Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02579586 2015-01-07
=
1
INSTALLATION FOR CONTINUOUS FIRE REFINING OF COPPER
FIELD
This invention relates to generally to an installation for the continuous fire
refining of
copper
BACKGROUND OF INVENTION
Smelting of copper concentrates produces matte and slag. Copper matte is
converted
into blister copper in Peirce-Smith, Hoboken converters or by continuous
converting
processes such as Kennecott-Outokumpu or Mitsubishi. Blister copper is
directed to fire
refining process prior to the electrorefining.
Fire refining of blister copper is carried out in stationary reverberatory or
vascular
furnaces, called anode furnaces due to the most common casting of refined
copper in
the form of anodes, which are transferred to the electrolytical refining.
Fire refining process is a classical discontinuous process (batch) that
consisting of four
stages: charging, oxidation and impurities slagging, reduction and anode
casting. The
total time of refining cycle without the stage of melting varies from 6 up to
24 hours.
Oxidized copper after oxidation stage contains from 5000 to 10000 ppm of
oxygen. The
copper is reduced by carboneous or amonia reductant. The most common reductant
in
use are the oil or natural gas. The oil or natural gas are injected with air
into the bath of
molten copper through a tuyere or tuyeres. Copper reduction faces significant
limitations
in the process rate and efficiency of reductant utilisation. Reduction stage
of the liquid
copper charge, which fluctuates from 150 to 400 t, varies in the range from
1.2 to 2.0
hours. Reported reductant efficiency is below 50%. Injection of liquid or
gaseous
CA 02579586 2015-01-07
= 2
reductant into the copper produces black fumes in off-gas due to thermal
decomposition
of hydrocarbons. Partial carbon utilisation in oxygen reduction from copper
causes the
presence of carbon particles in the reduction gases, which are partly
combusted if the
burner flame is oxidising. Carbon particles are transferred to the furnace off-
gas,
creating black fumes emitted through a chimney to the atmosphere.
Oxidation and reduction of liquid copper is practiced for centuries and it was
first
described by Georgious Agricola (G. Agricola: "De Re Metallica", translated
from Latin, la
edition 1556 by Hebert C. Hoover and Lou H. Hoover, Dover Publications, 1950,
535-
536). After copper oxidation with air in open hearth furnace and removal of
impurities,
the copper was reduced with wood. Said copper reduction with wood (poling) is
still
practiced in some smelters.
L. Klein presented a new idea of the use of gas reductant as a substitute of
wood
("Gaseous reduction of oxygen-containing copper", J. of Metals, Vol. 13, N 8,
August
1961, 545-547; U.S. Patent N 2.989.397, June 1961). The study showed that the
injection of natural gas with air states a better solution than the injection
of only natural
gas into a liquid copper. Method of deoxidization of copper with reformed
natural gas
and related apparatus have been patented by Phelps Dodge Corporation in USA
and
Canada (C. Kuzell, M. Fowler, S. Davis, and L. Klein: "Apparatus for reforming
gases"
U.S. Patent N 3.071.454, January 1963; "Gaseous reduction of oxygen
containing
copper", Canadian Patent N 668.598, August 1963).
R. Nenych, F. Kadler and V. Sedlacek replaced the conventional reduction with
wood by
ammonia, what allowed for production of high quality copper. Ammonia
consumption is
about 1 kg/t of copper, when oxygen is reduced from 4000 to 1000 ppm. (R.
Henych et
al., "Copper refining by gaseous ammonia", J. of Metals, Vol 17, No. 4, April
1955).
N. Themelis and P. Schmidt have patented the deoxidisation of a liquid copper
by
injection of various reformed hydrocarbons (methane, ethane, butane) with
steam,
leading to the formation of the gas containing carbon monoxide and hydrogen.
Patented
CA 02579586 2015-01-07
,
,
3
installation was based on vascular furnace. ("Apparatus and process for the
gaseous
deoxidisation of molten metal", Canadian Patent No 827.066, November 1969).
R. Beck, C.Andersen and M. Messner have patented the process of copper
deoxidisation with the mix of natural gas/air. ("Process for deoxidising
copper with
natural gas/air mixture", U.S. Patent N 3.619.177, November 1971).
Anaconda Company patented a process of copper deoxidisation in vascular
furnace by
injection through lances of the mix of natural gas or diesel oil and water
vapour (W.
Foard and R. Lear: "Refining copper" U.S. Patent N 3.529.956, September,
1970).
J. Henderson and W. Johnson have patented for ASARCO, "the method of copper
reduction in a vascular furnace by natural gas injection through tuyeres"
("Gas poling of
copper", U.S. Patent N 3.623.863, November 1971).
G. Mckerrow and D. PaneII reviewed in a paper "Gaseous deoxidization of anode
copper
at the Noranda smelter", Canadian Metallurgical Quarterly, Vol. 11, N 4,
1972, 629-633,
the evolution of methods of copper deoxidization in Noranda smelter using
natural gas
injected through tuyeres in a vascular furnace. J. Oudiz made a general review
of
copper reduction processes ("Poling processes for copper refining", J. of
Metals, Vol. 25,
December 1973, 35-38). Based on industrial data the consumption of reductant,
benefits and problems related with the use of various reductants, reforming
reactions
and red uctant efficiency have been analyzed.
L. Lavrov ("Deoxidization of anode copper by natural gas and steam mixture";
The
Soviet Journal of Non-Ferrous Metals, Vol. N 19, N 5, English translation,
May 1978,
25-26) verified the use of a mix of natural gas and steam injected through of
a lance.
C. Toro and V. Paredes ("SustituciOn parcial del petroleo diesel por Enap-6
como agente
reductor en el proceso de obtencion de cobre an6dico en la fundicion
Potrerillos", 34a
Convencion Anual IIMCh, Noviembre 1983, Rancagua) developed in industrial
scale and
CA 02579586 2015-12-22
4
demonstrated the possibilities of the use of heavy oil (ENAP-6), with higher
sulphur
content and lower price, in copper reduction.
J. Minoura ("Bunker fuel oil poling in anode furnace at Kosaka smelter", 114th
AIME
s Annual Meeting, 1985, NY, USA) describes the copper reduction with heavy
oil (Bunker
C), showing the advantages and lower costs with comparison of copper reduction
with
ammonia practiced since 1967.
SUMMARY
According to an aspect of the present invention, there is provided a system
for the
continuous fire refining of liquid copper. The system includes:
an oxidation reactor having an input launder for receiving liquid blister
copper by
continuous gravity feed to produce liquid oxidized copper;
a reduction reactor connected in series to the oxidation reactor, for
receiving the
liquid oxidized copper from the oxidation reactor by continuous gravity feed
and having
an output launder for egress of liquid reduced copper from the reduction
reactor;
wherein the oxidation reactor houses a packed bed of chemically neutral grains
through which the received liquid blister copper descends, the oxidation
reactor further
providing at least one opening for the introduction of oxidants consisting of
one of air and
a mix of fuel and air into the oxidation reactor; and
wherein the reduction reactor comprises a packed bed of reductants through
which the liquid oxidized copper received from the oxidization reactor
descends.
In some embodiments, the oxidation reactor includes a siphon located adjacent
the base
thereof for continuous discharge of liquid oxidized copper to the reduction
reactor, and
wherein the reduction reactor comprises a siphon located adjacent the base
thereof for
the collection of the liquid reduced copper.
CA 02579586 2015-01-07
In some embodiments, the at least one opening of the oxidation reactor is
located below
the input launder thereof.
In some embodiments, the oxidation reactor provides a bleed launder for egress
of slag
5 therefrom, the bleed launder being located opposite the location of the
siphon thereof.
According to another aspect of the present invention, there is provided an
installation for
continuous fire refining of copper, the installation comprising:
(a) an oxidation reactor comprising a settler for separating oxidized copper
and
1.0 slag;
(b) an input launder for transferring liquid blister copper from one of a
continuous
converting furnace and a retention furnace into the oxidation reactor;
(c) a reduction reactor;
(d) a launder for transferring oxidized copper from the oxidation reactor into
the
reduction reactor; and
(e) a launder for transferring reduced copper from the reduction reactor to
one of
a casting wheel and a retention-casting furnace.
In some embodiments, the flow of liquid blister copper is gravitational and
continuous
through the oxidation reactor, and the flow of oxidized copper from the
oxidation reactor
to the reduction reactor is gravitational and continuous.
In some embodiments, the oxidation reactor is one of a vertical, cylindrical
and
rectangular furnace made of a steel shell and refractories, the furnace having
a hearth
and equipped with tuyeres injecting one of air and a mix of fuel and air, the
furnace
having one of a siphon and inclined tapping hole for continuous evacuation of
oxidized
copper therefrom and a tapping hole for continuous tapping out of refining
slag, the
furnace being filled by a packed bed of chemically neutral grains, having a
size of 2 to
100 mm in diameter.
In some embodiments, the chemically neutral grains are ceramic grains.
CA 02579586 2015-01-07
6
In some embodiments, the oxidation furnace is equipped with a bean and
charging
apparatus for the addition of fluxes and an apparatus for the evacuation of
reaction
gases to a stack.
In some embodiments, oxidized copper and slag flow downwards in the furnace,
creating after phase separation in two layers on the hearth thereof, and
wherein slag and
copper are each separately evacuated through one of a siphon and tapping hole.
In some embodiments, oxidized copper and slag flow downwards in the furnace,
the
oxidized copper and slag being tapped out of the furnace together to be
separated in a
launder-settler.
In some embodiments, the reduction furnace is one of a vertical, cylindrical
and
rectangular furnace made of a steel shell and refractories, the furnace being
equipped
with tuyeres injecting one of air and a mix of fuel and air, the furnace
having one of a
siphon and inclined tapping hole for continuous evacuation of reduced copper,
the
furnace being filled by a packed bed of one of charcoal grains and coke grains
of low
sulphur content, having a size of 2 to 100 mm in diameter.
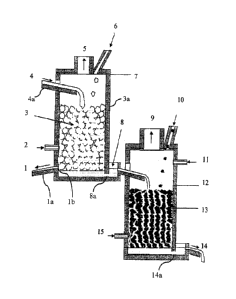
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic sectional view in elevation and profile of an
installation for
continuous fire refining of copper according to embodiments of the present
invention.
DETAILED DESCRIPTION
CA 02579586 2015-12-22
7
This invention relates to a system for continuous fire refining copper using
gravity flow of
liquid copper through two reactors in series.
Referring to Figure 1, there is shown an installation in accordance with an
embodiment
of the present invention. The installation includes an oxidation reactor 7 and
a reduction
reactor 12. The oxidation reactor includes a launder 4a, for transferring
liquid blister =
copper 4 from, for example, a continuous converting furnace or from a
retention furnace.
The liquid blister copper 4 flows into the oxidation reactor via launder 4a.
The oxidation reactor 7 may contain a number of chemically neutral grains 3
through
which the liquid blister copper may flow. The grains may be 2 to 100 mm in
diameter.
In various embodiments, the grains may be ceramic grains. In various
embodiments,
the chemically neutral grains may be inputted into the oxidation reactor 7
through
channel 6.
The grains 3 may be inputted into the reactor prior to the introduction of the
liquid blister
copper into the oxidation reactor 7, such that the grains 3 are already
present when the
liquid blister copper is introduced into the oxidation reactor 7.
As will be understood by those skilled in the art, the oxidation step in a
fire refining
process may serve to remove impurities from the liquid blister copper when air
is blown
through the molten metal. The impurities removed are contained in the slag
that is
generated. Given the function of oxidation reactor 7, an opening as at 2 are
provided in
the oxidation reactor 7 for the supply of air or the mix of fuel and air. As
shown in Figure
= 25 1, the opening 2 is located below input launder 4a to encourage
countercurrent
interaction within oxidation reactor 7.
As shown, oxidized copper and slag flow downwards in the oxidation reactor 7,
creating
after phase separation in two layers on the floor of the oxidation reactor 7.
As shown,
the liquid oxidized copper may settle at the bottom of the oxidation reactor
7, and the
slag may float above the liquid oxidized copper. An opening 8a (i.e., a
tapping hole)
CA 02579586 2015-01-07
8
may be provided at the base of the oxidation reactor 7, for the continuous
discharge of
liquid oxidized copper from the oxidation reactor 7.
The oxidation reactor 7 further provides a slag tapping hole lb for continuous
tapping
out of refining slag 1. A slag launder la may be connected to the slag tapping
hole 1 b,
to facilitate the egress of slag 1 from the oxidation reactor 7. The slag
tapping hole lb
and slag launder 1 a may be located through the shell 3a of oxidation reactor
7 opposite
the location of opening 8a.
As shown in Figure 1 and as will be readily apparent to those skilled in the
art, the level
difference between opening 8a and the slag tapping hole lb is such as to
encourage the
refining slag layer formed over the oxidized liquid copper to exit through the
slag tapping
hole lb and to reduce the likelihood of slag entering the opening 8a that is
intended for
receiving liquid oxidized copper.
The opening 8a may further be connected to a collector for collecting the
liquid oxidized
copper egressed from the oxidation reactor 7. As shown, the collector may be
connected to a launder 8 that may allow, by means of a gravity feed, liquid
oxidized
copper to be provided to the reduction reactor 12.
As per Figure 1, and given the function of oxidation reactor 7, an off-gas
flue 5 is
provided adjacent the top end of oxidation reaction 7. In some embodiments,
the
oxidation reactor 7 may be equipped with an apparatus for the evacuation of
reaction
gases to a stack (not shown). In some embodiments, the oxidation reactor 7 may
be
equipped with a bean and charging apparatus (not shown) for the addition of
fluxes.
As illustrated, the liquid oxidized copper is egressed from the oxidation
reactor 7 via an
opening 8a into a launder 8. The opening 8a and launder 8 may be considered a
siphon
or an inclined tapping hole.
CA 02579586 2015-12-22
9
In an alternative embodiment, instead of the oxidized copper and slag being
separately
tapped out of the oxidation reactor 7 via different openings, the oxidized
copper and slag
may be tapped out of the oxidation reactor 7 together via a single opening.
The oxidized
copper and slag may then be later separated in a launder-settler prior to the
oxidized
copper being fed into the reduction reactor 12.
In some embodiments, the oxidation reactor 7 may be a furnace in which the
shell 3a is
made of steel and refractories. The furnace may be vertical, cylindrical or
rectangular in
shape. The furnace may have a hearth where the liquidized copper flows, and
the
opening 2 may be a tuyere through which air or a mixture of fuel and air can
be injected
into the oxidation reactor 7.
As further illustrated in Figure 1, the reduction reactor 12 has a
construction and
structure similar to that of oxidation reactor 7. However, as is apparent from
the
drawings, the packed bed 13 of reduction reactor 12 is different than that of
oxidation
reactor 7. Given the function of reduction reactor 12, the packed bed 13
thereof consists
of reductants in the form of charcoal grains and coke grains of low sulphur
content. The
grains may have a size of 2 to 100mm in diameter. The reductants may be
introduced to
reduction reactor 12 by means of a feed tube 10 or the like.
Oxidized liquid copper entering the reduction reactor 12 by way of launder 8
thereby
flows downwardly through packed bed 13 of reductants thereof and the resulting
liquid
reduced copper is continuously egressed through an opening 14a, and collected
in a
collector located adjacent the base of reduction reactor 12. The collector may
be further
connected to a launder 14, by which the reduced copper may continuously flow
to a
casting wheel or a retention-casting furnace. The opening 14a and launder 14
may be
considered a siphon or an inclined tapping hole.
Given the function of reduction reactor 12, openings as at 11 and 15 are
provided in the
reduction reactor 12 for the supply of air and/or fuel flow. An off-gas flue 9
is provided at
the top of reduction reactor 12.
CA 02579586 2015-01-07
As with the oxidation reactor 7, the reduction reactor 12 may also be a
furnace in which
the shell is made of steel and refractories. The furnace may be vertical,
cylindrical or
rectangular in shape. The furnace may have a hearth where the reduced copper
may
5 flow, and one or more of the openings 11 and 15 may be a tuyere through
which air or a
mixture of fuel and air can be injected into the reduction reactor 12.
The scope of the claims should not be limited by the embodiments set forth in
the
examples, but should be given the broadest interpretation consistent with the
description
10 as a whole.