Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 146
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 146
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Albun-in Fusion Proteins
REFERENCE TO SEQUENCE LISTING ON COMPACT DISC
[0001] This application refers to a "Sequence Listing" listed below, which is
provided as an electronic document on three identical compact
discs (CD-R), labeled "Copy I," "Copy 2," and "Copy 3." These compact discs
each contain the file "PF617PCT Sequence Listing.txt" (1,192,036
bytes, created on July 28, 2006), which is incorporated by reference in its
entirety.
BACKGROUND OF THE INVENTION
[0002] The invention relates generally to Therapeutic proteins (including, but
not fimited to, at least one polypeptide, antlbody, peptide, or
fragment and variant thereof) fused to albumin or fragments or variants of
albumin. The invention encompasses polynucleotides encoding
therapeutic albumin fusion proteins, therapeutic alburnin fusion proteins,
compositions, pharmaceutical compositions, formulations and ldts. Host
cells transformed with the potynucleotides encoding therapeutic albumin fusion
proteins are also encompassed by the invention, as are methods of
making the albumin fusion proteins of the invention using these
polynucleotides, and/or host cells.
[0003] Human serum albumin (HSA, or HA), a protein of 585 anrino acids in its
mature form (as shown in Figure 1(SEQ ID NO:1)), is
responsible for a significant proportion of the osmotic pressure of serum and
also functions as a carrier of endogenous and exogenous ligands. At
present, HA for clinical use is produced by extraction from human blood. The
production of recombinant HA (rHA) in microorganisms has been
disclosed in EP 330 451 and EP 361 991.
[0004] Therapeutic proteins in their native state or when recombinantly
produced, such as interferons and growth hormones, are typically labile
molecules exhibiting short shelf-lives, particularly when formulated in
aqueous solutions. The instability in these molecules when formulated for
administration dictates that many of the molecules must be lyophilized and
refrigerated at all times during storage, thereby rendering the molecules
difficult to transport and/or store. Storage problems are particularly acute
when pharmaceutical formulations must be stored and dispensed outside
of the hospital environment.
[0005] Few practical solutions to the storage problems of labile protein
molecules have been proposed. Accordingly, there is a need for
stabilized, long lasting formulations of proteinaceous therapeutic molecules
that are easily dispensed, preferably with a simple formulation requiring
minimal post-storage manipulation.
[0006] Upon in vivo administration, therapeutic proteins in their native state
or when recombinantly produced, such as interferons and growth
hormones, exhibit a short plasma stability due to rapid clearance from the
bloodstream. Accordingly, the therapeutic effects provided by these
proteins are also short-lived. Thus, in order to sustain their desired
therapeutic effect in vivo, the rapid clearance of these proteins from the
blood
dictates_that the therapeuticmolecules must be administered--more frequently
or at a higher dose. -However, increasing the dosing schedule for
administration of the therapeutic protein often results in an increase in
injection site reactions, side-effects, and toxicity in the patient.
Similarly,
administration of the therapeutic protein at a higher dose also commonly
results in an increase in toxicity and side-effects in the patient.
[0007] The few practical solutions to increasing plasma stability of
therapeutic molecules that have been proposed, including chemical
conjugation, have provided limited benefit to the patient. Generally, in most
cases, these chemically modified therapeutic molecules are still
administered on a frequent dosing schedule, retaining significant injection
site reactions, side-effects, and toxicity in patients. Accordingly, there is
a need for an stabifized form of therapeutic molecules that retains a higher
plasma stability in vivo than the native or recombinantly produced
therapeutic alone and can be administered less frequently, thereby decreasing
potential side-effects to the patient.
SUIVIMARY OF THE INVENTION
[0008] The present invention encompasses albumin fusion proteins comprising a
Therapeutic protein (e.g., a polypeptide, antibody, or peptide,
or fragment or variant thereof) fused to albumin or a fragment (portion) or
variant of albumin. The present invention also encompasses
polynucleotides comprising, or alternatively consisting of, nucleic acid
molecules encoding a Therapeutic protein (e.g., a polypeptide, antibody, or
peptide, or fragment or variant thereof) fused to albumin or a fragment
(portion) or variant of albumin. The present invention also encompasses
polynucleotides, comprising, or alternatively consisting of, nucleic acid
molecules encoding proteins comprising a Therapeutic protein (e.g., a
polypeptide, antibody, or peptide, or fragment or variant thereof) fused to
albumin or a fragment (portion) or variant of albumin, that is sufficient to
prolong the shelf life of the Therapeutic protein, to increase the plasma
stability of the Therapeutic protein compared to its unfused state, and/or
stabilize the Therapeutic protein and/or its activity in solution (or in a
pharmaceutical composition) in vitro and/or in vivo. Albumin fusion proteins
encoded by a polynucleotide of the invention are also encompassed by the
invention, as are host cells transformed with polynucleotides of the
invention, and methods of making the albumin fusion proteins of the invention
and using these polynucleotides of the invention, and/or host cells.
[0009] In a preferred aspect of the invention, albumin fusion proteins
include, but are not limited to, those described in Table 2 and the
polynucleotides encoding such proteins.
[0010] The invention also encompasses pharmaceutical formulations comprising
an albumin fusion protein of the invention and a
1
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
u o.m,. ~..o ,. k,n.. r ~~,.... ..,,.in ... ...u..
pharmaceutically acceptable diluent or carrier. Such formulations may be in a
kit or container. Such kit or container may be packaged with
instructions pertaining to the extended shelf life of the Therapeutic protein.
Such formulations may be used in methods of treating, preventing,
ameliorating or diagnosing a disease or disease symptom in a patient,
preferably a mammal, most preferably a human, comprising the step of
administering the pharmaceutical formulation to the patient.
[0011] In other embodiments, the present invention encompasses methods of
preventing, treating, or ameliorating a disease or disorder. In
preferred embodiments, the present invention encompasses a method of treating
a disease or disorder listed in the "Preferred Indication: Y" column
of Table 1 comprising administering to a patient in which such treatment,
prevention or amelioration is desired an albumin fusion protein of the
invention that comprises a Therapeutic protein or portion corresponding to a
Therapeutic protein (or fragment or variant tliereof) disclosed in the
"Therapeutic Protein: X" column of Table 1 (in the same row as the disease or
disorder to be treated as listed in the "Preferred Indication: Y"
column of Table 1) in an amount effective to treat, prevent or ameliorate the
disease or disorder.
[0012] In one embodiment, an albumin fusion protein described in Table 1 or 2
has extended shelf life.
[0013] In a second embodiment, an albumin fusion protein described in Table 1
or 2 is more stable than the corresponding unfused Therapeutic
molecule described in Table 1.
[0014] The present invention further includes transgenic organisms modified to
contain the nucleic acid molecules of the invention (including,
but not limited to, the polynucleotides described in Tables 1 and 2),
preferably modified to express an albumin fusion protein of the invention.
BRIEF DESCRIPTION OF THE FIGURES
[0015] Figure 1A-D shows the amino acid sequence of the mature form of human
albumin (SEQ ID NO:1) and a polynucleotide encoding it
(SEQ ID NO:2). Nucleotides 1 to 1755 of SEQ ID NO:2 encode the mature form of
human albumin (SEQ ID NO:1).
[0016] Figure 2 shows the restriction map of the pPPC0005 cloning vector ATCC
deposit PTA-3278.
[0017] Figure 3 shows the restriction map of the pSAC35 yeast S. cerevisiae
expression vector (Sleep et al., BioTechnology 8:42 (1990)).
[0018] Figure 4 compares the anti-proliferative activity of IFN albumin fusion
protein encoded by CID 3165 (CID 3165 protein) and
recombinant IFNa (rIFNa) on Hs294T melanoma cells. The cells were cultured
with varying concentrations of either CID 3165 protein or rIFNa and
proliferation was measured by BrdU incorporation after 3 days of culture. CID
3165 protein caused measurable inhibition of cell proliferation at
concentrations above 10 ng/ml with 50% inhibition achieved at approximately
200 ng/ml. (M) = CID 3165 protein, (+) = rIFNa.
[0019] Figure 5 shows the effect of various dilutions of IFNa albumin fusion
proteins on SEAP activity in the ISRE-SEAP/293F reporter cells.
One preparation of IFNa fused upstream of albumin (+) was tested, as well as
two different preparations of 1FNa fused downstream of albumin (0)
and (M).
[0020] - Figure 6 shows the effect of time and-dose of IFNa albumin fusion
protein encoded-by DNA comprised in- construct -2249 (CID 2249
protein) on the mRNA level of OAS (p41) in treated monkeys (see Example 76).
Per time point: first bar = Vehicle control, 2 d bar = 30 ug/kg CID
2249 protein day 1 iv, third bar = 30 ug/kg CID 2249 protein day 1 sc, 4s' bar
= 300 ug/kg CID 2249 protein day 1 sc, 501 bar = 40 ug/kg
recombinant IFNa day 1, 3 and 5 sc.
[0021] Figure 7 shows the dose-response relationship of BNP albumin fusion
proteins encoded by DNA comprised in constructs CID 3691 and
3618 (CID 3691 and 3618 protein) on activating cGMP formation in NPR-A/293F
reporter cells (see Examples 78 and 79). Both BNP peptide (0),
as well as, two different preparations of BNP fused upstream of albumin (0)
and (0) were tested.
[0022] Figure 8 shows the effect of BNP albumin fusion protein on mean
arterial pressure in spontaneously hypertensive rats (see Example 78).
Vehicle (~), BNP peptide (0), or BNP albumin fusion protein (0) were delivered
via tail vein injection. Systolic and diastolic blood pressures
were recorded by cuff-tail method.
[0023] Figure 9 shows the plasma cGMP levels in eleven- to 12-week-old male
C57/BL6 mice after intravenous injection of recombinant BNP
peptide (0) or BNP albumin fusion protein (0 (see Example 78). oGMP levels
were determined from plasma prepared from tail bleeds collected
at several time points after intravenous injection.
[0024] Figure 10 shows the dose-response relationship of BNP peptide and BNP
albumin fusion proteins encoded by DNA comprised in
constructs CID 3796 and 3959 on activating cGMP formation in NPR-A/293F
reporter cells (see Example 80). Both BNP peptide (~), as well as,
two different preparations comprising BNP fused downstream of albumin, (0) and
(O) were tested.
[0025] Figure 11A shows the dose-response relationship of BNP and ANP peptides
with or without treatment of neprilysin for 24 hours on
activating cGMP formation in NPR-A/293F reporter cells (see Example 81).
[0026] Figure 11B shows the dose-reponse relationship of ANP peptide on
activating cGMP formation in NPR-A/293F reporter cells following
treatment of neprilysin or control MES buffer for 20 minutes, 1 hour, or 24
hours (see Example 81).
[0027] Figure 11C shows the dose-reponse relationship of ANP albumin fusion
protein comprising ANP fused upstream of albumin and
encoded by DNA comprised in construct CID3484 on activating cGMI' formation in
NPR-A/293F reporter cells following treatment of neprilysin or
control MES buffer for 20 minutes, 1 hour, or 24 hours (see Example 81).
2
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
[0028] Figure 11D shows the percentage of intact natriuretic peptides
following treatment with neprilysin for the specified time. Both ANP and
BNP peptides, as well as, two albumin fusion proteins comprising BNP fused
upstream of albumin via tripartite glycines (CID 3809) and ANP
fused upstream to albumin (CID 3484) were tested (see Example 81).
[0029] Figure 12 shows the reduction in HCV RNA titer, as measured by median
HCV RNA change (logio N/ml), in patients infected with
chronic hepatitis C genotype 1 and who have previously failed to respond to at
least one treatment regimen of pegylated interferon alpha in
combination with ribavirin (PEG-RBV) (nonresponders) following treatment with
HSA-IFNa2b in combination with ribavirin for 0 to 24 weeks.
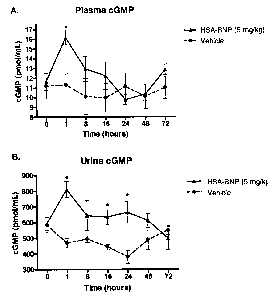
[0030] Figures 13A and B show the effect of HSA-BNP (Construct ID #3959) on
plasma and urine cGMP levels, respectively following
administration of an 5 mg/kg IV bolus in normal healthy pigs (n = 4-6/group).
Asterisks indicate significant differences in cGMP levels from
vehicle (p<0.05).
[0031] Figure 14A shows the effect of administration of an intravenous bolus
of 2 mg/kg or 6 nig/kg HSA-BNP (Construct ID #3959) on end-
diastolic diameter change in a porcine experimental heart failure model (n =
10/group). Heart failure was induced in the pig by ventricular pacing.
End diastolic diameter was measure by echocardiography. Significant changes
(p<0.05) from vehicle or baseline are indicated (& and #,
respectively).
[0032] Figure 14B shows the effect of administration of an intravenous bolus
of 2 mg/kg or 6 mg/kg HSA-BNP (Construct ID #3959) on
fractional shortening in a porcine experimental heart failure model (n =
10/group). Heart failure was induced in the pig by ventricular pacing.
Significant changes (p<0.05) from vehicle or baseline are indicated (& and #,
respectively).
[0033] Figures 15A-H show the hemodynamic effects of HSA-BNP (Construct ID
#3959) administered via a single intravenous bolus at 0.5
mg/kg or 5 mg/kg in a normal dog model. Cardiac output (CO), mean arterial
pressure (MAP), pulmonary capillary wedge pressure (PCWP) and
pulmonary arterial pressure (PAP) were measured at baseline prior to
intravenous bolus of 0.5 mg/kg or 5 mg/kg HSA-BNP (Construct ID #3959)
and at 30, 60, 90, 150, 210, and 270 post-infusion in anesthesized normal
mongrels (n = 8/group). Asterisks indicate statistically significant
changes from baseline (p<0.05).
[0034] Figures 16A-H show the renal effects of HSA-BNP (Construct ID #3959)
administered via a single intravenous bolus at 0.5 mg/kg or 5
mg/kg in a normal dog model. Urine flow (rate/30 minute collection), sodium
excretion, renal blood flow, and glomerular filtration rate (GFR) were
measured at baseline prior to intravenous bolus of 0.5 mg/kg or 5 mg/kg HSA-
BNP (Construct ID #3959) and at 30, 60, 90, 150, 210, and 270 post-
infusion in anesthesized normal mongrels (n = 8/group). Asterisks indicate
statistically significant changes from baseline (p<0.05).
[0035] Figures 17A-F show the hormonal effects of HSA-BNP (Construct ID #3959)
administered via a single intravenous bolus at 0.5 mg/kg
or 5 mg/kg in a normal dog model. Plasma aldosterone, renin, and angiotensin
II levels were measured at baseline prior to intravenous bolus of 0.5
mg/kg or 5 mg/kg HSA-BNP (Construct ID #3959) and at 30, 60, 90, 150, 210, and
270 post-infusion in anesthesized normal mongrels (n =
8/group): Asterisks indicate statistically significant changes from baseline
(p<0.05).
[0036] Figures 18A-C show the effect of a single intravenous bolus of 5 mg/kg
HSA-BNP (Construct ID #3959) on systolic and mean arterial
blood pressure in normal, healthy, awake beagles surgically implanted with a
Data Sciences International radiotelemetry transmitter, which had
systemic arterial blood pressure, heart rate and ECG data collection
capabilities. Change from baseline of systolic blood pressure (Figure 18A),
difference in mean systolic blood pressure (Figure 18B), and change from
baseline in mean arterial pressures (Figure 18C) over 48 hours of
continuous data recording following infusion are presented. Asterisks indicate
a statistically significant difference in baseline-adjusted mean values
for 5 mg/kg HSA-BNP (Construct ID #3959) compared to vehicle (p<0.05).
[0037] Figures 19A and B show a comparison of the effect of an intravenous
bolus of 0.02 mg/kg unfused BNP peptide and a subcutaneous
injection of 10 mg/kg HSA-BNP (Construct ID #3959) on systemic blood pressure
in normal, healthy, awake beagles surgically implanted with a
Data Sciences International radiotelemetry transmitter, which had systemic
arterial blood pressure, heart rate and ECG data collection capabilities.
Change from baseline of systolic blood pressure over 48 hours of continuous
data recording following administration of BNP (Figure 19A) and
HSA-BNP (Construct ID#3959) are presented. Asterisks indicate a statistically
significant difference in baseline-adjusted mean values for 5 mg/kg
HSA-BNP (Construct ID #3959) compared to vehicle (p<0.05).
DETAILED DESCRIPTION
Definitions
[0038] The following defmifions are provided to facilitate understanding of
certain terms used throughout this specification.
[0039] As used herein, "polynucleotide" refers to a nucleic acid molecule
having a nucleotide sequence encoding a fusion protein comprising, or
alternatively consisting of, at least one molecule of albumin (or a fragment
or variant thereof) joined in frame to at least one Therapeutic protein X
(or fragment or variant thereof); a nucleic acid molecule having a nucleotide
sequence encoding a fusion protein comprising, or alternatively
consisting of, the amino acid sequence of SEQ ID NO:Y (as described in column
6 of Table 2) or a fragment or variant thereof; a nucleic acid
molecule having a nucleotide sequence comprising or altematively consisting of
the sequence shown in SEQ ID NO:X; a nucleic acid molecule
having a nucleotide sequence encoding a fusion protein comprising, or
alternatively consisting of, the amino acid sequence of SEQ ID NO:Z; a
3
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
nucleic acid molecule having a nucleotide sequence encoding an albumin fusion
protein of the invention generated as described in Table 2 or in the
Examples; a nucleic acid molecule having a nucleotide sequence encoding a
Therapeutic albumin fusion protein of the invention, a nucleic acid
molecule having a nucleotide sequence contained in an albumin fusion construct
described in Table 2, or a nucleic acid molecule having a nucleotide
sequence contained in an albumin fusion construct deposited with the ATCC (as
described in Table 3).
[0040] As used herein, "albumin fusion construct" refers to a nucleic acid
niolecule comprising, or alternatively consisting of, a polynucleotide
encoding at least one molecule of albumin (or a fragment or variant thereof)
joined in frame to at least one polynucleotide encoding at least one
molecule of a Therapeutic protein (or fragment or variant thereof); a nucleic
acid molecule comprising, or altematively consisting of, a
polynucleotide encoding at least one molecule of albumin (or a fragment or
variant thereof) joined in frame to at least one polynucleotide encoding
at least one molecule of a Therapeutic protein (or fragment or variant
thereof) generated as described in Table 2 or in the Examples; or a nucleic
acid
molecule comprising, or alternatively consisting of, a polynucleotide encoding
at least one molecule of albumin (or a fragment or variant thereof)
joined in frame to at least one polynucleotide encoding at least one molecule
of a Therapeutic protein (or fragment or variant thereof), further
comprising, for example, one or more of the following elements: (1) a
functional self-replicating vector (including but not limited to, a shuttle
vector, an expression vector, an integration vector, and/or a replication
system), (2) a region for initiation of transcription (e.g., a promoter
region,
such as for example, a regulatable or inducible promoter, a constitutive
promoter), (3) a region for termination of transcription, (4) a leader
sequence, and (5) a selectable marker. The polynucleotide encoding the
Therapeutic protein and albumin protein, once part of the albumin fusion
construct, may each be referred to as a "portion," "region" or "moiety" of the
albumin fusion construct.
[0041] The present invention relates generally to polynucleotides encoding
albumin fusion proteins; albumin fusion proteins; and methods of
treating, preventing, or ameliorating diseases or disorders using albumin
fusion proteins or polynucleotides encoding albumin fusion proteins. As
used herein, "albumin fusion protein" refers to a protein formed by the fusion
of at least one molecule of albumin (or a fragment or variant thereof)
to at least one molecule of a Therapeutic protein (or fragment or variant
thereof). An albumin fusion protein of the invention comprises at least a
fraginent or variant of a Therapeutic protein and at least a fraginent or
variant of human serum albumin, which are associated with one another by
genetic fusion (i.e., the albumin fusion protein is generated by translation
of a nucleic acid in which a polynucleotide encoding all or a portion of a
Therapeutic protein is joined in-frame with a polynucleotide encoding all or a
portion of albumin). The Therapeutic protein and albumin protein,
once part of the albumin fusion protein, may each be referred to as a
"portion", "region" or "moiety" of the albumin fusion protein (e.g., a
"Therapeutic protein portion" or an "albumin protein portion"). In a highly
preferred embodiment, an albumin fusion protein of the invention
comprises at least one molecule of a Therapeutic protein X or fragment or
variant of thereof (including, but not limited to a mature form of the
Therapeutic protein X) and at least one moleoule of albumin or fragment or
variant thereof (including but not limited to a mature form of albumin).
[0042] In a further preferred embodiment, an albumin fusion protein of the
invention is processed by a host cell and secreted into the
surrounding culture medium. Processing of the nasoent albumin fusion proteig
that-occurs in the secretory patliwaysof the host used for expression-
_ _--
may include, but is not limited to signal peptide cleavage; formation of
disulfide bonds; proper folding; addition and processing of carbohydrates
(such as for example, N- and 0- linked glycosylation); specific proteolytic
cleavages; and assembly into multimeric proteins. An albumin fusion
protein of the invention is preferably in the processed form. In a most
preferred embodiment, the "processed form of an albumin fusion protein"
refers to an albumin fusion protein product which has undergone N- terminal
signal peptide cleavage, herein also referred to as a "mature albumin
fusion protein".
[0043] In several instances, a representative clone containing an albumin
fusion construct of the invention was deposited with the American
Type Culture Collection (herein referred to as "ATCC "). Furthermore, it is
possible to retrieve a given albumin fusion construct from the deposit
by techniques known in the art and described elsewhere herein. The ATCC is
located at 10801 University Boulevard, Manassas, Virginia 20110-
2209, USA. The ATCCO deposits were made pursuant to the terms of the Budapest
Treaty on the international recognition of the deposit of
microorganisms for the purposes of patent procedure.
[0044] In one embodiment, the invention provides a polynucleotide encoding an
albumin fusion protein comprising, or alternatively consisting
of, a Therapeutic protein and a serum albumin protein. In a further
embodiment, the invention provides an albumin fusion protein comprising, or
alternatively consisting of, a Therapeutic protein and a serum albumin
protein. In a preferred embodiment, the invention provides an albumin fusion
protein comprising, or alternatively consisting of, a Therapeutic protein and
a serum albumin protein encoded by a polynucleotide described in Table
2. In a further preferred embodiment, the invention provides a polynucleotide
encoding an albumin fusion protein whose sequence is shown as SEQ
ID NO:Y in Table 2. In other enibodiments, the invention provides an albumin
fusion protein comprising, or alternatively consisting of, a
biologically active and/or therapeutically active fragment of a Therapeutic
protein and a serum albumin protein. In other embodiments, the invention
provides an albumin fusion protein comprising, or alternatively consisting of,
a biologically active and/or therapeutically active variant of a
Therapeutic protein and a serum albumin protein. In preferred embodiments, the
serum albumin protein component of the albumin fusion protein is
the mature portion of serum albumin. The invention further encompasses
polynucleotides encoding these albumin fusion proteins.
[0045] In further embodiments, the invention provides an albumin fusion
protein comprising, or alternatively consisting of, a Therapeutic
protein, and a biologically active and/or therapeutically active fragment of
serum albumin. In fhrther embodiments, the invention provides an
4
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
. . . ... ~_. .,,. ,..,õ.
albumin fusion protein comprising, or altematively consisting of, a
Therapeutic protein and a biologically active and/or therapeutically active
variant
of serum albumin. In preferred embodiments, the Therapeutic protein portion of
the albumin fusion protein is the mature portion of the Therapeutic
protein. In a furtlier preferred enibodiment, the Therapeutic protein portion
of the albumin fusion protein is the extracellular soluble domain of the
Therapeutic protein. In an alternative embodiment, the Therapeutic protein
portion of the albumin fusion protein is the active form of the
Tlierapeutic protein. The invention further encompasses polynucleotides
encoding these albumin fusion proteins.
[0046] In further embodiments, the invention provides an albumin fusion
protein comprising, or alternatively consisting of, a biologically active
and/or therapeutically active fragmetit or variant of a Therapeutic protein
and a biologically active and/or therapeutically active fragment or variant
of serum albumin. In preferred embodiments, the invention provides an albumin
fusion protein comprising, or alternatively consisting of, the mature
portion of a Therapeutic protein and the mature portion of serum albumin. The
invention further encompasses polynucleotides encoding these
albumin fusion proteins.
Therapeutic proteins
[00471 As stated above, a polynucleotide of the invention encodes a protein
comprising or alternatively consisting of, at least a fragment or
variant of a Therapeutic protein and at least a fragment or variant of human
serum albumin, which are associated with one another, preferably by
genetic fusion.
[0048] An additional embodiment includes a polynucleotide encoding a protein
comprising or alternatively consisting of at least a fragment or
variant of a Therapeutic protein and at least a fragment or variant of human
serum albumin, which are linked with one another by chemical
conjugation.
[0049] As used herein, "Therapeutic protein" refers to proteins, polypeptides,
antibodies, peptides or fragments or variants thereof, having one
or more therapeutic and/or biological activities. Therapeutic proteins
encompassed by the invention include but are not limited to, proteins,
polypeptides, peptides, antibodies, and biologics. (The terms peptides,
proteins, and polypeptides are used interchangeably herein.) It is
specifically
contemplated that the term "Therapeutic protein" encompasses antibodies and
fragments and variants thereof. Thus a protein of the invention may
contain at least a fragment or variant of a Therapeutic protein, and/or at
least a fragment or variant of an antibody. Additionally, the term
"Therapeutic protein" nlay refer to the endogenous or naturally occurring
correlate of a Therapeutic protein.
[0050] By a polypeptide displaying a "therapeutic activity" or a protein that
is "therapeutically active" is meant a polypeptide that possesses one
or more known biological and/or therapeutic activities associated with a
therapeutic protein such as one or more of the Therapeutic proteins
described herein or otherwise known in the art. As a non-limiting example, a
"Therapeutic protein" is a protein that is useful to treat, prevent or
ameliorate a disease, condition or disorder. As a non-limiting example, a
"Therapeutic protein" may be one that binds specifically to a particular cell
type (normal (e.g., lymphocytes) or abnormal e.g., (cancer cells)) and
therefore may be used to target a compound (drug, or cytotoxic agent) to that
cell type specifrcally.
[0051] For example, a non-exhaustive list of "Therapeutic protein" portions
which may be comprised by an albumin fusion protein of the
invention includes, but is not limited to, IFNa, , ANP, BNP, LANP, VDP, KUP,
CNP, DNP, HCC-1, beta defensin-2, fractalkine, oxyntomodulin,
killer toxin peptide, TIMP-4, PYY, adrenomedullin, ghrelin, CGRP, IGF-1,
neuraminidase, hemagglutinin, butyrylcholinesterase, endothelin, and
mechano growth factor.
[0052] Interferon hybrids may also be fused to the amino or carboxy terminus
of albumin to form an interferon hybrid albumin fusion protein.
Interferon hybrid albumin fusion protein may have enhanced, or altematively,
suppressed interferon activity, such as antiviral responses, regulation
of cell growth, and modulation of immune response (Lebleu et al., PNAS USA,
73:3107-3111 (1976); Gresser et al., Nature, 251:543-545 (1974);
and Johnson, Texas Reports Biol Med, 35:357-369 (1977)). Each interferon
hybrid albumin fusion protein can be used to treat, prevent, or
ameliorate viral infections (e.g., hepatitis (e.g., HCV); or HIV), multiple
sclerosis, or cancer.
[0053] In one embodiment, the interferon hybrid portion of the interferon
hybrid albumin fusion protein comprises an interferon alpha-interferon
alpha hybrid (herein referred to as an alpha-alpha hybrid). For example, the
alpha-alpha hybrid portion of the interferon hybrid albumin fusion
protein consists, or altematively comprises, of interferon alpha A fused to
interferon alpha D. In a further embodiment, the A/D hybrid is fused at
the common BgIII restriction site to interferon alpha D, wherein the N-
terminal portion of the A/D hybrid corresponds to amino acids 1-62 of
interferon alpha A and the C-terminal portion corresponds to amino acids 64-
166 of interferon alpha D. For example, this A/D hybrid would
comprise the amino acid sequence:
CDLPQTHSLGS RRTLMLLAQMRXIISLFS CLKDRHDFGFPQEEFGNQFQKAETIPVLHEMIQQIFNLFTTKDS
SAAWDEDLLDKFCTELYQQL
NDLEACVMQEERVGETPLMNX2DSILAVKKYFRRITLYLTEKKYSPCAWEVVRAEIMRSLSLSTNLQERLRRKE
(SEQ ID NO:99), wherein
the X, is R or K and the XZ is A or V. In an additional embodiment, the A/D
hybrid is fused at the common PvuIII restriction site, wherein the N-
terminal portion of the A/D hybrid corresponds to amino acids 1-91 of
interferon alpha A and the C-terminal portion corresponds to amino acids 93-
166 of interferon alpha D. For example, this A/D hybrid would comprise the
amino acid sequence:
CDLPQTHSLGSRRTLMLLAQMRXIISLFSCLKDRHDFGFPQEEFGNQFQKAETIPVLHEMIQQIFNLFSTKDSSAAWDE
TLLDKFYTELYQQL
NDLEACVMQEERVGETPLMNX2DSILAVKKYFRRITLYLTEKKYSPCAWEVVRA.EIMRSLSLSTNLQERLRRKE
(SEQ ID NO:100), wherein
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
... .. ....... ...- .,.....
the X, is R or K and the sec.ond.. X2 is A or V. These hybrids are further
described in U.S. Patent No. 4,414,510, which is hereby incorporated by
reference in its entirety.
[0054] In an additional embodiment, the alpha-alpha hybrid portion of the
interferon hybrid albumin fusion protein consists, or alternatively
comprises, of interferon alpha A fused to interferon alpha F. In a further
embodiment, the A/F hybrid is fused at the common PvuIII restriction site,
wherein the N-terminal portion of the A/F liybrid corresponds to amino acids 1-
91 of interferon alpha A and the C-terminal portion corresponds to
amino acids 93-166 of interferon alpha F. For example, this A/F hybrid would
comprise the amino acid sequence:
CDLPQTHSLGSRRTLMLLAQMRXISLFSCLKDRHDFGFPQEEFGNQFQKAETIPVLHEMIQQIFNLFSTKDS
SAAWDETLLDKFYTELYQQL
NDMEACVIQEVGVEETPLMNVDSILAVKKYFQRITLYLTEKKYSPCAWEVVRAEIMRSFSLSKIFQERLRRKE (SEQ
ID NO:101), wherein X
is either R or K. These hybrids are furtlier described in U.S. Patent No.
4,414,510, which is hereby incorporated by reference in its entirety. In a
further embodiment, the alpha-alpha hybrid portion of the interferon hybrid
albumin fusion protein consists, or alternatively comprises, of interferon
alpha A fused to interferon alpha B. In an additional embodiment, the A/B
hybrid is fused at the common PvuIII restriction site, wherein the N-
terminal portion of the A/B hybrid corresponds to amino acids 1-91 of
interferon alpha A and the C-terminal portion corresponds to amino acids 93-
166 of interferon alpha B. For example, this A/B hybrid would comprise an
amino acid sequence:
CDLPQTHSLGSRRTLMLLAQMRXIISLFSCLKDRHDFGFPQEEFGNQFQKAETIPVLHEMIQQIFNLFSTKDSSAAWDE
TLLDKFYTELYQQL
NDLEXZX3XaX5QEVGVIESPLMYEDSILAVRKYFQRITLYLTEKKYSSCAWEVVRAEIMRSFSLSINLQKRLKSKE
(SEQ ID NO: 102), wherein
the Xt is R or K and X2 through X5 is SCVM or VLCD. These hybrids are further
described in U.S. Patent No. 4,414,510, which is hereby
incorporated by reference in its entirety.
[0055] In another embodiment, the interferon hybrid portion of the interferon
hybrid albumin fusion protein comprises an interferon beta-
interferon alpha hybrid (herein referred to as a beta-alpha hybrid). For
example, the beta-alpha hybrid portion of the interferon hybrid albumin
fusion protein consists, or alternatively comprises, of interferon beta-I
fused to interferon alpha D (also referred to as interferon alpha-1). In a
further embodiment, the beta-I/alpha D hybrid is fused wherein the N-terminal
portion corresponds to amino acids 1-73 of interferon beta-1 and the
C-terminal portion corresponds to amino acids 74-167 of interferon alpha D.
For example, this beta-1/alpha D hybrid would comprise an amino
acid sequence:
MSYNLLGFLQRS SNFQCQKLLWQLNGRLEYCLKDRMNFDIPEEIKQLQQFQKEDAALTIYEMLQNIFAIFRQDS
SAAWDEDLLDKFCTELY
QQLNDLEACVMQEERVGETPLMNXDSILAVKKYFRRITLYLTEKKYSPCAWEVVRAEIMRSLSLSTNLQERLRRKE
(SEQ ID NO:103),
wherein X is A or V. These hybrids are further described in U.S. Patent No.
4,758,428, which is hereby incorporated by reference in its entirety.
[00561 In another embodiment, the interferon hybrid portion of the interferon
hybrid albumin fusion protein comprises an interferon alpha-
interferon beta hybrid (herein referred to as a alpha-beta hybrid). For
example, the alpha-beta hybrid portion of the interferon hybrid albumin fusion
protein consists, or altematively comprises, of interferon alpha_ D(also
referred to_as interferon alpha-1) -fused to interferon- beta-1. In a further-
-
embodiment, the alpha Dlbeta-1 hybrid is fused wherein the N-terminal portion
corresponds to amino acids 1-73 of interferon alpha D and the C-
terminal portion corresponds to amino acids 74-166 of interferon beta-1. For
example, this alpha D/beta-1 hybrid would have an amino acid
sequence:
MCDLPETHSLDNRRTLMLLAQMSRISPSSCLMDRHDFGFPQEEFDGNQFQKAPAIS
VLHELIQQIFNLFTTKDSSSTGWNETIVENLLANVY
HQINHLKTVLEEKLEKEDFTRGKLMSSLHLKRYYGRILHYLKAKEYSHCAWTIVRVEILRNFYFINRLTGYLRN
(SEQ ID NO:104). These
hybrids are further described in U.S. Patent No. 4,758,428, which is hereby
incorporated by reference in its entirety.
[0057] In further embodiments, the interferon hybrid portion of the interferon
hybrid albumin fusion proteins may comprise additional
combinations of alpha-alpha interferon hybrids, alpha-beta interferon hybrids,
and beta-alpha interferon hybrids. In additional embodiments, the
interferon hybrid portion of the interferon hybrid albumin fusion protein may
be modified to include mutations, substitutions, deletions, or additions
to the amino acid sequence of the interferon hybrid. Such modifications to the
interferon hybrid albumin fusion proteins may be made, for example,
to improve levels of production, increase stability, increase or decrease
activity, or confer new biological properties.
[0058] The above-described interferon hybrid albumin fusion proteins are
encompassed by the invention, as are host cells and vectors containing
polynucleotides encoding the polypeptides. In one embodiment, a interferon
hybrid albumin fusion protein encoded by a polynucleotide as
described above has extended shelf life. In an additional embodiment, a
interferon hybrid albumin fusion protein encoded by a polynucleot3de
described above has a longer serum half-life and/or more stabilized activity
in solution (or in a pharmaceutical composition) in vitro and/or in vivo
than the corresponding unfused interferon hybrid molecule.
[0059] In another non-limiting example, a "Therapeutic protein" is a protein
that has a biological activity, and in particular, a biological activity
that is useful for treating, preventing or ameliorating a disease. A non-
inclusive list of biological activities that may be possessed by a Therapeutic
protein includes, inhibition of HIV-1 infection of cells, stimulation of
intestinal epithelial cell proliferation, reducing intestinal epithelial cell
permeability, stimulating insulin secretion, induction of bronchodilation and
vasodilation, uihibition of aldosterone and renin secretion, blood
pressure regulation, promoting neuronal growth, enhancing an inunune response,
enhancing inflammation, suppression of appetite, or any one or
6
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
_ ..... . ..~ .,. ,.. ,.- ..,,..
more of the biological activities described in the "Biological Activities"
section below and/or as disclosed for a given Therapeutic protein in Table I
(column 2).
[00601 In one embodiment, IFN-alpha-HSA fusions are used to inhibit viral
agents classified under Category A- Filo (Ebola), Arena (Pichende),
Category B- Toga (VEE) or Category C- Bunya (Punto toro), Flavi (Yellow fever,
West Nile). For example, CPE inhibition, neutral red staining and
virus yield assays were employed to evaluate the antiviral activities of INF-
alpha fused downstream of HSA (CID 3165 protein). The
pharmacokinetics and pharmacodynamic activity of CID 3165 protein in
cynomolgus monkeys and human subjects were evaluated. The results
indicate that antiviral activity was achieved against all the RNA viruses
evaluated with a favorable safety index. The IC50 values ranged from <0.1
ng/ml (Punta Toro A) to 19 ng/ml (VEE) in the CPE assay. In cynomolgus
monkeys, the half-life of CID 3165 protein was 90 hours and was
detectable up to 14 days post-dose. In human subjects, CID 3165 protein was
safe and well tolerated. C_ following single injection doses was
dose-proportional. The mean C,n. in the 500 ug cohort was 22 ng/ml, and the
mean t1/2 was 150 hours. Dosing once every 2-4 weeks or more is
supported by the pharmacokinetics. Antiviral response against Hepatitis C was
observed in the majority of subjects in the single injection cohorts
(120-500 ug).
[00611 In a further embodiment, IFN-alpha-HSA fusions are used to treat
patients with chronic Hepatitis C infection (HCV). Interferon alpha,
also known as interferon alfa or leukocyte interferon, is the standard of care
for treatment of patients infected with HCV. The term "interferon
alpha" refers to a family of highly homologous related polypeptides with anti-
viral activity. The interferon alpha portion of the IFN-alpha-HSA
fusion consists or alternatively comprises any interferon alpha or fragment
thereof known in the art. Non-limiting examples of the interferon alpha
portion of the IFN-alpha-HSA fusion proteins of the invention include, but are
not limited to, the interferon alpha proteins disclosed in the
Therapeutic protein column of Table 1. In particular embodiments, the
interferon alpha portion consists or alternatively comprises interferon alplia-
2a, interferon alpha-2b, interferon alpha-2c, consensus interferon, interferon
alfacon-1, interferon alpha-nl, interferon alpha-n3, any commercially
available form of interferon alpha, such as, for example, INTRONO A (Schering
Corp., Kenilwor0i, N.J.), ROFERON A(Hoffman-La Roche,
Nutley, N.J.), Berofor alpha inteferon (Boehringer Ingelheim Pharmaceutical,
Inc., Ridgefied, Conn.), OMNIFERONTM (Viragen, Inc., Plantation,
FL), MULTIFERONTM (Viragen, Inc., Plantation, FL) WELLFERON'D
(GlaxoSmithKline, London, Great Britian), INFERGEN (Amgen, Inc.,
Thousands Oaks, CA), SUMIFERON (Sumitomo, Japan), BELEROFON'~' (Nautilus
Biotech, France), hLkXY-ALPHATM (Maxygen, Redwood
City, CA 1 Hoffinan-La Roche, Nutley, N.J.), or any purified interferon alpha
product or a fragment thereof. In further embodiments, the interferon
alpha portion of the IFN-alpha-HSA fusion protein consists or alternatively
comprises interferon alpha modified or formulated for extended or
controlled release. For example, the interferon alpha portion consists, or
alternatively comprises commercially available extended release or
controlled release interferon alpha, including, but not limited to interferon-
alpha-XL (Flamel Technologies, France) and LOCTERONTM (BioLex
Therapeutics/OctoPlus, Pittsboro, NC). hi additional embodiments, the
interferon alpha portion of the IFN-alpha-HSA fusion protein may be
modified by the attachment of chemical moieties._ For example, the_inteferon
alpha portion may_bemodified by -pegylation. - Accordingly, in-
additional embodiments, the interferon alpha portion of the IFN-alpha-HSA
fusion protein consists or alternatively comprises pegylated forms of
interferon alpha-2a, 2b, or consensus interferon and include, but are not
limited to, a commercially available pegylated interferon alpha, such as, for
example, PEG-INTRON (Schering Corp., Kenilworth, N.J.), PEGASYS (Hoffman-La
Roche, Nutley, N.J.), PEG-OMNIFERONTM (Viragen, Inc.,
Plantation, FL) or a fragment thereof. However, as used herein, "IFN-alpha-
HSA" fusions refers to the HSA fused to any of the interferon alpha
proteins known in the art or a fragment thereof.
[0062] Patients infected with HCV may fall within two categories based on
previous exposure to an interferon regimen for treatment of the
HCV infection. "Treatment-naive patients" or "naYve patients" are those
patients who have never been treated with an interferon regimen.
"Treatment-experienced patients" or "experienced patients" are those patients
who have been treated or are currently being treated with an interferon
regimen. "Non-responders" are experienced patients who have been previously
treated with an interferon regimen but have failed to meet the
primary endpoint of treatment such as an early viral load reduction (EVR) or
an end-of-treatment response (ETR). "Relapsers" are experienced
patients who have previously been treated with an interferon regimen and have
a achieved primary endpoint of treatment such as EVR or ETR, but
become subsequently positive for HCV at later time points. However, as used
herein, an "HCV patient" refers to a patient who is infected with
HCV and who is either natve or experienced. In addition, as used herein, an
"HCV patient" who is "experienced" is either a non-responder or a
relapser.
[0063] In addition, the Hepatitis C virus can be classified into numerous
genotypes, with four genotypes, genotype 1, 2, 3, or 4, being the most
prevalent. Generally, the Hepatitis C virus that infects an HCV patient
comprises a single genotype. However, the Hepatitis virus can comprise a
combination of two or more genotypes. In addition, the genotype of Hepatitis C
virus may also be a variant of one of the known HCV genotypes. In
a further embodiment, the Hepatitis C virus of the HCV patient is genotype 1
or a variant thereof. However, as used herein, "HCV" refers to the
Hepatitis C virus of any genotype, or combination or variants thereof.
[0064] The standard treatment regimen for patients with HCV involves treatment
with interferon alpha in combination with an antiviral agent,
such as, ribavirin. In general, the interferon alpha is administered daily,
twice-a-week, or weekly and the ribavirin is administered daily. However,
recent studies have also used inteferon alpha in combination with other
antiviral agents known in the art for the treatment of HCV. Thus, in a
7
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
.~ ..,,,.. ,. ~ ,,,.,.,..,, ,w..~, .~- ... .. ....... further embodiment the
IFN-alpha-HSA fusion may be administered to the HCV patient either alone or in
combination with an antiviral agent, such
as, for example, ribavirin. In a more preferred embodiment, IFN-alpha-HSA
fusion may be administered to the HCV patient in combination with
one or more antiviral agents, such as, for example, ribavirin and an
additional antiviral agent.
[0065] As noted above, pharmokinetics of the CID 3165 protein support a dosing
schedule of once every 2-4 weeks or greater. Thus, in a
further embodiment, the HCV patients are treated with an IFN-alpha-HSA fusion
by administration once every 2-4 weeks alone or in combination
with an effective amount of an antiviral agent. hr a preferred embodiment, the
HCV patients are treated with an IFN-alpha-HSA fusion by
administration once every 2-4 weeks in combination with an effective amount of
one or more antiviral agents. In an additional preferred
embodiment, the IF'N-alpha-HSA fusion is administered to the HCV patient once
every 4 weeks. In an additional preferred embodiment, the IFN-
alpha-HSA fusion is administered to the HCV patient more than once every 4
weeks. In additional embodiments, the IFN-alpha-HSA fusion is
adminstered once every 4 weeks or more to an HCV patient, wherein the
treatment also includes administration of an effective amount of one or
more antiviral agents.
[0066] In a another embodiment, IFN-alpha-HSA fusions may be used as a low-
dose monotherapy for maintenance therapy of HCV. In a
further additional embodiment, IFN-alpha-HSA fusions may used in combination
with ribavirin and one or more other antiviral agents for the
treatement of HCV. Alternatively, in another embodiment, IFN-alpha-HSA fusions
may be used in combination with one or more antiviral agents,
other than ribavirin, for the treatment of HCV.
[0067] In an additional embodiment, IFN-alpha-HSA fusions may be used for the
treatment of other viral infections. For example, in one
embodiment, IFN-alpha-HSA fusions may be used for the treatment of Hepatitis B
(HBV). In an additional embodiment, IFN-alpha-HSA fusions
may be used for the treatment of Human Papilloma Virus (HPV). In a further
embodiment, IFN-alpha-HSA fusions may be used in the treatment of
cancer, including, but not limited to hairy cell leukemia, malignant melanoma,
follicular lymphoma, chronic myelogenous leukemia, AIDS related
Kaposi's Sarcoma, multiple myeloma, or renal cell cancer.
[0068] In another embodiment, HSA fusions with natriuretic peptides, including
but not limited to ANP-HSA fusions or BNP-HSA fusions,
may be used for the treatment of cardiovascular disorders. For example, in a
preferred embodiment, HSA fusions with natriuretic peptides,
including but not limited to ANP-HSA fusions or BNP-HSA fusions, may be used
for the treatment of congestive heart failure. In an additional
preferred embodiment, HSA fusions with natriuretic peptides, including but not
limited to ANP-HSA fusions or BNP-HSA fusions, may be used in
the treatement of post-myocardial infarction. In additional embodiments, HSA
fusions with natriuretic peptides, including but not limited to ANP-
HSA fusions or BNP-HSA fusions, may be used to additional cardiovascular
disorders, including, but not limited to hypertension, salt-sensitive
hypertension, angina pectoris, peripherial artery disease, hypotension,
cardiac volume overload, cardiac decompensation, cardiac failure, left
ventricular dysfunction, dyspnea, myocardial reperfusion injury, or left
ventricular remodeling. In another embodiment, HSA fusions with
natriuretic-peptides, -including but not limited -to ANP-HSA fusions or -BNP-
HSA fusions, may-be used inthe-treatment for elevated aldosterone
levels, which can lead to vasoconstriction, impaired cardiac ouput and/or
hypertension. In further embodiments, HSA fusions with natriuretic
peptides, including but not limited to ANP-HSA fusions or BNP-HSA fusions, may
be used in the treatment of renal diseases, including, but not
limited to diabetic nephrophathy; glomerular hypertrophy, glomerular injury,
renal glomerular disease, actute and/or chronic renal failure. In an
additional embodiment, HSA fusions with natriuretic peptides, including but
not limited to ANP-HSA fusions or BNP-HSA fusions, may be used to
treat stroke or excess fluid in tissues.
[0069] In an additional embodiment, HSA may be fused with natriuretic peptide
variants including, but not limited to, BNP-HSA fusions
wherein the BNP component of the fusion protein is BNP amino acid residues 1-
29. In one embodiment, the BNP component of the HSA fusion
protein consists of two BNP variants (e.g., BNP amino acid residues 1-29) in
tandem. In another embodiment, the BNP component of the HSA
fusion protein consists of three, four, five or more BNP variants (e.g., BNP
amino acid residues 1-29) in tandem. In a preferred embodiment, HSA
fusions with BNP variants (e.g., BNP amino acid residues 1-29) may be used for
the treatment of congestive heart failure. In an additional preferred
embodiment, HSA fusions with BNP variants (e.g., BNP amino acid residues 1-29)
may be used in the treatment of post-myocardial infarction. In
an additional embodiment, HSA fusions with BNP variants (e.g., BNP amino acid
residues 1-29) may be used to treat additional cardiovascular
disorders, including, but not limited to, hypertension, salt-sensitive
hypertension, angina pectoris, peripheral artery disease, hypotension, cardiac
volume overload, cardiac decompensation, cardiac failure, non-hemodynamic CHF,
left ventricular dysfunction, dyspnea, myocardial reperfusion
injury, or left ventricular remodeling. In another embodiment, HSA fusions
with BNP variants (e.g., BNP amino acid residues 1-29) may be used in
the treatment for elevated aldosterone levels, which can lead to
vasoconstriction, impaired cardiac output and/or hypertension. hi a preferred
embodiment, HSA fusion with BNP variants (e.g., BNP amino acid residues 1-29)
may be used in the treatment of renal disorders or diseases,
including, but not limited to, diabetic nephropathy; glomerular hypertrophy,
glomerular injury, renal glomerular disease, acute and/or chronic renal
failure. In an additional embodiment HSA fusions with BNP variants (e.g., BNP
amino acid residues 1-29) may be used to treat stroke or excess
fluid in tissues.
[0070] In related but distinct embodiments, the invention is directed to
natriuretic peptide variants including, but not limited to BNP amino acid
8
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
, =. .,, ,. . ..~, .,..,. .,,,,,, :. ... . : ..::. ...:u
residues 1-29, wherein the peptides are not fused with HSA. In one embodiment,
the BNP variants of the invention have the sequence of two BNP
variants (e.g., BNP amino acid residues 1-29) in tandem. In an additional
embodiment, the BNP variants of the invention have the sequence of
three, four, five or more BNP variants (e.g., BNP amino acid residues 1-29) in
tandem. In a preferred embodiment, the BNP variants (e.g., BNP
amino acid residues 1-29) of the invention may be used for the treatment of
congestive heart failure. In an additional preferred embodiment, the
BNP variants (e.g., BNP amino acid residues 1-29) of the invention may be used
in the treatment of post-myocardial infarction. In an additional
embodiment, the BNP variants (e.g., BNP amino acid residues 1-29) of the
invention may be used to treat additional cardiovascular disorders,
including, but not limited to, hypertension, salt-sensitive hypertension,
angina pectoris, peripheral artery disease, hypotension, cardiac volume
overload, cardiac decompensation, cardiac failure, non-hemodynamic CHF, left
ventricular dysfunction, dyspnea, myocardial reperfusion injury, or
left ventricular remodeling. In anotlier embodiment, the BNP variants (e.g.,
BNP amino acid residues 1-29) of the invention may be used in the
treatment for elevated aldosterone levels, which can lead to vasoconstriction,
impaired cardiac output and/or hypertension. In a further preferred
embodiment, the BNP variants (e.g., BNP amino acid residues 1-29) of the
invention may be used in the treatment of renal disorders or diseases,
including, but not limited to, diabetic nepliropathy; glomerular hypertrophy,
glomerular injury, renal glomerular disease, acute and/or chronic renal
failure. In an additional embodiment, the BNP variants (e.g., BNP amino acid
residues 1-29) of the invention may be used to treat stroke or excess
fluid in tissues.
[0071] In a further embodiment, the invention is directed to natriuretic
peptide variants including, but not limited to, BNP variants (e.g., BNP
amino acid residues 1-29), that have been modified in order to extend half-
life, biological activity, and/or to facilitate purification of the variant.
According to this embodiment, the natriuretic peptide variants (e.g., BNP
amino acid residues 1-29) may be pegylated, methylated, or otherwise
chemically modified or conjugated using techniques known in the art.
Alternatively, methods known in the art may be used to recombinantly fuse
the natriuretic peptide variants of the invention to other peptide sequences
known in the art to extend half-life, improve biological activity and/or
facilitate purification. For example, natriuretic peptide variants of the
invention may be fused or conjugated to an antibody Fc region, or portion
thereof. The antibody portion fused to a natriuretic variants (e.g., BNP amino
acid residues 1-29) of the invention may comprise the constant
region, hinge region, CH1 domain, CH2 domain, and CH3 domain or any
combination of whole domains or portions thereof. The natriuretic
variants may also be fused or conjugated to the above antibody portions to
form multimers. For example, Fc portions fused to the polypeptides of
the present invention (e.g., BNP amino acid residues 1-29) can form dimers
tlirough disulfide bonding between the Fc portions. Higher multimeric
forms can be made by fusing the variants to portions of IgA and IgM. Methods
for fusing or conjugating the variants of the present invention to
antibody portions are known in the art. See, e.g., U.S. Patent Nos. 5,336,603;
5,622,929; 5,359,046; 5,349,053; 5,447,851; 5,112,946; EP 307,434;
EP 367,166; PCT publications WO 96/04388; WO 91/06570; Ashkenazi et al., Proc.
Natl. Acad. Sci. USA 88:10535-10539 (1991); Zheng et al., J.
Immunol. 154:5590-5600 (1995); and Vil et al., Proc. Natl. Acad. Sci. USA
89:11337- 11341(1992) (said references incorporated by reference in
--their entireties): - In-an additional einbodirrient, the modified BNP
variants of the invention have the sequence of two BNP variants (e.g., BNP
amino
acid residues 1-29) in tandem. In an additional embodiment, the modified BNP
variants of the invention have the sequence of three, four, five or
more BNP variants (e.g., BNP amino acid residues 1-29) in tandem. In a
preferred embodiment, the modified BNP variants (e.g., BNP amino acid
residues 1-29) of the invention may be used for the treatment of congestive
heart failure. In a preferred embodiment, the modified BNP variants
(e.g., BNP amino acid residues 1-29) of the invention may be used in the
treatment of post-myocardial infarction. In an additional embodiment, the
modified BNP variants (e.g., BNP amino acid residues 1-29) of the invention
may be used to treat additional cardiovascular disorders, including, but
not limited to, hypertension, salt-sensitive hypertension, angina pectoris,
peripheral artery disease, hypotension, cardiac volume overload, cardiac
decompensation, cardiac failure, non-hemodynamic CHF, left ventricular
dysfunction, dyspnea, myocardial reperfusion injury, or left ventricular
remodeling. In another embodiment, the modified BNP variants (e.g., BNP amino
acid residues 1-29) of the invention may be used in the treatment
for elevated aldosterone levels, which can lead to vasoconstriction, impaired
cardiac output and/or hypertension. In a preferred embodiment, the
modified BNP variants (e.g., BNP amino acid residues 1-29) of the invention
may be used in the treatment of renal disorders or diseases, including,
but not limited to, diabetic nephropathy; glomerular hypertrophy, glomerular
injury, renal glomerular disease, acute and/or chronic renal failure. In
an additional embodiment, the modified BNP variants (e.g., BNP amino acid
residues 1-29) of the invention may be used to treat stroke or excess
fluid in tissues.
[0072] In another embodiment, CNP-HSA fusions may be used in the regulation of
endochodral ossification. For example, in a preferred
embodiment, CNP-HSA fusions may be used in the treatment of skeletal
dysplasias, including, but not limited to anchondroplasia,
hypochondroplasia, and thanatophoric dysplasia.
[0073] As used herein, "therapeutic activity" or "activity" may refer to an
activity whose effect is consistent with a desirable therapeutic
outcome in humans, or to desired effects in non-human mammals or in other
species or organisms. Therapeutic activity may be measured in vivo or
in vitro. For example, a desirable effect may be assayed in cell culture. Such
in vitro or cell culture assays are commonly available for many
Therapeutic proteins as described in the art. Examples of assays include, but
are not limited to those described herein in the Examples section or in
the "Exemplary Activity Assay" column (column 3) of Table 1.
9
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
.. , ,... . .,,~~..
[0074] Therapeutic proteins corresponding to a Therapeutic protein portion of
an albumin fusion protein of the invention, such as cell surface
and secretory proteins, are often modified by the attachment of one or more
oligosaccharide groups. The modification, referred to as glycosylation,
can dramatically affect the physical properties of proteins and can be
important in protein stability, secretion, and localization. Glycosylation
occurs
at specific locations along the polypeptide backbone. There are usually two
major types of glycosylation: glycosylation characterized by O-linked
oligosaccharides, which are attached to serine or threonine residues; and
glycosylation characterized by N-linked oligosaccharides, which are
attached to asparagine residues in an Asn-X-Ser or Asn-X-Thr sequence, where X
can be any amino acid except proline. N-acetylneuramic acid
(also known as sialic acid) is usually the terminal residue of both N-linked
and 0-linked oligosaccharides. Variables such as protein structure and
cell type influence the number and nature of the carbohydrate units within the
chains at different glycosylation sites. Glycosylation isomers are also
common at the same site within a given cell type.
[0075] Therapeutic proteins corresponding to a Therapeutic protein portion of
an albumin fusion protein of the invention, as well as analogs and
variants thereof, may be modified so that glycosylation at one or more sites
is altered as a result of manipulation(s) of their nucleic acid sequence, by
the host cell in which they are expressed, or due to other conditions of their
expression. For example, glycosylation isomers may be produced by
abolishing or introduoing glycosylation sites, e.g., by substitution or
deletion of amino acid residues, such as substitution of glutamine for
asparagine, or unglycosylated recombinant proteins may be produced by
expressing the proteins in host cells that will not glycosylate them, e.g. in
E.
coli or glycosylation-deficient yeast. These approaches are described in more
detail below and are known in the art.
[0076] Therapeutic proteins, particularly those disclosed in Table 1, and
their nucleic acid and amino acid sequences are well known in the art
and available in public databases such as Chemical Abstracts Services
Databases (e.g., the CAS Registry), GenBank, and subscription provided
databases such as GenSeq (e.g., Derwent). Exemplary nucleotide sequences of
Therapeutic proteins which may be used to derive a polynucleotide of
the invention are shown in column 7, "SEQ ID NO:X," of Table 2. Sequences
shown as SEQ ID NO:X may be a wild type polynucleotide sequence
encoding a given Therapeutic protein (e.g., either full length or mature), or
in some instances the sequence may be a variant of said wild type
polynucleotide sequence (e.g., a polynucleotide which encodes the wild type
Therapeutic protein, wherein the DNA sequence of said polynucleotide
has been optimized, for example, for expression in a particular species; or a
polynucleotide encoding a variant of the wild type Therapeutic protein
(i.e., a site directed mutant; an allelic variant)). It is well within the
ability of the skilled artisan to use the sequence shown as SEQ ID NO:X to
derive the construct described in the same row. For example, if SEQ ID NO:X
corresponds to a full length protein, but only a portion of that protein
is used to generate the specific CID, it is within the skill of the art to
rely on molecular biology techniques, such as PCR, to amplify the specific
fragment and clone it into the appropriate vector.
[0077] Additional Therapeutic proteins corresponding to a Therapeutic protein
portion of an albumin fusion protein of the invention include, but
are not limited to, one or more of the Therapeutic proteins or peptides
disclosed in the "Therapeutic Protein X" column of Table 1 (column 1), or
_fragmentor_variant-thereof._
[0078] Table 1 provides a non-exliaustive list of Therapeutic proteins that
correspond to a Therapeutic protein portion of an albumin fusion
protein of the invention, or an albumin fusion protein encoded by a
polynucleotide of the invention. The first column, "Therapeutic Protein X,"
discloses Therapeutic protein molecules that may be followed by parentheses
containing scientific and brand names of proteins that comprise, or
alternatively consist of, that Therapeutic protein molecule or a fragment or
variant thereof. "Therapeutic protein X" as used herein may refer either
to an individual Therapeutic protein molecule, or to the entire group of
Therapeutic proteins associated with a given Therapeutic protein molecule
disclosed in this column. The "Biological activity" column (column 2)
describes Biological activities associated with the Therapeutic protein
molecule. Column 3, "Exemplary Activity Assay," provides references that
describe assays which may be used to test the therapeutic and/or
biological activity of a Therapeutic protein:X or an albumin fusion protein
comprising a Therapeutic protein X (or fragment thereof) portion. Each of
the references cited in the "Exemplary Activity Assay" column are herein
incorporated by reference in their entireties, particularly with respect to
the description of the respective activity assay described in the reference
(see Methods section therein, for example) for assaying the corresponding
biological activity set forth in the "Biological Activity" column of Table 1.
The fourth column, "Preferred Indication: Y," describes disease,
disorders, and/or conditions that may be treated, prevented, diagnosed, and/or
ameliorated by Therapeutic protein X or an albumin fusion protein
comprising a Therapeutic protein X (or fragment thereof) portion. The
"Construct ID" column (column 5) provides a link to an exemplary albumin
fusion construct disclosed in Table 2 which encodes an albumin fusion protein
comprising, or alternatively consisting of the referenced Therapeutic
Protein X (or fragment thereof) portion.
CA 02618476 2008-02-06
2007/021494 PCT/US2006/029391
8N03 O a
a~i c " VWj =
.. a~ y0 U ,D O U
H N H N
~O o a ~iO c
z
r,i,--p ON=7O ~=ti vi
m M;3= 4N= dN= rn N
NNMMM~3'
O Ng M~O N O
NMM~4N~=NNN
NNNMMM'cF7ei= c=-~
~n 'Cf bq > .N"
o U y=...U' vi y ~'p L " o +. ~?
O in.~ 'D
p U; O,C~~= T vi =~ y~ a}'i O b~q ~ cC
N 44i cd'D > o vNi W a~. >C+ o~~ M~L: 4=, CC ~ ~ U
=põA o no.~ .c ~ vi y ., 'v =.a = ~ v i~r =~
a Y, v a~ ov~ Y~ ~ ~.
. c~tl U ca O Q .b U a~i V U O 0
O7 N r~
o c b o > v 'y n F a~ > t' =~ w = cb N 8
''~' '~ > 'd W w y ,2 vo y v - p o e 'v ~ o ,>:",o g
E
p
p
~ ~=~~w ~ > y a~> >~U cn
ayi yo N> o V c~ w a"i Q O a'tli :~ O.~ ani .d N~ G~ ~= ~'~ Fy ~. ~
y+ r~ p~ q v.C ~-~ ti o a F~~a n ty y a p~ ai s ' s' on e0 y i oa ao
G1 ,D ~dZ ;- -~ - a~ 'in a~ +. a> t U O D U
> N o ~roQ . .C ~ ~S~ ~,~=~~ ~~Zwo ~,~ ' m >
~ ~ v~
t a~a~i ~ ~" =a v ' ro Cj o '~' .c ~ ~ C
m
a C~ 'C ~ .~C vi = ti ~ ~ w ~ > ~
C a~ q C a'ct n"[''. v, co .p p C. N, ~ N U ~ _ o o~~d
vrr on. =~G c0 ~ c o ~~ o W a~ c e~+
=U U ~ > ~ ~ U N ~ . o n V
~ 4. ,
t" U~ U p O, 9 Q ~ q U~~ T c~'V U~ ro N Q~,=, O
ce aCi 'c3 pj ~~i 7~ A. ~ U
o5 v G 9 H .~~
o e o o Hb o ( N o o
> r~ . w b G> E a~ 3'S o~ n ti> a> ro U W ~ W~ v~~ an G ti v> 3
o0
E' ~h
p V v1 U~+ k v r~ O~
,~ ~O ly p=1-. O -f. N T3 ~,'~' M
'E
w + V O aJ cd
rn ~.~ N~=~i c~ G=, ~', U+-' O~ v' ~ O
p~s O LL .O =-= y L,. N p~ O
>
M N ~n EJ ctl _
G~i.U__ >>a~i...:_T1'..N _ _. _._._....._ ..__ ..._.,_..._... . . ,...._
....... _. .._..... . ... 0..~U_sU.__a. ~+_.n...= _..
.. . ._ _ ._ U ' ...==.'~ ~~õ x1'O aki V
r"'n rn y
p
c o a~ a a
~vio;a=y.SoP. cC
ai ' z
W ~a=~ arn ~ ~ ~~~
t~ ~=~rn ~x~
ty ca 1:
=U.~ O~
,~ =~ ~S ~ Oj S"+ Y
0 ~ v~i ~~/, .'J
$ W Id [~~ b ~=~ ~ >
'_ oc~o= -~ c v.cvz7~+o-p
~'d ? ~ = j h ~ 'b c~'y ti y
o
y
=O O C id CU'C .~ c'"' p.O~ .,G
p 4'.~ a. ~ p $ O P~ O U N p Uj p
V sU. t~d =.~. v, N O~ ~~ w
..C e) cn O
M z
,e ro 'a' O wz
(7
rC
zO
W ZOW a~d
wL 4 UO~.L Oa ~0 3C o
N~ o - ~4 ~ ~7 Z W w O Qa
Z W C7 ~ r o
~ w
ao~i a ~ioZpC70~H p.,
rs: e w~'4- c~ M
= c C~ ~ c a c c a ~i a~i ~~ vMi ~~y x~ p" Z z~'
o a a a o o~ C
' ~ w=~ N O O
O CW7 W
o o o O 0 0 F-~ a'O" ~ v~ a. 0 a~
~ uy
~~ 0.
~~~~a~
i UmN z~~CW./r0 C~N
11
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
U fy N cd y
=
cAV w >, w'D o q c~~V
FN v FN ~~y a~? F
u O o dl O ~ d)
V] z V V] z V> N._ Vj
~
pi " ti t+i h " 00 h O~ ~ ~D Oi N~O oo ~O N O T':
~o l r- oo oo oO 0\ O~i rn ~ ~ O O ~O ~O 'O h pp
q~"~ M M M M M M M M M M M M M M M M Ch ~j' ~I= '[~' d= 4 e,} M O
t"''
U o0 O N~O ~h %O O~, Ki l0 ~D 00 4'i lz V'i l~ O 00 O O pp"
pN O~ ~=-i N M' CO O\ oN 01 O Vn ' ~ 10 O Om O ~~ 00 00
k %D 1- h h I- h l- o0 0o ON O~ \ rn O\ O o0 00
M M M M M M M M M m M M m M M M'~3' Cl' d' 'ch 'c~= d= ~y M M
N V
U Q .R7. U ' 4 y = .>,
fV' p V = rn 'L c~ N.O y .'~ ~'tii(py W ~.~ v~
y U fU/-J U C
U N y~~ TJ O'i~'' >i y c,d V i., Q
y~~ ~~.D 'O O CJ N w V V N.p ~"' 'O O N N~~ 4"' U
b .~ uyi 'v; = c .~ c'"a =~ .c
ti =O 'Cl =rn N 'x O ,~ 'O ..V+ V =y 'x ~V-= y y V A U L,,' =~ ...p,
o N t o U .b b 1'!ilUilll
" a~ =~ =~a y ci r, o ~a w y y~ 47 0 o c~ "' ~' c
y+ o~ Y o Q ~~~?~ a>i c o a o o ~~, E b a>i o
v U ... N a o. s3~ F, ~
api ~ ~
yc~ P > > o o 2 a
C p S v d a o o~ w 3 .5 ~ ~
~e o~ a~ = o y y > C o y~ u o u
5 c c c ni a~ > a a~ = ~ o
~ E a ou ~' na a~i o 8 d ~ C~~~ q a~ > v
q E w; ' ' o ,b A ~
o o~ Ca ~~ o~ ~ a w~ ny ~
z~w~
a 2
y..,.V., ~ V O p E.~ ;,b ,~
> T ~ q
o~> x c'v ro c cu c o 0 3 0> xu ~ c on.c 'v =b o0 >
v
o
o tn ''"' ~ ' OJ N O " ~ +'~=' ..~. w
=~ X w O'IN , N p ..q CJ GJ p a) ~~D ~ O M q y. N bf)
~ ~ ~.D ~ ~/j 'i7 GD Vl y. ~ 01 =4,i ~ ~ .D
.~ V ty ~ CN U bA.~ N "6 EO
O m =-~=~ q .A .v = p O N .~ ~ y >i N p Q p ~
CJ w p O E U.C O
'A ~=~ y tV-~ ~ M~ .'..if"' V~ A v CV O N CI..C N ~ ~
O E N cd m o~ Z Y ~~ U OC O~C v'~ bA Ri ~~ Q CJ
' v a oE 8~ N 3~=~ M
~ w v p ~> w c o n c d~~ a~ ~ O abi ~~a o v~~ c oa
... u, u o
> f~
bD 0a.'~orn_~~oa~ Ai~a i~
0 N J. CY, .., ~-- O N p d= E N~ F
w -9 cA r ..~ N~, ~ x N ~ o ca ' = 'o v a ~
r- C N'~ 'a aAi v.~y =V 'V 7) a~i C +~ p a) N cC p C cd O ~j
O v~ ~~'" TJ p~ N ~~ M~' cd 'L7 ~p ~ V~ N~ O C' O V cy
s o e .~ m 0 . N ~ A 1 o o '
W ~ c 8 >> o' w' o c u~i =~ o.~ a o~ b~ o c
rn~ ~ a~ aa~w W Z Ew v '
0
0 ,,..co, ~ ~? v
o o =o ~ '' C ~ 6n
o= ~n C F. op p ca N, ca ~.O p='
w
c ~ a +=~ ~ xd 3 "a ~ a~i a "
$ ~ N ' : o o +cd
'~' ? ~ =~ on
'a'u ~.o~~ ~~~a. ~~a= ~, y ~
ot=
w - 69~'~ ~Ccp~N=>=~p~d fl
a~ a~ ~. o E w o
i%]
'pU' N
=D
N V ~ .
$Z' sy ~
C - V ~ O
w
~, t" yV., v /
~ ~ =~ ~
p ~ p O
~-=~ =G~ ~ r~r = ~, ctl
H H ia,z
>
12
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
N~v0 a'~ y J+O a
'C N U O N/~K-= U O N W U_ O"C N ~~m==
~ lga" 'or
~.K, o p' . T ~ c i y p' . . ~ c i ~ O' = ,~~ T U
~ e~ ~=~ c ~i ~o e~ ~.o=~ o ,o~o i~a~~=~
o 10
ce
>~ c
E. ~' A R ~tl N
N9 ~ F?. o,v F ~ y a W o F+ a fs nv
o ayi aci -~ w a Di O c~ ~ oo ai O c ai aci ~~ +
~ U9t~N=; cnZ c U9C4N,_~. vl Z U> v N.c
c~i
7 M
CN
00 00
G M M
0
U rn rn
00 00
M M
F. cd ~ ~ , .~.. F. .y~ ~ = .y~
U U_ > U UN .y+ s . ~
y b N~.o N y~ c~ ~.~ N =~ N N~GJ
tl '- C ~' o b 'cU~ o A atli ~ ~
c v o~ m o v> 3 si ~ o 0
~'n ~ 'v; ~ 'a~'- c~e ==-ai U =C c_ =D ci b; 'a = ~ .'"J- c~e '=c--. v c~d
=~ ai ~,i 'o a .~ .c
7E;~ o ird .2 aa o o a a.S o ~~~ v G
K~ Xi w N V] L'= y C1= U L'i c~ . CC .1 y Li y A~ U~. m. x U~ N U y,~r
~~ ~ ~~ o=E ~ ov a a~ cci
~~ o
c x a a o > v a m c x v z7 o y > as ~ x m zy o fl >
a= o c b c o U ~.? o c o v G o c c 4
b 3 0 ~ o a~ 'C b y b n o
~y ca> ~v> p~v~ ~caa~a' v P~ '~,~'cn>~o= ov
czt a a8=~o~~.~~~~ a' ~~o ~ ~ ca~ a nc~~o ~~
>;" o c> a~ .~ S; == o =~ o v .o ~=='~ ~"+ o cc .b o~
=~ y ~ ui 'c-=" o .~G ,= v~ a~ =~ 4'~ ai j v, '' m a~ =~ w ci > v, [
_ ~Gwc~~ ~om . E'~m~= > om
4r 7 U o C c~d bA 'in ~ y'~ cUi O m N U o~.~ ~ O yU
,.. a> c a > > > o , o~ c~a
wy a+ aai =~ d v uc ~ w>~ ~ a~ ~ .2 o ~~2 IL) v ~
m y.2 a ' va ai '~ C o
g a~ Bvi~ v~y~~+ a~ a c ei m> a~ , a ~
pp U w0 sN. t6 U w >i .fr p f DA U~ J.U. cNU b G ~U+0 Pl O y~ U ~'U-= K b U
~
tl a) 'tlU N =N. =Q ~ U G N ~ ~ N V aU. =N., V =~ ~ '~.' Y
ti t-+ v T ) ,~,~~ c C N E W 'O
- C cti N,a: ~~ Ui ~~-+ v'b C tl aKi ~
b I-~ O Yr \o m 'p ,
' .~ 'O _N =v, N c ~
y~, tl 'Lj =p K ~C v ~i N ~~ W c~ p .~ ty =p c6 =w e y =c tlC W ~
tla c +Ki
A i3. =
~.=~~ U cd U U U m y Cm ..N. ,~,= A. U~ O N m~ Lrj .~ L'. R. N tl U
wd
' ~'" w o v ' E a=~
m 2~ o o a.~ c v t~ Q Y~ ~ ~..o c
o n. , -- ~ 0 ~
~ op. o s~.2
tr
4 . a0i 7~~, a i , B 8 a ~' T 8 ~ o >,
c c e c un c v v 0 0> x a u u c nn c a u 3 0 0> x c v m c an ,a a o 3 0 0
i, c"~ ro oA ~ o'ro o o
ep 'd D ~ .M. on = o m ~ c eq a o q'a o ~ o
v v o c~
E.: o m~~ n U~r n x y v v m 8 'D caa m E m 8 c n
,~ U v c N~ U c~ ~ O U c~ U y K 0 O N at U y m U y m tl~ ~
on w' ~'' .~. C N c on w' ~' tl U 1~ c a~ = m'~
o a y v..~.. y N <'-' ~:; aKi '~ ~ p '~ ~ N =~ ~ rry =,~ ~ '~" p ~~'' 0 +~=' ,-
;
d bA bA .6
n y
oo U'C O tl ~M tlA tl vic
d d~ a~ ~.. o m o E~~ o d ~ .c~ ai m o o o y .
~ W N c ~ N a~ ~.~ ax o y N
U~ >. n ~ .8 a~.~ mc a~ N a~ o c c w c c c g v
n
rot~ ~ a . v o -
- -'~ aKi- a
C ~ >
v ~ v v
~
y ~ m N~, W c o av>i F U 7 0 0 O D
N a O
o
eS y ~y q m o tl y,+ ~ = ~ 2 'L7 ~ ~ w
m y .5 ~ ty
tl~ bA m o N O -al iC TJ b0 ~.~ t'.' ~ rn =b qEp ~.~, U tl~~+~ tl p
't)
K v~ o ' = Ts 'o yg ~' o b~o a3 m o o 2
CII
~ ~ a R P~ W y~ ~ 5~ -q 5 z '
SZ.
A ~ g N' Z mo N o v ~ d
y v o _~ 'a o 'a o ~ o o~ a
~ ~ "=' ~ =~ ' n a i o a b ~ >
cC, 0 .4o o y t)
tL,~ ~' m N=~ y'~ =>.= y .~, y. n L+ N[n 'i7 Y" N W ~'
oca o~ c o~ o a~
0
y cu c ~'== ~t ~ ,r~c ~ ==a 3 o
v, Q K ~ ~ ='~~-
d ~. o o a' .ubo o
=~~, :a 'i7 ,C v ..o = ,n ~ ~
Q N 's.," tl "3' ~'~" r~+= C . f.'
tl~ T cC ' cC q U ~ C y cC ~ tl O
C~ O
=C ~ K =m O ~ .U ~ ~ ~ ~ ~ =~ ~ ~ N =tl ~ O tl .
O rN
O .~c O C ,~a vv~i J '" ~ > 6i m C > N .s'0i > aNi =~ 'r~n
C t-~ O~=.. 61 y CC '~ r.. c~ y ~ at tl i. K tl cn
a) L], U w=t7 >~n 3~. ~+. .~ Ll..o ~.. R= p W"" L1 .O . O
=~ x a
L O ~O
a 00 . N v
(T O" '~ "~~~3'
K U
~'f = =~ ~ z
F H u ~A c. tJ
13
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
.t ~t .u u o 9.=.t' e= dn r4==u tLJt " ::ilt I....II ~iit . (f
?: A
tO N u1 O 'U U
a~
a, c VW] '-' ~ a'V cWn = VW] .~
o C C
a ~ a c
~ ' >_, > r c a~ ?? ' ' '
O U ~p =.- U U U O U
F"a HN ai af%;
(S v) , ; p ~ 0
~z~N~~ ~zs ~z
tf' lz o; ==~ 7 00 00 t~ o~, " cn v-i t~ vi vi
oo ~ ~ ~ ~ ~ oMO oM0 0~0 0~0 0~0 ~
p M d'<t'd'7 ~ NNNNNNMc+7M
U M~O 00 O M h i o0 O N V" , ~p 70)
' .-~i CG t+1 CP N 4 M'V' '~'Y V= 'cY %U N~ ~
Co ~ , p~ M CO OC CC oG Co O
N N N N N N N M M M
=~ N = y N ~ .~ N N =L7 bf)
>, ~ = w 9
W N... U N. o CJ
. o 'c~~d t '~ c"'a o ia ,-.~7 ~ a i
~ - =L'J .~ ~ .'I. O .f. y L1 y ~. ,cd ,Y 0 ~
>
o''a ~ 0- to
iUiThflflflU
> o a c ~- a a y ks >i o _ '= '~
sz b " .~
o 0
y' "
rIIflflUFOQ 11110
a ui c ws ro' :b v
o ~" ~ob~ a c1' ~3'u ~.5'a cip"
w o i~ C, 0 o ...,
'ky y p a~ N a) ~O G. U p~o =~ ~ v c '0 aUi c a i o
o c acli
a v x =- a i a i o ~ a i ,o w w ~ ~~
> y~ x.o 0 c ou a o'0 3 o U> F a~ S~, =~ a~ .~ a~ v
ti
a> pp r b9 c~a v' a z
U v a ~ 8 c a *a o
N ~ 2 o
.~ ' M ca .r'~" _= M -.4 z v, oo "o ?o 0 a~ $
~ 8 c v q 6 ~3 n ' c v c o O
a o s' .~ o L " ' E t~ 3 ~ U
F., .. aJ rn cc U U .~y N y A o ca . .. ,
o o i v ~ o 0 , ~ ~ o Z 4
~ o~ ti cn a: ~+ 8 c 0 a~ o N y ai 1a~
.? p o .o .. ttt v '+r ca A 'G ."r~- 'o U .9 U o s..
cc,.~ 'to 0 r'~ ob a~ c U s~. U caa
a~ W b C ~ .~..= ,C CA N N =U O N~~
. " .- ,-. ~ ... ~
~,W M N
.~_
~ ~ E =- ~- ~_
> ... ~ p -a
... .4 ., U' a~ M _ =õ_, ...
h0 ~ r.i N>~ l=NZ=W > U ~ -O ~ 0 ,~. . =? C > i-;
ti Ofl ~J' N -" U -!r ~O O ..O ctl ~ r'"ii c C C O W
'b o ~ y ~ c ~ b a 3 > ~
N cC p C cC O A y~~'~ ~ fn W CJ U ~ N
d z ~ ~ ~ ~ cUe pq ~ E y - j o ~' =~ ~ y ~ 'O õ~U, .X =.'C =n O
y
b n~u c o c~: d ~ 6'd o w
o
t l o 0 d 0
y N bj)
N U J v~, a .d
W~ a o Cl, P' ~ W~ 5 z ro 78 m~ a
,
N ~.:3 o O N cz.C C O
U U O n
N W U N'~ FU. .fl N N 0~ ~ N q c,: U U
w N N~' vi ,c~~C ,O C yu?
=M~ ' >~ cs'd v N c o ~ v a~,' 0 ~' a' "i ~ =t= ' y a
o a~ o. a~ ~. o. ~= c C~ r
H eppo
o o 0 o ~ =~ (~
= 6 ~n U
o c eou a a o C,~
'~ a0 w0 ~ ~ p o c~ y W vj v o
~ ~ Q ; _ i v n . w
z
=o ;n = =~ v~ 8 =a~ o =a. . c_ ~ c a~i : =~
a a a~ ~ > E v x ? 0
w a~
cl.
.--~ tn Y N Ti
?~ c c en - > ~ a t., ~~d o> 0 0
=8 a en cd CZ13 o~ ss. P.
or-
U N U =
L = = .~ c~j O r:. q ~--i
P~ ~ NPa W O
N W W o EU
d C Q Q p
GJ " O
a) O N Py N'u
~ sa 8a~
H H q Q 0.1 v Q x::
14
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~/
a ~ =~ ~'~y a
a a=~
nj (V
O y Q' a7 U C. N p,
F a 4 " " c, a 42 a ,o 0
FN F"N F"N E=N,~
z0 zR zv zR
V
O et ~O O M Vi [~ O N d~ O h N t+i
'n ~ o 0 l-
M M M rn rn oo .-i M v1
C M M M M 7 d' M iN1' ~JN" ~N,
h ~l N -lir %D
V N~ ~O O O O , d= N M n
f+l t+1 M c+l M M t+l CF !Y M CNt NNa' ~
~=
y 1
EJ =N _ =~ y y p U
4~1~C
o .L'_' F ~ Q a L~ = ~
"=~.~~~~, ~ ~ ~ ~õ
a a = ~= . JO La .S
.,x~~q "'.~-a- rn=~ a~>
a~' ~ a~
E <
x ? a ~ j y x = v
m ;~'=~ y i vy p .~
:Ly = ' Q.2 p R O cls
'O N wU N R. N N U O r=~J =~ c0 m ~0
~ o ~ ~ A u
o~ow'tt~~~~~~'
a a~ o ~Ga aUZx~O~5 U ~Ca
bD =o en n ..,
kn fi p C 'y o0
a ~ ~ v ~ Y c U a rn o
cC
~ =y (,1~ U N y~ y "
=~ O A
TJ ca y~ ~ d' at =4' iy
Y=o ~ o G y ti a a) o
' 5u ,~ ~ =5 , ~y 'b ,~, c a " .~ ~''"=~ o ~ ~ "c ~q ;~
,?i . .~ ~, U a~ = ,-: c ... -O = '~''' c~ ~
.2 ' eqZ ~ " ~3 ~ o a~i >o
N r.--
o
?~ k ca y~o o ca =~ 'C ~ -' p~~ c~ ~' ~
õ 0A~ 0 3 ~o ~~~ ~ o o
4 o E o c In
C U U y,~ O~, C ='y~' U q~ 0 TJ T..p ti.D A 7'T ~--~
O M ~ = t
8 x o
14, o
k E ~ eo : ~ 0 rn ~ > o ~
~ m.~. p\ ~ N_y y
w U. M n F v.~ ~. U E~. U~~ !~ F a cf Q
cn N ~ ~ ' ~
~~rs p5 c o N~~ ~o c c U~ n ai y vyi ~ o ~ y o on
U at . ~ pp V.~ .~_, rn .C =L .p.., r.. ~ Rt
v ~'~ x o ~a c a
8 ~ o a ~ aQi a gv
q 'en
> w
.~
p ~ ~ '~ ~ =~ A x o 9- ca ~ =- c ~ a~i v c ~ ~' ~an = ' ~ ~
~ 0 ~ ~ L A ~ y 0 =
u ~ U m y L .'f~' k ~ N -2 - .~-. .E = +~' m
U R. iG N U .O o N N ctl
2 .2 ~
o ~~~ ~~~ ot"ONc~~ o~o~~=
Oa y ~ '
w Ev cn~~~E
w
yG ~ o
o a =o 0
.~ =~ F ~
r-i y C C 'k v v o
8 0
J ~~', ~ o o F a~ <r o
w o O ~ a ~
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
' (Y~ ~
a7i O v] =~ C!J V1 V]
N~, p, p, N p,
7.. O . . .
U~ U O D U O D U O D O U
Eyp" ~'" ~' ~v" 4"
F N y F N~ F N~ F N y
N O U p U O U O
cozv ~z~ ~z~ ~zs
u
=F7.~,A OcNO~+i~ M~o d' d"7
~~ M M M V~' V~' V~ VN' ~.IN" d=
CO ~O O 00 O CO
U O o0 _ M ~ -11,10 d' ~O =V' V=
MrnMd=7CF Kl7 d"'3' q'd'
O =y .~ C U b0 p
0 F~7 =~ R7 V O~ p ~~'.-' N y ~ 7 rn o c~ ' o
.~qj _ ~y =N ~ 0 E y p P. 'K b abi ~! cq q
ca ~o N C ~r. U z ~" N '~' = " o =vCi eCn W
~ N O ~c'=.C
~ a ' aoa
c~i z v c a~ a J
;it
C cd R=.,.., y O,~ T(7 ~ T U p E. ,O ~~
~ =8 O ~, o w o o o
~ 3
v ~
w b ~~a 4 v o V. o Ca w ~ .~ o o y
o A o b o 99 y A o~ ~ $ 9 ' 2
en y
-~ b - ~ ~ & E ~
vw o s~ 3 E p k~
p +yJ= U~~ a) N a~ U>..~ p. F~' r~i~ > b G b
O ~ C
.
cd . ~=ti ~.~ A, yõ~+ F.~? y'C
y en
i =o a~ ~a F~ ~~' Q a o ai on o~~ , fl u a po y
o = " 2' o 0 =o
A v x ai a~ Q ai 0 o a c =~ ' o o ?
a
U~ o~ a*i ~~. y~ o x p'
p o 4..~ p=~ ~_ ~_ a ~ a o
-6 t~qa~i A A O a :3 w ~ o .~ c i ~ v o =3 ~ aki ~ -fl p v
abi w o ~~' n o ti m v.o o. _.N o ei o aXi A o o a api "r-, L= o~
2 O cd ~.. +r ~ ca y=~ b.
U U ~" .o' N N%'O O D U m>-.~.' p Y N y O aJ . 4" U O~".
c c b 'b . ., ~ =~ ~ o o
o=
d a~
~ a~ E w=~ '~ ~ w a~'i ~ ~~ a~ o; v a~ ci a~
pL =~ ~ ~
i~~SAAD O b a F x Ov Ov 00 xOy xca
a=o v
x=oi
x a~
>
a
vi ~ ~=O o ~ o
p vi U p. =,~ C. O ~
.O ctl p v~ b~. O \1 =O c~V ~ cptl
~ ~ cd ~ U =17
;b
Nr~ O O U 'N-.
~ O ~ cpC
M U C d' Q=I ~ W ~ rn U~ d' Ur ?~
~ ~ 'NO" = O b0 '>' ~ N
cl 0 cc a ' ~ 3 o yU on '
y N U pq N N~ p~ ti N U p N O=y,p~õ
'
2 U OC3
O x=x N O F'n N
i" O p
48
N p vi 0 . ~ ~'C cUd W
b~z CCo
J I R
y ' p~ U ' O c~ = ~. cd N
0 'p. e~ cls O~;~w~~
.. a :: py U - u P~'
kn C D a~ C O
p ~ y =~ .~ ~ U vpi o ~ 'O cd ~ 3 "O M
=~' m
o~'p"
.! a~ a, ca Rt ti cs. ca y c~
o a a~ a y o 0 o v n a v~ a
W U d E~ >cue Aa~ OE s cav
Y n p
A Iz, On
w ~ ~ o ~y ~ o o w=~ 9 > a i :3 ' y ~3 ;> o v
~ ~
N 'O CC N
O aJ UU
Op U ? yEi G O W ~ W
~.. O cC L1 O N
O w
~ = N'O N aU., N.wU+ '>=~" 0~ RS
cC = p
y ~Ei
TJ E A~ Gm .fl V] .= V] W U N O
4'" .'UU...
C d= .b ~. a,
C i~ cn ~rj Z 'O
= p y a~
ip. 5'' b p~ J' .7
CL iU.
W p+ p
a ~ en a ~ ~
a b.Wv~. b n
M "E! U
r onm ow'
e p
a az'i a m c,~ U~ rn o .C op.,
ce
">. cn C C7 U U
16
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
a a~
U
%
~=0 N L~. N N LL
d L "y y. L ~ y ti =
E-4~ "p ~O U p f0 U õp i0 UR
NN~ FN~ F~N y
O o a Ji 0 o v 0 0
cnzU ~z coz
C-i h
tf)
U n v~i vi
Nd= v a
." ."
0 o z z
In
C, x =~ 0 N N
'O y ~+ N N N
O y o~ ~ a
o y~ u
;0" " 3 O~ c q
y
CIS
o 2 M >m = -'
~. v y a n b o c ~n n
'"
Fy V ~ /1 C p
,.C a~ ~~,~ p C cd cd
O C O=s
L ,~U, =~ CJ d)
p > , UO O A. 'U =U -f.
m.2
"O rVi, c" 7~
~ y 0
a~ N
C
bJ3i d/] C~ =~= > f~/1 y ci
C.0 s = o 7 = '~'~' Y f~ .N.~ y +.C-C~
~ O o o .~ IZ
U
~
~ ~
co
> ao~ ~
JO o oc =
w o >,.= , U C c '6 o cf w o>, a
y N ~ m0 N , O . " ~ 0000 U ~ .0
cd LL U. O, w 'b
'_> O U ~ y O w ~_' ~_
O 7....>i >..=C O r:.~ .. ~.~ O
ro~ w M o
U
o~MO 8 c-, c~?N '
2~aNo~o ~"''=o c,
a 121 0 c,
C? =c
2 U 2 en
'd' rn ~4 m
o oo
U
~ ~ N
o aVi : C .~ ,~ .d ap~7 y H V: ~ 1-0 c V 1C -Z;
a~ G. =~ ' a~ =rJ a~ =O = ..-, C
E y=y a~ a~ r ,2: a~ v V C''~ p o j- = '"' >, a~i e~i~ " w ~ o~=~ a~ ctl =
.~
~ =~ o p c m El - >
.U. >-ctl+ U ~" A N 'ii M O ""' ~ t'.N. y ttl ~ cd CU.' =~ Q U y bO y
~ ~ y .T~'i+ Y
t~ ' a'~ u ~y" 8=c =c en U v n~ a a o ti> ~~a
a O .b a o o It~ fl~~ " o
a o= ~~= Q"~~
? Ca
bD ~ ~ O O N L1 ~= O ctl i~ . ~-+ Oc~ > yU, =.~~, C ~ ~., c~ y =~ ,q =C > O
t~ ' U !7-! C
= ~.C .-~'. bA .p . N vi ~.~ O C r F F N U in af inL G
U m a) b t~. ;.~ ie C m
T8a =y
~ = . w u o'~ 0 8a E C
aoi ~ d ? =8 > .~ v~~ F' E ' ~ L ~ 3 a~ a .o o . e a~ =~ ~ .5 . .
ee vo o
c c 3 > ~ ~ 8 g ~ a~ o a . C s ~ ~ z ~ ~ ~ o ? > ' j
=0 N vUi =k a. ~ ='= s y N ~ .c c o ~C N L. ,~ J ~ aNi ~o an C ~O N
g
C ~w ~ Z ~ ~ a~ c o [C ~ a~==~v a
y w .li y CC p U O hD .. i-~ 4J y~ .z br (>C .U+ =w_ O= ~ U '~~' 3 S'i U N
R1 0 0 o = o a~i ~ y c a~ C b ai ~ 0 U: o a v N i - C. r a~ r. > w
o a G~n a.b a c k z aoa~ a ti c 42 02 42 0 : w 0 0 ~ x.~ on=8
z
O ," C C N
C c" p ro:d W y -Sb
Z a U C;~
o C..) O ~7 ~ ~
QI 2 ~C
C U W(a N C
d bf
y'i 0
~ y -45 ~ o EOOw c
F+ C7 O
b~U
.0 CN 0 O aC, vi _ C m
F E+ 3~c7 01 z x>w
17
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
. ._ .. __ ...... .... _ .,,.~. .~e, ..~,,. ..~,. ,,.~~.
u
dU ~
a =~ ~;
N Q
a~i y N z
NN
N
Q N N
LI)~
o
.~ A v>
N
~
~ .~
N O
O
=U A
y N[]., =t+ N ~
O
O
Fa cC .?~ 0 3
p N U w
u o N (>xy
'1.~
b r- o
o
L ~, tC [1.~~-1 =.~
a ~k W-x4
0 8 a~
0o 0
q ~. v rn o
,v, o ei ~
_ 'b~>,.. =3x 0
O ,.C 0 = V N Q
cd ic .~ y,' ~.C a~i U y.C
>> C,= o'= pq rJ pQ ~n C =
0 n '.~
~, 3 ? c 3 ti
~L o o . m a~ iy
>, w
c
=a o a~ .5
o o ~ o_W
c ~3 c '~
0 ~ >
o
o a v o~~_ ~_~ c c~
d con 5nc7~0J
cd N ..C ~o O.. y > v~ 4~ ' U' '.cJ
W A d' L~ .C tU.. ~~C Jr' cUd ~.D C7 W~
v =' a o =o ~ 40
-cy cou o g . c J 8 ~ 0 ~ o
Y
~. ~ ' ~ 9
o 0 6 ' o [c- 0
A ~
'
o > .? ai ' o '" C v~ C
aoi ov 0 ~ U E" o o o o C7 c "o ~
o ~
o : abi o a ~ i c 3 n ~ ~ ~ wc ~w o a 'y :o rs o R e ~ ~ ~ ' o
7 V~ ~~ N cd ~
A. o
~ ~ ti i.:
u U ~~..o ~' > O. C U T1 U U,~ ~ N~.~ O 0~
> ctl o= ~ o~ o.o U G:d T~ o a rn ,~ A
= Q U Q~" x w W
W vi =~ 'b ~ N N U Q b ict G7 > v~i= v~ N.CJ' ~C~ = ..~+ U TJ -~I. x~-= 1040.
CtL o
o c0 GJ N .~ p..)
o~ C7 w ya ia o -zi y~~~37 =~
n o 0 8~ a~ bq UtUU ~ ak o v ~ .a o~ b a~ .o c~ a~
o - ' 7~ o Ir-oi U ba 0 E ~~ rUil ep
o ai ~ ai oE~ o a> > N v '
a. U
a o ai d A~~ ' 3.~ o y W >
W x
Q~
'ai a~ y W aoi o
xN~., ~.ca. vUi ~ o
ai .~o.. ~ z
.c ami ~ o
d o o .~ z
r, .S
a ~ U o
a o o
F E- Ci z~~= W Q
18
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
M ~ u a~ ti
CJ S~.' 00 ~J CJ .b t4 [y'V =~ ~ ~
Ra
N.
~AZ M M m r.
V M
m '~i' =~ C M N
M M ~y M
a N
Z, N N N N
.~.
A O
O R v~ ~~7C "' N N N
~
oz B
9 o a =,
ON
Rf ~y ~ U OA "~"
O R.yUy cC N ~."
=7'"' ~ O Q O
:a N c vC =,~ =C~J ~ t'yn M Ir yj
+~
U U U U "' m
va
a' a n' p
v a o ., :n
tM y 0 p w
CU v
cli ~
a :a ~ o o
=' '' ~ ~ ~ o o ' a 2y u ai o W o c~ .~ R ~ v ~ '
ro 8 a~ a ~~ m a a ~ a~ ~:~ q a
N
a, [W" ~ ~ ~ =y c
C"l
T o o~
2
IV
=1.'' c W r ~ cGi c''' c =~ :c o ' '~' a =~ " "" '~ 8 't, L 9 >, 0 E
~~~ ~ro~~rov~r~wFvv~-00
"Zt p O ia v
cEa
o rn cc m ai a>
_ .==~, ti O - !~7 ~'-U . . r'~n
cC U C '~3 cy 'fl ~ N yM-y N ~r ~ ~ cV b N O -' O q~ y .~u. cry ~ =,~y ~. (~
.b-,~
C' p~ ro'C Ug
I N r..," v
i p~iZi p w C%
s 'e aVi ti It cq
~~~~r C ~ a oo o' ~rs coot ~ o N o ~ cd ow ~ o
' 0.'r~ ,X Ca ~ N N ~ l0 -C ~~ U~ O ~ m O R. ~ R3 N~C O N 4r
~+M'.~NP.ia W
,~ 'tJ ~ x. O =a P-i cyv H y ~ ~ ~ cC
Z? c~ 8
4 e~' 21,~U ',~~,
=~ o'-~ ~ ~'d ,o~ a 7~ N
q y o t) 'o ~ o ~ ~ ~ =c cu Z m # < c~n
mo
cn
W ~aa 7 x ~"a
~ v ~ M M M t~C f~l tY~j
:' o~. U Ui rn V7 y d
a Fm ~ O~ u fl' C7. fJ. Rt R C.
u~ o
f ~ U
a caS U' L~ O O M M c.j N
~ u N N
U ~'[~. V v O
,G L=~.. Q, ~ w Z, N c~1 'ct V'~ ~D
19
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ V 1 L
~ ~y N~ N N N N N ~ N N N N N
gb
z C Cn V] O G O ~ ,~ C C C
~ x x U x U U~ U P. U c~ U x
o (1) fKl M z c'\Oll M m M z z Z f+l M M M KI M M
w~ (+1 M M ~I ~ M M m (+l z z ~i f+l O M M M M 00
a F'~ M d n O (- 00 Orn O .=. fN+ Hm tn 00 rn C.
p t~ t~ r t~ t- t- t- oo 00 00 00 00 00 00 00 00 00 rn
z N N N N N N N N N N N N N N N N N N
en -tY 47 ~o h 00 ~ O ~ N M d= wi 10 I- 00 rn o
Q N N N N N N N M M M m M m M M M m V'
00 Orn o N t+'1 v In .o r 00 Orn
rn rn C. o O O o O o 0 0 0
0 N N N N N N N N N N N N N N N
C
O
=v! V1 h N h ~--1 ~=-1 õ"i V'1 h
u M en M M M M
sae d U U e~n ~ ~ U C'5 w w W ~r 'r '~ ~~
v~ W W W U U U U U vs v~
zLI a, a. c. a, a a. a. a. a a rs~ o.
'b
~ _~ ~ y ~ \ M d' p. C/]
8 x yc a z o x
R ro x " v N _~ vs o 0 o
=v TJ =o
gb w o 0
r ~ ~w 'C1 2 ti U 0/J G 4+ Z 'U~ t~C cF.'tl C
o 0 3 ~~~ ~=~~~ v~ ~'~' 5 5 ~w
ai o~ o~
E =~ N ~ ..o o Z ~ ..o .n a o r ~n ~ P~ Ga !~ .
~n w '" ~ .~ v~ '" ~ ~ 3 3 o N o. ' = =
'~ ry T o o N~' ~ o ~~" =~ ~: o 0 0 0~ c~~~ 3~ 3~ 3 z
r
z w Zwo 9 Y''.d , wo w C7 a~ ; d~ ~
w ca cou .~' v v W.: u=+ a~ a~ z Y N wo 40
n R E Nvs y c zoo . c c c owv' Z' y E y E w' a~
p 3 7 ~ fl o. n n o - 1h01i01iHH
W o o 6. d' ;o c' rr~ ~ jy a~ cn ~: 4, .d N x o 0 E a~i v1 o w o o o w
0 ro 0 E
v b . o a o ~ Y ~ w e 2
o n o o y ~ ~
~ o a~ d d c Z 4
U a V c~ n o cu N~= +~'~ M o o O ?n an 8.~
N N o ~ ~ ~i c, W = ~ N ~i N ~ N G o 0.1 a, "'
v o 0 z Y v L~ 3 w o w 1 " o w o w o w o
o V CL .D 0 0 0 0 ... - y~ ~.~. 7 Y ~~-= b =~
=~ al ~ O ,b =O 7J N N N~' yJ ~p N N N N N N C 'O ~Sr N~ N N N GJ C~
= U Gj N .,,~,~ ~O 4J N 7 o
o N ~ 3~ c~.~ ~ o c c c c c 3 3 N N o
c o a~ a~ o= y o= o= o= a~ Q' abi abi
~ N vs o o N o N o N
< S.~UoU ~wx.w~
Bd
<
00 cl) cn UD
UD
col
P4 x L)
, x r~ ~q a a a a a
~yp, [~ O O ~~j M Pl FQ Pl Z~ O
z
~ x c7 p W V] C~] O a 0 U U xi cQ
'~
~ V, U r-~ v U U
y Fr: M M M M M P.
H a a U U
N FF~~ U U~ U U U .-Ni ,N-~ N.~ Pa V] Vl C/]
q v ~r Q Q o~ Q d v w W w ~r <r ~r ~r
U U va v) =~? v) v~ U U cn w W w U U U U U vs cn
U a c. a r~ ro c a s~ rs a a o cL c r~ o o a
u
-=~ N O ~/1 ~ O M d= V7 ~O 01 00 cn ~/l ~o O,
co ~="~ N N N M 10 h 00 N ) O
~o ~ I- l- I- l- l- GO
N N N m m M M m m m M m M M M M M C'l
U
N
~ =n O r 00 m In ~o e0 T O N M d=
cl ', ~ N N N N N
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
L U SV x YC S< X iC y~ X X iG a x Y. Y. X h' Y~
N N N N N O
a~ x x x x x x x x x x 0 x x x x x x
d C 'Oi," C C -Of. C G C C G M V1 l~ O~ .-~ t+l Vl
[/] ~"i "'~ z z y o-'~ "-i z Z Z Z M M M M M en M
a F~r C C fC C C C C C C N dl ~o 00 O N ch
C
r-~= 'Z z Z Z "'~ z z Z. z r 'y M M M K, KI M en
~ 1~ N M oo Ol O .. N m <h v7 ~o l~
Q Q~ Ol ON O% O, O, 01 O~ Q, O O C o C. C C
z N N N N N N N N N m cn M cM m ,n cn cn
a ~.~'. N m <Y ~n ~D [~ 00 Orn o N m -tF v-, ~o t~
0 V ~t 'c1= V= ~P <3= d' It -zY h vl v~ v', v7 v1 n v1
r- 00 % O N f+1 7 Vl ~ l- 00 O% O ~--~ N
A N N N N N N N N N N M cn m
Z N N N N N N N N N N N N N N N N N
C
O
In ~n ~n In v7 ~n ~n In ~n ~n ~n kn tn kn kn vn
m M M M f+1 M M M c+1 m M t+'1 m M M M
iL'e ~ U U ~.) U U U U U U U U U U ~.) U U
~ ~ ~ ~ d ~ Q ~ a= ~ Q ~ ~ ~
va rn vs on cs1 rn ra cn cn v) U rn cn Cn vs CIO v~
> r~ c. o. c r~ a, a aa a. a. a. a. z~ a. n~ c. o.
v' y 10 ti o v~o o m 41
b ' axi nxi o,
b z o b z o ~ a~i c abi aZi abi
o 0 o q o o o d
aD 8 x ai
0
~? w
0 0 ~ a~ a~i n a
~ ~
o 0
a > c ~ ~ a
~o >, a, o o >, >, >, >' a=vi '-~ avi '-~ abi o v n x ~ ~
0" >, o b
a a ~ .~ . b .~ .9 a õO .fl .fl .n b ,n C m N v~ ttS O a
=0 tz CU
0 t~ abi C abi abi C E o
o u 3 3 $ c 3 3 c ~ w o 0 0 w~o 0 0 U o U~ U b
o o o y U~ U~
o
~a~= o 0 o U U~~ U U o U ~
w ~w w y w w w w w x x~ x x~'" x u
o o w '''
, 1
a J a~ a~ a~ x' ' = ' U ' = a~ ' =
i. s.. _ ~-~. TJ 0 V] 0 0 V] ce .Ti N U]
~_ ~ a . c C~ ~ . ~- . . a~ aai z aai- -. ar x M x a~ M N y.,
rn ~ rn o a, o~ rn
2 F~7 o, 0 z r z w w z 8 Z E" Z
c a n rn y o 0 0 o ca o ca 0 1/
2 w ~'? ~cU 0 "! i0 c~a ~tl - C cC ia ~a 0 = oo O
~ rn c 8 ~ ~ ~ ~41 E ~ B c ~ c ~ ~ 8 ~u 0 1
r ~
ro N bA W N W w b q~A w
,h bD cC r7-~ =b~A ,~ =hD c3 ~J w'in WO N H cc H ca ~ ~U' C:J O C:J
m 8
0 ~ 0 8p N 0 8Q - o~ 8 ti 0 w " w cxy v w cy _ w c~ v ~ a
o ~ F~ ~ ~ ~ R ~ .~ ~ a a~ y ~ y ~ v = v ~ a~ ~ a~ ~ a P~ x rooc o i mo r c
o, u~ v~ a~ a~ a
~ a~ ~ o ca ~ cc a
0 a
~ == a~ ~ =~ ~ ~ =, o ~ =8 ~ =s E * Z c = o c v 'a _ 0 S a~ ._ - a~
~~, ~, 0
xZoxZx. Ca
a x x x x U x Q Cr!
~ a ~ W ~ ~L 6 X I~.~
rn v1 cn Z vs Z vD . o C7
a x > x x x U x Q x N z H N o ~
~ ~ ~ o ~ ~
Z ~n rn v~ cn ~ va v1 v~ v~ v~ U U x x x~
xy z x x Sy .T. x Oz
w M M c*1 t+'l en M M M M M Pr M M M M M M fy
U C', ~n _ v~
~ U U U U U U U U U U ~? U U Ux ~! Ux U N
O C/~ V] Go V] V] U] V] V] V] V] U V] V] V] C, V] ~ V] V]
U u a a a c a rs a o c a zi a a Z a Z aZ a.Z
U
[- 00 O, O .-~ N ~n 'd~ V1 00 'ch cn 7 I~ oo ~n
CO 00 00 00 O, 01 01 , O, O\ 00 M M d' In
00 00 o0 00 00 00 00 GO CO CO 10 O M M M M M M M M M c+1 M M
U
N
~ O O V1 ~ l~ 00 ol O .--~ N m "'h h ~O [- 00 O, O
~ =~ '~' N N N N N M M M M M m m M M M 7 ~=
H w
21
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
tN
x x x x x x x x x
ae 0 a O~i O C. O O ~ M ~ ~ ~ N N
7 CY <i' 'V' 7 d= d= 'ct d' d' 7 d'
en cn
00 O ~ 4 ~_D o_o O N
~~ z M M 'V' CY V d' dN'
a~ O 00 O ~-r 00 O, O N
~ t+l en t+1 t+1 M M M M M M M M M M
a ~! 00 O~ O N M 'ch h 10 t~ 00 G, O
~qo~
aA ~ M d' Vn 10 l~ 00 l O N M d' ~ ~
N N N N N N N N N N N N N N
C
kn n c c ~ n m
y t
s. w U U U
U U U V U U U V
d U
a
U V] C/] V] V1 Vl V] C/) V] V] C/) V] f/]
W~ a. a. o. a. a c. a a. o. rs, a. o. a s~
~ ~ w ca w ~
ob ab ab ab ab ab ob b wb a
~ w g w g w ~" uo m
41 o L w=..
pv
a o' o d N~ r w~' ~ a Z a ~ a
:,. vr .2x U 2 o o o'~ o'~
y' F w v i~y v~i u: a~
w co -W cn co coo Ln U~ con co
0 0 0
w w w 0 0 0
v~ ~ a~ - 8 =a ~ o a =w cw cw= =w cw ~w ~w
U x U~ U ' ~ U N o ~ o N o ~ o ~ o y o y o ~ o " o O1 0
w o E~ E ;5 H 'i~ E E a
U N U 'y U cd N cd N c3 U cC CJ ty c0
~ ~_ _ E- ~
M w M w M'~ oCn w -H- - _ __8
rn o . o o 3 d rn a~ a~ ~ ti ~_ a y ZBZg ZZti9 Ng
o" o 0
-o 0 -o 0 v0 -o0 ~ ov v0 0 , o
oo ~ v v v ~
00 ~ oo .d oo ~ c~V
N N ~ N R7 N 'rJ ~ TJ 'Lf 'O Tl ~ 'C 'O "c7 "O "O Ry
C7 3
rn m m tAJ ~,n G U v' ~ U U cn ~ U rn ~ U rn ~ U U U
o b o . a~ a~ v=y o w' x o~ x o~ x o~ o~ o~,' o~ xa' oa x o~' x o~ o
N y M M y,Uõ M
2 R7 roTJ N 8 ~"w GJ M h y M~D y M~ M~ M ~ i.U. M G M Ol ,~,'~
T M ~ M Ul M p~ M ~ M 01 ~1= 01 d'
ON T c7'
=fy .fJ Ua i 0 0 ~ Z
9 ~ c~ Z Z Z. Z
c ctl c
u V1 ~ C~/] V] ~~>~ w N U w N N w N w N~ W r U~ N U~ OM C? w N U v'
~~=8 Z o F-~ Z o w Z o r~ z o v~ Z o v~ z o'wy Z o r1. Z o Z o C7 Z M.
~ U ~r~ x x x x x x x.. ~" x
00 ON O1 (71 QMi ol O\ OM1 ~ OM1 00
x p v_
Z Z. Z. X. Z z Z. z C7
z ca ~ ~ N M kn ~o ~ ~ Z.
d c ~
N
U '~~ ~=' w v v
õ
z >~~ u
o ~ n x n~ v~ In vi v) tn vi
~ M M M M M M M M M M M M
~ U U~ Ux UxU U U U U U U U U x
o y d ~~~ ~o~ d ~ d d < en
d d d
U a a.oS aZ a.Z ~ a. a a c a c a a a a Z
O, l~ 00 , O N M ~h h a\
+~+ C ~ In t+7 M M '7 ~f= Ch d' '~I' ~l' 7
y F~ Q' M 'ch V' 00 00 00 00 00 00 cG 00 00 00
N N N N N N N N N N N N N
N
v O
O N t+l ~' ~/1 ~O l~ 00 Qi O N M d' ~n
E"'~ w Z 'd= 7 V' 'ch 7 7 d' ~Y V'~ v~ v~ V'~ V'~ h
22
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
w
r
t: r~ t: t:
c > > > > > > > > > > > .~
S c a ar ar S ~4 4 x x x x
W~ p N N N M M M M e,} 7 3 O O ~ 'r vi W)
~, 'cl' 'ch 'cl= 'd' ~h 7 V= 'd' V <I= ~t Z =-~ 7 V <!'
~'c1= ~O oo O N 'ch O 00 O N d= G O oo O N
A N N N M M M M M 'IY O O -It
z v ~r ~r ~r v a= v v <1 <1 z z
a N N M eh ~n ~O l~ 00 Os O m ~I= rn ~O [~ o0
~ A p N N N N N N N N m M m M M M M cn m
..~ m M m f'l M M t+1 M M M C1 M M M M M M
N M 00 Orn O ,--~ N m "d= In D l~ o0
t~ t- r cc o0 00 00 00 00 00 00 00
z~ . .. .. .,
00 Ol O N M d= h ~O l~ 00 O~ O N M
~qo~ ~ ) W~ ~, W) W, kn W, WI kn o
z N N N N N N N N N N N N N N N N N
V] Ln v1 vl vl N N try vl V1 vl vl vn N h Ul
~ L M M M M M M M m M M M m I M M M
L,u~n U U U U
co co N V~
W'J R. fs, Li, G
_ - O
4 '. o 0 0 0 o N Z
0 3 o rn o 0 0~ n S~~ cQ.S~.S~ Sd C7 o o~ a a
Z =~ ~ v .S v abi abi 3
Z o Z ~ o o Z 3 2 2 8 > 2 0 ti g ~ 3 - 3 3
00 co o0 O o w w
N Tj N i N~ O~ l~ N O 'd cd 'O =O ~'O cd 'O ~y U ce
q O O
C7 ~? C7 w C7 ~ _ a o yo 0o w w ~
v a c Ca7 b~~ ~ v
w
I's W wo 4
o o o E N
~?' 0 0 S r ~ n C_ .: o o 0~ 5 0
o t qy ,c ~o c3 c
cd =L7 0 w ~ 4"i cd w 0 3 cd 4~ aS ~ m+~ ~y cC 0 cd 0 cC
> ~ > o 9 o o > > c o a o 0 0
aw a~ o ~ O a o.n- a a tix 3 S 0
S .S S
a)rn o abi ve ~=S abi ~ ~z Y ~ ~~ bi~ abix aT~i~, ti' ~ 2 o M E E
31 o
3 o~ o~ 3~Z 3U 3U 3U 3U 3U w~ "M woO >'o >
o O~ o~ o~ o S S ~- ~ o n n
00 U. wo zySZ o -W yo ~~ . w w ~w ~ ceC O!Sd o ~~ 79.~
N N z~i N V v:I V N N V V.~ N N N v C N z ,--i U] V]
o~ ti~~ b~ v o v ;o o 0 v v= v v~ o c~ crn o V] p o x V] ?
a a 2 8i 0 i 0 i 0 a ai a~ ~ ~, N
0 z o: z oo: c
O ~ 0 w w w
o o = o o o
" N c~d a~i c~d a~i c~d
a V ~ ~~ M s w ~
~ = .?n~ ~n ~ = -n on ~ 9u'" v .~~ ~ ~Z ~~ o = b c
U' =o
p =~ C1 ~ y c1 =y y ln N=w N=rn ~.. V] m '~ 'v~ ~ii 'Zi' S O~ /-~ C ~~ ~ ~ G
010 r0 i~N rqN rOiiN Y~F7 ~N~D O G ~ ~
L a c o S~ a a c~ o v~ m C7 m W bq O C7 z n ~x o o n
~ 1a~ ;o a~ v Y ;o v Y n Y
> v > a~ > o ; o n o
a~ v~u v o a~
~
> U c~ o> c a o ~> --
. C 4~ b u o~n ~ ~
A a~ 9 E S e? w S~ u? .. ~ S: a E~ a S a 4 ~ a a~ ro~~ o~ ro c7 x v v x o~, v
x=v
~cy 6 n
~ N 7.= N/] r% CJ a N 00
cl)
~ ao C7 ~. !C7! C-.4
00 x ~a ~ a. c7 N x
x U C) U~ u U U u U b b b
~~~~ > >
~
k~i k~; CO c)
k~i k~; t~;
u
o ~ Q r ~ ~ ~ ~ ~ d ~
U a, c. m a, Z a. z ~ o. Z a. z a. Z ~ c. Z a. Z ~ c. Z a. Z a. Z c. Z a. c.
c. a.
U
%0 V1 d= ~n Cl N M <h V7 \O ON O N m d= W)
C. O ~ N -i .-M-~ ~ ~ .1/1 -a h- .-V1- %D ~ 1= O O O
p m M M M M M M M m M M M M M M N
N
U
N
ILI ~ C.
. L~ oo O1 O N tn 7 In l0 l~ 00 C. O N
F w~ v~ v n n ~ o 10 %c o 0 0 0 0 0 ~ h t~
23
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
g
~ x x >
x x x x x x x x x
a ~ V=1 l~ O~ O~ ti M U1 l~ Q~ M N N N
00 o 00 00 z~r ~r ~r n ' ~ n v~ n v~ n n z " ~n Z v O ~ Z z
d a~ a~ a~ a~
0' ~t' ~ eo co O N cP ~D o0 O N ~ q oo O q =
O h N N 'O l> l, l~ 00 00 0 0o GO r~ z
z h
'a' d= 7 V=~ -n tn Z tn z ~ va\
a N O~ O N M d' V'~ O t~ oo O~ O N M <t ~n ~O l~
rW ,'~ Q C1 ~= C~' en
M M M M M en
M 'ci' C~' '~= t} t} e,} ty d=
~ en M en tn k/1 Vl V1 kn 1n V1 4') kn Vl N n N1 V1 h
m d= ~n ~ t~ oo rn O N M 7 v'>
d~ p o o rn o ~ a o 10
0 0 0 0 0 ~ r t~ t~ t~ r r
z~ ~ ~t ~r a ~r v v v ~r v v v er ~r v ~r v
tn ' ~ ~ a, C, C, o 0 0 0 0 0 0 o 0 0
z N N N 7 V d= 'd= vl vn V) kn v'i vi v') tn tn vn N Ln
o ~ cl: 111
N V) Vl cdIt~-! v'1 h v7 V7 tf) 1n Iry In
M M i: G M m m y M M M M M M
~u U< A H U U U U U
<
cn co U) U~ U U~ v~ v~ vs v) v1 U v) cn vs v~ cn vz
W~ a a ss c.. a s~ .. a o o c a a c a a a. a o
w W o W p. ti o
a~ z z z ~ o o
d > o c~ n c~ M o W W W M rm v
4)=~ o 2 0 0 0 0 ~ ~ ,~
0 C "O b ~ ~ ~ =d 'b r m
x a~ m' 'A c~d .p = y o
~ vi ~ v, 4, V V v~
vi w C Ga V) rn rn c.~ ~ .=
2 Vy w w ca cd
~" 0=0 a) y S 8~ vi cn ci~ ~ o c
x x Z o "1 =~ 8
c E x
-0 ' Z
~z cn oa ~~, ox~,, ~
o - ~ ~ ~ Y a ~ o ~ a n a r; a)
o =o v y=o v~ ~ o~ o'~~~ 8 0 o rn o,> x
> 5 0 0~ o a a~ x a~ Z a~ o a~ p a~ ~ ' > a~ T m C aa
3 y
L. cn 2 3 y 3 c 3 8 3 ~" 3 8 3 c~~ n o 2
o w x o o~ o 0 0I c~a+ z o' o ai o ai riJ0 y ..O o cO a~i o w ttl 4 w w ' _
w
c 0 0~ 0n Y o ~ wo ~ w~ 5
ca w
.O
x
~ E a~ . w
w o
_ _o
~ o -e ca o B w o
3 v~ 3 ... o~ rn .'( a~ =~ a~ f a~ a~ o v y
o x o,."~ a~ a> cn ~ x ~'" ~ a c~ ~. a~ o y a~~ ~~ d m a~
p~o ~~p; coc~c cr. cQ' < oBoV cocr~o a
~ R -rw, ,~ o
0 w-~' = ~ c~C ~ i '~'~' i~3 N c~C N tl w w M O ~'"'~ x" N v~ ~ OM~1 ~ 4: CO
'~
N iC N c~tl ~ =~ q>, Q
Y N i~. .bD +' OA c~a ~D ~=-~ CO.C W rn q~ N
.d: =O u? T! y ~ O rn =~ p z =in 'm v~ =in 'v .n: O v, 'ln rn =
~ i s. >> N'~,i a~ (~j 'O (~] i3 'pp
0 ~ bA
'~y l~Ud o 0 cUa aki " aki .~ o
- 0.5 ~ ~ y ~ V) V 0 L C õ 0
y ~~~xa=~~a ioo?~ ~oE b v 0~ aEi a c ctou~~azc o 00
V] '.~ N >i ..~-. ai >. ~ ~ ~ ~ ~ N ~ ='" ~ y ~ 'i7 .., ..., N N .C N "" ~ N
a)
u 00 00 u
xvxv od~~ xw'xw~x x.~xYa; x 2.~ xx~x4
N
C/I ;~. c~n
U
oUo N o
0
z a a a ~ a z zx.~ ~~ M. ~~~>x 4 z~
~ A
h Vl x x x x~ N
w m M M ~ h CJ V'l V"I Vl Vl ~ ~ V) V]
U U~ U 0 0 ~ x x mV~M MVl M~y
U U M M M M y U U U U U~"~ U 0 v~i r~i~ r~i1 ~ U U U ~ ~ vi QF r~ r~n v~i N~
v~i M r~i~ o
U a a a x a a a a a~ a s~ a a a a ax a x c Z a a~
u
E o o ~ QNi ko (~ rn o
y v tn kn In 10 ~ ~ ~ ~ ~ 00 o 0
N N N ~D h l~ Cl O\ T N 01 ON O, ) 01 01 \ O O
U M M M M M M fM M M M M <+1 M M M f+'1 M
N
Z
cc ~Z n r r r r n r ~ ~ ~ ~ ~ ~ ~ 00 ~ ~ rn rn
E+ W
24
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ N N N N N N C p q y N N N
~ G axi ~ aYi axi axi aa ~ U y X x 1~s '~' x y~ jt
x x ~x~xx x s x x x
p ~ c c ~ ~ a c c c c a M n ~ rn ' '
zz Z z ~ 0 0 Z 2 Z Z Z z v~i ~ ~ z Z
C 0 d) 0 ~ C C C C C Q N 7 l0 00 O
W~ p o 0 0 0 0 0 0 0 0 0 0 rn o, rn o Z 0 z
zz z z z z z z z z z z~ v~ 10
d N oo rn o N m 10 t- oo rn o ,-
~ v~ ~n v, tn v, ~n ~n ~n n ~ ~ ~
ztn Ln kn v7 v> In
d ~.~. ~O h o0 0~ O N cn drn ~o t~ o0 0~ O N m
~~A p t~ n t~ t~ oo eo 0o co co eo 0o co co 0o rn rn rn rn
z'd= <I' V= V=d' ~t 'ch ~t ~Y 'd= V' V eh <1' d= 'ct 'y' 7
d~n ~.=N-~ ~ 7 V'~ ~O l~ oo ~ O
Z v
v'~ V'> V~'~ V1 ) V1 ~rl v) ~n W) vNj vN) v v
vNi Ni N') v h N') N
O ..~ .~ .. ..
vl l/'l l/'1 N Vl N Vl V~ V~ U') ro In =tCC td N
3n M M M M M m M M m G ~ ~ M M
c u U Z U U U U U U U U U U
~n U r~ v~ v~ ra v~ cn v~ n v~ U v~ U~ U~ U~ v~ co
W 9 a. a. c, c. a. a. c. a. a a. a n. a a. c. c. .. a. s~
O .O
G
i o a 2
v ~ a r M m a~ n a~ o o ~. .. E o a rv
w y ~ U o U o n a N a o o E c ~
o
=~ ~ ~n ~ o ;o v 'O ~n 'b v~ 7 c~d ca y v N o ~o .0 4" ~ c
'c c a a =~ E o ;o > N .o ~ w
~
~ N Q~ O rn q v~ O W N
Y~
p ~p, =~ 0 M N ~ y Y w aJ ttl 0 W WW
c '.7 N C~ CJ N z
r~i v U u. ~ i.. U ~= U w F'~ ..O
V] iO T ~p v~ <p ~n T T ~ ..., O U C ..~+ >+ ~ ~ U C
a~ x py, A ~ yp~ ~ a~ o 0 o a a U 0 8 a~ ~ n 8
=.'~. ~ f-~ 'L7 7 H V] 'Z U o U o ,,, U ~ a y w
o ~ 0.1 ~= pa ~ ~ R+ abi ' abi 4 a~i a i ~w 0. a i U~v 0
i ~i, a~i ~ o
+ v~ U o o~ ~ ' o 0 0 ~ 3. 3 0 . 0 0 3 a 3
o ~ o ~ o a U n ~ o W ~ 7~ o
w w= O .
O N wO vw O N O~ U O~ . .~
ct o'b~ w
~ o 0 8 a a m" o 0 0~ ~ o rny ~.y U~ or~is
~
o on on r' a -w c ~ c ~
. ...---. ._ . ~ ~ o =y z a~ a~ m a~ v, o ca c/~ y a~ ~ - N- 'z s.a~.
a e. c vs Q a a o 0 o W W a z o v o a ~y ~ x ~ c; v~ a v~
x 0 o c u,c N N c c ~ c g a~ o x c o~ x o x~ x= c~ c~C 11 y . a~ o~ y a~
c a~ ~ m v a~ a~ y N ro
~= c~ .v 3~ axi U o o aoi
> C ~ 0 CJ 0 "b w N R7 O
'z ~ cC pp O ~' ~ ~ ,~U, w0 .~,,,0 .~ ~ .~ N ~ p 0
v o o ~w o on ~n io G ~n a y en ~ bA
N 0 on~' bwZwZ v o ~. ~ ~+ c o yw ~ o c ~
N M."G. 0 cV U cV a~ N a) N N
Y r~ N W ~ N
j N ~ O O m v~ U r .~,~ ~
O O v O y
=EU~J ~ ~ ~ T O ~ ~
M ~ ~ ~ ~ O ~ O=+ ~ ~_ 7 = ~ ~_ ~ U ~~ ~ ~ U a ~'+Ni rZ
I'D
7~ W]" W Ll P~ S C~ x
V] C-0
0.~ ~_ ~" ~,'I'i, ~ Pn ~ 'c~' =~ .a N .y =~
M ~ W v 'm w N yN (n
N ~ -~r -r.
[/] ~ ~4 N N
x r~ a a a ~ r~
z
x Q) Q
cn < ' Q ~ x 8
e~ .~ a y U .~ O O~ V] Cn O Q~ co .,.~, .O N X N
E ~ J '~p., v~ v~ o v U '~ P' iNC x a ci c N .ryo
< z z
~ a? ~ x w a~ C? A ~' ~. x ~ x ~ x x x ~ x
n
y M x Q~ M M M M M f''I M M M
M < <
~ ~~ ~ Z N U U U U U U U U U cl) < ~ ~ ~
rn <
~o pU~MC~rn~ ~ vi ~Nr~i~ i ~x~ix xrilx
V L1 ~ CL LY N A. f3. i7. C1. !]. N i1 R. d' L1. i~. L1 R. R~ N L1 N C N A. N
u
o p c~ CD a\ CD ~ l~ ,=l~- ~ r,
~I= ~1= ~h ~i' d= '~h ~Y ~h V 'ch ~t 'd~ d= 'cl' ~P Vd= ~h
U
N
u o
00
O N M '7 h IO l~ ~ ~ O O O O O O O O O O
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
y N N N N N N N N
~ b 7
u k k X X x X o y ~ k X X
u q a> a~ a~ a> a~ a~ r. t:
U
a~ ~". x x x x x o ~ x
a =V~= C G C G G C C O N C C d' ~ C C C G
zZ 0 Z Z Z Z Z ~ ~ Z Z N N ~ z Z 0
a '~'~ c L 7 G CO.' -Of. .O!'. OC-~ 0~1 ., N N N N N
Q 0 0 O O O 0 0 N O 0 N N O N O 0
zz z z z z z z~ r z z~ z z
N~o l- O =-. N m d= [~ cc rn C. N M d=
Q 'D \D o0 00 00 00 00 00 00 00 00 CO O, Q~ O\ Qi O\
z Irl In ~O b b ~O %O ~O 10 %O 10 ~O %O \O \O
a ~ ~P In N m d= W~ 10 l- 00 rn O .r ~ m et ~n ~
z O O O O O O C. O .-+ =--~
~1= ~F ~D ~O ~D ~O ~O 10 ~D ~O \O
0 M d' N.y. M~. h ~O l, 00 O, O N M V' V1
'Ih 7 -It d= C= h v) kn vl
z N V7 ~O ~D ~D ~O %D ~O ~O ~O ID l0 ~O ~O ~O ~O ~O
C cOC cd cOa cCa
a " .. .. .,
'Vl v-> n W) xn ~n v-> vl v-) Cd 'n kn kn m m ,n
d O M M M M m M m M a M M M E M E
E
d 6 c~V Q Q~ Q~ =a= c~C '~1= c0
rn v~ vs v~ v1 cn ~n v~ U v~ U v~ v~ ry U v~ U~
> a c. a c. a. r~ a. a a a. o. o. c. a. s~. a
y 3 C A .2 o = ,~ = C 0
O O O c~~,tl y y O~ p ~O d. ~ ~Ø a~i ~Ø iC ~Ø M "cl
abi abi abi ~ a abi bo r a'~
.' q
r,
w~ ~, ~ ~ w w~ ~ o o ~ ~ v T N
< od :? ~ o ro U o QF a v 0
vs vs cn ~ a oa ~ d Q va ~ 'k ~ <r -o a va ~ =o p o
x x a~ v) 0 00 0 E pA = 0 x y a) a E
: :=~ ~'U ~ : ~r~x 8 s~ 8=~ ~ 2.k 15 c 0
-P- w
ro ~~~. 3 ro~ ~ c ~ y w g w
U P ~a 0
c, cd In w v r
0
C/D
> > U a > > v d o > '" > o
. a .. oa ow,n>, p w~ P. '".=~ ro
=o .o 44 .,y o .d v, ,=y o .1 a a .~ a~ >, o =o '~ =o '" o p., 'o rn ' V1 '
a~ o u aai d ~ aci - a r v 3
3 3 r~ 3- 3 a~ ~ 3 w o o ~= n v,
O -
~ O N O N O~ ~=-+ O O o
N N~+ 3 ~ N O U O ~ y ,0 y0=, ~ I D 0 0.~ o 0 o a~ o 0 3~ 3 a~i 0 3 v~ 0 ~
w w w. w u'~ v1 ~ vdi q " x o~b .. wo w y~ ~u o U E ~
z
aii 4~ = x m~ ~ a a~i cvi n 6 x
- ---- " 'o ,i.,'.'~-N OpN'.v- L.~ ~..y.:' U o. 0...-- -y-=- va ~- E--- No c~C
o N -N - -- -
~ ~ Ca ~ o y o = p ~ v~ o ~ ~ ~ ~, ~, ~
x o o B
c~ c~ c~ c o c~~ c~~ ro~~ v1 c ~
a) Y a7 N ce N N p N bA ~ ~.~ ',7 O~ O v+ Q3 a
~a~ yd~OO c cc~~Y
p U . U p ~~ ~ a~ ~ qa w
p m C p~~ C N~ ul vpi ~ pO..~'. 'b H L7 ~d U O Z M w y
qU .r tlU ~D .'L bA ~ b-A bA cC y id id p ... ~y .b .'. cC N cd cO O G
N~'v"i ~. G ~O ~ O C ,~ ~"' ~,~ O M O O rn in
c ~ O y o~b cn ~O~n c bn o ro B M o co z
p N CV ' N O fV O cV ~J N .~ U= a) = pp CV W
(V ;p - a) a)
k t k X u~ ye; ~ m~ m cd '~ .= a~ a~,bi " ~ ai aim E ica - 0 x.,.a, 'n
q a~ ~ y ~a/~ y n a~ ca a~ c. a~ o a~ a~ . a~ c v~ o~ o aoi r
=y 'm ~ N O ~x N ~ cO C~ 9 C ~.y at ~ wp O
c a~ a ~~ a C v C v : o C' a Ql Q1 ~.~ a ~ o b d o
vacv~~co=va8, co=o>~ > JaZi ';~ai> r6n ~ra~~v~ rE cn $az
ta x cx ?x x ~x~tax ~ a~wa~wawax~ ~xaxx ~xU axU
N
N .~ 'A
Q ~ U C C ~ x x' C1
~,=~' .o N W ;y ~ ~d i~GtL LL 7 ~-+ ~-Yi ~~cyy [.'=' J' ' ~
q N .~Ki N
Z A
Z t W d W cdo W d q U y L d ~[] N O ~C O
o W) vi c) vi ~' vi
~n
M M <+1 M M~ M= M M~ Go M x M~ M ~ M ~
m U U U O U O U O U O ~ D U~ x U~ zi U l~ U ".~y. U~Q
do < o ti ~x d' O dtn ~ d <r ~vsv
U a a a C7 c C7 a C7 a C7 c c c a 'nn W a a C7 a c c a a
u
M d= ~ ~n ~ l~ N cn V= kn ~o rn O
N M M M m M M 'd=
G N N N N N N N N N N N
<h 7 'ch 'ch d' V 'ch d' ~h <1= =d' ch 'ch =cP V 'cl= 'd=
U
d õ O~ ~ ~ ~ ~ ~O ~ 00 O~ O N en 7 h ~O
z ~ N N N N N ~
H w
26
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
y CV (V N N N N ~, N y N y N
d q Q ~ N N N N N N = d ti N N
V N y
a~ x x ~ ~ ~ ~ ~ ~ ~
U U ~ ~ ~
a p O~ .--i O N N N N N N N N N O N
C ~ C ~ ~ C ~ ~ C ci C Q
N M 0 0 0 0 O 0 0 O 0 0 0 0
~ '' z z z z z z z z z z z z
a ~ 00 O q C q C C [7 C ~ C ~ q
~
W A z~ ~ z z z, z z z z z z z z z
N O 00 O, O .-~ N m 7 h \O [~ 00
~ q p rn o rn o% rn o O O O o 0 0 o O
z a .~1 l~ oo O~ O N m V' h ~O 00 01 O
N N N N N N N N N N m
z ~O ~O %O D ~D ~O ~O ~O ~D %O %O
a ~. O l~ 00 01 O N c+l 1= h pp
~~ O yl V1 V'l h ~D ~O 10 %O l0 ~O ~O l0
z~O l0 b ~D ~O l0 ~D l0 ~O ~p ~O ~D ~O ~D
~ ~
C C cOa ~_
~ i~d+ Vl ~d V"~ ~0+ ~ ctl V7
=~ L C M U M M M M M
g U
a u E
m
W y c~ a c a a a a a~ a~ ~ a~ a
UD 0 O c~ w =O ~= ~ c00 c~0
o w axi o~ w 8 o y C~w7 y .
M c ~~ w v x w
~a e
8 8 ti ~ ~~+s ox a ~ - _a vw ~==~ ~'zx
T ~. oE Gj " U Q'' N V] cd Q rOi~ ~ {~. y~ O LL y Qi y
abi ~ abi rA U"C~ T" m va 0 " c>
x~ U v 8 0~ c o õ~ rn' o on - : a =~ ""~' G z
0 ~ m ~ 0 ca w
~~+ ~ ~ - a
o b 8 ~ iMZ
CJ 0 cC +O-' F~ td cd i G cC =~'' w f-i y O o ~ td g .'t Ry o - 'r" ,=' 'O o.a
M=~
m avi a z n O a aZi a~ a a a y~~ w~ n S o Y .
acri 0 ~ o, = abi oo :, a~'~" c:''~" =~ y~::
o 3~ 3 3~ 3 z 0 3 ~ 3 k7 3 ~ vM C 3 v Z
~~~~ o o o 0 0 o d o 0 o a a o ~ C7 o
b9 Y~ " ~ " " vy ti 9 42 o awi Z w ~' "' Z0 b ~7 ~ . a Z~ .ti Z w -o
U
rn U 0 ~, a) ~ m a) OL a) o a> N v v .~ =-, ('~ c0.~ N=p y a' N=Q rn
m v, o o cd o~ o ce o C O'~J a) O~=.. ~ G q o o~ o N
. + ~i ~ C. C. Y.," ~'" L' ='. 1: y -!"n ,y+ O p o Y C~ .. 'U N o =~ '~ FJ N O
=~ Vl .. - m
---
v
~
0, y o O O o a~ p~
~. c a aci O aai .~ ~ o i ~myC~7 aai .C a aCi~ c a aMY~
v U' ' C7 y o C7 rn o C7 r ~n v
U
~.'~.~. = rn " " v rn U~ ~ y y
N a~ ws ~ ~ cn = o C7 c o . ris o : vC7i r d
~ c ;o o '~ c o c c co
o
.o_n ~ c7 @u 4~ G C7 w k7 .~~
O.c cn gn ~u ou b o,e-q'~ 0
O o O o a, o n, rn o a, u, o n, u~ =_ v~ ~ z = ~'~ '~ U' v G id ~J =~ ~~
q tV '~ N O N'~ tV WO tV N
J 'C O p~ =C''
O CU T bt] W O'Ly ~=,~r N. v) . . v) . N E'L7
~ O v'~ c
~ N aki c~a aki .d v ~ v o aki wo -
' o ~-a p., "-O' p~ .=O' ~
o vOi 4r ~ (7 ~ ~ N N .m-~ r ~ vOi ~ M ~-O+ ~ O ~==~
0~ C ~~~ yC~7~Uo
~~ vaC7 rr~ ~
axaxxUx~xUxx~x~x doxw ..~~~~C7xxcxc ~ C7 7 ~~..w~~~Z
< < iz= a
w x w ~ x x v<
cl) x
tA M In
rn "'
00
u ~~y v c? o ~ z A W
z U ~ d -< d' N N cV CIO
u Q U ~."i U x x U <
o v1 vi ~n ~n ~ri ~n ~n vi U uj U M r.~
M M M M M t+1 t+l L4 M Pr w
Ci M W~ M
~ w U U U U U U ~!? G~ U
<U d= QUi 'cf' ~O 'ch M
U v~ v1 rn v) v1 rIo w U ~n U a rn U'~ cn
U c a s~ a a a. a. a. r~ c c~ ~ a a O a
L.~ N ~O l~ 00 ON N m d= ~n ~O h 00
~ d. d. .~i' 7 V= 7 ~n ~n v') ~n ~n In
C N N N N N N N N N N N N N N
O P ~}= ~i= 7 'ch V= 7 ~t =cY d' 7
U
N
I I~ CO O~ O .~ N M 7 V"> ~O h o0 O1 O
~ '= y z
N C', N M M M M m M m M M M 'V=
H w
27
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
d N fV N N N
~ U x x x x x x x x x x x x
p, a) (D
A C C q q t". C ~ q C C a, ="~ M h
z z z z z z z z z
d ~ C ~ C C q C C C C C C ~ 00 O N V=
z Z Z z z Z -7. Z Z Z Z ?-. r- i- t- r-
ON. O O ~i =-Mi .V"lr ~ õ1~,. C ~O (~ oo O~ O
z r r n r r r r n r r ~n n r r r
a~ Q M M M M M M M M M '~V" M M M M M M
\0 %0
d ~. O N m cF v~ ~ t~ oo rn vn
l- oo rn
z ~O ~O ~O ~D ~O ~O ~D ~O ~O b r r r n r r
F' c~d c~d cd
O ~' ~ ~
=y C~=tl+ C~tl+ ,~ ctl ,/"1 V1 V7 tn tP> V1 Vl V7 V1
Yn t=i G m m m M M M M M M M M M
CIS U U , U U U U U U U U U U
a a a. a. a o. a. a= a a a a
o
d~ "~" a~ a o o G U
=r~ ,. ~s .. ca ca v~ P. o
c c 10 m~ c 0 O
o=~o~79b d
~
. ~ ~ Cm o ~ ~ 8 8 8 w 8 8 o a) Q cn a~ o
o =~
>,.Y >, >, '~-~ ..o v~ .~ ==~' ~
S ca a 2 a~ a~ 3
oo
o a o
y a7., ~ a> o a 8 .fl
TJ V] 'O [/] y M at x W M O
.
o m ' 3~ 3~ 3 N rn o~ w yM. v
cyo 0 0~yP. v, o o 2 H oU oU ov ' '~' y., ~n
w ~ c d
o ~ o oon w ~ '8~+ 9n w t~ w
U
N G1 = ~ ~ G=~ N' .y''' m N' G U a y~ N tV N
vcVi a~di ~'a U U;S~ ~i ia o af o '~" v~ X
. .. - . .. m ~ _~. ~..5. .v a .iy_ ~ Q a a~ '~ ~. ~. . - _c c- .. c- ~- - ~ -
= P.+..- 'b ... _. ... - - -
cm w' ti c 8~_ x~ U ~ c c a a o o o C)
v y N aU+ N =r~õO N +.U+ N N U c-, OJ U N tu 0 'O ~ .'d CaJ x N L'
(J
aY U c~d 4õ~ iC iC id itl =3 U cC
bn w ~ =o id ~ . ~ .c ~ .c ~ a ~ .~ y' ~ o
o
o
v~ _=5 e~uroU .c~~ba 6 c~u en c~u c_n" ?A vr~ o 'ow
m Z o 1>1 o ro.~' o
4'= 7 '~ TJ , id ,--. N u~ r'~.. o N ~
a o
2 N O N M
~n v1 vs Xi
=r~i.
..2
I
a, It xU x" xUU x.. x 8 x~. x~i x 8. xx xrn '8a a=oo
~
N h ~ ~J w c~G ~ 00
N
f"
R w A~ a U A~' a ~ !y
'~-' O~ V] V] V] V] C~ V] Vl ~ ~i M C M
x x x x x x x x x '~y JPy' (~ x
U V]
7 x ~/] y~ Uj V1 k;~ Vl V7 Vl N In V7 f.. kn W) V7
w ~~ tiy M M M M M M M M M /' M M M
O W~ U U U U U U U U U p-1.
U U cn v~ co vs rn rn v1 v~ cn cn rn
U ay a a a a a a a a a a a a a a a
, N l~ O N ~ co O,
~n ~ G ~O O, 31 ol a1 Ol O O =-+ o0 00
= N N N N N N N N N =--~ .--~ N
ch C~= V' M M M M M
M oo rn O N M kn ~o
,C z 7 '~i' d= V V' 7 ~F 7 V = v'~ v~ v~ v'~ v~ v~ v7
G~.
28
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
r. u ~G X ~ N N
d C N Q) aJ f ~ d N
d C C
x x U
~AZz z ~ z z
O d 'o v 11
z z z z z
a N N M v c
0 10 10 'D 1.0 10
a ~.~. o N M ~t v1
o ..
Eo U U
o a ce). a a a.
~ Z c o
> 40 0 r w' y
a 7
o a~i E~ x x
cq T3 a3
N ~. .i o N c~a ~ c~c U
co
0 C) a c
o W
a a 4+
- T~ =o z =D _ ---
..---
. . _ .. 3 ,b ai a~ c~.~ C a~ C =8 ... ..-.. .. ._ __ _ ._ .
_
0 ~ c o c~_
t~.. o 'O
" a~i a~i ro~ c~' c c
C's =~ a ecn~ a~w' ,~qd~3y
O X~ y~ N N N A
a 8 a~ ~ a~ aXi p aki E
=7;, O O
cn
a x~xox~~~x=~x~
~~+ N
N ~~ O ~~' O P=~
O
7 r~-"i= O ~ d=
z
x
M h
~ 45 u ux~
U a vNi n. ~c ~ c, c a, c, a a
r C) 00 n n
o A M m c~+i ~ 7 yN=
U
N
00 ~ O N
H w
29
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
. .... ,_,,..
[0079] Table 2 provides a non-exhaustive list of polynucleotides of the
invention comprising, or alternatively consisting of, nucleic acid
molecules encoding an albumin fusion protein. The first column, "Fusion No."
gives a fusion number to each polynucleotide. Colunin 2, "Construct
ID" provides a unique numerical identifier for each polynucleotide of the
invention. The Construct IDs may be used to identify polynucleotides
which encode albumin fusion proteins comprising, or alternatively consisting
of, a Therapeutic protein portion corresponding to a given T7ierapeutic
Protein:X listed in the corresponding row of Table 1 wherein that Construct ID
is listed in column 5. The "Construct Name" column (column 3)
provides the name of a given albumin fusion construct or polynucleotide.
[0080] The fourth column in Table 2, "Description" provides a general
description of a given albumin fusion construct, and the fifth column,
"Expression Vector" lists the vector into which a polynucleotide comprising,
or alternatively consisting of, a nucleic acid molecule encoding a given
albumin fusion protein was cloned. Vectors are known in the art, and are
available commercially or described elsewhere. For example, as described
in the Examples, an "expression cassette" comprising, or alternatively
consisting ot one or more of (1) a polynucleotide encoding a given albumin
fusion protein, (2) a leader sequence, (3) a promoter region, and (4) a
transcriptional terminator, may be assembled in a convenient cloning vector
and subsequently be moved into an alternative vector, such as, for example, an
expression vector including, for example, a yeast expression vector
or a mammalian expression vector. In one embodiment, for expression in S. cer-
visiae, an expression cassette comprising, or altematively consisting
of, a nucleic acid molecule encoding an albumin fusion protein is cloned into
pSAC35. In another embodiment, for expression in CHO cells, an
expression cassette comprising, or alternatively consisting ot a nucleic acid
molecule encoding an albumin fusion protein is cloned into pC4. In a
further embodiment, a polynucleotide comprising or alternatively consisting of
a nucleic acid molecule encoding the Therapeutic protein portion of
an albumin fusion protein is cloned into pC4:HSA. In a still further
embodiment, for expression in NSO cells, an expression cassette comprising, or
alternatively consisting of, a nucleic acid molecule encoding an albumin
fusion protein is cloned into pEE12. Other useful cloning and/or expression
vectors will be known to the skilled artisan and are within the scope of the
invention.
[0081] Column 6, "SEQ ID NO:Y," provides the full length amino acid sequence
of the albumin fusion protein of the invention. In most
instances, SEQ ID NO:Y shows the unprocessed form of the albumin fusion
protein encoded - in other words, SEQ ID NO:Y shows the signal
sequence, a HSA portion, and a therapeutic portion all encoded by the
particular construct. Specifically contemplated by the present invention are
all polynucleotides that encode SEQ ID NO:Y. When these polynucleotides are
used to express the encoded protein from a cell, the cell's natural
secretion and processing steps produces a protein that lacks the signal
sequence listed in columns 4 and/or 11 of Table 2. The specific amino acid
sequence of the listed signal sequence is shown later in the specification or
is well known in the art. Thus, most preferred embodiments of the
present invention include the albumin fusion protein produced by a cell (which
would lack the leader sequence shown in columns 4 and/or 11 of
Table 2). Also most preferred are polypeptides comprising SEQ ID NO:Y without
the specific leader sequence listed in columns 4 and/or 11 of
Table 2. Compositions comprising these two preferred embodiments, including
pliarmaceutical compositions, are also preferred. Moreover, it is
well within the ability of the skilled artisan to replace the signal sequence
listed in columns 4 and/or 11 of Table 2 with a-different signal sequence;
____.
such as those described later in the specification to facilitate secretion of
the processed albumin fusion protein.
[00821 The seventh column, "SEQ ID NO:X," provides the parent nucleic acid
sequence from which a polynucleotide encoding a Therapeutic
protein portion of a given albumin fusion protein may be derived. In one
embodiment, the parent nucleic acid sequence from which a
polynucleotide encoding a Therapeutic protein portion of an albumin fusion
protein may be derived comprises the wild type gene sequence encoding
a Therapeutic protein sliown in Table 1. In an alternative embodiment, the
parent nucleic acid sequence from which a polynucleotide encoding a
Therapeutic protein portion of an albumin fusion protein may be derived
comprises a variant or derivative of a wild type gene sequence encoding a
Therapeutic protein shown in Table 1, such as, for example, a synthetic codon
optimized variant of a wild type gene sequence encoding a
Therapeutic protein.
[0083] The eighth column, "SEQ ID NO:Z," provides a predicted translation of
the parent nucleic acid sequence (SEQ ID NO:X). This parent
sequence can be a full length parent protein used to derive the particular
construct, the mature portion of a parent protein, a variant or fragment of a
wildtype protein, or an artificial sequence that can be used to create the
described construct. One of skill in the art can use this amino acid sequence
shown in SEQ ID NO:Z to determine which amino acid residues of an albumin
fusion protein encoded by a given construct are provided by the
therapeutic protein. Moreover, it is well within the ability of the skilled
artisan to use the sequence shown as SEQ ID NO:Z to derive the construct
described in the same row. For example, if SEQ ID NO:Z corresponds to a full
length protein, but only a portion of that protein is used to generate
the specific CID, it is within the skill of the art to rely on molecular
biology techniques, such as PCR, to amplify the specific fragment and clone it
into the appropriate vector.
[0084] Amplification primers provided in columns 9 and 10, "SEQ ID NO:A" and
"SEQ ID NO:B" respectively, are exemplary primers used to
generate a polynucleotide comprising or alternatively consisting of a nucleic
acid molecule encoding the Therapeutic protein portion of a given
albumin fusion protein. In one embodiment of the invention, oligonucleotide
primers having the sequences shown in columns 9 and/or 10 (SEQ ID
NOS:A and/or B) are used to PCR amplify a polynucleotide encoding the
Therapeutic protein portion of an albumin fusion protein using a nucleic
acid molecule comprising or alternatively consisting of the nucleotide
sequence provided in column 7 (SEQ ID NO:X)of the corresponding row as
the template DNA. PCR methods are well-established in the art. Additional
useful primer sequences could readily be envisioned and utilized by
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
... ,. _ . .... . _ _ _ . . ....... ..... ...~~. .,~,. aõ~,.
those of ordinary skill in the art.
[00851 In an alternative embodiment, oligonucleotide primers may be used in
overlapping PCR reactions to generate niutations within a
template DNA sequence. PCR methods are known in the art.
[0086] As shown in Table 3, certain albumin fusion constructs disclosed in
this application have been deposited with the ATCC .
Table 3
Construct ID Construct Name ATCC Deposit No./ Date
2249 pSAC35:IFNa2-HSA PTA-3763
also named pSAC23:IFNa2-HSA Oct. 4, 2001
2343 pSAC35.INV-IFNA2.HSA PTA-3940
Dec. 19, 2001
2381 pC4:HSA-IFNa2(C17-E181) PTA-3942
Dec. 19, 2001
2382 pC4:IFNa2-HSA PTA-3939
Dec. 19, 2001
3165 pSAC35:HSA.IFNa PTA-4670
also named CID 3165, pSAC35:HSA.INFa Sept. 16, 2002
[0087] It is possible to retrieve a given albumin fusion construct from the
deposit by techniques known in the art and described elsewhere herein
(see, Example 10). The ATCC is located at 10801 University Boulevard,
Manassas, Virginia 20110-2209, USA. The ATCC deposits were made
pursuant to the terms of the Budapest Treaty on the international recognition
of the deposit of microorganisms for the purposes of patent procedure.
[0088] In a firrther embodiment of the invention, an "expression cassette"
comprising, or alternatively consisting of one or more of (1) a
polynucleotide encoding a given albumin fusion protein, (2) a leader sequence,
(3) a promoter region, and (4) a transcriptional terminator can be
moved or "subcloned" from one vector into another. Fragments to be subcloned
may be generated by metliods well known in the art, such as, for
example, PCR amplification (e.g., using oligonucleotide primers having the
sequence shown in SEQ ID NO:A or B), and/or restriction enzyme
digestion.
[0089] In preferred embodiments, the albumin fusion proteins of the invention
are capable of a therapeutic activity and/or biologic activity
corresponding to the therapeutic activity and/or biologic activity of the
Therapeutic protein corresponding to the Therapeutic protein portion of the
albumin fusion protein listed in the corresponding row of Table 1. In further
preferred embodiments, the therapeutically active protein portions of
the albumin fusion proteins of the invention are fragments or variants of the
protein encoded by the sequence shown in SEQ ID NO:X column of
Table 2, and are capable of the therapeutic activity and/or biologic activity
of the corresponding Therapeutic protein.
PolyReptide and Polynucleotide Franrnexts and Variants
_
- Fragmeitts
[0090] The present invention is further directed to fragments of the
Therapeutic proteins described in Table 1, albumin proteins, and/or albumin
fusion proteins of the invention.
[0091] The present invention is also directed to polynucleotides encoding
fragments of the Therapeutic proteins described in Table 1, albumin
proteins, and/or albumin fusion proteins of the invention.
[0092] Even if deletion of one or more amino acids from the N-terminus of a
protein results in modification or loss of one or more biological
functions of the Therapeutic protein, albumin protein, and/or albumin fusion
protein of the invention, other Therapeutic activities and/or functional
activities (e.g., biological activities, ability to multimerize, ability to
bind a ligand) may still be retained. For example, the ability of polypeptides
with N-terminal deletions to induce and/or bind to antibodies which recognize
the complete or mature forms of the polypeptides generally will be
retained when less than the majority of the residues of the complete
polypeptide are removed from the N-terminus. Whether a particular
polypeptide lacking N-terminal residues of a complete polypeptide retains such
immunologic activities can readily be determined by routine methods
described herein and otherwise known in the art. It is not unlikely that a
mutein with a large number of deleted N-terminal amino acid residues may
retain some biological or immunogenic activities. In fact, peptides composed
of as few as six amino acid residues may often evoke an immune
response.
[0093] Accordingly, fragments of a Therapeutic protein corresponding to a
Therapeutic protein portion of an albumin fusion protein of the
invention, include the full length protein as well as polypeptides having one
or more residues deleted from the amino terminus of the amino acid
sequence of the reference polypeptide (i.e., a Therapeutic protein referred to
in Table 1, or a Therapeutic protein portion of an albumin fusion
protein encoded by a polynucleotide or albumin fusion construct described in
Table 2). In particular, N-terminal deletions may be described by the
general formula m to q, where q is a whole integer representiug the total
number of amino acid residues in a reference polypeptide (e.g., a
Therapeutic protein referred to in Table 1, or a Therapeutic protein portion
of an albumin fusion protein of the invention, or a Therapeutic protein
portion of an albumin fusion protein encoded by a polynucleotide or albumin
fusion construct described in Table 2), and m is defined as any integer
ranging from 2 to q minus 6. Polynucleotides encoding these polypeptides are
also encompassed by the invention.
31
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
[0094] In addition, fragments of serum albumin polypeptides corresponding to
an albumin protein portion of an albumin fusion protein of the
invention, include the full length protein as well as polypeptides having one
or more residues deleted from the amino terminus of the amino acid
sequence of the reference polypeptide (i.e., serum albumin, or a serum albumin
portion of an albumin fusion protein encoded by a polynucleotide or
albumin fusion construct described in Table 2). In preferred embodiments, N-
terminal deletions may be described by ttie general formula m to 585,
where 585 is a whole integer representing the total number of amino acid
residues in mature human serum albumin (SEQ ID NO:1), and m is
defined as any integer ranging from 2 to 579. Polynucleotides encoding these
polypeptides are also encompassed by the invention. In additional
embodiments, N-terminal deletions may be described by the general formula m to
609, wliere 609 is a whole integer representing the total number
of amino acid residues in full length human serum albumin (SEQ ID NO:3), and m
is defined as any integer ranging from 2 to 603. Polynucleotides
encoding these polypeptides are also encompassed by the invention.
[0095] Moreover, fragments of albumin fusion proteins of the invention,
include the full length albumin fusion protein as well as polypeptides
having one or more residues deleted from the amino terminus of the albumin
fusion protein (e.g., an albumin fusion protein encoded by a
polynucleotide or albumin fusion construct described in Table 2; or an albumin
fusion protein having the amino acid sequence disclosed in column 6
of Table 2). In particular, N-tenninal, deletions may be described by the
general formula m to q, where q is a whole integer representing the total
number of amino acid residues in the albumin fusion protein, and m is defined
as any integer ranging from 2 to q minus 6. Polynucleotides
encoding these polypeptides are also encompassed by the invention.
[0096] Also as mentioned above, even if deletion of one or more amino acids
from the N-terminus or C-terminus of a reference polypeptide
(e.g., a Therapeutic protein; serum albumin protein; or albumin fusion protein
of the invention) results in modification or loss of one or more
biological functions of the protein, other functional activities (e.g.,
biological activities, ability to multimerize, ability to bind a ligand)
and/or
Therapeutic activities may still be retained. For example the ability of
polypeptides with C-terminal deletions to induce and/or bind to antibodies
which recognize the complete or mature forms of the polypeptide generally will
be retained when less than the majority of the residues of the
complete or mature polypeptide are removed from the C-terminus. Whether a
particular polypeptide lacking the N-terminal and/or C-terminal
residues of a reference polypeptide retains Therapeutic activity can readily
be determined by routine methods described herein and/or otherwise
known in the art.
[00971 The present invention further provides polypeptides having one or more
residues deleted from the carboxy terminus of the amino acid
sequence of a Therapeutic protein corresponding to a Therapeutic protein
portion of an albumin fusion protein of the invention (e.g., a Therapeutic
protein referred to in Table 1, or a Therapeutic protein portion of an albumin
fusion protein encoded by a polynucleotide or albumin fusion construct
described in Table 2). In particular, C-terminal deletions may be described by
the general formula 1 to n, where n is any whole integer ranging from
6 to q minus 1, and where q is a whole integer representing the total number
of amino acid residues in a reference polypeptide (e.g., a Therapeutic
protein referred to in Table 1, or a Therapeutic protein portion of an albumin
fusion-protein encoded by a polynucleotide-oralbumin fusion construct
described in Table 2). Polynucleotides encoding these polypeptides are also
encompassed by the invention.
[00981 In addition, the present invention provides polypeptides having one or
more residues deleted from the carboxy terminus of the amino
acid sequence of an albumin protein corresponding to an albumin protein
portion of an albumin fusion protein of the invention (e.g., serum albumin
or an albumin protein portion of an albumin fusion protein encoded by a
polynucleotide or albumin fusion construct described in Table 2). In
particular, C-terminal deletions may be described by the general formula I to
n, where n is any whole integer ranging from 6 to 584, where 584 is
the whole integer representing the total number of amino acid residues in
mature human serum albumin (SEQ ID NO:1) minus 1. Polynucleotides
encoding these polypeptides are also encompassed by the invention. In
particular, C-terminal deletions may be described by the general formula 1 to
n, where n is any whole integer ranging from 6 to 608, where 608 is the whole
integer representing the total number of amino acid residues in serum
albumin (SEQ ID NO:3) minus 1. Polynucleotides encoding these polypeptides are
also encompassed by the invention.
[0099] Moreover, the present invention provides polypeptides having one or
more residues deleted from the carboxy terminus of an albumin
fusion protein of the invention. In particular, C-terminal deletions may be
described by the general formula 1 to n, where n is any whole integer
ranging from 6 to q minus 1, and where q is a whole integer representing the
total number of amino acid residues in an albumin fusion protein of the
invention. Polynucleotides encoding these polypeptides are also encompassed by
the invention.
10100] In addition, any of the above described N- or C-terminal deletions can
be combined to produce a N- and C-terminal deleted reference
polypeptide. The invention also provides polypeptides having one or more amino
acids deleted from both the amino and the carboxyl termini, which
may be described generally as having residues m to n of a reference
polypeptide (e.g., a Therapeutic protein referred to in Table 1, or a
Therapeutic
protein portion of an albumin fusion protein of the invention, or a
Therapeutic protein portion encoded by a polynucleotide or albumin fusion
construct described in Table 2, or serum albumin (e.g., SEQ ID NO:1), or an
albumin protein portion of an albumin fusion protein of the invention,
or an albumin protein portion encoded by a polynucleotide or albumin fusion
construct described in Table 2, or an albumin fusion protein, or an
albumin fusion protein encoded by a polynucleotide or albumin fusion construct
of the invention) where n and m are integers as described above.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[0101] The present application is also directed to proteins containing
polypeptides at least 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99%
32
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
_.. ..... . .... ....,. .,._ ~õ~.
identical to a reference polypeptide sequence (e.g., a Therapeutic protein
referred to in Table 1, or a Therapeutic protein portion of an albumin
fusion protein of the invention, or a Therapeutic protein portion encoded by a
polynucleotide or albumin fusion construct described in Table 2, or
serum albumin (e.g., SEQ ID NO: 1), or an albumin protein portion of an
albumin fusion protein of the invention, or an albumin protein portion
encoded by a polyuucleotide or albumin fusion construct described in Table 2,
or an albumin fusion protein, or an albumin fusion protein encoded
by a polynucleotide or albumin fusion construct of the invention) set forth
herein, or fragments tliereo~ In preferred embodiments, the application is
directed to proteins comprising polypeptides at least 80%, 85%, 90%, 95%, 96%,
97%, 98% or 99% identical to reference polypeptides having the
amino acid sequence of N- and C-terminal deletions as described above.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[01021 Preferred polypeptide fragments of the invention are fragments
comprising, or altematively, consisting of, an amino acid sequence that
displays a Therapeutic activity and/or functional activity (e.g, biological
activity) of the polypeptide sequence of the Therapeutic protein or serum
albumin protein of which the amino acid sequence is a fragment.
[0103] Other preferred polypeptide fragments are biologically active
fragments. Biologically active fragments are those exhibiting activity
similar, but not necessarily identical, to an activity of the polypeptide of
the present invention. The biological activity of the fragments may include
an improved desired activity, or a decreased undesirable activity.
isariaafs
[0104] "Variant" refers to a polynucleotide or nucleic acid differing from a
reference nucleic acid or polypeptide, but retaining essential
properties thereof. Generally, variants are overall closely similar, and, in
many regions, identical to the reference nucleic acid or polypeptide.
[0105] As used herein, "variant", refers to a Therapeutic protein portion of
an albumin fusion protein of the invention, albumin portion of an
albumin fusion protein of the invention, or albumin fusion protein of the
invention differing in sequence from a Therapeutic protein (e.g. see
"therapeutic" column of Table 1), albumin protein, and/or albumin fusion
protein, respectively, but retaining at least one functional and/or
therapeutic property thereof as described elsewhere herein or otherwise known
in the art. Generally, variants are overall very similar, and, in many
regions, identical to the amino acid sequence of the Therapeutic protein
corresponding to a Therapeutic protein portion of an albumin fusion protein,
albumin protein corresponding to an albumin protein portion of an albumin
fusion protein, and/or albumin fusion protein. Nucleic acids encoding
these variants are also encompassed by the invention.
[0106] The present invention is also directed to proteins which comprise, or
alternatively consist of, an amino acid sequence which is at least
80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% or 100%, identical to, for example, the
amino acid sequence of a Therapeutic protein corresponding to
a Therapeutic protein portion of an albumin fusion protein of the invention
(e.g., the amino acid sequence of a Therapeutic protein:X disclosed in
Table 1; or the amino acid sequence of a Therapeutic protein portion of an
albumin fusion protein encoded by a polynucleotide or albumin fusion
construct described in Table 1 and 2, or fragments or variants thereof),
albumin_proteins_correspondingto an albuniin protein portion of an albumin
fusion protein of the invention (e.g., the amino acid sequence of an albumin
protein portion of an albumin fusion protein encoded by a
polynucleotide or albumin fusion construct described in Table 1 and 2; the
amino acid sequence shown in SEQ ID NO: 1; or fragments or variants
thereof), and/or albumin fusion proteins. Fragments of these polypeptides are
also provided (e.g., those fragments described herein). Further
polypeptides encompassed by the invention are polypeptides encoded by
polynucleotides which hybridize to the complement of a nucleic acid
molecule encoding an albumin fusion protein of the invention under stringent
hybridization conditions (e.g., hybridization to filter bound DNA in
6X Sodium chloride/Sodium citrate (SSC) at about 45 degrees Celsius, followed
by one or more washes in 0.2X SSC, 0.1% SDS at about 50 - 65
degrees Celsius), under highly stringent conditions (e.g., hybridization to
filter bound DNA in 6X sodium chloride/Sodium citrate (SSC) at about 45
degrees Celsius, followed by one or more washes in 0.1X SSC, 0.2% SDS at about
68 degrees Celsius), or under other stringent hybridization
conditions which are known to those of skill in the art (see, for example,
Ausubel, F.M, et al., eds., 1989 Current protocol in Agolecular Biology,
Green publishing associates, Inc., and John Wiley & Sons Inc., New York, at
pages 6.3.1 - 6.3.6 and 2.10.3). Polynucleotides encoding these
polypeptides are also encompassed by the invention.
[0107] By a polypeptide having an amino acid sequence at least, for example,
95% "identical" to a query amino acid sequence, it is intended
that the amino acid sequence of the subject polypeptide is identical to the
query sequence except that the subject polypeptide sequence may include
up to five amino acid alterations per each 100 amino acids of the query amino
acid sequence. In other words, to obtain a polypeptide having an
amino acid sequence at least 95% identical to a query amino acid sequence, up
to 5% of the amino acid residues in the subject sequence may be
inserted, deleted, or substituted with another amino acid. These alterations
of the reference sequence may occur at the amino- or carboxy-terminal
positions of the reference amino acid sequence or anywhere between those
terminal positions, interspersed either individually among residues in the
reference sequence or in one or more contiguous groups within the reference
sequence.
[0108] As a practical matter, whether any particular polypeptide is at least
80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% identical to, for
instance, the amino acid sequence of an albumin fusion protein of the
invention or a fragment thereof (such as a Therapeutic protein portion of the
albumin fusion protein or an albumin portion of the albumin fusion protein),
can be determined conventionally using known computer programs. A
preferred method for determining the best overall match between a query
sequence (a sequence of the present invention) and a subject sequence,
33
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
also referred to as a global sequence alignment, can be determined using the
FASTDB computer program based on the algorithm of Brutlag et al.
(Comp. App. Biosci.6:237-245 (1990)). In a sequence alignment the query and
subject sequences are either both nucleotide sequences or both
aniino acid sequences. The result of said global sequence alignment is
expressed as percent identity. Prefarred parameters used in a FASTDB
amino acid alignment are: Matrix=PAM 0, k-tuple=2, Mismatch Penalty=l, Joining
Penalty=20, Randomization Group Length=0, Cutoff Score=l,
Window Size=sequence length, Gap Penalty=5, Gap Size Penalty=0.05, Window
Size=500 or the length of the subject amino acid sequence,
whichever is shorter.
[0109] If the subject sequence is shorter than the query sequence due to N- or
C-terminal deletions, not because of internal deletions, a manual
correction must be made to the results. This is because the FASTDB program
does not account for N- and C-terminal truncations of the subject
sequence when calculating global percent identity. For subject sequences
truncated at the N- and C-termini, relative to the query sequence, the
percent identity is corrected by calculating the number of residues of the
query sequence that are N- and C-terminal of the subject sequence, which
are not matched/aligned with a corresponding subject residue, as a percent of
the total bases of the query sequence. Whether a residue is
matched/aligned is determined by results of the FASTDB sequence alignment.
This percentage is then subtracted from the percent identity,
calculated by the above FASTDB program using the specified parameters, to
arrive at a final percent identity score. This final percent identity score
is what is used for the purposes of the present invention. Only residues to
the N- and C-termini of the subject sequence, which are not
matched/aligned with the query sequence, are considered for the purposes of
manually adjusting the percent identity score. That is, only query
residue positions outside the farthest N- and C- terminal residues of the
subject sequence.
[01101 For example, a 90 amino acid residue subject sequence is aligned with a
100 residue query sequence to determine percent identity. The
deletion occurs at the N-terminus of the subject sequetice and therefore, the
FASTDB alignment does not show a matching/alignment of the first 10
residues at the N-terminus. The 10 unpaired residues represent 10% of the
sequence (number of residues at the N- and C- termini not matched/total
number of residues in the query sequence) so 10% is subtracted from the
percent identity score calculated by the FASTDB program. If the
remaining 90 residues were perfectly matclied the final percent identity would
be 90%. In another example, a 90 residue subject sequence is
compared with a 100 residue query sequence. This time the deletions are
internal deletions so there are no residues at the N- or C-termini of the
subject sequence wliich are not matched/aligned with the query. In this case
the percent identity calculated by FASTDB is not manually corrected.
Once again, only residue positions outside the N- and C-terminal ends of the
subject sequence, as displayed in the FASTDB alignment, which are
not matched/aligned with the query sequence are manually corrected for. No
other manual corrections are to made for the purposes of the present
invention.
[01111 The variant will usually have at least 75% (preferably at least about
80%, 90%, 95% or 99%) sequence identity with a length of normal
HA or Therapeutic protein which is the same length as the variant. Homology or
identity at the nucleotide or amino acid sequence level is
determined by BLAST (Basic Local Alignment SearchTool)_ analysis using the
algorithm employed by the programs blastp,-blastn, blastx, tblastn - -
-- - -- - -
and tblastx (Karlin et al., Proc. Natl. Acad. Sci. USA 87: 2264-2268 (1990)
and Altschul, J. Mol. Evol. 36: 290-300 (1993), fully incorporated by
reference) which are tailored for sequence similarity searching.
[0112] The approach used by the BLAST program is to first consider similar
segments between a query sequence and a database sequence, then
to evaluate the statistical significance of all matches that are identified
and finally to summarize only those matches which satisfy a preselected
threshold of significance. For a discussion of basic issues in similarity
searching of sequence databases, see Altschul et al., (Nature Genetics 6:
119-129 (1994)) which is fully incorporated by reference. The search
parameters for histogram, descriptions, alignments, expect (i.e., the
statistical
significance threshold for reporting matches against database sequences),
cutoff, matrix and filter are at the default settings. The default scoring
matrix used by blastp, blastx, tblastn, and tblastx is the BLOSUM62 matrix
(Henikoff et al., Proc. Natl. Acad. Sci. USA 89: 10915-10919 (1992),
fully incorporated by reference). For blastn, the scoring matrix is set by the
ratios of M (i.e., the reward score for a pair of matching residues) to N
(i.e., the penalty score for mismatching residues), wherein the default values
for M and N are 5 and -4, respectively. Four blastn parameters may be
adjusted as follows: Q=10 (gap creation penalty); R=10 (gap extension
penalty); wink=l (generates word hits at every wink's position along the
query); and gapw=16 (sets the window width within which gapped alignments are
generated). The equivalent Blastp parameter settings were Q=9;
R=2; wink=l; and gapw=32. A Bestfit comparison between sequences, available in
the GCG package version 10.0, uses DNA parameters GAP=50
(gap creation penalty) and LEN=3 (gap extension penalty) and the equivalent
settings in protein comparisons are GAP=8 and LEN=2.
[0113] The polynucleotide variants of the invention may contain alterations in
the coding regions, non-coding regions, or both. Especially
preferred are polynucleotide variants containing alterations which produce
silent substitutions, additions, or deletions, but do not alter the properties
or activities of the encoded polypeptide. Nucleotide variants produced by
silent substitutions due to the degeneracy of the genetic code are
preferred. Moreover, polypeptide variants in which less than 50, less than 40,
less than 30, less than 20, less than 10, or 5-50, 5-25, 5-10, 1-5, or 1-2
amino acids are substituted, deleted, or added in any combination are also
preferred. Polynucleotide variants can be produced for a variety of
reasons, e.g., to optimize codon expression for a particular host (change
codons in the human mRNA to those preferred by a bacterial host, such as,
yeast or E. cali).
[0114] In a preferred embodiment, a polynucleotide of the invention which
encodes the albumin portion of an albumin fusion protein is
34
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
õ . õ~ .. . .... ..... . ....... ..... ..,'o ~tr .~ma,. ,.,ms~..
optim~zed for ..expression...... ..in yeast malian cells. In a further
preferred embodiment, a polynucleotide of the invention which encodes the
Therapeutic protein portion of an albumin fusion protein is optimized for
expression in yeast or mammalian cells. In a still further preferred
embodiment, a polynucleotide encoding an albumin fusion protein of the
invention is optimized for expression in yeast or mammalian cells.
[01151 In an altemative embodiment, a codon optimized polynucleotide which
encodes a Therapeutic protein portion of an albumin fusion
protein does not hybridize to the wild type polynucleotide encoding the
Therapeutic protein under stringent hybridization conditions as described
herein. In a further embodiment, a codon optimized polynucleotide which
encodes an albumin portion of an albumin fusion protein does not
hybridize to the wild type polynucleotide encoding the albumin protein under
stringent hybridization conditions as described herein. In another
embodiment, a codon optimized polynucleotide which encodes an albumin fusion
protein does not liybridize to the wild type polynucleotide
encoding the Therapeutic protein portion or the albumin protein portion under
stringent hybridization conditions as described herein.
[0116) In an additional embodiment, a polynucleotide which encodes a
Therapeutic protein portion of an albumin fusion protein does not
comprise, or alternatively consist of, the naturally occurring sequence of
that Therapeutic protein. In a further embodiment, a polynucleotide which
encodes an albumin protein portion of an albumin fusion protein does not
comprise, or alternatively consist of, the naturally occurring sequence of
albumin protein. In an alternative embodiment, a polynucleotide which encodes
an albumin fusion protein does not comprise, or alternatively
consist of, the naturally occurring sequence of a Therapeutic protein portion
or the albumin protein portion.
[0117] Naturally occurring variants are called "allelic variants," and refer
to one of several alternate forms of a gene occupying a given locus on
a chromosome of an organism. (Genes lI, Lewin, B., ed., John Wiley & Sons, New
York (1985)). These allelic variants can vary at either the
polynucleotide and/or polypeptide level and are included in the present
invention. Alternatively, non-naturally occurring variants may be produced
by mutagenesis techniques or by direct synthesis.
[0118] Using known methods of protein engineering and recombinant DNA
technology, variants may be generated to improve or alter the
characteristics of the polypeptides of the present invention. For instance,
one or more amino acids can be deleted from the N-terminus or C-
terrriinus of the polypeptide of the present invention witliout substantial
loss of biological function. As an example, Ron et al. (J. Biol. Chem. 268:
2984-2988 (1993)) reported variaut KGF proteins having heparin binding
activity even after deleting 3, 8, or 27 amino-terminal amino acid residues.
Similarly, Interferon gamma exhibited up to teu times higher activity after
deleting 8-10 amino acid residues from the carboxy terminus of this
protein. (Dobeli et al., J. Biotechnology 7:199-216 (1988).)
[01191 Moreover, ample evidence demonstrates that variants often retain a
biological activity similar to that of the naturally occurring protein.
For example, Gayle and coworkers (J. Biol. Chem. 268:22105-22111 (1993))
conducted extensive mutational analysis of human cytokine IL-la.
They used random mutagenesis to generate over 3,500 individual IL-la mutants
that averaged 2.5 amino acid changes per variant over the entire
length of the molecule. Multiple mutations were examined at every possible
amino acid position. The investigators found that "[m]ost of the
molecule could be altered with little effect on either [binding or
biological_activity]." In-fact, only 23-uniqueamino acid sequences, outofmore
than-
3,500 nucleotide sequences examined, produced a protein that significantly
differed in activity from wild-type.
[01201 Furthermore, even if deleting one or more amino acids from the N-
terminus or C-terminus of a polypeptide results in modification or
loss of one or more biological functions, other biological activities may
still be retained. For example, the ability of a deletion variant to induce
and/or to bind antibodies which recognize the secreted form will likely be
retained when less than the majority of the residues of the secreted form
are removed from the N-terminus or C-terminus. Whether a particular
polypeptide lacking N- or C-terminal residues of a protein retains such
immunogenic activities can readily be determined by routine methods described
herein and otherwise known in the art.
[0121) Thus, the invention further includes polypeptide variants which have a
functional activity (e.g., biological activity and/or therapeutic
activity). In one embodiment, the invention provides variants of albumin
fusion proteins that have a functional activity (e.g., biological activity
and/or therapeutic activity) that corresponds to one or more biological and/or
therapeutic activities of the Therapeutic protein corresponding to the
Therapeutic protein portion of the albumin fusion protein. In another
embodiment, the invention provides variants of albumin fusion proteins that
have a functional activity (e.g., biological activity and/or therapeutic
activity) that corresponds to one or more biological and/or therapeutic
activities
of the Therapeutic protein corresponding to the Therapeutic protein portion of
the albumin fusion protein. Such variants include deletions,
insertions, inversions, repeats, and substitutions selected according to
general rules known in the art so as have little effect on activity.
Polynucleotides encoding such variants are also encompassed by the invention.
[0122] In preferred embodinients, the variants of the invention have
conservative substitutions. By "conservative substitutions" is intended
swaps within groups such as replacement of the aliphatic or hydrophobic amino
acids Ala, Val, Leu and Ile; replacement of the hydroxyl residues
Ser and Thr; replacement of the acidic residues Asp and Glu; replacement of
the amide residues Asn and Gln, replacement of the basic residues Lys,
Arg, and His; replacement of the aromatic residues Phe, Tyr, and Trp, and
replacement of the small-sized amino acids Ala, Ser, Thr, Met, and Gly.
[0123) Guidance concerning how to make phenotypically silent amino acid
substitutions is provided, for example, in Bowie et al., "Deciphering
the Message in Protein Sequences: Tolerance to Amino Acid Substitutions,"
Science 247:1306-1310 (1990), wherein the authors indicate that there
are two main strategies for studying the tolerance of an amino acid sequence
to change.
[0124] The first strategy exploits the tolerance of amino acid substitutions
by natural selection during the process of evolution. By comparing
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
õ ,. -.1 - -,. ,...,,= ,.= .,,. . ... ... ,,.
amino acid sequences in different species, conserved amino acids can be
identified. These conserved amino acids are likely important for protein
function. In contrast, the amino acid positions where substitutions have been
tolerated by natural selection indicates that these positions are not
critical for protein function. Thus, positions tolerating amino acid
substitution could be modified while still maintaining biological activity of
the
protein.
[0125] The second strategy uses genetic engineering to introduce amino acid
changes at specific positions of a cloned gene to identify regions
critical for protein function. For example, site directed mutagenesis or
alanine-scanning mutagenesis (introduction of single alanine mutations at
every residue in the molecule) can be used. See Cunningham and Wells, Science
244:1081-1085 (1989). The resulting mutant molecules can then
be tested for biological activity.
[0126] As the authors state, these two strategies have revealed that proteins
are surprisingly tolerant of amino acid substitutions. The authors
further indicate whicli amino acid changes are likely to be permissive at
certain amino acid positions in the protein. For example, most buried
(within the tertiary structure of the protein) amino acid residues require
nonpolar side chains, whereas few features of surface side chains are
generally conserved. Moreover, tolerated conservative amino acid substitutions
involve replacement of the aliphatic or hydrophobic amino acids
Ala, Val, Leu and Ile; replacement of the hydroxyl residues Ser and Thr;
replacement of the acidic residues Asp and Glu; replacement of the amide
residues Asn and Gln, replacement of the basic residues Lys, Arg, and His;
replacement of the aromatic residues Plre, Tyr, and Trp, and replacement
of the small-sized amino acids Ala, Ser, Thr, Met, and Gly. Besides
conservative amino acid substitution, variants of the present invention
include
(i) polypeptides containing substitutions of one or more of the non-conserved
amino acid residues, where the substituted amino acid residues may or
may not be one encoded by the genetic code, or (ii) polypeptides containing
substitutions of one or more of the aniino acid residues having a
substituent group, or (iii) polypeptides which have been fused with or
chemically conjugated to another compound, such as a compound to increase
the stability and/or solubility of the polypeptide (for example, polyethylene
glycol), (iv) polypeptide containing additional amino acids, such as, for
example, an IgG Fc fusion region peptide. Such variant polypeptides are deemed
to be within the scope of those skilled in the art from the
teachings herein.
[01271 For example, polypeptide variants containing amino acid substitutions
of charged amino acids with other charged or neutral amino acids
may produce proteins with iniproved characteristics, such as less aggregation.
Aggregation of pharmaceutical formulations both reduces activity
and increases clearance due to the aggregate's immunogenic activity. See
Pinckard et a]., Clin. Exp. Immunol. 2:331-340 (1967); Robbins et al.,
Diabetes 36: 838-845 (1987); Cleland et al., Crit. Rev. Therapeutic Drug
Carrier Systems 10:307-377 (1993).
[0128] In specific embodiments, the polypeptides of the invention comprise, or
alternatively, consist of, fragments or variants of the amino acid
sequence of an albumin fusion protein, the amino acid sequence of a
Therapeutic protein and/or human serum albumin, wherein the fragments or
variants have 1-5, 5-10, 5-25, 5-50, 10-50 or 50-150, amino acid residue
additions, substitutions, and/or deletions when compared to the reference
amino acid sequence. In preferred embodiments,_the amino acid
substitutions_are conservative. - Nucleic acids encoding these polypeptides
are also
encompassed by the invention.
[0129] The polypeptide of the present invention can be composed of amino acids
joined to each other by peptide bonds or modified peptide
bonds, i.e., peptide isosteres, and may contain amino acids other than the 20
gene-encoded amino acids. The polypeptides may be modified by
either natural processes, such as post-translational processing, or by
chemical modification techniques which are well known in the art. Such
modifications are well described in basic texts and in more detailed
monographs, as well as in a voluminous researcir literature. Modifications can
occur anywhere in a polypeptide, including the peptide backbone, the amino
acid side-chains and the amino or carboxyl termini. It will be
appreciated that the same type of modification may be present in the same or
varying degrees at several sites in a given polypeptide. Also, a given
polypeptide may contain many types of modifications. Polypeptides may be
branched, for example, as a result of ubiquitination, and they may be
cyclic, with or without branching. Cyclic, branched, and branched cyclic
polypeptides may result from posttranslation natural processes or may be
made by synthetic methods. Modifications include acetylation, acylation, ADP-
ribosylation, amidation, covalent attachment of flavin, covalent
attachment of a heme moiety, covalent attachment of a nucleotide or nucleotide
derivative, covalent attachment of a lipid or lipid derivative, covalent
attachment of phosphotidylinositol, cross-linking, cyclization, disulfide bond
formation, demethylation, formation of covalent cross-links, formation
of cysteine, formation of pyroglutamate, formylation, gamma-carboxylation,
glycosylation, GPI anchor formation, hydroxylation, iodination,
methylation, myristylation, oxidation, pegylation, proteolytic processing,
phosphorylation, prenylation, racemization, selenoylation, sulfation,
transfer-RNA mediated addition of amino acids to proteins such as
arginylation, and ubiquitination. (See, for instance, PROTEINS - STRUCTURE
AND MOLECULAR PROPERTIES, 2nd Ed., T. E. Creighton, W. H. Freeman and Company,
New York (1993); POST-TRANSLATIONAL
COVALENT MODIFICATION OF PROTEINS, B. C. Johnson, Ed., Academic Press, New
York, pgs. 1-12 (1983); Seifter et al., Meth. Enzymol.
182:626-646 (1990); Rattan et al., Ann. N.Y. Acad. Sci. 663:48-62 (1992)).
Furrctional activily
[0130] "A polypeptide having functional activity" refers to a polypeptide
capable of displaying one or more known functional activities
associated with the fnll-length, pro-protein, and/or mature form of a
Therapeutic protein. Such functional activities include, but are not limited
to,
biological activity, antigenicity [ability to bind (or compete with a
polypeptide for binding) to an anti-polypeptide antibody], immunogenicity
36
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
,N,.,. . = - m.~, .~.~~. ,~,.~. ,. ~~~, . .~~~ ,~~.,
(ability to generate antibody which binds to a specific polypeptide of the
invention), ability to form multimers with polypeptides of the invention,
and ability to bind to a receptor or ligand for a polypeptide.
[0131] "A polypeptide having biological activity" refers to a polypeptide
exhibiting activity similar to, but not necessarily identical to, an activity
of a Therapeutic protein of the present invention, including mature forms, as
measured in a particular biological assay, with or without dose
dependency. In the case where dose dependency does exist, it need not be
identical to that of the polypeptide, but rather substantially similar to the
dose-dependence in a given activity as compared to the polypeptide of the
present invention (i.e., the candidate polypeptide will exhibit greater
activity or not more than about 25-fold less and, preferably, not more than
about tenfold less activity, and most preferably, not more than about
three-fold less activity relative to the polypeptide of the present
invention).
[0132] In preferred embodiments, an albumin fusion protein of the invention
has at least one biological and/or therapeutic activity associated
with the Therapeutic protein portion (or fragment or variant thereof) when it
is not fused to albumin.
[0133] In additional preferred embodiments, the albumin fusion protein of the
invention has an increased plasma stability compared to the
Therapeutic protein portion (or fragment or variant thereof) in an unfused
state. Plasma stability of the albumin fusion protein of the invention or of
the unfused Therapeutic protein portion (or fragment or variant thereof) can
be assayed using or routinely modifying assays known in the art.
[0134] The albumin fusion proteins of the invention can be assayed for
functional activity (e.g., biological activity) using or routinely modifying
assays known in the art, as well as assays described herein. Additionally, one
of skill in the art may routinely assay fragments of a Therapeutic
protein corresponding to a Therapeutic protein portion of an albumin fusion
protein, for activity using assays referenced in its corresponding row of
Table 1 (e.g., in column 3 of Table 1). Furtlier, one of skill in the art may
routinely assay fragments of an albumin protein corresponding to an
albumin protein portion of an albumin fusion protein, for activity using
assays known in the art and/or as described in the Examples section below.
[0135] For example, in one embodiment where one is assaying for the ability of
an albumin fusion protein to bind or compete with a
Therapeutic protein for binding to an anti-Therapeutic polypeptide antibody
and/or anti-albumin antibody, various immunoassays known in the art
can be used, including but not limited to, competitive and non-competitive
assay systems using techniques such as radioimmunoassays, ELISA
(enzyme linked immunosorbent assay), "sandwich" immunoassays,
immunoradiometric assays, gel diffusion precipitation reactions,
inununodiffusion assays, in situ immunoassays (using colloidal gold, enzyme or
radioisotope labels, for example), western blots, precipitation
reactions, agglutination assays (e.g., gel agglutination assays,
hemagglutination assays), complement fixation assays, immunofluorescence
assays,
proteha A assays, and immunoelectrophoresis assays, etc. In one embodiment,
antibody binding is detected by detecting a label on the primary
antibody. In another embodiment, the primary antibody is detected by detecting
binding of a secondary antibody or reagent to the primary antibody.
In a further embodiment, the secondary antibody is labeled. Many means are
known in the art for detecting binding in an immunoassay and are
within the scope of the present invention.
[0136] In a preferred embodiment, wherea binding partner-(e.g.,-areceptor- or-
a ligand) -of a-Therapeutic protein is identified,-binding to that
binding partner by an albumin fusion protein which comprises that Therapeutic
protein as the Therapeutic protein portion of the fusion can be
assayed, e.g., by means well-known in the art, such as, for example, reducing
and non-reducing gel chromatography, protein affmity
chromatography, and affinity blotting. See generally, Phizicky et al.,
Microbiol. Rev. 59:94-123 (1995). In another embodiment, the ability of
physiological correlates of an albumin fusion protein to bind to a
substrate(s) of the Therapeutic polypeptide con=esponding to the Therapeutic
protein portion of the fusion can be routinely assayed using techniques known
in the art.
[0137] In an alternative embodiment, where the ability of an albumin fusion
protein to multimerize is being evaluated, association with other
components of the multimer can be assayed, e.g., by means well-lmown in the
art, such as, for example, reducing and non-reducing gel
chromatography, protein affinity chromatography, and affmity blotting. See
generally, Phizicky et al., supra.
[0138] In preferred embodiments, an albumin fusion protein comprising all or a
portion of an antibody that binds a Therapeutic protein, has at
least one biological and/or therapeutic activity (e.g., to specifically bind a
polypeptide or epitope) associated with the antibody that binds a
Therapeutic protein (or fragment or variant thereof) when it is not fused to
albumin. In other preferred embodiments, the biological activity and/or
therapeutic activity of an albumin fusion protein comprising all or a portion
of an antibody that binds a Therapeutic protein is the inhibition (i.e.,
antagonism) or activation (i.e., agonism) of one or more of the biological
activities and/or therapeutic activities associated with the polypeptide that
is specifically bound by antibody that binds a Therapeutic protein.
[0139] Albumin fusion proteins comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein may be characterized in
a variety of ways. In particular, albumin fusion proteins comprising at least
a fragment or variant of an antibody that binds a Therapeutic protein
may be assayed for the ability to specifically bind to the same antigens
specifically bound by the antibody that binds a Therapeutic protein
corresponding to the Therapeutic protein portion of the albumin fusion protein
using techniques described herein or routinely modifying techniques
known in the art.
[0140] Assays for the ability of the albumin fusion proteins (e.g., comprising
at least a fragment or variant of an antibody that binds a
Therapeutic protein) to (specifically) bind a specific protein or epitope may
be performed in solution (e.g., Houghten, Bio/Techniques
13:412-421(1992)), on beads (e.g., Lam, Nature 354:82-84 (1991)), on chips
(e.g., Fodor, Nature 364:555-556 (1993)), on bacteria (e.g., U.S.
37
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Il..nc 1: a' fLl1.::df fL.F fllf d if:. '":ii[..::
Patent No. 5,223,409), on spores (e.g., Patent Nos. 5,571,698; 5,403,484; and
5,223,409), on plasmids (e.g., Cull et al., Proc. Nati. Acad. Sci. USA
89:1865-1869 (1992)) or on phage (e.g., Scott and Smith, Science 249:386-390
(1990); Devlin, Science 249:404-406 (1990); Cwirla et al., Proc.
Natl. Acad. Sci. USA 87:6378-6382 (1990); and Felici, J. Mol. Biol. 222:301-
310 (1991)) (each of these references is incorporated herein in its
entirety by reference). Albumin fusion proteins comprising at least a fragment
or variant of a Therapeutic antibody may also be assayed for their
specificity and affinity for a specific protein or epitope using or routinely
modifying techniques described herein or othenvise known in the art.
[01411 The albumin fusion proteins comprising at least a fragment or variant
of an antibody that binds a Therapeutic protein may be assayed for
cross-reactivity with other antigens (e.g., molecules that have
sequence/structure conservation with the molecule(s) specifically bound by the
antibody that binds a Therapeutic protein (or fragment or variant thereof)
corresponding to the Therapeutic protein portion of the albumin fusion
protein of the invention) by any method known in the art.
[01421 Immunoassays which can be used to analyze (immunospecific) binding and
cross-reactivity include, but are not limited to, competitive
and non-competitive assay systems using techniques such as western blots,
radioimmunoassays, ELISA (enzyme linked immunosorbent assay),
"sandwich" immunoassays, immunoprecipitation assays, precipitin reactions, gel
diffiasion precipitin reactions, immunodiffusion assays,
agglutination assays, complement-fixation assays, immunoradiometric assays,
fluorescent immunoassays, and protein A immunoassays, to name but
a few. Such assays are routine and well known in the art (see, e.g., Ausubel
et al, eds, 1994, Current Protocols in Molecular Biology, Vol. 1, John
Wiley & Sons, Inc., New York, which is incorporated by reference herein in its
entirety). Exemplary immunoassays are described briefly below
(but are not intended by way of limitation).
[0143] Immunoprecipitation protocols generally comprise lysing a population of
cells in a lysis buffer such as RIPA buffer (1% NP-40 or Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 0.15 M I3aC1, 0.01 M sodium
phospliate at pH 7.2, 1% Trasylol) supplemented with protein
phosphatase and/or protease inhibitors (e.g., EDTA, PMSF, aprotinin, sodium
vanadate), adding the albumin fusion protein of the invention (e.g.,
comprising at least a fragment or variant of an antibody that binds a
Therapeutic protein) to the cell lysate, incubating for a period of time
(e.g., I to
4 hours) at 40 degrees C, adding sepharose beads coupled to an anti-albumin
antibody, for example, to the cell lysate, incubating for about an hour
or more at 40 degrees C, washing the beads in lysis buffer and resuspending
the beads in SDS/sample buffer. The ability of the albumin fusion
protein to immunoprecipitate a particular antigen can be assessed by, e.g.,
western blot analysis. One of skill in the art would be knowledgeable as
to the parameters that can be modified to increase the binding of the albumin
fusion protein to an antigen and decrease the background (e.g., pre-
clearing the cell lysate with sepharose beads). For further discussion
regarding immunoprecipitation protocols see, e.g., Ausubel et al, eds, 1994,
Current Protocols in Molecular Biology, Vol. 1, John Wiley & Sons, Inc., New
York at 10.16.1.
[0144] Western blot analysis generally comprises preparing protein samples,
electrophoresis of the protein samples in a polyacrylamide gel (e.g.,
8%- 20% SDS-PAGE depending on the molecular weight of the antigen),
transferring the protein sample from the polyacrylamide gel to a
membrane such as nitrocellulose, PVDF or nylon,-blocking the membrane in
blocking solution (e.g.-; PBS with 3%BSA or non-fat milk), washing
the membrane in washing buffer (e.g., PBS-Tween 20), applying the albumin
fusion protein of the invention (diluted in blocking buffer) to the
membrane, washing the membrane in washing buffer, applying a secondary
antibody (which recognizes the albumin fusion protein, e.g., an anti-
human serum albumin antibody) conjugated to an enzymatic substrate (e.g.,
horseradish peroxidase or alkaline phosphatase) or radioactive molecule
(e.g., 32P or 1251) diluted in blocking buffer, washing the membrane in wash
buffer, and detecting the presence of the antigen. One of skill in the art
would be knowledgeable as to the parameters that can be modified to increase
the signal detected and to reduce the background noise. For further
discussion regarding western blot protocols see, e.g., Ausubel et al, eds,
1994, Current Protocols in Molecular Biology, Vol. 1, John Wiley & Sons,
Inc., New York at 10.8.1.
[0145] ELISAs comprise preparing antigen, coating the well of a 96-well
microtiter plate with the antigen, washing away antigen that did not
bind the wells, adding the albumin fusion protein (e.g., comprising at least a
fragment or variant of an antibody that binds a Therapeutic protein) of
the invention conjugated to a detectable compound such as an enzymatic
substrate (e.g., horseradish peroxidase or alkaline phosphatase) to the wells
and incubating for a period of time, washing away unbound or non-specifically
bound albumin fusion proteins, and detecting the presence of the
albumin fusion proteins specifically bound to the antigen coating the well. In
ELISAs the albumin fusion protein does not have to be conjugated to a
detectable compound; instead, a second antibody (which recognizes albumin
fusion protein) conjugated to a detectable compound may be added to
the well. Further, instead of coating the well with the antigen, the albumin
fusion protein may be coated to the well. In this case, the detectable
molecule could be the antigen conjugated to a detectable compound such as an
enzymatic substrate (e.g., horseradish peroxidase or alkaline
phosphatase). One of skill in the art would be knowledgeable as to the
parameters that can be modified to increase the signal detected as well as
other variations of ELISAs lniown in the art. For further discussion regarding
ELISAs see, e.g., Ausubel et al, eds, 1994, Current Protocols in
Molecular Biology, Vol. 1, John Wiley & Sons, Inc., New York at 11.2.1.
[01461 The binding affinity of an albumin fusion protein to a protein,
antigen, or epitope and the off-rate of an albumin fusion protein-
protein/antigen/epitope interaction can be determined by competitive binding
assays. One example of a competitive binding assay is a
radioimmunoassay comprising the incubation of labeled antigen (e.g., 3H or
1251) with the albumin fusion protein of the invention in the presence of
increasing amounts of unlabeled antigen, and the detection of the antibody
bound to the labeled antigen. The affinity of the albumin fusion protein
38
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
or , ,; ,~.~~,,.,,~, ~~,,,~. ,~,,,~= .. ~~,,,,, ,,,,, ,,,,,~ .,õ ~. ,.
a specific protein, antigen, or epitope and the binding off-rates can be
determined from the data by Scatchard plot analysis. Conipetition with a
second protein that binds the same protein, antigen or epitope as the albumin
fusion protein, can also be determined using radioinimunoassays. In
this case, the protein, antigen or epitope is incubated with an albumin fusion
protein conjugated to a labeled compound (e.g., 3H or 125I) in the
presence of increasing amounts of an unlabeled second protein that binds the
same protein, antigen, or epitope as the albumin fusion protein of the
invention.
[0147] In a preferred embodiment, BIAcore kinetic analysis is used to
determine the binding on and off rates of albumin fusion proteins of the
invention to a protein, antigen or epitope. BIAcore kinetic analysis comprises
analyzing the binding and dissociation of albumin fusion proteins, or
specific polypeptides, antigens or epitopes from chips with immobilized
specific polypeptides, antigens or epitopes or albumin fusion proteins,
respectively, on their surface.
[01481 Antibodies that bind a Therapeutic protein corresponding to the
Therapeutic protein portion of an albumin fusion protein may also be
described or specified in terms of their binding affinity for a given protein
or antigen, preferably the antigen which they specifically bind. Preferred
binding affinities include those with a dissociation constant or Kd less than
5 X 10'2 M, 10"2 M, 5 X 10 M, 10 M, 5 X 10"A M, 10-" M. More
preferred binding affinities include those with a dissociation constant or Kd
less than 5 X 10'S M, 10 M, 5 X 10'6 M, 10"6M, 5 X 10'7 M, 1W M, 5
X 10'$ M or 10'8 M. Even niore preferred binding affinities include those with
a dissociation constant or Kd less than 5 X 10"9 M, 10"9 M, 5 X 100
M, 100 M, 5 X 10'" M, 10-11 M, 5 X 10'12 M, 1042 M, 5 X 10'13 M, 10'" M, 5 X
10'" M, 10'14 M, 5 X 10'15 M, or 10'15 M. In preferred
embodiments, albumin fusion proteins comprising at least a fragment or variant
of an antibody that binds a Therapeutic protein, has an affinity for a
given protein or epitope similar to that of the corresponding antibody (not
fused to albumin) that binds a Therapeutic protein, taking into account the
valency of the albumin fusion protein (comprising at least a fragment or
variant of an antibody that binds a Therapeutic protein) and the valency of
the corresponding antibody. In addition, assays described herein (see Examples
and Table 1) and otherwise known in the art may routinely be
applied to measure the ability of albumin fusion proteins and fragments,
variants and derivatives thereof to elicit biological activity and/or
Therapeutic activity (either in vitro or in vivo) related to either the
Therapeutic protein portion and/or albumin portion of the albumin fusion
protein.
Other methods will be known to the skilled artisan and are within the scope of
the invention.
Albumin
[0149] As described above, an albumin fusion protein of the invention
comprises at least a fragment or variant of a Therapeutic protein and at
least a fragment or variant of human serum albumin, which are associated with
one another, preferably by genetic fusion.
[0150] An additional embodiment comprises at least a fragment or variant of a
Therapeutic protein and at least a fragment or variant of human
serum albumin, which are linked to one anotlier by chemical conjugation.
[0151] The terms, human serum albumin (HSA) and human albumin (HA) are used
interchangeably herein. The terms, "albumin and "serum
albumin" are broader, and encompass human serum. albumin (and-fragments and-
variants-thereof) as--well as-albumin from other species-(aiid
fragments and variants thereof).
[0152] As used herein, "albumin" refers collectively to albumin protein or
amino acid sequence, or an albumin fragment or variant, having one
or more functional activities (e.g., biological activities) of albumin. In
particular, "albumin" refers to human albumin or fragments thereof (see for
example, EP 201 239, EP 322 094 WO 97/24445, W095/23857) especially the mature
form of human albumin as shown in Figure 1 and SEQ ID
NO: 1, or albumin from other vertebrates or fragments thereof, or analogs or
variants of these molecules or fragments thereof.
[0153] In preferred embodiments, the human serum albumin protein used in the
albumin fusion proteins of the invention contains one or both of
the following sets of point mutations with reference to SEQ ID NO: 1: Leu-407
to Ala, Leu-408 to Val, Val-409 to Ala, and Arg410 to Ala; or Arg-
410 to A, Lys-413 to Gln, and Lys-414 to Gln (see, e.g., International
Publication No. W095/23857, hereby incorporated in its entirety by reference
herein). In even more preferred embodiments, albumin fusion proteins of the
invention that contain one or both of above-described sets of point
mutations have improved stability/resistance to yeast Yap3p proteolytic
cleavage, allowing increased production of recombinant albumin fusion
proteins expressed in yeast host cells.
[0154] As used herein, a portion of albumin sufficient to prolong the
therapeutic activity or plasma stability or shelf-life of the Therapeutic
protein refers to a portion of albumin sufficient in length or structure to
stabilize or prolong the therapeutic activity or plasma stability of the
protein
so that the shelf life or plasma stability of the Therapeutic protein portion
of the albumin fusion protein is prolonged or extended compared to the
shelf-life or plasma stability in the non-fusion state. The albumin portion of
the albumin fusion proteins may comprise the full length of the HA
sequence as described above, or may include one or more fragments thereof that
are capable of stabilizing or prolonging the therapeutic activity.
Such fragments may be of 10 or more amino acids in length or may include about
15, 20, 25, 30, 50, or more contiguous amino acids from the IiA
sequence or may include part or all of specific domains of HA. For instance,
one or more fragments of HA spanning the first two immunoglobulin-
like domains may be used. In a preferred embodiment, the HA fragment is the
mature form of HA.
[0155] The albumin portion of the albumin fusion proteins of the invention may
be a variant of normal HA. The Therapeutic protein portion of
the albumin fusion proteins of the invention may also be variants of the
Therapeutic proteins as described herein. The term "variants" includes
insertions, deletions and substitutions, either conservative or non
conservative, where such changes do not substantially alter one or more of the
39
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
..~.. .. .. ....... .... ....... .... .......
oncotic, useful ligand-binding and non-immunogenic properties of albumin, or
the active site, or active domain which confers the therapeutic
activities of the Therapeutic proteins.
[0156] In particular, the albumin fusion proteins of the invention may include
naturally occurring polymorphic variants of human albumin and
fragments of human albumin, for example those fragments disclosed in EP 322
094 (namely HA (Pn), where n is 369 to 419). The albumin may be
derived from any vertebrate, especially any mammal, for example human, cow,
sheep, or pig. Non-mammalian albumins include, but are not
limited to, hen and salmon. The albumin portion of the albumin fusion protein
may be from a different animal than the Therapeutic protein portion.
[0157] Generally speaking, an HA fragment or variant will be at least 100
amino acids long, preferably at least 150 amino acids long. The HA
variant may consist of or alternatively comprise at least one wliole domain of
HA, for example domains 1(amino acids 1-194 of SEQ ID NO: 1),
domain 2 (amino acids 195-387 of SEQ ID NO:1), domain 3 (amino acids 388-585
of SEQ ID NO:1), domains 1 and 2 (1-387 of SEQ ID NO:1),
domains 2 and 3 (195-585 of SEQ IDNO:1) or domains I and 3 (amino acids 1-194
of SEQ IDNO:1 and amino acids 388-585 of SEQ IDNO:1).
Each domain is itself made up of two homologous subdomains namely 1-105, 120-
194, 195-291, 316-387, 388-491 and 512-585, with flexible
inter-subdomain linker regions comprising residues Lys 106 to Glu119, G1u292
to Va1315 and G1u492 to A1a511.
[0158] Preferably, the albumin portion of an albumin fusion protein of the
invention comprises at least one subdomain or domain of HA or
conservative modifications thereof. If the fusion is based on subdomains, some
or all of the adjacent linker is preferably used to link to the
Therapeutic protein moiety.
Autibodies that Sneciricallt, bind Tizerapeutic proteins are also Theraneutic
nrateins
[0159] The present invention also encompasses albumin fusion proteins that
comprise at least a fragment or variant of an antibody that
specifically binds a Therapeutic protein disclosed in Table 1. It is
specifically contemplated that the term "Therapeutic protein" encompasses
antibodies that bind a Therapeutic protein (e.g., as Described in column I of
Table 1) and fragments and variants thereof. Thus an albumin fusion
protein of the invention may contain at least a fragment or variant of a
Therapeutic protein, and/or at least a fragment or variant of an antibody that
binds a Therapeutic protein.
Antibody structure and backgrourzd
[0160] The basic antibody structural unit is known to comprise a tetramer.
Each tetramer is composed of two identical pairs of polypeptide
chains, each pair having one "light" (about 25 kDa) and one "heavy" chain
(about 50-70 kDa). The amino-terminal portion of each chain includes a
variable region of about 100 to 110 or more amino acids primarily responsible
for antigen recognition. The carboxy-terminal portion of each chain
defines a constant region primarily responsible for effector function. Human
light chains are classified as kappa and lambda light chains. Heavy
chains are classified as mu, delta, gamma, alpha, or epsilon, and define the
antibody's isotype as IgM, IgD, 1gG, IgA, and IgE, respectively. See
generally, Fundanzental Immunology Chapters 3-5 (Paul, W., ed., 4th ed. Raven
Press, N.Y. (1998)) (incorporated by reference in its entirety for all
purposes . The variable regions of each lightlheavy chainpairfonn the antibody
binding site. -- ---- -- -- - -
- - --- -- -
[0161] Thus, an intact IgG antibody has two binding sites. Except in
bifunctional or bispecific antibodies, the two binding sites are the same.
[0162] The chains all exhibit the same general structure of relatively
conserved framework regions (FR) joined by three hypervariable regions,
also called complementarity determining regions or CDRs. The CDR regions, in
general, are the portions of the antibody which make contact with
the antigen and determine its specificity. The CDRs from the heavy and the
light chains of each pair are aligned by the framework regions, enabling
binding to a specific epitope. From N-terminal to C-terminal, both light and
heavy chains variable regions comprise the domains FRI, CDRl, FR2,
CDR2, FR3, CDR3 and FR4. The variable regions are connected to the heavy or
light chain constant region. The assignment of amino acids to each
domain is in accordance with the definitions of Kabat Segziences ofProteins
oflznrnunologicalInterest (National Institutes of Health, Bethesda, Md.
(1987 and 1991)), or Chothia & Lesk J Mol. Biol. 196:901-917 (1987); Chothia
et al. Nature 342:878-883 (1989).
[0163] As used herein, "antibody" refers to immunoglobulin molecules and
immunologically active portions of immunoglobulin molecules, i.e.,
molecules that contain an antigen binding site that specifically binds an
antigen (e.g., a molecule containing one or more CDR regions of an
antibody). Antibodies that may correspond to a Therapeutic protein portion of
an albumin fusion protein include, but are not limited to, monoclonal,
multispecific, human, humanized or chimeric antibodies, single chain
antibodies (e.g., single chain Fvs), Fab fragments, F(ab') fragments,
fragments produced by a Fab expression library, anti-idiotypic (anti-Id)
antibodies (including, e.g., anti-Id antibodies specific to antibodies of the
invention), and epitope-binding fragments of any of the above (e.g., VH
domains, VL domains, or one or more CDR regions).
Antibodies that bind Therapeutic Protefns
[0164] The present invention encompasses albumin fusion proteins that comprise
at least a fragment or variant of an antibody that binds a
Therapeutic Protein (e.g., as disclosed in Table 1) or fragment or variant
thereof.
[0165] Antibodies that bind a Therapeutic protein (or fragment or variant
thereof) may be from any animal origin, including birds and
manirnals. Preferably, the antibodies are human, murine (e.g., mouse and rat),
donkey, sheep, rabbit, goat, guinea pig, camel, horse, or chicken
antibodies. Most preferably, the antibodies are human antibodies. As used
herein, "human" antibodies include antibodies having the amino acid
sequence of a human immunoglobulin and include antibodies isolated from human
immunoglobulin libraries and xenomice or other organisms that
have been genetically engineered to produce human antibodies.
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
tL,n- tE a 11,.P n -iw tL.;.. R õB õ It it..
[01661 The antibody molecues tfiat bmd to a Therapeutic protein and that may
correspond to a Therapeutic protein portion of an albumin fusion
protein of the invention can be of any type (e.g., IgG, IgE, JgM, IgD, IgA and
IgY), class (e.g., IgGl, IgG2, IgG3, IgG4, IgAl and IgA2) or subclass
of immunoglobulin molecule. In preferred embodiments, the antibody molecules
that bind to a Therapeutic protein and that may correspond to a
Therapeutic protein portion of an albumin fusion protein are IgGI, In other
preferred embodiments, the immunoglobulin molecules that bind to a
Therapeutic protein and that may correspond to a Therapeutic protein portion
of an albumin fusion protein are IgG2. In other preferred
embodiments, the immunoglobulin molecules that bind to a Therapeutic protein
and that may correspond to a Therapeutic protein portion of an
albumin fusion protein are IgG4.
[01671 Most preferably the antibodies that bind to a Therapeutic protein and
that may correspond to a Therapeutic protein portion of an albumin
fusion protein are human antigen-binding antibody fragments of the present
invention and include, but are not limited to, Fab, Fab' and F(ab')2, Fd,
single-chain Fvs (scFv), single-chain antibodies, disulfide-linked Fvs (sdFv)
and fragments comprising either a VL or VH domain. Antigen-binding
antibody fragments, including single-chain antibodies, may comprise the
variable region(s) alone or in combination with the entirety or a portion of
the following: hinge region, CH1, CH2, and CH3 domains.
[01681 The antibodies that bind to a Therapeutic protein and that may
correspond to a Therapeutic protein portion of an albumin fusion protein
may be monospecific, bispecific, trispecific or of greater multispecificity.
Multispecific antibodies may be specific for different epitopes of a
Therapeutic protein or may be specific for both a Therapeutic protein as well
as for a heterologous epitope, such as a heterologous polypeptide or
solid support material. See, e.g., PCT publications WO 93/17715; WO 92/08802;
WO 91/00360; WO 92/05793; Tutt, et al., J. Immunol. 147:60-69
(1991); U.S. PatentNos. 4,474,893; 4,714,681; 4,925,648; 5,573,920; 5,601,819;
Kostelny et al., J. Immunol. 148:1547-1553 (1992).
[01691 Antibodies that bind a Therapeutic protein (or fragment or variant
thereof) may be bispecific or bifunctional which means that the
antibody is an artificial hybrid antibody having two different heavy/light
chain pairs and two different binding sites. Bispecific antibodies can be
produced by a variety of methods including fusion of hybridomas or linking of
Fab' fragments. See, e.g., Songsivilai & Lachmann Clin. Exp.
Iminunol. 79: 315-321 (1990), Kostelny et al. J Immunol. 148:1547 1553 (1992).
In addition, bispecific antibodies may be formed as "diabodies"
(Holliger et al. 'Diabodies': small bivalent and bispecific antibody
fragments" PNAS USA 90:6444-6448 (1993)) or "Janusins" (Traunecker et al.
"Bispecific single chain molecules (Janusins) target cytotoxic lympliocytes on
HIV infected cells" EA1BO J 10:3655-3659 (1991) and Traunecker et
al. "Janusin: new molecular design for bispecific reagents" Int JCaneer
Suppl7:51-52 (1992)).
[0170] The present invention also provides albumin fusion proteins that
comprise, fragments or variants (including derivatives) of an antibody
described herein or known elsewhere in the art. Standard techniques known to
those of skill in the art can be used to introduce mutations in the
nucleotide sequence encoding a molecule of the invention, including, for
example, site-directed mutagenesis and PCR-mediated mutagenesis which
result in amino acid substitutions. Preferably, the variants (including
derivatives) encode less than 50 amino acid substitutions, less than 40 amino
aeid substitutions, lessthan 30 amino acid_substitutions,.less than 25 amino
acid substitutions; less than 20 amino acid substitutions, less than 15
amino acid substitutions, less than 10 amino acid substitutions, less than 5
amino acid substitutions, less than 4 amino acid substitutions, less than 3
amino acid substitutions, or less than 2 amino acid substitutions relative to
the reference VH domain, VHCDRI, VHCDR2, VHCDR3, VL domain,
VLCDR1, VLCDR2, or VLCDR3. In specific embodiments, the variants encode
substitutions of VHCDR3. In a preferred embodiment, the
variants have conservative amino acid substitutions at one or more predicted
non-essential amino acid residues.
10171] Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein may
be described or specified in terms of the epitope(s) or portion(s) of a
Therapeutic protein which they recognize or specifically bind. Antibodies
which specifically bind a Therapeutic protein or a specific epitope of a
Therapeutic protein may also be excluded. Therefore, the present invention
encompasses antibodies that specifically bind Therapeutic proteins, and allows
for the exclusion of the same. In preferred embodiments, albumin
fusion proteins comprising at least a fragment or variant of an antibody that
binds a Therapeutic protein, binds the same epitopes as the unfused
fragment or variant of that antibody itself.
[01721 Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein may
also be described or specified in terms of their cross-reactivity. Antibodies
that do not bind any other analog, ortholog, or homolog of a Therapeutic
protein are included. Antibodies that bind polypeptides with at least 95%, at
least 90%, at least 85%, at least 80%, at least 75%, at least 70%, at
least 65%, at least 60%, at least 55%, and at least 50% sequence identity (as
calculated using methods larown in the art and described herein) to a
Therapeutic protein are also included in the present invention. In specific
embodiments, antibodies that bind to a Therapeutic protein and that may
correspond to a Therapeutic protein portion of an albumin fusion protein cross-
react with murine, rat and/or rabbit homologs of human proteins and
the corresponding epitopes thereof. Antibodies that do not bind polypeptides
with less than 95%, less than 90%, less than 85%, less than 80%, less
than 75%, less than 70%, less than 65%, less than 60%, less than 55%, and less
than 50% sequence identity (as calculated using methods known in
the art and described herein) to a Therapeutic protein are also included in
the present invention. In a specific embodiment, the above-described
cross-reactivity is with respect to any single specific antigenic or
imniunogenic polypeptide, or combination(s) of 2, 3, 4, 5, or more of the
specific
antigenic and/or immunogenic polypeptides disclosed herein. In preferred
embodiments, albumin fusion proteins comprising at least a fragment or
variant of an antibody that binds a Therapeutic protein, has similar or
substantially identical cross reactivity characteristics compared to the
fragment
41
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
sei,.
or,var~ ~p,.i=ant ~ ~,o ,., a t~ ,..rat"a.particuõn.lar,.,.an.,thnb.oyn it
:;a'
( .
[0173] Further included in the present invention are antibodies which bind
polypeptides encoded by polynucleotides which hybridize to a
polynucleotide encoding a Tlierapeutic protein under stringent hybridization
conditions (as described herein). Antibodies that bind to a Therapeutic
protein and that may correspond to a Therapeutic protein portion of an albumin
fusion protein of the invention may also be described or specified in
terms of their binding affinity to a polypeptide of the invention. Preferred
binding affinities include those with a dissociation constant or Kd less
than 5 X 10"2 M, 10'Z M, 5 X 10'3 M, 10'3 M, 5 X 104 M, 104 M. More preferred
binding affinities include those with a dissociation constant or Kd
less than 5 X 10'$ M, 10'S M, 5 X 10'6 M, 10'6M, 5 X 10-7 M, 10' M, 5 X 10's M
or 10'$ M. Even more preferred binding affinities include those
with a dissociation constant or Kd less than 5 X 10"9 M, 10'9 M, 5 X 10'10 M,
10'10 M, 5 X 10'" M, 10'" M, 5 X 10'12 M,tO'1Z M, 5 X 10'13 M, 10'l3
M, 5 X 10'14 M, 10-14 M, 5 X 10'15 M, or 10-15 M. In preferred embodiments,
albumin fusion proteins comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein, has an affinity for a given protein
or epitope similar to that of the corresponding antibody (not fused to
albumin) that binds a Therapeutic protein, taking into account the valency of
the albumin fusion protein (comprising at least a fragment or variant of
an antibody that binds a Therapeutic protein) and the valency of the
corresponding antibody.
[0174] The invention also provides antibodies that competitively inhibit
binding of an antibody to an epitope of a Therapeutic protein as
determined by any method known in the art for determining competitive binding,
for example, the immunoassays described herein. In preferred
embodiments, the antibody competitively inhibits binding to the epitope by at
least 95%, at least 90%, at least 85%, at least 80%, at least 75%, at
least 70%, at least 60%, or at least 50%. hi preferred embodiments, albumin
fusion proteins comprising at least a fragment or variant of an antibody
that binds a Therapeutic protein, competitively inhibits binding of a second
antibody to an epitope of a Therapeutic protein. In other preferred
embodiments, albumin fusion proteins comprising at least a fragment or variant
of an antibody that binds a Therapeutic protein, competitively
inhibits binding of a second antibody to an epitope of a Therapeutic protein
by at least 95%, at least 90%, at least 85 %, at least 80%, at least 75%,
at least 70%, at least 60%, or at least 50%.
[01751 Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein of
the invention may act as agonists or antagonists of the Therapeutic protein.
For example, the present invention includes antibodies which disrupt the
receptor/ligand interactions with the polypeptides of the invention either
partially or fully. The invention features both receptor-specific antibodies
and ligand-specific antibodies. The invention also features receptor-specific
antibodies which do not prevent ligand binding but prevent receptor
activation. Receptor activation (i.e., signaling) may be determined by
techniques described herein or otherwise known in the art. For example,
receptor activation can be determined by detecting the phosphorylation (e.g.,
tyrosine or serine/threonine) of the receptor or its substrate by
immunoprecipitation followed by westem blot analysis (for example, as
described supra). In specific embodiments, antibodies are provided that
inhibit ligand activity or receptor activity by at least 95%, at least 90%, at
least 85%, at least 80%, at least 75%, at least 70%, at least 60%, or at least
50% of the activity in absence of the antibody. In preferred embodiments,
albumin_fusion protenis comprising-at-least a fragment or variant of an
antibody that binds a Therapeutic protein, has similar or substantially
similar characteristics with regard to preventing ligand binding and/or
preventing receptor activation compared to an un-fused fragment or variant of
the antibody that binds the Therapeutic protein.
[0176] The invention also features receptor-specific antibodies which both
prevent ligand binding and receptor activation as well as antibodies
that recognize the receptor-ligand complex, and, preferably, do not
specifically recognize the unbound receptor or the unbound ligand. Likewise,
included in the invention are neutralizing antibodies which bind the ligand
and prevent binding of the ligand to the receptor, as well as antibodies
which bind the ligand, thereby preventing receptor ac.tivation, but do not
prevent the ligand from binding the receptor. Further included in the
invention are antibodies which activate -the receptor. These antibodies may
act as receptor agonists, i.e., potentiate or activate either all or a subset
of the biological activities of the ligand-mediated receptor activation, for
example, by inducing dimerization of the receptor. The antibodies may be
specified as agonists, antagonists or inverse agonists for biological
activities comprising the specific biological activities of the Therapeutic
proteins
(e.g. as disclosed in Table 1). The above antibody agonists can be made using
methods lmown in the art. See, e.g., PCT publication WO 96/40281;
U.S. Patent No. 5,811,097; Deng et al., Blood 92(6):1981-1988 (1998); Chen et
al., Cancer Res. 58(16):3668-3678 (1998); Harrop et al., J.
Irnmunol. 161(4):1786-1794 (1998); Zhu et al., Cancer Res. 58(15):3209-3214
(1998); Yoon et al., J. Immunol. 160(7):3170-3179 (1998); Prat et
al., J. Cell. Sci. 111(Pt2):237-247 (1998); Pitard et al., J. Immunol. Methods
205(2):177-190 (1997); Liautard et al., Cytokine 9(4):233-241 (1997);
Carlson et al., J. Biol. Chem. 272(17):11295-11301 (1997); Taryman et al.,
Neuron 14(4):755-762 (1995); Muller et al., Structure 6(9):1153-1167
(1998); Bartunek et al., Cytokine 8(1):14-20 (1996) (which are all
incorporated by reference herein in their entireties). In preferred
embodiments,
albumin fusion proteins comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein, have similar or substantially
identical agonist or antagonist properties as an un-fused fragment or variant
of the antibody that binds the Therapeutic protein.
[0177] Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein of
the invention may be used, for example, to purify, detect, and target
Therapeutic proteins, including both in in vitro and in vivo diagnostic and
therapeutic methods. For example, the antibodies have utility in immunoassays
for qualitatively and quantitatively measuring levels of the
Therapeutic protein in biological samples. See, e.g., Harlow et al.,
Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory Press, 2nd ed.
1988); incorporated by reference herein in its entirety. Likewise, albumin
fusion proteins comprising at least a fragment or variant of an antibody
42
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
...... .,,. .,.,. .,.. ..,...
that binds a Therapeutic protein, may be used, for example, to purify, detect,
and target Therapeutic proteins, including both in vitro and in vivo
diagnostic and therapeutic methods.
[0178] Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein
include derivatives that are modified, i.e., by the covalent attachment of any
type of molecule to the antibody. For example, but not by way of
limitation, the antibody derivatives include antibodies that have been
modified, e.g., by glycosylation, acetylation, pegylation, phosphorylation,
amidation, derivatization by known protecting/blocking groups, proteolytic
cleavage, linkage to a cellular ligand or other protein, etc. Any of
numerous chemical modifications may be carried out by known teclrniques,
including, but not limited to specific chemical cleavage, acetylation,
formylation, metabolic synthesis of tunicamycin, etc. Additionally, the
derivative may contain one or more non-classical amino acids. Albumin
fusion proteins of the invention may also be modified as described above.
Metleods ofProdacingAntibodies tleat bind Tberapeutic Proteites
[0179] The antibodies that bind to a Therapeutic protein and that may
correspond to a Therapeutic protein portion of an albumin fusion protein
of the invention may be generated by any suitable method known in the art.
Polyclonal antibodies to an antigen-of-interest can be produced by
various procedures well known in the art. For example, a Therapeutic protein
may be administered to various host animals including, but not
limited to, rabbits, mice, rats, etc. to induce the production of sera
containing polyclonal antibodies specific for the antigen. Various adjuvants
may
be used to increase the immunological response, depending on the host species,
and include but are not limited to, Freund's (complete and
incomplete), mineral gels such as aluminum hydroxide, surface active
substances such as lysolecithin, pluronic polyols, polyanions, peptides, oil
emulsions, keyhole limpet hemocyanins, dinitrophenol, and potentially useful
human adjuvants such as BCG (bacille Calmette-Guerin) and
corynebacterium parvum. Such adjuvants are also well known in the art.
[0180] Monoclonal antibodies can be prepared using a wide variety of
techniques known in the art including the use of hybridoma, recombinant,
and phage display technologies, or a combination thereof. For example,
monoclonal antibodies can be produced using hybridoma techniques
including those known in the art and taught, for example, in Harlow et al.,
Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory Press,
2nd ed. 1988); Hammerling, et al., in: Monoclonal Antibodies and T-Cell
Hybridomas 563-681 (Elsevier, N.Y., 1981) (said references incorporated
by reference in their entireties). The term "monoclonal antibody" as used
herein is not limited to antibodies produced through hybridoma
teclrnology. The term "monoclonal antibody" refers to an antibody that is
derived from a single clone, including any eukaryotic, prokaryotic, or
phage clone, and not the method by which it is produced.
[0181] Methods for producing and screening for specific antibodies using
hybridoma technology are routine and well known in the art. In a
non-limiting example, mice can be immunized with a Therapeutic protein or
fragment or variant thereof, an albumin fusion protein, or a cell
expressing such a Therapeutic protein or fragment or variant thereof or
albumin fusion protein. Once an immune response is detected, e.g.,
antibodies specific for the antigen are detected in the mouse serum, the mouse
spleen is harvested and splenocytes isolated. -The-splenocytes are -
then fused by well known techniques to any suitable myeloma cells, for example
cells from cell line SP20 available from the ATCC. Hybridomas
are selected and cloned by limited dilution. The hybridoma clones are then
assayed by methods known in the art for cells that secrete antibodies
capable of binding a polypeptide of the invention. Ascites fluid, which
generally contains high levels of antibodies, can be generated by immunizing
mice with positive hybridoma clones.
[0182] Accordingly, the present invention provides methods of generating
monoclonal antibodies as well as antibodies produced by the method
comprising culturing a hybridoma cell secreting an antibody wherein,
preferably, the hybridoma is generated by fusing splenocytes isolated from a
mouse inununized with an antigen of the invention with myeloma cells and then
screening the hybridomas resulting from the fusion for hybridoma
clones that secrete an antibody able to bind a polypeptide of the invention.
[0183] Another well known method for producing both polyclonal and monoclonal
human B cell lines is transformation using Epstein Barr
Virus (EBV). Protocols for generating EBV-transformed B cell lines are
commonly known in the art, such as, for example, the protocol outlined in
Chapter 7.22 of Current Protocols in Immunology, Coligan et al., Eds., 1994,
John Wiley & Sons, NY, which is hereby incorporated in its entirety
by reference. The source of B cells for transformation is commonly human
peripheral blood, but B cells for transformation may also be derived
from other sources including, but not limited to, lymph nodes, tonsil, spleen,
tumor tissue, and infected tissues. Tissues are generally made into
single cell suspensions prior to EBV transformation. Additionally, steps may
be taken to either physically remove or inactivate T cells (e.g., by
treatment with cyclosporin A) in B cell-containing samples, because T cells
from individuals seropositive for anti-EBV antibodies can suppress B
cell immortalization by EBV.
[0184] In general, the sample containing human B cells is innoculated with
EBV, and cultured for 3-4 weeks. A typical source of EBV is the
culture supematant of the B95-8 cell line (ATCC #VR-1492). Physical signs of
EBV transformation can generally be seen towards the end of the 3-
4 week culture period. By phase-contrast microscopy, transformed cells may
appear large, clear, hairy and tend to aggregate in tight clusters of
cells. Initially, EBV lines are generally polyclonal. However, over prolonged
periods of cell cultures, EBV lines may become monoclonal or
polyclonal as a result of the selective outgrowth of particular B cell clones.
Altematively, polyclonal EBV transformed lines may be subcloned (e.g.,
by limiting dilution culture) or fused with a suitable fusion parmer and
plated at limiting dilution to obtain monoclonal B cell lines. Suitable fusion
43
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
q.e4 B,. =xnV yoN , ll, a, ~1..,, ~1, ~
partners for E transformed= cell' iries'ihclude mouse myeloma cell lines
(e.g., SP2/0, X63-Ag8.653), heteromyeloma cell lines (human x mouse;
e.g, SPAM-8, SBC-H20, and CB-F7), and human cell lines (e.g., GM 1500, SKO-
007, RPMI 8226, and KR-4). Thus, the present invention also
provides a method of generating polyclonal or monoclonal human antibodies
against polypeptides of the invention or fragments thereof, comprising
EBV-transformation of human B cells.
[0185] Antibody fragments which recognize specific epitopes may be generated
by known techniques. For example, Fab and F(ab')2 fragments
of the invention may be produced by proteolytic cleavage of immunoglobulin
molecules, using enzymes such as papain (to produce Fab fragments)
or pepsin (to produce F(ab')2 fragments). F(ab')2 fragments contain the
variable region, the light chain constant region and the CHi domain of the
heavy chain.
[0186] For example, antibodies that bind to a Therapeutic protein can also be
generated using various phage display methods known in the art.
In phage display methods, functional antibody domains are displayed on the
surface of phage particles which carry the polynucleotide sequences
encoding them. In a particular embodiment, such phage can be utilized to
display antigen binding domains expressed from a repertoire or
combinatorial antibody library (e.g., human or murine). Phage expressing an
antigen binding domain that binds the antigen of interest can be
selected or identified with antigen, e.g., using labeled antigen or antigen
bound or captured to a solid surface or bead. Pliage used in these methods
are typically filamentous phage including fd and M13 binding domains expressed
from phage with Fab, Fv or disulfide stabilized Fv antibody
domains recombinantly fused to either the pliage gene III or gene VIII
protein. Examples of phage display methods that can be used to make
antibodies that bind to a Therapeutic protein include those disclosed in
Brinknian et al., J. Immunol. Methods 182:41-50 (1995); Ames et al., J.
Immunol. Methods 184:177-186 (1995); Kettleborough et al., Eur. S. Immunol.
24:952-958 (1994); Persic et al., Gene 187 9-18 (1997); Burton et
al., Advances in Jmmunology 57:191-280 (1994); PCT application No.
PCT/GB91/01134; PCT publications WO 90/02809; WO 91/10737; WO
92/01047; WO 92/18619; WO 93/11236; WO 95/15982; WO 95/20401; and U.S. Patent
Nos. 5,698,426; 5,223,409; 5,403,484; 5,580,717;
5,427,908; 5,750,753; 5,821,047; 5,571,698; 5,427,908; 5,516,637; 5,780,225;
5,658,727; 5,733,743 and 5,969,108; each of which is incorporated
herein by reference in its entirety.
[01871 As described in the above references, after phage selection, the
antibody coding regions from the phage can be isolated and used to
generate whole antibodies, including human antibodies, or any other desired
antigen binding fragment, and expressed in any desired host, including
mammalian cells, insect cells, plant cells, yeast, and bacteria, e.g., as
described in detail below. For example, techniques to recombinantly produce
Fab, Fab' and F(ab')2 fragments can also be employed using methods known in
the art such as those disclosed in PCT publication WO 92/22324;
Mullinax et al., BioTechniques 12(6):864-869 (1992); and Sawai et al., A3RI
34:26-34 (1995); and Better et al., Science 240:1041-1043 (1988)
(said references incorporated by reference in their entireties).
[0188] Examples of techniques which can be used to produce single-chain Fvs
and antibodies include those described in U.S. Patents 4,946,778
and 5,258,498; Huston et al., Methods in Enzymology_ 203;46-88 (1994);_ Shu et
al., PNAS -90:7995-7999-(1993); - and Skerra et al., Science
240:1038-1040 (1988). For some uses, including in vivo use of antibodies in
humans and in vitro detection assays, it may be preferable to use
chimeric, humanized, or human antibodies. A chimeric antibody is a molecule in
which different portions of the antibody are derived from different
animal species, such as antibodies having a variable region derived from a
murine monoclonal antibody and a human inimunoglobulin constant
region. Methods for producing chimeric antibodies are known in the art. See
e.g., Morrison, Science 229:1202 (1985); Oi et al., BioTechniques
4:214 (1986); Gillies et al., (1989) J. Immunol. Methods 125:191-202; U.S.
Patent Nos. 5,807,715; 4,816,567; and 4,816397, which are
incorporated herein by reference in their entirety. Humanized antibodies are
antibody molecules from non-human species antibody that binds the
desired antigen having one or more complementarity determining regions (CDRs)
from the non-human species and a framework regions from a
human imniunoglobulin molecule. Often, framework residues in the human
framework regions will be substituted with the corresponding residue
from the CDR donor antibody to alter, preferably improve, antigen binding.
These framework substitutions are identified by methods well known
in the art, e.g., by modeling of the interactions of the CDR and framework
residues to identify framework residues important for antigen binding
and sequence comparison to identify unusual framework residues at particular
positions. (See, e.g., Queen et al., U.S. Patent No. 5,585,089;
Rieohmann et al., Nature 332:323 (1988), which are incorporated lierein by
reference in their entireties.) Antibodies can be humanized using a
variety of techniques known in the art including, for example, CDR-grafting
(EP 239,400; PCT publication WO 91/09967; U.S. Patent Nos.
5,225,539; 5,530,101; and 5,585,089), veneering or resurfacing (EP 592,106; EP
519,596; Padlan, Molecular Immunology 28(4/5):489-498 (1991);
Studnicka et al., Protein Engineering 7(6):805-814 (1994); Roguska. et al.,
PNAS 91:969-973 (1994)), and chain shuffling (U.S. Patent No.
5,565,332).
[0189] Completely human antibodies are particularly desirable for therapeutic
treatment of human patients. Human antibodies can be made by a
variety of methods known in the art including phage display methods described
above using antibody libraries derived from human immunoglobulin
sequences. See also, U.S. Patent Nos. 4,444,887 and 4,716,111; and PCT
publications WO 98/46645, WO 98/50433, WO 98/24893, WO
98/16654, WO 96/34096, WO 96/33735, and WO 91/10741; each of which is
incorporated herein by reference in its entirety.
[0190] Human antibodies can also be produced using transgenic mice which are
incapable of expressing functional endogenous
inununoglobulins, but which can express human immunoglobulin genes. For
example, the human heavy and light chain immunoglobulin gene
44
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
õ .. .~.~~ ,~.,~. , , ,~,. .,
complexes may ~e ~nt.,roduced rariclom]y or by homologous recombination into
mouse embryonic stem cells. Altematively, the human variable
region, constant region, and diversity region may be introduced into mouse
embryonic stem cells in addition to the human heavy and light chain
genes. The mouse heavy and light chain immunoglobulin genes may be rendered
non-functional separately or simultaneously with the introduction
of human immunoglobulin loci by homologous recombination. In particular,
homozygous deletion of the JH region prevents endogenous antibody
production. The modified embryonic stem cells are expanded and microinjected
into blastocysts to produce chimeric mice. The chimeric mice are
tlien bred to produce homozygous offspring which express human antibodies. The
transgenic mice are immunized in the normal fashion with a
selected antigen, e.g., all or a portion of a polypeptide of the invention.
Monoclonal antibodies directed against the antigen can be obtained from the
immunized, transgenic mice using conventional hybridoma technology. The human
immunoglobulin transgenes harbored by the transgenic mice
rearrange during B cell differentiation, and subsequently undergo class
switching and somatic mutation. Thus, using such a technique, it is possible
to produce therapeutically useful IgG, IgA, IgM and IgE antibodies. For an
overview of this technology for producing human antibodies, see
Lonberg and Huszar, Int. Rev. Immunol. 13:65-93 (1995). For a detailed
discussion of this technology for producing human antibodies and human
monoclonal antibodies and protocols for producing such antibodies, see, e.g.,
PCT publications WO 98/24893; WO 92/01047; WO 96/34096; WO
96/33735; European Patent No. 0 598 877; U.S. Patent Nos. 5,413,923;
5,625,126; 5,633,425; 5,569,825; 5,661,016; 5,545,806; 5,814,318;
5,885,793; 5,916,771; 5,939,598; 6,075,181; and 6,114,598, whicli are
incorporated by reference herein in their entirety. In addition, companies
such as Abgenix, Inc. (Freemont, CA) and Genpharm (San Jose, CA) can be
engaged to provide human antibodies directed against a selected
antigen using technology similar to that described above.
[0191] Completely liuman antibodies which recognize a selected epitope can be
generated using a teclmique referred to as "guided selection."
In this approach a selected non-human monoclonal antibody, e.g., a mouse
antibody, is used to guide the selection of a completely human antibody
recognizing the same epitope. (Jespers et al., Bio/technology 12:899-903
(1988)).
Polyuucleotides Ei:codiugAutibodies
[01921 The invention further provides polynucleotides comprising a nucleotide
sequence encoding an antibody and fragments thereof. The
invention also encompasses polynucleotides that hybridize under stringent or
alternatively, under lower stringency hybridization conditions, e.g., as
defined supra, to polynucleotides that encode an antibody, preferably, that
specifically binds to a Therapeutic protein, and more preferably, an
antibody that binds to a polypeptide having the amino acid sequence of a
"Therapeutic protein:X" as disclosed in the "SEQ ID NO:Z" column of
Table 2.
[01931 The polynucleotides may be obtained, and the nueleotide sequence of the
polynucleotides determined, by any method known in the art.
For example, if the nucleotide sequence of the antibody is known, a
polynucleotide encoding the antibody may be assembled from chemically
synthesized oligonucleotides (e.g., as described in Kutmeier et al.,
BioTechniques 17:242 (1994)), which, briefly, involves the synthesis of
overlapping oligonucleotides containing portions of the secluence encoding_the
antibody, annealing and ligatingof those oligonucleotides, and-then-
_
amplification of the ligated oligonucleotides by PCR.
[01941 Alternatively, a polynucleotide encoding an antibody may be generated
from nucleic acid from a suitable source. If a clone containing a
nucleic acid encoding a particular antibody is not available, but the sequence
of the antibody molecule is known, a nucleic acid encoding the
immunoglobulin may be chemically synthesized or obtained from a suitable
source (e.g., an antibody eDNA library, or a cDNA library generated
from, or nucleic acid, preferably poly A+ RNA, isolated from, any tissue or
cells expressing the antibody, such as hybridoma cells selected to
express an antibody) by PCR amplification using synthetic primers hybridizable
to the 3' and 5' ends of the sequence or by cloning using an
oligonucleotide probe specific for the particular gene sequence to identify,
e.g., a cDNA clone from a cDNA library that encodes the antibody.
Amplified nucleic acids generated by PCR may then be cloned into replicable
cloning vectors using any method well known in the art (See Example
65).
[0195] Once the nucleotide sequence and corresponding amino acid sequence of
the antibody is determined, the nucleotide sequence of the
antibody may be manipulated using methods well known in the art for the
manipulation of nucleotide sequences, e.g., recombinant DNA techniques,
site directed mutagenesis, PCR, etc. (see, for example, the techniques
described in Sambrook et al., 1990, Molecular Cloning, A Laboratory Manual,
2d Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY and Ausubel et
al., eds., 1998, Current Protocols in Molecular Biology, John
Wiley & Sons, NY, which are both incorporated by reference herein in their
entireties ), to generate antibodies having a different amino acid
sequence, for example to create amino acid substitutions, deletions, and/or
insertions.
[0196] In a specific embodiment, the amino acid sequence of the heavy and/or
light chain variable domains may be inspected to identify the
sequences of the complementarity determining regions (CDRs) by methods that
are well know in the art, e.g., by comparison to known amino acid
sequences of other heavy and light chain variable regions to determine the
regions of sequence hypervariability. Using routine recombinant DNA
techniques, one or more of the CDRs may be inserted within framework regions,
e.g., into human framework regions to humanize a non-human
antibody, as described supra. The framework regions may be naturally occurring
or consensus framework regions, and preferably human
framework regions (see, e.g., Chothia et al., J. Mol. Biol. 278: 457-479
(1998) for a listing of human framework regions). Preferably, the
polynucleotide generated by the combination of the framework regions and CDRs
encodes an antibody that specifacally binds a polypeptide of the
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Il -- il...t. Id J; 'I=..L' ==' ll.r=. .o1 ..a.V n= tl+
invention. Prefera ly, as dtscussed's:rpra, one or more amino acid
substitutions may be made within the framework regions, and, preferably, the
amino acid substitutions improve binding of the antibody to its antigen.
Additionally, such methods may be used to make amino acid substitutions
or deletions of one or more variable region cysteine residues participating in
an intrachain disulfide bond to generate antibody molecules lacking one
or more intracliain disulfide bonds. Other alterations to the polynucleotide
are encompassed by the present invention and within the skill of the art.
[0197] In addition, tecliniques developed for the production of "chimeric
antibodies" (Morrison et at., Proc. Natl. Acad. Sci. 81:851-855 (1984);
Neuberger et al., Nature 312:604-608 (1984); Takeda et al., Nature 314:452-454
(1985)) by splicing genes from a mouse antibody molecule of
appropriate antigen specificity together with genes from a human antibody
molecule of appropriate biological activity can be used. As described
supra, a chimeric antibody is a molecule in which different portions are
derived from different animal species, such as those having a variable
region derived from a murine mAb and a liuman immunoglobulin constant region,
e.g., humanized antibodies.
[0198] Alternatively, techniques described for the production of single cltain
antibodies (U.S. Patent No. 4,946,778; Bird, Science 242:423- 42
(1988); Huston et al., Proc. Natl. Acad. Sci. USA 85:5879-5883 (1988); and
Ward et al., Nature 334:544-54 (1989)) can be adapted to produce
single chain antibodies. Single chain antibodies are formed by linking the
heavy and light chain fragments of the Fv region via an amino acid
bridge, resulting in a single chain polypeptide. Techniques for the assembly
of functional Fv fragments in E. coli may also be used (Skerra et al.,
Science 242:1038- 1041 (1988)).
Recombiriant Expression ofAntibodies
[0199] Recombinant expression of an antibody, or fragment, derivative or
analog thereof, (e.g., a heavy or light chain of an antibody or a single
chain antibody), requires construction of an expression vector containing a
polynucleotide that encodes the antibody. Once a polynucleotide
encoding an antibody molecule or a lieavy or light chain of an antibody, or
portion thereof (preferably containing the heavy or light chain variable
domain), of the invention has been obtained, the vector for the production of
the antibody molecule may be produced by recombinant DNA
technology using techniques well known in the art. Thus, methods for preparing
a protein by expressing a polynucleotide containing an antibody
encoding nucleotide sequence are described herein. Methods which are well
known to those skilled in the art can be used to construct expression
vectors containing antibody coding sequences and appropriate transcriptional
and translational control signals. These methods include, for example,
in vitro recombinant DNA techniques, synthetic techniques, and in vivo genetic
recombination. The invention, thus, provides replicable vectors
comprising a nucleotide sequence encoding an antibody molecule of the
invention, or a heavy or light chain thereof, or a heavy or light chain
variable domain, operably linked to a promoter. Such vectors may include the
nucleotide sequence encoding the constant region of the antibody
molecule (see, e.g., PCT Publication WO 86/05807; PCT Publication WO 89/01036;
and U.S. Patent No. 5,122,464) and the variable domain of
the antibody may be cloned into such a vector for expression of the entire
heavy or light chain.
[0200] The expression vector is transferred to a host cell by conventional
techniques and the transfected cells are then cultured by conventional
techniques to produce an antibody. Thus, the invention includes host cells
containing a polynucleotide encoding an-antibody of the invention, or a
heavy or light chain thereof, or a single chain antibody, operably linked to a
heterologous promoter. In preferred embodiments for the expression of
double-chained antibodies, vectors encoding both the heavy and light chains
may be co-expressed in the host cell for expression of the entire
immunoglobulin molecule, as detailed below.
[0201] A variety of host-expression vector systems may be utilized to express
the antibody molecules of the invention. Such host-expression
systems represent vehioles by which the coding sequences of interest may be
produced and subsequently purified, but also represent cells which
may, when transformed or transfected with the appropriate nucleotide coding
sequences, express an antibody molecule of the invention in situ.
These include but are not limited to microorganisms such as bacteria (e.g., E.
coli, B. subtilis) transformed with recombinant bacteriophage DNA,
plasmid DNA or cosmid DNA expression vectors containing antibody coding
sequences; yeast (e.g., Saccharomyces, Pichia) transformed with
recombinant yeast expression vectors containing antibody coding sequences;
insect cell systems infected with recombinant virus expression vectors
(e.g., baculovirus) containing antibody coding sequences; plant cell systems
infected with recombinant virus expression vectors (e.g., cauliflower
mosaic virus, CaMV; tobacco mosaic virus, TMV) or transformed with recombinant
plasmid expression vectors (e.g., Ti plasmid) containing
antibody coding sequences; or mammalian cell systems (e.g., COS, CHO, BHK,
293, 3T3 cells) harboring recombinant expression constructs
containing promoters derived from the genome of mammalian cells (e.g.,
metallothionein promoter) or from mammalian viruses (e.g., the
adenovirus late promoter; the vaccinia virus 7.5K promoter). Preferably,
bacterial cells such as Escherichia coli, and more preferably, eukaryotic
cells, especially for the expression of whole recombinant antibody molecule,
are used for the expression of a recombinant antibody molecule. For
example, mammalian cells such as Chinese hamster ovary cells (CHO), in
conjunction with a vector such as the major intermediate early gene
promoter element from human cytomegalovirus is an effective expression system
for antibodies (Foecking et al., Gene 45:101 (1986); Cockett et al.,
Bio/Technology 8:2 (1990)).
[0202] In bacterial systems, a number of expression vectors may be
advantageously selected depending upon the use intended for the antibody
molecule being expressed. For example, when a large quantity of such a protein
is to be produced, for the generation of pharmaceutical
compositions of an antibody molecule, vectors which direct the expression of
high levels of fusion protein products that are readily purified may be
desirable. Such vectors include, but are not limited, to the E. coli
expression vector pUR278 (Ruther et al., EMBO J. 2:1791 (1983)), in which the
46
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~s , , , ,,-. ..... .,.. ,. ,,. õ .-.,,.
antibody coding,,sequence,. .. may..be..Iigated individually into the vector
in frame with the lac Z coding region so that a fusion protein is produced;
pIN
vectors (Inouye & Inouye, Nucleic Acids Res. 13:3101-3109 (1985); Van Heeke &
Sclruster, J. Biol. Chem. 24:5503-5509 (1989)); and the like.
pGEX vectors may also be used to express foreign polypeptides as fusion
proteins with glutatirione S-transferase (GST). hi general, such fusion
proteins are soluble and can easily be purified from lysed cells by adsorption
and binding to matrix glutathione-agarose beads followed by elution in
the presence of free glutathione. The pGEX vectors are designed to include
thrombin or factor Xa protease cleavage sites so that the cloned target
gene product can be released from the GST moiety.
[0203] In an insect system, Autographa californica nuclear polyhedrosis virus
(AcNPV) is used as a vector to express foreign genes. The virus
grows in Spodoptera fi=ugiperda cells. The antibody coding sequence may be
cloned individually into non-essential regions (for example the
polyhedrin gene) of the virus and placed under control of an AcNPV promoter
(for example the polyhedrin promoter).
[0204] In mammalian host cells, a number of viral-based expression systems may
be utilized. hi cases where an adenovirus is used as an
expression vector, the antibody coding sequence of interest may be ligated to
an adenovirus transcription/translation control complex, e.g., the late
promoter and tripartite leader sequence. This chimeric gene may then be
inserted in the adenovirus genome by in vitro or in vivo recombination.
Insertion in a non- essential region of the viral genome (e.g., region El or
E3) will result in a recombinant virus that is viable and capable of
expressing the antibody molecule in infected hosts. (e.g., see Logan & Shenk,
Proc. Natl. Acad. Sci. USA 81:355-359 (1984)). Specific initiation
signals may also be required for efficient translation of inserted antibody
coding sequences. These signals include the ATG initiation codon and
adjacent sequences. Furthermore, the initiation codon must be in phase witli
the reading frame of the desired coding sequence to ensure translation
of the entire insert. These exogenous translational control signals and
initiation codons can be of a variety of origins, both natural and synthetic.
The efficiency of expression may be enhanced by the inclusion of appropriate
transcription enhancer elements, transcription terminators, etc. (see
Bittner et al., Methods in Enzymol. 153:51-544 (1987)).
[0205] In addition, a host cell strain may be chosen which modulates the
expression of the inserted sequences, or modifies and processes the
gene product in the specific fashion desired. Such modifications (e.g.,
glycosylation) and processing (e.g., cleavage) of protein products may be
important for the function of the protein. Different host cells have
characteristic and specific mechanisms for the post-translational processing
and
modification of proteins and gene products. Appropriate cell lines or host
systems can be chosen to ensure the correct modification and processing
of the foreign protein expressed. To this end, eukaryotie host cells which
possess the cellular machinery for proper processing of the primary
transcript, glycosylation, and phosphorylation of the gene product may be
used. Such mammalian host cells include but are not limited to CHO,
VERY, BHK, Hela, COS, MDCK, 293, 3T3, W138, and in particular, breast cancer
cell lines such as, for example, BT483, Hs578T, HTB2, BT20
and T47D, and normal mammary gland cell line such as, for example, CRL7030 and
Hs578Bst.
[02061 For long-term, high-yield production of recombinant proteins, stable
expression is preferred. For example, cell lines which stably
express the antibody molecule may be engineered._ Rather than using-expression
vectors which-contain viral origins of replication, host cells can be --
_
transformed with DNA controlled by appropriate expression control elements
(e.g., promoter, enhancer, sequences, transcription terminators,
polyadenylation sites, etc.), and a selectable marker. Following the
introduction of the foreign DNA, engineered cells may be allowed to grow for
1-2 days in an enriched media, and then are switched to a selective media. The
selectable marker in the recombinant plasmid confers resistance to
the selection and allows cells to stably integrate the plasmid into their
chromosomes and grow to form foci which in turn can be cloned and
expanded into cell lines. This method may advantageously be used to engineer
cell lines which express the antibody molecule. Such engineered
cell lines may be particularly useful in screening and evaluation of compounds
that interact directly or indirectly with the antibody molecule.
[02071 A number of selection systems may be used, including but not limited to
the herpes simplex virus thymidine kinase (Wigler et al., Cell
11:223 (1977)), hypoxanthine-guanine phosphoribosyltransferase (Szybalska &
Szybalski, Proc. Natl. Acad. Sci. USA 48:202 (1992)), and adenine
phosphoribosyltransferase (Lowy et al., Ce1122:817 (1980)) genes can be
employed in tk-, hgprt- or aprt- cells, respectively. Also, antimetabolite
resistance can be used as the basis of selection for the following genes:
dhfr, which confers resistance to methotrexate (Wigler et al., Natl. Acad.
Sci. USA 77:357 (1980); O'Hare et al., Proc. Nati. Acad. Sci. USA 78:1527
(1981)); gpt, wlrich confers resistance to mycophenolic acid (Mulligan
& Berg, Proc. Natl. Acad. Sci. USA 78:2072 (1981)); neo, which confers
resistance to the aminoglycoside G-418 Clinical Pharmacy 12:488-505;
Wu and Wu, Biotherapy 3:87-95 (1991); Tolstoshev, Ann. Rev. Pharmacol.
Toxicol. 32:573-596 (1993); Mulligan, Science 260:926-932 (1993);
and Morgan and Anderson, Ann. Rev. Biochem. 62:191-217 (1993); May, 1993, TIB
TECH 11(5):155-215 (1993)); and hygro, whiclr confers
resistance to hygromycin (Santerre et al., Gene 30:147 (1984)). Methods
conunonly known in the art of recombinant DNA technology may be
routinely applied to select the desired recombinant clone, and such methods
are described, for example, in Ausubel et al. (eds.), Current Protocols
in Molecular Biology, John Wiley & Sons, NY (1993); Kriegler, Gene Transfer
and Expression, A Laboratory Manual, Stockton Press, NY (1990);
and in Chapters 12 and 13, Dracopoli et al. (eds), Current Protocols in Human
Genetics, John Wiley & Sons, NY (1994); Colberre-Garapni et al., J.
Mol. Biol. 150:1 (1981), which are incorporated by reference herein in their
entireties.
[0208] The expression levels of an antibody molecule can be increased by
vector amplification (for a review, see Bebbington and Hentschel,
The use of vectors based on gene amplification for the expression of cloned
genes in mammalian cells in DNA cloning, Vol.3. (Academic Press,
New York, 1987)). When a marker in the vector system expressing antibody is
amplifiable, increase in the level of inhibitor present in culture of
47
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
=I ~ r ~ n ~i,.~~ ,~õ~, õ ,u.... ..,o= ,,.,~. .,,= ,..~~..
host cel wi1' increase the number of copies of the marker gene. Since the
amplified region is associated with the antibody gene, production of the
antibody will also increase (Crouse et al., Mol. Cell. Biol. 3:257 (1983)).
[02091 Vectors whicli use glutamine synthase (GS) or DHFR as the selectable
markers can be amplified in the presence of the drugs methionine
sulphoximine or methotrexate, respectively. An advantage of glutamine synthase
based vectors are the availability of cell lines (e.g., the murine
myeloma cell line, NSO) which are glutamine synthase negative. Glutamine
synthase expression systems can also function in glutamine synthase
expressing cells (e.g. Chinese Hamster Ovary (CHO) cells) by providing
additional inhibitor to prevent the functioning of the endogenous gene. A
glutamine synthase expression system and components thereof are detailed in
PCT publications: W087J04462; W086/05807; W089/01036;
W089/10404; and W091/06657 which are incorporated in their entireties by
reference herein. Additionally, glutamine synthase expression vectors
that may be used according to the present invention are commercially available
from suppliers, including, for example Lonza Biologics, Inc.
(Portsmouth, NH). Expression and production of monoclonal antibodies using a
GS expression system in murine myeloma cells is described in
Bebbington et al., Bio/techrzology 10:169(1992) and in Biblia and Robinson
BiotecluioT. Prog. 11:1 (1995) which are incorporated in their entireties
by reference herein.
[0210] The host cell may be co-transfected with two expression vectors of the
invention, the first vector encoding a heavy chain derived
polypeptide and the second vector encoding a liglit chain derived polypeptide.
The two vectors may contain identical selectable markers which
enable equal expression of heavy and light chain polypeptides. Alternatively,
a single vector may be used which encodes, and is capable of
expressing, both heavy and light chain polypeptides. In such situations, the
light chain should be placed before the heavy chain to avoid an excess
of toxic free heavy chain (Proudfoot, Nature 322:52 (1986); Kohler, Proc.
Natl. Acad. Sci. USA 77:2197 (1980)). The coding sequences for the
heavy and light chains may comprise oDNA or genomic DNA.
[0211] Once an antibody molecule of the invention has been produced by an
animal, chemically synthesized, or recombinantly expressed, it may
be purified by any method known in the art for purification of an
immunoglobulin molecule, for example, by chromatography (e.g., ion exchange,
affinity, particularly by affinity for the specific antigen after Protein A,
and sizing column chromatography), centrifugation, differential solubility, or
by any other standard technique for the purification of proteins. In addition,
the antibodies that bind to a Therapeutic protein and that may
correspond to a Therapeutic protein portion of an albumin fusion protein of
the invention or fragments thereof can be fused to heterologous
polypeptide sequences described herein or otherwise known in the art, to
facilitate purification.
Modifications ofAntibodies
[0212] Antibodies that bind a Therapeutic protein or fragments or variants can
be fused to marker sequences, such as a peptide to facilitate
purification. In preferred embodiments, the marker amino acid sequence is a
hexa-histidine peptide, such as the tag provided in a pQE vector
(QIAGEN, hic., 9259 Eton Avenue, Chatsworth, CA, 91311), among others, many of
which are commercially available. As described in Gentz et
al., Proo. Natl. Acad. Sci. USA 86:821-824 _(1989), for instance,_hexa
histidine- provides for convenient purification of the fusion protein. Other -
peptide tags useful for purification include, but are not limited to, the
hemagglutinin tag (also called the "HA tag"), which corresponds to an epitope
derived from the influenza hemagglutinin protein (Wilson et al., Cell 37:767
(1984)) and the "flag" tag.
[02131 The present invention further encompasses antibodies or fragments
thereof conjugated to a diagnostic or therapeutic agent. The
antibodies can be used diagnostically to, for example, monitor the development
or progression of a tumor as part of a clinical testing procedure to,
e.g., determine the efficacy of a given treatment regimen. Detection can be
facilitated by coupling the antibody to a detectable substance.
Examples of detectable substances include various enzymes, prosthetic groups,
fluorescent materials, luminescent materials, bioluminescent
materials, radioactive materials, positron emitting metals using various
positron emission tomographies, and nonradioactive paramagnetic metal
ions. The detectable substance may be coupled or conjugated either directly to
the antibody (or fragment thereof) or indirectly, through an
intermediate (such as, for example, a linker known in the art) using
techniques laiown in the art. See, for example, U.S. Patent No. 4,741,900 for
metal ions which can be conjugated to antibodies for use as diagnostics
according to the present invention. Examples of suitable enzymes include
horseradish peroxidase, alkaline phosphatase, beta-galactosidase, or
acetylcholinesterase; examples of suitable prosthetic group complexes include
streptavidin/biotin and avidinJbiotin; examples of suitable fluorescent
materials include umbelliferone, fluorescein, fluorescein isothiocyanate,
rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride or
phycoerythrin; an example of a luminescent material includes luminol; examples
of bioluminescent materials include luciferase, luciferin, and aequorin; and
exaniples of suitable radioactive material include 125I, 131I, 111In or
99Tc. Other examples of detectable substances have been described elsewhere
herein.
[02141 Further, an antibody of the invention may be conjugated to a
therapeutic moiety such as a cytotoxin, e.g., a cytostatic or cytocidal agent,
a therapeutic agent or a radioactive metal ion, e.g., alpha-emitters such as,
for example, 213Bi. A cytotoxin or cytotoxic agent includes any agent
that is detrimental to cells. Examples include paclitaxol, cytochalasin B,
gramicidin D, ethidium bromide, emetine, mitomycin, etoposide,
tenoposide, vincristine, vinblastine, colchicin, doxorubicin, daunorubicin,
dihydroxy anthracin dione, mitoxantrone, mithramycin, actinomycin D,
1-dehydrotestosterone, glucocorticoids, procaine, tetracaine, lidocaine,
propranolol, and puromycin and analogs or homologs thereof. Therapeutic
agents include, but are not limited to, antimetabolites (e.g., methotrexate, 6-
mercaptopurine, 6-thioguanine, cytarabine, 5-fluorouracil decarbazine),
all.ylating agents (e.g., mechlorethamine, thioepa chlorambucil, melphalan,
carmustine (BSNU) and lomustine (CCNU), cyclothosphamide,
48
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
õ w ~l,~ u ,,,..,, õ~ ... .. . . ..... ....,.,,~, :;;,,. ,~~~,. .
busulfan.~, ~iTiromomannrto , streptozotocin, mitomyoin C, and cis-
dichlorodiamine platinum (II) (DDP) cisplatin), anthracyclines (e.g.,
daunorubicin
(formerly daunomycin) and doxorubicin), antibiotics (e.g., dactinomycin
(formerly actinomycin), bleomycin, mithramycin, and anthramycin
(AMC)), and anti-mitotic agents (e.g., vincristine and vinblastine).
[0215] The conjugates of the invention can be used for modifying a given
biological response, the therapeutic agent or drug moiety is not to be
construed as limited to classical chemical therapeutic agents. For example,
the dmg moiety may be a protein or polypeptide possessing a desired
biological activity. Such proteins may include, for example, a toxin such as
abrin, ricin A, pseudomonas exotoxin, or diphtheria toxin; a protein
such as tumor necrosis factor, alpha-interferon, 13-interferon, nerve growtli
factor, platelet derived growth factor, tissue plasminogen activator, an
apoptotic agent, e.g., TNF-alpha, TNF-beta, AIM I (See, Intemational
Publication No. WO 97/33899), AIM II(See, International Publication No.
WO 97/34911), Fas Ligand (Takahashi et al., Int. ImmunoT., 6:1567-1574
(1994)), VEGI (See, International Publication No. WO 99/23105), a
thrombotic agent or an anti- angiogenic agent, e.g., angiostatin or
endostatin; or, biological response modifiers such as, for example,
lymphokines,
interleukin-1 interleukin-2 ("IL-2"), interleukin-6 ("IL-6"), granulocyte
macrophage colony stimulating factor ("GM-CSF"), granulocyte
colony stimulating factor ("G-CSF"), or other growth factors.
[0216] Antibodies may also be attached to solid supports, which are
particularly useful for immunoassays or purification of the target antigeh.
Such solid supports include, but are not limited to, glass, cellulose,
polyacrylamide, nylon, polystyrene, polyvinyl chloride or polypropylene.
[0217] Techniques for conjugating such therapeutic moiety to antibodies are
well known. See, for example, Arnon et al., "Monoclonal
Antibodies For Immunotargeting Of Drugs In Cancer Therapy", in Monoclonal
Antibodies And Cancer Therapy, Reisfeld et al. (eds.), pp. 243-56
(Alan R. Liss, Inc. 1985); Hellstrom et al., "Antibodies For Drug Delivery",
in Controlled Drug Delivery (2nd Ed.), Robinson et al. (eds.), pp. 623-
53 (Marcel Dekker, Inc. 1987); Thorpe, "Antibody Carriers Of Cytotoxic Agents
In Cancer Therapy: A Review", in Monoclonal Antibodies '84:
Biological And Clinical Applications, Pinchera et al. (eds.), pp. 475-506
(1985); "Analysis, Results, And Future Prospective Of The Therapeutic
Use Of Radiolabeled Antibody In Cancer Therapy", in Monoclonal Antibodies For
Cancer Detection And Therapy, Baldwin et al. (eds.), pp. 303-16
(Academic Press 1985), and Thorpe et al., "The Preparation And Cytotoxic
Properties Of Antibody-Toxin Conjugates", Immunol. Rev. 62:119-58
(1982).
[02181 Altematively, an antibody can be conjugated to a second antibody to
form an antibody heteroconjugate as described by Segal in U.S.
Patent No. 4,676,980, which is incorporated herein by reference in its
entirety.
[02191 An antibody, with or without a therapeutic moiety conjugated to it,
administered alone or in combination with cytotoxic factor(s) and/or
cytokine(s) can be used as a therapeutic.
Antibody-albumiu fusion
[02201 Antibodies that bind to a Therapeutic protein and that may correspond
to a Therapeutic protein portion of an albumin fusion protein of
the invention include, but are not limited to, antibodies_that-bind_a
Therapeutic protein disclosed-in the"Therapeutic Protein X" column of Table 1,
or a fragment or variant thereof.
[02211 In specific embodiments, the fragment or variant of an antibody that
immunospecifcally binds a Therapeutic protein and that
corresponds to a Therapeutic protein portion of an albumin fusion protein
comprises, or alternatively consists of, the VH domain. In other
embodiments, the fragment or variant of an antibody that immunospecifcally
binds a Therapeutic protein and that corresponds to a Therapeutic
protein portion of an albumin fusion protein comprises, or alternatively
consists of, one, two or three VH CDRs. In other embodiments, the
fragment or variant of an antibody that immunospecifcally binds a Therapeutic
protein and that corresponds to a Therapeutic protein portion of an
albumin fusion protein comprises, or alternatively consists of, the VH CDRI.
In other embodiments, the fragment or variant of an antibody that
immunospecifoally binds a Therapeutic protein and that corresponds to a
Therapeutic protein portion of an albumin fusion protein comprises, or
alternatively consists of, the VH CDR2. In other embodiments, the fragment or
variant of an antibody that immunospecifcally binds a Therapeutic
protein and that corresponds to a Therapeutic protein portion of an albumin
fusion protein comprises, or alternatively consists of, the VH CDR3.
[0222] In specific embodiments, the fragment or variant of an antibody that
immunospecifcally binds a Therapeutic protein and that
corresponds to a Therapeutic protein portion of an albumin fusion protein
comprises, or alternatively consists of, the VL domain. In other
embodiments, the fragment or variant of an antibody that immunospecifcally
binds a Therapeutic protein and that corresponds to a Therapeutic
protein portion of an albumin fusion protein comprises, or altematively
consists of, one, two or three VL CDRs. In other embodiments, the
fragment or variant of an antibody that immunospecifcally binds a Therapeutic
protein and that corresponds to a Therapeutic protein portion of an
albumin fusion protein comprises, or alternatively consists of, the VL CDR1.
In other embodiments, the fragment or variant of an antibody that
immunospecifcally binds a Therapeutic protein and that corresponds to a
Therapeutic protein portion of an albumin fusion protein coniprises, or
alternatively consists of, the VL CDR2. In other embodiments, the fragment or
variant of an antibody that inununospecifcally binds a Therapeutic
protein and that corresponds to a Therapeutic protein portion of an albumin
fusion protein comprises, or altematively consists o f the VL CDR3.
[02231 In other embodiments, the fragment or variant of an antibody that
immunospecifcally binds a Therapeutic protein and that corresponds
to a Therapeutic protein portion of an albumin fusion protein comprises, or
alternatively consists of, one, two, three, four, five, or six VH and/or VL
CDRs.
49
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
." +6...t. .r 't...l+ .'Tn ~dt q.Idete'irUed u'~ em'b IG:-". "..od.iPim ~.e::
t1nts; th.,.i0 .d6.
224] pre fragment or variant of an antibody that immunospecifically binds a
Therapeutic protein and that
corresponds to a Therapeutic protein portion of an albumin fusion protein
comprises, or alternatively consists of, an scFv comprising the VH domain
of the Therapeutic antibody, linked to the VL domain of the therapeutic
antibody by a peptide linker such as (Gly4Ser)3 (SEQ ID NO:4).
Iutntuuoplreuot} ping
[0225] The antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that
binds a Therapeutic protein (or fragment or variant thereof) may be utilized
for immunophenotyping of cell lines and biological samples.
Therapeutic proteins of the present invention may be useful as cell-specific
markers, or more specifically as cellular markers that are differentially
expressed at various stages of differentiation and/or maturation of particular
cell types. Monoclonal antibodies (or albumin fusion proteins
comprising at least a fragment or variant of an antibody that binds a
Therapeutic protein) directed against a specific epitope, or combination of
epitopes, will allow for the screening of cellular populations expressing the
marker. Various techniques can be utilized using monoclonal antibodies
(or albumin fusion proteins comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein) to screen for cellular
populations expressing the marker(s), and include magnetic separation using
antibody-coated magnetic beads, "panning" with antibody attached to a
solid matrix (i.e., plate), and flow cytometry (See, e.g., U.S. Patent
5,985,660; and Morrison et al., Cell, 96:737-49 (1999)).
[0226] These teclrniques allow for the screening of particular populations of
cells, such as might be found with hematological malignancies (i.e.
minimal residual disease (MRD) in acute leukemic patients) and "non-self'
cells in transplantations to prevent Graft-versus-Host Disease (GVHD).
Alternatively, these techniques allow for the screening of hematopoietic stem
and progenitor cells capable of undergoing proliferation and/or
differentiation, as might be found in human unibilical cord blood.
Characteriziug Antibodies tltat butd a Tlterapeutic Protein and Albumin Fusioa
Proteins Contprisiug a Fragutent or Variant of an
Aatibody that binds a Tlterapeutic Proteitt
[0227] The antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that
binds a Therapeutic protein (or fragment or variant thereof) may be
characterized in a variety of ways. In particular, Albumin fusion proteins of
the
invention comprising at least a fragment or variant of an antibody that binds
a Therapeutic protein may be assayed for the ability to specifically bind
to the same antigens specifically bound by the antibody that binds a
Therapeutic protein corresponding to the antibody that binds a Therapeutic
protein portion of the albumin fusion protein using techniques described
herein or routinely modifying techniques known in the art.
[0228] Assays for the ability of the antibodies of the invention or albumin
fusion proteins of the invention comprising at least a fragment or
variant of an antibody that binds a Therapeutic protein (or fragment or
variant thereof) to (specifically) bind a specific protein or epitope may be
performed in solution (e.g., Houghten, Bio/Techniques 13:412-421(1992)), on
beads (e.g., Lam, Nature 354:82-84 (1991)), on chips (e.g., Fodor,
Nature 364:555-556 (1993)), on bacteria (e.g., U.S. Patent No. 5,223,409), on
spores (e.g., Patent Nos. 5,571,698; 5,403,484; and 5,223,409), on
plasmids (e.g., Cull et al., Proc. Natl. Acad. Sci. USA 89_1865-
1869(1992))"oL.on phage (e.g., Scott-and Smith, Science 249:386-390 (1990);
Devlin, Science 249:404-406 (1990); Cwirla et al., Proc. Natl. Acad. Sci. USA
87:6378-6382 (1990); and Felici, J. Mol. Biol. 222:301-310 (1991))
(each of these references is incorporated herein in its entirety by
reference). The antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that binds
a Therapeutic protein (or fragment or variant thereof) may also be
assayed for their specificity and affniity for a specific protein or epitope
using or routinely modifying techniques described herein or otherwise
known in the art.
[0229] The albumin fusion proteins of the invention comprising at least a
fragment or variant of an antibody that binds a Therapeutic protein
may be assayed for cross-reactivity with other antigens (e.g., molecules that
have sequence/structure conservation with the molecule(s) specifically
bound by the antibody that binds a Therapeutic protein (or fragment or variant
thereof) corresponding to the Therapeutic protein portion of the
albumin fusion protein of the invention) by any method known in the art.
[0230] Tmmunoassays which can be used to analyze (immunospecific) binding and
cross-reactivity include, but are not limited to, competitive
and non-competitive assay systems using techniques such as western blots,
radioimmunoassays, ELISA (enzyme linked immunosorbent assay),
"sandwich" immunoassays, immunoprecipitation assays, precipitin reactions, gel
diffusion precipitin reactions, immunodiffusion assays,
agglutination assays, complement-fixation assays, immunoradiometric assays,
fluorescent immunoassays, and protein A immunoassays, to name but
a few. Such assays are routine and well krtown in the art (see, e.g., Ausubel
et al, eds, 1994, Current Protocols in Molecular Biology, Vol. 1, John
Wiley & Sons, Inc., New York, which is incorporated by reference herein in its
entirety). Exemplary immunoassays are described briefly below
(but are not intended by way of limitation).
[0231] Immunoprecipitation protocols generally comprise lysing a population of
cells in a lysis buffer such as RIPA buffer (1% NP-40 or Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCI, 0.01 M sodium phosphate
at pH 7.2, 1% Trasylol) supplemented with protein
phosphatase and/or protease inhibitors (e.g., EDTA, PMSF, aprotinin, sodium
vanadate), adding an antibody of the invention or albumin fusion
protein of the invention comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein (or fragment or variant thereof) to
the cell lysate, incubating for a period of time (e.g., 1 to 4 hours) at 40
degrees C, adding protein A and/or protein G sepharose beads (or beads
coated with an appropriate anti-idiotypic antibody or anti-albumin antibody in
the case when an albumin fusion protein comprising at least a
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
,. ,. ..,., .,.,,
fragment or variant of a Theiapeutic andoay) to the cell lysate, incubating
for about an hour or more at 40 degrees C, washing the beads in lysis
buffer and resuspending the beads in SDS/sample buffer. The ability of the
antibody or albumin fusion protein of the invention to
immunoprecipitate a particular antigen can be assessed by, e.g., western blot
analysis. One of skill in the art would be knowledgeable as to the
parameters that can be modified to increase the binding of the antibody or
albumin fusion protein to an antigen and decrease the background (e.g.,
pre-clearing the cell lysate with sepharose beads). For further discussion
regarding immunoprecipitation protocols see, e.g., Ausubel et al, eds,
1994, Current Protocols in Molecular Biology, Vol. 1, John Wiley & Sons, Inc.,
New York at 10.16.1.
[0232] Western blot analysis generally comprises preparing protein samples,
electrophoresis of the protein samples in a polyacrylamide gel (e.g.,
8%- 20% SDS-PAGE depending on the molecular weight of the antigen),
transferring the protein sample from the polyacrylamide gel to a
membrane such as nitrocellulose, PVDF or nylon, blocking the membrane in
blocking solution (e.g., PBS with 3% BSA or non-fat milk), washing
the membrane in washing buffer (e.g., PBS-Tween 20), applying the antibody or
albumin fusion protein of the invention (diluted in blocking buffer)
to the membrane, washing the membrane in washing buffer, applying a secondary
antibody (which recognizes the albumin fusion protein, e.g., an
anti-human serum albumin antibody) conjugated to an enzymatic substrate (e.g.,
horseradish peroxidase or alkaline phosphatase) or radioactive
molecule (e.g., 32P or125I) diluted in blocking buffer, washing the membrane
in wash buffer, and detecting the presence of the antigen. One of skill
in the art would be knowledgeable as to the parameters that can be modified to
increase the signal detected and to reduce the background noise. For
further discussion regarding western blot protocols see, e.g., Ausubel et al,
eds, 1994, Current Protocols in Molecular Biology, Vol. 1, John Wiley &
Sons, Inc., New York at 10.8.1.
[0233] ELISAs comprise preparing antigen, coating the well of a 96-well
microtiter plate with the antigen, washing away antigen that did not
bind the wells, adding the antibody or albumin fusion protein (comprising at
least a fragment or variant of an antibody that binds a Therapeutic
protein) of the invention conjugated to a detectable compound such as an
enzymatic substrate (e.g., horseradish peroxidase or alkaline phosphatase)
to the wells and incubating for a period of time, washing away unbound or non-
specifically bound albumin fusion proteins, and detecting the
presence of the antibody or albumin fusion proteins specifically bound to the
antigen coating the well. In ELISAs the antibody or albumin fusion
protein does not have to be conjugated to a detectable compound; instead, a
second antibody (which recognizes the antibody or albumin fusion
protein, respectively) conjugated to a detectable compound may be added to the
well. Further, instead of coating the well with the antigen, antibody
or the albumin fusion protein may be coated to the well. In this case, the
detectable molecule could be the antigen conjugated to a detectable
compound such as an enzymatic substrate (e.g., horseradish peroxidase or
alkaline pliosphatase). One of skill in the art would be knowledgeable as
to the parameters that can be modified to increase the signal detected as well
as other variations of ELISAs known in the art. For further discussion
regarding ELISAs see, e.g., Ausubel et al, eds, 1994, Current Protocols in
Molecular Biology, Vol. 1, John Wiley & Sons, Inc., New York at 11.2.1.
[02341 The binding affinity of an albumin fusion protein to a protein,
antigen, or epitope and the off-rate of an antibody- or albumin fusion
protein-protein/antigen/epitope interaction can be determined by competitive
binding -assays. -One-example of a competitive binding assay is a
radioimmunoassay comprising the incubation of labeled antigen (e.g., 3H or
1211) with the antibody or albumin fusion protein of the invention in the
presence of increasing amounts of unlabeled antigen, and the detection of the
antibody bound to the labeled antigen. The affinity of the antibody or
albumin fusion protein of the invention for a specific protein, antigen, or
epitope and the binding off-rates can be determined from the data by
Scatchard plot analysis. Competition with a second protein that binds the same
protein, antigen or epitope as the antibody or albumin fusion protein,
can also be determined using radioimmunoassays. In this case, the protein,
antigen or epitope is incubated with an antibody or albumin fusion
protein of the invention conjugated to a labeled compound (e.g.,'H or''51) in
the presence of increasing amounts of an unlabeled second protein that
binds the same protein, antigen, or epitope as the albumin fusion protein of
the invention.
[0235] In a preferred embodiment, BlAcore kinetic analysis is used to
determine the binding on and off rates of antibody or albumin fusion
proteins of the invention to a protein, antigen or epitope. BIAcore kinetic
analysis comprises analyzing the binding and dissociation of antibodies,
albumin fusion proteins, or specific polypeptides, antigens or epitopes from
chips with immobilized specific polypeptides, antigens or epitopes,
antibodies or albumin fusion proteins, respectively, on their surface.
Tl:erapeutic Uses
[0236] The present invention is further directed to antibody-based therapies
which involve administering antibodies of the invention or albumin
fusion proteins of the invention comprising at least a fragment or variant of
an antibody that binds a Therapeutic protein to an animal, preferably a
mammal, and most preferably a human, patient for treating one or more of the
disclosed diseases, disorders, or conditions. Therapeutic compounds
of the invention include, but are not limited to, antibodies of the invention
(including fragments, analogs and derivatives thereof as described
herein), nucleic acids encoding antibodies of the invention (including
fragments, analogs and derivatives thereof and anti-idiotypic antibodies as
described herein), albumin fusion proteins of the invention comprising at
least a fragment or variant of an antibody that binds a Therapeutic protein,
and nucleic acids encoding such albumin fusion proteins. The antibodies of the
invention or albumin fusion proteins of the invention comprising at
least a fragment or variant of an antibody that binds a Therapeutic protein
can be used to treat, inhibit or prevent diseases, disorders or conditions
associated with aberrant expression and/or activity of a Therapeutic protein,
including, but not limited to, any one or more of the diseases,
disorders, or conditions described herein. The treatment and/or prevention of
diseases, disorders, or conditions associated with aberrant expression
51
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ . ~.. ...I,. " ,.. õ.~ ..... .. . .... .......
and%r activity of a Therapeutic protein includes, but is not limited to,
alleviating symptoms associated with those diseases, disorders or conditions.
antibodies of the invention or albumin fusion proteins of the invention
comprising at least a fragment or variant of an antibody that binds a
Therapeutic protein may be provided in pharmaceutically acceptable
compositions as known in the art or as described herein.
[0237] In a specific and preferred embodiment, the present invention is
directed to antibody-based therapies which involve administering
antibodies of the invention or albumin fusion proteins of the invention
comprising at least a fragment or variant of an antibody that binds a
Therapeutic protein to an animal, preferably a niammal, and most preferably a
human, patient for treating one or more diseases, disorders, or
conditions, including but not limited to: neural disorders, immune system
disorders, muscular disorders, reproductive disorders, gastrointestinal
disorders, pulmonary disorders, cardiovascular disorders, renal disorders,
proliferative disorders, and/or cancerous diseases and conditions., and/or
as described elsewhere herein. Therapeutic compounds of the invention include,
but are not limited to, antibodies of the invention (e.g., antibodies
directed to the full length protein expressed on the cell surface of a
mammalian cell; antibodies directed to an epitope of a Therapeutic protein and
nucleic acids encoding antibodies of the invention (including fragments,
analogs and derivatives thereof and anti-idiotypic antibodies as described
herein). The antibodies of the invention can be used to treat, inhibit or
prevent diseases, disorders or conditions associated with aberrant expression
and/or activity of a Therapeutic protein, including, but not limited to, any
one or more of the diseases, disorders, or conditions described herein.
The treatment and/or prevention of diseases, disorders, or conditions
associated with aberrant expression and/or activity of a Therapeutic protein
includes, but is not limited to, alleviating symptoms associated witli those
diseases, disorders or conditions. Antibodies of the invention or albumin
fusion proteins of the invention comprising at least a fragment or variant of
an antibody that binds a Therapeutic protein may be provided in
pharmaceutically acceptable compositions as known in the art or as described
herein.
[0238] A summary of the ways in which the antibodies of the invention or
albumin fusion proteins of the invention comprising at least a
fragment or variant of an antibody that binds a Therapeutic protein may be
used therapeutically includes binding Therapeutic proteins locally or
systemically in the body or by direct cytotoxicity of the antibody, e.g. as
mediated by complement (CDC) or by effector cells (ADCC). Some of
these approaches are described in more detail below. Armed with the teachings
provided herein, one of ordinary skill in the art will know how to
use the antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that binds
a
Therapeutic protein for diagnostic, monitoring or therapeutic purposes without
undue experimentation.
[0239] The antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that
binds a Therapeutic protein may be advantageously utilized in combination with
other monoclonal or chimeric antibodies, or with lymphokines or
hematopoietic growth factors (such as, e.g., IL-2, IL-3 and IL-7), for
example, which serve to increase the number or activity of effector cells
which
interact with the antibodies.
[0240] The antibodies of the invention or albumin fusion proteins of the
invention comprising at least a fragment or variant of an antibody that
binds a Therapeutic protein may be administered_alone or in combination with
other_types of-treatments (e.g., radiation therapy,-chemotherapy,-
hormonal therapy, immunotherapy and anti-tumor agents). Generally,
administration of products of a species origin or species reactivity (in the
case of antibodies) that is the same species as that of the patient is
preferred. Thus, in a preferred embodiment, human antibodies, fragments
derivatives, analogs, or nucleic acids, are administered to a human patient
for therapy or prophylaxis.
[0241] It is preferred to use high affinity and/or potent in vivo inhibiting
and/or neutralizing antibodies against Therapeutic proteins, fragments
or regions thereof, (or the albumin fusion protein correlate of such an
antibody) for both immunoassays directed to and therapy of disorders related
to polynucleotides or polypeptides, including fragments thereof, of the
present invention. Such antibodies, fragments, or regions, will preferably
bave an affinity for polynucleotides or polypeptides of the invention,
including fragments thereof. Preferred binding affinities include dissociation
constants or Kd's less than 5 X 10-2 M, 10-2 M, 5 X 10-3 M, 10-3 M, 5 X 10
M, 104 M. More preferred binding affinities include those with a
dissociation constant or Kd less than 5 X 10-5 M, 10-5 M, 5 X 10-6 M, 10-6M, 5
X 10-' M, 10' M, 5 X 10-$ M or 10-8 M. Even more preferred
binding affinities include those with a dissociation constant or Kd less than
5 X 10-9 M, 10-9 M, 5 X 10-10 M, 10-10 M, 5 X 101 M, 10-11 M, 5 X 10-
12 M, 102M,5X10-i3M, 10-13M,5X10-14 M, 10-14 M,5X10'" M,or10-'sM.
Gene TJteranv
[0242] In a specific embodiment, nucleic acids comprising sequences encoding
antibodies that bind therapeutic proteins or albumin fusion
proteins comprising at least a fragment or variant of an antibody that binds a
Therapeutic protein are administered to treat, inhibit or prevent a
disease or disorder associated with aberrant expression and/or activity of a
Therapeutic protein, by way of gene therapy. Gene therapy refers to
therapy performed by the administration to a subject of an expressed or
expressible nucleic acid. In this embodiment of the invention, the nucleic
acids produce their encoded protein that mediates a therapeutic effect.
[0243] Any of the methods for gene therapy available in the art can be used
according to the present invention. Exemplary methods are
described in more detail elsewhere in this application.
Deinoustration afTlteraDeutic or Pronhylactic Activily
[0244] The compounds or pharmaceutical compositions of the invention are
preferably tested in vitro, and then in vivo for the desired
therapeutic or prophylactic activity, prior to use in humans. For example, in
vitro assays to demonstrate the therapeutic or prophylactic utility of a
52
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~.,.V =.,... .,..,,~ ....... .. ....... ..o. ,..a~ , .,~a= ,~ ,.
compound or pharmaceutical compos~tion mclude, the effect of a compound on a
cell line or a patient tissue sample. The effect of the compound or
composition on the cell line and/or tissue sample can be determined utilizing
tecliniques known to those of skill in the art including, but not limited
to, rosette formation assays and cell lysis assays. In accordance with the
invention, in vitro assays which can be used to determine whether
administration of a specific compound is indicated, include in vitro cell
culture assays in which a patient tissue sample is grown in culture, and
exposed to or otherwise administered a compound, and the effect of such
compound upon the tissue sample is observed.
TGeraperttic/PropirylacticAdministratioie and Composition
[0245] The invention provides methods of treatment, inhibition and prophylaxis
by administration to a subject of an effective amount of a
compound or pharmaceutical composition of the invention. In a preferred
embodiment, the compound is substantially purified (e.g., substantially
free from substances that limit its effect or produce undesired side-effects).
The subject is preferably an animal, including but not limited to
animals such as cows, pigs, horses, chickens, cats, dogs, etc., and is
preferably a mammal, and most preferably liuman.
[0246] Formulations and methods of administration that can be employed when
the compound comprises a nucleic acid or an immunoglobulin
are described above; additional appropriate formulations and routes of
administration can be selected from among those described herein below.
[0247] Various delivery systems are known and can be used to administer a
compound of the invention, e.g., encapsulation in liposomes,
microparticles, microcapsules, recombinant cells capable of expressing the
compound, receptor-mediated endocytosis (see, e.g., Wu and Wu, J.
Biol. Chem. 262:4429-4432 (1987)), construction of a nucleic acid as part of a
retroviral or other vector, etc. Metliods of introduction include but
are not limited to intradermal, intramuscular, intraperitoneal, intravenous,
subcutaneous, intranasal, epidural, and oral routes. The compounds or
compositions may be administered by any convenient route, for example by
infusion or bolus injection, by absorption through epithelial or
mucocutaneous linings (e.g., oral mucosa, rectal and intestinal mucosa, etc.)
and may be administered togetlier with other biologically active agents.
Administration can be systemic or local. In addition, it may be desirable to
introduce the pharmaceutical compounds or compositions of the
invention into the central nervous system by any suitable route, including
intraventricular and intrathecal injection; intraventricular injection may be
facilitated by an intraventricular catheter, for example, attached to a
reservoir, such as an Ommaya reservoir. Pulmonary administration can also be
employed, e.g., by use of an inhaler or nebulizer, and formulation with an
aerosolizing agent.
[02481 In a specific embodiment, it may be desirable to administer the
pharmaceutical compounds or compositions of the invention locally to
the area in need of treatment; this may be achieved by, for example, and not
by way of limitation, local infusion durnig surgery, topical application,
e.g., in conjunction with a wound dressing after surgery, by injection, by
means of a catheter, by means of a suppository, or by means of an implant,
said implant being of a porous, non-porous, or gelatinous material, including
membranes, such as sialastic membranes, or fibers. Preferably, when
administering a protein, including an antibody, of the invention, care must be
taken to use materials to which the protein does not absorb.
[0249] In another embodiment, the compound or composition can be delivered in
a vesicle, in particular a liposome (see Langer, Science
249:1527-1533 (1990); Treat et al., in Liposomes in the Therapy of Infectious
Disease and Cancer, Lopez-Beresteimand Fidler (eds:); Liss, New
York, pp. 353- 365 (1989); Lopez-Berestein, ibid., pp. 317-327; see generally
ibid.)
[0250] In yet another embodiment, the compound or composition can be delivered
in a controlled release system. In one embodiment, a pump
may be used (see Langer, supra; Sefton, CRC Crit. Ref. Biomed. Eng. 14:201
(1987); Buchwald et al., Surgery 88:507 (1980); Saudek et al., N.
Engl. J. Med. 321:574 (1989)). In another embodiment, polymeric materials can
be used (see Medical Applications of Controlled Release, Langer
and Wise (eds.), CRC Pres., Boca Raton, Florida (1974); Controlled Drug
Bioavailability, Drug Product Design and Performance, Smolen and Ball
(eds.), Wiley, New York (1984); Ranger and Peppas, J., Macromol. Sci. Rev.
Macromol. Chem. 23:61 (1983); see also Levy et al., Science
228:190 (1985); During et al., Ann. Neurol. 25:351 (1989); Howard et al.,
J.Neurosurg. 71:105 (1989)). In yet another embodiment, a controlled
release system can be placed in proximity of the therapeutic target, e.g., the
brain, thus requiring only a fraction of the systemic dose (see, e.g.,
Goodson, in Medical Applications of Controlled Release, s~~pra, vol. 2, pp.
115-138 (1984)).
10251] Other controlled release systems are discussed in the review by Langer
(Science 249:1527-1533 (1990)).
[0252] In a specific embodiment where the compound of the invention is a
nucleic acid encoding a protein, the nucleic acid can be administered
in vivo to promote expression of its encoded protein, by constructing it as
part of an appropriate nucleic acid expression vector and administering it
so that it becomes intracellular, e.g., by use of a retroviral vector (see
U.S. Patent No. 4,980,286), or by direct injection, or by use of microparticle
bombardment (e.g., a gene gun; Biolistic, Dupont), or coating with lipids or
cell-surface receptors or transfecting agents, or by administering it in
linkage to a homeobox- like peptide which is known to enter the nucleus (see
e.g., Joliot et al., Proc. Natl. Acad. Sci. USA 88:1864-1868 (1991)),
etc. Alternatively, a nucleic acid can be introduced intracellularly and
incorporated within host cell DNA for expression, by homologous
recombination.
[0253] The present invention also provides pharmaceutical compositions. Such
compositions comprise a therapeutically effective amount of a
compound, and a pharmaceutically acceptable carrier. In a specific embodiment,
the term "pharmaceutically acceptable" means approved by a
regulatory agency of the Federal or a state government or listed in the U.S.
Pharmacopeia or other generally recognized pharmacopeia for use in
animals, and more particularly in humans. The term "carrier" refers to a
diluent, adjuvant, excipient, or vehicle with which the therapeutic is
administered. Such pharmaceutical carriers can be sterile liquids, such as
water and oils, including those of petroleum, animal, vegetable or
53
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and
the like. Water is a preferred carrier when the pharmaceutical
composition is administered intravenously. Saline solutions and aqueous
dextrose and glycerol solutions can also be employed as liquid carriers,
particularly for injectable solutions. Suitable pharmaceutical excipients
include starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk,
silica gel, sodium stearate, glycerol monostearate, tale, sodium cliloride,
dried skim milk, glycerol, propylene, glycol, water, ethanol and the like.
The composition, if desired, can also contain minor amounts of wetting or
emulsifying agents, or pH buffering agents. These compositions can
take the form of solutions, suspensions, emulsion, tablets, pills, capsules,
powders, sustained-release formulations and the like. The composition
can be fomiulated as a suppository, with traditional binders and carriers such
as triglycerides. Oral formulation can include standard carriers such as
pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium
saccharine, cellulose, magnesium carbonate, etc. Examples of
suitable pharmaceutical carriers are described in "Remington's Pharmaceutical
Sciences" by E.W. Martin. Such compositions will contain a
therapeutically effective amount of the compound, preferably in purified form,
together with a suitable amount of carrier so as to provide the form
for proper administration to the patient. The formulation should suit the mode
of administration.
[0254] In a preferred embodiment, the composition is formulated in accordance
with routine procedures as a pharmaceutical composition
adapted for intravenous administration to human beings. Typically,
compositions for intravenous administration are solutions in sterile isotonic
aqueous buffer. Where necessary, the composition may also include a
solubilizing agent and a local anesthetic such as lignocaine to ease pain at
the
site of the injection. Generally, the ingredients are supplied either
separately or mixed together in unit dosage form, for example, as a dry
lyophilized powder or water free concentrate in a hermetically sealed
container such as an ampoule or sachette indicating the quantity of active
agent. Where the composition is to be administered by infusion, it can be
dispensed with an infusion bottle containing sterile pharmaceutical grade
water or saline. Where the composition is administered by injection, an
ampoule of sterile water for injection or saline can be provided so that the
ingredients may be mixed prior to administration.
[0255] The compounds of the invention can be formulated as neutral or salt
forms. Pharmaeeutically acceptable salts include those formed with
anions such as those derived from hydrochloric, phosphoric, acetic, oxalic,
tartaric acids, etc., and those formed with cations such as those derived
from sodium, potassium, animonium, calcium, ferric hydroxides, isopropylamine,
triethylamine, 2-ethylamino ethanol, histidine, procaine, etc.
[0256] The amount of the compound of the invention which will be effective in
the treatment, inhibition and prevention of a disease or disorder
associated with aberrant expression and/or activity of a Therapeutic protein
can be determined by standard clinical techniques. In addition, in vitro
assays may optionally be employed to help identify optimal dosage ranges. The
precise dose to be employed in the formulation will also depend on
the route of administration, and the seriousness of the disease or disorder,
and should be decided according to the judgment of the practitioner and
each patient's circumstances. Effective doses may be extrapolated from dose-
response curves derived from in vitro or animal model test systems.
[0257] For antibodies, the dosage administered to a patient is typically 0.1
mg/kg to 100 mg/kg of the patient's body weight. Preferably, the
dosage administered to a patient is between 0.1 mg/kg and 20_mg/kg of the-
patient's.body-weight, more-preferably 1 mg/kg to-10 mg/kg of the----
- - - - -
patient's body weight. Generally, human antibodies have a longer half-life
within the human body than antibodies from other species due to the
immune response to the foreign polypeptides. Thus, lower dosages of human
antibodies and less frequent administration is often possible. Further,
the dosage and frequency of administration of antibodies of the invention may
be reduced by enhancing uptake and tissue penetration (e.g., into the
brain) of the antibodies by modifications such as, for example, lipidation.
Diaenosis and I :aQi~zQ
[0258] Labeled antibodies and derivatives and analogs thereof that bind a
Therapeutic protein (or fragment or variant thereof) (including
albumin fusion proteins comprising at least a fragment or variant of an
antibody that binds a Therapeutic protein), can be used for diagnostic
purposes to detect, diagnose, or monitor diseases, disorders, and/or
conditions associated with the aberrant expression and/or activity of
Therapeutic
protein. The invention provides for the detection of aberrant expression of a
Therapeutic protein, comprising (a) assaying the expression of the
Therapeutic protein in cells or body fluid of an individual using one or more
antibodies specific to the polypeptide interest and (b) comparing the
level of gene expression with a standard gene expression level, whereby an
increase or decrease in the assayed Therapeutic protein expression level
compared to the standard expression level is indicative of aberrant
expression.
102591 The invention provides a diagnostic assay for diagnosing a disorder,
comprising (a) assaying the expression of the Therapeutic protein in
cells or body fluid of an individual using one or more antibodies specific to
the Therapeutic protein or albumin fusion proteins comprising at least a
fragment of variant of an antibody specific to a Therapeutic protein, and (b)
comparing the level of gene expression with a standard gene expression
level, wliereby an increase or decrease in the assayed Therapeutic protein
gene expression level compared to the standard expression level is
indicative of a particular disorder. With respect to cancer, the presence of a
relatively high amount of transcript in biopsied tissue from an individual
may indicate a predisposition for the development of the disease, or may
provide a means for detecting the disease prior to the appearance of actual
clinical symptoms. A more definitive diagnosis of this type may allow health
professionals to employ preventative measures or aggressive treatment
earlier thereby preventing the development or further progression of the
cancer.
[0260] Antibodies of the invention or albumin fusion proteins comprising at
least a fragment of variant of an antibody specific to a Therapeutic
protein can be used to assay protein levels in a biological sample using
classical immunohistological methods laiown to those of skill in the art
(e.g.,
54
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
see Jalkanen et al., J. Cell. Biol. 101:976-985 (1985); Jalkanen et al., J.
Cell . Biol. 105:3087-3096 (1987)). Other antibody-based methods useful
for detecting protein gene expression include immunoassays, such as the enzyme
linked immunosorbent assay (ELISA) and the radioimmunoassay
(RIA). Suitable antibody assay labels are known in the art and include enzyme
labels, such as, glucose oxidase; radioisotopes, such as iodine (1251,
1211), carbon (14C), sulfur (35S), tritium (3H), indium (1121n), and
technetium (99Tc); luminescent labels, such as luminol; and fluorescent
labels,
such as fluorescein and rhodamine, and biotin.
[0261] One facet of the invention is the detection and diagnosis of a disease
or disorder associated with aberrant expression of a Therapeutic
protein in an animal, preferably a mammal and most preferably a human. In one
embodiment, diagnosis comprises: a) administering (for example,
parenterally, subcutaneously, or intraperitoneally) to a subject an effective
amount of a labeled molecule which specifically binds to the polypeptide
of interest; b) waiting for a time interval following the administering for
permitting the labeled molecule to preferentially concentrate at sites in the
subject where the Therapeutic protein is expressed (and for unbound labeled
molecule to be cleared to background level); c) determining
background level; and d) detecting the labeled molecule in the subject, such
that detection of labeled molecule above the background level indicates
that the subject has a particular disease or disorder associated with aberrant
expression of the therapeutic protein. Background level can be
determined by various methods including, comparing the amount of labeled
molecule detected to a standard value previously determined for a
particular system.
[0262] It will be understood in the art that the size of the subject and the
imaging system used will determine the quantity of imaging moiety
needed to produce diagnostic images. In the case of a radioisotope moiety, for
a human subject, the quantity of radioactivity injected will normally
range from about 5 to 20 millicuries of 99mTc. The labeled antibody, antibody
fragment, or albumin fusion protein comprising at least a fragment
or variant of an antibody that binds a Therapeutic protein will then
preferentially accumulate at the location of cells which contain the specific
Therapeutic protein. In vivo tumor imaging is described in S.W. Burchiel et
al., "Immunopharmacokinetics of Radiolabeled Antibodies and Their
Fragments." (Chapter 13 in Tumor Imaging: The Radiochemical Detection of
Cancer, S.W. Burchiel and B. A. Rhodes, eds., Masson Publishing
Inc. (1982)).
[0263] Depending on several variables, including the type of label used and
the mode of administration, the time interval following the
administration for permitting the labeled molecule to preferentially
concentrate at sites in the subject and for unbound labeled molecule to be
cleared
to background level is 6 to 48 hours or 6 to 24 hours or 6 to 12 hours. In
another embodiment the time interval following administration is 5 to 20
days or 5 to 10 days.
[0264] In an embodiment, monitoring of the disease or disorder is carried out
by repeating the method for diagnosing the disease or disease, for
example, one month after initial diagnosis, six months after initial
diagnosis, one year after initial diagnosis, etc.
[0265] Presence of the labeled molecule can be detected in the patient using
methods known in the art for in vivo scanning. These methods
depend upon the type of label used. Skilled artisans will. be able to
detemiine_the appropriate method-for detecting- a particular label: Methods
and
devices that may be used in the diagnostic methods of the invention include,
but are not limited to, computed tomography (CT), whole body scan
such as position emission tomography (PET), magnetic resonance imaging (MRI),
and sonography.
[0266] In a specific embodiment, the molecule is labeled with a radioisotope
and is detected in the patient using a radiation responsive surgical
instrument (Thurston et al., U.S. Patent No. 5,441,050). In another
embodiment, the molecule is labeled with a fluorescent compound and is
detected in the patient using a fluorescence responsive scanning instrument.
In another embodiment, the molecule is labeled with a positron
emitting metal and is detected in the patent using positron emission-
tomography. In yet another embodiment, the molecule is labeled with a
paramagnetic label and is detected in a patient using magnetic resonance
imaging (MRI). Antibodies that specifically detect the albumin fusion
protein but not albumin or the therapeutic protein alone are a preferred
embodiment. These can be used to detect the albumin fusion protein as
described throughout the specification.
Kits
[0267] The present invention provides kits that can be used in the above
methods. In one embodiment, a kit comprises an antibody, preferably a
purified antibody, in one or more containers. In a specific embodiment, the
kits of the present invention contain a substantially isolated polypeptide
comprising an epitope which is specifically immunoreactive with an antibody
included in the kit. Preferably, the kits of the present invention f-urther
comprise a control antibody which does not react with the polypeptide of
interest. In another specific embodiment, the kits of the present invention
contain a means for detecting the binding of an antibody to a polypeptide of
interest (e.g., the antibody may be conjugated to a detectable substrate
such as a fluorescent compound, an enzymatic substrate, a radioactive compound
or a luminescent compound, or a second antibody which
recognizes the first antibody may be conjugated to a detectable substrate).
[0268] In another specific embodiment of the present invention, the kit is a
diagnostic kit for use in screening serum containing antibodies
specific against proliferative and/or cancerous polynucleotides and
polypeptides. Such a kit may include a control antibody that does not react
with
the polypeptide of interest. Such a kit may include a substantially isolated
polypeptide antigen comprising an epitope which is speciftcally
immunoreactive with at least one anti-polypeptide antigen antibody. Further,
such a kit includes means for detecting the binding of said antibody to
the antigen (e.g., the antibody may be conjugated to a fluorescent compound
such as fluorescein or rhodamine which can be detected by flow
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
cytometry). In specific embodiments, the kit may include a recombinantly
produced or chemically synthesized polypeptide antigen. The polypeptide
antigen of the kit may also be attached to a solid support.
[0269] In a more specific embodiment the detecting means of the above-
described kit includes a solid support to which said polypeptide antigen
is attached. Such a kit may also include a non-attached reporter-labeled anti-
human antibody. In this embodiment, binding of the antibody to the
polypeptide antigen can be detected by binding of the said reporter-labeled
antibody.
[02701 In an additional embodiment, the invention includes a diagnostic kit
for use in screening serum containing antigens of the polypeptide of
the invention. The diagnostic kit includes a substantially isolated antibody
specifically immunoreactive with polypeptide or polynucleotide antigens,
and means for detecting the binding of the polynucleotide or polypeptide
antigen to the antibody. In one embodiment, the antibody is attached to a
solid support. In a specific embodiment, the antibody may be a monoclonal
antibody. The detecting means of the kit may include a second, labeled
monoclonal antibody. Alternatively, or in addition, the detecting means may
include a labeled, competing antigen.
[0271] In one diagnostic configuration, test serum is reacted with a solid
phase reagent having a surface-bound antigen obtained by the methods
of the present invention. After binding with specific antigen antibody to the
reagent and removing unbound serum components by washing, the
reagent is reacted with reporter-labeled anti-human antibody to bind reporter
to the reagent in proportion to the amount of bound anti-antigen
antibody on the solid support. The reagent is again washed to remove unbound
labeled antibody, and the amount of reporter associated with the
reagent is determined. Typically, the reporter is an enzyme which is detected
by incubating the solid phase in the presence of a suitable
fluorometric, luminescent or colorimetric substrate (Sigma, St. Louis, MO).
[0272] The solid surface reagent in the above assay is prepared by known
techniques for attaching protein material to solid support material,
such as polymeric beads, dip sticks, 96-well plate or filter material. These
attachment methods generally include non-specific adsorption of the
protein to the support or covalent attachment of the protein, typically
through a free amine group, to a chemically reactive group on the solid
support,
such as an activated carboxyl, hydroxyl, or aldehyde group. Alternatively,
streptavidin coated plates can be used in conjunction with biotinylated
antigen(s).
[0273] Thus, the invention provides an assay system or kit for carrying out
this diagnostic method. The kit generally includes a support with
surface-bound recombinant antigens, and a reporter-labeled anti-human antibody
for detecting surface-bound anti-antigen antibody.
Albumiu Fusior: Proteius
[0274] The present invention relates generally to albumin fusion proteins and
methods of treating, preventing, or ameliorating diseases or
disorders. As used herein, "albumin fusion protein" refers to a protein formed
by the fusion of at least one molecule of albumin (or a fragment or
variant thereof) to at least one molecule of a Therapeutic protein (or
fragment or variant thereof). An albumin fusion protein of the invention
comprises at least a fragment or variant of a Therapeutic protein and at least
a fragment or variant of human serum albumin, which are associated
with one another, preferably by genetic fusion (i.e., the albumin fusion
pro.tein is generated by translation of a nucleic acid in which a
polynucleotide
encoding all or a portion of a Therapeutic protein is joined in-frame with a
polynucleotide encoding all or a portion of albumin) or to one another.
The Therapeutic protein and albumin protein, once part of the albumin fusion
protein, may each be referred to as a "portion", "region" or "moiety"
of the albumin fusion protein.
[0275] . In a preferred embodiment, the invention provides an albumin fusion
protein encoded by a polynucleotide or albumin fusion construct
described in Table 1 or Table 2. Polynucleotides encoding these albumin fusion
proteins are also encompassed by the invention.
[0276] Preferred albumin fusion proteins of the invention, include, but are
not limited to, albumin fusion proteins encoded by a nucleic acid
molecule comprising, or altternatively consisting of, a polynucleotide
encoding at least one molecule of albumin (or a fragment or variant thereof)
joined in frame to at least one polynucleotide encoding at least one molecule
of a Therapeutic protein (or fragment or variant thereof); a nucleic acid
molecule comprising, or alternatively consisting of, a polynucleotide encoding
at least one molecule of albumin (or a fragment or variant thereof)
joined in frame to at least one polynucleotide encoding at least one molecule
of a Therapeutic protein (or fragment or variant thereof) generated as
described in Table 1, Table 2 or in the Examples; or a nucleic acid molecule
comprising, or alternatively consisting of, a polynucleotide encoding at
least one molecule of albumin (or a fragment or variant thereof) joined in
frame to at least one polynucleotide encoding at least one molecule of a
Therapeutic protein (or fragment or variant thereof), further comprising, for
example, one or more of the following elements: (1) a functional self-
replicating vector (including but not limited to, a shuttle vector, an
expression vector, an integration vector, and/or a replication system), (2) a
region
for initiation of transcription (e.g., a promoter region, such as for example,
a regulatable or inducible promoter, a constitutive promoter), (3) a region
for termination of transcription, (4) a leader sequence, and (5) a selectable
marker.
102771 In one embodiment, the invention provides an albumin fusion protein
comprising, or alternatively consisting of, a Therapeutic protein
(e.g., as described in Table 1) and a serum albumin protein. In other
embodiments, the invention provides an albumin fusion protein comprising, or
alternatively consisting of, a biologically active and/or therapeutically
active fragment of a Therapeutic protein and a serum albumin protein. In other
embodiments, the invention provides an albumin fusion protein comprising, or
alternatively consisting of, a biologically active and/or therapeutically
active variant of a Therapeutic protein and a serum albumin protein. In
preferred embodiments, the serum albumin protein component of the
albumin fusion protein is the mature portion of serum albumin.
56
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~;.,, n . ~,=.n.,~õ, ,,.,~ õ
[d27 j Iri' fu' er em[iodiments, t}ie invention provides an albumin fusion
protein comprising, or alternatively consisting of, a Therapeutic
protein, and a biologically active and/or therapeutically active fragment of
serum albumin. In further embodiments, the invention provides an
albumin fusion protein comprising, or alternatively consisting of, a
Therapeutic protein and a biologically active and/or tlierapeutically active
variant
of serum albumin. In preferred embodiments, the Therapeutic protein portion of
the albumin fusion protein is the mature portion of the Therapeutic
protein.
[02791 In further embodiments, the invention provides an albumin fusion
protein comprising, or alternatively consisting of, a biologically active
and/or therapeutically active fragment or variant of a Therapeutic protein and
a biologically active and/or therapeutically active fragment or variant
of serum albumin. In preferred embodiments, the invention provides an albumin
fusion protein comprising, or alternatively consisting of, the mature
portion of a Therapeutic protein and the mature portion of serum albumin.
[0280] Preferably, the albumin fusion protein comprises HA as the N-terminal
portion, and a Therapeutic protein as the C-terminal portion.
Alternatively, an albumin fusion protein comprising HA as the C-terminal
portion, and a Therapeutic protein as the N-terminal portion may also be
used.
[02811 In other embodiments, the albumin fusion protein has a Therapeutic
protein fused to both the N-terminus and the C-terminus of albumin.
In a preferred embodiment, the Therapeutic proteins fused at the N- and C-
termini are the same Therapeutic proteins. In an alternative preferred
embodiment, the Therapeutic proteins fused at the N- and C- termini are
different Therapeutic proteins. In another preferred embodiment, the
Therapeutic proteins fused at the N- and C- tennini are different Therapeutic
proteins which may be used to treat or prevent the same or a related
disease, disorder, or condition (e.g. as listed in the "Preferred Indication
Y" column of Table 1). In another preferred embodiment, the Therapeutic
proteins fused at the N- and C- termini are different Therapeutic proteins
which may be used to treat, ameliorate, or prevent diseases or disorders
(e.g. as listed in the "Preferred Indication Y" column of Table 1) which are
known in the art to commonly occur in patients simultaneously,
concurrently, or consecutively, or which commonly occur in patients in
association with one another.
[0282] Albumin fusion proteins of the invention encompass proteins containing
one, two, three, four, or more molecules of a given Therapeutic
protein X or variant thereof fused to the N- or C- terminus of an albumin
fusion protein of the invention, and/or to the N- and/or C- terminus of
albumin or variant thereof. Molecules of a given Therapeutic protein X or
variants thereof may be in any number of orientations, including, but not
limited to, a'head to head' orientation (e.g., wherein the N-terminus of one
molecule of a Therapeutic protein X is fused to the N-terminus of
another molecule of the Therapeutic protein X), or a'head to tail' orientation
(e.g., wherein the C-terminus of one molecule of a Therapeutic protein
X is fused to the N-terminus of another molecule of Therapeutic protein X).
[02831 In one embodiment, one, two, three, or more tandemly oriented
Therapeutic protein X polypeptides (or fragments or variants thereof) are
fused to the N- or C- terminus of an albumin fusion protein of the invention,
and/or to the N- and/or C- terminus of albumin or variant thereof.
[02841 Albumin fusion proteins of the invention further encompass proteins
containing one, two, three, four, or more-molecules of-a-given-
__.
Therapeutic protein X or variant thereof fused to the N- or C- terminus of an
albumin fusion protein of the invention, and/or to the N- and/or C-
terminus of albumin or variant thereof, wherein the molecules are joined
through peptide linkers. Examples include those peptide linkers described
in U.S. Pat. No. 5,073,627 (hereby incorporated by reference). Albumin fusion
proteins comprising multiple Therapeutic protein X polypeptides
separated by peptide linkers may be produced using conventional recombinant
DNA technology. Linkers are particularly important when fusing a
small peptide to the large HSA molecule. The peptide itself can be a linker by
fusing tandem copies of the peptide or other known linkers can be
used. Constructs that incorporate linkers are described in Table 2 or are
apparent when examining SEQ ID NO:Y.
[0285] Further, albumin fusion proteins of the invention may also be produced
by fusing a Therapeutic protein X or variants thereof to the N-
terminal and/or C-terminal of albumin or variants thereof in such a way as to
allow the formation of intramolecular and/or intermolecular multimeric
forms. In one embodiment of the invention, albumin fusion proteins may be in
monomeric or multimeric forms (i.e., dimers, trimers, tetramers and.
higher multimers). In a further embodiment of the invention, the Therapeutic
protein portion of an albumin fusion protein may be in monomeric
form or multimeric form (i.e., dimers, trimers, tetramers and higher
multimers). In a specific embodiment, the Therapeutic protein portion of an
albumin fusion protein is in multimeric form (i.e., dimers, trimers, tetramers
and higher multimers), and the albumin protein portion is in
monomeric form.
[02861 In addition to albumin fusion protein in which the albumin portion is
fused N- terminal and/or C-terminal of the Therapeutic protein
portion, albumin fusion proteins of the invention may also be produced by
inserting the Therapeutic protein or peptide of interest (e.g., a Therapeutic
protein X as disclosed in Table 1, or an antibody that binds a Therapeutic
protein or a fragment or variant thereof) into an internal region of HA. For
instance, within the protein sequence of the HA molecule a number of loops or
turns exist between the end and beginning of a-helices, which are
stabilized by disulphide bonds. The loops, as determined from the crystal
structure of HA (PDB identifiers tA06, IBJ5, 1BK.E, 1BM0, tE7E to
11371 and 1UOR) for the most part extend away from the body of the molecule.
These loops are useful for the insertion, or intemal fusion, of
therapeutically active peptides, particularly those requiring a secondary
structure to be functional, or Therapeutic proteins, to essentially generate
an
albumin molecule with specific biological activity.
[0287] Loops in human albumin structure into which peptides or polypeptides
may be inserted to generate albumin fusion proteins of the
57
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~~õ -(E'inven~j~lr~!~tid[u[1~:;1VaI5 R l(s~4lt~lly"l[11Y~QKsp89, A1a92-G1u100,
G1n170-A1a176, His 247 - G1u252, Glu 266 - G1u277, Glu 280-His288, A1a362-
GIu368, Lys439-Pro447, Va1462-Lys475, Thr478-Pro486, and Lys560-Thr566, In
more preferred embodiments, peptides or polypeptides are
inserted into the Va154-Asn6l, G1n170-A1a176, and/or Lys560-Thr5661oops of
mature liuman albumin (SEQ ID NO:1).
[0288] Peptides to be inserted may be derived from either phage display or
synthetic peptide libraries screened for specific biological activity or
from the active portions of a molecule with the desired function.
Additionally, random peptide libraries may be generated witliin particular
loops or
by insertions of randomized peptides into particular loops of the HA molecule
and in which all possible combinations of amino acids are
represented.
[0289] Such library(s) could be generated on HA or domain fragments of HA by
one of the following metliods:
randomized mutation of amino acids within one or more peptide loops of HA or
HA domain fragments. Either one, more or all the
residues within a loop could be mutated in this manner;
replacement of, or insertion into one or more loops of HA or HA domain
fragments (i.e., internal fusion) of a randomized peptide(s) of
lengdi X. (where X is an amino acid and n is the number of residues;
N-, C- or N- and C- terminal peptide/protein fusions in addition to (a) and/or
(b).
[0290] The HA or HA domain fragment may also be made multifunctional by
grafting the peptides derived from different screens of different
loops against different targets into the same HA or HA domain fragment.
[0291] In preferred embodiments, peptides inserted into a loop of human serum
albumin are peptide fragments or peptide variants of the
Tlterapeutic proteins disclosed in Table 1. More particularly, the invention
encompasses albumin fusion proteins which comprise peptide fragments
or peptide variants at least 7 at least 8, at least 9, at least 10, at least
11, at least 12, at least 13, at least 14, at least 15, at least 20, at least
25, at least
30, at least 35, or at least 40 amino acids in length inserted into a loop of
human serum albumin. The invention also encompasses albumin fusion
proteins which comprise peptide fragments or peptide variants at least 7 at
least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at
least
14, at least 15, at least 20, at least 25, at least 30, at least 35, or at
least 40 amino acids fused to the N-terminus of human serum albumin. The
invention also encompasses albumin fusion proteins which comprise peptide
fragments or peptide variants at least 7 at least 8, at least 9, at least 10,
at least 11, at least 12, at least 13, at least 14, at least 15, at least 20,
at least 25, at least 30, at least 35, or at least 40 amino acids fused to the
C-
terminus of human serum albumin. For exanzple, short peptides described in
Table 1 and 2 (e.g., Therapeutic Y) can be inserted into the albumin
loops.
[0292] Generally, the albumin fusion proteins of the invention may have one HA-
derived region and one Therapeutic protein-derived region.
Multiple regions of each protein, however, may be used to make an albumin
fusion protein of the invention. Similarly, more than one Therapeutic
protein may be used to make an albumin fusion protein of the invention. For
instance, a Therapeutic protein may be fused to both the N- and C-
terminal ends of the HA. In such a configuration, the Therapeutic_protein
portions may be_the same or differentTherapeutic protein molecules. --The --
-._
stmeture of bifunctional albumin fusion proteins may be represented as: X-HA-Y
or Y-HA-X.
[0293] For example, an anti-BLySTM scFv-HA-IFP3a-2b fusion may be prepared to
modulate the immune response to IFNa-2b by anti-BLySTM
scFv. An altemative is making a bi (or even multi) functional dose of HA-
fusions e.g. HA-IFNa-2b fusion mixed with HA-anti-BLySTM scFv
fusion or other HA-fusions in various ratio's depending on function, half-life
etc.
[0294] Bi- or multi-functional albumin fusion proteins may also be prepared to
target the Therapeutic protein portion of a fusion to a target
organ or cell type via protein or peptide at the opposite terminus of HA.
102951 As an alternative to the fusion of k-nown therapeutic molecules, the
peptides could be obtained by screening libraries constructed as
fusions to the N-, C- or N- and C- termini of HA, or domain fragment of HA, of
typically 6, 8, 12, 20 or 25 or Xõ (where X is an amino acid (aa)
and n equals the number of residues) randomized amino acids, and in which all
possible combinations of amino acids were represented. A
particular advantage of this approach is that the peptides may be selected in
situ on the HA molecule and the properties of the peptide would
therefore be as selected for rather than, potentially, modified as might be
the case for a peptide derived by any other method then being attached to
HA.
[0296] Additionally, the albumin fusion proteins of the invention may include
a linker peptide between the fused portions to provide greater
physical separation between the moieties and thus maximize the accessibility
of the Therapeutic protein portion, for instance, for binding to its
cognate receptor. The linker peptide may consist of amino acids such that it
is flexible or more rigid.
[0297] The linker sequence may be cleavable by a protease or cheniically to
yield the growth hormone related moiety. Preferably, the protease
is one which is produced naturally by the host, for example the S. cerevisiae
protease kex2 or equivalent proteases.
[0298] Therefore, as described above, the albumin fusion proteins of the
invention may have the following formula R1-L-R2; R2-L-R1; or Rl-
L-R2-L-R1, wherein Rl is at least one Therapeutic protein, peptide or
polypeptide sequence, and not necessarily the same Therapeutic protein, L is a
linker and R2 is a serum albumin sequence.
[0299] In preferred embodiments, albumin fusion proteins of the invention
comprising a Therapeutic protein have a higher plasma stability
compared to the plasma stability of the same Therapeutic protein when not
fused to albumin. Plasma stability typically refers to the time period
58
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
administered in vivo and carried into the bloodstream and when the therapeutic
protein is degraded and
cleared from the bloodstream, into an organ, such as the kidney or liver, that
ultimately clears the Therapeutic protein from the body. Plasma
stability is calculated in terms of the half-life of the Therapeutic protein
in the bloodstream. The half-life of the Therapeutic protein in the
bloodstream can be readily determined by common assays known in the art.
[0300] In preferred embodiments, Albumin fusion proteins of the invention
comprising a Therapeutic protein have extended shelf life compared
to the shelf life the same Therapeutic protein when not fused to albumin.
Shelf-life typically refers to the time period over which the therapeutic
activity of a Therapeutic protein in solution or in some other storage
formulation, is stable without undue loss of therapeutic activity. Many of the
Therapeutic proteins are highly labile in their unfused state. As described
below, the typical shelf-life of these Therapeutic proteins is markedly
prolonged upon incorporation into the albumin fusion protein of the invention.
[0301] Albumin fusion proteins of the invention with "prolonged" or "extended"
shelf-life exhibit greater therapeutic activity relative to a
standard that has been subjected to the same storage and handling conditions.
The standard may be the unfused futl-length Therapeutic protein.
When the Therapeutic protein portion of the albumin fusion protein is an
analog, a variant, or is ottierwise altered or does not include the complete
sequence for that protein, the prolongation of therapeutic activity may
alternatively be compared to the unfused equivalent of that analog, variant,
altered peptide or incomplete sequence. As an example, an albumin fusion
protein of the invention may retain greater than about 100% of the
therapeutic activity, or greater than about 105%, 110%, 120%, 130%, 150% or
200% of the therapeutic activity of a standard when subjected to the
same storage and handling conditions as the standard when compared at a given
time point.
[0302] Shelf-life may also be assessed in terms of therapeutic activity
remaining after storage, normalized to therapeutic activity when storage
began. Albumin fusion proteins of the invention with prolonged or extended
shelf-life as exhibited by prolonged or extended tlierapeutic activity
may retain greater than about 50% of the therapeutic activity, about 60%, 70%,
80%, or 90% or more of the therapeutic activity of the equivalent
unfused Therapeutic protein when subjected to the same conditions.
Expression of Fitsion Proteins
[0303] The albumin fusion proteins of the invention may be produced as
recombinant molecules by secretion from yeast, a microorganism such
as a bacterium, or a human or animal cell line. Preferably, the polypeptide is
secreted from the host cells.
[03041 A particular embodiment of the invention comprises a DNA construct
encoding a signal sequence effective for directing secretion in
yeast, particularly a yeast-derived signal sequence (especially one which is
homologous to the yeast host), and the fused molecule of the first aspect
of the invention, there being no yeast-derived pro sequence between the signal
and the mature polypeptide.
[0305] The Saccharomyces cerevisiae invertase signal is a preferred example of
a yeast-derived signal sequence.
[0306] Conjugates of the kind prepared by Poznansky et al., (FEBS Lett. 239:18
(1988)), in which separately-prepared polypeptides are joined
by chemical cross-linking, are not contemplated.
[0307] The present invention also includes a cell, preferably a yeast cell
transformed to express an albumin fusion protein of the invention. In
addition to the transformed host cells themselves, the present invention also
contemplates a culture of those cells, preferably a monoclonal (clonally
homogeneous) culture, or a culture derived from a monoclonal culture, in a
nutrient medium. If the polypeptide is secreted, the medium will contain
the polypeptide, with the cells, or without the cells if they have been
filtered or centrifuged away. Many expression systems are known and may be
used, including bacteria (for example E. colt and Bacillus subtilis), yeasts
(for example Saccharomyces cerevisiae, Klu}rneromyces lactis and Pichia
pastoris, filamentous fungi (for example Aspergillus), plant cells, animal
cells and insect cells.
[0308] Preferred yeast strains to be used in the production of albumin fusion
proteins are D88, DXY1 and BXP10. D88 [leu2-3, leu2-122, cafzl,
pral, ubc4] is a derivative of parent strain AH22his (also known as DB1; see,
e.g., Sleep el al. Biotechnology 8:42-46 (1990)). The strain contains
a leu2 mutation which allows for auxotropic selection of 2 micron-based
plasmids that contain the LEU2 gene. D88 also exhibits a derepression of
PRB1 in glucose excess. The PRB1 promoter is normally controlled by two
checkpoints that monitor glucose levels and growth stage. The promoter
is activated in wild type yeast upon glucose depletion and entry into
stationary phase. Strain D88 exhibits the repression by glucose but maintains
the induction upon entry into stationary phase. The PRA.1 gene encodes a yeast
vacuolar protease, YscA endoprotease A, that is localized in the
ER The UBC4 gene is in the ubiquitination pathway and is involved in targeting
short lived and abnormal proteins for ubiquitin dependant
degradation. Isolation of this ubc4 mutation was found to increase the copy
number of an expression plasmid in the cell and cause an increased
level of expression of a desired protein expressed from the plasmid (see,
e.g., International Publication No. W099/00504, hereby incorporated in its
entirety by reference herein).
[0309] DXYI, a derivative of D88, has the following genotype: [leu2-3, leu2-
122, canl, pral, ubc4, ura3:: yap3]. hi addition to the mutations
isolated in D88, this strain also has a knockout of the YAP3 protease. This
protease causes cleavage of mostly di-basic residues (RR, RK, KR, KK)
but can also promote cleavage at single basic residues in proteins. Isolation
of this yap3 mutation resulted in higher levels of full length HSA
production (see, e.g., U.S. Patent No. 5,965,386 and Kerry-Williams et al.,
Yeast 14:161-169 (1998), hereby incorporated in their entireties by
reference herein).
[0310] BXP10 has the following genotype: leu2-3, leu2-122, canl, pral, ubc4,
ura3, yap3:: UIL43, lys2, hsp150::LYS2, pnitl:: URA3. In
59 1
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~{, , I~dditi~u~~t~l th~,a[S~CtiiphslS l$l~at~d,;)~ OnL this strain also has a
knockout of the PMT1 gene and the HSP150 gene. The PMTI gene is a member
of the evolutionarily conserved family of dolichyl-phosphate-D-mannose protein
O-mannosyltransferases (Pmts). The transmembrane topology of
Pmtlp suggests that it is an integral membrane protein of the endoplasmic
reticulum with a role in 0-linked glycosylation. This mutation serves to
reduce/eliminate 0-linked glycosylation of HSA fusions (see, e.g.,
Intemational Publication No. W000/44772, hereby incorporated in its entirety
by
reference herein). Studies revealed that the Hsp150 protein is inefficiently
separated from rHA by ion exchange chromatography. The mutation in
the HSP150 gene removes a potential contaminant that has proven difficult to
remove by standard purification techniques. See, e.g., U.S. Patent No.
5,783,423, hereby incorporated in its entirety by reference herein.
103111 The desired protein is produced in conventional ways, for example from
a coding sequence inserted in the host chromosome or on a free
plasmid. The yeasts are transformed with a coding sequence for the desired
protein in any of the usual ways, for example electroporation. Metliods
for transformation of yeast by electroporation are disclosed in Becker &
Guarente (1990) Methods En.yn:ol. 194, 182.
[03121 Successfully transformed cells, i.e., cells that contain a DNA
construct of the present invention, can be identified by well known
techniques. For example, cells resulting from the introduction of an
expression construct can be grown to produce the desired polypeptide. Cells
can be harvested and lysed and their DNA content examined for the presence of
the DNA using a method such as that described by Southern (1975)
J. Mol. Biol. 98, 503 or Berent et al. (1985) Biotech. 3, 208. Alternatively,
the presence of the protein in the supematant can be detected using
antibodies.
[0313] Useful yeast plasmid vectors include pRS403-406 and pRS413-416 and are
generally available from Stratagene Cloning Systems, La
Jolla, CA 92037, USA. Plasmids pRS403, pRS404, pRS405 and pRS406 are Yeast
Integrating plasmids (Ylps) and incorporate the yeast selectable
markers HIS3, 7RP1, LEU2 and URA3. Plasmids pRS413-416 are Yeast Centromere
plasmids (Ycps).
[0314] Preferred vectors for making albumin fusion proteins for expression in
yeast include pPPC0005, pScCHSA, pScNHSA, and pC4:HSA
which are described in detail in Example 1. Figure 2 shows a map of the
pPPC0005 plasmid that can be used as the base vector into which
polynucleotides encoding Therapeutic proteins may be cloned to form HA-
fusions. It contains a PRBI S. cerevisiae promoter (PRBIp), a Fusion
leader sequence (FL), DNA encoding HA (rHA) and an ADH1 S. cerevisiae
terminator sequence. The sequence of the fusion leader sequence
consists of the first 19 amino acids of the signal peptide of human serum
albumin (SEQ ID N0:3) and the last five amino acids of the mating factor
alpha 1 promoter (SLDKR, see EP-A-387 319 whioh is hereby incorporated by
reference in its entirety).
[03151 The plasmids, pPPC0005, pScCHSA, pScNHSA, and pC4:HSA were deposited on
April 11, 2001 at the American Type Culture
Collection, 10801 University Boulevard, Manassas, Virginia 20110-2209 and
given accession numbers ATCC PTA-3278, PTA-3276, PTA-3279,
and PTA-3277, respectively. Another vector useful for expressing an albumin
fusion protein in yeast the pSAC35 vector which is described in
Sleep et al., BioTechnology 8:42 (1990) which is hereby incorporated by
reference in its entirety.
--
[0316] A yeast promoter that can be used to express the albumin fusion protein
is the MET25 promoter, _See, for example, DominikMumburg,
__
Rolf Muller and Martin Funk. Nucleic Acids Research, 1994, Vol. 22, No. 25,
pp. 5767-5768. The Met25 promoter is 383 bases long (bases -382
to -1) and the genes expressed by this promoter are also known as Metl5,
Met17, and YLR303W. A preferred embodiment uses the sequence
below, where, at the 5' end of the sequence below, the.Not 1 site used in the
cloning is underlined and at the 3' end, the ATG start codon is
underlined:
GCGGCCGCCGGATGCAAGGGTTCGAATCCCTTAGCTCTCATTATTTTTTGCTTTTTCTCTTGAGGTCACATGATCGCAA
AATGGCAAA
TGGCACGTGAAGCTGTCGATATTGGGGAACTGTGGTGGTTGGCAAATGACTAATTAAGTTAGTCAAGGCGCCATCCTCA
TGAAAACT
GTGTAACATAATAACCGAAGTGTCGAAAAGGTGGCACCTTGTCCAATTGAACACGCTCGATGAAAAAAATAAGATATAT
ATAAGGTT
AAGTAAAGCGTCTGTTAGAAAGGAAGTTTTTCCTTTTTCTTGCTCTCTTGTCTTTTCATCTACTATTTCCTTCGTGTAA
TACAGGGTCGT
CAGATACATAGATACAATTCTATTACCCCCATCCATACAA_TC (SEQ ID N0:5)
[0317] Additional promoters that can be used to express the albumin fusion
protein in yeast include the following:
a) the cbhl promoter:
TCTAGAGTTGTGAAGTCGGTAATCCCGCTGTATAGTAATACGAGTCGCATCTAAATACTCCGAAGCTGCTGCGAACCCG
GA
GAATCGAGATGTGCTGGAAAGCTTCTAGCGAGCGGCTAAATTAGCATGAAAGGCTATGAGAAATTCTGGAGACGGCTTG
T
TGAATCATGGCGTTCCATTCTTCGACAAGCAAAGCGTTCCGTCGCAGTAGCAGGCACTCATTCCCGAAAAAACTCGGAG
A
TTCCTAAGTAGCGATGGAACCGGAATAATATAATAGGCAATACATTGAGTTGCCTCGACGGTTGCAATGCAGGGGTACT
G
AGCTTGGACATAACTGTTCCGTACCCCACCTCTTCTCAACCTTTGGCGTTTCCCTGATTCAGCGTACCCGTACAAGTCG
TAA
TCACTATTAACCCAGACTGACCGGACGTGTTTTGCCCTTCATTTGGAGAAATAATGTCATTGCGATGTGTAATTTGCCT
GCT
TGACCGACTGGGGCTGTTCGAAGCCCGAATGTAGGATTGTTATCCGAACTCTGCTCGTAGAGGCATGTTGTGAATCTGT
GT
CGGGCAGGACACGCCTCGAAGGTTCACGGCAAGGGAAACCACCGATAGCAGTGTCTAGTAGCAACCTGTAAAGCCGCAA
TGCAGCATCACTGGAAAATACAAACCAATGGCTAAAAGTACATAAGTTAATGCCTAAAGAAGTCATATACCAGCGGCTA
A
TAATTGTACAATCAAGTGGCTAAACGTACCGTAATTTGCCAACGGCTTGTGGGGTTGCAGAAGCAACGGCAAAGCCCCA
C
TTCCCCACGTTTGTTTCTTCACTCAGTCCAATCTCAGCTGGTGATCCCCCAATTGGGTCGCTTGTTTGTTCCGGTGAAG
TGA
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Ir .' 11 =Il !, AAdAAG=t10h't:6A;d4;r-,FAAlFi
~~ATGTCTGACTCGGA(3CGTTTTGCATACAACCAAGGGCAGTGATGGAAGACAGTGAAATGTT
GACATTCAAGGAGTATTTAGCCAGGGATGCTTGAGTGTATCGTGTAAGGAGGTTTGTCTGCCGATACGACGAATACTGT
AT
AGTCACTTCTGGTGAAGTGGTCCATATTGAAATGTAAGTCGGCACTGAACAGGCAAAAGATTGAGTTGAAACTGCCTAA
G
ATCTCGGGCCCTCGGGCCTTCGGCCTTTGGGTGTACATGTTTGTGCTCCGGGCAAATGCAAAGTGTGGTAGGATCGAAC
AC
ACTGCTGCCTTTACCAAGCAGCTGAGGGTATGTGATAGGCAAATGTTCAGGGGCCACTGCATGGTTTCGAATAGAAAGA
G
AAGCTTAGCCAAGAACAATAGCCGATAAAGATAGCCTCATTAAACGGAATGAGCTAGTAGGCAAAGTCAGCGAATGTGT
ATATATAAAGGTTCGAGGTCCGTGCCTCCCTCATGCTCTCCCCATCTACTCATCAACTCAGATCCTCCAGGAGACTTGT
AC
ACCATCTTTTGAGGCACAGAAACCCAATAGTCAACCGCGGACTGGCATC(SEQ ID NO:113)
b) the cysD promoter from Aspergillus nidulans:
AGATCTGGTTCCTGAGTACATCTACCGATGCGCCTCGATCCCCCTCTTAGCCGCATGAGATTCCTACCATTTATGTCCT
ATCG
TTCAGGGTCCTATTTGGACCGCTAGAAATAGACTCTGCTCGATTTGTTTCCATTATTCACGCAATTACGATAGTATTTG
GCTC
TTTTCGTTTGGCCCAGGTCAATTCGGGTAAGACGCGATCACGCCATTGTGGCCGCCGGCGTTGTGCTGCTGCTATTCCC
CGC
ATATAAACAACCCCTCCACCAGTTCGTTGGGCTTTGCGAATGCTGTACTCTATTTCAAGTTGTCAAAAGAGAGGATTCA
AAA
AATTATACCCCAGATATCAAAGATATCAAAGCCATC (SEQ ID NO: 114)
c) a modified cbhl promoter having the sequence:
TCTAGAGTTGTGAAGTCGGTAATCCCGCTGTATAGTAATACGAGTCGCATCTAAATACTCCGAAGCTGCTGCGAACCCG
GA
GAATCGAGATGTGCTGGAAAGCTTCTAGCGAGCGGCTAAATTAGCATGAAAGGCTATGAGAAATTCTGGAGACGGCTTG
T
TGAATCATGGCGTTCCATTCTTCGACAAGCAAA(3CGTTCCGTCGCAGTAGCAGGCACTCATTCCCGAAAAAACTCGGA
GA
TTCCTAAGTAGCGATGGAACCGGAATAATATAATAGGCAATACATTGAGTTGCCTCGACGGTTGCAATGCAGGGGTACT
G
AGCTTGGACATAACTGTTCCGTACCCCACCTCTTCTCAACCTTTGGCGTTTCCCTGATTCAGCGTACCCGTACAAGTCG
TAA
TCACTATTAACCCAGACTGACCGGACGTGTTTTGCCCTTCATTTGGAGAAATAATGTCATTGCGATGTGTAATTTGCCT
GCT
TGACCGACTGGGGCTGTTCGAAGCCCGAATGTAGGATTGTTATCCGAACTCTGCTCGTAGAGGCATGTTGTGAATCTGT
GT
CGGGCAGGACACGCCTCGAAGGTTCACGGCAAGGGAAACCACCGATAGCAGTGTCTAGTAGCAACCTGTAAAGCCGCAA
TGCAGCATCACTGGAAAATACAAACCAATGGCTAAAAGTACATAAGTTAATGCCTAAAGAAGTCATATACCAGCGGCTA
A
TAATTGTACAATCAAGTGGCTAAACGTACCGTAATTTGCCAACGGCTTGTGGGGTTGCAGAAGCAACGGCAAAGCCCCA
C
TTCCCCACGTTTGTTTCTTCACTCAGTCCAATCTCAGCTGGTGATCCCCCAATTGGGTCGCTTGTTTGTTCCGGTGAAG
TGA
AAGAAGACAGAGGTAAGAATGTCTGACTCGGAGCGTTTTGCATACAACCAAGGGCAGTGATGGAAGACAGTGAAATGTT
GACATTCAAGGAGTATTTAGCCAGGGATGCTTGAGTGTATCGTGTAAGGAGGTTTGTCTGCCGATACGACGAATACTGT
AT
AGTCACTTCTGGTGAAGTGGTCCATATTGAAATGTAAGTCGGCACTGAACAGGCAAAAGATTGAGTTGAAACTGCCTAA
G--
___
ATCTCGGGCCCTCGGGCCTTCGGCCTTTGGGTGTACATGTTTGTGCTCCGGGCAAATGCAAAGTGTGGTAGGATCGAAC
AC
ACTGCTGCCTTTACCAAGCAGCTGAGGGTATGTGATAGGCAAATGTTCAGGGGCCACTGCATGGTTTCGAATAGAAAGA
G
AAGCTTAGCCTGCAGCCTCTTATCGAGAA.AGAAATTACCGTCGCTCGTGATTTGTTTGCAAAAAGAACAAAACTGAAA
AA
ACCCAGACACGCTCGACTTCCTGTCTTCCTATTGATTGCAGCTTCCAATTTCGTCACACAACAAGGTCCTAGCTTAGCC
AA
GAACAATAGCCGATAAAGATAGCCTCATTAAACGGAATGAGCTAGTAGGCAAAGTCAGCGAATGTGTATATATAAAGGT
T
CGAGGTCCGTGCCTCCCTCATGCTCTCCCCATCTACTCATCAACTCAGATCCTCCAGGAGACTTGTACACCATCTTTTG
AGG
CACAGAAACCCAATAGTCAACCGCGGACTGGCATC (SEQ ID NO:115)
d) a cysD promoter from Aspergillus nidulans having the sequence:
AGATCTGGTTCCTGAGTACATCTACCGATGCGCCTCGATCCCCCTCTTAGCCGCATGAGATTCCTACCATTTATGTCCT
ATC
GTTCAGGGTCCTATTTGGACCGCTAGAAATAGACTCTGCTCGATTTGTTTCCATTATTCACGCAATTACGATAGTATTT
GGC
TCTTTTCGTTTGGCCCAGGTCAATTCGGGTAAGACGCGATCACGCCATTGTGGCCGCCGGCGCTGCAGCCTCTTATCGA
GA
AAGAAATTACCGTCGCTCGTGATTTGTTTGCAAAAAGAACAAAACTGAAAAAACCCAGACACGCTCGACTTCCTGTCTT
CC
TATTGATTGCAGCTTCCAATTTCGTCACACAACAAGGTCCTACGCCGGCGTTGTGCTGCTGCTATTCCCCGCATATAAA
CA
ACCCCTCCACCAGTTCGTTGGGCTTTGCGAATGCTGTACTCTATTTCAAGTTGTCAAAAGAGAGGATTCAAAAAATTAT
AC
CCCAGATATCAAAGATATCAAAGCCATC (SEQ ID NO:116)
[0318] A variety of methods have been developed to operably link DNA to
vectors via complementary cohesive termini. For instance,
complementary homopolymer tracts can be added to the DNA segment to be
inserted to the vector DNA. The vector and DNA segment are then
joined by hydrogen bonding between the complementary homopolymeric tails to
form recombinant DNA molecules.
[0319] Synthetic linkers containing one or more restriction sites provide an
alternative method of joining the DNA segment to vectors. The
DNA segment, generated by endonuclease restriction digestion, is treated with
bacteriophage T4 DNA polymerase or E. coli DNA polymerase I,
enzymes that remove protruding, gamma-single-stranded termini with their 3' 5'-
exonucleolytic activities, and fill in recessed 3'-ends with their
polymerizing activities.
61
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~' ~E.,, .~[03Z~}.~f ~~ :fiI~~'~~fi5ib9naYTdn~Yi~ tti~*se i4ot]l ities
therefore generates blunt-ended DNA segments. The blunt-ended segments are
then incubated with a
large molar excess of linker molecules in the presence of an enzyme that is
able to catalyze the ligation of blunt-ended DNA molecules, such as
bacteriophage T4 DNA ligase. Thus, the products of the reaction are DNA
segments carrying polymeric linker sequences at their ends. These DNA
segments are then cleaved with the appropriate restriction enzyme and ligated
to an expression vector that has been cleaved with an enzyme that
produces termini compatible with those of the DNA segment.
[0321] Synthetic linkers containing a variety of restriction endonuclease
sites are commercially available from a number of sources including
hiternational Biotechnologies Inc, New Haveti, CT, USA.
10322] A desirable way to modify the DNA in accordance with the invention, if,
for example, I-IA variants are to be prepared, is to use the
polymerase chain reaction as disclosed by Saiki et al. (1988) Scierice 239,
487-491. In this method the DNA to be enzymatically amplified is
flanked by two specific oligonucleotide primers which themselves become
incorporated into the amplified DNA. The specific primers may contain
restriction endonuclease recognition sites which can be used for cloning into
expression vectors using methods known in the art.
[0323] Exemplary genera of yeast contemplated to be useful in the practice of
the present invention as hosts for expressing the albumin fusion
proteins are Pichia (Hansenula), Saccltaromyces, Kluyveromyces, Candida,
Torulopsis, Torulaspora, SchLosaccharontyces, Citeroinyces,
Pachysolen, Debaronryces, MetscTmnikotivia, Rhadosporiditnn, Leucosporidium,
Botryoascus, Sporidiobolus, Ettdomycopsis, and the like. Preferred
genera are those selected from the group consisting of Saccharomyces,
SchfcosaccTtaromyces, Kluyveromyces, Pichia and Torulaspora. Examples
of Saccharornyces spp. are S. cerevisiae, S. italicus and S. rotctii.
[0324] Examples of Kluyveromyces spp. are K. fragilis, K. lactis and K.
marxianus. A suitable Torulaspora species is T. delbrueckii. Examples
of Pichia (Hansenula) spp. are P. angusta (formerly H. polymorpha), P. anomala
(formerly H. anomala) and P. pastoris. Methods for the
transformation of S. cerevisiae are taught generally in EP 251 744, EP 258 067
and WO 90/01063, all of which are incorporated herein by reference.
[03251 Preferred exemplary species of Saccharomyces include S. cerevisiae, S.
italicus, S. diastaticus, and Zygosaccharomyces rouxii.
Preferred exemplary species of Kluyveromyces include K. fragilis and K.
lactis. Preferred exemplary species of Hansenula include H. polymorpha
(now Pichia angusta), H. anomala (now Pichia anomala), and Pichia capsulata.
Additional preferred exemplary species of Pichia include P. pastoris.
Preferred exemplary species of Aspergillus include A. niger and A. nidulans.
Preferred exemplary species of Yarrowia include Y. lipolytica. Many
preferred yeast species are available from the ATCC. For example, the
following preferred yeast species are available from the ATCC and are
useful in the expression of albumin fusion proteins: Saccharomyces cerevisiae
Hansen, teleomorph strain BY4743 yap3 mutant (ATCC Accession
No. 4022731); Saccliaromyces cerevisiae Hansen, teleomorph strain BY4743
hsp150 mutant (ATCC Accession No. 4021266); Saccharomyces
cerevisiae Hansen, teleomorph strain BY4743 pmtl mutant (ATCC Accession No.
4023792); Saccharomyces cerevisiae Hansen, teleomorph
(ATCC Accession Nos. 20626; 44773; 44774; and 62995); Saccharomyces
diastaticus Andrews et Gilliland ex van der Walt, teleomorph (ATCC
Accession No. 62987); Kluyveromyces lactis (Dombrowski) vander Walt,
teleomorph- (ATCC_Accession No. 76492); Pichia angusta-(Teunisson-et-
---
al.) Kurtzman, teleomorph deposited as Hansenula polymorpha de Morais et Maia,
teleomorph (ATCC Accession No. 26012); Aspergillus niger van
Tieghem, anamorph (ATCC Accession No. 9029); Aspergillus niger van Tieghem,
anamorph (ATCC Accession No. 16404); Aspergillus nidulans
(Eidam) Winter, anamorph (ATCC Accession No. 48756); and Yarrowia lipolytica
(Wickerham et al.) van der Walt et von Arx, teleomorph (ATCC
Accession No. 201847).
[0326] Suitable promoters for S. cerevisiae include those associated with the
PGKI gene, GALl or GALIO genes, CYCI, PHO5, TRPI, ADHI,
ADH2, the genes for glyceraldehyde-3-phosphate dehydrogenase, hexokinase,
pyruvate decarboxylase, phosphofructokinase, triose phosphate
isomerase, phosphoglucose isomerase, glucokinase, alpha-mating factor
pheromone, [a mating factor pheromone], the PRBI promoter, the GUT2
promoter, the GPDI promoter, and hybrid promoters involving hybrids of parts
of 5' regulatory regions with parts of 5' regulatory regions of other
promoters or with upstream activation sites (e.g. the promoter of EP-A-258
067).
[0327] Convenient regulatable promoters for use in Schiosaccharomyces pontbe
are the thiamine-repressible promoter from the nmt gene as
described by Maundrell (1990) J. Biol. Chent. 265, 10857-10864 and the glucose
repressible jbpl gene promoter as described by Hoffman &
Winston (1990) Genetics 124, 807-816.
[0328] Methods of transfozming Pichia for expression of foreign genes are
taught in, for example, Cregg et al. (1993), and various Phillips
patents (e.g. US 4 857 467, incorporated herein by reference), and Pichia
expression kits are commercially available from Invitrogen BV, Leek,
Netherlands, and Invitrogen Corp., San Diego, California. Suitable promoters
include AOXI and AOX2. Gleeson et al. (1986) J. Gen. Microbiol.
132, 3459-3465 include information on Hansenula vectors and transformation,
suitable promoters being MOXI and FMD1; whilst EP 361 991,
Fleer et al. (1991) and other- publications from Rhone-Poulenc Rorer teach how
to express foreign proteins in Klu}rnerontyces spp., a suitable
promoter being PGKI.
[0329] The transcription termination signal is preferably the 3' flanking
sequence of a eukaryotic gane which contains proper signals for
transcription termination and polyadenylation. Suitable 3' flanking sequences
may, for example, be those of the gene naturally linked to the
expression control sequence used, i.e. may correspond to the promoter.
Alternatively, they may be different in which case the termination signal of
the S. cerevisiae ADHI gene is preferred.
62
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
...,, _ .
,,, 0390 ~. 11 ?;SYed alt9 i trI px tein may be initially expressed with a
secretion leader sequence, which may be any leader effective in the
yeast cliosen. Leaders useful in yeast include any of the following:
a) the MPIF-1 signal sequence (e.g., amino acids 1-21 of GenBank Accession
number AAB51134) IvIKVSVAALSCLMLVTALGSQA
(SEQ ID NO:6)
b) the stanniocalcin signal sequence (MLQNSAVLLLLVISASA, SEQ ID NO:7)
c) the pre-pro region of the HSA signal sequence (e.g.,
MKWVTFISLLFLFSSAYSRGVFRR, SEQ ID N0:8)
d) the pre region of the HSA signal sequence (e.g., MKWVTFISLLFLFSSAYS, SEQ ID
NO:9) or variants thereof, such as, for example,
MKWVSFISLLFLFSSAYS, (SEQ ID N0:10)
e) the invertase signal sequence (e.g., MLLQAFLFLLAGFAAKISA, SEQ ID NO: 11)
f) the yeast mating factor alpha signal sequence (e.g.,
MRFPSIFTAVLAFAASSALAAPVNTTTEDETAQIPAEAVIGYSDLEGDFDVAVLPFSNSTNNGLLFINTTIASIAAKEE
GVSLEKR,
SEQ ID NO:12 or
MRFPSIFTAVLAFAAS
SALAAPVNTTTEDETAQIPAEAVIGYSDLEGDFDVAVLPFSNSTNNGLLFINTTIASIAAKEEGVSLDKR,
SEQ ID NO: 12)
g) K. lactis killer toxin leader sequence
li) a hybrid signal sequence (e.g., MKWVSFISLLFLFSSAYSRSLEKR, SEQ ID NO:13)
i) an HSA/Wa-1 hybrid signal sequence (also known as HSA/kex2) (e.g.,
MKWVSFISLLFLFSSAYSRSLDKR, SEQ ID NO:14)
j) a P. lactis killer/ MFa-1 fusion leader sequence (e.g.,
MNIFYIFLFLLSFVQGSLDKR, SEQ ID NO:15)
k) the Immunoglobulin Ig signal sequence (e.g., MGWSCIILFLVATATGVHS, SEQ ID
NO:16)
1) the Fibulin B precursor signal sequence (e.g.,
MERAAPSRRVPLPLLLLGGLALLAAGVDA, SEQ ID NO: 17)
m) the clusterin precursor signal sequence (e.g., MMKTLLLFVGLLLTWESGQVLG, SEQ
ID NO:18)
n) the insulin-like growth factor-binding protein 4 signal sequence (e.g.,
MLPLCLVAALLLAAGPGPSLG, SEQ ID NO:19)
o) variants of the pre-pro-region of the HSA signal sequence such as, for
example,
MKWVSFISLLFLFSSAYSRGVFRR (SEQ ID NO:20),
MKWVTFISLLFLFAGVLG (SEQ ID NO:21),
MKWVTFISLLFLFSGVLG (SEQ ID NO:22),
MKWVTFISLLFLFGGVLG (SEQ ID NO:23),
Modified HSA leader HSA #64 - MKWVTFISLLFLFAGVSG (SEQ ID NO:24);
Modified HSA leader HSA #66 - MKW VTFISLLFLFGGVSG (SEQ ID NO:25);
Modified HSA (A14) leader - MKWVTFISLLFLFAGVSG (SEQ ID NO:26);
Modified HSA (S 14) leader (also known as modified HSA #65) -
MKWVTFISLLFLFSGVSG (SEQ ID NO:27),
Modified HSA (G14) leader - MKWVTFISLLFLFGGVSG (SEQ ID NO:28), or
MKWVTFISLLFLFGGVLGDLHKS (SEQ ID NO:29)
p) a consensus signal sequence (MPTWAWWLFLVLLLALWAPARG, SEQ ID NO:30)
q) acid phosphatase (PH05) leader (e.g., MFKSVVYSILAASLANA SEQ ID NO:31)
r) the pre-sequence of MFoz-1
s) the pre-sequence of 0 glucanase (BGL2)
t) killer toxin leader
u) the presequence of killer toxin
v) k. lactis killer toxin prepro (29 amino acids; 16 amino acids of pre and 13
amino acids of pro)
MNIFYIFLFLLSFVQGLEHTHRRGSLDKR (SEQ ID NO:32)
w) S. diastaticus glucoarnylase Il secretion leader sequence
x) S. carlsbergensis a-galactosidase (MELI) secretion leader sequence
y) Candida glucoarnylase leader sequence
z) The hybrid leaders disclosed in EP-A-387 319 (herin incorporated by
reference)
aa) the gp67 signal sequence (in conjunction with baculoviral expression
systems) (e.g., amino acids 1-19 of GenBank Accession Number
AAA72759) or
bb) the natural leader of the therapeutic protein X;
cc) S. cerevisiae invertase (SUC2) leader, as disclosed in JP 62-096086
(granted as 911036516, herein incorporate by reference); or
dd) Inulinase - MKLAYSLLLPLAGVSASVINYKR (SEQ ID NO:33).
ee) A modified TA57 propeptide leader variant # 1-
MKLKTVRSAVLSSLFASQVLGQPIDDTESQTTSVNLMADDTESAFATQTNSGGLDVVGLISMAKR (SEQ ID
NO:34)
63
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
"119 ,.-tlA'nyd'dified TW7"[Yro:&ifClUe,Ieader variant #2 -
MKLKTVRSAVLSSLFASQVLGQPIDDTESQTTSVNLMADDTESAFATQTNSGGLDWGLISMAEEGEPKR (SEQ ID
NO:35)
gg) A consensus signal peptide - MWWRLWWLLLLLLLLWPMVWA (SEQ IDNO:111)
hh) A modified HSA/kex2 signal sequence- MKWVSFISLLFLFSSAYSGSLDICR (SEQ ID NO:
112)
ii) A consensus signal peptide #2 - MRPTWAW WLFLVLLLALWAPARG (SEQ ID NO:105)
Additionalllletltods ofRecombirmrrt and Syutleetic Production ofAlhluuiit
Firsiort Proteins
[0331] The present invention also relates to vectors containing a
polynucleotide encoding an albumin fusion protein of the present invention,
host cells, and the production of albumin fusion proteins by synthetic and
recombinant techniques. The vector may be, for example, a phage,
plasmid, viral, or retroviral vector. Retroviral vectors may be replication
competent or replication defective. In the latter case, viral propagation
generally will occur only in complementing host cells.
[0332] The polynucleotides encoding albumin fusion proteins of the invention
may be joined to a vector containing a selectable marker for
propagation in a host. Generally, a plasmid vector is introduced in a
precipitate, such as a calcium phosphate precipitate, or in a complex with a
charged lipid. If the vector is a virus, it may be packaged in vitro using an
appropriate packaging cell line and then transduced into host cells.
[0333] The polynucleotide insert should be operatively linked to an
appropriate promoter, such as the phage lambda PL promoter, the E. coli
Iac, tip, phoA and tac promoters, the SV40 early and late promoters and
promoters of retroviral LTRs, to name a few. Other suitable promoters will
be ]mown to the skilled artisan. The expression constructs will further
contain sites for transcription initiation, termination, and, in the
transcribed
region, a ribosome binding site for translation. The coding portion of the
transcripts expressed by the constructs will preferably include a translation
initiating codon at the beginning and a termination codon (UAA, UGA or UAG)
appropriately positioned at the end of the polypeptide to be
translated.
[0334] As indicated, the expression vectors will preferably include at least
one selectable marker. Such markers include dihydrofolate reductase,
G418, glutamine synthase, or neomycin resistance for eukaryotic cell culture,
and tetracycline, kanamycin or ampicillin resistance genes for
culturing in E. coli and other bacteria. Representative examples of
appropriate hosts include, but are not limited to, bacterial cells, such as E.
coli,
Streptomyces and Salmonella typhin:urium cells; fungal cells, such as yeast
cells (e.g., Saccharomyces cerevisiae or Pichia pastoris (ATCC
Accession No. 201178)); insect cells such as Drosophila S2 and Spodoptera Sf9
cells; animal cells such as CHO, COS, NSO, 293, and Bowes
melanoma cells; and plant cells. Appropriate culture mediums and conditions
for the above-described host cells are known in the art.
[0335] Among vectors preferred for use in bacteria include pQE70, pQE60 and
pQE-9, available from QIAGEN, Inc.; pBluescript vectors,
Phagescript vectors, pNH8A, pNH16a, pNH18A, pNH46A, available from Stratagene
Cloning Systems, Inc.; and ptrc99a, pKK223-3, pKK233-3,
pDR540, pRIT5 available from Pharmacia Biotech, Inc. Among preferred
eukaryotic vectors are pWLNEO, pSV2CAT, pOG44, pXT1 and pSG
available from Stratagene; and pSVK3, pBPV, pMSG and pSVL available from
Pharmacia. Preferred expressionvectors for use in-yeast systems-_-_
include, but are not limited to pYES2, pYDt, pTEFt/Zeo, pYES2/GS, pPICZ,
pGAPZ, pGAPZalph, pPIC9, pPIC3.5, pHIL-D2, pHIL-S1,
pPIC3.5K, pPIC9K, and PA0815 (all available from Invitrogen, Carlbad, CA).
Other suitable vectors will be readily apparent to the skilled artisan.
[0336] In one embodiment, polynucleotides encoding an albumin fusion protein
of the invention may be fused to signal sequences which will
direct the localization of a protein of the invention to particular
comparlments of a prokaryotic or eukaryotic cell and/or direct the secretion
of a
protein of the invention from a prokaryotic or eukaryotic cell. For example,
in E. coli, one may wish to direct the expression of the protein to the
periplasmic space. Examples of signal sequences or proteins (or fragments
thereof) to which the albumin fusion proteins of the invention may be
fused in order to direct the expression of the polypeptide to the periplasmic
space of bacteria include, but are not limited to, the pe1B signal
sequence, the maltose binding protein (MBP) signal sequence, MBP, the ornpA
signal sequence, the signal sequence of the periplasmic E. colf heat-
labile enterotoxin B-subunit, and the signal sequence of alkaline phosphatase.
Several vectors are commercially available for the construction of
fusion proteins which will direct the localization of a protein, such as the
pMAL series of vectors (particularly the pMAL-p series) available from
New England Biolabs. In a specific embodiment, polynucleotides albumin fusion
proteins of the invention may be fused to the pe1B pectate lyase
signal sequence to increase the efficiency of expression and purification of
such polypeptides in Gram-negative bacteria. See, U.S. Patent Nos.
5,576,195 and 5,846,818, the contents of which are herein incorporated by
reference in their entireties.
[0337] Examples of signal peptides that may be fused to an albumin fusion
protein of the invention in order to direct its secretion in mammalian
cells include, but are not limited to:
a) the MPIF-1 signal sequence (e.g., amino acids 1-21 of GenBank Accession
number AAB51134) MKVSVAALSCLMLVTALGSQA
(SEQ ID NO:6)
b) the stanniocalcin signal sequence (MI.,QNSAVLLLLVISASA, SEQ ID NO:7)
c) the pre-pro region of the HSA signal sequence (e.g.,
MKWVTFISLLFLFSSAYSRGVFRR, SEQ ID NO:8)
d) the pre region of the HSA signal sequence (e.g., MKWVTFISLLFLFSSAYS, SEQ ID
NO:9) or variants thereof, such as, for example,
MKWVSFISLLFLFSSAYS, (SEQ ID NO:10)
e) the invertase signal sequence (e.g., MLLQAFLFLLAGFAAKISA, SEQ ID NO:11)
64
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ 't~~ig ~ . ,.~ faLt~
~)=lE nia~tor:UpR[i,sqgnal sequence (e.g.,
MRFPSIFTAVLAFAASSALAAP
VNTTTEDETAQIPAEAVIGYSDLEGDFDVAVLPFSNSTNNGLLFINTTIASIAAKEEGVSLEKR,
SEQ ID NO: 12 or
MRFPSIFTAVLAFAASSALAAPVNTTTEDETAQIPAEAVIGYSDLEGDFDVAVLPFSNSTNNGLLFINTTIASIAAKEE
GVSLDKR,
SEQ ID NO: 12)
g) K. lactis killer toxin leader sequence
h) a hybrid signal sequence (e.g., MKWVSFISLLFLFSSAYSRSLEKR, SEQ ID NO:13)
i) an HSA/MFa-1 hybrid signal sequence (also known as HSA/kex2) (e.g.,
MKWVSFISLLFLFSSAYSRSLDICR, SEQ ID NO:14)
j) a K lactis killer/ MFa-1 fusion leader sequence (e.g.,
MNIFYIFLFLLSFVQGSLDKR, SEQ ID NO:15)
k) the Immunoglobulin Ig signal sequence (e.g., MGWSCIILFLVATATGVHS, SEQ ID
NO:16)
I) the Fibulin B precursor signal sequence (e.g.,
MERAAPSRRVPLPLLLLGGLALLAAGVDA, SEQ ID NO:17)
m) the clusterin precursor signal sequence (e.g., MMKTLLLFVGLLLTWESGQVLG, SEQ
ID NO:18)
n) the insulin-like growth factor-binding protein 4 signal sequence (e.g.,
MLPLCLVAALLLAAGPGPSLG, SEQ ID NO:19)
o) variants of the pre-pro-region of the HSA signal sequence such as, for
example,
MKWVSFISLLFLFSSAYSRGVFRR (SEQ ID NO:20),
MKWVTFISLLFLFAGVLG (SEQ ID NO:21),
MKWVTFISLLFLFSGVLG (SEQ ID NO:22),
MKWVTFISLLFLFGGVLG (SEQ ID NO:23),
Modified HSA leader HSA #64 - MKWVTFISLLFLFAGVSG (SEQ ID NO:24);
Modified HSA leader HSA #66 - MKWVTFISLLFLFGGVSG (SEQ ID NO:25);
Modified HSA (A14) leader - MKWVTFISLLFLFAGVSG (SEQ ID NO:26);
Modified HSA (S 14) leader (also known as modified HSA #65) -
MKWVTFISLLFLFSGVSG (SEQ ID NO:27),
Modified HSA (G14) leader- MKWVTFISLLFLFGGVSG (SEQ ID NO:28), or
MKWVTFISLLFLFGGVLGDLHKS (SEQ ID NO:29)
p) a consensus signal sequence (MPTWAWWLFLVLLLALWAPARG, SEQ ID NO:30)
q) acid phosphatase (PH05) leader (e.g., MFKSVVYSILAASLANA SEQ ID NO:31)
r) the pre-sequence of MFoz-1
s) the pre-sequence of 0 glucanase (BGL2)
t) killer toxin leader
u) the presequence of killer toxin
v) k. lactis killer toxin prepro (29 amino acids; 16 amino acids of pre and 13
amino acids of pro)
MNIFYIFLPLLSFVQGLEHTHItRGSLDKR (SEQ ID NO:32)
w) S. diastaticzrs glucoamylase 11 secretion leader sequence
x) S. carlsbergensis (x-galactosidase (MEL1) secretion leader sequence
y) Candida glucoarnylaseleadersequence
z) The hybrid leaders disclosed in EP-A-387 319 (herin incorporated by
reference)
aa) the gp67 signal sequence (in conjunction with baculoviral expression
systems) (e.g., amino acids 1-19 of GenBank Accession Number
AAA72759) or
bb) the natural leader of the therapeutic protein X;
cc) S. cerevisiae invertase (SUC2) leader, as disclosed in JP 62-096086
(granted as 911036516, herein incorporate by reference); or
dd) Inulinase - MI{I.AYSLLLPLAGVSASVINYKR (SEQ ID NO:33).
ee) A modified TA57 propeptide leader variant # 1-
MKLKTVRSAVLSSLFASQVLGQPIDDTESQTTSVNLMADDTESAFATQTNSGGLDVVGLISMAKR (SEQ ID
NO:34)
ff) A modified TA57 propeptide leader variant #2 -
MIfLKTVRSAVLSSLFASQVLGQPIDDTESQTTSVNLMADDTESAFATQTNSGGLDWGLISMAEEGEPKR (SEQ ID
NO:35)
gg) A consensus signal peptide - IvIW WRLW WLLLLLLLLWPMV WA (SEQ ID NO:111)
jj) A modified HSA/kex2 signal sequence- MKWVSFISLLFLFSSAYSGSLDKR (SEQ ID NO:
112)
kk) A consensus signal peptide #2 - MRPTWAW WLFLVLLLALWAPARG (SEQ ID NO:105)
(0338] In a preferred embodiment, the modified HSA/kex2 signal sequence (SEQ
ID NO:112) is fused to the amino terminus of an albumin
fusion protein, including fusion proteins comprising albumin and a therapeutic
protein as described herein, as well as albumin fusion proteins
disclosed in W093/15199; W097/24445; W003/60071; W003/59934; and
PCT/USO4/01369, each of which are incorporated herein by reference
in their entireties. The modified HSA/kex2 signal sequence is based on the
HSA/kex2 signal sequence (SEQ ID NO: 14) disclosed, e.g., in Sleep et
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
t"' ~G, , Ilal,;'BPoieclifi~fldg~~7990,i1,uo1'.'"~,,=p~s."?S~,ACa; and US
Patent 5,302,697, both of which are incorporated herein by reference in their
entireties. The
modified HSA/kex2 leader sequence disclosed herein contains a non-conservative
amino acid substitution (Arg to Gly) at residue 19 of the parent
signal peptide. The modified HSA/kex2 signal peptide has been found to produce
unexpectedly better expression yield and/or better cleavage
efficiency of albumin fusion proteins when expressed in yeast than the
unmodified HSA/kex2 signal sequence. Variants of the modified HSA/kex2
signal peptide are also encompassed by the invention. In particular the Gly
residue at position 19 of SEQ ID NO: 112 may be substituted with a Pro
residue. Otlier conservative substitution variants of the modified HSA/kex2
signal sequence are also contemplated. Nucleic acids encoding the
modified HSA/kex2 signal sequence of SEQ ID NO: 112, as well as conservative
substitution variants thereof, are also encompassed by the
invention.
[0339] Vectors which use glutamine synthase (GS) or DHFR as the selectable
markers can be amplified in the presence of the drugs methionine
sulphoximine or methotrexate, respectively. An advantage of glutamine synthase
based vectors are the availability of cell lines (e.g., the murine
myeloma cell line, NSO) which are glutamine synthase negative. Glutamine
synthase expression systems can also function in glutamine synthase
expressing cells (e.g., Chinese Hamster Ovary (CHO) cells) by providing
additional inhibitor to prevent the functioning of the endogenous gene. A
glutamine synthase expression system and components thereof are detailed in
PCT publications: W087/04462; WO86/05807; WO89/01036;
W089/10404; and WO91/06657, tvhich are hereby incorporated in their entireties
by reference herein. Additionally, glutamine synthase expression
vectors can be obtained from Lonza Biologics, Inc. (Portsmouth, NH).
Expression and production of monoclonal antibodies using a GS expression
system in murine myeloma cells is described in Bebbington et al.,
Bio/techisolog,y 10:169(1992) and in Biblia and Robinson Biotechnol. Prog.
11:1
(1995) which are herein incorporated by reference.
[0340] The present invention also relates to host cells containing the above-
described vector constructs described herein, and additionally
encompasses host cells containing nucleotide sequences of the invention that
are operably associated with one or more heterologous control regions
(e.g., promoter and/or enhancer) using techniques known of in the art. The
host cell can be a higher eukaryotic cell, such as a mammalian cell (e.g.,
a human derived cell), or a lower eukaryotic cell, such as a yeast cell, or
the host cell can be a prokaryotic cell, such as a bacterial cell. A host
strain
may be chosen which modulates the expression of the inserted gene sequences,
or modifies and processes the gene product in the specific fashion
desired. Expression from certain promoters can be elevated in the presence of
certain inducers; thus expression of the genetically engineered
polypeptide may be controlled. Furtliermore, different host cells have
characteristics and specific mechanisms for the translational and post-
translational processing and modification (e.g., phosphorylation, cleavage) of
proteins. Appropriate cell lines can be chosen to ensure the desired
modifications and processing of the foreign protein expressed.
[0341] Introduction of the nucleic acids and nucleic acid constructs of the
invention into the host cell can be effected by calcium phosphate
transfection, DEAE-dextran mediated transfection, cationic lipid-mediated
transfection, electroporation, transduction, infection, or other methods.
Such methods are described in many standard laboratory manuals, such as Davis
et al., Basic Methods In Molecular Biology _(19_86). . It is__
specifically contemplated that the polypeptides of the present invention may
in fact be expressed by a host cell lacking a recombinant vector.
[0342] In addition to encompassing host cells containing the vector constructs
discussed herein, the invention also encompasses primary,
secondary, and immortalized host cells of vertebrate origin, particularly
mammalian origin, that have been engineered to delete or replace
endogenous genetic material (e.g., the coding sequence corresponding to a
Therapeutic protein may be replaced with an albumin fusion protein
corresponding to the Therapeutic protein), and/or to include genetic material
(e.g., heterologous polynucleotide sequences such as for example, an
albumin fusion protein of the invention corresponding to the Therapeutic
protein may be included). The genetic material operably associated with
the endogenous polynucleotide may activate, alter, and/or amplify endogenous
polynucleotides.
[0343] In addition, techniques known in the art may be used to operably
associate heterologous pol}mucleotides (e.g., polynucleotides encoding
an albumin protein, or a fragment or variant thereof) and/or heterologous
control regions (e.g., promoter and/or enhancer) with endogenous
polynucleotide sequences encoding a Therapeutic protein via homologous
recombination (see, e.g., US Patent Number 5,641,670, issued June 24,
1997; International Publication Number WO 96/29411; International Publication
Number WO 94/12650; Koller et al., Proc. Natl. Acad. Sci. USA
86:8932-8935 (1989); and Zijlstra et al., Nature 342:435-438 (1989), the
disclosures of each of which are incorporated by reference in their
entireties).
[0344] Albumin fusion proteins of the invention can be recovered and purified
from recombinant cell cultures by well-Imown methods including
ammonium sulfate or ethanol precipitation, acid extraction, anion or cation
exchange chromatography, phosphocellulose chromatography,
hydrophobic interaction chromatography, affinity chromatography,
hydroxylapatite chromatography, hydrophobic charge interaction
chromatography and lectin chromatography. Most preferably, high performance
liquid chromatography ("HPLC") is employed for purification.
[0345] In preferred embodiments the albumin fusion proteins of the invention
are purified using Anion Exchange Chromatography including,
but not limited to, chromatography on Q-sepharose, DEAE sepharose, poros HQ,
poros DEAE, Toyopearl Q, Toyopearl QAE, Toyopearl DEAE,
Resource/Source Q and DEAE, Fractogel Q and DEAE columns.
[0346] In specific embodiments the albumin fusion proteins of the invention
are purified using Cation Exchange Chromatography including, but
not limited to, SP-sepharose, CM sepharose, poros HS, poros CM, Toyopearl SP,
Toyopearl CM, Resource/Source S and CM, Fractogel S and CM
66
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
'} ~i= = = bol~imr~s=Ilarii~~hY~e~r~~e(4rival~nts a]id 'obii ]~ar~bles.
[0347] In specific embodiments the albumin fusion proteins of the invention
are purified using Hydrophobic hiteraction Chromatography
including, but not limited to, Phenyl, Butyl, Methyl, Oatyl, Hexyl-sepharose,
poros Phenyl, Butyl, Methyl, Octyl, Hexyl, Toyopearl Phenyl, Butyl,
Metiyl, Octyl, HexWl Resource/Source Phenyl, Butyl, Methyl, Octyl, Hexyl,
Fractogel Phenyl, Butyl, Methyl, Octyl, Hexyl columns and their
equivalents and comparables.
[03481 In specific embodiments the albumin fusion proteins of the invention
are purified using Size Exclusion Chromatography including, but
not limited to, sepharose S 100, S200, S300, superdex resin columns and their
equivalents and coniparables.
[0349] In specific embodiments the albumin fusion proteins of the invention
are purified using Affnity Chromatography including, but not
limited to, Mimetic Dye affinity, peptide affinity and antibody affinity
columns that are selective for either the HSA or the "fusion target"
molecules.
[0350] In preferred embodiments albumin fusion proteins of the invention are
purified using one or more Chromatography methods listed
above. In other preferred embodiments, albumin fusion proteins of the
invention are purified using one or more of the following Chromatography
columns, Q sepharose FF column, SP Sepharose FF column, Q Sepharose High
Performance Column, Blue Sepharose FF column , Blue Colunm,
Phenyl Sepharose FF column, DEAE Sepharose FF, or Methyl Column.
[03511 Additionally, albumin fusion proteins of the invention may be purified
using the process described in PCT International Publication WO
00/44772 which is herein incorporated by reference in its entirety. One of
skill in the art could easily modify the process described therein for use in
the purification of albumin fusion proteins of the invention.
[03521 Albumin fusion proteins of the present invention may be recovered from:
products of chemical synthetic procedures; and products
produced by recombinant techniques from a prokaryotic or eukaryotic host,
including, for example, bacterial, yeast, higher plant, insect, and
mammalian cells. Depending upon the host employed in a recombinant production
procedure, the polypeptides of the present invention may be
glycosylated or may be non-glycosylated. In addition, albumin fusion proteins
of the invention may also include an initial modified methionine
residue, in some cases as a result of host-mediated processes. Tlius, it is
well known in the art that the N-terminal methionine encoded by the
translation initiation codon generally is removed with high efficiency from
any protein after translation in all eukaryotic cells. While the N-terminal
methionine on most proteins also is efficiently removed in most prokaryotes,
for some proteins, this prokaryotic removal process is inefficient,
depending on the nature of the amino acid to which the N-terminal methionine
is covalently linked.
[03531 In one embodiment, the yeast Pichia pastoris is used to express albumin
fusion proteins of the invention in a eukaryotic system. Pichia
pastoris is a methylotrophic yeast which can metabolize methanol as its sole
carbon source. A main step in the methanol metabolization pathway is
the oxidation of methanol to formaldehyde using 02. This reaction is catalyzed
by the enzyme alcohol oxidase. In order to metabolize metlianol as
its sole carbon source, Pichia pastoris must generate high levels of alcohol
oxidase due, in part, to the relatively low affinity of alcohol oxidase for
02. Consequently, in a growth medium depending on methanol as a main carbon
source, the promoter region of.one of the-two alcohol oxidase -
genes (AOXI) is highly active. In the presence of methanol, alcohol oxidase
produced from the AOX1 gene comprises up to approximately 30% of
the total soluble protein in Pichia pastoris. See Ellis, S.B., et al., Mol.
Cell. Biol. 5:1111-21 (1985); Koutz, P.J, et al., Yeast 5:167-77 (1989);
Tschopp, J.F., et al., Nucl. Acids Res. 15:3859-76 (1987). Thus, a
heterologous coding sequence, such as, for example, a polynucleotide of the
present invention, under the transcriptional regulation of all or part of the
AOX1 regulatory sequence is expressed at exceptionally high levels in
Pichia yeast grown in the presence of methanol.
[0354] In one example, the plasmid vector pPIC9K is used to express DNA
encoding an albumin fusion protein of the invention, as set forth
herein, in a Pichea yeast system essentially as described in "Pichia
Protocols: Methods in Molecular Biology," D.R. Higgins and J. Cregg, eds. The
Humana Press, Totowa, NJ, 1998. This expression vector allows expression and
secretion of a polypeptide of the invention by virtue of the strong
AOXI promoter linked to the Pichia pastoris alkaline phosphatase (PHO)
secretory signal peptide (i.e., leader) located upstream of a multiple
cloning site.
[0355] Many other yeast vectors could be used in place of pPIC9K, such as,
pYES2, pYD1, pTEFl/Zeo, pYES2/GS, pPICZ, pGAPZ,
pGAPZalpha, pPIC9, pPIC3.5, pHIL-D2, pHIL-S l, pPIC3.51C, and PAO815, as one
skilled in the art would readily appreciate, as long as the
proposed expression construct provides appropriately located signals for
transcription, translation, secretion (if desired), and the like, including an
in-frame AUG as required.
[0356] In another embodiment, high-level expression of a heterologous coding
sequence, such as, for example, a polynucleotide encoding an
albumin fusion protein of the present invention, may be achieved by cloning
the heterologous polynucleotide of the invention into an expression
vector such as, for example, pGAPZ or pGAPZalpha, and growing the yeast
culture in the absence of methanol.
[0357] In addition, albumin fusion proteins of the invention can be chemically
synthesized using techniques known in the art (e.g., see
Creighton, 1983, Proteins: Structures and Molecular Principles, W.H. Freeman &
Co., N.Y., and Hunkapiller et al., Nature, 310:105-111 (1984)).
For example, a polypeptide corresponding to a fragment of a polypeptide can be
synthesized by use of a peptide synthesizer. Furthermore, if
desired, nonclassical amino acids or chemical amino acid analogs can be
introduced as a substitution or addition into the polypeptide sequence.
Non-classical amino acids include, but are not limited to, to the D-isomers of
the connnon amino acids, 2,4-diaminobutyric acid, a-amino isobutyric
67
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
" 1~ n4 alcid, 4~a~in~1~~~~~iclia~id 11Abu;~~2 a[n~ii~i =t]{~iyric acid, g-
Abu, e-Ahx, 6-amino hexanoic acid, Aib, 2-amino isobutyric acid, 3-amino
propionic acid,
omithine, norleucine, norvaline, hydroxyproline, sarcosine, citrulline,
homocitrulline, cysteic acid, t-butylglycine, t-butylalanine, phenylglycine,
cyclohexylalanine, b-alanine, fluoro-amino acids, designer amino acids such as
b-methyl amino acids, Ca-methyl amino acids, Na-methyl amino
acids, and amino acid analogs in general. Furthermore, the amino acid can be
D(dextrorotary) or L(levorotary).
[0358] The invention encompasses albumin fusion proteins of the present
invention which are differentially modified during or after translation,
e.g., by glycosylation, acetylation, phosphorylation, amidation,
derivatization by known protecting/blocking groups, proteolytic cleavage,
linkage to
an antibody molecule or other cellular ligand, etc. Any of numerous chemical
modifications may be carried out by known techniques, including but
not limited, to specific chemical cleavage by cyanogen bromide, trypsin,
chymotrypsin, papain, V8 protease, NaBHa; acetylation, formylation,
oxidation, reduction; metabolic synthesis in the presence of tunicamycin; etc.
[0359] Additional post-translational modifications encompassed by the
invention include, for example, e.g., N-linked or 0-linked carbohydrate
chains, processing of N-terminal or C-terminal ends), attachment of chemical
moieties to the amino acid backbone, chemical modifications of
N-linked or 0-linked carbohydrate chains, and addition or deletion of an N-
terminal methionine residue as a result of procaryotic host cell
expression. The albumin fusion proteins may also be modified with a detectable
label, such as an enzymatic, fluorescent, isotopic or affinity label to
allow for detection and isolation of the protein.
[0360] Examples of suitable enzymes include horseradish peroxidase, alkaline
phosphatase, beta-galactosidase, or acetylcholinesterase;
examples of suitable prosthetic group complexes include streptavidin/biotin
and avidin/biotin; examples of suitable fluorescent materials include
umbelliferone, fluorescein, fluorescein isothiocyanate, rhodamine,
dichlorotriazinylamine fluorescein, dansyl chloride or phycoerythrin; an
example
of a luminescent material includes luminol; examples of bioluminescent
materials include luciferase, luciferin, and aequorin; and examples of
suitable radioactive material include iodine (121I, 123I,125I,13'I), carbon
('"C), sulfllr (5S), tritium (3H), indium ("'In, 1121n,113mhl ]lsm1n\
technetium
(99To,99mTc), thallium (20'Ti), gallium (68Ga, 67Ga), palladium (103Pd),
molybdenum (99Mo), xenon (133Xe), fluorine ('$F),'53Sm, 177Lu, /'59Gd, 149Pm,
140La 175yb 166HO 90I, 47Sc 186Re 188Re 142Pr, losRh and 97Ru.
[0361] In specific embodiments, albumin fusion proteins of the present
invention or fragments or variants thereof are attached to macrocyclic
chelators that associate witli radiometal ions, including but not limited to,
17Lu, 90Y,'66Ho, and 153Sm, to polypeptides. In a preferred embodiment,
the radiometat ion associated with the macrocyclic chelators is "'hi. In
another preferred embodiment, the radiometal ion associated with the
macrocyclic chelator is 90Y. In specific embodiments, the macrocyclic chelator
is 1,4,7,10-tetraazacyclododecane-N,N',N",N"'-tetraacetic acid
(DOTA). In other specific embodiments, DOTA is attached to an antibody of the
invention or fragment thereof via linker molecule. Examples of
linker molecules useful for conjugating DOTA to a polypeptide are commonly
known in the art - see, for example, DeNardo et al., Clin Cancer Res.
4(10):2483-90 (1998); Peterson et al., Bioconjug. Chem. 10(4):553-7 (1999);
and Zimmerman et al, Nucl. Med. Biol. 26(8):943-50 (1999); which
are hereby incorporated by reference in their entirety.
[0362] As mentioned, the albumin fusion proteins of the invention may be
modified by either natural processes, such as post-translational
processing, or by chemical modification teohniques which are well known in the
art. It will be appreciated that the same type of modification may
be present in the same or varying degrees at several sites in a given
polypeptide. Polypeptides of the invention may be branched, for example, as a
result of ubiquitination, and they may be cyclic, with or without branching.
Cyclic, branched, and branched cyclic polypeptides may result from
posttranslation natural processes or may be made by synthetic methods.
Modifications include acetylation, acylation, ADP-ribosylation, amidation,
covalent attachment of flavin, covalent attachment of a heme moiety, covalent
attachment of a nucleotide or nucleotide derivative, covalent
attachment of a lipid or lipid derivative, covalent attachment of
phosphotidylinositol, cross-linking, cyclization, disulfide bond formation,
demethylation, formation of covalent cross-links, formation of cysteine,
formation of pyroglutamate, formylation, gamma-carbox.ylation,
glycosylation, GPI anchor formation, hydroxylation, iodination, methylation,
myristylation, oxidation, pegylation, proteolytic processing,
phosphorylation, prenylation, racemization, selenoylation, sulfation, transfer-
RNA mediated addition of amino acids to proteins such as arginylation,
and ubiquitination. (See, for instance, PROTEINS - STRUCTURE AND MOLECULAR
PROPERTIES, 2nd Ed., T. E. Creighton, W. H.
Freeman and Company, New York (1993); POST-TRANSLATIONAL COVALENT MODIFICATION
OF PROTEINS, B. C. Johnson, Ed.,
Academic Press, New York, pgs. 1-12 (1983); Sei$er et al., Meth. Enzymol.
182:626-646 (1990); Rattan et al., Ann. N.Y. Acad. Sci. 663:48-62
(1992)).
[0363] Albumin fusion proteins of the invention and antibodies that bind a
Therapeutic protein or fragments or variants thereof can be fused to
marker sequences, such as a peptide to facilitate purification. In preferred
embodiments, the marker amino acid sequence is a hexa-histidine
peptide, such as the tag provided in a pQE vector (QIAGEN, Inc., 9259 Eton
Avenue, Chatsworth, CA, 91311), among others, many of which are
commercially available. As described in Gentz et al., Proc. Natl. Acad. Sci.
USA 86:821-824 (1989), for instance, hexa-histidine provides for
convenient purification of the fusion protein. Other peptide tags useful for
purification include, but are not limited to, the "HA" tag, which
corresponds to an epitope derived from the influenza hemagglutinin protein
(Wilson et al., Cell 37:767 (1984)) and the "flag" tag.
[0364] Further, an albumin fusion protein of the invention may be conjugated
to a therapeutic moiety such as a cytotoxin, e.g., a cytostatic or
cytocidal agent, a therapeutic agent or a radioactive metal ion, e.g., alpha-
emitters such as, for example, 213Bi. A cytotoxin or cytotoxic agent
68
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
11-- Hindlu'4 an~ ~LMA6 i~ ~etr"i~nci]4al ~o ~ells. Examples include
paclitaxol, cytochalasin B, gramicidin D, ethidium bromide, emetine,
mitomycin,
etoposide, tenoposide, vincristine, vinblastine, colchicin, doxorubicin,
daunorubicin, dihydroxy anthracin dione, mitoxantrone, mithramycin,
actinomycin D, 1-dehydrotestosterone, glucocorticoids, procaine, tetracaine,
lidocaine, propranolol, and puromycin and analogs or homologs
thereof. Therapeutic agents include, but are not limited to, antimetabolites
(e.g., methotrexate, 6-mercaptopurine, 6-thioguanine, cytarabine, 5-
fluorouracil decarbazine), alkylating agents (e.g., mechlorethamine, thioepa
clilorambucil, melphalan, carmustine (BSNU) and lomustine (CCNU),
cyclotliosphamide, busulfan, dibromomannitol, streptozotocin, mitomycin C, and
cis- dichlorodiamine platinum (II) (DDP) cisplatin), anthracyclines
(e.g., daunorubicin (formerly daunomycin) and doxorubicin), antibiotics (e.g.,
dactinomycin (formerly actinomycin), bleomycin, mithramycin, and
anthramycin (AMC)), and anti-mitotic agents (e.g., vincristine and
vinblastine).
[0365] The conjugates of the invention can be used for modifying a given
biological response, the therapeutic agent or drug moiety is not to be
construed as limited to classical chemical therapeutic agents. For example,
the drug moiety may be a protein or polypeptide possessing a desired
biological activity. Such proteins may include, for example, a toxin such as
abrin, ricin A, pseudomonas exotoxin, or diphtheria toxin; a protein
such as tumor necrosis factor, alpha-interferon, fi-interferon, nerve growth
factor, platelet derived growth factor, tissue plasminogen activator, an
apoptotic agent, e.g., TNF-alpha, TNF-beta, AIM I (See, International
Publication No. WO 97/33899), AIM II(See, International Publication No.
WO 97/34911), Fas Ligand (Takahashi et al., Int. ImrnunoL, 6:1567-1574
(1994)), VEGI (See, Intemational Publication No. WO 99/23105), a
thrombotic agent or an anti- angiogenic agent, e.g., angiostatin or
endostatin; or, biological response modifiers such as, for example,
lymphokines,
interleukin-1 interleukin-2 ("IL-2"), interleukin-6 ("IL-6"), granulocyte
macrophage colony stimulating factor ("GM-CSF"), granulocyte
colony stimulating factor ("G-CSF"), or other growth factors. Techniques for
conjugating such therapeutic moiety to proteins (e.g., albumin fusion
proteins) are well known in the art.
[0366] Albumin fusion proteins may also be attached to solid supports, which
are particularly useful for immunoassays or purification of
polypeptides that are bound by, that bind to, or associate with albumin fusion
proteins of the invention. Such solid supports include, but are not
limited to, glass, cellulose, polyacrylamide, nylon, polystyrene, polyvinyl
chloride or polypropylene.
[03671 Albumin fusion proteins, with or without a therapeutic moiety
conjugated to it, administered alone or in combination with cytotoxic
factor(s) and/or cytokine(s) can be used as a therapeutic.
[0368] In embodiments where the albumin fusion protein of the invention
comprises only the VH domain of an antibody that binds a
Therapeutic protein, it may be necessary and/or desirable to coexpress the
fusion protein with the VL domain of the same antibody that binds a
Therapeutic protein, such that the VH-albumin fusion protein and VL protein
will associate (either covalently or non-covalently) post-translationally.
[0369] In embodiments where the albumin fusion protein of the invention
comprises only the VL domain of an antibody that binds a
Tlierapeutic protein, it may be necessary and/or desirable to coexpress the
fusion protein with the VH domain of the same antibody that binds a
Therapeutic protein, such that the VL-albumin fusion protein and VH protein
wiIl assoeiate (either-covalently or non-covalently) post-translationally. ---
--
[0370] Some Therapeutic antibodies are bispecific antibodies, meaning the
antibody that binds a Therapeutic protein is an artificial hybrid
antibody having two different heavy/light chain pairs and two different
binding sites. In order to create an albumin fusion protein corresponding to
that Therapeutic protein, it is possible to create an albumin fusion protein
which has an scFv fragment fused to both the N- and C- terminus of the
albumin protein moiety. More particularly, the scFv fused to the N-terminus of
albumin would correspond to one of the heavy/light (VIUVL) pairs
of the original antibody that binds a Therapeutic protein and the scFv fused
to the C-terminus of albumin would correspond to the other heavy/light
(VH/VL) pair of the original antibody that binds a Therapeutic protein.
[0371] Also provided by the invention are chemically modified derivatives of
the albumin fusion proteins of the invention which may provide
additional advantages such as increased solubility, stability and circulating
time of the polypeptide, or decreased immunogenicity (see U.S. Patent
No. 4,179,337). The chemical moieties for derivitization may be selected from
water soluble polymers such as polyethylene glycol, ethylene
glycollpropylene glycol copolymers, carboxymethylcellulose, dextran, polyvinyl
alcohol and the like. The albumin fusion proteins may be modified
at random positions within the molecule, or at predetermined positions within
the molecule and may include one, two, three or more attached
chemical moieties.
[0372] The polymer may be of any molecular weight, and may be branched or
unbranched. For polyethylene glycol, the preferred molecular
weight is between about lkDa and about 100 kDa (the term "about" indicating
that in preparations of polyethylene glycol, some molecules will
weigh more, some less, than the stated molecular weight) for ease in handling
and manufacturing. Other sizes may be used, depending on the
desired therapeutic profile (e.g., the duration of sustained release desired,
the effects, if any on biological activity, the ease in handling, the degree
or
lack of antigenicity and other known effects of the polyethylene glycol to a
Therapeutic protein or analog). For example, the polyethylene glycol may
have an average molecular weight of about 200, 500, 1000, 1500, 2000, 2500,
3000, 3500, 4000, 4500, 5000, 5500, 6000, 6500, 7000, 7500, 8000,
8500, 9000, 9500, 10,000, 10,500, 11,000, 11,500, 12,000, 12,500, 13,000,
13,500, 14,000, 14,500, 15,000, 15,500, 16,000, 16,500, 17,000,
17,500, 18,000, 18,500, 19,000, 19,500, 20,000, 25,000, 30,000, 35,000,
40,000, 45,000, 50,000, 55,000, 60,000, 65,000, 70,000, 75,000, 80,000,
85,000, 90,000, 95,000, or 100,000 kDa.
[0373] As noted above, the polyethylene glycol may have a branched structure.
Branched polyethylene glycols are described, for example, in
69
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~l'" 11~ iJ17S: 'Pa~~e~it~I~6~~~!~~1~,575~~#wloi~ur'~6 eilal~~=Appl.
Bioclrem. Biotecl:nol. 56:59-72 (1996); Vorobjev et al., Nucleosides
Nucleotides 18:2745-2750
(1999); and Caliceti et al., Biocoijug. Cliein. 10:638-646 (1999), the
disclosures of each of which are incorporated ]rerein by reference.
[0374] The polyethylene glycol molecules (or other chemical moieties) should
be attached to the protein with consideration of effects on
functional or antigenic domains of the protein. There are a number of
attachment metlrods available to those skilled in the art, such as, for
example,
the method disclosed in EP 0 401 384 (coupling PEG to G-CSF), herein
incorporated by reference; see also Malik et al., Gxp. Hematol.
20:1028-1035 (1992), reporting pegylation of GM-CSF using tresyl chloride. For
example, polyetlrylene glycol may be covalently bound through
amino acid residues via reactive group, such as a free amino or carboxyl
group. Reactive groups are those to which an activated polyethylene glycol
molecule may be bound. The amino acid residues having a free amino group may
include lysine residues and the N-terminal amino acid residues;
those having a free carboxyl group may include aspartic acid residues glutamic
acid residues and the C-terminal amino acid residue. Sulfhydryl
groups may also be used as a reactive group for attaching the polyethylene
glycol molecules. Preferred for therapeutic purposes is attachment at an
amino group, such as attachment at the N-terminus or lysine group. 1
[0375] As suggested above, polyethylene glycol may be attached to proteins via
linkage to any of a number of amino acid residues. For
example, polyethylene glycol can be linked to proteins via covalent bonds to
lysine, histidine, aspartic acid, glutamic acid, or oysteine residues. One
or more reaction chemistries may be employed to attach polyethylene glycol to
specific amino acid residues (e.g., lysine, histidine, aspartic acid,
glutamic acid, or cysteine) of the protein or to more than one type of amino
acid residue (e.g., lysine, histidine, aspartic acid, glutamic acid, cysteine
and combinations thereof) of the protein.
[0376] One may specifically desire proteins chemically modified at the N-
terminus. Using polyethylene glycol as an illustration of the present
composition, one may select from a variety of polyethylene glycol molecules
(by molecular weiglit, branching, etc.), the proportion of polyethylene
glycol molecules to protein (polypeptide) molecules in the reaction mix, the
type of pegylation reaction to be performed, and the method of obtaining
the selected N-terminally pegylated protein. The method of obtaining the N-
terminally pegylated preparation (i.e., separating this moiety from other
monopegylated moieties if necessary) may be by purification of the N-
terminally pegylated material from a population of pegylated protein
molecules. Selective proteins chemically modified at the N-terminus
modification may be accomplished by reductive alkylation which exploits
differential reactivity of different types of primary amino groups (lysine
versus the N-terminal) available for derivatization in a particular protein.
Under the appropriate reaction conditions, substantially selective
derivatization of the protein at the N-terminus with a carbonyl group
containing
polymer is achieved.
[0377] As indicated above, pegylation of the albumin fusion proteins of the
invention may be accomplished by any number of means. For
example, polyethylene glycol may be attached to the albumin fusion protein
either directly or by an intervening linker. Linkerless systems for
attaching polyethylene glycol to proteins are described in Delgado et al.,
Crit. Rev. Thera. Drug Carrier Sys. 9:249-304 (1992); Francis et al., Intern.
J. of Hematol. 68:1-18 (1998); U.S. Patent No. 4,002,531; U.S. Patent No.
5,349,052; WQ_95/06058; and WO-98/32466, the disclosures of each of
which are incorporated herein by reference.
[0378] One system for attaching polyethylene glycol directly to amino acid
residues of proteins without an intervening linker employs tresylated
MPEG, which is produced by the modification of monmethoxy polyethylene glycol
(MPEG) using tresylchloride (C1SO2CH2CF3). Upon reaction of
protein with tresylated MPEG, polyethylene glycol is directly attached to
amine groups of the protein. Thus, the invention includes protein-
polyethylene glycol conjugates produced by reacting proteins of the invention
with a polyethylene glycol molecule having a 2,2,2-trifluoreothane
sulphonyl group.
[0379] Polyethylene glycol can also be attached to proteins using a number of
different intervening linkers. For example, U.S. Patent No.
5,612,460, the entire disclosure of which is incorporated herein by reference,
discloses urethane linkers for connecting polyethylene glycol to
proteins. Protein-polyetlrylene glycol conjugates wherein the polyethylene
glycol is attached to the protein by a linker can also be produced by
reaction of proteins with compounds such as MPEG-succinimidylsuccinate, MPEG
activated with 1,1'-carbonyldiimidazole, MPEG-
2,4,5-trichloropenylcarbonate, MPEG-p-nitrophenolcarbonate, and various MPEG-
succinate derivatives. A number of additional polyethylene
glycol derivatives and reaction chemistries for aitaching polyethylene glycol
to proteins are described in Intemational Publication No. WO 98/32466,
the entire disclosure of which is incorporated herein by reference. Pegylated
protein products produced using the reaction chemistries set out herein
are included within the scope of the invention.
[0380] The number of polyethylene glycol moieties attached to each albumin
fusion protein of the invention (i.e., the degree of substitution) may
also vary. For example, the pegylated proteins of the invention may be linked,
on average, to 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 17, 20, or more
polyethylene glycol molecules. Similarly, the average degree of substitution
within ranges such as 1-3, 2-4, 3-5, 4-6, 5-7, 6-8, 7-9, 8-10, 9-11, 10-
12, 11-13, 12-14, 13-15, 14-16, 15-17, 16-18, 17-19, or 18-20 polyethylene
glycol moieties per protein molecule. Methods for determining the
degree of substitution are discussed, for example, in Delgado et al., Crit.
Rev. Thera. Drug Carrier Sys. 9:249-304 (1992).
[0381] The polypeptides of the invention can be recovered and purified from
chemical synthesis and recombinant cell cultures by standard
methods which include, but are not limited to, ammonium sulfate or ethanol
precipitation, acid extraction, anion or cation exchange
chromatography, phosphocellulose chromatography, hydrophobic interaction
chromatography, affinity chromatography, hydroxylapatite
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
'8hrbmNgY~~1~~~~a~~' lecti]x elii~rria~Eogi~p~y. Most preferably, high
performance liquid chromatography ("HPLC") is employed for purification.
Well known tecllniques for refolding protein may be employed to regenerate
active conformation when the polypeptide is denatured during isolation
and/or purification.
[0382] The presence and quantity of albumin fusion proteins of the invention
may be determined using ELISA, a well known immunoassay
known in the art. In one ELISA protocol that would be useful for
detecting/quantifying albumin fusion proteins of the invention, comprises the
steps
of coating an ELISA plate with an anti-human serum albumin antibody, blocking
the plate to prevent non-specific binding, washing the ELISA plate,
adding a solution containing the albumin fusion protein of the invention (at
one or more different concentrations), adding a secondary anti-
Therapeutic protein specific antibody coupled to a detectable label (as
described herein or otherwise known in the art), and detecting the presence of
the secondary antibody. In an alternate version of this protocol, the ELISA
plate might be coated with the anti-Therapeutic protein specific antibody
and the labeled secondary reagent might be the anti-human albumin specific
antibody.
Uses of tlte Polvitucleotides
[0383] Each of the polynucleotides identified herein can be used in numerous
ways as reagents. The following description should be considered
exemplary and utilizes known techniques.
[0384] The polynucleotides of the present invention are useful to produce the
albumin fusion proteins of the invention. As described in more
detail below, polynucleotides of the invention (encoding albumin fusion
proteins) may be used in recombinant DNA methods useful in genetic
engineering to make cells, cell lines, or tissues that express the albumin
fusion protein encoded by the polynucleotides encoding albumin fusion
proteins of the invention.
[0385] Polynucleotides of the present invention are also useful in gene
therapy. One goal of gene therapy is to insert a normal gene into an
organism having a defective gene, in an effort to correct the genetic defect.
The polynucleotides disclosed in the present invention offer a means of
targeting such genetic defects in a higlily accurate manner. Another goal is
to insert a new gene that was not present in the host genome, thereby
producing a new trait in the host cell. Additional non-limiting examples of
gene therapy methods encompassed by the present invention are more
thoroughly described elsewhere herein (see, e.g., the sections labeled "Gene
Therapy", and Examples 61 and 62).
Uses oftl:e Polypeotides
[0386] Each of the polypeptides identified herein can be used in numerous
ways. The following description should be considered exemplary
and utilizes known techniques.
[03871 Albumin fusion proteins of the invention are useful to provide
immunological probes for differential identification of the tissue(s) (e.g.,
immunohistochemistry assays suoh as, for example, ABC immunoperoxidase (Hsu et
al., J. Histochem. Cytochem. 29:577-580 (1981)) or cell
type(s) (e.g., immunocytochemistry assays).
[0388] Albumin fusion proteins can be used to assay levels of polypeptides
in_a_biological.sample using classical immunohistological methods -
known to those of skill in the art (e.g., see Jalkanen, et al., J. Cell. Biol.
101:976-985 (1985); Jalkanen, et al., J. Cell. Biol. 105:3087-3096 (1987)).
Other methods useful for detecting protein gene expression include
immunoassays, such as the enzynie linked immunosorbent assay (ELISA) and
the radioimmunoassay (RIA). Suitable assay labels are known in the art and
include enzyme labels, such as, glucose oxidase; radioisotopes, such as
iodine (131.,125I,123I,1211), carbon (14C), sulfur (35S), tritium (3H), indium
(15mIn 113min' 1121n ]llIn) and technetium (99Tc, 99mTc), thallium (201Ti),
gallium (68Ga, 67Ga), palladium (103Pd), molybdenum (94Mo), xenon (133Xe),
fluorine (aF), 153Sm, 17Lu,159Gd,'49Pm, 140La, 175~n 166Ho, 90Y, 47Sc,
186Re,1$8Re,142Pr, 105Rh, 97Ru; luminescent labels, such as luminol; and
fluorescent labels, such as fluorescein and rhodamine, and biotin.
[0389] Albumin fusion proteins of the invention can also be detected in vivo
by imaging. Labels or markers for in vivo imaging of protein
include those detectable by X-radiography, nuclear magnetic resonance (NMR) or
electron spin relaxtion (ESR). For X-radiography, suitable labels
include radioisotopes such as barium or cesium, which emit detectable
radiation but are not overtly harmful to the subject. Suitable markers for
NMR and ESR include those with a detectable characteristic spin, such as
deuterium, which may be incorporated into the albumin fusion protein by
labeling of nutrients given to a cell line expressing the albumin fusion
protein of the invention.
[0390] An albumin fusion protein which has been labeled with an appropriate
detectable imaging moiety, such as a radioisotope (for example,
131L 112in 99mTc (111I,125I,123I,1211), carbon (14C), sulfur (35S), tritium
(H), indium ("SmIn, "3mIn "2In,'1'In) and technetium (99Tc, 99mTc), thallium
(201Ti), gallium (68Ga, 67Ga), palladium (103Pd), molybdenum (99Mo), xenon
(33Xe), fluorine ('SF, '53Sm, "'Lu, '59Gd, 149Pm, '40La 175y~b 1e6Ho,
90Y, 47Sc, 186Re, 188Re, 142Pr, '05Rh, "'Ru), a radio-opaque substance, or a
material detectable by nuclear magnetic resonance, is introduced (for
example, parenterally, subcutaneously or intraperitoneally) into the mammal to
be examined for immune system disorder. It will be understood in
the art that the size of the subject and the imaging system used will
determine the quantity of imaging moiety needed to produce diagnostic images.
In the case of a radioisotope moiety, for a human subject, the quantity of
radioactivity injected will normally range from about 5 to 20 millicuries of
99i'Tc. The labeled albumin fusion protein will then preferentially accumulate
at locations in the body (e.g., organs, cells, extracellular spaces or
matrices) where one or more receptors, ligands or substrates (corresponding to
that of the Therapeutic protein used to make the albumin fusion
protein of the invention) are located. Altematively, in the case where the
albumin fusion protein comprises at least a fragment or variant of a
Therapeutic antibody, the labeled albumin fusion protein will then
preferentially accumulate at the locations in the body (e.g., organs, cells,
71
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ i l õn ~ ~s s o' c~i~.,rr . n3~acric' ,.
xirac I ul r )h"' t e polypeptides/epitopes corresponding to those bound by
the Therapeutie antibody (used to make the
albumin fusion protein of the invention) are located. In vivo tumor imaging is
described in S.W. Burchiel et al., "Immunopharmacokinetics of
Radiolabeled Antibodies and Their Fragments" (Chapter 13 in Tunlor Iinaging:
The Radioclieinical Detection of Cancer, S.W. Burchiel and B. A.
Rhodes, eds., Masson Publishing Inc. (1982)). The protocols described therein
could easily be modified by one of skill in the art for use with the
albumin fusion proteins of the invention.
[0391] In one embodiment, the invention provides a method for the specific
delivery of albumin fusion proteins of the invention to cells by
administering albumin fusion proteins of the invention (e.g., polypeptides
encoded by polynucleotides encoding albumin fusion proteins of the
invention and/or antibodies) that are associated with heterologous
polypeptides or nucleic acids. In one example, the invention provides a method
for delivering a Therapeutic protein into the targeted cell. In another
example, the invention provides a method for delivering a single stranded
nucleic acid (e.g., antisense or ribozymes) or double stranded nucleic acid
(e.g., DNA that can integrate into the cell's genome or replicate
episomally and that can be transcribed) into the targeted cell.
[0392] In another embodiment, the invention provides a method for the specific
destruction of cells (e.g., the destruction of tumor cells) by
administering albumin fusion proteins of the invention in association witli
toxins or cytotoxic prodrugs.
[0393] By "toxin" is meant one or more compounds that bind and activate
endogenous cytotoxic effector systems, radioisotopes, holotoxins,
modified toxins, catalytic subunits of toxins, or any molecules or enzymes not
normally present in or on the surface of a cell that under defined
conditions cause the cell's death. Toxins that may be used according to the
methods of the invention include, but are not limited to, radioisotopes
known in the art, compounds such as, for example, antibodies (or complement
fixing containing portions thereof) that bind an inherent or induced
endogenous cytotoxic effector system, thymidine kinase, endonuclease, RNAse,
alpha toxin, ricin, abrin, Pseudon:onas exotoxin A, diphtheria toxin,
saporin, momordin, gelonin, pokeweed antiviral protein, alpha-sarcin and
cholera toxin. "Toxin" also includes a cytostatic or cytocidal agent, a
tlierapeutic agent or a radioactive metal ion, e.g., alpha-emitters such as,
for example, 213Bi, or other radioisotopes such as, for example, }03Pd,133Xe,
131I, 68Ge, SICo, 65Zn, $SSr, 32P, 35S' 90Y 153sm 153Gd 169yb 51Cr, 54Mn,7SSe,
113Sn, 90Yttrlum, 117Tin, '6Rhenium, "56Holmium, and'SaRhenium;
luminescent labels, such as luminol; and fluorescent labels, such as
fluorescein and rhodamine, and biotin. In a specific embodiment, the invention
provides a method for the specific destruction of cells (e.g., the destruction
of tumor cells) by administering polypeptides of the invention or
antibodies of the invention in association with the radioisotope 9 Y. In
anotlier specific embodiment, the invention provides a method for the
specific destruction of cells (e.g., the destruction of tumor cells) by
administering polypeptides of the invention or antibodies of the invention in
association with the radioisotope 11tIn. In a further specific embodiment, the
invention provides a method for the specific destruction of cells (e.g.,
the destruction of tumor cells) by administering polypeptides of the invention
or antibodies of the invention in association with the radioisotope 131I.
[0394] Techniques known in the art may be applied to lable polypeptides of the
invention. Such techniques include, but are not limited to, the
use of bifunctional conjugating agents (see e.g., U.S. Patent Nos. 5,756,065;
5,714,631; 5,696,239; 5,652,361;-5,505,931; 5,489,425;-5,435;990;-
_.._..._
5,428,139; 5,342,604; 5,274;119; 4,994,560; and 5,808,003; the contents of
each of which are hereby incorporated by reference in its entirety).
[0395] The albumin fusion proteins of the present invention are useful for
diagnosis, treatment, prevention and/or prognosis of various disorders
in mammals, preferably humans. Such disorders include, but are not limited to,
those described herein under the section heading "Biological
Activities," below.
[0396] Thus, the invention provides a diagnostic method of a disorder, which
involves (a) assaying the expression level of a certain polypeptide
in cells or body fluid of an individual using an albumin fusion protein of the
invention; and (b) comparing the assayed polypeptide expression level
with a standard polypeptide expression level, whereby an increase or decrease
in the assayed polypeptide expression level compared to the standard
expression level is indicative of a disorder. With respect to cancer, the
presence of a relatively high amount of transcript in biopsied tissue from an
individual may indicate a predisposition for the development of the disease,
or may provide a means for detecting the disease prior to the appearance
of actual clinical symptoms. A more definitive diagnosis of this type may
allow health professionals to employ preventative measures or aggressive
treatment earlier thereby preventing the development or further progression of
the cancer.
[0397] Moreover, albumin fusion proteins of the present invention can be used
to treat or prevent diseases or conditions such as, for example,
neural disorders, immune system disorders, muscular disorders, reproductive
disorders, gastrointestinal disorders, pulmonary disorders,
cardiovascular disorders, renal disorders, proliferative disorders, and/or
cancerous diseases and conditions. For example, patients can be
administered a polypeptide of the present invention in an effort to replace
absent or decreased levels of the polypeptide (e.g., insulin), to supplement
absent or decreased levels of a different polypeptide (e.g., hemoglobin S for
hemoglobin B, SOD, catalase, DNA repair proteins), to inhibit the
activity of a polypeptide (e.g., an oncogene or tumor supressor), to activate
the activity of a polypeptide (e.g., by binding to a receptor), to reduce the
activity of a membrane bound receptor by competing with it for free ligand
(e.g., soluble TNF receptors used in reducing uiflammation), or to bring
about a desired response (e.g., blood vessel growth inhibition, enhancement of
the immune response to proliferative cells or tissues).
[0398] In particular, albumin fusion proteins comprising of at least a
fragment or variant of a Therapeutic antibody can also be used to treat
disease (as described supra, and elsewhere herein). For example,
administration of an albumin fusion protein comprising of at least a fragment
or
variant of a Therapeutic antibody can bind, and/or neutralize the polypeptide
to which the Therapeutic antibody used to make the albumin fusion
72
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
'l~~'" lprd~eiri slpe~f~C~11~ ~~inds~~ an~~or ~t a~i'be ~]YVerproduetion of
the polypeptide to which the Therapeutic antibody used to make the albumin
fusion
protein specifically binds. Similarly, administration of an albumin fusion
protein comprising of at least a fragment or variant of a Tlierapeutic
antibody can activate the polypeptide to which the Tlierapeutic antibody used
to make the albumin fusion protein specifically binds, by binding to
the polypeptide bound to a membrane (receptor).
[0399] At the very least, the albumin fusion proteins of the invention of the
present invention can be used as molecular weight markers on SDS-
PAGE gels or on molecular sieve gel filtration columns using methods well
known to those of skill in the art. Albumin fusion proteins of the
invention can also be used to raise antibodies, which in turn may be used to
measure protein expression of the Therapeutic protein, albumin protein,
and/or the albumin fusion protein of the invention from a recombinant cell, as
a way of assessing transformation of the host cell, or in a biological
sample. Moreover, the albumin fusion proteins of the present invention can be
used to test the biological activities described herein.
DiaPnostic Assa
[0400] The compounds of the present invention are useful for diagnosis,
treatment, prevention and/or prognosis of various disorders in
mammals, preferably humans. Such disorders include, but are not limited to,
those described for eacli Therapeutic protein in the corresponding row
of Table 1 and herein under the section headings "Immune Activity," "Blood
Related Disorders," "Hyperproliferative Disorders," "Renal
Disorders," "Cardiovascular Disorders," "Respiratory Disorders," "Anti-
Angiogenesis Activity," "Diseases at the Cellular Level," "Wound Healing
and Epithelial Cell Proliferation," "Neural Activity and Neurological
Diseases," "Endocrine Disorders," "Reproductive System Disorders,"
"Infectious Disease," "Regeneration," and/or "Gastrointestinal Disorders,"
infra.
[0401] For a number of disorders, substantially altered (increased or
decreased) levels of gene expression can be detected in tissues, cells or
bodily fluids (e.g., sera, plasma, urine, semen, synovial fluid or spinal
fluid) taken from an individual having such a disorder, relative to a
"standard"
gene expression level, that is, the expression level in tissues or bodily
fluids from an individual not having the disorder. Thus, the invention
provides a diagnostic method useful during diagnosis of a disorder, which
involves measuring the expression level of the gene encoding a
polypeptide in tissues, cells or body fluid from an individual and comparing
the measured gene expression level with a standard gene expression
level, whereby an increase or decrease in the gene expression level(s)
compared to the standard is indicative of a disorder. These diagnostic assays
may be performed in vivo or in vitro, such as, for example, on blood samples,
biopsy tissue or autopsy tissue.
[0402] The present invention is also useful as a prognostic indicator, whereby
patients exhibiting enhanced or depressed gene expression will
experience a worse clinical outcome.
[0403] By "assaying the expression level of the gene encoding a polypeptide"
is intended qualitatively or quantitatively measuring or estimating
the level of a particular polypeptide (e.g. a polypeptide corresponding to a
Therapeutic protein disclosed in Table 1) or the level of the mRNA
encoding the polypeptide of the invention in a first biological sample either
directly (e.g., by determining or estimating absolute protein level or
mRNA level) or relatively (e.g., by comparing to the polypeptide level or mRNA
level in a-second biological sample). - Preferably, the polypeptide-
expression level or mRNA level in the first biological sample is measured or
estimated and compared to a standard polypeptide level or mRNA
level, the standard being taken from a second biological sample obtained from
an individual not having the disorder or being determined by
averaging levels from a population of individuals not having the disorder. As
will be appreciated in the art, once a standard polypeptide level or
mRNA level is known, it can be used repeatedly as a standard for comparison.
[0404] By "biological sample" is intended any biological sample obtained from
an individual, cell line, tissue culture, or other source containing
polypeptides of the invention (including portions thereof) or mRNA. As
indicated, biological samples include body fluids (such as sera, plasma,
urine, synovial fluid and spinal fluid) and tissue sources found to express
the full length or fragments thereof of a polypeptide or mRNA. Methods
for obtaining tissue biopsies and body fluids from mammals are well known in
the art. Where the biological sample is to include mRNA, a tissue
biopsy is the preferred source.
(0405] Total cellular RNA can be isolated from a biological sample using any
suitable technique such as the single-step
guanidinium-thiocyanate-phenol-chloroform method described in Chomczynski and
Sacchi, Anal. Biochem. 162:156-159 (1987). Levels of mRNA
encoding the polypeptides of the invention are then assayed using any
appropriate method. These include Northem blot analysis, S1 nuclease
mapping, the polymerase chain reaction (PCR), reverse transcription in
combination with the polymerase chain reaction (RT-PCR), and reverse
transcription in combination with the ligase chain reaction (RT-LCR).
[0406] The present invention also relates to diagnostic assays such as
quantitative and diagnostic assays for detecting levels of polypeptides that
bind to, are bound by, or associate with albumin fusion proteins of the
invention, in a biological sample (e.g., cells and tissues), including
determination of normal and abnormal levels of polypeptides. Thus, for
instance, a diagnostic assay in accordance with the invention for detecting
abnormal expression of polypeptides that bind to, are bound by, or associate
with albumin fusion proteins compared to normal control tissue
samples may be used to detect the presence of tumors. Assay techniques that
can be used to determine levels of a polypeptide that bind to, are
bound by, or associate with albumin fusion proteins of the present invention
in a sample derived from a host are well-known to those of skill in the
art. Such assay methods include radioimmunoassays, competitive-binding assays,
Westem Blot analysis and ELISA assays. Assaying polypeptide
levels in a biological sample can occur using any art-known method.
73
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
11[04 . ;. , . ... .~~. 0 """' s yyl g''pol pepi dt "' wd s~t ' a biological
sample can occur using a variety of techniques. For example, polypeptide
expression in
tissues can be studied with classical immunoliistological methods (Jalkanen et
al., J. Cell. Biol. 101:976-985 (1985); Jalkanen, M., et al., J. Cell .
Biol. 105:3087-3096 (1987)). Other methods useful for detecting polypeptide
gene expression include immunoassays, such as the enzyme linked
immunosorbent assay (ELISA) and the radioimmunoassay (RIA). Suitable antibody
assay labels are known in the art and include enzyme labels,
such as, glucose oxidase, and radioisotopes, such as iodine (125I, 121I),
carbon (t"C), sulfur (35S), tritium (3H), indium ("In), and technetium (99i
Tc),
and fluorescent labels, such as fluorescein and rhodamine, and biotin.
[04081 The tissue or cell type to be analyzed will generally include those
which are known, or suspected, to express the gene of interest (such as,
for example, cancer). The protein isolation methods employed herein may, for
example, be such as those described in Harlow and Lane (Harlow, E.
and Lane, D., 1988, "Antibodies: A Laboratory Manual", Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, New York), which is
incorporated herein by reference in its entirety. The isolated cells can be
derived from cell culture or from a patient. The analysis of cells taken
from culture may be a necessary step in the assessment of cells that could be
used as part of a cell-based gene therapy technique or, altematively, to
test the effect of compounds on the expression of the gene.
[0409] For example, albumin fusion proteins may be used to quantitatively or
qualitatively detect the presence of polypeptides that bind to, are
bound by, or associate with albumin fusion proteins of the present invention.
This can be accomplished, for example, by immunofluorescence
techniques employing a fluorescently labeled albumin fusion protein coupled
with light microscopic, flow cytometric, or fluorimetric detection.
[0410] In a preferred embodiment, albumin fusion proteins comprising at least
a fragment or variant of an antibody that specifically binds at
least a Therapeutic protein disclosed herein (e.g., the Therapeutic proteins
disclosed in Table 1) or otherwise known in the art may be used to
quantitatively or qualitatively detect the presence of gene products or
conserved variants or peptide fragments thereof. This can be accomplished,
for example, by immunofluorescence techniques employing a fluorescently
labeled antibody coupled with light microscopic, flow cytometric, or
fluorimetric detection.
[0411] The albumin fusion proteins of the present invention may, additionally,
be employed histologically, as in immunofluorescence,
immunoelectron microscopy or non-immunological assays, for in situ detection
of polypeptides that bind to, are bound by, or associate with an
albumin fusion protein of the present invention. In situ detection may be
accomplished by removing a histological specimen from a patient, and
applying thereto a labeled antibody or polypeptide of the present invention.
The albumin fusion proteins are preferably applied by overlaying the
labeled albumin fusion proteins onto a biological sample. Through the use of
such a procedure, it is possible to determine not only the presence of
the polypeptides that bind to, are bound by, or associate with albumin fusion
proteins, but also its distribution in the examined tissue. Using the
present invention, those of ordinary skill will readily perceive that any of a
wide variety of histological methods (such as staining procedures) can be
modified in order to achieve such in situ detection.
[0412] Immunoassays and non-immunoassays that detect-polypeptides that-bindto,
are_bound by, or-associate with albumin fusion proteinswill-
--
typically comprise incubating a sample, such as a biological fluid, a tissue
extract, freshly harvested cells, or lysates of cells which have been
incubated in cell culture, in the presence of a detectably labeled antibody
capable of binding gene products or conserved variants or peptide
fragments thereof, and detecting the bound antibody by any of a number of
techniques well-known in the art.
[0413] The biological sample may be brought in contact with and immobilized
onto a solid phase support or carrier such as nitrocellulose, or
other solid support which is capable of immobilizing cells, cell particles or,
soluble proteins. The support may then be washed with suitable buffers
followed by treatment with the detectably labeled albumin fusion protein of
the invention. The solid phase support may then be washed with the
buffer a second time to remove unbound antibody or polypeptide. Optionally the
antibody is subsequently labeled. The amount of bound label on
solid support may then be detected by conventional means.
[0414] By "solid phase support or carrier" is intended any support capable of
binding a polypeptide (e.g., an albumin fusion protein, or
polypeptide that binds, is bound by, or associates with an albumin fusion
protein of the invention.) Well-known supports or carriers include glass,
polystyrene, polypropylene, polyethylene, dextrau, nylon, amylases, natural
and modified celluloses, polyacrylamides, gabbros, and magnetite. The
nature of the carrier can be either soluble to some extent or insoluble for
the purposes of the present invention. The support material may have
virtually any possible structural configuration so long as the coupled
molecule is capable of binding to a polypeptide. Thus, the support
configuration may be spherical, as in a bead, or cylindrical, as in the inside
surface of a test tube, or the external surface of a rod. Alternatively, the
surface may be flat such as a sheet, test strip, etc. Preferred supports
include polystyrene beads. Those skilled in the art will know many other
suitable carriers for binding antibody or antigen, or will be able to
ascertain the same by use of routine experimentation.
[0415] The binding activity of a given lot of albumin fusion protein may be
determined according to well lmown methods. Those skilled in the
art will be able to determine operative and optimal assay conditions for each
determination by employing routine experimentation.
[0416] In addition to assaying polypeptide levels in a biological sample
obtained from an individual, polypeptide can also be detected in vivo by
imaging. For example, in one embodiment of the invention, albumin fusion
proteins of the invention are used to image diseased or neoplastic cells.
[0417] Labels or markers for in vivo imaging of albumin fusion proteins of the
invention include those detectable by X-radiography, NMR,
MRI, CAT-scans or ESR. For X-radiography, suitable labels include
radioisotopes such as barium or cesium, which emit detectable radiation but
74
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
. , . .. .,,..
11""' r~ no "~ 1' 'fiil t~=the bj U.3 S itable markers for NMR and ESR include
those with a detectable characteristic spin, such as deuterium,
which may be incorporated into the albumin fusion protein by labeling of
nutrients of a cell line (or bacterial or yeast strain) engineered.
[0418] Additionally, albumin fusion proteins of the invention whose presence
can be detected, can be administered. For example, albumin
fusion proteins of the invention labeled with a radio-opaque or other
appropriate compound can be administered and visualized in vivo, as discussed,
above for labeled antibodies. Further, such polypeptides can be utilized for
in vitro diagnostic procedures.
[0419] A polypeptide-specific antibody or antibody fragment which has been
labeled with an appropriate detectable imaging moiety, such as a
radioisotope (for example, 1311, 112In, 99' Tc), a radio-opaque substance, or
a material detectable by nuclear magnetic resonance, is introduced (for
example, parenterally, subcutaneously or intraperitoneally) into the mammal to
be examined for a disorder. It will be understood in the art that the
size of the subject and the imaging system used will determine the quantity of
imaging moiety needed to produce diagnostic images. In the case of a
radioisotope moiety, for a human subject, the quantity of radioactivity
injected will normally range from about 5 to 20 millicuries of 99 'Te. The
labeled albumin fusion protein will then preferentially accumulate at the
locations in the body which contain a polypeptide or other substance that
binds to, is bound by or associates with an albumin fusion protein of the
present invention. In vivo tumor imaging is described in S.W. Burchiel et
al., "Immunopharmacokinetics of Radiolabeled Antibodies and Their Fragments"
(Chapter 13 in Tumor Imaging: The Radiochemical Detection of
Caiicer, S.W. Burchiel and B. A. Rhodes, eds., Masson Publishing Inc. (1982)).
[0420] One of the ways in which an albumin fusion protein of the present
invention can be detectably labeled is by linking the same to a reporter
enzyme and using the linked product in an enzyme immunoassay (EIA) (Voller,
A., "The Enzyme Linked Immunosorbent Assay (ELISA)", 1978,
Diagnostic Horizons 2:1-7, Microbiological Associates Quarterly Publication,
Walkersville, MD); Voller et al., J. Ctin. Pathol. 31:507-520 (1978);
Butler, J.E., Meth. En:yinol. 73:482-523 (1981); Maggio, E. (ed.), 1980,
Enzyme Immunoassay, CRC Press, Boca Raton, FL,; Ishikawa, E. et al.,
(eds.), 1981, Enzyme Immunoassay, Kgaku Shoin, Tokyo). The reporter enzyme
which is bound to the antibody will react with an appropriate
substrate, preferably a chromogenic substrate, in such a manner as to produce
a chemical moiety which can be detected, for example, by
spectrophotometric, fluorimetric or by visual means. Reporter enzymes which
can be used to detectably label the antibody include, but are not
limited to, malate dehydrogenase, staphylococcal nuclease, delta-5-steroid
isomerase, yeast alcohol dehydrogenase, alpha-glycerophosphate,
dehydrogenase, triose phosphate isomerase, horseradish peroxidase, alkaline
phosphatase, asparaginase, glucose oxidase, beta-galactosidase,
ribonuclease, urease, catalase, glucose-6-phosphate dehydrogenase,
glucoamylase and acetylcholinesterase. Additionally, the detection can be
accomplished by colorimetric methods which employ a chromogenic substrate for
the reporter enzyme. Detection may also be accomplished by
visual comparison of the extent of enzymatic reaction of a substrate in
comparison with similarly prepared standards.
[04211 Albumin fusion proteins may also be radiolabelled and used in any of a
variety of otlier immunoassays. For example, by radioactively
labeling the albumin fusion proteins, it is possible to the use the albumin
fusion proteins in a radioimmunoassay (RIA) (see, for example,
Weintraub, B., Principles of Radioimmunoassays, Seventh_Training- Course on
Radioligand Assay- Techniques; The Endocrine Society, -March,
1986, which is incorporated by reference herein). The radioactive isotope can
be detected by means including, but not limited to, a gamma counter,
a scintillation counter, or autoradiography.
[0422] Additionally, chelator molecules, are known in the art and can be used
to label the Albumin fusion proteins. Chelator molecules may be
attached Albumin fusion proteins of the invention to facilitate labeling said
protein with metal ions including radionuclides or fluorescent labels. For
example, see Subramanian, R. and Meares, C.F., "Bifunctional Chelating Agents
for Radiometal-labeled monoclonal Antibodies," in Cancer
Insaging with Radiolabeled Antibodies (D. M. Goldenberg, Ed.) Kluwer Academic
Publications, Boston; Saji, H., "Targeted delivery of radiolabeled
imaging and therapeutic agents: bifunctional radiopharmaceuticals." Crit. Rev.
Ther. Drug Carrier Syst. 16:209-244 (1999); Srivastava S.C. and
Mease R.C., "Progress in research on ligands, nuclides and techniques for
labeling monoclonal antibodies." Int. J. Rad. Appl. Instrura. B 18:589-
603 (1991); and Liu, S. and Edwards, D.S., "Bifunctional chelators for
therapeutic lanthanide radiopharmaceuticals." Bioconjug. Chens. 12:7-34
(2001). Any chelator which can be covalently bound to said Albumin fusion
proteins may be used according to the present invention. The chelator
may further comprise a linker moiety that connects the chelating moiety to the
Albumin fusion protein.
[0423] In one embodiment, the Albumin fusion protein of the invention are
attached to an acyclic chelator such as diethylene triamine-
N,N,N',N",N"-pentaacetic acid (DPTA), analogues of DPTA, and derivatives of
DPTA. As non-limiting examples, the chelator may be 2-(p-
isothiocyanatobenzyl)-6- methyldiethylenetriaminepentaacetic acid (1B4M-DPTA,
also Imown as MX-DTPA), 2-methyl-6-(rho-nitrobenzyl)-1,4,7-
triazaheptane-N,N,N',N",N"-pentaacetic acid (nitro-IB4M-DTPA or nitro-MX-
DTPA); 2-(p-isothiocyanatobenzyl)-
oyclohexyldiethylenetriaminepentaacetic acid (CHX-DTPA), or N-[2-amino-3-(rho-
nitrophenyl)propyl]-trans-cyclohexane-1,2-diamine-N,N',N"-
pentaacetic acid (nitro-CHX-A-DTPA).
[0424] In another embodiment, the Albumin fusion protein of the invention are
attached to an acyclic terpyridine chelator such as 6,6"-
bis[[N,N,N",N"- tetra(carboxymethyl)amino]methyl]-4'-(3-amino-4-methoxyphenyl)-
2,2':6',2 "- terpyridine (TMT-amine).
[0425] In specific embodiments, the macrocyclic chelator which is attached to
the the Albumin fusion protein of the invention is 1,4,7,10-
tetraazacyclododecane-N,N',N",N"'-tetraacetic acid (DOTA). In other specific
embodiments, the DOTA is attached to the the Albumin fusion protein
of the invention via a linker molecule. Examples of linker molecules useful
for conjugating DOTA to a polypeptide are commonly Imown in the art
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
11.1.H lt
- see, r oX~a I;]?!:N dv e' ak, ~ i ancer Res. 4(10):2483-90, 1998; Peterson
et aL, Brocor jzrg. Ghem. 10(4):553-7, 1999; and Zimmerman
et al., Nncl. Med. BroL 26(8):943-50, 1999 which are hereby incorporated by
reference in their entirety. In addition, U.S. Patents 5,652,361 and
5,756,065, which disclose chelating agents that may be conjugated to
antibodies, and methods for making and using them, are hereby incorporated
by reference in their entireties. Though U.S. Patents 5,652,361 and 5,756,065
focus on conjugating chelating agents to antibodies, one skilled in the
art could readily adapt the method disclosed therein in order to conjugate
chelating agents to other polypeptides.
[0426] Bifunctional chelators based on macrocyclic ligands in which
conjugation is via an activated arm, or functional group, attached to the
carbon backbone of the ligand can be employed as described by M. Moi et aZ, J.
Azner, Clzenz. Soc. 49:2639 (1989) (2 p-nitrobenzyl-1,4,7,10-
tetraazacyclododecane-N,N',N",N"'-tetraacetic acid); S. V. Deshpande et aL, J.
Nncl. Med 31:473 (1990); G. Ruser et aTõ Biocozj. Chem. 1:345
(1990); C. J. Broan et al., J. C. S. Chem. Comm. 23:1739 (1990); and C. J.
Anderson et al., J. Nucl. Med. 36:850 (1995).
[0427] In one embodiment, a macrocyclic chelator, such as polyazamacrocyclic
chelators, optionally containing one or more carboxy, amino,
hydroxamate, phosphonate, or phosphate groups, are attached to the Albumin
fusion protein of the invention. In another embodiment, the chelator
is a chelator selected from the group consisting of DOTA, analogues of DOTA,
and derivatives of DOTA.
[0428] In one embodiment, suitable chelator molecules that may be attached to
the the Albumin fusion protein of the invention include DOXA
(1-oxa-4,7,10-triazacyclododecanetriacetic acid), NOTA (1,4,7-
triazacyclononanetriacetic acid), TETA (1,4,8,1 1-
tetraazacyclotetradecanetetraacetic
acid), and THT (4'-(3-amino-4-methoxy-phenyl)-6,6"-bis(N',N'-dicarbo).ymethyl-
N-methylhydra zino)-2,2':6',2"-terpyridine), and analogs and
derivatives thereof. See, e.g., Ohmono et aG, J. Med. CTsern. 35: 157-162
(1992); Kung et al., J. Nucl. Mecl. 25: 326-332 (1984); Jurisson et al.,
Cliezn. Rev. 93:1137-1156 (1993); and U.S. Patent No. 5,367,080. Other
suitable chelators include chelating agents disclosed in U.S. Patent Nos.
4,647,447; 4,687,659; 4,885,363; EP-A-71564; W089/00557; and EP-A-232751.
[04291 In another embodiment, suitable macrocyclic carboaylic acid chelators
which can be used in the present invention include 1,4,7,10-
tetraazacyclododecane-N,N,N",N"-tetraacetic acid (DOTA); 1,4,8,12-
tetraazacyclopentadecane-N,N,N',N"-tetraacetic acid (15N4); 1,4,7-
triazacyclononane-N,N',N"-triacetic acid (9N3); 1,5,9-triazacyclododecane-
N,N',N"-triacetic acid (12N3); and 6-bromoacetamido-benzyl-1,4,8,11-
tetraazacyclotetradecane- N,N,N',N"'-tetraacetic acid (BAT).
[04301 A preferred chelator that can be attached to the Albumin Fusion protein
of the invention is a-(5-isothiocyanato- 2-methoxyphenyl)-
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, which is also known
as MeO-DOTA-NCS. A salt or ester of a-(5-isothiocyanato- 2-
methoxyphenyl)- 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid may
also be used.
[04311 Albumin fusion proteins of the invention to which chelators such as
those decribed are covalently attached may be labeled (via the
coordination site of the chelator) with radionuclides that are suitable for
therapeutic, diagnostic, or both therapeutic and diagnostic purposes.
Examples of appropriate metals include Ag, At, Au, Bi, Cu, Ga, Ho, In, Lu, Pb,
Pd, Pm, Pr, Rb, Re, Rh, Sc, Sr, Tc, Tl, Y, and Yb. Examples of the
radionuclide used for diagnostic purposes-are Fe, Gd,In, 67Ga, or 68Ga. In
another embodiment, the radionuclide used-for-diagnostic"purposes is
_. - "'In, or 67Ga. Examples of the radionuclide used for therapeutic purposes
are 166Ho, '65Dy 90Z, "smIn s2Fe, or'ZGa. In one embodiment, the
radionuclide used for diagnostic purposes is 's"Ho or 90Y. Examples of the
radionuclides used for both therapeutic and diagnostic purposes include
'53Sm, 177Lu,159Gd, I75Yb, or 47Sc. In one embodiment, the radionuclide is
153Sm,'77Lu, "$Yb, or'59Gd.
[0432] Preferred metal radionuclides include 90Y, 99mTc, "'In, 47SC, 67Ga,
$'Cr, "'mSn, 67Cu,167Tm, 97Ru,18SRe, 177Lu,'99Au, 47Sc, 67Ga, S'Cr,
177mS.n' 67C.n I67Tm' 95Rn lggRe 177Ln 199Au 203 Pb and 141 Ce.
[0433] In a particular embodiment, Albumin fusion proteins of the invention to
which chelators are covalently attached may be labeled with a
metal ion selected from the group consisting of 90Y, "'In, '77Lu,166Ho, 215Bi,
and ZZSAc.
[0434] Moreover, y-emitting radionuclides, such as 99niTc, "'In, 67Ga, and
1fi9Yb have been approved or under investigation for diagnostic
imaging, while (3-emitters, such as 67Cu, "'Ag, 186Re, and 90Y are useful for
the applications in tumor therapy. Also other useful radionuclides
include y-emitters, such as 99mTc, "'In, 67Ga, and169Yb, and (3-emitters, such
as 67Cu, "'Ag,'86Re,'S$Re and 90Y, as well as other radionuclides of
th 153Sm, 198Au,'49Pm, 85Sr, '42Pr, 214Pb, '09Pd, 166Ho, 208T1, and 44Sc.
Albumin fusion proteins of the
interest such as Z"At, 212Bi, 177Lu, 86Rb,1osP
invention to which chelators are covalently attached may be labeled with the
radionuclides described above.
[0435] In another embodiment, Albumin fusion proteins of the invention to
which chelators are covalently attached may be labeled with
paramagnetic metal ions including ions of transition and lanthanide metal,
such as metals having atomic numbers of 21-29, 42, 43, 44, or 57-71, in
particular ions of Cr, V, Mn, Fe, Co, Ni, Cu, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd,
Tb, Dy, Ho, Er, Tm, Yb, and Lu. The paramagnetic metals used in
compositions for magnetic resonance imaging include the elements having atomic
numbers of 22 to 29, 42, 44 and 58-70.
[04361 In another embodiment, Albumin fusion proteins of the invention to
which chelators are covalently attached may be labeled with
fluorescent metal ions including lanthanides, in particular La, Ce, Pr, Nd,
Pm, Sm, Eu (e.g., 'SZEu), Gd, Th, Dy, Ho, Er, Tm, Yb, and Lu.
[0437] In another embodiment, Albumin fusion proteins of the invention to
which chelators are covalently attached may be labeled with heavy
metal-containing reporters may include atoms of Mo, Bi, Si, and W.
[0438] It is also possible to label the albumin fusion proteins with a
fluorescent compound. When the fluorescently labeled antibody is exposed
to light of the proper wave length, its presence can then be detected due to
fluorescence. Among the most commonly used fluorescent labeling
76
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
com o rtd~ r'u resc irr isi5 hti byd at ; rhodamine, phycoerythrin,
phycocyanin, allophycocyanin, ophthaldehyde and fluorescamine.
[0439] The albumin fusion protein can also be detectably labeled using
fluorescence emitting metals such as 152Eu, or otirers of the lanthanide
series. These metals can be attached to the antibody using such metal
chelating groups as diethylenetriaminepentacetic acid (DTPA) or
ethylenediaminetetraacetic acid (EDTA).
[0440] The albumin fusion proteins can also can be detectably labeled by
coupling it to a chemiluminescent compound. The presence of the
chemiluminescent-tagged albumin fusion protein is then determined by detecting
the presence of luminescence that arises during the course of a
chemical reaction. Examples of particularly useful chemiluminescent labeling
compounds are luminol, isoluminol, theromatic acridinium ester,
imidazole, acridinium salt and oxalate ester.
[0441] Likewise, a bioluminescent compound may be used to label albumin fusion
proteins of the present invention. Bioluminescence is a type
of chemiluminescence found in biological systems in, which a catalytic protein
increases the efficiency of the chemiluminescent reaction. The
presence of a bioluminescent protein is determined by detecting the presence
of luminescence. Important bioluminescent compounds for purposes
of labeling are luciferin, luciferase and aequorin.
Trmtseenic Oreanisnrs
[0442] Transgenic organisms that express the albumin fusion proteins of the
invention are also included in the invention. Transgenic organisms
are genetically modified organisms into which recombinant, exogenous or cloned
genetic material has been transferred. Such genetic material is
often referred to as a transgene. The nucleic acid sequence of the transgene
may include one or more transcriptional regulatory sequences and other
nucleic acid sequences such as introns, that may be necessary for optimal
expression and secretion of the encoded protein. The transgene may be
designed to direct the expression of the encoded protein in a manner that
facilitates its recovery from the organism or from a product produced by
the organism, e.g. from the milk, blood, urine, eggs, hair or seeds of the
organism. The transgene may consist of nucleic acid sequences derived
from the genome of the same species or of a different species than the species
of the target animal. The transgene may be integrated either at a
locus of a genome where that particular nucleic acid sequence is not otherwise
normally found or at the normal locus for the transgene.
[0443] The term "germ cell line transgenic organism" refers to a transgenic
organism in which the genetic alteration or genetic information was
introduced into a germ line cell, thereby conferring the ability of the
transgenic organism to transfer the genetic information to offspring. If such
offspring in fact possess some or all of that alteration or genetic
information, then they too are transgenic organisms. The alteration or genetic
information may be foreign to the species of organism to which the recipient
belongs, foreign only to the particular individual recipient, or may be
geuetic information already possessed by the recipient. In the last case, the
altered or introduced gene may be expressed differently than the native
gene.
[0444] A transgenic organism may be a transgenic animal or a transgenic plant.
Transgenic animals can be produced by a variety of different
methods including transfection, electroporation, microinjection, gene
targeting in_embryonic-stem.cells.and recombinant viral and retroviral
infection
(see, e.g., U.S. Patent No. 4,736,866; U.S. Patent No. 5,602,307; Mullins et
al. (1993) Hypertension 22(4):630-633; Brenin et al. (1997) Surg.
Oncol. 6(2)99-110; Tuan (ed.), Recombinant Gene Expression Protocols, Methods
in Molecular Biology No. 62, Humana Press (1997)). The
method of introduction of nucleic acid fragments into recombination competent
mammalian cells can be by any method which favors
co-transformation of multiple nucleic acid molecules. Detailed procedures for
producing transgenic animals are readily available to one skilled in
the art, including the disclosures in U.S. Patent No. 5,489,743 and U.S.
Patent No. 5,602,307.
[0445] A number of recombinant or transgenic mice have been produced,
including those which express an activated oncogene sequence (U.S.
Patent No. 4,736,866); express simian SV40 T-antigen (U.S. Patent No.
5,728,915); lack the expression of interferon regulatory factor 1(IIZF-1)
(U.S. Patent No. 5,731,490); exhibit dopaminergic dysfunction (U.S. Patent No.
5,723,719); express at least one human gene which participates in
blood pressure control (U.S. Patent No. 5,731,489); display greater similarity
to the conditions existing in naturally occurring Alzheimer's disease
(U.S. Patent No. 5,720,936); have a reduced capacity to mediate cellular
adhesion (U.S. Patent No. 5,602,307); possess a bovine growth hormone
gene (Clutter et al. (1996) Genetics 143(4):1753-1760); or, are capable of
generating a fully human antibody response (McCarthy (1997) The Lancet
349(9049):405).
[0446] While mice and rats remain the animals of choice for most transgenic
experimentation, in some instances it is preferable or even
necessary to use alternative animal species. Transgenic procedures have been
successfully utilized in a variety of non-murine animals, including
sheep, goats, pigs, dogs, cats, monkeys, chimpanzees, hamsters, rabbits, cows
and guinea pigs (see, e.g., Kim et al. (1997) Mol. Reprod. Dev.
46(4):515-526; Houdebine (1995) Reprod. Nutr. Dev. 35(6):609-617; Petters
(1994) Reprod. Fertil. Dev. 6(5):643-645; Schnieke et al. (1997)
Science 278(5346):2130-2133; and Amoah (1997) J. Animal Science 75(2):578-
585).
[0447] To direct the secretion of the transgene-encoded protein of the
invention into the milk of transgenic mammals, it may be put under the
control of a promoter that is preferentially activated in mammary epithelial
cells. Promoters that control the genes encoding milk proteins are
preferred, for example the promoter for casein, beta lactoglobulin, whey acid
protein, or lactalbumin (see, e.g., DiTullio (1992) BioTechnology
10:74-77; Clark et al. (1989) BioTechnology 7:487-492; Gorton et al. (1987)
BioTechnology 5:1183-1187; and Soulier et a1. (1992) FEBS Letts.
297:13). The transgenic mammals of choice would produce large volumes of milk
and have long lactating periods, for example goats, cows, camels
77
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
1l ''
or'sheCCp.
[0448] An albumin fusion protein of the invention can also be expressed in a
transgenic plant, e.g. a plant in which the DNA transgene is
inserted into the nuclear or plastidic genome. Plant transformation procedures
used to introduce foreign nucleic acids into plant cells or protoplasts
are known in the art. See, in general, Methods in Enzymology Vol. 153
("Recombinant DNA Part D") 1987, Wu and Grossman Eds., Academic
Press and European Patent Application EP 693554. Methods for generation of
genetically engineered plants are further described in US Patent No.
5,283,184, US Patent No. 5, 482,852, and European Patent Application EP 693
554, all of wliich are hereby incorporated by reference.
Pharmaceutical or Tlteranerrtic Conrpositious
[0449] The albumin fusion proteins of the invention or formulations tliereof
may be administered by any conventional method including
parenteral (e.g. subcutaneous or intramuscular) injection or intravenous
infusion. The treatment may consist of a single dose or a plurality of doses
over a period of time.
[0450] While it is possible for an albumin fusion protein of the invention to
be administered alone, it is preferable to present it as a
pharmaceutical formulation, together with one or more acceptable carriers. The
carrier(s) must be "acceptable" in the sense of being compatible
with the albumin fusion protein and not deleterious to the recipients thereof.
Typically, the carriers will be water or saline which will be sterile and
pyrogen free. Albumin fusion proteins of the invention are particularly well
suited to formulation in aqueous carriers such as sterile pyrogen free
water, saline or other isotonic solutions because of their extended shelf-life
in solution. For instance, pharmaceutical compositions of the invention
may be formulated well in advance in aqueous form, for instance, weeks or
months or longer time periods before being dispensed.
[0451] For example, formulations containing the albumin fusion protein may be
prepared taking into account the extended shelf-life of the
albumin fusion protein in aqueous formulations. As discussed above, the shelf-
life of many of these Therapeutic proteins are markedly increased or
prolonged after fusion to HA.
[0452] In instances where aerosol administration is appropriate, the albumin
fusion proteins of the invention can be formulated as aerosols using
standard procedures. The term "aerosol" includes any gas-bome suspended phase
of an albumin fusion protein of the instant invention which is
capable of being inhaled into the bronchioles or nasal passages. Specifically,
aerosol includes a gas-borne suspension of droplets of an albumin
fusion protein of the instant invention, as may be produced in a metered dose
inhaler or nebulizer, or in a mist sprayer. Aerosol also includes a dry
powder composition of a compound of the instant invention suspended in air or
other carrier gas, which may be delivered by insufflation from an
inhaler device, for example. See Ganderton & Jones, Drug Delivety to the
Respiratory Tract, Ellis Horwood (19 87); Gonda (1990) Critical
Revieivs in Tlserapeutic Drug Carrier Systeans 6:273-313; and Raeburn et al,.
(1992) Plzarmacol. Toxicol. Methods 27:143-159.
[0453] The formulations of the invention are also typically non-immunogenic,
in part, because of the use of the components of the albumin
fusion protein being derived from the proper species. For instance, for human
use, both the Therapeutic protein and albumin portions of the
albumin fusion protein will-typicallybe-human:- Imsome cases;-wherein either
component-is non human-derived, that componentmay be humanized- -
by substitution of key amino acids so that specific epitopes appear to the
human immune system to be human in nature rather than foreign.
[0454] The formulations may conveniently be presented in unit dosage form and
may be prepared by any of the methods well known in the art
of pharmacy. Such methods include the step of bringing into association the
albumin fusion protein with the carrier that constitutes one or more
accessory ingredients. In general the formulations are prepared by uniformly
and intimately bringing into association the active ingredient with
liquid carriers or fmely divided solid carriers or both, and then, if
necessary, shaping the product.
[0455] Formulations suitable for parenteral administration include aqueous and
non-aqueous sterile injection solutions which may contain
anti-oxidants, buffers, bacteriostats and solutes which render the formulation
appropriate for the intended recipient; and aqueous and non-aqueous
sterile suspensions which may include suspending agents and thickening agents.
The formulations may be presented in unit-dose or multi-dose
containers, for example sealed ampules, vials or syringes, and may be stored
in a freeze-dried (lyophilised) condition requiring only the addition of
the sterile liquid carrier, for example water for injections, immediately
prior to use. Extemporaneous injection solutions and suspensions may be
prepared from sterile powders. Dosage formulations may contain the Therapeutic
protein portion at a lower molar concentration or lower dosage
compared to the non-fused standard formulation for the Therapeutic protein
given the extended serum half-life exhibited by many of the albumin
fusion proteins of the invention.
[0456] As an example, when an albumin fusion protein of the invention
comprises one of the proteins listed in the "Therapeutic Protein:X"
column of Table 1 as one or more of the Therapeutic protein regions, the
dosage form can be calculated on the basis of the potency of the albumin
fusion protein relative to the potency of the therapeutic protein alone, while
taking into account the prolonged serum half-life and shelf-life of the
albumin fusion proteins compared to that of native therapeutic protein. For
example, if the therapeutic protein is typically administered at 0.3 to
30.0 IU/kg/week, or 0.9 to 12.0 IU/kg/week, given in three or seven divided
doses for a year or more. In an albumin fusion protein consisting of full
length HA fused to a therpeutic protein, an equivalent dose in terms of units
would represent a greater weight of agent but the dosage frequency can
be reduced, for example to twice a week, once a week or less.
[0457] Formulations or compositions of the invention may be packaged together
with, or included in a kit with, instructions or a package insert
referring to the extended shelf-life of the albumin fusion protein component.
For instance, such instructions or package inserts may address
78
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
e8om 9en"t4e 8 o~dge co~'dit~dhs;' lrcti as ~ime, temperature and light,
taking into account the extended or prolonged shelf-life of the albumin fusion
proteins of the invention. Such instructions or package inserts may also
address the particular advantages of the albumin fusion proteins of the
inventions, such as the ease of storage for formulations that may require use
in the field, outside of controlled hospital, clinic or office conditions.
As described above, formulations of the invention may be in aqueous form and
may be stored under less than ideal circumstances without
significant loss of therapeutic activity.
[0458] Albumin fusion proteins of the invention can also be included in
nutraceuticals. For instance, certain albumin fusion proteins of the
invention may be administered in natural products, including milk or milk
product obtained from a transgenic mammal tvhich expresses albumin
fusion protein. Such compositions can also include plant or plant products
obtained from a transgenic plant whicli expresses the albumin fusion
protein. The albumin fusion protein can also be provided in powder or tablet
form, with or without other known additives, carriers, fillers and
diluents. Nutraceuticals are described in Scott Hegenhart, Food Product
Design, Dec. 1993.
[0459] The invention also provides methods of treatment and/or prevention of
diseases or disorders (such as, for example, any one or more of
the diseases or disorders disclosed herein) by administration to a subject of
an effective amount of an albumin fusion protein of the invention or a
polynucleotide encoding an albumin fusion protein of the invention ("albumin
fusion polynucleotide") in a pharmaceutically acceptable carrier.
[0460] The albumin fusion protein and/or polynucleotide will be formulated and
dosed in a fashion consistent with good medical practice, taking
into account the clinical condition of the individual patient (especially the
side effects of treatment with the albumin fusion protein and/or
polynucleotide alone), the site of delivery, the method of administration, the
scheduling of administration, and other factors known to practitioners.
The "effective amount" for purposes herein is thus determined by such
considerations.
[04611 As a general proposition, the total pharmaceutically effective amount
of the albumin fusion protein administered parenterally per dose
will be in the range of about lug/kg/day to 10 mg/kg/day of patient body
weight, although, as noted above, this will be subject to therapeutic
discretion. More preferably, this dose is at least 0.01 mg/kg/day, and most
preferably for humans between about 0.01 and 1 mg/kg/day for the
hormone. If given continuously, the albumin fusion protein is typically
administered at a dose rate of about 1 ug/kg/hour to about 50 ug/kg/hour,
either by 1-4 injections per day or by continuous subcutaneous infusions, for
example, using a mini-pump. An intravenous bag solution may also be
employed. The length of treatment needed to observe changes and the interval
following treatment for responses to occur appears to vary depending
on the desired effect.
[0462] As noted above, the albumin fusion protein of the invention has a
higher plasma stablity compared to the Therapeutic protein portion (or
fragment or variant thereof) alone. This increase in plasma stability should
be taken into account when determining the effective amount of the
albumin fusion protein to be administered per dose and the dosing
administration schedule. In particular, higher plasma stability may allow the
albumin fusion protein to be administered at a lower dose at the same
frequency of administrations, or alternatively, may allow the albumin fusion
protein to be administered_in fewer dosings. -Preferably, the-higher stability
allows the albumin-fusion protein of the invention to be administered
less often in fewer dosings. More preferably, the albumin fusion protein can
be administered once every two weeks. Still more preferably, the
albumin fusion protein can be administered once every three, four, five, or
more weeks depending on the pharmacokinetics of the albumin fusion
protein. For example, as discussed above, the pharmacokinetics of an IFN-alpha-
HSA fusion protein supports a dosing regimen of once every 2-4
weeks or more, and even dosing at intervals of 4 weeks or more than every 4
weeks.
[0463] The effective amount of the albumin fusion protein to be administered
per dose can also be denoted as the total formulated albumin
fusion protein concentration given per dose. In one embodiment, the total
formulated albumin fusion protein concentration administered to a patient
per dose is in the range of about 10 ug/dose to about 2000 ug/dose. More
preferably, the total concentration is in the range of about 100 ug/dose to
about 1000 ug/dose, or alternatively, about 1000 ug/dose to about 1200 ug/dose
or about 900 ug/dose to about 1800 ug/dose.
[04641 In a specific embodiment, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
dosed in a total formulated concentration of about 90 ug/dose to
about 2000 ug/dose. In more preferred embodiments, an IFN-alpha-HSA fusion
protein of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295,
or 4296) is dosed in a total formulated concentration of about 900
ug/dose to about 2000 ug/dose, about 900 ug/dose to about 1200 ug/dose, about
900 ug/dose to about 1800 ug/dose and most preferably in a total
formulated concentration of about 1200 ug/dose to about 1800 ug/dose. In
additional preferred embodiments, an IFN-alpha-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410,
3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296)
is dosed in a total formulated concentration of 600 ug/dose, 720 ug/dose, 800
ug/dose, 900 ug/dose, 1000 ug/dose, 1200 ug/dose, 1500 ug/dose,
1800 ug/dose, or 2000 ug/dose. In additional embodiments, the total formulated
dose of an IFN-alpha-HSA fusion protein of the invention (e.g.,
produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424,
3476, 3960, 4290, 4291, 4292, 4295, or 4296) is administered
either alone or in combination with an antiviral compound, such as ribavirin.
In additionally preferred embodiments, the total formulated dose of an
IFN-alpha-HSA fusion protein of the invention (e.g., produced by CIDs 2249,
2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960,
4290, 4291, 4292, 4295, or 4296) is administered in combination with one or
more antiviral compounds, including, but not limited to ribavirin.
[0465] In an additional embodiment, the total formulated concentration of an
IF'N-alpha-HSA fusion proteins of the invention (e.g., produced by
79
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
2'1E1~G3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered to treat a patient
infected with HCV. In a specific embodiment, the IFN-alpha-HSA fusion proteins
of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295,
or 4296) are administered to a Treatment naYve patient with HCV
either alone or in combination with an effective amount of an antiviral
compound, such as ribavirin, in a total formulated concentration of about 90
ug/dose to about 2000 ug/dose. In more preferred embodiments, the IFN-alpha-
HSA fusion protein of the invention (e.g., produced by CIDs 2249,
2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291,
4292, 4295, or 4296) is administered to a Treatment natve patient
with HCV either alone or in combination with an effective amount of antiviral
compound, such as ribavirin, in a total formulated concentration of
abodt 900 ug/dose to about 2000 ug/dose, about 900 ug/dose to about 1200
ug/dose, about 900 ug/dose to about 1800 ug/dose and most preferably
in a total formulated concentration of about 1200 ug/dose to about 1800
ug/dose. In additional preferred embodiments, an IFN-alpha-HSA fusion
protein of the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292,
4295, or 4296) is administered to a Treatment natve patient with HCV either
alone or in combination witli an effective amount of antiviral
compound, such as ribavirin, in a total formulated concentration of 600
ug/dose, 720 ug/dose, 800 ug/dose, 900 ug/dose, 1000 ug/dose, 1200
ug/dose, 1500 ug/dose, 1800 ug/dose, or 2000 ug/dose.
[0466] In an additional embodiment, the total formulated concentration of an
IFN-alpha-HSA fusion proteins of the invention are administered
to a Treatment naive patient with HCV in combination with an effective amount
of one or more antiviral compounds, including, for example,
ribavirin, in a total formulated concentration of about 90 ug/dose to about
2000 ug/dose. In additional preferred embodiments, the IFN-alpha-HSA
fusion protein of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291,
, 4292, 4295, or 4296) is administered to a Treatment naive patient with HCV
in combination with an effective amount of one or more antiviral
compounds, including, for example, ribavirin, in a total formulated
concentration of about 900 ug/dose to about 2000 ug/dose, about 900 ug/dose to
about 1200 ug/dose, about 900 ug/dose to about 1800 ug/dose and most
preferably in a total formulated concentration of about 1200 ug/dose to
about 1800 ug/dose. In additional preferred embodiments, an IFN-alpha-HSA
fusion protein of the invention (e.g., produced by CIDs 2249, 2343,
2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292,
4295, or 4296) is administered to a Treatment natve patient with
HCV in combination with an effective amount of one or more antiviral
compounds, including, for example, ribavirin, in a total formulated
concentration of 600 ug/dose, 720 ug/dose, 800 ug/dose, 900 ug/dose, 1000
ug/dose, 1200 ug/dose, 1500 ug/dose, 1800 ug/dose, or 2000 ug/dose.
[0467] In an additional embodiment, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered to a Treatment experienced patient with HCV either
alone or in combination with an effective amount of antiviral compound, such
as ribavirin, in a total formulated concentration of about 90 ug/dose to
about 2000 ug/dose. In more preferred embodiments, an IFN-alpha-HSA fusion
protein of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381; 2382, 2410, 3165; 3422, 3423; 3424, 3476, 3960; 4290, 4291,--4292,-4295,
or 4296) is administeredto aTreatment experienced patient-with
HCV either alone or in combination with an effective amount of antiviral
compound, such as ribavirin, in a total formulated concentration of about
900 ug/dose to about 2000 ug/dose, about 900 ug/dose to about 1200 ug/dose,
about 900 ug/dose to about 1800 ug/dose and most preferably in a
total formulated concentration of about 1200 ug/dose to about 1800 ug/dose. In
additional preferred embodiments, an IFN-alpha-HSA fusion
proteins of the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292,
4295, or 4296) is administered to a Treatment experienced patient with HCV
either alone or in combination with an effective amount of antiviral
compound, such as ribavirin, in a total formulated concentration of 600
ug/dose, 720 ug/dose, 800 ug/dose, 900 ug/dose, 1000 ug/dose, 1200
ug/dose, 1500 ug/dose, 1800 ug/dose, or 2000 ug/dose.
[0468] In an additional embodiment, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered to a Treatment experienced patient with HCV in
combination with an effective amount of one or more antiviral compounds,
including, for example, ribavirin, in a total formulated concentration of
about 90 ug/dose to about 2000 ug/dose. In more preferred embodiments, an IFN-
alpha-HSA fusion protein of the invention (e.g., produced by
CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960,
4290, 4291, 4292, 4295, or 4296) is administered to a Treatment
experienced patient with HCV in combination with an effective amount of one or
more antiviral compounds, including, for example, ribavirin, in a
total formulated concentration of about 900 ug/dose to about 2000 ug/dose,
about 900 ug/dose to about 1200 ug/dose, about 900 ug/dose to about
1800 ug/dose and most preferably in a total formulated concentration of about
1200 ug/dose to about 1800 ug/dose. In additional preferred
embodiments, an IFN-alpha-HSA fusion proteins of the invention (e.g., produced
by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423,
3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is administered to a
Treatment experienced patient with HCV in combination with an effective
amount of one or more antiviral compounds, including, for example, ribavirin,
in a total formulated concentration of 600 ug/dose, 720 ug/dose, 800
ug/dose, 900 ug/dose, 1000 ug/dose, 1200 ug/dose, 1500 ug/dose, 1800 ug/dose,
or 2000 ug/dose.
[0469] The total formulated concentration of the albumin fusion protein and
the dosing interval in which the dosing interval at which the
albumin fusion protein will administered will vary depending on the desired
effect and the particular therapeutic protein adminstered. In one
embodiment, the total formulated albumin fusion protein concentration
administered to a patient per dose is in the range of about 10 ug/dose to
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
abdut ZC1bb'u~ ~g~oseIoncei1a"week;onceevery two weeks, once every three
weeks, once every four weeks or more. More preferably, the total
concentration is in the range of about 100 ug/dose to about 1000 ug/dose once
a week, once every two weeks, once every three weeks, once every
four weeks or more, or altematively, about 1000 ug/dose to about 1200 ug/dose
or about 900 ug/dose to about 1800 ug/dose once a week, once every
two weeks, once every three weeks, once every four weeks or more.
[0470] In a specific embodiment, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered at a total formulated concentration of about 90
ug/dose to about 2000 ug/dose once every two, three, four, or five weeks. In
more preferred embodiments, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165,
3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
dosed in a total formulated concentration of about 900 ug/dose to about 2000
ug/dose once every one, two, three, four or five weeks; about 900
ug/dose to about 1200 ug/dose once every one, two, tliree, four or five weeks;
about 900 ug/dose to about 1800 ug/dose once every one, two, three,
four or five weeks; and most preferably in a total formulated concentration of
about 1200 ug/dose to 1800 ug/dose once every one, two, three, four
or five weeks. In additional embodiments, an IFN-alplra-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered at a total formulated concentration of about 600
ug/dose once every one, two, three, four or five weeks; 800 ug/dose once every
one, two, three, four or five weeks, 900 ug/dose once every one, two,
three, four or five weeks; 1000 ug/dose once every one, two, three, four or
five weeks; 1200 ug/dose once every one, two, three, four or five weeks;
1500 ug/dose once every one, two, three, four or five weeks; 1600 ug/dose once
every one, two, three, four or five weeks; 1800 ug/dose once every
one, two, three, four or five weeks; or 2000 ug/dose once every one, two,
three, four or five weeks. In more preferred embodiments, the IFN-alpha-
HSA fusion protein of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290,
4291, 4292, 4295, or 4296) is administered at a total formulated concentration
of 900 ug/dose once every two weeks, and more preferably at a total
concentration of 1200 ug/dose once every two weeks, 1200 ug/dose once every
four weeks, or 1800 ug/dose once every four weeks. In additional
embodiments, the total formulated dose of an IFN-alpha-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered either alone or in combination with an antiviral
compound, such as ribavirin. In additional preferred embodiments, the total
fonnulated dose of an IFN-alpha-HSA fusion protein of the invention
(e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423,
3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered in combination with one or more antiviral compounds, including,
for example, ribavirin.
[0471] In specific embodiments, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered to a Treatment natve HCV patient at a total
formulated concentration of about 90 ug/dose to about 2000 ug/dose once every
two, three, four, or five weeks either alone or in combination with
an antiviral compound, suchas ribavirin. - Inmore preferred embodiments, an-
IFN-alpha-HSA fusion protein-of the-invention (e.g., produced by
CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960,
4290, 4291, 4292, 4295, or 4296) is administered to a Treatment
naive HCV patient in a total formulated concentration of about 900 ug/dose to
about 2000 ug/dose once every one, two, three, four or five weeks;
about 900 ug/dose to about 1200 ug/dose once every one, two, three, four or
five weeks; about 900 ug/dose to about 1800 ug/dose once every one,
two, three, four or five weeks; and most preferably in a total formulated
concentration of about 1200 ug/dose to about 1800 ug/dose once every one,
two, three, four or five weeks either alone or in combination with an
antiviral compound, including, such as ribavirin. In additional embodiments,
an IFN-alpha-HSA fusion protein of the invention (e.g., produced by CIDs 2249,
2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476,
3960, 4290, 4291, 4292, 4295, or 4296) is administered to a Treatment naive
HCV patient at a total formulated concentration of about 600 ug/dose
once every one, two, three, four or five weeks; 800 ug/dose once every one,
two, three, four or five weeks, 900 ug/dose once every one, two, three,
four or five weeks; 1000 ug/dose once every one, two, three, four or five
weeks; 1200 ug/dose once every one, two, three, four or five weeks; 1500
ug/dose once every one, two, three, four or five weeks; 1600 ug/dose once
every one, two, three, four or five weeks; 1800 ug/dose once every one,
two, three, four or five weeks; or 2000 ug/dose once every one, two, three,
four or five weeks either alone or in combination with an antiviral
compound, such as ribavirin.
[0472] In preferred specific embodiments, an IFN-alpha-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment naYve HCV patient at a total
formulated concentration of about 90 ug/dose to about 2000 ug/dose once every
two, three, four, or five weeks in combination with one or more
antiviral compounds, including, for example, ribavirin. In more preferred
embodiments, an IFN-alpha-HSA fusion protein of the invention (e.g.,
produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424,
3476, 3960, 4290, 4291, 4292, 4295, or 4296) is administered to a
Treatment naYve HCV patient in a total formulated concentration of about 900
ug/dose to about 2000 ug/dose once every one, two, three, four or five
weeks; about 900 ug/dose to about 1200 ug/dose once every one, two, three,
four or five weeks; about 900 ug/dose to about 1800 ug/dose once every
one, two, three, four or five weeks; and most preferably in a total formulated
concentration of about 1200 ug/dose to about 1800 ug/dose once every
one, two, three, four or five weeks in combination with one or more antiviral
compounds, including, for example, ribavirin. In additional
embodiments, an IFN-alpha-HSA fusion protein of the invention (e.g., produced
by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423,
81
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
i .,,,. ,~~ ~~,.E
4z4, 4 ~, 6; , '~ 5,~~ 4~90, 4291; 29 , 4 9"or 4296) is administered to a
Treatment naive HCV patient at a total formulated concentration of about
600 ug/dose once every one, two, three, four or five weeks; 800 ug/dose once
every one, two, three, four or five weeks, 900 ug/dose once every one,
two, three, four or five weeks; 1000 ug/dose once every one, two, tlrree, four
or five weeks; 1200 ug/dose once every one, two, three, four or five
weeks; 1500 ug/dose once every one, two, three, four or five weeks; 1600
ug/dose once every one, two, tliree, four or five weeks; 1800 ug/dose
once every one, two, three, four or five weeks; or 2000 ug/dose once every
one, two, three, four or five weeks in combination witlr one or more
antiviral compounds, including, for example, ribavirin.
[0473] In more preferred embodiments, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment naive HCV patient at a total
formulated concentration of 900 ug/dose once every two weeks, and more
preferably at a total concentration of 1200 ug/dose once every two weeks,
1200 ug/dose once every four weeks, or 1800 ug/dose once every four weeks,
either alone or in combination with an antiviral compound, such as
ribavirin. In most preferred embodiments, an IFN-alpha-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment naYve HCV patient at a total
formulated concentration of 900 ug/dose once every two weeks, and more
preferably at a total concentration of 1200 ug/dose once every two weeks,
1200 ug/dose once every four weeks, or 1800 ug/dose once every four weeks, in
combination with one or more antiviral compounds, including, for
example, ribavirin.
[0474] In additional specific embodiments, an IFN-alpha-HSA fusion protein of
the invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment experienced HCV patient at a
total formulated concentration of about 90 ug/dose to about 2000 ug/dose once
every two, three, four, or five weeks eitlier alone or in combination
with an antiviral compound, such as ribavirin. In more preferred embodiments,
an IFN-alpha-HSA fusion protein of the invention (e.g., produced
by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476,
3960, 4290, 4291, 4292, 4295, or 4296) is administered to a
Treatment experienced HCV patient in a total formulated concentration of about
900 ug/dose to about 2000 ug/dose once every one, two, three, four
or five weeks; about 900 ug/dose to about 1200 ug/dose once every one, two,
three, four or five weeks; about 900 ug/dose to about 1800 ug/dose
once every one, two, three, four or five weeks; and most preferably in a total
formulated concentration of about 1200 ug/dose to about 1800 ug/dose
once every one, three, four or five weeks, or rtmost preferably every two
weeks either alone or in combination with an antiviral compound, such as
ribavirin. In additional embodiments, an IFN-alpha-HSA fusion proteins of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381, 2382,
2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or 4296) is
administered to a Treatment experienced HCV patient at a total
formulated concentration of about 600 ug/dose once every one, two, three, four
or five weeks; 800 ug/dose once every one, two, three, four or five
weeks, 900 ug/dose once every one, two, three, four or five weeks; 1000
ug/dose once every one, two, three, four or five weeks; 1200 ug/dose once
-every one; two; three, -four or five weeks; 1500 ug/dose once every one,-two,-
three, four or five weeks; 1600 ug/dose-once every one, two, three,
four or five weeks; 1800 ug/dose once every one, two, three, four or five
weeks; or 2000 ug/dose once every one, two, three, four or five weeks
either alone or in combination with an antiviral compound, such as ribavirin.
[0475] hi more specific embodiments, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment experienced HCV patient at a
total formulated concentration of about 90 ug/dose to about 2000 ug/dose once
every two, three, four, or five weeks in combination with one or more
antiviral compounds, including, for example, ribavirin. In more preferred
embodiments, an IFN-alpha-HSA fusion protein of the invention (e.g.,
produced by CIDs 2249, 2343, 2366, 2381, 2382, 2410, 3165, 3422, 3423, 3424,
3476, 3960, 4290, 4291, 4292, 4295, or 4296) is administered to a
Treatment experienced HCV patient in a total formulated concentration of about
900 ug/dose to about 2000 ug/dose once every one, two, three, four
or five weeks; about 900 ug/dose to about 1200 ug/dose once every one, two,
three, four or five weeks; about 900 ug/dose to about 1800 ug/dose
once every one, two, three, four or five weeks; and most preferably in a total
formulated concentration of about 1200 ug/dose to about 1800 ug/dose
once every one, three, four or five weeks, or most preferably every two weeks
in combination with one or more antiviral compounds, including, for
example, ribavirin. In additional embodiments, an IFN-alpha-HSA fusion
proteins of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295,
or 4296) is administered to a Treatment experienced HCV patient
at a total formulated concentration of about 600 ug/dose once every one, two,
three, four or five weeks; 800 ug/dose once every one, two, three, four
or five weeks, 900 ug/dose once every one, two, three, four or five weeks;
1000 ug/dose once every one, two, three, four or five weeks; 1200
ug/dose once every one, two, three, four or five weeks; 1500 ug/dose once
every one, two, three, four or five weeks; 1600 ug/dose once every one,
two, three, four or five weeks; 1800 ug/dose once every one, two, three, four
or five weeks; or 2000 ug/dose once every one, two, three, four or five
weeks in combination with one or more antiviral compounds, including, for
example, ribavirin.
[0476] In more preferred embodiments, an IFN-alpha-HSA fusion protein of the
invention (e.g., produced by CIDs 2249, 2343, 2366, 2381,
2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295, or
4296) is administered to a Treatment experienced HCV patient at a
total formulated concentration of 900 ug/dose once every two weeks, and more
preferably at a total concentration of 1200 ug/dose once every two
weeks, 1200 ug/dose once every four weeks, or 1800 ug/dose once every four
weeks, either alone or in combination with an antiviral compound,
82
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Il ,,. 1 ,'; i 11.J! ~i li;;ai iG; l: i i~,,;N
sucii as n avlrnl. "~ most YiPetb ed5 tY AIments, an IFN-alpha-HSA fusion
protein of the invention (e.g., produced by CIDs 2249, 2343, 2366,
2381, 2382, 2410, 3165, 3422, 3423, 3424, 3476, 3960, 4290, 4291, 4292, 4295,
or 4296) is administered to a Treatment experienced HCV patient
at a total formulated concentration of 900 ug/dose once every two weeks, and
more preferably at a total concentration of 1200 ug/dose once every
two weeks, 1200 ug/dose once every four weeks, or 1800 ug/dose once every four
weeks, in combination with one or more antiviral compounds,
including, for example, ribavirin.
[0477] Albumin fusion proteins and/or polynucleotides can be are administered
orally, rectally, parenterally, intracisternally, intravaginally,
intraperitoneally, topically (as by powders, ointments, gels, drops or
transdermal patch), bucally, or as an oral or nasal spray. "Pharmaceutically
acceptable carrier" refers to a non-toxic solid, semisolid or liquid filler,
diluent, encapsulating material or formulation auxiliary of any. The term
"parenteral" as used lierein refers to modes of administration which include
intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous
and intraarticular injection and infusion.
[0478] Albumin fusion proteins and/or polynucleotides of the invention are
also suitably administered by sustained-release systems. Examples
of sustained-release albumin fusion proteins and/or polynucleotides are
administered orally, rectally, parenterally, intracisternally, intravaginally,
intraperitoneally, topically (as by powders, ointments, gels, drops or
transdermal patch), bucally, or as an oral or nasal spray. "Pharmaceutically
acceptable carrier" refers to a non-toxic solid, semisolid or liquid filler,
diluent, encapsulating material or fonnulation auxiliary of any type. The
term "parenteral" as used herein refers to modes of administration which
include intravenous, intramuscular, intraperitoneal, intrastemal,
subcutaneous and intraarticular injection and infusion. Additional examples of
sustained-release albumin fusion proteins and/or polynucleotides
include suitable polymeric materials (such as, for example, semi-permeable
polymer matrices in the form of shaped articles, e.g., films, or
mirocapsules), suitable hydrophobic materials (for example as an emulsion in
an acceptable oil) or ion exchange resins, and sparingly soluble
derivatives (such as, for example, a sparingly soluble salt).
[0479] Sustained-release matrices include polylactides (U.S. Pat. No.
3,773,919, EP 58,481), copolymers of L-glutamic acid and
gamma-ethyl-L-glutamate (Sidman et al., Biopolymers 22:547-556 (1983)), poly
(2- hydroxyethyl methacrylate) (Langer et al., J. Biomed. Mater.
Res. 15:167-277 (1981), and Langer, Chem. Tech. 12:98-105 (1982)), ethylene
vinyl acetate (Langer et al., Id.) or poly-D- (-)-3-hydroxybutyric acid
(EP 133,988).
[0480] Sustained-release albumin fusion proteins and/or polynucleotides also
include liposomally entrapped albumin fusion proteins and/or
polynucleotides of the invention (see generally, Langer, Science 249:1527-1533
(1990); Treat et al., in Lrposomes in the Therapy of Irfectious
Disease and Cancer, Lopez-Berestein and Fidler (eds.), Liss, New York, pp. 317
-327 and 353-365 (1989)). Liposomes containing the albumin
fusion protein and/or polynucleotide are prepared by methods known per se: DE
3,218,121; Epstein et al., Proc. Natl. Acad. Sci. (USA)
82:3688-3692 (1985); Hwang et al., Proc. Natl. Acad. Sci.(USA) 77:4030-4034
(1980); EP 52,322; EP 36,676; EP 88,046; EP 143,949; EP
142,641;-Japanese Pat. Appl. 83-118008; U.S. Pat. Nos. 4,485,045 and
4,544,545; and EP 102,324-_ Ordinarily, the liposomes are of the small
(about 200-800 Angstroms) unilamellar type in which the lipid content is
greater than about 30 mol. percent cholesterol, the selected proportion
being adjusted for the optimal Therapeutic.
[0481] In yet an additional embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are delivered by way of a pump
(see Langer, supra; Sefton, CRC Crit. Ref. Biomed. Eng. 14:201 (1987);
Buchwald et al., Surgery 88:507 (1980); Saudek et al., N. Engl. J. Med.
321:574 (1989)).
[0482] Other controlled release systems are discussed in the review by Langer
(Science 249:1527-1533 (1990)).
[0483] For parenteral administration, in one embodiment, the albumin fusion
protein and/or polynucleotide is formulated generally by mixing it
at the desired degree of purity, in a unit dosage injectable form (solution,
suspension, or emulsion), with a pharmaceutically acceptable carrier, i.e.,
one that is non-toxic to recipients at the dosages and concentrations employed
and is compatible with other ingredients of the formulation. For
example, the formulation preferably does not include oxidizing agents and
other compounds that are known to be deleterious to the Therapeutic.
[0484] Generally, the formulations are prepared by contacting the albumin
fusion protein and/or polynucleotide uniformly and intimately with
liquid carriers or finely divided solid carriers or both. Then, if necessary,
the product is shaped into the desired formulation. Preferably the carrier
is a parenteral carrier, more preferably a solution that is isotonic with the
blood of the recipient. Examples of such carrier vehicles include water,
saline, Ringer's solution, and dextrose solution. Non-aqueous vehicles such as
fixed oils and ethyl oleate are also useful herein, as well as
liposomes.
[0485] The carrier suitably contains minor amounts of additives such as
substances that enhance isotonicity and chemical stability. Such
materials are non-toxic to recipients at the dosages and concentrations
employed, and include buffers such as phosphate, citrate, succinate, acetic
acid, and other organic acids or their salts; antioxidants such as ascorbic
acid; Iow molecular weight (less than about ten residues) polypeptides, e.g.,
polyarginine or tripeptides; proteins, such as serum albumin, gelatin, or
immunoglobulins; hydrophilic polymers such as polyvinylpyrrolidone;
amino acids, such as glycine, glutamic acid, aspartic acid, or arginine;
monosaccharides, disaccharides, and other carbohydrates including cellulose
or its derivatives, glucose, manose, or dextrins; chelating agents such as
EDTA; sugar alcohols such as mannitol or sorbitol; counterions such as
sodium; and/or nonionic surfactants such as polysorbates (including, for
example, Tween-20), poloxamers, or PEG.
83
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~r... q... i '' 'b..u
0486] he al uman t~l~foti pYt~ eitl i~" pacally formulated in such vehicles at
a concentration of about 0.1 mg/ml to 100 mg/mI, preferably 1-10
mg/ml, at a pH of about 3 to 8. It will be understood that the use of certain
of the foregoing excipients, carriers, or stabilizers will result in the
formation of polypeptide salts.
[0487] Any pharmaceutical used for therapeutic administration can be sterile.
Sterility is readily accomplished by filtration through sterile
filtration membranes (e.g., 0.2 micron membranes). Albumin fusion proteins
and/or polynuoleotides generally are placed into a container having a
sterile access port, for example, an intravenous solution bag or vial having a
stopper pierceable by a hypodermic injection needle.
[0488] Albumin fusion proteins and/or polynucleotides ordinarily will be
stored in unit or multi-dose containers, for example, sealed ampoules
or vials, as an aqueous solution or as a lyophilized formulation for
reconstitution. As an example of a lyophilized formulation, 10-m1 vials are
filled
with 5 ml of sterile-filtered 1% (w/v) aqueous albumin fusion protein and/or
polynucleotide solution, and the resulting mixture is lyophilized. The
infusion solution is prepared by reconstituting the lyophilized albumin fusion
protein and/or polynucleotide using bacteriostatic Water-for-Injection.
[0489] In a specific and preferred embodiment, the Albumin fusion protein
formulations comprises 0.01 M sodium phosphate, 0.15 mM sodium
cliloride, 0.16 micromole sodium octanoate/milligram of fusion protein, 15
micrograms/milliliter polysorbate 80, pH 7.2. In another specific and
preferred embodiment, the Albumin fusion protein formulations consists 0.01 M
sodium phosphate, 0.15 mM sodium chloride, 0.16 micromole
sodium octanoate/milligram of fusion protein, 15 micrograms/milliliter
polysorbate 80, pH 7.2. The pH and buffer are chosen to match
physiological conditions and the salt is added as a tonicifier. Sodium
octanoate has been chosen due to its reported ability to increase the thermal
stability of the protein in solution. Finally, polysorbate has been added as a
generic surfactant, which lowers the surface tension of the solution and
lowers non-specific adsorption of the albumin fusion protein to tiie container
closure system.
[0490] The invention also provides a pharmaceutical pack or kit comprising one
or more containers filled with one or more of the ingredients of
the albumin fusion proteins and/or polynucleotides of the invention.
Associated with such container(s) can be a notice in the form prescribed by a
govemmental agency regulating the manufacture, use or sale of pharmaceuticals
or biological products, which notice reflects approval by the agency
of manufacture, use or sale for human administration. In addition, the albumin
fusion proteins and/or polynucleotides may be employed in
conjunction with other therapeutic compounds.
[0491] The albumin fusion proteins and/or polynucleotides of the invention may
be administered alone or in combination with adjuvants.
Adjuvants that may be administered with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to, alum,
alum plus deoxycholate (ImmunoAg), MTP-PE (Biocine Corp.), QS21 (Genentech,
Inc.), BCG (e.g., THERACYSd), MPL and nonviable
preparations of Corynebacterium parvum. In a specific embodiment, albumin
fusion proteins and/or polynucleotides of the invention are
administered in combination with alum. In another specific embodiment, albumin
fusion proteins and/or polynucleotides of the invention are
administered in combination with QS-21. Further adjuvants that may be
administered with the albumin fusion proteins and/or polynucleotides of
the-invention include, but-are not limited to,--Monophosphoryl-lipid
immunomodulator; AdjuVax 100a; QS-21, QS-18, C-RL1005 Aluminum-salts;-
MF-59, and Virosomal adjuvant technology. Vaccines that may be administered
with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, vaccines directed toward protection
against MMR (measles, mumps, rubella), polio, varicella,
tetanus/diptheria, hepatitis A, hepatitis B, Haeinophilus influen.;ae B,
whooping cough, pneumonia, influenza, Lyme's Disease, rotavirus, cholera,
yellow fever, Japanese encephalitis, poliomyelitis, rabies, typhoid fever, and
pertussis. Combinations may be administered either concomitantly,
e.g., as an admixture, separately but simultaneously or concurrently; or
sequentially. This includes presentations in which the combined agents are
administered together as a therapeutic mixture, and also procedures in which
the combined agents are administered separately but simultaneously,
e.g., as through separate intravenous Iines into the same individual.
Administration "in combination" further includes the separate administration
of
one of the compounds or agents given first, followed by the second.
[0492] The albumin fusion proteins and/or polynucleotides of the invention may
be administered alone or in combination with other therapeutic
agents. Albumin fusion protein and/or polynucleotide agents that may be
administered in combination with the albumin fusion proteins and/or
polynucleotides of the invention, include but not limited to, chemotherapeutic
agents, antibiotics, steroidal and non-steroidal anti-inflammatories,
conventional immunotherapeutic agents, and/or therapeutic treatments described
below. Combinations may be administered either concomitantly,
e.g., as an admixture, separately but simultaneously or concurrently; or
sequentially. This includes presentations in which the combined agents are
administered together as a therapeutic mixture, and also procedures in which
the combined agents are administered separately but simultaneously,
e.g., as through separate intravenous lines into the same individual.
Administration "in combination" further includes the separate administration
of
one of the compounds or agents given first, followed by the second.
[0493] In one embodiment, the albumin fusion proteins and/or polynucleotides
of the invention are administered in combination with an
anticoagulant. Anticoagulants that may be administered with the compositions
of the invention include, but are not limited to, heparin, low
molecular weight heparin, warfarin sodium (e.g., COUIVIADIN@), dicumarol, 4-
hydroxycoumarin, anisindione (e.g., MIRADONTM),
acenocoumarol (e.g., nicoumalone, SINTHROMETM), indan-1,3-dione, phenprocoumon
(e.g., MARCUIvIARTM), ethyl biscoumacetate (e.g.,
TROMEXANTM), and aspirin. In a specific embodiment, compositions of the
invention are administered in combination with heparin and/or
warfarin. hi another specific embodiment, compositions of the invention are
administered in combination with warfarin. In another specific
84
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
1L., ll r'' !L dt :;",11tLl! 11::3t ': .li"n . Ill.
embodiment, composrtions of the invenlion are administered in combination with
warfarin and aspirin. In another specific embodiment,
compositions of the invention are administered in combination with heparin. In
another specific embodiment, compositions of the invention are
administered in combination with heparin and aspirin.
[0494] In another embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
thrombolytic drugs. Thrombolytic drugs that may be administered with the
compositions of the invention include, but are not limited to,
plasminogen, lys-plasminogen, alpha2-antiplasmin, streptokinae (e.g.,
KABII{INASETM), antiresplace (e.g., EMINASE''M), tissue plasminogen
activator (t-PA, altevase, ACTIVASETM), urokinase (e.g., ABBOKINASETM),
sauruplase, (Prourokinase, single chain urokinase), and aminocaproic
acid (e.g., AMICARTM). In a specific embodiment, compositions of the invention
are administered in combination with tissue plasminogen activator
and aspirin.
[0495] In another embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
antiplatelet drugs. Antiplatelet drugs that may be administered with the
compositions of the invention include, but are not limited to, aspirin,
dipyridamole (e.g., PERSANTINETM), and ticlopidine (e.g., TICLIDTM).
[0496] In specific embodiments, the use of anti-coagulants, thrombolytic
and/or antiplatelet drugs in combination witli albumin fusion proteins
and/or polynucleotides of the invention is contemplated for the prevention,
diagnosis, and/or treatment of tluombosis, arterial thrombosis, venous
thrombosis, thromboembolism, pulmonary embolism, atherosclerosis, myocardial
infaretion, transient ischemic attack, unstable angina. In specific
embodiments, the use of anticoagulants, thrombolytic drugs and/or antiplatelet
drugs in combination with albumin fusion proteins and/or
polynucleotides of the invention is contemplated for the prevention of
occulsion of saphenous grafts, for reducing the risk of periprocedural
thrombosis as might accompany angioplasty procedures, for reducing the risk of
stroke in patients with atrial fibrillation including nonrheumatic
atrial fibrillation, for reducing the risk of embolism associated with
mechanical heart valves and or mitral valves disease. Other uses for the
therapeutics of the invention, alone or in combination with antiplatelet,
anticoagulant, and/or thrombolytic drugs, include, but are not limited to, the
prevention of occlusions in extracorporeal devices (e.g., intravascular
canulas, vascular access shunts in hemodialysis patients, hemodialysis
machines, and cardiopulmonary bypass machines).
[0497] In certain embodiments, albumin fusion proteins and/or polynucleotides
of the invention are administered in combination with
antiretroviral agents, nucleoside/nucleotide reverse transcriptase inhibitors
(NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs),
and/or protease inhibitors (PIs). NRTIs that may be administered in
combination with the albumin fusion proteins and/or polynucleotides of the
invention, include, but are not limited to, RETROVIRT"' (zidovudine/AZT),
VIDEXT"" (didanosine/ddl), HIVIDT " (zalcitabine/ddC), ZERITT d
(stavudine/d4T), EPIVIRT"' (lamivudine/3TC), and COMBIVIRT"'
(zidovudine/lamivudine). NNRTIs that may be administered in combination with
the albumin fusion proteins and/or polynucleotides of the invention, include,
but are not liniited to, VIRAMUNET"' (nevirapine), RESCRIPTORT"'
(delavirdine), and-SUSTIVAT!"- (efavirenz).-- Protease inhibitors thatnaybe-
administered-in comb-inatioii with thd albumiii fusion proteins and/or
polynucleotides of the invention, include, but are not limited to, CRIXIVANT"'
(indinavir), NORVIRTM (ritonavir), INVIRASET"' (saquinavir), and
VIRACEPTT"' (nelfmavir). In a specific embodiment, antiretroviral agents,
nucleoside reverse transcriptase inhibitors, non-nucleoside reverse
transcriptase inhibitors, and/or protease inhibitors may be used in any
combination with albumin fusion proteins and/or polynucleotides of the
invention to treat AIDS and/or to prevent or treat HIV infection.
[0498] Additional NRTIs include LODENOSINET"' (F-ddA; an acid-stable adenosine
NRTI; Triangle/Abbott; COVIRACILT"'
(emtricitabine/FTC; structurally related to lamivudine (3TC) but with 3- to 10-
fold greater activity in vitro; Triangle/Abbott); dOTC (BCH-10652,
also structurally related to lamivudine but retains activity against a
substantial proportion of lamivudine-resistant isolates; Biochem Pharma);
Adefovir (refused approval for anti-HIV therapy by FDA; Gilead Sciences);
PREVEON (Adefovir Dipivoxil, the active prodrug of adefovir; its
active form is PMEA-pp); TENOFOVIRT"' (bis-POC PMPA, a PMPA prodrug; Gilead);
DAPD/DXG (active metabolite of DAPD;
Triangle/Abbott); D-D4FC (related to 3TC, with activity against AZT13TC-
resistant virus); GW420867X (Glaxo Wellcome); ZIAGENT "
(abacavir/159U89; Glaxo Wellcome Inc.); CS-87 (3'azido-2',3'-dideoxyuridine;
WO 99/66936); and S-acyl-2-thioethyl (SATE)-bearing prodrug
forms of (3-L-FD4C and (3-IrFddC (WO 98/17281).
[0499] Additional NNRTIs include COACTINONT"' (Emivirine/MKC-442, potent NNRTI
of the HEPT class; Triangle/Abbott);
CAPRAVIRINET"" (AG-1549/S-1153, a next generation NNRTI with activity against
viruses containing the K103N mutation; Agouron); PNU-
142721 (has 20- to 50-fold greater activity than its predecessor delavirdine
and is active against K103N mutants; Pharmacia & Upjohn); DPC-961
and DPC-963 (second-generation derivatives of efavirenz, designed to be active
against viruses with the K103N mutation; DuPont); GW-420867X
(has 25-fold greater activity than HBY097 and is active against K103N mutants;
Glaxo Wellcome); CALANOLIDE A (naturally occurring agent
fxom the latex tree; active against viruses containing either or both the
Y181C and K103N mutations); and Propolis (WO 99/49830).
[0500] Additional protease inhibitors include LOPINAVIRTM (ABT378/r; Abbott
Laboratories); BMS-232632 (an azapeptide; Bristol-Myres
Squibb); TIPRANAVIRTM' (PNU-140690, a non-peptic dihydropyrone; Phannacia &
Upjohn); PD-178390 (a nonpeptidic dihydropyrone; Parke-
Davis); BMS 232632 (an azapeptide; Bristol-Myers Squibb); L-756,423 (an
indinavir analog; Merck); DMP-450 (a cyclic urea compound; Avid &
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
!!.,. = If,,, . r' ~! !l :w. ! 11':, i~ .,f!.. ",:~i s~ . f! , ~ . .
upont); AUU- "~a peptidomnrietic"wr i rrr vitro activity against protease
inhibitor-resistant viruses; Agouron); VX-175/GW-433908 (phosphate
prodrug of amprenavir; Vertex & Glaxo Welcome); CGP61755 (Ciba); and
AGENERASETM (amprenavir; Glaxo Wellcome Inc.).
[0501] Additional antiretroviral agents include fusion inhibitors/gp4l
binders. Fusion inhibitors/gp4l binders include T-20 (a peptide from
residues 643-678 of the HN gp41 transmembrane protein ectodomain which binds
to gp41 in its resting state and prevents transformation to the
fusogenic state; Trimeris) and T-1249 (a second-generation fusion inhibitor;
Trimeris).
[0502] Additional antiretroviral agents include fusion iniiibitors/chemokine
receptor antagonists. Fusion inhibitors/chemokine receptor
antagonists include CXCR4 antagonists such as AMD 3100 (a bicyclam), SDF-1 and
its analogs, and ALX40-4C (a cationic peptide), T22 (an 18
amino acid peptide; Trimeris) and the T22 analogs T134 and T140; CCR5
antagonists such as RANTES (9-68), AOP-RANTES, NNY-RANTES,
and TAK-779; and CCR5/CXCR4 antagonists such as NSC 651016 (a distamycin
analog). Also included are CCR2B, CCR3, and CCR6
antagonists. Chemokine recpetor agonists such as RANTES, SDF-1, MIP-la, MIP-
10, etc., may also inhibit fusion.
[0503] Additional antiretroviral agents include integrase inhibitors.
Integrase inhibitors include dicaffeoylquinic (DFQA) acids; L-chicoric acid
(a dicaffeoyltartaric (DCTA) acid); quinalizarin (QLC) and related
anthraquinones; ZINTEVIRT"" (AR 177, an oligonucleotide that probably acts at
cell surface rather than being a true integrase inhibitor; Arondex); and
naphthols such as those disclosed in WO 98/50347.
[0504] Additional antiretroviral agents include hydror.yurea-like compunds
such as BCX-34 (a purine nucleoside phosphorylase inhibitor;
Biocryst); ribonucleotide reductase inhibitors such as DIDOXT"' (Molecules for
Health); inosine monophosphate dehydrogenase (IMPDH) inhibitors
sucha as VX-497 (Vertex); and mycopholic acids such as CellCept (mycophenolate
mofetil; Roche).
[0505] Additional antiretroviral agents include inhibitors of viral integrase,
inhibitors of viral genome nuclear translocation such as arylene
bis(methylketone) compounds; inhibitors of HIV entry such as AOP-RANTES, NNY-
RANTES, RANTES-IgG fusion protein, soluble complexes of
RANTES and glycosaminoglycans (GAG), and AMD-3100; nucleocapsid zinc finger
inhibitors such as dithiane compounds; targets of HIV Tat and
Rev; and pharmacoenhancers such as ABT-378.
[0506] Other antiretroviral therapies and adjunct therapies include cytokines
and lymphokines such as MIP-la, MIP-10, SDF-la, IL-2,
PROLEUKINTM (aldesleukin/L2-7001; Chiron), IL-4, IL-10, IL-12, and IL-13;
interferons such as IFN-alpha2a, IFN-alpha2b, or IFN-beta;
antagonists of TNFs, NFKB, GM-CSF, M-CSF, and IL-10; agents that modulate
immune activation such as cyclosporin and prednisone; vaccines
such as RemuneTM (HIV Immunogen), APL 400-003 (Apollon), recombinant gp120 and
fragments, bivalent (B/E) recombinant envelope
glycoprotein, rgp120CM235, MN rgp120, SF-2 rgp120, gp120/soluble CD4 complex,
Delta JR-FL protein, branched synthetic peptide derived from
discontinuous gp120 C3/C4 domain, fusion-competent immunogens, and Gag, Pol,
Nef, and Tat vaccines; gene-based therapies such as genetic
suppressor elements (GSEs; WO 98/54366), and intrakines (genetically modified
CC chemokines targetted to the ER to block surface expression of
newly synthesized CCR5 (Yang et a1., PNAS 94:11567-72 (1997); Chen et al.,
Nat. Med 3:1110-16 (1997)); antibodies such as the anti-CXCR4
antibody 12G5, the anti-CCR5 antibodies 2D7, 5C7, PA8, PA9, PA10, PAl l, PA12,
and PA14, the anti-CD4 antibodies Q4120 and RPA-T4, the
anti-CCR3 antibody 7B11, the anti-gp 120 antibodies 17b, 48d, 447-52D, 257-D,
268-D and 50.1, anti-Tat antibodies, anti-TNF-a antibodies, and
monoclonal antibody 33A; aryl hydrocarbon (AH) receptor agonists and
antagonists such as TCDD, 3,3',4,4',5-pentachlorobiphenyl, 3,3',4,4'-
tetrachlorobiphenyl, and a-naphthoflavone (WO 98/30213); and antioxidants such
as y-L-glutamyl-L-cysteine ethyl ester (y-GCE; WO 99/56764).
[0507] In a farther embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with one
or more antiviral agent. Antiviral agents that may be administered with the
albumin fusion proteins and/or polynucleotides of the invention include,
but are not limited to, acyclovir, ribavirin, ribavirin analog, amantadine,
remantidine, maxamine, or thymalfasin. Specifically, interferon albumin
fusion protein can be administered in combination with any of these agents.
Moreover, interferon alpha albumin fusion protein can also be
admistered with any of these agents, and preferably, interferon alpha 2a or 2b
albumin fusion protein can be administered with any of these agents.
Furthermore, interferon beta albumin fusion protein can also be admistered
with any of these agents. Additionally, any of the IFN hybrids albumin
fusion proteins can be administered in combination with any of these agents.
[0508] In a most preferred embodiment, an interferon albumin fusion protein of
the invention is administered in combination with ribavirin or a
ribavirin analog. In a preferred embodiment, the ribavirin or ribavirin
analogs that may be administered in combination with an interferon albumin
fusion protein include but are not limited to COPEGUS (HofBnan-La Roche,
Nutley, N.J.), REBETOL (Schering Corp., Kenilworth, N.J.),
VIR.AZOLE' (Valeant, Costa Mesa, CA), RIBAVINTM (Lupin, Baltimore, MD),
RIBAZIDTM (Epla, Kirachi, Pakistan), tribavirin, VIRAMIDINETM
(Valeant, Costa Mesa, CA), and RIBASPHERETM (Three Rivers Pharmaceuticals,
Cranberry Township, PA). In a further preferred embodiment,
interferon alpha albumin fusion protein is administered in combination with
ribavirin or ribavirin analog. In a further preferred embodiment,
interferon alpha 2a albumin fusion protein is administered in combination with
ribavirin or ribavirin analog. In a further preferred embodiment,
interferon alpha 2b albumin fusion protein is administered in combination with
ribavirin or ribavirin analog. In a further preferred embodiment,
interferon beta albumin fusion protein is administered in combination with
ribavirin or ribavirin analog. In a further preferred embodiment, hybrid
interferon albumin fusion protein is administered in combination with
ribavirin or ribavirin analog.
[0509] In a further embodiment, the albumin fusion proteins and/or
polynucleotides of the invention may be administered alone or in
86
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
ii IG. II:'fs =.1.= I K,"1 1,,,;U ;!i,.. I.. f
con wt one or ~iYOr~ ad19 ira( a ents for the treatment of viral infection. In
a preferred embodiment, an interferon-albumin fusion protein
of the invention may be administered in combination with one or more antiviral
agents. In an additional preferred embodiment, the viral infection
results from infection with a hepatitis virus. In a most preferred embodiment,
the liepatitis virus is hepatitis C virus (HCV). Antiviral agents that
may be administered with the albumin fusion proteins and/or polynucleotides of
the invention include, but are not limited to, small-molecule
inhibitors of viral enzymes, small-molecule inhibitors of RNA polymerase,
nucleic acid based antiviral agents, antisense oligonucleotide inhibitors,
thiazolides, novel immunomodulatory agents, and interferon enhancers. Anti-
viral enzynie inhibitors that may be administered in combination with
the albumin fusion proteins and/or polynucleotides of the invention include,
but are not limited to, VX-950 (protease inhibitor, Vertex, Cambridge,
MA), VX-497 (merimepodib, oral IMPDH inhibitor, Vertex, Cambridge, MA), BILB
1941 (protease inhibitor, Boehringer Ingelheim, Germany),
SCH7 (protease inhibitor, Schering Corp., Kenilworth, N.J.), MX-3253
(glucosidase inhibitor, Migenix, Vancouver, BC), IDN-6556 (caspase
inhibitor, Pfizer, New York, NY), UT231B (glucosidase inhibitor, United
Therapeutics, Silver Spring, MD), R1626 (viral protease inhibitor, F.
Hoffman-La Roche, Switzerland), ITMN-B (ITMN-191, protease inhibitor,
InterMune, Brisbane, CA), Celgosivir (MBI-3253, a-glucosidase
inhibitor, Migenix, Inc., Vancouver, B.C.), SCH 503034 (protease inhibitor,
Schering Corp., Kenilworth, N.J.), ACH 806 (GS9132, oral protease
inhibitor, Achillion, New Haven, CT / Gilead Sciences, Foster City, CA). Anti-
viral polymerase inhibitors that may be administered in combination
with the albumin fusion proteins and/or polynucleotides of the invention may
be nucleoside analogs or non-nucleoside inhibitors (NNIs). In a
preferred embodiment, the anti-viral polymerase inhibitors inhibit HCV RNA
polymerase. In one embodiment, the anti-viral polymerase inhibitors
may be nucleoside analogs including, but not limited to, NM283 (oral prodrug
of 23'-C-methyl-oytidine, Idenix, Cambride, MA), and 2'-C-methyl
nucleosides. In another emboidment, the anti-viral polymerase inhibitors may
be non-nucleoside inhibitors, including, but not limited to, JTK-103,
JTK-003, and JTK-109 (Japan Tabacco, Tokyo, Japan), R803 (Rigel, South San
Francisco, CA), HCV-371, HCV-086, and HCV-796 (ViroPharm,
Exton, PA / Wyeth, Madison, NJ), and XTL-2125 (BC2125, XTLbio, New York, NY).
Anti-viral nucleic acid based agents that may be
administered in combination with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to, antisense
oligonucleotides, ribozymes, and siRNAs or short hairpin RNAs (shRNA). Anti-
viral antisense oligonucleotide inhibitor agents that may be
administered in combination with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to, NEUGENE
AVI-4065 (AVI Biopharma, Portland, OR). In another embodiment, a thiazolide
may be administered in combination with the albumin fusion
proteins and/or polynucleotides of the invention. In a preferred embodiment,
thiazolides that may be administered in combination with the albumin
fusion proteins and/or polynucleotides of the invention include, but are not
limited to ALINIA (nitazoxanide, Romark Laboratories, L.C., Tampa,
FL). Anti-viral immunomodulatory agents that may be administered in
combination with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, ZADXNO (thymosin alpha 1,
thyrnalfasin, SciClone Pharmaceuticals Int'l, Hong Kong) and toll-like
receptor (TLR) agonists, including, but not limited to, ANA245 (TLR-7 agonist,
Anadys Pharmaceuticals, San Diego, CA), ANA975 (oral prodrug
of ANA245, Anadys Pharmaceuticals, San Diego, CA), and.CP_G-10101 (ACTILONTM,
TLR-9 agonist, Coley-P-harmaceutical Group, Wellesley,
MA). Interferon enhancers that may be administered in combination with the
albumin fusion proteins and/or polynucleotides of the invention
include, but are not limited to EMZ702 (Transition Therapeutics, Toronto,
Ontario). Moreover, anti-viral antibodies that may be administered in
combination with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to Tarvacin (humanized
monoclonal antibody that targets phosphatidylserine on the surface of tumor
endothelial cells, Peregrine Pharmaceuticals, Inc., Tustin, CA).
10510] In a preferred embodiment the albumin fusion protein that may be
administered alone or in combination with one or more of the antiviral
agents encompassed by the invention is an inteferon-albumin fusion protein. In
additional embodiment, the interferon portion of the interferon-
albumin fusion protein is an interferon alpha. Non-limiting examples of
interferon alpha encompassed by the invention include, but are not limited
to, the interferon alpha proteins disclosed in the Therapeutic protein column
of Table 1. In particular embodiments, the interferon alpha portion
consists or alternatively comprises interferon alpha-2a, interferon alpha-2b,
interferon alpha-2c, consensus interferon, interferon alfacon-1, interferon
alpha-nl, interferon alpha-n3, any commercially available form of interferon
alpha, such as, for example, INTRON A (Schering Corp., Kenilworth,
N.J.), ROFEROWA(Hoffinan-La Roche, Nutley, N.J.), Berofor alpha inteferon
(Boehringer Ingelheim Pharmaceutical, Inc., Ridgefied, Conn.),
OMNIFERONTM (Viragen, Inc., Plantation, FL), MULTIFERONTM (Viragen, Inc.,
Plantation, FL) WELLFERON (GlaxoSmithKline, London,
Great Britian), INFERGEN (Amgen, Inc., Thousands Oaks, CA), SUIvIIF=ERONO
(Sumitomo, Japan), BELEROFON" (Nautilus Biotech, France),
MAXY-ALPHATM (Maxygen, Redwood City, CA / Hoffinan-La Roche, Nutley, N.J.), or
any purified interferon alpha product or a fragment
thereof. In further embodiments, the interferon alpha portion of the IFN-alpha-
HSA fusion protein consists or alternatively comprises interferon
alpha modified or formulated for extended or controlled release. For example,
the interferon alpha portion consists, or alternatively comprises
commercially available extended release or controlled release interferon
alpha, including, but not limited to interferon-alpha-XL (Flamel
Technologies, France) and LOCTERONTM (BioLex Therapeutics/OctoPlus, Pittsboro,
NC). In additional embodiments, the interferon alpha portion
of the IFN-alpha-HSA fusion protein may be modified by the attachment of
chemical moieties. For example, the inteferon alpha portion may be
modified by pegylation. Accordingly, in additional embodiments, the interferon
alpha portion of the IFN-alpha-HSA fusion protein consists or
alternatively comprises pegylated forms of interferon alpha-2a, 2b, or
consensus interferon and include, but are not limited to, a commercially
available pegylated interferon alpha, such as, for example, PEG-INTRON
(Schering Corp., Kenilworth, N.J.), PEGASYS (Hoffinan-La Roche,
87
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
II~~' I~ ,. ii _,; = q
Nutley;'N.7: PE 0 MiV~E1~0~T M tV'iragen, Inc., Plantation, FL) or a fragment
thereo~In an additional preferred embodiment the interferon
portion of the albumin fusion protein is interferon alpha 2a or 2b interferon,
interferon albumin fusion protein can be administered in combination
with any of these agents. Moreover, in another embodiment, the interferon
portion of the interferon-albumin fusion protein is an interferon beta or
an interferon liybrids. In a further embodiment, the unfused interferon
portion of the inteferon-albumin fusion protein may be used alone or in
combination with one or more of the antiviral agents encompassed by the
invention.
[0511] In other embodiments, albumin fusion proteins and/or polynucleotides of
the invention may be administered in combination with anti-
opportunistic infection agents. Anti-opportunistic agents that may be
administered in combination with the albumin fusion proteins and/or
polynucleotides of the invention, include, but are not limited to,
TRIMETHOPRIM-SULFAMETHOXAZOLETM, DAPSONETM, PENTAMIDINETM,
ATOVAQUONETM, ISONIAZIDTM, RIFAMPINTM, PYRAZINAMIDETM, ETHAMBUTOLTM,
RIFABUTINTM, CLARITHROMYCINTM,
AZITHROMYCINTM, GANCICLOVIRTM, FOSCARNETTM, CIDOFOVIRTM, FLUCONAZOLETM,
ITRACONAZOLETM, KETOCONAZOLETM,
ACYCLOVIRTM, FAMCICOLVIRTM, PYRIMETI-IAMINETM, LEUCOVORINTM, NEUPOGENTM
(filgrastim/G-CSF), and LEUKINETM
(sargramostim/GM-CSF). In a specific embodiment, albumin fusion proteins
and/or polynucleotides of the invention are used in any combination
with TRIIv1ETHOPRIIv1-SULFAMETHOXAZOLETM, DAPSONETM, PENTAMIDINETM, and/or
ATOVAQUONETM to prophylactically treat or
prevent an opportunistic Prieumocystis carinii pneumonia infection. In another
specific embodiment, albumin fusion proteins and/or
polynucleotides of the invention are used in any combination with ISONIAZIDTM,
RIFAMPINTM, PYRAZINAMIDETM, and/or ETHAMBUTOLTM to
prophylactically treat or prevent an opportunistic Mycobacterium avium complex
infection. In another specific embodiment, albumin fusion
proteins and/or polynucleotides of the invention are used in any combination
with RIFABUTINTM, CLARITHROMYCINTM, and/or
AZTTHROMYCINTM to prophylactically treat or prevent an opportunistic
Mycobacterimn tuberculosis infection. ht another specific embodiment,
albumin fusion proteins and/or polynucleotides of the invention are used in
any combination with GANCICLOVIRTM, FOSCARNETTM, and/or
CIDOFOVIRTM to prophylactically treat or prevent an opportunistic
cytomegalovirus infection. In another specific embodiment, albumin fusion
proteins and/or polynucleotides of the invention are used in any combination
with FLUCONAZOLETM, ITRACONAZOLETM, and/or
KETOCONAZOLETM to prophylactically treat or prevent an opportunistic fungal
infection. In another specific embodiment, albumin fusion proteins
and/or polynucleotides of the invention are used in any combination with
ACYCLOVIRTM and/or FAMCICOLVIRTM to prophylactically treat or
prevent an opportunistic herpes simplex virus type I and/or type II infection.
In another specific embodiment, albumin fusion proteins and/or
polynucleotides of the invention are used in any combination with
PYRIMETHAMINETM and/or LEUCOVORINTM to prophylactically treat or
prevent an opportunistic Toxoplasina gondii infection. In another specific
embodiment, albumin fusion proteins and/or polynucleotides of the
invention are used in any combination with LEUCOVORINTM and/or NEUPOGENTM to
prophylactically treat or prevent an opportunistic bacterial
infection.
[0512] In a further embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with an
antibiotic agent. Antibiotic agents that may be administered with the albumin
fusion proteins and/or polynucleotides of the invention include, but are
not limited to, amoxicillin, beta-lactamases, aminoglycosides, beta-lactam
(glycopeptide), beta-lactamases, Clindamycin, chloramphenicol,
cephalosporins, ciprofloxacin, erythromycin, fluoroquinolones, macrolides,
metronidazole, penicillins, quinolones, rapamycin, rifampin,
streptomycin, sulfonamide, tetracyclines, trimethoprim, trimethoprim-
sulfamethoxazole, and vancomycin.
[0513] In other embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
immunestimulants. Immunostimulants that may be administered in combination
with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, levamisole (e.g., ERGAMISOLTM),
isoprinosine (e.g. INOSIPLEXTM), interferons (e.g. interferon alpha),
and interleukins (e.g., IL-2).
[0514] In other embodiments, albumin fusion proteins and/or polynucleotides of
the invention are administered in combination with
immunosuppressive agents. Immunosuppressive agents that may be administered in
combination with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to, steroids,
cyclosporine, cyclosporine analogs, cyclophosphamide methylprednisone,
prednisone, azathioprine, FK-506, 15-deoxyspergualin, and other
immunosuppressive agents that act by suppressing the function of responding T
cells. Other immunosuppressive agents that may be administered in combination
with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, prednisolone, methotrexate,
thalidomide, methoxsalen, rapamycin, leflunomide, mizoribine
(BREDININTM), brequinar, deoxyspergualin, and azaspirane (SKF 105685),
ORTHOCLONE OKT 3 (muromonab-CD3), SANDIIvIIvIUNETM,
NEORAT,TM, SANGDYATM (cyclosporine), PROGRAF (FK506, taerolimus), CELLCEPTm
(mycophenolate motefil, of which the active
metabolite is mycophenolic acid), IIvIURANTM (azathioprine),
glucocorticosteroids, adrenocortical steroids such as DELTASONETM (prednisone)
and HYDELTRASOLTM (prednisolone), FOLEXTM and MEXATETM (methotrxate),
OXSORALEN-ULTRATM (methoxsalen) and RAPAMUNETM
(sirolimus). In a specific embodiment, immunosuppressants may be used to
prevent rejection of organ or bone marrow transplantation.
[0515] In an additional embodiment, albumin fusion proteins and/or
polynucleotides of the invention are administered alone or in combination
with one or more intravenous immune globulin preparations. Intravenous immune
globulin preparations that may be administered with the albumin
88
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
1U, r.,.;It4n.1[ iLnU r.'~ 11.... ':~IIt .~JI ntlt ~r~
sion proteins and/or polynucYeotieles of the invention include, but not
limited to, GAMMART", IVEEGAMTA4, SANDOGLOBULINTM,
GAMMAGARD S/DT"", ATGAMTM (antithymocyte glubulin), and GAM1IvIUNET'". In a
specific embodiment, albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
intravenous immune globulin preparations in transplantation therapy (e.g.,
bone marrow transplant).
[0516] In another embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered alone or as part of a
combination therapy, either in vivo to patients or in vitro to cells, for the
treament of cancer. In a specific embodiment, the albumin fusion proteins,
particularly IL-2-albumin fusions, are administered repeatedly during passive
immunotlierapy for cancer, such as adoptive cell transfer therapy for
metastatic melanoma as described in Dudley el al. (Science Express, 19
September 2002., at www.scienceexpress.org, hereby incorporated by
reference in its entirety).
[0517] In certain embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered alone or in combination
with an anti-inflammatory agent. Anti-inflammatory agents that may be
administered with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, corticosteroids (e.g.
betamethasone, budesonide, cortisone, dexamethasone, hydrocortisone,
methylprednisolone, prednisolone, prednisone, and triamcinolone), nonsteroidal
anti-inflammatory drugs (e.g., diclofenac, diflunisal, etodolac,
fenoprofen, floctafenine, flurbiprofen, ibuprofen, indomethacin, ketoprofen,
meclofenamate, mefenamic acid, meloxicam, nabumetone, naproxen,
oxaprozin, phenylbutazone, piroxicam, sulindac, tenoxicam, tiaprofenic acid,
and tolmetin.), as well as antihistamines, aminoarylcarboxylic acid
derivatives, arylacetic acid derivatives, arylbutyric acid derivatives,
arylcarboxylic acids, arylpropionic acid derivatives, pyrazoles, pyrazolones,
salicylic acid derivatives, thiazinecarboxamides, e-acetamidocaproic acid, S-
adenosylmethionine, 3-amino-4-hydroxybutyric acid, amixetrine,
bendazac, benzydamine, bucolome, difenpiramide, ditazol, emorfazone,
guaiazulene, nabumetone, nimesulide, orgotein, oxaceprol, paranyline,
perisoxal, pifoxime, proquazone, proxazole, and tenidap.
[0518] In an additional embodiment, the compositions of the invention are
administered alone or in combination with an anti-angiogenic agent.
Anti-angiogenic agents that may be administered with the compositions of the
invention include, but are not limited to, Angiostatin (Entremed,
Rockville, MD), Troponin-1 (Boston Life Sciences, Boston, MA), anti-Invasive
Factor, retinoic acid and derivatives thereof, paclitaxel (Taxol),
Suramin, Tissue Inhibitor of Metalloproteinase-1, Tissue Inhibitor of
Metalloproteinase-2, VEGI, Plasminogen Activator Inhibitor-1, Plasminogen
Activator Inhibitor-2, and various forms of the lighter "d group" transition
metals.
[0519] Lighter "d group" transition metals include, for example, vanadium,
molybdenum, tungsten, titanium, niobium, and tantalum species.
Such transition metal species may form transition metal complexes. Suitable
complexes of the above-mentioned transition metal species include
oxo transition metal complexes.
[0520] Representative examples of vanadium complexes include oxo vanadium
complexes such as vanadate and vanadyl complexes. Suitable
vanadate complexes include-metavanadate and orthovanadate complexes -such as;
for example, -ammonium metavanadate, sodium metavanadate,--
and sodium orthovanadate. Suitable vanadyl complexes include, for example,
vanadyl acetylacetonate and vanadyl sulfate including vanadyl sulfate
hydrates such as vanadyl sulfate mono- and trihydrates.
[0521] Representative examples of tungsten and molybdenum complexes also
include oxo complexes. Suitable oxo tungsten complexes include
tungstate and tungsten oxide complexes. Suitable tungstate complexes include
ammonium tungstate, calcium tungstate, sodium tungstate dihydrate,
and tungstic acid. Suitable tungsten oxides include tungsten (IV) oxide and
tungsten (VI) oxide. Suitable oxo molybdenum complexes include
molybdate, molybdenum oxide, and molybdenyl complexes. Suitable molybdate
complexes include ammonium molybdate and its hydrates, sodium
molybdate and its hydrates, and potassium molybdate and its hydrates. Suitable
molybdenum oxides include molybdenum (VI) oxide, molybdenum
(VI) oxide, and molybdic acid. Suitable molybdenyl complexes include, for
example, molybdenyl acetylacetonate. Other suitable tungsten and
molybdenum complexes include hydroxo derivatives derived from, for example,
glycerol, tartaric acid, and sugars.
[0522] A wide variety of other anti-angiogenic factors may also be utilized
within the context of the present invention. Representative examples
include, but are not limited to, platelet factor 4; protamine sulphate;
sulphated chitin derivatives (prepared from queen crab shells), (Murata et
al.,
Cancer Res. 51:22-26, (1991)); Sulphated Polysaccharide Peptidoglycan Complex
(SP- PG) (the function of this compound may be enhanced by the
presence of steroids such as estrogen, and tamoxifen citrate); Staurosporine;
modulators of matrix metabolism, including for example, proline
analogs, cishydroxyproline, d,L-3,4-dehydroproline, Thiaproline, alpha,alpha-
dipyridyl, aminopropionitrile fumarate; 4-propyl-5-(4-pyridinyl)-
2(3H)-oxazolone; Methotrexate; Mitoxantrone; Heparin; Interferons; 2
Macroglobulin-serum; ChIIvII'-3 (Pavloff et al., J. Bio. Chem. 267:17321-
17326, (1992)); Chymostatin (Tomkinson et al., Biochem J. 286:475-480,
(1992)); Cyclodextrin Tetradecasulfate; Eponemycin; Camptothecin;
Fumagillin (Ingber et al., Nature 348:555-557, (1990)); Gold Sodium Thiomalate
("GST"; Matsubara and Ziff, J. Clin. Invest. 79:1440-1446,
(1987)); anticollagenase-serum; alpha2-antiplasmin (Holmes et al., J. Biol.
Chem. 262(4):1659-1664, (1987)); Bisantrene (National Cancer
Institute); Lobenzarit disodium (N-(2)-carboxyphenyl-4- chloroanthronilic acid
disodium or "CCA"; (Takeuchi et al., Agents Actions 36:312-316,
(1992)); and metalloproteinase inhibitors such as BB94.
[0523] Additional anti-angiogenic factors that may also be utilized within the
context of the present invention include Thalidomide, (Celgene,
Warren, NJ); Angiostatic steroid; AGM-1470 (H. Brem and J. Folkman JPediatr.
Surg. 28:445-51 (1993)); an integrin alpha v beta 3 antagonist
89
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
U417[154 (C. Storgar~ et al., J Chn. InvesC '~
(1999)); carboxynaminolmidazole; Carboxyamidotriazole (CAI) (National Cancer
Institute, Bethesda,
MD); Conbretastatin A-4 (CA4P) (OXiGENE, Boston, MA); Squalamine (Magainin
Pharmaceuticals, Plymouth Meeting, PA); TNP-470, (Tap
Pharmaceuticals, Deerfield, IL); ZD-0101 AstraZeneca (London, UK); APRA
(CT2584); Benefin, Byrostatin-1 (SC339555); CGP-41251 (PKC
412); CM101; Dexrazoxane (ICRF187); DMXAA; Endostatin; Flavopridiol;
Genestein; GTE; ImmTher; Iressa (ZD1839); Octreotide
(Somatostatin); Panretin; Penacillamine; Photopoint; PI-88; Prinomastat (AG-
3340) Purlytin; Suradista (FCE26644); Tamoxifen (Nolvadex);
Tazarotene; Tetrathiomolybdate; Xeloda (Capecitabine); and 5-Fluorouracil.
[0524] Anti-angiogenic agents that may be administed in combination witli the
compounds of the invention may work through a variety of
mechanisms including, but not limited to, inhibiting proteolysis of the
extracellular matrix, blocking the function of endothelial cell-extracellular
matrix adhesion molecules, by antagonizing the function of angiogenesis
inducers such as growth factors, and inhibiting integrin receptors expressed
on proliferating endothelial cells. Examples of anti-angiogenic inhibitors
that interfere with extracellular matrix proteolysis and which may be
administered in combination with the compositons of the invention include, but
are not limited to, AG-3340 (Agouron, La Jolla, CA), BAY-12-9566
(Bayer, West Haven, CT), BMS-275291 (Bristol Myers Squibb, Princeton, NJ), CGS-
27032A (Novartis, East Hanover, NJ), Marimastat (British
Biotech, Oxford, UK), and Metastat (Aeterna, St-Foy, Quebec). Examples of anti-
angiogenic inhibitors that act by blocking the function of
endothelial cell-extracellular matrix adhesion molecules and which may be
administered in combination with the compositons of the invention
include, but are not limited to, EMD-121974 (Merck KcgaA Darmstadt, Germany)
and Vitaxin (Ixsys, La Jolla, CA/Medimmune, Gaithersburg,
MD). Examples of anti-angiogenic agents that act by directly antagonizing or
inhibiting angiogenesis inducers and which may be administered in
combination with the compositons of the invention include, but are not limited
to, Angiozyme (Ribozyme, Boulder, CO), Anti-VEGF antibody
(Genentech, S. San Francisco, CA), PTK-787/ZK-225846 (Novartis, Basel,
Switzerland), SU-101 (Sugen, S. San Francisco, CA), SU-5416 (Sugen/
Pharmacia Upjohn, Bridgewater, NJ), and SU-6668 (Sugen). Other anti-angiogenic
agents act to indirectly inhibit angiogenesis. Examples of
indirect inhibitors of angiogenesis which may be administered in combination
with the compositons of the invention include, but are not limited to,
IM-862 (Cytran, Kirkland, WA), Iuterferon-alpha, IL-12 (Roche, Nutley, NJ),
and Pentosan polysulfate (Georgetown University, Washington, DC).
[0525] In particular embodiments, the use of compositions of the invention in
combination with anti-angiogenic agents is contemplated for the
treatment, prevention, and/or amelioration of an autoimmune disease, such as
for example, an autoimmune disease described herein.
[0526] In a particular embodiment, the use of compositions of the invention in
combination with anti-angiogenic agents is contemplated for the
treatment, prevention, and/or amelioration of arthritis. In a more particular
embodiment, the use of compositions of the invention in combination
with anti-angiogenic agents is contemplated for the treatment, prevention,
and/or amelioration of rheumatoid arthritis.
[0527] In another embodiment, the polynucleotides encoding a polypeptide of
the present invention are administered in combination with an
angiogenic protein, or polynucleotides encoding an angiogenic protein.
Examples of angiogenic proteins that may be administered with the
-compositions af the invention include, but are not limited to; acidic and-
basic fibroblast-growth factors,-VEGF-I,-VEGF-2; VEGF-3, epidermal
growth factor alpha and beta, platelet-derived endothelial cell growth factor,
platelet-derived growth factor, tumor necrosis factor alpha, hepatocyte
growth factor, insulin-like growth factor, colony stimulating factor,
macrophage colony stimulating factor, granulocyte/macrophage colony
stimulating factor, and nitric oxide synthase.
[0528] In additional embodiments, compositions of the invention are
administered in combination with a chemotherapeutic agent.
Chemotherapeutic agents that may be administered with the albumin fusion
proteins and/or polynucleotides of the invention include, but are not
limited to alkylating agents such as nitrogen mustards (for example,
Mechlorethamine, cyclophosphamide, Cyclophosphamide Ifosfamide,
Melphalan (L-sarcolysin), and Chlorambucil), ethylenimines and methylmelamines
(for example, Hexamethylmelamine and Thiotepa), alkyl
sulfonates (for example, Busulfan), nitrosoureas (for example, Carmustine
(BCNU), Lomustine (CCNU), Semustine (methyl-CCNU), and
Streptozocin (streptozotocin)), triazenes (for example, Dacarbazine (DTIC;
dimethyltriazenoimidazolecarboxamide)), folic acid analogs (for
example, Methotrexate (amethopterin)), pyrimidine analogs (for example,
Fluorouacil (5-fluorouracil; 5-FU), Floxuridine (fluorodeoxyuridine;
FudR), and Cytarabine (cytosine arabinoside)), purine analogs and related
inhibitors (for example, Mercaptopurine (6-mercaptopurine; 6-MP),
Thioguanine (6-thioguanine; TG), and Pentostatin (2'-deoxycoformycin)), vinca
alkaloids (for example, Vinblastine (VLB, vinblastine sulfate)) and
Vincristine (vincristine sulfate)), epipodophyllotoxins (for example,
Etoposide and Teniposide), antibiotics (for example, Dactinomycin
(actinomycin D), Daunorubicin (daunomycin; rubidomycin), Doxorubicin,
Bleomycin, Plicamycin (mithramycin), and Mitomycin (mitomycin C),
enzymes (for example, L-Asparaginase), biological response modifiers (for
example, Interferon-alpha and interferon-alpha-2b), platinum
coordination compounds (for example, Cisplatin (cis-DDP) and Carboplatin),
anthracenedione (Mitoxantrone), substituted ureas (for example,
Hydroxyurea), metliylhydrazine derivatives (for example, Procarbazine (N-
methylhydrazine; MIH), adrenocorticosteroids (for example,
Prednisone), progestins (for example, Hydroxyprogesterone caproate,
Medroxyprogesterone, Medroxyprogesterone acetate, and Megestrol acetate),
estrogens (for example, Diethylstilbestrol (DES), Diethylstilbestrol
diphosphate, Estradiol, and Ethinyl estradiol), antiestrogens (for example,
Tamoxifen), androgens (Testosterone proprionate, and Fluoxymesterone),
antiandrogens (for example, Flutamide), gonadotropin-releasing
horomone analogs (for example, Leuprolide), other hormones and hormone analogs
(for example, methyltestosterone, estramustine, estramustine
phosphate sodium, chlorotrianisene, and testolactone), and others (for
example, dicarbazine, glutamic acid, and mitotane).
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
It...ir .; ' trõ.':'"lt
[0529] In one embodiment, fhe cgnipositions of the invention are administered
in combination with one or more of the following drugs:
infliximab (also known as RemicadeTM Centocor, Inc.), Trocade (Roche, RO-32-
3555), Leflunomide (also known as AravaTM from IIoechst Marion
Roussel), KineretTM (an IL-1 Receptor antagonist also known as Anakinra from
Amgen, Inc.)
[0530] In a specific embodiment, compositions of the invention are
administered in combination with CHOP (cyclophosphamide, doxorubicin,
vincristine, and prednisone) or combination of one or more of the components
of CHOP. In one embodiment, the compositions of the invention are
administered in combination with anti-CD20 antibodies, human monoclonal anti-
CD20 antibodies. In another embodiment, the compositions of the
invention are administered in combination with anti-CD20 antibodies and CHOP,
or anti-CD20 antibodies and any combination of one or more of
the components of CHOP, particularly cyclophosphamide and/or prednisone. In a
specific embodiment, compositions of the invention are
administered in combination with Rituximab. In a further embodiment,
compositions of the invention are administered with Rituximab and CHOP,
or Rituximab and any combination of one or more of the components of CHOP,
particularly cyclophosphamide and/or prednisone. In a specific
embodiment, compositions of the invention are administered in combination with
tositumomab. In a further embodiment, compositions of the
invention are administered with tositumomab and CHOP, or tositumomab and any
combination of one or more of the components of CHOP,
particularly cyclophosphamide and/or prednisone. The anti-CD20 antibodies may
optionally be associated with radioisotopes, toxins or cytotoxic
prodrugs.
[0531] In another specific embodiment, the compositions of the invention are
administered in combination ZevalinTM. In a further embodiment,
compositions of the invention are administered with ZevalinTM and CHOP, or
ZevalinTM and any combination of one or more of the components of
CHOP, particularly cyclophosphamide and/or prednisone. ZevalinTM may be
associated with one or more radisotopes. Particularly preferred
isotopes are 90Y and'"In.
[05321 In an additional embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
cytokines. Cytokines that may be administered with the albumin fusion proteins
and/or polynucleotides of the invention include, but are not limited
to, IL2, 1L3, Iltl, IL5, IL6, IL7, IL10, IL12, IL13, 1L15, anti-CD40, CD40L,
IFN-gamma and TNF-alpha. In another embodiment, albumin fusion
proteins and/or polynucleotides of the invention may be administered with any
interleukin, including, but not limited to, IL-lalpha, 1L-lbeta, IL-2,
IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12,1L-13, IL-14, IL-
15, IL-16, IL-17,1L-18, IL-19, IL-20, and IL-21.
[0533] In one embodiment, the albumin fusion proteins and/or polynucleotides
of the invention are administered in combination with members
of the TNF family. TNF, TNF-related or TNF-like molecules that may be
administered with the albumin fusion proteins and/or polynucleotides of
the invention include, but are not limited to, soluble forms of TNF-alpha,
lymphotoxin-alpha (LT-alpha, also known as TNF-beta), LT-beta (found in
complex lieterotrimer LT-alpha2-beta), OPGL, FasL, CD27L, CD30L, CD40L, 4-
1BBL, DcR3, OX40L, TNF-gamma (International Publication No.
WO 96/14328), AIM-I (Intemational Publication No. WO 97/33899), endokine-alpha
(International Publication No. WO 98/07880), OPG, and
neutrokine-al -lia (lnternational Publicatiori No. W0 98/18921; OX40, and
nerve
p growth factor (NGF); and soluble fornis -of Fas, CD30, CD27,
CD40 and 4-1BB, TR2 (International Publication No. WO 96/34095), DR3
(International Publication No. WO 97/33904), DR4 (International
Publication No. WO 98/32856), TR5 (International Publication No. WO 98/30693),
TRANK, TR9 (International Publication No. WO
98/56892),TR10 (International Publication No. WO 98/54202), 312C2
(International Publication No. WO 98/06842), and TR12, and soluble forms
CD154, CD70, and CD153.
[0534] In an additional embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
angiogenic proteins. Angiogenic proteins that may be administered with the
albumin fusion proteins and/or polynucleotides of the invention
include, but are not limited to, Glioma Derived Growth Factor (GDGF), as
disclosed in European Patent Number EP-399816; Platelet Derived
Growth Factor-A (PDGF-A), as disclosed in European Patent Number EP-682110;
Platelet Derived Growth Factor-B (PDGF-B), as disclosed in
European Patent Number EP-282317; Placental Growth Factor (P1GF), as disclosed
in International Publication Number WO 92/06194; Placental
Growth Factor-2 (PIGF-2), as disclosed in Hauser et al., Growth Factors, 4:259-
268 (1993); Vascular Endothelial Growth Factor (VEGF), as
disclosed in International Publication Number WO 90/13649; Vascular
Endothelial Growth Factor-A (VEGF-A), as disclosed in European Patent
Number EP-506477; Vascular Endothelial Growth Factor-2 (VEGF-2), as disclosed
in International Publication Number WO 96/39515; Vascular
Endothelial Growth Factor B (VEGF-3); Vascular Endothelial Growth Factor B-186
(VEGF-B186), as disclosed in International Publication
Number WO 96/26736; Vascular Endothelial Growth Factor-D (VEGF-D), as
disclosed in International Publication Number WO 98/02543;
Vascular Endothelial Growth Factor-D (VEGF-D), as disclosed in International
Publication Number WO 98/07832; and Vascular Endothelial
Growth Factor-E (VEGF-E), as disclosed in German Patent Number DE19639601. The
above mentioned references are herein incorporated by
reference in their entireties.
[0535] In an additional embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
Fibroblast Growth Factors. Fibroblast Growth Factors that may be administered
with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, FGF-1, FGF-2, FGF-3, FGF-4, FGF-5,
FGF-6, FGF-7, FGF-8, FGF-9, FGF-10, FGF-1 1, FGF-12, FGF-13,
FGF-14, and FGF-15.
[0536] In an additional embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
91
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
hematopoietic growth factors. Hematopoietic growth factors that may be
administered with the albumin fusion proteins and/or polynucleotides of
the invention include, but are not limited to, granulocyte macrophage colony
stimulating factor (GM-CSF) (sargramostim, LEUICINETM,
PROKINETM), granulocyte colony stimulating factor (G-CSF) (filgrastim,
NEUPOGENT"'), macrophage colony stimulating factor (M-CSF, CSF-1)
erythropoietin (epoetin alfa, EPOGENTM, PROCRiTT"r), stem cell factor (SCF, c-
kit ligand, steel factor), megakaryocyte colony stimulating factor,
PI7CY321 (a GMCSF/IL-3 fusion protein), interleukins, especially any one or
more of IL-1 through IL-12, interferon-gamma, or thrombopoietin.
[0537] In certain embodiments, albumin fusion proteins and/or polynucleotides
of the present invention are administered in combination with
adrenergic blockers, such as, for example, acebutolol, atenolol, betaxolol,
bisoprolol, carteolol, labetalol, metoprolol, nadolol, oxprenolol, penbutolol,
pindolol, propranolol, sotalol, and timolol.
[0538] In another embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with an
antiarrhythmic drug (e.g., adenosine, amidoarone, bretylium, digitalis,
digoxin, digitoxin, diliazem, disopyramide, esmolol, flecainide, lidocaine,
mexiletine, moricizine, phenytoin, procainamide, N-acetyl procainamide,
propafenone, propranolol, quinidine, sotalol, tocainide, and verapamil).
[0539] In another embodiment, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
diuretic agents, such as carbonic anhydrase-inhibiting agents (e.g.,
acetazolamide, dichlorphenamide, and niethazolamide), osmotic diuretics (e.g.,
glycerin, isosorbide, mannitol, and urea), diuretics that inhibit Na+-K+-2C1"
symport (e.g., furosemide, bumetanide, azosemide, piretanide,
tripamide, ethacrynic acid, muzolimine, and torsemide), thiazide and thiazide-
like diuretics (e.g., bendroflumethiazide, benzthiazide, chlorothiazide,
hydrochlorothiazide, hydroflumethiazide, methyclothiazide, polythiazide,
trichormethiazide, chlorthalidone, indapamide, metolazone, and
quinethazone), potassium sparing diuretics (e.g., amiloride and triamterene),
and mineralcorticoid receptor antagonists (e.g., spironolactone,
canrenone, and potassium canrenoate).
[0540] In one embodiment, the albumin fusion proteins and/or polynucleotides
of the invention are administered in combination with treatments
for endocrine and/or hormone imbalance disorders. Treatments for endocrine
and/or hormone imbalance disorders include, but are not limited to,
127 I, radioactive isotopes of iodine such as 13'I and "3I; recombinant growth
hormone, such as HUMATROPETA1(recombinant somatropin); growth
hormone analogs such as PROTROPINT"' (somatrem); dopamine agonists such as
PARLODELT"" (bromocriptine); somatostatin analogs such as
SANDOSTATINTM' (octreotide); gonadotropin preparations such as PREGNYLT"",
A.P.L.T"' and PROFASIT"' (chorionic gonadotropin (CG)),
PERGONALT"' (menotropins), and METRODINT"' (urofollitropin (uFSH)); synthetic
human gonadotropin releasing hormone preparations such as
FACTRELT"' and LUTREPULSETM (gonadorelin hydrochloride); synthetic
gonadotropin agonists such as LUPRONT"" (leuprolide acetate),
SUPPRELINTAd (histrelin acetate), SYNARELT'" (nafarelin acetate), and
ZOLADEXT~" (goserelin acetate); syntlietic preparations of thyrotropin-
releasing hormone such as RELEFACT TRHTM' and THYPINONET"' (protirelin);
recombinant human TSH such as THYROGENr"'; synthetic
preparations of the sodium salts of the natural isomers of thyroid hormones
such as L-T4TM, SYNTHROIDT"" and LEVOTHROIDTM (levothyroxine
sodium), L-T3TM, CYTOMELTOA and TRIOSTAT" (liothyroine sodium), and
THYROLART"' (liotrix); antithyroid compounds such as 6-n-
propylthiouracil (propylthiouracil), 1-methyl-2-mercaptoimidazole and
TAPAZOLET"" (methimazole), NEO-MERCAZOLET'" (carbimazole); beta-
adrenergic receptor antagonists such as propranolol and esmolol; Ca'-+ channel
blockers; dexamethasone and iodinated radiological contrast agents
such as TELEPAQUET"' (iopanoic acid) and ORAGRAFINT"' (sodium ipodate).
[0541] Additional treatments for endocrine and/or hormone imbalance disorders
include, but are not limited to, estrogens or congugated
estrogens such as ESTRACETM (estradiol), ESTINYLT"' (ethinyl estradiol),
PREMARINT"", ESTRATABT"', ORTHO-ESTT"', OGENT"' and
estropipate (estrone), ESTROVIST"" (quinestrol), ESTRADERMT"" (estradiol),
DELESTROGENT"' and VALERGENTAd (estradiol valerate), DEPO-
ESTRADIOL CYPIONATET"~ and ESTROJECT LAT"' (estradiol cypionate);
antiestrogens such as NOLVADEXT"' (tamoxifen), SEROPHENETM
and CLOMIDT"" (clomiphene); progestins such as DURALUTINT"'
(hydroxyprogesterone caproate), MPAT'" and DEPO-PROVERAT"'
(medroxyprogesterone acetate), PROVERAT"' and CYCRINT"" (MPA), MEGACET"'
(megestrol acetate), NORLUTINT"' (norethindrone), and
NORLUTATETM and AYGESTINTM' (norethindrone acetate); progesterone implants
such as NORPLANT SYSTEMT"' (subdermal implants of
norgestrel); antiprogestins such as RU 486TM (mifepristone); hormonal
contraceptives such as ENOVIDT'" (norethynodrel plus mestranol),
PROGESTASERTT"' (intrauterine device that releases progesterone), LOESTRINT"',
BREVICONT"', MODICONT", GENORAT"', NELONAT'"
NORINYLT"", OVACON-35T"" and OVACON-50T " (ethinyl estradiol/norethindrone),
LEVLENT~", NORDETTETh', TRI-LEVLENT"" and
TRIPHASIL-21T"' (ethinyl estradiol/levonorgestrel) LO/OVRALT"' and OVRALT"'
(ethinyl estradiol/norgestrel), DEMULENT"' (ethinyl
estradiol/ethynodiol diacetate), NORINYLT"', ORTHO-NOVUMTA , NORETHINT"',
GENORATA1, and NELOVAT"' (noretliindrone/mestranol),
DESOGENT"' and ORTHO-CEPTT"' (ethinyl estradiol/desogestrel), ORTHO-CYCLENT'"
and ORTHO-TRICYCLENTh' (ethinyl
estradiol/norgestimate), MICRONORT"' and NOR QDT"' (norethindrone), and
OVRETTET"' (norgestrel).
[0542] Additional treatments for endocrine and/or hormone imbalance disorders
include, but are not limited to, testosterone esters such as
methenolone acetate and testosterone undecanoate; parenteral and oral
androgens such as TESTOJECT-50T"' (testosterone), TESTEXT"' (testosterone
propionate), DELATESTRYLT"' (testosterone enanthate), DEPO-TESTOSTERONET"'
(testosterone cypionate), DANOCRINET"' (danazol),
HALOTESTINT"' (fluoxymesterone), ORETON METHYLT"', TESTREDT"' and VIRII,ONT"'
(methyltestosterone), and OXANDRINT~'
92
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
ry4 r
(oxandrolone); testostero e transaernial systems such as TESTODERMTM; androgen
receptor antagonist and 5-alpha-reductase inhibitors such as
ANDROCURT'" (cyproterone acetate), EULEXINTM (flutamide), and PROSCART""
(finasteride); adrenocorticotropic hormone preparations such as
CORTROSYNTM (cosyntropin); adrenocortical steroids and their synthetic analogs
such as ACLOVATETM (alclometasone dipropionate),
CYCLOCORTT"' (amcinonide), BECLOVENTT"" and VANCERILTM (beclomethasone
dipropionate), CELESTONETM (betamethasone),
BENISONET'" and UTICORTTM (betamethasone benzoate), DIPROSONETM (betamethasone
dipropionate), CELESTONE PHOSPI-IATET""
(betamethasone sodium phosphate), CELESTONE SOLUSPANT'" (betamethasone, sodium
phosphate and acetate), BETA-VALT'" and
VALISONET"" (betamethasone valerate), TEMOVATETM (clobetasol propionate),
CLODERMT'" (clocortolone pivalate), CORTEFT"' and
HYDROCORTONETA1 (cortisol (hydrocortisone)), HYDROCORTONE ACETATETM (cortisol
(hydrocortisone) acetate), LOCOIDT"" (cortisol
(hydrocortisone) butyrate), HYDROCORTONE PHOSPHATET"" (cortisol
(Iiydrocortisone) sodium phosphate), A-HYDROCORTT"" and SOLU
CORTEFTM (cortisol (hydrocortisone) sodium succinate), WESTCORTT'" (cortisol
(hydrocortisone) valerate), CORTISONE ACETATETM
(cortisone acetate), DESOWENT"' and TRIDESILONTAd (desonide), TOPICORTTM
(desoximetasone), DECADRONTM (dexamethasone),
DECADRON LATM (dexamethasone acetate), DECADRON PHOSPHATET"' and HEXADROL
PHOSPHATET"' (dexamethasone sodium
phosphate), FLORONET"' and MAXIFLORTM (diflorasone diacetate), FLORINEF
ACETATET'" (fludrocortisone acetate), AEROBIDT"' and
NASALIDET"" (flunisolide), FLUONIDT"' and SYNALART"' (fluocinolone acetonide),
LIDEXTM (fluocinonide), FLUOR-OPT"' and FMLT'd
(fluorometholone), CORDRANT'" (flurandrenolide), HALOGT~' (halcinonide), HMS
LIZUIFILMT"' (medrysone), MEDROLTh' (methylprednisolone),
DEPO-MEDROLT"" and MEDROL ACETATETM (methylprednisone acetate), A-METHAPREDT""
and SOLUMEDROLTM (methylprednisolone
sodium succinate), ELOCONT"' (mometasone furoate), HALDRONET"' (paramethasone
acetate), DELTA-CORTEFT"' (prednisolone),
ECONOPREDT"' (prednisolone acetate), HYDELTRASOLT"' (prednisolone sodium
phosphate), HYDELTRA-T.B.AT"' (prednisolone tebutate),
DELTASONET"' (prednisone), ARISTOCORTT"' and KENACORTTM' (triamcinolone),
If.ENALOGT"" (triamoinolone acetonide), ARISTOCORTTM
and KENACORT DIACETATETM (triamcinolone diacetate), and ARISTOSPANT"'
(triamcinolone hexacetonide); inhibitors of biosynthesis and
action of adrenocortical steroids such as CYTADRENT"" (aminoglutethimide),
NIZORALT"' (ketoconazole), MODRASTANETM (trilostane), and
METOPIRONET " (metyrapone); bovine, porcine or human insulin or mixtures
thereof; insulin analogs; recombinant human insulin such as
HUMULINT"' and NOVOLINT"'; oral hypoglycemic agents such as ORAMIDET"' and
ORINASETM (tolbutamide), DIABINESETM (chlorpropamide),
TOLAMIDET"" and TOLINASETM (tolazamide), DYMELORT"' (acetohexamide),
glibenclamide, MICRONASETM, DIBETAT"' and GLYNASET""
(glyburide), GLUCOTROLT"" (glipizide), and DIAMICRONT"' (gliclazide),
GLUCOPHAGETM (metformin), ciglitazone, pioglitazone, and alpha-
glucosidase inhibitors; bovine or porcine glucagon; somatostatins such as
SANDOSTATINT"' (octreotide); and diazoxides such as PROGLYCEMTA
(diazoxide).
[0543] --In one embodiment, the albumin fusion proteins and/or polynucleotides
of the invention are administered in combination-with treatments
for uterine motility disorders. Treatments for uterine motility disorders
include, but are not limited to, estrogen drugs such as conjugated estrogens
(e.g., PREMARIN and ESTRATABO), estradiols (e.g., CLIMARA and ALORA ),
estropipate, and chlorotrianisene; progestin drugs (e.g.,
AMEN (medroxyprogesterone), MICRONOR (norethidrone acetate), PROMETRIIJM
progesterone, and megestrol acetate); and
estrogen/progesterone combination therapies such as, for example, conjugated
estrogens/medroxyprogesterone (e.g., PREMPROT"' and
PREMPHASE ) and norethindrone acetate/ethinyl estsradiol (e.g., FEMHRTT"").
[0544] In an additional embodiment, the albumiri fusion proteins and/or
polynucleotides of the invention are administered in combination with
drugs effective in treating iron deficiency and hypochromic anemias, including
but not limited to, ferrous sulfate (iron sulfata, FEOSOLTM), ferrous
fumarate (e.g., FEOSTATTM), ferrous gluconate (e.g., FERGONTM), polysaccharide-
iron complex (e.g., NIFEREXTM), iron dextran injection (e.g.,
INFEDTM), cupric sulfate, pyroxidine, riboflavin, Vitamin B12, cyancobalamin
injection (e.g., REDISOLTM, RUBRAMIN PCTM),
hydroxocobalamin, folic acid (e.g., FOLVITETM), leucovorin (folinic acid, 5-
CHOH4PteGlu, citrovorum factor) or WELLCOVORIN (Calcium salt
of leucovorin), transferrin or ferritin.
[0545] In certain embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with agents
used to treat psychiatric disorders. Psychiatric drugs that may be
administered with the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, antipsychotic agents (e.g.,
chlorpromazine, chlorprothixene, clozapine, fluphenazine, haloperidol,
loxapine,
mesoridazine, molindone, olanzapine, perphenazine, pimozide, quetiapine,
risperidone, thioridazine, thiothixene, trifluoperazine, and
triflupromazine), antimanic agents (e.g., carbamazepine, divalproex sodium,
lithium carbonate, and lithium citrate), antidepressants (e.g.,
amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine,
doxepin, fluvoxamine, fluoxetine, imipramine, isocarboxazid,
maprotiline, mirtazapine, nefazodone, nortriptyline, paroxetine, phenelzine,
protriptyline, sertraline, tranylcypromine, trazodone, trimipramine, and
venlafaxine), antianxiety agents (e.g., alprazolam, buspirone,
chlordiazepoxide, clorazepate, diazepam, halazepam, lorazepam, oxazepam, and
prazepam), and stimulants (e.g., d-amphetamine, methylphenidate, and
pemoline).
[0546] In other embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with agents
93
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
r' tf..tr M:;u n.,.n n;:;u ..'= II :; ,JI ..";li ., II :IG
used to treat neurological r]isorders. IVeurological agents that may be
administered witli the albumin fusion proteins and/or polynucleotides of the
invention include, but are not limited to, antiepileptic agents (e.g.,
carbamazepine, clonazepam, ethosuximide, phenobarbital, phenytoin, primidone,
valproic acid, divalproex sodium, felbamate, gabapentin, lamotrigine,
levetiracetam, oxcarbazepine, tiagabine, topiramate, zonisamide, diazepam,
lorazepam, and clonazepam), antiparkinsonian agents (e.g., levodopa/carbidopa,
selegiline, amantidine, bromocriptine, pergolide, ropinirole,
pramipexole, benztropine; biperiden; ethopropazine; procyclidine;
trihexyphenidyl, tolcapone), and ALS therapeutics (e.g. riluzole).
[0547] In another embodiment, albumin fusion proteins and/or polynucleotides
of the invention are administered in combination with
vasodilating agents and/or calcium channel blocking agents. Vasodilating
agents that may be administered with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to, Angiotensin
Converting Enzyme (ACE) inhibitors (e.g., papaverine, isoxsuprine,
benazepril, captopril, cilazapril, enalapril., enalaprilat, fosinopril,
lisinopril, moexipril, perindopril, quinapril, ramipril, spirapril,
trandolapril, and
nylidrin), and nitrates (e.g., isosorbide dinitrate, isosorbide mononitrate,
and nitroglycerin). Examples of calcium channel blocking agents that may
be administered in combination with the albumin fusion proteins and/or
polynucleotides of the invention include, but are not limited to amlodipine,
bepridil, diltiazem, felodipine, flunarizine, isradipine, nicardipine,
nifedipine, nimodipine, and verapamil.
[0548] In certain embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
treatments for gastrointestinal disorders. Treatments for gastrointestinal
disorders that may be administered with the albumin fusion protein and/or
polynucleotide of the invention include, but are not limited to, H2 histamine
receptor antagonists (e.g., TAGAMETTm (cimetidine), ZANTACTM
(ranitidine), PEPCIDTm (famotidine), and AXIDT'" (nizatidine)); inhibitors of
H, K+ ATPase (e.g., PREVACIDTm (lansoprazole) and PRILOSECTM
(omeprazole)); Bismuth compounds (e.g., PEPTO-BISMOOm (bismuth subsalicylate)
and DE-NOLm (bismuth subcitrate)); various antacids;
sucralfate; prostaglandin analogs (e.g. CYTOTECT"l (misoprostol)); muscarinic
cholinergic antagonists; laxatives (e.g., surfactant laxatives,
stimulant laxatives, saline and osmotic laxatives); antidiarrheal agents
(e.g., LOMOTILTM (diphenoxylate), MOTOFENm (diphenoxin), and
IIvIODIUMT"'t (loperamide hydrochloride)), synthetic analogs of somatostatin
such as SANDOSTATINTm (octreotide), antiemetic agents (e.g.,
ZOFRANTm (ondansetron), KYTRILTm (granisetron hydrochloride), tropisetron,
dolasetron, metoclopramide, chlorpromazine, perphenazine,
prochlorperazine, promethazine, thiethylperazine, triflupromazine,
domperidone, haloperidol, droperidol, trimethobenzamide, dexamethasone,
methylprednisolone, dronabinol, and nabilone); D2 antagonists (e.g.,
metoclopramide, trimethobenzamide and chlorpromazine); bile salts;
chenodeoxycholic acid; ursodeoxycholic acid; and pancreatic enzyme
preparations such as pancreatin and pancrelipase.
[0549] In additional embodiments, the albumin fusion proteins and/or
polynucleotides of the invention are administered in combination with
other therapeutic or prophylactic regimens, such as, for example, radiation
therapy.
[0550] The invention also provides a pharmaceutical pack or kit comprising one
or more containers filled with one or more of the ingredients of
the pharmaceutical compositions comprising albumin fusion proteins of the
invention. Optionally associated with such container(s) can be a notice
in the-form-prescribed by a- governmental agency regulating the manufacture,
use or sale of-pharmaceuticals or biological products, which notice
reflects approval by the agency of manufacture, use or sale for human
administration.
Gene Therany
[0551] Constructs encoding albumin fusion proteins of the invention can be
used as a part of a gene therapy protocol to deliver therapeutically
effective doses of the albumin fusion protein. A preferred approach for in
vivo introduction of nucleic acid into a cell is by use of a viral vector
containing nucleic acid, encoding an albumin fusion protein of the invention.
Infection of cells with a viral vector has the advantage that a large
proportion of the targeted cells can receive the nucleic acid. Additionally,
molecules encoded within the viral vector, e.g., by a cDNA contained in
the viral vector, are expressed efficiently in cells which have taken up viral
vector nucleic acid.
[0552] Retrovirus vectors and adeno-associated virus vectors can be used as a
recombinant gene delivery system for the transfer of exogenous
nucleic acid molecules encoding albumin fusion proteins in vivo. These vectors
provide efficient delivery of nucleic acids into cells, and the
transferred nucleic acids are stably integrated into the chromosomal DNA of
the host. The development of specialized cell lines (termed "packaging
cells") which produce only replication-defective retroviruses has increased
the utility of retroviruses for gene therapy, and defective retroviruses are
characterized for use in gene transfer for gene therapy purposes (for a review
see Miller, A.D. (1990) Blood 76:27 1). A replication defective
retrovirus can be packaged into virions which can be used to infect a target
cell through the use of a helper virus by standard techniques. Protocols
for producing recombinant retroviruses and for infecting cells in vitro or in
vivo with such viruses can be found in Current Protocols in Molecular
Biology, Ausubel, F.M. et al., (eds.) Greene Publishing Associates, (1989),
Sections 9.10-9.14 and other standard laboratory manuals.
[0553] Another viral gene delivery system useful in the present invention uses
adenovirus-derived vectors. The genome of an adenovirus can
be manipulated such that it encodes and expresses a gene product of interest
but is inactivated in terms of its ability to replicate in a normal lytic
viral life cycle. See, for example, Berlnier et al., BioTechniques 6:616
(1988); Rosenfeld et al., Science 252:431-434 (1991); and Rosenfeld et al.,
Ce1168:143-155 (1992). Suitable adenoviral vectors derived from the adenovirus
strain Ad type 5 d1324 or other strains of adenovirus (e.g., Ad2,
Ad3, Ad7 etc.) are lolown to those skilled in the art. Recombinant
adenoviruses can be advantageous in certain circumstances in that they are not
capable of infecting nondividing cells and can be used to infect a wide
variety of cell types, including epithelial cells (Rosenfeld et al., (1992)
cited
supra). Furthermore, the virus particle is relatively stable and amenable to
purification and concentration, and as above, can be modified so as to
94
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
õ N ., =i =r= u uai ii-u : u, ':;uc"'Ic = ll~ II
affect the spectrum of infectivity.,.Additiona'lly, introduced adenoviral DNA
(and foreign DNA contained tlierein) is not integrated into the genome
of a host cell but remains episomal, thereby avoiding potential problems that
can occur as a result of insertional mutagenesis in situations where
introduced DNA becomes integrated into the host genome (e.g., retroviral DNA).
Moreover, the carrying capacity of Ute adenoviral genome for
foreign DNA is large (up to 8 kilobases) relative to other gene delivery
vectors (Berkner et al., cited supra; Haj-Ahmand et al., J. Virol. 57:267
(1986)).
[05541 In another embodiment, non-viral gene delivery systems of the present
invention rely on endocytic pathways for the uptake of the subject
nucleotide molecule by the targeted cell. Exemplary gene delivery systems of
this type include liposomal derived systems, poly-lysine conjugates,
and artificial viral envelopes. In a representative embodiment, a nucleic acid
molecule encoding an albumin fusion protein of the invention can be
entrapped in liposomes bearing positive charges on their surface (e.g.,
lipofectins) and (optionally) which are tagged with antibodies against cell
surface antigens of the target tissue (Mizuno et al. (1992) No Sliiirkef Geka
20:547-5 5 1; PCT publication W091/06309; Japanese patent application
1047381; and European patent publication EP-A-43075).
[0555] Gene delivery systems for a gene encoding an albumin fusion protein of
the invention can be introduced into a patient by any of a
number of methods. For instance, a pharmaceutical preparation of the gene
delivery system can be introduced systemically, e.g. by intravenous
injection, and specific transduction of the protein in the target cells occurs
predominantly from specificity of transfection provided by the gene
delivery vehicle, cell-type or tissue-type expression due to the
transcriptional regulatory sequences controlling expression of the receptor
gene, or a
combination thereof. In other embodiments, initial delivery of the recombinant
gene is more limited with introduction into the animal being quite
localized. For example, the gene delivery vehicle can be introduced by
catheter (see U.S. Patent 5,328,470) or by Stereotactic injection (e.g. Chen
et
al. (1994) PNA3 91: 3 054-3 05 7). The pharmaceutical preparation of the gene
therapy construct can consist essentially of the gene delivery system
in an acceptable diluent, or can comprise a slow release matrix in which the
gene delivery vehicle is imbedded. Where the albumin fusion protein
can be produced intact from recombinant cells, e.g. retroviral vectors, the
pharmaceutical preparation can comprise one or more cells which produce
the albumin fusion protein.
Additional Gette Therany Met/tods
[0556] Also encompassed by the invention are gene therapy methods for treating
or preventing disorders, diseases and conditions. The gene
therapy methods relate to the introduction of nucleic acid (DNA, RNA and
antisense DNA or RNA) sequences into an animal to achieve expression
of an albumin fusion protein of the invention. This method requires a
polynucleotide which codes for an albumin fusion protein of the present
invention operatively linked to a promoter and any other genetic elements
necessary for the expression of the fusion protein by the target tissue.
Such gene therapy and delivery techniques are known in the art, see, for
example, W090/11092, which is herein incorporated by reference.
[0557] Thus, for example, cells from a patient may be engineered with a
polynucleotide (DNA or RNA) comprising a promoter operably linked
to-a polynucleotide-encoding an albumin fusionprotein of-the present-
invention ex-vivo with-the-engineered cells then being provided-to a patient
to be treated with the fusion protein of the present invention. Such methods
are well-known in the art. For example, see Belldegrun, A., et al., J.
Natl. Cancer Inst. 85: 207-216 (1993); Ferrantini, M. et al., Cancer Research
53: 1107-1112 (1993); Ferrantini, M. et al., J. Immunology 153: 4604-
4615 (1994); Kaido, T., et al., Int. J. Cancer 60: 221-229 (1995); Ogura, H.,
et al., Cancer Research 50: 5102-5106 (1990); Santodonato, L., et al.,
Human Gene Therapy 7:1-10 (1996); Santodonato, L., et al., Gene Therapy 4:1246-
1255 (1997); and Zhang, J.-F. et al., Cancer Gene Therapy 3:
31-38 (1996)), which are herein incorporated by reference. In one embodiment,
the cells which are engineered are arterial cells. The arterial cells
may be reintroduced into the patient through direct injection to the artery,
the tissues surrounding the artery, or through catheter injection.
[0558] As discussed in more detail below, the polynucleotide constructs can be
delivered by any method that delivers injectable materials to the
cells of an animal, such as, injection into the interstitial space of tissues
(heart, muscle, skin, lung, liver, and the like). The polynucleotide
constructs
may be delivered in a pharmaceutically acceptable liquid or aqueous carrier.
[0559] In one embodiment, polynucleotides encoding the albumin fusion proteins
of the present invention is delivered as a naked
polynucleotide. The term "naked" polynucleotide, DNA or RNA refers to
sequences that are free from any delivery vehicle that acts to assist,
promote or facilitate entry into the cell, including viral sequences, viral
particles, liposome formulations, lipofectin or precipitating agents and the
like. However, polynucleotides encoding the albumin fusion proteins of the
present invention can also be delivered in liposome formulations and
lipofectin formulations and the like can be prepared by methods well known to
those skilled in the art. Such methods are described, for example, in
U.S. Patent Nos. 5,593,972, 5,589,466, and 5,580,859, which are herein
incorporated by reference.
[0560] The polynucleotide vector constructs used in the gene therapy method
are preferably constructs that will not integrate into the host
genome nor will they contain sequences that allow for replication. Appropriate
vectors include pWLNEO, pSV2CAT, pOG44, pXT1 and pSG
available from Stratagene; pSVK3, pBPV, pMSG and pSVL available from
Pharmacia; and pEFI/V5, pcDNA3. 1, and pRc/CMV2 available from
hivitrogen. Other suitable vectors will be readily apparent to the skilled
artisan.
[0561] Any strong promoter known to those skilled in the art can be used for
driving the expression of the polynucleotide sequence. Suitable
promoters include adenoviral promoters, such as the adenoviral major late
promoter; or heterologous promoters, such as the cytomegalovirus (CMV)
promoter; the respiratory syncytial virus (RSV) promoter; inducible promoters,
such as the MMT promoter, the metallothionein promoter; heat
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
... .....~ .. õ ,.n.- ...,,n qnd= iF.d. / iLn.. .J,. ,..n1, :nP nd~õ
shock promoters; the albumin promoter; the ApoAI promoter; human globin
promoters; viral thymidine kinase promoters, such as the Herpes
Simplex thymidine kinase promoter; retroviral LTRs; the b-actin promoter; and
human growtli hormone promoters. The promoter also may be the
native promoter for the gene corresponding to the Therapeutic protein portion
of the albumin fusion proteins of the invention.
105621 Unlike other gene therapy techniques, one major advantage of
introducing naked nucleic acid sequences into target cells is the transitory
nature of the polynucleotide synthesis in the cells. Studies have shown that
non-replicating DNA sequences can be introduced into cells to provide
production of the desired polypeptide for periods of up to six months.
[0563] The polynucleotide construct can be delivered to the interstitial space
of tissues within the an animal, including of muscle, skin, brain,
lung, liver, spleen, bone marrow, tltymus, heart, lymph, blood, bone,
cartilage, pancreas, kidney, gall bladder, stomach, intestine, testis, ovary,
uterus, rectum, nervous system, eye, gland, and connective tissue.
Interstitial space of the tissues comprises the intercellular, fluid,
mucopolysaccharide matrix among the reticular fibers of organ tissues, elastic
fibers in the walls of vessels or chambers, collagen fibers of fibrous
tissues, or that same matrix within connective tissue ensheathing muscle cells
or in the lacunae of bone. It is similarly the space occupied by the
plasma of the circulation and the lymph fluid of the lymphatic channels.
Delivery to the interstitial space of muscle tissue is preferred for the
reasons
discussed below. They may be conveniently delivered by injection into the
tissues comprising these cells. They are preferably delivered to and
expressed in persistent, non-dividing cells which are differentiated, although
delivery and expression may be achieved in non-differentiated or less
completely differentiated cells, such as, for example, stem cells of blood or
skin fibroblasts. In vivo muscle cells are particularly competent in their
ability to take up and express polynucleotides.
[0564] For the naked nucleic acid sequence injection, an effective dosage
amount of DNA or RNA will be in the range of from about 0.05
mg/kg body weight to about 50 mg/kg body weight. Preferably the dosage will be
from about 0.005 mg/kg to about 20 mg/kg and more preferably
from about 0.05 mg/kg to about 5 mg/kg. Of course, as the artisan of ordinary
skill will appreciate, this dosage will vary according to the tissue site
of injection. The appropriate and effective dosage of nucleic acid sequence
can readily be determined by those of ordinary skill in the art and may
depend on the condition being treated and the route of administration.
[0565] The preferred route of administration is by the parenteral route of
injection into the interstitial space of tissues. However, other
parenteral routes may also be used, such as, inhalation of an aerosol
formulation particularly for delivery to lungs or bronchial tissues, throat or
mucous membranes of the nose. In addition, naked DNA constructs can be
delivered to arteries during angioplasty by the catheter used in the
procedure.
[0566] The naked polynucleotides are delivered by any method known in the art,
including, but not limited to, direct needle injection at the
delivery site, intravenous injection, topical administration, catheter
infusion, and so-called "gene guns". These delivery methods are k-nown in the
art.
[0567] - The-constructs may also be-delivered with delivery vehicles such-as
viral sequences; viral particles,-liposome formulations,-lipofectin,
precipitating agents, etc. Such methods of delivery are known in the art.
[0568] In certain embodiments, the polynucleotide constructs are complexed in
a liposome preparation. Liposomal preparations for use in the
instant invention include cationic (positively charged), anionic (negatively
charged) and neutral preparations. However, cationic liposomes are
particularly preferred because a tight charge complex can be formed between
the cationic liposome and the polyanionic nucleic acid. Cationic
liposomes have been shown to mediate intracellular delivery of plasmid DNA
(Felgner et al., Proc. Natl. Acad. Sci. USA (1987) 84:7413-7416,
which is herein incorporated by reference); mRNA (Malone et al., Proc. Natl.
Acad. Sci. USA (1989) 86:6077-6081, which is herein incorporated
by reference); and purified transcription factors (Debs et al., J. Biol. Chem.
(1990) 265:10189-10192, which is herein incorporated by reference), in
functional form.
[0569] Cationic liposomes are readily available. For example, N[1-2,3-
dioleyloxy)propyl]-N,N,N-triethylammonium (DOTMA) liposomes are
particularly useful and are available under the trademark Lipofectin, from
GIBCO BRL, Grand Island, N.Y. (See, also, Felgner et al., Proc. Natl
Acad. Sci. USA (1987) 84:7413-7416, which is herein incorporated by
reference). Other commercially available liposomes include transfectace
(DDAB/DOPE) and DOTAP/DOPE (Boehringer).
[0570] Other cationic liposomes can be prepared from readily available
materials using techniques well known in the art. See, e.g. PCT
Publication No. WO 90/11092 (which is herein incorporated by reference) for a
description of the synthesis of DOTAP (1,2-bis(oleoyloxy)-3-
(trimethylammonio)propane) liposomes. Preparation of DOTMA liposomes is
explained in the literature, see, e.g., P. Felgner et al., Proc. Natl.
Acad. Sci. USA 84:7413-7417, which is herein incorporated by reference.
Similar methods can be used to prepare liposomes from other cationic
lipid materials.
[0571] Similarly, anionic and neutral liposomes are readily available, such as
from Avanti Polar Lipids (Birmingham, Ala.), or can be easily
prepared using readily available materials. Such materials include
phosphatidyl, choline, cholesterol, phosphatidyl ethanolamine,
dioleoylphosphatidyl choline (DOPC), dioleoylphosphatidyl glycerol (DOPG),
dioleoylphoshatidyl ethanolamine (DOPE), among others. These
materials can also be mixed with the DOTMA and DOTAP starting materials in
appropriate ratios. Methods for making liposomes using these
materials are well known in the art.
96
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
rr ic. u, h. tr ,::a[ n...n 4;. U F' ...IL.
[0572] For example, commercially dioleoylphosphatidyl choline (DOPC),
dioleoylphosphatidyl glycerol (DOPG), and dioleoylphosphatidyl
ethanolamine (DOPE) can be used in various combinations to make conventional
liposomes, with or without the addition of cholesterol. Thus, for
example, DOPG/DOPC vesicles can be prepared by drying 50 mg each of DOPG and
DOPC under a stream of nitrogen gas into a sonication vial.
The sample is placed under a vacuum pump overnight and is hydrated the
following day with deionized water. The sample is then sonicated for 2
hours in a capped vial, using a Heat Systems mode1350 sonicator equipped with
an inverted cup (bath type) probe at the maximum setting while the
bath is circulated at 15 degrees celcius. Alternatively, negatively charged
vesicles can be prepared without sonication to produce multilamellar
vesicles or by extrusion through nucleopore membranes to produce unilamellar
vesicles of discrete size. Other methods are known and available to
those of skill in the art.
[0573] The liposomes can comprise multilamellar vesicles (MLVs), small
unilamellar vesicles (SUVs), or large unilamellar vesicles (LUVs),
with SUVs being preferred. The various liposome-nucleic acid complexes are
prepared using methods well known in the art. See, e.g., Straubinger
et al., Methods of hnmunology (1983), 101:512-527, which is herein
incorporated by reference. For example, MLVs containing nucleic acid can be
prepared by depositing a thin film of phospholipid on the walls of a glass
tube and subsequently hydrating with a solution of the material to be
encapsulated. SUVs are prepared by extended sonication of MLVs to produce a
homogeneous population of unilamellar liposomes. The material to
be entrapped is added to a suspension of preformed MLVs and then sonicated.
When using liposomes containing cationic lipids, the dried lipid film
is resuspended in an appropriate solution such as sterile water or an isotonic
buffer solution such as 10 mM Tris/NaCI, sonicated, and then the
preformed liposomes are mixed directly with the DNA. The liposome and DNA form
a very stable complex due to binding of the positively charged
liposomes to the cationic DNA. SUVs find use with small nucleic acid
fragments. LUVs are prepared by a number of methods, well known in the
art. Commonly used methods include CaZ+-EDTA chelation (Papahadjopoulos et
al., Biochim. Biophys. Acta (1975) 394:483; Wilson et al., Cell
17:77 (1979)); ether injection (Deamer, D. and Bangham, A., Biochim. Biophys.
Acta 443:629 (1976); Ostro et al., Biochem. Biophys. Res.
Commun. 76:836 (1977); Fraley et al., Proc. Natl. Acad. Sci. USA 76:3348
(1979)); detergent dialysis (Enoch, H. and Strittmatter, P., Proc. Natl.
Acad. Sci. USA 76:145 (1979)); and reverse-phase evaporation (REV) (Fraley et
al., J. Biol. Chem. 255:10431 (1980); Szoka, F. and
Papahadjopoulos, D., Proc. Natl. Acad. Sci. USA 75:145 (1978); Schaefer-Ridder
et al., Science 215:166 (1982)), which are herein incorporated
by reference.
[0574] Generally, the ratio of DNA to liposomes will be from about 10:1 to
about 1:10. Preferably, the ration will be from about 5:1 to about
1:5. More preferably, the ration will be about 3:1 to about 1:3. Still more
preferably, the ratio will be about 1:1.
[0575] U.S. Patent No. 5,676,954 (which is herein incorporated by reference)
reports on the injection of genetic material, complexed with
cationic liposomes carriers, into mice. U.S. Patent Nos. 4,897,355, 4,946,787,
5,049,386, 5,459,127, 5,589,466, 5,693,622, 5,580,859, 5,703,055,
and international publication no. WO 94/9469 (which are herein incorporated by
reference) provide cationic lipids for use in transfecting DNA into
cells-and mammals:---U.S. PatentNos: 51-589;466; 5;693,622;-5,580,859,
5;703,055; andintemational publication no WO 94/9469 provide methods
for delivering DNA-cationic lipid complexes to mammals.
[0576] In certain embodiments, cells are engineered, ex vivo or in vivo, using
a retroviral particle containing RNA which comprises a sequence
encoding an albumin fusion protein of the present invention. Retroviruses from
which the retroviral plasmid vectors may be derived include, but
are not limited to, Moloney Murine Leukemia Virus, spleen necrosis virus, Rous
sarcoma Virus, Harvey Sarcoma Virus, avian leukosis virus,
gibbon ape leukemia virus, human immunodeficiency virus, Myeloproliferative
Sarcoma Virus, and mammary tumor virus.
[0577] The retroviral plasmid vector is employed to transduce packaging cell
lines to form producer cell lines. Examples of packaging cells
which may be transfected include, but are not limited to, the PE501, PA317, R-
2, R-AM, PA12, T19-14X, VT-19-17-H2, RCRE, RCRIP, GP+E-86,
GP+envAml2, and DAN cell lines as described in Miller, Human Gene Therapy 1:5-
14 (1990), which is incorporated herein by reference in its
entirety. The vector may transduce the packaging cells through any means known
in the art. Such means include, but are not limited to,
electroporation, the use of liposomes, and CaPO4 precipitation. In one
alternative, the retroviral plasmid vector may be encapsulated into a
liposome, or coupled to a lipid, and then administered to a host.
[0578] The producer cell line generates infectious retroviral vector particles
which include polynucleotide encoding an albumin fusion protein
of the present invention. Such retroviral vector particles then may be
employed, to transduce eukaryotic cells, either irs vitro or in vivo. The
transduced eukaryotic cells will express a fusion protin of the present
invention.
[0579] In certain other embodiments, cells are engineered, ex vivo or in vivo,
with polynucleotide contained in an adenovirus vector.
Adenovirus can be manipulated such that it encodes and expresses fusion
protein of the present invention, and at the same time is inactivated in
terms of its ability to replicate in a normal lytic viral life cycle.
Adenovirus expression is achieved without integration of the viral DNA into
the host
cell chromosome, thereby alleviating concerns about insertional mutagenesis.
Furthermore, adenoviruses have been used as live enteric vaccines for
many years with an excellent safety profile (Schwartz et al. Am. Rev. Respir.
Dis.109:233-238 (1974)). Finally, adenovirus mediated gene transfer
has been demonstrated in a number of instances including transfer of alpha-l-
antitrypsin and CFTR to the lungs of cotton rats (Rosenfeld, M. A. et
al. (1991) Science 252:431-434; Rosenfeld et al., (1992) Cell 68:143-155).
Furthermore, extensive studies to attempt to establish adenovirus as a
causative agent in human cancer were uniformly negative (Green, M. et al.
(1979) Proc. Natl. Acad. Sci. USA 76:6606).
97
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
.:= ttõP Z::;Irii,.i
[0580] Suitab e adenoviral vectors :'useiul in the present invention are
described, for example, in Kozarsky and Wilson, Curr. Opin. Genet.
Devel. 3:499-503 (1993); Rosenfeld et al., Cell 68:143-155 (1992); Engelhardt
et al., Human Genet. Ther. 4:759-769 (1993); Yang et al., Nature
Genet. 7:362-369 (1994); Wilson et al., Nature 365:691-692 (1993); and U.S.
Patent No. 5,652,224, which are lierein incorporated by reference.
For example, the adenovirus vector Ad2 is useful and can be grown in human 293
cells. These cells contain the El region of adenovirus and
constitutively express Ela and Elb, which complement the defective
adenoviruses by providing the products of the genes deleted from the vector.
In
addition to Ad2, other varieties of adenovirus (e.g., Ad3, Ad5, and Ad7) are
also useful in the present invention.
[0581] Preferably, the adenoviruses used in the present invention are
replication deficient. Replication deficient adenoviruses require the aid of
a helper virus and/or packaging cell line to form infectious particles. The
resulting virus is capable of infecting cells and can express a
polynucleotide of interest which is operably linked to a promoter, but cannot
replicate in most cells. Replication deficient adenoviruses may be
deleted in one or more of all or a portion of the following genes: Ela, Elb,
E3, E4, E2a, or Ll through L5.
[0582] In certain other embodiments, the cells are engineered, ex vivo or in
vivo, using an adeno-associated virus (AAV). AAVs are naturally
occurring defective viruses that require helper viruses to produce infectious
particles (Muzyczka, N., Curr. Topics in Microbiol. Immunol. 158:97
(1992)). It is also one of the few viruses that may integrate its DNA into non-
dividing cells. Vectors containing as little as 300 base pairs of AAV
can be packaged and can integrate, but space for exogenous DNA is limited to
about 4.5 kb. Methods for producing and using such AAVs are
known in the art. See, for example, U.S. Patent Nos. 5,139,941, 5,173,414,
5,354,678, 5,436,146, 5,474,935, 5,478,745, and 5,589,377.
[0583] For example, an appropriate AAV vector for use in the present invention
will include all the sequences necessary for DNA replication,
encapsidation, and host-cell integration. The polynucleotide construct is
inserted into the AAV vector using standard cloning methods, such as those
found in Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold Spring
Harbor Press (1989). The recombinant AAV vector is then
transfected into packaging cells which are infected with a helper virus, using
any standard technique, including lipofection, electroporation, calcium
phosphate precipitation, etc. Appropriate helper viruses include adenoviruses,
cytomegaloviruses, vacoinia viruses, or herpes viruses. Once the
packaging cells are transfected and infected, they will produce infectious AAV
viral particles which contain the polynucleotide construct. These
viral particles are then used to transduce eukaryotic cells, either ex vivo or
in vivo. The transduced cells will contain the polynucleotide construct
integrated into its genome, and will express a fusion protein of the
invention.
[0584] Another method of gene therapy involves operably associating
heterologous control regions and endogenous polynucleotide sequences
(e.g. encoding a polypeptide of the present invention) via homologous
recombination (see, e.g., U.S. Patent No. 5,641,670, issued June 24, 1997;
International Publication No. WO 96/29411, published September 26, 1996;
International Publication No. WO 94/12650, published August 4, 1994;
Koller et al., Proc. Natl. Acad. Sci. USA 86:8932-8935 (1989); and Zijlstra et
al., Nature 342:435-438 (1989), which are herein encorporated by
reference. This method involves the activation of a gene which is present in
the target cells, but which is not normally expressed in the cells, or is
expressed at a lower-level-than desired.
[0585] Polynucleotide constructs are made, using standard techniques known in
the art, which contain the promoter with targeting sequences
flanking the promoter. Suitable promoters are described herein. The targeting
sequence is sufficiently complementary to an endogenous sequence
to permit homologous recombination of the promoter-targeting sequence with the
endogenous sequence. The targeting sequence will be sufficiently
near the 5' end of the desired endogenous polynucleotide sequence so the
promoter will be operably linked to the endogenous sequence upon
homologous recombination.
[0586] The promoter and the targeting sequences can be amplified using PCR.
Preferably, the amplified promoter contains distinct restriction
enzyme sites on the 5' and 3' ends. Preferably, the 3' end of the first
targeting sequence contains the same restriction enzyme site as the 5' end of
the
amplified promoter and the 5' end of the second targeting sequence contains
the same restriction site as the 3' end of the amplified promoter. The
amplified promoter and targeting sequences are digested and ligated together.
[0587] The promoter-targeting sequence construct is delivered to the cells,
either as naked polynucleotide, or in conjunction with transfection-
facilitating agents, such as liposomes, viral sequences, viral particles,
whole viruses, lipofection, precipitating agents, etc., described in more
detail
above. The P promoter-targeting sequence can be delivered by any method,
included direct needle injection, intravenous injection, topical
administration, catheter infusion, particle accelerators, etc. The methods are
described in more detail below.
[0588] The promoter-targeting sequence construct is taken up by cells.
Homologous recombination between the construct and the endogenous
sequence takes place, such that an endogenous sequence is placed under the
control of the promoter. The promoter then drives the expression of the
endogenous sequence.
[0589] The polynucleotide encoding an albumin fusion protein of the present
invention may contain a secretory signal sequence that facilitates
secretion of the protein. Typically, the signal sequence is positioned in the
coding region of the polynucleotide to be expressed towards or at the 5'
end of the coding region. The signal sequence may be homologous or
heterologous to the polynucleotide of interest and may be homologous or
heterologous to the cells to be transfected. Additionally, the signal sequence
may be chemically synthesized using methods laiown in the art.
[0590] Any mode of administration of any of the above-described
polynucleotides constructs can be used so long as the mode results in the
expression of one or more molecules in an amount sufficient to provide a
therapeutic effect. This includes direct needle injection, systemic
98
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
injection, catheter infusion, biolrsirc iiijec ors, particle accelerators
(i.e., "gene guns"), gelfoam sponge depots, otlier commercially available
depot
materials, osmotic pumps (e.g., Alza minipumps), oral or suppositorial solid
(tablet or pill) pharmaceutical formulations, and decanting or topical
applications during surgery. For example, direct injection of naked calcium
phosphate-precipitated plasmid into rat liver and rat spleen or a
protein-coated plasmid into the portal vein has resulted in gene expression of
the foreign gene in tiie rat livers (Kaneda et al., Science 243:375
(1989)).
[0591] A preferred method of local administration is by direct injection.
Preferably, an albumin fusion protein of the present invention
complexed with a delivery vehicle is administered by direct injection into or
locally within the area of arteries. Administration of a composition
locally within the area of arteries refers to injecting the composition
centimeters and preferably, millimeters within arteries.
[0592] Another method of local administration is to contact a polynucleotide
constnict of the present invention in or around a surgical wound.
For example, a patient can undergo surgery and the polynucleotide construct
can be coated on the surface of tissue inside the wound or the construct
can be injected into areas of tissue inside the wound.
[0593] Therapeutic compositions useful in systemic administration, include
fusion proteins of the present invention complexed to a targeted
delivery vehicle of the present invention. Suitable delivery vehicles for use
with systemic administration comprise liposomes coniprising ligands for
targeting the vehicle to a particular site. In specific embodiments, suitable
delivery vehicles for use with systemic administration comprise liposomes
comprising albumin fusion proteins of the invention for targeting the vehicle
to a particular site.
[0594] Preferred methods of systemic administration, include intravenous
injection, aerosol, oral and percutaneous (topical) delivery.
Intravenous injections can be performed using methods standard in the art.
Aerosol delivery can also be performed using methods standard in the art
(see, for example, Stribling et al., Proc. Nat1. Acad. Sci. USA 189:11277-
11281, 1992, which is incorporated herein by reference). Oral delivery can
be performed by complexing a polynucleotide construct of the present invention
to a carrier capable of withstanding degradation by digestive
enzymes in the gut of an animal. Examples of such carriers, include plastic
capsules or tablets, such as those known in the art. Topical delivery can
be performed by mixing a polynucleotide construct of the present invention
with a lipophilic reagent (e.g., DMSO) that is capable of passing into the
skin.
[0595] Determining an effective amount of substance to be delivered can depend
upon a number of factors including, for example, the chemical
structure and biological activity of the substance, the age and weight of the
animal, the precise condition requiring treatment and its severity, and the
route of administration. The frequency of treatments depends upon a number of
factors, such as the amount of polynucleotide constructs
administered per dose, as well as the health and history of the subject. The
precise amount, number of doses, and timing of doses will be
determined by the attending physician or veterinarian.
[0596] Albumin fusion proteins of the present invention can be administered to
any animal, preferably to mammals and birds. Preferred
mammals include humans, dogs, cats,-mice, rats, rabbits sheep, cattle, horses
and pigs, with humans beingparticularly preferred.
BioloQical AcBvities
[0597] Albumin fusion proteins and/or polynucleotides encoding albumin fusion
proteins of the present invention, can be used in assays to test
for one or more biological activities. If an albumin fusion protein and/or
polynucleotide exhibits an activity in a particular assay, it is likely that
the
Therapeutic protein corresponding to the fusion portein may be involved in the
diseases associated with the biological activity. Thus, the fusion
protein could be used to treat the associated disease.
[0598] In preferred embodiments, the present invention encompasses a method of
treating a disease or disorder listed in the "Preferred
Indication Y" column of Table 1 comprising administering to a patient in which
such treatment, prevention or amelioration is desired an albumin
fusion protein of the invention that comprises a Therapeutic protein portion
corresponding to a Therapeutic protein disclosed in the "Therapeutic
Protein X" column of Table 1(in the same row as the disease or disorder to be
treated is listed in the "Preferred Indication Y" column of Table 1) in
an amount effective to treat, prevent or ameliorate the disease or disorder.
[0599] In a further preferred embodiment, the present invention encompasses a
method of treating a disease or disorder listed for a particular
Therapeutic protein in the "Preferred Indication:Y" column of Table 1
comprising administering to a patient in which such treatment, prevention or
amelioration is desired an albumin fusion protein of the invention that
comprises a Therapeutic protein portion corresponding to the Therapeutic
protein for which the indications ni the Examples are related in an amount
effective to treat, prevent or ameliorate the disease or disorder.
[0600] Specifically contemplated by the present invention are albumin fusion
proteins produced by a cell when encoded by the polynucleotides
that encode SEQ ID NO:Y. When these polynucleotides are used to express the
encoded protein from a cell, the cell's natural secretion and
processing steps produces a protein that lacks the signal sequence explicitly
listed in columns 4 and/or 11 of Table 2. The specific amino acid
sequence of the listed signal sequence is shown in the specification or is
well known in the art. Thus, most preferred embodiments of the present
invention include the albumin fusion protein produced by a cell (which would
lack the leader sequence shown in columns 4 and/or 11 of Table 2).
Also most preferred are polypeptides comprising SEQ ID NO:Y without the
specific leader sequence listed in columns 4 and/or 11 of Table 2.
Compositions comprising these two preferred embodiments, including
pharmaceutical compositions, are also preferred. These albumin fusion
proteins are specifically contemplated to treat, prevent, or ameliorate a
disease or disorder listed for a particular Therapeutic protein in the
"Preferred
99
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
..., u ,. ,.,,, ..1111.o,.n '1 ,.,1"r 11.1., :aLt
Indication:Y" column of Table 1.
[0601] In preferred embodiments, fusion proteins of the present invention may
be used in the diagnosis, prognosis, prevention and/or treatment
of diseases and/or disorders relating to diseases and disorders of the
endocrine system (see, for example, "Endocrine Disorders" section below), the
nervous system (see, for example, "Neurological Disorders" section below), the
immune system (see, for example, "Immune Activity" section
below), respiratory system (see, for example, "Respiratory Disorders" section
below), cardiovascular system (see, for example, "Cardiovascular
Disorders" section below), reproductive system (see, for example,
"Reproductive System Disorders" section below) digestive system (see, for
example, "Gastrointestinal Disorders" section below), diseases and/or
disorders relating to cell proliferation (see, for example,
"Hyperproliferative
Disorders" section below), and/or diseases or disorders relating to the blood
(see, for example, "Blood-Related Disorders" section below).
[0602] In certain embodiments, an albumin fusion protein of the present
invention may be used to diagnose and/or prognose diseases and/or
disorders associated with the tissue(s) in which the gene corresponding to the
Therapeutic protein portion of the fusion protein of the invention is
expressed.
[0603] Thus, fusion proteins of the invention and polynucleotides encoding
albumin fusion proteins of the invention are useful in the diagnosis,
detection and/or treatment of diseases and/or disorders associated with
activities that include, but are not limited to, prohormone activation,
neurotransmitter activity, cellular signaling, cellular proliferation,
cellular differentiation, and cell migration.
[0604] More generally, fusion proteins of the invention and polynucleotides
encoding albumin fusion proteins of the invention may be useful for
the diagnosis, prognosis, prevention and/or treatment of diseases and/or
disorders associated with the following systems.
Inemrute Activity
[0605] Albumin fusion proteins of the invention and polynucleotides encoding
albumin fusion proteins of the invention may be useful in
treating, preventing, diagnosing and/or prognosing diseases, disorders, and/or
conditions of the immune system, by, for example, activating or
inhibiting the proliferation, differentiation, or mobilization (chemotaxis) of
immune cells. hnmune cells develop through a process called
hematopoiesis, producing myeloid (platelets, red blood cells, neutrophils, and
macrophages) and lymphoid (B and T lymphocytes) cells from
pluripotent stem cells. The etiology of these immune diseases, disorders,
and/or conditions may be genetic, somatic, such as cancer and some
autoimmune diseases, acquired (e.g., by chemotherapy or toxins), or
infectious. Moreover, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention can be used as a marker or
detector of a particular immune system disease or disorder.
[0606] In another embodiment, a fusion protein of the invention and/or
polynucleotide encoding an albumin fusion protein of the invention, may
be used to treat diseases and disorders of the immune system and/or to inhibit
or enhance an immune response generated by cells associated with the
tissue(s) in which the polypeptide of the invention is expressed.
[0607] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
-treating, preventing, diagnosing,and/or prognosing immunodeficiencies,
including both congenital and acquired-immunodeficiencies. -Examples of---
B cell immunodeficiencies in which immunoglobulin levels B cell function
and/or B cell numbers are decreased include: X-linked
agammaglobulinemia (Bruton's disease), X-linked infantile agammaglobulinemia,
X-linked immunodeficiency with hyper IgM, non X-linked
immunodeficiency with hyper IgM, X-linked lymphoproliferative syndrome (XLP),
agammaglobulinemia including congenital and acquired
agammaglobulinemia, adult onset agammaglobulinemia, late-onset
agammaglobulinemia, dysgammaglobulinemia, hypogammaglobulinemia,
unspecified hypogammaglobulinemia, recessive agammaglobulinemia (Swiss type),
Selective IgM deficiency, selective IgA deficiency, selective
IgG subclass deficiencies, IgG subclass deficiency (with or without IgA
deficiency), Ig deficiency with increased IgM, IgG and IgA deficiency with
increased IgM, antibody deficiency with normal or elevated Igs, Ig heavy chain
deletions, kappa chain deficiency, B cell lymphoproliferative
disorder (BLPD), common variable immunodeficiency (CVID), common variable
immunodeficiency (CVI) (acquired), and transient
hypogammaglobulinemia of infancy.
[0608] In specific embodiments, ataxia-telangiectasia or conditions associated
with ataxia-telangiectasia are treated, prevented, diagnosed,
and/or prognosing using the, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention.
[0609] Examples of congenital immunodeficiencies in which T cell and/or B cell
function and/or number is decreased include, but are not
limited to: DiGeorge anomaly, severe combined immunodeficiencies (SCID)
(including, but not limited to, X-linked SCID, autosomal recessive
SCID, adenosine deaminase deficiency, purine nucleoside phosphorylase (PNP)
deficiency, Class II MHC deficiency (Bare 13nnphocyte syndrome),
Wiskott-Aldrich syndrome, and ataxia telangiectasia), thymic hypoplasia, third
and fourth pharyngeal pouch syndrome, 22q11.2 deletion, chronic
mucocutaneous candidiasis, natural killer cell deficiency (NK), idiopathic
CD4+ T-lymphocytopenia, immunodeficiency with predominant T cell
defect (unspecified), and unspecified immunodeficiency of cell mediated
immunity.
[0610] In specific embodiments, DiGeorge anomaly or conditions associated with
DiGeorge anomaly are treated, prevented, diagnosed, and/or
prognosed using fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention.
[0611] Other immunodeficiencies that may be treated, prevented, diagnosed,
and/or prognosed using fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, include,
but are not limited to, chronic granulomatous disease, Chediak-Higashi
syndrome, myeloperoxidase deficiency, leukocyte glucose-6-phosphate
dehydrogenase deficiency, X-linked lymphoproliferative syndrome (XLP),
100
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
n it,.,. It r' I6.,11,.n 1111 H; ;; ":,;It ~..
leukocyte adhesion deficiency, compleinen component deficiencies (including
Cl, C2, C3, C4, C5, C6, C7, C8 and/or C9 deficiencies), reticular
dysgenesis, thymic alymphoplasia-aplasia, immunodeficiency with thymoma,
severe congenital leukopenia, dysplasia with immunodeficiency,
neonatal neutropenia, short limbed dwarfism, and Nezelof syndrome-combined
immunodeficiency with Igs.
[0612] In a preferred embodiment, the immunodeficiencies and/or conditions
associated with the immunodeficiencies recited above are treated,
prevented, diagnosed and/or prognosed using fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention.
[0613] In a preferred embodiment fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
could be used as an agent to boost immunoresponsiveness among immunodeficient
individuals. In specific embodiments, fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention could be used as an agent to boost immunoresponsiveness
among B cell and/or T cell immunodeficient individuals.
[0614] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
treating, preventing, diagnosing and/or prognosing autoimmune disorders. Many
autoimmune disorders result from inappropriate recognition of self
as foreign material by immune cells. This inappropriate recognition results in
an immune response leading to the destruction of the host tissue.
Therefore, the administration of fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention that can
inhibit an immune response, particularly the proliferation, differentiation,
or chemotaxis of T-cells, may be an effective therapy in preventing
autoimmune disorders.
[06151 Autoimmune diseases or disorders that may be treated, prevented,
diagnosed and/or prognosed by fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention include, but
are not limited to, one or more of the following: systemic lupus
erythematosus, rheumatoid arthritis, ankylosing spondylitis, multiple
sclerosis, autoimmune thyroiditis, Hashimoto's thyroiditis, autoimmune
hemolytic anemia, hemolytic anemia, thrombocytopenia, autoimmune
thrombocytopenia purpura, autoimmune neonatal thrombocytopenia,
idiopathic thrombocytopenia purpura, purpura (e.g., Henloch-Scoenlein
purpura), autoimmunocytopenia, Goodpasture's syndrome, Pemphigus
vulgaris, myasthenia gravis, Grave's disease (hyperthyroidism), and insulin-
resistant diabetes mellitus.
[0616] Additional disorders that are likely to have an autoimmune component
that may be treated, prevented, and/or diagnosed with the albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention include, but are not limited to, type II
collagen-induced arthritis, antiphospholipid syndrome, dermatitis, allergic
encephalomyelitis, myocarditis, relapsing polychondritis, rheumatic heart
disease, neuritis, uveitis ophthalmia, polyendocrinopathies, Reiter's Disease,
Stiff-Man Syndrome, autoimmune pulmonary inflammation, autism,
Guillain-Barre Syndrome, insulin dependent diabetes mellitus, and autoimmune
inflammatory eye disorders.
[0617] Additional disorders that are likely to have an autoimmune component
that may be treated, prevented, diagnosed and/or prognosed with
the albumin-fusion-proteins of the invention and/or polynucleotides encoding
albumin fusion proteins -of the invention include, but are not limited to;
scleroderma with anti-collagen antibodies (often characterized, e.g., by
nucleolar and other nuclear antibodies), mixed connective tissue disease
(often characterized, e.g., by antibodies to extractable nuclear antigens
(e.g., ribonucleoprotein)), polymyositis (often characterized, e.g., by
nonhistone ANA), pernicious anemia (often characterized, e.g., by antiparietal
cell, microsomes, and intrinsic factor antibodies), idiopathic
Addison's disease (often characterized, e.g., by humoral and cell-mediated
adrenal cytotoxicity, infertility (often characterized, e.g., by
antispermatozoal antibodies), glomerulonephritis (often characterized, e.g.,
by glomerular basement membrane antibodies or immune complexes),
bullous pemphigoid (often characterized, e.g., by IgG and complement in
basement membrane), Sjogren's syndrome (often characterized, e.g., by
multiple tissue antibodies, and/or a specific nonhistone ANA (SS-B)), diabetes
mellitus (often characterized, e.g., by cell-mediated and humoral islet
cell antibodies), and adrenergic drug resistance (including adrenergic drug
resistance with asthma or cystic fibrosis) (often characterized, e.g., by
beta-adrenergic receptor antibodies).
[0618] Additional disorders that may have an autoimmune component that may be
treated, prevented, diagnosed and/or prognosed with the
albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include, but are not limited to,
chronic active hepatitis (often characterized, e.g., by smooth muscle
antibodies), primary biliary cirrhosis (often characterized, e.g., by
mitochondria
antibodies), other endocrine gland failure (often characterized, e.g., by
specific tissue antibodies in some cases), vitiligo (often characterized,
e.g., by
melanocyte antibodies), vasculitis (often characterized, e.g., by Ig and
complement in vessel walls and/or low serum complement), post-MI (often
characterized, e.g., by myocardial antibodies), cardiotomy syndrome (often
characterized, e.g., by myocardial antibodies), urticaria (often
characterized, e.g., by IgG and IgM antibodies to IgE), atopic dermatitis
(often charaeterized, e.g., by IgG and IgM antibodies to IgE), asthma (often
characterized, e.g., by IgG and IgM antibodies to IgE), and many other
inflammatory, granulomatous, degenerative, and atrophic disorders.
[0619] In a preferred embodiment, the autoimmune diseases and disorders and/or
conditions associated with the diseases and disorders recited
above are treated, prevented, diagnosed and/or prognosed using for example,
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention. In a specific preferred embodiment,
rheumatoid arthritis is treated, prevented, and/or diagnosed using
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention.
[0620] hi another specific preferred embodiment, systemic lupus erythematosus
is treated, prevented, and/or diagnosed using fusion proteins of
101
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
s.... ~~, . hi ,. ~.,.~~ ~Mu 1i.,.u~r n ..1, ,1...n ~'b ,~l ~
e invention an yor polyriucfeofi' es"LnC tling albumin fusion proteins of the
invention. In another specific preferred embodiment, idiopathic
tlirombocytopenia purpura is treated, prevented, and/or diagnosed using fusion
proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention.
[0621] In another specific preferred embodiment IgA nephropatliy is treated,
prevented, and/or diagnosed using fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the invention.
[0622] In a preferred embodiment, the autoimmune diseases and disorders and/or
conditions associated with the diseases and disorders recited
above are treated, prevented, diagnosed and/or prognosed using fusion proteins
of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention.
[0623] In preferred embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention are
used as a immunosuppressive agent(s).
[06241 Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
treating, preventing, prognosing, and/or diagnosing diseases, disorders,
and/or conditions of hematopoietic cells. Albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention could be used to increase differentiation and proliferation of
hematopoietic cells, including the pluripotent stem cells, in an effort to
treat or prevent those diseases, disorders, and/or conditions associated with
a
decrease in certain (or many) types hematopoietic cells, including but not
limited to, leukopenia, neutropenia, anemia, and thrombocytopenia.
Alternatively, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention could be used to increase
differentiation and proliferation of hematopoietic cells, including the
pluripotent stem cells, in an effort to treat or prevent those diseases,
disorders,
and/or conditions associated with an increase in certain (or many) types of
hematopoietic cells, including but not limited to, histiocytosis.
[0625] Allergic reactions and conditions, such as asthma (particularly
allergic asthma) or other respiratory problems, may also be treated,
prevented, diagnosed and/or prognosed using fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention. Moreover, these molecules can be used to treat, prevent, prognose,
and/or diagnose anaphylaxis, hypersensitivity to an antigenic
molecule, or blood group incompatibility.
[0626] Additionally, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be used to
treat, prevent, diagnose and/or prognose IgE-mediated allergic reactions. Such
allergic reactions include, but are not limited to, asthma, rhinitis, and
eczema. In specific embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
used to modulate IgE concentrations in vitro or in vivo.
[0627] Moreover, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention have uses in the
diagnosis, prognosis, prevention, and/or treatment of inflammatory conditions.
For example, since fusion proteins of the invention and/or
polynucleotides encoding-albumin-fusion proteins-ofthe invention-may inhibit
the activation, proliferation-and/or differentiation of cells involved- in -
an inflammatory response, these molecules can be used to prevent and/or treat
chronic and acute inflammatory conditions. Such inflammatory
conditions include, but are not limited to, for example, inflammation
associated with infection (e.g., septic shock, sepsis, or systemic
inflammatory
response syndrome), ischemia-reperfusion injury, endotoxin lethality,
complement-mediated hyperacute rejection, nephritis, cytokine or chemokine
induced lung injury, inflammatory bowel disease, Crohn's disease, over
production of cytokines (e.g., TNF or IL-1.), respiratory disorders (e.g.,
asthma and allergy); gastrointestinal disorders (e.g., inflammatory bowel
disease); cancers (e.g., gastric, ovarian, lung, bladder, liver, and breast);
CNS disorders (e.g., multiple sclerosis; ischemic brain injury and/or stroke,
traumatic brain injury, neurodegenerative disorders (e.g., Parkinson's
disease and Alzheimer's disease); AIDS-related dementia; and prion disease);
cardiovascular disorders (e.g., atherosclerosis, myocarditis,
cardiovascular disease, and cardiopulmonary bypass complications); as well as
many additional diseases, conditions, and disorders that are
characterized by inflanunation (e.g., hepatitis, rheumatoid arthritis, gout,
trauma, pancreatitis, sarcoidosis, dermatitis, renal ischemia-reperfusion
injury, Grave's disease, systemic lupus erythematosus, diabetes mellitus, and
allogenic transplant rejection).
[0628] Because inflammation is a fundamental defense mechanism, inflammatory
disorders can effect virtually any tissue of the body.
Accordingly, fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, have uses in the treatment of
tissue-specific inflammatory disorders, including, but not limited to,
adrenalitis, alveolitis, angiocholecystitis, appendicitis, balanitis,
blepharitis,
bronchitis, bursitis, carditis, cellulitis, cervicitis, cholecystitis,
chorditis, cochlitis, colitis, conjunctivitis, cystitis, dermatitis,
diverticulitis,
encephalitis, endocarditis, esophagitis, eustachitis, fibrositis,
folliculitis, gastritis, gastroenteritis, gingivitis, glossitis,
hepatosplenitis, keratitis,
labyrinthitis, laryngitis, lymphangitis, mastitis, media otitis, meningitis,
metritis, mucitis, myocarditis, myosititis, myringitis, nephritis, neuritis,
orchitis, osteochondritis, otitis, pericarditis, peritendonitis, peritonitis,
pharyngitis, phlebitis, poliomyelitis, prostatitis, pulpitis, retinitis,
rhinitis,
salpingitis, scleritis, sclerochoroiditis, scrotitis, sinusitis, spondylitis,
steatitis, stomatitis, synovitis, syringitis, tendonitis, tonsillitis,
urethritis, and
vaginitis.
[0629] In specific embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, are
useful to diagnose, prognose, prevent, and/or treat organ transplant
rejections and graft-versus-host disease. Organ rejection occurs by host
immune
cell destruction of the transplanted tissue through an immune response.
Similarly, an immune response is also involved in GVHD, but, in this case,
102
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
11 ,..if ..Mii ~r:;olt,lE,
the foreign transplanted immune cells desiroy the host tissues. Polypeptides,
antibodies, or polynucleotides of the invention, and/or agonists or
antagonists thereof, that inhibit an immune response, particularly the
activation, proliferation, differentiation, or chemotaxis of T-cells, may be
an
effective therapy in preventing organ rejection or GVHD. In specific
embodiments, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, that inhibit an immune
response, particularly the activation, proliferation, differentiation, or
chemotaxis of T-cells, may be an effective tlierapy in preventing experimental
allergic and hyperacute xenograft rejection.
[0630] In other embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, are
useful to diagnose, prognose, prevent, and/or treat immune complex diseases,
including, but not limited to, serum sickness, post streptococcal
glomerulonephritis, polyarteritis nodosa, and immune complex-induced
vasculitis.
[0631] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention can be used to treat,
detect, and/or prevent infectious agents. For example, by increasing the
immune response, particularly increasing the proliferation activation and/or
differentiation of B and/or T cells, infectious diseases may be treated,
detected, and/or prevented. The immune response may be increased by either
enhancing an existing immune response, or by initiating a new immune response.
Alternatively, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may also
directly inhibit the infectious agent (refer to section of application listing
infectious agents, etc), without necessarily eliciting an immune response.
[0632] In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention are used as a vaccine adjuvant that enhances immune responsiveness
to an antigen. In a specific embodiment, albumin fusion proteins of
the invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as an adjuvant to enhance tumor-specific immune
responses.
[0633] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an adjuvant to enhance anti-viral immune responses.
Anti-viral immune responses that may be enhanced using the
compositions of the invention as an adjuvant, include virus and virus
associated diseases or symptoms described herein or otherwise known in the
art. In specific embodiments, the compositions of the invention are used as an
adjuvant to enhance an immune response to a virus, disease, or
symptom selected from the group consisting of: AIDS, meningitis, Dengue, EBV,
and hepatitis (e.g., hepatitis B). In another specific embodiment,
the compositions of the invention are used as an adjuvant to enhance an immune
response to a virus, disease, or symptom selected from the group
consisting of: HIV/AIDS, respiratory syncytial virus, Dengue, rotavirus,
Japanese B encephalitis, influenza A and B, parainfluenza, measles,
cytomegalovirus, rabies, Junin, Chikungunya, Rift Valley Fever, herpes
simplex, and yellow fever.
[0634] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an adjuvant to enhance anti-bacterial or anti-fungal
immune responses. Anti-bacterial or anti-fungal immune responses that
may be enhanced usingthe compositions of the invention as an adjuvant, include-
bacteria or fungus and bacteria or fungus associated diseases or -
symptoms described herein or otherwise known in the art. In specific
embodiments, the compositions of the invention are used as an adjuvant to
enhance an immune response to a bacteria or fungus, disease, or symptom
selected from the group consisting of: tetanus, Diphtheria, botulism, and
meningitis type B.
[0635] In another specific embodiment, the compositions of the invention are
used as an adjuvant to enhance an immune response to a bacteria
or fungus, disease, or symptom selected from the group consisting of: Vibrio
cholerae, Mycobacterium leprae, Salmonella typhi, Sabnonella
paratyphi, Meisseria meningitidis, Streptococcus pne:unoniae, Group B
streptococcus, Shigella spp., Enterotoxigenic Escherichia coli,
Enterohemorrhagic E. coli, and Borrelia burgdorferi.
[0636] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an adjuvant to enhance anti-parasitic immune
responses. Anti-parasitic immune responses that may be enhanced using the
compositions of the invention as an adjuvant, include parasite and parasite
associated diseases or symptoms described herein or otherwise known in
the art. In specific embodiments, the compositions of the invention are used
as an adjuvant to enhance an immune response to a parasite. In another
specific embodiment, the compositions of the invention are used as an adjuvant
to enhance an immune response to Plasmodium (malaria) or
Leishmania.
[0637] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may also be employed to treat infectious diseases including
silicosis, sarcoidosis, and idiopathic puhnonary fibrosis; for example, by
preventing the recruitment and activation of mononuclear phagocytes.
[0638] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an antigen for the generation of antibodies to
inhibit or enhance immune mediated responses against polypeptides of the
invention.
[0639] In one embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
are administered to an animal (e.g., mouse, rat, rabbit, hamster, guinea pig,
pigs, micro-pig, chicken, camel, goat, horse, cow, sheep, dog, cat, non-
human primate, and human, most preferably human) to boost the immune system to
produce increased quantities of one or more antibodies (e.g.,
103
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
19b, .... ..... ...... ~..,.. .,,,. ,.,..~, ..~, ~,~~,.
IgA IgM, and IgE), to induce higher affinity antibody production and
immunoglobulin class switching (e.g., IgG, IgA, IgM, and IgE), and/or to
increase an immune response.
[0640] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as a stimulator of B cell responsiveness to pathogens.
[0641] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an activator of T cells.
[0642] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an agent that elevates the immune status of an
individual prior to their receipt of immunosuppressive therapies.
[0643] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an agent to induce higlier affinity antibodies.
[0644] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an agent to increase serum immunoglobulin
concentrations.
[0645] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an agent to accelerate recovery of immunocompromised
individuals.
[0646] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an agent to boost immunoresponsiveness among aged
populations and/or neonates.
[0647] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as an immune system enhancer prior to, during, or after
bone marrow transplant and/or other transplants (e.g., allogeneic or
xenogeneic organ transplantation). With respect to transplantation,
compositions of the invention may be administered prior to, concomitant with,
and/or after transplantation. In a specific embodiment, compositions of the
invention are administered after transplantation, prior to the beginning of
recovery of T-cell populations. In another specific embodiment, compositions
of the invention are first administered after transplantation after the
beginning of recovery of T cell populations, but prior to full recovery of B
cell populations.
[0648] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the inveution are used as an agent to boost immunoresponsiveness among
individuals having an acquired loss of B cell function. Conditions
resulting in an acquired loss of B cell function that may be ameliorated or
treated by administering the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the invention,
include, but are not limited to, HN Infection, AIDS, bone marrow
transplant, and B cell chronic lymphocytic leukemia (CLL).
[0649] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used-as an agent-to boost immunoresponsiveness- among
individuals-having-a temporary -immune deficiency. Coriditions resulting-in a
temporary immune deficiency that may be ameliorated or treated by
administering the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, include,
but are not limited to, recovery from viral infections (e.g., influenza),
conditions associated with malnutrition, recovery from infectious
mononucleosis, or conditions associated with stress, recovery from measles,
recovery from blood transfusion, and recovery from surgery.
[0650] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a regulator of antigen presentation by monocytes,
dendritic cells, and/or B-cells. In one embodiment, albumin fusion proteins
of the invention and/or polynucleotides encoding'albumin fusion proteins of
the invention enhance antigen presentation or antagonize antigen
presentation in vitro or in vivo. Moreover, in related embodiments, this
enhancement or antagonism of antigen presentation may be useful as an
anti-tumor treatment or to modulate the immune system.
[0651] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as an agent to direct an individual's immune system towards
development of a humoral response.(i.e. TH2) as opposed to a THI
cellular response.
[0652] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a means to induce tumor proliferation and thus make it
more susceptible to anti-neoplastic agents. For example, multiple
myeloma is a slowly dividing disease and is thus refractory to virtually all
anti-neoplastic regimens. If these cells were forced to proliferate more
rapidly their susceptibility profile would likely change.
[0653] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a stimulator of B cell production in pathologies such as
AIDS, chronic lymphocyte disorder and/or Common Variable
Immunodificiency.
[0654] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a therapy for generation and/or regeneration of lymphoid
tissues following surgery, trauma or genetic defect. In another
specific embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention are used in
104
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
the pretreatment of bone marrow samples prior to transplant.
[0655] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a gene-based therapy for genetically inherited disorders
resulting in immuno-incompetence/immunodeficiency such as
observed among SCID patients.
[0656] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a means of activating monocytes/macrophages to defend
against parasitic diseases that effect monocytes such as Leishmania.
[0657] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a means of regulating secreted cytokines that are
elicited by polypeptides of the invention.
[06581 In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention are used in one or more of the applications decribed herein, as they
may apply to veterinary medicine.
[0659] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a means of blocking various aspects of immune responses
to foreign agents or self. Examples of diseases or conditions in
which blocking of certain aspects of innnune responses may be desired include
autoimmune disorders such as lupus, and artliritis, as well as
immunoresponsiveness to skin allergies, inflammation, bowel disease, injury
and diseases/disorders associated with pathogens.
[0660] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a therapy for preventing the B cell proliferation and Ig
secretion associated with autoimmune diseases such as idiopathic
thrombocytopenic purpura, systemic lupus erythematosus and multiple sclerosis.
[0661] In another specific embodiment, polypeptides, antibodies,
polynucleotides and/or agonists or antagonists of the present fusion proteins
of
the invention and/or polynucleotides encoding albumin fusion proteins of the
invention invention are used as a inhibitor of B and/or T cell migration
in endothelial cells. This activity disrupts tissue architecture or cognate
responses and is useful, for example in disrupting immune responses, and
blocking sepsis.
[0662] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used as a therapy for chronic hypergammaglobulinemia evident in
such diseases as monoclonal gammopathy of undetermined
significance (MGUS), Waldenstrom's disease, related idiopathic monoclonal
gammopathies, and plasmacytomas.
[0663] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention may be employed for instance to inhibit polypeptide chemotaxis and
activation of macrophages and their precursors, and of neutrophils,
basophils, B lymphocytes and some T-cell subsets, e.g., activated and CD8
cytotoxic T cells and natural killer cells, in certain autoimmune and
chronic inflammatory and infective diseases. Examples of autoimmune diseases
are described herein and include multiple sclerosis, and
msulin-dependent diabetes. - - - -
[0664] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may also be
employed to treat idiopathic hyper-eosinophilic syndrome by, for example,
preventing eosinophil production and migration.
[0665] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used to enhance or inhibit complement mediated cell lysis.
[0666] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used to enhance or inhibit antibody dependent cellular
cytotoxicity.
[0667] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may also be employed for treating atherosclerosis, for example,
by preventing monocyte infiltration in the artery wall.
[0668] hi another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be employed to treat adult respiratory distress syndrome
(ARDS).
[0669] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be useful for stimulating wound and tissue repair,
stimulating angiogenesis, and/or stimulating the repair of vascular or
lymphatic
diseases or disorders. Additionally, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may
be used to stimulate the regeneration of mucosal surfaces.
[0670] In a specific embodiment, albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention are used to diagnose, prognose, treat, and/or prevent a disorder
characterized by primary or acquired immunodeficiency, deficient serum
immunoglobulin production, recurrent infections, and/or immune system
dysfunction. Moreover, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be used
to treat or prevent infections of the joints, bones, skin, and/or parotid
glands, blood-borne infections (e.g., sepsis, meningitis, septic arthritis,
and/or osteomyelitis), autoimmune diseases (e.g., those disclosed herein),
inflammatory disorders, and malignancies, and/or any disease or disorder or
condition associated with these infections, diseases, disorders and/or
malignancies) including, but not limited to, CVID, other primary immune
deficiencies, HIV disease, CLL, recurrent bronchitis, sinusitis, otitis
media, conjunctivitis, pneumonia, hepatitis, meningitis, herpes zoster (e.g.,
severe herpes zoster), and/or pneumocystis carnii. Other diseases and
105
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
o-(! " IIJI,::::11 Ih..IF If;U t' K':õ ":;'U ::~4 ~..
disorders that may be prevented, diagriose , prognosed, and/or treated with
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention include, but are not limited to, HIV
infection, HTLV-BLV infection, lymphopenia, phagocyte bactericidal
dysfunction anemia, thrombocytopenia, and hemoglobinuria.
[06711 In anotlier embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention are used to treat, and/or diagnose an individual having common
variable immunodeficiency disease ("CVID"; also known as "acquired
agammaglobulinemia" and "acquired hypogammaglobulinemia") or a subset of this
disease.
[0672] hi a specific embodiment, albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be used to diagnose, prognose, prevent, and/or treat cancers or
neoplasms including immune cell or immune tissue-related cancers or
neoplasms. Examples of cancers or neoplasms that may be prevented, diagnosed,
or treated by fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention include, but
are not limited to, acute myelogenous leukemia, chronic
myelogenous leukemia, Hodgkin's disease, non-Hodgkin's lymphoma, acute
lymphocytic anemia (ALL) Chronic lymphocyte Ieukemia,
plasmacytomas, multiple myeloma, Burkitt's lymphoma, EBV-transformed diseases,
and/or diseases and disorders described in the section entitled
"Hyperproliferative Disorders" elsewhere herein.
[0673] In another specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as a therapy for decreasing cellular proliferation of
Large B-cell Lymphomas.
[0674] In anotlier specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used as a means of decreasing the involvement of B cells and
Ig associated with Chronic Myelogenous Leukemia.
[06751 In specific embodiments, the compositions of the invention are used as
an agent to boost immunoresponsiveness among B cell
immunodeficient individuals, such as, for example, an individual who has
undergone a partial or complete splenectomy.
Blood-Related Disorders
[0676] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be used to
modulate hemostatic (the stopping of bleeding) or thrombolytic (clot
dissolving) activity. For example, by increasing hemostatic or thrombolytic
activity, fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention could be used to treat or prevent
blood coagulation diseases, disorders, and/or conditions (e.g.,
afibrinogenemia, factor deficiencies, hemophilia), blood platelet diseases,
disorders,
and/or conditions (e.g., thrombocytopenia), or wounds resulting from trauma,
surgery, or other causes. Alternatively, fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention that can decrease hemostatic or thrombolytic activity could be
used to inhibit or dissolve clotting. These molecules could be important in
the treatment or prevention of heart attacks (infarction), strokes, or
scarring.
[0677] In--specific- embodiments,- the albumin-fusion--proteins of the
invention and/or polynucleotides encoding albumin fusion proteins-of the
invention may be used to prevent, diagnose, prognose, and/or treat thrombosis,
arterial thrombosis, venous thrombosis, thromboembolism,
pulmonary embolism, atherosclerosis, myocardial infarction, transient ischemic
attack, unstable angina. In specific embodiments, the albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention may be used for the prevention of
occulsion of saphenous grafts, for reducing the risk of periprocedural
thrombosis as might accompany angioplasty procedures, for reducing the risk
of stroke in patients with atrial fibrillation including nonrheumatic atrial
fibrillation, for reducing the risk of embolism associated with mechanical
heart valves and or mitral valves disease. Other uses for the albumin fusion
proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention, include, but are not limited to, the
prevention of occlusions in extrcorporeal devices (e.g., intravascular
canulas,
vascular access shunts in hemodialysis patients, liemodialysis machines, and
cardiopulmonary bypass machines).
[0678] In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention, may be used to prevent, diagnose, prognose, and/or treat diseases
and disorders of the blood and/or blood forming organs associated with
the tissue(s) in which the polypeptide of the invention is expressed.
[0679] The fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may be used to modulate
hematopoietic activity (the formation of blood cells). For example, the
albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may be used to increase the quantity
of all or subsets of blood cells, such as, for example, erytlirocytes,
lymphocytes (B or T cells), myeloid cells (e.g., basophils, eosinophils,
neutrophils, mast cells, macrophages) and platelets. The ability to decrease
the quantity of blood cells or subsets of blood cells may be useful in the
prevention, detection, diagnosis and/or treatment of anemias and
leukopenias described below. Alternatively, the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be used to decrease the quantity of aIl or subsets of blood
cells, such as, for example, erythrocytes, lymphocytes (B or T cells),
myeloid cells (e.g., basophils, eosinophils, neutrophils, mast cells,
macrophages) and platelets.. The ability to decrease the quantity of blood
cells or
subsets of blood cells may be useful in the prevention, detection, diagnosis
and/or treatment of leukocytoses, such as, for example eosinophilia.
[0680] The fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may be used to prevent,
treat, or diagnose blood dyscrasia.
106
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
[0G81]I..J'-Anemias~are co'nditionsiri'wli,cli the number of red blood cells
or amount of hemoglobin (the protein that carries oxygen) in them is
below normal. Anemia may be caused by excessive bleeding, decreased red blood
cell production, or increased red blood cell destruction
(hemolysis). The albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
useful
in treating, preventing, and/or diagnosing anemias. Anemias that may be
treated prevented or diagnosed by the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention include iron deficiency anemia, hypochromic anemia, microcytic
anemia, chlorosis, hereditary siderob;astic anemia, idiopathic acquired
sideroblastic anemia, red cell aplasia, megaloblastic anemia (e.g., pemicious
anemia, (vitamin B12 deficiency) and folic acid deficiency anemia), aplastic
anemia, hemolytic anemias (e.g., autoimmune helolytic anemia,
microangiopathic hemolytic anemia, and paroxysmal nocturnal hemoglobinuria).
The albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
useful in treating, preventing, and/or diagnosing anemias associated with
diseases including but not limited to, anemias associated with systemic lupus
erythematosus, cancers, lymphomas, chronic renal disease, and
enlarged spleens. The albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
useful in treating, preventing, and/or diagnosing anemias arising from drug
treatments such as anemias associated with methyldopa, dapsone, and/or
sulfadrugs. Additionally, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
useful in
treating, preventing, and/or diagnosing anemias associated with abnormal red
blood cell architecture including but not limited to, hereditary
spherocytosis, hereditary elliptocytosis, glucose-6-phosphate, dehydrogenase
deficiency, and sickle cell anemia.
[0682] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
treating, preventing, and/or diagnosing hemoglobin abnormalities, (e.g., those
associated with sickle cell anemia, hemoglobin C disease, hemoglobin
S-C disease, and hemoglobin E disease). Additionally, the albumin fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention may be useful in diagnosing, prognosing, preventing,
and/or treating thalassemias, including, but not limited to, major and
minor forms of alpha-thalassemia and beta-thalassemia.
[0683] In another embodiment, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or treating
bleeding disorders including, but not limited to, thrombocytopenia
(e.g., idiopathic thrombocytopenic purpura, and thrombotic thrombocytopenic
purpura), Von Willebrand's disease, hereditary platelet disorders
(e.g., storage pool disease such as Chediak-Higashi and Hernnansky-Pudlak
syndromes, thromboxane A2 dysfunction, thromboasthenia, and
Bemard-Soulier syndrome), hemolytic-uremic syndrome, hemophelias such as
hemophelia A or Factor Vll deficiency and Christmas disease or
Factor IX deficiency, Hereditary Hemorhhagic Telangiectsia, also known as
Rendu-Osler-Weber syndrome, allergic purpura (Henoch Schonlein
purpura) and disseminated intravascular coagulation.
[0684] The effect of the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention on
the clotting- time -of blood may be-monitored using any of the clotting tests
lmown in the art including, but not limited to, whole blood partial
thromboplastin time (PTT), the activated partial thromboplastin time (aPTT),
the activated clotting time (ACT), the recalcified activated clotting
time, or the Lee-White Clotting time.
[0685] Several diseases and a variety of drugs can cause platelet dysfunction.
Thus, in a specific embodiment, the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or
treating acquired platelet dysfunction such as platelet dysfunction
accompanying kidney failure, leukemia, multiple myeloma, cirrhosis of the
liver,
and systemic lupus erythematosus as well as platelet dysfunction associated
with drug treatments, including treatment with aspirin, ticlopidine,
nonsteroidal anti-inflammatory drugs (used for arthritis, pain, and sprains),
and penicillin in high doses.
[0686] In another embodiment, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or treating
diseases and disorders characterized by or associated with increased
or decreased numbers of white blood cells. Leukopenia occurs when the number
of white blood cells decreases below normal. Leukopenias
include, but are not limited to, neutropenia and lymphocytopenia. An increase
in the number of white blood cells compared to normal is known as
leukocytosis. The body generates increased numbers of white blood cells during
infection. Thus, leukocytosis may simply be a normal
physiological parameter that reflects infection. Alternatively, leukocytosis
may be an indicator of injury or other disease such as cancer.
Leokocytoses, include but are not limited to, eosinophilia, and accumulations
of macrophages. In specific embodiments, the albumin fusion proteins
of the invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be useful in diagnosing, prognosing, preventing,
and/or treating leukopenia. In other specific embodiments, the albumin fusion
proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention may be useful in diagnosing, prognosing,
preventing, and/or treating leukocytosis.
[0687] Leukopenia may be a generalized decreased in all types of white blood
cells, or may be a specific depletion of particular types of white
blood cells. Thus, in specific embodiments, the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be useful in diagnosing, prognosing, preventing, and/or
treating decreases in neutrophil numbers, known as neutropenia.
Neutropenias that may be diagnosed, prognosed, prevented, and/or treated by
the albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention include, but are not limited
to, infantile genetic agranulocytosis, familial neutropenia, cyclic
107
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
9...1' nõP qõu qJl. õ'- (1:..: ;ilt ,~~IL ill If
neutropenta, neutropenias resulttng from or'as'sociated with dietary
deficiencies (e.g., vitamin B 12 deficiency or folic acid deficiency),
neutropenias
resulting from or associated with drug treatments (e.g., antibiotic regimens
such as penicillin treatment, sulfonamide treatment, anticoagulant
treatment, anticonvulsant drugs, anti-thyroid drugs, and cancer chemotherapy),
and neutropenias resulting from increased neutrophil destruction that
may occur in association with some bacterial or viral infections, allergic
disorders, autoimmune diseases, conditions in which an individual has an
enlarged spleen (e.g., Felty syndrome, malaria and sarcoidosis), and some drug
treatment regimens.
[0688] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
diagnosing, prognosing, preventing, and/or treating lymphocytopenias
(decreased numbers of B and/or T lymphocytes), including, but not limited
to, lymphocytopenias resulting from or associated with stress, drug treatments
(e.g., drug treatment with corticosteroids, cancer chemotherapies,
and/or radiation therapies), AIDS infection and/or other diseases such as, for
example, cancer, rheumatoid arthritis, systemic lupus erythematosus,
chronic infections, some viral infections and/or hereditary disorders (e.g.,
DiGeorge syndrome, Wiskott-Aldrich Syndome, severe combined
immunodeficiency, ataxia telangiectsia).
[0689] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
diagnosing, prognosing, preventing, and/or treating diseases and disorders
associated with macrophage numbers and/or macrophage function
including, but not limited to, Gaucher's disease, Niemann-Pick disease,
Letterer-Siwe disease and Hand-Schuller-Christian disease.
[0690] In another embodiment, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or treating
diseases and disorders associated with eosinophil numbers and/or
eosinophil function including, but not limited to, idiopathic
hypereosinophilic syndrome, eosinophilia-myalgia syndrome, and Hand-Schuller-
Christian disease.
[0691] In yet another embodiment, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or treating
leukemias and lymphomas including, but not limited to, acute
lymphocytic (lymphpblastic) leukemia (ALL), acute myeloid (myelocytic,
myelogenous, myeloblastic, or myelomonocytic) leukemia, chronic
lymphocytic leukemia (e.g., B cell leukemias, T cell leukemias, Sezary
syndrome, and Hairy cell leukenia), chronic myelocytic (myeloid,
myelogenous, or granulocytic) leukemia, Hodgkin's lymphoma, non-hodgkin's
lymphoma, Burkitt's lymphoma, and mycosis fungoides.
[0692] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in diagnosing, prognosing, preventing, and/or treating
diseases and disorders of plasma cells including, but not limited to,
plasma cell dyscrasias, monoclonal gammaopatliies, monoclonal gammopathies of
undetermined significance, multiple myeloma,
macroglobulinemia, Waldenstrom's macroglobulinemia, cryoglobulinemia, and
Raynaud's phenomenon.
[0693] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in treating, preventing, and/or diagnosing
myeloproliferative disorders, including-but not limited to, polycythemia vera;
-
relative polycythemia, secondary polycythemia, myelofibrosis, acute
myelofibrosis, agnogenic myelod metaplasia, thrombocythemia, (including both
primary and seconday thrombocythemia) and chronic myelocytic leukemia.
[0694] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful as a treatment prior to surgery, to increase blood
cell production.
[0695] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful as an agent to enhance the migration, phagocytosis,
superoxide production, antibody dependent cellular cytotoxicity of
neutrophils, eosionophils and macrophages.
[0696] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful as an agent to increase the number of stem cells in
circulation prior to stem cells pheresis. In another specific embodiment,
the albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may be useful as an agent to
increase the number of stem cells in circulation prior to platelet pheresis.
[0697] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful as an agent to increase cytokine production.
[0698] In other embodiments, the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the
invention may be useful in preventing, diagnosing, and/or treating primary
hematopoietic disorders.
H,lperproliferatlve Disorders
[0699] In certain embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention can be
used to treat or detect hyperproliferative disorders, including neoplasms.
Albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may inhibit the proliferation of the
disorder through direct or indirect interactions. Alternatively, fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention may proliferate other cells which can inhibit the
hyperproliferative disorder.
[0700] For example, by increasing an immune response, particularly increasing
antigenic qualities of the hyperproliferative disorder or by
108
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
"i~f~ferentiatin"g~ '" o"rmo 'iliziri'g11
'T
royifera} in"-cells, hyperproliferative disorders can be treated. This immune
response may be increased by either
P ~
enhancing an existing immune response, or by initiating a new immune response.
Alternatively, decreasing an immune response may also be a
method of treating hyperproliferative disorders, such as a chemotherapeutic
agent.
[0701] Examples of hyperproliferative disorders that can be treated or
detected by fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention include, but are not limited
to neoplasms located in the: colon, abdomen, bone, breast, digestive
system, liver, pancreas, peritoneum, endocrine glands (adrenal, parathyroid,
pituitary, testicles, ovary, thymus, thyroid), eye, head and neck, nervous
(central and peripheral), lymphatic system, pelvis, skin, soft tissue, spleen,
thorax, and urogenital tract.
[0702] Similarly, other hyperproliferative disorders can also be treated or
detected by fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention. Examples of such
hyperproliferative disorders include, but are not limited to: Acute Childhood
Lymphoblastic Leukemia, Acute Lymphoblastic Leukemia, Acute Lymphocytic
Leukemia, Acute Myeloid Leukemia, Adrenocortical Carcinoma,
Adult (Primary) Hepatocellular Cancer, Adult (Primary) Liver Cancer, Adult
Acute Lymphocytic Leukemia, Adult Acute Myeloid Leukemia, Adult
Hodgkin's Disease, Adult Hodgkin's Lymphoma, Adult Lymphocytic Leukemia, Adult
Non-Hodgkin's Lymphoma, Adult Primary Liver Cancer,
Adult Soft Tissue Sarcoma, AIDS-Related Lymphoma, AIDS-Related Malignancies,
Anal Cancer, Astrocytoma, Bile Duct Cancer, Bladder Cancer,
Bone Cancer, Brain Stem Glioma, Brain Tumors, Breast Cancer, Cancer of the
Renal Pelvis and Ureter, Central Nervous System (Primary)
Lymphoma, Central Nervous System Lymphoma, Cerebellar Astrocytoma, Cerebral
Astrocytoma, Cervical Cancer, Childhood (Primary)
Hepatocellular Cancer, Childhood (Primary) Liver Cancer, Childhood Acute
Lymphoblastic Leukemia, Childhood Acute Myeloid Leukemia,
Childhood Brain Stem Glioma, Childhood Cerebellar Astrocytoma, Childhood
Cerebral Astrocytoma, Childhood Extracranial Germ Cell Tumors,
Childhood Hodgkin's Disease, Childhood Hodgkin's Lymphoma, Childhood
Hypothalamic and Visual Pathway Glioma, Childhood Lymphoblastic
Leukemia, Childhood Medulloblastoma, Childhood Non-Hodgkin's Lymphoma,
Childhood Pineal and Supratentorial Primitive Neuroectodermal
Tumors, Childhood Primary Liver Cancer, Childhood Rhabdomyosarcoma, Childhood
Soft Tissue Sarcoma, Childhood Visual Pathway and
Hypothalamic Glioma, Chronic Lymphocytic Leukemia, Chronic Myelogenous
Leukemia, Colon Cancer, Cutaneous T-Cell Lymphoma, Endocrine
Pancreas Islet Cell Carcinoma, Endometrial Cancer, Ependymoma, Epithelial
Cancer, Esophageal Cancer, Ewing's Sarcoma and Related Tumors,
Exocrine Pancreatic Cancer, Extracranial Germ Cell Tumor, Extragonadal Germ
Cell Tumor, Extrahepatic Bile Duct Cancer, Eye Cancer, Female
Breast Cancer, Gaucher's Disease, Gallbladder Cancer, Gastric Cancer,
Gastrointestinal Careinoid Tumor, Gastrointestinal Tumors, Germ Cell
Tumors, Gestational Trophoblastic Tumor, Hairy Cell Leukemia, Head and Neck
Cancer, Hepatocellular Cancer, Hodgkin's Disease, Hodgkin's
Lymphoma, Hypergammaglobulinemia, Hypopharyngeal Cancer, Intestinal Cancers,
Intraocular Melanoma, Islet Cell Carcinoma, Islet Cell
Pancreatic Cancer, Kaposi's Sarcoma, Kidney Cancer, Laryngeal Cancer, Lip and
Oral Cavity Cancer, Liver Cancer, Lung Cancer,
Lymphoproliferative Disorders, Macroglobulinemia, Male Breast Cancer,
Malignant Mesothelioma, Malignant Thymoma, Medulloblastoma,
Melanoma, Mesothelioma, -Metastatic Occult Prima -ry Squamous Neck Cancer,
Metastatic Primary Squamous Neck Cancer, Metastatic Squamous
Neck Cancer, Multiple Myeloma, Multiple Myeloma/Plasma Cell Neoplasm,
Myelodysplastic Syndrome, Myelogenous Leukemia, Myeloid
Leukemia, Myeloproliferative Disorders, Nasal Cavity and Paranasal Sinus
Cancer, Nasopharyngeal Cancer, Neuroblastoma, Non-Hodgkin's
Lymphoma Duruig Pregnancy, Nonmelanoma Skin Cancer, Non-Small Cell Lung
Cancer, Occult Primary Metastatic Squamous Neck Cancer,
Oropharyngeal Cancer, Osteo-/Malignant Fibrous Sarcoma, Osteosarcoma/Malignant
Fibrous Histiocytoma, Osteosarcoma/Malignant Fibrous
Histiocytoma of Bone, Ovarian Epithelial Cancer, Ovarian Germ Cell Tumor,
Ovarian Low Malignant Potential Tumor, Pancreatic Cancer,
Paraproteinemias, Purpura, Parathyroid Cancer, Penile Cancer,
Pheochromocytoma, Pituitary Tumor, Plasma Cell Neoplasm/Multiple Myeloma,
Primary Central Nervous System Lymphoma, Primary Liver Cancer, Prostate
Cancer, Rectal Cancer, Renal Cell Cancer, Renal Pelvis and Ureter
Cancer, Retinoblastoma, Rhabdomyosarcoma, Salivary Gland Cancer, Sarcoidosis
Sarcomas, Sezary Syndrome, Skin Cancer, Small Cell Lung
Cancer, Small Intestine Cancer, Soft Tissue Sarcoma, Squamous Neck Cancer,
Stomach Cancer, Supratentorial Primitive Neuroectodermal and
Pineal Tumors, T-Cell Lymphoma, Testicular Cancer, Thymoma, Thyroid Cancer,
Transitional Cell Cancer of the Renal Pelvis and Ureter,
Transitional Renal Pelvis and Ureter Cancer, Trophoblastic Tumors, Ureter and
Renal Pelvis Cell Cancer, Urethral Cancer, Uterine Cancer, Uterine
Sarcoma, Vaginal Cancer, Visual Pathway and Hypothalamic Glioma, Vulvar
Cancer, Waldenstrom's Macroglobulinemia, Wilms' Tumor, and any
other hyperproliferative disease, besides neoplasia, located in an organ
system listed above.
[0703] In another preferred embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used to diagnose, prognose, prevent, and/or treat
premalignant conditions and to prevent progression to a neoplastic or
malignant
state, including but not limited to those disorders described above. Such uses
are indicated in conditions known or suspected of preceding
progression to neoplasia or cancer, in particular, where non-neoplastic cell
growth consisting of hyperplasia, metaplasia, or most particularly,
dysplasia has occurred (for review of such abnormal growth conditions, see
Robbins and Angell, 1976, Basic Pathology, 2d Ed., W. B. Saunders
Co., Philadelphia, pp. 68-79.)
[0704] Hyperplasia is a form of controlled cell proliferation, involving an
increase in cell number in a tissue or organ, without significant
alteration in structure or function. Hyperplastic disorders which can be
diagnosed, prognosed, prevented, and/or treated with fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention include, but are not limited to, angiofollicular mediastinal lymph
109
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
"' 9.41 õ-:U Il..it liil .. r~ I1P-,.. ,~.a1l '!i "{It il ,
node hyperpfasta, angtolymphotd liyperpla'sia with eosinophilia, atypical
melanocytic hyperplasia, basal cell hyperplasia, benign giant lymph node
hyperplasia, cementum hyperplasia, congenital adrenal hyperplasia, congenital
sebaceous hyperplasia, cystic hyperplasia, cystic hyperplasia of the
breast, denture hyperplasia, ductal hyperplasia, endometrial hyperplasia,
fibromuscular hyperplasia, focal epithelial hyperplasia, gingival
hyperplasia, inflammatory fibrous hyperplasia, inflammatory papillary
hyperplasia, intravascular papillary endothelial liyperplasia, nodular
hyperplasia of prostate, nodular regenerative hyperplasia,
pseudoepitheliomatous hyperplasia, senile sebaceous hyperplasia, and verrucous
liyperplasia.
[0705] Metaplasia is a form of controlled cell growth in which one type of
adult or fully differentiated cell substitutes for another type of adult
cell. Metaplastic disorders which can be diagnosed, prognosed, prevented,
and/or treated with fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention include, but
are not limited to, agnogenic myeloid metaplasia, apocrine
metaplasia, atypical metaplasia, autoparenchymatous metaplasia, connective
tissue metaplasia, epithelial metaplasia, intestinal metaplasia,
metaplastic anemia, metaplastic ossification, metaplastic polyps, myeloid
metaplasia, primary myeloid metaplasia, secondary myeloid metaplasia,
squamous metaplasia, squamous metaplasia of amnion, and symptomatic myeloid
metaplasia.
[0706] Dysplasia is frequently a forerunner of cancer, and is found mainly in
the epithelia; it is the most disorderly fonn of non-neoplastic cell
growth, involving a loss in individual cell unifonnity and in the
architectural orientation of cells. Dysplastic cells often have abnormally
large, deeply
stained nuclei, and exhibit pleomorphism. Dysplasia characteristically occurs
where there exists chronic irritation or inflammation. Dysplastic
disorders which can be diagnosed, prognosed, prevented, and/or treated with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include, but are not limited to,
anhidrotic ectodermal dysplasia, anterofacial dysplasia, asphyxiating thoracic
dysplasia, atriodigital dysplasia, bronchopulmonary dysplasia, cerebral
dysplasia, cervical dysplasia, chondroectodermal dysplasia, cleidocranial
dysplasia, congenital ectodennal dysplasia, craniodiaphysial dysplasia,
craniocarpotarsal dysplasia, craniometaphysial dysplasia, dentin dysplasia,
diaphysial dysplasia, ectodermal dysplasia, enamel dysplasia, encephalo-
ophthalmic dysplasia, dysplasia epiphysialis hemimelia, dysplasia
epiphysialis multiplex, dysplasia epiphysialis punctata, epithelial dysplasia,
faciodigitogenital dysplasia, familial fibrous dysplasia ofjaws, familial
white folded dysplasia, fibromuscular dysplasia, fibrous dysplasia of bone,
florid osseous dysplasia, hereditary renal-retinal dysplasia, hidrotic
ectodermal dysplasia, hypohidrotic ectodermal dysplasia, lymphopenic thymic
dysplasia, mammary dysplasia, mandibulofacial dysplasia,
metaphysial dysplasia, Mondini dysplasia, monostotic fibrous dysplasia,
mucoepithelial dysplasia, multiple epiphysial dysplasia,
oculoauriculovertebral dysplasia, oculodentodigital dysplasia, oculovertebral
dysplasia, odontogenic dysplasia, ophthalmomandibulomelic dysplasia,
periapical cemental dysplasia, polyostotic fibrous dysplasia,
pseudoachondroplastic spondyloepiphysial dysplasia, retinal dysplasia, septo-
optic
dysplasia, spondyloepiphysial dysplasia, and ventriculoradial dysplasia.
[0707] Additional pre-neoplastic disorders which can be diagnosed, prognosed,
prevented, and/or treated with fusion proteins of the invention
--and/or--polynucleotides encoding albumin fusion- proteins -of the invention
include, but- are not limited to; benign dysproliferative disorders (e.g.,
benign tumors, fibrocystic conditions, tissue hypertrophy, intestinal polyps,
colon polyps, and esophageal dysplasia), leukoplakia, keratoses,
Bowen's disease, Farmer's Skin, solar cheilitis, and solar keratosis.
[0708] In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention, may be used to diagnose and/or prognose disorders associated with
the tissue(s) in which the polypeptide of the invention is expressed.
[0709] In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention conjugated to a toxin or a radioactive isotope, as described herein,
may be used to treat cancers and neoplasms, including, but not limited
to, those described herein. In a further preferred embodiment, albumin fusion
proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention conjugated to a toxin or a radioactive
isotope, as described herein, may be used to treat acute myelogenous leukemia.
[0710] Additionally, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may affect
apoptosis, and therefore, would be useful in treating a number of diseases
associated with increased cell survival or the inhibition of apoptosis. For
example, diseases associated with increased cell survival or the inhibition of
apoptosis that could be diagnosed, prognosed, prevented, and/or treated
by polynucleotides, polypeptides, and/or agonists or antagonists of the
invention, include cancers (such as follicular lymphomas, carcinomas with
p53 mutations, and hormone-dependent tumors, including, but not limited to
colon cancer, cardiac tumors, pancreatic cancer, melanoma,
retinoblastoma, glioblastoma, lung cancer, intestinal cancer, testicular
cancer, stomach cancer, neuroblastoma, myxoma, myoma, lymphoma,
endothelioma, osteoblastoma, osteoclastoma, osteosarcoma, chondrosarcoma,
adenoma, breast cancer, prostate cancer, Kaposi's sarcoma and
ovarian cancer); autoimmune disorders such as, multiple sclerosis, Sjogren's
syndrome, Hashimoto's thyroiditis, biliary cirrhosis, Behcet's disease,
Crohn's disease, polymyositis, systemic lupus erythematosus and immune-related
glomerulonephritis and rheumatoid arthritis) and viral infections
(such as herpes viruses, pox viruses and adenoviruses), inflammation, graft v.
host disease, acute graft rejection, and chronic graft rejection.
[0711] In preferred embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention are
used to inhibit growth, progression, and/or metastasis of cancers, in
particular those listed above.
[0712] Additional diseases or conditions associated with increased cell
survival that could be diagnosed, prognosed, prevented, and/or treated by
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention, include, but are not limited to,
110
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~ro ession";'and)oimetastases"oini0li hdtlhies and related disorders such as
leukemia ~ncludin acute leukemias (e.g., P g~' b (' g acute lympliocytic
leukemia,
acute niyelocytic leukemia (including myeloblastic, promyelocytic,
myelomonocytic, monocytic, and erythroleukemia)) and chronic leukemias (e.g.,
chronic myelocytic (granulocytic) leukemia and chronic lymphocytic leukemia)),
polycythemia vera, lymphomas (e.g., Hodgkin's disease and
non-Hodgkin's disease), multiple myeloma, Waldenstrom's macroglobulinemia,
liaavy cliain disease, and solid tumors including, but not limited to,
sarcomas and carcinomas such as fibrosarcoma, myxosarcoma, liposarcoma,
chondrosarcoma, osteogenic sarcoma, chordoma, angiosarcoma,
endotheliosarcoma, lymphangiosarcoma, lymphangioendotheliosarcoma, synovioma,
mesothelioma, Ewing's tumor, leiomyosarcoma,
rhabdomyosarcoma, colon carcinoma, pancreatic cancer, breast cancer, ovarian
cancer, prostate cancer, squamous cell carcinoma, basal cell
carcinoma, adenocaroinoma, sweat gland carcinoma, sebaceous gland carcinoma,
papillary carcinoma, papillary adenocarcinomas,
cystadenocareinoma, medullary carcinoma, bronchogenic carcinoma, renal cell
carcinoma, hepatoma, bile duct carcinoma, choriocarcinoma,
seminoma, embryonal carcinoma, Wilm's tumor, cervical cancer, testicular
tumor, lung carcinoma, small cell lung carcinoma, bladder carcinoma,
epithelial carcinoma, glioma, astrocytoma, medulloblastoma, craniopharyngioma,
ependymoma, pinealoma, emangioblastoma, acoustic neuroma,
oligodendroglioma, menangioma, melanoma, neuroblastoma, and retinoblastoma.
[0713] Diseases associated with increased apoptosis that could be diagnosed,
prognosed, prevented, and/or treated by fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention, include AIDS; neurodegenerative disorders (such as
Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis,
retinitis pigmentosa, cerebellar degeneration and brain tumor or prior
associated disease); autoimmune disorders (such as, multiple sclerosis,
Sjogren's syndrome, Hashimoto's thyroiditis, biliary cirrhosis, Behcet's
disease, Crohn's disease, polymyositis, systemic lupus erythematosus and
immune-related glomerulonephritis and rheumatoid arthritis)
myelodysplastic syndromes (such as aplastic anemia), graft v. host disease,
ischemic injury (such as that caused by myocardial infarction, stroke and
reperfusion injury), liver injury (e.g., hepatitis related liver injury,
ischemia/reperfusion injury, cholestosis (bile duct injury) and liver cancer);
toxin-
induced liver disease (such as that caused by alcohol), septic shock, cachexia
and anorexia.
[0714] Hyperproliferative diseases and/or disorders that could be diagnosed,
prognosed, prevented, and/or treated by fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention, include, but are not limited to, neoplasms located in the liver,
abdomen, bone, breast, digestive system, pancreas, peritoneum, endocrine
glands (adrenal, parathyroid, pituitary, testicles, ovary, thymus, thyroid),
eye, head and neck, nervous system (central and peripheral), lymphatic system,
pelvis, skin, soft tissue, spleen, thorax, and urogenital tract.
[0715] Similarly, other hyperproliferative disorders can also be diagnosed,
prognosed, prevented, and/or treated by fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention. Examples of such hyperproliferative disorders include, but are
not limited to: hypergammaglobulinemia, lymphoproliferative disorders,
paraproteinemias, purpura, sarcoidosis, Sezary Syndrome, Waldenstron's
macroglobulinemia, Gaucher's Disease, histiocytosis, and any other
hyperproliferative disease, besides neoplasia, located in an organ system
listed
-above. - [0716] Another preferred embodiment utilizes polynucleotides
encoding albumin fusion proteins of the invention to inhibit aberrant cellular
division, by gene therapy using the present invention, and/or protein fusions
or fragments thereof.
[0717] Thus, the present invention provides a method for treating cell
proliferative disorders by inserting into an abnormally proliferating cell a
polynucleotide encoding an albumin fusion protein of the present invention,
wherein said polynucleotide represses said expression.
[0718] Another embodiment of the present invention provides a method of
treating cell-proliferative disorders in individuals comprising
administration of one or more active gene copies of the present invention to
an abnormally proliferating cell or cells. In a preferred embodiment,
polynucleotides of the present invention is a DNA constmct comprising a
recombinant expression vector effective in expressing a DNA sequence
encoding said polynucleotides. In another preferred embodiment of the present
invention, the DNA construct encoding the fusion protein of the
present invention is inserted into cells to be treated utilizing a retrovirus,
or more preferably an adenoviral vector (See G J. Nabel, et. al., PNAS
1999 96: 324-326, which is hereby incorporated by reference). In a most
preferred embodiment, the viral vector is defective and will not transform
non-proliferating cells, only proliferating cells. Moreover, in a preferred
embodiment, the polynucleotides of the present invention inserted into
proliferating cells either alone, or in combination with or fused to other
polynucleotides, can then be modulated via an extemal stimulus (i.e.
magnetic, specific small molecule, chemical, or drug administration, etc.),
which acts upon the promoter upstream of said polynucleotides to induce
expression of the encoded protein product. As such the beneficial therapeutic
affect of the present invention may be expressly modulated (i.e. to
increase, decrease, or inhibit expression of the present invention) based upon
said external stimulus.
[0719] Polynucleotides of the present invention may be useful in repressing
expression of oncogenic genes or antigens. By "repressing
expression of the oncogenic genes " is intended the suppression of the
transcription of the gene, the degradation of the gene transcript (pre-message
RNA), the inhibition of splicing, the destruction of the messenger RNA, the
prevention of the post-translational modifications of the protein, the
destruction of the protein, or the inhibition of the normal function of the
protein.
[0720] For local administration to abnormally proliferating cells,
polynucleotides of the present invention may be administered by any method
known to those of skill in the art including, but not limited to transfection,
electroporation, microinjection of cells, or in vehicles such as liposomes,
lipofectin, or as naked polynucleotides, or any other method described
throughout the specification. The polynucleotide of the present invention
111
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
may Ibf:31nown'ene delivet II
y b by l.= g ry"systems such as, but not limited to, retroviral vectors
(Gilboa, J. Virology 44:845 (1982); Hocke, Nature
320:275 (1986); Wilson, et al., Proc. Natl. Acad. Sci. U.S.A. 85:3014),
vaccinia virus system (Chakrabarty et al., Mol. Cell Biol. 5:3403 (1985) or
other efficient DNA delivery systems (Yates et al., Nature 313:812 (1985))
known to those skilled in the art. These references are exemplary only
and are hereby incorporated by reference. In order to specifically deliver or
transfect cells which are abnormally proliferating and spare non-dividing
cells, it is preferable to utilize a retrovirus, or adenoviral (as described
in the art and elsewhere herein) delivery system known to those of skill in
the
art. Since host DNA replication is required for retroviral DNA to integrate
and the retrovirus will be unable to self replicate due to the lack of the
retrovirus genes needed for its life cycle. Utilizing such a retroviral
delivery system for polynucleotides of the present invention will target said
gene
and constructs to abnormally proliferating cells and will spare the non-
dividing normal cells.
[0721] The polynucleotides of the present invention may be delivered directly
to cell proliferative disorder/disease sites in internal organs, body
cavities and tlie like by use of imaging devices used to guide an injecting
needle directly to the disease site. The polynucleotides of the present
invention may also be administered to disease sites at the time of surgical
intervention.
[0722] By "cell proliferative disease" is meant any human or animal disease or
disorder, affecting any one or any combination of organs,
cavities, or body parts, which is characterized by single or multiple local
abnormal proliferations of cells, groups of cells, or tissues, whether benign
or malignant.
[0723] Any amount of the polynucleotides of the present invention may be
administered as long as it has a biologically inhibiting effect on the
proliferation of the treated cells. Moreover, it is possible to administer
more than one of the polynucleotide of the present invention simultaneously
to the same site. By "biologically inhibiting" is meant partial or total
growth inhibition as well as decreases in the rate of proliferation or growth
of
the cells. The biologically inhibitory dose may be determined by assessing the
effects of the polynucleotides of the present invention on target
malignant or abnormally proliferating cell growth in tissue culture, tumor
growth in animals and cell cultures, or any other method known to one of
ordinary skill in the art.
[07241 Moreover, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention of the present
invention are useful in inhibiting the angiogenesis of proliferative cells or
tissues, either alone, as a protein fusion, or in combination with other
polypeptides directly or indirectly, as described elsewhere herein. In a most
preferred embodiment, said anti-angiogenesis effect may be achieved
indirectly, for example, through the inhibition of hematopoietic, tumor-
specific cells, such as tumor-associated macrophages (See Joseph IB, et al. J
Natl Cancer Inst, 90(21):1648-53 (1998), which is hereby incorporated by
reference).
[07251 Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
inhibiting proliferative cells or tissues through the induction of apoptosis.
These fusion protieins and/or polynucleotides may act either directly, or
indirectly to induce apoptosis of proliferative cells and tissues, for example
in the activation of a death-domain receptor, such as tumor necrosis
factor (TNF) receptor-1, CD95 (Fas/-APO-1); TNF-receptor-related apoptosis-
mediated-protein-(T-RAMP) and TNF-related apoptosis-inducing
ligand (TRAIL) receptor-1 and -2 (See Schulze-Osthoff I{, et.al., Eur J
Biochem 254(3):439-59 (1998), which is hereby incorporated by reference).
Moreover, in another preferred embodiment of the present invention, these
fusion proteins and/or polynucleotides may induce apoptosis through
other mechanisms, such as in the activation of other proteins which will
activate apoptosis, or through stimulating the expression of these proteins,
either alone or in combination with small molecule drugs or adjuviants, such
as apoptonin, galectins, thioredoxins, anti-inflammatory proteins (See
for example, Mutat Res 400(1-2):447-55 (1998), Med Hypotheses.50(5):423-33
(1998), Chem Biol Interact. Apr 24;111-112:23-34 (1998), J Mol
Med.76(6):402-12 (1998), Int J Tissue React;20(1):3-15 (1998), which are all
hereby incorporated by reference).
[0726] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention are useful in
inhibiting the metastasis of proliferative cells or tissues. Inhibition may
occur as a direct result of administering these albumin fusion proteins
and/or polynucleotides, or indirectly, such as activating the expression of
proteins known to inhibit metastasis, for example alpha 4 integrins, (See,
e.g., Curr Top Microbiol Immunol 1998;231:125-41, which is hereby incorporated
by reference). Such thereapeutic affects of the present invention
may be achieved either alone, or in combination with small molecule drugs or
adjuvants.
[0727] In another embodiment, the invention provides a method of delivering
compositions containing the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention to targeted cells expressing the a polypeptide bound by, that
binds to, or associates with an albumin fuison protein of the invention.
Albumin fusion proteins of the invention may be associated with with
heterologous polypeptides, heterologous nucleic acids, toxins, or prodrugs via
hydrophobic, hydrophilic, ionic and/or covalent interactions.
[0728] Albumin fusion proteins of the invention are useful in enhancing the
immunogenicity and/or antigenicity of proliferating cells or tissues,
either directly, such as would occur if the albumin fusion proteins of the
invention 'vaccinated' the immune response to respond to proliferative
antigens and immunogens, or indirectly, such as in activating the expression
of proteins known to enhance the immune response (e.g. chemokines),
to said antigens and immunogens.
Renal Disorders
[0729] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be used to treat,
prevent, diagnose, and/or prognose disorders of the renal system. Renal
disorders which can be diagnosed, prognosed, prevented, and/or treated
112
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~yith co"mpositions"'o~f theinventfo~n iricl'ude, but are not limited to,
kidney failure, nephritis, blood vessel disorders of kidney, metabolic and
congenital kidney disorders, urinary disorders of the kidney, autoimmune
disorders, sclerosis and necrosis, electrolyte imbalance, and kidney
cancers.
[0730] Kidney diseases which can be diagnosed, prognosed, prevented, and/or
treated with compositions of the invention include, but are not
limited to, acute kidney failure, chronic kidney failure, atheroembolic renal
failure, end-stage renal disease, inflammatory diseases of the kidney
(e.g., acute glomerulonephritis, postinfectious glomerulonephritis, rapidly
progressive glomerulonephritis, nephrotic syndrome, membranous
glomerulonephritis, familial nephrotic syndrome, membranoproliferative
glomerulonephritis I and II, mesangial proliferative glomerulonephritis,
chronic glomerulonephritis, acute tubulointerstitial nephritis, chronic
tubulointerstitial nephritis, acute post-streptococcal glomerulonephritis
(PSGN), pyelonepliritis, lupus nephritis, chronic nephritis, interstitial
nephritis, and post-streptococcal glomerulonephritis), blood vessel disorders
of
the kidneys (e.g., kidney infaretion, atheroembolic kidney disease, cortical
necrosis, malignant nephrosclerosis, renal vein thrombosis, renal
underperfusion, renal retinopathy, renal ischemia-reperfusion, renal artery
embolism, and renal artery stenosis), and kidney disorders resulting form
urinary tract disease (e.g., pyelonephritis, hydronephrosis, urolithiasis
(renal litiiiasis, nephrolithiasis), reflux nephropathy, urinary tract
infections,
urinary retention, and acute or chronic unilateral obstructive uropathy.)
[0731] In addition, compositions of the invention can be used to diagnose,
prognose, prevent, and/or treat metabolic and congenital disorders of
the kidney (e.g., uremia, renal amyloidosis, renal osteodystrophy, renal
tubular acidosis, renal glycosuria, nephrogenic diabetes insipidus,
oystinuria,
Fanconi's syndrome, renal fibrocystic osteosis (renal rickets), Hartnup
disease, Bartter's syndrome, Liddle's syndrome, polycystic kidney disease,
medullary cystic disease, medullary sponge kidney, Alport's syndrome, nail-
patella syndrome, congenital nephrotic syndrome, CRUSH syndrome,
horseshoe kidney, diabetic nephropathy, nephrogenic diabetes insipidus,
analgesic nephropathy, kidney stones, and membranous nephropathy), and
autoimmune disorders of the kidney (e.g., systemic lupus erythematosus (SLE),
Goodpasture syndrome, IgA nephropathy, and IgM mesangial
proliferative glomerulonephritis).
[0732] Compositions of the invention can also be used to diagnose, prognose,
prevent, and/or treat sclerotic or necrotic disorders of the kidney
(e.g., glomerulosolerosis, diabetic nephropathy, focal segmental
glomerulosclerosis (FSGS), necrotizing glomerulonephritis, and renal papillary
necrosis), cancers of the kidney (e.g., nephroma, hypemephroma,
nephroblastoma, renal cell cancer, transitional cell cancer, renal
adenocarcinoma,
squamous cell cancer, and Wilm's tumor), and electrolyte imbalances (e.g.,
nephrocalcinosis, pyuria, edema, hydronephritis, proteinuria,
hyponatremia, hypematremia, hypokalemia, hyperkalemia, liypocalcemia,
hypercalcemia, hypophosphatemia, and hyperphosphatemia).
[0733] Compositions of the invention may be administered using any method
known in the art, including, but not limited to, direct needle
injection at the delivery site, intravenous injection, topical administration,
catheter infusion, biolistic injectors, particle accelerators, gelfoam sponge
depots, other commercially available depot materials, osmotic pumps, oral or
suppositorial solid pharmaceutical formulations, decanting or topical
-applications during surgery; aerosol delivery.- Such methods are laiown in
the art. Compositions-of the invention may be administered as part of a
Therapeutic, described in more detail below. Methods of delivering
polynucleotides of the invention are described in more detail herein.
Cardiovascular Disorders
[0734] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be used to treat,
prevent, diagnose, and/or prognose cardiovascular disorders, including, but
not limited to, peripheral artery disease, such as limb ischemia.
[0735] Cardiovascular disorders include, but are not limited to,
cardiovascular abnormalities, such as arterio-arterial fistula, arteriovenous
fistula, cerebral arteriovenous malformations, congenital heart defects,
pulmonary atresia, and Scimitar Syndrome. Congenital heart defects include,
but are not limited to, aortic coarotation, cor triatriatum, coronary vessel
anomalies, crisscross heart, dextrocardia, patent ductus arteriosus, Ebstein's
anomaly, Eisenmenger complex, hypoplastic left heart syndrome, levocardia,
tetralogy of fallot, transposition of great vessels, double outlet right
ventricle, tricuspid atresia, persistent truncus arteriosus, and heart septal
defects, such as aortopulmonary septal defect, endocardial cushion defects,
Lutembacher's Syndrome, trilogy of Fallot, ventricular heart septal defects.
[0736] Cardiovascular disorders also include, but are not limited to, heart
disease, such as arrhythmias, carcinoid heart disease, high cardiac
output, low cardiac output, cardiac tamponade, endocarditis (including
bacterial), heart aneurysm, cardiac arrest, congestive heart failure,
congestive
cardiomyopathy, paroxysmal dyspnea, cardiac edema, heart hypertrophy,
congestive cardiomyopathy, left ventricular hypertrophy, right ventricular
hypertrophy, post-infarction heart rupture, ventricular septal rupture, heart
valve diseases, myocardial diseases, myocardial ischemia, pericardial
effusion, pericarditis (including constrictive and tuberculous),
pneumopericardium, postpericardiotomy syndrome, pulmonary heart disease,
rheumatic heart disease, ventricular dysfunction, hyperemia, cardiovascular
pregnancy complications, Scimitar Syndrome, cardiovascular syphilis,
and cardiovascular tuberculosis.
[0737] Arrhythmias include, but are not limited to, sinus arrhythmia, atrial
fibrillation, atrial flutter, bradycardia, extrasystole, Adams-Stokes
Syndrome, bundle-branch block, sinoatrial block, long QT syndrome,
parasystole, Lown-Ganong-Levine Syndrome, Mahaim-type pre-excitation
syndrome, Wolff-Parkinson-White syndrome, sick sinus syndrome, tachycardias,
and ventricular fibrillation. Tachycardias include paroxysmal
tachycardia, supraventricular tachycardia, accelerated idioventricular rhythm,
atrioventricular nodal reentry tachycardia, ectopic atrial tachycardia,
ectopic junctional tachycardia, sinoatrial nodal reentry tachycardia, sinus
tachycardia, Torsades de Pointes, and ventricular tachycardia.
113
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
C" IL.. tl lt '.ea 11õ 1va'~ It" t.ve''
dt~eases ine ude, but are not limited to, aortic valve insufficiency, aortic
valve stenosis, hear murmurs, aortic valve prolapse,
mitral valve prolapse, tricuspid valve prolapse, mitral valve insufficiency,
mitral valve stenosis, pulmonary atresia, pulmonary valve insufficiency,
pulmonary valve stenosis, tricuspid atresia, tricuspid valve insufficiency,
and tricuspid valve stenosis.
[0739] Myocardial diseases include, but are not limited to, alcoholic
cardiomyopathy, congestive cardiomyopathy, hypertrophic cardiomyopathy,
aortic subvalvular stenosis, pulmonary subvalvular stenosis, restrictive
cardiomyopatliy, Chagas cardiomyopathy, endocardial fibroelastosis,
endomyocardial fibrosis, Keams Syndrome, myocardial reperfusion injury, and
myocarditis.
[0740] Myocardial iscliemias include, but are not limited to, coronary
disease, such as angina pectoris, coronary aneurysm, coronary
arteriosclerosis, coronary thrombosis, coronary vasospasm, myocardial
infarction and myocardial stunning.
[0741] Cardiovascular diseases also include vascular diseases such as
aneurysms, angiodysplasia, angiomatosis, bacillary angiomatosis, Hippel-
Lindau Disease, Klippel-Trenaunay-Weber Syndrome, Sturge-Weber Syndrome,
angioneurotic edema, aortic diseases, Takayasu's Arteritis, aortitis,
Leriche's Syndrome, arterial occlusive diseases, arteritis, enarteritis,
polyarteritis nodosa, cerebrovascular disorders, diabetic angiopathies,
diabetic
retinopathy, embolisms, thrombosis, erythromelalgia, hemorrhoids, hepatic veno-
occlusive disease, hypertension, hypotension, ischemia, peripheral
vascular diseases, phlebitis, pulmonary veno-occlusive disease, Raynaud's
disease, CREST syndrome, retinal vein occlusion, Scimitar syndrome,
superior vena cava syndrome, telangiectasia, atacia telangiectasia, hereditary
hemorrhagic telangiectasia, varicocele, varicose veins, varicose ulcer,
vasculitis, and venous insufficiency.
[0742] Aneurysms include, but are not limited to, dissecting aneurysms, false
aneurysms, infected aneurysms, ruptured aneurysms, aortic
aneurysms, cerebral aneurysms, coronary aneurysms, heart aneurysms, and iliac
aneurysms.
[0743] Arterial occlusive diseases include, but are not limited to,
arteriosclerosis, intermittent claudication, carotid stenosis, fibromuscular
dysplasias, mesenteric vascular occlusion, Moyamoya disease, renal artery
obstruction, retinal artery occlusion, and thromboangiitis obliterans.
[0744] Cerebrovascular disorders include, but are not limited to, carotid
artery diseases, cerebral amyloid angiopathy, cerebral aneurysm,
cerebral anoxia, cerebral arteriosclerosis, cerebral arteriovenous
malfbrmation, cerebral artery diseases, cerebral embolism and thrombosis,
carotid
artery thrombosis, sinus thrombosis, Wallenberg's syndrome, cerebral
hemorrhage, epidural hematoma, subdural hematoma, subaraxhnoid
hemorrhage, cerebral infarction, cerebral ischemia (including transient),
subclavian steal syndrome, periventricular leukomalacia, vascular headache,
cluster headache, migraine, and vertebrobasilar insufficiency.
[0745] Embolisms include, but are not limited to, air embolisms, amniotic
fluid embolisms, cholesterol embolisms, blue toe syndrome, fat
embolisms, pulmonary embolisms, and thromoboembolisms. Thrombosis include, but
are not limited to, coronary thrombosis, hepatic vein
thrombosis, retinal vein occlusion, carotid artery thrombosis, sinus
thrombosis, Wallenberg's syndrome, and thrombophlebitis.
[0746] Ischemic disorders include, but are not limited to, cerebral ischemia,
ischemic colitis, compartment syndromes, anterior compartment
-syndrome, myocardial ischemia, reperfusion injuries, and peripheral limb
ischemia. Vasculitis includes, but is not limited to, aortitis, arteritis,
Behcet's Syndrome, Churg-Strauss Syndrome, mucocutaneous lymph node syndrome,
thromboangiitis obliterans, hypersensitivity vasculitis,
Schoenlein-Henoch purpura, allergic cutaneous vasculitis, and Wegener's
granulomatosis.
[0747] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be administered
using any method known in the art, including, but not limited to, direct
needle injection at the delivery site, intravenous injection, topical
administration, catheter infusion, biolistic injectors, particle accelerators,
gelfoam sponge depots, other commercially available depot materials,
osmotic pumps, oral or suppositorial solid pharmaceutical formulations,
decanting or topical applications during surgery, aerosol delivery. Such
methods are known in the art. Methods of delivering polynucleotides are
described in more detail herein.
Respiratory Disorders
[0748] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be used to treat,
prevent, diagnose, and/or prognose diseases and/or disorders of the
respiratory system.
[0749] Diseases and disorders of the respiratory system include, but are not
limited to, nasal vestibulitis, nonallergic rhinitis (e.g., acute rhinitis,
chronic rhinitis, atrophic rhinitis, vasomotor rhinitis), nasal polyps, and
sinusitis, juvenile angiofibromas, cancer of the nose and juvenile papillomas,
vocal cord polyps, nodules (singer's nodules), contact ulcers, vocal cord
paralysis, laryngoceles, pharyngitis (e.g., viral and bacterial), tonsillitis,
tonsillar cellulitis, parapharyngeal abscess, laryngitis, laryngoceles, and
throat cancers (e.g., cancer of the nasopharynx, tonsil cancer, larynx
cancer),
lung cancer (e.g., squamous cell carcinoma, small cell (oat cell) carcinoma,
large cell carcinoma, and adenocarcinoma), allergic disorders
(eosinophilic pneumonia, hypersensitivity pneumonitis (e.g., extrinsic
allergic alveolitis, allergic interstitial pneumonitis, organic dust
pneumoconiosis, allergic bronchopulmonary aspergillosis, asthma, Wegener's
granulomatosis (granulomatous vasculitis), Goodpasture's syndrome)),
pneumonia (e.g., bacterial pneumonia (e.g., Streptococcus pneumoniae
(pneumoncoccal pneumonia), Staphylococcus aureus (staphylococcal
pneumonia), Gram-negative bacterial pneumonia (caused by, e.g., Klebsiella and
Pseudon:as spp.), Mycoplasma pzeumoniae pneumonia,
Hemophilus influenaae pneumonia, Legionella pneumophila (Legionnaires'
disease), and Clzlamydia psittaci (Psittacosis)), and viral pneumonia
(e.g., influenza, chickenpox (varicella).
[0750] Additional diseases and disorders of the respiratory system include,
but are not limited to bronchiolitis, polio (poliomyelitis), croup,
114
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
1! t:::it 1{,..I! If;:' ..
respiraiory syncytta~ vira iiifecti0' , dlui s, erythema infectiosum (fifth
disease), roseola infantum, progressive rubella panencephalitis, german
measles, and subacute solerosing panencephalitis), fungal pneumonia (e.g.,
Histoplasmosis, Coccidioidomycosis, Blastomycosis, fungal infections in
people with severely suppressed immune systems (e.g., cryptococcosis, caused
by Ciyptococcus neoforinans; aspergillosis, caused by Aspergilhts
spp.; candidiasis, caused by Candida; and mucormycosis)), Pneuwnocystis
carinii (pneumocystis pneumonia), atypical pneumonias (e.g.,
Mycoplasina and Chlainydia spp.), opportunistic infection pneumonia,
nosocomial pneumonia, chemical pneumonitis, and aspiration pneumonia,
pleural disorders (e.g., pleurisy, pleural effusion, and pneumothorax (e.g.,
simple spontaneous pneumothorax, complicated spontaneous
pneumothorax, tension pneumothorax)), obstructive airway diseases (e.g.,
asthma, chronic obstructive pulmonary disease (COPD), emphysema,
clironic or acute bronchitis), occupational lung diseases (e.g., silicosis,
black lung (coal workers' pneumoconiosis), asbestosis, berylliosis,
occupational asthsma, byssinosis, and benign pneumoconioses), Infiltrative
Lung Disease (e.g., pulmonary fibrosis (e.g., fibrosing alveolitis, usual
interstitial pneumonia), idiopathic pulmonary fibrosis, desquamative
interstitial pneumonia, lymphoid interstitial pneumonia, histiocytosis X
(e.g.,
Letterer-Siwe disease, Hand-Schuller-Christian disease, eosinophilic
granuloma), idiopathic pulmonary heniosiderosis, sarcoidosis and pulmonary
alveolar proteinosis), Acute respiratory distress syndrome (also called, e.g.,
adult respiratory distress syndrome), edema, pulmonary embolism,
bronchitis (e.g., viral, bacterial), bronchiectasis, atelectasis, lung abscess
(caused by, e.g., Staphylococcus aureus or Legionella pneurnophila), and
cystic fibrosis.
Auti Angiogenesis Activity
[0751] The naturally occurring balance between endogenous stimulators and
inhibitors of angiogenesis is one in which inhibitory influences
predominate. Rastinejad et al., Cell 56:345-355 (1989). In those rare
instances in which neovascularization occurs under normal physiological
conditions, such as wound healing, organ regeneration, embryonic development,
and female reproductive processes, angiogenesis is stringently
regulated and spatially and temporally delimited. Under conditions of
pathological angiogenesis such as that characterizing solid tumor growth,
these
regulatory controls fail. Uri'regulated angiogenesis becomes pathologic and
sustains progression of many neoplastic and non-neoplastic diseases. A
number of serious diseases are dominated by abnormal neovascularization
including solid tumor growth and metastases, arthritis, some types of eye
disorders, and psoriasis. See, e.g., reviews by Moses et al., Biotech. 9:630-
634 (1991); Folkman et al., N. Engl. J. Med., 333:1757-1763 (1995);
Auerbach et al., J. Microvasc. Res. 29:401-411 (1985); Folkman, Advances in
Cancer Research, eds. Klein and Weinhouse, Academic Press, New
York, pp. 175-203 (1985); Patz, An:. J. Optlzahnol. 94:715-743 (1982); and
Folkman el al., Science 221:719-725 (1983). In a number of
pathological conditions, the process of angiogenesis contributes to the
disease state. For example, significant data have accumulated which suggest
that the growth of solid tumors is dependent on angiogenesis. Folkman and
Klagsbrun, Science 235:442-447 (1987).
[0752] The present invention provides for treatment of diseases or disorders
associated with neovascularization by administration of fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention. Malignant and metastatic conditions which can
be treated-with the polynucleotides and polypeptides, or agonists or
antagonists of the invention include, -but are notlimited to, malignancies~
solid
tumors, and cancers described herein and otherwise known in the art (for a
review of such disorders, see Fishman el al., Medicine, 2d Ed., J. B.
Lippincott Co., Philadelphia (1985)).Thus, the present invention provides a
method of treating an angiogenesis-related disease and/or disorder,
comprising administering to an individual in need thereof a therapeutically
effective amount of an albumin fusion protein of the invention and/or
polynucleotides encoding an albumin fusion protein of the invention. For
example, fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention may be utilized in a variety of
additional methods in order to therapeutically treat a cancer or tumor.
Cancers which may be treated with fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
include, but are not limited to solid tumors, including prostate, lung,
breast, ovarian, stomach, pancreas, larynx, esophagus, testes, liver, parotid,
biliary tract, colon, rectum, cervix, uterus, endometrium, kidney, bladder,
thyroid cancer; primary tumors and metastases; melanomas; glioblastoma;
Kaposi's sarcoma; leiomyosarcoma; non- small cell lung cancer; colorectal
cancer; advanced malignancies; and blood born tumors such as
leukemias. For example, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
delivered
topically, in order to treat cancers such as skin cancer, head and neck
tumors, breast tumors, and Kaposi's sarcoma.
[0753] Within yet other aspects, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may
be utilized to treat superficial forms of bladder cancer by, for example,
intravesical administration. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may be
delivered directly into the tumor, or near the tumor site, via injection or a
catheter. Of course, as the artisan of ordinary skill will appreciate, the
appropriate mode of administration will vary according to the cancer to be
treated. Other modes of delivery are discussed herein.
[0754] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be useful in
treating other disorders, besides cancers, which involve angiogenesis. These
disorders include, but are not limited to: benign tumors, for example
hemangiomas, acoustic neuromas, neurofibromas, trachomas, and pyogenic
granulomas; artheroscleric plaques; ocular angiogenic diseases, for
example, diabetic retinopathy, retinopathy of prematurity, macular
degeneration, corneal graft rejection, neovascular glaucoma, retrolental
fibroplasia, rubeosis, retinoblastoma, uvietis and Pterygia (abnormal blood
vessel growth) of the eye; rheumatoid arthritis; psoriasis; delayed wound
healing; endometriosis; vasculogenesis; granulations; hypertrophic scars
(keloids); nonunion fractures; scieroderma; trachoma; vascular adhesions;
115
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
.... iG..rv la .lf 11.1
~. ~r::~l õ'= , u:~:;; =,is~F ,~:::~1
myocarU~a[ angiogenesis; coronary coilaferals; cerebral collaterals;
arteriovenous malformations; ischemic limb angiogenesis; Osler-Webber
Syndrome; plaque neovascularization; telangiectasia; hemopiiiliac joints;
angiofibroma; fibromuscular dysplasia; wound granulation; Crohn's
disease; and atherosclerosis.
[0755] For example, within one aspect of the present invention methods are
provided for treating hypertrophic scars and keloids, comprising the
step of administering albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention to a
hypertrophic scar or keloid.
[0756] Within one embodiment of the present invention fusion proteins of the
invention and/or polynucleotides encoding albumin fusion
proteins of the invention are directly injected'into a hypertrophic scar or
keloid, in order to prevent the progression of these lesions. This therapy is
of particular value in the prophylactic treatment of conditions wliich are
known to result in the development of hypertrophic scars and keloids (e.g.,
burns), and is preferably initiated after the proliferative phase has had time
to progress (approximately 14 days after the initial injury), but before
hypertrophic scar or keloid development. As noted above, the present invention
also provides methods for treating neovascular diseases of the eye,
including for example, comeal neovascularization, neovascular glaucoma,
proliferative diabetic retinopathy, retrolental fibroplasia and macular
degeneration.
[0757] Moreover, Ocular disorders associated with neovascularization which can
be treated with the albumin fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the invention
include, but are not limited to: neovascular glaucoma, diabetic retinopathy,
retinoblastoma, retrolental fibroplasia, uveitis, retinopathy of prematurity
macular degeneration, corneal graft neovascularization, as well as otlier eye
inflammatory diseases, ocular tumors and diseases associated with choroidal or
iris neovascularization. See, e.g., reviews by Waltman et al., Am. J.
Opl:tlial. 85:704-710 (1978) and Gartner et al., Surv. Oplithal. 22:291-312
(1978).
[07581 Thus, within one aspect of the present invention methods are provided
for treating neovascular diseases of the eye such as comeal
neovascularization (including corneal graft neovascularization), comprising
the step of administering to a patient a therapeutically effective amount
of a compound (e.g., fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention) to the cornea, such
that the formation of blood vessels is inhibited. Briefly, the cornea is a
tissue which normally lacks blood vessels. In certain pathological conditions
however, capillaries may extend into the cornea from the pericomeal vascular
plexus of the limbus. When the cornea becomes vascularized, it also
becomes clouded, resulting in a decline in the patient's visual acuity. Visual
loss may become complete if the cornea completely opacitates. A wide
variety of disorders can result in corneal neovascularization, including for
example, corneal infections (e.g., trachoma, herpes simplex keratitis,
leishmaniasis and onchocerciasis), immunological processes (e.g., graft
rejection and Stevens-Johnson's syndrome), alkali burns, trauma,
inflammation (of any cause), toxic and nutritional deficiency states, and as a
complication of wearing contact lenses.
[0759] Within particularly preferred embodiments of the invention, may be
prepared for topical administration in saline (combined with any of
the-preservatives and antimicrobial agents commonly used in ocular
preparations), and administered in eyedrop form: The solution-orsuspension
may be prepared in its pure form and administered several times daily.
Alternatively, anti-angiogenic compositions, prepared as described above,
may also be administered directly to the cornea. Within preferred embodiments,
the anti-angiogenic composition is prepared with a muco-adhesive
polymer which binds to cornea. Within further embodiments, the anti-angiogenic
factors or anti-angiogenic compositions may be utilized as an
adjunct to conventional steroid therapy. Topical therapy may also be useful
prophylactically in comeal lesions wliich are known to have a high
probability of inducing an angiogenic response (such as chemical burns). In
these instances the treatment, likely in combination with steroids, may
be instituted immediately to help prevent subsequent complications.
[0760] Within other embodiments, the compounds described above may be injected
directly into the corneal stroma by an ophthalmologist under
microscopic guidance. The preferred site of injection may vary with the
morphology of the individual lesion, but the goal of the administration
would be to place the composition at the advancing front of the vasculature
(i.e., interspersed between the blood vessels and the normal cornea). hi
most cases this would involve perilimbic corneal injection to "protect" the
cornea from the advancing blood vessels. This method may also be
utilized shortly after a comeal insult in order to prophylactically prevent
corneal neovascularization. In this situation the material could be injected
in the perilimbic cornea interspersed between the comeal lesion and its
undesired potential limbic blood supply. Such methods may also be utilized
in a similar fashion to prevent capillary invasion of transplanted comeas. In
a sustained-release form injections might only be required 2-3 times per
year. A steroid could also be added to the injection solution to reduce
inflammation resulting from the injection itself.
[0761] Within another aspect of the present invention, methods are provided
for treating neovascular glaucoma, comprising the step of
administering to a patient a therapeutically effective amount of an albumin
fusion protein of the invention and/or polynucleotides encoding an
albumin fusion protein of the invention to the eye, such that the formation of
blood vessels is inhibited. In one embodiment, the compound may be
administered topically to the eye in order to treat early forms of neovascular
glaucoma. Within other embodiments, the compound may be implanted
by injection into the region of the anterior chamber angle. Within other
embodiments, the compound may also be placed in any location such that
the compound is continuously released into the aqueous humor. Within another
aspect of the present invention, methods are provided for treating
proliferative diabetic retinopathy, comprising the step of administering to a
patient a therapeutically effective amount of an albumin fusion protein of
the invention and/or polynucleotides encoding an albumin fusion protein of the
invention to the eyes, such that the formation of blood vessels is
116
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
107621 Within particularly preferred embodiments of the invention,
proliferative diabetic retinopathy may be treated by injection into the
aqueous humor or the vitreous, in order to increase the local concentration of
the polynucleotide, polypeptide, antagonist and/or agonist in the retina.
Preferably, this treatment should be initiated prior to the acquisition of
severe disease requiring photocoagulation.
[0763] Witliin another aspect of the present invention, methods are provided
for treating retrolental fibroplasia, comprising the step of
administering to a patient a therapeutically effective amount of an albumin
fusion protein of the invention and/or polynucleotides encoding an
albumin fusion protein of the invention to the eye, such that the fonnation of
blood vessels is inhibited. The compound may be administered
topically, via intravitreous injection and/or via intraocular implants.
[0764] Additionally, disorders which can be treated with fusion proteins of
the invention and/or polynucleotides encoding albumin fusion
proteins of the invention include, but are not limited to, hemangioma,
arthritis, psoriasis, angiofibroma, atlierosclerotic plaques, delayed wound
healing, granulations, hemophilic joints, hypertrophic scars, nonunion
fractures, Osler-Weber syndrome, pyogenic granuloma, scleroderma,
trachoma, and vascular adhesions.
107651 Moreover, disorders and/or states, which can be treated, prevented,
diagnosed, and/or prognosed with the the albumin fusion proteins of
the invention and/or polynucleotides encoding albumin fusion proteins of the
invention of the invention include, but are not limited to, solid tumors,
blood born tumors such as leukemias, tumor metastasis, Kaposi's sarcoma,
benign tumors, for example hemangiomas, acoustic neuromas,
neurofibromas, tracliomas, and pyogenic granulomas, rheumatoid arthritis,
psoriasis, ocular angiogenic diseases, for example, diabetic retinopathy,
retinopathy of prematurity, macular degeneration, corneal graft rejection,
neovascular glaucoma, retrolental fibroplasia, rubeosis, retinoblastoma,
and uvietis, delayed wound healing, endometriosis, vascluogenesis,
granulations, hypertrophic scars (keloids), nonunion fractures, scleroderma,
trachoma, vascular adhesions, myocardial angiogenesis, coronary collaterals,
cerebral collaterals, arteriovenous malformations, ischemic limb
angiogenesis, Osler-Webber Syndrome, plaque neovascularization,
telangiectasia, hemophiliac joints, angiofibroma fibromuscular dysplasia,
wound
granulation, Crohn's disease, atherosclerosis, birth control agent by
preventing vascularization required for embryo implantation controlling
menstruation, diseases that have angiogenesis as a pathologic consequence such
as cat scratch disease (Rochele minalia quintosa), ulcers
(Helicobacter pylori), Bartonellosis and bacillary angiomatosis.
[0766] In one aspect of the birth control metliod, an amount of the compound
sufficient to block embryo implantation is administered before or
after intercourse and fertilization have occurred, thus providing an effective
method of birth control, possibly a"morning after" metliod. Albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention may also be used in controlling
menstruation or administered as either a peritoneal lavage fluid or for
peritoneal implantation in the treatment of endometriosis.
[0767] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be incorporated
into surgical sutures in order to prevent stitch granulomas.
[0768] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be utilized in a
wide variety of surgical procedures. For example, within one aspect of the
present invention a compositions (in the form of, for example, a spray or
film) may be utilized to coat or spray an area prior to removal of a tumor, in
order to isolate normal surrounding tissues from malignant tissue,
and/or to prevent the spread of disease to surrounding tissues. Within other
aspects of the present invention, compositions (e.g., in the form of a
spray) may be delivered via endoscopic procedures in order to coat tumors, or
inhibit angiogenesis in a desired locale. Within yet other aspects of
the present invention, surgical meshes which have been coated with anti-
angiogenic compositions of the present invention may be utilized in any
procedure wherein a surgical mesh might be utilized. For example, within one
embodiment of the invention a surgical mesh laden with an anti-
angiogenic composition may be utilized during abdominal cancer resection
surgery (e.g., subsequent to colon resection) in order to provide support
to the structure, and to release an amount of the anti-angiogenic factor.
[0769] Within further aspects of the present invention, methods are provided
for treating tumor excision sites, comprising administering
albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention to the resection margins of a
tumor subsequent to excision, such that the local recurrence of cancer and the
formation of new blood vessels at the site is inhibited. Within one
embodiment of the invention, the anti-angiogenic compound is administered
directly to the tumor excision site (e.g., applied by swabbing, brushing
or otherwise coating the resection margins of the tumor with the anti-
angiogenic compound). Alternatively, the anti-angiogenic compounds may be
incorporated into known surgical pastes prior to administration. Within
parNcularly preferred embodiments of the invention, the anti-angiogenic
compounds are applied after hepatic resections for malignancy, and after
neurosurgical operations.
[0770] Within one aspect of the present invention, fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention may be administered to the resection margin of a wide variety of
tumors, including for example, breast, colon, brain and hepatic
tumors. For example, within one embodiment of the invention, anti-angiogenic
compounds may be administered to the site of a neurological tumor
subsequent to excision, such that the formation of new blood vessels at the
site are inhibited.
[0771] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may also be
administered along with other anti-angiogenic factors. Representative examples
of other anti-angiogenic factors include: Anti-Invasive Factor,
117
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
q .u IE ..;' !Lr ;,'.";Ir tf,wit
re tinoic acid an derivatives thereo ,"paclitaxel, Suramin, Tissue Inhibitor
of Metalloproteinase-1, Tissue Inhibitor of Metalloproteinase-2,
Plasminogen Activator Inliibitor-1, Plasminogen Activator Inhibitor-2, and
various forms of the lighter "d group" transition metals.
[0772] Lighter "d group" transition metals include, for example, vanadium,
molybdenum, tungsten, titanium, niobium, and tantalum species.
Such transition metal species may form transition metal complexes. Suitable
complexes of the above-mentioned transition metal species include
oxo transition metal complexes.
[0773] Representative examples of vanadium complexes include oxo vanadium
complexes such as vanadate and vanadyl complexes. Suitable
vanadate complexes include metavanadate and orthovanadate complexes such as,
for example, ammonium metavanadate, sodium metavanadate,
and sodium orthovanadate. Suitable vanadyl complexes include, for example,
vanadyl acetylacetonate and vanadyl sulfate including vanadyl sulfate
hydrates such as vanadyl sulfate mono- and triliydrates.
[0774] Representative examples of tungsten and molybdenum complexes also
include oxo complexes. Suitable oxo tungsten complexes include
tungstate and tungsten oxide complexes. Suitable tungstate complexes include
ammonium tungstate, calcium tungstate, sodium tungstate dihydrate,
and tungstic acid. Suitable tungsten oxides include tungsten (IV) oxide and
tungsten (VI) oxide. Suitable oxo molybdenum complexes include
molybdate, molybdenum oxide, and molybdenyl complexes. Suitable molybdate
complexes include ammonium molybdate and its hydrates, sodium
molybdate and its hydrates, and potassium molybdate and its liydrates.
Suitable molybdenum oxides include molybdenum (VI) oxide, molybdenum
(VI) oxide, and molybdic acid. Suitable molybdenyl complexes include, for
example, molybdenyl acetylacetonate. Other suitable tungsten and
molybdenum complexes include hydroxo derivatives derived from, for example,
glycerol, tartaric acid, and sugars.
[0775] A wide variety of other anti-angiogenic factors may also be utilized
within the context of the present invention. Representative examples
include platelet factor 4; protamine sulphate; sulphated chitin derivatives
(prepared from queen crab shells), (Murata et al., Cancer Res. 51:22-26,
1991); Sulphated Polysaccharide Peptidoglycan Complex (SP- PG) (the function
of this compound may be enhanced by the presence of steroids
such as estrogen, and tamoxifen citrate); Staurosporine; modulators of matrix
metabolism, including for example, proline analogs,
cishydroxyproline, d,L-3,4-dehydroproline, Thiaproline, alpha,alpha-dipyridyl,
aminopropionitrile fumarate; 4-propyl-5-(4-pyridinyl)-2(3H)-
oxazolone; Methotrexate; Mitoxantrone; Heparin; Interferons; 2 Macroglobulin-
serum; ChIMP-3 (Pavloff et al., J. Bio. Chem. 267:17321-17326,
(1992)); Chymostatin (Tomkinson et al., Biochem J. 286:475-480, (1992));
Cyclodextrin Tetradecasulfate; Eponemycin; Camptothecin; Fumagillin
(Ingber et al., Nature 348:555-557, 1990); Gold Sodium Thiomalate ("GST";
Matsubara and Ziff, J. Clin. Invest. 79:1440-1446, (1987));
anticollagenase-serum; alpha2-antiplasmin (Holmes et al., J. Biol. Chem.
262(4):1659-1664, (1987)); Bisantrene (National Cancer Institute);
Lobenzarit disodium (N-(2)-carboxyphenyl-4- chloroanthronilic acid disodium or
"CCA"; Takeuchi et al., Agents Actions 36:312-316, (1992));
Thalidomide; Angostatic steroid; AGM-1470; carboxynaminolmidazole; and
metalloproteinase inhibitors such as BB94.
Diseases at the Cellular Level
[07761 - Diseases associated with increased-cell survival or -the- inhibition
of apoptosis that could -be treated, prevented, -diagnosed, and/or
prognosed using fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, include cancers (such as
follicular lymphomas, carcinomas with p53 mutations, and hormone-dependent
tumors, including, but not limited to colon cancer, cardiac tumors,
pancreatic cancer, melanoma, retinoblastoma, glioblastoma, lung cancer,
intestinal cancer, testicular cancer, stomach cancer, neuroblastoma,
myxoma, myoma, lymphoma, endothelioma, osteoblastoma, osteoclastoma,
osteosarcoma, chondrosarcoma, adenoma, breast cancer, prostate
cancer, Kaposi's sarcoma and ovarian cancer); autoimmune disorders (such as,
multiple sclerosis, Sjogren's syndrome, Hashimoto's thyroiditis,
biliary cirrhosis, Behcet's disease, Crohn's disease, polymyositis, systemic
lupus erythematosus and immune-related glomerulonephritis and
rheumatoid arthritis) and viral infections (such as herpes viruses, pox
viruses and adenoviruses), inflammation, graft v. host disease, acute graft
rejection, and chronic graft rejection.
[0777] In preferred embodiments, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention are
used to inhibit growth, progression, and/or metasis of cancers, in particular
those listed above.
[0778] Additional diseases or conditions associated with increased cell
survival that could be treated or detected by fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention include, but are not limited to, progression, and/or metastases of
malignancies and related disorders such as leukemia (including acute leukemias
(e.g., acute lymphocytic leukemia, acute myelocytic leukemia
(including myeloblastic, promyelocytic, myelomonocytic, monocytic, and
erythroleukemia)) and chronic leukemias (e.g., chronic myelocytic
(granulocytic) leukemia and chronic lymphocytic leukemia)), polycythemia vera,
lymphomas (e.g., Hodgkin's disease and non-Hodgkin's disease),
multiple myeloma, Waldenstrom's macroglobulinemia, heavy chain disease, and
solid tumors including, but not limited to, sarcomas and carcinomas
such as fibrosarcoma, myxosarcoma, liposarcoma, chondrosarcoma, osteogenic
sarcoma, chordoma, angiosarcoma, endotheliosarcoma,
lymphangiosarcoma, lymphangioendotheliosarcoma, synovioma, mesothelioma,
Ewing's tumor, leiomyosarcoma, rhabdomyosarcoma, colon
carcinoma, pancreatic cancer, breast cancer, ovarian cancer, prostate cancer,
squamous cell carcinoma, basal cell carcinoma, adenocarcinoma, sweat
gland carcinoma, sebaceous gland carcinoma, papillary carcinoma, papillary
adenocarcinomas, cystadenocarcinoma, medullary carcinoma,
bronchogenic carcinoma, renal cell carcinoma, hepatoma, bile duct carcinoma,
choriocarcinoma, seminoma, embryonal carcinoma, Wilm's tumor,
cervical cancer, testicular tumor, lung carcinoma, small cell lung carcinoma,
bladder carcinoma, epithelial carcinoma, glioma, astrocytoma,
118
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
medullobla toma~, 1f"craniot'Ti' I) aryngi oma; e'end
p p ymoma, pinealoma, hemangioblastoma, acoustic neuroma, oligodendroglioma,
menangioma,
melanoma, neuroblastoma, and retinoblastoma.
[07791 Diseases associated with increased apoptosis that could be treated,
prevented, diagnosed, and/or prognesed using fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention, include, but are not limited to, AIDS; neurodegenerative
disorders (sucli as Alzheimer's disease, Parkinson's disease, Amyotrophic
lateral sclerosis, Retinitis pigmentosa, Cerebellar degeneration and brain
tumor or prior associated disease); autoimmune disorders (such as, multiple
sclerosis, Sjogren's syndrome, Hashimoto's thyroiditis, biliary cirrliosis,
Behcet's disease, Crohn's disease, polymyositis, systemic lupus erythematosus
and immune-related glomenilonephritis and rheumatoid arthritis)
myelodysplastic syndromes (such as aplastic anemia), graft v. host disease,
ischemic injury (such as that caused by myocardial infarction, stroke and
reperfusion injury), liver injury (e.g., hepatitis related liver injury,
ischemia/reperfusion injury, cholestosis (bile duct injury) and liver cancer);
toxin-
induced liver disease (such as that caused by alcohol), septic shock, cachexia
and anorexia.
Wound Healiug aad Epit/telial Cell Proliferation
[0780] In accordance with yet a further aspect of the present invention, there
is provided a process for utilizing fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the invention, for
therapeutic purposes, for example, to stimulate epithelial cell
proliferation and basal keratinocytes for the purpose of wound healing, and to
stimulate hair follicle production and healing of dermal wounds.
Albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, may be clinically useful in
stimulating wound healing including surgical wounds, excisional wounds, deep
wounds involving damage of the dermis and epidermis, eye tissue
wounds, dental tissue wounds, oral cavity wounds, diabetic ulcers, dennal
ulcers, cubitus ulcers, arterial ulcers, venous stasis ulcers, bums resulting
from heat exposure or chemicals, and other abnormal wound healing conditions
such as uremia, malnutrition, vitamin deficiencies and
complications associated with systemic treatment with steroids, radiation
therapy and antineoplastic drugs and antimetabolites. Albumin fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention, could be used to promote dermal reestablishment
subsequent to dermal loss
[07811 Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could be used to
increase the adherence of skin grafts to a wound bed and to stimulate re-
epithelialization from the wound bed. The following are types of grafts that
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention, could be used to increase adherence to a
wound bed: autografts, artificial skin, allografts, autodermic graft,
autoepdennic grafts, avacular grafts, Blair-Brown grafts, bone graft,
brephoplastic
grafts, cutis graft, delayed graft, dermic graft, epidermic graft, fascia
graft, full thickness graft, heterologous graft, xenograft, homologous graft,
hyperplastic graft, lamellar graft, mesh graft, mucosal graft, Ollier-Thiersch
graft, omenpal graft, patch graft, pedicle graft, penetrating graft, split
skin graft, thick split graft. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, can
be-used-to strength promote-skin and-to improve the appearance of aged skin. -
[0782] It is believed that fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, will also
produce changes in hepatocyte proliferation, and epithelial cell proliferation
in the lung, breast, pancreas, stomach, small intestine, and large
intestine. Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could promote
proliferation of epithelial cells such as sebocytes, hair follicles,
hepatocytes, type II pneumocytes, mucin-producing goblet cells, and other
epithelia]
cells and their progenitors contained within the skin, lung, liver, and
gastrointestinal tract. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, may promote
proliferation of endothelial cells, keratinocytes, and basal
keratinocytes.
[0783] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could also be used to
reduce the side effects of gut toxicity that result from radiation,
chemotherapy treatments or viral infections. Albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention, may have a cytoprotective effect on the small intestine mucosa.
Albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, may also stimulate healing of
mucositis (mouth ulcers) that result from chemotherapy and viral infections.
[0784] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could further be used
in full regeneration of skin in full and partial thickness skin defects,
including burns, (i.e., repopulation of hair follicles, sweat glands, and
sebaceous
glands), treatment of other skin defects such as psoriasis. Albumin fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention, could be used to treat epidermolysis bullosa, a
defect in adherence of the epidermis to the underlying dermis which results
in frequent, open and painful blisters by accelerating reepithelialization of
these lesions. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, could also
be used to treat gastric and doudenal ulcers and help heal by scar
formation of the mucosal lining and regeneration of glandular mucosa and
duodenal mucosal lining more rapidly. Inflammatory bowel diseases,
such as Crohn's disease and ulcerative colitis, are diseases which result in
destruction of the mucosal surface of the small or large intestine,
respectively. Thus, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could be used to
promote the resurfacing of the mucosal surface to aid more rapid healing and
to prevent progression of inflammatory bowel disease. Treatment with
119
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
I~ .;;;v
sion'proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention, is expected to have a significant effect on
the production of mucus througliout the gastrointestinal tract and could be
used to protect the intestinal mucosa from injurious substances that are
ingested or following surgery. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention,
could be used to treat diseases associate with the under expression.
[0785] Moreover, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could be used to
prevent and heal damage to the lungs due to various pathological states.
Albumin fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, which could stimulate proliferation
and differentiation and promote the repair of alveoli and brochiolar
epithelium to prevent or treat acute or chronic lung damage. For example,
emphysema, which results in the progressive loss of aveoli, and
inhalation injuries, i.e., resulting from smoke inhalation and bums, that
cause necrosis of the bronchiolar epitlrelium and alveoli could be effectively
treated using polynucleotides or polypeptides, agonists or antagonists of the
present invention. Also fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, could be
used to stimulate the proliferation of and differentiation of type II
pneumocytes, which may help treat or prevent disease such as hyaline membrane
diseases, such as infant respiratory distress syndrome and
bronchopulmonary displasia, in premature infants.
[0786] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could stimulate the
proliferation and differentiation of hepatocytes and, thus, could be used to
alleviate or treat liver diseases and pathologies such as fulminant liver
failure caused by cirrhosis, liver damage caused by viral hepatitis and toxic
substances (i.e., acetaminophen, carbon tetraholoride and other
hepatotoxins known in the art).
[0787] In addition, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, could be used treat
or prevent the onset of diabetes mellitus. In patients with newly diagnosed
Types I and II diabetes, where some islet cell function remains, fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention, could be used to maintain the islet function so as
to alleviate, delay or prevent permanent manifestation of the disease. Also,
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, could be used as an auxiliary in
islet cell transplantation to improve or promote islet cell function.
Neural Activity m:d Neurological Diseases
[0788] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be used for
the diagnosis and/or treatment of diseases, disorders, damage or injury of the
brain and/or nervous system. Nervous system disorders that can be
treated with the compositions of the invention (e.g., fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of tlie
invention), include, but are not limited to, nervous system injuries, and
diseases or disorders which result in either a disconnection of axons, a
diminution or degeneration of neurons, or demyelination. Nervous system
lesions which may be treated in a patient (including human and non-
human-mammalian patients) according to the methods-of the invention; include-
but-are not-limited-ta,-the following lesions of either the-central--
(including spinal cord, brain) or peripheral nervous systems: (1) ischemic
lesions, in which a lack of oxygen in a portion of the nervous system
results in neuronal injury or death, including cerebral infarction or
ischemia, or spinal cord infarction or ischemia; (2) traumatic lesions,
including
lesions caused by physical injury or associated with surgery, for example,
lesions which sever a portion of the nervous system, or compression
injuries; (3) malignant lesions, in which a portion of the nervous system is
destroyed or injured by malignant tissue which is either a nervous system
associated malignancy or a malignancy derived from non-nervous system tissue;
(4) infectious lesions, in which a portion of the nervous system is
destroyed or injured as a result of infection, for example, by an abscess or
associated with infection by human immunodeficiency virus, herpes
zoster, or herpes simplex virus or with Lyme disease, tuberculosis, or
syphilis; (5) degenerative lesions, in which a portion of the-nervous system
is
destroyed or injured as a result of a degenerative process including but not
limited to, degeneration associated with Parkinson's disease, Alzheimer's
disease, Huntington's chorea, or amyotrophic lateral sclerosis (ALS); (6)
lesions associated with nutritional diseases or disorders, in which a portion
of the nervous system is destroyed or injured by a nutritional disorder or
disorder of metabolism including, but not limited to, vitamin B12
deficiency, folic acid deficiency, Wemicke disease, tobacco-alcohol amblyopia,
Marchiafava-Bignami disease (primary degeneration of the corpus
callosum), and alcoholic cerebellar degeneration; (7) neurological lesions
associated with systemic diseases including, but not limited to, diabetes
(diabetic neuropathy, Bell's palsy), systemic lupus erythematosus, carcinoma,
or sarcoidosis; (8) lesions caused by toxic substances including
alcohol, lead, or particular neurotoxins; and (9) demyelinated lesions in
which a portion of the nervous system is destroyed or injured by a
demyelinating disease including, but not limited to, multiple sclerosis, human
immunodeficiency virus-associated myelopathy, transverse
myelopathy or various etiologies, progressive multifocal leukoencephalopathy,
and central pontine myelinolysis.
[0789] In one embodiment, the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention are used to protect neural cells from the damaging effects of
hypoxia. In a further preferred embodiment, the albumin fusion proteins of
the invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used to protect neural cells from the damaging effects of
cerebral hypoxia. According to this embodiment, the compositions of the
invention are used to treat or prevent neural cell injury associated with
cerebral hypoxia. In one non-exclusive aspect of this embodiment, the albumin
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, are used to treat or prevent neural
cell injury associated with cerebral ischemia. In another non-exclusive
120
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
... ib i. I[ ,r+Aõ1i ,LL q:,dt 111;7c .: " Ii;::. -':itc,.wlr :;ii' ..di,.
aspect of this embodiment, the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
are used to treat or prevent neural cell injury associated with cerebral
infarction.
[0790] In another preferred embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used to treat or prevent neural cell injury associated with
a stroke. In a specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used to treat or prevent cerebral neural cell injury associated
with a stroke.
[0791] In another preferred embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of
the invention are used to treat or prevent neural cell injury associated with
a heart attack. In a specific embodiment, albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention are used to treat or prevent cerebral neural cell injury associated
with a heart attack.
[0792] The compositions of the invention which are useful for treating or
preventing a nervous system disorder may be selected by testing for
biological activity in promoting the survival or differentiation of neurons.
For example, and not by way of limitation, compositions of the invention
which elicit any of the following effects may be useful according to the
invention: (1) increased survival time of neurons in culture either in the
presence or absence of hypoxia or hypoxic conditions; (2) increased sprouting
of neurons in culture or in vivo; (3) increased production of a neuron-
associated molecule in culture or in vivo, e.g., choline acetyltransferase or
acetylcholinesterase with respect to motor neurons; or (4) decreased
symptoms of neuron dysfunction in vivo. Such effects may be measured by any
method known in the art. In preferred, non-limiting embodiments,
increased survival of neurons may routinely be measured using a method set
forth herein or otherwise known in the art, such as, for example, in
Zhang et al., Proc Natl Acad Sci USA 97:3637-42 (2000) or in Arakawa et al.,
J. Neurosci., 10:3507-15 (1990); increased sprouting of neurons may
be detected by methods known in the art, such as, for example, the methods set
forth in Pestronk et al., Exp. Neurol., 70:65-82 (1980), or Brown et
al., Aiin. Rev. Neuroscf., 4:17-42 (1981); increased production of neuron-
associated molecules may be measured by bioassay, enzymatic assay,
antibody binding, Northern blot assay, etc., using techniques known in the art
and depending on the molecule to be measured; and motor neuron
dysfunction may be measured by assessing the physical manifestation of motor
neuron disorder, e.g., weakness, motor neuron conduction velocity,
or functional disability.
[0793] In specific embodiments, motor neuron disorders that may be treated
according to the invention include, but are not limited to, disorders
such as infarction, infection, exposure to toxin, trauma, surgical damage,
degenerative disease or malignancy that may affect motor neurons as well
as other components of the nervous system, as well as disorders that
selectively affect neurons such as amyotrophic lateral sclerosis, and
including,
but not limited to, progressive spinal muscular atrophy, progressive bulbar
palsy, primary lateral sclerosis, infantile and juvenile muscular atrophy,
progressive bulbar paralysis of childhood (Fazio-Londe syndrome),
poliomyelitis and the post polio syndrome, and Hereditary Motorsensory
Neuropathy(Charcot-Marie-Tooth-Disease).
[0794] Further, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may play a role in
neuronal survival; synapse formation; conductance; neural differentiation,
etc. Thus, compositions of the invention (including fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention) may be used to diagnose and/or treat or prevent diseases or
disorders associated with these roles, including, but not limited to, learning
and/or cognition disorders. The compositions of the invention may also
be useful in the treatment or prevention of neurodegenerative disease states
and/or behavioural disorders. Such neurodegenerative disease states
and/or behavioral disorders include, but are not limited to, Alzheimer's
Disease, Parkinson's Disease, Huntington's Disease, Tourette Syndrome,
schizophrenia, mania, dementia, paranoia, obsessive compulsive disorder, panic
disorder, leaming disabilities, ALS, psychoses, autism, and altered
behaviors, including disorders in feeding, sleep patterns, balance, and
perception. In addition, compositions of the invention may also play a role in
the treatment, prevention and/or detection of developmental disorders
associated with the developing embryo, or sexually-linked disorders.
[0795] Additionally, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be useful in
protecting neural cells from diseases, damage, disorders, or injury,
associated with cerebrovascular disorders including, but not limited to,
carotid
artery diseases (e.g., carotid artery thrombosis, carotid stenosis, or
Moyamoya Disease), cerebral amyloid angiopathy, cerebral aneurysm, cerebral
anoxia, cerebral arteriosclerosis, cerebral arteriovenous malformations,
cerebral artery diseases, cerebral embolism and thrombosis (e.g., carotid
artery thrombosis, sinus thrombosis, or Wallenberg's Syndrome), cerebral
hemorrhage (e.g., epidural or subdural hematoma, or subarachnoid
hemorrhage), cerebral infarction, cerebral ischemia (e.g., transient cerebral
ischemia, Subolavian Steal Syndrome, or vertebrobasilar insufficiency),
vascular dementia (e.g., multi-infarct), leukomalacia, periventricular, and
vascular headache (e.g., cluster headache or migraines).
[0796] In accordance with yet a further aspect of the present invention, there
is provided a process for utilizing fusion proteins of the invention
and/or polynucleotides encoding albumin fusion proteins of the invention, for
therapeutic purposes, for example, to stimulate neurological cell
proliferation and/or differentiation. Therefore, fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention may be used to treat and/or detect neurologic diseases. Moreover,
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, can be used as a marker or detector
of a particular nervous system disease or disorder.
[0797] Examples of neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
121
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
a tan ,,.n
It,,.. Il lb u min ".:U t6.A ~I1:I[ ,,; Ii ,:, ::;o ..::;ii
~usion proteins of the invention include, brain diseases, such as metabolic
brain diseases which includes phenylketonuria such as maternal
phenylketonuria, pyruvate carboxylase deficiency, pyruvate dehydrogenase
complex deficiency, Wernicke's Encephalopathy, brain edema, brain
neoplasms such as cerebellar neoplasms which include infratentorial neoplasms,
cerebral ventricle neoplasms such as choroid plexus neoplasms,
hypothalamic neoplasms, supratentorial neoplasms, canavan disease, cerebellar
diseases such as cerebellar ataxia which include spinocerebellar
degeneration such as ataxia telangiectasia, cerebellar dyssynergia,
Friederich's Ataxia, Machado-Joseph Disease, -olivopontocerebellar atrophy,
cerebellar neoplasms such as infratentorial neoplasms, diffuse cerebral
sclerosis such as encephalitis periaxialis, globoid cell leukodystrophy,
metachromatic leukodystropliy and subacute sclerosing panencephalitis.
[0798] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include cerebrovascular disorders
(such as carotid artery diseases which include carotid artery thrombosis,
carotid stenosis and Moyamoya Disease), cerebral amyloid angiopathy, cerebral
aneurysm, cerebral anoxia, cerebral arteriosclerosis, cerebral
arteriovenous malformations, cerebral artery diseases, cerebral embolism and
thrombosis such as carotid artery thrombosis, sinus thrombosis and
Wallenberg's Syndrome, cerebral hemorrliage such as epidural liematoma,
subdural hematoma and subarachnoid hemorrhage, cerebral infarction,
cerebral ischemia sucli as transient cerebral ischemia, Subolavian Steal
Syndrome and vertebrobasilar insufficiency, vascular dementia such as
multi-infaret dementia, periventricular leukomalacia, vascular headache such
as cluster headache and migraine.
[0799] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include dementia such as AIDS
Dementia Complex, presenile dementia such as Alzheimer's Disease and
Creutzfeldt-Jakob Syndrome, senile dementia such as Alzheimer's Disease and
progressive supranuclear palsy, vascular dementia such as multi-
infarct dementia, encephalitis which include encephalitis periaxialis, viral
encephalitis such as epidemic encephalitis, Japanese Encephalitis, St.
Louis Encephalitis, tick-borne encephalitis and West Nile Fever, acute
disseminated encephalomyelitis, meningoencephalitis ~such as
uveomeningoencephalitic syndrome, Postencephalitic Parkinson Disease and
subacute sclerosing panencephalitis, encephalomalacia such as
periventricular leukomalacia, epilepsy such as generalized epilepsy which
includes infantile spasms, absence epilepsy, myoclonic epilepsy which
includes MERRF Syndrome, tonic-clonic epilepsy, partial epilepsy such as
complex partial epilepsy, frontal lobe epilepsy and temporal lobe
epilepsy, post-traumatic epilepsy, status epilepticus such as Epilepsia
Partialis Continua, and Hallervorden-Spatz Syndrome.
[0800] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include hydrocephalus such as Dandy-
Walker Syndrome and normal pressure hydrocephalus, hypothalamic
diseases such as hypothalamic neoplasms, cerebral malaria, narcolepsy which
includes cataplexy, bulbar poliomyelitis, cerebri pseudotumor, Rett
Syndrome, Reye's Syndrome, thalamic diseases, cerebral toxoplasmosis,
intracranial tuberculoma and Zellweger Syndrome, central nervous system
infections such as AIDS Dementia Complex, Brain Abscess, subdural empyema,
encephalomyelitis such as Equine Encephalomyelitis, Venezuelan
Equine Encephalomyelitis, Necrotizing Hemorrhagic Encephalomyelitis,-Visna and
cerebral malaria:
[0801] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include meningitis such as
arachnoiditis, aseptic meningtitis such as viral meningtitis which includes
lymphocytic choriomeningitis, Bacterial meningtitis which includes Haemophilus
Meningtitis, Listeria Meningtitis, Meningococcal Meningtitis such
as Waterhouse-Friderichsen Syndrome, Pneumococcal Meningtitis and meningeal
tuberculosis, fungal meningitis such as Cryptococcal Meningtitis,
subdural effusion, meningoencephalitis such as uvemeningoencephalitic
syndrome, myelitis such as transverse myelitis, neurosyphilis such as tabes
dorsalis, poliomyelitis which includes bulbar poliomyelitis and
postpoliomyelitis syndrome, prion diseases (such as Creutzfeldt-Jakob
Syndrome,
Bovine Spongiform Encephalopathy, Gerstmann-Straussler Syndrome, Kuru,
Scrapie), and cerebral toxoplasmosis.
[0802] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include central nervous system
neoplasms such as brain neoplasms that include cerebellar neoplasms such
as infratentorial neoplasms, cerebral ventricle neoplasms such as choroid
plexus neoplasms, hypothalamic neoplasms and supratentorial neoplasms,
meningeal neoplasms, spinal cord neoplasms which include epidural neoplasms,
demyelinating diseases such as Canavan Diseases, diffuse cerebral
sceloris which includes adrenoleukodystrophy, encephalitis periaxialis,
globoid cell leukodystrophy, diffuse cerebral sclerosis such as metachromatic
leukodystrophy, allergic encephalomyelitis, necrotizing hemorrhagic
encephalomyelitis, progressive multifocal leukoencephalopathy, multiple
sclerosis, central pontine myelinolysis, transverse myelitis, neuromyelitis
optica, Scrapie, Swayback, Chronic Fatigue Syndrome, Visna, High
Pressure Nervous Syndrome, Meningism, spinal cord diseases such as amyotonia
congenita, amyotrophic lateral sclerosis, spinal muscular atrophy
such as Werdnig-Hoffinann Disease, spinal cord compression, spinal cord
neoplasms such as epidural neoplasms, syringomyelia, Tabes Dorsalis,
Stiff-Man Syndrome, mental retardation such as Angelman Syndrome, Cri-du-Chat
Syndrome, De Lange's Syndrome, Down Syndrome,
Gangliosidoses such as gangliosidoses G(M1), Sandhoff Disease, Tay-Sachs
Disease, Hartnup Disease, homocystinuria, Laurence-Moon- Biedl
Syndrome, Lesch-Nyhan Syndrome, Maple Syrup Urine Disease, mucolipidosis such
as fucosidosis, neuronal ceroid-lipofuscinosis,
oculocerebrorenal syndrome, phenylketonuria such as maternal phenylketonuria,
Prader-Willi Syndrome, Rett Syndrome, Rubinstein-Taybi
Syndrome, Tuberous Sclerosis, WAGR Syndrome, nervous system abnormalities such
as holoprosencephaly, neural tube defects such as
anencephaly which includes hydrangencephaly, Arnold-Chairi Deformity,
encephalocele, meningocele, meningomyelocele, spinal dysraphism such
122
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~t,,, = 11,.w It li.H .. z n, u n;,u r= u-=:,: ';ilo :=11 :,~n , ~1.,
as spina iYi a cystica and spina bifida occulta.
[0803] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include hereditary motor and sensory
neuropathies which include Charcot-Marie Disease, Hereditary optic
atrophy, Refsum's Disease, hereditary spastic paraplegia, Werdnig-Hoffinann
Disease, Hereditary Sensory and Autonomic Neuropathies such as
Congenital Analgesia and Familial Dysautonomia, Neurologic manifestations
(such as agnosia that include Gerstmann's Syndrome, Amnesia such as
retrograde amnesia, apraxia, neurogenic bladder, cataplexy, communicative
disorders such as hearing disorders that includes deafness, partial
hearing loss, loudness recruitment and tinnitus, language disorders such as
aphasia which include agraphia, anomia, broca aphasia, and Wernicke
Aphasia, Dyslexia such as Acquired Dyslexia, language development disorders,
speech disorders such as aphasia which includes anomia, broca
aphasia and Wernicke Aphasia, articulation disorders, communicative disorders
such as speech disorders which include dysarthria, echolalia,
mutism and stuttering, voice disorders such as aphonia and hoarseness,
decerebrate state, delirium, fasciculation, hallucinations, meningism,
movement disorders such as angelman syndrome, ataxia, athetosis, chorea,
dystonia, hypokinesia, muscle hypotonia, myoclonus, tic, torticollis and
tremor, muscle hypertonia such as muscle rigidity such as stiff-man syndrome,
muscle spasticity, paralysis such as facial paralysis which includes
Herpes Zoster Oticus, Gastroparesis, Hemiplegia, ophthalmoplegia such as
diplopia, Duane's Syndrome, Homer's Syndrome, Chronic progressive
external ophthalmoplegia such as Kearns Syndrome, Bulbar Paralysis, Tropical
Spastic Paraparesis, Paraplegia such as Brown-Sequard Syndrome,
quadriplegia, respiratory paralysis and vocal cord paralysis, paresis, phantom
limb, taste disorders such as ageusia and dysgeusia, vision disorders
such as amblyopia, blindness, color vision defects, diplopia, hemianopsia,
scotoma and subnormal vision, sleep disorders such as hypersomnia
which includes Kleine-Levin Syndrome, insomnia, and somnambulism, spasm such
as trismus, unconsciousness such as coma, persistent vegetative
state and syncope and vertigo, neuromuscular diseases such as amyotonia
congenita, amyotrophic lateral sclerosis, Lambert-Eaton Myastlienic
Syndrome, motor neuron disease, muscular atropliy such as spinal muscular
atrophy, Charcot-Marie Disease and Werdnig-Hoffinann Disease,
Postpoliomyelitis Syndrome, Muscular Dystrophy, Myasthenia Gravis, Myotonia
Atrophica, Myotonia Confenita, Nemaline Myopathy, Familial
Periodic Paralysis, Multiplex Paramyloclonus, Tropical Spastic Paraparesis and
Stiff-Man Syndrome, peripheral nervous system diseases such as
acrodynia, amyloid neuropathies, autonomic nervous system diseases such as
Adie's Syndrome, Barre-Lieou Syndrome, Familial Dysautonomia,
Homer's Syndrome, Reflex Sympathetic Dystrophy and Shy-Drager Syndrome,
Cranial Nerve Diseases such as Acoustic Nerve Diseases such as
Acoustic Neuroma which includes Neurofibromatosis 2, Facial Nerve Diseases
such as Facial Neuralgia,Melkersson-Rosenthal Syndrome, ocular
motility disorders which includes amblyopia, nystagmus, oculomotor nerve
paralysis, ophthalmoplegia such as Duane's Syndrome, Homer's
Syndrome, Chronic Progressive External Ophthalmoplegia which includes Kearns
Syndrome, Strabismus such as Esotropia and Exotropia,
Oculomotor Nerve Paralysis, Optic Nerve Diseases such as Optic Atrophy which
includes Hereditary Optic Atrophy, Optic Disk Drusen, Optic
Neuritis such as Neuromyelitis Optica, Papilledema, Trigeminal Neuralgia,
Vocal Cord Paralysis, Demyelinating Diseases such as Neuromyelitis
Optica and Swayback and Diabetic neuropathies such as diabetic-foot. -
[0804] Additional neurologic diseases which can be treated or detected with
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention include nerve compression syndromes
such as carpal tunnel syndrome, tarsal tunnel syndrome, thoracic
outlet syndrome such as cervical rib syndrome, ulnar nerve compression
syndrome, neuralgia such as causalgia, cervico-brachial neuralgia, facial
neuralgia and trigeminal neuralgia, neuritis such as experimental allergic
neuritis, optic neuritis, polyneuritis, polyradiculoneuritis and radiculities
such as polyradiculitis, hereditary motor and sensory neuropathies such as
Charcot-Marie Disease, Hereditary Optic Atrophy, Refsum's Disease,
Hereditary Spastic Paraplegia and Werdnig-Hoffrnann Disease, Hereditary
Sensory and Autonomic Neuropathies which include Congenital
Analgesia and Familial Dysautonomia, POEMS Syndrome, Sciatica, Gustatory
Sweating and Tetany).
Endocrine Disorders
[0805] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be used to treat,
prevent, diagnose, and/or prognose disorders and/or diseases related to
hormone imbalance, and/or disorders or diseases of the endocrine system.
[0806] Hormones secreted by the glands of the endocrine system control
physical growth, sexual function, metabolism, and other functions.
Disorders may be classified in two ways: disturbances in the production of
hormones, and the inability of tissues to respond to hormones. The
etiology of these hormone imbalance or endocrine system diseases, disorders or
conditions may be genetic, somatic, such as cancer and some
autoimmune diseases, acquired (e.g., by chemotherapy, injury or toxins), or
infectious. Moreover, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention can be used
as a marker or detector of a particular disease or disorder related to
the endocrine system and/or hormone imbalance.
[0807] Endocrine system and/or hormone imbalance and/or diseases encompass
disorders of uterine motility including, but not limited to:
complications with pregnancy and labor (e.g., pre-term labor, post-term
pregnancy, spontaneous abortion, and slow or stopped labor); and disorders
and/or diseases of the menstrual cycle (e.g., dysmenorrhea and endometriosis).
[0808] Endocrine system and/or hormone imbalance disorders and/or diseases
include disorders and/or diseases of the pancreas, such as, for
example, diabetes mellitus, diabetes insipidus, congenital pancreatic
agenesis, pheochromocytoma--islet cell tumor syndrome; disorders and/or
diseases of the adrenal glands such as, for example, Addison's Disease,
corticosteroid deficiency, virilizing disease, hirsutism, Cushing's Syndrome,
123
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
tt ,. II. , lt u - 11.,,P ..;,,n n,.,u nn",: ':cin i; :;a' .,,6
hyperaldosteronism, pheochromocytoma; disorders and/or diseases of the
pituitary gland, such as, for example, hyperpituitarism, hypopituitarism,
pituitary dwarfism, pituitary adenoma, panhypopituitarism, acromegaly,
gigantism; disorders and/or diseases of the thyroid, including but not
limited to, hyperthyroidism, hypothyroidism, Plummer's disease, Graves'
disease (toxic diffuse goiter), toxic nodular goiter, thyroiditis (Hashimoto's
thyroiditis, subacute granulomatous thyroiditis, and silent lymphocytic
thyroiditis), Pendred's syndrome, myxedema, cretinism, thyrotoxicosis,
thyroid hormone coupling defect, thymic aplasia, IHurthle cell tumours of the
thyroid, thyroid cancer, thyroid carcinoma, Medullary thyroid
carcinoma; disorders and/or diseases of the parathyroid, such as, for example,
hyperparathyroidism, hypoparathyroidism; disorders and/or diseases
of the hypothalamus.
[0809] In addition, endocrine system and/or hormone imbalance disorders and/or
diseases may also include disorders and/or diseases of the
testes or ovaries, including cancer. Other disorders and/or diseases of the
testes or ovaries further include, for example, ovarian cancer, polycystic
ovary syndrome, Klinefelter's syndrome, vanishing testes syndrome (bilateral
anorchia), congenital absence of Leydig's cells, cryptorchidism,
Noonan's syndrome, myotonic dystrophy, capillary haemangioma of the testis
(benign), neoplasias of the testis and neo-testis.
[0810] Moreover, endocrine system and/or hormone imbalance disorders and/or
diseases may also include disorders and/or diseases such as, for
example, polyglandular deficiency syndromes, pheochromocytoma, neuroblastoma,
multiple Endocrine neoplasia, and disorders and/or cancers of
endocrine tissues.
[0811] In another embodiment, albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the
invention, may be used to diagnose, prognose, prevent, and/or treat endocrine
diseases and/or disorders associated with the tissue(s) in which the
Therapeutic protein corresponding to the Therapeutic protein portion of the
albumin protein of the invention is expressed,
Reproductive Systen: Disorders
[0812] The albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may be used for
the diagnosis, treatment, or prevention of diseases and/or disorders of the
reproductive system. Reproductive system disorders that can be treated by
the compositions of the invention, include, but are not limited to,
reproductive system injuries, infections, neoplastic disorders, congenital
defects,
and diseases or disorders wliich result in infertility, complications with
pregnancy, labor, or parturition, and postpartum difficulties.
[0813] Reproductive system disorders and/or diseases include diseases and/or
disorders of the testes, including testicular atrophy, testicular
feminization, cryptorchism (unilateral and bilateral), anorchia, ectopic
testis, epididymitis and orchitis (typically resulting from infections such
as,
for example, gonorrhea, mumps, tuberculosis, and syphilis), testicular
torsion, vasitis nodosa, germ cell tumors (e.g., seminomas, embryonal cell
carcinomas, teratocarcinomas, choriocarcinomas, yolk sac tumors, and
teratomas), stromal tumors (e.g., Leydig cell tumors), hydrocele, hematocele,
varicocele, spermatocele, inguinal hernia, and disorders of sperm production
(e.g., immotile cilia syndrome, aspermia, asthenozoospermia,
azoospermia, oligospermia, and teratozoospermia).
[0814] Reproductive-system disorders also-include disorders-ofthe prostate
gland, such as acute nori-bacterial prostatitis,"chronic non-bacterial-
prostatitis, acute bacterial prostatitis, chronic bacterial prostatitis,
prostatodystonia, prostatosis, granulomatous prostatitis, malacoplakia, benign
prostatic hypertrophy or hyperplasia, and prostate neoplastic disorders,
including adenocaroinomas, transitional cell carcinomas, ductal carcinomas,
and squamous cell carcinomas.
[08151 Additionally, the compositions of the invention may be useful in the
diagnosis, treatment, and/or prevention of disorders or diseases of
the penis and urethra, including inflammatory disorders, such as
balanoposthitis, balanitis xerotica obliterans, phimosis, paraphimosis,
syphilis,
herpes simplex virus, gonorrhea, non-gonococcal urethritis, chlamydia,
mycoplasma, trichomonas, HIV, AIDS, Reiter's syndrome, condyloma
acuminatum, condyloma latum, and pearly penile papules; urethral
abnormalities, such as hypospadias, epispadias, and phimosis; premalignant
lesions, including Erythroplasia of Queyrat, Bowen's disease, Bowenoid
paplosis, giant condyloma of Buscke-Lowenstein, and varrucous
carcinoma; penile cancers, including squamous cell carcinomas, carcinoma in
situ, verrucous carcinoma, and disseminated penile carcinoma;
urethral neoplastic disorders, including penile urethral carcinoma,
bulbomembranous urethral carcinoma, and prostatic urethral carcinoma; and
erectile disorders, such as priapism, Peyronie's disease, erectile
dysfunction, and impotence.
[0816] Moreover, diseases and/or disorders of the vas deferens include
vasculititis and CBAVD (congenital bilateral absence of the vas
deferens); additionally, the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may
be used in the diagnosis, treatment, and/or prevention of diseases and/or
disorders of the seminal vesicles, including hydatid disease, congenital
chloride diarrhea, and polycystic kidney disease.
[0817] Other disorders and/or diseases of the male reproductive system
include, for example, Klinefelter's syndrome, Young's syndrome,
premature ejaculation, diabetes mellitus, cystic fibrosis, Kartagener's
syndrome, high fever, multiple sclerosis, and gynecomastia.
[0818] Further, the polynucleotides, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
may be used in the diagnosis, treatment, and/or prevention of diseases and/or
disorders of the vagina and vulva, including bacterial vaginosis,
candida vaginitis, herpes simplex virus, chancroid, granuloma inguinale,
lymphogranuloma venereum, scabies, human papillomavirus, vaginal
trauma, vulvar trauma, adenosis, chlamydia vaginitis, gonorrhea, trichomonas
vaginitis, condyloma acuminatum, syphilis, molluscum contagiosum,
atrophic vaginitis, Paget's disease, lichen sclerosus, lichen planus,
vulvodynia, toxic shock syndrome, vaginismus, vulvovaginitis, vulvar
vestibulitis,
124
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~~= ~1õ,n (~ ~ ~1,,~~ :, =;~t 1~ va 11.~a ..'~ 1~~ . .: 11 ,,;::1t M~~I
,,.~d,.
anc~neop ast~c dI sorclers, suc1i as squamous cell hyperplasia, clear cell
carcinoma, basal cell carcinoma, melanomas, cancer of Bartholin's gland, and
vulvar intraepithelial neoplasia.
[0819] Disorders and/or diseases of the uterus include dysmenorrhea,
retroverted uterus, endometriosis, fibroids, adenomyosis, anovulatory
bleeding, amenorrhea, Cushing's syndrome, hydatidiform moles, Asherman's
syndrome, premature menopause, precocious puberty, uterine polyps,
dysfunctional uterine bleeding (e.g., due to aberrant hormonal signals), and
neoplastic disorders, such as adenocarcinomas, keiomyosarcomas, and
sarcomas. Additionally, the albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may
be useful as a marker or detector of, as well as in the diagnosis, treatment,
and/or prevention of congenital uterine abnormalities, such as bicornuate
uterus, septate uterus, simple unicornuate uterus, unicomuate uterus with a
noncavitary rudimentary horn, unicomuate uterus with a non-
communicating cavitary rudimentary horn, unicomuate uterus with a
communicating cavitary horn, arcuate uterus, uterine didelfus, and T-shaped
uterus.
[0820] Ovarian diseases and/or disorders include anovulation, polycystic ovary
syndrome (Stein-Leventhal syndrome), ovarian cysts, ovarian
hypofunction, ovarian insensitivity to gonadotropins, ovarian overproduction
of androgens, right ovarian vein syndrome, amenorrhea, hirutism, and
ovarian cancer (including, but not limited to, primary and secondary cancerous
growth, Sertoli-Leydig tumors, endometriod carcinoma of the ovary,
ovarian papillary serous adenocarcinoma, ovarian mucinous adenocarcinoma, and
Ovarian Krukenberg tumors).
[0821] Cervical diseases and/or disorders include cervicitis, chronic
cervicitis, mucopurulent cervicitis, cervical dysplasia, cervical polyps,
Nabothian cysts, cervical erosion, cervical incompetence, and cervical
neoplasms (including, for example, cervical carcinoma, squamous metaplasia,
squamous cell carcinoma, adenosquamous cell neoplasia, and columnar cell
neoplasia).
[0822] Additionally, diseases and/or disorders of the reproductive system
include disorders and/or diseases of pregnancy, including miscarriage
and stillbirth, such as early abortion, late abortion, spontaneous abortion,
induced abortion, therapeutic abortion, threatened abortion, missed
abortion, incomplete abortion, complete abortion, habitual abortion, missed
abortion, and septic abortion; ectopic pregnancy, anemia, Rh
incompatibility, vaginal bleeding during pregnancy, gestational diabetes,
intrauterine growth retardation, polyhydramnios, HELLP syndrome,
abruptio placentae, placenta previa, hyperemesis, preeclampsia, eclampsia,
herpes gestationis, and urticaria of pregnancy. Additionally, the albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention may be used in the diagnosis, treatment,
and/or prevention of diseases that can complicate pregnancy, including heart
disease, heart failure, rheumatic heart disease, congenital heart disease,
mitral valve prolapse, high blood pressure, anemia, kidney disease, infectious
disease (e.g., rubella, cytomegalovirus, toxoplasmosis, infectious
hepatitis, chlamydia, HIV, AIDS, and genital herpes), diabetes mellitus,
Graves' disease, thyroiditis, hypothyroidism, Hashimoto's thyroiditis,
chronic active hepatitis, cirrhosis of the liver, primary biliary cirrhosis,
asthma, systemic lupus eryematosis, rheumatoid arthritis, myasthenia gravis,
idiopathic thrombocytopenic purpura, appendicitis, ovarian cysts, gallbladder
disorders,and obstruction of the intestine.
[0823] Complications associated with labor and parturition include-premature
rupture of the membranes, -pre-term labor-a post-term pregnancy, -
postmaturity, labor that progresses too slowly, fetal distress (e.g., abnormal
heart rate (fetal or matemal), breathing problems, and abnormal fetal
position), shoulder dystocia, prolapsed umbilical cord, amniotic fluid
embolism, and aberrant uterine bleeding.
[0824] Further, diseases and/or disorders of the postdelivery period,
including endometritis, myometritis, parametritis, peritonitis, pelvic
thrombophlebitis, pulmonary embolism, endotoxemia, pyelonephritis, saphenous
thrombophlebitis, mastitis, cystitis, postpartum hemorrhage, and
inverted uterus.
[0825] Other disorders and/or diseases of the female reproductive system that
may be diagnosed, treated, and/or prevented by the albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention include, for example, Turner's syndrome,
pseudohermaphroditism, premenstrual syndrome, pelvic inflammatory disease,
pelvic congestion (vascular engorgement), frigidity, anorgasmia,
dyspareunia, ruptured fallopian tube, and Mittelschmerz.
Infectious Disease
[0826] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention can be used to treat or
detect infectious agents. For example, by increasing the immune response,
particularly increasing the proliferation and differentiation of B and/or T
cells, infectious diseases may be treated. The immune response may be
increased by either enhancing an existing immune response, or by initiating
a new immune response. Alternatively, fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
may also directly inhibit the infectious agent, without necessarily eliciting
an immune response.
[0827] Viruses are one example of an infectious agent that can cause disease
or symptoms that can be treated or detected by albumin fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention. Examples of viruses, include, but are not limited
to Examples of viruses, include, but are not limited to the following DNA and
RNA viruses and viral families: Arbovirus, Adenoviridae,
Arenaviridae, Arterivirus, Bimaviridae, Bunyaviridae, Caliciviridae,
Circoviridae, Coronaviridae, Dengue, EBV, HIV, Flaviviridae, Hepadnaviridae
(Hepatitis), Herpesviridae (such as, Cytomegalovirus, Herpes Simplex, Herpes
Zoster), Mononegavirus (e.g., Paramyxoviridae, Morbillivirus,
Rhabdoviridae), Orthomyxoviridae (e.g., Influenza A, Influenza B, and
parainfluenza), Papiloma virus, Papovaviridae, Parvoviridae, Picornaviridae,
Poxviridae (such as Smallpox or Vaccinia), Reoviridae (e.g., Rotavirus),
Retroviridae (HTLV-I, HTLV-Il, Lentivirus), and Togaviridae (e.g.,
125
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Rubiviru's~k. Vuusesi~alling iwitliin theselamilies can cause a variety of
diseases or symptoms, including, but not limited to: arthritis,
bronchiollitis,
respiratory syncytial virus, encephalitis, eye infections (e.g.,
conjunctivitis, keratitis), cltronic fatigue syndrome, hepatitis (A, B, C, E,
Chronic
Active, Delta), Japanese B encephalitis, Junin, Chikungunya, Rift Valley
fever, yellow fever, meningitis, opportunistic infections (e.g., AIDS),
pneumonia, Burkitt's Lyniphoma, chickenpox, heniorrhagic fever, Measles,
Mumps, Parainfluenza, Rabies, the common cold, Polio, leukemia,
Rubella, sexually transmitted diseases, skin diseases (e.g., Kaposi's, warts),
and viremia. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, can be used
to treat or detect any of these symptoms or diseases. In specific
embodiments, fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention are used to treat:
meningitis, Dengue, EBV, and/or hepatitis (e.g., hepatitis B). In an
additional specific embodiment fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention are used to
treat patients nonresponsive to one or more other commercially
available hepatitis vaccines. In a further specific embodiment fusion proteins
of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention are used to treat AIDS.
[0828] Similarly, bacterial and fungal agents that can cause disease or
symptoms and that can be treated or detected by albumin fusion proteins
of the invention and/or polynucleotides encoding albumin fusion proteins of
the invention include, but not limited to, the following Gram-Negative
and Gram-positive bacteria, bacterial families, and fungi: Actinomyces (e.g.,
Norcardia), Acinetobacter, 03Ptococcus neoforinans, Aspergillus,
Bacillaceae (e.g., Bacillus antla-asis), Bacteroides (e.g., Bacteroides
fi=agilis), Blastomycosis, Bordetella, Borrelia (e.g., Borrelia burgdoiferi),
Brucella, Candidia, Campylobacter, Chlamydia, Clostridium (e.g., Clostridium
botulinum, Clostridiznn difrcile, Clostridium perfringens,
Clostridiurn tetani), Coccidioides, Corynebacterium (e.g., Cwynebacterium
dipthertae), Cryptococcus; Dermatocycoses, E. coli (e.g.,
Enterotoxigenic E. coli and Enterohemorrhagic E. coli), Enterobacter (e.g.
Enterobacter aerogenes), Enterobacteriaceae (Klebsiella, Salmonella
(e.g., Sabnonella ryphi, Salmonella enteritidis, Salmonella typhi), Serratia,
Yersinia, Shigella), Erysipelothrix, Haemophilus (e.g., Haemophilus
infuenza type B), Helicobacter, Legionella (e.g., Legionella pneumophila),
Leptospira, Listeria (e.g., Listeria monocylogenes), Mycoplasma,
Mycobacterium (e.g., Mycobacterium leprae and Mycobacteriunt tuberculosis),
Vibrio (e.g., Vibrio cholerae), Neisseriaceae (e.g., Neisseria
gonorrhea, Neisseria rneningitidis), Pasteurellacea, Proteus, Pseudomonas
(e.g., Pseudomonas aeruginosa), Rickettsiaceae, Spirochetes (e.g.,
Treponema spp., Leptospira spp., Borrelia spp.), Shigella spp., Staphylococcus
(e.g., Staphylococcus am-eus), Meningiococcus, Pneumococcus and
Streptococcus (e.g., Streptococcus pneuinoniae and Groups A, B, and C
Streptococci), and Ureaplasmas. These bacterial, parasitic, and fungal
families can cause diseases or symptoms, including, but not limited to:
antibiotic-resistant infections, bacteremia, endocarditis, septicemia, eye
infections (e.g., conjunctivitis), uveitis, tuberculosis, gingivitis,
bacterial diarrhea, opportunistic infections (e.g., AIDS related infections),
paronychia, prostliesis-related infections, dental caries, Reiter's Disease,
respiratory tract infections, such as Whooping Cough or Empyema, sepsis,
Lyme Disease, Cat-Scratch Disease, dysentery, paratyphoid fever, food
poisoning, Legionella disease, chronic and acute inflammation, erythema,
yeast infections, typhoid, -pneumonia, gonorrhea, meningitis (e.g., mengitis
types- A-and B), chlamydia, syphillis, diphtheria, leprosy; brucellosis, -
peptic ulcers, anthrax, spontaneous abortions, birth defects, pneumonia, lung
infections, ear infections, deafness, blindness, lethargy, malaise,
vomiting, chronic diarrliea, Crohn's disease, colitis, vaginosis, sterility,
pelvic inflammatory diseases, candidiasis, paratuberculosis, tuberculosis,
lupus, botulism, gangrene, tetanus, impetigo, Rheumatic Fever, Scarlet Fever,
sexually transmitted diseases, skin diseases (e.g., cellulitis,
dennatocycoses), toxemia, urinary tract infections, wound infections,
noscomial infections. Albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention, can be used
to treat or detect any of these symptoms or diseases. In specific
embodiments, fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention are used to treat: tetanus,
diptheria, botulism, and/or meningitis type B.
[0829] Moreover, parasitic agents causing disease or symptoms that can be
treated, prevented, and/or diagnosed by fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention include, but not limited to, the following families or class:
Amebiasis, Babesiosis, Coccidiosis, Cryptosporidiosis, Dientamoebiasis,
Dourine, Ectoparasitic, Giardias, Helminthiasis, Leishmaniasis,
Schistisoma, Theileriasis, Toxoplasmosis, Trypanosomiasis, and Trichomonas and
Sporozoans (e.g., Plasmodittni virax, Plasn:odium falciparium,
Plasmodhnn n:alariae and Plasmodimn ovale). These parasites can cause a
variety of diseases or symptoms, including, but not limited to: Scabies,
Trombiculiasis, eye infections, intestinal disease (e.g., dysentery,
giardiasis), liver disease, lung disease, opportunistic infections (e.g., AIDS
related), malaria, pregnancy complications, and toxoplasmosis. Albumin fusion
proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention, can be used to treat, prevent, and/or
diagnose any of these symptoms or diseases. In specific embodiments, fusion
proteins of the invention and/or polynucleotides encoding albumin fusion
proteins of the invention are used to treat, prevent, and/or diagnose
malaria.
[0830] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention could either be by
administering an effective amount of an albumin fusion protein of the
invention to the patient, or by removing cells from the patient, supplying the
cells with a polynucleotide of the present invention, and returning the
engineered cells to the patient (ex vivo therapy). Moreover, the albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention can be used as an antigen in a vaccine to
raise an inunune response against infectious disease.
126
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
II. .r 11_1t 4N11 rakou IC:a(,r 11:.:. ":nll Z11 ":HII .,.lE.
[0831] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention can be used to
differentiate, proliferate, and attract cells, leading to the regeneration of
tissues. (See, Science 276:59-87 (1997)). The regeneration of tissues could
be used to repair, replace, or protect tissue damaged by congenital defects,
trauma (wounds, burns, incisions, or ulcers), age, disease (e.g.
osteoporosis, osteocarthritis, periodontal disease, liver failure), surgery,
including cosmetic plastic surgery, fibrosis, reperfusion injury, or systemic
cytokine damage.
[0832] Tissues that could be regenerated using the present invention include
organs (e.g., pancreas, liver, intestine, kidney, skin, endothelium),
muscle (smooth, skeletal or cardiac), vasculature (including vascular and
lymphatics), nervous, hematopoietic, and skeletal (bone, cartilage, tendon,
and ligament) tissue. Preferably, regeneration occurs without or decreased
scarring. Regeneration also may include angiogenesis.
[0833] Moreover, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may increase
regeneration of tissues difficult to heal. For example, increased
tendon/ligament regeneration would quicken recovery time after damage. Albumin
fusion proteins of the invention and/or polynucleotides encoding albumin
fusion proteins of the invention could also be used prophylactically in an
effort to avoid damage. Specific diseases that could be treated include of
tendinitis, carpal tunnel syndrome, and other tendon or ligament defects.
A further example of tissue regeneration of non-healing wounds includes
pressure ulcers, ulcers associated with vascular insufficiency, surgical, and
traumatic wounds.
[0834] Similarly, nerve and brain tissue could also be regenerated by using
fusion proteins of the invention and/or polynucleotides encoding
albumin fusion proteins of the invention, to proliferate and differentiate
nerve cells. Diseases that could be treated using this method include central
and peripheral nervous system diseases, neuropathies, or mechanical and
traumatic disorders (e.g., spinal cord disorders, head trauma,
cerebrovascular disease, and stoke). Specifically, diseases associated with
peripheral nerve injuries, peripheral neuropathy (e.g., resulting from
chemotherapy or other medical therapies), localized neuropathies, and central
nervous system diseases (e.g., Alzheimer's disease, Parkinson's
disease, Huntington's disease, amyotrophic lateral sclerosis, and Shy-Drager
syndrome), could all be treated using the albumin fusion proteins of the
invention and/or polynucleotides encoding albumin fusion proteins of the
invention.
Gastrointestiital Disorders
[0835] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention, may be used to treat,
prevent, diagnose, and/or prognose gastrointestinal disorders, including
inflammatory diseases and/or conditions, infections, cancers (e.g., intestinal
neoplasms (carcinoid tumor of the small intestine, non-Hodgkin's lymphoma of
the small intestine, small bowl lymphoma)), and ulcers, such as
peptic ulcers.
[0836] Gastrointestinal disorders include dysphagia, odynophagia, inflammation
of the esophagus, peptic esophagitis, gastric reflux, submucosal
fibrosis and- -stricturing, -Mallory-Weiss- lesions, - leiomyomas, - lipomas,
epiderrnal-- cancers, --adeoncarcinomas, gastric- retention - disorders;-
gastroenteritis, gastric atrophy, gastric/stomach cancers, polyps of the
stomach, autoimmune disorders such as pernicious anemia, pyloric stenosis,
gastritis (bacterial, viral, eosinophilic, stress-induced, chronic erosive,
atrophic, plasma cell, and Menetrier's), and peritoneal diseases (e.g.,
chyloperioneum, hemoperitoneum, mesenteric cyst, mesenteric lymphadenitis,
mesenteric vascular occlusion, panniculitis, neoplasms, peritonitis,
pneumoperitoneum, bubphrenic abscess,).
[0837] Gastrointestinal disorders also include disorders associated with the
small intestine, such as malabsorption syndromes, distension,
irritable bowel syndrome, sugar intolerance, celiac disease, duodenal ulcers,
duodenitis, tropical sprue, Whipple's disease, intestinal
lymphangiectasia, Crohn's disease, appendicitis, obstructions of the ileum,
Meckel's diverticulum, multiple diverticula, failure of complete rotation
of the small and large intestine, lymphoma, and bacterial and parasitic
diseases (such as Traveler's diarrhea, typhoid and paratyphoid, cholera,
infection by Roundworms (Ascariasis luntbricoides), Hookworms (Ancylostoma
duodenale), Threadworms (Enterobius verinicnlaris), Tapeworms
(Taenia saginata, Echinococcus granulosus, Diphyllobothrium spp., and T.
solium).
[0838] Liver diseases and/or disorders include intraliepatic cholestasis
(alagille syndrome, biliary liver cirrhosis), fatty liver (alcoholic fatty
liver,
reye syndrome), hepatic vein thrombosis, hepatolentricular degeneration,
hepatomegaly, hepatopulmonary syndrome, hepatorenal syndrome, portal
hypertension (esophageal and gastric varices), liver abscess (amebic liver
abscess), liver cirrhosis (alcoholic, biliary and experimental), alcoholic
liver diseases (fatty liver, hepatitis, cirrhosis), parasitic (hepatic
echinococcosis, fascioliasis, amebic liver abscess), jaundice (hemolytic,
hepatocellular, and cholestatic), cholestasis, portal hypertension, liver
enlargement, ascites, hepatitis (alcoholic hepatitis, animal hepatitis,
chronic
hepatitis (autoimmune, hepatitis B, hepatitis C, hepatitis D, drug induced),
toxic hepatitis, viral human hepatitis (hepatitis A, hepatitis B, hepatitis
C, hepatitis D, hepatitis E), Wilson's disease, granulomatous hepatitis,
secondary biliary cirrhosis, hepatic encephalopathy, portal hypertension,
varices, hepatic encephalopathy, primary biliary cirrhosis, primary sclerosing
cholangitis, hepatocellular adenoma, hemangiomas, bile stones, liver
failure (hepatic encephalopathy, acute liver failure), and liver neoplasms
(angiomyolipoma, calcified liver metastases, cystic liver metastases,
epithelial tumors, fibrolamellar hepatocarcinoma, focal nodular hyperplasia,
hepatic adenoma, hepatobiliary cystadenoma, hepatoblastoma,
hepatocellular carcinoma, hepatoma, liver cancer, liver hemangioendothelioma,
mesenchymal hamartoma, mesenchymal tumors of liver, nodular
regenerative hyperplasia, benign liver tumors (Hepatic cysts [Simple cysts,
Polycystic liver disease, Hepatobiliary cystadenoma, Choledochal cyst],
127
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
n' 41' ..,r 16..6 qo ..'u:::õ.':~I~; .,.(L"
Mesenchymal tumors [Mesenchymal hamartoma, htfantile hemangioendothelioma,
Hemangionia, Peliosis hepatis, Lipomas, Inflammatory
pseudotumor, Miscellaneous], Epithelial tumors [Bile duct epithelium (Bile
duct hamartoma, Bile duct adenoma), Hepatocyte (Adenoma, Focal
nodular hyperplasia, Nodular regenerative hyperplasia)], malignant liver
tumors [hepatocellular, hepatoblastoma, hepatocellular carcinoma,
cholangiocellular, cholangiocaroinoma, oystadenocarcinoma, tumors of blood
vessels, angiosarcoma, Karposi's sarcoma, hemangioendothelioma,
other tumors, embryonal sarcoma, fibrosarcoma, leiomyosarcoma,
rhabdomyosarcoma, carcinosarcoma, teratoma, carcinoid, squamous carcinoma,
primary lymphoma]), peliosis hepatis, erythrohepatic porphyria, hepatic
porphyria (acute intermittent porphyria, porphyria cutanea tarda), Zellweger
syndrome).
[0839] Pancreatic diseases and/or disorders include acute pancreatitis,
chronic pancreatitis (acute necrotizing pancreatitis, alcoholic
pancreatitis), neoplasnis (adenocarcinoma of the pancreas, cystadenocarcinoma,
insulinoma, gastrinoma, and glucagonoma, cystic neoplasms, islet-
cell tumors, pancreoblastoma), and other pancreatic diseases (e.g., cystic
fibrosis, cyst (pancreatic pseudocyst, pancreatic fistula, insufficiency)).
[0840] Gallbladder diseases include gallstones (cholelitliiasis and
choledocholithiasis), postcholecystectomy syndrome, diverticulosis of the
gallbladder, acute cholecystitis, chronic cholecystitis, bile duct tumors, and
mucocele.
[0841] Diseases and/or disorders of the large intestine include antibiotic-
associated colitis, diverticulitis, ulcerative colitis, acquired megacolon,
abscesses, fungal and bacterial infections, anorectal disorders (e.g.,
fissures, hemorrhoids), colonic diseases (colitis, colonic neoplasms [colon
cancer, adenomatous colon polyps (e.g., villous adenoma), colon carcinoma,
colorectal cancer], colonic diverticulitis, colonic diverticulosis,
megacolon [Hirschsprung disease, toxic megacolon]; sigmoid diseases
[proctocolitis, sigmoin neoplasms]), constipation, Crohn's disease, diarrhea
(infantile diarrhea, dysentery), duodenal diseases (duodenal neoplasms,
duodenal obstruction, duodenal ulcer, duodenitis), enteritis (enterocolitis),
HIV enteropathy, ileal diseases (ileal neoplasms, ileitis),
immunoproliferative small intestinal disease, inflammatory bowel disease
(ulcerative
colitis, Crohn's disease), intestinal atresia, parasitic diseases
(anisakiasis, balantidiasis, blastocystis infections, cryptosporidiosis,
dientamoebiasis,
amebic dysentery, giardiasis), intestinal fistula (rectal fistula), intestinal
neoplasms (cecal neoplasms, colonic neoplasms, duodenal neoplasms, ileal
neoplasms, intestinal polyps, jejunal neoplasms, rectal neoplasms), intestinal
obstruction (afferent loop syndrome, duodenal obstruction, impacted
feces, intestinal pseudo-obstruction [cecal volvulus], intussusception),
intestinal perforation, intestinal polyps (colonic polyps, gardner syndrome,
peutz-jeghers syndrome), jejunal diseases (jejunal neoplasms), malabsorption
syndromes (blind loop syndrome, celiac disease, lactose intolerance,
short bowl syndrome, tropical sprue, whipple's disease), mesenteric vascular
occlusion, pneumatosis cystoides intestinalis, protein-losing
enteropathies (intestinal lymphagiectasis), rectal diseases (anus diseases,
fecal incontinence, hemorrhoids, proctitis, rectal fistula, rectal prolapse,
rectocele), peptic ulcer (duodenal ulcer, peptic esophagitis, hemorrhage,
perforation, stomach ulcer, Zollinger-Ellison syndrome), postgastrectomy
syndromes (dumping syndrome), stomach diseases (e.g., achlorhydria,
duodenogastric reflux (bile reflux), gastric antral vascular ectasia, gastric
fistula, gastric outlet obstruction, gastritis (atrophic or hypertrophic),
gastroparesis, stomach dilatation, stomach diverticulum, stomach neoplasms
(gastric cancer, gastric polyps, gastricadenocarcinoma; hyperplastic gastric
polyp); stomach mpture, stomach ulcer, stomach volvulus), tuberculosis,
visceroptosis, vomiting (e.g., hematemesis, hyperemesis gravidarum,
postoperative nausea and vomiting) and hemorrhagic colitis.
[0842] Further diseases and/or disorders of the gastrointestinal system
include biliary tract diseases, such as, gastroschisis, fistula (e.g., biliary
fistula, esophageal fistula, gastric fistula, intestinal fistula, pancreatic
fistula), neoplasms (e.g., biliary tract neoplasms, esophageal neoplasms, such
as adenocarcinoma of the esophagus, esophageal squamous cell carcinoma,
gastrointestinal neoplasms, pancreatic neoplasms, such as
adenocaroinoma of the pancreas, mucinous cystic neoplasm of the pancreas,
pancreatic cystic neoplasms, pancreatoblastoma, and peritoneal
neoplasms), esophageal disease (e.g., bullous diseases, candidiasis,
glycogenic acanthosis, ulceration, barrett esophagus varices, atresia, cyst,
diverticulum (e.g., Zenker's diverticulum), fistula (e.g., tracheoesophageal
fistula), motility disorders (e.g., CREST syndrome, deglutition disorders,
achalasia, spasm, gastroesophageal reflux), neoplasms, perforation (e.g.,
Boerhaave syndrome, Mallory-Weiss syndrome), stenosis, esophagitis,
diaphragmatic hernia (e.g., hiatal hemia); gastrointestinal diseases, such as,
gastroenteritis (e.g., cholera morbus, norwalk virus infection),
hemorrhage (e.g., hematemesis, melena, peptic ulcer hemorrhage), stomach
neoplasms (gastric cancer, gastric polyps, gastric adenocarcinoma,
stomach cancer)), hemia (e.g., congenital diaphragmatic hemia, femoral hernia,
inguinal hernia, obturator hemia, umbilical hernia, ventral hernia),
and intestinal diseases (e.g., cecal diseases (appendicitis, cecal
neoplasms)).
Chemotaxis
[0843] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may have chemotaxis
activity. A chemotaxic molecule attracts or mobilizes cells (e.g., monocytes,
fibroblasts, neutrophils, T-cells, mast cells, eosinophils, epithelial
and/or endothelial cells) to a particular site in the body, such as
inflammation, infection, or site of hyperproliferation. The mobilized cells
can then
fight off and/or heal the particular trauma or abnormality.
[0844] Albumin fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention may increase
chemotaxic activity of particular cells. These chemotactic molecules can then
be used to treat inflammation, infection, hyperproliferative disorders,
or any immune system disorder by increasing the number of cells targeted to a
particular location in the body. For example, chemotaxic molecules
can be used to treat wounds and other trauma to tissues by attracting immune
cells to the injured location. Chemotactic molecules of the present
invention can also attract fibroblasts, which can be used to treat wounds.
128
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
108451 It is also contemplated that fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention may
inhibit chemotactic activity. These molecules could also be used to treat
disorders. Thus, fusion proteins of the invention and/or polynucleotides
encoding albumin fusion proteins of the invention could be used as an
inhibitor of chemotaxis.
BiudiegActivity
[0846] Albumin fusion proteins of the invention may be used to screen for
molecules that bind to the Therapeutic protein portion of the fusion
protein or for molecules to which the Tlierapeutic protein portion of the
fusion protein binds. The binding of the fusion protein and the molecule
may activate (agonist), increase, inhibit (antagonist), or decrease activity
of the fusion protein or the molecule bound. Examples of such molecules
include antibodies, oligonucleotides, proteins (e.g., receptors), or small
molecules.
[0847] Preferably, the molecule is closely related to the natural ligand of
the Therapeutic protein portion of the fusion protein of the invention,
e.g., a fragment of the ligand, or a natural substrate, a ligand, a structural
or functional mimetic. (See, Coligan et al., Current Protocols in
Immunology 1(2):Chapter 5 (1991)). Similarly, the molecule can be closely
related to the natural receptor to which the Therapeutic protein portion
of an albumin fusion protein of the invention binds, or at least, a fragment
of the receptor capable of being bound by the Therapeutic protein portion
of an albumin fusion protein of the invention (e.g., active site). In eitlier
case, the molecule can be rationally designed using known techniques.
[0848] Preferably, the screening for these molecules involves producing
appropriate cells which express the albumin fusion proteins of the
invention. Preferred cells include cells from mammals, yeast, Drosophila, or
E. colf.
[0849] The assay may simply test binding of a candidate compound to an albumin
fusion protein of the invention, wherein binding is detected
by a label, or in an assay involving competition with a labeled competitor.
Further, the assay may test whether the candidate compound results in a
signal generated by binding to the fusion protein.
[0850] Altematively, the assay can be carried out using cell-free
preparations, fusion protein/molecule affixed to a solid support, chemical
libraries, or natural product mixtures. The assay may also simply comprise the
steps of mixing a candidate compound with a solution containing an
albumin fusion protein, measuring fusion protein/molecule activity or binding,
and comparing the fusion protein/molecule activity or binding to a
standard.
[0851] Preferably, an ELISA assay can measure fusion protein level or activity
in a sample (e.g., biological sample) using a monoclonal or
polyclonal antibody. The antibody can measure fusion protein level or activity
by either binding, directly or indirectly, to the albumin fusion protein
or by competing with the albumin fusion protein for a substrate.
[0852] Additionally, the receptor to which a Therapeutic protein portion of an
albumin fusion protein of the invention binds can be identified by
numerous methods laiown to those of skill in the art, for example, ligand
panning and FACS sorting (Coligan, et al., Current Protocols in Immun.,
1(2), Chapter 5, (1991)). For example, in cases wherein the Therapeutic
protein portion of the fusion protein corresponds to FGF, expression
cloning may-be employed wherein polyadenylated RNA is prepared from a cell
responsive to the albumin fusion protein, for example,NIH3T3 cells
which are known to contain multiple receptors for the FGF family proteins, and
SC-3 cells, and a cDNA library created from this RNA is divided
into pools and used to transfect COS cells or other cells that are not
responsive to the albumin fusion protein. Transfected cells which are grown on
glass slides are exposed to the albumin fusion protein of the present
invention, after they have been labeled. The albumin fusion proteins can be
labeled by a variety of means including iodination or inclusion of a
recognition site for a site-specific protein kinase.
[0853] Following fixation and incubation, the slides are subjected to auto-
radiographic analysis. Positive pools are identified and sub-pools are
prepared and re-transfected using an iterative sub-pooling and re-screening
process, eventually yielding a single clones that encodes the putative
receptor.
[0854] As an alternative approach for receptor identification, a labeled
albumin fusion protein can be photoaffinity linked with cell membrane or
extract preparations that express the receptor molecule for the Therapeutoo
protein component of an albumin fusion protein of the invention, the
linked material may be resolved by PAGE analysis and exposed to X-ray film.
The labeled complex containing the receptors of the fusion protein
can be excised, resolved into peptide fragments, and subjected to protein
microsequencing. The amino acid sequence obtained from
microsequencing would be used to design a set of degenerate oligonucleotide
probes to screen a cDNA library to identify the genes encoding the
putative receptors.
[0855] Moreover, the techniques of gene-shuffling, motif-shuffling, exon-
shuffling, and/or codon-shuffling (collectively referred to as "DNA
shuffling") may be employed to modulate the activities of the fusion protein,
and/or Therapeutic protein portion or albumin component of an
albumin fusion protein of the present invention, thereby effectively
generating agonists and antagonists of an albumin fusion protein of the
present
invention. See generally, U.S. Patent Nos. 5,605,793, 5,811,238, 5,830,721,
5,834,252, and 5,837,458, and Patten, P. A., et al., Curr. Opinion
Biotechnol. 8:724-33 (1997); Harayama, S. Trends Biotechnol. 16(2):76-82
(1998); Hansson, L. 0., et al., J. Mo1. Biol. 287:265-76 (1999); and
Lorenzo, M. M. and Blasco, R. Biotechniques 24(2):308-13 (1998); each of these
patents and publications are hereby incorporated by reference).
In one embodiment, alteration of polynucleotides encoding albumin fusion
proteins of the invention and thus, the albumin fusion proteins encoded
thereby, may be achieved by DNA shuffling. DNA shuffling involves the assembly
of two or more DNA segments into a desired molecule by
homologous, or site-specific, recombination. In another embodiment,
polynucleotides encoding albumin fusion proteins of the invention and thus,
129
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
f1...W u ,r' 9..~6 =~.n iI.,e, .n.,n ,. ,~,,,. .~,. ~~.= .,tlre,~,....
tlre albumin fusion proteins encodedreby, may be altered by being subjected to
random mutagenesis by error-prone PCR, random nucleotide
insertion or other methods prior to recombination. In another embodiment, one
or more components, motifs, sections, parts, domains, fragments,
etc., of an albumin fusion protein of the present invention may be recombined
with one or more components, motifs, sections, parts, domains,
fragments, etc. of one or more heterologous molecules. hi preferred
embodiments, the heterologous molecules are family members. In further
preferred embodiments, the heterologous molecule is a growth factor such as,
for example, platelet-derived growth factor (PDGF), insulin-like
growth factor (IGF-1), transforming growth factor (TGF)-alpha, epidermal
growth factor (EGF), fibroblast growth factor (FGF), TGF-beta, bone
morphogenetic protein (BMP)-2, BMP-4, BMP-5, BMP-6, BMP-7, activins A and B,
decapentaplegic(dpp), 60A, OP-2, dorsalin, growth
differentiation factors (GDFs), nodal, MIS, inhibin-alpha, TGF-betal, TGF-
beta2, TGF-beta3, TGF-beta5, and glial-derived neurotrophic factor
(GDNF).
[0856] Other preferred fragments are biologically active fragments of the
Therapeutic protein portion and/or albumin component of the albumin
fusion proteins of the present invention. Biologically active fragments are
those exhibiting activity similar, but not necessarily identical, to an
activity of a Therapeutic protein portion and/or albumin component of the
albumin fusion proteins of the present invention. The biological activity
of the fragments may include an improved desired activity, or a decreased
undesirable activity.
[0857] Additionally, this invention provides a method of screening compounds
to identify those which modulate the action of an albumin fusion
protein of the present invention. An example of such an assay comprises
combining a mammalian fibroblast cell, an albumin fusion protein of the
present invention, and the compound to be screened and 3[H] thymidine under
cell culture conditions where the fibroblast cell would normally
proliferate. A control assay may be performed in the absence of the compound
to be screened and compared to the amount of fibroblast
proliferation in the presence of the compound to determine if the compound
stimulates proliferation by determining the uptake of 3[H] thymidine in
each case. The amount of fibroblast cell proliferation is measured by liquid
scintillation chromatograplry which measures the incorporation of 3[H]
thymidine. Both agonist and antagonist compounds may be identified by this
procedure.
[0858] In another method, a mammalian cell or membrane preparation expressing
a receptor for the Therapeutic protien component of a fusion
protine of the invention is incubated with a labeled fusion protein of the
present invention in the presence of the compound. The ability of the
compound to enhance or block this interaction could then be measured.
Alternatively, the response of a known second messenger system following
interaction of a compound to be screened and the receptor is measured and the
ability of the compound to bind to the receptor and elicit a second
messenger response is measured to determine if the compound is a potential
fusion protein. Such second messenger systems include but are not
limited to, cAMP guanylate cyclase, ion channels or phosphoinositide
hydrolysis.
[0859] All of these above assays can be used as diagnostic or prognostic
markers. The molecules discovered using these assays can be used to
treat disease or to bring about a particular result in a patient (e.g., blood
vessel growth) by activating or inhibiting the fusion protein/molecule.
Moreover, the assays can discover agents which may inhibit or enhance the
production of the albumin fusion proteins of the invention from suitably
manipulated cells or tissues.
[0860] Therefore, the invention includes a method of identifying compounds
which bind to an albumin fusion protein of the invention
comprising the steps of: (a) incubating a candidate binding compound with an
albumin fusion protein of the present invention; and (b) determining
if binding has occurred. Moreover, the invention includes a method of
identifying agonists/antagonists comprising the steps of: (a) incubating a
candidate compound with an albumin fusion protein of the present invention,
(b) assaying a biological activity, and (b) determining if a biological
activity of the fusion protein has been altered.
Targeted Delivery
[0861] In another embodiment, the invention provides a method of delivering
compositions to targeted cells expressing a receptor for a
component of an albumin fusion protein of the invention.
[0862] As discussed herein, fusion proteins of the invention may be associated
with heterologous polypeptides, heterologous nucleic acids,
toxins, or prodrugs via hydrophobic, hydrophilic, ionic and/or covalent
interactions. In one embodiment, the invention provides a method for the
specific delivery of compositions of the invention to cells by administering
fusion proteins of the invention (including antibodies) that are associated
with heterologous polypeptides or nucleic acids. In one example, the invention
provides a method for delivering a Therapeutic protein into the
targeted cell. In another example, the invention provides a method for
delivering a single stranded nucleic acid (e.g., antisense or ribozymes) or
double stranded nucleic acid (e.g., DNA that can integrate into the cell's
genome or replicate episomally and that can be transcribed) into the targeted
cell.
[0863] In another embodiment, the invention provides a method for the specific
destruction of cells (e.g., the destruction of tumor cells) by
administering an albumin fusion protein of the invention (e.g., polypeptides
of the invention or antibodies of the invention) in association with
toxins or cytotoxic prodrugs.
[08641 By "toxin" is meant compounds that bind and activate endogenous
cytotoxic effector systems, radioisotopes, holotoxins, modified toxins,
catalytic subunits of toxins, or any molecules or enzymes not normally present
in or on the surface of a cell that under defmed conditions cause the
130
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
I ~, 9"ar 1' .II u:"11 ' I1:: ",o1 ,a~ ,..A,.
e11's d~ t1'i: ]"oxins 4hat maybe usec~ acoording to the methods of the
invention include, but are not limited to, radioisotopes known in the art,
compounds such as, for example, antibodies (or complement fixing containing
portions thereof) that bind an inherent or induced endogenous
cytotoxic effector system, thymidine kinase, endonuclease, RNAse, alpha toxin,
ricin, abrin, Pseudoinonas exotoxin A, diphtheria toxin, saporin,
momordin, gelonin, pokeweed antiviral protein, alpha-sarcin and cholera toxin.
By "cytotoxic prodrug" is meant a non-toxic compound that is
converted by an enzyme, normally present in the cell, into a cytotoxic
compound. Cytotoxic prodrugs that may be used according to the methods of
the invention include, but are not limited to, glutamyl derivatives of benzoic
acid mustard alkylating agent, phosphate derivatives of etoposide or
mitomycin C, cytosine arabinoside, daunorubisin, and phenoxyacetamide
derivatives of doxorubicin.
Drug Screeaiug
[0865] Further contemplated is the use of the albumin fusion proteins of the
present invention, or the polynucleotides encoding these fusion
proteins, to screen for molecules which modify the activities of the albumin
fusion protein of the present invention or proteins corresponding to the
Therapeutic protein portion of the albumin fusion protein. Such a method would
include contacting the fusion protein with a selected compound(s)
suspected of having antagonist or agonist activity, and assaying the activity
of the fusion protein following binding.
[0866] This invention is particularly useful for screening therapeutic
compounds by using the albumin fusion proteins of the present invention,
or binding fragments thereof, in any of a variety of drug screening
techniques. The albumin fusion protein employed in such a test may be affixed
to
a solid support, expressed on a cell surface, free in solution, or located
intracellularly. One method of drug screening utilizes eukaryotic or
prokaryotic host cells which are stably transformed with recombinant nucleic
acids expressing the albumin fusion protein. Drugs are screened
against such transformed cells or supernatants obtained from culturing such
cells, in competitive binding assays. One may measure, for exainple, the
formulation of complexes between the agent being tested and an albumin fusion
protein of the present invention.
[0867] Thus, the present invention provides methods of screening for drugs or
any other agents whioh affect activities mediated by the albumin
fusion proteins of the present invention. These methods comprise contacting
such an agent with an albumin fusion protein of the present invention
or a fragment thereof and assaying for the presence of a complex between the
agent and the albumin fusion protein or a fragment tliereof, by
methods well known in the art. In such a competitive binding assay, the agents
to screen are typically labeled. Following incubation, free agent is
separated from that present in bound form, and the amount of free or
uncomplexed label is a measure of the ability of a particular agent to bind to
the albumin fusion protein of the present invention.
[0868] Another technique for drug screening provides high throughput screening
for compounds having suitable binding affinity to an albumin
fusion protein of the present invention, and is described in great detail in
European Patent Application 84/03564, published on September 13, 1984,
which is incorporated herein by reference herein. Briefly stated, large
numbers of different small peptide test compounds are synthesized on a solid
substrate, such as plastic pins or some other surface. The peptide test
compounds are reacted with an albumin fusion protein of the present invention
and washed. Bound peptides-are then detected by methods well known in the-art.-
Purified albumin fusion protein may be coated direetly onto plates
for use in the aforementioned drug screening techniques. In addition, non-
neutralizing antibodies may be used to capture the peptide and
immobilize it on the solid support.
[0869] This invention also contemplates the use of competitive drug screening
assays in which neutralizing antibodies capable of binding an
albumin fusion protein of the present invention specifically compete with a
test compound for binding to the albumin fusion protein or fragments
thereof. In this manner, the antibodies are used to detect the presence of any
peptide which shares one or more antigenic epitopes with an albumin
fusion protein of the invention.
BindinQ Peptides aud Otleer Molecules
[0870] The invention also encompasses screening methods for identifying
polypeptides and nonpolypeptides that bind albumin fusion proteins
of the invention, and the binding molecules identified thereby. These binding
molecules are useful, for example, as agonists and antagonists of the
albumin fusion proteins of the invention. Such agonists and antagonists can be
used, in accordance with the invention, in the therapeutic
embodiments described in detail, below.
[0871] This method comprises the steps of: contacting an albumin fusion
protein of the invention with a plurality of molecules; and identifying
a molecule that binds the albumin fusion protein.
[0872] The step of contacting the albumin fusion protein of the invention with
the plurality of molecules may be effected in a number of ways.
For example, one may contemplate immobilizing the albumin fusion protein on a
solid support and bringing a solution of the plurality of molecules
in contact with the immobilized polypeptides. Such a procedure would be akin
to an affinity chromatographic process, with the affinity matrix being
comprised of the immobilized albumin fusion protein of the invention. The
molecules having a selective affinity for the albumin fusion protein can
then be purified by affmity selection. The nature of the solid support,
process for attachment of the albumin fusion protein to the solid support,
solvent, and conditions of the affinity isolation or selection are largely
conventional and well known to those of ordinary skill in the art.
[0873] Alternatively, one may also separate a plurality of polypeptides into
substantially separate fractions comprising a subset of or individual
polypeptides. For instance, one can separate the plurality of polypeptides by
gel electrophoresis, column chromatography, or like method known to
those of ordinary skill for the separation of polypeptides. The individual
polypeptides can also be produced by a transformed host cell in such a way
131
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
It t ,,.tt ;:"1t t1."II li";Ic , % lt:' ":;t ; ,,,;tc ~a91 ,"tt..
ag to e expressecl on or about its outer surface (e.g., a recombinant phage).
Individual isolates can then be "probed" by an albumin fusion protein of
the invention, optionally in the presence of an inducer should one be required
for expression, to determine if any selective affinity interaction takes
place between the albumin fusion protein and the individual clone. Prior to
contacting the albumin fusion protein with each fraction comprising
individual polypeptides, the polypeptides could first be transferred to a
solid support for additional convenience. Such a solid support may simply be
a piece of filter membrane, such as one made of nitrocellulose or nylon. In
this manner, positive clones could be identified from a collection of
transformed host cells of an expression library, which harbor a DNA construct
encoding a polypeptide having a selective affinity for an albumin
fusion protein of the invention. Furthemiore, the amino acid sequence of the
polypeptide having a selective affinity for an albumin fusion protein of
the invention can be determined directly by conventional means or the coding
sequence of the DNA encoding the polypeptide can frequently be
determined more conveniently. The primary sequence can then be deduced from
the corresponding DNA sequence. If the amino acid sequence is to
be determined from the polypeptide itself, one may use microsequencing
techniques. The sequencing technique may include mass spectroscopy.
[0874] In certain situations, it may be desirable to wash away any unbound
polypeptides from a mixture of an albumin fusion protein of the
invention and the plurality of polypeptides prior to attempting to determine
or to detect the presence of a selective affinity interaction. Such a wash
step may be particularly desirable when the albumin fusion protein of the
invention or the plurality of polypeptides are bound to a solid support.
[0875] The plurality of molecules provided according to this method may be
provided by way of diversity libraries, such as random or
combinatorial peptide or nonpeptide libraries which can be screened for
molecules that specifically bind an albumin fusion protein of the invention.
Many libraries are Imown in the art that can be used, e.g., chemically
synthesized libraries, recombinant (e.g., phage display libraries), and in
vitro
translation-based libraries. Examples of chemically synthesized libraries are
described in Fodor et al., Science 251:767-773 (1991); Houghten et al.,
Nature 354:84-86 (1991); Lam et al., Nature 354:82-84 (1991); Medynski,
Bio/Technology 12:709-710 (1994); Gallop et al., J. Medicinal
Chemistry 37(9):1233-1251 (1994); Ohlmeyer et al., Proo. Natl. Acad. Sci. USA
90:10922-10926 (1993); Erb et al., Proc. Natl. Acad. Soi. USA
91:11422-11426 (1994); Houghten et al., Biotechniques 13:412 (1992);
Jayawickreme et al., Proc. Natl. Acad. Sci. USA 91:1614-1618 (1994);
Salmon et al., Proc. Natl. Acad. Sci. USA 90:11708-11712 (1993); PCT
Publication No. WO 93/20242; and Brenner and Lemer, Proo. Natl. Acad.
Sci. USA 89:5381-5383 (1992).
[0876] Examples of phage display libraries are described in Scoit et al.,
Science 249:386-390 (1990); Devlin et al., Science, 249:404-406
(1990); Cluistian et al., 1992, J. Mol. Biol. 227:711-718 1992); Lenstra, J.
Immunol. Meth. 152:149-157 (1992); Kay et al., Gene 128:59-65
(1993); and PCT Publication No. WO 94/18318 dated Aug. 18, 1994.
[0877] In vitro translation-based libraries include but are not limited to
those described in PCT Publication No. WO 91/05058 dated Apr. 18,
1991; and Mattheakis et al., Proc. Natl. Acad. Sci. USA 91:9022-9026 (1994).
[0878] By way of examples of nonpeptide libraries, a benzodiazepine library
(see e.g., Bunin et al., Proc. Natl. Acad. Sci. USA 91:4708-4712
(1994))-can be adapted for-use. Peptoid libraries (Simon-et-a1., Proc: Natl.
Acad -Sci. USA 89:9367-9371 (1992)) can also be used. Another
example of a library that can be used, in which the amide functionalities in
peptides have been permethylated to generate a chemically transformed
combinatorial library, is described by Ostresh et al. (Proc. Natl. Acad. Sci.
USA 91:11138-11142 (1994)).
[0879] The variety of non-peptide libraries that are useful in the present
invention is great. For example, Ecker and Crooke (Bio/Technology
13:351-360 (1995) list benzodiazepines, hydantoins, piperazinediones,
biphenyls, sugar analogs, beta-mercaptoketones, arylacetic acids,
acylpiperidines, benzopyrans, cubanes, xanthines, aminimides, and oxazolones
as among the chemical species that form the basis of various
libraries.
[0880] Non-peptide libraries can be classified broadly into two types:
decorated monomers and oligomers. Decorated monomer libraries employ
a relatively simple scaffold structure upon which a variety functional groups
is added. Often the scaffold will be a molecule with a known useful
pharmacological activity. For example, the scaffold might be the
benzodiazepine structure.
[0881] Non-peptide oligomer libraries utilize a large number of monomers that
are assembled together in ways that create new shapes that
depend on the order of the monomers. Among the monomer units that have been
used are carbamates, pyrrolinones, and morpholinos. Peptoids,
peptide-like oligomers in which the side chain is attached to the alpha amino
group rather than the alpha carbon, form the basis of another version of
non-peptide oligomer libraries. The first non-peptide oligomer libraries
utilized a single type of monomer and thus contained a repeating backbone.
Recent libraries have utilized more than one monomer, giving the libraries
added flexibility.
[0882] Screening the libraries can be accomplished by any of a variety of
commonly laiown methods. See, e.g., the following references, which
disclose screening of peptide libraries: Parmley et al., Adv. Exp. Med. Biol.
251:215-218 (1989); Scott et al,. Science 249:386-390 (1990); Fowlkes
et al., BioTechniques 13:422-427 (1992); Oldenburg et al., Proc. Natl. Acad.
Sci. USA 89:5393-5397 (1992); Yu et al., Cell 76:933-945 (1994);
Staudt et al., Science 241:577-580 (1988); Bock et al., Nature 355:564-566
(1992); Tuerk et al., Proc. Natl. Acad. Sci. USA 89:6988-6992 (1992);
Ellington et al., Nature 355:850-852 (1992); U.S. Pat. No. 5,096,815, U.S.
Pat. No. 5,223,409, and U.S. Pat. No. 5,198,346, all to Ladner et al.;
Rebar et al., Science 263:671-673 (1993); and PCT Publication No. WO 94/18318.
[0883] In a specific embodiment, screening to identify a molecule that binds
an albumin fusion protein of the invention can be carried out by
contacting the library members with an albumin fusion protein of the invention
immobilized on a solid phase and harvesting those library members
132
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
If... If ' ~tõIt ;;'wu ,,,u ir u , ' . nu : :; ,.;-u ;1~ .,.u '
th'at bmd to t e albumm fusion protem. hxamples of such screening methods,
termed "panning" techniques are described by way of example in
Parmley et al., Gene 73:305-318 (1988); Fowlkes et al., BioTechniques 13:422-
427 (1992); PCT Publication No. WO 94/18318; and in references
cited herein.
[0884] In another embodiment, the two-hybrid system for selecting interacting
proteins in yeast (Fields et al., Nature 340:245-246 (1989);
Chien et al., Proc. Natl. Acad. Sci. USA 88:9578-9582 (1991) can be used to
identify molecules that specifically bind to polypeptides of the
invention.
[0885] Where the binding molecule is a polypeptide, the polypeptide can be
conveniently selected from any peptide library, including random
peptide libraries, combinatorial peptide libraries, or biased peptide
libraries. The term "biased" is used herein to mean that the method of
generating
the library is manipulated so as to restrict one or more parameters tiiat
govern the diversity of the resulting collection of molecules, in this case
peptides.
[0886] Thus, a truly random peptide library would generate a collection of
peptides in which the probability of finding a particular amino acid at
a given position of the peptide is the same for all 20 amino acids. A bias can
be introduced into the library, however, by specifying, for example, that
a lysine occur every fifth amino acid or that positions 4, 8, and 9 of a
decapeptide library be fixed to include only arginine. Clearly, many types of
biases can be contemplated, and the present invention is not restricted to any
particular bias. Furtliermore, the present invention contemplates
specific types of peptide libraries, such as phage displayed peptide libraries
and those that utilize a DNA construct comprising a lambda phage
vector with a DNA insert.
[0887] As mentioned above, in the case of a binding molecule that is a
polypeptide, the polypeptide may have about 6 to less than about 60
amino acid residues, preferably about 6 to about 10 amino acid residues, and
most preferably, about 6 to about 22 amino acids. In another
embodiment, a binding polypeptide has in the range of 15-100 amino acids, or
20-50 amino acids.
[0888] The selected binding polypeptide can be obtained by chemical synthesis
or recombinant expression.
Otlaer Activities
[0889] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention, may be employed
in treatment for stimulating re-vascularization of ischemic tissues due to
various disease conditions such as thrombosis, arteriosclerosis, and other
cardiovascular conditions. The albumin fusion proteins of the invention and/or
polynucleotides encoding albumin fusion proteins of the invention
may also be employed to stimulate angiogenesis and limb regeneration, as
discussed above.
[0890] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also be
employed for treating wounds due to injuries, burns, post-operative tissue
repair, and ulcers since they are mitogenic to various cells of different
origins, such as fibroblast cells and skeletal muscle cells, and tlierefore,
facilitate the repair or replacement of damaged or diseased tissue.
[0891] An albumin-fusion protein of the- invention and/or polynucleotide -
encoding an albumin fusion protein of the invention may-also be
employed stimulate neuronal growth and to treat and prevent neuronal damage
which occurs in certain neuronal disorders or neuro-degenerative
conditions such as Alzheimer's disease, Parkinson's disease, and AIDS-related
complex. An albumin fusion protein of the invention and/or
polynucleotide encoding an albumin fusion protein of the invention may have
the ability to stimulate chondrocyte growth, therefore, they may be
employed to enhance bone and periodontal regeneration and aid in tissue
transplants or bone grafts.
[0892] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may be also be
employed to prevent skin aging due to sunburn by stimulating keratinocyte
growth.
[0893] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also be
employed for preventing hair loss. Along the same lines, an albumin fusion
protein of the invention and/or polynucleotide encoding an albumin
fusion protein of the invention may be employed to stimulate growth and
differentiation of hematopoietic cells and bone marrow cells when used in
combination with other cytokines.
[0894] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also be
employed to maintain organs before transplantation or for supporting cell
culture of primary tissues. An albumin fusion protein of the invention
and/or polynucleotide encoding an albumin fusion protein of the invention may
also be employed for inducing tissue of inesodermal origin to
differentiate in early embryos.
[0895] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also increase
or decrease the differentiation or proliferation of embryonic stem cells,
besides, as discussed above, hematopoietic lineage.
[0896] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also be used
to modulate mammalian characteristics, such as body height, weight, hair
color, eye color, skin, percentage of adipose tissue, pigmentation, size, and
shape (e.g., cosmetic surgery). Similarly, an albumin fusion protein of the
invention and/or polynucleotide encoding an albumin fusion protein of
the invention may be used to modulate mammalian metabolism affecting
catabolism, anabolism, processing, utilization, and storage of energy.
[0897] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may be used to
change a mammal's mental state or physical state by influencing biorhythms,
caricadic rhythms, depression (including depressive disorders),
133
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
,t. u". I~ : I}. IL ...~.ib k ~I" 1t . ii::::, ';It ..C:G st~ , il..
te d''encY"to'r" 1 nCO, tolerancd for pain, reproductive capabilities
(preferably by Activin or Inhibin-like activity), hormonal or endocrine
levels,
appetite, libido, memory, stress, or other cognitive qualities.
[0898] An albumin fusion protein of the invention and/or polynucleotide
encoding an albumin fusion protein of the invention may also be used
as a food additive or preservative, such as to increase or decrease storage
capabilities, fat content, lipid, protein, carbohydrate, vitamins, minerals,
cofactors or other nutritional components.
[0899] The above-recited applications have uses in a wide variety of hosts.
Such hosts include, but are not limited to, human, murine, rabbit,
goat, guinea pig, camel, horse, mouse, rat, hamster, pig, micro-pig, chicken,
goat, cow, sheep, dog, cat, non-human primate, and human. In specific
embodiments, the host is a mouse, rabbit, goat, guinea pig, chicken, rat,
hamster, pig, sheep, dog or cat. In preferred embodiments, the host is a
mammal. In most preferred embodiments, the host is a human.
[0900] I-Iaving generally described the invention, the same will be more
readily understood by reference to the following examples, which are
provided by way of illustration and are not intended as limiting.
[0901] Without further description, it is believed that one of ordinary skill
in the art can, using the preceding description and the following
illustrative examples, make and utilize the alterations detected in the
present invention and practice the claimed methods. The following working
examples therefore, specifically point out preferred embodiments of the
present invention, and are not to be construed as limiting in any way the
remainder of the disclosure.
EXAMPLES
EXAA9PLE 1: Geaeratiorr ofpScNHSA and nScCHSA.
[0902] The vectors pScNHSA (ATCC Deposit No. PTA-3279) and pScCHSA (ATCC
Deposit No. PTA-3276) are derivatives of pPPC0005
(ATCC Deposit No. PTA-3278) and are used as cloning vectors into which
polynucleotides encoding a therapeutic protein or fragment or variant
thereof is inserted adjacent to and in translation frame with polynucleotides
encoding human serum albumin "HSA". pScCHSA may be used for
generating Therapeutic protein-HSA fusions, while pScNHSA may be used to
generate HSA-Therapeutic protein fusions.
Generation ofpScCHSA: alburnin fusion with the albuinin rnoietv C-terminal to
the tkerapeutie portion.
[0903] A vector to facilitate cloning DNA encoding a Therapeutic protein N-
terminal to DNA encoding the mature albumin protein was made
by altering the nucleic acid sequence that encodes the chimeric HSA signal
peptide in pPPC0005 to include the Xho I and Cla I restriction sites.
[0904] First, the Xho I and Cla I sites inherent to pPPC0005 (located 3' of
the ADH1 terminator sequence) were eliminated by digesting
pPPC0005 with Xho I and Cla I, filling in the sticky ends witli T4 DNA
polymerase, and religating the blunt ends to create pPPC0006.
[0905] Second, the Xho I and Cla I restriction sites were engineered into the
nucleic acid sequence that encodes the signal peptide of HSA (a
chimera of the HSA leader and a kex2 site from mating_factor alpha, "MAF") in
pPPC0006 using two rounds of PCR. In the first round of PCR,
amplification with primers shown as SEQ ID NO:36 and SEQ ID NO:37 was
performed. The primer whose sequence is shown as SEQ ID NO:36
comprises a nucleic acid sequence that encodes part of the signal peptide
sequence of HSA, a kex2 site from the mating factor alpha leader
sequence, and part of the amino-terminus of the mature form of HSA. Four point
mutations were introduced in the sequence, creating the Xlso I and
Cla I sites found at the junction of the chimeric signal peptide and the
mature form of HSA. These four mutations are underlined in the sequence
shown below. In pPPC0005 the nucleotides at these four positions from 5' to 3'
are T, G, T, and G.
5'-GCCTCGA.QAAAAGAGATGCACACAAGAGTGAGGTTGCTCATCGATTTAAAGATTTGGG-3' (SEQ ID
NO:36) and
5'-AATCGATGAGCAACCTCACTCTTGTGTGCATCTCTTTTCTCGAGGCTCCTGGAATAAGC-3' (SEQ ID
NO:37). A second round of PCR
was then performed with an upstream flanking primer, 5'-
TACAAACTTAAGAGTCCAATTAGC-3' (SEQ ID NO:38) and a downstream flanking
primer 5'-CACTTCTCTAGAGTGGTTTCATATGTCTT-3' (SEQ ID NO:39). The resulting PCR
product was then purified and digested with Af1II
and h7ia I and ligated into the same sites in pPPC0006 creating pScCHSA. The
resulting plasmid has Xho I and Cla I sites engineered into the
signal sequence. The presence of the Xho I site creates a single amino acid
change in the end of the signal sequence from LDKR to LEKR. The D
to E change will not be present in the final albumin fusion protein expression
plasmid when a nucleic acid sequence comprising a polynucleotide
encoding the Therapeutic portion of the albumin fusion protein with a 5' Sal I
site (which is compatible with the Xho I site) and a 3' Cla I site is
ligated into the Xho I and Cla I sites of pScCHSA. Ligation of Sal I to Xho I
restores the original amino acid sequence of the signal peptide
sequence. DNA encoding the Therapeutic portion of the albumin fusion protein
may be inserted after the Kex2 site (Kex2 cleaves after the dibasic
amino acid sequence KR at the end of the signal peptide) and prior to the Cla
I site.
Generation of pScNHSA: albuinin fusion with the albumin n:oietv N-terminal to
the therapeutic portion.
[0906] A vector to facilitate cloning DNA encoding a Therapeutic protein
portion C-terminal to DNA encoding the mature albumin protein, was
made by adding three, eight-base-pair restriction sites to pScCHSA. The Asc I,
Fse I, and Pine I restriction sites were added in between the Bsu36 I
and Hind III sites at the end of the nucleic acid sequence encoding the mature
HSA protein. This was accomplished through the use of two
complementary synthetic primers containing the Asc I, Fse I, and Pme I
restriction sites underlined (SEQ ID NO:40 and SEQ ID NO:41).
5'-AAGCTGCCTTAGGCTTATAATAAGGCGCGCCGGCCGGCCGTTTAAACTAAGCTTAATTCT-3' (SEQ ID
NO:40) and
134
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
XGi. ~ ~-
1 TAA A ~AILCIiG GGCCGGCGCGCCTTATTATAAGCCTAAGGCAGCTT-3' (SEQ ID NO:41). These
primers were
annealed and digested with Bsu36I and Hirid III and ligated into the same
sites in pScCHSA creating pSeNHSA.
EXA1t1PLE 2: General Construct Getteration for Yeast Transformation.
[0907] The vectors pScNHSA and pScCHSA may be used as cloning vectors into
which polynucleotides encoding a tlierapeutic protein or
fragment or variant thereof is inserted adjacent to polynucleotides encoding
mature human serum albumin "HSA". pScCHSA is used for generating
Therapeutic protein-HSA fusions, while pScNHSA may be used to generate HSA-
Therapeutic protein fusions.
Generation ofalbttmin fusion constructs comprisinz HSA-Therapeuttc protein
usion products.
[0908] DNA encoding a Therapeutic protein (e.g., sequences shown in SEQ ID
NO:X or known in the art) may be PCR amplified using the
primers which facilitate the generation of a fusion construct (e.g., by adding
restriction sites, encoding seamless fusions, encoding linker sequences,
etc.) For example, one skilled in the art could design a 5' primer that adds
polynucleotides encoding the last four amino acids of the mature form of
HSA (and containing the Bsu361 site) onto the 5' end of DNA encoding a
Therapeutic protein; and a 3' primer that adds a STOP codon and
appropriate cloning sites onto the 3' end of the Therapeutic protein coding
sequence. For instance, the forward primer used to amplify DNA
encoding a Therapeutic protein might have the sequence, 5'-
aagctGCCTTAGGCTTA(N)l5-3' (SEQ ID NO:42) where the underlined sequence is a
Bsu361 site, the upper case nucleotides encode the last four amino acids of
the mature HSA protein (ALGL), and (N)15 is identical to the first 15
nucleotides encoding the Therapetic protein of interest. Similarly, the
reverse primer used to amplify DNA encoding a Therapeutic protein might
have the sequence, 5'-GCGCGCGTTTAAACGGCCGGCCGGCGCGC TTC A(N)15-3' (SEQ ID
NO:43) where the italicized sequence is a Pme I
site, the double underlined sequence is an Fse I site, the singly underlined
sequence is an Asc I site, the boxed nucleotides are the reverse
complement of two tandem stop codons, and (N)15 is identical to the reverse
complement of the last 15 nucleotides encoding the Therapeutic protein
of interest. Once the PCR product is amplified it may be cut with Bsu361 and
one of (Asc I, Fse I, or Pn:e I) and ligated into pScNHSA.
[0909] The presence of the Xho I site in the HSA chimeric leader sequence
creates a single amino acid change in the end of the chimeric signal
sequence, i.e. the HSA-kex2 signal sequence, from LDKR (SEQ ID NO:44) to LEKR
(SEQ ID NO:45).
Generation ofalbumin fusion constructs comprising gene-HSA fusion prodsicts.
[0910] Similar to the method described above, DNA encoding a Therapeutic
protein may be PCR amplified using the following primers: A 5'
primer that adds polynucleotides containing a Sall site and encoding the last
three amino acids of the HSA leader sequence, DKR, onto the 5' end of
DNA encoding a Therapeutic protein; and a 3' primer that adds polynucleotides
encoding the first few amino acids of the mature HSA containing a
Cla I site onto the 3' end of DNA encoding a Therapeutic protein. For
instance, the forward primer used to amplify the DNA encoding a
Therapeutic protein might have the sequence, 5'-aggagc c A AAAAGA(N)15-3' (SEQ
ID NO:46) where the underlined sequence is a Sal I site;
the upper case nucleotides encode the last three amino acids of the HSA leader
sequence (DKR), and (N)15 is identical to the first 15 nuoleotides
encoding the T-herapetic protein of interest. Similarly, the-reverse primer
used to-amplify-the DNA encoding a-Therapeutic protein might have the-
sequence, 5'-CTTTAAATCGA7GAGCAACCTCACTCTTGTGTGCATC(N)15-3'(SEQ ID NO:47) where
the italicized sequence is a Cla I site, the
underlined nucleotides are the reverse complement of the DNA encoding the
first 9 amino acids of the mature form of HSA (DAHKSEVAH, SEQ
ID NO:48), and (N)15 is identical to the reverse complement of the last 15
nucleotides encoding the Therapeutic protein of interest. Once the PCR
product is amplified it may be cut with Sal I and Cla I and ligated into
pScCHSA digested with Xho I and Cla I. A different signal or leader
sequence may be desired, for example, invertase "INV" (Swiss-Prot Accession
P00724), mating factor alpha "MAF" (Genbank Accession
AAA18405), MPIF (Geneseq AAF82936), Fibulin B (Swiss-Prot Accession P23142),
Clusterin (Swiss-Prot Accession P10909), Insulin-Like
Growth Factor- Binding Protein 4 (Swiss-Prot Accession P22692), and
permutations of the HSA leader sequence can be subcloned into the
appropriate vector by means of standard methods known in the art.
Generation ofalbmnin fusion construct compatible for expression in yeast S.
cerevisiae.
[0911] The Not I fragment containing the DNA encoding either an N-terminal or
C-terminal albumin fusion protein generated from pScNHSA
or pScCHSA may then be cloned into the Not I site of pSAC35 which has a LEU2
selectable marker. The resulting vector is then used in
transformation of a yeast S. eerevisiae expression system.
EXADIPLE 3: General Expression in Yeast S. cerevisiae.
[0912] An expression vector compatible with yeast expression can be
transformed into yeast S. cerevisiae by lithium acetate transformation,
electroporation, or other methods known in the art and or as described in part
in Sambrook, Fritsch, and Maniatis. 1989. "Molecular Cloning: A
Laboratory Manual, 2"d edition", volumes 1-3, and in Ausubel et al. 2000.
Massachusetts General Hospital and Harvard Medical School "Current
Protocols in Molecular Biology", volumes 1-4. The expression vectors are
introduced into S. cerevisiae strains DXY1, D88, or BXP10 by
transformation, individual transformants can be grown, for example, for 3 days
at 30 C in 10 mL YEPD (1% w/v yeast extract, 2 % w/v, peptone, 2
% w/v, dextrose), and cells can be collected at stationary phase after 60
hours of growth. Supernatants are collected by elarifying cells at 3000g for
minutes.
[0913] pSAC35 (Sleep et al., 1990, Biotechnology 8:42 and see Figure 3)
comprises, in addition to the LEU2 selectable marker, the entire yeast
2 m plasmid to provide replication functions, the PRB1 promoter, and the ADHI
termination signal.
135
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
l1,,,u II ,r' ~L,fI "N:Ir qn;;,u ,r : ',,.uõ
E.~4MPLE 4= Geueral Pztrifzcation of an Albumiu Fusion Proteiu Eapressed front
azt Albzunin Frrsiou in Yeast S. cerevisiae.
[0914] In preferred embodiments, albumin fusion proteins of the invention
comprise the mature form of HSA fused to either the N- or C-
terminus of tiie mature form of a therapeutic protein or portions thereof
(e.g., the mature form of a therapeutic protein listed in Table 1, or the
mature form of a therapeutic protein shown in Table 2 as SEQ ID NO:Z). In one
embodiment of the invention, albumin fusion proteins of the
invention further comprise a signal sequence which directs the nascent fusion
polypeptide in the secretory pathways of the host used for expression.
In a preferred embodiment, the signal peptide encoded by the signal sequence
is removed, and the mature albumin fusion protein is secreted
directly into the culture medium. Albumin fusion proteins of the invention
preferably comprise heterologous signal sequences (e.g., the non-native
signal sequence of a particular therapeutic protein) including, but not
limited to, MAF, 1NV, Ig, Fibulin B, Clusterin, Insulin-Like Growth Factor
Binding Protein 4, variant HSA leader sequences including, but not limited to,
a chimeric HSA/MAF leader sequence, or other heterologous signal
sequences k-nown in the art. Especially preferred as those signal sequence
listed in Table 2 and/or the signal sequence listed in the "Expression of
Fusion Proteins" and/or "Additional Methods of Recombinant and Synthetic
Production of Albumin Fusion Proteins" section of the specification,
above. In preferred embodiments, the fusion proteins of the invention further
comprise an N-terminal methionine residue. Polynucleotides
encoding these polypeptides, including fragments and/or variants, are also
encompassed by the invention.
[0915] Albumin fusion proteins expressed in yeast as described above can be
purified on a small-scale over a Dyax peptide affinity column as
follows. Supematants from yeast expressing an albumin fusion protein is
diafiltrated against 3 mM phosphate buffer pH 6.2, 20 mM NaCI and
0.01% Tween 20 to reduce the volume and to remove the pigments. The solution
is then filtered through a 0.22 gm device. The filtrate is loaded
onto a Dyax peptide affinity column. The column is eluted with 100 mM
Tris/HCl, pH 8.2 buffer. The peak fractions containing protein are
collected and analyzed on SDS-PAGE atter concentrating 5-fold.
[0916] For large scale purification, the following method can be utilized. The
supernatant in excess of 2 L is diafiltered and concentrated to 500
mL in 20 mM Tris/HCI pH 8Ø The concentrated protein solution is loaded onto
a pre-equilibrated 50 mI. DEAE-Sepharose Fast Flow column, the
column is washed, and the protein is eluted with a linear gradient of NaCI
from 0 to 0.4 M NaCI in 20 mM Tris/HCI, pH 8Ø Those fractions
containing the protein are pooled, adjusted to pH 6.8 with 0.5 M sodium
phosphate (NaH2PO4). A final concentration of 0.9 M (NH4)2S04 is added
to the protein solution and the whole solution is loaded onto a pre-
equilibrated 50 mL Buty1650S column. The protein is eluted with a linear
gradient of ammonium sulfate (0.9 to 0 M(NHb)ZSO4). Those fractions with the
albumin fusion are again pooled, diafiltered against 10 mM
NazHP04/citric acid buffer pH 5.75, and loaded onto a 50 mL pre-equilibrated
SP-Sepharose Fast Flow column. The protein is eluted with a NaCI
linear gradient from 0 to 0.5 M. The fractions containing the protein of
interest are combined, the buffer is changed to 10 mM NazHPO4/citric acid
pH 6.25 with an Amicon concentrator, the conductivity is < 2.5 mS/cm. This
protein solution is loaded onto a 15 mL pre-equilibrated Q-Sepharose
high performance column, the column is washed, and the protein is eluted with
a NaCI linear gradient from 0 to 0.15 M NaC1. The purified protein
can then-be -formulated into a specific buffer composition by buffer exchange.
EXAMPLE 5: General Construct Generation for Maznznalian Cell Transfection.
Generation of albmnin fusion construct compatible for expression in matnmalian
cell-lines.
[0917] Albumin fusion constructs can be generated in expression vectors for
use in mammalian cell culture systems. DNA encoding a
therapeutic protein can be cloned N-terminus or C-terminus to HSA in a
mammalian expression vector by standard methods known in the art (e.g.,
PCR amplification, restriction digestion, and ligation). Once the expression
vector has been constructed, transfection into a mammalian expression
system can proceed. Suitable vectors are known in the art including, but not
limited to, for example, the pC4 vector, and/or vectors available from
Lonza Biologics, Inc. (Portsmouth, NH).
[0918] The DNA encoding human serum albumin has been cloned into the pC4
vector which is suitable for mammalian culture systems,
creating plasmid pC4:HSA (ATCC Deposit # PTA-3277). This vector has a
DiHydroFolate Reductase, "DI-IFR", gene that will allow for selection
in the presence of methotrexate.
[0919] The pC4:HSA vector is suitable for expression of albumin fusion
proteins in CHO cells. For expression, in other mammalian cell culture
systems, it may be desirable to subclone a fragment comprising, or
alternatively consisting of, DNA which encodes for an albumin fusion protein
into an alternative expression vector. For example, a fragment comprising, or
alternatively consisting, of DNA which encodes for a mature albumin
fusion protein may be subcloned into another expression vector including, but
not limited to, any of the mammalian expression vectors described
herein.
[0920] In a preferred embodiment, DNA encoding an albumin fusion construct is
subcloned into vectors provided by Lonza Biologics, Inc.
(Portsmouth, NH) by procedures known in the art for expression in NSO cells.
Generation of albuznin fusion constructs comDrising HSA-Therapeutic Protein
zsion products.
[0921] Using pC4:HSA (ATCC Deposit # PTA-3277), albumin fusion constructs can
be generated in which the Therapeutic protein portion is C
terminal to the mature albumin sequence. For example, one can clone DNA
encoding a Therapeutic protein of fragment or variant thereof between
the Bsu 361 and Asc I restriction sites of the vector. When cloning into the
Bsu 361 and Asc I, the same primer design used to clone into the yeast
vector system (SEQ ID NO:42 and 43) may be employed (see Example 2).
136
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~~" ~' ~) ,. ~~ ='' ~f.,,~} .;:C11e~)7~) ~'t~b~9'of ki~)iiiiv~iu::~~sir~jv
~instrucis comprrsinggene-HSA fusioiz product.s.
[0922] Using pC4:HSA (ATCC Deposit # PTA-3277), albumin fusion constructs can
be generated in which a Therapeutic protein portion is
cloned N terminal to the mature albumin sequence. For example, one can clone
DNA encoding a Therapeutic protein that lias its own signal
sequence between the Barn HI (or Hrnd III) and Cla I sites of pC4:HSA. When
cloning into either the Baru HI or Hind III site, it is preferrable to
include a Kozak sequence (CCGCCACCATG, SEQ ID NO:49) prior to the
translational start codon of the DNA encoding the Therapeutic protein.
If a Therapeutic protein does not have a signal sequence, DNA encoding that
Therapeutic protein may be cloned in between the A7:o I and Cla I sites
of pC4:HSA. When using theX7zo I site, the following 5' (SEQ ID NO:50) and 3'
(SEQ ID NO:51) exemplary PCR primers may be used:
5'-CCGCCG TCGA GGGTGTGTTTCGTCGA(N)1$-3' (SEQ ID NO: 50)
5'-AGTCCCATCGATGAGCAACCTCACTCTTGTGTGCATC(N)1$-3' (SEQ ID NO:51)
[0923] In the 5' primer (SEQ ID NO:50), the underlined sequence is a Xho I
site; and the Xho I site and the DNA following the Xho I site code
for the last seven amino acids of the leader sequence of natural human serum
albumin. In SEQ ID NO:50, "(N)18" is DNA identical to the first 18
nucleotides encoding the Therapeutic protein of interest. In the 3' primer
(SEQ ID NO:51), the underlined sequence is a Cla I site; and the Cla I
site and the DNA following it are the reverse complement of the DNA encoding
the first 10 amino acids of the mature HSA protein (SEQ ID NO:1).
In SEQ ID NO:51 "(N)18" is the reverse complement of DNA encoding the last 18
nucleotides encoding the Therapeutic protein of interest. Using
these two primers, one may PCR amplify the Therapeutic protein of interest,
purify the PCR product, digest it with Xho I and Cla I restriction
enzymes and clone it into the Xho I and Cla I sites in the pC4:HSA vector.
[0924] If an alternative leader sequence is desired, the native albumin leader
sequence can be replaced with the chimeric albumin leader, i.e., the
HSA-kex2 signal peptide, or an alternative leader by standard methods known in
the art. (For example, one skilled in the art could routinely PCR
amplify an alternate leader and subclone the PCR product into an albumin
fusion construct in place of the albumin leader while maintaining the
reading frame).
EXAMPLE 6: Geaeral Expression in Mafnfnaliaa Cell-Liaes.
[0925] An albumin fusion construct generated in an expression vector
compatible with expression in mammalian cell-lines can be transfected
into appropriate cell-lines by calcium phosphate precipitation, lipofectamine,
electroporation, or other transfection methods known in the art and/or
as described in Sambrook, Fritsch, and Maniatis. 1989. "Molecular Cloning: A
Laboratory Manual, 2nd edition" and in Ausubel et al. 2000.
Massachusetts General Hospital and Harvard Medical School "Current Protocols
in Molecular Biology", volumes 1-4. The transfected cells are then
selected for by the presence of a selecting agent determined by the selectable
marker in the expression vector.
[0926] The pC4 expression vector (ATCC Accession No. 209646) is a derivative
of the plasmid pSV2-DHFR (ATCC Accession No. 37146).
pC4 contains the strong promoter Long Terminal Repeats "LTR" of the Rous
Sarcoma Virus (Cullen et al., March 1985, Molecular and Cellular
Biology, 438-4_47) anda fragment of#he CytoMegaloVirus"CMV"-enhancer (Boshart
et al., 1985,-Ce1141: 521-530). -Thevector-also contains-the-
3' intron, the polyadenylation and termination signal of the rat preproinsulin
gene, and the mouse DHFR gene under control of the SV40 early
promoter. Chinese hamster ovary "CHO" cells or other cell-lines lacking an
active DHFR gene are used for transfection. Transfection of an
albumin fusion construct in pC4 into CHO cells by methods laiown in the art
will allow for the expression of the albumin fusion protein in CHO
cells, followed by leader sequence cleavage, and secretion into the
supematant. The albumin fusion protein is then further purified from the
supernatant.
[0927] The pEE12.1 expression vector is provided by Lonza Biologics, Inc.
(Portsmouth, NH) and is a derivative of pEE6 (Stephens and
Cockett, 1989, Nucl. Acids Res. 17: 7110). This vector comprises a promoter,
enhancer and complete 5'-untranslated region of the Major
Immediate Early gene of the human CytoMegaloVirus, "hCMV-MIE" (International
Publication # W089/01036), upstream of a sequence of
interest, and a Glutamine Synthetase gene (Murphy et al., 1991, Biochem J.
227: 277-279; Bebbington et al., 1992, Bio/Technology 10:169-175;
US patent US 5,122,464) for purposes of selection of transfected cells in
selective methionine sulphoximine containing medium. Transfection of
albumin fusion constructs made in pEE12.1 into NSO cells (Intemational
Publication # W086/05807) by methods known in the art will allow for the
expression of the albumin fusion protein in NSO cells, followed by leader
sequence cleavage, and secretion into the supernatant. The albumin fusion
protein is then further purified from the supernatant using techniques
described herein or otherwise known in the art.
[0928] Expression of an albumin fusion protein may be analyzed, for example,
by SDS-PAGE and Western blot, reversed phase HPLC analysis,
or other methods known in the art.
[0929] Stable CHO and NSO cell-lines transfected with albumin fusion
constructs are generated by methods known in the art (e.g.,
lipofectamine transfection) and selected, for example, with 100 nM
methotrexate for vectors having the DiHydroFolate Reductase 'DHFR' gene as a
selectable marker or through growth in the absence of glutamine. Expression
levels can be examined for example, by immunoblotting, primarily,
with an anti-HSA serum as the primary antibody, or, secondarily, with serum
containing antibodies directed to the Therapeutic protein portion of a
given albumin fusion protein as the primary antibody.
[0930] Expression levels are examined by immunoblot detection with anti-HSA
serum as the primary antibody. The specific productivity rates
are determined via ELISA in which the capture antibody can be a monoclonal
antibody towards the therapeutic protein portion of the albumin fusion
137
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~' E~ ~and t'{u~~e~L'Ye~ti~~;8ntibdSy noclonal anti-HSA-biotinylated antibody
(or vice versa), followed by horseradish peroxidase/streptavidin
binding and analysis according to the manufacturer's protocol.
EX4MPLE 7: Exnression ofmt Albrunin Fusiou Proteiu in Mammaliart Ce11.s.
[0931] The albumin fusion proteins of the present invention can be expressed
in a mammalian cell. A typical mammalian expression vector
contains a promoter element, which mediates the initiation of transcription of
mRNA, a protein coding sequence, and signals required for the
termination of transcription and polyadenylation of the transcript. Additional
elements include enhancers, Kozak sequences and intervening
sequences flanked by donor and acceptor sites for RNA splicing. Higlily
efficient transcription is achieved with the early and late promoters from
SV40, the long terminal repeats (LTRs) from Retroviruses, e.g., RSV, HTLVI,
HIVI and the early promoter of the cytomegalovirus (CMV).
However, cellular elements can also be used (e.g., the human actin promoter).
[0932] Suitable expression vectors for use in practicing the present invention
include, for example, vectors such as, pSVL and pMSG
(Pharmacia, Uppsala, Sweden), pRSVcat (ATCC 37152), pSV2dhfr (ATCC 37146),
pBC12MI (ATCC 67109), pCMVSport 2.0, and pCMVSport
3Ø Mammalian host cells that could be used include, but are not limited to,
human Hela, 293, H9 and Jurkat cells, mouse NIH3T3 and C127 cells,
Cos 1, Cos 7 and CV1, quail QC1-3 cells, mouse L cells and Chinese hamster
ovary (CHO) cells.
[0933] Alternatively, the albumin fusion protein can be expressed in stable
cell lines containing the polynucleotide encoding the albumin fusion
protein integrated into a chromosome. The co-transfection with a selectable
marker such as DHFR, gpt, neomycin, or hygromycin allows the
identification and isolation of the transfected cells.
[0934] The transfected polynucleotide encoding the fusion protein can also be
amplified to express large amounts of the encoded fusion protein.
The DHFR (dihydrofolate reductase) marker is useful in developing cell lines
that carry several hundred or even several thousand copies of the gene
of interest. (See, e.g., Alt et al., J. Biol. Chem. 253:1357-1370 (1978);
Hamlin et al., Biochem. et Biophys. Acta, 1097:107-143 (1990); Page et al.,
Biotechnology 9:64-68 (1991)). Another useful selection marker is the enzyme
glutamine synthase (GS) (Murphy et al., Biochem J. 227:277-279
(1991); Bebbington et al., Bio/Technology 10:169-175 (1992). Using these
markers, the mammalian cells are grown in selective medium and the
cells with the highest resistance are selected. These cell lines contain the
amplified gene(s) integrated into a chromosome. Chinese hamster ovary
(CHO) and NSO cells are often used for the production of proteins.
[0935] Derivatives of the plasmid pSV2-dhfr (ATCC Accession No. 37146), the
expression vectors pC4 (ATCC Accession No. 209646) and
pC6 (ATCC Accession No.209647) contain the strong promoter (LTR) of the Rous
Sarcoma Virus (Cullen et al., Molecular and Cellular Biology,
438-447 (March, 1985)) plus a fragment of the CMV-enhancer (Boshart et al.,
Cell 41:521-530 (1985)). Multiple cloning sites, e.g., with the
restriction enzyme cleavage sites BamHI, XbaI and Asp718, facilitate the
cloning of the gene of interest. The vectors also contain the 3' intron, the
polyadenylation and termination signal of the rat preproinsulin gene, and the
mouse DHFR gene under control of the SV40 early promoter.
[0936] Specifically, the -plasmid -pC6, for -example, is digested with
appropriate -restriction enzymes -and--then- dephosphorylated -using calf
intestinal phosphates by procedures known in the art. The vector is then
isolated from a 1% agarose gel.
[0937] A polynucleotide encoding an albumin fusion protein of the present
invention is generated using techniques laiown in the art and this
polynucleotide is amplified using PCR technology known in the art. If a
naturally occurring signal sequence is used to produce the fusion protein of
the present invention, the vector does not need a second signal peptide.
Alternatively, if a naturally occurring signal sequence is not used, the
vector
can be modified to include a heterologous signal sequence. (See, e.g.,
International Publication No. WO 96/34891.)
[0938] The amplified fragment encoding the fusion protein of the invention is
isolated from a 1% agarose gel using a commercially available kit
("Geneclean," BIO 101 Inc., La Jolla, Ca.). The fragment then is digested with
appropriate restriction enzymes and again purified on a 1% agarose
gel.
[0939] The amplified fragment encoding the albumin fusion protein of the
invention is then digested with the same restriction enzyme and
purified on a 1% agarose gel. The isolated fragment and the dephosphorylated
vector are then ligated with T4 DNA ligase. E. coli HB 101 or XL-1
Blue cells are then transformed and bacteria are identified that contain the
fragment inserted into plasmid pC6 using, for instance, restriction enzyme
analysis.
[0940] Chinese hamster ovary cells lacking an active DHFR gene is used for
transfection. Five g of the expression plasmid pC6 or pC4 is
cotransfected with 0.5 g of the plasmid pSVneo using lipofectin (Felgner et
al., supra). The plasmid pSV2-neo contains a dominant selectable
marker, the rseo gene from Tn5 encoding an enzyme that confers resistance to a
group of antibiotics including G418. The cells are seeded in alpha
minus MEM supplemented with 1 mg/ml G418. After 2 days, the cells are
trypsinized and seeded in hybridoma cloning plates (Greiner, Germany)
in alpha minus MEM supplemented with 10, 25, or 50 ng/ml of methotrexate plus
1 mg/ml G418. After about 10-14 days single clones are
trypsinized and then seeded in 6-well petri dishes or 10 ml flasks using
different concentrations of methotrexate (50 nM, 100 nM, 200 nM, 400 nM,
800 nM). Clones growing at the highest concentrations of methotrexate are then
transferred to new 6-well plates containing even higher
concentrations of methotrexate (1 M, 2 M, 5 M, 10 mM, 20 mM). The same
procedure is repeated until clones are obtained which grow at a
concentration of 100 - 200 M. Expression of the desired fusion protein is
analyzed, for instance, by SDS-PAGE and Western blot or by reversed
phase HPLC analysis.
138
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
!Fli;AiRtI~'' PEEti~~ 6,iitei!~t' rD-lrX ,~!'icatiat of au Albrurefit Firsiou
Protein Expressed frora an Albunein Fusioar Corrstruct iu Mammalian
Cell-liue.c.
[0941] In preferred embodiments, albumin fusion proteins of the invention
comprise the mature form of HSA fused to eitlier the N- or C-
terminus of the mature form of a therapeutic protein or portions thereof
(e.g., the mature form of a therapeutic protein listed in Table 1, or the
mature form of a therapeutic protein shown in Table 2 as SEQ ID NO:Z). In one
embodiment of the invention, albumin fusion proteins of the
invention further comprise a signal sequence which directs the nascent fusion
polypeptide in the secretory pathways of the host used for expression.
In a preferred embodiment, the signal peptide encoded by the signal sequence
is removed, and the mature albumin fusion protein is secreted
directly into the culture medium. Albumin fusion proteins of the invention
preferably comprise heterologous signal sequences (e.g., the non-native
signal sequence of a particular therapeutic protein) including, but not
limited to, MAF, INV, Ig, Fibulin B, Clusterin, hisulin-Like Growth Factor
Binding Protein 4, variant HSA leader sequences including, but not limited to,
a chimeric HSA/MAF leader sequence, or other heterologous signal
sequences known in the art. Especially preferred as those signal sequence
listed in Table 2 and/or the signal sequence listed in the "Expression of
Fusion Proteins" and/or "Additional Methods of Recombinant and Synthetic
Production of Albumin Fusion Proteins" section of the specification,
above. In preferred embodiments, the fusion proteins of the invention further
comprise an N-terminal methionine residue. Polynucleotides
encoding these polypeptides, including fragments and/or variants, are also
encompassed by the invention.
[0942] Albumin fusion proteins from mammalian cell-line supematants are
purified according to different protocols depending on the
expression system used.
Puri rcatiora from CHO and 293T cell-lines.
[0943] Purification of an albumin fusion protein from CHO cell supematant or
from transiently transfected 293T cell supernatant may involve
initial capture with an anionic HQ resin using a sodium phosphate buffer and a
phosphate gradient elution, followed by affinity chromatography on a
Blue Sepharose FF column using a salt gradient elution. Blue Sepharose FF
removes the main BSA/fetuin contaminants. Further purification over
the Poros PI 50 resin with a phosphate gradient may remove and lower endotoxin
contamination as well as concentrate the albumin fusion protein.
Puri ication from NSO cell-line.
[0944] Purification of an albumin-fusion protein from NSO cell supernatant may
involve Q-Sepharose anion exchange chromatography,
followed by SP-sepharose purification with a step elution, followed by Phenyl-
650M purification with a step elution, and, ultimately, diafiltration.
[0945] The purified protein may then be formulated by buffer exchange.
EXAMPLE 9: Bacterial Exnression of an Albumia Fnsion Proteia.
[0946] A polynucleotide encoding an albumin fusion protein of the present
invention comprising a bacterial signal sequence is amplified using
PCR oligonucleotide primers corresponding to the 5' and 3' ends of the DNA
sequence, to synthesize insertion fragments. The primers used to
amplify thepolynucleotide encoding insert should-preferably contain-
restriction sites; such as BamHI- and Xbal; at the 5' end of the primers-in-
order -
to clone the amplified product into the expression vector. For example, BamHI
and XbaI correspond to the restriction enzyme sites on the bacterial
expression vector pQE-9. (Qiagen, Inc., Chatsworth, CA). This plasmid vector
encodes antibiotic resistance (Ampr), a bacterial origin of replication
(ori), an IPTG-regulatable promoter/operator (P/0), a ribosome binding site
(RBS), a 6-histidine tag (6-His), and restriction enzyme cloning sites.
[0947] The pQE-9 vector is digested with BamHI and Xbal and the amplified
fragment is ligated into the pQE-9 vector maintaining the reading
frame initiated at the bacterial RBS. The ligation mixture is then used to
transform the E. coli strain M15/rep4 (Qiagen, Inc.) which contains
multiple copies of the plasmid pREP4, which expresses the lacI repressor and
also confers kanamycin resistance (Kanr). Transformants are
identified by their ability to grow on LB plates and ampicillin/kanamycin
resistant colonies are selected. Plasmid DNA is isolated and confirmed by
restriction analysis.
[0948] Clones containing the desired constructs are grown overnight (O/N) in
liquid culture in LB media supplemented with both Amp (100
ug/ml) and Kan (25 ug/m1). The O/N culture is used to inoculate a large
culture at a ratio of 1:100 to 1:250. The cells are grown to an optical
density 600 (O.D.6oo) of between 0.4 and 0.6. IPTG (Isopropyl-B-D-thiogalacto
pyranoside) is then added to a fmal concentration of 1 mM. IPTG
induces by inactivating the IacI repressor, clearing the P/0 leading to
increased gene expression.
[0949] Cells are grown for an extra 3 to 4 hours. Cells are then harvested by
centrifugation (20 mins at 6000Xg). The cell pellet is solubilized
in the chaotropic agent 6 Molar Guanidine HCI or preferably in 8 M urea and
concentrations greater than 0.14 M 2-mercaptoethanol by stirring for
3-4 hours at 4 C (see, e.g., Burton et al., Eur. J. Biochem. 179:379-387
(1989)). The cell debris is removed by centrifugation, and the supernatant
containing the polypeptide is loaded onto a nickel-nitrilo-tri-acetic acid
("Ni-NTA") affmity resin column (available from QIAGEN, Inc., supra).
Proteins with a 6 x His tag bind to the Ni-NTA resin with high affmity and can
be purified in a simple one-step procedure (for details see: The
QlAexpressionist (1995) QIAGEN, Ine., supra).
[0950] Briefly, the supernatant is loaded onto the column in 6 M guanidine-
HCI, pH 8. The column is first washed with 10 volumes of 6 M
guanidine-HCI, pH 8, then washed with 10 volumes of 6 M guanidine-HCl pH 6,
and finally the polypeptide is eluted with 6 M guanidine-HCI, pH
5.
139
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
If~t~fl:;~..C~_ ~,.q f~~~~ -;1o1 r.f --~~-
OH51' Th~ i i~d'prd[ein =9 t1i9n i&red by dialyzing it against phosphate-
buffered saline (PBS) or 50 mM Na-acetate, pH 6 buffer plus
200 mM NaCI. Alternatively, the protein can be successfully refolded while
immobilized on the Ni-NTA column. Exemplary conditions are as
follows: renature using a linear 6M-1M urea gradient in 500 mM NaCI, 20%
glycerol, 20 mM Tris/HCI pH 7.4, containing protease inhibitors. The
renaturation should be performed over a period of 1.5 hours or more. After
renaturation the proteins are eluted by the addition of 250 mM
immidazole. Immidazole is removed by a final dialyzing step against PBS or 50
mM sodium acetate pH 6 buffer plus 200 mM NaCI. The purified
protein is stored at 4 C or frozen at -80 C.
[0952] In addition to the above expression vector, the present invention
further includes an expression vector, called pHE4a (ATCC Accession
Number 209645, deposited on February 25, 1998) which contains phage operator
and promoter elements operatively linked to a polynucleotide
encoding an albumin fusion protein of the present invention, called pHE4a.
(ATCC Accession Number 209645, deposited on February 25, 1998.)
This vector contains: 1) a neomycinphosphotransferase gene as a selection
marker, 2) an E. coli origin of replication, 3) a T5 phage promoter
sequence, 4) two lac operator sequences, 5) a Shine-Delgamo sequence, and 6)
the lactose operon repressor gene (laclq). The origin of replication
(oriC) is derived from pUC19 (LTI, Gaithersburg, MD). The promoter and
operator sequences are made synthetically.
[0953] DNA can be inserted into the pHE4a by restricting the vector with NdeI
and XbaI, BamHI, XhoI, or Asp718, running the restricted
product on a gel, and isolating the larger fragment (the stuffer fragment
should be about 310 base pairs). The DNA insert is generated according to
PCR protocols described herein or otherwise known in the art, using PCR
primers having restriction sites for Ndel (5' primer) and XbaI, BamHI,
XhoI, or Asp718 (3' primer). The PCR insert is gel purified and restricted
with compatible enzymes. The insert and vector are ligated according to
standard protocols.
[0954] The engineered vector may be substituted in the above protocol to
express protein in a bacterial system.
EXAMPLE 10: Isolation of a Selected cDNA Clone From tlze Deposited Sample.
[0955] Many of the albumin fusion constructs of the invention have been
deposited with the ATCC as shown in Table 3. The albumin fusion
constructs may comprise any one of the following expression vectors: the yeast
S. cerevisiae expression vector pSAC35, the mammalian expression
vector pC4, or the mammalian expression vector pEE12.1.
[0956] pSAC35 (Sleep et al., 1990, Biotechnology 8:42), pC4 (ATCC Accession
No. 209646; Cullen et al., Molecular and Cellular Biology,
438-447 (1985); Boshart et al., Cel141: 521-530 (1985)), and pEE12.1 (Lonza
Biologics, Inc.; Stephens and Cockett, NucI. Acids Res. 17: 7110
(1989); International Publication #W089/01036; Murphy et al., Biochem J. 227:
277-279 (1991); Bebbington et al., Bio/Technology 10:169-175
(1992); US patent US 5,122,464; International Publication #W086/05807) vectors
comprise an ampicillin resistance gene for growth in bacterial
cells. These vectors and/or an albumin fusion construct comprising them can be
transformed into an E. colf strain such as Stratagene XL-1 Blue
(Stratagene Cloning Systems, Inc., 11011 N. Torrey Pines Road, La Jolla, CA,
92037) using techniques described in the art such as Hanahan, spread
--onto Luria-Broth agar plates containing 100 g/mL ampicillin, and-grown
overnight at37 C:
[0957] The deposited material in the sample assigned the ATCC Deposit Number
cited in Table 3 for any given albumin fusion construct also
may contain one or more additional albumin fusion constructs, each encoding
different albumin fusion proteins. Thus, deposits sharing the same
ATCC Deposit Number contain at least an albumin fusion construct identified in
the corresponding row of Table 3.
[0958] Two approaches can be used to isolate a particular albumin fusion
construct from the deposited sample of plasmid DNAs cited for that
albumin fusion construct in Table 3.
Metliod 1: Screening
[0959] First, an albumin fusion construct may be directly isolated by
screening the sample of deposited plasmid DNAs using a polynucleotide
probe corresponding to SEQ ID NO:X for an individual construct ID number in
Table 1, using methods known in the art. For example, a specific
polynucleotide with 30-40 nucleotides may be synthesized using an Applied
Biosystems DNA synthesizer according to the sequence reported. The
oligonucleotide can be labeled, for instance, with 32P-y-ATP using T4
polynucleotide kinase and purified according to routine methods. (E.g.,
Maniatis et al., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor
Press, Cold Spring, NY (1982)). The albumin fusion construct from
a given ATCC deposit is transformed into a suitable host, as indicated above
(such as )9-1 Blue (Stratagene)) using techniques known to those of
skill in the art, such as those provided by the vector supplier or in related
publications or patents cited above. The transformants are plated on 1.5%
agar plates (containing the appropriate selection agent, e.g., ampicillin) to
a density of about 150 transformants (colonies) per plate. These plates are
screened using Nylon membranes according to routine methods for bacterial
colony screening (e.g., Sambrook et al., Molecular Cloning: A
Laboratory Manual, 2nd Edit., (1989), Cold Spring Harbor Laboratory Press,
pages 1.93 to 1.104), or other techniques Imown to those of skill in the
art.
Metl:od 2: PCR
[0960] Alternatively, DNA encoding a given albumin fusion protein may be
amplified from a sample of a deposited albumin fusion construct
with SEQ ID NO:X, for example, by using two primers of 17-20 nucleotides that
hybridize to the deposited albumin fusion construct 5' and 3' to
the DNA encoding a given albumin fusion protein. The polymerase chain reaction
is carried out under routine conditions, for instance, in 25 l of
reaction mixture with 0.5 ug of the above cDNA template. A convenient reaction
mixture is 1.5-5 mM MgC12, 0.01% (w/v) gelatin, 20 M each of
140
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~h.. tfdAT;' I w,.. IC'1~',L ;' n2' õ fioul'llof.'.
dT~1 P ,7iSn ach primer and 0.25 Unit of Taq polymerase. Thirty five cycles of
PCR (denaturation at 94 C for 1 min;
annealing at 55 C for I min; elongation at 72 C for 1 min) are performed with
a Perkin-Elmer Cetus automated thermal cycler. The amplified
product is analyzed by agarose gel electrophoresis and the DNA band with
expected molecular weight is excised and purified. The PCR product is
verified to be the selected sequence by subcloning and sequencing the DNA
product.
[09611 Several methods are available for the identification of the 5' or 3'
non-coding portions of a gene which may not be present in the
deposited clone. These methods include but are not limited to, filter probing,
clone enrichment using specific probes, and protocols similar or
identical to 5' and 3' "RACE" protocols wliich are known in the art. For
instance, a method similar to 5' RACE is available for generating the
missing 5' end of a desired full-length transcript. (Fromont-Racine et al.,
Nucleic Acids Res., 21(7):1683-1684 (1993)).
[0962] Briefly, a specific RNA oligonucleotide is ligated to the 5' ends of a
population of RNA presumably containing full-length gene RNA
transcripts. A primer set containing a primer specific to the ligated RNA
oligonuoleotide and a primer specific to a known sequence of the gene of
interest is used to PCR amplify the 5' portion of the desired full-length
gene. This amplified product may then be sequenced and used to generate
the full length gene.
[0963] This above method starts with total RNA isolated from the desired
source, although poly-A+ RNA can be used. The RNA preparation
can then be treated with phosphatase if necessary to eliminate 5' phosphate
groups on degraded or damaged RNA which may interfere with the later
RNA ligase step. The phosphatase should then be inactivated and the RNA
treated with tobacco acid pyrophosphatase in order to remove the cap
structure present at the 5' ends of messenger RNAs. This reaction leaves a 5'
phosphate group at the 5' end of the cap cleaved RNA which can then
be ligated to an RNA oligonucleotide using T4 RNA ligase.
[0964] This modified RNA preparation is used as a template for first strand
cDNA synthesis using a gene specific oligonucleotide. The first
strand synthesis reaction is used as a template for PCR amplification of the
desired 5' end using a primer specific to the ligated RNA oligonucleotide
and a primer specific to the known sequence of the gene of interest. The
resultant product is then sequenced and analyzed to confirm that the 5' end
sequence belongs to the desired gene.
EXAMPLE II: Multifusion Fiisions.
[0965] The albumin fusion proteins (e.g,. containing a Therapeutic protein (or
fragment or variant thereof) fused to albumin (or a fragment or
variant thereof)) may additionally be fused to other proteins to generate
"multifusion proteins". These multifusion proteins can be used for a variety
of applications. For example, fusion of the albumin fusion proteins of the
invention to His-tag, HA-tag, protein A, IgG domains, and maltose
binding protein facilitates purification. (See e.g,. EP A 394,827; Traunecker
et al., Nature 331:84-86 (1988)). Nuclear localization signals fused to
the polypeptides of the present invention can target the protein to a specific
subcellular localization, while covalent heterodimer or homodimers can
increase or decrease the activity of an albumin fusion protein. Furthermore,
the fusion of additional protein sequences to the albumin fusion
proteins of the -invention may further increase the solubility and/or
stability of the fusion-protein. - The fusion proteins described above can be
made---
using or routinely modifting techniques known in the art and/or by modifying
the following protocol, which outlines the fusion of a polypeptide to an
IgG molecule.
[0966] Briefly, the human Fc portion of the IgG molecule can be PCR amplified,
using primers that span the 5' and 3' ends of the sequence
described below. These primers also should have convenient restriction enzyme
sites that will facilitate cloning into an expression vector, preferably
a mammalian or yeast expression vector.
[0967] For example, if pC4 (ATCC Accession No. 209646) is used, the human Fc
portion can be ligated into the BamHI cloning site. Note that
the 3' BamHI site should be destroyed. Next, the vector containing the human
Fe portion is re-restricted with BamHI, linearizing the vector, and a
polynucleotide encoding an albumin fusion protein of the present invention
(generateed and isolated using techniques known in the art), is ligated
into this BamHI site. Note that the polynucleotide encoding the fusion protein
of the invention is cloned without a stop codon, otherwise a Fo
containing fusion protein will not be produced.
[0968] If the naturally occurring signal sequence is used to produce the
albumin fusion protein of the present invention, pC4 does not need a
second signal peptide. Altematively, if the naturally occurring signal
sequence is not used, the vector can be modified to include a heterologous
signal sequence. (See, e.g., International Publication No. WO 96/34891.)
[0969] Human IgG Fc region:
GGGATCCGGAGCCCAAATCTTCTGACAAAACTCACACATGCCCACCGTGCCCAGCACCTGAATTCGAGGGTGCACCGTC
AGTCTTCC
TCTTCCCCCCAAAACCCAAGGACACCCTCATGATCTCCCGGACTCCTGAGGTCACATGCGTGGTGGTGGACGTAAGCCA
CGAAGACC
CTGAGGTCAAGTTCAACTGGTACGTGGACGGCGTGGAGGTGCATAATGCCAAGACAAAGCCGCGGGAGGAGCAGTACAA
CAGCACG
TACCGTGTGGTCAGCGTCCTCACCGTCCTGCACCAGGACTGGCTGAATGGCAAGGAGTACAAGTGCAAGGTCTCCAACA
AAGCCCTC
CCAACCCCCATCGAGAAAACCATCTCCAAAGCCAAAGGGCAGCCCCGAGAACCACAGGTGTACACCCTGCCCCCATCCC
GGGATGA
GCTGACCAAGAACCAGGTCAGCCTGACCTGCCTGGTCAAAGGCTTCTATCCAAGCGACATCGCCGTGGAGTGGGAGAGC
AATGGGC
AGCCGGAGAACAACTACAAGACCACGCCTCCCGTGCTGGACTCCGACGGCTCCTTCTTCCTCTACAGCAAGCTCACCGT
GGACAAGA
GCAGGTGGCAGCAGGGGAACGTCTTCTCATGCTCCGTGATGCATGAGGCTCTGCACAACCACTACACGCAGAAGAGCCT
CTCCCTGT
141
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
fi ~CC:~C'i~i~l ~AJA~Y~PGA~iTGIGAGG&GCGACTCTAGAGGAT (SEQ ID NO:52)
EYAMPLE 12: Prodnction of arn Autibody from art Albrurriu Frtsiou Proteiu.
Hvbrieloma Tecimolog)?
[0970] Antibodies that bind the albumin fusion proteins of the present
invention and portions of the albumin fusion proteins of the present
invention (e.g., the Therapeutic protein portion or albumin portion of the
fusion protein) can be prepared by a variety of methods. (See, Current
Protocols, Chapter 2.) As one example of such methods, a preparation of an
albumin fusion protein of the invention or a portion of an albumin
fusion protein of the invention is prepared and purified to render it
substantially free of natural contaminants. Such a preparation is then
introduced
into an animal in order to produce polyclonal antisera of greater specific
activity.
[0971] Monoclonal antibodies specific for an albumin fusion protein of the
invention, or a portion of an albumin fusion protein of the invention,
are prepared using hybridoma technology (Kohler et al., Nature 256:495 (1975);
Kohler et al., Eur. J. Immunol. 6:511 (1976); Kohler et al., Eur. J.
Immunol. 6:292 (1976); Hammerling et al., in: Monoclonal Antibodies and T-Cell
Hybridomas, Elsevier, N.Y., pp. 563-681 (1981)). In general,
an animal (preferably a mouse) is immunized with an albumin fusion protein of
the invention, or a portion of an albumin fusion protein of the
invention. The splenocytes of such mice are extracted and fused with a
suitable myeloma cell line. Any suitable myeloma cell line may be
employed in accordance with the present invention; however, it is preferable
to employ the parent myeloma cell line (SP2O), available from the
ATCC. After fusion, the resulting hybridoma cells are selectively maintained
in HAT medium, and then cloned by limiting dilution as described by
Wands et al. (Gastroenterology 80:225-232 (1981)). The hybridoma cells
obtained through such a selection are then assayed to identify clones
which secrete antibodies capable of binding an albumin fusion protein of the
invention, or a portion of an albumin fusion protein of the invention.
[0972] Alternatively, additional antibodies capable of binding to an albumin
fusion protein of the invention, or a portion of an albumin fusion
protein of the invention can be produced in a two-step procedure using anti-
idiotypic antibodies. Such a method makes use of the fact that
antibodies are themselves antigens, and therefore, it is possible to obtain an
antibody which binds to a second antibody. In accordance with this
method, protein specific antibodies are used to immunize an animal, preferably
a mouse. The splenocytes of such an animal are then used to
produce hybridoma cells, and the hybridoma cells are screened to identify
clones which produce an antibody whose ability to bind to the an albumin
fusion protein of the invention (or portion of an albumin fusion protein of
the invention) -specific antibody can be blocked by the fusion protein of
the invention, or a portion of an albumin fusion protein of the invention.
Such antibodies comprise anti-idiotypic antibodies to the fusion protein of
the invention (or portion of an albumin fusion protein of the invention) -
specific antibody and are used to immunize an animal to induce formation
of further fusion protein of the invention (or portion of an albumin fusion
protein of the invention) -specific antibodies.
[0973] For in vivo use of antibodies in humans, an antibody is "humanized".
Such antibodies can be produced using genetic constructs derived
from hybridoma cells producing the monoclonal antibodies described above.
Methods for producing chimeric and humanized antibodies are known
in the art and are discussed-herein:- (See,-for review, Morrison -Science
229:1202 (1985); Oi et al.; BioTechniques 4:214 (1986); Cabilly et al., U.S.
Patent No. 4,816,567; Taniguchi et al., EP 171496; Morrison et al., EP 173494;
Neuberger et al., WO 8601533; Robinson et al., International
Publication No. WO 8702671; Boulianne et al., Nature 312:643 (1984); Neuberger
et al., Nature 314:268 (1985)).
[0974] Isolation OfAntiboa'y Fragments Directed Against an albumin fusion
protein of the invention, or a portion of an albumin fusion proteiaz
of the invention From A Library Of scFvs. Naturally occurring V-genes isolated
from human PBLs are constructed into a library of antibody
fragments which contain reactivities against an albumin fusion protein of the
invention, or a portion of an albumin fusion protein of the invention, to
which the donor may or may not have been exposed (see e.g., U.S. Patent
5,885,793 incorporated herein by reference in its entirety).
[0975] Rescue of the Library. A library of scFvs is constructed from the RNA
of human PBLs as described in Internaflorial Publication No.
WO 92/01047. To rescue phage displaying antibody fragments, approximately 109
E. coli harboring the phagemid are used to inoculate 50 ml of
2xTY containing 1% glucose and 100 g/ml of ampicillin (2xTY-AMP-GLU) and
grown to an O.D. of 0.8 with shaking. Five ml of this culture is
used to inoculate 50 ml of 2xTY-AMP-GLU, 2 x 108 TU of delta gene 3 helper
(M13 delta gene IIl, see International Publication No. WO
92/01047) are added and the culture incubated at 37 C for 45 minutes without
shaking and then at 37 C for 45 minutes with shaking. The culture
is centrifuged at 4000 r.p.m. for 10 min. and the pellet resuspended in 2
liters of 2xTY containing 100 gfml ampicillin and 50 ug/ml kanamycin
and grown overnight. Phage are prepared as described in International
Publication No. WO 92/01047.
[0976] M13 delta gene III is prepared as follows: M13 delta gene III helper
phage does not encode gene III protein, hence the phage(mid)
displaying antibody fragments have a greater avidity of binding to antigen.
Infectious M13 delta gene III particles are made by growing the helper
phage in cells harboring a pUC19 derivative supplying the wild type gene III
protein during phage morphogenesis. The culture is incubated for 1
hour at 37 C without shaking and then for a further hour at 37 C with
shaking. Cells are spun down (IEC-Centra 8,400 r.p.m. for 10 min),
resuspended in 300 ml 2xTY broth containing 100 g ampicillin/ml and 25 g
kanamycin/ml (2xTY-AMP-KAN) and grown overnight, shaking at
37 C. Phage particles are purified and concentrated from the culture medium by
two PEG-precipitations (Sambrook et al., 1990), resuspended in 2
ml PBS and passed through a 0.45 m filter (Minisart NML; Sartorius) to give a
final concentration of approximately 1013 transducing units/ml
(ampicillin-resistant clones).
[0977] Paiif:ing of the Libraiy. Immunotubes (Nunc) are coated overnight in
PBS with 4 ml of either 100 g/ml or 10 g/ml of an albumin
142
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
Uiorif'~iot'eiu"o~f flie invenliori~1 6"r~a p'orC oh of an albumin fusion
protein of the invention. Tubes are blocked with 2% Marvel-PBS for 2 hours at
37 C and then washed 3 times in PBS. Approximately 1013 TU of phage is applied
to the tube and incubated for 30 minutes at room temperature
tumbling on an over and under tumtable and then left to stand for another 1.5
hours. Tubes are washed 10 times with PBS 0.1% Tween-20 and 10
times with PBS. Phage are eluted by adding 1 ml of 100 niIvl triethylamine and
rotating 15 minutes on an under and over turntable after which the
solution is immediately neutralized with 0.5 ml of I.OM Tris-HCI, pH 7.4.
Phage are then used to infect 10 ml of mid-log E. coli TGI by incubating
eluted phage with bacteria for 30 minutes at 37 C. The E. coli are then plated
on TYE plates containing 1% glucose and 100 g/ml ampicillin. The
resulting bacterial library is then rescued with delta gene 3 helper phage as
described above to prepare phage for a subsequent round of selection.
This process is then repeated for a total of 4 rounds of affinity purification
with tube-washing increased to 20 times with PBS, 0.1% Tween-20 and
20 times with PBS for rounds 3 and 4.
[0978] Ckaracterization of Binders. Eluted phage from the 3rd and 4th rounds
of selection are used to infect E. coli HB 2151 and soluble scFv
is produced (Marks, et al., 1991) from single colonies for assay. ELISAs are
performed with microtitre plates coated with eitlier 10 pg/ml of an
albumin fusion protein of the invention, or a portion of an albumin fusion
protein of the invention, in 50 mM bicarbonate pH 9.6. Clones positive in
ELISA are further characterized by PCR fingerprinting (see, e.g.,
International Publication No. WO 92/01047) and then by sequencing. These
ELISA positive clones may also be further cliaracterized by techniques knovvn
in the art, such as, for example, epitope mapping, binding affinity,
receptor signal transduction, ability to block or competitively inhibit
antibody/antigen binding, and competitive agonistic or antagonistic activity.
EXAMPLE 13: eHI-Z-DeoxyQlucose Uptake Assay.
[0979] Adipose, skeletal muscle, and liver are insulin-sensitive tissues.
Insulin can stimulate glucose uptake/transport into these tissues. In the
case of adipose and skeletal muscle, insulin initiates the signal transduction
that eventually leads to the translocation of the glucose transporter 4
molecule, GLUT4, from a specialized intracellular compartment to the cell
surface. Once on the cell surface, GLUT4 allows for glucose
uptake/transport.
/3H7-2-Deoxvglncose U tza ake
[0980] A number of adipose and muscle related cell-lines can be used to test
for glucose uptake/transport activity in the absence or presence of a
combination of any one or more of the therapeutic drugs listed for the
treatment of diabetes mellitus. In particular, the 3T3-Ll murine fibroblast
cells and the L6 murine skeletal muscle cells can be differentiated into 3T3-
L1 adipocytes and into myotubes, respectively, to serve as appropriate in
vitro models for the [3 H]-2-deoxyglucose uptake assay (Urso et al., J Biol
Chem, 274(43): 30864-73 (1999); Wang et al., J Mol Endocrinol, 19(3):
241-8 (1997); Haspel et al., J Membr Biol, 169 (1): 45-53 (1999); Tsakiridis
et al., Endocrinology, 136(10): 4315-22 (1995)). Briefly, 2 x 105
cells/100 L of adipocytes or differentiated L6 cells are transferred to 96-
well Tissue-Culture, "TC", treated, i.e., coated with 50 g/mL of poly-L-
lysine, plates in post-differentiation medium and are incubated overnight at
37 C in 5% COZ. The cells are first washed once with serum free low
glucose DMEM medium-and are- then -starved with 100- L/well of the same medium
and with 100 L/well of either buffer or of a combinatiori of
any one or more of the therapeutic drugs listed for the treatment of diabetes
mellitus, for example, increasing concentrations of 1 nM, 10 nM, and
100 nM of the therapeutics of the subject invention (e.g., specific fusions
disclosed as SEQ ID NO:Y and fragments and variants thereof) for 16
hours at 37 C in the absence or presence of 1 nM insulin. The plates are
washed three times with 100 L/well of HEPES buffered saline. Insulin is
added at 1 nM in HEPES buffered saline for 30 min at 37 C in the presence of
10 M labeled [3 H]-2-deoxyglucose (Amersham, #TRK672) and 10
M unlabeled 2-deoxyglucose (SIGMA, D-3179). As control, the same conditions
are carried out except in the absence of insulin. A~nal
concentration of 10 }aM cytochalasin B (SIGMA, C6762) is added at 100 L/well
in a separate well to measure the non-specific uptake. The cells
are washed three times with HEPES buffered saline. Labeled, i.e., 10 pM of
[3H]-2-deoxyglucose, and unlabeled, i.e., 10 M of 2-deoxyglucose, are
added for 10 minutes at room temperature. The cells are washed three times
with cold Phosphate Buffered Sal ine, "PBS". The cells are lysed upon
the addition of 150 L/well of 0.2 N NaOH and subsequent incubation with
shaking for 20 minutes at room temperature. Samples are then
transferred to a scintillation vial to which is added 5 mL of scintillation
fluid. The vials are counted in a Beta-Scintillation counter. Uptake in
duplicate conditions, the difference being the absence or presence of insulin,
is determined with the following equation: [(Insulin counts per minute
"cpm" - Non-Specific cpm)/(No Insulin cpm - Non-Specific cpm)]. Average
responses fall within the limits of about 5-fold and 3-fold that of
controls for adipocytes and myotubes, respectively.
Differentiation ofCells
[0981] The cells are allowed to become fully confluent in a T-75 cm2 flask.
The medium is removed and replaced with 25 mL of pre-
differentiation medium for 48 hours. The cells are incubated at 37 C, in 5%
C02, 85% humidity. After 48 hours, the pre-differentiation medium is
removed and replaced with 25 mL differentiation medium for 48 hours. The cells
are again incubated at 37 C, in 5% CO2, 85% humidity. After 48
hours, the medium is removed and replaced with 30 mL post-differentiation
medium. Post-differentiation medium is maintained for 14-20 days or
until complete differentiation is achieved. The medium is changed every 2-3
days. Human adipocytes can be purchased from Zen-Bio, INC (# SA-
1096).
EXAMPLE 19: In vitro Assay of I3Hl-Tlzvmidine Incorporation into Pancreatic
Cell-lines.
143
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
li;.;: If ~ (~ o!~:iF ~ I ;, ;. = , ~n,~i ,.,~ ~.;i~ ..
[098 ] ~ a~e'cen~4y b~t n s otVn't 'ht GLP-1 induces differentiation of the
rat pancreatic ductal epithelial cell-line ARIP in a time- and dose-
dependent manner which is associated with an increase in Islet Duodenal
Homeobox-1 (IDX-1) and insulin mRNA levels (Hui et al., 2001,
Diabetes, 50(4): 785-96). The IDX-1 in turn increases mRNA levels of the GLP-1
receptor.
Cells TMes Tested
[0983] RIN-M cells: These cells are available from the American Type Tissue
Culture Collection (ATCC Cell Line Number CRL-2057 ). The
RIN-M cell line was derived from a radiation induced transplantable rat islet
cell tumor. The line was established from a nude mouse xenograft of
the tumor. The cells produce and secrete islet polypeptide hormones, and
produce L-dopa decarboxylase (a marker for cells having amine precursor
uptake and decarboaylation, or APUD, activity).
[0984] ARIP cells: These are pancreatic exocrine cells of epithelial
morpliology available from the American Type Tissue Culture Collection
(ATCC Cell Line Number CRL-1674). See also, references: Jessop, N.W. and Hay,
R.J., "Characteristics of two rat pancreatic exocrine cell lines
derived from transplantable tumors," In Vitro 16: 212, (1980); Cockell, M. et
al., "Identification of a cell-specific DNA-binding activity that
interacts with a transcriptional activator of genes expressed in the acinar
pancreas," Mol. Cell. Biol. 9: 2464-2476, (1989); Roux, E., et al. "The
cell-specific transcription factor PTF1 contains two different subunits that
interact with the DNA" Genes Dev. 3: 1613-1624, (1989); and, Hui, H.,
et al., "Glucagon-like peptide 1 induces differentiation of islet duodenal
homeobox-l-positive pancreatic ductal cells into insulin-secreting cells,"
Diabetes 50: 785-796 (2001).
Preparation of Cells
[0985] The RIN-M cell-line is grown in RPMI 1640 medium (Hyclone,
#SH300027.01) with 10% fetal bovine serum (HyClone, #SH30088.03)
and is subcultured every 6 to 8 days at a ratio of 1:3 to 1:6. The medium is
changed every 3 to 4 days.
[0986] The ARIP (ATCC #CRL-1674) cell-line is grown in Ham's F12K medium
(ATCC, #30-2004) with 2 mM L-glutamine adjusted to
contain 1.5 g/L sodium bicarbonate and 10% fetal bovine serum. The ARIP cell-
line is subcultured at a ratio of 1:3 to 1:6 twice per week. The
medium is changed every 3 to 4 days.
Assav Protocol
[0987] The cells are seeded at 4000 cells/well in 96-well plates and cultured
for 48 to 72 hours to 50% confluence. The cells are switched to
serum-free media at 100 L/well. After incubation for 48-72 hours, serum
and/or the therapeutics of the subject invention (e.g., albumin fusion
proteins of the invention and fragments and variants thereof) are added to the
well. Incubation persists for an additional 36 hours. [3H]-Thymidine
(5-20 Ci/mmol) (Amersham Pharmacia, #TRK120) is diluted to 1 microCuries/5
microliters. After the 36 hour incubation, 5 microliters is added
per well for a further 24 hours. The reaction is terminated by washing the
cells gently with cold Phosphate-Buffered Sal ine, "PBS", once. The cells
are then fixed with 100 microliters of 10% ice cold TCA for 15 min at 4 C. The
PBS is removed and 200 microliters of 0.2 N NaOH is added.
The plates are incubated for 1 hour at room temperature with shaking. The
solution is transferred to a scintillation vial and 5 mL of scintillation
fluid compatible with aqueous solutions is added and mixed vigorously. The
vials are counted in a beta scintillation counter. As negative control,
only buffer is used. As a positive control fetal calf serum is used.
EXAMPLE 15: Assaviue for Glvcosuria.
[0988] Glycosuria (i.e., excess sugar in the urine), can be readily assayed to
provide an index of the disease state of diabetes mellitus. Excess
urine in a patient sample as compared with a normal patient sample is
symptomatic of IDDM and NIDDM. Efficacy of treatment of such a patient
having IDDM and NIDDM is indicated by a resulting decrease in the amount of
excess glucose in the urine. In a preferred embodiment for IDDM
and NIDDM monitoring, urine samples from patients are assayed for the presence
of glucose using techniques known in the art. Glycosuria in
humans is defined by a urinary glucose concentration exceeding 100 mg per 100
ml. Excess sugar levels in those patients exhibiting glycosuria can
be measured even more precisely by obtaining blood samples and assaying serum
glucose.
EXAMPLE 16: Assays DetectinQ Stimulation or Inbibition of B cell Proliferation
and Differentiafion.
[0989] Generation of functional humoral immune responses requires both soluble
and cognate signaling between B-lineage cells and their
microenvironment. Signals may impart a positive stimulus that allows a B-
lineage cell to continue its programmed development, or a negative
stimulus that instructs the cell to arrest its current developmental pathway.
To date, numerous stimulatory and inhibitory signals have been found to
influence B cell responsiveness including IL-2, IL-4, IL-5, IL-6, IL-7, IL10,
IL-13, IL-14 and IL-15. Interestingly, these signals are by themselves
weak effectors but can, in combination with various co-stimulatory proteins,
induce activation, proliferation, differentiation, homing, tolerance and
death among B cell populations.
[0990] One of the best studied classes of B-cell co-stimulatory proteins is
the TNF-superfamily. Within this family CD40, CD27, and CD30
along with their respective ligands CD154, CD70, and CD153 have been found to
regulate a variety of immune responses. Assays which allow for
the detection and/or observation of the proliferation and differentiation of
these B-cell populations and their precursors are valuable tools in
determining the effects various proteins may have on these B-cell populations
in terms of proliferation and differentiation. Listed below are two
assays designed to allow for the detection of the differentiation,
proliferation, or inhibition of B-cell populations and their precursors.
[0991] In Vitro Assay- Albumin fusion proteins of the invention (including
fusion proteins containing fragments or variants of Therapeutic
144
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
11 ~, I! ' U, t"",.yr ' ti,..n ,. ~e11 ' f
pioteriS ~Sid1 ll~uli~in nr itõ (gm" nts o variants of albumin) can be
assessed for its ability to induce activation, proliferation, differentiation
or
inhibition and/or death in B-cell populations and their precursors. The
activity of an albumin fusion protein of the invention on purified human
tonsillar B cells, measured qualitatively over the dose range from 0.1 to
10,000 ng/mL, is assessed in a standard B-lymphocyte co-stimulation assay
in which purified tonsillar B cells are cultured in the presence of either
formalin-fixed Stapliylococcus am=eus Cowan I (SAC) or immobilized anti-
human IgM antibody as the priming agent. Second signals such as IL-2 and IL-15
synergize with SAC and IgM crosslinking to elicit B cell
proliferation as measured by tritiated-thymidine incorporation. Novel
synergizing agents can be readily identified using this assay. The assay
involves isolating human tonsillar B cells by magnetic bead (MACS) depletion
of CD3-positive cells. The resulting cell population is greater than
95% B cells as assessed by expression of CD45R(B220).
[0992] Various dilutions of each sample are placed into individual wells of a
96-well plate to which are added 105 B-cells suspended in culture
medium (RPMI 1640 containing 10% FBS, 5 X 10'SM 2ME, 100U/ml penicillin,
l0ug/mi streptomycin, and 10'5 dilution of SAC) in a total volume
of 150u1. Proliferation or inhibition is quantitated by a 20h pulse
(luCi/well) with 3H-thymidine (6.7 Ci/mM) beginning 72h post factor addition.
The positive and negative controls are IL2 and medium respectively.
[0993] In vivo Assay- BALB/c mice are injected (i.p.) twice per day witli
buffer only, or 2 mg/Kg of an albumin fusion protein of the invention
(including fusion proteins containing fragments or variants of Therapeutic
proteins and/or albumin or fragments or variants of albumin). Mice
receive this treatment for 4 consecutive days, at which time they are
sacrificed and various tissues and serum collected for analyses. Comparison of
H&E sections from normal spleens and spleens treated with the albumin fusion
protein of the invention identify the results of the activity of the
fusion protein on spleen cells, such as the diffusion of peri-arterial
lymphatic sheatlis, and/or significant increases in the nucleated cellularity
of the
red pulp regions, which may indicate the activation of the differentiation and
proliferation of B-cell populations. Immunohistochemical studies using
a B cell marker, anti-CD45R(B220), are used to determine whether any
physiological changes to splenic cells, such as splenic disorganization, are
due to increased B-cell representation within loosely defined B-cell zones
that infiltrate established T-cell regions.
[0994] Flow cytometric analyses of the spleens from mice treated with the
albumin fusion protein is used to indicate whether the albumin fusion
protein specifically increases the proportion of ThB+, CD45R(B220)dull B cells
over that which is observed in control mice.
[0995] Likewise, a predicted consequence of increased mature B-cell
representation in vivo is a relative increase in serum Ig titers. Accordingly,
serum IgM and IgA levels are compared between buffer and fusion protein
treated mice.
EXAMPLE 17: T Cell Proliferation Assav.
[0996] A CD3-induced proliferation assay is performed on PBMCs and is measured
by the uptake of'H-thymidine. The assay is performed as
follows. Ninety-six well plates are coated with 100 Uwell of mAb to CD3
(HIT3a, Phanningen) or isotype-matched control mAb (B33.1)
overnight at 4 degrees C (1 g/ml in .05M bicarbonate buffer, pH 9.5), then
washed three times with PBS. PBMC are isolated by F/H gradient
centrifugation from human peripheral blood and added to quadruplicate wells (5
x-10 /well) of mAb coated plates in RPMI containing 10% FCS and
P/S in the presence of varying concentrations of an albumin fusion protein of
the invention (including fusion proteins containing fragments or
variants of Therapeutic proteins and/or albumin or fragments or variants of
albumin) (total volume 200 ul). Relevant protein buffer and medium
alone are controls. After 48 hr. culture at 37 degrees C, plates are spun for
2 min. at 1000 rpm and 100 1 of supernatant is removed and stored -20
degrees C for measurement of IL-2 (or other cytokines) if effect on
proliferation is observed. Wells are supplemented with 100 ul of medium
containing 0.5 uCi of'H-thymidine and cultured at 37 degrees C for 18-24 hr.
Wells are harvested and incorporation of 3H-thymidine used as a
measure of proliferation. Anti-CD3 alone is the positive control for
proliferation. IL-2 (100 U/ml) is also used as a control which enhances
proliferation. Control antibody which does not induce proliferation of T cells
is used as the negative control for the effects of fusion proteins of the
invention.
EXAMPLE 18: Effect of Fusion Proteins of the Invention on the Expression of
MHC Class II, Costimulatory and Adltesiou
Molecules and Cell Differentiation ofMonocvtes and Monocyte-Derived Human
Dendritic Cells.
[0997] Dendritic cells are generated by the expansion of proliferating
precursors found in the peripheral blood: adherent PBMC or elutriated
monocytic fractions are cultured for 7-10 days with GM-CSF (50 ng/ml) and IL-4
(20 ng/ml). These dendritic cells have the characteristic
phenotype of immature cells (expression of CD1, CD80, CD86, CD40 and MHC class
II antigens). Treatment with activating factors, such as TNF-
a, causes a rapid change in surface phenotype (increased expression of MHC
class I and II, costimulatory and adhesion molecules, downregulation
of FCyRII, upregulation of CD83). These changes correlate with increased
antigen-presenting capacity and with functional maturation of the
dendritic cells.
[0998] FACS analysis of surface antigens is performed as follows. Cells are
treated 1-3 days with increasing concentrations of an albumin
fusion protein of the invention or LPS (positive control), washed with PBS
containing 1% BSA and 0.02 mM sodium azide, and then incubated with
1:20 dilution of appropriate FITC- or PE-labeled monoclonal antibodies for 30
minutes at 4 degrees C. After an additional wash, the labeled cells
are analyzed by flow cytometry on a FACScan (Becton Dickinson).
[0999] Effect on the production of c okines. Cytokines generated by dendritic
cells, in particular IL-12, are important in the initiation of T-cell
dependent inunune responses. IL-12 strongly influences the development of Th1
helper T-cell immune response, and induces cytotoxic T and NK
145
CA 02618476 2008-02-06
WO 2007/021494 PCT/US2006/029391
~i " i cell ~cl~ictib'ri 'PAn'~~LISA'is useih'to"m'e'~asure the II, 12 release
as follows. Dendritic cells (106/ml) are treated with increasing
concentrations of an
albumin fusion protein of the invention for 24 hours. LPS (100 ng/ml) is added
to the cell culture as positive control. Supernatants from the cell
cultures are then collected and analyzed for IL-12 content using commercial
ELISA kit (e.g., R & D Systems (Minneapolis, MN)). The standard
protocols provided with the kits are used.
[1000] Effect on the expression of MHC Class H. costimulatory and adhesion
molecules. Three major families of cell surface antigens can be
identified on monocytes: adhesion molecules, molecules involved in antigen
presentation, and Fc receptor. Modulation of the expression of MHC
class II antigens and other costimulatory molecules, such as B7 and ICAM-1,
may result in changes in the antigen presenting capacity of monocytes
and ability to induce T cell activation. Increased expression of Fc receptors
may correlate with improved monocyte cytotoxic activity, cytokine
release and phagocytosis.
[1001] FACS analysis is used to examine the surface antigens as follows.
Monocytes are treated 1-5 days with increasing concentrations of an
albumin fusion protein of the invention or LPS (positive control), washed with
PBS containing 1% BSA and 0.02 mM sodium azide, and then
incubated with 1:20 dilution of appropriate FITC- or PE-labeled monoclonal
antibodies for 30 minutes at 4 degrees C. After an additional wash, the
labeled cells are analyzed by flow cytometry on a FACScan (Becton Dickinson).
[1002] Monooyte activation and/or increased survival. Assays for molecules
that activate (or alternatively, inactivate) monocytes and/or increase
monocyte survival (or altematively, decrease monocyte survival) are known in
the art and may routinely be applied to determine whether a molecule
of the invention functions as an inhibitor or activator of monocytes. Albumin
fusion proteins of the invention can be screened using the three assays
described below. For each of these assays, Peripheral blood mononuclear cells
(PBMC) are purified from single donor leukopacks (American Red
Cross, Baltimore, MD) by centrifugation through a Histopaque gradient (Sigma).
Monocytes are isolated from PBMC by counterflow centrifugal
elutriation.
[1003] Monooyte Survival Assay. Human peripheral blood monocytes progressively
lose viability when cultured in absence of serum or other
stimuli. Their death results from internally regulated processes (apoptosis).
Addition to the culture of activating factors, such as TNF-alpha
dramatically improves cell survival and prevents DNA fragmentation. Propidium
iodide (PI) staining is used to measure apoptosis as follows.
Monocytes are cultured for 48 hours in polypropylene tubes in serum-free
medium (positive control), in the presence of 100 ng/ml TNF-alpha
(negative control), and in the presence of varying concentrations of the
fusion protein to be tested. Cells are suspended at a concentration of 2 x
106/ml in PBS containing PI at a final concentration of 5 g/ml, and then
incubated at room temperature for 5 minutes before FACScan analysis. PI
uptake has been demonstrated to correlate with DNA fragmentation in this
experimental paradigm.
[1004] Effect on cytokine release. An important function of
monocytes/macrophages is their regulatory activity on other cellular
populations of
the immune system through the release of cytokines after stimulation. An ELISA
to measure cytokine release is performed as follows. Human
monocytes are incubated at a density of5x105 cells/ml with increasing-
concentrations of-an albumin-fusion protein of the invention and under the--
same conditions, but in the absence of the fusion protein. For IL-12
production, the cells are primed ovemight with IFN (100 U/ml) in the presence
of the fusion protein. LPS (10 ng/ml) is then added. Conditioned media are
collected after 24h and kept frozen until use. Measurement of TNF-
alpha, IL-10, MCP-1 and IL-8 is then performed using a commercially available
ELISA kit (e.g., R & D Systems (Minneapolis, MN)) and applying
the standard protocols provided with the kit.
[1005] Oxidative burst. Purified monocytes are plated in 96-w plate at 2-1x105
cell/well. Increasing concentrations of an albumin fusion protein
of the invention are added to the wells in a total volume of 0.2 ml culture
medium (RPMI 1640 + 10% FCS, glutamine and antibiotics). After 3 days
incubation, the plates are centrifuged and the medium is removed from the
wells. To the macrophage monolayers, 0.2 ml per well of phenol red
solution (140 mM NaCI, 10 mM potassium phosphate buffer pH 7.0, 5.5 mM
dextrose, 0.56 mM phenol red and 19 U/ml of HRPO) is added,
together with the stimulant (200 nM PMA). The plates are incubated at 37 C for
2 hours and the reaction is stopped by adding 20 l 1N NaOH per
well. The absorbance is read at 610 nm. To calculate the amount of H202
produced by the macrophages, a standard curve of a HZOZ solution of
known molarity is performed for each experiment.
EXAMPLE79: The Effect ofAlbumin Fusion Proteins oftlze Invention on the Growth
of [jascular Endotkelial Cells.
[1006] On day 1, human umbilical vein endothelial cells (HUVEC) are seeded at
2-5x10" cells/35 mm dish density in M199 medium containing
4% fetal bovine serum (FBS), 16 units/ml heparin, and 50 units/ml endothelial
cell growth supplements (ECGS, Biotechnique, Inc.). On day 2, the
medium is replaced with M199 containing 10% FBS, 8 units/ml heparin. An
albumin fusion protein of the invention, and positive controls, such as
VEGF and basic FGF (bFGF) are added, at varying concentrations. On days 4 and
6, the medium is replaced. On day 8, cell number is determined
with a Coulter Counter.
[1007] An increase in the number of HUVEC cells indicates that the fusion
protein may proliferate vascular endothelial cells, while a decrease
in the number of HUVEC cells indicates that the fusion protein inhibits
vascular endothelial cells.
EXAMPLE 20: Rat Corzzeal Wouzzd Healinza ModeL
[1008] This animal model shows the effect of an albumin fusion protein of the
invention on neovascularization. The experimental protocol
includes:
146
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 146
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 146
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: