Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-1-
PHOSPHODIESTERASE 9 INHIBITION AS TREATMENT FOR OBESITY-
RELATED CONDITIONS
FIELD OF THE INVENTION
The present invention provides methods to decrease body weight and/or body
fat in the treatment, for example, of overweight or obese patients, and
methods to
treat eating disorders (e.g., binge eating disorder and bulimia), by
administering a
phosphodiesterase 9 (PDE9) inhibitor. The invention also features genetically-
modified mammalian cells, and genetically-modified mice, with a disruption of
the
PDE9 gene.
BACKGROUND
Individuals diagnosed as obese or overweight suffer increased risk for
developing other health conditions such as coronary heart disease, stroke,
hypertension, type 2 diabetes mellitus, dyslipidemia, sleep apnea,
osteoarthritis,
gall bladder disease, depression, and certain forms of cancer (e.g.,
endometrial,
breast, prostate, and colon). The negative health consequences of obesity make
it
the second leading cause of preventable death in the United States and impart
a
significant economic and psychosocial effect on society (see, e.g., McGinnis
and
Foege, JAMA 270: 2207-2212, 1993).
Obesity has become a major public health concern because of its increasing
prevalence, and it is now recognized as a chronic disease that requires
treatment to
reduce its associated health risks. Although weight loss itself is an
important
treatment outcome, one of the main goals of obesity management is to improve
cardiovascular and metabolic values to reduce obesity-related morbidity and
mortality. It has been shown that 5-10% loss of body weight can substantially
improve metabolic values, such as blood glucose, blood pressure, and lipid
concentrations. Hence, it is believed that a 5-10% intentional reduction in
body
weight may reduce morbidity and mortality.
Cyclic nucleotide phosphodiesterases (PDEs) catalyze the hydrolysis of cyclic
nucleotides, such as the second messengers cAMP (cyclic adenosine 3'S'-
monophosphate) and cGMP (cyclic guanine 3'S'-monophosphate). Thus, PDEs play
a pivotal regulatory role in a wide variety of signal transduction pathways
(Beavo,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-2-
Physiol. Rev. 75: 725-748, 1995). For example, PDEs mediate processes involved
in
vision (McLaughlin et al., Nat. Genet. 4: 130-134, 1993), olfaction (Yan et
al., Proc.
Natl. Acad. Sci. USA 92: 9677-81, 1995), platelet aggregation (Dickinson et
al.
Biochem. J. 323: 371-377, 1997), aldosterone synthesis (MacFarland et al., J.
Biol.
Chem. 266: 136-142, 1991), insulin secretion (Zhao et al., J. Clin. Invest.
102: 869-
873, 1998), T cell activation (Li et al., Science 283: 848-51, 1999), and
smooth
muscle relaxation (Boolell et al., Int. J. Impot. Res. 8: 47-52, 1996; Ballard
et al., J.
Urol. 159: 2164-171, 1998).
PDEs form a superfamily of enzymes that are subdivided into 11 major gene
families (Beavo, Physiol. Rev. 75: 725-748, 1995; Beavo et al., Mol.
Pharmacol. 46:
399-405, 1994; Soderling et al., Proc. Natl. Acad. Sci. USA 95: 8991-8996,
1998;
Fisher et al., Biochem. Biophys. Res. Commun. 246: 570-577, 1998; Hayashi et
al.,
Biochem. Biophys. Res. Commun. 250: 751-756, 1998; Soderling et al., J. Biol.
Chem. 273: 15553-58, 1998; Fisher et al., J. Biol. Chem. 273: 15559-15564,
1998;
Soderling et al., Proc. Natl. Acad. Sci. USA 96: 7071-7076, 1999; and Fawcett
et al.,
Proc. Natl. Acad. Sci. USA 97: 3702-3707, 2000).
Each PDE gene family encodes a phosphodiesterase distinguished
functionally by unique enzymatic characteristics and pharmacological profiles.
In
addition, each family exhibits distinct tissue, cell, and subcellular
expression patterns
(Beavo et al., Mol. Pharmacol. 46: 399 405, 1994; Soderling et al., Proc.
Natl. Acad.
Sci. USA 95: 8991-8996, 1998; Fisher et al., Biochem. Biophys. Res. Commun.
246:
570-577, 1998; Hayashi et al., Biochem. Biophys. Res. Commun. 250: 751-756,
1998; Soderling et al., J. Biol. Chem. 273: 15553-15558, 1998; Fisher et al.,
J. Biol.
Chem. 273: 15559-15564, 1998; Soderling et al., Proc. Natl. Acad. Sci. USA 96:
7071-7076, 1999; Fawcett et al., Proc. Natl. Acad. Sci. USA 97: 3702-3707,
2000;
Boolell et al., Int. J. Impot. Res. 8: 47-52, 1996; Ballard et al., J. Urol.
159: 2164-71,
1998; Houslay, Semin. Cell Dev. Biol. 9: 161-67, 1998; and Torphy et al.,
Pulm.
Pharmacol. Ther. 12: 131-135, 1999). Therefore, by administering a compound
that
selectively regulates the activity of one family or subfamily of PDE enzymes,
it is
possible to regulate cAMP andlor cGMP signal transduction pathways in a cell-
or
tissue-specific manner.
Fisher et al. (J. Biol. Chem. 273: 15559-15564, 1998) identified the PDE9
enzyme as a novel member of the PDE enzyme family that selectively hydrolyses
cGMP over cAMP. PDE9 is present in a variety of human tissues, including
testes,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-3-
brain, small intestine, skeletal muscle, heart, lung, thymus, and spleen. PDE9
inhibitors have bee reported as useful to treat cardiovascular disorders (UUO
03/037899), and insulin resistance syndrome, hypertension, and/or type 2
diabetes
(V1/0 03/037432).
SUMMARY OF THE INVENTION
In a first aspect, the invention features a method of treating an animal to
reduce body fat comprising administering to an animal in need thereof a
therapeutically effective amount of a PDE9 inhibitor. Preferably, the animal
is a
human or companion animal (e.g., dog, cat, horse) and is overweight, more
preferably, the animal is obese. In another preferred embodiment, the animal
is a
food stock animal (e.g., chicken, cattle, pig) and such treatment is rendered
to
produce leaner meat. In another preferred embodiment, the PDE9 inhibitor is a
PDE9 selective inhibitor or the PDE9 inhibitor is administered orally.
In a second aspect, the invention features a method of treating an animal for
an eating disorder comprising administering to an animal in need thereof a
therapeutically effective amount of a PDE9 inhibitor. Preferably, the eating
disorder is
binge eating disorder or bulimia, the PDE9 inhibitor is a PDE9 selective
inhibitor, or
the PDE9 inhibitor is administered orally.
In a third aspect, the invention features a method of treating an animal for
metabolic syndrome comprising administering to an animal in, need thereof a
therapeutically effective amount of a PDE9 inhibitor. Preferably, the PDE9
inhibitor is
a PDE9 selective inhibitor, or the PDE9 inhibitor is administered orally.
The invention also features a genetically-modified mouse, wherein the mouse
is homozygous for disruption of the PDE9 gene and wherein the mouse, following
a
six week high fat diet, exhibits reduced body weight or reduced fat mass in an
adipose depot, as compared to a wild type mouse following a six week high fat
diet.
In a preferred embodiment, the mouse expresses an exogenous reporter gene
under
the control of the regulatory sequences of the PDE9 gene or the mouse exhibits
nondetectable PDE9 activity. In a related aspect, the invention provides a
cultured
genetically-modified murine cell derived from the above-described mouse. In
another
related aspect, the invention provides a method for producing the above-
described
mouse comprising: (a) introducing a DNA sequence into a mouse ES cell, wherein
the DNA sequence comprises a PDE9 gene targeting construct, which, upon
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-4-
recombination with the PDE9 gene, disrupts the PDE9 gene; (b) selecting a
mouse
ES cell whose genome comprises a disruption of the PDE9 gene; (c) introducing
an
ES cell selected in step (b) into a mouse blastocyst or morulae; (d)
transplanting the
blastocyst or morulae of step (c) into a pseudopregnant mouse; (e) developing
the
transferred blastocyst or morulae to term to produce a chimeric mouse; and (f)
mating
sexually mature chimeric mice and mice heterozygous for the PDE9 disruption to
obtain a mouse homozygous for the PDE9 gene disruption; wherein the mouse,
following a six week high fat diet, exhibits reduced body weight or reduced
fat mass
in an adipose depot, as compared to a wild type mouse following a six week
high fat
diet.
The invention also features a genetically-modified cultured mammalian cell,
wherein the cell is homozygous for disruption of the PDE9 gene and wherein the
cell,
or a progeny cell derived from the cell, exhibits nondetectable PDE9
polypeptide
activity wherein the cell or progeny cell would exhibit PDE9 polypeptide
activity
absent the homozygous disruption. In a preferred embodiment, the cell is an
embryonic stem (ES) cell, more preferably, the cell is a murine ES cell or a
human
ES cell.
In another aspect, the invention provides an isolated nucleic acid molecule
comprising a PDE11 gene targeting construct, wherein, upon recombination with
the
PDE9 gene, the construct disrupts the PDE9 gene.
Those skilled in the art will fully understand the terms used herein in the
description and the appendant claims to describe the present invention.
Nonetheless, unless otherwise provided herein, the following terms are as
described
immediately below.
By "PDE9 inhibitor" is meant an agent that reduces or attenuates the
biological activity of the PDE9 polypeptide. Such agents may include proteins,
such
as anti-PDE9 antibodies, nucleic acids, e.g., PDE9 antisense or RNA
interference
(RNAi) nucleic acids, amino acids, peptides, carbohydrates, small molecules
(organic
or inorganic), or any other compound or composition which decreases the
activity of a
PDE9 polypeptide either by effectively reducing the amount of PDE9 present in
a cell,
or by decreasing the enzymatic activity of the PDE9 polypeptide. Compounds
that
are PDE9 inhibitors include all solvates, hydrates, pharmaceutically
acceptable salts,
tautomers, stereoisomers, and prodrugs of the compounds. Preferably, a small
molecule PDE9 inhibitor used in the present invention has an ICSO of less that
10 pM,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-5-
more preferably, less than 1 NM, and, even more preferably, less than 0.1 pM.
Any
PDE9 inhibitor used in the present invention is preferably also selective
against some
or all other PDEs, preferably, against PDE1A, PDE1B, PDE1C, PDE2, PDE3A,
PDE3B, PDE4A, PDE4B, PDE4C, PDE4D, PDES, PDE6, PDE7A, PDE7B, PDEBA,
PDEBB, PDE10, and/or PDE11.
By a "selective" PDE9 inhibitor is meant an agent that inhibits PDE9 activity
with an ICSO at least 10-fold less, preferably, at least 100-fold less, than
the ICSO for
inhibition of one or more other PDEs. Preferably, such agents are combined
with a
pharmaceutically acceptable delivery vehicle or carrier. An antisense
oligonucleotide
directed to the PDE9 gene or mRNA to inhibit its expression is made according
to
standard techniques (see, e.g., Agrawal et al. Methods in Molecular Biology:
Protocols for Oligonucleotides and Analogs, Vol. 20, 1993). Similarly, an RNA
molecule that functions to reduce the production of PDE9 enzyme in a cell can
be
produced according to standard techniques known to those skilled in the art
(see,
e.g., Hannon, Nature 418: 244-251, 2002; Shi, Trends in Genetics 19: 9-12,
2003;
Shuey et al., Drug Discovery Today 7: 1040-1046, 2002). Examples of PDE9
inhibitors are provided herein and in WO 03/037899, in WO 03/037432, and in
U.S.
Provisional Patent Appl. No. 60/466,639, filed April 30, 2003, incorporated
herein by
reference.
"Decreased PDE9 activity" means a manipulated decrease in the polypeptide
activity of the PDE9 enzyme as a result of genetic disruption or manipulation
of the
PDE9 gene function that causes a reduction in the level of functional PDE9
polypeptide in a cell, or as the result of administration of a pharmacological
agent that
inhibits PDE9 activity.
The phrase "pharmaceutically acceptable" indicates that the designated
carrier, vehicle, diluent, excipient(s), and/or salt is generally chemically
and/or
physically compatible with the other ingredients comprising the formulation,
and
physiologically compatible with the recipient thereof.
The term "prodrug" refers to a compound that is a drug precursor which,
following administration, releases the drug in vivo via a chemical or
physiological
process (e.g., upon being brought to physiological pH or through enzyme
activity). A
discussion of the synthesis and use of prodrugs is provided by Higuchi and
Stella,
Prodrugs as Novel Delivery Systems, vol. 14 of the ACS Symposium Series, and
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-6-
Bioreversible Carriers in Drug Design, ed. Edward B. Roche, American
Pharmaceutical Association and Pergamon Press, 1987.
The terms "salts" and "pharmaceutically acceptable salts" refer to organic and
inorganic salts of a compound, a stereoisomer of the compound, or a prodrug of
the
compound.
"Overweight" and the more severe "obese" conditions, in an adult person 18
years or older, constitute having greater than ideal body weight (more
specifically,
greater than ideal body fat) and are generally defined by body mass index
(BMI),
which is correlated with total body fat and the relative risk of suffering
from
premature death or disability due to disease as a consequence of the
overweight or
obese condition. The health risks increase with the increase in excessive body
fat.
BMI is calculated by weight in kilograms divided by height in meters squared
(kg/m2) or, alternatively, by weight in pounds, multiplied by 703, divided by
height in
inches squared (Ibs x 703/in~). "Overweight" typically constitutes a BMI of
between
25.0 and 29.9. "Obesity" is typically defined as a BMI of 30 or greater (see,
e.g.,
National Heart, Lung, and Blood Institute, Clinical Guidelines on the
Identification,
Evaluation, and Treatment of Overweight and Obesity in Adults, The Evidence
Report, Washington, DC: U.S. Department of Health and Human Services, NIH
publication no. 98-4083,1998). In heavily muscled individuals, the correlation
between BMI, body fat, and disease risk is weaker than in other individuals.
Therefore, assessment of whether such heavily muscled individuals are in fact
overweight or obese may be more accurately performed by another measure such
as direct measure of total body fat or waist-to-hip ratio assessment.
By a "high fat diet", as administered to a genetically-modified or wild type
mouse, is meant a diet composed of at least 45% kcal fat, and, preferably, at
least
58% fat. Exemplary diets include the Surwit diet (Surwit et al., Metabolism
47:
1354-1359; Surwit et al., Metabolism 47: 1089-1096, 1998; Surwit et al., J.
Biol.
Chem. 271: 9437-9440, 1996; and Surwit et al., Metabolism 44: 645-651, 1995),
D12451 Rodent Diet (45% kcal fat, Research Diets, Inc., New Brunswick, NJ),
and
D12331 Rodent Diet (58% kcal fat, Research Diets, Inc.)
"Metabolic syndrome", as defined herein, and as according to the Adult
Treatment Panel II I (ATP I II; National Institutes of Health: Third Report of
the National
Cholesterol Education Program Expert Panel on Detection, Evaluation, and
Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III),
Executive
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-7-
Summary; Bethesda~ MD, National Institutes of Health, National Heart, Lung and
Blood Institute, 2001 (NIH pub. no. 01-3670), occurs when a person has three
or
more of the following criteria:
1. Abdominal obesity: waist circumference >102 cm in men and >88 cm in women;
2. Hypertriglyceridemia: ~ 50 mg/dl (1.695 mmol/I);
3. Low HDL cholesterol: <40 mg/dl (1.036 mmol/I) in men and <50 mg/dl (1.295
mmol/I) in women;
4. High blood pressure: ~ 30/85 mmHg;
5. High fasting glucose: ~ 10 mg/dl ( ~i.1 mmol/l); or,
as according to World Health Organization criteria (Alberti and Zimmet,
Diabet. Med.
15: 539-53, 1998), when a person has diabetes, impaired glucose tolerance,
impaired
fasting glucose, or insulin resistance plus two or more of the following
abnormalities:
1. High blood pressure: ~ 60/90 mmHg;
2. Hyperlipidemia: triglyceride concentration ~ 50 mg/dl (1.695 mmol/I) and/or
HDL
cholesterol <35 mg/dl (0.9 mmol/I) in men and <39 mg/dl (1.0 mmol/I) in women;
3. Central obesity: waist-to-hip ratio of >0.90 for men and >0.85 in women
andlor
BMI >30 kg/m~;
4. Microalbuminuria: urinary albumin excretion rate ~O,ug/min or an albumin-to-
creatinine ratio ~0 mg/kg.
By "therapeutically effective" is meant resulting in a decrease in body fat.
A "disrupted PDE9 gene" refers to a PDE9 gene that is genetically-modified
such that the cellular activity of the PDE9 polypeptide encoded by the
disrupted gene
is decreased or, preferably, eliminated in cells that normally express a wild
type
version of the PDE9 gene. When the genetic modification effectively eliminates
all
wild type copies of the PDE9 gene in a cell (e.g., the genetically-modified,
non-human
mammal or animal cell is homozygous for the PDE9 gene disruption or the only
wild
type copy of the PDE9 gene originally present is now disrupted), the genetic
modification results in a reduction in PDE9 polypeptide activity as compared
to a
control cell that expresses the wild type PDE9 gene. This reduction in PDE9
polypeptide activity results from either reduced PDE9 gene expression (i.e.,
PDE9
mRNA levels are effectively reduced resulting in reduced levels of PDE9
polypeptide)
and/or because the disrupted PDE9 gene encodes a mutated polypeptide with
altered, e.g., reduced, function as compared to a wild type PDE9 polypeptide.
Preferably, the activity of PDE9 polypeptide in the genetically-modified, non-
human
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
_g_
mammal or animal cell is reduced to 50% or less of wild type levels, more
preferably,
to 25% or less, and, even more preferably, to 10% or less of wild type levels.
Most
preferably, the homozygous PDE9 gene disruption results in non-detectable PDE9
activity in cells of a type that demonstrate wild type PDE9 activity.
A "genetically-modified, non-human mammal" containing a disrupted PDE9
gene refers to a non-human mammal created by genetic engineering to contain a
disrupted PDE9 gene, as well as a progeny of such non-human mammal that
inherits
the disrupted PDE9 gene. A genetically-modified non-human mammal may be
produced, for example, by creating a blastocyst or embryo carrying the desired
genetic modification and then implanting the blastocyst or embryo in a foster
mother
for in utero development. The genetically-modified blastocyst or embryo can be
made, in the case of mice, by implanting a genetically-modified embryonic stem
(ES)
cell into a mouse blastocyst or by aggregating ES cells with tetraploid
embryos.
Alternatively, various species of genetically-modified embryos can be obtained
by
nuclear transfer. In the case of nuclear transfer, the donor cell is a somatic
cell or a
pluripotent stem cell, and it is engineered to contain the desired genetic
modification
that disrupts the PDE9 gene. The nucleus of this cell is then transferred into
a
fertilized or parthenogenetic oocyte that is enucleated; the resultant embryo
is
reconstituted and developed into a blastocyst. A genetically-modified
blastocyst
produced by either of the above methods is then implanted into a foster mother
according to standard methods well known to those skilled in the art. A
"genetically-
modified, non-human mammal" includes all progeny of the non-human mammals
created by the methods described above, provided that the progeny inherit at
least
one copy of the genetic modification that disrupts the PDE9 gene. It is
preferred that
all somatic cells and germline cells of the genetically-modified non-human
mammal
contain the modification. Preferred non-human mammals that are genetically-
modified to contain a disrupted PDE9 gene include rodents, such as mice and
rats,
cats, dogs, rabbits, guinea pigs, hamsters, sheep, pigs, and ferrets.
A "genetically-modified animal cell" containing a disrupted PDE9 gene refers
to an animal cell (preferably a mammalian cell), including a human cell,
created by
genetic engineering to contain a disrupted PDE9 gene, as well as daughter
cells and
cells differentiated from a genetically-modified parent ES or stem cell, that
inherit the
disrupted PDE9 gene. These cells may be genetically-modified in culture
according
to any standard method known in the art. As an alternative to genetically
modifying
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
_g_
the cells in culture, non-human mammalian cells may also be isolated from a
genetically-modified, non-human mammal that contains a PDE9 gene disruption.
The animal cells of the invention may be obtained from primary cell or tissue
preparations as well as culture-adapted, tumorigenic, or transformed cell
lines.
These cells and cell lines are derived, for example, from endothelial cells,
epithelial
cells, islets, neurons and other neural tissue-derived cells, mesothelial
cells,
osteocytes, lymphocytes, chondrocytes, hematopoietic cells, immune cells,
cells of
the major glands or organs (e.g., testicle, liver, lung, heart, stomach,
pancreas,
kidney, and skin), muscle cells (including cells from skeletal muscle, smooth
muscle,
and cardiac muscle), exocrine or endocrine cells, fibroblasts, and embryonic
and
other totipotent or pluripotent stem cells (e.g., ES cells, ES-like cells,
embryonic
germline cells, and other stem cells, such as progenitor cells and tissue-
derived stem
cells). The preferred genetically-modified cells are ES cells, more
preferably, mouse
or rat ES cells, and, most preferably, human ES cells, as well as cells
differentiated
from the genetically-modified ES cells.
A non-human mammal or a animal cell that is "genetically-modified" is
heterozygous or homozygous for a modification that is introduced irito the non-
human
mammal or animal cell, or into a progenitor non-human mammal or animal cell,
by
genetic engineering. The standard methods of genetic engineering that are
available
for introducing the modification include homologous recombination, viral
vector gene
t
trapping, irradiation, chemical mutagenesis, and the transgenic expression of
a
nucleotide sequence encoding antisense RNA alone or in combination with
catalytic
ribozymes. Preferred methods for genetic modification to disrupt a gene are
those
which modify an endogenous gene by inserting a "foreign nucleic acid sequence"
into
the gene locus, e.g., by homologous recombination or viral vector gene
trapping. A
"foreign nucleic acid sequence" is an exogenous sequence that is non-naturally
occurring in the gene. This insertion of foreign DNA can occur within any
region of
the PDE9 gene, e.g., in an enhancer, promoter, regulator region, noncoding
region,
coding region, intron, or exon. The most preferred method of genetic
engineering for
gene disruption is homologous recombination, in which the foreign nucleic acid
sequence is inserted in a targeted manner either alone or in combination with
a
deletion of a portion of the endogenous gene sequence.
"Homozygosity", when referring to PDE9 gene disruption in a non-human
mammal or an animal cell, means a non-human mammal or animal cell having
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-10-
disruption of all alleles of the PDE9 gene. However, the PDE9 gene sequences
of
each of these disrupted alleles need not be identical. For example, a non-
human
mammal may be homozygous for PDE9 disruption wherein one allele of PDE9 is
disrupted as a result of deletion of one region of the gene sequence and the
other
allele is disrupted as a result of deletion of another region of the gene
sequence.
"ES cell" or an "ES-like cell" means a pluripotent stem cell derived from an
embryo, from a primordial germ cell, or from a teratocarcinoma, that is
capable of
indefinite elf-renewal as well as differentiation into cell types that are
representative
of all three embryonic germ layers.
"Microarray" means an arrangement of distinct polynucleotides or
polypeptides on a substrate, as more fully described herein.
"Wild type", when referring to a non-human mammal or an animal cell, means
a non-human mammal or an animal cell, as the case may be, that does not
comprise
a disrupted PDE9 gene. For example, in a comparison of a particular
characteristic
of a non-human mammal of this invention to that characteristic in a wild type
mammal, the term wild type refers to non-human mammal that does not comprise a
disrupted PDE9 gene (i.e., a mammal whose PDE9 gene is wild type). Preferably,
a
wild type non-human mammal is substantially similar, and, more preferably,
substantially identical, to a non-human mammal of the invention, except for
the non-
disruption or disruption of the PDE9 gene, respectively. Likewise, for
example, in a
comparison of a particular characteristic of an animal cell of this invention
to that
characteristic in a wild type animal cell, the term wild type refers to an
animal cell that
does not comprise a disrupted PDE9 gene (i.e., a cell whose PDE9 gene is wild
type). Preferably, a wild type animal cell is substantially similar, and, more
preferably,
substantially identical, to an animal cell of the invention, except for the
non-disruption
or disruption of the PDE9 gene, respectively.
Other features and advantages of the invention will be even further apparent
from the following detailed description and from the claims. While the
invention is
described in connection with specific embodiments, it will be understood that
other
changes and modifications that may be practiced are also part of this
invention and
are also within the scope of the appendant claims. This application is
intended to
cover any equivalents, variations, uses, or adaptations of the,invention that
follow, in
general, the principles of the invention, including departures from the
present
disclosure that come within known or customary practice within the art, and
that are
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-11-
able to be ascertained without undue experimentation. Additional guidance with
respect to making and using nucleic acids and polypeptides is found in
standard
textbooks of molecular biology, protein science, and immunology (see, e.g.,
Davis et
al., Basic Methods in Molecular Biology, Elsevir Sciences Publishing, Inc.,
New York,
NY, 1986; Hames et al., Nucleic Acid Hybridization, IL Press, 1985; Molecular
Cloning, Sambrook et al., Current Protocols in Molecular Biology, Eds. Ausubel
et al.,
John Wiley and Sons, 2001; Current Protocols in Human Genetics, Eds. Dracopoli
et
al., John. Wiley and Sons, 1994; Current Protocols in Protein Science, Eds.
John E.
Coligan et al., John Wiley and Sons, 2002; and Current Protocols in
Immunology,
Eds. John E. Coligan et al., John Wiley and Sons, 1994). All publications,
including
published patent applications and issued patents, mentioned herein are
incorporated
by reference in their entireties.
BRIEF DESCRIPTION OF THE FIGURES
Fig. 1 is a schematic of the targeting construct used to disrupt the PDE9
gene. The 5' and 3' homology arms complementary to PDE9 genomic sequence, 0.9
kb and 4.3 kb in length, respectively, flanked a LacZ-Neo cassette. A portion
of the
genomic sequence of each homology arm is shown as SEQ ID NO: 1 and SEQ ID
NO: 2.
Fig. 2 shows the cDNA sequence for a murine PDE9 (SEQ ID NO: 3). Upon
homologous recombination with the targeting construct, the underlined
sequence,
base pairs 142-175, was deleted and replaced with LacZ-Neo.
Fig. 3 is a line graph detailing the body weight change in wild type (V1IT)
and
genetically-modified mice homozygous for disruption of the PDE9 gene (PDE9
knockout (KO) mice) during the course of a six week high fat diet.
Fig. 4A (male) and Fig. 4B (female) are bar graphs showing the mass of
several adipose depots in WT and PDE9 KO mice after a six week high fat diet.
SC - subcutaneous; TBW - total body weight.
Fig. 5 is a bar graph comparing the body weight of female WT and PDE9 KO
mice following a six week control chow diet.
Fig. 6A (baseline) and Fig. 6B (post-six week chow diet) are bar graphs
showing the mass of adipose depots in female WT and PDE9 KO mice. (Ing -
inguinal subcutaneous; Gon - gonadal; RP - retroperitoneal; Mes - mesenteric)
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-12-
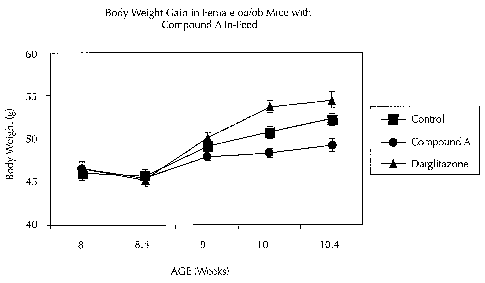
Fig. 7 is a line graph detailing the time course for body weight gain in
female
ob/ob mice in Control, Compound A-treated (100 mglkg/day), and Darglitazone-
treated groups.
Fig. 8A is a bar graph showing the Compound A dose effect on body weight
at Days 2 and 4 in female ob/ob mice. Fig. 8B is a bar graph showing the
Compound
A dose effect on food consumption at Days 2 and 4 in female ob/ob mice.
Fig. 9 is a bar graph comparing the time course for food consumption
between Control, Compound A-treated (100 mg/kg/day), and Darglitazone-treated
female ob/ob mice.
Fig. 10 is a line graph comparing plasma glucose in Control, Compound A-
treated (100 mg/kg/day), and Darglitazone-treated female ob/ob mice.
Fig. 11 is a line graph showing plasma triglycerides in Control and Compound
A-treated (50 and 100 mg/kg/day) female ob/ob mice at Days 1, 2, and 4.
Fig. 12 is a bar graph comparing plasma fructosamine in Control, Compound
A (100, mg/kg/day), and Darglitazone-treated female ob/ob mice at Day 16.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to methods to decrease body weight and/or
body fat in an animal, e.g., in the treatment of overweight or obese patients
(e.g.,
humans or companion animals), or as a means to produce leaner meat in food
stock
animals (e.g., cattle, chickens, pigs), and methods to treat eating disorders
(e.g.,
binge eating disorder and bulimia) in patients in need thereof by
administering a
PDE9 inhibitor. The invention also features biological tools to further study
PDE9
function, i.e., genetically-modified mice and animal cells having a PDE9 gene
disruption. As disclosed in the Examples herein, administration of a PDE9
inhibitor
reduces weight gain in the ob/ob mouse model of obesity, and PDE9 knockout
mice
are relatively resistant to developing increased body weight and increased
adiposity
subsequent to exposure to a high fat diet. Both Examples demonstrate that
causing
a decrease in PDE9 activity is an effective method to reduce body weight
and/or body
fat, and can be used, e.g., to treat animal patierits that are overweight,
obese, or
suffer from an eating disorder, and can be used in animal food stock species
to
produce leaner meat.
Exemplary PDE9 Inhibitors
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-13-
Any PDE9 inhibitor may be used in this invention. PDE9 inhibitors are
known to those skilled in the art and may be determined by standard assays
known
to those in the art, such as in WO 03/037899 and WO 03/037432. The PDE9
inhibitors used in the methods of the invention include those disclosed in WO
03/037899 and WO 03/037432, as well as in U.S. Provisional Appl. No.
60/466,639,
filed April 30, 2003, incorporated hereinbefore by reference. Compounds
disclosed
as PDE9 inhibitors in the above-discussed U.S. Provisional Patent Appl.
include:
3-isopropyl-5-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,6-dihydro-pyrazolo[4,3-
d] pyrimidin-7-one (hereinafter referred to as "Compound A");
1-{[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5-ylmethyl)-
phenoxy]-acetyl}-pyrrolidine-2-carboxylic acid;
3-isopropyl-5-[2-(2-oxo-2-piperazin-1-yl-ethoxy)-benzyl]-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one trifluoro acetate;
3-isopropyl-5-[2-(2-morpholin-4-yl-2-oxo-ethoxy)-benzyl]-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one;
3-isopropyl-5-[2-(2-oxo-2-pyrrolidin-1-yl-ethoxy)-benzyl]-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one;
N, N-d iethyl-2-[2-(3-isopropyl-7-oxo-6, 7-d ihyd ro-1 H-pyrazolo[4, 3-
d]pyrimid in-
5-ylmethyl)-phenoxy]-acetamide;
1-{[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5
ylmethyl)-phenoxy]-acetyl}-pyrrolidine-2-carboxylic acid methyl ester;
4-{[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5-
ylmethyl)-phenoxy]-acetyl}-piperazine-1-carboxylic acid tent-butyl ester;
N-(2-dimethylamino-ethyl)-2-[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-
pyrazolo[4,3-d]pyrimidin-5-ylmethyl)-phenoxy]-acetamide;
[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5-ylmethyl)-
phenoxy]-acetic;
3-isopropyl-5-[2-(5-chloro-2-morpholin-4-yl-ethoxy)-benzyl]-1,6-dihydro-
pyrazolo[4,3-d] pyrimidin-7-one;
3-isopropyl-5-[2-(2-pyrrolidin-1-yl-ethoxy)-benzyl]-1,6-dihydro-pyrazolo[4,3-
d]pyrimidin-7-one;
3-isopropyl-5-[2-(2-morpholin-4-yl-ethoxy)-cyclohexylmethyl]-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one;
5-[5-fluoro-2-(2-morpholin-4-yl-ethoxy)-benzyl]-3-isopropyl-1,6-dihydro-
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-14-
pyrazolo[4,3-d]pyrimidin-7-one;
3-cyclopentyl-5-[5-fluoro-2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one;
9-(1,2-dimethyl-propyl)-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-
purin-6-one;
2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-9-(tetrahydro-furan-3-yl)-1,9-dihydro-
purin-6-one;
5-[2-(2-diethylamino-ethoxy)-benzyl]-3-isopropyl-1,6-dihydro-pyrazolo[4,3-
d]pyrimidin-7-one;
3-cyclopentyl-5-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,6-dihydro-pyrazolo[4,3-
d]pyrimidin-7-one;
9-(1 (R),2-dimethyl-propyl)-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-
purin-6-one;
9-(2-methyl-butyl)-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-purin-
6-one;
9-cyclopentyl-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-purin-6-
one;
5-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-3-pyridin-3-yl-1,6-dihydro-
pyrazolo[4,3-d]pyrimidin-7-one;
9-(1,2-dimethyl-propyl)-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-
purin-6-one;
9-isop ropyl-2-[2-(2-morp hol i n-4-yl-ethoxy)-be nzyl]-1, 9-d i hyd ro-p a ri
n-6-on e;
2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-9-(tetrahydro-furan-2-ylmethyl)-1,9-
dihydro-purin-6-one;
9-(1-isopropyl-2-methyl-propyl)-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-
dihydro-purin-6-one;
9-(1-ethyl-propyl)-2-[2-(2-morpholin-4-yl-ethoxy)-benzyl]-1,9-dihydro-purin-6-
one; and
N-[2-(3-isopropyl-7-oxo-6,7-dihydro-1 H-pyrazolo[4,3-d]pyrimidin-5-ylmethyl)-
cyclohexyl]-2-pyrrolidin-1-yl-acetamide.
It will be understood by those skilled in the art that all sterioisomers,
tautomers, solvates, hydrates prodrugs, and pharmaceutically acceptable salts
of
the compounds listed above are also included.
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-15-
Therapeutic Methods
An agent identified as a PDE9 inhibitor is administered in a dose sufficient
to
reduce body weight or body fat, e.g., by reducing the mass of one or more
adipose
depots. Such therapeutically effective amounts will be determined using
routine
optimization techniques that are dependent on, for example, the condition of
the
patient or animal, the route of administration, the formulation, the judgment
of the
practitioner, and other factors evident to those skilled in the art in light
of this
disclosure.
The PDE9 inhibitors suitable for use in accordance with the present invention
can be administered alone but, in human therapy, will generally be
administered in
admixture with a suitable pharmaceutical excipient diluent or carrier selected
with
regard to the intended route of administration and standard pharmaceutical
practice.
For example, the PDE9 suitable for use in accordance with the present
invention or salts or solvates thereof can be administered orally, buccally or
sublingually in the form of tablets, capsules (including soft gel capsules),
multi-
particulate, gels, films, ovules, elixirs, solutions or suspensions, which may
contain
flavoring or coloring agents, for immediate-, delayed-, modified-, sustained-,
dual-,
controlled-release or pulsatile delivery applications. Such compounds may also
be
administered via fast dispersing or fast dissolving dosages forms or in the
form of a
high energy dispersion or as coated particles. Suitable pharmaceutical
formulations
may be in coated or un-coated form as desired.
Such solid pharmaceutical compositions, for example, tablets may contain
excipients such as microcrystalline cellulose, lactose, sodium citrate,
calcium
carbonate, dibasic calcium phosphate, glycine and starch (preferably corn,
potato or
tapioca starch), disintegrants such as sodium starch glycollate,
croscarmellose
sodium and certain complex silicates, and granulation binders such as
polyvinylpyrrolidone, hydroxypropylmethyl cellulose (HPMC),
hydroxypropylcellulose
(HPC), hydroxypropyl methylcellulose acetate succinate (HPMCAS), sucrose,
gelatin
and acacia. Additionally, lubricating agents such as magnesium stearate,
stearic
acid, glyceryl behenate and talc may be included.
Solid compositions of a similar type may also be employed as fillers in
gelatin
capsules or HPMC capsules. Preferred excipients in this regard include
lactose,
starch, a cellulose, milk sugar or high molecular weight polyethylene glycols.
For
aqueous suspensions andlor elixirs, the PDE9 inhibitor compounds may be
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-16-
combined with various sweetening or flavouring agents, colouring matter or
dyes,
with emulsifying and/or suspending agents and with diluents such as water,
ethanol,
propylene glycol and glycerin, and combinations thereof.
Modified release and pulsatile release dosage forms may contain excipients
such as those detailed for immediate release dosage forms together with
additional
excipients that act as release rate modifiers, these being coated on and/or
included
in the body of the device. Release rate modifiers include, but are not
exclusively
limited to, HPMC, HPMCAS, methyl cellulose, sodium carboxymethylcellulose,
ethyl
cellulose, cellulose acetate, polyethylene oxide, Xanthan gum, Carbomer,
ammonio
methacrylate copolymer, hydrogenated castor oil, carnauba wax, paraffin wax,
cellulose acetate phthalate, hydroxypropylmethyl cellulose phthalate,
methacrylic
acid copolymer and mixtures thereof. Modified release and pulsatile release
dosage forms may contain one or a combination of release rate modifying
excipients. Release rate modifying excipients maybe present both within the
dosage form, i.e., within the matrix, and/or on the dosage form, i.e., upon
the
surface or coating.
Fast dispersing or dissolving dosage formulations (FDDFs) may contain the
following ingredients: aspartame, acesulfame potassium, citric acid,
croscarmellose sodium, crospovidone, diascorbic acid, ethyl acrylate, ethyl
cellulose, gelatin, hydroxypropylmethyl cellulose, magnesium stearate,
mannitol,
methyl methacrylate, mint flavouring, polyethylene glycol, fumed silica,
silicon
dioxide, sodium starch glycolate, sodium stearyl fumarate, sorbitol, xylitol.
The
terms dispersing or dissolving as used herein to describe FDDFs are dependent
upon the solubility of the drug substance used i.e., in cases where the drug
substance is insoluble, a fast dispersing dosage form can be prepared, and, in
cases where the drug substance is soluble, a fast dissolving dosage form can
be
prepared.
The PDE9 inhibitors suitable for use in accordance with the present invention
can also be administered parenterally, for example, intracavernosally,
intravenously,
intra-arterially, intraperitoneally, intrathecally, intraventricularly,
intraurethrally,
intrasternally, intracranially, intramuscularly or subcutaneously, or they may
be
administered by infusion or needle-free techniques. For such parenteral
administration they are best used in the form of a sterile aqueous solution
which may
contain other substances, for example, enough salts or glucose to make the
solution
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-17-
isotonic with blood. The aqueous solutions should be suitably buffered
(preferably to
a pH of from about 3 to 9), if necessary. The preparation of suitable
parenteral
formulations under sterile conditions is readily accomplished by standard
pharmaceutical techniques well-known to those skilled in the art.
For oral and parenteral administration to human patients, the daily dosage
level of the PDE9 inhibitors for use in the present invention will usually be
from 1 to
500 mg (in single or divided doses). A preferred dosage range is about 1 mg to
about
100 mg. The dosage may by via single dose, divided daily dose, or multiple
daily
dose. Alternatively, continuous dosing, such as for example, via a controlled
release
dosage form wherein such continuous dosage form can be administered on a daily
basis or wherein such continuous dosing can be affected via a slow-release
formulation which doses for more than one day at a time.
Thus, for example, tablets or capsules of the PDE9 inhibitors suitable for use
in accordance with the present invention may contain from 1 mg to 250 mg of
active
compound for administration singly or two or more at a time, as appropriate.
Preferred tablets or capsules will contain about 1 mg to about 50 mg of active
compound for administration singly or two or more at a time, as appropriate.
The
physician in any event will determine the actual dosage which will be most
suitable
for any individual patient and it will vary with the age, weight and response
of the
particular patient. There can, of course, be individual instances where higher
or lower
dosage ranges are merited and such are within the scope of this invention.
The PDE9 inhibitors suitable for use in accordance with the present invention
can also be administered intranasally or by inhalation and are conveniently
delivered
in the form of a dry powder inhaler or an aerosol spray presentation from a
pressurized container, pump, spray or nebuliser.with the use of a suitable
propellant,
e.g. dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, a
hydrofluoroalkane such as 1,1,1,2-tetrafluoroethane (HFA 134ATM or
1,1,1,2,3,3,3-
heptafluoropropane (HFA 227EAT""), carbon dioxide or other suitable gas. In
the
case of a pressurised aerosol, the dosage unit may be determined by providing
a
valve to deliver a metered amount. The pressurised container, pump, spray or
nebuliser may contain a solution or suspension of the active compound, e.g.
using a
mixture of ethanol and the propellant as the solvent, which may additionally
contain a
lubricant, e.g., sorbitan trioleate. Capsules and cartridges (made, for
example, from
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-18-
gelatin) for use in an inhaler or insufflator may be formulated to contain a
powder mix
of a compound of the invention and a suitable powder base such as lactose or
starch.
Aerosol or dry powder formulations are preferably arranged so that each
metered dose or "puff' contains from 1 to 50 mg of a PDE9 inhibitor for
delivery to
the animal to be treated. The overall daily dose with an aerosol will be in
the range
of from 1 to 50 mg which may be administered in a single dose or, more
usually, in
divided doses throughout the day.
The PDE9 inhibitors suitable for use in accordance with the present invention
may also be formulated for delivery via an atomiser. Formulations for atomiser
devices may contain the following ingredients as solubilisers, emulsifiers or
suspending agents: water, ethanol, glycerol, propylene glycol, low molecular
weight
polyethylene glycols, sodium chloride, fluorocarbons, polyethylene glycol
ethers,
sorbitan trioleate, oleic acid.
Alternatively, the PDE9 inhibitors suitable for use in accordance with the
present invention can be administered in the form of a suppository or pessary,
or they
may be applied topically in the form of a gel, hydrogel, lotion, solution,
cream,
ointment or dusting powder. The PDE9 inhibitors suitable for use in accordance
with
the present invention may also be dermally or transdermally administered, for
example, by the use of a skin patch. They may also be administered by the
pulmonary or rectal routes.
The PDE9 inhibitors may also be administered by the ocular route. For
ophthalmic use, the compounds can be formulated as micronised suspensions in
isotonic, pH adjusted, sterile saline, or, preferably, as solutions in
isotonic, pH
adjusted, sterile saline, optionally in combination with a preservative such
as a
benzylalkonium chloride. Alternatively, they may be formulated in an ointment
such
as petrolatum.
For application topically to the skin, the PDE9 inhibitors suitable for use in
accordance with the present invention can be formulated as a suitable ointment
containing the active ingredient or agent suspended or dissolved in, for
example, a
mixture with one or more of the following: mineral oil, liquid petrolatum,
white
petrolatum, propylene glycol, polyoxyethylene polyoxypropylene compound,
emulsifying wax and water. Alternatively, they can be formulated as a suitable
lotion
or cream, suspended or dissolved in, for example, a mixture of one or more of
the
following: mineral oil, sorbitan monostearate, a polyethylene glycol, liquid
paraffin,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-19-
polysorbate 60, cetyl esters wax, cetearyl alcohol, 2-octyldodecanol, benzyl
alcohol
and water.
The PDE9 inhibitors suitable for use in accordance with the present invention
may also be used in combination with a cyclodextrin. Cyclodextrins are known
to
form inclusion and non-inclusion complexes with drug molecules. Formation of a
drug-cyclodextrin complex may modify the solubility, dissolution rate,
bioavailability
and/or stability property of a drug molecule. Drug-cyclodextrin complexes are
generally useful for most dosage forms and administration routes. As an
alternative
to direct complexation with the drug the cyclodextrin may be used as an
auxiliary
additive, e.g. as a carrier, diluent or solubiliser. Alpha-, beta- and gamma-
cyclodextrins are some of the most commonly used and suitable examples are
described in WO 91/11172, WO 94102518 and WO 98/55148.
Generally, in humans, oral administration is the preferred route, being the
most convenient. In circumstances where the recipient suffers from a
swallowing
disorder or from impairment of drug absorption after oral administration, the
drug
may be administered parenterally, sublingually, or buccally.
For veterinary use, a PDE9 inhibitor is administered as a suitably acceptable
formulation in accordance with normal veterinary practice and the veterinary
surgeon
will determine the dosing regimen and route of administration which will be
most
appropriate for a particular animal. Such animals include companion animals
who
are overweight, obese, or at risk of being overweight or obese. Other animals
that
may be treated according to the present invention are foodstock animals in
order to
obtain leaner meat than would be obtained absent treatment according to the
present
invention.
Therapeutic efficacy of such PDE9 inhibitors can be determined in light of
this
disclosure by standard therapeutic procedures in cell cultures or experimental
animals, e.g., for determining the EDSO (the dose therapeutically effective in
50% of
the population).
The data obtained from the cell culture assays and animal studies can be
used in formulating a range of dosage for use in humans. The dosage may vary,
for
example, depending upon the formulation and the route of administration. For
any
PDE9 inhibitor used in the method of the invention, the therapeutically
effective dose
can be estimated initially from cell culture assays. A dose may be formulated
in
animal models to achieve a circulating plasma concentration range that
includes the
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-20-
ICso as determined in cell culture. Such information can be used to more
accurately
determine useful doses in humans. Levels in plasma may be measured, for
example, by high performance liquid chromatography.
The PDE9 inhibitors used in accordance with the present invention may also
be used in conjunction with other pharmaceutical agents for the treatment of
the
diseases, conditions and/or disorders described herein. Therefore, methods of
treatment that include administering PDE9 inhibitors in combination with other
pharmaceutical agents are also provided. Suitable pharmaceutical agents that
may
be used in combination with the compounds of the present invention include
anti-
obesity agents such as ~i3 adrenergic receptor agonists, apolipoprotein-B
secretion/microsomal triglyceride transfer protein (apo-B/MTP) inhibitors,
peptide YY3_
ss and analogs thereof, MCR-4 agonists, cholecystokinin-A (CCI<-A) agonists,
monoamine reuptake inhibitors (e.g., sibutramine), sympathomimetic agents,
cannabinoid receptor antagonists (e.g., rimonabant (SR-141,716A)), dopamine
agonists (e.g., bromocriptine), melanocyte-stimulating hormone receptor
analogs,
5HT2c agonists, melanin concentrating hormone antagonists, leptin (the OB
protein),
leptin analogs, leptin receptor agonists, galanin antagonists, lipase
inhibitors (e.g.,
tetrahydrolipstatin, i.e., orlistat), anorectic agents (e.g., a bombesin
agonist),
Neuropeptide-Y antagonists, thyromimetic agents, dehydroepiandrosterones or
analogs thereof, glucocorticoid receptor agonists or antagonists, orexin
receptor
antagonists, glucagon-like peptide-1 receptor agonists, ciliary neurotrophic
factors
(e.g., AxokineT"' available from Regeneron Pharmaceuticals, Inc., Tarrytown,
NY and
Procter & Gamble Company, Cincinnati, OH), human agouti-related proteins
(AGRP),
ghrelin receptor antagonists, histamine 3 receptor antagonists or inverse
agonists,
neuromedin U receptor agonists, II/3-hydroxy steroid dehydrogenase-1
inhibitors and
the like. Other anti-obesity agents, including the preferred agents set forth
hereinbelow, are well known, or will be readily apparent in light of the
instant
disclosure, to one of ordinary skill in the art.
Especially preferred are anti-obesity agents selected from the group
consisting of orlistat; sibutramine, bromocriptine, ephedrine, leptin,
pseudoephedrine,
and peptide YY3_36 (including analogs thereof). Preferably, compounds of the
present
invention and combination therapies are administered in conjunction with
exercise
and a sensible diet.
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-21-
Representative anti-obesity agents for use in the combinations,
pharmaceutical compositions, and methods of the invention can be prepared
using
methods known to one of ordinary skill in the art, for example, sibutramine
can be
prepared, e.g., as described in U.S. Pat. No. 4,929,629; bromocriptine can be
prepared, e.g., as described in U.S. Pat. Nos. 3,752,814 and 3,752,888;
orlistat can
be prepared, e.g., as described in U.S. Pat. Nos. 5,274,143; 5,420,305;
5,540,917;
and 5,643,874; and PYY3~6 (including analogs) can be prepared, e.g., as
described in
U.S. Patent Appl. Publication No. 2002/0141985, and WO 03/027637.
The skilled artisan will appreciate that certain factors may influence the
dosage and timing required to effectively treat a mammal including, but not
limited to,
the severity of the disease or disorder, previous treatments, the general
health and/or
age of the mammal, and other diseases present. Moreover, treatment of a mammal
with a therapeutically effective amount of a PDE9 inhibitor can include a
single
treatment or, preferably, can include a series of treatments.
Genetically-modified Non-human Mammals and Cells
The genetically-modified, non-human mammals and genetically-modified
animal cells, including human cells, of the invention are heterozygous or
homozygous
for a modification that disrupts the PDE9 gene. The animal cells may be
derived by
genetically engineering cells in culture, or, in the case of non-human
mammalian
cells, the cells may be isolated from genetically-modified, non-human mammals.
Disruption of the PDE9 Gene
In order to create genetically-modified non-human mammals and mammal
cells of the invention, the PDE9 gene locus may be disrupted using techniques
for
genetic modification known in the art, including chemical mutagenesis
(Rinchik,
Trends in Genetics 7: 15-21, 1991, Russell, Environmental & Molecular
Mutagenesis 23 (Suppl. 24): 23-29, 1994), irradiation (Russell, supra),
transgenic
expression of PDE9 gene antisense RNA, either alone or in combination with a
catalytic RNA ribozyme sequence (Luyckx et al., Proc. Natl. Acad. Sci. 96:
12174-
79, 1999; Sokol et al., Transgenic Research 5: 363-71, 1996; Efrat et al.,
Proc.
Natl. Acad. Sci. USA 91: 2051-55, 1994; Larsson et al., Nucleic Acids Research
22: 2242-48, 1994) and, as further discussed below, the disruption of the PDE9
gene by the insertion of a foreign nucleic acid sequence into the PDE9 gene
locus.
Preferably, the foreign sequence is inserted by homologous recombination or by
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
_22_
the insertion of a viral vector. Most preferably, the method of PDE9 gene
disruption to create the genetically modified non-human mammals and animal
cells of the invention is homologous recombination and includes a deletion of
a
portion of the endogenous PDE9 gene sequence.
The integration of the foreign sequence disrupts the PDE9 gene through
one or more of the following mechanisms: by interfering with the PDE9 gene
transcription or translation process (e.g., by interfering with promoter
recognition,
or by introducing a transcription termination site or a translational stop
codon into
the PDE9 gene); or by distorting the PDE9 gene coding sequence such that it no
longer encodes a PDE9 polypeptide with normal function (e.g., by inserting a
foreign coding sequence into the PDE9 gene coding sequence, by introducing a
frameshift mutation or amino acids) substitution, or, in the case of a double
crossover event, by deleting a portion of the PDE9 gene coding sequence that
is
required for expression of a functional PDE9 protein).
To insert a foreign sequence into a PDE9 gene locus in the genome of a
cell to create the genetically modified non-human mammals and animal cells of
the
invention based upon the present description, the foreign DNA sequence is
introduced into the cell according to a standard method known in the art such
as
electroporation, calcium-phosphate precipitation, retroviral infection,
microinjection,
biolistics, liposome transfection, DEAE-dextran transfection, or
transferrinfection
(see, e.g., Neumann et al., EMBO J. 1: 841-845, 1982; Potter et al., Proc.
Natl.
Acad. Sci USA 81: 7161-65, 1984; Chu et al., Nucleic Acids Res. 15: 1311-26,
1987; Thomas and Capecchi, Cell 51: 503-12, 1987; Baum et al., Biotechniques
17: 1058-62, 1994; Biewenga et al., J. Neuroscience Methods 71: 67-75, 1997;
Zhang et al., Biotechniques 15: 868-72, 1993; Ray and Gage, Biotechniques 13:
598-603, 1992; Lo, Mol. Cell. Biol. 3: 1803-14, 1983; Nickoloff et al., Mol.
Biotech.
10: 93-101, 1998; Linney et al., Dev. Biol. (Orlando) 213: 207-16, 1999;
Zimmer
and Gruss, Nature 338: 150-153, 1989; and Robertson et al., Nature 323: 445-
48,
1986). The preferred method for introducing foreign DNA into a cell is
electroporation.
Homologous Recombination
The method of homologous recombination targets the PDE9 gene for
disruption by introducing a PDE9 gene targeting vector into a cell containing
a
PDE9 gene. The ability of the vector to target the PDE9 gene for disruption
stems
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-23-
from using a nucleotide sequence in the vector that is homologous, i.e.,
related, to
the PDE9 gene. This homology region facilitates hybridization between the
vector
and the endogenous sequence of the PDE9 gene. Upon hybridization, the
probability of a crossover event between the targeting vector and genomic
sequences greatly increases. This crossover event results in the integration
of the
vector sequence into the PDE9 gene locus and the functional disruption of the
PDE9 gene.
General principles regarding the construction of vectors used for targeting
are reviewed in Bradley et al. (Biotechnol. 10: 534, 1992). Two different
types of
vector can be used to insert DNA by homologous recombination: an insertion
vector or a replacement vector. An insertion vector is circular DNA which
contains
a region of PDE9 gene homology with a double stranded break. Following
hybridization between the homology region and the endogenous PDE9 gene, a
single crossover event at the double stranded break results in the insertion
of the
entire vector sequence into the endogenous gene at the site of crossover.
The more preferred vector to create the genetically modified non-human
mammals and animals cells of the invention by homologous recombination is a
replacement vector, which is colinear rather than circular. Replacement vector
integration into the PDE9 gene requires a double crossover event, i.e.,
crossing
over at two sites of hybridization between the targeting vector and the PDE9
gene.
This double crossover event results in the integration of a vector sequence
that is
sandwiched between the two sites of crossover into the PDE9 gene and the
deletion of the corresponding endogenous PDE9 gene sequence that originally
spanned between the two sites of crossover (see, e.g., Thomas and Capecchi et
al., Cell 51: 503-12, 1987; Mansour et al., Nature 336: 348-52, 1988; Mansour
et
al., Proc. Natl. Acad. Sci. USA 87: 7688-7692, 1990; and Mansour, GATA 7: 219-
227, 1990).
A region of homology in a targeting vector used to create the genetically
modified non-human mammals and animal cells of the invention is generally at
least 100 nucleotides in length. Most preferably, the homology region is at
least 1-
5 kilobases (kb) in length. Although there is no demonstrated minimum length
or
minimum degree of relatedness required for a homology region, targeting
efficiency for homologous recombination generally corresponds with the length
and the degree of relatedness between the targeting vector and the PDE9 gene
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-24-
locus. In the case where a replacement vector is used, and a portion of the
endogenous PDE9 gene is deleted upon homologous recombination, an additional
consideration is the size of the deleted portion of the endogenous PDE9 gene.
If
this portion of the endogenous PDE9 gene is greater than 1 kb in length, then
a
targeting cassette with regions of homology that are longer than 1 kb is
recommended to enhance the efficiency of recombination. Further guidance
regarding the selection and use of sequences effective for homologous
recombination, based on the present description, is described in the
literature (see,
e.g., Deng and Capecchi, Mol. Cell. Biol. 12: 3365-3371, 1992; Bollag et al.,
Annu.
Rev. Genet. 23: 199-225, 1989; and Waldman and Liskay, Mol. Cell. Biol. 8:
5350-
5357, 1988).
As those skilled in the art will recognize based upon the present invention,
a wide variety of cloning vectors may be used as vector backbones in the
construction of the PDE9 gene targeting vectors of the present invention,
including
pBluescript-related plasmids (e.g., Bluescript KS+11 ), pQE70, pQE60, pQE-9,
pBS, pDlO, phagescript, phiX174, pBK Phagemid, pNHBA, pNH16a, pNH18Z,
pNH46A, ptrc99a, pKK223-3, pKK233-3, pDR540, and pRIT5 PWLNEO,
pSV2CAT, pXT1, pSG (Stratagene), pSVK3, PBPV, PMSG, and pSVL, pBR322
and pBR322-based vectors, pMB9, pBR325, pKH47, pBR328, pHC79, phage
Charon 28, pKB11, pKSV-10, pK19 related plasmids, pUC plasmids, and the
pGEM series of plasmids. These vectors are available from a variety of
commercial sources (e.g., Boehringer Mannheim Biochemicals, Indianapolis, IN;
Qiagen, Valencia, CA; Stratagene, La Jolla, CA; Promega, Madison, WI; and New
England Biolabs, Beverly, MA). , However, any other vectors, e.g. plasmids,
viruses, or parts thereof, may be used as long as they are replicable and
viable in
the desired host. The vector may also comprise sequences which enable it to
replicate in the host whose genome is to be modified. The use of such a vector
can expand the interaction period during which recombination can occur,
increasing the efficiency of targeting (see Molecular Biology, ed. Ausubel et
al,
Unit 9.16, Fig. 9.16.1 ).
The specific host employed for propagating the targeting vectors of the
present invention is not critical. Examples include E. coli K12 RR1 (Bolivar
et al.,
Gene 2: 95, 1977), E. coli K12 HB101 (ATCC No. 33694), E. coli MM21 (ATCC
No. 336780), E. coli DH1 (ATCC No. 33849), E. coli strain DHSa, and E. coli
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-25-
STBL2. Alternatively, hosts such as C. cerevisiae or B. subtilis can be used.
The
above-mentioned hosts are available commercially (e.g., Stratagene, La Jolla,
CA;
and Life Technologies, Rockville, MD).
To create the targeting vector, a PDE9 gene targeting construct is added to
an above-described vector backbone. The PDE9 gene targeting constructs of the
invention have at least one PDE9 gene homology region. To make the PDE9
gene homology regions, a PDE9 genomic or cDNA sequence is used as a basis
for producing PCR primers. These primers are used to amplify the desired
region
of the PDE9 sequence by high fidelity PCR amplification (Mattila et al.,
Nucleic
Acids Res. 19: 4967, 1991; Eckert and ICunkel 1: 17, 1991; and U.S. Pat. No.
4,683, 202). The genomic sequence is obtained from a genomic clone library or
from a preparation of genomic DNA, preferably from the animal species that is
to
be targeted for PDE9 gene disruption. a PDE9 cDNA sequence can be used in
making a PDE9 targeting vector (e.g., GenBank~ NM008804 (murine) or
GenBank~ NM002606 (human)).
Preferably, the targeting constructs of the invention also include an
exogenous nucleotide sequence encoding a positive marker protein. The stable
expression of a positive marker after vector integration confers an
identifiable
characteristic on the cell, ideally, without compromising cell viability.
Therefore, in
the case of a replacement vector, the marker gene is positioned between two
flanking homology regions so that it integrates into the PDE9 gene following
the '
double crossover event in a manner such that the marker gene is positioned for
expression after integration.
It is preferred that the positive marker protein is a selectable protein; the
stable expression of such a protein in a cell confers a selectable phenotypic
characteristic, i.e., the characteristic enhances the survival of the cell
under
otherwise lethal conditions. Thus, by imposing the selectable condition, one
can
isolate cells that stably express the positive selectable marker-encoding
vector
sequence from other cells that have not successfully integrated the vector
sequence on the basis of viability. Examples of positive selectable marker
proteins (and their agents of selection) include neo (G418 or kanomycin), hyg
(hygromycin), hisD (histidinol), gpt (xanthine), ble (bleomycin), and hprt
(hypoxanthine) (see, e.g., Capecchi and Thomas, U.S. Pat. No. 5,464,764, and
Capecchi, Science 244: 1288-92, 1989). Other positive markers that may also be
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-26-
used as an alternative to a selectable marker include reporter proteins such
as ~3-
galactosidase, firefly luciferase, or green fluorescent protein (see, e.g.,
Current
Protocols in Cytometry, Unit 9.5, and Current Protocols in Molecular Biology,
Unit
9.6, John Wiley & Sons, New York, NY, 2000).
The above-described positive selection step does not distinguish between
cells that have integrated the vector by targeted homologous recombination at
the
PDE9 gene locus versus random, non-homologous integration of vector sequence
into any chromosomal position. Therefore, when using a replacement vector for
homologous recombination to make the genetically modified non-human mammals
and animal cells of the invention, it is also preferred to include a
nucleotide
sequence encoding a negative selectable marker protein. Expression of a
negative selectable marker causes a cell expressing the marker to lose
viability
when exposed to a certain agent (i.e., the marker protein becomes lethal to
the cell
under certain selectable conditions). Examples of negative selectable markers
(and their agents of lethality) include herpes simplex virus thymidine kinase
(gancyclovir or 1,2-deoxy-2-fluoro-a-d-arabinofuransyl-5-iodouracil), Hprt (6-
thioguanine or 6-thioxanthine), and diphtheria toxin, ricin toxin, and
cytosine
deaminase (5-fluorocytosine).
The nucleotide sequence encoding the negative selectable marker is
positioned outside of the two homology regions of the replacement vector.
Given
this positioning, cells will only integrate and stably express the negative
selectable marker if integration occurs by random, non-homologous
recombination; homologous recombination between the PDE9 gene and the two
regions of homology in the targeting construct excludes the sequence encoding
the negative selectable marker from integration. Thus, by imposing the
negative
condition, cells that have integrated the targeting vector by random, non
homologous recombination lose viability.
The above-described combination of positive and negative selectable
markers is preferred in a targeting construct used to make the genetically
modified
non-human mammals and animal cells of the invention because a series of
positive and negative selection steps can be designed to more efficiently
select
only those cells that have undergone vector integration by homologous
recombination, and, therefore, have a potentially disrupted PDE9 gene. Further
examples of positive-negative selection schemes, selectable markers, and
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-27-
targeting constructs are described, for example, in U.S. Pat. No. 5,464,764,
WO
94/06908; U.S. Pat. No. 5,859,312, and Valancius and Smithies, Mol. Cell.
Biol.
11: 1402, 1991.
For a marker protein to be stably expressed upon vector integration, the
targeting vector may be designed so that the marker coding sequence is
operably
linked to the endogenous PDE9 gene promoter upon vector integration.
Expression of the marker is then driven by the PDE9 gene promoter in cells
that
normally express the PDE9 gene. Alternatively, each marker in the targeting
construct of the vector may contain its own promoter that drives expression
independent of the PDE9 gene promoter. This latter scheme has the advantage of
allowing for expression of markers in cells that do not typically express the
PDE9
gene (Smith and Berg, Cold Spring Harbor Symp. Quant. Biol. 49: 171, 1984;
Sedivy and Sharp, Proc: Natl. Acad. Sci. (USA) 86: 227, 1989; Thomas and
Capecchi, Cell 51: 503, 1987).
Exogenous promoters that can be used to drive marker gene expression
include cell-specific or stage-specific promoters, constitutive promoters, and
inducible or regulatable promoters. Non-limiting examples of these promoters
include the herpes simplex thymidine kinase promoter, cytomegalovirus (CMV)
promoter/enhancer, SV40 promoters, PGK promoter, PMC1-neo, metallothionein
promoter, adenovirus late promoter, vaccinia virus 7.5K promoter, avian beta
globin promoter, histone promoters (e.g., mouse histone H3-614), beta actin
promoter, neuron-specific enolase, muscle actin promoter, and the cauliflower
mosaic virus 35S promoter (see generally, Sambrook et al., Molecular Cloning,
Vols. I-III, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
1989,
and Current Protocols in Molecular Biology, John Wiley & Sons, New York, NY,
2000; Stratagene, La Jolla, CA).
To confirm whether cells have integrated the vector sequence into the
targeted PDE9 gene locus while making the genetically modified non-human
mammals and animal cells of the invention, primers or genomic probes that are
specific for the desired vector integration event can be used in combination
with
polymerase chain reaction (PCR) or Southern blot analysis to identify the
presence
of the desired vector integration into the PDE9 gene locus (Erlich et al.,
Science
252: 1643-51, 1991; Zimmer and Gruss, Nature 338: 150, 1989; Mouellic et al.,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
_28_
Proc. Natl. Acad. Sci. (USA) 87: 4712, 1990; and Shesely et al., Proc. Natl.
Acad.
Sci. (USA) 88: 4294, 1991 ).
Gene Trapping
Another method available for inserting a foreign nucleic acid sequence into
the PDE9 gene locus to disrupt the PDE9 gene, based on the present
description,
is gene trapping. This method takes advantage of the cellular machinery
present
in all mammalian cells that splices exons into mRNA to insert a gene trap
vector
coding sequence into a gene in a random fashion. Once inserted, the gene trap
vector creates a mutation that may disrupt the trapped PDE9 gene. In contrast
to
homologous recombination, this system for mutagenesis creates largely random
mutations. Thus, to obtain a genetically-modified cell that contains a
disrupted
PDE9 gene, cells containing this particular mutation must be identified and
selected from a pool of cells that contain random mutations in a variety of
genes.
Gene trapping systems and vectors have been described for use in
genetically modifying murine cells and other cell types (see, e.g., Allen et
al.,
Nature 333: 852-55, 1988; Bellen et al., Genes Dev. 3: 1288-1300, 1989; Bier
et
al., Genes Dev. 3: 1273-1287, 1989; Bonnerot et al., J. Virol. 66: 4982-91,
1992;
Brenner et al., Proc. Nat. Acad. Sci. USA 86: 5517-21, 1989; Chang et al.,
Virology
193: 737-47, 1993; Friedrich and Soriano, Methods Enzymol. 225: 681-701, 1993;
Friedrich and Soriano, Genes Dev. 5: 1513-23, 1991; Goff, Methods Enzymol.
152: 469-81, 1987; Gossler et al., Science 244: 463-65, 1989; Hope, Develop.
113: 399-408, 1991; Kerr et al., Cold Spring Harb. Symp. Quant. Biol. 2: 767-
776,
1989; Reddy et al., J. Virol. 65: 1507-1515, 1991; Reddy et al., Proc. Natl.
Acad.
Sci. U.S.A. 89: 6721-25, 1992; Skarnes et al., Genes Dev. 6: 903-918, 1992;
von
Melchner and Ruley, J. Virol. 63: 3227-3233, 1989; and Yoshida et al.,
Transgen.
Res. 4: 277-87, 1995).
Individual mutant cell lines containing a disrupted PDE9 gene are identified
in a population of mutated cells using, for example, reverse transcription
(RT) and
PCR to identify a mutation in a PDE9 gene sequence. This process can be
streamlined by pooling clones. For example, to find an individual clone
containing
a disrupted PDE9 gene, RT-PCR is performed using one primer anchored in the
gene trap vector and the other primer located in the PDE9 gene sequence. A
positive RT-PCR result indicates that the vector sequence is encoded in the
PDE9
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
_29_
gene transcript, indicating that the PDE9 gene has been disrupted by a gene
trap
integration event (see, e.g., Sands et al., WO 98!14614, U.S. Pat. No.
6,080,576).
Temporal, Spatial, and Inducible PDE9 Gene Disruptions
In certain embodiments of the present invention, a functional disruption of
the endogenous PDE9 gene occurs at specific developmental or cell cycle stages
(temporal disruption) or in specific cell types (spatial disruption). In other
embodiments, the PDE9 gene disruption is inducible when certain conditions are
present. A recombinase excision system, such as a Cre-Lox system, may be used
to activate or inactivate the PDE9 gene at a specific developmental stage, in
a
particular tissue or cell type, or under particular environmental conditions.
Generally, methods utilizing Cre-Lox technology are carried out as described
by
Torres and Kuhn, Laboratory Protocols for Conditional Gene Targeting, Oxford
University Press, 1997. Methodology similar to that described for the Cre-Lox
system can also be employed utilizing the FLP-FRT system. Further guidance
regarding the use of recombinase excision systems for conditionally disrupting
genes by homologous recombination or viral insertion is provided, for example,
in
U.S. Pat. No. 5,626,159; U.S. Pat. No. 5,527,695; U.S. Pat. No. 5,434,066; WO
98129533; U.S. Pat. No. 6,228,639; Orban et al., Proc. Nat. Acad. Sci. USA 89:
6861-65, 1992; O'Gorman et al., Science 251: 1351-55, 1991; Sauer et al.,
Nucleic Acids Research 17: 147-61, 1989; Barinaga, Science 265: 26-28, 1994;
and Akagi et al., Nucleic Acids Res. 25: 1766-73, 1997. More than one
recombinase system can be used to genetically modify a non-human mammal or
animal cell of the present invention.
When using homologous recombination to disrupt the PDE9 gene in a
temporal, spatial, or inducible fashion, using a recombinase system such as
the
Cre-Lox system, a portion of the PDE9 gene coding region is replaced by a
targeting construct comprising the PDE9 gene coding region flanked by IoxP
sites.
Non-human mammals and animal cells carrying this genetic modification contain
a functional, IoxP-flanked PDE9 gene. The temporal, spatial, or inducible
aspect
of the PDE9 gene disruption is caused by the expression pattern of an
additional
transgene, a Cre recombinase transgene, that is expressed in the non-human
mammal or animal cell under the control of the desired spatially-regulated,
temporally-regulated, or inducible promoter, respectively. A Cre recombinase
targets the IoxP sites for recombination. Therefore, when Cre expression is
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-30-
activated, the LoxP sites undergo recombination to excise the sandwiched PDE9
gene coding sequence, resulting in a functional disruption of the PDE9 gene
(Rajewski et al., J. Clin. Invest. 98: 600-03, 1996; St.-Onge et al., Nucleic
Acids
Res. 24: 3875-77, 1996; Agah et al., J. Clin. Invest. 100: 169-79, 1997;
Brocard et
al., Proc. Natl. Acad. Sci. USA 94: 14559-63, 1997; Feil et al., Proc. Natl.
Acad.
Sci. USA 93: 10887-90, 1996; and Kuhn et al., Science 269: 1427-29, 1995).
A cell containing both a Cre recombinase transgene and IoxP-flanked
PDE9 gene can be generated through standard transgenic techniques or, in the
case of genetically-modified, non-human mammals, by crossing genetically-
modified, non-human mammals wherein one parent contains a IoxP flanked PDE9
gene and the other contairis a Cre recombinase transgene under the control of
the
desired promoter. Further guidance regarding the use of recombinase systems
and specific promoters to temporally, spatially, or conditionally disrupt the
PDE9
gene is found, for example, in Sauer, Meth. Enz. 225: 890-900, 1993; Gu et
al.,
Science 265: 103-06, 1994; Araki et al., J. Biochem. 122: 977-82, 1997;
Dymecki,
Proc. Natl. Acad. Sci. 93: 6191-96, 1996; and Meyers et al., Nature Genetics
18:
136-41, 1998. ,
An inducible disruption of the PDE9 gene can also be achieved by using a
tetracycline responsive binary system (Gossen and Bujard, Proc. Natl. Acad.
Sci.
USA 89: 5547-51, 1992). This system involves genetically modifying a cell to
introduce a Tet promoter into the endogenous PDE9 gene regulatory element and
a transgene expressing a tetracycline-controllable repressor (TetR). In such a
cell,
the administration of tetracycline activates the TetR which, in turn, inhibits
PDE9
gene expression and, therefore, disrupts the PDE9 gene (St.-Onge et al.,
Nucleic
Acids Res. 24: 3875-77, 1996; U.S. Patent No. 5,922,927).
The above-described systems for temporal, spatial, and inducible
disruptions of the PDE9 gene can also be adopted when using gene trapping as
the method of genetic modification, for example, as described, in WO 98/29533
and U.S. Pat. No. 6,288,639, for creating the genetically modified non-human
mammals and animal cells of the invention.
Creating Genetically-Modified, Non-Human Mammals and Animal Cells
The above-described methods for genetic modification can be used to
disrupt a PDE9 gene in virtually any type of somatic or stem cell derived from
an
animal to create the genetically modified animal cells of the invention.
Genetically-
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-31-
modified animal cells of the invention include, but are not limited to,
mammalian
cells, including human cells, and avian cells. These cells may be derived from
~ genetically engineering any animal cell line, such as culture-adapted,
tumorigenic,
or transformed cell lines, differentiated genetically-engineered ES cells, or
they
may be isolated from a genetically-modified, non-human mammal carrying the
desired PDE9 genetic modification.
The cells may be heterozygous or homozygous for the disrupted PDE9
gene. To obtain cells that are homozygous for the PDE9 gene disruption (-/-),
direct, sequential targeting of both alleles can be performed. This process
can be
facilitated by recycling a positive selectable marker. According to this
scheme the
nucleotide sequence encoding the positive selectable marker is removed
following
the disruption of one allele using the Cre-Lox P system. Thus, the same vector
can be used in a subsequent round of targeting to disrupt the second PDE9 gene
allele (Abuin and Bradley, Mol. Cell. Biol. 16: 1851-56, 1996; Sedivy et al.,
T.I.G.
15: 88-90, 1999; Cruz et al., Proc. Natl. Acad. Sci. (USA) 88: 7170-74, 1991;
Mortensen et al., Proc. Natl. Acad. Sci. (USA) 88: 7036-40, 1991; to Riele et
al.,
Nature (London) 348: 649-651, 1990).
An alternative strategy for obtaining ES cells that are PDE9-/- is the
homogenotization of cells from a population of cells that is heterozygous for
the
PDE9 gene disruption (PDE9+/-). The method uses a scheme in which PDE9+/-
targeted clones that express a selectable drug resistance marker are selected
against a very high drug concentration; this selection favors cells that
express two
copies of the sequence encoding the drug resistance marker and are, therefore,
homozygous for the PDE9 gene disruption (Mortensen et al., Mol. Cell. Biol.
12:
2391-95, 1992). In addition, genetically-modified animal cells can be obtained
from genetically-modified PDE9-/- non-human mammals that are created by
mating non-human mammals that are PDE9+/- in germline' cells, as further
discussed below.
Following the genetic modification of the desired cell or cell line, the PDE9
gene locus can be confirmed as the site of modification by PCR analysis
according
to standard PCR or Southern blotting methods known in the art (see, e.g., U.S.
Pat. No. 4,683,202; and Erlich et al., Science 252: 1643, 1991 ). Further
verification of the functional disruption of the PDE9 gene may also be made if
PDE9 gene messenger RNA (mRNA) levels and/or PDE9 polypeptide levels are
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-32-
reduced in cells that normally express the PDE9 gene. Measures of PDE9 gene
mRNA levels may be obtained by using RT-PCR, Northern blot analysis, or in
situ
hybridization. The quantification of PDE9 polypeptide levels produced by the
cells
can be made, for example, by standard immunoassay methods known in the art.
Such immunoassays include, but are not limited to, competitive and non-
competitive assay systems using techniques such as RIAs (radioimmunoassays),
ELISAs (enzyme-linked immunosorbent assays), "sandwich" immunoassays,
immunoradiometric assays, gel diffusion precipitin reactions, immunodiffusion
assays, in situ immunoassays (using, for example, colloidal gold, enzymatic,
or
~ radioisotope labels), Western blots, 2-dimensional gel analysis,
precipitation
reactions, immunofluorescence assays, protein A assays, and
immunoelectrophoresis assays.
Preferred genetically-modified animal cells of the invention are embryonic
stem (ES) cells and ES-like cells. These cells are derived from the
preimplantation
embryos and blastocysts of various species, such as mice (Evans et al., Nature
129:154-156, 1981; Martin, Proc. Natl. Acad. Sci., USA, 78: 7634-7638, 1981),
pigs and sheep (Notanianni et al., J. Reprod. Fert. Suppl., 43: 255-260, 1991;
Campbell et al., Nature 380: 64-68,1996) and primates, including humans
(Thomson et al., U.S. Patent No. 5,843,780, Thomson et al., Science 282: 1145-
1147, 1995; and Thomson et al., Proc. Natl. Acad. Sci. USA 92: 7844-7848,
1995).
Success at homologous recombination-mediated gene disruption in human ES
cells has been reported (Zwaka and Thomson, Nature Biotech. 21: 319-21, 2003).
These types of cells are pluripotent, that is, under proper conditions, they
differentiate into a wide variety of cell types derived from all three
embryonic germ
layers: ectoderm, mesoderm and endoderm. Depending upon the culture
conditions, a sample of ES cells can be cultured indefinitely as stem cells,
allowed
to differentiate into a wide variety of different cell types within a single
sample, or
directed to differentiate into a specific cell type, such as macrophage-like
cells,
neuronal cells, cardiomyocytes, chondrocytes, adipocytes, smooth muscle cells,
endothelial cells, skeletal muscle cells, keratinocytes, and hematopoietic
cells,
such as eosinophils, mast cells, erythroid progenitor cells, or
megakaryocytes.
Directed differentiation is accomplished by including specific growth factors
or
matrix components in the culture conditions, as further described, for
example, in
Keller et al., Curr. Opin. Cell Biol. 7: 862-69, 1995; Li et al., Curr. Biol.
8: 971,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-33-
1998; Klug et al., J. Clin. Invest. 98: 216-24, 1996; Lieschke et al., Exp.
Hematol.
23: 328-34, 1995; Yamane et al., Blood 90: 3516-23, 1997; and Hirashima et
al.,
Blood 93: 1253-63, 1999.
The particular embryonic stem cell line that is used for genetic modification
is not critical; exemplary murine ES cell lines include AB-1 (McMahon and
Bradley,
Cell 62:1073-85, 1990), E14 (Hooper et al., Nature 326: 292-95, 1987), D3
(Doetschman et al., J. Embryol. Exp. Morph. 87: 27-45, 1985), CCE (Robertson
et
al, Nature 323: 445-48, 1986), RW4 (Genome Systems, St. Louis, MO), and
DBAi1IacJ (Roach et al., Exp. Cell Res. 221: 520-25, 1995); an exemplary human
ES cell line is H1.1 cells (Zwaka and Thomson, Nature Biotech. 21: 319-321,
2003). Genetically-modified murine ES cells may be used to generate
genetically-
modified mice, according to published procedures (Robertson, 1987,
Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, Ed. E. J.
Robertson, Oxford: IRL Press, pp. 71-112, 1987; Zjilstra et al., Nature 342:
435-
438, 1989; and Schwartzberg et al., Science 246: 799-803, 1989).
Following confirmation that the ES cells contain the desired functional
disruption of the PDE9 gene, these ES cells are then injected into suitable
blastocyst hosts for generation of chimeric mice according to methods known in
the art (Capecchi, Trends Genet. 5: 70, 1989). The particular mouse
blastocysts
employed in the present invention are not critical. Examples of such
blastocysts
include those derived from C57BL6 mice, C57BL6 Albino mice, Swiss outbred
mice, CFLP mice, and MFI mice. Alternatively ES cells may be sandwiched
between tetraploid embryos in aggregation wells (Nagy et al., Proc. Natl.
Acad.
Sci. USA 90: 8424-8428, 1993).
The blastocysts or embryos containing the genetically-modified ES cells
are then implanted in pseudopregnant female mice and allowed to develop in
utero (Hogan et al., Manipulating the Mouse Embryo: A Laboratory Manual, Cold
Spring Harbor Laboratory press, Cold Spring Harbor, NY 1988; and
Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, E.J.
Robertson, ed., IRL Press, Washington, D.C., 1987). The offspring born to the
foster mothers may be screened to identify those that are chimeric for the
PDE9
gene disruption. Generally, such offspring contain some cells that are derived
from the genetically-modified donor ES cell as well as other cells derived
from the
original blastocyst. In such circumstances, offspring may be screened
initially for
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-34-
mosaic coat color, where a coat color selection strategy has been employed, to
distinguish cells derived from the donor ES cell from the other cells of the
blastocyst. Alternatively, DNA from tail tissue of the offspring can be used
to
identify mice containing the genetically-modified cells.
The mating of chimeric mice that contain the PDE9 gene disruption in germ
line cells produces progeny that possess the PDE9 gene disruption in all germ
line
cells and somatic cells. Mice that are heterozygous for the PDE9 gene
disruption
can then be crossed to produce homozygotes (see, e.g., U.S. Pat. No.
5,557,032;
and U.S. Pat. No. 5,532,158).
An alternative to the above-described ES cell technology for transferring a
genetic modification from a cell to a whole animal is to use nuclear transfer.
This
method can be employed to make other genetically-modified, non-human
mammals besides mice, for example, sheep (McCreath et al., Nature 29: 1066-69,
2000; Campbell et al., Nature 389: 64-66, 1996; and Schnieke et al., Science
278:
2130-33, 1997) and calves (Cibelli et al., Science 280: 1256-58, 1998).
Briefly,
somatic cells (e.g., fibroblasts) or pluripotent stem cells (e.g., ES-like
cells) are
selected as nuclear donors and are genetically-modified to contain a
functional
disruption of the PDE9 gene. When inserting a DNA vector into a somatic cell
to
mutate the PDE9 gene, it is preferred that a promoterless marker be used in
the
vector such that vector integration into the PDE9 gene results in expression
of the
marker under the control of the PDE9 gene promoter (Sedivy and Dutriaux, T.LG.
15: 88-90, 1999; McCreath et al., Nature 29: 1066-69, 2000). Nuclei from donor
cells which have the appropriate PDE9 gene disruption are then transferred to
fertilized or parthenogenetic oocytes that are enucleated (Campbell et al.,
Nature
380: 64, 1996; Wilmut et al., Nature 385: 810, 1997). Embryos are
reconstructed,
cultured to develop into the morula/blastocyst stage, and transferred into
foster
mothers for full term in utero development.
The present invention also encompasses the progeny of the genetically
modified, non-human mammals and genetically-modified animal cells. While the
progeny are heterozygous or homozygous for the genetic modification that
disrupts the PDE9 gene, they may not be genetically identical to the parent
non-
human mammals and animal cells due to mutations or environmental influences,
besides that of the original genetic disruption of the PDE9 gene, that may
occur in
succeeding generations.
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-35-
The cells from a non-human genetically modified animal can be isolated
from tissue or organs using techniques known to those of skill in the art. In
one
embodiment, the genetically modified cells of the invention are immortalized.
In
accordance with this embodiment, cells can be immortalized by genetically
engineering the telomerase gene, an oncogene, e.g., mos or v src, or an
apoptosis-inhibiting gene, e.g., bcl 2, into the cells. Alternatively, cells
can be
immortalized by fusion with a hybridization partner utilizing techniques known
to
one of skill in the art.
"Humanized" Non-human Mammals and Animal Cells
The genetically-modified non-human mammals and animal cells (non-
human) of the invention containing a disrupted endogenous PDE9 gene can be
further modified to express the human PDE9 sequence (referred to herein as
"humanized"). A preferred method for humanizing cells involves replacing the
endogenous PDE9 sequence with nucleic acid sequence encoding the human
PDE9 sequence (Jakobsson et al., Proc. Natl. Acad. Sci. USA 96: 7220-25, 1999)
by homologous recombination. The vectors are similar to those traditionally
used
as targeting vectors with respect to the 5' and 3' homology arms and
positive/negative selection schemes. However, the vectors also include
sequence
that, after recombination, either substitutes the human PDE9 coding sequence
for
the endogenous sequence, or effects base pair changes, exon substitutions, or
codon substitutions that modify the endogenous sequence to encode the human
PDE9. Once homologous recombinants have been identified, it is possible to
excise any selection-based sequences (e.g., neo) by using Cre or Flp-mediated
site directed recombination (Dymecki, Proc. Natl. Acad. Sci. 93: 6191-96,
1996).
When substituting the human PDE9 sequence for the endogenous
sequence, it is preferred that these changes are introduced directly
downstream of
the endogenous translation start site. This positioning preserves the
endogenous
temporal and spatial expression patterns of the PDE9 gene. The human
sequence can be the full length human cDNA sequence with a polyA tail attached
at the 3' end for proper processing or the whole genomic sequence (Shiao et
al.,
Transgenic Res. 8: 295-302, 1999). Further guidance regarding these methods of
genetically modifying cells and non-human mammals to replace expression of an
endogenous gene with its human counterpart is found, for example, in Sullivan
et
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-36-
al., J. Biol. Chem. 272: 17972-17980, 1997, Reaume et al., J. Biol. Chem. 271:
23380-23388, 1996, and Scott et al., U.S. Pat. No. 5,777,194).
Another method for creating such "humanized" organisms is a two step
process involving the disruption of the endogenous gene followed by the
introduction of a transgene encoding the human sequence by pronuclear
microinjection into the knock-out embryos.
Uses for the Genetically-Modified Non-human Mammals and Animal Cells
PDE9 function and therapeutic relevance can be further elucidated by
additional investigation into the phenotype of PDE9-l- non-human mammals and
animals cells of the invention, as illustrated, for example, in the Examples
hereinbelow. For example, the genetically-modified PDE9-/- non-human
mammals and animal cells can be used to determine whether the PDE9 plays a
role in causing or preventing symptoms or phenotypes to develop in certain
models of disease, e.g., obesity, eating disorders, cardiovascular disorders,
insulin
resistance syndrome, hypertension, and/or type 2 diabetes. If a symptom or
phenotype is different in a PDE9-/- non-human mammal or animal cell as
compared to a wild type (PDE9+/+) or PDE9+/- non-human mammal or animal
cell, then the PDE9 polypeptide plays a role in regulating functions
associated with
the symptom or phenotype. Examples of testing that can be used to assess PDE9
function include comparing PDE9-/- mice to wild type mice in terms of body
weight, body fat, blood pressure, glucose/insulin metabolism (e.g., glucose
uptake
in isolated tissues, alterations in the activity of glycogen metabolism
enzymes,
alterations in glycogen levels in liver or muscle, and/or alterations in body
composition), and changes in the activity or phosphorylation state of
components
' in the insulin signaling pathway.
In addition, under circumstances in which an agent has been identified as
a PDE9 agonist or antagonist (e.g., the agent significantly modifies one or
more of
the PDE9 polypeptide activities when the agent is administered to a PDE9+l+ or
PDE9+l- non-human mammal or animal cell), the genetically-modified PDE9-/-
non-human mammals and animal cells of the invention are useful to characterize
any other effects caused by the agent besides those known to result from the
(ant)agonism of PDE9 (i.e., the non-human mammals and animal cells can be
used as negative controls). For example, if the administration of the agent
causes
an effect in a PDE9+/+ non-human mammal or animal cell that is not known to be
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-37-
associated with PDE9 polypeptide activity, then one can determine whether the
agent exerts this effect solely or primarily through modulation of PDE9 by
administering the agent to a corresponding PDE9-/- non-human mammal or
animal cell. If this effect is absent, or is significantly reduced, in the
PDE9-/- non-
human mammal or animal cell, then the effect is mediated, at least in part, by
PDE9. However, if the PDE9-/- non-human mammal or animal cell exhibits the
effect to a degree comparable to the PDE9+/+ or PDE9+/- non-human mammal or
animal cell, then the effect is mediated by a pathway that does not involve
PDE9
signaling.
Furthermore, if an agent is suspected of possibly exerting an effect
predominantly via a PDE9 pathway, then the PDE9-/- non-human mammals and
animal cells are useful as negative controls to test this hypothesis. If the
agent is
indeed acting through PDE9, then the PDE9-/- non-human mammals and animal
cells, upon administration of the agent, should not demonstrate the same
effect
observed in the PDE9+/+ non-human mammals or animal cells.
The genetically modified non-human mamrrials and animal cells of the
invention can also be used to identify genes whose expression is
differentially
regulated in PDE9+/- or PDE9-/- non-human mammals or animal cells relative to
their respective wild type control. Techniques known to those of skill in the
art can
be used to identify such genes based upon the present description. For
example,
DNA arrays can be used to identify genes whose expression is differentially
regulated in PDE9+/- or PDE9-/- mice to compensate for a deficiency in PDE9
expression. DNA arrays are known to those of skill in the art (see, e.g.,
Aigner et
al., Arthritis and Rheumatism 44: 2777-89, 2001; U.S. Pat. No. 5,965,352;
Schena
et al., Science 270: 467-470, 1995; Schena et al., Proc. Natl. Acad. Sci. USA
93:
10614-10619, 1996; DeRisi et al., Nature Genetics 14: 457-460, 1996; Shalon et
al., Genome Res. 6: 639-645, 1996; and Schena et al., Proc. Natl. Acad. Sci.
(USA) 93: 10539-11286, 1995; U.S. Pat. No. 5,474,796; U.S. Pat. No. 5,605,662;
WO 95/25116; WO 95/35505; Heller et al., Proc. Natl. Acad. Sci. 94: 2150-2155,
1997).
A chemical coupling procedure and an ink jet device may be used to
synthesize array elements on the surface of a substrate. An array analogous to
a dot
or slot blot may also be used to arrange and link elements to the surface of a
substrate using thermal, UV, chemical, or mechanical bonding procedures. A
typical
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-38-
array may be produced by hand or using available methods and machines and
contain any appropriate number of elements. After hybridization, nonhybridized
probes are removed and a scanner used to determine the levels and patterns of
fluorescence. The degree of complementarity and the relative abundance of each
probe which hybridizes to an element on the microarray may be assessed through
analysis of the scanned images.
Full-length cDNAs, expressed sequence tags (ESTs), or fragments thereof
may comprise the elements of a microarray. Fragments suitable for
hybridization
may be selected using software well known in the art such as LASERGENE
software
(DNASTAR). Full-length cDNAs, ESTs, or fragments thereof corresponding to one
of
the nucleotide sequences of the present invention, or selected at random from
a
cDNA library relevant to the present invention, are arranged on an appropriate
substrate, e.g., a glass slide. The cDNA is fixed to the slide using, e.g.,
ultra-violet
cross-linking followed by thermal and chemical treatments and subsequent
drying.
Fluorescent probes are prepared and used for hybridization to the elements on
the
substrate. The substrate is analyzed by procedures well known in the art, for
example, by scanning and analyzing images of a microarray.
In addition, the genetically modified non-human mammals and animal cells
of the invention can also be used to identify proteins whose expression
profile or
postranslational modification is altered in PDE9+l- or PDE9-/- non-human
mammals or animal cells relative to their respective wild type control.
Techniques
known to those of skill in the art can be used to identify such proteins based
upon
the present description. For example, proteomic assays can be used to identify
proteins whose expression profile or postranslational modification is altered
in
PDE9+/- or PDE9-/- mice to compensate for a deficiency in PDE9 expression.
Proteomic assays are known to those of skill in the art (see, e.g., Conrads et
al.,
Biochem. Biophys. Res. Commun. 290: 896-890, 2002; Dongre et al., Biopolymers
60: 206-211, 2001; Van Eyk, Curr Opin Mol Ther 3: 546-553, 2001; Cole et al.,
Electrophoresis 21: 1772-1781, 2000; Araki et al., Electrophoresis 21: 180-
1889,
2000).
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-39-
Examples
1. Preparation of PDE9 Tar~etin Vq ector
A targeting vector construct was designed according to the scheme shown in
Fig. 1. The construct contained two arms homologous to the murine PDE9 genomic
sequence: a 0.9 kb 5' homology arm and a 4.3 kb 3' homology arm. These arms
sandwiched a LacZ-Neo construct. DNA containing the targeting construct was
inserted into ES R1 cells by electroporation (Deng et al., Dev. Biol. 185: 42-
54, 1997;
Udy et al., Exp. Cell Res. 231: 296-301, 1997). Upon homologous recombination,
base pairs 142-175 of the PDE9 cDNA coding sequence shown in Fig. 2 (base
pairs
142-175 are underlined) were deleted from the endogenous gene and replaced by
the LacZ Neo cassette. ES cells that were neomycin resistant were analyzed by
Southern blot to confirm disruption of a PDE9 gene. These targeted ES cells
were
then used for generation of chimeric mice by injecting the cells into
blastocysts and
implanting the blastocysts into pseudopregnant female mice (Capecchi et al.,
Trends
Genet. 5: 70, 1989, Hogan et al., Manipulating the Mouse Embryo: A Laboratory
Manual, Cold Spring Harbor Laboratory, 1988; and Teratoearcinomas and
Embryonic
Stem Cells: A Practical Approaeh, E.J. Robertson, ed., IRL Press, Washington,
D.C.,
1987). Chimeric mice were then bred with C57BL/6 (Jackson Laboratories, Bar
Harbor, ME) mice to create F1 PDE9+/- heterozygotes, which were in turn bred
to
produce F2 PDE9-/- homozygous mice (Charles River Laboratories, Wilmington,
MA). The functional disruption of the PDE9 gene in the heterozygotes and
homozygotes was confirmed by PCR and Southern blot analysis.
2. Effect of PDE9 Gene Disruption on Body Weight Body Composition and
Metabolites
Methods
Male and female PDE9 KO (-/-) mice, as previously described, and wild type
(+/+) littermate controls were allowed to acclimate for at least one week
prior to the
start of the study and were given free access to water and D11 mouse chow
(Purina, Brentwood, MO).
Male and female mice (aged 17-19 weeks) were divided into four
experimental groups with two to five mice per cage. One group of control mice
of
each gender remained on D11 mouse chow and the remaining groups of each
gender were switched to a diet composed of 58 kcal% fat (D12331 Rodent Diet,
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-40-
Research Diets, Inc., New Brunswick, NJ) for the duration of the 6 week study.
Body weight was determined on Day 0 and monitored weekly. Adipose depot mass
was analyzed on Day 0 and at the end of the study, as further described below.
On Day 1, plasma glucose was determined via retro-orbital blood samples.
25 wL of blood was added to 100 wL of 0.025 percent heparinized-saline in
microtubes (Denville Scientific, Inc., Metuchen, NJ). The tubes were spun at
the
highest setting in a Beckman Microfuge 12 for 2 minutes. Plasma was collected
for
plasma glucose and triglyceride determination, as further described below.
During
the course of the study, body weight and food consumption were assessed, and
blood samples were taken at approximately 8 am for plasma glucose and
triglyceride measures, as further described below.
On the morning of the last day of the study, blood samples were taken via
retro-orbital sinus for plasma glucose and triglyceride determination. The
mice were
then sacrificed and about one milliliter of blood was collected in
Microtainer~ plasma
separator tubes with lithium heparin (Becton-Dickinson, Inc., Franklin Lakes,
NJ).
The tubes were spun in a Beckman Microfuge 12 at the maximum setting for five
minutes. Plasma was collected in 1.5 ml Eppendorf tubes, snap frozen in liquid
nitrogen, and stored at -80°C until analyzed for insulin, fructosamine,
or cGMP levels.
Plasma glucose, triglycerides, and fructosamine were measured using the
Roche/Hitachi 912 Clinical Chemistry Analyzer (Roche Diagnostics Corp.,
Indianapolis, IN). Plasma cGMP was measured using the BioTrakT"~ enzyme-
immunoassay system (Amersham, Piscataway, NJ). Plasma insulin was assessed
via a similar technique using the Mercodia ELISA Insulin kit supplied by ALPCO
(Uppsala, Sweden). All assays were conducted according to each manufacturer's
instructions.
Quantification of adipose depot mass was done five days prior to the end of
the study. To assess the adipose depot mass, 360° radioscopic images of
the mice
were obtained using a commercially available micro computed tomography (CT)
system (MicroCAT~, ImTek Inc., Oak Ridge, TN) with a high-resolution
CCD/phosphor screen detector. The scanner consisted of a cylindrical
diameter/long
field view of 36mm/36mm with a spatial resolution of less than 50 pM. The X-
ray
source was biased at 40 KeV with the anode current set to 0.4 mA. Anesthetized
mice were placed on a radiotransparent mouse bed in an anatomically correct
supine
position, caudal end closest to the micro CT with the rostral end held in
place against
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-41-
an anesthesia delivery tube. An initial radiographic image was acquired at
90° to the
plane of the mouse bed to allow correct positioning of the mouse by centering
the
scan acquisition area at the level of the iliac crest of each mouse. Once
correct
alignment was assured, each animal was scanned. Each scan consisted of 196
individual projections with an exposure time of 250 ps/projection; total image
acquisition time was approximately 12 minutes at 145 pM resolution.
Image reconstruction, whereby the 196 projections acquired in the micro CT
scan of the mouse were manipulated to produce two-dimensional cross sectional
images of the mouse, was performed using the MicroCAT° Reconstruction,
Visualization, and Analysis Software (ImTek Inc., Oak Ridge, TN) (Paulus et
al.,
Neoplasia 2: 62-70, 2000). Two sets of reconstructed images per scan were
generated for each mouse for the determination of individual fat depot mass.
The
first set of six reconstructed images provided a montage for the analysis of
inguinal
and epididymal adipose tissue depot mass. The second reconstruction set
consisted
of nine slices, determined by both intervertebral and midvertebral landmarks,
and
was used to determine retroperitoneal and mesenteric adipose tissue depot
mass.
For image analysis, reconstructed bitmap images were converted to TIFF
images. The TIFF images were subsequently analyzed and fat depot mass
determined using Scion Image for Windows~ (Scion Corporation, Frederick MD).
Demarcation lines separating individual fat depots were placed using the
paintbrush
tool (pixel size #3) and total pixel counts of each adipose region determined
by the
Scion Image software. An upper and lower pixel intensity threshold was chosen,
in
this study, a look-up-table (LUT) of between 115-187 was determined to be
optimal
for capturing the adipose depot.
Average pixel number between each slice was calculated (sliCe~+Sllce~+,)/2).
Total pixel number, representing the individual fat depots, was calculated by
multiplying the average pixel number between each slice by the average pixel
number of each slice. Finally, the pixel count was converted into depot mass
with the
following equation: Depot mass (mg) = 0.000915g/ul x 0.000757 pl/voxel x
1000mg/g
x voxel count. The first factor corrects for specific gravity of glyceryl
trioleate,
representative of the density of the primary storage form of lipid in adipose
tissue, i.e.,
triglyceride. The second factor is the volume per pixel and the third factor
converts
the resulting mass into mg units.
Results
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-42-
PDE9 disruption resulted in a decreased body weight gain and reduced body
weight while on a high fat diet in both male and female KO mice as compared to
their
wild type counterparts (Fig. 3). Female mice also demonstrated a 6% decrease
in
body length. In conjunction with the decreased body weight, the male and
female KO
mice also demonstrated decreased fat mass in various adipose depots (Fig. 4).
In
male KO mice, significant decreases were seen in the retroperitoneal and
mesenteric
adipose depots; in female KO mice, significant decreases were seen in the
inguinal,
gonadal, and retroperitoneal depots. By comparison, in female mice fed a
standard
chow diet, no differences in body weight were observed between KO and wild
type
mice (Fig. 5) and he trend towards decreased adipose fat mass was significant
in
only the gonadal adipose depot (Fig. 6).
With respect to plasma metabolites following the high fat diet, female KO mice
demonstrated increased cGMP, decreased glucose, and decreased insulin (Table 1
);
male KO mice demonstrated a trend towards increased cGMP and a trend toward
decreased glucose (Table 1 ).
Table 1. Plasma Metabolites Following 6-week High-fat Diet
Female Male
WT KO p valueWT KO p value
cGMP 8.00.50 13.70.6 <0.01 8.21.0 11.51.8 0.14
mol/ml
Glucose 1758 1526 0.03 17978 1648 0.21
m
Insulin 1.580.250.880.15 0.03 3.530.48 3.280.48 0.72
n /ml
Triglycerides1116 1199 0.42 20013 1911 0.61
m
3. Effect of Pharmacologic PDE9 Inhibition on Body Wei hg t, Body Composition
and
Food Intake in ob/ob Mice
Methods
Female oblob mice obtained from Jackson Laboratories (Bar Harbor, ME)
were used at 6 to 10 weeks of age. Mice were housed five per cage and allowed
free
access to water and, initially, to D11 mouse chow. Following a one week
acclimation
period, mice were switched to a powdered diet (Mouse Breeder/Auto-JL K20 mouse
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
-43-
chow, PMI Feeds, Inc., St. Louis, MO) for three days and allowed to adapt to
the diet
prior to the start of the PDE9 inhibitor dosing period.
The PDE9 inhibitor compound (Compound A) was administered in powdered
mouse chow that was custom ground (Research Diets, Inc., New Brunswick, NJ) as
a
compound/chow admixture; compounds were mixed with the chow to achieve
consumption of the specified doses ranging from 1-200 mg/kg/day. In addition
to a
compound-free control group, a group consuming darglitazone (1 mg/kg/day) was
also included as a positive control.
Mice were randomly assigned to groups of ten with five mice per cage.
Body weight was determined on Day 0 and weekly thereafter. On Day 1, retro-
orbital blood samples were obtained and plasma glucose was determined as
previously described. On the final day of the study, blood samples were taken
for
glucose, triglyceride, insulin, and cGMP measurements, as previously
described.
Results
The results below represent results from several separate studies using the
same above-described protocol. Fig. 7 shows a reduced body weight gain in
ob/ob
mice fed 100 mg/kg/day of the PDE9 inhibitor Compound A as compared to the
mice fed either a compound-free control diet or a darglitazone-treated diet.
Compound A elicited a dose-dependent effect following 2 and 4 days of
treatment,
both in terms of reducing the normal body weight gain (Fig. 8A) and also in
terms of
reducing food intake (Fig. 8B). The PDE9 effect on food intake could be
transient,
given that no effect on food intake was observed in the later stages of the
study
(Fig. 9) with the intermediate dose of 100 mg/kg/day.
The intermediate dose of 100 mg/kg/day of Compound A also resulted in
decreased glucose, triglycerides and fructosamine. Representative results are
shown in Fig. 10, Fig. 11, and Fig. 12, respectively.
Both Examples demonstrate that causing a decrease in PDE9 activity is an
effective method to reduce body weight and/or body fat, and can be used, e.g.,
to
treat animal patients that are overweight, obese, or suffer from an eating
disorder,
and can be used in animal food stock species to produce leaner meat.
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
PC25667 foreign filing spee.ST25.txt
SEQUENCE LISTING
<110> Pfizer Products Inc.
<120> PHOSPHODIESTERASE 9 INHIBITION AS TREATMENT FOR OBESITY-RELATED
CONDITIONS
<130> PC25667A
<140> 60/516,213
<141> 2003-10-31
<160> 3
<170> Patentln version 3.2
<210> 1
<211> 200
<212> DNA
<213> Mus musculus
<400> 1
ttgagggaga acctctcact ggcctgggtg accagcgagt acatgagttc tagggactga 60
acttgggtca tcactcttag gtaggaagca cattactccc tgagccaact ctccagctca 120
acttctgtgt tttgcaggtg gttttcagca agtactgcaa ctccagtgac atcatggacc 180
tgttctgtat cgccaccggc 200
<2l0> 2
<211> 200
<212> DNA
<213> Mus musculus
<400> 2
cagacgacgc catggtctcc atcgatccca ccatgcctgc gaattcagag cggtaagcga 60
ctcctcctgg ggtgtctcac ttgtctatga tgtgtgccag ggagtctgca atttggcttt 120
gtttactact gctaagacgt gtgaagtttc agcagaagag ggaaggaggc agatgggagg 180
caggcggagg cttggttcac ~ 200
<210>
3
<211>
1929
<212>
DNA
<213> musculus
Mus
<400>
3
gcgggcgcgatgggggccggctcctcaagctaccggcccaaggccatctacctagacatc 60
gatggacgcatccagaaggtggttttcagcaagtactgcaactccagtgacatcatggac 120
ctgttctgtatcgccaccggcctgcctcggaacaccaccatctcccttttaaccacagac 180
gacgccatggtctccatcgatcccaccatgcctgcgaattcagagcgcactccctacaag 240
gtgagacctgtggctgtgaagcaagtgtctgaacgagaagaacttatccagggcgtgctg 300
gcccaggtggcggaacaattttccagagcgtttaagatcaacgagctgaaagccgaagtt 360
gcaaatcacctggccgtgctggagaaacgggtggaattggaaggacttaaagtggtggag 420
atcgaaaaatgcaagagtgacattaaaaagatgcgggaggagttggcagctaggaacagc 480
Page 1
CA 02543522 2006-04-24
WO 2005/041972 PCT/IB2004/003396
Pc25667
foreign
filing
spec.s'r25.txt
aggaccaactgtccatgtaaatacagttttttggataacaagaagttgacacctcgacgt 540
gatgtccccacttaccccaagtacctgctctccccagagaccatcgaagccctacggaag 600
cccacctttgatgtctggctttgggaacccaacgagatgctgagctgtttagaacatatg 660
taccacgaccttggtctggtcagggacttcagcatcaacccaatcacgctccgcaggtgg 720
ctgctctgtgtgcatgacaactacaggaacaaccccttccacaacttccggcactgcttc 780
tgtgtgacacagatgatgtacagtatggtctggctctgtggcctccaggagaagttttcc 840
cagatggacatcttggtcctgatgactgcagccatctgccatgacctggaccacccaggg 900
tacaacaatacataccagatcaatgcccgcacggaactcgctgtgcgctacaacgacatc 960
tcaccgctggagaaccaccactgcgccattgccttccagatcctggcaagacctgagtgc 1020
aacatcttcgccagtgtgccacccgagggcttcaggcagatcaggcaggggatgatcaca 1080
ttgatcctggctaccgacatggcaaggcatgctgaaattatggattctttcaaagaaaag 1140
atggagaattttgactacagcaacgaggagcacctgaccctgctgaagatgattctcata 1200
aaatgctgtgatatctccaatgaagtccgtcccatggaggtggcagaaccgtgggtggac 1260
tgtttactggaagaatattttatgcagagtgaccgtgagaagtccgaaggccttcctgtg 1320
gccccattcatggaccgagacaaagtgaccaaagcaacagcccaaattgggttcatcaag 1380
tttgtcctgatcccaatgtttgaaacagtgaccaagctcttccccgttgttgaggagacc 1440
atgctgcggccgctctgggagtcccgagaacactacgaggagctgaagcagctggacgat 1500
gccatgaaggagttgcagaagaagacagagagcttaacatctggggccccggaaaacacc 1560
acagagaagaacagagatgcaaaagacagtgaaggccattctcctccaaactgatggaca 1620
tctcaatgtgtgcacagactgtaccagttagagcagatgaattgtggcctgtgagtggac 1680
agagccaagcgaggcttcccaggatcttccacacaaggatggtcacgcccagacaaccct 1740
gatgacctgctacagaccatgttttctaagaaccattttgttccctgatataaaaacagg 1800
aatcccgatgctgttcagaatttttatttttaaactgttttttaaataat'atatttttac1860
acagaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa,aaaaaaaaaa1920
aaaaaaaaa . 1929
Page 2