Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
VISTA Antagonist and Methods of Use
Background
[0003] The immune system is tightly controlled by co-
stimulatory and co-inhibitory ligands and receptors. These
molecules provide not only a second signal for T cell
activation but also a balanced network of positive and
negative signals to maximize immune responses against
infection while limiting immunity to self.
[0004] Induction of an immune response requires T cell
expansion, differentiation, contraction and establishment of T
cell memory. T cells must encounter antigen presenting cells
(APCs) and communicate via T cell receptor (TCR)/major
histocompatibility complex (MHC) interactions on APCs. Once
the TCR/MHC interaction is established, other sets of
receptor-ligand contacts between the T cell and the APC are
-1-
Date Recue/Date Received 2021-06-18
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
required, i.e. co-stimulation via CD154/CD40 and CD28/B7.1-
B7.2. The synergy between these contacts results in a
productive immune response capable of clearing pathogens and
tumors, and may be capable of inducing autoimmunity.
[0005] Another level of control has been identified, namely
regulatory T cells (Treg). This specific subset of T cells is
generated in the thymus, delivered into the periphery, and is
capable of constant and inducible control of T cells
responses. Sakaguchi (2000) Cell 101(5):455-8; Shevach (2000)
Annu. Rev. Immunol. 18:423-49; Bluestone and Abbas (2003) Nat.
Rev. Immunol. 3(3):253-7. Treg are represented by a CD4+CD25+
phenotype and also express high levels of cytotoxic T
lymphocyte-associated antigen-4 (CTLA-4), OX-40, 4-1BB and the
glucocorticoid inducible TNF receptor-associated protein
(GITR). McHugh, et al. (2002) Immunity 16(2):311-23; Shimizu,
et al. (2002) Nat. Immun. 3(2):135-42. Elimination of Treg
cells by 5 day neonatal thymectomy or antibody depletion using
anti-0D25, results in the induction of autoimmune pathology
and exacerbation of T cells responses to foreign and self-
antigens, including heightened anti-tumor responses.
Sakaguchi, et al. (1965) J. Exp. Med. 161(1):72-67; Sakaguchi,
et al. (1995) J. Immunol. 155(3):1151-64; Jones, et al. (2002)
Cancer Immun. 2:1. In addition, Treg have also been involved in
the induction and maintenance of transplantation tolerance,
since depletion of Treg with anti-CD25 monoclonal antibodies
results in ablation of transplantation tolerance and rapid
graft rejection. Jarvinen, et al. (2003) Transplantation
76:1375-9. Among the receptors expressed by Treg GITR seems to
be an important component since ligation of GITR on the
surface of Treg with an agonistic monoclonal antibody results
-2-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
in rapid termination of Tõg activity, resulting in autoimmune
pathology and ablation of transplantation tolerance.
[0006] Costimulatory and co-inhibitory ligands and receptors
not only provide a "second signal" for T cell activation, but
also a balanced network of positive and negative signal to
maximize immune responses against infection while limiting
immunity to self. The best characterized costimulatory ligands
are 137.1 and 137.2, which are expressed by professional APCs,
and whose receptors are CD28 and CTLA-4.
Greenwald, et al.
(2005) Annu Rev Immunol 23, 515-548; Sharpe and Freeman (2002)
Nat Rev Immunol 2, 116-126. CD28 is
expressed by naive and
activated T cells and is critical for optimal T cell
activation. In
contrast, CTLA-4 is induced upon T cell
activation and inhibits T cell activation by binding to
87.1/B7.2, thus impairing 0D28-mediated costimulation. CTLA-4
also transduces negative signaling through its cytoplasmic
ITIM motif. Teft, et al. (2006). Annu Rev Immunol 24, 65-97.
B7.1/B7.2 KO mice are impaired in adaptive immune response
(Borriello, et al. (1997) Immunity 6, 303-313; Freeman, et al.
(1993) Science 262, 907-909), whereas CTLA-4 KO mice can not
adequately control inflammation and develop systemic
autoimmune diseases. Chambers, et al. (1997) Immunity 7, 885-
895; Tivol, et a]. (1995) Immunity 3, 541-547; Waterhouse, et
al. (1995) Science 270, 985-988. The B7
family ligands have
expanded to include costimulatory B7-H2 (ICOS Ligand) and B7-
H3, as well as co-inhibitory 137-H1 (PD-L1), 137-DC (PD-L2), B7-
H4 (B7S1 or 137x), and B7-H6. See Brandt, et al. (2009) J Exp
Med 206, 1495-1503; Greenwald, et al. (2005) Annu Rev Immunol
23: 515-548.
-3-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
[0007] Inducible costimulatory (1COS) molecule is expressed
on activated T cells and binds to 87-82. See
Yoshinaga, et
al. (1999) Nature 402, 827-832. ICOS is important for T cell
activation, differentiation and function, as well as essential
for T-helper-cell-induced B cell activation, Ig class
switching, and germinal center (GC) formation. Dong, et a/.
(2001) Nature 409, 97-101; Tafuri, et al. (2001) Nature 409,
105-109; Yoshinaga, et al. (1999) Nature 402, 827-832.
Programmed Death 1 (PD-1) on the other hand, negatively
regulates T cell responses. PD-1 KO mice develop lupus-like
autoimmune disease, or autoimmune dilated cardiomyopathy
depending upon the genetic background.
Nishimura, et al.
(1999) Immunity 11, 141-151. Nishimura, et al. (2001) Science
291: 319-322. The autoimmunity most likely results from the
loss of signaling by both ligands PD-Li and PD-L2. Recently,
CD80 was identified as a second receptor for PD-Li that
transduces inhibitory signals into T cells. Butte, et al.
(2007) Immunity 27: 111-122. The receptor for B7-83 and B7-H4
still remain unknown.
[0008] The best characterized co-stimulatory ligands are 87.1
and B7.2, which belong to the Ig superfamily and are expressed
on professional APCs and whose receptors are CD28 and CTLA-4
(Greenwald, et al. (2005) Annu. Rev. Immunol. 23:515-548).
CD28 is expressed by naive and activated T cells and is
critical for optimal T cell activation. In contrast, CTLA-4 is
induced upon T cell activation and inhibits T cell activation
by binding to B7.1/B7.2, impairing CD28-mediated cc-
stimulation. 87.1 and 87.2 KO mice are impaired in adaptive
immune response (Borriello, et al. (1997) Immunity 6:303-313),
whereas CTLA-4 knockout mice cannot adequately control
-4-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
inflammation and develop systemic autoimmune diseases (Tivol,
et al. (1995) Immunity 3:541-547; Waterhouse, et al. (1995)
Science 270:985-988; Chambers, et al. (1997) Immunity 7:885-
895).
[0009] The B7 family ligands have expanded to include co-
stimulatory B7-H2 (inducible T cell co-stimulator (ICOS)
ligand) and B7-H3, as well as co-inhibitory B7-H1 (PD-L1), B7-
DC (PD-L2), B7-H4 (B7S1 or B7x), and B7-H6 (Greenwald, et al.
(2005) supra; Brandt, et al. (2009) J. Exp. Med. 206:1495-
1503). Accordingly, additional CD28 family receptors have been
identified. ICOS is expressed on activated T cells and binds
to B7-H2 (Yoshinaga, et al. (1999) Nature 402:827-832). ICOS
is a positive coregulator, which is important for T cell
activation, differentiation, and function (Yoshinaga, et al.
(1999) supra; Dong, et al. (2001) Nature 409:97-101). In
contrast, PD-1 (programmed death 1) negatively regulates T
cell responses. PD-1 knockout mice develop lupus-like
autoimmune disease or autoimmune dilated cardiomyopathy
(Nishimura, et al. (1999) Immunity 11:141-151; Nishimura, et
al. (2001) Science 291:319-322). The autoimmunity most likely
results from the loss of signaling by both ligands PD-Li and
PD-L2. Recently, CD80 was identified as a second receptor for
PD-L1 that transduces inhibitory signals into T cells (Butte,
et al. (2007) Immunity 27:111-122).
[0010] The two inhibitory B7 family ligands, PD-Li and PD-L2,
have distinct expression patterns. PD-L2 is inducibly
expressed on DCs and macrophages, whereas PD-Li is broadly
expressed on both hematopoietic cells and nonhematopoietic
cell types (Okazaki & Honjo (2006) Immunology 27:195-201;
Keir, et al. (2008) Annu. Rev. Immunol. 26:677-704).
-5-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
Consistent with the immune-suppressive role of PD-1 receptor,
a study using PD-L1-1- and PD-L2-/- mice has shown that both
ligands have overlapping roles in inhibiting T cell
proliferation and cytokine production (Keir, et al. (2006) J.
Exp. Med. 203:883-895). PD-L1 deficiency enhances disease
progression in both the non-obese diabetic model of autoimmune
diabetes and the mouse model of multiple sclerosis
(experimental autoimmune encephalomyelitis (EAE); Anasari, et
al. (2003) J. Exp. Med. 198:63-69; Salama, et al. (2003) J.
Exp. Med. 198:71-78; Latchman, et al. (2004) Proc. Natl. Acad.
Sci. USA. 101:10691-10696). PD-L1-/- T cells produce elevated
levels of the proinflammatory cytokines in both disease
models. In addition, bone marrow chimera experiments have
demonstrated that the tissue expression of PD-L1 (i.e., within
pancreas) uniquely contributes to its capacity of regionally
controlling inflammation (Keir, et al. (2006) supra; Keir, et
al. (2007) J. Immunol. 179:5064-5070; Grabie, et al. (2007)
Circulation. 116:2062-2071). PD-Li is also highly expressed on
placental syncytiotrophoblasts, which critically control the
maternal immune responses to allogeneic fetus (Guleria, et al.
(2005) J. Exp. Med. 202:231-237).
[0011] Consistent with its immune-suppressive role, PD-Li
potently suppresses antitumor immune responses and helps
tumors evade immune surveillance. PD-Li can induce apoptosis
of infiltrating cytotoxic CDe T cells, which express a high
level of PD-1 (Dong, et al. (2002) Nature 409:97-101; Dong &
Chen (2003) J. Mol. Med. 81:281-287). Studies have shown that
blocking the PD-Li-PD-1 signaling pathway, in conjunction with
other immune therapies, prevents tumor progression by
enhancing antitumor cytotoxic T lymphocyte activity and
-6-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
cytokine production (Iwai, et al. (2002) Proc. Natl. Acad.
Sci. USA 99:12293-12297; Blank, et al. (2004) Cancer Res.
64:1140-1145; Blank, et al. (2005) Cancer Immunol. Immunother.
54:307-314; Geng, et al. (2006) Int. J. Cancer. 118:2657-
2664). In addition, it has been shown that PD-L1 expression on
dendritic cells promotes the induction of adaptive Foxp3+CD4+
regulatory T cells (aTreg cells), and PD-Li is a potent inducer
of aTreg cells within the tumor microenvironment (Wang, et al.
(2008) Proc. Natl. Acad. Sci. USA. 105:9331-9336).
[0012] An additional immune regulatory ligand, referred to as
V-domain Ig suppressor of T cell activation (VISTA) or PD-L3,
has been recently identified as an upregulated molecule in a T
cell transcriptional profiling screen. (Wang, et al. (2011) J.
Exp. Med. 208:577; WO 2011/120013). It has been shown that the
extracellular Ig domain of VISTA shares significant sequence
homology with the B7 family ligands PD-Li and PD-L2, albeit
with unique structural features that distinguish it from the
B7 family members.
[0013] VISTA is primarily expressed on hematopoietic cells,
and VISTA expression is highly regulated on myeloid antigen-
presenting cells (APCs) and T cells. Expression of VISTA on
antigen presenting cells (APCs) suppresses T cell responses by
engaging its counter-receptor on T cells during cognate
interactions between T cells and APCs. VISTA blockade enhances
T cell-mediated immunity in an autoimmune disease model,
suggesting its unique and non-redundant role in controlling
autoimmunity when compared with other inhibitory B7 family
ligands such as PD-Li and PD-L2. In addition, VISTA blockade
enhances anti-tumor immunity and suppressed tumor growth in
preclinical murine tumor models (WO 2011/120013). In this
-7-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
regard, therapeutic intervention of the VISTA inhibitory
pathway represents a novel approach to modulate T cell-
mediated immunity for treating diseases such as viral
infection and cancer.
Summary of the Invention
[0014] The present invention provides an isolated VISTA
antagonist that comprises a peptide that is identical to the
amino acid sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-
Ile-Lys-Arg-Ser-Cys-His), or a multimer, conjugate, analog,
derivative or mimetic thereof.
[0015] In one embodiment, the isolated VISTA antagonist
comprises a peptide which is identical to the amino acid
sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-Ile-Lys-Arg-
Ser-Cys-His), or which comprises a peptide having an amino
acid sequence that differs from SEQ ID NO:1 by at most 2 amino
acid residues, or an multimer, conjugate, analog, derivative
or mimetic thereof. In another embodiment, the isolated VISTA
antagonist comprises a peptide having an amino acid sequence
that differs from SEQ ID NO:1 by at most 1 amino acid residue,
or an multimer, conjugate, analog, derivative or mimetic
thereof. In yet another embodiment, the isolated VISTA
antagonist comprises a peptide which is identical to the amino
acid sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-Ile-Lys-
Arg-Ser-Cys-His), or a multimer, or conjugate thereof. In a
specific embodiment, the isolated VISTA antagonist consists of
the amino acid sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-
Trp-Ile-Lys-Arg-Ser-Cys-His).
-8-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
[0016] In one embodiment, the cysteine residues at positions
4 and 11 of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-Ile-Lys-Arg-
Ser-Cys-His), or their corresponding positions in a variant of
said peptide, form a disulfide bridge.
[0017] In another embodiment, the isolated VISTA antagonist
has been modified to improve binding affinity and/or
stability. In a specific embodiment, the isolated VISTA
antagonist has been modified by PEG, acetylation, XTEN,
albumin and/or multimerization.
[0018] In another embodiment, the isolated VISTA antagonist
is directly or indirectly attached to an immunoglobulin
polypeptide or a fragment thereof. The
immunoglobulin
polypeptide may comprise a human IgGl, IgG2, IgG3 or IgG4
constant region or fragment thereof.
Preferably, the
immunoglobulin polypeptide comprises a human IgG1 constant
region or fragment thereof.
[0019] In yet another embodiment, the isolated VISTA
antagonist comprises multiple, i.e., 2, 3, 4, 5, 6, 7 or more,
copies of said peptide.
[0020] In a further embodiment, the isolated VISTA antagonist
comprises another moiety that targets said peptide to a target
site. The targeting moiety may be selected from an antibody or
ligand that binds to an antigen, a receptor expressed by the
target cell or an infectious agent.
[0021] In yet a further embodiment, the isolated VISTA
antagonist is attached to another moiety or another copy of
said antagonist via a linker. The linker may be a peptide that
permits the antagonist to interact with VISTA expressed on the
surface of a target cell.
-9-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
[0022] In a further embodiment, the isolated VISTA antagonist
is directly or indirectly attached to a detectable label or
therapeutic agent.
[0023] In several of the embodiments, the isolated VISTA
antagonist binds to the extracellular domain of VISTA and
disrupts its interaction with a VISTA receptor and/or reduces
or inhibits VISTA-mediated T cell suppression.
[0024] In one embodiment, the isolated VISTA antagonist
elicits anti-tumor and/or anti-viral activity.
[0025] Additionally, the invention contemplates a composition
suitable for therapeutic, prophylactic or diagnostic use
comprising a therapeutically, prophylactically or
diagnostically effective amount of the isolated VISTA
antagonist.
[0026] In one embodiment, the composition further comprises a
pharmaceutically acceptable carrier, diluent, solubilizer,
preservative or mixture thereof.
[0027] In another embodiment, the composition further
comprises another therapeutic agent, e.g., an anti-cancer
agent, an anti-viral agent, a cytokine or an immune agonist.
In a particular embodiment, the other therapeutic agent is
selected from CTLA-4-Ig, anti-PD-1, PD-L1 or PD-L2 fusion
proteins, and EGFR antagonists.
[0028] In one embodiment, the composition is suitable for
subcutaneous administration or intravenous administration.
[0029] Moreover, the present invention further contemplates
an isolated nucleic acid sequence encoding a VISTA antagonist
peptide, analog, derivative or mimetic thereof disclosed
herein, a vector containing the isolated nucleic acid
-10-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
sequence, and a host cell comprising the isolated nucleic acid
sequence or the vector.
[0030] In one embodiment, the host cell is a mammalian cell,
a bacterial cell, a fungal cell, a yeast cell, an avian cell
or an insect cell.
[0031] The present invention further contemplates a method of
expressing a VISTA antagonist peptide, analog, derivative or
mimetic thereof comprising culturing the host cell under
conditions that provide for expression of said peptide,
analog, derivative or mimetic thereof.
[0032] Furthermore, the present invention contemplates
various uses of the isolated VISTA antagonist.
[0033] In one embodiment, the invention provides a method for
blocking, inhibiting or neutralizing VISTA-mediated T cell
suppression, comprising administering to a subject in need
thereof an effective amount of an isolated VISTA antagonist
disclosed herein or a composition containing said isolated
VISTA antagonist.
[0034] In another embodiment, the invention provides a method
for stimulating an immune response in a subject, comprising
administering to the subject in need thereof an effective
amount of an isolated VISTA antagonist disclosed herein or a
composition containing said isolated VISTA antagonist. Such a
method may be used for treating cancer in a subject.
[0035] The subject may have cancer and/or an infection
selected from the group consisting of bacterial, viral,
parasitic and fungal infections.
[0036] The bacterial infection may be caused by at least one
bacterium selected from the group consisting of Bordetella,
Borrelia, Brucella, Burkholderia, Campylobacter, Chlamydia,
-11-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
Clostridium, Corynebacterium, Enterobacter, Enterococcus,
Erwinia, Escherichia, Francisella, Haemophilus, Heliobacter,
Legionella, Leptospira, Listeria, Mycobacterium, Mycoplasma,
Neisseria, Pasteurella, Pelobacter, Pseudomonas, Rickettsia,
Salmonella, Serratia, Shigella, Staphylococcus, Streptococcus,
Treponema, Vibrio, Yersinia and Xanthomonas.
[0037] The viral infection may be caused by at least one
virus selected from the group consisting of Adenoviridae,
Papillomaviridae, Polyomaviridae, Herpesviridae, Poxviridae,
Hepadnaviridae, Parvoviridae, Astroviridae, Caliciviridae,
Piccrnaviridae, Coronoviridae, Flaviviridae, Retroviridae,
Togaviridae, Arenaviridae, Bunyaviridae,
Filoviridae,
Orthomyxoviridae, Paramyxoviridae, Rhabdoviridae, and
Reoviridae. More specifically, the virus may be adenovirus,
herpes simplex type I, herpes simplex type 2, Varicella-zoster
virus, Epstein-barr virus, cytomegalovirus, herpesvirus type
8, papillomavirus, BK virus, JC virus, smallpox, Hepatitis B,
bocavirus, parvovirus B19, astrovirus, Norwalk virus,
coxsackievirus, Hepatitis A, poliovirus, rhinovirus, severe
acute respiratory syndrome virus, Hepatitis C, yellow fever,
dengue virus, West Nile virus, rubella, Hepatitis E, human
immunodeficiency virus (HIV), influenza, guanarito virus,
Junin virus, Lassa virus, Machupo virus, Sabia virus, Crimean-
Congo hemorrhagic fever virus, ebola virus, Marburg virus,
measles virus, mumps virus, parainfluenza, respiratory
syncytial virus, human metapneumovirus, Hendra virus, Nipah
virus, rabies, Hepatitis D, rotavirus, orbivirus, coltivirus
or Banna virus.
[0038] The fungal infection may be selected from the group
consisting of thrush, candidiasis,
cryptococcosis,
-12-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
histoplasmosis, blastomycosis,
aspergillosis,
coccidioidomycosis, paracoccidiomycosis,
sporotrichosis,
zygomycosis, chromoblastomycosis, lobomycosis, mycetoma,
onychomycosis, piedra pityriasis versicolor, tinea barbae,
tinea capitis, tinea corporis, tinea cruris, tinea favosa,
tinea nigra, tinea pedis, otomycosis, phaeohyphomycosis, or
rhinosporidiosis.
[0039] The parasitic infection may be caused by at least one
parasite selected from the group consisting of Entamoeba
hystolytica, Giardia lamblia, Cryptosporidium muris,
Trypanosomatida gambiense, Trypanosomatida rhodesiense,
Trypanosomatida crusi, Leishmania mexicana, Leishmania
braziliensis, Leishmania tropica, Leishmania donovani,
Toxoplasma gondii, Plasmodium vivax, Plasmodium ovale,
Plasmodium malariae, Plasmodium falciparum, Trichomonas
vaginalis, Histomonas
meleagridis;
Secementea; Trichuris trichiura, Ascaris lumbricoides,
Enterobius vermicularis, Ancylostoma duodenale, Necator
americanus, Strongyloides stercoralis, Wuchereria bancrofti,
Dracunculus medinensis; blood flukes, liver flukes, intestinal
flukes, lung flukes; Schistosoma mansoni, Schistosoma
haematobium, Schistosoma japonicum, Fasciola hepatica,
Fasciola gigantica, Heterophyes heterophyes, and Paragonimus
westermani.
[0040] In another embodiment, the invention provides a method
for enhancing anti-cancer or anti-tumor immunity, comprising
administering to a subject in need thereof an effective amount
of an isolated VISTA antagonist disclosed herein or a
composition containing said isolated VISTA antagonist.
-13-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
[0041] In another embodiment, the invention provides a method
for treating or preventing cancer, inhibiting tumor invasion
and/or cancer metastasis, comprising administering to a
subject in need thereof an effective amount of an isolated
VISTA antagonist disclosed herein or a composition containing
said isolated VISTA antagonist.
[0042] The cancer may be selected from the group consisting
of carcinoma, lymphoma, blastoma, sarcoma, leukemia, lymphoid
malignancies, melanoma, squamous cell cancer, lung cancer
(including small-cell lung cancer, non-small cell lung cancer,
adenocarcinoma of the lung, and squamous carcinoma of the
lung), cancer of the peritoneum, hepatocellular cancer,
gastric or stomach cancer (including gastrointestinal cancer),
pancreatic cancer, glioblastoma, cervical cancer, ovarian
cancer, liver cancer, bladder cancer, hepatoma, breast cancer,
colon cancer, colorectal cancer, endometrial or uterine
carcinoma, salivary gland carcinoma, kidney or renal cancer,
liver cancer, prostate cancer, vulval cancer, thyroid cancer,
hepatic carcinoma, head and neck cancer, B-cell lymphoma
(including low grade/follicular non-Hodgkin's lymphoma (NHL);
small lymphocytic (SL) NHL; intermediate grade/follicular NHL;
intermediate grade diffuse NHL; high grade immunoblastic NHL;
high grade lymphoblastic NHL; high grade small non-cleaved
cell NHL; bulky disease NHL; mantle cell lymphoma; AIDS-
related lymphoma; and Waldenstrom's Macroglobulinemia);
chronic lymphocytic leukemia (CLL); acute lymphoblastic
leukemia (ALL); Hairy cell leukemia; chronic myeloblastic
leukemia; post-transplant lymphoproliferative disorder (PTLD),
abnormal vascular proliferation associated with phakomatoses,
-14-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
edema (such as that associated with brain tumors), and Meigs'
syndrome.
[0043] In yet another embodiment, the invention provides a
method for treating or preventing a viral infection,
comprising administering to a subject in need thereof an
effective amount of an isolated VISTA antagonist disclosed
herein or a composition containing said isolated VISTA
antagonist.
[0044] These methods may further comprise the administration
of another therapeutic agent, wherein said peptide and
therapeutic may be separately or jointly administered, at the
same or different times.
[0045] In one embodiment, the other therapeutic agent is an
anti-cancer agent, an anti-viral or other anti-infectious
agent, a cytokine or an immune agonist. Preferably, the other
therapeutic agent is selected from CTLA-4-Ig, anti-PD-1, PD-Li
or PD-L2 fusion proteins, and EGFR antagonists.
[0046] Finally, the present invention also contemplates a
method for mapping the active site of VISTA, comprising: (a)
incubating an isolated VISTA fusion protein with an isolated
VISTA antagonist comprising a peptide that is identical to the
amino acid sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-
Ile-Lys-Arg-Ser-Cys-His), or which comprises a peptide having
an amino acid sequence which differs from SEQ ID NO:1 by at
most 2 amino acid residues or an multimer, conjugate, analog,
derivative or mimetic thereof; and (b) determining the binding
site of the isolated VISTA antagonist.
[0047] In one embodiment, the active site of VISTA binds to a
VISTA receptor and mediates immune suppression.
-15-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/10S2015/012752
In another embodiment, step (b) comprises domain deletion,
domain swapping, amino acid mutagenesis, foot printing, NMR,
X-ray crystallography or homology modeling.
Brief Description of the Drawings
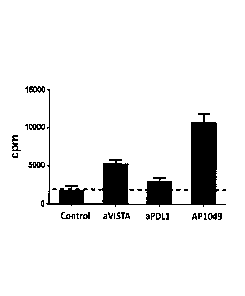
[0048] Figure 1 shows that a VISTA antagonist peptide (SEQ ID
NO:1) significantly enhances the proliferation of T cells as
compared to an anti-VISTA antibody (aVISTA) and an anti-PD--Li
antibody (aPDL1). Myeloid CD11B+ APCs were incubated with 0T2
CD4+ T cells, antigen, and a monoclonal antibody (aVISTA or
aPDL1) or AP1049. Proliferation of T cells was measured by
tritium incorporation at 72 hours.
[0049] Figure 2 shows that a VISTA antagonist peptide (SEQ ID
NO:1) significantly enhances anti-tumor immunity. Female mice
inoculated with MB49 tumors were treated with either PBS
(control) or AP1049. Tumor size was measured by caliper every
2-3 days.
Detailed Description of the Invention
[0050] In order that the invention herein described may be
fully understood, the following detailed description is set
forth. Various embodiments of the invention are described in
detail and may be further illustrated by the provided
examples.
[0051] Unless defined otherwise, all technical and scientific
terms used herein have the same meaning as those commonly
understood by one of ordinary skill in the art to which this
invention belongs. Although methods and materials similar or
equivalent to those described herein may be used in the
invention or testing of the present invention, suitable
-16-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
methods and materials are described herein. The
materials,
methods and examples are illustrative only, and are not
intended to be limiting.
Definitions
[0052] As used in the description herein and throughout the
claims that follow, the meaning of "a," "an," and "the"
includes plural reference unless the context clearly dictates
otherwise.
[0053] "Antagonist," as used herein, refers to a compound
(preferably a peptide) that opposed the physiological effects
of another compound. For example, at the receptor level, an
antagonist is a compound that opposes the receptor-associated
response normally induced by another agent that binds to and
activates the biological activity the receptor. Likewise, at
the ligand level, an antagonist is a compound that opposes the
ligand-associated response normally induced when the ligand
binds to its target receptor and/or accessory factors. In a
specific embodiment, a VISTA antagonist is a compound, e.g., a
peptide or analog, derivative or mimetic thereof, that binds
to VISTA and opposes one or more of its biological activities,
e.g., VISTA-mediated T cell suppression and/or VISTA-mediated
suppression of anti-tumor immunity, thereby enhancing T cell-
mediated immunity and/or anti-tumor immunity.
[0054] "Analog," as used herein, refers to a compound
(preferably a peptide) whose structure is related to that of a
given compound (preferably a peptide) but differs in chemical
and biological properties.
[0055] "Antigen presenting cell," as used herein, refers
broadly to professional antigen presenting cells (e.g., B
-17-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
lymphocytes, monocytes, dendritic cells, and Langerhans cells)
as well as other antigen presenting cells (e.g.,
keratinocytes, endothelial cells, astrocytes, fibroblasts, and
oligodendrocytes).
[0056] "Amino acid," as used herein refers broadly to
naturally occurring and synthetic amino acids, as well as
amino acid analogs and amino acid mimetics that function in a
manner similar to the naturally occurring amino acids.
Naturally occurring amino acids are those encoded by the
genetic code, as well as those amino acids that are later
modified (e.g., hydroxyproline, y-carboxyglutamate, and 0-
phosphoserine.) Amino acid analogs refers to compounds that
have the same basic chemical structure as a naturally
occurring amino acid (i.e., a carbon that is bound to a
hydrogen, a carboxyl group, an amino group), and an R group
(e.g., homoserine, norleucine, methionine
sulfoxide,
methionine methyl sulfonium.) Analogs
may have modified R
groups (e.g., norleucine) or modified peptide backbones, but
retain the same basic chemical structure as a naturally
occurring amino acid. Amino acid mimetics refers to chemical
compounds that have a structure that is different from the
general chemical structure of an amino acid, but that
functions in a manner similar to a naturally occurring amino
acid.
[0057] "Allergic disease," as used herein, refers broadly to
a disease involving allergic reactions. More specifically, an
"allergic disease" is defined as a disease for which an
allergen is identified, where there is a strong correlation
between exposure to that allergen and the onset of
pathological change, and where that pathological change has
-18-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
been proven to have an immunological mechanism. Herein, an
immunological mechanism means that leukocytes show an immune
response to allergen stimulation.
[0058] "Autoimmune disease" as used herein, refers broadly to
a disease or disorder arising from and directed against an
individual's own tissues or a co-segregate or manifestation
thereof or resulting condition therefrom.
[0059] "Cancer," as used herein, refers broadly to any
neoplastic disease (whether invasive or metastatic)
characterized by abnormal and uncontrolled cell division
causing malignant growth or tumor (e.g., unregulated cell
growth.)
[0060] "Conservatively modified variants," as used herein,
applies to both amino acid and nucleic acid sequences, and
with respect to particular nucleic acid sequences, refers
broadly to conservatively modified variants refers to those
nucleic acids which encode identical or essentially identical
amino acid sequences, or where the nucleic acid does not
encode an amino acid sequence, to essentially identical
sequences. Because of the degeneracy of the genetic code, a
large number of functionally identical nucleic acids encode
any given protein. "Silent
variations" are one species of
conservatively modified nucleic acid variations. Every
nucleic acid sequence herein which encodes a polypeptide also
describes every possible silent variation of the nucleic acid.
One of skill will recognize that each codon in a nucleic acid
(except AUG, which is ordinarily the only codon for
methionine, and TGG, which is ordinarily the only codon for
tryptophan) may be modified to yield a functionally identical
molecule.
-19-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
[0061] "Costimulatory receptor," as used herein, refers
broadly to receptors which transmit a costimulatory signal to
an immune cell, e.g., CD28 or ICOS.
[0062] "Cytoplasmic domain," as used herein, refers broadly
to the portion of a protein which extends into the cytoplasm
of a cell.
[0063] "Derivative" or "peptide derivative," as used herein,
contain a modification of one or more amino acid residues or a
linker group or other covalently linked group. Non-limiting
examples of derivatives include N-acyl derivatives of the
amino terminal or of another free amino group, esters of the
carboxyl terminal or of another free carboxyl or hydroxy
group, amides of the carboxyl terminal or of another free
carboxyl group produced by reaction with ammonia or with a
suitable amine, glycosylated derivatives, hydroxylated
derivatives, nucleotidylated derivatives, ADP-ribosylated
derivatives, pegylated derivatives,
phosphorylated
derivatives, derivatives conjugated to lipophilic moieties,
and derivatives conjugated to an antibody or other biological
ligand. Also included among the chemical derivatives are those
obtained by modification of the peptide bond -CO-NH-, for
example by reduction to -CH2-NH- or alkylation to -00-
N(alkyl)-. Preferred
derivatisation include, but are not
limited tom C-terminal amidation and N-terminal acetylation,
which removes the negative charge of the C terminus or removes
the positive charge at the N-terminus, respectively. Blocking
of the C- or N- terminus, such as by C-terminal amidation or
N-terminal acetylation, may improve proteolytic stability due
to reduced susceptibility to exoproteolytic digestion.
-20-
CA 029926 2016--1.4
WO 2015/109340 PCT/11S2015/012752
Peptide derivatives having a C-terminal amide are represented
with "NH2" at the C-terminus.
[0064] "Diagnostic," as used herein, refers broadly to
identifying the presence or nature of a pathologic condition.
Diagnostic methods differ in their sensitivity and
specificity. The "sensitivity" of a diagnostic assay is the
percentage of diseased individuals who test positive (percent
of "true positives"). Diseased
individuals not detected by
the assay are "false negatives." Subjects
who are not
diseased and who test negative in the assay are termed "true
negatives." The "specificity" of a diagnostic assay is 1
minus the false positive rate, where the "false positive" rate
is defined as the proportion of those without the disease who
test positive. While a particular diagnostic method may not
provide a definitive diagnosis of a condition, it suffices if
the method provides a positive indication that aids in
diagnosis.
[0065] "Diagnosing," as used herein refers broadly to
classifying a disease or a symptom, determining a severity of
the disease, monitoring disease progression, forecasting an
outcome of a disease and/or prospects of recovery. The term
"detecting" may also optionally encompass any of the
foregoing. Diagnosis
of a disease according to the present
invention may, in some embodiments, be affected by determining
a level of a polynucleotide or a polypeptide of the present
invention in a biological sample obtained from the subject,
wherein the level determined can be correlated with
predisposition to, or presence or absence of the disease. It
should be noted that a "biological sample obtained from the
-21-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
subject" may also optionally comprise a sample that has not
been physically removed from the subject.
[0066] "Effective amount," as used herein, refers broadly to
the amount of a compound, antibody, antigen, or cells that,
when administered to a patient for treating a disease, is
sufficient to effect such treatment for the disease. The
effective amount may be an amount effective for prophylaxis,
and/or an amount effective for prevention. The
effective
amount may be an amount effective to reduce, an amount
effective to prevent the incidence of signs/symptoms, to
reduce the severity of the incidence of signs/symptoms, to
eliminate the incidence of signs/symptoms, to slow the
development of the incidence of signs/symptoms, to prevent the
development of the incidence of signs/symptoms, and/or effect
prophylaxis of the incidence of signs/symptoms. The
"effective amount" may vary depending on the disease and its
severity and the age, weight, medical history, susceptibility,
and pre-existing conditions, of the patient to be treated.
The term "effective amount" is synonymous with
"therapeutically effective amount" for purposes of this
invention.
[0067] "Extracellular domain," as used herein refers broadly
to the portion of a protein that extend from the surface of a
cell.
[0068] "Expression vector," as used herein, refers broadly to
any recombinant expression system for the purpose of
expressing a nucleic acid sequence of the invention in vitro
or in vivo, constitutively or inducibly, in any cell,
including prokaryotic, yeast, fungal, plant, insect or
mammalian cell. The term
includes linear or circular
-22-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
expression systems. The term includes expression systems that
remain episomal or integrate into the host cell genome. The
expression systems can have the ability to self-replicate or
not, i.e., drive only transient expression in a cell. The
term includes recombinant expression cassettes which contain
only the minimum elements needed for transcription of the
recombinant nucleic acid.
[0069] "Homology," as used herein, refers broadly to a degree
of similarity between a nucleic acid sequence and a reference
nucleic acid sequence or between a polypeptide sequence and a
reference polypeptide sequence. Homology
may be partial or
complete. Complete homology indicates that the nucleic acid
or amino acid sequences are identical. A partially homologous
nucleic acid or amino acid sequence is one that is not
identical to the reference nucleic acid or amino acid
sequence. The
degree of homology can be determined by
sequence comparison. The term "sequence identity" may be used
interchangeably with 'homology."
[0070] "Host cell," as used herein, refers broadly to refer
to a cell into which a nucleic acid molecule of the invention,
such as a recombinant expression vector of the invention, has
been introduced. Host cells may be prokaryotic cells (e.g., E.
coli), or eukaryotic cells such as yeast, insect (e.g., SF9),
amphibian, or mammalian cells such as CHO, HeLa, HEK-293,
e.g., cultured cells, explants, and cells in vivo. The terms
"host cell" and "recombinant host cell" are used
interchangeably herein. It should be understood that such
terms refer not only to the particular subject cell but to the
progeny or potential progeny of such a cell. Because certain
modifications may occur in succeeding generations due to
-23-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
either mutation or environmental influences, progeny may not,
in fact, be identical to the parent cell, but are still
included within the scope of the term as used herein.
[0071] "Immune response," as used herein, refers broadly to T
cell-mediated and/or B cell-mediated immune responses that are
influenced by modulation of T cell costimulation. Exemplary
immune responses include B cell responses (e.g., antibody
production) T cell responses (e.g., cytokine production, and
cellular cytotoxicity) and activation of cytokine responsive
cells, e.g., macrophages. As used herein, the term "down
modulation" with reference to the immune response includes a
diminution in any one or more immune responses, while the term
"up modulation" with reference to the immune response includes
an increase in any one or more immune responses. It will be
understood that up modulation of one type of immune response
may lead to a corresponding downmodulation in another type of
immune response. For example, up modulation of the production
of certain cytokines (e.g., IL-10) can lead to downmodulation
of cellular immune responses.
[0072] "Inflammatory disease," as used herein, refers broadly
to chronic or acute inflammatory diseases.
[0073] "Detectable label" as used herein, refers broadly to a
composition detectable by spectroscopic, photochemical,
biochemical, immunochemical, chemical, or other physical
means.
[0074] "Mimetic" or "peptidomimetic," as used herein, refers
to a fully or partially synthetic peptide that has the
activity of a given peptide. Such a mimetic or peptidomimetic
comprises one or more amino acid residues that is an
artificial chemical mimetic of a corresponding naturally
-24-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
occurring amino acid, naturally occurring amino acid polymers
and non-naturally occurring amino acid polymers.
[0075] Modifications of the VISTA and VISTA conjugate
polypeptides described herein include, but are not limited to
N-terminus modification, C-terminus modification, peptide bond
modification (e.g., CH2-NH, CH2-S, CH2-S=C, 0=C-NH, CH2-0, CH2-
CH2, S=C-NH, CH=CH or CF=CH), backbone modifications, and
residue modification, e.g., by the addition of carbohydrate
residues to form glycoproteins, by the addition of chemical
residues such as PEG and/or XTEN, etc. Methods for preparing
peptidomimetic compounds are well known in the art. Martin,
(2010).
[0076] "Nucleic acid" or "nucleic acid sequence," as used
herein, refers broadly to a deoxy-ribonucleotide or
ribonucleotide oligonucleotide in either single- or double-
stranded form. The term
encompasses nucleic acids, i.e.,
oligonucleotides, containing known analogs of natural
nucleotides. The term
also encompasses nucleic-acid-like
structures with synthetic backbones. Unless
otherwise
indicated, a particular nucleic acid sequence also implicitly
encompasses conservatively modified variants thereof (e.g.,
degenerate codon substitutions) and complementary sequences,
as well as the sequence explicitly indicated. The term
nucleic acid is used interchangeably with gene, cONA, mRNA,
oligonucleotide, and polynucleotide.
[0077] "Polypeptide," "peptide" and "protein," are used
interchangeably and refer broadly to a polymer of amino acid
residues. The terms apply to amino acid polymers in which one
or more amino acid residue is an analog or mimetic of a
corresponding naturally occurring amino acid, as well as to
-25-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
naturally occurring amino acid polymers. The terms apply to
amino acid polymers in which one or more amino acid residue is
an artificial chemical mimetic of a corresponding naturally
occurring amino acid, as well as to naturally occurring amino
acid polymers and non-naturally occurring amino acid polymer.
Polypeptides can be modified, e.g., by the addition of
carbohydrate residues to form glycoproteins. The terms
"polypeptide," "peptide" and "protein" include glycoproteins,
as well as non-glycoproteins.
[0078] "Prophylactically effective amount," as used herein,
refers broadly to the amount of a compound that, when
administered to a patient for prophylaxis of a disease or
prevention of the reoccurrence of a disease, is sufficient to
effect such prophylaxis for the disease or reoccurrence. The
prophylactically effective amount may be an amount effective
to prevent the incidence of signs and/or symptoms. The
"prophylactically effective amount" may vary depending on the
disease and its severity and the age, weight, medical history,
predisposition to conditions, preexisting conditions, of the
patient to be treated.
[0079] "Prophylaxis," as used herein, refers broadly to a
course of therapy where signs and/or symptoms are not present
in the patient, are in remission, or were previously present
in a patient.
Prophylaxis includes preventing disease
occurring subsequent to treatment of a disease in a patient.
Further, prevention includes treating patients who may
potentially develop the disease, especially patients who are
susceptible to the disease (e.g., members of a patent
population, those with risk factors, or at risk for developing
the disease).
-26-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
[0080] "Recombinant" as used herein, refers broadly with
reference to a product, e.g., to a cell, or nucleic acid,
protein, or vector, indicates that the cell, nucleic acid,
protein or vector, has been modified by the introduction of a
heterologous nucleic acid or protein or the alteration of a
native nucleic acid or protein, or that the cell is derived
from a cell so modified. Thus, for example, recombinant cells
express genes that are not found within the native (non-
recombinant) form of the cell or express native genes that are
otherwise abnormally expressed, under expressed or not
expressed at all.
[0081] "Sequence identity," as used herein, refers broadly to
a degree of similarity between a nucleic acid sequence and a
reference nucleic acid sequence or between a polypeptide
sequence and a reference polypeptide sequence. Sequence
identity (also synonymous with "homology") may be partial or
complete. Complete
sequence identity indicates that the
nucleic acid or amino acid sequences are identical, i.e., 100%
sequence identity. A
partially homologous nucleic acid or
amino acid sequence is one that is not identical to the
reference nucleic acid or amino acid sequence. The degree of
homology can be determined by sequence comparison, e.g., 60%
identity, 70% identity, 80% identity, 90% identity, 95%
identity, 97% identity, 98% identity, or 99% identity.
[0082] "Signs" of disease, as used herein, refers broadly to
any abnormality indicative of disease, discoverable on
examination of the patient; an objective indication of
disease, in contrast to a symptom, which is a subjective
indication of disease.
-27-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
[0083] "Subject," as used herein, refers broadly to any
animal that is in need of treatment either to alleviate a
disease state or to prevent the occurrence or reoccurrence of
a disease state. Also,
"subject" as used herein, refers
broadly to any animal that has risk factors, a history of
disease, susceptibility, symptoms, and signs, was previously
diagnosed, is at risk for, or is a member of a patient
population for a disease. The
subject may be a clinical
patient such as a human or a veterinary patient such as a
companion, domesticated, livestock, exotic, or zoo animal.
The term "subject" may be used interchangeably with the term
"patient."
[0084] "Symptoms" of disease as used herein, refers broadly
to any morbid phenomenon or departure from the normal in
structure, function, or sensation, experienced by the patient
and indicative of disease.
[0085] "T cell," as used herein, refers broadly to CD4+ T
cells and CD8+ T cells. The term T cell also includes both T
helper 1 type T cells and T helper 2 type T cells.
[0086] "Therapy," "therapeutic," "treating," or "treatment",
as used herein, refers broadly to treating a disease,
arresting, or reducing the development of the disease or its
clinical symptoms, and/or relieving the disease, causing
regression of the disease or its clinical symptoms. Therapy
encompasses prophylaxis, treatment, remedy, reduction,
alleviation, and/or providing relief from a disease, signs,
and/or symptoms of a disease. Therapy
encompasses an
alleviation of signs and/or symptoms in patients with ongoing
disease signs and/or symptoms (e.g., inflammation, pain).
Therapy also encompasses "prophylaxis". The term "reduced",
-28-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/11S2015/012752
for purpose of therapy, refers broadly to the clinical
significant reduction in signs and/or symptoms. Therapy
includes treating relapses or recurrent signs and/or symptoms
(e.g., inflammation, pain). Therapy
encompasses but is not
limited to precluding the appearance of signs and/or symptoms
anytime as well as reducing existing signs and/or symptoms and
eliminating existing signs and/or symptoms. Therapy includes
treating chronic disease ("maintenance") and acute disease.
For example, treatment includes treating or preventing
relapses or the recurrence of signs and/or symptoms (e.g.,
inflammation, pain).
[0087] "Transmembrane domain," as used herein, refers broadly
to an amino acid sequence of about 15 amino acid residues in
length which spans the plasma membrane. More preferably, a
transmembrane domain includes about at least 20, 25, 30, 35,
40, or 45 amino acid residues and spans the plasma membrane.
Transmembrane domains are rich in hydrophobic residues, and
typically have an alpha-helical structure. In an embodiment,
at least 50%, 60%, 70%, 80%, 90%, 95% or more of the amino
acids of a transmembrane domain are hydrophobic, e.g.,
leucines, isoleucines, tyrosines, or
tryptophans.
Transmembrane domains are described in, for example, Zagotta,
et al. (1996) Annu. Rev. Neurosci. 19:235-263.
[0088] "Tumor," as used herein, refers broadly to at least
one cell or cell mass in the form of a tissue neoformation, in
particular in the form of a spontaneous, autonomous and
irreversible excess growth, which is more or less
disinhibited, of endogenous tissue, which growth is as a rule
associated with the more or less pronounced loss of specific
cell and tissue functions. This cell or cell mass is not
-29-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
effectively inhibited, in regard to its growth, by itself or
by the regulatory mechanisms of the host organism, e.g.,
melanoma or carcinoma. Tumor antigens not only include
antigens present in or on the malignant cells themselves, but
also include antigens present on the stromal supporting tissue
of tumors including endothelial cells and other blood vessel
components.
[0089] "Vector," as used herein, refers broadly to a nucleic
acid molecule capable of transporting another nucleic acid
molecule to which it has been linked. One type of vector is a
"plasmid", which refers to a circular double stranded DNA loop
into which additional DNA segments may be ligated. Another
type of vector is a viral vector, wherein additional DNA
segments may be ligated into the viral genome. Certain vectors
are capable of autonomous replication in a host cell into
which they are introduced (e.g., bacterial vectors having a
bacterial origin of replication and episomal mammalian
vectors). Other vectors (e.g., non-episomal mammalian vectors)
are integrated into the genome of a host cell upon
introduction into the host cell, and thereby are replicated
along with the host genome. Moreover, certain vectors are
capable of directing the expression of genes to which they are
operatively linked. Vectors are referred to herein as
"recombinant expression vectors" or simply "expression
vectors". In general, expression vectors of utility in
recombinant DNA techniques are often in the form of plasmids.
In the present specification, "plasmid" and "vector" may be
used interchangeably as the plasmid is the most commonly used
form of vector. However, the invention is intended to include
such other forms of expression vectors, such as viral vectors
-30-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
(e.g., replication defective retroviruses, adenoviruses and
adeno-associated viruses), which serve equivalent functions.
The techniques and procedures are generally performed
according to conventional methods well known in the art and as
described in various general and more specific references that
are cited and discussed throughout the present specification.
See, e.g., Sambrook, et al. (2001) Molec. Cloning: Lab. Manual
[3rd Ed] Cold Spring Harbor Laboratory Press. Standard
techniques may be used for recombinant DNA, oligonucleotide
synthesis, and tissue culture, and transformation (e.g.,
electroporation, lipofection). Enzymatic reactions and
purification techniques may be performed according to
manufacturer's specifications or as commonly accomplished in
the art or as described herein.
[0090] The nomenclatures utilized in connection with, and the
laboratory procedures and techniques of, analytical chemistry,
synthetic organic chemistry, and medicinal and pharmaceutical
chemistry described herein are those well known and commonly
used in the art. Standard techniques may be used for chemical
syntheses, chemical analyses, pharmaceutical preparation,
formulation, and delivery, and treatment of patients.
VISTA and VISTA Antagonists
[0091] This application relates to a peptide antagonist that
can recognize and suppress the inhibitory activity of VISTA.
This peptide, designated herein as AP1049, was discovered
through phage display and shown to exhibit superior
bioactivity when compared to an anti-VISTA monoclonal
antibody. Given its neutralizing activity, AP1049 can be used
to, e.g., treat cancer and/or pathogenic, i.e., bacterial,
-31-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
fungal, parasite or viral infections and enhance anti-tumor
immune responses and suppress tumor growth.
[0092] Accordingly, the present invention is a VISTA
antagonistic peptide, as well as multimers, conjugates,
analogs, derivatives and mimetics thereof and methods of using
this peptide to inhibit or suppress the activity of VISTA. As
used herein, the term "peptide" denotes an amino acid polymer
that is composed of at least two amino acids covalently linked
by an amide bond. Peptides of the present invention are
desirably 10 to 20 residues in length, or more desirably 12 to
15 residues in length. In certain embodiments, a VISTA
antagonistic peptide is a 12 to 20 residue peptide containing
the amino acid sequence of SEQ ID NO:l. In other embodiments
of the present invention, the isolated VISTA antagonist
comprises a peptide that is identical to the amino acid
sequence of SEQ ID NO:1 (Ser-Ser-Ala-Cys-Asp-Trp-Ile-Lys-Arg-
Ser-Cys-His), or which comprises a peptide having an amino
acid sequence that differs from SEQ ID NO:1 by at most 1 amino
acid residue or at most 2 amino acid residues, or an multimer,
conjugate, analog, derivative or mimetic thereof. In yet
other embodiments of the invention, the isolated VISTA
antagonist consists of the amino acid sequence of SEQ ID NO:1
(Ser-Ser-Ala-Cys-Asp-Trp-Ile-Lys-Arg-Ser-Cys-His).
[0093] In certain embodiments of the present invention,
cysteine residues at positions 4 and 11 of the VISTA
antagonistic peptide (or their corresponding positions in a
variant of the VISTA antagonist) form a disulfide bridge.
[0094] In accordance with the present invention, multimers,
conjugates, analogs, derivatives and mimetics of the peptide
of the invention are also provided.
-32-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/11S2015/012752
[0095] An analog is a molecule that differs in chemical
structure from a parent compound, for example a homolog
(differing by an increment in the chemical structure, such as
a difference one amino acid residue), a structure that differs
by one or more functional groups, or a change in ionization.
Structural analogs are often found using quantitative
structure activity relationships (QSAR), with techniques such
as those disclosed in Remington (The Science and Practice of
Pharmacology, 19th Edition (1995), chapter 28).
[0096] Analogs can be prepared by modifying the amino acids
sequence of SEQ ID NO:l. The simplest modifications involve
the substitution of one or more amino acids for amino acids
having similar physiochemical and/or structural properties.
These so-called conservative substitutions are likely to have
minimal impact on the activity and/or structure of the
resultant peptide. Examples of conservative substitutions
include substituting a serine with a threonine, substituting
alanine with a serine or valine, substituting aspartic acid
with glutamic acid, substituting tryptophan with a tyrosine,
substituting isoleucine with leucine or valine, substituting
arginine with lysine, and/or substituting histidine with
arginine or lysine. Conservative substitutions generally
maintain (a) the structure of the peptide backbone in the area
of the substitution, for example, as a helical conformation,
(b) the charge or hydrophobicity of the molecule at the target
site, or (c) the bulk of the side chain.
[0097] Amino acid substitutions are typically classified in
one or more categories, including polar, hydrophobic, acidic,
basic and aromatic, according to their side chains. Examples
of polar amino acids include those having side chain
-33-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
functional groups such as hydroxyl, sulfhydryl, and amide, as
well as the acidic and basic amino acids. Polar amino acids
include, without limitation, asparagine, cysteine, glutamine,
histidine, selenocysteine, serine, threonine, tryptophan and
tyrosine. Examples of hydrophobic or non-polar amino acids
include those residues having non-polar aliphatic side chains,
such as, without limitation, leucine, isoleucine, valine,
glycine, alanine, proline, methionine and phenylalanine.
Examples of basic amino acid residues include those having a
basic side chain, such as an amino or guanidino group. Basic
amino acid residues include, without limitation, arginine,
homolysine and lysine. Examples of acidic amino acid residues
include those having an acidic side chain functional group,
such as a carboxy group. Acidic amino acid residues include,
without limitation aspartic acid and glutamic acid. Aromatic
amino acids include those having an aromatic side chain group.
Examples of aromatic amino acids include, without limitation,
biphenylalanine, histidine, 2-
napthylalananine,
pentafluorophenylalanine, phenylalanine, tryptophan and
tyrosine. It is noted that some amino acids are classified in
more than one group, for example, histidine, tryptophan and
tyrosine are classified as both polar and aromatic amino
acids. Additional amino acids that are classified in each of
the above groups are known to those of ordinary skill in the
art.
[0098] As used herein, a peptide derivative is a molecule
which retains the primary amino acids of the peptide, however,
the N-terminus, C-terminus, and/or one or more of the side
chains of the amino acids therein have been chemically altered
or derivatized. Such derivatized peptides include, for
-34-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
example, naturally occurring amino acid derivatives, for
example, 4-hydroxyproline for proline, 5-hydroxylysine for
lysine, homoserine for serine, ornithine for lysine, and the
like. Other derivatives or modifications include, e.g., a
label, such as fluorescein or tetramethylrhodamine; or one or
more post-translational modifications such as acetylation,
amidation, formylation, hydroxylation,
methylation,
phosphorylation, sulfatation, glycosylation, or lipidation.
Indeed, certain chemical modifications, in particular N-
terminal glycosylation, have been shown to increase the
stability of peptides in human serum (Powell et al. (1993)
Pharma. Res. 10:1268-1273). Peptide derivatives also include
those with increased membrane permeability obtained by N-
myristoylation (Brand, et al. (1996) Am. J. Physiol. Cell.
Physiol. 270:C1362-C1369).
[0099] In addition, a peptide derivative of the invention can
include a cell-penetrating sequence which facilitates,
enhances, or increases the transmembrane transport or
intracellular delivery of the peptide into a cell. For
example, a variety of proteins, including the HIV-1 Tat
transcription factor, Drosophila Antennapedia transcription
factor, as well as the herpes simplex virus VP22 protein have
been shown to facilitate transport of proteins into the cell
(Wadia and Dowdy (2002) Curr. Opin. Biotechnol. 13:52-56).
Further, an arginine-rich peptide (Futaki (2002) Int. J.
Pharm. 245:1-7), a polylysine peptide containing Tat PTD
(Hashida, et al. (2004) Br. J. Cancer 90(6):1252-8), Pep-1
(Deshayes, et al. (2004) Biochemistry 43(6):1449-57) or an
HSP70 protein or fragment thereof (WO 00/31113) is suitable
-35-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
for enhancing intracellular delivery of a peptide or mimetic
of the invention into the cell.
[00100] While a peptide of the invention can be derivatized
with by one of the above indicated modifications, it is
understood that a peptide of this invention may contain more
than one of the above described modifications within the same
peptide.
[00101] A mimetic or peptidomimetic refers to a synthetic
chemical compound which has substantially the same structural
and/or functional characteristics of a peptide of the
invention. The mimetic can be entirely composed of synthetic,
non-natural amino acid analogues, or can be a chimeric
molecule including one or more natural peptide amino acids and
one or more non-natural amino acid analogs. The mimetic can
also incorporate any number of natural amino acid conservative
substitutions as long as such substitutions do not destroy the
activity of the mimetic. Routine testing can be used to
determine whether a mimetic has the requisite activity, e.g.,
that it can inhibit the activity of VISTA.
[00102] The phrase "substantially the same," when used in
reference to a mimetic or peptidomimetic, means that the
mimetic or peptidomimetic has one or more activities or
functions of the referenced molecule, e.g., the ability to
enhance T cell proliferation.
[00103] There are clear advantages for using a mimetic of a
given peptide. For example, there are considerable cost
savings and improved patient compliance associated with
peptidomimetics, since they can be administered orally
compared with parenteral administration for peptides.
-36-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/11S2015/012752
Furthermore, peptidomimetics are much cheaper to produce than
peptides.
[00104] Thus, a peptide of this invention has utility in the
development of such small chemical compounds with similar
biological activities and therefore with similar therapeutic
utilities. The techniques of developing peptidomimetics are
conventional. For example, peptide bonds can be replaced by
non-peptide bonds or non-natural amino acids that allow the
peptidomimetic to adopt a similar structure, and therefore
biological activity, to the original peptide. Further
modifications can also be made by replacing chemical groups of
the amino acids with other chemical groups of similar
structure. The development of peptidomimetics can be aided by
determining the tertiary structure of the original peptide by
NMR spectroscopy, crystallography and/or computer-aided
molecular modeling. These techniques aid in the development of
novel compositions of higher potency and/or greater
bioavailability and/or greater stability than the original
peptide (Dean (1994) BioEssays 16:683-687; Cohen & Shatzmiller
(1993) J. Mol. Graph. 11:166-173; Wiley & Rich (1993) Med.
Res. Rev. 13:327-384; Moore (1994) Trends Pharmacol. Sci.
15:124-129; Hruby (1993) Biopolymers 33:1073-1082; Bugg, et
al. (1993) Sci. Am. 269:92-98). Once a potential
peptidomimetic compound is identified, it may be synthesized
and assayed using an assay described herein or any other
appropriate assay for monitoring VISTA activity.
[00105] Peptide mimetic compositions can contain any
combination of non-natural structural components, which are
typically from three structural groups: residue linkage groups
other than the natural amide bond ("peptide bond") linkages;
-37-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
non-natural residues in place of naturally occurring amino
acid residues; residues which induce secondary structural
mimicry, i.e., induce or stabilize a secondary structure,
e.g., a beta turn, gamma turn, beta sheet, alpha helix
conformation, and the like; or other changes which confer
resistance to proteolysis. For example, a peptide can be
characterized as a mimetic when one or more of the residues
are joined by chemical means other than an amide bond.
Individual peptidomimetic residues can be joined by amide
bonds, non-natural and non-amide chemical bonds other chemical
bonds or coupling means including, for example,
glutaraldehyde, N-hydroxysuccinimide esters, bifunctional
maleimides, N,N'-dicyclohexylcarbodiimide (DCC) or N,N'-
diisopropyl-carbodiimide (DIC). Linking groups alternative to
the amide bond include, for example, ketomethylene (e.g., -
C(=0)-CH2- for -C(=0)-NH-), aminomethylene (CH2-NH), ethylene,
olefin (CH=CH), ether (CH2-0), thioether (CH2-S), tetrazole
(CN4-), thiazole, retroamide, thioamide, or ester (see, e.g.,
Spatola (1983) in Chemistry and Biochemistry of Amino Acids,
Peptides and Proteins, 7:267-357, "Peptide and Backbone
Modifications," Marcel Decker, NY).
[00106] As discussed, a peptide can be characterized as a
mimetic by containing one or more non-natural residues in
place of a naturally occurring amino acid residue. Non-natural
residues are known in the art. Particular non-limiting
examples of non-natural residues useful as mimetics of natural
amino acid residues are mimetics of aromatic amino acids
include, for example, D- or L-naphylalanine; D- or L-
phenylglycine; D- or L-2 thieneylalanine; D- or L-1, -2, 3-,
or 4-pyreneylalanine; D- or L-3 thieneylalanine; D- or L-(2-
-38-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
pyridiny1)-alanine; D- or L-(3-pyridiny1)-alanine; D- or L-(2-
pyraziny1)-alanine; D- or L-(4-isopropyl)-phenylglycine; D-
(trifluoromethyl)-phenylglycine; D-
(trifluoromethyl)-
phenylalanine; D-p-fluoro-phenylalanine; D- or L-p-
biphenylphenylalanine; D- or L-p-
methoxy-biphenyl-
phenylalanine; and D- or L-2-indole(alkyl)alanines, where
alkyl can be substituted or unsubstituted methyl, ethyl,
propyl, hexyl, butyl, pentyl, isopropyl, iso-butyl, sec-
isotyl, iso-pentyl, or a non-acidic amino acid. Aromatic rings
of a non-natural amino acid that can be used in place a
natural aromatic ring include, for example, thiazolyl,
thiophenyl, pyrazolyl, benzimidazolyl, naphthyl, furanyl,
pyrrolyl, and pyridyl aromatic rings.
[00107] Mimetics of acidic amino acids can be generated by
substitution with non-carboxylate amino acids while
maintaining a negative charge; (phosphono)alanine; and
sulfated threonine. Carboxyl side groups (e.g., aspartyl or
glutamyl) can also be selectively modified by reaction with
carbodiimides (R'-N-C-N-R') including, for example, 1-
cyclohexy1-3(2-morpholinyl-(4-ethyl)carbodiimide or 1-ethyl-
3(4-azonia-4,4-dimetholpentyl)carbodiimide. Aspartyl or
glutamyl groups can also be converted to asparaginyl and
glutaminyl groups by reaction with ammonium ions.
[00108] Lysine mimetics can be generated (and amino terminal
residues can be altered) by reacting lysinyl with succinic or
other carboxylic acid anhydrides. Lysine and other alpha-
amino-containing residue mimetics can also be generated by
reaction with imidoesters, such as methyl picolinimidate,
pyridoxal phosphate, pyridoxal,
chloroborohydride,
trinitrobenzenesulfonic acid, 0-methylisourea, 2,4,
-39-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
pentanedione, and transamidase-catalyzed reactions with
glyoxylate.
[00109] One or more residues can also be replaced by an amino
acid (or peptidomimetic residue) of the opposite chirality.
Thus, any amino acid naturally occurring in the L-
configuration (which can also be referred to as R or S,
depending upon the structure of the chemical entity) can be
replaced with the same amino acid or a mimetic, but of the
opposite chirality, referred to as the D-amino acid, but which
can additionally be referred to as the R- or S-form.
[00110] As will be appreciated by one skilled in the art, a
peptidomimetic of the present invention can also include one
or more of the modifications described herein for derivatized
peptides, e.g., a detectable label (such as an effector label
or a radionuclide), a therapeutic agent (such as a
chemotherapeutic agent), one or more post-translational
modifications, or cell-penetrating sequence.
[00111] For example, the VISTA antagonists described herein
may be modified post-translationally to add effector labels
such as chemical linkers, detectable labels such as for
example fluorescent dyes, enzymes, substrates, bioluminescent
materials, radioactive materials, and chemiluminescent labels,
or functional labels such as for example streptavidin, avidin,
biotin, a cytotoxin, a cytotoxic agent, and radioactive
materials. Further exemplary enzymes include, but are not
limited to, horseradish peroxidase, acetylcholinesterase,
alkaline phosphatase, P-galactosidase and luciferase. Further
exemplary fluorescent materials include, but are not limited
to, rhodamine, fluorescein, fluorescein isothiocyanate,
-40-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
umbelliferone, dichlorotriazinylamine, phycoerythrin and
dansyl chloride. Further
exemplary chemiluminescent labels
include, but are not limited to, luminol. Further exemplary
bioluminescent materials include, but are not limited to,
luciferin, luciferase, and aequorin. Further
exemplary
radioactive materials include, but are not limited to,
bismuth-213 (2133s), carbon-14 (14C), carbon-11 (11C), chlorine-
18 (C1"), chromium-51 (51Cr), cobalt-57 (57Co), cobalt-60
("Co), copper-64 ("Cu), copper-67 (57Cu), dysprosium-165
(165Dy), erbium-169 (169Er), fluorine-18 (19F), gallium-67
(67Ga), gallium-68 ("Ga), germanium-68 ("Ge), holmium-166
(166Ho), indium-111 (luIn), iodine-125 (1251) ,
iodine-123 (1241),
iodine-124 (1241), iodine-131 (1311), iridium-192 (192Ir), iron-
59 ("Fe), krypton-81 ("Kr), lead-212 (212Pb), lutetium-177
(177Lu), molybdenum-99 ("Mo), nitrogen-13 (13N), oxygen-15
(150), palladium-103 ('"Pd), phosphorus-32 (92P), potassium-42
(42K), rhenium-186 (3.86Ke), rhenium-188 (188Re), rubidium-81
("Rb), rubidium-82 ("Rb), samarium-153 (153Sm), selenium-75
(75Se), sodium-24 (24Na), strontium-82 ("Sr), strontium-89
("Sr), sulfur 35 (35S), technetium-99m ("Tc), thallium-201
(291T1), tritium (9H), xenon-133 (133Xe), ytterbium-169 (169yb),
ytterbium-177 (177Yb), and yttrium-90 ("Y).
[00112] Additionally, the VISTA antagonists provided herein
may be modified to add a therapeutic agent including, but not
limited to, chemotherapeutic agents such as carboplatin,
cisplatin, paclitaxel, gemcitabine,
calicheamicin,
doxorubicin, 5-fluorouracil, mitomycin C, actinomycin D,
cyclophosphamide, vincristine, bleomycin, VEGF antagonists,
EGFR antagonists, platins, taxols, irinotecan, 5-fluorouracil,
gemcytabine, leucovorine, steroids,
cyclophosphamide,
-41-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
melphalan, vinca alkaloids (e.g., vinblastine, vincristine,
vindesine and vinorelbine), mustines, tyrosine kinase
inhibitors, radiotherapy, sex hormone antagonists, selective
androgen receptor modulators, selective estrogen receptor
modulators, PDGF antagonists, TNF antagonists, IL-1
antagonists, interleukins (e.g., IL-12 or IL-2), IL-12R
antagonists, Toxin conjugated monoclonal antibodies, tumor
antigen specific monoclonal antibodies, Erbitux , Avastie,
Pertuzumab, anti-CD20 antibodies, Rituxan , ocrelizumab,
ofatumumab, DXL625, Herceptin , or any combination thereof.
Toxic enzymes from plants and bacteria such as ricin,
diphtheria toxin and Pseudomonas toxin may be conjugated to
the VISTA antagonists to generate cell-type-specific-killing
reagents. Youle, et al. (1980) Proc. Nat'l Acad. Sci. USA 77:
5483; Gilliland, et al. (1980) Proc. Nat'l Acad. Sci. USA 77:
4539; Krolick, et al. (1980) Proc. Nat'l Acad. Sci. USA 77:
5419.
[00113] Furthermore, the VISTA antagonists described herein
may be conjugated to a radionuclide that emits alpha or beta
particles (e.g., radioimmunoconjuagtes). Such
radioactive
isotopes include but are not limited to beta-emitters such as
phosphorus-32 (32P), scandium-47 (47Sc), copper-67 (67Cu),
gallium-67 (67Ga), yttrium-88 (HY), yttrium-90 (HY), iodine-
125 (3I), iodine-131 (1) 31I. r
samarium-153 (153Sm), lutetium-177
(rnLu), rhenium-186 (186Re) rhenium-168 (188Re,
) and alpha-
emitters such as astatine-211 (mAt) lead-212 (23.2pb)
bismuth-212 (212Bi,
) bismuth-213 (213Bi) or actinium-225 (225Ac)
[00114] Methods are known in the art for conjugating a VISTA
antagonist described herein to a label, such as those methods
described by Hunter, et all (1962) Nature 144: 945; David, et
-42-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
al. (1974) Biochemistry 13: 1014; Pain, et al. (1981) J.
Immunol. Meth. 40: 219; and Nygren (1982) Histochem and
Cytochem, 30: 407.
[00115] Additionally, the VISTA antagonists described herein
may comprise another moiety, i.e., a "targeting moiety," that
targets the antagonist peptide to a target site (such as a
cancer cell, a tumor, a virally-infected cell, etc). The
targeting moiety may be selected from an antibody or ligand
that binds to an antigen, a receptor expressed by the target
cell or an infectious agent.
[00116] The VISTA antagonist (as well as
multimers,
conjugates, analogs, derivatives and mimetics thereof) may
also be directly or indirectly attached to an immunoglobulin
polypeptide or a fragment thereof, e.g., a antibody constant
region.
[00117] A "conjugate," as used herein, refers to a compound
having at least one isolated VISTA antagonist peptide and one
immunoglobulin polypeptide or a fragment thereof, e.g.,
antibody constant region, joined at the polypeptide level,
with or without the use of a linker. A conjugate may be a
fusion polypeptide produced as the result of joining at the
nucleic acid level of genes encoding at least one natriuretic
peptide and one antibody constant region, with or without a
coding sequence for a peptide linker.
[00118] Such VISTA antagonist peptide-antibody conjugates may
have a higher serum stability, e.g., at least 20%, preferably
at least 30%, 50%, 80%, 100%, 200% or more, increase in the
serum half-life when compared with the antagonist peptide
without the antibody constant region under the same
conditions. A human antibody, e.g., a human IgG, such as IgGl,
-43-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
IgG2, IgG3 or IgG4, is frequently used to derive a constant
region or a fragment thereof for the purpose of making a
natriuretic peptide conjugate of this invention.
[00119] As used herein, an "antibody (or immunoglobuiin)
constant region" refers to a polypeptide that corresponds to
at least a portion of the constant region of an antibody heavy
chain or light chain, such portion including at least one
constant domain (e.g., the constant domain of CL or one of the
constant domains of CH). For example, an "antibody constant
region" used for making the conjugates of this invention may
be derived from an antibody heavy chain and include two out of
three (CH2 and CH3 for IgA, IgD, and IgG) or three out of four
(CH2, CH3, and 0H4, for IgE and IgM) constant domains; the
first constant domain (CHI) may be present in some cases but
may be excluded in others. Such an antibody constant region
can be obtained by a variety of means, e.g., by a recombinant
method or synthetic method, or by purification subsequent to
enzymatic digestion, for instance, pepsin or papain digestion
of an intact antibody or an antibody heavy or light chain.
[00120] Further encompassed by this term as used in this
application are polypeptides having a substantial sequence
identity (for instance, at least 80%, 85%, 90%, 95% or more)
to the corresponding amino acid sequence of an antibody heavy
or light chain constant region or a portion thereof that
contains at least one constant domain nearest to the C-
terminus of the antibody chain, so long as the presence of
such an "antibody constant region" in a VISTA antagonist
peptide-antibody constant region conjugate renders the
conjugate a higher serum stability.
-44-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
(00 1 21] Additionally, the peptide, multimer, conjugate,
analog, derivative or mimetic may be modified to increase
certain properties, e.g., biological half life. Various
approaches are possible including, but not limited to, N-
terminal modification/conjugation (e.g., lipidation or
acetylation), C-terminal modification/conjugation (e.g.,
lipidation or acetylation), amino acid substitutions (i.e.,
substitution of natural amino acid with unnatural amino acids,
such as D-conformation, N-methylation, tetra-substitution,
beta-amino acids, etc.), peptide backbone modifications (e.g.,
chemical modification of peptide bonds, such as simple
reductions or replacement of carbonyl or amide groups with
esters, sulfides and alkyls), side chain modifications and/or
cyclization (e.g., disulfide bond formation).
[00122] In one embodiment, the peptide may be pegylated to,
e.g., increase the biological (e.g., serum) half life of the
antibody. To pegylate a peptide, typically it is reacted with
polyethylene glycol (PEG), such as a reactive ester or
aldehyde derivative of PEG, under conditions in which one or
more PEG groups become attached to the peptide. Preferably,
the pegylation is carried out via an acylation reaction or an
alkylation reaction with a reactive PEG molecule (or an
analogous reactive water-soluble polymer.
[00123] Similarly, in another embodiment, a peptide, multimer,
conjugate, analog, derivative or mimetic may be modified by
conjugation of polysialic acid (PEA) to increase half-life.
[00124] Additionally, the peptide, multimer, conjugate,
analog, derivative or mimetic may be modified, e.g.,
genetically fused or chemically conjugated, to comprise
extended recombinant polypeptide (XTEN), through a process
-45-
called XTENylation, to improve its half life. XTEN is a long,
hydrophilic, and unstructured protein-based polymer of 864
amino acids. See, e.g., WO 2013/130683.
When
attached to a molecule of interest, greatly increases the
effective size of the molecule, thereby prolonging its
presence in serum by slowing kidney clearance in a manner
analogous to that of PEG. In addition to slowing kidney
clearance, attachment to XTEN can also inhibit receptor-
mediated clearance by reducing the ligand's affinity for its
receptor. XTEN coupling chemistries include, but are not
limited to, Thiol-XTEN; Maleimide-XTEN; Alkyne-XTEN; and
Iodoacetyl-XTEN.
[00125] Moreover, the peptide, multimer, conjugate, analog,
derivative or mimetic may be modified with recombinant
albumin, e.g., Novozymes RecombuminO, to improve half life.
The peptide can be genetically fused or chemically conjugated
to a recombinant albumin using standard protocols.
[00126] Furthermore, the peptide, multimer, conjugate, analog,
derivative or mimetic may be modified by the addition and/or
removal of specific amino acids to and/or from the peptide.
For example, a number of specific amino acids may be added to
the peptide, thereby strengthening or tightening its molecular
structure to make it less susceptible to biological
degradation and, thus, providing a longer life-span in the
blood, using, e.g., Zealand Structure Induced Probe (SIP )
tail technology.
[00127] Yet another exemplary method for improving the
stability and therapeutic potential of peptides, analogs,
derivatives or mimetics is multimerization. For example, a
-46-
Date Recue/Date Received 2021-06-18
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
multimer may comprise two or more copies, e.g., 2, 3, 4, 5, 6,
7 or more, of the isolated VISTA antagonist or variant
thereof. Multimers
include both homomultimers and
heteromultimers.
Multimerization can result in increased
peptide stability, higher binding strength (due to multiple
valencies in the molecule), and/or improved pharmacokinetic
properties.
[00128] Another exemplary approach for improving the stability
and, thus, therapeutic potential of the VISTA antagonist
peptides, multimers, conjugates, analogs, derivatives or
mimetics disclosed herein is the addition of acetyl groups to
the N and/or C terminus of the peptide.
Acetylation may
protect the peptide from exopeptidases, thereby extending the
half-life of the peptide.
Production of VISTA Antagonists
[00129] The peptide multimer, conjugate, analog, derivative or
mimetic can be produced and isolated using any method known in
the art. Peptides can be synthesized, whole or in part, using
chemical methods known in the art (see, e.g., Caruthers (1980)
Nucleic Acids Res. Symp. Ser. 215-223; Horn (1980) Nucleic
Acids Res. Symp. Ser. 225-232; and Banga (1995) Therapeutic
Peptides and Proteins, Formulation, Processing and Delivery
Systems, Technomic Publishing Co., Lancaster, Pa.). Peptide
synthesis can be performed using various solid-phase
techniques (see, e.g., Roberge (1995) Science 269:202;
Merrifield (1997) Methods Enzymol. 289:3-13) and automated
synthesis may be achieved, e.g., using the ABI 431A Peptide
Synthesizer (Perkin Elmer) in accordance with the
manufacturer's instructions.
-47-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
[00130] Individual synthetic residues and peptides
incorporating mimetics can be synthesized using a variety of
procedures and methodologies known in the art (see, e.g.,
Organic Syntheses Collective Volumes, Gilman, et al. (Eds)
John Wiley & Sons, Inc., NY). Peptides and peptide mimetics
can also be synthesized using combinatorial methodologies.
Techniques for generating peptide and peptidomimetic libraries
are well-known, and include, for example, multipin, tea bag,
and split-couple-mix techniques (see, for example, al-Obeidi
(1998) Mod.. Biotechnol. 9:205-223; Hruby (1997) Curr.
Chem. Biol. 1:114-119; Ostergaard (1997) Mod.. Divers. 3:17-27;
and Ostresh (1996) Methods Enzymol. 267:220-234). Modified
peptides can be further produced by chemical modification
methods (see, for example, Belousov (1997) Nucleic Acids Res.
25:3440-3444; Frenkel (1995) Free Radic. Biol. Med. 19:373-
380; and Blommers (1994) Biochemistry 33:7886-7896).
[00131] Alternatively, a peptide of this invention can be
prepared in recombinant protein systems using polynucleotide
sequences encoding the peptides. By way of illustration, a
nucleic acid molecule encoding a peptide of the invention is
introduced into a host cell, such as bacteria, yeast or
mallunalian cell, under conditions suitable for expression of
the peptide, and the peptide is purified or isolated using
methods known in the art. See, e.g., Deutscher et al. (1990)
Guide to Protein Purification: Methods in Enzymology Vol. 182,
Academic Press. In particular embodiments, the peptide, or
analog, derivative or mimetic thereof is isolated and/or
purified to homogeneity (e.g. greater than 90% purity).
[00132] It is contemplated that the peptide disclosed herein
can be used as a lead compound for the design and synthesis of
-48-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
compounds with improved efficacy, clearance, half-lives, and
the like.
[00133] One approach includes structure-activity relationship
(SAR) analysis (e.g., NMR analysis) to determine specific
binding interactions between the peptide and VISTA to
facilitate the development of more efficacious agents. Agents
identified in such SAR analysis or from agent libraries can
then be screened for their ability to, e.g., decrease the
activity of VISTA and/or enhance T cell proliferation.
Pharmaceutical Compositions
[00134] The VISTA antagonist peptide, multimer, conjugate,
analog, derivative and mimetic thereof described herein can be
provided in a pharmaceutical composition.
[00135] A "pharmaceutical composition" refers to a chemical or
biological composition suitable for administration to a
mammal. Such compositions may be specifically formulated for
administration via one or more of a number of routes,
including but not limited to buccal, epicutaneous, epidural,
inhalation, intraarterial,
intracardial,
intracerebroventricular, intradermal,
intramuscular,
intranasal, intraocular, intraperitoneal,
intraspinal,
intrathecal, intravenous, oral, parenteral, rectally via an
enema or suppository, subcutaneous, subdermal, sublingual,
transdermal, and transmucosal. In
addition, administration
may occur by means of injection, powder, liquid, gel, drops,
or other means of administration.
[00136] A "pharmaceutical excipient" or a "pharmaceutically
acceptable excipient" is a carrier, usually a liquid, in which
an active therapeutic agent is formulated. In one embodiment
-49-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
of the invention, the active therapeutic agent is a humanized
antibody described herein, or one or more fragments thereof.
The excipient generally does not provide any pharmacological
activity to the formulation, though it may provide chemical
and/or biological stability, and release characteristics.
Exemplary formulations may be found, for example, in Grennaro
(2005) [Ed.] Remington: The Science and Practice of Pharmacy
[21st Ed.]
[00137] Pharmaceutical compositions typically must be sterile
and stable under the conditions of manufacture and storage.
The invention contemplates that the pharmaceutical composition
is present in lyophilized form. The
composition may be
formulated as a solution, microemulsion, liposome, or other
ordered structure suitable to high drug concentration. The
carrier may be a solvent or dispersion medium containing, for
example, water, ethanol, polyol (for example, glycerol,
propylene glycol, and liquid polyethylene glycol), and
suitable mixtures thereof. The invention further contemplates
the inclusion of a stabilizer in the pharmaceutical
composition.
[00138] The VISTA antagonist peptide, multimer, conjugate,
analog, derivative and mimetic thereof described herein may be
formulated into pharmaceutical compositions of various dosage
forms. To
prepare the pharmaceutical compositions of the
invention, at least one VISTA antagonist as the active
ingredient may be intimately mixed with appropriate carriers
and additives according to techniques well known to those
skilled in the art of pharmaceutical formulations. See
Grennaro (2005) [Ed.] Remington: The Science and Practice of
Pharmacy [218t Ed.] For
example, the antagonists described
-50-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
herein may be formulated in phosphate buffered saline pH 7.2
and supplied as a 5.0 mg/mL clear colorless liquid solution.
[00139] Similarly, compositions for liquid
preparations
include solutions, emulsions, dispersions, suspensions,
syrups, and elixirs, with suitable carriers and additives
including but not limited to water, alcohols, oils, glycols,
preservatives, flavoring agents, coloring agents, and
suspending agents. Typical
preparations for parenteral
administration comprise the active ingredient with a carrier
such as sterile water or parenterally acceptable oil including
but not limited to polyethylene glycol, polyvinyl pyrrolidone,
lecithin, arachis oil or sesame oil, with other additives for
aiding solubility or preservation may also be included. In
the case of a solution, it may be lyophilized to a powder and
then reconstituted immediately prior to use. For dispersions
and suspensions, appropriate carriers and additives include
aqueous gums, celluloses, silicates, or oils.
[00140] For each of the recited embodiments, the VISTA
antagonist peptides, multimers, conjugates, analogs,
derivatives and mimetics thereof may be administered by a
variety of dosage forms. Any biologically-acceptable dosage
form known to persons of ordinary skill in the art, and
combinations thereof, are contemplated. Examples
of such
dosage forms include, without limitation, reconstitutable
powders, elixirs, liquids, solutions, suspensions, emulsions,
powders, granules, particles, microparticles, dispersible
granules, cachets, inhalants, aerosol inhalants, patches,
particle inhalants, implants, depot implants, injectables
(including subcutaneous, intramuscular, intravenous, and
intradermal), infusions, and combinations thereof.
-51-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
[00141] In many cases, it will be preferable to include
isotonic agents, e.g., sugars, polyalcohols such as mannitol,
sorbitol, or sodium chloride in the composition. Prolonged
absorption of the injectable compositions may be brought about
by including in the composition an agent which delays
absorption, e.g., monostearate salts and gelatin. Moreover,
the compounds described herein may be formulated in a time
release formulation, e.g. in a composition that includes a
slow release polymer. The VISTA and VISTA conjugate may be
prepared with carriers that will protect the compound against
rapid release, such as a controlled release formulation,
including implants and microencapsulated delivery systems.
Biodegradable, biocompatible polymers may be used, such as
ethylene vinyl acetate, polyanhydrides, polyglycolic acid,
collagen, polyorthoesters, polylactic acid and polylactic,
polyglycolic copolymers (PLG). Many
methods for the
preparation of such formulations are known to those skilled in
the art.
[00142] A person of skill in the art would be able to
determine an effective dosage and frequency of administration
through routine experimentation, for example guided by the
disclosure herein and the teachings in Goodman, et al. (2011)
Goodman & Gilman's The Pharmacological Basis of Therapeutics
[12th Ed.]; Howland, et al. (2005) Lippincott's Illustrated
Reviews: Pharmacology [2nd Ed.]; and Golan, (2008) Principles
of Pharmacology: The Pathophysiologic Basis of Drug Therapy
[2nd Edo See,
also, Grennaro (2005) [Ed.] Remington: The
Science and Practice of Pharmacy [210tEd.]
[00143] The compositions described herein may be administered
in any of the following routes: buccal, epicutaneous,
-52-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
epidural, infusion, inhalation, intraarterial, intracardial,
intracerebroventricular, intradermal,
intramuscular,
intranasal, intraocular, intraperitoneal,
intraspinal,
intrathecal, intravenous, oral, parenteral, pulmonary,
rectally via an enema or suppository, subcutaneous, subdermal,
sublingual, transdermal, and transmucosal. The
preferred
routes of administration are intravenous injection or
infusion. The
administration can be local, where the
composition is administered directly, close to, in the
locality, near, at, about, or in the vicinity of, the site(s)
of disease, e.g., tumor, or systemic, wherein the composition
is given to the patient and passes through the body widely,
thereby reaching the site(s) of disease. Local administration
(e.g., injection) may be accomplished by administration to the
cell, tissue, organ, and/or organ system, which encompasses
and/or is affected by the disease, and/or where the disease
signs and/or symptoms are active or are likely to occur (e.g.,
tumor site).
Administration can be topical with a local
effect, composition is applied directly where its action is
desired (e.g., tumor site).
[00144] For each of the recited embodiments, the compounds can
be administered by a variety of dosage forms as known in the
art. Any biologically-acceptable dosage form known to persons
of ordinary skill in the art, and combinations thereof, are
contemplated. Examples of such dosage forms include, without
limitation, chewable tablets, quick dissolve tablets,
effervescent tablets, reconstitutable powders, elixirs,
liquids, solutions, suspensions, emulsions, tablets, multi-
layer tablets, bi-layer tablets, capsules, soft gelatin
capsules, hard gelatin capsules, caplets, lozenges, chewable
-53...
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
lozenges, beads, powders, gum, granules, particles,
microparticles, dispersible granules, cachets, douches,
suppositories, creams, topicals, inhalants, aerosol inhalants,
patches, particle inhalants, implants, depot implants,
ingestibles, injectables (including
subcutaneous,
intramuscular, intravenous, and intradermal), infusions, and
combinations thereof.
[00145] Other compounds which can be included by admixture
are, for example, medically inert ingredients (e.g., solid and
liquid diluent), such as lactose, dextrosesaccharose,
cellulose, starch or calcium phosphate for tablets or
capsules, olive oil or ethyl oleate for soft capsules and
water or vegetable oil for suspensions or emulsions;
lubricating agents such as silica, talc, stearic acid,
magnesium or calcium stearate and/or polyethylene glycols;
gelling agents such as colloidal clays; thickening agents such
as gum tragacanth or sodium alginate, binding agents such as
starches, arabic gums, gelatin,
methylcellulose,
carboxymethylcellulose or polyvinylpyrrolidone; disintegrating
agents such as starch, alginic acid, alginates or sodium
starch glycolate; effervescing mixtures; dyestuff; sweeteners;
wetting agents such as lecithin, polysorbates or
laurylsulphates; and other therapeutically acceptable
accessory ingredients, such as humectants, preservatives,
buffers and antioxidants, which are known additives for such
formulations.
[00146] Liquid dispersions for oral administration can be
syrups, emulsions, solutions, or suspensions. The syrups can
contain as a carrier, for example, saccharose or saccharose
with glycerol and/or mannitol and/or sorbitol. The
-54-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
suspensions and the emulsions can contain a carrier, for
example a natural gum, agar, sodium alginate, pectin,
methylcellulose, carboxymethylceilulose, or polyvinyl alcohol.
[00147] In further embodiments, the present invention provides
kits including one or more containers comprising
pharmaceutical dosage units comprising an effective amount of
one or more VISTA antagonists of the present invention. Kits
may include instructions, directions, labels, marketing
information, warnings, or information pamphlets.
[00148] The amount of VISTA antagonist in a therapeutic
composition according to any embodiments of this invention may
vary according to factors such as the disease state, age,
gender, weight, patient history, risk factors, predisposition
to disease, administration route, pre-existing treatment
regime (e.g., possible interactions with other medications),
and weight of the individual. Dosage regimens may be adjusted
to provide the optimum therapeutic response. For example, a
single bolus may be administered, several divided doses may be
administered over time, or the dose may be proportionally
reduced or increased as indicated by the exigencies of
therapeutic situation.
[00149] It is especially advantageous to formulate parenteral
compositions in dosage unit form for ease of administration
and uniformity of dosage. Dosage
unit form as used herein
refers to physically discrete units suited as unitary dosages
for the mammalian subjects to be treated; each unit containing
a predetermined quantity of antibodies, and fragments thereof,
calculated to produce the desired therapeutic effect in
association with the required pharmaceutical carrier. The
specification for the dosage unit forms of the invention are
-55-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
dictated by and directly dependent on the unique
characteristics of the antibodies, and fragments thereof, and
the particular therapeutic effect to be achieved, and the
limitations inherent in the art of compounding such an
antibodies, and fragments thereof, for the treatment of
sensitivity in individuals. In therapeutic use for treatment
of conditions in mammals (e.g., humans) for which the
antibodies and fragments thereof of the present invention or
an appropriate pharmaceutical composition thereof are
effective, the antibodies and fragments thereof of the present
invention may be administered in an effective amount. The
dosages as suitable for this invention may be a composition, a
pharmaceutical composition or any other compositions described
herein.
[00150] The dosage may be administered as a single dose, a
double dose, a triple dose, a quadruple dose, and/or a
quintuple dose. The dosages may be administered singularly,
simultaneously, and sequentially.
[00151] The dosage form may be any form of release known to
persons of ordinary skill in the art. The compositions of the
present invention may be formulated to provide immediate
release of the active ingredient or sustained or controlled
release of the active ingredient. In a sustained release or
controlled release preparation, release of the active
ingredient may occur at a rate such that blood levels are
maintained within a therapeutic range but below toxic levels
over an extended period of time (e.g., 4 to 24 hours). The
preferred dosage forms include immediate release, extended
release, pulse release, variable release, controlled release,
-56-
CA 029926 2016--1.4
WO 2015/109340 PCT/1JS2015/012752
timed release, sustained release, delayed release, long
acting, and combinations thereof, and are known in the art.
[00152] As defined herein, a therapeutically effective amount
of VISTA antagonist peptide, analog, derivative or mimetic
thereof (i.e., an effective dosage) ranges from about 0.001 to
30 mg/kg body weight, preferably about 0.01 to 25 mg/kg body
weight, more preferably about 0.1 to 20 mg/kg body weight, and
even more preferably about 1 to 10 mg/kg, 2 to 9 mg/kg, 3 to 8
mg/kg, 4 to 7 mg/kg, or 5 to 6 mg/kg body weight.
[00153] The skilled artisan will appreciate that certain
factors may influence the dosage required to effectively treat
a subject, including but not limited to the severity of the
disease or disorder, previous treatments, the general health
and/or age of the subject, and other diseases present.
Moreover, treatment of a subject with a therapeutically
effective amount of a peptide can include a single treatment
or, preferably, can include a series of treatments.
[00154] In a preferred example, a subject is treated with
peptide, analog, derivative or mimetic thereof in the range of
between about 0.1 to 20 mg/kg body weight, one time per week
for between about 1 to 10 weeks, preferably between 2 to 8
weeks, more preferably between about 3 to 7 weeks, and even
more preferably for about 4, 5, or 6 weeks. It will also be
appreciated that the effective dosage of antibody, protein, or
polypeptide used for treatment may increase or decrease over
the course of a particular treatment. Changes in dosage may
result and become apparent from the results of diagnostic
assays as described herein.
[00155] It will be appreciated that the pharmacological
activity of the compositions may be monitored using standard
-57-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
pharmacological models that are known in the art.
Furthermore, it will be appreciated that the compositions
comprising a VISTA antagonist may be incorporated or
encapsulated in a suitable polymer matrix or membrane for
site-specific delivery, or may be functionalized with specific
targeting agents capable of effecting site specific delivery.
These techniques, as well as other drug delivery techniques
are well known in the art. Determination of optimal dosages
for a particular situation is within the capabilities of those
skilled in the art. See, e.g., Grennaro (2005) [Ed.]
Remington: The Science and Practice of Pharmacy [21st Ed.]
[00156] A peptide or analog, derivative or mimetic of this
invention can be co-formulated with and/or coadministered with
one or more additional therapeutic agents (e.g., an anti-
cancer agent, an anti-viral agent, a cytokine and/or an immune
agonist). Such combination therapies may require lower dosages
of the peptide or analog, derivative or mimetic and/or the co-
administered agents, thus avoiding possible toxicities or
complications associated with the various monotherapies. There
are a number of agents that may be advantageously combined
with peptide or analog, derivative or mimetic of the invention
and the selection of such agents will depend on the intended
disease or condition to be treated. For example, the present
invention includes combination therapies composed of a peptide
or multimer, conjugate, analog, derivative or mimetic of the
invention that is capable of inducing or promoting a response
against a cancerous or pre-cancerous condition and at least
one anti-cancer agent. Accordingly, in particular embodiments,
the instant peptide or analog, derivative or mimetic is used
as an adjuvant therapy in the treatment of cancer. As another
-58-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
example, the invention embraces combination therapies that
include a peptide or analog, derivative or mimetic of the
invention that is capable of inducing or promoting a
therapeutic response against a viral infection and at least
one anti-viral agent. Exemplary therapeutic agents that may be
contained in the compositions comprising the VISTA antagonist
peptide, multimer, conjugate, analog, derivative or mimetic
include, e.g., CTLA-4-Ig, anti-PD-1, PD-Li or PD-L2 fusion
proteins and EGFR antagonists.
[00157] Anti-cancer agents include, but are not limited to,
cytotoxic agents such as Vinca alkaloid, taxanes, and
topoisomerase inhibitors; antisense nucleic acids such as
augmerosen/G3139, LY900003 (ISIS 3521), ISIS 2503, OCX-011
(ISIS 112989), LE-AON/LEraf-AON (liposome encapsulated c-raf
antisense oligonucleotide/ISIS-5132), MG98, and other
antisense nucleic acids that target PKCa, clusterin, IGFBPs,
protein kinase A, cyclin D1, or Bc1-2; anticancer nucleozymes
such as angiozyme (Ribozyme Pharmaceuticals); tumor
suppressor-encoding nucleic acids such as a p53, BRCA1, RB1,
BRCA2, DPC4 (Smad4), MSH2, ML-I1, and DCC; oncolytic viruses
such as oncolytic adenoviruses and herpes viruses; anti-cancer
immunogens such as a cancer antigen/tumor-associated antigen,
e.g., an epithelial cell adhesion molecule (Ep-CAM/TACSTD1),
mucin 1 (MUC1), carcinoembryonic antigen (CEA), tumor-
associated glycoprotein 72 (TAG-72), gp100, Melan-A, MART-1,
KDR, RCAS1, MDA7, cancer-associated viral vaccines, tumor-
derived heat shock proteins, and the like; anti-cancer
cytokines, chemokines, or combination thereof; inhibitors of
angiogenesis, neovascularization, and/or other
vascularization; and/or any other conventional anticancer
-59-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
agent including fluoropyrimidiner carbamates, non-
polyglutamatable thymidylate synthase inhibitors, nucleoside
analogs, antifolates, topoisomerase inhibitors, polyamine
analogs, mTOR inhibitors, alkylating agents, lectin
inhibitors, vitamin D analogs, carbohydrate processing
inhibitors, anti-metabolism folate antagonists, thumidylate
synthase inhibitors, antimetabolites, ribonuclease reductase
inhibitors, dioxolate nucleoside analogs, and chemically
modified tetracyclines.
[00158] Anti-viral agents of use in the invention include, but
are not limited to, protease inhibitors (e.g., acyclovir) in
the context of HIV treatment or an anti-viral antibody (e.g.,
an anti-gp41 antibody in the context of HIV treatment; an
anti-CD4 antibody in the context of the treatment of CMV,
etc.). Numerous other types of anti-viral agents are known in
the art.
[00159] Toxicity and therapeutic efficacy of the peptide or
analog, derivative or mimetic can be determined by standard
pharmaceutical procedures in cell cultures or experimental
animals. The data obtained from the cell culture assays and
animal studies can be used in formulating a range of dosage
for use in humans. The dosage of such agents lies preferably
within a range of circulating concentrations that include the
ED50 with little or no toxicity. The dosage may vary within
this range depending upon the dosage form employed and the
route of administration utilized. For any agent used in the
methods of the invention, the therapeutically effective dose
can be estimated initially from cell culture assays. A dose
may be formulated in animal models to achieve a circulating
plasma concentration range that includes the ICH (i.e., the
-60-
CA 02936926 2016-07-1.4
W02015/109340 PCT/1JS2015/012752
concentration of the test compound which achieves a half-
maximal inhibition of symptoms) as determined in cell culture.
Such information can be used to more accurately determine
useful doses in humans. Levels in plasma may be measured, for
example, by high performance liquid chromatography.
Use of VISTA Antagonists and Compositions comprising the same
[00160] The peptide or analog, derivative or mimetic of this
invention finds use in inhibiting the activity of VISTA (i.e.,
PD-13) thereby upregulating immune responses. Opregulation of
immune responses can be in the form of enhancing an existing
immune response or eliciting an initial immune response. For
example, enhancing an immune response through inhibition of
VISTA activity is useful in the prevention and/or treatment of
infections with microbes, e.g., bacteria, viruses, or
parasites, or in cases of immunosuppression and cancer.
[00161] Accordingly, the present invention includes
prophylactic and therapeutic methods for the prevention and
treatment of cancer and infectious disease. Terms such as
"treat," "treating" and "treatment" herein refer to the
delivery of an effective amount of a peptide or analog,
derivative or mimetic of this invention with the purpose of
easing, ameliorating, or eradicating (curing) such symptoms or
disease states already developed. The terms "prevent,"
"preventing" and "prevention" refer to the delivery of an
effective amount of a peptide or analog, derivative or mimetic
of this invention with the purpose of preventing any symptoms
or disease state to develop. Thus, these terms are meant to
include prophylactic treatment.
-61-
CA 029926 2016--1.4
WO 2015/109340 PCT/11S2015/012752
[00162] Accordingly to one embodiment, the invention provides
a method of treating or preventing cancer, inhibiting tumor
invasion and/or cancer metastasis by administering to a
subject in need thereof, such as a mammalian subject,
preferably a human subject, an effective amount of an isolated
VISTA antagonist disclosed herein or a composition containing
said isolated VISTA antagonist.
Optionally the subject has
one or more precancerous lesions or is predisposed to cancer,
e.g., as a result of genetic mutation, family history or
exposure to a carcinogenic agent. In another embodiment the
invention provides a method of treating cancer in subject,
such as a mammalian subject, preferably a human subject, such
as a human subject, who optionally has a detectable level of
cancer cells. In accordance with these embodiments, the
subject is administered a peptide or analog, derivative or
mimetic of this invention in an amount sufficient to
detectably reduce the development or progression of the cancer
in the subject.
[00163] Cancers are generally composed of single or several
clones of cells that are capable of partially independent
growth in a host (e.g., a benign tumor) or fully independent
growth in a host (malignant cancer). Cancer cells are cells
that divide and reproduce abnormally with uncontrolled growth.
[00164] Cancer cells arise from host cells via neoplastic
transformation (i.e., carcinogenesis). Terms such as
"preneoplastic," "premalignant" and "precancerous" with
respect to the description of cells and/or tissues herein
refer to cells or tissues having a genetic and/or phenotypic
profile that signifies a significant potential of becoming
cancerous. Usually such cells can be characterized by one or
-62-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
more differences from their nearest counterparts that signal
the onset of cancer progression or significant risk for the
start of cancer progression. Such precancerous changes, if
detectable, can usually be treated with excellent results.
[00165] In general, a precancerous state will be associated
with the incidence of neoplasm(s) or preneoplastic lesion(s).
Examples of known and likely preneoplastic tissues include
ductal carcinoma in situ (DCIS) growths in breast cancer,
cervical intra-epithelial neoplasia (CIN) in cervical cancer,
adenomatous polyps of colon in colorectal cancers, atypical
adenomatous hyperplasia in lung cancers, and actinic keratosis
(AK) in skin cancers. Pre-neoplastic phenotypes and genotypes
for various cancers, and methods for assessing the existence
of a preneoplastic state in cells, have been characterized.
See, e.g., Medina (2000) J. Mammary Gland Biol. Neoplasia
5(4):393-407; Krishnamurthy, et al. (2002) Adv. Anat. Pathol.
9(3):185-97; Ponten (2001) Eur. J. Cancer Suppl 8:S97-113;
Niklinski, et al. (2001) Eur. J. Cancer Prey. 10(3):213-26;
Walch, et al. Pathobiology (2000) 68(1):9-17; Busch (1998)
Cancer Surv. 32:149-79.
[00166] Gene expression profiles can increasingly be used to
differentiate between normal, precancerous, and cancer cells.
For example, familial adenomatous polyposis genes prompt close
surveillance for colon cancer; mutated p53 tumor-suppressor
gene flags cells that are likely to develop into aggressive
cancers; osteopontin expression levels are elevated in
premalignant cells, and increased telomerase activity also can
be a marker of a precancerous condition (e.g., in cancers of
the bladder and lung). In one aspect, the invention relates to
the treatment of precancerous cells. In another aspect, the
-63-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
invention relates to the preparation of medicaments for
treatment of precancerous cells.
[00167] In general, a peptide or analog, derivative or mimetic
of this invention can be used to treat subjects suffering from
any stage of cancer (and to prepare medicaments for reduction,
delay, or other treatment of cancer). Effective treatment of
cancer (and thus the reduction thereof) can be detected by any
variety of suitable methods. Methods for detecting cancers and
effective cancer treatment include clinical examination
(symptoms can include swelling, palpable lumps, enlarged lymph
nodes, bleeding, visible skin lesions, and weight loss);
imaging (X-ray techniques, mammography, colonoscopy, computed
tomography (CT and/or CAT) scanning, magnetic resonance
imaging (MRI), etc.); immunodiagnostic assays (e.g., detection
of CEA, AFP, CA125, etc.); antibody-mediated radioimaging; and
analyzing cellular/tissue immunohistochemistry. Other examples
of suitable techniques for assessing a cancerous state and
effective cancer treatment include PCR and RT-PCR (e.g., of
cancer cell associated genes or "markers"), biopsy, electron
microscopy, positron emission tomography (PET), computed
tomography, magnetic resonance imaging (MRI), karyotyping and
other chromosomal analysis, immunoassay/immunocytochemical
detection techniques (e.g., differential antibody
recognition), histological and/or histopathologic assays
(e.g., of cell membrane changes), cell kinetic studies and
cell cycle analysis, ultrasound or other sonographic detection
techniques, radiological detection techniques, flow cytometry,
endoscopic visualization techniques, and physical examination
techniques.
-64-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
[00168] In general, delivering a peptide or analog, derivative
or mimetic of this invention to a subject (either by direct
administration or expression from a nucleic acid) can be used
to reduce, treat, prevent, or otherwise ameliorate any aspect
of cancer in a subject. In this respect, treatment of cancer
can include, e.g., any detectable decrease in the rate of
normal cells transforming to neoplastic cells (or any aspect
thereof), the rate of proliferation of pre-neoplastic or
neoplastic cells, the number of cells exhibiting a pre-
neoplastic and/or neoplastic phenotype, the physical area of a
cell media (e.g., a cell culture, tissue, or organ) containing
pre-neoplastic and/or neoplastic cells, the probability that
normal cells and/or preneoplastic cells will transform to
neoplastic cells, the probability that cancer cells will
progress to the next aspect of cancer progression (e.g., a
reduction in metastatic potential), or any combination
thereof. Such changes can be detected using any of the above-
described techniques or suitable counterparts thereof known in
the art, which typically are applied at a suitable time prior
to the administration of a therapeutic regimen so as to assess
its effectiveness. Times and conditions for assaying whether a
reduction in cancer has occurred will depend on several
factors including the type of cancer, type and amount of
peptide, related composition, or combination composition being
delivered to the host.
[00169] The methods of the invention can be used to treat a
variety of cancers. Forms of cancer that may be treated by the
delivery or administration of a peptide or analog, derivative
or mimetic of this invention and combination therapies
containing the same include squamous cell carcinoma, leukemia,
-65-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/11S2015/012752
acute lymphocytic leukemia, acute lymphoblastic leukemia, B-
cell lymphoma, T-cell lymphoma, Hodgkins lymphoma, non-
Hodgkins lymphoma, hairy cell lymphoma, Burketts lymphoma,
acute or chronic myelogenous leukemias, promyelocytic
leukemia, fibrosarcoma, rhabdomyoscarcoma, melanoma, seminoma,
teratocarcinoma, neuroblastoma, glioma,
astrocytoma,
neuroblastoma, glioma, schwannomas;
fibrosarcoma,
rhabdomyoscaroma, osteosarcoma, melanoma,
xeroderma
pigmentosum, keratoacanthoma, seminoma, thyroid follicular
cancer, and teratocarcinoma. The compositions of this
invention also can be useful in the treatment of other
carcinomas of the bladder, breast, colon, kidney, liver, lung,
ovary, prostate, pancreas, stomach, cervix, thyroid or skin.
Compositions of this invention also may be useful in treatment
of other hematopoietic tumors of lymphoid lineage, other
hematopoietic tumors of myeloid lineage, other tumors of
mesenchymal origin, other tumors of the central or peripheral
nervous system, and/or other tumors of mesenchymal origin.
Advantageously, the methods of the invention also may be
useful in reducing cancer progression in prostate cancer
cells, melanoma cells (e.g., cutaneous melanoma cells, ocular
melanoma cells, and/or lymph node-associated melanoma cells),
breast cancer cells, colon cancer cells, and lung cancer
cells. The methods of the invention can be used to treat both
tumorigenic and non-tumorigenic cancers (e.g., non-tumor-
forming hematopoietic cancers). The methods of the invention
are particularly useful in the treatment of epithelial cancers
(e.g., carcinomas) and/or colorectal cancers, breast cancers,
lung cancers, vaginal cancers, cervical cancers, and/or
squamous cell carcinomas (e.g., of the head and neck).
-66-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
Additional potential targets include sarcomas and lymphomas.
Additional advantageous targets include solid tumors and/or
disseminated tumors (e.g., myeloid and lymphoid tumors, which
can be acute or chronic).
[00170] The present invention also provides methods for
enhancing anti-cancer or anti-tumor immunity, comprising
administering to a subject in need thereof an effective amount
of an isolated VISTA antagonist or a composition containing
said isolated VISTA antagonist.
[00171] In addition to cancer treatment, the present invention
also features a method of treating a pathogen infection, i.e.,
a bacterial, viral, parasitic or fungal infection, in a
subject or host. This method involves administering or
otherwise delivering an effective amount of a peptide or
analog, derivative or mimetic of this invention so as to
reduce the severity, spread, symptoms, or duration of such
infection. Such pathogen infections include, but are not
limited to diseases caused by bacteria, protozoa, fungi,
parasites, or viruses.
[00172] In particular embodiments, a viral infection is
treated. Any virus normally associated with the activity of
effector lymphocytes can be treated by the method. For
example, such a method can be used to treat infection by one
or more viruses selected from hepatitis type A, hepatitis type
B, hepatitis type C, influenza, varicella, adenovirus, herpes
simplex type I (1-ISV-1), herpes simplex type 2 (HSV-2),
rinderpest, rhinovirus, echovirus, rotavirus, respiratory
syncytial virus, papilloma virus, papilloma virus,
cytomegalovirus (CMV, e.g., HCMV), echinovirus, arbovirus,
huntavirus, coxsackie virus, mumps virus, measles virus,
-67-
CA 029926 2016--1.4
WO 2015/109340 PCT/US2015/012752
rubella virus, polio virus, and/or human immunodeficiency
virus type I or type 2 (HIV-1, HIV-2). The practice of such
methods may result in a reduction in the titer of virus (viral
load), reduction of the number of virally infected cells, etc.
[00173] In addition to pathogen infections, a peptide or
analog, derivative or mimetic of this invention can be
administered or otherwise delivered to a subject in
association with the treatment of immunoproliferative
diseases, immunodeficiency diseases, autoimmune diseases,
inflammatory responses, and/or allergic responses.
Moreover, the invention also provides methods for blocking,
inhibiting or neutralizing VISTA-mediated T cell suppression
and/or stimulating an immune response in a subject, comprising
administering to the subject in need thereof an effective
amount of an isolated VISTA antagonist or a composition
containing said isolated VISTA antagonist. Such methods may
be useful for treating a subject with a one or more of a
bacterial, viral, parasitic and fungal infections and/or
cancer.
Examples
[00174] The invention now being generally described, it will
be more readily understood by reference to the following
examples, which are included merely for purposes of
illustration of certain aspects and embodiments of the present
invention, and are not intended to limit the invention.
Example 1: Materials and Methods
[00175] Peptide Synthesis. AP1049 (SSACDWIKRSCH-amide, wherein
Cys4-Cysll form a disulfide bridge; SEQ ID NO:1 (Ser-Ser-Ala-
-68-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/US2015/012752
Cys-Asp-Trp-Ile-Lys-Arg-Ser-Cys-His)) and scrambled negative
control sequence (SSACKSWRDICH-amide, wherein Cys4-Cysll form
a disulfide bridge; SEQ ID NO:2) were prepared using standard
Emoc-based solid-phase peptide synthesis (SPPS). The peptides
were purified via HPLC, and analyzed by mass spectrometric
using the liquid chromatography-mass spectrometry (LC-MS) and
matrix-assisted laser desorption/ionization (MALDI)
[00176] Peptide Discovery Using Phage Display. An M13 phage
peptide library was provided by Dr. Brian Kay (U. Illinois-
Chicago). The VISTA protein required for both the phage
display biopanning experiments and the confirmatory ELISA
binding experiments was prepared by conventional recombinant
protein techniques.
[00177] T Cell Proliferation Assay. An VISTA-Ig fusion protein
or control Ig fusion protein was co-absorbed to a cell-culture
plate together with the polyclonal T cell receptor (TCR)
stimuli (i.e., anti-CD3 antibody). To evaluate the activity of
VISTA-specific peptides, peptides (VISTA specific and
scrambled control) were added as a soluble reagent to the
culture on day 0, and T cell proliferation and cytokine
production were analyzed after 3-4 days.
[00178] T Cell Priming Assay. VISTA is known to suppress T
cell priming when expressed on antigen-presenting cells
(APCs). VISTA-expressing myeloid APCs (Cd11b111 MHCII-F myeloid
cells) were purified from mice spleen, FACS sorted, and
irradiated (2500 rads). To test the activity of VISTA-specific
peptides, transgenic T cells such as OT-II were stimulated ex
vivo with VISTA-expressing APCs and cognate antigen chicken
ovalbumin (15 ng/mL). VISTA-specific peptide or control
scramble peptide was added to the cell culture. T cell
-69-
CA 02936926 2016-07-14
WO 2015/109340 PCT/US2015/012752
proliferation and cytokine production was evaluated after 3-5
days of culture. As an additional specificity control, VISTA-
negative parent cell line A20 or APCs purified from VISTA
knockout mice were used.
[00179] Foxp34-CD4+ Regulatory T Cell (Treg) Suppression Assay.
VISTA plays a role in the suppressive function of Foxp3+ CD4+
regulatory T cells (Tregs), as VISTA-blocking monoclonal
antibody partially reverses Treg suppressive activity in the
in vitro Treg suppression assay. This assay includes antigen
presenting cells, purified Foxp3+ CD4+ Tregs, and Foxp3- 0D4+
naive T cells, which are stimulated by the polyclonal TCR
stimuli. To examine the activity of VISTA-specific peptides,
peptides (VISTA specific and scrambled control) were added to
the Treg suppression assay on day 0. T cell proliferation and
cytokine production were measured on day +3.
[00180]Model of Experimental Autoimmune Encephalomyelitis
(EAE), a Murine Autoimmune Inflammatory Disease Model for
Human Multiple Sclerosis. It has been shown that VISTA-
blocking monoclonal antibody significantly accelerates disease
onset, as well as exacerbates disease severity in a passive
transfer EAE model. In this model, MOG-specific
encephalitogenic CD4+ T cells are first primed in donor mice
upon immunization with MOG peptide, and then purified and ex
vivo expanded in the presence of MOG peptide and cytokines
(IL23, TGFp, IL6 and IL1b). Expanded encephalogenic CD4+ T
cells are transferred into naive recipients to induce disease.
To evaluate the activity of VISTA-specific peptides, peptides
(VISTA specific and scrambled control) are administered via
intraperitoneal injections to mice either prophylactically
(starting from day 2) or therapeutically (starting from day +7
-70-
CA 02936926 2016-07-1.4
WO 2015/109340 PCT/1JS2015/012752
during disease onset), and continuously every 2 days for the
entire duration of the experiment. Disease severity is
evaluated according to the established protocol.
[00181] Murine Tumor Models. It has been demonstrated that
VISTA suppresses tumor-specific T cell responses. VISTA
blockade via VISTA-specific monoclonal antibody significantly
enhances anti-tumor immune responses and inhibits tumor
progression in murine tumor models such as the B16 melanoma
model. The activity of VISTA-specific peptides can be
evaluated in vivo using this tumor model. Mice are inoculated
on the flank with B16 tumor cells (15,000 cells) on day 0.
Peptides (VISTA specific and scrambled control) are
administered via intraperitoneal injections to mice either
prophylactically (starting from day 2) or therapeutically
(i.e., when tumors are palpable), and continuously every 2
days for the entire duration of the experiment. Tumor growth
is measured every 2-3 days with a caliper.
Example 2: Enhancement of T Cell Proliferation
[00182] VISTAFCD11b+ monocytes were enriched from naive
splenocytes using CD11b magnetic beads (Miltenyi).
VISTA+CD1lbhi MHCII+ myeloid APCs were SACS sorted, irradiated
(2500 rads), and used as antigen-presenting cells to stimulate
OT-II transgenic CD4+ T cells in the presence of OVA peptide.
Control-ig, monoclonal antibody specific for VISTA and PD-Li
(30 pg/mL), or VISTA-specific peptide (100 pg/mL) were added
as indicated. Cell proliferation was measured by tritium
incorporation during the last 8 hours of a 72-hour assay. This
analysis indicated that T cell proliferation was enhanced in
the presence of VISTA or PD-Li neutralizing monoclonal
-71-
CA 02936926 2016-07-14
WO 2015/109340 PCT/1JS2015/012752
antibodies, or the AP1049 peptide (Figure 1). In fact, the
AP1049 peptide stimulated T cell proliferation much better
than either of the monoclonal antibodies, indicating that the
peptide possesses strong antagonistic activity against VISTA.
Example 3: Enhancement of anti-tumor immunity
[00183] Immunogenic bladder carcinoma tumors (MB49) were
inoculated in female mice. A21049 was tested for its ability
to slow tumor growth and/or facilitate tumor regression. The
readout for this assay was tumor growth.
[00184] MB49 tumors were inoculated in female mice (300k) via
intradermal (i.d.) inoculation, which facilitates measurement
of tumor size. Mice were treated with either PBS (control) or
VISTA antagonist peptide (AP1049), via daily injections around
tumor mass starting on day+1 and continuing for 2 weeks. Tumor
size was measured by caliper every 2-3 days.
[00185] Using these methods slowed tumor growth and/or tumor
regression in mice treated with AP1049 was obtained as
compared with mice treated with control.
[00186] As shown in Figure 2, AP1049 treatment reduced tumor
growth in the MB49 tumor model, indicating that the peptide
may bind to the critical/active site of VISTA and block the
immune-suppressive function of VISTA.
-72-