Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
METHODS OF TREATING INFLAMMATION
CROSS-REFERNCE
[0001] This application claims priority to US Provisional Application
61/245,214, filed September 23, 2009;
and to US Provisional Application 61/319,039, filed March 30, 2010; both of
which are incorporated herein by
reference.
BACKGROUND OF THE INVENTION
[0002] Inflammatory diseases, disorders, conditions and symptoms are
characterized, in part, by
the migration lymphocytes and monocytes into the affected tissue. The
migration of lymphocytes
and monocytes induces tissue damage and exacerbates inflammatory diseases,
disorders, conditions
and symptoms. Many leukocytes and monocytes follow a MIF gradient to the
affected tissue. In
general, MIF interacts with CXCR2 and CXCR4 receptors on leukocytes and
monocytes to trigger
and maintain leukocyte and monocyte migration.
SUMMARY OF THE INVENTION
[0003] There is a need for new methods of treating inflammatory diseases,
disorders, conditions
(e.g., atherosclerosis) and symptoms that do not interfere with (a) non-
inflammatory processes or (b)
desired-inflammatory processes. The inventors have discovered that undesired
and harmful
inflammation can be treated by inhibiting the ability of MIF to bind to CXCR2,
CXCR4, CD44, and
CD74. Further, the inventors have discovered that targeting precise regions of
MIF and CXCR2,
CXCR4, CD44, and CD74 will inhibit the ability of MIF to bind to CXCR2, CXCR4,
CD44, and
CD74 (thus, preventing undesired inflammation) without affecting other (e.g.,
desired and
beneficial) interactions of MIF, CXCR2, CXCR4, CD44, and CD74.
[0004] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one of the following domains of MIF: the N-terminal/pseudo-
ELR motif/domain,
the alpha-helix #1 motif/domain, the MIF N-loop motif/domain, the loop-barrel-
loop motif/domain,
the C-terminal motif/domain, or a combination thereof. In some embodiments,
the peptide that
competitively binds with a binding partner of one of the following domains: N-
terminal tail, the
pseudo ELR-loop, the alpha-helix #1 motif/domain, the PPQ-loop, the PDQ-loop,
the IGK-loop, the
NRS-helix, the SPDR-loop, the C-terminal tail, or the combination thereof. In
some embodiments,
the peptide competitively binds with a binding partner of the N-loop domain.
In some embodiments,
the peptide comprises an amino acid that competitively binds with a binding
partner of MIF 1eu47.
In some embodiments, the peptide competitively binds with a binding partner of
the pseudo-ELR
domain. In some embodiments, the peptide is selected from: LMAFGGSSEP (SEQ ID
NO. 18);
LMAFGGSS (SEQ ID NO. 20); cyclic CLMAFGGSSEPCALC (SEQ ID NO. 423);
VHVVPDQLMA (SEQ ID NO. 465); QLMAFGGSSE (SEQ ID NO. 468); VNTNVPRASVPDG
-1-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(SEQ ID NO. 437); NVPRASVPDG (SEQ ID NO. 172); cyclic CNVPRASVPDGC (SEQ ID NO.
440); NVPRASVPD (SEQ ID NO. 82); cyclic CLMAFGGSSEP[Abu]ALC (SEQ ID NO. 429),
wherein Abu is isosteric L-amino acid, alpha-aminobutyric acid; or cyclic
CLMAFGGSSEPSALC
(SEQ ID NO. 469).
[0005] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CXCR2. In some embodiments, the peptide
competitively
binds with a binding partner of one of the following domains: CXCR2
extracellular loop 1, CXCR2
extracellular loop 2, CXCR2 extracellular loop 3, or the CXCR2 N-
terminus/domain.
[0006] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CXCR4. In some embodiments, the peptide
competitively
binds with a binding partner of. SEADDRYICDRFYPNDLWVVV; or DDRYICDRFYPNDLW.
[0007] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CD44.
[0008] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CD74.
[0009] Disclosed herein, in certain embodiments, is a fusion peptide
comprising (a) a first peptide
that competitively binds with a binding partner of the N-loop motif of MIF;
and (b) a second peptide
that competitively binds with a binding partner of the pseudo ELR motif of
MIF; wherein the first
peptide and the second peptide retain their activity in the fusion peptide. In
some embodiments, the
fusion peptide comprises (a) a first peptide that competitively binds with a
binding partner of the N-
loop motif of MIF; (b) a second peptide that competitively binds with a
binding partner of the
pseudo ELR motif of MIF; and (c) a third peptide that competitively binds with
a binding partner of
the pseudo ELR motif of MIF; and wherein the first peptide and the second
peptide retain their
activity in the fusion peptide. In some embodiments, the fusion peptide
comprises a peptide selected
from: LMAFGGSSEP (SEQ ID NO. 18); LMAFGGSS (SEQ ID NO. 20); cyclic
CLMAFGGSSEPCALC (SEQ ID NO. 423); VHVVPDQLMA (SEQ ID NO. 465); QLMAFGGSSE
(SEQ ID NO. 468); VNTNVPRASVPDG (SEQ ID NO. 437); NVPRASVPDG (SEQ ID NO. 172);
cyclic CNVPRASVPDGC (SEQ ID NO. 440); NVPRASVPD (SEQ ID NO. 82); cyclic
CLMAFGGSSEP[Abu]ALC (SEQ ID NO. 429), wherein Abu is isosteric L-amino acid,
alpha-
aminobutyric acid; or cyclic CLMAFGGSSEPSALC (SEQ ID NO. 469). In some
embodiments, the
fusion peptide is given by Formula (IV):
Peptide 1 Linker Peptide 2
In some embodiments, the fusion peptide is given by Formula (V):
-2-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Peptide 1 Linker Peptide 2
Peptide 3
In some embodiments, the linker comprises an alkyl, a heteroalkyl, an
alkylene, an alkenylene, an
alkynylene, a heteroalkylene, a carbocycle, a heterocycle, an aromatic ring, a
non-aromatic ring, a
substituted ring, a monocyclic ring, a polycyclic ring, or a combination
thereof.
[0010] Disclosed herein, in certain embodiments, is a peptibody comprising (a)
an antibody, (b) a
peptide described herein, and (c) a linker binding the peptide to the Fab
region of the antibody;
wherein the peptide and the antibody retain their activity in the peptibody.
In some embodiments,
the linker binds the peptide to an antigen binding site. In some embodiments,
the linker comprises an
alkyl, a heteroalkyl, an alkylene, an alkenylene, an alkynylene, a
heteroalkylene, a carbocycle, a
heterocycle, an aromatic ring, a non-aromatic ring, a substituted ring, a
monocyclic ring, a
polycyclic ring, or a combination thereof. In some embodiments, the antibody
is an IgA, IgD, IgE,
IgG, or IgM.
Disclosed herein, in certain embodiments, is the use of a composition of
matter described herein for
treating an inflammatory disease, disorder or condition. In some embodiments,
the inflammatory
disease, disorder or condition is Atherosclerosis; Abdominal aortic aneurysm;
Acute disseminated
encephalomyelitis; Moyamoya disease; Takayasu disease; Acute coronary
syndrome; Cardiac-
allograft vasculopathy; Pulmonary inflammation; Acute respiratory distress
syndrome; Pulmonary
fibrosis; Acute disseminated encephalomyelitis; Addison's disease; Ankylosing
spondylitis;
Antiphospholipid antibody syndrome; Autoimmune hemolytic anemia; Autoimmune
hepatitis;
Autoimmune inner ear disease; Bullous pemphigoid; Chagas disease; Chronic
obstructive
pulmonary disease; Coeliac disease; Dermatomyositis; Diabetes mellitus type 1;
Diabetes mellitus
type 2; Endometriosis; Goodpasture's syndrome; Graves' disease; Guillain-Barre
syndrome;
Hashimoto's disease; Idiopathic thrombocytopenic purpura; Interstitial
cystitis; Systemic lupus
erythematosus (SLE); Metabolic syndrome; Multiple sclerosis; Myasthenia
gravis; Myocarditis;
Narcolepsy; Obesity; Pemphigus Vulgaris; Pernicious anaemia; Polymyositis;
Primary biliary
cirrhosis; Rheumatoid arthritis; Schizophrenia; Scleroderma; Sjogren's
syndrome; Vasculitis;
Vitiligo; Wegener's granulomatosis; Allergic rhinitis; Prostate cancer; Non-
small cell lung
carcinoma; Ovarian cancer; Breast cancer; Melanoma; Gastric cancer; Colorectal
cancer; Brain
cancer; Metastatic bone disorder; Pancreatic cancer; bladder cancer;
hepatocellular cancer; liver
cancer; adenocarcinoma of the lung; esophageal squamous cell carcinoma; CNS
tumors;
hematological tumors; a Lymphoma; Nasal polyps; Gastrointestinal cancer;
Ulcerative colitis;
Crohn's disorder; Collagenous colitis; Lymphocytic colitis; Ischaemic colitis;
Diversion colitis;
Behcet's syndrome; Infective colitis; Indeterminate colitis; Inflammatory
liver disorder; Endotoxin
shock; Septic shock; Rheumatoid spondylitis; Ankylosing spondylitis; Gouty
arthritis; Polymyalgia
-3-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
rheumatica; Alzheimer's disorder; Parkinson's disorder; Epilepsy; AIDS
dementia; Asthma; Adult
respiratory distress syndrome; Bronchitis; Cystic fibrosis; Acute leukocyte-
mediated lung injury;
Distal proctitis; Wegener's granulomatosis; Fibromyalgia; Bronchitis;
;Uveitis; Conjunctivitis;
Psoriasis; Eczema; Dermatitis; Smooth muscle proliferation disorders;
Meningitis; Shingles;
Encephalitis; Nephritis; Tuberculosis; Retinitis; Atopic dermatitis;
Pancreatitis; Periodontal
gingivitis; Coagulative Necrosis; Liquefactive Necrosis; Fibrinoid Necrosis;
Neointimal hyperplasia;
Myocardial infarction; Stroke; organ transplant rejection; influenza, or
combinations thereof. In
some embodiments, the inflammatory, disease, disorder, or condition is:
Prostate cancer; Non-small
cell lung carcinoma; Ovarian cancer; Breast cancer; Melanoma; Gastric cancer;
Colorectal cancer;
Brain cancer; Metastatic bone disorder; Pancreatic cancer; bladder cancer;
hepatocellular cancer;
liver cancer; adenocarcinoma of the lung; esophageal squamous cell carcinoma;
CNS tumors;
hematological tumors; a Lymphoma; or a combination thereof. In some
embodiments, the
inflammatory, disease, disorder, or condition is rehumatoid arthritis. In some
embodiments, the
inflammatory, disease, disorder, or condition is acute respiratory distress
syndrome. In some
embodiments, the inflammatory, disease, disorder, or condition is
glomerulonephritis. In some
embodiments, the inflammatory, disease, disorder, or condition is inflammatory
bowel disease. In
some embodiments, the inflammatory, disease, disorder, or condition is
abdominal aortic aneurysm
disease. In some embodiments, the inflammatory, disease, disorder, or
condition is chronic
obstructive pulmonary disease. In some embodiments, the inflammatory, disease,
disorder, or
condition is asthma. In some embodiments, the inflammatory, disease, disorder,
or condition is
lupus. In some embodiments, the inflammatory, disease, disorder, or condition
is sepsis.
[0011] Disclosed herein, in certain embodiments, is the use of a composition
of matter described
herein to treat, prevent or reduce angiogenesis.
[0012] Disclosed herein, in certain embodiments, is a pharmaceutical
composition for treating an
inflammatory disease, disorder, condition or symptom in an individual in need
thereof, comprising a
composition of matter described herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] The novel features of the invention are set forth with particularity in
the appended claims. A
better understanding of the features and advantages of the present invention
will be obtained by
reference to the following detailed description that sets forth illustrative
embodiments, in which the
principles of the invention are utilized, and the accompanying drawings of
which:
[0014] Figure 1 illustrates the crystal structure of a MIF trimer. The pseudo-
ELR motif/domains
form a ring in the trimer while the N-loop motif/domains extend outward from
the pseudo-ELR ring.
[0015] Figure 2 illustrates the nucleotide sequence of MIF annotated to show
the sequences that
correspond to the N-Loop motif/domain and the pseudo-ELR motif/domain.
-4-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0016] Figure 3 shows the nucleic acid sequence of human MIF and the
corresponding MIF
motif/domains.
[0017] FIGURE 4 shows that the peptide of SEQ ID No. 18 blocks chemotaxis in
human peripheral
blood mononuclear cells (PBMC).
[0018] FIGURE 5 shows that the peptide of SEQ ID NO. 423 significantly
antagonizes MIF-
induced chemotaxis in PMBCs.
[0019] FIGURE 6 shows that the peptide of SEQ ID NO. 423 significantly
antagonizes MIF-
induced chemotaxis in PMBCs in a dose dependent manner.
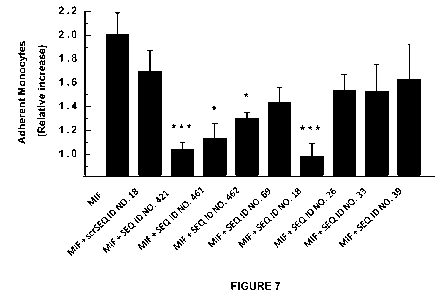
[0020] FIGURE 7 shows that peptides disclosed herein block MIF-mediated
monocyte arrest on
human aortic endothelial cells (under flow). 4nM of MIF were used. 5uM of
peptide were used. *p<
0.05; ** p< 0.01; *** = p< 0.005.
[0021] FIGURE 8 sgows that the peptide of SE ID NO. 18 blocks MIF-mediated
monocyte arrest
HuAoECs in a dose dependent manner. 4nM of MIF were used. *p< 0.05; ** p<
0.01; *** = p<
0.005.
[0022] FIGURE 9 presents the results of probing the MIF-MIF interface with
Peptide SPOT arrays.
luM of MIF was incubated overnight. Streptavidin-POD (1:10000) was incubated
for 2 hours at
room temperature.
[0023] FIGURE 10 presents the results of probing the MIF-CXCR2 interface with
Peptide SPOT
arrays. luM of MIF was incubated overnight. Streptavidin-POD (1:10000) was
incubated for 2
hours at room temperature.
[0024] FIGURE 11 presents the results of probing the MIF-CXCR2 interface with
Peptide SPOT
arrays using wildtype MIF (1 M Biotin-monoQ-MIF:Streptavidin-POD) and mutant
MIF (1 M
biotin-R 11A-D44A-MIF Streptavidin-POD). Streptavidin-POD (1:10000) was
incubated for 2 hours
at room temperature. Wildtype MIF and mutant MIF were incubated overnight at
room temperature.
[0025] FIGURE 12 presents the results of probing the MIF-CXCR4 interface with
Peptide SPOT
arrays using wildtype MIF (1 M Biotin- MIF: Streptavidin-POD). Streptavidin-
POD (1:10000) was
incubated for 2 hours at room temperature. Wildtype MIF was incubated
overnight at 4 C.
[0026] FIGURE 13 shows that SEQ ID NO. 18 inhibits leukocyte adhesion to
carotid arteries of
Apoe-deficient mice. Deficient and age matched controls on Western diet for 8
wks. n=5 for each
group; quantification from 10 high power fields (HPF) throughout carotid
artery. lx injection per
day of SEQ ID NO 18 or scrambled SEQ ID NO 18 for 3 days prior to adhesion
expt& IVM.
[0027] FIGURE 14 shows that SEQ ID NO 18 reduces TNFa and MCP-1 in mouse
peritonitis
model. Thioglycollate (TG): (3% IP). Vehicle, SEQ ID NO 18, SEQ ID NO 85 and
Dex: 30 min
prior to and 30 min post TG challenge. 2hr post TG, peritoneal lavage for cell
counts and
chemokines.
-5-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0028] FIGURE 15 shows that SEQ ID NO 18 reduces MCP-1 and monocyte levels in
mouse
peritonitis model. Thioglycollate (TG): (3% IP). Vehicle, SEQ ID NO. 422, SEQ
ID NO. 421, SEQ
ID NO. 45l and Dex: 30 min prior to and 30 min post TG challenge. 2hr post TG,
peritoneal lavage
for cell counts and chemokines.
[0029] FIGURE 16 presents a proposed mechanism of MIF signalling modulation.
[0030] FIGURE 17 illustrates the structure and surface exposure of the MIF-N-
loop and schematic
of the two-site binding model for MIF/CXCR2. A, 3 D-architectural homology
between CXCL8 and
MIF with a focus on the receptor interaction motifs. Binding of the canonical
ligand CXCL8 to
CXCR2 involves the N-loop and the ELR motif. MIF contains an N-like-loop
(sequence stretch 47-
56) and a pseudo-(E)LR motif (amino acids R12 and D45, constituting a 3D-ELR
motif). For clarity
reasons, only the monomeric structures of CXCL8 and MIF are depicted. B,
Schematic showing the
structure of MIF. C, Application of the two-site binding model of
chemokine/chemokine receptor
binding to MIF. The proposed interaction interface between MIF and CXCR2 (site
1: interaction
between the N-like-loop of MIF and the receptor N-terminus; site 2:
interaction between the pseudo-
(E)LR-motif of MIF and the receptor exoloops EL2 and 3). D, Trimeric structure
of MIF depicted in
surface mode.
[0031] FIGURE 18 is a peptide SPOT array analysis identifying the interaction
sites between MIF
and the extracellular domains of CXCR2 by peptide spot array analysis. Short
15-mer peptides
representing full-length human MIF (A) and the CXCR2 extracellular domains (B
and C) were
directly synthesized onto amino-cellulose membranes. CXCR2 peptides correspond
to the N-
terminus (N-term) and extracellular loops (EL) 1-3. Peptide strips were
incubated with 1 M biotin-
MIF (A and B) or biotin-Rl2A/D45A-MIF (C) and detected using streptavidin-POD.
A, MIFstrip
developed with biotin-MIF. B, CXCR2-strip developed with biotin-MIF. C, CXCR2-
strip developed
with biotin-R12A/D45A-MIF.
[0032] Figure 19 illustrates that MIF N-loop peptides inhibit the interaction
between MIF and
CXCR2. The effect of the peptides was examined by a competitive receptor
binding assay
measuring the reversal by the N-loop peptides of the inhibitory effect of MIF
on tracer binding.
HEK293 cells stably overexpressing CXCR2 were incubated with radioiodinated
I125CXCL8 tracer
together with 1 M human MIF and 100 M of the indicated N-like-loop peptides
of MIF as
competitor. Plots represent percent of specific 1125-CXCL8 binding. Tracer
binding in the absence
of MIF and peptide (buffer) was set at 100% and the competitive effect of MIF
in the absence of
peptide at 0%. Data represent means SEM of 3 independent experiments, each
performed in
duplicate measurements (* = p<0.01).
[0033] FIGURE 20 is a model depicting the interactions at the MIF/CXCR2
interface according to
the general two-site binding mechanism.
[0034] FIGURE 21 is a model depicting a peptibody.
-6-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
DETAILED DESCRIPTION OF THE INVENTION
[0035] Disclosed herein, in some embodiments, are peptides, small molecules,
antibodies, and
peptibodies (collectively, "compositions of matter") for treating inflammatory
diseases, disorders,
conditions and symptoms. Further disclosed herein are pharmaceutical
compositions and methods of
treating inflammatory diseases, disorders, conditions and symptoms. In some
embodiments, the
inflammatory disease, disorder, condition or symptom is characterized by
undesired MIF signaling.
In some embodiments, the inflammatory disease, disorder, condition or symptom
is characterized by
MIF-mediated leukocyte recruitment.
[0036] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one of the following domains of MIF: the N-terminal/pseudo-
ELR motif/domain,
the alpha-helix #1 motif/domain, the MIF N-loop motif/domain, the loop-barrel-
loop motif/domain,
the C-terminal motif/domain, or a combination thereof. In some embodiments,
the peptide that
competitively binds with a binding partner of one of the following domains: N-
terminal tail, the
pseudo ELR-loop, the alpha-helix #1 motif/domain, the PPQ-loop, the PDQ-loop,
the IGK-loop, the
NRS-helix, the SPDR-loop, the C-terminal tail, or the combination thereof. In
some embodiments,
the peptide competitively binds with a binding partner of the N-loop domain.
In some embodiments,
the peptide comprises an amino acid that competitively binds with a binding
partner of MIF 1eu47.
In some embodiments, the peptide competitively binds with a binding partner of
the pseudo-ELR
domain. In some embodiments, the peptide is selected from: LMAFGGSSEP (SEQ ID
NO. 18);
LMAFGGSS (SEQ ID NO. 20); cyclic CLMAFGGSSEPCALC (SEQ ID NO. 423);
VHVVPDQLMA (SEQ ID NO. 465); QLMAFGGSSE (SEQ ID NO. 468); VNTNVPRASVPDG
(SEQ ID NO. 437); NVPRASVPDG (SEQ ID NO. 172); cyclic CNVPRASVPDGC (SEQ ID NO.
440); NVPRASVPD (SEQ ID NO. 82); cyclic CLMAFGGSSEP[Abu]ALC (SEQ ID NO. 429),
wherein Abu is isosteric L-amino acid, alpha-aminobutyric acid; or cyclic
CLMAFGGSSEPSALC
(SEQ ID NO. 469).
[0037] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CXCR2. In some embodiments, the peptide
competitively
binds with a binding partner of one of the following domains: CXCR2
extracellular loop 1, CXCR2
extracellular loop 2, CXCR2 extracellular loop 3, or the CXCR2 N-
terminus/domain.
[0038] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CXCR4. In some embodiments, the peptide
competitively
binds with a binding partner of. SEADDRYICDRFYPNDLWVVV; or DDRYICDRFYPNDLW.
[0039] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CD44.
-7-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0040] Disclosed herein, in certain embodiments, are peptides that
competitively bind with a
binding partner of one motif/domain of CD74.
[0041] Disclosed herein, in certain embodiments, is a fusion peptide
comprising (a) a first peptide
that competitively binds with a binding partner of the N-loop motif of MIF;
and (b) a second peptide
that competitively binds with a binding partner of the pseudo ELR motif of
MIF; wherein the first
peptide and the second peptide retain their activity in the fusion peptide. In
some embodiments, the
fusion peptide comprises (a) a first peptide that competitively binds with a
binding partner of the N-
loop motif of MIF; (b) a second peptide that competitively binds with a
binding partner of the
pseudo ELR motif of MIF; and (c) a third peptide that competitively binds with
a binding partner of
the pseudo ELR motif of MIF; and wherein the first peptide and the second
peptide retain their
activity in the fusion peptide. In some embodiments, the fusion peptide
comprises a peptide selected
from: LMAFGGSSEP (SEQ ID NO. 18); LMAFGGSS (SEQ ID NO. 20); cyclic
CLMAFGGSSEPCALC (SEQ ID NO. 423); VHVVPDQLMA (SEQ ID NO. 465); QLMAFGGSSE
(SEQ ID NO. 468); VNTNVPRASVPDG (SEQ ID NO. 437); NVPRASVPDG (SEQ ID NO. 172);
cyclic CNVPRASVPDGC (SEQ ID NO. 440); NVPRASVPD (SEQ ID NO. 82); cyclic
CLMAFGGSSEP[Abu]ALC (SEQ ID NO. 429), wherein Abu is isosteric L-amino acid,
alpha-
aminobutyric acid; or cyclic CLMAFGGSSEPSALC (SEQ ID NO. 469). In some
embodiments, the
fusion peptide is given by Formula (IV):
Peptide 1 Linker Peptide 2
In some embodiments, the fusion peptide is given by Formula (V):
Peptide 1 Linker Peptide 2
Peptide 3
In some embodiments, the linker comprises an alkyl, a heteroalkyl, an
alkylene, an alkenylene, an
alkynylene, a heteroalkylene, a carbocycle, a heterocycle, an aromatic ring, a
non-aromatic ring, a
substituted ring, a monocyclic ring, a polycyclic ring, or a combination
thereof.
[0042] Disclosed herein, in certain embodiments, is a peptibody comprising (a)
an antibody, (b) a
peptide described herein, and (c) a linker binding the peptide to the Fab
region of the antibody;
wherein the peptide and the antibody retain their activity in the peptibody.
In some embodiments,
the linker binds the peptide to an antigen binding site. In some embodiments,
the linker comprises an
alkyl, a heteroalkyl, an alkylene, an alkenylene, an alkynylene, a
heteroalkylene, a carbocycle, a
heterocycle, an aromatic ring, a non-aromatic ring, a substituted ring, a
monocyclic ring, a
polycyclic ring, or a combination thereof. In some embodiments, the antibody
is an IgA, IgD, IgE,
IgG, or IgM.
-8-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0043] Disclosed herein, in certain embodiments, is the use of a composition
of matter described
herein for treating an inflammatory disease, disorder or condition. In some
embodiments, the
inflammatory, disease, disorder, or condition is a cancer. In some
embodiments, the inflammatory
disease, disorder or condition is Atherosclerosis; Abdominal aortic aneurysm;
Acute disseminated
encephalomyelitis; Moyamoya disease; Takayasu disease; Acute coronary
syndrome; Cardiac-
allograft vasculopathy; Pulmonary inflammation; Acute respiratory distress
syndrome; Pulmonary
fibrosis; Acute disseminated encephalomyelitis; Addison's disease; Ankylosing
spondylitis;
Antiphospholipid antibody syndrome; Autoimmune hemolytic anemia; Autoimmune
hepatitis;
Autoimmune inner ear disease; Bullous pemphigoid; Chagas disease; Chronic
obstructive
pulmonary disease; Coeliac disease; Dermatomyositis; Diabetes mellitus type 1;
Diabetes mellitus
type 2; Endometriosis; Goodpasture's syndrome; Graves' disease; Guillain-Barre
syndrome;
Hashimoto's disease; Idiopathic thrombocytopenic purpura; Interstitial
cystitis; Systemic lupus
erythematosus (SLE); Metabolic syndrome; Multiple sclerosis; Myasthenia
gravis; Myocarditis;
Narcolepsy; Obesity; Pemphigus Vulgaris; Pernicious anaemia; Polymyositis;
Primary biliary
cirrhosis; Rheumatoid arthritis; Schizophrenia; Scleroderma; Sjogren's
syndrome; Vasculitis;
Vitiligo; Wegener's granulomatosis; Allergic rhinitis; Prostate cancer; Non-
small cell lung
carcinoma; Ovarian cancer; Breast cancer; Melanoma; Gastric cancer; Colorectal
cancer; Brain
cancer; Metastatic bone disorder; Pancreatic cancer; bladder cancer;
hepatocellular cancer; liver
cancer; adenocarcinoma of the lung; esophageal squamous cell carcinoma; CNS
tumors;
hematological tumors; a Lymphoma; Nasal polyps; Gastrointestinal cancer;
Ulcerative colitis;
Crohn's disorder; Collagenous colitis; Lymphocytic colitis; Ischaemic colitis;
Diversion colitis;
Behcet's syndrome; Infective colitis; Indeterminate colitis; Inflammatory
liver disorder; Endotoxin
shock; Septic shock; Rheumatoid spondylitis; Ankylosing spondylitis; Gouty
arthritis; Polymyalgia
rheumatica; Alzheimer's disorder; Parkinson's disorder; Epilepsy; AIDS
dementia; Asthma; Adult
respiratory distress syndrome; Bronchitis; Cystic fibrosis; Acute leukocyte-
mediated lung injury;
Distal proctitis; Wegener's granulomatosis; Fibromyalgia; Bronchitis;
;Uveitis; Conjunctivitis;
Psoriasis; Eczema; Dermatitis; Smooth muscle proliferation disorders;
Meningitis; Shingles;
Encephalitis; Nephritis; Tuberculosis; Retinitis; Atopic dermatitis;
Pancreatitis; Periodontal
gingivitis; Coagulative Necrosis; Liquefactive Necrosis; Fibrinoid Necrosis;
Neointimal hyperplasia;
Myocardial infarction; Stroke; organ transplant rejection; influenza, or
combinations thereof.
[0044] Disclosed herein, in certain embodiments, is the use of a composition
of matter described
herein to treat, prevent or reduce angiogenesis.
[0045] Disclosed herein, in certain embodiments, is a pharmaceutical
composition for treating an
inflammatory disease, disorder, condition or symptom in an individual in need
thereof, comprising a
composition of matter described herein.
-9-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Definitions
[0046] The terms "individual," "subject," or "patient" are used
interchangeably. As used herein,
they mean any mammal (i.e. species of any orders, families, and genus within
the taxonomic
classification animalia: chordata: vertebrata: mammalia). In some embodiments,
the mammal is a
human. In some embodiments, the mammal is a non-human. In some embodiments,
the mammal is a
member of the taxonomic orders: primates (e.g. lemurs, lorids, galagos,
tarsiers, monkeys, apes, and
humans); rodentia (e.g. mice, rats, squirrels, chipmunks, and gophers);
lagomorpha (e.g. hares,
rabbits, and pika); erinaceomorpha (e.g. hedgehogs and gymnures); soricomorpha
(e.g. shrews,
moles, and solenodons); chiroptera (e.g., bats); cetacea (e.g. whales,
dolphins, and porpoises);
carnivora (e.g. cats, lions, and other feliformia; dogs, bears, weasels, and
seals); perissodactyla (e.g.
horse, zebra, tapir, and rhinoceros); artiodactyla (e.g. pigs, camels, cattle,
and deer); proboscidea
(e.g. elephants); sirenia (e.g. manatees, dugong, and sea cows); cingulata
(e.g. armadillos); pilosa
(e.g. anteaters and sloths); didelphimorphia (e.g. american opossums);
paucituberculata (e.g. shrew
opossums); microbiotheria (e.g. Monito del Monte); notoryctemorphia (e.g.
marsupial moles);
dasyuromorphia (e.g. marsupial carnivores); peramelemorphia (e.g. bandicoots
and bilbies); or
diprotodontia (e.g. wombats, koalas, possums, gliders, kangaroos, wallaroos,
and wallabies). In
some embodiments, the animal is a reptile (i.e. species of any orders,
families, and genus within the
taxonomic classification animalia: chordata: vertebrata: reptilia). In some
embodiments, the animal
is a bird (i.e. animalia: chordata: vertebrata: ayes). None of the terms
require or are limited to
situation characterized by the supervision (e.g. constant or intermittent) of
a health care worker (e.g.
a doctor, a registered nurse, a nurse practitioner, a physician's assistant,
an orderly, or a hospice
worker).
[0047] The phrase "specifically binds" when referring to the interaction
between a binding
molecule (i.e., the agent; e.g., a peptide or peptide mimetic) and a protein
or polypeptide or epitope,
typically refers to a binding molecule that recognizes and detestably
specifically binds with high
affinity to the target of interest. Preferably, under designated or
physiological conditions, the
specified antibodies or binding molecules bind to a particular polypeptide,
protein or epitope yet
does not bind in a significant or undesirable amount to other molecules
present in a sample. In other
words the specified antibody or binding molecule does not undesirably cross-
react with non-target
antigens and/or epitopes. A variety of immunoassay formats are used to select
antibodies or other
binding molecule that are immunoreactive with a particular polypeptide and
have a desired
specificity. For example, solid-phase ELISA immunoassays, BlAcore, flow
cytometry and
radioimmunoassays are used to select monoclonal antibodies having a desired
immunoreactivity and
specificity. See, Harlow, 1988, ANTIBODIES, A LABORATORY MANUAL, Cold Spring
Harbor
Publications, New York (hereinafter, "Harlow"), for a description of
immunoassay formats and
conditions that are used to determine or assess immunoreactivity and
specificity.
-10-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0048] "Selective binding," "selectivity," and the like refer the preference
of agent to interact with
one molecule as compared to another. Preferably, interactions between an agent
disclosed herein and
proteins are both specific and selective. Note that in some embodiments an
agent is designed to
"specifically bind" and "selectively bind" two distinct, yet similar targets
without binding to other
undesirable targets.
[0049] The terms "polypeptide," "peptide" and "protein" are used
interchangeably herein to refer to
a polymer of amino acid residues. The terms apply to naturally occurring amino
acid polymers as
well as amino acid polymers in which one or more amino acid residues is a non-
naturally occurring
amino acid (e.g., an amino acid analog). The terms encompass amino acid chains
of any length,
including full length proteins (i.e., antigens), wherein the amino acid
residues are linked by covalent
peptide bonds.
[0050] The terms "motif' and "domain" are used interchangeably. As used
herein, they mean a
discrete, contiguous or non-contiguous portion of a polypeptide that folds
independently of the rest
of the polypeptide and possesses its own function.
[0051] The term "disruption" means to interfere with the function of. For
example, to disrupt a
motif/domain means to interfere with the function of the motif/domain.
[0052] The term "antigen" refers to a substance that is capable of inducing
the production of an
antibody. In some embodiments an antigen is a substance that specifically
binds to an antibody
variable region.
[0053] The terms "antibody" and "antibodies" refer to monoclonal antibodies,
polyclonal
antibodies, bi-specific antibodies, multispecific antibodies, grafted
antibodies, human antibodies,
humanized antibodies, synthetic antibodies, chimeric antibodies, camelized
antibodies, single-chain
Fvs (scFv), single chain antibodies, Fab fragments, F(ab') fragments,
disulfide-linked Fvs (sdFv),
intrabodies, and anti-idiotypic (anti-Id) antibodies and antigen-binding
fragments of any of the
above. In particular, antibodies include immunoglobulin molecules and
immunologically active
fragments of immunoglobulin molecules, i.e., molecules that contain an antigen
binding site.
Depending on the amino acid sequence of the constant motif/domain of their
heavy chains,
immunoglobulins can be assigned to different classes. The heavy-chain constant
motif/domains (Fc)
that correspond to the different classes of immunoglobulins are called a., 6,
c, y, and , respectively.
The subunit structures and three-dimensional configurations of different
classes of immunoglobulins
are well known. Immunoglobulin molecules are of any type (e.g., IgG, IgE, IgM,
IgD, IgA and IgY),
class (e.g., IgG i, IgG 2, IgG 3, IgG 4, IgA 1 and IgA 2) or subclass. The
terms "antibody" and
"immunoglobulin" are used interchangeably in the broadest sense. In some
embodiments an
antibody is part of a larger molecule, formed by covalent or non-covalent
association of the antibody
with one or more other proteins or peptides.
-11-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00541 With respect to antibodies, the term "variable motif/domain" refers to
the variable
motif/domains of antibodies that are used in the binding and specificity of
each particular antibody
for its particular antigen. However, the variability is not evenly distributed
throughout the variable
motif/domains of antibodies. Rather, it is concentrated in three segments
called hypervariable
regions (also known as CDRs) in both the light chain and the heavy chain
variable motif/domains.
More highly conserved portions of variable motif/domains are called the
"framework regions" or
"FRs." The variable motif/domains of unmodified heavy and light chains each
contain four FRs
(FRI, FR2, FR3 and FR4), largely adopting a (3-sheet configuration
interspersed with three CDRs
which form loops connecting and, in some cases, part of the (3-sheet
structure. The CDRs in each
chain are held together in close proximity by the FRs and, with the CDRs from
the other chain,
contribute to the formation of the antigen-binding site of antibodies (see
Kabat et al., Sequences of
Proteins of Immunological Interest, 5th Ed. Public Health Service, National
Institutes of Health,
Bethesda, Md. (1991), pages 647-669).
[00551 The terms "hypervariable region" and "CDR" when used herein, refer to
the amino
acid residues of an antibody which are responsible for antigen-binding. The
CDRs comprise amino
acid residues from three sequence regions which bind in a complementary manner
to an antigen and
are known as CDRI, CDR2, and CDR3 for each of the VH and VL chains. In the
light chain variable
motif/domain, the CDRs typically correspond to approximately residues 24-34
(CDRL1), 50-56
(CDRL2) and 89-97 (CDRL3), and in the heavy chain variable motif/domain the
CDRs typically
correspond to approximately residues 31-35 (CDRHI), 50-65 (CDRH2) and 95-102
(CDRH3)
according to Kabat et al., Sequences of Proteins of Immunological Interest,
5th Ed. Public Health
Service, National Institutes of Health, Bethesda, Md. (1991)). It is
understood that the CDRs of
different antibodies may contain insertions, thus the amino acid numbering may
differ. The Kabat
numbering system accounts for such insertions with a numbering scheme that
utilizes letters
attached to specific residues (e.g., 27A, 27B, 27C, 27D, 27E, and 27F of CDRLI
in the light chain)
to reflect any insertions in the numberings between different antibodies.
Alternatively, in the light
chain variable motif/domain, the CDRs typically correspond to approximately
residues 26-32
(CDRL1), 50-52 (CDRL2) and 91-96 (CDRL3), and in the heavy chain variable
motif/domain, the
CDRs typically correspond to approximately residues 26-32 (CDRHI), 53-55
(CDRH2) and 96-101
(CDRH3) according to Chothia and Lesk, J. Mol. Biol., 196: 901-917 (1987)).
[00561 Constant motif/domains (Fc) of antibodies are not involved directly in
binding an
antibody to an antigen but, rather, exhibit various effector functions, such
as participation of the
antibody in antibody-dependent cellular toxicity via interactions with, for
example, Fc receptors
(FcR). Fc motif/domains can also increase bioavailability of an antibody in
circulation following
administration to a patient.
-12-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0057] As used herein, the term "affinity" refers to the equilibrium constant
for the reversible
binding of two agents and is expressed as Kd. Affinity of a binding protein to
a ligand such as
affinity of an antibody for an epitope can be, for example, from about 100
nanomolar (nM) to about
0.1 nM, from about 100 nM to about 1 picomolar (pM), or from about 100 nM to
about 1
femtomolar (fM). As used herein, the term "avidity" refers to the resistance
of a complex of two or
more agents to dissociation after dilution.
[0058] The term "peptibody" refers to a molecule comprising peptide(s) fused
either directly or
indirectly to an antibody or one or more antibody motif/domains (e.g., an Fc
motif/domain of an
antibody), where the peptide moiety specifically binds to a desired target.
The peptide(s) may be
fused to either an Fc region or inserted into an Fc- Loop, a modified Fc
molecule. The term
"peptibody" does not include Fc-fusion proteins (e.g., full length proteins
fused to an Fc
motif/domain).
[0059] The terms "isolated" and "purified" refer to a material that is
substantially or essentially
removed from or concentrated in its natural environment. For example, an
isolated nucleic acid is
one that is separated from at least some of the nucleic acids that normally
flank it or other nucleic
acids or components (proteins, lipids, etc.) in a sample. In another example,
a polypeptide is purified
if it is substantially removed from or concentrated in its natural
environment. Methods for
purification and isolation of nucleic acids and proteins are documented
methodologies.
Embodiments of "substantially" include at least 20%, at least 40%, at least
50%, at least 75%, at
least 85%, at least 90%, at least 95%, or at least 99%.
[0060] The terms "treat," "treating" or "treatment," and other grammatical
equivalents as used
herein, include alleviating, inhibiting or reducing symptoms, reducing or
inhibiting severity of,
reducing incidence of, prophylactic treatment of, reducing or inhibiting
recurrence of, preventing,
delaying onset of, delaying recurrence of, abating or ameliorating a disease
or condition symptoms,
ameliorating the underlying metabolic causes of symptoms, inhibiting the
disease or condition, e.g.,
arresting the development of the disease or condition, relieving the disease
or condition, causing
regression of the disease or condition, relieving a condition caused by the
disease or condition, or
stopping the symptoms of the disease or condition. The terms further include
achieving a therapeutic
benefit. By therapeutic benefit is meant eradication or amelioration of the
underlying disorder being
treated, and/or the eradication or amelioration of one or more of the
physiological symptoms
associated with the underlying disorder such that an improvement is observed
in the individual.
[0061] The terms "prevent," "preventing" or "prevention," and other
grammatical equivalents as
used herein, include preventing additional symptoms, preventing the underlying
metabolic causes of
symptoms, inhibiting the disease or condition, e.g., arresting the development
of the disease or
condition and are intended to include prophylaxis. The terms further include
achieving a
prophylactic benefit. For prophylactic benefit, the compositions are
optionally administered to an
-13-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
individual at risk of developing a particular disease, to an individual
reporting one or more of the
physiological symptoms of a disease, or to an individual at risk of
reoccurrence of the disease.
[0062] The terms "effective amount" or "therapeutically effective amount" as
used herein,
refer to a sufficient amount of at least one agent being administered which
achieve a desired result,
e.g., to relieve to some extent one or more symptoms of a disease or condition
being treated. In
certain instances, the result is a reduction and/or alleviation of the signs,
symptoms, or causes of a
disease, or any other desired alteration of a biological system. In specific
instances, the result is a
decrease in the growth of, the killing of, or the inducing of apoptosis in at
least one abnormally
proliferating cell, e.g., a cancer stem cell. In certain instances, an
"effective amount" for therapeutic
uses is the amount of the composition comprising an agent as set forth herein
required to provide a
clinically significant decrease in a disease. An appropriate "effective"
amount in any individual case
is determined using any suitable technique, such as a dose escalation study.
[0063] The terms "administer," "administering," "administration," and the
like, as used herein,
refer to the methods that are used to enable delivery of agents or
compositions to the desired site of
biological action. These methods include, but are not limited to oral routes,
intraduodenal routes,
parenteral injection (including intravenous, subcutaneous, intraperitoneal,
intramuscular,
intravascular or infusion), topical and rectal administration. Administration
techniques that are
optionally employed with the agents and methods described herein, include
e.g., as discussed in
Goodman and Gilman, The Pharmacological Basis of Therapeutics, current ed.;
Pergamon; and
Remington's, Pharmaceutical Sciences (current edition), Mack Publishing Co.,
Easton, Pa. In some
embodiments, the agents and compositions described herein are administered
orally.
[0064] The term "pharmaceutically acceptable" as used herein, refers to a
material that does not
abrogate the biological activity or properties of the agents described herein,
and is relatively
nontoxic (i.e., the toxicity of the material significantly outweighs the
benefit of the material). In
some instances, a pharmaceutically acceptable material is administered to an
individual without
causing significant undesirable biological effects or significantly
interacting in a deleterious manner
with any of the components of the composition in which it is contained.
1. Macrophage Migration Inhibitory Factor (MIF)
[0065] Disclosed herein, in some embodiments, are peptides, small molecules,
antibodies, and
peptibodies (collectively, "compositions of matter") for treating inflammatory
diseases, disorders,
conditions and symptoms. Further disclosed herein are pharmaceutical
compositions and methods of
treating inflammatory diseases, disorders, conditions and symptoms.
[0066] MIF is a pro-inflammatory cytokine. In certain instances, it is
secreted by activated immune
cells (e.g. a lymphocyte (T-cell)) in response to an infection, inflammation,
or tissue injury. In
certain instances, MIF is a ligand for the receptors CXCR2, CXCR4, CD44, and
CD74. In some
-14-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
inhibits (partially or fully) the activity of CXCR2 CXCR4, CD44, and/or CD74.
[0067] In certain instances, MIF induces chemotaxis in nearby leukocytes (e.g.
lymphocytes,
granulocytes, monocytes/macrophages, and TH- 17 cells) along a MIF gradient.
In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
prevents chemotaxis along a MIF gradient, or reduces chemotaxis along a MIF
gradient. In certain
instances, MIF induces the chemotaxis of a leukocyte (e.g. lymphocytes,
granulocytes,
monocytes/macrophages, and TH- 17 cells) to the site of an infection,
inflammation or tissue injury.
In some embodiments, a composition of matter, method and/or pharmaceutical
composition
disclosed herein prevents or decreases the chemotaxis of a leukocyte to the
site of an infection,
inflammation or tissue injury. In certain instances, the chemotaxis of a
leukocyte (e.g. lymphocytes,
granulocytes, monocytes/macrophages, and TH- 17 cells) along a MIF gradient
results in
inflammation at the site of infection, inflammation, or tissue injury. In some
embodiments, a
composition of matter, method and/or pharmaceutical composition disclosed
herein treats
inflammation at the site of infection, inflammation, or tissue injury. In
certain instances, the
chemotaxis of monocytes along a RANTES gradient results in monocyte arrest
(i.e., the deposition
of monocytes on epithelium) at the site of injury or inflammation. In some
embodiments, a
composition of matter, method and/or pharmaceutical composition disclosed
herein prevents or
decreases monocyte arrest at the site of injury or inflammation. In some
embodiments, a
composition of matter, method and/or pharmaceutical composition disclosed
herein inhibits treats a
lymphocyte mediated disorder. In some embodiments, a composition of matter,
method and/or
pharmaceutical composition disclosed herein treats a granulocyte mediated
disorder. In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
treats a macrophage mediated disorder. In some embodiments, a composition of
matter, method
and/or pharmaceutical composition disclosed herein treats a Th- 17 mediated
disorder. In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
treats a pancreatic beta-cell mediated disorder.
[0068] In certain instances, MIF is inducible by glucocorticoids, a mechanism
implicated in an
acceleration of atherosclerosis associated with many diseases requiring
glucocorticoid therapy.
Thus, in some embodiments, the compositions and methods described herein
inhibit the induction of
MIF expression by glucocorticoids.
[0069] Human MIF peptide is encoded by a nucleotide sequence located on
chromosome 22 at the
cytogenic band 22g11.23.
[0070] In certain instances, a mature MIF protein is a homotrimer comprising
three polypeptides of
about 114 amino acids; the first methionine having been removed during
translation from each of
the MIF peptide monomers.
-15-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0071] In certain instances, a human MIF peptide is encoded by the nucleic
acid sequence SEQ ID
No. 422:
GGTACCGGATCCCCCATGTTCATCGTGAACACCAACGTGCCCAGAGCCAGCGTGCCCGAC
GCTTCCTGAGCGAGCTGACACAGCAGCTGGCCCAGGCCACCGGCAAGCCCCCTCAGTAT
ATCGCCGTGCACGTGGTGCCCGACCAGCTGATGGCCTTCGGCGGCAGCAGCGAGCCTTGC
GCCCTGTGTAGCCTGCACAGCATCGGCAAGATCGGCGGAGCCCAGAACAGAAGCTACAGC
AAGCTGCTGTGCGGCCTGCTGGCCGAGAGACTGAGAATCAGCCCCGACAGAGTGTACATC
AACTACTACGACATGAACGCCGCCAACGTGGGCTGGAACAACAGCACCTTCGCCCTCGAG
CTC
[0072] In certain instances, a human MIF peptide is encoded by SEQ ID No. 1:
MPMFIVNTNVPRASVPDGFLSELTQQLAQATGKPPQYIAVHVVPDQLMA
FGGSSEPCALCSLHSIGKIGGAQNRSYSKLLCGLLAERLRISPDRVYINYY
DMNAANVGWNGSTFAL (SEQ ID 1).
[0073] In certain instances, a porcine MIF peptide is encoded by SEQ ID No. 2:
MPMFVVNTNVPRASVPDGFLSELTQQLVQAMGKPAQYIAVHVVPDQLM
AFGGSSEPCALCSLHSIGKIGGAQNRSYSKLLCGLLAERLRISPDRIYINYY
DMNAANVGWNGSTFAL (SEQ ID No. 2).
[0074] In certain instances, a bovine MIF peptide is encoded by SEQ ID No. 3:
MPMFV VNTNVPRASV PDGLLSELTQQLAQATGKPAQYIAVHV VPDQLM
TFGGSSEPCALCSLHSIGKIGGAQNRSYSKLLCGLLTERLRISPDRIYINFC
DMNAANVGWNGSTFAL (SEQ ID No. 3).
[0075] In certain instances, a murine MIF peptide is encoded by SEQ ID No. 4:
MPMFIVNTNVPRAS VPEGFLSELTQQLAQATGKPPAYIAVHV VPDQLMT
FS GTNDPCALC SLHSIGKIGGAQNRNYSKLLCGLLSDRLHISPDRVYINYY
DMNAANVGWGNSTFAL (SEQ ID No. 4).
[0076] In certain instances, a rat MIF peptide is encoded by SEQ ID No. 5:
MPMFIVNTNVPRAS VPEGFLSELTQQLAQATGKPPAYIAVHV VPDQLMT
FSGTSDPCALCSLHSIGKIGGAQNRNYSKLLCGLLSDRLHISPDRVYINYY
DMNAANVGWGNSTFAL (SEQ ID No. 5).
[0077] In some embodiments, a peptide disclosed herein comprises a sequence
that competitively
binds with a binding partner of the MIF pseudo ELR motif/domain. The pseudo
ELR motif/domain
comprises two nonadjacent but adequately spaced residues (Arg12 and Asp45 &
see Fig. 11). The
pseudo ELR motif/domain comprises the amino acid sequence from amino acid 12
to amino acid 45
(this numbering includes the first methionine residue). This is equivalent to
a pseudo ELR
motif/domain from amino acid 11 to amino acid 44 in which the first methionine
residue is not
counted (in such instances, the pseudo ELR motif/domain comprises Arg 11 and
Asp 44). In some
-16-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
inhibits binding of the pseudo ELR motif/domain to CXCR2 and/or CXCR4. In some
embodiments,
a composition of matter, method and/or pharmaceutical composition disclosed
herein treats
inflammatory diseases, disorders, conditions and symptoms by inhibiting
binding of the pseudo ELR
motif/domain to CXCR2 and/or CXCR4.
[0078] A MIF peptide comprises a 10- to 20-residue N-terminal Loop
motif/domain (N-loop). In
certain instances, a MIF N-loop mediates binding to a CXCR2 and/or CXCR4
receptor. In certain
instances, the N-loop motif/domain of MIF comprises the sequential residues 44-
57 of MIF (i.e.,
P45 D45 Q46 L47 M48 A49 F50 G51 G52 S53 S54 E55 P56 C57; see FIG. 11), where
the first
methionine is included. This is equivalent to amino acid 43 to amino acid 56
in which the first
methionine residue is not counted. In certain instances, the N-loop
motif/domain of MIF comprises
amino acids 45-60, where the first methionine is included. In certain
instances, the N-loop
motif/domain of MIF comprises amino acids 44-61, where the first methionine is
included. In
certain instances, the N-loop motif/domain of MIF comprises amino acids 43-62.
In certain
instances, the N-loop motif/domain of MIF comprises amino acids 42-63, where
the first methionine
is included. In certain instances, the N-loop motif/domain of MIF comprises
amino acids 41-64,
where the first methionine is included. In certain instances, the N-loop
motif/domain of MIF
comprises amino acids 40-65, where the first methionine is included. In
certain instances, the N-loop
motif/domain of MIF comprises amino acids 46-59, where the first methionine is
included. In
certain instances, the N-loop motif/domain of MIF comprises amino acids 47-59,
where the first
methionine is included. In certain instances, the N-loop motif/domain of MIF
comprises amino acids
48-59, where the first methionine is included. In certain instances, the N-
loop motif/domain of MIF
comprises amino acids 50-59, where the first methionine is included. In
certain instances, the N-loop
motif/domain of MIF comprises amino acids 47-58, where the first methionine is
included. In
certain instances, the N-loop motif/domain of MIF comprises amino acids 47-57,
where the first
methionine is included. In certain instances, the N-loop motif/domain of MIF
comprises amino acids
47-56, where the first methionine is included. In certain instances, the N-
loop motif/domain of MIF
comprises amino acids 48-58, where the first methionine is included. In some
embodiments the N-
loop motif/domain comprises amino acids 48-57, where the first methionine is
included. In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
inhibits binding of the N-loop motif/domain to CXCR2 and/or CXCR4. In some
embodiments, a
composition of matter, method and/or pharmaceutical composition disclosed
herein treats
inflammatory diseases, disorders, conditions and symptoms by inhibiting
binding of the N-loop
motif/domain to CXCR2 and/or CXCR4.
[0079] A MIF polypeptide comprises the following motifs/domains: an N-
terminal/pseudo-ELR
motif/domain (MIFi_17), an alpha-helix #1 motif/domain (i.e., MIF18_31), an
MIF N-loop
-17-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
motif/domain (i.e., MIF32_60), a loop-barrel-loop motif/domain (i.e.,
MIF64_93), and a C-terminal
motif/domain (i.e., MIF90_114). Alternatively, a MIF polypeptide comprises the
following
motifs/domains: an N-terminal tail (i.e., MIF1_7), a pseudo ELR-loop (i.e.,
MIF7_17), an alpha-helix
#1 motif/domain (i.e., MIF18_31), a PPQ-loop (i.e., MIF32-38), a PDQ-loop
(i.e., MIF43-56), an IGK-
loop (i.e., MIF64_71), an NRS-helix (i.e., MIF72_89), a SPDR-loop (i.e.,
MIF90_94), and a C-terminal tail
(i.e., MlF1o1_114). In some embodiments, a peptide disclosed herein
competitively binds with a
binding partner of one of the following domains: N-terminal/pseudo-ELR
motif/domain (MIF1_17),
the alpha-helix #1 motif/domain (i.e., M1F18-31), the MIF N-loop motif/domain
(i.e., MIF32-60), the
loop-barrel-loop motif/domain (i.e., MIF64_93), the C-terminal motif/domain
(i.e., MIF9o_114), or a
combination of any of the aforementioned domains. In some embodiments, a
peptide disclosed
herein competitively binds with a binding partner of one of the following
domains: N-terminal tail
(i.e., MIF1_7), the pseudo ELR-loop (i.e., MIF7_17), the alpha-helix #1
motif/domain (i.e., MIF18_31),
the PPQ-loop (i.e., MIF32-38), the PDQ-loop (i.e., MIF43-56), the IGK-loop
(i.e., MIF64.71), the NRS-
helix (i.e., MIF72_89), the SPDR-loop (i.e., MIF90_94), the C-terminal tail
(i.e., MIF101-114), or a
combination of any of the aforementioned domains. In some embodiments, a
peptide disclosed
herein competitively binds with a binding partner of MIF47 (leucine).
[0080] In some embodiments, a composition of matter, method and/or
pharmaceutical composition
disclosed herein inhibits (1) binding of the N-loop motif/domain to CXCR2
and/or CXCR4; and (2)
binding of the pseudo ELR motif/domain to CXCR2 and/or CXCR4. In some
embodiments, a
composition of matter, method and/or pharmaceutical composition disclosed
herein treats
inflammatory diseases, disorders, conditions and symptoms by inhibiting (1)
binding of the N-loop
motif/domain to CXCR2 and/or CXCR4; and (2) binding of the pseudo ELR
motif/domain to
CXCR2 and/or CXCR4.
II. Active Agents
[0081] Disclosed herein, in some embodiments, are peptides, small molecules,
antibodies, and
peptibodies (collectively, "compositions of matter") for treating inflammatory
diseases, disorders,
conditions and symptoms. Further disclosed herein are pharmaceutical
compositions and methods of
treating inflammatory diseases, disorders, conditions and symptoms.
[0082] Additionally disclosed herein, in some embodiments, are compositions of
matter, methods,
and pharmaceutical compositions that inhibit the ability of MIF to bind to
CXCR2, CXCR4, CD44,
CD74, or a combination thereof. Further disclosed herein, are compositions of
matter, methods, and
pharmaceutical compositions that treat inflammatory diseases, disorders,
conditions and symptoms
by inhibiting the ability of MIF to bind to CXCR2, CXCR4, CD44, CD74, or a
combination thereof.
In certain instances, occupying, masking, or otherwise disrupting
motif/domains on MIF does not
affect CXCR2, CXCR4, CD44 and/or CD74 signaling mediated by other
agonists/ligands (e.g.,
-18-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
CXCR2 interactions with IL-8/CXCL8, GROG/CXCL2, GROa., GRO-y, ENA78, NAP2; and
CXCR4 interactions with Stromal Cell-Derived Factor-la (SDF-la)/CXCL12, and
GP120).
[0083] Any amino acid in any of the peptides disclosed herein may be
substituted with an unnatural
or natural amino acid that corresponds to and functions as an effective
substitute for the original
amino acid, but does not substantially diminish binding or bioactivity
relative to the parent peptide
sequence. The phrase "does not substantially diminish binding or bioactivity
relative to the parent
peptide sequence" means the modified peptide sequence has about 60%, about
65%, about 70%,
about 75%, about 80%, about 85%, about 90%, or about 95% of the same activity
of the parent
peptide sequence. Unnatural amino acids include, but are not limited to: D-
amino acids such as D-
phenylalanine (D-F) and D-cyclohexyl alanine (D-CA); norLeucine (NLe), L-
cyclohexyl alanine (L-
CA), and L-amino acid, alpha-aminobutyric acid (Abu). In some embodiments, an
amino acid of a
peptide disclosed herein is substituted with a non-natural amino acid. In some
embodiments, an
amino acid of a peptide disclosed herein comprises N- and/or C-terminal
chemical modifications to
improve ADME-PK.
Disruption of MIF Motifs/Domains
[0084] In some embodiments, a composition of matter inhibits the ability of
MIF to interact with
CXCR2, CXCR4, CD74, CD44 or a combination thereof. In some embodiments, an
inflammatory
disease, disorder, condition and symptom is treated, diagnosed, or monitored
by disrupting the
ability of MIF to interact with CXCR2, CXCR4, CD74, CD44 or a combination
thereof. In some
embodiments, the ability of MIF to interact with CXCR2, CXCR4, CD74, CD44 or a
combination
thereof is inhibited by occupying, masking, or otherwise disrupting
motif/domains on MIF to which
CXCR2, CXCR4, CD74 and/or CD44 bind. In some embodiments, the ability of MIF
to interact
with CXCR2, CXCR4, CD74, CD44 or a combination thereof is inhibited by
occupying, masking,
or otherwise disrupting all or a portion of a motif/domain selected from: an N-
terminal/pseudo-ELR
motif/domain (MIF1_17), an alpha-helix #1 motif/domain (i.e., MIF18_31), an
MIF N-loop
motif/domain (i.e., MIF32_60), a loop-barrel-loop motif/domain (i.e.,
MIF64_93), and a C-terminal
motif/domain (i.e., MIF90_ii4). In some embodiments, the ability of MIF to
interact with CXCR2,
CXCR4, CD74, CD44 or a combination thereof is inhibited by occupying, masking,
or otherwise
disrupting all or a portion of a motif/domain selected from: an N-terminal
tail (i.e., MIF1_7), a pseudo
ELR-loop (i.e., MIF7_17), an alpha-helix #1 motif/domain (i.e., MIF18_31), a
PPQ-loop (i.e., MIF32-
38), a PDQ-loop (i.e., MIF43-56), an IGK-loop (i.e., MIF64_71), an NRS-helix
(i.e., MIF72_89), a
SPDR-loop (i.e., MIF90_94), and a C-terminal tail (i.e., MlFioi_114). In some
embodiments, the ability
of MIF to interact with CXCR2, CXCR4, CD74, CD44 or a combination thereof is
inhibited by
occupying, masking, or otherwise disrupting MIF47 (leucine).
[0085] In some embodiments, the ability of MIF to interact with CXCR2, CXCR4,
CD74, CD44 or
a combination thereof is inhibited by a small molecule, peptide, antibody,
and/or peptibody
-19-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
occupying, masking, or otherwise disrupting one or more motifs/domains on MIF
to which CXCR2,
CXCR4, CD74 and/or CD44 bind. In certain instances, occupying, masking, or
otherwise disrupting
one or more motifs/domains on MIF does not affect CXCR2 and CXCR4 signaling
mediated by
other agonists/ligands (e.g., IL-8/CXCL8, GRObeta/CXCL2 and/or Stromal Cell-
Derived Factor-la
(SDF-la)/CXCL12).
[0086] In certain instances, the pseudo-ELR motif/domain of MIF mediates
ligand (e.g., CD44,
CD74, CXCR2, CXCR4) binding to MIF. In some embodiments, the binding of a
small molecule,
peptide, antibody, and/or peptibody to all or a portion of thepseudo ELR
motif/domain of MIF
inhibits the ability of MIF to bind to CXCR2, CXCR4, CD74, CD44 or a
combination thereof. In
some embodiments, the binding of a small molecule, peptide, antibody, and/or
peptibody to all or a
portion of theN-terminal tail (i.e., MIF1_7) and/or all or a portion of
thepseudo ELR-loop (i.e., MIF7_
17) inhibits the ability of MIF to bind to CXCR2, CXCR4, CD74, CD44 or a
combination thereof.
[0087] In certain instances, the N-loop motif/domain of MIF mediates ligand
(e.g., CD44, CD74,
CXCR2, CXCR4) binding to MIF. In some embodiments, the binding of a small
molecule, peptide,
antibody, and/or peptibody to all or a portion of theN-loop motif/domain of
MIF inhibits the ability
of MIF to bind to CXCR2, CXCR4, CD74, CD44 or a combination thereof. In some
embodiments,
the binding of a small molecule, peptide, antibody, and/or peptibody to all or
a portion of thePPQ-
loop (i.e., MIF32_38) and/or all or a portion of thePDQ-loop (i.e., MIF43.56)
inhibits the ability of MIF
to to bind to CXCR2, CXCR4, CD74, CD44 or a combination thereof. In some
embodiments, the
binding of a small molecule, peptide, antibody, and/or peptibody to all or a
portion of theN-loop
motif/domain of MIF invokes a conformational change in MIF that prevents
receptor or substrate
interactions. In some embodiments, the binding of a small molecule, peptide,
antibody, and/or
peptibody to all or a portion of thePPQ-loop (i.e., MIF32_38) and/or all or a
portion of thePDQ-loop
(i.e., MIF43-56) invokes a conformational change in MIF that prevents receptor
or substrate
interactions.
[0088] In certain instances, amino acids 65-94 of MIF (e.g.,
IGKIGGAQNRSYSKLLCGLLAERLRISPDR (SEQ ID No. 8); numbering includes the first
methionine) mediate CXCR2 binding to MIF. In some embodiments, the binding of
a small
molecule, peptide, antibody, and/or peptibody to all or a portion of amino
acids 65-94 of MIF
inhibits the ability of MIF to bind to CXCR2. In some embodiments, the binding
of a peptide to all
or a portion of amino acids 65-94 of MIF inhibits the ability of MIF to bind
to CXCR2. In some
embodiments, the binding of an antibody to all or a portion of amino acids 65-
94 of MIF inhibits the
ability of MIF to bind to CXCR2. In some embodiments, the binding of a
peptibody to all or a
portion of amino acids 65-94 of MIF inhibits the ability of MIF to bind to
CXCR2. In some
embodiments, the binding of a small molecule to amino acids all or a portion
of 65-94 of MIF
inhibits the ability of MIF to bind to CXCR2.
-20-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0089] In certain instances, amino acids 80-95 of MIF (e.g., LCGLLAERLRISPDRV
(SEQ ID No.
9); numbering includes the first methionine) mediate ligand binding to MIF. In
some embodiments,
the binding of a small molecule, peptide, antibody, and/or peptibody to all or
a portion of amino
acids 80-95 of MIF inhibits the ability of MIF to bind to a ligand. In some
embodiments, the binding
of a peptide to all or a portion of amino acids 80-95 of MIF inhibits the
ability of MIF to bind to a
ligand. In some embodiments, the binding of an antibody to all or a portion of
amino acids 80-95 of
MIF inhibits the ability of MIF to bind to a ligand. In some embodiments, the
binding of a peptibody
to all or a portion of amino acids 80-95 of MIF inhibits the ability of MIF to
bind to a ligand. In
some embodiments, the binding of a small molecule to all or a portion of amino
acids 80-95 of MIF
inhibits the ability of MIF to bind to a ligand.
[0090] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed or monitored by administering a peptide that occupies, masks, or
otherwise disrupts all or
a portion of a motif/domain on MIF to which CXCR2, CXCR4, CD74 and/or CD44
binds. In some
embodiments, the peptide specifically binds to all or a portion of the pseudo
ELR motif/domain of
MIF. In some embodiments, the peptide specifically binds to all or a portion
of the N-loop
motif/domain of MIF. In some embodiments, the peptide specifically binds to
all or a portion of both
the pseudo-ELR and N-loop motifs.
[0091] In some embodiments, the agent is a peptide that specifically binds to
all or a portion of a
peptide sequence as follows: VNTNVPPRASVPDGFLSELTQQLAQATGKPPQYIAVHVVPDQL
(SEQ ID No. 10) and the corresponding feature/domain of at least one of a MIF
monomer or MIF
trimer; a peptide that specifically binds to all or a portion of a peptide
sequence as follows:
PDQLMAFGGSSEPCALCSL (SEQ ID No. 11) and the corresponding feature/domain of at
least
one of a MIF monomer or MIF trimer; a peptide that specifically binds to all
or a portion of a
peptide sequence as follows:
VNTNVPPRASVPDGFLSELTQQLAQATGKPPQYIAVHVVPDQLMAFGGSSEPCALCSL
(SEQ ID No. 12) and the corresponding feature/domain of at least one of a MIF
monomer or MIF
trimer; a peptide that specifically binds to all or a portion of a peptide
sequence as follows:
PDQLMAFGGSSEPCALCSLHSI (SEQ ID No. 13) and the corresponding feature/domain of
at
least one of a MIF monomer or MIF trimer; or combinations thereof.
[0092] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed or monitored by administering an antibody that occupies, masks, or
otherwise disrupts all
or a portion of amotif/domain on MIF to which CXCR2, CXCR4, CD74 and/or CD44
binds. In
some embodiments, the antibody specifically binds to all or a portion of the
pseudo ELR
motif/domain of MIF. In some embodiments, the antibody specifically binds to
all or a portion of the
N-loop motif/domain of MIF. In some embodiments, the antibody specifically
binds to all or a
portion of both the pseudo-ELR and N-loop motifs.
-21-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[0093] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed or monitored by administering a peptibody that occupies, masks, or
otherwise disrupts all
or a portion of a motif/domain on MIF to which CXCR2, CXCR4, CD74 and/or CD44
binds. In
some embodiments, the peptibody specifically binds to all or a portion of the
pseudo ELR
motif/domain of MIF. In some embodiments, the peptibody specifically binds to
all or a portion of
the N-loop motif/domain of MIF. In some embodiments, the peptibody
specifically binds to all or a
portion of both the pseudo-ELR and N-loop motifs.
[0094] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed or monitored by administering a small molecule that occupies, masks,
or otherwise
disrupts all or a portion of a motif/domain on MIF to which CXCR2, CXCR4, CD74
and/or CD44
binds. In some embodiments, the small molecule specifically binds to all or a
portion of the pseudo
ELR motif/domain of MIF. In some embodiments, the small molecule specifically
binds to all or a
portion of the N-loop motif/domain of MIF. In some embodiments, the small
molecule specifically
binds to all or a portion of both the pseudo-ELR and N-loop motifs.
Disruption of CXCR2 and CXCR4 Motifs/Domains
[0095] In some embodiments, a composition of matter disrupts all or a portion
of a motif/domain
on CXCR2 to which CXCR4, MIF, CD44 and/or CD74 bind. In some embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed,
or monitored by
administering an agent that occupies, masks, or otherwise disrupts all or a
portion of a motif/domain
on CXCR2 to which CXCR4, MIF, CD44 and/or CD74 bind.
[0096] In some embodiments, a composition of matter disrupts all or a portion
of a motif/domain
on CXCR4 to which CXCR2, MIF, CD44 and/or CD74 bind. In some embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed,
or monitored by
administering an agent that occupies, masks, or otherwise disrupts all or a
portion of a motif/domain
on CXCR4 to which CXCR2, MIF, CD44 and/or CD74 bind.
[0097] In some embodiments, the agent that inhibits the binding of CXCR4, MIF,
CD74 and/or
CD44 to CXCR2 is a peptide. In some embodiments, the agent that inhibits the
binding of CXCR2,
MIF, CD74 and/or CD44 to CXCR4 is a peptide.
[0098] In some embodiments, the agent that inhibits the binding of CXCR4, MIF,
CD74 and/or
CD44 to CXCR2 is an antibody. In some embodiments, the agent that inhibits the
binding of
CXCR2, MIF, CD74 and/or CD44 to CXCR4 is an antibody.
[0099] In some embodiments, the agent that inhibits the binding of CXCR4, MIF,
CD74 and/or
CD44 to CXCR2 is a peptibody. In some embodiments, the agent that inhibits the
binding of
CXCR2, MIF, CD74 and/or CD44 to CXCR4 is a peptibody.
-22-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00100] In some embodiments, the agent that inhibits the binding of MIF, CD74
and/or CD44 to
CXCR2 and/or CXCR4 is a derivative of hydroxycinnamate, Schiff-based
tryptophan analogs, or
imino-quinone metabolites of acetaminophen.
[00101] In some embodiments, the agent that inhibits the binding of MIF, CD74
and/or CD44 to
CXCR2 and/or CXCR4 is glyburide, probenicide, DIDS (4, 4-
diisothiocyanatostilbene-2, 2-
disulfonic acid), bumetanide, furosemide, sulfobromophthalein, diphenylamine-2-
carboxylic acid,
flufenamic acid, or combinations thereof.
[00102] In some embodiments, the agent that inhibits the binding of MIF, CD74
and/or CD44 to
CXCR2 is CXCL8(3_74)K11R/G31P; IL-8(4_72); IL-8 (6-72); recombinant IL-8 (rIL-
8); recombinant IL-
8,NMeLeu (rhIL-8 with an N-methylated leucine at position 25); (AAR)IL-8 (IL-8
with N-terminal
Ala4-Ala5 instead of Glu4-Leu5); GRO-alpha(1_73) (also known as CXCL1); GRO-
alpha(4_73); GRO-
alpha(5_73); GRO-alpha(6_73); recombinant GRO (rGRO); (ELR)PF4 (PF4 with an
ELR seq. at the N-
terminus); recombinant PF4 (rPF4); Antileukinate; Sch527123 (-hydroxy-N,N-
dimethyl-3-{2-[[(R)-
1 -(5-methyl-furan-2-yl)-propyl]amino] -3,4-dioxo-cyclobut-l-enylamino}-
benzamide); N-(3-
(aminosulfonyl)-4-chloro-2-hydroxyphenyl)-N'-(2,3-dichlorophenyl) urea; SB-
517785-M (GSK);
SB 265610 (N-(2-Bromophenyl)-N'-(7-cyano-lH-benzotriazol-4-yl)urea); SB225002
(N-(2-
Bromophenyl)-N'-(2-hydroxy-4-nitrophenyl)urea); SB455821 (GSK), SB272844
(GSK); DF2162
(4- [(1R)-2-amino-l-methyl-2-oxoethyl]phenyl trifluoromethanesulphonate);
Reparixin; or
combinations thereof.
[00103] In some embodiments, the agent that inhibits the binding of MIF, CD74
and/or CD44 to
CXCR4 is ALX40-4C (N-alpha-acetyl-nona-D-arginine amide acetate); AMD-070 (AMD
11070,
AnorMED); Plerixafor (AMD3 100); AMD3465(AnorMED); AMD8664 (1-pyridin-2-yl-N-
[4-
(1,4,7-triazacyclotetradecan-4-ylmethyl)benzyl]methanamine); KRH-1636 (Kureha
Chemical
Industry Co. Limited); KRH-2731 (Kureha Chemical Industry Co. Limited); KRH-
3955 (Kureha
Chemical Industry Co. Limited); KRH-3140 (Kureha Chemical Industry Co.
Limited); T134 (L-
citrullinel6-TW70 substituted for the C-terminal amide by a carboxylic acid);
T22 ([Tyr5"2, Lys7]-
polyphemusin II); TW70 (des-[Cys8,13, Tyr9,12]-[D-Lys10, Prol l]-T22); T140 (H-
Arg-Arg-Nal-
Cys-Tyr- Arg-Lys-DLys-Pro-Tyr-Arg-Cit-Cys-Arg-OH); TC14012 (R-R-Nal-C-Y-(L)Cit-
K-(D)Cit-
P-Y-R-(L)citrulline-C-R-NH2, where Nal=L-3-(2-naphthylalanine), Cit=citruline
and the peptide is
cyclized with the cysteines); TN14003; RCP168 (vMIP-II (11_71) with D-amino
acids added to the N
terminus); POL3026 (Arg(*)-Arg-Nal(2)-Cys(1x)-Tyr-Gln-Lys-(d-Pro)-Pro-Tyr-Arg-
Cit-Cys(lx)-
Arg-Gly-(d-Pro)(*)); POL2438; compound 3 (N-(1-methyl-l-phenylethyl)-N-[((3S)-
1-{2-[5-(4H-
1,2,4-triazol-4-yl)-1H-indol-3-yl]ethyl}pyrrolidin-3-yl)methyl]amine);
isothioureas la-lu (for
information regarding isothioureas la-lu see Gebhard Thoma, et al., Orally
Bioavailable
Isothioureas Block Function of the Chemokine Receptor CXCR4 In Vitro and In
Vivo, J. Med.
-23-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Chem., Article ASAP (2008), which is herein incorporated by reference for such
disclosures); or
combinations thereof.
[00104] In some embodiments, the agent that inhibits the binding of MIF, CD74
and/or CD44 to
CXCR2 and/or CXCR4 is MIF is COR100140 (Genzyme Corp/Cortical Pty Ltd.); ISO-1
((S,R)-3-
(4-Hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid, methyl ester); 4-IPP (4-
iodo-6-
phenylpyrimidine); or combinations thereof.
Disruption of CD74 Motifs/Domains
[00105] In some embodiments, a composition of matter disrupts all or a portion
of a motif/domain
on CD74 to which MIF, CD44, CXCR2, and/or CXCR4 bind. In some embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed,
or monitored by
administering an agent that occupies, masks, or otherwise disrupts all or a
portion of a motif/domain
on CD74 to which MIF, CD44, CXCR2, and/or CXCR4 bind.
[00106] In some embodiments, the agent that inhibits the binding of MIF, CD44,
CXCR2, CXCR4,
or a combination thereof to CD74 is a peptide.
[00107] In some embodiments, the agent that inhibits the binding of MIF, CD44,
CXCR2, CXCR4,
or a combination thereof to CD74 is an antibody. In some embodiments, the
agent that inhibits the
binding of MIF, CD44, CXCR2, CXCR4, or a combination thereof to CD74 is M-
B741, 555538
(BD Pharmingen).
[00108] In some embodiments, the agent that inhibits the binding of MIF, CD44,
CXCR2, CXCR4,
or a combination thereof to CD74 is a peptibody.
[00109] In some embodiments, the agent that inhibits the binding of MIF, CD44,
CXCR2, CXCR4,
or a combination thereof to CD74 is a small molecule.
[00110] In certain instances, occupying, masking, or otherwise disrupting all
or a portion of
motifs/domains on MIF does not affect CD74 signaling mediated by other
agonists/ligands (e.g., IL-
8/CXCL8, GRObeta/CXCL2 and/or Stromal Cell-Derived Factor-la (SDF-la)/CXCL12).
Disruption of CD44 Motifs/Domains
[00111] In some embodiments, a composition of matter disrupts all or a portion
of a motif/domain
on CD44 to which MIF, CD74, CXCR2, and/or CXCR4 bind. In some embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed,
or monitored by
administering an agent that occupies, masks, or otherwise disrupts all or a
portion of a motif/domain
on CD44 to which MIF, CD74, CXCR2, and/or CXCR4 bind.
[00112] In some embodiments, the agent that inhibits the binding of MIF, CD74,
CXCR2, CXCR4,
or a combination thereof to CD44 is a peptide.
[00113] In some embodiments, the agent that inhibits the binding of MIF, CD74,
CXCR2, CXCR4,
or a combination thereof to CD44 is an antibody.
-24-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00114] In some embodiments, the agent that inhibits the binding of MIF, CD74,
CXCR2, CXCR4,
or a combination thereof to CD44 is a peptibody.
[00115] In some embodiments, the agent that inhibits the binding of MIF, CD74,
CXCR2, CXCR4,
or a combination thereof to CD44 is a small molecule.
MIF Mimics
[00116] In some embodiments, a composition of matter disrupts the ability of
MIF to bind to
CXCR2, CXCR4, CD74, CD 44 or a combination thereof. In some embodiments, the
composition
of matter is a peptide that competitively binds with a binding partner of a
MIF motif/domain (e.g.,
the pseudo-ELR, or N-Loop motif/domains). In some embodiments, an inflammatory
disease,
disorder, condition and symptom is treated, diagnosed, or monitored by
disrupting the ability of MIF
to bind to CXCR2, CXCR4, CD74, CD 44 or a combination thereof. In some
embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed,
or monitored by
administering to an individual in need thereof a peptide that competitively
binds with a binding
partner of a MIF motif/domain (e.g., the pseudo-ELR, or N-Loop motif/domains).
In some
embodiments, the Peptide binds to CXCR2, CXCR4, CD74, CD44 or a combination
thereof and
thus prevents CXCR2, CXCR4, CD44 or CD74 from binding to MIF.
[00117] In some embodiments, the Peptide adopts structural or functional
features similar to the N-
Loop motif/domain of MIF. In some embodiments, the peptide comprises the
sequence of Formula
(I):
XI-X2-Q/A-X3-X4-X5-X6-G/S-X7-X8-X9-Xl0-P-X11
wherein:
X1 is selected from the group consisting of threonine, glycine, proline and
alanine;
X2 is selected from the group consisting of glycine, asparagine, aspartic
acid, and serine;
X3 is selected from the group consisting of methionine, isoleucine, leucine,
alanine, proline, lysine,
glutamine, arginine and lysine;
X4 is selected from the group consisting of methionine, isoleucine and
leucine;
X5 is selected from the group consisting of alanine, threonine, methionine,
serine and valine;
x 6 is selected from the group consisting of phenylalanine, histidine,
arginine and lysine;
X7 is selected from the group consisting of aspartic acid, glutamic acid,
threonine, glycine and
alanine;
X8 is selected from the group consisting of serine, threonine, lysine and
arginine;
X9 is selected from the group consisting of serine, asparagine, glycine,
threonine, aspartic acid,
glutamic acid, glutamine and histidine;
X10 is selected from the group consisting of aspartic acid, glutamic acid,
alanine and asparagine; and
X11 is selected from the group consisting of cysteine, alanine, serine,
threonine and valine.
-25-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00118] In some embodiments, X1 is proline. In some embodiments, X2 is
aspartic acid. In some
embodiments, X3 is leucine. In some embodiments, X4 is methionine. In some
embodiments, X5 is
alanine. In some embodiments, X6 is phenylalanine. In some embodiments, X7 is
glycine. In some
embodiments, X8 is serine. In some embodiments, X9 is serine. In some
embodiments, X10 is
glutamic acid. In some embodiments, X11 is serine cysteine.
[00119] In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of human
MIF44_57 (numbering includes the first methionine). In some embodiments, the
Peptide comprises 3
or more consecutive amino acids of murine MIF44_57. In some embodiments, the
Peptide comprises 3
or more consecutive amino acids of porcine MIF44_57. In some embodiments, the
Peptide comprises 3
or more consecutive amino acids of bovine MIF44_57. In some embodiments, the
Peptide comprises 3
or more consecutive amino acids of rat MIF44_57.
[00120] In some embodiments, the peptide is selected from Table 1. Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
LMAFGGSSEPCALC (SEQ SEPCAL (SEQ ID No. 62) cyclo(GSSEPCALC) (SEQ ID
ID No. 14) No. 110)
LMAFGGSSEPCAL (SEQ EPCALC (SEQ ID No. 63) cyclo(GSSEPCAL) (SEQ ID
ID No. 15) No. 111)
LMAFGGSSEPCA (SEQ ID QLMAFGGSSEPCALC (SEQ cyclo(GSSEPCA) (SEQ ID No.
No. 16) ID No. 64) 112)
LMAFGGSSEPC (SEQ ID QLMAFGGSSEPCAL (SEQ ID cyclo(GSSEPC) (SEQ ID No.
No. 17) No. 65) 113)
LMAFGGSSEP (SEQ ID No. QLMAFGGSSEPCA (SEQ ID cyclo(SSEPCALC) (SEQ ID
18) No. 66) No. 114)
LMAFGGSSE (SEQ ID No. QLMAFGGSSEPC (SEQ ID cyclo(SSEPCAL) (SEQ ID No.
19) No. 67) 115)
LMAFGGSS (SEQ ID No. QLMAFGGSSEP (SEQ ID No. cyclo(SSEPCA) (SEQ ID No.
20) 68) 116)
LMAFGGS (SEQ ID No. 21) QLMAFGGSSE (SEQ ID No. cyclo(SEPCALC) (SEQ ID No.
-26-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
69) 117)
LMAFGG (SEQ ID No. 22) QLMAFGGSS (SEQ ID No. cyclo(SEPCAL) (SEQ ID No.
70) 118)
MAFGGSSEPCALC (SEQ QLMAFGGS (SEQ ID No. 71) cyclo(EPCALC) (SEQ ID No.
ID No. 23) 119)
MAFGGSSEPCAL (SEQ ID QLMAFGG (SEQ ID No. 72) cyclo(QLMAFGGSSEPCALC)
No. 24) (SEQ ID No. 120)
MAFGGSSEPCA (SEQ ID QLMAFG (SEQ ID No. 73) cyclo(QLMAFGGSSEPCAL)
No. 25) (SEQ ID No. 121)
MAFGGSSEPC (SEQ ID No. CSSEPCALC (SEQ ID No. 74) cyclo(QLMAFGGSSEPCA)
26) (SEQ ID No. 122)
MAFGGSSEP (SEQ ID No. CFGGSSEPCALC (SEQ ID No. cyclo(QLMAFGGSSEPC)
27) 75) (SEQ ID No. 123)
MAFGGSSE (SEQ ID No. CLMAFGGSSEPCALC (SEQ cyclo(QLMAFGGSSEP) (SEQ
28) ID No. 76) ID No. 124)
MAFGGSS (SEQ ID No. 29) CAFGGSSC (SEQ ID No. 77) cyclo(QLMAFGGSSE) (SEQ
ID No. 125)
MAFGGS (SEQ ID No. 30) CLMAFGGSSEPCC (SEQ ID cyclo(QLMAFGGSS) (SEQ ID
No. 78) No. 126)
AFGGSSEPCALC (SEQ ID CAFGGSSEPCAC (SEQ ID cyclo(QLMAFGGS) (SEQ ID
No. 31) No. 79) No. 127)
AFGGSSEPCAL (SEQ ID CMAFGGSSEPC (SEQ ID No. cyclo(QLMAFGG) (SEQ ID
No. 32) 80) No. 128)
AFGGSSEPCA (SEQ ID No. CGGSSEPCAC (SEQ ID No. cyclo(QLMAFG) (SEQ ID No.
33) 81) 129)
AFGGSSEPC (SEQ ID No. NVPRASVPD (SEQ ID No. 82) cyclo(AFGGSSEPCALC)
34) (SEQ ID No. 130)
AFGGSSEP (SEQ ID No. 35) VPDGFLSEL (SEQ ID No. 83) cyclo(AFGGSSEPCAL) (SEQ
ID No. 131)
AFGGSSE (SEQ ID No. 36) CFGGSSEPC (SEQ ID No. 84) cyclo(AFGGSSEPCA) (SEQ
ID No. 132)
AFGGSS (SEQ ID No. 37) IAVHVVPDQLMAFGGSSEPC cyclo(AFGGSSEPC) (SEQ ID
(SEQ ID No. 85) No. 133)
FGGSSEPCALC (SEQ ID CLHSIGKIGGAQNRSYSKLL cyclo(AFGGSSEP) (SEQ ID
No. 38) (SEQ ID No. 86) No. 134)
-27-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
FGGSSEPCAL (SEQ ID No. PCALLCSLHSIGKIG (SEQ ID cyclo(AFGGSSE) (SEQ ID
39) No. 87) No. 135)
FGGSSEPCA (SEQ ID No. CSLHSIGKIGGAQNR (SEQ cyclo(AFGGSS) (SEQ ID No.
40) ID No. 88) 136)
FGGSSEPC (SEQ ID No. 41) IGKIGGAQNRSYSKL (SEQ cyclo(FGGSSEPCALC) (SEQ
ID No. 89) ID No. 137)
FGGSSEP (SEQ ID No. 42) GAQNRSYSKLLCGLLA cyclo(FGGSSEPCAL) (SEQ
(SEQ ID No. 90) ID No. 138)
FGGSSE (SEQ ID No. 43) CGLLAERLRISPDRV (SEQ cyclo(FGGSSEPCA) (SEQ ID
ID No. 91) No. 139)
GGSSEPCALC (SEQ ID No. ERLRISPDRVYINYY (SEQ ID cyclo(FGGSSEPC) (SEQ ID
44) No. 92) No. 140)
GGSSEPCAL (SEQ ID No. cyclo(LMAFGGSSEPCALC) cyclo(FGGSSEP) (SEQ ID No.
45) (SEQ ID No. 93) 141)
GGSSEPCA (SEQ ID No. 46) cyclo(LMAFGGSSEPCAL) cyclo(FGGSSE) (SEQ ID No.
(SEQ ID No. 94) 142)
GGSSEPC (SEQ ID No. 47) cyclo(LMAFGGSSEPCA) cyclo(GGSSEPCALC) (SEQ
(SEQ ID No. 95) ID No. 143)
GGSSEP (SEQ ID No. 48) cyclo(LMAFGGSSEPC) (SEQ cyclo(GGSSEPCAL) (SEQ ID
ID No. 96) No. 144)
GSSEPCALC (SEQ ID No. cyclo(LMAFGGSSEP) (SEQ ID cyclo(GGSSEPCA) (SEQ ID
49) No. 97) No. 145)
GSSEPCAL (SEQ ID No. 50) cyclo(LMAFGGSSE) (SEQ ID cyclo(GGSSEPC) (SEQ ID No.
No. 98) 146)
GSSEPCA (SEQ ID No. 51) cyclo(LMAFGGSS) (SEQ ID cyclo(GGSSEP) (SEQ ID No.
No. 99) 147)
GSSEPC (SEQ ID No. 52) cyclo(LMAFGGS) (SEQ ID No. cyclo(CSSEPCALC) (SEQ ID
100) No. 148)
SSEPCALC (SEQ ID No. 53) cyclo(LMAFGG) (SEQ ID No. cyclo(CFGGSSEPCALC)
101) (SEQ ID No. 149)
GSSEPCALC (SEQ ID No. cyclo(MAFGGSSEPCALC) cyclo(CFGGSSEPCC) (SEQ
54) (SEQ ID No. 102) ID No. 150)
GSSEPCAL (SEQ ID No. 55) cyclo(MAFGGSSEPCAL) cyclo(CFGGSSEPC) (SEQ ID
(SEQ ID No. 103) No. 151)
GSSEPCA (SEQ ID No. 56) cyclo(MAFGGSSEPCA) (SEQ cyclo(CGSSEPCALC) (SEQ
-28-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
ID No. 104) ID No. 152)
GSSEPC (SEQ ID No. 57) cyclo(MAFGGSSEPC) (SEQ cyclo(CAFGGSSEPCAC)
ID No. 105) (SEQ ID No. 153)
SSEPCALC (SEQ ID No. 58) cyclo(MAFGGSSEP) (SEQ ID cyclo(CLMAFGGSSEPCALC)
No. 106) (SEQ ID No. 154)
SSEPCAL (SEQ ID No. 59) cyclo(MAFGGSSE) (SEQ ID cyclo(CAFGGSSC) (SEQ ID
No. 107) No. 155)
SSEPCA (SEQ ID No. 60) cyclo(MAFGGSS) (SEQ ID No. VVPDQLMAFG (SEQ ID No.
108) 461)
SEPCALC (SEQ ID No. 61) cyclo(MAFGGS) (SEQ ID No. DQLMAFGGSSEPC (SEQ ID
109) NO. 462)
Table 1
[00121] In some embodiments, the peptide is cyclic: CLMAFGGSSEPC (SEQ ID No.
422);
CLMAFGGSSEPCALC (SEQ ID No. 423); CGLMAFGGSSEPGC (SEQ ID NO. 424);
CGGLMAFGGSSEPGGC (SEQ ID NO. 425); CGGSLMAFGGSSEPSGGC (SEQ ID NO. 426);
CGGSGLMAFGGSSEPGSGGC (SEQ ID NO. 427); CGGSGGLMAFGGSSEPGGSGGC (SEQ ID
NO. 428); CLMAFGGSSEP[Abu]ALC (SEQ ID NO. 429); wherein Abu is isosteric L-
amino acid,
alpha-aminobutyric acid; CFGGSSEPCALC (SEQ ID NO. 441); CSSEPCALC (SEQ ID NO.
443);
CFGGSSEPCC (SEQ ID NO. 444); CFGGSSEPC (SEQ ID NO. 445); CGSSEPCALCC (SEQ ID
NO. 446); CAFGGSSEPCAC (SEQ ID NO. 449); CAFGGSSC (SEQ ID NO. 450);
CLMAFGGSSEC (SEQ ID NO. 463); or cyclic CLMAFGGSSEPSALC (SEQ ID NO. 469).
[00122] In some embodiments, the peptide is linear: CLMAFGGSSEPCALC (SEQ ID
No. 442);
linera CAFGGSSC (SEQ ID No. 447); CAFGGSSEPCAC (SEQ ID NO. 448); CLMAFGGSSEC
(SEQ ID NO. 464).
[00123] In some embodiments, the peptide is: LMA[NLe]AFGGSSEPC[NLe] (SEQ ID
NO. 430),
wherein NLe is norLeucine; LMA[L-CA]AFGGSSEPC[L-CA] (SEQ ID NO. 431), wherein
L-CA is
L-cyclohexylalanine; LMA[D-CA]AFGGSSEPC[D-CA] (SEQ ID NO. 432), wherein D-CA
is D-
cyclohexylalanine; LMA[D-F]AFGGSSEPC[D-F] (SEQ ID NO. 433), wherein D-F is D-
phenylalanine; (D)-MAFGGSSEPC (SEQ ID NO. 434); (D)-CPESSGGFAML (SEQ ID NO.
435);
(L)-CPESSGGFAML (SEQ ID NO. 436); CLMAFGGSSEPCACG (SEQ ID NO. 452);
CLMAFGGSSEPCCGG (SEQ ID NO. 453); CLMAFGGSSEPCGGG (SEQ ID NO. 454);
CLMAFGGSSECGGGG (SEQ ID NO. 455); CLMAFGGSSCGGGGG (SEQ ID NO. 456);
CLMAFGGSCGGGGG (SEQ ID NO. 457); CLMAFGGCGGGGGGG (SEQ ID NO. 458);
CLMAFGCGGGGGGGG (SEQ ID NO. 459); or CLMAFGGSSEPCALG (SEQ ID NO. 460).
[00124] In some embodiments, a peptide disclosed herein competitively binds
with a binding partner
of of MIF40_49 (i.e., the peptide has the sequence VHVVPDQLMA (SEQ ID NO.
465)). In some
-29-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
embodiments, a peptide disclosed herein competitively binds with a binding
partner of MIF42-51 (i.e.,
the peptide has the sequence VVPDQLMAFG (SEQ ID NO. 466)). In some
embodiments, a peptide
disclosed herein competitively binds with all or a portion of MIF45.57 (i.e.,
the peptide has the
sequence DQLMAFGGSSEPC (SEQ ID NO. 467)). In some embodiments, a peptide
disclosed
herein competitively binds with a binding partner of MIF46-55 (i.e., the
peptide has the sequence
QLMAFGGSSE (SEQ ID NO. 468)). In some embodiments, the peptide has the
sequence:
VHVVPDQLMA (SEQ ID NO. 421), VVPDQLMAFG (SEQ ID NO. 461), DQLMAFGGSSEPC
(SEQ ID NO. 462), or QLMAFGGSSE (SEQ ID NO. 69).
[00125] In some embodiments, the peptide comprises the sequence of Formula
(II):
XI-X2-T/S-N-X3-X4-X5-X6-X7-Xg-P/S-X9-Xl
wherein:
X1 is selected from the group consisting of valine, isoleucine, threonine,
phenylalanine and leucine;
x2 is selected from the group asparagine, arginine, aspartic acid, glutamic
acid, serine and alanine;
x 3 is selected from the group valine, isoleucine, arginine, lysine and
leucine;
X4 is selected from the group proline, alanine, cysteine and leucine;
X5 is selected from the group arginine, lysine, glutamine, serine, alanine,
aspartic acid, glutamic acid
and asparagine;
x 6 is selected from the group alanine, aspartic acid, glutamic acid,
asparagine, serine and glutamine;
X7 is selected from the group serine, glutamic acid, aspartic acid,
asparagine, arginine, glycine,
lysine and arginine;
X8 is selected from the group valine, isoleucine and phenylalanine;
X9 is selected from the group aspartic acid, glutamic acid, valine, serine and
threonine; and
X10 is selected from the group glycine, alanine, threonine, aspartic acid and
glutamic acid.
[00126] In some embodiments, X1 is valine. In some embodiments, X2 is
asparagine. In some
embodiments, X3 is valine. In some embodiments, X4 is proline. In some
embodiments, X5 is
arginine. In some embodiments, X6 is alanine. In some embodiments, X7 is
serine. In some
embodiments, X8 is valine. In some embodiments, X9 is aspartic acid. In some
embodiments, X10 is
glycine.
[00127] In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of human
MIF1_45 (numbering includes the first methionine). In some embodiments, the
Peptide comprises 3 or
more consecutive amino acids of murine MIF1_45. In some embodiments, the
Peptide comprises 3 or
more consecutive amino acids of porcine MIF1-45. In some embodiments, the
Peptide comprises 3 or
more consecutive amino acids of bovine M1F1_45. In some embodiments, the
Peptide comprises 3 or
more consecutive amino acids of rat MIF1-45.
[00128] In some embodiments, the peptide is selected from Table 2 Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
-30-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
CTNVPRASVPDGC (SEQ ID No. 156) NVPRASVPD (SEQ ID No. 173)
CVPRASC (SEQ ID No. 157) NVPRASVP (SEQ ID No. 174)
VNTNVPRASVPDGFLSEL (SEQ ID No. VPRASVP (SEQ ID No. 175)
158)
NTNVPRASVPDGFLSEL (SEQ ID No. PRASVP (SEQ ID No. 176)
159)
TNVPRASVPDGFLSEL (SEQ ID No. 160) VPRASVPDGFL (SEQ ID No. 177)
NVPRASVPDGFLSEL (SEQ ID No. 161) VPRASVPDGF (SEQ ID No. 178)
VPRASVPDGFLSEL (SEQ ID No. 162) VPRASVPDG (SEQ ID No. 179)
PRASVPDGFLSEL (SEQ ID No. 163) VPRASVPD (SEQ ID No. 180)
RASVPDGFLSEL (SEQ ID No. 164) VPRASVP (SEQ ID No. 181)
ASVPDGFLSEL (SEQ ID No. 165) VPRAS (SEQ ID No. 182)
SVPDGFLSEL (SEQ ID No. 166) MPMFIVNTNVPRASVPDGFLSEC (SEQ
ID No. 183)
VPDGFLSEL (SEQ ID No. 167) MPMFIVNTNVPRASV (SEQ ID No. 184)
NVPRASVPDGFLSE (SEQ ID No. 168) FIVNTNVPRASVPDG (SEQ ID No. 185)
NVPRASVPDGFLS (SEQ ID No. 169) NTNVPRASVPDGFLS (SEQ ID No. 186)
NVPRASVPDGFL (SEQ ID No. 170) VPRASVPDGFLSELT (SEQ ID No. 187)
NVPRASVPDGF (SEQ ID No. 171) PRASVPDG (SEQ ID NO. 436)
NVPRASVPDG (SEQ ID No. 172) VNTNVPRASVPDG (SEQ ID NO. 437)
Table 2
[00129] In some embodiments, Peptide is cyclic: CPRASVPDGC (SEQ ID NO. 438),
CGGSGGPRASVPDGGGSGGC (SEQ ID NO. 439); or CNVPRASVPDGC (SEQ ID NO. 440).
[00130] In some embodiments, the Peptide adopts structural or functional
features similar to the
amino acid residues 65-94 (numbering includes the first methionine). In some
embodiments, the
Peptide comprises a peptide of Formula (III):
-31-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
I/L-G-Xl-X2-X3-X4-Xs-X6-N-X7-Xs-X9-X10-Xl1-X12-L/I-X13-X14-X'5-X16-X'7-X'8-X19-
L/V-X20-I-
X21-X22-X23 -X24
wherein:
X1 is selected from the group consisting of lysine, arginine, cysteine, serine
and alanine;
X2 is selected from the group consisting of isoleucine, valine and
phenylalanine;
X3 is selected from the group consisting of glycine, asparagine and serine;
X4 is selected from the group consisting of glycine, proline, alanine,
aspartic acid and glutamic acid;
X5 is selected from the group consisting of alanine, proline, lysine,
arginine, asparagine, aspartic
acid and glutamic acid;
x 6 is selected from the group consisting of glutamine, valine, lysine,
arginine, leucine, aspartic acid
and glutamic acid;
X7 is selected from the group consisting of lysine, arginine, asparagine,
isoleucine and valine;
X8 is selected from the group consisting of serine, asparagine, glutamine,
aspartic acid, glutamic
acid, lysine and arginine;
X9 is selected from the group consisting of tyrosine, histidine and
asparagine;
X10 is selected from the group consisting of serine, threonine and alanine;
X11 is selected from the group consisting of lysine, aspartic acid, glutamic
acid, alanine, serine and
glycine;
X12 is selected from the group consisting of leucine, glutamine, lysine,
arginine, leucine, serine and
alanine;
X13 is selected from the group consisting of cysteine, tyrosine,
phenylalanine, serine, alanine and
threonine;
X14 is selected from the group consisting of glycine, aspartic acid, glutamic
acid, lysine and
arginine;
X15 is selected from the group consisting of leucine, glutamine, isoleucine,
histidine and
phenylalanine;
X16 is selected from the group consisting of leucine, methionine, isoleucine
and cysteine;
X17 is selected from the group consisting of alanine, threonine, serine,
arginine, lysine, alanine,
glutamine and glycine;
X'8 is selected from the group consisting of glutamic acid, aspartic acid,
lysine and arginine;
X19 is selected from the group consisting of arginine, histidine, glutamine,
aspartic acid, glutamic
acid, glycine, threonine and lysine;
X20 is selected from the group consisting of arginine, histidine, glycine,
asparagine, lysine, arginine,
aspartic acid and glutamic acid;
X21 is selected from the group consisting of serine, aspartic acid, glutamic
acid, lysine, arginine and
proline;
-32-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
X22 is selected from the group consisting of proline, alanine, lysine,
arginine and glycine;
x 23 is selected from the group consisting of aspartic acid, glutamic acid,
asparagine and alanine; and
x 24 is selected from the group consisting of histidine, tyrosine, lysine and
arginine.
[00131] In some embodiments, X1 is lysine. In some embodiments, X2 is
isoleucine. In some
embodiments, X3 is glycine. In some embodiments, X4 is glycine. In some
embodiments, X5 is
alanine. In some embodiments, X6 is glutamine. In some embodiments, X7 is
arginine. In some
embodiments, X8 is serine. In some embodiments, X9 is tyrosine. In some
embodiments, X10 is
serine. In some embodiments, X11 is lysine. In some embodiments, X12 is
leucine. In some
embodiments, X13 is cysteine. In some embodiments, X14 is glycine. In some
embodiments, X15 is
leucine. In some embodiments, X16 is leucine. In some embodiments, X17 is
alanine. In some
embodiments, X'8 is glutamic acid. In some embodiments, X19 is arginine. In
some embodiments,
X20 is arginine. In some embodiments, X21 is serine. In some embodiments, X22
is proline. In some
embodiments, X23 is aspartic acid. In some embodiments, X24 is arginine.
[00132] In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of human
MIF65-94. In some embodiments, the Peptide comprises 3 or more consecutive
amino acids of murine
MIF65-94. In some embodiments, the Peptide comprises 3 or more consecutive
amino acids of porcine
MIF65-94. In some embodiments, the Peptide comprises 3 or more consecutive
amino acids of bovine
MIF65-94. In some embodiments, the Peptide comprises 3 or more consecutive
amino acids of rat
MIF65-94=
[00133] In some embodiments, the peptide is selected from Table 3. Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
CSLHSIGKIGGAQNR (SEQ ID IAVHVVPDQLMAFGGSSEPCALCSLHSIGKIGGAQNRSYSKLL
No. 188) (SEQ ID No. 218)
IGKIGGAQNRSYSKL (SEQ ID IAVHVVPDQLMAFGGSSEPCALCSLHSIGKIGGAQNRSY (SEQ
No. 189) ID No. 219)
HSIGKIGGAQNRSYSKLLCGLL IAVHVVPDQLMAFGGSSEPCALCSLHSIGKIGGAQ (SEQ ID
(SEQ ID No. 190) No. 220)
HSIGKIGGAQNRSYSKLLCG IAVHVVPDQLMAFGGSSEPCALCSLHSIGKI (SEQ ID No. 221)
-33-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(SEQ ID No. 191)
HSIGKIGGAQNRSYSKLL (SEQ IAVHVVPDQLMAFGGSSEPCALCSLHS (SEQ ID No. 222)
ID No. 192)
HSIGKIGGAQNRSYSK (SEQ ID IAVHVVPDQLMAFGGSSEPCALC (SEQ ID No. 223)
No. 193)
HSIGKIGGAQNRSYS (SEQ ID IAVHVVPDQLMAFGGSSEP (SEQ ID No. 224)
No. 194)
IGKIGGAQNRSYSKLLC (SEQ IAVHVVPDQLMAFGG (SEQ ID No. 225)
ID No. 195)
KIGGAQNRSYSKLLC (SEQ ID IAVHVVPDQLM (SEQ ID No. 226)
No. 196)
GGAQNRSYSKLLCGLLAERLRI IAVHVVPDQLMAFGGSSEPCALCSLHSIGKIGGAQNRSYSKLL
(SEQ ID No. 197) (SEQ ID No. 227)
AQNRSYSKLLCGLLAERLRI VVPDQLMAFGGSSEPCALCSLHSIGKIGGAQNRSYSKLL (SEQ
(SEQ ID No. 198) ID No. 228)
NRSYSKLLCGLLAERLRI (SEQ QLMAFGGSSEPCALCSLHSIGKIGGAQNRSYSKLL (SEQ ID
ID No. 199) No. 229)
SYSKLLCGLLAERLRI (SEQ ID FGGSSEPCALCSLHSIGKIGGAQNRSYSKLL (SEQ ID No. 230)
No. 200)
YSKLLCGLLAERLRI (SEQ ID SEPCALCSLHSIGKIGGAQNRSYSKLL (SEQ ID No. 231)
No. 201)
GAQNRSYSKLLCGLLAE (SEQ ALCSLHSIGKIGGAQNRSYSKLL (SEQ ID No. 232)
ID No. 202)
GAQNRSYSKLLCGLL (SEQ ID LHSIGKIGGAQNRSYSKLL (SEQ ID No. 233)
No. 203)
QNRSYSKLLCGLLAE (SEQ ID GKIGGAQNRSYSKLL (SEQ ID No. 234)
No. 204)
HSIGKIGGAQNRSY (SEQ ID IGGAQNRSYSKLL (SEQ ID No. 235)
No. 205)
HSIGKIGGAQNR (SEQ ID No. QNRSYSKLL (SEQ ID No. 236)
206)
HSIGKIGGAQNRSYSK (SEQ ID IGKIGGAQNRSYSKL (SEQ ID No. 237)
No. 207)
IGKIGGAQNRSYSKLLC (SEQ IGKIGGAQ (SEQ ID No. 238)
ID No. 208)
-34-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
KIGGAQNRSYSKLLC (SEQ ID linear (CIGKIGGAQC) (SEQ ID No. 239)
No. 209)
KIGGAQNRSYS (SEQ ID No. cyclo (CIGKIGGAQC) (SEQ ID No. 240)
210)
GAQNRSYSKLLCGLLAE (SEQ RSYSKLLCGLLAE (SEQ ID No. 241)
ID No. 211)
GAQNRSYSKLLCGLL (SEQ ID linear (CRSYSKLLCGLLAEC) (SEQ ID No. 242)
No. 212)
GAQNRSYSKLLCG (SEQ ID No. cyclo (CRSYSKLLCGLLAEC) (SEQ ID No. 243)
213)
GAQNRSYSKLL (SEQ ID No. CGLLAERLRISPDR (SEQ ID No. 244)
214)
QNRSYSKLLCGLLAE (SEQ ID linear(CGLLAERLRISPDRC) (SEQ ID No. 245)
No. 215)
RSYSKLLCGLLAE (SEQ ID No. Cyclo (CGLLAERLRISPDRC) (SEQ ID No. 246)
216)
YSKLLCGLLAE (SEQ ID No. VHVVPDQLMA (SEQ ID No. 421)
217)
Table 3
[00134] In some embodiments, Peptide is: CVHVVPDQLMAC (SEQ ID NO. 451).
CD74 Mimics
[00135] CD74 is transmembrane protein that binds MIF. In some embodiments,
CD74 is a receptor
for MIF. In some embodiments, a composition of matter, method and/or
pharmaceutical
composition disclosed herein inhibits binding of the CD74 to CXCR2, CXCR4,
MIF, CD44 or a
combination thereof. In some embodiments, a composition of matter, method
and/or pharmaceutical
composition disclosed herein treats inflammatory diseases, disorders,
conditions and symptoms by
inhibiting the binding of the CD74 to CXCR2, CXCR4, MIF, CD44.
[00136] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed, or monitored by administering to an individual in need thereof a
peptide that
competitively binds with a binding partner of a CD74 motif/domain (e.g., the C-
terminal/extracellular (lumenal) motif/domain). In some embodiments, the
peptide competitively
binds with MIF, CD44, CXCR2, and/or CXCR4 and thus prevents CD74 from binding
to MIF,
CD44, CXCR2, and/or CXCR4.
[00137] In some embodiments, the peptide- adopts structural or functional
features similar to CD74.
[00138] In some embodiments, the -peptide comprises 3 or more consecutive
amino acids of human
CD74. In some embodiments, the comprises 3 or more consecutive amino acids of
bovine CD74. In
-35-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
some embodiments, the peptide comprises 3 or more consecutive amino acids of
porcine CD74. In
some embodiments, the peptide comprises 3 or more consecutive amino acids of
murine CD74. In
some embodiments, the peptide comprises 3 or more consecutive amino acids of
rat CD74.
[00139] In some embodiments, the peptide is selected from Table 4. Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
AYFLYQQQ (SEQ ID NO. 247) TKYGNMTEDHVMHLL (SEQ ID NO. 280)
QQQGRLDKLTVTGRL (SEQ ID NO. 248) HVMHLLQNADPLKVY (SEQ ID NO. 281)
GRLDKLTVTSQNLQL (SEQ ID NO. 249) DPLKVYPPLKGSFPE (SEQ ID NO. 282)
SQNLQLENLRM (SEQ ID NO. 250) KGSFPENLRHLKNTM (SEQ ID NO. 283)
TVTGRLDKLTVTSQN (SEQ ID NO. 251) HLKNTMETIDWKVFE (SEQ ID NO. 284)
TVTSQNLQLENLRM (SEQ ID NO. 252) DWKVFESWMHHWLLF (SEQ ID NO. 285)
LENLRMKLPKPPKPV (SEQ ID NO. 253) HHWLLFEMSRHSLEQ (SEQ ID NO. 286)
KLPKPPKPVSKMRMA (SEQ ID NO. 254) RHSLEQKPTDAPPKE (SEQ ID NO. 287)
SKMRMATPL (SEQ ID NO. 255) DAPPKESLELEDPSS (SEQ ID NO. 288)
LMQALPMGALPQGPM (SEQ ID NO. 256) LEDPSSGLGVTKQDL (SEQ ID NO. 289)
LPQGPMQNATKYGNM (SEQ ID NO. 257) SGLGVTKQDLGPVPM (SEQ ID NO. 290)
TKYGNMTEDHVMHLL (SEQ ID NO. 258) MDDQRDLISNHEQLP (SEQ ID NO. 291)
HVMHLLQNADPLKVY (SEQ ID NO. 259) LPILGNRPREPERCS (SEQ ID NO. 292)
DPLKVYPPLKGSFPE (SEQ ID NO. 260) CSRGALYTGVSVLVA (SEQ ID NO. 293)
KGSFPENLRHLKNTM (SEQ ID NO. 261) VSVLVALLLAGQATT (SEQ ID NO. 294)
HLKNTMETIDWKVFE (SEQ ID NO. 262) AYFLYQQQGRLDKLT (SEQ ID NO. 295)
DWKVFESWMHHWLLF (SEQ ID NO. 263) LTITSQNLQLESLRM (SEQ ID NO. 296)
HHWLLFEMSRHSLEQ (SEQ ID NO. 264) RMKLPKSAKPVSQMR (SEQ ID NO. 297)
RHSLEQKPTDAPPKE (SEQ ID NO. 265) MRMATPLLMRPMSMD (SEQ ID NO. 298)
DAPPKESLELEDPSS (SEQ ID NO. 266) MDNMLLGPVKNVTKY (SEQ ID NO. 299)
LEDPSSGLGVTKQDL (SEQ ID NO. 267) KYGNMTQDHVMHLLT (SEQ ID NO. 300)
VTKQDLGPVPM (SEQ ID NO. 268) RSGPLEYPQLKGTFP (SEQ ID NO. 301)
MDDQRDLISNNEQLP (SEQ ID NO. 269) FPENLKHLKNSMDGV (SEQ ID NO. 302)
-36-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
LPMLGRRPGAPESKC (SEQ ID NO. 270) GVNWKIFESWMKQWL (SEQ ID NO. 303)
CSRGALYTGFSILVT (SEQ ID NO. 271) WLLFEMSKNSLEEKK (SEQ ID NO. 304)
FSILVTLLLAGQATT (SEQ ID NO. 272) EKKPTEAPPKVLTKC (SEQ ID NO. 305)
AYFLYQQQGRLDKLT (SEQ ID NO. 273) CQEEVSHIPAVYPGA (SEQ ID NO. 306)
GRLDKLTVTSQNLQL (SEQ ID NO. 274) GAFRPKCDENGNYLP (SEQ ID NO. 307)
SQNLQLENLRMKLPK (SEQ ID NO. 275) LPLQCHGSTGYCWCV (SEQ ID NO. 308)
KLPKPPKPVSKMRMA (SEQ ID NO. 276) CVFPNGTEVPHTKSR (SEQ ID NO. 309)
SKMRMATPLLMQALP (SEQ ID NO. 277) SRGRHNCSEPLDMED (SEQ ID NO. 310)
LMQALPMGALPQGPM (SEQ ID NO. 278) EDLSSGLGVTRQELG (SEQ ID NO. 311)
LPQGPMQNATKYGNM (SEQ ID NO. 279) SGLGVTRQELGQVTL (SEQ ID NO. 312)
Table 4
CXCR2/CXCR4 Mimics
[00140] In some embodiments, a composition of matter, method and/or
pharmaceutical composition
disclosed herein inhibits binding of the CXCR2 to CXCR4, MIF, CD44, CD74 or a
combination
thereof. In some embodiments, a composition of matter, method and/or
pharmaceutical composition
disclosed herein treats inflammatory diseases, disorders, conditions and
symptoms by inhibiting the
binding of the CXCR2 to CXCR4, MIF, CD44, CD74 or a combination thereof. In
some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
inhibits binding of the CXCR4 to CXCR2, MIF, CD44, CD74 or a combination
thereof. In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
treats inflammatory diseases, disorders, conditions and symptoms by inhibiting
the binding of the
CXCR4 to CXCR2, MIF, CD44, CD74 or a combination thereof.
[00141] In some embodiments, a peptide disclosed herein competitively binds
with a binding partner
of a CXCR2 domain/motif. In some embodiments, an inflammatory disease,
disorder, condition and
symptom is treated, diagnosed or monitored by administering to an individual
in need thereof a
peptide that competitively binds with a binding partner of a CXCR2
motif/domain. In some
embodiments, the peptide binds to MIF, CD74 and/or CD44 and thus prevents
CXCR2 from binding
to MIF, CD74 and/or CD44.
[00142] In some embodiments, a peptide disclosed herein competitively binds
with a binding partner
of the CXCR2 extracellular loop 1 (i.e., CXCR2108 120), the extracellular loop
2 (i.e., CXCR2184-212),
and/or the extracellular loop 3 (i.e., CXCR2286-300)= In some embodiments, a
peptide disclosed herein
competitively binds with a binding partner of the extracellular loop 2 (i.e.,
CXCR2184-212), and/or
CXCR2 extracellular loop 3 (i.e., CXCR2286-300)= In some embodiments, an
inflammatory disease,
disorder, condition and symptom is treated, diagnosed or monitored by
administering to an
individual in need thereof a peptide that competitively binds with a binding
partner of the CXCR2
extracellular loop 1 (i.e., CXCR2108-120), the extracellular loop 2 (i.e.,
CXCR2184-212), and/or the
-37-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
extracellular loop 3 (i.e., CXCR2286-300). In some embodiments, an
inflammatory disease, disorder,
condition and symptom is treated, diagnosed or monitored by administering to
an individual in need
thereof a peptide that competitively binds with a binding partner of the CXCR2
extracellular loop 2
(i.e., CXCR2i84-212), and/or CXCR2 extracellular loop 3 (i.e., CXCR2286-300)=
[00143] In some embodiments, a peptide disclosed herein competitively binds
with a binding partner
of CXCR2 N-terminus/domain (i.e., CXCR21-39). In some embodiments, an
inflammatory disease,
disorder, condition and symptom is treated, diagnosed or monitored by
administering to an
individual in need thereof a peptide that competitively binds with a binding
partner of the CXCR2
N-terminus/domain (i.e., CXCR21-39).
[00144] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed or monitored by administering to an individual in need thereof a
peptide that
competitively binds with a binding partner of a CXCR4 motif/domain. In some
embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed or
monitored by
administering to an individual in need thereof a peptide that competitively
binds with a binding
partner of the CXCR4 extracellular loop 1 and/or extracellular loop 2. In some
embodiments, an
inflammatory disease, disorder, condition and symptom is treated, diagnosed or
monitored by
administering to an individual in need thereof a peptide that competitively
binds with a binding
partner of CXCR4 amino acids 182-202 (SEADDRYICDRFYPNDLWVVV). In some
embodiments, an inflammatory disease, disorder, condition and symptom is
treated, diagnosed or
monitored by administering to an individual in need thereof a peptide that
competitively binds with
a binding partner of CXCR4 amino acids 185-199 (DDRYICDRFYPNDLW). In some
embodiments, the peptide binds to MIF, CD74 and/or CD44 and thus prevents
CXCR4 from binding
to MIF, CD74 and/or CD44.
[00145] In some embodiments, the peptide comprises 3 or more consecutive amino
acids of human
CXCR2. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of bovine
CXCR2. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of porcine
CXCR2. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of murine
CXCR2. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of rat
CXCR2.
[00146] In some embodiments, the peptide comprises 3 or more consecutive amino
acids of human
CXCR4. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of bovine
CXCR4. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of porcine
CXCR4. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of murine
CXCR4. In some embodiments, the peptide comprises 3 or more consecutive amino
acids of rat
CXCR4.
-38-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00147] In some embodiments, the peptide is selected from Table 5. Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
DLSNYSYSSTLPPFL (SEQ ID NO. 313) MRTQVIQ (SEQ ID NO. 336)
DLSNYSYSSTLPP (SEQ ID NO. 314) MRTQV (SEQ ID NO. 337)
DLSNYSYSSTL (SEQ ID NO. 315) CERRNHIDRALDA (SEQ ID NO. 338)
DLSNYSYSS (SEQ ID NO. 316) CERRNHIDRAL (SEQ ID NO. 339)
DLSNYSY (SEQ ID NO. 317) CERRNHIDR (SEQ ID NO. 340)
DLSNY (SEQ ID NO. 318) CERRNHI (SEQ ID NO. 341)
KVNGWIFGTFL (SEQ ID NO. 319) CERRN (SEQ ID NO. 342)
KVNGWIFGT (SEQ ID NO. 320) DRYICDRFYPNDL (SEQ ID NO. 343)
KVNGWIF (SEQ ID NO. 321) DRYICDRFYPN (SEQ ID NO. 344)
KVNGW (SEQ ID NO. 322) DRYICDRFY (SEQ ID NO. 345)
RRTVYSSNVSPAC (SEQ ID NO. 323) DRYICDR (SEQ ID NO. 346)
RRTVYSSNVSP (SEQ ID NO. 324) DRYIC (SEQ ID NO. 347)
RRTVYSSNV (SEQ ID NO. 325) ICDRFYPNDLWVV (SEQ ID NO. 348)
RRTVYSS (SEQ ID NO. 326) ICDRFYP (SEQ ID NO. 349)
RRTVY (SEQ ID NO. 327) ICDRF (SEQ ID NO. 350)
EDMGNNTANWRML (SEQ ID NO. 328) RFYPNDLWVVVFQ (SEQ ID NO. 351)
EDMGNNTANWR (SEQ ID NO. 329) RFYPNDLWVVV (SEQ ID NO. 352)
EDMGNNTAN (SEQ ID NO. 330) RFYPNDLWV (SEQ ID NO. 353)
EDMGNNT (SEQ ID NO. 331) RFYPNDL (SEQ ID NO. 354)
EDMGN (SEQ ID NO. 332) RFYPN (SEQ ID NO. 355)
MRTQVIQETCERR (SEQ ID NO. 333) ICDRFYPNDLW (SEQ ID NO. 356)
MRTQVIQETCE (SEQ ID NO. 334) ICDRFYPND (SEQ ID NO. 357)
MRTQVIQET (SEQ ID NO. 335)
Table 5
CD44 Mimics
-39-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00148] CD44 is a cell-surface glycoprotein involved in cell-cell
interactions, cell adhesion and
migration. In certain instances, human CD44 has the sequence:
MDKFWWHAAWGLCLVPLSLAQIDLNITCRFAGVFHVEKNGRYSISRTEA
ADLCKAFNSTLPTMAQMEKALSIGFETCRYGFIEGHVVIPRIHPNSICAAN
NTGVYILTSNTSQYDTYCFNASAPPEEDCTSVTDLPNAFDGPITITIVNRD
GTRYV QKGEYRTNPEDIYPSNPTDDDV S SGS S SERS STSGGYIFYTFSTVH
PIPDEDSPWITDSTDRIPATRDQDTFHPSGGSHTTHGSESDGHSHGSQEGG
ANTTSGPIRTPQIPEWLIILASLLALALILAVCIAVNSRRRCGQKKKLVINS
GNGAVEDRKPSGLNGEASKSQEMVHLVNKESSETPDQFMTADETRNLQ
NVDMKIGV (SEQ ID No. 358).
In certain instances, murine CD44 has the sequence:
MDKFWWHTAWGLCLLQLSLAHPHQQIDLNVTCRYAGVFHVEKNGRYSI
SRTEAADLCQAFNSTLPTMDQMKLALSKGFETCRYGFIEGNVVIPRIHPN
AICAANHTGVYILVTSNTSHYDTYCFNASAPPEEDCTSVTDLPNSFDGPV
TITIVNRDGTRYSKKGEYRTHQEDIDASNIIDDDVSSGSTIEKSTPESYILHT
YLPTEQPTGDQDDSFFIRSTLATRDRDSSKDSRGSSRTVTHGSELAGHSSA
NQDSGVTTTSGPMRRPQIPEWLIILASLLALALILAVCIAVNSRRRCGQKK
KLVINGGNGTVEDRKPSELNGEASKSQEMVHLVNKEPSETPDQCMTADE
TRNLQSVDMKIGV (SEQ ID No. 359).
[00149] In certain instances, CD44 forms a complex with CD74. In some
embodiments, inhibiting
the binding of CD44 and CD74 reduces or inhibits (partially or fully)
inflammation. In some
embodiments, inhibiting the binding of CD44 and MIF reduces or inhibits
(partially or fully)
inflammation.
[00150] In some embodiments, a composition of matter, method and/or
pharmaceutical composition
disclosed herein inhibits binding of the CD44 to CXCR2, CXCR4, MIF, CD74 or a
combination
thereof. In some embodiments, a composition of matter, method and/or
pharmaceutical composition
disclosed herein treats inflammatory diseases, disorders, conditions and
symptoms by inhibiting the
binding of the CD44 to CXCR2, CXCR4, MIF, CD74 or a combination thereof.
[00151] In some embodiments, an inflammatory disease, disorder, condition and
symptom is treated,
diagnosed, or monitored by administering to an individual in need thereof a
peptide that
competitively binds with a binding partner of a CD44 motif/domain. In some
embodiments, the
Peptide binds to MIF, CXCR2, CXCR4, CD74, or a combination thereof.
[00152] In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of human
CD44. In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of bovine
CD44. In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of porcine
CD44. In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of murine
-40-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
CD44. In some embodiments, the Peptide comprises 3 or more consecutive amino
acids of rat
CD44.
[00153] In some embodiments, the peptide is selected from Table 6. Any amino
acid in any of the
peptides disclosed herein may be substituted with an unnatural or natural
amino acid that
corresponds to and functions as an effective substitute for the original amino
acid, but does not
substantially diminish binding or bioactivity relative to the parent peptide
sequence. Unnatural
amino acids include, but are not limited to: D-amino acids such as D-
phenylalanine (D-F) and D-
cyclohexyl alanine (D-CA); norLeucine (NLe), L-cyclohexyl alanine (L-CA), and
L-amino acid,
alpha-aminobutyric acid (Abu). In some embodiments, an amino acid of a peptide
disclosed herein
is substituted with a non-natural amino acid. In some embodiments, an amino
acid of a peptide
disclosed herein comprises N- and/or C-terminal chemical modifications to
improve ADME-PK.
MDKFWWHAAWGLCLV (SEQ ID NO. 360) GVFHVEKNGRYSI (SEQ ID NO. 391)
LVPLSLAQIDLNITC (SEQ ID NO. 361) SISRTEAADLCQA (SEQ ID NO. 392)
CRFAGVFHVEKNGRY (SEQ ID NO. 362) QAFNSTLPTMDQM (SEQ ID NO. 393)
RYSISRTEAADLCKA (SEQ ID NO. 363) QMKLALSKGFETC (SEQ ID NO. 394)
KAAFNSTLPTMAQME (SEQ ID NO. 364) CRYGFIEGNVVIP (SEQ ID NO. 395)
KALSIGFETCRYGFI (SEQ ID NO. 365) IPRIHPNAICAAN (SEQ ID NO. 396)
FIEGHVVIPRIHPNS (SEQ ID NO. 366) ANHTGVYILVTSN (SEQ ID NO. 397)
NSICAANNTGVYILT (SEQ ID NO. 367) SNTSHYDTYCFNA (SEQ ID NO. 398)
LTSNTSQYDTYCFNA (SEQ ID NO. 368) NASAPPEEDCTSV (SEQ ID NO. 399)
NASAPPEEDCTSVTD (SEQ ID NO. 369) SVTDLPNSFDGPV (SEQ ID NO. 400)
TDLPNAFDGPITITI (SEQ ID NO. 370) PVTITIVNRDGTR (SEQ ID NO. 401)
TIVNRDGTRYVQKGE (SEQ ID NO. 371) TRYSKKGEYRTHQ (SEQ ID NO. 402)
GEYRTNPEDIYPSNP (SEQ ID NO. 372) HQEDIDASNIIDD (SEQ ID NO. 403)
NPTDDDVSSGSSSER (SEQ ID NO. 373) DDVSSGSTIEKST (SEQ ID NO. 404)
ERSSTSGGYIFYTFS (SEQ ID NO. 374) STPESYILHTYLP (SEQ ID NO. 405)
FSTVHPIPDEDSPWI (SEQ ID NO. 375) LPTEQPTGDQDDS (SEQ ID NO. 406)
WITDSTDRIPATRDQ (SEQ ID NO. 376) DSFFIRSTLATRD (SEQ ID NO. 407)
DQDTFHPSGGSHTTH (SEQ ID NO. 377) RDRDSSKDSRGSS (SEQ ID NO. 408)
THGSESDGHSHGSQE (SEQ ID NO. 378) SSRTVTHGSELAG (SEQ ID NO. 409)
QEGGANTTSGPIRTP (SEQ ID NO. 379) AGHSSANQDSGVT (SEQ ID NO. 410)
TPQIPEWLIILASLL (SEQ ID NO. 380) TTSGPMRRPQIPE (SEQ ID NO. 411)
LLALALILAVCIAVN (SEQ ID NO. 381) PEWLIILASLLAL (SEQ ID NO. 412)
VNSRRRCGQKKKLVI (SEQ ID NO. 382) ALALILAVCIAVN (SEQ ID NO. 413)
VINSGNGAVEDRKPS (SEQ ID NO. 383) VNSRRRCGQKKKL (SEQ ID NO. 414)
-41-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
PSGLNGEASKSQEMV (SEQ ID NO. 384) KLVINGGNGTVED (SEQ ID NO. 415)
MVHLVNKESSETPDQ (SEQ ID NO. 385) EDRKPSELNGEAS (SEQ ID NO. 416)
DQFMTADETRNLQNV (SEQ ID NO. 386) ASKSQEMVHLVNK (SEQ ID NO. 417)
DETRNLQNVDMKIGV (SEQ ID NO. 387) NKEPSETPDQCMT (SEQ ID NO. 418)
MDKFWWHTAWGLC (SEQ ID NO. 388) MTADETRNLQSVD (SEQ ID NO. 419)
LLQLSLAHPHQQI (SEQ ID NO. 389) TRNLQSVDMKIGV (SEQ ID NO. 420)
QIDLNVTCRYAGV (SEQ ID NO. 390)
Table 6
F. Fusion Peptide
[00154] In some embodiments, a composition of matter disrupts the ability of
MIF to bind to
CXCR2, CXCR4, CD74, or a combination thereof. In some embodiments, the
composition of matter
is a fusion peptide that binds both the N-loop motif/domain of MIF and the
pseudo-ELR
motif/domain of MIF. In some embodiments, an inflammatory disease, disorder,
condition, or
symptom is treated by disrupting the ability of MIF to bind to CXCR2, CXCR4,
CD74, or a
combination thereof. In some embodiments, an inflammatory disease, disorder,
condition, or
symptom is treated by administering to an individual in need thereof a fusion
peptide that binds both
the N-loop motif/domain of MIF and the pseudo-ELR motif/domain of MIF.
[00155] In some embodiments, the peptides that comprise the fusion peptide are
derived from human
MIF, bovine MIF, porcine MIF, murine MIF, rat MIF, or a combination thereof.
In some
embodiments, the peptides that comprise the fusion peptide are artificially
constructed.
[00156] In some embodiments, the fusion peptide comprises at least one peptide
that competitively
binds with a binding partner of the N-loop motif/domain of MIF, and at least
one peptide that
competitively binds with a binding partner of the pseudo ELR motif/domain of
MIF. In some
embodiments, the fusion peptide comprises (a) a first peptide that
competitively binds with a
binding partner of the N-loop motif/domain of MIF; and (b) a second peptide
that competitively
binds with a binding partner of the pseudo ELR motif/domain of MIF. In some
embodiments, the
fusion peptide comprise (a) a first peptide that competitively binds with a
binding partner of the N-
loop motif/domain of MIF; (b) a second peptide that competitively binds with a
binding partner of
the pseudo ELR motif/domain of MIF; and (c) a third peptide that competitively
binds with a
binding partner of the pseudo ELR motif/domain of MIF.
[00157] In some embodiments, the fusion peptide comprise (a) a first peptide
that competitively
binds with a binding partner of the N-loop motif/domain of MIF; and (b) a
second peptide that
competitively binds with a binding partner of the pseudo ELR motif/domain of
MIF; wherein the
first peptide and the second peptide are chemically linked. In some
embodiments, the fusion peptide
comprise (a) a first peptide that competitively binds with a binding partner
of the N-loop
-42-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
motif/domain of MIF; (b) a second peptide that that competitively binds with a
binding partner of
the pseudo ELR motif/domain of MIF; and (c) a third peptide that that
competitively binds with a
binding partner of the e pseudo ELR motif/domain of MIF; wherein the first
peptide, the second
peptide, and the third peptide are chemically linked.
[00158] In some embodiments, the fusion peptide comprises (a) a first peptide
having the sequence
MAFGGSSEPC; and (b) a second peptide having the sequence NVPRA. In some
embodiments, the
fusion peptide comprises (a) a first peptide having the sequence MAFGGSSEPC;
(b) a second
peptide having the sequence NVPRA; and (c) a third peptide having the sequence
SVPDG.
[00159] In some embodiments, the methods and compositions disclosed herein
comprise (a) a first
peptide having the sequence LQDP; and (b) a second peptide having the sequence
NVPRA.
[00160] In some embodiments, the first peptide and the second peptide are
directly bound to each
other (e.g., via a covalent or ionic bond).
Linkers
[00161] In some embodiments, at least one peptide that competitively binds
with a binding partner
of the N-loop motif/domain of MIF and at least one peptide that competitively
binds with a binding
partner of the pseudo ELR motif/domain of MIF are indirectly bound to each
other (e.g., via a
linker). In some embodiments, at least one peptide that competitively binds
with a binding partner of
the N-loop motif/domain of MIF and at least one peptide that competitively
binds with a binding
partner of the pseudo ELR motif/domain of MIF are bound by a linker.
[00162] In some embodiments, the linker binds (a) a first peptide that
competitively binds with a
binding partner of the N-loop motif/domain of MIF; and (b) a second peptide
that competitively
binds with a binding partner of the pseudo ELR motif/domain of MIF. In some
embodiments, the
fusion peptide is a peptide of Formula (IV):
Peptide 1 -Linker Peptide 2
Formula (IV)
wherein Peptide 1, and Peptide 2 are selected from any peptide disclosed
herein.
[00163] In some embodiments, the linker binds (a) a first peptide that
competitively binds with a
binding partner of the N-loop motif/domain of MIF; (b) a second peptide that
adopts structural or
functional features similar to a first portion of the pseudo ELR motif/domain
of MIF; and (c) a third
peptide that adopts structural or functional features similar to a second
portion of the pseudo ELR
motif/domain of MIF. In some embodiments, the fusion peptide is a peptide of
Formula (V):
Peptide 1 Linker Peptide 2
Peptide 3
Formula (V)
-43-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
wherein Peptide 1, Peptide 2, and Peptide 3 are selected from any peptide
disclosed herein.
[00164] As used herein, a "linker" is any molecule capable of binding (e.g.,
covalently) to multiple
peptides. In some embodiments, the linker binds to the peptide by a covalent
linkage. In some
embodiments, the covalent linkage comprises a ether bond, thioether bond,
amine bond, amide bond,
carbon-carbon bond, carbon-nitrogen bond, carbon-oxygen bond, or carbon-sulfur
bond.
[00165] In some embodiments, the linker is flexible. In some embodiments, the
linker is rigid. In
some embodiments, the linker is long enough to allow the fusion peptide to
bind to both the pseudo-
ELR and N-loop motif/domains of MIF.
[00166] In some embodiments, the linker binds to two peptides. In some
embodiments, the linker
binds to three peptides.
[00167] In some embodiments, a linker described herein binds to the C-terminus
of one or more of
the peptides that form the fusion peptide. In some embodiments, the linker
binds to the N-terminus
of one or more of the peptides that form the fusion peptide. In some
embodiments, a linker described
herein binds to the C-terminus of one or more of the peptides and the N-
terminus of any remaining
peptides.
[00168] In some embodiments, the linker comprises a linear structure. In some
embodiments, the
linker comprises a non-linear structure. In some embodiments, the linker
comprises a branched
structure. In some embodiments, the linker comprises a cyclic structure.
[00169] In some embodiments, the linker is an alkyl. In some embodiments, the
linker is heteroalkyl.
[00170] In some embodiments, the linker is an alkylene. In some embodiments,
the linker is an
alkenylene. In some embodiments, the linker is an alkynylene. In some
embodiments, the linker is a
heteroalkylene.
[00171] An "alkyl" group refers to an aliphatic hydrocarbon group. The alkyl
moiety may be a
saturated alkyl or an unsaturated alkyl. Depending on the structure, an alkyl
group can be a
monoradical or a diradical (i.e., an alkylene group).
[00172] The "alkyl" moiety may have 1 to 10 carbon atoms (whenever it appears
herein, a
numerical range such as "1 to 10" refers to each integer in the given range;
e.g., "1 to 10 carbon
atoms" means that the alkyl group may consist of 1 carbon atom, 2 carbon
atoms, 3 carbon atoms,
etc., up to and including 10 carbon atoms, although the present definition
also covers the occurrence
of the term "alkyl" where no numerical range is designated). The alkyl group
could also be a "lower
alkyl" having 1 to 6 carbon atoms. The alkyl group of the compounds described
herein may be
designated as "C1-C4 alkyl" or similar designations. By way of example only,
"C1-C4 alkyl"
indicates that there are one to four carbon atoms in the alkyl chain, i.e.,
the alkyl chain is selected
from the group consisting of methyl, ethyl, propyl, iso-propyl, n-butyl, iso-
butyl, sec-butyl, and t-
butyl. Typical alkyl groups include, but are in no way limited to, methyl,
ethyl, propyl, isopropyl,
butyl, isobutyl, tertiary butyl, pentyl, hexyl, ethenyl, propenyl, butenyl,
and the like.
-44-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00173] In some embodiments, the linker comprises a ring structure (e.g., an
aryl). As used herein,
the term "ring" refers to any covalently closed structure. Rings include, for
example, carbocycles
(e.g., aryls and cycloalkyls), heterocycles (e.g., heteroaryls and non-
aromatic heterocycles),
aromatics (e.g. aryls and heteroaryls), and non-aromatics (e.g., cycloalkyls
and non-aromatic
heterocycles). Rings can be optionally substituted. Rings can be monocyclic or
polycyclic.
[00174] As used herein, the term "aryl" refers to an aromatic ring wherein
each of the atoms
forming the ring is a carbon atom. Aryl rings can be formed by five, six,
seven, eight, nine, or more
than nine carbon atoms. Aryl groups can be optionally substituted. Examples of
aryl groups include,
but are not limited to phenyl, naphthalenyl, phenanthrenyl, anthracenyl,
fluorenyl, and indenyl.
Depending on the structure, an aryl group can be a monoradical or a diradical
(i.e., an arylene
group).
[00175] The term "cycloalkyl" refers to a monocyclic or polycyclic non-
aromatic radical, wherein
each of the atoms forming the ring (i.e. skeletal atoms) is a carbon atom.
Cycloalkyls may be
saturated, or partially unsaturated. Cycloalkyl groups include groups having
from 3 to 10 ring atoms.
Cycloalkyls include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl,
cycloheptyl, and cyclooctyl.
[00176] In some embodiments, the ring is a cycloalkane. In some embodiments,
the ring is a
cycloalkene.
[00177] In some embodiments, the ring is an aromatic ring. The term "aromatic"
refers to a planar
ring having a delocalized t-electron system containing 4n+2 t electrons, where
n is an integer.
Aromatic rings can be formed from five, six, seven, eight, nine, or more than
nine atoms. Aromatics
can be optionally substituted. The term "aromatic" includes both carbocyclic
aryl (e.g., phenyl) and
heterocyclic aryl (or "heteroaryl" or "heteroaromatic") groups (e.g.,
pyridine). The term includes
monocyclic or fused-ring polycyclic (i.e., rings which share adjacent pairs of
carbon atoms) groups.
[00178] In some embodiments, the ring is a heterocycle. The term "heterocycle"
refers to
heteroaromatic and heteroalicyclic groups containing one to four heteroatoms
each selected from 0,
S and N, wherein each heterocyclic group has from 4 to 10 atoms in its ring
system, and with the
proviso that the ring of said group does not contain two adjacent 0 or S
atoms. Non-aromatic
heterocyclic groups include groups having only 3 atoms in their ring system,
but aromatic
heterocyclic groups must have at least 5 atoms in their ring system. The
heterocyclic groups include
benzo-fused ring systems. An example of a 3-membered heterocyclic group is
aziridinyl. An
example of a 4-membered heterocyclic group is azetidinyl (derived from
azetidine). An example of
a 5-membered heterocyclic group is thiazolyl. An example of a 6-membered
heterocyclic group is
pyridyl, and an example of a 10-membered heterocyclic group is quinolinyl.
Examples of non-
aromatic heterocyclic groups are pyrrolidinyl, tetrahydrofuranyl,
dihydrofuranyl, tetrahydrothienyl,
tetrahydropyranyl, dihydropyranyl, tetrahydrothiopyranyl, piperidino,
morpholino, thiomorpholino,
-45-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
thioxanyl, piperazinyl, azetidinyl, oxetanyl, thietanyl, homopiperidinyl,
oxepanyl, thiepanyl,
oxazepinyl, diazepinyl, thiazepinyl, 1,2,3,6-tetrahydropyridinyl, 2-
pyrrolinyl, 3-pyrrolinyl, indolinyl,
2H-pyranyl, 4H-pyranyl, dioxanyl, 1,3-dioxolanyl, pyrazolinyl, dithianyl,
dithiolanyl,
dihydropyranyl, dihydrothienyl, dihydrofuranyl, pyrazolidinyl, imidazolinyl,
imidazolidinyl, 3-
azabicyclo[3.1.0]hexanyl, 3-azabicyclo[4.1.0]heptanyl, 3H-indolyl and
quinolizinyl. Examples of
aromatic heterocyclic groups are pyridinyl, imidazolyl, pyrimidinyl,
pyrazolyl, triazolyl, pyrazinyl,
tetrazolyl, furyl, thienyl, isoxazolyl, thiazolyl, oxazolyl, isothiazolyl,
pyrrolyl, quinolinyl,
isoquinolinyl, indolyl, benzimidazolyl, benzofuranyl, cinnolinyl, indazolyl,
indolizinyl, phthalazinyl,
pyridazinyl, triazinyl, isoindolyl, pteridinyl, purinyl, oxadiazolyl,
thiadiazolyl, furazanyl,
benzofurazanyl, benzothiophenyl, benzothiazolyl, benzoxazolyl, quinazolinyl,
quinoxalinyl,
naphthyridinyl, and furopyridinyl. The foregoing groups, may be C-attached or
N-attached where
such is possible. For instance, a group derived from pyrrole may be pyrrol-1-
yl (N-attached) or
pyrrol-3-yl (C-attached). Further, a group derived from imidazole may be
imidazol-l-yl or imidazol-
3-yl (both N-attached) or imidazol-2-yl, imidazol-4-yl or imidazol-5-yl (all C-
attached). The
heterocyclic groups include benzo-fused ring systems and ring systems
substituted with one or two
oxo (=O) moieties such as pyrrolidin-2-one. Depending on the structure, a
heterocycle group can be
a monoradical or a diradical (i.e., a heterocyclene group).
[00179] In some embodiments, the ring is fused. The term "fused" refers to
structures in which two
or more rings share one or more bonds. In some embodiments, the ring is a
dimer. In some
embodiments, the ring is a trimer. In some embodiments, the ring is a
substituted.
[00180] The term "carbocyclic" or "carbocycle" refers to a ring wherein each
of the atoms forming
the ring is a carbon atom. Carbocycle includes aryl and cycloalkyl. The term
thus distinguishes
carbocycle from heterocycle ("heterocyclic") in which the ring backbone
contains at least one atom
which is different from carbon (i.e., a heteroatom). Heterocycle includes
heteroaryl and
heterocycloalkyl. Carbocycles and heterocycles can be optionally substituted.
[00181] In some embodiments, the linker is substituted. The term "optionally
substituted" or
"substituted" means that the referenced group may be substituted with one or
more additional
group(s) individually and independently selected from Ci-C6alkyl, C3-
Cgcycloalkyl, aryl, heteroaryl,
C2-C6heteroalicyclic, hydroxy, Ci-C6alkoxy, aryloxy, Ci-C6alkylthio, arylthio,
Ci-C6alkylsulfoxide,
arylsulfoxide, Ci-C6alkylsulfone, arylsulfone, cyano, halo, C2-Cgacyl, C2-
Cgacyloxy, nitro, Ci-
C6haloalkyl, Ci-C6fluoroalkyl, and amino, including Ci-C6alkylamino, and the
protected derivatives
thereof. By way of example, an optional substituents may be LSRS, wherein each
Ls is independently
selected from a bond, -0-, -C(=O)-, -S-, -S(=O)-, -S(=0)2-, -NH-, -NHC(=O)-, -
C(=O)NH-,
S(=0)2NH-, -NHS(=0)2-, -OC(=O)NH-, -NHC(=O)O-, -(Ci-C6alkyl)-, or -(C2-
C6alkenyl)-; and each
Rs is independently selected from H, (Ci-C4alkyl), (C3-Cgcycloalkyl),
heteroaryl, aryl, and Ci-
C6heteroalkyl. Optionally substituted non-aromatic groups may be substituted
with one or more oxo
-46-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(=O). The protecting groups that may form the protective derivatives of the
above substituents are
known to those of skill in the art.
[00182] In some embodiments, the linker is an amino acid. In some embodiments,
the fusion peptide
is a peptide of Formula (VI):
O H
Peptide 1,N N,Pe Peptide 2
H p
r, r2
Formula (VI)
wherein Peptide 1, and Peptide 2 are selected from any peptide disclosed
herein.
[00183] In some embodiments, the linker is an artificial amino acid. In some
embodiments, the
linker is a (3-amino acid. In some embodiments, the linker is a y-amino acid.
[00184] In some embodiments, the linker is a polyethylene glycol (PEG). In
some embodiments, the
linker is a diamino acid. In some embodiments, the linker is diaminopropionic
acid.
[00185] In some embodiments, the linker is hydrolyzible.
[00186] By way of non-limiting example, the fusion peptide is:
O H
O Peptide 1,
~--~ NPeptide 2 H N N, Peptide 2
Peptide1v H
Peptide 3' NH
0 Peptide 1
Peptide 1 I \N HN O
/ ,Peptide 2 HNUN'Peptide 2
H I H
Peptide 3
H
Peptide 1' TO H
Peptide 1 I" N,
N-Peptide 2 Peptide 3
NzzN O 0
HN,Peptide 2
Peptide 1, NH
O H
N, N,Peptide 2
Peptide 1 Peptide 2 H
HN
Peptide 3
-47-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
O O Peptide 1 O
Peptide 1, Peptide 2 HN N N' H / H Peptide 2
HN N'
H
HN 0 Peptide 3
Peptide 3
O O Peptide 1
Peptide 1, Peptide 2 HN N N' H
H H HN N, Peptide 2
Peptide 3'NH Peptide 3
H H Peptide 1 O
Peptide 1'N N, Peptide 2 HN
HN N' Peptide 2
NH I O
Peptide 3' Peptide 3
wherein Peptide 1, Peptide 2, and Peptide 3 are selected from any peptide
disclosed herein.
E. MIF Trimerization Modulating Agents
[00187] In some embodiments, an inflammatory disease, disorder, condition, or
symptom is treated
by modulating the ability of MIF to form a homo-multimer. In some embodiments,
an inflammatory
disease, disorder, condition, or symptom is treated by disrupting the ability
of MIF to form a trimer.
In some embodiments, an inflammatory disease, disorder, condition, or symptom
is treated by
promoting MIF trimerization.
[00188] In certain instances, functionally-active (or, mature) MIF comprises
three MIF peptide
sequences (i.e., a trimer). In certain instances, the pseudo ELR motif/domains
of each MIF
polypeptide form a ring in the trimer. In certain instances, the N-loop
motifs/domains of each MIF
polypeptide extend outwards from the pseudo-ELR ring (see Figure 1).
[00189] In certain instances, residues 38-44 of one subunit interact with
residues 48-50 of a second
subunit. In certain instances, residues 96-102 of one subunit interact with
residues 107-109 of a
second subunit. In certain instances, a motif/domain on one subunit formed by
N73 R74 S77 K78
C81 (numbering includes the first methionine) interacts with N110 Sill T112
(numbering includes
the first methionine) of a second subunit.
[00190] In some embodiments, a MIF trimerization disrupting agent is derived
from and/or
incorporates any or all of amino acid residues 38-44 of MIF (e.g., human,
bovine, procine, murine,
or rat). In some embodiments, a MIF trimerization disrupting agent is a
peptide derived from and/or
incorporates any or all of amino acid residues 48-50 of MIF (e.g., human,
bovine, procine, murine,
or rat). In some embodiments, a MIF trimerization disrupting agent is a
peptide derived from and/or
-48-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
incorporates any or all of amino acid residues 57-66 of MIF (e.g., human,
bovine, procine, murine,
or rat). In some embodiments, a MIF trimerization disrupting agent is a
peptide derived from and/or
incorporates any or all of amino acid residues 61-70 of MIF (e.g., human,
bovine, procine, murine,
or rat). In some embodiments, a MIF trimerization disrupting agent is a
peptide derived from and/or
incorporates any or all of amino acid residues 96-102 of MIF (e.g., human,
bovine, procine, murine,
or rat). In some embodiments, a MIF trimerization disrupting agent is a
peptide derived from and/or
incorporates any or all of amino acid residues 107-109 of MIF (e.g., human,
bovine, procine,
murine, or rat). In some embodiments, a MIF trimerization disrupting agent is
a peptide derived
from and/or incorporates any or all of amino acid residues N73, R74, S77, K78,
and C81 of MIF
(e.g., human, bovine, procine, murine, or rat) (numbering includes the first
methionine). In some
embodiments, a MIF trimerization disrupting agent is a peptide derived from
and/or incorporates
any or all of amino acid residues N110, S111, and T112 of MIF (e.g., human,
bovine, procine,
murine, or rat) (numbering includes the first methionine).
[00191] In some embodiments, a MIF trimerization disrupting agent is a peptide
derived from and/or
incorporates any or all of amino acid residues 57-66 of MIF (numbering
includes the first
methionine). In some embodiments, a MIF trimerization disrupting agent is a
peptide of Formula
(VII):
Xl -XZ-X3-X4-X5-X6-X7-S/A-I-G
wherein:
X' is selected from the group consisting of cysteine, alanine, serine, and
threonine;
x2 is selected from the group consisting of alanine, proline, glycine and
cysteine;
X3 is selected from the group consisting of leucine, valine and
pheynylalanine;
X4 is selected from the group consisting of cysteine, glycine, threonine and
isoleucine;
X5 is selected from the group consisting of serine, valine, glutamine and
asparagine;
X6 is selected from the group consisting of leucine, valine, isoleucine and
methionine; and
X7 is selected from the group consisting of histidine, cysteine, lysine,
arginine, and leucine.
[00192] In some embodiments, the MIF trimerization disrupting agent comprises
3 or more
consecutive amino acids of human MIF57-66- In some embodiments, the MIF
trimerization disrupting
agent comprises 3 or more consecutive amino acids of murine MIF57-66- In some
embodiments, the
MIF trimerization disrupting agent comprises 3 or more consecutive amino acids
of porcine MIF57_
66= In some embodiments, the MIF trimerization disrupting agent comprises 3 or
more consecutive
amino acids of bovine MIF57-66- In some embodiments, the MIF trimerization
disrupting agent
comprises 3 or more consecutive amino acids of rat MIF57-66=
[00193] In some embodiments, a MIF trimerization disrupting agent is an
antibody that binds to any
or all of amino acid residues 38-44 of MIF. In some embodiments, a MIF
trimerization disrupting
agent is an antibody that binds to any or all of amino acid residues 48-50 of
MIF. In some
-49-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
embodiments, a MIF trimerization disrupting agent is an antibody that binds to
any or all of amino
acid residues 57-66 of MIF. In some embodiments, a MIF trimerization
disrupting agent is an
antibody that binds to any or all of amino acid residues 61-70 of MIF. In some
embodiments, a MIF
trimerization disrupting agent is an antibody that binds to any or all of
amino acid residues 96-102
of MIF. In some embodiments, a MIF trimerization disrupting agent is an
antibody that binds to any
or all of amino acid residues 107-109 of MIF. In some embodiments, a MIF
trimerization disrupting
agent is an antibody that binds to any or all of amino acid residues N73, R74,
S77, K78, and C81 of
MIF. In some embodiments, a MIF trimerization disrupting agent is an antibody
that binds to any or
all of amino acid residues N 110, S 111, and T 112 of MIF.
[00194] In some embodiments, a MIF trimerization disrupting agent is a small
molecule that binds to
any or all of amino acid residues 38-44 of MIF. In some embodiments, a MIF
trimerization
disrupting agent is a small molecule that binds to any or all of amino acid
residues 48-50 of MIF. In
some embodiments, a MIF trimerization disrupting agent is a small molecule
that binds to any or all
of amino acid residues 57-66 of MIF. In some embodiments, a MIF trimerization
disrupting agent is
a small molecule that binds to any or all of amino acid residues 61-70 of MIF.
In some
embodiments, a MIF trimerization disrupting agent is a small molecule that
binds to any or all of
amino acid residues 96-102 of MIF. In some embodiments, a MIF trimerization
disrupting agent is a
small molecule that binds to any or all of amino acid residues 107-109 of MIF.
In some
embodiments, a MIF trimerization disrupting agent is a small molecule that
binds to any or all of
amino acid residues N73, R74, S77, K78, and C81 of MIF. In some embodiments, a
MIF
trimerization disrupting agent is a small molecule that binds to any or all of
amino acid residues
N110, S111, and T112 of MIF.
[00195] In some embodiments, a MIF trimerization disrupting agent is a
peptibody that binds to any
or all of amino acid residues 38-44 of MIF. In some embodiments, a MIF
trimerization disrupting
agent is a peptibody that binds to any or all of amino acid residues 48-50 of
MIF. In some
embodiments, a MIF trimerization disrupting agent is a peptibody that binds to
any or all of amino
acid residues 57-66 of MIF. In some embodiments, a MIF trimerization
disrupting agent is a
peptibody that binds to any or all of amino acid residues 61-70 of MIF. In
some embodiments, a
MIF trimerization disrupting agent is a peptibody that binds to any or all of
amino acid residues 96-
102 of MIF. In some embodiments, a MIF trimerization disrupting agent is a
peptibody that binds to
any or all of amino acid residues 107-109 of MIF. In some embodiments, a MIF
trimerization
disrupting agent is a peptibody that binds to any or all of amino acid
residues N73, R74, S77, K78,
and C81 of MIF. In some embodiments, a MIF trimerization disrupting agent is a
peptibody that
binds to any or all of amino acid residues N110, S111, and T112 of MIF.
F. Peptide Mimetics
-50-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00196] In some embodiments, a peptide mimetic is used in place of the
peptides described herein,
including for use in the treatment or prevention of an inflammatory disorder.
[00197] Peptide mimetics (and peptide-based inhibitors) are developed using,
for example,
computerized molecular modeling. Peptide mimetics are designed to include
structures having one
or more peptide linkages optionally replaced by a linkage selected from the
group consisting of. -
CH2NH-, -CH2S-, -CH2 -CH2 -, -CH=CH-(cis and trans), -CH=CF-(trans), -CoCH2
-, -CH(OH)CH2 -, and -CH2SO-, by methods well known in the art. In some
embodiments
such peptide mimetics have greater chemical stability, enhanced
pharmacological properties (half-
life, absorption, potency, efficacy, etc.), altered specificity (e.g., a broad-
spectrum of biological
activities), reduced antigenicity, and are more economically prepared. In some
embodiments peptide
mimetics include covalent attachment of one or more labels or conjugates,
directly or through a
spacer (e.g., an amide group), to non-interfering positions(s) on the analog
that are predicted by
quantitative structure-activity data and/or molecular modeling. Such non-
interfering positions
generally are positions that do not form direct contacts with the receptor(s)
to which the peptide
mimetic specifically binds to produce the therapeutic effect. In some
embodiments, systematic
substitution of one or more amino acids of a consensus sequence with a D-amino
acid of the same
type (e.g., D-lysine in place of L-lysine) are used to generate more stable
peptides with desired
properties.
[00198] Phage display peptide libraries have emerged as a technique in
generating peptide mimetics
(Scott, J. K. et al. (1990) Science 249:386; Devlin, J. J. et al. (1990)
Science 249:404; US5,223,409,
U55,733,731; US5,498,530; US5,432,018;US5,338,665;US5,922,545; WO 96/40987and
WO
98/15833 (each of which is incorporated by reference for such disclosure). In
such libraries, random
peptide sequences are displayed by fusion with coat proteins of filamentous
phage. Typically, the
displayed peptides are affinity-eluted against an antibody-immobilized
extracellular motif/domain
(in this case PF4 or RANTES. In some embodiments peptide mimetics are isolated
by biopanning
(Nowakowski, G.S, et al. (2004) Stem Cells 22:1030-1038). In some embodiments
whole cells
expressing MIF are used to screen the library utilizing FACs to isolate phage
specifically bound
cells. The retained phages are enriched by successive rounds of biopanning and
repropagation. The
best binding peptides are sequenced to identify key residues within one or
more structurally related
families of peptides. The peptide sequences also suggest which residues to
replace by alanine
scanning or by mutagenesis at the DNA level. In some embodiments mutagenesis
libraries are
created and screened to further optimize the sequence of the best binders.
Lowman (1997)
Ann.Rev.Biophys.Biomol.Struct. 26:401-24.
[00199] In some embodiments structural analysis of protein-protein interaction
is used to suggest
peptides that competitiveky bind with a binding partners of polypeptides
described herein. In some
embodiments the crystal structure resulting from such an analysis suggests the
identity and relative
-51-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
orientation of critical residues of the polypeptide, from which a peptide is
designed. See, e.g.,
Takasaki, et al. (1997) Nature Biotech, 15: 1266-70.
[00200] In some embodiments, the agent is a peptide or polypeptide. In some
embodiments, the
peptide is: a peptide that competitively binds with a binding partner of
VNTNVPPRASVPDGFLSELTQQLAQATGKPPQYIAVHVVPDQL (SEQ ID No. 10); a peptide
that competitively binds with a binding partner of PDQLMAFGGSSEPCALCSL (SEQ ID
No. 11);
a peptide that competitively binds with a binding partner of
VNTNVPPRASVPDGFLSELTQQLAQATGKPPQYIAVHVVPDQLMAFGGSSEPCALCSL
(SEQ ID No. 12); a peptide that competitively binds with a binding partner of
PDQLMAFGGSSEPCALCSLHSI (SEQ ID No. 13); or combinations thereof.
G. Antibodies
[00201] In some embodiments, an inflammatory disease, disorder, condition, or
symptom is treated
by disrupting the ability of MIF to bind to CXCR2, CXCR4, CD74, or a
combination thereof. In
some embodiments, an inflammatory disease, disorder, condition, or symptom is
treated by
administering to an individual in need an antibody that binds to MIF, one or
more MIF motifs. In
some embodiments, an inflammatory disease, disorder, condition, or symptom is
treated by
administering to an individual in need an antibody that binds to CD44. In some
embodiments, an
inflammatory disease, disorder, condition, or symptom is treated by
administering to an individual in
need an antibody that binds to CD74. In some embodiments, an inflammatory
disease, disorder,
condition, or symptom is treated by administering to an individual in need an
antibody that binds to
CXCR2. In some embodiments, an inflammatory disease, disorder, condition, or
symptom is treated
by administering to an individual in need an antibody that binds to CXCR4.
[00202] In some embodiments, the antibody is a human antibody or a humanized
antibody. In some
embodiments, the antibody is a human IgG. In some embodiments, the antibody is
or comprises one
or more polypeptides derived from a human IgGI, IgG4, IgG2, IgD, IgA or IgM.
An antibody
disclosed herein is generated by any suitable method.
Antigen-Based Antibody Development
[00203] In some embodiments, an antibody disclosed herein is generated by
contacting a host (e.g., a
mouse or rabbit) with an antigen. In some embodiments, the antigen is a MIF
monomer. In some
embodiments, the antigen is a MIF trimer. In some embodiments, the antigen is
a fragment of a full-
length MIF polypeptide. In some embodiments, the antigen is a polypeptide that
encompasses all or
part of MIF50-65. In some embodiments, the antigen is a polypeptide that
encompasses all or part of
the MIF N-terminal/pseudo-ELR motif/domain (MIF1-17). In some embodiments, the
antigen is a
polypeptide that encompasses all or part of the MIF alpha-helix #1
motif/domain (i.e., MIF18-31). In
some embodiments, the antigen is a polypeptide that encompasses all or part of
the MIF N-loop
-52-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
motif/domain (i.e., MIF32_60). In some embodiments, the antigen is a
polypeptide that encompasses
all or part of the MIF loop-barrel-loop motif/domain (i.e., MIF64-93)= In some
embodiments, the
antigen is a polypeptide that encompasses all or part of the MIF C-terminal
motif/domain (i.e.,
MIF90_114). In some embodiments, the antigen is a polypeptide that encompasses
all or part of the
MIF N-terminal tail (i.e., M1F1_7). In some embodiments, the antigen is a
polypeptide that
encompasses all or part of the MIF pseudo ELR-loop (i.e., MIF7_17). In some
embodiments, the
antigen is a polypeptide that encompasses all or part of the MIF PPQ-loop
(i.e., MIF32-38)= In some
embodiments, the antigen is a polypeptide that encompasses all or part of the
MIF PDQ-loop (i.e.,
MIF43_56). In some embodiments, the antigen is a polypeptide that encompasses
all or part of the MIF
IGK-loop (i.e., MIF64_71). In some embodiments, the antigen is a polypeptide
that encompasses all or
part of the MIF NRS-helix (i.e., MIF72_89). In some embodiments, the antigen
is a polypeptide that
encompasses all or part of the MIF SPDR-loop (i.e., MIF90_94). In some
embodiments, the antigen is
a polypeptide that encompasses all or part of the MIF C-terminal tail (i.e.,
M1F1o1-114).
[00204] In some embodiments, an antibody disclosed herein is generated by
contacting a host (e.g., a
mouse or rabbit) with at least two antigens. In some embodiments, the antigens
are selected from: a
polypeptide that encompasses all or part of the MIF N-terminal/pseudo-ELR
motif; a polypeptide
that encompasses all or part of the MIF N-loop motif; a polypeptide that
encompasses all or part of
the MIF loop-barrel-loop motif; a polypeptide that encompasses all or part of
the MIF C-terminal
motif; a polypeptide that encompasses all or part of the MIF alpha-helix #1
motif; a polypeptide that
encompasses all or part of the MIF N-terminal tail; a polypeptide that
encompasses al or part of the
MIF pseudo ELR motif/domain; a polypeptide that encompasses all or part of the
MIF PPQ-loop; a
polypeptide that encompasses all or part of the MIF PDQ-loop; a polypeptide
that encompasses all
or part of the MIF IGK loop; a polypeptide that encompasses all or part of the
MIF NRS helix; a
polypeptide that encompasses all or part of the MIF SPDR loop; a polypeptide
that encompasses all
or part of the C-terminal tail; a polypeptide that encompasses all or part of
MIF50-65=
[00205] In some embodiments, an antibody disclosed herein is generated by
contacting a host (e.g., a
mouse or rabbit) with at least three antigens. In some embodiments, the
antigens are selected from: a
polypeptide that encompasses all or part of the MIF N-terminal/pseudo-ELR
motif; a polypeptide
that encompasses all or part of the MIF N-loop motif; a polypeptide that
encompasses all or part of
the MIF loop-barrel-loop motif; a polypeptide that encompasses all or part of
the MIF C-terminal
motif; a polypeptide that encompasses all or part of the MIF alpha-helix #1
motif; a polypeptide that
encompasses all or part of the MIF N-terminal tail; a polypeptide that
encompasses al or part of the
MIF pseudo ELR motif/domain; a polypeptide that encompasses all or part of the
MIF PPQ-loop; a
polypeptide that encompasses all or part of the MIF PDQ-loop; a polypeptide
that encompasses all
or part of the MIF IGK loop; a polypeptide that encompasses all or part of the
MIF NRS helix; a
-53-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
polypeptide that encompasses all or part of the MIF SPDR loop; a polypeptide
that encompasses all
or part of the C-terminal tail; and a polypeptide that encompasses all or part
of MIF50-65=
DNA-Based Antibody Development
[00206] In some embodiments, an antibody disclosed herein is generated by
contacting a host with a
nucleic acid sequence encoding part or all of a MIF polypeptide
(alternatively, "MIF nucleic acid
sequence").
[00207] In some embodiments, the MIF nucleic acid sequence has been cloned
into an expression
vector (e.g., a plasmid).
[00208] In some embodiments, the host is a mammal. In some embodiments, the
host is a mouse, a
rabbit, or a rat. In some embodiments, the host is a mammalian cell. In some
embodiments, the host
is a bacterial cell.
[00209] In some embodiments, the MIF nucleic acid sequence is contacted with
the host by injecting
the MIF nucleic acid sequence into the host intramuscularly or intradermally.
In some embodiments,
the contacting further comprises applying an electric current to the site of
injection (i.e.,
electroporation). In some embodiments, the MIF nucleic acid sequence is
contacted with the host by
use of a gene gun.
[00210] In some embodiments, the nucleic acid sequence encoding part or all of
a MIF polypeptide
is expressed by a host cell (or a plurality of host cells) to generate an
expressed MIF polypeptide. In
some embodiments, the expressed MIF polypeptide is cysteinylated. In some
embodiments, the
expressed MIF polypeptide is phosphorylated. In some embodiments, the
expressed MIF
polypeptide is glycosylated.
[00211] In some embodiments, a method of generating an antibody disclosed
herein further
comprises contacting the host with an adjuvant. In some embodiments, the
adjuvant is administered
as a nucleic acid sequence. In some embodiments, the adjuvant is administered
as a polypeptide or
polysaccharide. In some embodiments, the adjuvant is a cytokine, a lymphokine,
or a combination
thereof. In some embodiments, the adjuvant is an interleukin, a tumor necrosis
factor, GM-CSF, or a
combination thereof. In some embodiments, the adjuvant is B7-1, B7-2, CD40L,
or a combination
thereof. In some embodiments, the expression vector containing the MIF nucleic
acid sequence
further comprises a nucleic acid sequence encoding an adjuvant. In some
embodiments, the host is
contacted with a second expression vector encoding an adjuvant.
[00212] In some embodiments, the nucleic acid sequence encodes the MIF N-
terminal tail/pseudo-
ELR motif. In some embodiments, the nucleic acid sequence encodes MIF50-65. In
some
embodiments, the nucleic acid sequence encodes the MIF N-loop motif. In some
embodiments, the
nucleic acid sequence encodes the MIF loop-barrel-loop motif. In some
embodiments, the nucleic
acid sequence encodes the MIF C-terminal motif. In some embodiments, the
nucleic acid sequence
encodes the MIF alpha-helix #1 motif/domain (i.e.,
-54-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
TTCCTGAGCGAGCTGACACAGCAGCTGGCCCAGGCCACCGGC). In some embodiments, the
nucleic acid sequence encodes the MIF N-terminal tail (i.e.,
CCCATGTTCATCGTGAACACC). In
some embodiments, the nucleic acid sequence encodes the MIF pseudo ELR
motif/domain (i.e.,
AACGTGCCCAGAGCCAGCGTGCCCGACGGC). In some embodiments, the nucleic acid
sequence
encodes the MIF PPQ loop (i.e., AAGCCCCCTCAGTATATCGCC). In some embodiments,
the
nucleic acid sequence encodes the MIF PDQ loop (i.e.,
CCCGACCAGCTGATGGCCTTCGGCGGCAGCAGCGAGCCTTGC). In some embodiments, the
nucleic acid sequence encodes the MIF IGK-loop (i.e.,
ATCGGCAAGATCGGCGGAGCCCAG). In
some embodiments, the nucleic acid sequence encodes the MIF NRS-helix (i.e.,
AACAGAAGCTACAGCAAGCTGCTGTGCGGCCTGCTGGCCGAGAGACTGAGAATC). In some
embodiments, the nucleic acid sequence encodes the SPDR loop (i.e.,
AGCCCCGACAGAGTGTACATCAACTACTACGAC). In some embodiments, the nucleic acid
sequence encodes the C-terminal tail (i.e.,
ATGAACGCCGCCAACGTGGGCTGGAACAACAGCACCTTCGCC).
[00213] In some embodiments, an antibody disclosed herein is generated by
contacting a host with at
least two nucleic acid sequences selected from: a sequence encoding the MIF N-
terminal
tail/pseudo-ELR motif, a sequence encoding the MIF N-loop motif, a sequence
encoding the MIF
loop-barrel-loop motif, a sequence encoding the MIF C-terminal motif, a
sequence encoding the
MIF alpha-helix #1 motif, a sequence encoding the MIF N-terminal tail, a
sequence encoding the
MIF pseudo ELR motif/domain, a sequence encoding the MIF PPQ loop, a sequence
encoding the
MIF PDQ loop, a sequence encoding the MIF IGK loop, a sequence encoding the
MIF NRS helix, a
sequence encoding the MIF SPDR loop, a sequence encoding the MIF C-terminal
tail, and a
sequence encoding MIF50-65. In some embodiments, an antibody disclosed herein
is generated by
contacting a host with a nucleic acid sequence encoding at least two MIF
polypeptide motifs
selected from: a sequence encoding the MIF N-terminal tail/pseudo-ELR motif, a
sequence
encoding the MIF N-loop motif, a sequence encoding the MIF loop-barrel-loop
motif, a sequence
encoding the MIF C-terminal motif, a sequence encoding the MIF alpha-helix #1
motif, a sequence
encoding the MIF N-terminal tail, a sequence encoding the MIF pseudo ELR
motif/domain, a
sequence encoding the MIF PPQ loop, a sequence encoding the MIF PDQ loop, a
sequence
encoding the MIF IGK loop, a sequence encoding the MIF NRS helix, a sequence
encoding the MIF
SPDR loop, a sequence encoding the MIF C-terminal tail, and a sequence
encoding MIF50-65=
[00214] In some embodiments, an antibody disclosed herein is generated by
contacting a host with at
least three nucleic acid sequences selected from: a sequence encoding the MIF
N-terminal
tail/pseudo-ELR motif, a sequence encoding the MIF N-loop motif, a sequence
encoding the MIF
loop-barrel-loop motif, a sequence encoding the MIF C-terminal motif, a
sequence encoding the
MIF alpha-helix #1 motif, a sequence encoding the MIF N-terminal tail, a
sequence encoding the
-55-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
MIF pseudo ELR motif/domain, a sequence encoding the MIF PPQ loop, a sequence
encoding the
MIF PDQ loop, a sequence encoding the MIF IGK loop, a sequence encoding the
MIF NRS helix, a
sequence encoding the MIF SPDR loop, a sequence encoding the MIF C-terminal
tail, and a
sequence encoding MIF50-65. In some embodiments, an antibody disclosed herein
is generated by
contacting a host with a nucleic acid sequence encoding at least three MIF
polypeptide motifs
selected from: a sequence encoding the MIF N-terminal tail/pseudo-ELR motif, a
sequence
encoding the MIF N-loop motif, a sequence encoding the MIF loop-barrel-loop
motif, a sequence
encoding the MIF C-terminal motif, a sequence encoding the MIF alpha-helix #1
motif, a sequence
encoding the MIF N-terminal tail, a sequence encoding the MIF pseudo ELR
motif/domain, a
sequence encoding the MIF PPQ loop, a sequence encoding the MIF PDQ loop, a
sequence
encoding the MIF IGK loop, a sequence encoding the MIF NRS helix, a sequence
encoding the MIF
SPDR loop, a sequence encoding the MIF C-terminal tail, and a sequence
encoding MIF50-65==
Production of Antibodies
[00215] In some embodiments, an antibody disclosed herein is produced via the
use of a hybridoma.
As used herein, a "hybridoma" is an immortalized antibody producing cell. In
some embodiments, a
host (e.g., a mouse or a rabbit) is inoculated with an antigen or a nucleic
acid. In some embodiments,
B-cells from the host's spleen are extracted. In some embodiments, a hybridoma
is generated by
fusing (1) an extracted B-cell with (2) a myeloma cell (i.e., hypoxanthine-
guanine-phosphoribosyl
transferase negative, immortalized myeloma cells). In some embodiments, the B-
cell and the
myeloma cells are cultured together and exposed to an agent that renders their
cell membranes more
permeable (e.g., PEG).
[00216] In some embodiments, the culture comprises a plurality of hybridoma, a
plurality of
myeloma cells, and a plurality of B-cells. In some embodiments, the cells are
individual to culturing
conditions that select for hybridoma (e.g., culturing with HAT media).
[00217] In some embodiments, an individual hybridoma (i.e., the clone) is
isolated and cultured. In
some embodiments, the hybridoma are injected into a laboratory animal. In some
embodiments, the
hybridoma are cultured in a cell culture.
Humanized Antibodies
[00218] In some embodiments, the methods described herein comprise a humanized
monoclonal
antibody. In some embodiments, a humanized monoclonal antibody comprises heavy
and light chain
constant regions from a human source and variable regions from a murine
source.
[00219] In some embodiments, humanized immunoglobulins, including humanized
antibodies, are
constructed by genetic engineering. In some embodiments, humanized
immunoglobulins comprise a
framework that is identical to the framework of a particular human
immunoglobulin chain (i.e., an
acceptor or recipient), and three CDRs from a non-human (donor) immunoglobulin
chain. In some
embodiments, a limited number of amino acids in the framework of a humanized
immunoglobulin
-56-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
chain are identified and chosen to be the same as the amino acids at those
positions in the donor
rather than in the acceptor.
[00220] In some embodiments, a framework is used from a particular human
immunoglobulin that is
homologous to the donor immunoglobulin to be humanized. For example,
comparison of the
sequence of a mouse heavy (or light) chain variable region against human heavy
(or light) variable
regions in a data bank (for example, the National Biomedical Research
Foundation Protein
Identification Resource or the protein sequence database of the National
Center for Biotechnology
Information - NCBI) shows that the extent of homology to different human
regions can vary greatly,
for example from about 40% to about 60%, about 70%, about 80%, or higher. By
choosing as the
acceptor immunoglobulin one of the human heavy chain variable regions that is
most homologous to
the heavy chain variable region of the donor immunoglobulin, fewer amino acids
will be changed in
going from the donor immunoglobulin to the humanized immunoglobulin. By
choosing as the
acceptor immunoglobulin one of the human light chain variable regions that is
most homologous to
the light chain variable region of the donor immunoglobulin, fewer amino acids
will be changed in
going from the donor immunoglobulin to the humanized immunoglobulin.
[00221] In some embodiments, a humanized immunoglobulin comprises light and
heavy chains from
the same human antibody as acceptor sequences. In some embodiments, a
humanized
immunoglobulin comprises light and heavy chains from different human antibody
germline
sequences as acceptor sequences; when such combinations are used, one can
readily determine
whether the VH and VL bind an epitope of interest using conventional assays
(e.g., an ELISA). In
some embodiments, the human antibody will be chosen in which the light and
heavy chain variable
regions sequences, taken together, are overall most homologous to the donor
light and heavy chain
variable region sequences. In some embodiments, higher affinity is achieved by
selecting a small
number of amino acids in the framework of the humanized immunoglobulin chain
to be the same as
the amino acids at those positions in the donor rather than in the acceptor.
[00222] Any suitable method of modifying a framework region is contemplated
herein. In some
embodiments, the relevant framework amino acids to change are selected based
on differences in
amino acid framework residues between the donor and acceptor molecules. In
some embodiments,
the amino acid positions to change are residues known to be important or to
contribute to CDR
conformation (e.g., canonical framework residues are important for CDR
conformation and/or
structure). In some embodiments, the relevant framework amino acids to change
are selected based
on frequency of an amino acid residue at a particular framework position
(e.g., comparison of the
selected framework with other framework sequences within its subfamily can
reveal residues that
occur at minor frequencies at a particular position or positions). In some
embodiments, the relevant
framework amino acids to change are selected based on proximity to a CDR. In
some embodiments,
the relevant framework amino acids to change are selected based on known or
predicted proximity
-57-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
to the antigen-CDR interface or predicted to modulate CDR activity. In some
embodiments, the
relevant framework amino acids to change are framework residues that are known
to, or predicted
to, form contacts between the heavy (VH) and light (VL) chain variable region
interface. In some
embodiments, the relevant framework amino acids to change are framework
residues that are
inaccessible to solvent.
[00223] In some embodiments, amino acid changes at some or all of the selected
positions are
incorporated into encoding nucleic acids for the acceptor variable region
framework and donor
CDRs. In some embodiments, altered framework or CDR sequences are individually
made and
tested, or are sequentially or simultaneously combined and tested.
[00224] In some embodiments, the variability at any or all of the altered
positions is from a few to a
plurality of different amino acid residues, including all twenty naturally
occurring amino acids or
functional equivalents and analogues thereof. In some embodiments, non-
naturally occurring amino
acids are considered.
[00225] In some embodiments, the humanized antibody sequence is cloned into a
vector. In some
embodiments, any suitable vector is used. In some embodiments, the vector is a
plasmid, viral e.g.
`phage, or phagemid, as appropriate. For further details see, for example,
Molecular Cloning: a
Laboratory Manual: 2nd edition, Sambrook et al., 1989, Cold Spring Harbor
Laboratory Press.
Many known techniques and protocols for manipulation of nucleic acid, for
example in preparation
of nucleic acid constructs, mutagenesis, sequencing, introduction of DNA into
cells and gene
expression, and analysis of proteins, are described in detail in Short
Protocols in Molecular Biology,
Second Edition, Ausubel et al. eds., John Wiley & Sons, 1992. The disclosures
of Sambrook et al.
and Ausubel et al. are incorporated herein by reference for such disclosure.
[00226] In some embodiments, any suitable host cell is transformed with the
vector expressing the
humanized antibody sequence. In some embodiments, the host cell is bacteria,
mammalian cells,
yeast and baculovirus systems. The expression of antibodies and antibody
fragments in prokaryotic
cells such as E. coli is well established in the art. For a review, see for
example Pliickthun, A.
Bio/Technology 9: 545-551 (1991). Expression in eukaryotic cells in culture is
also available to
those skilled in the art as an option for production of the antibodies and
antigen-binding fragments
described herein, see for recent reviews, for example Raff, M.E. (1993) Curr.
Opinion Biotech. 4:
573-576; Trill J.J. et al. (1995) Curr. Opinion Biotech 6: 553-560, each of
which is which is
incorporated herein by reference for such disclosure.
[00227] In some embodiments, a mammalian expression system is used. In some
embodiments, the
mammalian expression system is dehydrofolate reductase deficient ("dhfr- ")
Chinese hamster ovary
cells. In some embodiments, dhfr- CHO cells are transfected with an expression
vector containing a
functional DHFR gene, together with a gene that encodes a desired humanized
antibody.
-58-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00228] In some embodiments, DNA is transformed by any suitable method. For
eukaryotic cells,
suitable techniques include, for example, calcium phosphate transfection, DEAE
Dextran,
electroporation, liposome-mediated transfection and transduction using
retrovirus or other virus,
e.g., vaccinia or, for insect cells, baculovirus. For bacterial cells,
suitable techniques include, for
example, calcium chloride transformation, electroporation and transfection
using bacteriophage.
[00229] In some embodiments, a DNA sequence encoding an antibody or antigen-
binding fragment
thereof is prepared synthetically rather than cloned. In some embodiments, the
DNA sequence is
designed with the appropriate codons for the antibody or antigen-binding
fragment amino acid
sequence. In general, one will select preferred codons for the intended host
if the sequence will be
used for expression. In some embodiments, the complete sequence is assembled
from overlapping
oligonucleotides prepared by standard methods and assembled into a complete
coding sequence.
See, e.g., Edge, Nature, 292:756 (1981); Nambair et al., Science, 223:1299
(1984); Jay et al., J. Biol.
Chem., 259:6311 (1984), each of which is which is incorporated herein by
reference for such
disclosure.
H. Peptibodies
[00230] In some embodiments, a composition of matter disrupts the ability of
MIF to bind to
CXCR2, CXCR4, CD74, or a combination thereof. In some embodiments, the
composition of matter
is a peptibody. In some embodiments, an inflammatory disease, disorder,
condition, or symptom is
treated by disrupting the ability of MIF to bind to CXCR2, CXCR4, CD74, or a
combination
thereof. In some embodiments, an inflammatory disease, disorder, condition, or
symptom is treated
by administering to an individual in need thereof a peptibody.
[00231] The term "peptibody" refers to a molecule comprising peptide(s) bound
(e.g., covalenly)
either directly or indirectly to an antibody or one or more antibody
motif/domains (e.g., an Fc
motif/domain of an antibody), where the peptide moiety specifically binds to a
desired target. The
peptide(s) may be fused to either an Fc region or inserted into an Fc- Loop, a
modified Fc molecule.
The term "peptibody" does not include Fc-fusion proteins (e.g., full length
proteins fused to an Fc
motif/domain).
[00232] In some embodiments, the peptibody comprises (a) an antibody, and (b)
a peptide disclosed
herein; wherein the peptide and the antibody retain their activity in the
peptibody. In some
embodiments, the peptide is bound (directly or indirectly) to the antibody. In
some embodiments,
the peptide is covalently bound (directly or indirectly) to the antibody. In
some embodiments, the
peptide is bound (directly or indirectly) to the Fab region of the antibody.
In some embodiments, the
peptide is bound (directly or indirectly) to the antigen binding site of the
antibody.
[00233] In some embodiments, the peptide binds to the antibody via a reactive
side chain. A reactive
side chain may be present naturally or may be placed in an antibody by
mutation. The reactive
residue of the antibody combining site may be associated with the antibody,
such as when the
-59-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
residue is encoded by nucleic acid present in the lymphoid cell first
identified to make the antibody.
Alternatively, the amino acid residue may arise by purposely mutating the DNA
so as to encode the
particular residue. The reactive residue may be a non-natural residue arising,
for example, by
biosynthetic incorporation using a unique codon, tRNA, and aminoacyl-tRNA as
discussed herein.
In another approach, the amino acid residue or its reactive functional groups
(e.g., a nucleophilic
amino group or sulfhydryl group) may be attached to an amino acid residue in
the antibody
combining site.
[00234] Catalytic antibodies are one source of antibodies that comprise one or
more reactive amino
acid side chains. Such antibodies include aldolase antibodies, beta lactamase
antibodies, esterase
antibodies, amidase antibodies, and the like.
[00235] In some embodiments, the peptide is indirectly bound to the antibody
via a linker. In some
embodiments, the linker comprises an alkyl, a heteroalkyl, an alkylene, an
alkenylene, an
alkynylene, a heteroalkylene, a carbocycle, a heterocycle, an aromatic ring, a
non-aromatic ring, a
substituted ring, a monocyclic ring, a polycyclic ring, or a combination
thereof.
[00236] In some embodiments, the antibody is an IgA, IgD, IgE, IgG, or IgM. In
some
embodiments, the antibody is a humanized antibody.
[00237] In some embodiments, the peptibody is a CovXTM body.
III. Assays for Identifying MIF motif/domain Disrupting Agents
[00238] In some embodiments, an agent that binds to a MIF motif/domain
disclosed herein is
identified. In some embodiments, an agent that binds to a MIF motif/domain
disclosed herein does
not influence MIF-independent signaling events at CXCR2 and CXCR4.
[00239] In some embodiments, a library of peptides covering the extracellular
N-terminal
motif/domain and/or the extracellular loops of CXCR2 and CXCR4 is generated.
In some
embodiments, the peptides range in size from about 5 amino acids to about 20
amino acid; from
about 7 amino acids to about 18 amino acids; from about 10 amino acids to
about 15 amino acids. In
some embodiments, the peptide library is screened for inhibition of MIF-
mediated signaling through
CXCR2 and CXCR4 using any suitable method (e.g., HTS GPCR screening
technology). In some
embodiments, the peptide library is further screened for inhibition of 11-8
and/or SDF-1 mediated
signaling on CXCR2 and CXCR4. In some embodiments, a peptide is identified as
a MIF
motif/domain disrupting peptide if it inhibits MIF- signaling through CXCR2
and CXCR4 but
allows SDF-1- and IL-8-mediated signaling through CXCR2 and CXCR4.
[00240] In some embodiments, peptide sequences from the extracellular N-
terminal motif/domain
and the extracellular loops of CXCR2 and CXCR4 are arrayed onto a membrane. In
some
embodiments, the peptide sequences from the extracellular N-terminal
motif/domain and the
extracellular loops of CXCR2 and CXCR4 are arrayed onto a membrane are probed
with full-length
-60-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
MIF. In some embodiments, the MIF is labeled (e.g., isotopically labeled,
radioactively labeled, or
fluorophore labeled). In some embodiments, peptide sequences to which labeled
MIF specifically
bound are assayed for inhibition of MIF-mediated signaling of CXCR2 and CXCR4.
In some
embodiments, the peptide sequences that inhibit MIF-mediated signaling of
CXCR2 and CXCR4 are
screened using any suitable method (e.g., GPCR screening assay).
[00241] In some embodiments, any of the aforementioned peptides and/or
polypeptides (e.g., a
peptide derived from a pseudo ELR motif/domain of MIF or an N-loop
motif/domain of MIF) is
used as a "model" to do structure-activity relationship (SAR) chemistry (as
provided in detail
herein). In some embodiments, the SAR chemistry yields smaller peptides. In
some embodiments,
the smaller peptides yield small molecules that disrupt the ability of MIF to
bind to CXCR2 and/or
CXCR4 (e.g., by determining the amino acid residues involved in disrupting the
ability of MIF to
bind to CXCR2 and/or CXCR4).
IV. Assays for Identifying MIF Trimerization Disrupting Agents
[00242] In some embodiments, a MIF trimerization disrupting peptide is
identified. In some
embodiments, a MIF motif/domain trimerization disrupting peptide does not
influence MIF-
independent signaling events at CXCR2 and CXCR4. In some embodiments, a
peptide and/or
polypeptide derived from any of the aforementioned amino acid sequences (e.g.,
amino acid residues
38-44 (beta-2 strand) of MIF, amino acid residues 48-50 (beta-3 strand) of
MIF, amino acid residues
96-102 (beta-5 strand) of MIF, amino acid residues 107-109 (beta-6 strand) of
MIF, amino acid
residues N73, R74, S77, K78, and C81 of MIF, and/or amino acid residues N110,
S111, and T112 of
MIF) is screened for inhibition of MIF-mediated signaling through CXCR2 and
CXCR4 using any
suitable method (e.g., HTS GPCR screening technology).
[00243] In some embodiments, a peptide and/or polypeptide derived from any of
the aforementioned
amino acid sequences (e.g., amino acid residues 3 8-44 (beta-2 strand) of MIF,
amino acid residues
48-50 (beta-3 strand) of MIF, amino acid residues 96-102 (beta-5 strand) of
MIF, amino acid
residues 107-109 (beta-6 strand) of MIF, amino acid residues N73, R74, S77,
K78, and C81 of MIF,
and/or amino acid residues N110, S 111, and T112 of MIF) is used as a "model"
to do structure-
activity relationship (SAR) chemistry. In some embodiments, the SAR chemistry
yields smaller
peptides. In some embodiments, the smaller peptides yield small molecules that
disrupt the ability of
MIF to form a homotrimer (e.g., by figuring out the amino acid residues
involved in disrupting the
ability of MIF to form a homotrimer).
[00244] In some embodiments, a MIF small molecule, peptide, and/or antibody
antagonist is derived
from and/or incorporates any or all of amino acid residues 1-45 of SEQ. ID.
NO. 1. In some
embodiments, a MIF small molecule, peptide, and/or antibody antagonist is a
peptide derived from
and/or incorporates any or all of amino acid residues 2-45 of SEQ. ID. NO. 1.
In some
-61-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
embodiments, a MIF small molecule, peptide, and/or antibody antagonist is a
peptide derived from
and/or incorporates any or all of amino acid residues 3-45 of SEQ. ID. NO. 1.
In some
embodiments, a MIF small molecule, peptide, and/or antibody antagonist is a
peptide derived from
and/or incorporates any or all of amino acid residues 4-45 of SEQ. ID. NO. 1.
In some
embodiments, a MIF small molecule, peptide, and/or antibody antagonist is a
peptide derived from
and/or incorporates any or all of amino acid residues 5-45 of SEQ. ID. NO. 1.
In some
embodiments, a MIF small molecule, peptide, and/or antibody antagonist is a
peptide derived from
and/or incorporates any or all of amino acid residues 6-45 of SEQ. ID. NO. In
some embodiments, a
MIF small molecule, peptide, and/or antibody antagonist is a peptide derived
from and/or
incorporates any or all of amino acid residues 7-45 of SEQ. ID. NO. In some
embodiments, a MIF
small molecule, peptide, and/or antibody antagonist is a peptide derived from
and/or incorporates
any or all of amino acid residues 8-45 of SEQ. ID. NO. In some embodiments, a
MIF small
molecule, peptide, and/or antibody antagonist is a peptide derived from and/or
incorporates any or
all of amino acid residues 9-45 of SEQ. ID. NO. In some embodiments, a MIF
small molecule,
peptide, and/or antibody antagonist is a peptide derived from and/or
incorporates any or all of amino
acid residues 10-45 of SEQ. ID. NO.
[00245] In some embodiments, a peptide and/or polypeptide derived from any of
the aforementioned
amino acid sequences (e.g., amino acid residues 1-45 of SEQ. ID. NO. 1; amino
acid residues 2-45
of SEQ. ID. NO. 1; amino acid residues 3-45 of SEQ. ID. NO. 1; amino acid
residues 4-45 of SEQ.
ID. NO. 1; amino acid residues 5-45 of SEQ. ID. NO. 1; amino acid residues 6-
45 of SEQ. ID. NO.
1; amino acid residues 7-45 of SEQ. ID. NO. 1; amino acid residues 8-45 of
SEQ. ID. NO. 1; amino
acid residues 9-45 of SEQ. ID. NO. 1; or amino acid residues 10-45 of SEQ. ID.
NO. 1) is used as a
"model" to do structure-activity relationship (SAR) chemistry. In some
embodiments, the SAR
chemistry yields smaller peptides. In some embodiments, the smaller peptides
yield small molecules
that disrupt the ability of MIF to form a homotrimer (e.g., by determining the
amino acid residues
involved in disrupting the ability of MIF to form a homotrimer).
[00246] In some embodiments, the antagonist of MIF is an siRNA molecule and/or
an antisense
molecule complementary to a MIF gene and/or MIF RNA sequence. In some
embodiments, the
siRNA and/or antisense molecule decreases the level or half-life of MIF mRNA
and/or protein by at
least about 5%, at least about 10%, at least about 20%, at least about 30%, at
least about 40%, at
least about 50%, at least about 60%, at least about 80%, at least about 90%,
at least about 95%, or
substantially 100%.
V. Cell Lines
[00247] Disclosed herein, in some embodiments, is a cell line that expresses a
recombinant human
CXCR4 plus human CD74. In some embodiments, the cell line that expresses a
recombinant human
-62-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
CXCR4 plus human CD74 is a human cell line (e.g., HEK293). In some
embodiments, the cell line
that expresses a recombinant human CXCR4 plus human CD74 is a non-human cell
line (e.g.,
CHO).
VI. Inflammation
[00248] In some embodiments, the methods and compositions described herein
treat an
inflammatory disease, disorder, condition, or symptom (e.g., acute or
chronic). In some
embodiments, the methods and compositions described herein treat an
inflammatory disease,
disorder, condition, or symptom resulting from (either partially or fully) an
infection. In some
embodiments, the methods and compositions described herein treat an
inflammatory disease,
disorder, condition, or symptom resulting from (either partially or fully)
damage to a tissue (e.g., by
a burn, by frostbite, by exposure to a cytotoxic agent, or by trauma). In some
embodiments, the
methods and compositions described herein treat an inflammatory disease,
disorder, condition, or
symptom resulting from (either partially or fully) an autoimmune disorder. In
some embodiments,
the methods and compositions described herein treat an inflammatory disease,
disorder, condition,
or symptom resulting from (either partially or fully) the presence of a
foreign body (e.g., a splinter).
In some embodiments, the methods and compositions described herein treat an
inflammatory
disease, disorder, condition, or symptom resulting from exposure to a toxin
and/or chemical irritant.
[00249] As used herein, "acute inflammation" refers to inflammation
characterized in that it
develops over the course of a few minutes to a few hours, and ceases once the
stimulus has been
removed (e.g., an infectious agent has been killed by an immune response or
administration of a
therapeutic agent, a foreign body has been removed by an immune response or
extraction, or
damaged tissue has healed). The short duration of acute inflammation results
from the short half-
lives of most inflammatory mediators.
[00250] In certain instances, acute inflammation begins with the activation of
leukocytes (e.g.,
dendritic cells, endothelial cells and mastocytes). In certain instances, the
leukocytes release
inflammatory mediators (e.g., histamines, proteoglycans, serine proteases,
eicosanoids, and
cytokines). In certain instances, inflammatory mediators result in (either
partially or fully) the
symptoms associated with inflammation. For example, In certain instances an
inflammatory
mediator dilates post capillary venules, and increases blood vessel
permeability. In certain instances,
the increased blood flow that follows vasodilation results in (either
partially or fully) rubor and
calor. In certain instances, increased permeability of the blood vessels
results in an exudation of
plasma into the tissue leading to edema. In certain instances, the latter
allows leukocytes to migrate
along a chemotactic gradient to the site of the inflammatory stimulant.
Further, In certain instances,
structural changes to blood vessels (e.g., capillaries and venules) occur. In
certain instances, the
structural changes are induced (either partially or fully) by monocytes and/or
macrophages. In
-63-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
certain instances, the structural changes include, but are not limited to,
remodeling of vessels, and
angiogenesis. In certain instances, angiogenesis contributes to the
maintenance of chronic
inflammation by allowing for increased transport of leukocytes. Additionally,
In certain instances,
histamines and bradykinin irritate nerve endings leading to itching and/or
pain.
[00251] In certain instances, chronic inflammation results from the presence
of a persistent stimulant
(e.g., persistent acute inflammation, bacterial infection (e.g., by
Mycobacterium tuberculosis),
prolonged exposure to chemical agents (e.g., silica, or tobacco smoke) and
autoimmune reactions
(e.g., rheumatoid arthritis)). In certain instances, the persistent stimulant
results in continuous
inflammation (e.g., due to the continuous recruitment of monocytes, and the
proliferation of
macrophages). In certain instances, the continuous inflammation further
damages tissues which
results in the additional recruitment of mononuclear cells thus maintaining
and exacerbating the
inflammation. In certain instances, physiological responses to inflammation
further include
angiogenesis and fibrosis.
[00252] In some embodiments, the methods and compositions described herein
treat an
inflammatory disease, disorder, condition, or symptom. By way of non-limiting
example,
inflammatory diseases, disorders and conditions include, but are not limited
to, Atherosclerosis;
Abdominal aortic aneurysm; Acute disseminated encephalomyelitis; Moyamoya
disease; Takayasu
disease; Acute coronary syndrome; Cardiac-allograft vasculopathy; Pulmonary
inflammation; Acute
respiratory distress syndrome; Pulmonary fibrosis; Acute disseminated
encephalomyelitis; Addison's
disease; Ankylosing spondylitis; Antiphospholipid antibody syndrome;
Autoimmune hemolytic
anemia; Autoimmune hepatitis; Autoimmune inner ear disease; Bullous
pemphigoid; Chagas
disease; Chronic obstructive pulmonary disease; Coeliac disease;
Dermatomyositis; Diabetes
mellitus type 1; Diabetes mellitus type 2; Endometriosis; Goodpasture's
syndrome; Graves' disease;
Guillain-Barre syndrome; Hashimoto's disease; Idiopathic thrombocytopenic
purpura; Interstitial
cystitis; Systemic lupus erythematosus (SLE); Metabolic syndrome, Multiple
sclerosis; Myasthenia
gravis; Myocarditis, Narcolepsy; Obesity; Pemphigus Vulgaris; Pernicious
anaemia; Polymyositis;
Primary biliary cirrhosis; Rheumatoid arthritis; Schizophrenia; Scleroderma;
Sjogren's syndrome;
Vasculitis; Vitiligo; Wegener's granulomatosis; Allergic rhinitis; Prostate
cancer; Non-small cell
lung carcinoma; Ovarian cancer; Breast cancer; Melanoma; Gastric cancer;
Colorectal cancer; Brain
cancer; Metastatic bone disorder; Pancreatic cancer; bladder cancer;
hepatocellular cancer; liver
cancer; adenocarcinoma of the lung; esophageal squamous cell carcinoma; CNS
tumors (e.g.,
Glioblastoma and neuroblastoma); hematological tumors; a Lymphoma; Nasal
polyps;
Gastrointestinal cancer; Ulcerative colitis; Crohn's disorder; Collagenous
colitis; Lymphocytic
colitis; Ischaemic colitis; Diversion colitis; Behcet's syndrome; Infective
colitis; Indeterminate
colitis; Inflammatory liver disorder, Endotoxin shock, Septic shock,
Rheumatoid spondylitis,
Ankylosing spondylitis, Gouty arthritis, Polymyalgia rheumatica, Alzheimer's
disorder, Parkinson's
-64-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
disorder, Epilepsy, AIDS dementia, Asthma, Adult respiratory distress
syndrome, Bronchitis, Acute
leukocyte-mediated lung injury, Distal proctitis, Wegener's granulomatosis,
Fibromyalgia,
Bronchitis, Cystic fibrosis, Uveitis, Conjunctivitis, Psoriasis, Eczema,
Dermatitis, Smooth muscle
proliferation disorders, Meningitis, Shingles, Encephalitis, Nephritis,
Tuberculosis, Retinitis, Atopic
dermatitis, Pancreatitis, Periodontal gingivitis, Coagulative Necrosis,
Liquefactive Necrosis,
Fibrinoid Necrosis, Neointimal hyperplasia, Myocardial infarction; Stroke;
organ transplant
rejection; influenza (e.g., H1N1 influenza A), or combinations thereof. In
some embodiments,
methods and compositions disclosed herein treat, reduce or prevent
angiogenesis.
[00253] In some embodiments, the inflammatory disease, disorder, or condition
is a cancer. In some
embodiments, the inflammatory disease, disorder or condition is Prostate
cancer; Non-small cell
lung carcinoma; Ovarian cancer; Breast cancer; Melanoma; Gastric cancer;
Colorectal cancer; Brain
cancer; Metastatic bone disorder; Pancreatic cancer; bladder cancer;
hepatocellular cancer; liver
cancer; adenocarcinoma of the lung; esophageal squamous cell carcinoma; CNS
tumors (e.g.,
Glioblastoma and neuroblastoma); hematological tumors; a Lymphoma;, or
combinations thereof.
[00254] In some embodiments, the inflammatory disease, disorder, or
conditionis is a cardiovascular
disorder. In some the inflammatory disease, disorder, or condition is:
Atherosclerosis, peripheral
vascular diseases, cerebrovascular disease (i.e., stroke), hypertension (i.e.,
high blood pressure),
heart failure, rheumatic heart disease, bacterial endocarditis,
cardiomyopathy, pulmonary circulation
diseases, vein & lympatics diseases, or combinations thereof.
Atherosclerosis
[00255] In some embodiments, the methods and compositions described herein
treat atherosclerosis.
As used herein, "atherosclerosis" means inflammation of an arterial wall and
includes all phases of
atherogenesis (e.g., lipid deposition, intima-media thickening, and subintimal
infiltration with
monocytes) and all atherosclerotic lesions (e.g., Type I lesions to Type VIII
lesions). In instance,
atherosclerosis results from (partially or fully) the accumulation of
macrophages. In some
embodiments, the methods and compositions described herein prevent the
accumulation of
macrophages, decrease the number of accumulated macrophages, and/or decrease
the rate at which
macrophages accumulate. In certain instances, atherosclerosis results from
(partially or fully) the
presence of oxidized LDL. In certain instances, oxidized LDL damages an
arterial wall. In some
embodiments, the methods and compositions described herein prevent oxidized
LDL-induced
damage to an arterial wall, decrease the portion of an arterial wall damaged
by oxidized LDL,
decrease the severity of the damage to an arterial wall, and/or decrease the
rate at which an arterial
wall is damaged by oxidized LDL. In certain instances, monocytes respond to
(i.e., follow a
chemotactic gradient to) the damaged arterial wall. In certain instances, the
monocytes differentiate
macrophages. In certain instances, macrophages endocytose the oxidized-LDL
(cells such as
macrophages with endocytosed LDL are called "foam cells"). In some
embodiments, the methods
-65-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
and compositions described herein prevent the formation of foam cells,
decrease the number of foam
cells, and/or decrease the rate at which foam cells are formed. In certain
instances, a foam cell dies
and subsequently ruptures. In certain instances, the rupture of a foam cell
deposits oxidized
cholesterol into the artery wall. In some embodiments, the methods and
compositions described
herein prevent the deposition of oxidized cholesterol deposited onto an artery
wall, decrease the
amount of oxidized cholesterol deposited onto an artery wall, and/or decrease
the rate at which
oxidized cholesterol is deposited onto an arterial wall. In certain instances,
the arterial wall becomes
inflamed due to the damage caused by the oxidized LDL. In some embodiments,
the methods and
compositions described herein prevent arterial wall inflammation, decrease the
portion of an arterial
wall that is inflamed, and/or decrease the severity of the inflammation. In
certain instances, the
inflammation of arterial walls results in (either partially or full) the
expression of matrix
metalloproteinase (MMP)-2, CD40 ligand, and tumor necrosis factor (TNF)-a. In
some
embodiments, the methods and compositions described herein prevent the
expression of matrix
metalloproteinase (MMP)-2, CD40 ligand, and tumor necrosis factor (TNF)-a, or
decrease the
amount of matrix metalloproteinase (MMP)-2, CD40 ligand, and tumor necrosis
factor (TNF)-a.
expressed. In certain instances, cells form a hard covering over the inflamed
area. In some
embodiments, the methods and compositions described herein prevent the
formation of the hard
covering, decrease the portion of an arterial wall affected by the hard
covering, and/or decrease the
rate at which the hard covering is formed. In certain instances, the cellular
covering narrows an
artery. In some embodiments, the methods and compositions described herein
prevent arterial
narrowing, decrease the portion of an artery that is narrowed, decrease the
severity of the narrowing,
and/or decrease the rate at which the artery is narrowed..
[00256] In certain instances, an atherosclerotic plaque results (partially or
fully) in stenosis (i.e., the
narrowing of blood vessel). In certain instances, stenosis results (partially
or fully) in decreased
blood flow. In some embodiments, the methods and compositions described herein
treat stenosis
and/or restinosis. In certain instances, the mechanical injury of stenotic
atherosclerotic lesions by
percutaneous intervention (e.g., balloon angioplasty or stenting) induces the
development of
neointimal hyperplasia. In certain instances, the acute injury of the vessel
wall induces acute
endothelial denudation and platelet adhesion, as well as apoptosis of SMCs in
the medial vessel
wall. In certain instances, the accumulation of phenotypically unique SMCs
within the intimal layer
in response to injury functions to restore the integrity of the arterial
vessel wall but subsequently
leads to the progressive narrowing of the vessel. In certain instances,
monocyte recruitment triggers
a more sustained and chronic inflammatory response. In some embodiments,
methods and
compositions disclosed herein inhibit the accumulation of phenotypically
unique SMCs within the
intimal layer. In some embodiments, methods and compositions disclosed herein
inhibit the
-66-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
accumulation of phenotypically unique SMCs within the intimal layer in an
individual treated by
balloon angioplasty or stenting.
[00257] In certain instances, the rupture of an atherosclerotic plaque results
(partially or fully) in an
infarction (e.g., myocardial infarction or stroke) to a tissue. In certain
instances, myocardial MIF
expression is upregulated in surviving cardiomyocytes and macrophages
following cute myocardial
ischemic injury. In certain instances, hypoxia and oxidative stress induce the
secretion of MIF from
cardiomyocytes through an atypical protein kinase C-dependent export mechanism
and result in
extracellular signal-regulated kinase activation. In certain instances,
increased serum concentrations
of MIF are detected in individuals with acute myocardial infarction. In
certain instances, MIF
contributes to macrophage accumulation in infarcted regions and to the
proinflammatory role of
myocyte-induced damage during infarction. In some embodiments, the methods and
compositions
described herein treat an infarction. In certain instances, reperfusion injury
follows an infarction. In
some embodiments, the methods and compositions described herein treat
reperfusion injury.
[00258] In some embodiments, an antibody disclosed herein is administered to
identify and/or locate
an atherosclerotic plaque. In some embodiments, the antibody is labeled for
imaging. In some
embodiments, the antibody is labeled for medical imaging. In some embodiments,
the antibody is
labeled for radio-imaging, PET imaging, MRI imaging, and fluorescent imaging.
In some
embodiments, the antibody localizes to areas of the circulatory system with
high concentrations of
MIF. In some embodiments, an area of the circulatory system with high
concentrations of MIF is an
atherosclerotic plaque. In some embodiments, the labeled antibodies are
detected by any suitable
method (e.g., by use of a gamma camera, MRI, PET scanner, x-ray computed
tomography (CT),
functional magnetic resonance imaging (fMRI), and single photon emission
computed tomography
(SPECT)).
Abdominal Aortic Aneurysm
[00259] In certain instances, an atherosclerotic plaque results (partially or
fully) in the development
of an aneurysm. In some embodiments, the methods and compositions described
herein are
administered to treat an aneurysm. In some embodiments, the methods and
compositions described
herein are administered to treat an abdominal aortic aneurysm ("AAA"). As used
herein, an
"abdominal aortic aneurysm" is a localized dilatation of the abdominal aorta
characterized by at
least a 50% increase over normal arterial diameter. In some embodiments, the
methods and
compositions described herein decrease the dilation of the abdominal aorta.
[00260] In certain instances, abdominal aortic aneurysms result (partially or
fully) from a breakdown
of structural proteins (e.g., elastin and collagen). In some embodiments, a
composition of matter,
method and/or pharmaceutical composition disclosed herein partially or fully
inhibits the breakdown
of a structural protein (e.g., elastin and collagen). In some embodiments, a
composition of matter,
method and/or pharmaceutical composition disclosed herein facilitates the
regeneration of a
-67-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
structural protein (e.g., elastin and collagen). In certain instances, the
breakdown of structural
proteins is caused by activated MMPs. In some embodiments, a composition of
matter, method
and/or pharmaceutical composition disclosed herein partially or fully inhibits
the activation of an
MMP. In some embodiments, a composition and/or method disclosed herein
inhibits the
upregulation of MMP-1, MMP-9 or MMP-12. In certain instances, MMPs are
activated following
infiltration of a section of the abdominal aorta by leukocytes (e.g.,
macrophages and neutrophils).
[00261] In some embodiments, the methods and compositions described herein
decrease the
infiltration of leukocytes. In certain instances, the MIF is upregulated in
early abdominal aortic
aneurysm. In certain instances, leukocytes follow a MIF gradient to a section
of the abdominal aorta
that is susceptible to the development of an AAA (e.g., the section of the
aorta affected by an
atherosclerotic plaque, infection, cystic medial necrosis, arteritis, trauma,
an anastomotic disruption
producing pseudoaneurysms). In some embodiments, a composition of matter,
method and/or
pharmaceutical composition disclosed herein partially or fully inhibits the
activity of MIF. In some
embodiments, a composition of matter, method and/or pharmaceutical composition
disclosed herein
partially or fully inhibits the ability of MIF to function as a chemokine for
macrophages and
neutrophils.
[00262] In some embodiments, an antibody disclosed herein is administered to
identify and/or locate
an AAA in an individual in need thereof. In some embodiments, an individual in
need thereof
displays one or more risk factors for developing an AAA (e.g., 60 years of age
or older; male;
cigarette smoking; high blood pressure; high serum cholesterol; diabetes
mellitus; atherosclerosis).
In some embodiments, the antibody is labeled for imaging. In some embodiments,
the antibody is
labeled for medical imaging. In some embodiments, the antibody is labeled for
radio-imaging, PET
imaging, MRI imaging, and fluorescent imaging. In some embodiments, the
antibody localizes to
areas of the circulatory system with high concentrations of MIF. In some
embodiments, an area of
the circulatory system with high concentrations of MIF is a AAA. In some
embodiments, the labeled
antibodies are detected by any suitable method (e.g., by use of a gamma
camera, MRI, PET scanner,
x-ray computed tomography (CT), functional magnetic resonance imaging (fMRI),
and single
photon emission computed tomography (SPECT)).
Miscellaneous Disorders
[00263] In some embodiments, the methods and compositions described herein
treat a T-cell
mediated autoimmune disorder. In certain instances, a T-cell mediated
autoimmune disorder is
characterized by a T-cell mediated immune response against self (e.g., native
cells and tissues).
Examples of T-cell mediated autoimmune disorders include, but are not limited
to colitis, multiple
sclerosis, arthritis, rheumatoid arthritis, osteoarthritis, juvenile
arthritis, psoriatic arthritis, acute
pancreatitis, chronic pancreatitis, diabetes, insulin-dependent diabetes
mellitus (IDDM or type I
diabetes), insulitis, inflammatory bowel disease, Crohn's disease, ulcerative
colitis, autoimmune
-68-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
hemolytic syndromes, autoimmune hepatitis, autoimmune neuropathy, autoimmune
ovarian failure,
autoimmune orchitis, autoimmune thrombocytopenia, reactive arthritis,
ankylosing spondylitis,
silicone implant associated autoimmune disease, Sjogren's syndrome, systemic
lupus erythematosus
(SLE), vasculitis syndromes (e.g., giant cell arteritis, Behcet's disease &
Wegener's granulomatosis),
vitiligo, secondary hematologic manifestation of autoimmune diseases (e.g.,
anemias), drug-induced
autoimmunity, Hashimoto's thyroiditis, hypophysitis, idiopathic thrombocytic
pupura, metal-induced
autoimmunity, myasthenia gravis, pemphigus, autoimmune deafness (e.g.,
Meniere's disease),
Goodpasture's syndrome, Graves' disease, HIV-related autoimmune syndromes and
Gullain-Barre
disease.
[00264] In some embodiments, the methods and compositions described herein
treat pain. Pain
includes, but is not limited to acute pain, acute inflammatory pain, chronic
inflammatory pain and
neuropathic pain.
[00265] In some embodiments, the methods and compositions described herein
treat
hypersensitivity. As used herein, "hypersensitivity" refers to an undesireable
immune system
response. Hypersensitivity is divided into four categories. Type I
hypersensitivity includes allergies
(e.g., Atopy, Anaphylaxis, or Asthma). Type II hypersensitivity is
cytotoxic/antibody mediated (e.g.,
Autoimmune hemolytic anemia, Thrombocytopenia, Erythroblastosis fetalis, or
Goodpasture's
syndrome). Type III is immune complex diseases (e.g., Serum sickness, Arthus
reaction, or SLE).
Type IV is delayed-type hypersensitivity (DTH), Cell-mediated immune memory
response, and
antibody-independent (e.g., Contact dermatitis, Tuberculin skin test, or
Chronic transplant rejection).
[00266] As used herein, "allergy" means a disorder characterized by excessive
activation of mast
cells and basophils by IgE. In certain instances, the excessive activation of
mast cells and basophils
by IgE results (either partially or fully) in an inflammatory response. In
certain instances, the
inflammatory response is local. In certain instances, the inflammatory
response results in the
narrowing of airways (i.e., bronchoconstriction). In certain instances, the
inflammatory response
results in inflammation of the nose (i.e., rhinitis). In certain instances,
the inflammatory response is
systemic (i.e., anaphylaxis).
[00267] In some embodiments, the methods and compositions described herein
treat angiogenesis.
As used herein, "angiogenesis" refers to the formations of new blood vessels.
In certain instances,
angiogenesis occurs with chronic inflammation. In certain instances,
angiogenesis is induced by
monocytes and/or macrophages. In some embodiments, a composition of matter,
method and/or
pharmaceutical composition disclosed herein inhibits angiogenesis. In certain
instances, MIF is
expressed in endothelial progenitor cells. In certain instances, MIF is
expressed in tumor-associated
neovasculature.
[00268] In some embodiments the present invention comprises a method of
treating a neoplasia. In
certain instances, a neoplastic cell induces an inflammatory response. In
certain instances, part of the
-69-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
inflammatory response to a neoplastic cell is angiogenesis. In certain
instances, angiogenesis
facilitates the development of a neoplasia. In some embodiments, the neoplasia
is: angiosarcoma,
Ewing sarcoma, osteosarcoma, and other sarcomas, breast carcinoma, cecum
carcinoma, colon
carcinoma, lung carcinoma, ovarian carcinoma, pharyngeal carcinoma,
rectosigmoid carcinoma,
pancreatic carcinoma, renal carcinoma, endometrial carcinoma, gastric
carcinoma, liver carcinoma,
head and neck carcinoma, breast carcinoma and other carcinomas, Hodgkins
lymphoma and other
lymphomas, malignant and other melanomas, parotid tumor, chronic lymphocytic
leukemia and
other leukemias, astrocytomas, gliomas, hemangiomas, retinoblastoma,
neuroblastoma, acoustic
neuroma, neurofibroma, trachoma and pyogenic granulomas.
[00269] Disclosed herein, in some embodiments, are methods of promoting
neovascularization
comprising administering to said individual MIF or a MIF analogue.
[00270] As used herein, "sepsis" is a disorder characterized by whole-body
inflammation. In certain
instances, inhibiting the expression or activity of MIF increases the survival
rate of individuals with
sepsis. In some embodiments, the methods and compositions described herein
treat sepsis. In certain
instances, sepsis results in (either partially or fully) myocardial
dysfunction (e.g., myocardial
dysfunction). In some embodiments, the methods and compositions described
herein treat
myocardial dysfunction (e.g., myocardial dysfunction) resulting from sepsis.
[00271] In certain instances, MIF induces kinase activation and
phosphorylation in the heart (i.e.,
indicators of cardiac depression). In some embodiments, the methods and
compositions described
herein treat myocardial dysfunction (e.g., myocardial dysfunction) resulting
from sepsis.
[00272] In certain instances, LPS induces the expression of MIF. In certain
instances, MIF is
induced by endotoxins during sepsis and functions as an initiating factor in
myocardial
inflammatory responses, cardiac myocyte apoptosis, and cardiac dysfunction.
[00273] In some embodiments, the methods and compositions described herein
inhibit myocardial
inflammatory responses resulting from endotoxin exposure. In some embodiments,
the methods and
compositions described herein inhibit cardiac myocyte apoptosis resulting from
endotoxin exposure.
In some embodiments, the methods and compositions described herein inhibit
cardiac dysfunction
resulting from endotoxin exposure.
[00274] In certain instances, inhibition of MIF results in (either partially
or fully) a significant
increase in survival factors (e.g., Bcl-2, Bax, and phospho-Akt) and an
improvement in
cardiomyocyte survival and myocardial function. In some embodiments, the
methods and
compositions described herein increase the expression of Bcl-2, Bax or phospho-
Akt.
[00275] In certain instances, MIF mediates the late and prolonged cardiac
depression after burn
injury associated and/or major tissue damage. In some embodiments, the methods
and compositions
described herein treat prolonged cardiac depression after burn injury. In some
embodiments, the
-70-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
methods and compositions described herein treat prolonged cardiac depression
after major tissue
damage.
[00276] In certain instances, MIF is released from the lungs during sepsis.
[00277] In certain instances, antibody neutralization of MIF inhibits the
onset of and reduced the
severity of autoimmune myocarditis. In some embodiments, the methods and
compositions
described herein treat autoimmune myocarditis.
VII. Combinations
[00278] Disclosed herein, in some embodiments, are methods and pharmaceutical
compositions for
modulating a disorder of a cardiovascular system, comprising a synergistic
combination of (a) agent
that inhibits (i) MIF binding to CXCR2 and CXCR4 and/or (ii) MIF-activation of
CXCR2 and
CXCR4; (iii) the ability of MIF to form a homomultimer; or a combination
thereof; and (b) a second
agent selected from an agent that treats inflammatory diseases, disorders,
conditions and symptoms
(the "MIF-mediated disorder agent").
[00279] Disclosed herein, in some embodiments, are methods and pharmaceutical
compositions for
modulating a disorder of a cardiovascular system, comprising a synergistic
combination of (a) agent
that inhibits (i) MIF binding to CXCR2 and CXCR4 and/or (ii) MIF-activation of
CXCR2 and
CXCR4; (iii) the ability of MIF to form a homomultimer; or a combination
thereof; and (b) a second
agent selected from an agent that treats a disorder a component of which is
inflammation.
[00280] Disclosed herein, in some embodiments, are methods and pharmaceutical
compositions for
modulating a disorder of a cardiovascular system, comprising a synergistic
combination of (a) agent
that inhibits (i) MIF binding to CXCR2 and CXCR4 and/or (ii) MIF-activation of
CXCR2 and
CXCR4; (iii) the ability of MIF to form a homomultimer; or a combination
thereof ; and (b) a
second agent selected from an agent a side-effect of which is undesired
inflammation. In certain
instances, statins (e.g., atorvastatin, lovastatin and simvastatin) induce
inflammation. In certain
instances, administration of a statin results (partially or fully) in
myositis.
[00281] As used herein, the terms "pharmaceutical combination," "administering
an additional
therapy," "administering an additional therapeutic agent" and the like refer
to a pharmaceutical
therapy resulting from the mixing or combining of more than one active
ingredient and includes
both fixed and non-fixed combinations of the active ingredients. The term
"fixed combination"
means that at least one of the agents described herein, and at least one co-
agent, are both
administered to an individual simultaneously in the form of a single entity or
dosage. The term
"non-fixed combination" means that at least one of the agents described
herein, and at least one co-
agent, are administered to an individual as separate entities either
simultaneously, concurrently or
sequentially with variable intervening time limits, wherein such
administration provides effective
levels of the two or more agents in the body of the individual. In some
instances, the co-agent is
-71-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
administered once or for a period of time, after which the agent is
administered once or over a
period of time. In other instances, the co-agent is administered for a period
of time, after which, a
therapy involving the administration of both the co-agent and the agent are
administered. In still
other embodiments, the agent is administered once or over a period of time,
after which, the co-
agent is administered once or over a period of time. These also apply to
cocktail therapies, e.g. the
administration of three or more active ingredients.
[00282] As used herein, the terms "co-administration," "administered in
combination with" and their
grammatical equivalents are meant to encompass administration of the selected
therapeutic agents to
a single individual, and are intended to include treatment regimens in which
the agents are
administered by the same or different route of administration or at the same
or different times. In
some embodiments the agents described herein will be co-administered with
other agents. These
terms encompass administration of two or more agents to an animal so that both
agents and/or their
metabolites are present in the animal at the same time. They include
simultaneous administration in
separate compositions, administration at different times in separate
compositions, and/or
administration in a composition in which both agents are present. Thus, in
some embodiments, the
agents described herein and the other agent(s) are administered in a single
composition. In some
embodiments, the agents described herein and the other agent(s) are admixed in
the composition.
[00283] Where combination treatments or prevention methods are contemplated,
it is not intended
that the agents described herein be limited by the particular nature of the
combination. For example,
the agents described herein are optionally administered in combination as
simple mixtures as well as
chemical hybrids. An example of the latter is where the agent is covalently
linked to a targeting
carrier or to an active pharmaceutical. Covalent binding can be accomplished
in many ways, such as,
though not limited to, the use of a commercially available cross-linking
agent. Furthermore,
combination treatments are optionally administered separately or
concomitantly.
[00284] In some embodiments, the co-administration of (a) agent disclosed
herein; and (b) a second
agent allows (partially or fully) a medical professional to increase the
prescribed dosage of the MIF-
mediated disorder agent. In certain instances, statin-induced myositis is dose-
dependent. In some
embodiments, prescribing the agent allows (partially or fully) a medical
professional to increase the
prescribed dosage of statin.
[00285] In some embodiments, the co-administration of (a) agent; and (b) a
second agent enables
(partially or fully) a medical professional to prescribe the second agent
(i.e., co-administration
rescues the MIF-mediated disorder agent).
[00286] In some embodiments, the second agent is an agent that targets HDL
levels by indirect
means (e.g. CETP inhibition). In some embodiments, combining a non-selective
HDL therapy with
agent disclosed herein; (2) a modulator of an interaction between RANTES and
Platelet Factor 4; or
-72-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(3) combinations thereof converts the second agent that targets HDL levels by
indirect means into a
more efficacious therapy.
[00287] In some embodiments, the second agent is administered before, after,
or simultaneously
with the modulator of inflammation.
VIII. Pharmaceutical Therapies
[00288] In some embodiments, the second agent is niacin, a fibrate, a statin,
a Apo-Al mimetic
peptide (e.g., DF-4, Novartis), an apoA-I transcriptional up-regulator, an
ACAT inhibitor, a CETP
modulator, Glycoprotein (GP) IIb/IIIa receptor antagonists, P2Y12 receptor
antagonists, Lp-PLA2-
inhibitors, an anti-TNF agent, an IL-1 receptor antagonist, an IL-2 receptor
antagonist, a cytotoxic
agent, an immunomodulatory agent, an antibiotic, a T-cell co-stimulatory
blocker, a disorder-
modifying anti-rheumatic agent, a B cell depleting agent, an immunosuppressive
agent, an anti-
lymphocyte antibody, an alkylating agent, an anti-metabolite, a plant
alkaloid, a terpenoids, a
topoisomerase inhibitor, an antitumor antibiotic, a monoclonal antibody, a
hormonal therapy (e.g.,
aromatase inhibitors), or combinations thereof.
[00289] In some embodiments, the second active is niacin, bezafibrate;
ciprofibrate; clofibrate;
gemfibrozil; fenofibrate; DF4 (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2);
DF5; RVX-
208 (Resverlogix); avasimibe; pactimibe sulfate (CS-505); CI-1011 (2,6-
diisopropylphenyl [(2, 4,6-
triisopropylphenyl)acetyl]sulfamate); CI-976 (2,2-dimethyl-N-(2,4,6-
trimethoxyphenyl)dodecanamide); VULM1457 (1-(2,6-diisopropyl-phenyl)-3-[4-(4'-
nitrophenylthio)phenyl] urea); CI-976 (2,2-dimethyl-N-(2,4,6-
trimethoxyphenyl)dodecanamide); E-
5324 (n-butyl-N'-(2-(3-(5-ethyl-4-phenyl-lH-imidazol-1-yl)propoxy)-6-
methylphenyl)urea); HL-
004 (N-(2,6-diisopropylphenyl) tetradecylthioacetamide); KY-455 (N-(4,6-
dimethyl-l-
pentylindolin-7-yl)-2,2-dimethylpropanamide); FY-087 (N-[2-[N'-pentyl-(6,6-
dimethyl-2,4-
heptadiynyl)amino]ethyl] -(2-methyl-l-naphthyl-thio)acetamide); MCC- 147
(Mitsubishi Pharma); F
12511 ((S)-2',3',5'-trimethyl-4'-hydroxy-alpha-dodecylthioacetanilide); SMP-
500 (Sumitomo
Pharmaceuticals); CL 277082 (2,4-difluoro-phenyl-N[[4-(2,2-
dimethylpropyl)phenyl]methyl]-N-
(hepthyl)urea); F-1394 ((ls,2s)-2-[3-(2,2-dimethylpropyl)-3-
nonylureido]aminocyclohexane-1-y13-
[N-(2,2,5,5-tetramethyl-1,3-dioxane-4-carbonyl)amino]propionate); CP- 113818
(N-(2,4-
bis(methylthio)-6-methylpyridin-3-yl)-2-(hexylthio)decanoic acid amide); YM-
750; torcetrapib;
anacetrapid; JTT-705 (Japan Tobacco/Roche); abciximab; eptifibatide;
tirofiban; roxifiban;
variabilin; XV 459 (N(3)-(2-(3-(4-formamidinophenyl)isoxazolin-5-yl)acetyl)-
N(2)-(1-
butyloxycarbonyl)-2,3-diaminopropionate); SR 121566A (3-[N- {4-[4-
(aminoiminomethyl)phenyl ]-
1 ,3-thiazol-2-yl}-N-(1 -carboxymethylpiperid-4-yl) aminol propionic acid,
trihydrochloride);
FK419 ((S)-2-acetylamino-3-[(R)-[1-[3-(piperidin-4-yl) propionyl] piperidin-3-
ylcarbonyl] amino]
propionic acid trihydrate); clopidogrel; prasugrel; cangrelor; AZD6140
(AstraZeneca); MRS 2395
-73-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)- 2-(2,2-
dimethyl-
propionyloxymethyl)-propyl ester); BX 667 (Berlex Biosciences); BX 048 (Berlex
Biosciences);
darapladib (SB 480848); SB-435495 (G1axoSmithKline); SB-222657
(G1axoSmithKline); SB-
253514 (G1axoSmithKline); alefacept, efalizumab, methotrexate, acitretin,
isotretinoin,
hydroxyurea, mycophenolate mofetil, sulfasalazine, 6-Thioguanine, Dovonex,
Taclonex,
betamethasone, tazarotene, hydroxychloroquine, sulfasalazine, etanercept,
adalimumab, infliximab,
abatacept, rituximab, trastuzumab, Anti-CD45 monoclonal antibody AHN-12 (NCI),
Iodine-131
Anti-B1 Antibody (Corixa Corp.), anti-CD66 monoclonal antibody BW 250/183
(NCI,
Southampton General Hospital), anti-CD45 monoclonal antibody (NCI, Baylor
College of
Medicine), antibody anti-anb3 integrin (NCI), BIW-8962 (BioWa Inc.), Antibody
BC8 (NCI),
antibody muJ591 (NCI), indium In 111 monoclonal antibody MN-14 (NCI), yttrium
Y 90
monoclonal antibody MN-14 (NCI), F105 Monoclonal Antibody (NIAID), Monoclonal
Antibody
RAV12 (Raven Biotechnologies), CAT-192 (Human Anti-TGF-Beta l Monoclonal
Antibody,
Genzyme), antibody 3F8 (NCI), 177Lu-J591 (Weill Medical College of Cornell
University), TB-403
(Biolnvent International AB), anakinra, azathioprine, cyclophosphamide,
cyclosporine A,
leflunomide, d-penicillamine, amitriptyline, or nortriptyline, chlorambucil,
nitrogen mustard,
prasterone, LJP 394 (abetimus sodium), LJP 1082 (La Jolla Pharmaceutical),
eculizumab,
belibumab, rhuCD40L (NIAID), epratuzumab, sirolimus, tacrolimus, pimecrolimus,
thalidomide,
antithymocyte globulin-equine (Atgam, Pharmacia Upjohn), antithymocyte
globulin-rabbit
(Thymoglobulin, Genzyme), Muromonab-CD3 (FDA Office of Orphan Products
Development),
basiliximab, daclizumab, riluzole, cladribine, natalizumab, interferon beta-
lb, interferon beta-la,
tizanidine, baclofen, mesalazine, asacol, pentasa, mesalamine, balsalazide,
olsalazine, 6-
mercaptopurine, AIN457 (Anti IL-17 Monoclonal Antibody, Novartis),
theophylline, D2E7 (a
human anti-TNF mAb from Knoll Pharmaceuticals), Mepolizumab (Anti-IL-S
antibody, SB
240563), Canakinumab (Anti-IL-1 Beta Antibody, NIAMS), Anti-IL-2 Receptor
Antibody
(Daclizumab, NHLBI), CNTO 328 (Anti IL-6 Monoclonal Antibody, Centocor),
ACZ885 (fully
human anti-interleukin-l beta monoclonal antibody, Novartis), CNTO 1275 (Fully
Human Anti-IL-
12 Monoclonal Antibody, Centocor), (3 S)-N-hydroxy-4-({4-[(4-hydroxy-2-
butynyl)oxy]phenyl} sulfonyl)-2,2-dimet- hyl-3-thiomorpholine carboxamide
(apratastat),
golimumab (CNTO 148), Onercept, BG9924 (Biogen Idec), Certolizumab Pegol
(CDP870, UCB
Pharma), AZD9056 (AstraZeneca), AZD5069 (AstraZeneca), AZD9668 (AstraZeneca),
AZD7928
(AstraZeneca), AZD2914 (AstraZeneca), AZD6067 (AstraZeneca), AZD3342
(AstraZeneca),
AZD8309 (AstraZeneca), ), [(1R)-3-methyl-l-({(2S)-3-phenyl-2-[(pyrazin-2-
ylcarbonyl)amino]propanoyl}amino)butyl]boronic acid (Bortezomib), AMG-714,
(Anti-IL 15
Human Monoclonal Antibody, Amgen), ABT-874 (Anti IL-12 monoclonal antibody,
Abbott Labs),
MRA(Tocilizumab, an Anti IL-6 Receptor Monoclonal Antibody, Chugai
Pharmaceutical), CAT-
-74-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
354 (a human anti-interleukin- 13 monoclonal antibody, Cambridge Antibody
Technology,
Medlmmune), aspirin, salicylic acid, gentisic acid, choline magnesium
salicylate, choline salicylate,
choline magnesium salicylate, choline salicylate, magnesium salicylate, sodium
salicylate,
diflunisal, carprofen, fenoprofen, fenoprofen calcium, flurobiprofen,
ibuprofen, ketoprofen,
nabutone, ketolorac, ketorolac tromethamine, naproxen, oxaprozin, diclofenac,
etodolac,
indomethacin, sulindac, tolmetin, meclofenamate, meclofenamate sodium,
mefenamic acid,
piroxicam, meloxicam, celecoxib, rofecoxib, valdecoxib, parecoxib, etoricoxib,
lumiracoxib, CS-
502 (Sankyo), JTE-522 (Japan Tobacco Inc.), L-745,337 (Almirall), NS398
(Sigma), betamethasone
(Celestone), prednisone (Deltasone), alclometasone, aldosterone, amcinonide,
beclometasone,
betamethasone, budesonide, ciclesonide, clobetasol, clobetasone, clocortolone,
cloprednol,
cortisone, cortivazol, deflazacort, deoxycorticosterone, desonide,
desoximetasone, desoxycortone,
dexamethasone, diflorasone, diflucortolone, difluprednate, fluclorolone,
fludrocortisone,
fludroxycortide, flumetasone, flunisolide, fluocinolone acetonide,
fluocinonide, fluocortin,
fluocortolone, fluorometholone, fluperolone, fluprednidene, fluticasone,
formocortal, formoterol,
halcinonide, halometasone, hydrocortisone, hydrocortisone aceponate,
hydrocortisone buteprate,
hydrocortisone butyrate, loteprednol, medrysone, meprednisone,
methylprednisolone,
methylprednisolone aceponate, mometasone furoate, paramethasone,
prednicarbate, prednisone,
rimexolone, tixocortol, triamcinolone, ulobetasol; cisplatin; carboplatin;
oxaliplatin;
mechlorethamine; cyclophosphamide; chlorambucil; vincristine; vinblastine;
vinorelbine; vindesine;
azathioprine; mercaptopurine; fludarabine; pentostatin; cladribine; 5-
fluorouracil (5FU); floxuridine
(FUDR); cytosine arabinoside; methotrexate; trimethoprim; pyrimethamine;
pemetrexed; paclitaxel;
docetaxel; etoposide; teniposide; irinotecan; topotecan; amsacrine; etoposide;
etoposide phosphate;
teniposide; dactinomycin; doxorubicin; daunorubicin; valrubicine; idarubicine;
epirubicin;
bleomycin; plicamycin; mitomycin; trastuzumab; cetuximab; rituximab;
bevacizumab; finasteride;
goserelin; aminoglutethimide; anastrozole; letrozole; vorozole; exemestane; 4-
androstene-3,6,17-
trione ("6-OXO' ; 1,4,6-androstatrien-3,17-dione (ATD); formestane;
testolactone; fadrozole;
milatuzumab; milatuzumab conjugated to doxorubicin; or combinations thereof.
Gene Therapy
[00290] Disclosed herein, in some embodiments, is a composition for modulating
an MIF-mediated
disorder, comprising a combination of (a) agent disclosed herein; and (b) gene
therapy. Disclosed
herein, in some embodiments, are methods for modulating an MIF-mediated
disorder, comprising
co-administering a combination of (a) agent disclosed herein; and (b) gene
therapy.
[00291] In some embodiments, the gene therapy comprises modulating the
concentration of a lipid
and/or lipoprotein (e.g., HDL) in the blood of an individual in need thereof.
In some embodiments,
modulating the concentration of a lipid and/or lipoprotein (e.g., HDL) in the
blood comprises
transfecting DNA into an individual in need thereof. In some embodiments, the
DNA encodes an
-75-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Apo Al gene, an LCAT gene, an LDL gene, an 11-4 gene, an IL-10 gene, an IL-
Ira gene, a galectin-
3 gene, or combinations thereof. In some embodiments, the DNA is transfected
into a liver cell.
[00292] In some embodiments, the DNA is transfected into a liver cell via use
of ultrasound. For
disclosures of techniques related to transfecting ApoAl DNA via use of
ultrasound see U.S. Patent
No. 7,211,248, which is hereby incorporated by reference for those
disclosures.
[00293] In some embodiments, an individual is administered a vector engineered
to carry the human
gene (the "gene vector"). For disclosures of techniques for creating an LDL
gene vector see U.S.
Patent No. 6,784,162, which is hereby incorporated by reference for those
disclosures. In some
embodiments, the gene vector is a retrovirus. In some embodiments, the gene
vector is not a
retrovirus (e.g. it is an adenovirus; a lentivirus; or a polymeric delivery
system such as
METAFECTENE, SUPERFECT , EFFECTENE , or MIRUS TRANSIT). In certain instances,
a
retrovirus, adenovirus, or lentivirus will have a mutation such that the virus
is rendered incompetent.
[00294] In some embodiments, the vector is administered in vivo (i.e., the
vector is injected directly
into the individual, for example into a liver cell), ex vivo (i.e., cells from
the individual are grown in
vitro and transduced with the gene vector, embedded in a carrier, and then
implanted in the
individual), or a combination thereof.
[00295] In certain instances, after administration of the gene vector, the
gene vector infects the cells
at the site of administration (e.g. the liver). In certain instances the gene
sequence is incorporated
into the individual's genome (e.g. when the gene vector is a retrovirus). In
certain instances the
therapy will need to be periodically re-administered (e.g. when the gene
vector is not a retrovirus).
In some embodiments, the therapy is re-administered annually. In some
embodiments, the therapy is
re-administered semi-annually. In some embodiments, the therapy is re-
administered when the
individual's HDL level decreases below about 60 mg/dL. In some embodiments,
the therapy is re-
administered when the individual's HDL level decreases below about 50 mg/dL.
In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 45 mg/dL. In some embodiments, the therapy is re-administered when the
individual's HDL
level decreases below about 40 mg/dL. In some embodiments, the therapy is re-
administered when
the individual's HDL level decreases below about 35 mg/dL. In some
embodiments, the therapy is
re-administered when the individual's HDL level decreases below about 30
mg/dL.
RNAi Therapies
[00296] Disclosed herein, in some embodiments, is composition for modulating
an MIF-mediated
disorder, comprising a combination of (a) agent disclosed herein; and (b) an
RNAi molecule
designed to silence the expression of a gene that participates in the
development and/or progression
of an MIF-mediated disorder (the "target gene"). Disclosed herein, in some
embodiments, are
methods for modulating an MIF-mediated disorder, comprising administering a
combination of (a)
agent disclosed herein; and (b)) an RNAi molecule designed to silence the
expression of a gene that
-76-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
participates in the development and/or progression of an MIF-mediated disorder
(the "target gene").
In some embodiments, the target gene is Apolipoprotein B (Apo B), Heat Shock
Protein 110 (Hsp
110), Proprotein Convertase Subtilisin Kexin 9 (Pcsk9), CyD1, TNF-a, IL-1(3,
Atrial Natriuretic
Peptide Receptor A (NPRA), GATA-3, Syk, VEGF, MIP-2, FasL, DDR-1, C5aR, AP-1,
or
combinations thereof.
[00297] In some embodiments, the target gene is silenced by RNA interference
(RNAi). In some
embodiments, the RNAi therapy comprises use of an siRNA molecule. In some
embodiments, a
double stranded RNA (dsRNA) molecule with sequences complementary to an mRNA
sequence of
a gene to be silenced (e.g., Apo B, Hsp 110 and Pcsk9) is generated (e.g by
PCR). In some
embodiments, a 20-25 bp siRNA molecule with sequences complementary to an mRNA
sequence of
a gene to be silenced is generated. In some embodiments, the 20-25 bp siRNA
molecule has 2-5 bp
overhangs on the 3' end of each strand, and a 5' phosphate terminus and a 3'
hydroxyl terminus. In
some embodiments, the 20-25 bp siRNA molecule has blunt ends. For techniques
for generating
RNA sequences see Molecular Cloning: A Laboratory Manual, second edition
(Sambrook et al.,
1989) and Molecular Cloning: A Laboratory Manual, third edition (Sambrook and
Russel, 2001),
jointly referred to herein as "Sambrook"); Current Protocols in Molecular
Biology (F. M. Ausubel et
al., eds., 1987, including supplements through 2001); Current Protocols in
Nucleic Acid Chemistry
John Wiley & Sons, Inc., New York, 2000) which are hereby incorporated by
reference for such
disclosure.
[00298] In some embodiments, an siRNA molecule is "fully complementary" (i.e.,
100%
complementary) to the target gene. In some embodiments, an antisense molecule
is "mostly
complementary" (e.g., 99%, 98%, 97%, 96%, 95%, 94%, 93%, 92%, 91%, 90%, 85%,
80%, 75%, or
70% complementary) to the target gene. In some embodiments, there is a 1 bp
mismatch, a 2 bp
mismatch, a 3 bp mismatch, a 4 bp mismatch, or a 5 bp mismatch.
[00299] In certain instances, after administration of the dsRNA or siRNA
molecule, cells at the site
of administration (e.g. the cells of the liver and/or small intestine) are
transformed with the dsRNA
or siRNA molecule. In certain instances following transformation, the dsRNA
molecule is cleaved
into multiple fragments of about 20-25 bp to yield siRNA molecules. In certain
instances, the
fragments have about 2bp overhangs on the 3' end of each strand.
[00300] In certain instances, an siRNA molecule is divided into two strands
(the guide strand and the
anti-guide strand) by an RNA-induced Silencing Complex (RISC). In certain
instances, the guide
strand is incorporated into the catalytic component of the RISC (i.e.
argonaute). In certain instances,
the guide strand specifically binds to a complementary RBI mRNA sequence. In
certain instances,
the RISC cleaves an mRNA sequence of a gene to be silenced. In certain
instances, the expression of
the gene to be silenced is down-regulated.
-77-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00301] In some embodiments, a sequence complementary to an mRNA sequence of a
target gene is
incorporated into a vector. In some embodiments, the sequence is placed
between two promoters. In
some embodiments, the promoters are orientated in opposite directions. In some
embodiments, the
vector is contacted with a cell. In certain instances, a cell is transformed
with the vector. In certain
instances following transformation, sense and anti-sense strands of the
sequence are generated. In
certain instances, the sense and anti-sense strands hybridize to form a dsRNA
molecule which is
cleaved into siRNA molecules. In certain instances, the strands hybridize to
form an siRNA
molecule. In some embodiments, the vector is a plasmid (e.g pSUPER;
pSUPER.neo;
pSUPER.neo+gfp).
[00302] In some embodiments, an siRNA molecule is administered to in vivo
(i.e., the vector is
injected directly into the individual, for example into a liver cell or a cell
of the small intestine, or
into the blood stream).
[00303] In some embodiments, a siRNA molecule is formulated with a delivery
vehicle (e.g., a
liposome, a biodegradable polymer, a cyclodextrin, a PLGA microsphere, a PLCA
microsphere, a
biodegradable nanocapsule, a bioadhesive microsphere, or a proteinaceous
vector), carriers and
diluents, and other pharmaceutically-acceptable excipients. For methods of
formulating and
administering a nucleic acid molecule to an individual in need thereof see
Akhtar et al., 1992,
Trends Cell Bio., 2, 139; Delivery Strategies for Antisense Oligonucleotide
Therapeutics, ed.
Akhtar, 1995; Maurer et al., 1999, Mol. Membr. Biol., 16, 129-140; Hofland and
Huang, 1999,
Handb. Exp. Pharmacol., 137, 165-192; Lee et al., 2000, ACS Symp. Ser., 752,
184-192; Beigelman
et al., U.S. Pat. No. 6,395,713; Sullivan et al., PCT WO 94/02595; Gonzalez et
al., 1999,
Bioconjugate Chem., 10, 1068-1074; Wang et al., International PCT publication
Nos. WO 03/47518
and WO 03/46185; U.S. Pat. No. 6,447,796; US Patent Application Publication
No. US
2002130430; O'Hare and Normand, International PCT Publication No. WO 00/53722;
and U.S.
Patent Application Publication No. 20030077829; U.S. Provisional patent
application No.
60/678,53 1, all of which are hereby incorporated by reference for such
disclosures.
[00304] In some embodiments, an siRNA molecule described herein is
administered to the liver by
any suitable manner (see e.g., Wen et al., 2004, World J Gastroenterol., 10,
244-9; Murao et al.,
2002, Pharm Res., 19, 1808-14; Liu et al., 2003, Gene Ther., 10, 180-7; Hong
et al., 2003, J Pharm
Pharmacol., 54, 51-8; Herrmann et al., 2004, Arch Virol., 149, 1611-7; and
Matsuno et al., 2003,
Gene Ther., 10, 1559-66).
[00305] In some embodiments, an siRNA molecule described herein is
administered
iontophoretically, for example to a particular organ or compartment (e.g., the
liver or small
intestine). Non-limiting examples of iontophoretic delivery are described in,
for example, WO
03/043689 and WO 03/030989, which are hereby incorporated by reference for
such disclosures.
-78-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00306] In some embodiments, an siRNA molecule described herein is
administered systemically
(i.e., in vivo systemic absorption or accumulation of an siRNA molecule in the
blood stream
followed by distribution throughout the entire body). Administration routes
contemplated for
systemic administration include, but are not limited to, intravenous,
subcutaneous, portal vein,
intraperitoneal, and intramuscular. Each of these administration routes
exposes the siRNA molecules
of the invention to an accessible diseased tissue (e.g., liver).
[00307] In certain instances the therapy will need to be periodically re-
administered. In some
embodiments, the therapy is re-administered annually. In some embodiments, the
therapy is re-
administered semi-annually. In some embodiments, the therapy is administered
monthly. In some
embodiments, the therapy is administered weekly. In some embodiments, the
therapy is re-
administered when the individual's HDL level decreases below about 60 mg/dL.
In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 50 mg/dL. In some embodiments, the therapy is re-administered when the
individual's HDL
level decreases below about 45 mg/dL. In some embodiments, the therapy is re-
administered when
the individual's HDL level decreases below about 40 mg/dL. In some
embodiments, the therapy is
re-administered when the individual's HDL level decreases below about 35
mg/dL. In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 30 mg/dL.
[00308] For disclosures of techniques related to silencing the expression of
Apo B and/or Hsp 110
see U.S. Pub. No. 2007/0293451 which is hereby incorporated by reference for
such disclosures. For
disclosures of techniques related to silencing the expression of Pcsk9 see
U.S. Pub. No.
2007/0173473 which is hereby incorporated by reference for such disclosures.
Antisense Therapies
[00309] Disclosed herein, in some embodiments, is a composition for modulating
an MIF-mediated
disorder, comprising a combination of (a) agent disclosed herein; and (b) an
antisense molecule
designed to inhibit the expression of and/or activity of a DNA or RNA sequence
that participates in
the development and/or progression of an MIF-mediated disorder (the "target
sequence"). Disclosed
herein, in some embodiments, are methods for modulating an MIF-mediated
disorder, comprising
co-administering (a) agent disclosed herein; and (b) an antisense molecule
designed to inhibit the
expression of and/or activity of a DNA or RNA sequence that participates in
the development and/or
progression of an MIF-mediated disorder (the "target sequence"). In some
embodiments, inhibiting
the expression of and/or activity of a target sequence comprises use of an
antisense molecule
complementary to the target sequence. In some embodiments, the target sequence
is microRNA- 122
(miRNA-122 or mRNA-122), secretory phospholipase A2 (sPLA2), intracellular
adhesion
molecule-1 (ICAM-1), GATA-3, NF-x B, Syk, or combinations thereof. In certain
instances,
-79-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
inhibiting the expression of and/or activity of miRNA-122 results (partially
or fully) in a decrease in
the concentration of cholesterol and/or lipids in blood.
[00310] In some embodiments, an antisense molecule that is complementary to a
target sequence is
generated (e.g. by PCR). In some embodiments, the antisense molecule is about
15 to about 30
nucleotides. In some embodiments, the antisense molecule is about 17 to about
28 nucleotides. In
some embodiments, the antisense molecule is about 19 to about 26 nucleotides.
In some
embodiments, the antisense molecule is about 21 to about 24 nucleotides. For
techniques for
generating RNA sequences see Molecular Cloning: A Laboratory Manual, second
edition
(Sambrook et al., 1989) and Molecular Cloning: A Laboratory Manual, third
edition (Sambrook and
Russel, 2001), jointly referred to herein as "Sambrook"); Current Protocols in
Molecular Biology (F.
M. Ausubel et al., eds., 1987, including supplements through 2001); Current
Protocols in Nucleic
Acid Chemistry John Wiley & Sons, Inc., New York, 2000) which are hereby
incorporated by
reference for such disclosure.
[00311] In some embodiments, the antisense molecules are single- stranded,
double- stranded,
circular or hairpin. In some embodiments, the antisense molecules contain
structural elements (e.g.,
internal or terminal bulges, or loops).
[00312] In some embodiments, an antisense molecule is "fully complementary"
(i.e., 100%
complementary) to the target sequence. In some embodiments, an antisense
molecule is "mostly
complementary" (e.g., 99%, 98%, 97%, 96%, 95%, 94%, 93%, 92%, 91%, 90%, 85%,
80%, 75%, or
70% complementary) to the target RNA sequence. In some embodiments, there is a
1 bp mismatch,
a 2 bp mismatch, a 3 bp mismatch, a 4 bp mismatch, or a 5 bp mismatch.
[00313] In some embodiments, the antisense molecule hybridizes to the target
sequence. As used
herein, "hybridize" means the pairing of nucleotides of an antisense molecule
with corresponding
nucleotides of the target sequence. In certain instances, hybridization
involves the formation of one
or more hydrogen bonds (e.g., Watson-Crick, Hoogsteen or reversed Hoogsteen
hydrogen bonding)
between the pairing nucleotides.
[00314] In certain instances, hybridizing results (partially or fully) in the
degradation, cleavage,
and/or sequestration of the RNA sequence.
[00315] In some embodiments, a siRNA molecule is formulated with a delivery
vehicle (e.g., a
liposome, a biodegradable polymer, a cyclodextrin, a PLGA microsphere, a PLCA
microsphere, a
biodegradable nanocapsule, a bioadhesive microsphere, or a proteinaceous
vector), carriers and
diluents, and other pharmaceutically-acceptable excipients. For methods of
formulating and
administering a nucleic acid molecule to an individual in need thereof see
Akhtar et al., 1992,
Trends Cell Bio., 2, 139; Delivery Strategies for Antisense Oligonucleotide
Therapeutics, ed.
Akhtar, 1995; Maurer et al., 1999, Mol. Membr. Biol., 16, 129-140; Hofland and
Huang, 1999,
Handb. Exp. Pharmacol., 137, 165-192; Lee et al., 2000, ACS Symp. Ser., 752,
184-192; Beigelman
-80-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
et al., U.S. Pat. No. 6,395,713; Sullivan et al., PCT WO 94/02595; Gonzalez et
al., 1999,
Bioconjugate Chem., 10, 1068-1074; Wang et al., International PCT publication
Nos. WO 03/47518
and WO 03/46185; U.S. Pat. No. 6,447,796; US Patent Application Publication
No. US
2002130430; O'Hare and Normand, International PCT Publication No. WO 00/53722;
and U.S.
Patent Application Publication No. 20030077829; U.S. Provisional patent
application No.
60/678,53 1, all of which are hereby incorporated by reference for such
disclosures.
[00316] In some embodiments, an siRNA molecule described herein is
administered to the liver by
any suitable manner (see e.g., Wen et al., 2004, World J Gastroenterol., 10,
244-9; Murao et al.,
2002, Pharm Res., 19, 1808-14; Liu et al., 2003, Gene Ther., 10, 180-7; Hong
et al., 2003, J Pharm
Pharmacol., 54, 51-8; Herrmann et al., 2004, Arch Virol., 149, 1611-7; and
Matsuno et al., 2003,
Gene Ther., 10, 1559-66).
[00317] In some embodiments, an siRNA molecule described herein is
administered
iontophoretically, for example to a particular organ or compartment (e.g., the
liver or small
intestine). Non-limiting examples of iontophoretic delivery are described in,
for example, WO
03/043689 and WO 03/030989, which are hereby incorporated by reference for
such disclosures.
[00318] In some embodiments, an siRNA molecule described herein is
administered systemically
(i.e., in vivo systemic absorption or accumulation of an siRNA molecule in the
blood stream
followed by distribution throughout the entire body). Administration routes
contemplated for
systemic administration include, but are not limited to, intravenous,
subcutaneous, portal vein,
intraperitoneal, and intramuscular. Each of these administration routes
exposes the siRNA molecules
of the invention to an accessible diseased tissue (e.g., liver).
[00319] In certain instances the therapy will need to be periodically re-
administered. In some
embodiments, the therapy is re-administered annually. In some embodiments, the
therapy is re-
administered semi-annually. In some embodiments, the therapy is administered
monthly. In some
embodiments, the therapy is administered weekly. In some embodiments, the
therapy is re-
administered when the individual's HDL level decreases below about 60 mg/dL.
In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 50 mg/dL. In some embodiments, the therapy is re-administered when the
individual's HDL
level decreases below about 45 mg/dL. In some embodiments, the therapy is re-
administered when
the individual's HDL level decreases below about 40 mg/dL. In some
embodiments, the therapy is
re-administered when the individual's HDL level decreases below about 35
mg/dL. In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 30 mg/dL.
[00320] For disclosures of techniques related to silencing the expression of
miRNA-122 see WO
07/027775A2 which is hereby incorporated by reference for such disclosures.
Device-Mediated Therapies
-81-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00321] In some embodiments, the device mediated strategy comprises removing a
lipid from an
HDL molecule in an individual in need thereof (delipification), removing an
LDL molecule from the
blood or plasma of an individual in need thereof (delipification), or a
combination thereof. For
disclosures of techniques for removing a lipid from an HDL molecule and
removing an LDL
molecule from the blood or plasma of an individual in need thereof see U.S.
Pub. No.
2008/0230465, which is hereby incorporated by reference for those disclosures.
[00322] In certain instances, the delipification therapy will need to be
periodically re-administered.
In some embodiments, the delipification therapy is re-administered annually.
In some embodiments,
the delipification therapy is re-administered semi-annually. In some
embodiments, the delipification
therapy is re-administered monthly. In some embodiments, the delipification
therapy is re-
administered semi-weekly. In some embodiments, the therapy is re-administered
when the
individual's HDL level decreases below about 60 mg/dL. In some embodiments,
the therapy is re-
administered when the individual's HDL level decreases below about 50 mg/dL.
In some
embodiments, the therapy is re-administered when the individual's HDL level
decreases below
about 45 mg/dL. In some embodiments, the therapy is re-administered when the
individual's HDL
level decreases below about 40 mg/dL. In some embodiments, the therapy is re-
administered when
the individual's HDL level decreases below about 35 mg/dL. In some
embodiments, the therapy is
re-administered when the individual's HDL level decreases below about 30
mg/dL.
Pharmaceutical Compositions
[00323] Disclosed herein, in some embodiments, is a pharmaceutical composition
for treating an
inflammatory disease, disorder, condition, or symptom comprising a
therapeutically-effective
amount of agent disclosed herein.
[00324] Pharmaceutical compositions herein are formulated using one or more
physiologically
acceptable carriers including excipients and auxiliaries which facilitate
processing of the agents into
preparations which are used pharmaceutically. Proper formulation is dependent
upon the route of
administration chosen. A summary of pharmaceutical compositions is found, for
example, in
Remington: The Science and Practice of Pharmacy, Nineteenth Ed (Easton, Pa.:
Mack Publishing
Company, 1995); Hoover, John E., Remington's Pharmaceutical Sciences, Mack
Publishing Co.,
Easton, Pennsylvania 1975; Liberman, H.A. and Lachman, L., Eds.,
Pharmaceutical Dosage Forms,
Marcel Decker, New York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug
Delivery
Systems, Seventh Ed. (Lippincott Williams & Wilkins, 1999).
[00325] In some embodiments, the pharmaceutical composition for modulating a
disorder of a
cardiovascular system further comprises a pharmaceutically acceptable
diluent(s), excipient(s), or
carrier(s). In some embodiments, the pharmaceutical compositions includes
other medicinal or
pharmaceutical agents, carriers, adjuvants, such as preserving, stabilizing,
wetting or emulsifying
-82-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
agents, solution promoters, salts for regulating the osmotic pressure, and/or
buffers. In addition, the
pharmaceutical compositions also contain other therapeutically valuable
substances.
[00326] The pharmaceutical formulations described herein are optionally
administered to an
individual by multiple administration routes, including but not limited to,
oral, parenteral (e.g.,
intravenous, subcutaneous, intramuscular), intranasal, buccal, topical,
rectal, or transdermal
administration routes. The pharmaceutical formulations described herein
include, but are not limited
to, aqueous liquid dispersions, self-emulsifying dispersions, solid solutions,
liposomal dispersions,
aerosols, solid dosage forms, powders, immediate release formulations,
controlled release
formulations, fast melt formulations, tablets, capsules, pills, delayed
release formulations, extended
release formulations, pulsatile release formulations, multiparticulate
formulations, and mixed
immediate and controlled release formulations.
[00327] The pharmaceutical compositions described herein are formulated into
any suitable dosage
form, including but not limited to, aqueous oral dispersions, liquids, gels,
syrups, elixirs, slurries,
suspensions and the like, for oral ingestion by an individual to be treated,
solid oral dosage forms,
aerosols, controlled release formulations, fast melt formulations,
effervescent formulations,
lyophilized formulations, tablets, powders, pills, dragees, capsules, modified
release formulations,
delayed release formulations, extended release formulations, pulsatile release
formulations,
multiparticulate formulations, and mixed immediate release and controlled
release formulations.
[00328] In some embodiments, the pharmaceutical compositions described herein
are formulated as
multiparticulate formulations. In some embodiments, the pharmaceutical
compositions described
herein comprise a first population of particles and a second population of
particles. In some
embodiments, the first population comprises an agent. In some embodiments, the
second population
comprises an agent. In some embodiments, the dose of agent in the first
population is equal to the
dose of agent in the second population. In some embodiments, the dose of agent
in the first
population is not equal to (e.g., greater than or less than) the dose of agent
in the second population.
[00329] In some embodiments, the agent of the first population is released
before the agent of the
second population. In some embodiments, the second population of particles
comprises a modified-
release (e.g., delayed-release, controlled-release, or extended release)
coating. In some
embodiments, the second population of particles comprises a modified-release
(e.g., delayed-release,
controlled-release, or extended release) matrix.
[00330] Coating materials for use with the pharmaceutical compositions
described herein include,
but are not limited to, polymer coating materials (e.g., cellulose acetate
phthalate, cellulose acetate
trimaletate, hydroxy propyl methylcellulose phthalate, polyvinyl acetate
phthalate); ammonio
methacrylate copolymers (e.g., Eudragit RS and RL); poly acrylic acid and
poly acrylate and
methacrylate copolymers (e.g., Eudragite S and L, polyvinyl acetaldiethylamino
acetate,
hydroxypropyl methylcellulose acetate succinate, shellac); hydrogels and gel-
forming materials
-83-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(e.g., carboxyvinyl polymers, sodium alginate, sodium carmellose, calcium
carmellose, sodium
carboxymethyl starch, poly vinyl alcohol, hydroxyethyl cellulose, methyl
cellulose, gelatin, starch,
hydoxypropyl cellulose, hydroxypropyl methylcellulose, polyvinylpyrrolidone,
crosslinked starch,
microcrystalline cellulose, chitin, aminoacryl-methacrylate copolymer,
pullulan, collagen, casein,
agar, gum arabic, sodium carboxymethyl cellulose, (swellable hydrophilic
polymers)
poly(hydroxyalkyl methacrylate) (m. wt. -5 k-5,000 k), polyvinylpyrrolidone
(m. wt. -10 k-360 k),
anionic and cationic hydrogels, polyvinyl alcohol having a low acetate
residual, a swellable mixture
of agar and carboxymethyl cellulose, copolymers of maleic anhydride and
styrene, ethylene,
propylene or isobutylene, pectin (m. wt. -30 k-300 k), polysaccharides such as
agar, acacia, karaya,
tragacanth, algins and guar, polyacrylamides, Polyox polyethylene oxides (m.
wt. -100 k-5,000 k),
AquaKeep acrylate polymers, diesters of polyglucan, crosslinked polyvinyl
alcohol and poly N-
vinyl-2-pyrrolidone, sodium starch; hydrophilic polymers (e.g.,
polysaccharides, methyl cellulose,
sodium or calcium carboxymethyl cellulose, hydroxypropyl methyl cellulose,
hydroxypropyl
cellulose, hydroxyethyl cellulose, nitro cellulose, carboxymethyl cellulose,
cellulose ethers,
polyethylene oxides, methyl ethyl cellulose, ethylhydroxy ethylcellulose,
cellulose acetate, cellulose
butyrate, cellulose propionate, gelatin, collagen, starch, maltodextrin,
pullulan, polyvinyl
pyrrolidone, polyvinyl alcohol, polyvinyl acetate, glycerol fatty acid esters,
polyacrylamide,
polyacrylic acid, copolymers of methacrylic acid or methacrylic acid, other
acrylic acid derivatives,
sorbitan esters, natural gums, lecithins, pectin, alginates, ammonia alginate,
sodium, calcium,
potassium alginates, propylene glycol alginate, agar, arabic gum, karaya gum,
locust bean gum,
tragacanth gum, carrageens gum, guar gum, xanthan gum, scleroglucan gum); or
combinations
thereof. In some embodiments, the coating comprises a plasticiser, a
lubricant, a solvent, or
combinations thereof. Suitable plasticisers include, but are not limited to,
acetylated
monoglycerides; butyl phthalyl butyl glycolate; dibutyl tartrate; diethyl
phthalate; dimethyl
phthalate; ethyl phthalyl ethyl glycolate; glycerin; propylene glycol;
triacetin; citrate; tripropioin;
diacetin; dibutyl phthalate; acetyl monoglyceride; polyethylene glycols;
castor oil; triethyl citrate;
polyhydric alcohols, glycerol, acetate esters, gylcerol triacetate, acetyl
triethyl citrate, dibenzyl
phthalate, dihexyl phthalate, butyl octyl phthalate, diisononyl phthalate,
butyl octyl phthalate,
dioctyl azelate, epoxidised tallate, triisoctyl trimellitate, diethylhexyl
phthalate, di-n-octyl phthalate,
di-i-octyl phthalate, di-i-decyl phthalate, di-n-undecyl phthalate, di-n-
tridecyl phthalate, tri-2-
ethylhexyl trimellitate, di-2-ethylhexyl adipate, di-2-ethylhexyl sebacate, di-
2-ethylhexyl azelate,
dibutyl sebacate.
[00331] In some embodiments, the second population of particles comprises a
modified release
matrix material. Materials for use with the pharmaceutical compositions
described herein include,
but are not limited to microcrytalline cellulose, sodium
carboxymethylcellulose,
hydoxyalkylcelluloses (e.g., hydroxypropylmethylcellulose and
hydroxypropylcellulose),
-84-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
polyethylene oxide, alkylcelluloses (e.g., methylcellulose and
ethylcellulose), polyethylene glycol,
polyvinylpyrrolidone, cellulose acteate, cellulose acetate butyrate, cellulose
acteate phthalate,
cellulose acteate trimellitate, polyvinylacetate phthalate,
polyalkylmethacrylates, polyvinyl acetate,
or combinations thereof.
[00332] In some embodiments, the first population of particles comprises a
cardiovascular disorder
agent. In some embodiments, the second population of particles comprises a (1)
a modulator of MIF;
(2) a modulator of an interaction between RANTES and Platelet Factor 4; or (3)
combinations
thereof. In some embodiments, the first population of particles comprises a
(1) a modulator of MIF;
(2) a modulator of an interaction between RANTES and Platelet Factor 4; or (3)
combinations
thereof. In some embodiments, the second population of particles comprises a
cardiovascular
disorder agent.
[00333] Dragee cores are provided with suitable coatings. For this purpose,
concentrated sugar
solutions are generally used, which optionally contain gum arabic, talc,
polyvinylpyrrolidone,
carbopol gel, polyethylene glycol, and/or titanium dioxide, lacquer solutions,
and suitable organic
solvents or solvent mixtures. Dyestuffs or pigments are optionally added to
the tablets or dragee
coatings for identification or to characterize different combinations of agent
doses.
[00334] In some embodiments, the solid dosage forms disclosed herein are in
the form of a tablet,
(including a suspension tablet, a fast-melt tablet, a bite-disintegration
tablet, a rapid-disintegration
tablet, an effervescent tablet, or a caplet), a pill, a powder (including a
sterile packaged powder, a
dispensable powder, or an effervescent powder) a capsule (including both soft
or hard capsules, e.g.,
capsules made from animal-derived gelatin or plant-derived HPMC, or "sprinkle
capsules"), solid
dispersion, solid solution, bioerodible dosage form, controlled release
formulations, pulsatile release
dosage forms, multiparticulate dosage forms, pellets, granules, or an aerosol.
In other embodiments,
the pharmaceutical formulation is in the form of a powder. In still other
embodiments, the
pharmaceutical formulation is in the form of a tablet, including but not
limited to, a fast-melt tablet.
Additionally, pharmaceutical formulations disclosed herein are optionally
administered as a single
capsule or in multiple capsule dosage form. In some embodiments, the
pharmaceutical formulation
is administered in two, or three, or four, capsules or tablets.
[00335] In another aspect, dosage forms include microencapsulated
formulations. In some
embodiments, one or more other compatible materials are present in the
microencapsulation
material. Exemplary materials include, but are not limited to, pH modifiers,
erosion facilitators, anti-
foaming agents, antioxidants, flavoring agents, and carrier materials such as
binders, suspending
agents, disintegration agents, filling agents, surfactants, solubilizers,
stabilizers, lubricants, wetting
agents, and diluents.
[00336] Exemplary microencapsulation materials useful for delaying the release
of the formulations
including a MIF receptor inhibitor, include, but are not limited to,
hydroxypropyl cellulose ethers
-85-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
(HPC) such as Klucel or Nisso HPC, low-substituted hydroxypropyl cellulose
ethers (L-HPC),
hydroxypropyl methyl cellulose ethers (HPMC) such as Seppifilm-LC, Pharmacoat
, Metolose SR,
Methocel -E, Opadry YS, PrimaFlo, Benecel MP824, and Benecel MP843,
methylcellulose
polymers such as Methocel -A, hydroxypropylmethylcellulose acetate stearate
Aqoat (HF-LS, HF-
LG,HF-MS) and Metolose , Ethylcelluloses (EC) and mixtures thereof such as E46
1, Ethocel ,
Aqualon -EC, Surelease , Polyvinyl alcohol (PVA) such as Opadry AMB,
hydroxyethylcelluloses
such as Natrosol , carboxymethylcelluloses and salts of
carboxymethylcelluloses (CMC) such as
Aqualon -CMC, polyvinyl alcohol and polyethylene glycol co-polymers such as
Kollicoat IR ,
monoglycerides (Myverol), triglycerides (KLX), polyethylene glycols, modified
food starch, acrylic
polymers and mixtures of acrylic polymers with cellulose ethers such as
Eudragit EPO, Eudragit
L30D-55, Eudragit FS 30D Eudragit L100-55, Eudragit L100, Eudragit 5100,
Eudragit
RD 100, Eudragit E l00, Eudragit L12.5, Eudragit S12.5, Eudragit NE30D,
and Eudragit
NE 40D, cellulose acetate phthalate, sepifilms such as mixtures of HPMC and
stearic acid,
cyclodextrins, and mixtures of these materials.
[00337] Liquid formulation dosage forms for oral administration are optionally
aqueous suspensions
selected from the group including, but not limited to, pharmaceutically
acceptable aqueous oral
dispersions, emulsions, solutions, elixirs, gels, and syrups. See, e.g., Singh
et al., Encyclopedia of
Pharmaceutical Technology, 2nd Ed., pp. 754-757 (2002). In addition to a MIF
receptor inhibitor,
the liquid dosage forms optionally include additives, such as: (a)
disintegrating agents; (b)
dispersing agents; (c) wetting agents; (d) at least one preservative, (e)
viscosity enhancing agents, (f)
at least one sweetening agent, and (g) at least one flavoring agent. In some
embodiments, the
aqueous dispersions further include a crystal-forming inhibitor.
[00338] In some embodiments, the pharmaceutical formulations described herein
are elf-emulsifying
drug delivery systems (SEDDS). Emulsions are dispersions of one immiscible
phase in another,
usually in the form of droplets. Generally, emulsions are created by vigorous
mechanical dispersion.
SEDDS, as opposed to emulsions or microemulsions, spontaneously form emulsions
when added to
an excess of water without any external mechanical dispersion or agitation. An
advantage of SEDDS
is that only gentle mixing is required to distribute the droplets throughout
the solution. Additionally,
water or the aqueous phase is optionally added just prior to administration,
which ensures stability of
an unstable or hydrophobic active ingredient. Thus, the SEDDS provides an
effective delivery
system for oral and parenteral delivery of hydrophobic active ingredients. In
some embodiments,
SEDDS provides improvements in the bioavailability of hydrophobic active
ingredients. Methods of
producing self-emulsifying dosage forms include, but are not limited to, for
example, U.S. Pat. Nos.
5,858,401, 6,667,048, and 6,960,563.
[00339] Suitable intranasal formulations include those described in, for
example, U.S. Pat. Nos.
4,476,116, 5,116,817 and 6,391,452. Nasal dosage forms generally contain large
amounts of water
-86-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
in addition to the active ingredient. Minor amounts of other ingredients such
as pH adjusters,
emulsifiers or dispersing agents, preservatives, surfactants, gelling agents,
or buffering and other
stabilizing and solubilizing agents are optionally present.
[00340] For administration by inhalation, the pharmaceutical compositions
disclosed herein are
optionally in a form of an aerosol, a mist or a powder. Pharmaceutical
compositions described
herein are conveniently delivered in the form of an aerosol spray presentation
from pressurized
packs or a nebuliser, with the use of a suitable propellant, e.g.,
dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other
suitable gas. In the case
of a pressurized aerosol, the dosage unit is determined by providing a valve
to deliver a metered
amount. Capsules and cartridges of, such as, by way of example only, gelatin
for use in an inhaler or
insufflator are formulated containing a powder mix and a suitable powder base
such as lactose or
starch.
[00341] Buccal formulations include, but are not limited to, U.S. Pat. Nos.
4,229,447, 4,596,795,
4,755,386, and 5,739,136. In addition, the buccal dosage forms described
herein optionally further
include a bioerodible (hydrolysable) polymeric carrier that also serves to
adhere the dosage form to
the buccal mucosa. The buccal dosage form is fabricated so as to erode
gradually over a
predetermined time period. Buccal drug delivery avoids the disadvantages
encountered with oral
drug administration, e.g., slow absorption, degradation of the agent by fluids
present in the
gastrointestinal tract and/or first-pass inactivation in the liver. The
bioerodible (hydrolysable)
polymeric carrier generally comprises hydrophilic (water-soluble and water-
swellable) polymers
that adhere to the wet surface of the buccal mucosa. Examples of polymeric
carriers useful herein
include acrylic acid polymers and co, e.g., those known as "carbomers"
(Carbopol , which is
obtained from B.F. Goodrich, is one such polymer). Other components also be
incorporated into the
buccal dosage forms described herein include, but are not limited to,
disintegrants, diluents, binders,
lubricants, flavoring, colorants, preservatives, and the like. For buccal or
sublingual administration,
the compositions optionally take the form of tablets, lozenges, or gels
formulated in a conventional
manner.
[00342] Transdermal formulations of a pharmaceutical compositions disclosed
here are administered
for example by those described in U.S. Pat. Nos. 3,598,122, 3,598,123,
3,710,795, 3,731,683,
3,742,951, 3,814,097, 3,921,636, 3,972,995, 3,993,072, 3,993,073, 3,996,934,
4,031,894, 4,060,084,
4,069,307, 4,077,407, 4,201,211, 4,230,105, 4,292,299, 4,292,303, 5,336,168,
5,665,378, 5,837,280,
5,869,090, 6,923,983, 6,929,801 and 6,946,144.
[00343] The transdermal formulations described herein include at least three
components: (1) an
agent; (2) a penetration enhancer; and (3) an aqueous adjuvant. In addition,
transdermal
formulations include components such as, but not limited to, gelling agents,
creams and ointment
bases, and the like. In some embodiments, the transdermal formulation further
includes a woven or
-87-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
non-woven backing material to enhance absorption and prevent the removal of
the transdermal
formulation from the skin. In other embodiments, the transdermal formulations
described herein
maintain a saturated or supersaturated state to promote diffusion into the
skin.
[00344] In some embodiments, formulations suitable for transdermal
administration employ
transdermal delivery devices and transdermal delivery patches and are
lipophilic emulsions or
buffered, aqueous solutions, dissolved and/or dispersed in a polymer or an
adhesive. Such patches
are optionally constructed for continuous, pulsatile, or on demand delivery of
pharmaceutical agents.
Still further, transdermal delivery is optionally accomplished by means of
iontophoretic patches and
the like. Additionally, transdermal patches provide controlled delivery. The
rate of absorption is
optionally slowed by using rate-controlling membranes or by trapping an agent
within a polymer
matrix or gel. Conversely, absorption enhancers are used to increase
absorption. An absorption
enhancer or carrier includes absorbable pharmaceutically acceptable solvents
to assist passage
through the skin. For example, transdermal devices are in the form of a
bandage comprising a
backing member, a reservoir containing an agent optionally with carriers,
optionally a rate
controlling barrier to deliver a an agent to the skin of the host at a
controlled and predetermined rate
over a prolonged period of time, and means to secure the device to the skin.
[00345] Formulations suitable for intramuscular, subcutaneous, or intravenous
injection include
physiologically acceptable sterile aqueous or non-aqueous solutions,
dispersions, suspensions or
emulsions, and sterile powders for reconstitution into sterile injectable
solutions or dispersions.
Examples of suitable aqueous and non-aqueous carriers, diluents, solvents, or
vehicles including
water, ethanol, polyols (propyleneglycol, polyethylene-glycol, glycerol,
cremophor and the like),
suitable mixtures thereof, vegetable oils (such as olive oil) and injectable
organic esters such as ethyl
oleate. Proper fluidity is maintained, for example, by the use of a coating
such as lecithin, by the
maintenance of the required particle size in the case of dispersions, and by
the use of surfactants.
Formulations suitable for subcutaneous injection also contain optional
additives such as preserving,
wetting, emulsifying, and dispensing agents.
[00346] For intravenous injections, an agent is optionally formulated in
aqueous solutions,
preferably in physiologically compatible buffers such as Hank's solution,
Ringer's solution, or
physiological saline buffer. For transmucosal administration, penetrants
appropriate to the barrier to
be permeated are used in the formulation. For other parenteral injections,
appropriate formulations
include aqueous or nonaqueous solutions, preferably with physiologically
compatible buffers or
excipients.
[00347] Parenteral injections optionally involve bolus injection or continuous
infusion. Formulations
for injection are optionally presented in unit dosage form, e.g., in ampoules
or in multi dose
containers, with an added preservative. In some embodiments, the
pharmaceutical composition
described herein are in a form suitable for parenteral injection as a sterile
suspensions, solutions or
-88-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
emulsions in oily or aqueous vehicles, and contain formulatory agents such as
suspending,
stabilizing and/or dispersing agents. Pharmaceutical formulations for
parenteral administration
include aqueous solutions of an agent in water soluble form. Additionally,
suspensions are
optionally prepared as appropriate oily injection suspensions.
[00348] In some embodiments, an agent disclosed herein is administered
topically and formulated
into a variety of topically administrable compositions, such as solutions,
suspensions, lotions, gels,
pastes, medicated sticks, balms, creams or ointments. Such pharmaceutical
compositions optionally
contain solubilizers, stabilizers, tonicity enhancing agents, buffers and
preservatives.
[00349] An agent disclosed herein is also optionally formulated in rectal
compositions such as
enemas, rectal gels, rectal foams, rectal aerosols, suppositories, jelly
suppositories, or retention
enemas, containing conventional suppository bases such as cocoa butter or
other glycerides, as well
as synthetic polymers such as polyvinylpyrrolidone, PEG, and the like. In
suppository forms of the
compositions, a low-melting wax such as, but not limited to, a mixture of
fatty acid glycerides,
optionally in combination with cocoa butter is first melted.
[00350] An agent disclosed herein is optionally used in the preparation of
medicaments for the
prophylactic and/or therapeutic treatment of inflammatory diseases, disorders,
conditions and
symptoms or conditions that would benefit, at least in part, from
amelioration. In addition, a method
for treating any of the diseases or conditions described herein in an
individual in need of such
treatment, involves administration of pharmaceutical compositions containing
an agent disclosed
herein, or a pharmaceutically acceptable salt, pharmaceutically acceptable N-
oxide,
pharmaceutically active metabolite, pharmaceutically acceptable prodrug, or
pharmaceutically
acceptable solvate thereof, in therapeutically effective amounts to said
individual.
[00351] In the case wherein the individual's condition does not improve, upon
the doctor's
discretion the administration of an agent disclosed herein is optionally
administered chronically, that
is, for an extended period of time, including throughout the duration of the
individual's life in order
to ameliorate or otherwise control or limit the symptoms of the individual's
disease or condition.
[00352] In the case wherein the individual's status does improve, upon the
doctor's discretion the
administration of an agent disclosed herein is optionally given continuously;
alternatively, the dose
of drug being administered is temporarily reduced or temporarily suspended for
a length of time
(i.e., a "drug holiday"). The length of the drug holiday optionally varies
between 2 days and 1 year,
including by way of example only, 2 days, 3 days, 4 days, 5 days, 6 days, 7
days, 10 days, 12 days,
15 days, 20 days, 28 days, 35 days, 50 days, 70 days, 100 days, 120 days, 150
days, 180 days, 200
days, 250 days, 280 days, 300 days, 320 days, 350 days, or 365 days. The dose
reduction during a
drug holiday includes from 10%-100%, including, by way of example only, 10%,
15%, 20%, 25%,
30%,35%,40%,45%,50%,55%,60%,65%,70%,75%,80%,85%,90%,95%, or 100%.
-89-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00353] Once improvement of the individual's conditions has occurred, a
maintenance dose is
administered if necessary. Subsequently, the dosage or the frequency of
administration, or both, is
reduced, as a function of the symptoms, to a level at which the improved
disease, disorder or
condition is retained. In some embodiments, individuals require intermittent
treatment on a long-
term basis upon any recurrence of symptoms.
[00354] In some embodiments, the pharmaceutical composition described herein
is in unit dosage
forms suitable for single administration of precise dosages. In unit dosage
form, the formulation is
divided into unit doses containing appropriate quantities of an agent
disclosed herein. In some
embodiments, the unit dosage is in the form of a package containing discrete
quantities of the
formulation. Non-limiting examples are packaged tablets or capsules, and
powders in vials or
ampoules. In some embodiments, aqueous suspension compositions are packaged in
single-dose
non-reclosable containers. Alternatively, multiple-dose reclosable containers
are used, in which case
it is typical to include a preservative in the composition. By way of example
only, formulations for
parenteral injection are presented in unit dosage form, which include, but are
not limited to
ampoules, or in multi dose containers, with an added preservative.
[00355] The daily dosages appropriate for an agent disclosed herein are from
about 0.01 to 3 mg/kg
per body weight. An indicated daily dosage in the larger mammal, including,
but not limited to,
humans, is in the range from about 0.5 mg to about 100 mg, conveniently
administered in divided
doses, including, but not limited to, up to four times a day or in extended
release form. Suitable unit
dosage forms for oral administration include from about 1 to 50 mg active
ingredient. The foregoing
ranges are merely suggestive, as the number of variables in regard to an
individual treatment regime
is large, and considerable excursions from these recommended values are not
uncommon. Such
dosages are optionally altered depending on a number of variables, not limited
to the activity of the
MIF receptor inhibitor used, the disease or condition to be treated, the mode
of administration, the
requirements of the individual, the severity of the disease or condition being
treated, and the
judgment of the practitioner.
[00356] Toxicity and therapeutic efficacy of such therapeutic regimens are
optionally determined in
cell cultures or experimental animals, including, but not limited to, the
determination of the LD50
(the dose lethal to 50% of the population) and the ED50 (the dose
therapeutically effective in 50%
of the population). The dose ratio between the toxic and therapeutic effects
is the therapeutic index,
which is expressed as the ratio between LD50 and ED50. An agent disclosed
herein exhibiting high
therapeutic indices is preferred. The data obtained from cell culture assays
and animal studies are
optionally used in formulating a range of dosage for use in human. The dosage
of such an agent
disclosed herein lies preferably within a range of circulating concentrations
that include the ED50
with minimal toxicity. The dosage optionally varies within this range
depending upon the dosage
form employed and the route of administration utilized.
-90-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
EXAMPLES
[00357] The following specific examples are to be construed as illustrative,
and not limiting of the
disclosure or the claims.
EXAMPLE 1
Cell Lines and Reagents
[00358] Human aortic (Schober, A., et al. (2004) Circulation 109, 380-385) and
umbilical vein
(Weber, K.S., et al. (1999) Eur. J. Immunol. 29, 700-712) endothelial cells
(PromoCell),
MonoMac6 cells (Weber, C., et al. (1993) Eur. J. Immunol. 23, 852-859) and
Chinese hamster
ovary (CHO) ICAM-1-transfectants (Ostermann, G., et al. (2002) Nat. Immunol.
3, 151-158) were
used as described. Jurkat cells and RAW264.7 macrophages were transfected with
pcDNA3-
CXCR2. HL-60 cells were transfected with pcDNA3.1/V5- HisTOPO-TA-CD74 or
vector control
(Nucleofector Kit V, Amaxa). L1.2 cells were transfected with pcDNA3-CXCRs or
pcDNA-CCR5
(UMR cDNA Resource Center) for assays on simian virus-40-transformed mouse
microvascular
endothelial cells (SVECs). Peripheral blood mononuclear cells were prepared
from buffy coats,
monocytes by adherence or immunomagnetic separation (Miltenyi), primary T
cells by
phytohaemaglutinin/interleukin-2 (Biosource) stimulation and/or immunomagnetic
selection
(antibody to CD3/ M-450 Dynabeads), and neutrophils by Ficoll gradient
centrifugation. Human
embryonal kidney-CXCR2 transfectants (HEK293-CXCR2) have been described
previously (Ben-
Baruch, A., et al. (1997) Cytokine 9, 37-45).
[00359] Recombinant MIF was expressed and purified as described (Bernhagen,
J., et al. (1993)
Nature 365, 756-759). Chemokines were from PeproTech. Human VCAM-1.Fc chimera,
blocking
antibodies to CXCRI (42705, 5A12), CXCR2 (48311), CXCR4 (44708, FABSP2
cocktail, R&D),
human MIF and mouse MIF (NIHIII.D.9) (Lan, H.Y., et al. (1997) J. Exp. Med.
185, 1455-1465),
CD74 (M-B741, Pharmingen), R2 integrin (TS 1/18), a4 integrin (HP2/1) (Weber,
C., et al. (1996) J.
Cell Biol. 134, 1063-1073) and CXCR2 (RII115), and antibody to aL integrin
(327C) (Shamri, R., et
al. (2005) Nat. Immunol. 6, 497-506) were used. PTX and B-oligomer were from
Merck.
Methods Used in Examples
Adhesion assays.
[00360] Arrest of calcein-AM (Molecular Probes)-labeled monocytes, T cells and
L1.2 transfectants
was quantified in parallel-wall chambers in flow (1.5 dynes/cm2, 5 min)
(Schober, A., et al. (2004)
Circulation 109, 380-385; Ostermann, G., et al. (2002) Nat. Immunol. 3, 151-
158; Weber, C., et al.
(1996) J. Cell Biol. 134, 1063-1073). Confluent endothelial cells, CHO-ICAM-1
cells, VCAM-
1.Fc-coated plates and leukocytes were pretreated with MIF, chemokines or
antibodies. CHO-
ICAM-1 cells incubated with MIF (2 h) were stained with antibody to MIF Ka565
(Leng, L., et al.
(2003) J. Exp. Med. 197, 1467-1476) and FITC-conjugated antibody.
-91-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Chemotaxis assays.
[00361] Using Transwell chambers (Costar), we quantified primary leukocyte
migration toward MIF
or chemokines by fluorescence microscopy or using calcein-AM labeling and
FluoroBlok filters
(Falcon). Cells were pretreated with PTX/B-oligomer, Ly294002, MIF (for
desensitization),
antibodies to CXCRs or CD74, or isotype IgG. Pore sizes and intervals were 5
m and 3 h
(monocytes), 3 m and 1.5 h (T cells), and 3 mm and 1 h (neutrophils).
Q-PCR and ELISA.
[00362] RNA was reverse-transcribed using oligo-dT primers. RTPCR was
performed using
QuantiTect Kit with SYBRGreen (Qiagen), specific primers and an MJ Opticon2
(Biozym). CXCL8
was quantified by Quantikine ELISA (R&D).
aL(32 integrin activation assay.
[00363] Monocytes stimulated with MIF or Mg2+/EGTA (positive control) were
fixed, reacted with
the agent 327C and an FITC-conjugated antibody to mouse IgG. LFA-1 activation
analyzed by flow
cytometry is reported as the increase in mean fluorescent intensity (MFI) or
relative to the positive
control (Shamri, R., et al. (2005) Nat. Immunol. 6, 497-506).
Calcium mobilization.
[00364] Neutrophils or L1.2 CXCR2 transfectants were labeled with Fluo-4 AM
(Molecular Probes).
After the addition of the first or a subsequent stimulus (MIF, CXCL8 or
CXCL7), MFI was
monitored as a measure of cytosolic Ca 2+ concentrations for 120 s using a BD
FACSAria. L1.2
controls showed negligible calcium influx.
Receptor-binding assays.
[00365] Because iodinated MIF is inactive (Leng, L., et al. (2003) J. Exp.
Med. 197, 1467-1476;
Kleemann, R., et al. (2002) J. Interferon Cytokine Res. 22, 351-363),
competitive receptor binding
(Hayashi, S., et al. (1995) J. Immunol. 154, 814-824) were performed using
radioiodinated tracers
(Amersham): [1125]CXCL8, reconstituted at 4 nM (80 &i/ml) to a final
concentration of 40 pM;
[1125]CXCL12, reconstituted at 5 nM (100 Ci/ml) to a final concentration of
50 pM. For
competition of [1125]CXCL8 with MIF for CXCR2 binding or competition of
[1125]CXCL12 with
MIF for CXCR4 binding in equilibrium binding assays, cold MIF and/or CXCL with
tracers to
HEK293-CXCR2 or CXCR4-bearing Jurkat cells were added. The analysis was
performed by liquid
scintillation counting. To calculate EC50 and Kd values, a one-site receptor-
ligand binding model was
assumed and the Cheng/Prusoff-equation and GraphPad Prism were used.
[00366] For pull-down of biotin-MIF-CXCR complexes, HEK293-CXCR2 transfectants
or controls
were incubated with biotin-labeled MIF (Kleemann, R., et al. (2002) J.
Interferon Cytokine Res. 22,
351-363), washed and lysed with coimmunoprecipitation (CoIP) buffer. Complexes
were isolated
from cleared lysates by streptavidin-coated magnetic beads (M280, Dynal) and
analyzed by western
blotting with antibody to CXCR2 or streptavidin-peroxidase. For flow
cytometry, HEK293-CXCR2
-92-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
transfectants or Jurkat cells pretreated with AMD3465 and/or a 20-fold excess
of unlabeled MIF
were incubated with fluorescein-labeled MIF and analyzed using a BD
FACSCalibur.
CXCR internalization assays.
[00367] HEK293-CXCR2 or Jurkat cells were treated with CXCL8 or CXCL12,
respectively,
treated with MIF, washed with acidic glycine-buffer, stained with antibodies
to CXCR2 or CXCR4,
and analyzed by flow cytometry. Internalization was calculated relative to
surface expression of
buffer-treated cells (100% control) and isotype control staining (0% control):
geometric
MFI[experimental]-MFI[0% control]/MFI[100% control]-MFI[0% control] x 100.
Co localization of CXCR2 and CD74.
[00368] RAW264.7-CXCR2 transfectants were co stained with CXCR2 and rat
antibody to mouse
CD74 (In-1, Pharmingen), followed by FITC-conjugated antibody to rat IgG and
Cy3-conjugated
antibody to mouse IgG, and were analyzed by confocal laser scanning microscopy
(Zeiss).
Coimmunoprecipitation of CXCR2 and CD74.
[00369] HEK293-CXCR2 cells transiently transfected with pcDNA3.1/V5-HisTOPO-TA-
CD74
were lysed in nondenaturing CoIP buffer. Supernatants were incubated with the
CXCR2 antibody
Rll115 or an isotype control, and were preblocked with protein G-sepharose
overnight. Proteins
were analyzed by western blots using agent to the His-tag (Santa Cruz).
Similarly, CoIPs and
immunoblots were performed with antibodies to the His-tag and CXCR2,
respectively. L1.2-CXCR2
cells were subjected to immunoprecipitation with antibody to CXCR2 and
immunoblotting with an
antibody to mouse CD74.
Ex vivo perfusion and intravital microscopy of carotid arteries.
[00370] Mil-I Ldlr /- mice and M I+Ldlr / littermate controls, crossbred from
Mil-1- (Fingerle-
Rowson, G., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 9354-9359) and Ldlr/
mice (Charles
River), and Apoe i- mice were fed an atherogenic diet (21% fat; Altromin) for
6 weeks. All single
knockout strains had been back-crossed in the C57BL/6 background ten times.
Mit i+ and M ' mice
were treated with TNF-a (intraperitoneally (i.p.), 4 h). Explanted arteries
were transferred onto the
stage of an epifluorescence microscope and perfused at 4 l/min with calcein-
AM-labeled
MonoMac6 cells treated with antibodies to CD74 or CXCR2, isotype control IgG,
or left untreated
(Huo, Y., et al. (2001) J. Clin. Invest. 108, 1307-1314). Untreated monocytic
cells were perfused
after blockade with antibody to MIF for 30 min. For intravital microscopy,
rhodamine-G (Molecular
Probes) was administered intravenously (i.v.), and carotid arteries were
exposed in anesthetized
mice. Arrest (>30 s) of labeled leukocytes was analyzed by epifluorescence
microscopy (Zeiss
Axiotech, 20x water immersion). All studies were approved by local authorities
(Bezirksregierung
Koln), and complied with German animal protection law Az: 50.203.2-AC 36,
19/05.
Mouse model of atherosclerotic disease progression.
-93-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00371] Apoe i- mice fed an atherogenic diet for 12 weeks were injected (3
injections per week, each
50 g) with antibodies to MIF (NIHIIID.9), CXCL12 (79014) or CXCLI (124014,
R&D) (n = 6-10
mice) for an additional 4 weeks. Aortic roots were fixed by in situ perfusion
and atherosclerosis was
quantified by staining transversal sections with Oil-Red-O. Relative
macrophage and T-cell contents
were determined by staining with antibodies to MOMA-2 (MCA519, Serotec) or to
CD3 (PC3/
188A, Dako) and FITC-conjugated antibody. In Mif4 Ldl and Mif~I+Ldlr 1 mice
fed a chow diet
for 30 weeks, the abundance of luminal monocytes and lesional macrophages in
aortic roots was
determined as described (Verschuren, L., et al. (2005) Arterioscler. Thromb.
Vasc. Biol. 25, 161-
167).
Cremaster microcirculation model.
[00372] Human MIF (1 g) was injected intra-scrotally and the cremaster muscle
was exteriorized in
mice treated with antibody to CXCR2 (100 g i.p.). After 4 h, intravital
microscopy (Zeiss
Axioplan; 20x) was performed in postcapillary venules (Gregory, J.L., et al.
(2004) Arthritis Rheum.
50, 3023-3034; Keane, M.P., et al. (2004) J. Immunol. 172, 2853-2860).
Adhesion was measured as
leukocytes stationary for more than 30 s, emigration as the number of
extravascular leukocytes per
field.
Bone marrow transplantation.
[00373] Femurs and tibias were aseptically removed from donor Il8rb_1-
(Jackson Laboratories) or
BALB/c mice. The cells, flushed from the marrow cavities, were administered
i.v. into Mifl+ or Mif
i mice 24 h after ablative whole-body irradiation (Zernecke, A., et al. (2005)
Circ. Res. 96, 784-
791).
Model of acute peritonitis.
[00374] Mice repopulated with Il8rb+i+ or Il8rb/- bone marrow were injected
i.p. with MIF (200 ng).
After 4 h, peritoneal lavage was performed and Gr-1+CD 115-F4/80- neutrophils
were quantified by
flow cytometry using the relevant conjugated antibodies.
Statistical analysis.
[00375] Statistical analysis was performed using either a one-way analysis of
variance (ANOVA)
and Newman-Keuls post-hoc test or an unpaired Student's t-test with Welch's
correction (GraphPad
Prism).
EXAMPLE 2:
Surface-bound MIF induced monocyte arrest through CXCR2
[00376] Monoclonal antibodies and pertussis toxin (PTX) were used to explore
whether MIF-
induced monocyte arrest depends on Ga,1-coupled activities of CXCR2. Human
aortic endothelial
cells that had been pretreated with recombinant MIF for 2 h substantially
increased the arrest of
primary human monocytes under flow conditions, an effect blocked by an
antibody to MIF (Fig. 1 a).
-94-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Notably, MIF-triggered, but not spontaneous, monocyte arrest was ablated by an
antibody to
CXCR2 or by PTX, implicating Ga,1-coupled CXCR2. The ability of MIF to induce
monocyte arrest
through CXCR2 was confirmed using monocytic Mono-Mach cells and this activity
was associated
with an immobilization of MIF on aortic endothelial cells (Fig. lb). This data
indicated that MIF
was presented on the endothelial cell surface and exerted a chemokine-like
arrest function as a
noncognate CXCR2 ligand. Blocking classical CXCR2 agonists (CXCL1/CXCL8)
failed to interfere
with these effects of MIF (Fig. I a).
[00377] Chinese hamster ovary (CHO) transfectants that express the R2 integrin
ligand, ICAM-1
(intercellular adhesion molecule 1), were used to dissect the mechanisms by
which MIF promotes
integrin-dependent arrest. As quantified under flow conditions, the exposure
of CHO transfectants to
MIF for 2 h resulted in its surface presentation (Fig. lb) and, like exposure
of the transfectants to
CXCL8, increased monocytic cell arrest (Fig. lc). This effect was fully
sensitive to PTX and an
antibody to R2 integrin (Fig. lc), confirming a role of Ga1 1 in R2 integrin-
mediated arrest induced by
MIF. Primary monocytes and MonoMac6 cells express both CXCRI and CXCR2 (Weber,
K.S., et
al. (1999) Eur. J. Immunol. 29, 700-712). Whereas blocking CXCR1 had no
effect, blocking
CXCR2 substantially but not fully impaired MIF-triggered and CXCL8-triggered
monocytic cell
arrest. Addition of antibodies to both CXCRI and CXCR2 completely inhibited
the arrest functions
of MIF or CXCL8 (Fig. I d & Fig. 8). The use of antibodies to CD74 implicated
this protein, along
with CXCR2, in MIF-induced arrest (Fig. 1 d). Spontaneous arrest was
unaffected (Fig. 8). Thus,
CXCR2 assisted by CD74 mediates MIF-induced arrest.
MIF induced T-Cell arrest through CXCR4
[00378] Either MIF or CXCL12 immobilized on aortic endothelial cells triggered
the arrest of
primary human effector T cells (Fig. 1 e). MIF-induced, but not spontaneous, T-
cell arrest was
sensitive to PTX and was inhibited by an antibody to CXCR4 (Fig. 1 e).
Although less pronounced
than in monocytes expressing CXCR2 (Fig. ld), presentation of MIF (or CXCL12)
on CHO
transfectants expressing ICAM-1 elicited aL(32-dependent arrest of Jurkat T
cells, an effect mediated
by CXCR4 (Fig. If).
[00379] Ectopic expression of CXCR2 in Jurkat T cells increased MIF-triggered
arrest (Fig. lg),
corroborating the idea that CXCR2 imparts responsiveness to MIF in leukocytes.
L1.2 pre-B
lymphoma transfectants expressing CXCR1, CXCR2 or CXCR3, and controls using
cells expressing
endogenous CXCR4 only were used in the presence of the CXCR4 antagonist
AMD3465. MIF
triggered the arrest of CXCR2 transfectants and CXCR4-bearing controls on
endothelial cells with a
similar efficacy to that of the canonical ligands CXCL8 and CXCL12, whereas
CXCR1 and CXCR3
transfectants were responsive to CXCL8 and CXCL10, respectively, but not to
MIF (Fig. lh). This
data established that CXCR2 and CXCR4, but not CXCRI or CXCR3, support MIF-
induced arrest.
-95-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
EXAMPLE 3
MIF-induced leukocyte chemotaxis through CXCR2/4 activation
[00380] Chemokines have been eponymously defined as inducers of chemotaxis
(Baggiolini, M., et
al. (1994) Adv. Immunol. 55, 97-179; Weber, C., et al. (2004) Arterioscler.
Thromb. Vasc. Biol. 24,
1997-2008). Paradoxically, MIF was initially thought to interfere with
`random' migration
(Calandra, T., et al. (2003) Nat. Rev. Immunol. 3, 791-800). Although this may
be attributable to
active repulsion or desensitization of directed emigration, specific
mechanisms evoked by MIF to
regulate migration remain to be clarified. Our results showing that MIF
induced Ga,1-mediated
functions of CXCR2 and CXCR4 prompted us to test if MIF directly elicits
leukocyte chemotaxis
through these receptors.
[00381] Using a transwell system, the promigratory effects of MIF and CXCL8
were compared on
primary human peripheral blood mononuclear cell-derived monocytes. CCL2 was
also used as a
prototypic chemokine for monocytes. Similar to CXCL8 and CCL2, adding MIF to
the lower
chamber induced migration, which followed a bell-shaped dose-response curve
typical for
chemokines, with an optimum at 25-50 ng/ml, albeit with a lower peak migratory
index (Fig. 2a).
Heat treatment or a neutralizing antibody to MIF abolished MIF-induced
transmigration. In contrast,
isotype-matched immunoglobulin (IgG) had no effect (Fig. 2b). When added to
the upper chamber,
MIF dose-dependently desensitized migration toward MIF in the lower chamber
(Fig. 2c) but did
not elicit migration when present in the upper chamber only, suggesting that
MIF evokes true
chemotaxis rather than chemokinesis. Consistent with Ga,1-dependent signaling
through
phosphoinositide-3-kinase, MIF-induced monocyte chemotaxis was sensitive to
PTX and abrogated
by Ly294002 (Fig. 2d). Both CXCR2 and CD74 specifically contributed to MIF-
triggered monocyte
chemotaxis (Fig. 2e). The role for CXCR2 was confirmed by showing MIF-mediated
cross-
desensitization of CXCL8-induced chemotaxis in CXCR2-transfected L1.2 cells.
The chemotactic
activity of MIF was verified in RAW264.7 macrophages (Fig. 8) and THP-1
monocytes. These data
demonstrate that MIF triggers monocyte chemotaxis through CXCR2.
[00382] To substantiate functional MIF-CXCR4 interactions, the transmigration
of primary CD3+ T
lymphocytes devoid of CXCRI and CXCR2 was evaluated. Similar to CXCL12, a
known CXCR4
ligand and T-cell chemoattractant, MIF dose-dependently induced
transmigration, a process that was
chemotactic and transduced through CXCR4, as shown by antibody blockade and
cross-
desensitization of CXCL12 (Fig. 2f & Fig. 8). Thus, MIF elicits directed T-
cell migration through
CXCR4. In primary human neutrophils, a major cell type bearing CXCR2, MIF
exerted CXCR2-
but not CXCR I -mediated chemotactic activity, exhibiting a bell-shaped dose-
response curve and
cross-densensitizing CXCL8 (Fig. 2g,h). The moderate chemotactic activity of
neutrophils towards
MIF is likely to be related to an absence of CD74 on neutrophils, as its
ectopic expression in CD74-
promyelocytic HL-60 cells enhanced MIF-induced migration (Fig. 8). Although
MIF, like other
-96-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
CXCR2 ligands, functions as an arrest chemokine, the present data revealed
that MIF also has
appreciable chemotactic properties on mononuclear cells and neutrophils.
EXAMPLE 4
MIF triggers rapid integrin activation and calcium flux
[00383] Arrest functions of MIF may reflect direct MIF/CXCR signaling, but it
cannot be entirely
excluded that MIF induces other arrest chemokines during the time required for
MIF
immobilization. To consolidate evidence that MIF directly induces leukocyte
arrest (Fig. 1), real-
time PCR and ELISAs were performed and found that 2-h-long preincubation of
human aortic (or
venous) endothelial cells with MIF failed to upregulate typical arrest
chemokines known to engage
CXCR2 (Fig. 3a).
[00384] Short-term exposure to chemokines present in solution or immobilized
in juxtaposition to
integrin ligands (for example, vascular cell adhesion molecule (VCAM)- 1) can
rapidly upregulate
integrin activity, which mediates leukocyte arrest (Laudanna, C., et al.
(2006) Thromb. Haemost. 95,
5-11). This is accomplished by clustering (for example, a4(3i) or
conformational changes (for
example, aL(32) immediately preceding ligand binding. Stimulation of monocytic
cells with MIF (or
CXCL8) for 1-5 min triggered aLR2-dependent arrest on CHO/ICAM-1 cells (Fig.
3b). To obtain
evidence for a direct stimulation of monocyte integrins, the reporter antibody
327C, which
recognizes an extended high-affinity conformation of aL(32, was used (Shamri,
R., et al. (2005) Nat.
Immunol. 6, 497-506). These assays revealed that aL(32 activation in MonoMac6
cells (Fig. 3c) and
human blood monocytes (Fig. 3d) occurred as early as 1 min after exposure to
MIF and persisted
over 30 min. To evaluate whether MIF's effects were restricted to aLR2, a4(3i-
dependent monocytic
cell arrest on VCAM-1 was studied. Exposure to MIF for 1-5 min induced marked
arrest, which was
mediated by CXCR2, CD74 and a4(3i (Fig. 3e). Similarly to the effect of
CXCL12, stimulation of
Jurkat T cells with MIF for 1-5 min triggered CXCR4-dependent adhesion on VCAM-
1 (Fig. 8).
[00385] As CXCR2 can mediate increases in cytosolic calcium elicited by CXCL8
(Jones, S.A., et
al. (1997) J. Biol. Chem. 272, 16166-16169), the ability of MIF to stimulate
calcium influx and
desensitize CXCL8 signals was tested. Indeed, like CXCL8, MIF induced calcium
influx in primary
human neutrophils and desensitized calcium transients in response to either
CXCL8 or MIF (Fig.
3f), confirming that MIF activates GPCR/G,,; signaling. The partial
desensitization of CXCL8
signaling by MIF seen in neutrophils parallels findings with other CXCR2
ligands (Jones, S.A., et al.
(1997) J. Biol. Chem. 272, 16166-16169) and reflects the presence of CXCR1. In
L1.2 transfectants
expressing CXCR2, MIF fully desensitized CXCL8-induced calcium influx, and in
neutrophils, MIF
desensitized transients induced by the selective CXCR2 ligand CXCL7 (and CXCL7
desensitized
transients induced by MIF) (Fig. 3f). In CXCR2 transfectants, MIF dose-
dependently induced
-97-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
calcium influx, and was slightly less potent and effective than CXCL8 or CXCL7
(Fig. 3g). In
conclusion, MIF acted on CXCR2 and CXCR4 to elicit rapid integrin activation
and calcium influx.
EXAMPLE 5
MIF interacts with CXCR2 and CXCR4
[00386] To assess the physical interactions of MIF with CXCR2 and CXCR4, we
performed
receptor-binding competition and internalization studies. In HEK293 cells
ectopically expressing
CXCR2, MIF strongly competed with 125I-labeled CXCL8 for CXCR2 binding under
equilibrium
conditions. Binding of the CXCL8 tracer to CXCR2 was inhibited by MIF with an
effector
concentration for half-maximum response (EC50) of 1.5 nM (Fig. 4a). The
affinity of CXCR2 for
MIF (Kd = 1.4 nM) was close to that for CXCL8 (Kd = 0.7 nM) and within the
range of the MIF
concentration that induced optimal chemotaxis (2-4 nM). To confirm binding to
CXCR2, we used a
receptor internalization assay that reports specific receptor-ligand
interactions. FACS analysis of
surface CXCR2 on stable HEK293 transfectants showed that MIF induced CXCR2
internalization
with a dose response resembling that of CXCL8 (Fig. 4b). Comparable data was
obtained in
CXCR2-transfected RAW264.7 macrophages (inset in Fig. 4b).
[00387] To verify an interaction of MIF with CXCR4, receptor-binding studies
were performed in
Jurkat T cells, which endogenously express CXCR4. MIF competed with 125I-
labeled CXCL12 for
CXCR4 binding (Kd for CXCL12 = 1.5 nM; EC50 = 19.9 nM, Kd for MIF = 19.8 nM)
(Fig. 4c). The
Kd was in accordance with MIF concentrations that induce T-cell chemotaxis.
Consistently, MIF,
like CXCL12, elicited CXCR4 internalization in a dose-dependent fashion (Fig.
4d). MIF-induced
internalization of CXCR2 and CXCR4 was specific to these receptors, as MIF,
unlike the cognate
ligand CCL5, was unable to induce CCR5 internalization in L1.2 CCR5
transfectants.
[00388] To corroborate its interactions with CXCRs, MIF was labeled with
biotin or fluorescein,
which, in contrast to iodinated MIF, allows for direct receptor-binding
assays. CXCR2 transfectants,
but not vector controls, supported direct binding of labeled MIF, as evidenced
by flow cytometry
(Fig. 4e), pull down with streptavidin beads (inset in Fig. 4e) and
fluorescence microscopy. In
addition, the specific binding of fluorescein-MIF to CXCR4-bearing Jurkat
cells was inhibited by
the CXCR4 antagonist AMD3465.
Complex formation between CXCR2 and CD74
[00389] Our data suggests the possibility that a functional MIF receptor
complex involves both
GPCRs and CD74. Thus, the colocalization of endogenous CD74 and CXCR2 was
visualized using
confocal fluorescence microscopy in RAW264.7 macrophages expressing human
CXCR2. Using
this technique, prominent colocalization was observed in a polarized pattern
in -50% of cells (Fig.
4f).
-98-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00390] In addition, coimmunoprecipitation assays revealed that CXCR2
physically interacts with
CD74. CXCR2/CD74 complexes were detected in HEK293 cells stably overexpressing
CXCR2 and
transiently expressing His-tagged CD74. These complexes were observed by
precipitation with an
antibody to CXCR2 and by detecting coprecipitated CD74 by western blot against
the His-tag.
Coprecipitation was also seen when the order of the antibodies used was
reversed (Fig. 4g).
Complexes were also detected with CD74 in L1.2 transfectants stably expressing
human CXCR2, as
assessed by coimmunoprecipitation with an antibody to CXCR2. In contrast, no
complexes were
observed with L1.2 controls or the isotype control (Fig. 4h). The data are
consistent with a model in
which CD74 forms a signaling complex with CXCR2 to mediate MIF functions.
EXAMPLE 6
CXCR2 mediates MIF-induced monocyte arrest in arteries
[00391] MIF promotes the formation of complex plaques with abundant cell
proliferation,
macrophage infiltration and lipid deposition (Weber, C., et al. (2004)
Arterioscler. Thromb. Vasc.
Biol. 24, 1997-2008; Morand, E.F., et al. (2006) Nat. Rev. Drug Discov. 5, 399-
4 10). This has been
related to the induction of endothelial MIF by oxLDL, triggering monocyte
arrest (Schober, A., et al.
(2004) Circulation 109, 380-385). The CXCR2 ligand CXCL1 can also elicit a4(3i-
dependent
monocyte accumulation in ex vivo-perfused carotid arteries of mice with early
atherosclerotic
endothelium (Huo, Y., et al. (2001) J. Clin. Invest. 108, 1307-1314). This
system was used to test
whether MIF acts via CXCR2 to induce recruitment. Monocyte arrest in carotid
arteries of Apoe i-
mice fed a high-fat diet was inhibited by antibodies to CXCR2, CD74 or MIF
(Fig. 5a & Fig. 9),
indicating that MIF contributed to atherogenic recruitment via CXCR2 and CD74.
Following the
blockade of MIF, CXCR2 and CD74 for 24 h, a similar pattern was observed for
monocyte arrest in
arteries of wild-type mice treated with tumor necrosis factor (TNF)- a,
mimicking acute vascular
inflammation (Fig. 5b). In arteries of TNF-a-treated Milmice, inhibitory
effects on CD74 were
attenuated and blocking MIF was ineffective, whereas there was residual CXCR2
inhibition,
implying the involvement of other inducible ligands (Fig. 5c). Compared to the
effect of MIF
deficiency observed with TNF- a stimulation, monocyte accumulation was more
clearly impaired by
MIF deficiency in arteries of Mii Ldlr / mice (compared to atherogenic M
1+Ldlr / mice; Fig.
5d,e). In the absence of MIF, there was no apparent contribution of CXCR2.
Moreover, blocking
MIF had no effect (Fig. 5d,e). The inhibitory effects of blocking CXCR2 were
restored by loading
exogenous MIF (Fig. 5f).
[00392] To provide further evidence for the idea that CXCR2 is required for
MIF-mediated
monocyte recruitment in vivo, intravital microscopy was performed on carotid
arteries of chimeric
wild-type Mif"+ and Mil-- mice reconstituted with wild-type or Il8rb-~- bone
marrow (Il8rb encodes
CXCR2; Fig. 5g,h). After treatment with TNF- a for 4 h, the accumulation of
rhodamine G-labeled
-99-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
leukocytes was attenuated in Mil'- mice reconstituted with wild-type bone
marrow compared to that
in wild-type mice reconstituted with wild-type bone marrow. The reduction in
leukocyte
accumulation due to deficiency in bone marrow CXCR2 was more marked in
chimeric wild-type
mice than in chimeric Mif mice (Fig. 5g,h).
EXAMPLE 7
MIF-induced inflammation in vivo relied on CXCR2
[00393] The importance of CXCR2 for MIF-mediated leukocyte recruitment under
atherogenic or
inflammatory diseases, disorders, conditions and symptoms was corroborated in
vivo. The adhesion
of monocytes to the luminal surface of aortic roots was reduced in Mif/ Ldlr /
versus M1+Ldlr
mice with primary atherosclerosis, and this was mirrored by a marked decrease
in lesional
macrophage content (Fig. 6a). Intravital microscopy of microcirculation in the
cremaster muscle
revealed that injecting MIF adjacent to the muscle caused a marked increase in
(mostly CD68+)
leukocyte adhesion and emigration in postcapillary venules, which was
inhibited by an antibody to
CXCR2 (Fig. 6b,c). Circulating monocyte counts were unaffected.
[00394] Next a model of MIF-induced peritonitis was used in chimeric mice
reconstituted with wild-
type or I18rb-/- bone marrow. Intraperitoneal injection of MIF elicited
neutrophil recruitment after 4
h in mice with wild-type bone marrow, which was abrogated in mice with Il8rb/-
bone marrow (Fig.
6d). Collectively, these results demonstrated that MIF triggers leukocyte
recruitment under
atherogenic and inflammatory diseases, disorders, conditions and symptoms in
vivo through
CXCR2.
Targeting MIF resulted in regression of atherosclerosis
[00395] As described herein, MIF acted through both CXCR2 and CXCR4. Given the
role of MIF
and CXCR2 in the development of atherosclerotic lesions, targeting MIF, rather
than CXCL1 or
CXCL 12, was investigated as a method to modify advanced lesions and their
content of CXCR2+
monocytes and CXCR4+ T cells. Apoe i- mice, which had received a high-fat diet
for 12 weeks and
had developed severe atherosclerotic lesions, were treated with neutralizing
antibodies to MIF,
CXCL1 or CXCL12 for 4 weeks. Immunoblotting and adhesion assays were used to
verify the
specificity of the MIF antibody. These assays confirmed that the MIF antibody
blocked MIF-
induced, but not CXCL1- or CXCL8-induced, arrest (Fig. 10).
[00396] Blockade of MIF, but not CXCL1 or CXCL12, resulted in a reduced plaque
area in the
aortic root at 16 weeks and a significant (P < 0.05) plaque regression
compared to baseline at 12
weeks (Fig. 6e,f). In addition, blockade of MIF, but not CXCL1 or CXCL12, was
associated with
less of an inflammatory plaque phenotype at 16 weeks, as evidenced by a lower
content of both
macrophages and CD3+ T cells (Fig. 6g,h). Therefore, by targeting MIF and
inhibiting the activation
of CXCR2 and CXCR4, therapeutic regression and stabilization of advanced
atherosclerotic lesions
-100-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
was achieved. In some embodiments, the present invention comprises a method of
reducing plaque
area in an individual in need thereof, comprising administering to said
individual one or more agents
that inhibit (i) MIF binding to CXCR2 and/or CXCR4 and/or (ii) MIF-activation
of CXCR2 and/or
CXCR4; or (iii) any combination of (i) and (ii).
EXAMPLE 8
Interference with CXCR4 aggravates atherosclerosis.
[00397] To explore the role of CXCR4 in atherosclerosis, Apoe-/- mice fed an
atherogenic diet are
continuously treated with the CXCR4 antagonist AMD3465 or vehicle (controls)
via osmotic
minipumps, and atherosclerotic plaque formation is analyzed after 12 weeks.
Compared with
controls, AMD3465 treatment significantly exacerbates lesion formation in oil
red O-stained aortic
root sections (Figure 9a) and in thoracoabdominal aortas prepared en face
(Figure 9b). In addition
continuous treatment of Apoe-/- mice with AMD3465 induces a pronounced
peripheral blood
leukocytosis within 2 days, which is sustained throughout the study period,
and an expansion in the
relative number of circulating neutrophils, which further increases during
disease progression
(Figure 9c).
EXAMPLE 9
Blocking Th-17 development in a mouse model of Multiple Sclerosis
[00398] Eight- to twelve-week-old C57BL/6 mice ( obtained from The Jackson
Laboratory, Bar
Harbor, Main, USA) are pretreated on day -1 and weekly thereafter with
intraperitoneal injections
of 5 mg/kg of either a control antibody (group 1), an antagonistic anti-mouse
MIF antibody (group
2), an antibody to CXCR2 that blocks MIF binding and/or activation of CXCR2
(group 3), an
antibody to CXCR4 that blocks MIF binding and/or activation of CXCR4 (group 4)
or an antibody
to CXCR4 that blocks MIF binding and/or activation of CXCR4 and an antibody to
CXCR2 that
blocks MIF binding and/or activation of CXCR2 (group 5). Mice (n = 30 per
group) are immunized
the following day (day 0) by two subcutaneous injections on the back totaling
200 l of an
emulsification of MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK; Bachem AG,
Bubendorf, Switzerland) in CFA. The final concentrations of peptide and M.
tuberculosis are 150
g/mouse and 1 mg/mouse, respectively. PTX (400 ng; LIST Biological
Laboratories Inc.,
Campbell, California, USA) is injected intraperitoneally on days 0 and 2. The
disease is monitored
daily by measuring paralysis on a 0-6 scale as described above. Average
maximal disease scores are
compared between groups using a one-way ANOVA.
[00399] Paralysis measurements are compared between group 2 mice and group 1
to determine the
efficacy of an antagonistic anti-MIF antibody, for treating or preventing EAE.
Group 5 mice are
compared to group 1 mice to determine the efficacy of an agent that blocks MIF
binding and/or
-101-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
activation of CXCR2 and CXCR4, for treating or preventing EAE. Group 5 mice
are compared to
groups 3 & 4 to determine the effect of blocking MIF binding and/or activation
of both CXCR2 and
CXCR4 to the effect of blocking CXCR2 or CXCR4 individually.
[00400] Mixed T cells are prepared from draining lymph nodes and spleen on day
7-11 after
immunization. Viable cells (3.75 x 106/ml) are cultured in complete medium
with (re-stimulated) or
without MOG peptide (amino acids 35-55) at various concentrations.
Supernatants from activated
cells are collected 72 h later and TNF, IFN--y, IL-23 & IL- 17 are measured by
ELISA (BD
Pharmingen). High IL-17 and IL-23 levels indicate the development of a Th-17
cells and a Th-17
mediated disease phenotype. Inhibition of these cytokines by treatment of mice
or cell cultures with
MIF blocking antibodies (group 2), or by blocking MIF binding and/or
activation of both CXCR2
and CXCR4 (group 5) illustrates a key regulatory role of MIF in the
development of Th- 17 cells and
in the progression of a Th- 17 mediated inflammatory disease (i.e. multiple
sclerosis).
[00401] For intracellular cytokine staining, spleen and lymph node cells from
immunized mice are
stimulated for 24 h with peptide antigen, and GolgiPlug (BD Pharmingen) is
added in the last 5 h or
GolgiPlug plus 500 ng/ml of ionomycin and 50 ng/ml of phorbol 12-myristate 13-
acetate (PMA;
Sigma-Aldrich) are added for 5 h. For cell staining, cells are permeabilized
with the
Cytofix/Cytoperm Plus Kit (BD Pharmingen) according to the manufacturer's
protocol. Gated CD4-
posivtive T-cells are analyzed for the presence of intracellular IL- 17, IL-23
or cell surface IL23
receptor (IL23R) by flow cytometry. The presence of CD4+, IL- 17+ double
positive T-cells
indicates development of a Th- 17 phenotype that is driving disease
progression. Further the up-
regulation of IL-23Rs on CD4+, IL-17 double positive cells provides supportive
evidence of a Th-17
phenotype. The presence of high intracellular IL-23 in CD4+, IL- 17 double
positive cells or in any
leukocyte provides additional supportive evidence for IL-23 driving Th- 17
cell expansion and/or
maintenance. Inhibition of Th-17 cell development, as determined by lower
levels of IL-17, IL-23R
or IL-23, as described in the above experiment, by treating mice with MIF
blocking agents (group 2
mice) or agents that block MIF binding/or activation of CXCR2 and CXCR4 (group
5 mice)
demonstrates a dominant role for MIF in driving the progression of Th- 17
mediated autoimmune
disease. The inhibition of Th- 17 cell development and the inhibition of the
progression of EAE in
mice by blocking MIF demonstrates the valuable utility of agents that inhibit
(i) MIF binding to
CXCR2 and/or CXCR4 and/or (ii) MIF-activation of CXCR2 and/or CXCR4; or (iii)
any
combination of (i) and (ii) for the treatment and/or prevention of Th- 17
mediated autoimmune
diseases such as multiple sclerosis.
EXAMPLE 10
Identification of an Agent that Disrupts MIF Signaling
-102-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00402] A library of peptides covering the extracellular N-terminal
motif/domain of CXCR2 is
generated. The peptides range in size from about 12 amino acids to about 15
amino acids.
[00403] The peptide library is screened for inhibition of MIF-mediated
signaling through CXCR2
using HTS GPCR screening technology.
[00404] The peptides that inhibit MIF-mediated signaling are next screened
from inhibition of 11-8
and/or SDF-1 mediated signaling on CXCR2.
[00405] Peptides that inhibit MIF- signaling through CXCR2 but allow SDF-1 and
IL-8-mediated
signaling through CXCR2 are selected for further investigation.
EXAMPLE 11
Identification of a MIF Trimerization Disrupting Agent
[00406] Polypeptides are generated that comprise amino acid residues 38-44
(beta-2 strand) of MIF.
[00407] The polypeptides are screened for inhibition of MIF-mediated signaling
through CXCR2
using HTS GPCR screening technology.
[00408] The polypeptides that inhibit MIF-mediated signaling are next screened
for inhibition of 11-8
and/or SDF-1 mediated signaling on CXCR2.
[00409] Peptides that inhibit MIF- signaling through CXCR2 but allow SDF-1-
and IL-8-mediated
signaling through CXCR2 are selected for further investigation.
EXAMPLE 12
Human Clinical Trial
[00410] Study Objective(s): The primary objective of this study is to assess
efficacy of (P1;
LMAFGGSSEP) (P1; 20 mg, 40 mg, 80 mg) in individuals with homozygous familial
hypercholesterolemia (HoFH).
METHODS
[00411] Study Design: This is a multi-center, open-label, single-group forced
titration study of fixed
combination P2 in male and female individuals >18 years of age with HoFH.
After initial screening,
eligible individuals enter a 4-week screening period, consisting of 2 visits
(Weeks -4 and -1), during
which all lipid-lowering drugs are discontinued (except for bile acid
sequestrants and cholesterol
absorption inhibitors) and therapeutic lifestyle change counseling (TLC)
according to National
Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP-III) clinical
guidelines or
equivalent are initiated. Individuals already on apheresis continue their
treatment regimen
maintaining consistent conditions and intervals during the study. At Visit 3
(Week 0), baseline
efficacy/safety values are determined and individuals begin treatment with the
initial dose of P2 (20
mg) once daily (QD) for 6 weeks. At Week 6 (Visit 4) doses are titrated to P2
40 mg QD for 6
weeks, and titrated again at Week 12 (Visit 5) to P2 80 mg QD, for 6 weeks, if
individuals tolerate
-103-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
the previous dose. Final visit (Visit 6) occurrs at Week 18. Study visits are
timed with individuals'
apheresis treatments to occur immediately before the visit procedures, where
applicable. When the
intervals between aphereses are misaligned with a study drug treatment period,
the individuals are
kept in the same drug treatment period until the next scheduled apheresis, and
until the intervals are
brought back to the original length of time. Efficacy measures are done at
least 2 weeks after the
previous apheresis and just before the apheresis procedure scheduled for the
day of study visit.
[00412] Number of Participants: Between 30 and 50 individuals.
[00413] Diagnosis and Main Criteria for Inclusion: Men and women 18 years of
age or older with
definite evidence of the familial hypercholesterolemia (FH) homozygote per
World Health
Organization guidelines, and with serum fasting triglyceride (TG) <400 mg/dL
(4.52 mmol/L) for
individuals aged >20 years and 200 mg/dL (2.26 mmol/L) for individuals aged 18-
20 years, are
screened for study participation.
[00414] Study Treatment: During the three 6-week open-label treatment periods,
individuals take 1
tablet QD, with food, immediately after the morning meal. No down titration is
permitted. If
individuals are unable to tolerate dose increases, they are discontinued from
the study.
[00415] Efficacy Evaluations: The primary endpoints are the mean percent
changes in HDL-C and
LDL-C from baseline to the end of each treatment period (ie, Weeks 6, 12 and
18). A lipid profile
which includes HDL-C and LDL-C is obtained at each study visit.
[00416] Safety Evaluations: Safety is assessed using routine clinical
laboratory evaluations
(hematology and urinalysis panels at Weeks -4, 0 and 18, and chemistry also at
Weeks 6 and 12).
Vital signs are monitored at every visit, and physical examinations and
electrocardiograms (ECGs)
are performed at Weeks 0 and 18. Urine pregnancy testing is carried out at
every visit except Week
-1. Individuals are monitored for adverse events (AEs) from Week 0 to Week 18.
Week 18 safety
assessments are completed at early termination if this took place.
[00417] Statistical Methods: The primary efficacy endpoints are the percent
changes in HDL-C and
LDL-C from baseline to the end of each treatment period (ie, Weeks 6, 12, and
18). The primary
efficacy analysis population is the full analysis set (FAS) which included all
individuals who
received at least 1 dose of study drug and had both a baseline and at least 1
valid post-baseline
measurement at each analysis period.
[00418] The primary efficacy endpoints are analyzed through the computation of
sample means of
percent (or nominal) changes, their 95% confidence intervals (CIs), 1-sample t-
test statistics, and
corresponding p-values. Incremental treatment differences between different
dose levels are also
estimated and 95% CIs obtained. Hypothesis testing is 2-sided with an overall
family-wise type I
error rate of 5% (ie, p = 0.05 significance level). Hochberg's procedure is
used to control the family-
wise error rate for multiple comparisons.
-104-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
EXAMPLE 13
Animal Model for Treatment of Abdominal Aortic Aneurysms (AAA)
[00419] Animal models are prepared as follows. An adult, male rat at is
subjected to infusion of
elastase for 2 hours. Histological analysis is performed 12-24 hours after
infusion to confirm
presence of fragmented and disorganized elastin. Ultrasound is performed daily
to identify and
monitor areas of aortic enlargement.
[00420] 2 weeks after administration of elastase, the rat is administered
Peptide 2
(PI;LMAFGGSSEP). The initial administration of P2 is infused into subject at a
rate of 0.5 mg/hr.
In the absence of infusion toxicity, increase infusion rate by 0.5 mg/hr
increments every 30 minutes,
to a maximum of 2.0 mg/hr. Each week thereafter, P2 is infused at a rate of
1.0 mg/hr. In the
absence of infusion toxicity, increase rate by 1.0 mg/hr increments at 30-
minute intervals, to a
maximum of 4.0 mg/hr.
Efficacy Evaluations: The primary endpoints are the mean percent changes in
AAA size (i.e., aortic
diameter) from baseline to weeks 3, 6, and 12.
EXAMPLE 14
Human Clinical Trial for Treatment of Abdominal Aortic Aneurysms (AAA)
[00421] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 1
(P1; LMAFGGSSEP) in individuals with early AAA.
METHODS
[00422] Study Design: This is a multi-center, open-label, single-group study
of P2in male and
female individuals >18 years of age with early AAA. Presence of early AAA is
confirmed with
serial cross-sectional imaging. At Week 0, baseline efficacy/safety values are
determined and
individuals begin treatment with the initial dose of P2. Subjects are
administered P2once a week for
12 weeks.
[00423] Number of Participants: Between 30 and 50 individuals.
[00424] Study Treatment: The initial administration of P2is infused into
subject at a rate of 50
mg/hr. In the absence of infusion toxicity, increase infusion rate by 50 mg/hr
increments every 30
minutes, to a maximum of 400 mg/hr. Each week thereafter, P2 is infused at a
rate of 100 mg/hr. In
the absence of infusion toxicity, increase rate by 100 mg/hr increments at 30-
minute intervals, to a
maximum of 400 mg/hr.
[00425] Efficacy Evaluations: The primary endpoints are the mean percent
changes in AAA size
(i.e., aortic diameter) from baseline to weeks 3, 6, and 12.
EXAMPLE 15: Human Clinical Trial for Treatment of Rheumatoid Arthritis
-105-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00426] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P2; cyclic CNVPRASVPDGC) in individuals with rheumatoid arthritis (RA).
Study Type:
[00427] Interventional
Study Design:
[00428] Allocation: Non-Randomized
[00429] Control: Uncontrolled
[00430] Endpoint Classification: Safety Study
[00431] Intervention Model: Single Group Assignment
[00432] Masking: Open Label
[00433] Primary Purpose: Treatment
Primary Outcome Measures:
[00434] Number of Subjects With American College of Rheumatology (ACR)
Criteria Improvement
Consisting of 20%, 50%, and 70% (ACR20/50/70 Responders, Respectively)
[00435] Number of responders with ACR criteria improvement consisting of 20%,
50%, and 70%
(ACR20/50/70, respectively) reduction in tender or swollen joint counts (TJC
or SJC, respectively)
and 20%, 50%, and 70% improvement, respectively, in 3 of the following 5
criteria: 1) physician's
global assessment of disease activity (PGA), 2) subject's assessment of
disease activity, 3) subject's
assessment of pain, 4) subject's assessment of functional disability via a
health assessment
questionnaire (DI-HAQ), and 5) C-reactive protein (CRP) at each visit.
Secondary Outcome Measures:
[00436] Mean Change From Baseline in Tender Joint Count (TJC, Max=68), a
Component of the
American College of Rheumatology (ACR) by Visit
[00437] Mean Change From Baseline in Swollen Joint Count (SJC, Max=66), a
Component of the
American College of Rheumatology (ACR) by Visit
[00438] Mean Change From Baseline in Physician Global Assessment of Disease
Activity (PGA), a
Component of the ACR Criteria by Visit
[00439] Mean Change From Baseline in Subject's Global Assessment of Disease
Activity Using a
Visual Analog Scale, a Component of the ACR Criteria by Visit
[00440] Mean Change From Baseline in Subject's Assessment of Pain Using a
Visual Analog Scale,
a Component of the ACR Criteria by Visit
[00441] Mean Change From Baseline in the Disability Index of the Health
Assessment Questionaire
(DI-HAQ, a Component of the American College of Rheumatology (ACR) Criteria by
Visit
[00442] Mean Change From Baseline in C-reactive Protein (CRP), a Component of
the American
College of Rheumatology (ACR) Criteria by Visit
[00443] Presence of Morning Stiffness
-106-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00444] Mean Change From Baseline in the Duration (Minutes) of Morning
Stiffness by Visit
[00445] Presence of Rheumatoid Factor (RF)
[00446] Mean Change From Baseline in Rheumatoid Factor (IU/ML) by Visit
Arms
[00447] Peptide 2 will be administered as a single oral dose (10 mg/Kg) once
per day
[00448] Placebo will be administered via oral administration once per day
Elegibility Criteria
[00449] 20 Years and older
[00450] Male and female
Inclusion Criteria:
[00451] Participation and completion until Week 24 of the prior adalimumab
dose-ranging study.
[00452] Females must be postmenopausal for at least 1 year, surgically
sterile, or practicing birth
control throughout the study and for 90 days after study completion.
[00453] Female subjects tested negative in pregnancy test (serum test) at Week
24 in prior
adalimumab study, if capable of pregnancy.
Exclusion Criteria:
[00454] A subject who experienced any of the following during prior study:
[00455] Advanced or poorly controlled diabetes
[00456] Joint surgery (joint evaluated in this study)
[00457] A subject who has been prescribed excluded medications during prior
study
[00458] History of following during prior study:
[00459] Clinically significant drug or alcohol abuse
[00460] Intravenous (iv) drug abuse
[00461] Active infection with listeria or tuberculosis (TB)
[00462] Lymphoma, leukemia
[00463] And, any malignancy with the exception of successfully treated non-
metastatic basal cell
carcinoma of the skin.
[00464] A subject who has been administered a live vaccine during prior study,
or subject scheduled
to complete the administration of a live vaccine during the study period
EXAMPLE 16: Human Clinical Trial for Treatment of Acute Respiratory Distress
Syndrome
(ARDS)
[00465] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P3; LMAFGGSS) in individuals with ARDS.
Study Type:
[00466] Interventional
-107-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Study Design:
[00467] Allocation: Randomized
[00468] Control: Placebo Control
[00469] Endpoint Classification: Safety/Efficacy Study
[00470] Intervention Model: Parallel Assignment
[00471] Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes
Assessor)
[00472] Primary Purpose: Treatment
Primary Outcome Measures:
[00473] Safety profile of the study drug [
[00474] Number of ventilator-free days at Day 28
Secondary Outcome Measures:
[00475] Mortality at Day 28
[00476] Length of hospitalization at Day 28
[00477] Length of ICU stay at Day 28
[00478] Number of Non-pulmonary organ failure free days at Day 28
[00479] Changes in physiological variables of lung injury
[00480] Changes in disease severity and lung injury scores
[00481] Effects of the study drug and the etiology of the disease (i.e.
pulmonary or extra-pulmonary
origin)
[00482] Pharmacokinetics & Pharmacodynamics
[00483] Immunogenicity
Arms
[00484] Peptide 3 will be administered as a single dose (0.06 mg/Kg) via
intravenous infusion over
15 minutes.
[00485] Placebo will be administered via intravenous infusion over 15 minutes
Elegibility Criteria
[00486] 18 years or older
[00487] Male and Female
[00488] Accepts Healthy Volunteers: No
INCLUSION CRITERIA:
[00489] Suspected or proven infection
[00490] Hypoxemia: Pa02/FiO2is <300 mm Hg
[00491] Bilateral infiltrates consistent with pulmonary edema
[00492] Positive-pressure mechanical ventilation through an endotracheal tube
[00493] No clinical evidence of left atrial hypertension to explain bilateral
infiltrates
-108-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00494] Presence of at least three of the four SIRS criteria. If only two
criteria are evidenced, one
must be temperature or WBC
[00495] Criteria 2 and 3 must occur within a 24-hour interval. The 48-hour
enrollment time window
begins when criteria 2, 3, and 4 are met.
EXCLUSION CRITERIA:
[00496] <18 years
[00497] Inability to obtain consent
[00498] Patient, surrogate, or physician not committed to full support
[00499] Moribund state in which death was perceived to be imminent
[00500] Morbid obesity
[00501] Malignancy or other irreversible disease or condition for which 6-
month mortality is
estimated to be >50%
[00502] Known HIV positive with known end stage processes
[00503] Prior cardiac arrest requiring CPR without fully demonstrated
neurological recovery; or
New York Heart Association Class IV
[00504] Pregnant or nursing
[00505] Mechanically or chemically-induced ALI/ARDS (including burns, trauma,
and near
drowning)
[00506] >48 hours since all inclusion criteria are met
[00507] Neuromuscular disease that impairs ability to ventilate without
assistance
[00508] Severe chronic respiratory disease, severe pulmonary hypertension, or
ventilator
dependency
[00509] Chest wall deformity resulting in severe exercise restriction,
secondary polycythemia, or
respirator dependent
[00510] History of organ transplant (including bone marrow)
[00511] Severe chronic liver disease, as determined by a Child-Pugh Score >10
[00512] Hemoglobin persistently < 8.0 g/dL
[00513] Platelet count <50,000/mm3
[00514] Prolonged INR >3
[00515] Bleeding disorders unless corrective surgery has been performed
[00516] Active internal bleeding
[00517] Major surgery within 48 hours before study drug infusion, or evidence
of active bleeding
postoperatively, or plan for any major surgery within 3 days after study drug
infusion.
[00518] Diffuse alveolar hemorrhage from vasculitis
[00519] Known bleeding diathesis
-109-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00520] Presence of an epidural catheter or lumbar puncture within 48 hours
before study drug
infusion or anticipation of receiving an epidural catheter or a lumbar
puncture within 48 hours after
study drug infusion
[00521] Stroke within 3 months of study entry
[00522] Trauma with an increased risk of life-threatening bleeding
[00523] A history of severe head trauma that required hospitalization, or
intracranial surgery within
two months of study entry
[00524] Any history of intracerebral arteriovenous malformation, cerebral
neurysm, or central
nervous system mass lesion
[00525] Uses of certain medications or treatment regimens such as
chemotherapy, unfractionated
heparin, low-molecular-weight heparin, Warfarin, antithrombin III,
acetylsalicylic acid, glycoprotein
IIb/IIIa antagonists, thrombolytic therapy, and activated Protein C are
restricted.
[00526] Participation in another experimental medication study within 30 days
of study entry with
the exception of the ARDSNet pharmaconutrient nutrition trial (OMEGA)
EXAMPLE 17: Human Clinical Trial for Treatment of Glomerulonephritis
[00527] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P4; VHVVPDLLMA) in individuals with Glomerulonephritis.
Study Type:
[00528] Interventional
Study Design:
[00529] Allocation: Randomized
[00530] Control: Active Control
[00531] Endpoint Classification: Efficacy Study
[00532] Intervention Model: Parallel Assignment
[00533] Masking: Open Label
[00534] Primary Purpose: Treatment
Primary Outcome Measures:
[00535] Initiation of acute dialysis or doubling of serum creatinine levels [
Time Frame: 3 months ] [
Designated as safety issue: Yes ]
Secondary Outcome Measures:
[00536] ESRD (defined by the need for long-term dialysis)
Detailed Description
[00537] This study is a randomized, open-label, comparative study.
-110-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00538] Group A is treated by three monthly standard intravenous pulse-dose
methylprednisolone
(15 mg/kg/day or a maximum of 1 g/day from days 1 to 3) followed by oral
prednisolone 0.5-1.0
mg/kg/day (from days 4-30).
[00539] Group B is treated by the same corticosteroid regimen plus intravenous
Peptide 4 (0.33-0.66
mg/kg/h from days 1 to 7) followed by oral Peptide 4 400-800 mg/day (from days
8-90). The dose
of intravenous pentoxifylline will be determined by estimated GFR, patients
whose GFRs are 30-59
ml/min/1.73 m2 will be given 0.66 mg/kg/h, and those below 30 ml/min/1.73 m2
will be given 0.33
mg/kg/h. The oral dose of Peptid 4 will also be determined by estimated GFR.
Patients whose
estimated GFRs are between 30-59 ml/min/1.73 m2 will be given 800 mg/day, and
those below 30
ml/min/1.73 m2 will be given 400 mg/day.
[00540] Serum and single-voided urine specimens will be collected at the
hospital before initiation
of therapy (day 0), and at days 8, 15, 30, and 90 after the commencement of
therapy. Renal function
will be calculated by Cockcroft-Gault and simplified MDRD formula. Serum and
urine samples will
be measured for inflammatory mediators such as TNF-alpha, IL-lbeta, IL-6, MCP-
1, CX3CL1
(fractalkine), IL-8 by using commercial ELISA kits.
Elegibility Criteria
[00541] 20 Years to 80 Years
[00542] Male and female
Inclusion Criteria:
[00543] Biopsied-proved crescentic glomerulonephritis, with rapidly
progressive renal failure
Exclusion Criteria:
[00544] Anti-GBM disease,
[00545] Dialysis-dependency or pulmonary hemorrhage,
[00546] Females are nursing or pregnant,
[00547] Congestive heart failure,
[00548] Unstable angina, myocardial infarction, coronary artery bypass graft
surgery, percutaneous
coronary intervention, within the past 6 months prior to signing the informed
consent form,
[00549] Cerebral hemorrhage within the past 6 months prior to signing the
informed consent form,
[00550] Retinal hemorrhage within the past 6 months prior to signing the
informed consent form,
[00551] Known or suspected secondary hypertension,
[00552] Uncontrolled hypertension or diabetes,
[00553] Liver cirrhosis or hepatic dysfunction as defined by ALT or AST > 2
times the upper limit
of the normal range,
[00554] Biliary obstructive disorders,
[00555] Active malignancy or infection
-111-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
EXAMPLE 18: Human Clinical Trial for Treatment of Inflammatory Bowel Disease
(IBD)
[00556] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P5; QLMAFGGSSE) in individuals with IBD.
[00557] This is an exploratory, open-label, uncontrolled, multi-center, 1-arm
study
[00558] A total of 24 patients will receive P5 tablets, 35 mg once daily for
12 weeks. First of all the
patients will undergo a screening period of 1 week and a follow-up visit will
be performed 4 weeks
after study drug discontinuation or earlier in case of relapse during follow
up. Total study duration
will be up to 17 weeks.
[00559] There will be 8 study visits: one screening visit, 6 visits during the
treatment period and one
follow-up visit. Two telephone visits will be performed at Week 6 and Week 10.
[00560] The duration of the entire study (first patient in till last patient
out) is expected to be about
13 months.
Primary Outcome Measures:
[00561] Efficacy of P5 at a dose of 35 mg once daily in patients with Crohn's
Disease (CD) or
Ulcerative Colitis (UC) after a 12 week therapy as measured by the number of
patients with
complete or partial response.
Secondary Outcome Measures:
[00562] The secondary objective of this study is to evaluate the safety and
tolerability of P5 at a dose
of 35 mg once daily in patients with CD or UC and to explore plasma levels
(trough values) of P5.
Elegibility Criteria
[00563] 18 Years to 70 Years
[00564] Male and femals
Inclusion Criteria:
[00565] Criteria regarding Crohn's Disease:
[00566] Established diagnosis of CD, confirmed by standard criteria (e.g.
endoscopy, ultrasound, X-
ray)
[00567] Patients must be in clinical remission (Crohn's Disease Activity Index
[CDAI] <150 points)
on steroid therapy for at least 2 weeks
[00568] Confirmed steroid-dependency of CD: patients who are either unable to
taper steroids
completely within 3 months of starting steroids, without recurrent active
disease, or who have a
relapse within 2 months of stopping steroids
[00569] Individual threshold* dose of previous relapses should be equal or
less than 20 mg/day
Prednisolone or equivalent steroid dose
[00570] Patients with stable glucocorticosteroid therapy between 20 and 40
mg/day Prednisolone or
equivalent steroid dose for the previous week
[00571] Criteria regarding Ulcerative Colitis:
-112-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00572] Established diagnosis of UC, confirmed by standard criteria (e.g.
endoscopy, ultrasound, X-
ray)
[00573] Patients must be in clinical remission (Clinical Activity Index [CAI]
<4 points) on steroid
therapy for at least 2 weeks
[00574] Confirmed steroid-dependency of UC: patients who are either unable to
taper steroids
completely within 3 months of starting steroids, without recurrent active
disease, or who have a
relapse within 2 months of stopping steroids
[00575] Individual threshold* dose of previous relapses should be equal or
less than 20 mg/day
Prednisolone or equivalent steroid dose
[00576] Patients with stable glucocorticosteroid therapy between 20 and 40
mg/day Prednisolone or
equivalent steroid dose for the previous week
Exclusion Criteria:
[00577] Short bowel syndrome
[00578] Ileostomy, colostomy or rectal pouch
[00579] Relapse during screening
EXAMPLE 19: Human Clinical Trial for Treatment of Sepsis
[00580] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P6; NVPRASVPDG) in individuals with sepsis.
Study Type:
[00581] Interventional
Study Design:
[00582] Allocation: Randomized
[00583] Control: Placebo Control
[00584] Endpoint Classification: Safety/Efficacy Study
[00585] Intervention Model: Single Group Assignment
[00586] Masking: Double-Blind
[00587] Primary Purpose: Treatment
Arms
[00588] P7 0.5 by IV infusion twice daily for 1 week
[00589] P7 1 mg by IV infusion twice daily for 1 week
[00590] Placebo by IV infusion twice daily for 1 week
Eligibility Criteria
[00591] 18 Years to 85 Years
[00592] Male and femal
Inclusion Criteria:
-113-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00593] Presently admitted, or about to be transferred, to the ICU.
[00594] Women of Child-bearing potential must have a negative serum (or urine)
hCG assay within
24 hours prior to drug administration.
[00595] Any Race.
[00596] Severe Sepsis [newly developed respiratory failure, refractory shock,
renal dysfunction,
hepatic dysfunction, or metabolic acidosis and at least three signs of SIRS
(systematic inflammatory
response syndrome)].
[00597] Objective signs of infection likely to be caused by a bacterial or
fungal pathogen.
[00598] Patients must receive study medication within 8 to 12 hours of
recognition of the initial
sepsis-related organ failure.
[00599] APACHE Predicted risk of mortality score between 20% and 80%.
[00600] An intent by physicians and family to aggressively treat the patient
for the 28 day study
period.
Exclusion Criteria:
[00601] Cardiogenic or hypovolemic shock.
[00602] Acute third degree burns involving >20% of body surface.
[00603] Recipients of non-autologous organ transplants within the past year.
[00604] Pregnancy.
[00605] Chronic vegetative state.
[00606] Uncontrolled serious hemorrhage (.2 units of blood/platelets in the
previous 24 hours).
Patients may be considered for enrollment if bleeding has stopped and patients
are still otherwise
qualified.
[00607] Unwilling or unable to be fully evaluated for all follow-up visits.
[00608] Patients who are classified as "Do not resusitate" or "Do not treat."
[00609] Patients who develop severe sepsis <36 hours post trauma or post-
surgery. Patients may be
considered for enrollment >36 hours post-trauma or post-surgery, if they meet
other inclusion
criteria.
[00610] Patients with a predicted risk of mortality score of <20% or >80%
after recognition of
qualifying organ failure.
[00611] Patients with a predicted risk of mortality of <51% for whom Xigris
use is planned.
EXAMPLE 20: Human Clinical Trial for Treatment of Lupus
[00612] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P7; NVPRASVPD) in individuals with lupus.
Study Type:
[00613] Interventional
-114-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Study Design:
[00614] Allocation: Randomized
[00615] Endpoint Classification: Safety/Efficacy Study
[00616] Intervention Model: Parallel Assignment
[00617] Masking: Double Blind (Subject, Investigator, Outcomes Assessor)
[00618] Primary Purpose: Treatment
Primary Outcome Measures:
[00619] Safety, Tolerability, Change in swollen and tender joint counts
Arms
[00620] P7 0.5 mg once daily for 12 weeks
[00621] P7 1 mg oral once daily for 12 weeks
[00622] Placebo oral once daily for 12 weeks
Eligibility Criteria
[00623] 18 Years to 75 Years
[00624] Male and female
Inclusion Criteria:
[00625] Subjects diagnosed with SLE.
[00626] Subjects with active lupus arthritis as evident by
[00627] At least 4 tender and 4 swollen joints
[00628] Active synovitis > 1 joint with some loss of functional range of
movement
Exclusion Criteria:
[00629] Subjects with severe renal impairment or dialysis
[00630] Severe, unstable and/or progressive CNS lupus
[00631] Subjects with a clinically significant or unstable medical or surgical
condition
[00632] Women who are pregnant or nursing or who intend to be during the study
period.
[00633] Women of child-bearing potential who do not practice an acceptable
method of birth control
EXAMPLE 21: Human Clinical Trial for Treatment of Asthma
[00634] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 2
(P8; cyclic CLMAFGGSSEPCALC) in individuals with asthma.
[00635] Study Type: Interventional
[00636] Study Design: Allocation: Randomized
[00637] Control: Placebo Control
[00638] Endpoint Classification: Safety/Efficacy Study
[00639] Intervention Model: Parallel Assignment
[00640] Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes
Assessor)
-115-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00641] Primary Purpose: Treatment
Primary Outcome Measures:
[00642] Airway reactivity will be measured with methacholine challenge testing
following ATS
guidelines
Secondary Outcome Measures:
[00643] Pulmonary function as measured by FEV 1 and FVC following ATS
guidelines
[00644] Asthma symptoms and control will be objectively monitored using the
Juniper
Questionnaire, Asthma Quality of Life Questionnaire, and St. George
Respiratory Questionnaire
Detailed Description:
[00645] Participants in this study will be randomly assigned to P8 or placebo
(an inactive pill). They
will be given study medication to take every day for 12 weeks (3 months).
[00646] Participants will complete a number of asthma-related questionnaires
and a variety of
pulmonary function tests. Participants will undergo physical exams, an
electrocardiogram, and blood
sampling to measure leptin, adiponectin, markers of inflammation, blood cell
counts, glucose levels,
BNP hormone levels, and liver function.
[00647] To monitor participants throughout the study, follow-up visits will be
done at 2, 6, and 12
weeks after starting study drug. At these visits many of the pulmonary
function tests and
questionnaires will be repeated.
Eligibility Criteria
[00648] 18 Years to 60 Years
[00649] Male and femal
Inclusion Criteria:
[00650] Asthma diagnosed by a physician at least 1 year prior to study
enrollment
[00651] Poorly-controlled asthma at study enrollment
[00652] Non smokers (stopped smoking at least 1 year ago) and limited lifetime
history of smoking
[00653] Body mass index 30-60
[00654] Responds to methacholine challenge test with PC20 of <16 mg/ml
[00655] On a stable dose of inhaled corticosteroid for at least 4 weeks prior
to study entry
[00656] FEV 1 > 60% predicted
[00657] Able to obtain weekly weights at home
Exclusion Criteria:
[00658] Systemic steroids within the past 4 weeks
[00659] Lung pathology other than asthma
[00660] Other significant non-pulmonary co-morbidities such as: coronary
artery disease, peripheral
vascular disease, cerebrovascular disease, congestive heart failure with an
ejection fraction <50%,
liver disease or elevated liver enzymes at baseline, malignancy (excluding non-
melanoma skin
-116-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
cancers), AIDS, renal failure with serum creatinine >3.0, or disorders
requiring steroid treatment
such as vasculitis, lupus, rheumatoid arthritis
[00661] B-type natriuretic peptide (BNP) >400pg/ml
[00662] Pregnant or lactating
[00663] Currently taking a beta blocker, a CYP2C8 inhibitor or inducer such as
gemfibrozil or
rifampin, a TZD (thiazolidinedione), or allergic to TZD
[00664] Taking antioxidants (if taking a multivitamin must be on a stable
regimen prior to
enrollment)
[00665] Illicit drug use within the past year
[00666] Current/active upper respiratory infection (if active URI, wait until
asymptomatic for 1
week to enroll)
[00667] Asthma exacerbation within the past 4 weeks (includes ER, urgent care,
or hospital visits
due to asthma resulting in an increase in asthma-related medications)
[00668] Undergoing evaluation for sleep apnea, or plans to institute treatment
for sleep apnea
(patients on a stable treatment regimen for sleep apnea for the last 3 months
will be allowed to
participate)
[00669] Clinically significant abnormalities present on screening 12-lead
electrocardiogram
[00670] Women of childbearing potential using oral contraceptives who are not
willing to use a
second method of contraception during the study
EXAMPLE 22: Human Clinical Trial for Treatment of Colon Cancer
[00671] Study Objective(s): The primary objective of this study is to assess
efficacy of Peptide 1
(P1; LMAFGGSSEP) in individuals with colon cancer
Study Type:
[00672] Interventional
Study Design:
[00673] Allocation: Non-Randomized
[00674] Control: Uncontrolled
[00675] Endpoint Classification: Efficacy Study
[00676] Intervention Model: Single Group Assignment
[00677] Masking: Open Label
[00678] Primary Purpose: Treatment
Arms
[00679] Oral peptide 1 (15 mg/kg), once daily, for 4 months
[00680] Oral placebo, once daily, for 4 months
-117-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
Elegibility Criteria
[00681] 18 Years and older
[00682] Male and female
Inclusion Criteria:
[00683] Has undergone complete resection of stage I or II adenocarcinoma of
the colon with curative
intent within the past year
[00684] Has undergone either a preoperative or postoperative colonoscopy to
the cecum (or small
bowel anastomosis) with adequate bowel preparation within the past 180 days
[00685] Distal border of the tumor located > 12 cm from the anal verge
[00686] No classic familial adenomatous polyposis, attenuated familial
adenomatous polyposis (i.e.,
> 20 adenomas, either synchronous or metachronous), or hereditary nonpolyposis
colorectal cancer
(Lynch syndrome)
PATIENT CHARACTERISTICS:
[00687] ECOG performance status 0-1
[00688] Serum creatinine < 1.5 times upper limit of normal (ULN)
[00689] AST and/or ALT < 3.0 times ULN
[00690] Total bilirubin < 1.5 times ULN
[00691] Not pregnant or nursing
[00692] Negative pregnancy test
[00693] Fertile patients must use effective contraception during and for > 3
months after completion
of study treatment
[00694] Able to swallow oral medication
[00695] No malabsorption syndrome, ulcerative colitis, inflammatory bowel
disease, resection of the
stomach or small bowel, or other disease significantly affecting
gastrointestinal (GI) function
[00696] No history of documented upper GI bleeding or upper GI ulcerative
disease
[00697] No hyperlipidemia with clinical indication for statin therapy
(determination of acceptable
fasting lipid values should be in accordance with current dyslipidemia
management guidelines)
[00698] No inadequately treated hypothyroidism, as determined by the
investigator
[00699] No history of myopathy or rhabdomyolysis
[00700] No other malignancy within the past 5 years except for in situ cancers
or basal cell or
squamous cell carcinoma of the skin
[00701] No hypersensitivity or intolerance to statins
[00702] No other non-malignant systemic disease that would preclude
rosuvastatin administration or
prolonged follow-up
PRIOR CONCURRENT THERAPY:
[00703] See Disease Characteristics
-118-
CA 02773978 2012-03-12
WO 2011/038149 PCT/US2010/050047
[00704] More than 30 days since prior statins
[00705] More than 30 days since prior investigational agents
[00706] No prior total colectomy or total proctocolectomy
[00707] No concurrent chronic use of NSAIDs
[00708] No concurrent chronic drug therapy with cyclosporine, coumarin
anticoagulants,
gemfibrozil, other lipid-lowering therapies (e.g., fibrates or niacin),
lopinavir/ritonavir, or drugs
(e.g., ketoconazole, spironolactone, or cimetidine) that lower levels or
activity of steroid hormones
[00709] While preferred embodiments of the present invention have been shown
and described
herein, it will be obvious to those skilled in the art that such embodiments
are provided by way of
example only. Numerous variations, changes, and substitutions will now occur
to those skilled in the
art without departing from the invention. It should be understood that various
alternatives to the
embodiments of the invention described herein may be employed in practicing
the invention. It is
intended that the following claims define the scope of the invention and that
methods and structures
within the scope of these claims and their equivalents be covered thereby.
-119-