Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
PROCESS FOR ISOLATING AND PURIFING OVINE HYALURONIDASE
Related Applications
This application claims the benefit of US Provisional Application No.
601463,516,
filed April 15, 2003, which is hereby incorporated by reference in its
entirety.
Field of the Invention
The disclosure relates to a process for preparing a mammalian testicular
hyaluronidase preparation suitable for pharmaceutical applications. In a
preferred
embodiment, the process is used to purify hyaluronidase from ovine testes and
includes the
use of viral filtration steps to increase the purity of the final product. The
process also
provides a method that enhances the purity of hyaluronidase preparations
presently
available in commerce. The methods are preferably used to purify hyaluronidase
from
mammalian sources. In an alternative embodiment, the methods disclosed can be
used to
purify recombinant hyaluronidase.
Background of the Invention
Hyaluroiudase is a versatile class of enzymes that are expressed in
vertebrates and
invertebrates alike. The mammalian hyaluronidase catalyzes the random
hydrolysis of 1,4-
linkages between 2-acetamido-2-deoxy-b-D-glucose and D-glucose residues in
hyaluronate.
The hyaluronidase from bovine testes has a reported molecular weight of 65,000
(Lathrop et al. 1990 J Cell Biol 111:2939). The bovine testicular
hyaluronidase hydrolyzes
the endo-N-acetylhexosaminic bonds of hyaluronic acid and chondroitin sulfuric
acids A
and C (but not B), primarily to tetrasaccharide residues (Ludovig et al. 1961
J Biol Chenz
236:333).
Typical purification protocols call for the use of standard chromatographic
techniques to produce a purified solution possessing hyaluronidase activity.
Tolksdorf, et
al. 1949 J Lab Clip Med 34:74; and Kass & Seastone, 1944 J Exp Med 79:319 have
articulated a generally accepted assay protocol to determine hyaluronidase
activity. These
purification protocols were sufficient to provide relatively crude
hyaluronidase
preparations.
Brief Description of the Drawings
Figures 1-11 show flow charts of the described hyaluronidase preparation
methodology.
-1-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Figure 12. Typical chromatograms: A. for Standard; B. for Sample.
Detailed Description of the Preferred Embodiment
Ovine hyaluronidase is an enzyme product purified from ovine testes and
capable of
hydrolyzing mucopolysaccharides of the type of hyaluronic acid.
Anaiyao Acid Sequehce - a form (SEQ ID NO: 1)
The consensus sites for glycosylation are underlined. The site of cleavage
that
yields the (3-form of hyaluronidase is assigned by homology with the bovine
sequence and
is indicated as bold and underlined.
Ovine 1 LDFRAPPLISNTSFLWAWNAPAERCVKIFKLPPDLRLFSVKGSPQKSATG
Ovine QFITLFYADRLGYYPHmEKTGNTVYGGIPQLGNLKNHLEKAKKDIAYYI
51
Ovine 101 PNDSVGLAVIDWENWRPTWARNVVKPKDVYRDESVELVLQKNPQLSFPEAS
Ovine 151 KIAKVDFETAGKSFMQETLKLGKLLRPNHLWGYYLFPDC~'NHNYNQPTYN
Ovine 201 GNCSDLEKRRNDDLDWLWKESTALFPSVYLNIKLKSTPKAAFYVRNRVQE
Ovine 251 AIRLSKIASVESPLPVFVYHRPVFTDGSSTYLSQGDLVNSVGEIVALGAS
Ovine GIIMWGSLNLSLTMQSCMNLGNYLNTTLNPYIINNVTLAAKMCSQVLCHDE
301
Ovine 351 GVCTRKQWNSSDYLHLNPMNFAIQTGKGGKYTVPGKVTLEDLQTFSDKFY
Ovine 401 CSCYANINCKKRVDIKNVHSVNVCMAEDICIEGPVKLQPSDHSSSQNEAS
Ovine 451 TTTVSSISPSTTATTVSPCTPEKQSPECLKVRCLEAIANVTQTGCQGVKW
Ovine 501 KNTSSQSQSSIQNIKNQTTY
Molecular Weight
The molecular weight based on mobility in 4-20% gradient reduced sodium
dodecylsulfate (SDS) polyacrylamide gels is 70-74 kDa for the a-form and 60-63
for the (3-
form (see hyaluronidase content assay below).
Identification test
The identification of hyaluronidase in samples of the drug substance is based
on
demonstration of a hyaluronidase enzymatic activity.
Activity ID Test:
Demonstration of hyaluronidase enzymatic activity is a non-quantitative
variation of
the activity assay (see "Potency" below). A solution of approximately 0.2
mg/mL drug
substance is prepared in the 20 mM sodium phosphate diluent buffer at pH 6.90.
In a
16x125 mm glass test tube, 1 mL of the drug substance solution is reacted with
1 mL of 0.5
sodium hyaluronate substrate solution and incubated at 37°C for 10
minutes. A blanlc
consisting of 1 mL of 20 mM sodium phosphate buffer with 1 mL of substrate
solution is
_2_
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
run simultaneously. After 10 minutes, the absorbance of the blank and drug
substance
solutions are read in the spectrophotometer at 600 nm. The value obtained by
subtracting
the absorbance of the sample from the absorbance of the blank must be greater
than 0.4 to
prove the presence of a hyaluronidase activity in the drug substance. This
demonstrates that
the hyaluronidase present is testicular in origin because only this enzyme of
the six known
mammalian hyaluronidases has significant enzymatic activity above pH 5Ø
MicYObial Limits Test
No E. coli, S. au~eus, P. aef°ugiyaosa, or Salmonella. Total microbial
contamination
less than 103 organisms per gram.
~: between 5.2 and 7.2, in a solution of three mg in one mL of deionized
water.
Impurity Tests
Process-related Impurities
Annexin II - The annexin II content of drug substance is determined by
electrophoretic content assay, quantitated against an internal standard curve
of in-house
a~mexin II reference standard. Specification: between 0.29 and 0.57 mg annexin
per mg
protein. The annexin II reference standard is purified from drug substance by
Protein G
affinity chromatography and size-exclusion chromatography. Batches of
reference standard
are qualified for use by testing for purity (SDS PAGE), identity (western
blot), protein
concentration and amino acid content (amino acid analysis) and in the
electrophoretic
content assay described here.
Assay Method:
Annexin II content is assessed with 12-well 4-20% gradient Tris-glycine sodium
dodecylsulfate (SDS) polyacrylamide gels. Samples of drug substance are
reduced and
denatured in 2X Tris-glycine SDS sample preparation buffer containing 2-
mercaptoethanol
at 500 ~,L per 10 mL. of buffer at a final concentration of 0.12 mg/mL. A
standard curve of
annexin II reference standard is prepared at 5 concentrations: 7.42, 14.84,
29.68, 44.52 and
74.2 ~g/mL (Stds 1-5, respectively). These are diluted 1:1 in reducing 2x
sample
preparation buffer (see above). A designated lot of Vitrase finished product
is used as a
system suitability standard, also prepared at 0.12 mg/mL. One sample lane is
used by a
sample of broad range reduced molecular weight standards. All samples and
standards are
loaded at a volume of 10 ~L of sample per well in the following order:
1 2 3 4 5 6 7 8 9 10 11 12
Std.lTS Std.2 TS Std.3 TS Std.4SSS Std.S SSS SSS MWS
-3-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
TS = DRUG SUBSTANCE test sample
SSS = system suitability standard lot
Std 1 thru Std 5 = standard curve of in-house asmexin II reference standard
MWS = broad range molecular weight standard
Gels are electrophoresed at a constant voltage of 120 V for 120 +/- 5 minutes,
or
until the bromphenol blue tracking dye line reaches the bottom of the gel.
When the run is
complete, the gel is carefully removed from its plastic cassette and placed in
50 mL of
colloidal Coomassie blue stain solution. Gels are left to stain 13-17 hours
with constant
mixing provided by a gel rocker platform.
Upon completion of the stain, the gel is destained over a period of not less
than 7
hours in deionized water on the gel rocker platform, using multiple changes of
water during
the process.
Following destaining, the gel is quantitated on a scanning laser densitometer.
The
densitometer yields a band density histogram that is integrated to give an
area under the
"peaks" in the lustogram.
Calculations:
1. The band density peak area data points for each annexin II concentration of
the
standard curve is plotted against their theoretical concentrations in
Microsoft Excel,
complete with correlation coefficient (r2) value and curve equation.
2. The amlexin II band mass (in ~.g) in all drug substance and system
suitability
samples is calculated from the quantitated band peals areas, and interpolated
from
the standard curve.
3. A mean annexin II mass for each set of three samples, (drug substance or
system
suitability) is calculated.
4. The mean annexin II mass for each sample is expressed as a percentage of
total
protein using the following equation:
%Annexin II = mean annexin II in sample (in ~,g) x 100/total protein loaded
(in fig).
The sample meets the requirement if the content is between 0.29 and 0.57 mg
annexin per mg protein.
Systefn Suitability Criteria:
Results from this assay are unacceptable if the following criteria are not
met:
1. The densitometer must pass its internal calibration check
-4-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
2. The annexin II band must migrate between the 31 and 36.5 kDa molecular
weight
standards in gel sample lane 12
3. The mean of system suitability samples tested in triplicate must be within
15% of
historically determined values
Acceptance C~itef~ia:
Data from this assay are unacceptable if the following criteria are not met.
1. The calculated r2 value for the standard curve must be > 0.98.
2. The percentage coefficient of variance for triplicate data points (SSS and
TS
samples) must be < 10%.
3. The mean calculated annexin II for samples must fall within the range of
0.076-0.39
~.g for drug substance.
I~G fragment - The IgG heavy chain fragment ("IgG") content of drug substance
is
quantitated with a high performance liquid chromatography (HPLC) method using
an
affinity column and IgG reference standard curve. Specification: < 0.23 mg IgG
per mg
protein.
The IgG reference standard is prepared in a one-step affinity purification by
Protein
G chromatography. Batches of IgG reference standard are qualified for use by
testing for
purity (SDS PAGE), identity (Western blot), concentration (colorirrietric
protein assay) and
content (HPLC method).
' The method uses a standard HPLC system with a tunable UV detector set at 280
nm. The column is a 4.6 x 100 mm Poros OH pre-column, attached in sequence to
a 4.6 x
100 mm Poros G affinity column. Samples of drug substance are loaded in a
mobile phase
of 0.05 M sodium phosphate, pH 7.2, supplemented with 0.15 M sodium chloride.
All IgG
is retained by the column, and removed by an elution buffer of 0.1 M glycine,
S% acetic
acid at pH 2.5. All chromatography is performed at ambient temperature. The
eluting
gradient has a cycle time of 10 minutes and tales the following shape:
Time % Solvent % Solvent
A B
~
0 100 0
3 100 0
7 0 100
10 100 0
Solvent A = 0.05 M sodium phosphate, pH 7.2, 0.15 M sodium chloride
Solvent B = 0.1 M glycine, 5% acetic acid, pH 2.5
P~eparatiofz of standards, samples and check samples:
-5-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
The IgG content is determined in comparison to an in-house purified ovine
testicular IgG reference standard. The IgG standards are prepared at
concentrations of 500,
250, 120, 60 and 30 ~.g/mL in Solvent A. The IgG standards are loaded onto the
column at
100 pL per injection.
Prepare drug substance samples by dissolving 10 mg of drug substance in 3.5 mL
of
Solvent A in a 5-mL volumetric flask. The samples are mixed briefly and
incubated for not
less than 12 hours at 2-8°C to ensure complete dissolution. Bring the
drug substance
sample to a final volume of 5.0 mL with more Solvent A, and mix. Samples are
filtered
with a 0.45-~m syringe filter fitted to a 3-mL syringe. All drug substance
samples are
analyzed with 100-~,L injections. The IgG content of a given drug substance is
measured in
3 separate weighings, analyzed at n = 2 injections of each. Samples of drug
substance may
be stored at 2-8°C for up to 3 days.
Check samples of Vitrase~ finished product are prepared by dissolving the
contents
of 2 vials in 0.5 mL (each) of Solvent A. Mix by vortexing briefly and then
allow them to
stand for not less than 30 minutes to ensure complete dissolution. Combine the
vial
contents and filter as described for drug substance. Each check sample
preparation is
analyzed with 2 injections of 100-~.L volume. Check sample preparations can be
stored at
2-8°C for up to 3 days.
Syste~ra Equilibration:
The system is equilibrated with Solvent A for 20 minutes, first at a flow rate
of 2
mL/minute for 5 minutes, and then at 4 mL/minute for 15 minutes. Four 100-~L
inj ections
of Solvent A are used to establish that the UV signal is stable.
Assay Method:
Once a stable baseline has been established, the standard curve of five IgG
standard
concentrations is run at one injection per standard concentration. This is
followed by all
drug substance sample injections and then the check sample injections. A final
pair of
injections of the 250-p,g/mL (or 25 ~,g) IgG standard are used to verify that
retention times
and signal amplitudes have not changed during the chromatography run.
Calculations:
To determine the concentration of IgG in a sample, first perform a linear
regression
analysis of the standard curve peak areas versus the theoretical mass injected
per standard
to derive the correlation coefficient (r2) value, the slope and the y-
intercept.
IgG mass inj ected (~.g) _ (Sample Peak Area - Intercept) / slope.
-6-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
IgG Concentration (~.g/mL) = IgG mass injected (~,g) / Injection Volume (mL)
The sample protein concentration is determined with a colorimetric protein
assay
and used to calculate the IgG content per mg of total protein in the sample.
IgG Concentration (~,g/mL) / Protein Concentration (~g/mL) _ (~,g IgG/~,g
protein) _
IgG content / ~.g total sample protein.
It meets the requirement if the content is -< 0.23 mg IgG per mg protein.
The % relative error is calculated by (use absolute value of subtraction
number):
%RE =100 x [Inj ection 1 (~,g) - Inj ection 2 (~,g)] / 2
The Relative Retention Time for IgG is calculated by:
RRT = RTsamp1~T250-pg/mL (or 25 pg) standard
System Suitability:
Results from this assay are unacceptable if the following chromatographic
conditions are not met.
1. The theoretical plate count for the 25-~.g IgG standard must be > 2300. e,
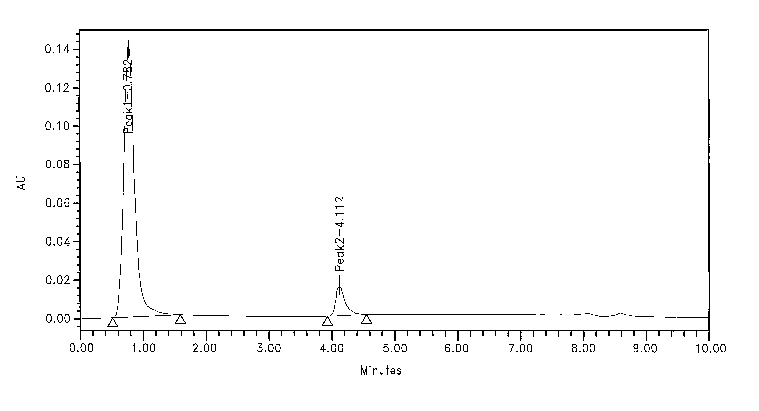
2. The peak retention time for IgG must be within 4.17 +0.5 minutes and non-
bound
proteins must elute at 0.79+0.5 minutes.
3. The baseline shift upon switching from loading to elution buffer must not
exceed
0.005 AZJ in the blank injections.
4. The % coefficient of variation for the 3 injections of the 250 ~,g/mL (or
25 ~,g) IgG
standard must be < 10%.
Acceptatace C~ite~ia:
Data from this assay are unacceptable if the following criteria are not met.
1. The r2 value for the standard curve must be > 0.99.
2. All sample concentrations must fall within the range of the IgG standard
curve.
3. Each injection of the 25-~.g standard must fall within the range of 22.5 -
27.5 ~.g (~
10%)
4. The % Relative Error for any two injections of the same sample must be <
5%,
5. The Relative Retention Time of IgG must be within 0.9 -1.1 minutes.
Loss on drying - Tare a glass-stoppered weighing vial that has been dried
overnight at 110°C. Place 150 milligrams + 10 milligrams of the drug
substance sample in
the bottle and accurately weigh the bottle and the contents. Place the bottle
in the drying
chamber, evacuate the chamber to less than 10 inches of Hg, and dry two (2)
hours + 1
minute at 60°C with the lid removed, but also in the chamber. Release
the vacuum, open
_7_
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
the chamber door and immediately replace the lid. Weight the dried container
and sample
after it has cooled to ambient temperature and subtract the weight obtained
from the tare
weight. Calculate the percent of the original weight that was lost on drying.
The loss on
drying should not be more than 5% of the original tare weight.
Product-related Ifnpurities
Quantitation
Hyaluronidase content - The hyaluronidase content of drug substance samples is
quantitated by an electrophoretic method. Hyaluronidase is quantitated against
a standard
curve of in-house hyaluronidase reference standard with a system suitability
sample
provided by Vitrase~ finished product. Specification: between 0.10 and 0.23 mg
hylauronidase per mg protein.
The ovine hyaluronidase standard is purified from drug substance by Protein G
affinity chromatography, followed by size-exclusion chromatography and
Concanavalin A
affinity chromatography. Batches of reference standard are qualified for use
by testing for
purity (SDS PAGE), identity (western blot), protein concentration and amino
acid content
(amino acid analysis) and in the electrophoretic content assay. Under reducing
conditions,
ovine testicular hyaluronidase migrates as a pair of discreet bands that
differ in apparent
mass by about 7 kDa. Both forms retain full enzymatic activity.
Assay Method:
Hyaluronidase content is assessed with 12-well 4-20% gradient Tris-glycine SDS
polyacrylamide gels. Samples of drug substance are reduced and denatured in 2X
Tris-
glycine SDS sample preparation buffer containing 2-mercaptoethanol at 500 ~.L
per 10 mL
of buffer, a final concentration of 0.1 mghnL. A standard curve of annexin II
reference
standard is prepared at 5 concentrations: 0.092, 0.0506, 0.0276, 0.0138 and
0.0092 pg/mL
(Stds 1-5, respectively). These are diluted 1:1 in reducing 2x sample
preparation buffer
(see above). A designated lot of Vitrase~ finished product is used as a system
suitability
standard, also prepared at 0.1 mg/mL. One sample lane is used by a sample of
broad range
reduced molecular weight standards. All samples and standards are loaded at a
volume of
10 ~L of sample per well.
1 2 3 4 5 6 7 8 9 10 11 12
MWS Std.lStd.2Std.3Std.4Std.STS TS TS SSS SSS SSS
TS = DRUG SUBSTANCE test sample
_g_
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
SSS = system suitability standard lot
Std 1 thru Std 5 = standard curve of in-house annexin II reference standard
MWS = broad range molecular weight standard
Gels are electrophoresed at a constant voltage of 120 V for 120 +/- 5 minutes,
or
until the bromphenol blue tracking dye line reaches the bottom of the gel.
When the run is
complete, the gel is carefully removed from its plastic cassette and placed in
50 mL of
colloidal Coomassie blue stain solution. Gels are left to stain 15-17 hours
with constant
mixing provided by a gel rocker platform.
Upon completion of the stain, the gel is destained over a period of not less
than 7
hours in deionized water on the gel rocker platform, using multiple changes of
water during
the process.
Following destaining, the gel is quantitated densitometrically on a scanning
laser
densitometer.
Calculations:
Plot the band density total peak area against the theoretical mass values for
each
standard in the standard curve, to yield an r2 value and equation for the
line.
Calculate the total mass of the summed hyaluronidase bands in each sample
using
the band density peak area of the sample and the equation from the standard
curve.
Calculate the mean hyaluronidase mass for each set of three hyaluronidase
samples.
The mean hyaluronidase mass is expressed as a percentage of the total protein
by:
%Hyaluronidase = 100 x [mean sample hyaluronidase (~,g)] / total protein (~.g)
loaded.
The sample meets the requirement if the content is between 0.10 and 0.23 mg
hyaluronidase per mg protein.
Systerra Suitability C~iteYia:
Results from this assay are unacceptable if the following criteria are not
met:
1. The densitometer must pass its internal calibration check.
2. The hyaluronidase bands must migrate between the phosphorylase b (97.4
l~Da) and
glutamic dehydrogenase (55.4 kDa) molecular weight standards in gel sample
lane
1.
3. The mean of system suitability samples tested in triplicate must fall
within 2
standard deviations the historically determined value.
-9-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Acceptaface Criteria:
Data from this assay are unacceptable if the following criteria are not met.
1. Each standard curve must be comprised of at least 5 points.
2. The calculated r2 value for each standard curve must be >0.98.
3. The % coefficient of variance for each set of triplicate data points must
not exceed
15%.
4. The mean hyaluronidase content of drug substance samples must fall between
0.092
and 0.32 p,g.
Total Protein - The total protein assay is a colorimetric method based on the
binding of Coomassie Brilliant Blue G-250 to proteins in solution. Proteins
are quantitated
relative to a standard curve of bovine serum albumin. Specification: between
0.55 and 0.83
mg protein per mg drug substance.
Solutiofzs:
The acidic dye concentrate, bovine serum albumin standard and 0.9% saline
solutions are obtained from commercial sources. The dye concentrate is diluted
5:1 in
deionized water and filtered through Whatman #1 filter paper prior to use.
This 5x-diluted
dye solution is stable for up to 14 days when stored at 2-8°C.
Samples of approximately 10 mg (~0.5 mg) of drug substance are weighed out and
diluted to approximately 1 mg/mL in 0.9% saline. Such drug substance
preparations are
left to stand for not less than 12 hours to ensure full dissolution prior to
use. The sample is
then vortexed gently to mix, and filtered through a 0.2-pm cellulose acetate
membrane.
Reconstituted drug substance sample solutions may be used for up to 7 days
when stored at
2-8°C.
Assay Met7aod:
The bovine serum albumin stock solution is 2.0 mglmL. Dilutions are prepared
in
0.9% saline to final concentrations of 0.9, 0.7, 0.5, 0.3 and 0.1 mg/mL in
volumes of 500
q.L. These standards are prepared fresh daily. A blank consisting solely of
0.9% saline is
included. All samples and standards are pipetted in triplicate into 13x100 mm
glass tubes
at 100 ~,L per tube.
The assay is initiated by pipetting 5.0 mL of dilute dye reagent into each
tube at
timed intervals of 20 seconds. After each addition, the tube is covered with a
plastic cap
and vortexed briefly to mix. The assays are then incubated at room temperature
for 10
minutes. The saline blank is used to blank the spectrophotometer at 595 nm -
the tubes are
-10-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
read directly in the spectrophotometer cuvette holder. After 10 minutes
incubation, the
samples are read in the same order as initiated, at the same 20-second
intervals.
Calculations:
Protein concentrations are interpolated from the regression line of standard
curve
absorbance at 595 nm (As9s) versus theoretical standard concentration. The
assay reporting
range is from 0.35 - 0.9 mg/mL - drug substance samples outside this range
must be re-
assayed with fresh dilutions to bring them into the standard curve range.
Because the drug substance contains proteins (in particular, the IgG fragment)
that
do not respond in the same way as BSA to the Coomassie Blue dye, a 1.2
correction factor
is applied to all concentrations obtained by this assay.
Concentrations of drug substance samples are reported in units of mg protein
per mg
drug substance. This is calculated from the concentration in mg/mL by the
following
expression:
mg protein/mg drug substance = (PC x 10 mL) / SW,
where PC is the protein concentration in mg/mL and SW is the original drug
substance
sample weight in mg.
The sample meets requirement if the protein content is between 0.55 and 0.83
mg
protein per mg drug substance.
Additionally, a coefficient of variance (%CV, or %RSD) is calculated for all
drug
substance samples run in triplicate.
System Suitability.'
Results from this assay are unacceptable if the following criteria are not
met:
1. The spectrophotometer calibration must be current.
2. The baseline reading on the blanl~ must be stable at 0.00 ~ 0.01 ALT for at
least 20
seconds.
Acceptance Criteria:
Data from this assay are unacceptable if the following criteria are not met.
1. The r2 value for the standard curve must be > 0.98.
2. All samples must fall within the 0.35 - 0.9 mg/mL reporting range of the
assay.
3. The %CV value for all drug substance samples run in triplicate must be <-
10%.
Potency Assay -
Potency is measured with a hyaluronidase activity assay in which enzyme is
incubated with hyaluronic acid substrate for a fixed time period and the non-
degraded
-11-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
substrate detected by the turbidity formed when reacted with an acidic albumin
solution.
Turbidity is measured spectrophotometrically at 600 nm. Hyaluronidase activity
is
quantitated relative to a USP hyaluronidase standard curve run simultaneously.
Specific
activity is calculated using the values from the activity and total protein
assays. Potency
specification: between 7.32 x 103 and 1.37 x 104 USP Units per mg drug.
Specific activity
specification: between 1.2 x 104 and 1.9 x 104 USP Units per mg protein.
Solutioyzs:
The sodium hyaluronate substrate solution is prepared at 0.5 mg/mL in 0.3 M
sodium phosphate at pH 5.30-5.35. All USP hyaluronidase standard, drug
substance and
check sample solutions axe prepared in a diluent buffer of 20 mM sodium
phosphate, pH
6.900.05 supplemented with 0.45% sodium chloride and 0.01% bovine serum
albumin.
The acidic albumin solution is 24 mM sodium acetate, pH 3.75 X0.05, with 0.1 %
bovine
serum albumin.
The USP hyaluronidase standard solution is prepared to yield a nominal
activity of
15 USP U/mL. For the current lot of USP hyaluronidase, this means dissolving
45.3 mg of
standard in a final volume of 25 mL of 20 mM sodium phosphate buffer.
Samples of drug substance are iutially diluted to approximately 1 mg/mL in
0.9%
saline in a 10-mL volume. This initial saline dilution is further diluted,
based upon the
manufacturer's release activity value for the particular drug substance lot to
be assayed, to
yield a final concentration of about 9 USP U/mL. This secondary dilution is
made in the 20
mM sodium phosphate buffer.
Check samples are made from lots of Vitrase~ finished product in the following
manner. A single vial of finished product is dissolved in 5.4 mL of 0.9%
saline, delivered
from a 10-mL syringe. This solution is filtered through a 5-~.m filter needle
and returned to
its original vial. Dilute 125 ~L of this solution to a final volume of 25 mL
of the 20 mM
sodium phosphate buffer to yield the check sample.
Assay Method.'
All assays consist of a 10-point standard curve, a check sample, and the drug
substance test samples. The standard curve contains USP hyaluronidase standard
at
concentrations of 1.5, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5, 12.0, 13.5 and 15.0 USP
U/mL in a final
volume of 1 mL of the 20 rnM sodium phosphate buffer in 16x125 mm glass test
tubes.
Check samples consist of 1 mL of the secondary dilution pipetted into the
glass test tubes.
Likewise, drug substance samples are of 1-mL volumes, pipetted into the test
tubes.
-12-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Typically, drug substance samples are assayed multiple times on multiple
preparations at n
= 2 assays per preparation, allowing for the natural variability of the assay
procedure. All
standards, check samples and drug substance samples are vortexed briefly to
mix, and the
tubes capped with plastic stoppers prior to being pre-incubated for 5 minutes
in a
circulating water bath at 37-38°C.
Assays are initiated by uncapping the tube, pipetting 1 mL of the 0.5 mg/mL
hyaluronate solution into the tube, recapping it and immediately vortexing
before returning
it to the water bath. Care should be taken to vortex at a relatively low speed
to avoid
foaming the solution. Assays are initiated every 30 seconds until all have
received the
substrate solution. The incubation time at 37-38°C is 45 minutes. At t
= 45 minutes, the
assays are quenched by the addition of 10 mL of acidic albumin solution: uncap
the tube,
add the acidic albumin, recap it, and mix by gently inverting. The assays are
quenched in
exactly the same order they were initiated, and left standing at room
temperature for 20
minutes before being read at 600 nm in the spectrophotometer at t = 65 minutes
(relative to
their respective start times). To read at 600 nm, the tube is first re-mixed
by gentle
inversion, and then the sample decanted into a disposable 4.5-mL polystyrene
cuvette.
Samples are read every 30 seconds, maintaining the temporal lockstep of the
process. The
spectrophotometer is initially blanked with a cuvette full of deionized water.
NOTE: the relative timing of these events throughout the assay is critical.
Turbidity
development is non-linear with respect to time, and improper timing causes
spurious or
inaccurate activities from the mistimed samples.
Calculations:
The standard curve is plotted as a third-order polynomial function of USP U/mL
versus the observed absorbance at 600 mn (A6oo). Check sample and drug
substance
sample activity values are then interpolated from this standard curve from
their A6oo values.
For the purpose of simplicity and reproducibility, analysts may find it most
convenient to
set up spreadsheet activity calculation tables whereby entering the raw A6oo
data, the
polynomial coefficient values from the standard curve equation, the mass value
(drug
substance samples only) and the sample dilution factors for drug substance and
check
samples yields calculated activity values for these samples from pre-entered
equations in
fixed cells of the spreadsheet.
The sample meets the potency requirement if the activity content is between
7.32 x
103 and 1.37 x 104 USP Units per mg drug substance.
-13-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
System Suitability Criteria:
Results from this assay are unacceptable if the following criteria are not
met:
1. Calibration of the spectrophotometer must be current.
2. The correlation coefficient (r2 value) of the USP standard curve must be >
0.999.
3. The check sample activity value must fall within +/- 6% of the historically
established value for that lot of finished product.
4. The % relative error between replicate assays (e.g. check samples) must be
< 6%.
Acceptance Criteria:
Data from this assay are unacceptable if the following criteria are not met.
1. The measured concentration of each sample assay must fall within the range
of 3.0 -13.5 USP U/mL.
2. The % relative error between replicate assays must be < 6%.
Specific Activity - Specific activity is calculated as the quotient of the
activity and
total protein assay results. The sample meets the requirement if the specific
activity is
between 1.2 x 104 and 1.9 x 104 USP Units per mg protein.
Bacterial endotoxins - less than 60 eu per milligram drug substance.
Hyaluronidase Preparation Methodology
The disclosure relates to a process for preparing a hyaluronidase preparation
suitable for pharmaceutical applications. In a preferred embodiment, the
process includes
the use of viral .filtration steps to increase the safety level of the final
product. The process
provides a method that enhances the purity of hyaluronidase preparations
presently
available in commerce. The methods are preferably used to purify hyaluronidase
from
mammalian sources. In an alternative embodiment, the methods disclosed can be
used to
purify recombinant hyaluronidase.
Tissue Sources
Purified preparations of mammalian beta-glucuronidase enzymes are prepared
preferably from mammalian testes. Examples of preferred of mammalian sources
include
ovine, bovine, porcine, and equine. However, any mammalian source can be used
with the
described methods. There are currently 4,629 currently recognized species of
mammals. A
taxonomic hierarchy that includes Order, Family, Subfamily, and Genus is found
in Wilson,
D. E., and D. M. Reeder (eds.) 1993 Mammal Species of the World, Smithsonian
Institution Press, 1206 p. (Available from Smithsonian Institution Press),
which is hereby
incorporated by reference.
-14-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Extraction of hyaluronidase
Typically a preliminary step of the purification of hyaluronidase is to
isolate testes
from a preferred mammalian source. The testes can be processed immediately or
preferably
are frozen for later use. When frozen, the testes should be thawed at 2-
8°C for 40-44 hours.
The testes are then minced and filtered to extract the hyaluronidase. The
isolated material
is then precipitated for a first time, preferably at 15% saturated ammonium
sulphate. The
temperature of the buffers is preferably maintained at 2-8°C. The pH of
the precipitation
step is preferably maintained at a pH of 3.60 ~ 0.1. The precipitate is
filtered, preferably
for less than 5 hours. Following filtration, the material is assayed for
hyaluronidase activity
and protein concentration, and the total units are calculated. The isolated
material is then
subjected to a second salt cut or precipitation. Ammonium sulphate is added to
the level of
approximately 85% saturation while maintaining the solution at pH 3.60 ~ 0.1.
These
processes are described more completely in Figures 1 and 2.
Figures 3 and 4 illustrate the second step in the preparation of hyaluronidase
from
mammalian testes. The product of the purification Step 1 (15/85) purification
is thawed
and ammonium sulphate is added to 35% saturation. The solution is stirred and
filtered.
The pH of the filtrate is adjusted to 4.10 ~ 0.20 and a sample is typically
taken for quality
control analysis. The solution is then brought to an 85% saturated
concentration of
ammonium sulphate and stirred. The solution is then filtered and the
precipitate (35/85
precipitate) is collected and stored.
Figures 5 through 8 illustrate the third step of the purification protocol.
The 35/85
precipitate is thawed and resuspended for further processing. The solution is
dialyzed
against 20mM potassium phosphate solution. The dialyzed solution is then
filtered and
concentrated. The concentrate is stored at 2-8°C overnight. Samples are
typically taken as
indicated in Figure 5 for quality control purposes. The concentrate is then
subjected to
DEAF sephadex fractionation. As described in Figure 6, fractions are collected
and
assayed for hyaluronidase activity. Selected fractions are combined and then
precipitated
with 85% saturated ammonium sulphate. This solution is stirred and then
filtered. The
precipitate (0/85) is collected, weighed, filtered, and clarified. The
solution is precipitated
with PEG6000 to a concentration of 20%. This suspension is centrifuged and the
precipitate is collected. The precipitate is then resuspended and filtered.
Quality control
testing is typically performed. The product is dried and stored for further
processing.
-15-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
The fourth step of the procedure is illustrated in Figure 9 through 11. The
product
is dissolved for CM sephadex column fractionation. The sample is applied to
the column
and fractions are collected. Active fractions are pooled and precipitated with
ammonium
sulphate to 85%. The precipitate (0/85) is collected and weighed. The sample
is dialyzed
against a 20 mM potassium phosphate. As illustrated in Figure 10, the
dialysate is then
filtered and the pH is adjusted.
As depicted in Figure 11 the product is filtered to remove potential viral
contaminants, freeze-dried and tested.
Hyaluronidase activity
Hyaluronidase activity was measured using a turbidity assay. This assay is
used to
determine the activity of hyaluronidase in the final product, the drug
substance [active
pharmaceutical ingredient (API)], and in-process intermediates during
manufacture of the
API or final product. Hyaluronidase activity is determined using a
modification of the
turbidity assay described in USP 26 for Hyaluronidase for Injection. Briefly,
the dissolved
hyaluronidase enzyme is allowed to react with the substrate, hyaluronic acid,
for a set
period of time followed by inactivation of the enzyme and precipitation of non-
degraded
hyaluronic acid by an acidic albumin solution. The degree of resultant
turbidity is
measured by absorbance determination at 600 nrn using a UV-Visual
Spectrophotometer.
Enzyme activity is inversely proportional to the turbidity of the solution.
Quantitation is
based on comparison to turbidity data from a primary USP bovine hyaluronidase
standard
of lrnown enzyme activity, run under the same conditions. The hyaluronidase
preparation
has 7.32 x 103 - 1.37 x 104 USP Units/mg. Typically the hyaluronidase
preparation has a
pH from 5.2 to 7.2.
Total protein
Total protein of the hyaluronidase preparation is measured by generally
accepted
protein concentration assays. These assays are used to determine the protein
concentration
of in-process intermediates, active pharmaceutical ingredient (API), and final
product. This
assay is based on the method of Bradford for protein quantitation. It is a dye-
binding assay
in which a proportional color change of the dye occurs in response to various
concentrations of protein. The absorbance maximum for an acidic solution of
the dye,
Coomassie Brilliant Blue G-250, shifts from 465 nm to 595 nm when binding to
protein
occurs. The Coomassie blue dye binds to primarily amine containing amino acid
residues,
especially arginine. The color yield for an individual protein may depend on
its amino acid
-16-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
composition. Total protein of the hyaluronidase preparation is 0.55 - 0.83
mg/mg protein.
Specific activity of the preparation ranges from 1.2 x 104 -1.9 x 104. USP
Units per mg
protein.
Water Content
The hyaluronidase preparation typically has a water content of < 12% by Karl
Fischer analysis. This assay is used to determine the amount of water present
within in-
process intermediates, active pharmaceutical ingredient (API), and final
product using the
Karl Fischer Coulometric method as delineated in the U.S. Pharmacopoeia 25
(United
States Pharmacopoeia), which is hereby incorporated by reference in its
entirety. The Karl
Fischer Coulometric assay is based on the titration of iodine against water
and sulfur
dioxide in the presence of a base and an alcohol. When all the water is used
up excess
iodine is generated and detected at the double platinum electrode. The Karl
Fischer
coulometer calculates and prints the percent (%) water of the injected sample
based on
weight. Alternatively, the water content of the hyaluronidase preparation
typically is < 5%
loss on drying.
Concentration of Bacterial Endotoxins and Microbial Limits
The hyaluronidase preparations disclosed possess a limited concentration of
bacterial endotoxins. The Limulus amoebocyte lysate (LAL) test is used to
determine the
concentration of bacterial endotoxins in a given sample. The LAL test is based
on the
observation that bacterial endotoxiris react with a lysate derived from
circulating cells
associated with the blood clotting mechanism of the horseshoe crab, Lirnulus
polyphemus.
Bacterial endotoxins are present in the hyaluronidase preparation at < 60
endotoxin units
per mg.
Microbial limits are determined using the assay outlined in USP 61. This test
is
designed to demonstrate that the viable aerobic microorganisms present in the
product are
free of E. coli, S. au~~eus, P. aeruginosa or Salmonella. Total microbial
contamination less
than 103 organisms per gram.
puantitation of Annexin II and I~G in the Final Product
The hyaluronidase preparations for use.in the disclosed method have a
prescribed
concentration of annexin II. To quantitate annexin II in final product, active
pharmaceutical
ingredient (API) and HYOSA in-process lyophilized intermediate (HYOSA) by
sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). A preferred
embodiment
of the purified solution typically contains four major protein components
(alpha
-17-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
hyaluronidase, beta hyaluronidase, annexin H, and an IgG fragment), as well as
lactose and
other buffer components. SDS-PAGE separates proteins based on their apparent
molecular
weight. This method describes the procedure for running SDS-PAGE gels,
scanning gels
by densitometry, and quantitating annexin II by ImageQuant~ densitometry
software.
Annexin II content determined by SDS-PAGE analysis from 0.29 - 0.57 mg annexin
per
mg protein.
The hyaluronidase preparations for use with the disclosed methods contain a
particular level of immunoglobulin. The amount of immunoglobulin (IgG) heavy
chain
fragment, referred to as "IgG," in drug substance [active pharmaceutical
ingredient -
(API)], in-process intermediates and final product was measured by high
performance
liquid chromatography (HPLC). A preferred embodiment is a formulation
containing
several proteins, lactose, and phosphate buffer. The formulation contains
hyaluronidase
(the active ingredient) and two major impurities, annexin II and IgG. In order
to
characterize the drug product, set product specifications and provide process
controls, the
amounts of various components need to be determined.
The HPLC procedure used for quantitation of IgG is discussed below. The
procedure uses an affinity column in which separation is achieved based on the
binding
affinity of the protein of interest to a ligand attached to the stationary
phase. This procedure
utilizes protein G as the ligand. During HPLC separation, the IgG is bound to
the stationary
phase using a buffer at neutral pH, and then is eluted in a step-wise manner
with a buffer of
low pH. The critical factor in the elution step is low pH. Salt is present to
reduce
nonspecific binding. IgG content determined by HPLC analysis is approximately
< 0.23
mg IgG per mg protein.
Hyaluronidase content is determined by SDS-PAGE analysis
To quantitate hyaluronidase protein content by running sodium dodecyl sulfate-
polyacrylamide gel electrophoresis (SDS-PAGE) for final product, various
process
intermediates, and active pharmaceutical ingredient (API). A preferred
embodiment
typically contains four (4) major protein components [alpha hyaluronidase,
beta
hyaluronidase, annexin H, and an irnrnunoglobulin (IgG) fragment], as well as
lactose and
other buffer components. SDS-PAGE separates proteins based on their apparent
molecular
weight. This method describes the procedure for running SDS-PAGE gels,
scanning gels
by densitometry, and quantitating hyaluronidase by ImageQuant~ densitometry
software.
-18-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Typically the hyaluronidase preparations contain 0.1-0.23 mg hyaluroW dase per
mg protein.
EDTA content is 23 - 33 ~,g per mg.
A Preferred Hyaluronidase Preparation
The disclosed hyaluronidase preparations are highly purified preparations
typically
containing 0.10-0.23 mg hyaluronidase per mg protein determined by SDS-PAGE
analysis.
The preparation typically has a specific activity ranging from 1.2 x 104 - 1.9
x 104 USP
Uiuts per mg protein and a total protein concentration of 0.55 - 0.~3 mg/mg
protein. As
adminstered, 7.32 x 103 - 1.37 x 104 USP Units/mg as measured by turbidity
assay, a pH
from5.2 to 7.2, and a water content of < 12% by Karl Fischer analysis or _< 5%
loss on
drying. The preparation will comprise bacterial endotoxins < 60 endotoxin
units per mg
drug substance. The composition will have an absence of E. coli, S.
auf°eus, P. aeruginosa
of~ Salmoraella, and a total microbial contamination of less than 103
organisms per gram
protein. Other protein components of the preparation include an annexin
content of about
0.29 - 0.57 mg annexin per mg protein as determined by SDS-PAGE analysis, and
an IgG
content of approximately <_ 0.23 mg IgG per mg protein as determined by HPLC
analysis.
EXAMPLE 1
Ophtlialmic Toxicities of Thimerosal, Hyaluronidase (ACS) and Hyaluronidase
(Wydase~) in Rabbits
Certain types of enzymes, when contacted with the vitreous humor following
hemorrhage thereinto, will accelerate the rate at which the hemorrhagic blood
is cleared
from the vitreous humor.
In this regard, a method is provided for accelerating clearance of hemorrhagic
blood
from the vitreous of the eye, said method generally comprising the step of
contacting, with
the vitreous humor, a quantity of hyaluronidase at a dose which is sufficient
to accelerate
the clearance of hemorrhagic blood from the vitreous without causing damage to
the retina
or other tissues of the eye. Preferably, the hyaluronidase is selected to have
a molecular
weight distribution which allows the hyaluronidase to be administered
intravitreally at
doses above 1 ICT, and preferably above 15 IU, and advantageously above 75 IU,
in the
absence of thimerosal, without causing toxic damage to the retina or other
tissues of the
eye. This hemorrhage-clearing method may be performed without any vitrectomy
or other
surgical manipulation or removal of the vitreous humor, thereby avoiding the
potential risl~s
and complications associated with such vitrectomy procedures.
-19-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
The preferred route of administration of these hemorrhage-clearing enzymes is
by
intraocular injection directly into the vitreous body. Alternatively, however,
the
hemorrhage-clearing enzymes) may be administered by any other suitable route
of
administration (e.g., topically) which results in sufficient distribution of
the enzymes) to
the vitreous body to cause the desired hemorrhage-clearing effect.
The preferred injectable solution may contain a hyaluronidase wluch has a
molecular weight distribution which allows it to be administered
intravitreally at doses
above 1 1U, and preferably above 15 IU, and advantageously above 75 ILJ,
without causing
toxic damage to the eye, along with inactive ingredients which cause the
solution to be
substantially isotonic, and of a pH which is suitable for injection into the
eye. This
preferred hyaluronidase preparation is preferably devoid of thimerosal. Such
solution for
injection may be initially lyophilized to a dry state and, thereafter, may be
reconstituted
prior to use.
Under USP 6,610,292 and 6,551,590, which are hereby expressly incorporated by
reference in their entireties, the term "hyaluronidase (ACS)" as used herein
describes a
hyaluronidase solution for intravitreal injection which is devoid of
thimerosal and which is
devoid of hyaluronidase molecular weight fractions above 100,000, between
50,000-60,000
and below 20,000, as determined by electrophoresis gel (4-20% gradient SDS-
PAGE).
Such hyaluronidase may be derived from ovine testicles and is available
commercially from
Biozyme Laboratories Limited, San Diego, California, which source may be a
starting
material for the disclosed process for isolating and purifying ovine
hyaluronidase. This
specific molecular weight distribution of the hyaluronidase (ACS) results in
less
ophthalmic toxicity than other hyaluronidase preparations, while exhibiting
desirable
therapeutic efficacy in a number of ophthalmic applications.
As described in the following examples, hyaluronidase (ACS) may be injected
directly into the posterior chamber of the eye at dosage levels which bring
about desirable
therapeutic affects, including but not necessarily limited to the intravitreal
hemorrhage
clearing effect, without causing significant toxicity to the eye or associated
anatomical
structures.
Fifty Two (52) healthy rabbits of the New Zealand Cross variety (26 male, 26
female) weighing 1.5 kg to 2.5 kg, were individually marked for identification
and were
housed individually in suspended cages. The animals received a commercially
available
pelleted rabbit feed on a daily basis, with tap water available ad libitum.
-20-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
The animals were divided into thirteen groups of 4 animals each (2 male, 2
female).
Two animals in each group (1 male, 1 female) were selected for pretreatment
fundus
photography and fluorescein angiography.
The fundus photography was performed by restraining the animals and
visualizing
the optic nerve, retinal arcades and fundas with a KOWA~ RC-3 Fundus Camera
loaded
with Kodak Gold 200 ASA film.
The fluorescein angiography involved a 1.5 ml injection of 2% sterile
fluorescein
solution via the marginal ear vein. Approximately 30 seconds post-injection
the fluorescein
was visualized upon localization of the optic nerve, retinal vessels and
fundas.
The following day, each animal was anesthetized by intravenous administration
of a
combination of 34 mg/kg of ketamine hydrochloride and 5 mg/kg xylazine. The
eyelids
were retracted using a lid speculum, and the eyes were disinfected with an
iodine-providone
wash.
Experimental treatments of either balanced salt solution (BSS),
BSS+thimerosal,
hyaluronidase (Wydase~) or hyaluronidase (ACS) were administered by injection
using a 1
cc tuberculin syringe with a 30 gauge, 0.5 inch needle attached thereto. The
hyaluronidase
(ACS) solution utilized in this example was free of thimerosal and constituted
the specific
formulation set forth in Table 5.
Table 5. Specific Formulation
Ingredient Quantity
Hyaluronidase (ACS) 7,200 LU.
Lactose USP 13.3 mg
Phosphate USP 5 mmole
Table 6. The experimental treatments administered to each animal group were as
follows:
Group # Treatment
1 BSS
2 BSS + 0.0075 mg Thimerosal
3 BSS + 0.025 mg Thimerosal
4 Hyaluronidase (Wydase~) 1
LU.
5 Hyaluronidase (Wydase~) 15
LU.
-21-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Group # Treatment
6 Hyaluronidase (Wydase~) 30
LU.
7 Hyaluronidase (Wydase~) 50
LU.
Hyaluronidase (Wydase~) 150
LU.
9 Hyaluronidase (ACS) 1 LU.
Hyaluronidase (ACS) 15 LU.
11 Hyaluronidase (ACS) 30 LU.
12 Hyaluronidase (ACS) 50 LU.
13 Hyaluronidase (ACS) 150 LU.
The day following the inj ections (Day 1 ), the 26 animals which were subj
ected to
the fundus photography and fluorescein angiography were observed using the
same
methods as for the pre-dose examination.
On Day 2 following the injections, the 13 male rabbits that had received the
fundus
5 photography and fluorescein angiography at pre-dose and Day 1, as well as
the 13 female
rabbits that were not selected for photography were euthanized with a sodium
pentobarbital
based drug. The eyes were then surgically removed and placed in a fixture
solution of 2.5%
glutaraldehyde with 0.1 M phosphate buffered saline at pH 7.37.
Alternatively, one randomly selected rabbit was euthanized by pentobarbital
10 injection but then fixed by intracardiac injection of the of the
glutaraldehyde solution into
the left ventricle to determine the effect of the fixation procedure on the
histology findings
within the enucleated eyes.
On Day 7, the 13 female rabbits that had been previously photographed and
angiography performed were subjected to the same observations following the
methods
previously described.
The remaining 26 animals were euthanized as described above 7 days after
dosing.
The eyes were fixed in the same manner as those which had been fixed on day 2.
Also, one
randomly selected rabbit was subjected to the same intracardiac glutaraldehyde
fixation
procedure described hereabove for the previously randomly selected animal.
The eyes of the animals treated in this example were examined grossly and
microscopically for evidence of treatment-related toxicities. A table setting
forth a
summary of the histological evidence of toxicity or non-toxicity in each
treatment group, is
set forth in Table 1.
-22-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
In summary, the eyes of the BSS-treated control group were free of toxicity at
2 and
7 days post dose.
The eyes of the Group No. 2 animals treated with BSS+thimerosal (0.0075 mg)
were free of toxicity at day 2, but exhibited evidence that there was a
breakdown of the
blood-retinal barrier at day 7.
The Group No. 3 animals treated with BSS+thimerosal (0.025 mg) exhibited
severe
treatment-related toxic effects, at days 2 and 7 post dose.
The Group No. 4 animals treated with Wydase~ at the 1 LU. dose were free of
toxicity at days 2 and 7, however, the eyes of the animals in Group Nos. 5-8
treated with
Wydase~ at dosages ranging from 15 LU.-150 LU. exhibited generally dose-
related toxic
effects at days 2 and 7 post dose.
The eyes of animals in treatment Groups Nos. 9-13 treated with the
hyaluronidase
(ACS) at dosages ranging from 1 LU. through 150 LU., were free of evidence of
toxic
effects at days 2 and 7 post dose.
Accordingly, it is concluded that thimerosal and the thimerosal-containing
Wydase~ formulation do cause toxic effects in the eyes of rabbits at the
dosages tested,
however, the hyaluronidase (ACS) caused no toxic effects in these animals at
the dosages
tested.
The results of the examinations conducted on day 7 are summarized in Table 1.
As
shown, in Table 1, significant toxic effects were observed on day 7 in the
eyes of rabbits
heated with BSS plus thimerosal (0.0075 mg.) and hyaluronidase (Wydase~) at
all doses
between 1 LU.-150 LU. In contrast, no toxic effects were observed in the eyes
of animals
treated with the hyaluronidase (ACS) at doses between 1 and 150 LU.
EXAMPLE 2
Safety and Efficacy of the Hyaluronidase (ACS) Injected Intravitreally in
Rabbit Eyes
In this example, 12 healthy rabbits of the New Zealand Cross variety were
marked
for identification and individually housed in suspended cages. The animals
received
commercially pelleted rabbit feed on a daily basis and tap water was available
ad libitum.
The animals were randomly divided into four (4) treatment groups of three (3)
animals each.
Initially, the eyes of each animal were examined by dilation with 1-2 drops of
10%
Tropicanide followed by gross examination, indirect ophthalinoscopy using a 20
diopter
lens, and slit lamp examination of the anterior anatomy of the eye.
-23-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Following the initial examination of the animals eyes, 100 ~.1 or 10 p,1 of
blood was
inj ected intravitreally into each eye of each animal.
On day 2, the animals of each treatment group received a single intravitreal
injection
of either BSS or the hyaluronidase (ACS) into the right eye, in accordance
with the
following treatment schedule:
G ~ Treatment
#
roup Left E Right Eye
a
A None BSS (30 1) x 1
B None 25 LU. Hyaluronidase (ACS)
in 30 1 x 1
C None 50 LU. Hyaluronidase (ACS)
in 30 1 x 1
D None 75 LU. Hyaluronidase (ACS)
in 30 1 x 1
The hyaluronidase (ACS) preparation used in this experiment was the preferred
formulation described hereabove and shown in Table 5.
On days 3, 5, 7, 14 and 21 the eyes of each animal were again examined by slit-
lamp to evaluate the cornea, anterior chamber and iris. In addition, the eyes
of each animal
were dilated with 10% tropicamide solution and the retina was examined by
indirect
ophthalmoscopy with a 20 diopter lens.
The observed hemorrhage-clearing efficacy of the hyaluronidase (ACS) is
summarized in Table 2. In general, the left eye (untreated) of each animal in
each treatment
group contained hazy vitreous and some blood clots, due to the quantity of
blood which had
been injected therein. The right eyes of the BSS treated (control) animals of
Group A also
contained hazy vitreous and some blood clots, while the right eyes of all
hyaluronidase-
treated animals in Treatment Groups B-D contained vitreous which was clear and
through
which transvitreal visualization of the retina was possible. Furthermore, the
retinas of the
rights eyes of all animals in Treatment Groups B-D appeared normal and free of
treatment-
related toxicity.
The results of this experiment indicate that intravitreally administered
hyaluronidase
(ACS) was effective at single doses of 25-75 LU. to accelerate the rate at
which blood was
cleared from the eyes of the treated animals and further that such single
doses of
hyaluronidase (ACS) administered in this experiment did not cause observable
toxic effects
in the eyes of the rabbits treated in this experiment.
The observations following each dose were consistent and are summarized in
Table
3. In general, the left eye (untreated) of each animal in each treatment
group, contained
hazy vitreous humor and some blood clots, due to the quantity of blood which
had been
-24-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
injected therein. The right eyes of the BSS treated (control) animals of Group
A also
contained hazy vitreous and some blood clots, while the right eyes of all
animals in
treatment Groups B-E (i.e., the animals treated with hyaluronidase (ACS))
contained clear
vitreous through which transvitreal visualization of the retina was possible.
Furthermore,
the retinas of the right eyes of all animals in treatment Groups B-D appeared
to be normal
and free of treatment-related toxicity, even after multiple doses of the
hyaluronidase (ACS).
The results of this experiment indicate that intravitreally administered
hyaluronidase
(ACS) was effective, at single doses of 25-75 LU. x 4, to accelerate the rate
at which blood
was cleared from the eyes of rabbits and that such doses of the hyaluronidase
(ACS) did not
cause observable toxic effects in the eyes of the treated rabbits, even after
four (4)
consecutive doses of the hyaluronidase (ACS) administered at 2 week intervals.
EXAMPLE 3
Safety and Efficacy of the Hyaluronidase (ACS) Injected Intravitreally in
Human
Eyes
The primary objective of this study was to determine if a balanced salt
solution
contaiung a highly purified hyaluronidase extract from ovine testicular tissue
could be
injected into the vitreous of visually impaired eyes without eliciting any
serious ocular
adverse effects.
Materials and Methods
Balanced Salt Solution (BSS) was employed as the placebo control, and was
obtained from Allergan Pharmaceuticals (Irvine, Calif.). The BSS contained
0.64% sodium
chloride, 0.075% potassium chloride, 0.045% calcium chloride dihydrate, 0.03%
magnesium chloride hexahydrate, 0.39% sodium acetate trihydrate, 0.17% sodium
citrate
dihydrate, sufficient sodium hydroxide/hydrochloric acid for adjustment of pH
to 7.1-7.2,
and water for injection (q.s. 100%). Thirty microliter aliquots of BSS or
hyaluronidase
specific formulation X (Table 7) were loaded into a 300 ~.1 microsyringe
fitted with a 29
gauge needle 0.5 inches in length. The loaded microsyringes were then used to
inj ect the
material into the vitreous of the patient's eye.
Table 7. Specific formulation X
Ingredient Quantity
Hyaluronidase (ACS) 6,500 LU.
Lactose USP 5.0 mg
Phosphate USP 0.02 mmoles
-25-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Initially, eight human subjects with at least one visually impaired eye were
randomly assigned to receive intravitreally either 50 ~,1 of 50 LU. of the
hyaluronidase
(ACS) in BSS or BSS alone (3:1 ratio). After one month of follow-up to assure
the 50 LU.
dosage was well-tolerated, a second group of six visually impaired subjects
were enrolled in
the study and randomly assigned to a higher hyaluronidase (ACS) dosage group
(100 LU.)
or the BSS control in a 2:1 ratio.
Procedures used to evaluate the safety of the test articles were completed at
various
intervals throughout the study, and included indirect ophthalmoscopy, fttndus
photography,
fluorescein angiography, electroretinography, external eye examination, slit
lamp
biomicroscopy, applanation tonometry, pachymetry, and autorefraction.
A concurrent placebo control group was included in the study so that adverse
events
peculiarly related to hyaluronidase (ACS) could be distinguished from those
attributable to
the vehicle (BSS)/injection procedure. Only visually impaired eyes were
treated, moreover,
since the test articles were injected proximate to the retina and any untoward
responses of a
serious nature could have been sight threatening. Patients were assigned to
treatment using
a computer generated randomization scheme beginning with the number 601 for
the first
phase of the study, and 701 for the second. Neither the patients nor
investigators were
aware of whether it was the BSS vehicle or hyaluronidase (ACS)/BSS solution
that was
being inj ected intravitreally.
Following establishment of a baseline for each patient, the subjects were
injected
with either the enzyme or the placebo control. Patients were placed in a
sitting position on
a comfortable chair. One or two drops of a local anesthetic were topically
instilled into the
eye that was to be treated, after which the patient was asked to look down and
a sterile
cotton swab soaked in Proparacaine Hydrochloride Ophthalmic solution was
applied for 10
seconds to an area on the sclera approximately 4-5 mm above the cornea
(superior
position/12:00 meridian). The test article was then injected into the vitreous
through a 29
gauge needle attached to a 200 ~,1 microsyringe that was inserted up to the
full length of the
needle at the site of application of the second anesthetic.
Results
Although only infrequently attaining statistical significance, the slit lamp
biomicroscopy data suggested that a substantially higher proportion of
patients treated with
the hyaluronidase (ACS)/BSS preparations as opposed to BSS alone exhibited
anterior
segment pathologic changes, the most prominent being the presence of cells and
flare in the
-26-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
anterior chamber. After the sixth (one month post treatment) visit, however,
no intergroup
differences were observed for any of the slit lamp assessed variables.
Retinal/cortical responses, as measured by electroretinography/visual evoked
potential, deteriorated over time in one patient treated with BSS and two who
were given
50 LU. of hyaluronidase (ACS)BSS. However, alterations in electroretinographic
patterns
were always bilateral and did not occur in either the treated or untreated
eyes of the patients
assigned to high dose (100 LU.) hyaluronidase (ACS)BSS, nor did fluorescein
angiographic test results indicate that retinal ischemia was present in any
eye irrespective of
treatment.
The indirect ophthalinoscopic exams revealed liquefaction and the
establishment of
posterior vitreal detachment (PVD) in the eyes of the test subjects. The
vitreous was
characterized as exhibiting a high degree of motility and/or liquefaction soon
after injecting
the test articles, which was expected for the hyaluronidase (ACS)-containing
preparations.
Certain test eyes injected with BSS control showed liquefaction and PVD, which
was lileely
present before treatment, since the latter did not possess any enzymatic
activity and was
given in very small volume (30 ~,l).
Concerning PVD, in the first group of patients, four of the six patients to be
treated
with hyaluronidase (ACS) displayed the absence of PVD by slit lamp
biomicroscopy (i.e.,
601, 602, 604, and 606) (See Table 8 below). After treatment, each of these
subjects
showed the presence of PVD. The results from the second group of patients were
less
clear, due to difficulties in imaging the vitreous using slit lamp microscopy.
Table 8. Human Safety Study with 50 ~.1 and 100 ~.1 Hyaluronidase (ACS)
Injection
Intravitreally
Number Enzyme Treated Split Day Vitreous
Dose Eye Lamp for Motility
Biomicroscopy PVD
of
Presence
of PVD
BaselineTreated
601 50 OD NO YES 2 Days +3/+4
602 50 OD NO YES 1 Da +4
603 BSS OD YES YES -- +3
604 50 OD NO YES 1 Day +3
605 BSS OS YES YES -- +3/+4
606 50 OD NO YES 14 Days+3/+4
607 50 OD YES YES -- +3/+4
608 SO OD YES YES -- +3
701 100 OS NO ? -- +2
702 100 OD NO ? +4
-27-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
703 BSS OD YES YES --
704 100 OS NO ?
705 100 OD NO Yes 1 Day
706 BSS OS NO NO
Given the results from Example 2 where injection of hyaluronidase (ACS) into
the
vitreous of rabbits at various doses up to 150 LU. did not result in any
significant
histopathologic changes in an earlier preclinical study, it was expected that
doses below
150 LU. would be well-tolerated in humans. Consistent with this expectation,
the
intravitreal administration of hyaluronidase (ACS)/BSS into visually impaired
eyes in the
current trial elicited few symptoms, all of which were believed attributable
to the injection
procedure itself as they occurred with comparable frequency in each of the
study groups,
and treatment-related adverse sequelae were relatively mild and of short
duration.
Furthermore, treatment of human eyes with hyaluronidase (ACS) was observed to
increase the incidence of observed posterior vitreal detachment. The observed
increase in
PVD in patients injected intravitreally with hyaluronidase (ACS) shows that
the methods
described herein are effective in inducing liquefaction and detaclmnent of the
vitreal humor.
Thus, the results of the present study indicate that hyaluronidase (ACS) can
be injected into
the vitreous of humans without eliciting any serious or long-term ocular
complications.
EXAMPLE 4
iTse of Hyaluronidase to Accelerate the Clearance of Hemorrhagic Blood from
the
Vitreous of the Eye
The Example set forth herebelow describes cases in which intravitreal
hyaluronidase (ACS) was used to accelerate the clearance of hemorrhagic blood
from the
vitreous of the eye. The hyaluronidase used was the thimerosal-free
hyaluronidase (ACS)
formulation described above and shown in Table 9.
In this experiment, six (6) human patients (5 female, 1 male) who presented
with
vitreous hemorrhage were treated with single intravitreal injections of
hyaluronidase (ACS)
at dosages of 50-200 LU.
The hyaluronidase (ACS) administered in this experiment was prepared by the
formulation, described hereabove and shown in Table 9.
Table 9. Specific Formulation Z
Ingredient Quantity
Hyaluronidase (ACS) 7,200 LU.
_2g_
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Lactose USP 5.0 mg
Phosphate USP 0.02 rmnoles
All of the patients treated in this experiment had a history of diabetic
retinopathy,
and were found to have vitreous hemorrhages of varying duration. In each
patient, the
amount of blood present in the vitreous was sufficient to prevent viewing of
the retina by
standard funduscopic means.
Each patient received a single intravitreal injection of hyaluronidase (ACS).
Four
(4) patients received a dose of 50 LU., one (1) patient received a dose of 70
LU., and one
(1) patient received a dose of 200 LU.
The observed results of this experiment are summarized in Table 4.
In the six (6) patients treated in this. example, the hemorrhagic vitreous
became
sufficiently clear to permit trans-vitreal viewing of the retina within 6-16
days of the single
intravitreal injection of the hyaluronidase (ACS). Such clearing of the
vitreous was
subjectively determined to have occurred significantly faster than that which
would have
been expected to occur in these patients without hyaluroiudase treatment.
It should be noted that unlike the fluorescein leakage observed at higher
doses of
hyaluronidase (ACS) in rabbits, no toxicity was observed in the present human
based study.
EXAMPLE 5
Use of Hyaluronidase to Treat Other Ophthalmological Disorders
Even a single intravitreal administration of hyaluronidase (ACS), at an
experimental
dose, is efficacious in treating certain ophthalmological disorders. Patients
suffering from
previously diagnosed disorders of the eye, including proliferative diabetic
retinopathy, age-
related macular degeneration, amblyopia, retinitis pigmentosa, macular holes,
macular
exudates and cystoid macular edema, have exhibited improvement in the clinical
symptoms
of these disorders upon treatment with hyaluronidase (ACS).
Hyaluronidase (ACS) is capable of being administered intravitreally at doses
of or
in excess of 1 LU. without causing toxic damage to the eye and thus is useable
to effect
prompt liquefaction of the vitreous body and concomitantly the disconnection
or
detachment of the vitreous body from the retina and other tissues (e.g.,
epiretinal
membranes, macula). As a result of this vitreal liquefaction and detachment,
the physical
pulling forces of the vitreous on the retina and other tissues axe minimized
and the rate of
natural turnover of fluids within the vitreous is accelerated. Accordingly,
hyaluronidase
(ACS) is particularly suitable for the treatment of many disorders (e.g.,
proliferative
-29-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
diabetic retinopathy, age-related macular degeneration, amblyopia, retinitis
pigmentosa,
macular holes, macular exudates and cystoid macular edema) which benefit from
liquefactionldisconnection of the vitreous and/or accelerated clearance of
toxins or other
deleterious substances (e.g., angiogenic factors, edema fluids, etc.) from the
posterior
chamber of the eye and/or from tissues adjacent the posterior chamber (e.g.,
the retina or
macula). Moreover, liquefaction of the vitreous is also believed to remove the
matrix, in
the form of the polymerized vitreous, necessary to support neovascularization.
Thus, the
present method is useful in preventing or reducing the incidence of retinal
neovascularization.
Furthermore, many ophthalmic disorders have as a causative component, a
destabilization of the blood-retina membrane. This destabilization nern,it~
~~r;~",~
components (e.g., serum components, lipids, proteins) of the choriocapillaries
to enter the
vitreal chamber and damage the retinal surface. This destabilization is also a
precursor to
vascular infiltration of the vitreal chamber, known as neovascularization.
Accordingly, embodiments of the present method are directed toward the
prevention
and treatment of various disorders of the mammalian eye which result from
damage or
pathology to the vascularization of the eye or which result in damage to the
blood-retinal
barrier. Examples of such diseases include but are not limited to
proliferative diabetic
retinopathy, age-related macular degeneration, amblyopia, retiutis pigmentosa,
macular
holes, macular exudates, and cystoid macular edema, and others in which the
clinical
symptoms of these disorders respond to the hyaluronidase (ACS) treatment.
EXAMPLE 6
Hyaluronidase Treatment of Proliferative Diabetic Retinopathy (PDR)
Diabetic retinopathy is the leading cause of blindness in working age
Americans.
The incidence of retinopathy increases with the time of the disease state,
from a level of
about 50% manifestation in diabetics with the disease for 7 years to
approximately 90% of
those with the disease for more than 20 years. It is estimated that PDR
affects an estimated
700,000 Americans.
The retinovascular consequences of diabetes essentially consist, in part, of
microvascular leakage and capillary nonperfusion resulting from chronic
hyperglycemia.
Microvascular leakage may in turn result in retinal edema, lipid exudates and
intraretinal
hemorrhages. Capillary nonperfusion results in the formation of intraretinal
microvascular
-3 0-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
abnormalities (IRMA). These abnormalities are arteriovenous shunts formed to
perfuse
retinal regions deprived of vascularization by diabetes-mediated arteriole
degeneration.
Expression of vascular endothelial growth factor from a hypoxic retina in
areas of
capillary nonperfusion is thought to result in the development of extraretinal
neovascularization. Such neovascularization and its associated fibrous
components may
spontaneously involute or be complicated by vitreous hemorrhage or traction
retinal
detachment. Neovascularization may be easily seen on fluorescein angiogram by
the
profuse leakage of dye from these new vessels since they lack the tight
endothelial
junctions of the retinal vasculature. Impaired axoplasmic flow in areas of
retinal hypoxia
result in cotton wool spots.
Proliferative diabetic retinopathy (PDR) requires careful screening of
diabetics for
early identification and treatment since PDR remains largely asymptomatic in
the early
stages. Proliferative diabetic retinopathy can be classified into three
subgroups: (1)
nonproliferative retinopathy; (2) preproliferative retinopathy; (3)
proliferative retinopathy.
Each classification has certain morphological characteristics. Features of
nonproliferative
retinopathy include capillary microangiopathy (microvascular obstructions and
permeability
changes, nonperfusion of capillaries, retinal capillary microaneurysms,
basement membrane
thiclcening, and internal microvascular abnormalities (IRMA)); intraretinal
hemorrhages;
exudates; and macular changes. Preproliferative retinopathy is indicated by
any or all of the
changes described for nonproliferative retinopathy and the following:
significant venous
beading, cotton-wool exudates, extensive IRMA and extensive retinal ischemia.
Proliferative retinopathy is indicated by extraretinal neovascularization and
fibrous tissue
proliferation, vitreous alterations and hemorrhage, macular disease, and
retinal detachment.
The creation of fibrovascular tissue is an especially important complication
of PDR
since it often will lead to retinal damage mediated by the vitreous. The
fibrovascular tissue
may form preretinal membranes that create dense adhesions with the posterior
hyaloid
membrane. These adhesions are responsible for transmitting the forces of
vitreous traction
to the retina, which may result in retinal detachments.
The vitreous base is normally firmly attached to the adj acent retina and to
the outer
circumference of the optic nerve head, known as the ring of Martegiani. The
attachment of
the vitreous to the retina in all other sites between the ring of Martegiani
and the vitreous
base is much less firm. Neovascularization from the retina leads to the
formation of
-31-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
vascular strands extending into the vitreous from the nerve head or elsewhere
in the fundus.
Contraction of these strands may cause partial or complete retinal detachment.
Retinal detachment at the macula is a major complication of PDR. Most retinal
detachments resulting from PDR begin as fractional detachments without holes,
but they
may become rhegmatogenous by the formation of retinal holes at some later
point in the
disease. The fractional detachments are caused by abnormal vitreoretinal
adhesions or
vitreal traction with subsequent shrinkage of the fibrous bands and elevation
of the retina.
The present method contemplates treatment of PDR in the preproliferative and
proliferative states using hyaluronidase (ACS) intravitreal injections.
Without being
limited to a particular mechanism, it is believed that the effect of
intravitreal hyaluronidase
(ACS) injection is to promote the clearance of the liquid phase of the
vitreous. The rate of
transfer of intravitreally inj ected tritiated water from the mid vitreous to
the choroid was
significantly increased after depolymerization of vitreous hyaluronic acid by
inj ected
hyaluronidase (ACS). The present method capitalizes upon this observation to
liquefy the
vitreous, for example, in order to promote the clearance of various growth
inducing factors
and other serum products leaked into the vitreous due to the presence of PDR.
It is further
contemplated that the hyaluronidase (ACS) treatment of the present method may
be
performed alone or in combination with other treatments of PDR.
EXAMPLE 7
Treatment of Non-Proliferative Diabetic Retinopathy
Purpose: To determine the effect of hyaluronidase (ACS) on progression of
moderately severe to severe non-proliferative diabetic retinopathy (NPDR in
the presence
or absence of an induced posterior vitreous detachment (PVD). Methods: sixty
patients
evaluated by ultrasonography and masked fiuldus photography were randomly
assigned to:
saline (0.05 ml), hyaluronidase (ACS) (75 LU., 0.05 ml), SF6 gas (0.3 ml) or
hyaluronidase
(ACS) plus SF6 gas 4 weeks later. PVD was assessed through week 16; seven-
field fundus
photographs were obtained as surrogate baseline and 12 months later. Results:
Of all eyes
treated, without regard to PVD, those with stable ETDRS scores were: saline
38% (6/16),
hyaluronidase (ACS) 67% (10/15), SF6 40% (6/15) and hyaluronidase (ACS) plus
SF6
43% (6/14). Worsening of ETDRS scores were: saline 38% (6/16), hyaluronidase
(ACS)
13% (2/15), SF6 20% (3/15) and hyaluronidase (ACS) plus SF6 21% (3/14).
Percent of
eyes with a complete PVD on or prior to 16 weelcs and stable ETDRS scores
were: saline
0% (0/14), hyaluronidase (ACS) 50% (6/12), SF6 30% (3/10) and hyaluronidase
(ACS)
-32-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
plus SF6 40% (4/10). Reduced visual acuity across all groups was the most
frequently
reported ocular adverse event. Conclusions: Eyes treated with hyaluronidase
(ACS) had
stable ETDRS retinopathy scores compared to saline. The same number of saline-
treated
eyes had stable ETDRS scores as worsened scores. The percent of eyes with a
complete
PVD and stable ETDRS scores was highest in hyaluronidase (ACS) group. These
data
indicate that induction of a PVD and/or enzymatic liquefaction of the vitreous
has the
ability to affect the progression of diabetic retinopathy.
EXAMPLE 8
Treatment of Preproliferative Diabetic Retinopathy
W this Example, a diabetic patient manifesting preproliferative diabetic
retinopathy
is treated for this complication of diabetes mellitus through the intravitreal
injection of
hyaluronidase (ACS). The purpose of this treatment is to reduce or prevent the
development of proliferative diabetic retinopathy maufested by extraretinal
neovascularization and fibrous tissue proliferation, vitreous alterations and
hemorrhage,
macular disease, and retinal detachment.
Once a patient has been diagnosed with diabetes, increased ophthalmic
surveillance
is performed, given the high percentage of individuals suffering from this
disease later
developing proliferative diabetic retinopathy (PDR). This increased
surveillance should
include periodic retinal examinations and fluorescein angiograms to monitor
the extent of
venous beading, IRMA, and retinal ischemia.
When preproliferative diabetic retinopathy begins reaching the proliferative
stage,
the hyaluronidase (ACS) treatment is commenced. This stage is defined as the
presence of
venous beading in 2 or more quadrants, IRMA in one or more quadrants, and/or
microaneurysm and dot hemorrhages in all quadrants. Once these indicia are
present,
hyaluronidase (ACS) method of treatment is initiated.
The patient is to receive a full ophthalmic examination to establish a
baseline of
ocular health. The ophthalmic examination includes indirect ophthalmoscopy,
slit-lamp
biomicroscopy, peripheral retinal examination, intraocular pressure
measurements, visual
acuity (unaided and best corrected) symptomatology, fundus photography,
fluorescein
angiography, electroretinography and A-scan measurements.
Following the preliminary examination, an intravitreal inj ection of
hyaluronidase
(ACS) is given to the patient's affected eye. If both eyes are affected, they
may be treated
separately. The eye to be treated is injected with 50 ~,l of SO LU. of the
hyaluronidase
-33-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
(ACS) ophthalmic solution described above intravitreally to promote the
depolymerization
of vitreous hyaluronic acid, resulting in the liquefaction of the vitreous.
After treatment, the patients' eyes are to be examined on days one (1), two
(2), seven
(7), fifteen (15), thirty (30) and sixty (60). On each examination day the
patient is
monitored for vitreous liquefaction. Additionally, the patient is monitored
for posterior
vitreous detachments using indirect ophthalinoscopy with scleral depression.
Finally, the
extent of PDR presented by the patient is continuously monitored through
periodic retinal
examinations and fluorescein angiograms to monitor the extent of venous
beading, IRMA,
and retinal ischemia.
EXAMPLE 9
Treatment of Proliferative Retinopathy
In this Example, a diabetic patient manifesting proliferative diabetic
retinopathy is
treated by the intravitreal injection of hyaluronidase (ACS). The purpose of
this treatment
is to reduce the extent of proliferative diabetic retinopathy, to prevent
fixrther manifestations
of the disease after removal of any extraretinal neovascularized tissue, and
to reduce the
likelihood of retinal detachment.
A patient presenting proliferative diabetic retinopathy is to receive the
hyaluronidase (ACS) method of treatment in combination with surgical treatment
of the
neovascularized tissue. The proliferation usually begins with the formation of
new vessels
with very little fibrous tissue component. They arise from primitive
mesenchymal elements
that differentiate into vascular endothelial cells. The newly formed vascular
channels then
undergo fibrous metaplasia; that is, the angioblastic buds are transformed
into fibrous
tissue.
The new vessels leak fluorescein, so the presence of proliferation is
especially
noticeable during angiography. The new vessels and fibrous tissue brealc
through the
internal limiting membrane and arborize at the interface between the internal
limiting
membrane and the posterior hyaloid membrane. The fibrovascular tissue may form
preretinal membranes that create dense adhesions with the posterior hyaloid
membrane.
These adhesions are extremely important because they are responsible for
transmitting the
forces of vitreous traction to the retina during the later stage of vitreous
shrinkage.
The proliferative stage of PDR is defined as the presence of three or more of
the
following characteristics: new vessels, new vessels on or within one disc
diameter of the
optic nerve, severe new vessels (as defined by one-third disc area
neovascularization at the
-34-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
optic nerve or one-half disc area neovascularization at the optic nerve or one-
half disc area
neovascularization elsewhere), and preretinal or vitreous hemorrhage.
Once diagnosed as entering the proliferative stage, the patient is to receive
a full
ophthalmic examination to establish a baseline of ocular health. The
ophthalmic
examination includes indirect ophthalinoscopy, slit-lamp biomicroscopy,
peripheral retinal
examination, intraocular pressure measurements, visual acuity (unaided and
best corrected
visual acuity) symptomatology, fundus photography, fluorescein angiography,
electroretinography and A-scan measurements.
Following the preliminary examination, an intravitreal injection of
hyaluronidase
(ACS) is given to patient's affected eyes. If both eyes are affected, they may
be treated
separately. The eye is injected with 50 ~.l of 50 LIJ. of the hyaluronidase
(ACS) ophthalmic
solution intravitreally to promote the depolymerization of vitreous hyaluronic
acid,
resulting in the liquefaction of the vitreous. In addition to depolymerization
of the vitreous,
the neovascularized tissue is also treated directly to minimize subsequent
damage to the
retina using panretinal photocoagulation.
Panretinal photocoagulation (PRP) may be used to treat patients presenting PDR
in
conjunction with the hyaluronidase (ACS) method of treatment. Panretinal
photocoagulation is a form of laser photocoagulation. Currently lasers such as
the argon
green (614 nm), argon blue-green (488 and 514 nm), krypton red (647 nm),
tunable dye,
diode and xenon arc lasers, are used for retinal surgery. Laser energy is
absorbed
predominantly by tissues containing pigment (melanin, xanthophyll, or
hemoglobin)
producing thermal effects on adjacent structures. Krypton red lasers are the
preferred
method of treatment, as they are better able to penetrate nuclear sclerotic
cataracts and
vitreous hemorrhage than the argon lasers, which require more energy to
produce equal
levels of penetration.
The parameters used during laser retinal surgery may be modified depending on
the
goal of the photocoagulation. At lower power setting, using longer durations
of treatment
and producing larger spot sizes, the laser has a coagulative effect on small
vessels. Focal
laser photocoagulation is used in diabetes to stop lealcage of microaneurysms.
The laser
spot is place directly over the microaneurysm to achieve a slight whitening
and closure of
the aneurysm. When applied as a grid over an edematous area of retina, the
laser may
reduce microvascular leakage. At higher energy levels, laser ablation of
tissue is possible.
Panretinal photocoagulation is thought to be effective by destroying tissue,
reducing the
-35-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
amount of ischemic tissue in the eye. Confluent laser spots may be used over a
neovascular
membrane to obliterate the abnormal vessels.
It should be understood that the present method does not require a particular
order
of treatment. In one embodiment, the patient is first treated with
hyaluronidase (ACS) and
then laser treatment. In another embodiment the patient is first undergoes
laser treatment
followed by the hyaluronidase (ACS) treatment.
After treatment, the patients' eyes are to be examined on days one (1), two
(2), seven
(7), fifteen (15), thirty (30) and sixty (60). On each examination day the
patient is
monitored for vitreous liquefaction. Additionally, the patient is monitored
for posterior
vitreous detachments using indirect ophthalmoscopy with scleral depression.
Finally, the
extent of PDR presented by the patient is continuously monitored through
periodic retinal
examinations and fluorescein angiograms to monitor the extent of venous
beading, IRMA,
retinal ischemia, neovascularization, and vitreal hemorrhage. Evidence of new
neopolymerization or incomplete depolymerization of the vitreous would warrant
a repeat
treatment of the patient as described above.
EXAMPLE 10
Hyaluronidase Treatment of Age-Related Macular Degeneration
The present methodology also contemplates utility in the treatment of age-
related
macular degeneration (AMD). Age-related macular degeneration consists of a
gradual,
often bilateral decrease of vision. It is the most common cause of legal
blindness in adults.
It is probably caused by aging and vascular disease in the choriocapillaries
or the afferent
retinal vessels. There are basically two morphologic types of AMD: "dry" and
"wet".
The underlying abnormality of AlVID is the development of involutional changes
at
the level of Bruch's membrane and the retinal pigment epithelium (RPE). The
hallmark
lesion of such changes is the druse. Clinically, drusen (the plural form of
druse) appear as
small, yellow-white deposits at the level of the RPE. Drusen may be
categorized as hard,
soft or basal laminar drusen.
The present method is directed both to the treatment and prevention of wet and
dry
forms of AMD. In the wet form the disease, the condition is thought to affect
the
choriocapillaries. The choriocapillaries are a component of the choroid which
serves to
vascularize the globe. The choriocapillaries consists of a rich capillary
network that supply
most of the nutrition for the pigment epithelium and outer layers of the
retina. Damage to
-3 6-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
the choriocapillaries is thought to result ultimately in neovascular
complications, a cause of
macular degeneration.
In the dry form, nondisciform macular degeneration results from a partial or
total
obliteration of the underlying choriocapillaries. Ophthalmoscopically,
degeneration of the
retinal pigment epithelium and hole formation may be observed. Also,
subpigment
epithelial deposits of material such as calcium chelates or proteinaceous
material and others
may be observed. In dry AMD, secondary retinal changes generally occur
gradually,
resulting in the gradual loss of visual acuity. Nevertheless, in some
percentage of patients,
a severe loss of vision results.
The present method contemplates utility in treating dry AMD and preventing
macular degeneration through liquefaction of the vitreous. It is contemplated
that the
liquefaction of the vitreous would result in an increase in the rate of
clearance from the
retina of deposited material that later results in macular degeneration.
Wet AMD most frequently results from choriocapillary insufficiency, leading to
subsequent subpigment epithelial neovascularization. Neovascularization also
is thought to
occur as an adaptation of retinal vascularization to inadequate oxygenation as
a result of
vesicular damage. Neovascularization may also cause several other disorders
such as
detachment of the pigment epithelium and sensory retina. Typically the disease
usually
begins after 60 years of age, manifesting in both sexes equally and in
patients presenting the
disease, bilaterally.
Perhaps the most important complication of age-related macular degeneration is
the
development of defects in Bruch's membranes of the globe through which new
vessels
grow. This epithelial neovascularization may result in the production of
exudative deposits
in and under the retina. The neovascularization may also lead to hemorrhage
into the
vitreous, which may lead to degeneration of the retina's rods and cones, and
cystoid macular
edema (discussed below). A macular hole may form which results in irreversible
visual
loss.
Although affecting only 10% of patients with AMD, neovascular complications of
AMD account for the overwhelming majority of cases of severe visual loss. Risk
factors
include increasing age, soft drusen, nongeographic atrophy, family history,
hyperopia, and
retinal pigment epithelial detachments. Symptoms of choroidal
neovascularization in AMD
include metamorphopsia, paracentral scotomas or diminished central vision.
Ophthalinoscopic findings include subretinal fluid, blood, exudates, RPE
detachment,
-37-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
cystic retinal changes, or the presence of grayish green subretinal
neovascular membrane.
Fluorescein angiography is often an effective method of diagnosis. During this
diagnostic
procedure, progressive pooling of the dye in the subretinal space, seen as
blurring of the
boundaries of the lesion or leakage from undetermined sources are indicators
of the disease.
Other components of choroidal neovascular membranes as delineated by
fluorescein
angiography include elevated blocked fluorescence, flat blocked fluorescence,
blood, and
disciform scar.
The present understanding of neovascular AMD suggests that classic choroidal
neovascularization is the lesion component most strongly associated with rapid
visual
deterioration. Accordingly, treatment of AMD must encompass all neovascular
and
fibrovascular components of the lesion. At present, treatment is only
indicated when
classic neovascularization has boundaries that are well demarcated, and
photocoagulation
has been shown to be beneficial.
In eyes with extrafoveal choroidal neovascularization (>-200 microns from the
foveal center), argon laser photocoagulation diminished the incidence of
severe visual loss,
($6 lines) at 5 years from 64% to 46%. Recurrent neovascularization developed
in one-half
of laser-treated eyes, usually in the first year after treatment. Recurrent
neovasculaxization
was invariably associated with the development of severe visual loss.
In eyes with juxtafoveal choroidal neovascularization (1 to 199 microns from
the
foveal center), krypton laser photocoagulation diminished the incidence of
severe visual
loss from 45% to 31% at 1 year, although the difference between untreated and
treated
groups was less marked at 5 years.
Laser treatment remains an essential therapeutic method for the treatment of
AlVm,
however, the present method would augment this approach by reducing the
reoccurrence of
neovascularization.
Treatment of Age-Related Macular Degeneration
In this Example, a patient manifesting age-related macular degeneration is
treated
with an intravitreal injection of hyaluronidase (ACS). The purpose of this
treatment is to
reduce or prevent the development of neovascularization, macular disease, and
retinal
damage.
Once a patient reaches the age of 60, increased ophthalmic surveillance is
performed to detect the presence of AMD. This increased surveillance should
include
periodic retinal examinations and fluorescein angiograms to monitor for the
presence of
-3 8-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
subretinal fluid, blood, exudates, RPE detachment, cystic retinal changes, or
the presence of
grayish green subretinal neovascular membrane.
When AMID is diagnosed, a regime of hyaluronidase (ACS) treatment is
commenced coupled with or without other treatments such as photocoagulation.
As the
first step of treatment, the patient is to receive a full ophthalmic
examination to establish a
baseline of ocular health. The ophthalmic examination includes indirect
ophthalinoscopy,
slit-lamp biomicroscopy, peripheral retinal examination, intraocular pressure
measurements, visual acuity (unaided and best corrected) syrnptomatology,
fundus
photography, fluorescein 'angiography, electroretinography and A-scan
measurements.
Following the preliminary examination, an intravitreal injection of
hyaluronidase
(ACS) is given to the patient's affected eye manifesting AMD. If both eyes are
affected,
they may be treated separately. The eye to be treated is injected with 50 ~.1
of 50 LU. of the
hyaluronidase (ACS) ophthalmic solution (described above) intravitreally to
promote the
depolymerization of vitreous hyaluronic acid, resulting in the liquefaction of
the vitreous.
Laser photocoagulation treatment of the hyaluronidase (ACS) injected eyes may
be
required. The laser treatment protocol described in Examples 8 and 9 should be
followed
when treating AMD. In an alternative embodiment, photocoagulation treatment
occurs
before the enzyme treatment of the present method.
After treatment, the patients' eyes are to be examined on days one (1), two
(2), seven
(7), fifteen (15), thirty (30) and sixty (60). Because of the possibility of
reoccurrence, the
patient should return for periodic examinations on a monthly basis thereafter.
On each
examination day the patient is monitored for vitreous liquefaction.
Additionally, the patient
is monitored for posterior vitreous detachments using indirect ophthalmoscopy
with scleral
depression. Finally, the extent of AMD presented by the patient is
continuously monitored
through periodic retinal examinations and fluorescein angiograms to monitor
for the
presence of subretinal fluid, blood, exudates, RPE detachment, cystic retinal
changes, or the
presence of grayish green subretinal neovascular membrane. Additional
hyaluronidase
(ACS) and/or laser treatments may be required if indicia of reoccurring
neovascularization
are observed.
The following Example demonstrates the efficacy of the present method, even
without the use of photocoagulation.
-39-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Improvement in Symptoms of Macular Degeneration Following Intravitreal
Hyaluronidase Infection
A greater than seventy-nine (79) year old male human being presented with a
lustory of age-related macular degeneration, and uncorrected vision of 20:400
in his right
eye. A single dose of 100 LU. of the hyaluronidase (ACS) was injected
intravitreally into
his right eye. The other eye remained untreated.
The patient was repeatedly examined post-dose and the vision in his left
(untreated)
eye remained unchanged, while the vision in his right (treated) eye was
observed to
improve as follows:
Time Post-Dose Vision uncorrectedVision corrected
Baseline 20:400 none
3 days cf 1 ft. none
1 wk 20:400 none
2 wk 20:400 none
' 4 wk ~ 20:300 I none
*cf denotes finger counting
No adverse effects of the hyaluronidase (ACS) were observed in this
experiment.
EXAMPLE 11
Hyaluronidase Treatment of Amblyopia
The term amblyopia is derived from Greek and means dull vision (amblys--dull,
ops--eye). Poor vision is caused by abnormal development in visual areas of
the brain,
which is in turn caused by abnormal visual stimulation during early visual
development.
The pathology associated with amblyopia is not specific to the eye, rather, it
is located in
the visual areas of the brain including the lateral geniculate nucleus and the
striate cortex.
This abnormal development is caused by three mechanisms: (1) blurred retinal
image called
pattern distortion; (2) cortical suppression, or (3) both cortical suppression
plus pattern
distortion. The present method is primarily concerned with pattern distortions
caused by
media opacity. More specifically, the present method addresses issues of
vitreous opacity.
Amblyopic vision is usually defined as a difference of at least two Snellen
lines of
visual acuity. Critical to the treatment of amblyopia is early detection and
early
intervention. The strategy for treating amblyopia caused by vitreous opacity
is to provide a
clear retinal image by altering the opacity of the vitreous so that clear
vision results.
In this Example, a patient manifesting amblyopia resulting from vitreal
opacity was
treated with an intravitreal injection of hyaluronidase (ACS). The purpose of
this treatment
-40-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
was to reduce the opacity of the vitreous by increasing the exchange rate of
the liquid in the
vitreous.
A forty (40) year old female human being having a history of amblyopia
presented
with uncorrected vision of 20:400 in her right eye and corrected vision in
that eye of
20:200. A single 100 LTJ. dose of the hyaluronidase (ACS) was injected
intravitreally into
her right eye. The other eye remained untreated.
The patient was examined repeatedly post-dose and the vision in her left
(untreated)
eye remained unchanged while the vision in her right (treated) eye was
observed to improve
as follows:
Vision Post-DoseTime uncorrected Vision corrected
4 wk. 20:200 20:70(-1)
8 wk. 20:200 20:60 -2)
12 wk. 20:200 20:60(-1)
52 wk. ~ 20:200 20:60(-1)
No adverse effects of the hyaluronidase (ACS) were observed in this patient.
EXAMPLE 12
Hyaluronidase Treatment of Retinitis Pigmentosa
Retinitis pigmentosa (RP) is the name given to a group of heritable disorders
of
progressive retinal degeneration characterized by bilateral nyctalopia
constricted visual
fields and abnormality of the electroretinogram. Early symptoms include
difficulty with
darlc adaptation and midperipheral visual field loss. As the disease
progresses, visual field
loss advances, typically leaving a small central field of vision until
eventually even central
vision is affected. Central acuity may also be affected earlier in the course
of disease either
by cystoid macular edema, macular atrophy, or development of a posterior
subcapsular
cataract. RP represents a varied group of diseases whose common thread is the
abnormal
production of at least one protein in photoreceptor outer segments critical to
light
transduction.
One clinical result of RP is the destabilization of the blood-retinal barrier
of the
perifoveal capillaries and the optic nerve head. This destabilization results
in leakage of
fluorescein dye observed by angiography. In addition to leakage, accumulation
of fluid as
microcycts in the outer plexiform layer may occur and be observed. These fluid
filled cysts
may eventually burst, resulting in damage to the retinal layer. The present
method
contemplates treating RP related damage to the retina by promoting the
accelerated
clearance of the tissue fluid accumulating in the microcycts.
-41-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
A fifty-nine (59) year old male human being presented with a history of
retinitis
pigmentosa. The uncorrected vision in his left eye was 20:400 and with
correction was also
20:400. A single intravitreal injection of 100 LU. of the hyaluronidase (ACS)
was
administered to the left eye of the patient. The other eye remained untreated.
The patient was examined repeatedly following the dose of hyaluronidase (ACS)
and the vision in the patient's right (untreated) eye remained unchanged,
while the vision in
the patient's left (treated) eye was observed to improve as follows:
Time Intraocular Unaided VisionBest Corrected
Pressure Acuit Vision Acuit
Baseline 17 mm 20/400 20/400
1 Day Post Treatment26 mm HM*/1 ft HM/1 ft
1 wk. -- HM/1 ft HM/1 ft
2 wk. 14 mm cf'x* 3 ft. cf 3 ft.
3 wk. 14 mm 20:300 20:300
4 wk. 12 mm 20:200 20:80
8 wk. 15 mm 20:80 20:40
9 wk. 15 mm 20:60 20:40
wk. 18 mm I 20:80 I 20:40
HM* denotes "hand movement"
cf** denotes "finger counting"
10 The study results demonstrate that there were significant improvements in
the visual
performance of the subject, both with respect to Unaided Visual Acuity
(improving from
20:400 to 20:80) and Best Corrected Visual Acuity (improving from 20:400 to
20:40).
Also, while changes in the intraoculax pressure of the subject during the
treatment period
were observed, the intraoculax pressure appeared to return to baseline levels
approximately
two weeks after the time of injection. Although these results are from a
single patient, they
appear sufficiently promising to warrant further studies.
EXAMPLE 13
Hyaluronidase Treatment of Macular Holes
A rupture or bursting open of the macula is known as a macular hole.
Interestingly,
this condition usually occurs in women in their sixth through eighth decades,
or after
trauma such as lightening injury, solar injury, scleral buclcling, or in
staphylomatous eyes.
Symptoms include metamorphopsia and diminished visual acuity.
Macular hole formation is thought to result from tangential traction across
the
retinal surface induced by the posterior cortical vitreous with involvement of
fluid
movement within a posterior vitreous synergetic cavity. The posterior vitreous
syneresis
-42-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
cavity is present in the vast majority of patients presenting macular holes.
It is thought that
as the posterior vitreal gel retreats from the retinal surface, the resulting
gap between the
two surfaces creates an area wherein movement of the vitreous humor may
negatively
interact with the retinal surface. The tangential movement of the vitreous
humor within the
space of the posterior vitreous syneresis cavity is thought to promote tears
of the retinal
membrane, resulting in the creation of macular holes.
The present method contemplates the use of hyaluronidase (ACS) to
depolylnerize
the vitreous so as to eliminate the conditions which result in macular hole
formation. Upon
depolymerization of the vitreous, the posterior vitreous syneresis cavity is
eliminated as a
result of hyaluronidase (ACS)-mediated reorganization of the vitreous. The
elimination of
this cavity permits the fluid between the vitreous and the retina to move
freely about the
vitreal chamber, dispersing any harmful forces that would have otherwise have
been
directed against the retinal surface.
A patient presenting the early signs of macular hole formation is treated with
an
intravitreal injection of hyaluronidase (ACS). The patient to be treated
presents the various
signs of premacular hole formation. These include loss of the foveal
depression associated
with a yellow foveal spot or ring. The fovea has begun to thin in the region
of hole
formation and the lesion may obtain a reddish appearance. Fluorescein
angiography at this
stage may appear normal or show faint hyperfluorescence. The appearance of an
eccentric
full thicl~ness dehiscence denotes an advanced early stage of the disease.
Upon observance
of these symptoms hyaluronidase (ACS) treatment is commenced.
The hyaluronidase (ACS) treatment of the present method is commenced when the
formation of a macular hole is diagnosed. The patient is to receive a full
ophthalinic
examination to establish a baseline of ocular health. The ophthalmic
examination included
indirect ophthalmoscopy, slit-lamp biomicroscopy, peripheral retinal
examination,
intraocular pressure measurements, visual acuity (unaided and best corrected)
symptomatology, fundus photography, fluorescein angiography,
electroretinography and A-
scan measurements.
Following the preliminary examination, an intravitreal injection of
hyaluronidase
(ACS) is given to the patient's affected eye. If both eyes are affected, they
may be treated
separately. The eye to be treated is injected with 50 ~.1 of 50 LU. of the
hyaluronidase
(ACS) ophthalnic solution described above intravitreally to promote the
depolymerization
of vitreous hyaluronic acid, resulting in the liquefaction of the vitreous.
-43-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
After treatment, the patients' eyes are to be examined on days one (1), two
(2), seven
(7), fifteen (15), thirty (30) and sixty (60). On each examination day the
patient is
monitored for vitreous liquefaction. Fluorescein angiography, considered a
particularly
effect method of monitoring the course of the treatment, is also performed.
Additionally,
the patient is monitored for posterior vitreous detachments using indirect
ophthalinoscopy
with scleral depression.
EXAMPLE 14
Hyaluronidase Treatment of Macular Exudates
Macular exudates are material that penetrates the blood-retina barrier and
seeps
through the macula into the vitreal chamber. There are two lcinds, soft
exudates and hard
exudates. The soft exudates are actually not exudates but clusters of ganglion
cell axons in
the nerve fiber layer that have undergone a bulbous dilation at a site of
ischemic damage or
infarction. Hard exudates are commonly exuded as a result of microvascular
changes in
background retinopathy. Hard exudates appear yellow and waxy are often
deposited in a
circular fashion about the macula. They consist of lipid and proteinaceous
material derived
from the exudation of serum components from leaking vessels or from the lipid
products of
degenerating neural elements within the retina. Adsorption of hard exudates is
primarily
mediated by macrophagic resorption, however, the rate of this process may be
slow since
exudation often occurs in the outer plexiform layer within the avasculax zone
of the retina.
The present method is particularly useful in reducing the extent of exudative
accumulation
resulting from the destabilization of the retinal membrane since hyaluronidase
(ACS)
depolymerization of the vitreous promotes an increased turn-over rate of the
aqueous
components of the vitreous.
A patient presenting macular exudates is treated with hyaluronidase (ACS)
injection
method of treatment. The patient is to receive a full ophthalmic examination
to establish a
baseline of ocular health. The ophthalmic examination included indirect
ophthalmoscopy,
slit-lamp biomicroscopy, peripheral retinal examination, intraocular pressure
measurements, visual acuity (unaided and best corrected) symptomatology,
fundus
photography, fluorescein angiography, electroretinography and A-scan
measurements.
Following the preliminary examination, an intravitreal injection of
hyaluronidase
(ACS) is given to the patient's affected eye. If both eyes are affected, they
may be treated
separately. The eye to be treated is injected with 50 ~,l of 50 LU. of the
hyaluronidase
-44-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
(ACS) ophthalmic solution described above intravitreally to promote the
depolymerization
of vitreous hyaluronic acid, resulting in the liquefaction of the vitreous.
After treatment, the patients' eyes are to be examined on days one (1), two
(2), seven
(7), fifteen (15), thirty (30) and sixty (60). On each examination day the
patient is
monitored for vitreous liquefaction. Fluorescein angiography, considered a
particularly
effect method of monitoring the course of the treatment, is also performed.
Additionally,
the patient is monitored for posterior vitreous detachments using indirect
ophthalmoscopy
with scleral depression.
EXAMPLE 15
Treatment of Cystoid Macular Edema
Cystoid macular edema is a common ocular abnormality resulting form a diverse
group of etiologies. Most the causes of this condition stem from a disturbance
of the blood-
retinal barrier of the perifoveal capillaries and the optic nerve head that
result in fluid
leakage which accumulates in microcysts of the outer plexiform layer. This
region is a
relatively thin and under vascularized area of the retina. Clinically, a
cystoid macular
edema produces a honey-comb appearance when examined with fluorescein
angiography.
As the edema progresses, the outer plexifonn layer may rupture, producing a
lamellar hole.
The hole may be confined to the inner layer of the retina or it may eventually
progress to a
complete macular hole.
The present method contemplates the treatment of cystoid macular edema and the
prevention of macular hole formation through the hyaluronidase (ACS)-mediated
depolymerization of the vitreous.
A patient presenting the indicia of cystoid macular edema is treated with an
intravitreal hyaluroudase (ACS) injection as described in Examples 13 and 14.
EXAMPLE 16
Other Pharmacological Uses of Hyaluronidase
Hyaluronidase has been used therapeutically for many years now. Its proven and
diverse effects, which occur mainly in intercellular connective tissue, are
primarily
attributable to the breakdown of hyaluronic acid in the tissue. The
therapeutically useful
consequences of this action, i.e. reduced viscosity of intercellular cement
and increased
membrane and vascular permeability, are due to its "spreading effect". The
permeability-
enhancing effect of hyaluronidase after administration of fluids and/or
radiopaque media is
of therapeutic significance.
-45-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Hyaluronidase - The Spreading Effect:
~ Reduces the viscosity of intercellular cement.
~ Increases membrane and vascular permeability.
_> Enhances spatial processes and leads to temporal acceleration when injected
via
intracutaneous, subcutaneous, intradermal, intravenous or intramuscular route.
Results: Improves diffusion and penetration of solutions, suspensions and
emulsions into
the surrounding tissue.
Hyaluronidase accelerates and enhances the absorbtion of injected drugs
(antibiotics, cytostatic agents, local anesthesia, chemotherapeutic agents,
antivirals, etc.) by
the tissue, even when large volumes of the medications are administered in
solution,
suspension or emulsion form.
Hyaluronidase has been successfully used in orthopedics, surgery,
ophthalmology,
internal medicine, oncology, gynecology, dermatology, etc. for many years.
The experimental use of hyaluronidase was tested in numerous areas of
medicine.
The substance has been administered clinically in various indications and
therapies.
Ophthalmology / peri- and retro-bulbar anesthesia: Ophthalmology is now an
important and well documented area of indication for hyaluronidase (Farr C. et
al. 1997
Wieh Med Wschr 147:1-~).
Hyaluronic acid is often applied during ophthalmic surgery (e.g., cataract
surgery),
for example, to keep the anterior chamber of the eye intact or to protect the
corneal
endothelium during lens implantation. Tlis results in an increase in
intraocular pressure.
Measurements have shown that introduction of hyaluronidase in the anterior
chamber of the eye can effectively decrease the intraocular pressure
postoperatively.
Hyaluronidase was also found to be effective in reducing the intraocular
pressure in
patients who underwent trabeculectomy for treatment of wide-angle glaucoma
(doses of
300 IU were administered as a subconjunctival injection). The authors
concluded that
hyaluronidase reduced the number of complications and improved the prognosis
of
trabeculectomy. Hyaluronidase can also be helpful in retro- and peribulbar
anesthesia for
cataract surgery when used in combination with local anesthetics such as
lidocaine and
bupivacaine (with or without adrenaline). The effects of hyaluronidase in
local anesthesia
of the eye were reported back in 1949. Later research studied the effects of
the combined
use of procaine + adrenaline + hyaluronidase for retrobulbar anesthesia in
patients.
-46-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Improvement of akinesia of the eye muscles was observed in subjective
assessments. The hyaluronidase-induced improvement of the action of anesthesia
may be
due to the fact that the orbita is an optimal inj ection site.
In a double-blind comparison of bupivacaine versus lidocaine with and without
hyaluronidase in groups of 50 patients each, investigators observed a
significantly larger
number of patients with complete anesthesia in the hyaluronidase group. It was
also found
that none of these patients required additional anesthetics, because the
induced muscular
blockade persisted throughout the entire operation. These findings were
unequivocally
confirmed in a later study.
The onset of effect of local anesthesia in conjunction with hyaluronidase was
investigated in 1990. Investigators found that, in all patients in whom the
enzyme
supplement was injected together with an anesthetic, the anesthetic effect
required for
surgery occurred within 5 minutes after the injection. In the control groups,
on the other
hand, an additional dose of anesthesia was required, and the onset of the
anesthetic effect
occurred later.
The addition of hyaluronidase to local anesthesia improved the conditions for
surgery. Hyaluronidase was found to be highly effective when added to
lidocaine and
noradrenaline in retrobulbar anesthesia in senile cataract operations.
In one clinical study, the addition of 75 IU of hyaluronidase to 0.75%
bupivacaine
and 2% mepivacaine lead to improved motor blockade, analgesia and hypotonus of
the
eyeball.
Studies on the use of hyaluronidase in strabotomies demonstrated that a
sufficient
anesthetic effect of mepivacaine and lidocaine can be achieved if supplemented
with
hyaluronidase. Even small volumes of hyaluronidase displayed the desired
spreading
effect.
The findings for retrobulbax anesthesia generally apply for peribulbar and
subconjunctival applications as well. The combination of local anesthetics
with
hyaluronidase leads to a reliable blockade of the ocular muscles and, thus, to
an
improvement of operation conditions.
The effect of hyaluronidase on local anesthesia was again studied in another
prospective, randomized, controlled, double-blind study. This study also
showed that the
addition of hyaluronidase can lead to additive effects.
-47-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
These results were confirmed when 80 patients with senile cataracts were
administered 150 1U of hyaluronidase as a supplement to anesthesia. Their
results were
later confirmed in another group of 70 patients who received 250 ILJ of
hyaluronidase.
Investigators demonstrated that ocular circulatory changes occur during the
combined use of lidocaine / bupivacaine and hyaluronidase. They detected
significant
reduction in the ocular pulsation volume and a significant reduction in
intraocular pressure.
Local anesthesia with the addition of 150 ICT of hyaluronidase proved to be a
reliable alternative for cataract operations.
Regarding peribulbar anesthesia, investigators recommended that a mixture of
2%
lidocaine, 0.5% bupivacaine, and 1550 ICT hyaluronidase be warmed to body
temperature to
eliminate symptoms of pain in the target areas. In a separate study, two
concentrations of
hyaluroudase were used as a supplement to peribulbar anesthesia. Three groups
received
either 0, 50 or 150 ICT of the hyaluronidase supplement, and the onset of
effect, analgesia
and akinesia were assessed. No statistically significant differences between
the 50 ICT and
150 IU hyaluroiudase groups could be detected. The authors concluded that the
addition of
hyaluronidase did not lead to any complications.
After comparing the results of peribulbar anesthesia with retrobulbar
anesthesia,
other authors concluded that the addition of hyaluronidase is useful in both
cases, that
hyaluronidase is extremely effective in preventing the vitreous body from
bulging into the
posterior chamber of the eye, and that it significantly reduces the occurrence
of vis a tergo.
Hyaluronidase makes is possible for lidocaine and bupivacaine to spread more
rapidly within the peribulbar space.
The injection pressure of local anesthesia administered prior to cataract
operations
was investigated in 50 patients in a double-blind study. The study concluded
that
significant (sufficient) akinesia of the extraocular muscles can be achieved
by
administration of 1% etidocaine, 0.5% bupivacaine, and 50 ILJ hyaluronidase.
Glaucoma: Hyaluronidase is useful for treatment of glaucoma or to alleviate
intraocular pressure.
Combination with local anesthetics: When hyaluronidase was combined with a
local anesthetic, e.g., procaine or lidocaine, the onset of effect was
quicker, the analgesic
region was enlarged, and postoperative pain was significantly reduced.
One study showed that the anesthetized area after subcutaneous injection of a
combination of procaine and hyaluronidase was almost twice as large as that of
procaine
-48-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
alone. This study also demonstrated that the duration of anesthesia after
administration of
combined preparations of hyaluronidase and procaine / adrenaline led to a 6-
fold extension
of the anesthetic effect.
The effects of bupivacaine plus adrenaline with or without the addition of
hyaluronidase on blockade of the axillary plexus brachialis were compared in a
double-
blind study. The enzyme neither influenced the time of occurrence of
anesthesia, nor the
occurrence of inadequate blockade of the plexus, nor the level of the plasma
bupivacaine
level. The study demonstrated that the duration of anesthesia was reduced.
Orthopedics, diseases of the supportive and locomotive apparatus: For many
years now, hyaluronidase has been successfully used for treatment of various
diseases of the
supportive and locomotive apparatus, e.g., acute conditions of the synovial
sheath,
surrounding connective tissue and varied inflammations in these areas
(paratendinitis
crepitans, humeroscapular periarthritis, humeral epicondylitis, tibial
condylitis, radial
styloiditis, etc.). Good treatment results can usually be achieved (especially
in combination
with exercise or physiotherapy) if hyaluronidase therapy is started as early
as possible, even
if the affected limb cannot be immobilized. Investigators successfully treated
patients with
acute tendovaginitis crepitans with hyaluronidase in 1968.
Combination therapy with hyaluronidase (300 IU) and Vitamin B12 injections for
treatment of peritendinitis and periarthritis humeroscapularis has been
reported. The
efficacy of hyaluronidase in the treatment of epicondylitis and tendovaginitis
was studied in
a total of 53 patients. Daily intravenous doses of 1500 to 3000 units of
hyaluronidase led to
a decrease in symptoms or clear relief from complaints.
The findings from other studies show the same treatment success in
paratendinitis,
humeral epicondylitis, humeroscapular periarthritis, and radial and ulnar
styloiditis; a dose
of 3000 ICT of hyaluronidase every two days was used in most cases.
Hyaluronidase was successfully used in combination with mephenesin for
treatment
of arthrosis, primarily in patients with gonarthrosis. The success rate in
chronic cases was
particularly impressive.
The findings that i.v. administration of hyaluronidase in patients with
Bechterew's
disease produced long-lasting effects is of great therapeutic significance.
Administration of
an average 1500 to 9000 ICT of hyaluronidase i.v., every 3 days increased the
mobility of
patients studied.
-49-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Regarding its mode of action, investigators assume that hyaluxonidase
depolymerizes mucopolysaccharides deposited in the connective tissue matrix
during
extraosseous calcification processes that occur due to increased mesenchymal
metabolism.
If calcium salts are present, they can be depolymerized as long as calcium
does not occur as
tertiary calcium phosphate. This can restrict the formation of extraosseous
calcification.
The improvement of flexibility (e.g., in the affected spinal segment) could be
attributed to
the presumed depolymerization resulting from the loosening of previously
formed
connective structures. It is assumed that hyaluronidase is able to influence
the composition
and structure of the dermis, and that it can promote the re-synthesis of the
proteoglycans.
In the treatment of joint stiffness, which often occurs as a complication of
supracondylar
fractures, prior or simultaneous administration of hyaluronidase can provide
the patient
quicker relief from symptoms.
Treatment of malignant diseases: When used as a supplement to chemotherapy of
malignant tumors, hyaluronidase can dissolve hyaluronic acid-containing areas
around
tumor cells and tumor cell conglomerates, thereby enabling a higher
concentration of the
cytostatic agent to take effect in the desired target area. Moreover,
hyaluronidase may
induce a related enhancement of immunological defensive processes, e.g., by
creating direct
contact between immunocompetent cells ("natural killer cells") and antigens on
the tumor
surface.
The usefulness of hyaluronidase (i.v., i.m. and intravesical) for treatment of
malignant diseases (hematological systemic diseases, carcinomas of the breast,
cerebral
metastases, glioma, squamous cell carcinomas in the ENT region,
adenocarcinomas of the
lung and colon, and carcinomas of the bladder) is the subject of various
clinical studies. In
isolated cases, hyaluronidase was usually found to increase the response rate
to cytostatic
agents if high doses of the enzyme are administered prior to administration of
the cytostatic
agent. Hyaluronidase supplements can improve the patient's response to
chemotherapy
when used in therapy-resistant patients with malignant hematological diseases.
In oncology, hyaluronidase has proven to be particularly useful when
administered
as a supplement to cytostatic agents like doxorubicin and adriamycin. The
enzyme
improves the penetration of doxorubicin in the cells and increases the
activity of adriamycin
in breast cancer.
The combined administration of cisplatin, vindesin, hyaluronidase and
radiation
therapy for treatment of advanced squamous cell carcinoma in the head and neck
region
-50-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
was found to be highly effective: a high rate of remissions arid improved
tolerance of
cytostatic therapy was observed in carcinoma patients.
Hyaluronidase led to a decrease in adhesion-related multicellular drug
resistance in
carcinomas of the breast. This mechanism of action is based on the reduction
of cell-
contact-dependent inhibition of growth and on the sensitization of cells for
the cytostatic
agents.
Hyaluronidase in tumor treatment:
~ Hyaluronidase increases the effects of cytostatic agents used for treatment
of
such malignant diseases as hematological systemic diseases, carcinomas of the
breast, cerebral metastases, glioma, squamous cell carcinomas in the ear, nose
and throat region, adenocarcinomas of the lung and colon, and carcinomas of
the
bladder.
~ In clinical studies, hyaluronidase was found to induce cessation of growth
(remission) of various tumors.
~ Therapy-resistant patients respond better to cytostatic agents if an
intravesical
dose of the enzyme is instilled prior to the cytostatic drug.
~ Hyaluronidase can improve the subjective well-being and the quality of life
of
tumor patients.
Dermatology: Investigators have concluded that hyaluronidase is useful in
certain
dermatological diseases, such as, for example, progressive scleroderma, which
is a systemic
disorder of the entire vascular connective tissue system, with its most
important
characteristic being the displacement of collagen fractions. Of
pathomechanistic
significance is the fact that the histomorphological skin changes that occur
in scleroderma
begin with a dermal edema rich in acidic mucopolysaccharides (hyaluronic acid,
chondroitin sulphate). Histopathological and chemical tests have shown that
part of the
ground substa~ice occurs as cement in the collagen fibers. It would therefore
appear that
acidic mucopolysaccharides, soluble collagen, and polymeric collagen are
responsible for
the sclerosis.
Investigators published further findings on high-dose i.v. hyaluronidase
therapy in
patients with primary diseases such as keloids and localized scleroderma. The
substance
achieved good results in both diseases. The same applies for progressive
scleroderma, but
the increase in active flexibility of the joint was not permanent. High-dose
i.v.
-51-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
administration of hyaluronidase for treatment of progressive scleroderma was
therefore
recommended only as a supplementary medication, as no influence on older areas
of
sclerosis could be demonstrated. Assessment of the selected routes of
administration
showed that local administration of the substance to the affected areas of the
skin did not
S lead to treatment success. Only intravenous injection or infusion achieved,
at minimum,
improvement of symptoms in most patients.
Treatment of myocardial infarction: The use of hyaluronidase for treatment of
acute myocardial infarction was first described in 1959. Studies have shown
that the
admiiustration of hyaluronidase in the acute stage, i.e., in the early stage
of fresh
myocardial infarction (2 to 4 hours after the onset of infarction) can reduce
the size of the
necrotic area in the heart.
Investigators studied medications that lead to a reduction in infarction size,
e.g.,
beta blockers, nitrates, calcium antagonists, etc. Hyaluronidase was found to
have a
favorable effect on concomitantly administered thrombolytic agents such as
streptokinase.
This appears to be attributable to the ability of hyaluronidase to entrap
oxygen radicals.
Statisticians performed meta-analyses in which they studied the role of
hyaluronidase and other cardiovascular drugs with a potential for reducing the
size of
myocardial infarction.
They found that hyaluronidase supplements reduced the mortality rate by 15 to
20
percent.
Investigators also recommended the use of hyaluroiudase, in addition to
conventional agents for treatment of acute myocardial infarction (nitrates,
beta receptor
blockers, calcium antagonists).
Despite differences in the data and, in some case, contradictory findings, it
appears
that the use of hyaluronidase for treatment of myocardial infarction has been
definitively
proven and confirmed.
Miscellaneous indications: Another indication is for treatment of submucosal
fibrosis. Experience with 150 patients over a 10-year period has shown that
the
combination of hyaluronidase and dexamethasone is able to reduce symptoms over
a long
period of time in most cases.
Local injection of chymotrypsin, hyaluronidase and dexamethasone was also
reported to induce good treatment results. Surgical excision was, however,
performed in
therapy-resistant patients.
-52-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
326 patients with oral submucosal fibrosis were randomized into 2 groups and
treated with either steroids and hyaluronidase or with topical vitamin A
steroid and oral
iron preparations. Treatment with steroids and hyaluronidase was found to be
much less
problematic.
Hyaluronidase has also been used in hair transplants and in therapeutic and
cosmetic
surgery of the scalp. It was found effective in improving the diffusion of
local anesthetics.
The use of hyaluronidase for prevention of postoperative adhesions following
surgery has also been reported.
In cerebral abscess, hyaluronidase was alternatively combined with
dexamethasone
or antibiotics, primarily to eliminate edema in high-risk patients.
Hyaluronidase was found to improve the absorption of locally administered
drugs
and to reduce the risk of progression of skin necrosis in patients treated
with intravenously
admiiustered Vinca alkaloids.
The use of hyaluronidase as an antidote for the extravasation of
chemotherapeutic
agents has also been described. Hyaluronidase is one of the few antidotes that
can be used
as an antidote for Vinca alkaloids or epipodophyllotoxins such as etoposide.
Gynecology is another area of application for hyaluronidase. When injected in
the
perineal region prior to the expulsive stage of labor, hyaluronidase was found
to soften the
consistency of the birth canal of first-time mothers, which often eliminated
the need for
episiotomy.
Hyaluronidase is also useful for facilitation of partial and complete
aspiration of
viscous joint effusions and pleural effusions, i.e., it liquefies the
effusions. The enzyme is
also used for treatment of edema of various origins and for treatment of
arthritic joint
changes.
Hyaluronidase is a treatment for corneaplasty, corneal scars, opacification,
and haze,
and cornea in need of delamination.
Hyaluronidase can be used as an alternative or adjunct to conventional
mechaiucal
vitrectomy.
Hyaluronidase is also useful for the induction of retinal detachments.
Hyaluronidase is indicated as an adjuvant to increase the absorption and
dispersion
of other injected drugs; for hypodermoclysis; and as an adjunct in
subcutaneous urography
for improving resorption of radiopaque agents.
-53-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Summary: The proven and diverse activity of hyaluronidase occurs mainly in the
intercellular connective tissue. This action is clearly attributable to the
breakdown of
hyaluronic acid in the tissue. The therapeutically useful consequences of this
action
(reduced viscosity of intercellular cement, increased the permeability of
membranes and
vessels) occur due to a "spreading effect."
The hyaluronidase-related enhancement of diffusion and increase in
permeability
that occurs after administration of liquids and/or radiopaque media is of
therapeutic
significance. The substance is able to accelerate and increase the absorption
of drugs
(antibiotics, cytostatics, local anesthetics, etc.) by the tissue, even when
large volumes of
the medication are injected in solution, suspension or emulsion form. If it is
not possible to
administer a certain drug intravenously in cases where a rapid onset of effect
is necessary, a
"pre-injection" of hyaluronidase can accelerate (by 200 to 300%) the
absorption of
subcutaneous or intramuscular doses of the drug in the bloodstream, which is
of particular
significance for internal medicine.
The efficacy of hyaluronidase in treatment of disorders of the supportive and
locomotive system can be attributed to the so-called "softening effect" of the
enzyme.
When administered early and, especially, when coupled with additional exercise
and/or physiotherapy measures, the "antiphlogistic" effect of hyaluronidase
makes it
possible to control acute symptoms involving the synovial sheaths and the
surrounding
connective tissue (peritendinitis crepitans, humeroscapular periarthritis,
humeral
epicondylitis, tibial condylitis, radial styloiditis, etc.)
Joint stiffness (e.g., due to supracondylar fracture) can also be treated
successfully.
Due to its diffusion potential, hyaluronidase can also be used for treatment
of
posttraumatic hematomas or edemas of any origin, and for liquefaction of joint
and pleural
effusions in orthopedics.
Hyaluronidase is indicated to be useful as an anti-edema and anti-inflammatory
agent in the prevention of transplant rejection. It has been shown in pre-
clinical
experiments to lend itself to this role, because it breaks down hyaluronan in
damaged
tissues. Hyaluronan, a glucosaminoglycan with unique water-binding capacity,
draws water
into some transplanted organs causing edema. This in turn impairs organ
function which
may lead to the transplanted organ failure and being rejected. In addition to
attracting
water, hyaluronan attracts certain cells of the immune system and therefore
may be
instrumental in initiating inflammatory reactions. Studies have confirmed that
-54-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
hyaluronidase treatment can be used to reduce edema and inflammation after
organ
transplantation.
When hyaluronidase is administered prior to administration of a local
anesthetic
such as procaine ("pre-injection"), the onset of effect of the anesthetic is
quicker, the
anesthetic region is larger, and the pain after completion of the procedure is
significantly
lower.
Optimal and efficient combinations of hyaluronidase and local anesthetics are
now
widely used, particularly in ophthalinology and especially in cataract
surgery. The
preoperative administration of hyaluronidase with certain local anesthetics
(procaine,
lidocaine, bupivacaine, etc.) for retro- and peribulbar anesthesia is useful
in various
ophthalinologic operations. The enzyme accelerates the onset of effect of the
anesthetic
agent and causes reliable blockade of the eye muscles which, in turn, creates
excellent
conditions for surgery. In combination with vasopressors such as adrenaline,
hyaluronidase
increases the duration of anesthesia in the treated area and prevents the
rapid diminishment
of local anesthesia. Hyaluronidase is also effective for treatment of
postoperatively
increased internal eye pressure due to the administration of viscoelastic
substances such as
sodium hyaluronate during ophthalmic surgery.
Hyaluronidase is also widely used in dermatology, i.e., in selected skin
disorders
involving the connective tissue system and characterized by degeneration of it
(scleroderma, keloid formation, psoriasis, chronic varicose ulcer, etc.).
Hyaluronidase is also useful in gynecology, i.e., for prevention of
episiotomy.
The usefulness of hyaluronidase has been validated for treatment of myocardial
infarction.
The most recent clinical studies have shown that hyaluronidase is helpful as a
supplement to chemotherapy in patients with cancer (myeloma, Hodgkin's
disease, non-
Hodgkin's lymphoma, breast cancer [also with concomitant cerebral metastasis],
cerebral
lymphomas, gliomas, squamous cell carcinomas in the ear, nose and throat
region, and
carcinomas of the bladder). The enzyme not only increases the patient's
response to the
cytostatic agents, but also drastically improves the patient's overall
subjective feeling of
well-being and the remission rate.
Preliminary evidence of the usefulness of this therapy principal has also been
found
in colon carcinoma, adenocarcinoma of the lung, bronchial carcinoma,
hypernephroma,
carcinomas of the stomach, pancreas and ovaries, myelosarcoma, and neurinoma.
In
-55-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
different tumor types, the administration of hyaluronidase was found to induce
temporary
cessation of tumor growth, and it improved the response of therapy-resistant
patients to the
cytostatic agent. Furthermore, it is assumed that hyaluronidase stimulates the
immwie
system. Positive findings after administration of the enzyme as a supplement
to
chemotherapy of malignant tumors give one reason to expect that hyaluronidase
may one
day be able to expand the potentials of anti-tumor chemotherapy and improve
the results of
therapy.
From a toxicological point of view and in light of the wide range of available
data
on the various applications of hyaluronidase, there does not seem to be any
reason to
prohibit the use of the enzyme in humans.
No serious pathological organ changes were detected in an acute toxicity test
with
administration of a single dose of hyaluronidase, nor after long-term
administration of the
substance.
However, one should always remember that the enzyme is antigenic in nature,
when
used alone or mixed with other substances. These effects can never be
completely excluded
due to the complicated nature of the process used to.isolate the enzyme. Thus,
there is a
need for a hyaluronidase preparation suitable for pharmaceutical applications,
wluch need is
met by the disclosed process for isolating and purifying ovine hyalurondase.
In light of the positive clinical findings on hyaluronidase, one may conclude
that
hyaluronidase is a therapeutically versatile enzyme that promises to be a
therapeutically
useful agent in new areas of medical practice now and in the future.
EXAMPLE 17
Hyaluronidase for Injection
In this Example for use of hyaluronidase as a spreading agent, hyaluronidase
for
inj ection dehydrated in the solid state under high vacuum with the inactive
ingredients
listed below, is supplied as a sterile, nonpreserved, white, odorless,
amorphous solid. The
product is to be reconstituted with Sodium Chloride Injection, USP, before
use.
Each vial of 6200 USP units contains 5 mg lactose, 1.92 mg potassium phosphate
dibasic, and 1.22 mg potassium phosphate monobasic.
The USP/NF hyaluronidase unit is equivalent to the turbidity-reducing (TR)
unit
and equal to 0.81 International Units (IUD.
The reconstituted solution is clear and colorless, with an approximate pH of
6.7 and
osmolality of 290 to 310 mOsm.
-56-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
Hyaluronidase for injection is to be reconstituted in a vial to a
concentration of 1000
Units/mL of Sodium Chloride Injection, USP by adding 6.2 mL of solution to the
vial.
Prior to administration, the reconstituted solution should be further diluted
to the desired
concentration, commonly 150 Units/mL, see table below. The resulting solution
should be
used immediately after preparation.
A 1mL syringe and a 5-micron filter needle are supplied in a hyaluronidase for
injection kit. Following reconstitution of hyaluronidase for injection, as
described above,
apply the 5-micron filter needle to the 1mL syringe. Draw the desired amount
of
hyaluronidase for injection into the syringe, and dilute according to the
table below.
Remove the filter needle and apply a needle appropriate for the intended
injection.
Desired Amount of hyaluronidase reconstitutedAdditional Saline
Concentration solution (1000 Units/mL)
50 Units/mL 0.05 mL 0.95 mL
75 Units/mL 0.075 mL 0.925 mL
150 Units/mL 0.15 mL 0.85 mL
300 Units/mL 0.3 mL 0.7 mL
-57-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
a~
b, v~ O v~
O ~ O ~ ~ O
O . p ~ ,.~ bA . cwn
_ U '~~' O O
° ~ z ~ '~ '~ o ~ ~ '~ z z z z z
x
r~ ~ . ~b . ~ ~ rx
0
z
''
~ ~,
w
o ~ ~ ~'~ ~ ~ '~ '~
o ' Gn ~ . ~ '~ '~ ,~a
0
o 'd ~ o ~ ~ ~ ~ ~ ~ ~ ~ °
o ~,~~ '~ ~ ~ z z z z
a~ z ~ ~ o ,.o ~, ~ ~ ~a
'a
,p
f3'~ '~ ~ r i ~. . ~ . °~ a
jF ~ '.~ p N N p
pq s0..~ U . ~ . ~ U
0
0
U
~ H
O ~ ~ ~ ~ O ~ ~ ~ ~ O O O
b4~ ~ ~ i i
o N ~ ~ ~ ~ ~ ~ '"' M ~n ,--i
~°O °00~ ~~~~~~jr% r% ~;
0
A ~ °' pa
w
o ~ H ~ 'b :b ~d ~ 0 0 0 0 0
'" ~ + + O o 0 0 ~ ~ ~ ~ '~ ~ o
x ~i
H
O ,-, N M ~Y ~n ~O l~ 00 01 O '-' N M
E~
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
+~
0
W .~ .~
o
~
U U U
a
o o o o
~ ~ ~
o o o o
~, ~, ~, ~,
~ ~ ~
o o o .
o
a
xa xa xa xa
0
0
b
~
y ~, a
~
o ~ ~ ~
o o o
-, ,-, ,-,
M M M
U
p ~ ~ ~ ~ ~
.-~ r r 01
M
O O O
H 1-1 H
C/~ W N o
~
4-, b
O
U
U
.
W
z z z z
y
~
U O
U m
b
M M M M
N
N C% C% C~ p
N
N
H o o o o
~ ~ ~
~ ~
b b
Pq
O N O ~ ~
i ~ ~ ~
.i U
fYl U
~G
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
a~
,~ o ,~ o ~ o
o ~ ~ ~ .~ vV, ~ .
0 0 0
o ~ ~ o ~ ~ o
~ o ~ ~ o ~ ~ o
x a
~ b
o ~ o ~ o ~ o
o ~, o ~, o ~, o
U W
a
x ~ x ~ x ~
a a a a
0
b
o ~- o ~- o
°' ~ o ~ o ~ o
N '9' ~ r, M ,-r M ,_., M
4~ ~ x ~
O U O U O U o
i
O G~ r.-~ ~-i i-i
~' H N
Q, ~a
N N N N
O ~ O O O O
z z z z
a
U
C
.~,~ r~ ~ M M M M
N
M
N ~ r~ r~
U U U
H
o a, 0 0 0
b ~ b
G4
~°. N .~ o .~
p4 U A
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
0
bA
° ~ ~ a~
~ ~. ~ o ~, ~ ca ~, ~, ca
~ o a A ~ '-a ~a 'd .~ b
H ~ ~ o ~° ~ ~ o0
x~
A U
..-,
0 0 0 0 0
°
° .~
o ~ o ~ o'~ o
v ~ ~ ~ N ~ N N N
U U U U U ~
~ ~ cG
M ~ ~
i~ ~ ~ ~ ~ ~ ~ N ~ N O
v
'~,'i N O N ~ N : ~ .~ .~~"' . O
cd CC '.,.~., ~' O ~-
N U
~' ~ ° ~ N ~ °
p~ ~~', ~ ~x' ~
w w
o
o ~ ,b
'U
N
H ~i ~i H H
'-'
~0~~0 0 0 X00
V
°
0
'w, o ° o
0
~, ~ b -'-~' V
V ° w ~d b N ~ ~ ~ ~ N O
o ~ aWd a~ 'd o
~, p" ..~ .--i
c~
b0 ~ d' bA N a~ ~ '° p ~, ~ a~
° ~, ~ a~ ~ o ;~
.N a~ a ~ ~~ , o °
U ~ a a
a
0 0 0 0 0 0
W a~ a~ a~ a~ a~ N
p ~ ~ ~ ~ ~ _O
H
w w w w w
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
It will be appreciated by those skilled in the art that the invention has been
described hereabove with reference to certain presently preferred embodiments
and
examples only, and no effort has been made to exhaustively describe all
embodiments in
which the invention may take physical form or be practiced. Indeed, various
modifications may be made to the specific embodiments and examples described
here
above, without departing from the intended spirit and scope of the present
invention.
Accordingly, it is intended that all such reasonable modifications to the
above be included
within the scope of the following claims.
-62-
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
ISTA.076VPCSEQLIST.TXT
SEQUENCE LISTING
<110> ISTA Pharmaceuticals
Biozyme Laboratories, Ltd.
Craig, William 5.
Chesham, john
<120> PROCEDURES FOR ISOLATING AND PURIFYING
OVINE HYALURONIDASE
<130> ISTA.076vPC
<150> US 60/463,516
<151> 2003-04-15
<160> 1
<170> FastSEQ for Windows Version 4.0
<210> 1
<211> 520
<212> PRT
<213> ovis ovis
<400> 1
Leu Asp Phe Arg Ala Pro Pro Leu Ile Ser Asn Thr Ser Phe Leu Trp
1 5 10 15
Ala Trp Asn Ala Pro Ala Glu Arg Cys Val Lys Ile Phe Lys Leu Pro
20 25 30
Pro Asp Leu Arg Leu Phe Ser val Lys Gly Ser Pro Gln Lys Ser Ala
35 40 45
Thr Gly Gln Phe Ile Thr Leu Phe Tyr Ala Asp Arg Leu Gly Tyr Tyr
50 55 60
Pro His Ile Asp Glu Lys Thr Gly Asn Thr Val Tyr Gly Gly Ile Pro
65 70 75 80
Gln Leu Gly Asn Leu Lys Asn His Leu Glu Lys Ala Lys Lys Asp Ile
85 90 95
Ala Tyr Tyr Ile Pro Asn Asp Ser Val Gly Leu Ala Val Ile Asp Trp
100 105 110
Glu Asn Trp Arg Pro Thr Trp Ala Arg Asn Trp Lys Pro Lys Asp Val
115 120 125
Tyr Arg Asp Glu Ser Val Glu Leu Val Leu Gln Lys Asn Pro Gln Leu
130 135 140
Ser Phe Pro Glu Ala Ser Lys Ile Ala Lys Val Asp Phe Glu Thr Ala
145 150 155 160
Gly Lys Ser Phe Met Gln Glu Thr Leu Lys Leu Gly Lys Leu Leu Arg
165 170 175
Pro Asn His Leu Trp Gly Tyr Tyr Leu Phe Pro Asp Cys Tyr Asn His
180 185 190
Asn Tyr Asn Gln Pro Thr Tyr Asn Gly Asn Cys Ser Asp Leu Glu Lys
195 200 205
Arg Arg Asn Asp Asp Leu Asp Trp Leu Trp Lys Glu Ser Thr Ala Leu
210 215 220
Phe Pro Ser Val Tyr Leu Asn Ile Lys Leu Lys Ser Thr Pro Lys Ala
225 230 235 240
Ala Phe Tyr Val Arg Asn Arg Val Gln Glu Ala Ile Arg Leu Ser Lys
245 250 255
Ile Ala Ser Val Glu Ser Pro Leu Pro Val Phe Val Tyr His Arg Pro
260 265 270
Val Phe Thr Asp Gly Ser Ser Thr Tyr Leu Ser Gln Gly Asp Leu Val
275 280 285
Asn Ser Val Gly Glu Ile Val Ala Leu Gly Ala Ser Gly Ile Ile Met
290 295 300
Trp Gly Ser Leu Asn Leu Ser Leu Thr Met Gln Ser Cys Met Asn Leu
305 310 315 320
Gly Asn Tyr Leu Asn Thr Thr Leu Asn Pro Tyr Ile Ile Asn Val Thr
325 330 335
Page 1
CA 02522544 2005-10-14
WO 2004/092361 PCT/US2004/011692
ISTA.076VPCSEQLIST.TXT
LeuAlaAlaLys MetCysSer GlnValLeu CysHisAsp GluGly Val
340 345 350
CysThrArgLys GlnTrpAsn SerSerAsp TyrLeuHis LeuAsn Pro
355 360 365
MetAsnPheAla IleGlnThr GlyLysGly GlyLysTyr ThrVal Pro
370 375 380
GlyLysValThr LeuGluAsp LeuGlnThr PheSerAsp LysPhe Tyr
385 390 395 400
CysSerCysTyr AlaAsnIle AsnCysLys LysArgVal AspIle Lys
405 410 415
AsnValHisSer ValAsnVal CysMetAla GluAspIle CysIle Glu
420 425 430
GlyProValLys LeuGlnPro SerAspHis SerSerSer GlnAsn Glu
435 440 445
AlaSerThrThr ThrValSer SerIleSer ProSerThr ThrAla Thr
450 455 460
ThrValSerPro CysThrPro GluLysGln SerProGlu CysLeu Lys
465 470 475 480
ValArgCysLeu GluAlaIle AlaAsnVal ThrGlnThr GlyCys Gln
485 490 495
GlyValLysTrp LysAsnThr SerSerGln SerGlnSer SerIle Gln
500 505 510
AsnIleLysAsn GlnThrThr Tyr
515 520
Page 2