Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02724413 2015-12-22
COMPOSITIONS AND METHODS RELATING TO HEAT SHOCK
TRANSCRIPTION FACTOR ACTIVATING COMPOUNDS AND TARGETS
THEREOF
FIELD OF THE INVENTION
The present invention relates to Heat Shock Transcription Factor (HSF)
activating
compounds, methods for their discovery, and their research and therapeutic
uses. In
particular, the present invention provides compounds capable of facilitating
HSF I activation,
and methods of using such compounds as therapeutic agents to treat a number of
conditions
associated with diseases and other pathophysiological states caused by or
associated with
defective protein folding.
BACKGROUND OF THE INVENTION
For a long time, protein folding was regarded as simply a theoretical problem.
Researchers investigated the mechanisms of protein folding to close the huge
gap in our
knowledge between the genetic blueprint of a protein and its biological
function. Only in the
1990s did it become clear that wrongly folded proteins are involved in the
development of
many diseases. Protein folding has become a focus of attention in
pharmaceutical research: it
is probable that new approaches to the treatment of diseases such as
Parkinson's disease and
Alzheimer's disease are to be found within its complex pathways.
CA 02724413 2015-12-22
Protein folding diseases can be divided into two groups: in the first,
excessive
quantities of incorrectly folded proteins collect in the form of uncontrolled
piles of molecular
rubbish. This is a group of diseases known as amyloidoses, of which
Alzheimer's disease is a
well-known example. In the other, a small error in the genetic blueprint leads
to incomplete
folding of a protein, which affects its function.
A common characteristic of all amyloidoses is the collection of aggregates or
plaques
of insoluble protein in the extracellular tissue, which cannot be broken down
by enzymes.
Their ordered structure gives them crystal-like properties: they are made up
of long filaments
(fibrils) that are formed from densely packed 13-pleated sheets of identical
proteins. There are
at least 20 different proteins that can act as the building blocks of these
fibrils, each of which
is associated with a different disease. In so-called systemic amyloidoses, the
precursors of
these plaques are transported through the bloodstream from their point of
origin to their point
of deposition. Localized amyloidoses are of greater clinical significance, as
they affect the
central nervous system, which is particularly susceptible to damage, as well
as the heart and
other organs and tissues.
What are needed are improved compositions and methods for treating diseases
associated with improper protein folding, aggregation and/or the clearance of
damaged
proteins.
SUMMARY
The present invention relates to HSF1 activating compounds, methods for their
discovery, and their research and therapeutic uses. In particular, the present
invention
provides compounds capable of facilitating HSF1 activation (e.g.,
homotrimerization), and
methods of using such compounds as therapeutic agents to treat a number of
conditions
associated with protein misfolding.
Experiments conducted during the course of development of embodiments for the
present invention developed a specialized high throughput screen for
identifying small
molecules capable of activating the human Heat Shock Transcription Factor 1
(HSF1) protein
from complex chemical libraries. In addition, experiments conducted during the
course of
developing embodiments for the present invention identified molecules capable
of activating
the human Heat Shock Transcription Factor 1. It was shown that these molecules
activate
2
CA 02724413 2015-12-22
human HSF1 when added to cells, and activate expression of heat shock proteins
(e.g.,
HSP70, HSP25). In addition, it was shown that these molecules reduce
aggregation of poly-
glutamine proteins in neuronal cells.
As such, in certain embodiments, the present invention provides compositions
capable
of HSF activation. The compositions are not limited to a particular type of
HSF. In some
embodiments, the HSF is HSF1, HSF2 or HSF4. The compositions are not limited
by the
manner in which they result in HSF activation. In some embodiments, HSF
activation
includes, but is not limited to, activation of HSF1 homo-trimerization,
activation of HSF
target protein expression (e.g., Heat Shock Proteins including but not limited
to HSP70 and
HSP25), activation of protein chaperone activity (e.g., increased protein
folding, increased
protein solubilization, protein degradation), ancUor reducing protein
aggregation (e.g.,
aggregation of poly-glutamine proteins in neuronal cells).
In certain embodiments, the composition comprises a compound described by the
R1 el
,N
N
R4
Xi
X2t-X3
R9 HN R6
R5
R7 /\ p
following formula: .
including salts, esters, and prodrugs thereof. The compound is not limited to
particular definitions of R1 through R9 groups. In some embodiments, the R1
through R9
groups define a compound capable of HSF activation, identifiable using
screening techniques
CH3 ___________________________________________________________________ /
CH3
1-0CH3 N
described herein. In some embodiments, R1 is 0 , CH,
0
________ N/7
0-, -OH, H, or halogen (e.g., chlorine). In some embodiments, Xi is
3
CA 02724413 2015-12-22
0
VNA
H , or X1 is absent. In some embodiments, X2 is S or C. In
some
embodiments, X3 is S or C. In some embodiments, R9 is ¨OCH3. In some
embodiments, R2
(
0, ___________________________ I) 0 III
'--CF3 I
1S Ri
NH
ill
/N¨CH3 _______________________
( ____________________________________ ) ____ ( ___ )
H3C
_____________________________ -NH CI
NH
it
0 , 41 , or ¨CF3. In some embodiments,
R3V N
N N , NN
is
N
/ %
R4 or C . In some embodi
,-.
is IN
ments, R5 is S or C. In
some embodiments, R6 is H or =0 . In some embodiments, R7 is H or =0 . In
4
CA 02724413 2015-12-22
\o
411
some embodiments, R8 is or
Ri
. In some embodiments, R10 is chlorine or CH3. In some
embodiments, R11 is substituted or unsubstituted alkyl such as, for example,
or
N
CA 02724413 2015-12-22
1411I ,N S
N N
)-
HNI
0= =0
14111
In some embodiments, the compound is
CI
4111, c
NN
NH
0
=
6
CA 02724413 2015-12-22
o, 7N
H,Cõo
*
NH
0 ____________ cs.
0 = 1-1,3C\
FIX
)FN 0 CH3
H3C-N 0
CH3
S N
11111. N
TH,
4101
CH
3
H,C
S N
7
CA 02724413 2015-12-22
S
F N
)i. 0 N
11+
41 I.
F 0 N
Alb
`0
WI
H,c¨N
.. \
S N CH,
CH
1 3
0 grik 0..,_
SIIW -CH3
S-1< Vir 1
N 0 N
N S N
0
11 0
0 *
CH3
i
IMP CH3 0 OH
N N
S.õ,...(/
N-----N= ss
N
F.-7----=-7
, or
In certain embodiments, the composition comprises a compound described by the
compounds shown in Figure 7.
8
CA 02724413 2015-12-22
In certain embodiments, the composition comprises a functional derivative of a
411 NNN 410
HN
0=-S=0
compound capable of HSF activation (e.g., as a functional
derivative of HSF 1A).
In certain embodiments, the composition comprises a compound described by the
R1 40
D R2
rµti
HN\ R6
0/
following formula: R7 R8 ; including salts, esters, and
prodrugs
thereof. The compound is not limited to a particular definitions of R1 through
R8 groups. In
some embodiments, the R1 through R8 groups define a compound capable of HSF
activation,
identifiable using screening techniques described herein. In some embodiments,
R1 is
1-0CH3
, H, or halogen (e.g., chlorine). In some embodiments, R2 is
/
N,
R
N9 R37 NNR4 is C
m
, or . In some embodiments, or
9
CA 02724413 2015-12-22
N,
C. In some embodiments, R5 is S or C. In some embodiments, R6 is H or
. In some embodiments, R7 is H or =0 . In some embodiments, R8 is
o
or ro\Rio
. In some embodiments, R9 is chlorine or CH3. In
some embodiments, R10 is substituted or unsubstituted alkyl such as, for
example,
IN ________________
, or . In certain embodiments, the compound is
S s
411, oi
I NV NX
NN
HNI NH
0=3=0
0
1.1
oI
XN *
NH
c
or
CA 02724413 2015-12-22
In certain embodiments, the present invention provides methods for treating a
condition associated with protein misfolding. The present invention is not
limited to a
particular method for treating a condition associated with abnormal protein
folding. In some
embodiments, the methods comprise administering to a subject (e.g., human
being, cat, dog,
mouse, rat, ape, monkey) having misfolded proteins a composition capable of
facilitating
HSF (e.g., HSF1) activation. The methods are not limited to treating a
particular condition
associated with protein misfolding. In some embodiments, conditions associated
with
irregular HSF (e.g., HSF1) activity include, but are not limited to,
Alzheimer's disease,
Parkinson's disease, Huntington disease, Amyotrophic Lateral Sclerosis, a
prion-based
disease, cataract, age-related cataract, glaucoma, macular degeneration, age-
related macular
degeneration, retinitis pigmentosa, cardiovascular disease and stroke, heat
stroke,
spinocerebellar ataxia, Machado Joseph disease, stress-related neuronal
degeneration, aging,
cancer, and type 2 diabetes mellitus. In some embodiments, the methods are
directed towards
crystallins in age-related cataracts. In some embodiments, the methods are
directed towards
myocillin in glaucoma. In some embodiments, the composition is co-administered
with one
or more therapeutic agents (e.g., anticonvulsant agents, antipsychotic agents,
rauwolfia
alkaloids, antidepressants, dopamine prodrugs, dopamine agonists, catechol-0-
methyltransferase (COMT) inhibitors, anticholinergics, MAO-B inhibitors, N-
methyl-D-
aspartic acid inhibitors, AChE inhibitors, NMDA antagonists, free-radical
scavengers,
glutamate pathway antagonists, antispastic agents, Congo red and its analogs,
anthracyclines,
amphotericin B and its analogs, sulfated polyanions, tetrapyrroles,
sulfonylurea agents,
meglitinides, biguanides, thiazolidinediones, dipeptidyl peptidase IV (DPP-4)
inhibitors,
incretin mimetics, amylin analogs, and alpha-glucosidase inhibitors,
Levobunolol (Betagan),
Timolol maleate/hemihydrate (Timoptic Timoptic XE, Betimol, Istalol),
Carteolol (Cartrol,
Ocupress), Betaxolol (Betoptic-S), Metipranolol hydrochloride (OptiPranolol),
Levobetaxolol
(Betaxon), Brimonidine (Alphagan-P), Apraclonidine (Iopidine), Dipivefrin
(AKPro,
Propine), Epinephrine (Epifrin), Memantine (Namenda, Axura), Dorzolamide
HC1(Trusopt),
Brinzolamide (Azopt), Acetazolamide (Diamox), Methazolamide (Neptazane),
Dorzolamide
HC1/timolol maleate (Cosopt), Latanoprost (Xalatan), Bimatoprost (Lumigan),
Travoprost
ophthamic solution (Travatan), Unoprostone (Rescula), Pilocarpine (Pilocar,
Pilagan, Pilogel,
Ocusert), Isosorbide (Ismotic), Mannitol (Osmitrol, Resectisol), Glycerin
(Ophthalgan,
11
CA 02724413 2015-12-22
Osmoglyn), Brimonidine/timolol (Combigan), Phenylephrine HCI (Neo-Synephrine),
Prednisolone acetate (AK-Pred, Pred Forte), Dexamethasone (Ocu-Dex),
Ciprofloxacin (Ciloxan), Erythromycin (E-Mycin), Nepafenac ophthalmic
(Nevanac),
Verteporfin (Visudyne), Pegaptanib (Macugen), Ranibizumab (Lucentis), Vitamin
A
(Aquasol A, Del-Vi-A), Vitamin E (Aquasol E, Vitec), Ascorbic acid (Cebid,
Ascorbicap,
Cevalin, Cecon), Lutein or Zeaxanthin, Bilberry, Beta-carotene, Diltiazem
(Cardizem,
Dilacor, Tiamate), Acetazolamide (Diamox, Diamox Sequels), and Methazolamide
(Neptazane)).
In certain embodiments, the present invention provides methods for identifying
HSF1
activating agents. The present invention is not limited to a particular method
for identifying
HSF1 activating agents. In some embodiments, the method comprises a) providing
a yeast
yhsfd strain expressing human HSF1, wherein the yhsfd strain comprises a yeast
HSF gene
coupled with an inducible promoter (e.g., GAL promoter); b) growing the yhsfd
strain on a
medium having the inducer (e.g., galactose); c) exposing the yhsfzI strain to
a candidate
compound; d) switching the yhsfd strain to a repressive growth medium; e)
assessing the
growth of the yhsf21 strain; and 0 characterizing the candidate compound as a
HSF1
activating agent if the yh.5121 strain grows on the non-inducer medium. In
some embodiments,
the human HSF is expressed via a pRS424-GPD-hHSF1 plasmid. In some
embodiments, the
repressive medium is a glucose medium.
BRIEF DESCRIPTION OF THE DRAWINGS
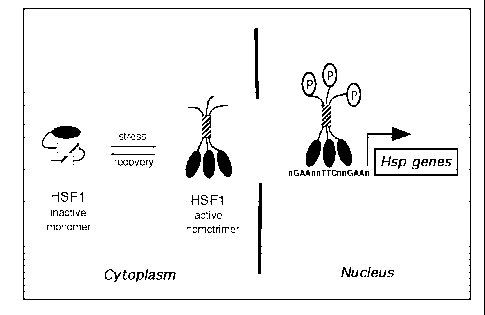
Figure 1 shows a model for the activation of human HSF1. HSF1 is shown as an
inactive monomer in the cytoplasm in the absence of stress. HSF1 is localized
to the
cytoplasm as an inactive monomer. Upon stress-dependent stimulation, HSF1
homotrimerizes, localizes to the nucleus, binds to DNA Heat Shock Elements
(HSEs)
becomes hyperphosphorylated, and activates gene transcription. Black oval: the
HSF1 DNA
binding domain; hatched rectangles: LZ1-3 (large), LZ4 (small), which form
intermolecular
coiled-coils in the homo-trimer; P: phosphorylation. HSF11z4m harbors a point
mutation in
LZ4 that renders HSF1 constitutively homo-trimerized, perhaps by breaking
intramolecular
coiled-coil interactions.
12
CA 02724413 2015-12-22
Figure 2 shows yeast-based screen for small molecule activators of human HSF1
(hHSF I). Yeast cells expressing the essential yeast Heat Shock Transcription
Factor (yHSF)
under control of the repressible GAL1 promoter are dependent on galactose for
growth.
Upon shifting the cells to glucose containing growth media, where the GAL1
promoter is
shut off, the cells become dependent on activation of hHSF1 for growth. Small
molecules
able to activate hHSF1 allow growth on glucose.
Figure 3 shows microtiter plate analysis of human HSF1 activation. Yeast hsfA
cells
harboring the GAL/-yeast HSF (yHSF) plasmid. On galactose (gal) all cells were
viable due
to galactose-inducible expression of yHSF. On glucose (glu) expression of yHSF
was
extinguished and cells were inviable when they express wild type human HSF1 or
the empty
vector. However, yhsfz1 cells expressing a constitutively trimerized human
HSF1 protein
(HSF11z4m) were viable in the absence of yeast HSF. All wells within a given
row of the
microtiter plate section contained the same yeast strain to show consistency.
Plasmids
transformed into the strain and carbon sources were indicated.
Figures 4 show growth of the yhsfA: human HSF1 yeast strain in the presence of
library compounds (Figure 4A) and in the presence of HSF1A derivatives (Figure
4B). Yeast
cells expressing hHSF1 were seeded into 96-well plates at a concentration of
¨1,000
cells/well in glucose and treated with 10 micromolar of various compounds from
a chemical
library or the DMSO solvent control. Growth was monitored by determining the
optical
density (0.D.600) for 96 hours.Note that compounds in the 1391 and 1393 series
are
structurally related but distinct from the 1261 series.
Figure 5 shows structures of three independent compounds positive in the yeast
screen
for human HSF1 activator molecules, designated HSF1-A, HSF1-B and HSF1-C.
Yeast cell
growth in the presence of these molecules at 10 uM is shown in Figure 4.
Figure 6 shows synthetic routes for HSF I A, HSF1B and HSF1C. The structural
relatedness of the three lead compounds simplified synthesis as a two-step
reaction.Figure 7
shows HSF1 activating compounds.
Figure 7 shows compounds identified as HSF1 activating compounds.
Figure 8 shows HSF1 activating compounds identified thorugh screens conducted
with compounds from the LOPAC and Prestwick chemical libraries
13
CA 02724413 2015-12-22
Figure 9 shows that HSF1A activates the expression of the HSP70 and HSP25 heat
shock protein (chaperone) genes in Mouse Embryonic Fibroblast (MEF) cells.
Figure 10 shows HSF1A dependent activation of HSP70 is dependent on the
presence
of the gene encoding HSF1.
Figure 11 shows HSF1A acts synergistically with heat shock to activate
expression of
the HSP70 protein chaperone.Figure 12A and 12B show that HSF1A promotes
expression of
HSP70 and reduces the aggregation of poly-glutamine (polyQ) proteins in rat
neuronal
precursor (PC-12) cells.
Figure 12 shows that HSF1A promotes expression of HSP70 and reduces
aggregation
of poly-glutamine (polyQ) proteins in rat neuronal precursor (PC-12) cells.
Figure 13 shows that HSF1A functions to ameliorate eye degeneration in a fruit
fly
model of poly glutamine disease (Example VIII).
Figure 14 shows that compositions of some embodiments of the present invention
activate HSF1 independently of HSP90 binding (Example IX).
DETAILED DESCRIPTION OF THE INVENTION
The proper synthesis, folding, trafficking, modifications, interactions,
biochemical
activities and eventual clearance of cellular proteins is essential for normal
growth,
development and maintenance during the life cycle of all organisms.
Inappropriate folding,
aggregation and accumulation of abnormal proteins is proteo-toxic to cells due
to their
dominant affects of insolubility, inappropriate interactions and long half-
lives (see, e.g.,
Johnson, J.L., and Craig, E.A. (1997) Cell 90(2): 201-204; Bukau, B., et al.,
(2000) Cell
101(2): 119-122; Hartl, F.U. (1996) Nature 381: 571-580; Deuerling, E., and
Bukau, B.
(2004) Crit. Rev. Biochem. Mol. Bio. 39: 261-277; Bukau, B., et al., (2006)
Cell 125(3): 443-
451; Dickey, C.A., etal., (2007) Trends in Mol. Med. 13(1): 32-38). Neuronal
tissues and
cells are exquisitely sensitive to defects in protein folding, aggregation and
clearance and
these defects are causally or correlatively associated with diseases that
include Huntington's
disease, Parkinson's disease, Alzheimer's disease, Amyotropic Lateral
Sclerosis, prion
diseases and other neurodegenerative disorders (see, e.g., Bonini, N. M.
(2002) Proc. Natl.
Acad. Sci., USA 99:16407-16411; Muchowski, P.J. (2002) Neuron 35: 9-12;
Morimoto, R. I.
(2006)
14
CA 02724413 2015-12-22
New England J. Med. 355: 2254-2255; Finkbeiner, S., et al., (2006) J.
Neurosci. 26(41):
10349-10357; Furukawa, Y., et al., (2006) PNAS 103(18): 7148-7153; Gidalevitz,
T., etal.,
(2006) Science 311:1471-1474).
Many of these are diseases occur frequently in the elderly and result in a
variety of
symptoms due to loss of function of motor, dopaminergic and other neurons
essential for a
normal healthy life (see, e.g., Cummings, C.J. and Zoghbi, H.Y. (2000) Hum.
Mol. Genet. 9:
909-916). Defects in protein folding, aggregation and clearance have also been
implicated in
type 2 diabetes mellitus (see, e.g., Chung, J., et al. (2008) PNAS 105(5) 1739-
1744).
While many aspects of these complex processes are incompletely understood, a
variety of individual protein chaperones and co-chaperone complexes function
to fold,
process, mature and degrade cellular proteins (see, e.g., Johnson, J.L., and
Craig, E.A. (1997)
Cell 90(2): 201-204; Bukau, B., et al., (2000) Cell 101(2): 119-122; Hartl,
F.U. (1996) Nature
381: 571-580; Deuerling, E., and Bukau, B. (2004) Crit. Rev. Biochem. Mol.
Bio. 39: 261-
277; Bukau, B., etal., (2006) Cell 125(3): 443-451; Dickey, C.A., et al.,
(2007) Trends in
Mol. Med. 13(1): 32-38). Many neurodegenerative diseases are caused, for
example, by
genetically programmed changes in specific proteins, such as through the
addition of poly
glutamine (polyQ) coding sequences, by genetic defects in the protein folding
and processing
machinery, or by as yet poorly understood mechanisms by which abnormal protein
conformations can be propagated in a protein-catalyzed fashion (see, e.g.,
Bonini, N. M.
(2002) Proc. Natl. Acad. Sci., USA 99:16407-16411; Muchowski, P.J. (2002)
Neuron 35: 9-
12; Morimoto, R. I. (2006) New England J. Med. 355: 2254-2255; Finkbeiner, S.,
et al.,
(2006) J. Neurosci. 26(41): 10349-10357; Furukawa, Y., etal., (2006) PNAS
103(18): 7148-
7153; Gidalevitz, T., etal., (2006) Science 311:1471-1474; Cummings, C.J. and
Zoghbi,
H.Y. (2000) Hum. Mol. Genet. 9: 909-916).
Protein chaperones facilitate the folding, stabilization, solubilization and
degradation
of cellular proteins and are often included in the group of Heat Shock
Proteins (Hsps)
because
CA 02724413 2015-12-22
their synthesis is elevated in response to heat and other stresses known to
induce protein
unfolding, aggregation and degradation (see, e.g., Morimoto, R.I., Tissieres,
A., and
Georgopoulos, C. (1994) The Biology of Heat Shock Proteins and Molecular
Chaperones,
Cold Springs Harbor Laboratory Press, Cold Springs Harbor New York; Lindquist,
S. (1992)
Curr. Opinion in Genet. and Develop. 2: 748-755; Feige, U., et al., (eds.)
Stress-inducible
cellular responses. Vol 77, Birkhauser, Verlag, Boston; Lindquist, S. and
Craig, E.A. (1988)
Ann. Rev. Genet. 22: 631-677). Recent evidence in cellular or organismal model
systems of
neurodegenerative diseases strongly support the notion that protein chaperones
act both
independently and in concert to ameliorate biochemical hallmarks or symptoms
of the disease
associated with unfolded or aggregated proteins. For example, in mammalian
cell culture,
mouse or Drosophila models of polyQ aggregation or alpha-synuclein toxicity,
expression of
the Hsp70 or Hsp40 chaperones can significantly suppress protein aggregation,
increase
protein solubility and turnover and ameliorate neuronal loss (see, e.g.,
Bailey, C.K., et al.,
(2002) Hum. Mol. Genet. 11(5): 515-523; Kitamura, A., et al., (2006) Nat. Cell
Bio.
8(10):1163-1170; Pavan, K., et al., (2002) Science 295: 865-868; Chai, Y., et
al., (1999) J.
Neurosci. 19(23): 10338-10347; Muchowski, P.J, et al., (2000) Proc. Natl.
Acad. Sci. USA
97: 7841-7846; Jana, N.R., et al., (2000) Hum. Mol. Genet. 9: 2009-2018;
Wyttenbach, A., et
al., (2000) Proc. Natl. Acad. Sci. 97: 2898-2903; Adachi, H., et al., (2003)
J. Neurosci. 23:
2203-2211; Cummings, C.J., et al., (2001) Hum. Mol. Genet. 10:1511-1518).
Additional
studies suggest that elevated expression of both Hsp70 and Hsp40 can synergize
in the
suppression of polyQ-mediated neuronal degeneration and that arimoclomal, an
inducer of
Hsp synthesis, significantly delays disease progression in a mouse model of
ALS (see, e.g.,
Kieran, D., et al., (2004) Nature Medicine 10: 402-405). From bacteria to
human cells, Hsp
synthesis is coordinately induced in response to stress conditions that result
in protein
unfolding, aggregation and proteolysis by stress-responsive transcription
factors.
In cells from yeast to humans, the transcription of genes encoding Hsps is
induced in
response to stresses such as increased temperature through cis-acting promoter
elements
called Heat Shock Elements (HSEs), composed of variations of the inverted
repeated
pentameric consensus sequence 5"-nGAAnnTTCnnGAAn-3' (SEQ ID NO:01) (see, e.g.,
16
CA 02724413 2015-12-22
Morimoto, R.I., Tissieres, A., and Georgopoulos, C. (1994) The Biology of Heat
Shock
Proteins and Molecular Chaperones, Cold Springs Harbor Laboratory Press, Cold
Springs
Harbor New York; Lindquist, S. and Craig, E.A. (1988) Ann. Rev. Genet. 22: 631-
677). In
response to stress the Heat Shock Transcription Factor, HSF, binds as a homo-
trimer to HSEs
and activates target gene transcription. Indeed, HSFs and their cognate DNA
binding site
HSEs are two highly structurally and functionally conserved cis- and trans-
acting regulatory
factors (see, e.g., Wu, C. (1995) Ann. Rev. Cell Dev. Biol. 11:441-469;
Pirkkala, L., et al.,
(2001) FASEB J. 15: 1118-1131). The baker's yeast Saccharomyces cerevisiae
harbors a
single gene encoding HSF that is essential for cell viability under all
conditions tested (see,
e.g., Sorger, P.K., and Pelham, H.R.B. (1988) Cell 54: 855-864; Wiederrecht,
G., etal.,
(1988) Cell 54: 841-853. Recent genome-wide expression and chromatin-
immuoprecipitation
experiments demonstrate that yeast HSF directly activates a broad range of
genes encoding
proteins that function as chaperones, in protein turnover and a variety of
additional stress
protection roles (see, e.g., Hahn, J.-S., et al., (2004) Molecular and
Cellular Biology 24:5249-
5256).
In mammals, Drosophila and C. elegans HSF1 responds to stress to activate
transcription of genes encoding a family of protein chaperones (Wu, C. (1995)
Ann. Rev.
Cell Dev. Biol. 11: 441-469; Pirkkala, L., Nykanen, et al., (2001) FASEB J.
15: 1118-1131;
Hsu, A.L., (2003) Science 300: 1142-1145; Morley, J.F., and Morimoto, R.I.
(2004) Mol.
Bio. Cell 15: 657-664). While the precise mechanisms whereby HSF1 from humans
and other
organisms sense and respond to stress have not been elucidated, a model that
summarizes
current understanding of this process in human cells is shown in Figure 1.
HSF1 activation is
a multi-step process that occurs posttranslationally in response to elevated
temperatures, the
accumulation of unfolded proteins and other stressful conditions (see, e.g.,
Wu, C. (1995)
Ann. Rev. Cell Dev. Biol. 11: 441-469; Pirkkala, L., et al., (2001) FASEB J.
15: 1118-1131;
Baler, R., etal., (1993) Mol. Cell. Biol. 13: 2486-2496; Sarge, K.D., et al.,
(1993) Mol. Cell.
Biol. 13: 1392-1407; Zuo, J., et al., (1995) Mol. Cell. Biol. 15: 4319-4330).
In the absence of
acute stress, HSF1 is present largely in the cytoplasm as a monomer, and is
thought to be
associated with Hsp90,
17
CA 02724413 2015-12-22
Hsp70 and other proteins (see, e.g., Zuo, J., et al., (1998) Cell 94: 471-480;
Ali, A., et al.,
(1998) Mol. Cell. Biol. 18: 4949-4960; Guo, Y., et al., (2001) J. Biol. Chem.
276: 45791-
45799). In vitro and in vivo experiments suggest that HSF1 is retained in the
momomeric
state through intramolecular interactions between two coiled coil regions,
Leucine Zipper 1-3
(LZ1-3) and Leucine Zipper 4 (LZ4) (see, e.g., Rabindran, S.K., et al., (1993)
Science 259:
230-234; Zuo, J., et al., (1994) Mol. Cell. Biol. 14: 7557-7568). Indeed,
point mutations in
Leucine Zipper 4 (HSF11z4m) cause constitutive HSF1 homo-trimerization in
mammalian
cells (see, e.g., Rabindran, S.K., et al., (1993) Science 259: 230-234). In
response to stress,
HSF1 is converted to a homo-trimer that is thought to be stabilized by inter-
molecular coiled
coil interactions and accumulates in the nucleus, where it engages in high
affinity binding to
HSEs within target gene promoters and activates target gene transcription.
Heat shock
induced Hsp target gene activation by HSF1 is transient, and correspondingly,
HSF1 is
ultimately converted back to the low affinity DNA binding monomeric form in
the cytosol.
HSF1 is phosphorylated both under basal conditions where this modification is
thought to
maintain the protein in an inactive state and in response to stress, with this
latter modification
having functional consequences that are not well understood (see, e.g., Cotto,
J. J., et al.,
(1996) J. Biol. Chem. 271: 3355-3358; Guettouche, T., (2005) BMC Bochem. 6:1-
14).
Experiments conducted during the course of development of embodiments for the
present invention developed a specialized high throughput screen for
identifying small
molecules capable of activating the human Heat Shock Transcription Factor 1
(HSF1) protein
from complex chemical libraries. In addition, experiments conducted during the
course of
developing embodiments for the present invention identified molecules capable
of activating
the human Heat Shock Transcription Factor 1. It was shown that these molecules
activate
human HSF1 when added to cultured cells, and activate expression of heat shock
proteins
(e.g., HSP70, HSP25, and reduce aggregation of poly-glutamine proteins in
neuronal cells).
Accordingly, the present invention provides small molecules (e.g., compounds)
capable of
activating heat shock factors (e.g., facilitating HSF1 homo-trimerization),
activating heat
shock factor (e.g., HSF1) target gene expression (e.g., Heat Shock genes) and
protein
18
CA 02724413 2015-12-22
expression (e.g., Heat Shock Proteins), methods for their discovery, and their
therapeutic
and/or research uses. Exemplary compositions and methods of the present
invention are
described in more detail in the following sections: I. HSF Activating Compound
Screens; II.
HSF Activating Compounds; III. Pharmaceutical Compositions; and IV.
Therapeutic
Applications.
The practice of the present invention employs, unless otherwise indicated,
conventional techniques of organic chemistry, pharmacology, molecular biology
(including
recombinant techniques), cell biology, biochemistry, and immunology, which are
within the
skill of the art. Such techniques are explained fully in the literature, such
as, "Molecular
cloning: a laboratory manual" Second Edition (Sambrook etal., 1989);
"Oligonucleotide
synthesis" (M.J. Gait, ed., 1984); "Animal cell culture" (R.I. Freshney, ed.,
1987); the series
"Methods in enzymology" (Academic Press, Inc.); "Handbook of experimental
immunology"
(D.M. Weir & C.C. Blackwell, eds.); "Gene transfer vectors for mammalian
cells" (J.M.
Miller & M.P. Cabs, eds., 1987); "Current protocols in molecular biology"
(F.M. Ausubel et
al., eds., 1987, and periodic updates); "PCR: the polymerase chain reaction"
(Mullis etal.,
eds., 1994); and "Current protocols in immunology" (J.E. Coligan etal., eds.,
1991.
I. HSF Activating Compound Screens
In some embodiments, the present invention provides screens for identifying
activators of heat shock factor (e.g., activators capable of facilitating HSF1
function), and for
identifying activators of heat shock factor (e.g., HSF1) target gene
expression (e.g., Heat
Shock genes) and protein expression (e.g., Heat Shock Proteins). The screen is
not limited to
identifying activators of a particular heat shock factor. In some embodiments,
the screens
identify HSF1 activators, HSF2 activators, and/or HSF4 activators. The present
invention is
not limited to identifying a particular type of heat shock factor activator.
Examples of
activators include, but are not limited to, small molecules (see, e.g., the
compounds provided
in Section II ¨ Exemplary Compounds).
The present invention is not limited to a particular type of screen for
identifying heat
shock factor (e.g., HSF1) activators. In some embodiments, the present
invention provides a
yeast based screen. The present invention is not limited to use of a
particular type of yeast.
19
CA 02724413 2015-12-22
In some embodiments, the screen comprises genetically modified yeast. The
screen is not
limited to a particular type of genetically modified yeast. In some
embodiments, the screens
provide yeast that are genetically modified such that expression of yeast HSF
is regulated. In
some embodiments, the screens provide yeast that are genetically modified such
that the yeast
express human HSF1. While human HSF1 and yeast HSF have similar structures,
bind as
homo-trimers to conserved HSEs and activate functionally common Hsp genes,
expression of
wild type human HSF1 cannot suppress the viability defect associated with
yeast HSF
deletion (yhsfA) cells (see, e.g., Liu, X.D., et al., (1997) EMBO J. 16: 6466-
6477).
Biochemical analysis of human HSF1 demonstrated that human HSF1 exists in
yeast as a
monomer and is not able to homo-trimerize under basal or stress conditions.
Indeed,
expression in yhsfA cells of the human HSF11z4m mutant, which is
constitutively trimerized
in culturized human cells, is able to rescue the yhsfA viability defect, bind
to and activate
stress-inducible target gene transcription such as from the yeast Hsp70 gene,
and exist as a
homo-trimer in yeast (see, e.g., Liu, X.D., et al., (1997) EMBO J. 16: 6466-
6477; Liu, P.C.C.,
and Thiele, D.J. (1999) Journal of Biological Chemistry 274: 17219-17225). The
present
invention is not limited to any mechanism of action. Indeed, an understanding
of the
mechanism is not necessary to practice the present invention. Nonetheless, it
is contemplated
that human HSF1 fails to function in yeast due to a homotrimerization
inability. In some
embodiments, the screens of the present invention identify activators (e.g.,
compounds)
capable of facilitating homotrimerization of HSF1.
The screens are not limited to a particular manner of genetically modifying
yeast HSF
expression. In some embodiments, genetically modified yeast HSF expression
occurs
through deleting the HSF gene open reading frame, thereby rendering a yhsfA
strain that is
inviable. In some embodiments, the yhsfA strains have a yeast HSF gene coupled
with an
inducible promoter (e.g., GAL1-10) thereby rendering growth of such yhsfA
strains viable on
a selectable medium (e.g., galactose). In some embodiments wherein yhsfA
strains express a
yeast HSF gene coupled with an GAL promoter, the strain is inviable at any
temperature or
under any condition tested when cells are shifted to a glucose medium thereby
extinguishing
yeast HSF espression. In some embodiments, the yhsfz1 strains expressing a
yeast HSF gene
coupled with a inducible promoter additionally harbor a plasmid configured for
human HSF1
CA 02724413 2015-12-22
(hHSF1) expression. In some embodiments, the plasmid configured for expression
of hHSF is
pRS424-GPD-hHSF1 (wherein GPD is the constitutively expressed glucose
phosphate
dehydrogenase promoter).
In some embodiments, as shown in Figure 2, the yhsfzl strains expressing a
yeast HSF
gene coupled with a inducible promoter configured for expression hHSF1 are
used for
identifying activators of hHSF1 (e.g., activators capable of facilitating HSF1
homotrimerization), identifying activators of HSF1 target gene expression
(e.g., Heat Shock
Proteins) and/or activation or inhibition of protein chaperone activity (e.g.,
increased protein
folding, increased protein solubilization, protein degradation). This screen
has several
features, including but not limited to, (1) when yeast HSF expression is
extinguished, only
cells in which human HSF1 has been activated are viable, providing a screen
with a very low
background; (2) this strain allows for positive selection of human HSF1
activator molecules;
(3) this strain in conjunction with an additional strain lacking hHSF1
expression permits
identification of molecules that act exclusively in a human HSF1-dependent
manner rather
than via the prevention of yeast HSF repression by glucose in yeast; and (4)
the screen is
amenable to automated liquid handling and optical density determination and is
therefore
high throughput in nature.
The screens are not limited to a particular method for identifying activators
of hHSF1.
In some embodiments, for example, a yhsfzI strain expressing hHSF1 is exposed
to a small
molecule under conditions inviable for growth absent homotrimerization or
other means of
activation of hHSF1. Growth in such conditions indicates that the small
molecule is an
activator of hHSF1 (see, Figure 2). A lack of growth indicates that the small
molecule is not
an activator of hHSF1 (see, Figure 2).
In some embodiments, the yeast strains used in the screens are genetically
modified to
maximize small molecule accumulation. The yeast strains are not limited to a
particular
manner of genetic modification to maximize small molecule accumulation. In
some
embodiments, genetic modification to maximize small molecule accumulation is
accomplished through, for example, sequential deletion of the PDR5, SNQ2 and
ERG6 genes
in the yhs14 background. PDR5 and SNQ2 encode ATP binding cassette integral
plasma
membrane transport proteins which mediate multidrug resistance by exporting
compounds
with a broad range of structures and relatively low specificity (see, e.g.,
Emter, R., (2002)
21
CA 02724413 2015-12-22
FEBS Letters 521: 57-61). As such, yeast cells lacking Pdr5 and Snq2
accumulate organic
molecules to a significantly higher steady state level than wild type strains.
The ERG6 gene,
encoding delta(24)-sterol C-methyltransferase, is a key enzyme in ergosterol
biosynthesis.
Erg6 mutants exhibit enhanced diffusion rates of lipophilic molecules across
the plasma
membrane (see, e.g., Emter, R., (2002) FEBS Letters 521: 57-61). In some
embodiments,
yeast strains having sequential deletion of PDR5, SNQ2 and ERG6 genes in the
yhsfA
background hyper-accumulate organic compounds.
HSF Activating Compounds
Experiments conducted during the course of development of embodiments for the
present invention identified HSF activating compounds (e.g., compounds capable
of
facilitating HSF1 homotrimerization, compounds capable of activating HSF1
target gene
expression (e.g., Heat Shock Elements), compounds capable of activating HSF1
protein
function (e.g., Heat Shock Proteins), compounds capable of activating or
inhibiting protein
chaperone activity (e.g., increased protein folding, increased protein
solubilization, protein
degradation)). An understanding of the mechanism by which the compounds
activate HSF
proteins is not required to practice the present invention.
Exemplary HSF1 activating compounds of the present invention are provided
below.
In certain embodiments, the composition comprises a compound described by the
R1
10111
R21,¨ R2
Xi
2---x3
R9 HN R6
R5
R7 /\ p
following formula: .
including salts, esters, and prodrugs thereof. The compound is not limited to
particular definitions of R1 through R9 groups. In some embodiments, the R1
through R9
22
CA 02724413 2015-12-22
groups define a compound capable of HSF activation, identifiable using
screening techniques
CH3 CH3
( /
1-0CH3 ¨N
\
described herein. In some embodiments, R1 is , 0 , CH3
1
0
- N'7
\ LNA
0-, -OH, H, or halogen (e.g., chlorine). In some embodiments, X1 is H ,
0
VNA
H , or Xi is absent. In some embodiments, X2 is S or C. In some
embodiments, X3 is S or C. In some embodiments, R9 is ¨OCH3. In some
embodiments, R2
foS( ) .
Ri0 S __ CF3 I
NH
=
N¨CH3 ________________________
H3C/
( ____________________________________ ) ____ ( ____ )
NH CI
NH
=
0 .
, , or ¨CF3. In some embodiments,
23
CA 02724413 2015-12-22
Nx y
N
R3 R4 is C
IN or C .
In some embodiments, R5 is S or C. In
some embodiments, R6 is H or =0 . In some embodiments, R7 is H or =0 . In
__________________________ OI \o
some embodiments, R8 is or
?SS
Ri
. In some embodiments, R10 is chlorine or CH3. In some
embodiments, R11 is substituted or unsubstituted alkyl such as, for example,
or
1¨N
24
CA 02724413 2015-12-22
S
N7 'Ix
HN
0= =0
In certain embodiments, the compound is
101 _Cr e
N7NN l c,
/NN
HN
NH
0=S=0
0
=
CA 02724413 2015-12-22
0
NNN
NH
0 ___________ c
_____________________ * = N
HC
=CH3
H3C-N 0\
CH3
S N
N
CH
1 3
=
CH3
H3C
S N
26
CA 02724413 2015-12-22
S
F N __ (,,
F 0 . N 0
F II+
0 N'''0
a
H3C¨ IV\
cH,
CH
I 3
0 Ail 0.õ
'VP -CH3
0 ..-----
----- at sõ..õ.f./N
, N
S--I( IIIt 1 0 N
N S N
0
11 0
41101 =
CH
i 3
---
IMP
0 afth 0,
'CH3 0 OH
N N
NI-----;s
N
4110 CI F F
, or .
In some embodiments, the present invention provides HSF activating compounds
described by any of the the compounds shown in Figure 7.
In certain embodiments, the present invention provides HSF activating
compounds
described by the following formulas:
27
CA 02724413 2015-12-22
R1
R3 RR24
\Hi
HN\ /R6
D.
1-µ6
1.7 1.8
including salts, esters, and prodrugs thereof; and
including both R and S enantiomeric forms and racemic mixtures thereof;
1-0CH3
wherein R1 is , H, or halogen (e.g., Chlorine);
_FoS ______________________________________ /
R9
wherein R2 is , or =
t\L
R3 i
V N R4 c or
N N7N
wherein s C (i.e., R3 and R4 are C or
N,
but R3 and R4 are not the same);
wherein R5 is S or C;
wherein R6 is H or
wherein R7 is H or =-0;
28
CA 02724413 2015-12-22
0
sNsri
4410
wherein R8 is or R 0 =
wherein R9 is Chlorine or CH3; and wherein R10 is or
IN _______________
In certain embodiments, the present invention provides the following HSF1
activating
compounds:
CI
s
* ci
4111 N7 NN
V XN
NH
0= =0
0
=
(HSF1-A), (HSF1-B),
and
oI ei
iN *
NH
0 _______________ c
(HSF1-C). In certain embodiments, the composition
29
CA 02724413 2015-12-22
comprises a functional derivative of a compound capable of HSF activation
(e.g.,
NN
HN
O-S-0
1.1
as a functional derivative of HSF1A).
In certain embodiments, the present invention provides any of the compounds
described herein with further functionalization. For example, in some
embodiments, the
present invention provides any of the compounds functionalized with a biotin
moiety (e.g.,
HSF1A-biotin)
0
,s
,,o
HN
N
\
0
NH
0 /H
).For
example, such functionalized compounds can be used for identification of
agents (e.g.,
protein(s)) with which they interact.
30
CA 02724413 2015-12-22
Additional exemplary compounds are provided in the figures and experimental
section, below.
III. Pharmaceutical compositions, formulations, and exemplary
administration
routes and dosing considerations
Exemplary embodiments of various contemplated medicaments and pharmaceutical
compositions are provided below.
A. Preparing Medicaments
It is contemplated that the compounds of the present invention are useful in
the
preparation of medicaments to treat a variety of conditions associated with
misfolded proteins
and/or deficient protein chaperone activity.
In addition, it is contemplated that the compounds are also useful for
preparing
medicaments for treating other disorders wherein the effectiveness of the
compounds are
known or predicted. Such disorders include, but are not limited to,
neurological disorders.
The methods and techniques for preparing medicaments of a compound of the
present
invention are well-known in the art. Exemplary pharmaceutical formulations and
routes of
delivery are described below.
One of skill in the art will appreciate that any one or more of the compounds
described herein, including the many specific embodiments, are prepared by
applying
standard pharmaceutical manufacturing procedures. Such medicaments can be
delivered to
the subject by using delivery methods that are well-known in the
pharmaceutical arts.
B. Exemplary pharmaceutical compositions and formulation
In some embodiments of the present invention, the compositions are
administered
alone, while in some other embodiments, the compositions are preferably
present in a
pharmaceutical formulation comprising at least one active ingredient/agent, as
defined above,
together with a solid support or alternatively, together with one or more
pharmaceutically
acceptable carriers and optionally other therapeutic agents. Each carrier must
be "acceptable"
in the sense that it is compatible with the other ingredients of the
formulation and not
injurious to the subject.
31
CA 02724413 2015-12-22
Contemplated formulations include those suitable oral, rectal, nasal, topical
(including
transdermal, buccal and sublingual), vaginal, parenteral (including
subcutaneous,
intramuscular, intravenous and intradermal) and pulmonary administration. In
some
embodiments, formulations are conveniently presented in unit dosage form and
are prepared
by any method known in the art of pharmacy. Such methods include the step of
bringing into
association the active ingredient with the carrier which constitutes one or
more accessory
ingredients. In general, the formulations are prepared by uniformly and
intimately bringing
into association (e.g., mixing) the active ingredient with liquid carriers or
finely divided solid
carriers or both, and then if necessary shaping the product.
Formulations of the present invention suitable for oral administration may be
presented as discrete units such as capsules, cachets or tablets, wherein each
preferably
contains a predetermined amount of the active ingredient; as a powder or
granules; as a
solution or suspension in an aqueous or non-aqueous liquid; or as an oil-in-
water liquid
emulsion or a water-in-oil liquid emulsion. In other embodiments, the active
ingredient is
presented as a bolus, electuary, or paste, etc.
In some embodiments, tablets comprise at least one active ingredient and
optionally
one or more accessory agents/carriers are made by compressing or molding the
respective
agents. In some embodiments, compressed tablets are prepared by compressing in
a suitable
machine the active ingredient in a free-flowing form such as a powder or
granules, optionally
mixed with a binder (e.g., povidone, gelatin, hydroxypropylmethyl cellulose),
lubricant, inert
diluent, preservative, disintegrant (e.g., sodium starch glycolate, cross-
linked povidone,
cross-linked sodium carboxymethyl cellulose)surface-active or dispersing
agent. Molded
tablets are made by molding in a suitable machine a mixture of the powdered
compound (e.g.,
active ingredient) moistened with an inert liquid diluent. Tablets may
optionally be coated or
scored and may be formulated so as to provide slow or controlled release of
the active
ingredient therein using, for example, hydroxypropylmethyl cellulose in
varying proportions
to provide the desired release profile. Tablets may optionally be provided
with an enteric
coating, to provide release in parts of the gut other than the stomach.
Formulations suitable for topical administration in the mouth include lozenges
comprising the active ingredient in a flavored basis, usually sucrose and
acacia or tragacanth;
pastilles comprising the active ingredient in an inert basis such as gelatin
and glycerin, or
32
CA 02724413 2015-12-22
sucrose and acacia; and mouthwashes comprising the active ingredient in a
suitable liquid
carrier.
Pharmaceutical compositions for topical administration according to the
present
invention are optionally formulated as ointments, creams, suspensions,
lotions, powders,
solutions, pastes, gels, sprays, aerosols or oils. In alternatively
embodiments, topical
formulations comprise patches or dressings such as a bandage or adhesive
plasters
impregnated with active ingredient(s), and optionally one or more excipients
or diluents. In
some embodiments, the topical formulations include a compound(s) that enhances
absorption
or penetration of the active agent(s) through the skin or other affected
areas. Examples of
such dermal penetration enhancers include dimethylsulfoxide (DMSO) and related
analogues.
If desired, the aqueous phase of a cream base includes, for example, at least
about
30% w/w of a polyhydric alcohol, i.e., an alcohol having two or more hydroxyl
groups such
as propylene glycol, butane-1,3-diol, mannitol, sorbitol, glycerol and
polyethylene glycol and
mixtures thereof.
In some embodiments, oily phase emulsions of this invention are constituted
from
known ingredients in a known manner. This phase typically comprises a lone
emulsifier
(otherwise known as an emulgent), it is also desirable in some embodiments for
this phase to
further comprises a mixture of at least one emulsifier with a fat or an oil or
with both a fat
and an oil.
Preferably, a hydrophilic emulsifier is included together with a lipophilic
emulsifier
so as to act as a stabilizer. It some embodiments it is also preferable to
include both an oil
and a fat. Together, the emulsifier(s) with or without stabilizer(s) make up
the so-called
emulsifying wax, and the wax together with the oil and/or fat make up the so-
called
emulsifying ointment base which forms the oily dispersed phase of the cream
formulations.
Emulgents and emulsion stabilizers suitable for use in the formulation of the
present
invention include Tween 60, Span 80, cetostearyl alcohol, myristyl alcohol,
glyceryl
monostearate and sodium lauryl sulfate.
The choice of suitable oils or fats for the formulation is based on achieving
the
desired properties (e.g., cosmetic properties), since the solubility of the
active
compound/agent in most oils likely to be used in pharmaceutical emulsion
formulations is
very low. Thus creams should preferably be a non-greasy, non-staining and
washable
33
CA 02724413 2015-12-22
products with suitable consistency to avoid leakage from tubes or other
containers. Straight
or branched chain, mono- or dibasic alkyl esters such as di-isoadipate,
isocetyl stearate,
propylene glycol diester of coconut fatty acids, isopropyl myristate, decyl
oleate, isopropyl
palmitate, butyl stearate, 2-ethylhexyl palmitate or a blend of branched chain
esters known as
Crodamol CAP may be used, the last three being preferred esters. These may be
used alone
or in combination depending on the properties required. Alternatively, high
melting point
lipids such as white soft paraffin and/or liquid paraffin or other mineral
oils can be used.
Formulations suitable for topical administration to the eye also include eye
drops
wherein the active ingredient is dissolved or suspended in a suitable carrier,
especially an
aqueous solvent for the agent.
Formulations for rectal administration may be presented as a suppository with
suitable
base comprising, for example, cocoa butter or a salicylate.
Formulations suitable for vaginal administration may be presented as
pessaries,
creams, gels, pastes, foams or spray formulations containing in addition to
the agent, such
carriers as are known in the art to be appropriate.
Formulations suitable for nasal administration, wherein the carrier is a
solid, include
coarse powders having a particle size, for example, in the range of about 20
to about 500
microns which are administered in the manner in which snuff is taken, i.e., by
rapid
inhalation (e.g., forced) through the nasal passage from a container of the
powder held close
up to the nose. Other suitable formulations wherein the carrier is a liquid
for administration
include, but are not limited to, nasal sprays, drops, or aerosols by
nebulizer, an include
aqueous or oily solutions of the agents.
Formulations suitable for parenteral administration include aqueous and non-
aqueous
isotonic sterile injection solutions which may contain antioxidants, buffers,
bacteriostats and
solutes which render the formulation isotonic with the blood of the intended
recipient; and
aqueous and non-aqueous sterile suspensions which may include suspending
agents and
thickening agents, and liposomes or other microparticulate systems which are
designed to
target the compound to blood components or one or more organs. In some
embodiments, the
formulations are presented/formulated in unit-dose or multi-dose sealed
containers, for
example, ampoules and vials, and may be stored in a freeze-dried (lyophilized)
condition
requiring only the addition of the sterile liquid carrier, for example water
for injections,
34
CA 02724413 2015-12-22
immediately prior to use. Extemporaneous injection solutions and suspensions
may be
prepared from sterile powders, granules and tablets of the kind previously
described.
Preferred unit dosage formulations are those containing a daily dose or unit,
daily
subdose, as herein above-recited, or an appropriate fraction thereof, of an
agent.
It should be understood that in addition to the ingredients particularly
mentioned
above, the formulations of this invention may include other agents
conventional in the art
having regard to the type of formulation in question, for example, those
suitable for oral
administration may include such further agents as sweeteners, thickeners and
flavoring
agents. It also is intended that the agents, compositions and methods of this
invention be
combined with other suitable compositions and therapies. Still other
formulations optionally
include food additives (suitable sweeteners, flavorings, colorings, etc.),
phytonutrients (e.g.,
flax seed oil), minerals (e.g., Ca, Fe, K, etc.), vitamins, and other
acceptable compositions
(e.g., conjugated linoelic acid), extenders, and stabilizers, etc.
In some embodiments, the compounds of the present invention are provided in
unsolvated form or are in non-aqueous solutions (e.g., ethanol). The compounds
may be
generated to allow such formulations through the production of specific
crystalline
polymorphs compatible with the formulations. In some embodiments, the
compounds of the
present invention are provided in conjunction with thermal therapy such as a
thermal bath.
In certain embodiments, the present invention provides instructions for
administering
said compound to a subject. In certain embodiments, the present invention
provides
instructions for using the compositions contained in a kit for the treatment
of conditions
characterized by the dysregulation of apoptotic processes in a cell or tissue
(e.g., providing
dosing, route of administration, decision trees for treating physicians for
correlating patient-
specific characteristics with therapeutic courses of action). In certain
embodiments, the
present invention provides instructions for using the compositions contained
in the kit to treat
a variety of medical conditions associated with misfolded proteins or
irregular HSF1
activitiy (e.g., medical conditions involving misfolded proteins or irregular
HSF1 activity)
(e.g., medical conditions involving irregular chaperone activity) (e.g.,
Alzheimer's disease,
Parkinson's disease, Huntington disease, Amyotrophic Lateral Sclerosis, a
prion-based
disease, cataract, age-related cataract, glaucoma, macular degeneration, age-
related macular
degeneration, retinitis pigmentosa, cardiovascular disease and stroke, heat
stroke,
CA 02724413 2015-12-22
spinocerebellar ataxia, Machado Joseph disease, stress-related neuronal
degeneration, aging,
cancer, and type 2 diabetes mellitus.) In some embodiments, the methods are
directed
towards crystallins in age-related cataracts. In some embodiments, the methods
are directed
towards myocillin in glaucoma.
C. Exemplary administration routes and dosing considerations
Various delivery systems are known and can be used to administer therapeutic
agents
(e.g., exemplary compounds as described in Section III above) of the present
invention, e.g.,
encapsulation in liposomes, microparticles, microcapsules, receptor-mediated
endocytosis,
and the like. Methods of delivery include, but are not limited to, intra-
arterial, intra-
muscular, intravenous, intranasal, and oral routes. In specific embodiments,
it may be
desirable to administer the pharmaceutical compositions of the invention
locally to the area in
need of treatment; this may be achieved by, for example, and not by way of
limitation, local
infusion during surgery, injection, or by means of a catheter.
It is contemplated that the agents identified can be administered to subjects
or
individuals susceptible to or at risk of developing pathological growth of
target cells and
correlated conditions. When the agent is administered to a subject such as a
mouse, a rat or a
human patient, the agent can be added to a pharmaceutically acceptable carrier
and
systemically or topically administered to the subject. To determine patients
that can be
beneficially treated, a tissue sample is removed from the patient and the
cells are assayed for
sensitivity to the agent.
Therapeutic amounts are empirically determined and vary with the pathology
being
treated, the subject being treated and the efficacy and toxicity of the agent.
When delivered
to an animal, the method is useful to further confirm efficacy of the agent.
In some embodiments, in vivo administration is effected in one dose,
continuously or
intermittently throughout the course of treatment. Methods of determining the
most effective
means and dosage of administration are well known to those of skill in the art
and vary with
the composition used for therapy, the purpose of the therapy, the target cell
being treated, and
the subject being treated. Single or multiple administrations are carried out
with the dose
level and pattern being selected by the treating physician.
36
CA 02724413 2015-12-22
Suitable dosage formulations and methods of administering the agents are
readily
determined by those of skill in the art. Preferably, the compounds are
administered at about
0.01 mg/kg to about 200 mg/kg, more preferably at about 0.1 mg/kg to about 100
mg/kg,
even more preferably at about 0.5 mg/kg to about 50 mg/kg. When the compounds
described
herein are co-administered with another agent (e.g., as sensitizing agents),
the effective
amount may be less than when the agent is used alone.
The pharmaceutical compositions can be administered orally, intranasally,
parenterally or by inhalation therapy, and may take the form of tablets,
lozenges, granules,
capsules, pills, ampoules, suppositories or aerosol form. They may also take
the form of
suspensions, solutions and emulsions of the active ingredient in aqueous or
nonaqueous
diluents, syrups, granulates or powders. In addition to an agent of the
present invention, the
pharmaceutical compositions can also contain other pharmaceutically active
compounds or a
plurality of compounds of the invention.
More particularly, an agent of the present invention also referred to herein
as the
active ingredient, may be administered for therapy by any suitable route
including, but not
limited to, oral, rectal, nasal, topical (including, but not limited to,
transdermal, aerosol,
buccal and sublingual), vaginal, parental (including, but not limited to,
subcutaneous,
intramuscular, intravenous and intradermal) and pulmonary. It is also
appreciated that the
preferred route varies with the condition and age of the recipient, and the
disease being
treated.
Ideally, the agent should be administered to achieve peak concentrations of
the active
compound at sites of disease. This may be achieved, for example, by the
intravenous
injection of the agent, optionally in saline, or orally administered, for
example, as a tablet,
capsule or syrup containing the active ingredient.
Desirable blood levels of the agent may be maintained by a continuous infusion
to
provide a therapeutic amount of the active ingredient within disease tissue.
The use of
operative combinations is contemplated to provide therapeutic combinations
requiring a
lower total dosage of each component antiviral agent than may be required when
each
individual therapeutic compound or drug is used alone, thereby reducing
adverse effects.
37
CA 02724413 2015-12-22
D. Exemplary co-administration routes and dosing considerations
The present invention also includes methods involving co-administration of the
compounds described herein with one or more additional active agents. Indeed,
it is a further
aspect of this invention to provide methods for enhancing prior art therapies
and/or
pharmaceutical compositions by co-administering a compound of this invention.
In co-
administration procedures, the agents may be administered concurrently or
sequentially. In
one embodiment, the compounds described herein are administered prior to the
other active
agent(s). The pharmaceutical formulations and modes of administration may be
any of those
described above. In addition, the two or more co-administered chemical agents,
biological
agents or radiation may each be administered using different modes or
different formulations.
The agent or agents to be co-administered depends on the type of condition
being
treated. For example, when the condition being treated is a neurological
disorder (e.g.,
Huntington Disease), the additional agent can be an anticonvulsant medication.
The
additional agents to be co-administered can be any of the well-known agents in
the art for a
particular disorder, including, but not limited to, those that are currently
in clinical use and/or
experimental use.
IV. Therapeutic Application
In certain embodiments, the present invention provides methods (e.g.,
therapeutic
applications) for treating conditions associated with protein misfolding. The
present
invention is not limited to a particular type of method. In some embodiments,
the methods
for treating conditions associated with protein misfolding comprise a)
providing: i. target
cells having misfolded proteins; and ii. a composition (e.g., a composition
comprising
exemplary HSF1 activating compounds as described in Section III above); and b)
exposing
the target cells to the composition under conditions such that the exposure
results in enhanced
HSF1 activity. The methods are not limited to treating a particular condition
associated with
protein misfolding. In some embodiments, the condition associated with protein
misfolding
is a medical condition involving deficient chaperone activity. In some
embodiments, the
condition associated with protein misfolding is enhanced aging, Alzheimer's
disease,
Parkinson's disease, Huntington disease, Amyotrophic Lateral Sclerosis, and
prion-based
38
CA 02724413 2015-12-22
disease (e.g., transmissible spongiform encephalopathy, Bovine spongiform
encephalopathy,
Creutzfeldt-Jakob disease, and Kuru). In some embodiments, the condition
associated with
protein misfolding is type 2 diabetes mellitus (see, e.g., Chung, J., et al.,
(2008) PNAS 105(5)
1739-1744; herein incorporated by refernece in its entirety). The methods are
not limited to a
particular type of target cells. In some embodiments, the target cells are
neurological cells.
In some embodiments, the target cells are within a living mammal (e.g., human,
horse, dog,
cat, pig, rat, mouse, ape, monkey).
Additionally, any one or more of these compounds can be used in combination
with at
least one other therapeutic agent (e.g., potassium channel openers, calcium
channel blockers,
sodium hydrogen exchanger inhibitors, anticonvulsant agents, antiarrhythmic
agents,
antiatherosclerotic agents, anticoagulants, antithrombotic agents,
prothrombolytic agents,
fibrinogen antagonists, diuretics, antihypertensive agents, ATPase inhibitors,
mineralocorticoid receptor antagonists, phospodiesterase inhibitors,
antidiabetic agents, anti-
inflammatory agents, antioxidants, angiogenesis modulators, antiosteoporosis
agents,
hormone replacement therapies, hormone receptor modulators, oral
contraceptives,
antiobesity agents, antidepressants, antianxiety agents, antipsychotic agents,
antiproliferative
agents, antitumor agents, antiulcer and gastroesophageal reflux disease
agents, growth
hormone agents and/or growth hormone secretagogues, thyroid mimetics, anti-
infective
agents, anti-spastic agents, antiviral agents, antibacterial agents,
antifungal agents,
cholesterol/lipid lowering agents and lipid profile therapies, and agents that
mimic ischemic
preconditioning and/or myocardial stunning, antiatherosclerotic agents,
anticoagulants,
antithrombotic agents, antihypertensive agents, antidiabetic agents, and
antihypertensive
agents selected from ACE inhibitors, AT-1 receptor antagonists, ET receptor
antagonists,
dual ET/AII receptor antagonists, and vasopepsidase inhibitors, or an
antiplatelet agent
selected from GPIIb/IlIa blockers, P2Y1 and P2Y12antagonists, thromboxane
receptor
antagonists, aspirin) in along with a pharmaceutically-acceptable carrier or
diluent in a
pharmaceutical composition. Additional therapeutic agents for Huntington
disease include,
but are not limited to, anticonvulsant agents (e.g., valproic acid,
clonazepam), antipsychotic
agents (e.g., risperidone, haloperidol), rauwolfia alkaloids (e.g.,
reserpine), antidepressants
(e.g., proxetine). Additional therapeutic agents for Parkinson's disease
include, but are not
limited to, dopamine prodrugs (e.g., levodopa/carbidopa), dopamine agonists
(e.g.,
39
CA 02724413 2015-12-22
apomorphine, bromocriptine, pergolide, pramipexole, ropinirole, rotigotine),
catechol-0-
methyltransferase (COMT) inhibitors (e.g., tolcapone, entacapone, levodopa,
carbidopa,
entacapone), anticholinergics (e.g., trihexyphenidyl, benztropine mesylate),
MAO-B
inhibitors (e.g., selegiline, rasagiline), and N-methyl-D-aspartic acid
inhibitors (e.g.,
amantadine). Additional therapeutic agents for Alzheimer's disease include,
but are not
limited to, centrally acting AChE inhibitors (e.g., rivastigmine), NMDA
antagonists (e.g.,
memantine), and free-radical scavengers (e.g., tocopherol). Additional
therapeutic agents for
Amyotrophic Lateral Sclerosis include, but are not limited to, glutamate
pathway antagonists
(e.g., riluzole), antispastic agents (e.g., baclofen). Additional therapeutic
agents for prion
diseases include, but are not limited to, Congo red and its analogs,
anthracyclines,
amphotericin B and its analogs, sulfated polyanions, and tetrapyrroles.
Additional
therapeutic agents for type 2 diabetes mellitus include, but are not limited
to, sulfonylurea
agents (e.g., glipizide, glyburide, glimepiride), meglitinides (e.g.,
repaglinide, nateglinide),
biguanides (e.g., metformin), thiazolidinediones (e.g., pioglitazone,
rosiglitazone), dipeptidyl
peptidase IV (DPP-4) inhibitors (e.g., sitagliptin), incretin mimetics (e.g.,
exenatide), amylin
analogs (e.g., pramlintide acetate), and alpha-glucosidase inhibitors (e.g.,
acarbose, miglitol).
Additional agents for glaucoma, cataract, retinitis pigmentosa, and/or macular
degeneration
include, but are not limited to, Levobunolol (Betagan), Timolol
maleate/hemihydrate
(Timoptic Timoptic XE, Betimol, Istalol), Carteolol (Cartrol, Ocupress),
Betaxolol (Betoptic-
S), Metipranolol hydrochloride (OptiPranolol), Levobetaxolol (Betaxon),
Brimonidine
(Alphagan-P), Apraclonidine (Iopidine), Dipivefrin (AKPro, Propine),
Epinephrine (Epifrin),
Memantine (Namenda, Axura), Dorzolamide HC1(Trusopt), Brinzolamide (Azopt),
Acetazolamide (Diamox), Methazolamide (Neptazane), Dorzolamide HCl/timolol
maleate
(Cosopt), Latanoprost (Xalatan), Bimatoprost (Lumigan), Travoprost ophthamic
solution
(Travatan), Unoprostone (Rescula), Pilocarpine (Pilocar, Pilagan, Pilogel,
Ocusert),
Isosorbide (Ismotic), Mannitol (Osmitrol, Resectisol), Glycerin (Ophthalgan,
Osmoglyn),
Brimonidine/timolol (Combigan), Phenylephrine HC1(Neo-Synephrine),
Prednisolone
acetate (AK-Pred, Pred Forte), Dexamethasone (Ocu-Dex), Ciprofloxacin
(Ciloxan),
Erythromycin (E-Mycin), Nepafenac ophthalmic (Nevanac), Verteporfin
(Visudyne),
Pegaptanib (Macugen), Ranibizumab (Lucentis), Vitamin A (Aquasol A, Del-Vi-A),
Vitamin
E (Aquasol E, Vitec), Ascorbic acid (Cebid, Ascorbicap, Cevalin, Cecon),
Lutein or
CA 02724413 2015-12-22
Zeaxanthin, Bilberry, Beta-carotene, Diltiazem (Cardizem, Dilacor, Tiamate),
Acetazolamide
(Diamox, Diamox Sequels), and Methazolamide (Neptazane)). Additional
treatments for
other diseases of protein misfolding include, but are not limited to,
hyperthermia.
EXPERIMENTAL
The following examples are provided to demonstrate and further illustrate
certain
preferred embodiments of the present invention and are not to be construed as
limiting the
scope thereof.
Example I.
This example describes the optimization of growth conditions for the DTY512
strain.
As demonstrated in Figure 3, the growth conditions for a yhsfzI strain
harboring 1) a yeast
HSF gene coupled with a GAL promoter and 2) a pRS424-GPD-hHSF1 plasmid for
hHSF I
expression (the DTY512 strain) were optimized from petri dishes to 96 well
microtiter dish
format. Cells were grown in Synthetic Complete medium lacking uracil and
tryptophan to
select for plasmid maintenance, in the presence of the non-inducing/non-
repressing carbon
source raffinose (2%). Galactose concentrations (0.01%) were empirically
identified that
induce sufficient levels of yeast HSF for robust yeast cell viability, while
rendering cells
sensitive to strong glucose repression of yeast HSF expression after the
addition of 4%
glucose. The screen cells are grown to midlog phase in selective synthetic
complete medium
with 2% raffinose and 0.01% galactose. The culture was then diluted to ¨5,000
cells/ml in the
same growth medium in which 4% glucose was substituted for the galactose, to
initiate
glucose repression of yeast HSF expression. Cells (200 microliters) were
seeded into 96 well
microtiter dishes at ¨1,000 cells/well and compound or carrier solvent
(dimethylsulfoxide,
DMSO) distributed independently to each well using a Beckman Biomek FX liquid
handling
robot under sterile conditions. Plates were incubated at 30 C and optical
density measured
over time using an attached SpectraMax Plus Plate Reader. . As shown in Figure
3, when
grown in galactose, yHSF1 is expressed allowing for cell growth. However, upon
shifting
the cells to a glucose containing media, expression of yHSF1 is repressed,
allowing only cells
expressing the constitutively active hHSF11z4m allele to grow. Activation of
hHSF I by small
molecules will also allow cell growth.Yeast culture growth curves were
generated for each
41
CA 02724413 2015-12-22
microtiter well and slopes calculated over the course of 96 hours. As shown in
Figure 4, the
growth of cells was quantitatively followed in each microtiter well, the
background growth of
cells expressing wild type human HSF1 with either no addition or DMSO alone is
very low
and allowed for facile qualitative detection of positive candidates in the
screen.
Example II.
This example describes validation of candidate HSF1 activating compounds. As
shown in Figure 3 appropriate growth conditions, galactose induction and
glucose repression
parameters for application of the yeast-based human HSF1 activator screen to a
high
throughput 96-well format was identified. This screen evaluated a
combinatorial chemistry
library of ¨10,500 diverse compounds built from approximately 75 unique
scaffold
structures in the PPD library (PPD Discovery Research, Research Triangle Park,
NC). While
this was a modest-sized screen, ¨50 distinct library components were
identified that allowed
modest to robust yeast cell growth within 48-96 hours post-seeding at a
concentration of 10
micromolar. Figure 4A shows a small sample of five compounds that stimulated
yeast cell
growth with different efficacy, the DMSO solvent control and one compound that
was
negative from this screen. Subsequent analysis of 32 of the more potent
compounds
demonstrated that the efficacy of all of these molecules in the yeast-based
screen was
dependent on human HSF1. The structural features of three unique compounds
were
evaluated, denoted HSF1-A, HSF1-B and HSF1-C, that were among the most
effective in
stimulating cell growth in the humanized yeast-based screen. Figure 4B shows
two non-
42
CA 02724413 2015-12-22
NNN
HN
functional derivatives (HSF1AD2 ); and HSF1AD3
1.1
NN =
HN
0--=S=0
) that were incapable of stimulating yeast cell growth in
comparison to HSF1A. Experiments conducted during the course of developing
embodiments for the present invention determined that HSF1AD1 is a functional
derivative
43
CA 02724413 2015-12-22
#10
HN
0=----3=--0
41111
of HSF1A (e.g., HSF1AD1 ( ) is
capable of stimulating yeast
cell growth similar to HSF1A).
As shown in Figure 5 the three chemically distinct compounds HSF1A, HSF1B, and
HSF1C exhibited common structural features including the conservation of aryl
moieties
typical of pyrazol benzamides, as well as polarized bonds to oxygen near the
center of each
molecule. Figure 6 shows synthetic routes for HSF1A, HSF1B, and HSF1C. Figure
7 shows
additional compounds, including HSF1-A, HSF1-B, and HSF-1C, identified as HSF1
activating compounds identified from the PPD library. It should be understood
that derivates
of these compounds may also be used in the compositions and methods described
herein (see,
e.g., HSF1AD1).
Additional screens were conducted with compounds from the LOPAC chemical
library and the Prestwick chemical library, which resulted in the
identification of additional
HSF1 activating compounds. Figure 8 shows HSF1 activating compounds identified
thorugh
screens conducted with compounds from the LOPAC and Prestwick chemical
libraries. It
should be understood that derivates of these compounds may also be used in the
compositions
and methods described herein.
Example III.
This example describes materials and methods for Examples IV-VII.
Cell Culture. Wild-type and hsfl -/- mouse embryonic fibroblast (MEF) cells
were
grown in DMEM + 10%FBS (37 C, 5% CO2). PC-12 cells were grown in DMEM + 5%
FBS/10%horse serum (37 C, 10% CO2). For MEF cells, at the time of the
experiment 6X105
44
CA 02724413 2015-12-22
cells were seeded into each well of a 6-well plate in serum containing media.
The cells were
incubated under those conditions for an additional 12 hr upon which time the
cells were
washed twice in IX PBS and the growth media was changed to OptiMEM media
(Invitrogen)
without serum. For PC-12 cell, 5X105 cells were seeded into each well of a 6-
well plate, in 2
ml OptiMEM media without serum and incubated under those conditions for an
additional 12
hrs. After 12 hr, HSF1A or DMSO was added to either the MEF or PC-12 cells and
incubated for 15 hr at 37 C.
Western Blotting. After 15 hr treatment, the cells were washed twice in 1X PBS
and
harvested by scraping. The cells were lysed using cell lysis buffer (25mM
Tris, 150mM
NaC1, 1% Triton X-100, 0.1% SDS, 1mM EDTA) and soluble proteins were
quantified using
BCA assay. Equal amounts of total protein was separated on 10%-20% gradient
gel,
transferred to a nitrocellulose membrane and analyzed for expression of HSP70,
mHSP25,
HSF1, Q74-GFP and SOD1 using anti-HSP70 (SC-24, Santa Cruz), anti-mHSP25 (SPA-
801,
Stressgen), anti-HSF1 (Bethyl), anti-GFP (SC-8334) and anti-SOD! (SOD-100,
Stressgen)
antibodies respectively. Secondary antibodies were either anti-mouse or anti-
rabbit- HRP
conjugated antibodies from GE Healthcare Life Sciences. Proteins were
visualized using the
Pico chemioluminescence kit (Pierce).
Example IV.
This example demonstrates that HSF1A activates the expression of the HSP70 and
HSP25 heat shock protein (chaperone) genes in Mouse Embryonic Fibroblast (MEF)
cells.
As shown in Figure 9, MEF cells were treated with the indicated concentrations
of HSF lA
(in micromolar) or the DMSO solvent control in OptiMEM media without serum for
15 hours
or heat shocked (HS) in OptiMEM media without serum for 2 hours at 42 C and
allowed to
recover for 15 hr at 37 C. Total cellular protein extracts were prepared,
fractionated by SDS-
polyacrylamide gel electrophoresis, transferred to a solid membrane and the
Hsp70, Hsp25
and SOD1 proteins detected by probing with their respective specific
antibodies followed by
standard immunoblotting techniques to identify the proteins.
45
CA 02724413 2015-12-22
Example V.
This example demonstrates HSF1A dependent activation of HSP70 is dependent on
the presence of the gene encoding HSF1. As shown in Figure 10, wild-type and
hsfl -/- MEF
cells were treated with the indicated concentrations of HSF1A or the DMSO
solvent as
control in OptiMEM media without serum for 15 hr or heat shocked (HS) for 2 hr
in
OptiMEM media without serum at 42 C and allowed to recover for 15 hr at 37 C.
The Hsp70,
HSF1 and SOD1 proteins were detected by immunoblotting as described in Figure
9.
Example VI.
This example demonstrates that HSF1A can act synergistically with heat shock
to
activate expression of the HSP70 protein chaperone. As shown in Figure 11, MEF
cells were
treated with the indicated concentrations of HSF1A or the solvent DMSO as
control in
OptiMEM media without serum for 15 hr at 37 C, or with 30 micromolar HSF1A for
lhr at
37 C, followed by a 1 hr heat shock at a sub-optimal heat shock temperature of
40 C
followed by a 15 hr recovery at 37 C. Independently, cells were treated at the
optimal heat
shock temperature of 42 C for two hours followed by a 15 hr recovery. The
HSP70 and
SOD1 proteins were detected by immunoblotting as described in Figure 9. Note
that 30
micromolar HSF1A treatment synergizes with a sub-optimal heat shock
temperature to result
in higher levels of expression of the HSP70 protein chaperone.
Example VII.
This example demonstrates that HSF1A promotes expression of HSP70 and reduces
the aggregation of poly-glutamine (polyQ) proteins in rat neuronal precursor
(PC-12) cells.
As shown in Figure 12A, PC-12 cells were treated with the indicated
concentrations of
HSF1A or the DMSO solvent as control in OptiMEM media without serum for 15 hr
or heat
shocked in OptiMEM media without serum for 2 hr at 42 C and allowed to recover
for 15 hr
at 37 C (HS). HSP70 and SOD1 proteins were detected by immunoblotting as
described in
Figure 9. Figure 12B shows PC-12 cells, stably expressing the Huntington Q74-
GFP fusion
protein were treated with 20 micromolar HSF1A or the solvent DMSO (0.5%) as
control in
OptiMEM media without serum for 15 hr to stimulate HSP70 expression. After 15
hr
incubation, expression of the Q74-GFP protein was induced via the addition of
lmicrogram
46
CA 02724413 2015-12-22
per milliliter doxycyclin. The cells were incubated in the presence of
doxycyclin and HSF1A
for an additional 48 hrs after which the cells were harvested and soluble
proteins (S) were
isolated using cell lysis buffer. Insoluble proteins (P) were pelleted by
centrifugation. The
pellet was washed once in 1 milliliter cell lysis buffer and solubilized via a
mixture urea
buffer (cell lysis buffer + 5M Urea) and Laemmli buffer (1:2 ratio). The
polyglutamine-GFP
protein (Q74-GFP) was detected by immunoblotting with anti-GFF' antibody in a
manner
similar to that described in Figure 9.
Example VIII.
An example of a neurodegenerative disease that results from protein misfolding
is
called Huntington's disease, a progressive neurodegenerative disease that
leads to neuronal
cell death. This disease is due to the aggregation of a protein called
Huntingtin, where those
patients with this disease gene express a Huntingtin protein that harbors a
genetically
inherited expansion of the amino acid glutamine (abbreviated Q). The polyQ
Huntingtin
protein mis-folds and aggregates, thereby causing neuron death and the disease
symptoms. A
fly model of polyQ protein-mediated neuronal degeneration has been used to
test if HSF1A-
mediated elevation in protein chaperone production can ameliorate eye cell
degeneration in a
fly in which polyQ protein is strongly expressed in the eye. Figure 13 shows
that HSF1A
functions to ameliorate eye degeneration in a fruit fly model of poly
glutamine disease.
As shown in Figure 13, UAS-MJDtrQ78 flies were crossed to gmr-GAL4 flies in
the
chronic presence of food supplemented with DMSO, 200 M HSF1A or 5 M 17-AAG
(positive control). Reductions in eye morphological defects and de-
pigmentation, caused by
polyQ-protein expression, were observed with HSF1A and 17-AAG treatment.
Control flies
were UAS-MJDtrQ78 flies lacking the Ga14 transcription factor.
Example IX.
Many chemical activators of mammalian HSF1 have, as their mechanism the
binding
and inhibition of the Hsp90 protein, a protein that represses HSF I activity.
Figure 14 shows
that the novel humanized yeast HSF1 activation screen identifies molecules
that act
independently of Hsp90. As shown in Figure 14, HSF1A did not bind to the Hsp90
protein
nor did it compete for binding with a known HSF1 activating chemical,
geldanamycin, that
47
CA 02724413 2015-12-22
activates by binding and inhibiting Hsp90. These data distinguish HSF IA from
other known
HSF1 activating molecules.
As shown in Figure 14A, purified Hsp90 (AssayDesigns) was incubated with
either
17-AAG, a known Hsp90 binding chemical, or HSF1A at the indicated
concentration for 30
mm at 4 C. 1 uM Geldanamycin, a known Hsp90 binding chemical, was coupled to
the
affinity matrix biotin (Geldanamycin-biotin) and was added for 1 hr at 4 C to
displace 17-
AAG and HSF1A from Hsp90. Geldanmycin-biotin bound Hsp90 was captured by the
addition of neutravidin-agarose beads (Pierce) at 4 C for 30 mm. Hsp90 was
eluted from
beads by heating to 95 C for 5 min and analyzed by immunoblotting utilizing
and anti-Hsp90
antibody. As shown in Figure 14B, purified Hsp90 was incubated with either 10
uM
Geldanamycin-biotin (GD-B) or 100 uM HSF1A-biotin for 60 mm at 4 C and bound
Hsp90a
was captured by the addition of neutravidin-agarose beads at 4 C for 30 min.
48
CA 02724413 2010-11-12
SEQUENCE LISTING IN ELECTRONIC FORM
This description contains a sequence listing in electronic form in ASCII
text format (file no. 84012-170 ca seglist_v1_12Nov2010.txt).
A copy of the sequence listing in electronic form is available from the
Canadian Intellectual Property Office.
The sequences in the sequence listing in electronic form are reproduced in
the following Table.
SEQUENCE LISTING
<110> Duke University
<120> Compositions and Methods Relating to Heat Shock Transcription
Factor Activating Compounds and Targets Thereof
<130> 84012-170
<150> PCT/US 2009/044186
<151> 2009-05-15
<150> US 61/053,513
<151> 2008-05-15
<160> 1
<170> PatentIn version 3.5
<210> 1
<211> 15
<212> DNA
<213> Artificial Sequence
<220>
<223> Synthetic
<220>
<221> misc feature
<222> (1)..(1)
<223> n is a, c, g, or t
<220>
<221> misc feature
<222> (5)..(6)
<223> n is a, c, g, or t
<220>
<221> misc_feature
<222> (10)..(11)
<223> n is a, c, g, or t
<220>
<221> misc feature
<222> (15)..(15)
<223> n is a, c, g, or t
48a
CA 02724413 2010-11-12
<400> 1
ngaannt t cn ngaan 15
48b