Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
STABLE DUAL VARIABLE DOMAIN IMMUNOGLOBULIN PROTEIN
FORMULATIONS
RELATED APPLICATIONS
This application claims the benefit of priority to US Provisional Appin. No.
61/721364, filed on November 1, 2012. This application also claims the benefit
of priority
to US Provisional Appin. No. 61/794231, filed on March 15, 2013. The contents
of both the
priority applications are hereby incorporated by reference.
BACKGROUND OF THE INVENTION
A basic principle of pharmaceutical protein formulations is that certain
instabilities,
e.g., chemical instability and physical instability, must be overcome.
Chemical instabilities
often lead to the modification of the protein through bond formation or
cleavage. Examples
of problems associated with chemical instability include deamidation,
racemization,
hydrolysis, oxidation, beta elimination and disulfide exchange. While physical
instabilities
do not lead to covalent changes in proteins, they are just as problematic and
difficult to
overcome. Physical instabilities involve changes in the higher order structure
(secondary and
above) of proteins, which can result in denaturation, adsorption to surfaces,
aggregation,
and/or precipitation (Manning et al. (1989) Pharm. Res. 6:903). For
therapeutic proteins,
chemical and physical instabilities can create significant challenges in
formulating the protein
for delivery to a patient. Aggregation is often considered the most common
type of physical
instability. For example, exposure to hydrophobic interfaces fosters physical
instability by
alignment of protein molecules at the interface, unfolding the protein and
maximizing
exposure of hydrophobic residues to air, and initiating aggregation.
Highly concentrated protein formulations, especially those in liquid form, are
often
desirable for therapeutic purposes since they allow for dosages with smaller
volumes, and
provide for the possibility of subcutaneous delivery. The development of high
protein
concentration formulations, however, presents many challenges. For example, a
high protein
concentration often results in increased protein aggregation, insolubility and
degradation (for
review, see Shire et al. (2004) J. Pharm. Sci. 93:1390).
1
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
To date, the majority of approved therapeutic proteins are antibodies. The
development of commercially viable antibody pharmaceutical formulations has
not, however,
been straightforward despite the fact that antibodies generally have the same
structure (see
Wang et al. (2007) J. Pharm. Sci. 96:1). Concentration dependent aggregation
is considered
one of the greatest challenges in formulating antibodies (see Shire et al.
(2004) J. Pharm. Sci.
93:1390).
Dual Variable Domain Immunoglobulin (DVD-Ig) proteins are multivalent binding
proteins that are engineered to combine the function and specificity of two
monoclonal
antibodies into one molecular entity (See Wu et al., US Patent No. 7,612,181).
Given the
multivalent nature of DVD-Ig proteins, these molecules hold tremendous promise
as
therapeutics. However, DVD-Ig proteins present a new formulation challenge
given their
much larger size (approximately 200 kDa) and complexity, compared to
antibodies.
SUMMARY OF THE INVENTION
While the majority of Dual Variable Domain Immunoglobulin (DVD-Ig) proteins
are prone to destabilization (e.g., aggregation) in aqueous formulations, a
subset of DVD-Ig
proteins can be stably formulated. Some DVD-Ig proteins in the aqueous state
suffer from
certain problems, such as aggregation and/or fragmentation of the DVD-Ig
protein monomer.
Unpredictably, a subset of DVD-Ig proteins can be stably formulated in the
aqueous state
even at high concentrations. Such stable DVD-Ig proteins are referred to
herein as Aqueous
Stable Dual Variable Domain Immunoglobulin proteins or "AS-DVD-Ig" proteins.
In certain embodiments, the disclosure provides stable aqueous formulations
comprising AS-DVD-Ig proteins, including high concentration AS-DVD-Ig
formulations.
AS-DVD-Ig proteins are a subpopulation of DVD-Ig proteins characterized by
their ability to
remain stable, e.g., in concentrations 50 mg/ml or greater, during storage
(e.g., exhibit an
aggregation increase of less than 3 % (determined by size exclusion
chromatography (SEC))
following accelerated storage (40 C) in an aqueous formulation at a
concentration of at least
50 mg/ml). In certain embodiments, the AS-DVD-Ig protein is characterized as a
DVD-Ig
protein having less than about 15% loss in relative percentage monomers as
determined by size
exclusion chromatography (SEC) when formulated in a histidine or citrate
phosphate buffer at a
concentration of at least about 50 mg/ml, following 14 days storage at about
40 C. In certain
embodiments, the AS-DVD-Ig protein is characterized as a DVD-Ig protein having
less than about
2
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
10% loss in relative percentage monomers as determined by SEC when formulated
in a histidine or
citrate phosphate buffer at a concentration of at least about 50 mg/ml,
following 14 days storage at
about 40 C. In certain embodiments, the AS-DVD-Ig protein is characterized as
a DVD-Ig
protein having less than about 5% loss in relative percentage monomers as
determined by SEC when
formulated in a histidine or citrate phosphate buffer at a concentration of at
least about 50 mg/ml,
following 14 days storage at about 40 C. In certain embodiments, an AS-DVD-Ig
protein is
characterized as having less than 10% aggregation as determined by SEC when
formulated in
a citrate phosphate buffer at a concentration of at least 50 mg/ml following
14 days of storage
at 40 C. In certain embodiments, the AS-DVD-Ig protein is characterized as
having 6% or
less aggregation as determined by SEC following 14 days of storage at 40 C,
wherein the AS-
DVD-Ig protein at a concentration of at least 50 mg/ml is stored in a citrate
phosphate buffer
or a histidine buffer. In certain embodiments, the AS-DVD-Ig protein is
characterized as
having 5% or less aggregation as determined by SEC following 14 days of
storage at 40 C,
wherein the AS-DVD-Ig protein at a concentration of at least 50 mg/ml is
stored in a citrate
phosphate buffer or a histidine buffer. In certain embodiments, the AS-DVD-Ig
protein is
characterized as having 4% or less aggregation as determined by SEC following
14 days of
storage at 40 C, wherein the AS-DVD-Ig protein at a concentration of at least
50 mg/ml is
stored in a citrate phosphate buffer or a histidine buffer. In certain
embodiments, the AS-
DVD-Ig protein is characterized as having 3% or less aggregation as determined
by SEC
following 14 days of storage at 40 C, wherein the AS-DVD-Ig protein at a
concentration of at
least 50 mg/ml is stored in a citrate phosphate buffer or a histidine buffer.
In certain
embodiments, the AS-DVD-Ig protein is characterized as having 2% or less
aggregation as
determined by SEC following 14 days of storage at 40 C, wherein the AS-DVD-Ig
protein at
a concentration of at least 50 mg/ml is stored in a citrate phosphate buffer
or a histidine
buffer. In certain embodiments, the AS-DVD-Ig protein is characterized as
having 1% or less
aggregation as determined by SEC following 14 days of storage at 40 C, wherein
the AS-
DVD-Ig protein at a concentration of at least 50 mg/ml is stored in a citrate
phosphate buffer
or a histidine buffer. In certain embodiments, the AS-DVD-Ig protein is
characterized as a DVD-Ig
protein having about a 10% relative (rel.) peak area or less change in
monomers at about 40 C after
21 days of storage at a concentration of about 100 mg/ml in an aqueous
formulation at a pH between
about 5.5 to about 6.5. In other embodiments, the AS-DVD-Ig protein is
characterized as a DVD-Ig
protein having about a 1% rel. peak area or less change in monomers at about 5
C after 21 days of
storage at a concentration of about 100 mg/ml at a pH between about 5.5 to
about 6.5 in an aqueous
3
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
formulation. Examples of AS-DVD-Ig proteins that may be included in the
formulations of
the disclosure include, but are not limited to, an AS-DVD-Ig protein having
binding
specificity for IL4 and IL13; ILla and ILlp; TNFcc and IL17; and DLL4 and
VEGF.
In certain embodiments, the formulation comprises less than about 10%
aggregate
AS-DVD-Ig protein. In certain embodiments, the formulation comprises less than
about 9%
aggregate AS-DVD-Ig protein. In certain embodiments, the formulation comprises
less than
about 8% aggregate AS-DVD-Ig protein. In certain embodiments, the formulation
comprises less than about 7% aggregate AS-DVD-Ig protein. In certain
embodiments, the
formulation comprises less than about 6% aggregate AS-DVD-Ig protein. In
certain
embodiments, the formulation comprises less than about 5% aggregate AS-DVD-Ig
protein.
In certain embodiments, the formulation comprises less than about 4% aggregate
AS-DVD-Ig
protein. In a further embodiment, the formulation comprises less than about 3%
aggregate
AS-DVD-Ig protein. Aggregation may be determined by SEC analysis.
In certain embodiments, the AS-DVD-Ig protein is characterized as a DVD-Ig
protein
having a about 10% relative (rel.) peak area or less change in monomers at
about 40 C after
21 days of storage at a concentration of about 100 mg/ml in an aqueous
formulation at a pH
between about 5.0 to about 6.5, e.g., about 5.5 to about 6Ø In certain
embodiments, the AS-
DVD-Ig protein is characterized as a DVD-Ig protein having a about 1% rel.
peak area or less
change in monomers at about 5 C after 21 days of storage at a concentration
of about 100
mg/ml at a pH between about 5.0 to about 6.5, e.g., about 5.5 to about 6.0, in
an aqueous
formulation.
In certain embodiments, the disclosure provides an aqueous formulation
comprising
an AS-DVD-Ig protein and a buffer. For example, the disclosure provides an
aqueous
formulation comprising an AS-DVD-Ig protein and a buffer having a molarity of
about 5 to
about 50 mM, wherein the formulation has a pH of about 4.5 to about 7.5, e.g.,
a pH of about
to about 6.5. In certain embodiments, the AS-DVD-Ig protein is characterized
as a DVD-Ig
protein having less than about 15% loss in relative percentage monomers as
determined by size
exclusion chromatography (SEC) when formulated in a histidine or citrate
phosphate buffer at a
concentration of at least about 50 mg/ml, following 14 days storage at about
40 degrees C. In certain
embodiments, the AS-DVD-Ig protein is characterized as a DVD-Ig protein having
less than about
10% loss in relative percentage monomers as determined by SEC when formulated
in a histidine or
citrate phosphate buffer at a concentration of at least about 50 mg/ml,
following 14 days storage at
4
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
about 40 degrees C. In certain embodiments, the AS-DVD-Ig protein is
characterized as a DVD-
Ig protein having less than about 5% loss in relative percentage monomers as
determined by SEC
when formulated in a histidine or citrate phosphate buffer at a concentration
of at least about 50
mg/ml, following 14 days storage at about 40 degrees C. In certain
embodiments, the formulation
comprises about 6% or less aggregation as determined by SEC analysis. In other
embodiment, the
formulation comprises about 5% or less aggregation as determined by SEC
analysis. In certain
embodiments, the AS-DVD-Ig protein is characterized as a DVD-Ig protein having
about a 10%
relative (rel.) peak area or less change in monomers at about 40 C after 21
days of storage at a
concentration of about 100 mg/ml in an aqueous formulation at a pH between
about 5.5 to about 6.5.
In other embodiments, the AS-DVD-Ig protein is characterized as a DVD-Ig
protein having about a
1% rel. peak area or less change in monomers at about 5 C after 21 days of
storage at a concentration
of about 100 mg/ml at a pH between about 5.5 to about 6.5 in an aqueous
formulation. In certain
embodiments, the formulation comprises about 1 to about 250 mg/ml, about 1 to
200 mg/ml,
about 10 to about 230 mg/ml, about 20 to about 210 mg/ml, about 30 to about
190 mg/ml,
about 40 to about 170 mg/ml, about 50 to about 150 mg/ml, about 60 to about
130 mg/ml,
about 70 to about 110 mg/ml, or about 80 to about 105 mg/ml of the AS-DVD-Ig
protein. In
certain embodiments, the buffer used in the formulation is acetate, histidine,
glycine, arginine,
phosphate, citrate, or citrate / phosphate buffer. In certain embodiments, the
molarity of the buffer
ranges from 5 to 50 mM, e.g., 10 to 20 mM.
In certain embodiments, the disclosure provides an aqueous formulation
comprising
an AS-DVD-Ig protein, a buffer and a surfactant. For example, the disclosure
provides an
aqueous formulation comprising an AS-DVD-Ig protein, a buffer having a
molarity of about
to about 50 mM, and a surfactant, wherein the formulation has a pH of about
4.5 to about
7.5. In certain embodiments, the formulation comprises about 1 to about 250
mg/ml, about
to about 230 mg/ml, about 20 to about 210 mg/ml, about 30 to about 190 mg/ml,
about 40
to about 170 mg/ml, about 50 to about 150 mg/ml, about 60 to about 130 mg/ml,
about 70 to
about 110 mg/ml, or about 80 to about 105 mg/ml of the AS-DVD-Ig protein. In
certain
embodiments, the surfactant is a polysorbate, e.g., polysorbate 80 or
polysorbate 20. In
certain embodiments, the concentration of polysorbate in the formulation is
about 0.001% to
about 1%, about 0.005% to about 0.05%, about 0.01% to about 0.05%, or about
0.1%. In
certain embodiments, the concentration of polysorbate 80 or polysorbate 20 in
the
formulation is about 0.005% to about 0.02%. In certain embodiments, the buffer
used in the
5
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
formulation is acetate, histidine, glycine, arginine, phosphate, citrate, or
citrate / phosphate buffer. In
certain embodiments, the molarity of the buffer ranges from 5 to 50 mM, e.g.,
10 to 20 mM.
In certain embodiments, the disclosure includes an aqueous formulation
comprising
an AS-DVD-Ig protein, a buffer, and a polyol. For example, the disclosure
provides an
aqueous formulation comprising an AS-DVD-Ig protein, a buffer having a
molarity of about
to about 50 mM, and a polyol, wherein the formulation has a pH of about 4.5 to
about 7.5.
In certain embodiments, the formulation comprises about 1 to about 250 mg/ml,
about 10 to
about 230 mg/ml, about 20 to about 210 mg/ml, about 30 to about 190 mg/ml,
about 40 to
about 170 mg/ml, about 50 to about 150 mg/ml, about 60 to about 130 mg/ml,
about 70 to
about 110 mg/ml, or about 80 to about 105 mg/ml of the AS-DVD-Ig protein. In
certain
embodiments, the polyol is sorbitol. In certain embodiments, the concentration
of sorbitol in
the formulation is about 20 to about 60 mg/ml sorbitol, about 25 to about 55
mg/ml, about 30
to about 50 mg/ml, or about 35 to about 45 mg/ml. In certain embodiments, the
polyol is
sucrose. In certain embodiments, the concentration of sucrose in the
formulation is about 60
to about 100 mg/ml, about 65 to about 95 mg/ml, about 70 to about 90 mg/ml, or
about 75 to
about 85 mg/ml. In certain embodiments, the polyol is mannitol. In certain
embodiments,
the concentration of mannitol in the formulation is about 10 to about 100
mg/ml, or about 20
to about 80, about 20 to about 70, about 30 to about 60, or about 30 to about
50 mg/ml. In
certain embodiments, the buffer used in the formulation is acetate, histidine,
glycine, arginine,
phosphate, citrate, or citrate / phosphate buffer. In certain embodiments, the
molarity of the buffer
ranges from 5 to 50 mM, e.g., 10 to 20 mM.
In certain embodiments, the disclosure provides an aqueous formulation
comprising
an AS-DVD-Ig protein, a buffer, a polyol, and a surfactant. For example, the
disclosure
provides an aqueous formulation comprising an AS-DVD-Ig protein, a buffer
having a
molarity of about 5 to about 50 mM, a surfactant, and a polyol, wherein the
formulation has a
pH of about 4.5 to about 7.5. In certain embodiments, the formulation
comprises about 1 to
about 250 mg/ml, about 10 to about 230 mg/ml, about 20 to about 210 mg/ml,
about 30 to
about 190 mg/ml, about 40 to about 170 mg/ml, about 50 to about 150 mg/ml,
about 60 to
about 130 mg/ml, about 70 to about 110 mg/ml, or about 80 to about 105 mg/ml
of the AS-
DVD-Ig. In certain embodiments, the polyol is sorbitol. In certain embodiment,
the sorbitol
concentration in the formulation is about 20 to about 60 mg/ml, about 25 to
about 55 mg/ml,
about 30 to about 50 mg/ml, or about 35 to about 45 mg/ml. In certain
embodiments, the
6
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
polyol is sucrose. In certain embodiments, the concentration of sucrose in the
formulation is
about 60 to about 100 mg/ml, about 65 to about 95 mg/ml, about 70 to about 90
mg/ml, or
about 75 to about 85 mg/ml. In certain embodiments, the polyol is mannitol. In
certain
embodiments, the concentration of mannitol in the formulation is about 10 to
about 100
mg/ml, or about 20 to about 80, about 20 to about 70, about 30 to about 60, or
about 30 to
about 50 mg/ml. In certain embodiments, the surfactant is a polysorbate, e.g.,
polysorbate 80
or polysorbate 20. In certain embodiments, the concentration of polysorbate in
the
formulation is about 0.001% to about 1%, about 0.005% to about 0.05%, about
0.01% to
about 0.05%, or about 0.1%. In certain embodiments, the concentration of
polysorbate 80 or
polysorbate 20 in the formulation is about 0.005% to about 0.02%. In certain
embodiments, the
buffer used in the formulation is acetate, histidine, glycine, arginine,
phosphate, citrate, or citrate /
phosphate buffer. In certain embodiments, the molarity of the buffer ranges
from 5 to 50 mM, e.g., 10
to 20 mM.
In certain embodiment, the disclosure provides a formulation comprising an AS-
DVD-Ig
protein, a polyol (e.g., sorbitol, mannitol, or sucrose), a buffer (e.g.,
acetate, histidine, glycine,
arginine, phosphate, citrate, or citrate / phosphate), and a surfactant (e.g.,
a polysorbate), wherein said
formulation has a pH of about 5 to about 7, and wherein the AS-DVD-Ig protein
is characterized as a
DVD-Ig protein having less than about 15% loss in relative percentage monomers
as determined by
SEC when formulated in a histidine or citrate phosphate buffer at a
concentration of about 60 mg/ml
following 14 days storage at about 40 degrees C. In certain embodiments, the
polysorbate is
polysorbate 80 or polysorbate 20. In certain embodiments, the concentration of
polysorbate 80 or
polysorbate 20 is about 0.005% to about 0.02%.
In certain embodiments, the disclosure provides a formulation comprising an AS-
DVD-Ig protein, a polyol, histidine buffer, and a polysorbate, wherein said
formulation has a
pH of about 5 to about 7, and wherein the AS-DVD-Ig protein is characterized
as having 6%
aggregation or less as determined by SEC, where the AS-DVD-Ig protein is
formulated in a
citrate phosphate buffer or histidine buffer at a concentration of at least 60
mg/ml, following
14 days storage at 40 C.
In certain embodiments, the disclosure provides a formulation comprising an AS-
DVD-Ig protein and a buffer having a molarity of about 5 to about 50 mM,
wherein the AS-
DVD-Ig protein is characterized as a DVD-Ig protein having less than about 10%
loss in
relative percentage monomers as determined by SEC when formulated in a
histidine or citrate
phosphate buffer at a concentration of about 60 mg/ml, following 14 days
storage at about 40
7
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
degrees C, and the formulation has a pH of 4.5 to 7.5. In certain embodiments,
the
formulation further comprises a surfactant, a polyol, or combinations thereof.
The disclosure is also based, in part, on the surprising discovery that while
the
majority of DVD-Ig Tm proteins are prone to destabilization in a lyophilized
state, a subset of
DVD-Ig proteins are able to be stably formulated in a lyophilized form. Such
stable DVD-Ig
proteins are referred to herein as Lyophilized Stable Dual Variable Domain
Immunoglobulin
proteins or "LS-DVD-Ig" proteins. Formulations wherein the DVD-Ig protein was
in the
lyophilized state suffered from certain problems such as aggregation and/or
fragmentation of
the DVD-Ig protein monomer. Unpredictably, a subset of DVD-Ig proteins can be
stably
formulated in the lyophilized state even at high concentrations.
In certain embodiments, the disclosure is a lyophilized formulation comprising
a
Lyophilized-Stable DVD Immunoglobulin (LS-DVD-Ig) protein, wherein when said
formulation is reconstituted said formulation comprises about 1 to about 100
mg/ml of the
LS-DVD-Ig protein, about 10 to about 50 mM of a buffer, a polyol, about 0.01
to about 0.2
mg/ml of a polysorbate, and has a pH of about 5 to about 7, e.g., about 5.5 to
about 6.5. In
certain embodiments, the LS-DVD-Ig protein has more than 10% rel. peak area
change in
monomers observed, following accelerated storage at a pH between about 5.5 and
about 6.5
in an aqueous formulation for 21 days at about 40 C, when formulated at a
concentration
over about 100 mg/ml.
In certain embodiments, the LS-DVD-Ig protein has more than about 10% rel.
peak
area change in monomers observed, following accelerated storage at a pH
between about 5.0
to about 6.5, e.g., about 5.5 to about 6.0, in an aqueous formulation for 21
days at about 40
C, when formulated at a concentration of about 100 mg/ml or more.
In certain embodiments, the disclosure provides a lyophilized formulation
prepared by
lyophilizing an aqueous formulation comprising a buffer have a molarity (e.g.,
histidine,
succinate or citrate and/or phosphate) of about 5 to about 50 mM, a
surfactant, and a polyol
(e.g., mannitol, sorbitol, sucrose, or trehalose), wherein the formulation has
a pH of about 4.5
to about 7.5, e.g., about 5.5 to about 6.5.
In certain embodiments, the disclosure provides LS-DVD-Ig proteins or AS-DVD-
Ig
proteins that are stable in formulations having a pH of about 4.5 to about
7.5. In certain
embodiments, the formulation has a pH of 5 to 6.5. In certain embodiments, the
formulation
8
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
has a pH of about 5.7 to about 6.3. In certain embodiments, the formulation
the formulation
of the disclosure has a pH of about 5.5 to about 6.5. In certain embodiments,
the formulation
of the disclosure has a pH of 5.8 to about 6.2, or a pH of 6.
In certain embodiments, the disclosure provides a formulation comprising an AS-
DVD-Ig protein or an LS-DVD-Ig protein, a polyol, histidine buffer, and a
polysorbate,
wherein said formulation has a pH of about 5 to about 7, and wherein the AS-
DVD-Ig protein
or an LS-DVD-Ig protein is characterized as having 15% aggregation or less as
determined
by SEC, where the AS-DVD-Ig protein or an LS-DVD-Ig protein is formulated in a
citrate
phosphate buffer or histidine buffer at a concentration of at least 60 mg/ml,
following 14 days
storage at 40 C. Such formulations may be either in a lyophilized or an
aqueous state, as AS-
DVD-Ig protein or an LS-DVD-Ig protein identified as having 15% aggregation or
less as
determined by SEC, where the AS-DVD-Ig protein or an LS-DVD-Ig protein is
formulated in
a citrate phosphate buffer or a histidine buffer at a concentration of at
least 60 mg/ml,
following 14 days storage at 40 C.
One advantage of the compositions of the disclosure is that the AS-DVD-Ig
protein or
LS-DVD-Ig protein can be stably formulated in liquid form at a high
concentration. In
certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein has a
concentration of
about 1 to about 200 mg/ml. In certain embodiments, the AS-DVD-Ig protein or
LS-DVD-Ig
protein has a concentration of about 20 to about 100 mg/ml. In certain
embodiments, the
formulation comprises about 1 to about 250 mg/ml, about 10 to about 230 mg/ml,
about 20 to
about 210 mg/ml, about 30 to about 190 mg/ml, about 40 to about 170 mg/ml,
about 50 to
about 150 mg/ml, about 60 to about 130 mg/ml, about 70 to about 110 mg/ml, or
about 80 to
about 105 mg/ml of the AS-DVD-Ig protein or LS-DVD-Ig.
Examples of buffers that may be used in the formulations of the disclosure
include,
but are not limited to, acetate, histidine, glycine, arginine, phosphate, and
citrate. In certain
embodiments, the molarity of the buffer in the formulation is about 5 to about
50 mM. In
certain embodiments, the buffer molarity is about 10 mM to about 20 mM.
Examples of polyols that may be used in the formulations of the disclosure
include,
but are not limited to, sorbitol, mannitol, and sucrose. In certain
embodiments, the polyol is
sorbitol. In certain embodiments, about 30 to about 50 mg/ml of sorbitol is
used in the
formulation. In certain embodiments, the polyol is sucrose. In certain
embodiments, about
9
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
70 to about 90 mg/ml of sucrose is used in the formulation. In a further
embodiment, the
polyol is mannitol. In certain embodiments, about 30 to about 50 mg/ml of
mannitol is used
in the formulation.
Examples of surfactants that may be used in the formulations of the disclosure
include, but are not limited to, polysorbates and poloxamers. In certain
embodiments, the
surfactant is a polysorbate, examples of which are polysorbate 80 and
polysorbate 20. Other
examples include poloxamer Pluronic F-68, albumin, lecithin, and
cyclodextrins. In certain
embodiments, the polysorbate has a concentration of about 0.05 mg/ml to about
2mg/ml. In a
further embodiment, the polysorbate has a concentration of about 0.01 to about
0.2 mg/ml.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, each independently comprising VD1-(X1)n-
VD2-C-
(X2)n, wherein VD1 is a first variable domain; VD2 is a second variable
domain; C is a
constant domain; X1 is a linker with the proviso that it is not CH1; X2 is an
Fc region; n is 0
or 1, wherein the VD1 domains on the first and second polypeptide chains form
a first
functional target binding site and the VD2 domains on the first and second
polypeptide chains
form a second functional target binding site. In a further embodiment, the
first polypeptide
chain comprises a first VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first heavy
chain
variable domain; VD2 is a second heavy chain variable domain; C is a heavy
chain constant
domain; X1 is a linker with the proviso that it is not CH1; X2 is an Fc
region; n is 0 or 1, and
wherein the second polypeptide chain comprises a second VD1-(X1)n-VD2-C-(X2)n,
wherein VD1 is a first light chain variable domain; VD2 is a second light
chain variable
domain; C is a light chain constant domain; X1 is a linker with the proviso
that it is not CH1;
X2 does not comprise an Fc region; n is 0 or 1, wherein the VD1 domains on the
first and
second polypeptide chains form a first functional target binding site and the
VD2 domains on
the first and second polypeptide chains form a second functional target
binding site. In
certain embodiments, the two first polypeptide chains and two second
polypeptide chains,
wherein the binding protein comprises four functional target binding sites. In
certain
embodiments, X1 is not CL.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises a
polypeptide chain comprising VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a first
variable
domain, VD2 is a second variable domain, C is a constant domain, X1 represents
an amino
acid or polypeptide, X2 represents an Fc region and n is 0 or 1.
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein used in the
compositions and methods of the disclosure comprises four polypeptide chains,
wherein two
polypeptide chains comprise VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first
heavy chain
variable domain, VD2 is a second heavy chain variable domain, C is a heavy
chain constant
domain, X1 is a linker with the proviso that it is not CH1, and X2 is an Fc
region; and two
polypeptide chains comprise VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first
light chain
variable domain, VD2 is a second light chain variable domain, C is a light
chain constant
domain, X1 is a linker with the proviso that it is not CH1, and X2 does not
comprise an Fc
region; and n is 0 or 1; and wherein said four polypeptide chains of said
binding protein form
four functional antigen binding sites.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises a
polypeptide chain wherein the polypeptide chain comprises VD1-(X1)n-VD2-C-
(X2)n,
wherein VD1 is a first heavy chain variable domain; VD2 is a second heavy
chain variable
domain; C is a heavy chain constant domain; X1 is a linker with the proviso
that it is not
CH1; X2 is an Fc region; and n is 0 or 1. In certain embodiments, the AS-DVD-
Ig protein or
LS-DVD-Ig protein comprises a polypeptide chain, wherein the polypeptide chain
comprises
VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first light chain variable domain; VD2
is a
second light chain variable domain; C is a light chain constant domain; X1 is
a linker with the
proviso that it is not a CH1 or CL; X2 does not comprise an Fc region; and n
is 0 or 1. In a
further embodiment, (Xl)n on the heavy and/or light chain is (X1)0 and/or
(X2)n on the
heavy and/or light chain is (X2)0.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, wherein the first polypeptide chain
comprises a first
VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first heavy chain variable domain; VD2
is a
second heavy chain variable domain; C is a heavy chain constant domain; X1 is
a first linker
with the proviso that it is not CH2; X2 is an Fc region; n is 0 or 1; and
wherein the second
polypeptide chain comprises a second VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a
first light
chain variable domain; VD2 is a second light chain variable domain; C is a
light chain
constant domain; X1 is a second linker with the proviso that it is not CH1 or
CL; X2 does
not comprise an Fc region; and n is 0 or 1.
11
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the VD1 of the first polypeptide chain and the VD1 of
the second
polypeptide chain are from different first and second parent antibodies,
respectively, or
binding portions thereof. In certain embodiments, the VD2 of the first
polypeptide chain and
the VD2 of the second polypeptide chain are from different first and second
parent
antibodies, respectively, or binding portions thereof. In certain embodiments,
the first and
the second parent antibodies bind different epitopes on the same target or
different targets. In
certain embodiments, the first parent antibody or binding portion thereof
binds the first target
with a potency that is different from the potency with which the second parent
antibody or
binding portion thereof binds the second target. In certain embodiments, the
first parent
antibody or binding portion thereof binds the first target with an affinity
different from the
affinity with which the second parent antibody or binding portion thereof
binds the second
target.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprise,
two
first polypeptide chains and two second polypeptide chains.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, each independently comprising VD1-(X1)n-
VD2-C-
(X2)n, wherein VD1 is a first variable domain; VD2 is a second variable
domain; C is a
constant domain; X1 is a linker with the proviso that it is not CH1; X2 is an
Fc region; n is 0
or 1, and wherein the VD1 domains on the first and second polypeptide chains
form a first
functional target binding site and the VD2 domains on the first and second
polypeptide chains
form a second functional target binding site. IN a further embodiment, the
first polypeptide
chain comprises a first VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first heavy
chain
variable domain; VD2 is a second heavy chain variable domain; C is a heavy
chain constant
domain; X1 is a linker with the proviso that it is not CH1; X2 is an Fc
region; n is 0 or 1, and
wherein the second polypeptide chain comprises a second VD1-(X1)n-VD2-C-(X2)n,
wherein VD1 is a first light chain variable domain; VD2 is a second light
chain variable
domain; C is a light chain constant domain; X1 is a linker with the proviso
that it is not CH1;
X2 does not comprise an Fc region; n is 0 or 1, wherein the VD1 domains on the
first and
second polypeptide chains form a first functional target binding site and the
VD2 domains on
the first and second polypeptide chains form a second functional target
binding site.
In certain embodiments, the formulation of the disclosure comprises a DVD-Ig
protein comprising a heavy or light chain amino acid sequence as set forth in
Table 58 or 65.
12
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the formulation of the disclosure comprises a DVD-Ig
protein comprising a heavy or light chain variable region amino acid sequence
as set forth in
Table 58 or 65 (SEQ ID NOs: 28-75). Alternatively, the formulation of the
disclosure
comprises a DVD-Ig protein comprising CDRs as set forth in the heavy or light
chain
variable region amino acid sequences as set forth in Table 58 or 65 (SEQ ID
NOs: 28-75).
In certain embodiments, the formulation of the disclosure comprises an anti-
TNF/IL-
17 DVD-Ig protein. In certain embodiments, the anti-TNF/IL-17 DVD-Ig protein
comprises
a heavy and light chain sequences having an amino acid sequence as set forth
in SEQ ID
NOs: 62 and 63, respectively.
In certain embodiments, the formulation of the disclosure comprises an anti-
ILlcc/ILlp DVD-Ig. In certain embodiments, the anti-ILlcc/ILlp DVD-Ig protein
comprises
a heavy and light chain sequences having an amino acid sequence as set forth
in SEQ ID
NOs: 66 and 67, respectively.
In certain embodiments, the DVD-Ig protein used in the formulation of the
disclosure
binds one of the following target combinations (in either target order):
CD20/CD80,
VEGF/Her2, TNF/RANKL, TNF/DKK, CD2O/RANKL, DLL4/PLGF, TNF/SOST (S2), IL-
9(52)/IgE, IL-12/IL-18, TNF/IL-17, TNF/PGE2, ILlcc/ILlp, or DLL4/VEGF.
In certain embodiments, the formulation of the disclosure is a pharmaceutical
formulation, including a pharmaceutical aqueous formulation or a
pharmaceutical lyophilized
formulation.
Also included in the disclosure are methods of making and using AS-DVD-Ig
protein
or LS-DVD-Ig protein formulations.
In certain embodiments, the formulations of the disclosure are used for
treating a
disorder in a subject.
A further embodiment of the disclosure is a method of identifying either an AS-
DVD-
Ig protein or an LS-DVD-Ig protein. Such methods include aggregation testing
(e.g., by SEC
analysis) following accelerated storage (e.g., 14 days at about 40 degrees C)
of a liquid
formulation comprising the DVD-Ig protein, a citrate/phosphate buffer, and a
high
concentration of DVD-Ig protein (e.g., about 50 mg/ml or greater).
13
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
BRIEF DESCRIPTION OF DRAWINGS
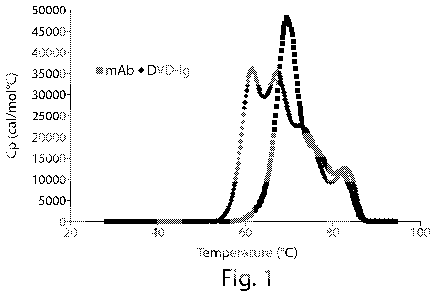
Figure 1 shows a graphic description of a comparison of DSC profiles of an
IgG1 antibody
(Briakinumab) to that of a DVD-Ig protein (TNF/PGE2; DVD-B) showing the
difference in
three vs. four domain unfolding, respectively. The sample composition of the
DVD-Ig
solution used was 1 mg/ml DVD-Ig protein, 1 mM ionic strength Histidine pH 6,
1 C/minute
scan rate.
Figure 2A shows a graphic description of serum stability of various DVD-Ig
proteins.
Figure 2B shows the domain orientation concept for different variable domain
combinations.
Figure 3 shows a graphic description of the correlation between
pharmacokinetic parameters
of different DVD-Ig proteins and high molecular weight (HMW) aggregate
formation.
Figure 4 provides a graphic description of a thermodynamic comparison of 14
monoclonal
antibodies and 16 DVD-Ig proteins showing the melting temperatures of the
molecules
relative to the number of molecules (y axis) for each temperature. The mean
onset melting
temperature for the antibodies was 59.2 C + 3.4 C. The mean onset melting
temperature for
the DVD-Ig proteins was 53.6 C + 3.6 C.
Figure 5 graphically depicts the molar ellipticity in the DVD-Ig proteins as
measured using
near UV-CD scans between 250-320 nm wavelengths.
DETAILED DESCRIPTION
I. Definitions
The term "multivalent binding protein" is used to denote a binding protein
comprising
two or more target binding sites. The multivalent binding protein may be
engineered to have
the three or more antigen binding sites, and is generally not a naturally
occurring antibody.
The term "multispecific binding protein" refers to a binding protein capable
of
binding two or more related or unrelated targets. An example of a multivalent
binding
protein is a Dual Variable Domain (DVD) binding protein, such as a DVD-Ig. In
certain
embodiments, DVD binding proteins comprise two or more antigen binding sites
and are
tetravalent or multivalent binding proteins. DVDs may be monospecific, i.e.,
capable of
binding one target, or multispecific, i.e., capable of binding two or more
targets.
The term "Dual Variable Domain Immunoglobulin" or "DVD-Ig" or "DVD-Ig
14
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
protein" refers to a DVD binding protein comprising two heavy chain DVD
polypeptides and
two light chain DVD polypeptides. Each half of a DVD-Ig comprises a heavy
chain DVD
polypeptide and a light chain DVD polypeptide, and two target binding sites.
Each binding
site comprises a heavy chain variable domain and a light chain variable domain
with a total of
6 CDRs involved in target binding. Each variable domain (VD) in a DVD-Ig
protein may be
obtained from one or more "parent" monoclonal antibodies (mAbs) that bind one
or more
desired antigens or epitopes. In certain embodiments, the resulting DVD-Ig
molecule retains
activities of both parental mAbs. The term "DVD-Ig protein" is inclusive of
the terms AS-
DVD-Ig protein and LS-DVD-Ig protein described below.
The term "Aqueous Stable Dual Variable Domain Immunoglobulin" or "AS-DVD-Ig"
or "AS-DVD-Ig protein" refers to a subset of DVD¨Ig proteins that have low
aggregation or
a low change in monomer content due to physical degradation following
stability tests at a
given temperature and time period, e.g., 5 C or 40 C for 14 to 21 days, at a
concentration
ranging from about 1 to about 100 mg/ml (e.g., about 1-10 mg/ml or about 50-
100 mg/ml)
and at a pH between about 5.0 to about 6.5, e.g, about 5.5 to about 6Ø
Different stability
tests may be used to define an AS-DVD-Ig. In certain embodiments, an AS-DVD-Ig
protein
is defined as a DVD-Ig protein that has about 1% relative peak area or less
change in
monomers as determined by SEC analysis at about 5 C after 21 days of storage
at a
concentration of about 50 mg/ml to about 100 mg/ml and at a pH between about
5.0 to about
6.5, e.g, about 5.5 to about 6.0, in a buffered aqueous formulation. In
certain embodiments,
an AS-DVD-Ig protein has 10% relative (rel.) peak area or less change in
monomers as
determined by SEC analysis following accelerated storage for 21 days at about
40 C at a
concentration of about 50 mg/ml to about 100 mg/ml and at a pH between about
5.0 to about
6.5, e.g, about 5.5 to about 6.0, in a buffered aqueous formulation. In
certain embodiments,
stability testing for AS-DVD-Ig proteins may be performed by testing the
stability, e.g., loss
of monomers, of a solution having a DVD-Ig protein concentration of 50 to 100
mg/ml in a
citrate phosphate buffer or histidine buffer at a pH between 5.0 ¨ 6.5, e.g,
about 5.5 to about
6Ø
The term "aqueous formulation" refers to a liquid solution in which the
solvent is
water. In certain embodiments, the term "aqueous formulation" refers to a
liquid formulation
in which the solvent is water wherein the formulation was not previously
lyophilized (i.e.,
does not result from reconstitution of a lyophilized formulation).
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
The term "Lyophilized Stable Dual Variable Domain Immunoglobulin" or "LS-DVD-
Ig" or "LS-DVD-Ig protein" refers to a subset of DVD¨Ig proteins that have low
aggregation
or elevated levels of change in monomers (i.e., loss of monomers) in the
liquid state.
Different stability tests may be used to define an LS-DVD-Ig. In certain
embodiments, an
LS-DVD-Ig protein has more than 10% rel. peak area change in monomers
observed,
following accelerated storage, e.g., 21 days at about 40 C, when formulated
at a
concentration of about 50 to 100 mg/ml at a pH between about 5.0 and about
6.5, e.g, about
5.5 to about 6.0, in an aqueous formulation. DVD-Ig proteins may be tested in
aqueous
formulations containing citrate and phosphate buffer, or histidine buffer.
Change in
monomers can be determined according to methods known in the art, including,
but not
limited to, SEC. In certain embodiments, the accelerated storage conditions
include storing
the DVD-Ig protein in the absence of light at about 40 C. In certain
embodiments, an LS-
DVD-Ig protein has 50% rel. peak area or less change in monomers as determined
by SEC
analysis following accelerated storage for 14 days at about 40 C, where the
LS-DVD-Ig
protein is formulated at a concentration of at least 50 mg/ml in a citrate
phosphate buffer in
an aqueous formulation. In certain embodiments, stability testing for LS-DVD-
Ig proteins
may be performed by testing the stability, e.g., loss of monomers, of a
solution having a
DVD-Ig protein concentration of about 50 mg/ml to 100 mg/ml) in a citrate
phosphate buffer
or histidine buffer at a pH between about 5.0 ¨ about 6.5, e.g, about 5.5 to
about 6Ø
The term "pharmaceutical formulation" refers to preparations that are in such
a form
as to permit the biological activity of the active ingredients to be effective
and, therefore, may
be administered to a subject for therapeutic use. In certain embodiments, the
disclosure
provides an aqueous pharmaceutical formulation comprising an AS-DVD-Ig. In
other
embodiments, the disclosure provides a lyophilized pharmaceutical formulation
comprising
an LS-DVD-Ig.
A "stable" formulation is one in which the DVD-Ig protein therein essentially
retains
its physical stability and/or chemical stability and/or biological activity
upon storage. Various
analytical techniques for measuring protein stability are available in the art
and are reviewed
in, e.g., Peptide and Protein Drug Delivery, pp. 247-301, Vincent Lee Ed.,
Marcel Dekker,
Inc., New York, N.Y., Pubs. (1991) and Jones (1993) Adv. Drug Delivery Rev.
10: 29-90. In
certain embodiments, the stability of the DVD-Ig protein is determined
according to the
percentage of monomer protein in the solution, with a low percentage of
degraded (e.g.,
16
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
fragmented) and/or aggregated protein. For example, an aqueous formulation
comprising a
stable DVD-Ig protein may include at least 95% monomer DVD-Ig protein, e.g.,
AS-DVD-Ig
protein. Alternatively, an aqueous formulation of the disclosure may include
no more than
5% aggregate and/or degraded DVD-Ig protein, e.g., AS-DVD-Ig protein.
A DVD-Ig protein "retains its physical stability" in a pharmaceutical
formulation if it
shows substantially no signs of aggregation, precipitation and/or denaturation
upon visual
examination of color and/or clarity, or as measured by UV light scattering or
by size
exclusion chromatography. In certain embodiments of the disclosure, a stable
aqueous
formulation is a formulation having less than about 10% aggregation, and less
than about 5%
AS-DVD-Ig protein aggregation in the formulation.
A DVD-Ig protein "retains its chemical stability" in a pharmaceutical
formulation if
the chemical stability at a given time is such that the DVD-Ig protein is
considered to still
retain its biological activity as defined below. Chemical stability can be
assessed by
detecting and quantifying chemically altered forms of the DVD-Ig. Chemical
alteration may
involve size modifications (e.g., clipping) which can be evaluated using size
exclusion
chromatography, SDS-PAGE and/or matrix-assisted laser desorption ionization /
time of
flight mass spectrometry (MALDI/TOF MS), for example. Other types of chemical
alternation include charge alteration (e.g., occurring as a result of
deamidation), which can be
evaluated by, e.g., ion-exchange chromatography.
A DVD-Ig protein "retains its biological activity" in a pharmaceutical
formulation, if
the protein in a pharmaceutical formulation is biologically active for its
intended purpose.
For example, biological activity of a DVD-Ig protein is retained if the
biological activity of
the DVD-Ig protein in the pharmaceutical formulation is within about 30%,
about 20%, or
about 10% (within the errors of the assay) of the biological activity
exhibited at the time the
pharmaceutical formulation was prepared (e.g., as determined in an antigen
binding assay).
The term "surfactant", as used herein, refers to organic substances having
amphipathic structures; namely, they are composed of groups of opposing
solubility
tendencies, typically an oil-soluble hydrocarbon chain and a water-soluble
ionic group.
Surfactants can be classified, depending on the charge of the surface-active
moiety, into
anionic, cationic, and nonionic surfactants. Surfactants are often used as
wetting, emulsifying,
solubilizing, and dispersing agents for various pharmaceutical compositions
and preparations
of biological materials. Examples of suitable surfactants include, but are not
limited to,
17
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
sodium lauryl sulfate, polysorbates such as polyoxyethylene sorbitan
monooleate,
monolaurate, monopalmitate, monostearate or another ester of polyoxyethylene
sorbitan (e.g.,
the commercially available Tweensm4, such as, TweenTh4 20 and TweenTm 80 (ICI
Speciality
Chemicals)), sodium dioctylsulfosuccinate (DOSS), lecithin, stearylic alcohol,
cetostearylic
alcohol, cholesterol, polyoxyethylene ricin oil, polyoxyethylene fatty acid
glycerides,
poloxamers (e.g., Pluronics F68 TM and F108 TM, which are block copolymers of
ethylene
oxide and propylene oxide); polyoxyethylene castor oil derivatives or mixtures
thereof. In
certain embodiments, a formulation of the disclosure comprises Polysorbate 20,
Polysorbate
40, Polysorbate 60, or Polysorbate 80.
The term "tonicity modifier" or "tonicity agent" refers to a compound that can
be used
to adjust the tonicity of a liquid formulation. Examples of tonicity modifiers
include glycerin,
lactose, mannitol, dextrose, sodium chloride, magnesium sulfate, magnesium
chloride,
sodium sulfate, sorbitol, trehalose, sucrose, raffinose, maltose and others
known to those or
ordinary skill in the art.
The term "polyol" refers to a substance with multiple hydroxyl groups, and
includes
sugars (reducing and nonreducing sugars), sugar alcohols and sugar acids. In
certain
embodiments, polyols have a molecular weight that is less than about 600 kD
(e.g., in the
range from about 120 to about 400 kD). A "reducing sugar" is one that contains
a free
aldehyde or ketone group and can reduce metal ions or react covalently with
lysine and other
amino groups in proteins. A "nonreducing sugar" is one that lacks a free
aldehyde or ketonic
group and is not oxidised by mild oxidising agents such as Fehling's or
Benedict's solutions..
Examples of reducing sugars are fructose, mannose, maltose, lactose,
arabinose, xylose,
ribose, rhamnose, galactose and glucose. Nonreducing sugars include sucrose,
trehalose,
sorbose, melezitose and raffinose. Mannitol, xylitol, erythritol, threitol,
sorbitol and glycerol
are examples of sugar alcohols. As to sugar acids, these include L-gluconate
and metallic
salts thereof. The polyol may also act as a tonicity agent. In certain
embodiments of the
disclosure, one ingredient of the formulation is sorbitol in a concentration
of about 10 to
about 70 mg/ml. In a particular embodiment of the disclosure, the
concentration of sorbitol is
about 30 to about 50 mg/ml. In certain embodiments, the concentration of
sucrose is about
60 to about 100 mg/ml. In a particular embodiment of the disclosure, the
concentration of
sucrose is about 70 to about 90 mg/ml.
The term "buffer" refers to a buffered solution that resists changes in pH by
the action
18
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
of its acid-base conjugate components. A buffer used in this disclosure has a
pH in the range
from about 4.5 to about 7.5. Examples of buffers that will control the pH in
this range
include acetate (e.g., sodium acetate), succinate (such as sodium succinate),
gluconate,
methionine, imidazole, histidine, glycine, arginine, citrate, phosphate,
citrate and phosphate,
Tris, and other organic acid buffers. In certain embodiments, the buffer used
in the
formulation of the disclosure is histidine, glycine, arginine, acetate,
citrate, and/or phosphate
buffered saline (PBS).
A "reconstituted" formulation is one which has been prepared by dissolving a
lyophilized protein formulation in a diluent such that the protein is
dispersed in the
reconstituted formulation. The reconstituted formulation is suitable for
administration (e.g.
parenteral administration) to a patient to be treated with the protein of
interest (e.g., LS-
DVD-Ig).
A "diluent" of interest herein is one which is pharmaceutically acceptable
(safe and
non-toxic for administration to a human) and is useful for the preparation of
a liquid
formulation, such as a formulation reconstituted after lyophilization.
Exemplary diluents
include sterile water, bacteriostatic water for injection (BWFI), a pH
buffered solution (e.g.
phosphate-buffered saline), sterile saline solution, Ringer's solution or
dextrose solution. In
an alternative embodiment, diluents can include aqueous solutions of salts
and/or buffers.
A "therapeutically effective amount" or "effective amount" of a binding
protein refers
to an amount effective in the prevention or treatment of a disorder for the
treatment of which
the antibody is effective.
The term "disorder" refers to any condition that would benefit from treatment
with the
formulations of the disclosure. This includes chronic and acute disorders or
diseases
including those pathological conditions that predispose the subject to the
disorder in question.
The term "treatment" refers to both therapeutic treatment and prophylactic or
preventative measures. Those patients in need of treatment include those
already with the
disorder as well as those in which the disorder is to be prevented.
The terms "parenteral administration" and "administered parenterally" means
modes
of administration other than enteral and topical administration, usually by
injection, and
includes, without limitation, intravenous, intramuscular, intraarterial,
intrathecal,
intracapsular, intraorbital, intracardiac, intradermal, intraperitoneal,
transtracheal,
subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid,
intraspinal and
19
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
intrasternal injection and infusion. The phrases "systemic administration,"
"administered
systemically," "peripheral administration" and "administered peripherally"
mean the
administration of a compound, drug or other material other than directly into
the central
nervous system, such that it enters the patient's system and is subject to
metabolism and other
like processes, for example, subcutaneous administration.
The term "antibody" broadly refers to an immunoglobulin (Ig) molecule,
generally
comprised of four polypeptide chains, two heavy (H) chains and two light (L)
chains, or any
functional fragment, mutant, variant, or derivative thereof, that retains the
essential target
binding features of an Ig molecule.
In a full-length antibody, each heavy chain is comprised of a heavy chain
variable
region (abbreviated herein as HCVR or VH) and a heavy chain constant region.
The heavy
chain constant region is comprised of three domains, CH1, CH2 and CH3. Each
light chain is
comprised of a light chain variable region (abbreviated herein as LCVR or VL)
and a light
chain constant region. The light chain constant region is comprised of one
domain, CL. The
VH and VL regions can be further subdivided into regions of hypervariability,
termed
complementarity determining regions (CDR), interspersed with regions that are
more
conserved, termed framework regions (FR). Each VH and VL is composed of three
CDRs
and four FRs, arranged from amino-terminus to carboxy-terminus in the
following order:
FR1, CDR1, FR2, CDR2, FR3, CDR3, FR4. Immunoglobulin molecules can be of any
type
(e.g., IgG, IgE, IgM, IgD, IgA and IgY) and class (e.g., IgGl, IgG2, IgG 3,
IgG4, IgAl and
IgA2) or subclass.
The term "Fc region" means the C-terminal region of an immunoglobulin heavy
chain, which may be generated by papain digestion of an intact antibody. The
Fc region may
be a native sequence Fc region or a variant sequence Fc region. The Fc region
of an
immunoglobulin generally comprises two constant domains, a CH2 domain and a
CH3
domain, and optionally comprises a CH4 domain.
The term "antigen-binding portion" refers to one or more fragments of a
binding
protein that specifically binds to a target or an antigen. Such embodiments
may be
monospecific, or may be bispecific, dual specific, or multi-specific (may
specifically bind
two or more different antigens).
A "functional antigen binding site" of a binding protein is one that is
capable of
binding a target antigen. The antigen binding affinity of the functional
antigen binding site is
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
not necessarily as strong as the parent antibody from which the antigen
binding site is
derived, but the ability to bind antigen must be measurable using a known
method for
evaluating antibody binding to an antigen. Moreover, the antigen binding
affinity of each of
the functional antigen binding sites of a multivalent binding protein need not
be
quantitatively the same.
The term "linker" denotes polypeptides comprising two or more amino acid
residues
joined by peptide bonds that are used to link one or more antigen binding
portions. Such
linker polypeptides are well known in the art (see, e.g., Holliger et al.
(1993) Proc. Natl.
Acad. Sci. USA 90:6444-6448; Poljak et al. (1994) Structure 2:1121-1123).
An "immunoglobulin constant domain" refers to a heavy or light chain constant
domain. Human heavy chain and light chain (e.g., IgG) constant domain amino
acid
sequences are known in the art.
The term "monoclonal antibody" refers to an antibody obtained from a
population of
substantially homogeneous antibodies, i.e., the individual antibodies
comprising the
population are identical except for possible naturally occurring mutations
that may be present
in minor amounts. Monoclonal antibodies are highly specific, being directed
against a single
antigen. Furthermore, in contrast to polyclonal antibody preparations that
typically include
different antibodies directed against different epitopes, each monoclonal
antibody is directed
against a single epitope on the antigen. The modifier "monoclonal" is not to
be construed as
requiring production of the antibody by any particular method.
The term "human antibody" includes antibodies that have variable and constant
regions derived from human germline immunoglobulin sequences. The human
antibodies
may include amino acid residues not encoded by human germline immunoglobulin
sequences
(e.g., mutations introduced by random or site-specific mutagenesis in vitro or
by somatic
mutation in vivo). However, the term "human antibody" is not intended to
include antibodies
in which CDR sequences derived from the germline of another mammalian species,
such as a
mouse, have been grafted onto human framework sequences.
The term "chimeric antibody" means antibodies that comprise heavy and light
chain
variable region sequences from one species and constant region sequences from
another
species, such as antibodies having murine heavy and light chain variable
regions linked to
human constant regions.
21
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
The term "CDR-grafted antibody" means antibodies that comprise heavy and light
chain variable region sequences from one species but in which the sequences of
one or more
of the CDR regions of their VH and/or VL are replaced with the CDR sequences
of another
species, such as antibodies having human heavy and light chain variable
regions in which one
or more of the murine CDRs (e.g., CDR3) has been replaced with murine CDR
sequences.
The term "humanized antibody" means an antibody that comprises heavy and light
chain variable region sequences from a non-human species (e.g., a mouse) but
in which at
least a portion of the VH and/or VL sequence has been altered to be more
"human-like", i.e.,
more similar to human germline variable sequences. One type of humanized
antibody
comprises non-human CDR sequences and human framework sequences.
The term "CDR" means the complementarity determining region within antibody
variable sequences. There are three CDRs in each of the variable regions of
the heavy chain
and the light chain, which are designated CDR1, CDR2 and CDR3, for each of the
variable
regions. The term "CDR set" as used herein refers to a group of three CDRs
that occur in a
single variable region capable of binding the target. The exact boundaries of
these CDRs
have been defined differently according to different systems.
The terms "Kabat numbering", "Kabat definitions and "Kabat labeling" are used
interchangeably herein. These terms refer to a system of numbering amino acid
residues that
are more variable (i.e., hypervariable) than other amino acid residues in the
heavy and light
chain variable regions of an antibody, or an antigen binding portion thereof
(Kabat et al.
(1971) Ann. NY Acad. Sci. 190:382-391 and Kabat et al. (1991) Sequences of
Proteins of
Immunological Interest, Fifth Edition, U.S. Department of Health and Human
Services, NIH
Publication No. 91-3242). For the heavy chain variable region, the
hypervariable region
generally ranges from amino acid positions 31 to 35 for CDR1, amino acid
positions 50 to 65
for CDR2, and amino acid positions 95 to 102 for CDR3. For the light chain
variable region,
the hypervariable region generally ranges from amino acid positions 24 to 34
for CDR1,
amino acid positions 50 to 56 for CDR2, and amino acid positions 89 to 97 for
CDR3.
Chothia and coworkers (Chothia and Lesk (1987) J. Mol. Biol. 196:901-917 and
Chothia et al. (1989) Nature 342:877-883) found that certain sub-portions
within Kabat
CDRs adopt nearly identical peptide backbone conformations, despite having
great diversity
at the level of amino acid sequence. These sub-portions were designated as Li,
L2 and L3 or
H1, H2 and H3 where the "L" and the "H" designates the light chain and the
heavy chains
22
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
regions, respectively. These regions may be referred to as Chothia CDRs, which
have
boundaries that overlap with Kabat CDRs. Other boundaries defining CDRs
overlapping with
the Kabat CDRs have been described by Padlan (1995) FASEB J. 9:133-139 and
MacCallum
(1996) J. Mol. Biol. 262(5):732-45. Still other CDR boundary definitions may
not strictly
follow one of the above systems, but will nonetheless overlap with the Kabat
CDRs, although
they may be shortened or lengthened in light of prediction or experimental
findings that
particular residues or groups of residues or even entire CDRs do not
significantly impact
antigen binding. The methods used herein may utilize CDRs defined according to
any of
these systems, although embodiments use Kabat or Chothia defined CDRs.
The term "framework" or "framework sequence" refers to the remaining sequences
of
a variable region minus the CDRs. Because the exact definition of a CDR
sequence can be
determined by different systems, the meaning of a framework sequence is
subject to
correspondingly different interpretations. The six CDRs (CDR-H1, -H2, and -H3
of the heavy
chain and CDR-L1, -L2, and -L3 of the light chain) also divide the framework
regions on the
light chain and the heavy chain into four sub-regions (FR1, FR2, FR3 and FR4)
on each
chain, in which CDR1 is positioned between FR1 and FR2, CDR2 between FR2 and
FR3,
and CDR3 between FR3 and FR4. The term "activity" includes activities such as
the binding
specificity and binding affinity of a DVD-Ig protein for two or more antigens.
The term "epitope" includes any polypeptide determinant capable of specific
binding
to an immunoglobulin or T-cell receptor. In certain embodiments, epitope
determinants
include chemically active surface groupings of molecules such as amino acids,
sugar side
chains, phosphoryl, or sulfonyl, and, in certain embodiments, may have
specific three
dimensional structural characteristics, and/or specific charge
characteristics. In certain
embodiments, an epitope is a region of an antigen that is bound by an antibody
or
multispecific binding protein.
The term "surface plasmon resonance" or "SPR", refers to an optical phenomenon
that
allows for the analysis of real-time biospecific interactions by detection of
alterations in
protein concentrations within a biosensor matrix, for example using the
BIAcore system
(Pharmacia Biosensor AB, Uppsala, Sweden and Piscataway, N.J.). For further
descriptions,
see Jonsson et al. (1993) Ann. Biol. Clin. 51:19-26; Jonsson et al. (1991)
Biotechniques
11:620-627; Jonsson et al. (1995) J. Mol. Recognit. 8:125-131; and Johnsson et
al. (1991)
Anal. Biochem. 198:268-277.
23
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
The term "Kon" refers to the on rate constant for association of a binding
protein to the
antigen to form the binding protein /antigen complex as is known in the art.
The term "Koff" refers to the off rate constant for dissociation of a binding
protein
from the binding protein/antigen complex as is known in the art.
The term "Kd" refers to the dissociation constant of a particular antibody-
antigen
interaction as is known in the art.
The terms "specific binding", "specifically binding" or "specifically binds",
as used
herein, in reference to the interaction of a binding protein with a second
chemical species,
mean that the interaction is dependent upon the presence of a particular
structure (e.g., an
antigenic determinant or epitope) on the chemical species; for example, a DVD-
Ig protein
recognizes and binds to a specific protein structure rather than to proteins
generally. If a
DVD-Ig protein is specific for epitope "A", the presence of a molecule
containing epitope A
(or free, unlabeled A), in a reaction containing labeled "A" and the DVD-Ig
protein, will
reduce the amount of labeled A bound to the DVD-Ig protein.
II. Dual Variable Domain Immunoglobulin (DVD-Ig) Proteins for Use in
Formulations of the Disclosure
The disclosure pertains to formulations, and uses thereof, of DVD-Ig proteins,
particularly those identified as AS-DVD-Ig protein or LS-DVD-Ig protein
(described in more
detail below).
A. General DVD-Ig Protein Structure
In certain embodiments, the DVD-Ig protein used in the formulations and
methods of
the disclosure comprises a polypeptide chain, wherein said polypeptide chain
comprises
VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first variable domain, VD2 is a second
variable
domain, C is a constant domain, X1 represents an amino acid or polypeptide, X2
represents
an Fc region and n is 0 or 1.
In certain embodiments, a DVD-Ig protein contains two polypeptide chains,
wherein a
first polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a
first heavy
chain variable domain, VD2 is a second heavy chain variable domain, C is a
heavy chain
constant domain, X1 is a linker with the proviso that it is not CH1, and X2 is
an Fc region;
and a second polypeptide chain comprises VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is
a first
24
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
light chain variable domain, VD2 is a second light chain variable domain, C is
a light chain
constant domain, X1 is a linker with the proviso that it is not CH1, and X2
does not comprise
an Fc region; and n is 0 or 1; and wherein said two polypeptide chains of said
binding protein
form two functional antigen binding sites.
In certain embodiments, a DVD-Ig protein contains four polypeptide chains,
wherein
two polypeptide chains comprise VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first
heavy
chain variable domain, VD2 is a second heavy chain variable domain, C is a
heavy chain
constant domain, X1 is a linker with the proviso that it is not CH1, and X2 is
an Fc region;
and two polypeptide chains comprise VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a
first light
chain variable domain, VD2 is a second light chain variable domain, C is a
light chain
constant domain, X1 is a linker with the proviso that it is not CH1, and X2
does not comprise
an Fc region; and n is 0 or 1; and wherein said four polypeptide chains of
said binding protein
form four functional antigen binding sites.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises a
polypeptide chain wherein the polypeptide chain comprises VD1-(X1)n-VD2-C-
(X2)n,
wherein VD1 is a first heavy chain variable domain; VD2 is a second heavy
chain variable
domain; C is a heavy chain constant domain; X1 is a linker with the proviso
that it is not
CH1; X2 is an Fc region; and n is 0 or 1. In certain embodiments, the AS-DVD-
Ig protein or
LS-DVD-Ig protein comprises a polypeptide chain, wherein the polypeptide chain
comprises
VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first light chain variable domain; VD2
is a
second light chain variable domain; C is a light chain constant domain; X1 is
a linker with the
proviso that it is not a CH1 or CL; X2 does not comprise an Fc region; and n
is 0 or 1. In a
further embodiment, (Xl)n on the heavy and/or light chain is (X1)0 and/or
(X2)n on the
heavy and/or light chain is (X2)0.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, wherein the first polypeptide chain
comprises a first
VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a first heavy chain variable domain; VD2
is a
second heavy chain variable domain; C is a heavy chain constant domain; X1 is
a first linker
with the proviso that it is not CH2; X2 is an Fc region; n is 0 or 1; and
wherein the second
polypeptide chain comprises a second VD1-(Xl)n-VD2-C-(X2)n, wherein VD1 is a
first light
chain variable domain; VD2 is a second light chain variable domain; C is a
light chain
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
constant domain; X1 is a second linker with the proviso that it is not CH1 or
CL; X2 does
not comprise an Fc region; and n is 0 or 1.
In certain embodiments, the VD1 of the first polypeptide chain and the VD1 of
the second
polypeptide chain are from different first and second parent antibodies,
respectively, or
binding portions thereof. In certain embodiments, the VD2 of the first
polypeptide chain and
the VD2 of the second polypeptide chain are from different first and second
parent
antibodies, respectively, or binding portions thereof. In certain embodiments,
the first and
the second parent antibodies bind different epitopes on the same target or
different targets. In
certain embodiments, the first parent antibody or binding portion thereof
binds the first target
with a potency that is different from the potency with which the second parent
antibody or
binding portion thereof binds the second target. In certain embodiments, the
first parent
antibody or binding portion thereof binds the first target with an affinity
different from the
affinity with which the second parent antibody or binding portion thereof
binds the second
target.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises,
two first polypeptide chains and two second polypeptide chains.
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, each independently comprising VD1-(X1)n-
VD2-C-
(X2)n, wherein VD1 is a first variable domain; VD2 is a second variable
domain; C is a
constant domain; X1 is a linker with the proviso that it is not CH1; X2 is an
Fc region; n is 0
or 1, wherein the VD1 domains on the first and second polypeptide chains form
a first
functional target binding site and the VD2 domains on the first and second
polypeptide chains
form a second functional target binding site. In a further embodiment, the
first polypeptide
chain comprises a first VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first heavy
chain
variable domain; VD2 is a second heavy chain variable domain; C is a heavy
chain constant
domain; X1 is a linker with the proviso that it is not CH1; X2 is an Fc
region; n is 0 or 1, and
wherein the second polypeptide chain comprises a second VD1-(Xl)n-VD2-C-(X2)n,
wherein VD1 is a first light chain variable domain; VD2 is a second light
chain variable
domain; C is a light chain constant domain; X1 is a linker with the proviso
that it is not CH1;
X2 does not comprise an Fc region; n is 0 or 1, wherein the VD1 domains on the
first and
second polypeptide chains form a first functional target binding site and the
VD2 domains on
the first and second polypeptide chains form a second functional target
binding site.
26
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the AS-DVD-Ig protein or LS-DVD-Ig protein comprises
first and second polypeptide chains, each independently comprising VD1-(X1)n-
VD2-C-
(X2)n, wherein VD1 is a first variable domain; VD2 is a second variable
domain; C is a
constant domain; X1 is a linker with the proviso that it is not CH1; X2 is an
Fc region; n is 0
or 1, and wherein the VD1 domains on the first and second polypeptide chains
form a first
functional target binding site and the VD2 domains on the first and second
polypeptide chains
form a second functional target binding site. In a further embodiment, the
first polypeptide
chain comprises a first VD1-(X1)n-VD2-C-(X2)n, wherein VD1 is a first heavy
chain
variable domain; VD2 is a second heavy chain variable domain; C is a heavy
chain constant
domain; X1 is a linker with the proviso that it is not CH1; X2 is an Fc
region; n is 0 or 1, and
wherein the second polypeptide chain comprises a second VD1-(X1)n-VD2-C-(X2)n,
wherein VD1 is a first light chain variable domain; VD2 is a second light
chain variable
domain; C is a light chain constant domain; X1 is a linker with the proviso
that it is not CH1;
X2 does not comprise an Fc region; n is 0 or 1, wherein the VD1 domains on the
first and
second polypeptide chains form a first functional target binding site and the
VD2 domains on
the first and second polypeptide chains form a second functional target
binding site.
Examples of DVD-Ig proteins are described in US Patent No. 7,612,181, which is
incorporated by reference herein.
Examples of DVD-Ig proteins that may be used in the methods and compositions
of
the disclosure are described below, including Tables 58 and 65. Further
examples of DVD-Ig
proteins that may be used in the methods and compositions of the disclosure
are described in
SEQ ID NOs: 28 to 75.
B. Generation of DVD-Ig Proteins
The variable domains of a DVD-Ig protein can be obtained from parent
antibodies,
including polyclonal and monoclonal antibodies capable of binding targets of
interest. These
antibodies may be naturally occurring or may be generated by recombinant
technology.
Examples of antibodies that may be used in making DVD-Ig proteins include
chimeric
antibodies, human antibodies, and humanized antibodies. Monoclonal antibodies
can be
prepared using a wide variety of techniques known in the art including, for
example, the use
of hybridoma, recombinant, and phage display technologies, or any combination
thereof.
Monoclonal antibodies may also be produced by immunizing a non-human animal
27
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
comprising some, or all, of the human immunoglobulin locus with an antigen of
interest, such
as, for example, XENOMOUSETh4 transgenic mouse, an engineered mouse strain
that
comprises large fragments of the human immunoglobulin loci and is deficient in
mouse
antibody production. Methods of generating DVD-Ig proteins are described in US
Patent No.
7,612,181, the teachings of which are incorporated by reference herein. DVD-Ig
proteins
used in the compositions and methods of the disclosure may be made from
antibodies capable
of binding specific targets and well known in the art. These include, but are
not limited to an
anti-TNF antibody (U.S. Patent No. 6,258,562), anti-IL-12 and or anti-IL-12p40
antibody
(U.S. Patent No. 6,914,128); anti-IL-18 antibody (US Patent Publication No.
20050147610),
as well as anti-05, anti-CBL, anti-CD147, anti-gp120, anti-VLA4, anti-CD11a,
anti-CD18,
anti-VEGF, anti-CD40L, anti-Id, anti-ICAM-1, anti-CXCL13, anti-CD2, anti-EGFR,
anti-
TGF-beta 2, anti-E-selectin, anti-Fact VII, anti-Her2/neu, anti-F gp, anti-
CD11/18, anti-
CD14, anti-ICAM-3, anti-CD80, anti-CD4, anti-CD3, anti-CD23, anti-beta2-
integrin, anti-
alpha4beta7, anti-CD52, anti-HLA DR, anti-CD22, anti-CD20, anti-MIF, anti-CD64
(FcR),
anti-TCR alpha beta, anti-CD2, anti-Hep B, anti-CA 125, anti-EpCAM, anti-
gp120, anti-
CMV, anti-gpllbIlla, anti-IgE, anti-CD25, anti-CD33, anti-HLA, anti-
VNRintegrin, anti-IL-
1 alpha, anti-IL- lbeta, anti-IL-1 receptor, anti-IL-2 receptor, anti-IL-4,
anti-1L4 receptor, anti-
1L5, anti-IL-5 receptor, anti-IL-6, anti-IL-8, anti-IL-9, anti-IL-13, anti-IL-
13 receptor, anti-
IL-17, and anti-IL-23 antibodies (see Presta (2005) J. Allergy Clin. Immunol.
116:731-6 and
Clark "Antibodies for Therapeutic Applications," Department of Pathology,
Cambridge
University, UK (2000), published online at M. Clark's home page at the website
for the
Department of Pathology, Cambridge University.
Parent monoclonal antibodies may also be selected from various therapeutic
antibodies approved for use, in clinical trials, or in development for
clinical use. Such
therapeutic antibodies include, but are not limited to: rituzimab (RITUXAN TM
Biogen Idec,
Genentech/Roche) (see for example U.S. Patent No. 5,736,137) a chimeric anti-
CD20
antibody approved to treat non-Hodgkin's lymphoma; ofatumumab (HUMAX-CD20 TM
Genmab, GlaxoSmithKlein) (described in U.S. Patent 5,500,362) an anti-CD20
antibody
approved to treat chronic lymphocytic leukemia that is refractory to
fludarabine and
alemtuzumab; AME-133v (Mentrik Biotech) an anti-CD20 antibody; veltuzumab
(hA20)
(Immunomedics) an anti-CD20 antibody; HumaLYM (Intracel); PR070769
(Genentech/Roche) (PCT/U52003/040426) an anti-CD20 antibody; trastuzumab
28
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
(HERCEPTIN TM Genentech/Roche) (described in U.S. Patent No. 5,677,171) a
humanized
anti-Her2/neu antibody approved to treat breast cancer; pertuzumab (rhuMab-
2C4,
OMNITARG TM Genentech/Roche) (described in U.S. Patent No. 4,753,894) ;
cetuximab
(ERBITUX TM Imclone) (described in U.S. Patent No. 4,943,533; PCT WO 96/40210)
a
chimeric anti-EGFR antibody approved to treat colorectal and head and neck
cancer;
panitumumab (ABX-EGF VECTIBIX Amgen) (described in U.S. Patent No. 6,235,883)
an
anti-EGFR antibody approved to treat colorectal cancer; zalutumumab (HUMAX-
EGFR TM
Genmab) (described in U.S. Patent Application Serial No. 10/172,317) an anti-
EGFR
antibody ; EMD55900 (Mab 425 Merck) an anti-EGFR antibody; EMD62000 and
EMD72000 (Mab 425 Merck) anti-EGFR antibodies (described in U.S. Patent No.
5,558,864;
Murthy et al. (1987) Arch. Biochem. Biophys. 252(2):549-60; Rodeck et al.
(1987) J. Cell.
Biochem. 35(4):315-20; Kettleborough et al. (1991) Protein Eng. 4(7):773-83;
ICR62
(Institute of Cancer Research) an anti-EGFR antibody (described in PCT
Publication No. WO
95/20045; Modjtahedi et al. (1993) J. Cell. Biophys. 22(1-3):129-46;
Modjtahedi et al.
(1993) Br. J. Cancer 67(2):247-53; Modjtahedi et al. (1996) Br. J. Cancer
73(2):228-35;
Modjtahedi et al. (2003) Int. J. Cancer 105(2):273-80); nimotuzumab
(TheraCIIVI hR3,
THERALOC YM Biosciences, Oncoscience AG) (described in U.S. Patent No.
5,891,996;
U.S. Patent No. 6,506,883; Mateo et al. (1997) Immunotechnol. 3(1):71-81) an
anti-EGFR
antibody; ABT-806 (Ludwig Institute for Cancer Research, Memorial Sloan-
Kettering)
(Jungbluth et al. (2003) Proc. Natl. Acad. Sci. USA 100(2):639-44) an anti-
EGFR antibody;
KSB-102 (KS Biomedix); MR1-1 (IVAX, National Cancer Institute) (PCT
Publication No.
WO 0162931A2) an anti-EGFRvIII antibody; SC100 (Scancell) (PCT Publication No.
WO
01/88138) an anti-EGFR antibody; alemtuzumab (CAMPATHTm Genzyme/Sanofi) an
anti-
CD52 antibody approved to treat B-cell chronic lymphocytic leukemia; muromonab-
CD3
(Orthoclone OKT3 TM Johnson and Johnson) an anti-CD3 antibody approved to
treat organ
transplant rejection; ibritumomab tiuxetan (ZEVALIN TM Spectrum
Pharmaceuticals) an anti-
CD20 antibody approved to treat non-Hogkins Lymphoma; gemtuzumab ozogamicin
(hP67.6
MYLOTARG TM Pfizer) an anti-CD33 antibody conjugated to calicheamicin;
alefacept
(AMEVIVE TM Astellas Pharma) an anti-CD2 LFA-3 Fc fusion; abciximab (REOPRO TM
Centocor Ortho Biotech Products, Lilly) a chimeric human-mouse anti-
glycoprotein IIb/IIIa
receptor and anti-vitronectic avI33 receptor antibody approved as an adjunct
to percutaneous
coronary intervention to prevent cardiac ishemia; basiliximab (SIMULECT TM
Novartis) an
29
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
anti-CF25 antibody approved to treat organ transplant rejection; palivizumab
(SYNAGIS TM
Medimmune) an antibody to the A antigenic site of F protein of RSV approved to
treat RSV
infection; infliximab (REMICADE TM Janssen Biotech) an anti-TNFalphaa antibody
approved to treat Crohn's disease, ulcerative colitis, arthritis, ankylosing
spondylitis, psoriatic
arthritis, and plaque psoriasis; adalimumab (HUMIRATh4 AbbVie) an anti-TNFa
antibody
approved to treat rheumatoid arthritis, juvenile idiopathic arthritis,
psoriatic arthritis,
ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis;
CDP571
(HUMICADETm Celltech, Biogen IDEC) an anti-TNFa antibody ; etanercept (ENBREL
TM
Amgen, Pfizer) an anti-TNFa Fc fusion antibody approved to treat rheumatoid
arthritis,
juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis,
plaque psoriasis;
certolizumab pegol (CIMZIA)UCB Pharma) an anti-TNFa antibody approved to treat
rheumatoid arthritis and Crohn's disease; ustekinumab (STELARA Janssen
Biotech) a human
anti-p40 subunit of IL-12 and IL-23 antibody approved to treat plaque
psoriasis; galilimomab
(ABX-CBL Abgenix) a mouse anti-CD147 antibody; ABX-1L8 (Abgenix) an anti-1L8
antibody ; ABX-MA1 (Abgenix) an anti-MUC18 antibody ; pemtumomab (Theragyn,
R1549,
90Y-muHMFG1Antisoma) a mouse anti-MUC1-Yttrium 90 antibody conjugate; Therex
(R1550 Antisoma) an anti-MUC1 antibody; AngioMab (muBC-1, AS1405 Antisoma);
HuBC-1 (Antisoma); Thioplatin (AS1407 Antisoma); natalizumab (TYSABRI Biogen
Idec,
Elan) an anti-a4 integrin antibody approved to treat multiple sclerosis and
Crohn's disease;
VLA-1 (Santarus) a humanized anti-VLA-1 antibody ; LTBR mAb (Biogen Idec) an
anti-
lymphotoxin 0 receptor antibody; lerdelimumab (CAT-152 Cambridge Antibody
Technology/Abbott) an anti-TGF-132 antibody ; briakinumab (AbbVie) an anti-IL-
12 and 23
antibody ; metelimumab (CAT-192 Cambridge Antibody Technology, Genzyme) an
anti-
TGF131 antibody ; bertilimumab (CAT-213, iC0-008 Cambridge Antibody
Technology, iCo
Therapeutics, Immune Pharmaceuticals) an anti-eotaxinl antibody ; belimumab
(BENLYSTA GlaxoSmithKline) an anti-B lymphocyte stimulator protein antibody
approved to treat systemic lupus erythematosus; maputumumab (HGS-ETR1
Cambridge
Antibody Technology, Human Genome Sciences) an anti-TRAIL-R1 antibody;
bevacizumab
(AVASTIN TM Genentech/Roche) an anti-VEGF antibody approved to treat
metastatic
colorectal cancer, non-squamous non-small cell lung cancer, glioblastoma,
metastatic renal
cell cancer; anti-HER3/EGFR antibody (Genentech/Roche); an Anti-Tissue Factor
antibody
(Genentech/Roche); omalizumab (XOLAIR TM Genentech/Roche, Novartis) an anti-
IgE
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
antibody approved to treat severe allergic asthma; efalizumab (RAPTIVA TM
Genentech/Roche, Merck Serono) an anti-CD1la antibody; MLN-02 (Millenium,
Genentech/Roche) an anti-a4137 integrin antibody; zanolimumab (HUMAX CD4m4
Emergent
BioSolutions) an anti-CD4 antibody; HUMAX -IL1517\4 (AMG-714 Genmab, Amgen) an
anti-1L15 antibody; HuMax-1L8 (HUMAX -InflamTm, MDX-018 Genmab, Cormorant
Pharmaceuticals) an anti-1L8 antibody; HUMAX TM -Cancer, (Genmab, Medarex,
Oxford
GlycoSciences) an anti-Heparanase I antibody; HUMAX TM ¨Lymphoma (Genmab) an
anti-
1L8 antibody; HUMAX TM ¨TAC (Genmab) an anti-IL-2Ra, CD25 antibody ;
daratumumab
(HuMax -CD38, Genmab, Janssen Biotech) an anti-CD38 antibody; toralizumab
(IDEC-131
Biogen Idec) an anti-CD4OL antibody ; clenolimimab (IDEC-151 Biogen Idec) an
anti-CD4
antibody; glaiximab (IDEC-114 Biogen Idec) an anti-CD80 antibody; lumilixmab
(IDEC-
152 Biogen Idec) an anti-CD23 ; anti-macrophage migration factor (MIF)
antibodies (Biogen
Idec, Taisho Pharmaceutical); mitumomab (BEC2 Imclone) a mouse anti-idiotypic
antibody;
IMC-1C11 (Imclone) a chimeric anti-VEGFR2 antibody; DC101 (Imclone) murine
anti-
VEGFR2 antibody;; anti-VE cadherin antibody (Imclone); labetuzumab (CEA-CIDE
TM
Immunomedics) an anti-carcinoembryonic antigen antibody; epratuzumab
(LYMPHOCIDE
TM
Immunomedics) an anti-CD22 antibody ; yttrium (90Y) tacatuzumab tetraxetan
(AFP-
Cide Immunomedics) an anti-afetoprotein antibody; milatuzumab (MyelomaCide
Immunomedics) an anti-CF74 antibody; LeukoCide (Immunomedics); ProstaCide
(Immunomedics); ipilimumab (YervoyTM, MDX-010 Bristol-Myers Squibb) an anti-
CTLA4
antibody approved to treat melanoma; iratumumab (MDX-060 Medarex) an anti-CD30
antibody ; MDX-070 (Medarex) an anti-prostate specific membrane antigen;
OSIDEM TM
(IDM-1 Medarex, Immuno-Designed Molecules) an anti-Her2 antibody; HUMAX TM -
CD4,
an anti-CD4 antibody being developed by Medarex and Genmab; HuMax-1L15, an
anti-IL15
antibody being developed by Medarex and Genmab; golimumab (SIMPONff Janssen
Biotech) an anti-TNFa antibody approved to treat rheumatoid arthritis,
psoriatic arthritis,
ankylosing spondylitis; ustekinumab (STELARA , CNTO 1275 Janssen Biotech) an
anti-IL-
12 antibody approved to treat plaque psoriasis; MOR101 and MOR102 (MorphoSys)
anti-
intercellular adhesion molecule-1 (ICAM-1) (CD54) antibodies; MOR201
(MorphoSys) an
anti-fibroblast growth factor receptor 3 antibody ; visilizumab (NUVION TM PDL
BioPharma) an anti-CD3 antibody ; fontolizumab (HUZAF TM PDL BioPharma) an
anti-
INFy antibody ; volociximab (M200 PDL BioPharma, Biogen Idec) an anti-a5131
integrin
31
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
antibody ; SMART IL-12 (PDL BioPharma) an anti-IL-12 ; ING-1 (Xoma) an anti-
Ep-CAM
antibody; omalizumab (XOLAIRTm Genentech/Roche, Novartis) an anti-IgE antibody
approved to treat allergic asthma; MLNO1 (Xoma) an anti-I3 integrin antibody;
and
tocilizumab (ACTEMRATh4 Genentech/Roche) an anti-1L6 antibody approved to
treat
rhemuatoid arthritis and systemic juvenile idiopathic arthritis.
C. Construction of DVD-Ig Proteins
A DVD-Ig protein is formed by combining two heavy chain DVD polypeptides and
two light chain DVD polypeptides. The dual variable domain immunoglobulin (DVD-
Ig)
heavy chain comprises two heavy chain variable domains (VH) linked in tandem,
directly or
by a linker, followed by the constant domain CH1 and Fc region. The dual
variable domain
immunoglobulin (DVD-Ig) light chain is designed such that two light chain
variable domains
(VL) from the two parent mAbs are linked in tandem, directly or via a linker,
followed by the
light chain constant domain (CL) (see Figure lA of U.S. Patent No. 7,612,181,
incorporated
by reference herein). Methods of making DVD-Ig proteins are also described in
U.S. Patent
No. 7,612,181, incorporated by reference herein.
The variable domains of the DVD-Ig protein can be obtained using recombinant
DNA
techniques from a parent antibody generated by any one of the methods
described above. In
certain embodiments, the variable domain is a CDR grafted or a humanized
variable heavy or
light chain domain. In certain embodiments, the variable domain is a human
heavy or light
chain variable domain.
The linker sequence may be a single amino acid or a polypeptide sequence.
Examples
of linker sequences that may be used to link variable domains include, but are
not limited to,
AKTTPKLEEGEFSEAR (SEQ ID NO:1); AKTTPKLEEGEFSEARV (SEQ ID NO:2);
AKTTPKLGG (SEQ ID NO:3); SAKTTPKLGG (SEQ ID NO:4); SAKTTP (SEQ ID NO:5);
RADAAP (SEQ ID NO:6); RADAAPTVS (SEQ ID NO:7); RADAAAAGGPGS (SEQ ID
NO:8); RADAAAA(G4s).4 (SEQ ID NO:9), SAKTTPKLEEGEFSEARV (SEQ ID NO:10);
ADAAP (SEQ ID NO:11); ADAAPTVSIFPP (SEQ ID NO:12); TVAAP (SEQ ID NO:13);
TVAAPSVFIFPP (SEQ ID NO:14); QPKAAP (SEQ ID NO:15); QPKAAPSVTLFPP (SEQ
ID NO:16); AKTTPP (SEQ ID NO:17); AKTTPPSVTPLAP (SEQ ID NO:18); AKTTAP
(SEQ ID NO:19); AKTTAPSVYPLAP (SEQ ID NO:20); ASTKGP (SEQ ID NO:21);
ASTKGPSVFPLAP (SEQ ID NO:22); GGGGSGGGGSGGGGS (SEQ ID NO:23);
32
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
GENKVEYAPALMALS (SEQ ID NO:24); GPAKELTPLKEAKVS (SEQ ID NO:25);
GHEAAAVMQVQYPAS (SEQ ID NO:26); and GGGGSGGGGS (SEQ ID NO: 27). Other
examples of linkers are described in U.S. Patent Publication No. 20100226923,
incorporated
by reference herein. The choice of linker sequences may be determined based on
crystal
structure analysis of several antibody Fab molecules. There is a natural
flexible linkage
between the variable domain and the CH1/CL constant domain in Fab or antibody
molecular
structure. This natural linkage comprises approximately 10-12 amino acid
residues,
contributed by 4-6 residues from C-terminus of V domain and 4-6 residues from
the N-
terminus of the CL or CH1 domain. DVD-Ig proteins of the disclosure were
generated using
N-terminal 5-6 amino acid residues, or 11-12 amino acid residues, of CL or CH1
as the linker
in the light chain and the heavy chain of the DVD-Ig protein, respectively.
The N-terminal
residues of the CL or the CH1 domains, particularly the first 5-6 amino acid
residues, adopt a
loop conformation without strong secondary structure, and therefore can act as
flexible
linkers between the two variable domains. The N-terminal residues of the CL or
CH1
domains are natural extensions of the variable domains, as they are part of
the Ig sequences,
and therefore immunogenicity potentially arising from the linkers or junctions
is minimized.
Further examples of linkers are described in Table 65 (see underlined amino
acids).
Other linker sequences may include a sequence of any length of the CL or CH1
domain but not all residues of a CL/CH1 domain; for example the first 5-12
amino acid
residues of the CL or CH1 domain; the light chain linkers can be from Cx or
CX; and the
heavy chain linkers can be derived from CH1 of any isotype, including Cyl,
Cy2, Cy3, Cy4,
Cal, Ccc2, C8, Cc, and Cll. Linker sequences may also be derived from other
proteins such
as Ig-like proteins, (e.g., TCR, FcR, KR); G/S based sequences (e.g., G45
repeats); hinge
region-derived sequences; and other natural sequences from proteins.
In certain embodiments, a constant domain is linked to the two linked variable
domains using recombinant DNA techniques. For example, a sequence comprising
linked
heavy chain variable domains is linked to a heavy chain constant domain and
sequence
comprising linked light chain variable domains is linked to a light chain
constant domain. In
certain embodiments, the constant domains are a human heavy chain constant
domain and a
human light chain constant domain, respectively. In certain embodiments, the
DVD-Ig heavy
chain is further linked to an Fc region. The Fc region may comprise a native
Fc region
sequence, or a variant Fc region sequence. In certain embodiments, the Fc
region is a human
33
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Fc region. For example, the Fc region comprises an Fc region from an IgGl,
IgG2, IgG3,
IgG4, IgA, IgM, IgE, or IgD.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure is a dual-specific tetravalent binding protein. In certain
embodiments, the
DVD-Ig protein used in the methods and compositions of the disclosure binds
CD20 and
CD80 In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure binds VEGF and HER2. In certain embodiments, the DVD-Ig protein
used in
the methods and compositions of the disclosure binds TNF and RANKL. In certain
embodiments, the DVD-Ig protein used in the methods and compositions of the
disclosure
binds TNF and DKK. In certain embodiments, the DVD-Ig protein used in the
methods and
compositions of the disclosure binds CD20 and RANKL. In certain embodiments,
the DVD-
Ig protein used in the methods and compositions of the disclosure binds DLL4
and PLGF. In
certain embodiments, the DVD-Ig protein used in the methods and compositions
of the
disclosure binds DLL4 and VEGF. In certain embodiments, the DVD-Ig protein
used in the
methods and compositions of the disclosure binds TNF and SOST. In certain
embodiments,
the DVD-Ig protein used in the methods and compositions of the disclosure
binds IL-9 and
IgE. In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure binds IL-12 and IL-18. An example of an IL-12 and IL-18 DVD-Ig
protein is
described in U.S. Patent No.7,612,181. In certain embodiments, the DVD-Ig
protein used in
the methods and compositions of the disclosure binds TNF and IL-17. In certain
embodiments, the DVD-Ig protein used in the methods and compositions of the
disclosure
binds TNF and PGE2. Examples of PGE2 DVD-Ig proteins are provided in U.S.
Patent
Publication No. 20100074900. In certain embodiments, the DVD-Ig protein used
in the
methods and compositions of the disclosure binds IL-la and IL-10. An example
of an IL-la
and IL-10 DVD-Ig protein is described in U.S. Patent No. 7,612,181. In certain
embodiments, the DVD-Ig protein used in the methods and compositions of the
disclosure
binds IL-4 and IL-1. An example of an IL-4 and IL-13 DVD-Ig protein is
described in U.S.
Publication No. 20100226923. The amino acid and nucleic acid sequences of the
DVD-Ig
proteins described in the aforementioned patents and patent applications are
incorporated by
reference herein. Amino acid sequences of DVD-Ig proteins that may be used in
the methods
and compositions of the disclosure are described in SEQ ID NOs: 28-75.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
34
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
the disclosure specifically binds CD20/CD80, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 30
and 31. In
certain embodiments, the anti-CD20/CD80 DVD-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 30 and
31. In certain embodiments, the anti-CD20/CD80 DVD-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
30 and 31.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds CD80/CD20, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 32
and 33. In
certain embodiments, the anti-CD80/CD20 DVD-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 32 and
33. In certain embodiments, the anti-CD80/CD20 DVD-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
32 and 33.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds VEGF/HER2, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 34
and 35. In
certain embodiments, the anti-VEGF/HER2-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 34 and
35. In certain embodiments, the anti-VEGF/HER2 DVD-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
34 and 35.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds HER2/VEGF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 36
and 37. In
certain embodiments, the anti-HER2/VEGF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 36 and
37. In certain embodiments, the anti-HER2/VEGF DVD-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
36 and 37.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds TNF/RANKL, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 38
and 39. In
certain embodiments, the anti-TNF/RANKL-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 38 and
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
39. In certain embodiments, the anti-TNF/RANKL DVD-Ig protein comprises the
amino
acid sequences corresponding to the heavy and light chains set forth in SEQ ID
NOs: 38 and
39.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds RANKL/TNF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 40
and 41. In
certain embodiments, the anti-RANKL/TNF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 40 and
41. In certain embodiments, the anti-RANKL/TNF DVD-Ig protein comprises the
amino
acid sequences corresponding to the heavy and light chains set forth in SEQ ID
NOs:40 and
41.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds TNF/DKK, and comprises amino acid sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 42
and 43. In
certain embodiments, the anti-TNF/DKK-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 42 and
43. In certain embodiments, the anti-TNF/DKK-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
42 and 43.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds DKK/TNF, and comprises amino acid sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 44
and 45. In
certain embodiments, the anti-DKK/TNF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 44 and
45. In certain embodiments, the anti-DKK/TNF-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
44 and 45.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds CD2O/RANKL, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 46
and 47. In
certain embodiments, the anti-CD2O/RANKL-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 46 and
47. In certain embodiments, the anti-CD2O/RANKL-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
46 and 47.
36
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds RANKL/CD20, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 48
and 49. In
certain embodiments, the anti-RANKL/CD20-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 48 and
49. In certain embodiments, the anti-RANKL/CD20-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
48 and 49.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds DLL4/PLGF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 50
and 51. In
certain embodiments, the anti-DLL4/PLGF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 50 and
51. In certain embodiments, the anti-DLL4/PLGF-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
50 and 51.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds PLGF/DLL4, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 52
and 53. In
certain embodiments, the anti-PLGF/DLL4-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 52 and
53. In certain embodiments, the anti-PLGF/DLL4-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
52 and 53.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds TNF/SOST (S2), and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 54
and 55. In
certain embodiments, the anti-TNF/SOST (52)-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 54 and
55. In certain embodiments, the anti-TNF/SOST (52)-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
54 and 55.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds SOST (52)/TNF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 56
and 57. In
certain embodiments, the anti-SOST (52)/TNF-Ig protein comprises amino acid
sequences
37
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 56 and
57. In certain embodiments, the anti-SOST (52)/TNF-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
56 and 57.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds IL-9 (52)/IgE, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 58
and 59. In
certain embodiments, the anti-IL-9 (52)/IgE-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 58 and
59. In certain embodiments, the anti-IL-9 (52)/IgE-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
58 and 59.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds IgE/IL-9 (S2), and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 60
and 61. In
certain embodiments, the anti-IgE/IL-9 (52)-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 60 and
61. In certain embodiments, the anti-IgE/IL-9 (52)-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
60 and 61.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds TNF/IL-17, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 62
and 63. In
certain embodiments, the anti-TNF/IL-17-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 62 and
63. In certain embodiments, the anti-TNF/IL-17-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
62 and 63.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds TNF/PGE2, and comprises amino acid sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 64
and 65. In
certain embodiments, the anti-TNF/PGE2-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 64 and
65. In certain embodiments, the anti-TNF/PGE2-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
64 and 65.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
38
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
the disclosure specifically binds IL-lcc/IL-113, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 66
and 67. In
certain embodiments, the anti-IL-lcc/IL-113-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 66 and
67. In certain embodiments, the anti-IL-lcc/IL-113-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
66 and 67.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds DLL4/VEGF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 28
and 29. In
certain embodiments, the anti-DLL4/VEGF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 28 and
29. In certain embodiments, the anti-DLL4/VEGF-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
28 and 29.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds DLL4/VEGF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 72
and 73. In
certain embodiments, the anti-DLL4/VEGF-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 72 and
73. In certain embodiments, the anti-DLL4/VEGF-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
72 and 73.
In certain embodiments, the DVD-Ig protein used in the methods and
compositions of
the disclosure specifically binds IL12/IL18, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 70
and 71. In
certain embodiments, the anti-IL12/IL18-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 70 and
71. In certain embodiments, the anti-IL12/IL18-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
70 and 71.
D. Expression of DVD-Ig Proteins
DVD-Ig proteins of the present disclosure may be produced by any of a number
of
techniques known in the art. For example, expression from host cells, wherein
expression
vector(s) encoding the DVD heavy and DVD light chains is (are) transfected
into a host cell
39
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
by standard techniques. The various forms of the term "transfection" are
intended to
encompass a wide variety of techniques commonly used for the introduction of
exogenous
DNA into a prokaryotic or eukaryotic host cell, e.g., electroporation, calcium-
phosphate
precipitation, DEAE-dextran transfection and the like.
Mammalian host cells for expressing the recombinant antibodies of the
disclosure
include Chinese Hamster Ovary (CHO cells) (including dhfr-CHO cells, described
in Urlaub
and Chasin (1980) Proc. Natl. Acad. Sci. USA 77:4216-4220, used with a DHFR
selectable
marker, e.g., as described in Kaufman and Sharp (1982) J. Mol. Biol. 159:601-
621) and
DG44 or DUXB11 cells (Urlaub et al. (1986) Som. Cell Molec. Genet. 12:555;
Haynes et al.
(1983) Nuc. Acid. Res. 11:687-706; Lau et al. (1984) Mol. Cell. Biol. 4:1469-
1475), NSO
myeloma cells, monkey kidney line (e.g., CVI and COS, such as a COS 7 cell),
5P2 cells,
human embryonic kidney (HEK) cells, such as a HEK-293 cell, Chinese hamster
fibroblast
(e.g., R1610), human cervical carcinoma (e.g., HELA), murine fibroblast (e.g.,
BALBc/3T3),
murine myeloma (P3x63-Ag3.653; NSO; 5P2/0), hamster kidney line (e.g., HAK),
murine L
cell (e.g., L-929), human lymphocyte (e.g., RAJI), human kidney (e.g., 293 and
293T). Host
cell lines are typically commercially available (e.g., from BD Biosciences,
Lexington, Ky.;
Promega, Madison, Wis.; Life Technologies, Gaithersburg, Md.) or from the
American Type
Culture Collection (ATCC, Manassas, Va.).
When recombinant expression vectors encoding DVD-Ig proteins are introduced
into
mammalian host cells, the DVD-Ig proteins are produced by culturing the host
cells for a
period of time sufficient to allow for expression of the DVD-Ig proteins in
the host cells or
secretion of the DVD-Ig proteins into the culture medium in which the host
cells are grown.
DVD-Ig proteins can be recovered from the culture medium using standard
protein
purification methods.
In an exemplary system for recombinant expression of DVD-Ig proteins, a
recombinant expression vector encoding both the DVD-Ig heavy chain and the DVD-
Ig light
chain is introduced into dhfr-CHO cells by calcium phosphate-mediated
transfection. Within
the recombinant expression vector, the DVD-Ig heavy and light chain cDNAs are
each
operatively linked to CMV enhancer/AdMLP promoter regulatory elements to drive
high
levels of transcription of the cDNAs. The recombinant expression vector also
carries cDNA
encoding DHFR, which allows for selection of CHO cells that have been
transfected with the
vector using methotrexate selection/amplification. The selected transformant
host cells are
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
cultured to allow for expression of the DVD-Ig heavy and light chains and
intact DVD-Ig
protein is recovered from the culture medium. Standard molecular biology
techniques are
used to prepare the recombinant expression vector, transfect the host cells,
select for
transformants, culture the host cells and recover the DVD-Ig protein from the
culture
medium. Still further, the disclosure provides a method of synthesizing a DVD-
Ig protein of
the disclosure by culturing a host cell of the disclosure in a suitable
culture medium until a
DVD-Ig protein of the disclosure is synthesized. The method can further
comprise isolating
the DVD-Ig protein from the culture medium. An important feature of DVD-Ig
protein is
that it can be produced and purified in a similar way as a conventional
antibody.
E. Methods for Identifying Aqueous Stable DVD-Ig (AS-DVD-Ig) Proteins and
Lyophilized Stable DVD-Ig (LS-DVD-Ig) Proteins
An unexpected and surprising finding is that a certain subset of DVD-Ig
proteins
(referred to as AS-DVD-Ig protein and LS-DVD-Ig proteins) are stable ¨ even at
high
concentrations - in aqueous formulations, while a large number of DVD-Ig
proteins are
unstable and prone to aggregation. In addition, while the majority of DVD-Ig
proteins have
been found not to be stable in a lyophilized state, a certain subset of DVD-Ig
proteins
(referred to as LS-DVD-Ig proteins) are stable and can be successfully
lyophilized using the
formulations of the disclosure. Notably, DVD-Ig proteins identified as AS-DVD-
Ig proteins
are also LS-DVD-Ig proteins given that AS-DVD-Ig proteins are generally more
stable than
LS-DVD-Ig proteins. In addition, a non-AS-DVD Ig protein can be an LS DVD-Ig
protein.
The distinction between the two subpopulations may be based on the level of
aggregation,
freeze/thaw characteristics, or monomer content following storage, as
described in the below
assays. Generally, an increase in aggregation indicates a decrease in monomer
content.
Thus, in certain embodiments, the disclosure comprises a method for
distinguishing
between AS-DVD-Ig proteins and non-AS-DVD-Ig proteins. The disclosure also
comprises
a method for distinguishing between LS-DVD-Ig proteins and non-LS-DVD-Ig
proteins. In
another alternative, the disclosure provides methods for distinguishing AS-DVD-
Ig proteins
from LS-DVD-Ig proteins. Following identification, AS-DVD-Ig protein and LS-
DVD-Ig
proteins may be successfully formulated in the compositions of the disclosure,
while non-AS-
DVD-Ig protein and non-LS-DVD-Ig proteins fail to remain stable in such
formulations and
are prone to aggregation and/or loss of monomer content. Generally, an
increase in
41
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
aggregation indicates a decrease in monomer content.
In certain embodiments, a freeze/thaw (F/T) (e.g., - 80 C / 30 C) test may
be used to
identify DVD-Ig proteins that are AS-DVD-Ig proteins or LS-DVD-Ig proteins.
Such a
method relies upon determining the percentage of high molecular weight (HMW)
species in a
solution having a high concentration of DVD-Ig protein (e.g., 100 mg/ml). In
one
embodiment, an AS-DVD-Ig protein or an LS-DVD-Ig protein is defined as a DVD-
Ig
protein that shows 1% or less increase in high molecular weight (HMW) species
(aggregates)
(e.g., 0.5% or less aggregate) or less than a 1 % increase (e.g., 0.5%) in
change in relative %
monomer as determined by SEC following a F/T cycle. A F/T cycle includes
freezing the
DVD-Ig protein solution, thawing the solution, optionally repeating, and
testing the thawed
solution for aggregate levels using, e.g., SEC analysis. Freeze/thaw testing
may be
performed on a solution comprising a DVD-Ig protein at a concentration of
about 1 mg/ml to
about 10 mg/ml. In certain embodiments, the F/T testing includes measuring
aggregation or
alternatively change in monomer content by SEC after 4 freeze/thaw cycles from
about 30 C
to ¨ 80 C. If testing results in more than about a 1 % increase in relative
percent aggregates
or change in relative % monomer after 4 cycles, then the DVD-Ig protein would
be
considered a non- LS DVD Ig.
In certain embodiments, the unfolding temperature of a DVD-Ig protein may be
used
to determine whether the DVD-Ig protein is an AS-DVD-Ig protein or an LS-DVD-
Ig
protein. As described in Figure 4, a DVD-Ig protein that shows a temperature
of unfolding of
less than about 45 C is generally not characterized as an AS-DVD-Ig protein
or an LS-DVD-
Ig protein ("A" as described in Figure 4). In certain other embodiments, a DVD-
Ig protein
that shows a temperature of unfolding of about 45-50 C is unlikely to be an
AS-DVD-Ig
protein but may be an LS-DVD-Ig protein ("B" as described in Figure 4). In
certain other
embodiments, a DVD-Ig protein that shows a temperature of unfolding that is
higher than 50
C is generally considered to be an AS-DVD-Ig protein and an LS-DVD-Ig protein
("C" as
described in Figure 4). Tests to determine DVD-Ig protein unfolding
temperatures are known
in the art (see also Example 1 describing thermodynamic testing) and are
generally performed
at a pH of between about 5 and about 7, e.g., about 5.5 to about 6.5.
In order to determine whether a DVD-Ig protein is an AS-DVD-Ig protein or an
LS-
DVD-Ig, storage testing of the solution can be performed. For example, storage
testing may
be performed at 5 C or 40 C for 14 to 21 days at a DVD-Ig protein in
solution at a
42
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
concentration ranging from 1 to 100 mg/ml, e.g., about 50-100 mg/ml.
Testing at temperatures greater than ambient temperatures, e.g., 40 C, are
often
referred to as accelerated conditions. In certain embodiments, the accelerated
storage
conditions include storing the DVD-Ig protein in the absence of light at 40
C. In certain
embodiments, testing is based on a solution's DVD-Ig protein aggregation
levels at a high
temperature (e.g., 40 C) and a high concentration (e.g., 50 mg/ml) as
determined by SEC.
For example, the DVD-Ig protein may be formulated at a concentration of at
least about 50
mg/ml, e.g., 50 to 100 mg/ml, in an aqueous formulation using a citrate
phosphate buffer or a
histidine buffer, and stored under accelerated conditions. Accelerated
conditions may include
temperatures higher than room temperature, e.g., storage temperatures of about
35 to about
45 Celsius (C), for extended periods of time, e.g., about 10 to about 21
days. In certain
embodiments, the accelerated storage conditions used to screen for an AS-DVD-
Ig protein or
LS-DVD-Ig protein are 14 days of storage at a temperature of 40 C at a DVD-
Ig protein
concentration of 50 mg/ml or greater, e.g., about 60 mg/ml or 50-100 mg/ml.
Following
accelerated storage testing at a concentration of 50 mg/ml or greater, e.g. 50-
100 mg/ml, the
solution may be tested for signs of DVD-Ig protein aggregation or change in
monomer
content.
In certain embodiments, DVD-Ig proteins may be tested to determine whether
they
are an AS-DVD-Ig protein or an LS-DVD-Ig protein in buffered solutions (e.g.,
histidine or
citrate / phosphate buffer) having a pH of 5.0 to 6.5, e.g., a pH of about 6,
and a concentration
of about 50 mg/ml to about 100 mg/ml.
Notably, lower levels of DVD-Ig protein concentration (e.g., 1 mg/ml) may also
be
used to test the protein, wherein lower levels of aggregate would be expected
for an AS-
DVD-Ig protein or an LS-DVD-Ig. For example, an AS-DVD-Ig protein is a DVD-Ig
that
has 3% or less aggregation when stored at about 40 C after 21 days at a
concentration of 1
mg/ml in an aqueous formulation.
Protein aggregation may be determined according to methods known in the art,
including, but not limited to, Size Exclusion Chromatography (SEC). Generally,
an increase
in aggregation indicates a decrease in monomer content, which can also be
determined using
SEC analysis.
In certain embodiments, the DVD-Ig protein is considered an AS-DVD-Ig protein
if
the solution has 10% or less aggregation of the DVD-Ig protein as determined
by Size
43
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Exclusion Chromatography (SEC) analysis following accelerated storage at a
concentration
of 1-100 mg/ml, preferably about 50 mg/ml to about 100 mg/ml. In certain
embodiments, the
DVD-Ig protein is considered an AS-DVD-Ig protein if the solution has 6% or
less
aggregation of the DVD-Ig protein as determined by SEC analysis following
accelerated
storage at a concentration of about 50 mg/ml to about 100 mg/ml. In certain
embodiments,
the DVD-Ig protein is considered an AS-DVD-Ig protein if the DVD-Ig protein
has less than
10%, alternatively less than 9%, less than 8 %, less than 7%, less than 6%,
less than 5%, less
than 4%, less than 3%, less than 2%, or less than 1% aggregation as determined
by SEC
analysis following accelerated storage at a concentration of about 50 mg/ml to
about 100
mg/ml. In certain embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig
that has less
than 8% aggregation following 14 days of accelerated storage (at, for example,
about 40 C).
In certain embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig that has
6% or less
aggregation following 14 days of accelerated storage (at, for example, about
40 C). In a
further embodiment, an AS-DVD-Ig protein is defined as a DVD-Ig that has less
than 5%
aggregation following 14 days of accelerated storage (at, for example, about
40 C). In
certain embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig that has 10%
or less
aggregation at about 40 C after 21 days of storage at a concentration of 100
mg/ml in an
aqueous formulation or has 10% or less aggregate following accelerated storage
after 14 days
at about 40 C, when formulated at a concentration of 50 mg/ml or more in an
aqueous
formulation.
In certain embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig protein
that
has 10% or less aggregation at about 40 C after 21 days of storage at a
concentration of 100
mg/ml in an aqueous formulation or, alternatively, a DVD-Ig protein that has
1% or less
aggregation at about 5 C after 21 days of storage at a concentration of 100
mg/ml in an
aqueous formulation. Alternatively, an AS-DVD-Ig protein is a DVD-Ig protein
that has
1.5% or less aggregation at about 5 C after 21 days of storage at a
concentration of 1 mg/ml
in an aqueous formulation or 3% or less aggregation at about 40 C after 21
days of storage at
a concentration of 1 mg/ml in an aqueous formulation. In certain embodiments,
an AS-
DVD-Ig protein is defined as a DVD-Ig protein that has less than 10% aggregate
formed
following accelerated storage after 14 days at about 40 C, when formulated at
a
concentration over 50 mg/ml in an aqueous formulation. In certain embodiments,
an AS-
DVD-Ig protein is defined as a DVD-Ig protein that has less than 10%
aggregation following
44
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
14 days of accelerated storage (at, for example, about 40 C). In certain
embodiments, an
AS-DVD-Ig protein is defined as a DVD-Ig protein that has less than 8%
aggregation
following 14 days of accelerated storage (at, for example, about 40 C). In
certain
embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig protein that has 6%
or less
aggregation following 14 days of accelerated storage (at, for example, about
40 C). In a
further embodiment, an AS-DVD-Ig protein is defined as a DVD-Ig protein that
has less than
5% aggregation following 14 days of accelerated storage (at, for example,
about 40 C).
Aggregation can be determined according to methods known in the art,
including, but not
limited to, size exclusion chromatography (SEC). In certain embodiments, the
accelerated
storage conditions include storing the DVD-Ig protein in the absence of light
at 40 C. DVD-
Ig proteins may be tested in aqueous formulations containing citrate and
phosphate buffer, or
histidine buffer. In certain embodiments, an AS-DVD-Ig protein has 10% or less
aggregation
as determined by SEC analysis following accelerated storage for 21 days at
about 40 C,
where the AS-DVD-Ig protein is formulated at a concentration of 50 to 100
mg/ml in a citrate
phosphate buffer or histidine buffer in an aqueous formulation. In certain
embodiments, an
AS-DVD-Ig protein has less than 6% aggregation as determined by SEC analysis
following
accelerated storage for 14 days at about 40 C, where the AS-DVD-Ig protein is
formulated at
a concentration of at least 50 mg/ml in a citrate phosphate buffer or
histidine buffer in an
aqueous formulation.
While percent aggregation may be used to determine whether aggregation is
present
following accelerated storage, monomer content of the DVD-Ig protein solution
may also be
used. Generally, an increase in percent aggregation indicates a decrease in
monomer content.
Alternatively, a DVD-Ig protein may be considered an AS-DVD-Ig protein if the
protein has
6% or less monomer loss (determined by SEC) after 14 days at 40 C or 3% or
less monomer
loss (determined by SEC) after 7 days at 40 C in a solution having a
concentration of 50
mg/ml or more DVD-Ig protein at pH about 5.0 to about 6.5, e.g., about 5.5 to
about 6.0 in 15
mM histidine. Monomer content may be used under any testing conditions,
including, but
not limited to, storage at 40 C and/or at a pH of about 5.0 to about 6.5,
e.g., about 5.5 to
about 6Ø
In certain embodiments, an AS-DVD-Ig protein is a DVD-Ig protein that has
about
10% relative (rel.) peak area or less change in monomers at about 40 C after
21 days of
storage at a concentration of about 100 mg/ml in an aqueous formulation or,
alternatively, a
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig protein that has about 1% rel. peak area or less change in monomers at
about 5 C
after 21 days of storage at a concentration of about 100 mg/ml and at a pH
between about 5.5
to about 6.5 in an aqueous formulation. Alternatively, an AS-DVD-Ig protein is
a DVD-Ig
protein that has about 1.5% rel. peak area or less change in monomers at about
5 C after 21
days of storage at a concentration of about 1 mg/ml in an aqueous formulation
or about 3%
rel. peak area or less change in monomers at about 40 C after 21 days of
storage at a
concentration of 1 mg/ml and at a pH between about 5.5 to about 6.5 in an
aqueous
formulation. In certain embodiments, an AS-DVD-Ig protein is defined as a DVD-
Ig protein
that has a change in monomers less than about 10% rel. peak area following
accelerated
storage after 14 days at about 40 C, when formulated at a concentration over
about 50 mg/ml
and at a pH between about 5.5 and about 6.5 in an aqueous formulation. In
certain
embodiments, an AS-DVD-Ig protein is defined as a DVD-Ig protein that has less
than 8%
rel. peak area change in monomers following 14 days of accelerated storage
(at, for example,
about 40 C) when formulated at a concentration over 60 mg/ml and at a pH
between 5.5 ¨
6.5 in an aqueous formulation. In certain embodiments, an AS-DVD-Ig protein is
defined as
a DVD-Ig protein that has 6% rel. peak area or less change in monomers
following 14 days of
accelerated storage (at, for example, about 40 C). In a further embodiment,
an AS-DVD-Ig
protein is defined as a DVD-Ig protein that has less than 5% rel. peak area
change in
monomers following 14 days of accelerated storage (at, for example, about 40
C).
In certain embodiments, an AS-DVD-Ig protein has 10% rel. peak area or less
change
in monomers as determined by SEC analysis following accelerated storage for 21
days at
about 40 C, where the AS-DVD-Ig protein is formulated at a concentration of
50 to 100
mg/ml in a citrate phosphate buffer or histidine buffer at a pH between about
5.0 to about 6.5,
e.g., about 5.5 to about 6.0, in an aqueous formulation. In certain
embodiments, an AS-
DVD-Ig protein has less than 6% rel. peak area change in monomers as
determined by SEC
analysis following accelerated storage for 14 days at about 40 C, where the
AS-DVD-Ig
protein is formulated at a concentration of at least 50 mg/ml in a citrate
phosphate buffer or
histidine buffer in an aqueous formulation.
In another alternative, AS-DVD-Ig proteins are identified based on a
solution's
stability aggregation and/or monomer content at a low temperature (e.g., 5 C)
and a high
concentration (e.g., 50 mg/ml) of DVD-Ig as determined by SEC. For example, a
solution
containing 50 mg/ml of an AS-DVD-Ig protein at a pH of about 5.0 to about 6.5,
e.g., about
46
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
5.5 to about 6.0 in 15 mM histidine may have 1% or less monomer (determined by
SEC) loss
after 7 days at 5 C (determined by SEC). In another example, a solution
containing 50
mg/ml of an AS-DVD-Ig protein at a pH of about 5.0 to about 6.5, e.g., about
5.5 to about 6.0
in 15 mM histidine may have 2% or less monomer loss after 14 days at 5 C.
Alternatively,
an AS-DVD-Ig has 1% or less aggregation at about 5 C after 21 days of storage
at a
concentration of 100 mg/ml in an aqueous formulation, or 1.5% or less
aggregation at about
C after 21 days of storage at a concentration of 1 mg/ml in an aqueous
formulation. In
certain embodiments, monomer loss is determined at a pH of about 5.0 to about
6.5, e.g.,
about 5.5 to about 6Ø
In certain embodiments, the test solution conditions described herein also
contain
0.02% (w/v) sodium azide as a bacteriostatic.
In certain embodiments, the DVD-Ig protein is considered an LS-DVD-Ig protein
if
the solution has 15% or less aggregation of the DVD-Ig protein as determined
by Size
Exclusion Chromatography (SEC) analysis. In certain embodiments, the LS-DVD-Ig
protein
is considered an LS-DVD-Ig protein if the DVD-Ig protein has 15% or less,
alternatively less
than 14%, less than 13%, less than 12%, less than 11%, less than aggregation
as determined
by SEC analysis.
In certain embodiments, an LS-DVD-Ig protein is defined as having less than
15%
aggregate formed, following accelerated storage, e.g., 14 days of accelerated
storage at about
40 C, when formulated at a concentration over 50 mg/ml in an aqueous
formulation. In
certain embodiments, an LS-DVD-Ig protein has 15% or less aggregation
following 14 days
of accelerated storage at, for example, 40 C. In certain embodiments, an LS-
DVD-Ig
protein has less than 14% aggregation following 14 days of accelerated
storage, at, for
example, about 40 C. In a further embodiment, an LS-DVD-Ig protein has less
than 13%
aggregation following 14 days of accelerated storage, at, for example, about
40 C.
Alternatively, an LS-DVD-Ig protein is defined as a DVD-Ig protein that has 1%
or less
aggregation following 4 freeze thaw cycles. DVD-Ig proteins may be tested in
aqueous
formulations containing citrate and phosphate buffer, or histidine buffer. In
certain
embodiments, an LS-DVD-Ig protein has 15% or less aggregation as determined by
SEC
analysis following accelerated storage for 14 days at about 40 C, where the
LS-DVD-Ig
protein is formulated at a concentration of at least 50 mg/ml in a citrate
phosphate buffer in
an aqueous formulation. As described above, aggregation can be determined
according to
47
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
methods known in the art, including, but not limited to, SEC.
In certain embodiments, an LS-DVD-Ig protein has more than 10% rel. peak area
change in monomers observed, following accelerated storage, e.g., 21 days at
about 40 C,
when formulated at a concentration of about 50 to 100 mg/ml at a pH between
about 5.0 and
about 6.5, e.g, about 5.5 to about 6.0, in an aqueous formulation. In certain
embodiments, an
LS-DVD-Ig protein has 50% rel. peak area or less change in monomers as
determined by
SEC analysis following accelerated storage for 14 days at about 40 C, where
the LS-DVD-Ig
protein is formulated at a concentration of at least 50 mg/ml in a citrate
phosphate buffer in
an aqueous formulation. In certain embodiments, an LS-DVD-Ig protein has 20 %
rel. peak
area or less change in monomers following 21 days of accelerated storage at
about 40 C,
when formulated at a concentration over 100 mg/ml at a pH between about 5.0 to
about 6.5,
e.g., about 5.5 to about 6.0 in an aqueous formulation. In certain
embodiments, an LS-
DVD-Ig protein has less than 18% rel. peak area change in monomers following
14 days of
accelerated storage, at, for example, about 40 C. In a further embodiment, an
LS-DVD-Ig
protein has less than 13% rel. peak area change in monomers following 14 days
of
accelerated storage, at, for example, about 40 C. Alternatively, an LS-DVD-Ig
protein is
defined as a DVD-Ig protein that has 1% rel. peak area or less change in
monomers following
4 freeze thaw cycles. Alternatively, an LS-DVD-Ig protein is defined as a DVD-
Ig protein
that has 4% rel. peak area or less change in monomers following 7 days at
about 25 C at a
concentration between 1-100 mg/mL, e.g., about 1 to 10 mg/ml or about 50 to
100 mg/ml, in
aqueous solution at the most stable pH. Alternatively, an LS-DVD-Ig protein is
defined as a
DVD-Ig protein that has 1 % rel. peak area or less change in monomers
following 7 days at
about 5 C in aqueous solution at the most stable
Notably, the assays described herein used to determine whether a DVD-Ig
protein is
an AS-DVD-Ig protein or an LS-DVD-Ig protein are not exclusive, meaning that,
for
example, an AS-DVD-Ig may be characterized as having an unfolding temperature
of at least
50 C and also have about a 1% relative peak area or less change in monomers
at about 5 C
after 21 days of storage at a concentration of about 50 to about 100 mg/ml and
at a pH
between about 5.5 to about 6.5 in an aqueous, buffered formulation. In certain
embodiments,
an AS-DVD-Ig protein has 10% rel. peak area or less change in monomers as
determined by
SEC analysis following accelerated storage for 21 days at about 40 C and has
an unfolding
temperature of at least 50 C.
48
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Once the DVD-Ig protein is identified as being an AS-DVD-Ig protein or LS-DVD-
Ig
protein according to the aforementioned tests, the AS-DVD-Ig protein or LS-DVD-
Ig protein
can be stably formulated. Further identification of AS-DVD-Ig protein and LS-
DVD-Ig
proteins is described below in Example 4.
III. Aqueous Stable Dual Variable Domain Immunoglobulin (AS-DVD-Ig)
Formulations of the Disclosure
The disclosure provides stable aqueous formulations comprising AS-DVD-Ig
proteins. The present disclosure features aqueous formulations having improved
properties
as compared to art-recognized formulations, in that AS-DVD-Ig proteins can be
stably
formulated, even at high concentrations.
Thus, the disclosure is based, at least in part, on the discovery that a
subpopulation of
DVD-Ig proteins can be stably formulated in an aqueous formulation having a pH
of about
4.5 to about 7.5, and containing a buffer, a surfactant, and/or a polyol.
These "Aqueous
Stable DVD-Ig proteins" are referred to as AS-DVD-Ig proteins and can be
identified using
an accelerated storage assay where the DVD-Ig protein is formulated in a
liquid form at a
concentration greater than 50 mg/ml, e.g., 50 mg/ml to 100 mg/ml (see also
Section II.E.
above).
In certain embodiments, the aqueous formulation of the disclosure has a pH of
about
4.5 to about 7.5. As described in the working examples, pH was found to have
an impact on
the stability of the AS-DVD-Ig protein in a buffered formulation. In certain
embodiments,
the pH of the formulation containing the AS-DVD-Ig protein ranges from about
4.5 to about
7.5; alternatively, the pH of the AS-DVD-Ig protein formulation ranges from
about 5.0 to
about 7.0; alternatively the pH may range from about 5 to about 6.5;
alternatively the pH of
the formulation may range from about 5.5 to about 6.5. In a further
embodiment, the pH
ranges from about 5.8 to about 6.2. The ranges intermediate to the
aforementioned pH
values, e.g., about 5.6 to about 6.4, are also intended to be part of the
disclosure. Ranges of
values using a combination of any of the aforementioned values as upper /
lower limits are
also intended to be included, e.g., a pH range of about 5.5 to about 6.2. In
certain
embodiments, the pH of the formulation of the disclosure is about 6Ø
In certain embodiments, the aqueous formulation of the disclosure includes an
AS-
DVD-Ig protein and a buffer. Examples of buffers that may be used in the
formulation of the
49
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
disclosure include, but are not limited to, acetate, histidine, glycine,
arginine, phosphate, Tris,
and citrate. The molarity of the buffer used in the formulation of the
disclosure may range
from about 1 to about 50 mM. In certain embodiments, the aqueous formulation
of the
disclosure has a buffer with a molarity of about 5 to about 50 mM.
Alternatively, the
molarity of the buffer is about 10 to about 20 mM.
In certain embodiments of the disclosure, the buffer system comprises about 1
to
about 200 mM histidine (e.g., about 2 to about 100 mM; about 5 to about 70 mM;
about 5 to
about 60 mM; about 5 to about 50 mM; about 10 to about 40 mM, about 10 to
about 30 mM,
or about 10 to about 20 mM) with a pH of about 4.5 to about 7.5, e.g., a pH of
about 5 to
about 7, or a pH of about 5.5 to about 6.5. In certain embodiments, the buffer
system of the
disclosure comprises about 15 mM histidine with a pH of about 4.5 to about
7.5, e.g., a pH of
about 5 to about 7, or a pH of about 5.5 to about 6.5.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
(e.g.,
about 5 to about 40 mM) glycine with a pH of about 4.5 to about 7.5. In a
particular
embodiment, the buffer system comprises glycine at a concentration of about 20
mM. In a
more particular embodiment, the buffer system comprises glycine at a
concentration of about
20 mM, and glycerol at a concentration of about 20 to about 30 mg/ml, e.g.,
about 26 mg/ml,
with a pH of about 4.5 to about 7.5, e.g., a pH of about 5 to about 7, or a pH
of about 5.5 to
about 6.5.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
acetate
(e.g., about 5 to about 50 mM, about 2 to about 40 mM; about 5 to about 30 mM;
or about 2
to about 15 mM) with a pH of about 4.5 to about 7.5, e.g., a pH of about 5 to
about 7, or a pH
of about 5.5 to about 6.5. In a particular embodiment, the buffer system
comprises acetate at
a concentration of about 2 to about 15 mM.
In certain embodiments of the disclosure, the buffer system comprises about 1
to
about 50 mM (e.g., about 5 to about 50 mM, about 2 to about 40 mM; about 5 to
about 30
mM; or about 2 to about 15 mM) arginine with a pH of about 4.5 to about 7.5,
e.g., a pH of
about 5 to about 7, or a pH of about 5.5 to about 6.5. In a particular
embodiment, the buffer
system comprises arginine at a concentration of about 15 mM.
In still another embodiment of the disclosure, the buffer system comprises
about 1 to
about 50 mM (e.g., about 5 to about 50 mM) citrate with a pH of about 4.5 to
about 7.5, e.g.,
a pH of about 5 to about 7, or a pH of about 5.5 to about 6.5. In a particular
embodiment, the
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
buffer system comprises citrate at a concentration of about 15 mM.
In still another embodiment of the disclosure, the buffer system comprises
about 1 to
about 50 mM (e.g., about 5 to about 50 mM) phosphate with a pH of about 4.5 to
about 7.5,
e.g., a pH of about 5 to about 7, or a pH of about 5.5 to about 6.5. In a
particular
embodiment, the buffer system comprises phosphate at a concentration of about
10 mM. In a
one embodiment, the buffer system comprises phosphate at a concentration of
about 10 mM,
and sodium chloride at a concentration of about 125 mM.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
(e.g.,
about 5 to about 50 mM) Tris with a pH of about 4.5 to about 7.5, e.g., a pH
of about 5 to
about 7, or a pH of about 5.5 to about 6.5. In a particular embodiment, the
buffer system
comprises Tris at a concentration of about 2 to about 10 mM.
In yet another embodiment, the buffer system comprises phosphate and citrate,
e.g.,
phosphate (e.g., sodium hydrogen phosphate) at a concentration of about 1 to
about 50 mM
(e.g., about 5 to about 50 mM, about 5 to about 10 mM), and citrate (citric
acid) at a
concentration of about 1 to about 50 mM (e.g., about 5 to about 10 mM). In a
particular
embodiment, the buffer system comprises phosphate at a concentration of about
5 mM and
citrate (citric acid) at a concentration of about 5mM. In certain embodiments,
the buffer
system comprises phosphate at a concentration of about 10 mM and citrate
(citric acid) at a
concentration of about 10 mM.
In addition to the buffer, a polyol may be added to the aqueous formulation,
e.g., for
added stability. The polyol may be added to the formulation in an amount that
may vary with
respect to the desired isotonicity of the formulation. In certain embodiments,
the aqueous
formulation is isotonic.
Examples of polyols that may be used in the aqueous formulations of the
disclosure
include, but are not limited to, sorbitol, mannitol, and sucrose fructose,
mannose, maltose,
lactose, arabinose, xylose, ribose, rhamnose, galactose and glucose.
Nonreducing sugars
include sucrose, trehalose, sorbose, melezitose, raffinose, mannitol, xylitol,
erythritol,
threitol, sorbitol and glycerol. The amount of polyol added may also vary with
respect to the
molecular weight of the polyol. For example, a lower amount of a
monosaccharide (e.g.,
mannitol) may be added, compared to a disaccharide (e.g., trehalose).
In certain embodiments, the concentration of a polyol such as sorbitol is
about 30 to
about 50 mg/ml. In a one embodiment, the composition comprises about 20 to
about 60
51
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
mg/ml sorbitol, about 25 to about 55 mg/ml, about 30 to about 50 mg/ml, about
35 to about
45 mg/ml, and ranges in between, e.g., about 33 to about 48 mg/ml of sorbitol.
In certain embodiments, sucrose has a concentration of about 70 to about 90
mg/ml.
In certain embodiments, the composition comprises about 60 to about 100 mg/ml
sucrose,
about 65 to about 95 mg/ml, about 70 to about 90 mg/ml, about 75 to about 85
mg/ml, and
ranges in between, e.g., about 72 to about 84 mg/ml of sucrose.
In certain embodiments, the polyol is mannitol. In certain embodiments, the
composition comprises about 10 to about 100 mg/ml, or about 20 to about 80,
about 20 to
about 70, about 30 to about 60, about 30 to about 50 mg/ml of mannitol, for
example, about
10, about 20, about 30, about 40, about 50, about 60, about 70, about 80,
about 90, or about
100 mg/ml.
In certain embodiments, the aqueous formulation of the disclosure includes an
AS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, and a polyol,
wherein the
formulation has a pH of about 4.5 to about 7.5.
In addition to the buffer, a surfactant may be added to the aqueous
formulations, e.g.,
for added stability. Exemplary surfactants include nonionic detergents such as
polysorbates
(e.g., polysorbates 20, 80) or poloxamers (e.g., poloxamer 188). In certain
embodiments, the
amount of surfactant added is such that it reduces aggregation of the
formulated AS-DVD-Ig
protein and/or minimizes the formation of particulates in the formulation
and/or reduces
adsorption.
In certain embodiments, the aqueous formulation contains the detergent
polysorbate
80 or Tween 80. Tween 80 is a term used to describe polyoxyethylene (20)
sorbitan
monooleate. In certain embodiments, the formulation contains about 0.001 to
about 1%
polysorbate 80, or about 0.005 and about 0.05% polysorbate 80, for example,
about 0.001%,
about 0.005, about 0.01%, about 0.05%, or about 0.1% polysorbate 80. In
certain
embodiments, about 0.01% polysorbate 80 is found in the formulation of the
disclosure.
In certain embodiments, the aqueous formulation of the disclosure includes an
AS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, and a
surfactant, wherein the
formulation has a pH of about 4.5 to about 7.5. In certain embodiments, the
surfactant is a
polysorbate, e.g., polysorbate 80 or polysorbate 20. In certain embodiments,
the polysorbate
has a concentration of about 0.005% to about 0.02%.
52
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the aqueous formulation of the disclosure includes an
AS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, a surfactant,
and a polyol,
wherein the formulation has a pH of about 4.5 to about 7.5. In certain
embodiments, the
formulation includes an AS-DVD-Ig, a buffer (e.g., histidine), a polysorbate,
e.g., polysorbate
80, and a sugar alcohol, e.g., mannitol or sorbitol. In certain embodiments,
the formulation
includes an AS-DVD-Ig, a buffer (e.g., histidine), a polysorbate, e.g.,
polysorbate 80, and a
non-reducing sugar, e.g., sucrose.
One advantage of the aqueous formulation of the disclosure is that high
concentrations of AS-DVD-Ig proteins may be stably maintained in an aqueous
solution.
Thus, in certain embodiments, the formulations of the disclosure comprise a
high protein
concentration, including, for example, a protein concentration greater than
about 10 mg/ml,
greater than about 20 mg/ml, greater than about 30 mg/ml, greater than about
40 mg/ml,
greater than about 50 mg/ml, greater than about 100 mg/ml, greater than about
110 mg/ml,
greater than about 120 mg/ml, greater than about 130 mg/ml, greater than about
140 mg/ml,
greater than about 150 mg/ml, greater than about 160 mg/ml, greater than about
170 mg/ml,
greater than about 180 mg/ml, greater than about 190 mg/ml, or greater than
about 200
mg/ml.
In various embodiments of the disclosure, the concentration of the AS-DVD-Ig
protein in the aqueous formulation is about 0.1-250 mg/ml, about 0.5-220
mg/ml, about 1-
210 mg/ml, about 5-200 mg/ml, about 10-195 mg/ml, about 15-190 mg/ml, about 20-
185
mg/ml, about 25-180 mg/ml, about 30-175 mg/ml, about 35-170 mg/ml, about 40-
165 mg/ml,
about 45-160 mg/ml, about 50-155 mg/ml, about 55-150 mg/ml, about 60-145
mg/ml, about
65-140 mg/ml, about 70-135 mg/ml, about 75-130 mg/ml, about 80-125 mg/ml,
about 85-120
mg/ml, about 90-H5 mg/ml, about 95-110 mg/ml, about 95-105 mg/ml, or about 100
mg/ml.
Ranges intermediate to the above recited concentrations, e.g., about 31-174
mg/ml, are also
intended to be part of this disclosure. For example, ranges of values using a
combination of
any of the above recited values as upper and/or lower limits are intended to
be included.
The present disclosure features aqueous formulations having improved
properties as
compared to art-recognized formulations. For example, the formulations of the
disclosure
have an AS-DVD-Ig protein aggregation level of less than 7% aggregate, less
than 6%
aggregate, or less than 5% aggregate.
53
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the AS-DVD-Ig protein used in the aqueous formulation
of
the disclosure is an anti-TNF/IL-17 DVD-Ig protein having heavy and light
chain sequences
having an amino acid sequence as set forth in SEQ ID NOs: 62 and 63,
respectively.
In certain embodiments, the aqueous formulation of the disclosure comprises an
anti-
TNF/IL-17 DVD-Ig protein (e.g., an anti-TNF/IL-17 DVD-Ig protein comprising a
heavy and
a light chain comprising amino acid sequences corresponding to the heavy and
light chain
CDRs set forth in SEQ ID NOs: 62 and 63; or an anti-TNF/IL-17 DVD-Ig protein
comprising
a heavy and a light chain comprising amino acid sequences corresponding to the
heavy and
light chain variable regions set forth in SEQ ID NOs: 62 and 63; or DVD-A),
histidine or
acetate buffer, and sucrose or sorbitol, wherein the formulation has a pH of
about 5 to about 6
and wherein the protein concentration is about 50 mg/ml or more. In further
embodiments,
the aqueous formulation has a pH ranging from about 5 to about 5.5, e.g.,
about 5.2. In a
further embodiment, the aqueous formulation comprises a surfactant, e.g., a
polysorbate.
Ranges for the recited buffers, excipients, and pH are described above.
In certain embodiments, the AS-DVD-Ig protein is an anti-ILlcc/IL-lp DVD-Ig
comprising an anti-ILlcc/ILlp DVD-Ig protein having a heavy and light chain
sequences
having an amino acid sequence as set forth in SEQ ID NOs: 66 and 67,
respectively.
In certain embodiments, the aqueous formulation of the disclosure comprises an
anti-
ILlcc/ILlp DVD-Ig protein (e.g., an anti-IL1WILl3 DVD-Ig protein comprising a
heavy and
a light chain comprising amino acid sequences corresponding to the heavy and
light chain
CDRs set forth in SEQ ID NOs: 66 and 67; or an anti-TNF/IL-17 DVD-Ig protein
comprising
a heavy and a light chain comprising amino acid sequences corresponding to the
heavy and
light chain variable regions set forth in SEQ ID NOs: 66 and 67; or DVD-C),
histidine buffer,
and sucrose or sorbitol, wherein the formulation has a pH of about 5 to about
6 and wherein
the protein concentration is about 50 mg/ml or more. In further embodiments,
the aqueous
formulation has a pH ranging from about 5 to about 5.5, e.g., about 5.4. In a
further
embodiment, the aqueous formulation comprises a surfactant, e.g., a
polysorbate. Ranges for
the recited buffers, excipients, and pH are described above.
In certain embodiments, the aqueous formulation of the disclosure comprises an
anti-
DLL4/VEGF DVD-Ig protein (e.g., an anti-DLL4/VEGF DVD-Ig protein comprising a
heavy and a light chain comprising amino acid sequences corresponding to the
heavy and
54
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
light chain CDRs set forth in SEQ ID NOs: 28 and 29 or SEQ ID NOs: 72 and 73;
or an anti-
DLL4/VEGF DVD-Ig protein comprising a heavy and a light chain comprising amino
acid
sequences corresponding to the heavy and light chain variable regions set
forth in SEQ ID
NOs: 28 and 29 or SEQ ID NOs: 72 and 73), histidine buffer, and sucrose or
arginine,
wherein the formulation has a pH of about 5 to about 6 and wherein the protein
concentration
is about 20 mg/mL to about 50 mg/ml. In further embodiments, the aqueous
formulation has
a pH ranging from about 5.5 to about 6 In a further embodiment, the aqueous
formulation
comprises a surfactant, e.g., a polysorbate. Ranges for the recited buffers,
excipients, and pH
are described above.
IV. Lyophilized Stable Dual Variable Domain Immunoglobulin (LS-DVD-Ig)
Protein
Formulations of the Disclosure
The disclosure further provides stable lyophilized formulations comprising LS-
DVD-
Ig proteins. Thus, the disclosure is based, at least in part, on the discovery
that a
subpopulation of DVD-Ig proteins can be stably formulated in a lyophilized
formulation
having a pH of about 4.5 to about 7.5, and containing a buffer, a surfactant,
and/or a polyol.
These "Lyophilized Stable DVD-Ig proteins" or "LS-DVD-Ig proteins" can be
identified
using an accelerated storage assay (described above) where the DVD-Ig protein
is formulated
in a liquid form at a concentration greater than 50 mg/ml.
The below-mentioned features of the lyophilized formulations of the disclosure
may
refer to the solution prior to lyophilization or, alternatively, the
reconstituted formulation, for
example, where liquid concentrations (mg/ml) are referenced.
In certain embodiments, the lyophilized formulation of the disclosure has a pH
of
about 4.5 to about 7.5. In certain embodiments, the pH of the formulation
containing the LS-
DVD-Ig protein ranges from about 4.5 to about 7.5; alternatively, the pH of
the LS-DVD-Ig
protein formulation ranges from about 5.0 to about 7.0; alternatively the pH
may range from
about 5 to about 6.5; alternatively the pH of the formulation may range from
about 5.5 to
about 6.5. In a further embodiment, the pH ranges from about 5.8 to about 6.2.
The ranges
intermediate to the aforementioned pH values, e.g., about 5.6 to about 6.4,
are also intended
to be part of the disclosure. Ranges of values using a combination of any of
the
aforementioned values as upper / lower limits are also intended to be
included, e.g., a pH
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
range of about 5.5 to about 6.2. In certain embodiments, the pH of the
formulation of the
disclosure is about 6Ø
In certain embodiments, the lyophilized formulation of the disclosure includes
an LS-
DVD-Ig protein and a buffer. Examples of buffers that may be used in the
formulation of the
disclosure include, but are not limited to, acetate, histidine, glycine,
arginine, phosphate, Tris,
and citrate. The molarity of the buffer used in the formulation of the
disclosure may range
from about 1 to about 50 mM. In certain embodiments, the aqueous formulation
of the
disclosure has a buffer with a molarity of about 5 to about 50 mM.
Alternatively, the
molarity of the buffer is about 10 to about 20 mM.
In certain embodiments of the disclosure, the buffer system comprises about 1
to
about 200 mM histidine (e.g., about 2 to about 100 mM; about 5 to about 70 mM;
about 5 to
about 60 mM; about 5 to about 50 mM; about 10 to about 40 mM, about 10 to
about 30 mM,
or about 10 to about 20 mM) with a pH of about 4.5 to about 7.5, e.g., a pH of
about 5 to
about 7, or a pH of about 5.5 to about 6.5. In certain embodiments, the buffer
system of the
disclosure comprises about 15 mM histidine with a pH of about 4.5 to about
7.5, e.g., a pH of
about 5 to about 7, or a pH of about 5.5 to about 6.5.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
(e.g.,
about 5 to about 40 mM) glycine with a pH of about 4.5 to about 7.5. In a
particular
embodiment, the buffer system comprises glycine at a concentration of about 20
mM. In a
more particular embodiment, the buffer system comprises glycine at a
concentration of about
20 mM, and glycerol at a concentration of about 20 to about 30 mg/ml, e.g.,
about 26 mg/ml,
with a pH of about 4.5 to about 7.5, e.g., a pH of about 5 to about 7, or a pH
of about 5.5 to
about 6.5.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
acetate
(e.g., about 5 to about 50 mM, about 2 to about 40 mM; about 5 to about 30 mM;
or about 2
to about 15 mM) with a pH of about 4.5 to about 7.5, e.g., a pH of about 5 to
about 7, or a pH
of about 5.5 to about 6.5. In a particular embodiment, the buffer system
comprises acetate at
a concentration of about 2 to about 15 mM.
In certain embodiments of the disclosure, the buffer system comprises about 1
to
about 50 mM (e.g., about 5 to about 50 mM, about 2 to about 40 mM; about 5 to
about 30
mM; or about 2 to about 15 mM) arginine with a pH of about 4.5 to about 7.5,
e.g., a pH of
about 5 to about 7, or a pH of about 5.5 to about 6.5. In a particular
embodiment, the buffer
56
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
system comprises arginine at a concentration of about 15 mM.
In still another embodiment of the disclosure, the buffer system comprises
about 1 to
about 50 mM (e.g., about 5 to about 50 mM) citrate with a pH of about 4.5 to
about 7.5, e.g.,
a pH of about 5 to about 7, or a pH of about 5.5 to about 6.5. In a particular
embodiment, the
buffer system comprises citrate at a concentration of about 15 mM.
In still another embodiment of the disclosure, the buffer system comprises
about 1 to
about 50 mM (e.g., about 5 to about 50 mM) phosphate with a pH of about 4.5 to
about 7.5,
e.g., a pH of about 5 to about 7, or a pH of about 5.5 to about 6.5. In a
particular
embodiment, the buffer system comprises phosphate at a concentration of about
10 mM. In a
one embodiment, the buffer system comprises phosphate at a concentration of
about 10 mM,
and sodium chloride at a concentration of about 125 mM.
In certain embodiments, the buffer system comprises about 1 to about 50 mM
(e.g.,
about 5 to about 50 mM) Tris with a pH of about 4.5 to about 7.5, e.g., a pH
of about 5 to
about 7, or a pH of about 5.5 to about 6.5. In a particular embodiment, the
buffer system
comprises Tris at a concentration of about 2 to about 10 mM.
In yet another embodiment, the buffer system comprises phosphate and citrate,
e.g.,
phosphate (e.g., sodium hydrogen phosphate) at a concentration of about 1 to
about 50 mM
(e.g., about 5 to about 50 mM, about 5 to about 10 mM), and citrate (citric
acid) at a
concentration of about 1 to about 50 mM (e.g., about 5 to about 10 mM). In a
particular
embodiment, the buffer system comprises phosphate at a concentration of about
5 mM and
citrate (citric acid) at a concentration of about 5mM. In certain embodiments,
the buffer
system comprises phosphate at a concentration of about 10 mM and citrate
(citric acid) at a
concentration of about 10 mM.
In addition to the buffer, a polyol may be added to the formulation, e.g., for
added
stability. The polyol may be added to the formulation in an amount that may
vary with
respect to the desired isotonicity of the formulation. In certain embodiments,
the lyophilized
formulation is isotonic upon reconstitution.
Examples of polyols that may be used in the lyophilized formulations of the
disclosure include, but are not limited to, mannitol, sucrose, trehalose and
raffinose. The
amount of polyol added may also vary with respect to the molecular weight of
the polyol. For
example, a lower amount of a monosaccharide (e.g., mannitol) may be added,
compared to a
disaccharide (e.g., trehalose).
57
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
In certain embodiments, the concentration of a polyol such as sorbitol is
about 30 to
about 50 mg/ml. In a one embodiment, the composition comprises about 20 to
about 60
mg/ml sorbitol, about 25 to about 55 mg/ml, about 30 to about 50 mg/ml, about
35 to about
45 mg/ml, and ranges in between, e.g., about 33 to about 48 mg/ml of sorbitol.
In certain embodiments, sucrose has a concentration of about 70 to about 90
mg/ml.
In certain embodiments, the composition comprises about 60 to about 100 mg/ml
sucrose,
about 65 to about 95 mg/ml, about 70 to about 90 mg/ml, about 75 to about 85
mg/ml, and
ranges in between, e.g., about 72 to about 84 mg/ml of sucrose.
In certain embodiments, the polyol is mannitol. In certain embodiments, the
composition comprises about 10 to about 100 mg/ml, or about 20 to about 80,
about 20 to
about 70, about 30 to about 60, about 30 to about 50 mg/ml of mannitol, for
example, about
10, about 20, about 30, about 40, about 50, about 60, about 70, about 80,
about 90, or about
100 mg/ml.
In certain embodiments, the lyophilized formulation of the disclosure includes
an AS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, and a polyol,
wherein the
formulation has a pH of about 4.5 to about 7.5.
In addition to the buffer, a surfactant may be added to the lyophilized
formulations,
e.g., for added stability. Exemplary surfactants include nonionic detergents
such as
polysorbates (e.g., polysorbates 20, 60, 80,) or poloxamers (e.g., poloxamer
188). In certain
embodiments, the amount of surfactant added is such that it reduces
aggregation of the
formulated LS-DVD-Ig protein and/or minimizes the formation of particulates in
the
formulation and/or reduces adsorption.
In certain embodiments, the lyophilized formulation contains the detergent
polysorbate 80 or Tween 80. Tween 80 is a term used to describe
polyoxyethylene (20)
sorbitan monooleate. In certain embodiments, the formulation contains about
0.001 to about
0.1% polysorbate 80, or about 0.005 and about 0.05%, 20 polysorbate 80, for
example, about
0.001, about 0.005, about 0.01, about 0.05, or about 0.1% polysorbate 80. In
certain
embodiments, about 0.01% polysorbate 80 is found in the formulation of the
disclosure.
In certain embodiments, the lyophilized formulation of the disclosure includes
an LS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, and a
surfactant, wherein the
formulation has a pH of about 4.5 to about 7.5. In certain embodiments, the
surfactant is a
polysorbate, e.g., polysorbate 80 or polysorbate 20. In certain embodiments,
the polysorbate
58
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
has a concentration of about 0.005% to about 0.02%.
In certain embodiments, the lyophilized formulation of the disclosure includes
an LS-
DVD-Ig, a buffer having a molarity of about 5 to about 50 mM, a surfactant,
and a polyol,
wherein the formulation has a pH of about 4.5 to about 7.5. In certain
embodiments, the
formulation includes an LS-DVD-Ig, a buffer (e.g., histidine), a polysorbate
(e.g., polysorbate
80), and a sugar alcohol (e.g., mannitol or sorbitol). In certain embodiments,
the formulation
includes an LS-DVD-Ig, a buffer (e.g., histidine), a polysorbate, e.g.,
polysorbate 80, and a
non-reducing sugar, e.g., sucrose.
The lyophilized formulation described herein is initially made as a "pre-
lyophilized
formulation," which is the formulation prior to the lyophilization process.
The amount of
protein present in the pre-lyophilized formulation is determined taking into
account the
desired dose volumes, mode(s) of administration etc. For example, the starting
concentration
of an LS-DVD-Ig protein can be from about 2 mg/ml to about 50 mg/ml.
In certain embodiments, the DVD-Ig protein used in the lyophilized
formulations of
the disclosure specifically binds TNF/IL-17, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 62
and 63. In
certain embodiments, the anti-TNF/IL-17-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 62 and
63. In certain embodiments, the anti-TNF/IL-17-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
62 and 63.
In certain embodiments, the lyophilized formulation of the disclosure
comprises an
anti-TNF/IL-17 DVD-Ig protein ((e.g., an anti-TNF/IL-17 DVD-Ig protein
comprising a
heavy and a light chain comprising amino acid sequences corresponding to the
heavy and
light chain CDRs set forth in SEQ ID NOs: 62 and 63; or an anti-TNF/IL-17 DVD-
Ig protein
comprising a heavy and a light chain comprising amino acid sequences
corresponding to the
heavy and light chain variable regions set forth in SEQ ID NOs: 62 and 63; or
DVD-A),
histidine or acetate buffer, and sucrose or sorbitol, where the formulation
has a pH of about 5
to about 6 and wherein the protein concentration is greater than about 50
mg/ml upon
reconstitution..
In certain embodiments, the DVD-Ig protein used in the lyophilized
formulations of
the disclosure specifically binds ILlcc/IL-113 and comprises a heavy and a
light chain
59
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
sequence comprising an amino acid sequence as set forth in SEQ ID NOs: 66 and
67,
respectively. In certain embodiments, the DVD-Ig protein used in the
lyophilized
formulations specifically binds IL-lcc/IL-113, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 66
and 67. In
certain embodiments, the anti-IL-lcc/IL-113-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 66 and
67. In certain embodiments, the anti-IL-lcc/IL-113-Ig protein comprises the
amino acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
66 and 67.
In certain embodiments, the lyophilized formulation of the disclosure
comprises an
anti-anti-ILlcc/ILlp DVD-Ig protein (e.g., an anti-ILlcc/ILlp DVD-Ig protein
comprising a
heavy and a light chain comprising amino acid sequences corresponding to the
heavy and
light chain CDRs set forth in SEQ ID NOs: 66 and 67; or an anti-TNF/IL-17 DVD-
Ig protein
comprising a heavy and a light chain comprising amino acid sequences
corresponding to the
heavy and light chain variable regions set forth in SEQ ID NOs: 66 and 67; or
DVD-C),
histidine buffer, and sucrose or sorbitol, where the formulation has a pH of
about 5 to about 6
and wherein the protein concentration is greater than about 50 mg/ml upon
reconstitution. In
certain embodiments, the aqueous formulation has a pH ranging from about 5 to
about 5.5,
e.g., about 5.4.
In certain embodiments, the DVD-Ig protein used in the lyophilized
formulations of
the disclosure specifically binds TNF/PGE2, and comprises amino acid sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 64
and 65. In
certain embodiments, the anti-TNF/PGE2-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 64 and
65. In certain embodiments, the anti-TNF/PGE2-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
64 and 65.
In certain embodiments, the lyophilized formulation of the disclosure
comprises a
DVD-Ig protein that specifically binds DLL4/VEGF, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 28
and 29 or SEQ
ID NOs: 72 and 73. In certain embodiments, the anti-DLL4/VEGF-Ig protein
comprises
amino acid sequences corresponding to the heavy and light chain variable
regions set forth in
SEQ ID NOs: 28 and 29 or SEQ ID NOs: 72 and 73. In certain embodiments, the
anti-
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DLL4/VEGF-Ig protein comprises the amino acid sequences corresponding to the
heavy and
light chains set forth in SEQ ID NOs: 28 and 29 or SEQ ID NOs: 72 and 73.
In certain embodiments, the lyophilized formulation of the disclosure
comprises a
DVD-Ig protein that specifically binds IL12/IL18, and comprises amino acid
sequences
corresponding to the heavy and light chain CDRs set forth in SEQ ID NOs: 70
and 71. In
certain embodiments, the anti-IL12/IL18-Ig protein comprises amino acid
sequences
corresponding to the heavy and light chain variable regions set forth in SEQ
ID NOs: 70 and
71. In certain embodiments, the anti-IL12/IL18-Ig protein comprises the amino
acid
sequences corresponding to the heavy and light chains set forth in SEQ ID NOs:
70 and 71.
Lyophilization may be performed according to methods known in the art. Many
different freeze-dryers are available for this purpose such as Hu115017\4
(Hull, USA) or
GT20.TM. (Leybold-Heraeus, Germany) freeze-dryers. Freeze-drying is
accomplished by
freezing the formulation and subsequently subliming ice from the frozen
content at a
temperature suitable for primary drying. Under this condition, the product
temperature is
below the eutectic point or the collapse temperature of the formulation.
Typically, the shelf
temperature for the primary drying will range from about -30 to 25 C.
(provided the product
remains frozen during primary drying) at a suitable pressure, ranging
typically from about 50
to 250 mTorr. The formulation, size and type of the container holding the
sample (e.g., glass
vial) and the volume of liquid will mainly dictate the time required for
drying, which can
range from a few hours to several days (e.g. 40-60 hours). Optionally, a
secondary drying
stage may also be performed depending upon the desired residual moisture level
in the
product. The temperature at which the secondary drying is carried out ranges
from about 0-
40 C, depending primarily on the type and size of container and the type of
protein
employed. For example, the shelf temperature throughout the entire water
removal phase of
lyophilization may be from about 15-30 C (e.g., about 20 C). The time and
pressure required
for secondary drying will be that which produces a suitable lyophilized cake,
dependent, e.g.,
on the temperature and other parameters. The secondary drying time is dictated
by the desired
residual moisture level in the product and typically takes at least about 5
hours (e.g. 10-15
hours). The pressure may be the same as that employed during the primary
drying step.
Freeze-drying conditions can be varied depending on the formulation and vial
size.
61
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Prior to administration to the patient, the lyophilized formulation is
reconstituted with
a pharmaceutically acceptable diluent such that the protein concentration in
the reconstituted
formulation is at least about 2 mg/ml, for example from about 2 mg/ml to about
100 mg/ml,
alternatively from about 10 mg/ml to about 90 mg/ml, alternatively from about
30 mg/ml to
about 50 mg/ml. Such high protein concentrations in the reconstituted
formulation are
considered to be particularly useful where subcutaneous delivery of the
reconstituted
formulation is intended. However, for other routes of administration, such as
intravenous
administration, lower concentrations of the protein in the reconstituted
formulation may be
desired (for example from about 2-50 mg/ml, or from about 3-40 mg/ml protein
in the
reconstituted formulation). In certain embodiments, the protein concentration
in the
reconstituted formulation is significantly higher than that in the pre-
lyophilized formulation.
Reconstitution generally takes place at a temperature of about 25.degree C to
ensure complete
hydration, although other temperatures may be employed as desired. The time
required for
reconstitution will depend, e.g., on the type of diluent, amount of
excipient(s) and protein.
Exemplary diluents include sterile water, bacteriostatic water for injection
(BWFI), a pH
buffered solution (e.g. phosphate-buffered saline), sterile saline solution,
Ringer's solution or
dextrose solution. The diluent optionally contains a preservative. Exemplary
preservatives
have been described above, with aromatic alcohols such as benzyl or phenol
alcohol being
the preferred preservatives. The amount of preservative employed is determined
by assessing
different preservative concentrations for compatibility with the protein and
preservative
efficacy testing. For example, if the preservative is an aromatic alcohol
(such as benzyl
alcohol), it can be present in an amount from about 0.1-2.0% and preferably
from about 0.5-
1.5%, but most preferably about 1.0-1.2%.
V. Uses of the Disclosure
The formulations of the disclosure may be used both therapeutically, i.e., in
vivo, or
as reagents for in vitro or in situ purposes. The methods of the disclosure
may also be used to
make a water-based formulation having characteristics that are advantageous
for therapeutic
use. The aqueous formulation may be used as a pharmaceutical formulation to
treat a disorder
in a subject.
The formulation of the disclosure may be used to treat any disorder for which
the
62
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
therapeutic protein is appropriate for treating. A "disorder" is any condition
that would
benefit from treatment with the protein. This includes chronic and acute
disorders or diseases
including those pathological conditions which predispose the mammal to the
disorder in
question. In the case of an anti-TNF DVD-Ig, a therapeutically effective
amount of the DVD-
Ig protein may be administered to treat an autoimmune disease, such as
rheumatoid arthritis,
an intestinal disorder, such as Crohn's disease, a spondyloarthropathy, such
as ankylosing
spondylitis, or a skin disorder, such as psoriasis. In the case of an anti-IL-
12 DVD-Ig, a
therapeutically effective amount of the DVD-Ig protein may be administered to
treat a
neurological disorder, such as multiple sclerosis, or a skin disorder, such as
psoriasis. Other
examples of disorders in which the formulation of the disclosure may be used
to treat include
cancer, including breast cancer, leukemia, lymphoma, and colon cancer.
The term "subject" is intended to include living organisms, e.g., prokaryotes
and
eukaryotes. Examples of subjects include mammals, e.g., humans, dogs, cows,
horses, pigs,
sheep, goats, cats, mice, rabbits, rats, and transgenic non-human animals. In
specific
embodiments of the disclosure, the subject is a human.
The term "treatment" refers to both therapeutic treatment and prophylactic or
preventative measures. Those in need of treatment include those already with
the disorder, as
well as those in which the disorder is to be prevented.
The aqueous formulation may be administered to a mammal, including a human, in
need of treatment in accordance with known methods of administration. Examples
of
methods of administration include parenteral, subcutaneous, intramuscular,
intravenous,
intraarticular, intrabronchial, intraabdominal, intracapsular,
intracartilaginous, intracavitary,
intracelial, intracerebellar, intracerebroventricular, intracolic,
intracervical, intragastric,
intrahepatic, intramyocardial, intraosteal, intrapelvic, intrapericardiac,
intraperitoneal,
intrapleural, intraprostatic, intrapulmonary, intrarectal, intrarenal,
intraretinal, intraspinal,
intrasynovial, intrathoracic, intrauterine, intravesical, bolus, vaginal,
rectal, buccal,
sublingual, intranasal, and transdermal.
The appropriate dosage ("therapeutically effective amount") of the protein
will
depend, for example, on the condition to be treated, the severity and course
of the condition,
whether the protein is administered for preventive or therapeutic purposes,
previous therapy,
the patient's clinical history and response to the protein, the type of
protein used, and the
discretion of the attending physician. The protein is administered to the
patient at one time or
63
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
over a series of treatments and may be administered to the patient at any time
from diagnosis
onwards. The protein may be administered as the sole treatment or in
conjunction with other
drugs or therapies useful in treating the condition in question.
Actual dosage levels of the AS-DVD-Ig protein or LS-DVD-Ig protein, the active
ingredient, in the pharmaceutical formulation of this disclosure may be varied
so as to obtain
an amount of the active ingredient that is effective to achieve the desired
therapeutic response
for a particular patient, composition, and mode of administration, without
being toxic to the
patient.
The selected dosage level will depend upon a variety of factors including the
activity
of the AS-DVD-Ig protein or LS-DVD-Ig protein found in the formulation, the
route of
administration, the time of administration, the rate of excretion of the
particular compound
being employed, the duration of the treatment, other drugs, compounds and/or
materials used
in combination with the particular compound employed, the age, sex, weight,
condition,
general health and prior medical history of the patient being treated, and
like factors well
known in the medical arts.
In certain embodiments of the disclosure, the dosage of the AS-DVD-Ig protein
in the
formulation is about 1 to about 250 mg. In certain embodiments, the dosage of
the AS-DVD-
Ig protein in the formulation is about 30 to about 140 mg, about 40 to about
120 mg, about 50
to about 110 mg, about 60 to about 100 mg, or about 70 to about 90 mg. In a
further
embodiment, the composition includes an AS-DVD-Ig protein dosage of about 1,
10, 20, 30,
40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200,
210, 220, 230,
240 or 250 mg.
In certain embodiments of the disclosure, the dosage of the LS-DVD-Ig protein
in the
formulation (upon reconstitution) is about 1 to about 250 mg. In a further
embodiment, the
dosage of the LS-DVD-Ig in the formulation is about 30 to about 140 mg, about
40 to about
120 mg, about 50 to about 110 mg, about 60 to about 100 mg, or about 70 to
about 90 mg. In
a further embodiment, the composition includes an LS-DVD-Ig dosage of about 1,
10, 20, 30,
40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200,
210, 220, 230,
240 or 250 mg.
The formulations of the disclosure overcome common problems known in
formulation development, including the problem of protein aggregation often
associated with
high concentrations of protein, particularly complex proteins such as DVD-Ig
proteins. Thus,
64
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
in certain embodiments, the formulations of the disclosure provide a new means
by which
high levels of this new therapeutic protein format may be administered to a
patient.
EXAMPLES
The Examples presented herein describe formulations containing dual variable
domain immunoglobulin (DVD-Ig) proteins that have unexpected stability
characteristics.
The experiments were surprising in that certain DVD-Ig proteins, referred to
as Aqueous
Stable DVD-Ig (AS-DVD-Ig) proteins or Lyophilized Stable DVD-Ig (LS-DVD-Ig)
proteins,
were stable in aqueous or lyophilized formulations, respectively, whereas
other DVD-Ig
proteins showed aggregation and instability when similarly formulated. The
experiments
exemplify methods for identifying AS-DVD-Ig proteins and LS-DVD-Ig proteins,
as well as
stable formulations thereof.
Materials and Methods
The methods described herein were used in experiments performed to assess and
monitor the stability of DVD-Ig proteins in aqueous and lyophilized
formulations.
General Methods
DVD-Ig protein formulations were tested for general quality parameters (e.g.,
pH),
parameters of physical stability (e.g., clarity, color, particle contamination
and purity), and
parameters of chemical stability (e.g., deamidation, oxidation, and general
chemical stability).
Exemplary tests included tests for visible particulate contamination, for
light obscuration
particle count for subvisible particles, and for purity such as size exclusion
high pressure
liquid chromatography (also referred to herein as size exclusion
chromatography (SEC)) and
ion exchange chromatography (IEC).
Particulate contamination (e.g., visible particles) was determined by visual
inspection.
Subvisible particles were monitored by light obscuration assays according to
the United
States Pharmacopeia (USP). The physicochemical stability of formulations was
assessed by
SEC, which allows for the detection of fragments and aggregates. To monitor
chemical
stability, SEC (for the detection of fragments and hydrolysis) and IEC were
performed.
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig Proteins Tested
The DVD-Ig proteins that were tested in the Examples provided herein are
listed in
Table 1. The sequences of the DVD-Ig proteins described in Table 1 are
provided in Table
65.
Table 1: Dual Variable Domain Immunoglobulin (DVD-Ig) Proteins and Their
Targets
DVD-Ig Protein Name Targets
DVD 5 CD20/CD80
DVD 6 CD80/CD20
DVD 37 VEGF/HER2
DVD 38 HER2/VEGF
DVD 53 TNF/RANKL
DVD 54 RANKL/TNF
DVD 65 TNF/DKK
DVD 66 DKK/TNF
DVD 165 CD2O/RANKL
DVD 166 RANKL/CD20
DVD 257 DLL4/PLGF
DVD 258 PLGF/DLL4
DVD 277 TNF/SOST (S2)
DVD 278 SOST(S2)/TNF
DVD 281 IL-9(S2)/IgE
DVD 282 IgE/IL-9(S2)
IL12IL18 IL-12/IL-18
DVD-A TNF/IL-17
DVD-B TNF/PGE2
DVD-C IL-la/IL-113
DVD-Ig protein as starting material was provided following purification and
was > 95
% monomeric form.
Cation Exchange HPLC Methods
Cation exchange HPLC, a form of IEC, was used to determine the identity and
purity
of the DVD-Ig protein formulations. The assay was performed with the
parameters detailed
below.
A Dionex ProPac WCX-10 analytical column (Dionex Corp., Sunnyvale, CA),
66
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
combined with a Dionex WCX-10G guard column (Dionex Corp., Sunnyvale, CA), was
run
with upper column pressure being limited to 210 bar. The mobile phase A
consisted of 10
mM Na2HPO4, pH 6Ø This buffer was created by dissolving 4.97 g anhydrous
disodium
hydrogen phosphate in approximately 3300 mL Milli-Q water, adjusting the pH to
7.0 using 1
M phosphoric acid, increasing the buffer volume to 3500 mL with Milli-Q water
and filtering
the solution through a membrane filter. The mobile phase B consisted of 10 mM
Na2HPO4,
500 mM NaC1, pH 6Ø This buffer was created by dissolving 2.56 g anhydrous
disodium
hydrogen phosphate in approximately 1500 ml Milli-Q water, adjusting the pH to
6.0 using 1
M phosphoric acid, increasing the buffer volume to 1800 ml with Milli-Q water
and filtering
the solution through a membrane filter. A summary of the cation exchange HPLC
methods is
described in Table 2.
Table 2: Summary of Cation Exchange HPLC Methods
Item
Description/Operating Conditions
Guard column ProPac WCX-10G, 4.0 x 50 mm
Column ProPac WCX-10, 4.0 x 250 mm
Mobile phase A* 10 mM disodium
hydrogen phosphate, pH 6.0
Mobile phase B* 10 mM disodium
hydrogen phosphate/500 mM
sodium chloride, pH 6.0
Binary Gradient
Time (minute) Mobile Phase
B%
0.01 15.0
30.00 30.0
32.00 100.0
37.00 100.0
39.00 15.0
44.00 15.0
Gradient 44.10 Stop
Flow rate 1.0 ml/minute
Detector wavelength 280 nm
Autosampler temperature Nominal 4 C
Column oven temperature 35 C
Sample load 100 ilL/100 jig
Run time 44.0 minutes
67
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
For the IL12IL18 and DVD 66 DVD-Ig proteins, the mobile phases used were
changed as follows:
Mobile phase A: 10 mM MES, pH 5.6; and
Mobile phase B: 10 mM MES + 500 mM NaC1, pH 5.6.
For ILlccIL13, the mobile phases used were changed as follows:
Mobile phase A: 20 mM Phosphate, pH 8.0; and
Mobile phase B: 20 mM Phosphate + 500 mM NaC1, pH 8Ø
Table 3. Gradient for ILlccILI3
Time (minutes) % B
0 7
7
25 25
27 100
32 100
34 7
37 7
Similar versions of IEC were used for various other DVD-Ig proteins.
Size Exclusion HPLC Methods
Size exclusion HPLC was used to determine the purity of DVD-Ig protein
solutions.
The assay was performed as outlined below.
A TSK gel guard (VWR Scientific, Bridgeport, NJ; cat. no. 08543, 6.0 mm x 4.0
cm,
7 lam), was combined with a TSK gel G3000SW (VWR Scientific, Bridgeport, NJ;
cat. no.
08541, 7.8 mm x 30 cm, 5 p.m) and run with an upper column pressure limit of
70 bar. The
mobile phase consisted of 100 mM Na2HPO4 / 200 mM Na2504, pH 7Ø This buffer
was
created by dissolving 49.68 g anhydrous disodium hydrogen phosphate and 99.44
g
anhydrous sodium sulfate in approximately 3300 ml Milli-Q water, adjusting the
pH to 7.0
using 1 M phosphoric acid, increasing the buffer volume to 3500 ml with Milli-
Q water and
filtering the solution through a membrane filter.
The experimental parameters were listed as follows:
Flow rate: 0.3 ml/minute;
68
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Injection volume (equivalent to 20 lug sample): 20 pi;
Column temperature: room temperature;
Autosampler temperature: 2 to 8 C;
Run time: 50 minute;
Elution: isocratic gradient.
Detection was performed using a diode array detector using a 214 nm wavelength
(>
0.1 min peak width and 8 nm band width) and a 360 nm reference wavelength (100
nm band
width).
Test samples were injected in duplicate. Purity was determined by comparing
the
area of DVD-Ig protein peak to the total area of all 214 nm absorbing
components in the
sample, excluding buffer-related peaks. High molecular weight aggregates and
antibody
fragments were resolved from intact DVD-Ig protein using this method.
Freeze/Thaw Assays
The stability of DVD-Ig protein solutions was measured using repeated
freeze/thaw
assays. The DVD-Ig proteins were frozen at -80 C and then thawed at 30 C in a
water bath
and the resulting solutions were analyzed for aggregation by SEC and/or for
subvisible
particle formation by light obscuration assays.
Accelerated and Real Time Storage Stability Studies
The pH and storage temperature of formulations are two important factors
influencing
protein stability during long-term storage of protein liquid and lyophilisate
formulations. To
assess the impact of these factors, the protein formulations were exposed to
short-term
storage at elevated temperatures, e.g., 40 C, (accelerated storage) in order
to mimic long term
storage and quickly gain insight in the formulation feasibility for long-term
storage at lower
temperatures (e.g., 2-8 C). To assess the real time storage stability, the
samples were also
kept at 2-8 C.
Light Obscuration Assays
Light obscuration assays were performed to measure the insoluble particulate
content
of aggregating DVD-Ig protein solutions. Light obscuration measurement
equipment
(particle counter, model syringe, Klotz Bad Liebenzell, Germany, series
S20037) was
69
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
equipped with laminar air hood (Thermo Electron Corp., Asheville, NC; Model
No.
ULT2586-9-A40) to minimize foreign particle contamination during measurements.
Light
obscuration analysis was performed as follows. A 3.5 ml sample was placed in a
5 ml round-
bottom tube under laminar air flow conditions. Measurements were performed
according to
manufacturer's specifications in n=3 mode (0.8 mL per single measurement),
after an initial
0.8 ml rinse.
Differential Scanning Calorimetry (DSC)
Prior to DSC analysis, DVD-Ig proteins were dialyzed into a suitable buffer
system
using Slide-A-Lyzer Cassettes (Thermo Fisher Scientific, Waltham, MA). This
buffer system
(e.g., 5 mM phosphate/5 mM citrate was also used as a reference/blank for the
DSC
measurement. The antibody was analyzed at 1-2 mg/ml. An automated VP-Capillary
DSC
System (MicroCal, Piscataway, NJ) was used. Unfolding of the molecules was
studied by
applying a 1 C/minute scan rate over a 25 C - 95 C temperature range. Other
measurement
parameters were as follows: fitting period: 16 seconds; pre-scan wait: 10
minutes; feedback
mode: none.
Air/Liquid Interface Shaking Studies
Shaking studies were conducted at a concentration of 1 mg/ml in 6R glass vials
on an
HS 260 IKA shaker (Wilmington, NC) at a speed of 150 rpms (revolutions per
minute). The
optical density of samples was evaluated following shaking for various
periods. Similarly,
SEC was also done for samples pulled at various time points.
PEG Solubility
PEG 3000 was used for solubility studies. A 50 % w/v solution of PEG 3000 was
prepared in water. Small aliquots of this solution were added to a stock
solution of protein in
buffer at 0.5 mg/ml concentration. The total volume required at the time first
signs of
precipitation originated was noted down.
Real Solubility
For real solubility evaluations, the DVD-Ig protein was concentrated and
stored
overnight at 5 C. The solution was visually inspected for precipitates, phase
separation,
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
turbidity, etc. The supernatant (or both phases) was checked for dissolved
concentration.
Near UV-CD
The near UV-CD scans were taken at 1 mg/ml concentrations using 2 ml vial fill
on a
Jasco spectrometer (JASCO Analytical Instruments, Easton, MD) between 250 and
320 nm.
The scan rate was 50 nm/minute and an average of 3 scans was taken. The
spectrometer was
allowed to equilibrate by turning on the lamp before data acquisition.
ATR-FTIR Spectrometry
FTIR scans were taken at 1 mg/ml concentrations using 10 pi solutions on a
Bruker
ATR-FTIR (Bruker Optics, Billerica, MA). The scans were collected between 400-
4000 cm -1
and area normalized and second derivatized before being curve fitted using
Origin software
(OriginLab, Northampton, MA).
Light Scattering
Light scattering studies were done on a Malvern zetasizer (Malvern Instruments
Ltd.,
Worcestershire, UK) using a backscattering angle of 173 . Toluene was used as
a standard
and a buffer (e.g., acetate, histidine, and Tris) was used as a blank. An
automatic mode was
used.
Dynamic Scanning Fluorimetry (DSF)
DSF was employed to assess the propensity of a protein to unfold. The
technique
involved the addition of dye Sypro Orange to the protein samples. This
fluorescent dye is
sensitive to hydrophobic surfaces and shows increased fluorescence in such
environments.
The sample with dye was then heated and the fluorescence signal as a function
of temperature
was monitored. As the temperature increased, the protein started to unfold and
exposed its
hydrophobic interior. This lead to dye binding to this region and a greater
fluorescence
signal. The temperature at which the signal begins to increase is the onset
temperature (Ton).
Proteins that have less intrinsic stability are more prone to unfold and have
lower Ton values
than proteins with greater intrinsic stability. DSF also provided a high
throughput tool for
rapid screening of clones in a 96 well format and eliminated potential
limitations of larger
quantities of samples and longer run times in DSC. 6 pi of the 0.4X SYPRO
Orange dye
71
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
(Invitrogen, Carlsbad, CA) was added to 27 pi of the DVD-Ig protein solution.
The scan rate
was 1 C/minute and scans were taken from 25-75 C.
Lyophilization Methods
The DVD-Ig proteins were lyophilized according to standard methods and the
stability of the resulting lyophilisates were investigated.
Sample Preparation and Lyophilization
The vials were stoppered with autoclaved and dried lyo stoppers. Afterwards
the vials
were lyophilized with a freeze dryer. A typical cycle is shown below in Table
4. The
samples were reconstituted to a 100 mg/ml DVD-Ig protein solution.
Table 4: Typical Freeze-Dry Cycle Used To Lyophilize DVD-Ig Proteins
Time /
Shelf Step Step Pressure
temperature time
Step [ C]
[hh:min] [min] [mbar]
0 Start 20 0:00:00 0 1000
1 Loading 20 0:00:00 0 1000
2 Freezing I (ramp) 0 0:20:00 20 1000
3 Freezing I 0 2:10:00 130 1000
4 Freezing II (ramp) -45 1:20:00 80 1000
Freezing II -45 3:00:00 180 1000
6 Adjust vacuum -45 1:00:00 60 0,066
Primary drying I
7 (ramp) -25 1:00:00 60 0,066
8 Primary drying I -25 70:00:00 4200 0,066
Secondary drying II
9 (ramp) 25 2:00:00 120 0,066
Secondary drying II 25 0:15:00 15 0,036
11 Secondary drying II 25 8:00:00 480 0,036
12 Holding step (ramp) 5 0:30:00 30 0,036
13 Holding step 5 0:00:00 0 0,036
Venting N2
14 atmosphere 5 0:00:00 0 500
Total 5375
72
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
I. STABILITY OF DVD-IG PROTEINS
Examples 1-3 demonstrate that DVD-Ig proteins are less stable, e.g.,
aggregating
more easily and having a lower melting temperature, than antibodies due to the
increased
structural complexity of DVD-Ig proteins.
EXAMPLE 1. Thermodynamic Comparison of DVD-Ig Proteins and Antibodies
An experiment was performed to determine the stability of a DVD-Ig protein in
comparison to an antibody. Differential scanning calorimetry (DSC) of an IgG1
molecule (a
monoclonal antibody, mAb) and a DVD-Ig was performed to determine the
differences in the
thermodynamic properties of the two molecules. Specifically, DSC was performed
to
compare the thermodynamic properties of an exemplary antibody (Briakinumab, an
anti-1L12
monoclonal antibody) to those of a DVD-Ig protein (TNF/PGE2; DVD-B).
Formulation
information and DSC conditions are provided below in Example 2. Comparison of
the DSC
profiles of Briakinumab to those of TNF/PGE2 (DVD-B) shows the differences in
three
versus four domain unfolding (Figure 1). It is also clear from Figure 1 that
the thermal
unfolding of the DVD-Ig protein started earlier than that of the antibody,
which indicates that
the overall thermodynamic stability (or the intrinsic stability) of the DVD-Ig
molecule is
lower than that of the antibody. It should be noted that while the DVD-Ig
protein in Figure 1
(DVD-B) had a DSC profile indicating an initial unfolding at about 50 C, this
same DVD-Ig
protein was characterized as an LS-DVD-Ig protein given results from further
stability
testing.
The thermodynamic stability (e.g., onset of unfolding as determined by dynamic
scanning calorimetry) of mAbs is in general approximately about 5 C higher
compared to
the panel of DVD-Ig proteins listed in Table 1. Although there is generally no
direct
correlation between the temperature of unfolding and the aggregation
propensity of a group
of DVD-Ig proteins, the values can be used as a screen to assess the stability
of a given group
of DVD-Ig proteins. Therefore, the onset temperature of unfolding can be
utilized to identify
AS-DVD-Ig proteins or LS-DVD-Ig proteins. As described in Figure 4, the
overall
distribution shows that DVD-Ig proteins generally have a lower unfolding
temperature in
comparison to antibodies. Figure 4 provides the compiled results from 16
different DVD-Ig
proteins and 14 different antibodies. The assays performed in the studies
described in Figure
4 are similar to those described above and in Figure 1.
73
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
EXAMPLE 2. Impact of pH on the Stability of DVD-Ig proteins in Solution
The thermodynamic stability (intrinsic stability) of DVD-Ig proteins
formulated in
solutions having a pH of 4, 6, or 8 was evaluated using differential scanning
calorimetry
(DSC). All formulations had a DVD-Ig protein concentration of 1 mg/ml in 5 mM
citrate/5mM phosphate buffer. Heating was performed at a scan rate of 1
C/minute. Results
showing the impact of pH on the stability of multiple DVD-Ig proteins are
provided in Table
below. Tml-Tm4 described in Table 5 represent the thermal melting/unfolding
temperatures of various domains, e.g., CH1, CH2 and CH3 domains, etc. The
stabilities of
two antibodies (Adalimumab and Briakinumab) are also described for comparison.
Table 5: Impact of pH on Stability of DVD-Ig Protein Formulations with pH 4,
6, or 8, as
Assessed by Differential Scanning Calorimetry (DSC)
DVD-Ig pH Tml C Tm2 C Tm3 C Tm4 C Onset C
5 4 62.3 63.9 73.9 80.7 45
6 4 61.5 69.5 75.4 82.2 45
37 4 61.4 66.7 77.1 82.7 52
38 4 68.4 70.4 75.8 81.6 56
53 4 58.6 67.9 76.5 82.7 46
54 4 66 67.9 75.2 82.6 52.5
65 4 61.3 69 73.9 81.3 52.5
66 4 65.5 67.6 74.7 81.9 55
165 4 63 67.4 75.4 82.1 47.5
166 4 67.3 71.7 74.9 82.4 55
257 4 66.2 69.2 76.6 83.5 52.5
258 4 68.2 69.6 78.8 83.7 55
277 4 61.4 64.8 75.3 82.6 50
278 4 55.8 67.5 76.1 82.8 45
281 4 65.8 68.4 79.1 83.1 55
282 4 73.1 75 77.4 83.8 57.5
5 6 61.9 65.1 76 81.8 50
6 6 61.6 69.8 76.2 81.9 48
37 6 60.4 67.5 77.2 83.1 51
38 6 69.07 70.3 75.9 82 57.5
53 6 58.3 67.05 76.4 82 49
54 6 65.8 67.6 74.9 82 56
65 6 61.2 67.3 73.4 82.4 51.5
66 6 65.3 67.5 74.8 81.9 55
165 6 62.6 67.3 75.7 82.1 50
166 6 67.3 71 74 82 54
257 6 66.6 69.2 75.8 82.9 57
258 6 67.8 70 77.5 79.5 55
74
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig pH Tml C Tm2 C Tm3 C Tm4 C Onset C
277 6 61.4 65.8 74.7 82.23 52.5
278 6 56.6 66.7 75.8 82.3 45
281 6 65.8 67.8 78.6 81.7 53
282 6 69.8 75 77.9 83.4 57
8 65.16 68.35 77 82.48 45
6 8 62 69.07 73.08 82.61 45
38 8 68.4 70.7 74.8 82.8 50
53 8 57.6 68.7 75 83 40
54 8 65.5 67.6 74.5 82.8 52.5
65 8 61 69.6 72.5 82.8 48
66 8 64.8 67.1 72.4 82.9 52.5
165 8 63.9 68.6 74.1 82.6 47.5
166 8 64.3 69.3 74 82.7 45
257 8 67.2 69.3 76.7 83.6 55
258 8 69.5 71.4 78.1 83.9 53
277 8 60.18 68.98 74.5 83 50
278 8 52.8 68.8 75.8 83.02 44
281 8 65.2 67.1 78.3 83.2 51
282 8 71.9 74.5 77.4 83.9 55
mAbs
Adalimumab 6 72 76 84 NA 62
Briakinumab 6 69 76 83 NA 59
As shown in Table 5, DVD-Ig proteins in general have unfolding onset
temperatures
of greater than about 50 C, and the melting temperatures are therefore
slightly lower than
those of antibodies and other stable proteins. Example 2 shows that the onset
temperature for
Briakinumab and Adalimumab is around 60 C at pH 6, whereas, for DVD-Ig
proteins, the
average is around 53 C.
The data also shows that the melting temperatures of DVD-Ig proteins are
higher at
pH 6 and pH 8 than at pH 4. Thus, pH affects the physico-chemical stability of
DVD-Ig
proteins, and stability appears best at approximately pH 6.
To further assess the impact of solution pH on the stability of DVD-Ig protein
formulations during long-term storage, DVD-Ig protein formulations were
analyzed using
SEC before being subjected to storage (TO) or after being subjected to 3
months of
accelerated storage (T3m). Storage stability of the DVD-Ig proteins in
solutions (1 mg/ml
DVD-Ig protein in 5 mM citrate/5mM phosphate buffer with the presence of 80
mg/ml
sucrose) formulated at pH of 4, 6, or 8 was evaluated. For accelerated
storage, samples were
filled into sterile vials (approx. 500 pi each) and stored under controlled
conditions (in
temperature chambers and in the absence of light) at 40 C. The percentage of
DVD-Ig
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
protein monomers (Mon) aggregates (Agg), and fragments (Frag) was determined
using SEC
and the results are presented in Table 6.
Table 6: Impact of pH on the Stability of 1 mg/ml DVD-Ig Protein Solutions
Before and
After Accelerated Storage
DVD-Ig pH Mon/TO Mon/T3m Agg/TO Agg/T3m Frag/TO Frag/T3m
4 91.14 57.51 5 0.79 3.85 41.6
6 4 97.38 62.46 2.29 4.11 0.31 33.4
38 4 94.9 56.52 3.58 22.02 1.5 21.44
53 4 97.61 28.11 0.99 53.94 1.38 17.93
54 4 96.57 53.89 1.93 15.2 1.48 30.89
65 4 94.46 50.35 1.33 26.21 4.21 23.42
66 4 97.99 58.39 0.9 17.72 1.1 23.87
165 4 96.2 68.38 2.25 3.18 1.53 28.42
166 4 97.09 66.3 0.76 0.66 2.14 30.55
258 4 98.61 45.42 0.46 30.56 0.91 24
277 4 97.05 61.26 1.55 11.48 1.38 27.23
278 4 98.33 45.58 0.9 28.13 0.76 26.27
5 6 90.46 81.75 7.02 3.04 2.51 12.77
6 6 97.02 85.44 2.78 2.71 0.18 11.82
38 6 95.31 87.89 3.48 5.48 1.2 6.61
53 6 97.75 86.68 1.02 6.21 1.22 7.08
54 6 96.48 88.03 2.07 5.37 1.43 6.59
65 6 94.89 87.13 0.89 5.78 4.21 7.06
66 6 97.99 88.93 0.9 5.13 1.09 5.91
165 6 96.21 86.31 2.24 5.84 1.53 7.82
166 6 98.6 89.37 1.39 0.92 0 7.38
258 6 98.98 84.76 0.2 9.41 0.81 5.81
277 6 97.48 83.96 1.48 5.11 1.03 10.91
278 6 98.65 79.81 0.78 9.52 0.56 10.65
5 8 90.21 67.86 7.26 4.51 2.51 22.75
6 8 97.36 76.33 2.44 8.89 0.18 14.76
38 8 95.09 80.11 3.47 9.37 1.42 10.5
53 8 97.81 79.63 0.96 9.82 1.22 10.53
54 8 96.69 81.13 1.93 9.44 1.37 9.41
65 8 93.12 79.5 1.27 9.88 5.59 10.59
66 8 97.69 81.37 0.99 9.17 1.31 9.44
165 8 96.57 71.02 2.03 18.85 1.39 10.11
166 8 97.23 79.76 0.76 1.92 1.99 11.3
258 8 98.67 84.22 0.24 5.91 1.07 9.84
277 8 97.06 70.08 1.72 16.13 1.2 13.78
278 8 98.18 43.34 0.98 45.59 0.83 11.04
The results in Table 6 showed that DVD-Ig proteins can be initially formulated
in a
pH range from pH 4 to pH 8 and that the initial values of the freshly prepared
samples at TO
do not show major differences. However, following accelerated storage, the DVD-
Ig proteins
76
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
at pH 4 and pH 8 showed significant loss in monomer and a corresponding
increase in
aggregates or fragmentation.
The stability of the tested DVD-Ig proteins was highest at around pH 6. All of
the
DVD-Ig proteins tested (including DVD 5, DVD 6, DVD 38, DVD 53, DVD 54, DVD
65,
DVD 66, DVD 165, DVD 166, DVD 258, DVD 277, and DVD 278) had a greater
percentage
of monomers and a lower percentage of fragments at pH 6 than at pH 4 or at pH
8.
Nine of the twelve DVD-Ig proteins tested showed a lower percentage of
aggregates
at pH 6 than at pH 4, and for the three DVD-Ig proteins that showed the
reverse pattern, the
difference in the percentage of aggregates at pH 6 and pH 4 was very small
(difference of less
than 2.7%). Also, eleven of the twelve DVD-Ig proteins tested showed a lower
percentage of
aggregates at pH 6 than at pH 8. Thus, accelerated storage resulted in
increased aggregate
formation. However, the increase was less than anticipated, particularly at pH
6.
Impact of Solution pH on the Storage Stability of IL12IL18 DVD-Ig Protein
Formulations
To assess the impact of solution pH on the stability of DVD-Ig protein
formulations
during storage, DVD-Ig protein formulations with solution pH of 4, 6, or 8
were analyzed
using SEC and IEC before being subjected to storage (TO) or after being
subjected to 4 days
(4d), 7 days (7d), or 21 days (21d) of storage at 5 C, 40 C, or 50 C (See
Tables 6, 7 and 8).
The solutions evaluated had an IL12-1L18 DVD-Ig concentration of 2 mg/ml and
were in a
buffer of 10 mM citrate and 10 mM phosphate. Samples were filled into sterile
vials (approx.
500 pi each) and stored under controlled conditions (in temperature chambers
and in the
absence of light). The percentage of DVD-Ig protein monomers, aggregates, and
fragments
was determined using SEC (see Table 7) and the numbers of main, acidic and
basic species
were assessed using IEC (see Table 8).
Table 7: Storage Stability of IL12-1L18 DVD-Ig Protein Formulations with pH 4,
6, or 8 as
Measured Using SEC
Monomer Aggregates Fragments
pH 4 pH 6 pH 8 pH 4 pH 6 pH 8 pH 4 pH 6 pH 8
TO 97.64 97.61 97.84 1.35 1.36 1.23 1.01 1.03 0.94
Storage at 5 C
4d, 5 C 97.98 97.64 97.95 1.07 1.31 1.19 0.95 1.06
0.87
7d, 5 C 97.68 98.02 97.8 1.25 1.17 1.08 1.07 0.81
1.11
21d, 5 C 98.13 97.81 97.64 1.23 1.17 1.08 0.88 0.96
1.28
77
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Accelerated Storage at 40 C
4d, 40 C 97.21 97.32 96 0.56 1.4 1.68 2.24 1.28
2.32
7d, 40 C 96.15 97.32 94.57 0.71 1.35 1.96 3.15 1.33
3.48
21d, 40 C 91.99 96.39 91.31 1.16 1.49 3.15 6.85 2.12
5.54
Accelerated Storage at 50 C
4d, 50 C 90.2 97.07 91.73 5.54 1.46 2.78 4.26 1.47
5.49
7d, 50 C 83.19 96.35 81.32 9.66 1.42 10.68 7.15 2.24
8.2
21d, 50 C 52.98 91.36 46.57 33.1 4.17 33.35 13.91 4.47
17.18
The SEC data show that the IL12IL18 DVD-Ig protein formulations were most
stable
at pH 6. At pH 6, the stored IL12IL18 DVD-Ig protein formulations generally
showed >95%
monomers and <2% aggregates. Even under accelerated storage conditions of 50 C
for 21
days, the formulation retained >90% monomers and <5% aggregates. IL12IL18 DVD-
Ig
formulations were more stable at pH 6 than pH 4 and pH 8, particularly in the
longer duration
and higher temperature storage conditions (e.g., in the 21 day, 50 C
condition). According to
these results the IL12/IL18 DVD-Ig protein would be considered an AS-DVD-Ig
protein
given the stability results following storage at 50 C.
Table 8: Storage Stability of IL12IL18 DVD-Ig Protein Formulations with pH 4,
6, or 8 as
Measured Using IEC
Main Species Acidic Basic
pH 4 pH 6 pH 8 pH 4 pH 6 pH 8 pH 4 pH 6 pH 8
TO 69.98 71.6 69.13 14.96 15.21 18.12 15.06 13.19 12.75
4d, 5 C 70.32 71 68.46 15.08 15.54 18.84 14.6
13.46 12.75
7d, 5 C 69.74 70.69 67.14 15.43 15.91 19.71
14.83 13.41 13.15
21d, 5 C 69.67 71.32 66.78 15.65 16.26 20.95
14.68 12.42 12.26
4d, 40 C 56.43 68.09 43.52 21.14 18.73 45.51
22.33 13.19 10.97
7d, 40 C 49.1 65.11 26.55 26.08 22.03 59.93
24.83 12.86 13.53
4d, 50 C 36.58 57.09 25.88 29.47 29.48 48.23
33.95 13.43 25.89
The IEC data for the IL12IL18 DVD-Ig protein formulations at pH 4, 6 and 8
show
that the chemical degradation is dependent on the pH and the storage
temperature. The
highest chemical stability was observed for samples stored at 5 C. The minor
changes in
stability detected after 21 days indicated that the IL12IL18 DVD-Ig protein
was stable at this
storage temperature, which is considered common for commercial
biotherapeutics. Although
the differences in stability were small, the highest chemical stability was
observed for
formulations at pH 6. The lack of chemical stability was even more pronounced
at the
elevated storage temperatures of 40 C and 50 C.
78
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
EXAMPLE 3. DVD-Ig Proteins Aggregate More Easily Than Monoclonal Antibodies
The impact of shaking on the aggregation of antibodies versus DVD-Ig proteins
was
examined. Shaking is a stress that can lead to the aggregation of molecules.
The
susceptibility of a DVD-Ig protein, TNF/PGE2 (DVD-B), to aggregation following
shaking
was compared with a monoclonal antibody, Briakinumab, using solutions having a
protein
concentration of 1 mg/ml (solutions at pH 6, 10 mM citrate/10 mM phosphate) in
6R vials.
The 6R vials were filled with samples of 5 ml of the protein solution and
shaken on an HS
260 IKA shaker (Wilmington, NC) at a speed of 150 revolutions per minute (rpm)
for various
lengths of time (0, 5, 24, 48, or 96 hours). The samples were checked for
optical density at
500 nm, which provides a measurement of the turbidity of the solutions. Higher
turbidity
indicates greater aggregation and less stability. The results are shown in
Table 9, revealing
that the DVD-Ig protein aggregates more readily than the monoclonal antibody.
Table 9: Impact of Shaking on the OD500 of 1 mg/ml Solutions of TNF / PGE2
DVD-Ig
Protein and Briakinumab
Optical Density (OD) at
500nM
TNF /
PGE2
Time (h) Briakinumab (DVD-B)
0 0 -0.0095
0.001 0.01175
24 0.01 0.0949
48 0.025 0.295
96 0.045 0.58
As indicated by 0D500 measurements which show turbidity of the solution,
shaking
caused the DVD-Ig protein to form more sub-visible and visible aggregates than
the
monoclonal antibody. Thus, after 48 hours of shaking, the DVD-Ig protein was
more prone
to colloidal instability and, therefore, less stable than the monoclonal
antibody. The greater
formation of visible aggregates of DVD-Ig protein compared with monoclonal
antibody
indicates that the DVD-Ig protein is generally less stable than the monoclonal
antibody
against shear stress in solution. Also, these results suggest that not all DVD-
Ig proteins are as
stable at pH 6 as a monoclonal antibody.
79
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
II. ASSAY FOR IDENTIFYING AN AQUEOUS STABLE DVD-IG PROTEIN
(AS-DVD-IG)
EXAMPLE 4. DVD-Ig Proteins Can be Characterized as "Aqueous Stable" or
"Aqueous Non-Stable"
As discussed above, the inventors found that formulations wherein the DVD-Ig
protein was in the aqueous state suffered from certain problems such as
aggregation and/or
fragmentation of the DVD-Ig protein monomer. The inventors conducted
experiments and
discovered, surprisingly and unpredictably, that a subset of DVD-Ig proteins
can be stably
formulated in the aqueous state even at high concentrations. The following
example
describes an SEC study showing that, surprisingly, DVD-Ig proteins can be
characterized as
either aqueous stable, e.g., the DVD-Ig protein shows low change in percent
rel. peak area in
monomers or aqueous non-stable, e.g., the DVD-Ig protein is prone to
aggregation and or
fragmentation. Notably, many of the DVD-Ig proteins tested were found to be
aqueous non-
stable or lyophilized non-stable. Due to the structural complexity of DVD-Ig
proteins and the
prominence of hydrophobic interactions at high concentrations, it was not
expected that
DVD-Ig proteins would be stable in formulations at high concentrations.
To assess the impact of storage temperature during accelerated or long-term
storage of
protein liquid formulations on protein stability, various DVD-Ig proteins were
exposed to
short-term storage at elevated temperatures in order to quickly gain insight
in the formulation
feasibility for long-term storage at lower temperatures (e.g., 2-8 C).
Stability Screen at a High Concentration for 14 Days
DVD-Ig protein formulations with concentrations of 60 mg/ml were analyzed
using
SEC before being subjected to storage (T=0) or after being subjected to 14
days of
accelerated storage (T=14days) (Table 10). Storage stability of the DVD-Ig
proteins in
solution (60 mg/ml, 10 mM citrate/10mM phosphate buffer with 80 mg/ml sucrose)
was
evaluated at 40 C. After defined storage periods, samples were pulled and the
impact of
storage time on DVD-Ig protein stability was evaluated. Briefly, samples were
filled into
sterile vials (approx. 500 [t.L each) and stored under controlled conditions
(in temperature
chambers and in the absence of light) at 40 C. At predefined points of time,
samples of
prepared solutions were pulled for analysis according to the sample pull
scheme. The
percentages of DVD-Ig protein monomers (Mon), aggregates (Agg), and fragments
(Frag)
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
were determined using SEC, and the results are presented in Table 10.
Table 10: Impact of High Concentration on the Storage Stability of DVD-Ig
Protein Solutions
DVD-
Ig Mon/TO Mon/T14d Agg/TO Agg/T14d Frag/TO Frag/T14d
79.1 73.28 19.12 24.26 1.77 2.44
6 84.01 89.46 12.69 9.81 3.29 0.71
37 92.85 74.35 6.34 23.42 0.8 2.21
65 95.4 92.79 1.31 5.39 3.28 1.79
66 96.56 94.49 1.08 3.88 2.35 1.6
165 90.17 64.09 8.99 33.44 0.83 2.45
166 98.17 94.93 1.09 3.11 0.72 1.93
257 94.83 76.47 4.77 21.78 0.39 1.72
277 97.46 85.06 1.77 13.19 0.76 1.73
278 98.45 73.01 1.06 25.69 0.48 1.27
281 93.92 68.01 2.78 30.5 3.29 1.46
282 98.34 95.39 1.03 3.05 0.62 1.54
Surprisingly, as shown in Table 10, the inventors discovered that a subset of
the
DVD-Ig proteins tested was stable when formulated in the aqueous state. Ten of
the twelve
DVD-Ig proteins tested (DVD 5, DVD 6, DVD 37, DVD 65, DVD 66, DVD 166, DVD
257,
DVD 277, DVD 278, and DVD 282) showed less than 26% aggregate formation and
had
greater than 73% monomers following 14 days of accelerated storage. Five of
the DVD-Ig
proteins tested (DVD 6, DVD 65, DVD 66, DVD 166, and DVD 282) showed aggregate
formation of less than 10%, and three of these (DVD 66, DVD 166, and DVD 282)
showed
aggregate formation of less than 5%.
As described above, certain DVD-Ig proteins ("Aqueous Stable DVD-Ig" proteins
or
"AS-DVD-Ig" proteins) remain stable (e.g., less than 6 % relative (rel.) peak
area change in
monomers or less than 10% relative (rel.) peak area change in monomers as
determined by
SEC) following accelerated storage in 14 days at 40 C, even when formulated
at high
concentration (e.g., concentrations of 60 mg/ml or higher). The majority of
DVD-Ig proteins
tended to aggregate during accelerated storage (non-AS-DVD-Ig proteins), as
would be
expected based on the general structure of DVD-Ig proteins and the stability
studies
described in Examples 1-3. Thus, in certain embodiments, the cutoff for
separating the AS-
DVD-Ig proteins from the non-AS-DVD-Ig proteins was taken as the formation of
10 % net
aggregates or less in 14 days at 40 C when stored at > 50 mg/ml at pH 6, as
seen in the
above non-limiting example where five of the twelve DVD-Ig proteins tested
showed
81
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
aggregate formation at this level. In certain embodiments, the cutoff for
separating the AS-
DVD-Ig proteins and the non-AS-DVD-Ig proteins was taken as the formation of 6
% net
aggregates or less in 14 days at 40 C when stored at > 50 mg/ml at pH 6, as
seen in the
above non-limiting example where four of the twelve DVD-Ig proteins tested
showed
aggregate formation at this level.
Many of DVD-Ig proteins do not show low aggregation, e.g., 1% or less
aggregation
at 5 C after 21 days or 10% or less aggregation at 40 C following 21 days of
storage. For
example, in an assay which examined monomer loss after 7 days in a solution
having a
TNF/IL13 DVD-Ig protein concentration of 50 mg/ml at either 4 C or 40 C,
many of DVD-
Ig proteins showed an increase in monomer loss as determined by SEC. In some
cases the
amount of monomer loss was negative as the monomer level increased in these
cases (e.g.,
some of the aggregates dissociated and formed back monomer and hence the
apparent
decrease in loss). A third experiment tested TNF/SOST DVD-Ig proteins in a
solution
having a DVD-Ig protein concentration of 50 mg/ml at 4 C. As in the
experiment relating to
TNF/IL13 DVD-Ig proteins, many of the DVD-Ig proteins showed an increase in
monomer
loss (determined by SEC).
Notably, the above assays can also be used to distinguish Lyophilized-Stable
DVD-
Immunoglubulin (LS-DVD-Ig) proteins. The cutoff for separating the LS-DVD-Ig
proteins
and the non-LS-DVD-Ig proteins was taken as the formation of 15 % net or less
aggregates in
14 days at 40 C when stored at > 50 mg/ml at pH 6. Thus, DVD-Ig proteins
tested in the
above assay that result in less than 10% or less than 6% aggregation or less
than 10 % or less
than 6 % relative (rel.) peak area change in monomers are considered both AS-
DVD-Ig
protein and LS-DVD-Ig proteins, and DVD-Ig proteins resulting in less than 15%
aggregation or less than 15 % relative (rel.) peak area change in monomers are
considered
LS-DVD-Ig proteins. Both AS-DVD-Ig protein and LS-DVD-Ig proteins represent
only a
percentage of the overall DVD-Ig proteins tested.
III. STABILITY OF NON-AS-DVD-IG PROTEINS IN FORMULATIONS
The following examples provide data showing the stability of non-AS-DVD-Ig
proteins (which fail the aggregation test, i.e., show more than, for example,
6% aggregation,
described in Example 4 above), in formulations, in comparison to AS-DVD-Ig
proteins
(described in Sections IV to VIII).
82
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
EXAMPLE 5: Impact of Concentration on the Storage Stability of an Exemplary
Non-
AS-DVD-Ig Protein
To assess the impact of protein concentration on long term storage stability,
formulations of an exemplary non-AS-DVD-Ig protein with concentrations of 1,
2, 5, 10, 25,
50, and 75 mg/ml were subjected to storage for 14 days at 40 C. The
formulations had a pH
of 6 and were in 15mM histidine buffer alone. The samples were filled into
sterile vials
(approx. 500 pi each) and stored under controlled conditions (in temperature
chambers and in
the absence of light). The samples were analyzed using SEC to determine the
percentage of
aggregates following storage. The resulting data is provided in Table 11.
Table 11: The Total Percent Aggregates in the DVD-B protein as Measured Using
SEC
Following Storage at 40 C for 14 Days
Concentration of Aggregates
DVD-B %
1 mg/ml 0
2 mg/ml 0.2
mg/ml 3.64
mg/ml 6.76
25 mg/ml 18.02
50 mg/ml 32.82
75 mg/ml 48.28
The data in Table 11 indicate that under the tested conditions (i.e., pH 6,
15mM
histidine buffer) DVD-B becomes unstable, namely, a high proportion of
aggregates form
after 14 days of storage at 40 C when high concentrations are reached. The
percentage of
aggregates formed exceeded 18% at a concentration of 25 mg/ml or more. Thus,
DVD-B, a
non-AS-DVD-Ig protein, was not stable in histidine buffer as evidenced by
increased
aggregation during storage.
EXAMPLE 6: Impact of pH, Ionic Strength, and Concentration on the Storage
Stability
of an Exemplary Non-AS-DVD-Ig Protein
To assess the impact of pH, ionic strength, and concentration on the storage
stability
of a DVD-Ig protein in solution, various formulations of DVD-B (5 mg/ml and
100 mg/ml)
were evaluated at 40 C and 5 C. After defined storage periods, samples were
pulled and the
83
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
impact of storage time on DVD-Ig protein stability was evaluated. The
following buffers
were used: acetate for pH 4.5, histidine for pH 6 and Tris for pH 8. A 2 mM
concentration of
buffer was used for 1 mM ionic strength solutions and a 10 mM concentration of
buffer for
20 and 100 mM ionic strength solutions (sodium chloride was used to further
maintain ionic
strength). Samples were filled into sterile vials, approx. 500 pi each, and
stored under
controlled conditions in a temperature chamber and in the absence of light.
After 3 months at
C (5C, 3m) or 21 days at 40 C (40C, 21d), samples of prepared solutions were
analyzed
using SEC. The numbers of net aggregates measured using SEC are presented in
Table 12.
Tables 13 and 14 further show that the addition of different
stabilizers/excipients (e.g.,
sucrose and Tween80) did not result in significant improvement in percent
monomer
remaining after defined time points (results were obtained using the
methodology described
above). The negative values of net aggregate values at the initial time points
for the low
concentration samples indicate also the initial formation of reversible
aggregates.
Table 12: Impact of Storage of DVD-B Protein Under Various Formulation
Conditions on the
Amount of Aggregates Formed as Measured By SEC
Added
DVD-Ig Protein Concentration Ionic
and Storage Condition pH strength Aggregate (Net)
DVD-B, 100 mg/ml, 5 C, 3m 4.5 1 49.51
20 54.79
100 56.64
6 0 11.68
1 6.6
20 9.4
100 7.85
8 1 5.66
20 8.08
100 7.87
DVD-B, 5 mg/ml, 40 C, 21d 6 0 -6.56
1 -3.3
20 10.79
100 9.99
DVD-B, 100 mg/ml, 40 C, 21d 6 0 73.37
1 70.39
20 77.39
100 65.4
Table 13: Polyol and Polysorbate Has Little to No Effect on Stability of DVD-B
at 1 mg/ml
in Histidine Buffer, pH 6
84
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Sample No. Monomer Aggregate Fragment AUC
Histidine Buffer
TO 98.97 0.34 0.67 79787
T4, 40 C 1 98.13 0.82 1.04 78552
2 97.98 0.87 1.13 78622
Average 98.055 0.845 1.085 78587
T7, 40 C 1 97.45 1.15 1.39 77836
2 97.42 1.2 1.36 78137
Average 97.435 1.175 1.375
77986.5
T21, 40 C 1 92.21 4.34 3.44 170875
2 93.19 3.7 3.1 72149
Average 92.7 4.02 3.27 121512
T4, 50 C 1 94.06 4.15 1.77 39698
2 93.37 4.44 2.18 43002
Average 93.715 4.295 1.975 41350
T7, 50 C 1 93.09 3.9 2.99 26451
2 91.95 4.81 3.22 30158
Average 92.52 4.355
3.105 28304.5
30 mM Hist, 80 mg/ml Sucrose, 0.02 % Tween 80
TO 97.5 1.61 0.88 62902
T21, 40 C 1 95.19 1.47 2.95 79362
2 95.56 1.67 3.13 79445
Average 95.375 1.57 3.04
79403.5
T4, 50 C 1 94.94 3.38 1.66 80742
2 94.98 3.3 1.62 79436
Average 94.96 3.34 1.64 80089
T7, 50 C 1 91.06 6.71 2.21 79672
2 91.01 6.6 2.37 79820
Average 91.035 6.655 2.29 79746
The addition of polyol to the formulation resulted in a slight improvement in
monomer content and a decrease in the levels of aggregates of DVD-B at lmg/ml.
Table 14: Polysorbate Has Little to No Effect on Stability of DVD-B at 100
mg/ml in
Histidine Buffer, pH 6
Buffer: 15 mM Histidine
Monomer Aggregate Fragment AUC
TO 96.26 2.43 1.3 73681
T7 (Viall) 41.4 56.1 2.34 64692
T7 (Vial2) 42.5 55.2 2.14 63246
T7 (Avg.) 41.95 55.65 2.24 63969
T21(Viall) 37.2 60.03 2.76 52389
T21(Vial2) 38.8 58.05 3.13 50722
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
T21(Avg.) 38 59.04 2.945 51555.5
Buffer: 15 mM Histidine + 0.02 % Tween 80
Monomer Aggregate Fragment AUC
TO 96.22 2.43 1.33 72007
T7 (Viall) 42.9 54.8 2.2 65403
T7 (Vial2) 47 50.8 2.09 58048
T7 (Avg.) 44.95 52.8 2.145 61725.5
T21(Viall) 40.32 54.54 5.12 32321
T21(Vial2) 38.38 55.9 5.7 30927
T21(Avg.) 39.35 55.22 5.41 31624
The addition of polyol to the formulation resulted in a slight improvement in
monomer content and a decrease in the levels of aggregates of DVD-B at
100mg/ml.
Table 15: Polyol or Surfactant Does Not Improve Stability (1 mg/ml) of DVD-B
Monomer (%) Aggregate (%) Fragment (%)
pH 6 Formulation
TO 1M 3M TO 1M 3M TO 1M 3M
15 mM Na Phos. 96.45 90.03 80.83 1.41 5 9.09 2.12
4.95 10.06
15 mM Na Cit. 96.51 90.72 85.3 1.44 5.3 6.57 2.04
3.97 8.11
15 mM Na Succ. 96.06 87.41 78.17 1.53 5.46 8.24 2.39
7.11 13.57
15 mM Na Acet. 96.14 89.62 81.8 1.48 4.76 7.52 2.36
5.6 10.66
15 mM Arg. 96.12 92.48 85.72 1.65 3.39 5.59 2.21
4.11 8.68
15 mM Hist. 96.42 91.82 81.6 1.29 3.85 6.39 2.28
4.32 12
Self Buff. 96.03 88.79 81.44 1.57 3.91 4.18 2.38
7.29 14.37
UB 10 mg/ml Mannitol 95.86 90.18 84.08 2.01 5.74 8.09
2.11 4.07 7.81
UB 10 mg/ml Sorbitol 96.49 89.36 84.09 1.38 6.56 7.68
2.11 4.07 8.21
UB 10 mg/ml Sucrose 96.12 90.03 84.26 1.58 5.92 7.62
2.28 4.03 8.11
UB 10 mg/ml Trehalose 96.34 89.97 84.31 1.5 5.98 7.59 2.14
4.03 8.09
UB 2.5% Gly 96.43 87.22 1.42 8.59 2.13 4.17
UB 15 mM (NI1.)2SO4 96.69 90.92 85.76 1.22 4.97 5.68
2 4.1 8.55
UB 20 mM NaC1 96.44 90.35 84.36 1.41 5.5 6.74 2.13
4.13 8.88
UB 200 mM NaC1 96.37 91.85 85.2 1.52 3.24 3.62 2.09
4.89 11.16
UB = citrate / phosphate buffer
The data show that although various solution conditions were analyzed, DVD-B
was
not very stable even at pH 6 (see, for example Table 13). Also, at pH 6, the
ionic strength did
not show a consistent relationship with net aggregate formation. The poor
stability is
86
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
indicated by the formation of high amounts of aggregates even in the 5 C
storage condition.
Furthermore, the addition of a polyol and/or a surfactant did not improve
aggregation of
DVD-B (see Tables 13, 14, and 15).
As described above in Examples 1 to 3, many DVD-Ig proteins are intrinsically
unstable. However, surprisingly, certain DVD-Ig proteins can be characterized
as being
stable, as described in Example 4. The experiments described in the Examples
below
demonstrate that AS-DVD-Ig proteins can unexpectedly be stably formulated,
even at high
concentrations, despite the differences in amino acid sequence. The below
examples stand in
contrast to Examples 5 and 6, which show the failure of non-AS-DVD-Ig proteins
to be
formulated.
IV. AS-DVD-IG PROTEINS ARE STABLE IN FORMULATIONS CONTAINING
A BUFFER AT PH RANGE OF 4.5-7.5
EXAMPLE 7: Effect of Buffer Concentrations on the Stability of DVD-Ig Proteins
The concentration of a buffer, e.g., histidine, is one of the important
factors that may
influence protein stability during accelerated/long-term storage of protein
liquid formulations.
To assess the impact, the protein was exposed to short-term storage at
elevated and real time
temperatures in order to quickly gain insight into stable formulations for
long-term storage at
lower temperatures (e.g., 2-8 C).
Storage stability of DVD-Ig proteins in solution was evaluated at 40 C and 5
C.
After defined storage periods, samples were pulled and the impact of storage
time on DVD-Ig
protein stability was evaluated. The concentrations of histidine that were
evaluated include 0,
5, 15, 50, and 200 mM.
Samples were filled into sterile vials (approx. 500 pi each) and stored under
controlled conditions (in temperature chambers and in the absence of light).
After 7days and
21 days, samples of the prepared solutions were analyzed using SEC and IEC.
87
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Table 16: Impact of Storage of Various DVD-Ig Proteins at Low and High
Concentrations
under Various Histidine Concentrations on SEC
TO
DVD-A, pH 5.2, DVD-A, pH 5.2,
1 mg/ml Agg Mon Frag Precipitation* 75 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 1.89 97.21 0.88 N 0 Histidine 2.63
96.39 0.97 N
mM Histidine 0.93 98.19 0.86 N 5 mM Histidine 0.52
98.27 1.19 N
mM Histidine 1.41 97.64 0.94 N 15 mM Histidine 1.69
97.38 0.92 N
50 mM Histidine 1.83 96.84 1.31 N 50 mM Histidine 2.01
96.86 1.11 N
200 mM
Histidine 2.09 96.96 0.94 N 200 mM Histidine 2.27
96.78 0.94 N
40C, 7d
DVD-A, pH 5.2, Agg DVD-A, pH 5.2,
1 mg/ml r Mon Frag 75 mg/ml Agg Mon Frag
0 Histidine 1.55 96.4 2.03 N 0 Histidine 10.52
87.77 1.7 N
5 mM Histidine 1.19 97.17 1.62 N 5 mM Histidine 1.26
95.01 3.71 N
15 mM Histidine 2.47 95.49 2.03 N 15 mM Histidine
13.76 83.35 2.88 N
50 mM Histidine 2.31 95.42 2.25 N 50 mM Histidine
16.62 80.82 2.54 N
200 mM
Histidine 2.14 95.92 1.92 N 200 mM Histidine 7.47
89.68 2.83 Y
40C, 21d
DVD-A, pH 5.2, DVD-A, pH 5.2,
1 mg/ml Agg Mon Frag Precipitation* 75 mg/ml Agg Mon
Frag Precipitation*
0 Histidine 0 Histidine 15.77 82.2
2.02 Y
5 mM Histidine 2.52 95.3 2.16 N 5 mM Histidine 14.07
83.8 2.12 N
15 mM Histidine 3.39 93.85 2.74 N 15 mM Histidine
18.07 79.49 2.42 N
50 mM Histidine 2.74 94.4 2.84 N 50 mM Histidine
13.41 83.71 2.86 Y
200 mM
Histidine 1.9 94.91 3.18 N 200 mM Histidine 8.35
87.66 3.97 Y
5C, 21d
DVD-A, pH 5.2, DVD-A, pH 5.2,
1 mg/ml Agg Mon Frag Precipitation* 75 mg/ml Agg Mon
Frag
0 Histidine 1.24 97.62 1.12 N 0 Histidine 1.78
97.16 1.04 N
5 mM Histidine 1 97.86 1.12 N 5 mM Histidine 1.74
97.33 0.91 N
15 mM Histidine 1.08 97.43 1.47 N 15 mM Histidine 2.01
96.77 1.21 N
50 mM Histidine 1.25 97.36 1.37 N 50 mM Histidine 2.31
96.45 1.22 N
200 mM
Histidine 1.61 97.08 1.3 N 200 mM
Histidine N
TO
DVD-C, pH 5.4, DVD-C, pH 5.4,
1 mg/ml Agg Mon Frag Precipitation* 100 mg/ml
Agg Mon Frag Precipitation*
0 Histidine 2.31 96.35 1.33 N 0 Histidine 3.05
95.18 1.75 N
5 mM Histidine 1.82 96.64 1.52 N 5 mM Histidine 2.55
95.84 1.6 N
15 mM Histidine 1.83 96.53 1.63 N 15 mM Histidine 2.2
96.1 1.68 N
50 mM Histidine 1.87 96.67 1.44 N 50 mM Histidine 1.8
96.54 1.64 N
200 mM
Histidine 2.17 96.14 1.68 N 200 mM Histidine 1.7
96.49 1.8 N
40C, 7d
DVD-C, pH 5.4, DVD-C, pH 5.4,
Precipitation*
1 mg/ml Agg Mon Frag Precipitation* 100 mg/ml Agg Mon
Frag
0 Histidine 2.41 92.11 5.47 N 0 Histidine 3.18
94.49 2.31 N
5 mM Histidine 1.62 95.93 2.43 N 5 mM Histidine 2.65
95.2 2.13 N
15 mM Histidine 1.49 95.9 2.59 N 15 mM Histidine 2.46
94.96 2.57 N
50 mM Histidine 1.38 96.16 2.45 N 50 mM Histidine 2.33
95.16 2.49 N
200 mM 1.47 96.12 2.39 N 200 mM Histidine 1.82
95.19 2.97 N
88
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Histidine
1 1 1 1 1 1 1 1 1
40C, 21d
DVD-C, pH 5.4, DVD-C, pH 5.4,
1 mg/ml Agg Mon Frag Precipitation* 100 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 1.38 95.5 3.11 N 0 Histidine 3.45 93.64
2.89 N
mM Histidine 1.5 95.39 3.1 N 5 mM Histidine 2.8
94.27 2.91 N
mM Histidine 1.26 96.01 2.71 N 15 mM Histidine
2.55 94.56 2.87 N
50mM Histidine 1.34 95.83 2.82 N 50mM Histidine
2.26 94.9 2.83 N
200 mM
Histidine 1.38 95.72 2.88 N 200 mM Histidine 2.84
93.29 3.86 N
5C, 21d
DVD-C, pH 5.4, DVD-C, pH 5.4,
1 mg/ml Agg Mon Frag Precipitation* 100 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 2.08 96.12 1.78 N 0 Histidine 2.77 94.77
2.44 N
5 mM Histidine 1.56 96.72 1.71 N 5 mM Histidine
2.35 95.43 2.2 N
15 mM Histidine 1.27 97.08 1.63 N 15 mM Histidine
2.18 95.42 2.38 N
50mM Histidine 1.4 96.67 1.91 N 50mM Histidine 1.95
96.07 1.96 N
200 mM
Histidine 1.59 96.62 1.77 N 200 mM Histidine 1.95
96.1 1.94 N
TO
IL121L18, pH IL121L18, pH 5.4,
5.4, 1 mg/ml Agg Mon Frag Precipitation* 150 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 2.95 95.23 1.8 N 0 Histidine 3.48 94.22
2.29 N
5 mM Histidine 1.92 96.28 1.79 N 5 mM Histidine
4.64 93.26 2.09 N
15 mM Histidine 2.16 95.71 2.12 N 15 mM Histidine
4.33 93.53 2.12 N
50mM Histidine 2.07 96 1.91 N 50mM Histidine 3.93
94.2 1.88 N
200 mM
Histidine 2.46 95.65 1.87 N 200 mM Histidine 3.55
94.48 1.96 N
40C, 7d
IL121L18, pH IL121L18, pH 5.4,
5.4, 1 mg/ml Agg Mon Frag Precipitation* 150 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 2.88 93.82 3.29 N 0 Histidine 4.71 91.46
3.82 N
5 mM Histidine 1.32 95.82 2.85 N 5 mM Histidine
5.79 90.44 3.75 N
15 mM Histidine 1.02 96.12 2.85 N 15 mM Histidine
4.87 91.81 3.3 N
50mM Histidine 1.57 95.23 3.19 N 50mM Histidine
8.58 87.7 3.71 N
200 mM
Histidine 1.43 95.56 3 N 200 mM Histidine 6.78
89.81 3.4 N
40C, 21d
IL121L18, pH IL1211L18, pH 5.4,
5.4, 1 mg/ml Agg Mon Frag Precipitation* 150 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 1.51 94.69 3.79 N 0 Histidine 5.18 91.21
3.6 N
5 mM Histidine 1.08 95.87 3.04 N 5 mM Histidine
6.76 89.67 3.56 N
15 mM Histidine 1.07 95.85 3.06 N 15 mM Histidine
5.7 91.19 3.09 N
50mM Histidine 1.2 95.56 3.32 N 50mM Histidine 12.87
83.35 3.76 N
200 mM
Histidine 1.42 95.42 3.15 N 200 mM Histidine 7.21
89.88 2.89 N
5C, 21d
IL121L18, pH IL121L18, pH 5.4,
5.4, 1 mg/ml Agg Mon Frag Precipitation* 150 mg/ml Agg
Mon Frag Precipitation*
0 Histidine 1.71 96.03 2.24 N 0 Histidine 5.96 91.35
2.67 N
5 mM Histidine 1.25 96.44 2.3 N 5 mM Histidine
6.94 90.97 2.07 N
15 mM Histidine 1.4 96.13 2.46 N 15 mM Histidine
5.69 92.19 2.1 N
50mM Histidine 1.78 96.03 2.18 N 50mM Histidine
10.07 88.13 1.78 N
200 mM
Histidine 2.1 95.7 2.19 N 200 mM Histidine 1.32
95.56 3.11 N
* Precipitation indicates if insoluble visible aggregates were observed (Yes
or No)
89
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Table 17: Impact of Storage of Various DVD-Ig Proteins at Low and High
Concentrations
Under Various Histidine Concentrations on IEC
TO
DVD-A, pH 5.2,
1 mg/ml Acidic
Main Basic DVD-A, pH 5.2, 75 mg/ml Acidic Main Basic
0 Histidine 15.66 49.3 35.02 0 Histidine 19.71
50.72 29.55
mM Histidine 13.15 50.9 35.93 5 mM Histidine 19.11
50.8 30.08
mM Histidine 15.11 46.74 38.13 15 mM Histidine 18.58
44.52 36.88
50mM Histidine 13.27 52.38 34.33 50mM Histidine 15.69
52.06 32.24
200 mM Histidine 14.55 52.01 33.42 200 mM
Histidine 16.79 45.36 37.84
40C, 7d
DVD-A, pH 5.2, 1
mg/ml Acidic
Main Basic DVD-A, pH 5.2, 75 mg/ml Acidic Main Basic
0 Histidine 24.97 40.98 34.03 0 Histidine 21.7
43.6 34.68
5 mM Histidine 22.21 44.35 33.43 5 mM Histidine 28.31
43.99 27.69
15 mM Histidine 19.79 44.91 35.29 15 mM Histidine
21.21 41.34 37.44
50mM Histidine 18.19 46.11 35.68 50mM
Histidine 18.42 42.84 38.73
200 mM Histidine 18.08 48.08 33.83 200 mM Histidine
16.71 42.09 41.19
5C, 21d
DVD-A, pH 5.2, 1
mg/ml Acidic
Main Basic DVD-A, pH 5.2, 75 mg/ml Acidic Main Basic
0 Histidine 15.66 46.47 37.86 0 Histidine 16.41
48.16 35.41
5 mM Histidine 18.76 49.37 31.85 5 mM
Histidine 16.19 49.44 34.36
15 mM Histidine 14.34 52.8 32.85 15 mM Histidine
16.91 45.77 37.3
50mM Histidine 15.06 50.33 34.59 50mM
Histidine 17.17 47.45 35.37
200 mM Histidine 14.49 48.8 36.7 200 mM
Histidine 15.77 46.18 38.03
TO
DVD-C, pH 5.4, 1 DVD-C, pH 5.4, 100
mg/ml mg/ml
0 Histidine 26.28 59.4 14.3 0 Histidine 24.53
66.44 9.01
5 mM Histidine 23.01 69.42 7.56 5 mM Histidine 24.17
65.96 9.86
15 mM Histidine 22.05 67.79 10.14 15 mM Histidine
23.67 67.77 8.55
50mM Histidine 21.9 69.09 9 50mM Histidine 22.71
67.65 9.63
200 mM Histidine 20.99 70.74 8.26 200 mM Histidine
40C, 7d
DVD-C, pH 5.4, 1 DVD-C, pH 5.4, 100
mg/ml mg/ml
0 Histidine 34.05 55.74 10.2 0 Histidine 28.68
61.69 9.61
5 mM Histidine 31.26 59.07 9.65 5 mM Histidine 29.05
61.57 9.37
15 mM Histidine 27.8 61.86 10.33 15 mM Histidine 29.2
61.25 9.54
50mM Histidine 25.97 59.33 14.49 50mM Histidine 27.43
61.86 10.69
200 mM Histidine 26.35 62.28 11.36 200 mM
Histidine 28.04 59.58 12.37
5C, 21d
DVD-C, pH 5.4, 1 DVD-C, pH 5.4, 100
mg/ml mg/ml
0 Histidine 25.05 65.7 9.23 0 Histidine 23.43
66.85 9.71
5 mM Histidine 23.66 66.17 10.15 5 mM Histidine 22.3
68.58 9.11
15 mM Histidine 22.11 69.96 8.91 15 mM Histidine
23.39 67.31 9.29
50mM Histidine 21.79 68.96 9.23 50mM Histidine 23.67
67.96 8.35
200 mM Histidine 21.96 70.23 7.8 200 mM Histidine
22.6 69.68 7.65
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
TO
IL121L18, pH 5.4, IL121L18, pH 5.4, 150
1 mg/ml mg/ml
0 Histidine 30.24 51.83 17.92 0 Histidine 31.05
50.74 18.2
mM Histidine 29.11 54.71 16.16 5 mM Histidine 30.3
53.53 16.16
15 mM Histidine 28.36 57.23 14.39 15 mM Histidine 30.02 53.9
16.07
50mM Histidine 29.15 53.29 17.55 50mM Histidine 28.42
51.31 20.26
200 mM Histidine 35.59 52.31 12.09 200 mM Histidine 28.85
55.24 15.9
40C, 7d
IL121L18, pH 5.4, IL121L18, pH 5.4, 150
1 mg/ml mg/ml
0 Histidine 37.24 45.3 17.45 0 Histidine 34.95
44.85 20.19
5 mM Histidine 36 47.57 16.42 5 mM Histidine 31.94
45.7 22.34
15 mM Histidine 36.17 50.01 13.81 15 mM Histidine 32.39
45.54 22.05
50mM Histidine 35.39 49.12 15.47 50mM Histidine 37 41.76 21.23
200 mM Histidine 38.48 45.03 16.47 200 mM Histidine 30.86
47.34 21.78
5C, 21d
IL121L18, pH 5.4, IL121L18, pH 5.4, 150
1 mg/ml mg/ml
0 Histidine 30.72 51.83 17.44 0 Histidine 30.91
50.49 18.59
5 mM Histidine 30.25 51 18.74 5 mM
Histidine 29.14 50.47 20.37
15 mM Histidine 30.07 56.33 13.58 15 mM Histidine 28.45
50.95 20.58
50mM Histidine 30.51 55.5 13.97 50mM Histidine
27.91 52.66 19.42
200 mM Histidine 42.9 49 8.08 200 mM
Histidine 27.96 48.84 22.19
Tables 16 and 17 show that the amount of monomer remaining at different time
points
and at the formation of insoluble aggregates indicates that histidine
concentrations in the
range of about 5 to about 50 mM provided optimum stability. A concentration of
about 200
mM histidine resulted in the formation of insoluble aggregates in some cases
(see indication
of precipitation in Table 16). 0 mM histidine formulations showed enhanced
aggregation as
indicated by formation of insoluble and soluble aggregates in some cases (in
some cases
soluble aggregates were higher at 0 than that at 5 mM histidine
concentrations). Secondly,
the pH is expected to be well maintained for longer storage times in
formulations containing
histidine.,
The error in IEC measurements is usually higher (2-3 % variation) compared to
SEC
measurements with the same formulation. Hence taking that into account, no
significant
differences were observed within formulations as assayed by IEC indicating
that the chemical
stability was not as affected by the molarity of histidine, hence the chemical
stability is
independent of the buffer concentration (generally, in contrast to aggregation
propensity).
91
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Example 8: Example of the Stability of an AS-DVD Ig Protein (DVD-CAnti-IL1
alpha/beta DVD-Ig Protein) in Solution
An anti-IL1 alpha/beta DVD-Ig protein (DVD-C) was assessed for stability over
time
at both 100 mg/ml and 1 mg/ml in different buffers, at different pHs and at
different
temperatures. Buffers that were tested at 100 mg/ml of DVD-C included 15 mM
acetate pH
4; 15 mM acetate pH 5; 15 mM histidine pH 5.5; 15 mM succinate pH 5.5; 15 mM
histidine
pH 6.0; Water (no buffer) pH 6.0; 15 mM citrate pH 6.0; 15 mM histidine pH
6.5; and 15
mM Tris pH 8Ø Buffers that were tested at 1 mg/ml of DVD-C included 10 mM
citrate + 10
mM phosphate buffer at pH 3, 4, 5, 6, 7, or 8.
The samples were stored at 50 C, 40 C, 25 C, and 5 C. At certain time points,
samples were pulled and evaluated for stability. Physical stability was
evaluated by size
exclusion chromatography (SE-HPLC or SEC), including % aggregate, % monomer, %
fragment, and total species recovered were quantitated. Chemical stability was
evaluated by
weak cation exchange chromatography (IEX-HPLC or IEC), including % acidic, %
main, and
% basic species quantitated.
Tables 18 and 19 describe stability of DVD-C at 100 mg/ml and 1 mg/ml,
respectively, in various buffers and pH. In both Tables 17 and 18, size
exclusion
chromatography (SEC) data and ion-exchange chromatography (IEC) data is
displayed.
Formulation and abbreviation keys are given below each table.
Table 18. Stability at Various Temperatures of DVD-C at 100 mg/ml in Different
Buffers and
at Different pHs.
SEC data IEX data
Time Temp( C) Formulation pH
% HMW % M LMW AUC % Acidic % Main % Basic
TO ace 4 0.88 98.03 1.09 45941 19.65
71.68 8.67
TO ace 5 0.94 98.19 0.87 45789 19.79
71.60 8.61
TO his 5.5 0.98 98.15 0.86 48085 20.40
71.90 7.70
TO succ 5.5 1.04 97.96 1.00 49317 19.70
71.54 8.76
TO his 6 1.15 97.93 0.92 48468 20.68
70.94 8.38
TO water 6 1.87 97.27 0.86 48356 18.95
72.35 8.70
TO citrate 6 1.38 97.54 1.09 44457 19.35
72.37 8.28
92
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
SEC data IEX data
Time Temp( C) Formulation pH %
% HMW % M LMW AUC % Acidic % Main % Basic
TO --- his 6.5 1.16 97.93 0.91 47102 21.17
70.45 8.38
TO --- tris 8 2.40 96.65 0.95 53889 21.71
69.12 9.17
T7d 40 ace 4 0.92 97.03 2.05 49124 19.16
71.61 9.23
T7d 40 ace 5 1.12 97.31 1.57 49077 21.62
71.09 7.29
T7d 40 his 5.5 1.17 97.27 1.56 49662 19.48
73.60 6.92
T7d 40 succ 5.5 1.46 97.06 1.48 48821 23.18
69.51 7.31
T7d 40 his 6 1.72 96.96 1.32 42872 20.99
73.36 5.64
T7d 40 water 6 2.48 96.22 1.30 37817 21.67
72.02 6.31
T7d 40 citrate 6 1.79 96.83 1.37 43022 21.70
71.52 6.78
T7d 40 his 6.5 2.08 96.65 1.27 48176 23.68
70.89 5.43
T7d 40 tris 8 4.58 93.87 1.55 48306 40.86
52.70 6.44
Tlmo 40 ace 4 1.32 94.29 4.38 52518 34.78
32.07 33.16
Tlmo 40 ace 5 1.64 95.36 3.00 53762 36.48
52.11 11.41
Tlmo 40 his 5.5 1.61 95.38 3.01 52489 33.12
55.52 11.36
Tlmo 40 succ 5.5 2.25 94.73 3.01 51508 40.94
46.88 12.19
Tlmo 40 his 6 2.81 94.53 2.66 52229 34.46
56.00 9.54
Tlmo 40 water 6 3.66 93.75 2.58 52285 33.46
56.53 10.01
Tlmo 40 citrate 6 2.67 94.91 2.42 51968 36.54
52.80 10.67
Tlmo 40 his 6.5 3.61 93.69 2.71 50276 39.06
51.20 9.74
Tlmo 40 tris 8 7.49 85.24 7.28 53081 52.90
15.51 31.58
Tlmo 25 ace 4 0.98 97.30 1.72 56026 23.39
59.02 17.59
Tlmo 25 ace 5 1.15 97.31 1.54 55264 22.44
69.01 8.55
Tlmo 25 his 5.5 1.16 97.41 1.43 54356 22.05
69.43 8.51
Tlmo 25 succ 5.5 1.42 97.07 1.51 52417 23.00
67.63 9.37
Tlmo 25 his 6 1.75 96.74 1.51 52220 23.80
67.67 8.52
Tlmo 25 water 6 2.66 95.94 1.40 51198 23.68
67.35 8.98
Tlmo 25 citrate 6 1.91 96.59 1.49 51137 22.41
68.14 9.45
Tlmo 25 his 6.5 1.93 96.58 1.49 50594 25.93
65.50 8.56
Tlmo 25 tris 8 4.35 93.87 1.78 51644 39.99
50.14 9.87
93
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
SEC data IEX data
Time Temp( C) Formulation pH %
% HMW % M LMW AUC % Acidic % Main % Basic
T3mo 40 ace 4
2.19 85.66 12.15 54546 45.24 29.61 25.15
T3mo 40 ace 5 2.69 89.70 7.61 63493 52.09
31.52 16.39
T3mo 40 his 5.5 2.57 89.79 7.64 64361 48.68
35.18 16.14
T3mo 40 succ 5.5 3.53 88.89 7.58 57855 60.00
24.73 15.28
T3mo 40 his 6 4.08 90.70 5.21 60062 50.85
37.10 12.04
T3mo 40 water
6 4.80 90.50 4.70 58908 47.55 43.39 9.06
T3mo 40 citrate 6 3.87 91.38 4.75 55505 56.39
30.85 12.76
T3mo 40 his 6.5 5.56 89.08 5.37 56243 58.77
32.28 8.95
T3mo 40 tris 8
14.20 75.16 10.64 59362 63.76 22.12 14.12
T3mo 25 ace 4 1.15 96.39 2.46 60821 25.24
61.82 12.94
T3mo 25 ace 5 1.29 96.85 1.86 53513 23.62
66.47 9.91
T3mo 25 his 5.5 1.34 96.84 1.82 56041 23.05
67.70 9.25
T3mo 25 succ 5.5 1.69 96.36 1.95 53657 27.27
61.25 11.48
T3mo 25 his 6 2.04 96.23 1.73 51684 26.17
63.16 10.67
T3mo 25 water 6 2.81 95.49 1.70 52442 25.01
64.07 10.91
T3mo 25 citrate 6 2.08 96.11 1.80 51993 24.48
64.34 11.18
T3mo 25 his 6.5 2.26 96.01 1.72 50988 30.52
59.06 10.42
T3mo 25 tris 8 5.61 91.87 2.51 52070 59.46
31.54 8.99
T3mo 5 ace 4 0.85 98.01 1.14 50571 18.16
71.76 10.09
T3mo 5 ace 5 1.06 97.91 1.04 49173 18.44
71.54 10.02
T3mo 5 his 5.5 1.33 97.65 1.02 51947 19.97
70.28 9.75
T3mo 5 succ 5.5 1.32 97.59 1.09 50358 19.12
70.88 10.00
T3mo 5 his 6 2.18 96.79 1.03 49875 22.34
67.47 10.19
T3mo 5 water 6 8.21 90.70 1.09 48448 18.89
67.56 13.55
T3mo 5 citrate 6 2.20 96.65 1.15 48907 19.08
70.77 10.16
T3mo 5 his 6.5 1.86 97.12 1.02 47895 22.91
67.10 9.99
T3mo 5 tris 8 5.93 93.02 1.05 49015 24.67
63.29 12.04
Formulation and abbreviation key:
ace = 15 mM acetate
his = 15 mM histidine
94
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
succ = 15 mM succinate
water = formulated in water by dialysis
citrate = 15 mM citrate
tris = 15 mM TRIS
% HMW = percentage of high molecular weight species quantitated by SEC
% M = percentage of monomer quantitated by SEC
% LMW = percentage of low molecular weight species (fragments) quantitated by
SEC
AUC = total integrated area of the SEC curve
% acidic = percentage of acidic species relative to the main species
quantitated by
IEC
% main = percentage of main species quantitated by IEC
% basic = percentage of basic species relative to the main species quantitated
by
IEX
TO = time zero
T7d = 7 days of storage
Tlmo = 1 month of storage
T3mo = 3 months of storage
Table 19. Stability at various temperatures of DVD-C at 1.0 mg/ml in 10 mM
citrate + 10
mM phosphate buffer and at different pHs.
SEC data IEX data
Time Temp( C) pH % % %
%
%M AUC . %
Basic
HMW LMW Acidic Main
0 3 ND ND ND ND ND ND ND
0 4
0.78 97.97 1.25 106805 10.14 73.38 16.48
0 5
1.07 97.71 1.21 104165 9.80 73.84 16.35
0 6
1.23 97.62 1.15 99838 10.80 73.52 15.69
0 7
1.37 97.43 1.21 98566 10.45 73.79 15.76
0 8
1.48 97.30 1.22 93914 10.33 74.41 15.26
7d 40 3 ND ND ND ND ND ND ND
7d 40 4
0.70 94.51 4.79 99177 38.59 41.06 20.35
7d 40 5
1.09 97.43 1.48 105735 24.92 57.07 18.01
7d 40 6
1.30 97.16 1.54 101459 21.48 62.95 15.57
7d 40 7
1.53 96.38 2.09 94903 16.60 69.48 13.93
7d 40 8
2.00 95.74 2.25 95090 50.88 36.05 13.07
7d 50 3 ND ND ND ND ND ND ND
7d 50 4 ND ND ND ND ND ND ND
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
SEC data IEX data
Time Temp( C) pH % % % %
%M AUC . %
Basic
HMW LMW Acidic Main
7d 50 5
2.72 93.91 3.37 102195 49.52 32.87 17.61
7d 50 6
2.23 95.55 2.22 99767 37.88 47.12 15.00
7d 50 7
1.67 94.59 3.74 96751 54.67 32.09 13.24
7d 50 8
1.82 94.13 4.05 85005 61.11 13.48 25.41
21d 5 3 ND ND ND ND ND ND ND
21d 5 4
0.01 96.54 3.45 105701 11.64 73.47 14.89
21d 5 5 0.00
96.29 3.71 102947 11.40 73.19 15.41
21d 5 6
0.01 96.33 3.66 98166 11.56 73.70 14.74
21d 5 7
0.01 96.24 3.75 96077 17.02 67.39 15.60
21d 5 8
0.01 95.63 4.36 91507 17.87 68.99 13.15
21d 25 3 ND ND ND ND ND ND ND
21d 25 4 0.08
95.89 4.02 107054 32.05 49.45 18.51
21d 25 5 0.07
96.85 3.08 104609 20.27 62.85 16.88
21d 25 6
0.05 96.86 3.09 100308 18.61 66.21 15.18
21d 25 7 0.08 96.40 3.52 97790 18.83 67.03 14.14
21d 25 8 0.07 95.82 4.12 92666 41.03 45.81 13.16
21d 40 3 ND ND ND ND ND ND ND
21d 40 4
0.09 88.58 11.33 79690 61.27 18.98 19.74
21d 40 5 0.20
95.13 4.67 105902 46.17 37.31 16.52
21d 40 6
0.23 95.96 3.81 101455 31.13 54.02 14.85
21d 40 7 0.33 94.32 5.35 98972 53.39 34.34 12.27
21d 40 8 0.37 93.05 6.57 91576 59.60 15.00 25.40
21d 50 3 ND ND ND ND ND ND ND
21d 50 4 ND ND ND ND ND ND ND
21d 50 5 0.20 91.14 8.66 90993 56.65 10.23 33.13
21d 50 6
0.34 93.57 6.09 100229 66.40 21.90 11.70
21d 50 7 0.54 89.92 9.54 91695 50.18 26.86 22.97
21d 50 8
0.47 87.11 12.41 67323 47.09 17.82 35.09
3mo 40 3 ND ND ND ND ND ND ND
96
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
SEC data IEX data
Time Temp( C) pH % % % %
%M AUC . %
Basic
HMW LMW Acidic Main
3mo 40 4 27.59 40.51 31.91 61759 NR NR NR
3mo 40 5 5.12 85.68 9.19 119476 NR NR NR
3mo 40 6 2.79 91.55 5.67 113431 NR NR NR
3mo 40 7 3.40 88.33 8.27 106409 NR NR NR
3mo 40 8 5.41 80.86 13.73 98142 NR NR NR
3mo 25 3 ND ND ND ND ND ND ND
3mo 25 4 1.10 91.64 7.26 109681 53.69 23.99 22.32
3mo 25 5 1.24 96.19 2.57 106230 37.33 41.02 21.65
3mo 25 6 1.56 96.65 1.79 102674 27.46 53.25 19.29
3mo 25 7 1.85 95.59 2.56 100526 17.69 65.85 16.46
3mo 25 8 2.37 94.80 2.83 95500 65.32 20.08 14.59
3mo 5 3 ND ND ND ND ND ND ND
3mo 5 4 0.83 97.90 1.28 104383 18.59 60.63 20.78
3mo 5 5 1.14 97.86 1.00 101554 16.91 58.78 24.30
3mo 5 6 1.31 97.75 0.95 97897 17.38 63.16 19.46
3mo 5 7 1.54 97.43 1.03 96067 18.48 63.33 18.19
3mo 5 8 1.67 97.27 1.06 92532 20.44 61.36 18.21
6.5mo 25 3 ND ND ND ND ND ND ND
6.5mo 25 4 1.67 85.69 12.64 7753 70.60 10.11 19.28
6.5mo 25 5 1.35 94.61 4.04 7865 50.36 28.97 20.67
6.5mo 25 6 1.69 95.80 2.51 7719 36.64 46.73 16.63
6.5mo 25 7 2.12 94.15 3.74 7523 55.68 28.89 15.44
6.5mo 25 8 2.71 93.02 4.26 7155 68.76 16.22 15.01
6.5mo 5 3 ND ND ND ND ND ND ND
6.5mo 5 4 0.76 97.78 1.46 7508 19.30 59.53 21.17
6.5mo 5 5 1.07 97.92 1.00 7315 17.96 60.34 21.70
6.5mo 5 6 1.38 97.75 0.87 6969 19.99 60.32 19.69
6.5mo 5 7 1.61 97.38 1.02 6859 19.46 62.30 18.24
6.5mo 5 8 1.71 97.17 1.11 6501 21.06 59.64
19.30
97
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Abbreviation key:
% HMW = percentage of high molecular weight species quantitated by SEC
% M = percentage of monomer quantitated by SEC
% LMW = percentage of low molecular weight species (fragments) quantitated by
SEC
AUC = total integrated area of the SEC curve
% acidic = percentage of acidic species relative to the main species
quantitated by
IEX
% main = percentage of main species quantitated by IEX
% basic = percentage of basic species relative to the main species quantitated
by
IEX
ND = no species detected
NR = assay not performed
0 = time zero
7d = 7 days of storage
21d = 1 month of storage
3mo = 3 months of storage
6.5mo = 6.5 months of storage
The molecule completely degraded during dialysis at pH 3. No species were
detected
by SEC or IEC after dialysis at this condition at time zero. Also, storage at
50 C at pH 4
yielded the same result. Both results are indicated by ND.
In addition, the thermal stability of DVD-C was assessed by differential
scanning
calorimetry (DSC). The thermal stability was evaluated at 1.0 mg/ml of the
molecule
formulated in 10 mM citrate + 10 mM phosphate buffer at pH 4, 5, 6, 7, or 8. A
higher onset
temperature of unfolding or higher domain midpoint temperature of unfolding
means greater
thermal stability. The thermal stability at different pHs is often correlated
with the long-term
stability of the molecule formulated at those pHs. Therefore, the DSC data can
help identify
the pHs at which the molecule is most and least stable.
Table 20. Differential Scanning Calorimetry Data of DVD-C at 1.0 mg/ml in 10
mM Citrate
+ 10 mM Phosphate Buffer and at Different pHs*
pH Onset ( C) Tml ( C) Tm2 ( C) Tm3 ( C) Tm4 ( C)
4 49.2 64.4 67.3 74.2 78.8
55.0 68.1 69.9 76.2 82.0
6 54.8 67.5 69.4 75.7 83.1
98
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
7 55.0 67.2 69.0 74.4 82.7
8 54.4 67.1 68.8 73.8 82.5
* Numbered Tm values indicate the midpoint of the unfolding transitions.
As described above, the stability of DVD-C was tested in a number of different
buffers and pHs, when stored at three different temperatures (40 C, 25 C, or 5
C). The
concentration of DVD-C ranged from 1 mg/ml to 100 mg/ml. At 100 mg/ml, buffers
included
acetate, histidine, succinate, citrate, and tris. DVD-C was also formulated in
plain water. The
pH of the formulations ranged from 4 to 8. At 1 mg/ml, the protein was
formulated in citrate-
phosphate buffer with the pH ranging from 3 to 8. Once formulated, the samples
of the DVD-
Ig proteins were stored at the aforementioned temperatures. At specific time
points, aliquots
were taken and assessed for physical stability by SEC and chemical stability
by weak cation
exchange (WCX).
Overall, the data indicated DVD-C is stable except at pH 3 and pH 4.
Specifically, the
data suggest that physical stability of DVD-C is greatest at a pH near 5.5 and
that chemical
stability is highest at a pH near 6Ø Histidine and succinate were determined
to be
appropriate buffers for these pHs.
In addition, the thermal stability of DVD-C was assessed by differential
scanning
calorimetry (DSC), as described in Table 18. The thermal stability was
evaluated at 1.0
mg/ml of DVD-C formulated in citrate-phosphate buffer at pHs 4 to 8. A higher
onset
temperature of unfolding (Ton) or higher domain midpoint temperature of
unfolding (Tm)
means greater thermal stability. Thermal stability is likely correlated with
the long-term
stability of the DVD-Ig protein. The data indicate similar thermal stability
in the pH range of
to 8.
EXAMPLE 9: Effect of a Polyol on the Stability of AS-DVD-Ig Proteins
Dynamic scanning fluorescence (DSF) was employed to assess the propensity of a
protein to unfold. The impact of polyols, e.g., sucrose and sorbitol, was
investigated in order
to assess the effect of these polyols on the stability of the protein in
solution. DVD-A, DVD-
C and an IL12/IL18 DVD-Ig protein were used as exemplary AS-DVD-Ig proteins.
As
shown in Table 21 below, in general, AS-DVD-Ig proteins at various
concentrations (1, 100,
99
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
150 mg/ml) are stable with the presence of sucrose (e.g., 40-160 mg/ml) and
sorbitol (e.g.,
20-80 mg/ml). Moreover, an increase in the concentration of sorbitol and
sucrose provided
resulted in a slight increased stability. Hence addition of sugars to a buffer
(e.g., 15 mM
histidine) containing protein formulation enhances the stability of AS-DVD-Ig
proteins
slightly
Table 21: Impact Of Polyols On The Thermodynamic Stability Of Various AS-DVD-
Ig
Proteins As Assessed By Dynamic Scanning Fluorescence At pH 6
Onset of
IL121L18 DVD-Ig Protein Conc. Unfolding
( C)
lmg/ml, No Sorbitol 62.8
lmg/ml, 20 mg/ml Sorbitol 63.2
lmg/ml, 40 mg/ml Sorbitol 63.1
lmg/ml, 80 mg/ml Sorbitol 63.5
150 mg/ml, No Sorbitol 57.5
150 mg/ml, 20 mg/ml Sorbitol 57.6
150 mg/ml, 40 mg/ml Sorbitol 57.6
150 mg/ml, 80 mg/ml Sorbitol 58.5
lmg/ml, No Sucrose 62.8
lmg/ml, 40 mg/ml Sucrose 63.1
lmg/ml, 80 mg/ml Sucrose 64.1
lmg/ml, 160 mg/ml Sucrose 64.3
150 mg/ml, No Sucrose 57.5
150 mg/ml, 40 mg/ml Sucrose 58.2
150 mg/ml, 80 mg/ml Sucrose 58.3
150 mg/ml, 160 mg/ml Sucrose 58.3
DVD-C
lmg/ml, No Sorbitol 62
lmg/ml, 20 mg/ml Sorbitol 62.3
lmg/ml, 40 mg/ml Sorbitol 62
lmg/ml, 80 mg/ml Sorbitol 62.1
100 mg/ml, No Sorbitol 47
100 mg/ml, 20 mg/ml Sorbitol 47.5
100 mg/ml, 40 mg/ml Sorbitol 47
100 mg/ml, 80 mg/ml Sorbitol 48.1
lmg/ml, No Sucrose 62
lmg/ml, 40 mg/ml Sucrose 62.1
lmg/ml, 80 mg/ml Sucrose 62
lmg/ml, 160 mg/ml Sucrose 62.2
100 mg/ml, No Sucrose 47
100 mg/ml, 40 mg/ml Sucrose 46.9
100 mg/ml, 80 mg/ml Sucrose 48.1
100 mg/ml, 160 mg/ml Sucrose 48
DVD-A
100
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
lmg/ml, No Sorbitol 55.8
lmg/ml, 20 mg/ml Sorbitol 56
lmg/ml, 40 mg/ml Sorbitol 56
lmg/ml, 80 mg/ml Sorbitol 56.1
75 mg/ml, No Sorbitol 49.5
75 mg/ml, 20 mg/ml Sorbitol 50
75 mg/ml, 40 mg/ml Sorbitol 50.5
75 mg/ml, 80 mg/ml Sorbitol 50.2
lmg/ml, No Sucrose 55.8
lmg/ml, 40 mg/ml Sucrose 56
lmg/ml, 80 mg/ml Sucrose 56.4
lmg/ml, 160 mg/ml Sucrose 56.1
75 mg/ml, No Sucrose 49.5
75 mg/ml, 40 mg/ml Sucrose 49.5
75 mg/ml, 80 mg/ml Sucrose 50
75 mg/ml, 160 mg/ml Sucrose 51.1
As evidenced by the above data in Table 21, polyols (e.g., sorbitol and
sucrose) can
improve stability of AS-DVD-Ig proteins in solution in a broad range (20 to
160 mg/ml
sucrose and 20 to 80/ mg/ml sorbitol). Furthermore, the data reveal a decrease
in the onset of
unfolding, hence a decrease in the thermodynamic stability, with increasing
DVD-Ig protein
concentration. These data correlate with the observed instability, such as
aggregation
observed at high concentration liquid formulations.
V. AS-DVD-IG PROTEINS ARE STABLE IN FORMULATIONS CONTAINING
A BUFFER AND A POLYOL AT PH RANGE OF 4.5-7.5
EXAMPLE 10: Effect of A Buffer on the Storage Stability of DVD-A in A
Formulation
Containing A Polyol
To assess the effect of a buffer on the storage stability of DVD-A in
different buffers,
the stability of the formulations was assessed before storage (TO) or after 7
days (7d) or 21
days (21d) of storage at 40 C (accelerated storage). DVD-A (85 mg/ml), an AS-
DVD-Ig
protein, was formulated in various buffers (15 mM citrate, 15 mM histidine, 15
mM arginine,
15 mM acetate, or water) at a pH of 5.2. Samples were filled into sterile
vials (approx. 500 pi
each) and stored under controlled conditions in temperature chambers and in
the absence of
light. The samples were analyzed using SEC and the results are provided in
Tables 22 and
23.
101
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 22: Effect of Buffer Type on Storage Stability of DVD-A As Measured By
SEC
Formulation Time Point Mon Agg Frag
15 mM acetate, 80 mg/ml sucrose, TO 95.96 2.75 1.28
pH 4.0 7d, 40C 71.84 24.93
3.21
21d, 40C 66.62 26.91 6.45
15 mM acetate, 80 mg/ml sucrose, TO 96.06 2.78 1.14
pH 5.2 7d, 40C 89.19 8.76 2.03
21d, 40C 90.93 4.41 4.65
15 mM arginine, 80 mg/ml sucrose, TO 95.84 3.07 1.08
pH 5.2 7d, 40C 89.62 8.54 1.82
21d, 40C 81.03 12.41 6.54
15 mM citrate, 80 mg/ml sucrose, TO 95.72 3.19 1.08
pH 5.2 7d, 40C 90.54 6.96 2.48
21d, 40C 87.39 7.74 4.86
15 mM histidine, 80 mg/ml sucrose, TO 96 2.9 1.09
pH 5.2 7d, 40C 91.04 6.91 2.03
21d, 40C 87.58 8.63 3.78
Water, 80 mg/ml sucrose, pH 5.2 TO 94.89 4.04 1.05
7d, 40C 88.4 9.76 1.82
21d, 40C 81.41 14.39 4.18
Table 23: Effect of Buffer Type on Storage Stability of DVD-A As Measured By
IEC
Formulation Time Point Main Acidic
Basic
TO 56.88 9.43 33.68
15 mM acetate, 80 mg/ml sucrose, pH 4.0 7d, 40C 46.91 18.74
34.33
21d, 40C 34.62 24.82 40.54
15 mM acetate, 80 mg/ml sucrose, pH 5.2 TO 57.2 9.45
33.34
7d, 40C 47.61 18.74 33.64
21d, 40C 35.29 33.35 31.35
15 mM arginine, 80 mg/ml sucrose, pH 5.2 TO 63.44 9.93
26.62
7d, 40C 49.22 19.57 31.2
21d, 40C 37.87 25.3 36.81
15 mM citrate, 80 mg/ml sucrose, pH 5.2 TO 59.59 7.88
35.51
7d, 40C 45.92 20.82 33.24
21d, 40C 31.97 31.81 36.21
15 mM histidine, 80 mg/ml sucrose, pH 5.2 TO 58.29 8.18
33.52
7d, 40C 44.97 16.84 38.17
21d, 40C 36.33 24.88 38.77
Water, 80 mg/ml sucrose, pH 5.2 TO 57.05 9.53 33.41
7d, 40C 48.23 19.96 37.15
21d, 40C 37.69 25.15 37.15
SEC results provided in Table 22 show that a pH 5.2 histidine formulation
compared
to the pH 4 histidine formulation had the lowest level of aggregates, although
citrate and
acetate buffers showed low levels of aggregates as well. Although citrate and
acetate showed
102
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
less soluble aggregates as measured by SEC, the solutions were visibly turbid
indicating
formation of insoluble aggregates. The visual turbidity was not significant,
however, given
the SEC measurements. Overall, citrate and acetate formulations were only
slightly less
stable than the histidine formulations. Given that the noise/error in IEC
measurement is
usually higher, no significant differences in the chemical stability were
observed within the
formulations presented in Table 23. The polyol only formulation was also
slightly less stable
as compared to the histidine formulation. Given the overall stability
characteristics of
proteins identified as AS-DVD-Ig proteins, the slight differences observed in
the SEC and
IEC analysis of Tables 22 and 23 showed that each of the tested buffers at pH
5.2 was stable.
Thus, the differences observed between the tested buffers at pH 5.2 indicated
that each could
be used to provide stable formulations for AS-DVD-Ig proteins, including those
at high
concentrations.
VI. AS-DVD-IG PROTEINS ARE STABLE IN FORMULATIONS CONTAINING
A BUFFER, A POLYOL, AND A SURFACTANT AT PH RANGE OF 4.5-7.5
EXAMPLE 11: AS-DVD-Ig Proteins Are Stable in A Range Of Histidine Formulations
Containing Surfactants and Sugars
The following example describes the impact of pH, buffers, and excipients
(including
surfactants and polyols) on the physico-chemical stability of DVD-Ig proteins
at low and
high concentration formulations during accelerated / real time stability
testing. In order to
demonstrate that DVD-Ig proteins have distinctly different protein properties
compared to
monoclonal antibodies, five different formulation conditions that are widely
known and used
to maintain the stability of monoclonal antibodies were tested.
Using size exclusion chromatography (SEC) and ion exchange chromatography
(IEC), the storage stability of low concentration (1 mg/ml) and higher
concentration (100
mg/ml) AS-DVD-Ig protein formulations was evaluated following three storage
conditions:
no storage (TO), 1 month at a controlled temperature of 5 C (1m, 5C), and 1
month at a
controlled temperature of 40 C (1m, 40C). Formulations with varying pH (a
range of 5.25 to
7.2 was selected), buffers, and excipients were tested, according to the
following conditions:
103
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
1) pH 5.25 and pH 6, 15 mM histidine, 80 mg/ml sucrose, 0.01% Tween 80;
2) pH 6, 15 mM histidine, 40 mg/ml sorbitol, 0.01% Tween 80;
3) PBS (10 mM phosphate, 125 mM NaC1) at pH 6 and 7.2;
4) 20 mM glycine, 26 mg/ml glycerol pH 6; and
5) Water, 0.01% Tween 80 pH 5 and 6.
The results are presented in Tables 24-27 below. The data show that not all
DVD-Ig
proteins are stable in all tested pH and formulation conditions. DVD-B formed
high amounts
of aggregates under all the solution conditions tested and were classified as
non-AS-DVD-Ig
proteins (in accordance with the assay presented in Example 4). All the other
DVD-Ig
proteins (previously selected as being AS-DVD-Ig proteins) behaved well and
were stable.
Sucrose, sorbitol, glycerol, and glycine were used to evaluate the effect of
these
excipients. Tween 80 (polysorbate 80), a surfactant that provides
stabilization against
shaking stress, was also used to evaluate its impact on the stability of high
concentration
solutions. The impact of salt concentration was evaluated by varying the ionic
strength using
sodium chloride.
In general, a formulation at pH 6 or pH 5.2 in a histidine buffer was
effective for all
AS-DVD-Ig proteins. Both sorbitol and sucrose each improved stability. Sucrose
resulted in
the formation of slightly less monomer after defined time points. The presence
of salt resulted
in increased instability (defined as less monomer remaining), especially in
the case of the
IL12/IL18 DVD-Ig protein of Table 24 at 100 mg/mL and to a lesser extent at 1
mg/mL, as
shown in Table 27. The presence of glycerol and glycine resulted in less
monomer remaining
following shelf stability as compared to other formulations. For example,
according to Table
24, the IL12IL18 DVD-Ig protein was slightly more stable at pH 6 in the
presence of sucrose
than sorbitol. The data also demonstrate that the formulations with either
none or very little
ionic strength showed comparable stability over time.
SEC data showed the physical stability of the DVD-Ig proteins, wherein the
rate of
formation of aggregates and/or fragments was evaluated (see Table 24 & 26). As
shown in
Table 26 all molecules showed no significant tendency to aggregation at low
concentrations.
IEC data is an indicator of the chemical stability of a DVD-Ig protein.
Deamidation, for
example, results in formation of acidic species (conversion of the main
species to acidic
species). Generation of positively charged variants would lead to an increase
in the basic
104
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
species. Formation of any of the two acidic or basic species indicates
instability, as the
formation of these two species results in an overall decrease in the % of main
species (see
Table 25 & 27).
The results in Tables 24 to 27 suggest that an AS-DVD-Ig protein is stable in
formulations comprising a surfactant alone, e.g., 0.01% Tween 80 at pH 5 - 6.
Table 24: 100 mg/ml SEC Data for Various Stored Formulations
DVD-Ig Formulation Time Point, temp Mon Agg Frag
IL12IL18 15 mM Histidine, 80 mg/ml TO 96.53 1.1 2.36
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 95.97 1.75
2.26
lm, 40C 92.58 2.86
4.55
IL12IL18 15 mM Histidine, 80 mg/ml TO 96.31 1.15
2.52
Sucrose, 0.01 % Tween, pH 6 lm, 5C 96 1.71 2.27
lm, 40C 92.11 3.5 4.38
IL12IL18 15 mM Histidine, 40 mg/ml TO 96.61 1.25
2.13
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 95.71 1.78 2.5
lm, 40C 91.62 4.04
4.33
6 15 mM Histidine, 80 mg/ml TO 89.61 10.38 0
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 89.48 10.51 0
lm, 40C 85.85 9.96
4.18
6 15 mM Histidine, 80 mg/ml TO 88.58 11.41 0
Sucrose, 0.01 % Tween, pH 6 lm, 5C 86.8 13.19 0
lm, 40C 83.09 12.68
4.21
65 15 mM Histidine, 80 mg/ml TO 93.08 0.93
5.98
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 92.59 1.67
5.72
lm, 40C 85.25 11.47
3.27
65 15 mM Histidine, 80 mg/ml TO 93.34 0.85
5.79
Sucrose, 0.01 % Tween, pH 6 lm, 5C 92.45 1.71
5.82
lm, 40C 86.15 10.69
3.14
66 15 mM Histidine, 80 mg/ml TO 97.57 0.62 1.8
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 97.6 0.92 1.47
lm, 40C 91.65 5 3.34
66 15 mM Histidine, 80 mg/ml TO 97.41 0.71
1.87
Sucrose, 0.01 % Tween, pH 6 lm, 5C 97.69 1.07
1.22
lm, 40C 92.68 4.3 3.01
66 15 mM Histidine, 40 mg/ml TO 97.65 0.61
1.73
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 97.52 1.04
1.42
lm, 40C 91.53 3.98
4.47
IL12IL18 10 mM Phosphate, 125 mM TO 96.13 1.57
2.28
NaCl, pH 6 lm, 5C 94.2 3.34 2.44
lm, 40C 88.48 7.3 4.2
IL12IL18 10 mM Phosphate, 125 mM TO 96.07 1.66
2.26
NaC1, pH 7.2 lm, 5C 94.64 2.91
2.43
lm, 40C 87.71 8.03
4.25
DVD-B 10 mM Phosphate, 125 mM TO 96.24 2.11
1.64
NaC1, pH 6 lm, 5C 94.66 3.85
1.48
lm, 40C 41.53 53.68
4.77
105
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig Formulation Time Point, temp Mon Agg Frag
DVD-B 10 mM Phosphate, 125 mM TO 95.75 2.45
1.79
NaC1, pH 7.2 lm, 5C 95.11 3.62
1.26
lm, 40C 33.96 60.56
5.46
IL12IL18 20 mM Glycine, 26 mg/ml TO 96.48 1.27
2.24
Glycerol, pH 6.0 lm, 5C 95.06 2.07
2.86
lm, 40C 90.33 4.7 4.95
DVD-B 20 mM Glycine, 26 mg/ml TO 96.29 1.88
1.81
Glycerol, pH 6.0 lm, 5C 95.93 2.62
1.43
lm, 40C 23.32 73.53
3.14
IL12IL18 Water, 0.01% Tween 80, pH 5.0 TO 95.06
2.55 2.37
lm, 5C 94.12 2.97
2.89
lm, 40C 90.99 4.93
4.07
IL12IL18 Water, 0.01% Tween 80, pH 6.0 TO 94.59
2.88 2.52
lm, 5C 94.22 3.28
2.48
lm, 40C 90.9 4.82 4.26
Water, 0.01% Tween 80, pH 5.0 TO 65.33 31.34 3.31
lm, 5C 17.02 79.12
3.84
lm, 40C 82.54 13.41
4.03
5 Water, 0.01% Tween 80, pH 6.0 TO 43.43
53.33 3.23
lm, 5C 17.53 79.15
3.3
lm, 40C 74.6 21.38
4.03
Table 25: 100 mg/ml IEC Data for Various Stored Formulations
DVD-Ig Formulation Time Point, temp Main Acidic Basic
IL12IL18 15 mM Histidine, 80 mg/ml TO 58.32 27.98
13.68
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 57.54 26.72
15.73
lm, 40C 44.91 37.65
17.43
IL12IL18 15 mM Histidine, 80 mg/ml TO 58.63 27.84
13.51
Sucrose, 0.01 % Tween, pH 6 lm, 5C 56.89 29.15
13.94
lm, 40C 41.47 44.38
14.14
IL12IL18 15 mM Histidine, 40 mg/ml TO 58.1 28.09
13.79
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 60.53 27.48
11.98
lm, 40C 41.02 44.69
14.29
6 15 mM Histidine, 80 mg/ml TO 42.99 11.66
45.34
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 42.53 10.26
47.19
lm, 40C 34.99 25.55
39.45
6 15 mM Histidine, 80 mg/ml TO 41.03 11.84
47.12
Sucrose, 0.01 % Tween, pH 6 lm, 5C 41.73 10.91
47.34
lm, 40C 36.18 25.94
37.86
65 15 mM Histidine, 80 mg/ml TO 49.64 39.02
11.33
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 45.48 38.77
15.73
lm, 40C 51.17 30.64
18.18
65 15 mM Histidine, 80 mg/ml TO 50 39.1
10.88
Sucrose, 0.01 % Tween, pH 6 lm, 5C 49.31 38.95
11.72
lm, 40C 29.83 52.65
17.5
66 15 mM Histidine, 80 mg/ml TO 63.78 24.78
11.43
106
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Formulation Time Point, temp Main
Acidic Basic
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 60.03 25.27
14.69
lm, 40C 42.13 41.93
15.93
66 15 mM Histidine, 80 mg/ml TO 61.5 26.21
12.27
Sucrose, 0.01 % Tween, pH 6 lm, 5C 59.06 25.85
15.07
lm, 40C 42.31 43.47
41.2
66 15 mM Histidine, 40 mg/ml TO 57.18 24.54
18.26
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 61.9 26.5
11.59
lm, 40C 41.24 45.29
13.46
IL12IL18 10 mM Phosphate, 125 mM TO 58.2 28.1
13.69
NaC1, pH 6 lm, 5C 57.43 27.35
15.21
lm, 40C 40.68 42.04
17.27
IL12IL18 10 mM Phosphate, 125 mM TO 54.51 27.99
17.48
NaC1, pH 7.2 lm, 5C 53.79 28.08
18.11
lm, 40C 27.08 58.25
14.65
DVD-B 10 mM Phosphate, 125 mM TO 52.94 27.32
19.72
NaC1, pH 6 lm, 5C 54.38 27.19
18.42
lm, 40C 29.14 38.63
32.21
DVD-B 10 mM Phosphate, 125 mM TO 27.93 52.59
19.46
NaC1, pH 7.2 lm, 5C 54.07 26.57
19.35
lm, 40C 19.79 48.61
31.59
IL12IL18 20 mM Glycine, 26 mg/ml TO 57.95 28.99
13.05
Glycerol, pH 6.0 lm, 5C 57.85 29.95
12.18
lm, 40C 37.55 47.49
14.94
DVD-B 20 mM Glycine, 26 mg/ml TO 30.77 51 18.22
Glycerol, pH 6.0 lm, 5C 50 33.32
16.67
lm, 40C 27.41 58.52
14.05
IL12IL18 Water, 0.01% Tween 80, pH 5.0 TO 56.03
27.31 16.64
lm, 5C 57.67 28.06
14.26
lm, 40C 44.97 41.34
13.68
IL12IL18 Water, 0.01% Tween 80, pH 6.0 TO 58.4
28.57 13.02
lm, 5C 58.93 26.36
14.7
lm, 40C 45.37 39.4
15.21
Water, 0.01% Tween 80, pH 5.0 TO 46.39 19.48
34.12
lm, 5C 16.75 8.54
74.69
lm, 40C 44.57 36.64
18.78
5 Water, 0.01% Tween 80, pH 6.0 TO 32.31
14.89 52.78
lm, 5C 16.32 8.27
75.4
lm, 40C 39.7 35.23
25.06
Table 26: 1 mg/ml SEC Data for Various Stored Formulations
DVD-Ig Formulation Time Point, temp Mon Agg Frag
IL12IL18 15 mM Histidine, 80 mg/ml TO 97.87 0.73 1.39
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 97.83 0.57 1.59
lm, 40C 95.55 0.74 3.7
IL12IL18 15 mM Histidine, 80 mg/ml TO 98.04 0.32
1.62
Sucrose, 0.01 % Tween, pH 6 lm, 5C 97.95 0.24
1.79
107
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Formulation Time Point, temp Mon Agg Frag
lm, 40C 95.83 0.58 3.58
IL12IL18 15 mM Histidine, 40 mg/ml TO 96.2 0.56 3.22
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 96.41 0.47 3.1
lm, 40C 94.21 0.66 5.12
6 15 mM Histidine, 80 mg/ml TO 92.05 7.94 0
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 99.23 0.76 0
lm, 40C 95.52 0.53 3.94
6 15 mM Histidine, 80 mg/ml TO 93.59 6.4 0
Sucrose, 0.01 % Tween, pH 6 lm, 5C 99.33 0.66 0
lm, 40C 95.3 0.59 4.1
65 15 mM Histidine, 80 mg/ml TO 94.74 0.51 4.73
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 94.34 0.49 5.15
lm, 40C 93.06 0.91 6.02
65 15 mM Histidine, 80 mg/ml TO 94.33 0.37 5.29
Sucrose, 0.01 % Tween, pH 6 lm, 5C 93.98 0.36 5.65
lm, 40C 92.71 0.87 6.41
66 15 mM Histidine, 80 mg/ml TO 98.76 0.51 0.72
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 98.59 0.51 0.89
lm, 40C 96.37 1.02 2.59
66 15 mM Histidine, 80 mg/ml TO 98.82 0.2 0.96
Sucrose, 0.01 % Tween, pH 6 lm, 5C 98.9 0.16 0.92
lm, 40C 96.82 0.66 2.51
66 15 mM Histidine, 40 mg/ml TO 97.87 0.29 1.83
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 97.3 0.23 2.45
lm, 40C 95.5 1.02 3.48
IL12IL18 10 mM Phosphate, 125 mM TO 95.38 1.54 3.07
NaC1, pH 6 lm, 5C 95.29 1.54 3.15
lm, 40C 91.87 1.95 6.17
IL12IL18 10 mM Phosphate, 125 mM TO 94.36 2.73 2.89
NaC1, pH 7.2 lm, 5C 93.78 2.99 3.22
lm, 40C 86.45 3.64 9.9
DVD-B 10 mM Phosphate, 125 mM TO 95.82 1.66 2.5
NaC1, pH 6 lm, 5C 95.54 1.53 2.91
lm, 40C 91.39 2.31 6.29
DVD-B 10 mM Phosphate, 125 mM TO 94.87 2.43 2.68
NaC1, pH 7.2 lm, 5C 95.01 2.3 2.68
lm, 40C 88.42 3.24 8.32
IL12IL18 20 mM Glycine, 26 mg/ml TO 96.48 0.36 3.14
Glycerol, pH 6.0 lm, 5C 96.51 0.29 3.18
lm, 40C 93.82 0.4 5.77
DVD-B 20 mM Glycine, 26 mg/ml TO 96.05 1.33 2.6
Glycerol, pH 6.0 lm, 5C 95.47 1.15 3.37
lm, 40C 94.14 1.22 4.63
IL12IL18 Water, 0.01% Tween 80, pH 5.0 TO 89.16 2.42 8.41
lm, 5C 95.1 1.86 3.02
lm, 40C 89.69 4.11 6.18
IL12IL18 Water, 0.01% Tween 80, pH 6.0 TO 94.24 2.88 2.86
lm, 5C 94.89 2.6 2.49
lm, 40C 90.66 3.38 5.94
108
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig Formulation Time Point, temp Mon Agg Frag
Water, pH 5.0 TO 69.49 26.36 4.13
lm, 5C 68.87 24.88
6.23
lm, 40C 91.35 3.6
5.08
5 Water, pH 6.0 TO 49.15 46.65
4.19
lm, 5C 51.96 43.27
4.76
lm, 40C 92.56 2.45
4.97
Table 27: 1 mg/ml IEC Data for the Various Stored Formulations
DVD-Ig Formulation Time Point, temp Main Acidic Basic
IL12IL18 15 mM Histidine, 80 mg/ml TO 61.83 24
14.14
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 61.76
26.77 11.46
lm, 40C 41.58
39.69 18.72
IL12IL18 15 mM Histidine, 80 mg/ml TO 61.31
26.12 12.55
Sucrose, 0.01 % Tween, pH 6 lm, 5C 59.67 25.3
15.02
lm, 40C 42.98
45.85 11.15
IL12IL18 15 mM Histidine, 40 mg/ml TO 60.81
25.86 13.31
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 59.07
27.12 13.8
lm, 40C 38.22
47.55 14.21
6 15 mM Histidine, 80 mg/ml TO 41.13 10.3
48.56
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 46.96
10.38 42.64
lm, 40C 36 24.57
39.42
6 15 mM Histidine, 80 mg/ml TO 44.43 9.92
45.63
Sucrose, 0.01 % Tween, pH 6 lm, 5C 46.33
11.55 42.11
lm, 40C 37.38
26.09 36.52
65 15 mM Histidine, 80 mg/ml TO 50.6
39.58 9.81
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 49.57
37.75 11.66
lm, 40C 33.38
50.27 16.33
65 15 mM Histidine, 80 mg/ml TO 51.74
38.73 9.52
Sucrose, 0.01 % Tween, pH 6 lm, 5C 53.21
36.58 10.2
lm, 40C 35.11
49.96 14.92
66 15 mM Histidine, 80 mg/ml TO 59.89
25.96 14.14
Sucrose, 0.01 % Tween, pH 5.25 lm, 5C 58.1
25.05 16.84
lm, 40C 45.43
37.21 17.34
66 15 mM Histidine, 80 mg/ml TO 58.18
24.86 16.94
Sucrose, 0.01 % Tween, pH 6 lm, 5C 61.69
24.94 13.36
lm, 40C 44.83
41.39 13.77
66 15 mM Histidine, 40 mg/ml TO 61.49
24.96 13.53
Sorbitol, 0.01 % Tween, pH 6 lm, 5C 60.34
24.96 16.69
lm, 40C 38.32
50.92 10.75
IL12IL18 10 mM Phosphate, 125 mM TO 58.52
26.18 15.29
NaCI, pH 6 lm, 5C 58.98
27.17 13.84
lm, 40C 37.54
47.49 14.96
IL12IL18 10 mM Phosphate, 125 mM TO 63.15
23.13 13.71
NaCI, pH 7.2 lm, 5C 58.58
26.07 15.33
lm, 40C 16.92
73.33 9.74
DVD-B 10 mM Phosphate, 125 mM TO 54.38
27.35 18.25
NaCI, pH 6 lm, 5C 56.48
27.42 16.09
109
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig Formulation Time Point, temp Main
Acidic Basic
lm, 40C 30.99 50.68
18.32
DVD-B 10 mM Phosphate, 125 mM TO 54.86 30
15.12
NaC1, pH 7.2 lm, 5C 52.66 31.63
15.69
lm, 40C 8.64 60.51
30.84
IL12IL18 20 mM Glycine, 26 mg/ml TO 57.58 27.8
14.6
Glycerol, pH 6.0 lm, 5C 58.02 29.5
12.46
lm, 40C 28.8 60.9
10.29
TNFPGE TO 53.05 30.46
16.48
2 20 mM Glycine, 26 mg/ml lm, 5C 44.21 33.97
21.8
Glycerol, pH 6.0
lm, 40C 5.52 81.17
13.3
IL12IL18 Water, 0.01% Tween 80, pH 5.0 TO 57.76 26.51
15.71
lm, 5C 58.44 26.13
15.41
lm, 40C 36.16 44.81
19.02
IL12IL18 Water, 0.01% Tween 80, pH 6.0 TO 59.24 26.09
14.66
lm, 5C 58.66 26.96
14.36
lm, 40C 35.97 44.16
19.85
Water, 0.01% Tween 80, pH 5.0 TO 51.73 19.4 28.85
lm, 5C 48.63 20.14
31.21
lm, 40C 44.52 41.73
13.73
5 Water, 0.01% Tween 80, pH 6.0 TO 34.34 14
51.62
lm, 5C 32.27 15.38
47.33
lm, 40C 42.03 44.28
13.67
EXAMPLE 12. Impact of Storage on the Stability of an AS-DVD-Ig Protein, (DVD-
C),
In Various Formulations
The pH and the storage temperature of a protein formulation are two important
factors
influencing protein stability during accelerated/long-term storage. To assess
the impact of
these factors, the DVD-Ig protein was exposed to short-term storage at
elevated and real time
temperatures in order to gain insight into the formulation feasibility of long-
term storage at
lower temperatures (e.g., 2-8 C).
The storage stability of DVD-C in solution (100 mg/ml) was evaluated in
formulations at 40 C. After defined storage periods, samples were pulled and
the impact of
storage time on DVD-Ig protein stability was evaluated. Samples were filled
into sterile vials
(approx. 500 pi each) and stored under controlled conditions, in a temperature
chamber and
in the absence of light. At predefined points of time, samples of prepared
solutions were
pulled for analysis according to the sample pull scheme. The resulting data is
provided in
Tables 28 and 29.
110
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 28: Impact of Storage of DVD-C at 100 mg/ml Concentrations in Various
Conditions
As Measured By SEC
Formulation Time Point Mon Agg Frag
TO 97.66 1.09 1.20
15 mM acetate 80 mg/ml sucrose
7d, 40 C 96.32 1.70 1.98
0.02 % Tween 80 pH 5
lm, 40 C 94.18 2.43 3.39
15 mM histidine 80 mg/ml TO 97.66 1.091 1.20
sucrose 0.02 % Tween 80 pH 6 7d, 40 C 95.92 2.09 1.99
lm, 40 C 93.64 2.72 3.64
Table 29: Impact of Storage of DVD-C at 100 mg/ml Concentrations in Various
Conditions
As Measured By IEC
Formulation Time Point Main Acidic Basic
15 mM acetate 80 mg/ml sucrose TO 73.84 9.80 16.35
0.02 % Tween 80 pH 5 T2m, 5 C 69.18 10.88 19.95
15 mM histidine 80 mg/ml sucrose TO 73.84 9.80 16.35
0.02 % Tween 80 pH 6 T2m, 5 C 67.99 10.59 21.42
The data provided in Tables 28 and 29 show that DVD-C was very stable
(compared to
some unstable DVD-Ig proteins, for example, in Example 5) in that only minimal
loss in
monomer levels occurred during the test storage conditions and hence would be
classified as
an AS-DVD-Ig protein.
EXAMPLE 13: Effect of a Surfactant on the Stability of AS-DVD-Ig Proteins in
Buffer
and Polyol Containing Formulations
It is generally beneficial to set a formulation pH more than 1 unit from the
protein's
isoelectric point (pI). The more a formulation pH approximates the pI,
generally, the more
the overall surface of the protein is regarded as uncharged, thus contributing
to protein-
protein attraction of non-polar groups, and thus enhancing non-covalent
aggregation and
instability. Shaking and stirring foster physical instability, creating
hydrophobic air/water
interfaces, which result in alignment of protein molecules at these
interfaces, and eventually
result in aggregation. Given that air is more hydrophobic than water, the
interface between
air and liquid is deemed to be a denaturing surface at which aggregation,
especially of
(partially) unfolded proteins can originate. The effective air-water interface
can be increased
by shaking or stirring.
In the following example, the effect of various concentrations of a surfactant
(e.g.,
Tween 80) on the instability of exemplary DVD-Ig proteins was evaluated. The
study was
111
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
done in the absence or presence of the polyol sucrose. The data presented in
Tables 30 and
31 compare various surfactant concentrations (0 to 2 mg/ml) in a histidine
buffer at pH 5.2 or
5.4 with and without sucrose (80 mg/ml). Results of turbidity measurements
show that a
surfactant (Tween 80) in a concentration range of 0.05 mg/ml - 2 mg/ml
provided stability
against shear/ denaturation stress to AS-DVD-Ig proteins in general. The
turbidity increased
upon lowering the surfactant concentration to 0.01 mg/ml. Similar observations
were made
for AS-DVD-Ig proteins in the presence of sucrose. All studies were conducted
at 15 mM
histidine at a DVD-Ig protein concentration of 1 mg/ml. The corresponding SEC
analysis
showed that, in general, a significant loss in rel. % monomer for samples that
contained 0.05 -
0.1 mg/mL Tween80, indicating that the amount of Tween 80 that yielded the
most stable
formulations is between 0.5 mg/mL and 2 mg/mL. Turbidity measurements at 500
nm using
UV are listed in Table 30 and the SEC analysis of the samples is listed in
Table 31. Ranges
of pH were also tested in the formulations described below.
Table 30: Effect of Tween on the Stability of Various DVD-Ig Proteins as
Assessed by
Air/Liquid Interface Denaturation Study/Shaking Study* by measurements at 500
nm using
UV
DVD Form: DVD-A, 15 mM DVD-C, 15 mM IL12IL18, 15 mM
Histidine,
Histidine, pH 5.2, 80 Histidine, pH 5.4, 80 pH 5.4, 80 mg/ml
Sucrose
mg/ml Sucrose mg/ml Sucrose
Tween 80 TO T24h T96h TO T24h T96h TO T24h T96h
0 0.11 0.07 0.55 0.085 0.08 0.107 0.051 0.051 0.051
2 mg/ml 0.069 0.018 0.004 0.085 0.038 0.003 0.069 0.072 0.017
0.5 mg/ml 0.014 0.014 0.002 0.025 0.036 0.004 0.09 0.006 0.008
0.1 mg/ml 0.061 0.02 0.001 0.043 0.007 0.002 0.055
0.011 0.0109
0.05 mg/ml 0.018 0.006 0.001 0.019 0.008 0.002 0.022
0.008 0.0208
0.01 mg/ml 0.007 0.007 1.03 0.03 0.033 0.22 0.057
0.081 0.082
DVD-A, 15 mM DVD-C, 15 mM IL12IL18, 15 mM
Histidine,
Histidine, pH 5.2 Histidine, pH 5.4 pH 5.4
Tween 80 TO T24h T96h TO T24h T96h TO T24h T96h
0 0.045 0.038 0.29 0.015 0.045 0.038 0.046 0.046 0.038
2 mg/ml 0.049 0.024 0.004 0.015 0.02 0.003 0.097 0.03 0.018
112
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
0.5 mg/ml 0.016 0.058 0.004 0.03 0.019 0.003
0.049 0.031 0.013
0.1 mg/ml 0.005 0.005 0.002 0.004 0.005 0.002
0.012 0.021 0.017
0.05 mg/ml 0.004 0.011 0.002 0.005 0.001 0.009 0.005
0.015 0.017
0.01 mg/ml 0.005 0.03 0.66 0.01 0.015 0.022
0.05 0.022 0.041
* The OD 500 was measured using UV
Table 31: Effect of Tween on the Stability of Various DVD-Ig Proteins As
Assessed By
Air/Liquid Interface Denaturation Study/Shaking Study*
DVD Form: DVD-A, 15 mM Histidine, pH 5.2, 80 mg/ml Sucrose DVD Form: DVD-C,
15 mM Histidine, pH 5.4, 80 mg/ml Sucrose
Tween TO T96h TO T96h
80 Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC
mg/ml
0 1.7 97.25 1.03 73550 1.35 97.55 1.08 39881 1.96
96.79 1.23 64166 1.35 97.55 1.08 39881
2 1.67 97.58 0.74 759057 2.33 96.67 0.99 74778
2.16 96.4 1.43 72649 /33 96.67 0.99 74778
0.5 1.76 97.43 0.8 75635 2.35 96.42 1.21 74782
2 96.66 1.33 71044 /35 96.42 1.21 74782
0.1 1.65 97.66 0.68 73158 3.85 95.06 1.07 72816
2.09 96.43 1.47 70277 3.85 95.06 1.07 72816
0.05 1.66 97.61 0.71 77474 18.86 80.36 0.76 76431
2.01 96.56 1.41 700701 18.86 80.36 0.76 76431
0.01 1.68 97.5 0.81 77410 9.32 83.25 7.41 11267
2.11 96.54 1.33 70397 9.32 83.25 7.41 11267
DVD Form: IL121L18, 15 mM Histidine, pH 5.4, 80 mg/ml Sucrose DVD Form: DVD-
A, 15 mM Histidine, pH 5.2
Tween TO T96h TO T96h
80 Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC
mg/ml
7.15 91.18 1.66 76420 6.11 9204. 1.83 72538 1.59 97.58 0.81 76481 1.09 98.02
0.87 60575
0 7.29 90.64 /05 77867 6.09 91.94 1.96 76447 1.55 97.65 0.79 85152 1.63 97.49
0.87 84633
2 7.04 91.4 1.54 77164 6.14 91.98 1.87 75844 1.65 97.64 0.7 88543 na na na
na
0.5 7.08 91.23 1.68 72875 6.4 90.9 /68 71645 1.69 97.57 0.73 85244 1.61 97.66
0.72 87583
0.1 7.03 91.3 1.65 69962 8.8 86.44 4.75 72941 1.72 97.57 0.7 86031 2.22 96.67
1.09 86215
0.05 6.81 91.57 1.61 77682 1/77 85.19 /03 70341 1.63 97.63 0.72 81730 28.99
69.37 1.63 15513
DVD Form: DVD-C, 15 mM Histidine, pH 5.4 DVD Form: IL121L18, 15 mM
Histidine, pH 5.4
Tween TO T96h TO T96h
80 Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC Agg Mon Frag AUC
mg/ml
/05 96.56 1.37 71752 1.71 97.3 0.98 67869 6.9 91.47 1.61 75651 6.34 91.6 /05
73693
0 /08 96.63 1.28 75988 2.03 96.58 1.37 75354 6.77
91.76 1.46 75316 6.43 91.61 1.95 75143
2 2.1 96.44 1.45 77324 2 96.49 1.5 76488 6.88 91.57 1.53 75439 6.12 91.84 2.03
74397
0.5 2.11 96.33 1.55 77859 1.86 96.59 1.54 76809
7.02 91.18 1.78 74981 7.06 90.81 /11 74698
0.1 /21 96.28 1.5 79134 2.38 96.09 1.52 77153 7.04 91.37 1.57 76385 5.61 9/44
1.93 75375
0.05 /08 96.44 1.47 76726 2.95 95.52 1.51 74056 6.66 91.69 1.64 65218 10.76
87.29 1.93 62074
113
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
* The SEC data corresponds to 0 and 96 h shaking samples of Table 30.
Example 14: Effect of Surfactant Concentration on the Stability of IL121L18
DVD-Ig
Protein as Measured in Shaking Studies
The following example describes the effect of different concentrations (a
range of
concentration) of polysorbate 80 and poloxamer on the shaking stability of an
IL12IL18
DVD-Ig protein at concentrations of 1 mg/mL at pH 6 (15 mM histidine + 80
mg/mL
sucrose) as measured using optical density at two different wavelengths of 350
and 500 nm.
Samples were taken after 0, 24, 48, 120, and 240 hours.
Table 32: Effect of Surfactants on Formulation with Buffer and Polyol
1L121L18 lmg/m1;15 mM Histidine + 80 mg/mL sucrose pH 6
Surfactant 0D500 nm 0D350 nm
IShake 0 24 48 120 240 0 24 48 120 240
Time (H)
0 0.003 0.06 0.085 0.137 0.387 0.01
0.097 0.135 0.22 0.574
0.01 0.0036 0.029 0.011 0.065 0.108 0.01 0.01 0.03
0.167 0.227
Tween 80 0.05 0.0056 0.003 0.01 0.087 0.113 0.013
0.01 0.027 0.17 0.22
(mg/ml) 0.1 0.0036 0.002 0.0022 0.067 0.004 0.01
0.01 0.009 0.135 0.011
0.5 0.003 0.002 0.002 0.04 0.001 0.01
0.009 0.009 0.086 0.009
2 0.035 0.002 0.0025 0.0025 0.002 0.062
0.012 0.013 0.014 0.013
1L121L18 lmg/m1;15 mM Histidine + 80 mg/mL sucrose pH 6
500 nM 350 nM
IShake 0 24 48 120 240 0 24 48 120 240
Time (H)
0 0.003 0.035 0.044 0.236 0.226 0.01
0.06 0.08 0.4 0.374
0.01 0.004 0.004 0.007 0.013 0.0126 0.01 0.012 0.019
0.031 0.034
Poloxam
er 0.05 0.003 0.014 0.002 0.027 0.002 0.01
0.02 0.011 0.009 0.011
(% w/v) 0.1 0.003 0.035 0.0037 0.0038 0.003 0.01
0.01 0.012 0.012 0.012
0.5 0.003 0.003 0.003 0.003 0.004 0.01
0.01 0.011 0.011 0.013
2 0.0036 0.003 0.003 0.005 0.004 0.011
0.01 0.01 0.013 0.013
114
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
As described in Table 32, the addition of surfactants increased the shaking
stability of
the IL12/IL18 DVD-Ig protein. Polysorbate concentration in the range of 0.05-2
mg/mL was
determined to be the most effective for stability of the IL12/IL18 DVD-Ig
protein, and
poloxamer was determined to be most effective in the range of 0.1-2 % w/v. DVD-
B was
also tested and showed similar stability when tested in this particular
shaking assay.
Example 15: Effect of Polyol (Sucrose and Sorbitol) Concentration on the
Stability of
IL121L18 and DVD-B DVD-Ig Protein
The following example shows the effect of polyols sucrose and sorbitol in the
presence of polysorbate 80 on the stability of the IL12IL18 DVD-Ig protein as
measured at
3mg/mL, at pH 6 by intrinsic fluorescence using an automated high throughput
instrument
Optim-1000 from Avacta (York, UK) as described in the methods section. 9 p1
MCA's were
used for the study. Thermal scans were obtained using a scan rate of 1
C/minute and scans
were taken from 25-75 C. All samples were freshly prepared from stock
solutions for the
sucrose and sorbitol excipients.
Table 33: Effect of polyols on 3 mg/ml IL12IL18 and DVD-B DVD-Ig protein
formulations
with 15 mM His buffer at pH 6 and polysorbate
DSF
Sucrose Sorbitol DSF ( C) ( C)
(mg/ml) (mg/ml) Tween IL12IL18 DVD-B
0 0.01% 60 54
0.01% 61 55
40 0.01% 61 55
70 0.01% 60 56
100 0.01% 60 57
0 0.01% 61 53
10 0.01% 62 54
0.01% 61 55
40 0.01% 62 55
60 0.01% 62 56
Based on the data in Table 33, the stability of the DVD-B Ig protein increased
with
an increase in the concentration of either polyol (sucrose or sorbitol). It
was determined that
100 mg/mL for sucrose and 60 mg/mL for sorbitol was about the maximum
concentrations
115
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
that would achieve osmolality and hence were investigated. Sucrose in the
range of 60-100
mg/mL was determined to be effective, while sorbitol in the range of 20-60
mg/mL was
effective for stability. In contrast, the results shown in Table 33 for the
IL12/IL18 DVD-Ig
protein did not show an improvement in the stability as measured by DSF.
Example 16: Impact of pH and Histidine Concentration (at pH 6) on the Shelf
Stability
of DVD-B and IL121L18 DVD-Ig Protein at 100mg/m1
The following examples show the effect of histidine concentration (ranging
from 0 -
200 mM) on the shelf stability of the DVD-B and the IL12IL18 DVD-Ig protein as
measured
using size exclusion chromatography (SEC). Samples were prepared and storage
stability of
the DVD-Ig proteins in solution at 100 mg/mL was evaluated at 5 C and 40 C.
After
defined storage periods, samples were pulled and the impact of storage time on
DVD-Ig
protein stability was evaluated. Briefly, samples were filled into sterile
vials (approx. 500 [t.L
each) and stored under controlled conditions (in temperature chambers and in
the absence of
light) at 40 C. At predefined points of time, samples of prepared solutions
were pulled for
analysis according to the sample pull scheme.
Table 34: Effect of pH on Stability of IL12IL18 DVD-Ig Protein and DVD-B DVD-
Ig
Protein at 100 mg/mL in Solution
C Sample Time (D) Formulation Agg Mon Frag
N/A IL12-18 0 0 mM His pH 6+
tween+ sucrose 3.08 95.12 1.8
N/A IL12-18 0 5 mM His pH 6+
tween+ sucrose 2.8 95.18 2.02
N/A IL12-18 0 10 mM His pH 6+
tween+ sucrose 2.92 95.06 2.02
N/A IL12-18 0 50 mM His pH 6+
tween+ sucrose 2.94 94.93 2.13
N/A IL12-18 0 200 mM His pH 6+
tween+ sucrose 3.11 94.63 2.25
N/A IL12-18 0 15 mM Ace pH 4.50+
tween+ sucrose 2.88 95.06 2.06
N/A IL12-18 0 15 mM His pH 6+
tween+ sucrose 2.54 95.4 2.06
N/A IL12-18 0 15 mM Pho pH 7.40+
tween+ sucrose 3.16 95.53 1.31
N/A DVDB 0 0 mM His pH 6+
tween+ sucrose 3.96 94.2 1.84
N/A DVDB 0 5 mM His pH 6+
tween+ sucrose 4.55 93.66 1.79
N/A DVDB 0 10 mM His pH 6+
tween+ sucrose 4.61 93.64 1.75
N/A DVDB 0 50 mM His pH 6+
tween+ sucrose 5.52 92.6 1.88
N/A DVDB 0 200 mM His pH 6+
tween+ sucrose 4.94 93.24 1.83
N/A DVDB 0 15 mM Ace pH 4.50+
tween+ sucrose 11.19 86.86 1.95
N/A DVDB 0 15 mM His pH 6+
tween+ sucrose 5.92 92.9 1.18
N/A DVDB 0 15 mM Pho pH 7.40+
tween+ sucrose 3.26 94.9 1.73
IL12-18 7 0 mM His pH 6+ tween+ sucrose 2.82 94.98 2.19
5 IL12-18 7 5 mM His pH 6+ tween+ sucrose 2.53 95.36 2.1
116
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
C Sample Time (D) Formulation Agg Mon Frag
IL12-18 7 10 mM His pH 6+ tween+ sucrose 2.63 95.9 1.48
5 IL12-18 7 50 mM His pH 6+ tween+ sucrose 2.7 95.57 1.73
5 IL12-18 7 200 mM His pH 6+ tween+ sucrose 3.03 95.08 1.9
5 IL12-18 7 15 mM Ace pH 4.50+ tween+ sucrose 2.23 95.73 2.05
5 IL12-18 7 15 mM His pH 6+ tween+ sucrose 2.38 95.46 2.16
5 IL12-18 7 15 mM Pho pH 7.40+ tween+ sucrose 2.76 94.66 2.56
5 DVDB 7 0 mM His pH 6+ tween+ sucrose 4.76 93.43 1.82
5 DVDB 7 5 mM His pH 6+ tween+ sucrose 5.41 92.75 1.85
5 DVDB 7 10 mM His pH 6+ tween+ sucrose 5.15 93.11 1.74
5 DVDB 7 50 mM His pH 6+ tween+ sucrose 6.44 91.64 1.92
5 DVDB 7 200 mM His pH 6+ tween+ sucrose 5.99 92.27 1.74
5 DVDB 7 15 mM Ace pH 4.50+ tween+ sucrose 14.69 83.4 1.91
5 DVDB 7 15 mM His pH 6+ tween+ sucrose 7.97 90.15 1.87
5 DVDB 7 15 mM Pho pH 7.40+ tween+ sucrose 4.06 94.24 1.69
5 IL12-18 21 0 mM His pH 6+ tween+ sucrose 4.01 91.56 4.43
5 IL12-18 21 5 mM His pH 6+ tween+ sucrose 3.08 92.37 4.55
5 IL12-18 21 10 mM His pH 6+ tween+ sucrose 2.95 92.69 4.35
5 IL12-18 21 50 mM His pH 6+ tween+ sucrose 2.86 92.71 4.43
5 IL12-18 21 200 mM His pH 6+ tween+ sucrose 3.28 92.22 4.5
5 IL12-18 21 15 mM Ace pH 4.50+ tween+ sucrose 2.18 93.18 4.65
5 IL12-18 21 15 mM His pH 6+ tween+ sucrose 2.42 92.36 5.22
5 IL12-18 21 15 mM Pho pH 7.40+ tween+ sucrose 3.95 91.84 4.2
5 DVDB 21 0 mM His pH 6+ tween+ sucrose 5.21 91.17 3.62
5 DVDB 21 5 mM His pH 6+ tween+ sucrose 6.68 89.81 3.51
5 DVDB 21 10 mM His pH 6+ tween+ sucrose 6.26 89.85 3.89
5 DVDB 21 50 mM His pH 6+ tween+ sucrose 8.13 87.88 3.99
5 DVDB 21 200 mM His pH 6+ tween+ sucrose 8.29 87.66 4.06
5 DVDB 21 15 mM Ace pH 4.50+ tween+ sucrose 19.23 77.03 3.73
5 DVDB 21 15 mM His pH 6+ tween+ sucrose 9.54 86.75 3.71
5 DVDB 21 15 mM Pho pH 7.40+ tween+ sucrose 6.25 89.65 4.09
40 IL12-18 7 0 mM His pH 6+ tween+
sucrose 2.56 95.01 2.44
40 IL12-18 7 5 mM His pH 6+ tween+
sucrose 2.06 95.45 2.5
40 IL12-18 7 10 mM His pH 6+ tween+
sucrose 1.93 95.59 2.48
40 IL12-18 7 50 mM His pH 6+ tween+
sucrose 1.82 95.3 2.92
40 IL12-18 7 200 mM His pH 6+
tween+ sucrose 2.18 95.04 2.78
40 IL12-18 7 15 mM Ace pH 4.50+
tween+ sucrose 1.97 95.27 2.76
40 IL12-18 7 15 mM His pH 6+ tween+
sucrose 1.85 95.61 2.54
40 IL12-18 7 15 mM Pho pH 7.40+
tween+ sucrose 4.35 92.57 3.08
40 DVDB 7 0 mM His pH 6+ tween+
sucrose 44.35 54.38 1.28
40 DVDB 7 5 mM His pH 6+ tween+
sucrose 51.66 46.62 1.72
40 DVDB 7 10 mM His pH 6+ tween+
sucrose 50.76 47.45 1.79
40 DVDB 7 50 mM His pH 6+ tween+
sucrose 53.53 44.78 1.69
40 DVDB 7 200 mM His pH 6+
tween+ sucrose 43.21 54.95 1.84
40 DVDB 7 15 mM Ace pH 4.50+
tween+ sucrose 64.17 33.34 2.49
40 DVDB 7 15 mM His pH 6+ tween+
sucrose 50.51 46.87 2.5
40 DVDB 7 15 mM Pho pH 7.40+
tween+ sucrose 36.56 60.79 2.65
40 IL12-18 21 0 mM His pH 6+ tween+
sucrose 4.09 90.71 5.19
40 IL12-18 21 5 mM His pH 6+ tween+
sucrose 3.16 91.26 5.58
40 IL12-18 21 10 mM His pH 6+
tween+ sucrose 3.15 91.78 5.07
117
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
C Sample Time (D) Formulation Agg Mon Frag
40 IL12-18 21 50 mM His pH 6+ tween+ sucrose 2.53
91.99 5.48
40 IL12-18 21 200 mM His pH 6+ tween+ sucrose 2.88
91.52 5.61
40 IL12-18 21 15 mM Ace pH 4.50+ tween+ sucrose 2.52
91.59 5.89
40 IL12-18 21 15 mM His pH 6+ tween+ sucrose 2.29
91.97 5.74
40 IL12-18 21 15 mM Pho pH 7.40+ tween+ sucrose 5.35
88.56 6.09
40 DVDB 21 0 mM His pH 6+ tween+ sucrose 55.03
39.46 5.51
40 DVDB 21 5 mM His pH 6+ tween+ sucrose 57.67
37.17 5.16
40 DVDB 21 10 mM His pH 6+ tween+ sucrose 55.56
39.33 5.11
40 DVDB 21 50 mM His pH 6+ tween+ sucrose 58.62
36.27 5.11
40 DVDB 21 200 mM His pH 6+ tween+ sucrose 56.6
37.74 5.66
40 DVDB 21 15 mM Ace pH 4.50+ tween+ sucrose 67.47
26.81 5.71
40 DVDB 21 15 mM His pH 6+ tween+ sucrose 63.14
31.52 5.34
40 DVDB 21 15 mM Pho pH 7.40+ tween+ sucrose 49.52
44.7 5.78
Table 34 also shows the effect of pH range from 4.5-7.4 (pH 4.5 is 15 mM
Acetate,
pH 6 is 15 mM Histidine and pH 7.4 is 15 mM Phosphate) on the stability of the
two DVD-Ig
proteins. The data indicate that the stability of IL1IL18 DVD-Ig protein, an
AS-DVD-Ig
protein, is maintained between the pH of about 4.5 to about 7.4, while the non-
AS DVD-Ig
protein DVD-B is unstable over pH 4.5 as indicated by the much higher level of
aggregate
formation over time. The amount of the buffering agent histidine seems to have
almost no
effect on stability, indicating that the stability issues for non-AS DVD-Ig
proteins can be
mitigated by formulation parameters, as might have been the case for
monoclonal antibodies.
The data indicates that the stability of IL12IL18 DVD-Ig protein is maintained
between the pH range of 4.5-7.4, while DVD-B is unstable at either pH 4.5 or
pH 7.4 in
solution. The stability of the two DVD-Ig proteins show similar stability
profiles, however,
between the 0-200 mM histidine concentration range at pH 6 given the above
conditions in
solution.
Example 17: Effect of Citrate Concentration at pH 6 on the Shelf Stability of
DVD-B
and IL121L18 DVD-Ig Proteins at 100 mg/ml
The following example describes the impact of citrate buffer concentration
(ranging
from 0 - 100 mM) on the shelf stability of the DVD-B and IL12/IL18 DVD-Ig
protein.
Samples were prepared and storage stability of the DVD-Ig proteins in solution
at 100
mg/mL was evaluated at 5 C and 40 C. After defined storage periods, samples
were pulled
118
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
and the impact of storage time on DVD-Ig protein stability was evaluated.
Briefly, samples
were filled into sterile vials (approx. 500 [t.L each) and stored under
controlled conditions (in
temperature chambers and in the absence of light) at 40 C. At predefined
points of time,
samples of prepared solutions were pulled for analysis according to the sample
pull scheme.
Table 35: Effect of pH on Stability of DVD-B and IL121L18 DVD-Ig Proteins
C Sample Time (D) Formulation Agg Mon Frag
1L12/18 0 0 mM Cit pH 6 3.2 94.98
1.82
1L12/18 0 5 mM Cit pH 6 3.3 94.68 2.2
1L12/18 0 10 mM Cit pH 6 3.06 94.76
2.18
1L12/18 0 50 mM Cit pH 6 2.52 95.28
2.19
1L12/18 0 100 mM Cit pH 6 2.82 95.05
2.13
1L12/18 0 0 mM Cit pH 6+ Sucrose+tween 2.73
95.11 2.16
1L12/18 0 5 mM Cit pH 6+ Sucrose+tween 2.47
95.55 1.97
1L12/18 0 10 mM Cit pH 6+ Sucrose+tween 2.67 95.1
2.23
1L12/18 0 50 mM Cit pH 6+ Sucrose+tween 2.57
95.37 2.05
1L12/18 0 100 mM Cit pH 6+ Sucrose+tween 2.55
95.24 2.21
1L12/18 7 0 mM Cit pH 6 2.91 94.51 2.58
5 1L12/18 7 5 mM Cit pH 6 3.16 94.76
2.08
5 1L12/18 7 10 mM Cit pH 6 2.77 94.77
2.46
5 1L12/18 7 50 mM Cit pH 6 3.16 94.67
2.17
5 1L12/18 7 100 mM Cit pH 6 2.84 95.81
1.36
5 1L12/18 7 0 mM Cit pH 6+ Sucrose+tween 2.58
95.21 2.21
5 1L12/18 7 5 mM Cit pH 6+ Sucrose+tween 2.98
94.84 2.18
5 1L12/18 7 10 mM Cit pH 6+ Sucrose+tween 2.78
94.82 2.4
5 1L12/18 7 50 mM Cit pH 6+ Sucrose+tween 2.87
94.79 2.35
5 1L12/18 7 100 mM Cit pH 6+ Sucrose+tween 2.77
94.97 2.25
5 1L12/18 21 0 mM Cit pH 6 3.22 94.21
2.57
5 1L12/18 21 5 mM Cit pH 6 3.64 95.31
1.05
5 1L12/18 21 10 mM Cit pH 6 3.4 93.9 2.7
5 1L12/18 21 50 mM Cit pH 6 3.66 94.53
1.81
5 1L12/18 21 100 mM Cit pH 6 3.3 94.47
2.23
5 1L12/18 21 0 mM Cit pH 6+ Sucrose+tween 2.93
94.44 2.68
5 1L12/18 21 5 mM Cit pH 6+ Sucrose+tween 3.19
94.71 2.1
5 1L12/18 21 10 mM Cit pH 6+ Sucrose+tween 3.2 94.54
2.26
5 1L12/18 21 50 mM Cit pH 6+ Sucrose+tween 3.12
94.51 2.37
5 1L12/18 21 100 mM Cit pH 6+ Sucrose+tween 2.88
94.94 2.19
40 1L12/18 7 0 mM Cit pH 6 3.06 94.81
2.12
40 1L12/18 7 5 mM Cit pH 6 3.4 94.33
2.27
40 1L12/18 7 10 mM Cit pH 6 3.28 94.47
2.24
40 1L12/18 7 50 mM Cit pH 6 3.81 94.13
2.06
40 1L12/18 7 100 mM Cit pH 6 3.55 94.07
2.38
40 1L12/18 7 0 mM Cit pH 6+ Sucrose+tween 2.7 96.87
0.44
40 1L12/18 7 5 mM Cit pH 6+ Sucrose+tween 2.84
94.61 2.55
40 1L12/18 7 10 mM Cit pH 6+ Sucrose+tween 2.98
94.61 2.4
40 1L12/18 7 50 mM Cit pH 6+ Sucrose+tween 2.88
94.84 2.27
40 1L12/18 7 100 mM Cit pH 6+ Sucrose+tween 2.96
94.64 2.4
119
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
C Sample Time (D) Formulation Agg Mon Frag
40 IL12/18 21 0 mM Cit pH 6 4.48 91.27
4.26
40 IL12/18 21 5 mM Cit pH 6 4.92 90.99 4.1
40 IL12/18 21 10 mM Cit pH 6 4.62 91.2
4.18
40 IL12/18 21 50 mM Cit pH 6 4.69 91.53
3.78
40 IL12/18 21 100 mM Cit pH 6 4.24 92.23 3.52
40 IL12/18 21 0 mM Cit pH 6+ Sucrose+tween 3.92 91.87 4.21
40 IL12/18 21 5 mM Cit pH 6+ Sucrose+tween 3.92 92.05 4.03
40 IL12/18 21 10 mM Cit pH 6+ Sucrose+tween 4.01 91.63
4.36
40 IL12/18 21 50 mM Cit pH 6+ Sucrose+tween 3.66 92.52
3.81
40 IL12/18 21 100 mM Cit pH 6+ Sucrose+tween 3.78 91.89
4.33
3 Month 0 mM Cit pH 6 2.53 94.36 3.11
5 3 Month 5 mM Cit pH 6 2.89 94.33
2.77
5 IL12/18 3 Month 10 mM Cit pH 6 3 94.35
2.64
5 IL12/18 3 Month 50 mM Cit pH 6 3.78 93.83
2.39
5 IL12/18 3 Month 100 mM Cit pH 6 3.58 94.45 1.98
5 IL12/18 3 Month 0 mM Cit pH 6+ Sucrose+tween 2.94 94.71
2.35
5 IL12/18 3 Month 5 mM Cit pH 6+ Sucrose+tween 3.58 94.29
2.13
5 IL12/18 3 Month 10 mM Cit pH 6+ Sucrose+tween 2.95 95.16
1.89
5 IL12/18 3 Month 50 mM Cit pH 6+ Sucrose+tween 3.44 94.58
1.98
5 IL12/18 3 Month 100 mM Cit pH 6+ Sucrose+tween 3.24 94.54
2.21
5 IL12/18 6 Month 0 mM Cit pH 6 6.4 90.99 2.61
5 IL12/18 6 Month 5 mM Cit pH 6 6.18 91.58
2.24
5 IL12/18 6 Month 10 mM Cit pH 6 6.04 92.12
1.84
5 IL12/18 6 Month 50 mM Cit pH 6 5.72 92.9
1.38
5 IL12/18 6 Month 100 mM Cit pH 6 5.98 92.86 1.16
5 IL12/18 6 Month 0 mM Cit pH 6+ Sucrose+tween 4.98 94.02
1
5 IL12/18 6 Month 5 mM Cit pH 6+ Sucrose+tween 5.47 92.96
1.5
5 IL12/18 6 Month 10 mM Cit pH 6+ Sucrose+tween 4.51 94.56
0.9
5 IL12/18 6 Month 50 mM Cit pH 6+ Sucrose+tween 4.58 93.44
1.9
5 IL12/18 6 Month 100 mM Cit pH 6+ Sucrose+tween 4.63 93.54
1.82
5 IL12/18 6 Month 0 mM Cit pH 6 6.4 90.99 2.61
DVD-B 0 0 mM Cit pH 6 2.6 95.63 1.77
0 5 mM Cit pH 6 2.6 95.49 1.87
DVD-B 0 10 mM Cit pH 6 2.41 95.88
1.72
DVD-B 0 50 mM Cit pH 6 1.76 96.62
1.62
DVD-B 0 100 mM Cit pH 6 1.34 97 1.66
DVD-B 0 0 mM Cit pH 6+ Sucrose+tween 1.54 97.31 1.15
DVD-B 0 5 mM Cit pH 6+ Sucrose+tween 1.52 96.74 1.73
DVD-B 0 10 mM Cit pH 6+ Sucrose+tween 1.5 96.8 1.71
DVD-B 0 50 mM Cit pH 6+ Sucrose+tween 1.5 96.74 1.75
DVD-B 0 100 mM Cit pH 6+ Sucrose+tween 1.11 97.16
1.73
5 DVD-B 7 0 mM Cit pH 6 2.61 95.54
1.85
5 DVD-B 7 5 mM Cit pH 6 3.01 95.37
1.62
5 DVD-B 7 10 mM Cit pH 6 3.2 95.12
1.68
5 DVD-B 7 50 mM Cit pH 6 2.85 95.5
1.64
5 DVD-B 7 100 mM Cit pH 6 2.14 96.22 1.64
120
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
C Sample Time (D) Formulation Agg Mon Frag
DVD-B 7 0 mM Cit pH 6+ Sucrose+tween 2.32 95.95
1.73
5 DVD-B 7 5 mM Cit pH 6+ Sucrose+tween 2.86 95.47
1.66
5 DVD-B 7 10 mM Cit pH 6+ Sucrose+tween 2.71 95.61
1.68
5 DVD-B 7 50 mM Cit pH 6+ Sucrose+tween 2.75 95.62
1.64
5 DVD-B 7 100 mM Cit pH 6+ Sucrose+tween 1.97 96.35
1.68
5 DVD-B 21 0 mM Cit pH 6 3.33 94.44
2.23
5 DVD-B 21 5 mM Cit pH 6 4.97 92.72
2.31
5 DVD-B 21 10 mM Cit pH 6 5.52 92.56
1.92
5 DVD-B 21 50 mM Cit pH 6 4.88 93.82
1.3
5 DVD-B 21 100 mM Cit pH 6 3.7 94.76 1.54
5 DVD-B 21 0 mM Cit pH 6+ Sucrose+tween 3 95.28 1.72
5 DVD-B 21 5 mM Cit pH 6+ Sucrose+tween 4.88 93.06
2.06
5 DVD-B 21 10 mM Cit pH 6+ Sucrose+tween 5 93.41
1.59
5 DVD-B 21 50 mM Cit pH 6+ Sucrose+tween 5.01 93.14
1.84
5 DVD-B 21 100 mM Cit pH 6+ Sucrose+tween 3.77 94.38
1.85
40 DVD-B 7 0 mM Cit pH 6 51.75 46.63
1.62
40 DVD-B 7 5 mM Cit pH 6 58.47 39.22
2.31
40 DVD-B 7 10 mM Cit pH 6 58.39 38.8
2.8
40 DVD-B 7 50 mM Cit pH 6 47.99 49.69
2.32
40 DVD-B 7 100 mM Cit pH 6 39.78 58.14 2.09
40 DVD-B 7 0 mM Cit pH 6+ Sucrose+tween 47.61 49.83
2.56
40 DVD-B 7 5 mM Cit pH 6+ Sucrose+tween 56.88 40.48
2.64
40 DVD-B 7 10 mM Cit pH 6+ Sucrose+tween 46.46 50.97
2.57
40 DVD-B 7 50 mM Cit pH 6+ Sucrose+tween 45.57 52.03
2.4
40 DVD-B 7 100 mM Cit pH 6+ Sucrose+tween 34.2 63.53
2.27
40 DVD-B 21 0 mM Cit pH 6 60.28 36.18
3.54
40 DVD-B 21 5 mM Cit pH 6 nia nia
nia
40 DVD-B 21 10 mM Cit pH 6 nia nia
nia
40 DVD-B 21 50 mM Cit pH 6 nia nia
nia
40 DVD-B 21 100 mM Cit pH 6 nia nia nia
40 DVD-B 21 0 mM Cit pH 6+ Sucrose+tween 56.73 39.59
3.67
40 DVD-B 21 5 mM Cit pH 6+ Sucrose+tween 59.87 36.82
3.3
40 DVD-B 21 10 mM Cit pH 6+ Sucrose+tween 53.36 43.59
3.05
40 DVD-B 21 50 mM Cit pH 6+ Sucrose+tween 51.27 45.72
3.01
40 DVD-B 21 100 mM Cit pH 6+ Sucrose+tween 44.11 52.84
3.05
5 DVD-B 3 Month 0 mM Cit pH 6 7.25 90.86
1.9
5 DVD-B 3 Month 5 mM Cit pH 6 13.89 84.29
1.82
5 DVD-B 3 Month 10 mM Cit pH 6 18.99 79.33
1.68
5 DVD-B 3 Month 50 mM Cit pH 6 16.07 82.2
1.73
5 DVD-B 3 Month 100 mM Cit pH 6 12.87 85.6 1.52
5 DVD-B 3 Month 0 mM Cit pH 6+ Sucrose+tween 7.43 90.74
1.83
5 DVD-B 3 Month 5 mM Cit pH 6+ Sucrose+tween 15.08 83.23
1.69
5 DVD-B 3 Month 10 mM Cit pH 6+ Sucrose+tween 16.03 82.02
1.95
5 DVD-B 3 Month 50 mM Cit pH 6+ Sucrose+tween 17.09 81.17
1.74
5 DVD-B 3 Month 100 mM Cit pH 6+ Sucrose+tween 10.89 87.64
1.46
5 DVD-B 6 Month 0 mM Cit pH 6 15.5 82.31
2.2
5 DVD-B 6 Month 5 mM Cit pH 6 27.45 71.13
1.43
5 DVD-B 6 Month 10 mM Cit pH 6 34.37 63.99
1.63
5 DVD-B 6 Month 50 mM Cit pH 6 29.11 69.6
1.29
121
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
C Sample Time (D) Formulation Agg Mon Frag
DVD-B 6 Month 100 mM Cit pH 6
5 DVD-B 6 Month 0 mM Cit pH 6+ Sucrose+tween 15.24
83.22 1.54
5 DVD-B 6 Month 5 mM Cit pH 6+ Sucrose+tween 27.28
71.19 1.53
5 DVD-B 6 Month 10 mM Cit pH 6+ Sucrose+tween 27.69
70.8 1.51
5 DVD-B 6 Month 50 mM Cit pH 6+ Sucrose+tween 27.18
70.78 2.04
5 DVD-B 6 Month 100 mM Cit pH 6+ Sucrose+tween 21.8
76.46 1.74
The two DVD-Ig proteins in Table 35 showed different stability profiles
between the
0-100 mM citrate concentrations at pH 6. While AS-DVD-Ig protein IL12IL18
showed a
high stability with only a minor increase in aggregates overtime, especially
at 5 C, the
opposite was true for the non AS-DVD-Ig protein DVD-B. This suggests that a
high
concentration liquid formulation would be feasible for the IL12IL18 DVD-Ig
protein but not
for DVD-B under those conditions.
VIII. STABLE LYOPHILIZED DVD-IG (LS-DVD-IG) PROTEIN FORMULATIONS
Examples 18 and 19 describe the stability of LS-DVD-Ig proteins in the
lyophilized
form. Example 18 describes surprising results that demonstrate freeze/ thaw
(F/T) stability of
LS-DVD-Ig proteins. Example 19 describes studies showing stable lyophilized
formulations
containing LS-DVD-Ig proteins. Freezing is the first step in lyophilization
and hence
molecules that do not have freeze thaw stability are susceptible to
instability during
lyophilization.
EXAMPLE 18: Impact of Solution pH on the Stability of DVD-Ig Proteins
Subjected to
Repeated Freeze/Thaw Cycles
The freeze thaw behavior of DVD-Ig proteins at a protein concentration of 1
mg/ml in
5 mM citrate/5mM phosphate buffer was evaluated by cycling the protein
solution up to 2
times between the frozen state and the liquid state at pH 4, pH 6, and pH 8.
Freezing was
performed using temperature controlled -80 C freezer, and thawing was
performed using a
30 C temperature controlled water bath. Samples were pulled after the second
freeze/thaw
(F/T) cycle and analyzed by SEC. Table 36 shows the effect of freeze/thaw
processing on the
amount of monomer (Mon) of remaining and the amount of fragments (Frag) and
aggregates
(Agg) formed in the samples formulated at these pH levels.
122
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Table 36: Numbers Of DVD-Ig Protein Monomers, Aggregates, And Fragments as
Determined by SEC Before and After Repeated Freeze/Thaw Cycles of DVD-Ig
Protein
Formulations with Solution pH Values of 4, 6, or 8
DVD-Ig pH Mon/TO Agg/TO Frag/TO Mon/T2 Agg/T2 Frag/T2
4 95.06 0.44 4.49 93.57 1.52 4.89
6 4 95.26 1.21 3.52 94.94 1.72 3.33
37 4 97.32 1.89 0.78 96.64 2.48 0.86
38 4 94.46 3.81 1.72 94.91 3.55 1.52
53 4 97.47 1 1.52 97.41 1.06 1.52
54 4 96.06 1.87 2.06 95.87 1.85 2.26
65 4 94.44 1.2 4.35 93.82 1.15 5.01
66 4 98.01 0.91 1.07 97.78 1.03 1.18
165 4 96.62 2.17 1.2 95.45 2.34 2.19
166 4 97.74 0.91 1.34 97.53 0.91 1.54
257 4 97.63 1.8 0.56 96.73 2.73 0.53
258 4 98.84 0.19 0.95 96.73 2.28 0.97
277 4 95.53 1.43 3.03 95.45 1.6 2.93
278 4 95.75 1.56 2.68 95.4 1.99 2.59
281 4 98.72 0.63 0.63 97.47 1.83 0.68
282 4 98.63 0.61 0.74 97.88 1.38 0.72
5 6 94.8 3.13 2.06 94.96 3.37 1.67
6 6 95.79 1.99 2.2 93.71 4.02 2.26
37 6 96.73 2.52 0.74 94.88 4.42 0.68
38 6 95.01 3.74 1.23 95.5 3.29 1.2
53 6 97.56 1 1.43 97.51 1.08 1.4
54 6 95.86 2.07 2.06 95.68 2.18 2.13
65 6 94.17 1.09 4.72 94.13 1.16 4.7
66 6 97.99 0.85 1.15 98.03 0.83 1.13
165 6 96.86 2.12 1.01 96.03 2.38 1.57
166 6 97.75 0.91 1.33 97.82 0.9 1.26
257 6 97.51 1.97 0.51 97.31 2.19 0.49
258 6 99.07 0.18 0.73 98.52 0.76 0.7
277 6 96.74 1.79 1.46 96.64 1.92 1.43
278 6 97.65 1.22 1.12 97.62 1.32 1.04
281 6 95.67 0.92 3.4 95.7 0.98 3.3
282 6 98.55 0.83 0.61 98.49 0.89 0.6
5 8 93.23 3.86 2.89 93.7 3.56 2.73
6 8 95.35 1.93 2.7 94.3 2.94 2.74
37 8 94.88 4.27 0.84 95.64 3.54 0.8
38 8 95.52 3.14 1.32 96.09 2.61 1.28
53 8 97.56 1.04 1.39 97.66 1.02 1.31
54 8 95.77 1.91 2.3 95.91 1.9 2.17
65 8 94.1 1.14 4.74 94.29 1.14 4.56
66 8 97.84 0.95 1.2 97.74 0.95 1.3
165 8 96.63 2.23 1.12 96.07 2.32 1.59
166 8 97.55 0.9 1.54 97.77 0.85 1.37
257 8 97.12 2.19 0.67 96.62 2.7 0.67
123
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-Ig pH Mon/TO Agg/TO Frag/TO Mon/T2 Agg/T2 Frag/T2
258 8 98.81 0.28 0.9 98.71 0.39 0.89
277 8 95.48 2.23 2.27 95.52 2.24 2.22
278 8 95.94 1.83 2.21 96.08 1.67 2.23
281 8 95.61 1 3.38 95.94 0.98 3.06
282 8 98.28 1.04 0.67 98.26 1.05 0.67
DVD 5, DVD 6, DVD 37, DVD 38, DVD 53, DVD 54, DVD 65, DVD 66, DVD 165,
DVD 166, DVD 257, DVD 258, DVD 277, DVD 278, DVD 281, and DVD 282
demonstrated stability after being subjected to repeated freeze thaw cycles.
These data
indicate that DVD-Ig proteins that are formulated in a pH range of about 4 to
about 8 remain
stable after repeated FIT processing. The high stability of the DVD-Ig protein
formulations
tested (all showed greater than 93% monomer content and 11/16 formulations
showed greater
than 95% monomer content) was unexpected, because DVD-Ig proteins are much
more
complex than IgGs. Complex molecules such as DVD-Ig proteins would be expected
to
aggregate and fragment easily when exposed to freezing and thawing.
Impact of Solution pH On the Stability of DVD-B Subjected to Repeated
Freeze/Thaw Cycles
The freeze thaw behavior of DVD-B at a protein concentration of 2 mg/ml in 10
mM
citrate/10mM phosphate buffer was evaluated by cycling the protein solution up
to 4 times
between the frozen state and the liquid state at pH 4-9. Freezing was
performed by means of
a temperature controlled -80 C freezer, and thawing was performed by means of
a 30 C
temperature controlled water bath. Samples were pulled after each freeze/thaw
(FIT) cycle
and analyzed by light obscuration and SEC. Table 37 shows the effect of
freeze/thaw
processing on the number of sub-visible particles formed as determined using
light
obscuration measurements at various pH values.
Table 37: Numbers of Subvisible Particles Per mL as Determined by Light
Obscuration
Assays After 0-4 Freeze/Thaw (FIT) Cycles of DVD-B Formulations with a pH
Value of 4, 5,
6,7, 8, Or 9
(A) Numbers of particles 1 micron in size.
Number
of FIT pH=4 pH= 5 pH= 6 pH= 7 pH= 8 pH = 9
cycles
0 39.16 55 44 41 55 46
1 334.5 1291 38127 31896 28444 25970
124
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
2 6728.6 7935 80064 61592 58562 46863
3 13658 18733 128448 95775 89934 67225
4 5702 37930 175024 132768 120339 89389
(B) Numbers of particles 10 microns in size.
Number
of FIT pH = 4 pH = 5 pH = 6 pH = 7 pH = 8 pH = 9
cycles
0 1.33 1.5 2.6 2.6 5.5 1.83
1 15.16 3.16 28 45 27.16 16.83
2 207.5 62 142 165 148 134
3 342 136 440 740 773 375
4 572 269 2418 4099 4537 1425
(C) Numbers of particles 25 microns in size.
Number
of F/T pH = 4 pH = 5 pH = 6 pH = 7 pH = 8 pH = 9
cycles
0 0.33 0.33 0.16 0.5 1.16 0.16
1 3.3 0.5 3.83 2 4.33 1.33
2 40.16 10.83 2.33 6.33 1.16 3.16
3 71.16 6.67 13.5 39.83 39.33 5.33
4 118.83 67.63 198.67 476.16 641.5 142.83
The results of the light obscuration assays show that the numbers of particles
formed
by DVD-B formulations with a pH of 4 to 9 was low. The numbers of particles
formed
increased with increasing pH and were at a maximum at around the pI of the
molecule (pI
8.5). However, with only one exception, the protein solutions tested satisfied
the
requirements of the International Conference on Harmonization of Technical
Requirements
for Registration of Pharmaceuticals for Human Use (ICH) guidelines, which
requires less
than 600 particles of size 25 microns or higher per ml.
Table 38 shows SEC measurements of the stability of DVD-B after freeze/thaw
processing. These measurements include the percentage of monomers, aggregates,
and
fragments, as well as the area under the curve (AUC).
Table 38: Stability Of DVD-B as Determined by SEC After 0-4 Freeze/Thaw Cycles
of
DVD-B Formulations with Solution pH Values of 4, 5, 6, 7, 8, Or 9
(A) SEC measurements of solutions not subjected to F/T cycles
Mon Agg Frag AUC
pH 4 Vial 1 96.38 1.44 2.16 76861
pH 4 Vial 2 95.61 1.56 2.82 76123
pH 4 Mean 95.995 1.5 2.49 76492
pH 5 Vial 1 96.1 1.57 2.32 73704
125
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
pH 5 Vial 2 96.16 1.55 2.28 74196
pH 5 Mean 96.13 1.56 2.3 73950
pH 6 Vial 2 95.09 1.69 3.2 77475
pH 7 Vial 2 95.61 1.81 2.56 75863
pH 8 Vial 2 95.84 1.87 2.27 74943
pH 9 Vial 1 95.66 2.11 2.21 82103
pH 9 Vial 2 95.43 2.14 2.41 81843
pH 9 Mean 95.545 2.125 2.31 81973
(B) SEC measurements after one F/T cycle
pH 4 Vial 1 96.37 1.68 1.94 74694
pH 4 Vial 2 95.39 1.74 2.86 76213
pH 4 Mean 95.88 1.71 2.4 75453.5
pH 5 Vial 1 96.18 1.82 1.98 71511
pH 5 Vial 2 96.08 1.6 2.31 76345
pH 5 Mean 96.13 1.71 2.145 73928
pH 6 Vial 1 96.37 1.6 2.02 75448
pH 6 Vial 2 95.24 1.6 3.14 77009
pH 6 Mean 95.805 1.6 2.58 76228.5
pH 7 Vial 1 95.88 1.86 2.24 75821
pH 7 Vial 2 95.28 1.96 2.74 76470
pH 7 Mean 95.58 1.91 2.49 76145.5
pH 8 Vial 1 95.64 2.01 2.34 74757
pH 8 Vial 2 95.59 2.08 2.31 74270
pH 8 Mean 95.81536 1.777143 2.3925 75306.68
pH 9 Vial 1 95.62 2.15 2.22 81981
pH 9 Vial 2 95.57 2.13 2.29 81516
pH 9 Mean 95.595 2.14 2.255 81748.5
(C) SEC measurements after two F/T cycles
pH 4 Vial 1 95.75 1.98 2.25 75402
pH 4 Vial 2 95.29 1.98 2.71 74567
pH 4 Mean 95.52 1.98 2.48 74984.5
pH 5 Vial 1 96.07 1.85 2.06 71454
pH 5 Vial 2 95.92 1.88 2.19 71525
pH 5 Mean 95.995 1.865 2.125 71489.5
pH 6 Vial 1 96.33 1.58 2.08 74666
pH 6 Vial 2 95.43 1.62 2.93 75080
pH 6 Mean 95.88 1.6 2.505 74873
pH 7 Vial 1 95.77 1.87 2.34 74813
pH 7 Vial 2 95.72 1.84 2.42 74584
pH 7 Mean 95.745 1.855 2.38 74698.5
pH 8 Vial 1 95.72 2.02 2.24 73460
pH 8 Vial 2 95.78 2 2.21 68769
pH 8 Mean 95.75 2.01 2.225 71114.5
pH 9 Vial 1 95.58 2.1 2.3 73356
pH 9 Vial 2 95.57 2.12 2.3 77337
pH 9 Mean 95.575 2.11 2.3 75346.5
(D) SEC measurements after three F/T cycles
pH 4 Vial 1 95.62 2.14 2.22 72539 1
126
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
pH 4 Vial 2 95.01 2.23 2.75 72480
pH 4 Mean 95.315 2.185 2.485 72509.5
pH 5 Vial 1 95.9 1.99 2.09 68689
pH 5 Vial 2 95.8 1.98 2.21 65381
pH 5 Mean 95.85 1.985 2.15 67035
pH 6 Vial 2 95.55 1.63 2.81 71195
pH 7 Vial 1 96.03 1.84 2.12 70617
pH 7 Vial 2 95.77 1.86 2.35 71405
pH 7 Mean 95.9 1.85 2.235 71011
pH 8 Vial 1 95.86 2.04 2.08 69364
pH 8 Vial 2 95.59 2.12 2.28 69308
pH 8 Mean 95.725 2.08 2.18 69336
pH 9 Vial 1 95.03 2.42 2.54 77679
pH 9 Vial 2 95.59 2.22 2.18 76113
pH 9 Mean 95.31 2.32 2.36 76896
(E) SEC measurements after four F/T cycles
pH 4 Vial 1 94.38 2.53 3.08 53902
pH 4 Vial 2 94.08 2.4 3.5 72943
pH 4 Mean 94.23 2.465 3.29 63422.5
pH 5 Vial 1 96.01 1.88 2.1 66962
pH 5 Vial 2 95.85 1.96 2.18 67786
pH 5 Mean 95.93 1.92 2.14 67374
pH 6 Vial 1 96.26 1.62 2.11 70371
pH 6 Vial 2 95.41 1.67 2.9 71086
pH 6 Mean 95.835 1.645 2.505 70728.5
pH 7 Vial 1 95.87 1.82 2.29 67317
pH 7 Vial 2 96.03 1.81 2.15 67869
pH 7 Mean 95.95 1.815 2.22 67593
pH 8 Vial 1 95.62 2.07 2.3 69629
pH 8 Vial 2 95.31 2.15 2.52 69401
pH 8 Mean 95.465 2.11 2.41 69515
pH 9 Vial 1 95.44 2.27 2.28 77495
pH 9 Vial 2 95.58 2.18 2.22 73373
pH 9 Mean 95.51 2.225 2.25 75434
The results in Table 38 indicate that the DVD-Ig protein does not form
significant
amounts of aggregates even after 4 FIT cycles. The good FIT stability is
surprising as it was
anticipated that due to their higher structural complexity DVD-Ig proteins
would be more
prone to undergo degradation at the liquid lice interface or even cold
denaturation upon
multiple freeze /thaw cycles. This is a common observation for proteins and
many other
DVD-Ig proteins show a significant amount of aggregation upon freeze/thawing.
Since
freezing is the initial, and a critical, step of the lyophilizing process, the
data suggests that
DVD-B can be lyophilized and hence converted into a stable biotherapeutic
dosage form.
The results in Table 37 indicate that the DVD-Ig protein does not form
significant
127
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
aggregates even after 4 F/T cycles. The good F/T stability was surprising as
it was
anticipated that the stability might not be as good as was observed given the
intrinsic lack of
stability and increased structural complexity relative to an antibody.
EXAMPLE 19: Impact of Lyophilization on the Stability of DVD-Ig Proteins
Aggregates may form during the process of lyophilization as well as later on
during
shelf stability of the solid protein. The aggregates formed during
lyophilization are generally
measured following immediate reconstitution.
Storage stability of DVD-Ig proteins was evaluated for prolonged periods of
time at
controlled temperature conditions. After defined storage periods, samples were
pulled and
the impact of storage time and storage temperature on the stability of
lyophilized DVD-Ig
proteins was evaluated by size exclusion chromatography (SEC) and ion exchange
chromatography (IEC). Three DVD-Ig proteins were studied: DVD-B (TNF-PGE2),
DVD-A
(TNF-1L17), and DVD-C (IL1cc¨IL1p). Of the three, DVD-A and DVD-C are AS-DVD-
Ig
proteins. DVD-B is a non-AS-DVD-Ig protein, but was identified as an LS-DVD-Ig
protein
as it (as well as LS-DVD-Ig proteins DVD-A and DVD-C) was found to be stable
in a
lyophilized formulation. The formulations were lyophilized in solutions as
shown in Table
39 in a formulation containing a buffer, a polyol, and a surfactant.
Table 39: Composition of Lyophilized Formulations
Component DVD-A DVD-B DVD-C
55 mg/ml 55 mg/ml 48 mg/ml
Histidine 2.33 (15mM) 2.33 (15mM) 2.33 (15mM)
Sucrose 46 (4.6%) 46 (4.6%) 46 (4.6%)
Polysorbate 80 0.2 (0.02%) 0.2 (0.02%) 0.1 (0.01%)
0.1 M HC1 q.s. (pH 5.25) q.s. (pH 5.25) q.s. (pH 6.0)
Table 40 describes the percentages of monomers, aggregates, and fragments that
were
measured using SEC before storage (TO) or following storage of the lyophilized
formulations
at either 5 C or 40 C for the given storage periods.
128
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 40: Stability of Stored Lyophilized Formulations as Assessed Using SEC
DVD-Ig Storage Condition Monomer Aggregate Fragment
DVD-A TO 94.22 5.09 0.67
1 Week 40 C 94.12 5.23 0.64
4 Week 40 C 94.41 5.14 0.43
3m 40C 93.31 6.24 0.43
3m 40C + 3h RT 93.17 6.38 0.43
DVD-B TO 96.59 2.11 1.28
1 Week 40 C 96.11 2.59 1.28
4 Week 40 C 95.44 3.19 1.36
4 Week 5 C 96.47 2.2 1.31
4 Week 40 C + 3h RT 95.35 3.29 1.34
DVD-C TO 96.55 2.94 0.5
1 Week 40 C 97.165 1.59 1.23
lm 40 C 96.88 1.93 1.18
3m 5 C 97.42 1.36 1.21
The SEC data in Table 40 shows that lyophilized LS-DVD-Ig proteins remain
stable
for periods of up to 3 months of storage because they show high percentages of
monomers
and low percentages of aggregates and fragments. After accelerated storage for
3 months at
40 C and 3 hours of reconstitution time, lyophilized DVD-A had more than 93%
monomers,
less than 6.4% aggregates, and only about 0.4% fragments. After 4 weeks of
accelerated
storage at 40 C, lyophilized DVD-B had more than 95% monomers and only about
3.2%
aggregates and about 1.4% fragments. After 1 month of accelerated storage at
40 C,
lyophilized DVD-C had approximately 97% monomers, 2% aggregates, and 1%
fragments.
Thus, lyophilized formulations of DVD-A, DVD-B, and DVD-C showed long term
stability,
as assessed by SEC.
Table 41 below provides data regarding the stability of stored formulations
measured
using IEC. The impact of chemical stability was not significant as observed
from formation
of acidic and basic species with time. Therefore, all tested molecules do not
show significant
chemical degradation in the lyophilized state. Storage stability of
lyophilized DVD-Ig
proteins was evaluated at 40 C and 5 C. After defined storage periods, the
lyophilized
samples were pulled and the impact of storage time on DVD-Ig protein stability
was
evaluated. Samples were reconstituted with water for injection and upon
complete
reconstitution analyzed using SEC and IEC.
129
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 41: Stability of Stored Lyophilized Formulations as Assessed Using IEC
DVD-Ig Storage condition Main Acidic Basic X
DVD-A TO 73.28 3.6 20.78 2.32
1 Week 40 C 72.59 3.38 21.91 2.1
4 Week 40 C 70.73 3.6 23.44 2.21
3m 40 C 71.75 3.84 22 2.39
3m 40 C + 3h RT 71.8 3.71 22.03 2.44
DVD-B TO 48.84 38.54 12.6
1 Week 40 C 48.18 38.72 13.09
4 Week 40 C 45.87 37.6 16.53
4 Week 5 C 48.17 38.16 13.56
4 Week 40 C + 3h RT 45.7 37.79 16.5
DVD-C 1 Week 40 C 54.82 28.82 16.35
lm 40 C 53.61 28.84 17.53
3m 5 C 55.43 28.57 15.99
The impact of chemical stability seen above in Table 40 was not significant as
observed from formation of acidic and basic species with time.
Table 42 below provides the reconstitution times of the stored lyophilized
formulations. One basic criterion for characterizing the feasibility of a
lyophilized dosage
form is the time it takes for a lyophilized sample to be converted in a
homogenous, clear
liquid after reconstitution with a solvent, usually water for injection.
Ideally, the
reconstitution procedure takes less than 10 minutes. As shown in Table 41 all
tested samples
showed complete reconstitution after a maximum of 5 minutes. This indicates
that AS-DVD-
Ig proteins (e.g., DVD-Ig proteins A & C) can be formulated as stable
lyophilized
formulations. Moreover, the above examples show that LS-DVD-Ig proteins -
which are not
stable in liquid formulations - can be stabilized using lyophilization (e.g.,
DVD-B).
Table 42: Reconstitution Time (RT) Of the Lyophilized Formulations (With Water
for
Injection)
Storage
DVD-Ig
condition RT
DVD-A TO 30s
Ti week 40 C 26s
T4 week 40 C 48s
T3 month 40 C 300s
DVD-B TO 48s
Ti week 40 C 60s
T4 week 5 C 69s
T4 week 40 C 89s
130
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Storage
DVD-Ig
condition RT
DVD-C TO 47s
Ti week 40 C 76s
3m 5 C 62s
lm 40 C 53s
IX. CHARACTERIZATION OF DVD-IG PROTEINS
Examples 20-23 provide further characterization of DVD-Ig proteins generally.
Example 20: Solubility Assessment of DVD-Ig Proteins Using PEG 3000
Polyethylene glycols (PEG) are often used as "crowders," which force the
protein in a
smaller amount of water, because some of the available solvent is bound to or
occupied by
the crowder, typically a polymer. PEGs are used to assess the true solubility
of a protein by
utilizing micro amounts of the protein material available at early stages of
development. In
general, the greater the amount of PEG is required to induce precipitation,
the greater is the
anticipated true solubility of the protein in solution.
The following studies were carried out using small aliquots of PEG solution
(50%
w/v) added to a stock solution of protein (0.5 mg/ml) in a buffer (5 mM
citrate and 5 mM
phosphate) at pH 6. Table 43 shows the data for various DVD-Ig proteins.
Table 43. Amount of PEG 3000 Required To Induce Precipitation in a 0.5 mg/ml
Protein
Solution of DVD-Ig Proteins
% PEG 3000 required to induce
DVD-Ig precipitation
10
6 9.37
37 10
38 9.33
53 8.12
54 9.37
65 7.5
66 7.5
165 10.625
166 11.875
257 9.37
258 10.62
277 12.5
278 13.75
131
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
281 8.12
282 7.5
These data show that the amount of PEG 3000 required to induce precipitation
of
DVD-Ig proteins is typical of highly soluble proteins (i.e., those proteins
with a true
solubility that exceeds about 100 mg/ml). Although the PEG precipitation assay
is a standard
assay in antibody formulation assessment to provide information about its
solubility, it would
not be sufficient to predict whether a DVD-Ig protein would be classified as
AS or LS or
even non LS, indicating the complexity of DVD-Ig proteins and the challenging
formulation
efforts compared to monoclonal antibodies.
Example 21: Assessment of the Tertiary Structure of DVD-Ig Proteins Using Near
UV
CD Scans
The structure of a protein is one of the important factors influencing protein
stability
during accelerated/long-term storage of protein liquid and lyophilizate
formulations. To
assess the tertiary structure of the DVD-Ig proteins, near UV CD scan between
250-320 nm
was taken on a Jasco Spectrometer with a scan rate of 50 nm/minute at a
concentration of
lmg/ml. An average of 3 scans/ DVD-Ig protein was taken. The pH used in the
study was 6
in 5 mM citrate and 5 mM phosphate conditions. UV CD scans of monoclonal
antibodies
were also obtained for comparison. The data presented in Figure 5 indicate
that the DVD-Ig
proteins have folded structures as indicated by the presence of featured
ellipticity profiles in
the 250-320 nm region.
The DVD-Ig ellipticity values presented in Figure 5 are similar to those
observed for
known stable monoclonal antibodies. Therefore, both the DVD-Ig proteins and
the mAbs are
expected to show similar storage stability. However, LS-DVD-Ig proteins are
actually less
stable than the mAbs. Therefore, UV-CD data alone cannot be used to accurately
predict the
stability issues observed for LS-DVD-Ig proteins.
Example 22: Assessment of the Secondary Structure of DVD-Ig Proteins Using ATR-
FTIR
To assess the secondary structure of DVD-Ig proteins, the second derivative,
area
normalized FTIR scans taken on an ATR-FTIR instrument from Bruker (Tensor 27)
in the
132
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
region 1600-1700 cm-1 were curve fitted and the various peaks were analyzed
and added up
to get total % beta sheet structure in the molecule. Especially, peaks such as
that at 1638 cm
-
1
, which are an indicator of the beta sheet arrangement, were taken into
account. This is
because beta sheets are known to comprise the majority of the secondary
structure of mAbs.
Therefore it is expected that DVD-Ig proteins which are derived from mAbs
should also
contain mostly beta sheets as their secondary structure composition. Any
deviation from this
may indicate that the overall structural integrity of the molecule is
compromised. The studies
were done in 5 mM citrate/5 mM phosphate buffer at a pH of 4, 6, or 8. The
concentration of
the DVD-Ig protein was lmg/ml. The total percentage of beta structure was
assessed for 16
DVD-Ig proteins (see Table 44).
Table 44: The Total % Beta Structure in DVD-Ig Proteins Measured Using ATR-
FTIR
DVD-Ig pH 6 pH 4 pH 8
89.8 86.5 95.8
6 94.1 78.2 92.3
37 89.6 85.9 90.9
38 96.5 92 95.6
53 NA NA 96.4
54 97.4 87.7 96.8
65 95.2 95.8 94.8
66 94.6 94.2 94.2
165 NA NA 95.2
166 95.1 90 95.2
257 95.8 89.6 90.8
258 95.6 88.3 96.6
277 93.6 85.3 95
278 93.8 77.4 94.3
281 94.4 84.4 85
282 93 84.7 84.6
The results presented in Table 45 show that all of the 16 DVD-Ig proteins
studied have a
folded secondary structure that is composed primarily of beta elements. The
proportion of
beta elements ranged from about 85% to about 97% which is similar to that
observed for
standard antibodies. This was unexpected since DVD-Ig proteins are engineered
molecules
and are assumed to have a lower percentage of beta sheets as compared to mAbs
(although
they still are expected to contain beta sheets as their predominant secondary
structure). These
findings suggest that secondary structure composition is not a factor for the
overall lower
133
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
storage stability of DVD-Ig proteins as compared to antibodies. The proportion
of beta
elements ranged from about 85% to about 97%.
EXAMPLE 23: Impact of Ionic Strength and pH on the Second Virial Coefficient
of
DVD-B Formulations
Second virial coefficient (B22) is a thermodynamic parameter and an indicator
of the
protein-protein attractive or repulsive interactions in solutions. A positive
value indicates
repulsive interactions and a negative value indicates attractive interactions.
Repulsive
interactions usually translate into better long term storage. The scattered
light intensity is
related to the molecular weight and B22 by the following equation.
Kr
¨ = ¨ .+ 2 c
iRe M.
Where K is optical constant and is given by
\
[27.47n
K
N,
Ro is the excess Rayleigh ratio, a measure of light scattered by the solute, n
is the
solvent refractive index, dn/dc is the refractive index increment of the
solute, NA is the
Avogadro's number, and X is the wavelength of the incident light. Since for
most dilute
solutions, higher order virial coefficients have negligible values, the
following equation
(Debye) is used to obtain the second virial coefficients.
Kc 1
¨ = ¨ 2EL c
R
The scattered intensities were measured on a Malvern Zetasizer Nano. The
second
virial coefficient values were all positive and indicate that DVD-Ig proteins
behave as typical
protein molecules with respect to this calculation at least under dilute
conditions. The
buffers used were acetate for pH 4.5, histidine for pH 6 and Tris for pH 8. 2
mM
concentration of buffer was used for 1 mM ionic strength solutions and 10 mM
for 20 and
100 mM ionic strength solutions. The rest of the ionic strength was maintained
by sodium
chloride. The results are shown in Tables 33 and 34. The values of the second
virial
coefficients were higher on average at pH 4.5 and pH 6.0 than at pH 8.0,
suggesting that
134
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
DVD-B would store better at pH 4.5 or pH 6.0 than at pH 8Ø Also, the values
of the second
virial coefficients were higher at lower ionic strength, suggesting that lower
ionic strength
may also be associated with stability of TNF-PGE2 DVD-Ig protein.
Ds is the self-diffusion coefficient of the molecule at infinite dilution. kd
is a
parameter describing the interaction between the molecules in solution. A
positive value for
kd indicates intermolecular repulsion and vice versa.
Table 45: Virial Coefficient Values for DVD-B protein at 0 mm Ionic Strength
pH B22 X iO4 B222 X 10-2 B2222 X 10-1 B22222 X 102 Mw (X
(mol mL/gm2) (mol mL2/gm3) (mol mL3/gm4) (mol mL4/gm5) 1000)
6.0 136 5.77 -273.8 16.6 3376.1 339 -221 35.8 189
14
Table 46: Second Virial Coefficients Under Various Conditions for DVD-B
Protein
pH Ionic Strength D X le kd (mL/gm)t B X 104 ML, (X
(mM) cn' (mol mL/gm2)*
1000)
4.5 1 3.42 0.04 434.90 15.31 26.09 0.82 195 1
20 3.66 0.004 5.96 0.32 5.08 0.03 168
2.89
100 3.60 0.03 -13.33 0.88 3.09 0.07 160
3.38
6 1 3.41 0.06 379.28 51.34 14.71 1.09 183
0.57
20 3.75 0.02 -11.04 1.37 3.37 0.06 163
2.35
100 3.70 .002 -23.05 0.02 2.51 0.19 170
3.40
8 1 3.81 0.01 -4.74 0.06 3.50 0.05 155 0.78
20 3.73 0.02 -27.31 0.29 2.14 0.03 162
5.08
100 3.66 0.03 -25.33 0.90 2.34 0.07 164
4.59
As mentioned, if B22 and kD are positive in sign and have a large magnitude,
it is an
indication of strong repulsive interactions among the DVD-Ig proteins in
solution. This is an
ideal condition for the long term storage stability of a DVD-Ig protein liquid
formulation
because there is a lowered probability of DVD-Ig proteins encountering each
other in
solution to form aggregates. The above data suggest that low ionic strength
and low pH as
part of a formulation will result in greater long term DVD-Ig protein
stability than a
formulation with high ionic strength and high pH. Also, since ionic strength
and pH
135
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
contribute highly to the B22 value, it indicates that electrostatic repulsion
in general comprises
a significant portion of the B22 value.
With respect to comparing DVD-Ig proteins and antibodies, it may be that a
more
positive B22 value is required for a DVD-Ig protein formulation to have a
similar long term
stability profile as that of an antibody formulation. This is because DVD-Ig
proteins are
larger than antibodies and the hard-sphere contribution to repulsion (and B22)
is greater for
DVD-Ig proteins. Therefore, if both DVD-Ig proteins and antibodies have the
same
electrostatic repulsions contributing to B22, the B22 term for DVD-Ig proteins
will be more
positive to reflect the same degree of protein-protein repulsion in solution.
EXAMPLE 24: Pharmacokinetic Study of DVD-Ig Proteins
The following example describes pharmacokinetic studies of various DVD-Ig
proteins.
Variable Domain Combination and Orientation Impacts Serum Stability
As described in Figure 2, certain variable domain combinations provide a more
stable DVD-Ig protein than other combinations. Figure 2A describes the serum
stability for
certain DVD-Ig proteins in two different domain orientations, i.e., "outer /
inner" and "inner /
outer". For example, the DVD-Ig protein TNF/SOST has a high molecular weight
(HMW) %
of about 16% in the "outer / inner" orientation, but has about a 75% HMW in
the opposite
orientation. The domain orientation concepts are set forth in Figure 2B. The
DVD-Ig
proteins in Figure 2 include short/short linker combinations and are huIgG1
isotypes.
In Vitro Pharmacokinetic Study
The pharmacokinetic (PK) properties of various biologic therapeutics were
assessed
following 4 mg/kg single intravenous doses in male Sprague-Dawley rats. Blood
samples
were collected throughout the 28 day studies. Serum samples were analyzed
using an MSD
assay employing anti-human Ig capture and Sulfo-Tag labeled goat anti-human
IgG antibody
for chemiluminescent detection. Pharmacokinetic parameters for each animal
were
calculated using WinNonlin software by non-compartmental analysis.
Figure 3 describes results from a pharmacokinetic study using rats. The study
136
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
examined the correlation between clearance (CL) and T 1/2 with high molecular
weight
(HMW) aggregate formation in vitro in rat serum. As shown in Figure 3, DVD-Ig
proteins
with less than 10% HMW aggregate in vitro are more likely to have low (< 0.3
ml / hour / kg)
clearance and long (> 10 days) half life. The outliers included DVD 257 and
DVD 258. The
DLL4/VEGF DVD-Ig protein recognized the rat target, and the PK was affected by
TMD.
DVD 037 (VEGF/HER2; SEQ ID NOs: 34 and 35) showed bad in vitro properties, but
good
PK in vivo. The amino acid sequences for the heavy and light chains of
DLL4/VEGF DVD-
Ig protein are provided in SEQ ID NOs: 68 and 69 of Table 65.
EXAMPLE 25: Viscosity Study of DVD-Ig Proteins
The following example describes viscosity studies for an exemplary AS-DVD-Ig
protein (DVD-A).
Viscosity was measured on m-VROC low volume viscometer from Rheosense
(Redwood, CA). m-VROC measure viscosity from the pressure drop of a test
liquid as it
flows through a rectangular slit. As the test liquid is pumped to flow through
the flow
channel, pressure is measured at increasing distance from the inlet. Plot of
the straight line in
the pressure vs. position of the sensor is proportional to the viscosity.
The instrument was evacuated beforehand to minimize the usage of material and
subsequently recover the material. Air was hence used to clean the instrument
before a
sample measurement was made. An initial flow rate of 40 ill/minute ¨ 200
ill/minute was
used to obtain the required pressure differential. Saturation of the pressure
chamber quickly
stabilizes the viscosity reading, and twenty microliters of sample achieved
stabilization. Once
the instrument has been primed with the sample, less than 5 microliters of
additional sample
was enough to give a stable second reading. A total of less than thirty
microliters (<35
microliters) of sample was thus enough to give readings in triplicate.
Viscosity of all samples was also measured on a rolling ball viscometer from
Anton
Paar (X, X). 1.8 mm capillary was used for samples of viscosity range 2-70 cP
and 1.6 mm
capillary was used for samples of minimal viscosity (less than 2 cP). The
instrument was pre-
calibrated and run at any of the various possible angles (70 , 50 and/or 40
).
Viscosity of DVDA was determined in histidine formulations having different
molarity (i.e., 0 mM to 30 mM histidine) and pH (i.e., pH 4.8 to pH 8.3) in
various DVD-A
concentrations. Results from the measurements are provided below in Tables 47
to 49.
137
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Table 47: Viscosity Measurements of 34 mg/ml DVD-A at Various pH and Histidine
Molarity
DVDA 34mg/m1
Anton Paar Viscosity readings (mPa.s.)
pH 4.8 pH 5.4 pH 6 pH 6.6 pH 8.3
0 Mm Histidine 1.83 2.9 5.22 Na na
Mm Histidine 1.59 1.81 2.46 3.86 1.83
30 Mm Histidine 1.33 1.71 2.03 3.5 1.97
Rheosense Viscosity measurments
pH 4.8 pH 5.4 pH 6 pH 6.6 pH 8.3
0 Mm Histidine
1 3.07 4.11 6.03 Na 2.35
2 3.04 4.04 5.84 Na 2.36
3 2.93 4.15 6.08 Na 2.40
Average Viscosity 3.01 4.10 5.98 Na 2.37
5 Mm Histidine
1 1.58 1.73 2.56 3.87 2.22
2 1.52 1.71 2.55 3.87 2.12
3 1.59 1.72 2.53 3.86 2.13
Average Viscosity 1.56 1.72 2.55 3.86 2.16
30 Mm Histidine
1 1.37 1.57 1.88 3.34 2.84
2 1.39 1.58 1.91 3.37 2.93
3 1.33 1.58 1.86 3.50 2.73
Average Viscosity 1.36 1.58 1.88 3.40 2.83
138
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 48: Viscosity Measurements of 15 mg/ml DVD-A at 0 Mm Histidine at pH 4.8
To 8.3
DVDA 15mg/ml0mM
pH 4.8 pH 5.4 pH 6 pH 6.6 pH 8.3
0 Mm Histidine
1 1.66 1.30 1.28 Na 1.85
2 1.64 1.34 1.31 Na 1.82
3 1.64 1.30 1.29 Na 1.80
Average Viscosity 1.65 1.31 1.29 Na 1.82
Table 49: Viscosity Measurements of Various Concentrations of DVD-A at Various
pH And
Histidine Molarity
DVDA
5mM 30mM
Anton Paar Rheosense Anton Paar Rheosense
pH4.8 (95mg/m1) 1 4.63 6.924 5.29 4.179
2 4.67 6.925 5.3 4.241
Average 4.65 6.9245 5.295 4.21
Ph5.4 (77mg/m1) 1 10.45 10.09 6.3 6.027
2 10.5 10.1 6.29 6.016
Average 10.475 10.095 6.295 6.0215
pH6 (47.8mg/me 1 6.23 5.721 3.643 3.34
2 6.3 5.695 3.648 3.36
Average 6.265 5.708 3.6455 3.35
5mM 30mM
The results described above in Tables 47-49 show the impact of ionic strength,
pH
and protein concentration on the viscosity of the DVD-Ig protein solutions.
DVD-A (SEQ ID
NOs: 62 and 63) is an AS-DVD-Ig protein and the results above show that the
viscosity
values can be modulated by formulation means to accommodate a syringeable
liquid
formulation at high concentrations that would be appropriate for
pharmaceutical
compositions and in-vivo use. Since DVD-Ig proteins have not only a much
higher
molecular weight of about 200kD compared to standard monoclonal antibodies of
about 150
kD, their structure is also more complex. Therefore, it was surprising that
the viscosity of
mAbs and DVD-Ig is relatively similar and is even at high concentration below
about 10 cPs.
139
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
EXAMPLE 26: Thermal Stability of DVD-Ig Proteins
The following example describes results from three different tests examining
thermal
stability of DVD-Ig proteins, including an exemplary AS-DVD-Ig protein and an
exemplary
LS-DVD-Ig protein, versus monoclonal antibodies, such as Adalimumab.
Dynamic Scanning Fluorescence
An automated high throughput instrument Optim-1000 from Avacta (York, UK) was
used for the study. 9 microliter micro cubic arrays (MCAs) were used for the
study. For
preparation of stock samples, 3 microliter Sypro orange (Invitrogen,
Cambridge, MA) was
added to 27 microliter sample solution in order to obtain a final 1X
concentration of the dye.
The dye is available as 5000X commercial product, although any dye would be
suitable.
Thermal scans were obtained from 26 C to 95 C at a scan rate of 60 C/hour.
Baseline was
fitted for linearity and the first point (the temperature) whose inclusion
decreased the R2
below 0.95 was taken as the onset temperature. Repeat studies confirmed that
the variation in
onset temperatures was less than 5 %.
Intrinsic Fluorescence
Tryptophan fluorescence was used to evaluate the unfolding temperatures.
Hitachi
FL-4500 instrument from Hitachi (Tokyo, Japan) was used for the study. The
temperatures
were maintained using a water bath. The temperature in the cuvette was
monitored using a
thermocouple and a temperature monitor CSi32 from Omega Inc. (Stamford, CT). A
front
surface triangular quartz cuvette from VWR (MA) was used as this minimized the
inner filter
effects and hence resulted in strong emission signals. An excitation
wavelength of 295 nm
was used. Emission was monitored between 328-338 nm. Although the Xmax was
observed at
332 nm, the intensity was monitored at 335 nm for comparison. The thermal
scans were
obtained from 30 C to 70 C at a scan rate of 78 C/hour. Baseline was fitted
for linearity and
the first point (the temperature) whose inclusion decreased the R2 below 0.95
was taken as
the onset temperature. Repeat studies confirmed that the variation in onset
temperatures was
less than 5 %. The increased scan rate did not significantly affect the onset
temperatures.
140
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Differential Scanning Calorimetry (DSC)
DSC was used to characterize the thermodynamic stability of the proteins under
various solution conditions. An automated cap DSC instrument from Microcal
(Northampton,
MA) was used. The thermal scans were obtained from 25 C to 65 C at a scan rate
of
60 C/hour. Since, aggregation and precipitation that follows unfolding in high
concentration
samples can lead to blocking of the cap DSC cells which than become rather
difficult to
clean, the scans were obtained only until --:--, 5 C beyond the onset
temperature to prevent any
such occurrence. A prescan equilibration thermostat of 10 minutes was applied
before each
scan. A corresponding buffer scan was taken immediately following the sample
scan. The
difference in onset was less than 2 C between repeat scans. Baseline was
fitted for linearity
and the first point (the temperature) whose inclusion decreased the R2 below
0.95 was taken
as the onset temperature. Repeat studies confirmed that the variation in onset
temperatures
was less than 5 %.
Results from the study are provided in Table 50.
Table 50: Results from Thermal Stability Studies Comparing DVD-Ig Proteins to
mAbs
Intrinsic fluorescence Extrinsic fluorescence DSC
1 mg/mL 75 mg/mL 1 mg/mL 75 mg/mL 1 mg/mL 75 mg/mL
mAbl 66 64 63 57 59 57
mAb2 74 69 73 68 63 59
mAb3 65 62 65 63 59 55
mAb4 72 67 64 58 61 59
mAb5 71 64 66 61 59 56
69 67 64 61 62 59
IL121L18
DVD-B 56 53 53 49 54 50
TNF/PGE2
141
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
The results described in Table 50 show the impact of protein concentration on
the
thermal stability of the protein solution. DVD1 (IL12IL18), an AS DVD and DVD2
(also
referred to herein as DVD-B), an LS-DVD-Ig protein, and other mAbs all show
that protein
concentration only has a slight impact on the thermal stability of the
protein. So the feasibility
of a liquid high concentration formulation may be independent of the impact of
protein
concentration on the thermal stability of the protein; however, high
concentration liquid
formulations present other well-known types of instabilities, e.g., shelf
instabilities.
Generally, as described in Examples 1-3, DVD-Ig proteins have a lower melting
temperature
than antibodies. In some instances, e.g., DVD1, similar melting temperatures
are observed,
but generally this is not the case.
X. ANTI-DLL4 / ANTI-VEGF DVD-IG FORMULATIONS (AQUEOUS AND
LYOPHILIZED)
EXAMPLE 27: Preformulation Characterization of Anti-DLL4/Anti-VEGF DVD-Ig
Protein h1A11.1SL-Av
The storage stability (5 C) and accelerated stability (40 C) of an anti-
DLL4/anti-VEGF DVD
(h1A11.1-SL-Av, Table 41) was evaluated in the formulations and protein
concentrations listed
below. Stability was evaluated by size exclusion chromatography (SEC) and %
aggregate, %
monomer, % fragment, and total species recovered were quantitated. Overall,
the formulations cover a
pH range of 5 to 7 and a protein concentration range of 1.0 to 118 mg/ml.
At 5 C and 40 C temperatures and at protein concentrations of 50, 30, and 10
mg/ml,
formulations were: 15 mM acetate pH 5; 15 mM phosphate pH 7; 30 mM acetate, 80
mg/ml sucrose,
0.02% Tween 80 at pH 5; 30 mM histidine, 80 mg/ml sucrose, 0.02% Tween 80 at
pH 6; PBS
(phosphate buffered saline). All formulations contained 0.02% sodium azide to
prevent microbial
growth during storage. At 5 C and 40 C temperatures and at protein
concentrations of 60, 50, 30, and
mg/ml, the formulation was 15 mM histidine pH 6 (also containing 0.02% sodium
azide to prevent
microbial growth during storage). At 5 C and at a protein concentration of 118
mg/ml, the
formulation was 15 mM histidine pH 6 (also containing 0.02% sodium azide to
prevent microbial
growth during storage). At 40 C and at a protein concentration of 1.0 mg/ml,
the formulations were
10 mM citrate and 10 mM phosphate at pHs 5, 6, and 7. Formulations with
protein were filtered to
remove possible microbes.
142
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Freeze-thaw stability was performed by subjecting the protein in formulation
to four cycles of
freezing at -80 C for at least 20 hours and thawing in a 30 C water bath. The
formulations that were
tested for freeze-thaw stability are listed below. Stability was evaluated by
SE-HPLC and %
aggregate, % monomer, % fragment, and total species recovered were
quantitated. The formulations
were 15 mNI histidine pH 6 at 60 mg/ml protein (also containing 0.02% sodium
azide to prevent
microbial growth) and 10 mNI citrate and 10 mNI phosphate at pHs 5, 6, and 7
and 1.0 mg/ml protein
(filtered to remove possible microbes).
Finally, differential scanning calorimetry to measure thermal stability was
performed on the
protein in 10 mNI citrate and 10 mNI phosphate buffer at pHs 5, 6, and 7 and
1.0 mg/ml protein. The
onset temperature of unfolding and the midpoint temperatures of unfolding (Tm)
of each protein
domain were quantitated.
Table 51: Accelerated Stability at 40 C of H1a11.1-SL-Av at Different
Concentrations and In
Different Buffers, Excipients, and pHs
Protein time temp buffer PH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/mi)
pre- --- --- --- 2.71 96.31 0.98 53058
dialysis
50, 30, 10 TO --- ace 5 2.89 96.08 1.03
48033
50, 30, 10 TO --- his 6 2.81 96.23 0.96
46995
50, 30, 10 TO --- phos 7 2.91 96.09 1.00
52571
50, 30, 10 TO --- ace-suc-tw 5 2.54 96.50
0.96 50185
50, 30, 10 TO --- his-suc-tw 6 2.37 96.62
1.01 50771
50, 30, 10 TO --- PBS 7 2.90 96.08 1.01
49170
50 T7d 40 ace 5 5.19 93.32 1.49 49028
30 T7d 40 ace 5 3.86 94.68 1.47 48171
T7d 40 ace 5 2.60 95.97 1.43 48379
50 T7d 40 his 6 5.25 93.46 1.29 47731
30 T7d 40 his 6 4.13 94.58 1.29 46684
10 T7d 40 his 6 2.73 95.84 1.42 46877
50 T7d 40 phos 7 9.02 89.52 1.46 53429
30 T7d 40 phos 7 6.11 92.40 1.49 51923
10 T7d 40 phos 7 3.94 94.57 1.49 53098
50 T7d 40 ace-suc-tw 5 5.42 92.85 1.73
50373
30 T7d 40 ace-suc-tw 5 4.07 94.06 1.87
48768
10 T7d 40 ace-suc-tw 5 2.66 95.20 2.14
49396
50 T7d 40 his-suc-tw 6 3.44 95.02 1.54
50040
30 T7d 40 his-suc-tw 6 4.16 94.14 1.70
48715
10 T7d 40 his-suc-tw 6 2.86 95.24 1.90
49871
50 T7d 40 PBS 7 8.13 90.28 1.60 49207
30 T7d 40 PBS 7 5.82 92.55 1.63 48853
10 T7d 40 PBS 7 3.62 94.82 1.56 48166
50 T21d 40 ace 5 6.65 90.83 2.51 48536
143
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein time temp buffer pH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/mi)
30 T21d 40 ace 5 4.55 92.91 2.54 48520
T21d 40 ace 5 2.71 94.70 2.59 48395
50 T21d 40 his 6 7.01 90.71 2.27 46729
30 T21d 40 his 6 4.69 93.10 2.21 46687
10 T21d 40 his 6 2.77 94.93 2.30 46866
50 T21d 40 phos 7 13.39 83.83 2.78 52244
30 T21d 40 phos 7 9.38 87.76 2.86 53556
10 T21d 40 phos 7 4.77 92.32 2.91 52536
50 T21d 40 ace-suc-tw 5 6.37 90.34 3.30 48268
30 T21d 40 ace-suc-tw 5 4.27 91.91 3.82 47211
10 T21d 40 ace-suc-tw 5 2.26 93.02 4.72 46322
50 T21d 40 his-suc-tw 6 6.84 89.82 3.34 47140
30 T21d 40 his-suc-tw 6 4.60 91.90 3.50 47416
10 T21d 40 his-suc-tw 6 2.67 93.66 3.67 48166
50 T21d 40 PBS 7 12.13 84.81 3.06 49845
30 T21d 40 PBS 7 8.09 88.78 3.13 48108
10 T21d 40 PBS 7 4.20 92.63 3.17 48803
Buffer key (all buffers contain 0.02% sodium azide to prevent microbial
growth):
ace = 15 mM acetate pH 5; his = 15 mM histidine pH 6; phos = 15 mM phosphate
pH
7
ace-suc-tw = 30 mM acetate, 80 mg/ml sucrose, 0.02% Tw80
his-suc-tw = 30 mM histidine, 80 mg/ml sucrose, 0.02% Tw80
PBS = phosphate buffered saline
Table 52. Storage Stability at 5 C of H1a11.1-SL-Av at Different
Concentrations and In Different
Buffers, Excipients, and pHs (Buffer Key Same as In Table 53)
Protein time temp buffer pH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/mi)
--- pre- --- --- --- 2.71 96.31 0.98 53058
dialysis
50, 30, 10 TO --- ace 5 2.89 96.08 1.03
48033
50, 30, 10 TO --- his 6 2.81 96.23 0.96
46995
50, 30, 10 TO --- phos 7 2.91 96.09 1.00
52571
50, 30, 10 TO --- ace-suc-tw 5 2.54 96.50
0.96 50185
50, 30, 10 TO --- his-suc-tw 6 2.37 96.62
1.01 50771
50, 30, 10 TO --- PBS 7 2.90 96.08 1.01
49170
50 T7d 5 ace 5 2.96 95.99 1.05 49118
30 T7d 5 ace 5 2.74 96.21 1.06 48434
10 T7d 5 ace 5 2.62 96.23 1.15 48915
50 T7d 5 his 6 2.93 95.87 1.20 47967
30 T7d 5 his 6 2.75 96.06 1.19 47182
10 T7d 5 his 6 2.55 96.31 1.13 47395
50 T7d 5 phos 7 3.15 95.64 1.21 53843
144
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein time temp buffer pH % % % Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/mi)
30 T7d 5 phos 7 3.10 95.76 1.14 53372
T7d 5 phos 7 2.91 95.96 1.13 53269
50 T7d 5 ace-suc-tw 5 2.75 96.13 1.12 50236
30 T7d 5 ace-suc-tw 5 2.62 96.11 1.27 50026
10 T7d 5 ace-suc-tw 5 2.56 96.18 1.26 49290
50 T7d 5 his-suc-tw 6 2.84 96.10 1.07 50129
30 T7d 5 his-suc-tw 6 2.58 96.19 1.23 49272
10 T7d 5 his-suc-tw 6 2.64 96.08 1.28 50926
50 T7d 5 PBS 7 3.26 95.59 1.15 49502
30 T7d 5 PBS 7 3.07 95.64 1.29 49724
10 T7d 5 PBS 7 2.83 95.87 1.29 49563
50 T21d 5 ace 5 2.57 95.76 1.67 49722
30 T21d 5 ace 5 2.37 96.03 1.60 48882
10 T21d 5 ace 5 2.22 96.09 1.69 49255
50 T21d 5 his 6 2.63 95.63 1.74 44884
30 T21d 5 his 6 2.42 95.95 1.62 47510
10 T21d 5 his 6 2.19 96.08 1.73 47015
50 T21d 5 phos 7 3.06 94.96 1.98 53449
30 T21d 5 phos 7 2.69 95.46 1.85 52938
10 T21d 5 phos 7 2.35 95.84 1.81 52703
50 T21d 5 ace-suc-tw 5 2.25 95.76 1.99 50960
30 T21d 5 ace-suc-tw 5 2.08 95.90 2.02 49042
10 T21d 5 ace-suc-tw 5 1.97 95.84 2.19 49851
50 T21d 5 his-suc-tw 6 2.24 95.62 2.14 49983
30 T21d 5 his-suc-tw 6 2.09 95.86 2.05 48813
10 T21d 5 his-suc-tw 6 1.97 95.83 2.19 49984
50 T21d 5 PBS 7 2.84 95.07 2.09 50641
30 T21d 5 PBS 7 2.27 95.62 2.12 48441
10 T21d 5 PBS 7 1.99 95.94 2.07 48978
50 TlOmo 5 his 6 8.05 91.04 0.91 45552
30 TlOmo 5 his 6 5.81 93.29 0.90 46607
10 TlOmo 5 his 6 3.62 95.46 0.92 46207
50 TlOmo 5 his-suc-tw 6 8.08 90.26 1.67 45430
30 TlOmo 5 his-suc-tw 6 5.98 92.43 1.58 42967
10 TlOmo 5 his-suc-tw 6 3.95 94.25 1.80 42567
Table 53. Storage Stability at 5 C, Accelerated Stability at 40 C, and Freeze-
Thaw Stability Of
H1a11.1-SL-Av at Different Concentrations and In Different Buffers and pHs
Protein
Concentration temp %
Total
(mg/mi) time/FT ( C) buffer pH % Aggregate % Monomer
Fragment Area
1 TO --- cit-phos 5 7.07 92.14 0.80
46824
1 T8d 40 cit-phos 5 2.23 96.39 1.38
47090
1 T22d 40 cit-phos 5 7.10 89.62 3.28
47956
1 FT2 --- cit-phos 5 7.91 90.75 1.34
46502
1 FT4 --- cit-phos 5 7.41 92.18 0.41
52181
145
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Protein
Concentration temp %
Total
(mg/mi) time/FT ( C) buffer pH % Aggregate % Monomer
Fragment Area
1 TO cit-phos 6 7.17 92.33 0.50
45809
1 T8d 40 cit-phos 6 2.56 96.03 1.42
46783
1 T22d 40 cit-phos 6 5.79 91.73 2.48
47401
1 FT2 cit-phos 6 7.14 91.48 1.38
45256
1 FT4 cit-phos 6 7.09 92.56 0.34
45004
1 TO cit-phos 7 6.82 92.67 0.51
47025
1 T8d 40 cit-phos 7 2.52 95.95 1.53
48080
1 T22d 40 cit-phos 7 5.52 91.58 2.90
48706
1 FT2 cit-phos 7 7.23 91.52 1.25
46732
1 FT4 cit-phos 7 7.15 92.49 0.36
46561
60 and 118 TO his 6 8.03 91.15 0.82
43528
60 T7d 40 his 6 7.17 91.76 1.07
45333
60 T21d 40 his 6 15.77 82.13 2.10
44729
60 T7d 5 his 6 3.83 95.32 0.86
46774
60 T26d 5 his 6 7.14 92.56 0.30
63982
118 T5mo 5 his 6 12.82 86.65 0.53
55869
60 T5mo 5 his 6 9.46 90.03 0.51
64573
60 FT2 his 6 6.71 92.59 0.70
42259
60 FT4 his 6 6.33 93.62 0.05
41054
Key:
FT = freeze thaw
FT2 = analysis after two cycles of freeze and thaw; freezing at -80 C and
thawing in a
30 C water bath
FT4 = analysis after four cycles of freeze and thaw; freezing at -80 C and
thawing in
a 30 C water bath
cit-phos = 10 mM citrate and 10 mM phosphate
his = 15 mM histidine and 0.02% sodium azide (azide for preventing microbial
growth)
Table 54. Differential Scanning Calorimetry Data of H1a11.1-SL-Av at 1 mg/ml
in 10 mm Citrate +
Mm Phosphate at Different pHs
pH Onset ( C) Tml ( C) Tm2 ( C) Tm3
( C) Tm4 ( C)
5 55 68.2 68.86 75.56 81.18
6 58 69.04 70.47 75.24 82.04
7 59 69.52 70.94 74.44 82.06
Overall, the data in Tables 51 to 54 suggest that h1A11.1-SL-Av has a
degradation
profile typically observed for stable monoclonal antibodies. The aggregation
and
fragmentation is greater at elevated temperatures (40 C) than at lower
temperatures (5 C).
146
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
At 5 C, there is no apparent increase in aggregation or fragmentation after 21
days of
storage. At 40 C, the amount of degradation was higher than those values
typically observed
for stable mAbs. However, it was not so great as to preclude it from further
development.
Overall, this DVD-Ig protein qualified as an AS-DVD-Ig protein based on its
stability.
EXAMPLE 28: Formulation Selection for Anti-DLL4/Anti-VEGF DVD-Ig Protein
Materials and Methods. The stability of anti-DLL4/anti-VEGF DVD-Ig h1A11.1-SL-
Av
protein was evaluated in the five formulations listed in Table 55. All
formulations were
prepared in 15 mM histidine buffer. Formulations F 1 to F4 were prepared at 50
mg/ml
protein concentration. In these formulations, the pH ranged from 5.5 to 6.0,
polysorbate 80
concentration ranged from 0 to 0.05% w/v, sucrose concentration ranged from 0
to 7.5% w/v,
and arginine concentration ranged from 0 to 1% w/v. Formulation F4 was
prepared in 15 mM
histidine buffer at pH 6.0 without any stabilizers and served as a study
control for the 50
mg/ml liquid formulation stability assessment. In addition, one formulation
was prepared at
25 mg/ml protein concentration at pH 6.0 (Formulation F5). In this
formulation, the
polysorbate 80 concentration was 0.025% w/v and sucrose concentration was 3.8%
w/v.
Table 55. Formulation Composition Description
anti-DLL4 / anti-
Polysorbate 80
Formulation VEGF DVD-Ig Sucrose Arginine
Buffer pH (Tween 80) (%
Identifier Concentration (% w/v) (% w/v)
w/v)
(mg/mL)
15 mM
Fl 50 6.0 0.05 7.5 0
Histidine
15 mM
F2 50 5.5 0.05 7.5 0
Histidine
15 mM
F3 50 6.0 0.05 7.5 1
Histidine
15 mM
F4 50 6.0 0 0 0
Histidine
15 mM
F5 25 6.0 0.025 3.8 0
Histidine
In the above formulations, 15 mM histidine buffer was selected because it
provides
adequate buffering capacity to maintain the target formulation pH. Sucrose was
evaluated as
a stabilizer against freeze-thaw stress (cryoprotectant) and lyophilization
process-induced
stress (lyoprotectant). Polysorbate 80 (surfactant) and arginine were added to
potentially
stabilize the formulation against aggregates and particulates formation.
147
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Freeze-thaw and Liquid Formulation Stability Testing. The stability of all
liquid
formulations was evaluated after three cycles of freeze/thaw (F/T) stress, and
after 1 month
storage at -80, 5, 25 and 40 C. Stability was tested by a broad panel of
analytical assays
including Visual appearance, % Aggregates by Size Exclusion Chromatography (SE-
HPLC),
Charge heterogeneity by Cation Exchange Chromatography (CEX-HPLC),
Fragmentation by
reduced SDS-Capillary Electrophoresis (CE-SDS), Sub-visible particles by Micro
Flow
Imaging (MFI) and DLL4/ VEGF binding potency using ELISA.
The freeze/thaw and liquid stability testing results are provided in Table 56.
Table 56. Freeze-Thaw and Liquid Formulation Stability Results
Binding
Sub-visible Particles by
% CEX-HPLC
Potency by
MFI
Formulation Time ________________ Visual Aggregates % ELISA
Identifier Points Appearance by SEC- % % % Purity .._ 2 >
10 > 25
HPLC Acidic Main Basic
DLL4 VEGF
m/mL m/mL m/mL
region peak region
TO EFVP 1.0 21.6 61.7 16.7 97.7 3333 5
0 93 113
3FT EFVP 1.1 21.5 61.8 16.6 97.7 2388 50 5 NP NP
1M at - EFVP 1364 15 5
1.1 21.0 62.1 16.8 97.8 NP
NP
80 C
1M at EFVP 1064 0 0
Fl 2.0 21.2 62.4 16.3 97.8 NP NP
C
1M at EFVP 1559 5 5
4.3 23.5 62.2 14.2 97.4 NP
NP
25 C
1M at EFVP 1219 15 0
7.2 35.8 43.0 21.2 95.0 94
104
40 C
TO EFVP 1.3 21.4 61.8 16.8 97.5 1589 15
0 93 113
3FT EFVP 1.3 21.4 61.8 16.9 97.6 435 5 5 NP NP
1M at - EFVP 315 0 0
1.4 21.0 62.0 17.0 97.8 NP
NP
80 C
1M at EFVP 230 5 5
F2 2.1 20.9 62.3 16.8 97.8 NP NP
5 C
1M at EFVP 1149 5 0
4.4 22.8 61.6 15.6 97.4 NP
NP
25 C
1M at EFVP 655 5 0
7.9 33.8 40.9 25.3 95.1 95
97
40 C
TO EFVP 1.1 21.5 61.7 16.7 97.6 784 0 0
93 113
3FT EFVP 1.1 21.3 61.8 16.9 97.5 490 10 0 NP NP
1M at - EFVP 250 0 0
1.1 21.0 61.9 17.1 97.9 NP
NP
80 C
1M at EFVP 225 5 0
F3 2.0 20.8 62.0 17.2 97.8 NP NP
5 C
1M at EFVP 834 10 0
4.9 21.5 58.3 20.2 97.3 NP
NP
25 C
1M at EFVP 1464 5 0
11.3 32.1 42.3 25.6 95.0 97
101
40 C
TO TMTC 1.2 21.6 61.8 16.6 97.4 23707 370
5 93 113
F4 3FT TMTC 1.5 21.4 61.8 16.8 97.4 105467
5906 30 NP NP
___________ 1M at - TMTC 1.2 21.1 62.0 16.9
97.8 42024 1329 60 NP NP
148
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Binding
Sub-visible Particles by
CEX-HPLC MFI
Potency by
Formulation Time ___________________________________________ Visual Aggregates
ELISA
Identifier Points Appearance by SEC- % % % Purity 2 > 10
>25
HPLC Acidic Main Basic
DLL4 VEGF
m/mL m/mL m/mL
region peak region
80 C
1M at EFVP 1189 0 0
2.0 21.3 62.3 16.5 97.8 NP NP
C
1M at EFVP 89299 3053 165
4.5 23.5 61.8 14.7 97.2 NP NP
25'C
1M at EFVP 61754 5051 670
7.7 34.9 44.2 20.9 94.8 101 97
40 C
TO EFVP 0.9 21.6 61.6 16.7 97.7 2808 5 5
93 113
3FT EFVP 1.2 21.5 61.8 16.8 97.6 1949 0 0 NP NP
1M at - EFVP 270 5 0
1.1 21.0 62.2 16.8 97.8 NP NP
80 C
1M at EFVP 709 10 0
F5 1.5 21.2 61.9 16.8 97.8 NP
NP
5 C
1M at EFVP 944 15 0
2.6 23.5 62.0 14.6 97.2 NP NP
25'C
1M at EFVP 974 20 0
3.9 37.0 44.4 18.6 95.0 92 95
40 C
Key. EFVP: Essentially Free of Visible Particles, TMTC: Too Many To Count, NP:
Not
Performed
As seen in the above table, Freeze-thaw stress resulted in the formation of
visible
particles and significantly higher sub-visible particle counts in Formulation
F4 that was
formulated without any stabilizers (e.g., polysorbate 80, sucrose, and
arginine). Relative to
the other formulations, this formulation also showed a trend of higher sub-
visible particle
counts after 1 month storage at 25 and 40 C. Formulation F5 with 25 mg/mL
protein
concentration showed significantly lower aggregation relative to the 50 mg/mL
formulations
over 1 month storage at 5, 25 and 40 C.
Lyophilized Formulation Stability Testing. The stability of select
formulations was also
evaluated after the formulations were lyophilized. The lyophilized drug
product stability was
assessed for all sucrose-containing formulations (F1, F2, F3, and F5).
Stability was assessed
after 2 weeks storage at 55 C. Stability was tested by a broad panel of
analytical assays
including Visual appearance (before and after reconstitution), Reconstitution
time, %
Aggregates by Size Exclusion Chromatography (SE-HPLC), Charge heterogeneity by
Cation
Exchange Chromatography (CEX-HPLC), Fragmentation by reduced SDS-Capillary
Electrophoresis (CE-SDS), Sub-visible particles by Micro Flow Imaging (MFI),
and Water
Content by Karl Fischer titration.
149
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
The lyophilized formulation stability testing results are provided in Table
57.
Reconstitution time for all evaluated formulations was approximately 1 minute.
A slight
increase in aggregation by SEC and % basic region by CEX was observed for all
formulations under the stressed storage condition of 55 C. Minimal changes
were observed in
all other measured product stability attributes.
Table 57. Lyophilized Formulation Stability Results
Visual CEX-HPLC % Sub-visible
Particles
Formulati Appearance Purity by MFI
__________________________ Aggreg _____________
on Time by R > 2 > 10 > 25
Before After by SEC-
Identifier HPLC Acidic Main Basic CE- gm/ gin! pm/
Recon Recon
region peak region SDS nil, miõ nil,
White
to off-
TO EFVP 1.1 21.3 61.9 16.8 97.6 749 20
10
white
Fl cake
2 White
week to off-
EFVP 1.6 20.6 58.1 21.3 97.5 1639
15 0
s at white
55 C cake
White
to off-
TO EFVP 1.3 21.2 61.8 17.0 97.6 1254
15 0
white
F2 cake
2 White
week to off-
EFVP 2.0 20.4 57.8 21.8 97.6 1609
10 0
s at white
55 C cake
White
to off-
TO EFVP 1.1 21.2 61.9 16.9 97.6 719 5
0
white
F3 cake
2 White
week to off-
EFVP 1.4 20.8 59.5 19.8 97.5 475 10
5
s at white
55 C cake
White
to off-
TO EFVP 1.0 21.3 61.8 16.9 97.5 844 35
5
white
F5 cake
2 White
week to off-
EFVP 1.5 20.5 58.0 21.5 97.7 270 5
0
s at white
_______ 55 C cake
Key. EFVP = Essentially Free of Visible Particles; NP = Not Performed
The sequence of the anti-DLL4 / anti-VEGF DVD-Ig protein H1A11.1-SL-Av is set
forth in Table 58 (DVD-Ig protein described in Examples 28 and 29).
150
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Table 58: Full Length Sequence For DVD h1A11.1-SL-Av
N Sequence
ame
123456789012345678901234567890123456789012345678901234567890
DIQMTQSPSSLSASVGDRVTITCRASEDIYSNLAWYQQKPGKAPKLLIYDTNNLADGVPS
h1A11.1-
RFSGSGSGTDFTLTISSLQPEDFATYYCQQYNNYPPTFGQGTKLEIKRTVAAPSVFIFPP
SL-Av DIQMTQSPSSLSASVGDRVTITCSASQDISNYLNWYQQKPGKAPKVLIYFTSSLHSGVPS
RFSGSGSGTDFTLTISSLQPEDFATYYCQQYSTVPWTFGQGTKVEIKRTVAAPSVFIFPP
light
SDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT
chain LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC (SEQ ID NO: 28)
h1A11.1-
EVQLVESGGGLVQPGGSLRLSCAASGFTFSNFPMAWVRQAPGKGLEWVATISSSDGTTYY
SL-Av RDSVKGRFTISRDNAKNSLYLQMNSLRAEDTAVYYCARGYYNSPFAYWGQGTLVTVSSAS
TKGPEVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNWVRQAPGKGLEWVGWINTYTG
heavy
EPTYAADFKRRFTFSLDTSKSTAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWGQG
chain TLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTF
PAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCP
APEAAGGPSVFLEPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTK
PREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYT
LPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKL
TVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK (SEQ ID NO: 29)
Table 58 provides the full-length heavy and light chain sequences for DVD
h1A11.1-SL-Av.
Linker sequences are underlined, while CDRs and constant region sequences are
in bold.
EXAMPLE 29: Extended Preformulation Characterization of Second Anti-DLL4/Anti-
VEGF DVD-Ig Protein (h1A11.1-LS-Av)
Extended preformulation characterization on anti-DLL4/-antiVEGF DVD-Ig
proteins
was performed to explore how different formulations conditions impact the
stability of the
DVD-Ig proteins. Data for h1A11.1-LS-Av is presented in Tables 59 and 60. The
storage
stability (5 C) and accelerated stability (40 C) of the DVD-Ig protein was
evaluated in the
formulations and protein concentrations listed below. Stability was evaluated
by SEC and %
aggregate, % monomer, % fragment, and total species recovered were
quantitated. Overall,
the formulations cover a pH range of 5 to 7 and a protein concentration range
of 10 to 50
mg/ml.
At 5 C and 40 C temperatures and at concentrations of 50, 30, and 10 mg/ml the
following formulations were evaluated: 15 mM acetate pH 5, 15 mM histidine pH
6, 15 mM
phosphate pH 7, 30 mM acetate, 80 mg/ml sucrose, 0.02% Tween 80 at pH 5, 30 mM
151
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
histidine, 80 mg/ml sucrose, 0.02% Tween 80 at pH 6, and PBS (phosphate
buffered saline).
All formulations contained 0.02% sodium azide to prevent microbial growth
during storage.
Table 59. Accelerated Stability at 40 C of h1A11.1-LS-Av
Protein
Concentration temp % % % Total
(mg/mi) Time ( C) buffer pH
Aggregate Monomer Fragment Area
dialysis pre-
0.21 98.42 1.36 56054
50, 30, 10 TO ace 5 0.28 98.41 1.31 56381
50, 30, 10 TO his 6 0.46 98.23 1.31 54316
50, 30, 10 TO phos 7 0.74 97.86 1.40 53212
50, 30, 10 TO ace-suc-tw 5 0.24 98.16 1.60 56244
50, 30, 10 TO his-suc-tw 6 0.30 98.11 1.59 54076
50, 30, 10 TO PBS 7 0.52 98.05 1.43 50085
50 T7d 40 ace 5 1.63 96.74 1.63 55563
30 T7d 40 ace 5 1.13 97.24 1.62 55194
T7d 40 ace 5 0.84 97.49 1.67 55029
50 T7d 40 his 6 2.00 96.62 1.38 53566
30 T7d 40 his 6 1.17 97.46 1.38 52443
10 T7d 40 his 6 0.60 98.00 1.40 53812
50 T7d 40 phos 7 4.31 94.02 1.67 52934
30 T7d 40 phos 7 2.85 95.46 1.69 52663
10 T7d 40 phos 7 1.20 97.11 1.69 52411
50 T7d 40 ace-suc-tw 5 1.10 96.23 2.66 54837
30 T7d 40 ace-suc-tw 5 0.77 96.40 2.83 52474
10 T7d 40 ace-suc-tw 5 0.43 96.39 3.17 50855
50 T7d 40 his-suc-tw 6 1.69 96.27 2.05 53017
30 T7d 40 his-suc-tw 6 1.14 96.84 2.02 52153
10 T7d 40 his-suc-tw 6 0.59 97.30 2.11 52208
50 T7d 40 PBS 7 2.77 95.30 1.93 51623
30 T7d 40 PBS 7 1.73 96.28 1.99 49973
10 T7d 40 PBS 7 0.78 97.25 1.97 50851
50 T21d 40 ace 5 3.66 94.30 2.04 55920
30 T21d 40 ace 5 2.56 95.33 2.10 54188
10 T21d 40 ace 5 1.85 96.00 2.15 55213
50 T21d 40 his 6 4.14 94.28 1.58 54807
30 T21d 40 his 6 2.67 95.79 1.54 53071
10 T21d 40 his 6 1.59 96.82 1.58 54053
50 T21d 40 phos 7 8.52 89.32 2.16 53273
30 T21d 40 phos 7 5.58 92.54 1.89 53162
10 T21d 40 phos 7 3.01 94.89 2.10 52747
50 T21d 40 ace-suc-tw 5 4.12 93.78 2.10 56278
30 T21d 40 ace-suc-tw 5 2.93 94.94 2.13 55481
10 T21d 40 ace-suc-tw 5 1.99 95.75 2.26 54696
50 T21d 40 his-suc-tw 6 4.94 93.21 1.85 54034
30 T21d 40 his-suc-tw 6 n/a n/a n/a n/a
10 T21d 40 his-suc-tw 6 2.00 96.30 1.70 52686
50 T21d 40 PBS 7 8.44 89.65 1.90 51697
152
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein
Concentration temp % % % Total
(mg/ml) Time ( C) buffer pH
Aggregate Monomer Fragment Area
30 T21d 40 PBS 7 5.54 92.43 2.03
50282
T21d 40 PBS 7 2.89 95.05 2.06 51580
Buffer key (all buffers contain 0.02% sodium azide to prevent microbial
growth): ace = 15 mNI acetate
pH 5; his = 15 mNI histidine pH 6; phos = 15 mNI phosphate pH 7; ace-suc-tw =
30 mNI acetate, 80
mg/ml sucrose, 0.02% Tween80; his-suc-tw = 30 mNI histidine, 80 mg/ml sucrose,
0.02% Tween80;
PBS = phosphate buffered saline
Table 60. Storage Stability At 5 C of h1A11.1-LS-Av
Protein
Concentration temp % % Total
(mg/ml) time ( C) buffer pH Aggregate
% Monomer Fragment Area
pre-
0.21 98.42 1.36 56054
dialysis
50, 30, 10 TO ace 5 0.28 98.41 1.31
56381
50, 30, 10 TO his 6 0.46 98.23 1.31
54316
50, 30, 10 TO phos 7 0.74 97.86 1.40
53212
50, 30, 10 TO
ace-suc-
0.24 98.16 1.60 56244
tw 5
50, 30, 10 TO his-suc-tw 6 0.30 98.11
1.59 54076
50, 30, 10 TO PBS 7 0.52 98.05 1.43
50085
50 T7d 5 ace 5 0.18 98.17 1.64 57599
30 T7d 5 ace 5 0.16 98.21 1.64 55889
10 T7d 5 ace 5 0.13 98.17 1.70 53289
50 T7d 5 his 6 0.18 98.14 1.68 55742
30 T7d 5 his 6 0.12 98.06 1.82 53603
10 T7d 5 his 6 0.13 98.07 1.80 53505
50 T7d 5 phos 7 0.23 97.72 2.05 54355
30 T7d 5 phos 7 0.18 97.77 2.04 53561
10 T7d 5 phos 7 0.13 97.72 2.15 53151
50 T7d
ace-suc-
0.09 97.40 2.51 57158
5 tw 5
30 T7d
ace-suc-
0.08 97.43 2.49 55025
5 tw 5
10 T7d
ace-suc-
0.08 97.34 2.58 53882
5 tw 5
50 T7d 5 his-suc-tw 6 0.10 97.48 2.43
55272
30 T7d 5 his-suc-tw 6 0.08 97.63 2.29
52763
10 T7d 5 his-suc-tw 6 0.05 97.41 2.53
52903
50 T7d 5 PBS 7 0.12 97.31 2.58 51698
30 T7d 5 PBS 7 0.09 97.24 2.67 50144
10 T7d 5 PBS 7 0.08 97.28 2.64 50428
50 T21d 5 ace 5 0.87 98.45 0.68 57706
30 T21d 5 ace 5 0.80 98.55 0.65 56566
10 T21d 5 ace 5 0.83 98.47 0.70 54226
50 T21d 5 his 6 1.05 98.29 0.66 55911
30 T21d 5 his 6 0.92 98.40 0.68 54225
153
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Protein
Concentration temp % % Total
(mg/mi) time ( C) buffer pH Aggregate %
Monomer Fragment Area
T21d 5 his 6 0.90 98.41 0.70 54128
50 T21d 5 phos 7 1.25 98.09 0.66 54980
30 T21d 5 phos 7 1.20 98.11 0.69 53903
10 T21d 5 phos 7 1.01 98.29 0.69 53271
ace-suc-
0.92 98.36 0.72 61574
50 T21d 5 tw 5
ace-suc-
0.89 98.39 0.72 55532
30 T21d 5 tw 5
ace-suc-
0.83 98.46 0.71 55841
10 T21d 5 tw 5
50 T21d 5 his-suc-tw 6 1.00 98.27 0.73 55484
30 T21d 5 his-suc-tw 6 0.92 98.37 0.70 53335
10 T21d 5 his-suc-tw 6 0.82 98.49 0.69 53736
50 T21d 5 PBS 7 1.49 97.79 0.71 52405
30 T21d 5 PBS 7 1.29 98.02 0.70 51284
10 T21d 5 PBS 7 1.12 98.18 0.70 51377
The buffer key for Table 59 is the same as in Table 60.
Example 30: Formulation Studies of Additional DLL4-VEGF DVD-Ig Proteins
h1A11.1.A6-LS-Av and h1A11.1.A6-SL-Av
Extended preformulation characterization on additional DLL4/VEGF DVD-Ig
proteins was performed to explore how different formulations conditions impact
the stability.
Data for h1A11.1.A6-LS-Av and h1A11.1.A6-SL-Av DLL4/VEGF DVD-Ig proteins are
presented below. These DVD-Ig proteins proved to be unstable and failed the
screening
criteria to be considered AS-DVD-Ig proteins.
The storage stability (5 C) and accelerated stability (40 C) of h1A11.1.A6-LS-
Av and
h1A11.1.A6-SL-Av were evaluated in the formulations and protein concentrations
listed
below. Stability was evaluated by SEC and % aggregate, % monomer, % fragment,
and total
species recovered were quantitated. Overall, the formulations cover a pH range
of 5 to 7 and
a protein concentration range of 10 to 50 mg/ml.
At 5 C and 40 C temperatures and at 50, 30, and 10 mg/ml, the following
conditions
were tested: 15 mM acetate pH 5; 15 mM histidine pH 6; 15 mM phosphate pH 7;
30 mM
acetate, 80 mg/ml sucrose, 0.02% Tween 80 at pH 5; 30 mM histidine, 80 mg/ml
sucrose,
154
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
0.02% Tween 80 at pH 6; and PBS (phosphate buffered saline). All formulations
contained
0.02% sodium azide to prevent microbial growth during storage
Overall, the data provided in Tables 61-63 suggests the two DVD-Ig proteins
have an
atypical degradation profile not observed for stable monoclonal antibodies.
The aggregation
rate was actually greater at 5 C than at 40 C.
At 5 C, there is a rapid increase in aggregation after 21 days of storage. At
40 C, the
amount of degradation also increases, but not at the rate observed at 5 C. In
both cases, the
aggregation is concentration dependent.
Overall, these DVD-Ig proteins failed the screen based on their 5 C
instability and are
examples of non-AS-DVD-Ig proteins.
Table 61. Accelerated Stability at 40 C of H1a11.1.A6-LS-Av at Different
Concentrations
and In Different Buffers, Excipients, and pHs
Protein Time temp buffer pH % % %
Total Area
Concentration ( C) Aggregate Monomer Fragment
(mg/ml)
--- pre- --- --- --- 0.66 98.64 0.70 48425
dialysis
50, 30, 10 TO --- ace 5 1.24 98.05 0.72
50944
50, 30, 10 TO --- his 6 1.49 97.74 0.76
46462
50, 30, 10 TO --- phos 7 1.76 97.67 0.58
43322
50, 30, 10 TO --- ace-suc- 5 1.66 97.69 0.65
57038
tw
50, 30, 10 TO --- his-suc- 6 1.62 97.87
0.52 54299
tw
50, 30, 10 TO --- PBS 7 2.22 97.25 0.53
48116
50 T7d 40 ace 5 4.40 94.52 1.08 43246
30 T7d 40 ace 5 2.76 96.17 1.08 46266
T7d 40 ace 5 1.54 97.41 1.05 52178
50 T7d 40 his 6 5.11 94.02 0.87 50471
30 T7d 40 his 6 3.17 95.97 0.87 40875
10 T7d 40 his 6 1.60 97.52 0.88 48759
50 T7d 40 phos 7 12.24 86.69 1.07 44327
30 T7d 40 phos 7 7.81 91.09 1.11 42284
10 T7d 40 phos 7 3.46 95.42 1.12 44023
50 T7d 40 ace-suc- 5 5.46 93.48 1.07 63100
tw
30 T7d 40 ace-suc- 5 3.42 95.55 1.03 59243
tw
10 T7d 40 ace-suc- 5 1.83 97.03 1.14 60361
tw
50 T7d 40 his-suc- 6 4.90 94.14 0.96
56706
tw
155
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein Time temp buffer pH % % %
Total Area
Concentration ( C) Aggregate
Monomer Fragment
(mg/ml)
30 T7d 40 his-suc- 6 3.65 95.50 0.84
53538
tw
T7d 40 his-suc- 6 1.78 97.31 0.91 55447
tw
50 T7d 40 PBS 7 14.78 84.08 1.14 58511
30 T7d 40 PBS 7 9.20 89.56 1.24 46917
10 T7d 40 PBS 7 4.08 94.73 1.19 49978
50 T21d 40 ace 5 5.58 92.75 1.67 53248
30 T21d 40 ace 5 3.59 94.67 1.74 51038
10 T21d 40 ace 5 1.90 96.38 1.72 50617
50 T21d 40 his 6 6.25 92.37 1.37 46908
30 T21d 40 his 6 3.82 94.77 1.41 46075
10 T21d 40 his 6 1.93 96.61 1.47 47827
50 T21d 40 phos 7 13.12 84.98 1.90 40311
30 T21d 40 phos 7 9.11 88.98 1.91 42037
10 T21d 40 phos 7 4.05 93.96 1.99 43009
50 T21d 40 ace-suc- 5 6.45 91.73 1.82
56752
tw
30 T21d 40 ace-suc- 5 4.21 93.92 1.87
56330
tw
10 T21d 40 ace-suc- 5 2.11 95.88 2.00
57757
tw
50 T21d 40 his-suc- 6 6.69 91.83 1.49
54091
tw
30 T21d 40 his-suc- 6 4.22 94.30 1.48
52932
tw
10 T21d 40 his-suc- 6 2.09 96.45 1.47
54118
tw
50 T21d 40 PBS 7 15.74 82.23 2.03 52080
30 T21d 40 PBS 7 9.98 87.82 2.19 47188
10 T21d 40 PBS 7 4.71 93.04 2.25 47400
Buffer key (all buffers contain 0.02% sodium azide to prevent microbial
growth):
ace = 15 mM acetate pH 5; his = 15 mM histidine pH 6; phos = 15 mM phosphate
pH
7
ace-suc-tw = 30 mM acetate, 80 mg/ml sucrose, 0.02% Tw80
his-suc-tw = 30 mM histidine, 80 mg/ml sucrose, 0.02% Tw80
PBS = phosphate buffered saline
Table 62. Storage Stability at 5 C of H1a11.1.A6-LS-Av at Different
Concentrations and In
Different Buffers, Excipients, and pHs (Buffer Key Same as In Table 63)
Protein Time temp buffer pH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/ml)
--- pre- --- --- --- 0.66 98.64 0.70 48425
dialysis
156
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Protein Time temp buffer pH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/ml)
50, 30, 10 TO --- ace 5 1.24 98.05 0.72
50944
50, 30, 10 TO --- his 6 1.49 97.74 0.76
46462
50, 30, 10 TO --- phos 7 1.76 97.67 0.58
43322
50, 30, 10 TO --- ace-suc- 5 1.66 97.69 0.65
57038
tw
50, 30, 10 TO --- his-suc-tw 6 1.62 97.87
0.52 54299
50, 30, 10 TO --- PBS 7 2.22 97.25 0.53
48116
50 T7d 5 ace 5 4.68 94.74 0.58 54446
30 T7d 5 ace 5 2.77 96.62 0.62 51286
T7d 5 ace 5 1.75 97.62 0.63 51444
50 T7d 5 his 6 7.46 91.88 0.66 46683
30 T7d 5 his 6 4.83 94.49 0.68 46282
10 T7d 5 his 6 2.36 96.95 0.69 47844
50 T7d 5 phos 7 1.84 97.46 0.71 34008
30 T7d 5 phos 7 1.89 97.48 0.63 36992
10 T7d 5 phos 7 2.05 97.30 0.65 41962
50 T7d 5 ace-suc- 5 4.74 94.65 0.61 57937
tw
30 T7d 5 ace-suc- 5 N/A N/A N/A N/A
tw
10 T7d 5 ace-suc- 5 1.95 97.41 0.64 58618
tw
50 T7d 5 his-suc-tw 6 7.97 91.45 0.59
54735
30 T7d 5 his-suc-tw 6 4.85 94.49 0.66
53379
10 T7d 5 his-suc-tw 6 2.34 97.01 0.65
54187
50 T7d 5 PBS 7 5.68 93.65 0.67 46544
30 T7d 5 PBS 7 5.20 94.13 0.67 45219
10 T7d 5 PBS 7 3.56 95.76 0.68 47653
50 T21d 5 ace 5 9.33 89.97 0.70 52020
30 T21d 5 ace 5 4.25 95.04 0.70 51223
10 T21d 5 ace 5 1.95 97.36 0.69 50950
50 T21d 5 his 6 19.35 79.71 0.94 44105
30 T21d 5 his 6 9.91 89.29 0.80 46096
10 T21d 5 his 6 3.11 96.15 0.73 47777
50 T21d 5 phos 7 1.65 97.49 0.86 25059
30 T21d 5 phos 7 1.49 97.75 0.76 29723
10 T21d 5 phos 7 2.00 97.09 0.90 38517
50 T21d 5 ace-suc- 5 9.41 89.79 0.79
56438
tw
30 T21d 5 ace-suc- 5 4.72 94.50 0.79
56230
tw
10 T21d 5 ace-suc- 5 2.13 97.01 0.86
58579
tw
50 T21d 5 his-suc-tw 6 20.57 78.32 1.11
53114
30 T21d 5 his-suc-tw 6 10.17 88.99 0.85
53155
10 T21d 5 his-suc-tw 6 3.20 95.94 0.86
54028
50 T21d 5 PBS 7 5.65 93.38 0.98 34294
30 T21d 5 PBS 7 2.99 96.04 0.96 36457
157
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein Time temp buffer pH % % %
Total
Concentration ( C)
Aggregate Monomer Fragment Area
(mg/ml)
T21d 5 PBS 7 5.14 93.95 0.91 46566
Table 63. Accelerated Stability at 40 C of Hlal 1.1.A6-SL-Av at Different
Concentrations
and In Different Buffers, Excipients, and pHs
Protein time temp ( C) buffer pH % % %
Total
Concentration
Aggregate Monomer Fragment Area
(mg/ml)
--- pre-dialysis --- --- --- 1.19 97.75 1.06
58988
50, 30, 10 TO --- ace 5 1.23 97.85 0.92
47138
50, 30, 10 TO --- his 6 1.30 97.84 0.87
44711
3.4 TO --- phos 7 0.86 98.00 1.14
57373
50, 30, 10 TO --- ace-suc- 5 1.30 97.85
0.85 52129
tw
50, 30, 10 TO --- his-suc- 6 1.28 97.85
0.87 47563
tw
6.6 TO --- PBS 7 1.35 97.76 0.89
71146
50 T7d 40 ace 5 7.66 90.88 1.46
48331
30 T7d 40 ace 5 4.05 94.43 1.52
45731
10 T7d 40 ace 5 1.45 97.05 1.51
47455
50 T7d 40 his 6 8.68 90.23 1.09
45897
30 T7d 40 his 6 4.74 94.15 1.11
44807
10 T7d 40 his 6 1.62 97.07 1.31
45567
3.4 T7d 40 phos 7 2.04 96.51 1.45
56002
50 T7d 40 ace-suc- 5 9.10 89.27 1.62
52696
tw
30 T7d 40 ace-suc- 5 5.23 93.14 1.64
50591
tw
10 T7d 40 ace-suc- 5 1.91 96.09 2.00
51790
tw
50 T7d 40 his-suc- 6 8.55 90.18 1.27
48458
tw
30 T7d 40 his-suc- 6 4.82 93.19 1.99
46963
tw
10 T7d 40 his-suc- 6 1.78 96.78 1.45
46676
tw
6.6 T7d 40 PBS 7 4.53 93.83 1.64
70277
50 T21d 40 ace 5 8.27 89.20 2.53
47139
30 T21d 40 ace 5 4.41 93.03 2.56
45779
10 T21d 40 ace 5 1.60 95.68 2.72
46794
50 T21d 40 his 6 9.26 88.56 2.18
44423
30 T21d 40 his 6 5.19 92.86 1.96
43874
10 T21d 40 his 6 1.74 95.71 2.55
45249
3.4 T21d 40 phos 7 2.43 95.14 2.42
54476
50 T21d 40 ace-suc- 5 9.45 87.66 2.89
51846
tw
30 T21d 40 ace-suc- 5 5.35 91.28 3.37
50456
tw
158
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Protein time temp ( C) buffer pH % % %
Total
Concentration
Aggregate Monomer Fragment Area
(mg/ml)
T21d 40 ace-suc- 5 1.94 94.29 3.78 50414
tw
50 T21d 40 his-suc- 6 8.72 89.02
2.26 46410
tw
30 T21d 40 his-suc- 6 5.08 92.63
2.29 43210
tw
10 T21d 40 his-suc- 6 1.95 95.68
2.37 46539
tw
6.6 T21d 40 PBS 7 4.71 92.43 2.86
68811
Buffer key (all buffers contain 0.02% sodium azide to prevent microbial
growth):
ace = 15 mM acetate pH 5; his = 15 mM histidine pH 6; phos = 15 mM phosphate
pH
7
ace-suc-tw = 30 mM acetate, 80 mg/ml sucrose, 0.02% Tw80
his-suc-tw = 30 mM histidine, 80 mg/ml sucrose, 0.02% Tw80
PBS = phosphate buffered saline
Table 64. Storage Stability at 5 C of H1a11.1.A6-SL-Av at Different
Concentrations and In
Different Buffers, Excipients, and pHs
Protein Time temp buffer pH % % %
Total Area
Concentration ( C) Aggregate Monomer Fragment
(mg/ml)
--- pre- --- --- --- 1.19 97.75 1.06 58988
dialysis
50, 30, 10 TO --- ace 5 1.23 97.85 0.92
47138
50, 30, 10 TO --- his 6 1.30 97.84 0.87
44711
3.4 TO --- phos 7 0.86 98.00 1.14 57373
50, 30, 10 TO --- ace-suc- 5 1.30 97.85
0.85 52129
tw
50, 30, 10 TO --- his-suc-tw 6 1.28 97.85
0.87 47563
6.6 TO --- PBS 7 1.35 97.76 0.89 71146
50 T7d 5 ace 5 3.52 95.40 1.08 46507
30 T7d 5 ace 5 2.35 96.55 1.10 40267
10 T7d 5 ace 5 1.46 97.48 1.07 47761
50 T7d 5 his 6 3.48 95.24 1.27 46306
30 T7d 5 his 6 3.63 95.34 1.03 46581
10 T7d 5 his 6 2.01 97.01 0.98 45843
3.4 T7d 5 phos 7 1.42 97.41 1.17 52150
50 T7d 5 ace-suc- 5 3.97 94.99 1.04 53439
tw
30 T7d 5 ace-suc- 5 2.65 96.35 1.00 52119
tw
10 T7d 5 ace-suc- 5 1.60 97.23 1.17 52681
tw
159
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
Protein Time temp buffer pH % % %
Total Area
Concentration ( C) Aggregate Monomer Fragment
(mg/ml)
50 T7d 5 his-suc-tw 6 4.81 94.10 1.09 48136
30 T7d 5 his-suc-tw 6 3.38 95.49 1.13 48266
T7d 5 his-suc-tw 6 1.88 96.90 1.22 47966
6.6 T7d 5 PBS 7 2.25 96.78 0.97 68563
50 T21d 5 ace 5 7.82 92.06 0.11 47402
30 T21d 5 ace 5 4.22 95.68 0.10 45736
10 T21d 5 ace 5 1.77 98.13 0.09 47900
50 T21d 5 his 6 7.36 92.48 0.17 47111
30 T21d 5 his 6 6.51 93.34 0.15 43911
10 T21d 5 his 6 2.92 96.98 0.10 45446
3.4 T21d 5 phos 7 0.05 99.53 0.42 45856
50 T21d 5 ace-suc- 5 9.13 90.73 0.14 52424
tw
30 T21d 5 ace-suc- 5 4.79 95.07 0.15 51575
tw
10 T21d 5 ace-suc- 5 1.98 97.87 0.15 51870
tw
50 T21d 5 his-suc-tw 6 9.50 90.30 0.20
46588
30 T21d 5 his-suc-tw 6 6.20 93.64 0.16
46023
10 T21d 5 his-suc-tw 6 2.61 97.27 0.12
47285
6.6 T21d 5 PBS 7 3.12 96.76 0.13 62856
Buffer key (all buffers contain 0.02% sodium azide to prevent microbial
growth):
ace = 15 mM acetate pH 5; his = 15 mM histidine pH 6; phos = 15 mM phosphate
pH
7
ace-suc-tw = 30 mM acetate, 80 mg/ml sucrose, 0.02% Tw80
his-suc-tw = 30 mM histidine, 80 mg/ml sucrose, 0.02% Tw80
PBS = phosphate buffered saline
Overall, the data in Tables 61 to 64 suggest the two DVD-Ig proteins have an
atypical
degradation profile not observed for stable monoclonal antibodies. The
aggregation rate is
actually greater at 5 C than at 40 C. At 5 C, there is a rapid increase in
aggregation after 21
days of storage. At 40 C, the amount of degradation also increases, but not at
the rate
observed at 5 C. In both cases, the aggregation is concentration dependent.
Overall, these
DVD-Ig proteins did not qualify as AS-DVD-Ig proteins based on their 5 C
instability and
would be considered non-aqueous stable.
Sequences
Amino acid sequences of the heavy and light chains for other DVD-Ig proteins
described herein are provided below in Table 65. Linker sequences are
underlined, while
160
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
CDR sequences are in bold. "L" below represents the light chain, and "H" below
represents
the heavy chain.
Table 65: DVD-Ig protein sequences
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light e e
Chain Name Domain Domain 1234567890123456789012345678901234567890
Name Name
DVD005H ABOO1VH ABOO7VH QVQLQQPGAELVKPGASVKMSCKASGYTFTSYNMHWVKQT
PGRGLEWIGAIYPGNGDTSYNQKFKGKATLTADKSSSTAY
CD20/CD80 MQLSSLTSEDSAVYYCARSTYYGGDWYFNVWGAGTTVTVS
AASTKGPQVQLQESGPGLVKPSETLSLTCAVSGGSISGGY
GWGWIRQPPGKGLEWIGSFYSSSGNTYYNPSLKSQVTIST
DTSKNQFSLKLNSMTAADTAVYYCVRDRLFSVVGMVYNNW
FDVWGPGVLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:30)
DVD005L ABOOlVL ABOO7VL QIVLSQSPAILSPSPGEKVTMTCRASSSVSYIHWFQQKPG
SSPKPWIYATSNLASGVPVRFSGSGSGTSYSLTISRVEAE
CD20/CD80 DAATYYCQQWTSNPPTFGGGTKLEIKRTVAAPESALTQPP
SVSGAPGQKVTISCTGSTSNIGGYDLHWYQQLPGTAPKLL
IYDINKRPSGISDRFSGSKSGTAASLAITGLQTEDEADYY
CQSYDSSLNAQVFGGGTRLTVLG
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSENRGEC(SEQ ID NO:31)
DVD006H ABOO7VH ABOO1VH QVQLQESGPGLVKPSETLSLTCAVSGGSISGGYGWGWIRQ
PPGKGLEWIGSFYSSSGNTYYNPSLKSQVTISTDTSKNQF
CD80/CD20 SLKLNSMTAADTAVYYCVRDRLFSVVGMVYNNWFDVWGPG
VLVTVSSASTKGPQVQLQQPGAELVKPGASVKMSCKASGY
TFTSYNMHWVKQTPGRGLEWIGAIYPGNGDTSYNQKFKGK
ATLTADKSSSTAYMQLSSLTSEDSAVYYCARSTYYGGDWY
FNVWGAGTTVTVSA
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:32)
DVD006L ABOO7VL ABOOlVL ESALTQPPSVSGAPGQKVTISCTGSTSNIGGYDLHWYQQL
PGTAPKLLIYDINKRPSGISDRFSGSKSGTAASLAITGLQ
161
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
CD80/CD20 TEDEADYYCQSYDSSLNAQVFGGGTRLTVLGQPKAAPQIV
LSQSPAILSPSPGEKVTMTCRASSSVSYIHWFQQKPGSSP
KPWIYATSNLASGVPVRFSGSGSGTSYSLTISRVEAEDAA
TYYCQQWTSNPPTFGGGTKLEIKR
QPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVA
WKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKS
HRSYSCQVTHEGSTVEKTVAPTECS(SEQ ID NO:33)
DVD037H AB014VH
ABOO4VH EVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNWVRQA
PGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDTSKSTAY
LQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWGQGTLVT
VEGF/HER2 VSSASTKGPEVQLVESGGGLVQPGGSLRLSCAASGFNIKD
TYIHWVRQAPGKGLEWVARTYPTNGYTRYADSVKGRFTIS
ADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAMDYWG
QGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:34)
DVD037L AB014VL
ABOO4VL DIQMTQSPSSLSASVGDRVTITCSASQDISNYLNWYQQKP
GKAPKVLIYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQP
VEGF/HER2 EDFATYYCQQYSTVPWTFGQGTKVEIKRTVAAPDIQMTQS
PSSLSASVGDRVTITCRASQDVNTAVAWYQQKPGKAPKLL
IYSASFLYSGVPSRFSGSRSGTDFTLTISSLQPEDFATYY
CQQHYTTPPTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:35)
DVD038H ABOO4VH
AB014VH EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQA
PGKGLEWVARIYPTNGYTRYADSVKGRFTISADTSKNTAY
Her2/VEGF LQMNSLRAEDTAVYYCSRWGGDGFYAMDYWGQGTLVTVSS
ASTKGPEVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGM
NWVRQAPGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDT
SKSTAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWG
QGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:36)
DVD038L ABOO4VL
AB014VL DIQMTQSPSSLSASVGDRVTITCRASQDVNTAVAWYQQKP
162
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
GKAPKLLIYSASFLYSGVPSRFSGSRSGTDFTLTISSLQP
Her2/VEGF EDFATYYCQQHYTTPPTFGQGTKVEIKRTVAAPDIQMTQS
PSSLSASVGDRVTITCSASQDISNYLNWYQQKPGKAPKVL
IYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQPEDFATYY
CQQYSTVPWTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:37)
DVD053H AB017VH
AB018VH EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
TNF/RANKL LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
SASTKGPEVQLLESGGGLVQPGGSLRLSCAASGFTFSSYA
MSWVRQAPGKGLEWVSGITGSGGSTYYADSVKGRFTISRD
NSKNTLYLQMNSLRAEDTAVYYCAKDPGTTVIMSWFDPWG
QGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:38)
DVD053L AB017VL
AB018VL DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
GKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
TNF/RANKL EDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPEIVLTQS
PGTLSLSPGERATLSCRASQSVRGRYLAWYQQKPGQAPRL
LIYGASSRATGIPDRFSGSGSGTDFTLTISRLEPEDFAVF
YCQQYGSSPRTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:39)
DVD054H AB018VH
AB017VH EVQLLESGGGLVQPGGSLRLSCAASGFTFSSYAMSWVRQA
PGKGLEWVSGITGSGGSTYYADSVKGRFTISRDNSKNTLY
RANKL/TNF LQMNSLRAEDTAVYYCAKDPGTTVIMSWFDPWGQGTLVTV
SSASTKGPEVQLVESGGGLVQPGRSLRLSCAASGFTFDDY
AMHWVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTISR
DNAKNSLYLQMNSLRAEDTAVYYCAKVSYLSTASSLDYWG
QGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:40)
163
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
DVD054L AB018VL
AB017VL EIVLTQSPGTLSLSPGERATLSCRASQSVRGRYLAWYQQK
PGQAPRLLIYGASSRATGIPDRFSGSGSGTDFTLTISRLE
RANKL/TNF PEDFAVFYCQQYGSSPRTFGQGTKVEIKRTVAAPDIQMTQ
SPSSLSASVGDRVTITCRASQGIRNYLAWYQQKPGKAPKL
LIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVATY
YCQRYNRAPYTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:41)
DVD065H AB017VH
AB023VH EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
TNF/DKK LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
SASTKGPEVQLVESGGGLVQPANSLKLSCAASGFTFSDYA
MAWVRQSPKKGLEWVATIIYDGSSTYYRDSVKGRFTISRD
NAKSTLYLQMDSLRSEDTATYYCATGLGIATDYFDYWGQG
VLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:42)
DVD065L AB017VL
AB023VL DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
GKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
TNF/DKK EDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPDIRMTQS
PASLSASLGETVNIECLASEDIYSDLAWYQQKPGKSPQLL
IYNANSLQNGVPSRFSGSGSGTQYSLKINSLQSEDVATYF
CQQYNNYPPTFGGGTKLELKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:43)
DVD066H AB023VH
AB017VH EVQLVESGGGLVQPANSLKLSCAASGFTFSDYAMAWVRQS
PKKGLEWVATIIYDGSSTYYRDSVKGRFTISRDNAKSTLY
DKK/TNF LQMDSLRSEDTATYYCATGLGIATDYFDYWGQGVLVTVSS
ASTKGPEVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAM
HWVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTISRDN
AKNSLYLQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQG
TLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
164
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
QKSLSLSPGK(SEQ ID NO:44)
DVD066L AB023VL
AB017VL DIRMTQSPASLSASLGETVNIECLASEDIYSDLAWYQQKP
GKSPQLLIYNANSLQNGVPSRFSGSGSGTQYSLKINSLQS
DKK/TNF EDVATYFCQQYNNYPPTFGGGTKLELKRTVAAPDIQMTQS
PSSLSASVGDRVTITCRASQGIRNYLAWYQQKPGKAPKLL
IYAASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVATYY
CQRYNRAPYTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:45)
DVD165H ABOO1VH
AB018VH QVQLQQPGAELVKPGASVKMSCKASGYTFTSYNMHWVKQT
PGRGLEWIGAIYPGNGDTSYNQKFKGKATLTADKSSSTAY
CD20/RANKL MQLSSLTSEDSAVYYCARSTYYGGDWYFNVWGAGTTVTVS
AASTKGPEVQLLESGGGLVQPGGSLRLSCAASGFTFSSYA
MSWVRQAPGKGLEWVSGITGSGGSTYYADSVKGRFTISRD
NSKNTLYLQMNSLRAEDTAVYYCAKDPGTTVIMSWFDPWG
QGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:46)
DVD165L ABOO1VL
AB018VL QIVLSQSPAILSPSPGEKVTMTCRASSSVSYIHWFQQKPG
SSPKPWIYATSNLASGVPVRFSGSGSGTSYSLTISRVEAE
CD20/RANKL DAATYYCQQWTSNPPTFGGGTKLEIKRTVAAPEIVLTQSP
GTLSLSPGERATLSCRASQSVRGRYLAWYQQKPGQAPRLL
IYGASSRATGIPDRFSGSGSGTDFTLTISRLEPEDFAVFY
CQQYGSSPRTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:47)
DVD166H AB018VH
ABOO1VH EVQLLESGGGLVQPGGSLRLSCAASGFTFSSYAMSWVRQA
PGKGLEWVSGITGSGGSTYYADSVKGRFTISRDNSKNTLY
RANKL/CD20 LQMNSLRAEDTAVYYCAKDPGTTVIMSWFDPWGQGTLVTV
SSASTKGPQVQLQQPGAELVKPGASVKMSCKASGYTFTSY
NMHWVKQTPGRGLEWIGAIYPGNGDTSYNQKFKGKATLTA
DKSSSTAYMQLSSLTSEDSAVYYCARSTYYGGDWYFNVWG
AGTTVTVSA
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
165
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:48)
DVD166L AB018VL
ABOO1VL EIVLTQSPGTLSLSPGERATLSCRASQSVRGRYLAWYQQK
PGQAPRLLIYGASSRATGIPDRFSGSGSGTDFTLTISRLE
RANKL/CD20 PEDFAVFYCQQYGSSPRTFGQGTKVEIKRTVAAPQIVLSQ
SPAILSPSPGEKVTMTCRASSSVSYIHWFQQKPGSSPKPW
IYATSNLASGVPVRFSGSGSGTSYSLTISRVEAEDAATYY
CQQWTSNPPTFGGGTKLEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:49)
DVD257H DLL4H P1GFH
EVQLVESGGGLVQPGGSLRLSCAASGFTFTDNWISWVRQA
PGKGLEWVGYISPNSGFTYYADSVKGRFTISADTSKNTAY
DLL4/PLGF LQMNSLRAEDTAVYYCARDNFGGYFDYWGQGTLVTVSSAS
TKGPQVQLQQSGAELVKPGASVKISCKASGYTFTDYYINW
VKLAPGQGLEWIGWIYPGSGNTKYNEKFKGKATLTIDTSS
STAYMQLSSLTSEDTAVYFCVRDSPFFDYWGQGTLLTVSS
(SEQ ID NO:50)
DVD257L DLL4L P1GFL
DIQMTQSPSSLSASVGDRVTITCRASQDVSTAVAWYQQKP
GKAPKLLIYSASFLYSGVPSRFSGSGSGTDFTLTISSLQP
DLL4/PLGF EDFATTYYCQQSYTGTVTFGQGTKVEIKRTVAAPDIVLTQ
SPDSLAVSLGERVTMNCKSSQSLLNSGMRKSFLAWYQQKP
GQSPKLLIYWASTRESGVPDRFTGSGSGTDFTLTISSVQA
EDVAVYYCKQSYHLFTFGSGTKLEIKR
(SEQ ID NO:51)
DVD258H P1GFH DLL4H
QVQLQQSGAELVKPGASVKISCKASGYTFTDYYINWVKLA
PGQGLEWIGWIYPGSGNTKYNEKFKGKATLTIDTSSSTAY
PLGF/DLL4 MQLSSLTSEDTAVYFCVRDSPFFDYWGQGTLLTVSSASTK
GPEVQLVESGGGLVQPGGSLRLSCAASGFTFTDNWISWVR
QAPGKGLEWVGYISPNSGFTYYADSVKGRFTISADTSKNT
AYLQMNSLRAEDTAVYYCARDNFGGYFDYWGQGTLVTVSS
(SEQ ID NO:52)
DVD258L P1GFL DLL4L
DIVLTQSPDSLAVSLGERVTMNCKSSQSLLNSGMRKSFLA
WYQQKPGQSPKLLIYWASTRESGVPDRFTGSGSGTDFTLT
PLGF/DLL4 ISSVQAEDVAVYYCKQSYHLFTFGSGTKLEIKRTVAAPDI
QMTQSPSSLSASVGDRVTITCRASQDVSTAVAWYQQKPGK
APKLLIYSASFLYSGVPSRFSGSGSGTDFTLTISSLQPED
FATTYYCQQSYTGTVTFGQGTKVEIKR
(SEQ ID NO:53)
DVD277H AB017VH
ABO5OVH EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
TNF/SOST(S
LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
2)
SASTKGPEVQLQQSGPELMKPGASVKMSCKASGYTFTDYN
166
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
MHWMKQNQGKSLEWIGEINPNSGGSGYNQKFKGKATLTVD
KSSSTAYMELRSLTSEDSAVYYCARLGYYGNYEDWYFDVW
GAGTTVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:54)
DVD277L AB017VL
ABO5OVL DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
GKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
TNF/SOST(S
EDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPDLQMTQT
2)
TSSLSASLGDRVTISCRASQDISNYLNWYQQKPDGTVKLL
IFYTSTLQSGVPSRFSGSGSGTNYSLTITNLEQDDAATYF
CQQGDTLPYTFGGGTKLEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:55)
DVD278H ABO5OVH
AB017VH EVQLQQSGPELMKPGASVKMSCKASGYTFTDYNMHWMKQN
QGKSLEWIGEINPNSGGSGYNQKFKGKATLTVDKSSSTAY
SOST(S2)/T
MELRSLTSEDSAVYYCARLGYYGNYEDWYFDVWGAGTTVT
NF
VSSASTKGPEVQLVESGGGLVQPGRSLRLSCAASGFTFDD
YAMHWVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTIS
RDNAKNSLYLQMNSLRAEDTAVYYCAKVSYLSTASSLDYW
GQGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:56)
DVD278L ABO5OVL
AB017VL DLQMTQTTSSLSASLGDRVTISCRASQDISNYLNWYQQKP
DGTVKLLIFYTSTLQSGVPSRFSGSGSGTNYSLTITNLEQ
SOST(52)/T
DDAATYFCQQGDTLPYTFGGGTKLEIKRTVAAPDIQMTQS
NF
PSSLSASVGDRVTITCRASQGIRNYLAWYQQKPGKAPKLL
IYAASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVATYY
CQRYNRAPYTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:57)
DVD281H AB056VH
AB053VH QVQLQQSGAELMKPGASVKLSCKATGYTFTGSWIEWIKQR
PGHGLEWIGQILPGSGSAYYNEKFKGKATFTADTSSKTVY
IL-
IQLISLTTEDSAIYYCAREDNYGSSSLAYWGQGTLLTVSA
167
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
9(S2)/IgE ASTKGPEVQLVESGGGLVQPGGSLRLSCAVSGYSITSGYS
WNWIRQAPGKGLEWVASITYDGSTNYNPSVKGRITISRDD
SKNTFYLQMNSLRAEDTAVYYCARGSHYFGHWHFAVWGQG
TLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:58)
DVD281L AB056VL
AB053VL DILLTQSPAILSVSPGERVSFSCRASQSIGTNIHWYQQRT
NGSPRLLIKYASESISGIPSRFSGGGSGTDFTLSINSVES
IL-
EDIADYYCQQSNNWPLTFGAGTKLELKRTVAAPDIQLTQS
9(S2)/IgE
PSSLSASVGDRVTITCRASQSVDYDGDSYMNWYQQKPGKA
PKLLIYAASYLESGVPSRFSGSGSGTDFTLTISSLQPEDF
ATYYCQQSHEDPYTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO:59)
DVD282H AB053VH
AB056VH EVQLVESGGGLVQPGGSLRLSCAVSGYSITSGYSWNWIRQ
APGKGLEWVASITYDGSTNYNPSVKGRITISRDDSKNTFY
IgE/IL-
LQMNSLRAEDTAVYYCARGSHYFGHWHFAVWGQGTLVTVS
9(S2)
SASTKGPQVQLQQSGAELMKPGASVKLSCKATGYTFTGSW
IEWIKQRPGHGLEWIGQILPGSGSAYYNEKFKGKATFTAD
TSSKTVYIQLISLTTEDSAIYYCAREDNYGSSSLAYWGQG
TLLTVSA
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:60)
DVD282L AB053VL
AB056VL DIQLTQSPSSLSASVGDRVTITCRASQSVDYDGDSYMNWY
QQKPGKAPKLLIYAASYLESGVPSRFSGSGSGTDFTLTIS
IgE/IL-
SLQPEDFATYYCQQSHEDPYTFGQGTKVEIKRTVAAPDIL
9(S2)
LTQSPAILSVSPGERVSFSCRASQSIGTNIHWYQQRTNGS
PRLLIKYASESISGIPSRFSGGGSGTDFTLSINSVESEDI
ADYYCQQSNNWPLTFGAGTKLELKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO: 61)
TNF/IL-17H TNFH IL-17H
EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
(DVD-A)
PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
168
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain
1234567890123456789012345678901234567890
Name Name
LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
SGGGGSGGGGSEVQLVQSGAEVKKPGSSVKVSCKASGGSF
GGYGIGWVRQAPGQGLEWMGGITPFFGFADYAQKFQGRVT
ITADESTTTAYMELSGLTSDDTAVYYCARDPNEFWNGYYS
THDFDSWGQGTTVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDE
LTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK
(SEQ ID NO:62)
TNF/IL-17L TNFL IL-17L
DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
(DVD-A)
GKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDVATYYCQRYNRAPYTFGQGTKVEIKRGGSGGGGSGSEI
VLTQSPDFQSVTPKEKVTITCRASQDIGSELHWYQQKPDQ
PPKLLIKYASHSTSGVPSRFSGSGSGTDFTLTINGLEAED
AGTYYCHQTDSLPYTFGPGTKVDIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSENRGEC(SEQ ID NO: 63)
TNF/PGE2H TNFH PGE2 EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQA
(DVD-B) PGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVS
SASTKGPEVQLVQSGAEVKKPGASVKVSCKASGYTFTKYW
LGWVRQAPGQGLEWMGDIYPGYDYTHYNEKFKDRVTLTTD
TSTSTAYMELRSLRSDDTAVYYCARSDGSSTYWGQGTLVT
VSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDE
LTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK
(SEQ ID NO:64)
TNF/PGE2L TNF PGE2
DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKP
(DVD-B)
GKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPDVLMTQT
PLSLPVTPGEPASISCTSSQNIVHSNGNTYLEWYLQKPGQ
SPQLLIYKVSNRFSGVPDRFSGSGSGTDFTLKISRVEAED
VGVYYCFQVSHVPYTFGGGTKVEIKR
169
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain 1234567890123456789012345678901234567890
Name Name
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQ ID NO: 65)
IL-la/IL- IL-1aH IL-1bH EVQLVESGGGVVQPGRSLRLSCSASGFIFSRYDMSWVRQA
1bH PGKGLEWVAYISHGGAGTYYPDSVKGRFTISRDNSKNTLF
(DVD-C) LQMDSLRPEDTGVYFCARGGVTKGYFDVWGQGTPVTVSSA
STKGPQVQLVESGGGVVQPGRSLRLSCTASGFTFSMFGVH
WVRQAPGKGLEWVAAVSYDGSNKYYAESVKGRFTISRDNS
KNILFLQMDSLRLEDTAVYYCARGRPKVVIPAPLAHWGQG
TLVTFSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK(SEQ ID NO:66)
IL-la/IL- IL-1aL IL-1bL DIQMTQSPSSLSASVGDRVTITCRASGNIHNYLTWYQQTP
1bL GKAPKLLIYNAKTLADGVPSRFSGSGSGTDYTFTISSLQP
(DVD-C) EDIATYYCQHFWSIPYTFGQGTKLQITRTVAAPDIQMTQS
PSSVSASVGDRVTITCRASQGISSWLAWYQQKPGKAPKLL
IYEASNLETGVPSRFSGSGSGSDFTLTISSLQPEDFATYY
CQQTSSFLLSFGGGTKVEHKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC(SEQIDNO:67)
DLL4/VEGFH EVQLVESGGGLVQPGGSLRLSCAASGFTFRHFPMAWVRQA
C(h1A11.1. PGKGLEWVATISSSDAWPSYRDSVKGRFTISRDNAKNSLY
A6-L5-Av) LQMNSLRAEDTAVYYCSRGYYNSPFAYWGQGTLVTVSSAS
TKGPSVFPLAPEVQLVESGGGLVQPGGSLRLSCAASGYTF
TNYGMNWVRQAPGKGLEWVGWINTYTGEPTYAADFKRRFT
FSLDTSKSTAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWY
FDVWGQGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK*(SEQ ID NO:68)
DLL4/VEGFL DIQMTQSPSSLSASVGDRVTITCRASEDIYSNLAWYQQKP
C(h1A11.1. GKAPKLLIYDTNNLADGVPSRFSGSGSGTDFTLTISSLQP
A6-LS-Av) EDFATYYCQQYNNYPPTFGQGTKLEIKRTVAAPDIQMTQS
PSSLSASVGDRVTITCSASQDISNYLNWYQQKPGKAPKVL
170
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain 1234567890123456789012345678901234567890
Name Name
IYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQPEDFATYY
CQQYSTVPWTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC*(SEQIDNO:69)
DLL4/VEGFH EVQLVESGGGLVQPGGSLRLSCAASGFTFRHFPMAWVRQA
C(h1A11.1. PGKGLEWVATISSSDAWPSYRDSVKGRFTISRDNAKNSLY
A6-SL-Av) LQMNSLRAEDTAVYYCSRGYYNSPFAYWGQGTLVTVSSAS
TKGPEVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNW
VRQAPGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDTSK
STAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWGQG
TLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK (SEQ ID NO:74)
DLL4/VEGFL DIQMTQSPSSLSASVGDRVTITCRASEDIYSNLAWYQQKP
C(h1A11.1. GKAPKLLIYDTNNLADGVPSRFSGSGSGTDFTLTISSLQP
A6-SL-Av) EDFATYYCQQYNNYPPTFGQGTKLEIKRTVAAPSVFIFPP
DIQMTQSPSSLSASVGDRVTITCSASQDISNYLNWYQQKP
GKAPKVLIYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQYSTVPWTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC
(SEQ ID NO:75)
IL12/IL18H EVTLRESGPALVKPTQTLTLTCTFSGFSLSKSVMGVSWIR
QPPGKALEWLAHIYWDDDKYYNPSLKSRLTISKDTSKNQV
VLTMTNMDPVDTATYYCARRGIRSAMDYWGQGTTVTVSSA
STKGPEVQLVQSGTEVKKPGESLKISCKGSGYTVTSYWIG
WVRQMPGKGLEWMGFIYPGDSETRYSPTFQGQVTISADKS
FNTAFLQWSSLKASDTAMYYCARVGSGWYPYTFDIWGQGT
MVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK (SEQ ID NO:70)
IL12/IL18 DIVMTQSPDSLAVSLGERATINCKASQSVSNDVAWYQQKP
LC GQPPKLLIYYASNRYTGVPDRFSGSGSGTDFTLTISSLQA
171
CA 02889488 2015-04-23
WO 2014/071212
PCT/US2013/068110
DVD-Ig Outer Inner Sequence, with Line Break Between
Heavy or Variabl Variabl Variable and Constant Regions
Light
Chain Name Domain Domain 1234567890123456789012345678901234567890
Name Name
EDVAVYYCQQDYNSPWTFGGGTKVEIKRTVAAPEIVMTQS
PATLSVSPGERATLSCRASESISSNLAWYQQKPGQAPRLF
IYTASTRATDIPARFSGSGSGTEFTLTISSLQSEDFAVYY
CQQYNNWPSITFGQGTRLEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC (SEQ ID
NO: 71)
DLL4/VEGF EVQLVESGGGLVQPGGSLRLSCAASGFTESNFPMAWVRQA
HC PGKGLEWVATISSSDGTTYYRDSVEGRFTISRDNAKNSLY
h1A11.1- LQMNSLRAEDTAVYYCARGYYNSPFAYWGQGTLVTVSSAS
LS-AV TEGPSVFPLAPEVQLVESGGGLVQPGGSLRLSCAASGYTE
TNYGMNWVRQAPGKGLEWVGWINTYTGEPTYAADFERRFT
FSLDTSKSTAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWY
FDVWGQGTLVTVSS
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQT
YICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPEAAGG
PSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNW
YVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREE
MTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPV
LDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYT
QKSLSLSPGK (SEQ ID NO:72)
DLL4/VEGF DIQMTQSPSSLSASVGDRVTITCRASEDIYSNLAWYQQKP
LC GKAPKLLIYDTNNLADGVPSRFSGSGSGTDFTLTISSLQP
h1A11.1- EDFATYYCQQYNNYPPTFGQGTKLEIKRTVAAPDIQMTQS
LS-AV PSSLSASVGDRVTITCSASQDISNYLNWYQQKPGKAPKVL
IYFTSSLHSGVPSRFSGSGSGTDFTLTISSLQPEDFATYY
CQQYSTVPWTFGQGTKVEIKR
TVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQW
KVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEK
HKVYACEVTHQGLSSPVTKSFNRGEC (SEQ ID
NO: 73)
172
CA 02889488 2015-04-23
WO 2014/071212 PCT/US2013/068110
Incorporation by Reference
The contents of all cited references (including literature references,
patents, patent
applications, and websites) that may be cited throughout this application are
hereby expressly
incorporated by reference in their entirety, as are the references cited
therein. The practice of
the present disclosure will employ, unless otherwise indicated, conventional
techniques of
immunology, molecular biology and cell biology, which are well known in the
art.
Equivalents
The disclosure may be embodied in other specific forms without departing from
the
spirit or essential characteristics thereof. The foregoing embodiments are
therefore to be
considered in all respects illustrative rather than limiting of the disclosure
described herein.
Scope of the disclosure is thus indicated by the appended claims rather than
by the foregoing
description, and all changes that come within the meaning and range of
equivalency of the
claims are therefore intended to be embraced herein.
173