Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02815847 2013-05-13
1
Title: VECTORS AND METHODS FOR ENHANCING RECOMBINANT
PROTEIN EXPRESSION IN PLANTS
Field of the Disclosure
[0001] The present application relates to a set of expression vectors
designed for enhancing the production of recombinant proteins in plants and
methods of using same.
Background of the Disclosure
[0002] The gene-silencing machinery of plants is involved in regulating
expression of endogenous gene transcripts as well as reducing or eliminating
the
effects of invading pathogens such as viruses (Baulcombe, 2004; Reinhart et
al.,
2002). As a countermeasure to this defense mechanism, viruses encode for
proteins that act as suppressors of gene-silencing (SGS). P19 from the Tomato
Bushy Stunt Virus (TBSV) is an example of proteins known to function as a
potent
suppressor of gene-silencing in plants as well as in animals (Scholthof, 2006;
Voinnet et al., 1999). Plants also react to most transfer DNA (T-DNA)
transgenes
that invade their genomes by initiating a post-transcriptional gene silencing
response (Baulcombe, 2004; Brodersen and Voinnet, 2006). The inhibitory effect
of P19 on the gene-silencing pathway has been exploited to enhance expression
levels of recombinant proteins in plants (Voinnet et al., 2003), but its use
has been
limited to transient expression only, mainly due to the deleterious effects of
this
protein when expressed constitutively at high levels in a transgenic setting
(Siddiqui et al., 2008).
[0003] There are two main cellular gene-silencing mechanisms in plants,
the small interfering RNA (siRNA) and the micro RNA (miRNA) silencing pathways
(Carthew and Sontheimer, 2009), which are collectively referred to as
interfering
RNA (RNAi). The two systems show a great deal of similarity in their
mechanisms
of action, as they share some key enzymes (Brodersen and Voinnet, 2006). Both
systems identify their target nucleic acid (viral RNA, viral DNA, transgene
mRNA,
endogenous mRNA) by a complex known as RNA-induced silencing complex
CA 02815847 2013-05-13
2
(RISC). RISC carries a complementary single stranded RNA probe for its target,
which upon binding, is either blocked or degraded.
[0004] The mechanism of action of P19 in suppressing gene-silencing
at the
molecular level has become better understood in recent times (Burgyan et al.,
2004), but certain aspects still remain unclear. P19 is a multifunctional
protein that
is active as a dimer and found in both the cytosol and the nucleus (Park et
al.,
2004). It is capable of binding siRNA and miRNA molecules in a non-specific
fashion (Dunoyer et at., 2004). Since there is a rise in virus-derived siRNA
levels in
plants in response to infection (Scholthof et at., 1995), P19 acts to reduce
the
amount of free siRNA duplexes through non-specific binding and represses the
silencing response by interfering with siRNA loading of RISC (Hsieh et al.,
2009).
Studies on TBSV mutants with lowered levels of P19 have shown that a high
titer
of the protein is critical for exerting its biological activity (Qiu et al.,
2002; Scholthof
et al., 1999). Despite P19's non-specific siRNA binding, the effects brought
about
by this protein show host-specificity (Ahn et al., 2011; Angel et at., 2011;
Siddiqui
et at., 2008).
[0005] Nicotiana species display a variation in induction of the
hypersensitive response (HR) to the P19 protein of TBSV, which is indicated by
an
initial leaf discoloration that leads to necrosis at the site of infection
(Angel et at.,
2011). In N. tabacum cv. Samsun, discoloration from HR develops 2-3 days after
infiltration of leaves with P19, leading to fully dehydrated spots on day 7,
while the
same treatment yields no necrosis or discoloration in N. benthamiana. Stable
transgenic expression of P19, however, does not elicit HR in either N. tabacum
cv.
Xanthi or N. benthamiana, indicating that high titers of P19 are required for
triggering this response (Siddiqui et al., 2008). The difference in the HR
generated
in N. tabacum and N. benthamiana in response to P19 has been attributed to a
host protein that is the product of a putative resistance (R) gene (Jovel et
at.,
2011). The putative R gene product is thought to identify P19 and trigger a
cascade of events that lead to local necrosis for slowing down the spread of
virus.
Although the R gene product that specifically interacts with P19 has not been
CA 02815847 2013-05-13
. ,
3
identified, experiments show that the resistance conferred by this gene
product is
inherited in a dominant fashion (Jovel et al., 2011).
[0006] The extent to which P19 increases expression seems to
vary for
different recombinant proteins. Several reports indicate that the expression
of
Green Fluorescent Protein (GFP), a commonly used reporter, is boosted
approximately 50-fold when co-expressed with P19 (Voinnet et al., 2003; Zheng
et
al., 2009). Expression of antibodies and other therapeutic proteins, on the
other
hand, have only been enhanced by five-fold (Saxena et al., 2011; Zheng et al.,
2009).
[0007] In the context of plant-derived therapeutic proteins, another very
important consideration is their glycan profile, since this post-translational
modification impacts the efficacy of therapeutic proteins and can be a major
factor
in batch-to-batch variability of recombinant therapeutic proteins (Gomord et
al.,
2010; Schiestl et al., 2011). Plant-specific sugar residues on the N-glycan
core,
namely core a1,3-fucose and 131,2-xylose, are immunogenic in mammals (Bardor
et al., 2003; Jin et al., 2008). As a result, a great deal of effort has been
directed
towards creating plants with modified humanized glycosylation patterns (Cox et
al.,
2006; Sourrouille et al., 2008; Strasser et al., 2008). For the most part,
glycomodified plants have been created through RNAi gene-silencing technology,
mainly due to the existence of multiple endogenous fucosyltransferase and
xylosyltransferase genes in most plants (Cox et al., 2006; Sourrouille et al.,
2008;
Strasser et al., 2008). Consequently, interference in the siRNA pathway by P19
becomes a concern when RNAi-generated genetic backgrounds are to be used as
expression hosts for producing therapeutic proteins, especially since in an
unrelated case, P19 was shown to repress the knockdown of a previously
established RNAi transgenic line (Ahn et al., 2011).
Summary of the Disclosure
[0008] The present inventors have designed and tested a suite of
plant
expression vectors which are suitable for enhancing expression of recombinant
protein in both transient expression and stable transgenic plants. The unique
CA 02815847 2013-05-13
. .
4
combination of promoter, 5' UTR, and 3' UTR/terminator in these vectors drives
high levels of heterologous protein expression in plants, including Nicotiana
benthamiana and Nicotiana tabacum.
[0009] Accordingly, the present application provides an
expression vector
comprising:
(a) a promoter selected from (i) the 35S promoter of the Cauliflower Mosaic
Virus (CaMV) or (ii) the promoter of the ribulose bisphosphate carboxylase
(rbc)
small subunit gene of Chrysanthemum morifolium;
(b) a 5' untranslated region (UTR) selected from (i) the 35S 5' UTR of
CaMV or (ii) the 5' UTR of the rbc small subunit gene of C. morifolium; and
(c) a 3' UTR and terminator sequence selected from (i) the 3' UTR and
terminator sequence of the nopaline synthase (nos) gene of Agrobacterium, (ii)
the
3' UTR and terminator sequence of the osmotin (osm) gene of Oryza sativa,
(iii)
the 3' UTR and terminator sequence from the rbc small subunit gene of C.
morifolium or (iv) a truncated version, by 162 bp as defined by a BspEl
recognition site, of the 3' UTR and terminator sequence from the rbc small
subunit
gene of C. morifolium.
[0010] In one embodiment, a nucleic acid sequence encoding a
recombinant protein is cloned in the above-mentioned vectors.
[0011] The present application further provides a method of enhancing the
production of a recombinant protein in a plant comprising:
(i) introducing an expression vector comprising
(a) a promoter selected from (i) the 35S promoter of the Cauliflower
Mosaic Virus (CaMV) or (ii) the promoter of the ribulose bisphosphate
carboxylase
(rbc) small subunit gene of Chrysanthemum morifolium;
(b) a 5' untranslated region (UTR) selected from (i) the 35S 5' UTR
of CaMV or (ii) the 5' UTR of the rbc small subunit gene of C. morifolium;
(c) a 3' UTR and terminator sequence selected from (i) the 3' UTR
and terminator sequence of the nopaline synthase (nos) gene of Agrobacterium,
(ii) the 3' UTR and terminator sequence of the osmotin (osm) gene of Oryza
CA 02815847 2013-05-13
sativa, (iii) the 3' UTR and terminator sequence from the rbc small subunit
gene of
C. morifolium or (iv) a truncated version, by 162 bp as defined by a BspEl
recognition site, of the 3' UTR and terminator sequence from the rbc small
subunit
gene of C. morifolium; and
5 (d) a nucleic acid sequence encoding a recombinant protein
into a plant or plant cell; and
(ii) growing the plant or plant cell to obtain a plant that expresses the
recombinant protein.
[0012] In one embodiment, the recombinant protein is co-expressed
with
the P19 suppressor of gene-silencing protein from tomato bushy stunt virus
(TBSV).
[0013] Other features and advantages of the present invention will
become
apparent from the following detailed description. It should be understood,
however,
that the detailed description and the specific examples while indicating
preferred
embodiments of the invention are given by way of illustration only, since
various
changes and modifications within the spirit and scope of the invention will
become
apparent to those skilled in the art from this detailed description.
Brief description of the drawings
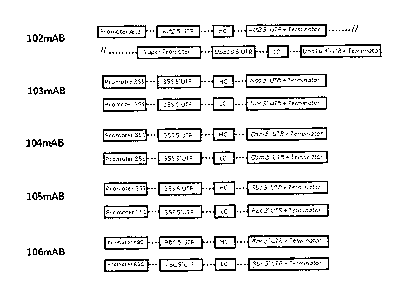
[0014] Figure 1: Diagram of the expression cassettes used in Example
1.
The expression cassettes shown here were situated on the T-DNA region of
binary
vectors. Vector 102mAb was the only vector carrying the heavy (HC) and light
chains (LC) of trastuzumab on the same T-DNA. When expressing trastuzumab
with vectors 103mAb-106mAb, the HC and LC were co-expressed to produce fully
assembled IgG molecules.
[0015] Figure 2: Western blot analysis of trastuzumab expressed transiently
with different plant expression vectors in N. benthamiana. Expression of the
103-
106mAb vectors was analyzed over 6 days. Plants were treated by vacuum
infiltration. Each lane represents a pooled sample, created by mixing three
leaf
samples. The vectors were either expressed alone (A), or together with P19
(B).
All vector sets carried the same codes for the HC and LC of trastuzumab
coupled
CA 02815847 2013-05-13
6
with different UTRs. Different expression dynamics were observed when vectors
were expressed alone or together with P19, as determined by ELISA (C).
[0016] Figure 3: Western blot analysis of trastuzumab expressed
transiently
with (A) 102mAb and with (B) TMV/PVX (virus-based) expression vectors in N.
benthamiana. Plants were treated by spot infiltration. Pooled samples were
generated by combining three infiltrated spots. Two pooled samples (harvested
5
d.p.i.) are shown for each treatment. Similar to 106mAb, co-expression of P19
did
not affect the level of trastuzumab expressed with either vector.
[0017] Figure 4: Western blot analysis showing the dose-dependent
effect
of P19 on enhancing recombinant antibody expression in N. benthamiana.
103mAb vectors were co-expressed with P19 at three different concentrations of
Agrobacterium, 0D600=0.2, 0.02, and 0.002. Plants were treated by spot
infiltration. Pooled sampled were generated by combining three infiltrated
spots.
Each lane represents a pooled sample. The boosting effect of P19 was most
prominent when applied at the higher concentration, regardless of the
concentration of 103mAb. P19 had no boosting effect on antibody expression
when applied at 0D600=0.002.
[0018] Figure 5: Differential response of N. tabacum and N.
benthamiana to
P19. Western blot analysis showing transient expression of trastuzumab alone
or
together with P19 in N. benthamiana and five different N. tabacum cultivars
(A)
and N. tabacum crosses (B). Plants were treated by spot infiltration. Samples
were
pooled by combining three infiltrated spots. Each lane represents a pooled
sample. Co-expression of 103mAb with P19 resulted in a significant reduction
in
antibody expression in all tobacco cultivars except in LCR. The drop in
antibody
expression indicates an intensified state of RNAi silencing. Crosses between
N.
tabacum 1-64 and LCR, and N. benthamiana and N. tabacum 1-64 (NBT) showed a
similar drop in antibody expression when P19 was co-expressed with 103mAb (B).
All Nicotiana species that showed a drop in antibody expression in the
presence of
P19 also displayed discoloration at the site of infection, which lead to
necrosis
about day 3 days post-infection (C). Images here show infiltrated spots at 5
days
CA 02815847 2013-05-13
7
post-infection. XAN, N. tabacum cv. Xanthi; PH, N. tabacum cv. Petite Havana
H4;
LCR, N. tabacum cv. Little Crittenden; BEN, N. benthamiana; NBT, N. benthamian
x N. tabacum cv. 1-64.
[0019] Figure 6: N-Glycan profiles of 103mAb expressed in N.
benthamiana
WT (A) and in AXTFT without (B) and with P19 (C). N-glycan analyses were
carried out by liquid-chromatography-electrospray ionization-mass spectrometry
(LC-ESI-MS) of tryptic glycopeptides as described previously (Stadlmann et
al.,
2008; Strasser et al., 2008). Note, that incomplete tryptic digest results in
the
generation of two glycopeptides that differ by 482 Da. Glycopeptide 1 is
indicated
with asterisks (*). See http://www.proglycan.com for N-glycan abbreviations.
[0020] Figure 7: Transient co-infiltration of P19 expression vector
greatly
enhances the expression of two more therapeutic antibodies in Nicotiana
benthamiana. Coding sequences for both anti-HIV mAb 4E10 and bevacizumab
were spot-infiltrated, with and without P19, into N.benthamiana leaf tissue
and
harvested after 7 days. Each Agrobacterium strain was applied at a final 0D600
of
0.2. Tissue harvests for each treatment included 3 or 4 spots, which were
pooled
and total soluble protein (TSP) was extracted as described in Garabagi et al.
(2012a,b). A 10% non-reducing SDS-PAGE gel was run that included 10 1,1g TSP
for each plant sample. Electrophoretically separated proteins were
subsequently
electrotransferred to PVDF membrane and probed with combined anti--y and anti-
K
antibody probes conjugated to alkaline phosphatase (also described in Garabagi
et al., 2012a). Results indicate that co-expression of P19 greatly enhances
expression of both antibodies irrespective of antibody expression vector. The
gel
loading scheme is tabulated above the immunoblot image, and the size of each
molecular weight marker band in lane 1 is given on the left in kDa. The human
serum immunoglobulin quantification standard in lane 10 is 500 ng.
mAb=monoclonal antibody; HC=heavy chain vector; LC=light chain vector. Note
that vector p105T contains a truncated 3'UTR compared with the original p105
vector, with 162 less base pairs (bps) due to a BspEl-BspEl deletion.
CA 02815847 2013-05-13
. ,
8
[0021] Figure 8: Transient expression of human
butyrylcholinesterase
(BChE) in N. benthamiana is greatly enhanced by co-expression of P19. Two
different BChE expression vectors were constructed in p105T: (1) with the
Arabidopsis basic chitinase (abc) signal sequence and a synthetic BChE coding
sequence optimized for expression in plants referred to as E2, i.e., abc-
BChE2;
and (2) with the native human BChE signal sequence (hSS) and another synthetic
BChE coding sequence optimized for expression in plants referred to as E3,
i.e.,
hSS-BChE3. These were introduced into whole N. benthamiana plants by vacuum
infiltration (Garabagi et al., 2012a,b), either with or without the P19
expression
vector described in this application. Samples were taken at 5, 7 and 9 days
post-
infiltration (DPI) and BChE activity was measured by Ellman Assay (Ellman et
al.,
1961). All histogram bars present amounts of BChE in mg BChE/kg leaf tissue,
by
converting activity measurements to mass of enzyme using the specific activity
conversion factor of 718.3 activity units per mg of BChE determined in Weber
et
al. (2010). Note that background readings (as determined with untreated plant
extracts) have been subtracted for the presentation of these data. Histogram
bars
indicate the mean BChE activity measured in 2 samples from 2 plants (4 total
repeats each) with associated standard error bars.
[0022] Figure 9: Diagram of one of the expression cassettes used
in Figure
7 and in Figure 8. The 105 mAb expression cassette from Figure 1 is shown at
the
top of the figure. Insertion of a coding sequence for a recombinant protein to
be
expressed in plants is performed by using the restriction endonuclease BspEl
for
ligation of the 3' end of that coding sequence, resulting in a 162 bp deletion
of the
5' end of the Rbc 3' UTR and terminator. The resulting 105T (T=truncated)
expression cassette is shown at the bottom of the figure.
Detailed description of the Disclosure
[0023] As previously described, the present inventors have
designed and
tested a suite of plant expression vectors which are suitable for enhancing
expression of recombinant protein in plants. The unique combination of
promoter,
5' UTR, and 3' UTR/terminator in these vectors drives high levels of
heterologous
CA 02815847 2013-05-13
. =
9
protein expression in plants, including Nicotiana benthamiana and Nicotiana
tabacum.
I. Expression vectors
[0024] In one embodiment, the application provides an expression
vector
comprising:
(a) a promoter selected from (i) the 35S promoter of the Cauliflower
Mosaic Virus (CaMV) or (ii) the promoter of the ribulose bisphosphate
carboxylase
(rbc) small subunit gene of Chrysanthemum morifolium,
(b) a 5' untranslated region (UTR) selected from (i) the 35S 5' UTR
of CaMV or (ii) the 5' UTR of the rbc small subunit gene of C. morifolium; and
(c) a 3' UTR and terminator sequence selected from (i) the 3' UTR
and terminator sequence of the nopaline synthase (nos) gene of Agrobacterium,
(ii) the 3' UTR and terminator sequence of the osmotin (osm) gene of Oryza
sativa, (iii) the 3' UTR and terminator sequence from the rbc small subunit
gene of
C. morifolium or (iv) a truncated version, by 162 bp as defined by a BspEl
recognition site, of the 3' UTR and terminator sequence from the rbc small
subunit
gene of C. morifolium.
[0025] As used herein, the term "expression vector" means a
nucleic acid
molecule, such as a plasmid, comprising regulatory elements and a site for
introducing transgenic DNA, which is used to introduce said transgenic DNA
into a
host cell. The transgenic DNA can encode a heterologous protein, which can be
expressed in and isolated from plant cells.
[0026] Regulatory elements include promoters, 5' and 3'
untranslated
regions (UTRs) and terminator sequences or truncations thereof. The regulatory
elements of the present invention can be selected from the 35S promoter and
5'UTR of the Cauliflower Mosaic Virus (CaMV; Genbank accession: AF140604),
the promoter and 5' UTR of ribulose bisphosphate carboxylase (rbc) small
subunit
gene from Chrysanthemum morifolium (Genbank accession: AY163904.1), the
heat-shock (Hsp81.1) promoter from Arabidopsis thaliana, the 3' UTR and
terminator sequences from the nopaline synthase (nos) gene of Agrobacterium
CA 02815847 2013-05-13
(Genbank accession: V00087.1), the 3' UTR and terminator sequences from the
osmotin (osm) gene of Oryza sativa (Genbank accession: L76377.1) and the 3'
UTR and terminator sequences from the rbc gene of C. morifolium. In one
embodiment, the Hsp81.1 promoter from Arabidopsis is placed directly upstream
5 of one of the other promoters.
[0027] In one embodiment, the expression vector comprises the 35S
promoter of CaMV, operably linked to the 35S 5' UTR of CaMV and the 3' UTR
and terminator sequence of the nos gene of Agrobacterium. This expression
vector may also be referred to as p103.
10 [0028] In another embodiment, the expression vector comprises
the 35S
promoter of CaMV, operably linked to the 35S 5' UTR of CaMV and the 3' UTR
and terminator sequence of the osm gene of Oryza sativa. This expression
vector
may also be referred to as p104.
[0029] In another embodiment, the expression vector comprises the
35S
promoter of CaMV, operably linked to the 35S 5' UTR of CaMV and the 3' UTR
and terminator sequence of the rbc small subunit gene of C. morifolium. This
expression vector may also be referred to as p105.
[0030] In another embodiment, the expression vector comprises the
35S
promoter of CaMV, operably linked to the 35S 5' UTR of CaMV and a truncated
version, by 162 bp as defined by a BspEl recognition site, of the 3' UTR and
terminator sequence from the rbc small subunit gene of C. morifolium. This
expression vector may also be referred to as p105T.
[0031] In another embodiment, the expression vector comprises the
promoter of the rbc small subunit gene of C. morifolium, operably linked to
the 5'
UTR of the rbc small subunit gene of C. morifolium and the 3' UTR and
terminator
sequence of the rbc small subunit gene of C. morifolium. This expression
vector
may also be referred to as p106.
[0032] As used herein, the term "nucleic acid molecule" means a
sequence
of nucleoside or nucleotide monomers consisting of naturally occurring bases,
sugars and intersugar (backbone) linkages. The term also includes modified or
CA 02815847 2013-05-13
. .
11
substituted sequences comprising non-naturally occurring monomers or portions
thereof. The nucleic acid sequences of the present invention may be
deoxyribonucleic acid sequences (DNA) or ribonucleic acid sequences (RNA) and
may include naturally occurring bases including adenine, guanine, cytosine,
thymidine and uracil. The sequences may also contain modified bases. Examples
of such modified bases include aza and deaza adenine, guanine, cytosine,
thymidine and uracil; and xanthine and hypoxanthine.
[0033]
In one embodiment, the application provides an expression vector
comprising:
(a) a promoter selected from (i) the 35S promoter of the Cauliflower
Mosaic Virus (CaMV) or (ii) the promoter of the ribulose bisphosphate
carboxylase
(rbc) small subunit gene of Chrysanthemum morifolium;
(b) a 5' untranslated region (UTR) selected from (i) the 35S 5' UTR
of CaMV or (ii) the 5' UTR of the rbc small subunit gene of C. morifolium;
(c) a 3' UTR and terminator sequence selected from (i) the 3' UTR
and terminator sequence of the nopaline synthase (nos) gene of Agrobacterium,
(ii) the 3' UTR and terminator sequence of the osmotin (osm) gene of Oryza
sativa, (iii) the 3' UTR and terminator sequence from the rbc small subunit
gene of
C. morifolium, or (iv) a truncated version, by 162 bp as defined by a BspEl
recognition site, of the 3' UTR and terminator sequence from the rbc small
subunit
gene of C. morifolium and
(d) a nucleic acid sequence encoding a recombinant protein.
[0034]
As used herein, the term "recombinant protein" means any
polypeptide that can be expressed in a plant cell, wherein said polypeptide is
encoded by transgenic DNA introduced into the plant cell via use of an
expression
vector. In a preferred embodiment, the expression vector is p103. In another
preferred embodiment, the expression vector is p105 or p105T.
[0035]
In one embodiment, the recombinant protein is an antibody or
antibody fragment. In a specific embodiment, the antibody is trastuzumab or a
modified form thereof, consisting of 2 heavy chains (HC) and 2 light chains
(LC).
CA 02815847 2013-05-13
. '
12
Trastuzumab (Herceptin Genentech Inc., San Francisco, CA) is a humanized
murine immunoglobulin G1K antibody that is used in the treatment of metastatic
breast cancer.
[0036]
In another specific embodiment, the antibody is bevacizumab or a
modified form thereof, consisting of 2 heavy chains (HC) and 2 light chains
(LC).
Bevacizumab (trade name Avastin, Genentech/Roche) is an angiogenesis
inhibitor, a drug that slows the growth of new blood vessels. It is licensed
to treat
various cancers, including colorectal, lung, breast, glioblastoma, kidney and
ovarian.
[0037] In
another specific embodiment, the recombinant protein is an
enzyme such as a therapeutic enzyme. In a specific embodiment, the therapeutic
enzyme is butyrylcholinesterase. Butyrylcholinesterase (also known as
pseudocholinesterase, plasma cholinesterase, BCHE, or BuChE) is a non-specific
cholinesterase enzyme that hydrolyses many different choline esters. In
humans, it
is found primarily in the liver and is encoded by the BCHE gene. It is being
developed as an antidote to nerve-gas poisoning.
[0038]
The nucleic acid molecules encoding the HC and LC of an antibody
or antibody fragment or the coding sequence of a therapeutic enzyme can be
incorporated separately into one expression vector each or incorporated
together
into a single expression vector comprising multiple expression cassettes.
[0039]
As used herein, the term "expression cassette" means a single,
operably linked set of regulatory elements that includes a promoter, a 5' UTR,
an
insertion site for transgenic DNA, a 3' UTR and a terminator sequence.
[0040]
As used herein, the term "antibody fragment" includes, without
limitation, Fab, Fab', F(ab1)2, scFv, dsFv, ds-scFv, dimers, minibodies,
diabodies,
and multimers thereof and bispecific antibody fragments.
[0041]
In one embodiment, a signal peptide that directs the polypeptide to
the secretory pathway of plant cells may be placed at the amino termini of
recombinant proteins, including antibody HCs and/or LCs.
In a specific
embodiment, the Arabidopsis thaliana basic chitinase signal peptide (SP),
namely
CA 02815847 2013-05-13
=
13
MAKTNLFLFLIFSLLLSLSSA (SEQ ID NO:2), is placed at the amino- (N-) termini
of both the HC and LC (Samac et al., 1990).
[0042] In another embodiment, the native human butyrylcholinesterase
signal peptide (SP), namely MHSKVTIICIRFLFWFLLLCMLIGKSHT (SEQ ID
NO:3), is placed at the amino- (N-) termini of a therapeutic enzyme such as
butyrylcholinesterase (GenBank: AAA99296.1).
[0043] Other signal peptides can be mined from GenBank
[http://www.ncbi.nlm.nih.gov/genbanki or other such databases, and their
sequences added to the N-termini of the HC or LC, nucleotides sequences for
these being optimized for plant preferred codons as described above and then
synthesized. The functionality of a SP sequence can be predicted using online
freeware such as the SignalP program
[http://www.cbs.dtu.dk/services/SignalP/].
[0044] In a specific embodiment, the nucleic acid constructs encoding
recombinant proteins, including antibody HCs and/or LCs, and therapeutic
proteins
such as enzymes, are optimized for plant codon usage. In particular, the
nucleic
acid sequence encoding the heavy chain and light chain can be modified to
incorporate preferred plant codons. In a specific embodiment, coding sequences
for both the HC and LC, including the SP in both cases, were optimized for
expression in Nicotiana species. The first goal of this procedure was to make
the
coding sequences more similar to those of Nicotiana species. Codon
optimizations
were performed utilizing online freeware, i.e., the Protein Back Translation
program (Entelchon), and Nicotiana coding sequence preferences. Codons with
the highest frequencies for each amino acid in Nicotiana species (Nakamura,
2005) were thereby incorporated. Furthermore, potential intervening sequence
splice-site acceptor and donor motifs were identified (Shapiro et al., 1987;
CNR
National Research Council) and subsequently removed by replacement with
nucleotides that resulted in codons encoding the same amino acids. Inverted
repeat sequences were analyzed using the Genebee RNA Secondary Structure
software package (Brodsky et al.; GeneBee Molecular Biology Server);
nucleotides were changed to reduce the free energy (kilocalories per mole) of
CA 02815847 2013-05-13
. '
14
potential secondary structure while maintaining the polypeptide sequence.
Likewise, repeated elements were analyzed (CNR National Research Council) and
replaced where present. Potential methylation sites (i.e., CXG and CpG;
Gardiner-
Garden et al.) were replaced where possible and always without changing the
encoded amino acid sequence. A Kozak (Kozak, 1984) optimized translation start
site was engineered. Plant polyadenylation sites (i.e., AATAAA, AATGAA,
AAATGGAAA, and AATGGAAATG; Li et al.; Rothnie) and ATTTA RNA instability
elements (Ohme-Takagi et al.) were likewise avoided.
[0045] Seletectable marker genes can also be linked on the T-
DNA, such as
kanamycin resistance gene (also known as neomycin phonphatase gene II, or
nptI1), Basta resistance gene, hygromycin resistance gene, or others.
II. P19 suppressor of ciene-silencing
[0046] In one embodiment the recombinant protein, such as an
antibody or
therapeutic enzyme, is co-expressed with the P19 protein from Tomato Bushy
Stunt Virus (TBSV; Genbank accession: M21958). In a preferred embodiment, the
P19 protein from TBSV is expressed from a nucleic acid molecule which has been
modified to optimize expression levels in tobacco plants. In a specific
embodiment,
the modified P19-encoding nucleic acid molecule has the sequence shown in SEQ
ID NO:1.
[0047] In one embodiment, the P19-encoding nucleic acid is incorporated
into one of the expression vectors of the present invention. In a preferred
embodiment, the expression vector is p103, p105 or p105T.
[0048] The P19 protein can be expressed from an expression
vector
comprising a single expression cassette or from an expression vector
containing
one or more additional cassettes, wherein the one or more additional cassettes
comprise transgenic DNA encoding one or more recombinant proteins.
III. Method of plant transformation
[0049] The inventors have demonstrated that they can achieve
high levels
of expression of recombinant proteins using the vectors described herein.
CA 02815847 2013-05-13
[0050] Accordingly, the present application provides a method of
enhancing
the production of a recombinant protein in a plant comprising:
(i) introducing an expression vector comprising
(a) a promoter selected from (i) the 35S promoter of the Cauliflower
5 Mosaic Virus (CaMV) or (ii) the promoter of the ribulose bisphosphate
carboxylase
(rbc) small subunit gene of Chrysanthemum morifolium;
(b) a 5' untranslated region (UTR) selected from (i) the 35S 5' UTR
of CaMV or (ii) the 5' UTR of the rbc small subunit gene of C. morifolium;
(c) a 3' UTR and terminator sequence selected from (i) the 3' UTR
10 and terminator sequence of the nopaline synthase (nos) gene of
Agrobacterium,
(ii) the 3' UTR and terminator sequence of the osmotin (osm) gene of Oryza
sativa, (iii) the 3' UTR and terminator sequence from the rbc small subunit
gene of
C. morifolium, or (iv) a truncated version, by 162 bp as defined by a BspEl
recognition site, of the 3' UTR and terminator sequence from the rbc small
subunit
15 gene of C. morifolium; and
(d) a nucleic acid sequence encoding a recombinant protein
into a plant or plant cell; and
(ii) growing the plant or plant cell to obtain a plant that expresses the
recombinant protein.
[0051] In one embodiment, the recombinant protein is the only heterologous
protein expressed in the plant or plant cell. In a preferred embodiment, the
recombinant protein is co-expressed with the P19 protein from TBSV. The P19
protein and expression vectors for expressing it are described above.
[0052] In one embodiment, the recombinant protein is an antibody or
antibody fragment, comprising a heavy chain variable region and a light chain
variable region. In a specific embodiment, the antibody is trastuzumab. In
another
specific embodiment, the antibody is bevacizumab.
[0053] In another specific embodiment, the recombinant protein is an
enzyme such as butyrylcholinesterase.
CA 02815847 2013-05-13
16
[0054] In one embodiment, the nucleic acid molecule encoding the
heavy
chain variable region and the nucleic acid molecule encoding the light chain
variable region may be introduced into the plant cell on separate expression
vectors. In another embodiment, the nucleic acid molecule encoding the heavy
chain variable region and the nucleic acid molecule encoding the light chain
variable region may be introduced into the plant cell on the same expression
vector. In such an embodiment, the heavy chain and the light chain would be
expressed separately and then combine in the plant cell in order to prepare
the
desired antibody or antibody fragment.
[0055] The phrase "introducing" an expression vector into a plant or plant
cell" includes both the stable integration of the recombinant nucleic acid
molecule
into the genome of a plant cell to prepare a transgenic plant as well as the
transient integration of the recombinant nucleic acid into a plant or part
thereof.
[0056] The expression vectors may be introduced into the plant cell
using
techniques known in the art including, without limitation, electroporation, an
accelerated particle delivery method, a cell fusion method or by any other
method
to deliver the expression vectors to a plant cell, including Agrobacterium
mediated
delivery, or other bacterial delivery such as Rhizobium sp. NGR234,
Sinorhizobium meliloti and Mesorhizobium loti (Chung et al, 2006).
[0057] The plant cell may be any plant cell, including, without limitation,
tobacco plants, tomato plants, maize plants, alfalfa plants, Nicotiana
benthamiana,
Nicotiana tabacum, Nicotiana tabacum of the cultivar cv. Little Crittenden,
rice
plants, Lemna major or Lemna minor (duckweeds), safflower plants or any other
plants that are both agriculturally propagated and amenable to genetic
modification for the expression of recombinant or foreign proteins.
[0058] In one embodiment, the recombinant protein is expressed
transiently, with or without P19, in N. benthamiana. In another embodiment,
the
nucleic acid molecule encoding the recombinant protein is integrated into the
genome of an N. tabacum plant, which can be used thereafter for transgenic
expression. In a preferred embodiment, the N. tabacum plant is of the cultivar
cv.
CA 02815847 2013-05-13
17
Little Crittenden (LCR) and the recombinant protein is co-expressed with P19.
As
described in Example 1, LCR was the only cultivar identified that does not
induce
hypersensitive response (HR) in the presence of P19. This tobacco cultivar can
thus be utilized effectively in conjunction with P19-based transgenic
expression
systems.
[0059] In one embodiment, the recombinant protein and P19 are co-
expressed in an RNAi-based glycomodified tobacco plant. In a preferred
embodiment, the plant is an N. benthamiana plant. In a more preferred
embodiment the N. benthamiana plant exhibits RNAi-induced gene-silencing of
endogenous fucosyftransferase (FT) and xylosyltransferase (XT) genes. As shown
in Example 1, P19 can safely be used with RNAi-based glycomodifed N.
benthamiana expression hosts for the production of recombinant proteins such
as
antibodies without altering the glycan profile of the recombinant protein.
[0060] As used herein, the phrase "RNAi-based glycomodified tobacco
plant" means a tobacco plant that expresses polypeptides with altered glycan
profiles, wherein the altered profiles result from the use of interfering RNA
(RNAi)
gene-silencing technology. Plant-specific sugar residues on the N-glycan core,
namely core a1,3-fucose and f31,2-xylose, are immunogenic in mammals (Bardor
et al., 2003; Jin et al., 2008). Because of the existence of multiple
endogenous FT
and XT genes in most plants, modified glycosylation patterns are preferably
created with the use of RNAi technology (Cox et al., 2006; Sourrouille et al.,
2008;
Strasser et al., 2008).
[0061] The phrase "growing a plant or plant cell to obtain a plant
that
expresses a recombinant protein" includes both growing transgenic plant cells
into
a mature plant as well as growing or culturing a mature plant that has
received the
nucleic acid molecules encoding the recombinant protein. One of skill in the
art
can readily determine the appropriate growth conditions in each case.
[0062] In a specific embodiment, expression vectors containing the
recombinant nucleic acid molecules are introduced into A. tumefaciens strain
by
electroporation procedures. The N. benthamiana plants can be vacuum
infiltrated
CA 02815847 2013-05-13
. ,
18
according to the protocol described by Marillonnet et al. (2005) and Giritch
et al.
(2006) with several modifications. Briefly, all cultures can be grown at 28 C
and
220 rpm to a final optical density at 600 nm (0D600) of 1.8. Equal volumes are
combined and pelleted by centrifugation at 8,000 rpm for 4 minutes,
resuspended
and diluted by 103 in infiltration buffer (10 mM 1-(N-
morpholino)ethanesulphonic
acid (MES) pH 5.5, 10 mM MgSO4). Alternatively, each of the 5 Agrobacterium
cultures could be grown to lower OD values and Beer's Law could be applied to
determine the volumes of each culture required to make a bacterial suspension
cocktail whereby the concentrations of each bacterial strain were equivalent.
Alternatively, lower or higher concentrations of expression vectors could be
used
to optimize the expression of recombinant protein. Alternatively, higher or
lower
dilutions with infiltration buffer could be used.
[0063] The aerial parts of six-week-old N. benthamiana plants
are
submerged in a chamber containing the Agrobacterium tumefaciens resuspension
solution, after which a vacuum (0.5 to 0.9 bar) is applied for 90 seconds
followed
by a slow release of the vacuum, after which plants were returned to the
greenhouse for 8 days before being harvested. Alternatively, longer or shorter
periods under vacuum, and/or vacuum release, could either/or/and be used.
Alternatively, longer or shorter periods of growth in greenhouse could be
used.
Alternatively, standard horticultural improvement of growth, maximized for
recombinant protein production could be used (see Colgan et al., 2010).
[0064] Alternately, instead of transient introduction of
expression vectors
containing the HC and LC coding sequences of an antibody, or the coding
sequence of butyrylcholinesterase, stable transgenic plants could be made
using
one vector on which the nucleic acid molecule encoding the heavy chain
variable
region and the nucleic acid molecule encoding the light chain variable region
may
be introduced together in the same construct. In one embodiment, the nucleic
acid
molecule encoding the heavy chain variable region may be attached to the
nucleic
acid molecule encoding the light chain variable region by a linker in order to
prepare a single chain variable region fragment (scFv).
CA 02815847 2013-05-13
19
[0065] In another embodiment, the nucleic acid molecule encoding the
heavy chain and the nucleic acid molecule encoding the light chain may be
introduced into the plant cell on separate expression vector nucleic acid
constructs. In such an embodiment, the heavy chain and the light chain would
be
expressed from separate transgenic loci and then combine in the plant cell in
order
to prepare the antibody or antibody fragment.
[0066] Expression vector(s) containing antibody HC and LC genes would
be
introduced into Agrobacterium tumefaciens At542 or other suitable
Agrobacterium
isolates or other suitable bacterial species capable of introducing DNA to
plants for
transformation such as Rhizobium sp., Sinorhizobium meliloti, Mesorhizobium
loti
and other species (Broothaerts et al. 2005; Chung et al., 2006), by
electroporation
or other bacterial transformation procedures. Agrobacterium clones containing
expression vectors would be propagated on Luria-Bertani (LB) plates containing
rifampicin (30 mg/I) and kanamycin (50 mg/I), or other selectable media,
depending on the nature of the selectable marker genes on the vector.
Agrobacterium-mediated leaf disk transformation (Horsch et al. 1985; Gelvin,
2003), or similar protocols involving wounded tobacco (N. tabacum, variety
81V9
or tissue of other tobacco varieties such as are listed in Conley et al, 2009)
or N.
benthamiana or other plant species such as those of the Solanaceae, maize,
safflower, Lemna spp.,etc. would be infected with the Agrobacterium culture
(0D600= 0.6) and plated on Murashige and Skoog plus vitamins medium (MS;
Sigma), supplemented with agar (5.8%; Sigma) and containing kanamycin (100
mg/I) or 500 cefotaxime (mg/L) or other selectable media, depending on the
nature
of the selectable marker genes on the expression vector, for selection of
transformed plant cells. Production of shoots would be induced with
naphthalene
acetic acid (NAA; 0.1 mg/I; Sigma) and benzyl adenine (BA; 1 mg/I; Sigma) in
the
medium. For induction of roots, the newly formed shoots were moved to Magenta
boxes (Sigma-Aldrich, Oakville, ON) on MS medium (as above) that was lacking
NAA and BA. After roots are formed, plants would be transplanted to soil and
could be raised in greenhouse culture. For plant transformation, as many as
CA 02815847 2013-05-13
possible or at least 25 primary transgenic plants would be produced. ELISA and
quantitative immunoblots would be performed on each plant to characterize
levels
of total and active antibody produced by the plants, respectively (Almquist et
al.,
2004; 2006; McLean et at., 2007; Olea-Popelka et at., 2005; Makvandi-Nejad et
5 al., 2005).
[0067] After selection of antibody-expressing primary transgenic
plants, or
concurrent with selection of antibody expressing plants, derivation of
homozygous
stable transgenic plant lines would be performed. Primary transgenic plants
would
be grown to maturity, allowed to self-pollinate, and produce seed.
Homozygosity
10 would be verified by the observation of 100% resistance of seedlings on
kanamycin plates (50 mg/L), or other selectable drug as indicated above. A
homozygous line with single T-DNA insertions, that are shown by molecular
analysis to produce most amounts of antibody, would be chosen for breeding to
homozygosity and seed production, ensuring subsequent sources of seed for
15 homogeneous production of antibody by the stable transgenic or
genetically
modified crop (Olea-Popelka et al., 2005; McLean et at., 2007; Yu et al.,
2008).
[0068] Alternatively, the expression vector with both HC and LC
genes, or 2
expression vectors (one with a HC gene and the other with a LC gene), could be
used to transiently infect a plant or plant tissues, as described above, and
tissue
20 harvested as described above for subsequent purification of antibody.
[0069] The antibody or antibody fragment or the enzyme may be
purified or
isolated from the plants using techniques known in the art, including
homogenization, clarification of homogenate and affinity purification.
Homogenization is any process that crushes or breaks up plant tissues and
cells
and produces homogeneous liquids from plant tissues, such as using a blender,
or
juicer, or grinder, or pulverizer such as mortar and pestle, etc.
Clarification
involves either / and / or centrifugation, filtration, etc. Affinity
purification uses
Protein A or Protein G or Protein L or antibodies that bind antibodies;
affinity
purification for enzymes uses ligands that bind them, such as procainamide or
huprine.
CA 02815847 2013-05-13
. .
21
[0070] The following non-limiting examples are illustrative of
the present
invention:
EXAMPLE 1
Experimental Procedures
Plant Expression Vectors
[0071] Four expression cassettes, namely 103-106, were
synthesized and
cloned into the T-DNA region of pICH14011, creating plasmids p103-p106 for
producing recombinant proteins in Nicotiana species. The structures of the
expression cassettes are depicted in Figure 1. Expression cassettes 103-105
contain the 35S promoter and 5'UTR of the Cauliflower Mosaic Virus (CaMV;
Genbank accession: AF140604), while 106 contains the promoter and 5' UTR of
ribulose bisphosphate carboxylase (rbc) small subunit gene from Chrysanthemum
morifolium (Genbank accession: AY163904.1). Cassette 103 contains the 3' UTR
and terminator sequences from the nopaline synthase (nos) gene of
Agrobacterium (Genbank accession: V00087.1), cassette 104 contains the 3' UTR
and terminator sequences from the osmotin (osm) gene of Oryza sativa (Genbank
accession: L76377.1), and cassettes 105 and 106 carry the 3' UTR and
terminator
sequences from the rbc gene of C. morifolium (Genbank accession: AY163904.1).
The structures of another expression cassette is depicted in Figure 9. This
expression cassette was derived from p105 by insertion of a coding sequence
for
a recombinant protein to be expressed in plants using the restriction
endonuclease
BspEl for ligation of the 3' end of that coding sequence, resulting in a 162
bp
deletion of the 5' end of the Rbc 3' UTR and terminator. The resulting plasmid
is
known as p105T.
[0072] The P19 protein from Tomato Bushy Stunt Virus (TBSV; Genbank
accession: M21958) was cloned in cassette 103. The heavy and light chains of
trastuzumab (Grohs et al., 2010) were cloned separately in expression
cassettes
103-106. The heavy and light chains were both fused to the signal sequence
from
the basic chitinase gene of Arabidopsis thaliana (Genbank accession: AY054628)
for secretion into the apoplast. All protein sequences were codon-optimized
for
CA 02815847 2013-05-13
=
22
expression in N. benthamiana. The codon-optimized nucleic acid molecule
encoding P19 has the sequence shown in SEQ ID NO:1. The heavy and light
chains of trastuzumab were also cloned in a single binary vector, designated
as
102mAb, in which the heavy chain was driven by actin2 promoter from
Arabidopsis
thaliana (Genbank accession: NM 112764) with A. thaliana actin2 UTRs and
terminator region, and the light chain driven by the chimeric octopine and
mannopine synthase promoter (Genbank accession: EU181146.1) with UTRs and
terminator region of A. thaliana ubiquitin 10 (ubq10) gene (Genbank accession:
L05361).
Bacterial Transformation and Culture
[0073] Competent Agrobacterium tumefaciens A136 cells were
transformed
with the above-mentioned expression cassettes by a standard heat-shock method.
Bacterial cultures were grown overnight at 28 C in YEP medium (10 g Bacto
peptone, 10 g yeast extract, and 5 g sodium chloride per liter, pH 7.0)
supplemented with antibiotics. Unless otherwise stated, dense overnight
cultures
(up to 0D600=2.5) for each vector were adjusted to an 0D600 of 0.2 in Agro-
infiltration buffer (AIB) containing 10mM 2-(4-morpholino)-ethanesulfonic acid
(MES), 10 mM MgSO4, pH 5.5, and mixed together to create an Agrobacterium
infiltration cocktail (AIC) for plant treatment.
Transient Expression Assay and Sample Preparation
[0074] Plants were grown in the greenhouse and fed high nitrate
fertilizer
(N:P:K = 20:8:20) daily at 1 g/I (Plant Products, Brampton, Ontario, Canada),
adjusted to pH 6.0 with 20% H3PO4. To transiently express trastuzumab, an AIC
containing two Agrobacterium strains, each harboring one of the antibody
chains,
were used to infiltrate plant leaves. All plants were treated at the 4- to 6-
week
stage, either by spot or whole-plant infiltration. Shortly after treatment,
the plants
were placed back in the greenhouse for a certain period of time prior to
harvest,
depending on the expression vector. During this period, plants were only fed
water. When harvesting wholly infiltrated plants, newly emerged leaves were
discarded and the infiltrated leaves were separated from the stems and stored
at -
CA 02815847 2013-05-13
23
80 C until further processing. For spot infiltrations, 100 mg of leaf tissue
from the
infiltrated area was weighed and stored at -80 C until further processing.
[0075] Approximately 100 mg of leaf tissue was mixed with 300 pl of
extraction buffer containing phosphate buffered saline (PBS: 8 g NaCI, 0.2 g
KCI,
1.44 g Na2HPO4, and 0.24 g KH2PO4 per liter) and 10mM EDTA, pH 6.8 (PBSE
buffer). Samples were disrupted in a 2 ml microfuge tube containing two
stainless
steel ball bearings for 5 minutes using a TissueLyser (Qiagen) to prepare a
crude
protein extract, which was aliquoted into small volumes and stored at -20 C.
Western Blotting
gel. The separated proteins were blotted on a polyvinylidene fluoride (PVDF)
membrane and probed for antibody presence with a mix of alkaline phosphatase
conjugated anti-human y and K antibodies (Sigma Aldrich, Cat# A3312 and
A3813), diluted to 1:10,000 in PBS (pH 7.4) using NBT/BCIP (Thermo Scientific,
Cat# 34042) as substrate. Blots were developed for 2-5 minutes, depending on
the
experiment.
ELISA
[0077] Enzyme-linked immunosorbent assay (ELISA) was used to
quantitate the amount of antibody present in the crude protein extract of
treated
plants. ELISA plates were coated overnight at 4 C with a mouse polyclonal anti-
human IgG1 (Sigma Aldrich, Cat# 15885) capture antibody at 0.6 pg/ml in PBS.
Human IgG1 standard (Athens Research and Technology, Cat# 16-16-090707)
spiked in 5 pg of untreated crude protein extract was used as a standard. The
standard curve was generated using human IgG1, which allowed for antibody
detection over a range spanning three orders of magnitude (0.1-100 ng/well).
Crude extract from each treated sample was loaded on an ELISA plate as two-
fold
CA 02815847 2013-05-13
24
dilutions in triplicate. Final antibody concentration was calculated by
averaging the
mean antibody concentration for three crude extract dilutions. A second anti-
human antibody conjugated to HRP from rabbit (Abcam, Cat # ab6759) was used
for detection, using TMB-ELISA (Thermo Scientific, Cat# 34022) as substrate.
The
plates were allowed to develop for 15 minutes before the reaction was stopped
with 0.5M H2SO4.
Antibody Purification
[0078] Antibodies were purified essentially as described by Grohs et
al.
(2010).
N-glycosylation Analyses
[0079] N-glycan analyses of purified mAbs were carried out by liquid-
chromatography electrospray ionization-mass spectrometry (LC-ESI-MS) of
tryptic
glycopeptides as recently described (Stadlmann et al., 2008). Briefly, the
purified
samples were submitted to reducing SDS PAGE and the 55 kD band
corresponding to the HC was cut from the gel, S-alkylated, digested with
trypsin,
eluted from the gel fragment with 50% acetonitril and separated on a Biobasic
018
column (150 x 0.32 mm, Thermo Electron) with a gradient of 1%-80% acetonitrile
containing 65 mM ammonium formate pH 3Ø Positive ions were detected with a
Q TOF Ultima Global mass spectrometer (Waters, Milford, MA, USA). Summed
and deconvoluted spectra of the glycopeptides elution range were used for
identification of glycoforms. This method generates two glycopeptides that
differ by
482 Da (glycopeptide 1, EEQYNSTYR; glycopeptide 2 TKPREEQYNSTYR).
Results
The Untranslated Regions Used in Recombinant Expression of Trastuzumab
Significantly Impact Antibody Accumulation
[0080] Trastuzumab is a therapeutic antibody used in the treatment of
HER2+ breast cancer (BaseIga et al., 1998; Lewis et al., 1993). To produce
this
antibody in Nicotiana hosts, its heavy (HC) and light (LC) chains were cloned
into
plant expression cassettes and placed either on a single binary vector
(102mAb),
or on separate binary vectors (103-106HC and 103-106LC), in which case they
CA 02815847 2013-05-13
were co-expressed (referred to as vector sets 103mAb-106mAb) to produce the
fully assembled antibody. The different expression cassettes were designed to
carry different combinations of promoters, 5'UTRs, and 3' UTR/terminators
(Figure
1). Trastuzumab was transiently expressed in N. benthamiana using vector sets
5 103mAb-106mAb to compare the levels of recombinant antibody production. A
7-
day expression time-course with whole-plant vacuum infiltration showed a
considerable difference in the dynamics and maximal antibody expression among
the four vector sets (Figure 2A). Vectors 105mAb and 106mAb resulted in higher
maximal antibody accumulation compared to 103mAb and 104mAb. Antibody
10 expression peaked at 3-4 days post-infection (d.p.i.) for 103mAb and
104mAb, and
at 4-5 d.p.i. for 105mAb, whereas 106mAb showed a steady increase in
expression up to 7 days post-infection. Maximal antibody levels were achieved
with the 105mAb and 106mAb vector sets, estimated by ELISA at =-=.1% of TSP.
The protein content of tobacco leaves has been estimated at approximately 2%
of
15 the fresh weight (Stevens et al., 2000), therefore antibody expression at
1% of
TSP translates into 2%200 mg of antibody per kg of fresh weight (FW). A 20-
fold
range in antibody accumulation was observed when the different vectors were
compared. Therefore, different UTR elements fused to the codes for the heavy
and light chains of trastuzumab significantly affect the accumulation of the
fully
20 assembled antibody when transiently expressed. In addition, N-
glycosylation
profiles of mAbs were determined by LC-ESI-MS (liquid-chromatography
electrospray ionization-mass spectrometry). Typically mAbs exhibited a largely
homogeneous GnGnXF3 oligosaccharide pattern with plant specific (31,2-xylose
and core a1,3-fucose residues (see last section of Results).
25 P19 Does Not Have a Similar Boosting Effect on the Expression of
Trastuzumab
from Different Expression Vectors
[0081] The effect of co-expressing P19 with vectors 103mAb-106mAb was
analyzed over 7 days in N. benthamiana (Figure 2B and 2C) to determine whether
the different UTR combinations had any effect on the ability of P19 to boost
antibody expression, as previously reported (Saxena et al., 2011; Vezina et
al.,
CA 02815847 2013-05-13
. .
26
2009). All Agrobacterium cultures used in this experiment were adjusted to an
0D600 of 0.2. Not all vector sets were positively affected by P19 (Figure 20,
Table
1). For 103mAb and 104mAb, P19 resulted in a 15-fold increase in the
concentration of trastuzumab, although maximal expression was almost 3-fold
greater with 103mAb compared to 104mAb, both without and with P19. For
105mAb, P19 only resulted in a =2-fold increase in the concentration of
trastuzumab, from 1 /0 to approximately 2.1% of TSP. Antibody accumulation
with
vector set 106mAb, which contained only plant-derived UTRs, was unaffected by
P19. Antibody expression peaked for 103mAb and 105mAb when co-expressed
with P19 at just over 2% of TSP. It was also noted that P19 changed the peak
time
of expression for the vectors 103mAb, 104mAb, and 105mAb (Figure 20). The
ability of P19 to boost expression of trastuzumab with vector 102mAb was also
tested. 102mAB is similar to 106mAb, i.e., it only contains plant-derived UTRs
(Figure 1). Similar to 106mAb, P19 did not affect the antibody expression
level of
102nnAb (Figure 3A). P19 was also found to have no boosting effect on
trastuzumab expressed with Tobacco Mosaic Virus- (TMV) and Potato X Virus-
based (PVX) deconstructed vectors (Grohs et al., 2010) (Figure 3B).
Boosting Effect of P19 on Recombinant Antibody Expression is Concentration
Dependent
[0082] It is believed that in order for P19 to exert its biological
function for a
successful TBSV infection, it has to accumulate beyond a certain threshold
concentration in plant cells (Qiu et al., 2002; Scholthof et al., 1999). When
expressed transiently, co-expression of P19 with 103mAb at an Agrobacterium
concentration of 013600=0.2 significantly boosted antibody production. Since
the
expression level of recombinant proteins are, for the most part, lower for a
transgenic versus transient expression, co-infiltration of 103mAb with P19 was
examined at lower Agrobacterium concentrations to simulate transgenic-like
levels
of P19 accumulation in N. benthamiana. Infiltration of 103mAb vectors alone at
0D600 values of 0.2, 0.02, and 0.002 resulted in different levels of antibody
production, with a direct correlation between the applied Agrobacterium
CA 02815847 2013-05-13
=
27
concentration and antibody expression level (Figure 4). The ability of P19 to
boost
antibody expression with 103mAb progressively diminished when P19 was applied
at lower concentrations, while co-expressing P19 at 0D600=0.2 resulted in a
significant increase in antibody expression irrespective of 103mAb
concentration
(Figure 4). Thus, unless P19 can be expressed at very high levels, its utility
in
transgenic production of recombinant proteins may be limited due to its
concentration-dependent mode of action.
Tobacco Varieties Respond Differently to P19
[0083] To utilize P19 for transgenic production of recombinant
proteins in
plants, it is imperative that P19 does not adversely affect plant growth, and
more
importantly, gene expression. As discussed, when expressed transiently at high
levels in N. benthamiana, P19 effectively boosts recombinant protein levels.
However, due to having a much greater biomass, N. tabacum is often selected
over N. benthamiana for transgenic production. The downside of using N.
tabacum
is the development of necroses at the site of infection by 7 days after the
introduction of recombinant P19 to leaf cells via Agroinfiltration (Angel et
al.,
2011). The reaction that is triggered by P19 in N. tabacum, also known as the
hypersensitive response, has been well documented but only tested in two
tobacco cultivars (Angel et al., 2011; Jovel et al., 2011; Siddiqui et al.,
2008). To
identify a tobacco that could be used with P19 in a transgenic expression
system,
five cultivars were screened for the development of the hypersensitive
response.
Trastuzumab was transiently expressed with 103mAb, alone or together with P19
by spot infiltration of 5-week-old plants. Four out of five tobacco cultivars,
namely
1-64, TI-95, Xanthi, and Petite Havana H4, showed a marked decrease in
antibody
expression at day 6 when co-expressed with P19, compared to antibody
expression without P19 (Figure 5A). Conversely, the co-expression of P19 with
103mAb in N. tabacum cv. Little Crittenden (LCR) resulted in a significant
boost in
trastuzumab expression (Figure 5B). Furthermore, the tobacco cultivars that
showed a decrease in antibody expression in the presence of P19 also showed a
marked discoloration of the treated areas after 3 days, while the infiltrated
areas of
CA 02815847 2013-05-13
28
N. benthamiana and N. tabacum cv. LCR were unaffected up to 10 days post-
infection (shown in Figure 50 at 5 d.p.i). These results indicate that N.
tabacum cv.
Little Crittenden can be used as a host for transgenic production of
recombinant
proteins using P19.
[0084] Reciprocal crosses were made between tobacco cultivars 1-64 and
LCR to look at the manner in which induction of the hypersensitive response by
P19 is inherited in N. tabacum. The induction of this response in a sterile
cross
between N. benthamiana and N. tabacum, named NBT, was also tested. Five-
week-old seedlings were infiltrated with 103mAb, with or without P19. The
level of
trastuzumab was reduced in all the crosses at 5 d.p.i. when 103mAb was co-
expressed with P19 (Figure 5B). This reduction in antibody expression
correlated
with discoloration of the treated leaves, followed by necrosis (Figure 5C).
These
results indicate that the putative R gene responsible for triggering the HR is
nuclear, and that LCR is homozygous recessive for that gene.
P19 Does Not Affect the Silencing of Fucosyltransferase and Xylosyltransferase
Activity in RNAi-based Glycomodified Nicotiana benthamiana
[0085] RNAi based silencing is a commonly used method to modify the N-
glycosylation pattern towards human like structures in plants (Cox et al.,
2006;
Sourrouille et al., 2008; Strasser et al., 2008). This strategy was also
applied to
eliminate plant specific N-glycan residues (i.e. xylose and core a1,3 fucoce)
in
Nicotiana benthamiana (AXTFT) by the down-regulation of the respective
enzymes fucosyltransferase and xylosyltransferase (Cox et al., 2006;
Sourrouille
et al., 2008; Strasser et al., 2008). The possible adverse effects of P19 in
such
plants had yet to be determined. Thus, AXTFT mutants were vacuum infiltrated
with the mAb103 vectors with or without P19. Trastuzumab was purified 5 days
post-infiltration and analyzed by LC-ESI-MS to determine the N-glycosylation
pattern (Figure 6 B, C). Interestingly, both 103mAb versions carried an
identical N-
glycosylation profile, lacking plant specific oligosaccharides. This suggests
that
P19 does not affect the RNAi silencing pathway responsible for the silencing
of FT
and XT in AXTFT while it interferes with the gene-silencing pathway that
reduces
CA 02815847 2013-05-13
'
29
transgene expression. These results indicate that P19 can be used for boosting
recombinant protein levels expressed in an RNAi-based expression host without
altering the protein's glycan profile.
Transient co-infiltration of P19 expression vector greatly enhances the
expression
of two more therapeutic antibodies in Nicotiana benthamiana.
[0086] To demonstrate that P19 inhances the expression of other
antibodies, coding sequences for the light and heavy chains of both anti-HIV
mAb
4E10 and bevacizumab were synthesized for expression in plants (as above).
Anti-HIV mAb 4E10 was first described in Buchacher et at. (1994) with its
light
chain sequence being available in the GenBank (http://www.ncbi.nlm.nih.gov/)
as
entry GI:122920218 and its heavy chain as a Fd/VH in entry GI: 61680025. This
antibody may be used as an anti-HIV vaccine or as a diagnostic reagent. The
light
and heavy chain sequences of bevacizumab are available from Drugbank
(http://www.drugbank.ca/) as entry DB00112. Bevacizumab is used with standard
chemotherapy for metastatic colon cancer; it also been approved for use in
certain
lung cancers, renal cancers, and glioblastoma multiforme of the brain. The
heavy
chain coding sequence for mAb 4E10, and both the light and heavy chain coding
sequences for bevacizumab were cloned downstream of the Arabidopsis basic
chitinase signal peptide (as above) and inserted into the 103 and 105 vectors.
The
light chain coding sequence for mAb 4E10 was also cloned downstream of the
Arabidopsis basic chitinase signal peptide and inserted into the 105 vector
and
into a version of the 105 vector in which a 162 base-pair deletion of the 5'
end of
the C. morifolium rbc gene terminator sequence was caused by cleavage at a
BspEl site; this latter vector is referred to as p105T (i.e., 105-truncated).
All eight
vectors were introduced into Agrobacterium tumefaciens strain At542 (as
above),
then spot-infiltrated, with and without P19 (as above), into N.benthamiana
leaf
tissue in the combinations indicated in Figure 7 and harvested after 7 days.
Each
Agrobacterium strain was applied at a final 0D600 of 0.2. Tissue harvests for
each
treatment included 3 or 4 spots, which were pooled and total soluble protein
(TSP)
was extracted as described in Garabagi et at. (2012a,b). A 10% non-reducing
CA 02815847 2013-05-13
SDS-PAGE gel was run that included 10 mg TSP for each plant sample.
Electrophoretically separated proteins were subsequently electrotransferred to
PVDF membrane and probed with combined anti-7 and anti-x antibody probes
conjugated to alkaline phosphatase (also described in Garabagi et al., 2012a).
5 Results indicate that co-expression of P19 greatly enhances expression of
both
antibodies irrespective of antibody expression vector. In Figure 7, the gel
loading
scheme is tabulated above the immunoblot image, and the size of each molecular
weight marker band in lane 1 is given on the left in kDa; the human serum
immunoglobulin quantification standard in lane 10 is 500 ng; mAb=monoclonal
10 antibody; HC=heavy chain vector; LC=light chain vector.
Transient expression of a therapeutic enzyme, human butyrylcholinesterase
(BChE) is greatly enhanced by co-expression of P19.
[0087]
Butyrylcholinesterase is a non-specific cholinesterase enzyme that
hydrolyses many different choline esters. In humans, it is found primarily in
the
15 liver and is encoded by the BCHE gene. It is being developed as an
antidote to
nerve-gas poisoning. Two different BChE expression vectors were constructed in
p105T (described above): (1) with the Arabidopsis basic chitinase (abc) signal
sequence and a synthetic BChE coding sequence optimized for expression in
plants referred to as E2, i.e., abc-BChE2; and (2) with the native human BChE
20 signal sequence (hSS) and another synthetic BChE coding sequence
optimized
for expression in plants referred to as E3, hSS-BChE3. These were
introduced
into whole N. benthamiana plants by vacuum infiltration (according to Garabagi
et
al., 2012a,b), either with or without the P19 (as above). Samples were taken
at 5,
7 and 9 days post-infiltration (DPI) and BChE activity was measured by Ellman
25 Assay (Ellman et at., 1961). Results of this experiment are presented in
Figure 8
where histogram bars present amounts of BChE in mg BChE/kg leaf tissue, by
converting activity measurements to mass of enzyme using the specific activity
conversion factor of 718.3 activity units per mg of BChE determined in Weber
et
al. (2010). Note that background readings (as determined with untreated plant
30 extracts) have been subtracted for the presentation of these data.
Histogram bars
CA 02815847 2013-05-13
. =
31
in Figure 8 indicate the mean BChE activity measured in 2 samples from 2
plants
(4 total repeats each) with associated standard error bars. These data
indicate that
co-expression of P19 greatly enhances expression of BChE.
Discussion
[0088] In this example, three important issues are addressed regarding the
application of P19 to improve recombinant protein production in plants: P19's
ability to sufficiently boost recombinant protein expression; its potential
adverse
physiological effects in the expression host; and interference with the state
of
gene-silencing in RNAi-based glycomodified plants. The results described
herein
indicate that all three requirements are met and that P19 can be effectively
utilized
for transient expression of recombinant glycoproteins in RNAi-based expression
hosts.
[0089] The highest antibody expression reports using suppressors
of gene
silencing to date have been on transient expression of mAb 2G12 with P19 at
400
mg/kg FW (Saxena et al., 2011) and mAb C5-1 with HcPro at 757 mg/kg FW
(Vezina et al., 2009). Both of these mAbs were retained in the ER by the
addition
of auxiliary C-terminal tags. In the context of biosimilar therapeutic
antibodies,
however, ER retention signals are problematic since they add extra amino acids
to
the primary sequence of the innovator protein. In addition, ER typical
oligomannisidic N-glycsoylation is for most therapeutic proteins untypical and
thus
unwanted.
[0090] P19 is herein shown to enhance expression of the model
therapeutic
mAb trastuzumab that is targeted to the apoplast using classical binary
vectors at
about 2.3% of TSP, or =460 mg/kg FW. Furthermore, regulatory genetic elements
used in recombinant constructs determine whether or not P19 can boost
expression. This was demonstrated by co-expressing P19 together with the heavy
and light chains of trastuzumab cloned in five different expression vectors
containing different 5' and 3' UTRs . The results described herein indicate
that
transcripts with at least one virus-derived UTR, such as in 103mAb, 104mAb,
and
105mAb, were boosted by P19. This suggests that the transcripts of these
CA 02815847 2013-05-13
32
expression cassettes are subjected to RNAi silencing, albeit to different
extents,
and therefore are boosted in the presence of a suppressor of a gene silencing.
In
contrast, transcripts that only contained plant derived UTRs, such as 106mAb
and
102mAb, were unaffected by P19, suggesting they were not subjected to any
significant RNAi silencing during the observation period. These findings may
have
direct implications in the design of expression vectors.
[0091] P19 is herein also shown to enhance expression of another
therapeutic mAb, namely bevacizumab, using two of the vectors described in
this
application. P19 is herein also shown to enhance expression of another
therapeutic mAb, namely anti-HIV mAb 4E10, using three of the vectors
described
in this application. These three examples illustrate the potential for P19 to
enhance
the expression of most any antibody using the vector system presented in this
application.
[0092] P19 is herein also shown to enhance expression of a potential
therapeutic enzyme, namely butyrylcholinesterase, using one of the vectors
described in this application with either the Arabidopsis basic chitinase
signal
sequence or with the native human butyrylcholinesterase signal sequence, and
using either of two synthetic coding sequences optimized for plant expression
of
the same identical butyrylcholinesterase polypeptide. This example illustrates
the
potential for P19 to enhance the expression of therapeutic proteinsother than
antibodies, such as enzymes.
[0093] It is apparent that the vectors described in this application
can
produce different therapeutic proteins, and that expression of proteins from
these
vectors in planta can be enhanced by co-expression of the P19 gene.
[0094] It is also apparent from several reports on constitutive transgenic
expression of P19 that the onset of adverse effects occurs only when the
protein is
used in high titers, especially since high-level constitutive expression of
P19 is
known to be lethal in Arabidopsis (Dunoyer et al., 2004). This dose-dependent
functionality of P19 is supported by the fact that transgenic tobacco cv.
Xanthi is
tolerant to P19 at low levels (Siddiqui et al., 2008), while the protein
generates
CA 02815847 2013-05-13
. .
33
necrosis in tobacco cvs. Samsun and NC95 leaves when transiently expressed at
high titers (Angel et al., 2011). Inducible expression systems that are
capable of
producing high levels of recombinant protein have been employed to circumvent
the lethality that is caused by producing high titers of P19 in transgenic
systems.
Nonetheless, unfavorable effects such as malformed leaves and flowers appear
when P19 is expressed with such systems at high levels, such as with the
pOp/LhG4 transactivation systems described by Stay et al. (2009). Since
transgenes generally express higher in transient as opposed to transgenic
settings
(Garabagi et at. 2012, in press), the amount of P19 protein produced by
transient
infiltration at concentrations of 0D600=0.02 and lower was shown not to
significantly enhance trastuzumab levels. On the other hand, higher P19
concentrations (0D600=0.2) caused a 15-fold increase in antibody levels
(Figure
4). These results support the idea of dose-dependent functionality of P19 in
the
context of boosting recombinant protein expression. However, the titer at
which
P19 exerts its boosting effect causes a hypersensitive response in most
tobacco
cultivars (Figure 5A).
[0095] Recent reports on the induction of the hypersensitive
response in N.
tabacum cvs. Samsun and NC95 describe a physiological response that involves
local induction of RNAi silencing (Jovel et al., 2011) followed by necrosis
(Angel et
al., 2011). This response is likely triggered by the product of a putative R
gene
(Angel et at., 2011). As described in this example, a tobacco cultivar, Little
Crittenden, was identified which did not trigger a hypersensitive response
when
exposed to high titers of P19 (Figure 5A and 5C). All other tested tobacco
cultivars
showed a marked decrease in antibody production in the presence of P19
compared to the expression of the antibody vectors alone, indicating the
induction
of an RNAi silencing mechanism that overrides the suppression of gene-
silencing
by P19. These findings are in line with recent reports on the induction of the
hypersensitive response in N. tabacum that includes a local induction of RNAi
silencing (Angel et al., 2011; Jovel et al., 2011). The results described
herein also
indicate a dominant mode of inheritance for this trait, also in accordance
with
CA 02815847 2013-05-13
34
previously described characteristics of R genes (Moffett, 2009), and that the
LCR
cultivar has a recessive mutation in this gene. Thus, this tobacco genotype
and
others that have similar R gene mutations may lend themselves to transgenic
expression of recombinant proteins using P19 when used with a system capable
of generating high titers of the protein. LCR can be used as a model for
determining the number of genes involved in the hypersensitive response to
P19.
[0096] Despite the ability of plants to carry out complex
glycosylation, a
possible bottleneck for their use as a versatile expression platform for
therapeutic
antibodies is the presence of plant specific N-glycan residues, i.e. xylose
and core
a1,3-fucose. Such non-mammalian oligosaccharides might change the biological
activity of a given protein or might even induce unwanted adverse side effects
upon therapeutic application. As expected, during the experiments described
herein, plant-typical N-glycosylation was detected on trastuzumab. To
circumvent
this problem RNAi technology had been used to generate a plant line that lacks
unwanted plant specific N-glycan residues (Strasser et al., 2008). Monoclonal
antibodies produced in this mutant (AXTFT) carry complex human-like N-glycans
lacking plant-specific glycosylation. Moreover, mAbs with such a
glycoengineered
profile have also shown increased effector functions compared to their
mammalian
cell-derived counterparts (Forthal et al., 2010; Zeitlin et al., 2011). Thus,
such
glycosylation mutant plants may serve as valuable expression platforms for the
generation of therapeutic mAbs. However, whether the use of P19 would perturb
the silencing of XT and FT in AXTFT mutants has not been investigated yet.
Based on previous reports on the cellular targets of P19, including a
documented
case of interference with the siRNA silencing pathway in a transgenic plant
line
(Ahn et al., 2011), P19 was expected to interfere with the RNAi-induced gene-
silencing of XT and FT in glycomodified N. benthamiana. Surprisingly, mAbs
expressed in AXTFT with and without P19 were in both cases completely devoid
of xylose and fucose residues. The virtually identical glycan profiles of the
two
antibodies indicate that the silencing of FT and XT is unaffected by P19
(Figure 6).
Thus, it appears that P19 can be used effectively in combination with RNAi-
based
CA 02815847 2013-05-13
. ,
glycomodified hosts for the production of therapeutic glycoproteins in both
transient and stable expression systems.
[0097] While the present invention has been described with
reference to
what are presently considered to be the preferred examples, it is to be
understood
5 that the invention is not limited to the disclosed examples. To the
contrary, the
invention is intended to cover various modifications and equivalent
arrangements
included within the spirit and scope of the appended claims.
[0098] All publications, patents and patent applications are
herein
incorporated by reference in their entirety to the same extent as if each
individual
10 publication, patent or patent application was specifically and
individually indicated
to be incorporated by reference in its entirety.
CA 02815847 2013-05-13
. .
36
Table 1 ¨ Maximal trastuzumab level produced with different expression
vectors.
Vector Max Expression Max Expression Expression
Set (VoTSP) with P19 (%TSP) increase with
P19
103mAb 0.15 0.01 2.35 0.13 15.6x
104mAb 0.05 -0,01 0.75 0.04 15.0x
105mAb 1.02 0.07 2.17 0.31 2.1x
106mAb 0.98 0.04 0.85 0.08 1.0x
CA 02815847 2013-05-13
. ,
37
SEQUENCE LISTING
SEQ ID NO:1 P19 nucleotide sequence codon-optimized for expression in
Nicotiana.
ATGGAAAGGGCTATTCAGGGAAATGATGCTAGAGAGCAGGCTAATTCTGAAA
GATGGGATGGTGGATCTGGTGGAACTACTTCTCCATTCAAGCTTCCAGATGA
GTCTCCATCTTGGACTGAGTGGAGGCTTCATAACGATGAGACTAACTCCAAT
CAGGATAACCCACTCGGATTCAAAGAATCTTGGGGATTCGGAAAGGTTGTGT
TCAAGCGTTACCTTAGGTATGATAGGACTGAGGCTTCACTTCATAGGGTTCTC
GGATCTTGGACTGGTGATTCTGTTAACTACGCTGCTTCTCGTTTTTTTGGATT
CGATCAGATCGGATGCACTTACTCTATTAGGTTCAGGGGAGTGTCTATTACTG
TTTCTGGTGGATCTAGGACTCTTCAACACCTTTGCGAGATGGCTATTAGGTCT
AAGCAAGAGCTTCTTCAGCTTGCTCCAATTGAGGITGAGICTAACGTTTCAAG
AGGATGTCCAGAAGGTACTGAGACTTTCGAGAAAGAATCCGAGTGA
CA 02815847 2013-05-13
= ,
38
FULL CITATIONS FOR REFERENCES REFERRED TO IN THE
SPECIFICATION
Ahn JW, Lee JS, Davarpanah SJ, Jeon JH, Park YI, Liu JR and Jeong WJ (2011)
Host-dependent suppression of RNA silencing mediated by the viral
suppressor p19 in potato. Planta.
Almquist K. C., Niu Y., McLean M. D., Mena F. L., Yau K. Y., Brown K., Brandle
J.
E. and Hall J. C. (2004) Immunomodulation confers herbicide resistance in
plants. Plant Biotechnol J 2, 189-97.
Angel CA, Hsieh YC and Schoelz JE (2011) Comparative analysis of the capacity
of tombusvirus P22 and P19 proteins to function as avirulence determinants
in Nicotiana species. Mol Plant Microbe Interact 24:91-99.
Bardor M, Faveeuw C, Fitchette AC, Gilbert D, Galas L, Trottein F, Faye L and
Lerouge P (2003) lmmunoreactivity in mammals of two typical plant glyco-
epitopes, core alpha(1,3)-fucose and core xylose. Glycobiology 13:427-434.
BaseIga J, Norton L, Albanell J, Kim YM and Mendelsohn J (1998) Recombinant
humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity
of paclitaxel and doxorubicin against HER2/neu overexpressing human
breast cancer xenografts. Cancer Res 58:2825-2831.
Baulcombe D (2004) RNA silencing in plants. Nature 431:356-363.
Brodersen P and Voinnet 0 (2006) The diversity of RNA silencing pathways in
plants. Trends Genet 22:268-280.
Brodsky, L. I.; lvanov, V. V.; Kalaidzidis, Y. L.; Leontovich, A. M.;
Nikolaev, V. K.,
Feranchuk, S. I.; Drachev, V. A. GeneBee-NET: internet-based server for
analyzing biopolymers structures. Biochemestry 1995, 60, 923-928.
Broothaerts W, Mitchell H J, Weir B, Kaines S, Smith L M A, Yang W, Mayer J E,
Roa-Rodriguez C, Jefferson R A (2005) Gene transfer to plants by diverse
species of bacteria, Nature 433:629-633.
Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M.
Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, and H. Katinger.
1994. Generation of human monoclonal antibodies against HIV-1 proteins;
electrofusion and Epstein-Barr virus transformation for peripheral blood
lymphocyte immortalization. AIDS Research and Human Retroviruses
10:359-69.
CA 02815847 2013-05-13
. .
39
Burgyan J, Lakatos L, Szittya G and Silhavy D (2004) Molecular mechanism of
RNA silencing suppression mediated by p19 protein of tombusviruses.
Embo Journal 23:876-884.
Carthew RW and Sontheimer EJ (2009) Origins and Mechanisms of miRNAs and
siRNAs. Cell 136:642-655.
Chung S. M., Vaidya M. and Tzfira T. (2006) Agrobacterium is not alone: gene
transfer to plants by viruses and other bacteria. Trends Plant Sci 11, 1-4.
Colgan R., Atkinson C. J., Paul M., Hassan S., Drake P. M., Sexton A. L.,
Santa-
Cruz S., James D., Hamp K., Gutteridge C. and Ma J. K. Optimisation of
contained Nicotiana tabacum cultivation for the production of recombinant
protein pharmaceuticals. Transgenic Res 19, 241-56.
Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, Black A,
Passmore D, Moldovan-Loomis C, Srinivasan M, Cuison S, Cardarelli PM
and Dickey LF (2006) Glycan optimization of a human monoclonal antibody
in the aquatic plant Lemna minor. Nature biotechnology 24:1591-1597.
Dunoyer P, Lecellier CH, Parizotto EA, Himber C and Voinnet 0 (2004) Probing
the microRNA and small interfering RNA pathways with virus-encoded
suppressors of RNA silencing. Plant Cell 16:1235-1250.
Ellman, G.L., Courtney, K.D., Andres, V., Jr., Featherstone, R.M., A new and
rapid
colorimetric determination of acetylcholinesterase activity. Biochem.
Pharmacol. 7:
88-95, 1961.
Forthal DN, Gach JS, Landucci G, Jez J, Strasser R, Kunert R and Steinkeliner
H
(2010) Fc-glycosylation influences Fcgamma receptor binding and cell-
mediated anti-HIV activity of monoclonal antibody 2G12. Journal of
immunology 185:6876-6882.
Gardiner-Garden, M.; Frommer, M. CpG islands in vertebrate genomes. J. Mol.
Biol. 1987, 196, 261-282.
Garabagi, F., E. Gilbert, A. Loos, M.D. McLean, and J.C. Hall. 2012a. Utility
of the
P19 suppressor of gene-silencing protein for production of therapeutic
antibodies in Nicotiana expression hosts. Plant Biotechnol J 10:1118-28.
Garabagi, F., M.D. McLean, and J.C. Hall. 2012b. Transient and stable
expression
of antibodies in Nicotiana species. Methods Mol Biol 907:389-408.
CA 02815847 2013-05-13
Gelvin S. B. (2003) Agrobacterium-mediated plant transformation: the biology
behind the "gene-jockeying" tool. Microbiol Mol Biol Rev 67, 16-37.
5
Giritch A., Marillonnet S., Engler C., van Eldik G., Botterman J., Klimyuk V.
and
Gleba Y. (2006) Rapid high-yield expression of full-size IgG antibodies in
plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci U S A
1 03,. 14701-6.
Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C,
Michaud D and Faye L (2010) Plant-specific glycosylation patterns in the
context of therapeutic protein production. Plant Biotechnol J 8:564-587.
Grohs BM, Niu Y, Veldhuis LJ, Trabelsi S, Garabagi F, Hassell JA, McLean MD
and Hall JC (2010) Plant-produced trastuzumab inhibits the growth of HER2
positive cancer cells. J Agric Food Chem 58:10056-10063.
Horsch R.B., Fry J.E., Hoffmann N.L., Eichholtz D., Rogers S.G. and Fraley
R.T.
1985. A simple and general method for transferring genes into plants.
Science 227: 1229-1231.
Hsieh YC, Omarov RT and Scholthof HB (2009) Diverse and newly recognized
effects associated with short interfering RNA binding site modifications on
the Tomato bushy stunt virus p19 silencing suppressor. J Virol 83:2188-
2200.
Jin C, Altmann F, Strasser R, Mach L, Schahs M, Kunert R, Rademacher T, Glossl
J and Steinkellner H (2008) A plant-derived human monoclonal antibody
induces an anti-carbohydrate immune response in rabbits. Glycobiology
18:235-241.
Jovel J, Walker M and Sanfacon H (2011) Salicylic acid-dependent restriction
of
Tomato ringspot virus spread in tobacco is accompanied by a
hypersensitive response, local RNA silencing, and moderate systemic
resistance. Mol Plant Microbe Interact 24:706-718.
Kozak M. (1984) Compilation and analysis of sequences upstream from the
translational start site in eukaryotic mRNAs. Nucleic Acids Res 12, 857-72.
Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C and Shepard HM
(1993) Differential responses of human tumor cell lines to anti-p185HER2
monoclonal antibodies. Cancer Immunol lmmunother 37:255-263.
CA 02815847 2013-05-13
41
Li, Q.; Hunt, A. A near-stream element in a plant polyadenylation signal
consist of
more than six nucleotides. Plant Mol. Biol. 1995, 28, 927-934.
Moffett P (2009) Mechanisms of recognition in dominant R gene mediated
resistance. Adv Virus Res 75:1-33.
Makvandi-Nejad S., McLean M. D., Hirama T., Almquist K. C., Mackenzie C. R.
and Hall J. C. (2005) Transgenic tobacco plants expressing a dimeric
single-chain variable fragment (scfv) antibody against Salmonella enterica
serotype Paratyphi B. Transgenic Res 14, 785-92.
Marillonnet, S., Thoeringer, C., Kandzia, R., Klimyuk, V., and Gleba, Y.
(2005).
Systemic Agrobacterium tumefaciens-mediated transfection of viral
replicons for efficient transient expression in plants. Nat. Biotechnol 23,
718-723.
McLean, M. D., Almquist, K. C., Niu, Y., Kimmel, R., Lai, Z., Schreiber, J.
R., and
Hall, J. C. (2007). A Human Anti-Pseudomonas aeruginosa Serotype 06ad
lmmunoglobulin Cl Expressed in Transgenic Tobacco Is Capable of
Recruiting Immune System Effector Function In Vitro. Antimicrob. Agents
Chemother. 51, 3322-3328.
Nakamura, Y. Codon usage database. Available on-line at
www.kazusa.or.jp/codon; 2005.
Ohme-Takagi, M.; Taylor, C.; Newman, T.; Green, P. The effect of sequences
with
high AU content on mRNA stability intobacco. Proc. Natl. Acad. Sci. U.S.A.
1993, 90, 11811-11815.
Olea-Popelka, F., McLean, M. D., Horsman, J., Almquist, K., Brandle, J. E.,
and
Hall, J. C. (2005). Increasing expression of an anti-picloram single-chain
variable fragment (ScFv) antibody and resistance to picloram in transgenic
tobacco (Nicotiana tabacum). J. Agric. Food Chem 53, 6683-6690.
Park JW, Faure-Rabasse S, Robinson MA, Desvoyes B and Scholthof HB (2004)
The multifunctional plant viral suppressor of gene silencing P19 interacts
with itself and an RNA binding host protein. Virology 323:49-58.
Qiu W, Park JW and Scholthof HB (2002) Tombusvirus P19-mediated suppression
of virus-induced gene silencing is controlled by genetic and dosage features
that influence pathogenicity. Mat Plant Microbe Interact 15:269-280.
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B and Bartel DP (2002)
MicroRNAs in plants. Genes Dev 16:1616-1626.
CA 02815847 2013-05-13
42
Rothnie, H. M. Plant mRNA 3E-end formation. Plant Mol. Bio/.1996, 32, 43-61.
Samac, D. A., Hironaka, C. M., Yallaly, P. E., and Shah, D. M. (1990).
Isolation
and Characterization of the Genes Encoding Basic and Acidic Chitinase in
Arabidopsis thaliana. Plant Physiol 93, 907-914.
Saxena P, Hsieh YC, Alvarado VY, Sainsbury F, Saunders K, Lomonossoff GP
and Scholthof HB (2011) Improved foreign gene expression in plants using
a virus-encoded suppressor of RNA silencing modified to be
developmentally harmless. Plant Biotechnol J 9:703-712.
Schiestl M, Stangler T, ToreIla C, Cepeljnik T, Toll H and Grau R (2011)
Acceptable changes in quality attributes of glycosylated
biopharmaceuticals. Nature Biotechnology 29:310-312.
Scholthof HB (2006) The Tombusvirus-encoded P19: from irrelevance to
elegance. Nat Rev Microbiol 4:405-411.
Scholthof HB, Desvoyes B, Kuecker J and Whitehead E (1999) Biological activity
of two tombusvirus proteins translated from nested genes is influenced by
dosage control via context-dependent leaky scanning. Mol Plant Microbe In
12:670-679.
Scholthof KBG, Scholthof HB and Jackson AO (1995) The Effect of Defective
Interfering Rnas on the Accumulation of Tomato Bushy Stunt Virus Proteins
and Implications for Disease Attenuation. Virology 211:324-328.
Shapiro, M. B.; Senapathy, P. RNA splice junctions of different classes of
eukaryotes: sequence statistics and functional implications in gene
expression. Nucleic Acids Res. 1987, 5, 7155-7174.
Siddiqui SA, Sarmiento C, Truve E, Lehto H and Lehto K (2008) Phenotypes and
functional effects caused by various viral RNA silencing suppressors in
transgenic Nicotiana benthamiana and N. tabacum. Mol Plant Microbe
Interact 21:178-187.
Sourrouille C, Marquet-Blouin E, D'Aoust MA, Kiefer-Meyer MC, Seveno M,
Pagny-Salehabadi S, Bardor M, Durambur G, Lerouge P, Vezina L and
Gomord V (2008) Down-regulated expression of plant-specific
glycoepitopes in alfalfa. Plant Biotechnol J 6:702-721.
Stadlmann J, Pabst M, Kolarich D, Kunert R and Altmann F (2008) Analysis of
immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and
oligosaccharides. Proteomics 8:2858-2871.
CA 02815847 2013-05-13
43
Stevens LH, Stoopen GM, Elbers IJ, Molthoff JW, Bakker HA, Lommen A, Bosch
D and Jordi W (2000) Effect of climate conditions and plant developmental
stage on the stability of antibodies expressed in transgenic tobacco. Plant
Physiol 124:173-182.
Strasser R, Stadlmann J, Schahs M, Stiegler G, Quendler H, Mach L, Gloss! J,
Weterings K, Pabst M and Steinkeliner H (2008) Generation of glyco-
engineered Nicotiana benthamiana for the production of monoclonal
antibodies with a homogeneous human-like N-glycan structure. Plant
Biotechnol J 6:392-402.
Vezina LP, Faye L, Lerouge P, D'Aoust MA, Marquet-Blouin E, Burel C, Lavoie
PO, Bardor M and Gomord V (2009) Transient co-expression for fast and
high-yield production of antibodies with human-like N-glycans in plants.
Plant Biotechnol J 7:442-455.
Voinnet 0, Pinto YM and Baulcombe DC (1999) Suppression of gene silencing: a
general strategy used by diverse DNA and RNA viruses of plants. Proc Natl
Acad Sci U S A 96:14147-14152.
Voinnet 0, Rivas S, Mestre P and Baulcombe D (2003) An enhanced transient
expression system in plants based on suppression of gene silencing by the
p19 protein of tomato bushy stunt virus. Plant J 33:949-956.
Weber, A., H. Butterweck, U. Mais-Paul, W. Teschner, L. Lei, E.M. Muchitsch,
D.
Kolarich, F. Altmann, H.J. Ehrlich, and H.P. Schwarz. 2010. Biochemical,
molecular and preclinical characterization of a double-virus-reduced human
butyrylcholinesterase preparation designed for clinical use. Vox Sang
100:285-97.
Yu D., McLean M. D., Hall J. C. and Ghosh R. (2008) Purification of a human
immunoglobulin G1 monoclonal antibody from transgenic tobacco using
membrane chromatographic processes. J Chromatogr A 1187, 128-37.
Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt A, Ngo L,
Steinkellner H, Whaley KJ and Olinger GG (2011) Enhanced potency of a
fucose-free monoclonal antibody being developed as an Ebola virus
immunoprotectant. Proc Natl Acad Sci U S A 108:20690-20694.
Zheng N, Xia R, Yang C, Yin B, Li Y, Duan C, Liang L, Guo H and Xie Q (2009)
Boosted expression of the SARS-CoV nucleocapsid protein in tobacco and
its immunogenicity in mice. Vaccine 27:5001-5007.