Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02915419 2015-12-14
BHC123073 FC
- 1 -
Substituted pheny1-2,3-benzodiazepines
The present invention relates to BET protein-inhibitory, especially BRD4-
inhibitory, substituted
pheny1-2,3-benzodiazepines, to pharmaceutical compositions comprising the
compounds according
to the invention, and to the prophylactic and therapeutic use thereof for
hyperproliferative
disorders, especially for neoplastic disorders. The present invention further
relates to the use of
BET protein inhibitors in benign hyperplasias, atherosclerotic disorders,
sepsis, autoimmune
disorders, vascular disorders, viral infections, in neurodegenerative
disorders, in inflammatory
disorders, in atherosclerotic disorders and in male fertility control.
The human BET family (bromo domain and extra C-terminal domain family) has
four members
(BRD2, BRD3, BRD4 and BRDT) containing two related bromo domains and one
extraterminal
domain (Wu and Chiang, J. Biol. Chem., 2007, 282:13141-13145). The bromo
domains are protein
regions which recognize acetylated lysine residues. Such acetylated lysines
are often found at the
N-terminal end of histones (e.g. histone 3 or histone 4), and they are
features of an open chromatin
structure and active gene transcription (Kuo and Allis, Bioessays, 1998,
20:615-626). The different
acetylation patterns recognized by BET proteins in histones were investigated
in depth (Umehara et
al., J. Biol. Chem., 2010, 285:7610-7618; Filippakopoulos et al., Cell, 2012,
149:214-231). In
addition, bromo domains can recognize further acetylated proteins. For
example, BRD4 binds to
RelA, which leads to stimulation of NF-03 and transcriptional activity of
inflammatory genes
(Huang et al., Mol. Cell. Biol., 2009, 29:1375-1387; Zhang et al., J. Biol.
Chem., 2012, 287:
28840-28851; Zou et al., Oncogene, 2013, doi:10.1038/onc.2013.179). The
extraterminal domain
of BRD2, BRD3 and BRD4 interacts with several proteins involved in chromatin
modulation and
the regulation of gene expression (Rahman et al., Mol. Cell. Biol., 2011,
31:2641-2652).
In mechanistic terms, BET proteins play an important role in cell growth and
in the cell cycle. They
are associated with mitotic chromosomes, suggesting a function in epigenetic
memory (Dey et al.,
Mol. Biol. Cell, 2009, 20:4899-4909; Yang et al., Mol. Cell. Biol., 2008,
28:967-976). BRD4 is
important for post-mitotic reactivation of gene transcription (Zhao et al.,
Nat. Cell. Biol., 2011,
13:1295-1304). It has been shown that BRD4 is essential for transcription
elongation and for the
recruitment of the elongation complex P-TEFb consisting of CDK9 and cyclin T1,
which leads to
activation of RNA polymerase II (Yang et al., Mol. Cell, 2005, 19:535-545;
Schroder et al., J. Biol.
Chem., 2012, 287:1090-1099). Consequently, the expression of genes involved in
cell proliferation
is stimulated, for example of c-Myc and aurora B (You et al., Mol. Cell.
Biol., 2009, 29:5094-5103;
Zuber et al., Nature, 2011, 478:524-528). BRD2 and BRD3 bind to transcribed
genes in
hyperacetylated chromatin regions and promote transcription by RNA polymerase
II (LeRoy et al.,
Mol. Cell, 2008, 30:51-60).
CA 02915419 2015-12-14
BHC123073FC
- 2 -
Knock-down of BRD4 or the inhibition of the interaction with acetylated
histones in various cell
lines leads to G1 arrest and to cell death apoptosis (Mochizuki et al., J.
Biol. Chem., 2008,
283:9040-9048; Mertz et al., Proc. Natl. Acad. Sci. USA, 2011, 108:16669-
16674). It has also been
shown that BRD4 binds to promoter regions of several genes which are activated
in the G1 phase,
for example cyclin D1 and D2 (Mochizuki et al., J. Biol. Chem., 2008, 283:9040-
9048). In
addition, inhibition of the expression of c-Myc, an essential factor in cell
proliferation, after BRD4
inhibition has been demonstrated (Dawson et al., Nature, 2011, 478:529-533;
Delmore et al., Cell,
2011, 146:1-14; Mertz et al., Proc. Natl. Acad. Sci. USA, 2011, 108:16669-
16674).
BRD2 and BRD4 knockout mice die early in embryogenesis (Gyuris et al.,
Biochim. Biophys.
Acta, 2009, 1789:413-421; Houzelstein et al., Mol. Cell. Biol., 2002, 22:3794-
3802). Heterozygotic
BRD4 mice have various growth defects attributable to reduced cell
proliferation (Houzelstein et
al., Mol. Cell. Biol., 2002, 22:3794-3802).
BET proteins play an important role in various tumour types. Fusion between
the BET proteins
BRD3 or BRD4 and NUT, a protein which is normally expressed only in the
testes, leads to an
aggressive form of squamous cell carcinoma, called NUT midline carcinoma
(French, Cancer
Genet. Cytogenet., 2010, 203:16-20). The fusion protein prevents cell
differentiation and promotes
proliferation (Yan et al., J. Biol. Chem., 2011, 286:27663-27675; Grayson et
al., 2013, doi:10-
1038/onc.2013.126). The growth of in vivo models derived therefrom is
inhibited by a BRD4
inhibitor (Filippakopoulos et al., Nature, 2010, 468:1067-1073). Screening for
therapeutic targets in
an acute myeloid leukaemia cell line (AML) showed that BRD4 plays an important
role in this
tumour (Zuber et al., Nature, 2011, doi:10.1038). Reduction in BRD4 expression
leads to a
selective arrest of the cell cycle and to apoptosis. Treatment with a BRD4
inhibitor prevents the
proliferation of an AML xenograft in vivo. Amplification of the DNA region
containing the BRD4
gene was detected in primary breast tumours (Kadota et al., Cancer Res, 2009,
69:7357-7365). For
BRD2 too, there are data relating to a role in tumours. A transgenic mouse
which overexpresses
BRD2 selectively in B cells develops B cell lymphomas and leukaemias
(Greenwall et al., Blood,
2005, 103:1475-1484).
BET proteins are also involved in viral infections. BRD4 binds to the E2
protein of various
papillomaviruses and is important for the survival of the viruses in latently
infected cells (Wu et al.,
Genes Dev., 2006, 20:2383-2396; Vosa et al., J. Virol., 2012, 86:348-357;
McBride und Jang,
Viruses, 2013, 5:1374-1394). The herpes virus, which is responsible for
Kaposi's sarcoma, also
interacts with various BET proteins, which is important for disease survival
(Viejo-Borbolla et al.,
J. Virol., 2005, 79:13618-13629; You et al., J. Virol., 2006, 80:8909-8919).
Through binding to P-
TEFb, BRD4 also plays an important role in the replication of HIV (Bisgrove et
al., Proc. Natl.
Acad. Sci. USA, 2007, 104:13690-13695).
BET proteins are additionally involved in inflammation processes. BRD2-
hypomorphic mice show
reduced inflammation in adipose tissue (Wang et al., Biochem. J., 2009, 425:71-
83). Infiltration of
macrophages in white adipose tissue is also reduced in BRD2-deficient mice
(Wang et al.,
CA 02915419 2015-12-14
BHC123073FC
- 3 -
,
Biochem. J., 2009, 425:71-83). It has also been shown that BRD4 regulates a
number of genes
involved in inflammation. In LPS-stimulated macrophages, a BRD4 inhibitor
prevents the
expression of inflammatory genes, for example IL-1 or IL-6 (Nicodeme et al.,
Nature, 2010,
468:1119-1123).
BET proteins also regulate the expression of the ApoAl gene which plays an
important role in
atherosclerosis and inflammatory processes (Chung et al., J. Med. Chem, 2011,
54:3827-3838).
Apolipoprotein Al (ApoAl) is a major component of high density lipoproteins
(HDL), and
increased expression of ApoAl leads to elevated blood cholesterol values
(Degoma and Rader,
Nat. Rev. Cardiol., 2011, 8:266-277). Elevated HDL values are associated with
a reduced risk of
atherosclerosis (Chapman et al., Eur. Heart J., 2011, 32:1345-1361).
CA 02915419 2015-12-14
BHC123073FC
-4_
Prior art
The nomenclature employed in the assessment of the structural prior art is
illustrated by the
following figure:
2
1 0
2 \ l 4
is 6
--"N
4-phenyl-6H-thieno[3,2-j]- 6-pheny1-4H-isoxazolo-[5,4-
a][1,4]diazepine d] [2]benzazepine
R4R5R1 R200
R7
410 1 2 3N¨R3
R1 NR2R3
R8
2
Ar R6 R4
substituted 3-amino-2,3-dihydro-1H-1- substituted 3,5-dihydro-4H-2,3-
ben7a7epin-2-ones benzodiazepin-4-ones
5
Based on the chemical structure, only very few types of BRD4 inhibitors have
been described to
date (Chun-Wa Chung et al., Progress in Medicinal Chemistry 2012, 51, 1-55).
The first published BRD4 inhibitors are phenylthienotriazolo-1,4-diazepines (4-
pheny1-6H-
thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepines) as described in
W02009/084693 (Mitsubishi
Tanabe Pharma Corporation) and as compound JQ1 in W02011/143669 (Dana Farber
Cancer
Institute). Replacement of the thieno moiety by a benzo moiety also leads to
active inhibitors (J.
Med. Chem. 2011, 54, 3827 ¨ 3838; E. Nicodeme et al., Nature 2010, 468, 1119).
These and one
further publication show that the pyrazole unit fused to the 1,4-
benzodiazepine or thieno-1,4-
diazepine ring system is actively involved in binding of the target protein
BRD4 (P.
Filippakopoulos et al., Nature 2010, 468, 1067). Further 4-pheny1-6H-
thieno[3,2-
[1,2,4]triazolo[4,3-a][1,4]diazepines and related compounds having alternative
rings as a fusion
partner rather than the benzo unit are described both in a general manner and
in some cases as
examples in W02012/075456 (Constellation Pharmaceuticals). W02012/075383
(Constellation
Pharmaceuticals) describes 6-substituted 4H-isoxazolo[5,4-d][2]benzazepines
and 4H-
isoxazolo[3,4-4[2]benzazepines, including compounds which have optionally
substituted phenyl at
position 6 as BRD4 inhibitors, and also analogues with alternative
heterocyclic fusion partners
rather than the benzo unit, for example thieno- or pyridoazepines.
CA 02915419 2015-12-14
BHC123073FC
- 5
Another structural class of BRD4 inhibitors described is that of 7-
isoxazoloquinolines and related
quinolone derivatives (W02011/054843, Bioorganic & Medicinal Chemistry Letters
22 (2012)
2963-2967, GlaxoSmithKline).
The compounds according to the invention are novel substituted phenyl-2,3-
benzodiazepines (1-
pheny1-4,5-dihydro-3H-2,3-benzodiazepines) which, inter alia, are not fused at
the benzodiazepine
skeleton to a second heterocyclic moiety, specifically an isoxazole or
triazole, and are still,
surprisingly, BET, in particular BRD4, inhibitors.
The compounds according to the invention differ from known 2,3-benzodiazepines
which have
been described as A_MPA receptor antagonists. Thus, US 5,536,832 / EP 0492485,
US 5,639,751,
US 5,459,137 (Gyogyszerkutato Intezet Kft) disclose substituted 1-phenyl-2,3-
benzodiazepines
which have a mandatory methylenedioxy bridge condensed to the benzo moiety of
the
benzodiazepine.
US 2004/0152693 / WO 2004/069197, US 2007/0027143 and HU 2004000338 (Sandor
Solyom et
al.) disclose substituted 1-phenyl-2,3-benzodiazepines which have a mandatory
heterocyclic group
as a substituent at the nitrogen in position 3. The exemplary compounds
disclosed and the
intermediates not having the abovementioned heterocyclic group at N-3 are
substituted at the 1-
phenyl group inter alia by -NH2, acetamido or nitro groups, but not by the
ether, amide or
substituted amino groups present in the compounds according to the invention.
HU 199700688 only describes 2,3-benzodiazepines in a very general manner as
AMPA
antagonists, without disclosing any specific exemplary compounds.
The exemplary compounds disclosed in WO 1997/028135 (Schering AG), WO
2001/098280
(Annovis, Inc.) and EP 0802195 / US 5,807,851 (EGIS Gyogysergyar, Rt) have
nitro or -NH2
groups at the 1-phenyl group, but not substituted amino groups or acylamines;
the ether, amino and
amide substituents claimed in a generic manner likewise differ from the
corresponding substituents
in the compounds according to the invention.
The compounds according to the invention furthermore differ from the known
psychopharmacological 1-pheny1-2,3-benzodiazepine derivatives which are
inhibitors of the
adenosine transporter and the MT2 receptor (W02008/124075, Teva Pharmaceutical
Industries,
Inc). The exemplary compounds disclosed therein are substituted at the 1-
phenyl group inter alia
by NH2- acetamido, methoxy or nitro groups, but not by the ether, amide or
substituted amino
groups present in the compounds according to the invention. The amino and
amide substituents
claimed in a generic manner also differ from the corresponding substituents in
the compounds
according to the invention.
CA 02915419 2015-12-14
BHC123073FC
- 6
W094/26718/EP0703222A1 (Yoshitomi Pharmaceutical Industries) describes
substituted 3-amino-
2,3-dihydro-1H-1-benzazepin-2-ones or the corresponding 2-thiones and
analogues in which the
benzo unit has been replaced by alternative monocyclic systems, and in which
the 2-ketone or the
2-thione together with the substituted nitrogen atom in the azepine ring may
form a heterocycle, as
CCK and gastrin antagonists for the treatment of CNS disorders, such as states
of anxiety and
depression, and of pancreatic disorders and of gastrointestinal ulcers.
Ligands of the gastrin and the cholecystokinin receptor are described in
W02006/051312 (James
Black Foundation). They also include substituted 3,5-dihydro-4H-2,3-
benzodiazepin-4-ones which
differ from the compounds according to the invention mainly by the obligatory
oxo group in
position 4 and by an obligatory carbonyl group-containing alkyl chain in
position 5.
Finally, substituted 3,5-dihydro-4H-2,3-benzodiazepin-4-ones are also
described as AMPA
antagonists in W097/34878 (Cocensys Inc.). The generic claim is very wide with
respect to the
possible substitution patterns at the benzodiazepine skeleton; however, the
working examples are
limited to just a narrow range.
In spite of the multifarious compounds of the prior art, there is still a need
for further highly
effective compounds, and it is therefore desirable to provide novel compounds
having prophylactic
and therapeutic properties.
Accordingly, it is an object of the present invention to provide compounds and
pharmaceutical
compositions comprising these compounds used for prophylactic and therapeutic
applications for
hyperproliferative disorders, in particular for tumour disorders, and as BET
protein inhibitors for
viral infections, for neurodegenerative disorders, for inflammatory disorders,
for atherosclerotic
disorders and for male fertility control.
The structurally most similar compounds of the prior art have not been
disclosed in the context of
the prophylaxis and therapy of tumour disorders.
From the prior art described above, there was no reason to modify the
structures of the prior art
such that structures suitable for the prophylaxis and therapy of neoplastic
disorders are obtained.
Surprisingly, the compounds according to the invention inhibit the interaction
between BET
proteins, in particular BRD4, and an acetylated histone 4 peptide and inhibit
the growth of cancer
cells. Accordingly, they provide novel useful and effective compounds for the
therapy of human
and animal disorders, in particular of cancers.
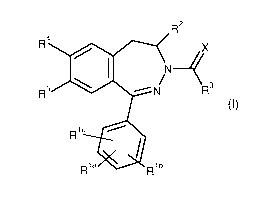
It has now been found that compounds of the general formula (I)
CA 02915419 2015-12-14
, BHC123073FC
- 7 -
,
R2
R4
X
R5 14111 ¨N" R3
Ric elk
Rla Rlb
(I)
in which
X represents an oxygen or sulphur atom,
Ria
represents -0R6 or -NR71e,
Rib and Ric independently of one another represent hydrogen, halogen, hydroxy,
cyano, nitro or
represent a C1-C6-alkyl, C1-C6-alkoxy, C1-C6-alkoxy-C1-C6-alkyl, halo-C1-C6-
alkyl,
halo-Ci-C6-alkoxy, C3-C10-cycloallcyl radical or a monocyclic heterocyclyl
radical
having 3 to 8 ring atoms,
le represents a C1-C3-alkyl or trifluoromethyl or a C3- or C4-cycloalkyl
radical,
R3 represents C1-C3-alkyl-, Ci-C3-alkoxy-, amino- or C1-
C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
nitro, amino,
aminocarbonyl-, fluorine, chlorine, bromine,
or
represent Ci-C6-alkyl-, C1-C6-alkoxy-, C1-C6-alkylamino-, C1-C6-
alkylcarbonylamino-,
C1-C6-alkylaminocarbonyl- or C1-C6-alkylaminosulphonyl- which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, carboxy, hydroxy-C1-C6-alkyl-, C1-C6-
alkoxY-,
Ci-C6-alkoxy-C1-C6-alkyl-, CI-C6-alkylamino-, amino-Ci-C6-alkyl-, a monocyclic
heterocyclyl radical having 3 to 8 ring atoms and a monocyclic heteroaryl
radical
having 5 or 6 ring atoms where the monocyclic heterocyclyl and heteroaryl
radicals
mentioned may for their part optionally be monosubstituted by C1-C3-alkyl-,
or
represent C3-Circycloalkyl- which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of halogen,
amino,
hydroxy, Ci-C6-alkyl-, C1-C6-alkoxy-, Ci-C6-alkoxy-CI-C6-alkyl-, Cr-C6-
allcylamino-,
amino-Ci-C6-alkyl-, C1-C6-alkylamino-C1-C6-alkyl, halo-Ci-C6-alkyl-, halo-C1-
05-
alkoxy- and a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be
CA 02915419 2015-12-14
BHC123073FC
- 8
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, nitro, carboxy, C1-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxY-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, C1-C6-allcylamino-,
amino-
Ci-C6-alkyl-, C1-C6-allcylamino-CI-C6-alkyl, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxy-,
C3-C10-cycloalkyl and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
or
represent monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, oxo, carboxy, Ci-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-alkylamino-, amino-C1-C6-alkyl-, C1-
C6-
alkylamino-C1-C6-alkyl-, hydroxy-C,-C6-alkyl-, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxy-
, C3-Cio-cycloallcyl- and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
Or
represent phenyl- which may optionally be mono- or polysubstituted by
identical or
different substituents from the group consisting of halogen, amino, hydroxy,
cyano,
nitro, carboxy, Ci-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkoxy-CI-C6-allcyl-, C1-C6-
alkylamino-, amino-Ci-C6-alkyl-, Ci-C6-alkylaminocarbonyl-, C1-C6-
alkylaminosulphonyl-, Ci-C6-alkylamino-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-,
halo-C1-
C6-a1lcy1-, halo-C1-C6-alkoxy-, C3-C10-cycloalkyl- and a monocyclic
heterocyclyl
radical having 3 to 8 ring atoms,
R6 represents C3-C7-cycloalkyl- or C2-C6-alkyl- monosubstituted by Ci-
C6-alkylamino-,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms which
may
optionally be monosubstituted by oxo, CI-C3-alkyl-, Ci-C3-alkylcarbonyl-, CI-
Cr
alkoxycarbonyl-, phenyl-C1-C3-alkyl- or C3-C7-cycloalkyl-,
or
represents a mono- or bicyclic aryl or heteroaryl radical, where the radicals
mentioned
may optionally be mono- or disubstituted by identical or different
substituents from
the group consisting of halogen, hydroxy, cyano, fluoro-C1-C3-alkyl,
hydroxy-Ci-C3-alkyl, C1-C3-alkoxy-, CI-C3-alkylamino-, amino-Ci-C3-alkyl-, C1-
C3-
alkylaminocarbonyl-, Ci-C3-alkylaminosulphonyl-, C1-C3-alkylcarbonylamino-, C1-
C3-allcylsulphonylamino-, Ci-C3-alkylcarbonyl-, C1-C3-alkylsulphonyl- and
trifluoromethoxy-,
or
represents a benzyl radical,
where the phenyl radical contained therein may optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of
halogen, hydroxy, cyano, C1-C3-alkyl, fluoro-C1-C3-allcyl, hydroxy-C1-C3-
CA 02915419 2015-12-14
= BHC123073FC
- 9
alkyl, CI-C3-alkoxy-, CI -C3-alkylarnino-, amino-CI-C3-alkyl-, C1-C3-
alkylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, C1-C3-
alkylcarbonylamino-, C1-C3-alkylsulphonylamino-, C1-C3-alkylcarbonyl-, CI-
C3-alkylsulphonyl- and trifluoromethoxy-,
and where the methylene group contained therein may optionally be
substituted by a hydroxy group or one or two Ci-C3-alkyl groups,
R7 represents C3-C7-cycloalkyl- or C2-C6-alkyl-
monosubstituted by - RNR9
or
represents a -C(=0)It'1 group,
or
represents a -S(=0)2R'2 group,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
C12-heterocycloallcyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-
C12-
heterobicycloalkyl radical, where the radicals mentioned may optionally be
mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, C1-C4-alkoxycarbonyl-, phenyl-CI-C3-alkyl-
and
C3-C7-cycloalkyl-,
Or
represents a mono- or bicyclic aryl- or heteroaryl radical, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the group consisting of halogen, hydroxy, cyano, CI-C3-
alkyl,
fluoro-C1-C3-alkyl, hydroxy-C1-C3-alkyl, Ci-C3-alkoxy-, CI-C3-alkylamino-,
amino-
CI-C3-alkyl-, C1-C3-alkylaminocarbonyl-, CI-C3-alkylaminosulphonyl-, C1-C3-
alkylcarbonylamino-, CI-C3-allcylsulphonylamino-, C1-C3-alkylcarbonyl-, C,-C3-
allcylsulphonyl- and trifluoromethoxy-,
or
represents fluoro-C1-C3-alkyl or CI-C3-alkyl monosubstituted by a phenyl or a
monocyclic heteroaryl radical, where the phenyl and heteroaryl radicals
mentioned
may be mono- or disubstituted by identical or different substituents from the
group
consisting of Ci-C3-alkyl-, halogen and C1-C3-alkoxy-,
represents hydrogen or CI-C6-alkyl,
R9 and RI independently of one another represent hydrogen or Ci-C6-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 3 to 8 ring atoms which may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, C1-C3-alkylcarbonyl-, Ci-
C4alkoxycarbonyl-,
phenyl-C1-C3-alkyl- or C3-C7-cycloalkyl-,
CA 02915419 2015-12-14
BHC123073FC
- 10 -
Rii
represents C3-C7-cycloalkyl- or CI-C6-alkyl- monosubstituted by - RNR9
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
Cp-heterocycloalkyl radical, a C5-Cu-heterospirocycloalkyl radical or a C6-C12-
heterobicycloalkyl radical, where the radicals mentioned may optionally be
mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
C1-C3-alkyl-, C1-C3-alkylcarbonyl-, CI-C4alkoxycarbonyl-, phenyl-Ci-C3-alkyl-
and
C3-C7-cycloalkyl-,
or
represents a mono- or bicyclic aryl or heteroaryl radical, where the radicals
mentioned
may optionally be mono- or disubstituted by identical or different
substituents from
the group consisting of halogen, hydroxy, cyano, CI-C3-alkyl, fluoro-CI-C3-
alkyl,
hydroxy-Ci-C3-alkyl, CI-C3-alkoxy-, CI-C3-alkylamino-, amino-C1-C3-alkyl-, C1-
C3-
alkylaminocarbonyl-, CI-C3-alkylaminosulphonyl-, CI-C3-alkylcarbonylamino-, CI-
C3-alkylsulphonylamino-, Ci-C3-alkylcarbonyl-, Ci-C3-alkylsulphonyl- and
trifluoromethoxy-, and
R12
represents CI-C6-alkyl- which may optionally be mono- or disubstituted by
identical or
different substituents from the group consisting of halogen, hydroxy, carboxy,
cyano,
Ci-C6-alkoxy-, -NR91e, phenyl, a monocyclic heteroaryl radical having 5 or 6
ring
atoms and a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
where
phenyl and the monocyclic heteroaryl radical having 5 or 6 ring atoms for
their part may be mono- to trisubstituted by identical or different
substituents from the group consisting of halogen, cyano, Ci-C3-alkyl,
trifluoromethyl, Ci-C3-alkoxy- and trifluoromethoxy-,
and in which
the monocyclic heterocyclyl radical for its part may optionally be mono- or
disubstituted by identical or different substituents from the group
consisting of oxo, Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, CI-C4-
alkoxycarbonyl-, phenyl-Ci-C3-alkyl- and C3-C7-cycloalkyl-,
Or
represents fluoro-C1-C3-alkyl-,
or
represents C3-Clo-cycloalkyl- which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of fluorine,
hydroxy, oxo,
cyano, Ci-C3-alkyl-, Ci-C3-alkoxy- and -NR9RM,
Or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
CA 02915419 2015-12-14
BHC123073FC
- 11 -
C17-heterocycloalkyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-
Cir
heterobicycloalkyl radical, where the radicals mentioned may optionally be
mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
Ci-C3-alkyl-, C1-C3-alkylcarbonyl-, Ci-C4-alkoxycarbonyl-, phenyl-Ci-C3-alkyl-
and
C3-C7-cycloalkyl-,
or
represents an aryl- or heteroaryl radical, where the radicals mentioned may
optionally
be mono- or disubstituted by identical or different substituents from the
group
consisting of halogen, hydroxy, cyano, Ci-C3-alkyl, fluoro-Ci-C3-alkyl,
hydroxy-C1-
C3-alkyl, Ci-C3-alkoxy-, Ci-C3-alkylamino-, amino-Ci-C3-alkyl-, C1-C3-
alkylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, Ci-C3-alkylcarbonylamino-, CI-
C3-alkylsulphonylamino-, Ci-C3-alkylcarbonyl-, Ci-C3-alkylsulphonyl- and
trifluoromethoxy-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts are particularly suitable for a
large number of
prophylactic and therapeutic applications, in particular for
hyperproliferative disorders, for
neoplastic disorders and as BET protein inhibitors for viral infections, for
neurodegenerative
disorders, for inflammatory disorders, for atherosclerotic disorders and for
male fertility control.
CA 02915419 2015-12-14
BHC123073FC
- 12 -
The invention is based on the following definitions:
Alkyl:
Alkyl represents a straight-chain or branched saturated monovalent hydrocarbon
radical having
generally 1 to 6 carbon atoms (C1-C6-alkyl), preferably 1 to 4 (C1-C4-alkyl),
2 to 4 (C2-C4-alkyl) or 1 to
3 carbon atoms (C1-C3-alkyl).
Preferred examples include:
methyl-, ethyl-, propyl-, butyl-, pentyl-, hexyl-, isopropyl-, isobutyl-, sec-
butyl, tert-butyl-,
isopentyl-, 2-methylbutyl-, 1-methylbutyl-, 1-ethylpropyl-, 1,2-
dimethylpropyl, neopentyl-,
1,1-dimethylpropyl, 4-methylpentyI, 3-methylpentyl, 2-methylpentyl, 1-
methylpentyl,
2-ethylbutyl, 1-ethylbutyl, 3,3-dimethylbutyl, 2,2-dimethylbutyl, 1,1-
dimethylbutyl,
2,3-dimethylbutyl-, 1,3-dimethylbutyl-, 1,2-dimethylbutyl-.
Particular preference is given to a methyl, ethyl, propyl, isopropyl or tert-
butyl radical.
Very particular preference is given to a methyl radical.
Cycloalkyl:
Cycloalkyl represents a mono- or bicyclic saturated monovalent hydrocarbon
radical having
generally 3 to 10 (C3-Cio-cycloalkyl), preferably 3 to 8
(C3-C9-cycloalkyl) and particularly preferably 3 to 7 (C3-C7-cycloalkyl)
carbon atoms.
Preferred examples of monocyclic cycloalkyl radicals include:
cyclopropyl-, cyclobutyl-, cyclopentyl-, cyclohexyl- and cycloheptyl-.
Particular preference is given to a cyclopropyl, cyclopentyl or cyclohexyl
radical.
Examples of bicyclic cycloalkyl radicals include:
perhydropentalenyl-, decalinyl-.
Phenylalkyl:
Phenyl-Ci-C3-alkyl is understood to mean a group composed of an optionally
substituted phenyl
radical and a CI-C3-alkyl group, and bonded to the rest of the molecule via
the Ci-C3-alkyl group. The
alkyl radical here is as defined above under alkyl.
Examples include benzyl, phenethyl, phenylpropyl, particular preference being
given to benzyl.
Alkoxy:
Alkoxy represents a straight-chain or branched saturated alkyl ether radical
of the formula ¨0-alkyl
having generally I to 6 (Ci-C6-alkoxy-), preferably 1 to 3 (C1-C3-alkoxy-)
carbon atoms.
Preferred examples include:
methoxy-, ethoxy-, n-propoxy, isopropoxy-, tert-butoxy-, n-pentyloxy- and n-
hexyloxy-.
CA 02915419 2015-12-14
BHC123073FC
- 13 -
Alkoxyalkyl:
Alkoxyalkyl represents an alkyl radical which is substituted by alkoxy.
Here, C1-C6-alkoxy-C1-C6-alkyl- means that the bond to the remainder of the
molecule is via the
alkyl moiety.
Oxo:
Oxo, an oxo group or an oxo substituent, is understood to mean a double-bonded
oxygen atom =0.
Oxo may be bonded to atoms of suitable valency, for example to a saturated
carbon atom or to
sulphur.
Preference is given to the bond to carbon with formation of a carbonyl group -
C(r=0)- and to the
bond of two double-bonded oxygen atoms to sulphur with formation of a
sulphonyl group -(S=0)2-.
Allglamino:
Alkylamino represents an amino radical having one or two (independently
selected) alkyl
substituents having generally 1 to 6 (C1-C6-alkylamino) and preferably 1 to 3
(C1-C3-allcylamino)
carbon atoms.
(C1-C3)-Alkylamino represents, for example, a monoalkylamino radical having 1
to 3 carbon atoms or
a dialkylamino radical having 1 to 3 carbon atoms each per alkyl substituent.
Examples include:
methylamino-, ethylamino-, n-propylamino-, isopropylamino-, tert-butylamino-,
n-pentylamino-,
n-hexylamino-, N,N-dimethylamino-, N,N-diethylamino-, N-ethyl-N-methylamino-,
N-methyl-N-n-
propylamino-, N-isopropyl-N-n-propylamino-, N-tert-butyl-N-methylamino-, N-
ethyl-N-n-
pentylamino- and N-n-hexyl-N-methylamino-.
Particular preference is given to methylamino and N,N-dimethylamino-.
Alkvlaminocarbonyl:
Alkylaminocarbonyl represents the alkylamino-C(=0)¨ group having one or two
(independently
selected) alkyl substituents having generally 1 to 6 (Ci-C6-
allcylaminocarbonyl-) and preferably 1 to 3
(C1-C3-alkylaminocarbonyl-) carbon atoms.
Aminocarbonvl:
Aminocarbonyl represents the group H2N-C(=0)¨.
Alkvicarbonyl:
Allcylcarbonyl represents the ¨C(=0)-alkyl group having generally 1 to 3
carbon atoms in the alkyl
moiety.
Examples include acetyl- and propanoyl-. Preference is given to acetyl-.
CA 02915419 2015-12-14
BHC123073FC
- 14
Alkylcarbonylamino:
Alkylcarbonylamino represents the alkyl-C(=0)-NH¨ group having generally 1 to
6
(C1-C6-alkylcarbonylamino-), preferably 1 to 3 carbon atoms in the alkyl
moiety.
Alkoxycarbonyl:
Alkoxycarbonyl represents the ¨C(=0)-0-alkyl group having generally I to 6,
preferably 1 to 4 carbon
atoms in the alkyl moiety.
Examples include:
methoxycarbonyl-, ethoxycarbonyl-, propoxycarbonyl-, isopropoxycarbonyl-, tert-
butoxycarbonyl, n-
pentyloxycarbonyl- and n-hexyloxycarbonyl-.
Particular preference is given to tert-butoxycarbonyl-.
Alkylsulphonyl:
Alkylsulphonyl represents a straight-chain or branched saturated radical of
the formula ¨S(=0)2-
alkyl having generally 1 to 3 (C1-C3-alkylsulphonyl-) carbon atoms.
Preferred examples include:
methylsulphonyl-, ethylsulphonyl-, propylsulphonyl-.
Preference is given to methylsulphonyl-.
Alkylsulphonylamino:
Alkylsulphonylamino represents a straight-chain or branched saturated radical
of the formula -NH¨
S(0)2-alkyl having generally 1 to 3 (Ci-C3-alkylsulphonylamino-) carbon atoms
in the alkyl
group.
Preferred examples include:
methylsulphonylamino-, ethylsulphonylamino-, propylsulphonylamino-.
Alkylaminosulphonyl:
Allcylaminosulphonyl represents the alkylamino-S(=--0)2¨ group having one or
two (independently
selected) alkyl substituents having generally 1 to 6 (C1-C6-
alkylaminosulphonyl) and preferably 1 to 3
carbon atoms.
Preferred examples include:
methylaminosulphonyl-, ethylaminosulphonyl-, N,N-dimethylaminosulphonyl-.
Heteroatoms:
Heteroatoms are understood to mean oxygen, nitrogen or sulphur atoms.
Aryl
An aryl radical or aryl- means a monovalent mono- or bicyclic aromatic ring
system which consists
CA 02915419 2015-12-14
BHC123073FC
- 15 -
..
of carbon atoms. Examples are naphthyl-, biphenyl- and phenyl-.
Preference is given to phenyl-.
Heteroaryl:
A heteroaryl radical or heteroaryl- means a monovalent mono- or bicyclic
aromatic ring system
having at least one heteroatom. The heteroatoms may be nitrogen atoms, oxygen
atoms and/or
sulphur atoms. The bonding valency may be located at any aromatic carbon atom
or at a nitrogen
atom.
A monocyclic heteroaryl radical according to the present invention has 5 or 6
ring atoms.
Heteroaryl radicals having 5 ring atoms include, for example, the following
rings:
thienyl-, thiazolyl-, furyl-, pyrrolyl-, oxazolyl-, imidazolyl-, pyrazolyl-,
isoxazolyl-, isothiazolyl-,
oxadiazolyl-, triazolyl-, tetrazolyl- and thiadiazolyl-.
Heteroaryl radicals having 6 ring atoms include, for example, the following
rings:
pyridyl-, pyridazinyl-, pyrimidinyl-, pyrazinyl- and triazinyl-.
A bicyclic heteroaryl radical in accordance with the present invention has 9
or 10 ring atoms.
Heteroaryl radicals having 9 ring atoms include, for example, the following
rings:
phthalidyl-, thiophthalidyl-, indolyl-, isoindolyl-, indazolyl-,
benzothiazolyl-, benzofuryl-,
benzothienyl-, benzimidazolyl-, benzoxazolyl-, azocinyl-, indolizinyl-,
purinyl-.
Heteroaryl radicals having 10 ring atoms include, for example, the following
rings:
isoquinolinyl-, quinolinyl-, quinolizinyl-, quinazolinyl-, quinoxalinyl-,
cinnolinyl-, phthalazinyl-,
1,7- and 1,8-naphthyridinyl-, pteridinyl-.
Monocvclic heterocyclyl:
Monocyclic heterocyclyl- or a monocyclic heterocyclyl radical means a non-
aromatic monocyclic
ring system having at least one heteroatom or a hetero group. The heteroatoms
may be nitrogen
atoms, oxygen atoms and/or sulphur atoms.
A monocyclic heterocyclyl ring according to the present invention may have 3
to 8, preferably 4 to
7, especially preferably 5 or 6 ring atoms.
Preferred examples of monocyclic heterocyclyl radicals having 3 ring atoms are
as follows:
aziridinyl-.
Preferred examples of monocyclic heterocyclyl radicals having 4 ring atoms are
as follows:
CA 02915419 2015-12-14
BHC123073FC
- 16
azetidinyl-, oxetanyl-.
Preferred examples of monocyclic heterocyclyl radicals having 5 ring atoms are
as follows:
pyrrolidinyl-, imidazolidinyl-, pyrazolidinyl-, dioxolanyl- and
tetrahydrofuranyl-.
Preferred examples of monocyclic heterocyclyl radicals having 6 ring atoms are
as follows:
piperidinyl-, piperazinyl-, morpholinyl-, dioxanyl-, tetrahydropyranyl- and
thiomorpholinyl-.
Preferred examples of monocyclic heterocyclyl radicals having 7 ring atoms are
as follows:
azepanyl-, oxepanyl-, 1,3-diazepanyl-, 1,4-diazepanyl-.
Preferred examples of monocyclic heterocyclyl radicals having 8 ring atoms are
as follows:
oxocanyl-, azocanyl-.
From among the monocyclic heterocyclyl radicals, preference is given to 4- to
7-membered
saturated heterocyclyl radicals having up to two heteroatoms from the group
consisting of 0, N and
S.
Particular preference is given to morpholinyl-, piperidinyl-, piperazinyl- and
pyrrolidinyl-.
Heterospirocycloalkyl:
C5-C12-Heterospirocycloalkyl- is to be understood as meaning a fusion of two
saturated ring
systems sharing a common atom, where C5-C12 denotes the number of ring
members, where 1-4
carbon atoms are replaced by heteroatoms as defined above in any combination.
Examples are
azaspiro[2.3]hexyl-, azaspiro[3.3]heptyl-, oxaa72spiro[3.3]heptyl-,
thiaazaspiro[3.3]heptyl-,
oxaspiro[3.3]heptyl-, oxazaspiro[3.5]nonyl-, oxazaspiro[3.4]octyl-,
oxazaspiro[5.5]undecyl-,
diazaspiro[3.3]heptyl-, thiazaspiro[3.3]heptyl-, thia72spiro[3.4]octyl-,
azaspiro[5.5]decyl-, and the
further homologous spiro[3.4]-, spiro[4.4]-, spiro[5.5]-, spiro[6.6]-,
spiro[2.4]-, spiro[2.5]-,
spiro[2.6]-, spiro[3.5]-, spiro[3.6]-, spiro[4.5]-, spiro[4.6]- and spiro[5.6]-
systems in which 1-4
carbon atoms are replaced by heteroatoms. Preference is given to C6-C8-
heterospirocycloalkyl-.
Bicycloalkyl and heterobicycloalkyl:
C5-C12-Heterobicycloallcyl- is to be understood as meaning a fusion of two
saturated ring systems
jointly sharing two directly adjacent atoms, where C6-C12 denotes the number
of ring members,
where 1-4 carbon atoms are replaced by heteroatoms as defined above in any
combination.
Examples are systems derived from bicyclo[2.2.0]hexyl, bicyclo[3.3.0]octyl,
bicyclo[4.4.0]clecyl,
bicyclo[5.4.0]undecyl, bicyclo[3.2.0]heptyl, bicyclo[4.2.0]octyl,
bicyclo[5.2.0]nonyl,
bicyclo[6.2.0]decyl, bicyclo[4.3.0]nonyl, bicyclo[5.3.0]decyl,
bicyclo[6.3.0]undecyl and
bicyclo[5.4.0]undecyl in which 1-4 carbon atoms are replaced by heteroatoms,
for example
CA 02915419 2015-12-14
BHC123073FC
- 17 -
azabicyclo[3.3.0]octyl, azabicyclo[4.3.0]nonyl, diazabicyclo[4.3.0)nonyl,
oxazabicyclo[4.3.0]nonyl, thia7abicyclo[4.3.0]nonyl or azabicyclo[4.4.0]decyl,
and the further
possible combinations as per the definition. Preference is given to C6-C10-
heterobicycloalkyl.
Bridged heterocycloalkyl:
Bridged C6-C12-heterocycloalkyl- is to be understood as meaning a fusion of at
least two saturated
rings sharing two atoms which are not directly adjacent to one another, where
C6-C12 denotes the
number of ring members, and where 1-4 carbon atoms are replaced by heteroatoms
as defined
above in any combination. Examples are azabicyclo[2.2.1]heptyl-,
oxazabicyclo[2.2.1]heptyl-,
thiazabicyclo[2.2.1]heptyl-, diazabicyclo[2.2.1]heptyl-,
azabicyclo[2.2.2]octyl-,
dia7nbicyclo[2.2.2]octyl-, oxazabicyclo[2.2.2]octyl-,
thiazabicyclo[2.2.2]octyl-,
azabicyclo[3.2.1]octyl-, diazabicyclo[3.2.1]octyl-, oxazabicyclo[3.2.1]octyl-,
thiazabicyclo[3.2.1]octyl-, azabicyclo[3.3.1]nonyl, diazabicyclo[3.3.1]nonyl-
oxazabicyclo[3.3.1]nonyl-, thiazabicyclo[3.3.1]nonyl-, azabicyclo[4.2.1]nonyl-
,
diazabicyclo[4.2.1]nonyl-, oxazabicyclo[4.2.1]nonyl-,
thiazabicyclo[4.2.1]nonyl-,
a7abicyclo[3.3.2]decyl-, diazabicyclo[3.3.2]decyl-, oxazabicyclo[3.3.2]decyl-,
thiazabicyclo[3.3.2]decyl- or azabicyclo[4.2.2]decyl- and the further possible
combinations
according to the definition. Preference is given to bridged C6-Cio-
heterocycloalkyl-, by way of
example and with particular preference azabicyclo[2.2.2]octyl-.
Halogen
The term "halogen" includes fluorine, chlorine, bromine and iodine.
Preference is given to fluorine and chlorine.
Haloalkyl:
Haloalkyl represents an alkyl radical having at least one halogen substituent.
A halo-C1-C6-alkyl radical is an alkyl radical having 1-6 carbon atoms and at
least one halogen
substituent. If two or more halogen substituents are present, these may also
be different from one
another. Preference is given to fluoro-Ci-C3-alkyl radicals.
Preferred examples include:
the trifluoromethyl, 2,2,2-trifluoroethyl, pentafluoroethyl, 4,4,5,5,5-
pentafluoropentyl or
3,3,4,4,5,5,5-heptafluoropentyl group.
Particular preference is given to trifluoromethyl-.
Halogenalkoxy:
Haloalkoxy represents an alkoxy radical having at least one halogen
substituent.
A halo-C1-C6-alkoxy radical is an alkoxy radical having 1-6 carbon atoms and
at least one halogen
substituent. If two or more halogen substituents are present, these may also
be different from one
CA 02915419 2015-12-14
. BHC123073FC
- 18 -
,.
another. Preference is given to fluoro-C1-C3-alkoxy radicals.
Particularly preferred examples include:
trifluoromethoxy- or 2,2,2-trifluoroethoxy-.
Hydroxyalkyl:
Hydroxyalkyl represents an alkyl radical having at least one hydroxyl
substituent
A hydroxy-C1-C6-alkyl radical is an alkyl radical having 1-6 carbon atoms and
at least one
hydroxyl substituent
Aminoalkyl:
Aminoalkyl represents an alkyl radical having at least one amino substituent
An amino-C1-C6-alkyl radical is an alkyl radical having 1-6 carbon atoms and
at least one amino
substituent.
Alkylaminoalkyl:
Alkylaminoalkyl represents an alkyl radical having at least one alkylamino
substituent.
A Ci-C6-alkylamino-C1-C6-alkyl radical is an alkyl radical having 1-6 carbon
atoms and at least
one C1-C6-alkylamino substituent as defined above.
Preference is given to those compounds of the general formula I in which
X represents an oxygen atom,
R1a represents -0R6 or -NR7R8,
Rib and Ric independently of one another represent hydrogen, halogen, hydroxy,
cyano, or
represent a CI-C3-alkyl-, C1-C3-alkoxy-, fluoro-C1-C3-alkyl- or fluoro-C1-C3-
alkoxy-
radical,
R2 represents methyl- or ethyl-,
R3 represents Ci-C3-alkyl-, Ci-C3-alkoxy-, amino- or C1-
C3-alkylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
nitro, amino,
aminocarbonyl-, fluorine, chlorine, bromine,
Or
represent C1-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkylamino-, C1-C6-
alkylcarbonylamino-,
C1-C6-alkylaminocarbonyl- or Ci-C6-alkylaminosulphonyl- which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, carboxy, hydroxy-CI-C6-alkyl-, C1-C6-
alkoxy-,
CI-C6-alkoxy-Ci-C6-alkyl-, Ci-C6-alkylamino- or amino-C1-C6-alkyl-, a
monocyclic
heterocyclyl radical having 3 to 8 ring atoms and a monocyclic heteroaryl
radical
having 5 or 6 ring atoms, where the monocyclic heterocyclyl and heteroaryl
radicals
mentioned may for their part optionally be monosubstituted by CI-C3-alkyl-,
CA 02915419 2015-12-14
BHC123073FC
- 19
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, nitro, carboxy, C1-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, C1-C6-alkylamino-,
amino-
C1-C6-alkyl-, C1-C6-alkylamino-C1-C6-alkyl, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxY-,
C3-C10-cycloalkyl and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
or
represent monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, oxo, carboxy, C1-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-alkylamino-, amino-C1-C6-alkyl-, C1-
C6-
alkylamino-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, halo-Ci-C6-alkyl-, halo-C1-C6-
alkoxy-
, C3-Ci0-cycloalkyl- and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
R6 represents C3-C7-cycloalkyl- or C2-C6-alkyl- monosubstituted by C1-C3-
alkylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by oxo, Ci-C3-alkyl-, C1-C3-alkylcarbonyl-, C1-
C4-
alkoxycarbonyl-, benzyl- or C3-C7-cycloalkyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of CrC3-alkyl-, halogen and
CI-C3-
alkoxy-,
or
represents a benzyl radical, where the phenyl radical contained therein may
optionally
be mono- or disubstituted by identical or different substituents from the
group
consisting of Ci-C3-alkyl-, halogen and C1-C3-alkoxy-,
R7 represents C3-C7-cycloalkyl- or C2-C6-alkyl- monosubstituted by -
NR9R16,
or
represents a -C(=0)R11 group,
Or
represents a -S(-0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C12-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, C1-C3-alkylcarbonyl-, benzyl- or CI-Cr
alkoxycarbonyl-,
CA 02915419 2015-12-14
= BHC123073FC
- 20
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of Ci-C3-alkyl-, halogen and
C1-C3-
alkoxy-,
or
represents fluoro-C1-C3-alkyl or C1-C3-alkyl monosubstituted by a phenyl- or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may optionally be mono- or disubstituted by
identical or
different substituents from the group consisting of C1-C3-alkyl-, halogen and
C1-C3-
alkoxy-,
R8 represents hydrogen or Ci-C3-alkyl,
R9 and le independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, benzyl- or C1-C4-
alkoxycarbonyl-,
R" represents C3-C7-cycloallcyl- or C1-C6-alkyl-
monosubstituted by -NR9R10
,
Or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
Ci2-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, phenyl-Ci-C3-alkyl-
or
CI -C4-alkoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of Ci-C3-alkyl-, halogen and
C1-C3-
alkoxy-, and
R12 represents C1-C6-alkyl- which may optionally be mono- or disubstituted
by identical or
different substituents from the group consisting of fluorine, hydroxy, Ci-C3-
alkoxy-
and -NR9R10
,
or
represents fluoro-C1-C3-alkyl-,
or
represents C3-C7-cycloalkyl- which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of fluorine,
hydroxy, oxo,
cyano, C1-C3-alkyl-, C1-C3-alkoxy- and -NR9R10
,
CA 02915419 2015-12-14
BHC123073FC
- 21
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C12-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo, C1-C3-alkylcarbonyl-, CI-C4-
alkoxycarbonyl-
and phenyl-C1-C3-alkyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of halogen, CI-C3-alkyl- and
CI-C3-
alkoxy-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is given to those compounds of the general formula I in
which
X represents an oxygen atom,
Rja represents -0R6 or -WIZ' and is located in the meta- or para-
position with respect to
the benzodiazepine,
Rib
represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-, methoxy- or
trifluoromethyl-,
Ric represents hydrogen,
R2 represents methyl-,
R3 represents Ci-C3-alkylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
R6 represents C2-C4-alkyl- monosubstituted by C1-C3-alkylamino-,
Or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by methyl-, ethyl-, acetyl- or tert-
butoxycarbonyl-,
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different substituents from the group consisting of methyl-,
ethyl-,
fluorine, chlorine, bromine, methoxy- and ethoxy-,
or
represents a benzyl radical where the phenyl radical contained therein may
optionally
be mono- or disubstituted by identical or different substituents from the
group
consisting of methyl-, ethyl-, fluorine, chlorine, bromine, methoxy- and
ethoxy-,
represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -NR9R10
,
CA 02915419 2015-12-14
BHC123073FC
- 22 -
,
or
represents a -C(=0)R" group,
or
represents a -S(=0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine,
bromine, methoxy and ethoxy-,
or
represents fluoro-Ci-C3-alkyl or Ci-C3-alkyl monosubstituted by a phenyl- or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may optionally be mono- or disubstituted by
identical or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine
and bromine,
R8 represents hydrogen or C1-C3-alkyl,
R9 and 12.1 independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
R" represents CI-Ca-alkyl monosubstituted by -NR9R10,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine, and
Ri2
represents Ci-C3-alkyl-,
or
represents fluoro-Ci-C3-alkyl-,
CA 02915419 2015-12-14
BHC123073FC
- 23 -
or
represents C3-C7-cycloallcyl, which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of fluorine,
hydroxy, oxo,
methyl-, ethyl-, methoxy-, ethoxy- and N,N-dimethylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
R'a represents -0R6 and is located in the meta- or para-position with
respect to the
benzodiazepine,
Rib represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-,
methoxy- or
trifluoromethyl-,
Ric represents hydrogen,
R2 represents methyl-,
represents C1-C3-alkylamino-,
R4 and 12.5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-, and
R6 represents C2-C4-alkyl- monosubstituted by Ci-C3-alkylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by methyl-, ethyl-, acetyl- or tert-
butoxycarbonyl-,
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different substituents from the group consisting of methyl-,
ethyl-,
fluorine, chlorine, bromine, methoxy- and ethoxy-,
represents a benzyl radical where the phenyl radical contained therein may
optionally
be mono- or disubstituted by identical or different substituents from the
group
CA 02915419 2015-12-14
= BHC123073FC
- 24 -
consisting of methyl-, ethyl-, fluorine, chlorine, bromine, methoxy- and
ethoxy-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Ria represents -NleR8 and is located in the meta- or para-
position with respect to the
benzodiazepine,
Rlb represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-,
methoxy- or
trifluoromethyl-,
R represents hydrogen,
represents methyl-,
represents CI-C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
represents a -C(=0)R11 group,
R8 represents hydrogen or CI-C3-alkyl,
R9 and Rm independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-, and
R" represents Ci-C4-alkyl monosubstituted by -N-R9Rio,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
CI o-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those compounds of the general
formula I in which
CA 02915419 2015-12-14
= BHC123073FC
- 25 -
X represents an oxygen atom,
R1a represents -NR7R8 and is located in the meta- or para-
position with respect to the
benzodiazepine,
Rib
represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-, methoxy- or
trifluoromethyl-,
R1` represents hydrogen,
R2 represents methyl-,
represents C1-C3-alkylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
R7 represents a -S(=0)2R12 group,
R8 represents hydrogen or C1-C3-alkyl-, and
R12
represents C1-C3-alkyl-,
or
represents fluoro-C1-C3-alkyl-,
or
represents C3-C7-cycloalkyl which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of fluorine,
hydroxy, oxo,
methyl-, ethyl-, methoxy-, ethoxy- and N,N-dimethylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
Cio-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Ria represents -NR7R8 and is located in the meta- or para-position with
respect to the
benzodiazepine,
Rib represents hydrogen, fluorine, chlorine, bromine, cyano,
methyl-, methoxy- or
trifluoromethyl-,
CA 02915419 2015-12-14
BHC123073FC
- 26 -
Rlc represents hydrogen,
R2 represents methyl-,
represents C1-C3-alkylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
R7 represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -
NR9R10,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine,
bromine, methoxy and ethoxy-,
or
represents fluoro-Ci-C3-alkyl or Ci-C3-allcyl monosubstituted by a phenyl- or
a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may be mono- or disubstituted by identical or
different
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine
and
bromine,
R8 represents hydrogen or CI-C3-alkyl-, and
R9 and le independently of one another represent hydrogen or C1-C3-alkyl-,
Or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Very particular preference is given to those compounds of the general formula
I in which
X represents an oxygen atom,
Ria represents -0R6 or -NR7R8 and is located in the meta- or para-
position with respect to
the benzodiazepine,
Rib represents hydrogen or fluorine,
Ric represents hydrogen,
represents methyl-,
CA 02915419 2015-12-14
= BHC123073FC
- 27 -
R3 represents rnethylamino-,
R4 and le independently of one another represent hydrogen, chlorine, methoxy
or
trifluoromethoxy,
R6 represents N,N-dimethylaminoethyl-,
or represents a monosubstituted monocyclic heterocyclyl radical selected from
* __________________________ CN¨CH3 ( /N¨CH3
or represents a phenyl radical which may optionally be substituted by a
fluorine atom,
or represents a benzyl radical,
represents N,N-dimethylaminoethyl- or N,N-dimethylaminopropyl-,
or
represents a -C(=-0)R11 group,
or
represents a -S(=0)2R12 group,
or represents a radical selected from
\ / __ \
/
C N¨ CH3 N\ /N ¨CH3 N N¨CH3
N¨ CH3
0 \ 0
* _______________________ C * __ (
N , CH3 , 0¨ tButyl CF:
H3C
or
represents fluorophenyl-, pyridyl-, fluoropyridyl-, dimethyloxazolyl-,
methylpyrazolyl-, methoxyoxadiazolyl-, pyridazinyl- or methylimidazolyl-,
represents hydrogen or methyl, and
R'' represents -CH2-NH(CH3), -CH2-N(CH3)2, methylpiperidinyl-,
methylpyrrolyl-,
thiadiazolyl-,
or represents a radical selected from
CA 02915419 2015-12-14
BHC123073FC
- 28 -
(N 0 /
* _______________________ ( \ N N/ 0 N N¨ CH3
/ OtButyl \ __ /
\ _____________________________________________________________ /
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
R12 represents methyl-, trifluoromethyl-, phenyl-, benzyl-, cyclopropyl-,
tetrahydropyran-
4-y1 or pyrid-3-y1-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Exceptional preference is given to those compounds of the general formula I in
which
X represents an oxygen atom,
Ria represents -0R6 or -Nlele and is located in the para-position with
respect to the
benzodiazepine,
Rib and Ri c represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
represents methoxy- or trifluoromethoxy-,
R6 represents C2-C4-alkyl- which is monosubstituted by CI-C3-alkylamino- or
represents
a monocyclic heterocyclyl radical having 6 ring atoms which may optionally be
monosubstituted by methyl-,
R7 represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -
NR9R10
,
or
represents a -C(=0)R11 group,
or
represents a -S(=0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 6 ring atoms or represents
azabicyclo[2.2.2]oct-3-y1-, where the radicals mentioned may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
CA 02915419 2015-12-14
BHC123073FC
- 29 -
different substituents from the group consisting of methyl-, fluoro or methoxy-
,
R8 represents hydrogen or methyl-,
R9 and R1 independently of one another represent hydrogen or CI-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
RH
represents C1-C2-alkyl monosubstituted by -NR9R10
,
or
represents a monocyclic heterocyclyl radical having 5 or 6 ring atoms which
may
optionally be monosubstituted by methyl-, benzyl-, acetyl- or tert-
butoxycarbonyl-,
and
R12
represents Ci-C3-alkyl, fluoro-Ci-C3-alkyl- or C3-C7-cycloalkyl-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by methyl-, ethyl-, acetyl-, benzyl or tert-
butoxycarbonyl-,
or
represents a phenyl or pyridyl radical, where the radicals mentioned may
optionally be
mono- or disubstituted by identical or different substituents from the group
consisting
of methyl-, ethyl-, fluorine, chlorine or bromine,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Ria represents -0R6 and is located in the para-position with respect
to the benzodiazepine,
Rib and RI c represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-, and
R6 represents C2-C4-allcyl- which is monosubstituted by CI-C3-
alkylamino- or represents
a monocyclic heterocyclyl radical having 6 ring atoms which may optionally be
CA 02915419 2015-12-14
. BHC123073FC
- 30 -
,
monosubstituted by methyl-,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to 11.2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Rla represents -NR7R8 and is located in the para-position
with respect to the
benzodiazepine,
Rlb and Rle represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-,
R7 represents a -C(=0)R1' group,
or
represents a -S(=0)2R12 group,
R8 represents hydrogen,
R9 and 11.1 independently of one another represent hydrogen or Cl-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
R11
represents C1-C2-alkyl monosubstituted by -
NR R9 io,
or
represents a monocyclic heterocyclyl radical having 5 or 6 ring atoms which
may
optionally be monosubstituted by methyl-, benzyl-, acetyl- or tert-
butoxycarbonyl-,
and
R12 represents CI-C3-alkyl, fluoro-Ci-C3-alkyl- or C3-C7-
cycloalkyl-,
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by methyl-, ethyl-, acetyl-, benzyl or tert-
butoxycarbonyl-,
or
represents a phenyl or pyridyl radical, where the radicals mentioned may
optionally be
mono- or disubstituted by identical or different substituents from the group
consisting
CA 02915419 2015-12-14
BHC123073FC
- 31 -
,
of methyl-, ethyl-, fluorine, chlorine or bromine,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Rla
represents -NR7R8 and is located in the para-position with respect to the
benzodiazepine,
Rib and R lc represent hydrogen,
R2 represents methyl-,
le represents methylamino-,
R4 represents hydrogen or methoxy-,
R5 represents methoxy- or trifluoromethoxy-,
represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -NR9R10
,
or
represents a monocyclic heterocyclyl radical having 6 ring atoms or represents
azabicyclo[2.2.2]oct-3-y1-, where the radicals mentioned may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, fluoro or methoxy-
,
R8 represents hydrogen or methyl, and
R9 and RI independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
CA 02915419 2015-12-14
BHC123073FC
- 32
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Rla represents -0R6 or -NR7R8 and is located in the para-position with
respect to the
benzodiazepine,
RH' and R1c represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
R5 represents methoxy- or trifluoromethoxy-,
R6 represents a radical selected from
/ 3
( 7¨CH3 CH
\CH3
R7 represents a radical selected from
/ \
/N¨CH3
.-0¨N N¨CH,
\ ______________________________________________ /
CH
/ 3
CH3 ,
or
represents a -C(=0)R" group,
or
represents a -S(=0)2R12 group,
or
represents a radical selected from
(k)
= _______________________________________ ( 7¨CH3 CH,CN
0 ' N
or
represents a radical selected from
CA 02915419 2015-12-14
' BHC123073FC
-33..
H3C H3C
\
H,C
NN .¨ m ,CH, \
* _____________________ u
*--- 1 --N
,
N" '
H3C
N F-0
*--___, 1 z_N
-- N
*% ____________________________________________________________ ? ,
H3C¨O
F
N
* /= , * \\
____,si , . ____ _ \ _ \
/7N , *---C ,!`l
N '
F F
* ,
N¨N
or represents the radical
F F
F
*
.CH3
/ N
/
R8 represents hydrogen or methyl, and
R" represents a radical selected from
,CH,
H3C
N¨CH, N
*_./ N
,
or
represents a radical selected from
_______________________________________________________________ III
* ( )N¨CH, , * ( )N¨CH,/NH ,
\/\
CH,
( _______________________________________ \ p (CH,
* N CH,
/ 0
CA 02915419 2015-12-14
BHC123073FC
- 34 -
,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
R12
represents methyl-, trifluoromethyl-, phenyl-, benzyl-, cyclopropyl-,
tetrahydropyran-
4-y1 or pyrid-3-y1-,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Ria represents -0R6 and is located in the para-position
with respect to the benzodiazepine,
Rib and R lc represent hydrogen,
represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-, and
R6 represents a radical selected from
CH
*_<\7¨CH3 N/ 3
CH,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
X represents an oxygen atom,
Rla represents -INFIele and is located in the para-position
with respect to the
benzodiazepine,
CA 02915419 2015-12-14
BHC123073FC
- 35 -
Rib and R lc represent hydrogen,
R2 represents methyl-,
represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-,
R7 represents a -C(---0)R11 group,
or
represents a -S(=0)2R12 group,
R8 represents hydrogen,
R13 represents a radical selected from
CH,
i0)
H3C,
or
represents a radical selected from
= ( /N¨CH, NH N
CH,
0 ( CH,
= (/N CH,
0
and
R12 represents methyl-, trifluoromethyl-, phenyl-, benzyl-,
cyclopropyl-, tetrahydropyran-
4-y1 or pyrid-3-y1-,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those compounds of the general
formula I in which
CA 02915419 2015-12-14
BHC123073FC
- 36 -
X represents an oxygen atom,
Rla
represents -NR7R8 and is located in the para-position with respect to the
benzodiazepine,
leb and R 1c represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
R5 represents methoxy- or trifluoromethoxy-,
R7 represents a radical selected from
/ \
/ ______________________ NI\ 7¨CH3
/
N¨CH3
CH,
CH3
or
represents a radical selected from
_______________________ p¨CH, _______ CH3 * __ <\/6
0 ' N
or
represents a radical selected from
H C H 3C
3 \ H 3C
N¨ ,CH3
* *
/
I
N"
H3C
N-
0
* N /_N
111 * F __
* ___________________________________________________ '
H3C¨
* * * * __ C
N '
N
*
N¨N
or represents the radical
CA 02915419 2015-12-14
BHC123073FC
- 37 -
F F
CH
3
and
represents hydrogen or methyl-,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
A subgroup of compounds of the general formula I are those in which
X represents an oxygen or sulphur atom,
Ria represents -OR' or -NR7R8,
Rib and R'' independently of one another represent hydrogen, halogen, hydroxy,
cyano, nitro or
represent a C1-C6-alkyl-, C1-C6-alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, halo-C1-C6-
alkyl-,
halo-C1-C6-alkoxy-, C3-C10-cycloalkyl radical or a monocyclic heterocyclyl
radical
having 3 to 8 ring atoms,
R2 represents a C1-C3-alkyl or trifluoromethyl or a C3- or
Crcycloalkyl radical,
R3 represents C3-C3-alkyl-, C1-C3-alkoxy-, amino- or C1-C3-alkylamino-
,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
nitro, amino,
aminocarbonyl-, fluorine, chlorine, bromine,
or
represent C1-C6-alkyl-, CI-C6-alkoxy-, Ci-C6-alkylamino-, C1-C6-
alkylcarbonylamino-,
C1-C6-alkylaminocarbonyl- or C1-C6-alkylaminosulphonyl- which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, carboxy, hydroxy-C1-C6-alkyl-, C1-C6-
alkoxy-,
C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-alkylamino-, amino-Ci-C6-alkyl-, a monocyclic
heterocyclyl radical having 3 to 8 ring atoms and a monocyclic heteroaryl
radical
having 5 or 6 ring atoms where the monocyclic heterocyclyl and heteroaryl
radicals
mentioned may for their part optionally be monosubstituted by C1-C3-alkyl,
CA 02915419 2015-12-14
BHC123073FC
- 38 -
or
represent C3-C10-cycloallcyl- which may optionally be mono- or polysubstituted
by
identical or different substituents from the group consisting of halogen,
amino,
hydroxy, C1-C6-alkyl-, C1-C6-alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-
alkylamino-,
amino-C1-C6-alkyl-, C1-C6-alkylamino-C1-C6-alkyl, halo-CI-C6-alkyl-, halo-C1-
C6-
alkoxy- and a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, nitro, carboxy, Ci-C6-alkyl-, Ci-
C6-
alkoxy-, Ci-C6-alkoxy-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, Ci-C6-alkylamino-,
amino-
Ci-C6-alkyl-, C1-C6-alkylamino-C1-C6-alkyl, halo-Ci-C6-alkyl-, halo-CI-C6-
alkoxY-,
C3-Clo-cycloalkyl and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
or
represent monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, oxo, carboxy, C1-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-alkylamino-, amino-Ci-C6-alkyl-, C1-
C6-
alkylamino-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxy-
, C3-Ci0-cycloalkyl- and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
or
represent phenyl- which may optionally be mono- or polysubstituted by
identical or
different substituents from the group consisting of halogen, amino, hydroxy,
cyano,
nitro, carboxy, C1-C6-alkyl-, Cj-C6-alkoxy-, C,-C6-alkoxy-Ci-C6-alkyl-, C1-C6-
alkylamino-, amino-C1-C6-alkyl-, C1-C6-alkylaminocarbonyl-, Ci-C6-
alkylaminosulphonyl-, Ci-C6-alkylamino-Ci-C6-alkyl-, hydroxy-C1-C6-alkyl-,
halo-Ci-
C6-alkyl-, halo-C1-C6-alkoxy-, C3-C10-cycloalkyl- and a monocyclic
heterocyclyl
radical having 3 to 8 ring atoms,
R6 represents C3-C7-cycloalkyl- or C2-C6-alkyl- monosubstituted by C1-
C6-alkylamino-,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms which
may
optionally be monosubstituted by oxo, CI-C3-alkyl-, Ci-C3-allcylcarbonyl-, C1-
C4-
alkoxycarbonyl-, phenyl-Ci-C3-alkyl- or C3-C7-cycloallcyl-,
or
represents a mono- or bicyclic aryl or heteroaryl radical, where the radicals
mentioned
may optionally be mono- or disubstituted by identical or different
substituents from
the group consisting of halogen, hydroxy, cyano, Ci-C3-alkyl, fluoro-Ci-C3-
alkyl,
hydroxy-Ci-C3-alkyl, C1-C3-alkoxy-, C1-C3-alkylamino-, amino-Ci-C3-alkyl-, C1-
C3-
CA 02915419 2015-12-14
, BHC123073FC
,
- 39 -
alkylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, C1-C3-alkylcarbonylamino-, C1-
C3-alkylsulphonylamino-, C1-C3-alkylcarbonyl-, C1-C3-alkylsulphonyl- and
trifluoromethoxy-,
R7 represents C3-C7-cycloalkyl- or C2-C6-alkyl-
monosubstituted by -NR9R10
,
or
represents a -C(=0)RI I group,
or
represents a -S(=0)2RI2 group,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
C12-heterocycloalkyl radical, a C5-C,2-heterospirocycloalkyl radical or a C6-
C12-
heterobicycloalkyl radical, where the radicals mentioned may optionally be
mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
CI-C3-alkyl-, CI -C3-alkylcarbonyl-, C1-C4-alkoxycarbonyl-, phenyl-Ci-C3-alkyl-
and
C3-C7-cycloalkyl-,
or
represents a mono- or bicyclic aryl- or heteroaryl radical, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the group consisting of halogen, hydroxy, cyano, C1-C3-
alkyl,
fluoro-Ci-C3-alkyl, hydroxy-C1-C3-alkyl, Ci-C3-alkoxy-, CI-C3-alkylamino-,
amino-
C1-C3-alkyl-, CI-C3-allcylaminocarbonyl-, C1-C3-allcylaminosulphonyl-, CI-C3-
alkylcarbonylamino-, C1-C3-alkylsulphonylamino-, CI-C3-alkylcarbonyl-, C1-C3-
alkylsulphonyl- and trifluoromethoxy-,
or
represents fluoro-Ci-C3-alkyl or CI-C3-alkyl monosubstituted by a phenyl or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may be mono- or disubstituted by identical or
different
substituents from the group consisting of CI-C3-alkyl-, halogen and CI-C3-
alkoxY-,
R8 represents hydrogen or Ci-C6-alkyl,
R9 and RI independently of one another represent hydrogen or Ci-C6-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 3 to 8 ring atoms which may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, Ci-C4-
alkoxycarbonyl-,
phenyl-Ci-C3-alkyl- or C3-C7-cycloallcyl-,
R11 represents C3-C7-cycloalkyl- or C,-C6-alkyl-
monosubstituted by -NR9R10,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
CA 02915419 2015-12-14
. BHC123073FC
.
- 40 -
C12-heterocycloalkyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-
C12-
heterobicycloalkyl radical, where the radicals mentioned may optionally be
mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
C1-C3-alkyl-, C1-C3-alkylcarbonyl-, CI-C4-alkoxycarbonyl-, phenyl-Ci-C3-alkyl-
and
C3-C7-cycloalkyl-,
or
represents a mono- or bicyclic aryl or heteroaryl radical, where the radicals
mentioned
may optionally be mono- or disubstituted by identical or different
substituents from
the group consisting of halogen, hydroxy, cyano, Ci-C3-alkyl, fluoro-Ci-C3-
alkyl,
1 0 hydroxy-Ci-C3-allcyl, C1-C3-alkoxy-, C1-C3-alkylamino-, amino-CI-C3-
alkyl-, C1-C3-
alkylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, C1-C3-alkylcarbonylamino-, CI
-
C3-alkylsulphonylamino-, Ci-C3-alkylcarbonyl-, Ci-C3-alkylsulphonyl- and
trifluoromethoxy-, and
R12 represents Ci-C6-alkyl- which may optionally be mono-
or disubstituted by identical or
1 5 different substituents from the group consisting of halogen,
hydroxy, carboxy, cyano,
C1-C6-alkoxy-, -N11.1 R11, phenyl, a monocyclic heteroaryl radical having 5 or
6 ring
atoms or a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
where
phenyl and the monocyclic heteroaryl radical having 5 or 6 ring atoms for
20 their part may be mono- to trisubstituted by identical or
different
substituents from the group consisting of halogen, cyano, Ci-C3-alkyl,
trifluoromethyl, C1-C3-alkoxy- and trifluoromethoxy-,
and in which
the monocyclic heterocyclyl radical for its part may optionally be mono- or
25 disubstituted by identical or different substituents from
the group
consisting of oxo, Cj-C3-alkyl-, CI-C3-alkylcarbonyl-, C1-C4-
alkoxycarbonyl-, phenyl-Ci-C3-alkyl- and C3-C7-cycloalkyl-,
or
represents C3-Cio-cycloalkyl- which may optionally be mono- or polysubstituted
by
30 identical or different substituents from the group consisting of
fluorine, hydroxy, oxo,
cyano, C1-C3-alkyl-, C1-C3-alkoxy- and -NR10R1 I,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-
C12-heterocycloalkyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-
C12-
35 heterobicycloalkyl radical, where the radicals mentioned may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of oxo,
C1-C3-alkyl-, C1-C3-alkylcarbonyl-, C1-C4-alkoxycarbonyl-, phenyl-CI-C3-allcyl-
and
C3-C7-cycloalkyl-,
CA 02915419 2015-12-14
BHC123073FC
- 41
or
represents an aryl or heteroaryl radical, where the radicals mentioned may
optionally
be mono- or disubstituted by identical or different substituents from the
group
consisting of halogen, hydroxy, cyano, C1-C3-alkyl, fluoro-C1-C3-alkyl,
hydroxy-C1-
C3-alkyl, Ci-C3-alkoxy-, C1-C3-allcylamino-, amino-C1-C3-alkyl-, C1-C3-
alkylaminocarbonyl-, C1-C3-allcylaminosulphonyl-, C1-C3-alkylcarbonylamino-,
C1-
C3-alkylsulphonylamino-, C1-C3-alkylcarbonyl-, C1-C3-alkylsulphonyl- and
trifluoromethoxy-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
A further subgroup thereof are those compounds of the general formula I in
which
X represents an oxygen atom,
R1a represents -0R6 or -Nlele,
RI6 and RI' independently of one another represent hydrogen, halogen, hydroxy,
cyano, or
represent a Ci-C3-alkyl-, C1-C3-alkoxy-, fluoro-C1-C3-alkyl- or fluoro-C1-C3-
alkoxy-
radical,
represents methyl- or ethyl-,
le represents Ci-C3-alkyl-, C1-C3-alkoxy-, amino- or Ci-C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
nitro, amino,
aminocarbonyl-, fluorine, chlorine, bromine,
or
represent Ci-C6-alkyl-, C1-C6-alkoxy-, CI-C6-alkylamino-, C1-C6-
alkylcarbonylamino-,
C1-C6-alkylaminocarbonyl- or C1-C6-alkylaminosulphonyl- which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, carboxy, hydroxy-Ci-C6-alkyl-,
C1-C6-alkoxy-Ci-C6-alkyl-, CI-C6-alkylamino- or amino-Ci-C6-alkyl-, a
monocyclic
heterocyclyl radical having 3 to 8 ring atoms and a monocyclic heteroaryl
radical
having 5 or 6 ring atoms where the monocyclic heterocyclyl and heteroaryl
radicals
mentioned may for their part optionally be monosubstituted by Ci-C3-alkyl,
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, nitro, carboxy, C1-C6-
alkoxy-, Ci-C6-alkoxy-Ci-C6-alkyl-, hydroxy-Ci-C6-alkyl-, Ci-C6-alkylamino-,
amino-
Ci-C6-alkyl-, Ci-C6-alkylamino-Ci-C6-alkyl, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxy-,
C3-Cio-cycloalkyl and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
CA 02915419 2015-12-14
' BHC123073FC
- 42 -
,
or
represent monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be
mono- or polysubstituted by identical or different substituents from the group
consisting of halogen, amino, hydroxy, cyano, oxo, carboxy, C1-C6-alkyl-, C1-
C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1-C6-alkylamino-, amino-C1-C6-alkyl-, CI-
C6-
alkylamino-C1-C6-alkyl-, hydroxy-C1-C6-alkyl-, halo-C1-C6-alkyl-, halo-C1-C6-
alkoxy-
, C3-Cio-cycloalkyl- and a monocyclic heterocyclyl radical having 3 to 8 ring
atoms,
R6 represents C3-C7-cycloallcyl- or C2-C6-alkyl-
monosubstituted by C1-C3-alkylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may
optionally be monosubstituted by oxo, Ci-C3-alkyl-, Ci-C3-alkylcarbonyl-, C1-
C4-
alkoxycarbonyl-, benzyl- or C3-C7-cycloallcyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of C1-C3-alkyl-, halogen and
C1-C3-
alkoxy-,
R7 represents C3-C7-cycloalkyl- or C2-C6-alkyl-
monosubstituted by -NR9R10
,
or
represents a -C(=0)R" group,
or
represents a -S(=0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C12-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo, C1-C3-alkyl-, CI-C3-alkylcarbonyl-, benzyl- or CI-C4-
alkoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of Ci-C3-alkyl-, halogen and
C1-C3-
alkoxy-,
or
represents fluoro-C1-C3-alkyl or Ci-C3-alkyl monosubstituted by a phenyl or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may be mono- or disubstituted by identical or
different
substituents from the group consisting of CI-C3-alkyl-, halogen and CI-C3-
alkoxy-,
R8 represents hydrogen or CI-C3-alkyl,
CA 02915419 2015-12-14
BHC123073FC
- 43 -
R9 and le independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, C1-C3-alkylcarbonyl-, benzyl- or Ci-C4-
alkoxycarbonyl-,
R" represents C3-C7-cycloallcyl- or CI-C6-alkyl- monosubstituted by -
NR9R16,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C12-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, Ci-C3-allcylcarbonyl-, phenyl-Ci-C3-
alkyl- or
C1-C4-alkoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of C1-C3-alkyl-, halogen and
CI-C3-
alkoxy-, and
represents Ci-C6-alkyl- which may optionally be mono- or disubstituted by
identical or
different substituents from the group consisting of fluorine, hydroxy, C1-C3-
alkoxy-
and -NR10R11,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Also of interest are subgroups of the general formula I in which
X represents an oxygen atom,
Rla
represents -0R6 or -NR7R8 and is located in the meta- or para-position with
respect to
the benzodiazepine,
Rib
represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-, methoxy- or
trifluoromethyl-,
R16 represents hydrogen,
R2 represents methyl-,
represents C,-C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C,-C3-alkoxy-, fluoro-Ci-C3-alkoxy-,
R6 represents C2-C4-alkyl- which is monosubstituted by C,-C3-
alkylamino- or represents
a monocyclic heterocyclyl radical having 4 to 7 ring atoms which may
optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
CA 02915419 2015-12-14
BHC123073FC
- 44
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different substituents from the group consisting of methyl-,
ethyl-,
fluorine, chlorine, bromine, methoxy- and ethoxy-,
R.' represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -
NR9Rio,
or
represents a -C(=0)R11 group,
or
represents a -S(=0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine,
bromine, methoxy and ethoxy-,
or
represents fluoro-C1-C3-alkyl or C1-C3-alkyl monosubstituted by a phenyl- or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may be mono- or disubstituted by identical or
different
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine
and
bromine,
R8 represents hydrogen or CI-C3-alkyl,
R9 and R1 independently of one another represent hydrogen or Ci-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
R."
represents CI-Ca-alkyl monosubstituted by -NR9RIO,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
C10-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
CA 02915419 2015-12-14
BHC123073FC
- 45
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine, and
R12
represents C1-C3-alkyl-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those subgroups of compounds of
the general formula
I in which
X represents an oxygen atom,
Rla
represents -0R6 and is located in the meta- or para-position with respect to
the
benzodiazepine,
Rib
represents hydrogen, fluorine, chlorine, bromine, cyano, methyl-, methoxy- or
trifluoromethyl-,
Ric represents hydrogen,
R2 represents methyl-,
R3 represents CI-C3-allcylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
R6 represents C2-C4-allcyl- which is monosubstituted by C1-C3-
allcylamino- or represents
a monocyclic heterocyclyl radical having 4 to 7 ring atoms which may
optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different substituents from the group consisting of methyl-,
ethyl-,
fluorine, chlorine, bromine, methoxy- and ethoxy-,
R9 and R1 independently of one another represent hydrogen or Ci-C3-allcyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to those subgroups of compounds of
the general formula
I in which
CA 02915419 2015-12-14
4 BHC123073FC
.
- 46 -
X represents an oxygen atom,
Rla represents -NR7R8 and is located in the meta- or para-
position with respect to the
benzodiazepine,
RIb represents hydrogen, fluorine, chlorine, bromine,
cyano, methyl-, methoxy- or
trifluoromethyl-,
RIC represents hydrogen,
R2 represents methyl-,
R3 represents C1-C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, C1-C3-alkoxy-, fluoro-CI-C3-alkoxy-,
represents a -C(=O)R11 group,
R8 represents hydrogen or C1-C3-alkyl,
R9 and RI independently of one another represent hydrogen or Ci-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-, and
R" represents Ci-C4-alkyl monosubstituted by -NR9R10
,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6'
C10-heterocycloallcyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine or
bromine,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particular preference is furthermore given to a subgroup of compounds of the
general formula I in
which
X represents an oxygen atom,
RI.
represents -N12.71t8 and is located in the meta- or para-position with respect
to the
benzodiazepine,
Rib represents hydrogen, fluorine, chlorine, bromine,
cyano, methyl-, methoxy- or
trifluoromethyl-,
CA 02915419 2015-12-14
. BHC123073FC
. - 47 -
R1` represents hydrogen,
R2 represents methyl-,
R3 represents C1-C3-alkylamino-,
R4 and R5 independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, Ci-C3-alkoxy-, fluoro-C1-C3-alkoxy-,
R7 represents a -S(=0)2R12 group,
R8 represents hydrogen or C1-C3-alkyl,
R9 and le independently of one another represent hydrogen or Ci-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-, and
R12 represents C1-C3-alkyl-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Particularly preferred subgroups are furthermore those compounds of the
general formula I in
which
X represents an oxygen atom,
Rla represents -NR7R8 and is located in the meta- or para-
position with respect to the
benzodiazepine,
Rib represents hydrogen, fluorine, chlorine, bromine,
cyano, methyl-, methoxy- or
trifluoromethyl-,
Ric represents hydrogen,
R2 represents methyl-,
R3 represents C1-C3-alkylamino-,
R4 and le independently of one another represent hydrogen, hydroxy, cyano,
fluorine, chlorine,
bromine, Ci-C3-alkoxy-, fluoro-Ci-C3-alkoxy-,
le represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -NR91e,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-
Ci0-heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine,
CA 02915419 2015-12-14
. BHC123073FC
-, - 48 -
bromine, methoxy and ethoxy-,
or
represents fluoro-C1-C3-alkyl or C1-C3-alkyl monosubstituted by a phenyl or a
monocyclic heteroaryl radical having 5 or 6 ring atoms, where the phenyl and
heteroaryl radicals mentioned may be mono- or disubstituted by identical or
different
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine
and
bromine,
R8 represents hydrogen or C1-C3-alkyl-, and
R9 and It'9 independently of one another represent hydrogen or Ci-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Very particular preference is given to those subgroups of compounds of the
general formula I in
which
X represents an oxygen atom,
Ria represents -0R6 or -NR7R8 and is located in the meta-
or para-position with respect to
the benzodiazepine,
RI b represents hydrogen or fluorine,
Rk represents hydrogen, ,
R2 represents methyl-,
R3 represents methylamino-,
R4 and R5 independently of one another represent hydrogen, chlorine, methoxy
or
trifluoromethoxy,
R6 represents NN-dimethylaminoethyl-,
or represents a monosubstituted monocyclic heterocyclyl radical selected from
* _______________________________ C N¨CH3N¨CH
\
* _____________________________________________________ ( 3
or represents a phenyl radical which may optionally be substituted by a
fluorine atom,
R7 represents N,N-dimethylaminoethyl- or N,N-dimethylaminopropyl-,
or
CA 02915419 2015-12-14
4 BHC123073FC
- 49
represents a -C(=0)R" group,
or
represents a -S(=0)2CH3 group,
or represents a radical selected from
/ \\
/
N- CH,
/ __________________________ N __ /N- CH3 *--0---N N- CH3 __ * ( N -
CH,
, *
*¨( 0 CF, \/b * ___________ CN 04
* < __________________________________________ \/ )
CH, , 0¨ tButyl
H,C
or
represents fluorophenyl-, pyridyl-, fluoropyridyl-, dimethyloxazoly1-,
methylpyrazolyl-, methoxyoxadiazolyl-, pyridazinyl- or methylimidazolyl-,
R8 represents hydrogen or methyl, and
R" represents -CH2-NH(CH3), -CH2-N(CH3)2, rnethylpiperidinyl-,
methylpyrrolyl-,
thiadiazolyl-,
or represents a radical selected from
* = 0 * ________________________________________ \N¨ CH
"N zr--N 0 / 3
OtButyl \ __ /
\ ___________________________________________________________________
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, enantiomers, diastereomers, racemates, tautomers,
solvates, physiologically
acceptable salts and solvates of these salts.
Exceptional preference is given to those subgroups of compounds of the general
formula I in which
X represents an oxygen atom,
Rla represents -0R6 or -NR7R8 and is located in the para-
position with respect to the
benzodiazepine,
Rib and Ric represent hydrogen,
CA 02915419 2015-12-14
BHC123073FC
- 50 -
R2 represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
represents methoxy- or trifluoromethoxy-,
R6 represents C2-C4-alkyl- which is monosubstituted by CI-C3-alkylamino- or
represents
a monocyclic heterocyclyl radical having 6 ring atoms which may optionally be
monosubstituted by methyl-,
represents C3-C7-cycloalkyl- or C2-C4-alkyl- monosubstituted by -NR9R10
,
or
represents a -C(=0)RI group,
or
represents a -S(=0)2R'2 group,
or
represents a monocyclic heterocyclyl radical having 6 ring atoms or represents
azabicyclo[2.2.2]oct-3-y1-, where the radicals mentioned may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, fluor or methoxy-
,
R8 represents hydrogen or methyl-,
R9 and le independently of one another represent hydrogen or Ci-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
R" represents Cl-C2-alkyl monosubstituted by -NR9R10,
or
represents a monocyclic heterocyclyl radical having 5 or 6 ring atoms which
may
optionally be monosubstituted by methyl-, benzyl-, acetyl- or tert-
butoxycarbonyl-,
and
R'2 represents CI-C3-alkyl,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
CA 02915419 2015-12-14
BHC123073FC
- 51
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
Ria represents -0R6 and is located in the para-position with respect to the
benzodiazepine,
Rib and Ric represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-,
R6 represents C2-C4-alkyl- which is monosubstituted by C1-C3-
allcylamino- or represents
a monocyclic heterocyclyl radical having 6 ring atoms which may optionally be
monosubstituted by methyl-, and
R9 and RI independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
Ria represents -NR7R8 and is located in the para-position with respect
to the
benzodiazepine,
Rib and Ric represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
represents methoxy-,
R7 represents a -C(-0)R11 group,
or
represents a -S(=0)2R12 group,
R8 represents hydrogen,
CA 02915419 2015-12-14
BHC123073FC
*
- 52 -
,
R9 and R1 independently of one another represent hydrogen or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-, and
Rn
represents C1-C7-alkyl monosubstituted by -NR
9R10,
or
represents a monocyclic heterocyclyl radical having 5 or 6 ring atoms which
may
optionally be monosubstituted by methyl-, benzyl-, acetyl- or tert-
butoxycarbonyl-,
and
R12 is Ci-C3-alkyl;
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
Rla represents -NR7R8 and is located in the para-position
with respect to the
benzodiazepine,
Rib and Ric represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
12. represents methoxy- or trifluoromethoxy-,
R7 represents C3-C7-cycloalkyl- or C2-C4-alkyl-
monosubstituted by -NR9R10
,
or
represents a monocyclic heterocyclyl radical having 6 ring atoms or represents
azabicyclo[2.2.2]oct-3-y1-, where the radicals mentioned may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where
the radicals mentioned may optionally be mono- or disubstituted by identical
or
different substituents from the group consisting of methyl-, fluoro or methoxy-
,
R8 represents hydrogen or methyl, and
CA 02915419 2015-12-14
BHC123073FC
- 53
R9 and le independently of one another represent hydrogen or C1-C3-alkyl-,
Or
together with the nitrogen atom to which they are attached represent a
monocyclic
heterocyclyl radical having 4 to 7 ring atoms which may optionally be
monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula 1 in which
X represents an oxygen atom,
Ria represents -0R6 or -NR7R8 and is located in the para-position with
respect to the
benzodiazepine,
Rib and Ric represent hydrogen,
R2 represents methyl-,
R3 represents methylamino-,
represents hydrogen or methoxy-,
R5 represents methoxy- or trifluoromethoxy-,
R6 represents a radical selected from
CH
( /N¨CH, \ __ NI 3
CH3
represents a radical selected from
/ \
7¨CH3 \N¨CH,
\ ______________________________________________ /
,CH,
N\
CH, ,
or
represents a -C(=0)R11 group,
or
represents a radical selected from
CA 02915419 2015-12-14
BHC123073FC
,
- 54 -
,
CH3 _______________________________________________________
*-( \ C
N-CH, * N
or
represents a radical selected from
H 3C\
H C H3C
N.- N,... ,CH, 3 \
___________________________________________________________________ ?
* _________________________ Ul , _ 11 N-...,.
*
*-i l ---N
N---
H,C
N._ F
0
* ___________________________ / 1
--N , * 4/1
* 40 F . _____________________________________________________ c) ,
,
H,C-C)
F
* N
____1 _________________________ ) /
*-- *
/7 , *
_____________________________________________________________ N '
F F
* µ ,
N-N
or represents the radical
F F
F
*
,CH,
/ N
/
R8 represents hydrogen or methyl, and
R" represents a radical selected from
CH,
0 Ni
H,C
N-CH, N--)
_____/ N
õ
,
or
represents a radical selected from
CA 02915419 2015-12-14
BHC123073FC
- 55
( 7¨CH3 (\ NH
/ ' (
CH,
\ 0 ( CH,
7,1¨ CH3
o
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
Ria represents -0R6 and is located in the para-position with respect to the
benzodiazepine,
Rib and Ric represent hydrogen,
represents methyl-,
R3 represents methylamino-,
R4 represents methoxy-,
R5 represents methoxy-, and
R6 represents a radical selected from
\_ CH3
* 7¨CH,
CH3
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
CA 02915419 2015-12-14
BHC123073FC
- 56 -
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
Ria represents -NR7R8 and is located in the para-position with respect
to the
benzodiazepine,
R and R1c represent hydrogen,
R2 represents methyl-,
le represents methylamino-,
R4 represents methoxy-,
represents methoxy-,
12.2 represents a -C(=0)RI I group,
or
represents a -S(----0)2R12 group,
R8 represents hydrogen,
R11
represents a radical selected from
CH3
0\
H3C\
N¨CH3
'
or
represents a radical selected from
N¨CH3 NH ' \N
/
CH3
0 __ CH,
CH3
0
and
R12
represents methyl-,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
CA 02915419 2015-12-14
BHC123073FC
- 57 -
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
Exceptional preference is furthermore given to those subgroups of compounds of
the general
formula I in which
X represents an oxygen atom,
RI a represents -NR7R8 and is located in the para-position with respect
to the
benzodiazepine,
Rib and Ric represent hydrogen,
represents methyl-,
R3 represents methylamino-,
R4 represents hydrogen or methoxy-,
R5 represents methoxy- or trifluoromethoxy-,
le represents a radical selected from
\
/¨N\ /N¨CH3 \N¨CH,
/
CH,
\
CH, ,
OT
represents a -C(=0)R11 group,
or
represents a radical selected from
CH
\N¨CH3 CN
0 N
or
represents a radical selected from
CA 02915419 2015-12-14
BHC123073FC
- 58 -
H3C H3C
\ H C
,CF13 3 \
* ________________ SI , * 11 N,
* I * __
N"--- '
H3C
N-... F
0
*1___I 1
--N , * , . = F, * _______ c¨N_? ,
H3C ¨
F
N
* *
* N * ____ K /N
F F
* ,
N¨N
or represents the radical
F F
F
/ N
/
and
R8 represents hydrogen or methyl-,
where "*" in each case indicates the point of attachment to the remainder of
the
molecule,
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts,
with the proviso that the stereocentre represented by the carbon atom,
attached to R2, of the
benzodiazepine skeleton is present either in racemic form or predominantly or
completely in the (S)
configuration.
The following compounds are of interest:
- ( )-7,8-dimethoxy-N,4-dimethy1-1-{4-[(1-methylpiperidin-4-
yDamino]phenyl 1 -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4.9-7,8-dimethoxy-N,4-dimethy1-1-{4-[(1-methylpiperidin-4-
yDamino]phenyl} -4,5-
dihydro-3H-2,3-benzodiazepine-3-earboxamide,
CA 02915419 2015-12-14
BHC123073FC
-59-
- (4R)-7,8-dimethoxy-N,4-dimethy1-1- {4-[(1-methylpiperidin-4-
y0amino]phenyll -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- { 4-[methyl(pyridin-3-
yDamino]phenyl 1 -4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1-{4-[(2-fluorophenyl)amino]phenyl } -7,8-dimethoxy-N,4-di methy1-4,5-
dihydro-3H-
2,3-benzodiazepine-3-carboxami de,
- (4R)-1-{4-[(2-fluorophenypamino]phenyll -7,8-dimethoxy-N,4-dimethy1-
4,5-dihydro-3H-
2,3-benzodi azepine-3 -carboxami de,
- (4S)-1- {4-[(2-fl uorophenypamino]phenyll-7,8-dimethoxy-N,4-dimethyl-
4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide,
- ( )-1- {4-[(3,5-dimethylisoxazol-4-yDamino]phenyl 1 -7,8-dimethoxy-
N,4-dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4R)-1- {4-[(3,5-dimethyli soxazol-4-yDamino]phenyl 1 -7,8-dimethoxy-
N,4-dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-1- {4-[(3,5-dimethylisoxazol-4-yDamino]phenyl } -7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-1-(4-{ [2-(dimethylamino)ethyl] amino { pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-1-(4-{ [2-(dimethylamino)ethyl]amino{ pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1-14-[(4-fluorophenypmethylamino]phenyll -7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-{4-[(1-methyl-1H-pyrazol-5-
yDamino]phenyl} -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4R)-7,8-dimethoxy-N,4-dimethy1-1- {4-[(1-methy1-1H-pyrazol-5-y0amino]phenyl
} -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1- 14-[(1-methy1-1H-pyrazol-5-
yflamino]phenyll -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-1-[4-(1-azabicyclo[2.2.2]oct-3-ylamino)pheny1]-7,8-dimethoxy-N,4-
dimethyl-4,5-
CA 02915419 2015-12-14
BHC123073FC
=
- 60 -
=
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (45)-144-(1-azabicycl o [2.2.2] oct-3-ylamino)pheny1]-7,8-
dimethoxy-N,4-dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-1- {4-[(4-methoxy-1,2,5-oxadi azol-3-
yDamino]phenyll-N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-7,8-dimethoxy-1- {4-[(4-methoxy-1,2,5-oxadiazol-3-
yDamino]phenyl } -N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- (4S)-7,8-dimethoxy-1- {4-[(4-methoxy-1,2,5-oxadi azol-3-
yDamino]phenyll-N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-144-(pyridazin-4-ylamino)pheny1]-4,5-dihydro-
3H-2,3-
benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-144-(pyridazin-3-ylamino)pheny1]-4,5-dihydro-
3H-2,3-
benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {4-[methyl(1-methyl-1H-
imidazol-2-yDamino]phenyl } -
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4R)-7,8-dimethoxy-N,4-dimethy1-1-14-[methyl(1-methyl-1H-imidazol-2-
yl)amino]phenyl 1 -4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4,5)-7,8-dimethoxy-N,4-dimethy1-1-{4-[methyl(1-methyl-1H-imidazol-2-
yDamino]phenyl } -4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1 -14-[(1-methyl-1H-pyrazol-3-yDamino]pheny11-
4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1- {4-[(1-methy1-1H-
pyrazol-3-yDamino]phenyl 1 -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-1- {4[(2-fluoropyri din-3-y Daminolpheny11-7,8-
dimethoxy-N,4-dimethy1-4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-1-{4-[(2-fluoropyridin-3-yDamino]phenyll-7,8-dimethoxy-N,4-dimethyl-4,5-
dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-1- {4- [(2-fluoropyri din-3-y Damino]pheny11-7,8-
dimethoxy-N,4-dimethy1-4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
CA 02915419 2015-12-14
BHC123073FC
- 61 -
- ( )-1-14-[(3-fluoropyridin-4-yDamino]phenyl -7,8-dimethoxy-N,4-dimethy1-4,5-
dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1-{4-[(3-fluoropyridin-2-yDamino]pheny11-7,8-dimethoxy-N,4-dimethyl-4,5-
dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-1- {4-[(3-fluoropyridin-2-yDamino]phenyl -7,8-dimethoxy-N,4-dimethy1-
4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxami de,
- (45)-1-14-[(3-fluoropyri din-2-yDamino]pheny11-7,8-dimethoxy-N,4-
dimethy1-4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [2,2,2-tri fluor-1-(1-methy1-1H-
pyrrol-2-
ypethyl] amino} phenyl)-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1 -(4- { [2-(dimethylamino)ethyl] methylamino} pheny1)-7,8-
dimethoxy-N,4-dimethy1-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (45)-144- [2-(dimethylamino)ethyl] methylamino} pheny1)-7,8-dimethoxy-
N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [2-(4-methylpiperazin-1-ypethyl]
amino} pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [2-(4-methylpiperazin-1-
ypethyl]amino pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {44methyl(1-methylpiperidin-4-
yDamino]phenyll -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-7,8-dimethoxy-N,4-di methyl-1- 14-[methyl(1-methylpiperidin-4-y
Damino]phenyll-
4,5-dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- (45)-7,8-dimethoxy-N,4-di methyl-1- {4- [methyl(1-methylpiperi din-4-
yDamino]phenyll-
4,5-dihydro-3H-2,3-benzo diazepine-3-carboxami de,
- ( )-tert-butyl 4-[ {4-[7,8-dimethoxy-4-methy1-3-(methylcarbamoy1)-4,5-
dihydro-3H-2,3-
benzodiazepin-l-yliphenyllmethylamino]piperidine-1-carboxylate,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {4-[(1-methyl azetidin-3-
yl)aminolphenyl -4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1- {4-[(1-acetylazeti din-3-y parnino]phenyl -7,8-dimethoxy-N,4-
dimethy1-4, 5-dihydro-
CA 02915419 2015-12-14
BHC123073FC
- 62 -
3H-2,3-benzodi azepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [trans-4-(4-methylpiperazin-1-
yl)cyclohexyl]amino pheny1)-4,5-dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {3-[methyl(pyridin-3-
yl)amino]phenyl } -4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-{3-[(1-methylpiperidin-4-
yDamino]phenyl} -4,5-
dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- ( )-1-(3-1[3-(dimethylamino)propylimethyl amino } -4-fluoropheny1)-
7,8-dimethoxy-N,4-
dimethy1-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1-(3- [2-(dimethylamino)ethyl]methylamino}-4-fluoropheny1)-7,8-dimethoxy-
N,4-
dimethyl-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-1-(4-{ [(dimethylamino)acetyl] amino } pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-1-(4-1[(dimethylamino)acetyl]amino} pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-1-(4- [(dimethylamino)acetyl]amino} pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [(1-methylpiperidin-4-
yl)carbonyl]amino pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [(1-methylpiperi di n-4-yl)carbonyl]
amino} pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (4R)-7,8-dimethoxy-N,4-dimethy1-1-(4-{[(1-methylpiperidin-4-
yl)carbonyllamino}pheny1)-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {4-[(piperi din-4-ylcarbonyl)amino]
phenyl } -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-14-[(morpholin-4-
ylacetyl)amino]phenyl } -4,5-dihydro-
3H-2,3-benzodi a zi-pine-3-carboxami de,
- ( )-1-(4-{ [(1-benzylpiperidin-4-y Dcarbonyl] amino pheny1)-7,8-
dimethoxy-N,4-dimethy1-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
CA 02915419 2015-12-14
BHC123073FC
-63-
- (4S)-1-(4- [(1-benzy lpiperidin-4-yl)carbonyl] amino pheny1)-7,8-
dimethoxy-N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- (4R)-1-(4- {[(1-benzylpiperidin-4-Acarbonyl]amino}phenyl)-7,8-
dimethoxy-N,4-dimethyl-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (+)-7,8-dimethoxy-N,4-dimethy1-1-(4-1 methyl [(methylamino)acetyl]amino
pheny1)-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [(4-methylpiperazin-1-
yDacetyl]aminol pheny1)-
4,5-dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- (4R)-7,8-dimethoxy-N,4-dimethy1-1-(4- [(4-methylpiperazin-1-
ypacetyl]am i no } pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1-(4-{[(4-methylpiperazin-1-
y1)acetyllaminolpheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-tert-butyl 4-({447,8-dimethoxy-4-methy1-3-(methylcarbamoy1)-4,5-dihydro-
3H-2,3-
benzodiazi-pin-1-yl]phenylIcarbamoyDpiperidine-1-carboxylate,
- (4S)-tert-butyl {447,8-dimethoxy-4-methy1-3-(methy lcarbamoy1)-4,5-di
hydro-3H-2,3-
benzodiazepin-1 -yl] phenyl } carbamoyl)piperidine-1-carboxyl ate,
- (4R)-tert-butyl 4-({447,8-dimethov-4-methyl-3-(methylcarbamoy1)-4,5-dihydro-
3H-2,3-
benzodiazepin-1-yllphenyl} carbamoyl)piperidine-1-carboxyl ate,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {4-[(1-methylpiperidin-4-
ypoxy]phenyl -4,5-dihydro-
3H-2,3-benzo di azepine-3-carboxami de,
- (45)-7,8-dimethoxy-N,4-dimethy1-1- { 4-[(1-methy lpiperi din-4-y
Doxy]phenyll -4,5-di hydro-
3H-2,3 -benzodi azepine-3 -carboxamide,
- ( )-1- {4[2-(dimethyl am ino)ethoxylphenyll -7,8-dimethoxy-N,4-
dimethy1-4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide,
- (4R)-1- {4[2-(dimethyl amino)ethoxy]phenyl I -7,8-di methoxy-N,4-dimethy1-
4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxami de,
- (4S)-1- {442-(dimethylamino)ethoxy]pheny1}-7,8-dimethoxy-N,4-dimethy1-
4,5-dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-{4-[(1-methylazetidin-3-ypoxy]phenyll-4,5-
dihydro-
CA 02915419 2015-12-14
BHC123073FC
- 64 -
3H-2,3 -benzodiazepine-3-carboxami de,
- (+)-7,8-dimethoxy-N,4-dimethy1-1-(4-phenoxypheny1)-4,5-dihydro-3H-2,3-
benzodiazepine-3-carboxamide,
- ( )-144-(4-fluorophenoxy)pheny1]-7,8-dimethoxy-N,4-dimethy1-4,5-dihydro-3H-
2,3-
benzodiazepine-3 -carboxamide,
- ( )-8-chloro-1- {4-[(2-fluoropyri din-3-yDamino]phenyl 1 -N,4-
dimethy1-7-
(trifluoromethoxy)-4,5-dihydro-3H-2,3-benzodi azepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-14-[methyl(1-methylpiperidin-4-
yl)amino]phenyll-8-
(tri fluoromethoxy)-4,5-dihydro-3H-2,3-benzodiazep ine-3-carboxami de,
- (4S)-8-methoxy-N,4-dimethy1-1-(4-{ [(1-methy1-1H-pyrrol-2-
ypcarbonyl]amino}pheny1)-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- (45)-8-methoxy-N,4-dimethy1-1- {4-[(1,2,3-thi adiazol-4-
ylcarbonypamino]phenyl 1 -4,5-
dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- ( )-1-(4-{ [(dimethylamino)acetyl] amino} pheny1)-8-methoxy-N,4-
dimethy1-4,5-dihydro-
3H-2,3-benzodia7epine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-{44(methyl sulphonyDamino]phenyl 1 -
4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1- {4-[(pyri din-3-y lsulphony
Damino]pheny11-4,5-
dihydro-3H-2,3-benzodi azepine-3-carboxami de,
- (4S)-7,8-dimethoxy-N,4-dimethy1-1- {4-[(tetrahydro-2H-pyran-4-
ylsulphonyl)amino]phenyl 1 -4,5-dihydro-3H-2,3-benzodiazepine-3-carboxami de,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {4-
[methyl(methylsulphonyl)amino]phenyl} -4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1- {41(phenylsulphonyl)aminolphenyl 1 -
4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide,
- ( )-1-{4-[(benzylsulphonyDamino]phenyll-7,8-dimethoxy-N,4-dimethyl-4,5-
dihydro-3H-
2,3-benzodiazepine-3-carboxamide,
- ( )-7,8-dimethoxy-N,4-dimethy1-1-(4-{ [(trifluoromethypsulphonyl]
amino 1 pheny1)-4,5-
dihydro-3H-2,3-benzo diazepine-3-carboxami de,
CA 02915419 2015-12-14
BHC123073FC
- 65 -
- ( )-1-{44(cyclopropylsulphonyl)aminolpheny11-7,8-dimethoxy-N,4-dimethyl-4,5-
dihydro-
3H-2,3-benzodiazepine-3-carboxamide,
- ( )-144-(berizyloxy)pheny1]-7,8-dimethoxy-N,4-dimethy1-4,5-dihydro-3H-2,3-
benzodiazepine-3-carboxamide,
- ( )-1- {4-[(N,N-dimethylglycyl)(methyDamino] phenyl 1 -4-ethy1-7,8-
dimethoxy-N-methy1-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
and
- ( )-4-isopropy1-7,8-dimethoxy-N-methyl-1-14-[methyl(1-methyl-1H-imidazol-2-
yDamino]phenyl}-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
and their polymorphs, tautomers, solvates, physiologically acceptable salts
and solvates of these
salts.
In the general formula (I), X may represent an oxygen or sulphur atom.
In the general formula (I), X preferably represents an oxygen atom.
In the general formula (I), Rla may represent -OR' or -NR7R8.
In the general formula (I), Rla preferably represents -OR'.
In the general formula (I), R." preferably represents -NR7R8.
In the general formula (I), R'" preferably represents hydrogen, halogen,
hydroxy, cyano, or
represents a C1-C3-alkyl-, C1-C3-alkoxy-, fluoro-C1-C3-alkyl- or fluoro-C1-C3-
alkoxy radical.
In the general formula (I), RI" particularly preferably represents hydrogen,
fluorine, chlorine,
bromine, cyano, methyl-, methoxy- or trifluoromethyl-.
In the general formula (I), RI" particularly preferably represents hydrogen,
fluorine, chlorine,
bromine or cyano.
In the general formula (I), RI" particularly preferably represents hydrogen,
fluorine, methyl-,
methoxy- or trifluoromethyl-.
In the general formula (I), Rth particularly preferably represents hydrogen,
fluorine or chlorine.
CA 02915419 2015-12-14
BHC123073FC
- 66 -
In the general formula (I), Rib very particularly preferably represents
hydrogen or fluorine.
In the general formula (I), Rib very particularly preferably represents
hydrogen.
In the general formula (I), leb very particularly preferably represents
fluorine.
In the general formula (I), Ric preferably represents hydrogen, halogen,
hydroxy, cyano, or
represents a C1-C3-alkyl-, C1-C3-alkoxy-, fluoro-C1-C3-alkyl- or fluoro-C1-C3-
alkoxy radical.
In the general formula (I), RIC particularly preferably represents hydrogen.
In the general formula (I), R2 may represent a C1-C3-alkyl- or trifluoromethyl-
or a C3- or C4-
cycloalkyl radical.
In the general formula (I), R2 preferably represents methyl- or ethyl-.
In the general formula (I), R2 particularly preferably represents methyl-.
In the general formula (I), R3 may represent Ci-C3-alkyl-, C1-C3-alkoxy-,
amino- or
C1-C3-alkylamino-.
In the general formula (I), R3 particularly preferably represents CI-C3-
alkylamino-.
In the general formula (I), R3 particularly preferably represents CI-C2-
alkylamino-.
In the general formula (I), R3 very particularly preferably represents
methylamino-.
In the general formula (I), R4 and le independently of one another may
represent hydrogen,
hydroxy, cyano, nitro, amino, aminocarbonyl-, fluorine, chlorine, bromine,
or
represent C1-C6-alkyl-, C/-C6-alkoxy-, C1-C6-alkylamino-, C1-C6-
alkylcarbonylamino-, C1-C6-
alkylaminocarbonyl- or Ci-C6-alkylaminosulphonyl- which may optionally be mono-
or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, carboxy, hydroxy-C1-C6-alkyl-, C1-C6-alkoxy-, Ci-C6-alkoxy-Ci-C6-
alkyl-, C1-C6-
CA 02915419 2015-12-14
BHC123073FC
- 67 -
allcylamino-, amino-C1-C6-alkyl-, a monocyclic heterocyclyl radical having 3
to 8 ring atoms and a
monocyclic heteroaryl radical having 5 or 6 ring atoms where the monocyclic
heterocyclyl and
heteroaryl radicals mentioned may for their part optionally be monosubstituted
by C1-C3-alkyl,
or
represent C3-C10-cycloalkyl- which may optionally be mono- or polysubstituted
by identical or
different substituents from the group consisting of halogen, amino, hydroxy,
C1-C6-alkyl-, C1-C6-
alkoxy-, C1-C6-alkoxy-C1-C6-alkyl-, C1 amino-C1-
C6-alkyl-, C1-C6-alkylamino-C1-
C6-alkyl, halo-Ci-C6-alkyl-, halo-C1-C6-alkoxy- and a monocyclic heterocyclyl
radical having 3 to
8 ring atoms,
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be mono- or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, cyano, nitro, carboxy, C1-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkoxy-Ci-
C6-alkyl-, hydroxy-
C1-C6-alkyl-, C1-C6-alkylamino-, amino-Ci-C6-alkyl-, C1-C6-alkylamino-C1-C6-
alkyl, halo-C1-C6-
1 5 alkyl-, halo-Ci-C6-alkoxy-, C3-C10-cycloalkyl and a monocyclic
heterocyclyl radical having 3 to 8
ring atoms,
or
represents monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be mono- or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, cyano, oxo, carboxy, C1-C6-alkyl-, CI -C6-alkoxy-, C1-C6-alkoxy-Ci-C6-
alkyl-, CI-C6-
alkylamino-, amino-Ci-C6-alkyl-, Ci-C6-alkylamino-Ci-C6-alkyl-, hydroxy-Ci-C6-
alkyl-, halo-C1-
C6-alkyl-, halo-C1-C6-alkoxy-, C3-C10-cycloalkyl- and a monocyclic
heterocyclyl radical having 3
to 8 ring atoms,
or
represents phenyl- which may optionally be mono- or polysubstituted by
identical or different
substituents from the group consisting of halogen, amino, hydroxy, cyano,
nitro, carboxy, C1-C6-
alkyl-, Ci-C6-alkoxy-, C1-C6-alkoxy-CI-C6-alkyl-, Ci-C6-alkylamino-, amino-CI-
C6-alkyl-, C1-C6-
allcylaminocarbonyl-, C1-C6-alkylaminosulphonyl-, CI-C6-alkylamino-C1-C6-alkyl-
, hydroxy-C1-
C6-alkyl-, halo-Ci-C6-alkyl-, halo-C1-C6-alkoxy-, C3-C10-cycloallcyl- and a
monocyclic heterocyclyl
radical having 3 to 8 ring atoms.
In the general formula (I), le and R5 independently of one another preferably
represent hydrogen,
hydroxy, cyano, nitro, amino, aminocarbonyl-, fluorine, chlorine, bromine,
or
represent C1-C6-alkyl-, C1-C6-alkoxy-, Ci-C6-alkylamino-, Ci-C6-
alkylcarbonylamino-, Ci-C6-
alkylaminocarbonyl- or Ci-C6-alkylaminosulphonyl- which may optionally be mono-
or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, carboxy, hydroxy-CI-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkoxy-Ci-C6-
alkyl-, C1-C6-
CA 02915419 2015-12-14
BHC123073FC
- 68 -
allcylamino- or amino-C1-C6-alkyl-, a monocyclic heterocyclyl radical having 3
to 8 ring atoms and
a monocyclic heteroaryl radical having 5 or 6 ring atoms where the monocyclic
heterocyclyl and
heteroaryl radicals mentioned may for their part optionally be monosubstituted
by C1-C3-alkyl,
or
represent monocyclic heteroaryl- having 5 or 6 ring atoms which may optionally
be mono- or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, cyano, nitro, carboxy, CI-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkoxy-C1-
C6-alkyl-, hydroxy-
Ci-C6-alkyl-, C1-C6-allcylamino-, amino-Ci-C6-alkyl-, CI-C6-alkylamino-Ci-C6-
alkyl, halo-C1-C6-
alkyl-, halo-C1-C6-alkoxy-, C3-Cio-cycloalkyl and a monocyclic heterocyclyl
radical having 3 to 8
ring atoms,
or
represents monocyclic heterocyclyl- having 3 to 8 ring atoms which may
optionally be mono- or
polysubstituted by identical or different substituents from the group
consisting of halogen, amino,
hydroxy, cyano, oxo, carboxy, Ci-C6-alkyl-, Ci-C6-alkoxy-, Ci-C6-alkoxy-C1-C6-
alkyl-, Ci-C6-
allcylamino-, amino-Ci-C6-alkyl-, C1-C6-allcylamino-C1-C6-alkyl-, hydroxy-C1-
C6-alkyl-, halo-C1-
C6-alkyl-, halo-Ci-C6-alkoxy-, C3-Cio-cycloallcyl- and a monocyclic
heterocyclyl radical having 3
to 8 ring atoms.
In the general formula (I), R4 and le independently of one another
particularly preferably represent
hydrogen, hydroxy, cyano, fluorine, chlorine, bromine, Ci-C3-alkoxy-, fluoro-
C1-C3-alkoxy-.
In the general formula (I), R4 particularly preferably represents hydrogen,
hydroxy, cyano, fluorine,
chlorine, bromine, C1-C3-alkoxy-, fluoro-C1-C3-alkoxy-.
In the general formula (I), le particularly preferably represents hydrogen,
hydroxy, cyano, fluorine,
chlorine, bromine, Ci-C3-alkoxy-, fluoro-CI-C3-alkoxy-.
In the general formula (I), R4 and le independently of one another very
particularly preferably
represent hydrogen, chlorine, methoxy- or trifluoromethoxy-.
In the general formula (I), R4 very particularly preferably represents
hydrogen, chlorine, methoxy-
or trifluoromethoxy-.
In the general formula (I), R5 very particularly preferably represents
hydrogen, chlorine, methoxy-
or trifluoromethoxy-.
In the general formula (I), R4 exceptionally preferably represents hydrogen or
methoxy-.
CA 02915419 2015-12-14
BHC123073FC
- 69 -
In the general formula (I), R4 exceptionally preferably represents hydrogen.
In the general formula (I), R4 exceptionally preferably represents methoxy-.
In the general formula (I), R5 very particularly preferably represents methoxy-
or trifluoromethoxy-.
In the general formula (I), R5 very particularly preferably represents methoxy-
.
In the general formula (I), R5 very particularly preferably represents
trifluoromethoxy-.
In the general formula (I), R6 may represent C3-C7-cycloalkyl- or C2-C6-alkyl-
which is
monosubstituted by C1-C6-alkylamino-,
or
a monocyclic heterocyclyl radical having 3 to 8 ring atoms which may
optionally be
monosubstituted by oxo, C1-C3-alkyl-, C1-C3-alkylcarbonyl-, C1-C4-
alkoxycarbonyl-, phenyl-C1-C3-
alkyl- or C3-C7-cycloalkyl-,
or
a mono- or bicyclic aryl- or heteroaryl radical, where the radicals mentioned
may optionally be
mono- or disubstituted by identical or different substituents from the group
consisting of halogen,
hydroxy, cyano, C1-C3-alkyl, fluoro-C1-C3-alkyl, hydroxy-C1-C3-alkyl, C1-C3-
alkoxy-, C1-C3-
alkylamino-, amino-C1-C3-alkyl-, C1-C3-alkylaminocarbonyl-, Ci-C3-
alkylaminosulphonyl-, C1-C3-
alkylcarbonylamino-, C1-C3-alkylsulphonylamino-, C1-C3-alkylcarbonyl-, C1-C3-
alkylsulphonyl-
and trifluoromethoxy-.
In the general formula (I), R6 may also represent C3-C7-cycloalkyl- or C2-C6-
alkyl- which is
monosubstituted by CI-C6-alkylamino-,
or
a monocyclic heterocyclyl radical having 3 to 8 ring atoms which may
optionally be
monosubstituted by oxo, C1-C3-alkyl-, C1-C3-alkylcarbonyl-, Ci-
C4alkoxycarbonyl-, phenyl-C1-C3-
alkyl- or C3-C7-cycloalkyl-,
or
a mono- or bicyclic aryl- or heteroaryl radical, where the radicals mentioned
may optionally be
mono- or disubstituted by identical or different substituents from the group
consisting of halogen,
hydroxy, cyano, C1-C3-alkyl, fluoro-C1-C3-alkyl, hydroxy-C1-C3-alkyl, C1-C3-
alkoxy-, C1-C3-
alkylamino-, amino-CI-C3-alkyl-, C1-C3-alkylaminocarbonyl-, CI-C3-
alkylaminosulphonyl-, C1-C3-
alkylcarbonylamino-, C1-C3-alkylsulphonylamino-, Ci-C3-alkylcarbonyl-, C1-C3-
alkylsulphonyl-
and trifluoromethoxy-,
or
CA 02915419 2015-12-14
BHC123073FC
- 70 -
a benzyl radical,
where the phenyl radical contained therein may optionally be mono- or
disubstituted by
identical or different substituents from the group consisting of halogen,
hydroxy, cyano,
C1-C3-alkyl, fluoro-Ci-C3-alkyl, hydroxy-C1-C3-alkyl,C1-C3-alkoxy-, C1-C3-
alkylamino-,
amino-C1-C3-alkyl-, C1-C3-allcylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, C1-
C3-
alkylcarbonylamino-, CrC3-allcylsulphonylamino-, C1-C3-alkylcarbonyl-, C1-C3-
alkylsulphonyl- and trifluoromethoxy-,
and where the methylene group contained therein may optionally be substituted
by a
hydroxy group or one or two Cl-C3-alkyl groups.
In the general formula (I), R6 preferably represents C3-C7-cycloalkyl- or C2-
C6-alkyl- which is
monosubstituted by CI-C3-alkylamino-,
or
represent a monocyclic heterocyclyl radical having 4 to 7 ring atoms which may
optionally be
monosubstituted by oxo, Ci-C3-alkyl-, CI-C3-alkylcarbonyl-, Ci-
C4alkoxycarbonyl-, benzyl- or C3-
C7-cycloalkyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of Ci-C3-alkyl-, halogen and C1-C3-alkoxy-.
In the general formula (I), R6 furthermore preferably represents C3-C7-
cycloallcyl- or C2-C6-alkyl-
which is monosubstituted by C1-C3-alkylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may optionally be
monosubstituted by oxo, Ci-C3-alkyl-, Ci-C3-alkylcarbonyl-, C1-C4-
alkoxycarbonyl-, benzyl- or C3-
C7-cycloalkyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of CrC3-alkyl-, halogen and Ci-C3-alkoxy-,
or
represents a benzyl radical, where the phenyl radical contained therein may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of Ci-C3-alkyl-,
halogen and Ci-C3-alkoxy-.
In the general formula (I), R6 particularly preferably represents C2-C4-alkyl-
which is
monosubstituted by Ci-C3-alkylamino- or represents a monocyclic heterocyclyl
radical haying 4 to
CA 02915419 2015-12-14
BHC123073FC
- 71 -
7 ring atoms which may optionally be monosubstituted by methyl-, ethyl-,
acetyl- or tert-
butoxycarbonyl-,
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine,
bromine, methoxy- and
ethoxy-.
In the general formula (I), R6 particularly preferably represents C2-C4-alkyl-
which is
monosubstituted by C1-C3-allcylamino- or represents a monocyclic heterocyclyl
radical having 4 to
7 ring atoms which may optionally be monosubstituted by methyl-, ethyl-,
acetyl- or tert-
butoxycarbonyl-,
or
represents a phenyl radical which may optionally be mono- or disubstituted by
identical or different
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine,
bromine, methoxy- and
ethoxy-,
or
represents a benzyl radical where the phenyl radical contained therein may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of methyl-, ethyl-,
fluorine, chlorine, bromine, methoxy- and ethoxy-.
In the general formula (I), R6 particularly preferably represents C2-C4-alkyl-
which is
monosubstituted by Ci-C3-alkylamino- or represents a monocyclic heterocyclyl
radical having 4 to
7 ring atoms which may optionally be monosubstituted by methyl-, ethyl-,
acetyl- or tert-
butoxycarbony1-.
In the general formula (I), R6 particularly preferably represents C2-C4-alkyl
which is
monosubstituted by C1-C3-alkylamino-,
In the general formula (I), R6 particularly preferably represents a monocyclic
heterocyclyl radical
having 4 to 7 ring atoms which may optionally be monosubstituted by methyl-,
ethyl-, acetyl- or
tert-butoxycarbonyl-.
In the general formula (I), R6 particularly preferably represents a phenyl
radical which may
optionally be mono- or disubstituted by identical or different substituents
from the group consisting
of methyl-, ethyl-, fluorine, chlorine, bromine, methoxy- and ethoxy-.
In the general formula (I), R6 particularly preferably represents a benzyl
radical where the phenyl
radical contained therein may optionally be mono- or disubstituted by
identical or different
CA 02915419 2015-12-14
BHC123073FC
- 72
substituents from the group consisting of methyl-, ethyl-, fluorine, chlorine,
bromine, methoxy- and
ethoxy-.
In the general formula (I), R6 very particularly preferably represents /V,N-
dimethylaminoethyl-,
or represents a monosubstituted heterocyclyl radical selected from
* ________________________________ N¨CH3 * (
N¨CH3
where "*" in each case indicates the point of attachment to the remainder of
the molecule,
or represents a phenyl radical which may optionally be substituted by a
fluorine atom.
In the general formula (I), R6 very particularly preferably represents /V,N-
dimethylaminoethyl-
or represents a monosubstituted heterocyclyl radical selected from
* C N¨CH3* N¨CH
3
where "*" in each case indicates the point of attachment to the remainder of
the molecule,
or represents a phenyl radical which may optionally be substituted by a
fluorine atom,
or represents a benzyl radical.
In the general formula (I), le very particularly preferably represents N,N-
dimethylaminoethyl-.
In the general formula (I), R6 very particularly preferably represents a
monosubstituted heterocyclyl
radical selected from
* ________________________________ C N¨CH3* ( N¨CH
3
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R6 very particularly preferably represents a
phenyl radical which may
optionally be substituted by a fluorine atom.
In the general formula (I), R6 very particularly preferably represents a
benzyl radical.
In the general formula (I), R6 exceptionally preferably represents C2-C4-alkyl-
which is
monosubstituted by C1-C3-alkylamino- or represents a monocyclic heterocyclyl
radical having 6
ring atoms which may optionally be monosubstituted by methyl-.
CA 02915419 2015-12-14
BHC123073FC
- 73 -
,
In the general formula (I), R6 exceptionally preferably represents a
monocyclic heterocyclyl radical
having 6 ring atoms which may optionally be monosubstituted by methyl-.
In the general formula (I), R6 furthermore exceptionally preferably represents
a radical selected
from
CH
( 7¨CH, 3
N
CH,
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R7 may represent C3-C7-cycloalkyl- or C2-C6-alkyl-
which is
monosubstituted by -
NR9Rio,
or
represents a -C(0)R" group,
or
represents a -S(=0)2R12 group,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-C12-
heterocycloalkyl radical, a C5-C)2-heterospirocycloalkyl radical or a C6-C12-
heterobicycloallcyl
radical, where the radicals mentioned may optionally be mono- or disubstituted
by identical or
different substituents from the group consisting of oxo, C1-C3-alkyl--, C1-C3-
alkylcarbonyl-, CI-C4-
alkoxycarbonyl-, phenyl-C1-C3-alkyl- and C3-C7-cycloalkyl-,
or
a mono- or bicyclic aryl- or heteroaryl radical, where the radicals mentioned
may optionally be
mono- or disubstituted by identical or different substituents from the group
consisting of halogen,
hydroxy, cyano, Ci-C3-alkyl, fluoro-CI-C3-aIkyl, hydroxy-Ci-C3-alkyl, C1-C3-
alkoxy-, C1-C3-
allcylamino-, amino-C1-C3-alkyl-, C1-C3-alkylaminocarbonyl-, C1-C3-
allcylaminosulphonyl-, CI-C3-
alkylcarbonylamino-, C1-C3-alkylsulphonylamino-, C,-C3-alkylcarbonyl-, C1-C3-
alkylsulphonyl-
and trifluoromethoxy-,
or
represents fluoro-C1-C3-alkyl or Ci-C3-alkyl monosubstituted by a phenyl- or a
monocyclic
heteroaryl radical having 5 or 6 ring atoms, where the phenyl and heteroaryl
radicals mentioned
may be mono- or disubstituted by identical or different substituents from the
group consisting of
CI-C3-alkyl-, halogen and CI-C3-alkoxy-.
In the general formula (I), le preferably represents C3-C7-cycloallcyl- or C2-
C6-alkyl- which is
monosubstituted by -NR9R1 ,
CA 02915419 2015-12-14
BHC123073FC
- 74
or
represents a -C(=0)R11 group,
or
represents a -S(=0)2RI2 group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-Cu-
heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo,
C1-C3-alkyl-, C1-C3-alkylcarbonyl-, benzyl- or CI-C4alkoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of Ci-C3-alkyl-, halogen and CI-C3-alkoxy-,
or
represents fluoro-C1-C3-alkyl or CI-C3-alkyl monosubstituted by a phenyl- or a
monocyclic
heteroaryl radical having 5 or 6 ring atoms, where the phenyl and heteroaryl
radicals mentioned
may be mono- or disubstituted by identical or different substituents from the
group consisting of
C1-C3-alkyl-, halogen and CI-C3-alkoxy-.
In the general formula (I), R7 preferably represents C3-C7-cycloalkyl- or C2-
C6-alkyl- which is
monosubstituted by -NR9R' .
In the general formula (I), R7 preferably represents a -C(=0)0RII group.
In the general formula (I), R7 preferably represents a -S(=0)2RI2 group.
In the general formula (I), R7 preferably represents a monocyclic heterocyclyl
=radical having 4 to 7
ring atoms or a bridged C6-C12-heterocycloallql radical, where the radicals
mentioned may
optionally be monosubstituted by oxo, CI-C3-alkyl-, CI-C3-alkylcarbonyl-,
benzyl- or CI-Cr-
alkoxycarbonyl-.
In the general formula (I), R7 preferably represents phenyl- or a monocyclic
heteroaryl radical
having 5 or 6 ring atoms, where the radicals mentioned may optionally be mono-
or disubstituted
by identical or different substituents from the group consisting of Ci-C3-
allcyl-, halogen and CI-C3-
alkoxy-.
In the general formula (I), R7 preferably represents fluoro-CI-C3-alkyl or CI-
C3-alkyl
monosubstituted by a phenyl- or a monocyclic heteroaryl radical having 5 or 6
ring atoms, where
the phenyl and heteroaryl radicals mentioned may be mono- or disubstituted by
identical or
CA 02915419 2015-12-14
BHC123073FC
- 75 -
different substituents from the group consisting of C1-C3-alkyl-, halogen and
C1-C3-alkoxy-.
In the general formula (I), R7 particularly preferably represents C3-07-
cycloalkyl- or C2-C4-alkyl-
which is monosubstituted by -NR9R10
,
or
represents a -C(---0)R1' group,
or
represents a -S(=0)21C group,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-Cio-
heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by
methyl-, ethyl-, acetyl- or tert-butoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of methyl-, ethyl-, fluorine, chlorine, bromine, methoxy and
ethoxy-,
or
represents fluoro-C1-C3-alkyl or C1-C3-alkyl monosubstituted by a phenyl- or a
monocyclic
heteroaryl radical having 5 or 6 ring atoms, where the phenyl and heteroaryl
radicals mentioned
may be mono- or disubstituted by identical or different substituents from the
group consisting of
methyl-, ethyl-, fluorine, chlorine and bromine.
In the general formula (I), R7 particularly preferably represents C3-C7-
cycloalkyl- or C2-C4-alkyl-
which is monosubstituted by -NR9RI .
In the general formula (I), R7 particularly preferably represents a monocyclic
heterocyclyl radical
having 4 to 7 ring atoms or a bridged C6-C10-heterocycloalkyl radical, where
the radicals mentioned
may optionally be monosubstituted by methyl-, ethyl-, acetyl- or tert-
butoxycarbonyl-.
In the general formula (I), le particularly preferably represents a phenyl or
a monocyclic heteroaryl
radical having 5 or 6 ring atoms, where the radicals mentioned may optionally
be mono- or
disubstituted by identical or different substituents from the group consisting
of methyl-, ethyl-,
fluorine, chlorine, bromine, methoxy and ethoxy-.
In the general formula (I), le particularly preferably represents fluoro-C1-C3-
alkyl or CI-C3-alkyl
monosubstituted by a phenyl- or a monocyclic heteroaryl radical having 5 or 6
ring atoms, where
the phenyl and heteroaryl radicals mentioned may be mono- or disubstituted by
identical or
different substituents from the group consisting of methyl-, ethyl-, fluorine,
chlorine and bromine.
CA 02915419 2015-12-14
BHC123073FC
- 76 -
In the general formula (I), It7 very particularly preferably represents /V,N-
dimethylaminoethyl or
/V,N-dimethylaminopropyl,
or
represents a -C(----0)R11 group,
or
represents a -S(---0)2R12 group,
or represents a radical selected from
= \ CN¨CH3 _________________ /¨N\ /N¨CH3 --0--N /N¨CH3
N¨ CH3
=
, \ , 7 \ \
0 0
, CF3
N
*-0 * __ CN - __ ( \N ) CH3 , / 0¨
tButyl , * N---
/
H3C
where "*" in each case indicates the point of attachment to the remainder of
the molecule,
or
represents fluorophenyl-, pyridyl-, fluoropyridyl-, dimethyloxazoly1-,
methylpyrazolyl-,
methoxyoxadiazolyl-, pyridazinyl- or methylimidazolyl-.
In the general formula (I), lee very particularly preferably represents N,N-
dimethylaminoethyl- or
N,N-dimethylaminopropyl-.
In the general formula (I), le very particularly preferably represents a
radical selected from
/ \ / \
\
= ______________ * __ / / __________________ N CN CH, \ /N¨CH3 *-0---N
/N¨ CH3 , . 7 \
N¨CH3
,
*---0 * CN __ * __ K \N / CF3
) _____________________________________________________________ -0
N , CH3 , / 0¨ tButyl , - N--
/
H3c
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), le very particularly preferably represents
fluorophenyl-, pyridyl-,
fluoropyridyl-, dimethyloxazolyl-, methylpyrazolyl-, methoxyoxadiazolyl-,
pyridazinyl- or
methylimidazolyl-.
In the general formula (I), le exceptionally preferably represents C3-C2-
cycloalkyl- or C2-C4-alkyl-
which is monosubstituted by -1\11ele ,
CA 02915419 2015-12-14
BHC123073FC
- 77 -
.
or
represents a -C(=0)12.11 group,
or
represents a -S(=0)12.12 group,
or
represents a monocyclic heterocyclyl radical having 6 ring atoms or represents
azabicyclo[2.2.2]oct-3-y1-, where the radicals mentioned may optionally be
monosubstituted by
methyl-, acetyl- or tert-butoxycarbonyl-,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of methyl-, fluoro or methoxy-.
In the general formula (I), R7 exceptionally preferably represents a
monocyclic heterocyclyl radical
having 6 ring atoms or represents azabicyclo[2.2.2]oct-3-y1-, where the
radicals mentioned may
optionally be monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-.
In the general formula (I), R.7 exceptionally preferably represents a phenyl
or a monocyclic
heteroaryl radical having 5 or 6 ring atoms, where the radicals mentioned may
optionally be mono-
or disubstituted by identical or different substituents from the group
consisting of methyl-, fluorine
and methoxy-.
In the general formula (I), R7 furthermore exceptionally preferably represents
a radical selected
from
\
N¨CH3\N¨CH3
/ \ __ /
CH
/ 3
f-N\
CH3
or
represents a -C(--=0)R" group,
Or
represents a radical selected from
(
CH3 '\/0
0 N
or N¨CH3 *
represents a radical selected from
CA 02915419 2015-12-14
BHC123073FC
- 78 -
HC H3C
N ,CH3 3\
* ________________________________ I N
N"
H3C
N-
0
* I
* 111 * 411 *
\
H3C ¨
*
* N /jµi
N % N
*<N
or represents the radical
F F
CH
= 3
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R7 furthermore exceptionally preferably represents
a radical selected
from
/ \
/ ________________________________________ \
N\ 71¨CH3 N¨CH,
CH3
/"--N\/
CH3
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (1), R7 furthermore exceptionally preferably represents
a radical selected
from
CH
* ______________ ( \N¨CH3 * _____ CN 3 * __ (\/0
0 N
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
CA 02915419 2015-12-14
BHC123073FC
- 79 -
In the general formula (I), R7 furthermore exceptionally preferably represents
a radical selected
from
H3C H C
HC
N¨ CH3
0
I
N
N"
H3C
N-
__________ 0
N
= ________________________________________ F
H3C ¨
¨
*
¨N
N '
N¨N
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R7 furthermore exceptionally preferably represents
a radical
F F
,CH3
N
where "*" denotes the point of attachment to the remainder of the molecule.
In the general formula (I), R8 may represent hydrogen or C1-C6-alkyl-.
In the general formula (I), R8 preferably represents hydrogen or C1-C3-alkyl.
In the general formula (I), R8 particularly preferably represents hydrogen or
C1-C2-alkyl.
In the general formula (I), R8 particularly preferably represents hydrogen.
In the general formula (I), R8 particularly preferably represents methyl-.
In the general formula (I), R8 particularly preferably represents ethyl-.
CA 02915419 2015-12-14
BHC123073FC
- 80 -
In the general formula (1), R9 and RI independently of one another may
represent hydrogen or C1-
C6-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic heterocyclyl
radical having 3 to 8 ring atoms which may optionally be monosubstituted by
oxo, C1-C3-alkyl-,
C1-C3-alkylcarbonyl-, C1-C4-alkoxycarbonyl-, phenyl-C1-C3-alkyl- or C3-C7-
cycloalkyl-.
In the general formula (I), R9 and R.1 independently of one another
preferably represent hydrogen
or C1-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic heterocyclyl
radical having 4 to 7 ring atoms which may optionally be monosubstituted by
oxo, Ci-C3-alkyl-,
C1-C3-alkylcarbonyl-, benzyl- or C1-C4-alkoxycarbonyl-.
In the general formula (1), R9 and le independently of one another preferably
represent hydrogen
or CI-C3-alkyl-.
In the general formula (I), R9 and R.1 preferably together with the nitrogen
atom to which they are
attached represent a monocyclic heterocyclyl radical having 4 to 7 ring atoms
which may
optionally be monosubstituted by oxo, C1-C3-alkyl-, Ci-C3-alkylcarbonyl-,
benzyl- or CI-Cr
alkoxycarbonyl-.
In the general formula (I), R9 and RI independently of one another
particularly preferably
represent hydrogen or CI-C3-alkyl-,
or
together with the nitrogen atom to which they are attached represent a
monocyclic heterocyclyl
radical having 4 to 7 ring atoms which may optionally be monosubstituted by
methyl-, ethyl-,
acetyl- or tert-butoxycarbonyl-.
In the general formula (I), R9 and RI particularly preferably together with
the nitrogen atom to
which they are attached represent a monocyclic heterocyclyl radical having 4
to 7 ring atoms which
may optionally be monosubstituted by methyl-, ethyl-, acetyl- or tert-
butoxycarbonyl-.
In the general formula (I), R9 and RI particularly preferably together with
the nitrogen atom to
which they are attached represent a monocyclic heterocyclyl radical having 4
to 7 ring atoms which
may optionally be monosubstituted by methyl-, acetyl- or tert-butoxycarbonyl-.
CA 02915419 2015-12-14
BHC123073FC
- 81 -
In the general formula (I), R9 and 12.1 independently of one another
particularly preferably
represent hydrogen or C1-C2-alkyl-.
In the general formula (I), R9 and RI very particularly preferably represent
hydrogen or methyl-.
In the general formula (I), R9 very particularly preferably represents methyl.
In the general formula (I), RI very particularly preferably represents
methyl.
In the general formula (I), R9 very particularly preferably represents
hydrogen.
In the general formula (I), RI very particularly preferably represents
hydrogen.
In the general formula (I), R9 and RI very particularly preferably represent
hydrogen or methyl-.
In the general formula (I), R9 and le very particularly preferably together
with the nitrogen atom
to which they are attached represent morpholinyl- or N-methylpiperazinyl.
In the general formula (I), R11 may represent C3-C7-cycloalkyl- or Ci-C6-alkyl-
which is
monosubstituted by - RN-R9
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-C12-
heterocycloalkyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-C,2-
heterobicycloalkyl
radical, where the radicals mentioned may optionally be mono- or disubstituted
by identical or
different substituents from the group consisting of oxo, Ci-C3-alkyl-, C,C3-
alkylcarbonyl-, CI-C4-
alkoxycarbonyl-, phenyl-CI-C3-alkyl- and C3-C7-cycloalkyl-,
Or
a mono- or bicyclic aryl- or heteroaryl radical, where the radicals mentioned
may optionally be
mono- or disubstituted by identical or different substituents from the group
consisting of halogen,
hydroxy, cyano, CI-C3-alkyl, fluoro-Ci-C3-alkyl, hydroxy-C1-C3-alkyl, Ci-C3-
alkoxy-, CI-C3-
alkylamino-, amino-Ci-C3-allcyl-, C,C3-alkylaminocarbonyl-, CI-C3-
alkylaminosulphonyl-, CI-C3-
alkylcarbonylamino-, CI-C3-alkylsulphonylamino-, CI-C3-alkylcarbonyl-, CI-C3-
alkylsulphonyl-
and trifluoromethoxy-.
In the general formula (I), R" preferably represents C3-C7-cycloalkyl- or Ci-
C6-alkyl- which is
monosubstituted by -NR9R10
,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-C12-
.
CA 02915419 2015-12-14
BHC123073FC
- 82 -
heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo,
C1-C3-alkyl-, Ci-C3-alkylcarbonyl-, phenyl-C1-C3-alkyl- or C1-Ca-
alkoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of CI-C3-alkyl-, halogen and CI-C3-alkoxy-,
In the general formula (I), R" preferably represents C3-C7-cycloalkyl- or Ci-
C6-alkyl- which is
monosubstituted by -
NR R9 io.
In the general formula (I), R" preferably represents a monocyclic heterocyclyl
radical having 4 to 7
ring atoms or a bridged C6-C12-heterocycloallcyl radical, where the radicals
mentioned may
optionally be monosubstituted by oxo, CI-C3-alkyl-, CI-C3-alkylcarbonyl-,
phenyl-Ci-C3-alkyl- or
CI-Ca-alkoxycarbonyl-.
In the general formula (I), Rn preferably represents a phenyl or a monocyclic
heteroaryl radical
having 5 or 6 ring atoms, where the radicals mentioned may optionally be mono-
or disubstituted
by identical or different substituents from the group consisting of Ci-C3-
alkyl-, halogen and C1-C3-
alkoxy-.
In the general formula (I), R" particularly preferably represents CI-Ca-alkyl
which is
monosubstituted by -NR
9R' ,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-C10-
heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by
methyl-, ethyl-, acetyl-, benzyl- or
tert-butoxycarbonyl,
or
represents a phenyl or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of methyl-, ethyl-, fluorine, chlorine or bromine.
In the general formula (I), R" particularly preferably represents CI-Ca-alkyl
which is
monosubstituted by -
NR R9 io.
In the general formula (I), R" particularly preferably represents a monocyclic
heterocyclyl radical
having 4 to 7 ring atoms or a bridged C6-Cio-heterocycloalkyl radical, where
the radicals mentioned
may optionally be monosubstituted by methyl-, ethyl-, acetyl-, benzyl- or tert-
butoxycarbonyl-.
CA 02915419 2015-12-14
BHC123073FC
- 83 -
In the general formula (1), R11 particularly preferably represents a phenyl or
a monocyclic
heteroaryl radical having 5 or 6 ring atoms, where the radicals mentioned may
optionally be mono-
or disubstituted by identical or different substituents from the group
consisting of methyl-, ethyl-,
fluorine, chlorine, or bromine.
In the general formula (I), R11 very particularly preferably represents -CH2-
NH(CH3),
-CH2-N(CH3)2, -S(-0)2.-CH3, methylpyrrolyl- or thiadiazolyl-,
or
represents a radical selected from
( (0 / \
N¨ CH3
N-1( 0
/- N\
/¨N"
OtButyl
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R" very particularly preferably represents -CH2-
NH(CH3),
-CH2-N(CH3)2, -S(=0)2-CH3, methylpyrrolyl- or thiadiazolyl-.
In the general formula (I), Ril very particularly preferably represents a
radical selected from
0
( ('N 0 N¨CH3
/- \/ //- N/ \ \
OtButyl
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R" exceptionally preferably represents CI-C2-alkyl
which is
monosubstituted by -NR9Rio,
or
represents a monocyclic heterocyclyl radical having 5 or 6 ring atoms which
may optionally be
monosubstituted by methyl-, benzyl-, acetyl- or tert-butoxycarbonyl-.
In the general formula (I), R" exceptionally preferably represents CI-C2-alkyl
which is
monosubstituted by -NR9R1 .
In the general formula (I), R" exceptionally preferably represents a
monocyclic heterocyclyl
radical having 5 or 6 ring atoms which may optionally be monosubstituted by
methyl-, benzyl-,
CA 02915419 2015-12-14
BHC123073FC
- 84 -
acetyl- or tert-butoxycarbonyl-.
In the general formula (I), Ril furthermore exceptionally preferably
represents a radical selected
from
/CH,
cO\
H,C\
N-CH,
N
or
represents a radical selected from
\N¨CH3 \N
' /
CH,
0 (CH,
CH,
0
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), R1' furthermore exceptionally preferably
represents a radical selected
from
CH,
cO\ NI
H,C
\N-CH,
*_/ N
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
In the general formula (I), Ru furthermore exceptionally preferably represents
a radical selected
from
= N-CH, NH
/ ____________________________________________ /
CH,
0 __ (CH,
= ( N CH,
/ \O
where "*" in each case indicates the point of attachment to the remainder of
the molecule.
CA 02915419 2015-12-14
BHC123073FC
- 85 -
In the general formula (I), RI2 may represent CI-C6-alkyl- which may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of halogen, hydroxy,
carboxy, cyano, Cl-C6-alkoxy-, -NRI R11, phenyl, a monocyclic heteroaryl
radical having 5 or 6
ring atoms or a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
where
phenyl and the monocyclic heteroaryl radical having 5 or 6 ring atoms for
their part may be
mono- to trisubstituted by identical or different substituents from the group
consisting of
halogen, cyano, CI-C3-alkyl, trifluoromethyl, CI-C3-alkoxy- and
trifluoromethoxy-,
and in which
the monocyclic heterocyclyl radical for its part may optionally be mono- or
disubstituted
by identical or different substituents from the group consisting of oxo, CI-C3-
alkyl-, C,-C3-
alkylcarbonyl-, CI-C4alkoxycarbonyl-, phenyl-C1-C3-alkyl- and C3-C7-cycloalkyl-
,
or
represents C3-C10-cycloalkyl- which may optionally be mono- or polysubstituted
by identical or
different substituents from the group consisting of fluorine, hydroxy, oxo,
cyano, C1-C3-alkyl-, CI-
C3-alkoxy- and -NRI RI I,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-C12-
heterocycloalkyl radical, a C5-Cu-heterospirocycloallcyl radical or a C6-C12-
heterobicycloallcyl
radical, where the radicals mentioned may optionally be mono- or disubstituted
by identical or
different substituents from the group consisting of oxo, Ci-C3-alicyl-, CI-C3-
allcylcarbonyl-, C1-C4-
alkoxycarbonyl-, phenyl-C,-C3-alkyl- and C3-C7-cycloalkyl-,
or
represents an aryl- or heteroaryl radical, where the radicals mentioned may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of halogen, hydroxy,
cyano, C1-C3-alkyl, fluoro-C1-C3-alkyl, hydroxy-C1-C3-alkyl, CI-C3-alkoxy-, C1-
C3-alkylamino-,
amino-CI-C3-alkyl-, CI-C3-alkylaminocarbonyl-, CI-C3-alkylaminosulphonyl-, CI-
C3-
alkylcarbonylamino-, CI-C3-alkylsulphonylamino-, Cl-C3-alkylcarbonyl-, CI-C3-
alkylsulphonyl-
and trifluoromethoxy-.
In the general formula (I), RI' may represent CI-C6-alkyl- which may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of halogen, hydroxy,
carboxy, cyano, CI-C6-alkoxy-, -NR9e, phenyl, a monocyclic heteroaryl radical
having 5 or 6 ring
atoms or a monocyclic heterocyclyl radical having 3 to 8 ring atoms,
where
phenyl and the monocyclic heteroaryl radical having 5 or 6 ring atoms for
their part may be
CA 02915419 2015-12-14
BHC123073FC
- 86 -
mono- to trisubstituted by identical or different substituents from the group
consisting of
halogen, cyano, C1-C3-alkyl, trifluoromethyl, C1-C3-alkoxy- and
trifluoromethoxy-,
and in which
the monocyclic heterocyclyl radical for its part may optionally be mono- or
disubstituted
by identical or different substituents from the group consisting of oxo, C1-C3-
alkyl-, C1-C3-
alkylcarbonyl-, C1-C4-alkoxycarbonyl-, phenyl-C1-C3-alkyl- and C3-C7-
cycloalkyl-,
or
represents fluoro-C1-C3-alkyl-,
or
represents C3-C10-cycloalkyl- which may optionally be mono- or polysubstituted
by identical or
different substituents from the group consisting of fluorine, hydroxy, oxo,
cyano, C1-C3-alkyl-, CI-
C3-alkoxy- and -NR9R1 ,
or
represents a monocyclic heterocyclyl radical having 3 to 8 ring atoms, a
bridged C6-C12-
heterocycloalkyl radical, a C5-C12-heterospirocycloalkyl radical or a C6-C12-
heterobicycloalkyl
radical, where the radicals mentioned may optionally be mono- or disubstituted
by identical or
different substituents from the group consisting of oxo, Ci-C3-alkyl-, CI-C3-
alkylcarbonyl-, CI-C4-
alkoxycarbonyl-, phenyl-CI-C3-alkyl- and C3-C7-cycloalkyl-,
or
represents an aryl or heteroaryl radical, where the radicals mentioned may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of halogen, hydroxy,
cyano, C1-C3-alkyl, fluoro-Ci-C3-alkyl, hydroxy-Ci-C3-alkyl, CI-C3-alkoxy-, CI-
C3-allcylamino-,
amino-Ci-C3-alkyl-, CI-C3-alkylaminocarbonyl-, C1-C3-alkylaminosulphonyl-, C1-
C3-
alkylcarbonylamino-, CI-C3-alkylsulphonylamino-, C1-C3-allcylcarbonyl-, Ci-C3-
alkylsulphonyl-
and trifluoromethoxy-.
In the general formula (I), R12 preferably represents Ci-C6-alkyl- which may
optionally be mono-
or disubstituted by identical or different substituents from the group
consisting of fluorine,
hydroxy, Ci-C3-alkoxy- and
-NRIGRII.
In the general formula (I), R12 preferably represents CI-C6-alkyl- which may
optionally be mono-
or disubstituted by identical or different substituents from the group
consisting of fluorine,
hydroxy, Ci-C3-alkoxy- and
_N-R9Rio.
In the general formula (I), R12 preferably represents CI-C6-alkyl- which may
optionally be mono-
or disubstituted by identical or different substituents from the group
consisting of fluorine,
CA 02915419 2015-12-14
BHC123073FC
- 87
hydroxy, C1-C3-alkoxy- and
-NR9R1 ,
or
represents fluoro-C1-C3-alkyl-,
or
represents C3-C7-cycloalkyl- which may optionally be mono- or polysubstituted
by identical or
different substituents from the group consisting of fluorine, hydroxy, oxo,
cyano, C1-C3-alkyl-, C1-
C3-alkoxy- and -NR9R1 ,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-C12-
heterocycloallcyl radical, where the radicals mentioned may optionally be
monosubstituted by oxo,
Ci-C3-alkyl-, Ci-C3-alkylcarbonyl-, CI-C3-alkoxycarbonyl- and phenyl-Ci-C3-
alkyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of halogen, C1-C3-alkyl- and CI-C3-alkoxy-.
In the general formula (I), R12 particularly preferably represents Ci-C3-alkyl-
.
In the general formula (I), R12 particularly preferably represents Ci-C3-alkyl-
,
or
represents fluoro-C1-C3-alkyl-,
or
represents C3-C7-cycloallcyl which may optionally be mono- or polysubstituted
by identical or
different substituents from the group consisting of fluorine, hydroxy, oxo,
methyl-, ethyl-,
methoxy-, ethoxy- and N,N-dimethylamino-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms or a
bridged C6-C10-
heterocycloalkyl radical, where the radicals mentioned may optionally be
monosubstituted by
methyl-, ethyl-, acetyl-, benzyl- or tert-butoxycarbonyl-,
or
represents phenyl- or a monocyclic heteroaryl radical having 5 or 6 ring
atoms, where the radicals
mentioned may optionally be mono- or disubstituted by identical or different
substituents from the
group consisting of methyl-, ethyl-, fluorine, chlorine or bromine.
In the general formula (I), R.12 very particularly preferably represents
methyl.
CA 02915419 2015-12-14
BHC123073FC
- 88
In the general formula (I), 12.12 very particularly preferably represents
methyl-, trifluoromethyl-,
phenyl-, benzyl-, cyclopropyl-, tetrahydropyran-4-y1 or pyrid-3-y1-.
In the general formula (I), R'2 exceptionally preferably represents CI-C3-
alkyl, fluoro-C1-C3-alkyl-
or C3-C7-cycloalkyl-,
OT
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms which
may optionally be
monosubstituted by methyl-, ethyl-, acetyl-, benzyl or tert-butoxycarbonyl-,
or
represents a phenyl or pyridyl radical, where the radicals mentioned may
optionally be mono- or
disubstituted by identical or different substituents from the group consisting
of methyl-, ethyl-,
fluorine, chlorine or bromine.
In the general formula (I), R'2 furthermore exceptionally preferably
represents CI-C3-alkyl, fluoro-
CI-C3-alkyl- or C3-C7-cycloallcyl-,
or
represents a monocyclic heterocyclyl radical having 4 to 7 ring atoms,
or
represents a phenyl or pyridyl radical.
The specific radical definitions given in the particular combinations or
preferred combinations of
radicals are, irrespective of the particular combinations of radicals
specified, also replaced as
desired by radical definitions of other combination.
Very particular preference is given to combinations of two or more of the
abovementioned
preferred ranges.
Compounds according to the invention are the compounds of the formula (I) and
their salts,
solvates and solvates of the salts, the compounds, comprised by formula (I),
of the formulae
mentioned below and their salts, solvates and solvates of the salts and the
compounds comprised by
the formula (I), mentioned below as embodiments and their salts, solvates and
solvates of the salts
if the compounds, comprised by the formula (I), mentioned below are not
already salts, solvates
and solvates of the salts.
The present invention is likewise considered to encompass the use of the salts
of the compounds
according to the invention.
Preferred salts in the context of the present invention are physiologically
acceptable salts of the
CA 02915419 2015-12-14
BHC123073FC
- 89 -
..
compounds according to the invention. However, the invention also encompasses
salts which
themselves are unsuitable for pharmaceutical applications but which can be
used, for example, for the
isolation or purification of the compounds according to the invention.
Physiologically acceptable salts of the compounds according to the invention
include acid addition
salts of mineral acids, carboxylic acids and sulphonic acids, for example
salts of hydrochloric acid,
hydrobromic acid, sulphuric acid, phosphoric acid, methanesulphonic acid,
ethanesulphonic acid,
toluenesulphonic acid, benzenesulphonic acid, naphthalenedisulphonic acid,
acetic acid, trifluoroacetic
acid, propionic acid, lactic acid, tartaric acid, malic acid, citric acid,
fumaric acid, maleic acid and
benzoic acid.
Physiologically acceptable salts of the compounds according to the invention
furthermore include base
addition salts, for example of alkali metals such as sodium or potassium, of
alkaline earth metals such
as calcium or magnesium, or of ammonium salts derived from ammonia or organic
amines having 1 to
16 carbon atoms, for example methylamine, ethylamine, diethylamine,
triethylamine,
ethyldiisopropylamine, monoethanolamine, diethanolamine, triethanolamine,
dicyclohexylamine,
dimethylaminoethanol, procaine, dibenzylamine, N-methylmorpholine, arginine,
lysine,
ethylenediamine, N-methylpiperidine, N-methylglucamine, dimethylglucamine,
ethylglucamine, 1,6-
hexadiamine, glucosamine, sarcosine, serinol, tris(hydroxymethyDaminomethane,
aminopropanediol,
Sovak base and/or 1-amino-2,3,4-butanetriol. Furthermore, the compounds
according to the invention
may form base addition salts with quaterary ammonium ions which can be
obtained, for example, by
quatemization of corresponding amines with agents such as lower alkyl halides,
for example methyl,
ethyl, propyl and butyl chlorides, methyl, ethyl, propyl and butyl bromides,
and methyl, ethyl, propyl
and butyl iodides, dialkyl sulphates such as dimethyl, diethyl, dibutyl and
diamyl sulphate, long-chain
halides such as decyl, lauryl, myristyl and stearyl chlorides, decyl, lauryl,
myristyl and stearyl
bromides, and decyl, lauryl, myristyl and stearyl iodides, or arylalkyl
halides such as benzyl bromide
or phenethyl bromide. Examples of such quaternary ammonium ions are
tetramethylarnmonium,
tetraethylammonium, tetra(n-propyl)ammonium, tetra(n-butyl)ammonium and also
benzyltrimethylammonium.
The present invention furthermore provides all the possible crystalline and
polymorphous forms of the
compounds according to the invention, where the polymorphs may be present
either as single
polymorphs or as a mixture of a plurality of polymorphs in all concentration
ranges.
The present invention furthermore provides medicaments comprising the
compounds according to
the invention and at least one or more further active compounds, in particular
for the prophylaxis
and/or therapy of neoplastic disorders.
CA 02915419 2015-12-14
BHC123073FC
- 90 -
Solvates in the context of the invention are described as those forms of the
compounds according to
the invention which form a complex in the solid or liquid state by
coordination with solvent molecules.
Hydrates are a specific form of the solvates in which the coordination is with
water. Solvates preferred
in the context of the present invention are hydrates.
The compounds according to the invention may, depending on their structure,
exist in different
stereoisomeric forms, i.e. in the form of configurational isomers or else
optionally as conformational
isomers. The compounds according to the invention may have a centre of
asymmetry at the carbon
atom to which R2 is attached (C-4). They may therefore take the form of pure
enantiomers, racemates,
or else of diastereomers or mixtures thereof when one or more of the
substituents described in the
formula (I) contains a further element of asymmetry, for example a chiral
carbon atom. The present
invention therefore also encompasses enantiomers and diastereomers, and the
respective mixtures
thereof The pure enantiomers and diastereomers can be isolated from such
mixtures in a known
manner; chromatography processes are preferably used for this, in particular
HPLC chromatography
on a chiral or achiral phase.
In general, the enantiomers according to the invention inhibit the target to
different degrees and
have different activity in the cancer cell lines studied. The more active
enantiomer is preferred,
which is often that in which the centre of asymmetry represented by the carbon
atom bonded to R2
has (S) configuration.
If the compounds according to the invention can occur in tautomeric forms, the
present invention
encompasses all the tautomeric forms.
The present invention also encompasses all suitable isotopic variants of the
compounds according to
the invention. An isotopic variant of a compound according to the invention is
understood here as
meaning a compound in which at least one atom within the compound according to
the invention has
been exchanged for another atom of the same atomic number, but with a
different atomic mass than
the atomic mass which usually or predominantly occurs in nature. Examples of
isotopes which can be
incorporated into a compound according to the invention are those of hydrogen,
carbon, nitrogen,
oxygen, phosphorus, sulphur, fluorine, chlorine, bromine and iodine, such as
2H (deuterium), 314
(tritium), 13C, 14C, 1 sN, 170, 180, 32F, 33F, 33s, 34s, 35s, 36s, 18F, 36C1,
82Br, 123/, 1241, 129/ and 131/.
Particular isotopic variants of a compound according to the invention,
especially those in which one or
more radioactive isotopes have been incorporated, may be beneficial, for
example, for the examination
of the mechanism of action or of the active ingredient distribution in the
body; due to comparatively
easy preparability and detectability, especially compounds labelled with 3H or
14C isotopes are suitable
for this purpose. In addition, the incorporation of isotopes, for example of
deuterium, can lead to
particular therapeutic benefits as a consequence of greater metabolic
stability of the compound, for
CA 02915419 2015-12-14
BHC123073FC
- 91 -
example an extension of the half-life in the body or a reduction in the active
dose required; such
modifications of the compounds according to the invention may therefore in
some cases also constitute
a preferred embodiment of the present invention. Isotopic variants of the
compounds according to the
invention can be prepared by the processes known to those skilled in the art,
for example by the
methods described further below and the procedures described in the working
examples, by using
corresponding isotopic modifications of the respective reagents and/or
starting compounds.
In addition, the present invention also encompasses prodrugs of the compounds
according to the
invention. The term "prodrugs" encompasses compounds which for their part may
be biologically
active or inactive but are converted during their residence time in the body
into compounds according
to the invention (for example by metabolism or hydrolysis).
The compounds according to the invention can act systemically and/or locally.
For this purpose,
they can be administered in a suitable manner, for example by the oral,
parenteral, pulmonary,
nasal, sublingual, lingual, buccal, rectal, dermal, transdermal, conjunctival
or otic route, or as
implant or stent.
The compounds according to the invention can be administered in suitable
administration forms for
these administration routes.
Suitable administration forms for oral administration are those which function
according to the
prior art and deliver the compounds according to the invention rapidly and/or
in modified fashion,
and which contain the compounds according to the invention in crystalline
and/or amorphized
and/or dissolved form, for example tablets (uncoated or coated tablets, for
example having enteric
coatings or coatings which are insoluble or dissolve with a delay and control
the release of the
compound according to the invention), tablets which disintegrate rapidly in
the mouth, or
films/wafers, films/Iyophilizates, capsules (for example hard or soft gelatin
capsules), sugar-coated
tablets, granules, pellets, powders, emulsions, suspensions, aerosols or
solutions.
Parenteral administration can be accomplished with avoidance of a resorption
step (for example by
an intravenous, intraarterial, intracardiac, intraspinal or intralumbar route)
or with inclusion of a
resorption (for example by an intramuscular, subcutaneous, intracutaneous,
percutaneous or
intraperitoneal route). Administration forms suitable for parenteral
administration include
preparations for injection and infusion in the form of solutions, suspensions,
emulsions,
lyophilizates or sterile powders.
Suitable administration forms for the other administration routes are, for
example, pharmaceutical
forms for inhalation (including powder inhalers, nebulizers), nasal drops,
solutions or sprays;
CA 02915419 2015-12-14
BHC123073FC
- 92 -
tablets for lingual, sublingual or buccal administration, films/wafers or
capsules, suppositories,
preparations for the ears or eyes, vaginal capsules, aqueous suspensions
(lotions, shaking mixtures),
lipophilic suspensions, ointments, creams, transdermal therapeutic systems
(for example patches),
milk, pastes, foams, dusting powders, implants or stents.
The compounds according to the invention can be converted to the
administration forms
mentioned. This can be accomplished in a manner known per se by mixing with
inert, nontoxic,
pharmaceutically suitable excipients. These excipients include carriers (for
example
microcrystalline cellulose, lactose, mannitol), solvents (e.g. liquid
polyethylene glycols),
emulsifiers and dispersing or wetting agents (for example sodium
dodecylsulphate,
polyoxysorbitan oleate), binders (for example polyvinylpyrrolidone), synthetic
and natural
polymers (for example albumin), stabilizers (e.g. antioxidants, for example
ascorbic acid),
colourants (e.g. inorganic pigments, for example iron oxides) and flavour
and/or odour correctants.
The present invention furthermore provides medicaments which comprise the
compounds
according to the invention, typically together with one or more inert,
nontoxic, pharmaceutically
suitable auxiliaries, and the use thereof for the aforementioned purposes.
The compounds according to the invention are formulated to give pharmaceutical
preparations in a
manner known per se, by converting the active compound(s) to the desired
administration form
with the excipients customary in the pharmaceutical formulation.
The excipients used may, for example, be carrier substances, fillers,
disintegrants, binders,
humectants, glidants, absorbents and adsorbents, diluents, solvents,
cosolvents, emulsifiers,
solubilizers, taste correctors, colourants, preservatives, stabilizers,
wetting agents, salts for
modifying the osmotic pressure or buffers. Reference should be made to
Remington's
Pharmaceutical Science, 15th ed. Mack Publishing Company, East Pennsylvania
(1980).
The pharmaceutical formulations can be present
in solid form, for example as tablets, sugar-coated tablets, pills,
suppositories, capsules,
transdermal systems or
in semisolid form, for example as ointments, creams, gels, suppositories,
emulsions or
in liquid form, for example as solutions, tinctures, suspensions or emulsions.
Excipients in the context of the invention may, for example, be salts,
saccharides (mono-, di-, tri-,
oligo- and/or polysaccharides), proteins, amino acids, peptides, fats, waxes,
oils, hydrocarbons and
derivatives thereof, and the excipients may be of natural origin or be
obtained by synthetic or
partially synthetic means.
CA 02915419 2015-12-14
BHC123073FC
- 93 -
Useful forms for oral or peroral administration are especially tablets, sugar-
coated tablets,
capsules, pills, powders, granules, pastilles, suspensions, emulsions or
solutions.
Useful forms for parenteral administration are especially suspensions,
emulsions, and particularly
solutions.
The present invention relates to the compounds according to the invention.
They can be used for the prophylaxis and therapy of human disorders, in
particular neoplastic
disorders.
The compounds according to the invention can be used in particular for
inhibiting or reducing cell
proliferation and/or cell division and/or to induce apoptosis.
The compounds according to the invention are suitable in particular for the
prophylaxis and/or
therapy of hyper-proliferative disorders such as, for example,
- psoriasis,
- keloids and other skin hyperplasias,
- benign prostate hyperplasias (BPH),
- solid tumours and
- haematological tumours.
Solid tumours that can be treated in accordance with the invention are, for
example, tumours of the
breast, the respiratory tract, the brain, the reproductive organs, the
gastrointestinal tract, the
urogenital tract, the eye, the liver, the skin, the head and the neck, the
thyroid gland, the
parathyroid gland, the bones, and the connective tissue and metastases of
these tumours.
Haematological tumours which can be treated are, for example,
- multiple myelomas
- lymphomas or
- leukaemias
Breast tumours which can be treated are, for example:
- breast carcinomas with positive hormone receptor status
- breast carcinomas with negative hormone receptor status
- Her-2 positive breast carcinomas
- hormone receptor and Her-2 negative breast carcinomas
- BRCA¨associated breast carcinomas
- inflammatory breast carcinomas.
Tumours of the respiratory tract which can be treated are, for example,
CA 02915419 2015-12-14
BHC123073FC
.
- 94 -
a
- non-small-cell bronchial carcinomas such as, for example,
squamous cell carcinoma,
adenocarcinoma, large-cell carcinoma and
- small-cell bronchial carcinomas.
Tumours of the brain which can be treated are, for example,
- gliomas,
- glioblastomas,
- astrocytomas,
- meningiomas and
- medulloblastomas.
Tumours of the male reproductive organs which can be treated are, for example:
- prostate carcinomas,
- malignant epididymal tumours
- malignant testicular tumours and
- penis carcinomas.
Tumours of the female reproductive organs which can be treated are, for
example:
- endometrial carcinomas
- cervix carcinomas
- ovarian carcinomas
- vaginal carcinomas
- vulvar carcinomas
Tumours of the gastrointestinal tract which can be treated are, for example:
- colorectal carcinomas
- anal carcinomas
- stomach carcinomas
- pancreas carcinomas
- oesophagus carcinomas
- gall bladder carcinomas
- carcinomas of the small intestine
- salivary gland carcinomas
- neuroendocrine tumours
- gastrointestinal stroma tumours
Tumours of the urogenital tract which can be treated are, for example:
- urinary bladder carcinomas
CA 02915419 2015-12-14
BHC123073FC
- 95 --
A
- kidney cell carcinomas
- carcinomas of the renal pelvis and lower urinary tract
Tumours of the eye which can be treated are, for example:
- retinoblastomas
- intraocular melanomas
Tumours of the liver which can be treated are, for example:
- hepatocellular carcinomas
- cholangiocellular carcinomas
Tumours of the skin which can be treated are, for example:
- malignant melanomas
- basaliomas
- spinaliomas
- Kaposi sarcomas
- Merkel cell carcinomas
Tumours of the head and neck which can be treated are, for example:
- larynx carcinomas
- carcinomas of the pharynx and the oral cavity
- carcinomas of the middle line structures (e.g. NMC, C.A.
French, Annu. Rev. Pathol. 2012,
7:247-265)
Sarcomas which can be treated are, for example:
- soft tissue sarcomas
- osteosarcomas
Lymphomas which can be treated are, for example:
- non-Hodgkin lymphomas
- Hodgkin lymphomas
- cutaneous lymphomas
- lymphomas of the central nervous system
- AIDS-associated lymphomas
Leukaemias which can be treated are, for example:
- acute myeloid leukaemias
- chronic myeloid leukaemias
CA 02915419 2015-12-14
BHC123073FC
A
- 96 -
a
- acute lymphatic leukaemias
- chronic lymphatic leukaemias
- hairy cell leukaemias
Advantageously, the compounds according to the invention can be used for
prophylaxis and/or
treatment of leukaemia, especially acute myeloid leukaemia, prostate
carcinoma, especially
androgen receptor-positive prostate carcinoma, cervical carcinoma, mammary
carcinoma,
especially hormone receptor-negative, hormone receptor-positive or BRCA-
associated mammary
carcinoma, pancreatic carcinoma, renal cell carcinoma, hepatocellular
carcinoma, melanoma and
other skin tumours, non-small-cell bronchial carcinoma, endometrial carcinoma
and colorectal
carcinoma.
Particularly advantageously, the compounds according to the invention can be
used for prophylaxis
and/or treatment of leukaemia, especially acute myeloid leukaemia, prostate
carcinoma, especially
androgen receptor-positive prostate carcinoma, mammary carcinoma, especially
oestrogen receptor
alpha-negative mammary carcinoma, melanoma or multiple myeloma.
The compounds according to the invention are also suitable for prophylaxis
and/or treatment of
benign hyperproliferative diseases, for example endometriosis, leiomyoma and
benign prostate
hyperplasia.
The compounds according to the invention are also suitable for male fertility
control.
The compounds according to the invention are also suitable for prophylaxis
and/or treatment of
systemic inflammatory diseases, especially LPS-induced endotoxic shock and/or
bacteria-induced
sepsis.
The compounds according to the invention are also suitable for prophylaxis
and/or treatment of
inflammatory or autoimmune disorders, for example:
- pulmonary disorders associated with inflammatory, allergic and/or
proliferative processes:
chronic obstructive pulmonary disorders of any origin, particularly bronchial
asthma;
bronchitis of different origin; all forms of restrictive pulmonary disorders,
particularly
allergic alveolitis; all forms of pulmonary oedema, particularly toxic
pulmonary oedema;
sarcoidoses and granulomatoses, particularly Boeck's disease,
- rheumatic disorders/autoimmune disorders/joint disorders associated with
inflammatory,
allergic and/or proliferative processes: all forms of rheumatic disorders,
especially
rheumatoid arthritis, acute rheumatic fever, polymyalgia rheumatica; reactive
arthritis;
inflammatory soft-tissue disorders of other origin; arthritic symptoms in the
case of
CA 02915419 2015-12-14
BHC123073FC
=
- 97
degenerative joint disorders (arthroses); traumatic arthritides; collagenoses
of any origin,
e.g. systemic lupus erythematosus, scleroderma, polymyositis, dermatomyositis,
Sjogren's
syndrome, Still's syndrome, Felty's syndrome
- allergies associated with inflammatory and/or proliferative
processes: all forms of allergic
reactions, e.g. angiooedema, hay fever, insect bites, allergic reactions to
medicaments,
blood derivatives, contrast agents, etc., anaphylactic shock, urticaria,
contact dermatitis
- vascular inflammation (vasculitis): panarteritis nodosa,
temporal arteritis, erythema
nodosum
- dermatological disorders associated with inflammatory,
allergic and/or proliferative
processes: atopic dermatitis; psoriasis; pityriasis rubra pilaris;
erythematous disorders
triggered by different noxae, for example radiation, chemicals, burns, etc.;
bullous
dermatoses; lichenoid disorders; pruritus; seborrhoeic eczema; rosacea;
pemphigus
vulgaris; erythema exsudativum multiforme; balanitis; vulvitis; hair loss,
such as alopecia
areata; cutaneous T-cell lymphoma
- renal disorders associated with inflammatory, allergic and/or proliferative
processes:
nephrotic syndrome; all nephritides
- hepatic disorders associated with inflammatory, allergic
and/or proliferative processes:
acute hepatic disintegration; acute hepatitis of different origin, for example
viral, toxic,
medicament-induced; chronic aggressive and/or chronic intermittent hepatitis
- gastrointestinal disorders associated with inflammatory, allergic and/or
proliferative
processes: regional enteritis (Crohn's disease); ulcerative colitis;
gastritis; reflux
oesophagitis; gastroenteritides of other origin, e.g. indigenous sprue
- proctological disorders associated with inflammatory,
allergic and/or proliferative
processes: anal eczema; fissures; haemorrhoids; idiopathic proctitis
- ocular disorders associated with inflammatory, allergic and/or proliferative
processes:
allergic keratitis, uveitis, iritis; conjunctivitis; blepharitis; optic
neuritis; chlorioditis;
sympathetic ophthalmia
- disorders of the ear-nose-throat region associated with
inflammatory, allergic and/or
proliferative processes: allergic rhinitis, hay fever; otitis externa, for
example caused by
contact eczema, infection, etc.; otitis media
- neurological disorders associated with inflammatory, allergic
and/or proliferative
processes: cerebral oedema, particularly tumour-related cerebral oedema;
multiple
sclerosis; acute encephalomyelitis; meningitis; various forms of seizure, for
example
West's syndrome
- haematological disorders associated with inflammatory, allergic and/or
proliferative
processes: acquired haemolytic anaemia; idiopathic thrombocytopenia
- neoplastic disorders associated with inflammatory, allergic
and/or proliferative processes:
acute lymphatic leukaemia; malignant lymphomas; lymphogranulomatoses;
CA 02915419 2015-12-14
BHC123073FC
- 98 ¨
Is
lymphosarcoma; extensive metastases, particularly in the case of mammary,
bronchial and
prostate carcinoma
- endocrine disorders associated with inflammatory,
allergic and/or proliferative processes:
endocrine orbitopathy; thyrotoxic crisis; de Quervain's thyroiditis;
Hashimoto's thyroiditis;
Basedow's disease
- organ and tissue transplants, graft-versus-host disease
- severe states of shock, for example anaphylactic shock,
systemic inflammatory response
syndrome (SIRS)
- substitution therapy in the case of: congenital primary
adrenal insufficiency, for example
congenital adrenogenital syndrome; acquired primary adrenal insufficiency, for
example
Addison's disease, autoimmune adrenalitis, postinfectious, tumours,
metastases, etc;
congenital secondary adrenal insufficiency, for example congenital
hypopituitarism;
acquired secondary adrenal insufficiency, for example postinfectious, tumours,
etc.
- emesis associated with inflammatory, allergic and/or proliferative
processes, for example
in combination with a 5-HT3 antagonist in the case of cytostatic-induced
vomiting
- pain of inflammatory origin, for example lumbago.
The compounds according to the invention are also suitable for the treatment
of viral disorders, for
example infections caused by papilloma viruses, herpes viruses, Epstein-Barr
viruses, hepatitis B or
C viruses, and human immunodeficiency viruses.
The compounds according to the invention are also suitable for the treatment
of atherosclerosis,
dyslipidaemia, hypercholesterolaemia, hypertriglyceridaemia, peripheral
vascular disorders,
cardiovascular disorders, angina pectoris, ischaemia, stroke, myocardial
infarction, angioplastic
restenosis, hypertension, thrombosis, obesity, endotoxaemia.
The compounds according to the invention are also suitable for the treatment
of neurodegenerative
diseases, for example multiple sclerosis, Alzheimer's disease and Parkinson's
disease.
These disorders are well characterized in man, but also exist in other
mammals.
The present application furthermore provides the compounds according to the
invention for use as
medicaments, in particular for the prophylaxis and/or therapy of tumour
disorders.
The present application furthermore provides the compounds according to the
invention for
prophylaxis and/or therapy of leukaemia, especially acute myeloid leukaemia,
prostate carcinoma,
especially androgen receptor-positive prostate carcinoma, cervical carcinoma,
mammary
carcinoma, especially hormone receptor-negative, hormone receptor-positive or
BRCA-associated
mammary carcinoma, pancreatic carcinoma, renal cell carcinoma, hepatocellular
carcinoma,
CA 02915419 2015-12-14
BHC123073FC
- 99 -
.4
melanoma and other skin tumours, non-small-cell bronchial carcinoma,
endometrial carcinoma and
colorectal carcinoma.
The present application furthermore provides the compounds according to the
invention for
prophylaxis and/or therapy of leukaemias, especially acute myeloid leukaemias,
prostate
carcinomas, especially androgen receptor-positive prostate carcinomas, mammary
carcinomas,
especially oestrogen receptor alpha-negative mammary carcinomas, melanomas or
multiple
myelomas.
The invention furthermore provides for the use of the compounds according to
the invention for
production of a medicament.
The present application furthermore provides for the use of the compounds
according to the
invention for production of a medicament for prophylaxis and/or therapy of
neoplastic disorders.
The present application furthermore provides for the use of the compounds
according to the
invention for production of a medicament for prophylaxis and/or therapy of
leukaemias, especially
acute myeloid leukaemias, prostate carcinomas, especially androgen receptor-
positive prostate
carcinomas, cervical carcinomas, mammary carcinomas, especially hormone
receptor-negative,
hormone receptor-positive or BRCA-associated mammary carcinomas, pancreatic
carcinomas,
renal cell carcinomas, hepatocellular carcinomas, melanomas and other skin
tumours, non-small-
cell bronchial carcinomas, endometrial carcinomas and colorectal carcinomas.
The present application furthermore provides for the use of the compounds
according to the
invention for production of a medicament for prophylaxis and/or therapy of
leukaemias, especially
acute myeloid leukaemias, prostate carcinomas, especially androgen receptor-
positive prostate
carcinomas, mammary carcinomas, especially oestrogen receptor alpha-negative
mammary
carcinomas, melanomas or multiple myelomas.
The present application furthermore provides for the use of the compounds
according to the
invention for prophylaxis and/or therapy of neoplastic disorders.
The present application furthermore provides for the use of the compounds
according to the
invention for prophylaxis and/or therapy of leukaemias, especially acute
myeloid leukaemias,
prostate carcinomas, especially androgen receptor-positive prostate
carcinomas, cervical
carcinomas, mammary carcinomas, especially hormone receptor-negative, hormone
receptor-
positive or BRCA-associated mammary carcinomas, pancreatic carcinomas, renal
cell carcinomas,
hepatocellular carcinomas, melanomas and other skin tumours, non-small-cell
bronchial
CA 02915419 2015-12-14
BHC123073FC
4
- 100 -
%
carcinomas, endometrial carcinomas and colorectal carcinomas.
The present application furthermore provides for the use of the compounds
according to the
invention for prophylaxis and/or therapy of leukaemias, especially acute
myeloid leukaemias,
prostate carcinomas, especially androgen receptor-positive prostate
carcinomas, mammary
carcinomas, especially oestrogen receptor alpha-negative mammary carcinomas,
melanomas or
multiple myelomas.
The present application furthermore provides pharmaceutical formulations in
the form of tablets
comprising one of the compounds according to the invention for prophylaxis
and/or therapy of
leukaemias, especially acute myeloid leukaemia, prostate carcinoma, especially
androgen receptor-
positive prostate carcinoma, cervical carcinoma, mammary carcinoma, especially
hormone
receptor-negative, hormone receptor-positive or BRCA-associated mammary
carcinoma, pancreatic
carcinoma, renal cell carcinoma, hepatocellular carcinoma, melanoma and other
skin tumours, non-
small-cell bronchial carcinoma, endometrial carcinoma and colorectal
carcinoma.
The present application furthermore provides pharmaceutical formulations in
the form of tablets
comprising one of the compounds according to the invention for prophylaxis
and/or therapy of
leukaemias, especially acute myeloid leukaemias, prostate carcinomas,
especially androgen
receptor-positive prostate carcinomas, mammary carcinomas, especially
oestrogen receptor alpha-
negative mammary carcinomas, melanomas or multiple myelomas.
The invention furthermore provides for the use of the compounds according to
the invention for
treatment of disorders associated with proliferative processes.
The invention furthermore provides for the use of the compounds according to
the invention for
treatment of benign hyperplasias, inflammation disorders, autoimmune
disorders, sepsis, viral
infections, vascular disorders and neurodegenerative disorders.
The compounds according to the invention can be used alone or, if required, in
combination with
one or more other pharmacologically active substances, provided that this
combination does not
lead to undesirable and unacceptable side effects. The present invention
therefore furthermore
provides medicaments comprising an inventive compound and one or more further
active
ingredients, especially for prophylaxis and/or treatment of the disorders
mentioned above.
For example, the compounds according to the invention can be combined with
known
antihyperproliferative, cytostatic or cytotoxic substances for treatment of
cancer. The combination
of the compounds according to the invention with other substances commonly
used for cancer
CA 02915419 2015-12-14
BHC123073FC
- 101
treatment, or else with radiotherapy, is particularly appropriate.
An illustrative but nonexhaustive list of suitable combination active
ingredients is as follows:
abiraterone acetate, abraxane, acolbifene, Actimmune, actinomycin D
(dactinomycin), afatinib,
affinitak, Afinitor, aldesleukin, alendronic acid, alfaferone, alitretinoin,
allopurinol, Aloprim,
Aloxi, alpharadin, altretamine, aminoglutethimide, aminopterin, amifostine,
amrubicin, amsacrine,
anastrozole, anzmet, apatinib, Aranesp, arglabin, arsenic trioxide, Aromasin,
arzoxifen, asoprisnil,
L-asparaginase, atamestane, atrasentane, avastin, axitinib, 5-azacytidine,
azathioprine, BCG or Tice
BCG, bendamustine, bestatin, beta-methasone acetate, betamethasone sodium
phosphate,
bexarotene, bicalutamide, bleomycin sulphate, broxuridine, bortezomib,
bosutinib, busulfan,
cabazitaxel, calcitonin, campath, camptothecin, capecitabine, carboplatin,
carfilzomib, carmustine,
casodex, CCI-779, CDC-501, cediranib, cefesone, celebrex, celmoleukin,
cerubidine, cediranib,
chlorambucil, cisplatin, cladribine, clodronic acid, clofarabine, colaspase,
corixa, crisnatol,
crizotinib, cyclophosphamide, cyproterone acetate, cytarabine, dacarbazine,
dactinomycin,
dasatinib, daunorubicin, DaunoXome, Decadron, Decadron Phosphate, decitabine,
degarelix,
delestrogen, denileukin diftitox, depomedrol, deslorelin, dexrazoxane,
diethylstilbestrol, diflucan,
2",2"-difluorodeoxycytidine, DN-101, docetaxel, doxifluridine, doxorubicin
(Adriamycin),
dronabinol, dSLIM, dutasteride, DW-166HC, edotecarin, eflornithine, Eligard,
Elitek, Ellence,
Emend, enzalutamide, epirubicin, epoetin-alfa, Epogen, epothilone and
derivatives thereof,
eptaplatin, ergamisol, erlotinib, erythro-hydroxynonyladenine, estrace,
oestradiol, oestramustine
sodium phosphate, ethinyloestradiol, Ethyol, etidronic acid, etopophos,
etoposide, everolimus,
exatecan, exemestane, fadrozole, farston, fenretinide, filgrastim,
finasteride, fligrastim, floxuridine,
fluconazole, fludarabine, 5-fluorodeoxyuridine monophosphate, 5-fluorouracil
(5-FU),
fluoxymesterone, flutamide, folotin, formestane, fosteabine, fotemustine,
fulvestrant, Ganunagard,
gefitinib, gemcitabine, gemtuzumab, Gleevec, Gliadel, goserelin, gossypol,
granisetrone
hydrochloride, hexamethylmelamine, histamine dihydrochloride, histrelin,
holmium-166-DOTPM,
hycamtin, hydrocortone, erythro-hydroxynonyladenine, hydroxyurea,
hydroxyprogesterone
caproate, ibandronic acid, ibritumomab tiuxetan, idarubicin, ifosfamide,
imatinib, iniparib,
interferon-alpha, interferon-alpha-2, interferon-alpha-2a, interferon-a1pha-
21, interferon-alpha-nl,
interferon-alpha-n3, interferon-beta, interferon-gamma-la, interleukin-2,
intron A, iressa,
irinotecan, ixabepilone, keyhole limpet haemocyanin, lcytril, lanreotide,
lapatinib, lasofoxifene,
lentinan sulphate, lestaurtinib, letrozole, leucovorin, leuprolide, leuprolide
acetate, levamisole,
levofolic acid calcium salt, levothroid, levoxyl, Libra, liposomal MTP-PE,
lomustine, lonafarnib,
lonidamine, marinol, mechlorethamine, mecobalamine, medroxyprogesterone
acetate, megestrol
acetate, melphalan, Menest, 6-mercaptopurine, mesna, methotrexate, metvix,
miltefosine,
minocycline, minodronate, miproxifen, mitomycin C, mitotan, mitoxantrone,
modrenal, MS-209,
MX-6, myocet, nafarelin, nedaplatin, nelarabine, nemorubicin, neovastat,
neratinib, neulasta,
neumega, neupogen, nilotimib, nilutamide, nimustine, nolatrexed, nolvadex, NSC-
631570,
CA 02915419 2015-12-14
BHC123073FC
- 102 -
obatoclax, oblimersen, OCT-43, octreotide, olaparib, ondansetron
hydrochloride, Onco-TCS,
Orapred, Osidem, oxaliplatin, paclitaxel, pamidronate disodium, pazopanib,
pediapred,
pegaspargase, pegasys, pemetrexed, pentostatin, N-phosphonoacetyl-L-aspartate,
picibanil,
pilocarpine hydrochloride, pirarubicin, plerixafor, plicamycin, PN-401,
portimer sodium,
prednimustine, prednisolone, prednisone, Premarin, procarbazine, Procrit, QS-
21, quazepam, R-
1589, raloxifene, raltitrexed, ranpirnas, RDEA119, Rebif, regorafenib, 13-cis-
retinoic acid,
rhenium-186 etidronate, rituximab, roferon-A, romidepsin, romurtide,
ruxolitinib, salagen,
salinomycin, sandostatin, sargramostim, satraplatin, semaxatinib, semustine,
seocalcitol,
sipuleucel-T, sizofiran, sobuzoxan, Solu-Medrol, sorafenib, streptozocin,
strontium-89 chloride,
sunitinib, Synthroid, T-138067, tamoxifen, tamsulosin, Tarceva, tasonermin,
tastolactone,
Taxoprexin, Taxoter, teceleukin, temozolomide, temsirolimus, teniposide,
testosterone propionate,
Testred, thalidomide, thymosin alpha-1, thioguanine, thiotepa, thyrotropin,
tiazorufin, tiludronic
acid, tipifarnib, tirapazamine, TLK-286, toceranib, topotecan, toremifen,
tositumomab, tastuzumab,
teosulfan, transMID-107R, tretinoin, Trexall, trimethylmelamine, trimetrexate,
triptorelin acetate,
triptorelin pamoate, trofosfamide, UFT, uridine, valrubicin, valspodar,
vandetanib, vapreotide,
vatalanib, vemurafinib, verte-porfin, vesnarinone, vinblastine, vincristine,
vindesine, vinflumine,
vinorelbine, virulizin, vismodegib, Xeloda, Z-100, Zinecard, zinostatin
stimalamer, zofran,
zoledronic acid.
The combination of the compound according to the invention with a P-TEFb or
CDK9 inhibitor, or
with a BCL6 inhibitor, is likewise particularly preferred.
In a promising manner, the compounds according to the invention can also be
combined with
biologics such as antibodies (for example aflibercept, alemtuzumab,
bevacizumab, brentuximumab,
catumaxomab, cetuximab, denosumab, edrecolomab, gemtuzumab, ibritumomab,
ipilimumab,
ofatumumab, panitumumab, pertuzumab, rituximab, tositumumab, trastuzumab) and
recombinant
proteins.
The compounds according to the invention can also achieve positive effects in
combination with
other therapies directed against angiogenesis, for example with bevacizumab,
axitinib, regorafenib,
cediranib, sorafenib, sunitinib or thalidomide. Combinations with antihormones
and steroidal
metabolic enzyme inhibitors are particularly suitable because of their
favourable profile of side
effects.
Generally, the following aims can be pursued with the combination of the
compounds according to
the invention with other cytostatically or cytotoxically active agents:
CA 02915419 2015-12-14
BHC123073FC
- 103 -
= improved efficacy in slowing the growth of a tumour, in reducing its size
or even in
completely eliminating it, compared with treatment with an individual active
ingredient;
= the possibility of using the chemotherapeutics used in a lower dosage
than in the case of
monotherapy;
= the possibility
of a more tolerable therapy with fewer side effects compared with individual
administration;
= the possibility of treatment of a broader spectrum of tumours;
= the achievement of a higher rate of response to the therapy;
= a longer survival time of the patient compared with present-day standard
therapy.
In addition, the compounds according to the invention can also be used in
conjunction with
radiotherapy and/or surgical intervention.
CA 02915419 2015-12-14
=
BHC123073FC
- 104 -
Preparation of the compounds of the general formula I according to the
invention
Synthesis routes for preparing the compounds of the general formula (I)
The following schemes and general procedures illustrate general synthetic
routes to the compounds
of the formula (I) according to the invention; however, this should not be
interpreted as meaning
that the synthesis of the compounds according to the invention is limited to
these.
4,5-Dihydro-3H-2,3-benzodiazepines of the general formula (I) can be prepared
analogously to
processes described in the literature. Depending on the substituents present,
protective group
strategies may optionally be required; however, these are known to the person
skilled in the art (T.
W. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, 3.
Edition, Wiley 1999).
Scheme 1 shows the synthesis of 4,5-dihydro-3H-2,3-benzodiazepines using a 3,4-
dihydro-1H-2-
benzopyran intermediate (III), where the radicals Rla, Rlb, RC, R2,
R4 and R5 have the meanings
given in the general formula (I). Corresponding approaches are described, for
example, in F. Gatta
et al. 11 Farmaco ¨ Ed. Sc. 1985, 40, 942, and in WO 1997028135, W02008124075
or
W0200198280.
The benzaldehydes of the formula (Ilb) used are commercially available, or
their preparation is
known to the person skilled in the art. R1a can also be introduced at a later
stage of the synthesis,
for example as described in Scheme 5.
The substituted phenethyl alcohols (II) used are either commercially available
or are prepared in a
manner generally known to the person skilled in the art, for example by
reduction of the
corresponding ketones (Ha), e.g. by reduction with lithium aluminium hydride
in THF;
alternatively, they can also be obtained by reacting the corresponding
phenylacetaldehydes with
organomagnesium compounds of the formula R2Mg-halogen where halogen represents
chlorine,
bromine or iodine (see, for example, Organic Letters 2007, 2103-2106; for the
preparation of the
corresponding phenylacetaldehydes see, for example, Monatshefte fur Chemie
2004, 1289-1295).
This synthesis route is preferably used for phenethyl alcohols (II) having
electron-rich substituents
(e.g. with alkoxy).
3,4-Dihydro-1H-2-benzopyrans (III) are obtained by condensation of the
substituted phenethyl
alcohols (11) with benzaldehydes (1Ib) under acidic conditions. The reaction
is preferably carried
out in solvents such as toluene or dioxane in the presence of hydrochloric
acid or anhydrous zinc
chloride. Further conversion of the 3,4-dihydro-1H-2-benzopyrans (III) can be
by various routes:
oxidative ring-opening using chromium(VI) oxide/sulphuric acid affords the
diketone (IV) which
CA 02915419 2015-12-14
=
BHC123073FC
- 105 -
can be cyclized with hydrazine to give 4-methyl-1-pheny1-5H-2,3-benzodiazepine
(V) (cf.
US5288863). Reduction, for example with sodium cyanoborohydride (Synthetic
Communications,
2002, 32, 527), then yields the desired 4,5-dihydro-3H-2,3-benzodiazepine
derivative (VI).
Oxidation of (III) with atmospheric oxygen affords the 1-ary1-3,4-dihydro-1H-2-
benzopyran-1-o1
(VII) which, under elimination of water, can be reacted with a monoprotected
hydrazine, for
example H2NNHBoc, to give the corresponding hydrazone derivatives, such as the
N-Boc-
hydrazone (VIII). This can be cyclized, for example by mesylation and
subsequent treatment with
base, to give the Boc-protected 4,5-dihydro-3H-2,3-benzodiazepine derivative,
such as (IX), which
in turn can be converted by deprotection, for example removal of Boc in the
presence of an acid, in
a generally known manner into the corresponding 4,5-dihydro-3H-2,3-
benzodiazepine derivative
(VI).
CA 02915419 2015-12-14
BHC123073FC
,
- 106 -
Scheme I: 4,5-Dihydro-3H-2,3-benzodiazepines via 3,4-dihydro-1H-2-benzopyrans
R4 R2 R4 R2
Rs 0Reduction
0 R5 SI OH
Ila II 0
I R'e * ell
Rla Ilb
R
R4 2
R2 0
R4 R2
0
Jones R5 Atmospheric
all 0
Oxidation oxidation Rs
R4 11111 VII
0 .ii---- OH
Rs ir 1101
Ric 111 Rib
IV * Ric
lit Feb
R" Ria
Rib Ria
III
Rie
1 H2N ¨NHBoc
N2114 i
R2
R4 R2 OHO H C
\ N R4 A
3)<CH3
HN 0 CH3
I
R5 * -- r4 v , N
VIII
RS =
Ric
ill Rib Ric * R15
Ri'
NaCNB\H3
Ria
1 1. MsCI
2. Base
R2 R2
R4 R4 0
NH ,N-
4 CH3
Rs .I --- Ni HCI RS = "*". N'l4 O---
CH3
-0---
VI
CH3
Ric * Ric elit Ix
R15 R 1 5
Ria Ria
CA 02915419 2015-12-14
BHC123073FC
- 107 -
Scheme 2 describes the synthesis of 4,5-dihydro-3H-2,3-benzodiazepines from
indanones (X).
Scheme 2: 4,5-Dihydro-3H-2,3-benzodiazepines from indanones
R4
FF FF (js, IP. R2 Ric it B(OH)2
F)c)Ci(4`F R 5
FFFF 0
r Ilc
F
F F F F
4
R2 ioe R2
MgHal F F 5 so R2
X 0R4
Rie * Rlb *le R2 Olip Rib
R" Ild R5
OH p-Ts0H R
R* ,OK
R"
R1'
XIII
RuCI, / Na104 R2 0 R2
or R4 R4
0s04 Ma 104 Si N2H4
-101 NaCNBH3NH
o N
R5
Fee * Rie
R1 * Rib R Rib
IV R" la
R VI
V
The radicals RI., Rib, Ric, R2, lean a = R5
in Scheme 2 have the meanings given in the general
formula (I).
The indanone (X) can be converted into the corresponding 3-phenyl-1H-indene
(XII). To this end,
the following processes may be used:
- the indanone derivative (X) can, for example, be converted in a generally
known manner into
the corresponding enol nonaflate (XI) and then be converted by palladium-
catalysed Suzuki
coupling with the appropriate boronic acid derivatives (Hc) into the indene
(XII).
- the indanone derivative (X) can be converted by addition of
organomagnesium reagents (Hd) in
a generally known manner into the corresponding indanols (XIII) which, via
acid-catalysed
elimination, readily form the corresponding indenes (XII).
The 3-phenyl-1H-indenes (XII) can be converted by oxidative methods using, for
example,
ruthenium(III) chloride/sodium periodate (Bioorganic and Medicinal Chemistry
Letters, 2011, 21,
2554) into the corresponding diketones (IV). These can be converted
analogously to Scheme 1 into
the corresponding 4,5-dihydro-3H-2,3-benzodiazepine derivatives (VI).
CA 02915419 2015-12-14
BHC123073FC
- 108 -
The indanones used for preparing the working examples are either commercially
available or can
be prepared as shown, for example, in Scheme 3, where the radicals R2, R4 and
125 have the
meanings given in the general formula (I).
Scheme 3: Synthesis of indanones
Br
EtO¨P=0
H,C OH
OEt
0
0
0 0RZ sio OH _____________
Rz Hydrogenation
: Base
R'
XV
CH_
0 0
CH, =
1.T.y
R2I R HCI
4110 0 0
0
A-C, 0
IR4
411
OH
R' 2. Reduction R5=0 0 H R' 4i 0
XIV CH, CH_
XVI XVII XVIII
Polyphosphoric acid
Or
\1/4 chlorosulphoric acid//,
1. SOCI,
R4
2. AICI,
O. R2
R'
5 X 0
Using processes known from the literature, e.g. via Perkin reaction (Medicinal
Chemistry Research,
2004, Vol. 13, 660) or Wittig reaction (Journal of Organic Chemistry, 2001,
Vol. 66, 3682), it is
possible to prepare the 2-methyl-3-phenylpropanoic acids (XVIII) from the
corresponding aromatic
aldehydes (XIV) . These can be cyclized using, for example, chlorosulphonic
acid or
polyphosphoric acid, giving the corresponding indanones (X) (cf. Synthesis
2009, 627 and Org.
Process Res. Dev. 2011, 15, 570-580, J. Org. Chem. 2005, 70, 1316 and Bioorg.
Med. Chem. Lett.
2011, 21, 2554-2558).
Scheme 4 illustrates the preparation of the exemplary compounds according to
the invention
starting with 4,5-dihydro-3H-2,3-benzodiazepines (VI) using generally known
reactions, for
example with acid chlorides, anhydrides, chloroformates or isocyanates or
isothiocyanates, where
the radicals RI, aIR b, Ric, R2, R3, I(-4
and R5 have the meanings giving in general formula (I). The
corresponding alkylureas (lb) can also be obtained by reacting a reactive
intermediate such as, for
example, the 4-nitrophenyl carbamate, with alkylamines.
CA 02915419 2015-12-14
BHC123073FC
,
- 109 -
Scheme 4: Synthesis of 4,5-dihydro-3H-2,3-benzodiazepine-3-carbonyl compounds
R2 0 R2
R4 R3¨ R4 0
VI NH a N-4
Rs * ¨1\11
Or R5 I. --14 R3 la
0
Ric * R3¨g Ric *
0
Rlb 0--
Rlb
Rla R3 Rla
R2
R2
R4 1 . R4
0 -,0 .
R5 0 -,NH 0 ci p-4 R = Alkyl
R5 * --- N N- R
H
vi Ric Rlb 02N * Ric *
______________________________________________ IN
Rla 2. RNH2 Rla Rlb lb
R2 R2
R4= R R4 X X =
o,s
NH Alkyl isocyanate 5 ,N-4 R = Alkyl
R5 --- N -- N N- R
Or H
alkyl isothiocyanate
VI Ric ___________________ 0-- Ric * ic
Rlb Rlb
Rla Rla
Rla can also be introduced at a later stage of the synthesis, for example as
described in Scheme 5.
The starting materials (XXI), (XXII) and (XXIII) used can be prepared using
the synthesis methods
discussed above and illustrated in Schemes 1 to 4. The radicals RI', Rlb, Ric,
R2, R3, R4, R5, R6, R7,
R8, RH, R12 in Scheme 5 have the meanings given in the general formula (I).
CA 02915419 2015-12-14
. BHC123073FC
- 110 -
Scheme 5:
R2
R2
Fe 0 R4 0
N4 /
R5 101 ¨ 4 R3 R7R8NH R5 44
* --- N R3
)0(1 ____________________________ r le
R" *Pd catalyst complex R" *
RTh Rib
Y R7¨ N
1
Y = CI, Br, I, OTf R8
R2 R2
R4 0 R4 N4
0
1\14
R5 (16 --- N R3
NH(R8)-00R11 R5 (III/ 3
¨ N
R if
XXII ____________________________ 3.-
Fec * Rib Cu catalyst complex
R" *
R"
Y R8¨N
Y = Cl. Br, I 0
R11
R2 R2
R4 0 R4 0
R5 4 N4
¨ ,N
N Fe 11 11 R6-OH R5 10 --- 4
R3 1g
XXII ,
Fee * Pd catalyst complex
R" *
R" or Cu catalyst complex
Rib
Y C
R-0
Y = CI, Br, I
R2 R2
R4 r" 0
XXIII R5 R4 *I 0
N-4 N4
R12-S02-NH(R8) i 3
-- N R
A
I h
Ric *Pd catalyst complex Ric *
Rib Feb
8
Br R¨N
µs=-=:,'0
R121 (3
Scheme 5 illustrates the preparation of working examples which can be prepared
by palladium-
catalysed coupling reactions generally known to the person skilled in the art
starting, for example,
with brominated intermediates (XXI) by reaction with the appropriate amines
(Chem. Sci. 2011, 2,
27; Angew. Chem. 2008, 47, 6338, Accounts of Chemical Research 2008, 41,
1534). Using copper-
catalysed reactions, it is possible to convert intermediates of type XXII by
reaction with amides
into the corresponding coupled derivatives (If) (JACS 2001, 123, 7727; JACS
2002, 124, 7421).
Via palladium-catalysed reactions, intermediates of type XXII can be converted
by reaction with
CA 02915419 2015-12-14
BHC123073FC
- 111 -
alcohol derivatives or by copper-catalysed couplings by reaction with phenol
derivatives into the
respective corresponding ether derivatives (Ig) (JACS 1997, 119, 3395;
Angewandte Chemie,
International Edition 2006, 45, 1276). N-Arylated sulphonamides (Ih) can be
prepared from
intermediates of type XXIII by palladium-catalysed reaction with sulphonamides
(Org. Lett. 2011,
2564). Intermediates XXI, XXII and XXIII can be prepared analogously to the
synthesis routes
shown.
Scheme 6 illustrates the preparation of working examples which can be prepared
by palladium-
catalysed coupling reactions generally known to the person skilled in the art
starting, for example,
with brominated intermediates (XXIV, XXV) by reaction with the appropriate
amines (Chem. Sci.
2011, 2, 27; Angew. Chem. 2008, 47, 6338, Accounts of Chemical Research 2008,
41, 1534,
Journal of Organometallic Chemistry (1999), 576(1-2), 125-146). The starting
materials (XXIV)
and (XXV) used can be prepared using the synthesis methods discussed above and
illustrated in
Schemes 1 to 4. Starting with XXIV and XXV, it is possible to obtain, by
palladium-catalysed
reaction with boronic acid derivatives, the corresponding coupling products Ii
and lj, respectively
(Chem. Rev. 1995, 95, 2457-2483; Angewandte Chemie, International Edition
(2002), 41(22),
4176-4211).
Scheme 6: Synthesis of 4,5-dihydro-3H-2,3-benzodiazepine-3-carbonyl compounds
R2
R2
Y 0
Boronic acid
derivative , 3
=
R5 111-1 N R3 or amine R5 N R
Ii
XXIV
R" Pd catalyst complex
Ric it
Rib
R 1 b
Rla R1 a
Y= Br, Cl, I
R2
R2
=R 11101 4 0
R4 N 3
Boronic acid
derivative 5 ,N.-4 3
y N R or amine N R lj
XXV
Ric Pd catalyst complex Ric =
R1 b
Rib
Rla R1 a
Y= Br. Cl, I
CA 02915419 2015-12-14
BHC123073FC
- 112 -
Boronic acid derivatives are commercially available or can be prepared in a
generally known
manner; for a review, see, for example, D. G. Hall, Boronic Acids, VCH-Wiley-
Verlag GmbH &
Co. KGaA, Weinheim 2005, ISBN 3-527-30991-8, and the literature cited therein.
Scheme 7 illustrates a method for preparing Working Examples (lk) from amino
intermediates
()OCVI) by amide coupling reactions generally known to the person skilled in
the art, using
carboxylic acids of the formula It11-C(=0)0H in the presence of coupling
agents familiar to the
person skilled in the art, for example propanephosphonic acid cyc/o-anhydride
or (benzotriazol-1-
yloxy)bisdimethylaminomethylium fluoroborate.
The preparation of amino intermediates (Xm) is known to the person skilled in
the art (see, for
example, WO 1997/028135); carboxylic acids of the formula R11-C(=0)0H are
likewise known to
the person skilled in the art and commercially available in many different
structures.
Scheme 7:
R2 R2
=4
0 R R4 0 5
p-4 ,N4
R11_C(=0)0H
R5 1101 N R3
XXVI p.
Ric *
Coupling agent
R" *
lk
Rlb Rib
H2N HN
0
Ril
CA 02915419 2015-12-14
BHC123073FC
- 113 -
Abbreviations:
ACN acetonitrile
Boc tert-butoxycarbonyl
CDC13 deuterochloroform
CO2 carbon dioxide
day
DMF dimethylformamide
DMSO dimethyl sulphoxide
ESI electrospray ionization (in MS)
sat. saturated
hour
HPLC high-pressure, high-performance liquid chromatography
conc. concentrated
LC-MS liquid chromatography-coupled mass spectrometry
min minutes
MS mass spectrometry
MW molecular weight [g/mol]
NMP N-methylpyrrolidone
NMR nuclear magnetic resonance spectroscopy
RT room temperature
Rt retention time (in HPLC)
SFC supercritical fluid chromatography
THF tetrahydrofuran
LC-MS Methods:
Method 1: Instrument: Waters Acquity LCT; column: Phenomenex Kinetex C18, 50
mm x 2.1 mm,
2.6 ft; mobile phase A: water/0.05% FA, mobile phase B: ACN/0.05% FA;
gradient: 0.0 min 98%
A 4 0.2 min: 98% A 1.7 min: 10% A - 1.9 min: 10% A 2 min: 98% A 4 2.5 min:
98% A;
flow rate: 1.3 ml/min; column temperature: 60 C; UV detection: 200-400 nm.
Method 2: Instrument: Waters Acquity Platform ZQ4000; column: Waters BEHC 18,
50 mm x 2.1
mm, 1.7 II; mobile phase A: water/0.05% FA, mobile phase B: ACN/0.05% FA;
gradient: 0.0 min
98% A -) 0.2 min: 98% A -4 1.7 min: 10% A -4 1.9 min: 10% A - 2 min: 98% A
2.5 min:
98% A; flow rate: 1.3 ml/min; column temperature: 60 C; UV detection: 200-400
nm.
CA 02915419 2015-12-14
BHC123073FC
- 114 -
Method 3: UPLC-SQD-HCOOH; instrument: Waters Acquity UPLC-MS SQD; column:
Acquity
LTPLC BEH C18 1.7 50x2.1 mm; mobile phase A: water + 0.1% by volume of formic
acid (99%),
mobile phase B: acetonitrile; gradient: 0-1.6 min 1-99% B, 1.6-2.0 min 99% B;
flow rate 0.8
ml/min; temperature: 60 C; injection: 2 ul; DAD scan: 210-400 nm.
Preparative HPLC methods:
Method III: System: Dionex Pump P 580, Gilson Liquid Handler 215, Knauer UV
detector K-2501;
column: Chiralpak IC 5i.tm 250x30 mm; mobile phase: hexane / ethanol 70:30
(v/v); flow rate: 50
ml/min; column temperature: 25 C; detection: UV 254 nm.
Method X: System: Agilent: Prep 1200, 2xPrep Pump, DLA, MWD, Prep FC; column:
Chiralpak
IC 5 m 250x30 mm; mobile phase: ethanol/methanol/diethylamine 50:50:0.1
(v/v/v); flow rate: 30
ml/min; temperature: RT; detection: UV 280 nm.
Method XI: System: Agilent: Prep 1200, 2xPrep Pump, DLA, MWD, Prep FC; column:
Chiralpak
IC 51.tm 250x20 mm; mobile phase: methanol/diethylamine 100:0.1 (v/v); flow
rate: 20 ml/min;
temperature: RT; detection: UV 280 nm.
Method XII: System: Agilent: Prep 1200, 2xPrep Pump, DLA, MWD, Prep FC;
column: Chiralpak
IC 51.tm 250x30 mm; mobile phase: hexane/ethanol/diethylamine 70:30:0.1
(v/v/v); flow rate: 50
ml/min; temperature: RT; detection: UV 280 nm.
Method XIII: System: Sepiatec: Prep SFC 100, Prep FC; column: Chiralpak IC Sum
250x20 mm;
mobile phase: CO2 / ethanol 7/3; flow rate: 80 ml/min; temperature: 40 C;
detection: UV 254 nm.
Method XIV: System: Agilent: Prep 1200, 2xPrep Pump, DLA, MWD, Prep FC;
column:
Chiralpak ID 51.1m 250x20 mm; mobile phase: hexane/2-propanol/diethylamine
70:30:0.1 (v/v/v);
flow rate: 25 ml/min; temperature: RT; detection: UV 280 nm.
Analytical HPLC methods:
Method C: System: Waters Alliance 2695, DAD 996; column: Chiralpak IC 31.tm
100x4.6 mm;
mobile phase: hexane / ethanol 70:30 (v/v); flow rate: 1.0 ml/min; column
temperature: 25 C;
detection: DAD 254 nm.
Method F: System: Waters Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak ID 3 m
CA 02915419 2015-12-14
BHC123073FC
- 115-
100x4.6 mm; mobile phase: hexane / 2-propanol 70:30 (v/v) +0.1% DEA; flow
rate: 1.0 ml/min;
column temperature: 25 C; detection: DAD 254 nm.
Method G: System: Waters Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IC 3 m
100x4.6 ntm; mobile phase: ethanol/methanol/DEA 50:50:0.1 (v/v/v); flow rate:
1.0 ml/min;
column temperature: 25 C; detection: DAD 254 nm.
Method H: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IA 5p.m
150x4.6 mm; mobile phase: hexane/2-propanol/diethylamine 70:30:0.1 (v/v/v);
flow rate: 1.0
ml/min; column temperature: 25 C; detection: DAD 210 nm.
Method I: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IC 5 m
150x4.6 mm; mobile phase: methanol 100 (v); flow rate: 1.0 ml/min; column
temperature: 25 C;
detection: DAD 280 nm.
Method J: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IC 51tm
150x4.6 mm; mobile phase: hexane / ethanol 70:30 (v/v); flow rate: 1.0 ml/min;
column
temperature: 25 C; detection: DAD 280 nm.
Method K: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IA 5 m
150x4.6 mm; mobile phase: methanol 100 (v); flow rate: 1.0 ml/min; column
temperature: 25 C;
detection: DAD 280 nm.
Method L: System: Agilent: 1260 AS, MWD, Aurora SFC module; column: Chiralpak
1D 5 m
100x4.6 mm; mobile phase: CO2 / 2-propanol / diethylamine 6:4:0.2; flow rate:
4.0 ml/min;
pressure (outlet): 100 bar; column temperature: 37.5 C; detection: UV 254 nm.
Method M: System: Agilent: 1260 AS, MWD, Aurora SFC module; column: Chiralpak
IC 5 m
100x4.6 mm; mobile phase: CO2 / methanol 70:30; flow rate: 4.0 ml/min;
pressure (outlet): 100
bar; column temperature: 37.5 C; detection: DAD 254 nm.
Method N: System: Agilent: 1260 AS, MWD, Aurora SFC module; column: Chiralpak
ID 51.1m
100x4.6 mm; mobile phase: CO2 / 2-propanol 70:30; flow rate: 4.0 ml/min;
pressure (outlet): 100
bar; column temperature: 37.5 C; detection: DAD 254 nm.
Method 0: System: Alliance 2695, DAD 996, ESA: Corona; column: Chiralpak AD-H
Sum
150x4.6 mm; mobile phase: hexane/ethanol 70:30 (v/v) +0.1% DEA; flow rate: 1
ml/min;
temperature: 25 C; detection: DAD 280 nm.
Method P: System: Alliance 2695, DAD 996, ESA: Corona; column: Chiralpak IC
3nm 100x4.6
mm; mobile phase: methanol/diethylamine 100:0.1 (v/v); flow rate: 1 ml/min;
temperature: 25 C;
detection: DAD 280 nm.
Method Q: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak IC 3 m
100x4.6 mm; mobile phase: hexane/ethanol/diethylamine 70:30:0.1 (v/v/v); flow
rate: 1.0 ml/min;
column temperature: 25 C; detection: DAD 280 nm.
Method R: System: Agilent: 1260 AS, MWD, Aurora SFC module; column: Chiralpak
IC 31.1m
100x4.6 mm; mobile phase: CO2 / ethanol 70:30; flow rate: 4.0 ml/min; pressure
(outlet): 100 bar;
column temperature: 37.5 C; detection: DAD 254 nm.
CA 02915419 2015-12-14
BHC123073FC
- 116 -
Method S: System: Waters: Alliance 2695, DAD 996, ESA: Corona; column:
Chiralpak ID 311m
100x4.6 mm; mobile phase: hexane/2-propanol/diethylamine 70:30:0.1 (v/v/v);
flow rate: 1.0
ml/min; column temperature: 25 C; detection: DAD 280 nm.
Preparation of the intermediates
Example 1A
(2E)-3[4-Chloro-3-(trifluoromethoxy)pheny1]-2-methylacrylic acid
O
F
OH
CH3
CI
10.0 g (44.5 mmol) of 4-chloro-3-(trifluoromethoxy)benzaldehyde (CAS [886499-
59-8]), 7.53 g
(57.9 mmol) of propionic anhydride and 4.49 g (46.8 mmol) of sodium propionate
(CAS [137-40-
6]) were combined under argon and stirred at 150 C for 5 h. Water was added to
the warm mixture
and the mixture was extracted 3x with ethyl acetate. The combined organic
phases were dried with
sodium sulphate and the solvent was removed on a rotary evaporator. This gave
11.68 g (88% of
theory) of the crude product which was converted further without further
purification.
LCMS (Method 2): R, = 1.31 min; ni/z [ES] = 279 (M-H)-
1H-NMR (300MHz, CDC13): ö = 2.13 (d, 3H), 7.30 (dd, 1H), 7.38 (s, 1H), 7.52
(d, 1H), 7.73 (s,
1H).
Example 2A
( )-3[4-Chloro-3-(trifluoromethoxy)pheny1]-2-methylpropanoic acid
O
Fl
CH3 OH
CI
In the presence of 289 mg of palladium catalyst (10% Pd on activated carbon,
0.27 mmol), 10.0 g
(35.6 mmol) of (2E)-3-[4-chloro-3-(trifluoromethoxy)pheny1]-2-methylacrylic
acid (Example 1A)
in 200 ml of ethyl acetate were hydrogenated at RT for 12 h with vigorous
shaking (1 atm
hydrogen atmosphere). The catalyst was then filtered off, the filtercake was
washed with ethyl
acetate and the filtrate was concentrated on a rotary evaporator. This gave
11.04 g (99%) of crude
product which is reacted without further purification.
CA 02915419 2015-12-14
3
BHC123073FC
- 117 -
LCMS (Method 2): R, = 1.27 min; m/z [ES] = 281 (M-H)-
1H-NMR (400MHz, CDC13): 5 = 1.21 (d, 3H), 2.67-2.81 (m, 2H), 2.98-3.09 (m,
1H), 7.08 (dd, 1H),
7.16 (s, 1H), 7.38 (d, 1H).
Example 3A
( )-6-Chloro-2-methy1-5-(trifluoromethoxy)indan-1-one
F F
IP. CH3
C I
0
With ice bath cooling, 26.3 g (225.7 mmol) of chlorosulphonic acid (CAS [7790-
94-5]) were added
carefully to 10.0 g (35.38 mmol) of ( )-344-chloro-3-(trifluoromethoxy)pheny1]-
2-
methylpropanoic acid (Example 2A), and the mixture was stirred in the ice bath
for a further 3 h.
Since the conversion was still incomplete, the mixture was stirred at 7 C for
a further 12 h. The
reaction was then terminated by careful additon of crushed ice, a little at a
time. The mixture was
extracted three times with ethyl acetate and the combined organic phases were
washed with sat.
sodium bicarbonate solution and dried with sodium sulphate. The solvents were
removed on a
rotary evaporator and the residue (10 g) was used for the next synthesis step
without further
purification.
LCMS (Method 2): Rt = 1.36 min; m/z = 306 (M+ACN)+
'H-NMR (300MHz, CDC13): 5 = 1.32 (d, 3H), 2.68-2.84 (m, 2H), 3.33-3.47 (m,
1H), 7.40 (s, 1H),
7.85 (s, 1H).
Example 4A
2,2-Dimethy1-5[4-(trifluoromethoxy)benzy1)-1,3-dioxane-4,6-dione
CH,
0
CH,
F 101
0
0
25.4 g (134 mmol) of 4-(trifluoromethoxy)benzaldehyde (CAS [659-28-9]), 19.3 g
(134 mmol) of
Meldrum's acid (2,2-dimethy1-1,3-dioxane-4,6-dione, CAS [2033-24-1]) and 1.93
g (13.4 mmol) of
piperidinium acetate (CAS [4540-33-4]) were dissolved in 500 ml of ethanol,
and the mixture was
stirred at RT for 30 min. The reaction solution was cooled to 0 C using an ice
bath and stirred for a
CA 02915419 2015-12-14
BHC123073FC
- 118 -
further 10 min. 12.6 g (200 mmol) of sodium cyanoborohydride were introduced a
little at a time
and the mixture was allowed to warm to RT and stirred for a further 1.5 h. 250
ml of 2M
hydrochloric acid were then added carefully and stirring was continued until
the evolution of gas
had ceased completely (about 30 min). The ethanol was removed on a rotary
evaporator, the
residue was taken up in 2M hydrochloric acid and the mixture was extracted
repeatedly with
dichloromethane. The combined organic phases were dried with sodium sulphate
and the solvent
was removed on a rotary evaporator. This gave 32.7 g (41% of theory) of crude
product as a white
solid which was converted further without further purification.
LCMS (Method 1): R = 1.33 min; m/z = 319 (M+H)+
Example 5A
2,2,5-Trimethy1-5[4-(trifluoromethoxy)benzy1]-1,3-dioxane-4,6-dione
CH,
FF>r0 = 0 0
CH3
0
CH3
0
At RT, 32.7 g (103 mmol) of 2,2-dimethy1-544-(trifluoromethoxy)benzy1]-1,3-
dioxane-4,6-dione
(Example 4A) and 21.3 g (154 mmol) of potassium carbonate were initially
charged in 400 ml of
DMF, and 72.9 g (514 mmol, 32.0 ml) of iodomethane were slowly added dropwise.
The mixture
was stirred vigorously at RT for 1.5 h and then added to water. The mixture
was extracted 3x with
ethyl acetate, the combined organic phases were washed with sat. sodium
chloride solution and
dried with sodium sulphate. The solvents were removed on a rotary evaporator
and the crude
product (32.5 g colourless oil) was purified by flash chromatography (Si02,
hexane/ethyl acetate).
This gave 20.0 mg (55% of theory) of the desired product as a colourless oil.
1H-NMR (300MHz, DMSO-d6): 8 = 0.99 (s, 3H), 1.57 (s, 3H), 1.63 (s, 3H), 3.22
(s, 2H), 7.12 (d,
2H), 7.31 (s, 2H).
Example 6A
( )-2-Methyl-3[4-(trifluoromethoxy)phenyl]propanoic acid
F0 40 0 OH
19.0 g (57.2 mmol) of 2,2,5-trimethy1-5[4-(trifluoromethoxy)benzy1]-1,3-
dioxane-4,6-dione
(Example 5A) were taken up in 90 ml of dioxane and 35 ml of conc. aqueous
hydrochloric acid and
CA 02915419 2015-12-14
BHC123073FC
- 119 -
heated under reflux at 125 C for 2 h. The mixture was allowed to cool and the
solvents were
removed on a rotary evaporator. The residue (19.5 g of a colourless resin) was
heated at 200 C for
1 h. The crude product obtained was reacted further without further
purification.
LCMS (Method 2): R, = 1.21 min; m/z [ES-] = 247 (M-H)-
'H-NMR (300MHz, DMSO-d6): = 1.12 (s, 3H), 3.06 (s, 2H), 7.21 - 7.27 (m, 4H).
Example 7A
( )-2-Methy1-6-(trifluoromethoxy)indan-1-one
F la* CH3
0
0
17.2 g (69.3 mmol) of crude ( )-2-methyl-3[4-
(trifluoromethoxy)phenyl]propanoic acid (Example
6A) were dissolved in 100 ml of dichloromethane, and 12.1 ml (16.6 g, 166
mmol) of thionyl
chloride and 0.16 ml of DMF were added dropwise at RT. The mixture was then
heated under
reflux for about 30 min until the evolution of gas had ceased. The solution
was allowed to cool and
the solvents were removed on a rotary evaporator. The residue (yellow solid)
was taken up in 35 ml
of dichloromethane and, at RT, added dropwise to a suspension of 10.2 g (76.2
mmol) of
anhydrous aluminium chloride in 200 ml of dichloromethane. The dark-red
solution was stirred for
30 min and then added to water and the phases were separated. The aqueous
phase was extracted
3x with dichloromethane. The combined organic phases were washed with water,
sat. sodium
bicarbonate solution and sat. sodium chloride solution and dried with sodium
sulphate. The
solvents were removed and the residue (10.0 g) was purified by flash
chromatography (Si02,
hexane/dioxane). This gave 5.84 mg (14% of theory) of the product as a yellow
oil.
LCMS (Method 2): R, = 1.27 min; m/z = 231 (M+H)'; 272 (M+ACN1H-H)+
CA 02915419 2015-12-14
BHC123073FC
- 120 -
Example 8A
5-Chloro-3-(4-chloropheny1)-2-methy1-6-(trifluoromethoxy)-1H-indene
F F
CH3
CI
CI
Under argon, 10.6 g (40.06 mmol) of ( )-6-chloro-2-methy1-5-
(trifluoromethoxy)indan-1-one
(Example 3A) were initially charged in 30 ml of THF, and 60.1 ml of 4-
chlorophenylmagnesium
bromide (1M in diethyl ether, 60.1 mmol) were slowly added dropwise at RT such
that the
temperature of the solution stayed below 30 C. The solution was left to stir
at RT for a further 16 h,
and water was then added. The mixture was extracted 3x with ethyl acetate and
the combined
organic phases were dried with sodium sulphate and the solvent was removed on
a rotary
evaporator. The residue was taken up in 300 ml of dichloromethane, 55 mg of 4-
toluenesulphonic
acid monohydrate were added and the mixture was stirred at RT for 16 h. The
reaction mixture was
added to sat. sodium bicarbonate solution and extracted lx with
dichloromethane and 2x with ethyl
acetate, the combined organic phases were washed with sat. sodium chloride
solution and dried
with sodium sulphate and the solvent was removed on a rotary evaporator. The
residue was purified
by flash chromatography (Si02, hexane/ethyl acetate). This gave 11.19 g (65%
of theory) of the
product.
LCMS (Method 2): R, = 1.81 min; m/z = 360 (M H)+
11-1-NMR (400MH.z, CDC13): 5 = 2.13 (s, 3H), 3.46 (s, 2H), 7.20 (s, 1H), 7.29
(d, 2H), 7.38 (s, br,
1H), 7.46 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 121 -
Analogously to Example 8A, the following compound was prepared from Example
7A:
No. Structure Name Analytical data
1 11011 CH, 3-(4-Chloropheny1)-2-
methyl-5- LCMS
(Method 1): Rt = 1.76 min;
9A F
(trifluoromethoxy)-1H- miz = 325 (M+H)+
indene
CI
Example 10A
144-Chloro-2-(4-chlorobenzoy1)-5-(trifluoromethoxy)phenyl]propan-2-one
H3C
F F 411
0
C I
C'
11 g (30.63 mmol) of 5-chloro-3-(4-chloropheny1)-2-methyl-6-(trifluoromethoxy)-
1H-indene
(Example 8A) were initially charged in 206 ml of a 2:2:3 mixture of n-hexane,
acetonitrile and
water, and 138 mg (0.61 mmol) of ruthenium(III) chloride hydrate (CAS 1j14898-
67-0]) were
added. The mixture was cooled to 0 C, and 13.1 g (61.3 mmol) of sodium
periodate were added a
little at a time over a period of 1 h. After 3 h of stirring with ice bath
cooling, water was added and
the mixture was extracted 3x with ethyl acetate. The combined organic phases
were filtered
through a water-separating filter and the solvents were then removed on a
rotary evaporator. The
residue was purified by flash chromatography. This gave 2.24 g (19% of theory)
of the product.
LCMS (Method 1): R = 1.54 min; miz = 391, 393 (C1 isotope pattern, M+H)+
11-I-NMR (300MHz, CDC13): 8 -= 2.20 (s, 3H), 4.04 (s, 2H), 7.21 (s, br, 1H),
7.45-7.53 (m, 3H),
7.77 (d, 2H).
CA 02915419 2015-12-14
,
BHC123073FC
- 122 -
Analogously to Example 10A, the following compound was prepared from the
corresponding 2-
methy1-1H-indene 9A:
No. Structure Name Analytical data
0 CH, 1H-NMR (400MHz, DMSO-
d6): 8
= 2.05 (s, 3H), 3.98 (s, 2H), 7.31 ¨
*
0 1-[2-(4-
Chlorobenzoy1)-4- 7.33 (m, 1H), 7.46 (d,
1H), 7.50 ¨
11A F-0F 7.56 (m, 1H), 7.60 (d,
2H), 7.68 (d,
(trifluoromethoxy)phen
141 yl]propan-2-one 2H).
LCMS (Method 1): R, = 1. 45 min;
01 m/z = 357 (M+H)+
Example 12A
( )-1-(3,4-Dimethoxyphenyl)propan-2-ol
0 CH3
H3C
H3C 1101 OH
0
At 0 C, 147 mg (3.86 mmol) of lithium aluminium hydride were initially charged
in 30 ml of THF,
and 1.00 g (5.15 mmol) of 1-(3,4-dimethoxyphenyl)propan-2-one (CAS [776-99-
81), dissolved in
10 ml of Tiff, were added dropwise. The mixture was stirred at 0 C for 2 h,
and 0.1 ml of water,
0.1 ml of 2M aqueous sodium hydroxide solution and a further 0.3 ml of water
were then added
carefully. After a further 30 min of stirring at RT, the mixture was filtered
through silica
gel/sodium sulphate, the filter cake was washed with ethyl acetate and the
filtrate was concentrated
on a rotary evaporator. This gave 950 mg of product (82% of theory) which was
directly reacted
further.
LCMS (Method 2): It, = 0.82 min; m/z = 197 (M+H)+; 179 (M-H2O+H)'
111-NMR (300MHz, DMSO-d6): 8 = 0.98 (d, 3H), 2.43 (dd, 1H), 2.59 (dd, 1H),
3.67 (s, 3H), 3.69
(s, 3H), 3.70 ¨ 3.79 (m, 1H), 4.43 (d, 1H), 6.65 (dd, 1H), 6.75 (d, 1H), 6.79
(d, 1H).
CA 02915419 2015-12-14
BHC123073FC
- 123 -
Example 13A
( )-1-(4-Bromopheny1)-3,4-dihydro-6,7-dimethoxy-3-methy1-1H-2-benzopyran
CH3
H3C
H C
3 0
Br
At RT, 349.2 g (1.779 mol) of ( )-1-(3,4-dimethoxyphenyl)propan-2-ol (Example
12A) and 329.2
g (1.779 mol) of 4-bromobenzaldehyde (CAS [1122-91-4]) were initially charged
in 3 1 of toluene,
140 ml of hydrochloric acid (36% strength aqueous solution) were added and the
mixture was
stirred at RT for 2 days. The mixture was then poured into 2 1 of water and
extracted 2x with in
each case 2 1 of ethyl acetate, and the combined organic phases were washed lx
with sat. aqueous
sodium bicarbonate solution and lx with 2 1 of water and dried with sodium
sulphate. The solvent
was reduced on a rotary evaporator. The product precipitated as a colourless
solid. Just before
dryness, 1 1 of hexane was added and the mixture was cooled in an ice bath.
The solid was filtered
off with suction, washed with hexane and then dried under reduced pressure at
50 C. This gave
598.9 g (93% of theory) of the product (isomer mixture) which was directly
converted further
without further purification.
LCMS (Method 2): R = 1.44 min; m/z = 363; 365 (Br isotope pattern, M+H)+
Analogously to Example 13A, the following compounds were prepared from Example
12A and 3-
bromobenzaldehyde or 3-bromo-4-fluorobenzaldehyde:
No. Structure Name Analytical data
CH3 ( )-1-(3-
H C
H33C,..o 0 Bromopheny1)-3,4- LCMS (Method
3): R, = 1.40 min;
14A dihydro-6,7- m/z = 363; 365 (M+H, Br
isotope
dimethoxy-3-methyl- pattern)+
Br 1H-2-benzopyran
CA 02915419 2015-12-14
BHC123073FC
- 124 -
No. Structure Name Analytical data
CH3
H3C ( )-1-(3-bromo-4-
H3C.,o 0 fluoropheny1)-6,7- LCMS
(Method 3): R, = 1.44 min;
15A dimethoxy-
3-methyl- m/z = 381; 383 (Br isotope pattern,
1.1 3,4-dihydro-1H-2- M+H)+
Br
benzopyran
Example 16A
1-[2-(4-Bromobenzoy1)-4,5-dimethoxyphenyl]propan-2-one
0
0
(10
0
0
14111
Br
Preparation ofJones reagent:
267g of chromium-VI oxide (Cr03) were carefully introduced into 230 ml of
sulphuric acid (95-
97%). Using water ice and water, the reaction was cooled such that the
internal temperature was
35-40 C. Initially, orange crystals were formed, which slowly dissolved with
addition of water.
After 500 ml of water had been added, everything apart from a small sediment
had gone into
solution. The mixture was stirred at RT for 30 min and then transferred into a
bottle and made up to
1000 ml with water. This gave an about 2.6 M solution.
496.5 g (1.367 mol) of ( )-1-(4-bromopheny1)-3,4-dihydro-6,7-dimethoxy-3-
methyl-IH-2-
benzopyran (Example 13A) were initially charged in 5 1 of acetone, the mixture
was cooled to 0 C
and 50 g of silica gel were added. 1.9 1 of chromium trioxide in sulphuric
acid (Jones reagent) were
then added dropwise over 4 h and the mixture was stirred at RT for 1 h. After
the reaction had gone
to completion, 4 1 of water were added slowly to the reaction mixture. The
mixture was extracted
3x with 4 1 of ethyl acetate each time. The combined organic phases were
washed with 4 1 of sat.
sodium bicarbonate solution and 3x with in each case 4 1 of aqueous sodium
chloride solution and
dried with sodium sulphate. The solvent was reduced on a rotary evaporator.
The product
precipitated as a colourless solid. Just before dryness, 500 ml of hexane were
added and the
mixture was cooled in an ice bath. The solid was filtered off with suction,
washed with hexane and
then dried under reduced pressure at 50 C. This gave 334.1 g (65% of theory)
of the product which
was directly converted further without further purification.
CA 02915419 2015-12-14
BHC123073FC
- 125 -
LCMS (Method 2): R, = 1.26 min; m/z = 377; 379 (Br isotope pattern, M+H)+
'H-NMR (300MHz, DMSO-d6): 8 = 2.07 (s, 3H), 3.66 (s, 3H), 3.83 (s, 3H), 3.89
(s, 2H), 6.91 (s,
1H), 6.97 (s, 1H), 7.61 (d, 2H), 7.72 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 126 -
Analogously to Example 16A, Examples 14A and 15A were used to prepare the
following
diketones:
No. Structure Name Analytical data
H3C 0
14243-
H,C 140 LCMS
(Method 3): Rt = 1.21 min;
Bromobenzoy1)-4,5-
17A H3c,..0 0 m/z = 377;
379 (M-+H, Br isotope
dimethoxyphenyl]prop
a
pattern)
n-2-one
Br
H3C 0
H
H CCO 142-(3-Bromo-4- LCMS
(Method 3): R, = 1.25 min;
fluorobenzoy1)-4,5-
18A 0 m/z = 395;
397 (Br isotope pattern,
dimethoxyphenyl]prop
14I
M+H)
an-2-one +
Br
Example 19A
1-(4-Bromopheny1)-7,8-dimethoxy-4-methy1-5H-2,3-benzodiazepine
CH3
o
H3C N
H3C
0 " N
41110
Br
At 0 C, 471 g (1.249 mol) of 142-(4-bromobenzoy1)-4,5-dimethoxyphenyl]propan-2-
one (Example
16A) were initially charged in 4.5 1 of ethanol, and 402 ml of hydrazine
hydrate (6.62 mol) were
added dropwise. The mixture was allowed to come to RT and stirred at this
temperature for 2 days.
The mixture was decanted off from the solid and the clear supernatant was
concentrated on a rotary
evaporator. 8 I of ice-water were added, resulting in the precipitation of a
beige solid. The
suspension was stirred for 2 days, and the precipitate was filtered off with
suction, washed with
water and then dried under reduced pressure at 50 C. This gave 409.8 g (88% of
theory) of the
product which was directly converted further without further purification.
CA 02915419 2015-12-14
BHC123073FC
- 127 -
LCMS (Method 2): 12, = 1.20 min; m/z = 373; 375 (Br isotope pattern, M+H)
11-1-NMR (300MHz, DMSO-d6): 5 = 2.04 (s, 3H), 2.72 (d, 1H), 3.44 (d, 1H), 3.62
(s, 3H), 3.85 (s,
3H), 6.72 (s, 1H), 7.09 (s, 1H), 7.53 (d, 2H), 7.64 (d, 2H).
Analogously to Example 19A, Examples 10A, 11A, 17A and 18A were used to
prepare the
following 5H-2,3-benzodiazepines:
No. Structure Name Analytical data
'H-NMR (300MHz, CDC13): 6 =
2.15 (s, 3H), 2.98 (d, 1H), 3.27 (d,
CH,
4;) 1H), 3.75 (s, 3H), 3.97 (s, 3H),
H3C 40/\ 1-(3-Bromopheny1)-
N 6.73 (s, 1H), 6.75 (s, 1H), 7.27 (dd,
/
H3C0 ---"N 7,8-dimethoxy-4-
20A 1H), 7.55 (dbr, 1H), 7.61 (dbr,
1H),
methyl-5H-2,3-
= benzodiazepine 7.86 (m, 1H).
LCMS (Method 3): R, = 1.15 min;
Br
111/Z = 373; 375 (M+H, Br isotope
pattern)+
'H-NMR (400MHz, CDC13): 6 =
CH3
2.16 (s, 3H), 2.99 (d, 1H), 3.29 (d,
,0
H,C * \
1 -(3-bromo-4- 1H), 3.77 (s, 3H), 3.98 (s, 3H),
H3C,.
0 ---N fluoropheny1)-7,8- 6.74 (s, 2H), 7.16 (dd, 1H), 7.62
21A
dimethoxy-4-methyl- (ddd, 1H), 7.94 (dd, 1H).
5H-2,3-benzodiazepine LCMS (Method 3): 12, = 1.21 min;
Br = 381; 383 (Br isotope pattern,
M+H)+
'H-NMR (300MHz, CDC13): 8 =
CH3
F
8-Chl oro-1 -(4- 2.20 (s, 3H), 3.10 (d, 1H), 3.40
(d,
F N/N chloropheny1)-4- 1H), 7.29 (s, br, 2H), 7.45 (d, 2H),
Ci
22A methyl-7- 7.62 (d, 2H).
4410 (trifluoromethoxy)-5H- LCMS (Method 1): R, = 1.55
min;
2,3-benzodiazepine m/z = 387 (C1 isotope pattern,
Cl M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 128 -
No. Structure Name Analytical data
CH, 11-1-NMR
(400MHz, DMSO-d6): 8
F1 40 \ N 1-(4-
chloropheny1)-4- = 2.05 (s, 3H), 2.89 (d, 1H), 3.61
23A
F4.0N methyl-8- (d, 1H),
7.20 (s, br, 1H), 7.49 ¨
F
(trifluoromethoxy)-5H- 7.54 (m, 4H), 7.59 ¨ 7.66 (m, 2H).
2,3-benzodiazepine LCMS
(Method 1): Rt = 1.44 min;
ci m/z = 353 (M+H)
Example 24A
( )-1-(4-Bromopheny1)-7,8-dimethoxy-4-methy1-4,5-dihydro-3H-2,3-benzodiazepine
CH,
0
FI,C
NH
0 -N
Br
At RT, 1.99 g (5.33 mmol) of 1-(4-bromopheny1)-7,8-dimethoxy-4-methyl-5H-2,3-
benzodiazepine
(Example 19A) were initially charged in 200 ml of methanol, 3.0 ml of 2M
hydrochloric acid were
added and 1.68 g (26.6 mmol) of sodium cyanoborohydride were introduced. The
mixture was
stirred at RT for 1 h and then made alkaline with 2M aqueous sodium hydroxide
solution (pH about
8). Most of the methanol was removed on a rotary evaporator, and the residue
was partitioned
between water and dichloromethane. The phases were separated and the aqueous
phase was
extracted with dichloromethane. The combined organic phases were washed with
sat. sodium
chloride solution and dried with sodium sulphate and the solvent was removed
on a rotary
evaporator. The residue was purified by flash chromatography (Si02,
hexane/ethyl acetate). This
gave 1.56 g(78% of theory) of the product as a yellow resin which
crystallized.
LCMS (Method 2): R, = 0.96 min; m/z = 375; 377 (Br isotope pattern, M+H)
11-1-NMR (400MHz, DMSO-d6): 8 = 1.09 (d, 3H), 2.58 (dd, 1H), 2.83 (dd, 1H),
3.27 (s, 3H), 3.51
(s, 3H), 3.77 ¨ 3.82 (m, 1H), 6.47 (s, 1H), 6.85 (s, 1H), 7.01 (d, 1H), 7.33
(d, 2H), 7.47 (d, 2H).
CA 02915419 2015-12-14
BHC I 23073FC
- 129 -
Analogously to Example 24A, Examples 20A, 21A, 22A and 23A were used to
prepare the
following 4,5-dihydro-3H-2,3-benzodiazepines:
No. Structure Name Analytical data
'H-NMR (300MHz, CDC13): ö =
1.28 (d, 3H), 2.62 (dd, 1H), 2.89
CH3
/10 ( )-1-(3- (dd, 1H), 3.71 (s, 3H), 3.94 (s, 3H),
NH
H C Bromopheny1)-7,8- 4.11 (m, 1H), 6.59 (s, 1H), 6.76
(s,
N
0
25A dimethoxy-4-methyl- 1H), 7.22 (dd, 1H), 7.45 (dbr,
1H),
4,5-dihydro-3H-2,3- 7.48 (dbr, 1H), 7.75 (m, 1H).
benzodiazepine LCMS (Method 3): R, = 0.99 min;
Br
rniz 375; 377 (M+H, Br isotope
pattern)+
'H-NMR (400MHz, CDC13): ö =
0-13 1.29 (d, 3H), 2.61 (dd, 1H), 2.89
H3C.- *NH
( )-1-(3-Bromo-4- (dd, 1H), 3.72 (s, 3H), 3.95 (s, 3H),
H3C, fluoropheny1)-7,8- 4.12 (m, 1H), 6.57 (s, 1H),
6.77 (s,
0 N
26A dimethoxy-4-methyl- 1H), 7.10 (dd, 1H), 7.45 (ddd,
1H),
= 4,5-dihydro-3H-2,3- 7.81 (dd, 1H).
benzodiazepine LCMS (Method 3): R, = 1.03 min;
Br
rniz = 393; 395 (Br isotope pattern,
M+H)+
'H-NMR (300MHz, CDCI3): 8 =
CH3 ( )-8-Chloro-1-(4- 1.34 (d, 3H), 2.72 (dd, 1H),
2.99
Fe.õ0
NH chloropheny1)-4- (dd, 1H), 4.07-4.21 (m, 1H),
7.24
F 401:1
ci N methyl-7- (s, br, 2H), 7.39 (d, 2H), 7.49 (d,
27A
(trifluoromethoxy)-4,5- 2H).
dihydro-3H-2,3- LCMS (Method 1): R, = 1.63 min;
01 benzodiazepine m/z = 389 (C1 isotope pattern,
M+H)+
III-NMR (400MHz, DMSO-d6):
CH3 ( )-1-(4-
= 1.10 (d, 3H), 2.75 (dd, 1H), 2.99
NH Chloropheny1)-4-
(dd, 1H), 3.76 ¨ 3.83 (m, 1H), 6.84
F4=0 =--N methyl-8-
28A F (s, br, 1H), 7.21 ¨ 7.24 (m, 1H),
411k1 (trifluoromethoxy)-4,5-
dihydro-3H-2,3- 7.32 ¨ 7.38 (m, 5H), 7.64 (s, br,
1H).
benzodiazepine
Cl LCMS (Method 2): R, = 1.50 min;
CA 02915419 2015-12-14
BHC123073FC
- 130 -
No. Structure Name Analytical data
m/z = 355 (WH)'
Example 29A
( )-1-(4-Bromopheny1)-7,8-dimethoxy-N,4-dimethyl-4,5-dihydro-3H-2,3-
benzodiazepine-3-
carboxamide
CH3
0
H3C
H3C 101
0 N NH
H3C
Br
At RT, 1.56 g (4.16 mmol) of ( )-1-(4-bromopheny1)-7,8-dimethoxy-4-methy1-4,5-
dihydro-3H-
2,3-benzodiazepine (Example 24A) were dissolved in 50 ml of THF, 1.68 g (8.31
mmol) of 4-
nitrophenyl chloroformate (CAS [7693-46-1]) were added dropwise and the
mixture was stirred at
RT for 1 h. During this time, the clear yellow solution slowly became turbid.
20.8 ml (41.6 mmol)
of a 2M solution of methylamine in THF were added dropwise and the mixture was
stirred at 60 C
for 5 h. The mixture was allowed to cool to RT, concentrated on a rotary
evaporator and partitioned
between water and ethyl acetate and the phases were separated. The aqueous
phase was extracted
with ethyl acetate. The combined organic phases were washed with sat. sodium
chloride solution
and dried with sodium sulphate and the solvent was removed on a rotary
evaporator. Since the
reaction was incomplete (monitored by UPLC/MS), the reaction was carried out
once more in an
analogous manner using the crude product/intermediate/starting material
mixture obtained to
achieve complete conversion. The crude product then obtained was purified by
flash
chromatography (Si02, hexane/ethyl acetate). This gave 1.90 g (100% of theory)
of the desired
product as a yellow foam.
LCMS (Method 2): R, = 1.33 min; m/z = 432; 434 (Br isotope pattern, M+H)
1H-NMR (400MHz, DMSO-d6): 5 = 0.92 (d, 3H), 2.64 (d, 3H), 2.67 (dd, 1H), 2.91
(dd, 1H), 3.53
(s, 3H), 3.80 (s, 3H), 5.03 ¨ 5.11 (m, 1H), 6.47 (s, 1H), 6.60 (q, 1H), 6.98
(s, 1H), 7.56 (s, 4H).
Enantiomer separation
19.9 g of the compound prepared under 29A were separated into the enantiomers
by chiral
preparative HPLC under the following conditions:
System: SFC Prep 400; column: Chiralpak AZ-H 5 im 250x50 mm; mobile phase: CO2
/
CA 02915419 2015-12-14
BHC123073FC
- 131 -
isopropanol 75:25 (v/v); flow rate: 300 ml/min; temperature: 38 C; pressure 80
bar; solution: 5 g /
100 ml of methanol / acetonitrile 50:50 (v/v); detection: UV 220 nm.
CA 02915419 2015-12-14
BHC123073FC
- 132 -
Example 29-1A:
(4R)-1-(4-Bromopheny1)-7,8-dimethoxy-N,4-dimethy1-4,5-dihydro-3H-2,3-
benzodiazepine-3-
carboxamide
9.29 g, light-yellow solid, HPLC (Method F): R = 3.29 min, purity > 99%
optical rotation: [a]D2 = -89.3 (c = 1.00; methanol)
Example 29-2A:
(45)-1-(4-Bromopheny1)-7,8-dimethoxy-N,4-dimethy1-4,5-dihydro-3H-2,3-
benzodiazepine-3-
carboxamide
9.9 g, light-yellow solid, HPLC (Method F): Rt = 4.55 min, purity 96%
optical rotation: [a]D2 = +81.3 (c = 1.00; methanol)
Analogously to Example 29A, the following compounds were prepared from the
corresponding
4,5-dihydro-3H-2,3-benzodiazepines 25A, 26A, 27A und 28A. Completeness of the
conversion
into the 4-nitrophenylcarbamate formed as an intermediate or to the methylurea
was checked by
UPLC/MS. If the conversion was incomplete, the reaction was once more carried
out analogously
using the crude product mixture.
No. Structure Name Analytical data
1H-NMR (500MHz, CDC13): =
0.95 (d, 3H), 2.86 (dd, 1H), 2.90
( )-1-(3-
(d, 3H), 3.12 (dd, 1H), 3.66 (s,
N¨<
Bromopheny1)-7,8-
I-1,C
dimethoxy-N,4- 3H), 3.93 (s, 3H), 5.48 (m, 1H),
H,c,
0 ¨N NH6.50 (m, 1H), 6.54 (s, 1H), 6.71 (s,
30A
H3C/ dimethy1-4,5-dihydro-
1H), 7.26 (dd, 1H), 7.39 (dbr, 1H),
3H-2,3-
benzodiazepine-3- 7.52 (dbr, 1H), 7.64 (m, 1H).
Br LCMS (Method 3): Rt = 1.27 min;
carboxamide
m/z = 432; 434 (M+H, Br isotope
pattern)+
CA 02915419 2015-12-14
BHC123073FC
- 133 -
No. Structure Name Analytical data
1H-NMR (400MHz, CDC13): =
0.95 (d, 3H), 2.86 (dd, 1H), 2.90
(+)-1-(3-bromo-4-
CH3 (d, 3H), 3.10 (dd, 1H), 3.67 (s,
H:o' * dimethoxy-N,4-
o fluoropheny1)-7,8-
3H), 3.93 (s, 3H), 5.48 (m, 1H),
H C
0 N NH 6.44 (m, 1H), 6.52 (s, 1H), 6.71 (s,
31A H,C dimethy1-4,5-dihydro-
* 3H-2,3- 1H), 7.14 (dd, 1H), 7.39 (ddd, 1H),
7.69 (dd, 1H).
benzodiazepine-3-
Br
LCMS (Method 3): R, = 1.31 min;
carboxamide
m/z = 450; 452 (Br isotope pattern,
WH)'
1H-NMR (400MHz, CDC13): 5 =
( )-8-Chloro-1-(4- 0.95 (d, 3H), 2.92 (d, 3H), 2.96
CH,
FO 0 chloropheny1)-N,4- (dd, 1H), 3.16 (dd, 1H), 5.51-
5.60
1.1 714
dimethy1-7- (m, 1H), 6.50 (q, 1H), 7.22 (s, 1H),
Cl N NH
32A H,C (trifluoromethoxy)-4,5- 7.23 (s, 1H), 740 (d, 2H),
7.43 (d,
= dihydro-3H-2,3- 2H).
benzodiazepine-3- LCMS (Method 1): R, = 1.61 min;
Cl
carboxamide m/z = 446 (CI isotope pattern,
M+H)+
1H-NMR (300MHz, CDC13): 5 =
0.91 (d, 3H), 2.90 (d, 3H), 2.96
cH3 ( )-1-(4-chloropheny1)-
o (dd, 1H), 3.14 (dd, 1H), 5.46 -5.55
7-4 N,4-dimethy1-8-
(m, 1H), 6.47 - 6.52 (m, 1H), 6.94
--N /NH (trifluoromethoxy)-4,5-
33A F H3C (s, br, 1H), 7.17 - 7.29 (m, 2H),
dihydro-3H-2,3-
benzodiazepine-3- 7.39 (s, 4H).
LCMS (Method 2): Rt = 1.53 min;
Cl carboxamide
m/z = 412;414 (CI isotope pattern,
M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 134 -
Example 34A
( )-1-(4-Aminopheny1)-8-methoxy-N,4-dimethyl-4,5-dihydro-3H-2,3-benzodiazepine-
3-
carboxamide
CH3
0
H3C =7¨<
140
0 N NH
H3C
H2N
The preparation of the title compound is described in W097/28135 Al (Schering
AG) as Example
5.
UPLC/MS (Method 3): Rt = 0.92 min; miz = 339 (M+H)
1H-NMR (300MHz, DMSO-d6): 5 = 1.07 (d, 3H), 2.37 (dd, 1H), 2.60 (d, 3H), 2.81
(dd 1H), 3.69
(s, 3H), 4.74 (m, 1H), 5.70 (sbr, 2H), 6.19 (qbr, 1H), 6.53 (d, 1H), 6.57 (d,
2H), 6.98 (dd, 1H), 7.28
(d, 1H), 7.45 (d, 2H).
Enantiomer separation (Preparative Method III)
Example 34-1A: (4R)-1-(4-Aminopheny1)-8-methoxy-N,4-dimethy1-4,5-dihydro-3H-
2,3-
benzodi __ 71-pine-3-carboxamide
1.64 g, yellow solid, HPLC (Method C): R = 5.05 min, purity 99%
optical rotation: [01]32 = -637.8 0.12 (c = 1.040; Me0H)
Example 34-2A: (4S)-1-(4-Aminopheny1)-8-methoxy-N,4-dimethy1-4,5-dihydro-3H-
2,3-
benzodiazepine-3-carboxamide
1.71 g, yellow solid, HPLC (Method C): R, = 6.75 min, purity 95%
optical rotation: [a]D2 = +604.9 0.100 (c = 1.030; Me0H)
CA 02915419 2015-12-14
BHC123073FC
- 135 -
The examples which follow describe the preparation of the compounds according
to the invention,
without restricting the invention to these examples.
Working examples
Example 1
( )-7,8-Dimethoxy-N,4-dimethy1-1- {4-[(1-methylpiperidin-4-yl)amino]phenyII-
4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide
CH,
H CO
[00
IN40
N NH
0
H,C
HN
0 c.3
Under argon, 100 mg (231 mol) of ( )-1-(4-bromopheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide (Example 29A) was initially
charged in 5 ml of
degassed toluene. 32 ul (29 mg, 254 mol) of 4-amino-l-methylpiperidine, 31 mg
(324 mop of
sodium tert-butoxide and 9 mg (12 mop of chloro-(2-dicyclohexylphosphino-
2,4,6-triisopropyl-
1,1-bipheny1)[2-(2-amino-1,1-biphenyl)]palladium(11) (CAS [1310584-14-5]) were
added. The
mixture was degassed again, saturated with argon and then stirred at 110 C for
16 hours. After
cooling, the mixture was partitioned between 15 ml of sat. sodium bicarbonate
solution and 15 ml
of ethyl acetate, and the phases were separated. The aqueous phase was
extracted with ethyl
acetate, and then the combined organic phases were washed with water and sat.
sodium chloride
solution and dried with sodium sulphate. The solvents were removed on a rotary
evaporator and the
residue (126 mg, yellow oil) was purified by flash chromatography (Si02,
hexane/ethyl acetate).
This gave 79 mg (73% of theory) of the desired product as a yellow solid.
LCMS (Method 2): 11, = 0.70 min; m/z = 466 (M+H)+
1H-NMR (500MHz, CDC13): 8 = 1.20 (d, 3H), 1.55 ¨ 1.67(m, 4H), 2.17 ¨ 2.24 (m,
2H), 2.09 ¨
2.17 (m, 2H), 2.37 (s, 31{), 2.73 (dd, 1H), 2.84 ¨ 2.91 (m, 1H), 2.90 (d, 3H),
2.93 (dd, 1H), 3.40 (s,
br, 1H), 3.76 (s, 3H), 3.98 (s, 3H), 5.23 ¨ 5.30 (m, 1H), 5.98 (q, 1H), 6.63
(d, 2H), 6.70 (s, 1H),
CA 02915419 2015-12-14
BHC123073FC
- 136 -
6.80 (s, 1H), 7.49 (d, 2H).
Enantiomer separation
78 mg of ( )-7,8-dimethoxy-N,4-dimethy1-1-{4-[(1-methy1piperidin-4-
y1)amino]pheny11-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide (Example 1) were separated into
the enantiomers
by chiral preparative HPLC under the following conditions:
System: Dionex: pump P 580, Gilson: Liquid Handler 215, Knauer: UV detector K-
2501; column:
Chiralpak IC 5m 250x30 mm; mobile phase: ethanol/methanol/diethylamine
50:50:0.1 (v/v/v);
flow rate: 40 ml/min; temperature: RT; solution: 78 mg / 3.3 ml of Me0H;
injection: 3 x 1.1 ml;
detection: UV 254 nm.
Example 1-1: (45)-7,8-Dimethoxy-N,4-dimethy1-1-{4-[(1-methylpiperidin-4-
yl)amino]phenyll-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
mg, yellowish solid, HPLC (Method G): Rt = 2.38 min, purity >99.9%
optical rotation: [4)2 = 362.3 0,55 (c = 1.00; methanol)
15 Example 1-2: (4R)-7,8-Dimethoxy-N,4-dimethy1-1-{4-[(1-methylpiperidin-4-
yDamino]pheny1}-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
24 mg, yellowish solid, HPLC (Method G): Rt = 2.86 min, purity 98.5%
optical rotation: [0E]02 = -360,4 0.44 (c = 1.00; methanol)
20 Analogously to Example 1, the racemic Example 29A (optionally with
subsequent enantiomer
separation) or the enantiomerically pure Example 29-2A and the appropriate
commercially
available amines gave the following exemplary compounds:
No. Structure Name Analytical data
CH,
( )..78-Dimethoxy- 1H-NMR (300MHz, DMS0-4): 8
,o
H,C 401)
N,4-dimethy1-1-{4- = 1.00 (d, 3H), 2.43 - 2.53 (m, 1H),
H3c
[methyl(pyridin-3- 2.60 (d, 3H), 2.83 (dd, 1H), 3.33 (s,
=cH3
2
yl)amino]pheny1}-4,5- 3H), 3.58 (s, 3H), 3.80 (s,
3H),
dihydro-3H-2,3- 4.85 -
4.97 (m, 1H), 6.33 (q, 1H),
H3C--N
benzodiazepine-3- 6.53 (s, 1H), 6.94 (d, 2H), 6.99 (s,
carboxamide 1H), 7.34
(dd, 1H), 7.54 - 7.58 (m,
CA 02915419 2015-12-14
BHC123073FC
- 137 -
No. Structure Name Analytical data
1H), 7.60 (d, 2H), 8.24 (dd, 1H),
8.42 (d, 1H).
LCMS (Method 2): R, = 0.87 min;
rniz = 460 (M+H)
1H-NMR (300MHz, DMSO-d6):
= 1.02 (d, 3H), 2.40 - 2.50 (m, 1H),
CH3 (+)-1-{4-[(2- 2.60 (d, 3H), 2.82 (dd, 1H), 3.58
(s,
H3c,o =7,4
Fluorophenyl)amino]ph 3H), 3.80 (s, 3H), 4.82 ¨ 4.92 (m,
¨N NH
H3C eny1}-7,8-dimethoxy- 1H), 6.27 (q, 1H), 6.53 (s, 1H),
=CH3
3
N,4-dimethy1-4,5- 6.93 (d, 2H), 6.99 (s, 1H), 6.98 -
F dihydro-3H-2,3- 7.04 (m, 111),
7.11 (dt, 1H), 7.22
HN
benzodiazepine-3- (ddd, 1H), 7.34 (dt, 1H), 7.57 (d,
carboxamide 2H), 8.29 (s, 1H).
LCMS (Method 2): R, = 1.28 min;
m/z = 463 (M-FH)+
cH3 (4R)-1-{4-[(2-
H3c,o
Fluorophenyl)amino]ph
N H3C/NH eny1}-7,8-dimethoxy-
Chiral HPLC (Method K):
3-1
N,4-dimethy1-4,5-
= 3.58 min
dihydro-3H-2,3 R.(-
HN
benzodiazepine-3-
carboxamide
cH3 (4S)-1-{4-[(2-
o 0
H3C, /1,1-- Fluorophenypamino]ph
N H3C /NH enyl 1-7,8-dimethoxy-
CH3 Chiral HPLC (Method K):
3-2
410 N,4-dimethy1-4,5-
dihydro-3H-2,3-
= 7.10 min
HN
benzodiazepine-3-
carboxamide
CA 02915419 2015-12-14
BHC123073FC
- 138 -
, No. Structure Name Analytical data
1H-N (300MHz, DMSO-d6): 8
= 1.03 (d, 3H), 2.03 (s, 3H), 2.22
( )-1-{4-[(3,5-
CH, (s,
3H), 2.41 (dd, 1H), 2.58 (d,
,0
N4 Dimethylisoxazol-4-
3H), 2.78 (dd, 1H), 3.58 (s, 3H),
H3C o ¨ N1 NH yl)amino]phenyl} -7,8-
1 / 3.79 (s, 3H), 4.77 -
4.89 (m, 1H),
CH, H3C
4
4#1 dimethoxy-N,4-
dimethy1-4,5-dihydro- 6.18 (q, 1H), 6.53 (s, 1H), 6.94 (d,
2H), 6.99 (s, 1H), 6.52 (d, 2H),
FINCH,
3H-2,3-
6.53 (s, 1H), 6.98 (s, 1H), 7.53 (d,
o benzodiazepine-3-
H3C N 2H), 7.63 (s, 1H).
carboxamide
LCMS (Method 2): R, = 1.10 min;
m/z = 464 (M+H)+
(4R)-1-{4-[(3,5-
CH,
,0 Dimethylisoxazol-4-
H3C 0
N 4
O --- N1 NH yl)amino]pheny1}-7,8-
1 /
dim
CH,
4-1 H3C Chiral HPLC (Method M):
40 dimethoxy-N,4-
ethy1-4,5-dihydro- R, = 2.96 min
HNz(CH,
3H-2,3-
o benzodiazepine-3-
,.
H3C N,
carboxamide
(4S)-1-14-[(3,5-
õcH3
H3C' 4111 ___0 Dimethylisoxazol-4-
N
O --- N1 NH ypaminolpheny1}-7,8-
1 /
CH,
4-2 H3C Chiral RPLC (Method M):
* dim dimethoxy-N,4-
ethy1-4,5-dihydro- R, = 3.95 min
HN,.....z...,<CH3
3H-2,3-
o benzodiazepine-3-
--.,
H3C N,
carboxamide
1H-NMR (300MHz, DMSO-d6): 8
( )-1-(4- { [2-
CH, = 1.04
(d, 3H), 1.19¨ 1.22 (m,
H3C' 0 ____ (Dimethylamino)ethyl]
N 2H),
2.15 (s, 6H), 2.38 - 2.42 (m,
/
o 11 11 ----N NH aminolpheny1)-7,8-
1 /
CH, H3C 1H),
2.58 (d, 3H), 2.77 (dd, 1H),
5
* dimethoxy-N,4-
dimethy1-4,5-dihydro- 3.11 (q, 2H), 3.58 (s, 3H), 3.80 (s,
3H), 4.76 - 4.84 (m, 1H), 5.94 ¨
j--NH 3H-2,3-
1-1,c\ 5.98 (m, 1H), 6.13 (q,
1H), 6.51 (s,
N benzodiazepine-3-
/
H3C 1H), 6.57 (d, 2H), 6.98
(s, 1H),
carboxamide
7.47 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 139 -
No. Structure Name Analytical data
LCMS (Method 2): 12, = 0.68 min;
ink = 440 (M+H)+
(4S)-1-(4-1[2-
.õ CH,
H, Co 4111 4 (Dimethylamino)ethyl]
0 N NH aminolpheny1)-7,8-
cH3
5-1 =H3c
dimethoxy-N,4-
dimethy1-4,5-dihydro- [a]D2 = 434.9 0.36
(c = 1.00; methanol)
NH 3H-2,3-
H3C,
benzodiazepine-3-
H3C
carboxamide
1H-NMR (300MHz, DMSO-d6): 8
cH3 ( )-1-{4-[(4-
= 1.01 (d, 3H), 2.40 - 2.44 (m, 1H),
,o
H3c
Fluorophenyl)methyla
2.59 (d, 3H), 2.81 (dd, 1H), 3.57 (s,
0 N NH minolpheny11-7,8-
CH3 =H3C 3H), 3.80 (s, 3H), 4.82 ¨ 4.92 (m,
dimethoxy-N,4-
dimethy1-4,5-dihydro- 1H), 6.25 (q, 1H), 6.52 (s, 1H),
6
6.75 (d, 2H), 6.99 (s, 1H), 7.18 ¨
H3c¨N1 3H-2,3-
benzodiazepine-3- 7.26 (m, 4H), 7.55 (d, 2H).
LCMS (Method 2): 12, = 1.39 min;
carboxamide
m/z = 477 (M+H)+
1H-NMR (400MHz, DMSO-d6): 5
= 1.02 (d, 3H), 2.43 - 2.47 (m, 1H),
CH, ( )-7,8-Dimethoxy-
,o =714 2.60 (d, 3H), 2.81 (dd, 1H), 3.58
(s,
H3C
N,4-dimethy1-1-{4-[(1-
3H), 3.62 (s, 3H), 3.80 (s, 3H),
N /NH methyl-1H-pyrazol-5-
CH, H3C 4.82 ¨ 4.91 (m, 1H), 6.03 (d, 1H),
7
4110 yl)aminolpheny11-4,5-
6.25 (q, 1H), 6.52 (s, 1H), 6.81 (d,
dihydro-3H-2,3-
CH, 2H), 6.99 (s, 1H), 7.57 (d, 2H),
HN
benzodiazepine-3-
t
8.32 (s, 1H).
I / carboxamide
LCMS (Method 2): 12, = 1.00 min;
rn/z = 449 (M+H)+
CA 02915419 2015-12-14
BHC123073FC
=
- 140 -
No. Structure Name Analytical
data
CH3 (4R)-7,8-Dimethoxy-
, 0
H,Co 0
,N4 N,4-dimethy1-1-{4-[(1-
o
I ---- N /NH methyl-1H-
pyrazol-5-
cH, H3c Chiral HPLC
(Method N):
7-1
40 yl)amino]pheny11-4,5-
R, = 3.06 min
dihydro-3H-2,3-
HN /CH,
benzodiazepine-3-
\C\N
I / carboxamide
, CH3(4S)-7,8-Dimethoxy-
,
H,C0 0
ini40 N,4-dimethy1-1-{4-[(1-
o
I -----N /NH methyl-1H-
pyrazol-5-
cH3 H3c Chiral HPLC
(Method N):
7-2
. ypaminolphenyll-4,5-
R, = 4.27 min
dihydro-3H-2,3-
HN /CH3
benzodiazepine-3-
t z.N\N
I / carboxamide
'H-NMR (300MHz, DMSO-d6): 8
= 1.03 (d, 3H), 1.23 ¨ 1.38 (m,
1H), 1.55 ¨ 1.66 (m, 2H), 1.70 -
( )-1-[4-(1-
0-13 1.83 (m, 1H), 1.85 ¨ 1.93 (m, 1H),
,o
H3 C 4111 Azabicyclo[2.2.21oct-
p-- 2.31 ¨2.45 (m, 1H), 2.57 (d, 3H),
o ¨N NH 3-ylamino)pheny1]-7,8-
I /
2.66 - 2.87 (m, 5H), 3.17 ¨ 3.29
CH, H3C
* dimethoxy-N,4-
dimethy1-4,5-dihydro- (m, 2H), 3.43 ¨ 3.52 (m, 1H), 3.58
8
(s, 3H), 3.79 (s, 3H), 4.73 - 4.85
HN 3H-2,3-
(m, 1H), 6.14 (q, 1H), 6.30 ¨ 6.33
benzodiazepine-3-
N (m, 1H), 6.51 (s, 1H), 6.56 (d, 2H),
carboxamide
6.98 (s, 1H), 7.47 (d, 2H).
LCMS (Method 2): R, = 0.73 min;
m/z = 478 (M+H)+
(4S)-1-[4-(1 -
CH
,0
H3C 110 Azabicyclo[2.2.2]oct-
N--
0 -- N/ NH 3-ylamino)pheny1]-7,8-
I /
cH,
8-1 * H3c
[4)2 = 279.2 + 0.28
dimethoxy-N,4-
dimethy1-4,5-dihydro- (c = 1.00; methanol)
HN 3H-2,3-
benzodiazepine-3-
N
carboxamide
CA 02915419 2015-12-14
e
BHC123073FC
- 141 -
No. Structure Name Analytical data
11-1-NMR (300MHz, DMSO-d6): 6
(+)-7,8-Dimethoxy-1-
CH, = 0.99 (d, 3H), 2.53 (dd, 1H), 2.62
,o o {4-[(4-methoxy-1,2,5-
,N_ (d, 3H), 2.84 (dd, 1H), 3.55 (s,
H3c iii
o ¨N NH oxadiazol-3-
I / 3H), 3.80 (s, 3H), 4.12
(s, 3H),
CH, H3C yl)amino]pheny1}-N,4-
9
#1 dimethy1-4,5-dihydro-
4.88 ¨ 5.00 (m, 1H), 6.40 (q, 1H),
6.50 (s, 1H), 6.99 (s, 1H), 7.60 (d,
HN 3H-2,3-
-----Nl'o benzodiazepine-3- 2H), 7.67 (d, 2H),
9.59 (s, 1H).
,0 ----,
LCMS (Method 2): R, = 1.16 min;
H3C N
carboxamide
m/z = 467 (M+H)
(4R)-7,8-Dimethoxy-1-
CH3
H3c, 410 0 0 14-[(4-methoxy-1,2,5-
IN--
O ---N / NH oxadiazol-3-
I
CH, H3C yl)amino]phenyll-N,4- Chiral HPLC
(Method I):
9-1
40 dimethy1-4,5-dihydro- It, = 2.40 min
HN 3H-2,3-
,Z,....-N\0
benzodiazepine-3-
H3c,0 ----N,
carboxamide
(45)-7,8-Dimethoxy-1-
cH3
,o o {44(4-[(4-1,2,5-
H3c
o O¨N/NNH
/ oxadiazol-3-
I
cH, H3c yl)amino]pheny1}-N,4- Chiral HPLC
(Method I):
9-2
. dimethy1-4,5-dihydro- R, = 3.08 min
HN 3H-2,3-
benzodiazepine-3-
H3c,,0õ),-..---.N,
carboxamide
1H-NMR (400MHz, DMSO-d6): 6
= 1.02 (d, 3H), 2.58 (dd, 1H), 2.65
CH3 ( )-7,8-Dimethoxy-
,o o (d, 3H), 2.89 (dd, 1H), 3.60 (s,
H,C /10
N-- N,4-dimethy1-144-[4
/
3H), 3.83 (s, 3H), 4.94¨ 5.03 (m,
o ¨ N NH (pyr
I / idazin-4-
cH3 H3c 1H), 6.47 (q, 1H), 6.55
(s, 1H),
. ylamino)pheny1]-4,5-
7.03 (s, 111), 7.22 (dd, 1H), 7.28 (d,
dihydro-3H--2,3-
HN 2H), 7,71 (d, 2H), 8.73
(d, 1H),
C-- benzodiazepine-3-
N 8.90 (d, 1H), 9.39 (s, 1H).
141 carboxamide
LCMS (Method 2): R, = 0.74 min;
m/z = 447 (M+H)+
CA 02915419 2015-12-14
4
BHC123073FC
,
- 142 -
No. Structure Name I Analytical
data
1H-NMR (400MHz, DMSO-d6): 8
= 1.02 (d, 3H), 2.52 (dd, 1H), 2.63
CH, ( )-7,8-Dimethoxy-
H3C,0 7 N,4-dimethy1-1-[4-
0 0 (d, 3H), 2.85 (dd, 1H), 3.57 (s,
O ¨N NH
3H), 3.81 (s, 3H), 4.88 ¨ 4.97 (m,
I / (pyridazin-3-
CH, H,C 1H), 6,38 (q, 1H),
6,54 (s, 1H),
11
. ylamino)pheny1]-4,5-
7.01 (s, 1H), 7.15 (dd, 1H), 7.45
dihydro-3H-2,3-
HN (dd, 1H), 7.67 (d, 2H), 7.81 (d,
N benzodiazepine-3-
\
2H), 8.68 (dd, 1H), 9.51 (s, 1H). /
carboxamide
LCMS (Method 2): R, = 0.87 min;
m/z = 447 (M+H)+
1H-NMR (300MHz, DMSO-d6): 8
( )-7,8-Dimethoxy- = 1.01 (d, 3H), 2.46 - 2.50 (m, 1H),
CH,
-0 0 N,4-dimethy1-1-14- 2.59 (d, 3H), 2.82 (dd,
1H), 3.21 (s,
H,C 0
___ N1N¨NH [methyl(1-methyl-1H- 3H), 3.34 (s, 3H), 3.56 (s, 3H),
o
I
/
CH, H3C imidazol-2-
3.80 (s, 3H), 4.83 ¨ 4.94 (m, 1H),
12
40 yl)aminolpheny11-4,5-
6.26 (q, 1H), 6.50 (d, 2H), 6.51 (s,
cH3 dihydro-3H-2,3- 1H), 6.83 (d,
1H), 6.99 (s, 1H),
H3c¨N /
)01 benzodiazepine-3- 7.10 (d, 1H), 7.57 (d, 2H).
carboxamide
LCMS (Method 2): R, = 0.76 min;
m/z = 463 (M+H)+
(4R)-7,8-Dimethoxy-
CH,
-0 0 N,4-dimethy1-1-14-
o N NH
H3c 0
N¨
/ [methyl (1-methy1-1 H-
---
I /
CH, H,C imidazol-2- Chiral HPLC
(Method 1-1):
12-1
= yflaminolpheny11-4,5-
R, = 2.97 min
3CH dihydro-3H-2,3-
H3C -- N is\f/--,)N/
benzodiazepine-3-
carboxamide
(4S)-7,8-Dimethoxy-
µ CH3
H,C.-C) - el , N,4-dimethy1-1 -{ 4-
----1--% H [methyl(1-methy1-1 H-
o
I /
cH, H3c imidazol-2- Chiral HPLC
(Method H):
12-2
. yl)aminolpheny11-4,5-
R, = 4.68 min
cH3 dihydro-3H-2,3-
H3c¨N /
benzodiazepine-3-
carboxamide
CA 02915419 2015-12-14
4
BHC123073FC
- 143 -
No. Structure Name Analytical data
1H-NIvIR (300MHz, DMSO-d6): 8
= 1.03 (d, 3H), 2.46 - 2.50 (m, 1H),
CH, ( )-7,8-Dimethoxy-
1-1,Co Ili o
2.60 (d, 3H), 2.80 (dd, 1H), 3.57 (s,
/N- N,4-dimethy1-1-{4-[(1-
3H), 3.71 (s, 3H), 3.80 (s, 3H),
o
I --- N /NH methyl-1H-pyrazol-3-
CH, H3C
4.79 ¨ 4.89 (m, 1H), 5.79 (d, 1H),
13
40 yl)aminolphenyl} -4,5-
6.23 (q, 1H), 6.52 (s, 1H), 6.99 (s,
dihydro-3H-2,3-
HN 1H), 7.32 (d, 2H), 7.50
(d, 1H),
N) benzodiazepine-3-
carboxamide H)
7.54 (d, 2H), 8.76 (s, 1.
LCMS (Method 2): R, = 1.03 min;
m/z = 449 (M+H)+
CH (4S)-7,8-Dimethoxy-
,o 0
H3C el714 N,4-dimethy1-1- {44(1-
o
I -- N /NH methyl-1H-pyrazol-3-
cH3 H3c [4)2
= 289.3 1.03
13-1
. yl)amino]pheny1}-4,5-
(c = 1.00; methanol)
dihydro-3H-2,3-
HN
\ ...c...._;\ benzodiazepine-3-
CH, carboxamide
11-1-NMR (300MHz, DMS046): 8
( )-1-{4-[(2- =
1.02 (d, 3H), 2.43 - 2.53 (m, 1H),
CH,
,0 0 Fluoropyridin-3-
2.61 (d, 3H), 2.83 (dd, 1H), 3.57 (s,
tc
0 N-
O
-- Ni NH yl)amino]pheny11-7,8-
3H), 3.80 (s, 3H), 4.85 ¨ 4.96 (m,
/
1-1,
H3C dimethoxy-N,4- 1H), 6.32 (q, 1H), 6.53 (s, 1H),
14
= dimethy1-4,5-dihydro- 7.00 (s, 1H), 7.04 (d, 2H), 7.24 (m,
F
HN 3H-2,3-
1H), 7.60 (d, 2H), 7.74 (m, 1H),
benzodiazepine-3- 7.81
(m, 1H), 8.47 (s, 1H).
carboxamide
LCMS (Method 2): R, = 1.13 min;
m/z = 464 (M+H)-'
(4R)-1-{4-[(2-
CH,
,0 0 Fluoropyridin-3-
H3c
N-
el
0 --- N/ NH yl)amino]phenyl} -7,8-
CH, /
H3C
dimethoxy-N,4-
Chiral HPLC (Method J):
14-1
* dimethy1-4,5-dihydro- R., = 3.05 min
F
HN 3H-2,3-
--
1N benzodiazepine-3-
\
carboxamide
CA 02915419 2015-12-14
BHC123073FC
- 144 -
No. Structure Name Analytical data
(4S)-1-{4-[(2-
, CH3
H3C 0
0
N
,0 Fluoropyridin-3-
i
O ¨n NH ypamino]pheny11-7,8-
I /
dimethoxy-N,4-
CH, H3C Chiral HPLC (Method J):
14-2
. dimethy1-4,5-dihydro- R, = 6.01 min
F
HN 3H-2,3-
--
\ /N benzodiazepine-3-
carboxamide
11-1-NMR (300MHz, DMSO-d6): 8
= 1.00 (d, 3H), 2.54-(dd, 1H), 2.62
( )-1-{4-[(3-
CI-13 (d, 3H), 2.86
(dd, 1H), 3.57 (s,
o o Fluoropyridin-4-
H3c-- 40
N- 3H), 3.80 (s,
3H), 4.89 ¨ 5.01 (m,
o ¨1,1/ NH yl)amino]pheny11-7,8-
I / 1H), 6.42 (q,
1H), 6.53 (s, 1H),
CH3 H,C
dimethoxy-N,4-
. dimethy1-4,5-dihydro- 7.00 (s, 1H),
7.23 ¨ 7.27 (m, 1H),
F 7.25 (d, 2H),
7.67 (d, 2H), 8.08 -
HN 3H-2,3-
-- 8.10 (m, 1H),
8.33 (d, 1H), 8.92 (s,
, / benzodiazepine-3-
, N 1H).
carboxamide
LCMS (Method 2): R., = 0.76 min;
m/z = 464 (M+H)+
11-1-NMR (300MHz, DMSO-d6): 8
( )-1-{4-[(3- = 1.01 (d,
3H), 2.44 - 2.54 (m, 1H),
CH,
O 0 Fluoropyridin-
2- 2.62 (d, 3H), 2.84 (dd, 1H), 3.56 (s,
H3c' .
N--
O -4 NN
yl)aminolpheny11-7,8- 3H), 3.80 (s, 3H), 4.86 ¨ 4.97 (m,
I
/
cH3 H3c dimethoxy-N,4- 1H), 6.37 (q,
1H), 6.52 (s, 1H),
16
= dimethy1-4,5-dihydro- 6.84 (m, 1H), 7.00 (s, 1H), 7.53 -
F
HN 3H-2,3- 7.58 (m, 1H),
7.62 (d, 2H), 7.85 (d,
N /
\ benzodiazepine-3- 2H), 7.99 (d,
1H), 9.10 (s, 1H).
carboxamide LCMS (Method
2): R, = 1.21 min;
m/z = 464 (M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 145 -
No. Structure Name Analytical data
_
(4R)-1- {44(3-
CH,
H3C o 4111 Fluoropyridin-2-
N--?
o ¨ Ni NH ypamino]pheny11-7,8-
1 /
CH,
16-1 H,C Chiral HPLC (Method J):
. dimethoxy-N,4-
dimethy1-4,5-dihydro- R, = 4.91 min
F
HN 3H-2,3-
_
N 1
\ benzodiazepine-3-
carboxamide
(4S)-1-{4-[(3-
_ CH,
, 0 Fluoropyridin-2-
H3c 40 r, j_.,c)
o ¨NI NH ypamino]phenyll -7,8-
I /
CH3
16-2 H3C Chiral linc (Method J):
. dimethoxy-N,4-
dimethy1-4,5-dihydro- R, = 6.85 min
F
HN 3H-2,3-
N /
\ benzodiazepine-3-
carboxamide
'H-NMR (600MHz, DMSO-d6): 8 .
= 1.07 (dd, 3H), 2.441(dd, 1H),
CH, ( )-7,8-Dimethoxy-
,o o 2.62 (d, 3H), 2.81 (dd, 114), 3.57
H3c 40 N,4-dimethy1-1-(4-
/N4
(d. 3H), 3.61 (s,13H), 3.83 (s, 3H),
o ¨ N /NH { [2,2,2-trifluor-1-(1-
I
CH, H3C 4.87 (dquin, 1H), 5.65 - 5.74 (m,
= methy1-1H-pyrrol-2-
yDethyllaminolphenyl) 1H), 5.961- 6.01 (m, 1H), 6.21 -
17
F 6.27 (m, 2H), 6.55 (d, 1H), 6.75 -
HN F -4,5-dihydro-3H-2,3-
F 6.811(m, 2H), 6.95 (dd, 2H), 7.02
H3C,
N \ benzodiazepine-3-
(d, 1H), 7.54 (dd, 2H).
carboxamide
LCMS (Method 2): Rt = 1.29 min;
miz ¨ 530 (M+H)+
_
1H-NMR (400MHz, CDC13): 8 =
CH3 ( )-1-(4-{ [2-
,o o 1.17 (d, 3H), 2.36 (s, 6H), 2.51 ¨
H3C is
N4 (Dimethylamino)ethyl]
/ 2.63 (m, 2H), 2.68 (dd, 1H), 2.86
o
I ---N H3C/NH methylaminolpheny1)-
cH3 (d, 3H), 2.88 (dd, 1H), 3.04 (s,
18 7,8-dimethoxy-N,4-
3H), 3.53 ¨ 3.62 (m, 2H), 3.73 (s,
dimethy1-4,5-dihydro-
3H), 3.94 (s, 3H), 5.17 ¨ 5.26 (m,
H3c¨N) 3H-2,3-
1H), 5.91 (q, 1H), 6.67 (s, 1H),
C= N.¨ CH3 benzodiazepine-3-
i 6.70 (d, 2H), 6.76 (s, 1H), 7.52
(d,
H3C carboxamide
2H).
CA 02915419 2015-12-14
=
BHC123073FC
- 146 -
No. Structure Name Analytical data
LCMS (Method 2): R, = 0.73 min;
in/z = 454 (M+H)
CH3 (4S)-1-(4- { [2-
HsC,0 = NH
(Dimethylamino)ethyl]
01 H,C/ methylaminolpheny1)-
CH3
18-1 =7,8-dimethoxy-N,4- [432
= 352.1 0.78
dimethy1-4,5-dihydro- (c = 0.503;
methanol)
Ft3c¨N)
3H-2,3-
CN--CH3 benzodiazepine-3-
H3C carboxamide
1H-NMR (400MHz, CDC13): 8 =
CH3
1.16 (d, 3H), 1.44¨ 1.94 (m, br,
,
H3co
N.4 ( )-7,8-Dimethoxy- 2H),
2.38 (s, 3H), 2.44 ¨ 2.77 (m,
N,4-dimethy1-1-(4-{[2-
8H), 2.69 (dd, IH), 2.86 (d, 3H),
cH3
(4-methylpiperazin-1- 2.88 (dd, 1H), 3.20
¨3.28 (m, 2H),
19
ypethyl]amino}phenyl)
3.72 (s, 3H), 3.93 (s, 3H), 5.18 ¨
HN
-4,5-dihydro-3H-2,3- 5.27 (m, 1H), 4.63 (s,
br, 1H), 5.94
benzodiazepine-3- (q, 1H), 6.62 (d, 2H), 6.66 (s, 1H),
N carboxamide 6.76 (s, 1H), 7.46
(d, 2H).
µCH3 LCMS (Method 2): R, =
0.69 min;
m/z = 495 (M+H)-1
CH3
0 H3c 0
H3C el 71 (4S)-7,8-Dimethoxy-
¨11 /NH N,4-dimethy1-1-(4- {[2-
cH3
19-1 (4-methylpiperazin-l-
[c]p2o = 384.6 0.38
ypethyljamino}phenyl)
HN= 1.00; methanol)
-4,5-dihydro-3H-2,3-
benzodiazepine-3-
7--)carboxamide
CH3
CA 02915419 2015-12-14
BHC123073FC
- 147 -
No. Structure Name Analytical data
'H-NMR (300MHz, DMSO-d6): 5
= 1.03 (d, 3H), 1.50 ¨ 1.60 (m,
CH, ( )-7,8-Dimethoxy- 2H), 1.67¨ 1.83
(m, 2H), 1.96 -
o o
H3c' 410 p4 N,4-dimethy1-1-{4- 2.08 (m, 2H),
2.16 (s, 3H), 2.33 ¨
o --- N /NH [methyl(1-
2.52 (m, 2H), 2.58 (d, 3H), 2.76 (s,
I
cH3 40 H3c
methylpiperidin-4- 3H), 2.78 - 2.86 (m, 2H), 3.59 ¨
yl)amino]pheny11-4,5- 3.69 (m, 1H),
3.57 (s, 3H), 3.80 (s,
H,C"-N dihydro-3H-2,3- 3H), 4.75 -
4.88 (m, 1H), 6.19 (q,
benzodiazepine-3- 1H), 6.52 (s, 1H), 6.75 (d, 2H),
CH,
carboxamide 6.99 (s, 1H), 7.54 (d, 2H).
LCMS (Method 2): R, = 0.74 min;
m/z = 480 (M+H)+
CH, (4R)-7,8-Dimethoxy-
, 0
H3C0 p4 0 N,4-dimethy1-1-{4-
o ¨ N /NH [methyl(1-
20-1
i
cH3 1-13c
.methylpiperidin-4-
yl)amino]phenyl} -4,5- Chiral HPLC (Method L):
R, = 1.72 min
H,C"-N \r\1 dihydro-3H-2,3-
C
rs benzodiazepine-3-
CH,
carboxamide
s CH, le 11¨
(4S)-7,8-Dimethoxy-
..
o
H,C N,4-dimethy1-1-{4-
1
O ¨ N / NH [methyl(1-
20-2
I
cH3 H3c
.methylpiperidin-4-
HP
LC
-4,5- Chiral LC (Method L):
Ri = 2.76 min
H3C --1µ10 dihydro-3H-2,3-
benzodiazepine-3-
CH3 carboxamide
N--
,
CH ( )-tert-Butyl 4-[{4- '1-1-NMR
(300MHz, CDC13): 8 =
H,C.-(3 Ns 0
[7,8-dimethoxy-4- 1.15 (d, 3H), 1.48 (s, 9H), 1.21 ¨
/
0 ¨ N NH I
HaCi methyl-3- 1.29 (m, 2H),
1.65 ¨ 1.80 (m, 4H),
CH3
21 4* (methylcarbamoyI)- 2.74 ¨ 2.82
(m, 2H), 2.68 (dd, 1H),
4,5-dihydro-3H-2,3- 2.84 (s, 3H), 2.85 (d, 3H), 2.89 (dd,
1-130¨N
H,C xf., H3 benzodiazepin-1- 1H), 3.78 ¨
3.85 (m, 1H), 3.72 (s,
\iro yl]phenyl}methylamin 3H), 3.93 (s,
3H), 5,19 - 5.26 (m,
0 o]piperidine-1- 1H), 5.95 (q, 1H), 6.66 (s, 1H),
CA 02915419 2015-12-14
BHC123073FC
- 148 -
No. Structure Name Analytical data
carboxylate 6.75 - 6.79 (m,
3H), 7.52 (d, 2H).
LCMS (Method 1): R= 1.36 min;
m/z = 566 (M+H)+
1H-NMR (400MHz, CDC13): 5 =-
1.16 (d, 3H), 2.41 (s, 3H), 2.71 (dd,
1H), 2.88 (d, 3H), 2.91 (dd, 1H),
CH3 ( oxy-
)-7,8-Dimeth
,o 2.98 (dd, 2H),
3.73 (s, 3H), 3.78
H3C
140 714H N,4-dimethy1-1-{4-[(1-
(dd, 2H), 3.95 (s, 3H), 4.11-4.20
methylazetidin-3-
H3C
CH (m, 1H), 4.30 (d, 1H),
5.21-5.32
22 3
=yl)amino]pheny1}-4,5-
(m, 1H), 6.01 (q, 1H), 6.55 (d, 2H),
dihydro-3H-2,3-
HN 6.66 (s, 1H), 6.77 (s, 1H), 7.46 (d,
1_1
benzodiazepine-3-
2H).
cH, carboxamide
LCMS (Method 1): R = 0.68 min;
m/z = 438 (M-i-HY
1H-NMR (400MHz, CDC13): 6 =
1.13 (d, 3H), 1.93 (s, 3H), 2.74 (dd,
cH3 ( )-1-{4-[(1- 1H), 2.88 (d, 3H), 2.96 (dd, 1H),
,o 0
,N4 Acetylazetidin-3- 3.73 (s, 3H),
3.88 (d, 1H), 3.96 (s,
H3C
3H), 3.97 (m, 1H), 4.32-4.40 (m,
=H3c/NH ypamino]pheny1}-7,8-
23 dimethoxy-N,4- 2H), 4.41-4.57
(m, 2H), 5.25-5.35
(m, 1H), 6.09 (q, 1H), 6.55 (d, 2H),
dimethy1-4,5-dihydro-
HN
3H-2,3- 6.65 (s, 1H), 6.77 (s, 1H), 7.48 (d,
2H).
sr benzodiazepine-3-
H3C carboxamide
LCMS (Method 1): R = 0.88 min;
m/z = 466 (M+H)+
111-NMR (300MHz, CDC13): 8 =
( )-7,8-Dimethoxy-
1.18 (d, 3H), 1.53-1.71 (m, 4H),
N,4-dimethy1-1-(4-
1.71-1.83 (m, 2H), 1.89-2.01 (m,
00
N-4
FI,C, 0
Ni NH [trans-4-(4-
2H), 2.21-2.30 (m, 1H), 2.32 (s,
H,C
40 methylpiperazin-1-
3H), 2.41-2.56 (m, 4H), 2.56-2.67
24
Acyclohexyllamino}p
(m, 4H), 2.69 (dd, 1H), 2.82-2.94
heny1)-4,5-dihydro-3H-
(m, 1H), 2.87 (d, 3H), 3.63-2.72
2,3-benzodiazepine-3-
C", (m, 1H), 3.75
(s, 3H), 3.95 (s, 3H),
carboxamide
4.12 (d, 1H), 5.16-5.30 (m, 1H),
CA 02915419 2015-12-14
BHC123073FC
- 149 -
No. Structure Name Analytical data
5.92 (q, 1H), 6.59 (d, 2H), 6.68 (s,
1H), 6.78 (s, 1H), 7.48 (d, 2H).
LCMS (Method 2): R, = 0.60 min;
m/z 549 (M+H)+
CA 02915419 2015-12-14
,
BHC123073FC
..
- 150 -
Analogously to Example 1, Example 30A or 31A and the appropriate commercially
available
amines gave the following exemplary compounds:
No. Structure Name Analytical
data
'H-NMR (300MHz, CDC13): 8 =
0.97 (d, 3H), 2.83 (dd, 1H), 2.86
CH, ( )-7,8-Dimethoxy-N,4- (d, 3H), 3.10 (dd, 1H), 3.35 (s,
-o
H3C 0 0
1N-- dimethyl-1- {3- 3H), 3.69 (s, 3H), 3.92 (s, 3H),
0 ---N NH
I / [methyl(pyridin-3-
5.44 (m, 1H), 6.44 (q, 1H), 6.62 (s,
CH, H,C
= yl)aminolpheny1}-4,5-
1H), 6.70 (s, 1H), 7.12 (d, 1H),
N-CH, dihydro-3H-2,3- 7,19 (m, 3H), 7.28 (m, 1H), 7.36
abenzodiazepine-3- (dd, 1H), 8.15 (dd, 1H), 8.33 (d, carboxamide 1H).
LCMS (Method 3): R, = 0.85 min;
m/z = 460 (M+H)+
'H-NMR (400MHz, CDC13): 6 =
1.01 (d, 3H), 1.53 (m, 2H), 2.07 (d,
CH, 2H), 2.15 (m, 2H), 2.32 (s, 3H),
-o o ( )-7,8-Dimethoxy-N,4-
,3c mol
/71¨ 2.81 (dd, 111), 2.84 (m, 2H), 2.87
o ¨N NH
dimethy1-1-{3-[(1-
I / (d, 3H), 3.07 (dd,
1H), 3.31 (m,
CH, H3C methylpiperidin-4-
26 40 ypamino]pheny11-4,5- 1H), 3.67 (s,
3H), 3.93 (s, 3H),
NH 5.40 (m, 1H), 6.44
(m, 1H), 6.64
adihydro-3H-2,3-
benzodiazepine-3- H)
(dd, 1, 6.65 (s, 1H), 6.71 (s, 1H),
6.72 (dd, 1H), 6.79 (d, 1H), 7.18
N
/ carboxamide
H3C (dd, 1H).
LCMS (Method 3): R, = 0.76 min;
m/z = 466 (M+H)
'H-NMR (300MHz, CDCI3): 6 =
CH, (1)-143-1[3- 1.02 (d, 3H), 1.89 (m, 2H), 2.40
,o o
itc 4111
7-4 (Dimethylamino)propyl] (m, 8H), 2.81
(s, 3H), 2.85 (m,
o ¨N NH
I / methylamino}-4- 1H),
2.88 (d, 3H), 3.08 (dd, 1H),
CH, H3C
27 . fluoropheny1)-7,8-
3.18 (m, 2H), 3.67 (s, 3H), 3.93 (s,
N_-CH3 dimethoxy-N,4-dimethyl- 3H), 5.43 (m, 1H), 6.41 (m, 11-1),
F
N--CH. 4,5-dihydro-3H-2,3-
6.58 (s, 1H), 6.72 (s, 1H), 6.97 ¨
benzodiazepine-3- 7.05 (m, 3H).
/
H3C carboxamide LCMS (Method 3): R,
= 0.81 min;
m/z = 486 (M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 151 -
No. Structure Name Analytical data
11-1-NMR (400MHz, CDC13): 5 =
CH, (+)-1-(3-{[2- 1.01 (d, 3H), 2.29 (s, 6H), 2.56 (m,
0
H,C 410
(Dimethylamino)ethyl]m 2H), 2.85 (s, 3H), 2.82 (dd, 1H),
¨N NH ethylamino} -4- 2.88 (d, 31-1), 3.08 (dd,
1H), 3.29
CH, H,C
28
fluoropheny1)-7,8- (m, 2H), 3.67 (s, 3H), 3.93 (s, 3H),
N--CH3 dimethoxy-N,4-dimethyl- 5.42 (m, 1H), 6.42 (m, 1H), 6.58
F 4,5-dihydro-3H-2,3- (s, 1H), 6.72
(s, 1H), 6.96 ¨ 7.06
benzodiazepine-3- (m, 3H).
nLA 3, ,--N
CH3
carboxamide LCMS (Method 3): It, = 0.79 min;
m/z = 472 (M+H)+
Example 29
( )-1-(4-{ [(Dimethylamino)acetyljamino} pheny1)-7,8-dimethoxy-N,4-dimethy1-
4,5-dihydro-3H-
2,3-benzodiazepine-3-carboxamide
C H3
0 0
H3C
0 NH
H/
CH 3 C
HN
o
CH3
H3 C
Under argon, 150 mg (0.347 mmol) of ( )-1-(4-bromopheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide (Intermediate 29A), 39 mg (0.382
mmol) of N2,N2-
dimethylglycinamide, 147 mg (0.69 mmol) of potassium phosphate and 132 mg
(0.69 mmol) of
copper(I) iodide were initially charged in 4 ml of degassed dioxane. 122 mg
(1.39 mmol) of NA-
dimethylethylenediamine were then added under argon and the mixture was
degassed again and
heated at 130 C for 3 hours. After cooling, 2M aqueous hydrochloric acid was
added, and after
brief stirring the mixture was made alkaline with 25% strength ammonia
solution. The mixture was
CA 02915419 2015-12-14
BHC123073FC
- 152 -
extracted twice with ethyl acetate, and the combined organic phases were dried
with sodium
sulphate. The solvent was removed on a rotary evaporator and the residue was
purified by column
chromatography (amino phase). This gave 115 mg (70% of theory) of the desired
product as a
solid.
LCMS (Method 2): R = 0.7 min; m/z = 454 (M+H)+
1H-NMR (300MHz, CDC13): 5 = 1.01 (d, 3H), 2.04 (s, 2H), 2.59 (s, br, 6H), 2.72-
2.88 (m, 1H),
2.88 (d, 3H), 3.07 (dd, 1H), 3.66 (s, 3H), 3.93 (s, 3H), 5.34-5.48 (m, 1H),
6.40 (q, 1H), 6.59 (s,
1H), 6.72 (s, 1H), 7.48 (d, 2H), 7.68 (d, 2H), 9.71 (s, br, 1H).
Enantiomer separation:
91 mg of ( )-1-(4-{ [(dimethylamino)acetyl]amino}pheny1)-7,8-dimethoxy-N,4-
dimethy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide (Example 29) were separated into
the enantiomers
by chiral preparative HPLC under the following conditions:
System: Agilent: Prep 1200, 2xPrep Pump, DLA, MWD, Prep FC; column: Chiralpak
AD-H 5p.m
250x30 mm; mobile phase: hexane/ethanol 70:30 (v/v) +0.1% DEA; flow rate: 50
ml/min;
temperature: RT; detection: UV 280 nm.
Example 29-1: (4R)-1-(4- [(Dimethylamino)acetyl]amino} pheny1)-7,8-dimethoxy-
N,4-di methyl-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
36 mg, solid, HPLC (Method 0): R, = 4.66 min, purity >99%
optical rotation: [a]D2 = -181,7 0,59 (c = 1,00; methanol)
Example 29-2: (45)-1-(4-{[(Dimethylamino)acetyl]aminolpheny1)-7,8-dimethoxy-
N,4-dimethy1-
4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
36 mg, solid, HPLC (Method 0): R, = 6.25 min, purity >99%
optical rotation: [c]u2 = 165,7 0,38 (c = 1,00; methanol)
. CA 02915419 2015-12-14
BHC123073FC
,
- 153 -
Analogously to Example 29, the racemic Intermediate 29A (optionally with
subsequent enantiomer
separation) and the appropriate commercially available carboxamides gave the
following
exemplary compounds:
No. Structure Name Analytical
data
'H-NMR (300M1-1z, CDCI3): 5 =-
CH,
1.01 (d, 3H), 1.85-2.05 (m, 4H),
,o o
H,C 40
N4 ( )-7,8-Dimethoxy-N,4-
2.05-2.20 (m, 2H), 2.25-2.40 (m,
/
o ¨ N /NH dimethy1-1-(4-{[(1-
1H), 2.33 (s, 3H), 2.78 (dd, 1H),
C
I
CH, H,
40 methylpiperidin-4-
2.86 (d, 3H), 2.95-3.09 (m, 3H),
30 yl)carbonyliaminolphen
3.64 (s, 3H), 3.92 (s, 3H), 5.30-
HN
Z o y1)-4,5-dihydro-3H-2,3- 5.44 (m, 1H),
6.36 (q, 1H), 6.57 (s,
benzodiazepine-3- 1H), 6.71 (s, 1H), 7.46 (d, 2H),
Cr--1 carboxamide 7.59 (d, 2H), 9.80
(s, br, 1H).
/
Ito
LCMS (Method 2): R, = 0.72 min;
m/z - 494 (M+H)+
,cH3
-0 = 0
H,C 0
,N4 (4S)-7,8-Dimethoxy-N,4-
o ¨ N /NH dimethy1-1-(4-{[(1-
prep. 1-IPLC: Method XII
I H,C
CH,
=methylpiperidin-4-
analyt. HPLC (Method Q):
30-1 yOcarbonyllaminolphen
HN R, = 10.83 min
zo y1)-4,5-dihydro-3H-2,3-
benzodiazepine-3-
(N) carboxamide
/
H,C
CH,
-o 0
H3C 0--- N- (4R)-7,8-Dimethoxy- prep. HPLC:
Method XX110 N /NH
N,4-dimethy1-1-(4-1[(1-
C1H, H,C analyt. HPLC
(Method Q):
40 methylpiperidin-4-
R, 9.49 min
yOcarbonyljaminolphen =
30-2
HN
optical rotation: [a]20 = -233.2
zo yI)-4,5-dihydro-3H-2,3-
0.36 (c = 1.00; methanol)
benzodiazepine-3-
c) carboxamide
/
H,C
. CA 02915419 2015-12-14
' BHC123073FC
,
- 154 -
No. Structure Name Analytical
data
11-1-NMR (300MHz, CDC13): 8 =
cH3
1.01 (d, 3H), 1.73-2.05 (m, 4H),
,o
/2 ( )-7,8-Dimethoxy-N,4-
H,C 010
,N,
2.31-2.55 (m, 4H), 2.67-2.83 (m,
o ¨N , NH dimethy1-1- {4-
1
2H), 2.86 (d, 3H), 3.04 (d, 1H),
H3C
31 O [(piperidin-4-
ylcarbonyl)amino]phenyl 3.26 (d, 1H), 3.64 (s, 3H), 3.92 (s,
CH,
3H), 5.36 (m, 1H), 6.38 (q, 1H),
HN }-4,5-dihydro-3H-2,3-
zo
6.57 (s, 1H), 6.71 (s, 1H), 7.47 (d,
benzodiazepine-3-
2H), 7.59 (d, 2H), 7.91 (s, br, 1H).
carboxamide
4')
H
LCMS (Method 2): R, = 0.72 min;
nth = 480 (M-PH)
111-NMR (600MHz, DMSO-d6): 8
cH3
= 1.02 (d, 3H), 2.51-2.54 (m, 4H),
, 0 o
H3c
( )-7,8-Dimethoxy-N,4-
N----4
2.59 (dd, 1H), 2.66 (d, 3H), 2.89
o ---- =
N NH dimethy1-1-{4-
1 H,C1
(dd, 111), 3.16 (s, 2H), 3.58 (s, 3H),
CH, [(morpholin-4-
32 ( cl, FO, (
3.64 d 4 3.84 s, 3H), 4.97-5-
O ylacetypamino]phenyll-
04 (m, 1H), 6.46 (q, 1H), 6.52 (s,
HN 4,5-dihydro-3H-2,3-
r benzodiazepine-3- 1H), 7.02 (s, 1H), 7.65 (d, 2H),
7.71 (d, 2H), 9.92 (s, 1H).
IN--)
carboxamide
LCMS (Method 2): R, = 0.76 min;
m/z = 496 (M+H)+
11-1-NMR (400MHz, CDC13): 8 =
cH3
1.04 (d, 3H), 1.88-2.02 (m, 41),
,o 40 o
Hsc
IN4
2.03-2.17 (m, 2H), 2.25-2.37 (m,
o .¨N NH ( )-1-(4-{[(1-
1 H3c
1H), 2.82 (dd, 1H), 2.90 (d, 3H),
cit ifi Benzy1piperidin-4-
2.99-3.12 (m, 3H), 3.57 (s, 2H),
yl)carbonyljamino}phen
3.68 (s, 3H), 3.95 (s, 3H), 5.38-
33 HN z y1)-7,8-dimethoxy-N,4-
o 5.47 (in, 1H), 6.40 (q, 1H), 6.61 (s,
dimethy1-4,5-dihydro-
1H), 6.74 (s, 1H), 7.30-7.34 (m,
c)
3H-2,3-benzodiazepine-
2H), 7.36 (m, 4H), 7.50 (d, 2H),
3-carboxamide
7.59 (d, 2H).
. LCMS (Method 2): R, = 0.85 min;
m/z = 570 (M+1-1)+
_
s CA 02915419 2015-12-14
BHC123073FC
,
- 155 -
No. Structure Name Analytical
data
CH
,. 3
H3C-. 0 .N40
O _N NH (4S)-1-(4-{[(1-
prep. HPLC: Method X
1 H3c,
CH Benzylpiperidin-4-
3 it
yl)carbonyl]aminolphen analyt. HPLC
(Method G):
R, ---- 2.78 min
33-1 HN y1)-7,8-dimethoxy-N,-
zo
4optical rotation: [a]D2 = 211.00
dimethy1-4,5-dihydro-
0.470 (c = 1.00; methanol)
3H-2,3-benzodiazepine-
(nri
3-carboxamide
lit
cH3
o 0
H3C-- 0
IN4
O --N NH (4R)-1-(4-{[(1-
prep. HPLC: Method X
1 H,C1
CH3 OBenzylpiperidin-4-
analyt. HPLC (Method G):
yl)carbonyljamino}phen
Rt = 2.49 min
33-2 HN y1)-7,8-dimethoxy-N,4-
optical rotation: [a]D2 = -192.5
dimethy1-4,5-dihydro-
0.38 (c ¨ 1.00; methanol)
3H-2,3-benzodiazepine-
ci
3-carboxamide
401
1H-NMR (600MHz, DMSO-d6): 5
= 0.98 (d, 3H), 2.20 (s, br, 3H),
,0 gair--r CH3o ( )-7,8-Dimethoxy-N,4-
2.68 (d, 3H), 2.70 (dd, 1H), 2.94
H3C
N-- dimethy1-1-(4-
? µP ¨N NH
3C (dd, 1H), 3.08 (s, br, 2H), 3.24
(s,
H, {methyl[(methylamino)a
CH,
3H), 3.58 (s, 3H), 3.84 (s, 3H),
34
O cetyl] amino} phenyI)-
5.05-5.12 (m, 1H), 6.52 (s, 1H),
4,5-dihydro-3H-2,3-
H,C"'" N =\,. 0 6.61 (q, 1H), 7.03 (s, 1H),
7.37 (d,
benzodiazepine-3-
2H), 7.71 (d, 2H).
CN--CH3 carboxamide
H
LCMS (Method 2): Rt = 0.75 min;
miz = 454 (M+H)+
. CA 02915419 2015-12-14
BHC123073FC
- 156 -
No. Structure Name Analytical data
_
1H-NMR (300MHz, CDC13): 8 =
cH3
1.01 (d, 3H), 2.43 (s, 3H2.61-
oH3c- . o
N4 ( )-7,8-Dimethoxy-N,4-
2.71 (m, 3H), 2.72-2.81 (m, 5H),
/
o --- N /NH
dimethy1-1-(4- {[(4- 2.83 (d, 1H), 2.88 (d, 3H), 3.07
1
CH3 H3C
thylpiperazin-1- (dd, 1H), 3.20 (s, 2H), 3.66 (s, 3H),
yl)acetyl]aminolphenyly 3.93 (s, 3H), 5.35-5.46 (m, 1H),
HN
\r0 4,5-dihydro-3H-2,3- 6.40 (q, 1H), 6.59 (s, 1H), 6.72
(s,
benzodiazepine-3- 1H), 7.49 (d, 2H), 7.61 (d, 2H),
/NMcarboxamide 9.16 (s, 1H).
s CH3 LCMS (Method 2): Rt =
0.75 min;
m/z = 509 (M+H)+
CH3
O 0
I-1,C ei
11,14 (4R)-7,8-Dimethoxy-
prep. HPLC: Method XI
o --- N NH N,4-dimethy1-1-
(4-{[(4-
I it g
analyt. HPLC (Method P):
cH3
. methylpiperazin-l-
R, = 4.54 min
ypacetyl]aminolpheny1)-
35-1
HN optical rotation: [ode' = -227.5
,ro 4,5-dihydro-3H-2,3-
benzodiazepine-3- 0.300 (c = 1.00; methanol)
N
c...,-N carboxamide
CH3
CH3
... 0
IV, o ill .7,4 (4S)-7,8-Dimethoxy-N,4- prep. HPLC: Method XI
O ¨ N /NH dimethy1-1-(4- {[(4-
cH3
I H,C
analyt. HPLC (Method P):
35-2 O methylpiperazin-1-
yl) acetyl] amino } phenyl)- Rt = 5.70 min
HN optical rotation: loc1D2 = 213.1
ro 4,5-dihydro-3H-2,3-
0.490 (c= 1.00; methanol)
benzodiazepine-3-
N
carboxamide
C,, N
s CH3
, CA 02915419 2015-12-14
BHC123073FC
,
- 157 -
No. Structure Name Analytical
data
11I-NMR (300MHz, CDCI3): 5 =
CH3
,0 0 1.06 (d, 3H), 1.49
(s, 9H), 1.60-
FI,C /10
1N-4
o ---N NH ( )-tert-Butyl
44{447,8- 2.01 (m, 6H), 2.76-2.87 (m, 3H),
i
CH3 H3C/
*
dimethoxy-4-methyl-3- 2.89 (d, 3H), 3.07 (dd, 1H), 3.69 (s,
(methylcarbamoy1)-4,5- 3H), 3.96 (s, 3H), 4.16-4.29 (m,
36 HN
ZO dihydro-3H-2,3- 1H), 5.34-5.46
(m, 1H), 6.37 (m,
benzodiazepin-1- 1H), 6.61 (s, 1H),
6.76 (s, 1H),
yliphenyl}carbamoyDpip 7.53 (d, 2H), 7.64 (d, 2H), 7.71 (s,
(nri
o--"o eridine-l-
carboxylate br, 1H).
El3CCH3
LCMS (Method 2): Rt = 1.25 min;
H3C
m/z = 580 (M+H)+
., CH3
itc,0 ga 'IV 40
/ (45)-tert-Butyl 4-({4-
o 1111111111 ¨ N NH
CI H3 H3g [7,8-dimethoxy-4- prep. HPLC:
Method XIII
Ili methy1-3-
analyt. HPLC (Method R):
(methylcarbamoy1)-4,5-
36-1 HN
Zo R = 4.14 min
dihydro-3H-2,3-
optical rotation: [a]02 = 189.9 E
benzodiazepin-1-
0.14 (c = 1.00; methanol)
1\ N-)
yl]phenylIcarbamoyl)pip
0--ko eridine-l-carboxylate
H3C4CH3
H3C
CH3
,0 0
H3C
el
O -- N1N4NH (4R)-tert-Butyl 4-({4-
prep. HPLC: Method XIII
1 it CI [7,8-dimethoxy-4-
CH,
=methy1-3- analyt. HPLC (Method R):
(methylcarbamoy1)-4,5- Rt = 3.46 min
36-2 HN
Z 0
dihydro-3H-2,3-
optical rotation: [402 = -190.9
benzodiazepin-1- 0.21 (c = 1.00;
methanol)
c--)
yllphenyllcarbamoyl)pip
o¨ko eridine-l-carboxylate
H3C CH3
H3C
CA 02915419 2015-12-14
BHC123073FC
- 158 -
Example 37
( )-7,8-Dimethoxy-N,4-dimethy1-1-14-[(1-methylpiperidin-4-ypoxy]phenyl -4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide
CH3
H3C
714
O N NH
1
H3C
CH3
0
CH3
In a microwave vessel, 18.5 mg (0.463 mmol) of sodium hydride (60%) were
initially charged in 1
ml of toluene, and 32 mg (0.278 mmol) of 1-methylpiperidin-4-ol were added and
the suspension
was degassed with argon and then heated at 70 C for 15 min. After cooling, 3
mg (0.003 mmol) of
Pd2(dba)3 [CAS No: 51364-51-3], 6 mg (0.008 mmol) of (S)-Tol-B1NAP (CAS
[100165-88-6]) and
100 mg (0.231 mmol) of ( )-1-(4-bromopheny1)-7,8-dimethoxy-N,4-dimethy1-4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide (Example 29A) were added and the mixture was
heated under
argon in a microwave oven at 100 C for 5 h. The reaction was then acidified
with 2N hydrochloric
acid and then made alkaline using 25% aqueous ammonia solution. The blue
solution was extracted
twice with ethyl acetate. The combined organic phases were dried with sodium
sulphate. The
solvent was removed on a rotary evaporator and the residue was purified by
prep. HPLC. This gave
44.7 mg (41% of theory) of the desired product as a solid.
LCMS (Method 2): R, = 0.74 min; ink = 467 (M+H)
111-NMR (400MHz, CDC13): 8 = 1.05 (d, 3H), 1.91-2.02 (m, 2H), 2.11-2.22 (m,
2H), 2.44 (s, 3H),
2.51-2.63 (m, 2H), 2.75 (dd, 1H), 2.79-2.85 (m, 2H), 2.87 (d, 3H), 2.99 (dd,
1H), 3.68 (s, 3H), 3.93
(s, 3H), 4.43-4.52 (m, 1H), 5.28-5.38 (m, 1H), 6.24 (q, 1H), 6.60 (s, 1H),
6.73 (s, 1H), 6.91 (d, 2H),
7.47 (d, 2H).
CA 02915419 2015-12-14
=
BHC123073FC
,
- 159 -
Analogously to Example 37, the racemic Example 29A (optionally with subsequent
enantiomer
separation) or the enantiomerically pure Example 29-2A and the appropriate
commercially
available alcohols gave the following exemplary compounds:
1, No. Structure Name Analytical data
.' CH' , (45)-7,8-Dimethoxy-N,4-
H3c- 40 4,-,
/ dimethy1-1-{4-[(1-
o --- N-
N NH LCMS (Method 2): R,
= 0.78 min;
I H,C/ methylpiperidin-4-
CH, ink - 467
(M+H)+
37-1
411 yl)oxylpheny11-4,5-
optical rotation: [4)2 - 314.1
dihydro-3H-2,3-
o
nzodiazepine-3-
be
o
0.22 (c = 1.00; methanol)
carboxamide
CH,
_
'H-NMR (300MHz, CDC13): 5 =
cH3
,o o ( )-I-{4-[2- 1.05 (d, 3H), 2.51
(s, 6H), 2.76 (dd,
H,C el
1N4 ,
0 ---N NH (Dimethylammo)ethoxy]
1H), 2.87 (d, 3H), 2.91-3.06 (m,
1 /
cH3 H3c phenyl}-7,8-dimethoxy-
3H), 3.68 (s, 3H), 3.93 (s, 3H),
38 #1 N,4-dimethy1-4,5- 4.24 (t, 2H),
5.28-5.41 (m, 1H),
o dihydro-3H-2,3-
6.26 (q, 1H), 6.58 (s, 1H), 6.73 (s,
Zbenzodiazepine-3- 1H), 6.93 (d, 2H), 7.47 (d, 2H).
N--- CH3
i carboxamide LCMS (Method 2): R,
= 0.74 min;
H,C
miz = 441 (M+H)
cii3
H,C prep. HPLC: Method XIV
o
,o is N4
o (4R)-1-{4-[2-
____NNH (Dimethylamino)ethoxy] analyt. HPLC
(Method S):
1 1
CH, phenyl }-7,8-dimethoxy- R., - 5.79
min
38-1 =H,C N,4-dimethy1-4,5- LCMS (Method 2): R, =
0.73 min;
o dihydro-3H-2,3- rn/z = 441 (M+H)+
benzodiazepine-3-
.,N.-c1-13 optical rotation:
[]D2 = -237.6
/ carboxamide
H3c 0.87 (c = 1.00;
methanol)
. CA 02915419 2015-12-14
BHC123073FC
.
- 160 -
No. Structure Name Analytical
data
CH
, 0
H3C el ' N-4 0 (4S)- I -{4-[2- prep.
HPLC: Method XIV
o ¨ N NH /
(Di methylarnino)ethoxy] analyt. HPLC
(Method S):
1 t
CH, =
phenyl}-7,8-dimethoxy- R, = 8.40 min
38-2 O H,C N,4-dimethy1-4,5-
LCMS (Method 2): R, = 0.72 min;
o dihydro-3H-2,3- m/z ¨ 441 (M+H)
Z
N--- cit benzodiazepine-3-
optical rotation: [(1]132 = 218.5
/ carboxamide
Itc 0.41 (c = 1.00;
methanol)
111-NMR (400MHz, CDC13): 5 =
1.07 (d, 3H), 2.45 (s, 3H), 2.78 (dd,
( )-7,8-Dimethoxy-N,4-
CH, 1H), 2.89 (d, 3H),
3.03 (dd, 1H),
H3C
,o 401) o dimethy1-1-{4-[(1-
3.18 (dd, 2H), 3.70 (s, 3H), 3.84-
/
4
o ¨ N
N NH H3C methylazetidin-3-
1 / 3.90 (m, 2H), 3.95
(s, 3H), 4.78-
39 C H3
=yl)oxylpheny11-4,5-
dihydro-3H-2,3- 4.86 (m, 1H), 5.32-
5.42 (m, 1H),
6.28 (q, 1H), 6.61 (s, 1H), 6.75 (s,
benzodiazepine-3-
1H), 6.81 (d, 2H), 7.49 (d, 2H).
carboxamide
LCMS (Method 1): R, = 0.80 min;
m/z = 439 (M+H)+
Example 40
( )-7,8-Dimethoxy-N,4-dimethy1-1-(4-phenoxypheny1)-4,5-dihydro-3H-2,3-benzodi
azepine-3-
carboxamide
CH3
1 CH3 H
0 N¨ CH3
H
71
C"
3 , el
0 ----. N 0
ES
O=
In a microwave vessel, 100 mg (0.231 mmol) of ( )-1-(4-bromopheny1)-7,8-
dimethoxy-N,4-
= CA 02915419 2015-12-14
BHC123073FC
- 161 -
dimethy1-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide (Example 29A), 43.5
mg (0.463
mmol) of phenol, 226 mg (0.694 mmol) of caesium carbonate, 9.2 mg (0.093 mmol)
of copper(I)
chloride and 8.5 mg (0.046 mmol) of 2,2,6,6-tetramethylheptane-3,5-dione were
initially charged
in 4 ml of NMP, and the reaction solution was degassed carefully and heated at
205 C under argon
for 20 min. The crude mixture was filtered and the filtrate was purified by
prep. HPLC. This gave
15.8 mg (15% of theory) of the desired product as a solid.
LCMS (Method 2): 1Z, = 1.33 min; m/z = 446 (M+H)
1H-NMR (300MHz, CDCI3): = 1.07 (d, 3H), 2.82 (dd, 1H), 2.90 (d, 3H), 3.08 (dd,
1H), 3.72 (s,
3H), 3.96 (s, 3H), 5.36-5.50 (m, 1H), 6.33-6.43 (m, 1H), 6.64 (s, 1H), 6.76
(s, 1H), 7.04 (d, 2H),
7.10 (d, 2H), 7.19 (t, 1H), 7.41 (t, 2H), 7.53 (d, 2H).
Analogously to Example 40, Intermediate 29A and the appropriate commercially
available phenol
derivative gave the following exemplary compound:
No. Structure Name Analytical data
'H-NMR (300M1Hz, CDCI3): 8
CH3
CI) CH3 H ( )-1-[4-(4- 1.07 (d, 3H), 2.82
(dd, 1H), 2.90
N-CH3 (d, 3H), 3.08 (dd, 1H),
3.72 (s,
Fluorophenoxy)phenyli-
H3C.,o =¨N 0 3H), 3.96 (s, 3H), 5.35-5.50 (m,
7,8-dimethoxy-N,4-
41
111101 dimethy1-4,5-dihydro-3H- 1H), 6.31-6.43
(m, 1H), 6.63 (s,
1H), 6.76 (s, 1H), 7.00 (d, 2H),
2,3-benzodiazepine-3-
0 a
7.05-7.15 (m, 4H), 7.53 (d, 2H).
carboxamide
"WI F LCMS (Method 2): R, =
1.34 min;
m/z = 464 (M+H)+
Example 42
( )-8-Chloro-1- {4-[(2-fluoropyridin-3-yDamino]phenyl)-N,4-dimethyl-7-
(trifluoromethoxy)-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide
CA 02915419 2015-12-14
BHC123073FC
- 162 -
F
F F
\./'"
CH3
ON¨
CI
¨N ,NH
H30
HN
Fr¨)
Under argon, 100 mg (0.224 mmol) of ( )-8-chloro-1-(4-chloropheny1)-N,4-
dimethy1-7-
(trifluoromethoxy)-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide (Example
32A) were
initially charged in 5 ml of degassed toluene. 50.2 mg (0.448 mmol) of 2-
fluoropyridin-3-amine, 30
mg (0.314 mmol) of sodium tert-butoxide and 9 mg (0.011 mmol) of chloro-(2-
dicyclohexylphosphino-2,4,6-triisopropy1-1,1-bipheny1)[2-(2-amino-1,1-
biphenyl)Jpalladium(ll)
(CAS [1310584-14-5]) were added. The mixture was degassed again and then
stirred at 120 C for 1
hour. After cooling, sat. aqueous ammonium chloride solution and water were
added and the
mixture was extracted with ethyl acetate. The mixture was filtered off with
suction through a
WhatmanTM filter, and the filter was rinsed with ethyl acetate. The solvent
was removed on a rotary
evaporator and the residue was purified by prep. HPLC. This gave 24 mg (20% of
theory) of the
desired product as a solid.
LCMS (Method 1): R = 1.42 min; miz = 522 (M+H)+
'H-NMR (600MHz, CDC13): 5 = 1.02 (d, 3H), 2.84 - 2.91 (m, 4H), 3.08 (dd, 1H),
5.39 - 5.47 (m,
1H), 6.07 (br. s., 1H), 6.32 (br. s., 1H), 7.12 (dd, 1H), 7.16 (d, 2H), 7.22
(s, 1H), 7.29 (s, 1H), 7.47
(d, 2H), 7.72 - 7.78 (m, 2H).
Analogously to Example 42, Example 33A and the appropriate commercially
available amine gave
the following exemplary compound:
= CA 02915419 2015-12-14
BHC123073FC
-163-
-
No. Structure Name Analytical data
_
1H-NMR (300MHz, CDC13): 8 =
1.13 (d, 3H), 1.77 (m, 2H), 1.95
CH3 (-7,8-Dimethoxy-N,4-
(m, 2H), 2.14 (m, 2H), 2.37 (s,
el N-/dimethy1-1-{4-[methyl(1-
/ \ 3H), 2.74 (dd, 1H),
2.85 (d, 3H),
o ¨ N /NH methylpiperidin-4-
H3C H)
2.88 (s, 3, 2.94 (dd, 1H), 3.03
F4'T yOamino]pheny1}-8-
43 F e (m, 2H), 3.69 (m, 1H),
5.23 (m,
(trifluoromethoxy)-4,5-
1H), 5.95 (m, 1H), 6.76 (d, 2H),
3
oidihydro-3H-2,3-
7.04 (d, 1H), 7.20 (dd, 1H), 7.28
benzodiazepine-3-
(d, 1H), 7.47 (d, 2H).
CH3 carboxamide
LCMS (Method 3): Rt = 0.96 min;
m/z = 504 (M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 164 -
Example 44
(4S)-8-Methoxy-N,4-dimethy1-1-(4-{[(1-methy1-1H-pyrrol-2-
ypcarbonyl]amino}pheny1)-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide
C H3
0
HC N
0 N /NH
H3 C
HN
)r
0
C H3
100 mg (0.295 mmol) of (4S)-1-(4-aminopheny1)-8-methoxy-N,4-dimethy1-4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide (Example 34-2A) and 40.7 mg (0.325 mmol) of 1-
methy1-1H-
pyrrole-2-carboxylic acid (CAS [6973-60-0]), 114 mg (0.355 mmol) of
(benzotriazol-1-
yloxy)bisdimethylaminomethylium fluoroborate (CAS [130312-02-6]) and 61.8 ul
(0.355 mmol) of
N,N-diisopropylethylamine in 2 ml of N,N-dimethylformamide were stirred at 80
C for 23 h. After
cooling, the mixture was concentrated under reduced pressure and the residue
was purified by prep.
HPLC. This gave 68 mg (50% of theory) of the desired product as a solid.
LCMS (Method 3): R = 1.16 min; m/z = 446 (M+H)+
'H-NMR (300MHz, CDC13): = 0.99 (d, 3H), 2.81 (dd, 1H), 2.88 (d, 3H), 3.04 (dd,
1H), 3.69 (s,
3H), 4.00 (s, 3H), 5.36 (m, 1H), 6.16 (dd, 1H), 6.37 (br. q., 1H), 6.67 (d,
1H), 6.74 (dd, 1H), 6.81
(dd, 1H), 6.88 (dd, 1H), 7.14 (d, 1H), 7.51 (d, 2H), 7.59 (d, 2H), 7.70 (br.
s., 1H).
CA 02915419 2015-12-14
BHC123073FC
- 165 -
Analogously to Example 44, Example 34-2A and 1,2,3-thiadiazole-4-carboxylic
acid (CAS [4100-
13-4]) gave the following exemplary compound:
No. Structure Name Analytical data
LCMS (Method 3): 124 = 1.08 min;
CH (45)-8-Methoxy-N,4- m/z = 451 (M+H)+
H.,C =
dimethy1-1-{44(1,2,3-[(1,2,3 'H-NMR
(300MHz, CDCI3): 8 =
- N NH thiadiazol-4- 0.98 (d,
3H), 2.84 (dd, 1H), 2.90
H,C
ylcarbonypamino]phenyl (d, 3H), 3.08 (dd, IH), 3.70 (s,
}-4,5-dihydro-3H-2,3- 3H), 5.39
(m, 1H), 6.44 (m, 1H),
benzodiazepine-3- 6.67 (d, 1H), 6.89 (dd, 1H), 7.15
carboxamide (d, 1H),
7.57 (d, 2H), 7.78 (d, 2H),
9.36 (s, 1H), 9.46 (br. s., 1H).
5 Example 46
( )-1-(4-{[(Dimethylamino)acetyl]aminolpheny1)-8-methoxy-N,4-dimethy1-4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide
C H3
0
71¨(
H3 C0
0 N NH
H3 C
HN
N--CH3
0 /
H3C
At 0 C, 998 mg (2.95 mmol) of ( )-1-(4-aminopheny1)-8-methoxy-N,4-dimethy1-4,5-
dihydro-3H-
10 2,3-benzodiazepine-3-carboxamide (Example 34A) and 304 mg (2.95 mmol) of
N,N-
dimethylglycine (CAS [1118-68-9]) and 1.28 ml (7.39 mmol) of N,N-
diisopropylethylamine were
initially charged in 20 ml of tetrahydrofuran. With ice bath cooling, 2.07 ml
(3.54 mmol) of a 50%
strength solution of propanephosphonic acid cyc/o-anhydride in N,N-
dimethylformamide (CAS
[68957-94-8]) were added dropwise. The reaction mixture was then stirred at 60
C for 3 days. For
CA 02915419 2015-12-14
BHC123073FC
- 166 -
workup, saturated aqueous sodium bicarbonate solution was added and the
mixture was extracted
three times with ethyl acetate. The combined organic phases were dried,
filtered and concentrated
under reduced pressure. The residue was purified by flash chromatography. This
gave 647 mg
(50% of theory) of the desired product as a solid.
LCMS (Method 3): 124 = 0.74 min; m/z = 424 (M+H)+
'H-NMR (300MHz, CDC13): = 0.98 (d, 3H), 2.44(s, 6H), 2.81 (dd, 1H), 2.88 (d,
3H), 3.05 (dd,
1H), 3.68 (s, 3H), 5.36 (m, 1H), 3.16 (s, 2H), 6.38 (m, 1H), 6.65 (d, 1H),
6.88 (dd, 1H), 7.14 (d,
1H), 7.50 (d, 2H), 7.64 (d, 2H), 9.32 (br. s., 1H).
Example 47
( )-7,8-Dimethoxy-N,4-dimethyl -1- {4-[(methylsulphonypaminolphenyll -4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide
CH
I 3 CH3
,N4
H C
3 41111
N NH
H3C
HN
,S 3
Cr' "
o
100 mg (0.231 mmol) of ( )-1-(4-bromopheny1)-7,8-dimethoxy-N,4-dimethy1-4,5-
dihydro-3H-2,3-
benzodiazepine-3-carboxamide (Example 29A), 26 mg (0.278 mmol) of
methanesulphonamide, 64
mg (0.46 mmol) of potassium carbonate and 13 mg (0.023 mmol) of
allylchloropalladium dimer
(CAS [12012-95-2]) were initially charged in 3 ml of 2-methyltetrahydrofuran,
and the suspension
was degassed with argon for 10 min. 39 mg (0.093 mmol) of di-tert-
buty1(2',4',6'-
triisopropylbipheny1-2-yOphosphane (CAS [564483-19-8]) were then added, and
the mixture was
degassed again with argon and heated at 80 C for 16 h. The crude mixture was
filtered, the solvent
was then removed and the residue obtained was purified by prep. HPLC. This
gave 16 mg (16% of
theory) of the desired product as a solid.
LCMS (Method 2): R, = 0.84 min; m/z = 447 (M+H)+
CA 02915419 2015-12-14
BHC123073FC
- 167 -
'11-NMR (300MHz, CDC13): = 1.02 (d, 3H), 2.84 (dd, 1H), 2.91 (d, 3H), 3.06-
3.15 (m, 1H), 3.10
(s, 3H), 3.69 (s, 3H), 3.96 (s, 311), 5.38-5.51 (m, 1H), 6.44 (q, 1H), 6.59
(s, 1H), 6.75 (s, 1H), 7.04
(s, br, 1H), 7.28 (d, 2H), 7.53 (d, 2H).
Analogously to Example 47, racemic Example 29 or enantiomerically pure Example
29-2A and the
appropriate sulphonamides gave the following exemplary compounds.
No. Structure Name Analytical data
LCMS (Method 1): R = 1.03 min;
m/z = 510 (M+H)+
cH3 (4S)-7,8-Dimethoxy-N,4-
O cH,c) 'H-NMIR
(300MHz, CDC13): 8 =
dimethy1-1-14-[(pyridin-
40'N4 1.00 (d, 3H), 2.83 (dd, 1H), 2.91
¨No N- CH, 3-
4840 (d, 3H), 3.09 (dd, 1H), 3.66 (s,
ylsulphonyl)amino]pheny
3H), 3.95 (s, 3H), 5.38-5.51 (m,
11-4,5-dihydro-3H-2,3-
HNõs 0 1H), 6.42 (q, 1H), 6.49 (s, 1H),
,
benzodiazepine-3-
6.73 (s, 1H), 7.17 (d, 2H), 7.28 (m,
carboxamide
IH), 7.41-7.51 (m, 3H), 8.16 (m,
1H), 8.80 (d, 1H), 9.04 (d, 1H).
LCMS (Method 1): R, = 1.01 min;
m/z = 517 (M+H)+
CH, (4S)-7,8-Dimethoxy-N,4- 1H-NMR
(400MHz, CDC13): =
O aghm ,cH30
dimethy1-1-{4- 1.04 (d, 3H), 1.96-2.10 (m, 4H),
H3c.,0 niN4N-cH, [(tetrahydro-2H-pyran-4- 2.85
(dd, 1H), 2.92 (d, 3H), 3.10
49
40
ylsulphonyDaminolpheny (dd, 1H), 3.29-3.42 (m, 3H), 3.70
11-4,5-dihydro-3H-2,3- (s, 3H),
3.96 (s, 3H), 4.07-4.15 (m,
HN,s,0
benzodiazepine-3- 2H), 5.40-
5.49 (m, 1H), 6.37-6.45
carboxamide (m, 1H),
6.60 (s, 1H), 6.76 (s, 1H),
6.80 (s, br, 1H), 7.28 (d, 2H), 7.52
(d, 2H).
- CA 02915419 2015-12-14
BHC123073FC
,
- 168 -
No. Structure Name Analytical
data
LCMS (Method 2): R, = 0.94 min;
( )-7,8-Dimethoxy-N,4- m/z = 461
(M+H)+
CH,
I CH,
dimethy1-1-{4- 'H-NMR (400MHz., CDCI3): 8 =
1-130,0 MPIP _ N [methyl(methylsulphonyl 1.02 (d, 3H),
2.86 (dd, 1H), 2.92 (s,
50 H
IP )amino]pheny1}-4,5-
dihydro-3H-2,3- 6H), 3.12 (dd, 1H), 3.40 (s, 3H),
3.70 (s, 3H), 3.96 (s, 3H), 5.42-
,N .0
H3C
N.
benzodiazepine-3- 5.53 (m, 1H), 6.44-
6.54 (m, 1H),
o '-'3
carboxamide 6.61 (s, 1H), 6.75 (s, 1H), 7.73 (d,
2H), 7.55 (d, 2H).
LCMS (Method 2): R, = 1.05 min;
in/z = 509 (M+H)+
CH, 1H-NMR (300MHz,
CDC13): 8 =
O abh cH30 ( )-7,8-Dimethoxy-N,4-
1.02 (d, 3H), 2.83 (dd, 1H), 2.90
H3c.,0 1111V _...;
DIN4N-C It dimethy1-1- {4-
(d, 3H), 3.08 (dd, 1H), 3.64 (s,
51 H [(phenylsulphonyl)amino
1110
]phenyl}-4,5-dihydro-3H- 3H), 3.95 (s, 3H), 5.37-5.51 (m,
1H), 6.34-6.44 (m, 1H), 6.49 (s,
HN - 0 2,3-benzodiazepine-3-
-s'
g 40 carboxamide 1H), 6.73 (s, 1H), 6.99 (m, 1H),
7.14 (d, 2H), 7.43 (d, 2H), 7.46-
7.54 (m, 2H), 7.55-7.63 (m, 1H),
7.88 (d, 2H).
_
LCMS (Method 2): Rt = 1.07 min;
m/z = 523.8 (M+H)+
( )-1-{4-
cH
CH,
, 'H-NMR (300MHz,
CDC13): 8 =
I
0 0 [(Benzylsulphonyl)amino
1.05 (d 3H) 2.86 (dd 1H) 2.93
gi ¨ rsiN4 ' ' ' "
N_CH, ]pheny1}-7,8-dunethoxy-
52 0
H ' (d, 3H), 3.11 (dd, 1H), 3.71 (s,
benzodiazepine-3-
1101 N,4-dimethy1-4,5-
dihydro-3H-2,3- 3H), 3.96 (s, 3H), 4.42 (s, 2H),
HNõF 0 el 5.39-5.52 (m, 1H),
6.39-6.47 (m,
o 1H), 6.50 (s, br, 1H), 6.61 (s, 1H),
carboxamide
6.76 (s, 1H), 7.18 (d, 2H), 7.30-
7.45 (m, 5H), 7.53 (d, 2H).
. CA 02915419 2015-12-14
BHC123073FC
- 169 -
No. Structure Name Analytical data
LCMS (Method 2): R, = 1.13 min;
( )-7,8-Dimethoxy-N,4- m/z = 501.8 (M+H)+
CH3
oI CH3
o
N dimethy1-1-(4- 'H-NMR (300MHz, CDC13): 8 =
4
¶3 0 .--- 14 N-CH3 {
[(trifluoromethyl)sulpho 1.00 (d, 3H), 2.87 (dd, 1H), 2.92
53 H
40 nyl]aminolpheny1)-4,5-
(d, 3H), 3.14 (dd, 1H), 3.67 (s,
dihydro-3H-2,3-
3H), 3.96 (s, 3H), 5.41-5.54 (m,
HN,_...,0
g.,.,, FF benzodiazepine-3- 1H), 6.51
(q, 1H), 6.54 (s, 1H),
F carboxamide 6.74 (s, 1H), 7.35 (d, 2H),
7.53 (d,
2H).
LCMS (Method 2): R., = 0.93 min;
m/z = 473 (M+H)+
( )-1-{4-
CH
cH3
,
'H-NMR (400MHz, CDC13): 8 =
oI
o [(Cyclopropylsulphonyl)a
N4 1.00-1.08 (m, 5H), 1.24-1.30
(m,
H3c-0 VI ¨ rsi N-CH3 mino]pheny11-7,8-
54 H
2H), 2.54-2.62 (m, 1H), 2.85 (dd,
SI dimethoxy-N,4-dimethy1-
4,5-dihydro-3H-2,3-
1H), 2.92 (d, 3H), 3.10 (dd, 1H),
HN,o 3.69 (s, 3H), 3.96 (s, 3H),
5.40-
benzodiazepine-3-
8 V 5.50 (m, 1H), 6.39-6.47 (m,
1H),
carboxamide
6.60 (s, 1H), 6.72 (s, br, 1H), 6.76
(s, 1H), 7.30 (d, 2H), 7.52 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 170 -
Example 55
( )-1-[4-(Benzyloxy)pheny1]-7,8-dimethoxy-N,4-dimethyl-4,5-dihydro-3H-2,3-
benzodiazepine-3-
carboxamide
CH
i 3 CH3
0
0
0 '14 N¨CH3
CH3
0
Analogously to Example 37, 100 mg (0.162 mmol) of racemic Example 29A gave, by
reaction with
27 mg (0.243 mmol) of phenylmethanol and subsequent purification by reversed-
phase prep.
HPLC, 32 mg (42% of theory) of the desired product as a solid.
LCMS (Method 2): R, = 1.32 min; m/z = 460.8 (M+H)+
1H-NMR (300MHz, CDC13): 6 = 1.10 (d, 3H), 2.79 (dd, 1H), 2.89 (d, 3H), 3.03
(dd, 1H), 3.71 (s,
3H), 3.96 (s, 3H), 5.16 (s, 2H), 5.31-5.44 (m, 1H), 6.27 (q, 1H), 6.63 (s,
1H), 6.77 (s, 1H), 7.03 (d,
2H), 7.34-7.51 (m, 5H), 7.53 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 171 -
Example 56
(1)-1 - {4-[(N,N-Dimethylglycyl)(methypamino] phenyl } -4-ethy1-7,8-dimethoxy-
N-methy1-4,5-
dihydro-3H-2,3-benzodiazepine-3-carboxamide
H3 C
CH
i 3
0 41,
N40
N-
0 CH H 3
C H3 fk
0
H3C, CH3
H3 C
Example 56 was prepared analogously to the synthesis sequence described for
Example 29. The 1-
(3,4-dimethoxyphenyl)butan-2-ol (cf. Formula II in Scheme 1) used for this
purpose was prepared
from (3,4-dimethoxyphenyl)acetaldehyde by reaction with ethylmagnesium bromide
(Journal of
Organic Chemistry 1976, 3201 - 3204). (3,4-Dimethoxyphenyl)acetaldehyde was
prepared from
commercial 2-(3,4-dimethoxyphenypethanol (CAS[7417-21-2]) by oxidation with
Dess-Martin
reagent (CAS[87413-09-0]) (cf. Monatshefte fijr Chemie 2004, 1289¨ 1295).
LCMS (Method 2): It, = 0.68 min; m/z = 482 (M+H)+
11-1-NMIt (300M1-Iz, CDC13): 5 = 0.88 (t, 3H), 1.04-1.21 (m, 1H), 1.32-1.48
(m, 1H), 2.38 (s, br,
6H), 2.64 (s, 2H), 2.93 (d, 3H), 3.02-3.15 (m, 2H), 3.36 (s, 3H), 3.68 (s,
3H), 3.96 (s, 3H), 5.26-
5.37 (m, 1H), 6.60 (s, 1H), 6.59-6.67 (m, 1H), 6.75 (s, I H), 7.25 (d, 2H),
7.55 (d, 2H).
CA 02915419 2015-12-14
BHC123073FC
- 172 -
Example 57
( )-4-Isopropyl-7,8-dimethoxy-N-methy1-1-{4-[methyl(1-methyl-IH-imidazol-2-
yDamino]phenyll-4,5-dihydro-3H-2,3-benzodiazepine-3-carboxamide
H3C
CH3
H3CC) 0
0 N NH
CH3 H,C
CH3
H,C'N
\\ N"
Example 57 was prepared analogously to the synthesis sequence described for
Example 12. The 1-
(3,4-dimethoxyphenyI)-3-methylbutan-2-ol (cf. Formula II in Scheme 1) used for
this purpose was
prepared from (3,4-dimethoxyphenypacetaldehyde by reaction with 2-
propylmagnesium chloride
(cf. Organic Letters 2007, 2103 ¨ 2106). (3,4-Dimethoxyphenyl)acetaldehyde was
prepared from
commercial 2-(3,4-dimethoxyphenyl)ethanol (CAS[7417-21-2]) by oxidation with
Dess-Martin
reagent (CAS[87413-09-0]) (cf. Monatshefte fiir Chemie 2004, 1289¨ 1295).
LCMS (Method 2): R, = 0.75 min; m/z = 491 (M+H)
'H-NMR (400MHz, CDC13): 8 = 0.88 (t, 6H), 1.48-1.60 (m, 1H), 2.87 (d, 3H),
2.91 (dd, 1H), 3.01
(dd, 1H), 3.41 (s, 3H), 3.43 (s, 3H), 3.69 (s, 3H), 3.94 (s, 3H), 5.08-5.16
(m, 1H), 6.35 (q, 1H), 6.61
(d, 2H), 6.68 (s, 1H), 6.75 (s, 1H), 6.86 (d, 1H), 7.03 (d, 1H), 7.43 (d, 2H).
CA 02915419 2015-12-14
= BHC123073FC
- 173 -
Biological efficacy of the compounds according to the invention
1. Assays
1.1 Protein-Protein Interaction Assay
Binding assay BRD4/acetylated peptide H4 ("PRO")
To assess the BRD4 binding strength of the substances described in this
application, the ability
thereof to inhibit the interaction between BRD4 (BD1) and acetylated histone
H4 in a dose-
dependent manner was quantified (Filippakopoulos et al., Cell, 2012, 149:214-
231).
For this purpose, a time-resolved fluorescence resonance energy transfer (TR-
FRET) assay was
used, which measures the binding between N-terminally His6-tagged BRD4 (BD1)
(amino acids
67-152, longer constructs also being possible, preferably amino acids 44-168)
and a synthetic
acetylated histone H4 (Ac-H4) peptide with sequence
GRGK(Ac)GGK(Ac)GLGK(Ac)GGAK(Ac)RHGSGSK-biotin. The recombinant BRD4 protein
produced in-house according to Filippakopoulos et al., Cell, 2012, 149:214-231
was expressed in
E. coli and purified by means of (Ni-NTA) affinity and (Sephadex G-75) size
exclusion
chromatography. The Ac-H4 peptide can be purchased, for example, from
Biosyntan (Berlin,
Germany).
In the assay, typically 11 different concentrations of each substance (0.1 nM,
0.33 nM, 1.1 nM,
3.8 nM, 13 nM, 44 nM, 0.15 M, 0.51 M, 1.7 M, 5.9 M and 20 M) were
analysed as
duplicates on the same microtitre plate. For this purpose, 100-fold
concentrated solutions in DMSO
were prepared by serial dilutions (1:3.4) of a 2 mM stock solution into a
clear, 384-well microtitre
plate (Greiner Bio-One, Frickenhausen, Germany). From this, 50 nl were
transferred into a black
test plate (Greiner Bio-One, Frickenhausen, Germany). The test was started by
the addition of 2 I
of a 2.5-fold concentrated BRD4 solution (final concentration typically 10 nM
in the 5 .1 of
reaction volume) in aqueous assay buffer [50 mM HEPES pH 7.5, 50 mM sodium
chloride (NaC1),
0.25 mM CHAPS and 0.05% serum albumin (BSA)] to the substances in the test
plate. This was
followed by a 10-minute incubation step at 22 C for the pre-equilibration of
putative complexes
between BRD4 and the substances. Subsequently, 3 1 of a 1.67-fold
concentrated solution (in
assay buffer) consisting of Ac-H4 peptide (83.5 nM) and TR-FRET detection
reagents [16.7 nM
anti-6His-XL665 and 3.34 nM streptavidin cryptate (both from Cisbio Bioassays,
Codolet, France),
and 668 mM potassium fluoride (KF)] were added.
The mixture was then incubated in the dark at 22 C for one hour and then at 4
C for at least 3
hours and for no longer than overnight. The formation of BRD4/Ac-H4 complexes
was determined
by the measurement of the resonance energy transfer from the streptavidin-Eu
cryptate to the anti-
CA 02915419 2015-12-14
BHC123073FC
- 174 -6His-XL665 antibody present in the reaction. For this purpose, the
fluorescence emission was
measured at 620 nm and 665 nm after excitation at 330-350 nm in a TR-FRET
measuring
instrument, for example a Rubystar or Pherastar (both from BMG Lab
Technologies, Offenburg,
Germany) or a Viewlux (Perkin-Elmer). The ratio of the emissions at 665 nm and
at 622 nm was
taken as an indicator of the amount of BRD4/Ac-H4 complexes formed.
The data (ratios) obtained were normalized, with 0% inhibition corresponding
to the mean from the
measurements for a set of controls (typically 32 data points) in which all the
reagents were present.
In these, in place of test substances, 50 n1 of DMS0 (100%) were used.
Inhibition of 100%
corresponded to the mean from the measurements for a set of controls
(typically 32 data points) in
which all the reagents except BRD4 were present. The IC50 was determined by
regression analysis
based on a 4-parameter equation (minimum, maximum, IC50, Hill; Y = max + (min -
max) / (1 +
(X/ICõ)}")).
1.2 Cell assays
Cell proliferation assays
In accordance with the invention, the ability of the substances to inhibit
cell proliferation was
determined. Cell viability was determined by means of the alamarBlue reagent
(Invitrogen) in a
Victor X3 Multilabel Reader (Perkin Elmer). The excitation wavelength was 530
nm and the
emission wavelength 590 nM.
The MOLM-13 cells (DSMZ, ACC 554) were sown at a concentration of 4000
cells/well in 100 1.11
of growth medium (RPMI1640, 10% FCS) on 96-well microtitre plates.
The Bl6F10 cells (ATCC, CRL-6475) were sown at a concentration of 300-500
cells/well in
100 [11 of growth medium (DMEM with phenol red, 10% FCS) on 96-well microtitre
plates.
The LOX-IMVI cells (NCI-60) were sown at a concentration of 1000 cells/well in
100 1 of growth
medium (RPMI1640, 10% FCS) on 96-well microtitre plates.
The CHL-1 cells (ATCC, CRL -9446) were sown at a concentration of 1000
cells/well in 100 I of
growth medium (DMEM, 10% FCS) on 96-well microtitre plates.
The MOLP-8 cells (DSMZ, ACC 569) were sown at a concentration of 4000
cells/well in 100 I of
growth medium (RPMI1640, 20% FCS) on 96-well microtitre plates.
The KMS-12-PE cells (DSMZ, ACC 606) were sown at a concentration of 4000
cells/well in 100
I of growth medium (RPMI1640, 20% FCS) on 96-well microtitre plates.
The LAPC-4 cells (ATCC, PTA-1441TM) were sown at a concentration of 4000
cells/well in
100 I of growth medium (RPMI1640, 2 mM L-glutamine, 10% cFCS) on 96-well
microtitre
plates. One day later, the LAPC-4 cells were treated with 1 nM
methyltrienolone and various
substance dilutions.
The MDA-MB-231 cells (DSMZ, ACC 732) were sown at a concentration of 4000
cells/well in
CA 02915419 2015-12-14
BHC123073FC
- 175 -
100 I of growth medium (DMEM/Ham's F12 medium, 10% FCS) on 96-well microtitre
plates.
After overnight incubation at 37 C, the fluorescence values (CI values) were
determined. Then the
plates were treated with various substance dilutions (1E-5 M, 3E-6 M, 1E-6 M,
3E-7 M, 1E-7 M,
3E-8 M, 1E-8 M) and incubated at 37 C for 72 (LOX-IMVI cells), 96 (MOLM-13,
B16F10, CHL-
1, MDA-MB-431 cells), 120 (MOLP-8, KMS-12-PE cells) or 168 (LAPC-4 cells)
hours.
Subsequently, the fluorescence values were determined (CO values). For the
data analysis, the CI
values were subtracted from the CO values and the results were compared
between cells which had
been treated with various dilutions of the substance or only with buffer
solution. The IC50 values
(substance concentration needed for 50% inhibition of cell proliferation) were
calculated therefrom.
The substances were tested in the cell lines in Table 1, which represent the
indications specified by
way of example:
Cell line Source Indication
MOLM-13 DSMZ acute myeloid leukaemia
Bl6F10 ATCC melanoma (BRAE
wild-type)
LOX IMVI NCI-60 melanoma (BRAF mutated)
CHL-1 ATCC melanoma (BRAE
wild-type)
MOLP-8 DSMZ multiple myeloma
KMS-12-PE DSMZ multiple myeloma
LAPC-4 ATCC prostate cancer
MDA-MB-231 DSMZ mammary carcinoma
2. Results
2.1 Bindin2 assay
Table 2 shows the results from the BRD4 (BD1) binding assay.
Table 2
IC 50 (BRD4) IC50 (BRD4)
Example ( Example
umol/l) (ttmol/l)
1 0.04 20-2 0.02
1-1 0.04 21 0.18
1-2 5.13 22 0.16
2 0.03 23 0.09
CA 02915419 2015-12-14
BHC123073FC
..
- 176 -
IC50 (BRD4)
1050 (BRD4)
Example Example
(pmol/l)
()molt()
3 0.05 24 0.05
3-1 1.02 25 0.08
3-2 0.02 26 0.12
4 0.02 27 0.22
4-1 1.99 28. 0.62
4-2 0.02 29 0.04
5 0.05 29-1 3.45
5-1 0.11 29-2 0.03
6 0.04 30 0.04
7 0.02 30-1 0.06
7-1 0.63 30-2 11.75
7-2 0.02 31 0.05
8 0.03 32 0.05
8-1 0.02 33 0.07
9 0.04 33-1 0.06
9-1 5.66 33-2 3.64
9-2 0.02 34 0.72
10 0.07 35 0.15
11 0.05 35-1 19.16
12 0.02 35-2 0.09
12-1 5.42 36 0.21
12-2 0.01 36-1 0.07
13 0.03 36-2 3.05
13-1 0.03 37 0.08
14 0.02 37-1 0.06
14-1 4.73 38 0.22
14-2 0.01 38-1 3.37
15 0.05 38-2 0.11
16 0.03 39 0.30
_
16-1 5.49 40 0.21
16-2 0.03 41 0.19
, 17 0.03 42 1.76
18 0.07 43 0.03
18-1 0.06 44 0.21
19 0.04 45 0.12
19-1 0.03 46 0.15
_ 20 0.03 47 0.05
20-1 1.71 48 0.02
CA 02915419 2015-12-14
BHC123073FC
_
- 177 -
IC50 (BRD4) IC50 (BRD4)
Example Example
( mo1/1) ( mo1/1)
49 0.06 54 0.06
50 0.14 55 0.40
51 0.03 56 >10.0
52 0.09 57 9.68
53 0.04
2.2 Cell assays
Table 3 shows the results from various cell proliferation assays.
Table 3
breast cancer
leukaemia melanoma multiple myeloma prostate
cancer
Exa MOLM-13 B16-F10 LOX IMVI CHL-1 MOLP-8 ICMS-12-PE LAPC-4 MDA-
MB-
mple ICso ICso ICso IC50 1050 1050
1050 231
( mo1/1) ( mo1/1) ( mo1/1) ( mo1/1)
( mo1/1) ( mo1/1) ( mo1/1) IC50
(am o1/1)
1 0.15 0.43 0.06 0.06
1-1 0.12 0.33 0.07 0.08
1-2 >10.0 >10.0 >10.0 >10.0
2 0.14 ' 0.09 0.04
3 0.08 0.07
3-1 3.01 1.75 1.90
3-2 0.08 0.04 0.05
4 0.06 0.06 0.21 0.05 0.05 0.02
0.06
4-1 >10.0 7.34 5.60
4-2 0.02 0.02 0.01
CA 02915419 2015-12-14
_
BHC123073FC
_
- 178 -
Leukaemia Melanoma
Multiple myeloma
Exampl MOLM-13 B16-F10 CHL-1 MOLP-8
e IC 50 (nmoUl) IC 50 (Ftmol/l) 1050 (umoUl) IC 50 (Imola)
5 0.57
5-1 0.22 0.20 0.10
6 0.15 0.33 0.15 0.22
7 0.19 0.21 0.12 0.13
7-1 2.40 1.74 1.48
7-2 0.12 0.01
0.06
8 0.15 0.40 0.20 0.11
8-1 0.07 0.14 0.03
_
9 0.28 0.18 0.16
9-1 3.37 1.80 1.67
9-2 0.15 0.09
0.07
10 0.85 1.45 0.29 0.69
11 0.49 0.75 0.28 0.49
12 0.15 0.36 0.10 0.11
12-1 >10.0 >10.0
>10.0
12-2 0.15 0.17 0.04
0.05
13 0.12 0.23 0.12 0.13
13-1 0.08 0.07
0.05
14 0.14
14-1 - >10.0 >10.0 >10.0
14-2 - 0.09 0.04 0.04
15 0.31 0.56 0.23 0.31
16 0.23
CA 02915419 2015-12-14
-
BHC123073FC
,
- 179 -
Exampl MOLM-13 B16-F10 CHL-1
MOLP-8
e IC50 (amo1/1) 1050 (pmo1/1)
1050 (=61/1) 1050 (pmo1/1)
16-1 ' 8.72 5.34 8.47
16-2 0.10 0.08 0.05 0.07
' 17 0.10 0.15 0.07 0.09
18 0.36 0.58 0.14 0.27
18-1 0.14 0.15 0.07
19 0.22 0.46 0.14 0.14
19-1 0.12 0.18 0.05
20 0.13 0.26 0.07 0.09
_
20-1 8.94 >10.0 >10.0
>10.0
_
20-2 0.08 0.08 0.03
21 0.21 0.27 0.15
22 0.33 0.39 0.16
_
23 0.61 0.57 0.30 0.57
_
24 0.10 0.26 0.07 0.06
' 25 0.63
' 26 0.65 0.73 0.30
' 27 ' 1.00 1.08 0.55
' 28 6.47 4.80 4.15
29 ' 0.24 0.60 0.23 0.22
' 29-1 9.46 >10.0 6.90
>10.0
29-2 0.13 0.15 0.07
30 0.27
-
30-1 0.20 0.29 0.12
30-2 ' >10.0 >10.0
>10.0
_
31 0.05 0.07 0.06
32 0.50 0.45 0.33
33 ' 0.31 0.39 0.23
33-1 0.19 0.22 0.09
_
33-2 7.53 9.93 8.57
=
34 2.78 3.14 1.71
,
35 0.36 0.43 0.15
CA 02915419 2015-12-14
_
BHC123073FC
- 180 -
Exampl MOLM-13 B16-F10 CHL-1 MOLP-8
e ICso ( mo1/0 1050 (innol/1) IC50
(ttmo1/1) IC50 (tunol/B
35-1 >10.0 >10.0 >10.0
35-2 0.19 0.18 0.08
36 0.55 0.44 0.38
36-1 0.24 0.21 0.18
36-2 >10.0 >10.0 >10.0
37 0.36
37-1 0.19 0.21 0.08
38 0.57 0.59 0.37 0.40
38-1 >10.0 9.87 9.70
38-2 0.18 0.09
39 0.37 0.36 0.18
40 0.46 0.47 0.23 0.32
41 0.38 0.43 0.36
42 1.42 0.50 0.99
43 0.06 0.12 0.03 0.04
44 0.10
45 0.23
CA 02915419 2015-12-14
BHC123073FC
- 181 -
MOLM-13 816-F10 CHL-1
MOLP-8
Exampl
e ICso (Amo1/1) ICso (iumo1/1) IC50 (Amo1/1)
IC50 (Amon)
46 0.62 0.69 0.36
47 0.72 0.58 0.46
48 0.27 0.26 0.22 0.16
49 0.17 0.26 0.07 0.13
50 0.59 0.92 0.47
51 1.33 0.24 0.17
52 0.25 0.40 0.26
53 2.18 2.26 1.65
54 1.14 0.34 0.17
55 0.73 0.83 0.44 0.70
56 >10.0 >10.0 >10.0 >10.0
57 >10.0 >10.0