Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
1
CHEMICAL PROCESS
The present invention relates to a process for the production of an nitrogen
containing epihalohydrin polymer from a nitrogen-containing precursor
comprising a
purification step to remove organic impurities, particularly reaction products
of
epihalohydrin. The invention further relates to a process for purifying a
solution of a
nitrogen containing epihalohydrin polymer made from nitrogen-containing
precursor from
organic impurities.
At preparation of epihalohydrin polymers from nitrogen-containing precursors
organic by-products may be obtained, some of which frequently are halogenated.
Further,
unreacted epihalohydrin may also remain in the final product. A typical
example is the
preparation of polyaminoamide epichlorohydrin polymers that are useful as wet
strength
resins in paper making. However, many halogenated organic compounds are toxic
and/or
questionable from an environmental point of view and for many applications,
such as
paper making, it is desirable to decrease the content thereof.
Various purifications methods for removing chlorinated contaminants from
polyaminoamide epihalohydrin polymers have been disclosed, for example ion
exchange
in US 5516885, electrodialysis in US 5643430, ultra-filtration in WO 00/34358,
treatment
with activated carbon in WO 01/18093, treatment with a base in WO 99/33901 and
enzymatic action in WO 02/50163. US 6576687 discloses a method of producing
polycondensate solutions based on polyamide amine epichlorohydrin resins
claimed to
have a very low content of organic chlorine compounds. However, these
preparation and
purification methods are either complicated to operate or insufficiently
effective.
It has been disclosed to remove impurities or residual monomers from polymers
by extracting with carbon dioxide, see e.g. US 5034132 and US 6180755. It has
also
been disclosed to extract impurities with carbon dioxide from polymer
dispersions, as
described in EP 374879.
CN 1350795 discloses removal of DCP and MCP from a hydrolytic liquid of plant
protein by treating with liquefied or supercritical carbon dioxide.
US 2005/0037932 discloses a perFluoropolyether lubricant, the preparation of
which includes a supercritical fluid extraction purification step.
It is an object of the invention to provide a process for the production of a
nitrogen containing epihalohydrin polymer from a nitrogen-containing precursor
comprising
an effective purification step for removing organic impurities, particularly
halogen
containing impurities.
It is another object of the invention to provide a process for efficiently
removing
organic impurities, particularly halogen containing impurities, from a
solution of a nitrogen
containing epihalohydrin polymer made from a nitrogen-containing precursor.
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
2
It is still another object of the invention to provide for efficient removal
of
epihalohydrin or reaction products thereof from a solution of a nitrogen
containing
epihalohydrin polymer without significantly negatively affecting its desired
properties.
It is a further object of the invention to provide for efficient removal of
1,2-, or 1,3-
dihalo-2-propanol or 3-halo-1,2-propanediol from a polyaminoamide
epihalohydrin
polymer without significantly negatively affecting its desired properties.
Through the present invention it has been found possible to achieve these
objects.
One aspect of the invention concerns a process for the production of a
nitrogen
containing epihalohydrin polymer involving reaction of a nitrogen-containing
precursor
selected from the group consisting of amines, poly-amines, polyaminoamides,
polyurethanes and mixtures thereof with epihalohydrin to form of a solution of
said
epihalohydrin polymer, the process comprising a purification step to remove
organic
impurities from the formed solution of the epihalohydrin polymer, said
purification step
comprising contacting the solution of the epihalohydrin polymer with a fluid
under liquid,
supercritical or near supercritical conditions to effect extraction of organic
impurities from
the solution of the epihalohydrin polymer to the fluid, withdrawing fluid
enriched in organic
impurities from the solution of the epihalohydrin polymer, and separating
extracted
impurities from the withdrawn fluid, wherein the fluid comprises a substance
that at
atmospheric pressure and room temperature (about 25 C) is gaseous.
Another aspect of the invention concerns a process for purifying a solution of
a
nitrogen containing epihalohydrin polymer formed by reaction of a nitrogen-
containing
precursor selected from the group consisting of amines, poly-amines,
polyaminoamides,
polyurethanes and mixtures thereof with epihalohydrin comprising the steps of
contacting
the solution of the epihalohydrin polymer with a fluid under liquid,
supercritical or near
supercritical conditions to effect extraction of organic impurities from the
solution of the
epihalohydrin polymer to the fluid, withdrawing fluid enriched in organic
impurities from
the solution of the epihalohydrin polymer, and separating extracted impurities
from the
withdrawn fluid, wherein the fluid comprises a substance that at atmospheric
pressure
and room temperature (about 25 C) is gaseous.
Extracting with a fluid under supercritical or near supercritical conditions
has
been found to efficiently remove unwanted organic compounds, particularly
halogen
containing compounds, from the polymer in a comparatively simple procedure
without
significantly negatively affecting its desired properties, for example as
additive in paper
making and particularly as wet strength resin. Normally such fluid may also be
re-used
after separation of the extracted impurities. Preferred fluids are those that
have a critical
temperature (Tcr) from about 20 C to about 100 C, most preferably from about
30 C to
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
3
about 60 C, and a critical pressure (Pcr) from about 30 bar to about 500 bar,
most
preferably from about 70 bar to about 300 bar. Examples of fluids include
carbon dioxide,
nitrous oxide, ethane, ethane and propane. Carbon dioxide is particularly
preferred as it is
readily available, non-toxic and non-flammable. Further, carbon dioxide has
been found
not to give any negative impact to the treated polymer. The fluid may by used
in
substantially pure form or mixed with small amounts of one or more co-
solvents,
surfactants, complexing agents or the like.
Nitrogen containing epihalohydrin polymers in the process of the present
invention include those that have been prepared with epihalohydrin, e.g.
epichlorohydrin,
as a reactant, either during the polymerisation or in the modification of an
existing
polymer. The invention is particularly advantageous for nitrogen containing
epihalodydrin
polymers that are water soluble.
Nitrogen containing epihalohydrin polymers include those formed by reactions
of
nitrogen containing precursors selected from amines, poly-amines,
polyaminoamides,
polyurethanes and mixtures thereof. Most preferably, the polymers are
polyaminoamide-
epihalohydrin polymers, which also may be referred to as polyamidoamine-
epihalohydrin
polymers, and are useful as wet strength resins in paper making.
Epihalohydrins that can be
used include epibromohydrin and epichlorohydrin, preferably epichlorohydrin.
Suitably, the
polymers are produced using from about 0.5 to about 2 moles of epihalohydrin
per mole of
basic nitrogen in the nitrogen-containing precursor.
The nitrogen-containing precursor is preferably the polyaminoamide reaction
product of a polycarboxylic acid or a derivative thereof, suitably a
dicarboxylic acid or
derivative thereof, and a polyamine. Derivatives of carboxylic acids include
e.g. anhydrides,
esters and half esters. Suitable poly-carboxylic acids include saturated or
unsaturated
aliphatic or aromatic dicarboxylic acids. Preferably, the polycarboxylic acids
contains less
than 10 carbon atoms.
Preferred polycarboxylic acids include oxalic acid, malonic acid, succinic
acid,
glutaric acid, adipic acid, azelaic acid, sebacic acid and derivatives and
mixtures thereof, of
which adipic acid is particularly preferred.
Preferred polyamines include polyalkylene polyamines, or mixtures thereof,
having
the following formula:
H2N-(CR' H)a-(C:R2H)b-N(R3)-(C:R4H)c (CR5H)d-NH2 (I)
in which R1-R5 represent hydrogen or lower alkyl, preferably up to C3 and a-d
represent
integers of from 0 to 4. Preferred polyalkylene polyamines include diethylene
triamine, tri-
ethylene tetra amine, tetraethylene penta amine, dipropylene triamine, and
mixtures thereof.
The polyamines of formula (I) can be combined with other polyamines or
mixtures
of other amines. Preferably, these amines have any of the following formulae
II-VII:
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
4
r ---------- 1
H-(-NH-(CH2)e CR6H-)f-NCH2CH2NH (II)
R'R$N-(-(CH2)9 CR9H-(CH2),,-N(R10)-);-H (III)
HR" N-(CH2)j-CR12H-(CH2)k-OH (IV)
HNR13R'a (V)
H2N-(CH2)1-COOH (VI)
I I
(CH2)r,; NH-CO (VII)
in which R6-R14 represent hydrogen or lower alkyl, preferably up to C3, e-I
represent integers
of from 0 to 4, and m represents an integer of from 0 to 5.
The polycarboxylic acid and the polyamine may, for example, be applied in a
molar
ratio from about 1:0.5 to about 1:1.5.
The nitrogen-containing epihalohydrin-based polymer is preferably present in
an
aqueous solution, that further may comprise a water-miscible solvent such as
methanol,
ethanol or dimethyl formamide. The aqueous polymer solution is preferably
prepared from
an aqueous solution of a nitrogen-containing precursor. The molecular weights
of the
polymers are not critical and may, for example, be within the range of from
about 50000 to
about 1000000 or higher.
Preparation of epihalodydrin polymers can be performed by any known process,
such as those disclosed in any one of US 4450045, US 3311594, US 4336835,
US 3891589, US 2926154, US4857586, US4975499, US5017642, US 5019606
US 5093470 and US 5516885, but additionally including a purification step
comprising
extraction with a fluid under supercritical or near supercritical conditions
as described
herein. According to the invention it is also possible to subject commercially
or otherwise
readily available nitrogen containing epihalohydrin polymers to such a
purification step.
The solution of the epihalohydrin polymer is preferably an aqueous solution
and it
has been found that only small amount of water are withdrawn in the
purification step.
The solids content of the solution to be purified is preferably as high as
possible
without causing handling problems and can in many cases be as high as about 35
wt% or
more, but is preferably from about 5 to about 30 wt%, most preferably from
about 10 to
about 25 wt%. The viscosity of the solution is preferably from about 1 to
about 250 mPas,
most preferably from about 5 to about 200 mPas. For example, the viscosity may
be from
about 1 to about 100 mPas or from about 5 to about 60 mPas.
The invention is particularly advantageous for removing halogen containing
organic impurities. Halogen containing impurities that can be removed include
epihalohydrin as such, particularly epichlorohydrin, and reactions products
thereof such
as 1,2- and 1,3-dihalo-2-propanol (DXP) and 3-halo-1,2-propanediol (XPD),
particularly
1,2- and 1,3-dichloro-2-propanol (DCP) and 3-chloro-1,2-propanediol (CPD). By
the
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
purification according to the present invention, DXP and XPD as well as any
remaining
epihalohydrin can be removed and eventually destructed. For example, it is
possible to
obtain polyaminoamide epichlorohydrin polymers with a content of organic
chlorines
below about 0.01 wt% and even below about 0.0001 wt% or less.
5 The extraction takes place under conditions in which the fluid is in a
liquid,
supercritical or near supercritical state. Near supercritical means that the
temperature is
slightly below T, and/or the pressure is slightly below P,, but the properties
of the fluid are
substantially equivalent to the case when the temperature and the pressure
exceeds T, and
P,. respectively. The temperature in K is preferably at least about 0.9 x T,,
most preferably
at least 0.95 x T, The pressure is preferably at least about 0.9 x P,, most
preferably at least
0.95 x P, For practical reasons and to avoid decomposition of the
epihalohydrin polymer it
is usually preferred to operate at a temperature from about 10 C to about 200
C most
preferably from about 40 C to about 120 C. For the same reasons it is usually
preferred to
operate at a pressure from about 40 bar to about 500 bar, most preferably from
about 75 bar
to about 400 bar. The optimal conditions may vary depending on which
epihalohydrin
polymer that is purified and which impurities that are most desirable to
remove. For
example, it has been found that DCP is efficiently removed from polyaminoamide
epichlorohydrin at a preferred temperature from about 30 C to about 180 C,
most preferably
from about 40 C to about 100 C and a preferred pressure from about 60 bar to
about 500
bar, most preferably from about 75 bar to about 300 bar, while CPD is
efficiently removed
from polyaminoamide epichlorohydrin at a preferred temperature from about 30 C
to about
180 C, most preferably from about 50 C to about 100 C and a preferred pressure
from
about 60 bar to about 500 bar, most preferably from about 150 bar to about 400
bar
The extraction may be a one stage process, but can also be performed in two or
more stages with the same or different conditions, the latter being of
interest if different
optimal conditions for extracting different kinds of halogen containing
impurities apply. The
average residence time for the solution of the epihalohydrin polymer in
contact with fluid
under supercritical or near supercritical conditions is preferably from about
5 minutes to
about 120 minutes, most preferably from about 15 minutes to about 60 minutes.
The purification through an extraction may be performed batchwise,
continuously
or a combination thereof.
In one embodiment the extraction is performed by maintaining a solution of the
epihalohydrin polymer in vessel, preferably under agitation, and contacting it
with a
continuous flow of fluid under liquid, supercritical or near supercritical
conditions during a
time sufficient to achieve removal of halogen containing impurities to a
satisfactory
degree. The temperature and pressure may remain substantially constant during
the
entire time but may also be changed stepwise or continuously. The purified
solution of the
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
6
polymer can then withdrawn as a product or be transferred to a further
extraction step or
any other treatment.
In another embodiment the extraction is performed by contacting the solution
of
the epihalohydrin polymer with the fluid under liquid, supercritical or near
supercritical
conditions in a through-flow vessel or column, in each case with or without a
packaging.
Both the solution of the polymer and the fluid flow continuously through the
vessel or
column, preferably counter-currently, but also co-current flow could come into
question. If
appropriate, part of the purified polymer solution may be recirculated. The
temperature
and pressure may be the same or different in various parts of the vessel or
column. Any
kind of vessels or columns commonly used for extraction can be used, such as
tray-type
columns or packed columns with structured or non-structured packings made of
e.g.
glass, metal or ceramic materials.
The separation of extracted impurities from the withdrawn fluid is suitably
done
by altering the temperature and/or pressure thereof to change the solubility
properties so
the impurities are precipitated. Usually this is easiest done by lowering the
pressure to a
level below Pcr. If necessary the extracted impurities can removed from the
carbon
dioxide by purification with water or active carbon. After the separation the
fluid can be re-
used after restoring the temperature and pressure to conditions suitable for
the
extraction. The separated impurities may be destructed by any suitable means.
The invention will be further described in connection with the following
examples,
which, however, are not intended to limit the scope thereof. Unless otherwise
stated, all
parts and percentages refer to parts and percent by weight.
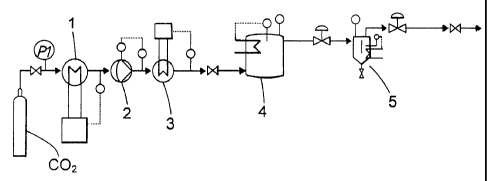
Figure 1 is a flow sheet for batchwise extraction while Figure 2 is a flow
sheet for
continuous extraction.
Example 1: An aqueous solution of polyaminoamide epichlorohydrin polymer
(PAAE) having a dry content of 20.7 wt% was purified by batch-wise extraction
with
supercritical carbon dioxide in an experimental setup according to Fig. 1.
Before the
purification the concentrations of DCP was 267 pg/g and of CPD 289 pg/g. The
solution
of PAAE was maintained under agitation in a 250 ml extraction vessel 4 and
subjected to
a continuous flow of supercritical carbon dioxide CO2 brought from a vessel
via a cooler
1, a pump 2 and a heat exchanger 3 under various conditions in respect of
temperature,
pressure and time. In a separator 5 the pressure was released, resulting in
precipitation
of the extracted components from the carbon dioxide. After the extraction the
content of
DCP and CPD in the purified PAAE solution was determined by gas
chromatography.
The results are shown in Table 1:
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
7
Table 1 Batch extraction
Conditions pg DCP/g pg CPD/g
Temperature, pressure,
redidence time, COZ flow
40 C, 150 bar, 30 min, 1.2 kg/h 69.9 249.4
70 C, 150 bar, 30 min, 1.2 kg/h 30.8 252
80 C, 150 bar, 30 min, 1.2 kg/h 45.8 141.8
80 C, 250 bar, 60 min, 1.2 kg/h < 5 227
Examples 2-4: An aqueous solution of polyaminoamide epichlorohydrin polymer
(PAAE) having a dry content of 21.2 wt% was purified by continuous extraction
with
supercritical carbon dioxide in an experimental setup according to Fig. 2.
Before the
purification the concentrations of DCP was 269.7 pg/g and of CPD 308.1 pg/g.
The
polymer solution F was brought via a pump 6, and a pre-heater 7 to a packed
column 4
where it was contacted by a counter-current flow of carbon dioxide circulating
via a cooler
1, a pump 2 a heat exchanger 3 and, after the column 4, a separator 5. The
purified
polymer solution was brought from the column 4 to a raffinate vessel R. In a
separator 5
the pressure was released and after purification with active carbon the carbon
dioxide
was recycled. In Examples 2 and 3 were run as bubble extraction with the
column 4 filled
with polymer solution, while Example 4 was run as film extraction with the
polymer
solution flowing as a film on the packings in the column 4. The results are
shown in Table
2.
Table 2 Continuous extraction
Ex. Conditions pg DCP/g pg CPD/g
Temperature, pressure, Feed flow, COZ flow
2 50 C, 200 bar, 1.8 kg/h Feed, 10kg/h CO2 16.3 159.7
3 50 C, 200 bar, 1.2 kg/h Feed, 10kg/h CO2 2.9 174.3
4 50 C, 200 bar, 1.2 kg/h Feed, 10kg/h CO2 0.8 202.2
Example 5: The treated polymers obtained in Examples 3 and 4 as well as the
commercially available polyaminoamide epichlorohydrin polymer solution Eka WS
320
were tested as wet strength agents in paper making on a pilot paper machine.
The
following conditions were applied:
Pulp: 40 wt% eucalyptus, 40 wt% birch, 20 wt% pine sulfate
Paper density: 70 g/m2
Freeness: about 34 SR
Consistency (chest): 1.5%
CA 02612134 2007-12-13
WO 2007/004972 PCT/SE2006/050188
8
Addition levels: 6, 9 and 12 kg/ tonne paper
pH (head box): 7.2-7.5
Stock temperature: 30 C
After curing for 30 minutes at 100 C the wet strength of the different papers
made were
tested and no differences could be found between the wet strength agents.
Thus, it was
found that the content of DCP and CPD in a polyaminoamide epichlorohydrin
polymer
solution could be significantly reduced without affecting its performance as
wet strength
agent in paper making.