Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02710489 2010-07-20
PN0965
Processing Crude Iodixanol Mixture By Nanofiltration
CROSS REFERENCE TO RELATED APPLICATIONS
The present application claims benefit of priority under 35 U.S.C. 119(e) to
United
States Provisional Application number 61/227,101 filed July 21, 2009, the
entire disclosure of
which is hereby incorporated by reference.
TECHNICAL FIELD
This invention relates generally to industrial preparation of iodixanol (1,3-
bis(acetamido)-N,N'-bis [3,5-bis(2,3-dihydroxypropylaminocarbonyl)-2,4,6-
triiodophenyl] -2-
hydroxypropane), a non-ionic X-ray contrasting agent. It further relates to an
improved
method in the purification of iodixanol. In particular, it relates to reducing
the salt content
and the alcoholic solvent content using a nanofiltration system prior to the
crystallization of
iodixanol.
BACKGROUND OF THE INVENTION
lodixanol is the non-proprietary name of the chemical drug substance, 1,3-
bis(acetamido)-N,N'-bis[3,5-bis(2,3-dihydroxypropylaminocarbonyl)-2,4,6-
triiodophenyl]-2-
hydroxypropane). It is a one of the most used agents in diagnostic X-ray
procedures and
marketed under the trade name Visipaque . It is produced in large quantities
by GE
Healthcare in Lindesnes, Norway. The manufacture of iodixanol involves the
production of
the chemical drug substance (referred to as primary production) followed by
formulation into
the drug product (referred to as secondary production). Primary production of
iodixanol
involves a multistep chemical synthesis and a thorough purification process.
For a
commercial drug product, it is important for the primary production to be
efficient and
economical and to provide a drug substance fulfilling the regulatory
specifications, such as
those mandated by US Pharmacopeia. In addition, the cost and efficiency of the
secondary
production depend on the synthesis and purification processes in the primary
production. It is
therefore critical to optimize each process in the primary production of
iodixanol.
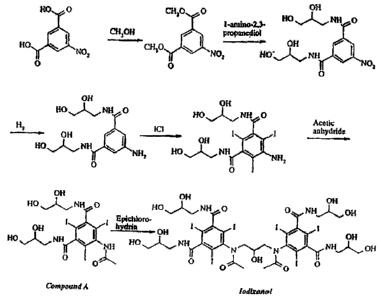
The industrial synthesis of iodixanol involves dimerisation of intermediate 5-
acetamido-N,N'-bis(2,3-d ihydroxypropyl)-2,4,6-triiodoisophthalamide
("Compound A") as
the final synthetic step. See Scheme I below and U.S. Patent No. 6,974,882.
1
CA 02710489 2010-07-20
PN0965
HO o CHjo 0 obi
Ho ,)"M 0
H{) on
W, NO=
0 0
n
on 014
00 rõ NH 0 H0 , , N U 0
y Acetic
off
014
HO N
H, HCi~Nfi
0
OH 014
144", ,NH 0 no",'NH o O NH OH
')''OH
Egid9oro
0i t h p ! i t i
pouMA ludixaomf
Scheme 1
The reaction mixture after the dimerisation of Compound A to iodixanol
contains a
considerable amount of salt, mainly in the form of NaCl. The sources of
chloride are
epichlorohydrin, which is added to the reaction in an amount of about 0.33
mole/mole
Compound A, and hydrochloric acid, which is used to adjust the pH before
epichlorohydrin
addition and to precipitate unreacted Compound A after the reaction. The
source of sodium
cations is the NaOH used to dissolve Compound A in the reaction solvent. More
than one
equivalent of NaOH is required to dissolve Compound A in the anionic form. In
addition, the
dimerisation reaction mixture contains a large amount of alcoholic solvent,
such as 2-
methoxyethanol, methanol, propylene glycol, or 1-methoxy-2-propanol. It is
thus desirable
to devise a cost effective procedure to work up the crude dimerisation
reaction mixture for
subsequent purification steps.
SUMMARY OF THE INVENTION
The present invention is directed to an industrial procedure for processing a
crude
reaction mixture following the dimerisation of Compound A to iodixanol in an
alcoholic
solvent. Such reaction mixture typically contains more than about 12 weight %
of salt
content relative to iodixanol content. The instant process comprises a series
of sequential
2
CA 02710489 2010-07-20
PN0965
steps to prime the crude reaction mixture prior to the crystallization of
iodixanol. These steps
include feeding the dimerisation reaction mixture containing Compound A and
iodixanol in
an alcoholic solvent into a filtration system, passing the filtrate through a
nanofiltration
system wherein water is added to the retentate side; and recovering the
retentate wherein the
salt content is less than about 0.6 weight % to iodixanol content and is
substantially free of
the alcoholic solvent used in the dimerisation reaction. The alcoholic
dimerisation solvent
may include 2-methoxyethanol, methanol, propylene glycol, and I -methoxy-
propanol.
DETAILED DESCRIPTION OF THE INVENTION
In the final step of the primary production process, iodixanol has to be
purified. A
common method for purification is by crystallisation. In order to crystallise
iodixanol in
accordance with the regulatory purity requirement, we have found that the salt
content prior
to the crystallisation process should be less than about 0.6 w/w % relative to
iodixanol.
It has also been found that the presence of alcoholic solvent used in the
dimerisation
has a detrimental effect on the crystallization yield of iodixanol. We have
found that even a
small amount of alcoholic solvent increases the solubility of iodixanol in the
crystallisation
mixture and hence reduces the crystallisation yield considerably.
To achieve the low salt and low alcoholic solvent levels, many attempts have
been to
devise a process to effectively and efficiently reduce large salt content
following the
dimerisation reaction. It has been found that a combination of concentration
and diafiltration
with an aqueous solvent can be successfully used to simultaneously reduce the
salt content
and the alcoholic solvent content to the desired levels in a cost-effective
manner. Only a
minimal amount of iodixanol is lost during the instant process.
The above process addresses the specific need of the current iodixanol
synthesis
methodology, where a large amount of salt is generated as a result of the
dimerisation reagent
and the quenching reagent selected. The instant process also represents a
significant
improvement over the alternative of employing ion exchange resins. For
example, two ion
exchange resins, one anionic and one cationic, are needed to remove the
significant amount
of salts present following the dimerisation reaction. The requirement of two
resins, both in
large quantities, causes significant loss of Compound A and iodixanol. In
addition, the
operation of large scale purifications using ion exchange resins (such as
separation time,
3
CA 02710489 2010-07-20
PN0965
energy consumption, cost of resin regeneration and replacement, and equipment
requirement)
is lengthy, complex, and expensive.
In addition, the instant process is superior to the alternative of using
evaporation to
reduce alcoholic solvent content. For example, distillation or any other form
of evaporation
is both energy consuming and potentially thermally stressing to the iodixanol
product.
The invention is illustrated further by the following examples that are not to
be
construed as limiting the invention in scope to the specific procedures
described in them.
EXAMPLES
EXAMPLE 1
Compound A (600 kg) is reacted with epichlorohydrin (0.33 eq) in an alcoholic
solvent in the presence of sodium hydroxide at a pH of about 11.9 at 15 C.
About 55 %
conversion to iodixanol is obtained. Most of unreacted Compound A is
precipitated from the
reaction mixture by addition of hydrochloric acid followed by filtration. The
aqueous filtrate
contains about 340 kg iodixanol, 100 kg Compound A and 20 kg iohexol. The pH
is
measured to about 4-6. The NaCl content is about 12-14 w/w % relative to
iodixanol. The
solution is then subjected to nanofiltration. Water is added continuously to
facilitate
diafiltration followed by volume reduction. A final salt concentration of
about 0.60 w/w %
relative to iodixanol (2.0 kg NaCl in 340 kg iodixanol) is obtained. The
alcoholic solvent is
removed through the membrane in the diafiltration process, resulting in an
aqueous process
solution ready for the next process step.
All patents, journal articles, publications and other documents discussed
and/or cited
above are hereby incorporated by reference.
4