Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 28
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 28
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Enhancement of Carotenoids in Plants.
Field of Invention:
The present invention relates to a method of enhancing the content of
carotenoids and
other isoprenoids, preferably of lycopene and or 0-carotene, in a plant, plant
cell, callus,
tissue, fruit, root or other part of a plant, and/or a method of increasing
the height in a
plant, to a plant, plant cell, callus, tissue, root or fruit produced by such
method, to a
method of obtaining carotenoids, preferably lycopene and/or 0-carotene, to a
nucleic acid
construct and to the use of such nucleic acid construct.
Background of the Invention:
Among modern diseases and ailments, both cancer and cardiovascular diseases
rank close
to the top and affect a significant proportion of the population. These
diseases have been
linked to factors such as reactive oxygen species which cause damage to
membranes,
DNA and other cellular functions. Consequently anti-oxidants have become very
popular
dietary supplements among all age-groups a number of products such as fresh
fruits,
juices, plant extracts and tablets are available and consumed on a daily basis
towards the
maintenance of health. This category of chemicals includes vitamins C and E,
polyphenols, flavonols and carotenoids. As humans do not synthesize any of
these, these
are obtained from plant of microbial sources. One of the key groups involved
in reactive
oxygen quenching are poly-ols and poly-enes. It is well known that lycopene,
an acyclic
isomer of (3-carotene, is one of the most potent anti-oxidants with twice the
singlet
oxygen quenching capacity of (3-carotene and 10 times that of a-tocopherol.
Further it is
the most predominant carotenoid in human plasma (Kaliora et al, 2005).
Lycopene's stability in serum and its superior quenching ability have made it
an
extensively studied compound in terms of its production and disease treatment.
Its 11
1
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
conjugated and 2 non-conjugated double bonds give it its characteristic red
colour (peak
absorbance at 472-476 nm). Further of the possible 1056 isomers about 12 are
found in
nature with the linear all trans isomer being the most stable and the one
found in plants.
This compound is a polyunsaturated hydrocarbon, lipophylic, and sensitive to
light, heat
and oxidation (Faulks and Southon, 2005).
Lycopene and diet
Lycopene is abundant in several fruits and vegetables such as grapefruit,
guava juice,
watermelon and tomatoes. In tomatoes, the red pigmentation is largely
accounted for by
lycopene and high-lycopene fruits exhibit enhanced redness. In plants lycopene
is found
in the all trans- form and is poorly absorbed from the diet without cooking.
Heating in
the presence of oil has shown better extractability and certain tomato
products such as
juice, puree, paste and sauce have been found to have better, i.e. higher
amounts of
lycopene. Cooking has shown to isomerize lycopene to a cis-configuration that
increases
its bioavailability. Further in serum the preponderance of lycopene is in the
cis-form. It
has been estimated that 35 mg of lycopene is required on a daily basis and may
not be
currently part of the current intake of consumers (Faulks and Southon, 2005).
Lycopene and health
Reactive oxygen species (ROS) are generated by a number of pathways whereby
energy
is transferred to a ground-state triplet oxygen making it a highly reactive
excited singlet
oxygen. This species is capable of lipid, protein and DNA damage. ROS are thus
associated with symptoms of aging, with cancer and cardiovascular diseases. By
virtue of
the abundance of unsaturations and the conjugation of these linkages,
carotenoid
molecules can quench the energy from the singlet oxygen. Concomitant with this
step,
the carotenoid molecule attains an excited state, which is subsequently
dissipated as heat
by interaction with the solvent milieu. After completing one quenching
reaction, the
carotenoid is regenerated for another reaction. Certain estimates reveal that
carotenoids
can quench around 1000 singlet oxygens before they break down (Krinsky, 1998,
Bhuvaneswari and Nagini 2005, Gruszecki and Strzalka, 2005).
2
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
ROS have been linked with a number of chronic diseases, due to the oxidative
damage
caused. In turn antioxidants have been associated with protecting lipids,
membranes, low-
density lipoproteins, proteins and DNA from damage. More specifically,
lycopene has
been demonstrated to prevent cancer and cardiovascular diseases. Several
studies have
found that lycopene, by binding to lipophylic moieties, protects such
complexes from
oxidative damage. Further it is proposed to prevent the carcinogen-induced
phosphorylation of p53 and Rb anti-oncogens, and stop cell division as the GO-
G1 phase.
It is also an inhibitor of HMG CoA reductase, the committed step in
cholesterol
biosynthesis. Additionally by binding to LDL and VLDL, it prevents the
formation of
cholesterol oxides and hence CVD (Kaliora et al 2005, Agarwal and Rao 2000).
While several transgenic approaches have resulted in the enhancement of
lycopene in
tomatoes, few natural high-lycopene varieties have been found. Spontaneous
mutations historically identified in tomato by the Campbell Soup company in
1917 (hp-
1), the hp-2 modified in the San Marzano variety (1975) and dg (Manapal
variety 1973)
have been associated with elevated lycopene levels and thus used in breeding
programs
(For review Bramley 2002).
Metabolic plant engineering
The quest for plants and plant products with enhanced carotenoids and
specifically
lycopene towards better nutrition has taken plant biology through very novel
and ground-
breaking science. Understanding and mapping the pathway of carotenoid
biosynthesis
(Fraser et al , 1994, Hirschberg 2001, Fraser et al 2002 and Bramley 2002 for
review) has
yielded a wealth of information enabling the metabolic engineering of plants
towards
enhanced carotenoid production. Key to recent advances was the discovery that
unlike
other organisms, plants synthesize carotenoids via the 1-deoxy-D-xylulose-5-
phosphate
(DOXP) pathway rather than the mevalonic acid pathway. While both pathways
produce
isopentenyl pyrophosphate, the mevalonate pathway channels carbon into the
sterol,
sesquiterpenoid and triterpenoid pathway while the DOXP leads to carotenoid,
phytol,
plastoquinone-9, and diterpene formation (Bramley 2002, Romer and Fraser
2005).
3
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
To begin, IPP is isomerized to dimethylallyl pyrophophate (DMAPP), an
activated
monomer that is eventually oligomerized into geranyl geranyl pyrophosphate,
the
precursor to C40 carotenoids and the first compound in the pathway, phytoene.
Phytoene
is desaturated to phytofluene by phytoene desaturase and further to ~-
carotene. Lycopene
is next produced via neurosporene by ~-carotene desaturase. Interestingly, the
bacterial
crtl (from Erwinia uredovora) converts phytoene to all trans-lycopene (Ye et
al 2000,
Bramley 2002). While beyond the scope of this work, it is to be noted that all
trans-
lycopene is further converted to a- and 0-carotene of which 0-carotene is a
pro-vitamin
A.
Engineering plants and or identifying plants with enhanced lycopene in view of
its health
benefits has been carried out for a great number of years with some of the
earliest dating
back to 1917 when the Campbell soup company identified hp-1 as a highly
pigmented
mutant of tomato (Reynard 1956). An extension of the hp-I phenomenon was
demonstrated by Liu et al (2004), who showed that the modulation of hp-I
homologs
DDB1, LeHY5 and LeCOP1LIKE affect carotenoid biosynthesis (Bramley 1997).
hp-2 and dg were also identified from natural populations and linked to
enhanced
carotenoid levels (for review Levin). Drawing from these results, hp2 and dg
were
determined to be tomato homologs of the Arabidopsis DET1 (DE-ETIOLATEDI). The
constitutive silencing of this gene resulted in elevated levels of 0-carotene
and lycopene
in tomato (Davuluri et al 2004). As with hp-2, these mutants had severe
development
defects in that, they were stunted, bushy and dwarf. Davuluri and coworker
(2005)
further, reduced the level of DET1 using an RNA interference strategy that
significantly
increased the level of carotenoids, resulting in a doubling of lycopene and a
10-fold
increase in 0-carotene. In a similar strategy, over-expression of the blue
light
photoreceptor cry2 increased lycopene contents by decreasing the expression of
lycopene
0 cyclases (Giliberto et al, 2004).
Modulation of enzymes involved in the biosynthetic pathway of carotenoids has
yielded
mixed results. Fray et al. (1995) over-expressed a tomato phytoene synthase
gene in
4
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
tomatoes resulting in increased lycopene and a simultaneous dwarfing due to a
reduction
in gibberellic acid biosynthesis. Fraser et al. (2002), further demonstrated
that a fruit
specific over-expression of phytoene synthase (crtB from Erwinia uredovora)
using a
polygalacturonase promoter resulted in a 1.8 fold increase in lycopene along
with
phytoene, (3-carotene and lutein (2.4, and 2.4 fold increases). The over-
expression of
bacterial phytoene desaturase (crtl form Erwinia uredovora) in tomatoes
however
(Romer et al. 2000) resulted in a 3-fold increase in P-carotene while the
lycopene content
was halved. Mehta et al. (2002) engineered polyamine accumulation in tomatoes
using
yeast S-adenosylmethionine decarboxylase gene driven by a ripening-induced E8
promoter. Fruit extracts revealed a yield of 120 gg/mg of lycopene (about 300
%
increase). Rosati et al. (2000) used a different approach, whereby lycopene
cyclase was
inactivated by antisense technology resulting in an increase in lycopene.
Breeders have long toiled with breeding for high lycopene, Liu et al. (2003)
reported that
around 16 QTLs determine tomato colour and thus lycopene by looking at 75
introgression lines between Lycopersicon pennellii and M82 (L. esculentum).
Lycopene has attracted the attention of health specialists and plant scientist
alike for it
beneficial properties. The 90 or so years of interest in developing new
strategies to
increase the carotenoid levels in tomatoes goes to show its importance and
that despite
the difficulties in developing such technologies, the need for the hour
continually directs
attention towards lycopene.
Summary of the invention
The object of this invention is to provide means to enhance carotenoid levels
in plants
and/or plant parts. A further object of the present invention is to provide a
method for
producing enhanced levels of carotenoids, in particular lycopene, in plants
and/or plant
parts. Yet a further object of the present invention is to provide a plant or
plant part
having enhanced levels of carotenoids, in particular lycopene.
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
The objects of the present invention are solved by a method of enhancing the
content of carotenoids and other isoprenoids, preferably of lycopene and or 0-
carotene, in
a plant, plant cell, callus, tissue, fruit, root or other part of a plant,
and/or increasing the
height in a plant, said method comprising:
impairment of mitochondrial function, preferably impairment of mitochondrial
complex
I, II, III and /or IV, more preferably mitochondrial complex I in said plant,
plant cell,
callus, tissue, fruit, root or other part of a plant.
2 In one embodiment of the method according to the present invention, said
impairment occurs using a modified protein component of mitochondrial complex
I of
said plant cell, preferably a protein component of mitochondrial complex I
that is the
translation product of an unedited coding sequence.
3 Preferably, said impairment occurs by transforming said plant cell with a
nucleic
acid construct, preferably a DNA-construct, or by transforming said plant cell
with a
nucleic acid construct via Agrobacterium species - mediated transformation,
preferably
Agrobacterium tumefaciens, or by viral transfection using a suitable plant
virus such as
Tobacco Mosaic Virus, or by protoplast transformation.
4 In one embodiment of the present invention, said nucleic acid construct,
preferably said DNA-construct, or said Agrobacterium comprises, preferably in
a binary
vector, a nucleic acid encoding said modified protein component of said
mitochondrial
complex I.
In a preferred embodiment of the present invention, said modified protein
component of mitochondrial complex I is a dysfunctional protein from another
plant
species than said plant cell or a dysfunctional protein of the same plant
species as said
plant cell.
6
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
6 In another embodiment of the method according to the present invention, said
modified protein component of mitochondrial complex I is a dysfunctional
protein
selected from the group comprising NAD 1, 2, 3, 4, 4L, 5, 6, 7, 9, nuclear
mitochondrial
proteins 76 Kda, 55 Kda, 28.5 Kda, 22 Kda and Acyl carrier protein.
7 Preferably said nucleic acid encoding said modified protein component is
selected
from the group comprising SEQ ID NO: 1- 3
8 In one embodiment of the method according to the present invention, said
nucleic
acid construct, preferably said DNA-construct, or said Agrobacterium,
preferably said
Agrobacterium binary vector, additionally comprises a nucleic acid encoding a
mitochondrial transit peptide, operably linked to said nucleic acid encoding
said modified
protein component of said mitochondrial complex I.
9 In a preferred embodiment of the invention, wherein said nucleic acid
construct,
preferably said DNA-construct, or said Agrobacterium, preferably said
Agrobacterium
binary vector, additionally comprises a promoter and a terminator, and said
promoter and
terminator are operably linked to said nucleic acid encoding said modified
protein
component of said mitochondrial complex I.
In one embodiment of the invention, wherein said nucleic acid construct,
preferably said DNA-construct, or said Agrobacterium, preferably said
Agrobacterium
binary vector, comprises said promoter and said terminator, said nucleic acid
encoding
mitochondrial transit peptide and said nucleic acid encoding said modified
protein
component of said mitochondrial complex I, all as defined before, all of them
being
operably linked.
11 Preferably said impairment occurs by mutating said plant cell with respect
to at
least one of the components of said mitochondrial complex I in said plant
cell.
7
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
12 In a preferred embodiment of the present invention, said mutating occurs by
mutating said plant cell at random using a chemical and/or physical mutagenic
agent
being applied to at least one plant cell, preferably a plurality of plant
cells of the same
plant, said chemical mutagenic agent preferably being selected from the group
comprising ethyl methane sulfonate and said physical mutagenic agent being
selected
from the group comprising fast neutron bombardment, X-ray, gamma ray and other
mutagenic irradiation.
13 In one embodiment of the method according to the present invention, the
method,
after mutating, further comprises the additional step of screening for a
modified protein
component of mitochondrial complex I or other mitochondrial functions of said
plant cell
in said plant cell or plurality of plant cells.
14 In one embodiment of the method according to the present invention, said
impairment occurs by applying a chemical inhibitor of mitochondrial function,
preferably
a chemical inhibitor of mitochondrial complex I of said plant cell, to said
plant cell, plant,
callus, tissue, a part of said plant, said plant in its entirety, fruit, root
and / or other plant
organ.
15 In a preferred embodiment of the present invention, said chemical inhibitor
is
selected from the group comprising rotenone, antimycin A, oxyfluorfen,
violaxanthin,
piericidin, piericidine A, pyrazoles, pyridaben, quinazolines, acetogenins,
thiangazoles
and fenaza.
16 In a preferred embodiment of the invention, said impairment is an
inhibition of
said of mitochondrial complex I.
17 In one embodiment of the method according to the present invention, said
method
further comprises the step of raising said plant cell, plant part, tissue,
seed or organ
having undergone the method of any of the foregoing claims, to produce a plant
callus,
tissue, plant, root and/or fruit.
8
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
18 The objects of the present invention are solved by a plant cell, callus,
tissue, plant,
root or fruit produced by the method according to the present invention.
19 Preferably the plant cell, callus, tissue, plant, root or fruit is/are
derived from a
plant origin selected from the group comprising solanaceous species, including
tomato,
pepper, capsicum, potato, petunia and or tobacco.
20 Preferably, in the plant cell, callus, tissue, plant, root or fruit, the
amount of
carotenoid, preferably lycopene, is enhanced to > 10mg, preferably > 15 mg,
even more
preferably >16 mg and most preferably >20 mg/100g fresh weight of plant cells,
callus
tissue, plant, root and/or fruit.
21 Preferably, in the plant cell, callus, tissue, plant, root or fruit, the
amount of
carotenoid, preferably lycopene is enhanced by at least two-fold, preferably
three-fold, in
relation to a plant cell/callus/tissue/plant, root or fruit not having
undergone the method
according to the present invention.
22 The objects of the present invention are solved by a method of obtaining
carotenoids, preferably lycopene and /or 0-carotene, comprising the steps:
Producing a plant cell, callus, tissue, plant, root or fruit according to the
present
invention,
Purifying carotenoids, preferably lycopene and /or (3-carotene, from said
plant cell, callus, tissue, plant, root or fruit, preferably by solvent
extraction and
purification or by supercritical carbon dioxide extraction or any other method
typically
employed by someone skilled in the art.
9
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
23 The objects of the present invention are also solved by a nucleic acid
construct
comprising a nucleic acid sequence encoding a modified protein component of
mitochondrial complex I.
24 Preferably, said modified protein component of mitochondrial complex I is
selected
from the group comprising NAD 1, 2, 3, 4, 4L, 5, 6, 7 and 9 or other
proteinaceous
component of said complex.
25 In one embodiment of the nucleic acid construct according to the present
invention, said modified protein component of mitochondrial complex I is from
a species
selected from the group comprising tomato, potato, tobacco, rice, maize,
petunia,
Arabidopsis, and or Lotus, Medicago, wheat and/or Sorghum.
26 Preferably, said modified protein component of mitochondrial complex I has
a
sequence selected from the group comprising SEQ ID NO: 4, 5, and 6.
27 The objects of the present invention are solved by the use of a nucleic
acid
construct according to the present invention for enhancing the content of
carotenoids and
other isoprenoids, preferably of lycopene and /or (3-carotene, in a plant
cell, plant, callus,
tissue, fruit, root or other part of said plant and/or for increasing the
height in a plant.
Definitions
A "transgenic or transformed plant" refers to a plant which contains a
recombinant
polynucleotide introduced by transformation. Transformation means the
introduction into
a plant of a polynucleotide sequence in a manner so as to cause a stable
integration of the
nucleotide sequence or a transient expression of the sequence. This may be
achieved by
particle bombardment (biolistic), Agrobacterium-mediated (using a suitably
developed
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
plasmid vector), transfection with viral DNA or vectors, introduction of DNA
by
electroporation or lipofection. Plant transformation may be carried out on
plant cells,
pollen, on plant seeds, on plant protoplasts, or any other type of plant
tissue, intact plant
or plant part under sterile or non-sterile conditions. A transformed plant may
refer to a
whole plant, any plant part, plant cell, plant organ, plant tissue, seed,
root, flower, fruit,
root or shoot. It may also refer to the progeny thereof.
A "vector" is a polynucleic acid construct, generated recombinantly,
artificially or
chemically, comprising nucleic acid elements that may encode genes, proteins,
promoters, terminators and transit peptides. These segments will be operably
linked so as
to enable the expression of the gene encoded or the complete execution of the
process
encoded. The promoter region may include constitutive or tissue-specific,
tissue-active,
developmental stage-active/specific, or inducible promoters such as but not
limited to the
cauliflower mosaic virus 35S promoter, the cassava vein mosaic virus promoter
or the
maize ubiquitin promoter.
A nucleotide sequence is "operably linked" when adjacent segments of DNA
sequence
are linked in such a manner so as to enable a cellular/biological function as
encoded by
the gene sequence.
Carotenoid .
Carotenoids are a class of hydrocarbons (carotenes) and their oxygenated
derivatives
(xanthophylls) consisting of eight isoprenoid units joined in such a manner
that the
arrangement of isoprenoid units is reversed at the centre of the molecule so
that the two
central methyl groups are in a 1,6-positional relationship and the remaining
non-terminal
methyl groups are in a 1,5-positional relationship. All carotenoids may be
formally
derived from the acyclic C4oH56 structure, having a long central chain of
conjugated
double bonds, by (i) hydrogenation, (ii) dehydrogenation, (iii) cyclization or
(iv)
oxidation or any combination of these processes.
11
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Impairment
Impairment refers to the partial or complete loss of function of the
organelle, functional
complex, enzyme or other functional entity. A complete loss of function as
used herein is
also sometimes referred to as inhibition.
Mitochondrial complex
Mitochondria complexes refer to the four electron transport chain complexes of
mitochondria called I, II, III and IV respectively, where complex I is NADH-
dehydrogenase, Complex II is succinate dehydrogenase, Complex III is
cytochrome c
reductase and complex IV is cytochrome c oxidase. These are multipolypeptide
complexes involved in ATP production by oxidation of NADH+H} and FADH2 to
NAD+ and FAD respectively and water.
Mitochondrial complex I proteins
This complexes comprises about 43 polypeptide chains including the
mitochondrial
encoded nadl, nad2, nad3, nad4, nad4Lm, nad5, nad6, nad7, nad9 and the nuclear
encoded 76 Kda, 55 Kda, 28.5 Kda, 22 Kda and Acyl carrier protein.
"Modified protein component" refers to a protein component that differs from
the native
functional protein in its amino acid sequence, structure or function and may
be non-
functional. It is to be noted that in plant mitochondria, the amino acid
sequence of the
native (functional) protein differs from the hypothetical translation
production of the
native gene sequence that encodes the protein due to one or more post-
translational RNA
editing events.
"Unedited" refers to a gene sequence identical to the gene sequence present
naturally in
the mitochondrial genome. It is often different from the mRNA product that
encodes the
native (functional) protein due to post-transcriptional RNA editing whereby
certain C
ribonucleotides are modified to U ribonucleotides and rarely certain U
ribonucleotides to
C ribonucleotides. For example the "translation product of an unedited coding
sequence"
12
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
is a protein that would be expected to be produced if no editing events at a
post-
transcriptional level occurred.
Dysfunctional: partially or completely non-functional
Transit peptide refers to an N-terminal presequence, which directs
mitochondria-bound
proteins encoded by the nucleus to the mitochondrion. The transit peptide is
required for
the transport of such proteins across the relevant membranes from their site
of synthesis
in the cytoplasm.
Inhibitors of mitochondria include but are not limited to Rotenone, Antimycin
A,
Cyanide, malonate (succinate dehydrogenase inhibitor), 2,4-Dinitrophenol
(DNP),
Carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone (FCCP), Oligomycin,
oxyfluorfen, violaxanthin, piereicidin A, pyrazoles, pyridaben, quinazolines,
acetogenins,
thiangazoles, fenaza, thenoxyltrifluoroacetone, carfboxin, oxycarboxin,
fenfuran, DDT,
chlorproham, propanil, dinoseb, ioxynil, cyclodiene, paraquat, dinoseb,
diafenthiuron,
methomyl, Bongkrekic acid and hydramethylnon.
Agrobacterium refers to a bacterium of Agrobacterium species which is used to
transform
plants with gene(s) of interest using a suitable recombinant Agrobacterium
binary vector.
Likewise Agrobacterium binary vector refers to a recombinant DNA construct,
which
with other gene(s) of interest will be transformed into Agrobacterium cells.
These cells in
turn will be used for the transformation of plant cells, parts, seeds, or
intact entire plants.
The inventors have surprisingly found that enhanced levels of carotenoids may
be
achieved by impairing mitochondrial function in plants, by way of genetic
engineering or
other means. Using the present invention the inventors were able to produce
surprisingly
high levels of carotenoids in a plant, most notably lycopene, in comparison to
a plant not
having undergone the method according to the present invention (table 1).
Similar levels
of carotenoids may only be encountered in processed and artificially enriched
foods such
as ketchup or concentrated tomato paste (see also Table 2).
13
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
For example, said objective may be achieved by assembling a polynucleotide
construct
encoding a maize ubiquitin promoter (SEQ ID No 7), an Arabidopsis At-mRBPla
mitochondrial targeting transit peptide (SEQ ID No 8-nucleotide sequence, SED
ID No
9- polypeptide sequence), an unedited nad9 gene from rice mitochondria peptide
(SEQ
ID No 1-nucleotide sequence, SED ID No 2- polypeptide sequence) and a Nopaline
synthase (NOS) terminator. This assembly may be carried out so as to render
the
construct transcriptionally and translationally competent in plants, and
additionally allow
the protein product to be translocated to the mitochondrion. A plant
cell/plant/plant part
transformed by such or similar construct can be observed to show the
aforementioned.
high levels of carotenoids, most notably lycopene and/or B-carotene.
This invention thus relates to a method of developing high carotenoid plants,
plant cell,
tissues or plant parts (comprising leaves, stems, roots, fruits and flowers).
This invention also relates to a method of developing plants with enhanced
levels of
lycopene and or/(3-carotene.
This invention furthermore relates to a method of enhancing the levels of
plant
compounds derived from the isoprenoid pathway including but not limited to
gibberellic
acid, abscisic acid, pigments, sterols.
In the following reference is made to the examples which are meant to
illustrate not to
limit the present invention. Reference is also made to the figures 1- 5
wherein:
Figure 1 shows: Plasmid pBS(SK-) construct used as a basic construct for
transformation.
Targeting sequence indicates the coding regions of At-mRBP 1 a cloned in
between Sac I
and Xba I restriction sites to get pNG1.
14
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Figure 2 shows: Plasmid pBS(SK-) construct used as a basic construct for
transformation.
TS and unedited nad9 indicates the coding regions of At-mRBP 1 a and edited
nad9 gene
cloned in between Xba I and BamHI restriction sites to get pNG3.
Figure 3 shows: Plasmid pBS(SK-) construct used as a basic construct for
transformation.
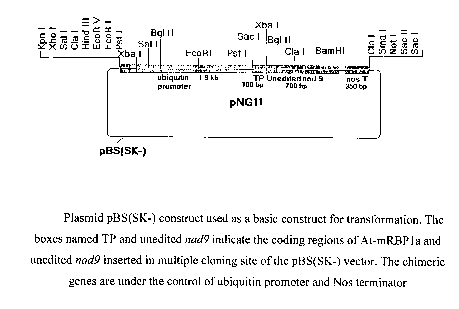
The boxes named TP and unedited nad9 indicate the coding regions of At-mRBP1a
and
unedited nad9 inserted in multiple cloning site of the pBS(SK-) vector. The
chimeric
genes are under the control of ubiquitin promoter and Nos terminator.
Figure 4 shows: Plasmid pLAU6.hph encoding the hygromycin resistance gene
(hph)
driven by a Cassava vein Mosaic Virus promoter (CVMV) with a NOS terminator.
Figure 5 shows: The Standard curve for Lycopene used for determining the
lycopene
content in one embodiment of the present invention.
Figure 6 shows: A typical chromatogram obtained by resolving a lycopene
extract as
described herein.
Furthermore reference is made to the sequences wherein:
SEQ ID NO 1: Rice (Oryza sativa) mitochondrial nad9 gene for NADH
dehydrogenase
subunit 9 (GenBank Accession number D50099 [RICMTNAD9]).
SEQ ID NO 2: Potato (Solanum tuberosum) mitochondrial nad9 gene for NADH
dehydrogenase subunit 9 (GenBank Accession number X79774 [STMINAD9]).
SEQ ID NO 3: Tobacco (Nicotiana tabacum) mitochondrial nad9 gene for NADH
dehydrogenase subunit 9 (GenBank Accession number YP 173479 [YP 173479]).
SEQ ID NO 4: Rice (Oryza sativa) mitochondrial nad9 protein for NADH
dehydrogenase
subunit 9 (translation of SEQ ID NO 1).
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
0.1 %BSA
4.0 mM 2-Mercaptoethanol
2 mM DTT
Wash Buffer (extraction Buffer without BSA & DTT
2 X Gradient buffer
0.5 M Sucrose
100 mM Tris HCL pH 7.5
6.0 mM EDTA
Percoll gradient prepared in 2 X gradient buffer
Rice mitochondria (Approximately 75 ug) was resuspended in resuspension buffer
and
lysed with 1/4 volumes of lysis buffer. After gentle mixing by inversion,
phenol was
added, followed by chloroform. Phenol chloroform extraction was carried out 3
times
followed by chloroform extraction. DNA was precipitated from the aqueous phase
with
2.5 volumes of ethanol and 1/10th volumes of 3 M sodium acetate, by
centrifugation at
13, 000 rpm for 20 minutes after a 30 minute incubation at -20 C. The DNA
pellet was
washed with 70 % ethanol, dried and dissolved in water.
The mitochondrial targeting sequence (At-mRBP 1 a, see SEQ ID No 8 for DNA and
SEQ
ID NO 9 for peptide) was cloned by polymerase chain reaction (PCR) from
Arabidopsis
thaliana cDNA, using the following set of primers:
Forward Primer: 5' AAGAGCTCCCATGGTCTTCTGTAACAAACTCG 3'
Reverse Primer: 5' AATCTAGACTTGGTAGACATCAACCGG 3'
The forward and the reverse primers have the SacI site and the Xbal site
incorporated
within them respectively and this facilitated the cloning of the PCR product
into pBS
(SK-) and the resulting vector was named pNGl (Figure 1).
16
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Lysis of mitochondria
Rice mitochondria (Approximately 75 g) was resuspended in resuspension buffer
and
lysed with 1/4 volumes of lysis buffer. After gentle mixing by inversion,
phenol was
added, followed by chloroform. Phenol chloroform extraction was carried out 3
times
followed by chloroform extraction. DNA was precipitated from the aqueous phase
with
2.5 volumes of ethanol and 1/10 th volumes of 3 M sodium acetate, by
centrifugation at
13, 000 rpm for 20 minutes after a 30 minute incubation at -20 C. The DNA
pellet was
washed with 70 % ethanol, dried and dissolved in water.
pNG3: Mitochonria-directed Unedited NAD9
Unedited nad9 (SEQ ID NO 1 for DNA and 4 for peptide) is obtained by PCR from
rice
mitochondrial DNA. The following primer combination was used for the
amplification:
Forward primer: 5' AATCTAGAATGGATAACCAATCCATTTTCCAA 3'
Reverse primer: 5' AAGGATCCGGGATTATCCGTCGCTACG 3'
The forward primer has the Xba I site and the reverse primer has the BamHI
site with
which the unedited nad9 gene was cloned adjacent to the mitochondrial
targeting
sequence and the vector was named as pNG3 (fig 2).
pNG11:
The unedited nad9 gene was excised out of pNG3 with SacI and BamHl and was
ligated
with the ubiquitin promoter upstream (SEQ ID NO 7) and the Nos terminator
downstream. The resulting construct was called pNGl 1(fig 3).
Basic transformation work done and tissue culture procedure at laboratory
level has been
standardized for tomato (Lycopersicon esculentum) and transformation protocols
are as
follows:
Protocol for transformation of tomato:
Seeds:
Tomato (Lycopersicon esculentum Mill, variety S-22 (Arka Vikas) seeds were
obtained
from Indian Institute of Horticultural Research (IIHR), Hesarghatta,
Bangalore.
~ The seeds were washed twice with double distilled autoclaved water.
17
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
~ The seeds were rinsed in 70% Ethanol for 2 minutes.
~ The seeds are immersed in a 70% solution of commercial bleach for 30
minutes,
in a shaker.
~ They are washed till all the traces of bleach are removed.
~ The seeds are dried on autoclaved tissue papers.
~ Steps 'd' and 'e' are carried out in the laminar hood to avoid
contamination.
Preparation of explant for bombardment:
The seeds are germinated in-vitro on half strength MS media. The 10-day old
seedlings
are uprooted the cotyledonary leaves are cut out and the center portion of the
leaves are
used for bombardments. About 40 such explants are placed at the center of a
Petri plate
containing osmoticum medium. After 4hrs incubation on this medium the calli
were
immediately subjected to microprojectile bombardment using the particle
accelerator,
PDS-1000/He.
Preparation of Gold Suspension:
The size of gold particles used was between 1.5-3.0 , 6mg of gold particles
was weighed
in a 0.5 ml eppendorf tube. 100 1 of autoclave double distilled water was
added to the
gold particles and vortexed for 30 seconds in a microfuge. It is centrifuged
for 30
seconds. 100 1 of 100% ethanol was added and vortexed for a minute. This was
centrifuged for 30seconds in a microfuge at 10000 rpm. The supernatant was
pipetted
out. The ethanol wash is repeated again. 100 l of sterile distilled water was
added to the
pellet. It is vortexed and 50 l of the suspension is transferred into another
0.5 ml tube.
Each of these tubes contains 50 1 of gold suspension and was stored at room
temperature
or at 4 C until DNA coating was done.
Particle Coating Protocol:
To 50 1 of the gold suspension the two plasmids that is plasmid-containing
gene of
interest (plasmid containing the unedited nad9 gene or edited nad9 or unedited
nad9 in
antisense version) & pLAU6hph (the selectable marker hygromycin containing
Plasmid,
see Figure 4) were added in the ratio of 3:1 so as to give a total
concentration of 10 g of
18
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
DNA. To this, 20 l of 0.1M spermidine (Sigma, Aldrich) was added and mixed at
low
speed on the vortex. 50 l of 2.5M CaC12 was then added and mixed well. The
mix was
left at room temperature for 10 minutes. It was later centrifuged for 30
seconds in a
microfuge. The supernatant is removed in the laminar hood.
Preparation Microcarriers, Rupture Discs and Screens for Biolistic
Transformation:
Macrocarriers
Pre-assemble and pre-sterilize the macrocarrier set in a macrocarrier holder
prior to
performing sample cell/tissue bombardments. The macrocarrier was first
immersed in
100% ethanol and then dried on autoclaved tissue paper. Then macrocarrier was
left
under UV light for surface sterilization for 10 minutes in the laminar hood.
Rupture disks
Transfer selected rupture disks to individual Petri dishes for easier
handling. Sterilize
rupture disks by briefly dipping them in 70% isopropanol just prior to
insertion in the
Retaining Cap. Do not soak for more than a few seconds. Extensive soaking may
delaminate the disks, resulting in premature rupture. All disks, with the
exception of
those rated at 450, 650 and 1,100 psi are laminated. Autoclaving is not
recommended
because of potential delamination.
Stopping screens
Transfer selected stopping screens to individual Petri dishes for easier
handling.
Sterilization by autoclaving is recommended. Alternatively, these parts can be
sterilized
by soaking in 70% ethanol, followed by drying in a sterile environment. The
stopping
screens can be double autoclaved and reused.
Coating of Microcarrier onto Macrocarrier:
19
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
The supernatant is removed from the DNA coated gold particles. Based on the
number of
plates to be bombarded, absolute ethanol is added to the DNA coated gold
pellet - 10.0
1 is added for every macrocarrier to be coated, i.e. for every plate to be
bombarded. The
mixture is vortexed until a homogenous suspension is obtained and 10.0 1 is
pipetted out
and coated onto the center of the macrocarrier disc. The ethanol is allowed to
dry away
leaving the gold particles on the macrocarrier disc.
Performing a Bombardment:
Before the Bombardment
l. The bombardment parameters for gap distance between rupture disk retaining
cap and
microcarrier assembly are selected and adjusted. The bombardment was carried
out at
900-psi rupture pressure and at a distance of 9cm. The stopping screen is
supported in
proper position inside fixed nest of microcarrier launch assembly.
2. The helium supply is adjusted to 200 psi in excess of the desired rupture
pressure.
3. The rupture disk retaining cap, microcarrier launch assembly, and the
entire apparatus is
wiped clean with 70% ethanol.
4. The macrocarriers coated with DNA and load onto sterile macrocarrier holder
the day of
the experiment
Firing the Device
1. Plug in power cord form main unit to electrical outlet.
2. Power ON.
3. Sterilize chamber walls with 70% ethanol.
4. Load sterile rupture disk into sterile retaining cap.
5. Secure retaining cap to end of gas acceleration tube (inside, top of
bombardment
chamber) and tighten with torque wrench.
6. Load macrocarrier and stopping screen into microcarrier launch assembly.
7. Place microcarrier launch assembly and target cell in chamber and close
door.
8. Evacuate chamber, hold vacuum at desired level (minimum 5 inches of
mercury).
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
9. Bombard sample: Fire button continuously depressed until rupture disk
bursts and
helium pressure drops to zero
10.Release Fire button.
After the Bombardment
1. Release vacuum from chamber.
2. Target cells removed from chamber.
3. Unload macrocarrier and stopping screen from macrocarrier launch assembly.
4. Unload spent rupture disk.
5. Remove helium pressure from the system (after all experiments completed for
the
day).
Growth & Selection of Bombarded Cells:
After 16 hrs the calli were transferred to tomato regeneration medium
containing BAP
(4.5 mg/L) and IBA (0.2 mg/L - selection media containing lOmg/L hygromycin)
medium for selection & incubated at 250C in light for 15 days. The resistant
calli are
subculture every 15 days onto fresh media. After regeneration the shoots are
cut out and
place on MS media containing BAP (4.5 mg/L), IBA (0.2 mg/L) and GA (1.0 mg/L),
for
elongation if only if the shoots have not elongated in the regeneration
medium. The
elongated shoots are shifted to MS media containing IAA (0.1 m/L) for rooting.
The
rooted plantlets are shifted to autoclaved distilled water for hardening for a
couple of
days and then into vermiculite. The plantlets are then shifted to red soil.
Plant Growth
Plantlets on soil are transferred to the greenhouse. Plants are raised under
standard
conditions for the variety and fruits harvested. Cross-fertilization may be
carried out for
experimental or breeding purposes. Genetic studies may also be carried out on
plants in
the greenhouse.
Quantitative analysis of Lycopene content in the tomato samples by HPLC method
21
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Chromatographic system:
1. Waters 2695 XE Separations Module equipped with auto sampler, degasser, PDA
detector and Millennium32 software
2. HPLC grade solvents Methanol, Dichloromethane (filtered through 0.22micron
membrane filter)
3. HPLC sample Vials
For Sample preparation:
Rotor Evaporator, Weighing Balance, Centrifuge, Tubes, Test Tube Stand and
Filters.
Extraction Solvent: Hexane : Acetone : Methanol (2:1:1) with 2.5% BHT
Sample Solvent: Dichloromethane : Methanol (45:55)
Extraction of Lycopene from Tomato samples:
One gram of fresh tomato (harvested from the greenhouse) sample was weighed
and
ground in lOml of extraction solvent using motor and pestle. Transferred to
centrifuge
tubes, sonicated for 6 minutes and centrifuged at 7500 rpm for 5 minutes. The
clear
organic layer is collected carefully and dried in the rotor evaporator, stored
at -20 C until
use. All the operations are done under dim light. Avoid direct light and high
temperature.
Sample Preparation:
The dried samples are redissolved in 1 ml of Sample solvent and 1:10 dilution
was made
from this stock. This diluted sample was passed through 0.22-micron filter
before
subjecting to HPLC analysis. Sample preparation should be carried out quickly
to avoid
loss of solvent.
Chromatographic conditions:
Mobile Phase: Methanol : Dichloromethane (95:5)
Methanol (HPLC Grade solvents were used for HPLC analysis)
22
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Pump Mode: Isocratic (MeOH:DCM ::95:5)
Column: BondapakTM C18, 4.6 x 150mm, 5 m
Detector: Waters 2996 PDA detector
Flow rate: 1.5m1/min
Injection volume: 20 l
No. of Injections: 2
Run time: 15 min
Detection wavelength : 476 nm
A binary solvent system of methanol : methylene chloride (95:5) was used to
resolve the
Lycopene from the samples over 15 minutes. The run time was reduced to 15
minutes
with a flow rate of 1.5m1/min to accommodate more samples. The detection
wavelength
for lycopene is 476nm, which was quantitated using standard curve.
Standard Curve: Standard Lycopene (Sigma) in the range of 10 to 80 g/ml was
prepared in HPLC grade Sample solvent with 0.1% BHT and subjected to analysis.
A
calibration curve was plotted -'Lycopene 17Jun05' Calibration ID 1729, Date:
17/06/2005, R=0.9948 and R2 = 0.9897. (See figure 5 for standard curve and
figure 6 for
a typical chromatographic profile).
23
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
Table 1. Levels of lycopene obtained -from transgenic and commercial varieties
of
tomato: Expressed in mg of lycopene per 100 g tomato fruit.
SI.No. Nature of the tomato Sample Name Lycopene Content
source (mg/100 gm fo
fresh weight)
1 Transgenic line Tl 1T1A101 26.9
2 Transgenic line T11T1A102 16.5
3 Transgenic line T11T1C107 17.4
4 Transgenic line T11T1B107 14.3
Transgenic line TI1'rIG115 23.5
6 Transgenic line Tl1T1B104 16.7
7 Commercial variety Arka Vikas (control) 5.5
8 Commercial variety Arka Vikas (control) 6.2
9 Commercial variety Mruthunjayam M-2 5.8
Commercial variety Agni 6.7
11 Commercial variety Abhinava 4.1
Table 1: Levels of lycopene analysed including transgenic lines and commercial
varieties.
As can be seen the levels of carotenoids, most notably lycopene, achieved by
the present
invention may be as high as 6.5 (26.9:4.1), and range from 2.1 (14.3:6.7) to
6.5, and
include ratios such as 4.2 (17.4:4.1), 3.0 (16.7:5.5), and 5.7 (23.5:4.1).
In absolute terms such high levels are elsewhere only achieved in processed
foods such as
concentrated tomato sauces, as can be seen from Table 2 below.
24
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
ood ~]Food Form m /100 ~
pricots 1risli 10.005
pricots ICanned, drained 0.065
~ ~
pricots 7 Ibried
1Chili 1.082.62
~][Processed
Grapefruit IPink, fresh 3.36
Guava--71 ink, fresh _ - _ ~~ F.40
jGuava uice lprocessed 3.34
fKetchup IProcessed j16.60
apaya _ ~ ed, fresh j.005.30
rizza sauce Canned jl2.7l
_-___.____..__._..
izza sauce [Prom pizza j2.89
osehip puree 1Canned ~~~~~ 10.78
Salsa 9.28
[Processed ...~...~W......_. -...~....._.._._.
Spaghetti sauce [Pocessed 17.50
;Y;_....... _ _.._.-.____-_-._._._...
jTomatoes fRe , fresh j3.17.74
Tomatoes Whole, peeled, processed 11.21
_........~~ ~
Tomato juice [P.0ce55ed 17.83
Tomato soup ICanned, condensed i [3.99
ITomato paste ICanned To.07
IWatermelon Rfresh Fl
'uice rocessed 7.28
~ ~....._.-..-_...__ Table 2: Levels of lycopene in food and food products
(ref Nguyen, M.L., Schwartz, S.J.
1999. Lycopene: Chemical and biological properties. Food Technol. 53:38-45.)
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
References:
1. Agarwal S, Rao AV (2000) tomato lycopene and its role in human health and
chronic diseases. Can Med Assoc Journ 163: 739-744
2. Bertram JS, Vine AL (2005) Cancer prevention by retinoids and carotenoids:
independent action on a common target. Biochim Biophys Acta 1740: 170-178
3. Bhuvaneswari V, Nagini S (2005) Lycopene: a review of its potential as an
anticancer agent. Curr Med Chem Anti-Canc Agents 5: 627-635
4. Bramley PM (1997) The regulation and genetic manipulation of carotenoid
biosynthesis in tomato fruit. Pure & Appl Chem 69: 2159-2162
5. Bramley PM (2002) Regulation of carotenoid formation during tomato fruit
ripening and development J Exp Bot 53: 2107-2113
6. Davuluri GR, van Tuinen A et al (2004) Manipulation of DET1 expression in
tomato results in photomorphogenic phetotypes caused by post-transcriptional
gene silencing. Plant Journal 40: 344-354
7. Davuluri GR, van Tuinen A, et al (2005) Fruit-specific RNAi-mediated
suppression of DETI enhances carotenoid and flavonoid content in tomatoes. Nat
Biotech 23: 890-895
8. Faulks RM, Southon S (2005) Challenges to understanding and measuring
carotenoid bioavailability, Biochim Biophys Acta 1740: 95-100
9. Fraser PD, Bramley P et al (2001) Effect of the Cnr mutation on carotenoid
formation during tomato fruit ripening. Phytochem 58: 75-79
10. Fraser PD, Truesdale MR et al (1994) Carotenoid biosynthesis during tomato
fruit
development. Plant Physiol 105: 405-413
11. Fraser PD, Romer S et al (2002) Evaluation of transgenic tomato plants
expressing an additional phytoene synthase in a fruit-specific manner. Proc
Nat
Acad Sci USA 99: 1092-1097
12. Fray RG, Wallace A et al (1995) Constitutive expressioni of a fruit
phytoene
synthase gene in transgenic tomatoes causes dwarfism by redirecting
metabolites
from the gibberellin pathway. Plant Journal 8: 693-701
13. Giege P, Sweetlove LJ et al (2003) Identification of mitochondrial
proteini
complexes in Arabidopsis using two-dimensional blue-native polyacrylamide gel
electrophoresis. Plant Mol Biol Rep 21: 133-144
26
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
14. Giliberto L, Perrotta G et al (2004) Manipulation of the blue light
photoreceptor
cryptochrome 2 in tomato affects vegetative development, flowering time, and
fruit antioxidant content. Plant Physio1137: 199-208
15. Giovannoni JJ, DellaPenna D et al (1989) Expression of a chimeric
polygalacaturonase gene in transgenic rin (Ripening inhibitor) tomato fruit
results
in polyuronide degradation but not fruit softening. Plant Cell 1: 53-63
16. Giuliano G, Aquilani R et al (2000) Metabolic engineering of plant
carotenoids.
Trends in Plant Sci 5: 405-407
17. Gruszecki WI, Strzalka K (2005) Carotenoids as modulators of lipid
membrane
physical proerties. Biochim Biophys Acta 1740: 108-115
18. Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Op
Plan
Bio14: 210-218
19. Kaliora AC, Dedoussis GVZ et al (2005) Dietary antioxidants in preventing
atherogenesis. Atherosclerosis 11: 1-17
20. Levin I, Frankel P et al (2003) the tomato dark green mutation is a novel
allel of
the tomato homolog of the DEETIOLATEDI gene. Theor Appl Genet 106: 454-
460
21. Liu Y-S, Gur A et al (2003) There is more to tomato fruit colour than
candidate
carotenoid genes. Plant Biotechnology Journal (2003) 1: 195-207
22. Liu Y, Roof S et al (2004) Manipulation of light signal transduction as a
means of
modifying fruit nutritional quality in tomato. Proc Nat Acad Sci USA 101: 9897-
9902
23. Lummen P (1998) Complex I inhibitos as insecticides and acaricides.
Biochim
Biophys Acta 1364: 287-296
24. Mackenzie S,1V1cIntosh L (1999) Higher plant mitochondria. Plant Cell 11:
571-
585
25. Mehta RA, Cassol T et al (2002) Engineered polyamine accumulation in
tomato
enhances phytonutrent content, juice quality, and vine life. Nature Biotech
20:
613-ppppppppp
26. Muir SR, Collins GJ (2001) Overexpression of petunia chalcone isomerase in
tomato results in fruit containing increased levels of flavonols. Nature
Biotech 19:
470-474
27
CA 02638818 2008-07-03
WO 2007/072110 PCT/IB2005/003995
27. Nguyen ML, Schwartz SJ (1999) Lycopene: Chemical and biological
properties.
Food techno153: 38-45.
28. Reynard GB (1956) Origin of Webb Special (Black Queen) in tomato. Rep
Tomato Genet Coop 40: 44-64
29. Romer S, Fraser PD et al (2000) Elevation of the provitamin A content of
transgenic tomato plants. Nature Biotech 18: 666-669
30. Romer S, Fraser PD (2005) recent advances in carotenoid biosynthesis,
regulation
and manipulation. Planta 221: 305-308
31. Rosati C, Aquilani R et al. (2000) Metabolic engineering of beta-carotene
and
lycopene content in tomato fruit. Plant Journal 24: 413-419
32. Washam C (2005) An ounce of prevention from a ton of tomatoes.
Environmental
Health Perspectives 113: A178-A181
33. Ye X, Al-Babili S et al (2000) Engineering the provitamin A (B-carotene)
biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287: 303-
305
34. Geifman et al (2005) Clear tomato concentrate as a taste enhancer US
Patent
6,890,574
35. Zelkha et al (2004) Carotenoid Extraction Process US Patent 6,797,
28
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 28
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 28
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: