Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1
COMBUSTION ENGINE AIR SUPPLY
RELATED APPLICATIONS
This application claims priority benefit of and is a continuation of U.S.
Serial
Number 13/019,387, filed February 2,2011.
This application also claims priority benefit of U.S. Serial Number
61/300,561,
filed February 2, 2010.
BACKGROUND OF THE DISCLOSURE
Filed of the Disclosure
This disclosure relates to a dry cell system for separating water into
hydrogen and
oxygen in combination with catalytic-type chemicals and materials. The
separated
hydrogen/oxygen are provided into the air intake system of an internal
combustion engine
and used therein to greatly improve the operation of said internal combustion
engine,
both in regards to fuel consumption as well as detrimental exhaust products.
Background Art
This disclosure improves upon known work concerning the use of hydrogen
added to gasoline to improve fuel consumption; as well as the known work
concerning
the use of Boron in a hydrogen generating cell. These works have been improved
upon
via inspiration and testing in the apparatus and method disclosed herein.
CA 2766757 2019-10-18
2
SUMMARY OF THE DISCLOSURE
In one example of the disclosed apparatus, as a dry cell system, the
reservoir, including the electrolyte and the water reservoir, is kept separate
from
the hydrogen generator. As the gas is created in the hydrogen generator, it is
expelled therefrom and utilized in the engine almost immediately. This
increases
safety, among other benefits, as there is not an accumulated quantity of
hydrogen gas or oxygen gas, which is potentially explosive in this
environment.
In most wet cell HHO generators, the distance between the anode and
cathode is approximately .125", and in dry cell generators the distance
between
plates is approximately .025". Thus, it follows that a dry cell generator will
also
take up less space, although the gas created must either be used or be removed
for storage, as there is no region within the generator for storage.
Furthermore,
the system disclosed herein operates without any substantial pressure
differential
between the produced gas and the surrounding atmosphere. A Venturi effect is
utilized to draw off the HHO gas, and thus the HHO gas need not pass through
any potentially dangerous pump.
The disclosed generator has also proven to operate at a relatively low
temperature, less than 212 F.
In one form, electrolyte chemicals, including boron in the form of boric
acid, which may be combined with potassium hydroxide as a catalyst, have been
found to greatly increase the HHO production in the HHO generator. An
unexpected result has been found in that the boron decreases the foaming
effect
of the dry cell, which increases the efficiency of the stabilization tank. In
addition,
the boric acid decreases the freezing temperature of the water electrolyte,
acts
as a refrigerant, and functions as an electric conductivity stabilizer. The
use of
potassium hydroxide (KOH) has been found to be exemplary in several
embodiments as a catalyst in the disclosed system.
CA 2766757 2018-06-27
3
BRIEF DESCRIPTION OF THE DRAWINGS
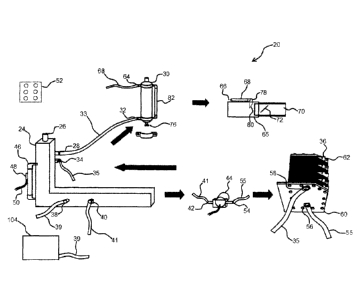
Fig. 1 is a highly schematic view of the improved combustion engine air
supply system, in one form.
Fig. 2 is an exploded view of the HHO generator, in one form.
Fig. 3 is a view of the components of the HHO generator, in one form.
CA 2766757 2018-06-27
4
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Tests have shown that the disclosed examples increase the gas efficiency
and lower carbon emissions in both gas and diesel engines.
In order to provide a sufficient amount of hydrogen and oxygen gas in a
dry cell environment, the combustion engine air supply system 20, as shown in
Fig. 1, in one example comprises a plurality of interoperating parts. While
the
fluid system substantially comprises a recycling system, we will begin with a
description of the electrolyte stabilization tank 24, which includes a
chemical
solution fill tube and safety relief diaphragm 26, and an HHO gas outlet 28,
which
is coupled by way of a piece of tubing 33 to a scrubber 30, such as by way of
an
HHO inlet 32. The electrolyte stabilization tank 24 may further comprise at
least
one HHO inlet 34, also coupled by way of a section of tubing 33 to a hydrogen
generator 36, which will be described in some detail. Furthermore, the
electrolyte stabilization tank 24 in one example comprises a (water) supply
inlet
38 coupled by way of a section of tubing to an electrolyte stabilization pump.
Furthermore, a water supply outlet 40 may be provided, which couples by way of
a section of tubing 41 to a water supply inlet 42 on an energy HHO circulation
pump 44. To allow for determination of the volume of fluid within the
electrolyte
stabilization tank 24, a sight glass 46 or equivalent measuring apparatus may
be
utilized. A fill switch 48 may also be utilized, which includes signal wires
50 that
are coupled, in one form, to an electronic control box 52, which controls the
electrolyte stabilization pump and other devices. Grounding the stabilization
tank
had been found to eliminate a negative build-up of electric charge within the
tank,
increasing the overall effectiveness of the apparatus.
CA 2766757 2018-06-27
5
The HHO circulation pump 44 not only comprises the water supply inlet
42, but also comprises a water supply outlet 54, which couples by way of a
section of tubing 55 to a water supply inlet 56 of the hydrogen generator 36.
The
hydrogen generator 36 also comprises a hydrogen product supply outlet 58,
which is coupled by way of a section of tubing 35to the HHO inlet 34 on the
electrolyte stabilization tank 24. These inlets 56 and outlets 58 may be
formed
on both the first side 60 and second side 62 of the hydrogen generator 36.
The HHO scrubber unit 30 comprises the HHO inlet 32 as well as an HHO
outlet 64, which couples by way of a length of tubing 68 to an HHO inlet 66 of
a
feed injector 78, which in one form is in line between an automotive air
cleaner
and a throttle body 70, so as to provide the HHO gas from the scrubber unit 30
to
the combustion chambers of the internal combustion engine. In one form, the
throttle body 70 comprises a throttle plate 72, which controls the throttle
position
sensor (TPS), which regulates the pulse width of a wave signal powering the
hydrogen generator 36. In this form, a pulse wave modulator (PWM) may be
utilized to provide a correct waveform to the hydrogen generator 36. In this
embodiment, as the throttle is opened wider, a signal is sent to the hydrogen
generator 36 to produce more HHO gas which is delivered to the feed injector
78.
In another form, a Hall effect sensor is coupled to the fuel injector input.
The output from the Hall effect sensor is then used to regulate the pulse
width of
the wave signal controlling the hydrogen generator 36. At idle, the positive
portion of the wave sent to the injector will be quite short in duration, and
when
power is applied the engine, the positive portion of the wave will be
considerably
longer. The fuel injector signal therefore defines the power/control signal
sent to
the hydrogen generator either directly to the anode/cathode or to the control
box
52, which in turn powers the hydrogen generator 36. While many common Hall
effect sensors will not be capable of surviving the heat generated by an
internal
CA 2766757 2018-06-27
6
combustion engine, Hall effect sensors built for industrial or military use
are
normally capable of handling such environments.
In one form, the electrolyte stabilization tank 24 contains a chemical
solution, which is pumped from the water supply outlet 40 through the hydrogen
generator 36 to release HHO gas. The HHO gas released by the hydrogen
generator 36 and any remaining liquid therein is then returned to the
stabilization
tank 24 through the supply outlet 58 to the inlet 34.
For optimum performance, the position of the HHO inlet 34 is above the
normal operating fluid level in the stabilization tank 24. As the HHO gas
outlet 28
is vertically above the HHO inlet 34, the electrolyte fluid is thus returned
to the
stabilization tank 24 and the HHO gas can be withdrawn therefrom. As
previously mentioned, in one example the liquid level is maintained by the
electrolyte fill switch 48, which in turn operates the electrolyte
stabilization pump,
which maintains the proper electrolyte level in the stabilization tank 24 by
.. pumping in water/electrolyte as needed. As with most electrolysis systems,
distilled water may be preferred.
The automatic fill system comprises the fill switch 48, control box 52, and
electrolyte stabilization pump, and the automatic fill system maintains the
chemical electrolyte at the desired concentration. As the system is in
operation,
the water is consumed in the gas (HHO) making process; however, the
electrolyte chemicals are not consumed in the gas making process and generally
cycle between the stabilization tank 24 and the hydrogen generator 36. This
system provides a stabilized reservoir for the chemical solution. The hydrogen
and oxygen (HHO) produced in the hydrogen generator 36 and reentering the
stabilization tank 24 are heated through the electrolysis process.
CA 2766757 2019-04-03
7
The HHO circulation pump 44, in one form, is a diaphragm-style pump that
maintains pressure when the air supply system 20 is engaged. In one form, the
constant positive pressure of up to 24 PSI is maintained between the
circulation
pump 44 and the feed injector 78. In one form, if and when the maximum output
pressure of the circulation pump 44 is reached, such as by an occlusion of the
line, the circulation pump 44 will turn off. During use, a very low pressure
differential will be maintained to enable flow of the fluid between the
circulation
pump 44 and the feed injector 78. The circulation pump 44 pumps the
electrolyte
from the electrolyte stabilization tank 24, forcing it through the hydrogen
generator 36, which causes the electrolyte and gas to flow back into the
stabilization tank 24, where the gas and liquid are then separated. This
constant
flow prevents the accumulation of contaminants and chemicals on the generator
plates shown in Fig. 3.
Operation of the air supply system 20 is facilitated by the above-described
fluid flow, which removes the gas bubbles off of the generator plates 74,
shown
in Figs. 2 and 3. This removal of the gas bubbles in one example is
accomplished as quickly as they are formed, thus increasing the cell
efficiency of
the hydrogen generator 36. Production is also improved by cooling the liquid
electrolyte stabilizing production fluctuations due to temperature changes
within
the system. As previously described, this cooling is accomplished by contact
of
the oxygen gas being discharged into the stainless steel headspace of the
electrolyte stabilization tank 26, causing an oxygen-to-stainless-steel
reaction
referred to as an element refrigeration action.
CA 2766757 2019-04-03
8
The common term fluid is used herein to define gasses, liquids, and
combinations thereof.
This described constant flow through the system ensures a constant
stirring action of the fluid flow, such that the chemicals are well mixed with
the
water being added by the chemical stabilization pump. The scrubber 30 is
designed to protect the appliance/internal combustion engine from damage
caused from actual droplets of liquid, should the gas-creating equipment for
some reason become overfilled in the stabilization tank 24, or some other
malfunction occur.
Another sight glass 82 provides visual observation should liquid
accumulate in the scrubber. A petcock 76 may be provided near the lower end of
the sight glass, through which the sight glass is easily emptied.
The input 66 comprises a delivery siphon or feed injector 78, which in one
form comprises a 900 hose barb fitting with one portion tapered 80, as shown
in
Fig. 1. The siphon 78 is inserted into the engine air intake system 65 as
previously described, allowing the engine vacuum to draw the HHO gas through
the tube leading from the gas scrubber outlet 64, thus decreasing any
potential
pressure in the entire gas production system. Utilizing the vacuum pressure of
the engine lowers the overall vapor pressure, which then allows for a
significant
increase in gas production.
As shown in Fig. 2, the hydrogen generator 36 comprises a plurality of
plates 74, including at least one front cover plate 84, which includes the
inlet 56
and outlet 58, previously described. A back cover plate 86 is also included,
as
well as a plurality of gaskets 88. In one form, the cover plate 84, back plate
86,
and gaskets 88 are formed of a non-conductive, hydrophilic material. Testing
has
shown that the commercial product Polyoxymethylene (Delrin) is exemplary for
construction of the cover plate 84 and back plate 86. Prior applications using
CA 2766757 2018-06-27
=
9
insulating materials that were not hydrophilic absorbed a small volume of
water/catalyst and deformed after extended use. While only a few of the
gaskets
88 are labeled in Fig, 2, it can be appreciated that in most embodiments
between
every layer of the plates 74 will be a gasket 88 to ensure fluid retention. At
least
one cathode 90 will also be utilized. Where it is desired to have the fluid
electrolyte pass through the cathode 90, a plurality of water (fluid) ports 92
will be
included, as shown in Fig. 3. The fluid ports 92 allow fluid to travel between
one
HHO generator cell 96 and another. At least one intermediate cathode 94 may
also be used, which may not include a fluid port where it is not desired to
allow
the fluid to flow between adjacent HHO generator cells. Each HHO generator
cell
96 will also include at least one anode 98. The use of intermediate anodes 95
and intermediate cathodes 94 is commonly known in the art of HHO generation.
To increase the volume of each HHO generator cell 96, a plurality of neutral
plates 100 may also be included. As shown and previously described, each of
the plates 74 will have a gasket 88 therebetween. To ease in manufacture of
the
hydrogen generator 36, each of the plates and gaskets may include a plurality
of
voids 102 through which a plurality of fasteners, such as (stainless steel)
bolts,
may be passed. By tensioning nuts onto these bolts, the hydrogen generator 36
is drawn together, compressing the gaskets 88 into the final, fluid-tight
form.
As with other electrolysis systems, electric voltage is applied between the
anode(s) and the associated cathode(s). In one form, the voltage is applied as
a
monopole square wave into a resonant circuit which is tuned to the specific
Q value of the solution used as an electrolyte.
In one embodiment, a water reservoir 104 is fluidly coupled to the
electrolyte stabilization tank through conduit 39 so as to maintain the fluid
level
within the electrolyte stabilization tank. The fill switch 48 may be coupled
to a
pump or valve between the water reservoir 104 and the electrolyte
stabilization
CA 2766757 2018-06-27
10
tank so as to allow water to flow into the electrolyte stabilization tank when
the
volume drops below a preset limit.
Testing has shown that marine grade, 316L stainless steel exhibits
substantially better production and corrosion resistance than other tested
.. materials.
The HHO generating electrolysis system may be arranged wherein a
square wave of direct current is provided to the power coupling and this
square
wave is tuned to a "Q" of the system, according to Faraday's electrolysis law.
While the present invention is illustrated by description of several
embodiments and while the illustrative embodiments are described in detail, it
is
not the intention of the applicants to restrict or in any way limit the scope
of the
appended claims to such detail. Additional advantages and modifications within
the scope of the appended claims will readily appear to those sufficed in the
art.
The invention in its broader aspects is therefore not limited to the specific
details,
representative apparatus and methods, and illustrative examples shown and
described. Accordingly, departures may be made from such details without
departing from the spirit or scope of applicants' general concept.
CA 2766757 2018-06-27