Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
. = CA 02695829 2010-02-08
Method for reducing bitumen odors
The invention relates to a method for reducing bitumen odors by admixing
active
agents.
Bitumen is a highly-viscous liquid or a solid that consists of hydrocarbons
and
derivatives thereof. It is soluble in trichloroethylene and softens at
temperatures
above 100 C and becomes thin fluid above approximately 150 C. Bitumen is used
on an industrial scale, e.g., as a road topping, whereby a mixture of bitumen
and
major amounts of stones is applied; moreover, it is used as insulating layer
against
the penetration of water and moisture into buildings, and for the production
of
sealing sheeting and tar paper.
In all said applications, bitumen is processed in liquefied form above
approximately 140 C. At these temperatures, most of the volatile substances
present in bitumen evaporate, e.g. sulfur and nitrogen compounds that may lead
to
foul odors.
US-A 5,271,767 describes an odor-free asphalt composition, in which 0.5 to
1 wt.-% of a mixture of
to 15 wt.-% limonene,
85 to 90 wt.-% of a vegetable oil, and
approximately 2 wt.-% of a silicone oil
are admixed into liquid asphalt (meaning bitumen).
In this context, relatively large amounts of active agents have to be used.
EP-A 1 235 768 describes a composition for odor suppression for hydrocarbons,
e.g. asphalt and bitumen, consisting of an aldehyde or ketone and 10 to 90 wt.-
%
of a carbonic acid alkyl ester. The composition in the example contains a
total of
31 wt.-% ester and 60 wt.-% benzaldehyde. Since benzaldehyde is detrimental to
CA 02695829 2010-02-08
_
2
health (see material safety data sheet of Merck of 31 March 2004), this
composition is not harmless.
WO 2004/099352 describes additives for odor reduction, e.g. for liquid asphalt
cement (meaning bitumen), preferably containing
35 to 45 vol.- /0 limonene,
25 to 35 yol.-% pine extract or pine oil,
1 to 5 vol.-% Pinus sylvestris oil,
3 to 8 vol.-% anise seed oil, and
20 to 25 vol.- /0 of other oils.
Said composition can be diluted with a carrier oil, e.g. a mineral oil, a
vegetable oil
or a fatty acid alkyl ester. However, it showed that said composition is not
optimal
for reducing bitumen odors, too.
EP-A 1 772 158 describes a method for reducing foul-smelling substances in
tank
containers for bitumen, whereby active agents are guided into the gas space
over
the liquid level together with an inert gas. The active agents can be, e.g.,
longer-
chain aldehydes, ketones, esters or alcohols, e.g. C7 to 012 aldehydes or
natural
oily essences such as resin oils, and, moreover, perfume-like substances such
as
vanillin, menthol or alpha-pinene. More detailed specifications concerning the
composition of the active agents are not provided therein.
It was therefore the object of the invention to provide a method for reducing
bitumen odors, in which relatively small amounts of active agents that are non-
objectionable in terms of health are used.
This object is met by the mixture of active agents according to claim 1. It is
self-
evident that said agents as such should not be foul-smelling, i.e. the
selected
substances should not bear any, e.g., mercaptan or amino groups.
CA 02695829 2010-02-08
3
According to the present invention, the mixture of active agents is not
admixed into
the gas space, but rather directly into the liquid bitumen which results in
higher
efficiency.
In the context of the present invention common commercial bitumen is used.
Said
bitumen might contain residues from crude oil distillation as well as residues
that
were obtained by means of a cracking process. Naturally occurring bitumen or
mixtures of various types of bitumen may be used as well. Examples of bitumen
of
this type include straight-run asphaltic bitumen, precipitation bitumen, e. g.
propane bitumen, oxidized bitumen, naphthene-base bitumen and mixtures
thereof. Well-suited bitumen can also be produced by mixing with a naphthene-
base or paraffin base flux oil or a vegetable oil. It can just as well contain
a
polymer such that a polymer-modified bitumen is generated. Well-suited
polymers
include thermoplastic elastomers or plastomers, such as, e.g. styrene block
copolymers and/or olefin copolymers such as ethylene/vinylacetate copolymer.
The aldehyde of component A is selected from
Al. alpha-hexyl cinnamaldehyde (jasmonal H),
A2. 2-(4-tert.-butylbenzyl)propionaldehyde (Lilial), and
A3. 2-benzylidene heptanal (jasminal)
or mixtures thereof. In a particularly preferred embodiment, component A
contains
all three of the aldehydes specified above.
The other optional aldehydes of component B are preferably selected from:
B1. 3-(4-iso-propylphenyI)-2-methylpropanal (cyclamaldehyde), particularly in
amounts of from 2 to 12 wt.-%, and
B2. 2-methylundecanal, particularly in amounts of from 2 to 8 wt.-%
B3. 2-methyl-3-(3,4-methylene-dioxyphenyl)propanal (helional), particularly in
amounts of from 0.5 to 5 wt.-%
= CA 02695829 2010-02-08
4
or mixtures thereof. In a particularly preferred embodiment, component B
contains
all three of the aldehydes specified above. Moreover, the aldehydes listed in
EP-A
1 235 768 are also well-suited. The amounts of benzaldehyde and citral that
are
present should preferably be at most 5 wt.-%.
Preferably, naturally occurring alcohols are used as component C. These are
preferably selected from
Cl. linalol having a boiling point of 198 C, particularly in amounts of from
5
to 25 wt.-%,
C2. eugenol having a boiling point of 253 C, particularly in amounts of from
3 to 12 wt.-%,
03. geraniol having a boiling point of 230 C, particularly in amounts of from
3 to 12 wt.-%, and
C4. alpha-terpineol having a boiling point of 219 C, particularly in amounts
of
from 0.5 to 5 wt.- /0
or mixtures thereof. In a particularly preferred embodiment, component C
contains
all four of the alcohols specified above. Other alcohols, such as vanillin and
thymol, are also well-suited.
The optional terpenes of component D are preferably selected from
Dl. limonene having a boiling point of 176 C, particularly in amounts of from
0.5 to 5 wt.-%, and
D2. alpha-pinene having a boiling point of 156 C, particularly in amounts of
from 0,5 to 5 wt.-%
or mixtures thereof. In a particularly preferred embodiment, component D
contains
both of the terpenes specified above. Terpenes that are present in natural
essential oils as well as the terpenes listed in WO-A 2004/099 352 are also
well-
suited.
CA 02695829 2014-11-18
Well-suited as ketones in optional component E are e.g.: benzophenone, butyl-
methyl-ketone, and 1,8-cineol, as well as other ketones that are present in
natural
essential oils. The ketones listed in WO-A 2004/099 352 are also well-suited.
Well-suited as carbonic acid esters of optional component F are e.g.:
benzylsalicylate, amylbutyrate, ethylbutyrate, and amylacetate as well as
other
carbonic acid esters listed in EP-A 1 235 768.
Preferably a mixture of the active agents and a diluting agent is admixed to
the
liquid bitumen. Said diluting agent simultaneously acts as mixing mediator
during
the mixing of the individual components.
The mixture preferably contains 5 to 50, in particular 10 to 40, wt.-% active
agents
and 95 to 50, in particular 90 to 60, wt.-% diluting agent.
Preferred diluting agents include mineral oils, i.e. hydrocarbon middle
distillates,
such as, e.g. CATENEXTm made by Shell, as well as vegetable oils such as
sunflower oil which consists mainly of linoleic acid. A mixture of CATENEXTm
and
sunflower oil that is characterized by a particularly high flash point, which
reduces
the risk of ignition, has also proven to be useful.
Preferably the mixture of active agents and diluting agent is admixed to the
liquid
bitumen in amounts of from 10 to 200 ppm, in particular from 20 to 180 ppm,
and
especially in amounts of from 50 to 150 ppm. This is preferably effected at
temperatures above 140 C, in particular at temperatures between 150 and 250 C.
Generally, the mixture can be introduced directly into the liquid bitumen that
is
present in a tank container or a truck. However, since liquid bitumen is
usually
delivered by truck and filled into the tank container through a hose or
preferably a
pipe, it is advantageous to introduce the mixture from a reservoir container
via a
CA 02695829 2010-02-08
6
dosing pump through a side line into the pipe. In this context, the pipe can
be split
into two channels at one site in the form of a bypass whereby the main amount
of
the liquid bitumen flows into the channel with the larger diameter, whereas
the
mixture of active agents and diluting agent is introduced into the narrower
bypass
channel. This simplifies the dosing and the mixture is better homogenized.
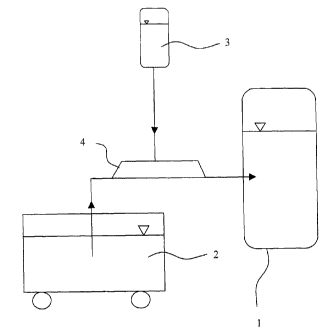
This
dosing is shown schematically in the drawing. While 1 denotes the bitumen
tank, 2
is the truck from which the bitumen is transferred to the tank, 3 is the
reservoir
container for the mixture containing the active agents, and 4 is the bypass.
It is assumed that the active agents specified above, in particular the
aldehydes,
undergo a chemical reaction with the foul-smelling substances, e.g. with
mercaptans and amines, and thus remove and/or neutralize them.
Using the method according to the invention, the foul smell during the
processing
of liquid bitumen can be reduced by 60 to 90%, in some cases even by more than
90%, which can be detected by means of olfactory measurement.
Example 1
A mixture of active agents is produced from:
22.9 parts by weight alpha-hexylcinnamaldehyde
19.1 parts by weight 2-benzylidene heptanal
9.8 parts by weight 2-(4-tert.-butylbenzyl)propionaldehyde
16.5 parts by weight linalol
7.7 parts by weight eugenol
7.7 parts by weight geraniol
7.7 parts by weight 3-(4-iso-propylpheny1)-2-methylpropanal
3.1 parts by weight 2-methylundecanal
1.9 parts by weight alpha-terpineol
1.2 parts by weight limonene
1.2 parts by weight 2-methy1-3-(3,4-methylene-dioxypheny1)-propanal
=.
CA 02695829 2010-02-08
7
1.2 parts by weight 2-methyl-3-(3,4-methylene-dioxypheny1)-propanal
1.2 parts by weight alpha-pinene.
30 parts by weight of this mixture were mixed with 70 parts by weight CATENEX
as diluting agent. The flash point (open cup) of this mixture was 165 C.
A total of 3.75 kg (150 ppm) of the mixture specified above were introduced
into a
truck containing 25 to liquid bitumen at a temperature of 170 C.
Example 2
30 parts by weight of the mixture from Example 1 were mixed with 70 parts by
weight of a mixture of CATENEX and sunflower oil at a ratio of 85 :15 as
diluting
agent. The flash point (open cup) of this mixture was 168 C.
Liquid bitumen was filled from a truck through a pipe into a tank with a
capacity of
25 to. Said pipe was divided into two channels with internal diameters of 200
mm
and 20 mm, respectively. A total of 2.5 kg (100 ppm) of the mixture specified
above were introduced into the narrower channel of the pipe.