Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02645883 2008-12-05
SELECTIVE DETECTION OF HUMAN RHINOVIRUS
= GOVERNMENT INTEREST
100011 The invention described herein may be manufactured, used, and
licensed by or for the
United States Government.
FIELD OF THE INVENTION
100021 This invention relates generally to processes for detection of
virus in fluid samples.
More specifically, the instant invention-relates-to-selective detection of-
human-rhinovirus- (1-IRV)-
in biological or other fluid media. Processes are described for rapid and
sensitive detection of
1-1RV in human and animal biological samples and quantification thereof
Diagnostic kits are
provided for detection of HRV in a clinical, laboratory, or field setting.
BACKGROUND OF THE INVENTION
[00031 Human rhinovirus (I-IRV) infections are among- the most frequent
cause of the
common cold. (Pitkaranta, A., and F. G. Hayden. 1998. Ann. Med. 30:529-537).
Recently
FIRVs have been linked to severe lower respiratory illnesses in young children
(Miller E.K.,
2007. J. Infect. Dis. 195:773-781, Monto A.S., Clin. Ther. 2001; 23:1615-
1627), the elderly
(Hicks, L.A., I...4177. Geriatr. Soc., 2006; 54:284-289, Nicholson, K.G., Br.
Med. J., 1996;
= 3 ] 3:1119-1123, Wald, T., Ann. Intern. Med, 1995; 123:588-593) and the
immunocompromised
(Gosh, S.R., Clin, Infect. Dis., 1999; 29:528-532, Ison, M.G., Clin. Infect.
Dis., 2003; 36:1139-
1143). Persons with underlying respiratory disease, like asthma, chronic
bronchitis and cystic
fibrosis may also have increased risk of severe HRV-associated complications.
(Friedlander, S.
CA 02645883 2008-12-05
L., and W. W. Busseõ1 Allergy. Cl/n. Inununol., 2005; 116:267-273;
Khetsuriani, N, Alleiv.
hninunol., 2007; 119:314-321; Smyth, AR., Arch. Dis. Child, 1995; 73:117-120).
[00041 The
family Picornaviridde contains HRVs together with the human enteroviruses
(HEVs) (King, A.M., et al. 2000. Picornaviridae, p. 657-678. In Virus
Taxonomy. Seventh
Report of the International Committee for the Taxonomy of Viruses. Academic
Press, San
Diego, CA). At least 100 distinct HR.V serotypes of this family are assigned
to two phylogenetic
groups, A and B (Andries, K., J. Vera, 1990; 64:1117-1123), and new genetic
variants of HRV
have recently been reported. (Lamson, D.õ/. Infect. Dis., 2006; 194:1398-1402;
McErlean, P., J.
Virol, 2007; 39:67-75.)
[00051
Clinically, presentation of 11RV infection is of little diagnostic value due
to
symPtomatic similarity with numerous other infectious agents. Compounding
problems with
HRV identification, laboratory diagnosis suffers from the failure of some
strains to grow in cell
culture and by their extreme antigenic variability, precluding routine use of
antigen detection
methods or serology. (Lti, X., J
119icrobiol. 2008; 46(2):533-9.) FIRV identification with
prior art methods is difficult, and distinguishing HRVs from HEVs in the same
clinical sample
using acid liability is ineffective for many strains. Thus, modern efforts
have attempted to use
reverse-transcription polymerase chain reaction (RT-PCR) assays to increase
the detection
sensitivity and differentiation of IIRVs from co-existing infectious agents.
NON Nucleic
acid assays for IIRV typically target the 5'-noncoding region (5'NCR) of the
viral genome. The 5'NCR is preferred due to the availability of highly
conserved sequences that
support the complex secondary structures of the HRV/HEV internal ribosome
entry site (Witwer,
C., Nucleic. Acids Res., 2001; 29:5079-5089). Whereas the locations of these
conserved
sequences offer considerable flexibility for designing targeted primer/probes
for HEV real-time
2
CA 02645883 2008-12-05
= RT-PCR assays (Kares, S., J. ain. Virol., 2004; 29:99-104, Nijhuis, M.,
J. Clin. Microbiol.,
2002; 40:3666-3670; Verstrepen, W. A., .1. Ciin. Microbiol.; 2001; 39:4093-
4096), development
of comparable assays for FIRVs is hampered by their greater genetic
variability and the paucity
of published I-IRV sequence data from the 5'-NCR. In addition, prior art
nucleic acid assays
require post-amplification processing of the amplicon by gel electrophoresis,
probe
hybridization, sequencing or restriction analysis to confirm and differentiate
IIRVs from HEVs
(Andeweg, A.C., J. Clin. Microbia, 1999; 37:524-530; Atmar, R.L., and
Georghiou, Clin.
Microbiol., 1993; 31:2544-2546; Billaud, 0., Virol.
Methods, 2003; 108:223-228; Blomqvist,
S,,J. Clin. Microbiol., 1999; 37:2813-2816; Halonen, P.,]. Clin. Microbiol.,
1995; 33:648-653;
Kfimmerer, U., J. ain. Microbiot, 1994; 32:285-291; Loens, K., J. Chn.
Microbiol., 2006;
44:166-171; Miller, E.K., J. Infect. Dis., 2007; 195:773-781; Papadopoulos, N.
G., J. Virol.
Methods., 1999; 80:179-85).
[0007] More
recently, real-time RT-PCR assays have been described for HRV/IIEVs
(Dagher, 11., Virol.
Methods., 2004; 117:113-121; Deffernez, C., J. Clin. Microbiol., 2004;
42:3212-3218; Kares, S., 6/in. Virol., 2004; 29:99-104, Nijhuis, M., J. ain.
Microbiol., 2002;
40:366613670; Scheltinga, S.A., .1: ain. Virol., 2005; 33:306-311). These
assays did not detect
all known HRV serotypes (Dagher, H., J. Virol. Methods., 2004; 117:113-121;
Deffernez, C., J.
Clin. Microbiol., 2004; 42:3212-3218; Scheltinga, S.A., J. Clin. Virol., 2005;
33:306-311;
Wright, P.F., I. Clin. Microbiol., 2007; 45:2126-2129) or used difficult to
interpret SYBR Green
detection (Dagher, H., J. Virol. Methods., 2004; 117:113-121; Wittwer, CT.,
Biotechniques,
1997; 22:130-131, 134-138). Moreover, these prior art assays are inaccurate
due to the extensive
genetic variability of the HRVs and lack of available sequence data in the
public domain. Thus,
no real-time RT-PCR assays specifically identify all FIRVs relative to HEVs or
other viral fluid
3
CA 02645883 2008-12-05
components (Dagher, H., J. Virol. Methods., 2004; 117:113-121; Deffernez, C.,
J. Clin.
Microbiol., 2004; 42:3212-3218, Scheltinga, S.A., J. Clin. Virol., 2005;
33:306-311; Wright,
P.F., J Gun. Micro')tol., 2007; 45:2126-2129). Finally, no prior art assay has
successfully
detected viral prototype strains. Thus, there is a need for a rapid,
sensitive, and discriminatory
assay for detection of HRV in complex clinical or laboratory samples in the
presence or absence
of other viral agents..
SUMMARY OF THE INVENTION
[0008] A process for detecting human rhinovirus in a biological sample
includes producing
an amplification product by amplifying a human rhinovirus nucleotide sequence
using a forward
primer homologous to a region within nucleotides 356-563 of human rhinovirus
and a reverse
primer homologous to a region within nucleotides 356-563 of human rhinovirus
and measuring
the amplification product under conditions for a polymerase chain reaction to
detect human
rhinovirus in the biological sample. The forward primer is illustratively of
SEQ 11) NO: 1 and
the reverse primer is illustratively of SEQ ID NO: 2. Measuring the
amplification product may
illustratively be by using a probe complementary to a sequence of human
rhinovirus. The probe
may illustratively be of SEQ ID NO: 3. The inventive process is operable for
detection of human
rhinovirus infection in a biological sample.
[0009] The inventive process detects a first, second, or third detection
signal by a variety of
techniques such as liquid chromatography, mass spectrometry, liquid
chromatography/mass
spectrometry, static fluorescence, dynamic fluorescence, high performance
liquid
chromatography, ultra-high performance liquid chromatography, enzyme-linked
4
CA 02645883 2008-12-05
immunoadsorbent assay, real-time RT-PCR, RT-PCR, nucleotide sequencing, or
combinations
thereof
[0010] The inventive process allows for diagnoses human rhinovirus
infection in a human
subject. By comparing the first detection signal to a second detection signal,
where the second
detection signal results from the hybridization of a probe complementary to a
sequence from a
human rhinovirus.
10011] A second detection signal is optionally obtained by detection within
the same or a
parallel biological sample possibly containing human enterovirus, polio virus,
respiratory
syncytial virus, human metapneumovirus, human parainfluenza viruses 1-4,
adenovirus,
coronaviruses 229E and 0C43, influenza viruses A and B, and human bocavirus,
and the
hybridization of a probe complementary to a sequence from one or more viruses
of said group.
[0012] An inventive process optionally also or independently detects the
presence of human
enterovirus in a biological sample that illustratively includes producing an
amplification product
by amplifying a human enterovirus nucleotide sequence using a forward primer
Homologous to a
region within 356-563 of human enterovirus and a reverse primer homologous to
a region within
356-563 of human enterovirus and measuring the amplification product .under
conditions for a
polymerase chain reaction to detect human enterovirus in the biological sample
[0013] A process is provided in which the second detection signal is
generated in parallel
with, prior to, or following the first detection Signal. The complementary
amplification product
is illustratively generated by PCR amplification of a purified and titered
human rhinovirus
. solution. The first detection signal is also optionally compared to a third
detection signal from a
nucleic acid calibrator extracted in parallel to the biological sample to
provide further
CA 02645883 2008-12-05
quantification data, with nucleic acid calibrator containing a known amount of
human rhinovirus
and a known amount of a medium similar to the biological sample.
[00141 A kit for detecting human rhinovirus infection is provided that
includes a forward
primer with sequence SEQ ID NO: 1, a reverse primer with SEQ ID NO: 2, and a
non-
degenerate probe. An exemplary non-degenerate probe has the sequence SEQ ID
NO: 3.
100151 Also provided is a nucleotide sequence of SEQ ID NO: 1, SEQ ID NO:
2, or SEQ ID
NO: 3.
BRIEF DESCRIPTION OF THE DRAWINGS
100161 FIG. 1 represents alignment of partial 5'NCR sequences of 100 FIRV
and 52 HEV
serotypes in regions corresponding to primers (SEQ ID NOS: 1 and 2) and probe
(SEQ ID NO.
3) used for the inventive HRV real-time RT-PCR assay;
[0017] FIG. 2 represents a representative inventive real-time RT-PCR
amplification plot
obtained with serial 10-fold dilutions (5x101 to 5x107 copies per reaction) of
HRV14 RNA
_ transcript demonstrating sensitivity and robustness of the inventive
assay; and
[0018] FIG. 3 represents III2V detected by the inventive real-time RT-PCR
assay in serial
nasal and throat swab specimens from 4 FIRV positive donors (A, B, C and D)
with acute
respiratory illness.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
100191 The high genetic variability of FIRV and the presence of numerous
other potential
infectious agents capable of producing similar clinical symptoms highlights
the need for a rapid,
sensitive, and discriminatory assay and reagents for the detection and
quantification of1112V in a
6
=
CA 02645883 2015-05-15
fluid sample. The instant invention has utility for detection of HRV in
biological samples,
diagnosis of disease associated therewith, and discrimination against other
viral pathogens such
as HEV.
[0020] Several details of the current invention were published by Lu, X.õI
Clin. Microbiol.
2008; 46(2):533-9.
[0021] The following definitional terms are used throughout the
specification without regard
to placement relative to these terms.
[0022] As used herein, the term "variant" defines either a naturally
occurring genetic mutant
of HRV or a recombinantly prepared variation of HRV, each of which contain one
or more
mutations in its genome compared to the HRV of HRV1B (accession no. D00239).
The term
"variant" may also refer to either a naturally occurring variation of a given
peptide or a
recombinantly prepared variation of a given peptide or protein in which one or
more amino acid
residues have been modified by amino acid substitution, addition, or deletion.
100231 As used herein, the term "analog" in the context of a non-
proteinaceous analog
defines a second organic or inorganic molecule that possesses a similar or
identical function as a
first organic or inorganic molecule and is structurally similar to the first
organic or inorganic
molecule.
[0024] As used herein, the term "derivative" in the context of a non-
proteinaceous derivative
defines a second organic or inorganic molecule that is formed based upon the
structure of a first
organic or inorganic molecule. A derivative of an organic molecule includes,
but is not limited
to, a molecule modified, e.g., by the addition or deletion of a hydroxyl,
methyl, ethyl, carboxyl or
7
CA 02645883 2008-12-05
amine group. An organic molecule may also be esterified, alkylated and/or
phosphorylated. A
derivative also defined as a degenerate base mimicking a C/T mix such as that
from Glen
Research Corporation, Sterling, VA, illustratively LNA-dA or LNA-dT, or other
nucleotide
modification known in the art or otherwise.
100251 As used herein, the term "mutant" defines the presence of mutations
in the nucleotide
sequence of an organism as compared to a wild-type organism.
100261 A "purified" nucleic acid molecule is one that is separated from
other nucleic acid
molecules that are present in the natural source of the nucleic acid molecule
and is often
substantially free of other cellular material, or culture medium when produced
by recombinant
techniques, or substantially free of chemical precursors or other chemicals
when chemically
synthesized. This term is exclusive of a nucleic acid that is a member of a
library that has not
been purified away from other library clones containing other nucleic acid
molecules.
100271 As used herein, the term "hybridizes under stringent conditions"
describes conditions
for hybridization and washing under which nucleotide sequences having at least
30%, 35%, 40%,
45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, or 95% identity to each
other typically
remain hybridized to each other. Such hybridization conditions are described
in, for example but
not limited to, Current Protocols in Molecular Biology, John Wiley & Sons,
N.Y. (1989), 6.3.1
6.3.6.; Basic Methods in Molecular Biology, Elsevier Science Publishing Co.,
Inc., N.Y. (1986),
pp. 75-78, and 84-87; and Molecular Cloning, Cold Spring Harbor Laboratory,
N.Y. (1982), pp.
387-389, and are well known to those skilled in the art. A preferred, non-
limiting example of
stringent hybridization conditions is hybridization in 6x sodium
chloride/sodium citrate (SSC),
0.5% SDS at about 68 C followed by one or more washes in 2xSSC, 0.5% SDS at
room
temperature. Another preferred, non-limiting example of stringent
hybridization conditions is
8
CA 02645883 2008-12-05
hybridization in 6x SSC at about 45 C followed by one or more washes in 0.2x
SSC, 0.1% SDS
at 50 to 65 C.
[0028] An
"isolated" or "purified" nucleotide or oligonucleotide sequence is
substantially
free of cellular material or other contaminating proteins from the cell or
tissue source from which
the nucleotide is derived, or is substantially free of chemical precursors or
other chemicals when .
chemically synthesized. The
language "substantially free of cellular material" includes
preparations of a nucleotide/oligonucleotide in which the
nucleotide/oligonucleotide is separated
from cellular components of the cells from which it is isolated or produced.
Thus, a
nucleotide/oligonucleotide that is substantially free of cellular material
includes preparations of
the nucleotide having less than about 30%, 20%, 10%, 5%, 2.5%, or 1% (by dry
weight) of
contaminating material. When nucleotide/oligonucleotide is produced by
chemical synthesis, it
is preferably substantially free of chemical precursors or other chemicals,
i.e., it is separated
from chemical precursors or other chemicals which are involved in the
synthesis of the protein.
Accordingly, such preparations of the nucleotide/oligonucleotide have less
than about 30%, 20%,
10%, 5% (by dry weight) of chemical precursors or compounds other than the
nucleotide/oligonucleotide of interest. In a preferred embodiment of the
present invention, the
nucleotide/oligonucleotide are isolated or purified.
[0029] As used
herein, the term "isolated" virus or virus-like particle (VLP) is one which is
separated from other organisms which are present in the natural source of the
virus, e.g.,
biological material such as cells, blood, serum, plasma, saliva, urine, stool,
sputum,
nasopharyngeal aspirates, and so forth. The isolated virus or VII' can be used
to infect a subject
cell.
9
CA 02645883 2008-12-05
= 10030] As used herein, the term "biological sample" is defined as
sample obtained from a
biological organism, a tissue, cell, cell culture medium, or any medium
suitable for mimicking
biological conditions, or from the environment. Non-limiting examples include,
saliva, gingival
secretions, cerebrospinal fluid, gastrointestinal fluid, mucous, urogenital
secretions, synovial
fluid, blood, serum, plasma, urine, cystic fluid, lymph fluid, ascites,
pleural effusion, interstitial
fluid, intracellular fluid, ocular fluids, seminal fluid, mammary secretions,
and vitreal fluid, and
nasal secretions, throat or nasal materials. In a preferred embodiment, viral
agents are contained
in serum, whole blood, nasopharyngeal fluid, throat fluid, other respiratory
fluid.
[0031]. As used herein, the term "medium" refers to any liquid or fluid
biological sample in
the presence or absence of virus. Non-limiting examples include buffered
saline solution, cell
culture medium, acetonitrile, trifluoroacetic acid, combinations thereof, or
any other fluid
recognized in the art as suitable for combination with virus or cells, or for
dilution of a biological
sample or amplification product for analysis.
100321 To determine the percent identity of two nucleic acid sequences, the
sequences are
aligned for optimal comparison purposes (e.g., gaps can be introduced in the
sequence of a first
amino acid or nucleic acid sequence for optimal alignment with a second amino
acid or nucleic
acid sequence). The nucleotides at corresponding nucleotide positions are then
compared.
When a position in the first sequence is occupied by the same nucleotide as
the corresponding
position in the second sequence, then the molecules are identical at that
position. The percent
identity between the two sequences is a function of the number of identical
positions shared by
the sequences (i.e., % identity¨number of identical overlapping
positions/total number of
positions x 100%). In one embodiment, the two sequences are the same length.
CA 02645883 2008-12-05
[00331 The
determination of percent identity between two sequences can also be
accomplished using a mathematical algorithm. A preferred, nonlimiting example
of a
mathematical algorithm utilized for the comparison of two sequences is the
algorithm of Karlin
and Altschul, 1990, PNAS 87:2264 2268, modified as in Karlin and Altschul,
1993, PNAS.
90:5873 5877. Such an algorithm is incorporated into the NBLAST and XBLAST
programs of
Altschul et al., 1990, Mol. Biol. 215:403. BLAST nucleotide searches are
performed with the
NBLAST nucleotide program parameters set, e.g., for score-100, wordlength=12
to obtain
nucleotide sequences homologous to a nucleic acid molecules of the present
invention. BLAST
protein searches are performed with the XBLAST program parameters set, e.g.,
to score 50,
wordlength=3 to obtain amino acid sequences homologous to a protein molecule
of the present
invention. To obtain gapped alignments for comparison purposes,- Gapped BLAST
are utilized
as described in Altschul et al., 1997, Nucleic Acids Res. 25:3389 3402.
Alternatively, PSI
BLAST is used to perform an iterated search which detects distant
relationships between
molecules (Id.). When utilizing BLAST, Gapped BLAST, and PSI Blast programs,
the default
parameters of the respective programs (e.g., of ximAsT and NBLAST) are used
(see, e.g., the
NCBI website). Another preferred, non-limiting example of a mathematical
algorithm utilized
for the comparison of sequences is the algorithm of Myers and Miller, 1988, GA
BIOS 4:11 17.
Such an algorithm is incorporated in the ALIGN program (version 2.0) which is
part of the GCG
sequence alignment software package. When utilizing the ALIGN program for
comparing
amino acid sequences, a PAM120 weight residue table, a gap length penalty of
12, and a gap
penalty of 4 is used.
= 11
CA 02645883 2008-12-05
[0034] The
percent identity between two sequences is determined using techniques similar
to
those described above, with or without allowing gaps. In calculating percent
identity, typically
only exact matches are counted.
[00351 As used
herein, the terms "subject" and "patient" are synonymous and refer to a
human or non-human animal, preferably a mammal including a human, non-primate
such as
cows, pigs, horses, goats, sheep, cats, dogs, avian species and rodents; and a
non-human primate
such as monkeys, chimpanzees, and apes; and a human, also denoted specifically
as a "human
subject".
[0036] The
instant inventive process provides a rapid, specific, and sensitive assay
process
for detection of HRV in biological samples by amplifying one or more
nucleotide sequences with
greater specificity to strains of IIRV than HEV or other viral agents and are
present in a
= biological sample by processes similar to the polymerase chain reaction
(PCR).
[0037] An
oligonucleotide forward primer with a nucleotide sequence complementary to a
unique sequence in an IIRV nucleotide sequence illustratively in the 5'-NCR is
hybridized to its
complementary sequence and extended.
Similarly, a reverse oligonucleotide primer
compleme tary to a second strand of IIRV DNA in the same or an alternate HRV
region is
hybridized and extended. This system allows for amplification of specific gene
sequences and is
suitable for simultaneous or sequential detection systems.
10038) The
present invention relates to the use of the sequence information of HRV for
diagnostic process. In particular, the present invention provides a process
for detecting the
presence or absence of nucleic acid molecules of HRV, natural or artificial
variants, analogs, or
derivatives thereof, in a biological sample. The process involves obtaining a
biological sample
12
CA 02645883 2008-12-05
from various sources and contacting the sample with a compound or an agent
capable of
detecting a nucleic acid sequence of HRV, natural or artificial variants,
analogs, or derivatives
thereof, such that the presence of IIRV, natural or artificial variants,
analogs, or derivatives
thereof, is detected in the sample. In a preferred specific embodiment, the
presence of FIRV,
natural or artificial variants, analogs, or derivatives thereof, is detected
in the sample by a reverse
transcription polymerase chain reaction (RT-PCR) using the primers that are
constructed based
on a partial nucleotide sequence of the HRV virus. In a non-limiting specific
embodiment,
preferred forward primer to be used in a RT-PCR process is 5'-CPXGCCZGCGTGGY-
3' (SEQ
ID NO: 1) where P= pyrimidine derivative, a degenerate base mimicking a C/T
mix (Glen
Research Corporation, Archive Report 8.1), X=1,NA-dA, Z=LNA-dT (Glen Research
Corporation, Archive Report 20.1, Y=C or T, and a reverse primer
5'-GAAACACCiGACACCCAAAGTA-3 (SEQ ID NO: 2). LNA denotes a locked nucleic acid
and X includes a deoxyadenosine or deoxythymidine bonded thereto. LNAs were
first detailed
in Koshkin et al., Tetrahedron 54, 3607-3630 (1998). In preferred embodiments,
the primers
comprise the nucleic acid sequence of SEQ ID NOS: I and 2. A preferred agent
for detecting
FIRV nucleic acid sequences is a labeled nucleic acid probe capable of
hybridizing thereto. In a
preferred embodiment, the nucleic acid probe is a nucleic acid molecule
comprising or consisting
of the nucleic acid sequence of 5'-"FCCTCCGGCCCCTGAATOYO0C -3' (SEQ ID NO: 3),
which sufficiently specifically hybridizes under stringent conditions to an I-
IRV nucleic acid
sequence when Y is C or T.
[00391 The
process of the present invention can involve a real-time quantitative PCR
assay.
In a preferred embodiment, the quantitative PCR used in the present invention
is TaqMan assay
(Holland et al., PNAS 88(16):7276 (1991)). It is appreciated that the current
invention is
13
CA 02645883 2008-12-05
amenable to performance on other RT-PCR systems and protocols that use
alternative reagents
illustratively including, but not limited to Molecular Beacons probes,
Scorpion probes, multiple
reporters for multiplex PCR, combinations thereof, or other DNA detection
systems.
[0040] The assays are performed on an instrument designed to perform such
assays, for
example those available from Applied Biosystems (Foster City, CA). In more
preferred specific
embodiments, the present invention provides a real-time quantitative PCR assay
to detect the
presence of I-112V, natural or artificial variants, analogs, or derivatives
thereof, in a biological
sample by subjecting the HRV nucleic acid from the sample to PCR reactions
using specific
primers, and detecting the amplified product using a probe. In preferred
embodiments, the probe
is a TaqMan probe which consists of an oligonucleotide with a 51-reporter dye
and a 31-quencher =
dye.
[00411 A fluorescent reporter dye, such as FAM dye (illustratively 6-
carboxylluoreseein), is
covalently linked to the 5' end of the oligonueleotide probe. Other dyes
illustratively include
such TAMRA, AlexaFluor dyes such as AlexaFluor 495 or 590, Cascade Blue,
Marina Blue,
Pacific Blue, Oregon Green, Rhodamine, Fluoroseein, TET, HEX, Cy5, Cy3, and
Tetramethylrhodamine. Each of the reporters is quenched by a dye at the 3' end
or other
non-fluorescent quencher. Quenching molecules are suitably matched to the
fluorescence
maximum of the dye. Any suitable fluorescent probe for use in real-time PCR
(RT-PCR)
detection systems is illustratively operable in the instant invention.
Similarly, any quenching
molecule for use in RT-PCR systems is illustratively operable. In a preferred
embodiment a
6-carboxyfluorescein reporter dye is present at the 5'-end and matched to
Black Hole Quencher
(BHQ1, Biosearch Technologies, Inc., Novato, CA). The fluorescence signals
from these
reactions are captured at the end of extension steps as PCR product is
generated over a range of
14 =
CA 02645883 2015-05-15
the thermal cycles, thereby allowing the quantitative determination of the
viral load in the sample
based on an amplification plot.
10042]
The HRV virus nucleic acid sequences are optionally amplified before being
detected.
The term "amplified" defines the process of making multiple copies of the
nucleic acid from a
single or lower copy number of nucleic acid sequence molecule. The
amplification of nucleic
acid sequences is carried out in vitro by biochemical processes known to those
of skill in the art.
The amplification agent may be any compound or system that will function to
accomplish the
synthesis of primer extension products, including enzymes. Suitable enzymes
for this purpose
include, for example, E. coli DNA polymerase I, Taq polymerase, Klenow
fragment of E. coli
DNA polymerase I, T4 DNA polymerase, AmpliTaq GoldTM DNA Polymerase [trademark
of
Hoffman-LaRoche Limited]. other available DNA polymerases, reverse
transcriptase (preferably
iScript RNase H+ reverse transcriptase), ligase, and other enzymes, including
heat-stable
enzymes (i.e., those enzymes that perform primer extension after being
subjected to temperatures
sufficiently elevated to cause denaturation). In a preferred embodiment, the
enzyme is hot-start
iTaq DNA polymerase from Bio-rad (Hercules, CA).
Suitable enzymes will facilitate
combination of the nucleotides in the proper manner to form the primer
extension products that
are complementary to each mutant nucleotide strand. Generally, the synthesis
is initiated at the
3'-end of each primer and proceed in the 5'-direction along the template
strand, until synthesis
terminates, producing molecules of different lengths. There may be
amplification agents,
however, that initiate synthesis at the 5'-end and proceed in the other
direction. using the same
process as described above. In any event, the process of the invention is not
to be limited to the
embodiments of amplification described herein.
CA 02645883 2008-12-05
100431 One process of in vitro amplification, which is used according to
this invention, is the
polymerase chain reaction (PCR) described in U.S. Patent Nos. 4,683,202 and
4,683,195. The
term "polymerase chain reaction" refers to a process for amplifying a DNA base
sequence using
a heat-stable DNA polymerase and two ofigonucleotide primers, one
complementary to the (+)
strand at one end of the sequence to be amplified and the other complementary
to the (-)-strand
at the other end. Because the newly synthesized DNA strands can subsequently
serve as
additional templates for the same primer sequences, successive rounds of
primer annealing,
strand elongation, and dissociation produce rapid and highly specific
amplification of the desired
sequence. Many polymerase chain processes are known to those of skill in the
art and may be
used in the process of the invention. For example, DNA is subjected to 30 to
35 cycles of
amplification in a thermocycler as follows: 95 C for 30 see, 52 to 60 C for I
min, and 72 C for 1
min, with a final extension step of 72 C for 5 min. For another example, DNA
is subjected to 35
polymerase chain reaction cycles in a thermocyeler at a denaturing temperature
of 95 C for 30
see, followed by varying annealing temperatures ranging from 54 to 58 C for l
min, an
extension step at 70 C for 1 min, with a final extension step at 70 C for 5
mm.
10044] The primers for use in amplifying the mRNA or genomic RNA of I-IRV
may be
prepared using any suitable process, such as conventional phosphotriester and
phosphodiester
processes or automated embodiments thereof so long as the primers are capable
of hybridizing to
the nucleic acid sequences of interest. One process for synthesizing
oligonucleotides on a
modified solid support is described in U.S. Patent No. 4,458,066. The exact
length of primer
will depend on many factors, including temperature, buffer, and nucleotide
composition. The
primer must prime the synthesis of extension products in the presence of the
inducing agent for
amplification.
16
CA 02645883 2008-12-05
[06451 Primers
used according to the process of the invention are complementary to each
strand of nucleotide sequence to be amplified. The term "complementary" means
that the
primers must hybridize with their respective strands under conditions, which
allow the agent for
polymerization to function. In other words, the primers that are complementary
to the 'flanking
sequences hybridize with the flanking sequences and permit amplification of
the nucleotide
sequence. Preferably, the 3' terminus of the primer that is extended is
perfectly base paired with
the complementary flanking strand.
Preferably, probes possess nucleotide sequences
complementary to one or more strands of the 5'-NCR of HRV. More preferably,
the primers are
complementary to HRV genetic sequences encompassing positions 300-600. Most
preferably,
primers contain the nucleotide sequences of SEQ ID NOS: 1 and 2. It is
appreciated that the
complement of SEQ ID NOS: 1 and 2 are similarly suitable for use in the
instant invention. It is
further appreciated that oligonucleotide sequences that hybridize with SEQ ID
NO: 1 or 2 are
also similarly suitable. Finally, multiple positions are available for
hybridization on the HRV
genome and will be also suitable hybridization with a probe when used with the
proper forward
and reverse primers.
100461 Those of
ordinary skill in the art will know of various amplification processes that
can also be utilized to increase the copy number of target HRV nucleic acid
sequence. The
nucleic acid sequences detected in the process of the invention are optionally
further evaluated,
detected, cloned, sequenced, and the like, either in solution or after binding
to a solid support, by
any process usually applied to the detection of a specific nucleic acid
sequence such as another
polymerase chain reaction, oligomer restriction (Saiki et al., BioTechnology
3:1008 1012
(1985)), allele-specific oligonucleotide (ASO) probe analysis (Conner et al.,
RIVAS 80: 278
(1983)), oligonucleotide ligation assays (OLAs) (Landegren et al., Science
241:1077 (1988)),
17
CA 02645883 2008-12-05
RNase Protection Assay and the like. Molecular techniques for DNA analysis
have been
reviewed (Landegren et al., Science 242:229 237 (1988)). Following DNA
amplification, the
reaction product may be detected by Southern blot analysis, without using
radioactive probes. In
such a process, for example, a small sample of DNA containing the nucleic acid
sequence
obtained from the tissue or subject is amplified, and analyzed via a Southern
blotting technique.
The use of non-radioactive probes or labels is facilitated by the high level
of the amplified signal.
In one embodiment of the invention, one nucleoside triphosphate is
radioactively labeled,
thereby allowing direct visualization of the amplification product by
autoradiography. In another
embodiment, amplification primers are fluorescently labeled and run through an
electrophoresis
system. Visualization of amplified products is by laser detection followed by
computer assisted
graphic display, without a radioactive signal.
[0047] Other methods of detection amplified oligonucleotide
illustratively include gel
electrophoresis, mass spectrometry, liquid chromatography, fluorescence,
luminescence, gel
mobility shift assay, fluorescence resonance energy transfer, nucleotide
sequencing, enzyme-
linked immunoadsorbent assay, affinity chromatography, chromatography,
immunoenzymatic
methods (Ortiz, A and Ritter, E, Nucleic Acids Res., 1996; 24:3280-3281),
streptavidin-
.
conjugated enzymes, DNA branch migration (Lishanski, A, et al., Nucleic Acids
Res., 2000;
28(9):e42), enzyme digestion (U.S. Patent No. 5,580,730), calorimetric methods
(Lee, K.,
Biotechnology Letters, 2003; 25:1739-1742), or combinations thereof
10048] The term "labeled" with regard to the probe is intended to
encompass direct labeling
of the probe by coupling (i.e., physically linking) a detectable substance to
the probe, as well as
indirect labeling of the probe by reactivity with another reagent that is
directly labeled.
Examples of indirect labeling include detection of a probe using a
fluorescently labeled antibody
18
CA 02645883 2008-12-05
and end-labeling or centrally labeling of a DNA probe with biotin Such that it
can be detected
with fluoreseently labeled streptavidin. The detection method of the invention
can be used to
detect RNA (particularly mRNA) or gcnomic nucleic acid in a sample in vitro as
well as in vivo.
For example, in vitro techniques for detection of nucleic acid include
northern hybridizations, in
situ hybridizations, RT-PCR, real-time RT-PCR, and DNase protection. In vitro
techniques for
detection of genomic nucleic acid include northern hybridizations, RT-PCR,
real-time RT-PCR,
and DNase protection. Furthermore, in vivo techniques for detection of HRV
include
introducing into a subject organism a labeled antibody directed against a
capsid or polypeptide
component or directed against a particular nucleic acid sequence of HRV. For
example, the
antibody can be labeled with a radioactive marker whose presence and location
in the subject
organism can be detected by standard imaging techniques, including
autoradiography.
100491 The size of the primers used to amplify a portion of the nucleic
acid sequence of FIRV
is at least 5, and often 10, 15, 20, 25, or 30 nucleotides in length.
Preferably, the GC ratio should
be above 30%, 35%, 40%, 45%, 50%, 55%, or 60% so as to prevent hair-pin
structure on the
primer. Furthermore, the amplicon should be sufficiently long enough to be
detected by standard
molecular biology methodologies. The forward primer is preferably shorter than
the reverse
primer. Techniques for modifying the Tõ, of either primer are operable herein.
An illustrative
forward primer contains LNA-dA and LNA-dT (Glen Research Corporation) so as to
match
with a corresponding alternate primer.
[0050] An inventive process uses a polymerization reaction which employs a
nucleic acid
polymerizing enzyme, illustratively a DNA polymerase, RNA polymerase, reverse
transcriptasc,
or mixtures thereof. It is further appreciated that accessory proteins or
molecules are present to
form the replication machinery. In a preferred embodiment the polymerizing
enzyme is a
19
CA 02645883 2008-12-05
thermostable polymerase or thermodcgradable polymerase. Use of thermostable
polymerases is
well known in the art such as Taq polymerase available from Invitrogen
Corporation.
Thermostable polymerases allow a polymerization reaction to be initiated or
shut down by
changing the temperature other condition in the reaction mixture without
destroying activity of
the polymerase.
100511 Accuracy of the base pairing in the preferred embodiment of DNA
sequencing is
provided by the specificity of the enzyme. Error rates for Taq polymerase tend
to be false base
incorporation of 10-5 or less. (Johnson, Annual Reviews of Biochemistry, 1993:
62:685-713;
= Kunkel, Journal of Biological Chemistry, 1992; 267:18251-18254). Specific
examples of
thermostable polymerases illustratively include those isolated from Therms
aquaticus, Therms =
thermophilus, Pyrococcus woesei, Pyrococcu s furiosus, Thermococcus litoralis
and Thermotoga
maritima. Thermodegradable polymerases illustratively include E. coli DNA
polymerase, the
Klenow fragment of E. coli DNA polymerase, T4 DNA polymerase, T7 DNA
polymerase and
other examples known in the art. It is recognized in the art that other
polymerizing enzymes are
similarly suitable illustratively including E. coli, T7, T3, SP6 RNA
polymerases and AMV,
M-MLV, and HIV reverse transcriptases.
[00521 The polymerases are optionally bound to the primer. When the HRV is
a single-
stranded DNA molecule due to heat denaturing the polymerase is bound at the
primed end of the
single-stranded nucleic acid at an origin of replication. A binding site for a
suitable polymerase
is optionally created by an accessory protein or by any primed single-stranded
nucleic acid.
100531 In a further embodiment detection of PCR products is achieved by
mass
spectrometry. Mass spectrometry has several advantages over RT-PCR or real-
time RI=PCR
systems in that it can be used to simultaneously detect the presence of IIRV
and decipher
CA 02645883 2008-12-05
mutations in target nucleic acid sequences allowing identification and
monitoring of emerging
strains. Further, mass spectrometers are prevalent in the clinical laboratory.
Similar to
fluorescence based detection systems mass spectrometry is capable of
simultaneously detecting
multiple amplification products for a multiplexed and controlled approach to
accurately
quantifying components of biological or environmental samples.
100541 Multiple mass spectrometry platforms are suitable for use in the
instant invention
illustratively including matrix assisted laser desorption ionization time of
flight mass
spectrometry (MALDI), electrospray mass spectrometry, clectrospray ionization-
Fourier
transform ion cyclotron resonance mass spectrometry (ESI-FTICR), multi-stage
mass
spectrometry fragmentation analysis (MS/MS), mass spectrometry coupled with
liquid
chromatography such as high performance liquid chromatography mass
spectrometry (11PLC)
and ultra performance liquid chromatography isotope dilution tandem mass
spectrometry (UPLC-
ID/MS/MS), and variations thereof.
100551 It is appreciated that numerous other detection processes are
similarly suitable for
measuring an amplification product by detecting a detection signal.
Illustrative examples include,
but are not limited to, liquid chromatography, mass spectrometry, liquid
chromatography/mass
spectrometry, static fluorescence, dynamic fluorescence, high performance
liquid chromatography,
ultra-high performance liquid chromatography, enzyme-linked immunoadsorbent
assay, real-time
PCR (RT-PCR), gel electrophoresis, or combinations thereof
100561 Preferably, PCR amplification products are generated using
complementary forward
and reverse oligonucleotide primers. In a non-limiting example, FIRV genetic
sequences or
fragments thereof are amplified by the primer pair SEQ ID NOS: 1 and 2 that
amplify a
conserved sequence in the FIRV 5'-NCR encompassing nucleotides 356-563. The
resulting
21
CA 02645883 2008-12-05
amplification product is processed and prepared for detection by processes
known in the art. It is
appreciated that the complements of SEQ ID NOS: 1 and 2 are similarly suitable
for use in the
instant invention. It is further appreciated that oligonueleotide sequences
that hybridize with
SEQ ID NO: 1 or 2 are also similarly suitable. Finally, multiple positions are
available for
hybridization on the FIRV genome and will be also suitable hybridization with
forward and
reverse primers that may or may not be used with a probe for real-time RT-PCR.
10057] Optionally, multiple amplification products are simultaneously
produced in a PCR
reaction that is then available for simultaneous detection and quantification.
Thus, multiple
detection signals are inherently produced or emitted that are separately and
uniquely detected in
one or more detection systems. It is appreciated that multiple detection
signals are optionally
produced in parallel. Preferably, a single biological sample is subjected to
analysis for the
simultaneous or sequential detection of 1-IRV genetic sequences. It is
appreciated that three or
more independent or overlapping sequences are simultaneously or sequentially
measured in the
instant inventive process. Oligonucleotide Matched primers (illustratively SEQ
ID NOS: 1 and
2) are simultaneously or sequentially added and the biological sample is
subjected to proper
thermocycling reaction parameters. For detection by mass spectrometry a single
sample of the
amplification products from each gene arc simultaneously analyzed allowing for
rapid and
accurate determination of the presence of I-IRV. Optionally, analysis by real-
time RT-PCR is
employed capitalizing on multiple probes with unique fluorescent signatures.
Thus, each gene is
detected without interference by other amplification products. This, multi-
target approach
increases confidence in quantification and provides for additional internal
control.
100581 In a specific embodiment, the processes further involve obtaining a
control sample
from a control subject, contacting the control sample with a compound or agent
capable of
22
CA 02645883 2008-12-05
. detecting the presence of IIRV nucleic acid in the sample, and comparing
the presence of mRNA
or genomic RNA in the control sample with the presence of mRNA or genomic DNA
in the test
sample.
100591 The invention also encompasses kits for detecting the presence of
HRV viral nucleic
acids in a test sample. The kit, for example, includes a labeled compound or
agent capable of
detecting a nucleic acid molecule in a test sample and, in certain
embodiments, for determining
the titer in the sample.
100601 For oligonucleotide-based kits, the kit includes for example: (1) an
oligonucleotide,
e.g., a detectably labeled oligonucleotide, which hybridizes to a nucleic acid
sequence of the
HRV virus and/or (2) a pair of primers (one forward and one reverse) useful
for amplifying a
nucleic acid molecule containing the HRV viral sequence. The kit can also
comprise, e.g., a
buffering agent, a preservative, or a protein stabilizing agent. The kit can
also comprise
components necessary for detecting the detectable agent (e.g., an enzyme or a
substrate). The kit
can also contain a control sample or a series of control samples which is
assayed and compared
to the test sample contained. Each component of the kit is usually enclosed
within an individual
container and all of the various containers are usually enclosed within a
single package along
with instructions for use.
100611 The instant inventive processes are amenable to use for diagnosis of
FIRV infection in
a subject, insects, and any inclusive other organism capable of infection or
transfection by or
with HRV.
10062] To increase confidence and to serve as an internal or external
control, a purified and
titered HRV solution is used as a biological sample. By amplification of a
single sample with
known quantities of FIRV or of a set of samples representing a titration of
HRV, the level of
23
CA 02645883 2008-12-05
FIRV in the unknown biological sample is determined. Preferably, the purified
and titered HRV
solution is analyzed in parallel with the unknown biological sample to reduce
inter assay error or
to serve as a standard curve for quantitation of unknown HRV in the biological
sample. Using
purified and titered HRV solution provides for a similar complete genetic base
DNA strand for
amplification.
100631 In another embodiment, a subgenomic fragment is cloned into a
plasmid for
amplification, purification, and use as a quantitative comparator or nucleic
acid calibrator. In a
non-limiting example, a DNA subgenomic fragment of I-IRV is optionally
amplified from a
positive nasal swab using primers bracketing the RT-PCR target regions in the
5'-NCR of HRV.
It is appreciated that other sequences are similarly suitable for use as a
quantitative control. The
known concentration of the subgenomic fragment is used to create a standard
curve for
quantitative determinations and to access amplification efficiency.
100641 Also provided is a kit for detecting HRV infection that contains
reagents for the
amplification; or direct detection of HRV or portions thereof. An exemplary
kit illustratively
includes a forward and reverse primer pair, a non-degenerate probe. In a
preferred embodiment,
the forward and reverse primers have the oligonucleotide sequence SEQ ID NOS:
1 and 2 and a
nondegenerate probe of the sequence SEQ ID NO: 3. It is appreciated that a
diagnostic kit may
optionally contain primers and probes that are the complements of SEQ ID NOS:
1-3 or that
hybridize with oligonucleotides SEQ ID NOS: 1-3. It is further appreciated
that a diagnostic kit
optionally includes ancillary reagents such as buffers, solvents, thermostable
polymerases,
nucleotides, and other reagents necessary and recognized in the art for
amplification and
detection of FIRV in a biological sample.
24
CA 02645883 2008-12-05
[00651 The invention provides a host cell containing a nucleic acid
sequences according to
the invention as an alternative to synthetic primer sequence generation.
Plasmids containing the
polymerase components of the HRV virus are generated in prokaryotic cells for
the expression of
the components in relevant cell types (bacteria, insect cells, eukaryotic
cells). Preferably, the cell
line is a primate cell line. These cell lines may be cultured and maintained
using known cell
culture techniques such as described in Cells, Julio, ed., 1994, Cell Biology
Laboratory
Handbook, Academic. Press, NY. Various culturing conditions for these cells,
including media
formulations with regard to specific nutrients, oxygen, tension, carbon
dioxide and reduced
serum levels, can be selected and optimized by one of skill in the art.
100661 The preferred cell line of the present invention is a eukaryotic
cell line, preferably an
insect cell line, such as Sf9 per, transiently or stably expressing one Or
more full-length or partial
HRV proteins. Such cells can be made by transfection (proteins or nucleic acid
vectors),
infection (viral vectors) or transduction (viral vectors). The cell lines for
use in the present
invention are cloned using known cell culture techniques familiar to one
skilled in the art. The
cells are cultured and expanded from a single cell using commercially
available culture media
under known conditions suitable for propagating cells.
100671 A host cell is a cell derived from a mammal, insect, yeast,
bacteria, or any other
single or multicellular organism recognized in the art. Host cells are
optionally primary cells or
immortalized derivative cells. Immortalized cells are those which can be
maintained in-vitro for
several replication passages.
100681 In a most preferred embodiment, an HRV antigen such as an amino acid
sequence
representative of a capsid protein is used as a control for a PCR based assay
for the detection and
measurement of the presence of HRV in a biological sample. The process of
detecting HRV
=
CA 02645883 2008-12-05
antibodies in a biological sample is optionally performed in parallel with the
same or control
biological samples that are used to detect HRV genetic sequences.
[0069] A kit for detection of HRV infection in a patient optionally
contains reagents for PCR
based detection of HRV genetic sequences, either structural or non-structural,
and optionally for
detection of antibodies directed to structural I-IRV proteins, The components
of the kits are any
of the reagents described above or other necessary and non-necessary reagents
known in the art
for solubilization, detection, washing, storage, or other need for in a
diagnostic assay kit.
[0070] The present invention is further illustrated with respect to the
following non-limiting
examples. The following examples are for illustrative purposes only and are
not a limitation on
. the practice or scope of the invention.
[0071] Example 1: Obtaining viral strains and clinical specimens. One
hundred HRV
prototype strains (strains identification 1A, 1B, 2-86, 88-100) (numbering
refers to strain
assignment number as illustrated in 'Fla 1 and the description thereof) are
kindly obtainable
from ViroPharma Inc. (Ledford, R.M.õ/. Virol., 2004; 78:3663-3674) and 85 HRV
field isolates
obtained from several sources between 1999 and 2007 were available for study.
IIRV isolates
are either sequenced directly or subjected to a single passage in HeLa Ohio
cells.
100721 For cell culture, infected cells are incubated at 35 C in 5% CO2
with gentle rocking
until reaching full cytopathic effect. Isolates are freeze-thawed twice,
clarified by low speed
centrifugation and supernatants collected and stored at -70 C. In one study 48
HEV laboratory
strains were grown in primary monkey kidney or human RD cells and prepared as
above. The
studied strains included echoviruses 1-6, 8, 9, 11-25, 29-31; coxsackievirus
types A2, A4-6, A8-
10, A16, A21, A24, 131-6; enterovirus types 68, 70, 71; and poliovirus types
1,2 and 3. Other
respiratory viruses are subjected to testing for specificity including
respiratory syncytial virus,
26
CA 02645883 2008-12-05
human metapneumovirus, human parainfluenza viruses 1-4, adenovirus,
coronaviruscs 229E and
0C43, influenza viruses A and B, and human bocavirus, (1_,u, X.,
Microbia, 2006;
44:3231-3235). Coded respiratory specimens that were culture positive for HRV
or HEV were
provided by California. Department of Health Services, University of
Washington, Vanderbilt
Medical Center, and University of Rochester Medical Center for clinical
validation studies.
Nasal and throat swab specimens are self-obtained by symptomatic volunteers or
obtained
clinically. These specimens are expressed in 2 ml of chilled viral transport
media (Hank's
buffered salt solution with 0.5% gelatin) and frozen at -70 C prior to
testing.
100731 Example
2: Preparation of nucleic acid and sequencing of viral strains. To
identify conserved regions of the sample viral strains all IIRV and HEV viral
strains obtained as
in Example 1 are subjected to nucleotide sequencing.
100741 Total
nucleic acid extracts from all samples collected or obtained as in Example 1
are
prepared from 100 pl of infected cell culture lysate or 200 1.t1 of clinical
specimen using the
NucliSense casyMAGTm extraction system following manufacturer's instructions
(bioMerieux,
Durham, NC).
. (00751 The
5'NCR of viral strains was sequenced to identify conserved .regions. Extracted
viral RNA is reverse transcribed using random hexamer primers (Promcga,
Madison, WI) at
52 C for 60 min with Superscript-rm III Reverse Transcriptase (Invitrogen,
Carlsbad, CA)
following manufacturer's instructions. Five }.11 of the obtained cDNA is
amplified in two
separate PCR reactions using IIRV species A (SEQ ID NOS: 4 and 5) and B
specific
amplification primer sets (SEQ II) NOS: 6 and 7) (Table 1) with the HotStarTaq
Master Mix Kit
(Qiagen, Chatsworth, CA). PCR cycling conditions are as follows: initial
activation step at 95 C
for 15 min followed by 35 cycles of 95 C for I min, 55 C for I min and 72 C
for 1 min, with a
27
CA 02645883 2008-12-05
final extension of 72 C for 5 min on a GeneAmp PCR System 9700 (Applied
Biosystems).
Amplified products are subjected to purification with the Q1Aquick0 PCR
Purification Kit
(Qiagen). Sequencing is performed in both directions using the amplification
primers and the
ABI Prism Bigdyem" Terminator Cycle Sequencing Ready Reaction Kit ver. 3.1 on
an ABI
3100 DNA Sequencer (Applied Biosystems). Sequence assembly and editing is
accomplished -
using Sequencher.rm ver. 3.1.1 software (Gene Codes, Ann Arbor, MI).
28
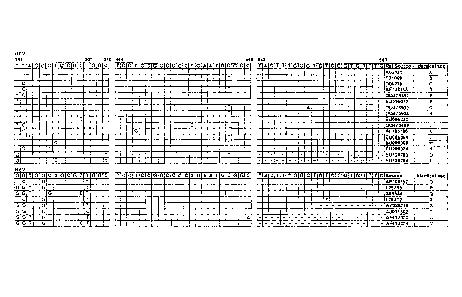
Table 1. HRV/HEV primers and probes.
Primer/Probea Sequence (5'- 3.)b
Position
Real-time RT-PCR
: ...:.... =:. . --=
:
Primer, fwd CPXGCCZGCGTGGY (SEQ ID NO: 1) where Y is C
356-369'
Primer, rev GAAACACGGACACCCAAAGTA (SEQ ID NO: 2)
563-543'
Probe TCCTCCGGCCCCTGAATGYGGC (SEQ ID NO: 3)
444-465'
FigvA 5'NCR:seqUeriCing= :.= = : s : =
= . : = : = .
. . - . . . . . .
. .
Primer, fwd GTACTCTGTTATTCCGGTAACTTTGYAYGCCA (SEQ ID NO: 4)
49-80'
Primer, rev CCAACATTCTGTCTAGATACYTGDGCVCCCAT (SEQ ID NO: 5)
655-623'
ugvB:5'INICR sequencing. . : : : : :
..!...1. ,
Primer, fwd A.CTCTGGTACTATGTACC-fTTGTACGCCTGTT (SEQ ID NO: 6)
48-80d
(xi
co
Primer, rev CCACTCTTCTGTGTAGACACYTGDGCDCCCAT (SEQ ID NO: 7)
661-629d co
Hini..14. RNA transcript' = = = = = = = =
0
Primer, fwd - T7 TAATACGACTCACTATAGGGCAAGCACTTCTGTTT (SEQ ID NO: 8)
179-193d
Primer, rev - SP6 ATTTAGGTGACACTATAGAAGCATCTGGTAATTTCC (SEQ ID NO: 9)
1089-1074d 0
(xi
.1-IEV68 RNA transcript
. =:. : . : =
=. . : .
. .
Primer, fwd - T7 TAATACGACTCACTATAGGOTCTTATGAGCAAGCACT (SEQ ID NO: 10)
52-68'
Primer, rev - SP6 ATTTAGGTGACACTATAGAAATTACTTCAAAATAACTCAG (SEQ ID NO: 11)
573-554'
'Probes 5'-end-labeled with 6-carboxyfluorescein (FAM) and 3'-end-labeled with
Black Hole Quencher' TM
bY=dC or dl. D=dA, dT or dG, dA, dC or dG, P= pyrimidine derivative, a
degenerate base mimicking a C/T mix (Glen Research
Corporation, 'Archive Report 8.1), X=LNA-dA, Z=LNA-dT (Glen Research
Corporation, Archive Report 20.1); underlined sequences
are T7 and SP6 promoter sites
Nucleotide numbering based on HRV1B (accession no. D00239)
dNucleotide numbering based on HRV14 (accession no. K02121)
'Nucleotide numbering based on HEV68 (formally HRV87) (accession no. AY062273)
29
CA 02645883 2008-12-05
[0076] All obtained sequences are aligned along with previously
identified sequences of
representative FIRWHEV strains available from CienBank (NIII). As demonstrated
in FIG. 1,
the alignment identified a conserved region within the 5'NCR between
nucleotide positions 356
and 563 (numbering relative to I-IRV B accession no. 1)00239). Subregions were
identified to
design real-time RT-PCR primer pairs for subsequent evaluation. The most
preferred primer pair
are represented by SEQ ID NOS: I and 2. The forward primer (SEQ ID NO: 1) is
located in a
variable region that contains a signature "f" indel at nt position 367. This
indel distinguishes all
ITRVs from HEVs and is exploited for differential amplification and
identification. The forward
primer is necessarily shorter in length due to the lower conservation of this
region of the 5'NCR.
To compensate LNA-dA and LNA-dT is introduced into the primer at positions 3
and 7
respectively to achieve a balanced T,õ with the reverse primer. It is
appreciated that other
modifications including sequence length, chemical and other modifications arc
similarly
operable. Real-time RT-PCR is optimally achieved using the iScriptTm One-step
RT-PCR Kit
for Probes (BioRad). Other commercial real-time RT-PCR reagent kits performed
equally or
less optimally. The QuantiTect Probe PCR Kit (Qiagen) and Ag-Path-IDTNA One-
Step RT-PCR
Kit (Applied Biosystems) performs comparably, whereas amplification is less
efficient with the
TaqMane One-Step RT-PCR Master Mix (Applied Biosystems). Real-time RT-PCR is
= unsuccessful using SuperScriptTm Ill Platinum One-Step qRT-PCR Kit
(Invitrogen).
100771 Example 3: Real-time RT-PCR to identify HRV and distinguish HEV.
The real-
time RT-PCR assay is optimally performed using iScriptTM One-Step RT-PCR Kit
for Probes
(Bio-Rad, Hercules, CA). The reaction is performed in 25 p.1 final volume
containing 1 plvl
forward and reverse primers, 0.1 p.M probe, and 5 p.1 of nucleic acid extract
with the remaining
volume made of buffer. Amplification is performed on an iCycler iQ Real-Time
Detection
CA 02645883 2008-12-05
=
System (Bio-Rad) using the following thermocycling conditions: 10 min at. 48 C
for reverse
transcription; 3 min at 95 C for polymerase activation; and 45 cycles of 15 s
at 95 C; and 1 min
at 60 C.
100781 Undiluted RNA extracts of all FIRV prototype strains and field
isolates produce
strongly positive reactions [median cycle threshold (Ct) value 13.7, range 9.3
¨ 25.3]. The assay
is specific and robust for IIRV in that 34 HEVs are nonreactive and 14 (Echol,
3, 5, 6, 13, 21;
Poliol, 2; EV68, 71; CoxA4, 6, 24; CoxB1) produce only weakly positive
reactions (median Ct
value 34, range 33 ¨ 34.8); and may be related to virus titer.
100791 Assay sensitivity is determined by comparison of serial dilutions of
representative
HRV strains and other viral strains. Serial ten-fold dilutions of IIRV 14 RNA
transcripts that
show 100% sequence identity to the real-time RT-PCR primers and probe set (SEQ
ID NOS: 1-
3) are compared to HEN and other representative viruses. With HRV14 linear
amplification is
achieved over a 7-log dynamic range from 5x101 to 5x107 copies per reaction.
The assay's
detection limit with 24 replicates of is 100% positive at 50 copies; at 5
copies, 37.5 % positive at =
9 copies; and at 1 copy, 2 (8.3%) were positive. In contrast, the 11EV68
transcript is
undetectable below approximately 5 x 105 copies per reaction. Nucleic acid
extracts of other
respiratory viruses, including human respiratory syncytial virus, human
metapneumovirus,
parainfluenza viruses 1 - 4, adenovirus, coronaviruses 229E and 0C43,
influenza A and B, and
human bocavirus are negative by the inventive real-time RT-PCR assay.
100801 Over the linear range of the assay, the coefficient of variation of
the mean Ct values =
ranged from 0.24% to 0.94% within runs, and from 0.91% to 2.68% between runs
demonstrating
robust reproducibility. (FIG. 2.)
31
CA 02645883 2008-12-05
10081] Example 4: Use of the real-time RT-PCR assay for identification of
HRV in
clinical samples. Extracts of ill coded respiratory specimens previously
determined to be
culture positive for HRV or MEV are prepared and tested simultaneously by the
inventive HRV
real-time RT-PCR assay and compared to results obtained from two independent
laboratories
using different in-house 1-1RV/IIEV RT-PCR assays. Of 87 HRV culture-positive
specimens
tested, all are identified as HRV by the inventive real-time RT-PCR assay
(median Ct value 26.3;
range 14.9-38.5); HRV is also identified in all 87 specimens by one or both of
the reference in-
house RT-PCR assays. Of 24 HF,V culture-positive specimens, 4 are positive for
HRV by the
real-time RT-PCR assay (median Ct value 28.8; range 26.2 - 32.1); 1 of these 4
was also
identified as FIRV by laboratory B. HEN isolates available from 3 of the 4 HRV
positive
specimens were not amplified by the inventive real-time RT-PCR assay, whereas
amplicon
sequences obtained from all 4 clinical specimens were HRV positive suggesting
that both LIRV
and HEV were present in these specimens.
100821 To access the inventive real-time RT-PCR assay in clinical specimens
of nasal or
throat swabs, volunteers who developed respiratory illnesses characterized by
one or more of the
following symptoms: cough, congestion, myalgia, chills or fever, donated self-
collected samples.
The inventive real-time RT-PCR identified 5 eases with IIRV infection.
Collection began 2 and
6 days after onset of symptoms and continued until at least 2 consecutive
specimens tested
negative (FIG. 3). The duration of detectable HRV ranged from 11 to 21 days
(median 12.5
days). With the exception of case A, where HRV Was detected at comparable
levels from both
throat and nasal. swabs, throat swabs were either consistently negative for
HRV (cases B and C)
or became negative earlier than from nasal swabs (ease D). The duration of
symptoms for five
HRV positive cases ranged from 12 to 24 days (median 16 days); one case (D)
had a prolonged
32
CA 02645883 2015-05-15
paroxysmal cough that persisted for 24 days. The duration of reported symptoms
exceeded the
duration of detectable HRV by the inventive real-time RT-PCR assay for all
cases. Sequencing
of a partial region of the HRV VP 1 gene from the specimens obtained from the
5 cases identified
two genetically distinct HRV strains that showed the closest sequence
identities to HRV86
(amino acid identity score 83.5%) and HRV69 (amino acid identity score 84.6%),
respectively.
[0083] Example 5: Detection of HRV amplicons via mass spectroscopy.
Detection of
amplification products obtained as in Example 3 was performed essentially as
described by Blyn,
L, et al. I Clin. Microbial. 2008; 46(2):644-651. Following amplification each
PCR mixture is
desalted and purified using a weak anion-exchange protocol based on the method
of Jiang and
Hofstadler (Jiang, Y., and S. A. Hofstadler. Anal. Biachem. 2003; 316:50-57).
ESI-TOF is used
to obtain accurate-mass (+1 ppm), high-resolution (M/AM, >10,000 full width
half maximum)
mass spectra. For each sample, approximately 1.5 j.tl of analyte solution is
consumed during the
spectral acquisition. Raw mass spectra are postcalibrated with an internal
mass standard and
deconvolved to average molecular masses. Quantitative results are obtained by
comparing the
peak heights with an internal PCR calibration standard present in every PCR
well at 300
molecules unless otherwise indicated.
100841 Patent applications and publications mentioned in the specification
are indicative of
the levels of those skilled in the art to which the invention pertains. The
foregoing description is
illustrative of particular embodiments of the invention, but is not meant to
be a limitation upon
the practice thereof. The following claims, including all equivalents thereof,
are intended to
define the scope of the invention.
100851 The invention is hereby described with relation to the following
references and
those otherwise identified in the instant specification. Each reference is
mentioned for
33
CA 02645883 2015-05-15
the individual point referred to in the specification as well as for all
information
contained therein and not explicitly identified in the specification. All
references are
representative of the knowledge of a person of skill in the art and illustrate
other aspects
of the present invention as envisioned by the inventors.
REFERENCE LIST
1. Andeweg, A. C., T. M. Bestebroer, M. Huybreghs, I. G. Kimman, and J. C.
de Jong.
1999. Improved detection of rhinoviruses in clinical samples by using a newly
developed
nested reverse transcription-PCR assay. J. Clin. Microbiol. 37:524-530
2. Andries, K., B. Dewindt, J. Snoeks, L. Wouters, H. Moereels, P. J. Lewi,
and P. A.
Janssen. 1990. Two groups of rhinoviruses revealed by a panel of antiviral
compounds
present sequence divergence and differential pathogenicity. I Virol. 64:1117-
1123.
3. Atmar, R. L., and P. R. Georghiou. 1993. Classification of respiratory
tract picornavirus
isolates as enteroviruses or rhinoviruses by using reverse transcription-
polymerase chain
reaction. J. Clin. Microbiol. 31:2544-2546.
4. Billaud, G., S. Peny, V. Legay, B. Lina, and M. Valette. 2003. Detection
of rhinovirus
and enterovirus in upper respiratory tract samples using a multiplex nested
PCR. J. Virol.
Methods 108:223-228.
5. Blomqvist, S., A. Skytta, M. Roivainen, and T. Hovi. 1999. Rapid
detection of human
rhinoviruses in nasopharyngeal aspirates by a microwell reverse transcription-
PCR-
hybridization assay. J. Clin. Microbiol. 37:2813-2816.
6. Blomqvist, S., C. Savolainen, L. Raman, M. Roivainen, and T. Hovi. 2002.
Human
rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus
and
enterovirus features. I Clin. Microbiol. 40:4218-4223.
7. Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, A. J. Fox, E. B.
Kaczmarski,
and K. J. Mutton. 2002. Development and evaluation of a 'real-time RT-PCR for
the
34
CA 02645883 2008-12-05
detection of enterovirus and parechovirus RNA in CSF and throat swab samples.
Med.
Virol. 67:555-562.
8. Dagher, H., H. Donninger, P. Hutchinson, R. Cihildyal, and P. Bardin.
2004. Rhinovirus
detection: comparison of real-time and conventional PCR. J. 'lira Methods.
117:113-
121.
9. Deffernez, C., W. Wunderli, V. Thomas, S. Yerly, L. Perrin, and L.
Kaiser. 2004.
Amp!icon sequencing and improved detection of human rhinovirus in respiratory
samples../ Clin. Microbiol. 42:3212-3218.
10. Emmy, S. L., D. D. Erdman, M. D. Bowen, B. R. Newton, J. M. Winchell,
R. F. Meyer,
S. Tong, B. T. Cook, B. P. Holloway, K. A. Mccaustland, P. A. Rota, B. Ba
kamp, L. E.
Lowe, T. 0. Ksiazek, W. J. Bellini, and L. J. Anderson. 2004. Real-time
reverse
transcription-polymerase chain reaction assay for SARS-associated coronavirus.
Emerg.
Infect. Dis. 10:311-316.
11. Fox, J. P., M. K. Cooney, C.E. Hall, and H.M. Foy. 1985. Rhinoviruses
in Seattle
families, 1975-1979. Am. Epidemiol. 122:830-846.
12. Friedlander, S. L., and W. W. Busse. 2005. The role of rhinovirus in
asthma
exacerbations. J. Allergy. Clin. Immunol. 116:267-273.
13. Ghosh, S., R. Champlin, R. Couch, J. England, I. Raad, S. Malik, M.
Luna, and E.
Whimbey. 1999. Rhinovirus infections in myelosuppressed adult blood and marrow
transplant recipients. Clin. Infect Di.. 29:528-532.
14. Halonen, P., E. Rocha, J. Hierholzer, B. Holloway, T. Hyypia, 13.
Hurskainen, and M.
Pallansch. 1995. Detection of -enteroviruses and rhinoviruses in clinical
specimens by
PCR and liquid-phase hybridization. J. (?in. Ailicrobiol. 33:648-653.
15. Fiendley, J. 0., and J. M. Gwaltney 1988. Mechanisms of transmission of
rhinovirus
infections. Epidemiol. Rev. 10:243-258.
16. Hicks, L. A., COW. Sheppard, P. H. Britz, a D. Erdman, M. Fischer, B.
L. Flannery, A.
J. Peck, X. Lu, W. L. Thacker, R. F. Benson, M. I,. Tondella, M. E. Moll, C.
G. Whitney,
CA 02645883 2008-12-05
L. J. Anderson, and D. R. Feikin. 2006. Two outbreaks of severe respiratory
disease in
nursing homes associated with rhinovirus. J. Am. Gericnr. Soc. 54:284-289.
. 17. Hyypia, T., T. Puhakka, 0. Ruuskanen, M. Makela, A. Arola, and P.
Arstila. 1998.
Molecular diagnosis of human rhinovirus infections: comparison with virus
isolation. J.
Microbla 36:2081-2081
18. Ireland, D. C., J. Kent, and KØ Nicholson. 1993. Improved detection
of rhinoviruses in
nasal and throat swabs by seminested RT-PCR. J. Med. Virol. 40:96-101.
19. Ison, M. G., F. 0. Hayden, L. Kaiser, L. Corey, and M. Boeckh. 2003.
Rhinovirus
infections in hematopoietic stem cell transplant recipients with pneumonia.
din.
Div. 36:1139-1143.
20. Jartti, T., P. Lehtinen, T. Vuorinen, M. .Koskenvuo, and 0. Ruuskanen.
2004. Persistence
of rhinovirus and enterovirus RNA after acute respiratory illness in children.
J. Med.
Vero'. 72:695-699.
21. Johnston, S. L., and D. A. J. Tyrreil. 1995. Rhinoviruscs, p. 553-563.
In E. H. Lennette,
D. A. Lennette, and E. T. Lennette (ed.), Diagnostic Procedures for Viral,
Rickettsia], and
Chlamydial Infections, 7th ed. American public Health Association, Washington,
DC.
22. Joki-Korpela, P., and T. Hyypia. 2001. Parechoviruses, a novel group of
human
picomaviruses. Ann. Med. 33:466-471.
23. Kaiser, L., J. D. Aubert, J. C. Pachc, C. Deffernez, T. Rochat, J.
Garbino, W. Wunderli, P.
Meylan, S. Yerly, I,. Perrin, I. Letovanec, L. Nicod, C. Tapparel, and P. M.
Soccal. 2006.
Chronic rhinoviral infection in lung transplant recipients. Am. J. Re6pir.
Crii. Care. Ivied.
174:1392-1399.
24. Mimmerer, U., B. Kunkel, and K. Korn. 1994. Nested PCR for specific
detection and
rapid identification of human picornaviruses. J. Cl/n. Microbiol. 32:285-291.
25. Kares, S., M. Lonnrot, P. Vuorincn, S. Oikarinen, S. Taurianen, and H.
Hyoty. 2004,
Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using
LightCycler.
J. Gin. Virol. 29:99-104.
36
CA 02645883 2008-12-05
26. Khetsuriani, N., N. N. Kazerouni, D. D. Erdman, X. Lu, S. C. Redd, L.
J. Anderson, and
W. G. Teague. 2007. Prevalence of viral respiratory tract infections in
children with
asthma. J. Allergy, Clin. Ininninol, 119:314-321.
27. King, A. M. Q., F. Brown, P. Christian, T. Hovi, T. flyypi5, N. J.
Knowles, S. M. Lemon,
P. D. Minor, A. C. Palmcnberg, T. Skern, and G. Stanway. 2000. Picornaviridae,
p. 657-
678../n. M. H. V. van Regenmortel, C. M. Fauquct, D. H. L. Bishop, E. B.
Carstens, M. K.
Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and
R. B.
Wickner (ed.), Virus Taxonomy. Seventh Report of the International Committee
for the
Taxonomy of Viruses. Academic Press, San Diego, CA.
28. Kling, S., H. Donninger, Z. Williams, J. Vermeulen, E. Weinberg, K.
Latiff, R. Ghildyal,
and P. Bardin. 2005. Persistence of rhinovirus RNA after asthma exacerbation
in children.
Clin. Exp. Allergy 35:672-678.
29. Lamson, D., N. Renwick ,V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean,
K. St George, T.
Briese, and W.I. Lipkin. 2006. MassTag polymerasc-chain-reaction detection of
respiratory pathogens, including a new rhinovirus genotype that caused
influenza-like
illness in New York State during 2004-2005..J. Infect. Dis. 194:1398-1402.
30. Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz,
M. S. Collett,
and D. C. Pevear, 2004. VP1 sequencing of all human rhinovirus serotypes:
insights into
genus phylogeny and susceptibility to antiviral capsid-binding compounds. J.
Virol.
78:3663-3674.
31. Loens, K., H. Goossens, C. de Laat , U. Foolen, P. Oudshoorn, S.
Pattyn, P. Sillekens, and
M. leven. 2006. Detection of rhinoviruses by tissue culture and two
independent
amplification techniques, nucleic acid sequence-based amplification and
reverse
transcription-PCR, in children with acute respiratory infections during a
winter season-1
Clin. Microbiol. 44:166-171.
32. Lu, X., M. Chittaganpitch, S. J. Olsen, I. M. Mackay, T. P. Sloots, A.
M. Fry, and D. D.
Erdman. 2006. Real-time PCR assays for detection of bocavirus in human
specimens../.
Clin. Microbiol. 44:3231-3235.
37
CA 02645883 2008-12-05
33, McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P.
Sloots, and 1. M.
Mackay. 2007. Characterisation of a newly identified human rhinovirus, FIRV-
QPM,
discovered in infants with bronchiolitis. J. Cl/n. Virol. 39:67-75.
34. Miller, E. K., X. Lu, D. D. Erdman, K. A. Pochling, Y. Zhu, M.. R.
Griffin, T. V. Hartert,
L. J. Anderson, G. A. Weinberg, C. B. Hall, M. K. lwane, K. M. Edwards, and
New
Vaccine Surveillance Network, 2007. Rhinovirus-associated hospitalizations in
young
children. J. Dis. 195:773-781.
35. Monto, A. S., A. M. Fendrick, and M. W. Sarnes. 2001. Respiratory
illness caused by
picornavirus infection: a review of clinical outcomes. Clin. Ther. 23:1615-
1627.
36. Nicholson, K. G., J. Kent, V. Hammersley, and E. Cancio. 1996. Risk
factors for lower
respiratory complications of rhinovirus infections in elderly people living in
the
community: prospective cohort study. Br. Med. 1 313:1119-1123.
37. Nielsen, C. B., S. K. Singh, .1. Wengel, and J. P. Jacobsen. 1999. The
solution structure of
a locked nucleic acid (LNA) hybridized to DNA../. Bio,noi. Shwa Dyn. 17:175-
191.
38. Nijhuis, M., N. van Maarseveen, R. Schuurman, S. Verkuijlen, M. de Vos,
K.
Hendriksen, and A. M. van Loon. 2002. Rapid and sensitive routine detection of
all
members of the genus enterovirus in different clinical specimens by real-time
PCR. .1.
Cl/n. Microblol. 40:3666-3670.
39. Nokso-Koivisto, .1., T. J. Kinnari, P. Lindahl, T. Hovi, and A.
Pitkiiranta. 2002. Human
picornavirus and coronavirus RNA in nasopharynx of children without concurrent
respiratory symptoms. 1 Med VirOl. 66:417-420.
40: Pallansch, M. A., and R. P. Roos. 2001. Enteroviruses: polioviruses,
coxsackieviruses,
echoviruses, and newer enterovirues. p. 723-775. In D. M. Knipe, P.M. Howley,
D. E.
Griffin, R. A. Lamb, M. A. Martin, 13. Roizman, and S. E. Straus (ed.), Fields
Virology.
Philadelphia, PA.
41. Papadopoulos, N. G., J. Hunter, G. Sanderson, J. Meyer, and S. L.
Johnston. 1999.
R.hinovirus identification by Bgll digestion of picornavirus RT-PCR amplicons.
Virol.
Methods. 80:179-85.
38
CA 02645883 2008-12-05
42. Pitkaranta, A., and F. G. Hayden. 1998. Rhinoviruses:, important
respiratory pathogens.
Ann. Med. 30:529-537.
43. Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi 2002. Genetic
clustering of all
102 human rhinovirus prototype strains: serotype 87 is close to human
enterovirus 70.1
Gen. Virol. 83:333-340.
44. Scheltinga, S. A., K. E. Templeton, M. F. Beersma, and E. C. Claas.
2005. Diagnosis of
human metapneumovirus and rhinovirus in patients with respiratory tract
infections by an
internally controlled multiplex real-time RNA PCR..1. Clin. Prof. 33:306-311.
45. Sehrag, S. J., J. T. Brooks, C. Van Beneden, U. D. Parashar, P. M.
Griffin, L. J.
Anderson, W..1. Bellini, R. F. Benson, D. D. Erdman, A. Klimov, T. O. Ksiazek,
T. C.
Peret, D. F. Talkington, W. L. Thacker, M. L. Tondella, J. S. Sampson, A. W.
Hightower,
D. F. Nordenberg, B. D. Plikaytis, A. S. Khan, N. E. Rosenstein, T. A.
Treadwell, C. G.
Whitney, A. E. Fiore, T. M. Durant, J. F. Perz, A. Wasley, D. Feikin, J. L.
Herndon, W.
A. Bower, B. W. Kliboum, D. A. Levy, V. (3. Coronado, J. Buffington, C. A.
Dykewicz,
R. F. Khabbaz, and M. E. Chamberland. 2004. SARS surveillance during emergency
= public health response, United States, March-July 2003. Ernerg. Infect.
Dis. 10:185-194.
46. Smyth, A. R., R. L. Smyth, C. Y. Tong, C. A. Hart, and D. P. Heaf.
1995. Effect of
respiratory virus infections including rhinovirus on clinical status in cystic
fibrosis. Arch.
Div. Child. 73: I 17-120.
47. Verstrepen, W. A., S. Kuhn, M. M. Kockx, M. E. Van De Vyvere, and A. H.
Mertens.
2001. Rapid detection of enterovirus RNA in cerebrospinal fluid specimens with
a novel
single-tube real-time reverse transcription-PCR assay. .1 Cl/n. It/ficrobiol.
39:4093-4096.
48. Wald, T., P. Shutt, P. Krause, B. Miller, P. Drinka, and S.
Gravenstein. 1995. A
rhinovirus outbreak among residents of a long-term care facility. Ann. Intern.
Med.
123:588-593.
49. Wittwer, C. T., M. G. Hermann, A. A. Moss, and R. P. Rasmussen. 1997.
Continuous
fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 22:130-
131,
134-138.
39
CA 02645883 2008-12-05
50. Witwer, C., S. Rauscher, 1. L. Hofacker, and P. F. Stadler. 2001.
Conserved RNA
secondary structures in Picornaviridae gcnomes. Nucleic Acids Res. 29:5079-
5089.
51. Wright, P. F., A. M. Dcatly, R. A. Karron, R. B. Belshe, J. R. Shi, W. C.
Gruber, Y. Zhu,
and V. B. Randolph. 2007. Comparison of results of detection of rhinovirus by
PCR and
viral culture in human nasal wash specimens from subjects with and without
clinical
symptoms of respiratory illness. J. Oin. Microbial. 45:2126-2129.
52. Allander, T., M. Tammi, M. Eriksson, A. Bjerkner, .A. Tiveljung-
Lindell, and B.
Andersson. 2005. Cloning of a human parvovirus by molecular screening of
respiratory
tract samples. ]WAS
102:12891-
12896.http://jcm.asm.org/cgi/iilink?linkType¨ABST&journalCode=pnas&resid-
102/36/1
2891
53. Bastien, N., K. Brandt, K. Dust, D. Ward, and Y. Li. 2006. Human
bocavirus infection,
Canada. Emerg. Infect. Dis. 12:848-850.
54. Bustin, S. A., and T. Nolan. 2004. A-Z of quantitative PCR.
International University
Line, La Jolla, Calif
= 55. Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson,
D. G. Higgins, and j. D.
Thompson. 2003. Multiple sequence alignment with the Clustal series of
programs.
Nucleic Acids Res. 31:3497-3500.
56. Emery, S. L., D. D.. Erdman, M. I). Bowen, B. R. Newton, J. M.
Winchell, R. F. Meyer,
S. Tong, B. T. Cook, B. P. Holloway, K. A. McCaustland, P. A. Rota, B.
Bankamp, L. E.
Lowe, T. G. Ksiazek, W. J. Bellini, and L. J. Anderson, 2004. Real-time
reverse
transcription-polymerase chain reaction assay for SARS-associated coronavirus.
Emerg.
Infect. Div. 10:311-316.
57. Foulongne, V., M. Rodiere, and M. Segondy. 2006. Human bocavirus in
children. Emerg.
Infect. Dir. 12:862-863.
58. Ma, X., R. Endo, N. 1shiguro, T. Ebihara, H. Ishiko, T. Ariga, and II.
Kikuta, 2006.
Detection of human bocavirus in Japanese children with lower respiratory tract
infections.
J. Clin. Microbial. 44:1132-1134.
CA 02645883 2008-12-05
59. Sloots, T. P., P. MeErlean, D. J. Speicher, K. E. Arden, M. D. Nissen,
and I, M. Mackay.
2006. Evidence of human coronavirus HMI I and human boeavirus in Australian
children. I Clin. Virot 35:99-102.
=
60. Tattersall, P. 2005. Family Parvoviridae, p. 353-369. In C. M. Fauquet,
M. A. Mayo, J.
Maniloff, U. Desselberger, and I,. A. Ball (ed.), Virus taxonomy:
classification and
nomenclature of viruses. Eighth report of the International Committee on the
Taxonomy
of Viruses. Elsevier Academic Press, London, United Kingdom.
61. Xiaoyan Lu, Malinee Chittaganpitch, Sonja J. Olsen, Ian M. Mackay, Theo
P. Sloots,
Alicia M. Fry, and Dean D. Erdman. 2006. Real-Time PCR Assays for Detection of
Bocavirus in Human Specimens-1 Gun. MicrobioL, Vol. 44, p. 3231-3235.
62. Lawrence 13, Blyn, Thomas A. Hall, Brian Libby, Raymond Ranken,
Rangarajan
Sampath, Karl Rudnick, Emily Moradi, Anjali Desai, David Metzgar, Kevin L.
Russell,
Nikki E. Freed, Melinda Balansay, Michael P. Broderick, Miguel A. Osuna,
Steven A.
Hofitadler, and David J. Ecker 2008. Rapid Detection and Molecular Serotyping
of
Adenovirus by Use of PCR Followed by Electrospray Ionization Mass Spectrometry
MicrobioL 46: 644-651.
63. Jiang Y, Hofstadler SA. 2003.A highly efficient and automated method of
purifying and
desalting PCR. products for analysis by electrospray ionization mass
spectrometry. Anal.
Biochem. 316: 50-57.
64. Karlin and Altschul, 1990, PNAS 87:2264 2268.
65. Karlin and Altschul, 1993, PNAS 90:5873 5877.
66. Altschul et al., 1990,1. MoL Biol, 215:403.
67. Altschul et al., 1997, Nucleic Acids Res. 25:3389 3402.
68. Myers and Miller, 1988, CAB./OS 4:11 17.
69. Holland et al., PNAS 88(16):7276 (1991).
70. Saiki et al., 13ionchnology 3:1008 1012 (1985).
71. Conner et al., PNAS 80: 278 (1983).
41
CA 02645883 2008-12-05
72. Landegren et al., Science 241:1077 (1988).
73. Ortiz, A and Ritter, E, Nucleic Acids Res., 1996; 24:3280-3281,
74. Lishanski, A, et al., Nucleic Acids Res., 2000; 28(9):e42.
75. Lee, K., Biotechnology Letters, 2003; 25:1739-1742.
76. Johnson, Annual Reviews of. Biochemistry, 1993: 62:685-713.
77. Kunkel, Journal of Biological Chemistry, 1992; 267:18251-18254.
=
42