and peptide mimics.
Ligands can include a naturally occurring substance, such as a protein (e.g.,
human serum
albumin (HSA), low-density lipoprotein (LDL), or globulin); carbohydrate
(e.g., a dextran,
pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid); or a
lipid. The ligand may
83
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
also be a recombinant or synthetic molecule, such as a synthetic polymer,
e.g., a synthetic
polyamino acid. Examples of polyamino acids include polyamino acid is a
polylysine (PLL),
poly L-aspartic acid, poly L-glutamic acid, styrene-maleic acid anhydride
copolymer, poly(L-
lactide-co-glycolied) copolymer, divinyl ether-maleic anhydride copolymer, N-
(2-
hydroxypropyl)methacrylamide copolymer (HMPA), polyethylene glycol (PEG),
polyvinyl
alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-isopropylacrylamide
polymers, or
polyphosphazine. Example of polyamines include: polyethylenimine, polylysine
(PLL),
spermine, spermidine, polyamine, pseudopeptide-polyamine, peptidomimetic
polyainine,
dendrimer polyamine, arginine, amidine, protamine, cationic lipid, cationic
porphyrin,
lo quaternary salt of a polyamine, or an alpha helical peptide.
Ligands can also include targeting groups, e.g., a cell or tissue targeting
agent, e.g., a
lectin, glycoprotein, lipid or protein, e.g., an antibody, that binds to a
specified cell type such as a
liver cell. A targeting group can be a thyrotropin, melanotropin, lectin,
glycoprotein, surfactant
protein A, Mucin carbohydrate, multivalent lactose, multivalent galactose, N-
acetyl-
galactosamine, N-acetyl-gulucosamine multivalent mannose, multivalent fucose,
glycosylated
polyaminoacids, multivalent galactose, transferrin, bisphosphonate,
polyglutamate,
polyaspartate, a lipid, cholesterol, a steroid, bile acid, folate, vitamin
B12, biotin, or an RGD
peptide or RGD peptide mimetic.
Other examples of ligands include dyes, intercalating agents (e.g. acridines),
cross-linkers
(e.g. psoralene, mitomycin C), porphyrins (TPPC4, texaphyrin, Sapphyrin),
polycyclic aromatic
hydrocarbons (e.g., phenazine, dihydrophenazine), artificial endonucleases
(e.g. EDTA),
lipophilic molecules, e.g, cholesterol, cholic acid, adamantane acetic acid, 1-
pyrene butyric acid,
dihydrotestosterone, 1,3-Bis-O(hexadecyl)glycerol, geranyloxyhexyl group,
hexadecylglycerol,
borneol, menthol, 1,3-propanediol, heptadecyl group, palmitic acid, myristic
acid,03-
(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid, dimethoxytrityl, or
phenoxazine)and peptide
conjugates (e.g., antennapedia peptide, Tat peptide), alkylating agents,
phosphate, amino,
mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]2, polyamino, alkyl, substituted
alkyl,
radiolabeled markers, enzymes, haptens (e.g. biotin), transport/absorption
facilitators (e.g.,
aspirin, vitamin E, folic acid), synthetic ribonucleases (e.g., imidazole,
bisimidazole, histamine,
84
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO I
imidazole clusters, acridine-imidazole conjugates, Eu3+ complexes of
tetraazamacrocycles),
dinitrophenyl, HRP, or AP.
Ligands can be proteins, e.g., glycoproteins, or peptides, e.g., molecules
having a specific
affinity for a co-ligand, or antibodies e.g., an antibody, that binds to a
specified cell type such as
a cancer cell, endothelial cell, or bone cell. Ligands may also include
hormones and hormone
receptors. They can also include non-peptidic species, such as lipids,
lectins, carbohydrates,
vitamins, cofactors, multivalent lactose, multivalent galactose, N-acetyl-
galactosamine, N-acetyl-
gulucosamine multivalent mannose, or multivalent fucose. The ligand can be,
for example, a
lipopolysaccharide, an activator of p38 MAP kinase, or an activator of NF-KB.
The ligand can be a substance, e.g, a drug, which can increase the uptake of
the iRNA
agent into the cell, for example, by disrupting the cell's cytoskeleton, e.g.,
by disrupting the cell's
microtubules, microfilaments, and/or intermediate filaments. The drug can be,
for example,
taxon, vincristine, vinblastine, cytochalasin, nocodazole, japlakinolide,
latrunculin A, phalloidin,
swinholide A, indanocine, or myoservin.
The ligand can increase the uptake of the iRNA agent into the cell by
activating an
inflammatory response, for example. Exemplary ligands that would have such an
effect include
tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or gamma
interferon.
In one aspect, the ligand is a lipid or lipid-based molecule. Such a lipid or
lipid-based
molecule preferably binds a serum protein, e.g., human serum albumin (HSA). An
HSA binding
ligand allows for distribution of the conjugate to a target tissue, e.g., a
non-liver target tissue of
the body. Preferably, the target tissue is the liver, preferably parenchymal
cells of the liver.
Other molecules that can bind HSA can also be used as ligands. For example,
neproxin or
aspirin can be used. A lipid or lipid-based ligand can (a) increase resistance
to degradation of the
conjugate, (b) increase targeting or transport into a target cell or cell
membrane, and/or (c) can be
used to adjust binding to a serum protein, e.g., HSA.
A lipid based ligand can be used to modulate, e.g., control the binding of the
conjugate to
a target tissue. For example, a lipid or lipid-based ligand that binds to HSA
more strongly will
be less likely to be targeted to the liver and therefore less likely to be
cleared from the body.
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment, the lipid based ligand binds HSA. Preferably, it
binds HSA
with a sufficient affinity such that the conjugate will be preferably
distributed to a non-kidney
tissue. However, it is preferred that the affinity not be so strong that the
HSA-ligand binding
cannot be reversed.
In another aspect, the ligand is a moiety, e.g., a vitamin, which is taken up
by a target cell,
e.g., a proliferating cell. These are particularly useful for treating
disorders characterized by
unwanted cell proliferation, e.g., of the malignant or non-malignant type,
e.g., cancer cells.
Exemplary vitamins include vitamin A, E, and K. Other exemplary vitamins
include are B
vitamin, e.g., folic acid, B 12, riboflavin, biotin, pyridoxal or other
vitamins or nutrients taken up
by cancer cells. Also included are HSA and low density lipoprotein (LDL).
In another aspect, the ligand is a cell-permeation agent, preferably a helical
cell-
permeation agent. Preferably, the agent is amphipathic. An exemplary agent is
a peptide such as
tat or antennopedia. If the agent is a peptide, it can be modified, including
a peptidylmimetic,
invertomers, non-peptide or pseudo-peptide linkages, and use of D-amino acids.
The helical
agent is preferably an alpha-helical agent, which preferably has a lipophilic
and a lipophobic
phase.
The ligand can be a peptide or peptidomimetic. A peptidomimetic (also referred
to
herein as an oligopeptidomimetic) is a molecule capable of folding into a
defined three-
dimensional structure similar to a natural peptide. The attachment of peptide
and
peptidomimetics to iRNA agents can affect pharmacokinetic distribution of the
iRNA, such as by
enhancing cellular recognition and absorption. The peptide or peptidomimetic
moiety can be
about 5-50 amino acids long, e.g., about 5, 10, 15, 20, 25, 30, 35, 40, 45, or
50 amino acids long
(see Table 2, for example).
86
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Table 2. Exemplary Cell Permeation Peptides
Cell Amino acid Sequence Reference
Permeation
Peptide
Penetratin RQIKIWFQNRRMKWKK (SEQ ID NO:6700) Derossi et al., J. Biol.
Chem. 269:10444,
1994
Tat fragment GRKKRRQRRRPPQC (SEQ ID NO:6701) Vives et al., J. Biol.
(48-60) Chem., 272:16010,
1997
Signal GALFLGWLGAAGSTMGAWSQPKKKRKV Chaloin et al.,
Sequence- (SEQ ID NO:6702) Biochem. Biophys.
based peptide Res. Commun.,
243:601, 1998
PVEC LLIILRRRTRKQAHAHSK (SEQ ID NO:6703) Elmquist et al., Exp.
Cell Res., 269:237,
2001
Transportan GWTLNSAGYLLKINLKALAALAKKIL Pooga et al., FASEB
(SEQ ID NO:6704) J., 12:67, 1998
87
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Amphiphilic KLALKLALKALKAALKLA (SEQ ID Oehlke et al., Mol.
model peptide NO:6705) Ther., 2:339, 2000
Arg9 RRRRRRRRR (SEQ ID NO:6706) Mitchell et al., J.
Pept. Res., 56:318,
2000
Bacterial cell KFFKFFKFFK (SEQ ID NO:6707)
wall
permeating
LL-37 LLGDFFRKSKEKIGKEFKRIVQRIKDFLRN
LVPRTES (SEQ ID NO:6708)
Cecropin P1 SWLSKTAKKLENSAKKRISEGIAIAIQGGP
R (SEQ ID NO:6709)
a-defensin ACYCRIPACIAGERRYGTCIYQGRLWAFC
C (SEQ IDNO:6710)
b-defensin DHYNCVSSGGQCLYSACPIFTKIQGTCYR
GKAKCCK (SEQ IDNO:6711)
Bactenecin RKCRIVVIRVCR (SEQ ID NO:6712)
PR-39 RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPP
RFPPRFPGKR-NH2 (SEQ ID NO:6713)
Indolicidin ILPWKWPWWPWRR-NH2 (SEQ ID
NO:6714)
A peptide or peptidomimetic can be, for example, a cell permeation peptide,
cationic
peptide, amphipathic peptide, or hydrophobic peptide (e.g., consisting
primarily of Tyr, Trp or
Phe). The peptide moiety can be a dendrimer peptide, constrained peptide or
crosslinked
peptide. The peptide moiety can be an L-peptide or D-peptide. In another
alternative, the
88
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO I
peptide moiety can include a hydrophobic membrane translocation sequence
(MTS). An
exemplary hydrophobic MTS-containing peptide is RFGF having the amino acid
sequence
AAVALLPAVLLALLAP (SEQ ID NO:6715). An RFGF analogue (e.g., amino acid sequence
AALLPVLLAAP (SEQ ID NO:6716)) containing a hydrophobic MTS can also be a
targeting
moiety. The peptide moiety can be a "delivery" peptide, which can carry large
polar molecules
including peptides, oligonucleotides, and protein across cell membranes. For
example,
sequences from the HIV Tat protein (GRKKRRQRRRPPQ (SEQ ID NO:6717)) and the
Drosophila Antennapedia protein (RQIKIWFQNRRMKWKK (SEQ ID NO:6718)) have been
found to be capable of functioning as delivery peptides. A peptide or
peptidomimetic can be
lo encoded by a random sequence of DNA, such as a peptide identified from a
phage-display
library, or one-bead-one-compound (OBOC) combinatorial library (Lam et al.,
Nature, 354:82-
84, 1991). Preferably the peptide or peptidomimetic tethered to an iRNA agent
via an
incorporated monomer unit is a cell targeting peptide such as an arginine-
glycine-aspartic acid
(RGD)-peptide, or RGD mimic. A peptide moiety can range in length from about 5
amino acids
to about 40 amino acids. The peptide moieties can have a structural
modification, such as to
increase stability or direct conformational properties. Any of the structural
modifications
described below can be utilized.
An RGD peptide moiety can be used to target a tumor cell, such as an
endothelial tumor
cell or a breast cancer tumor cell (Zitzmann et al., Cancer Res., 62:5139-43,
2002). An RGD
peptide can facilitate targeting of an iRNA agent to tumors of a variety of
other tissues, including
the lung, kidney, spleen, or liver (Aoki et al., Cancer Gene Therapy 8:783-
787, 2001). The
RGD peptide can be linear or cyclic, and can be modified, e.g., glycosylated
or methylated to
facilitate targeting to specific tissues. For example, a glycosylated RGD
peptide can deliver an
iRNA agent to a tumor cell expressing as,B3 (Haubner et al., Jour. Nucl. Med.,
42:326-336,
2001).
Peptides that target markers enriched in proliferating cells can be used.
E.g., RGD
containing peptides and peptidomimetics can target cancer cells, in particular
cells that exhibit an
a,(33 integrin. Thus, one could use RGD peptides, cyclic peptides containing
RGD, RGD
peptides that include D-amino acids, as well as synthetic RGD mimics. In
addition to RGD, one
can use other moieties that target the a,-(33 integrin ligand. Generally, such
ligands can be used
89
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
to control proliferating cells and angiogeneis. Preferred conjugates of this
type include an iRNA
agent that targets PECAM-1, VEGF, or other cancer gene, e.g., a cancer gene
described herein.
A "cell permeation peptide" is capable of permeating a cell, e.g., a microbial
cell, such as
a bacterial or fungal cell, or a mammalian cell, such as a human cell. A
microbial cell-
permeating peptide can be, for example, an a-helical linear peptide (e.g., LL-
37 or Ceropin P1), a
disulfide bond-containing peptide (e.g., a -defensin, ,6-defensin or
bactenecin), or a peptide
containing only one or two dominating amino acids (e.g., PR-39 or
indolicidin). A cell
permeation peptide can also include a nuclear localization signal (NLS). For
example, a cell
permeation peptide can be a bipartite amphipathic peptide, such as MPG, which
is derived from
1o the fusion peptide domain of HIV-1 gp4l and the NLS of SV40 large T antigen
(Simeoni et al.,
Nucl. Acids Res. 31:2717-2724, 2003).
In one embodiment, a targeting peptide tethered to an RRMS can be an
amphipathic a-
helical peptide. Exemplary amphipathic a-helical peptides include, but are not
limited to,
cecropins, lycotoxins, paradaxins, buforin, CPF, bombinin-like peptide (BLP),
cathelicidins,
ceratotoxins, S. clava peptides, hagfish intestinal antimicrobial peptides
(HFIAPs), magainines,
brevinins-2, dermaseptins, melittins, pleurocidin, H2A peptides, Xenopus
peptides, esculentinis-
1, and caerins. A number of factors will preferably be considered to maintain
the integrity of
helix stability. For example, a maximum number of helix stabilization residues
will be utilized
(e.g., leu, ala, or lys), and a minimum number helix destabilization residues
will be utilized (e.g.,
proline, or cyclic monomeric units. The capping residue will be considered
(for example Gly is
an exemplary N-capping residue and/or C-terminal amidation can be used to
provide an extra H-
bond to stabilize the helix. Formation of salt bridges between residues with
opposite charges,
separated by i 3, or i 4 positions can provide stability. For example,
cationic residues such as
lysine, arginine, homo-arginine, ornithine or histidine can form salt bridges
with the anionic
residues glutamate or aspartate.
Peptide and petidomimetic ligands include those having naturally occurring or
modified
peptides, e.g., D or L peptides; a, (3, or y peptides; N-methyl peptides;
azapeptides; peptides
having one or more amide, i.e., peptide, linkages replaced with one or more
urea, thiourea,
carbamate, or sulfonyl urea linkages; or cyclic peptides.
CA 02521464 2011-06-30
51912-8
Methods for making iRNA agents
iRNA agents can include modified or non-naturally occuring bases, e.g., bases
described
in copending and coowned United States Provisional Application Serial No.
60/463,772, filed on
April 17, 2003, and/or in copending and coowned
United States Provisional Application Serial No. 60/465,802, filed on April
25, 2003.
Monomers and iRNA agents which include such bases can be
made by the methods found in United States Provisional Application Serial No.
60/463,772, filed
on April 17, 2003, and/or in United States Provisional Application Serial No.
60/465,802, filed
on April 25, 2003.
In addition, the invention includes iRNA agents having a modified or non-
naturally
occuring base and another element described herein. E.g., the invention
includes an iRNA agent
described herein, e.g., a palindromic iRNA agent, an M NA agent having a non
canonical pairing,
an iRNA agent which targets a gene described herein, e.g., a gene active in
the liver, an iRNA
agent.having an architecture or structure described herein, an iRNA associated
with an
amphipathic delivery agent described herein, an iRNA associated with a drug
delivery module
described herein, an iRNA agent administered as described herein, or an iRNA
agent formulated
as described herein, which also incorporates a modified or non-naturally
occuring base.
The synthesis and purification of oligonucleotide peptide conjugates can be
performed by
established methods. See, for example, Trufert et al., Tetrahedron, 52:3005,
1996; and
Manoharan, "Oligonucleotide Conjugates in Antisense Technology," in Antisense
Drug
Technoloay ed. S.T. Crooke, Marcel Dekker, Inc., 2001.
In one embodiment of the invention, a peptidomimetic can be modified to create
a
constrained peptide that adopts a distinct and specific preferred
conformation, which can
increase the potency and selectivity of the peptide. For example, the
constrained peptide can be
an azapeptide (Gante, Synthesis, 405-413, 1989). An azapeptide is synthesized
by replacing the
a-carbon of an amino acid with a nitrogen atom without changing the structure
of the amino acid
side chain. For example, the azapeptide can be synthesized by using hydrazine
in traditional
peptide synthesis coupling methods, such as by reacting hydrazine with a
"carbonyl donor," e.g.,
phenylchloroformate.
91
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be an N-methyl peptide. N-methyl
peptides are
composed of N-methyl amino acids, which provide an additional methyl group in
the peptide
backbone, thereby potentially providing additional means of resistance to
proteolytic cleavage.
N-methyl peptides can by synthesized by methods known in the art (see, for
example, Lindgren
et al., Trends Pharmacol. Sci. 21:99, 2000; Cell Penetrating Peptides:
Processes and
Applications, Langel, ed., CRC Press, Boca Raton, FL, 2002; Fische et al.,
Bioconjugate. Chem.
12: 825, 2001; Wander et al., J. Am. Chem. Soc., 124:13382, 2002). For
example, an Ant or Tat
peptide can be an N-methyl peptide.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be a (3-peptide. ,6-peptides form
stable secondary
structures such as helices, pleated sheets, turns and hairpins in solutions.
Their cyclic derivatives
can fold into nanotubes in the solid state. /3-peptides are resistant to
degradation by proteolytic
enzymes. i3-peptides can be synthesized by methods known in the art. For
example, an Ant or
Tat peptide can be a (3-peptide.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be a oligocarbamate. Oiigocarbamate
peptides are
internalized into a cell by a transport pathway facilitated by carbamate
transporters. For
example, an Ant or Tat peptide can be an oligocarbamate.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be an oligourea conjugate (or an
oligothiourea
conjugate), in which the amide bond of a peptidomimetic is replaced with a
urea moiety.
Replacement of the amide bond provides increased resistance to degradation by
proteolytic
enzymes, e.g., proteolytic enzymes in the gastrointestinal tract. In one
embodiment, an oligourea
conjugate is tethered to an iRNA agent for use in oral delivery. The backbone
in each repeating
unit of an oligourea peptidomimetic can be extended by one carbon atom in
comparison with the
natural amino acid. The single carbon atom extension can increase peptide
stability and
lipophilicity, for example. An oligourea peptide can therefore be advantageous
when an iRNA
agent is directed for passage through a bacterial cell wall, or when an iRNA
agent must traverse
92
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
the blood-brain barrier, such as for the treatment of a neurological disorder.
In one embodiment,
a hydrogen bonding unit is conjugated to the oligourea peptide, such as to
create an increased
affinity with a receptor. For example, an Ant or Tat peptide can be an
oligourea conjugate (or an
oligothiourea conjugate).
The siRNA peptide conjugates of the invention can be affiliated with, e.g.,
tethered to,
RRMSs occurring at various positions on an iRNA agent. For example, a peptide
can be
terminally conjugated, on either the sense or the antisense strand, or a
peptide can be
bisconjugated (one peptide tethered to each end, one conjugated to the sense
strand, and one
conjugated to the antisense strand). In another option, the peptide can be
internally conjugated,
1 o such as in the loop of a short hairpin iRNA agent. In yet another option,
the peptide can be
affiliated with a complex, such as a peptide-carrier complex.
A peptide-carrier complex consists of at least a carrier molecule, which can
encapsulate
one or more iRNA agents (such as for delivery to a biological system and/or a
cell), and a
peptide moiety tethered to the outside of the carrier molecule, such as for
targeting the carrier
complex to a particular tissue or cell type. A carrier complex can carry
additional targeting
molecules on the exterior of the complex, or fusogenic agents to aid in cell
delivery. The one or
more iRNA agents encapsulated within the carrier can be conjugated to
lipophilic molecules,
which can aid in the delivery of the agents to the interior of the carrier.
A carrier molecule or structure can be, for example, a micelle, a liposome
(e.g., a cationic
liposome), a nanoparticle, a microsphere, or a biodegradable polymer. A
peptide moiety can be
tethered to the carrier molecule by a variety of linkages, such as a disulfide
linkage, an acid
labile linkage, a peptide-based linkage, an oxyamino linkage or a hydrazine
linkage. For
example, a peptide-based linkage can be a GFLG peptide. Certain linkages will
have particular
advantages, and the advantages (or disadvantages) can be considered depending
on the tissue
target or intended use. For example, peptide based linkages are stable in the
blood stream but are
susceptible to enzymatic cleavage in the lysosomes.
93
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Targeting
The iRNA agents of the invention are particularly useful when targeted to the
liver. An
iRNA agent can be targeted to the liver by incorporation of an RRMS containing
a ligand that
targets the liver. For example, a liver-targeting agent can be a lipophilic
moiety. Preferred
lipophilic moieties include lipids, cholesterols, oleyl, retinyl, or
cholesteryl residues. Other
lipophilic moieties that can function as liver-targeting agents include cholic
acid, adamantane
acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol,
geranyloxyhexyl group, hexadecylglycerol, borneol, menthol, 1,3-propanediol,
heptadecyl group,
palmitic acid, myristic acid,03-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic
acid,
dimethoxytrityl, or phenoxazine.
An iRNA agent can also be targeted to the liver by association with a low-
density
lipoprotein (LDL), such as lactosylated LDL. Polymeric carriers complexed with
sugar residues
can also function to target iRNA agents to the liver.
A targeting agent that incorporates a sugar, e.g., galactose and/or analogues
thereof, is
particularly useful. These agents target, in particular, the parenchymal cells
of the liver. For
example, a targeting moiety can include more than one or preferably two or
three galactose
moieties, spaced about 15 angstroms from each other. The targeting moiety can
alternatively be
lactose (e.g., three lactose moieties), which is glucose coupled to a
galactose. The targeting
moiety can also be N-Acetyl-Galactosamine, N-Ac-Glucosamine. A mannose or
mannose-6-
phosphate targeting moiety can be used for macrophage targeting.
Conjugation of an iRNA agent with a serum albumin (SA), such as human serum
albumin, can also be used to target the iRNA agent to a non-kidney tissue,
such as the liver.
An iRNA agent targeted to the liver by an RRMS targeting moiety described
herein can
target a gene expressed in the liver.
An iRNA agent targeted to the liver by an RRMS targeting moiety described
herein can
target a gene expressed in the liver. For example, the iRNA agent can target
p21(WAF1/DIP1),
P27(KIP1), the a-fetoprotein gene, beta-catenin, or c-MET, such as for
treating a cancer of the
liver. In another embodiment, the iRNA agent can target apoB-100, such as for
the treatment of
94
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
an HDL/LDL cholesterol imbalance; dyslipidemias, e.g., familial combined
hyperlipidemia
(FCHL), or acquired hyperlipidemia; hypercholesterolemia; statin-resistant
hypercholesterolemia; coronary artery disease (CAD); coronary heart disease
(CHD); or
atherosclerosis. In another embodiment, the iRNA agent can target forkhead
homologue in
rhabdomyosarcoma (FKHR); glucagon; glucagon receptor; glycogen phosphorylase;
PPAR-
Gamma Coactivator (PGC-1); fructose-l,6-bisphosphatase; glucose-6-phosphatase;
glucose-6-
phosphate translocator; glucokinase inhibitory regulatory protein; or
phosphoenolpyruvate
carboxykinase (PEPCK), such as to inhibit hepatic glucose production in a
mammal, such as a
human, such as for the treatment of diabetes. In another embodiment, an iRNA
agent targeted to
1o the liver can target Factor V, e.g., the Leiden Factor V allele, such as to
reduce the tendency to
form a blood clot. An iRNA agent targeted to the liver can include a sequence
which targets
hepatitis virus (e.g., Hepatitis A, B, C, D, E, F, G, or H). For example, an
iRNA agent of the
invention can target any one of the nonstructural proteins of HCV: NS3, 4A,
4B, 5A, or 5B. For
the treatment of hepatitis B, an iRNA agent can target the protein X (HBx)
gene, for example.
Preferred ligands on RRMSs include folic acid, glucose, cholesterol, cholic
acid, Vitamin
E, Vitamin K, or Vitamin A.
Definitions
The term "halo" refers to any radical of fluorine, chlorine, bromine or
iodine.
The term "alkyl" refers to a hydrocarbon chain that may be a straight chain or
branched
chain, containing the indicated number of carbon atoms. For example, C1-C12
alkyl indicates
that the group may have from 1 to 12 (inclusive) carbon atoms in it. The term
"haloalkyl" refers
to an alkyl in which one or more hydrogen atoms are replaced by halo, and
includes alkyl
moieties in which all hydrogens have been replaced by halo (e.g.,
perfluoroalkyl). Alkyl and
haloalkyl groups may be optionally inserted with 0, N, or S. The terms
"aralkyl" refers to an
alkyl moiety in which an alkyl hydrogen atom is replaced by an aryl group.
Aralkyl includes
groups in which more than one hydrogen atom has been replaced by an aryl
group. Examples of
"aralkyl" include benzyl, 9-fluorenyl, benzhydryl, and trityl groups.
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The term "alkenyl" refers to a straight or branched hydrocarbon chain
containing 2-8
carbon atoms and characterized in having one or more double bonds. Examples of
a typical
alkenyl include, but not limited to, allyl, propenyl, 2-butenyl, 3-hexenyl and
3-octenyl groups.
The term "alkynyl" refers to a straight or branched hydrocarbon chain
containing 2-8 carbon
atoms and characterized in having one or more triple bonds. Some examples of a
typical alkynyl
are ethynyl, 2-propynyl, and 3-methylbutynyl, and propargyl. The sp2 and spa
carbons may
optionally serve as the point of attachment of the alkenyl and alkynyl groups,
respectively.
The term "alkoxy" refers to an -0-alkyl radical. The term "aminoalkyl" refers
to an alkyl
substituted with an aminoThe term "mercapto" refers to an -SH radical. The
term "thioalkoxy"
refers to an -S-alkyl radical.
The term "alkylene" refers to a divalent alkyl (i.e., -R-), e.g., -CH2-, -
CH2CH2-, and -
CH2CH2CH2-. The term "alkylenedioxo" refers to a divalent species of the
structure -O-R-O-,
in which R represents an alkylene.
The term "aryl" refers to an aromatic monocyclic, bicyclic, or tricyclic
hydrocarbon ring
system, wherein any ring atom capable of substitution can be substituted by a
substituent.
Examples of aryl moieties include, but are not limited to, phenyl, naphthyl,
and anthracenyl.
The term "cycloalkyl" as employed herein includes saturated cyclic, bicyclic,
tricyclic,or
polycyclic hydrocarbon groups having 3 to 12 carbons, wherein any ring atom
capable of
substitution can be substituted by a substituent. The cycloalkyl groups herein
described may also
contain fused rings. Fused rings are rings that share a common carbon-carbon
bond. Examples
of cycloalkyl moieties include, but are not limited to, cyclohexyl,
adarnantyl, and norbornyl.
The term "heterocyclyl" refers to a nonaromatic 3-10 membered monocyclic, 8-12
membered bicyclic, or 11-14 membered tricyclic ring system having 1-3
heteroatoms if
monocyclic, 1-6 heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said
heteroatoms
selected from 0, N, or S (e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms
of N. 0, or S if
monocyclic, bicyclic, or tricyclic, respectively), wherein any ring atom
capable of substitution
can be substituted by a substituent. The heterocyclyl groups herein described
may also contain
fused rings. Fused rings are rings that share a common carbon-carbon bond.
Examples of
96
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
heterocyclyl include, but are not limited to tetrahydrofuranyl,
tetrahydropyranyl, piperidinyl,
morpholino, pyrrolinyl and pyrrolidinyl.
The term "heteroaryl" refers to an aromatic 5-8 membered monocyclic, 8-12
membered
bicyclic, or 11-14 membered tricyclic ring system having 1-3 heteroatoms if
monocyclic, 1-6
heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said heteroatoms
selected from 0, N, or S
(e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms of N, 0, or S if
monocyclic, bicyclic, or
tricyclic, respectively), wherein any ring atom capable of substitution can be
substituted by a
substituent.
The term "oxo" refers to an oxygen atom, which forms a carbonyl when attached
to
1 o carbon, an N-oxide when attached to nitrogen, and a sulfoxide or sulfone
when attached to sulfur.
The term "acyl" refers to an alkylcarbonyl, cycloalkylcarbonyl, arylcarbonyl,
heterocyclylcarbonyl, or heteroarylcarbonyl substituent, any of which may be
further substituted
by substituents.
The term "substituents" refers to a group "substituted" on an alkyl,
cycloalkyl, alkenyl,
alkynyl, heterocyclyl, heterocycloalkenyl, cycloalkenyl, aryl, or heteroaryl
group at any atom of
that group. Suitable substituents include, without limitation, alkyl, alkenyl,
alkynyl, alkoxy,
halo, hydroxy, cyano, nitro, amino, SO3H, sulfate, phosphate, perfluoroalkyl,
perfluoroalkoxy,
methylenedioxy, ethylenedioxy, carboxyl, oxo, thioxo, imino (alkyl, aryl,
aralkyl), S(O)r,alkyl
(where n is-0-2), S(O),, aryl (where n is 0-2), S(O)n heteroaryl (where n is 0-
2), S(O)n
heterocyclyl (where n is 0-2), amine (mono-, di-, alkyl, cycloalkyl, aralkyl,
heteroaralkyl, and
combinations thereof), ester (alkyl, aralkyl, heteroaralkyl), amide (mono-, di-
, alkyl, aralkyl,
heteroaralkyl, and combinations thereof), sulfonamide (mono-, di-, alkyl,
aralkyl, heteroaralkyl,
and combinations thereof), unsubstituted aryl, unsubstituted heteroaryl,
unsubstituted
heterocyclyl, and unsubstituted cycloalkyl. In one aspect, the substituents on
a group are
independently any one single, or any subset of the aforementioned
substituents.
The terms "adeninyl, cytosinyl, guaninyl, thyminyl, and uracilyl" and the like
refer to
radicals of adenine, cytosine, guanine, thymine, and uracil.
As used herein, an "unusual" nucleobase can include any one of the following:
97
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
2-methyladeninyl,
N6-methyladeninyl,
2-methylthio-N6-methyladeninyl,
N6-isopentenyladeninyl,
2-methylthio-N6-isopentenyladeninyl,
N6-(cis-hydroxyisopentenyl)adeninyl,
2-methylthio-N6-(cis-hydroxyisopentenyl) adeninyl,
N6-glycinylcarbamoyladeninyl,
N6-threonylcarbamoyladeninyl,
2-methylthio-N6-threonyl carbamoyladeninyl,
N6-methyl-N6-threonylcarbamoyladeninyl,
N6-hydroxynorvalylcarbamoyladeninyl,
2-methylthio-N6-hydroxynorvalyl carbamoyladeninyl,
N6,N6-dimethyladeninyl,
3-methylcytosinyl,
5-methylcytosinyl,
2-thiocytosinyl,
5-formylcytosinyl,
NH
COOH N
H2N N N
H ' 98
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
N4-methylcytosinyl,
5-hydroxymethylcytosinyl,
1-methylguaninyl,
N2-methylguaninyl,
7-methylguaninyl,
N2,N2-dimethylguaninyl,
NHCOOCH3 NHCOOCH3 NHCOOCH3
H3000C H300OC OH H3000C OOH
0 0 0
N N N
H3C H3C / ` > H 3 `>
N N N N N N N N N
CH3 CH3 CH3
NH2
C 0
HOOC OH 0 H 3
O N N N N
N N H3CN \) H31' \> H3C \> N N N N N N
NN
CH3 CH3
CH3
99
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
O 0 HO HO
HN N H3C\N N HO HO 0
N) ~.N N p NH O NH
HN HN
H2NN NN H2N'N N
HO HO
beta-galactosylO O beta-mannosyl0 O
O NH 0 NH p HN NH2
, HN HN
HN
,I)s
H2NL,~- N N H2N)--I N N H2NN N
N2,7-dimethylguaninyl,
N2,N2,7 -trimethyl guaninyl,
1-methylguaninyl,
7-cyano-7-deazaguaninyl,
7-aminomethyl-7-deazaguaninyl,
pseudouracilyl,
dihydrouracilyl,
5-methyluracilyl,
1 -methylp s eudouracilyl,
2-thiouracilyl,
4-thiouracilyl,
100
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
2-thiothyminyl
5-methyl-2-thiouracilyl,
3-(3 -amino-3-carboxypropyl)uracilyl,
5-hydroxyuracilyl,
5-methoxyuracilyl,
uracilyl 5-oxyacetic acid,
uracilyl 5-oxyacetic acid methyl ester,
5-(carboxyhydroxymethyl)uracilyl,
5-(carboxyhydroxymethyl)uracilyl methyl ester,
5-methoxycarbonylmethyluracilyl,
5-methoxycarbonylmethyl-2-thiouracilyl,
5-aminomethyl-2-thiouracilyl,
5-methylaminomethyluracilyl,
5-methylaminomethyl-2-thiouracilyl,
5-methylaminomethyl-2-selenouracilyl,
5-carbamoylmethyluracilyl,
5-carboxymethylaminomethyluracilyl,
5-carboxymethylaminomethyl-2-thiouracilyl,
3-methyluracilyl,
1-methyl-3-(3-amino-3-carboxypropyl) pseudouracilyl,
101
CA 02521464 2011-06-30
51912-8
5-carboxymethyluracilyl,
5-methyldihydrouracilyl, or
3 -methylpseudouracilyl.
Palindromes
An RNA, e.g., an iRNA agent, can have a palindrome structure as described
herein and
those described in one or more of United States Provisional Application Serial
No. 60/452,682,
filed March 7, 2003; United States Provisional Application Serial No.
60/462,894, filed April
14,2003; and International Application No. PCT/USO4/07070, filed March 8,
2004.
The iRNA agents of the invention can target more than
one RNA region. For example, an iRNA agent can include a first and second
sequence that are
sufficiently complementary to each other to hybridize. The first sequence can
be complementary
to a first target RNA region and the second sequence can be complementary to a
second target
RNA region. The first and second sequences of the iRNA agent can be on
different RNA
strands, and the mismatch between the first and second sequences can be less
than 50%, 40%,
30%, 20%, 10%, 5%, or 1%. The first and second sequences of the iRNA agent are
on the same
RNA strand, and in a related embodiment more than 50%, 60%, 70%, 80%, 90%,
95%, or 1% of
the iRNA agent can be in bimolecular form. The first and second sequences of
the iRNA agent
can be fully complementary to each other.
The first target RNA region can be encoded by a first gene and the second
target RNA
region can encoded by a second gene, or the first and second target RNA
regions can be different
regions of an RNA from a single gene. The first and second sequences can
differ by at least 1
nucleotide.
The first and second target RNA regions can be on transcripts encoded by first
and
second sequence variants, e.g., first and second alleles, of a gene. The
sequence variants can be
mutations, or polymorphisms, for example. The first target RNA region can
include a nucleotide
substitution, insertion, or deletion relative to the second target RNA region,
or the second target
RNA region can a mutant or variant of the first target region.
102
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The first and second target RNA regions can comprise viral or human RNA
regions. The
first and second target RNA regions can also be on variant transcripts of an
oncogene or include
different mutations of a tumor suppressor gene transcript. In addition, the
first and second target
RNA regions can correspond to hot-spots for genetic variation.
The compositions of the invention can include mixtures of iRNA agent
molecules. For
example, one iRNA agent can contain a first sequence and a second sequence
sufficiently
complementary to each other to hybridize, and in addition the first sequence
is complementary to
a first target RNA region and the second sequence is complementary to a second
target RNA
region. The mixture can also include at least one additional iRNA agent
variety that includes a
third sequence and a fourth sequence sufficiently complementary to each other
to hybridize, and
where the third sequence is complementary to a third target RNA region and the
fourth sequence
is complementary to a fourth target RNA region. In addition, the first or
second sequence can be
sufficiently complementary to the third or fourth sequence to be capable of
hybridizing to each
other. The first and second sequences can be on the same or different RNA
strands, and the third
and fourth sequences can be on the same or different RNA strands.
The target RNA regions can be variant sequences of a viral or human RNA, and
in
certain embodiments, at least two of the target RNA regions can be on variant
transcripts of an
oncogene or tumor suppressor gene. The target RNA regions can correspond to
genetic hot-
spots.
Methods of making an iRNA agent composition can include obtaining or providing
information about a region of an RNA of a target gene (e.g., a viral or human
gene, or an
oncogene or tumor suppressor, e.g., p53), where the region has high
variability or mutational
frequency (e.g., in humans). In addition, information about a plurality of RNA
targets within the
region can be obtained or provided, where each RNA target corresponds to a
different variant or
mutant of the gene (e.g., a region including the codon encoding p53 248Q
and/or p53 249S).
The iRNA agent can be constructed such that a first sequence is complementary
to a first of the
plurality of variant RNA targets (e.g., encoding 249Q) and a second sequence
is complementary
to a second of the plurality of variant RNA targets (e.g., encoding 249S), and
the first and second
sequences can be sufficiently complementary to hybridize.
103
CA 02521464 2011-06-30
51912-8
Sequence analysis, e.g., to identify common mutants in the target gene, can be
used to
identify a region of the target gene that has high variability or mutational
frequency. A region of
the target gene having high variability or mutational frequency can be
identified by obtaining or
providing genotype information about the target gene from a population.
Expression of a target gene can be modulated, e.g., downregulated or silenced,
by
providing an iRNA agent that has a first sequence and a second sequence
sufficiently
complementary to each other to hybridize. In addition, the first sequence can
be complementary
to a first target RNA region and the second sequence can be complementary to a
second target
RNA region.
An iRNA agent can include a first sequence complementary to a first variant
RNA target
region and a second sequence complementary to a second variant RNA target
region. The first
and second variant RNA target regions can correspond to first and second
variants or mutants of
a target gene, e.g., viral gene, tumor suppressor or oncogene. The first and
second variant target
RNA regions can include allelic variants, mutations (e.g., point mutations),
or polymorphisms of
the target gene. The first and second variant RNA target regions can
correspond to genetic hot
spots.
A plurality of iRNA agents (e.g., a panel or bank) can be provided.
Other than Canonical Watson-Crick Duplex Structures
An RNA, e.g., an iRNA agent can include monomers which can form other than a
canonical Watson-Crick pairing with another monomer, e.g., a monomer on
another strand, such
as those described herein and those described in United States Provisional
Application Serial No.
60/465,665, filed April 25, 2003, and International Application No.
PCT/US04/07070, filed
March 8, 2004.
The use of "other than canonical Watson-Crick pairing" between monomers of a
duplex
can be used to control, often to promote, melting of all or part of a duplex.
The iRNA agent can
include a monomer at a selected or constrained position that results in a
first level of stability in
the iRNA agent duplex (e.g., between the two separate molecules of a double
stranded iRNA
agent) and a second level of stability in a duplex between a sequence of an
iRNA agent and
104
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
another sequence molecule, e.g., a target or off-target sequence in a subject.
In some cases the
second duplex has a relatively greater level of stability, e.g., in a duplex
between an anti-sense
sequence of an iRNA agent and a target mRNA. In this case one or more of the
monomers, the
position of the monomers in the iRNA agent, and the target sequence (sometimes
referred to
herein as the selection or constraint parameters), are selected such that the
iRNA agent duplex is
has a comparatively lower free energy of association (which while not wishing
to be bound by
mechanism or theory, is believed to contribute to efficacy by promoting
disassociation of the
duplex iRNA agent in the context of the RISC) while the duplex formed between
an anti-sense
targeting sequence and its target sequence, has a relatively higher free
energy of association
(which while not wishing to be bound by mechanism or theory, is believed to
contribute to
efficacy by promoting association of the anti-sense sequence and the target
RNA).
In other cases the second duplex has a relatively lower level of stability,
e.g.; in a duplex
between a sense sequence of an iRNA agent and an off-target mRNA. In this case
one or more
of the monomers, the position of the monomers in the iRNA agent, and an off-
target sequence,
are selected such that the iRNA agent duplex is has a comparatively higher
free energy of
association while the duplex formed between a sense targeting sequence and its
off-target
sequence, has a relatively lower free energy of association (which while not
wishing to be bound
by mechanism or theory, is believed to reduce the level of off-target
silencing by contribute to
efficacy by promoting disassociation of the duplex formed by the sense strand
and the off-target
sequence).
Thus, inherent in the structure of the iRNA agent is the property of having a
first stability
for the intra-iRNA agent duplex and a second stability for a duplex formed
between a sequence
from the iRNA agent and another RNA, e.g., a target mRNA. As discussed above,
this can be
accomplished by judicious selection of one or more of the monomers at a
selected or constrained
position, the selection of the position in the duplex to place the selected or
constrained position,
and selection of the sequence of a target sequence (e.g., the particular
region of a target gene
which is to be targeted). The iRNA agent sequences which satisfy these
requirements are
sometimes referred herein as constrained sequences. Exercise of the constraint
or selection
parameters can e, e.g., by inspection, or by computer assisted methods.
Exercise of the
105
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
parameters can result in selection of a target sequence and of particular
monomers to give a
desired result in terms of the stability, or relative stability, of a duplex.
Thus, in another aspect, the invention features, an iRNA agent which includes:
a first
sequence which targets a first target region and a second sequence which
targets a second target
region. The first and second sequences have sufficient complementarity to each
other to
hybridize, e.g., under physiological conditions, e.g., under physiological
conditions but not in
contact with a helicase or other unwinding enzyme. In a duplex region of the
iRNA agent, at a
selected or constrained position, the first target region has a first monomer,
and the second target
region has a second monomer. The first and second monomers occupy
complementary or
I o corresponding positions. One, and preferably both monomers are selected
such that the stability
of the pairing of the monomers contribute to a duplex between the first and
second sequence will
differ form the stability of the pairing between the first or second sequence
with a target
sequence.
Usually, the monomers will be selected (selection of the target sequence may
be required
as well) such that they form a pairing in the iRNA agent duplex which has a
lower free energy of
dissociation, and a lower Tm, than will be possessed by the paring of the
monomer with its
complementary monomer in a duplex between the iRNA agent sequence and a target
RNA
duplex.
The constraint placed upon the monomers can be applied at a selected site or
at more
than one selected site. By way of example, the constraint can be applied at
more than 1, but less
than 3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA agent
duplex. E.g., a constrained or selected site can be present within 3, 4, 5, or
6 positions from
either end, 3' or 5' of a duplexed sequence. A constrained or selected site
can be present in the
middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
addition to the selected or constrained site or sites. Preferably it will have
no more than 1, 2, 3,
106
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
Overhangs are discussed in detail elsewhere herein but are preferably about 2
nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
sequences can also be joined, e.g., by additional bases to form a hairpin, or
by other non-base
linkers.
The monomers can be selected such that: first and second monomers are
naturally
occurring ribonuceotides, or modified ribonucleotides having naturally
occurring bases, and
when occupying complemetary sites either do not pair and have no substantial
level of H-
1 o bonding, or form a non canonical Watson-Crick pairing and form a non-
canonical pattern of H
bonding, which usually have a lower free energy of dissociation than seen in a
canonical
Watson-Crick pairing, or otherwise pair to give a free energy of association
which is less than
that of a preselected value or is less, e.g., than that of a canonical
pairing. When one (or both) of
the iRNA agent sequences duplexes with a target, the first (or second) monomer
forms a
canonical Watson-Crick pairing with the base in the complemetary position on
the target, or
forms a non canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tm than seen in the paring in the iRNA agent. The classical Watson-
Crick parings are as
follows: A-T, G-C, and A-U. Non-canonical Watson-Crick pairings are known in
the art and
can include, U-U, G-G, G-Atrans, G-Acis, and GU.
The monomer in one or both of the sequences is selected such that, it does not
pair, or
forms a pair with its corresponding monomer in the other sequence which
minimizes stability
(e.g., the H bonding formed between the monomer'at the selected site in the
one sequence and its
monomer at the corresponding site in the other sequence are less stable than
the H bonds formed
by the monomer one (or both) of the sequences with the respective target
sequence. The
monomer is one or both strands is also chosen to promote stability in one or
both of the duplexes
made by a strand and its target sequence. E.g., one or more of the monomers
and the target
sequences are selected such that at the selected or constrained position,
there is are no H bonds
formed, or a non canonical pairing is formed in the iRNA agent duplex, or
otherwise they
otherwise pair to give a free energy of association which is less than that of
a preselected value
or is less, e.g., than that of a canonical pairing, but when one (or both)
sequences form a duplex
107
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
with the respective target, the pairing at the selected or constrained site is
a canonical Watson-
Crick paring.
The inclusion of such a monomers will have one or more of the following
effects: it will
destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off-target
sequences, and duplex
interactions between the a sequence and the intended target will not be
destabilized.
By way of example:
The monomer at the selected site in the first sequence includes an A (or a
modified base
which pairs with T), and the monomer in at the selected position in the second
sequence is
1o chosen from a monomer which will not pair or which will form a non-
canonical pairing, e.g., G.
These will be useful in applications wherein the target sequence for the first
sequence has a T at
the selected position. In embodiments where both target duplexes are
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes U (or a
modified base
which pairs with A), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G. These will be useful in applications wherein the target sequence for the
first sequence has
a T at the selected position. In embodiments where both target duplexes are
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes a G (or a
modified base
which pairs with C), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G,
A,;S, Atrans, or U. These will be useful in applications wherein the target
sequence for the first
sequence has a T at the selected position. In embodiments where both target
duplexes are
stabilized it is useful wherein the target sequence for the second strand has
a monomer which
108
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
will form a canonical Watson-Crick pairing with the monomer selected for the
selected position
in the second strand.
The monomer at the selected site in the first sequence includes a C (or a
modified base
which pairs with G), and the monomer in at the selected position in the second
sequence is
chosen a monomer which will not pair or which will form a non-canonical
pairing. These will be
useful in applications wherein the target sequence for the first sequence has
a T at the selected
position. In embodiments where both target duplexes are stabilized it is
useful wherein the target
sequence for the second strand has a monomer which will form a canonical
Watson-Crick
pairing with the monomer selected for the selected position in the second
strand.
A non-naturally occurring or modified monomer or monomers can be chosen such
that
when a non-naturally occurring or modified monomer occupies a positions at the
selected or
constrained position in an iRNA agent they exhibit a first free energy of
dissociation and when
one (or both) of them pairs with a naturally occurring monomer, the pair
exhibits a second free
energy of dissociation, which is usually higher than that of the pairing of
the first and second
monomers. E.g., when the first and second monomers occupy complementary
positions they
either do not pair and have no substantial level of H-bonding, or form a
weaker bond than one of
them would form with a naturally occurring monomer, and reduce the stability
of that duplex, but
when the duplex dissociates at least one of the strands will form a duplex
with a target in which
the selected monomer will promote stability, e.g., the monomer will form a
more stable pair with
a naturally occurring monomer in the target sequence than the pairing it
formed in the iRNA
agent.
An example of such a pairing is 2-amino A and either of a 2-thio pyrimidine
analog of U
or T.
When placed in complementary positions of the iRNA agent these monomers will
pair
very poorly and will minimize stability. However, a duplex is formed between 2
amino A and
the U of a naturally occurring target, or a duplex is between 2-thio U and the
A of a naturally
occurring target or 2-thio T and the A of a naturally occurring target will
have a relatively higher
free energy of dissociation and be more stable. This is shown in the FIG. 1.
109
CA 02521464 2011-06-30
51912-8
The pair shown in FIG. 1 (the 2-amino A and the 2-s U and T) is exemplary. In
another
embodiment, the monomer at the selected position in the sense strand can be a
universal pairing
moiety. A universal pairing agent will form some level of H bonding with more
than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-nitro pyrrole. (Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447.
Examples can also be found in the section on Universal Bases below.) In these
cases
the monomer at the corresponding position of the anti-sense strand can be
chosen for its ability to
form a duplex with the target and can include, e.g., A, U, G, or C.
1 o iRNA agents of the invention can include:
A sense sequence, which preferably does not target a sequence in a subject,
and an anti-
sense sequence, which targets a target gene in a subject. The sense and anti-
sense sequences
have sufficient complementarity to each other to hybridize hybridize, e.g.,
under physiological
conditions, e.g., under physiological conditions but not in contact with a
helicase or other
unwinding enzyme. In a duplex region of the iRNA agent, at a selected or
constrained position,
the monomers are selected such that:
The monomer in the sense sequence is selected such that, it does not pair, or
forms a pair
with its corresponding monomer in the anti-sense strand which minimizes
stability (e.g., the H
bonding formed between the monomer at the selected site in the sense strand
and its monomer at
the corresponding site in the anti-sense strand are less stable than the H
bonds formed by the
monomer of the anti-sense sequence and its canonical Watson-Crick partner or,
if the monomer
in the anti-sense strand includes a modified base, the natural analog of the
modified base and its
canonical Watson-Crick partner);
The monomer is in the corresponding position in the anti-sense strand is
selected such
that it maximizes the stability of a duplex it forms with the target sequence,
e.g., it forms a
canonical Watson-Crick paring with the monomer in the corresponding position
on the target
stand;
110
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Optionally, the monomer in the sense sequence is selected such that, it does
not pair, or
forms a pair with its corresponding monomer in the anti-sense strand which
minimizes stability
with an off-target sequence.
The inclusion of such a monomers will have one or more of the following
effects: it will
destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off-target
sequences, and duplex
interactions between the anti-sense strand and the intended target will not be
destabilized.
The constraint placed upon the monomers can be applied at a selected site or
at more
than one selected site. By way of example, the constraint can be applied at
more than 1, but less
1 o than 3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA agent
duplex. E.g., a constrained or selected site can be present within 3, 4, 5, or
6 positions from
either end, 3' or 5' of a duplexed sequence. A constrained or selected site
can be present in the
middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
addition to the selected or constrained site or sites. Preferably it will have
no more than 1, 2, 3,
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
Overhangs are discussed in detail elsewhere herein but are preferably about 2
nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
sequences can also be joined, e.g., by additional bases to form a hairpin, or
by other non-base
linkers.
The monomers can be selected such that: first and second monomers are
naturally
occurring ribonuceotides, or modified ribonucleotides having naturally
occurring bases, and
when occupying complemetary sites either do not pair and have no substantial
level of H-
bonding, or form a non canonical Watson-Crick pairing and form a non-canonical
pattern of H
bonding, which usually have a lower free energy of dissociation than seen in a
canonical
111
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Watson-Crick pairing, or otherwise pair to give a free energy of association
which is less than
that of a preselected value or is less, e.g., than that of a canonical
pairing. When one (or both) of
the iRNA agent sequences duplexes with a target, the first (or second) monomer
forms a
canonical Watson-Crick pairing with the base in the complemetary position on
the target, or
forms a non canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tm than seen in the paring in the iRNA agent. The classical Watson-
Crick parings are as
follows: A-T, G-C, and A-U. Non-canonical Watson-Crick pairings are known in
the art and
can include, U-U, G-G, G-Atar,5, G-Aci,, and GU.
The monomer in one or both of the sequences is selected such that, it does not
pair, or
lo forms a pair with its corresponding monomer in the other sequence which
minimizes stability
(e.g., the H bonding formed between the monomer at the selected site in the
one sequence and its
monomer at the corresponding site in the other sequence are less stable than
the H bonds formed
by the monomer one (or both) of the sequences with the respective target
sequence. The
monomer is one or both strands is also chosen to promote stability in one or
both of the duplexes
made by a strand and its target sequence. E.g., one or more of the monomers
and the target
sequences are selected such that at the selected or constrained position,
there is are no H bonds
formed, or a non canonical pairing is formed in the iRNA agent duplex, or
otherwise they
otherwise pair to give a free energy of association which is less than that of
a preselected value
or is less, e.g., than that of a canonical pairing, but when one (or both)
sequences form a duplex
with the respective target, the pairing at the selected or constrained site is
a canonical Watson-
Crick paring.
The inclusion of such a monomers will have one or more of the following
effects: it will
destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off-target
sequences, and duplex
interactions between the a sequence and the intended target will not be
destabilized.
By way of example:
The monomer at the selected site in the first sequence includes an A (or a
modified base
which pairs with T), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G.
112
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
These will be useful in applications wherein the target sequence for the first
sequence has a T at
the selected position. In embodiments where both target duplexes are
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes U (or a
modified base
which pairs with A), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G. These will be useful in applications wherein the target sequence for the
first sequence has
a T at the selected position. In embodiments where both target duplexes are
stabilized it is useful
lo wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes a G (or a
modified base
which pairs with C), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G,
Aci,, Atrans, or U. These will be useful in applications wherein the target
sequence for the first
sequence has a T at the selected position. In embodiments where both target
duplexes are
stabilized it is useful wherein the target sequence for the second strand has
a monomer which
will form a canonical Watson-Crick pairing with the monomer selected for the
selected position
in the second strand.
The monomer at the selected site in the first sequence includes a C (or a
modified base
which pairs with G), and the monomer in at the selected position in the second
sequence is
chosen a monomer which will not pair or which will form a non-canonical
pairing. These will be
useful in applications wherein the target sequence for the first sequence has
a T at the selected
position. In embodiments where both target duplexes are stabilized it is
useful wherein the target
sequence for the second strand has a monomer which will form a canonical
Watson-Crick
pairing with the monomer selected for the selected position in the second
strand.
A non-naturally occurring or modified monomer or monomers can be chosen such
that
when a non-naturally occurring or modified monomer occupies a positions at the
selected or
constrained position in an iRNA agent they exhibit a first free energy of
dissociation and when
113
11 11
CA 02521464 2011-06-30
51912-8
one (or both) of them pairs with a naturally occurring monomer, the pair
exhibits a second free
energy of dissociation, which is usually higher than that of the pairing of
the first and second
monomers. E.g., when the first and second monomers occupy complementary
positions they
either do not pair and have no substantial level of H -bonding, or form a
weaker bond than one of
them would form with a naturally occurring monomer, and reduce the stability
of that duplex, but
when the duplex dissociates at least one of the strands will form a duplex
with a target in which
the selected monomer will promote stability, e.g., the monomer will form a
more stable pair with
a naturally occurring monomer in the target sequence than the pairing it
formed-in the iRNA
agent.
An example of such a pairing is 2-amino A and either of a 2-thio pyrinudine
analog of U
or-T.
When placed in complementary positions of the iRNA agent these monomers will
pair
very poorly and will minimize stability. However, a duplex is formed between 2
amino A and
the U of a naturally occurring target, or a duplex is between 2-thio U and the
A of a naturally
occurring target or 2-thio T and the A of a naturally occurring target will
have a relatively higher
free energy of dissociation and be more stable.
The monomer at the selected position in the sense strand can be a universal
pairing
moiety. A universal pairing agent will form some level of H bonding with more
than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-nitro pyrrole. (Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447.
Examples can also be found in the section on Universal Bases below.) In these
cases
the monomer at the corresponding position of the anti-sense strand can be
chosen for its ability to
form a duplex with the target and can include, e.g., A, U, G, or C.
iRNA agents of the invention can include:
A sense sequence, which preferably does not target a sequence in a subject,
and an anti-
sense sequence, which targets a target gene in a subject. The sense and anti-
sense sequences
have sufficient complementarity to each other to hybridize hybridize, e.g.,
under physiological
114
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
conditions, e.g., under physiological conditions but not in contact with a
helicase or other
unwinding enzyme. In a duplex region of the iRNA agent, at a selected or
constrained position,
the monomers are selected such that:
The monomer in the sense sequence is selected such that, it does not pair, or
forms a pair
with its corresponding monomer in the anti-sense strand which minimizes
stability (e.g., the H
bonding formed between the monomer at the selected site in the sense strand
and its monomer at
the corresponding site in the anti-sense strand are less stable than the H
bonds formed- by the
monomer of the anti-sense sequence and its canonical Watson-Crick partner or,
if the monomer
in the anti-sense strand includes a modified base, the natural analog of the
modified base and its
canonical Watson-Crick partner);
The monomer is in the corresponding position in the anti-sense strand is
selected such
that it maximizes the stability of a duplex it forms with the target sequence,
e.g., it forms a
canonical Watson-Crick paring with the monomer in the corresponding position
on the target
stand;
Optionally, the monomer in the sense sequence is selected such that, it does
not pair, or
forms a pair with its corresponding monomer in the anti-sense strand which
minimizes stability
with an off-target sequence.
The inclusion of such a monomers will have one or more of the following
effects: it will
destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off-target
sequences, and duplex
interactions between the anti-sense strand and the intended target will not be
destabilized.
The constraint placed upon the monomers can be applied at a selected site or
at more
than one selected site. By way of example, the constraint can be applied at
more than 1, but less
than 3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA agent
duplex. E.g., a constrained or selected site can be present within 3, 4, 5, or
6 positions from
either end, 3' or 5' of a duplexed sequence. A constrained or selected site
can be present in the
115
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
The iRNA agent can be selected to target a broad spectrum of genes, including
any of
the genes described herein.
In a preferred embodiment the iRNA agent has an architecture (architecture
refers to
one or more of overall length, length of a duplex region, the presence,
number, location, or
length of overhangs, sing strand versus double strand form) described herein.
E.g., the iRNA agent can be less than 30 nucleotides in length,'e.g., 21-23
nucleotides.
Preferably, the iRNA is 21 nucleotides in length and there is a duplex region
of about 19 pairs.
1 o In one embodiment, the iRNA is 21 nucleotides in length, and the duplex
region of the iRNA is
19 nucleotides. In another embodiment, the iRNA is greater than 30 nucleotides
in length.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
addition to the selected or constrained site or sites. Preferably it will have
no more than 1, 2, 3,
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
Overhangs are discussed in detail elsewhere herein but are preferably about 2
nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
sequences can also be joined, e.g., by additional bases to form a hairpin, or
by other non-base
linkers.
One or more selection or constraint parameters can be exercised such that:
monomers at
the selected site in the sense and anti-sense sequences are both naturally
occurring
ribonucleotides, or modified ribonucleotides having naturally occurring bases,
and when
occupying complementary sites in the iRNA agent duplex either do not pair and
have no
substantial level of H-bonding, or form a non-canonical Watson-Crick pairing
and thus form a
non-canonical pattern of H bonding, which generally have a lower free energy
of dissociation
than seen in a Watson-Crick pairing, or otherwise pair to give a free energy
of association which
is less than that of a preselected value or is less, e.g., than that of a
canonical pairing. When one,
usually the anti-sense sequence of the iRNA agent sequences forms a duplex
with another
116
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
sequence, generally a sequence in the subject, and generally a target
sequence, the monomer
forms a classic Watson-Crick pairing with the base in the complementary
position on the target,
or forms a non-canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tin than seen in the paring in the iRNA agent. Optionally, when the
other sequence of the
iRNA agent, usually the sense sequences forms a duplex with another sequence,
generally a
sequence in the subject, and generally an off-target sequence, the monomer
fails to forms a
canonical Watson-Crick pairing with the base in the complementary position on
the off target
sequence, e.g., it forms or forms a non-canonical Watson-Crick pairing having
a lower free
energy of dissociation and a lower Tm.
By way of example:
the monomer at the selected site in the anti-sense stand includes an A (or a
modified base
which pairs with T), the corresponding monomer in the target is a T, and the
sense strand is
chosen from a base which will not pair or which will form a noncanonical pair,
e.g., G;
the monomer at the selected site in the anti-sense stand includes a U (or a
modified base
which pairs with A), the corresponding monomer in the target is an A, and the
sense strand is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G;
the monomer at the selected site in the anti-sense stand includes a C (or a
modified base
which pairs with G), the corresponding monomer in the target is a G, and the
sense strand is
chosen a monomer which will not pair or which will form a non-canonical
pairing, e.g., G, Acis,
Atrans, or U; or
the monomer at the selected site in the anti-sense stand includes a G (or a
modified base
which pairs with C), the corresponding monomer in the target is a C, and the
sense strand is
chosen from a monomer which will not pair or which will form a non-canonical
pairing.
In another embodiment a non-naturally occurring or modified monomer or
monomers is
chosen such that when it occupies complementary a position in an iRNA agent
they exhibit a
first free energy of dissociation and when one (or both) of them pairs with a
naturally occurring
monomer, the pair exhibits a second free energy of dissociation, which is
usually higher than that
117
CA 02521464 2011-06-30
51912-8
of the pairing of the first and second monomers. E.g., when the first and
second monomers
occupy complementary positions they either do not pair and have no substantial
level of H-
bonding, or form a weaker bond than one of them would form with a naturally
occurring
monomer, and reduce the stability of that duplex, but when the duplex
dissociates at least one of
the strands will form a duplex with a target in which the selected monomer
will promote
stability, e.g., the monomer will form a more stable pair with a naturally
occurring monomer in
the target sequence than the pairing it formed in the iRNA agent.
An example of such a pairing is 2-amino A and either of a 2-thio pyrimidine
analog of U
or T. As is discussed above, when placed in complementary positions of the
iRNA agent these
monomers will pair very poorly and will minimize stability. However, a duplex
is formed
between 2 amino A and the U of a naturally occurring target, or a duplex is
formed between 2-
thio U and the A of a naturally occurring target or 2-thio T and the A of a
naturally occurring
target will have a relatively higher free energy of dissociation and be more
stable.
The monomer at the selected position in the sense strand can be a universal
pairing
moiety. A universal pairing agent will form some level of H bonding with more
than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-nitro pyrrole. Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447.
In these cases the monomer at the corresponding position of the anti-sense
strand can
be chosen for its ability to form a duplex with the target and can include,
e.g., A, U, G, or C.
In another aspect, the invention features, an iRNA agent which includes:
a sense sequence, which preferably does not target a sequence in a subject,
and an anti-
sense sequence, which targets a plurality of target sequences in a subject,
wherein the targets
differ in sequence at only 1 or a small number, e.g., no more than 5, 4, 3 or
2 positions. The
sense and anti-sense sequences have sufficient complementarity to each other
to hybridize, e.g.,
under physiological conditions, e.g., under physiological conditions but not
in contact with a
helicase or other unwinding enzyme. In the sequence of the anti-sense strand
of the iRNA agent
is selected such that at one, some, or all of the positions which correspond
to positions that
differe in sequence between the target sequences, the anti-sense strand will
include a monomer
118
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
which will form H-bonds with at least two different target sequences. In a
preferred example the
anti-sense sequence will include a universal or promiscuous monomer, e.g., a
monomer which
includes 5-nitro pyrrole, 2-amino A, 2-thio U or 2-thio T, or other universal
base referred to
herein.
In a preferred embodiment the iRNA agent targets repeated sequences (which
differ at
only one or a small number of positions from each other) in a single gene, a
plurality of genes, or
a viral genome, e.g., the HCV genome.
An embodiment is illustrated in the FIGs. 2 and 3.
In another aspect, the invention features, determining, e.g., by measurement
or
1o calculation, the stability of a pairing between monomers at a selected or
constrained positoin in
the iRNA agent duplex, and preferably determining the stability for the
corresponding pairing in
a duplex between a sequence form the iRNA agent and another RNA, e.g., a taret
sequence. The
determinations can be compared. An iRNA agent thus analysed can be used in the
devolopement
of a further modified iRNA agent or can be administered to a subject. This
analysis can be
performed successively to refine or desing optimized iRNA agents.
In another aspect, the invention features, a kit which inlcudes one or more of
the
folowing an iRNA described herein, a sterile container in which the iRNA agent
is discolsed,
and instructions for use.
In another aspect, the invention features, an iRNA agent containing a
constrained
sequence made by a method described herein. The iRNA agent can target one or
more of the
genes referred to herein.
iRNA agents having constrained or selected sites, e.g., as described herein,
can be used
in any way described herein. Accordingly, they iRNA agents having constrained
or selected
sites, e.g., as described herein, can be used to silence a target, e.g., in
any of the methods
described herein and to target any of the genes described herein or to treat
any of the disorders
described herein. iRNA agents having constrained or selected sites, e.g., as
described herein, can
be incorporated into any of the formulations or preparations, e.g.,
pharmaceutical or sterile
119
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
preparations described herein. iRNA agents having constrained or selected
sites, e.g., as
described herein, can be administered by any of the routes of administration
described herein.
The term "other than canonical Watson-Crick pairing" as used herein, refers to
a pairing
between a first monomer in a first sequence and a second monomer at the
corresponding position
in a second sequence of a duplex in which one or more of the following is
true: (1) there is
essentially no pairing between the two, e.g., there is no significant level of
H bonding between
the monomers or binding between the monomers does not contribute in any
significant way to
the stability of the duplex; (2) the monomers are a non-canonical paring of
monomers having a
naturally occurring bases, i.e., they are other than A-T, A-U, or G-C, and
they form monomer-
monomer H bonds, although generally the H bonding pattern formed is less
strong than the
bonds formed by a canonical pairing; or(3) at least one of the monomers
includes a non-naturally
occurring bases and the H bonds formed between the monomers is, preferably
formed is less
strong than the bonds formed by a canonical pairing, namely one or more of A-
T, A-U, G-C.
The term "off-target" as used herein, refers to as a sequence other than the
sequence to>be
silenced.
Universal Bases: "wild-cards"; shape-based complementarity
Bi-stranded, multisite replication of a base pair between difluorotoluene and
adenine: confirmation by
`inverse' sequencing. Liu, D.; Moran, S.; Kool, E. T. Chem. Biol., 1997, 4,
919-926)
F CH3
H3C \ H N
HO I F HO N I /
O
OH F OH z
(Importance of terminal base pair hydrogen-bonding in 3'-end proofreading by
the Klenow fragment of
DNA polymerase I. Morales, J. C.; Kool, E. T. Biochemistry, 2000, 39, 2626-
2632)
(Selective and stable DNA base pairing without hydrogen bonds. Matray, T, J.;
Kool, E. T. J. Am. Chem.
Soc., 1998, 120, 6191-6192)
120
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
F
H3C
HO
F
O
OH
(Difluorotoluene, a nonpolar isostere for thyrnine, codes specifically and
efficiently for adenine in DNA
replication. Moran, S. Ren, R. X.-F.; Rumney IV, S.; Kool, E. T. J. Am. Chem.
Soc., 1997,119,2056-2057)
(Structure and base pairing properties of a replicable nonpolar isostere for
deoxyadenosine. Guckian, K.
M.; Morales, J. C.; Kool, E. T. J. Org. Chem., 1998, 63, 9652-9656)
F CH3
H3C N
HO F HO \N I /
O
OH F OH
Z
121
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
N02
HO C N\
O
ls~
OH
3-nitropyrrole
NO2
HO N /
O
OH
5-nitroindole
N O N O N O
MICS PIM SMICS
(
(Universal bases for hybridization, replication and chain termination. Berger,
M.; Wu. Y.; Ogawa, A. K.;
McMinn, D. L.; Schultz, P.G.; Romesberg, F. E. Nucleic Acids Res., 2000, 28,
2911-2914)
TM DM ICS PIGS
N N
2MN DMN 7AI 2Np 3MN
122
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(1. Efforts toward the expansion of the genetic alphabet: Information storage
and replication with unnatural
hydrophobic base pairs. Ogawa, A. K.; Wu, Y.; McMinn, D. L.; Liu, J.; Schultz,
P. G.; Romesberg, F. E. J. Am.
Chem. Soc., 2000, 122, 3274-3287. 2. Rational design of an unnatural base pair
with increased kinetic
selectivity. Ogawa, A. K.; Wu. Y.; Berger, M.; Schultz, P. G.; Romesberg, F.
E. J. Am. Chem. Soc., 2000, 122,
8803-8804)
N
N
7AI
(Efforts toward expansion of the genetic alphabet: replication of DNA with
three base pairs. Tae, E. L.;
Wu, Y.; Xia, G.; Schultz, P. G.; Romesberg, F. E. J. Am. Chem. Soc., 2001,
123, 7439-7440)
JI~
N /
~N
HO I /
O
OH
(1. Efforts toward expansion of the genetic alphabet: Optimization of
interbase hydrophobic interactions.
Wu, Y.; Ogawa, A. K.; Berger, M.; McMinn, D. L.; Schultz, P. G.; Romesberg, F.
E. J. Am. Cliem. Soc., 2000, 122,
7621-7632. 2. Efforts toward expansion of genetic alphabet: DNA polymerase
recognition of a highly stable, self-
pairing hydrophobic base. McMinn, D. L.; Ogawa. A. K.; Wu, Y.; Liu, J.;
Schultz, P. G.; Romesberg, F. E. J. Am.
Chem. Soc., 1999,121,11585-11586)
(A stable DNA duplex containing a non-hydrogen-bonding and non-shape
complementary base couple:
Interstrand stacking as the stability determining factor. Brotschi, C.;
Haberli, A.; Leumann, C, J. Angew. Chem. Int.
Ed., 2001, 40, 3012-3014)
(2,2'-Bipyridine Ligandoside: A novel building block for modifying DNA with
infra-duplex metal
complexes. Weizman, H.; Tor, Y. J. Am. Chem. Soc., 2001, 123, 3375-3376)
123
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
NH2 NHZ
N
N
HO I / HO N
O
OH OH
d2APy d2APm
(Minor groove hydration is critical to the stability of DNA duplexes. Lan, T.;
McLaughlin, L. W. J. Am.
Chem. Soc., 2000, 122, 6512-13)
NO2
0" HO
N
O
OH
(Effect of the Universal base 3-nitropyrrole on the selectivity of neighboring
natural bases. Oliver, J. S.;
Parker, K. A.; Suggs, J. W. Organic Lett., 2001, 3, 1977-1980. 2. Effect of
the 1-(2'-deoxy-(3-D-ribofuranosyl)-3-
nitropyrrol residue on the stability of DNA duplexes and triplexes. Amosova,
0.; George J.; Fresco, J. R. Nucleic
Acids Res., 1997, 25, 1930-1934.3. Synthesis, structure and deoxyribonucleic
acid sequencing with a universal
nucleosides: 1-(2'-deoxy-(3-D-ribofuranosyl)-3-nitropyrrole. Bergstrom, D. E.;
Zhang, P.; Toma, P. H.; Andrews, P.
C.; Nichols, R. J. Am. Chem. Soc., 1995, 117, 1201-1209)
OH
H\ OH
HO N-H............ 0 O
N%\
N-HN
0 "'- ~ D- "I/ I\\ ' \ ,
HO I I N
N HO
FI
O ~N H H
H H N 0
OH Bu'N N
/ / I OH
O
(Model studies directed toward a general triplex DNA recognition scheme: a
novel DNA base that binds a
CG base-pair in an organic solvent. Zimmerman, S. C.; Schmitt, P. J. Am. Chem.
Soc., 1995, 117, 10769-10770)
124
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
/-0
O
DNA
O
O NO
2
O1
DNA
(A universal, photocleavable DNA base: nitropiperonyl 2'-deoxyriboside. J.
Org. Chem., 2001, 66, 2067-
2071)
oR2
/ N NH
R N
H ~O
H N
/ N
H E N-\N / .f N \ H
(Recognition of a single guanine bulge by 2-acylamino-l,8-naphthyridine.
Nakatani, K.; Sando, S.; Saito, I.
J. Am. Chem. Soc., 2000, 122, 2172-2177. b. Specific binding of 2-amino-1,8-
naphthyridine into single guanine
bulge as evidenced by photooxidation of GC doublet, Nakatani, K.; Sando, S.;
Yoshida, K.; Saito, I. Bioorg. Med.
Chem. Lett., 2001, 11, 335-337)
R2
O
lN' H
N H
O- :O
O IN N O
H_N /N O
F_N 0
O O H
O O'
OAP O 0
U
0-0111
-_oP00
0-
Other universal bases can have the following formulas:
125
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R53
R54
R46 R51
"IQ MQ
Q. Q. i v / / Q i v / / R55
Q Q ~N R52 \N
R48 r t R55
R62 0
R61 R63 ni Q ivQ
N `2 \ \ and
Rho R57 R64 R67
59 58 R65 R66
::iciv1
R70 R69
wherein:
Q is N or CR44;
Q'isNorCR45;
Q" is N or CR47;
126
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Q"' is N or CR49;
T is N or CR50;
R44 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR ,
C1-C6
alkyl, C6-C10 aryl, C6-Cio heteroaryl, C3-C8 heterocyclyl, or when taken
together with R45 forms
-OCH2O-;
R45 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbRc,
C1-C6
alkyl, C6-C10 aryl, C6-C10 heteroaryl, C3-C8 heterocyclyl, or when taken
together with R44 or R46
forms -OCH2O ;
R46 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR ,
C1-C6
alkyl, C6-C10 aryl, C6-C10 heteroaryl, C3-C8 heterocyclyl, or when taken
together with R45 or R47
forms -OCH2O-;
R47 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR ,
C1-C6
alkyl, C6-C10 aryl, C6-C10 heteroaryl, C3-C8 heterocyclyl, or when taken
together with R46 or R48
forms -OCH2O-;
R48 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR ,
C1-C6
alkyl, C6-C10 aryl, C6-C10 heteroaryl, C3-C8 heterocyclyl, or when taken
together with R47 forms
-OCH2O-;
R49 R50, R51, R52, R53' R54, R57, R58, R59, R60, R61' R62, R63' R64' R65' R66,
R67, R68' R69'
R70, R71, and R72 are each independently selected from hydrogen, halo,
hydroxy, nitro, protected
hydroxy, -H2, NHRb, or NRbR , C1-C6 alkyl, C2-C6 alkynyl, C6-C10 aryl, C6-C10
heteroaryl, C3-
C8 heterocyclyl, NC(O)R17, or NC(O)R ;
R55 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR ,
C1-C6
alkyl, C2-C6 alkynyl, C6-C10 aryl, C6-C10 heteroaryl, C3-C8 heterocyclyl,
NC(O)R17, or NC(O)R ,
or when taken together with R56 forms a fused aromatic ring which may be
optionally
substituted;
127
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R56 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbRc,
Cl-C6
alkyl, C2-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl,
NC(O)R17, or NC(O)R ,
or when taken together with R55 forms a fused aromatic ring which may be
optionally
substituted;
R17 is halo, NH2, NHRb, or NRbRc;
Rb is C1-C6 alkyl or a nitrogen protecting group;
Rc is C1-C6 alkyl; and
R is alkyl optionally substituted with halo, hydroxy, nitro, protected
hydroxy, NH2,
NHRb, or NRbR, CI-C6 alkyl, C2-C6 alkynyl, C6-CIO aryl, C6-C10 heteroaryl, C3-
C8 heterocyclyl,
NC(O)R17, or NC(O)R .
128
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Examples of universal bases include:
F CH3 NH2 NH2
02N
H3C \ \ N> N N~N
\
F N
NO2 \ 0 I \ 0
~J BuHN~ / N N N--\ H N - 02N 0
H N
CH3 O CH3 O N!~; 0 0 H3C CH3
\ N~ I \ I \ jN!~
H3C CH3
I~ II
0 CH3
H3C'' CH3
/ I N N CH3
CH3
and N
CH3
129
CA 02521464 2011-06-30
51912-8
Asymmetrical Modifications
An RNA, e.g., an iRNA agent, can be asymmetrically modified as described
herein, and
as described in International Application Serial No. PCT/USO4/07070, filed
March 8, 2004.
In addition, the invention includes iRNA agents having asymmetrical
modifications and
another element described herein. E.g., the invention includes an iRNA agent
described herein,
e.g., a palindromic iRNA agent, an iRNA agent having a non canonical pairing,
an iRNA agent
which targets a gene described herein, e.g., a gene active in the liver, an
iRNA agent having an
architecture or structure described herein, an iRNA associated with an
amphipathic delivery
1o agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
which also incorporates an asymmetrical modification.
An asymmetrically modified iRNA agent is one in which a strand has a
modification
which is not present on the other strand. An asymmetrical modification is a
modification found
on one strand but not on the other strand. Any modification, e.g., any
modification described
herein, can be present as an asymmetrical modification. An asymmetrical
modification can
confer any of the desired properties associated with a modification, e.g.,
those properties
discussed herein. E.g., an asymmetrical modification can: confer resistance to
degradation, an
alteration in half life; target the iRNA agent to a particular target, e.g.,
to a particular tissue;
modulate, e.g., increase or decrease, the affinity of a strand for its
complement or target
sequence; or hinder or promote modification of a terminal moiety, e.g.,
modification by a kinase
or other enzymes involved in the RISC mechanism pathway. The designation of a
modification
as having one property does not mean that it has no other property, e.g., a
modification referred
to as one which promotes stabilization might also enhance targeting.
While not wishing to be bound by theory or any particular mechanistic model,
it is
believed that asymmetrical modification allows an iRNA agent to be optimized
in view of the
different or "asymmetrical" functions of the sense and antisense strands. For
example, both
strands can be modified to increase nuclease resistance, however, since some
changes can inhibit
RISC activity, these changes can be chosen for the sense stand . In addition,
since some
130
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
modifications, e.g., targeting moieties, can add large bulky groups that,
e.g., can interfere with
the cleavage activity of the RISC complex, such modifications are preferably
placed on the sense
strand. Thus, targeting moieties, especially bulky ones (e.g. cholesterol),
are preferentially added
to the sense strand. In one embodiment, an asymmetrical modification in which
a phosphate of
the backbone is substituted with S, e.g., a phosphorothioate modification, is
present in the
antisense strand, and a 2' modification, e.g., 2' OMe is present in the sense
strand. A targeting
moiety can be present at either (or both) the 5' or 3' end of the sense strand
of the iRNA agent. In
a preferred example, a P of the backbone is replaced with S in the antisense
strand, 2'OMe is
present in the sense strand, and a targeting moiety is added to either the 5'
or 3' end of the sense
lo strand of the iRNA agent.
In a preferred embodiment an asyrarnetrically modified iRNA agent has a
modification
on the sense strand which modification is not found on the antisense strand
and the antisense
strand has a modification which is not found on the sense strand.
Each strand can include one or more asymmetrical modifications. By way of
example:
one strand can include a first asymmetrical modification which confers a first
property on the
iRNA agent and the other strand can have a second asymmetrical modification
which confers a
second property on the iRNA. E.g., one strand, e.g., the sense strand can have
a modification
which targets the iRNA agent to a tissue, and the other strand, e.g., the
antisense strand, has a
modification which promotes hybridization with the target gene sequence.
In some embodiments both strands can be modified to optimize the same
property, e.g.,
to increase resistance to nucleolytic degradation, but different modifications
are chosen for the
sense and the antisense strands, e.g., because the modifications affect other
properties as well.
E.g., since some changes can affect RISC activity these modifications are
chosen for the sense
strand.
In an embodiment one strand has an asymmetrical 2' modification, e.g., a 2'
OMe
modification, and the other strand has an asymmetrical modification of the
phosphate backbone,
e.g., a phosphorothioate modification. So, in one embodiment the antisense
strand has an
asymmetrical 2' OMe modification and the sense strand has an asymmetrical
phosphorothioate
modification (or vice versa). In a particularly preferred embodiment the RNAi
agent will have
131
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
asymmetrical 2'-O alkyl, preferably, 2'-OMe modifications on the sense strand
and
asymmetrical backbone P modification, preferably a phosphothioate modification
in the
antisense strand. There can be one or multiple 2'-OMe modifications, e.g., at
least 2, 3, 4, 5, or
6, of the subunits of the sense strand can be so modified. There can be one or
multiple
phosphorothioate modifications, e.g., at least 2, 3, 4, 5, or 6, of the
subunits of the antisense
strand can be so modified. It is preferable to have an iRNA agent wherein
there are multiple 2'-
OMe modifications on the sense strand and multiple phophorothioate
modifications on the
antisense strand. All of the subunits on one or both strands can be so
modified. A particularly
preferred embodiment of multiple asymmetric modification on both strands has a
duplex region
about 20-21, and preferably 19, subunits in length and one or two 3' overhangs
of about 2
subunits in length.
Asymmetrical modifications are useful for promoting resistance to degradation
by
nucleases, e.g., endonucleases. iRNA agents can include one or more
asymmetrical
modifications which promote resistance to degradation. In preferred
embodiments the
modification on the antisense strand is one which will not interfere with
silencing of the target,
e.g., one which will not interfere with cleavage of the target. Most if not
all sites on a strand are
vulnerable, to some degree, to degradation by endonucleases. One can determine
sites which are
relatively vulnerable and insert asymmetrical modifications which inhibit
degradation. It is often
desirable to provide asymmetrical modification of a UA site in an iRNA agent,
and in some
cases it is desirable to provide the UA sequence on both strands with
asymmetrical modification.
Examples of modifications which inhibit endonucleolytic degradation can be
found herein.
Particularly favored modifications include: 2' modification, e.g., provision
of a 2' OMe moiety
on the U, especially on a sense strand; modification of the backbone, e.g.,
with the replacement
of an 0 with an S, in the phosphate backbone, e.g., the provision of a
phosphorothioate
modification, on the U or the A or both, especially on an antisense strand;
replacement of the U
with a C5 amino linker; replacement of the A with a G (sequence changes are
preferred to be
located on the sense strand and not the antisense strand); and modification of
the at the 2', 6', 7',
or 8' position. Preferred embodiments are those in which one or more of these
modifications are
present on the sense but not the antisense strand, or embodiments where the
antisense strand has
fewer of such modifications.
132
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Asymmetrical modification can be used to inhibit degradation by exonucleases.
Asymmetrical modifications can include those in which only one strand is
modified as well as
those in which both are modified. In preferred embodiments the modification on
the antisense
strand is one which will not interfere with silencing of the target, e.g., one
which will not
interfere with cleavage of the target. Some embodiments will have an
asymmetrical
modification on the sense strand, e.g., in a 3' overhang, e.g., at the 3'
terminus, and on the
antisense strand, e.g., in a 3' overhang, e.g., at the 3' terminus. If the
modifications introduce
moieties of different size it is preferable that the larger be on the sense
strand. If the
modifications introduce moieties of different charge it is preferable that the
one with greater
lo charge be on the sense strand.
Examples of modifications which inhibit exonucleolytic degradation can be
found herein.
Particularly favored modifications include: 2' modification, e.g., provision
of a ?' OMe moiety
in a 3' overhang, e.g., at the 3' terminus (3' terminus means at the 3' atom
of the molecule or at
the most 3' moiety, e.g., the most 3' P or 2' position, as indicated by the
context); modification
of the backbone, e.g., with the replacement of a P with an S, e.g., the
provision of a
phosphorothioate modification, or the use of a methylated P in a 3' overhang,
e.g., at the 3'
terminus; combination of a 2' modification, e.g., provision of a 2' 0 Me
moiety and
modification of the backbone, e.g., with the replacement of a P with an S,
e.g., the provision of a
phosphorothioate modification, or the use of a methylated P, in a 3' overhang,
e.g., at the 3'
terminus; modification with a 3' alkyl; modification with an abasic pyrolidine
in a 3' overhang,
e.g., at the 3' terminus; modification with naproxene, ibuprofen, or other
moieties which inhibit
degradation at the 3' terminus. Preferred embodiments are those in which one
or more of these
modifications are present on the sense but not the antisense strand, or
embodiments where the
antisense strand has fewer of such modifications.
Modifications, e.g., those described herein, which affect targeting can be
provided as
asymmetrical modifications. Targeting modifications which can inhibit
silencing, e.g., by
inhibiting cleavage of a target, can be provided as asymmetrical modifications
of the sense
strand. A biodistribution altering moiety, e.g., cholesterol, can be provided
in one or more, e.g.,
two, asymmetrical modifications of the sense strand. Targeting modifications
which introduce
moieties having a relatively large molecular weight, e.g., a molecular weight
of more than 400,
133
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W O1
500, or 1000 daltons, or which introduce a charged moiety (e.g., having more
than one positive
charge or one negative charge) can be placed on the sense strand.
Modifications, e.g., those described herein, which modulate, e.g., increase or
decrease,
the affinity of a strand for its compliment or target, can be provided as
asymmetrical
modifications. These include: 5 methyl U; 5 methyl C; pseudouridine, Locked
nucleic acids ,2
thio U and 2-amino-A. In some embodiments one or more of these is provided on
the antisense
strand.
iRNA agents have a defined structure, with a sense strand and an antisense
strand, and in
many cases short single strand overhangs, e.g., of 2 or 3 nucleotides are
present at one or both 3'
lo ends. Asymmetrical modification can be used to optimize the activity of
such a structure, e.g.,
by being placed selectively within the iRNA. E.g., the end region of the iRNA
agent defined by
the 5' end of the sense strand and the 3'end of the antisense strand is
important for function.
This region can include the terminal 2, 3, or 4 paired nucleotides and any 3'
overhang. In
preferred embodiments asymmetrical modifications which result in one or more
of the following
are used: modifications of the 5' end of the sense strand which inhibit kinase
activation of the
sense strand, including, e.g., attachments of conjugates which target the
molecule or the use
modifications which protect against 5' exonucleolytic degradation; or
modifications of either
strand, but preferably the sense strand, which enhance binding between the
sense and antisense
strand and thereby promote a "tight" structure at this end of the molecule.
The end region of the iRNA agent defined by the 3' end of the sense strand and
the 5'end
of the antisense strand is also important for function. This region can
include the terminal 2, 3,
or 4 paired nucleotides and any 3' overhang. Preferred embodiments include
asymmetrical
modifications of either strand, but preferably the sense strand, which
decrease binding between
the sense and antisense strand and thereby promote an "open" structure at this
end of the
molecule. Such modifications include placing conjugates which target the
molecule or
modifications which promote nuclease resistance on the sense strand in this
region. Modification
of the antisense strand which inhibit kinase activation are avoided in
preferred embodiments.
134
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Exemplary modifications for asymmetrical placement in the sense strand include
the
following:
(a) backbone modifications, e.g., modification of a backbone P, including
replacement of
P with S, or P substituted with alkyl or allyl, e.g., Me, and dithioates (S-
P=S); these
modifications can be used to promote nuclease resistance;
(b) 2'-O alkyl, e.g., 2'-OMe, 3'-O alkyl, e.g., 3'-OMe (at terminal and/or
internal
positions); these modifications can be used to promote nuclease resistance or
to enhance binding
of the sense to the antisense strand, the 3' modifications can be used at the
5' end of the sense
lo strand to avoid sense strand activation by RISC;
(c) 2'-5' linkages (with 2'-H, 2'-OH and 2'-OMe and with P=O or P=S) these
modifications can be used to promote nuclease resistance or to inhibit binding
of the sense to the
antisense strand, or can be used at the 5' end of the sense strand to avoid
sense strand activation
by RISC;
(d) L sugars (e.g., L ribose, L-arabinose with 2'-H, 2'-OH and 2'-OMe); these
modifications can be used to promote nuclease resistance or to inhibit binding
of the sense to the
antisense strand, or can be used at the 5' end of the sense strand to avoid
sense strand activation
by RISC;
(e) modified sugars (e.g., locked nucleic acids (LNA's), hexose nucleic acids
(HNA's)
and cyclohexene nucleic acids (CeNA's)); these modifications can be used to
promote nuclease
resistance or to inhibit binding of the sense to the antisense strand, or can
be used at the 5' end of
the sense strand to avoid sense strand activation by RISC;
(f) nucleobase modifications (e.g., C-5 modified pyrimidines, N-2 modified
purines, N-7
modified purines, N-6 modified purines), these modifications can be used to
promote nuclease
resistance or to enhance binding of the sense to the antisense strand;
(g) cationic groups and Zwitterionic groups (preferably at a terminus), these
modifications can be used to promote nuclease resistance;
135
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(h) conjugate groups (preferably at terminal positions), e,g., naproxen,
biotin, cholesterol,
ibuprofen, folic acid, peptides, and carbohydrates; these modifications can be
used to promote
nuclease resistance or to target the molecule, or can be used at the 5' end of
the sense strand to
avoid sense strand activation by RISC.
Exemplary modifications for asymmetrical placement in the antisense strand
include the
following:
(a) backbone modifications, e.g., modification of a backbone P, including
replacement of
P with S, or P substituted with alkyl or allyl, e.g., Me, and dithioates (S-
P=S);
(b) 2'-O alkyl, e.g., 2'-OMe, (at terminal positions);
(c) 2'-5' linkages (with 2'-H, 2'-OH and 2'-OMe) e.g., terminal at the 3'
end); e.g., with
P=O or P=S preferably at the 3'-end, these modifications are preferably
excluded from the 5' end
region as they may interfere with RISC enzyme activity such as kinase
activity;
(d) L sugars (e.g, L ribose, L-arabinose with 2'-H, 2'-OH and 2'-OMe); e.g.,
terminal at
the 3' end; e.g., with P=O or P=S preferably at the 3'-end, these
modifications are preferably
excluded from the 5' end region as they may interfere with kinase activity;
(e) modified sugars (e.g., LNA's, HNA's and CeNA's); these modifications are
preferably excluded from the 5' end region as they may contribute to unwanted
enhancements of
paring between the sense and antisense strands, it is often preferred to have
a "loose" structure in
the 5' region, additionally, they may interfere with kinase activity;
(f) nucleobase modifications (e.g., C-5 modified pyrimidines, N-2 modified
purines, N-7
modified purines, N-6 modified purines);
(g) cationic groups and Zwitterionic groups (preferably at a terminus);
cationic groups and Zwitterionic groups at 2'-position of sugar; 3'-position
of the sugar;
as nucleobase modifications (e.g., C-5 modified pyrimidines, N-2 modified
purines, N-7
modified purines, N-6 modified purines);
136
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
conjugate groups (preferably at terminal positions), e,g., naproxen, biotin,
cholesterol,
ibuprofen, folic acid, peptides, and carbohydrates, but bulky groups or
generally groups which
inhibit RISC activity should are less preferred.
The 5'-OH of the antisense strand should be kept free to promote activity. In
some
preferred embodiments modifications that promote nuclease resistance should be
included at the
3' end, particularly in the 3' overhang.
In another aspect, the invention features a method of optimizing, e.g.,
stabilizing, an
iRNA agent. The method includes selecting a sequence having activity,
introducing one or more
asymmetric modifications into the sequence, wherein the introduction of the
asymmetric
lo modification optimizes a property of the iRNA agent but does not result in
a decrease in activity.
The decrease in activity can be less than a preselected level of decrease. In
preferred
embodiments decrease in activity means a decrease of less than 5, 10, 20, 40,
or 50 % activity, as
compared with an otherwise similar iRNA lacking the introduced modification.
Activity can,
e.g., be measured in vivo, or in vitro, with a result in either being
sufficient to demonstrate the
required maintenance of activity.
The optimized property can be any property described herein and in particular
the
properties discussed in the section on asymmetrical modifications provided
herein. The
modification can be any asymmetrical modification, e.g., an asymmetric
modification described
in the section on asymmetrical modifications described herein. Particularly
preferred
asymmetric modifications are 2'-O alkyl modifications, e.g., 2'-OMe
modifications, particularly
in the sense sequence, and modifications of a backbone 0, particularly
phosphorothioate
modifications, in the antisense sequence.
In a preferred embodiment a sense sequence is selected and provided with an
asymmetrical modification, while in other embodiments an antisense sequence is
selected and
provided with an asymmetrical modification. In some embodiments both sense and
antisense
sequences are selected and each provided with one or more asymmetrical
modifications.
137
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Multiple asymmetric modifications can be introduced into either or both of the
sense and
antisense sequence. A sequence can have at least 2, 4, 6, 8, or more
modifications and all or
substantially all of the monomers of a sequence can be modified.
Table 3 shows examples having strand I with a selected modification and strand
II with a
selected modification.
Table 3. Exemplary strand I- and strand II-modifications
Strand I Strand II
Nuclease Resistance (e.g., 2'-OMe) Biodistribution (e.g., P=S)
Biodistribution conjugate Protein Binding Functionality
(e.g., Lipophile) (e.g., Naproxen)
Tissue Distribution Functionality Cell Targeting Functionality
(e.g., Carbohydrates) (e.g., Folate for cancer cells)
Tissue Distribution Functionality Fusogenic Functionality
(e.g., liver Cell Targeting moieties) (e.g., Polyethylene imines)
Cancer Cell Targeting Fusogenic Functionality
(e.g., RGD peptides and imines) (e.g., peptides)
2-
Nuclease Resistance '-OMe) Increase in binding Affinity (5-Me-C, 5-Me-U, 2-
(e.g., 2 thio-U, 2-amino-A, G-clamp, LNA)
Tissue Distribution Functionality
RISC activity improving Functionality
Helical conformation changing Tissue Distribution Functionality
138
CA 02521464 2011-06-30
51912-8
Functionalities (P-S; lipophile, carbohydrates)
Z-X-Y Architecture
An RNA, e.g., an iRNA. agent, can have a Z-X-Y architecture or structure such
as those
described herein and those described in copending, co-owned United States
Provisional
Application Serial No. 60/510,246, filed on October 9, 2003,
copending, co-owned United States Provisional Application Serial No.
60/510,318,
filed on October 10, 2003, and copending, co-owned
International Application No. PCT/USO4/07070, filed March 8, 2004.
In addition, the invention includes iRNA agents having a Z-X-Y structure and
another
element described herein. E.g., the invention includes an iRNA agent described
herein, e.g., a
1o palindromic iRNA agent, an iRNA agent having a non canonical pairing, an
iRNA agent which
targets a gene described herein, e.g., a gene active in the liver, an iRNA
associated with an
amphipathic delivery agent described herein, an iRNA associated with a drug
delivery module
described herein, an iRNA agent administered as described herein, or an iRNA
agent formulated
as described herein, which also incorporates a Z-X-Y architecture.
. Thus, an iRNA agent can have a first segment, the Z region, a second
segment, the X
region, and optionally a third region, the Y region:
zr--X-Y.
It may be desirable to modify subunits in one or both of Zand/or Y on one hand
and X on
I
the other hand. In some cases they will have the same modification or the same
class of
modification but it will more often be the case that the modifications made in
Z and/or Y will
differ from those made in X.
The Z region typically includes a terminus of an iRNA agent. The length of the
Z region
can vary, but will typically be from 2-14, more preferably 2-10, subunits in
length. It typically is
single stranded, i.e., it will not base pair with bases of another strand,
though it may in some
embodiments self associate, e.g., to form a loop structure. Such structures
can be formed by the
139
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
end of a strand looping back and forming an intrastrand duplex. E.g., 2, 3, 4,
5 or more intra-
strand bases pairs can form, having a looped out or connecting region,
typically of 2 or more
subunits which do not pair. This can occur at one or both ends of a strand. A
typical
embodiment of a Z region is a single strand overhang, e.g., an over hang of
the length described
elsewhere herein. The Z region can thus be or include a 3' or 5' terminal
single strand. It can be
sense or antisense strand but if it is antisense it is preferred that it is a
3- overhang. Typical
inter-subunit bonds in the Z region include: P=O; P=S; S-P=S; P-NR2; and P-
BR2. Chiral P=X,
where X is S, N, or B) inter-subunit bonds can also be present. (These inter-
subunit bonds are
discussed in more detail elsewhere herein.) Other preferred Z region subunit
modifications (also
1o discussed elsewhere herein) can include: 3'-OR, 3'SR, 2'-OMe, 3'-OMe, and
2'OH
modifications and moieties; alpha configuration bases; and 2' arabino
modifications.
The X region will in most cases be duplexed, in the case of a single strand
iRNA agent,
with a corresponding region of the single strand, or in the case of a double
stranded iRNA agent,
with the corresponding region of the other strand. The length of the X region
can vary but will
typically be between 10-45 and more preferably between 15 and 35 subunits.
Particularly
preferred region X's will include 17, 18, 19, 29, 21, 22, 23, 24, or 25
nucleotide pairs, though
other suitable lengths are described elsewhere herein and can be used. Typical
X region subunits
include 2'-OH subunits. In typical embodiments phosphate inter-subunit bonds
are preferred
while phophorothioate or non-phosphate bonds are absent. Other modifications
preferred in the
X region include: modifications to improve binding, e.g., nucleobase
modifications; cationic
nucleobase modifications; and C-5 modified pyrimidines, e.g., allylamines.
Some embodiments
have 4 or more consecutive 2'OH subunits. While the use of phosphorothioate is
sometimes non
preferred they can be used if they connect less than 4 consecutive 2'OH
subunits.
The Y region will generally conform to the the parameters set out for the Z
regions.
However, the X and Z regions need not be the same, different types and numbers
of
modifications can be present, and infact, one will usually be a 3' overhang
and one will usually
be a 5' overhang.
In a preferred embodiment the iRNA agent will have a Y and/or Z region each
having
ribonucleosides in which the 2'-OH is substituted, e.g., with 2'-OMe or other
alkyl; and an X
140
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
region that includes at least four consecutive ribonucleoside subunits in
which the 2'-OH
remains unsubstituted.
The subunit linkages (the linkages between subunits) of an iRNA agent can be
modified,
e.g., to promote resistance to degradation. Numerous examples of such
modifications are
disclosed herein, one example of which is the phosphorothioate linkage. These
modifications
can be provided bewteen the subunits of any of the regions, Y, X, and Z.
However, it is
preferred that their occureceis minimized and in particular it is preferred
that consecutive
modified linkages be avoided.
In a preferred embodiment the iRNA agent will have a Y and Z region each
having
1o ribonucleosides in which the 2'-OH is substituted, e.g., with 2'-OMe; and
an X region that
includes at least four consecutive subunits, e.g., ribonucleoside subunits in
which the 2'-OH
remains unsubstituted.
As mentioned above, the subunit linkages of an iRNA agent can be modified,
e.g., to
promote resistance to degradation. These modifications can be provided between
the subunits of
any of the regions, Y, X, and Z. However, it is preferred that they are
minimized and in
particular it is preferred that consecutive modified linkages be avoided.
Thus, in a preferred embodiment, not all of the subunit linkages of the iRNA
agent are
modified and more preferably the maximum number of consecutive subunits linked
by other than
a phospodiester bond will be 2, 3, or 4. Particulary preferred iRNA agents
will not have four or
more consecutive subunits, e.g., 2'-hydroxyl ribonucleoside subunits, in which
each subunits is
joined by modified linkages - i.e. linkages that have been modified to
stabilize them from
degradation as compared to the phosphodiester linkages that naturally occur in
RNA and DNA.
It is particularly preferred to minimize the occurrence in region X. Thus, in
preferred
embodiments each of the nucleoside subunit linkages in X will be
phosphodiester linkages, or if
subunit linkages in region X are modified, such modifications will be
minimized. E.g., although
the Y and/or Z regions can include inter subunit linkages which have been
stabilized against
degradation, such modifications will be minimized in the X region, and in
particular consecutive
modifications will be minimized. Thus, in preferred embodiments the maximum
number of
141
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
consecutive subunits linked by other than a phospodiester bond will be 2, 3,
or 4. Particulary
preferred X regions will not have four or more consecutive subunits, e.g., 2'-
hydroxyl
ribonucleoside subunits, in which each subunits is joined by modified linkages
- i.e. linkages
that have been modified to stabilize them from degradation as compared to the
phosphodiester
linkages that naturally occur in RNA and DNA.
In a preferred embodiment Y and /or Z will be free of phosphorothioate
linkages, though
either or both may contain other modifications, e.g., other modifications of
the subunit linkages.
In a preferred embodiment region X, or in some cases, the entire iRNA agent,
has no
more than 3 or no more than 4 subunits having identical 2' moieties.
In a preferred embodiment region X, or in some cases, the entire iRNA agent,
has no
more than 3 or no more than 4 subunits having identical subunit linkages.
In a preferred embodiment one or more phosphorothioate linkages (or other
modifications of the subunit linkage) are present in Y and/or Z, but such
modified linkages do
not connect two adjacent subunits, e.g., nucleosides, having a 2'
modification, e.g., a 2'-O-alkyl
moiety. E.g., any adjacent 2'-O-alkyl moieties in the Y and/or Z, are
connected by a linkage
other than a a phosphorothioate linkage.
In a preferred embodiment each of Y and/or Z independently has only one
phosphorothioate linkage between adjacent subunits, e.g., nucleosides, having
a 2' modification,
e.g., 2'-O-alkyl nucleosides. If there is a second set of adjacent subunits,
e.g., nucleosides,
having a 2' modification, e.g., 2'-O-alkyl nucleosides, in Y and/or Z that
second set is connected
by a linkage other than a phosphorothioate linkage, e.g., a modified linkage
other than a
phosphorothioate linkage.
In a prefered embodiment each of Y and/orZ independently has more than one
phosphorothioate linkage connecting adjacent pairs of subunits, e.g.,
nucleosides, having a 2'
modification, e.g., 2'-O-alkyl nucleosides, but at least one pair of adjacent
subunits, e.g.,
nucleosides, having a 2' modification, e.g., 2'-O-alkyl nucleosides, are be
connected by a linkage
other than a phosphorothioate linkage, e.g., a modified linkage other than a
phosphorothioate
linkage.
142
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a prefered embodiment one of the above recited limitation on adjacent
subunits in Y
and or Z is combined with a limitation on the subunits in X. E.g., one or more
phosphorothioate
linkages (or other modifications of the subunit linkage) are present in Y
and/or Z, but such
modified linkages do not connect two adjacent subunits, e.g., nucleosides,
having a 2'
modification, e.g., a 2'-O-alkyl moiety. E.g., any adjacent 2'-O-alkyl
moieties in the Y and/or Z,
are connected by a linkage other than a a phosporothioate linkage. In
addition, the X region has
no more than 3 or no more than 4 identical subunits, e.g., subunits having
identical 2' moieties or
the X region has no more than 3 or no more than 4 subunits having identical
subunit linkages.
A Y and/or Z region can include at least one, and preferably 2, 3 or 4 of a
modification
1 o disclosed herein. Such modifications can be chosen, independently, from
any modification
described herein, e.g., from nuclease resistant subunits, subunits with
modified bases, subunits
with modified intersubunit linkages, subunits with modified sugars, and
subunits linked to
another moiety, e.g., a targeting moiety. In a preferred embodiment more than
1 of such subunits
can be present but in some emobodiments it is prefered that no more than 1, 2,
3, or 4 of such
modifications occur, or occur consecutively. In a preferred embodiment the
frequency of the
modification will differ between Yand /or Z and X, e.g., the modification will
be present one of
Y and/or Z or X and absent in the other.
An X region can include at least one, and preferably 2, 3 or 4 of a
modification disclosed
herein. Such modifications can be chosen, independently, from any modification
described
herein, e.g., from nuclease resistant subunits, subunits with modified bases,
subunits with
modified intersubunit linkages, subunits with modified sugars, and subunits
linked to another
moiety, e.g., a targeting moiety. In a preferred embodiment more than 1 of
such subunits can b
present but in some emobodiments it is prefered that no more than 1, 2, 3, or
4 of such
modifications occur, or occur consecutively.
An RRMS (described elswhere herein) can be introduced at one or more points in
one or
both strands of a double-stranded iRNA agent. An RRMS can be placed in a Y
and/or Z region,
at or near (within 1, 2, or 3 positions) of the 3' or 5' end of the sense
strand or at near (within 2
or 3 positions of) the 3' end of the antisense strand. In some embodiments it
is preferred to not
have an RRMS at or near (within 1, 2, or 3 positions of) the 5' end of the
antisense strand. An
143
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
RRMS can be positioned in the X region, and will preferably be positioned in
the sense strand or
in an area of the antisense strand not critical for antisense binding to the
target.
Differential Modification of Terminal Duplex Stability
In one aspect, the invention features an iRNA agent which can have
differential
modification of terminal duplex stability (DMTDS).
In addition, the invention includes iRNA agents having DMTDS and another
element
described herein. E.g., the invention includes an iRNA agent described herein,
e.g., a
palindromic iRNA agent, an iRNA agent having a non canonical pairing, an iRNA
agent which
targets a gene described herein, e.g., a gene active in the liver, an iRNA
agent having an
1o architecture or structure described herein, an iRNA associated with an
amphipathic delivery
agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
which also incorporates DMTDS.
iRNA agents can be optimized by increasing the propensity of the duplex to
disassociate
or melt (decreasing the free energy of duplex association), in the region of
the 5' end of the
antisense strand duplex. This can be accomplished, e.g., by the inclusion of
subunits which
increase the propensity of the duplex to disassociate or melt in the region of
the 5' end of the
antisense strand. It can also be accomplished by the attachment of a ligand
that increases the
propensity of the duplex to disassociate of melt in the region of the 5'end .
While not wishing to
be bound by theory, the effect may be due to promoting the effect of an enzyme
such as helicase,
for example, promoting the effect of the enzyme in the proximity of the 5' end
of the antisense
strand.
The inventors have also discovered that iRNA agents can be optimized by
decreasing the
propensity of the duplex to disassociate or melt (increasing the free energy
of duplex
association),'in the region of the 3' end of the antisense strand duplex. This
can be
accomplished, e.g., by the inclusion of subunits which decrease the propensity
of the duplex to
disassociate or melt in the region of the 3' end of the antisense strand. It
can also be
144
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
accomplished by the attachment of ligand that decreases the propensity of the
duplex to
disassociate of melt in the region of the 5'end.
Modifications which increase the tendency of the 5' end of the duplex to
dissociate can
be used alone or in combination with other modifications described herein,
e.g., with
modifications which decrease the tendency of the 3' end of the duplex to
dissociate. Likewise,
modifications which decrease the tendency of the 3' end of the duplex to
dissociate can be used
alone or in combination with other modifications described herein, e.g., with
modifications
which increase the tendency of the 5' end of the duplex to dissociate.
Decreasing the stability of the AS 5' end of the duplex
Subunit pairs can be ranked on the basis of their propensity to promote
dissociation or
melting (e.g., on the free energy of association or dissociation of a
particular pairing, the simplest
approach is to examine the pairs on an individual pair basis, though next
neighbor or similar
analysis can also be used). In terms of promoting dissociation:
A:U is preferred over G:C;
G:U is preferred over G:C;
I:C is preferred over G:C (I=inosine);
mismatches, e.g., non-canonical or other than canonical pairings (as described
elsewhere herein) are preferred over canonical (A:T, A:U, G:C) pairings;
pairings which include a universal base are preferred over canonical pairings.
A typical ds iRNA agent can be diagrammed as follows:
S 5' Rt NI N2 N3 N4 N5 [N] N-5 N-4 N-3 N-2 N_1 R2 3'
AS 3' R3 NI N2 N3 N4 N5 [N] N-5 N-4 N-3 N-2 N-1 R4 5'
S:AS PI P2 P3 P4 P5 [N] P-5P-4P-3P-2P-1 5'
145
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
S indicates the sense strand; AS indicates antisense strand; R1 indicates an
optional (and
nonpreferred) 5' sense strand overhang; R2 indicates an optional (though
preferred) 3' sense
overhang; R3 indicates an optional (though preferred) 3' antisense sense
overhang; R4 indicates
an optional (and nonpreferred) 5' antisense overhang; N indicates subunits;
[N] indicates that
additional subunit pairs may be present; and P,,, indicates a paring of sense
N,, and antisense N.
Overhangs are not shown in the P diagram. In some embodiments a 3' AS overhang
corresponds
to region Z, the duplex region corresponds to region X, and the 3' S strand
overhang corresponds
to region Y, as described elsewhere herein. (The diagram is not meant to imply
maximum or
minimum lengths, on which guidance is provided elsewhere herein.)
It is preferred that pairings which decrease the propensity to form a duplex
are used at 1
or more of the positions in the duplex at the 5' end of the AS strand. The
terminal pair (the most
5' pair in terms of the AS strand) is designated as P-1, and the subsequent
pairing positions
(going in the 3' direction in terms of the AS strand) in the duplex are
designated, P-2, P-3, P-4, P_5,
and so on. The preferred region in which to modify to modulate duplex
formation is at P-5
through P-1, more preferably P-4 through P-1 , more preferably P-3 through P-
1. Modification at P_
1, is particularly preferred, alone or with modification(s) other position(s),
e.g., any of the
positions just identified. It is preferred that at least 1, and more
preferably 2, 3, 4, or 5 of the
pairs of one of the recited regions be chosen independently from the group of
A:U
G:U
I:C
mismatched pairs, e.g., non-canonical or other than canonical pairings or
pairings which
include a universal base.
In preferred embodiments the change in subunit needed to achieve a pairing
which
promotes dissociation will be made in the sense strand, though in some
embodiments the change
will be made in the antisense strand.
146
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the at least 2, or 3, of the pairs in P_1, through P-
4, are pairs
which promote disociation.
In a preferred embodiment the at least 2, or 3, of the pairs in P_1, through P-
4, are A:U.
In a preferred embodiment the at least 2, or 3, of the pairs in P-1, through P-
4, are G:U.
In a preferred embodiment the at least 2, or 3, of the pairs in P-1, through P-
4, are I:C.
In a preferred embodiment the at least 2, or 3, of the pairs in P-1, through P-
4, are
mismatched pairs, e.g., non-canonical or other than canonical pairings
pairings.
In a preferred embodiment the at least 2, or 3, of the pairs in P-1, through
P_4, are pairings
which include a universal base.
Increasing the stability of the AS 3' end of the duplex
Subunit pairs can be ranked on the basis of their propensity to promote
stability and
inhibit dissociation or melting (e.g., on the free energy of association or
dissociation of a
particular pairing, the simplest approach is to examine the pairs on an
individual pair basis,
though next neighbor or similar analysis can also be used). In terms of
promoting duplex
stability:
G:C is preferred over A:U
Watson-Crick matches (A:T, A:U, G:C) are preferred over non-canonical or other
than
canonical pairings
analogs that increase stability are preferred over Watson-Crick matches (A:T,
A:U,
G:C)
2-amino-A:U is preferred over A:U
2-thio U or 5 Me-thio-U:A are preferred over U:A
G-clamp (an analog of C having 4 hydrogen bonds):G is preferred over C:G
147
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
guanadinium-G-clamp:G is preferred over C:G
psuedo uridine:A is preferred over U:A
sugar modifications, e.g., 2' modifications, e.g., 2'F, ENA, or LNA, which
enhance
binding are preferred over non-modified moieties and can be present on one or
both strands to
enhance stability of the duplex. It is preferred that pairings which increase
the propensity to
form a duplex are used at 1 or more of the positions in the duplex at the 3'
end of the AS strand.
The terminal pair (the most 3' pair in teens of the AS strand) is designated
as P1, and the
subsequent pairing positions (going in the 5' direction in terms of the AS
strand) in the duplex
are designated, P2, P3, P4, P5, and so on. The preferred region in which to
modify to modulate
lo duplex formation is at P5 through P1, more preferably P4 through P1 , more
preferably P3 through
P1. Modification at PI, is particularly preferred, alone or with
mdification(s) at other position(s),
e.g.,any of the positions just identified. It is preferred that at least 1,
and more preferably 2, 3, 4,
or 5 of the pairs of the recited regions be chosen independently from the
group of:
G:C
a pair having an analog that increases stability over Watson-Crick matches
(A:T, A:U,
G:C)
2-amino-A:U
2-thio U or 5 Me-thio-U:A
G-clamp (an analog of C having 4 hydrogen bonds):G
guanadinium-G-clamp : G
psuedo uridine:A
a pair in which one or both subunits has a sugar modification, e.g., a 2'
modification,
e.g., 2'F, ENA, or LNA, which enhance binding.
148
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the at least 2, or 3, of the pairs in P_1, through P-
4, are pairs
which promote duplex stability.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are G: C.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are a pair
having an analog that increases stability over Watson-Crick matches.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are 2-amino-
A:U.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are 2-thio U
or 5 Me-thio-U:A.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are G-
clamp:G.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are
guanidinium-G-clamp : G.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are psuedo
uridine:A.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are a pair in
which one or both subunits has a sugar modification, e.g., a 2' modification,
e.g., 2'F, ENA, or
LNA, which enhances binding.
G-clamps and guanidinium G-clamps are discussed in the following references:
Holmes
and Gait, "The Synthesis of 2'-O-Methyl G-Clamp Containing Oligonucleotides
and Their
Inhibition of the HIV-1 Tat-TAR Interaction," Nucleosides, Nucleotides &
Nucleic Acids,
22:1259-1262, 2003; Holmes et al., "Steric inhibition of human
immunodeficiency virus type-1
Tat-dependent trans-activation in vitro and in cells by oligonucleotides
containing 2'-O-methyl
G-clamp ribonucleoside analogues," Nucleic Acids Research, 31:2759-2768, 2003;
Wilds, et al.,
"Structural basis for recognition of guanosine by a synthetic tricyclic
cytosine analogue:
Guanidinium G-clamp," Helvetica Chimica Acta, 86:966-978, 2003; Rajeev, et
al., "High-
149
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Affinity Peptide Nucleic Acid Oligomers Containing Tricyclic Cytosine
Analogues," Organic
Letters, 4:4395-4398, 2002; Ausin, et al., "Synthesis of Amino- and Guanidino-
G-Clamp PNA
Monomers," Organic Letters, 4:4073-4075, 2002; Maier et al., "Nuclease
resistance of
oligonucleotides containing the tricyclic cytosine analogues phenoxazine and 9-
(2-
aminoethoxy)-phenoxazine ("G-clamp") and origins of their nuclease resistance
properties,"
Biochemistry, 41:1323-7, 2002; Flanagan, et al., "A cytosine analog that
confers enhanced
potency to antisense oligonucleotides," Proceedings Of The National Academy Of
Sciences Of
The United States Of America, 96:3513-8, 1999.
150
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Simultaneously decreasing the stability of the AS 5'end of the duplex and
increasing the
stability of the AS 3' end of the duplex
As is discussed above, an iRNA agent can be modified to both decrease the
stability of
the AS 5'end of the duplex and increase the stability of the AS 3' end of the
duplex. This can be
effected by combining one or more of the stability decreasing modifications in
the AS 5' end of
the duplex with one or more of the stability increasing modifications in the
AS 3' end of the
duplex. Accordingly a preferred embodiment includes modification in P-5
through P_1, more
preferably P-4 through P_1 and more preferably P_3 through P-1. Modification
at P-1, is particularly
preferred, alone or with other position, e.g., the positions just identified.
It is preferred that at
least 1, and more preferably 2, 3, 4, or 5 of the pairs of one of the recited
regions of the AS 5'
end of the duplex region be chosen independently from the group of:
A:U
G:U
I:C
mismatched pairs, e.g., non-canonical or other than canonical pairings which
include a
universal base; and
a modification in P5 through P1, more preferably P4 through P1 and more
preferably P3
through P1. Modification at P1, is particularly preferred, alone or with other
position, e.g., the
positions just identified. It is preferred that at least 1, and more
preferably 2, 3, 4, or 5 of the
pairs of one of the recited regions of the AS 3' end of the duplex region be
chosen independently
from the group of:
G:C
a pair having an analog that increases stability over Watson-Crick matches
(A:T, A:U,
G:C)
2-amino-A:U
151
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
2-thio U or 5 Me-thio-U:A
G-clamp (an analog of C having 4 hydrogen bonds):G
guanadinium- G-clamp : G
psuedo uridine:A
a pair in which one or both subunits has a sugar modification, e.g., a 2'
modification,
e.g., 2'F, ENA, or LNA, which enhance binding.
The invention also includes methods of selecting and making iRNA agents having
DMTDS. E.g., when screening a target sequence for candidate sequences for use
as iRNA
agents one can select sequences having a DMTDS property described herein or
one which can be
lo modified, preferably with as few changes as possible, especially to the
AS strand, to provide a desired level of DMTDS.
The invention also includes, providing a candidate iRNA agent sequence, and
modifying
at least one P in P_5 through P-1 and/or at least one P in P5 through P1 to
provide a DMTDS
iRNA agent.
DMTDS iRNA agents can be used in any method described herein, e.g., to silence
any
gene disclosed herein, to treat any disorder described herein, in any
formulation described herein,
and generally in and/or with the methods and compositions described elsewhere
herein. DMTDS
iRNA agents can incorporate other modifications described herein, e.g., the
attachment of
targeting agents or the inclusion of modifications which enhance stability,
e.g., the inclusion of
nuclease resistant monomers or the inclusion of single strand overhangs (e.g.,
3' AS overhangs
and/or 3' S strand overhangs) which self associate to form intrastrand duplex
structure.
Preferably these iRNA agents will have an architecture described herein.
Other Embodiments
An RNA, e.g., an iRNA agent, can be produced in a cell in vivo, e.g., from
exogenous
DNA templates that are delivered into the cell. For example, the DNA templates
can be inserted
152
CA 02521464 2012-03-22
51912-8
into vectors and used as gene therapy vectors. Gene therapy vectors can be
delivered to a subject
by, for example, intravenous injection, local administration (U.S. Pat. No.
5,328,470), or by
stereotactic injection (see, e.g., Chen et al., Proc. Natl. Acad. Sci. USA
91:3054-3057, 1994).
The pharmaceutical preparation of the gene therapy vector can include the gene
therapy vector in
an acceptable diluent, or can comprise a slow release matrix in which the gene
delivery vehicle is
imbedded. The DNA templates, for example, can include two transcription units,
one that
produces a transcript that includes the top strand of an iRNA agent and one
that produces a
transcript that includes the bottom strand of an iRNA agent. When the
templates are transcribed,
the iRNA agent is produced, and processed into sRNA agent fragments that
mediate gene
silencing.
In vivo Delivery
An iRNA agent can be linked, e.g., noncovalently linked to a polymer for the
efficient
delivery of the iRNA agent to a subject, e.g., a mammal, such as a human. The
iRNA agent can,
for example, be complexed with cyclodextrin. Cyclodextrins have been used as-
delivery
vehicles of therapeutic compounds. Cyclodextrins can form inclusion complexes
with drugs that
are able to fit into the hydrophobic cavity of the cyclodextrin. In other
examples, cyclodextrins
form non-covalent associations with other biologically active molecules such
as oligonucleotides
and derivatives thereof. The use of cyclodextrins creates a water-soluble drug
delivery complex,
that can be modified with targeting or other functional groups. Cyclodextrin
cellular delivery
system for oligonucleotides described in U.S. Pat. No. 5,691,316,
are suitable for use in methods of the invention. In this system, an
oligonucleotide
is noncovalently complexed with a cyclodextrin, or the oligonucleotide is
covalently bound to
adamantine which in turn is non-covalently associated with a cyclodextrin.
The delivery molecule can include a linear cyclodextrin copolymer or a linear
oxidized
cyclodextrin copolymer having at least one ligand bound to the cyclodextrin
copolymer.
Delivery systems, as described in U.S. Patent No. 6,509,323,
are suitable for use in methods of the invention. An iRNA agent can be bound
to the linear
cyclodextrin copolymer and/or a linear oxidized cyclodextrin copolymer. Either
or both of the
153
CA 02521464 2011-06-30
51912-8
cyclodextrin or oxidized cyclodextrin copolymers can be crosslinked to another
polymer and/or
bound to a ligand.
A composition for iRNA delivery can employ an "inclusion complex," a molecular
compound having the characteristic structure of an adduct. In this structure,
the "host
molecule" spatially encloses at least part of another compound in the delivery
vehicle. The
enclosed compound (the "guest molecule") is situated in the cavity of the host
molecule without
affecting the framework structure of the host. A "host" is preferably
cyclodextrin, but can be any
of the molecules suggested in U.S. Patent Publ. 2003/00088 18.
Cyclodextrins can interact with a variety of ionic and molecular species, and
the resulting
1 o inclusion compounds belong to the class of "host guest" complexes. Within
the host guest
relationship, the binding sites of the host and guest molecules should be
complementary in the
stereoelectronic sense. A composition of the invention can contain at least
one polymer and at
least one therapeutic agent, generally in the form of a particulate composite
of the polymer and
therapeutic agent, e.g., the ERNA agent. The iRNA agent can contain one or
more complexing
agents. At least one polymer of the particulate composite can interact with
the complexing agent
in a host-guest or a guest-host interaction to form an inclusion complex
between the polymer and
the complexing agent. The polymer and, more particularly, the complexing agent
can be used to
introduce functionality into the composition. For example, at least one
polymer of the particulate
composite has host functionality and forms an inclusion complex with a
complexing agent
having guest functionality. Alternatively, at least one polymer of the
particulate composite has
guest functionality and forms an inclusion complex with a complexing agent
having host
functionality. A polymer of the particulate composite can also contain both
host and guest
fimctionalities and form inclusion complexes with guest complexing agents and
host complexing
agents. A polymer with functionality can, for example, facilitate cell
targeting and/or cell
contact (e.g., targeting or contact to a liver cell), intercellular
trafficking, and/or cell entry and
release.
Upon forming the particulate composite, the iRNA agent may or may not retain
its
biological or therapeutic activity. Upon release from the therapeutic
composition, specifically,
from the polymer of the particulate composite, the activity of the iRNA agent
is restored.
154
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072 W O 1
Accordingly, the particulate composite advantageously affords the iRNA agent
protection
against loss of activity due to, for example, degradation and offers enhanced
bioavailability.
Thus, a composition may be used to provide stability, particularly storage or
solution stability, to
an iRNA agent or any active chemical compound. The iRNA agent may be further
modified
with a ligand prior to or after particulate composite or therapeutic
composition formation. The
ligand can provide further functionality. For example, the ligand can be a
targeting moiety.
Physiological Effects
The iRNA agents described herein can be designed such that determining
therapeutic
toxicity is made easier by the complementarity of the iRNA agent with both a
human and a non-
1o human animal sequence. By these methods, an iRNA agent can consist of a
sequence that is
fully complementary to a nucleic acid sequence from a human and a nucleic acid
sequence from
at least one non-human animal, e.g., a non-human mammal, such as a rodent,
ruminant or
primate. For example, the non-human mammal can be a mouse, rat, dog, pig,
goat, sheep, cow,
monkey, Pan paniscus, Pan troglodytes, Macaca mulatto, or Cynomolgus monkey.
The sequence
of the iRNA agent could be complementary to sequences within homologous genes,
e.g.,
oncogenes or tumor suppressor genes, of the non-human mammal and the human. By
determining the toxicity of the iRNA agent in the non-human mammal, one can
extrapolate the
toxicity of the iRNA agent in a human. For a more strenuous toxicity test, the
iRNA agent can
be complementary to a human and more than one, e.g., two or three or more, non-
human
animals.
The methods described herein can be used to correlate any physiological effect
of an
iRNA agent on a human, e.g., any unwanted effect, such as a toxic effect, or
any positive, or
desired effect.
Delivery Module
An RNA, e.g., an iRNA agent described herein, can be used with a drug delivery
conjugate or module, such as those described herein and those described in
copending, co-owned
United States Provisional Application Serial No. 60/454,265, filed on March
12, 2003, and
155
CA 02521464 2011-06-30
51912-8
International Application Serial No. PCT/USO4/07070, filed march 8,2004.
In addition, the invention includes iRNA agents described herein, e.g., a
palindromic
iRNA agent, an iRNA agent living a non canonical pairing, an iRNA agent which
targets a gene
described herein, e.g., a gene active in the liver, an iRNA agent having a
chemical modification
described herein, e.g., a modification which enhances resistance to
degradation, an iRNA agent
having an architecture or structure described herein, an iRNA agent
administered as described
herein, or an iRNA agent formulated as described herein, combined with,
associated with, and
delivered by such a drug delivery conjugate or module.
The iRNA agents can be complexed to a delivery agent that features a modular
complex.
The complex can include a carrier agent linked to one or more of (preferably
two or more, more
preferably all three of): (a) a condensing agent (e.g., an agent capable of
attracting, e.g., binding,
a nucleic acid, e.g., through ionic or electrostatic interactions); (b) a
fusogenic agent (e.g., an
agent capable of fusing and/or being transported through a cell membrane,
e.g., an endosome
membrane); and (c) a targeting group, e.g., a cell or tissue targeting agent,
e.g., a lectin,
glycoprotein,. lipid or protein, e.g., an antibody, that binds to a specified
cell type such as a liver
cell.
An iRNA agent, e.g., iRNA agent or sRNA agent described herein, can be linked,
e.g.,
coupled or bound, to the modular complex. The iRNA agent can interact with the
condensing
agent of the complex, and the complex can be used to deliver an iRNA agent to
a cell, e.g., in
vitro or in vivo. For example, the complex can be used to deliver an iRNA
agent to a subject in
need thereof, e g., to deliver an iRNA agent to a subject having a disorder,
e.g., a disorder
described herein, such as a disease or disorder of the liver.
The fusogenic agent and the condensing agent can be different agents or the
one and the
same agent. For example, a polyamino chain, e.g., polyethyleneimine (PEI), can
be the
fusogenic and/or the condensing agent.
The delivery agent can be a modular complex. For example, the complex can
include a
carrier agent linked to one or more of (preferably two or more, more
preferably all three of):
156
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
(a) a condensing agent (e.g., an agent capable of attracting, e.g., binding, a
nucleic acid,
e.g., through ionic interaction),
(b) a fusogenic agent (e.g., an agent capable of fusing and/or being
transported through a
cell membrane, e.g., an endosome membrane), and
(c) a targeting group, e.g., a cell or tissue targeting agent, e.g., a lectin,
glycoprotein, lipid
or protein, e.g., an antibody, that binds to a specified cell type such as a
liver cell. A targeting
group can be a thyrotropin, melanotropin, lectin, glycoprotein, surfactant
protein A, Mucin
carbohydrate, multivalent lactose, multivalent galactose, N-acetyl-
galactosamine, N-acetyl-
gulucosamine multivalent mannose, multivalent fucose, glycosylated
polyaminoacids,
1o multivalent galactose, transferrin, bisphosphonate, polyglutamate,
polyaspartate, a lipid,
cholesterol, a steroid, bile acid, folate, vitamin B12, biotin, Neproxin, or
an RGD peptide or
RGD peptide mimetic.
Carrier agents
The carrier agent of a modular complex described herein can be a substrate for
attachment of one or more of. a condensing agent, a fusogenic agent, and a
targeting group. The
carrier agent would preferably lack an endogenous enzymatic activity. The
agent would
preferably be a biological molecule, preferably a macromolecule. Polymeric
biological carriers
are preferred. It would also be preferred that the carrier molecule be
biodegradable..
The carrier agent can be a naturally occurring substance, such as a protein
(e.g., human
serum albumin (HSA), low-density lipoprotein (LDL), or globulin); carbohydrate
(e.g., a
dextran, pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid);
or lipid. The carrier
molecule can also be a recombinant or synthetic molecule, such as a synthetic
polymer, e.g., a
synthetic polyamino acid. Examples of polyamino acids include polylysine
(PLL),
poly L-aspartic acid, poly L-glutamic acid, styrene-maleic acid anhydride
copolymer, poly(L-
lactide-co-glycolied) copolymer, divinyl ether-maleic anhydride copolymer, N-
(2-
hydroxypropyl)methacrylamide copolymer (HMPA), polyethylene glycol (PEG),
polyvinyl
alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-isopropylacrylamide
polymers, or
polyphosphazine. Other useful carrier molecules can be identified by routine
methods.
157
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
A carrier agent can be characterized by one or more of (a) is at least 1 Da in
size; (b) has
at least 5 charged groups, preferably between 5 and 5000 charged groups; (c)
is present in the
complex at a ratio of at least 1:1 carrier agent to fusogenic agent; (d) is
present in the complex at
a ratio of at least 1:1 carrier agent to condensing agent; (e) is present in
the complex at a ratio of
at least 1:1 carrier agent to targeting agent.
Fusogenic agents
A fusogenic agent of a modular complex described herein can be an agent that
is
responsive to, e.g., changes charge depending on, the pH environment. Upon
encountering the
pH of an endosome, it can cause a physical change, e.g., a change in osmotic
properties which
lo disrupts or increases the permeability of the endosome membrane.
Preferably, the fusogenic
agent changes charge, e.g., becomes protonated, at pH lower than physiological
range. For
example, the fusogenic agent can become protonated at pH 4.5-6.5. The
fusogenic agent can
serve to release the iRNA agent into the cytoplasm of a cell after the complex
is taken up, e.g.,
via endocytosis, by the cell, thereby increasing the cellular concentration of
the iRNA agent in
the cell.
In one embodiment, the fusogenic agent can have a moiety, e.g., an amino
group, which,
when exposed to a specified pH range, will undergo a change, e.g., in charge,
e.g., protonation.
The change in charge of the fusogenic agent can trigger a change, e.g., an
osmotic change, in a
vesicle, e.g., an endocytic vesicle, e.g., an endosome. For example, the
fusogenic agent, upon
being exposed to the pH environment of an endosome, will cause a solubility or
osmotic change
substantial enough to increase the porosity of (preferably, to rupture) the
endosomal membrane.
The fusogenic agent can be a polymer, preferably a polyamino chain, e.g.,
polyethyleneimine (PEI). The PEI can be linear, branched, synthetic or
natural. The PEI can be,
e.g., alkyl substituted PEI, or lipid substituted PEI.
In other embodiments, the fusogenic agent can be polyhistidine, polyimidazole,
polypyridine, polypropyleneimine, mellitin, or a polyacetal substance, e.g., a
cationic polyacetal.
In some embodiment, the fusogenic agent can have an alpha helical structure.
The fusogenic
agent can be a membrane disruptive agent, e.g., mellittin.
158
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
A fusogenic agent can have one or more of the following characteristics: (a)
is at least
iDa in size; (b) has at least 10 charged groups, preferably between 10 and
5000 charged groups,
more preferably between 50 and 1000 charged groups; (c) is present in the
complex at a ratio of
at least 1:1 fusogenic agent to carrier agent; (d) is present in the complex
at a ratio of at least 1:1
fusogenic agent to condensing agent; (e) is present in the complex at a ratio
of at least 1:1
fusogenic agent to targeting agent.
Other suitable fusogenic agents can be tested and identified by a skilled
artisan. The
ability of a compound to respond to, e.g., change charge depending on, the pH
environment can
be tested by routine methods, e.g., in a cellular assay. For example, a test
compound is
1o combined or contacted with a cell, and the cell is allowed to take up the
test compound, e.g., by
endocytosis. An endosome preparation can then be made from the contacted cells
and the
endosome preparation compared to an endosome preparation from control cells. A
change, e.g.,
a decrease, in the endosome fraction from the contacted cell vs. the control
cell indicates that the
test compound can function as a fusogenic agent. Alternatively, the contacted
cell and control
cell can be evaluated, e.g., by microscopy, e.g., by light or electron
microscopy, to determine a
difference in endosome population in the cells. The test compound can be
labeled. In another
type of assay, a modular complex described herein is constructed using one or
more test:or
putative fusogenic agents. The modular complex can be constructed using a
labeled nucleic acid
instead of the iRNA. The ability of the fusogenic agent to respond to, e.g.,
change charge
depending on, the pH environment, once the modular complex is taken up by the
cell, can be
evaluated, e.g., by preparation of an endosome preparation, or by microscopy
techniques, as
described above. A two-step assay can also be performed, wherein a first assay
evaluates the
ability of a test compound alone to respond to, e.g., change charge depending
on, the pH
environment; and a second assay evaluates the ability of a modular complex
that includes the test
compound to respond to, e.g., change charge depending on, the pH environment.
Condensing agent
The condensing agent of a modular complex described herein can interact with
(e.g.,
attracts, holds, or binds to) an iRNA agent and act to (a) condense, e.g.,
reduce the size or charge
of the iRNA agent and/or (b) protect the iRNA agent, e.g., protect the iRNA
agent against
159
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
degradation. The condensing agent can include a moiety, e.g., a charged
moiety, that can
interact with a nucleic acid, e.g., an iRNA agent, e.g., by ionic
interactions. The condensing
agent would preferably be a charged polymer, e.g., a polycationic chain. The
condensing agent
can be a polylysine (PLL), spermine, spermidine, polyamine, pseudopeptide-
polyamine,
peptidomimetic polyamine, dendrimer polyamine, arginine, amidine, protamine,
cationic lipid,
cationic porphyrin, quarternary salt of a polyamine, or an alpha helical
peptide.
A condensing agent can have the following characteristics: (a) at least 1Da in
size; (b)
has at least 2 charged groups, preferably between 2 and 100 charged groups;
(c) is present in the
complex at a ratio of at least 1:1 condensing agent to carrier agent; (d) is
present in the complex
1 o at a ratio of at least 1:1 condensing agent to fusogenic agent; (e) is
present in the complex at a
ratio of at least 1:1 condensing agent to targeting agent.
Other suitable condensing agents can be tested and identified by a skilled
artisan, e.g., by
evaluating the ability of a test agent to interact with a nucleic acid, e.g.,
an iRNA agent. The
ability of a test agent to interact with a nucleic acid, e.g., an iRNA agent,
e.g., to condense or
protect the iRNA agent, can be evaluated by routine techniques. In one assay,
a test agent is
contacted with a nucleic acid, and the size and/or charge of the contacted
nucleic acid is
evaluated by a technique suitable to detect changes in molecular mass and/or
charge. Such
techniques include non-denaturing gel electrophoresis, immunological methods,
e.g.,
immunoprecipitation, gel filtration, ionic interaction chromatography, and the
like. A test agent
is identified as a condensing agent if it changes the mass and/or charge
(preferably both) of the
contacted nucleic acid, compared to a control. A two-step assay can also be
performed, wherein
a first assay evaluates the ability of a test compound alone to interact with,
e.g., bind to, e.g.,
condense the charge and/or mass of, a nucleic cid; and a second assay
evaluates the ability of a
modular complex that includes the test compound to interact with, e.g., bind
to, e.g., condense
the charge and/or mass of, a nucleic acid.
Amphipathic Delivery Agents
An RNA, e.g., an iRNA agent, described herein can be used with an amphipathic
delivery
conjugate or module, such as those described herein and those described in
copending, co-owned
United States Provisional Application Serial No. 60/455,050, filed on March
13, 2003, and
160
CA 02521464 2011-06-30
51912-8
International Application Serial No. PCT/US04/07070, filed March 8, 2004.
In addition, the invention includes an iRNA agent described herein, e.g., a
palindromic
iRNA agent, an iRNA agent having a noncanonical pairing, an iRNA agent which
targets a gene
described herein, e.g., a gene active in the liver, an iRNA agent having a
chemical modification
described herein, e.g., a modification which enhances resistance to
degradation, an iRNA agent
having an architecture or structure described herein, an iRNA'agent
administered as described
herein,, or an iRNA agent formulated as described herein, combined with,
associated with, and
delivered by such an amphipathic delivery conjugate.
An amphipathic molecule is a molecule having a hydrophobic and a hydrophilic
region.
Such molecules can interact with (e.g., penetrate or disrupt) lipids, e.g., a
lipid bylayer of a cell.
As such, they can serve as delivery agent for an associated (e.g., bound) iRNA
(e.g., an iRNA or
sRNA described herein). A preferred amphipathic molecule to be used in the
compositions
described herein (e.g., the amphipathic iRNA constructs descriebd herein) is a
polymer. The
polymer may have a secondary structure, e.g., a repeating secondary structure.
One example of an amphipathic polymer is an amphipathic polypeptide, e.g., a
polypeptide having a secondary structure such that the polypeptide has a
hydrophilic and a
hybrophobic face. The design of amphipathic peptide structures (e.g., alpha-
helical polypeptides)
is routine to one of skill in the at. For example, the following references
provide guidance:
Grell et al. (2001) "Protein design and folding: template trapping of self-
assembled helical
bundles" J Pept Sci 7(3):146-51; Chen et al. (2002) "Determination of
stereochemistry stability
coefficients of amino acid side-chains in an amphipathic alpha-helix" J Pept
Res 59(l):18-33;
Iwata et al. (1994) "Design and synthesis of amphipathic 3(10)-helical
peptides and their
interactions with phospholipid bilayers and ion channel formation" J Biol Chem
269(7):4928-33;
Comut et al. (1994) "The.amphipathic alpha helix concept. Application to the
de novo design of
ideally amphipathic Leu, Lys peptides with hemolytic activity higher than that
of meittin"
FEBS Lett 349(1):29-33; Negrete et al. (1998) "Deciphering the structural code
for proteins:
helical propensities in domain classes and statistical multiresidue
information in alpha-helices,"
Protein Sci 7(6):1368-79.
161
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Another example of an amphipathic polymer is a polymer made up of two or more
amphipathic subunits, e.g., two or more subunits containing cyclic moieties
(e.g., a cyclic moiety
having one or more hydrophilic groups and one or more hydrophobic groups). For
example, the
subunit may contain a steroid, e.g., cholic acid; or a aromatic moiety. Such
moieties preferably
can exhibit atropisomerism, such that they can form opposing hydrophobic and
hydrophilic faces
when in a polymer structure.
The ability of a putative amphipathic molecule to interact with a lipid
membrane, e.g., a
cell membrane, can be tested by routine methods, e.g., in a cell free or
cellular assay. For
example, a test compound is combined or contacted with a synthetic lipid
bilayer, a cellular
1o membrane fraction, or a cell, and the test compound is evaluated for its
ability to interact with,
penetrate or disrupt the lipid bilayer, cell membrane or cell. The test
compound can labeled in
order to detect the interaction with the lipid bilayer, cell membrane or cell.
In another type of
assay, the test compound is linked to a reporter molecule or an iRNA agent
(e.g., an iRNA or
sRNA described herein) and the ability of the reporter molecule or iRNA agent
to penetrate the
lipid bilayer, cell membrane or cell is evaluated. A two-step assay can also
be performed,
wherein a first assay evaluates the ability of a test compound alone to
interact with a lipid
bilayer, cell membrane or cell; and a second assay evaluates the ability of a
construct (e.g., a
construct described herein) that includes the test compound and a reporter or
iRNA agent to .
interact with a lipid bilayer, cell membrane or cell.
An amphipathic polymer useful in the compositions described herein has at
least 2,
preferably at least 5, more preferably at least 10, 25, 50, 100, 200, 500,
1000, 2000, 50000 or
more subunits (e.g., amino acids or cyclic subunits). A single amphipathic
polymer can be
linked to one or more, e.g., 2, 3, 5, 10 or more iRNA agents (e.g., iRNA or
sRNA agents
described herein). In some embodiments, an amphipathic polymer can contain
both amino acid
and cyclic subunits, e.g., aromatic subunits.
The invention features a composition that includes an iRNA agent (e.g., an
iRNA or
sRNA described herein) in association with an amphipathic molecule. Such
compositions may
be referred to herein as "amphipathic iRNA constructs." Such compositions and
constructs are
useful in the delivery or targeting of iRNA agents, e.g., delivery or
targeting of iRNA agents to a
162
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
cell. While not wanting to be bound by theory, such compositions and
constructs can increase
the porosity of, e.g., can penetrate or disrupt, a lipid (e.g., a lipid
bilayer of a cell), e.g., to allow
entry of the iRNA agent into a cell.
In one aspect, the invention relates to a composition comprising an iRNA agent
(e.g., an
iRNA or sRNA agent described herein) linked to an amphipathic molecule. The
iRNA agent and
the amphipathic molecule may be held in continuous contact with one another by
either covalent
or noncovalent linkages.
The amphipathic molecule of the composition or construct is preferably other
than a
phospholipid, e.g., other than a micelle, membrane or membrane fragment.
The amphipathic molecule of the composition or construct is preferably a
polymer. The
polymer may include two or more amphipathic subunits. One or more hydrophilic
groups and
one or more hydrophobic groups may be present on the polymer. The polymer may
have a
repeating secondary structure as well as a first face and a second face. The
distribution of the
hydrophilic groups and the hydrophobic groups along the repeating secondary
structure can be
such that one face of the polymer is a hydrophilic face and the other face of
the polymer is a
hydrophobic face.
The amphipathic molecule can be a polypeptide, e.g., a polypeptide comprising
an
a-helical conformation as its secondary structure.
In one embodiment, the amphipathic polymer includes one or more subunits
containing
one or more cyclic moiety (e.g., a cyclic moiety having one or more
hydrophilic groups and/or
one or more hydrophobic groups). In one embodiment, the polymer is a polymer
of cyclic
moieties such that the moieties have alternating hydrophobic and hydrophilic
groups. For
example, the subunit may contain a steroid, e.g., cholic acid. In another
example, the subunit
may contain an aromatic moiety. The aromatic moiety may be one that can
exhibit
atropisomerism, e.g., a 2,2'-bis(substituted)-l-l'-binaphthyl or a 2,2'-
bis(substituted) biphenyl.
A subunit may include an aromatic moiety of Formula (M):
163
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
R3
R4 R2
R4 RI
R3
(M)
The invention features a composition that includes an iRNA agent (e.g., an
iRNA or
sRNA described herein) in association with an amphipathic molecule. Such
compositions may
be referred to herein as "amphipathic iRNA constructs." Such compositions and
constructs are
useful in the delivery or targeting of iRNA agents, e.g., delivery or
targeting of iRNA agents to a
cell. While not wanting to be bound by theory, such compositions and
constructs can increase
the porosity of, e.g., can penetrate or disrupt, a lipid (e.g., a lipid
bilayer of a cell), e.g., to allow
entry of the iRNA agent into a cell.
In one aspect, the invention relates to a composition comprising an iRNA agent
(e.g., an
iRNA or sRNA agent described herein) linked to an amphipathic molecule. The
iRNA agent and
the amphipathic molecule may be held in continuous contact with one another by
either covalent
or noncovalent linkages.
The amphipathic molecule of the composition or construct is preferably other
than a
phospholipid, e.g., other than a micelle, membrane or membrane fragment.
The amphipathic molecule of the composition or construct is preferably a
polymer. The
polymer may include two or more amphipathic subunits. One or more hydrophilic
groups and
164
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO 1
one or more hydrophobic groups maybe present on the polymer. The polymer may
have a
repeating secondary structure as well as a first face and a second face. The
distribution of the
hydrophilic groups and the hydrophobic groups along the repeating secondary
structure can be
such that one face of the polymer is a hydrophilic face and the other face of
the polymer is a
hydrophobic face.
The amphipathic molecule can be a polypeptide, e.g., a polypeptide comprising
an
a-helical conformation as its secondary structure.
In one embodiment, the amphipathic polymer includes one or more subunits
containing
one or more cyclic moiety (e.g., a cyclic moiety having one or more
hydrophilic groups and/or
one or more hydrophobic groups). In one embodiment, the polymer is a polymer
of cyclic
moieties such that the moieties have alternating hydrophobic and hydrophilic
groups. For
example, the subunit may contain a steroid, e.g., cholic acid. In another
example, the subunit
may contain an aromatic moiety. The aromatic moiety may be one that can
exhibit
atropisomerism, e.g., a 2,2'-bis(substituted)-1-1'-binaphthyl or a 2,2'-
bis(substituted) biphenyl.
A subunit may include an aromatic moiety of Formula (M):
R3
R4 R2
R4 R,
R3
(M)
165
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Referring to Formula M, R1 is C1-C100 alkyl optionally substituted with aryl,
alkenyl,
alkynyl, alkoxy or halo and/or optionally inserted with 0, S, alkenyl or
alkynyl; C1-C10O
perfluoroalkyl; or OR5.
R2 is hydroxy; nitro; sulfate; phosphate; phosphate ester; sulfonic acid; OR6;
or C1-C10O
alkyl optionally substituted with hydroxy, halo, nitro, aryl or alkyl
sulfinyl, aryl or alkyl sulfonyl,
sulfate, sulfonic acid, phosphate, phosphate ester, substituted or
unsubstituted aryl, carboxyl,
carboxylate, amino carbonyl, or alkoxycarbonyl, and/or optionally inserted
with 0, NH, S, S(O),
SO2, alkenyl, or alkynyl.
R3 is hydrogen, or when taken together with R4 froms a fused phenyl ring.
R4 is hydrogen, or when taken together with R3 froms a fused phenyl ring.
R5 is C1-C100 alkyl optionally substituted with aryl, alkenyl, alkynyl, alkoxy
or halo
and/or optionally inserted with 0, S, alkenyl or alkynyl; or C1-C100
perfluoroalkyl; and R6 is C1-
C100 alkyl optionally substituted with hydroxy, halo, nitro, aryl or alkyl
sulfinyl, aryl or alkyl
sulfonyl, sulfate, sulfonic acid, phosphate, phosphate ester, substituted or
unsubstituted aryl,
carboxyl, carboxylate, amino carbonyl, or alkoxycarbonyl, and/or optionally
inserted with 0,
NH, S, S(O), SO2, alkenyl, or alkynyl.
Increasing cellular uptake of dsRNAs
A method of the invention that can include the administration of an iRNA agent
and a
drug that affects the uptake of the iRNA agent into the cell. The drug can be
administered
before, after, or at the same time that the iRNA agent is administered. The
drug can be
covalently linked to the iRNA agent. The drug can be, for example, a
lipopolysaccharide, an
activator of p38 MAP kinase, or an activator of NF-KB. The drug can have a
transient effect on
the cell.
The drug can increase the uptake of the iRNA agent into the cell, for example,
by
disrupting the cell's cytoskeleton, e.g., by disrupting the cell's
microtubules, microfilaments,
and/or intermediate filaments. The drug can be, for example, taxon,
vincristine, vinblastine,
166
CA 02521464 2011-06-30
51912-8
cytochalasin, nocodazole, japlakinolide, latrunculin A, phalloidin, swinholide
A, indanocine, or
myoservin.
The drug can also increase the uptake of the iRNA agent into the cell by
activating an
inflammatory response, for example. Exemplary drug's that would have such an
effect include
tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or gamma
interferon.
iRNA conjugates
An iRNA agent can be coupled, e.g., covalently coupled, to a second agent. For
example,
an iRNA agent used to treat a particular disorder can be coupled to a second
therapeutic agent,
eg., an agent other than the lENA agent. The second therapeutic agent can be
one which is.
1 o directed to the treatment of the same disorder. For example, in the case
of an iRNA used to treat
a disorder characterized by unwanted cell proliferation, e.g., cancer, the
iRNA agent can be
coupled to a second agent which has an anti-cancer effect. For example, it can
be coupled to an
agent which stimulates the immune system, e.g., a CpG motif, or more generally
an agent that
activates a toll-like receptor and/or increases the production of gamma
interferon.
IRNA Production
An iRNA can be produced, e.g.; in bulk, by a variety of methods. Exemplary
methods
include: organic synthesis and RNA cleavage, e.g., in vitro cleavage.
Organic Synthesis
An iRNA can be made by separately synthesizing each respective strand of a
double-
stranded RNA molecule. The component strands can then be annealed.
A large bioreactor, e.g., the OligoPilot 3I "from Pharmacia Biotec AB (Uppsala
Sweden),
can be used to produce a large amount of a particular RNA strand for a given
iRNA. The
OligoPilotII reactor can efficiently couple a nucleotide using only a 1.5
molar excess of a
phosphoramidite nucleotide. To make an RNA strand, ribonucleotides amidites
are used.
Standard cycles of monomer addition can be used to synthesize the 21 to 23
nucleotide strand for
the iRNA. Typically, the two complementary strands are produced separately and
then annealed,
e.g., after release from the solid support and deprotection.
* Trade-mark 167
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Organic synthesis can be used to produce a discrete iRNA species. The
complementary
of the species to a particular target gene can be precisely specified. For
example, the species
may be complementary to a region that includes a polymorphism, e.g., a single
nucleotide
polymorphism. Further the location of the polymorphism can be precisely
defined. In some
embodiments, the polymorphism is located in an internal region, e.g., at least
4, 5, 7, or 9
nucleotides from one or both of the termini.
dsRNA Cleavage
iRNAs can also be made by cleaving a larger ds iRNA. The cleavage can be
mediated in
vitro or in vivo. For example, to produce iRNAs by cleavage in vitro, the
following method can
lo be used:
In vitro transcription. dsRNA is produced by transcribing a nucleic acid (DNA)
segment
in both directions. For example, the HiScribeTM RNAi transcription kit (New
England Biolabs)
provides a vector and a method for producing a dsRNA for a nucleic acid
segment that is cloned
into the vector at a position flanked on either side by a T7 promoter.
Separate templates are
generated for T7 transcription of the two complementary strands for the dsRNA.
The templates
are transcribed in vitro by addition of T7 RNA polymerase and dsRNA is
produced. Similar
methods using PCR and/or other RNA polymerases (e.g., T3 or SP6 polymerase)
can also be
used. In one embodiment, RNA generated by this method is carefully purified to
remove
endotoxins that may contaminate preparations of the recombinant enzymes.
In vitro cleavage. dsRNA is cleaved in vitro into iRNAs, for example, using a
Dicer or
comparable RNAse III-based activity. For example, the dsRNA can be incubated
in an in vitro
extract from Drosophila or using purified components, e.g. a purified RNAse or
RISC complex
(RNA-induced silencing complex ). See, e.g., Ketting et al. Genes Dev 2001 Oct
15;15(20):2654-9. and Hammond Science 2001 Aug 10;293(5532):1146-50.
dsRNA cleavage generally produces a plurality of iRNA species, each being a
particular
21 to 23 nt fragment of a source dsRNAmolecule. For example, iRNAs that
include sequences
complementary to overlapping regions and adjacent regions of a source dsRNA
molecule may be
present.
168
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Regardless of the method of synthesis, the iRNA preparation can be prepared in
a
solution (e.g., an aqueous and/or organic solution) that is appropriate for
formulation. For
example, the iRNA preparation can be precipitated and redissolved in pure
double-distilled
water, and lyophilized. The dried iRNA can then be resuspended in a solution
appropriate for the
intended formulation process.
Synthesis of modified and nucleotide surrogate iRNA agents is discussed below.
FORMULATION
The iRNA agents described herein can be formulated for administration to a
subject
For ease of exposition the formulations, compositions and methods in this
section are
I o discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention.
A formulated iRNA composition can assume a variety of states. In some
examples, the
composition is at least partially crystalline, uniformly crystalline, and/or
anhydrous (e.g., less
than 80, 50, 30, 20, or 10% water). In another example, the iRNAis in an
aqueous phase, e.g., in
a solution that includes water.
The aqueous phase or the crystalline compositions can, e.g., be incorporated
into a
delivery vehicle, e.g., a liposome (particularly for the aqueous phase) or a
particle (e.g., a
microparticle as can be appropriate for a crystalline composition). Generally,
the iRNA
composition is formulated in a manner that is compatible with the intended
method of
administration (see, below).
In particular embodiments, the composition is prepared by at least one of the
following
methods: spray drying, lyophilization, vacuum drying, evaporation, fluid bed
drying, or a
combination of these techniques; or sonication with a lipid, freeze-drying,
condensation and
other self-assembly.
A iRNA preparation can be formulated in combination with another agent, e.g.,
another
therapeutic agent or an agent that stabilizes a iRNA, e.g., a protein that
complexes with iRNA to
169
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
form an iRNP. Still other agents include chelators, e.g., EDTA (e.g., to
remove divalent cations
such as Mgz+), salts, RNAse inhibitors (e.g., a broad specificity RNAse
inhibitor such as
RNAsin) and so forth.
In one embodiment, the iRNA preparation includes another iRNA agent, e.g., a
second
iRNA that can mediated RNAi with respect to a second gene, or with respect to
the same gene.
Still other preparation can include at least 3, 5, ten, twenty, fifty, or a
hundred or more different
iRNA species. Such iRNAs can mediated RNAi with respect to a similar number of
different
genes.
In one embodiment, the iRNA preparation includes at least a second therapeutic
agent
(e.g., an agent other than an RNA or a DNA). For example, a iRNA composition
for the
treatment of a viral disease, e.g. HIV, might include a known antiviral agent
(e.g., a protease
inhibitor or reverse transcriptase inhibitor). In another example, a iRNA
composition for the
treatment of a cancer might further comprise a chemotherapeutic agent.
Exemplary formulations are discussed below:
Liposomes
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA s agents, and such practice is within the invention. An
iRNA agent, e.g., a
double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent, or a DNA which encodes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, or precursor thereof) preparation can be
formulated for
delivery in a membranous molecular assembly, e.g., a liposome or a micelle. As
used herein, the
term "liposome" refers to a vesicle composed of amphiphilic lipids arranged in
at least one
bilayer, e.g., one bilayer or a plurality of bilayers. Liposomes include
unilamellar and
multilamellar vesicles that have a membrane formed from a lipophilic material
and an aqueous
interior. The aqueous portion contains the iRNA composition. The lipophilic
material isolates
the aqueous interior from an aqueous exterior, which typically does not
include the iRNA
170
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
composition, although in some examples, it may. Liposomes are useful for the
transfer and
delivery of active ingredients to the site of action. Because the liposomal
membrane is
structurally similar to biological membranes, when liposomes are applied to a
tissue, the
liposomal bilayer fuses with bilayer of the cellular membranes. As the merging
of the liposome
and cell progresses, the internal aqueous contents that include the iRNA are
delivered into the
cell where the iRNA can specifically bind to a target RNA and can mediate
RNAi. In some
cases the liposomes are also specifically targeted, e.g., to direct the iRNA
to particular cell types,
e.g., to cells of the liver, such as those described herein.
A liposome containing a iRNA can be prepared by a variety of methods.
In one example, the lipid component of a liposome is dissolved in a detergent
so that
micelles are formed with the lipid component. For example, the lipid component
can be an
amphipathic cationic lipid or lipid conjugate. The detergent can have a high
critical micelle
concentration and may be nonionic. Exemplary detergents include cholate,
CHAPS,
octylglucoside, deoxycholate, and lauroyl sarcosine. The iRNA preparation is
then added to the
micelles that include the lipid component. The cationic groups on the lipid
interact with the
iRNA and condense around the iRNA to form a liposome. After condensation, the
detergent is
removed, e.g., by dialysis, to yield a liposomal preparation of iRNA.
If necessary a carrier compound that assists in condensation can be added
during the
condensation reaction, e.g., by controlled addition. For example, the carrier
compound can be a
polymer other than a nucleic acid (e.g., spermine or spermidine). pH can also
adjusted to favor
condensation.
Further description of methods for producing stable polynucleotide delivery
vehicles,
which incorporate a polynucleotide/cationic lipid complex as structural
components of the
delivery vehicle, are described in, e.g., WO 96/37194. Liposome formation can
also include one
or more aspects of exemplary methods described in Feigner, P. L. et al., Proc.
Natl. Acad. Sci.,
USA 8:7413-7417, 1987; U.S. Pat. No. 4,897,355; U.S. Pat. No. 5,171,678;
Bangham, et al. M.
Mol. Biol. 23:23 8, 1965; Olson, et al. Biochim. Biophys. Acta 557:9, 1979;
Szoka, et al. Proc.
Natl. Acad. Sci. 75: 4194, 1978; Mayhew, et al. Biochim. Biophys. Acta
775:169, 1984; Kim, et
al. Biochim. Biophys. Acta 728:339, 1983; and Fuklmaga, et al. Endocrinol.
115:757, 1984.
171
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Commonly used techniques for preparing lipid aggregates of appropriate size
for use as delivery
vehicles include sonication and freeze-thaw plus extrusion (see, e.g., Mayer,
et al. Biochim.
Biophys. Acta 858:161, 1986). Microfluidization can be used when consistently
small (50 to 200
nm) and relatively uniform aggregates are desired (Mayhew, et al. Biochim.
Biophys. Acta
775:169, 1984). These methods are readily adapted to packaging iRNA
preparations into
liposomes.
Liposomes that are pH-sensitive or negatively-charged, entrap nucleic acid
molecules
rather than complex with them. Since both the nucleic acid molecules and the
lipid are similarly
charged, repulsion rather than complex formation occurs. Nevertheless, some
nucleic acid
1 o molecules are entrapped within the aqueous interior of these liposomes. pH-
sensitive liposomes
have been used to deliver DNA encoding the thymidine kinase gene to cell
monolayers in
culture. Expression of the exogenous gene was detected in the target cells
(Zhou et al., Journal
of Controlled Release, 19, (1992) 269-274).
One major type of liposomal composition includes phospholipids other than
naturally-
derived phosphatidylcholine. Neutral liposome compositions, for example, can
be formed from
dimyristoyl phosphatidylcholine (DMPC) or dipalmitoyl phosphatidylcholine
(DPPC). Anionic
liposome compositions generally are formed from dimyristoyl
phosphatidylglycerol, while
anionic fusogenic liposomes are formed primarily from dioleoyl
phosphatidylethanolamine
(DOPE). Another type of liposomal composition is formed from
phosphatidylcholine (PC) such
as, for example, soybean PC, and egg PC. Another type is formed from mixtures
of
phospholipid and/or phosphatidylcholine and/or cholesterol.
Examples of other methods to introduce liposomes into cells in vitro and in
vivo include
U.S. Pat. No. 5,283,185; U.S. Pat. No. 5,171,678; WO 94/00569; WO 93/24640; WO
91/16024;
Feigner, J. Biol. Chem. 269:2550, 1994; Nabel, Proc. Natl. Acad. Sci.
90:11307, 1993; Nabel,
Human Gene Ther. 3:649, 1992; Gershon, Biochem. 32:7143, 1993; and Strauss
EMBO J.
11:417, 1992.
In one embodiment, cationic liposomes are used. Cationic liposomes possess the
advantage of being able to fuse to the cell membrane. Non-cationic liposomes,
although not able
172
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
to fuse as efficiently with the plasma membrane, are taken up by macrophages
in vivo and can be
used to deliver iRNAs to macrophages.
Further advantages of liposomes include: liposomes obtained from natural
phospholipids
are biocompatible and biodegradable; liposomes can incorporate a wide range of
water and lipid
soluble drugs; liposomes can protect encapsulated iRNAs in their internal
compartments from
metabolism and degradation (Rosoff, in "Pharmaceutical Dosage Forms,"
Lieberman, Rieger and
Banker (Eds.), 1988, volume 1, p. 245). Important considerations in the
preparation of liposome
formulations are the lipid surface charge, vesicle size and the aqueous volume
of the liposomes.
A positively charged synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-
N,N,N-
1o trimethylammonium chloride (DOTMA) can be used to form small liposomes that
interact
spontaneously with nucleic acid to form lipid-nucleic acid complexes which are
capable of
fusing with the negatively charged lipids of the cell membranes of tissue
culture cells, resulting
in delivery of iRNA (see, e.g., Feigner, P. L. et al., Proc. Natl. Acad. Sci.,
USA 8:7413-7417,
1987 and U.S. Pat. No. 4,897,355 for a description of DOTMA and its use with
DNA).
A DOTMA analogue, 1,2-bis(oleoyloxy)-3-(trimethylammonia)propane (DOTAP) can
be
used in combination with a phospholipid to form DNA-complexing vesicles.
LipofectinTM
Bethesda Research Laboratories, Gaithersburg, Md.) is an effective agent for
the delivery of
highly anionic nucleic acids into living tissue culture cells that comprise
positively charged
DOTMA liposomes which interact spontaneously with negatively charged
polynucleotides to
form complexes. When enough positively charged liposomes are used, the net
charge on the
resulting complexes is also positive. Positively charged complexes prepared in
this way
spontaneously attach to negatively charged cell surfaces, fuse with the plasma
membrane, and
efficiently deliver functional nucleic acids into, for example, tissue culture
cells. Another
commercially available cationic lipid, 1,2-bis(oleoyloxy)-3,3-
(trimethylammonia)propane
("DOTAP") (Boehringer Mannheim, Indianapolis, Indiana) differs from DOTMA in
that the
oleoyl moieties are linked by ester, rather than ether linkages.
Other reported cationic lipid compounds include those that have been
conjugated to a
variety of moieties including, for example, carboxyspermine which has been
conjugated to one
of two types of lipids and includes compounds such as 5-carboxyspermylglycine
173
CA 02521464 2011-06-30
51912-8
dioctaoleoylamide ("DOGS") (TransfectammM, Promega, Madison, Wisconsin) and
dipalmitoylphosphatidylethanolamine 5-carboxyspermyl-amide ("DPPES") (see,
e.g., U.S. Pat.
No. 5,171,678).
Another cationic lipid conjugate includes derivatization of the lipid with
cholesterol
("DC-Chol") which has been formulated into liposomes in combination with DOPE
(See, Gao,
X. and Huang, L., Biochim. Biophys. Res. Commun.179:280,1991). Lipopolylysine,
made by
conjugating polylysine to DOPE, has been reported to be effective for
transfection in the
presence of serum (thou, X. et al., Biochim. Biophys. Acta 1065:8, 1991). For
certain cell lines,
these liposomes containing conjugated cationic lipids, are said to, exhibit
lower toxicity and
I o provide more efficient transfection than the DOTMA-containing
compositions. Other
commercially available cationic lipid products include DMRIE and DMRIE-HP
(Vical, La Jolla,
California) and Lipofectamine (DOSPA) (Life Technology, Inc., Gaithersburg,
Maryland).
Other cationic lipids suitable for the delivery of oligonucleotides are
described in WO 98/39359
and WO 96/37194.
Liposomal formulations are particularly suited for topical administration,
liposomes
present several advantages over other formulations. Such advantages include
reduced side
effects related to high systemic absorption of the administered drug,
increased accumulation of
the administered drug at the desired target, and the ability to administer
iRNA, into the skin. In
some implementations, liposomes are used for delivering 04A to epidermal cells
and also to
enhance the penetration of iRNA into dermal tissues, eg., into skin. For
example, the liposomes
can be applied topically. Topical delivery of drugs formulated as liposomes to
the skin has been
documented (see, e.g., Weiner et at., Journal of Drug Targeting, 1992, vol.
2,405-410 and du
Plessis et al., Antiviral Research, 18, 1992, 259-265; Mannino, R. J. and
Fould-Fogerite, S.,
Biotechniques 6:682-690, 1988; Itani, T. et al. Gene 56:267-276. 1987;
Nicolau, C. et al. Meth.
E=. 149:157-176,1987; Straubinger, R. M. and Papahadjopoulos, D. Meth.
Enz.101:512-527,
1983; Wang, C. Y. and Huang, L., Proc. Natl. Acad. Sci. USA 84:7851-
7855,1987).
Non-ionic liposomal systems have also been examined to determine their utility
in the
delivery of drugs to the skin, in particular systems comprising non-ionic
surfactant and
cholesterol. Non-ionic liposomal formulations comprising Novasome I (glyceryl
* Trade-mark
174
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
dilaurate/cholesterol/polyoxyethylene-10-stearyl ether) and Novasome II
(glyceryl distearate/
cholesterol/polyoxyethylene-10-stearyl ether) were used to deliver a drug into
the dermis of
mouse skin. Such formulations with iRNA are useful for treating a
dermatological disorder.
Liposomes that include iRNA can be made highly deformable. Such deformability
can
enable the liposomes to penetrate through pore that are smaller than the
average radius of the
liposome. For example, transfersomes are a type of deformable liposomes.
Transferosomes can
be made by adding surface edge activators, usually surfactants, to a standard
liposomal
composition. Transfersomes that include iRNA can be delivered, for example,
subcutaneously
by infection in order to deliver iRNA to keratinocytes in the skin. In order
to cross intact
1 o mammalian skin, lipid vesicles must pass through a series of fine pores,
each with a diameter less
than 50 nm, under the influence of a suitable transdermal gradient. In
addition, due to the lipid
properties, these transferosomes can be self-optimizing (adaptive to the shape
of pores, e.g., in
the skin), self-repairing, and can frequently reach their targets without
fragmenting, and often
self-loading. The iRNA agents can include an RRMS tethered to a moiety which
improves
association with a liposome.
Surfactants
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention.
Surfactants find wide
application in formulations such as emulsions (including microemulsions) and
liposomes (see
above). iRNA (or a precursor, e.g., a larger dsRNA which can be processed into
a iRNA, or a
DNA which encodes a iRNA or precursor) compositions can include a surfactant.
In one
embodiment, the iRNA is formulated as an emulsion that includes a surfactant.
The most
common way of classifying and ranking the properties of the many different
types of surfactants,
both natural and synthetic, is by the use of the hydrophile/lipophile balance
(HLB). The nature
of the hydrophilic group provides the most useful means for categorizing the
different surfactants
used in formulations (Rieger, in "Pharmaceutical Dosage Forms," Marcel Dekker,
Inc., New
York, NY, 1988, p. 285).
175
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072 WO I
If the surfactant molecule is not ionized, it is classified as a nonionic
surfactant.
Nonionic surfactants find wide application in pharmaceutical products and are
usable over a
wide range of pH values. In general their HLB values range from 2 to about 18
depending on
their structure. Nonionic surfactants include nonionic esters such as ethylene
glycol esters,
propylene glycol esters, glyceryl esters, polyglyceryl esters, sorbitan
esters, sucrose esters, and
ethoxylated esters. Nonionic alkanolamides and ethers such as fatty alcohol
ethoxylates,
propoxylated alcohols, and ethoxylated/propoxylated block polymers are also
included in this
class. The polyoxyethylene surfactants are the most popular members of the
nonionic surfactant
class.
If the surfactant molecule carries a negative charge when it is dissolved or
dispersed in
water, the surfactant is classified as anionic. Anionic surfactants include
carboxylates such as
soaps, acyl lactylates, acyl amides of amino acids, esters of sulfuric acid
such as alkyl sulfates
and ethoxylated alkyl sulfates, sulfonates such as alkyl benzene sulfonates,
acyl isethionates,
acyl taurates and sulfosuccinates, and phosphates. The most important members
of the anionic
surfactant class are the alkyl sulfates and the soaps.
If the surfactant molecule carries a positive charge when it is dissolved or
dispersed in
water, the surfactant is classified as cationic. Cationic surfactants include
quaternary ammonium
salts and ethoxylated amines. The quaternary ammonium salts are the most used
members of
this class.
If the surfactant molecule has the ability to carry either a positive or
negative charge, the
surfactant is classified as amphoteric. Amphoteric surfactants include acrylic
acid derivatives,
substituted alkylamides, N-alkylbetaines and phosphatides.
The use of surfactants in drug products, formulations and in emulsions has
been reviewed
(Rieger, in "Pharmaceutical Dosage Forms," Marcel Dekker, Inc., New York, NY,
1988, p. 285).
Micelles and other Membranous Formulations
For ease of exposition the micelles and other formulations, compositions and
methods in
this section are discussed largely with regard to unmodified iRNA agents. It
should be
understood, however, that these micelles and other formulations, compositions
and methods can
176
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
be practiced with other iRNA agents, e.g., modified iRNA agents, and such
practice is within the
invention. The iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g., a
precursor, e.g., a larger iRNA agent which can be processed into a sRNA agent,
or a DNA which
encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or
precursor
thereof)) composition can be provided as a micellar formulation. "Micelles"
are defined herein
as a particular type of molecular assembly in which amphipathic molecules are
arranged in a
spherical structure such that all the hydrophobic portions of the molecules
are directed inward,
leaving the hydrophilic portions in contact with the surrounding aqueous
phase. The converse
arrangement exists if the environment is hydrophobic.
A mixed micellar formulation suitable for delivery through transdermal
membranes may
be prepared by mixing an aqueous solution of the iRNA composition, an alkali
metal C8 to C22
alkyl sulphate, and a micelle forming compounds. Exemplary micelle forming
compounds
include lecithin, hyaluronic acid, pharmaceutically acceptable salts of
hyaluronic acid, glycolic
acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linoleic
acid, linolenic acid,
monoolein, monooleates, monolaurates, borage oil, evening of primrose oil,
menthol, trihydroxy
oxo cholanyl glycine and pharmaceutically acceptable salts thereof, glycerin,
polyglycerin,
lysine, polylysine, triolein, polyoxyethylene ethers and analogues thereof,
polidocanol alkyl
ethers and analogues thereof, chenodeoxycholate, deoxycholate, and mixtures
thereof The
micelle forming compounds may be added at the same time or after addition of
the alkali metal
alkyl sulphate. Mixed micelles will form with substantially any kind of mixing
of the ingredients
but vigorous mixing is preferred in order to provide smaller size micelles.
In one method a first micellar composition is prepared which contains the iRNA
composition and at least the alkali metal alkyl sulphate. The first micellar
composition is then
mixed with at least three micelle forming compounds to form a mixed micellar
composition. In
another method, the micellar composition is prepared by mixing the iRNA
composition, the
alkali metal alkyl sulphate and at least one of the micelle forming compounds,
followed by
addition of the remaining micelle forming compounds, with vigorous mixing.
Phenol and/or m-cresol may be added to the mixed micellar composition to
stabilize the
formulation and protect against bacterial growth. Alternatively, phenol and/or
m-cresol may be
177
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
added with the micelle forming ingredients. An isotonic agent such as glycerin
may also be
added after formation of the mixed micellar composition.
For delivery of the micellar formulation as a spray, the formulation can be
put into an
aerosol dispenser and the dispenser is charged with a propellant. The
propellant, which is under
pressure, is in liquid form in the dispenser. The ratios of the ingredients
are adjusted so that the
aqueous and propellant phases become one, i.e. there is one phase. If there
are two phases, it is
necessary to shake the dispenser prior to dispensing a portion of the
contents, e.g. through a
metered valve. The dispensed dose of pharmaceutical agent is propelled from
the metered valve
in a fine spray.
The preferred propellants are hydrogen-containing chlorofluorocarbons,
hydrogen-
containing fluorocarbons, dimethyl ether and diethyl ether. Even more
preferred is HFA 134a
(1,1,1,2 tetrafluoroethane).
The specific concentrations of the essential ingredients can be determined by
relatively
straightforward experimentation. For absorption through the oral cavities, it
is often desirable to
increase, e.g. at least double or triple, the dosage for through injection or
administration through
the gastrointestinal tract.
The iRNA agents can include an RRMS tethered to a moiety which improves
association
with a micelle or other membranous formulation.
Particles
For ease of exposition the particles, formulations, compositions and methods
in this
section are discussed largely with regard to unmodified iRNA agents. It should
be understood,
however, that these particles, formulations, compositions and methods can be
practiced with
other iRNA agents, e.g., modified iRNA agents, and such practice is within the
invention. In
another embodiment, an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, (e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof) preparations may be incorporated into a particle, e.g., a
microparticle. Microparticles
can be produced by spray-drying, but may also be produced by other methods
including
178
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
lyophilization, evaporation, fluid bed drying, vacuum drying, or a combination
of these
techniques. See below for further description.
Sustained-Release Formulations. An iRNA agent, e.g., a double-stranded iRNA
agent, or
sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof) described herein can be formulated for
controlled, e.g., slow release.
Controlled release can be achieved by disposing the iRNA within a structure or
substance which
impedes its release. E.g., iRNA can be disposed within a porous matrix or in
an erodable matrix,
either of which allow release of the iRNA over a period of time.
Polymeric particles, e.g., polymeric in microparticles can be used as a
sustained-release
reservoir of iRNA that is taken up by cells only released from the
microparticle through
biodegradation. The polymeric particles in this embodiment should therefore be
large enough to
preclude phagocytosis (e.g., larger than 10 m and preferably larger than 20
m). Such particles
can be produced by the same methods to make smaller particles, but with less
vigorous mixing of
the first and second emulsions. That is to say, a lower homogenization speed,
vortex mixing
speed, or sonication setting can be used to obtain particles having a diameter
around 100 m
rather than 10 m. The time of mixing also can be altered.
Larger microparticles can be formulated as a suspension, a powder, or an
implantable
solid, to be delivered by intramuscular, subcutaneous, intradermal,
intravenous, or intraperitoneal
injection; via inhalation (intranasal or intrapulmonary); orally; or by
implantation. These
particles are useful for delivery of any iRNA when slow release over a
relatively long term is
desired. The rate of degradation, and consequently of release, varies with the
polymeric
formulation.
Microparticles preferably include pores, voids, hollows, defects or other
interstitial
spaces that allow the fluid suspension medium to freely permeate or perfuse
the particulate
boundary. For example, the perforated microstructures can be used to form
hollow, porous spray
dried microspheres.
179
CA 02521464 2011-06-30
51912-8
Polymeric particles containing 11 NA (e.g., a sRNA) can be made using a double
emulsion technique, for instance. First, the polymer is dissolved in an
organic solvent. A
preferred polymer is polylactic-co-glycolic acid (PLGA), with a
lactic/glycolic acid weight ratio
of 65:35, 50:50, or 75:25. Next, a sample of nucleic acid suspended in aqueous
solution is added
to the polymer solution and the two solutions are mixed to form a first
emulsion. The solutions
can be mixed by vortexing or shaking, and in a preferred method, the mixture
can be sonicated.
Most preferable is any method by which the nucleic acid receives the least
amount of damage in
the form of nicking, shearing, or degradation, while still allowing the
formation of an appropriate
emulsion. For example, acceptable results can be obtained with a Vibra cell'
model VC-250
1o sonicator with a 1/8" microtip probe, at setting #3.
Spray-Drying. An iRNA agent, eg., a double-stranded iRNA agent, or sRNA agent,
(e.g., a precursor, e.g., a larger iRNA agent which can be processed into a
sRNA agent, or a DNA
which encodes an iRNA agent, eg., a double-stranded iRNA agent, or sRNA agent,
or precursor
thereof)) can be prepared by spray drying. Spray dried iRNA can be
administered to a subject or
be subjected to further formulation. A pharmaceutical composition of iRNA can
be prepared by
spray drying a homogeneous aqueous mixture that includes a iRNA under
conditions sufficient
to provide a dispersible powdered composition, e.g., a pharmaceutical
composition. The material
for spray drying can also include one or more of a pharmaceutically acceptable
excipient, or a
=.dispersibility-enhancing amount of a,physiologically acceptable, water-
soluble protein. The
spray-dried product can be a dispersible powder that includes the iRNA.
Spray drying is a process that converts a liquid or slurry material to a dried
particulate
form. 'Spray drying can be used to provide powdered material for various
administrative routes
including inhalation. See, for example, M. Sacchetti and M. M. Van Oort in:
Inhalation Aerosols:
Physical and Biological Basis for Therapy, A. J. Hickey, ed. Marcel Dekkar,
New York, 1996.
Spray drying can include atomizing a solution, emulsion, or suspension to form
a fine
mist of droplets and drying the droplets. The mist can be projected into a
drying chamber (e.g., a
vessel, tank, tubing, or coil) where it contacts a drying gas. The mist can
include solid or liquid
pore forming agents. The solvent and pore forming agents evaporate from the
droplets into the
* Trade-mark
180
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
drying gas to solidify the droplets, simultaneously forming pores throughout
the solid. The solid
(typically in a powder, particulate form) then is separated from the drying
gas and collected.
Spray drying includes bringing together a highly dispersed liquid, and a
sufficient volume
of air (e.g., hot air) to produce evaporation and drying of the liquid
droplets. The preparation to
be spray dried can be any solution, course suspension, slurry, colloidal -
dispersion, or paste that
may be atomized using the selected spray drying apparatus. Typically, the feed
is sprayed into a
current of warm filtered air that evaporates the solvent and conveys the dried
product to a
collector. The spent air is then exhausted with the solvent. Several different
types of apparatus
may be used to provide the desired product. For example, commercial spray
dryers manufactured
lo by Buchi Ltd. or Niro Corp. can effectively produce particles of desired
size.
Spray-dried powdered particles can be approximately spherical in shape, nearly
uniform
in size and frequently hollow. There may be some degree of irregularity in
shape depending
upon the incorporated medicament and the spray drying conditions. In many
instances the
dispersion stability of spray-dried microspheres appears to be more effective
if an inflating agent
(or blowing agent) is used in their production. Particularly preferred
embodiments may comprise
an emulsion with an inflating agent as the disperse or continuous phase (the
other phase being
aqueous in nature). An inflating agent is preferably dispersed with a
surfactant solution, using,
for instance, a commercially available microfluidizer at a pressure of about
5000 to 15,000 psi.
This process forms an emulsion, preferably stabilized by an incorporated
surfactant, typically
comprising submicron droplets of water immiscible blowing agent dispersed in
an aqueous
continuous phase. The formation of such dispersions using this and other
techniques are common
and well known to those in the art. The blowing agent is preferably a
fluorinated compound (e.g.
perfluorohexane, perfluorooctyl bromide, perfluorodecalin, perfluorobutyl
ethane) which
vaporizes during the spray-drying process, leaving behind generally hollow,
porous
aerodynamically light microspheres. As will be discussed in more detail below,
other suitable
blowing agents include chloroform, freons, and hydrocarbons. Nitrogen gas and
carbon dioxide
are also contemplated as a suitable blowing agent.
Although the perforated microstructures are preferably formed using a blowing
agent as
described above, it will be appreciated that, in some instances, no blowing
agent is required and
181
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
an aqueous dispersion of the medicament and surfactant(s) are spray dried
directly. In such cases,
the formulation may be amenable to process conditions (e.g., elevated
temperatures) that
generally lead to the formation of hollow, relatively porous microparticles.
Moreover, the
medicament may possess special physicochemical properties (e.g., high
crystallinity, elevated
melting temperature, surface activity, etc.) that make it particularly
suitable for use in such
techniques.
The perforated microstructures may optionally be associated with, or comprise,
one or
more surfactants. Moreover, miscible surfactants may optionally be combined
with the
suspension medium liquid phase. It will be appreciated by those skilled in the
art that the use of
I o surfactants may further increase dispersion stability, simplify
formulation procedures or increase
bioavailability upon administration. Of course combinations of surfactants,
including the use of
one or more in the liquid phase and one or more associated with the perforated
microstructures
are contemplated as being within the scope of the invention. By "associated
with or comprise" it
is meant that the structural matrix or perforated microstructure may
incorporate, adsorb, absorb,
be coated with or be formed by the surfactant.
Surfactants suitable for use include any compound or composition that aids in
the
formation and maintenance of the stabilized respiratory dispersions by forming
a layer at the
interface between the structural matrix and the suspension medium. The
surfactant may comprise
a single compound or any combination of compounds, such as in the case of co-
surfactants.
Particularly preferred surfactants are substantially insoluble in the
propellant, nonfluorinated, and
selected from.the group consisting of saturated and unsaturated lipids,
nonionic detergents,
nonionic block copolymers, ionic surfactants, and combinations of such agents.
It should be
emphasized that, in addition to the aforementioned surfactants, suitable (i.e.
biocompatible)
fluorinated surfactants are compatible with the teachings herein and may be
used to provide the
desired stabilized preparations.
Lipids, including phospholipids, from both natural and synthetic sources may
be used in
varying concentrations to form a structural matrix. Generally, compatible
lipids comprise those
that have a gel to liquid crystal phase transition greater than about 400 C.
Preferably, the
incorporated lipids are relatively long chain (i.e. C6 -C22) saturated lipids
and more preferably
182
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
comprise phospholipids. Exemplary phospholipids useful in the disclosed
stabilized preparations
comprise egg phosphatidylcholine, dilauroylphosphatidylcholine,
dioleylphosphatidylcholine,
dipalmitoylphosphatidyl-choline, disteroylphosphatidylcholine, short-chain
phosphatidylcholines, phosphatidylethanolamine,
dioleylphosphatidylethanolamine,
phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, glycolipids,
ganglioside GM1,
sphingomyelin, phosphatidic acid, cardiolipin; lipids bearing polymer chains
such as,
polyethylene glycol, chitin, hyaluronic acid, or polyvinylpyrrolidone; lipids
bearing sulfonated
mono-, di-, and polysaccharides; fatty acids such as palmitic acid, stearic
acid, and oleic acid;
cholesterol, cholesterol esters, and cholesterol hemisuccinate. Due to their
excellent
biocompatibility characteristics, phospholipids and combinations of
phospholipids and
poloxamers are particularly suitable for use in the stabilized dispersions
disclosed herein.
Compatible nonionic detergents comprise: sorbitan esters including sorbitan
trioleate
(SpansTM 85), sorbitan sesquioleate, sorbitan monooleate, sorbitan
monolaurate, polyoxyethylene
(20) sorbitan monolaurate, and polyoxyethylene (20) sorbitan monooleate, oleyl
polyoxyethylene
(2) ether, stearyl polyoxyethylene (2) ether, lauryl polyoxyethylene (4)
ether, glycerol esters, and,
sucrose esters. Other suitable nonionic detergents can be easily identified
using McCutcheon's
Emulsifiers and Detergents (McPublishing Co., Glen Rock, N.J.). Preferred
block copolymers
include diblock and triblock copolymers of polyoxyethylene and
polyoxypropylene, including
poloxamer 188 (Pluronic® F68), poloxamer 407 (Pluronic® F-127), and
poloxamer
338. Ionic surfactants such as sodium sulfosuccinate, and fatty acid soaps may
also be utilized. In
preferred embodiments, the microstructures may comprise oleic acid or its
alkali salt.
In addition to the aforementioned surfactants, cationic surfactants or lipids
are preferred
especially in the case of delivery of an iRNA agent, e.g., a double-stranded
iRNA agent, or
sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof). Examples of suitable cationic lipids include:
DOTMA, N-[-(2,3-
dioleyloxy)propyl]-N,N,N-trimethylammonium-chloride; DOTAP,1,2-dioleyloxy-3- .
(trimethylammonio)propane; and DOTB, 1,2-dioleyl-3-(4'-
trimethylammonio)butanoyl-sn-
glycerol. Polycationic amino acids such as polylysine, and polyarginine are
also contemplated.
183
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
For the spraying process, such spraying methods as rotary atomization,
pressure
atomization and two-fluid atomization can be used. Examples of the devices
used in these
processes include "Parubisu [phonetic rendering] Mini-Spray GA-32" and
"Parubisu Spray Drier
DL-41 ", manufactured by Yamato Chemical Co., or "Spray Drier CL-8," "Spray
Drier L-8,"
"Spray Drier FL-12," "Spray Drier FL-16" or "Spray Drier FL-20," manufactured
by Okawara
Kakoki Co., can be used for the method of spraying using rotary-disk atomizer.
While no particular restrictions are placed on the gas used to dry the sprayed
material, it
is recommended to use air, nitrogen gas or an inert gas. The temperature of
the inlet of the gas
used to dry the sprayed materials such that it does not cause heat
deactivation of the sprayed
lo material. The range of temperatures may vary between about 50 C to about
200 C, preferably
between about 50 C and 100 C. The temperature of the outlet gas used to dry
the sprayed
material, may vary between about 0 C and about 150 C, preferably between 0 C
and 90 C, and
even more preferably between 0 C and 60 C.
The spray drying is done under conditions that result in substantially
amorphous powder
of homogeneous constitution having a particle size that is respirable, a low
moisture content and
flow characteristics that allow for ready aerosolization. Preferably the
particle size of the
resulting powder is such that more than about 98% of the mass is in particles
having a diameter
of about 10 m or less with about 90% of the mass being in particles having a
diameter less than
5 m. Alternatively, about 95% of the mass will have particles with a diameter
of less than 10
m with about 80% of the mass of the particles having a diameter of less than 5
m.
The dispersible pharmaceutical-based dry powders that include the iRNA
preparation
may optionally be combined with pharmaceutical carriers or excipients which
are suitable for
respiratory and pulmonary administration. Such carriers may serve simply as
bulking agents
when it is desired to reduce the iRNA concentration in the powder which is
being delivered to a
patient, but may also serve to enhance the stability of the iRNA compositions
and to improve the
dispersibility of the powder within a powder dispersion device in order to
provide more efficient
and reproducible delivery of the iRNA and to improve handling characteristics
of the iRNA such
as flowability and consistency to facilitate manufacturing and powder filling.
184
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Such carrier materials maybe combined with the drug prior to spray drying,
i.e., by
adding the carrier material to the purified bulk solution. In that way, the
carrier particles will be
formed simultaneously with the drug particles to produce a homogeneous powder.
Alternatively,
the carriers may be separately prepared in a dry powder form and combined with
the dry powder
drug by blending. The powder carriers will usually be crystalline (to avoid
water absorption), but
might in some cases be amorphous or mixtures of crystalline and amorphous. The
size of the
carrier particles may be selected to improve the flowability of the drug
powder, typically being in
the range from 25 ttm to 100 m. A preferred carrier material is crystalline
lactose having a size
in the above-stated range.
Powders prepared by any of the above methods will be collected from the spray
dryer in a
conventional manner for subsequent use. For use as pharmaceuticals and other
purposes, it will
frequently be desirable to disrupt any agglomerates which may have formed by
screening or
other conventional techniques. For pharmaceutical uses, the dry powder
formulations will
usually be measured into a single dose, and the single dose sealed into a
package. Such packages
are particularly useful for dispersion in dry powder inhalers, as described in
detail below.
Alternatively, the powders may be packaged in multiple-dose containers.
Methods for spray drying hydrophobic and other drugs and components are
described in
U.S. Pat. Nos. 5,000,888; 5,026,550; 4,670,419, 4,540,602; and 4,486,435.
Bloch and Speison
(1983) Pharm. Acta Helv 58:14-22 teaches spray drying of hydrochlorothiazide
and
chlorthalidone (lipophilic drugs) and a hydrophilic adjuvant (pentaerythritol)
in azeotropic
solvents of dioxane-water and 2-ethoxyethanol-water. A number of Japanese
Patent application
Abstracts relate to spray drying of hydrophilic-hydrophobic product
combinations, including JP
806766; JP 7242568; JP 7101884; JP 7101883; JP 71018982; JP 7101881; and JP
4036233.
Other foreign patent publications relevant to spray drying hydrophilic-
hydrophobic product
combinations include FR 2594693; DE 2209477; and WO 88/07870.
LYOPHILIZATION.
An iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof)
185
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
preparation can be made by lyophilization. Lyophilization is a freeze-drying
process in which
water is sublimed from the composition after it is frozen. The particular
advantage associated
with the lyophilization process is that biologicals and pharmaceuticals that
are relatively unstable
in an aqueous solution can be dried without elevated temperatures (thereby
eliminating the
adverse thermal effects), and then stored in a dry state where there are few
stability problems.
With respect to the instant invention such techniques are particularly
compatible with the
incorporation of nucleic acids in perforated microstructures without
compromising physiological
activity. Methods for providing lyophilized particulates are known to those of
skill in the art and
it would clearly not require undue experimentation to provide dispersion
compatible
lo microstructures in accordance with the teachings herein. Accordingly, to
the extent that
lyophilization processes may be used to provide microstructures having the
desired porosity and
size, they are conformance with the teachings herein and are expressly
contemplated as being
within the scope of the instant invention.
Tar eg ting
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNAs. It should be understood,
however, that
these formulations, compositions and methods can be practiced with other iRNA
agents, e.g.,
modified iRNA agents, and such practice is within the invention.
In some embodiments, an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA
agent, (e.g., a precursor, e.g., a larger iRNA agent which can be processed
into a sRNA agent, or
a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof) is targeted to a particular cell. For example, a liposome
or particle or other
structure that includes a iRNA can also include a targeting moiety that
recognizes a specific
molecule on a target cell. The targeting moiety can be a molecule with a
specific affinity for a
target cell. Targeting moieties can include antibodies directed against a
protein found on the
surface of a target cell, or the ligand or a receptor-binding portion of a
ligand for a molecule
found on the surface of a target cell. For example, the targeting moiety can
recognize a cancer-
specific antigen of the liver or a viral antigen, thus delivering the iRNA to
a cancer cell or a
virus-infected cell. Exemplary targeting moieties include antibodies (such as
IgM, IgG, IgA,
186
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
IgD, and the like, or a functional portions thereof), ligands for cell surface
receptors (e.g.,
ectodomains thereof).
An antigen, such as a-feto protein, can be used to target an iRNA to a liver
cell.
In one embodiment, the targeting moiety is attached to a liposome. For
example, US
Patent 6,245,427 describes a method for targeting a liposome using a protein
or peptide. In
another example, a cationic lipid component of the liposome is derivatized
with a targeting
moiety. For example, WO 96/37194 describes converting N-
glutaryldioleoylphosphatidyl
ethanolamine to a N-hydroxysuccinimide activated ester. The product was then
coupled to an
RGD peptide.
GENES AND DISEASES
In one aspect, the invention features, a method of treating a subject at risk
for or afflicted
with unwanted cell proliferation, e.g., malignant or nonmalignant cell
proliferation. The method
includes:
providing an iRNA agent, e.g., an sRNA or iRNA agent described herein, e.g.,
an iRNA
having a structure described herein, where the iRNA is homologous to and can
silence, e.g., by
cleavage, a gene which promotes unwanted cell proliferation;
administering an iRNA agent, e.g., an sRNA or iRNA agent described herein to a
subject,
preferably a human subject,
thereby treating the subject.
In a preferred embodiment the gene is a growth factor or growth factor
receptor gene, a
kinase, e.g., a protein tyrosine, serine or threonine kinase gene, an adaptor
protein gene, a gene
encoding a G protein superfamily molecule, or a gene encoding a transcription
factor.
In a preferred embodiment the iRNA agent silences the PDGF beta gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted PDGF beta
expression, e.g., testicular and lung cancers.
187
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In another preferred embodiment the iRNA agent silences the Erb-B gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted Erb-B
expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the Src gene, and thus can
be used to
treat a subject having or at risk for a disorder characterized by unwanted Src
expression, e.g.,
colon cancers.
In a preferred embodiment the iRNA agent silences the CRK gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
CRK expression, e.g.,
colon and lung cancers.
In a preferred embodiment the iRNA agent silences the GRB2 gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
GRB2 expression,
e.g., squamous cell carcinoma.
In another preferred embodiment the iRNA agent silences the RAS gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted RAS
expression, e.g., pancreatic, colon and lung cancers, and chronic leukemia.
In another preferred embodiment the iRNA agent silences the MEKK gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted MEKK
expression, e.g., squamous cell carcinoma, melanoma or leukemia.
In another preferred embodiment the iRNA agent silences the JNK gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted JNK expression,
e.g., pancreatic or breast cancers.
In a preferred embodiment the iRNA agent silences the RAF gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
RAF expression, e.g.,
lung cancer or leukemia.
In a preferred embodiment the iRNA agent silences the Erkl/2 gene, and thus
can be used
to treat a subject having or at risk for a disorder characterized by unwanted
Erkl/2 expression,
e.g., lung cancer.
188
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In another preferred embodiment the iRNA agent silences the PCNA(p21) gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted PCNA
expression, e.g., lung cancer.
In a preferred embodiment the iRNA agent silences the MYB gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
MYB expression,
e.g., colon cancer or chronic myelogenous leukemia.
In a preferred embodiment the iRNA agent silences the c-MYC gene, and thus can
be
used to treat a subject having or at risk for a disorder characterized by
unwanted c-MYC
expression, e.g., Burkitt's lymphoma or neuroblastoma.
In another preferred embodiment the iRNA agent silences the JUN gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted JUN expression,
e.g., ovarian, prostate or breast cancers.
In another preferred embodiment the iRNA agent silences the FOS gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted FOS expression,
e.g., skin or prostate cancers.
In a preferred embodiment the iRNA agent silences the BCL-2 gene, and thus can
be
used to treat a subject having or at risk for a disorder characterized by
unwanted BCL-2
expression, e.g., lung or prostate cancers or Non-Hodgkin lymphoma.
In a preferred embodiment the iRNA agent silences the Cyclin D gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted Cyclin D
expression, e.g., esophageal and colon cancers.
In a preferred embodiment the iRNA agent silences the VEGF gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
VEGF expression,
e.g., esophageal and colon cancers.
In a preferred embodiment the iRNA agent silences the EGFR gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
EGFR expression,
e.g., breast cancer.
189
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In another preferred embodiment the iRNA agent silences the Cyclin A gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted Cyclin A
expression, e.g., lung and cervical cancers.
In another preferred embodiment the iRNA agent silences the Cyclin E gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted Cyclin E
expression, e.g., lung and breast cancers.
In another preferred embodiment the iRNA agent silences the WNT-1 gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted WNT-1
expression, e.g., basal cell carcinoma.
In another preferred embodiment the iRNA agent silences the beta-catenin gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted beta-
catenin expression, e.g., adenocarcinoma or hepatocellular carcinoma.
In another preferred embodiment the iRNA agent silences the c-MET gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted c-MET
expression, e.g., hepatocellular carcinoma.
In another preferred embodiment the iRNA agent silences the PKC gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted PKC
expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the NFKB gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
NFKB expression,
e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the STAT3 gene, and thus can
be
used to treat a subject having or at risk for a disorder characterized by
unwanted STAT3
expression, e.g., prostate cancer.
In another preferred embodiment the iRNA agent silences the survivin gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted survivin
expression, e.g., cervical or pancreatic cancers.
190
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In another preferred embodiment the iRNA agent silences the Her2/Neu gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted
Her2/Neu expression, e.g., breast cancer.
In another preferred embodiment the iRNA agent silences the topoisomerase I
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
topoisomerase I expression, e.g., ovarian and colon cancers.
In a preferred embodiment the iRNA agent silences the topoisomerase II alpha
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
topoisomerase II expression, e.g., breast and colon cancers.
In a preferred embodiment the iRNA agent silences mutations in the p73 gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted p73
expression, e.g., colorectal adenocarcinoma.
In a preferred embodiment the iRNA agent silences mutations in the
p21(WAF1/CIP1)
gene, and thus can be used to treat a subject having or at risk for a disorder
characterized by
unwanted p21(WAF1/CIP1) expression, e.g., liver cancer.
In a preferred embodiment the iRNA agent silences mutations in the p27(KIP1)
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted'
p27(KIP1) expression, e.g., liver cancer.
In preferred embodiments the iRNA agent silences mutations in tumor suppressor
genes,
and thus can be used as a method to promote apoptotic activity in combination
with
chemotherapeutics.
In another aspect, the invention features, a method of treating a subject,
e.g., a human, at
risk for or afflicted with a disease or disorder that may benefit by
angiogenesis inhibition e.g.,
cancer. The method includes:
providing an iRNA agent, e.g., an iRNA agent having a structure described
herein, which
iRNA agent is homologous to and can silence, e.g., by cleavage, a gene which
mediates
angiogenesis;
191
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
administering the iRNA agent to a subject,
thereby treating the subject.
In another aspect, the invention features a method of treating a subject
infected with a
virus or at risk for or afflicted with a disorder or disease associated with a
viral infection. The
method includes:
providing an iRNA agent, e.g., and iRNA agent having a structure described
herein,
which iRNA agent is homologous to and can silence, e.g., by cleavage, a viral
gene of a cellular
gene which mediates viral function, e.g., entry or growth;
administering the iRNA agent to a subject, preferably a human subject,
thereby treating the subject.
Thus, the invention provides for a method of treating patients infected by the
Human
Papilloma Virus (HPV) or at risk for or afflicted with a disorder mediated by
HPV, e.g, cervical
cancer. HPV is linked to 95% of cervical carcinomas and thus an antiviral
therapy is an
attractive method to treat these cancers and other symptoms of viral
infection.
In a preferred embodiment, the expression of a HPV gene is reduced. In another
preferred embodiment, the HPV gene is one of the group of E2, E6, or E7.
In a preferred embodiment the expression of a human gene that is required for
HPV
replication is reduced.
The invention also includes a method of treating patients infected by the
Human
Immunodeficiency Virus (HIV) or at risk for or afflicted with a disorder
mediated by HIV, e.g.,
Acquired Immune Deficiency Syndrome (AIDS).
In a preferred embodiment, the expression of a HIV gene is reduced. In another
preferred
embodiment, the HIV gene is CCR5, Gag, or Rev.
In a preferred embodiment the expression of a human gene that is required for
HIV
replication is reduced. In another preferred embodiment, the gene is CD4 or
Tsg101.
192
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The invention also includes a method for treating patients infected by the
Hepatitis B
Virus (HBV) or at risk for or afflicted with a disorder mediated by HBV, e.g.,
cirrhosis and
heptocellular carcinoma.
In a preferred embodiment, the expression of a HBV gene is reduced. In another
preferred embodiment, the targeted HBV gene encodes one of the group of the
tail region of the
HBV core protein, the pre-cregious (pre-c) region, or the cregious (c) region.
In another
preferred embodiment, a targeted HBV-RNA sequence is comprised of the poly(A)
tail.
In preferred embodiment the expression of a human gene that is required for
HBV
replication is reduced.
The invention also provides for a method of treating patients infected by the
Hepatitis A
Virus (HAV), or at risk for or afflicted with a disorder mediated by HAV.
In a preferred embodiment the expression of a human gene that is required for
HAV
replication is reduced.
The present invention provides for a method of treating patients infected by
the Hepatitis
C Virus (HCV), or at risk for or afflicted with a disorder mediated by HCV,
e.g., cirrhosis
In a preferred embodiment, the expression of a HCV gene is reduced.
In another preferred embodiment the expression of a human gene that is
required for
HCV replication is reduced.
The present invention also provides for a method of treating patients infected
by the any
of the group of Hepatitis Viral strains comprising hepatitis D, E, F, G. or H,
or patients at risk for
or afflicted with a disorder mediated by any of these strains of hepatitis.
In a preferred embodiment, the expression of a Hepatitis, D, E, F, G, or H
gene is
reduced.
In another preferred embodiment the expression of a human gene that is
required for
hepatitis D, E, F, G or H replication is reduced.
193
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Methods of the invention also provide for treating patients infected by the
Respiratory
Syncytial Virus (RSV) or at risk for or afflicted with a disorder mediated by
RSV, e.g, lower
respiratory tract infection in infants and childhood asthma, pneumonia and
other complications,
e.g., in the elderly.
In a preferred embodiment, the expression of a RSV gene is reduced. In another
preferred embodiment, the targeted HBV gene encodes one of the group of genes
N, L, or P.
In a preferred embodiment the expression of a human gene that is required for
RSV
replication is reduced.
Methods of the invention provide for treating patients infected by the Herpes
Simplex
Virus (HSV) or at risk for or afflicted with a disorder mediated by HSV, e.g,
genital herpes and
cold sores as well as life-threatening or sight-impairing disease mainly in
immunocompromised
patients.
In a preferred embodiment, the expression of a HSV gene is reduced. In another
preferred embodiment, the targeted HSV gene encodes DNA polymerase or the
helicase-
primase.
In a preferred embodiment the expression of a human gene that is required for
HSV
replication is reduced.
The invention also provides a method for treating patients infected by the
herpes
Cytomegalovirus (CMV) or at risk for or afflicted with a disorder mediated by
CMV, e.g.,
congenital virus infections and morbidity in immunocompromised patients.
In a preferred embodiment, the expression of a CMV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
CMV
replication is reduced.
Methods of the invention also provide for a method of treating patients
infected by the
herpes Epstein Barr Virus (EBV) or at risk for or afflicted with a disorder
mediated by EBV,
e.g., NK/T-cell lymphoma, non-Hodgkin lymphoma, and Hodgkin disease.
194
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment, the expression of a EBV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
EBV
replication is reduced.
Methods of the invention also provide for treating patients infected by
Kaposi's Sarcoma-
associated Herpes Virus (KSHV), also called human herpesvirus 8, or patients
at risk for or
afflicted with a disorder mediated by KSHV, e.g., Kaposi's sarcoma,
multicentric Castleman's
disease and AIDS-associated primary effusion lymphoma.
In a preferred embodiment, the expression of a KSHV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
KSHV
lo replication is reduced.
The invention also includes a method for treating patients infected by the JC
Virus (JCV)
or a disease or disorder associated with this virus, e.g., progressive
multifocal
leukoencephalopathy (PML).
In a preferred embodiment, the expression of a JCV gene is reduced.
In preferred embodiment the expression of a human gene that is required for
JCV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
myxovirus or
at risk for or afflicted with a disorder mediated by myxovirus, e.g.,
influenza.
In a preferred embodiment, the expression of a myxovirus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
myxovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
rhinovirus or at
risk for of afflicted with a disorder mediated by rhinovirus, e.g., the common
cold.
In a preferred embodiment, the expression of a rhinovirus gene is reduced.
195
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: I 4174-072 W 01
In preferred embodiment the expression of a human gene that is required for
rhinovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
coronavirus or
at risk for of afflicted with a disorder mediated by coronavirus, e.g., the
common cold.
In a preferred embodiment, the expression of a coronavirus gene is reduced.
In preferred embodiment the expression of a human gene that is required for
coronavirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
flavivirus West
Nile or at risk for or afflicted with a disorder mediated by West Nile Virus.
In a preferred embodiment, the expression of a West Nile Virus gene is
reduced. In
another preferred embodiment, the West Nile Virus gene is one of the group
comprising E, NS3,
or NS5.
In a preferred embodiment the expression of a human gene that is required for
West Nile
Virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
St. Louis
Encephalitis flavivirus, or at risk for or afflicted with a disease or
disorder associated with this
virus, e.g., viral haemorrhagic fever or neurological disease.
In a preferred embodiment, the expression of a St. Louis Encephalitis gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
St. Louis
Encephalitis virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
Tick-borne
encephalitis flavivirus, or at risk for or afflicted with a disorder mediated
by Tick-borne
encephalitis virus, e.g., viral haemorrhagic fever and neurological disease.
In a preferred embodiment, the expression of a Tick-borne encephalitis virus
gene is
reduced.
196
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the expression of a human gene that is required for
Tick-
borne encephalitis virus replication is reduced.
Methods of the invention also provide for methods of treating patients
infected by the
Murray Valley encephalitis flavivirus, which commonly results in viral
haemorrhagic fever and
neurological disease.
In a preferred embodiment, the expression of a Murray Valley encephalitis
virus gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Murray
Valley encephalitis virus replication is reduced.
The invention also includes methods for treating patients infected by the
dengue
flavivirus, or a disease or disorder associated with this virus, e.g., dengue
haemorrhagic fever.
In a preferred embodiment, the expression of a dengue virus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
dengue
virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
Simian Virus
40,(SV40) or at risk for or afflicted with a disorder mediated by SV40, e.g.,
tumorigenesis.
In a preferred embodiment, the expression of a SV40 gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
SV40
replication is reduced.
The invention also includes methods for treating patients infected by the
Human T Cell
Lymphotropic Virus (HTLV), or a disease or disorder associated with this
virus, e.g., leukemia
and myelopathy.
In a preferred embodiment, the expression of a HTLV gene is reduced. In
another
preferred embodiment the HTLV1 gene is the Tax transcriptional activator.
197
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the expression of a human gene that is required for
HTLV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
Moloney-
Murine Leukemia Virus (Mo-MuLV) or at risk for or afflicted with a disorder
mediated by Mo-
MuLV, e.g., T-cell leukemia.
In a preferred embodiment, the expression of a Mo-MuLV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
Mo-MuLV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
lo encephalomyocarditis virus (EMCV) or at risk for or afflicted with a
disorder mediated by
EMCV, e.g. myocarditis. EMCV leads to myocarditis in mice and pigs and is
capable of
infecting human myocardial cells. This virus is therefore a concern for
patients undergoing
xenotransplantation.
In a preferred embodiment, the expression of a EMCV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
EMCV
replication is reduced.
The invention also includes a method for treating patients infected by the
measles virus
(MV) or at risk for or afflicted with a disorder mediated by MV, e.g. measles.
In a preferred embodiment, the expression of a MV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
MV
replication is reduced.
The invention also includes a method for treating patients infected by the
Vericella zoster
virus (VZV) or at risk for or afflicted with a disorder mediated by VZV, e.g.
chicken pox or
shingles (also called zoster).
In a preferred embodiment, the expression of a VZV gene is reduced.
198
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment the expression of a human gene that is required for
VZV
replication is reduced.
The invention also includes a method for treating patients infected by an
adenovirus or at
risk for or afflicted with a disorder mediated by an adenovirus, e.g.
respiratory tract infection.
In a preferred embodiment, the expression of an adenovirus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
adenovirus
replication is reduced.
The invention includes a method for treating patients infected by a yellow
fever virus
(YFV) or at risk for or afflicted with a disorder mediated by a YFV, e.g.
respiratory tract
infection.
In a preferred embodiment, the expression of a YFV gene is reduced. In another
preferred embodiment, the preferred gene is one of a group that includes the
E, NS2A, or NS3
genes.
In a preferred embodiment the expression of a human gene that is required for
YFV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
poliovirus'or at
risk for or afflicted with a disorder mediated by poliovirus, e.g., polio.
In a preferred embodiment, the expression of a poliovirus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
poliovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by a
poxvirus or at
risk for or afflicted with a disorder mediated by a poxvirus, e.g., smallpox
In a preferred embodiment, the expression of a poxvirus gene is reduced.
199
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
In a preferred embodiment the expression of a human gene that is required for
poxvirus
replication is reduced.
In another, aspect the invention features methods of treating a subject
infected with a
pathogen, e.g., a bacterial, amoebic, parasitic, or fungal pathogen. The
method includes:
providing a iRNA agent, e.g., a siRNA having a structure described herein,
where siRNA
is homologous to and can silence, e.g., by cleavage of a pathogen gene;
administering the iRNA agent to a subject, prefereably a human subject,
thereby treating the subject.
The target gene can be one involved in growth, cell wall synthesis, protein
synthesis,
1o transcription, energy metabolism, e.g., the Krebs cycle, or toxin
production.
Thus, the present invention provides for a method of treating patients
infected by a
plasmodium that causes malaria.
In a preferred embodiment, the expression of a plasmodium gene is reduced. In
another
preferred embodiment, the gene is apical membrane antigen 1 (AMAl).
In a preferred embodiment the expression of a human gene that is required for
plasmodium replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
ulcerans, or a disease or disorder associated with this pathogen, e.g. Buruli
ulcers.
In a preferred embodiment, the expression of a Mycobacterium ulcerans gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium ulcerans replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
tuberculosis, or a disease or disorder associated with this pathogen, e.g.
tuberculosis.
200
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment, the expression of a Mycobacterium tuberculosis gene
is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium tuberculosis replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
leprae, or a disease or disorder associated with this pathogen, e.g. leprosy.
In a preferred embodiment, the expression of a Mycobacterium leprae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium leprae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Staphylococcus aureus, or a disease or disorder associated with this pathogen,
e.g. infections of
the skin and muscous membranes. ,
In a preferred embodiment, the expression of a Staphylococcus aureus gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Staphylococcus aureus replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Streptococcus pneumoniae, or a disease or disorder associated with this
pathogen, e.g.
pneumonia or childhood lower respiratory tract infection.
In a preferred embodiment, the expression of a Streptococcus pneumoniae gene
is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Streptococcus pneumoniae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Streptococcus pyogenes, or a disease or disorder associated with this
pathogen, e.g. Strep throat
or Scarlet fever.
201
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In a preferred embodiment, the expression of a Streptococcus pyogenes gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Streptococcus pyogenes replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Chlamydia pneumoniae, or a disease or disorder associated with this pathogen,
e.g. pneumonia
or childhood lower respiratory tract infection
In a preferred embodiment, the expression of a Chlamydia pneumoniae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Chlamydia
pneumoniae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Mycoplasma pneumoniae, or a disease or disorder associated with this pathogen,
e.g. pneumonia
or childhood lower respiratory tract infection
In a preferred embodiment, the expression of a Mycoplasma pneumoniae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycoplasma pneumoniae replication is reduced.
The loss of heterozygosity (LOH) can result in hemizygosity for sequence,
e.g., genes, in
the area of LOH. This can result in a significant genetic difference between
normal and disease-
state cells, e.g., cancer cells, and provides a useful difference between
normal and disease-state
cells, e.g., cancer cells. This difference can arise because a gene or other
sequence is
heterozygous in euploid cells but is hemizygous in cells having LOH. The
regions of LOH will
often include a gene, the loss of which promotes unwanted proliferation, e.g.,
a tumor suppressor
gene, and other sequences including, e.g., other genes, in some cases a gene
which is essential
for normal function, e.g., growth. Methods of the invention rely, in part, on
the specific cleavage
or silencing of one allele of an essential gene with an iRNA agent of the
invention. The iRNA
agent is selected such that it targets the single allele of the essential gene
found in the cells
having LOH but does not silence the other allele, which is present in cells
which do not show
LOH. In essence, it discriminates between the two alleles, preferentially
silencing the selected
202
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
allele. In essence polymorphisms, e.g., SNPs of essential genes that are
affected by LOH, are
used as a target for a disorder characterized by cells having LOH, e.g.,
cancer cells having LOH.
E.g., one of ordinary skill in the art can identify essential genes which are
in proximity to
tumor suppressor genes, and which are within a LOH region which includes the
tumor
suppressor gene. The gene encoding the large subunit of human RNA polymerase
II, POLR2A,
a gene located in close proximity to the tumor suppressor gene p53, is such a
gene. It frequently
occurs within a region of LOH in cancer cells. Other genes that occur within
LOH regions and
are lost in many cancer cell types include the group comprising replication
protein A 70-kDa
subunit, replication protein A 32-kD, ribonucleotide reductase, thymidilate
synthase, TATA
1o associated factor 2H, ribosomal protein S14, eukaryotic initiation factor
5A, alanyl tRNA
synthetase, cysteinyl tRNA synthetase, NaK ATPase, alpha-1 subunit, and
transferrin receptor.
Accordingly, the invention features, a method of treating 'a disorder
characterized by
LOH, e.g., cancer. The method includes:
optionally, determining the genotype of the allele of a gene in the region of
LOH and
preferably determining the genotype of both alleles of the gene in a normal
cell;
providing an iRNA agent which preferentially cleaves or silences the allele
found in the
LOH cells;
administerning the iRNA to the subject,
thereby treating the disorder.
The invention also includes a iRNA agent disclosed herein, e.g, an iRNA agent
which
can preferentially silence, e.g., cleave, one allele of a polymorphic gene
In another aspect, the invention provides a method of cleaving or silencing
more than one
gene with an iRNA agent. In these embodiments the iRNA agent is selected so
that it has
sufficient homology to a sequence found in more than one gene. For example,
the sequence
AAGCTGGCCCTGGACATGGAGAT (SEQ ID NO:6719) is conserved between mouse lamin
B1, lamin B2, keratin complex 2-gene 1 and lamin A/C. Thus an iRNA agent
targeted to this
sequence would effectively silence the entire collection of genes.
203
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The invention also includes an iRNA agent disclosed herein, which can silence
more
than one gene.
ROUTE OF DELIVERY
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention. A
composition that
includes a iRNA can be delivered to a subject by a variety of routes.
Exemplary routes include:
intravenous, topical, rectal, anal, vaginal, nasal, pulmonary, ocular.
The iRNA molecules of the invention can be incorporated into pharmaceutical
compositions suitable for administration. Such compositions typically include
one or more
species of iRNA and a pharmaceutically acceptable carrier. As used herein the
language
"pharmaceutically acceptable carrier" is intended to include any and all
solvents, dispersion
media, coatings, antibacterial and antifungal agents, isotonic and absorption
delaying agents, and
the like, compatible with pharmaceutical administration. The use of such media
and agents for
pharmaceutically active substances is well known in the art. Except insofar as
any conventional
media or agent is incompatible with the active compound, use thereof in the
compositions is
contemplated. Supplementary active compounds can also be incorporated into the
compositions.
The pharmaceutical compositions of the present invention may be administered
in a
number of ways depending upon whether local or systemic treatment is desired
and upon the
area to be treated. Administration may be topical (including ophthalmic,
vaginal, rectal,
intranasal, transdermal), oral or parenteral. Parenteral administration
includes intravenous drip,
subcutaneous, intraperitoneal or intramuscular injection, or intrathecal or
intraventricular
administration.
The route and site of administration may be chosen to enhance targeting. For
example, to
target muscle cells, intramuscular injection into the muscles of interest
would be a logical choice.
Lung cells might be targeted by administering the iRNA in aerosol form. The
vascular
204
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
endothelial cells could be targeted by coating a balloon catheter with the
iRNA and mechanically
introducing the DNA.
Formulations for topical administration may include transdermal patches,
ointments,
lotions, creams, gels, drops, suppositories, sprays, liquids and powders.
Conventional
pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the
like may be necessary
or desirable. Coated condoms, gloves and the like may also be useful.
Compositions for oral administration include powders or granules, suspensions
or
solutions in water, syrups, elixirs or non-aqueous media, tablets, capsules,
lozenges, or troches.
In the case of tablets, carriers that can be used include lactose, sodium
citrate and salts of
1o phosphoric acid. Various disintegrants such as starch, and lubricating
agents such as magnesium
stearate, sodium lauryl sulfate and talc, are commonly used in tablets. For
oral administration in
capsule form, useful diluents are lactose and high molecular weight
polyethylene glycols. When
aqueous suspensions are required for oral use, the nucleic acid compositions
can be combined
with emulsifying and suspending agents. If desired, certain sweetening and/or
flavoring agents
can be added.
Compositions for intrathecal or intraventricular administration may include
sterile
aqueous solutions which may also contain buffers, diluents and other suitable
additives.
Formulations for parenteral administration may include sterile aqueous
solutions which
may also contain buffers, diluents and other suitable additives.
Intraventricular injection may be
facilitated by an intraventricular catheter, for example, attached to a
reservoir. For intravenous
use, the total concentration of solutes should be controlled to render the
preparation isotonic.
For ocular administration, ointments or droppable liquids may be delivered by
ocular
delivery systems known to the art such as applicators or eye droppers. Such
compositions can
include mucomimetics such as hyaluronic acid, chondroitin sulfate,
hydroxypropyl
methylcellulose or poly(vinyl alcohol), preservatives such as sorbic acid,
EDTA or
benzylchronium chloride, and the usual quantities of diluents and/or carriers.
205
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Topical Delivery
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention. In a
preferred embodiment, an
iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor, e.g., a larger
iRNA agent which can be processed into a sRNA agent, or a DNA which encodes an
iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof) is delivered to a
subject via topical administration. "Topical administration" refers to the
delivery to a subject by
lo contacting the formulation directly to a surface of the subject. The most
common form of topical
delivery is to the skin, but a composition disclosed herein can also be
directly applied to other
surfaces of the body, e.g., to the eye, a mucous membrane, to surfaces of a
body cavity or to an
internal surface. As mentioned above, the most common topical delivery is to
the skin. The term
encompasses several routes of administration including, but not limited to,
topical and
transdermal. These modes of administration typically include penetration of
the skin's
permeability barrier and efficient delivery to the target tissue or stratum.
Topical administration
can be used as a means to penetrate the epidermis and dermis and ultimately
achieve systemic
delivery of the composition. Topical administration can also be used as a
means to selectively
deliver oligonucleotides to the epidermis or dermis of a subject, or to
specific strata thereof, or to
an underlying tissue.
The term "skin," as used herein, refers to the epidermis and/or dermis of an
animal.
Mammalian skin consists of two major, distinct layers. The outer layer of the
skin is called the
epidermis. The epidermis is comprised of the stratum corneum, the stratum
granulosum, the
stratum spinosum, and the stratum basale, with the stratum corneum being at
the surface of the
skin and the stratum basale being the deepest portion of the epidermis. The
epidermis is between
50 m and 0.2 mm thick, depending on its location on the body.
Beneath the epidermis is the dermis, which is significantly thicker than the
epidermis.
The dermis is primarily composed of collagen in the form of fibrous bundles.
The collagenous
206
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
bundles provide support for, inter alia, blood vessels, lymph capillaries,
glands, nerve endings
and immunologically active cells.
One of the major functions of the skin as an organ is to regulate the entry of
substances
into the body. The principal permeability barrier of the skin is provided by
the stratum corneum,
which is formed from many layers of cells in various states of
differentiation. The spaces
between cells in the stratum corneum is filled with different lipids arranged
in lattice-like
formations that provide seals to further enhance the skins permeability
barrier.
The permeability barrier provided by the skin is such that it is largely
impermeable to
molecules having molecular weight greater than about 750 Da. For larger
molecules to cross the
1 o skin's permeability barrier, mechanisms other than normal osmosis must be
used.
Several factors determine the permeability of the skin to administered agents.
These
factors include the characteristics of the treated skin, the characteristics
of the delivery agent,
interactions between both the drug and delivery agent and the drug and skin,
the dosage of the
drug applied, the form of treatment, and the post treatment regimen. To
selectively target the
epidermis and dermis, it is sometimes possible to formulate a composition that
comprises one or
more penetration enhancers that will enable penetration of the drug to a
preselected stratum.
Transdermal delivery is a valuable route for the administration of lipid
soluble
therapeutics. The dermis is more permeable than the epidermis and therefore
absorption is much
more rapid through abraded, burned or denuded skin. Inflammation and other
physiologic
conditions that increase blood flow to the skin also enhance transdermal
adsorption. Absorption
via this route may be enhanced by the use of an oily vehicle (inunction) or
through the use of one
or more penetration enhancers. Other effective ways to deliver a composition
disclosed herein
via the transdermal route include hydration of the skin and the use of
controlled release topical
patches. The transdermal route provides a potentially effective means to
deliver a composition
disclosed herein for systemic and/or local therapy.
In addition, iontophoresis (transfer of ionic solutes through biological
membranes under
the influence of an electric field) (Lee et al., Critical Reviews in
Therapeutic Drug Carrier
Systems, 1991, p. 163), phonophoresis or sonophoresis (use of ultrasound to
enhance the
207
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
absorption of various therapeutic agents across biological membranes, notably
the skin and the
cornea) (Lee et al., Critical Reviews in Therapeutic Drug Carrier Systems,
1991, p. 166), and
optimization of vehicle characteristics relative to dose position and
retention at the site of
administration (Lee et al., Critical Reviews in Therapeutic Drug Carrier
Systems, 1991, p. 168)
maybe useful methods for enhancing the transport of topically applied
compositions across skin
and mucosal sites.
The compositions and methods provided may also be used to examine the function
of
various proteins and genes in vitro in cultured or preserved dermal tissues
and in animals. The
invention can be thus applied to examine the function of any gene. The methods
of the invention
1o can also be used therapeutically or prophylactically. For example, for the
treatment of animals
that are known or suspected to suffer from diseases such as psoriasis, lichen
planus, toxic
epidermal necrolysis, ertythema multiforme, basal cell carcinoma, squamous
cell carcinoma,
malignant melanoma, Paget's disease, Kaposi's sarcoma, pulmonary fibrosis,
Lyme disease and
viral, fungal and bacterial infections of the skin.
Pulmonary Delivery
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention. A composition
that includes an
iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor, e.g., a larger
iRNA agent which can be processed into a sRNA agent, or a DNA which encodes an
iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof) can be
administered to a subject by pulmonary delivery. Pulmonary delivery
compositions can be
delivered by inhalation by the patient of a dispersion so that the
composition, preferably iRNA,
within the dispersion can reach the lung where it can be readily absorbed
through the alveolar
region directly into blood circulation. Pulmonary delivery can be effective
both for systemic
delivery and for localized delivery to treat diseases of the lungs.
Pulmonary delivery can be achieved by different approaches, including the use
of
nebulized, aerosolized, micellular and dry powder-based formulations. Delivery
can be achieved
208
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
with liquid nebulizers, aerosol-based inhalers, and dry powder dispersion
devices. Metered-dose
devices are preferred. One of the benefits of using an atomizer or inhaler is
that the potential for
contamination is minimized because the devices are self contained. Dry powder
dispersion
devices, for example, deliver drugs that may be readily formulated as dry
powders. A iRNA
composition may be stably stored as lyophilized or spray-dried powders by
itself or in
combination with suitable powder carriers. The delivery of a composition for
inhalation can be
mediated by a dosing timing element which can include a timer, a dose counter,
time measuring
device, or a time indicator which when incorporated into the device enables
dose tracking,
compliance monitoring, and/or dose triggering to a patient during
administration of the aerosol
lo medicament.
The term "powder" means a composition that consists of finely dispersed solid
particles
that are free flowing and capable of being readily dispersed in an inhalation
device and
subsequently inhaled by a subject so that the particles reach the lungs to
permit penetration into
the alveoli. Thus, the powder is said to be "respirable." Preferably the
average particle size is
less than about 10 pm in diameter preferably with a relatively uniform
spheroidal shape
distribution. More preferably the diameter is less than about 7.5 am and most
preferably less than
about 5.0 m. Usually the particle size distribution is between about 0.1 pm
and about 5 pm in
diameter, particularly about 0.3 m to about 5 pm.
The term "dry" means that the composition has a moisture content below about
10% by
weight (% w) water, usually below about 5% w and preferably less it than about
3% w. A dry
composition can be such that the particles are readily dispersible in an
inhalation device to form
an aerosol.
The term "therapeutically effective amount" is the amount present in the
composition that
is needed to provide the desired level of drug in the subject to be treated to
give the anticipated
physiological response.
The term "physiologically effective amount" is that amount delivered to a
subject to give
the desired palliative or curative effect.
209
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The term "pharmaceutically acceptable carrier" means that the carrier can be
taken into
the lungs with no significant adverse toxicological effects on the lungs.
The types of pharmaceutical excipients that are useful as carrier include
stabilizers such
as human serum albumin (HSA), bulking agents such as carbohydrates, amino
acids and
polypeptides; pH adjusters or buffers; salts such as sodium chloride; and the
like. These carriers
may be in a crystalline or amorphous form or maybe a mixture of the two.
Bulking agents that are particularly valuable include compatible
carbohydrates,
polypeptides, amino acids or combinations thereof. Suitable carbohydrates
include
monosaccharides such as galactose, D-mannose, sorbose, and the like;
disaccharides, such as
lactose, trehalose, and the like; cyclodextrins, such as 2-hydroxypropyl-
.beta.-cyclodextrin; and
polysaccharides, such as raffinose, maltodextrins, dextrans, and the like;
alditols, such as
mannitol, xylitol, and the like. A preferred group of carbohydrates includes
lactose, threhalose,
raffinose maltodextrins, and mannitol. Suitable polypeptides include
aspartame. Amino acids
include alanine and glycine, with glycine being preferred.
Additives, which are minor components of the composition of this invention,
may be
included for conformational stability during spray drying and for improving
dispersibility of the
powder. These additives include hydrophobic amino acids such as tryptophan,
tyrosine, leucine,
phenylalanine, and the like.
Suitable pH adjusters or buffers include organic salts prepared from organic
acids and
bases, such as sodium citrate, sodium ascorbate, and the like; sodium citrate
is preferred.
Pulmonary administration of a micellar iRNA formulation may be achieved
through
metered dose spray devices with propellants such as tetrafluoroethane,
heptafluoroethane,
dimethylfluoropropane, tetrafluoropropane, butane, isobutane, dimethyl ether
and other non-CFC
and CFC propellants.
Oral or Nasal Delivery
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
210
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention. Both
the oral and nasal
membranes offer advantages over other routes of administration. For example,
drugs
administered through these membranes have a rapid onset of action, provide
therapeutic plasma
levels, avoid first pass effect of hepatic metabolism, and avoid exposure of
the drug to the hostile
gastrointestinal (GI) environment. Additional advantages include easy access
to the membrane
sites so that the drug can be applied, localized and removed easily.
In oral delivery, compositions can be targeted to a surface of the oral
cavity, e.g., to
sublingual mucosa which includes the membrane of ventral surface of the tongue
and the floor of
the mouth or the buccal mucosa which constitutes the lining of the cheek. The
sublingual mucosa
is relatively permeable thus giving rapid absorption and acceptable
bioavailability of many
drugs. Further, the sublingual mucosa is convenient, acceptable and easily
accessible.
The ability of molecules to permeate through the oral mucosa appears to be
related to
molecular size, lipid solubility and peptide protein ionization. Small
molecules, less than 1000
daltons appear to cross mucosa rapidly. As molecular size increases, the
permeability decreases
rapidly. Lipid soluble compounds are more permeable than non-lipid soluble
molecules.
Maximum absorption occurs when molecules are un-ionized or neutral in
electrical charges.
Therefore charged molecules present the biggest challenges to absorption
through the oral
mucosae.
A pharmaceutical composition of iRNA may also be administered to the buccal
cavity of
a human being by spraying into the cavity, without inhalation, from a metered
dose spray
dispenser, a mixed micellar pharmaceutical formulation as described above and
a propellant. In
one embodiment, the dispenser is first shaken prior to spraying the
pharmaceutical formulation
and propellant into the buccal cavity.
Devices
For ease of exposition the devices, formulations, compositions and methods in
this
section are discussed largely with regard to unmodified iRNA agents. It should
be understood,
however, that these devices, formulations, compositions and methods can be
practiced with other
211
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
iRNA agents, e.g., modified iRNA agents, and such practice is within the
invention. An iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) can be
disposed on or in a
device, e.g., a device which implanted or otherwise placed in a subject.
Exemplary devices
include devices which are introduced into the vasculature, e.g., devices
inserted into the lumen of
a vascular tissue, or which devices themselves form a part of the vasculature,
including stents,
catheters, heart valves, and other vascular devices. These devices, e.g.,
catheters or stents, can be
placed in the vasculature of the lung, heart, or leg.
Other devices include non-vascular devices, e.g., devices implanted in the
peritoneum, or
in organ or glandular tissue, e.g., artificial organs. The device can release
a therapeutic substance
in addition to a iRNA, e.g., a device can release insulin.
Other devices include artificial joints, e.g., hip joints, and other
orthopedic implants.
In one embodiment, unit doses or measured doses of a composition that includes
iRNA
are dispensed by an implanted device. The device can include a sensor that
monitors a parameter
within a subject. For example, the device can include pump, e.g., and,
optionally, associated
electronics.
Tissue, e.g., cells or organs, such as the liver, can be treated with an iRNA
agent, e.g., a
double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent, or a DNA which encodes an iRNA agents
e.g., a double-
stranded iRNA agent, or sRNA agent, or precursor thereof) ex vivo and then
administered or
implanted in a subject.
The tissue can be autologous, allogeneic, or xenogeneic tissue. For example,
tissue (e.g.,
liver) can be treated to reduce graft v. host disease. In other embodiments,
the tissue is
allogeneic and the tissue is treated to treat a disorder characterized by
unwanted gene expression
in that tissue, such as in the liver. In another example, tissue containing
hematopoietic cells, e.g.,
bone marrow hematopoietic cells, can be treated to inhibit unwanted cell
proliferation.
212
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Introduction of treated tissue, whether autologous or transplant, can be
combined with
other therapies.
In some implementations, the iRNA treated cells are insulated from other
cells, e.g., by a
semi-permeable porous barrier that prevents the cells from leaving the
implant, but enables
molecules from the body to reach the cells and molecules produced by the cells
to enter the body.
In one embodiment, the porous barrier is formed from alginate.
In one embodiment, a contraceptive device is coated with or contains an iRNA
agent,
e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a
larger iRNA agent
which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g., a
double-stranded iRNA agent, or sRNA agent, or precursor thereof). Exemplary
devices include
condoms, diaphragms, IUD (implantable uterine devices, sponges, vaginal
sheaths, and birth
control devices. In one embodiment, the iRNA is chosen to inactive sperm or
egg. In another
embodiment, the iRNA is chosen to be complementary to a viral or pathogen RNA,
e.g., an RNA
of an STD. In some instances, the iRNA composition can include a spermicide.
DOSAGE
In one aspect, the invention features a method of administering an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent, to a subject (e.g., a human
subject). The method
includes administering a unit dose of the iRNA agent, e.g., a sRNA agent,
e.g., double stranded
sRNA agent that (a) the double-stranded part is 19-25 nucleotides (nt) long,
preferably 21-23 nt,
(b) is complementary to a target RNA (e.g., an endogenous or pathogen target
RNA), and,
optionally, (c) includes at least one 3' overhang 1-5 nucleotide long. In one
embodiment, the unit
dose is less than 1.4 mg per kg of bodyweight, or less than 10, 5, 2, 1, 0.5,
0.1, 0.05, 0.01, 0.005,
0.001, 0.0005, 0.0001, 0.00005 or 0.00001 mg per kg of bodyweight, and less
than 200 nmole of
RNA agent (e.g. about 4.4 x 1016 copies) per kg of bodyweight, or less than
1500, 750, 300, 150,
75, 15, 7.5, 1.5, 0.75, 0.15, 0.075, 0.015, 0.0075, 0.0015, 0.00075, 0.00015
nmole of RNA agent
per kg of bodyweight.
The defined amount can be an amount effective to treat or prevent a disease or
disorder,
e.g., a disease or disorder associated with the target RNA, such as an RNA
present in the liver.
213
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The unit dose, for example, can be administered by injection (e.g.,
intravenous or intramuscular),
an inhaled dose, or a topical application. Particularly preferred dosages are
less than 2, 1, or 0.1
mg/kg of body weight.
In a preferred embodiment, the unit dose is administered less frequently than
once a day,
e.g., less than every 2, 4, 8 or 30 days. In another embodiment, the unit dose
is not administered
with a frequency (e.g., not a regular frequency). For example, the unit dose
may be administered
a single time.
In one embodiment, the effective dose is administered with other traditional
therapeutic
modalities. In one embodiment, the subject has a viral infection and the
modality is an antiviral
lo agent other than an iRNA agent, e.g., other than a double-stranded iRNA
agent, or sRNA agent,.
In another embodiment, the subject has atherosclerosis and the effective dose
of an iRNA agent,
e.g., a double-stranded iRNA agent, or sRNA agent, is administered in
combination with, e.g.,
after surgical intervention, e.g., angioplasty.
In one embodiment, a subject is administered an initial dose and one or more
maintenance doses of an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA agent, (e.g:,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof). The maintenance dose or doses are generally lower than the initial
dose, e.g., one-half
less of the initial dose. A maintenance regimen can include treating the
subject with a dose or
doses ranging from 0.01 g to 1.4 mg/kg of body weight per day, e.g., 10, 1,
0.1, 0.01, 0.001, or
0.00001 mg per kg of bodyweight per day. The maintenance doses are preferably
administered
no more than once every 5, 10, or 30 days. Further, the treatment regimen may
last for a period
of time which will vary depending upon the nature of the particular disease,
its severity and the
overall condition of the patient. In preferred embodiments the dosage may be
delivered no more
than once per day, e.g., no more than once per 24, 36, 48, or more hours,
e.g., no more than once
for every 5 or 8 days. Following treatment, the patient can be monitored for
changes in his
condition and for alleviation of the symptoms of the disease state. The dosage
of the compound
may either be increased in the event the patient does not respond
significantly to current dosage
214
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
= levels, or the dose may be decreased if an alleviation of the symptoms of
the disease state is
observed, if the disease state has been ablated, or if undesired side-effects
are observed.
The effective dose can be administered in a single dose or in two or more
doses, as
desired or considered appropriate under the specific circumstances. If desired
to facilitate
repeated or frequent infusions, implantation of a delivery device, e.g., a
pump, semi-permanent
stent (e.g., intravenous, intraperitoneal, intracisternal or intracapsular),
or reservoir may be
advisable.
In one embodiment, the iRNA agent pharmaceutical composition includes a
plurality of
iRNA agent species. In another embodiment, the iRNA agent species has
sequences that are
non-overlapping and non-adjacent to another species with respect to a
naturally occurring target
sequence. In another embodiment, the plurality of iRNA agent species is
specific for different
naturally occurring target genes. In another embodiment, the iRNA agent is
allele specific.
In some cases, a patient is treated with a iRNA agent in conjunction with
other
therapeutic modalities. For example, a patient being treated for a liver
disease can be
administered an iRNA agent specific for a target gene known to enhance the
progression of the
disease in conjunction with a drug known to inhibit activity of the target
gene product. For
example, a patient being treated for a cancer of the liver can be administered
an iRNA agent
specific for a target essential for tumor cell proliferation in conjunction
with a chemotherapy.
Following successful treatment, it may be desirable to have the patient
undergo
maintenance therapy to prevent the recurrence of the disease state, wherein
the compound of the
invention is administered in maintenance doses, ranging from 0.01 g to 100 g
per kg of body
weight (see US 6,107,094).
The concentration of the iRNA agent composition is an amount sufficient to be
effective
in treating or preventing a disorder or to regulate a physiological condition
in humans. The
concentration or amount of iRNA agent administered will depend on the
parameters determined
for the agent and the method of administration, e.g. nasal, buccal, pulmonary.
For example,
nasal formulations tend to require much lower concentrations of some
ingredients in order to
215
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
avoid irritation or burning of the nasal passages. It is sometimes desirable
to dilute an oral
formulation up to 10-100 times in order to provide a suitable nasal
formulation.
Certain factors may influence the dosage required to effectively treat a
subject, including
but not limited to the severity of the disease or disorder, previous
treatments, the general health
and/or age of the subject, and other diseases present. Moreover, treatment of
a subject with a
therapeutically effective amount of an iRNA agent, e.g., a double-stranded
iRNA agent, or sRNA
agent, (e.g., a precursor, e.g., a larger iRNA agent which can be processed
into a sRNA agent, or
a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof) can include a single treatment or, preferably, can include
a series of
treatments. It will also be appreciated that the effective dosage of a iRNA
agent such as a sRNA
agent used for treatment may increase or decrease over the course of a
particular treatment.
Changes in dosage may result and become apparent from the results of
diagnostic assays as
described herein. For example, the subject can be monitored after
administering a iRNA agent
composition. Based on information from the monitoring, an additional amount of
the iRNA
agent composition can be administered.
Dosing is dependent on severity and responsiveness of the disease condition to
be treated,.
with the course of treatment lasting from several days to several months, or
until a cure is
effected or a diminution of disease state is achieved. Optimal dosing
schedules can be calculated
from measurements of drug accumulation in the body of the patient. Persons of
ordinary skill can
easily determine optimum dosages, dosing methodologies and repetition rates.
Optimum dosages
may vary depending on the relative potency of individual compounds, and can
generally be
estimated based on EC50s found to be effective in in vitro and in vivo animal
models. In some
embodiments, the animal models include transgenic animals that express a human
gene, e.g. a
gene that produces a target RNA. The transgenic animal can be deficient for
the corresponding
endogenous RNA. In another embodiment, the composition for testing includes a
iRNA agent
that is complementary, at least in an internal region, to a sequence that is
conserved between the
target RNA in the animal model and the target RNA in a human.
216
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The inventors have discovered that iRNA agents described herein can be
administered to
mammals, particularly large mammals such as nonhuman primates or humans in a
number of
ways.
In one embodiment, the administration of the iRNA agent, e.g., a double-
stranded iRNA
agent, or sRNA agent, composition is parenteral, e.g. intravenous (e.g., as a
bolus or as a
diffusible infusion), intradermal, intraperitoneal, intramuscular,
intrathecal, intraventricular,
intracranial, subcutaneous, transmucosal, buccal, sublingual, endoscopic,
rectal, oral, vaginal,
topical, pulmonary, intranasal, urethral or ocular. Administration can be
provided by the
subject or by another person, e.g., a health care provider. The medication can
be provided in
lo measured doses or in a dispenser which delivers a metered dose. Selected
modes of delivery are
discussed in more detail below.
The invention provides methods, compositions, and kits, for rectal
administration or
delivery of iRNA agents described herein.
Accordingly, an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes a an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof) described herein, e.g., a therapeutically effective amount
of a iRNA agent
described herein, e.g., a iRNA agent having a double stranded region of less
than 40, and
preferably less than 30 nucleotides and having one or two 1-3 nucleotide
single strand 3'
overhangs can be administered rectally, e.g., introduced through the rectum
into the lower or
upper colon. This approach is particularly useful in the treatment of,
inflammatory disorders,
disorders characterized by unwanted cell proliferation, e.g., polyps, or colon
cancer.
The medication can be delivered to a site in the colon by introducing a
dispensing device,
e.g., a flexible, camera-guided device similar to that used for inspection of
the colon or removal
of polyps, which includes means for delivery of the medication.
The rectal administration of the iRNA agent is by means of an enema. The iRNA
agent
of the enema can be dissolved in a saline or buffered solution. The rectal
administration can also
217
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
by means of a suppository, which can include other ingredients, e.g., an
excipient, e.g., cocoa
butter or hydropropylmethylcellulose.
Any of the iRNA agents described herein can be administered orally, e.g., in
the form of
tablets, capsules, gel capsules, lozenges, troches or liquid syrups. Further,
the composition can
be applied topically to a surface of the oral cavity.
Any of the iRNA agents described herein can be administered buccally. For
example, the
medication can be sprayed into the buccal cavity or applied directly, e.g., in
a liquid, solid, or gel
form to a surface in the buccal cavity. This administration is particularly
desirable for the
treatment of inflammations of the buccal cavity, e.g., the gums or tongue,
e.g., in one
1o embodiment, the buccal administration is by spraying into the cavity, e.g.,
without inhalation,
from a dispenser, e.g., a metered dose spray dispenser that dispenses the
pharmaceutical
composition and a propellant.
Any of the iRNA agents described herein can be administered to ocular tissue.
For
example, the medications can be applied to the surface of the eye or nearby
tissue, e.g., the inside
of the eyelid. They can be applied topically, e.g., by spraying, in drops, as
an eyewash, or an
ointment. Administration can be provided by the subject or by another person,
e.g., a health care
provider. The medication can be provided in measured doses or in a dispenser
which delivers a
metered dose. The medication can also be administered to the interior of the
eye, and can be
introduced by a needle or other delivery device which can introduce it to a
selected area or
structure. Ocular treatment is particularly desirable for treating
inflammation of the eye or
nearby tissue.
Any of the iRNA agents described herein can be administered directly to the
skin. For
example, the medication can be applied topically or delivered in a layer of
the skin, e.g., by the
use of a microneedle or a battery of microneedles which penetrate into the
skin, but preferably
not into the underlying muscle tissue. Administration of the iRNA agent
composition can be
topical. Topical applications can, for example, deliver the composition to the
dermis or
epidermis of a subject. Topical administration can be in the form of
transdermal patches,
ointments, lotions, creams, gels, drops, suppositories, sprays, liquids or
powders. A composition
for topical administration can be formulated as a liposome, micelle, emulsion,
or other lipophilic
218
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
molecular assembly. The transdermal administration can be applied with at
least one penetration
enhancer, such as iontophoresis, phonophoresis, and sonophoresis.
Any of the iRNA agents described herein can be administered to the pulmonary
system.
Pulmonary administration can be achieved by inhalation or by the introduction
of a delivery
device into the pulmonary system, e.g., by introducing a delivery device which
can dispense the
medication. A preferred method of pulmonary delivery is by inhalation. The
medication can be
provided in a dispenser which delivers the medication, e.g., wet or dry, in a
form sufficiently
small such that it can be inhaled. The device can deliver a metered dose of
medication. The
subject, or another person, can administer the medication.
Pulmonary delivery is effective not only for disorders which directly affect
pulmonary
tissue, but also for disorders which affect other tissue.
iRNA agents can be formulated as a liquid or nonliquid, e.g., a powder,
crystal, or aerosol
for pulmonary delivery.
Any of the iRNA agents described herein can be administered nasally. Nasal
administration can be achieved by introduction of a delivery device into the
nose, e.g., by
introducing a delivery device which can dispense the medication. Methods of
nasal delivery
include spray, aerosol, liquid, e.g., by drops, or by topical administration
to a surface of the nasal
cavity. The medication can be provided in a dispenser with delivery of the
medication, e.g., wet
or dry, in a form sufficiently small such that it can be inhaled. The device
can deliver a metered
dose of medication. The subject, or another person, can administer the
medication.
Nasal delivery is effective not only for disorders which directly affect nasal
tissue, but
also for disorders which affect other tissue
iRNA agents can be formulated as a liquid or nonliquid, e.g., a powder,
crystal, or for
nasal delivery.
An iRNA agent can be packaged in a viral natural capsid or in a chemically or
enzymatically produced artificial capsid or structure derived therefrom.
219
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
The dosage of a pharmaceutical composition including a iRNA agent can be
administered
in order to alleviate the symptoms of a disease state, e.g., cancer or a
cardiovascular disease. A
subject can be treated with the pharmaceutical composition by any of the
methods mentioned
above.
Gene expression in a subject can be modulated by administering a
pharmaceutical
composition including an iRNA agent.
A subject can be treated by administering a defined amount of an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent) composition that is in a powdered form,
e.g., a collection of
microparticles, such as crystalline particles. The composition can include a
plurality of iRNA
agents, e.g., specific for one or more different endogenous target RNAs. The
method can include
other features described herein.
A subject can be treated by administering a defined amount of an iRNA agent
composition that is prepared by a method that includes spray-drying, i. e.
atomizing a liquid
solution, emulsion, or suspension, immediately exposing the droplets to a
drying gas,'and
collecting the resulting porous powder particles. The composition can include
a plurality of
iRNA agents, e.g., specific for one or more different endogenous target RNAs.
The method can
include other features described herein.
The iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof), can be
provided in a powdered, crystallized or other finely divided form, with or
without a carrier, e.g.,
a micro- or nano-particle suitable for inhalation or other pulmonary delivery.
This can include
providing an aerosol preparation, e.g., an aerosolized spray-dried
composition. The aerosol
composition can be provided in and/or dispensed by a metered dose delivery
device.
The subject can be treated for a condition treatable by inhalation, e.g., by
aerosolizing a
spray-dried iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g., a precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
220
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO I
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof)
composition and inhaling the aerosolized composition. The iRNA agent can be an
sRNA. The
composition can include a plurality of iRNA agents, e.g., specific for one or
more different
endogenous target RNAs. The method can include other features described
herein.
A subject can be treated by, for example, administering a composition
including an
effective/defined amount of an iRNA agent, e.g., a double-stranded iRNA agent,
or sRNA agent,
(e.g., a precursor, e.g., a larger iRNA agent which can be processed into a
sRNA agent, or a
DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof), wherein the composition is prepared by a method that
includes spray-drying,
I o lyophilization, vacuum drying, evaporation, fluid bed drying, or a
combination of these
techniques
In another aspect, the invention features a method that includes: evaluating a
parameter
related to the abundance of a transcript in a cell of a subject; comparing the
evaluated parameter
to a reference value; and if the evaluated parameter has a preselected
relationship to the reference
value (e.g., it is greater), administering a iRNA agent (or a precursor, e.g.,
a larger iRNA agent
which can be processed into a sRNA agent, or a DNA which encodes a iRNA agent
or precursor
thereof) to the subject. In one embodiment, the iRNA agent includes a sequence
that is
complementary to the evaluated transcript. For example, the parameter can be a
direct measure
of transcript levels, a measure of a protein level, a disease or disorder
symptom or
characterization (e.g., rate of cell proliferation and/or tumor mass, viral
load).
In another aspect, the invention features a method that includes:
administering a first
amount of a composition that comprises an iRNA agent, e.g., a double-stranded
iRNA agent, or
sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof) to a subject, wherein the iRNA agent includes a
strand substantially
complementary to a target nucleic acid; evaluating an activity associated with
a protein encoded
by the target nucleic acid; wherein the evaluation is used to determine if a
second amount should
be administered. In a preferred embodiment the method includes administering a
second amount
221
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
of the composition, wherein the timing of administration or dosage of the
second amount is a
function of the evaluating. The method can include other features described
herein.
In another aspect, the invention features a method of administering a source
of a double-
stranded iRNA agent (ds iRNA agent) to a subject. The method includes
administering or
implanting a source of a ds iRNA agent, e.g., a sRNA agent, that (a) includes
a double-stranded
region that is 19-25 nucleotides long, preferably 21-23 nucleotides, (b) is
complementary to a
target RNA (e.g., an endogenous RNA or a pathogen RNA), and, optionally, (c)
includes at least
one 3' overhang 1-5 nt long. In one embodiment, the source releases ds iRNA
agent over time,
e.g. the source is a controlled or a slow release source, e.g., a
microparticle that gradually
lo releases the ds iRNA agent. In another embodiment, the source is a pump,
e.g., a pump that
includes a sensor or a pump that can release one or more unit doses.
In one aspect, the invention features a pharmaceutical composition that
includes an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) including a
nucleotide
sequence complementary to a target RNA, e.g., substantially and/or exactly
complementary. The
target RNA can be a transcript of an endogenous human gene. In one embodiment,
the iRNA
agent (a) is 19-25 nucleotides long, preferably 21-23 nucleotides, (b) is
complementary to an
endogenous target RNA, and, optionally, (c) includes at least one 3' overhang
1-5 nt long. In one
embodiment, the pharmaceutical composition can be an emulsion, microemulsion,
cream, jelly,
or liposome.
In one example the pharmaceutical composition includes an iRNA agent mixed
with a
topical delivery agent. The topical delivery agent can be a plurality of
microscopic vesicles. The
microscopic vesicles can be liposomes. In a preferred embodiment the liposomes
are cationic
liposomes.
In another aspect, the pharmaceutical composition includes an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent, or a DNA which encodes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, or precursor thereof) admixed with a
topical penetration
222
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
enhancer. In one embodiment, the topical penetration enhancer is a fatty acid.
The fatty acid can
be arachidonic acid, oleic acid, lauric acid, caprylic acid, capric acid,
myristic acid, palmitic acid,
stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monolein,
dilaurin, glyceryl 1-
monocaprate, 1-dodecylazacycloheptan-2-one, an acylcarnitine, an acylcholine,
or a C1-lo alkyl
ester, monoglyceride, diglyceride or pharmaceutically acceptable salt thereof.
In another embodiment, the topical penetration enhancer is a bile salt. The
bile salt can
be cholic acid, dehydrocholic acid, deoxycholic acid, glucholic acid,
glycholic acid,
glycodeoxycholic acid, taurocholic acid, taurodeoxycholic acid,
chenodeoxycholic acid,
ursodeoxycholic acid, sodium tauro-24,25-dihydro-fusidate, sodium
glycodihydrofusidate,
lo polyoxyethylene-9-lauryl ether or a pharmaceutically acceptable salt
thereof.
In another embodiment, the penetration enhancer is a chelating agent. The
chelating
agent can be EDTA, citric acid, a salicyclate, a N-acyl derivative of
collagen, laureth-9, an N-
amino acyl derivative of a beta-diketone or a mixture thereof.
In another embodiment, the penetration enhancer is a surfactant, e.g., an
ionic or nonionic
surfactant. The surfactant can be sodium lauryl sulfate, polyoxyethylene-9-
lauryl ether,
polyoxyethylene-20-cetyl ether, a perfluorchemical emulsion or mixture
thereof.
In another embodiment, the penetration enhancer can be selected from a group
consisting
of unsaturated cyclic ureas, 1-alkyl-alkones, 1-alkenylazacyclo-alakanones,
steroidal anti-
inflammatory agents and mixtures thereof. In yet another embodiment the
penetration enhancer
can be a glycol, a pyrrol, an azone, or a terpenes.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a form
suitable for oral
delivery. In one embodiment, oral delivery can be used to deliver an iRNA
agent composition to
a cell or a region of the gastro-intestinal tract, e.g., small intestine,
colon (e.g., to treat a colon
cancer), and so forth. The oral delivery form can be tablets, capsules or gel
capsules. In one
embodiment, the iRNA agent of the pharmaceutical composition modulates
expression of a
223
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
cellular adhesion protein, modulates a rate of cellular proliferation, or has
biological activity
against eukaryotic pathogens or retroviruses. In another embodiment, the
pharmaceutical
composition includes an enteric material that substantially prevents
dissolution of the tablets,
capsules or gel capsules in a mammalian stomach. In a preferred embodiment the
enteric
material is a coating. The coating can be acetate phthalate, propylene glycol,
sorbitan monoleate,
cellulose acetate trimellitate, hydroxy propyl methylcellulose phthalate or
cellulose acetate
phthalate.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
a penetration enhancer. The penetration enhancer can be a bile salt or a fatty
acid. The bile salt
can be ursodeoxycholic acid, chenodeoxycholic acid, and salts thereof. The
fatty acid can be
capric acid, lauric acid, and salts thereof.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
an excipient. In one example the excipient is polyethyleneglycol. In another
example the
excipient is precirol.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
a plasticizer. The plasticizer can be diethyl phthalate, triacetin dibutyl
sebacate, dibutyl phthalate
or triethyl citrate.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent and a delivery vehicle. In one embodiment, the iRNA agent is (a) is 19-
25 nucleotides
long, preferably 21-23 nucleotides, (b) is complementary to an endogenous
target RNA, and,
optionally, (c) includes at least one 3' overhang 1-5 nucleotides long.
In one embodiment, the delivery vehicle can deliver an iRNA agent, e.g., a
double-
stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA
agent which can be
processed into a sRNA agent, or a DNA which encodes an iRNA agent, e.g., a
double-stranded
iRNA agent, or sRNA agent, or precursor thereof) to a cell by a topical route
of administration.
The delivery vehicle can be microscopic vesicles. In one example the
microscopic vesicles are
liposomes. In a preferred embodiment the liposomes are cationic liposomes. In
another example
the microscopic vesicles are micelles.In one aspect, the invention features a
pharmaceutical
224
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
composition including an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA agent, (e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof) in an injectable dosage form. In one embodiment, the injectable
dosage form of the
pharmaceutical composition includes sterile aqueous solutions or dispersions
and sterile
powders. In a preferred embodiment the sterile solution can include a diluent
such as water;
saline solution; fixed oils, polyethylene glycols, glycerin, or propylene
glycol.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in oral
dosage form.. In one
embodiment, the oral dosage form is selected from the group consisting of
tablets, capsules and
gel capsules. In another embodiment, the pharmaceutical composition includes
an enteric
material that substantially prevents dissolution of the tablets, capsules or
gel capsules in a
mammalian stomach. In a preferred embodiment the enteric material is a
coating. The coating
can be acetate phthalate, propylene glycol, sorbitan monoleate, cellulose
acetate trimellitate,
hydroxy propyl methyl cellulose phthalate or cellulose acetate phthalate. In
one embodiment,
the oral dosage form of the pharmaceutical composition includes a penetration
enhancer, e.g., a
penetration enhancer. described herein.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a rectal
dosage form. In
one embodiment, the rectal dosage form is an enema. In another embodiment, the
rectal dosage
form is a suppository.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a
vaginal dosage form.
225
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
In one embodiment, the vaginal dosage form is a suppository. In another
embodiment, the
vaginal dosage form is a foam, cream, or gel.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a
pulmonary or nasal
dosage form. In one embodiment, the iRNA agent is incorporated into a
particle, e.g., a
macroparticle, e.g., a microsphere. The particle can be produced by spray
drying, lyophilization,
evaporation, fluid bed drying, vacuum drying, or a combination thereof. The
microsphere can be
1o formulated as a suspension, a powder, or an implantable solid.
In one aspect, the invention features a spray-dried iRNA agent, e.g., a double-
stranded
iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which
can be processed
into a sRNA agent, or a DNA which encodes an iRNA agent, e.g., a double-
stranded iRNA
agent, or sRNA agent, or precursor thereof) composition suitable for
inhalation by a subject,
including: (a) a therapeutically effective amount of a iRNA agent suitable for
treating a condition
in the subject by inhalation; (b) a pharmaceutically acceptable excipient
selected from the group
consisting of carbohydrates and amino acids; and (c) optionally, a
dispersibility-enhancing
amount of a physiologically-acceptable, water-soluble polypeptide.
In one embodiment, the excipient is a carbohydrate. The carbohydrate can be
selected
from the group consisting of monosaccharides, disaccharides, trisaccharides,
and
polysaccharides. In a preferred embodiment the carbohydrate is a
monosaccharide selected from
the group consisting of dextrose, galactose, mannitol, D-mannose, sorbitol,
and sorbose. In
another preferred embodiment the carbohydrate is a disaccharide selected from
the group
consisting of lactose, maltose, sucrose, and trehalose.
In another embodiment, the excipient is an amino acid. In one embodiment, the
amino
acid is a hydrophobic amino acid. In a preferred embodiment the hydrophobic
amino acid is
selected from the group consisting of alanine, isoleucine, leucine,
methionine, phenylalanine,
proline, tryptophan, and valine. In yet another embodiment the amino acid is a
polar amino acid.
In a preferred embodiment the amino acid is selected from the group consisting
of arginine,
226
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
histidine, lysine, cysteine, glycine, glutamine, serine, threonine, tyrosine,
aspartic acid and
glutamic acid.
In one embodiment, the dispersibility-enhancing polypeptide is selected from
the group
consisting of human serum albumin, a-lactalbumin, trypsinogen, and
polyalanine.
In one embodiment, the spray-dried iRNA agent composition includes particles
having a
mass median diameter (MMD) of less than 10 microns. In another embodiment, the
spray-dried
iRNA agent composition includes particles having a mass median diameter of
less than 5
microns. In yet another embodiment the spray-dried iRNA agent composition
includes particles
having a mass median aerodynamic diameter (MMAD) of less than 5 microns.
In certain other aspects, the invention provides kits that include a suitable
container
containing a pharmaceutical formulation of an iRNA agent, e.g., a double-
stranded iRNA agent,
or sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof). In certain embodiments the individual components
of the
pharmaceutical formulation may be provided in one container. Alternatively, it
may be desirable
to provide the components of the pharmaceutical formulation separately in two
or more
containers, e.g., one container for an iRNA agent preparation, and at least
another for a carrier
compound. The kit may be packaged in a number of different configurations such
as one or
more containers in a single box. The different components can be combined,
e.g., according to
instructions provided with the kit. The components can be combined according
to a method
described herein, e.g., to prepare and administer a pharmaceutical
composition. The kit can also
include a delivery device.
In another aspect, the invention features a device, e.g., an implantable
device, wherein the
device can dispense or administer a composition that includes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA
agent which can be
processed into a sRNA agent, or a DNA which encodes an iRNA agent, e.g., a
double-stranded
iRNA agent, or sRNA agent, or precursor thereof), e.g., a iRNA agent that
silences an
endogenous transcript. In one embodiment, the device is coated with the
composition. In
another embodiment the iRNA agent is disposed within the device. In another
embodiment, the
227
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 141 74-072W01
device includes a mechanism to dispense a unit dose of the composition. In
other embodiments
the device releases the composition continuously, e.g., by diffusion.
Exemplary devices include
stents, catheters, pumps, artificial organs or organ components (e.g.,
artificial heart, a heart
valve, etc.), and sutures.
As used herein, the term "crystalline" describes a solid'having the structure
or
characteristics of a crystal, i.e., particles of three-dimensional structure
in which the plane faces
intersect at definite angles and in which there is a regular internal
structure. The compositions of
the invention may have different crystalline forms. Crystalline forms can be
prepared by a
variety of methods, including, for example, spray drying.
The invention is further illustrated by the following examples, which should
not be
construed as further limiting.
EXAMPLES
Example 1: apoB protein as a therapeutic target for lipid-based diseases
Apolipoprotein B (apoB) is a candidate target gene for the development of
novel
therapies for lipid-based diseases.
Methods described herein can be used to evaluate the efficacy of a particular
siRNA as a
therapeutic tool for treating lipid metabolism disorders resulting elevated
apoB levels. Use of
siRNA duplexes to selectively bind and inactivate the target apoB mRNA is an
approach totreat
these disorders.
Two approaches:
i) Inhibition of apoB in ex-vivo models by transfecting siRNA duplexes
homologous to
human apoB mRNA in a human hepatoma cell line (Hep G2) and monitor the level
of the protein
and the RNA using the Western blotting and RT-PCR methods, respectively. siRNA
molecules
that efficiently inhibit apoB expression will be tested for similar effects in
vivo.
ii) In vivo trials using an apoB transgenic mouse model (apoB 100 Transgenic
Mice,
C57BL/6NTac-TgN (APOB 100), Order Model Ps: 1004-T (hemizygotes), B6
(control)). siRNA
duplexes are designed to target apoB-100 or CETP/apoB double transgenic mice
which express
both cholesteryl ester transfer protein (CETP) and apoB. The effect of the
siRNA on gene
expression in vivo can be measured by monitoring the HDL/LDL cholesterol level
in serum. The
228
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
results of these experiments would indicate the therapeutic potential of
siRNAs to treat lipid-
based diseases, including hypercholesterolemia, HDL/LDL cholesterol imbalance,
familial
combined hyperlipidemia, and acquired hyperlipidemia.
Background Fats, in the form of triglycerides, are ideal for energy storage
because they are
highly reduced and anhydrous. An adipocyte (or fat cell) consists of a
nucleus, a cell membrane,
and triglycerides, and its function is to store triglycerides.
The lipid portion of the human diet consists largely of triglycerides and
cholesterol (and
its esters). These must be emulsified and digested to be absorbed.
Specifically, fats
(triacylglycerols) are ingested. Bile (bile acids, salts, and cholesterol),
which is made in the
liver, is secreted by the gall bladder. Pancreatic lipase digests the
triglycerides to fatty acids, and
also digests di-, and mono-acylglycerols, which are absorbed by intestinal
epithelial cells and
then are resynthesized into triacylglycerols once inside the cells. These
triglycerides and some
cholesterols are combined with apolipoproteins to produce chylomicrons.
Chylomicrons consist
of approximately 95% triglycerides. The chylomicrons transport fatty acids to
peripheral tissues.
Any excess fat is stored in adipose tissue.
Lipid transport and clearance from the blood into cells, and from the cells
into the blood
and the liver, is mediated by the lipoprotein transport proteins. This class
of approximately 17
proteins can be divided into three groups: Apolipoproteins, lipoprotein
processing proteins, and
lipoprotein receptors.
Apolipoproteins coat lipoprotein particles, and include the A-I, A-II, A-IV,
B, CI, CII,
CIII, D, E, Apo(a) proteins. Lipoprotein processing proteins include
lipoprotein lipase, hepatic
lipase, lecithin cholesterol acyltransferase and cholesterol ester transfer
protein. Lipoprotein
receptors include the low density lipoprotein (LDL) receptor, chylomicron-
remnant receptor (the
LDL receptor like protein or LDL receptor related protein - LRP) and the
scavenger receptor.
Lipoprotein Metabolism Since the triglycerides, cholesterol esters, and
cholesterol absorbed
into the small intestine are not soluble in aqueous medium, they must be
combined with suitable
proteins (apolipoproteins) in order to prevent them from forming large oil
droplets. The resulting
lipoproteins undergo a type of metabolism as they pass through the bloodstream
and certain
organs (notably the liver).
229
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Also synthesized in the liver is high density lipoprotein (HDL), which
contains the
apoproteins A- 1, A-2, C-1, and D; HDL collects cholesterol from peripheral
tissues and blood
vessels and returns it to the liver. LDL is taken up by specific cell surface
receptors into an
endosome, which fuses with a lysosome where cholesterol ester is converted to
free cholesterol.
The apoproteins (including apo B-100) are digested to amino acids. The
receptor protein is
recycled to the cell membrane.
The free cholesterol formed by this process has two fates. First, it can move
to the
endoplasmic reticulum (ER), where it can inhibit HMG-CoA reductase, the
synthesis of HMG-
CoA reductase, and the synthesis of cell surface receptors for LDL. Also in
the ER, cholesterol
lo can speed up the degradation of HMG-CoA reductase. The, free cholesterol
can also be
converted by acyl-CoA and acyl transferase (ACAT) to cholesterol esters, which
form oil
droplets.
ApoB is the major apolipoprotein of chylomicrons of very low density
lipoproteins
(VLDL, which carry most of the plasma triglyceride) and low density
lipoprotein (LDL, which
carry most of the plasma cholesterol). ApoB exists in human plasma in two
isoforms, apoB-48
and apoB-100.
ApoB-100 is the major physiological ligand for the LDL receptor. The ApoB
precursor
has 4563 amino acids, and the mature apoB-100 has 4536 amino acid residues.
The LDL-binding
domain of ApoB-100 is proposed to be located between residues 3129 and 3532.
ApoB-100 is
synthesized in the liver and is required for the assembly of very low density
lipoproteins VLDL
and for the preparation of apoB-100 to transport triglycerides (TG) and
cholesterol from the liver
to other tissues. ApoB-100 does not interchange between lipoprotein particles,
as do the other
lipoproteins, and it is found in IDL and LDL particles. After the removal of
apolipoproteins A, E
and C, apoB is incorporation into VLDL by hepatocytes. ApoB-48 is present in
chylomicrons
and plays an essential role in the intestinal absorption of dietary fats. ApoB-
48 is synthesized in
the small intestine. It comprises the N-terminal 48% of apoB-100 and is
produced by a
posttranscriptional apoB-100 mRNA editing event at codon 2153 (C to U). This
editing event is
a product of the apoBEC-lb enzyme, which is expressed in the intestine. This
editing event
creates a stop codon instead of a glutamine codon, and therefore apoB-48,
instead of apoB- 100 is
3o expressed in the intestine (apoB-100 is expressed in the liver).
230
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
There is also strong evidence that plasma apoB levels maybe a better index of
the risk of
coronary artery disease (CAD) than total or LDL cholesterol levels. Clinical
studies have
demonstrated the value of measuring apoB in hypertriglyceridemic,
hypercholesterolemic and
normalipidemic subjects.
231
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Table 4. Reference Range Lipid level in the Blood
Lipid Range (mmols/ L)
Plasma Cholesterol 3.5-6.5
Low density lipoprotein 1.55-4.4
Very low density lipoprotein 0.128-0.645
High density lipoprotein/ triglycerides 0.5-2.1
Total lipid 4.0-10 / L
Molecular genetics of lipid metabolism in both humans and induced mutant mouse
models
Elevated plasma levels of LDL and apoB are associated with a higher risk for
atherosclerosis and
coronary heart disease, a leading cause of mortality. ApoB is the mandatory
constituent of LDL
particles. In addition to its role in lipoprotein metabolism, apoB has also
been implicated as a
factor in male infertility and fetal development. Furthermore, two
quantitative trait loci
regulating plasma apoB levels have been discovered, through the use of
transgenic mouse
1o models. Future experiments will facilitate the identification of human
orthologous genes
encoding regulators of plasma apoB levels. These loci are candidate
therapeutic targets for
human disorders characterized by altered plasma apoB levels. Such disorders
include non-apoB
linked hypobetalipoproteinemia and familial combined hyperlipidemia. The
identification of
these genetic loci would also reveal possible new pathways involved in the
regulation of apoB
secretion, potentially providing novel sites for pharmacological therapy.
Diseases and Clinical Pharmacology Familial combined hyperlipemia (FCHL)
affects an
estimated one in 10 Americans. FCHL can cause premature heart disease.
Familial Hypercholesterolemia (high level of apo B) A common genetic disorder
of lipid
metabolism. Familial hypercholesterolemia is characterized by elevated serum
TC in association
with xanthelasma, tendon and tuberous xanthomas, accelerated atherosclerosis,
and early death
from myocardial infarction (MI). It is caused by absent or defective LDL cell
receptors,
resulting in delayed LDL clearance, an increase in plasma LDL levels, and an
accumulation of
LDL cholesterol in macrophages over joints and pressure points, and in blood
vessels.
232
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Atherosclerosis (high level of apo B) Atherosclerosis develops as a deposition
of cholesterol and
fat in the arterial wall due to disturbances in lipid transport and clearance
from the blood into
cells and from the cells to blood and the liver.
Clinical studies have demonstrated that elevation of total cholesterol (TC),
low- density
lipoprotein cholesterol (LDL-C) and apoB-100 promote human atherosclerosis.
Similarly,
decreased levels of high - density lipoprotein cholesterol (HDL-C) are
associated with the
development of atherosclerosis.
ApoB may be a factor in the genetic cause of high cholesterol.
The risk of coronary artery disease (CAD) (high level of apo B) Cardiovascular
disease,
1o including coronary heart disease and stroke, is a leading cause of death
and disability. The major
risk factors include age, gender, elevated low-density lipoprotein cholesterol
blood levels,
decreased high-density lipoprotein cholesterol levels, cigarette smoking,
hypertension, and
diabetes. Emerging risk factors include elevated lipoprotein (a), remnant
lipoproteins, and C
reactive protein. Dietary intake, physical activity and genetics also impact
cardiovascular risk.
Hypertension and age are the major risk factors for stroke.
Abetalipoproteinemia, an inherited human disease characterized by a near-
complete
absence of apoB-containing lipoproteins in the plasma, is caused by mutations
in the gene for
microsomal triglyceride transfer protein (MTP).
Model for human atherosclerosis (Lipoprotein A transgenic mouse) Numerous
studies have
demonstrated that an elevated plasma level of lipoprotein(a) (Lp(a)) is a
major independent risk
factor for coronary heart disease (CHD). Current therapies, however, have
little or no effect on
apo(a) levels and the homology between apo(a) and plasminogen presents
barriers to drug
development. Lp(a) particles consist of apo(a) and apoB-100 proteins, and they
are found only
in primates and the hedgehog. The development of LPA transgenic mouse requires
the creation
of animals that express both human apoB and apo(a) transgenes to achieve
assembly of LP(a).
An atherosclerosis mouse model would facilitate the study of the disease
process and factors
influencing it, and further would facilitate the development of therapeutic or
preventive agents.
There are several strategies for gene-oriented therapy. For example, the
missing or non-
functional gene can be replaced, or unwanted gene activity can be inhibited.
233
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Model for lipid Metabolism and Atherosclerosis DNX Transgenic Sciences has
demonstrated
that both CETP/ApoB and ApoB transgenic mice develop atherosclerotic plaques.
Model for apoB-100 overexpression The apoB-100 transgenic mice express high
levels of
human apoB-100. They consequently demonstrate elevated serum levels of LDL
cholesterol.
After 6 months on a high-fat diet, the mice develop significant foam cell
accumulation under the
endothelium and within the media, as well as cholesterol crystals and fibrotic
lesions.
lo Model for Cholestetyl ester transfer protein over expression The apoB-100
transgenic mice
express the human enzyme, CETP, and consequently demonstrate a dramatically
reduced level of
serum HDL cholesterol.
Model for apoB-100 and CETP overexpression The apoB-100 transgenic mice
express both
CETP and apoB-100, resulting in mice with a human like serum HDL/LDL
distribution.
Following 6 months on a high-fat diet these mice develop significant foam cell
accumulation
underlying the endothelium and within the media, as well as cholesterol
crystals and fibrotic
lesions.
ApoB100 Transgenic Mice (Order Model #'s:1004-T (hemizygotes), B6 (control))
These mice express high levels of human apoB-100, resulting in mice with
elevated serum levels
of LDL cholesterol. These mice are useful in identifying and evaluating
compounds to reduce
elevated levels of LDL cholesterol and the risk of atherosclerosis. When fed a
high fat
cholesterol diet, these mice develop significant foam cell accumulation
underly the endothelium
and within the media, and have significantly more complex atherosclerotic
lesions than control
animals.
Double Transgenic Mice, CETP/ApoB100 (Order Model #: 1007-TT) These mice
express both
CETP and apoB-100, resulting in a human-like serum HDL/LDL distribution. These
mice are
useful for evaluating compounds to treat hypercholesterolemia or HDL/LDL
cholesterol
imbalance to reduce the risk of developing atherosclerosis. When fed a high
fat high cholesterol
234
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
diet, these mice develop significant foam cell accumulation underlying the
endothelium and
within the media, and have significantly more complex atherosclerotic lesions
than control
animals.
ApoE gene knockout mouse Homozygous apoE knockout mice exhibit strong
hypercholesterolemia, primarily due to elevated levels of VLDL and IDL caused
by a defect in
lipoprotein clearance from plasma. These mice develop atherosclerotic lesions
which progress
with age and resemble human lesions (Zhang et al., Science 258:46-71, 1992;
Plump et al., Cell
71:343-353, 1992; Nakashima et al., Arterioscler Thromp. 14:133-140, 1994;
Reddick et al.,
1o Arterioscler Tromb. 14:141-147, 1994). These mice are a promising model for
studying the
effect of diet and drugs on atherosclerosis.
Low density lipoprotein receptor (LDLR) mediates lipoprotein clearance from
plasma
through the recognition of apoB and apoE on the surface of lipoprotein
particles. Humans, who
lack or have a decreased number of the LDL receptors, have familial
hypercholesterolemia and
develop CHD at an early age.
ApoE Knockout Mice (Order Model #: APOE-M) The apoE knockout mouse was created
by
gene targeting in embryonic stem cells to disrupt the apoE gene. ApoE, a
glycoprotein, is a
structural component of very low density lipoprotein (VLDL) synthesized by the
liver and
intestinally synthesized chylomicrons. It is also a constituent of a subclass
of high density
lipoproteins (HDLs) involved in cholesterol transport activity among cells.
One of the most
important roles of apoE is to mediate high affinity binding of chylomicrons
and VLDL particles
that contain apoE to the low density lipoprotein (LDL) receptor. This allows
for the specific
uptake of these particles by the liver which is necessary for transport
preventing the
accumulation in plasma of cholesterol-rich remnants. The homozygous
inactivation of the apoE
gene results in animals that are devoid of apoE in their sera. The mice appear
to develop
normally, but they exhibit five times the normal serum plasma cholesterol and
spontaneous
atherosclerotic lesions. This is similar to a disease in people who have a
variant form of the
apoE gene that is defective in binding to the LDL receptor and are at risk for
early development
of atherosclerosis and increased plasma triglyceride and cholesterol levels.
There are indications
that apoE is also involved in immune system regulation, nerve regeneration and
muscle
235
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
differentiation. The apoE knockout mice can be used to study the role of apoE
in lipid
metabolism, atherogenesis, and nerve injury, and to investigate intervention
therapies that
modify the atherogenic process.
Apoe4 Targeted Replacement Mouse (Order Model #: 001549-M) ApoE is a plasma
protein
involved in cholesterol transport, and the three human isoforms (E2, E3, and
E4) have been
associated with atherosclerosis and Alzheimer's disease. Gene targeting of 129
ES cells was
used to replace the coding sequence of mouse apoE with human APOE4 without
disturbing the
murine regulatory sequences. The E4 isoform occurs in approximately 14% of the
human
population and is associated with increased plasma cholesterol and a greater
risk of coronary
artery disease. The Taconic apoE4 Targeted Replacement model has normal plasma
cholesterol
and triglyceride levels, but altered quantities of different plasma
lipoprotein particles. This
model also has delayed plasma clearance of cholesterol-rich lipoprotein
particles (VLDL), with
only half the clearance rate seen in the apoE3 Targeted Replacement model.
Like the apoE3
model, the apoE4 mice develop altered plasma lipoprotein values and
atherosclerotic plaques on
an atherogenic diet. However, the atherosclerosis is more severe in the apoE4
model, with larger
plaques and cholesterol apoE and apoB-48 levels twice that seen in the apoE3
model. The
Taconic apoE4 Targeted Replacement model, along with the apoE2 and apoE3
Targeted
Replacement Mice, provide an excellent tool for in vivo study of the human
apoE isoforms.
CETP Transgenic Mice (Order Model #: 1003-T) These animals express the human
plasma
enzyme, CETP, resulting in mice with a dramatic reduction in serum HDL
cholesterol. The mice
can be useful in identifying and evaluating compounds that increase the levels
of HDL
cholesterol for reducing the risk of developing atherosclerosis
Transgene/Promoter: human apolipoprotein A-I These mice produce mouse HDL
cholesterol
particles that contain human apolipoprotein A-I. Transgenic expression is life-
long in both sexes
(Biochemical Genetics and Metabolism Laboratory, Rockefeller University, NY
City).
A Mouse Model for Abetalipoproteinemia Abetalipoproteinemia, an inherited
human disease
characterized by a near-complete absence of apoB-containing lipoproteins in
the plasma, is
caused by mutations in the gene for microsomal triglyceride transfer protein
(MTP). Gene
236
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072WO I
targeting was used to knock out the mouse MTP gene (Mttp). In heterozygous
knockout mice
(Mttp+i ), the MTP mRNA, protein, and activity levels were reduced by 50% in
both liver and
intestine. Recent studies with heterozygous MTP knockout mice have suggested
that half-
normal levels of MTP in the liver reduce apoB secretion. They hypothesized
that reduced apoB
secretion in the setting of half-normal MTP levels might be caused by a
reduced MTP:apoB ratio
in the endoplasmic reticulum, which would reduce the number of apoB-MTP
interactions. If
this hypothesis were true, half-normal levels of MTP might have little impact
on lipoprotein
secretion in the setting of half-normal levels of apoB synthesis (since the
ratio of MTP to apoB
would not be abnormally low) and might cause an exaggerated reduction in
lipoprotein secretion
in the setting of apoB overexpression (since the ratio of MTP to apoB would be
even lower). To
test this hypothesis, they examined the effects of heterozygous MTP deficiency
on apoB
metabolism in the setting of normal levels of apoB synthesis, half-normal
levels of apoB
synthesis (heterozygous Apob deficiency), and increased levels of apoB
synthesis (transgenic
overexpression of human apoB). Contrary to their expectations, half-normal
levels of MTP
reduced plasma apoB-100 levels to the same extent (-25-35%) at each level of
apoB synthesis.
In addition, apoB secretion from primary hepatocytes was reduced to a
comparable extent at
each level of apoB synthesis. Thus, these results indicate that the
concentration of MTP within
the endoplasmic reticulum, rather than the MTP:apoB ratio, is the critical
determinant of
lipoprotein secretion. Finally, heterozygosity for an apoB knockout mutation
was found to lower
plasma apoB-100 levels more than heterozygosity for an MTP knockout allele.
Consistent with
that result, hepatic triglyceride accumulation was greater in heterozygous
apoB knockout mice
than in heterozygous MTP knockout mice. Cre/loxP tissue-specific recombination
techniques
were also used to generate liver-specific Mttp knockout mice. Inactivation of
the Mttp gene in
the liver caused a striking reduction in very low density lipoprotein (VLDL)
triglycerides and
large reductions in both VLDL/low density lipoproteins (LDL) and high density
lipoprotein
cholesterol levels. Histologic studies in liver-specific knockout mice
revealed moderate hepatic
steatosis. Currently being tested is the hypothesis that accumulation of
triglycerides in the liver
renders the liver more susceptible to injury by a second insult (e.g.,
lipopolysaccharide).
Human apo B (apolipoprotein B) Transgene mice show apo B locus may have a
causative role
male infertility The fertility of apoB (apolipoprotein B) (+/-) mice was
recorded during the
course of backcrossing (to C57BL/6J mice) and test mating. No apparent
fertility problem was
237
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
observed in female apoB (+/-) and wild-type female mice, as was documented by
the presence of
vaginal plugs in female mice. Although apoB (+/-) mice mated normally, only
40% of the
animals from the second backcross generation produced any offspring within the
4-month test
period. Of the animals that produced progeny, litters resulted from < 50% of
documented
matings. In contrast, all wild-type mice (616--i. e., 100%) tested were
fertile. These data suggest
genetic influence on the infertility phenotype, as a small number of male
heterozygotes were not
sterile. Fertilization in vivo was dramatically impaired in male apoB (+/-)
mice. 74% of eggs
examined were fertilized by the sperm from wild-type mice, whereas only 3% of
eggs examined
were fertilized by the sperm from apoB (+/-) mice. The sperm counts of apoB
(+/-) mice were
mildly but significantly reduced compared with controls. However, the
percentage of motile
sperm was markedly reduced in the apoB (+/-) animals compared with that of the
wild-type
controls. Of the sperm from apoB (+/-) mice, 20% (i.e., 4.9% of the initial
20% motile sperm)
remained motile after 6 hr of incubation, whereas 45% (i.e., 33.6% of the
initial 69.5%) of the
motile sperm retained motility in controls after this time. In vitro
fertilization yielded no
fertilized eggs in three attempts with apo B (+/-) mice, while wild-type
controls showed a
fertilization rate of 53%. However, sperm from apoB (+/-) mice fertilized 84%
of eggs once the
zona pellucida had been removed. Numerous sperm from apoB (+/-) mice were seen
binding to
zona-intact eggs. However, these sperm lost their motility when observed 4-6
hours after
binding, showing that sperm from apoB (+/-) mice were unable to penetrate the
zona pellucida
but that the interaction between sperm and egg was probably not direct. Sperm
binding to zona-
free oocytes'was abnormal. In the apoB (+/-) mice, sperm binding did not
attenuate, even after
pronuclei had clearly formed, suggesting that apoB deficiency results in
abnormal surface
interaction between the sperm and egg.
Knockout of the mouse apoB gene resulted in embryonic lethality in
homozygotes,
protection against diet-induced hypercholesterolemia in heterozygotes, and
developmental
abnormalities in mice.
Model of insulin resistance, dyslipidemia & overexpression of human apoB It
was shown that
the livers of apoB mice assemble and secrete increased numbers of VLDL
particles.
238
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Example 2. Treatment of Diabetes Type-2 with iRNA
Introduction The regulation of hepatic gluconeogenesis is an important process
in the
adjustment of the blood glucose level. Pathological changes in the glucose
production of the
liver are a central characteristic in type-2-diabetes. For example, the
fasting hyperglycemia
observed in patients with type-2-diabetes reflects the lack of inhibition of
hepatic
gluconeogenesis and glycogenolysis due to the underlying insulin resistance in
this disease.
Extreme conditions of insulin resistance can be observed for example in mice
with a liver-
specific insulin receptor knockout ('LIRKO'). These mice have an increased
expression of the
two rate-limiting gluconeogenic enzymes, phosphoenolpyruvate carboxykinase
(PEPCK) and the
glucose-6-phosphatase catalytic subunit (G6Pase). Insulin is known to repress
both PEPCK and
G6Pase gene, expression at the transcriptional level and the signal
transduction involved in the
regulation of G6Pase and PEPCK gene expression by insulin is only partly
understood. While
PEPCK is involved in a very early step of hepatic gluconeogenesis (synthesis
of
phosphoenolpyruvate from oxaloacetate), G6Pase catalyzes the terminal step of
both,
gluconeogenesis and glycogenolysis, the cleavage of glucose-6-phosphate into
phosphate and
free glucose, which is then delivered into the blood stream.
The pharmacological intervention in the regulation of expression of PEPCK and
G6Pase
can be used for the treatment of the metabolic aberrations associated with
diabetes. Hepatic
glucose production can be reduced by an iRNA-based reduction of PEPCK and
G6Pase
enzymatic activity in subjects with type-2-diabetes.
Targets for iRNA
Glucose-6-phosphatase (G6Pase)
G6Pase mRNA is expressed principally in liver and kidney, and in lower amounts
in the
small intestine. Membrane-bound G6Pase is associated with the endoplasmic
reticulum. Low
activities have been detected in skeletal muscle and in astrocytes as well.
G6Pase catalyzes the terminal step in gluconeogenesis and glycogenolysis. The
activity
of the enzyme is several fold higher in diabetic animals and probably in
diabetic humans.
Starvation and diabetes cause a 2-3-fold increase in G6Pase activity in the
liver and a 2-4-fold
increase in G6Pase mRNA.
239
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Phosphoenolpyruvate carboxykinase (PEPCK)
Overexpression of PEPCK in mice results in symptoms of type-2-diabetes
mellitus.
PEPCK overexpression results in a metabolic pattern that increases G6Pase mRNA
and results in
a selective decrease in insulin receptor substrate (IRS)-2 protein, decreased
phosphatidylinositol
3-kinase activity, and reduced ability of insulin to suppress gluconeogenic
gene expression.
Table 5. Other targets to inhibit hepatic glucose production
Target Comment
FKHR good evidence for antidiabetic phenotype
(Nakae et al., Nat Genetics 32:245(2002)
Glucagon
Glucagon receptor
Glycogen phosphorylase
regulates the cAMP response (and
PGC-1 (PPAR-Gamma probably the PKB/FKHR-regulation) on
Coactivator) PEPCK/G6Pase
Fructose-1, 6-bisphosphatase
Glucose-6-phospate translocator
Glucokinase inhibitory
regulatory protein
lo Materials and Methods
Animals: BKS.Cg-m +/+ Lepr db mice, which contain a point mutation in the
leptin receptor
gene are used to examine the efficacy of iRNA for the targets listed above.
BKS.Cg-m +/+ Lepr db are available from the Jackson Laboratory (Stock Number
000642). These animals are obese at 3-4 weeks after birth, show elevation of
plasma insulin at
10 to 14 days, elevation of blood sugar at 4 to 8 weeks, and uncontrolled rise
in blood sugar.
Exogenous insulin fails to control blood glucose levels and gluconeogenic
activity increases.
The following numbers of male animals (age>12 weeks) could be tested with the
following iRNAs:
PEPCK, 2 sequences, 5 animals per sequence
G6Pase, 2 sequences, 5 animals per sequence
1 nonspecific sequence, 5 animals
1 control group (only injected, no siRNA), 5 animals
1 control group (not injected, no siRNA), 5 animals
240
CA 02521464 2011-06-30
51912-8
Reagents: Necessary reagents would ideally include a Glucometer Elite XL
(Bayer, Pittsburgh,
PA) for glucose quantification, and an Insulin Radioimmunoassay (RIA) kit
(Amersham,
Piscataway, NJ) for insulin quanitation.
Assays:
G6P enzyme assays and PEPCK enzyme assays are used to measure the activity of
the enzymes.
Northern blotting is used to detect levels of G6Pase and PEPCK mRNA. Antibody-
based
techniques (e.g., immunoblotting, inununofluorescence) are used to detect
levels of G6Pase and
PEPCK protein. Glycogen staining is used to detect levels of glycogen in the
liver. Histological
lo analysis is performed to analyze tissues.
Gene information:
G6Pase GenBank No.: NM 008061,Mus musculus glucose-6-phosphatase, catalytic
(G6pc),
mRNA 1..2259, ORF 83..1156;
GenBank No: U00445,Mus musculus glucose-6-phosphatase mRNA, complete cds
1..2259,
ORF 83.1156
GenBank No: BC013448
PEPCK
GenBank No: NM 011044, Mus musculus phosphoenolpyruvate carboxyidnase 1,
cytosolic
(Pckl), mRNA.1..2618, ORF 141..2009
GenBank No: AF009605.1
Administration of iRNA:
iRNA corresponding to the genes described above could be administered to mice
with
hydrodynamic injection. One control group of animals would be treated with
Metformin as a
positive control for reduction in hepatic glucose levels.
Experimental Protocol
Mice could be housed in a facility in which there is light from 7:00 AM to
7:00 PM.
Mice would be fed ad libidum from 7:00 PM to 7:00 AM and fast from 7:00 AM to
7:00 PM.
* Trade-mark
241
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
Day 0: 7:00 PM: Approximately 100 l blood would be drawn from the tail. Serum
could be
isolated to measure glucose, insulin, HbAlc (EDTA-blood), glucagon, FFAs,
lactate,
corticosterone, serum triglycerides.
Day 1-7: Blood glucose could be measured daily at 8:00 AM and 6:00 PM (approx.
3-5 l;
measured with a Haemoglucometer)
Day 8: Blood glucose could be measured daily at 8:00 AM and 6:00 PM. iRNA
would be
injected between 10:00 AM and 2:00 PM
Day 9-20: Blood glucose could be measured daily at 8:00 AM and 6:00 PM.
Day 21: Mice could be sacrificed after 10 hours of fasting.
Blood would be isolated. Glucose, insulin, HbAlc (EDTA-blood), glucagon, FFAs,
lactate,
corticosterone, serum triglycerides would be measured. Liver tissue would be
isolated for
histology, protein assays, RNA assays, glycogen quantitation, and enzyme
assays.
Example 3: Inhibition of Glucose-6-Phosphatase iRNA in vivo
iRNA targeted to the Glucose-6-Phosphatase (G6P) gene was used to examine the
effects
of inhibition of G6P expression on glucose metabolism in vivo.
Female mice, 10 weeks of age, strain BKS.Cg-m +/+ Lepr db (The Jackson
Laboratory)
were used for in vivo analysis of enzymes of the hepatic glucose production.
Mice were housed
under conditions where it was light from 6:30 am to 6:30 pm. Mice were fed (ad
libidum) during
the night period and fasted during the day period.
On day 1, approximately l00 l of blood was collected from test animals by
puncturing
the retroorbital plexus. On days 1-7, blood glucose was measured in blood
obtained from tail
veins (approximately 3-5 l) using a Glucometer (Elite XL, Bayer). Blood
glucose was sampled
daily at 8 am and 6 pm.
On day 7 at approximately 2pm, GL3 plasmid (10 g) and siRNAs (100 gg G6Pase
specific, Renilla nonspecific or no siRNA control) were delivered to animals
using
hydrodynamic coinjection.
242
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
On day 8, GL3 expression was analyzed by injection of luceferin (3 mg) after
anaesthesia
with avertin and imaging. This was done to control for successful hydrodynamic
delivery.
On days 8-10, blood glucose was measured in blood obtained from tail veins
(approximately 3-5 ml) using a Glucometer (Elite XL, Bayer).
On day 10, mice were sacrificed after 10 hours of fasting. Blood and liver
were isolated
from sacrificed animals.
Table 6 lists blood glucose levels (mg/dl) for mice injected with GL3 plasmid
and
G6Pase iRNA (G6P4), Renilla nonspecific iRNA (RL), or no iRNA (no). Days on
which nucleic
acids were injected are shaded.
Table 6. Blood glucose levels in mice
plasmid GL3 GL3 GL3 GL3 GL3 GL3 GL3 GL3
sIRNA G6P4 G6P4 G6P4 no G6P4 RL RL no
mouse 03 mouse 04 mouse 05 mouse 07 mouse 09 mouse 14 mouse 15 mouse 17
day BG BG BG BG BG BG BG BG
1 512 250 537 241 196 275 538 437
2 555 437 339 556 408 315 524 386
3 483 446 356 567 283 491 600 459
4 579 543 552 423 404 457 548 375
5 600 501 600 277 198 441 533 430
6 464 600 408 454 461 412 490 301
7 214 201 245 -
260 494 600 429 injeclion
8 600 566 246 521 277 600 576 404
9, 350 448 438 536 600 459 injection
10 369 600 446
average
day 1to 6 532 463 465 420 325 399 539 398
Table 7 lists average blood glucose levels (mg/dl) on days 1-6 or day 7 for
mice injected
with GL3 plasmid and G6Pase iRNA (G6P4), Renilla nonspecific iRNA (RL), no
iRNA (no), or
for mice that were not injected, or for which injection failed.
Table 7. Average blood glucose levels
G6P4 RL no RL and no (combined)
mouse 03,04,05,09 mouse 14, 15 mouse 07, 17 mouse 14, 15, 07, 17
average (dl-6) 446 469 409 439
stddev(dl-6) 124 96 101 101
average (d7) 230 547 369 458
stddev (d7) 27 122
243
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Attorney's Docket No.: 14174-072W01
FIGs. 6A, 6B, and 6C show graphs depicting blood glucose levels of animals
injected
with control or no siRNA, G6Pase RNA, or non-injected mice (respectively) at
days 1-6 and day
7. FIG. 7 contains a graph of average blood glucose levels for mice injected
with G6Pase RNA
(solid line) and mice injected with, Renilla nonspecific iRNA (RL) or no iRNA
(no) (dashed
line).
Table 8 lists average blood glucose levels for mice injected with G6Pase iRNA
or Renilla
nonspecific iRNA (RL) and no iRNA.
Table 8. Average blood glucose levels
RNA G6P4 RL and no (combined)
mouse 03,04,05,09 mouse 14, 15, 07,17
average stddev average stddev
day
1 374 176 373 139
2 435 90 445 114
3 392 90 529 65
4 520 79 451 73
5 475 190, 420 106
6 483 82 414 82
7 230 27 458 122.
8 422 187 525 87
9 412 54 532 71
10 472 118
Example 4: Selected Palindromic Sequences
Tables 9-14 below provide selected palindromic sequences from the following
genes: human
ApoB, human glucose-6-phosphatase, rat glucose-6-phosphatase, (3-catenin, and
hepatitis C virus
(HCV).
244
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
Table 9. Selected palindromic sequences from human ApoB
Source Start End Match Start End # B
Index Index Index Index
SEQ ID 1 ggccattccagaagggaag 509 528 SEQ ID 1004 cttccgttctgtaatggcc 5795 5814
19
NO: NO:
SEQ ID 2 tgccatctcgagagttcca 1099 1118 SEQ ID 1005 tggaactctctccatggca 10876
10895 18
NO: NO:
SEQ ID 3 catgtcaaacactttgtta 7056 7075 SEQ ID 1006 taacaaattccttgacatg 7358
7377 181
NO: NO:
SEQ ID 4 tttgttataaatcttattg 7068 7087 SEQ ID 1007 caataagatcaatagcaaa 8990
9009 18
NO: NO:
SEQ ID 5 tctggaaaagggtcatgga 8880 8899= SEQ ID 1008 tccatgtcccatttacaga 11356
11375 18
NO: NO:
SEQ ID 6 cagctcttgttcaggtcca 10900 10919 SEQ ID 1009 tggacctgcaccaaagctg 13952
13971 18
NO: NO: 11
SEQ ID 7 ggaggttccccagctctgc 356 375 SEQ ID 1010 gcagccctgggaaaactcc 6447 6466
17
NO: NO:
SEQ ID 8 ctgttttgaagactctcca 1081 1100 SEQ ID 1011 tggagggtagtcataacag 10327
10346 17
NO: NO:
SEQ ID 9 agtggctgaaacgtgtgca 1297 1316 SEQ ID 1012 tgcagagctttctgccact 13508
13527 17
NO: NO:
SEQ ID 10 ccaaaatagaagggaatct 2068 2087 SEQ ID 1013 agattcctttgccttttgg 4000
4019 1171
NO: NO:
SEQ ID 11 tgaagagaagattgaattt 3620 3639 SEQ ID 1014 aaattctcttttcttttca 9212,
9231 17
NO: NO:
SEQ ID 12 agtggtggcaacaccagca 1230 1249 SEQ ID 1015 tgctagtgaggccaacact 10649
10668 17
NO: NO:
SEQ ID 13 aaggctccacaagtcatca 5950 5969 SEQ ID 1016 tgatgatatctggaacctt 10724
10743 17
NO: NO:
SEQ ID 14 gtcagccaggtttatagca 7725 7744 SEQ ID 1017 tgctaagaaccttactgac 7781'
7800 1171
NO: NO:
SEQ ID 15 tgatatctggaaccttgaa 10727 10746 SEQ ID 1018 ttcactgttcctgaaatca 7863
7882 17
NO: NO:
SEQ ID 16 gtcaagttgagcaatttct 13423 13442 SEQ ID 1019 agaaaaggcacaccttgac
11072 11091 17
NO: NO:
SEQ ID 17 atccagatggaaaagggaa 13480 13499 SEQ ID 1020 ttccaatttccctgtggat 3680
3699 17
NO: NO: 11
SEQ ID 18 atttgtttgtcaaagaagt 543 562 SEQ ID 1021 acttcagagaaatacaaat 11401
11420 46
NO: NO:
SEQ ID 19 ctggaaaatgtcagcctgg 204 223 SEQ ID 1022 ccagacttccgtttaccag 8235
8254 26
NO: NO:
SEQ ID 20 accaggaggttcttcttca 1729 1748 SEQ ID 1023 tgaagtgtagtctcctggt 5089
5108 26
NO: NO:
SEQ ID 21 aaagaagttctgaaagaat 1956 1975 SEQ ID 1024 attccatcacaaatccttt 9661
9680 26
NO: NO:
SEQ ID 122,gctacagcttatggctcca 3570 3589 SEQ ID 1025 tggatctaaatgcagtagc 11623
11642 26
NO: NO:
SEQ ID 23 atcaatattgatcaatttg 6414 6433 SEQ ID 1026 caaagaagtcaagattgat 4553
4572 26
NO: NO:
SEQ ID 24 gaattatcttttaaaacat 7326 7345 SEQ ID 1027 atgtgttaacaaaatattc 11494
11513 26
NO: NO:
SEQ ID 25 cgaggcccgcgctgctggc 130 149 SEQ ID 1028 gccagaagtgagatcctcg 3507
3526 16
NO: NO:
SEQ ID 26 acaactatgaggctgagag 71 290 SEQ ID 1029 ctctgagcaacaaatttgt 10309
10328 16
NO: NO:
245
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
SEQ ID 27 gctgagagttccagtggag 282 301 SEQ ID 1030 ctccatggcaaatgtcagc 10885
10904 1161
NO: NO:
SEQ ID 28 tgaagaaaaccaagaactc 48 67 SEQ ID 1031 gagtcattgaggttcttca 4929 4948
1 6
NO: NO:
SEQ ID 29 cctacttacatcctgaaca 558 577 SEQ ID 1032 tgttcataagggaggtagg 12766
12785 1 6
NO: NO:
SEQ ID 30 ctacttacatcctgaacat 559 578 SEQ ID 1033 atgttcataagggaggtag 12765
12784 16
NO: NO:
SEQ ID 31 gagacagaagaagccaagc 615 634 SEQ ID 1034 gcttggttttgccagtctc 2459
2478 16
NO: NO:
SEQ ID 132,cactcactttaccgtcaag 671 690 SEQ ID 1035 cttgaacacaaagtcagtg 6000
6019 16
NO: NO:
SEQ ID 33 ctgatcagcagcagccagt 822 841 SEQ ID 1036 actgggaagtgcttatcag 5237
5256 16
NO: NO:
SEQ ID 34 actggacgctaagaggaag 854 873 SEQ ID 1037 cttccccaaagagaccagt 2890
2909 Ifl6
NO: NO:
246
CA 02521464 2005-10-04
WO 2004/091515 PCT/US2004/011255
SEQ ID NO: 35 agaggaagcatgtggcaga 865 884 SEQ ID 1038 tctggcatttactttctct 5921
5940 16
NO:
SEQ ID NO:36 tgaagactctccaggaact 1087 1106 SEQ ID 1039 agttgaaggagactattca
7216 7235 16
NO:
SEQ ID NO: 37 ctctgagcaaaatatccag 1121 1140 SEQ ID 1040 ctggttactgagctgagag
1161 1180 16
NO:
SEQ ID NO:38 atgaagcagtcacatctct 1189 1208 SEQ ID 1041 agagctgccagtccttcat
10016 10035 16
NO:
SEQ ID NO: 39 ttgccacagctgattgagg 1209 1228 SEQ ID 1042 cctcctacagtggtggcaa
4222 4241 16
NO:
SEQ ID NO:40 agctgattgaggtgtccag 1216 1235 SEQ ID 1043 ctggattccacatgcagct
11847 11866 16
NO:
SEQ ID NO:41 tgctccactcacatcctcc 1278 1297 SEQ ID 1044 ggaggctttaagttcagca
7601 7620 16
NO:
SEQ ID NO:42 tgaaacgtgtgcatgccaa 1303 1322 SEQ ID '1045 ttgggagagacaagtttca
6500 6519 16
NO:
SEQ ID NO: 43 gacattgctaattacctga 1503 1522 SEQ ID 1046 tcagaagctaagcaatgtc
7232 7251 16
NO:
SEQ ID NO: 44 ttcttcttcagactttcct 1738 1757 SEQ ID 1047 aggagagtccaaattagaa
8498 8517 16
NO:
SEQ ID NO:45 ccaatatcttgaactcaga 1903 1922 SEQ ID 1048 tctgaattcattcaattgg
6485 6504 16
NO:
SEQ ID NO:46 aaagttagtgaaagaagtt 1946 1965 SEQ ID 1049 aactaccctcactgccttt
2132 2151 16
NO:
SEQ ID NO:47 aagttagtgaaagaagttc 1947 1966 SEQ ID 1050 gaacctctggcatttactt
5916 5935 16
NO:
SEQ ID NO: 48 aaagaagttctgaaagaat 1956 1975 SEQ ID 1051 attctctggtaactacttt
5482 5501" 16
NO:
SEQ ID NO:49 tttggctataccaaagatg 322 2341 SEQ ID 1052 catcttaggcactgacaaa 4997
5016 11
NO:
SEQ ID NO: 50 tgttgagaagctgattaaa 2381 2400 SEQ ID 1053 tttagccatcggctcaaca
5700 5719 16
NO:
SEQ ID NO:51 caggaagggctcaaagaat 2561 2580 SEQ ID 1054 attcctttaacaattcctg
9492 9511 16
NO:
SEQ ID NO: 52 aggaagggctcaaagaatg 2562 2581 SEQ ID 1055 cattcctttaacaattcct
9491 9510 16
NO:
SEQ ID NO: 53 gaagggctcaaagaatgac 2564 2583 SEQ ID 1056 gtcagtcttcaggctcttc
7914 7933 16
NO:
SEQ ID NO: 54 caaagaatgacttttttct 2572 2591 SEQ ID 1057 agaaggatggcattttttg
14000 14019 16
NO:
SEQ ID NO: 55 catggagaatgcctttgaa 2603 2622 SEQ ID 1058 ttcagagccaaagtccatg
7119, 7138 16
NO:
SEQ ID NO: 56 ggagccaaggctggagtaa 2679 2698 SEQ ID 1059 ttactccaacgccagctcc
3050 3069 16
NO:
SEQ ID NO:57 tcattccttccccaaagag 2884 2903 SEQ ID 1060 ctctctggggcatctatga
5139 5158 16
NO:
SEQ ID NO:58 acctatgagctccagagag 3165 3184 SEQ ID 1061 ctctcaagaccacagaggt
12976 12995 1.6
NO:
SEQ ID NO: 59 gggcaaaacgtcttacaga 3365 3384 SEQ ID 1062 tctgaaagacaacgtgccc
12317 12336 16
NO:
SEQ ID NO: 60 accctggacattcagaaca 3387 3406 SEQ ID 1063 tgttgctaaggttcagggt
5675 5694 16
NO:
SEQ ID NO: 61 atgggcgacctaagttgtg 3429 3448 SEQ ID 1064 cacaaattagtttcaccat
8941 8960 1 6
NO:
SEQ ID NO: 62 gatgaagagaagattgaat 3618 3637 SEQ ID 1065 attccagcttccccacatc
8330 8349 1 6
247
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 5
NOTE: For additional volumes please contact the Canadian Patent Office.