Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
ANTIBODIES REACTIVE WITH AN EPITOPE LOCATED IN THE N-
TERMINAL REGION OF MUC5AC COMPRISING CYSTEINE-RICH
SUBDOMAIN 2 (CYS2)
Related Applications
1011 This application is a continuation-in-part of U.S. Patent Application
14/632,480, filed
February 26, 2015. This application claims the benefit under 35 U.S.C. 119(e)
of provisional
U.S. Patent Application Serial Nos. 62/018,989, filed June 30, 2014,
62/091,932, filed
December 15, 2014, and 62/148,428, filed April 16, 2015, the entire text of
each priority
application incorporated herein by reference.
Sequence Listing
1021 The instant application contains a Sequence Listing which has been
submitted in
ASCII format via EFS-Web and is hereby incorporated by reference in its
entirety. Said
ASCII copy, created on June 25, 2015, is named IMM352WO1_SL.txt and is 56,457
bytes in
size.
Field of the Invention
1031 This invention relates to anti-pancreatic cancer antibodies and antigen-
binding
fragments thereof that bind to MUC5AC mucin in pancreatic cancer. Preferably,
the
antibodies or fragments thereof bind to an epitope located within the second
to fourth
cysteine-rich subdomains of MUC5AC (amino acid residues 1575-2052, Cys2-Cys4).
More
preferably, the antibodies bind to an epitope located in amino acid residues
1575-1725 and
1903-2052 (Cys2 and Cys4). Even more preferably, the antibodies bind to an
epitope located
in amino acid residues 1575-1725 (Cys2+). Most preferably, the antibodies bind
to an epitope
located in the Cys2 subdomain of MUC5AC. In preferred embodiments, the anti-
pancreatic
cancer antibody binds to the same epitope as, or competes for binding to
MUC5AC with a
PAM4 antibody comprising the light chain variable region complementarity-
determining
region (CDR) sequences CDR I (SASSSVSSSYLY, SEQ ID NO:!); CDR2 (STSNLAS, SEQ
ID NO:2); and CDR.3 (IIQWNR.YPYT, SEQ ID NO:3); and the heavy chain CDR
sequences
1
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
CDR1 (SYVLII, SEQ ID NO:4); CDR2 (YINPYNDGTQYNEKFKG, SEQ ID NO:5)and
CDR3 (GFGGSYGFAY, SEQ ID NO:6). The subject antibodies or antibody fragments
bind
with high selectivity to pancreatic cancer cells to allow detection and/or
diagnosis of
pancreatic adenocarcinoma at the earliest stages of the disease. Most
preferably, antibody-
based assays are capable of detecting about 85% or more of pancreatic
adenocarcinomas,
with a false positive rate of about 5% or less for healthy controls. In
particular embodiments,
the methods and compositions can be used to detect and/or diagnose pancreatic
adenocarcinoma by screening serum samples from subjects and preferably can
detect 60% or
more of stage I pancreatic cancers and 80% or more of stage II cancers by
serum sample
analysis. In still other embodiments, reactivity with the anti-pancreatic
cancer antibody can
be used to detect occult pancreatic cancer or neoplastic precursor lesions
against a
background of pancreatitis or benign pancreatic hyperplasia.
1041 The anti-pancreatic cancer antibody is of use for diagnosis, detection
and/or treatment
of pancreatic cancer. For therapy, the antibody or fragment thereof may be
administered as a
naked antibody, as an antibody complex, as an antibody fusion protein, or
conjugated to at
least one therapeutic agent, such as a radionuclide, an immunomoduiator, a
hormone, a
hormone antagonist, an enzyme, an oligonucleotide such as an anti-sense
oligonucleotide or a
siRNA, an enzyme inhibitor, a photoactive therapeutic agent, a cytotoxic agent
such as a drug
or toxin, an angiogenesis inhibitor and a pro-apoptotic agent. Most
preferably, the antibody is
a 90Y-11PAM4 antibody and the radiolabeled antibody may be administered in
fractionated
dosages for treating pancreatic cancer.
BACKGROUND
1051 The number of patients who succumb to pancreatic ductal adenocarcinoma
(PDAC)
each year continues to rise, unlike other leading cancers where surveillance
and/or screening
technologies have led to a decrease in cancer-related mortality rates (Cardin
& Berlin, 2013, J
Nat! Cancer Inst 105:1675-6; Ma et al., 2013, J Nati Cancer Inst 105:1694-
1700; Siegel et al.
2012, Cancer statistics, 62:10-29). Unfortunately, the mortality rate for PDAC
is nearly equal
to the incidence. The overall survival rate for all stages of pancreatic
cancer diagnosed
between 2001 and 2007 is only 20% after one year, and about 6% after 5 years
(Siegel et al.
2012, Cancer statistics, 62:10-29). With the alarming increase in PDAC
incidence, it is
projected that by the year 2030, pancreatic cancer will become the second
leading cause of
cancer deaths in the United States (Rahib et al., 2014, Cancer Res 74:2913-
21). The major
reason for this poor prognosis is the inability to detect the disease at an
early stage, when
2
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
curative measures may have a greater opportunity to provide successful
outcomes.
1061 Biomarkers, whether they are biological, chemical, or physical in nature,
have proven
of significant value in providing information leading to the earlier detection
and diagnosis of
cancer, such as breast (Goldhirsch et al., 2003, Ann Oncol 14:1212-4), colon
(Mandel et al.,
1993, N Engl J Med 328:1365-71), and prostate (Jacobsen et al., 1995, JAMA
274:1445-9),
resulting in improved patient outcomes. Unfortunately, this has not been the
case for PDAC.
Despite considerable attention directed towards discovery of biomarkers for
PDAC (Lennon
et al., 2014, Cancer Research 74:1-9), to date no FDA-approved means for early
detection/diagnosis exists. A need exists for more effective compositions and
methods for
early detection and/or diagnosis of prostate cancer, preferably at the
earliest stages of the
disease.
1071 In addition to more effective and earlier means of detection, a need also
exists for
better therapeutic treatments for pancreatic cancer. The outlook for patients
with advanced
pancreatic adenocarcinoma remains poor (Hidalgo, 2010, N Engl j Med 362:1605-
17). In the
frontline, median survival was 6.2-6.7 months with gemeitabine alone (Burris
et al., 1997, J
Clin Oncol 15:2403-13) or with erlotinib (Moore et al., 2007, J Clin Oncol
25:1960-6), 8.5
months combined with albumin-bound paclitaxel (Von Hoff et al., 2013, N Engl J
Med
369:1691-1703), and 11.1 months for those able to tolerate combination
chemotherapy
(FOLFIRINOX) (Conroy et al., 2011, N Engl J Med 364:1817-25). Beyond 1st line,
the
survival advantage with chemotherapy remains limited (Rahma et al., 2013, Ann
Oncol
24:1972-9; Oettle et al., 2014, .1 Clin ()rico] 32:2423-9) and after two prior
treatments (one
usually gemcitabine-based, the other fluoropyrimidine-based), there are no
accepted
treatments (Seufferlein et al., 2012, Ann Oncol 23(suppl 7):vii33-40; Almhanna
& Kim,
2008, Oncology (Williston Park) 22:1176-83).
1081 There is an unmet need for more effective therapies in pancreatic cancer
patients who
have received and shown resistance to or relapsed from two or more prior
therapies.
SUMMARY
1091 In various embodiments, the present invention concerns antibodies,
antigen-binding
antibody fragments and fusion proteins that bind to the MUC5AC pancreatic
cancer mucin.
Preferably, the antibodies or fragments thereof bind to an epitope located
within the second to
fourth cysteine-rich subdomains of MUC5AC (amino acid residues 1575-2052, Cys2-
Cys4).
More preferably, the antibodies bind to an epitope located in amino acid
residues 1575-1725
3
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
and 1903-2052 (Cys2 and Cys 4). Even more preferably, the antibodies bind to
an epitope
located in amino acid residues 1575-1725 (Cys2+). Most preferably, the
antibodies bind to an
epitope located in Cys2.
10101 In preferred embodiments, the subject antibodies or fragments thereof
bind
specifically to pancreatic cancer cells, with little or no binding to normal
or non-neoplastic
pancreatic cells. The antibodies are capable of binding to the earliest stages
of pancreatic
cancer, with detection rates of about 50-60% for PanIN-1A, 70-80% for Pan1B
and 80-90%
for PanIN-2. More preferably, the antibodies bind to 80 to 90% or more of
human invasive
pancreatic adenocarcinoma, intraductal papillary mucinous neoplasia, PanIN-1A,
PanIN-1B
and PanIN-2 lesions. Most preferably, the antibodies can distinguish between
early stage
pancreatic cancer and non-malignant conditions such as pancreatitis.
PM Such antibodies are of particular use for early detection of cancer and
differential
diagnosis between early stage pancreatic cancer and benign pancreatic
conditions. In
preferred embodiments, such antibodies are of use for in vivo or ex vivo
analysis of samples
from individuals suspected of having early stage pancreatic or certain other
cancers. More
preferably, the antibodies are of use for detection and diagnosis of early
stage pancreatic
cancer by analysis of serum samples.
10121 In alternative embodiments, the antibodies, antibody fragments or fusion
proteins are
capable of binding to synthetic peptide sequences, for example to phage
display peptides,
such as WTWNITKAYPLP (SEQ ID NO:7) and ACPEWWG'FTC (SEQ ID NO:8). Such
synthetic peptides may be linear or cyclic and may or may not compete with
antibody binding
to the endogenous pancreatic cancer antigen. Amino acids in certain positions
of the synthetic
peptide sequences may be less critical for antibody binding than others. For
example, in SEQ
ID NO:7 the residues K, A and L at positions 7, 8 and 11 of the peptide
sequence may be
varied while still retaining antibody binding. Similarly, in SEQ ID NO:8 the
threonine
residues at positions 8 and 9 of the sequence may be varied, although
substitution of the
threonine at position 9 may significantly affect antibody binding to the
peptide.
10131 Binding of the antibodies to a target pancreatic cancer antigen may be
inhibited by
treatment of the target antigen with reagents such as dithiothreitol (DTF)
and/or periodate.
Thus, binding of the antibodies to a pancreatic cancer antigen may be
dependent upon the
presence of disulfide bonds and/or the glycosylation state of the target
antigen. In more
preferred embodiments, the epitope recognized by the subject antibodies is not
cross-reactive
with other reported mucin-specific antibodies, such as the MA5 antibody, the
CLII2-2
4
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
antibody and/or the 45M1 antibody (see, e.g., Major et al., J Histochem
Cytochem. 35:139-
48, 1987; Dion et al., Hybridoma 10:595-610, 1991).
[014] The subject antibodies or fragments may be naked antibodies or fragments
or
preferably are conjugated to at least one therapeutic and/or diagnostic agent
for delivery of
the agent to target tissues. In alternative embodiments, the subject
antibodies or fragments
may be part of a bispecific antibody with a first binding site for an epitope
of MUC5AC as
discussed above and a second binding site for a hapten conjugated to a
targetable construct.
The targetable construct may in turn be attached to at least one therapeutic
and/or diagnostic
agent, of use in pretargeting techniques.
[015] In preferred embodiments, the subject antibody, antibody fragment or
fusion protein
is a humanized PAM4 antibody or fragment, comprising the light chain variable
region CDR
sequences CDR1 (SASSSVSSSYLY, SEQ ID NO:1); CDR2 (STSNLAS, SEQ ID NO:2);
and CDR3 (HQWNRYPYT, SEQ ID NO:3); and the heavy chain variable region CDR
sequences CDR.1 (SYVLH, SEQ ID NO:4); CDR2 (YINPYNDGTQYNEKFKG, SEQ ID
NO:5) and CDR3 (GFGGSYGFAY, SEQ ID NO:6) and human antibody framework region
(FR) and constant region sequences. More preferably, the FRs of the light and
heavy chain
variable regions of the humanized PAM4 antibody or fragment thereof comprise
at least one
amino acid substituted from amino acid residues 5, 27, 30, 38, 48, 66, 67 and
69 of the
murine PAM4 heavy chain variable region (SEQ ID NO:12) and/or at least one
amino acid
selected from amino acid residues 21, 47, 59, 60, 85, 87 and 100 of the murine
PAM4 light
chain variable region (SEQ ID NO:1.0). Most preferably, the antibody or
fragment thereof
comprises the hPAM4 VH amino acid sequence of SEQ ID NO:19 and the hPAM4 VK
amino
acid sequence of SEQ ID NO:16.
[016] In alternative embodiments, the anti-pancreatic cancer antibody may be a
chimeric,
humanized or human antibody that binds to the same antigenic determinant
(epitope) as, or
competes for binding to MUC5AC with, a chimeric PAM4 (cPAM4) antibody. As
discussed
below, the cPAM4 antibody is one that comprises the light chain variable
region CDR
sequences CDR.1 (SASSSVSSSYLY, SEQ ID NO:!); CDR2 (STSNLA.S, SEQ ID NO:2);
and CDR3 (IIQWNR.YPYT, SEQ ID NO:3); and the heavy chain variable region CDR
sequences CDR1 (SYVLH, SEQ ID NO:4); CDR2 (YINPYNDGTQYNEKFKG, SEQ ID
NO:5)and CDR3 (GFGGSYGFAY, SEQ ID NO:6). Antibodies that bind to the same
antigenic determinant may be identified by a variety of techniques known in
the art, such as
by competitive binding studies using the cPAM4 antibody as the competing
antibody and
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
human pancreatic mucin or MUC5AC as the target antigen. Antibodies that block
(compete
for) binding to human pancreatic mucin by a cPAM4 antibody are referred to as
cross
blocking antibodies. Preferably, such cross-blocking antibodies are ones that
bind to an
epitope located within the second to fourth cysteine-rich subdomains of
MUC5AC, or that
compete for binding to amino acid residues 1575-2052 with a PAM4 antibody.
More
preferably, the antibodies bind to an epitope located in Cys2.
[0171 Other embodiments concern cancer cell-targeting therapeutic
immunoconjugates
comprising an antibody or fragment thereof or fusion protein bound to at least
one therapeutic
agent. Preferably, the therapeutic agent is selected from the group consisting
of a
radionuclide, an immunomodulator, a hormone, a hormone antagonist, an enzyme,
an
oligonucleotide such as an anti-sense oligonucleotide or a siRNA, an enzyme
inhibitor, a
photoactive therapeutic agent, a cytotoxic agent such as a drug or toxin, an
angiogenesis
inhibitor and a pro-apoptotic agent. In embodiments where more than one
therapeutic agent is
used, the therapeutic agents may comprise multiple copies of the same
therapeutic agent or
else combinations of different therapeutic agents. More preferably, the
therapeutic agent is a
radionuclide, such as 90Y. The labeled antibody may be administered alone, or
in combination
with one or more other therapeutic agents, such as low-dose gemcitabine.
10181 In certain embodiments, the therapeutic agent is a cytotoxic agent, such
as a drug or a
toxin. Also preferred, the drug is selected from the group consisting of
nitrogen mustards,
ethylenimine derivatives, alkyl sulfonates, nitrosoureas, gemcitabine,
triazenes, folic acid
analogs, anthracyclines, taxanes. COX-2 inhibitors, pyrimidine analogs, purine
analogs,
antibiotics, enzyme inhibitors, epipodophyllotoxins, platinum coordination
complexes, virica
alkaloids, substituted ureas, methyl hydrazine derivatives, adrenocortical
suppressants,
hormone antagonists, endostatin, taxols, camptothecins, SN-38, doxombicins and
their
analogs, antimetabolites, alkylating agents, antimitotics, anti-arigiogenic
agents, tyrosine
kinase inhibitors, Bruton tyrosine kinase inhibitors, mTOR inhibitors, heat
shock protein
(HSP90) inhibitors, proteosome inhibitors, HDAC inhibitors, pro-apoptotic
agents,
methotrexate, CPT-11, SN-38, 2-PDOX, pro-2-PDOX, and a combination thereof.
[0191 In another preferred embodiment, the therapeutic agent is a toxin
selected from the
group consisting of ricin, abrin, alpha toxin, saporin, ribonuclease (RNase),
DNase I,
Staphylococcal enterotoxin-A, pokeweed antiviral protein, gelonin, diphtheria
toxin,
Pseudomonas exotoxin, and Pseudomonas endotoxin and combinations thereof. Or
an
immunomodulator selected from the group consisting of a cytokine, a stem cell
growth
6
CA 02948013 2016-11-03
WO 2016/003869 PCT/US2015/038252
factor, a lymphotoxin, a hematopoietic factor, a colony stimulating factor
(CSF), an
interferon (IFN), a stem cell growth factor, erythropoietin, thrombopoietin
and a
combinations thereof.
10201 Alternatively, the therapeutic agent is an enzyme selected from the
group consisting
of malate dehydrogenase, staphylococcal nuclease, delta-V-steroid isomerase,
yeast alcohol
dehydrogenase, alpha-glycerophosphate dehydrogenase, triose phosphate
isomerase,
horseradish peroxidase, alkaline phosphata.se, asparaginase, glucose oxidase,
beta-
galactosidase, ribonuclease, urease, catalase, glucose-6-phosphate
dehydrogenase,
glucoamylase and acetylcholinesterase. Such enzymes may be used, for example,
in
combination with prodnigs that are administered in relatively non-toxic form
and converted
at the target site by the enzyme into a cytotoxic agent. In other
alternatives, a drug may be
converted into less toxic form by endogenous enzymes in the subject but may be
reconverted
into a cytotoxic form by the therapeutic enzyme.
[0211 Other therapeutic agents include radionuclides such as I4C, I3N, ISO,
32P, 33P, 47Sc,
5ICr, 57Co, "Co, 59Fe, 62Cu, 67Cu, 67Ga, 670a, 75Br, 75Se, "Se, 76Br, 77AS,
7713r, 8 93T,
89 90 95 97 99 99m 103m 103 105 105 107 109
109
Sr, Y, Ru, Ru, Mo, Tc, Rh, Ru, Rh, Ru, IIg, Pd, Pt,
11 IA g, I I 'in , 13m1n, I I9Sb, I 2ImTe, 122mTo, 1251, 12501o, 1261, 1311,
1331, 142pr, 143pr, 149pm,
152Dy, 153SM, 16IHO, 161Tb, 165Tm, 166Dy, 166//0, 167Tin, 1681-m, 169Er, 169--
Yb ,
177Lu, 186Re,
188Re, 189m05, 189Re, 1921r, 1941r, 197Pt, 198Au, 199Au, 201n, 263/1g,
211m, 21lBi,
211pb, 212Bi5 212pb, 213Bi, 215po, 217m, 219Ro, 221Fr, 223Ra, 224Ac, 225Ao,
255Fm or 777
Th.
10221 A variety of tyrosine kinase inhibitors are known in the art and any
such known
therapeutic agent may be utilized. Exemplary tyrosine kinase inhibitors
include, but are not
limited to canertinib, dasatinib, erlotinib, gefitinib, imatinib, lapatinib,
leflunomide, nilotinib,
pazopanib, semaxinib, sorafenib, sunitinib, sutent and vatalanib. A specific
class of tyrosine
kinase inhibitor is the Bruton tyrosine kinase inhibitor. Bruton tyrosine
kinase (Btk) has a well-
defined role in B-cell development Bruton kinase inhibitors include, but are
not limited to, PCI-
32765 (ibrutinib), PCI-45292, GDC-0834, LFM-A13 and RN486.
10231 The subject antibody or fragment may be conjugated to at least one
diagnostic (or
detection) agent. Preferably, the diagnostic agent is selected from the group
consisting of a
radionuclide, a contrast agent, a fluorescent agent, a chemiluminescent agent,
a
bioluminescent agent, a paramagnetic ion, an enzyme and a photoactive
diagnostic agent.
Still more preferred, the diagnostic agent is a radionuclide with an energy
between 20 and
4,000 keV or is a radionuclide selected from the group consisting of I win,
"In, I77Lu, I8F,
7
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
62cu, Cu, btu, 1201, 1231, 1241, 1251,
1311,
52Fe, 67Ga, 68Ga, 86Y, 9 Y, 89' , 94
mTc, 94Tc, 99mTc,
Im-158Gd, 32P, "C, 13.N1, 150, 186Re, 188Re, 51Mn, "mMn, "Co, 72As, 75Br,
76Br, 82mRb, "Sr, or
other gamma-, beta-, or positron-emitters. In a particularly preferred
embodiment, the
diagnostic radionuclide 18F is used for labeling and PET imaging, as described
in the
Examples below. The 18F may be attached to an antibody, antibody fragment or
peptide by
complexation to a metal, such as aluminum, and binding of the 18F-metal
complex to a
chelating moiety that is conjugated to a targeting protein, peptide or other
molecule.
10241 Also preferred, the diagnostic agent is a paramagnetic ion, such as
chromium (III),
manganese (11), iron (111), iron (II), cobalt (11), nickel (I1), copper (II),
neodymium (III),
samarium (III), ytterbium (III), gadolinium (III), vanadium (II), terbium
(III), dysprosium
(III), holmium (Iii) and erbium (III), or a radiopaque material, such as
barium, diatrizoate,
ethiodized oil, gallium citrate, iocarmic acid, iocetamic acid, iodamide,
iodipamide,
iodoxamic acid, iogulamide, iohexol, iopamidol, iopanoic acid, ioprocemic
acid, iosefamic
acid, ioseric acid, iosulamide meglumine, iosemetic acid, iotasul, iotetric
acid, iothalamic
acid, iotroxic acid, ioxaglic acid, ioxotrizoic acid, ipodate, meglumine,
metrizamide,
metrizoate, propyliodone, and thallous chloride.
10251 In still other embodiments, the diagnostic agent is a fluorescent
labeling compound
selected from the group consisting of fluorescein isothiocyanate, rhodamine,
phycoerytherin,
phycocyanin, allophycocyanin, o-phthaldehyde and fluorescamine, a
chemiktminescent
labeling compound selected from the group consisting of luminol, isoluminol,
an aromatic
acridinium ester, an imidazole, an actidinium salt and an oxalate ester, or a
bioluminescent
compound selected from the group consisting of luciferin, luciferase and
aequorin. In another
embodiment, the diagnostic inununoconjugates are used in intraoperative,
endoscopic, or
intravascular tumor diagnosis.
10261 Also contemplated are multivalent, multispecific antibodies or fragments
thereof
comprising at least one binding site that binds to an epitope of MUC5AC as
discussed above
and one or more hapten binding sites having affinity towards hapten molecules.
Preferably,
the antibody or fragment thereof is a chimeric, humanized or fully human
antibody or
fragment thereof. The hapten molecule may be conjugated to a targetable
construct for
delivery of one or more therapeutic and/or diagnostic agents. In certain
preferred
embodiments, the multivalent antibodies or fragments thereof may be prepared
by the
DOCK-AND-LOCKTm (DNLTM) technique, as described below. An exemplary DNLTM
construct incorporating hPAM4 antibody fragments is designated TF10, as
described below.
8
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
10271 Also contemplated is a bispecific antibody or fragment thereof
comprising at least one
binding site with an affinity toward an epitope of MUC5AC as discussed above
and at least
one binding site with an affinity toward a targetable construct which is
capable of carrying at
least one diagnostic and/or therapeutic agent. Targetable constructs suitable
for use are
disclosed, for example, in U.S. Patent Nos. 6,576,746; 6,962,702; 7,052,872;
7,138,103;
7,172,751; 7,405,320; 7,597,876; 7,563,433; 7,993,626; ,147,799; 8,153,100;
8,153,101;
8,202,509; 8,343,460; 8,444,956, 8,496,912; 8,545,809; 8,617,518; and
8,632,752, the
Examples section of each of which is incorporated herein by reference.
[028] Other embodiments concern fusion proteins comprising at least two anti-
pancreatic
cancer antibodies and fragments thereof as described herein. Alternatively,
the fusion protein
or fragment thereof may comprise at least one first antibody or fragment
thereof that binds to
an epitope of MUC5AC as discussed above and at least one second MAb or
fragment thereof.
Preferably, the second MAb binds to a tumor-associated antigen, for example
selected from
the group consisting of CA19.9, DUPAN2, SPAN1, Nd2, B72.3, CC49, CEA
(CEACAM5),
CEACAM6, Lea, the Lewis antigen Le(y), CSAp, insulin-like growth factor (Iff),
epithelial
glycoprotein-1 (EGP-1), epithelial glycoprotein-2 (EGP-2), CD-80, placental
growth factor
(P1GF), carbonic anhydrase IX, tenascin, IL-6, HLA-DR, CD40, CD74 (e.g.,
milatuzumab),
CD 138 (syndecan-1), CTLA-4, PD-1, PD-L I, TIM-3, LAG-3, matrix
metalloproteinase- I
(MMP-1), MMP-2, MMP-7, IVEVIP-14, MUC1, MUC2, MUC3, MUC4, MUC5AC,
MUC16, M'UC17, TAG-72, EGFR, platelet-derived growth factor (PDGF),
angiogenesis
factors (e.g., VEGF and P1GF), products of oncogenes (e.g., bc1-2, Kras, p53),
cME'F,
HER2/neu, and antigens associated with gastric cancer and colorectal cancer.
The second
MAb may also bind to a different epitope of MUC5AC than the first MAb. The
antibody
fusion protein or fragments thereof may further comprise at least one
diagnostic and/or
therapeutic agent.
[029] Also described herein are DNA sequences comprising a nucleic acid
encoding an anti-
pancreatic cancer antibody, fusion protein, multispecific antibody, bispecific
antibody or
fragment thereof as described herein. Other embodiments concern expression
vectors and/or
host cells comprising the antibody-encoding DNA sequences. In certain
preferred
embodiments, the host cell may be an Sp2/0 cell line transformed with a mutant
Bc1-2 gene,
for example with a triple mutant Bc1-2 gene (T69E, S70E, S87E), that has been
adapted to
cell transformation and growth in serum free medium. (See, e.g., U.S. Patent
Nos. 7,531,327;
7,537,930; and 7,608,425, the Examples section of each of which is
incorporated herein by
9
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
reference.)
[030] Another embodiment concerns methods of delivering a diagnostic or
therapeutic
agent, or a combination thereof, to a target comprising (i) providing a
composition that
comprises an anti-pancreatic cancer antibody or fragment that binds to an
epitope located
within the second to fourth cysteine-rich subdomains of MUC5AC (amino acid
residues
1575-2052), more preferably to an epitope located in amino acid residues 1575-
1725 and
1903-2052 (Cys2 and Cys 4), even more preferably to an epitope located in
amino acid
residues 1575-1725 (Cys2-+), most preferably, to an epitope located in Cys2,
conjugated to at
least one diagnostic and/or therapeutic agent and (ii) administering to a
subject in need
thereof the diagnostic or therapeutic conjugate of any one of the antibodies,
antibody
fragments or fusion proteins claimed herein.
[031] Also contemplated is a method of delivering a diagnostic agent, a
therapeutic agent,
or a combination thereof to a target, comprising: (a) administering to a
subject any one of the
multivalent, multispecific or bispecific antibodies or fragments thereof that
have an affinity
toward an epitope of MUC5AC as discussed above and comprising one or more
hapten
binding sites; (b) waiting a sufficient amount of time for antibody that does
not bind to
MUC5AC to clear the subject's blood stream; and (c) administering to said
subject a carrier
molecule comprising a diagnostic agent, a therapeutic agent, or a combination
thereof, that
binds to a binding site of the antibody. Preferably, the carrier molecule
binds to more than
one binding site of the antibody.
[032] Described herein is a method for diagnosing or treating cancer,
comprising: (a)
administering to a subject any one of the multivalent, multispecific
antibodies or fragments
thereof claimed herein that have an affinity toward an epitope of MUC5AC as
discussed
above and comprising one or more hapten binding sites; (b) waiting a
sufficient amount of
time for an amount of the non-bound antibody to clear the subject's blood
stream; and (c)
administering to said subject a carrier molecule comprising a diagnostic
agent, a therapeutic
agent, or a combination thereof, that binds to a binding site of the antibody.
In a preferred
embodiment the cancer is pancreatic cancer. Also preferred, the method can be
used for
intTaoperative identification of diseased tissues, endoscopic identification
of diseased tissues,
or intravascular identification of diseased tissues.
[033] Another embodiment is a method of treating a malignancy in a subject
comprising
administering to said subject a therapeutically effective amount of an
antibody or fragment
thereof that binds to an epitope of MUC5AC as discussed above, optionally
conjugated to at
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
least one therapeutic agent, such as 9 Y. The antibody or fragment thereof may
alternatively
be a naked antibody or fragment thereof. In more preferred embodiments, the
antibody or
fragment is administered either before, simultaneously with, or after
administration of
another therapeutic agent as described above.
[034] Contemplated herein is a method of diagnosing a malignancy in a subject,
particularly
a pancreatic cancer, comprising (a) administering to said subject a diagnostic
conjugate
comprising an antibody or fragment thereof that binds to an epitope of MUC5AC
as
discussed above, wherein said IvIAb or fragment thereof is conjugated to at
least one
diagnostic agent, and (b) detecting the presence of labeled antibody bound to
pancreatic
cancer cells or other malignant cells, wherein binding of the antibody is
diagnostic for the
presence of pancreatic cancer or another malignancy. In preferred embodiments,
the antibody
or fragment binds to pancreatic cancer and not to normal pancreatic tissue,
pancreatitis or
other non-malignant conditions. In less preferred embodiments, the antibody or
fragment
binds at a significantly higher level to cancer cells than to non-malignant
cells, allowing
differential diagnosis of cancer from non-malignant conditions. In a most
preferred
embodiment, the diagnostic agent may be an F-18 labeled molecule that is
detected by PET
imaging.
[035] In more preferred embodiments, the use of anti-pancreatic cancer
antibodies that bind
to an epitope located within the second to fourth cysteine-rich subdomains of
MUC5AC
(amino acid residues 1575-2052), more preferably to an epitope located in
amino acid
residues 1575-1725 and 1903-2052 (Cys2 and Cys 4), even more preferably to an
epitope
located in amino acid residues 1575-1725 (Cys2+), most preferably, to an
epitope located in
Cys2, allows the detection and/or diagnosis of pancreatic cancer with high
specificity and
sensitivity at the earliest stages of malignant disease. Preferably, the
diagnostic antibody or
fragment is capable of labeling at least 70%, more preferably at least 80%,
more preferably at
least 90%, more preferably at least 95%, most preferably about 100% of well
differentiated,
moderately differentiated and poorly differentiated pancreatic cancer and 90%
or more of
invasive pancreatic adenocarcinomas. The anti-pancreatic cancer antibody of
use is
preferably capable of detecting 85% or more of PanIN-1A, PanIN-1B, PanIN-2,
TPMN and
MCN precursor lesions. Most preferably, immunoassays using the anti-pancreatic
cancer
antibody are capable of detecting 89% or more of total PanIN, 86% or more of
IPMN, and
92% or more of MCN.
[036] An alternative embodiment is a method of detecting the presence of PAM4-
binding
11
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
MUC5AC and/or diagnosing pancreatic cancer in an individual by in vitro
analysis of blood,
plasma or serum samples. Preferably, the sample is subjected to an organic
phase extraction,
using an organic solvent such as butanol, before it is processed for
immunodetection using an
anti-pancreatic cancer antibody, such as a PAM4 antibody. Following organic
phase
extraction, the extracted aqueous phase is analyzed for the presence of the
epitope of
MUC5AC to which PAM4 binds in the sample, using any of a variety of
immunoassay
techniques known in. the art, such as EL1SA., sandwich immunoassay, solid
phase RIA., and
similar techniques. Surprisingly, the organic phase extraction results in the
removal of an
inhibitor of PAM4 binding to MUC5AC, allowing detection of MUC5AC in fresh
serum
samples. More surprisingly, using the in vitro analysis techniques described
herein, serum
samples may be analyzed to detect and/or diagnose pancreatic cancer in a
subject at the
earliest stages of pancreatic adenocarcinoma. These unexpected results provide
the first
serum-based assay technique that is diagnostic for the presence of early stage
pancreatic
cancer.
[037] Another embodiment is a method of treating a cancer cell in a subject
comprising
administering to said subject a composition comprising a naked antibody or
fragment thereof
or a naked antibody fusion protein or fragment thereof that binds to an
epitope of MUC5A.0
as discussed above. Preferably, the method further comprises administering a
second naked
antibody or fragment thereof selected from the group consisting of CA19.9,
DUPAN2,
SPAN!, Nd2, B72.3, CC49, anti-CEA, anti-CEACAM6, anti-EGP-1, anti-EGP-2, anti-
Le,
antibodies defined by the Lewis antigen Le(y), and antibodies against CSAp,
MUC1, MUC2,
MUC3, MIJC4, MUC5AC, MUC16, MIJC17, TAG-72, ECiFR, CD40, IILA.-DR, CD74,
CD138, angiogenesis factors (e.g., VEGF and placenta-like growth factor
(P1GF),
growth factor OGF), tenascin, platelet-derived growth factor, 1L-6, products
of oncogenes,
cMET, and IIER2/neu.
[038] Still other embodiments concern a method of diagnosing a malignancy in a
subject
comprising (i) performing an in vitro diagnosis assay on a specimen from said
subject with a
composition comprising an antibody or fragment thereof that binds to an
epitope of
MUC5AC as discussed above; and (ii) detecting the presence of antibody or
fragment bound
to malignant cells in the specimen. Preferably, the malignancy is a cancer.
More preferably,
the cancer is pancreatic cancer.
BRIEF DESCRIPTION OF THE DRAWINGS
[039] The following drawings are provided to illustrate preferred embodiments
of the
12
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
invention. However, the claimed subject matter is in no way limited by the
illustrative
embodiments disclosed in the drawings.
[040] FIG. 1. Co-localization of PAM4 antigen with MUC5AC by cytoflurometric
staining. Mucin-expressing cell lines (Capan-1 , BxPC-3, HT-29, and MCF-7)
were stained
with DAPI, 2-11M1 (anti-MUC5AC), hPAM4, and examined by immunofluorescence
microcopy. BxPC-3 and HT-29 cells were also stained with anti-MUCl. PAM4
antigen was
shown to co-localize with MUC5A.C, not MUC 1.
[041] FIG. 2. Co-knockdown of PAM4 antigen and MUC5AC by MUC5AC-specific
siRNA. CFPAC-1 cells were treated with a MUC5AC-specific siRNA, followed by
immunostaining with DAPI, hPAM4, and 2-11M1 (anti-MUC5AC), or with DAPI,
hPA.M.4
and anti-MUC 1. Untreated Cells or cells treated with only the transfection
agent (Mock)
served as controls. Cells treated with MUC5AC-specific siRNA lost the binding
to anti-
MUC5AC and hPAM4 concurrently, with little effect on the binding to anti-MUC
I.
[042] FIG. 3A. Immunoreactivity of fractions eluted from SEPHAROS.E CL-2B.
Capan-I cell culture supernatant was separated on a SEP11 AROSE CL2B column
with the
eluted fractions analyzed by hPAM4 and anti-MUCI.
[043] FIG. 3B. Immunoreactivity of fractions eluted from SEPHAROSF, CL-2B.
The
void-volume (Vo) fractions of Capan- I reacted positively with three anti-
MUC5AC
antibodies (45M1, 1-13M1 and H-160), but not with 2Q445, which recognizes the
unglycosylated tandem repeat region of MUC5AC.
[044] FIG. 3C. Immunoreactivity of fractions eluted from SEPHAROS.E CL-2B.
The
Capan-I void-volume peak, following capture by 2-11M1, could be detected
directly by
HRP-hPAM4, or indirectly by biotin-45M1 plus SA-HRP.
[045] FIG. 4A. Agarose gel electrophoresis. The Capan-I void-volume peak
displayed the
characteristic banding pattern of MUC5AC as revealed by Western blot analysis
with
hPAM4, 45M1, and MAN-5ACI. In the left panel, samples in the lanes marked as!,
1/2, 1/4,
and 1/8 were tested undiluted, 2-, 4- and 8-fold diluted, respectively. In the
far right panel,
the monomeric and dimeric MUC5A.0 were indicated as M and D, respectively.
[046] FIG. 4B. Agarose gel electrophoresis. The serum from a pancreatic cancer
patient
(PS) tested positive for hPAM4-reactive substance was differentially detected
by hPAM4 and
three anti-MUC5AC antibodies (2-11M1, 45M1, and H-160). The Capan-1 void-
volume peak
(Vo) and normal serum sample (NS) were included as controls.
[047] FIG. 5A. Mapping the PAM4-reactive epitope on human MUC5AC. Schematic
13
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
diagram of different MUC5AC recombinant fragments (a-h) generated in PANC-1
cells for
mapping PAM4 epitope; Numbers are AA positions in the MUC5AC protein sequence
(UniProtKB/Swiss-Prot: P98088).
[048] FIG. 5B. Mapping the PAM4-reactive epitope on human MUC5AC. Western blot
of a-fragment (AA3992-5030), b-fragment (AA1-1218) and c-fragment (AA1218-
2199),
which spans the five N-terminal cysteine-rich subdomains (Cys1-2-3-4-5), with
hPAM4 and
45M1 antibodies. Lane m indicates samples from untransfected cells.
[049] FIG. 5C. Mapping the PAM4-reactive epitope on human MUC5AC. Western blot
of c-fragment (AA1218-2199), d-fragment (AA1218-1517) and e-fragment (AA1575-
2052),
with hPAM4, H160 and 45M1 antibodies. Lane m indicates samples from
untransfected
cells.
[050] FIG. 5D. Mapping the PAM4-reactive epitope on human MUC5AC. Western blot
of e-fragment (AA1575-2052), f-fragment (AA1725-2052), g-fragment (AA1575-1725
and
1903-2052) and h-fragment (AA1575-1853) with hPAM4 and 45M.1 antibodies. Lane
m
indicates samples from untransfected cells.
[051] FIG. 5E. Mapping the PAM4-reactive epitope on human MUC5AC. Western blot
of GFP-fused e*-fragment (AA1575-2052), e'-fragment (AA1725-2052), g*-fragment
(AA1575-1725 and 1903-2052) and h*-fragment (AA1575-1853) with hPAM4 and anti-
CM'
antibodies. Lane m indicates samples from untransfected cells.
[052] FIG. 6A. SDS-PAGE and Western blot analyses of recombinant MUC5AC
fragments expressed in E. coli. Four gels were run under similar conditions of
SDS-PAGE.
Gel was stained with coomassie blue. Samples, either reduced (R) or non-
reduced (NR), were
loaded at 500 ng/well; lane M, markers; lanes 1 & 3, Cys2-3-4 (AA1575-2052);
lanes 2 &4,
Cys2+ (AA1575-1725).
[053] FIG. 6B. SDS-PAGE and Western blot analyses of recombinant MUC5AC
fragments expressed in E. coll. Four gels were run under similar conditions of
SDS-PAGE.
Gel was transferred onto nitrocellulose membrane and stained with anti-Myc.
Samples, either
reduced (R) or non-reduced (NR), were loaded at 500 ng/well; lane M, markers;
lanes 1 & 3,
Cys2-3-4 (AA1575-2052); lanes 2 &4, Cys2+ (AA1575-1725).
[054] FIG. 6C. SDS-PAGE and Western blot analyses of recombinant MUC5AC
fragments expressed in E. coli. Four gels were run under similar conditions of
SDS-PAGE.
Gel was transferred onto nitrocellulose membrane and stained with hPAM4.
Samples, either
reduced (R) or non-reduced (NR), were loaded at 500 ng/well; lane M, markers;
lanes 1 & 3,
14
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Cys2-3-4 (AA1575-2052); lanes 2 &4, Cys2+ (AA1575-1725).
[055] FIG. 6D. SDS-PAGE and Western blot analyses of recombinant MUC5AC
fragments expressed in E. coli. Four gels were rim under similar conditions of
SDS-PAGE.
Gel was transferred onto nitrocellulose membrane and stained with 1-13M1.
Samples, either
reduced (R) or non-reduced (NR), were loaded at 500 ng/well; lane M, markers;
lanes 1 & 3,
Cys2-3-4 (AA1575-2052); lanes 2 &4, Cys2+ (AA1575-1725).
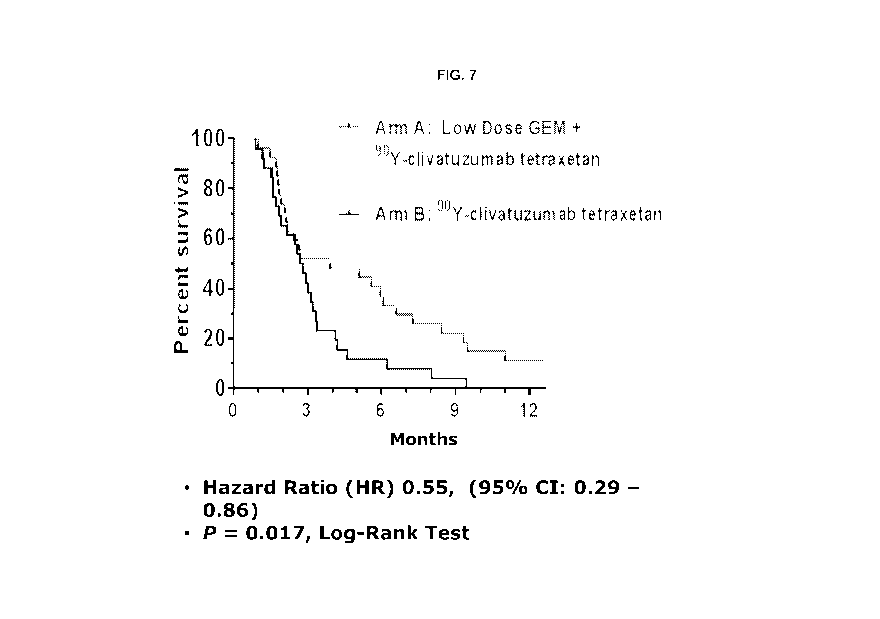
[056] FIG. 7. Overall survival. Kaplan-Meier curves and time-point analyses
for all 29
patients in Arm A ("Y-clivatuzumab tetraxetan combined with low-dose
gemcitabine) and
all 29 patients in Arm B ("Y-clivatuzumab tetraxetan alone).
[057] FIG. 8A. Variable region cDNA sequences (SEQ ID NO:9) and the deduced
amino
acid sequences (SEQ ID NO:10) of the murine PAM4 Vk. Amino acid sequences
encoded by
the corresponding DNA sequences are given as one-letter codes below the
nucleotide
sequence. Numbering of the nucleotide sequence is on the right side. The amino
acid residues
in the CDR regions are shown in bold and underlined. Kabat's Ig molecule
numbering is used
for amino acid residues as shown by the numbering above the amino acid
residues. The
amino acid residues numbered by a letter are the insertion residues defined by
Kabat
numbering scheme. The insertion residues have the same preceding digits as
that of the
previous residue.
[058] FIG. 8B. Variable region cDNA sequence (SEQ ID NO:!!) and the deduced
amino
acid sequence (SEQ ID NO:12) of the murine PAM4 VH. Amino acid sequences
encoded by
the corresponding DNA sequences are given as one-letter codes below the
nucleotide
sequence. Numbering of the nucleotide sequence is on the right side. The amino
acid residues
in the CDR regions are shown in bold and underlined. Kabat's Ig molecule
numbering is used
for amino acid residues as shown by the numbering above the amino acid
residues. The
amino acid residues numbered by a letter are the insertion residues defined by
Kabat
numbering scheme. The insertion residues have the same preceding digits as
that of the
previous residue.
[059] FIG. 9A. Amino acid sequence (SEQ ID NO:13) of the chimeric PA.M.4
(cPAM4)
Vk. The sequences are given as one letter codes. The amino acid residues in
the CDR regions
are shown in bold and underlined. Kabat's Ig molecule number scheme is used to
number the
residues.
[060] FIG. 9B. Amino acid sequence (SEQ ID NO:14) of the cPAM4 VH. The
sequences
are given as one letter codes. The amino acid residues in the CDR regions are
shown in bold
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
and underlined. Kabat's Ig molecule number scheme is used to number the
residues.
[061] FIG. 10A. Alignment of the Vic amino acid sequences of the human
antibody Walker
(SEQ ID NO:15) with PAM4 (SEQ ID NO:10) and hPAM4 (SEQ ID NO:16). Dots
indicate
the residues of PAM4 that are identical to the corresponding residues of the
human or
humanized antibodies. Boxed regions represent the CDR regions. Both N- and C-
terminal
residues (underlined) of hPAM4 are fixed by the staging vectors used. Kabat's
Ig molecule
number scheme is used to number the residues.
[062] FIG. 10B. Alignment of the VII amino acid sequences of the human
antibody Wi12
(FR1-3) (SEQ ID NO:17) and NEWM (FR4) (SEQ ID NO:18) with PAM4 (SEQ ID NO:12)
and hPA.M.4 (SEQ ID NO:19). Dots indicate the residues of PAM4 that are
identical to the
corresponding residues of the human or humanized antibodies. Boxed regions
represent the
CDR regions. Both N- and C-terminal residues (underlined) of hPAM4 are fixed
by the
staging vectors used. Kabat's Ig molecule number scheme is used to number the
residues.
[063] FIG. 11A. DNA. (SEQ ID NO:20) and amino acid (SEQ ID NO:16) sequences of
the
humanized PAM4 (hPAM4) Vk. Numbering of the nucleotide sequence is on the
right side.
Amino acid sequences encoded by the corresponding DNA sequences are given as
one-letter
codes. The amino acid residues in the CDR regions are shown in bold and
underlined. Kabat's
Ig molecule numbering scheme is used for amino acid residues.
[064] FIG. 11B. DNA (SEQ ID NO:21) and amino acid (SEQ ID NO:19) sequences of
the
hPAM4 VH. Numbering of the nucleotide sequence is on the right side. Amino
acid
sequences encoded by the corresponding DNA. sequences are given as one-letter
codes. The
amino acid residues in the CDR regions are shown in bold and underlined.
Kabat's Ig
molecule numbering scheme is used for amino acid residues.
[065] FIG. 12. Binding activity of humanized PAM4 antibody, hPAM4, as compared
to the
chimeric PAM4, cPAM4. hPAM4 is shown by diamonds and cPAM4 is shown by closed
circles. Results indicate comparable binding activity of the ITAM4 antibody
and cPAM4
when competing with 125I-cPAM4 binding to CaPanl antigens.
[066] FIG. 13. PET/CT fusion images for a patient with inoperable metastatic
pancreatic
cancer treated with fractionated 90Y-hPAM4 plus gemcitabine, before therapy
(left side) and
post-therapy (right side). The circle indicates the location of the primary
lesion, which shows
a significant decrease in PET/CT intensity following therapy.
[067] FIG. 14. 3D PET images for a patient with inoperable metastatic
pancreatic cancer
treated with fractionated 9 Y-hPAM4 plus gemcitabine, before therapy (left
side) and post-
16
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
therapy (right side). Arrows point to the locations of the primary lesion (on
right) and
metastases (on left), each of which shows a significant decrease in PET image
intensity after
therapy with radiolabeled hPAM4 plus gemcitabine.
[068] FIG. 15A. In vivo imaging of tumors using an "In-labeled diIISG peptide
(IMP 288)
with or without pretargeting TF10 bispecific anti-pancreatic cancer MUC5AC
antibody. FIG.
15A illustrates mice showing the location of tumors (arrow).
10691 FIG. 15B In vivo imaging of tumors using an "In-labeled diHSG peptide
(IMP 288)
with or without pretargeting IF 10 bispecific anti-pancreatic cancer WiC5AC
antibody. FIG.
15B shows the detected tumors with 111In4abeled IMP 288 in the presence
(above) or
absence (below) of TF10 bispecific antibody.
[070] FIG. 16. Exemplary binding curves for TF10, PAM4-IgG, PAM4-F(ab.)2and a
monovalent bsPAM4 chemical conjugate (PAM4-Fab' x anti-DTPA-Fab'). Binding to
target
mucin antigen was measured by ELISA assay.
[071] FIG. 17A. Immunoscintigraphy of CaPanl human pancreatic cancer
xenografts
(-0.25 g). An image of mice that were injected with bispecific TFIO (80 rig,
5.07 x 10-1 mol)
followed 16 h later by administration of 111In-IMP-288 (30 jiCi, 5.07 x 10-11
mol). The image
was taken 3 h later. The intensity of the image background was increased to
match the
intensity of the image obtained when "In-IMP-288 was administered alone (30
!Xi, 5.07 x
1011 mol).
[072] FIG. 17B. Immunoscintigraphy of CaPanl human pancreatic cancer
xenografts
(-0.25 g). No targeting was observed in mice given "In-IMP-288 alone.
[073] FIG. 17C. Immunoscintigraphy of CaPanl human pancreatic cancer
xenografts
(-0.25 g). An image of mice that were given "In-DOTA-PAM4-IgG (20 iCi, 5014)
with
imaging done 24 h later. Although tumors are visible, considerable background
activity is
still present at this time point.
[074] FIG. 18A. Extended biodistribution of111Tn-DOTA-PAM4-IgG (20 p.Ci, 50
lig) and
TF10-pretargeted "In-IMP-288 (80 lig, 5.07 x 10-10 mol TF10 followed 16 h
later with 30
!Xi, 5.07 x 10-11 mol "In-IMP-288) in nude mice bearing CaPanl human
pancreatic cancer
xenografts (mean tumor weight +1- SD, 0.28 +1- 0.21 and 0.10 1- 0.06 g for
the pretargeting
and IgG groups of animals, respectively) . FIG. 18A shows percent of initial
dose per gram
of tissue in tumor with PAM4 IgG (open circles), blood with PAM4 IgG (open
squares),
tumor with pretargeted peptide (closed circles) and blood with pretargeted
peptide (closed
squares).
17
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
[075] FIG. 18B. Extended biodistribution of I"In-DOTA-PAM4-IgG (20 11Ci, 50
ilg) and
TF10-pretargeted I IIn-IMP-288 (80 pg, 5.07 x 10-I mol TF10 followed 16 h
later with 30
pei, 5.07 x mol' I 'in-IMP-288) in nude mice bearing CaPani human
pancreatic cancer
xenografts (mean tumor weight +1- SD, 0.28 +/- 0.21 and 0.10 +/- 0.06 g for
the pretargeting
and IgG groups of animals, respectively). FIG. 18B shows percent of initial
dose per per
gram of tissue in liver with PAM4 IgG (open triangles), kidney with PAM4 IgG
(open
diamonds), liver with pretargeted peptide (closed triangles) and kidney with
pretargeted
peptide (closed diamonds).
[076] FIG. 18C. Extended biodistribution of' "In-DOTA-PAM4-IgG (20 !Xi, 50
ilg) and
TF10-pretargeted "In-IMP-288 (80 Itg, 5.07 x 10-I mol TF10 followed 16 h
later with 30
p.Ci, 5.07 x 1041 mol "In-IMP-288) in nude mice bearing CaPanl human
pancreatic cancer
xenogralls (mean tumor weight +/- SD, 0.28 +/- 0.21 and 0.10 +/- 0.06 g for
the pretargeting
and IgG groups of animals, respectively). FIG. 18C shows microcuries per gram
of tissue in
tumor with PAM4 IgG (open circles), blood with PAM4 IgG (open squares), tumor
with
pretargeted peptide (closed circles) and blood with pretargeted peptide
(closed squares).
[077] FIG. 18D. Extended biodistribution of I I lIn-DOTA-PAM4-IgG (20 pei, 50
gig) and
TF10-pretargeted "In-IMP-288 (80 Itg, 5.07 x 10-I mol TF10 followed 16 h
later with 30
p.Ci, 5.07 x 1041 mol "In-IMP-288) in nude mice bearing CaPanl human
pancreatic cancer
xenogralls (mean tumor weight +/- SD, 0.28 +/- 0.21 and 0.10 +/- 0.06 g for
the pretargeting
and IgG groups of animals, respectively). FIG. 18D shows microcuries per per
gram of tissue
in liver with PAM4 IgG (open triangles), kidney with PAM4 IgG (open diamonds),
liver with
pretargeted peptide (closed triangles) and kidney with pretargeted peptide
(closed diamonds).
[078] FIG. 19. Therapeutic activity of a single treatment of established (-0.4
cm3) CaPanl
tumors with 0.15 mCi of 90Y-hPAM4 IgG, or 0.25 or 0.50 mCi of TF10-pretargeted
90Y-IMP-
288.
[079] FIG. 20. Effect of gemcitabine potentiation of PT-RAIT therapy.
[080] FIG. 21. Effect of combination of cetuximab with gemcitabine and PT-
RAIT.
[081] FIG. 22. Differential diagnosis of pancreatic cancer using PAM4-based
immunoassay. The horizontal line shows the cutoff level selected for a
positive result, based
on ROC analysis.
[082] FIG. 23. Frequency distribution of PAM4 antigen in patient sera from
healthy
volunteers and individuals with varying stages of pancreatic cancer.
18
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
[083] FIG. 24. ROC curve for PAM4 serum immunoassay, showing sensitivity for
detection of 81.6% and specificity of 84.6%.
[084] FIG. 25. Accuracy of the PAM4-immunoassay was determined to be within
10% of
the nominal concentrations examined at or above the cutoff value of 2.40
units/mL. A linear
trend was calculated with an equation of y = 0.965x 4-0.174, and goodness of
fit r2 ¨ 0.999.
[085] FIG. 26. Frequency distribution of PAM4-reactive antigen in patient sera
by stage of
disease. Cutoff value = 2.4 units/mt., (horizontal line). The median values
(units/mi.) are
shown for each study group.
[086] FIG. 27. Receiver Operator Characteristics (ROC) curve for the
performance of the
PAM4-based immunoassay; pancreatic aderiocarcinoma vs. healthy adults. Values
for the
area under the curves (AIX) and 95% confidence limits are provided.
[087] FIG. 28A. Circulating PAM4 antigen levels correlated with
progression/regression of
tumor volume (CT) following treatment with 9 Y-PAM4-IgG plus gemcitabine.
Patient 076-
001 was responsive to therapy and serum PAM4 antigen decreased. Serum PAM4
levels
correlated with tumor volume.
[088] FIG. 28B. Circulating PAM4 antigen levels correlated with
progression/regression of
tumor volume (CT) following treatment with 90Y-PAM4-IgG plus gemcitabine.
Patient
1810002 showed an initial response to therapy, followed by recurrence of the
tumor. Serum
PAM4 levels correlated with tumor volume.
[089] FIG. 29. Reactivity of PAM4 with mucin standards in the presence or
absence of
palmitic acid.
10901 FIG. 30A. Sensitivity and specificity for PAM4 detection of PDAC vs.
chronic
pancreatitis (CP).
[091] FIG. 30B. Sensitivity and specificity for PAM4 detection of PDAC vs. all
benign
tissue samples.
[092] FIG. 31. Comparative labeling of PDAC vs. non-neoplastic prostate tissue
with
PAM4 vs. antibodies against MUC1, MUC,'4, CEACAM6 and CA19-9.
[093] FIG. 32. Reactivity of several anti-mucin MAbs with a high molecular
weight mucin
containing fraction (CPM1) isolated from the Capan-1, human pancreatic
adenocarcinoma.
MAbs are identified by clone name with reactive species of mucin indicated by
horizontal
bars beneath MAb clone names. In addition to PAM4, substantial reactions were
observed for
anti-WJC1, MIJC5AC, and CEACAM6 antibodies. All MAbs were employed at a
constant
flg/mL.
19
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
[094] FIG. 33. Reaction of several anti-mucin MAbs with PAM4-captured antigen.
Mucin
antigens were captured on hPAM4 coated plates, and then probed with several
murine anti-
mucin MA.bs for reaction signal. Both anti-MUC5AC MAbs (2-11 MI and 45M1)
bound to
the hPAM4-captured mucin, whereas the anti-MUC1 MAbs (MA.5 and KC4) did not
bind.
The homologous hPAM4/mPAM4, capture/probe immunoassay gave no signal,
suggesting
the density of PAM4 epitopes within the mucin may be low, possibly only a
single site. A
rabbit polyclonal anti-CPM I, IgG, was used as a positive control for reaction
with hPAM4-
captured antigen.
[095] FIG. 34A. Inhibition of hPAM4/antigen binding reaction by murine anti-
mucin
MAbs. Anti-mucin mMAbs (purified IgG) were added to CPM1-coated plates as
potential
inhibitors prior to addition of hPAM4. mPAM4 provided almost complete
inhibition of the
reaction between hPAM4 and antigen with the 45M1 anti-MUC5AC providing limited
inhibitory affect (IC.,õ = 25.5%). Neither 2-11M1, anti-MUC5AC nor MA5 and
KC,'4, anti-
MUC1 MAbs were able to inhibit the specific hPAM4/antigen reaction.
[096] FIG. 34B. Inhibition of hPAM4/antigen binding reaction by murine anti-
mucin
MAbs. A similar inhibition study was performed with several anti-MUC5AC MAbs
obtained
as ascites fluids. MA.bs 21M1, 62M1, and 463M1, anti-MUC5AC provided
substantial
inhibitory affect similar to that observed with TriPAM4, TgG, self-inhibition.
The ascites form
of 45M1 yielded an inhibitory affect similar to that of the purified IgG.
Ascites containing
anti-alpha fetoprotein was employed as a negative control.
[097] FIG. 35. Representation of the domains of the MUC5AC glycoprotein with
reactive
epitopes indicated for several anti-MUC5AC MAbs. Data derived by tansfection
with
plasmid vectors containing the cDNA of the 3'-end of MUC5AC, along with
derivative
cDNA. vectors obtained by restriction enzyme digestion, have identified the
location of
specific epitopes for anti-MUC5AC MAbs employed in the current studies.
Specific blocking
studies suggest the PAM4-epitope resides within the cysteine-rich C-terminus
domain.
DETAILED DESCRIPTION
Definitions
[098] Unless otherwise specified, "a" or "an" means one or more.
10991 As used herein, "about" means plus or minus 100/o. For example, "about
100" would
include any number between 90 and 110.
101001 As described herein, the term "PAM4 antibody" includes murine,
chimeric,
humanized and human PAM4 antibodies. In preferred embodiments, the PAM4
antibody or
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
antigen-binding fragment thereof comprises the CDR sequences of SEQ ID NO:1 to
SEQ ID
NO:6.
101011 As used herein, an "anti-pancreatic cancer antibody" is an antibody
that exhibits the
same diagnostic, therapeutic and binding characteristics as the PAM4 antibody.
In preferred
embodiments, the "anti-pancreatic cancer antibody" binds to an epitope located
within the
second to fourth cysteine-rich subdomains of MUC5AC (amino acid residues 1575-
2052),
more preferably to an epitope located in amino acid residues 1575-1725 and
1903-2052
(Cys2 and Cys 4), even more preferably to an epitope located in amino acid
residues 1575-
1725 (Cys2+), most preferably, to an epitope located in Cys2.
101021 A "non-endocrine pancreatic cancer" generally refers to cancers arising
from the
exocrine pancreatic glands. The term excludes pancreatic insulinomas and
includes
pancreatic carcinoma, pancreatic adenocarcinoma, adenosquamous carcinoma,
squamous cell
carcinoma and giant cell carcinoma and precursor lesions such as pancreatic
intra-epithelial
neoplasia (PanIN), mucinous cyst neoplasms (MCN) and intrapancreatic mucinous
neoplasms (IPMN), which are neoplastic but not yet malignant. The terms
"pancreatic
cancer" and "non-endocrine pancreatic cancer" are used interchangeably herein.
101031 An antibody, as described herein, refers to a full-length (i.e.,
naturally occurring or
formed by normal immunoglobulin gene fragment recombinatorial processes)
immunoglobulin molecule (e.g., an IgG antibody) or an immunologically active
(i.e.,
specifically binding) portion of an immunoglobulin molecule, like an antibody
fragment.
101041 An antibody fragment is a portion of an antibody such as F(ab1)2, Fab',
Fab, Fv, sf:v
and the like. Regardless of structure, an antibody fragment binds with the
same antigen that is
recognized by the full-length antibody. The term "antibody fragment" also
includes isolated
fragments consisting of the variable regions of antibodies, such as the "Fv"
fragments
consisting of the variable regions of the heavy and light chains and
recombinant single chain
polypeptide molecules in which light and heavy variable regions are connected
by a peptide
linker ("scFv proteins"). Another form of antibody fragment is a single domain
antibody
(nanobody).
101051 A naked antibody is an antibody or fragment thereof that is not
conjugated to a
therapeutic or diagnostic agent. Generally, the Fc portion of the antibody
molecule provides
effector functions, such as complement-mediated cytotoxicity (CDC) and ADCC
(antibody-
dependent cellular cytotoxicity), which set mechanisms into action that may
result in cell
lysis. However, the Fe portion may not be required for therapeutic function,
with other
21
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
mechanisms, such as signaling-induced apoptosis, coming into play. Naked
antibodies
include both polyclonal and monoclonal antibodies, as well as fusion proteins
and certain
recombinant antibodies, such as chimeric, humanized or human antibodies.
101061 A chimeric antibody is a recombinant protein that contains the variable
domains
including the complementarily determining regions (CDRs) of an antibody
derived from one
species, preferably a rodent antibody, while the constant domains of the
antibody molecule
are derived from those of a human antibody. For veterinary applications, the
constant
domains of the chimeric antibody may be derived from that of other species,
such as a cat or
dog.
101071 A humanized antibody is a recombinant protein in which the CDRs from an
antibody
from one species; e.g., a rodent antibody, are transferred from the heavy and
light variable
chains of the rodent antibody into human heavy and light variable domains
(e.g., framework
region sequences). The constant domains of the antibody molecule are derived
from those of
a human antibody. In certain embodiments, a limited number of framework region
amino
acid residues from the parent (rodent) antibody may be substituted into the
human antibody
framework region sequences.
101081 A human antibody is, e.g., an antibody obtained from transgenic mice
that have been
"engineered" to produce specific human antibodies in response to antigenic
challenge. In this
technique, elements of the human heavy and light chain loci are introduced
into strains of
mice derived from embryonic stem cell lines that contain targeted disruptions
of the
endogenous murine heavy chain and light chain loci. The transgenic mice can
synthesize
human antibodies specific for particular antigens, and the mice can be used to
produce human
antibody-secreting hybridomas. Methods for obtaining human antibodies from
transgenic
mice are described by Green et al., Nature Genet. 7:13 (1994), Lonberg et al.,
Nature 368:856
(1994), and Taylor et al., Int. Immun. 6:579 (1994). A fully human antibody
also can be
constructed by genetic or chromosomal transfection methods, as well as phage
display
technology, all of which are known in the art. See for example, McCafferty et
al., Nature
348:552-553 (1990) for the production of human antibodies and fragments
thereof in vitro,
from immunoglobulin variable domain gene repertoires from unimmunized donors.
In this
technique, antibody variable domain genes are cloned in-frame into either a
major or minor
coat protein gene of a filamentous bacteriophage, and displayed as functional
antibody
fragments on the surface of the phage particle. Because the filamentous
particle contains a
single-stranded DNA copy of the phage genome, selections based on the
functional properties
22
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
of the antibody also result in selection of the gene encoding the antibody
exhibiting those
properties. In this way, the phage mimics some of the properties of the B
cell. Phage display
can be performed in a variety of formats, for review, see e.g. Johnson and
Chiswell, Current
Opinion in Structural Biology 3:5564-571 (1993). Human antibodies may also be
generated
by in vitro activated B cells. See U.S. Pat. Nos. 5,567,610 and 5,229,275, the
Examples
sections of which are incorporated herein by reference.
[0109] A therapeutic agent is a compound, molecule or atom which is
administered
separately, concurrently or sequentially with an antibody moiety or conjugated
to an antibody
moiety, i.e., antibody or antibody fragment, or a subfragment, and is useful
in the treatment
of a disease. Examples of therapeutic agents include antibodies, antibody
fragments,
cytotoxic agents, drugs, toxins, nucleases, hormones, immunomodulators, pro-
apoptotic
agents, anti-angiogenic agents, boron compounds, photoactive agents or dyes
and
radioisotopes. Therapeutic agents of use are described in more detail below.
(0110] A diagnostic agent is a molecule, atom or other detectable moiety which
may be
administered conjugated to an antibody moiety or targetable construct and is
useful in
detecting or diagnosing a disease by locating cells containing the target
antigen. Useful
diagnostic agents include, but are not limited to, radioisotopes, dyes,
contrast agents,
fluorescent compounds or molecules and enhancing agents (e.g., paramagnetic
ions) for
magnetic resonance imaging (MRI) or positron emission tomography (PET)
scanning.
Preferably, the diagnostic agents are selected from the group consisting of
radioisotopes,
enhancing agents for use in magnetic resonance or PET imaging, and fluorescent
compounds.
In order to load an antibody component with radioactive metals, paramagnetic
ions or other
diagnostic cations, it may be necessary to react it with a reagent having a
long tail to which
are attached a multiplicity of chelating groups for binding the ions. Such a
tail can be a
polymer such as a polylysine, polysaccharide, or other derivatized or
derivatizable chain
having pendant groups to which can be bound chelating groups such as, e.g.,
ethylenediaininetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid
(DTPA),
DOTA, NOTA, NETA, porphyrins, polyamines, crown ethers, bis-
thiosemicarbazones,
polyoximes, and like groups known to be useful for this purpose. Chelates are
coupled to the
antibodies using standard chemistries. The chelate is normally linked to the
antibody by a
group which enables formation of a bond to the molecule with minimal loss of
immunoreactivity and minimal aggregation and/or internal cross-linking. Other,
more
unusual, methods and reagents for conjugating chelates to antibodies are
disclosed in U.S.
23
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Pat. No. 4,824,659, the Examples section of which is incorporated herein by
reference.
Particularly useful metal-chelate combinations include 2-benzyl-DTPA and its
monomethyl
and cyclohexyl analogs, used with diagnostic isotopes in the general energy
range of 60 to
4,000 keV, such as 1251, l311, 1231, 12.4i, 62L.,.-,u,
¨CU, 18F, "1Tn, 670a, 68Ga,99mTc, 94mTc, "C, 13N,
150, 76Br, for radioimaging. The same chelates, when complexed with non-
radioactive metals,
such as manganese, iron and gadolinium are useful for MR1, when used along
with the
antibodies of the invention. Macrocyclic chelates such as NOTA (1,4,7-
triaz.acyclononane-
NN,N"-triacetic acid), DOTA (1,4,7,10-tetraazacyclododecanetetraacetic acid),
and TETA
(p-bromoacetamido-benzyl-tetraethylaminetetraacetic acid) are of use with a
variety of
metals and radiometals, most particularly with radionuclides of gallium,
yttrium and copper,
respectively. Such metal-chelate complexes can be made very stable by
tailoring the ring size
to the metal of interest. Other ring-type chelates such as macrocyclic
polyethers, which are of
interest for stably binding nuclides, such as 223Ra for radioimmunotherapy
(RA1T) are
encompassed by the invention. More recently, techniques of general utility for
labeling
virtually any molecule with an 18F atom of use in PET imaging have been
described in U.S.
Patent Nos. 7,563,433; 7,597,876 and 7,993,626, the Examples section of each
incorporated
herein by reference.
101111 An immunoconjugate is an antibody, antibody fragment or antibody fusion
protein
conjugated to at least one therapeutic and/or diagnostic agent. The diagnostic
agent and/or
therapeutic agent are as defmed above.
10112] An expression vector is a DNA molecule comprising a gene that is
expressed in a host
cell. Typically, gene expression is placed under the control of certain
regulatory elements,
including constitutive or inducible promoters, tissue-specific regulatory
elements and
enhancers. Such a gene is said to be "operably linked to" the regulatory
elements.
101131 A recombinant host may be any prokaryotic or eukaryotic cell that
contains either a
cloning vector or expression vector. This term also includes those prokatyotic
or eukaryotic
cells, as well as transgenic animals, that have been genetically engineered to
contain the
cloned gene(s) in the chromosome or genome of the host cell. Suitable
mammalian host cells
include myeloma cells, such as SP2/0 cells, and NSO cells, as well as Chinese
Hamster Ovary
(CHO) cells, hybridoma cell lines and other mammalian host cell useful for
expressing
antibodies. Also particularly useful to express mAbs and other fusion proteins
are Sp2/0 cells
transfected with an apoptosis inhibitor, such as a Bel-EEE gene, and adapted
to grow and be
further transfected in serum free conditions, as described in U.S. Patent Nos.
7,531,327;
24
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
7,537,930; and 7,608,425, the Examples section of each of which is
incorporated herein by
reference.
Anti-Pancreatic Cancer Antibodies
101141 Various embodiments of the invention concern antibodies that react with
very high
selectivity with pancreatic cancer as opposed to normal or benign pancreatic
tissues. The anti-
pancreatic cancer antibodies and fragments thereof are preferably raised
against a crude
mucin preparation from a tumor of the human pancreas, although partially
purified or even
purified MUC5AC may be utilized. A non-limiting example of such antibodies is
the PAM4
antibody.
101151 The marine PAM4 (mPAM4) antibody was developed by employing pancreatic
cancer mucin derived from the xenografted RIP-1 human pancreatic carcinoma as
immunogen. (Gold et al., Int. J. Cancer, 57:204-210, 1994.) As discussed
below, antibody
cross-reactivity and immunohistochemical staining studies indicate that the
PAM4 antibody
recognizes a unique and novel epitope on MUC5AC. Immunohistochemical staining
studies
have shown that the PAM4 MAb binds to an antigen expressed by breast, pancreas
and other
cancer cells, with limited binding to normal human tissue. However, the
highest expression is
usually by pancreatic cancer cells. Thus, the PAM4 antibodies are relatively
specific to
pancreatic cancer and preferentially bind pancreatic cancer cells. The PAM4
antibody is
reactive with a target epitope which can be internalized. This epitope is
expressed primarily
by antigens associated with pancreatic cancer and not with focal pancreatitis
or normal
pancreatic tissue. Binding of PAM4 antibody to the PAM4 epitope is inhibited
by treatment
of the antigen with DTT or periodate. Localization and therapy studies using a
radiolabeled
PAM4 MAb in animal models have demonstrated tumor targeting and therapeutic
efficacy.
101161 The PAM4 antibodies bind to an epitope located within the second to
fourth cysteine-
rich subdomains of MUC5AC (amino acid residues 1575-2052), more preferably to
an
epitope located in amino acid residues 1575-1725 and 1903-2052 (Cys2 and Cys
4), even
more preferably to an epitope located in amino acid residues 1575-1725
(Cys2+), most
preferably, to an epitope located in Cys2. The PAW epitope is expressed by
many organs
and tumor types, but is preferentially expressed in pancreatic cancer cells.
Studies with a
PAM4 MAb, as in the Examples below, indicate that the antibody exhibits
several important
properties, which make it a good candidate for clinical diagnostic and
therapeutic
applications. The epitope provides a useful target for diagnosis and therapy
of pancreatic and
other cancers. The PAM4 antibody apparently recognizes an epitope of MUC5AC
that is
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
distinct from the epitopes recognized by non-PAM4 anti-pancreatic cancer
antibodies (e.g.,
CA19.9, DUPAN2, SPAN!, Nd2, CEACAM5, B72.3, anti-Lea, and other anti-Lewis
antigens).
101171 Surprisingly, the Examples below indicate that the MIJC5AC epitope to
which PAM4
binds is present in detectable concentrations in serum of patients with very
early stage
pancreatic cancer. Also surprisingly, it appears that an endogenous inhibitor
of PAM4
antibody binding to MUC5AC is present in fresh human serum. The inhibitor is
removed by
long-term frozen storage of serum samples, or by organic phase extraction of
fresh serum.
These unexpected results provide the basis of a relatively non-invasive, early
detection test
for pancreatic cancer, using blood, serum or plasma samples. In alternative
embodiments, the
PAM4 antibody may be used alone, or else in conjunction with one or more other
antibodies,
such as CA19.9 antibody, to detect pancreatic cancer markers in serum.
101181 At the tissue level, the reactivity of PAM4 is highly restricted to
PDAC, with the
biomarker expressed (or becomes accessible) at the earliest stages of
neoplastic development
(Gold et al., 1994, Int J Cancer 57:204-10; Gold et al., 2007, Clin Cancer Res
13:7380-7),
including pancreatic intraepithelial neoplasia (PanIN), and intraductal
papillary mucinous
neoplasm (IPMN). Notably, the PAM4-biomarker is absent from normal pancreas
and
benign, non-neoplastic lesions. In over 50 surgical specimens of chronic
pancreatitis, the
PAM4-biomarker was identified only within associated PanIN lesions and not by
the
inflamed parenchyma, including ducts, acinar cells, and acinar-ductal
metaplasia (Shi et al.,
2014, Arch Pathol Lab Med 138:220-8).
101191 Preclinical studies have demonstrated the potential applications of
PAM4 for
radioimmunoimaging and radioiinmunotherapy of pancreatic carcinoma (Gold et
al., 2002,
Crit Rev Oncol Hematol 39:147-54; Gold et al., 2004, Int j Cancer 109: 618-
26). In patients,
90Y-labeled, humanized PAM4 (9 Y-clivatuzumab tetraxetan, hereafter referred
to as 90Y-
hPAM4) was well tolerated with manageable hematologic toxicity under maximal
tolerated
90Y dosing, and produced objective responses in both chemotherapy-naive and -
refractory
patients with advanced PDAC (Gulec et al., 2011, Clin Cancer Res 17:4091-100).
Further,
90Y-hPAM4 in combination with low-dose gemcitabine showed enhanced therapeutic
efficacy in patients with metastatic pancreatic cancer (Ocean et al., 2012,
Cancer 118:5497-
506). In a recently completed phase lb study (Picozzi et al., 2014, J Clin
Oncol 132:4026)
involving 58 patients with metastatic PDAC who had at least 2 prior therapies,
multiple
cycles of fractionated 90Y-hPAM4 in combination with low radiosensitizing
doses of
26
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
gemcitabine significantly (P - 0.004) improved the Kaplan-Meier median overall
survival of
this difficult-to-treat population to 7.9 months, compared to those receiving
only 90Y-hPAM4
(3.4 months). These promising results led to the ongoing phase III
registration trial of 90Y-
hPAM4 in combination with gemcitabine (NCT01956812).
101201 In addition, PAM4 or hPAM4-based ELISA has been devised and evaluated
for
detection of PDAC, showing that nearly two-thirds of patients having confirmed
stage-1
disease had elevated PAM4 antigen in their serum (Gold et al., 2010, Cancer
Epidemiol
Biomarkers Prey 19:2786-94). However, the current assay, which employs hPAM4
as the
capture antibody and a polyclonal rabbit anti-mucin antisemm (IgG fraction) as
a probe, is
not optimal, because the polyclonal probe is available in only limited
quantities and, more
importantly, is not itself specific for the PAM4 antigen. Another concern for
further
development of the assay has been the unknown nature of the antigen marker to
which PAM4
is reactive. Given the clinical merit and ongoing evaluation of hPAM4 as a
potential
diagnostic and therapeutic agent for PDAC, there is an urgent need to identify
the PAM4
epitope. Towards this end, we recently proposed (Gold et al., 2013, Mol Cancer
12:143) that
PAM4 was reactive with the human MUC5AC, a polymeric gel-forming mucin with
the
monomeric form consisting of more than 5,000 amino acid residues organized
into three
major regions (Thornton et al., 2008, Annu Rev Physiol 70:459-86): a signal
peptide and four
von Willebrand factor (vWF)-like cysteine-rich domains (DI, D2, D' and D3) in
the N-
terminal region, a MUC11p15-type domain preceding the heavily 0-glycosylated
mucin
domain in the central region, and a cluster of vWF-like cysteine-rich domains
(1)4, B, C, and
CK) in the C-terminal region. In addition, 9 cysteine-rich subdomains
(designated Cysl,
Cys2, Cys3, Cys4, Cys5, Cys6, Cys7, Cys8, and Cys9) are interspersed within
the mucin
domain. Herein we present further evidence to support MUC5AC as the PAM4-
reactive
mucin and, importantly, have mapped the PAM4 epitope to Cys2.
101211 For therapeutic use, antibodies suitable for use in combination or
conjunction with
PAM4 antibodies include, for example, the antibodies CA19.9, DUPAN2, SPAN1,
Nd2,
B72.3, CC49, anti-CEA, anti-CEACAM6, anti-Le, anti-HLA-DR, anti-CD40, anti-
CD74,
anti-CD138, and antibodies defined by the Lewis antigen Le(y), or antibodies
against colon-
specific antigen-p (CSAp), CTLA-4, PD-1, PD-Li, TIM-3, LAG-3, matrix
metalloproteinase-1 (MMP-1), MMP-2, MMP-7, MMP-9, MMP-14, M'UC1, MUC2, MUC3,
M1.JC4, MUC5AC, MUC16, MUC17, EGP-1, ECiP-2, HER2ineu, EGFR, angiogenesis
factors (e.g., VEGF and PIGF), insulin-like growth factor (IGF), tenascin,
platelet-derived
27
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
growth factor, and IL-6, as well as products of oncogenes (bc1-2, Kras, p53),
cMET, and
antibodies against tumor necrosis substances, such as described in patents by
Epstein et al.
(U.S. Pat. Nos. 6,071,491, 6.017,514, 5,019,368 and 5,882,626). Such
antibodies would be
useful for complementing PAM4 antibody immunodetection and immunotherapy
methods.
These and other therapeutic agents could act synergistically with anti-
pancreatic cancer
antibodies, such as PAM4 antibody, when administered before, together with or
after
administration of PAM4 antibody.
101221 In therapeutic applications, antibodies that are agonistic or
antagonistic to
immunomodulators involved in effector cell function against tumor cells could
also be useful
in combination with PAM4 antibodies alone or in combination with other tumor-
associated
antibodies, one example being antibodies against CD40. Todiyk et al., J.
Immunol Methods,
248:139-147 (2001); Turner et al., J. Immunol, 166:89-94 (2001). Also of use
are antibodies
against markers or products of oncogenes (e.g., bc1-2, Kras, p53, cMET), or
antibodies
against angiogenesis factors, such as VEGFR and placenta-like growth factor
(PIGF).
101231 The availability of another PAM4-like antibody that binds to a
different epitope of
MUC5AC is important for the development of a double-determinant enzyme-linked
immunosorbent assay (ELISA), of use for MUC5AC in clinical samples. ELISA
experiments
are described in the Examples below.
101241 The murine, chimeric, humanized and fully human PAM4 antibodies and
fragments
thereof described herein are exemplary of anti-pancreatic cancer antibodies of
use for
diagnostic and/or therapeutic methods. The Examples below disclose a preferred
embodiment
of the construction and use of a humanized PAM4 antibody. Because non-human
monoclonal
antibodies can be recognized by the human host as a foreign protein, and
repeated injections
can lead to harmful hypersensitivity reactions, humanization of a murine
antibody sequences
can reduce the adverse immune response that patients may experience. For
murine-based
monoclonal antibodies, this is often referred to as a Human Anti-Mouse
Antibody (HAMA)
response. Preferably some human residues in the framework regions of the
humanized anti
pancreatic cancer antibody or fragments thereof are replaced by their murine
counterparts. It
is also preferred that a combination of framework sequences from two different
human
antibodies is used for VH. The constant domains of the antibody molecule are
derived from
those of a human antibody.
Antibody Preparation
101251 Monoclonal antibodies for specific antigens may be obtained by methods
known to
28
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
those skilled in the art. See, for example, Kohler and Milstein, Nature 256:
495 (1975), and
Coligan et al. (eds.), CURRENT PROTOCOLS IN IMMUNOLOGY, VOL. 1, pages 2.5.1-
2.6.7 (John Wiley & Sons 1991) (hereinafter "Coligan"). Briefly, anti-
pancreatic cancer
MAbs can be obtained by injecting mice with a composition comprising a mixture
of
pancreatic cancer mucins comprising MUC5AC, or a purified MUC5AC, or a peptide
or
protein corresponding to an epitope located within the second to fourth
cysteine-rich domains
of MUC5AC (amino acid residues 1575-2052), more preferably to an epitope
located in
amino acid residues 1575-1725 and 1903-2052 (Cys2 and Cys 4), even more
preferably to an
epitope located in amino acid residues 1575-1725 (Cys2+), most preferably, to
an epitope
located in Cys2, verifying the presence of antibody production by removing a
serum sample,
removing the spleen to obtain B-lymphocytes, fusing the B-lymphocytes with
myeloma cells
to produce hybridomas, cloning the hybridomas, selecting positive clones which
produce
antibodies to MUC5AC, culturing the clones that produce antibodies to an
epitope of
MUC5AC as discussed above, and isolating anti-pancreatic cancer antibodies
from the
hybridoma cultures.
101261 After the initial raising of antibodies to the immunogen, the
antibodies can be
sequenced and subsequently prepared by recombinant techniques to produce
chimeric or
humanized antibodies. Chimerization of murine antibodies and antibody
fragments are well
known to those skilled in the art The use of antibody components derived from
chimerized
monoclonal antibodies reduces potential problems associated with the
immunogenicity of
murine constant regions.
101271 General techniques for cloning murine immunoglobulin variable domains
are
described, for example, by the publication of Orlandi et al., Proc Nat'l Acad.
Sci. USA 86:
3833 (1989), incorporated herein by reference. In general, the VK (variable
light chain) and
VH (variable heavy chain) sequences for murine antibodies can be obtained by a
variety of
molecular cloning procedures, such as RT-PCR, 5'-RACE, and cDNA library
screening.
Specifically, the VH and VK sequences of the murine PAM4 MAb were cloned by
PCR
amplification from the hybridoma cells by RT-PCR, and their sequences
determined by DNA
sequencing. To confirm their authenticity, the cloned VK and V11 genes can be
expressed in
cell culture as a chimeric Ab as described by Orlandi et al., (Proc Natl.
Acad. Sci., USA, 86:
3833, 1989).
101281 In a preferred embodiment, a chimerized PAM4 antibody or antibody
fragment
comprises the complementarity-determining regions (CDRs) and framework regions
(FR) of
29
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
a murine PAM4 MAb and the light and heavy chain constant regions of a human
antibody,
wherein the CDRs of the light chain variable region of the chimerized PAM4
comprises
CDR.1 (SASSSVSSSYLY, SEQ ID NO:1); CDR2 (STSNLA.S, SEQ ID NO:2); and CDR3
(HQWNRYPYT, SEQ ID NO:3); and the CDRs of the heavy chain variable region of
the
chimerized PAM4 MAb comprises CDR1 (SYVLII, SEQ ID NO:4); CDR2
(YINPYNDGTQYNEKFKG, SEQ ID NO:5) and CDR3 (GFGGSYGFAY, SEQ ID NO:6).
The use of antibody components derived from chimerized monoclonal antibodies
reduces
potential problems associated with the immunogenicity of murine constant
regions.
101291 Humanization of murine antibodies and antibody fragments is also well
known to
those skilled in the art. Techniques for producing humanized MAbs are
disclosed, for
example, by Carter et al., Proc Nat'l Acad. Sci. USA. 89: 4285 (1992), Singer
et al., J. Immun.
150: 2844 (1992), Mountain et al. Biotechnol Genet Eng Rev. 10:1 (1992), and
Coligan at
pages 10.19.1-10.19.11, each of which is incorporated herein by reference. For
example,
humanized monoclonal antibodies may be produced by transferring murine
complementary
determining regions from heavy and light variable chains of the mouse
immunoglobulin into
a human variable domain, and then substituting selected human residues in the
framework
regions with their the murine FR. counterparts. The use of human framework
region
sequences, in addition to human constant region sequences, further reduces the
chance of
inducing HAMA reactions.
101301 Humanized antibodies can be designed and constructed as described by
Leung et al.
(Mol immunol. 32: 1413 (1995)). Example 3 describes the humanization process
utilized for
construction of the hPAM4 MAb.
101311 The nucleotide sequences of the primers used to prepare the hPAM4
antibodies are
discussed in Example 3, below. In a preferred embodiment, a humanized PAM4
antibody or
antibody fragment comprises the light and heavy chain CDR sequences (SEQ TD
NO:! to
SEQ ID NO:6) disclosed above. Also preferred, the FRs of the light and heavy
chain variable
regions of the humanized antibody comprise at least one amino acid substituted
from said
corresponding FRs of the murine PAM4 MAb.
101321 A. fully human antibody, e.g., human PAM4 can be obtained from a
transgenic non-
human animal. See, e.g., Mendez et al., Nature Genetics, 15: 146-156 (1997);
U.S. Pat. No.
5,633,425. For example, a human antibody can be recovered from a transgenic
mouse
possessing human immunoglobulin loci. The mouse humoral immune system is
humanized
by inactivating the endogenous immunoglobulin genes and introducing human
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
immunoglobulin loci. The human immunoglobulin loci are exceedingly complex and
comprise a large number of discrete segments which together occupy almost 0.2%
of the
human genome. To ensure that transgenic mice are capable of producing adequate
repertoires
of antibodies, large portions of human heavy- and light-chain loci must be
introduced into the
mouse genome. This is accomplished in a stepwise process beginning with the
formation of
yeast artificial chromosomes (YACs) containing either human heavy- or light-
chain
immunoglobulin loci in germline configuration. Since each insert is
approximately 1 Mb in
size, YAC construction requires homologous recombination of overlapping
fragments of the
immunoglobulin loci. The two YACs, one containing the heavy-chain loci and one
containing
the light-chain loci, are introduced separately into mice via fusion of YAC-
containing yeast
spheroblasts with mouse embryonic stem cells. Embryonic stem cell clones are
then
microinjected into mouse blastocysts. Resulting chimeric males are screened
for their ability
to transmit the YAC through their germline and are bred with mice deficient in
murine
antibody production. Breeding the two transgenic strains, one containing the
human heavy-
chain loci and the other containing the human light-chain loci, creates
progeny which produce
human antibodies in response to immunization. However, these techniques are
not limiting
and other methods known in the art for producing human antibodies, such as the
use of phage
display, may also be utilized to produce human anti-pancreatic cancer
antibodies.
101331 Antibodies can be produced by cell culture techniques using methods
known in the
art. In one example transfectoma cultures are adapted to serum-free medium.
For production
of humanized antibody, cells may be grown as a 500 ml culture in roller
bottles using HSFM.
Cultures are centrifuged and the supernatant filtered through a 0.2 gm
membrane. The filtered
medium is passed through a protein-A column (1 x 3 cm) at a flow rate of 1
ml/min. The
resin is then washed with about 10 column volumes of PBS and protein A-bound
antibody is
eluted from the column with 0.1 M glycine buffer (pH 3.5) containing 10 mM
EDTA.
Fractions of 1.0 ml are collected in tubes containing 10 gl of 3 M Tris (pH
8.6), and protein
concentrations determined from the absorbance at 280/260 nm. Peak fractions
are pooled,
dialyzed against PBS, and the antibody concentrated, for example, with a
Centricon 30 filter
(Amicon, Beverly, MA). The antibody concentration is determined by ELISA and
its
concentration adjusted to about 1 mg/ml using PBS. Sodium azide, 0.01% (w/v),
is
conveniently added to the sample as preservative.
101341 Antibodies can be isolated and purified from hybridoma cultures by a
variety of well-
established techniques. Such isolation techniques include affinity
chromatography with
31
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Protein-A SEPHAROSECO, size-exclusion chromatography, and ion-exchange
chromatography. See, for example, Coligan at pages 2.7.1-2.7.12 and pages
2.9.1-2.9.3. Also,
see Baines et al., "Purification of imrnunoglobulin 0 (IgG)," in METHODS IN
MOLECULAR BIOLOGY, VOL. 10, pages 79-104 (The Humana Press, Inc. 1992).
101351 Anti-pancreatic cancer MAbs can be characterized by a variety of
techniques that are
well-known to those of skill in the art. For example, the ability of an
antibody to bind to an
epitope of MUC5AC as discussed above can be verified using an indirect enzyme
immunoassay, flow cytometry analysis, ELISA or Western blot analysis.
Antibody Fragments
101361 Antibody fragments are antigen binding portions of an antibody, such as
F(ab') 2, Fab',
F(ab)2, Fab, Fv, sFv, say and the like. Antibody fragments which recognize
specific epitopes
can be generated by known techniques. F(abe)2 fragments, for example, can be
produced by
pepsin digestion of the antibody molecule. These and other methods are
described, for
example, by Goldenberg, U.S. Pat. Nos. 4,036,945 and 4,331,647 and references
contained
therein. Also, see Nisonoff et al., Arch Biochem. Biophys. 89: 230 (1960);
Porter, Biochem.
J. 73: 119 (1959), Edelman et al., in METHODS IN ENZYMOLOGY VOL. 1, page 422
(Academic Press 1967), and Coligan at pages 2.8.1-2.8.10 and 2.10.-2.10.4.
Alternatively,
Fab' expression libraries can be constructed (Huse et al., 1989, Science,
246:1274-1281) to
allow rapid and easy identification of monoclonal Fab' fragments with the
desired specificity.
101371 A single chain Fv molecule (scFv) comprises a VL domain and a VH
domain. The VL
and VII domains associate to form a target binding site. These two domains are
further
covalently linked by a peptide linker (L). A say molecule is denoted as either
V1,-L-VH if the
Vr, domain is the N-terminal part of the scFv molecule, or as VH-L-VL if the
VH domain is the
N-terminal part of the scFv molecule. Methods for making scFv molecules and
designing
suitable peptide linkers are described in U.S. Pat. No. 4,704,692, U.S. Pat.
No. 4,946,778, R.
Raag and M. Whitlow, "Single Chain Fvs." FASEB Vol 9:73-80 (1995) and R. E.
Bird and B.
W. Walker, Single Chain Antibody Variable Regions, TIBTECH, Vol 9: 132-137
(1991).
101381 Other antibody fragments, for example single domain antibody fragments,
are known
in the art and may be used in the claimed constructs. Single domain antibodies
(VIII!) may
be obtained, for example, from camels, alpacas or llamas by standard
immunization
techniques. (See, e.g., Muyldermans et al., TIBS 26:230-235, 2001; Yau et al.,
j Immunol
Methods 281:161-75, 2003; Maass et al., J Immunol Methods 324:13-25, 2007).
The VITH
may have potent antigen-binding capacity and can interact with novel epitopes
that are
32
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
inaccessible to conventional VH-V[. pairs. (Muyldermans et al., 2001). Alpaca
serum IgG
contains about 50% camelid heavy chain only IgG antibodies (HCAbs) (Maass et
al., 2007).
Alpacas may be immunized with known antigens, such as TN F-a, and VHHs can be
isolated
that bind to and neutralize the target antigen (Maass et al., 2007). PCR
primers that amplify
virtually all alpaca VIIII coding sequences have been identified and may be
used to construct
alpaca VHH phage display libraries, which can be used for antibody fragment
isolation by
standard biopanning techniques well known in the art (Maass et al., 2007).
Commercially
available single domain antibodies, also known as nanobodies, may be purchased
for example
from Ablynx (Ghent, Belgium).
101391 An antibody fragment can also be prepared by proteolytic hydrolysis of
a full-length
antibody or by expression in E. coli or another host of the DNA coding for the
fragment. An
antibody fragment can be obtained by pepsin or papain digestion of full-length
antibodies by
conventional methods. For example, an antibody fragment can be produced by
enzymatic
cleavage of antibodies with pepsin to provide an approximate 100 Kd fragment
denoted
F(a1:02. This fragment can be further cleaved using a thiol reducing agent,
and optionally a
blocking group for the sulthydryl groups resulting from cleavage of disulfide
linkages, to
produce an approximate 50 Kd Fab' monovalent fragment. Alternatively, an
enzymatic
cleavage using papain produces two monovalent Fab fragments and an Fe fragment
directly.
MA Other methods of cleaving antibodies, such as separation of heavy chains to
form
monovalent light-heavy chain fragments, further cleavage of fragments, or
other enzymatic,
chemical or genetic techniques may also be used, so long as the fragments bind
to the antigen
that is recognized by the intact antibody.
Antibody Fusion Proteins and Multivalent Antibodies
10141.1 Fusion proteins comprising the anti-pancreatic cancer antibodies of
interest can be
prepared by a variety of conventional procedures, ranging from glutaraldehyde
linkage to
more specific linkages between functional groups. The antibodies and/or
antibody fragments
that comprise the fusion proteins described herein are preferably covalently
bound to one
another, directly or through a linker moiety, through one or more functional
groups on the
antibody or fragment, e.g., amine, carboxyl, phenyl, thiol, or hydroxyl
groups. Various
conventional linkers in addition to glutaraldehyde can be used, e.g.,
diisocyanates,
diiosothiocyanates, bis(hydroxysuccinimide)esters, carbodiimides,
maleimidehydroxy
succinimide esters, and the like.
33
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
101421 A simple method for producing fusion proteins is to mix the antibodies
or fragments
in the presence of glutaraldehyde. The initial Schiff base linkages can be
stabilized, e.g., by
borohydride reduction to secondary amines. A diiosothiocyanate or carbodiimide
can be used
in place of glutaraldehyde as a non-site-specific linker. In one embodiment,
an antibody
fusion protein comprises an anti-pancreatic cancer MAb, or fragment thereof,
wherein the
MAb binds to an epitope located within the second to fourth cysteine-rich
domains of
MUC5AC (amino acid residues 1575-2052), more preferably to an epitope located
in amino
acid residues 1575-1725 and 1903-2052 (Cys2 and Cys 4), even more preferably
to an
epitope located in amino acid residues 1575-1725 (Cys2+), most preferably, to
an epitope
located in Cys2. This fusion protein and fragments thereof preferentially bind
pancreatic
cancer cells. This monovalent, monospecific MAb is useful for direct targeting
of an antigen,
where the IvIAb is attached to a therapeutic agent, a diagnostic agent, or a
combination
thereof, and the protein is administered directly to a patient.
101431 The fusion proteins may instead comprise at least two anti-pancreatic
cancer MAbs
that bind to distinct epitopes of MUC5AC. For example, the MAbs can produce
antigen
specific diabodies, triabodies and tetrabodies, which are multivalent but
monospecific to the
MUC5AC. The non-covalent association of two or more scFv molecules can form
functional
diabodies, triabodies and tetrabodies. Monospecific diabodies are homodimers
of the same
scFv, where each scFv comprises the VH domain from the selected antibody
connected by a
short linker to the VE, domain of the same antibody. A diabody is a bivalent
dimer formed by
the non-covalent association of two scFvs, yielding two Fv binding sites. A
triabody results
from the formation of a trivalent timer of three says, yielding three binding
sites, and a
tetrabody is a tetravalent tetramer of four scFvs, resulting in four binding
sites. Several
monospecific diabodies have been made using an expression vector that contains
a
recombinant gene construct comprising V1-11-linker-VL1 . See IIolliger et al.,
Proc Natl. Acad.
Sci USA 90: 6444-6448 (1993); Atwell et al., Molecular Immunology 33: 1301-
1302 (1996);
Holliger et al., Nature Biotechnology 15: 632-631(1997); Helfrich et al., int
j Cancer 76:
232-239 (1998); Kipriyanov et al., Int j Cancer 77: 763-772 (1998); Holiger et
al., Cancer
Research 59: 2909-2916(1999)). Methods of constructing says are disclosed in
U.S. Pat. No.
4,946,778 (1990) and U.S. Pat. No. 5,132,405 (1992), the Examples section of
each of which
is incorporated herein by reference. Methods of producing multivalent,
monospecific
antibodies based on say are disclosed in U.S. Pat. No. 5,837,242 (1998), U.S.
Pat. No.
5,844,094 (1998) and WO-98/44001 (1998), the Examples section of each of which
is
34
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
incorporated herein by reference. The multivalent, monospecific antibody
fusion protein
binds to two or more of the same type of epitopes that can be situated on the
same antigen or
on separate antigens. The increased valency allows for additional interaction,
increased
affinity, and longer residence times. These antibody fusion proteins can be
utilized in direct
targeting systems, where the antibody fusion protein is conjugated to a
therapeutic agent, a
diagnostic agent, or a combination thereof, and administered directly to a
patient in need
thereof.
101441 A preferred embodiment is a multivalent, multispecific antibody or
fragment thereof
comprising one or more antigen binding sites having an affinity toward a PAM4
target
epitope and one or more additional binding sites for other epitopes associated
with pancreatic
cancer. This fusion protein is multispecific because it binds at least two
different epitopes,
which can reside on the same or different antigens. For example, the fusion
protein may
comprise more than one antigen binding site, the first with an affinity toward
an epitope of
MUC5AC as discussed above and the second with an affinity toward another
target antigen
such as TAG-72 or CEA. Another example is a bispecific antibody fusion protein
which may
comprise a CA19.9 MAb (or fragment thereof) and a PAM4 MAb (or fragment
thereof).
Such a fusion protein will have an affinity toward CA19.9 as well as MLIC5AC.
The
antibody fusion proteins and fragments thereof can be utilized in direct
targeting systems,
where the antibody fusion protein is conjugated to a therapeutic agent, a
diagnostic agent, or
a combination thereof, and administered directly to a patient in need thereof
101451 Another preferred embodiment is a multivalent, multispecific antibody
comprising at
least one binding site having affinity toward a PAM4 target epitope and at
least one hapten
binding site having affinity towards hapten molecules. For example, a
bispecific fusion
protein may comprise the 679 MAb (or fragment thereof) and the PAM4 MAb (or
fragment
thereof). The monoclonal 679 antibody binds with high affinity to molecules
containing the
ni-peptide moiety histamine succinyl glycyl (HSG). Such a bispecific PAM4
antibody fusion
protein can be prepared, for example, by obtaining a F(ab')2 fragment from
679, as described
above. The interchain disulfide bridges of the 679 F(á1:2 fragment are gently
reduced with
DTT, taking care to avoid light-heavy chain linkage, to form Fab'-SH
fragments. The SH
group(s) is (are) activated with an excess of bis-maleimide linker (1,1'-
(methylenedi-4,1-
phenylene)b-is-maleimide). The PAM4 MAb is converted to Fab'-SH and then
reacted with
the activated 679 Fab'-SH fragment to obtain a bispecific antibody fusion
protein. Bispecific
antibody fusion proteins such as this one can be utilized in affinity
enhancing systems, where
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
the target antigen is pretargeted with the fusion protein and is subsequently
targeted with a
diagnostic or therapeutic agent attached to a carrier moiety (targetable
construct) containing
one or more HSG haptens. In alternative preferred embodiments, a DN1,1110-
based hPAM4-
679 construct, such as TF10, may be prepared and used as described in the
Examples below.
101461 Bispecific antibodies can be made by a variety of conventional methods,
e.g.,
disulfide cleavage and reformation of mixtures of whole IgG or, preferably
F(ab') 2 fragments,
fusions of more than one hybiidoma to form polyomas that produce antibodies
having more
than one specificity, and by genetic engineering. Bispecific antibody fusion
proteins have
been prepared by oxidative cleavage of Fab' fragments resulting from reductive
cleavage of
different antibodies. This is advantageously carried out by mixing two
different F(ab1) 2
fragments produced by pepsin digestion of two different antibodies, reductive
cleavage to
form a mixture of Fab' fragments, followed by oxidative reformation of the
disulfide linkages
to produce a mixture of F(abl) 2 fragments including bispecific antibody
fusion proteins
containing a Fab' portion specific to each of the original epitopes. General
techniques for the
preparation of antibody fusion proteins may be found, for example, in Nisonoff
et al., Arch
Biochem Biophys. 93: 470 (1961), Hammerling et al., J Exp Med. 128: 1461
(1968), and
U.S. Pat. No. 4,331,647.
101471 More selective linkage can be achieved by using a heterobifunctional
linker such as
maleimidehydroxysuccinimide ester. Reaction of the ester with an antibody or
fragment will
derivatize amine groups on the antibody or fragment, and the derivative can
then be reacted
with, e.g., an antibody Fab fragment having free sulthydryl groups (or, a
larger fragment or
intact antibody with sulfhydryl groups appended thereto by, e.g., Traut's
Reagent). Such a
linker is less likely to crosslink groups in the same antibody and improves
the selectivity of
the linkage.
101481 It is advantageous to link the antibodies or fragments at sites remote
from the antigen-
binding sites. This can be accomplished by, e.g., linkage to cleaved
interchain sulfhydryl
groups, as noted above. Another method involves reacting an antibody having an
oxidized
carbohydrate portion with another antibody that has at lease one five amine
fimction. This
results in an initial Schiff base linkage, which is preferably stabilized by
reduction to a
secondary amine, e.g., by borohydride reduction, to form the fmal composite.
Such site-
specific linkages are disclosed, for small molecules, in U.S. Pat. No.
4,671,958, and for larger
addends in U.S. Pat. No. 4,699,784, the Examples section of each of which is
incorporated
herein by reference.
36
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
101491 ScFvs with linkers greater than 12 amino acid residues in length (for
example, 15-or
18-residue linkers) allow interactions between the VH and VL domains on the
same chain and
generally form a mixture of monomers, dimers (termed diabodies) and small
amounts of
higher mass multimers, (Kortt et al., Eur J Biochem. (1994) 221: 151-157).
ScFvs with
linkers of 5 or less amino acid residues, however, prohibit intramolecular
pairing of the VH
and VL domains on the same chain, forcing pairing with VH and VL domains on a
different
chain. Linkers between 3- and 12-residues form predominantly dimers (Atwell et
al., Protein
Engineering (1999) 12: 597-604). With linkers between 0 and 2 residues,
trimeric (termed
triabodies), tetrameric (termed tetrabodies) or higher oligomeric structures
of scFvs are
formed; however, the exact patterns of oligomeri.zation appear to depend on
the composition
as well as the orientation of the V-domains, in addition to the linker length.
101501 More recently, a novel technique for construction of mixtures of
antibodies, antibody
fragments and/or other effector moieties in virtually any combination has been
described in
U.S. Patent Nos. 7,550,143; 7,521,056; 7,534,866; 7,527,787; and 7,666,400,
the Examples
section of each of which is incorporated herein by reference. The technique,
known generally
as DOCK-AND-LOCK m (DNLTIvi) involves the production of fusion proteins that
comprise
at their N- or C-terminal ends one of two complementary peptide sequences,
called
dimetization and docking domain (DDD) and anchoring domain (AD) sequences. In
preferred embodiments, the DDD sequences are derived from the regulatory
subunits of
cAMP-dependent protein kinase and the Al) sequence is derived from the
sequence of A-
kinase anchoring protein (AKAP). The DDD sequences form dimers that bind to
the Al)
sequence, which allows formation of timers, tetramers, hex amers or any of a
variety of other
complexes. By attaching effector moieties, such as antibodies or antibody
fragments, to the
DDD and Al) sequences, complexes may be formed of any selected combination of
antibodies or antibody fragments. The DNLTM complexes may be covalently
stabilized by
formation of disulfide bonds or other linkages.
Pretargeting
101511 Bispecific or multispecific antibodies may be utilized in pre-targeting
techniques. Pre-
targeting is a multistep process originally developed to resolve the slow
blood clearance of
directly targeting antibodies, which contributes to undesirable toxicity to
normal tissues such
as bone marrow. With pre-targeting, a radionuclide or other therapeutic agent
is attached to a
small delivery molecule (targetable construct or targetable conjugate) that is
cleared within
minutes from the blood. A pre-targeting bispecific or multispecific antibody,
which has
37
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
binding sites for the targetable construct as well as a target antigen, is
administered first, free
antibody is allowed to clear from circulation and then the targetable
construct is administered.
101521 Pre-targeting methods are well known in the art, for example, as
disclosed in
Goodwin et al., U.S. Pat. No. 4,863,713; Goodwin et al., J. Nucl. Med. 29:226,
1988;
tinatowich et al., J. Nucl. Med. 28:1294, 1987; Oehr et al., J. Nucl. Med.
29:728, 1988;
Klibanov et al., J. Nucl. Med. 29:1951, 1988; Sinitsyn et al., J. Nucl. Med.
30:66, 1989;
Kalofonos et al., J. Nucl. Med. 31:1791, 1990; Schechter et al., Int. J.
Cancer 48:167, 1991;
Paganelli et al., Cancer Res. 51:5960, 1991; Paganelli et al., Nucl. Med.
Commun. 12:211,
1991; U.S. Pat. No. 5,256,395; Stickney et al., Cancer Res. 51:6650, 1991;
Yuan et al.,
Cancer Res. 51:3119, 1991; U.S. Pat. No. 6,077,499; U.S. Pat. No. 6,090,381;
U.S. Pat. No.
6,472,511; and U.S. Patent No. 6,962,702.
101531 A pre-targeting method of treating or diagnosing a disease or disorder
in a subject
may be provided by: (1) administering to the subject a bispecific antibody or
antigen binding
antibody fragment; (2) optionally administering to the subject a clearing
composition, and
allowing the composition to clear the antibody from circulation; and (3)
administering to the
subject the targetable construct, containing one or more chelated or
chemically bound
therapeutic or diagnostic agents. The technique may also be utilized for
antibody dependent
enzyme prodrug therapy (ADEPT) by administering an enzyme conjugated to a
targetable
construct, followed by a proding that is converted into active form by the
enzyme.
Known Antibodies
10154i In various embodiments, the claimed methods and compositions may
utilize any of a
variety of antibodies known in the art, for example for combination antibody
therapy.
Antibodies of use may be commercially obtained from a number of known sources.
For
example, a variety of antibody secreting hybridoma lines are available from
the American
Type Culture Collection (ATCC, Manassas, VA). A large number of antibodies
against
various disease targets, including but not limited to tumor-associated
antigens, have been
deposited at the ATCC and/or have published variable region sequences and are
available for
use in the claimed methods and compositions. See, e.g., U.S. Patent Nos.
7,312,318;
7,282,567; 7,151,164; 7,074,403; 7,060,802; 7,056,509; 7,049,060; 7,045,132;
7,041,803;
7,041,802; 7,041,293; 7,038,018; 7,037,498; 7,012,133; 7,001,598; 6,998,468;
6,994,976;
6,994,852; 6,989,241; 6,974,863; 6,965,018; 6,964,854; 6,962,981; 6,962,813;
6,956,107;
6,951,924; 6,949,244; 6,946,129; 6,943,020; 6,939,547; 6,921,645; 6,921,645;
6,921,533;
6,919,433; 6,919,078; 6,916,475; 6,905,681; 6,899,879; 6,893,625; 6,887,468;
6,887,466;
38
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
6,884,594; 6,881,405; 6,878,812; 6,875,580; 6,872,568; 6,867,006; 6,864,062;
6,861,511;
6,861,227; 6,861,226; 6,838,282; 6,835,549; 6,835,370; 6,824,780; 6,824,778;
6,812,206;
6,793,924; 6,783,758; 6,770,450; 6,767,711; 6,764,688; 6,764,681; 6,764,679;
6,743,898;
6,733,981; 6,730,307; 6,720,155; 6,716,966; 6,709,653; 6,693,176; 6,692,908;
6,689,607;
6,689,362; 6,689,355; 6,682,737; 6,682,736; 6,682,734; 6,673,344; 6,653,104;
6,652,852;
6,635,482; 6,630,144; 6,610,833; 6,610,294; 6,605,441; 6,605,279; 6,596,852;
6,592,868;
6,576,745; 6,572;856; 6,566,076; 6,562,618; 6,545,130; 6,544,749; 6,534,058;
6,528,625;
6,528,269; 6,521,227; 6,518,404; 6,511,665; 6,491,915; 6,488,930; 6,482,598;
6,482,408;
6,479,247; 6,468,531; 6,468,529; 6,465,173; 6,461,823; 6,458,356; 6,455,044;
6,455,040,
6,451,310; 6,444,206; 6,441,143; 6,432,404; 6,432,402; 6,419,928; 6,413,726;
6,406,694;
6,403,770; 6,403,091; 6,395,276; 6,395,274; 6,387,350; 6,383,759; 6,383,484;
6,376,654;
6,372,215; 6,359,126; 6,355,481; 6,355,444; 6,355,245; 6,355,244; 6,346,246;
6,344,198;
6,340,571; 6,340,459; 6,331,175; 6,306,393; 6,254,868; 6,187,287; 6,183,744;
6,129,914;
6,120,767; 6,096,289; 6,077,499; 5,922,302; 5,874,540; 5,814,440; 5,798,229;
5,789,554;
5,776,456; 5,736,119; 5,716,595; 5,677,136; 5,587,459; 5,443,953; 5,525,338,
the Examples
section of each of which is incorporated herein by reference. These are
exemplary only and a
wide variety of other antibodies and their hybridomas are known in the art.
The skilled artisan
will realize that antibody sequences or antibody-secreting hybridomas against
almost any
disease-associated antigen may be obtained by a simple search of the ATCC,
NCBI and/or
USPTO databases for antibodies against a selected disease-associated target of
interest. The
antigen binding domains of the cloned antibodies may be amplified, excised,
ligated into an
expression vector, transfected into an adapted host cell and used for protein
production, using
standard techniques well known in the art (see, e.g., U.S. Patent Nos.
7,531,327; 7,537,930;
7,608,425 and 7,785,880, the Examples section of each of which is incorporated
herein by
reference).
101551 Particular antibodies that may be of use for therapy of cancer within
the scope of the
claimed methods and compositions include, but are not limited to, LL I (anti-
CD74), LL2 or
RFB4 (anti-CD22), veltuzumab (hA20, anti-CD20), rituxumab (anti-CD20),
obinutuzumab
(GA101, anti-CD20), lambrolizumab (anti-PD-1 receptor), nivolumab (anti-PD-1
receptor),
ipilimumab (anti-CTLA-4), RS7 (anti-epithelial glycoprotein-1 (EGP-1, also
known as
TROP-2)), KC4 (anti-mucin), MN-14 (anti-carcinoembryonic antigen (anti-CEA,
also known
as CD66e or CEACAM5), MN-15 or MN-3 (anti-CEACAM6), Mu-9 (anti-colon-specific
antigen-p), Immu 31 (an anti-alpha-fetoprotein), R1 (anti-IGF-1R), Al9 (anti-
CD19), TAG-
39
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
72 (e.g., CC49), Tn, J591 or HuJ591 (anti-PSMA (prostate-specific membrane
antigen)), AB-
PG1-XG1-026 (anti-PSMA dimer), D2/13 (anti-PSMA), G250 (an anti-carbonic
anhydrase IX
MAb), L243 (anti-HLA-DR) alemtuzumab (anti-C1)52), bevacizumab (anti-VEGI-7),
cetuximab (anti-EGFR), gemtuzumab (anti-CD33), ibritumomab tiuxetan (anti-
CD20);
panitunnunab (anti-EGFR); tositumomab (anti-CD20); PAM4 (aka clivatuzumab,
anti-
MUC5AC) and trastuzumab (anti-ErbB2). Such antibodies are known in the art
(e.g., U.S.
Patent Nos. 5,686,072; 5,874,540; 6,107,090; 6,183,744; 6,306,393; 6,653,104;
6,730.300;
6,899,864; 6,926,893; 6,962,702; 7,074,403; 7,230,084; 7,238,785; 7,238,786;
7,256,004;
7,282,567; 7,300,655; 7,312,318; 7,585,491; 7,612,180; 7,642,239; and U.S.
Patent
Application Pub!. No. 20050271671; 20060193865; 20060210475; 20070087001; the
Examples section of each incorporated herein by reference.) Specific known
antibodies of
use include hPAM4 (U.S. Patent No. 7,282,567), hA20 (U.S. Patent No.
7,251,164), hAl9
(U.S. Patent No. 7,109,304), hIMMU-31 (U.S. Patent No. 7,300,655), tall (U.S.
Patent No.
7,312,318,), h112 (U.S. Patent No. 7,074,403), hMu-9 (U.S. Patent No.
7,387,773), hl..243
(U.S. Patent No. 7,612,180), hMN-14 (U.S. Patent No. 6,676,924), b.MN-15 (U.S.
Patent No.
7,541,440), hR1 (U.S. Patent Application 12/772,645), hRS7 (U.S. Patent No.
7,238,785),
hMN-3 (U.S. Patent No. 7,541,440), AB-PG1-XG1-026 (U.S. Patent Application
11/983,372,
deposited as ATCC PTA-4405 and PTA-4406), D2/B (WO 2009/130575), BWA-3 (anti-
histone II4), LG2-1 (anti-histone II3) and LG2-2 (anti-histone II2B) (U.S.
Patent Application
Serial No. 14/180,646, filed 2/14/14) the text of each recited patent or
application is
incorporated herein by reference with respect to the Figures and Examples
sections.
101561 Other useful antigens that may be targeted using the described
conjugates include
carbonic anhydrase IX, B7, CCL19, CCL21, CSAp, HER-2/neu, BrE3, CD!, CD1a,
CD2,
CD3, CD4, CD5, CD8, CD 1 1A, CD14, CD15, CD16, CD18, CD19, CD20 (e.g., C2B8,
hA20, 1F5 MAbs), CD21, CD22, CD23, CD25, CD29, CD30, CD32b, CD33, CD37, CD38,
CD40, CD4OL, CD44, CD45, CD46, CD47, CD52, CD54, CD55, CD59, CD64, CD67,
CD70, CD74, CD79a, CD80, CD83, CD95, CD126, CD133, CD138, CD147, CD154,
CEACAM5, CEACAM6, CTLA-4, CXCR4, alpha-fetoprotein (AFP), VEGF (e.g.,
AVASTIN , fibronectin splice variant), ED-B fibmectin (e.g., L19), EGP-1 (TROP-
2),
EGP-2 (e.g., 17-1A), EGF receptor (ErbB1) (e.g., ERBITUXO), ErbB2, ErbB3,
Factor H,
FHL-1, Flt-3, folate receptor, Ga 733,GRO-13, HMGB-1, hypoxia inducible factor
(HIF),
HM1.24, HER-2/neu, histone H2B, histone 113, histone 114, insulin-like growth
factor
(ILGF), IFN-y, IFN-a, IFN-(3, IFN-k, IL-2R, IL-4R, IL-6R, IL-13R, IL-15R, IL-
17R, IL-18R,
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
IL-2, IL-6, IL-8, IL-12, IL-15, IL-17, 1L-18, IL-25, IP-10, IGF-1R, Ia,
HM1.24, gangliosides,
HCG, the HLA-DR antigen to which L243 binds, CD66 antigens, i.e., CD66a-d or a
combination thereof, MAGE, mCRP, MCP-1, MIP-1A, MIP-1B, macrophage migration-
inhibitory factor (MIF), MTJC1, MIJC2, MUC3, MUC4, MUC5AC, placental growth
factor
(P1GF), PSA (prostate-specific antigen), PSIvIA, PD-1, PD-L1, TIM-3, LAG-3,
matrix
metalloproteinase-1 (MMP-1), MMP-7, MMP-9, MMP-14, NCA-95, NCA-90, A3,
A33, Ep-CAM, KS-1, Le(y), mesothelin, S100, tenascin, TAC, in antigen, Thomas-
Friedenreich antigens, tumor necrosis antigens, tumor angiogenesis antigens,
TNF-a, TRAIL
receptor (R1 and R2), TROP-2, VEGFR, RANTES, T101, as well as cancer stem cell
antigens, complement factors C3, C3a, C3b, C5a, C5, and an oncogene product.
101571 A comprehensive analysis of suitable antigen (Cluster Designation, or
CD) targets on
hematopoietic malignant cells, as shown by flow cytometry and which can be a
guide to
selecting suitable antibodies for drug-conjugated iinmunotherapy, is Craig and
Foon, Blood
prepublished online January 15, 2008; DOL 10.1182/blood-2007-11-120535.
101581 The CD66 antigens consist of five different glycoproteins with similar
structures,
CD66a-e, encoded by the carcinoembryonic antigen (CEA) gene family members,
BCG,
CGM6, NCA, CGM1 and CEA, respectively. These CD66 antigens (e.g., CEACAM6) are
expressed mainly in granulocytes, normal epithelial cells of the digestive
tract and tumor cells
of various tissues. Also included as suitable targets for cancers are cancer
testis antigens, such
as NY-ES0-1 (Theurillat et al., Int. J. Cancer 2007; 120(11):2411-7), as well
as CD79a in
myeloid leukemia (Kozlov et al., Cancer Genet. Cytogenet. 2005; 163(1):62-7)
and also B-
cell diseases, and CD79b for non-Hodgkin's lymphoma (Poison et al., Blood
110(2):616-
623). A number of the aforementioned antigens are disclosed in U.S.
Provisional Application
Serial No. 60/426,379, entitled "Use of Multi-specific, Non-covalent Complexes
for Targeted
Delivery of Therapeutics," filed November 15, 2002. Cancer stem cells, which
are ascribed to
be more therapy-resistant precursor malignant cell populations (Hill and
Perris, J. Natl.
Cancer Inst. 2007; 99:1435-40), have antigens that can be targeted in certain
cancer types,
such as CD133 in prostate cancer (Maitland et al., Ernst Schering Found
Sympos. Proc.
2006; 5:155-79), non-small-cell lung cancer (Donnenberg et al., J. Control
Release 2007;
122(3):385-91), and glioblastoma (Beier et al., Cancer Res. 2007; 67(9):4010-
5), and CD44
in colorectal cancer (Dalerba er al., Proc. Natl. Acad. Sci. USA 2007;
104(24)10158-63),
pancreatic cancer (Li et al., Cancer Res. 2007; 67(3):1030-7), and in head and
neck squamous
cell carcinoma (Prince et al., Proc. Natl. Acad. Sci. USA 2007; 104(3)973-8).
Another useful
41
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
target for breast cancer therapy is the LIV-1 antigen described by Taylor et
al. (Biochem. J.
2003; 375:51-9).
101591 For multiple myeloma therapy, suitable targeting antibodies have been
described
against, for example, CD38 and CD138 (Stevenson, Mol Med 2006; 12(11-12):345-
346;
Tassone et al., Blood 2004; 104(12):3688-96), CD74 (Stein et al., ibid.), CS!
(Tai et al.,
Blood 2008; 112(4):1329-37, and CD40 (Tai et al., 2005; Cancer Res.
65(13):5898-5906).
101601 Macrophage migration inhibitory factor (MIF) is an important regulator
of innate and
adaptive immunity and apoptosis. It has been reported that CD74 is the
endogenous receptor
for MIF (Leng et al., 2003, J Exp Med 197:1467-76). The therapeutic effect of
antagonistic
anti-CD74 antibodies on MIF-mediated intracellular pathways may be of use for
treatment of
a broad range of disease states, such as cancers of the bladder, prostate,
breast, lung, colon
and chronic lymphocytic leukemia (e.g., Meyer-Siegler et al., 2004, B.MC
Cancer 12:34;
Shachar & Haran, 2011, Leuk Lymphoma 52:1446-54); autoimmune diseases such as
rheumatoid arthritis and systemic lupus erythematosus (Morand & Leech, 2005,
Front Biosci
10:12-22; Shachar & Haran, 2011, Leuk Lymphoma 52:1446-54); kidney diseases
such as
renal allograft rejection (Lan, 2008, Nephron Exp Nephrol. 109:e79-83); and
numerous
inflammatory diseases (Meyer-Siegler et al., 2009, Mediators Inflamm epub
March 22, 2009;
Takahashi et al., 2009, Respir Res 10:33; Milatuzumab (IILL1) is an exemplary
anti-CD74
antibody of therapeutic use for treatment of IA:IF-mediated diseases.
101611 Anti-TNF-a antibodies are known in the art and may be of use to treat
immune
diseases, such as autoimmune disease, iMMUlle dysfunction (e.g., graft-versus-
host disease,
organ transplant rejection) or diabetes. Known antibodies against TNF-a
include the human
antibody CDP571 (Ofei et al., 2011, Diabetes 45:881-85); murine antibodies
MTNFAI,
M2TNFAI, M3TNFAI, M3INFABI, M302B and M303 (Thermo Scientific, Rockford, IL);
infliximab (Centocor, Malvern, PA); certolizumab pegol (1JCB, Brussels,
Belgium); and
adalimumab (Abbott, Abbott Park, IL). These and many other known anti-TNF-a
antibodies
may be used in the claimed methods and compositions. Other antibodies of use
for therapy of
immune dysregulatory or autoimmune disease include, but are not limited to,
anti-B-cell
antibodies such as veltuzumab, epratuzumab, milatuzumab or hL243; tocilizumab
(anti-1L-6
receptor); basiliximab (anti-CD25); daclizumab (anti-CD25); efalizumab (anti-
CD11a);
muromonab-CD3 (anti-CD3 receptor); anti-CD4OL (UCB, Brussels, Belgium);
natalizumab
(anti-a4 integrin) and omalizumab (anti-IgE).
42
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
101621 Checkpoint inhibitor antibodies have been used primarily in cancer
therapy. Immune
checkpoints refer to inhibitory pathways in the immune system that are
responsible for
maintaining self-tolerance and modulating the degree of immune system response
to
minimize peripheral tissue damage. However, tumor cells can also activate
immune system
checkpoints to decrease the effectiveness of immune response against tumor
tissues.
Exemplary checkpoint inhibitor antibodies against cytotoxic T-lymphocyte
antigen 4 (CTLA-
4, also known as CD 152), programmed cell death protein 1 (P1)-1, also known
as CD279)
and programmed cell death 1 ligand 1 (PD-L1, also known as CD274), may be used
in
combination with one or more other agents to enhance the effectiveness of
immune response
against disease cells, tissues or pathogens. Exemplary anti-PD1 antibodies
include
lambrolizumab (MK-3475, MERCK), nivolumab (BMS-936558, BRISTOL-MYERS
SQUIBB), AMP-224 (MERCK), and pidilizumab (CT-011, CURETECH LTD.). Anti-PD1
antibodies are commercially available, for example from ABCAMIt.) (AB137132),
BIOLEGEND (EH12.2H7, RMP1-14) and AFFYMETRIX EBIOSCIENCE (J1.05, J.116,
MIII4). Exemplary anti-PD-L I antibodies include MDX-1105 (MEDAREX), MEDI4736
(MEDIMMUNE) MPDL3280A (GENENTECH) and BMS-936559 (BRISTOL-MYERS
SQUIBB). Anti-PD-L1 antibodies are also commercially available, for example
from
AFFYMETRIX EBIOSCIENCE (MIII1). Exemplary anti-CTLA4 antibodies include
ipilimumab (Bristol-Myers Squibb) and tremelimumab (PFIZER). Anti-PD1
antibodies are
commercially available, for example from ABCAM63) (AB134090), SINO BIOLOGICAL
INC. (11159-H03H, 11159-H08H), and THERMO SCIENTIFIC PIER. .CE (PA.5-29572,
PA5-
23967, PA5-26465, MA1-12205, MA1-35914). Ipilimumab has recently received FDA
approval for treatment of metastatic melanoma (Wada et al., 2013, J Transl Med
11:89).
More recently, other checkpoint inhibitory receptors have been identified,
including TIM-3
and LAG-3 (Stagg, 2013, Ther Adv Med Oncol 5:169-81). Antibodies against TIM-3
and
LAG-3 may also be used in combination with the anti-MUC5AC antibodies
disclosed herein.
101631 Antibodies against matrix metalloproteinases, for example matrix
metalloproteinase-1
(MMP-1), MMP-2, MMP-7, MMP-9 and MMP-14, are also of use in combination anti-
cancer therapies. (See, e.g., Agarwal A, et al., Mol Cancer Ther 2008;7:2746-
57; Freije JM,
et al. Adv Exp Med Biol 2003;532:91-107; Coticchia CM, et al.Gynecol Oncol
2011;123:295-300; Boiire D, et al., Cell 2005;120:303-13; Belotti D, et al.,
Cancer Res
2003;63:5224-9; Barbolina MV, et al., J Biol Chem 2007;282:4924-31;Kaimal R,
etal.,
Cancer Res 2013;73:2457-67; Denzel S. et al, Int J Exp Pathol 2012; 93:341-
53.)
43
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
101641 Other antibodies of use may include anti-histone antibodies and/or
antigen-binding
fragments thereof, such as the BWA-3 (anti-H4), LG2-1 (anti-H3) and LG2-2
(anti-H2B)
antibodies. Exemplary anti-histone antibodies are disclosed, for example, in
U.S. Patent
Application Serial No. 14/180,646, filed 2/14/14 (the Examples section of
which is
incorporated herein by reference).
101651 In another preferred embodiment, antibodies are used that internalize
rapidly and are
then re-expressed, processed and presented on cell surfaces, enabling
continual uptake and
accretion of circulating conjugate by the cell. An example of a most-preferred
antibody/antigen pair is LL I, an anti-CD74 MAb (invariant chain, class II-
specific
chaperone, Ii) (see, e.g., U.S. Patent Nos. 6,653,104; 7,312,318; the Examples
section of each
incorporated herein by reference). The CD74 antigen is highly expressed on B-
cell
lymphomas (including multiple myeloma) and leukemias, certain T-cell
lymphomas,
melanomas, colonic, lung, and renal cancers, glioblastomas, and certain other
cancers (Ong et
al., Immunology 98:296-302 (1999)). A review of the use of CD74 antibodies in
cancer is
contained in Stein et al., Clin Cancer Res. 2007 Sep 15;13(18 Pt 2):5556s-
5563s,
incorporated herein by reference.
101661 The diseases that are preferably treated with anti-CD74 antibodies
include, but are not
limited to, non-Hodgkin's lymphoma, Hodgkin's disease, melanoma, lung, renal,
colonic
cancers, glioblastome multiforme, histiocytomas, myeloid leukemias, and
multiple myeloma.
Continual expression of the CD74 antigen for short periods of time on the
surface of target
cells, followed by internalization of the antigen, and re-expression of the
antigen, enables the
targeting LL1 antibody to be internalized along with any chemotherapeutic
moiety it canies.
This allows a high, and therapeutic, concentration of LL I-chemotherapeutic
drug conjugate
to be accumulated inside such cells. Internalized LL1-chemotherapeutic drug
conjugates are
cycled through lysosomes and endosomes, and the chemotherapeutic moiety is
released in an
active form within the target cells.
Antibody Use for Treatment and Diagnosis
101671 Certain embodiments concern methods of diagnosing or treating a
malignancy in a
subject, comprising administering to the subject an anti-pancreatic cancer
MAb, fusion
protein or fragment thereof, wherein the MAb, fusion protein or fragment is
bound to at least
one diagnostic and/or therapeutic agent. The antibody preferably binds to an
epitope located
within the second to fourth cysteine-rich domains of M1.JC5AC (amino acid
residues 1575-
2052), more preferably to an epitope located in amino acid residues 1575-1725
and 1903-
44
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
2052 (Cys2 and Cys 4), even more preferably to an epitope located in amino
acid residues
1575-1725 (Cys2+), most preferably, to an epitope located in Cys2
101681 Also preferred is a method for diagnosing or treating cancer,
comprising
administering to a subject a multivalent, multispecific antibody or fragment
thereof
comprising one or more antigen binding sites toward an epitope of MUC5AC as
discussed
above and one or more hapten binding sites, waiting a sufficient amount of
time for non-
bound antibody to clear the subject's blood stream; and then administering to
the subject a
carrier molecule comprising a diagnostic agent, a therapeutic agent, or a
combination thereof,
that binds to the hapten-binding site of the localized antibody. In a more
preferred
embodiment, the cancer is a non-endocrine pancreatic cancer.
101691 The use of MAbs for in vitro diagnosis is well-known. See, for example,
Carlsson et
al., Bio/Technology 7 (6): 567 (1989). For example, MAbs can be used to detect
the presence
of a tumor-associated antigen in tissue from biopsy samples. MAbs also can be
used to
measure the amount of tumor-associated antigen in clinical fluid samples, such
as blood or
serum, using techniques such as radioimmunoassay, enzyme-linked immunosorbent
assay,
and fluorescence immunoassay. In vitro and in vivo methods of diagnosis are
discussed in
further detail below.
101701 Conjugates of tumor-targeted MAbs and toxins can be used to selectively
kill cancer
cells in vivo (Spalding, Bio/Technology 9(8): 701(1991); Goldenberg,
Scientific American
Science & Medicine 1(1): 64 (1994)). For example, therapeutic studies in
experimental
animal models have demonstrated the anti-tumor activity of antibodies carrying
cytotoxic
radionuclides. (Goldenberg et al., Cancer Res. 41: 4354 (1981), Cheung et al.,
J. Nat'l Cancer
Inst. 77: 739 (1986), and Senekowitsch et al., J. Nucl. Med. 30: 531 (1989)).
In a preferred
embodiment, the conjugate comprises a "Y-labeled hPAM4 antibody. The conjugate
may
optionally be administered in conjunction with one or more other therapeutic
agents. In a
preferred embodiment, "Y-labeled hPAM4 is administered together with
gemcitabine or 5-
fluorouracil to a patient with pancreatic cancer. In a further preferred
embodiment, "Y is
conjugated to a DOTA chelate for attachment to hPAM4. In a more preferred
embodiment,
the 9 Y-DOTA-hPAM4 is combined with gemcitabine in fractionated doses
comprising a
treatment cycle, such as with repeated, lower, less-toxic doses of gemcitabine
combined with
lower, fractionated doses of 90Y-DOTA-hPAM4. Alternatively, a radiolabeled or
other
conjugated PAM4 antibody may be administered in combination with another
immunoconjugate, such as an SN-38 conjugated antibody. A particularly
preferred
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
combination is 90Y-11PAM4 and SN-38-hRS7 (anti-TROP2 antibody) (see, e.g.,
U.S. Patent
No. 8,586,050, the Examples section incorporated herein by reference).
10171.1 As tolerated, repeated cycles of a fractionated dose schedule are
indicated. By way of
example, 4 weekly doses of 200 mg/m2 of gemcitabine are combined with three
weekly doses
of 8 mg/m2 of"Y-DOTA-hPAM4, with the latter commencing in the second week of
gemcitabine administration, constitutes a single therapy cycle. Still other
doses, higher or
lower of each component, may constitute a fractionated dose, which is
determined by
conventional means of assessing hematopoietic toxicity (see, e.g., U.S. Patent
Nos.
6,649,352; 7,112,409; 7,279,289; 7,465,551), since myelosuppressive effects of
both agents
can be cumulative. A skilled physician in such therapy interventions can
adjust these doses
based on the patient's bone marrow status and general health status based on
many factors,
including prior exposure to myelosuppressive therapeutic agents. These
principles can also
apply to combinations of radiolabeled hPAM4 with other therapeutic agents,
including
radiosensitizing drugs such as 5-fluorouracil and cisplatin.
101721 Chimeric, humanized and human antibodies and fragments thereof have
been used for
in vivo therapeutic and diagnostic methods. Accordingly contemplated is a
method of
delivering a diagnostic or therapeutic agent, or a combination thereof, to a
target comprising
(i) providing a composition that comprises an anti-pancreatic cancer antibody
or fragment
thereof, such as a chimeric, humanized or human PAM4 antibody, conjugated to
at least one
diagnostic and/or therapeutic agent and (ii) administering to a subject the
diagnostic or
therapeutic antibody conjugate. In a preferred embodiment, the anti-pancreatic
cancer
antibodies and fragments thereof are humanized or fully human.
101731 Another embodiment concerns a method for treating a malignancy
comprising
administering a naked or conjugated anti-pancreatic cancer antibody, antibody
fragment or
fusion protein that binds to an epitope located within the second to fourth
cysteine-rich
domains of MUC5AC (amino acid residues 1575-2052), more preferably to an
epitope
located in amino acid residues 1575-1725 and 1903-2052 (Cys2 and Cys 4), even
more
preferably to an epitope located in amino acid residues 1575-1725 (Cys2+),
most preferably,
to an epitope located in Cys2, such as a PAM4 antibody, either alone or in
conjunction with
one or more other therapeutic agents. The other therapeutic agent may be added
before,
simultaneously with or after the antibody. In a preferred embodiment, the
therapeutic agent is
gemcitabine, and in a more preferred embodiment, gemcitabine is given with the
hPAM4
radioconjugate in a fractionated dose schedule at lower doses than the
conventional 800-
46
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
1,000 mg/m2 doses of gemcitabine given weekly for 6 weeks. For example, when
combined
with fractionated therapeutic doses of 90Y-PAM4, repeated fractionated doses
intended to
function as a radiosensitizing agent of 200-380 mg/m2gemcitabine are infused.
The skilled
artisan will realize that the antibodies, fusion proteins and/or fragments
thereof described and
claimed herein may be administered with any known or described therapeutic
agent,
including but not limited to heat shock protein 90 (Hsp90).
[01741 In another form of multimodal therapy, subjects receive
immunoconjugates in
conjunction with standard cancer chemotherapy. For example, "CVB" (1.5 g/m2
cyclophosphamide, 200-400 mg/m2 etoposide, and 150-200 mg/m2 carmustine) is a
regimen
used to treat non-Hodgkin's lymphoma. Patti et al., Eur. J. Haematol. 51: 18
(1993). Other
suitable combination chemotherapeutic regimens are well-known to those of
skill in the art.
See, for example, Freedman et al., "Non-Hodgkin's Lymphomas," in CANCER
MEDICINE,
VOLUME 2, 3rd Edition, Holland etal. (eds.), pages 2028-2068 (Lea & Febiger
1993). As
an illustration, first generation chemotherapeutic regimens for treatment of
intermediate-
grade non-Hodgkin's lymphoma (NHL) include C-MOPP (cyclophosphamide,
vincristine,
procarbazine and prednisone) and CHOP (cyclophosphamide, doxorubicin,
vincristine, and
prednisone). A useful second generation chemotherapeutic regimen is m-BACOD
(methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine,
dexamethasone and
leucovorin), while a suitable third generation regimen is MACOP-B
(methotrexate,
doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin and
leucovorin).
Additional useful drugs include phenyl butyrate, bendamustine, and bryostatin-
1.
101751 The present invention contemplates the administration of anti-
pancreatic cancer
antibodies and fragments thereof, including fusion proteins and fragments
thereof, alone, as a
naked antibody or antibody fragment, or administered as a multimodal therapy.
Preferably,
the antibody is a humanized or fully human PAM4 antibody or fragment thereof.
Multimodal
therapies further include immunotherapy with a naked anti-pancreatic cancer
antibody
supplemented with administration of other antibodies in the form of naked
antibodies, fusion
proteins, or as immunoconjugates. For example, a humanized or fully human PAM4
antibody
may be combined with another naked antibody, or a humanized PAM4 or other
antibody
conjugated to an isotope, one or more chemotherapeutic agents, cytokines,
toxins or a
combination thereof For example, the present invention contemplates treatment
of a naked or
conjugated PAM4 antibody or fragments thereof before, in combination with, or
after other
pancreatic tumor associated antibodies such as CAI 9.9, DUPAN2, SPAN!, Nd2,
B72.3,
47
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
CC49, anti-Lea antibodies, and antibodies to other Lewis antigens (e.g.,
Le(y)), as well as
antibodies against carcinoembryonic antigen (CEA or CEACAM5), CEACAM6, colon-
specific antigen-p (CSAp), MUC I, MUC2, MUC3, MUC4, MUC5AC, MUC16, MUCI7,
[ILA-DR, CD40, CD74, CD138, FIER2/neu, EGFR, EGP-I, EGP-2, angiogenesis
factors
(e.g., VEGF, P1GF), insulin-like growth factor (IGF), tenascin, platelet-
derived growth factor,
and 1L-6, as well as products of oncogenes (e.g., bc1-2, Kras, p53), cMET, and
antibodies
against tumor necrosis substances.
101761 These solid tumor antibodies may be naked or conjugated to, inter alia,
drugs, toxins,
isotopes, radionuclides or immunomodulators. Many different antibody
combinations may be
constructed and used in either naked or conjugated form. Alternatively,
different naked
antibody combinations may be employed for administration in combination with
other
therapeutic agents, such as a cytotoxic drug or with radiation, given
consecutively,
simultaneously, or sequentially.
101771 Administration of the antibodies and their fragments can be effected by
intravenous,
intraarterial, intraperitoneal, intramuscular, subcutaneous, intrapleural,
intrathecal, perfusion
through a regional catheter, or direct intralesional injection. When
administering the antibody
by injection, the administration may be by continuous infusion or by single or
multiple
boluses.
101781 The immunoconjugate of the present invention can be formulated for
intravenous
administration via, for example, bolus injection or continuous infusion.
Preferably, the
antibody of the present invention is infused over a period of less than about
4 hours, and more
preferably, over a period of less than about 3 hours. For example, the first
25-50 mg could be
infused within 30 minutes, preferably even 15 min, and the remainder infused
over the next
2-3 hrs. Formulations for injection can be presented in unit dosage form,
e.g., in ampoules or
in multi-dose containers, with an added preservative. The compositions can
take such forms
as suspensions, solutions or emulsions in oily or aqueous vehicles, and can
contain
formulatory agents such as suspending, stabilizing and/or dispersing agents.
Alternatively,
the active ingredient can be in powder form for constitution with a suitable
vehicle, e.g.,
sterile pyrogen-free water, before use.
101791 Additional pharmaceutical methods may be employed to control the
duration of action
of the therapeutic conjugate. Control release preparations can be prepared
through the use of
polymers to complex or adsorb the immunoconjugate. For example, biocompatible
polymers
include matrices of poly(ethylene-co-vinyl acetate) and matrices of a
polyanhydride
48
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
copolymer of a stearic acid dimer and sebacic acid. Sherwood et al.,
Bioirechnology 10:
1446 (1992). The rate of release of an inmmnoconjugate or antibody from such a
matrix
depends upon the molecular weight of the immunoconjugate or antibody, the
amount of
immunoconjugate or antibody within the matrix, and the size of dispersed
particles. Saltzman
et al., Biophys. J. 55: 163 (1989); Sherwood et al., supra. Other solid dosage
forms are
described in Ansel et al., PHARMACEUTICAL DOSAGE FORMS AND DRUG
DELIVERY SYSTEMS, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.),
REMINGTON'S PHARMACEUTICAL SCIENCES, 18th Edition (Mack Publishing
Company 1990), and revised editions thereof.
101801 More generally, the dosage of an administered immunoconjugate for
humans will
vary depending upon such factors as the patient's age, weight, height, sex,
general medical
condition and previous medical history. It may be desirable to provide the
recipient with a
dosage of immunoconju gate, antibody fusion protein that is in the range of
from about 1
mg/kg to 25 mg/kg as a single intravenous infusion, although a lower or higher
dosage also
may be administered as circumstances dictate. A dosage of 1-20 mg/kg for a 70
kg patient,
for example, is 70-1,400 mg, or 41-824 mg/m2 for a 1.7-m patient. The dosage
may be
repeated as needed, for example, once per week for 4-10 weeks, once per week
for 8 weeks,
or once per week for 4 weeks. It may also be given less frequently, such as
every other week
for several months, or monthly or quarterly for many months, as needed in a
maintenance
therapy.
101811 Alternatively, an antibody may be administered as one dosage every 2 or
3 weeks,
repeated for a total of at least 3 dosages. Or, the antibodies may be
administered twice per
week for 4-6 weeks. If the dosage is lowered to approximately 200-300
mg/m2(340 mg per
dosage for a 1.7-m patient, or 4.9 mg/kg for a 70 kg patient), it may be
administered once or
even twice weekly for 4 to 10 weeks. Alternatively, the dosage schedule may be
decreased,
namely every 2 or 3 weeks for 2-3 months. It has been determined, however,
that even higher
doses, such as 20 mg/kg once weekly or once every 2-3 weeks can be
administered by slow
i.v. infusion, for repeated dosing cycles. The dosing schedule can optionally
be repeated at
other intervals and dosage may be given through various parenteral routes,
with appropriate
adjustment of the dose and schedule.
Immunoconjugates
101821 Anti-pancreatic cancer antibodies and fragments thereof may be
conjugated to at least
one therapeutic and/or diagnostic agent for therapy or diagnosis. For
immunotherapy, the
49
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
objective is to deliver cytotoxic doses of radioactivity, toxin, antibody
and/or drug to target
cells, while minimizing exposure to non-target tissues. Preferably, anti-
pancreatic cancer
antibodies are used to diagnose and/or treat pancreatic tumors.
101831 Any of the antibodies, antibody fragments and fusion proteins can be
conjugated with
one or more therapeutic or diagnostic agents, using a variety of techniques
known in the art.
One or more therapeutic or diagnostic agents may be attached to each antibody,
antibody
fragment or fusion protein, for example by conjugating an agent to a
carbohydrate moiety in
the Fe region of the antibody. If the Fe region is absent (for example with
certain antibody
fragments), it is possible to introduce a carbohydrate moiety into the light
chain variable
region of either an antibody or antibody fragment to which a therapeutic or
diagnostic agent
may be attached. See, for example, Leung et al., J Immunol. 154: 5919 (1995);
I-Tansen etal.,
U.S. Pat. No. 5,443,953 (1995), Leung et al., U.S. Pat. No. 6,254,868, the
Examples section
of each patent incorporated herein by reference.
101841 Methods for conjugating peptides to antibody components via an antibody
carbohydrate moiety are well-known to those of skill in the art See, for
example, Shih et al.,
Int J Cancer 41: 832 (1988); Shih et al., Int J Cancer 46: 1101 (1990); and
Shih et al., U.S.
Pat. No. 5,057,313, the Examples section of which is incorporated herein by
reference. The
general method involves reacting an antibody component having an oxidized
carbohydrate
portion with a carrier polymer that has at least one free amine function and
that is loaded with
a plurality of therapeutic agents, such as peptides or drugs. This reaction
results in an initial
Schiff base (imine) linkage, which can be stabilized by reduction to a
secondary amine to
form the final conjugate.
101851 Antibody fusion proteins or multispecific antibodies comprise two or
more antibodies
or fragments thereof, each of which may be attached to at least one
therapeutic agent and/or
diagnostic agent. Accordingly, one or more of the antibodies or fragments
thereof of the
antibody fusion protein can have more than one therapeutic and/or diagnostic
agent attached.
Further, the therapeutic agents do not need to be the same but can be
different therapeutic
agents, for example, one can attach a drug and a radioisotope to the same
fusion protein. For
example, an IgG can be radiolabeled with 1311 and attached to a drug. The 1311
can be
incorporated into the tyrosine of the IgG and the drug attached to the epsilon
amino group of
the IgG lysines. Both therapeutic and diagnostic agents also can be attached
to reduced SH
groups and to the carbohydrate side chains of antibodies. Alternatively, a
bispecific antibody
may comprise one antibody or fragment thereof against a disease antigen and
another against
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
a hapten attached to a targetable construct, for use in pretargeting
techniques as discussed
above.
101861 A therapeutic or diagnostic agent can be attached at the hinge region
of a reduced
antibody component via disulfide bond formation. As an alternative, such
agents can be
attached to the antibody component using a heterobifunctional cross-linker,
such as N-
succinyl 3-(2-pyridyldithio)proprionate (SPDP). Yu et al., Int. J. Cancer 56:
244 (1994).
General techniques for such conjugation are well-known in the art. See, for
example, Wong,
CIIEMISTRY OF PROTEIN CONJUGATION AND CROSS-LINKING (CRC Press 1991);
Upeslacis et al., "Modification of Antibodies by Chemical Methods," in
MONOCLONAL
ANTIBODIES: PRINCIPLES AND APPLICATIONS, Birch et al. (eds.), pages 187-230
(Wiley-Liss, Inc. 1995); Price, "Production and Characterization of Synthetic
Peptide-
Derived Antibodies," in MONOCLONAL ANTIBODIES: PRODUCTION, ENGINEERING
AND CLINICAL APPLICATION, Ritter et al. (eds.), pages 60-84 (Cambridge
University
Press 1995).
Click Chemistry
101871 An alternative method for attaching chelating moieties, drugs or other
functional
groups to an antibody, fragment or fusion protein involves use of click
chemistry reactions.
The click chemistry approach was originally conceived as a method to rapidly
generate
complex substances by joining small subunits together in a modular fashion.
(See, e.g., Kolb
et al., 2004, Angew Chem Int Ed 40:3004-31; Evans, 2007, Aust J Chem 60:384-
95.)
Various forms of click chemistry reaction are known in the art, such as the
Huisgen 1,3-
dipolar cycloaddition copper catalyzed reaction (Tornoe et al., 2002, J
Organic Chem
67:3057-64), which is often referred to as the "click reaction." Other
alternatives include
cycloaddition reactions such as the Diels-Alder, nucleophilic substitution
reactions
(especially to small strained rings like epoxy and aziridine compounds),
carbonyl chemistry
formation of urea compounds and reactions involving carbon-carbon double
bonds, such as
alkynes in thiol-yne reactions.
101881 The azide alkyne Huisgen cycloaddition reaction uses a copper catalyst
in the
presence of a reducing agent to catalyze the reaction of a terminal alkyne
group attached to a
first molecule. In the presence of a second molecule comprising an azide
moiety, the azide
reacts with the activated alkyne to form a 1,4-disubstituted 1,2,3-triazole.
The copper
catalyzed reaction occurs at room temperature and is sufficiently specific
that purification of
the reaction product is often not required. (Rostovstev et al., 2002, Angew
Chem Int Ed
51
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
41:2596; Tomoe et al., 2002, .1 Org Chem 67:3057.) The azide and alkyne
functional groups
are largely inert towards biomolecules in aqueous medium, allowing the
reaction to occur in
complex solutions. The triazole formed is chemically stable and is not subject
to enzymatic
cleavage, making the click chemistry product highly stable in biological
systems. Although
the copper catalyst is toxic to living cells, the copper-based click chemistry
reaction may be
used in vitro for immunoconjugate formation.
101891 A copper-free click reaction has been proposed for covalent
modification of
biomolecules. (See, e.g., Agard et al., 2004, J Am Chem Soc 126:15046-47.) The
copper-
free reaction uses ring strain in place of the copper catalyst to promote a [3
+ 2] azide-alkyne
cycloaddition reaction (Id.) For example, cyclooctyne is a 8-carbon ring
structure
comprising an internal alkyne bond. The closed ring structure induces a
substantial bond
angle deformation of the acetylene, which is highly reactive with azide groups
to form a
triazole. Thus, cyclooctyne derivatives may be used for copper-free click
reactions (Id.)
101901 Another type of copper-free click reaction was reported by Ning et al.
(2010, Angew
Chem Int Ed 49:3065-68), involving strain-promoted alkyne-nitrone
cycloaddition. To
address the slow rate of the original cyclooctyne reaction, electron-
withdrawing groups are
attached adjacent to the triple bond (Id.) Examples of such substituted
cyclooctynes include
difluotinated cyclooctynes, 4-dibenzocyclooetynol and azacyclooctyne (Id.) An
alternative
copper-free reaction involved strain-promoted alkyne-nitrone cycloaddition to
give N-
alkylated isoxazolines (Id.) The reaction was reported to have exceptionally
fast reaction
kinetics and was used in a one-pot three-step protocol for site-specific
modification of
peptides and proteins (Id.) Nitrones were prepared by the condensation of
appropriate
aldehydes with N-methylhydroxylamine and the cycloaddition reaction took place
in a
mixture of acetonitrile and water (Id.) These and other known click chemistry
reactions may
be used to attach chelating moieties to antibodies or other targeting
molecules in vitro.
Therapeutic Agents
101911 A wide variety of therapeutic reagents can be administered concurrently
or
sequentially, or advantageously conjugated to the antibodies of the invention,
for example,
drugs, toxins, oligonucleotides (e.g., siRNA), immunomodulators, hormones,
hormone
antagonists, enzymes, enzyme inhibitors, radionuclides, angiogenesis
inhibitors, pro-
apoptotic agents, etc. The therapeutic agents recited here are those agents
that are useful for
either conjugated to an antibody, fragment or fusion protein or for
administration separately
with a naked antibody as described above.
52
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
101921 Therapeutic agents include, for example, chemotherapeutic drugs such as
vinca
alkaloids, anthracyclines, gemcitabine, epipodophyllotoxins, taxanes,
antimetabolites,
alkylating agents, antibiotics, SN-38, COX-2 inhibitors, antimitotics,
antiangiogenic and
apoptotic agents, particularly doxorubicin, methotrexate, taxol, CPT-11,
camptothecans,
proteosome inhibitors, mTOR inhibitors, HDAC inhibitors, tyrosine kinase
inhibitors, and
others from these and other classes of anticancer agents.
101931 Other useful cancer chemotherapeutic drugs include nitrogen mustards,
alkyl
sulfonates, nitrosoureas, triazenes, folic acid analogs, COX-2 inhibitors,
antimetabolites,
pyrimidine analogs, purine analogs, platinum coordination complexes, mTOR
inhibitors,
tyrosine lcinase inhibitors, proteosome inhibitors, HDAC inhibitors,
camptothecins and
hormones. Suitable chemotherapeutic agents are described in REMINGTON'S
PHARMACEUTICAL SCIENCES, 19th Ed. (Mack Publishing Co. 1995), and in
GOODMAN AND GILMAN'S THE PHARMACOLOGICAL BASIS OF
THERAPEUTICS, 7th Ed. (MacMillan Publishing Co. 1985), as well as revised
editions of
these publications. Other suitable chemotherapeutic agents, such as
experimental drugs, are
known to those of skill in the art.
101941 Specific drugs of use may include 5-fluorouracil, afatinib, aplidin,
azatibine,
anastrozole, anthracyclines, axitinib, AVL-101, AVL-291, bendamustine,
bleomycin,
bortezomib, bosutinib, bryostatin-1, busulfan, calicheamycin, camptothecin,
carboplatin, 10-
hydroxycamptothecin, carmustine, celebrex, chlorambucil, eisplatin (CDDP), Cox-
2
inhibitors, irinotecan (CPT-11), SN-38, carboplatin, cladribine,
camptothecans, crizotinib,
cyclophosphamide, cytarabine, dacarbazine, dasatinib, dinaciclib, docetaxel,
dactinomycin,
daunorubicin, doxorubicin, 2-pyrrolinodoxonthicine (2-PDOX), pro-2PDOX, cyano-
morpholino doxorubicin, doxorubicin glucuronide, epirubicin glucuronide,
erlotinib,
estramustine, epidophyllotoxin, erlotinib, entinostat, estrogen receptor
binding agents,
etoposide (VP16), etoposide glucuronide, etoposide phosphate, exemestane,
fingolimod,
floxuridine (FUdR), 3`,5'-O-dioleoyl-FudR (FlUdR-d0), fludarabine, flutamide,
farnesyl-
protein transferase inhibitors, flavopiridol, fostamatinib, ganetespib, GDC-
0834, GS-1101,
gefitinib, gemcitabine, hydroxyurea, ibrutinib, idarubicin, idelalisib,
ifosfamide, imatinib, L-
asparaginase, lapatinib, lenolidamide, leucovorin, LFM-A13, lomustine,
mechlorethamine,
melphalan, mercaptopurine, 6-mercaptopurine, methotrexate, mitoxantrone,
mithramycin,
mitomycin, mitotane, navelbine, neratinib, nilotinib, nitrosurea, olaparib,
plicomycin,
procarbazine, paclitaxd, PCI-32765, pentostatin, PSI-341, raloxifene,
semustine, sorafenib,
53
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
streptozocin, SU11248, sunitinib, tamoxifen, temazolomide (an aqueous form of
DTIC),
transplatinum, thalidomide, thioguanine, thiotepa, teniposide, topotecan,
uracil mustard,
vatalanib, vinorelbine, vinblasfine, vincristine, vinca alkaloids and ZD1839.
101951 In a preferred embodiment, conjugates of camptothecins and related
compounds, such
as SN-38, may be conjugated to hPAM4 or other anti-pancreatic cancer
antibodies, for
example as disclosed in U.S. Patent No. 7,591,994; and U.S. Patent Application
Serial No.
11/388,032, filed March 23, 2006, the Examples section of each of which is
incorporated
herein by reference.
101961 In another preferred embodiment, prodrug forms of 2-PDOX, as disclosed
in U.S.
Patent Application Serial No. 14/175,089 (the Examples section of which is
incorporated
herein by reference) may be used as an immunoconjugate with an anti-pancreatic
cancer
antibody that binds to an epitope of MUC5AC as discussed above.
101971 In another preferred embodiment, an hPAM4 antibody is given with
gemcitabine,
which may be given before, after, or concurrently with a naked or conjugated
chimeric,
humanized or human PAM4 antibody. Preferably, the conjugated hPAM4 antibody or
antibody fragment is conjugated to a radionuclide.
101981 A toxin can be of animal, plant or microbial origin. A toxin, such as
Pseudomonas
exotoxin, may also be complexed to or form the therapeutic agent portion of an
immunoconjugate of the anti-pancreatic cancer and hPAM4 antibodies. Other
toxins suitably
employed in the preparation of such conjugates or other fusion proteins,
include ricin, abrin,
ribonuclea.se (RNase), DNase I, Staphylococcal enterotoxin-A, pokeweed
antiviral protein,
gelonin, diphtheria toxin, ranpimase, Pseudomonas exotoxin, and Pseudomonas
endotoxin.
See, for example, Pastan et al., Cell 47:641(1986), Goldenberg, CA--A Cancer
Journal for
Clinicians 44:43 (1994), Sharkey and Goldenberg, CA--A Cancer journal for
Clinicians
56:226 (2006). Additional toxins suitable for use are known to those of skill
in the art and are
disclosed in U.S. Pat. No. 6,077,499, the Examples section of which is
incorporated herein by
reference.
101991 An immunomodulator, such as a cytokine, may also be conjugated to, or
form the
therapeutic agent portion of the immunoconjugate, or may be administered with,
but
unconjugated to, an antibody, antibody fragment or fusion protein. The fusion
protein may
comprise one or more antibodies or fragments thereof binding to different
antigens. For
example, the fusion protein may bind to an epitope of MUC5AC as discussed
above as well
as to immunomodulating cells or factors. Alternatively, subjects can receive a
naked
54
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
antibody, antibody fragment or fusion protein and a separately administered
cytokine, which
can be administered before, concurrently or after administration of the naked
antibodies. As
used herein, the term "immunomodulator" includes a cytokine, a lymphokine, a
monokine, a
stem cell growth factor, a lymphotoxin, a hematopoietic factor, a colony
stimulating factor
(CSF), an interferon (IFN), parathyroid hormone, thyroxine, insulin,
proinsulin, relaxin,
prorelaxin, follicle stimulating hormone (FSH), thyroid stimulating hormone
(TSH),
luteinizing hormone (LH), hepatic growth factor, prostaglandin, fibroblast
growth factor,
prolactin, placental lactogen, OB protein, a transforming growth factor (TGF),
TGF-a, TGF-
insulin-like growth factor (1GF), erythropoietin, thrombopoietin, tumor
necrosis factor
(TNF), TNF- a, TNF-13, a mullerian-inhibiting substance, mouse gonadotropin-
associated
peptide, inhibin, activin, vascular endothelial growth factor, integrin,
interleukin (IL),
granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony
stimulating
factor (GM-CSF), interferon- a, interferon-13, interaeron-y, SI factor, IL-1,
IL-Ice, IL-2, IL-
3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, 1L-13, 1L-14, IL-
15, 11,-16, 1L-17,
IL-18 IL-21 and IL-25, LIF, FLT-3, angiostatin, thrombospondin, endostatin
and
lymphotoxin.
102001 The therapeutic agent may comprise one or more radioactive isotopes
useful for
treating diseased tissue. Particularly useful therapeutic radionuclides
include, but are not
limited to "'In, 177Lu, 212Bi,213Bi, 21 im, 6201, 64cu, 67cu, 90y, 1251, 131i,
32p, 33p, 47s,c, lAg,
67Ga, I42pr, 1535m, 161Tb, 166....y, 166 IR6 'RR
V - -Ho, - - -Re, - --Re, itoRe, 2.12pb, 223Ra, 225A. c,
.
59Fe, 75Se,
77As, 89sr, 99mo, 105Rb, 109pd, I43pr, I49pm, , I69-r
E 1941r, 198A11, 199AU, 211Pb and 22711.i The
therapeutic radionuclide preferably has a decay energy in the range of 20 to
6,000 keV,
preferably in the ranges 60 to 200 keV for an Auger emitter, 100-2,500 keV for
a beta
emitter, and 4,000-6,000 keV for an alpha emitter. Maximum decay energies of
useful beta-
particle-emitting nuclides are preferably 20-5,000 keV, more preferably 100-
4,000 keV, and
most preferably 500-2,500 keV. Also preferred are radionuclides that
substantially decay
with Auger-emitting particles. For example, Co-58, Ga-67, Br-80m, Tc-99m, Rh-
103m, Pt-
109, In-111, Sb-119, 1-125, Ho-161, Os-189m and ir-192. Decay energies of
useful beta-
particle-emitting nuclides are preferably <1,000 keV, more preferably <100
keV, and most
preferably <70 keV. Also preferred are radionuclides that substantially decay
with generation
of alpha-particles. Such radionuclides include, but are not limited to: Dy-
I52, At-211, Bi-
212, Ra-223, Rn-219, Po-215, Bi-211, Ac-225, Fr-221, At-217, Bi-213, Th-227
and Fm-255.
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Decay energies of useful alpha-particle-emitting radionuclides are preferably
2,000-10,000
keV, more preferably 3,000-8,000 keV, and most preferably 4,000-7,000 keV.
102011 For example, 67Cu, considered one of the more promising radioisotopes
for
radioimmunotherapy due to its 61.5-hour half-life and abundant supply of beta
particles and
gamma rays, can be conjugated to an antibody using the chelating agent, p-
bromoacetamido-
benzyl-tetraethylaminetetraacetic acid (TETA). Alternatively, 90Y, which emits
an energetic
beta particle, can be coupled to an antibody, antibody fragment or fusion
protein, using
diethylenetriaminepentaacetic acid (DTPA), or more preferably using DOTA.
Methods of
conjugating 90Y to antibodies or targetable constructs are known in the art
and any such
known methods may be used. (See, e.g., U.S. Patent No. 7,259,249, the Examples
section of
which is incorporated herein by reference. See also Linden et al., Clin Cancer
Res. 11:5215-
22, 2005; Sharkey et al., J Nucl Med. 46:620-33, 2005; Sharkey et al., J. Nucl
Med. 44:2000-
18, 2003.)
102021 Additional potential therapeutic radioisotopes include "C, '3N, 150,
75Br, 198AU,
224 AC, 1261, 133r, 7713r, "3mIri, 95Ru, 97Ru, io3Ru, losRu, 203Hg, 12ImTe,
l22mTe, 125inTe,
165Tru, 167,rm, 168Tru, 197pt, I 09pd, 105Rh, 142- r,
P I43Pr, 16ITb, 16611o, 199Au, "CO, "CO, 5ICr,
59Fe, 75se, 20111, 225Ac, 76Br, '69Y
b, and the like.
102031 In another embodiment, a radiosensitizer can be used in combination
with a naked or
conjugated antibody or antibody fragment. For example, the radiosensitizer can
be used in
combination with a radiolabeled antibody or antibody fragment. The addition of
the
radiosensitizer can result in enhanced efficacy when compared to treatment
with the
radiolabeled antibody or antibody fragment alone. Radiosensitizers are
described in D. M.
Goldenberg (ed.), CANCER THERAPY WITH RADIOLABELED ANTIBODIES, CRC
Press (1995). Other typical radionsensitizers of interest for use with this
technology include
gemcitabine, 5-fluorouracil, and cisplatin, and have been used in combination
with external
irradiation in the therapy of diverse cancers, including pancreatic cancer.
Therefore, we have
studied the combination of gemcitabine at what is believed to be
radiosensitizing doses (once
weekly 200 mg/m2 over 4 weeks) of gemcitabine combined with fractionated doses
of90Y-
hPAM4, and have observed objective evidence of pancreatic cancer reduction
after a single
cycle of this combination that proved to be well-tolerated (no grade 3-4
toxicities by NCI-
CTC v. 3 standard).
102041 Antibodies or fragments thereof that have a boron addend-loaded carrier
for thermal
neutron activation therapy will normally be affected in similar ways. However,
it will be
56
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
advantageous to wait until non-targeted immunoconjugate clears before neutron
irradiation is
performed. Clearance can be accelerated using an anti-idiotypic antibody that
binds to the
anti-pancreatic cancer antibody. See U.S. Pat. No. 4,624,846 for a description
of this general
principle. For example, boron addends such as carboranes, can be attached to
antibodies.
Carboranes can be prepared with carboxyl functions on pendant side chains, as
is well-known
in the art. Attachment of carboranes to a carrier, such as aminodextran, can
be achieved by
activation of the carboxyl groups of the carboranes and condensation with
amines on the
carrier. The intermediate conjugate is then conjugated to the antibody. After
administration of
the antibody conjugate, a boron addend is activated by thermal neutron
irradiation and
converted to radioactive atoms which decay by alpha-emission to produce highly
toxic, short-
range effects.
Interference RNA
102051 Another type of therapeutic agent is RNAi or siRNA. RNA interference
(RNAi) is
mediated by the RNA-induced silencing complex (RISC) and is initiated by short
double-
stranded RNA molecules that interact with the catalytic RISC component
argonaute (Rand et
al., 2005, Cell 123:621-29). Types of RNAi molecules include microRNA (miRNA)
and
small interfering RNA (siRNA). RNAi species can bind with messenger RNA (mRNA)
through complementary base-pairing and inhibits gene expression by post-
transcriptional
gene silencing. Upon binding to a complementary mRNA species, RNAi induces
cleavage of
the mRNA molecule by the argonaute component of RISC. Among other
characteristics,
miRNA and siRNA differ in the degree of specificity for particular gene
targets, with siRNA.
being relatively specific for a particular target gene and miRNA inhibiting
translation of
multiple mRNA species.
102061 Therapeutic use of RNAi by inhibition of selected gene expression has
been attempted
for a variety of disease states, such as macular degeneration and respiratory
syncytial virus
infection (Sah, 2006, Life Sci 79:1773-80). It has been suggested that siRNA
functions in
host cell defenses against viral infection and siRNA has been widely examined
as an
approach to anti-viral therapy (see, e.g., Zhang et al., 2004, Nature Med
11:56-62; Novina et
al., 2002, Nature Med 8:681-86; Palliser etal., 2006, Nature 439:89-94). The
use of siRNA
for cancer therapy has also been attempted. Fujii et al. (2006, Int J Oncol
29:541-48)
transfected HPV positive cervical cancer cells with siRNA against HPV E6 and
E7 and
suppressed tumor growth. siRNA-mediated knockdown of metadherin expression in
breast
57
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
cancer cells was reported to inhibit experimental lung metastasis (Brown and
Ruoslahfi,
2004, Cancer Cell 5:365-74).
102071 Attempts have been made to provide targeted delivery of siRNA to reduce
the
potential for off-target toxicity. Song et al. (2005, Nat Biotechnol 23:709-
17) used protamine-
conjugated Fab fragments against HIV envelope protein to deliver siRNA to
circulating cells.
Schiffelers et al. (2004, Nucl Acids Res 32:e149) conjugated RGD peptides to
nanoparticles
to deliver anti-VEGFR2 siRNA to tumors and inhibited tumor angiogenesis and
growth rate
in nude mice. Dickerson et al. (2010, Cancer 10:10) used nanogels
functionalized with anti-
EphA2 receptor peptides to chemosensitize ovarian cancer cells with siRNA
against EGFR.
Dendrimer-conjugated magnetic nanoparticles have been applied to the targeted
delivery of
antisense survivin oligodeoxynucleotides (Pan et al., 2007, Cancer Res 67:8156-
63).
102081 The skilled artisan will realize that any siRNA or interference RNA
species may be
attached to the subject antibodies. siRNA and RNAi species against a wide
variety of targets
are known in the art, and any such known oligonucleotide species may be
utilized in the
claimed methods and compositions.
102091 Known siRNA species of potential use include those specific for IKK-
gamma (U.S.
Patent 7,022,828); VEGF, Flt-1 and Flk-1/KDR (U.S. Patent 7,148,342); BcI2 and
EGFR
(U.S. Patent 7,541,453); CDC20 (U.S. Patent 7,550,572); transducin (beta)-like
3 (U.S.
Patent 7,576,196); KRAS (U.S. Patent 7,576,197); carbonic anhydrase II (U.S.
Patent
7,579,457); complement component 3 (U.S. Patent 7,582,746); interleukin-1
receptor-
associated kinase 4 (IRAK4) (U.S. Patent 7,592,443); survivin (U.S. Patent
7,608,7070);
superoxide dismutase 1 (U.S. Patent 7,632,938); MET proto-oncogene (U.S.
Patent
7,632,939); amyloid beta precursor protein (APP) (U.S. Patent 7,635,771); IGF-
1R (U.S.
Patent 7,638,621); ICAM1 (U.S. Patent 7,642,349); complement factor B (U.S.
Patent
7,696,344); p53 (7,781,575), and apolipoprotein B (7,795,421), the Examples
section of each
of which is incorporated herein by reference.
102101 Additional siRNA species are available from known commercial sources,
such as
Sigma-Aldrich (St Louis, MO), Invitrogen (Carlsbad, CA), Santa Cruz
Biotechnology (Santa
Cruz, CA), Ambion (Austin, TX), Dharrnacon (Thermo Scientific, Lafayette, CO),
Promega
(Madison, WI), Mirus Bio (Madison, WI) and Qiagen (Valencia, CA), among many
others.
Other publicly available sources of siRNA species include the siRNAdb database
at the
Stockholm Bioinformatics Centre, the MIT/ICBP siRNA Database, the RNAi
Consortium
shRNA Library at the Broad Institute, and the Probe database at NCBI. For
example, there
58
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
are 30,852 siRNA species in the NCBI Probe database. The skilled artisan will
realize that for
any gene of interest, either an siRNA species has already been designed, or
one may readily
be designed using publicly available software tools. Any such siRNA. species
may be
delivered using the subject DNLTM complexes.
102111 Exemplary siRNA species that have been reported are listed in Table 1.
Although
siRNA is delivered as a double-stranded molecule, for simplicity only the
sense strand
sequences are shown in Table 1.
Table 1. Exemplary siRNA Sequences
Target Sequence SEQ ID NO
VEGF R2 AATGCGGCGGTGGTGACAGTA. SEQ ID NO:22
VEGF R2 AAGCTCAGCACACAGAAA.GAC SEQ ID NO:23
CXCR4 UAAAAUCUUCCUGCCCACCdTdT SEQ ID NO:24
CXCR4 GGAAGCUGUUGGCUGAAAAdTdT SEQ ID NO:25
PPARC1. AAGA CCAGCCUCUUUGCCC AG SEQ ID NO:26
Dynamin 2 GGACCAGGCAGAAAACGAG SEQ ID NO:27
Catenin CUAUCAGGAUGACGCGG SEQ ID NO:28
El A binding protein LIGACA.CA.GGCAGGCUUGACUU SEQ ID NO:29
Plasminogen GGTGAA.GAAGGGCGTCCAA SEQ ID NO:30
activator
K-ras GATCCGTMGAGCTGTTGGCGTAGIT SEQ ID NO:31
CAAGAGACTCGCCAACA.GCTCCAAC'F
TTTGGAAA
Sortilin 1 AGGTGGTGTTAACAGCAGAG SEQ ID NO:32
Apolipoprotein E AA.GGTGGAGCAAGCGGTGGAG SEQ ID NO:33
Apolipoprotein E AAGGAGTTGAAGGCCGACAAA SEQ ID NO:34
BcI-X UAUGGAGCUGCAGAGGAUGdTdT SEQ ID NO:35
Raf-1 TTTGAATATCTGTGCTGAGAACACA SEQ ID NO:36
GTFCTCAGCACAGATATFCITITT
Heat shock aatgagaaaagcaaaaggtgccctgtcte SEQ ID NO:37
transcription factor 2
IGFBP3 AAUCAUCAUCAAGAAAGGGCA SEQ ID NO:38
Thioredoxin A.UGACUGUCAGGAUGULJGCdTdI SEQ ID NO:39
CD44 GAACGAAUCCUGAAGACAUCU SEQ ID NO:40
59
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
MMP14 AAGCCTGGCTACAGCAATATGCCTGTCTC SEQ ID NO:41
MAPKAPK2 UGACCAUCACCGAGUUUAUdTdT SEQ ID NO:42
FGFR1 AA.GTCGGACGCAACAGA.GAAA SEQ ID NO:43
ERBB2 CUACCUUUCUACGGACGUGdTdT SEQ ID NO:44
BCL2L1 CTGCCTAAGGCGGATTTGAAT SEQ ID NO:45
ABL1 TTALTUCCU'UCU'UCGGGAAGUC SEQ ID NO:46
CEA.CA.M1 AACCITCTGGAA.CCCGCCCA.0 SEQ ID NO:47
CD9 GAGCATCTTCGAGCAAGAA SEQ ID NO:48
CD151 CATGTGGCACCGTTTGCCT SEQ ID NO:49
Caspase 8 AA.CTACCAGAAAGGTATACCT SEQ ID NO:50
BR CA1 UCACAGUGUCCUUUAUGUA.dTdT SEQ ID NO:51
p53 GCAUGAACCGGAGGCCCAUTT SEQ ID NO:52
CEACAM6 CCGGACAGTTCCATGTATA SEQ ID NO:53
Diagnostic Agents
102121 In the context of this application, the terms "diagnosis" or
"detection" can be used
interchangeably. Whereas diagnosis usually refers to defining a tissues
specific histological
status, detection recognizes and locates a tissue, lesion or organism
containing a particular
antigen.
102131 The subject antibodies and fragments can be detectably labeled by
linking the
antibody to an enzyme. When the antibody-enzyme conjugate is incubated in the
presence of
the appropriate substrate, the enzyme moiety reacts with the substrate to
produce a chemical
moiety which can be detected, for example, by spectrophotometric, fluorometric
or visual
means. Examples of enzymes that can be used to detectably label antibody
include malate
dehydrogenase, staphylococcal nuclease, delta-V-steroid isomerase, yeast
alcohol
dehydrogenase, alpha-glycerophosphate dehydrogenase, triose phosphate
isomerase,
horseradish peroxidase, alkaline phosphatase, asparaginase, glucose oxidase,
alpha-
galactosidase, ribonuclease, urease, catalase, glucose-6-phosphate
dehydrogenase,
glucoamylase and acetyleholinesterase.
102141 The immunoconjugate may comprise one or more radioactive isotopes
useful for
detecting diseased tissue. Particularly useful diagnostic radionuclides
include, but are not
limited to, 0In, 1111n,
1771,u, 18F, 2Fe, 62Cu, "CU, 67CU, 670a, 68Ga, "Y, 9 Y, 89Z17, 94mTC,
94TC, 99111TC, 120j, 1231, 124j, 1251, 131j, 154-158Gd, 32p, 11C, 13N, 1.50,
186- e,
K I88Re, 5IMn, 52mMn,
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
55Co, 72As, 75Br, 76Br, 82mRb, 83Sr, or other gamma-, beta-, or positron-
emitters, preferably
with a decay energy in the range of 20 to 4,000 keV, more preferably in the
range of 25 to
4,000 keV, and even more preferably in the range of 25 to 1,000 keV, and still
more
preferably in the range of 70 to 700 keV. Total decay energies of useful
positron-emitting
radionuclides are preferably <2,000 keV, more preferably under 1,000 keV, and
most
preferably <700 keV. Radionuclides useful as diagnostic agents utilizing gamma-
ray
detection include, but are not limited to: 51Cr, 57Co, 58Co, 59Fe, 67Cu, 67Ga,
75Se, 97Ru, 99mTc,
111In, 114mIn, 1231, 1251, 1311, 169'"Ir 1971-Ig, and 201T1. Decay energies of
useful gamma-ray
emitting radionuclides are preferably 20-2000 keV, more preferably 60-600 keV,
and most
preferably 100-300 keV.
102151 Methods of diagnosing cancer in a subject may be accomplished by
administering a
diagnostic immunoconjugate and detecting the diagnostic label attached to an
immunoconjugate that is localized to a cancer or tumor. The antibodies,
antibody fragments
and fusion proteins may be conjugated to the diagnostic agent or may be
administered in a
pretargeting technique using targetable constructs attached to a diagnostic
agent. Radioactive
agents that can be used as diagnostic agents are discussed above. A suitable
non-radioactive
diagnostic agent is a contrast agent suitable for magnetic resonance imaging,
X-rays,
computed tomography or ultrasound. Magnetic imaging agents include, for
example, non-
radioactive metals, such as manganese, iron and gadolinium, complexed with
metal-chelate
combinations that include 2-benzyl-DTPA and its monomethyl and cyclohexyl
analogs. See
U.S. Ser. No. 09/921,290 (now abandoned) filed on Oct. 10, 2001, the Examples
section of
which is incorporated herein by reference. Other imaging agents such as PET
scanning
nucleotides, preferably 18F, may also be used.
102161 Contrast agents, such as MR1 contrast agents, including, for example,
gadolinium
ions, lanthanum ions, dysprosium ions, iron ions, manganese ions or other
comparable labels,
CT contrast agents, and ultrasound contrast agents may be used as diagnostic
agents.
Paramagnetic ions suitable for use include chromium (l11), manganese (I1),
iron (III), iron
(11), cobalt 01), nickel (II), copper (11), neodymium (III), samarium (III),
ytterbium (111),
gadolinium (III), vanadium 0I), terbium (iiI), dysprosium (III), holmium MI)
and erbium
(III), with gadolinium being particularly preferred.
102171 Ions useful in other contexts, such as X-ray imaging, include but are
not limited to
lanthanum (III), gold (III), lead (ID and bismuth 0II). Fluorescent labels
include rhodamine,
fluorescein and renographin. Rhodamine and fluorescein are often linked via an
61
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
isothiocyanate intermediate.
102181 Metals are also useful in diagnostic agents, including those for
magnetic resonance
imaging techniques. These metals include, but are not limited to: gadolinium,
manganese,
iron, chromium, copper, cobalt, nickel, dysprosium, rhenium, europium,
terbium, holmium
and neodymium. In order to load an antibody with radioactive metals or
paramagnetic ions, it
may be necessary to react it with a reagent having a long tail to which are
attached a
multiplicity of chelating groups for binding the ions. Such a tail can be a
polymer such as a
polylysine, polysaccharide, or other derivatized or derivatizable chain having
pendant groups
to which can be bound chelating groups such as, e.g.,
ethylenediaminetetraacetic acid
(EDTA), diethylenetriaminepentaacetic acid (DTPA), porphyrins, polyamines,
crown ethers,
bis-thiosemicarbazones, polyoximes, and like groups known to be useful for
this purpose.
102191 Chelates are coupled to an antibody, fusion protein, or fragments
thereof using
standard chemistries. The chelate is normally linked to the antibody by a
group which enables
formation of a bond to the molecule with minimal loss of immunoreactivity and
minimal
aggregation and/or internal cross-linking. Other, more unusual, methods and
reagents for
conjugating chelates to antibodies are disclosed in U.S. Pat. No. 4,824,659 to
Hawthorne,
entitled "Antibody Conjugates", issued Apr. 25, 1989, the Examples section of
which is
incorporated herein by reference. Particularly useful metal-chelate
combinations include 2-
benzyl-DTPA and its monomethyl and cyclohexyl analogs, used with diagnostic
isotopes in
the general energy range of 20 to 2,000 keV. The same chelates, when complexed
with non-
radioactive metals, such as manganese, iron and gadolinium are useful for MRI.
Macrocyclic
chelates such as NOTA, DOTA, and TETA are of use with a variety of metals and
radiometals, most particularly with radionuclides of gallium, yttrium and
copper,
respectively. Such metal-chelate complexes can be made very stable by
tailoring the ring size
to the metal of interest. Other ring-type chelates such as macrocyclic
polyethers, which are of
interest for stably binding nuclides, such as 223Ra for RAIT are encompassed
by the
invention.
102201 Radiopaque and contrast materials are used for enhancing X-rays and
computed
tomography, and include iodine compounds, barium compounds, gallium compounds,
thallium compounds, etc. Specific compounds include barium, diatrizoate,
ethiodized oil,
gallium citrate, iocarmic acid, iocetamic acid, iodamide, iodipamide,
iodoxamic acid,
iogulamide, iohexol, iopamidol, iopanoic acid, ioprocemic acid, iosefamic
acid, ioseric acid,
iosulamide meglumine, iosemetic acid, iotasul, iotetric acid, iothalamic acid,
iotroxic acid,
62
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
ioxaglic acid, ioxotrizoic acid, ipodate, meglumine, metrizamide, metrizoate,
propyliodone,
and thallous chloride.
102211 The antibodies, antibody fragments and fusion proteins also can be
labeled with a
fluorescent compound. The presence of a fluorescent-labeled MAb is determined
by exposing
the antibody to light of the proper wavelength and detecting the resultant
fluorescence.
Fluorescent labeling compounds include Alexa 350, Alexa 430, AMCA,
aminoacridine,
BOD1PY 630/650, BODIPY 650/665, BODIPY-FL, BODIPY-R6G, BODIPY-TMR,
BODIPY-TRX, 5-carboxy-4',5`-dichloro-2',7`-dimethoxy fluorescein, 5-carboxy-
2`,4',5',7'-
tetrachlomfluorescein, 5-carboxyfluorescein, 5-carboxyrhodamine, 6-
carboxyrhodamine, 6-
carboxytetramethyl amino, Cascade Blue, Cy2, Cy3, Cy5,6-FAM, dansyl chloride,
Fluorescein, fluorescein isothiocyanate, fluorescamine, HEX, 6-JOE, NBD (7-
nitrobenz-2-
oxa-1,3-diazole), Oregon Green 488, Oregon Green 500, Oregon Green 514,
Pacific Blue,
phthalic acid, terephthalic acid, isophthalic acid, cresyl fast violet, cresyl
blue violet, brilliant
cresyl blue, para-aminobenzoic acid, eiythrosine, phthalocyanines,
phthaldehyde,
azomethines, cyanines, xanthines, succinylfluoresceins, rare earth metal
cryptates, europium
trisbipyridine diamine, a europium cryptate or chelate, diamine, dicyanins, La
Jolla blue dye,
allopycocyanin, allococyanin B, phycocyanin C, phycocyanin R, thiamine,
phycoerythrocyanin, phycoerythtin R, REG, Rhodamine Green, rhodamine
isothiocyanate,
Rhodamine Red, ROX, TAMRA, TET, TRIT (tetramethyl rhodamine isothiol),
Tetramethylrhodamine, and Texas Red. Fluorescently-labeled antibodies are
particularly
useful for flow cytometry analysis, but can also be used in endoscopic and
intravascular
detection methods..
102221 Alternatively, the antibodies, antibody fragments and fusion proteins
can be
detectably labeled by coupling the antibody to a chemiluminescent compound.
The presence
of the chemiluminescent-tagged MAb is determined by detecting the presence of
luminescence that arises during the course of a chemical reaction. Examples of
chemiluminescent labeling compounds include luminol, isoluminol, an aromatic
acridinium
ester, an imidazole, an acridinium salt and an oxalate ester.
102231 Similarly, a bioluminescent compound can be used to label the
antibodies and
fragments there. Bioluminescence is a type of chemiluminescence found in
biological
systems in which a catalytic protein increases the efficiency of the
chemiluminescent
reaction. The presence of a bioluminescent protein is determined by detecting
the presence of
63
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
luminescence. Bioluminescent compounds that are useful for labeling include
luciferin,
luciferase and aequorin.
102241 Accordingly, a method of diagnosing a maligiancy in a subject is
described,
comprising performing an in vitro diagnosis assay on a specimen (fluid, tissue
or cells) from
the subject with a composition comprising an anti-pancreatic cancer MAb,
fusion protein or
fragment thereof. Immunohistochemistry can be used to detect the presence of
PAM4 antigen
in a cell or tissue by the presence of bound antibody. Preferably, the
malignancy that is being
diagnosed is a cancer. Most preferably, the cancer is pancreatic cancer.
102251 Additionally, a chelator such as DTPA, DOTA, TETA, or NOTA or a
suitable
peptide, to which a detectable label, such as a fluorescent molecule, or
cytotoxic agent, such
as a heavy metal or radionuclide, can be conjugated to a subject antibody. For
example, a
therapeutically useful immunoconjugate can be obtained by conjugating a
photoactive agent
or dye to an antibody fusion protein. Fluorescent compositions, such as
fluomchrome, and
other chromogens, or dyes, such as porphyrins sensitive to visible light, have
been used to
detect and to treat lesions by directing the suitable light to the lesion. In
therapy, this has been
termed photoradiation, phototherapy, or photodynamic therapy (Joni et al.
(eds.),
PHOTODYNAM1C THERAPY OF TUMORS AND OTHER DISEASES (Libreria Progetto
1985); van den Bergh, Chem. Britain 22:430 (1986)). Moreover, monoclonal
antibodies have
been coupled with photoactivated dyes for achieving phototherapy. Mew et al.,
J. Immunol.
130:1473 (1983); idem., Cancer Res. 45:4380 (1985); Oseroff et al., Pmc Natl.
Acad. Sci.
USA 83:8744 (1986); idem., Photochem. Photobiol. 46:83 (1987); Ha.san et al.,
Prog. Clin.
Biol. Res. 288:471 (1989); Tatsuta etal., Lasers Surg. Med. 9:422 (1989);
Pelegrin et al.,
Cancer 67:2529 (1991).
102261 Fluorescent and radioactive agents conjugated to antibodies or used in
bispecific,
pretargeting methods, are particularly useful for endoscopic, intraoperative
or intravascular
detection of the targeted antigens associated with diseased tissues or
clusters of cells, such as
malignant tumors, as disclosed in Goldenberg U.S. Pat. Nos. 5,716,595;
6,096,289 and
6,387,350, the Examples section of each incorporated herein by reference,
particularly with
gamma-, beta- and positron-emitters. Endoscopic applications may be used when
there is
spread to a structure that allows an endoscope, such as the colon.
Radionuclides useful for
positron emission tomography include, but are not limited to: F-18, Mn-51, Mn-
52m, Fe-52,
Co-55, Cu-62, Cu-64, Ga-68, As-72, Br-75, Br-76, Rb-82m, Sr-83, Y-86, Zr-89,
Tc-94m, In-
110, 1-120, and 1-124. Total decay energies of useful positron-emitting
radionuclides are
64
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
preferably <2,000 keV, more preferably under 1,000 key, and most preferably
<700 keV.
Radionuclides useful as diagnostic agents utilizing gamma-ray detection
include, but are not
limited to: Cr-51, Co-57, Co-58, Fe-59, Cu-67, Ga-67, Se-75, Ru-97, Tc-99m, In-
111, In-
114m, 1-123, 1-125, I-131, Yb-169, Hg-197, and TI-201. Decay energies of
useful gamma-ray
emitting radionuclides are preferably 20-2000 keV, more preferably 60-600 keV,
and most
preferably 100-300 keV.
In Vitro Diagnosis
102271 The present invention contemplates the use of anti-pancreatic cancer
antibodies to
screen biological samples in vitro for the presence of the PAM4 antigen. In
such
immunoassays, the antibody, antibody fragment or fusion protein may be
utilized in liquid
phase or bound to a solid-phase carrier, as described below. For purposes of
in vitro
diagnosis, any type of antibody such as murine, chimeric, humanized or human
may be
utilized, since there is no host immune response to consider.
102281 One example of a screening method for determining whether a biological
sample
contains MUC5AC is the radioimmunoassay (RIA). For example, in one form of
RIA, the
substance under test is mixed with PAM4 MAb in the presence of radiolabeled
MUC5AC. In
this method, the concentration of the test substance will be inversely
proportional to the
amount of labeled MUC5AC bound to the MM, and directly related to the amount
of free,
labeled MUC5AC. Other suitable screening methods will be readily apparent to
those of skill
in the art.
102291 Alternatively, in vitro assays can be performed in which an anti-
pancreatic cancer
antibody, antibody fragment or fusion protein is bound to a solid-phase
carrier. For example,
MAbs can be attached to a polymer, such as aminodextran, in order to link the
MM, to an
insoluble support such as a polymer-coated bead, a plate or a tube.
102301 Other suitable in vitro assays will be readily apparent to those of
skill in the art. The
specific concentrations of detectably labeled antibody and MUC5AC, the
temperature and
time of incubation, as well as other assay conditions may be varied, depending
on various
factors including the concentration of MUC5AC in the sample, the nature of the
sample, and
the like. The binding activity of a sample of anti-pancreatic cancer antibody
may be
determined according to well-known methods. Those skilled in the art will be
able to
determine operative and optimal assay conditions for each determination by
employing
routine experimentation.
102311 The presence of the PAM4 antigen in a biological sample can be
determined using an
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
enzyme-linked immunosorbent assay (ELISA) (e.g., Gold et al. J Clin Oncol.
24:252-58,
2006). In the direct competitive ELISA, a pure or semipure antigen preparation
is bound to a
solid support that is insoluble in the fluid or cellular extract being tested
and a quantity of
detectably labeled soluble antibody is added to permit detection and/or
quantitation of the
binary complex formed between solid-phase antigen and labeled antibody.
102321 In contrast, a "double-determinant" ELISA, also known as a "two-site
ELISA" or
"sandwich assay," requires small amounts of antigen and the assay does not
require extensive
purification of the antigen. Thus, the double-determinant ELISA is preferred
to the direct
competitive EL1SA for the detection of an antigen in a clinical sample. See,
for example, the
use of the double-determinant ELISA for quantitation of the c-myc oncoprotein
in biopsy
specimens. Field et al., Oncogene 4: 1463 (1989); Spandidos et al., AntiCancer
Res. 9: 821
(1989).
102331 In a double-determinant ELISA, a quantity of unlabeled MAb or antibody
fragment
(the "capture antibody") is bound to a solid support, the test sample is
brought into contact
with the capture antibody, and a quantity of detectably labeled soluble
antibody (or antibody
fragment) is added to permit detection and/or quantitation of the ternary
complex formed
between the capture antibody, antigen, and labeled antibody. In the present
context, an
antibody fragment is a portion of an anti-pancreatic cancer MAb that binds to
an epitope of
MUC5AC. Methods of performing a double-determinant ELISA are well-known. See,
for
example, Field et al., supra, Spandidos et al., supra, and Moore et al., "Twin-
Site EL1SAs for
fos and myc Oncoproteins Using the AMPAK System," in METHODS IN MOLECULAR
BIOLOGY, VOL. 10, pages 273-281 (The Humana Press, Inc. 1992).
102341 In the double-determinant ELISA, the soluble antibody or antibody
fragment must
bind to a MUC5AC epitope that is distinct from the epitope recognized by the
capture
antibody. The double-determinant ELISA can be performed to ascertain whether
the PAM4
antigen is present in a biopsy sample. Alternatively, the assay can be
performed to quantitate
the amount of MUC5AC that is present in a clinical sample of body fluid. The
quantitative
assay can be performed by including dilutions of purified MUC5AC.
102351 The anti-pancreatic cancer MAbs, fusion proteins, and fragments thereof
also are
suited for the preparation of an assay kit. Such a kit may comprise a carrier
means that is
compartmentalized to receive in close confinement one or more container means
such as
vials, tubes and the like, each of said container means comprising the
separate elements of the
immunoassay.
66
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
102361 The subject antibodies, antibody fragments and fusion proteins also can
be used to
detect the presence of the PAM4 antigen in tissue sections prepared from a
histological
specimen. Such in situ detection can be used to determine the presence of
MUC5AC and to
determine the distribution of MUC5AC in the examined tissue. In situ detection
can be
accomplished by applying a detectably-labeled antibody to frozen tissue
sections. Studies
indicate that the PAM4 antigen is preserved in paraffin-embedded sections.
General
techniques of in situ detection are well-known to those of ordinary skill.
See, for example,
Ponder, "Cell Marking Techniques and Their Application," in MAMMALIAN
DEVELOPMENT: A PRACTICAL APPROACH 113-38 Monk (ed.) (IRL Press 1987), and
Coligan at pages 5.8.1-5.8.8.
102371 Antibodies, antibody fragments and fusion proteins can be detectably
labeled with any
appropriate marker moiety, for example, a radioisotope, an enzyme, a
fluorescent label, a
dye, a cluomogen, a chemiluminescent label, a bioluminescent labels or a
paramagnetic label.
102381 The marker moiety can be a radioisotope that is detected by such means
as the use of
a gamma counter or a scintillation counter or by autoradiogr, aphy. In a
preferred embodiment,
the diagnostic conjugate is a gamma-, beta- or a positron-emitting isotope. A
marker moiety
in the present description refers to a molecule that will generate a signal
under predetermined
conditions. Examples of marker moieties include radioisotopes, enzymes,
fluorescent labels,
chemiluminescent labels, bioluminescent labels and paramagnetic labels.
102391 The binding of marker moieties to anti-pancreatic cancer antibodies can
be
accomplished using standard techniques known to the art. Typical methodology
in this regard
is described by Kennedy et al., Clin Chim Acta 70: 1 (1976), Schurs et al.,
Clin. Chim. Acta
81: 1(1977), Shih et al., Int J Cancer 46: 1101 (1990).
102401 The above-described in vitro and in situ detection methods may be used
to assist in
the diagnosis or staging of a pathological condition. For example, such
methods can be used
to detect tumors that express the PAM4 antigen such as pancreatic cancer.
In Vivo Diagnosis/Detection
102411 Various methods of in vivo diagnostic imaging with radiolabeled MAbs
are well-
known. In the technique of immunoseintigraphy, for example, antibodies are
labeled with a
gamma-emitting radioisotope and introduced into a patient. A gamma camera is
used to
detect the location and distribution of gamma-emitting radioisotopes. See, for
example,
Srivastava (ed.), RADIOLABELED MONOCLONAL ANTIBODIES FOR IMAGING AND
TIIERAPY (Plenum Press 1988), Chase, "Medical Applications of Radioisotopes,"
in
67
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
REMINGTON'S PHARMACEUTICAL SCIENCES, 18th Edition, Gennaro et al. (eds.), pp.
624-652 (Mack Publishing Co., 1990), and Brown, "Clinical Use of Monoclonal
Antibodies,"
in BIOTECHNOLOGY AND PHARMACY 227-49, Pezzuto et al. (eds.) (Chapman & Hall
1993).
102421 For diagnostic imaging, radioisotopes may be bound to antibody either
directly or
indirectly by using an intermediary functional group. Useful intermediary
functional groups
include chelators such as ethylenediaminetetraacetic acid and
diethylenetriaminepentaacefic
acid. For example, see Shih et al., supra, and U.S. Pat. No. 5,057,313.
102431 The radiation dose delivered to the patient is maintained at as low a
level as possible
through the choice of isotope for the best combination of minimum half-life,
minimum
retention in the body, and minimum quantity of isotope which will permit
detection and
accurate measurement. Examples of radioisotopes that can be bound to anti-
pancreatic cancer
antibody and are appropriate for diagnostic imaging include 99mTc, "In and
18F.
102441 The subject antibodies, antibody fragments and fusion proteins also can
be labeled
with paramagnetic ions and a variety of radiological contrast agents for
purposes of in vivo
diagnosis. Contrast agents that are particularly useful for magnetic resonance
imaging
comprise gadolinium, manganese, dysprosium, lanthanum, or iron ions.
Additional agents
include chromium, copper, cobalt, nickel, rhenium, europium, terbium, holmium,
or
neodymium. Antibodies and fragments thereof can also be conjugated to
ultrasound
contrast/enhancing agents. For example, one ultrasound contrast agent is a
liposome. Also
preferred, the ultrasound contrast agent is a liposome that is gas filled.
102451 In a preferred embodiment, a bispecific antibody can be conjugated to a
contrast
agent. For example, the bispecific antibody may comprise more than one image-
enhancing
agent for use in ultrasound imaging. In another preferred embodiment, the
contrast agent is a
liposome. Preferably, the liposome comprises a bivalent DTPA-peptide
covalently attached to
the outside surface of the liposome.
Pharmaceutically Suitable Excipients
102461 Additional pharmaceutical methods may be employed to control the
duration of action
of an anti-pancreatic cancer antibody in a therapeutic application. Control
release
preparations can be prepared through the use of polymers to complex or adsorb
the antibody,
antibody fragment or fusion protein. For example, biocompatible polymers
include matrices
of poly(ethylene-co-vinyl acetate) and matrices of a polyanhydride copolymer
of a stearic
acid dimer and sebacic acid. Sherwood et al., Bio/Technology 10: 1446 (1992).
The rate of
68
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
release of an antibody, antibody fragment or fusion protein from such a matrix
depends upon
the molecular weight of the antibody, antibody fragment or fusion protein, the
amount of
antibody within the matrix, and the size of dispersed particles. Saltzman et
al., Biophys..E. 55:
163 (1989); Sherwood et al., supra. Other solid dosage forms are described in
Ansel et al.,
PHARMACEUTICAL DOSAGE FORMS AND DRUG DELIVERY SYSTEMS, 5th
Edition (Lea & Febiger 1990), and Gennaro (ed.), REMINGTON'S PHARMACEUTICAL
SCIENCES, 18th Edition (Mack Publishing Company 1990), and revised editions
thereof.
102471 The antibodies, fragments thereof or fusion proteins to be delivered to
a subject can
comprise one or more pharmaceutically suitable excipients, one or more
additional
ingredients, or some combination of these. The antibody can be formulated
according to
known methods to prepare pharmaceutically useful compositions, whereby the
immunoconjugate or naked antibody is combined in a mixture with a
pharmaceutically
suitable excipient. Sterile phosphate-buffered saline is one example of a
pharmaceutically
suitable excipient. Other suitable excipients are well-known to those in the
art. See, for
example, Ansel et al., PHARMACEUTICAL DOSAGE FORMS AND DRUG DELIVERY
SYSTEMS, 5th Edition (Lea & Febiger 1990), and Gennaro (ed.), REMINGTON'S
PHARMACEUTICAL SCIENCES, 18th Edition (Mack Publishing Company 1990), and
revised editions thereof.
102481 The immunoconjugate or naked antibody can be formulated for intravenous
administration via, for example, bolus injection or continuous infusion.
Formulations for
injection can be presented in unit dosage form, e.g., in ampules or in multi-
dose containers,
with an added preservative. The compositions can take such forms as
suspensions, solutions
or emulsions in oily or aqueous vehicles, and can contain thrmulatory agents
such as
suspending, stabilizing and/or dispersing agents. Alternatively, the active
ingredient can be in
powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-
free water, before
use.
102491 The immunoconjugate, naked antibody, fragment thereof or fusion protein
may also
be administered to a mammal subcutaneously or by other parenteral routes. In a
preferred
embodiment, the antibody or fragment thereof is administered in a dosage of 20
to 2000
milligrams protein per dose. Moreover, the administration may be by continuous
infusion or
by single or multiple boluses. In general, the dosage of an administered
immunoconjugate,
fusion protein or naked antibody for humans will vary depending upon such
factors as the
patient's age, weight, height, sex, general medical condition and previous
medical history.
69
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Typically, it is desirable to provide the recipient with a dosage of
immunoconjugate, antibody
fusion protein or naked antibody that is in the range of from about 1 mg/kg to
20 mg/kg as a
single intravenous or infusion, although a lower or higher dosage also may be
administered as
circumstances dictate. This dosage may be repeated as needed, for example,
once per week
for four to ten weeks, preferably once per week for eight weeks, and more
preferably, once
per week for four weeks. It may also be given less frequently, such as every
other week for
several months, or more frequently, such as two- or three-time weekly. The
dosage may be
given through various parenteral routes, with appropriate adjustment of the
dose and
schedule.
Kits
102501 Various embodiments may concern kits containing components suitable for
treating or
diagnosing diseased tissue in a patient. Exemplary kits may contain at least
one antibody,
antigen binding fragment or fusion protein as described herein. If the
composition containing
components for administration is not formulated for delivery via the
alimentary canal, such as
by oral delivery, a device capable of delivering the kit components through
some other route
may be included. One type of device, for applications such as parenteral
delivery, is a syringe
that is used to inject the composition into the body of a subject. Inhalation
devices may also
be used. In certain embodiments, an anti-pancreatic cancer antibody or antigen
binding
fragment thereof may be provided in the form of a prefilled syringe or
autoirkjection pen
containing a sterile, liquid formulation or lyophilized preparation of
antibody (e.g., Kivitz et
al., Clin. Then 2006, 28:1619-29).
102511 The kit components may be packaged together or separated into two or
more
containers. In some embodiments, the containers may be vials that contain
sterile, lyophilized
formulations of a composition that are suitable for reconstitution. A kit may
also contain one
or more buffers suitable for reconstitution and/or dilution of other reagents.
Other containers
that may be used include, but are not limited to, a pouch, tray, box, tube, or
the like. Kit
components may be packaged and maintained sterilely within the containers.
Another
component that can be included is instructions for use of the kit.
EXAMPLES
102521 The examples below are illustrative of embodiments of the current
invention and are
not limiting to the scope of the claims. The examples discuss studies
employing an exemplary
anti-pancreatic cancer monoclonal antibody (e.g., PAM4). Clinical studies with
the PAM4
MAb have shown that a majority of pancreatic cancer lesions were targeted in
patients and
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
there was no indication of uptake in normal tissues. Dosimetry indicated that
it was possible
to deliver 10 to 20 eGy/mCi to tumors, with a tumor to red marrow dose ratio
of 3:1 to 10:1.
The data show that PAM4 is useful for the treatment of pancreatic cancer.
Example I. Epitope of MUC5AC That Binds to hPAM4 Antibody
102531 PAM4 is a =rine monoclonal antibody showing high specificity for
pancreatic
ductal adenocarcinoma (PDAC) compared with normal tissues and other cancers.
Humanized
PAM4 labeled with 90Y in combination with low-dose gemcitabine has shown
promising
therapeutic activity in patients with metastatic PDAC, and is being evaluated
in a phase III
registration trial. Prior efforts have suggested the mucin species recognized
by PAM4 is
human MUC5AC, a secretory mucin expressed de novo in early pancreatic
intraepithelial
neoplasia and retained throughout disease progression. In the present study,
we provide
further evidence validating MUC5AC as the PAM4 antigen, and locate the PAM4-
reactive
epitope within the N-terminal cysteine-rich su.bdomain 2 (Cys2), thus
differentiating PAM4
from anti-MUC5AC antibodies known to-date. Specifically, we show (i) PAM4-
antigen and
MUC5AC were co-localized in the immunocytochemical analysis of multiple human
cancer
cell lines, including Capan-1, BxPC-3, HT-29, and MCF-7; (ii) MUC5AC-specific
siRNA
prominently reduced the expression of both MUC5A.0 and PAM4-antigen in CFPAC-1
cells;
(iii) ELTSA performed on Capan-1 culture supernatants following SEPITAROSEO-
CL2B
chromatography depicted the preferential binding of PAM4 to the void-volume
fractions,
which were further revealed by agarose gel electrophoresis and Western blot to
display the
ladder pattern characteristic of oligomeric MUC5AC; and (iv) by testing the
reactivity of
PAM4 with a panel of recombinant fragments of MUC5AC, we demonstrated the N-
terminal
region comprising Cys2 is essential for binding to PAM4.
MATERIALS and METHODS
102541 Antibodies and Reagents - Humanized PAM4 (hPAM4) was provided by
Immunomedics, Inc. Horseradish peroxidase (HRP)-IPAM4 conjugate was generated
using
the SureLINK HRP Conjugation Kit (Kirkegaard & Perry Laboratories). MAN-5ACI,
a
rabbit antiserum. against MUC5AC (Thornton et al., 1996, Biochem j 316:967-75)
was a
generous gift from Dr. David J. Thornton (University of Manchester).
Commercially
available antibodies acquired include the following: four mouse mAbs against
MUC5AC
(45M1, 2-11M1, 2-12M1, and 1-13M1) from Thermo Fisher Scientific, one mouse
monoclonal (2Q445) and one rabbit polyclonal (H-160) antibodies against
MIJC5AC from
Santa Cruz Biotechnology, one mouse mAb against human MUC1 (MAB6298, hereafter
71
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
referred to as a-MUC1) from R&D Systems, one rabbit polyclonal antibody
against full-
length CM' (a-GFP) from Clontech Laboratories, one rabbit polyclonal Myc-tag
antibody (a-
Myc) from Cell Signaling Technology, one FITC-labeled goat anti-human IgG
(FITC-GAH)
from Jackson ImmunoResearch Laboratories, and one Cy3-labeled goat anti-mouse
IgG
(Cy3-GAM) from EMD Millipore. The MUC5AC double-strand siRNA targeting
sequence
5'-GGAGCCTGATCATCCAGCA-3' (SEQ ID NO:54) was synthesized by GenScript.
SEPHAROSEe CL-2B was purchased from Sigma-Aldrich.
102551 Cell Culture - All cell lines were obtained from the American Type
Culture Collection
(ATCC) and have been authenticated by Promega using Short Tandem Repeat (STR)
analysis. BxPC-3, HT-29, LS174T, MCF-7, and Calu-3 were grown in RPM]. 1640
medium
(Life Technologies) with 10% fetal bovine serum (FBS, Thermo Scientific
HyClone); Capan-
1 was grown in RPMI 1640 medium with 20% FBS; CFPAC-1 was grown in ATCC-
formulated Iscove's Modified Dulbecco's Medium (IMDM) with 10% FBS; SW1990 was
grown in ATCC-formulated Leibovitz's L-15 Medium with 10% FBS; and PANC-1 was
grown in Dulbecco's Modified Eagle Medium (Life technologies) plus 10% FBS.
All cell
lines were incubated at 37 C in 5% CO2 except SW1990, which was cultured in
100% air.
102561 Immunocytochemistry - Cells were plated on 8-chamber slides (Thermo
Fisher
Scientific) at approximately 2 x 104 cells/chamber and incubated overnight at
37 C.
Following removal of the medium, cells were fixed in 4% formalin (Sigma-
Aldrich) for 15
min at RT, and then treated with 0.1% Triton X-100 in PBS for another 15 min.
After
washing twice with PBS, cells were incubated with 10 Ag/m1 of either hPAM4 or
a murine
mAb against MUC5AC or MIJC1 in PBS plus 1% BSA for 45 min at RT. Afterwards,
cells
were washed twice and incubated with a mixture of FITC-GMI and Cy3-GAM in PBS
plus
1% BSA for 30 min at RT. After three washes, chambers were dissembled. Slides
were
mounted with an antifade solution (VectaShield, Vector Laboratories)
containing the nuclear
counterstain, 4, 6-diamidino-2-phenylindole (DAPI). Image acquisition and
analyses were
performed using an Olympus fluorescence microscope with a Kodak camera system.
102571 RNA. Interference - CFPAC-1 cells grown to 90% confluence were used for
transfection. MIJC5AC SiRNA or PBS alone (Mock) was 1:100 diluted into Opti-
MEM I
Medium (Life Technologies) prior to the addition of 1/100 volume of
LIPOFECTAMINECR)
RNAiMAX Reagent (Life Technologies). After 20 mm incubation at RT, the siRNA
or Mock
mixture was dispersed onto 8-chamber slides (80 i1/chamber). Meanwhile, cells
were
trypsinized, washed, diluted in complete growth medium, and then added at
8x103 cells/400
72
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
RI/chamber. The final RNA concentration was 15.6 nM in a total volume of 480
tl. After 48-
h incubation, cells were stained with hPAM4 and anti-MUC5AC mAbs and examined
under
fluorescence microscope as described above.
102581 Gel Chromatography of Cell Culture Supernatant - Capan-1 cells were
cultured for 3-
4 days to reach over 90% confluence. The spent media were collected, mixed
with an equal
volume of 8 M guanidine hydrochloride (GdmCI) in 20 mM sodium phosphate buffer
(pH 7),
and 10-fold concentrated using the Amicon ultrafiltration membrane with 30 kDa
normal
molecular weight limit (EMD Millipore). Gel chromatography was performed on a
SEPHAROSER.) CL-2B column (78 cmx2.6 cm) using 4 M GdmC1 as the eluent and a
flow
rate of 40 ml/h. Fractions of 8 mi., were collected and each analyzed for
reactivity with
hPAM4 and a-MUC-1 by ELISA as follows. Briefly, MaxiSotp 96-well plates (Nunc,
Roskilde, Denmark) were coated with CL-2B-eluted fractions (100 Al/well) at 37
'C
overnight, washed twice with PBS, and blocked with Casein Blocking Buffers
(Thermo
Fisher Scientific) for 1 h. HRP-hPAM4 or a-MUC1 was diluted in PBS and added
at 100
ill/well. After 1-h incubation at RI, plates with a-MUC1 were washed and
incubated further
with HRP-GAM for 1 h. Plates were washed and bound HRP-hPAM4 or HRP-GAM was
detected with o-phenylenediamine dihydrochloride (0.4 mg/m1) in PBS plus 0.03%
hydrogen
peroxide as a substrate. The optical density was read at 490 nm using the
EnVision 2100
Multilabel Reader (PerkinElmer). The fractions eluted in the void-volume peak
were also
pooled, dialyzed against the PBS-AG buffer (35.2 mM Na2PO4.7H20; 0.4 M NaCl;
6.5 mM
Nall/PO4.H20; 150 mM arginine; 150 mM monosodium glutamate, pH 8.0), and
concentrated with 30 kDa Amicon Ultra centrifugal filters (EMD Millipore) for
further
analysis.
102591 MUC5AC Sandwich EL1SA MaxiSorp 96-well plates were coated with 100 pi
of 2-
11M1 (20 ig/m1) in PBS and incubated at 4 C overnight. After blocking with
casein buffer,
a 5-fold concentrated void-volume peak pooled from the CL-2B fractionation of
Capan-1
supernatant (hereafter referred to as the Capan-I void-volume peak) was 2-fold
serially
diluted and added to the plate at 100 p.1/well. After overnight incubation at
R'F, plates were
washed and detected by IIRP-PAM4, or by Biotin-45M1 plus HRP-streptavidin as a
positive
control.
102601 Agarose Gel Electrophoresis Agarose gel electrophoresis was performed
as
described (Sheehan et al., 2000, Biochem .1347:37-44), with modifications.
Briefly, the
Capan-1 void-volume peak was concentrated in PBS-AG buffer and diluted with
gel running
73
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
buffer (40 inM Tris-acetate/lmM EDTA, 0.1%SDS, pII 8.0). In selective
experiments, serum
samples from normal subjects or pancreatic cancer patients were mixed with an
equal volume
of 8 M guanidine hydrochloride (GdmCI) and dialyzed into gel running buffer.
All samples
were supplemented with 1 M urea, 3% glycerol and 0.02% bromophenol blue before
loading
into thin wells shaped with a 0.8 mm-thick comb in 0.7% agarose gel (5.7 cm x
8.3 cm).
Electrophoresis was performed at 30 V for 4 to 8 h in the Horizon 58
Electrophoresis
Apparatus (LABRepCo).
102611 Construction of Expression Vectors for MUC5AC Recombinant Fragments -
The
ASM-MUC5AC-CH-long expression vector (Lidell et al., 2008, FEBS J 275:481-9;
Lidell &
Hansson, 2006, Biochem .1399: 121-9), which encodes a signal sequence, a Myc
tag
(EQKLISEEDL, SEQ TD NO:55), the human MUC5AC (Swiss-Prot accession no. P98088)
C-terminal cysteine-rich part (AA3993-5030), and a histidine tag, was kindly
provided by Dr.
Gunner Hansson of Gothenburg University (Gothenburg, Sweden). Additional
vectors were
constructed from pSM-MUC5AC-CH-long by replacing the DNA sequence of AA3993-
5030
with that of AA1-1217, AA1218-2199, AA1218-1517, AA1575-2052, AA1725-2052,
AA1575-1723/1903-2052, AA1575-1853, and AA1575-1725, to express D1-D2-D'-D3 (b-
fragment), 11P15-Cys1-2-3-4-5 (c-fragment), 11P15-Cysl (d-fragment), Cys2-3-4
(e-
fragment), Cys3-4 (f-fragment), Cys2/4 (g-fragment), Cys2-3 (h-fragment), and
Cys2+ (I-
fragment), respectively, as listed in Table 2. In addition, four GFP-fused
fragments were
produced by replacing the Myc tag with a full GFP sequence in the vectors
encoding Cys2-3-
4, Cys3-4, Cys2/4, and Cys2-3, resulting in the e*-, f*-, g*- and h*-fragment,
respectively.
Myc-tagged Cys2-3-4 and Cys2+ were also expressed in E. coil, and purified
from the
inclusion body using HIS-Select Nickel Affinity Gel (Sigma-Aldrich), and
refolded.
Table 2. Recombinant MUC5AC fragments
Frag Tag MUC5AC AA # (P98088) MW Expressio
meat Domains 8(Da)
PANC-1 E. coli
a Myc Cys9-D4-B-C- 3992-5030 116,140
CK
b Myc D1-D2-D'-D3 1-1217 136,727
c Myc 11P15-Cys1-2-3- 1218-2199 109,380
74
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
4-5
d Myc 11P15-Cysl 1218-1517 35,704 4-
+--
e Mc Cys2-3-4 1575-2052 56,444
Myc Cys3-4 1725-2052 39,686
g Myc Cys2/4 1575-1725/1903- 37,171
2052
h Myc Cys2-3 1575-1853 35,452
I Myc Cys2+ 1575-1725 20,504
OF? Cys2-3-4 1575-2052 81,742
re GIP Cys3-4 1725-2052 64,983
g*GFP Cys2/4 1575-1725/1903- 62,468
2052
h* OF? Cys2-3 1575-1853 60,749
102621 Transient Expression of Recombinant MUC5AC Fragments - One day prior to
transfection, PANC-1 cells were seeded in a 24-well plate at 2x105/well and
held at 37 'V
overnight. Transfection was performed using Lipofectamine 2000 (Life
Technologies) with
and without the recombinant plasmid DNA of interest. After 72 h, the spent
media were
collected and analyzed by Western blot following gel electrophoresis.
102631 Western Blot - Samples were electrophoresed in the same gel or
different gels under
the same conditions. After electrophoresis, samples were transferred (100V, 1
h) onto a
nitrocellulose membrane using the Mini TRANS-BLOT cell system (Bio-Rad
Laboratories)
and probed with hPAM4, an anti-MUC5AC antibody, a-GFP, or a-Myc, as indicated.
The
signals were developed with SUPER.SIGNALThi West Dura Chemiluminescent
Substrate
(Thermo Fisher Scientific).
RESULTS
102641 Co-localization of the hPAM4 Antigen and MUC5AC in Different Cell Lines
-
Several cell lines were subjected to cytofluorometry in order to evaluate
localization patterns
(heterogeneous and/or homogenous) of MUC1, MUC5AC, and/or MUC17, as detected
by
hPAM4 and other mucin-specific mAbs. The cell lines examined included those
derived from
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
human pancreatic (CaPan-I, BxPC3, CFPAC-1, and AsPC-I), colorectal (HT-29 and
LS174
T), breast (MCF-7), and lung (A549) carcinomas. As shown in FIG. 1, in each of
the cell
lines examined, hPAM4 exclusively co-localized with MUC5AC (as identified by
two anti-
MUC5AC mAbs, 2-11M1 and 2-12M1), but not with MIX] or MUCI7 (data not shown),
suggesting that MUC5AC is the ITAM4-reactive antigen.
102651 Co-knockdown of the hPAM4 Antigen and MUC5AC by MUC5AC-specific siRNA -
The disparate localization between PAM4 and anti-MUCI or anti-MUCI7 indicates
that
PAM4 reacts with neither MUC I nor MUC17. On the other hand, the co-
localization of
PAM4 and the two anti-MUC5AC mAbs (2-11M1 and 2-12M1) is consistent with PAM4
being specific for MUC5AC (Gold et al., 2013, Mol Cancer 12:143). To
investigate if
hPAM4 associates with MUC5AC, we employed the RNAi method to specifically
knockdown MUC5AC. As shown in the upper panel of FIG. 2, hPAM4 and 2-11M1 are
co-
localized in untreated CFPAC-1 cells, as well as the mock-treated
(transfection agent alone)
cells. In contrast, treatment with MUC5AC-specific siRNA resulted in
substantially reduced
immunostaining for both 2-11M l and hPAM4. Moreover, as shown in the bottom
panel of
FIG. 2, siRNA knockdown of MUC5AC did not alter the anti-MUC1 immunostaining,
providing further evidence that hPAM4 is not reactive with MUCl.
102661 Presence of the hPAM4 Antigen in the Culture Supernatant of Mucin-
producing
Carcinoma Cell Lines - MUC5AC is a highly oligomeric secretory mucin that has
been
isolated from cell culture and in vivo mucous secretions (Sheehan et al.,
2000, Biochem J
347:37-44; Hovenberg et al., 1996, Glycoconj J 13:839-47). Our early studies
showed that
hPAM4 reacts with mucin derived from the CaPari-1 xenografted human PDAC (Gold
et al.,
1994, Int J Cancer 57:204-10). In the current study, we used SEPHAROSE CL-2B
molecular sieve chromatography to separate the mucin species secreted into the
supernatant
of CaPan-I. The eluted fractions were then examined for immunoreactivity with
hPAM4 and
a-MUCI. As shown in FIG. 3A, PAM4-reactive substance was present predominantly
in the
void-volume peak, whereas only subsequently eluted fractions were found
reactive with a-
MUC I . When the Capan-I void-volume peak was probed with anti-MUC5AC
antibodies, we
found a positive response with 45M1, -13M1, and 11-160, but not 20.445, as
shown in FIG.
3B. It is noted that the void-volume peaks obtained from other cancer cell
lines known to
secret MUC5AC, such as HT-29 (Sheehan et al., 2000, Biochem J 347:37-44), LS
174T
(Asker et al., 1998, Biochem J 335:381-7), SW1990 (IIoshi et al, 2011, Int J
Oncol 38:619-
27), CFPAC-1 (Luka et al., 2011, J Biomed Biotechnol 2011:93475728), and Calu-
3 (Rose et
76
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
al., 2000, J Aerosol Med 13:245-61), all tested positive for reactivity with
hPAM4 (data not
shown).
102671 Direct evidence that correlates the hPAM4-reactive substance in the
Capan-1 void-
volume peak with MUC5AC is provided by a sandwich ELISA formatted to quantify
the
MUC5AC captured by 2-11M1, which reacts with the N-terminal domains of MUC5AC
(Nollet et al., 2004, Hybrid Hybridomics 23:93-930). As shown in FIG. 3C, 2-
11M1-
captured MUC5AC could be detected by hPAM4 in a dose-dependent marmer,
demonstrating
that hPAM4 binds to a different region of MUC5AC from 2-11M1. The additional
results
obtained with 45M1, which serves as a positive control, also support the
previous conclusions
that the epitopes of 45M1 (Udell etal., 2008, FEBS J 275:481-9) and 2-11M1 on
MUC5AC
are non-overlapping.
102681 Electronhoretic Resolution of the hPAM4-reactive Void-volume Fractions
on Agarose
Gel - To further verify that the hPAM4-reactive substance is MUC5AC, the Capan-
1 void-
volume peak was separated by electrophoresis on 0.7% agarose gel, and
subsequently probed
with hPAM4 (FIG. 4A, left panel), 45M1 (FIG. 4A, middle panel), or MAN-SAC
(FIG.
4A, right panel) by Western blotting. Under non-reducing conditions, a group
of bands
resembling the ladder-like pattern reported for MUC5AC (Sheehan et al., 2000,
Biochem J
347:37-44, Sheehan et al., 2004, J Biol Chem 279:15698-705) was clearly
discerned by all
three antibodies in the same gel. In contrast, under reducing conditions, two
bands were
revealed by MAN-SAC!, but undetectable by either hPAM4 or 45M1, which
corroborate the
previous findings that the predominant fast-migrating band and the minor band
trailing
behind represent the MUC5AC monomer and a reduction-resistant dimer,
respectively
(Sheehan et al., 2000, Biochem J 347:37-44), and that neither 45M1 (Lidell et
al., 2008,
FEBS j 275:481-9) nor hPAM4 (Gold et al., 1994, Int j Cancer 57:204-10) should
react with
a reduced mucin.
102691 Detection of hPAM4-reactive Substance in Serum Samples of Pancreatic
Cancer
Patients - The visualization of MUC5AC by hPAM4 as a characteristic ladder in
Western blot
following agarose gel electrophoresis prompted us to examine whether such a
pattern could
be demonstrated for patient serum found positive with the presently formulated
PAM4-based
assay (Picozzi etal., 2014, J Clin Oncol 132:4026; Gold et al., 2010, Cancer
Epidemiol
Biomarkers Prey 19:2786-94). As shown in FIG. 4B, a broad band migrating
faster than
MUC5AC monomer was detected by hPAM4 and several MUC5AC-specific antibodies,
such as 2-11M and H-160, but not by 45M1, suggesting the PAM4-reactive antigen
in patient
77
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
serum could be derived from an immature MUC5AC variant, or a breakdown product
of
mature MUC5AC.
102701 Mapping of the hPAM4 Epitope on MUC5AC - The disparity in the
reactivity of
hPAM4 and 2Q445 with the Capan-1 void-volume peak, as noted in FIG. 3B,
suggests that
the hPAM4 epitope is not in the tandem repeat region of MUC5AC recognized by
2Q445
(Perez-Vilar et al., 2006, J Biol Chem 281:4844-55). Therefore, we excluded
the tandem
repeat region (AA2199-3992) and decided to express in PANC-1 cells three large
recombinant fragments (designated as a, b, and c) that comprise the remainder
of MUC5AC
(FIG. 5A). We found that hPAM4 did not react with the C-terminal a-fragment
(AA3992-
5030) or the N-terminal b-fragment (AA1-1217), suggesting its epitope was
located outside
the N-terminal DI-D2-D'-D3 domains and the C-terminal region encompassing Cys9-
D4-B-
C-CK domains. In contrast, the c-fragment (AA1218-2199), which spans the five
N-terminal
cysteine-rich subdomains (Cys1-2-3-4-5), reacted with 1IPAM4 as shown by
Western blot
(FIG. 5B, left panel). Expectedly, the c-fragment was found to react also with
1-13M1 (data
not shown) and 45M1 (FIG. 5B, right panel), which recognize cysteine-rich
subdomains of
class-2 (Cys2 and Cys4) and class-3 (Cys3, 5, 6, 7 and 9), respectively. We
next expressed
two sub-fragments (d and e) within the c-fragment and showed (FIG. 5C, left
panel) that
hPAM4 failed to react with the d-fragment (AA1218-1517) comprising 11P15-Cysl,
but
strongly stained the e-fragment (AA! 575-2052)comprising Cys2-3-4. We then
expressed
three overlapping sub-fragments (f, g, and h) of the e-fragment and showed
(FIG. 5ll, left
panel) hPAM4 stained the g-fragment (AA1575-1725 joined to AA1903-2052,
comprising
Cys2 and Cys4 with Cys3 deleted), but barely the f-fragment (AA1725-2052,
comprising
Cys3-4) or the h-fragment (AA1575-1853, comprising Cys2-3). The differential
reactivity of
hPAM4 observed for the e-, f-, g-, and II-fragments was confirmed (FIG. 5E,
left panel) with
the respective (-RP-fused counterparts (the e*-, g*- and h*-fragrnents);
the expression of
each was clearly shown by Western blot with anti-GFP (FIG. 5E, right panel).
Together,
these results indicate that (i) the hPAM4 epitope resides within the e-
fragment, which
contains the Cys2-3-4 region; (ii) the presence of Cys2 or Cys4, or both, is
needed for
recognition by hPAM4; (iii) Cys3 is essential for the binding of 45M1, since
it stained each
of the c- (FIG. 5B, right panel; FIG. 5C, rightmost panel), e-, f-, and h-
fragments (FIG. 5C,
rightmost panel; FIG. 5D, right panel), all of which contains Cys3; but not
the g- fragment
(Figures 5D, right panel), which lacks Cys3; and (iv) the validity of the d-
fragment was
78
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
supported by its positive staining with H-160 (FIG. 5C, middle panel), whose
epitope was
reported to reside in AA1214-1373 (33) contained in the d-fragment.
102711 The successful expression of Cys2-3-4 (AA1575-2052) and Cys2+ (AA1575-
1725) in
E. con, as evidenced by the coomassie blue staining (FIG. 6A) and Western blot
using anti-
Myc (FIG. 6B), was instrumental in further defining the location of the hPAM4
epitope to
the Cys2 subdomain. The unglycosylated Cys2-3-4 and Cys2+ were isolated
predominantly
as monomeric species of 55.4 and 20.5 kDa, respectively. As shown in FIG. 6C,
hPAM4
reacts with non-reduced, but not the reduced, Cys2-3-4 and Cys2+. Although 1-
13M1 also
targets Cys2 or Cys4, its binding to both non-reduced and reduced Cys2+ (FIG.
6D)
differentiates it from hPAM4. Thus, we further establish that the hPAM4
epitope, being
reduction-sensitive, is conformational, located within the Cys2 subdomain, and
unlikely
involving carbohydrates. We speculate that the weakly positive bands observed
for hPAM4
in lanes 3 and 4 of FIG. 6C could result from reformation of the disulfide
bond to a varying
degree in the process of blotting, which would restore the hPAM4 epitope.
DISCUSSION
102721 In the past decade, concerted efforts in the search of biomarkers for
PDAC have
produced compelling evidence that mucins are aberrantly expressed in this
devastating
malignancy, and have diverse biological functions in tumor development,
progression,
metastasis, and drug resistance (Kaur et al., 2013, Nat Rev Gastroenterol
Hepatol 10:607-20).
Moreover, a number of studies (Kaur et al., 2013, Nat Rev Gastroenterol
Hepatol 10:607-20;
Lau et al., 2004, Am .1. Clin Pathol 122:61-9; Remmers et al., 2013, Clin
Cancer Res 19:1981-
93) have shown that both cell-tethered and secreted mucins display different
expression
profiles in pancreatic cancer when compared to normal pancreas. As a de novo
mucin in
pancreatic cancer, MUC5AC could be detected as early as the pre-
malignantidysplastic stages
(Nagata et al., 2007, J IIepatobiliary Pancreat Surg 14:243-54), and was
identified in a high
percentage of PDAC (Remmers et al., 2013, Clin Cancer Res 19:1981-93; Yamazoe
et al.,
2011. Pancreas 40:896-904; Kanno et al., 2006, Pancreas 33:391-6).
102731 Our own endeavors for over 20 years have focused on the exploration of
mucin-
reactive PAM4 as a potential diagnostic and therapeutic agent for PDAC.
Although we have
recently proposed MUC5AC to be the PAM4 antigen (Gold et al., 2013, Mol Cancer
12:143),
the identification of the PAM4 epitope has lagged behind its clinical
development, mainly
due to the challenges encountered in characterizing MUC5AC, which is
polymeric, heavily
0-glycosylated, and present in several variant forms (Thornton et al., 2008,
Annu Rev
79
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Physiol 70:459-86; Silverman et al., 2001, Glycobiology 2001;11:459-71; Guo et
al., 2014,
Am .1 Respir Cell Mol Biol 50:223-32).
102741 In the current Example, we provide additional evidence from
immunocytochemistry,
RNA interference, and biochemical studies that authenticates MUC5AC as the
hPAM4
antigen; and more importantly, have located the PAM epitope to the N-terminal
region
comprising Cys2 through the recombinant expression of MUC5AC domains
(Backstrom et
al., 2013, Mol Biotechnol 54:250-6). We should note that DEGYTFCESPR (SEQ ID
NO:56),
one of the 6 MUC5AC peptides most frequently detected in the pancreatic cystic
lesions with
malignant potential, and not in the benign lesions, is located in the Cys2 and
Cys4
subdomains, as reported in a very recent study of mucin proteomics (jabbar et
al., 2014,
Nat! Cancer Inst 106:djt439).
102751 Based on their sequence similarity (Escande et al., 2001, Biochem J
358:763-72), the
9 Cys subdomains of MUC SAC have been characterized (Guo et al., 2014, Am j
Respir Cell
Mol Biol 50:223-32) as Class 1 (Cysl), Class II (Cys2, Cys4; 98% identical),
and Class 111
(Cys3, Cys5-9; 96% identical). Whereas each subdomain contains about 110 amino
acid
residues, including 10 remarkably conserved cysteine residues involved in
intramolecular
disulfide bonds, there is only one potential 0-glycosylation site and no
potential N-
glycosylation site. These structural features appear to match the
characteristics of the hPAM4
epitope. Earlier work (Gold et al., Int J Cancer 1994, 57:204-10) showed that
the reactivity
between PAM4 and its mucin antigen was negatively affected by heating,
reduction of
disulfide bonds, or certain protease digestion, suggesting that the PAM4
epitope is a
conformational glycopeptide. While we have confirmed that reduced MUC5AC no
longer
reacts with hPAM4, the results obtained from the unglycosylated Cys2-3-4 and
Cys2+ of this
study also indicate that the hPAM epitope is retained under denaturing
conditions (or can be
readily restored following blotting or immobilization and washing), and
unlikely to involve
carbohydrates. Because Cys2 and Cys4 are 98% identical in amino acid sequence,
including
all of the 10 conserved cysteine residues, we expect the hPAM4 epitope is
present on Cys4
also.
102761 It is worthy of note that among the various anti-M1.JC5AC antibodies
with mapped
epitopes, which include the mouse mAbs of the M1 series: 1-13M1 (Rose et al.,
2000, J
Aerosol Med 13:245-61), 2-11M1 (Rose et al., 2000), 9-13M1 (Rose et al.,
2000), 19M1
(Rose et at, 2000), 21M1 (Rose et at, 2000) , 62M1 (Rose etal., 2000), 45M1
(Sheehan et
al., 2000, Biochem J 347:37-44), and 2-12M1 (Sheehan et al., 2000); other
murine mAbs
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
such as CLII2 (Reis et al., 1997, Int J Cancer 74:112-21), SOMU1 (Rose et al.,
2000, J
Aerosol Med 13:245-61), 2Q445 (Sheehan et al., 2004, J Biol Chem 279:15698-
705), and
NPC-1C (US patent 7,763,720); and two rabbit polyclonal antibodies, H-160
(Perez-Vilar et
al., 2006, J Biol Chem 281:4844-55) and MAN-5AC1 (Thornton et al., 1996,
Biochem J
316:967-75), 1-13M1 is the only mAb reported to react with Cys2/4 subdomains
of
MUC5AC. Our data, however, indicate that 1-13M1 binds to a reduction-
insensitive epitope,
thus being different from that of hPAM4.
102771 Because the Cys2, Cys3 and Cys4 subdomains are flanked by
threonine/serine/proline
(TSP)-rich sequences, which contain numerous 0-glycosylation sites, we further
note that the
accessibility of hPAM4 to its epitope on Cys2 (or Cys4) could be masked by the
surrounding
oligosaccharides either structurally or in a conformation-dependent manner, or
both.
Accordingly, hPAM4 would prevail for underglycosylated MUC5AC, whose
expression in
epithelial cancers in general, and PDAC in particular, including the early-
stage pancreatic
cancer precursors, has not been as well studied as that of underglycosylated
MUC1 (Reis et
al., 1998, Int J Cancer 79:402-10).
102781 In conclusion, we have located the hPAM4 antigen to the N-terminal Cys2
of
MUC5AC and characterized it as a reduction-sensitive, carbohydrate-free
epitope, whose
access may be restricted by the surrounding oligosaccharides in the flanking
TSP-domains.
We believe the ultimate delineation of the hPAM4 epitope may lead to its
exploration as a
candidate for vaccine development, while providing valuable insight for
diagnosis and
treatment of MUC5AC-expressing cancers, such as biliary tract cancer (Wongkham
et al.,
2003, Cancer Lett 195:93-9), colorectal cancer (Bu et al., 2010, World J
Gastroenterol
16:4089-94), and gastric cancer (Wang et al., 2003, J Surg Oncol 83:453-60),
in addition to
PDAC.
Example 2. Therapeutic Dosages of "Y-Labeled Anti-MUC5AC Antibody for
Human Pancreatic Cancer
102791 For patients with metastatic pancreatic adenocarcinoma, there are no
approved or
established treatments beyond 2nd line. A study of fractionated
radioimmunotherapy was
undertaken, administering "Y-clivatuzumab tetraxetan (yttrium-90-radiolabeled
hPAM4
anti-MUC5AC antibody) with or without low radiosensitizing doses of
gemcitabine.
102801 Methods: Fifty-eight patients with 3 median (range 2-7) prior
treatments were treated
on Arm A (N=29, "Y-clivatuzumab tetraxetan, weekly 6.5 mCi/m2 doses X 3, plus
gemeitabine, weekly 200 mg/m2doses X 4 starting one week earlier) or Arm B
(N=29, 90Y-
81
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
clivatuzumab tetraxetan alone, weekly 6.5 mCilm2 doses X 3), repeating cycles
after 4-week
delays. Safety and efficacy were evaluated.
102811 Results: Cytopenias (predominantly transient thrombocytopenia) were the
only
significant toxicities. Fifty-three patients (27 Arm A, 26 Arm B, 91% overall)
completed >1
full treatment cycles, with 23 (12 Arm A, 11 Arm B; 40%) receiving multiple
cycles,
including 7 (6 Arm A, 1 Arm B; 12%) given 3-9 cycles. Two patients in Arm A
had partial
responses by RECIST criteria. Kaplan-Meier overall survival (OS) appeared
improved in
Arm A v B (hazard ratio [FIR] 0.55, 95% CI: 0.29-0.86; P-0.017, log-rank) and
the median
OS for Arm A v Arm B increased to 7.9 v 3.4 months with multiple cycles (HR
0.32,
P=0.004), including 3 patients in Ann A surviving >1 year.
102821 Conclusions: With these surprising results, clinical use of "Y-
clivatuzumab
tetraxetan and low-dose gemcitabine is feasible in metastatic pancreatic
cancer patients
beyond 2nd line. A Phase III trial is now underway in this setting.
Introduction
102831 The outlook for patients with advanced pancreatic adenocarcinoma
remains poor
(Hidalgo, 2010, N Engl j Med 362:1605-17). In the frontline, median survival
was 6.2-6.7
months with gemcitabine alone (Burris etal., 1997, J Clin Oncol 15:2403-13) or
with
erlotinib (Moore et al., 2007, J Clin Oncol 25:1960-6), 8.5 months combined
with albumin-
bound paclitaxel (Von Hoff et al., 2013, N Engl J Med 369:1691-1703), and 11.1
months for
those able to tolerate combination chemotherapy (FOLFIRINOX) (Conroy et al.,
2011, N
Engl J Med 364:1817-25). Beyond 1st line, the survival advantage with
chemotherapy
remains limited (Rahma et al., 2013, Ann Oncol 24:1972-9; Oettle et al., 2014,
J Clin Oncol
32:2423-9) and after two prior treatments (one usually gemcitabine-based, the
other
fluoropyrimidine-based), there are no accepted treatments (Seufferlein et al.,
2012, Ann
Oncol 23(suppl 7):vii33-40; Almhanna & Kim, 2008, Oncology (Williston Park)
22:1176-
83).
102841 We pursued radioimmunotherapy to target and directly irradiate tumor
sites without
needing to physically overcome transport barriers in pancreatic cancer (high
interstitial
pressure, dense stromal reaction) or be incorporated into the tumor cells to
be effective (Koay
et al., 2014, j Clin Invest 124:1525-36). PAM4, an anti-MUC5AC monoclonal
antibody
selectively binding to pancreatic adenocarcinoma mucin, proved active when
radiolabeled in
preclinical models of human pancreatic cancer (Cardillo et al., 2001, Clin
Cancer Res 7:3186-
92; Gold et al., 1997, Int J Cancer 71:660-7). However, dosage studies in
animal model
82
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
systems are generally not predictive of effective therapeutic dosages in
humans (Reagan-
Shaw et al., 2007, FASEB J 22:659-61), requiring clinical studies in human
subjects to
establish safe and effective therapeutic dosages.
102851 After humanization and conjugation with DOTA (1,4,7,10-
tetraazacyclododecane-
NN,N",Nm-tetraacetic acid), the chelate-hPAM4 conjugate (clivatuzumab
tetraxetan) was
labeled with 90-yttrium (90Y), a beta-emitting radionuclide with a radiation
path-length of
mm suitable for bulky tumors. "Y-clivatuzumab tetraxetan was initially
administered as a
single dose (Gulec et al., 2011, Clin Cancer Res 17:4091-100), but
fractionated doses should
be more effective (DeNardo et al., 2002, Cancer 94:1332-48). Gemcitabine is a
known
radiosensitizer (Morgan et al., 2008, Clin Cancer Res 14:6744-50), tolerated
clinically at low
doses with external radiotherapy (Pauwels et al., 2005, Oncologist 10:34-51),
and preclinical
studies showed enhanced anti-tumor activity combining 90Y-labeled PAM4 with
gemcitabine
(Cardillo et al., 2002, Int J Cancer 97:386-92; Gold et al., 2003, Clin Cancer
Res 9:3929S-
375; Gold et al., 2004, int J Cancer 109:618-26).
102861 In the frontline, fractionated doses of 90Y-clivatuzumab tetraxetan
combined with 200
mg/m2 doses of gemcitabine achieved 11.8 months median survival for those
patients given
repeated treatment cycles; manageable myelosuppression was the principal side-
effect
(Ocean et al., 2012, Cancer 118:5497-505). There is an unmet medical need for
further
therapy in pancreatic cancer patients who have received and shown resistance
to or relapsed
from two or more prior therapies. Radioimmunotherapy may be particularly
attractive for
patients considering continued treatment, but unable or unwilling to tolerate
the side effects
of further chemotherapy.
Methods
102871 This Example reports the results of an open-label, multicenter phase lb
study of9 Y-
clivatuzumab tetraxetan administered with or without 200 mg/m2 gemcitabine in
patients
with metastatic pancreatic adenocarcinoma after >2 prior therapies. Primary
study objectives
included evaluating treatment safety and tolerability in this setting.
Additional objectives
were to obtain evidence of efficacy based on survival, CT imaging and CA19-9
serum levels,
assess the contribution of gemcitabine to this treatment regimen, and evaluate
any
immunogenicity toward this antibody-based regimen.
102881 Population. Adults 18 years old with metastatic pancreatic
adenocarcinoma had
received ;?: 2 prior chemotherapy regimens for their advanced disease and had
measureable
disease by CT imaging, but no CNS metastases or bulky disease (no single mass?
10 cm).
83
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Other requirements included Karnofsky performance status 270%, hemoglobin 29
g/dL,
neutrophils [ANC]? 1500/mm3, platelets > 100,000/mm3, creatinine and bilimbin
< 1.5 x
IULN (institutional upper limit of normal), AST and ALT 5 2.0 x IULN, with any
prior
external radiation therapy <2000 eGy to lungs and kidneys, <3000 eGy to liver,
<30% of red
marrow, and with < Grade 2 nausea/vomiting, anorexia, or signs of intestinal
obstruction.
102891 Treatment and assessments. Immunomedics, Inc., (Morris Plains, NJ)
provided
clivatuzumab tetraxetan to local commercial radiopharmacies for "Y-
radiolabeling. Based
on the prior study (Ocean et al., 2012, Cancer 118:5497-5506), a 6.5 mCilm2
dose was
selected for this population, with "Y-clivatuzumab tetraxetan administered in
the hospital
nuclear medicine department by slow injection over 5-10 minutes. Commercially
available
gemeitabine prepared by the hospital pharmacy was given intravenously over 30
minutes.
102901 Patients were alternately assigned to treatment aims, without
consideration of prior
history other than meeting eligibility criteria. In Arm A, patients received
6.5 mCi/m2"Y-
clivatuzumab tetraxetan once-weekly for 3 weeks together with 200 mg/m2
gemcitabine
once-weekly for 4 weeks starting 5 days before beginning "Y-clivatuzumab
tetraxetan and
then 2 days after each dose. In Arm B, patients received only 6.5 mCi/m290Y-
clivatuzumab
tetraxetan once-weekly for 3 weeks. For both arms, treatment cycles were
repeated after 4
weeks following the last dose until unacceptable toxicity, progressive
disease, or patient
withdrawal. The full "Y-clivatuzumab tetraxetan dose was administered for ANC
21000/mm3 and platelets >100,000/mm3; otherwise, a 75% dose for ANC 2750/mm3
and
platelets 275,000/mm3, or a 50% dose for lower values with ANC ?.500/mm3 and
platelets
250,000/mm3, with the dose held if ANC <500/mm3 or platelets <50,000/mm3 and
treatment
delayed on a weekly basis until blood levels permitted dosing to continue.
Gemcitabine doses
were held if "Y-clivatuzumab tetraxetan doses were held, but were otherwise
given without
reduction.
102911 Adverse events were graded by NCI-CTCAE v4.0 (National Cancer Institute
(NCI).
Common terminology criteria for adverse events (CTCAE) version 4.0). Vital
signs, physical
examination, blood counts, serum chemistries, and CA19-9 serum levels were
evaluated
during treatment cycles, then 4, 8, and 12 weeks after last cycle. Serum
samples were
evaluated by enzyme immunoassay for any human anti-hPAM4 antibodies (I-IMIA).
CT
scans were interpreted by local radiologists every 4 weeks after each cycle
until progression
or 12 weeks post-treatment. Tumor lesion changes were categorized as a
complete response
84
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(CR), partial response (PR), stable (SD), or progressive disease (PD) by
RECIST v1.0 [27].
18F-FDG-PET or PET/CT imaging was optional. All patients were followed for
survival.
102921 Statistical analysis. Overall survival (OS) from first dose to death or
last contact was
analyzed by Kaplan-Meier methods. Other results were summarized by descriptive
statistics.
To determine if this approach is sufficiently active in this population, and
if low-dose
gemcitabine adds to the therapeutic activity, a SWOG two-stage design was used
with a
treatment target of? 25% disease control (PR or CR, or else Si) for at least 8
weeks from
treatment initiation) versus > 5% for an inactive therapy, resulting in a
sample size of >50
patients (25 per arm) for this study.
Results
102931 Patients and study treatment. Filly-eight patients with a median of 3
(range, 2-7) prior
chemotherapy regimens were enrolled. All had received gemcitabine-containing
regimens
(21% with nab-paclitaxel) while 97% had received fluoropyrimidine-containing
regimens
(50% with FOLFIRINOX). Twenty-nine patients were treated in both Arm A
(gemcitabine)
and Arm B (no gemcitabine). Table 3 summarizes patient demographics and
baseline
characteristics for each arm.
Table 3. Demographics and baseline characteristics
Arm A Arm B
Patients, N 29 29
Sex M/F 19/10 14/15 ________
Age, median (range) 62 (39-73) 66(51-80)
Kamofsky performance status, N
=
90 ¨ 100 14 10
70 ¨ 80 15 19
Hematology, median (range)
Platelets (x 1000/1iL) 211(100-577) 224(113-570)
Neutrophils (x 1000/4) 5.2 (1.9-15.1) 5.4 (3.0-13.4)
Hemoglobin (g/dL) 11.3 (9.2-15.1) 11.6 (9.4-15.8)
Years from diagnosis, median (range) 1.8 (0.3-4.1) 1.5 (0.4-
3.7)
Stage IV disease, N (%) 29 (100%) 29 (100%)
Extent of disease, median (range)
CA19-9 (1.17mL) 863 (0.8 ¨ UL*) 2720 (6.5 ¨ UL*)
Sum of index lesions (cm) 9.8 (1.7 ¨ 19.0) 7.1 (1.2 ¨ 22.2)
Tumor location, N (%)
Pancreas, including resection bed 11(38%) 15 (52%)
Liver 14 (48%) 17 (59%)
Other abdomen sites 13 (45%) 16 (55%)
Lung 13 (45%) 12 (41%)
Prior therapies
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Pancreatectomy, N (%) 15 (52%) 9 (31%)
External radiation, N (%) 10 (34%) 8 (28%)
Chemotherapy regimens
Median (range) 3 (2-7) 3(2-6)
Most frequent, N (%)
Gemcitabine-containing 29 (100%) 29 (100%)
Geincitabinelnab-paclitaxel 6 (21%) 6 (21%)
Fluoropyrimidine-containing 28 (97%) 28 (97%)
FOLEIRINOXI 17 (59%) 12 (41%)
*TM = upper limit of quantitation, nominally >200,000 UlmL.
thinotecan, oxaliplatin, and 5-fluorouracillleucovorin combination.
102941 No drug-related interruptions, discontinuations or adverse reactions
occurred with
treatment administrations. Five patients rapidly deteriorated before finishing
one cycle
(stroke, entered hospice, biliary obstruction, pulmonary embolism, severe
constipation).
Patients terminated further treatment due to disease progression/clinical
deterioration or other
treatment-unrelated events, with 30/58 (52%) patients (15 per arm) completing
only one
cycle, and 23 (40%) patients (12 Arm A, 11 Arm B) receiving multiple cycles,
including 16
(6 Arm A. 10 Arm B) given 2 cycles and 7 (6 Arm A, 1 Arm B) given 3 to 9
cycles. For cycle
1, 8/58 (14%) patients had >1 doses of 90Y- clivatuzumab tetraxetan reduced to
75%; but,
besides the 5 patients who rapidly deteriorated, no doses were held. For
repeated cycles,
16/23 (70%) patients had _>_1 doses reduced to 75% (N-7) or 50% (N-9),
including 11(48%)
who also had >1 doses held.
102951 Adverse Events. Events considered at least possibly treatment-related
were
thrombocytopenia, 50% of patients; fatigue, 26%; anemia, 22%; nausea, 16%;
leukopenia,
neutropenia, 12% each; abdominal pain, anorexia, vomiting, diarrhea, 9% each;
bleeding,
fever, chills, 7% each; dyspnea, hyperbilirubinemia, headache, 5% each; others
< 5%. These
included Grade ?.3 events of thrombocytopenia, 19%; anemia, leukopenia,
neutropenia, 7%
each; others < 2%.
102961 Comparison of events regardless of assumed treatment relationship shows
limited
differences between treatment arms, and AEs occurring more frequently (>10%
difference) in
arm A (fatigue, neutropenia, leukopenia, nausea, diarrhea, dyspnea, alkaline
phosphatase,
headache) or arm B (ascites, asthenia, gastrointestinal pain or tenderness)
were primarily
limited to Grade 1-2 events (Table 4).
86
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Table 4, Patient Incidence of Most Frequent Adverse Events Regardless of
Assumed
Relationship to Treatment,*
Arm A Arm B
All
All Grade
Adverse event, % Grade :=:3
Grades >3 Grades
,
Laboratories .
Thrombocytopenia 62 71 52 17
Anemia 45 14 18 10
Nentropenia 28 10 3 1
Leukopenia 24 14 3 1
Alkaline phosphatase 24 3 10 µ 0
'
Hyponatremia 21 , 14 µ 7
' Aspartate aminotransferase , 17 0 7 7
' .
Lymphopenia 14 10 17 10
Hyperbilirubinemia 14 3 21 3
"
Hyperglycemia 14 3 14 7
"
Hypoalbumemia 14 0 1 -;
Clinical Events
Fatigue 76 1 38 7
Nausea 41 0 74 3
Anorexia 31 0 24 --;
, .
Abdominal/gastrointestinal
31 -; 48 10
pain or tenderness
. .
,
Constipation 28 0 28 1
,
Diarrhea 28 0 10 0 .
Dyspnea 28 7 14 0 .
Infection 24 7 17 7
Vomiting 21 1 28 3
Abdominal distension 17 0 ,7
, 0
Bleeding 17 1 14 7
Back Pain 17 7 14 0 .
Cough 17 0 , 0 .
Dehydration 14 3 17 3
Headache 14 0 0 0
Hypertension 14 7 1 0
Fever 14 0 14 0
Pleural effusion 10 7 17 7
!
Peripheral edema 10 0 14 0
Ascites 0 0 14 3
Asthenia 0 0 14 3
*Events occurring in >10% of the 29 patients in either treatment arm
[0297l Six patients (3 per arm) had serious events considered at least
possibly treatment--
related. Two patients had cerebrovascular accidents due to thromboembolic or
watershed
87
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
events, one after the first dose and the other one month after cycle 1, and
two other patients
developed consumptive coagulopathies, one after cycle 1 with thrombocytopenia,
deep
venous thrombosis and sub-acute cerebral infarcts, the other after cycle 2
with
thrombocytopenia, acute renal failure and fatal gastrointestinal hemorrhage.
One patient
developed fever after cycle 3 with negative cultures but responded to
antibiotics, while
another patient with a history of severe infections developed fatal Gram-
negative bacteremia
during cycle 2; both had undergone recent biliary stent placements and were
not neutropenic
at time of event.
102981 Overall, 20/58 (34%) patients (10 per arm) developed Grade >3
thrombocytopenia (4
given platelets), 14 (11 Arm A, 3 Arm B; 24% overall) developed Grade ?.3
neutropenia (4
given cytokine support), and 11 (7 Arm A, 4 Arm B; 19% overall) developed
Grade >3
anemia (8 transfused). In patients with follow-up data, only 4 events (all
thrombocytopenia)
remained at Grade 4 levels >7 days, while all Grade 3 cytopenias recovered to
Grade 2 levels
within 12 weeks. Grade ?.3 cytopenias generally increased with repeated cycles
but Grade 4
occurrences generally remaining limited (Table 5).
Table 5. Grade 3 and 4 Hematol9..gical Toxicity by Treatment Cycle
Cycle Thrombocytopenia I Neutropenia Anemia
Grade Grade
Grade 3 Grade 4 Grade 3 Grade 4
3 4
Cycle 1 58 4(7%) 6 (10%)1 (2%) 5 (9%) 0(0%)
0 7%)
Cycle 2 23 7 (30%) 2 (9%) 3 (13%) 0(0%) 4 (17%) 0 (0%)
Cycle 3- 1
7 3 (43%) 2 (29%) 1 (14%)(14%) 3 (43%) 0 0%)
9
102991 Infections occurred in 11(19%) patients, including 4 serious events
[fatal septic
entercolitis from pre-study pancreatectomy (Sump syndrome); fatal septic
bacteremia from
unidentified source; post-interventional Grade 3 acute cholangitis; Grade 3
pneumonia
responding to antibiotics]; and 10 Grade 1-2 events [upper respiratory
infection (URI) x 4,
urinary tract infection (UTI) x 3, superficial fungal infection x 2,
pneumonia, Lyme disease).
The fatal bacteremia was considered possibly-related although the patient had
a history of
severe infections and recent biliary stent placement; other infections were
considered
unrelated by the investigators. Furthermore, only 3 patients were neutropenic
(700 - 900
cells/4) at time of infection (pneumonia, UTI, URI).
103001 Bleeding occurred in 9 (16%) patients, including 3 serious events
(fatal GI bleeding
from consumptive coagulopathy, Grade 3 melena from concomitant medications,
Grade 3 GI
88
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
bleeding from underlying disease), and 7 minor Grade 1 events (bruising x 4,
epistaxis,
hemorrhoids, conjunctival). Minor bruising was considered at least possibly
study drug
related, but the other bleeding events were all considered unrelated by the
investigators, and
only 3 patients had Grade 3-4 thrombocytopenia at time of event (consumptive
coagulopathy,
bruising x 2).
103011 Efficacy. The median overall survival (OS) for all 58 patients was 2.7
months, with 2
patients (both Arm A) currently alive 22 and 23 months from treatment
initiation. Kaplan-
Meier survival curves (FIG. 7) showed improvement in Arm A v B beginning at
about 3
months, with the relative number of patients remaining alive in Arm A v B then
progressively
increasing with time (hazard ratio [HR] 0.55, 95% CI: 0.29 -0.86; P = 0.017,
log-rank test).
Median OS for Arm A. v B (Table 6) was only 2.7 v 2.6 months overall, but
increased to 7.9 v
3.4 months for those patients who received multiple cycles (FIR 0.32, P
0.004), including 3
patients in Arm A surviving > 1 year (one for 1.5 years, the others still
alive). OS generally
increased with better performance status and lower CA19-9 serum levels at
study entry, and
to a lesser degree with fewer prior therapies and smaller tumor burden
estimated by summing
lengths of index lesions (Table 61).
Table 6. Dependence of overall survival (OS) on treatment and patient
factors
Median (range), months
Treatment
Arm A, Overall 29 2.7 (0.4 - 22.8+)
Single Cycle 17 1.9 (0.4 - 11.0)
Multiple Cycles 12 7.9 (3.9- 22.8+)
________________________________________________________________ =
Arm B, Overall 29 2.6 (0.7 - 9.4)
Single Cycle 18 1.7 (0.7 - 4.1)
Multiple Cycles 11 3.4 (1.7 - 9.4)
Patient Factors
Karnofsky Performance Status
90- 100 24 4.0 (0.4- 22.8+)
70 - 80 34 2.0 (0.7 - 21.7+)
Number of Prior Systemic Treatments
2 19 2.9 (0.4 -21.7+)
3 18 3.1 (0.9- 22.8+)
>3 21 2.4 (0.7 17.5)
Sum of Index Lesions (cm)
89
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
1.2 - 8.1 29 2.9 (0.7¨ 22.8+)
8.3 ¨22.2 29 2.6 (0.4 21.7+)
Serum CA19-9 (Ij/mL)
5,1257 29 3.9 (0.4 ¨ 22.8+)
>1257 28* 2.1 (0.7 ¨ 8.4)
f.n. + indicates value from patient currently alive
*Baseline CA19-9 unavailable in one patient.
103021 There was >25% disease control (PR + SD) in both treatment arms at
interim
evaluation, thus meeting the SWOG two-stage criteria to complete enrollment.
By RECIST
criteria, there were 2 PRs (both Arm A) and 22 SDs (10 Arm A, 12 Arm B) as
best response,
with other patients having progressed by first CT evaluation 4 weeks after
cycle 1. Median
OS for those with PR.. SD and PD was 11.5, 5.1 and 1.8 months, respectively.
Eleven
patients had elevated CA19-9 levels at baseline that decreased with treatment,
either 20-50%
and considered a minor response (N=8), or >50% and considered an objective
response
(N=3). Median OS for patients with CA19-9 responses was 3.9 v 2.5 months for
the
remaining population.
103031 Immunogenicity. Five patients (1 arm A, 4 arm B) with baseline serum
samples that
were HAT-IA-negative (<50 ng/mL) became HAT-IA-positive after their first
(N=3) or second
(N-2) cycle, developing maximum titers of 135 21,611 ng/mL. These were
isolated
laboratory findings without event and of uncertain clinical significance.
DISCUSSION
103041 Despite having 3 median (2 to 7) prior chemotherapies, 58 patients with
metastatic
pancreatic cancer were enrolled within 8 months, demonstrating the feasibility
of using "Y-
clivatuzumab tetraxetan in this setting. All had received gemcitabine-
containing regimens
(21% with nab-paclitaxel), and 97% had received fluoropyrimidine-containing
regimens
(50% with FOLFIRINOX), underscoring the importance of developing treatments
beyond
2nd line for pancreatic cancer.
103051 There were no infusion reactions and, as expected, cytopenias
(predominantly
thrombocytopenia) were the only significant toxicities. Even in this
population, these were
mostly transient and reversible events, with infrequent hematologic support
required.
Treatment-related myelosuppression may have exacerbated two cases of
consumptive
coagulopathy, but otherwise, the few infections or major bleeding events that
occurred could
be attributed to complications of underlying disease. Most other AEs were mild-
moderate
constitutional and gastrointestinal events also expected in advanced
pancreatic cancer, and
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
comparison of events between treatment arms showed no substantial differences.
Thus this
combination approach appears to be an acceptable regimen in this advanced
population.
103061 Although median survival was only 2.7 months overall, the addition of
low-dose
gemcitabine to this regimen showed survival progressively improving with time
(48% v 35%
alive at 3 months, 35% v 10% at 6 months, 21% v 3% at 9 months, 10% v 0% at 1
year). For
patients receiving only one cycle, gemcitabine made little difference, but
with multiple cycles
median OS increased (3.4 v 7.9 months), including 3 patients surviving >1
year. Patients
undergoing multiple cycles could reflect a healthier population, but this
would not explain
survival results favoring combining radioimmunotherapy with low-dose
gemcitabine.
Although more limited than previously seen with "Y-clivatuzumab tetraxetan in
less heavily
treated patients (Ciulec et al., 2011, Clin Cancer Res 17:4091-100; Ocean et
al., 2012, Cancer
118:5497-505), tumor response assessed by CT imaging or CA19-9 levels still
showed
treatment activity, and the improvement of survival with better responses and
patient risk
factors also supported the consistency of results in this advanced population.
However, to
additionally examine the role of low-dose gemcitabine in the treatment regimen
before
pursuing a large, randomized, controlled trial, the study was powered to
demonstrate
prespecified criteria for treatment arm activity, not survival.
103071 In conclusion, this trial demonstrated the feasibility of using "Y-
clivatuzumab
tetraxetan in metastatic pancreatic cancer patients after >2 prior
chemotherapy regimens (3rd
line and beyond). This is important, because the benefits of current second-
line therapies
appear modest, at the cost of drug toxicity (Rahma et al., 2013, Ann Oncol
24:1972-9;
Seufferlein et al., 2012, Ann Oncol 23(suppl 7):vii33-40; Almharma & Kim,
2008, Oncology
(Williston Park) 22:1176-83). "Y-clivatuzumab tetraxetan combined with low-
dose
gemcitabine appears promising in this difficult-to-treat population.
Example 3. Humanized PAM4 MAb
103081 In preferred embodiments, the claimed methods and compositions utilize
the antibody
hPAM4 which is a humanized IgG of the murine PAM4 MAb raised against
pancreatic
cancer mucin. Humanization of the murine PAM4 sequences was utilized to reduce
the
human antimouse antibody (FIAMA) response. To produce the humanized PAM4,
murine
complementarily determining regions (CDR) were transferred from heavy and
light variable
chains of the mouse immunoglobulin into human framework region (FR) antibody
sequences,
followed by the replacement of some human FR residues with their murine
counterparts.
91
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Humanized monoclonal antibodies are suitable for use in in vitro and in vivo
diagnostic and
therapeutic methods.
103091 Comparison of the variable region framework sequences of the murine
PAM4 MAb
(FIG. 8A and FIG. 8B) to known human antibodies in the Kabat database showed
that the
Fits of PAM4 VK and VH exhibited the highest degree of sequence homology to
that of the
human antibodies Walker Vic (FIG. 10A) and Wil2 VH (FIG. 10B), respectively.
Therefore,
the Walker VK (FIG. 10A) and Win VH (FIG. 10B) FRs were selected as the human
frameworks into which the murine CDRs for PAM4 VK and VH were grafted,
respectively.
The FR4 sequence of the human antibody, NEWM, however, was used to replace the
Wi12
FR4 sequence for the humanization of the PAM4 heavy chain (FIG. 10B). A few
amino acid
residues in PAM4 FRs that flank the putative CDRs were maintained in hPAM4
based on the
consideration that these residues have more impact on Ag binding than other FR
residues.
These residues were 21M, 47W, 59P, 60A, 85S, 87F, and 100G of Vic (FIG. 10A)
and 27Y,
30P, 38K, 481, 66K, 67A, and 69L of VII (FIG. 10B). The DNA and amino acid
sequences of
hPAM4 VK (SEQ. ID NO:16) and VH (SEQ ID NO:19) are shown in FIG. 11.A and 11B,
respectively.
103101 A modified strategy as described by Leung et al. (Leung et al., 1994))
was used to
construct the designed VK (FIG. 11A) and VH (FIG. 11B) genes for hPAM4 using a
combination of long oligonucleotide syntheses and PCR. For the construction of
the hPAM4
VH domain, two long oligonucleotides, hPAM4 VH A (173-iner) and hPAM4 VH B
(173-mer)
were synthesized on an automated DNA. synthesizer (Applied Biosystems). hPAM4
VH A
represents nt 17 to 189 of the hPAM4 VH domain.
AGTCTGGGGC TGAGGTGAAG AAGCCTGGGG CCTCAGTGAA GGTCTCCTGC
GAGGCTXTG GATACACA'FT CCCTAGCTAT GMTGCACT GGGTGAAGCA
GGCCCCTGGA CAAGGGCTTG A.GTOCiA.TIGG ATATA.TIAAT CCTTACAATG
ATGGTACTCA GTACAATGAG AAG-3' (SEQ ID NO:57)
103111 hPAM4 VHB represents the minus strand of the hPAM4 VH domain
complementary
to nt 169 to 341.
A.GGGTFCCCT CiGCCCCAGIA AGCAAA.TCCG TAGCTACCAC CCiAAGCCTCT
TGCACAGTAA TACACGGCCG TGTCGTCAGA TCTCAGCCTG CTCAGCTCCA
TGTAGGCTGT GTTGATGGAC GTGTCCCTGG TCAGTGTGGC CTTGCCTTTG
AACTTCTCAT TGTACTGAGT ACC-3' (SEQ ID NO:58)
92
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
103121 The 3'-terminal sequences (21 nt residues) of hPAM4 VHA and VHB are
complementary to each other. Under defined PCR condition, the 3'-ends of hPAM4
VHA and
VHB anneal to form a short double stranded DNA flanked by the rest of the long
oligonucleotides. Each annealed end serves as a primer for the transcription
of the single
stranded DNA, resulting in a double strand DNA composed of the nt 17 to 341 of
hPAM4
VH. This DNA was further amplified in the presence of two short
oligonucleotides, hPAM4
VHBACK and hPAM4 VHFOR. to form the fill-length hPA.M.4 VH. The underlined
portions
are restriction sites for subcloning as shown in FIG. 11B.
hPAM4 VHBACK 5'-CAG GTG CAG CTG CAG CAG TCT GGG GCT GAG GTG A-3'
(SEQ ID NO:59)
hPAM4 VHFOR 5'-TGA GGA GAC GOT GAC CAG GOT TCC CTG GCC CCA-3' (SEQ
ID NO:60)
103131 A minimal amount of hPAM4 VHA and VHB (determined empirically) was
amplified
in the presence of 10111.. of 10X PCR. Buffer (500 mM KCI, 100 mM Tris HCI
buffer, pH 8.3,
15 mM MgCl2), 2 p.mol of 1IPAM4 VHBACK and hPAM4 VKFOR, and 2.5 units of Taq
DNA polymerase (Perkin Elmer Cetus, Norwalk, Conn.). This reaction mixture was
subjected to three cycles of polymerase chain. reaction (PCR) consisting of
denaturation at
94 C for 1 minute, annealing at 45 C for 1 minute, and polymerization at 72 C
for 1.5
minutes. This procedure was followed by 27 cycles of PCR reaction consisting
of
denaturation at 94 C for 1 minute, annealing at 55 C for 1 minute, and
polymerization at
72 C for 1 minute. Double-stranded PCR-amplified product for hPAM4 VH was gel-
purified,
restriction-digested with Pstl and BstEII restriction sites and cloned into
the complementary
Pstl/BstEII restriction sites of the heavy chain staging vector, VHpBS2, in
which the VII
sequence was fully assembled with the DNA sequence encoding the translation
initiation
codon and a secretion signal peptide in-frame ligated at the 5.-end and an
intron sequence at
the 3`-end. VHpBS2 is a modified staging vector of VHpBS (Leung et al.,
IIybridoma, 13:469,
1994), into which a Xhol restriction site was introduced at sixteen bases
upstream of the
translation initiation codon to facilitate the next subcloning step. The
assembled Vu gene was
subcloned as a XhoI-BamITI restriction fragment into the expression vector,
pdHL2, which
contains the expression cassettes for both human IgG heavy and light chains
under the control
of IgH enhancer and MT1 promoter, as well as a mouse d/fr gene as a marker for
selection
and amplification. Since the heavy chain region of pdIIL2 lacks a Hamill
restriction site, this
ligation requires use of a linker to provide a bridge between the Hamill site
of the variable
93
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
chain and the Hind111 site present in the pdIIL2 vector. The resulting
expression vectors were
designated as hPAM4 VHpdHL2.
103141 For constructing the full length DNA of the humanized VK sequence (FIG.
11A),
hPAM4 VKA (157-mer) and hPAM4 VKB (156-mer) were synthesized as described
above.
hPAM4 VKA and VKB were amplified by two short oligonucleotides hPAM4 VKBACK
and
hPAM4 VKFOR as described above. hPAM4 VKA represents nt 16 to 172 of the hPAM4
VK
domain.
5`-CAGTCTCCAT CCTCCCTGTC TGCATCTGTA GGAGACAGAG TCACCATGAC
CTGCAGTGCC AGCTCAAGTG TAAGTTCCAG CTACTTGTAC TGGTACCAAC
AGAAACCAGG GAAAGCCCCC AAACTCTGGA TTIATAGCAC ATCCAACCTG
GCTTCTG-3' (SEQ ID NO:61)
103151 hPAM4 VKB represents the minus strand of the hPAM4 VK domain
complementary
to nt 153 to 308.
5'-GTCCCCCCTC CGAACGTGTA. CGGGTACCTA TTCCACTGAT GGCAGAAATA
AGAGGCAGAA TCTTCAGGTT GCA.GACTGCT GATGGTGAGA GTGAAGTCTG
TCCCAGATCC ACTGCCACTG AAGCGAGCAG GGACTCCAGA AGCCAGGTTG
GATGTG-3' (SEQ ID NO:62)
103161 The 3c-terminal sequences (20 nt residues) of hPAM4 VKA and VKB are
complementary to each other. Under defined PCR condition, the 3'-ends of hPAM4
VKA and
VKB anneal to form a short double-stranded DNA flanked by the rest of the long
oligonucleotides. Each annealed end served as a primer for the transcription
of the single
stranded DNA, resulting in a double strand DNA composed of nt 16 to 308 of
hPAM4 VK.
This DNA was further amplified in the presence of two short oligonucleotides,
hPAM4
VKBACK and hPAM4 VKFOR to form the full-length 1IPAM4 W. The underlined
portions
are restriction sites for subeloning as described below.
hPAM4 VKBACK 5'-GAC ATC CAG CTG ACC CAG TCT CCA TCC TCC CTG-3' (SEQ
ID NO:63)
hPAM4 VKFOR 5'- TFA OAT cry, CAG TCG TOT CCC CCC TCC GAA CGT-3' (SEQ ID
NO:64)
103171 Gel-purified PCR products for ITAM4 Vic were restriction-digested with
PvulI and
BglII and cloned into the complementary PvulUBc11 sites of the light chain
staging vector,
VKpBR2. VKpBR2 is a modified staging vector of VKpBR (Leung et al., Hybiidoma,
13:469,
1994), into which a Xbal restriction site was introduced at sixteen bases
upstream of the
94
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
translation initiation codon. The assembled Vx genes were subcloned as Xbal-
BainHI
restriction fragments into the expression vector containing the VH sequence,
hPAM4
VlipdHL.2. The resulting expression vectors were designated as hPAM4pdH.L2.
103181 Approximately 30 ttg of hPAM4pdHL2 was linearized by digestion with
Sall and
transfected into Sp2/0-Ag14 cells by electroporation at 450 V and 25 11F. The
transfected
cells were plated into 96-well plates and incubated in a CO, cell culture
incubator for two
days and then selected for MTX resistance. Colonies surviving selection
emerged in two to
three weeks and were screened for human antibody secretion by ELISA assay.
Briefly,
supernatants (-100 ul) from the surviving colonies were added into the wells
of an ELISA
microplate precoated with goat anti-human IgG F(ab1)2 fragment-specific A.b.
The plate was
incubated for one hour at room temperature. Unbound proteins were removed by
washing
three times with wash buffer (PBS containing 0.05% Tween-20). Horseradish
peroxidase-
conjugated goat anti-human IgG Fc fragment-specific Ab was added to the wells.
Following
incubation for one hour, a substrate solution (100 utlwell.) containing 4 mM o-
phenylenediamine dihydrochloride (OPD) and 0.04%11202in PBS was added to the
wells
after washing. Color was allowed to develop in the dark for 30 minutes and the
reaction was
stopped by the addition of 501.11, of 4 N II2SO4 solution. The bound human IgG
was
measured by reading the absorbance at 490 nm on an ELISA reader. Positive cell
clones were
expanded and hPAM4 was purified from cell culture supernatant by affinity
chromatography
on a Protein A column.
103191 The Ag-binding activity of hPAM4 was confirmed by ELISA assay in a
microtiter
plate coated with pancreas cancer cell extracts. An ELISA competitive binding
assay using
PAM4-antigen coated plates was developed to assess the Ag-binding affinity of
hPAM4 in
comparison with that of a chimeric PAM4 composed of murine V and human C
domains.
Constant amounts of the IIRP-conjugated cPAM4 mixed with varying
concentrations of
cPAM4 or hPAM4 were added to the coated wells and incubated at room
temperature for 1-2
h. The amount of HRP-conjugated cPAM4 bound to the CaPanl Ag was revealed by
reading
the absorbance at 490 nm after the addition of a substrate solution containing
4 mM o-
phenylenediamine dihydrochloride and 0.04% H202. As shown by the competition
assays in
FIG. 12, hPAM4 and cPAM4 antibodies exhibited similar binding activities.
Example 4. Immunohistochemistry Staining Studies
103201 Immunohistochemistry on normal adult tissues showed that the PAM4
reactive
epitope was restricted to the gastrointestinal tract where staining was weak,
yet positive
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(Table 7). Normal pancreatic tissue, including ducts, ductules, acini, and
islet cells, were
negative for staining. A PAM4 based enzyme immunoassay with tissue homogenates
as
antigens generally supported the immunohistology data (Table 8). The PAM4
epitope was
absent from normal pancreas and other non-gastrointestinal tissues. In
neoplastic tissues,
PAM4 was reactive with twenty one out of twenty five (85%) pancreatic cancers
(Table 9
and Table 10) and ten out of twenty six colon cancers, but only limited
reactivity with tumors
of the stomach, lung, breast, ovary, prostate, liver or kidney (Table 10).
PAM4 reactivity
appeared to correlate with the stage of tumor differentiation, with a greater
percentage of
staining observed in well differentiated pancreatic cancers than in moderately
differentiated
or poorly differentiated tumors. Generally, poorly differentiated tumors
represent less than
10% of all pancreatic cancers.
103211 These studies have shown the PAM4 reactivity and tissue distribution
(both normal
and cancer) to be unlike that reported for the CA19.9, DUPAN2, SPAN1, Nd2 and
B72.3
antibodies and antibodies against the Lewis antigens. Together with
crossblocking studies
performed with certain of these MAbs, the data suggests that the PAM4 MAb
recognizes a
unique and novel epitope. When compared to the antigens recognized by the
CA19.9,
DLIPAN2, and anti-Lea antibodies, the PAM4 antigen appears to be more
restricted in its
tissue distribution and is reactive with a higher percentage of pancreatic
tumors. Moreover, it
gives a greater overall intensity of reaction at equivalent concentrations and
is reactive with a
higher percentage of cells within the pancreatic tumors. Finally, PAM4 was
found to be only
weakly reactive with three out of twelve chronic pancreatitis specimens,
whereas CA19.9 and
DIRAN2 were strongly reactive with all twelve specimens. Although it is
recognized that
specificity is dependent in part upon the type of assay employed and the range
and number of
tissues examined, the ability of PAM4 to discriminate between normal and
neoplastic
pancreatic tissue, its ability to react with a large percentage of the cancer
specimens, the high
intensity of the reactions, and the ability to distinguish between early stage
pancreatic cancer
and benign conditions such as pancreatitis are important characteristics of
this exemplary
anti-pancreatic cancer antibody.
Table 7. Immunoperoxidase Staining of N ormal Adult Tissues with MAb PAM4
Tissue Staining
Reaction
Pancreas (22)a
Ducts
96
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Acini
Islets
Submaxillary gland (2)
Esophagus (2)
Stomach (3) + mucus secreting cells
Duodenum (3) + goblet cells
Jejunum (3) + goblet cells
Ileum (3) + goblet cells
Colon (5) + Goblet cells
Liver (3)
Gallbladder (2)
Bronchus (3)
Lung (3)
Heart (3)
Spleen (3)
Kidney (3)
Bladder (3)
Prostate (2)
Testes (2)
-Uterus (2)
Ovary (2)
a
¨ number of individual specimens examined in parentheses
Table 8. Monoclonal Antibody PAM4 Reactivity with Normal Adult Tissue
Homogenates by E'AA
Tissue gig tissue
Pancreas 6.4
Esophagus 8.1
Stomach 61.3
Duodenum 447
Jejunum 60.6
Colon 74.5
Liver 00
97
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Gallbladder 5.6
Heart 3.7
Spleen 3,4
Kidney 6.6
Bladder 4.9
Thyroid 3.5
Adrenal 1.3
Ureter 2.6
Testes 3.9
CaPanl Pancreatic Tumor 569
a ¨ values are mean from two autopsy specimens
Table 9. Immunobistochemical Reactivity of Several Monoclonal Antibodies with
Pancreatic Tumors
Differentiation PAM4 CA19.9 Lea DUPAN2
1 W +++ - - E I 1
2 M ++ +++ +++ +
3 M + - -I- +
4 M +++ ++4- -H -- f +
M-1-H- + -
-
6 M + ND Ni) ND
! M* ++4- ++4- +++ +++
8 NI 4- -
9 NI F f + -H- -
1 0 Ma 4-+ ++ 4-4- +++
ii M ++ +-H-- -i -- I -- 1- +
12 NI ++ + + +++
13 NI + +++ +++ +
14 Ni ++ + + +4-
NI -F-H- 4- + ++
16 NI + + -H- -
17 M - + -I- -
18 M ++ -1-H--1-+ ++
98
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
19 M 4--H- 4- -H--F -1-4-
21 M. -F++
22
23
24
TOTAL 21/25 17/24 18/24 16/24
: Negative; : 5-20% of tissue is stained; ++ : 21-50% of tissue is stained;
-H-+: >50% of tissue is stained; W,M,P : Well, moderate, or poor
differentiation;
: Metastatic tissue; ND : Not Done
Table 10. immunoperoxidase Staining of Neoplastic Tissues with MAb PAM4
Cancer Tissue Positiveaotal
Pancreas 21/25
Colon 10/26
Stomach 1/5
Lung 1/15
Breast 0/30
Ovarian 0/10
Prostate 0/4
Liver 0/10
Kidney 0/4
Example 5. In Vivo Biodistribution and Tumor Targeting of Radiolabeled PAM4
[0322j Initial biodistribution studies of PAM4 were carried out in a series of
four different
xenografted human pancreatic tumors covering the range of expected
differentiation. Each of
the four tumor lines employed, A.sPc1., BxPc3, Hs766T and CaPanl, exhibited
concentrations
of 131I-PAM4 within the tumors (range: 21%-48% ID/g on day three) that were
significantly
(P<0.01-0.001) higher than concomitantly administered nonspecific, isotype-
matched Ag8
antibody (range: 3.6%-9.3% ID/g on day three). The biodistribution data were
used to
estimate potential radiation doses to the tumor of 12,230; 10,684; 6,835; and
15,843 eGy/mCi
of injected dose to AsPcl, BxPc3, 11s766T and CaPanl, respectively. With an
actual
99
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
maximum tolerated dose (MTD) of 0.7 mCi, PAM4 could provide substantial rad
dose to
each of the xenografted tumor models. In each tumor line the blood levels of
radiolabeled
PAM4 were significantly (P<0.01-0.001) lower than the nonspecific Ag8.
Potential radiation
doses to the blood from PAM4 were 1.4-4.4 fold lower than from Ag8. When
radiation doses
to the tumor from PAM4 were normalized to the blood doses from PAM4, the
tumors
received doses that were 2.2; 3.3; 3.4; and 13.1-fold higher than blood,
respectively.
Importantly, potential radiation doses to non-tumor tissues were minimal.
103231 The biodistribution of PAM4 was compared with an anti-CEA antibody, MN-
14,
using the CaPanl tumor model. The concentration of PAM4 within the tumor was
much
greater than MN-14 at early timepoints, yielding tumor:blood ratios at day
three of 12.7 2.3
for PAM4 compared to 2.7 1.9 for MN-14. Although PAM4 uptake within the
tumor was
significantly higher than for MN-14 at early timepoints (day one--P<0.001; day
three--
P<0.01), dosimetry analyses indicated only a 3.2-fold higher dose to the tumor
from PAM4
as compared to MN-14 over the fourteen day study period. This was due to a
rapid clearance
of PAM4 from the tumor, such that at later timepoints similar concentrations
of the two
antibodies were present within the tumors. A rapid clearance of PAM4 from the
tumor was
also noted in the BxPc3 and Hs766T but not AsPc1 tumor models. These
observations were
unlike those reported for other anti-muein antibodies, as for example Ci9 and
B72.3 in
colorectal cancer, where each exhibited longer retention times as compared to
the MN-14
antibody. Results from studies on the metabolism of PAM4, indicate that after
initial binding
to the tumor cell, antibody is rapidly released, possibly being catabolized or
being shed as an
antigen:antibody complex. The blood clearance is also very rapid. These data
suggest that 13IT
may not be the appropriate choice of isotope for therapeutic applications. A
short-lived
isotope, such as 9 Y or I88Re, which can be administered frequently may be a
more effective
reagent.
103241 PAM4 showed no evidence of targeting to normal tissues, except in the
CaPan I tumor
model, where a small but statistically significant splenic uptake was observed
(range 3.1-
7.5% ID/g on day-3). This type of splenic targeting has been observed in the
clinical
application of the anti-muein antibodies B72.3 and CC49. Importantly, these
studies also
reported that splenic targeting did not affect tumor uptake of antibody nor
did it interfere with
interpretation of the nuclear scans. These studies suggested that splenic
targeting was not due
to crossreactive antigens in the spleen, nor to binding by Fe receptors, but
rather to one or
more of the following possibilities: direct targeting of antigen trapped in
the spleen, or
100
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
indirect uptake of antigen:antibody complexes formed either in the blood or
released from the
tumor site. The latter would require the presence of immune complexes in the
blood.
However, these were not observed when specimens as early as five minutes and
as late as
seven days were examined by gel filtration (IIPLC, GF-250 column);
radiolabeled antibody
eluted as native material. The former explanation seems more likely in view of
the fact that
the CaPanl tumor produced large quantities of PAM4reactive antigen, 100- to
1000-fold
higher than for the other tumor cell lines examined. The lack of splenic
targeting by PAM4 in
these other tumor lines suggests that this phenomenon was related to excessive
antigen
production. Splenic targeting can be overcome by increasing the protein dose
to 10 lig from
the original 2 pg dose. A greater amount of the splenic entrapped antigen
presumably was
complexed with unlabeled PAM4 rather than radiolabeled antibody. Increasing
the protein
dose had no adverse effect upon targeting of PAM4 to the tumor or nontumor
tissues. In fact,
an increase of the protein dose to 100 pg more than doubled the concentration
of radiolabeled
PAM4 within the CaPanl tumor.
Example 6. Development of Orthotopic Pancreatic Tumor Model in Athymic
Nude Mice.
103251 In order to resemble the clinical presentation of pancreatic cancer in
an animal model
more closely, we developed an orthotopic model by injecting tumor cells
directly into the
head of the pancreas. Orthotopic CaPanl tumors grew progressively without
overt symptoms
until the development of ascites and death at ten to fourteen weeks. By three
to four weeks
post-implantation, animals developed a palpable tumor of approximately 0.2 g.
Within eight
weeks of growth, primary tumors of approximately 1.2 g along with metastases
to the liver
and spleen were observed (1-3 metastatic tumors/animal; each tumor <0.1 g). At
ten to
fourteen weeks seeding of the diaphragm with development of ascites were
evident. Ascites
formation and occasional jaundice were usually the first overt indications of
tumor growth.
At this time tumors were quite large, 1 to 2 g, and animals had at most only
three to four
weeks until death occurred.
103261 Radiolabeled 1311-PAM4, administered to animals bearing four week old
orthotopic
tumors (approximately 0.2 g) showed specific targeting to the primary tumor
with
localization indices of 7.9 I 3.0 at day one increasing to 22.8 15.3 at day
fourteen. No
evidence of specific targeting to other tissues was noted. In one case where
tumor metastases
to the liver and spleen were observed, both metastases were targeted, and had
high
concentrations of radiolabeled antibody. In addition, approximately half of
the animals
101
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
developed a subcutaneous tumor at the incision site. No significant
differences were noted in
the targeting of orthotopic and subcutaneous tumors within the same animal,
and no
significant differences were observed in the targeting of orthotopic tumor
whether or not the
animal had an additional subcutaneous tumor. The estimated radiation doses
from PAM4
were 6,704 and 1,655 eGy/mCi to the primary tumor and blood, respectively.
Example 7. Radioimmunotherapy of Pancreatic Cancer
103271 The initial studies on the use of 131I-PAM4 for therapy were carried
out with the
CaPanl tumor, which was grown as a subcutaneous xenograft in athymic mice.
Animals
bearing a 0.25 g tumor were administered 350 !Xi, 131I-PAM4 in an experiment
that also
compared the therapeutic effects of a similar dose of nonspecific Ag8. The MID
for
administration of 131I-PAM4 to animals bearing 1 cm3 tumors is 700 p.Ci. By
weeks five and
six, the PAM4 treated animals showed a dramatic regression of tumor, and even
at week
twenty seven, five out of eight remained tumor free. The untreated, as well as
Ag8-treated
animals, showed rapid progression of tumor growth although a significant
difference was
noted between these two control groups. At seven weeks, tumors from the
untreated group
had grown 20.0 14.6-fold from the initial timepoint whereas the 131I-Ag8-
treated tumors
had grown only 4.9 1.8-fold. At this time point, the PAM4 tumors had
regressed to 0.1
0.1-fold of their original size, a significant difference from both untreated
(P<0.001) and
nonspecific Ag8-treated (P<0.01) animals.
103281 These data show that CaPanl tumors were sensitive to treatment with
131I-PAM4. The
outcome, that is, regression or progression of the tumor, was dependent upon
several factors
including initial tumor size. Thus, groups of animals bearing CaPanl tumor
burdens of 0.25
g, 0.5 g, 1.0 g, or 2.0 g were treated with a single dose of the 350 1.1.Ci
1311-PAM4. The
majority of animals having tumors of initial size 0.25 g and 0.5 g (nine of
ten animals in each
group) showed tumor regression or growth inhibition for at least sixteen weeks
post
treatment. In the 1.0 g tumor group five out of seven showed no tumor growth
for the sixteen
week period and in the 2.0 g tumor group six out of nine showed no tumor
growth for a
period of six weeks before progression occurred. Although a single 350 !Xi
dose was not as
effective against larger tumors, a single dose may not be the appropriate
regimen for large
tumors.
103291 Toxicity studies indicate the ability to give multiple cycles of
radioimmunotherapy,
which may be more effective with a larger tumor burden. Animals bearing
CaParil tumors
averaging 1.0 g, were given either a single dose of 350 1./Ci 1311-PAM4, two
doses given at
102
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
times zero and four weeks or were left untreated. The untreated group had a
mean survival
time of 3.7 1.0 weeks (survival defined as time for tumor to reach 5 cm3).
Animals died as
early as three weeks, with no animal surviving past six weeks. A. single dose
of 350 Ci 13II-
PAM4 produced a significant increase in the survival time to 18.8 4.2 weeks
(P<0.0001).
The range of animal deaths extended from weeks thirteen to twenty five. None
of the animals
were alive at the end of the study period of twenty six weeks.
103301 A. significant increase in survival time was observed for the two dose
group as
compared to the single dose group. Half of the animals were alive at the
twenty six week
timepoint with tumor sizes from 1.0-2.8 cm3, and a mean tumor growth rate of
1.6 0.7 fold
from initial tumor size. For those animals that were non-survivors at twenty
six weeks, the
mean survival time (17.7 5.3 weeks) was similar to the single dose group.
103311 Therapy studies with PAM4 were also conducted using the orthotopic
tumor model.
Groups of animals bearing four week old orthotopic tumors (estimated tumor
weight of 0.25
g) were either left untreated or treated with a single dose of either 350 !Xi
1311-PAM4 or 350
!Xi of "II-nonspecific Ag8. The untreated animals had a 50% death rate by week
ten with no
survivors at week fifteen. Animals administered nonspecific 131I-Ag8 at four
weeks of tumor
growth, showed a 50% death rate at week seven with no survivors at week
fourteen.
Although statistically (logrank analysis) there were no differences between
these two groups,
it is possible that radiation toxicity had occurred in the Ag8 treated
animals. Radiolabeled
PAM4 provided a significant survival advantage (P 0.001) as compared to the
untreated or
Ag8 treated animals, with 70% survival at sixteen weeks, the end of the
experiment. At this
time the surviving animals were sacrificed to determine tumor size. All
animals had tumor
with an average weight of 1.2 g, as well as one or two small (<0.1 g)
metastases evident in
four of the seven animals. At sixteen weeks of growth, these tumors were more
representative
of an eight-week-old tumor.
Example 8. Combined Modality GEMZAR Chemotherapy and 1311-PAM4
Experimental Radioimmunotherapy
103321 Initial studies into the combined use of gem.citabine (GEMZARO) with
1311-PAM4
radioimmunotherapy were performed as a checkerboard array; a single dose of
gemeitabine
(0, 100, 200, 500 mg/kg) versus a single dose of '311-PAM4 ([MTD-700 !Xi]
100%, 75%,
50%, 0% of the MTD). The combined MTD was found to be 500 mg/kg gemcitabine
with
350 pei 131I-PAM4 (50% M.'FD). Toxicity, as measured by loss of body weight,
went to the
maximum considered as nontoxic; that is 20% loss in body weight. Although the
combined
103
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
treatment protocol was significantly more effective than gemcitabine alone,
the treatment was
no more effective than radioinununotherapy alone. The next studies were
performed at a low
dose of gemcitabine and radioimrnunotherapy to examine if a true synergistic
therapeutic
effect would be observed. Athymic nude mice bearing tumors of approximately 1
cm3
(approximately 5% of body weight) were administered gemcitabine, 100 mg/kg on
days zero,
three, six, nine, and twelve, with 100 1iCi of 311-PAM4 given on day zero. A
therapeutic
effect was observed with statistically significant (P<0.0001) regression (two
of five tumors
less than 0.1 cm3) and/or growth inhibition of the tumors compared to
gemcitabine alone.
Thus, at lower dosages of therapeutic agent, there surprisingly appears to be
a synergistic
effect of the combination of gemcitabine and radioimmunotherapy. Of additional
note, in
terms of body weight, toxicity was not observed. The combination treatment
protocol can, if
necessary, be delivered in multiple cycles, with the second treatment cycle
beginning in
week-four, as was done with the radioimmunotherapy-alone studies described
above.
Example 9. Effects of Reagent Treatment on I mmunoreactivity of PAM4
Antigen
103331 Treatment of pancreatic mucin with DTT (15 min at room temp),
completely
abolished reactivity with PAM4 (DTT-EC50, 0.60 + 0.00 tiM). The only cysteines
(cystine
bridges) within MUC-1 are present within the transmembrane domain and should
not be
accessible to DTT. The secreted form of MUC-1 does not contain the
transmembrane domain
and therefore has no intrainolecular cystine bridges. Data from periodate
oxidation treatment
of pancreatic cancer mucin with 0.05 M sodium periodate for 2 his at room
temperature
yielded 40% loss of immunoreactivity with PAM4 antibody (not shown). Further
periodate
studies have shown as high as a 60% loss of immunoreactivity with PAM4
antibody (not
shown). The results of periodate and DTT studies suggest that the PAM4 epitope
is
conformationally dependent upon some minimal form of glycosylation, and may be
affected
by intermolecular disulfide bond formation.
Example 10. Distribution and Cross-Reactivity of the PAM4 Antigen
103341 The expression of the PAM4-epitope within Patiffis is atypical for MUC-
1. It is
similar to the expression reported for MUC5AC as detected by the commercially
available
MAb-CLH2-2. However, an attempted sandwich immunoassay with PAM4 capture and
MAb-CLH2-2 as probe gave negative results. Although this possibly suggests the
PAM4 and
CLI12-2 epitopes may overlap and thus block each other, the CLF12-2 was
reported to be
104
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
reactive with 42/66 (64%) gastric carcinomas whereas the PAM4 MAb showed
reactivity
with only 6/40 (15%) of gastric carcinomas and, of these, only in focal
reactivity.
103351 Use of the commercially available 45M1, an anti-MUC5A.0 MM,, as a probe
reagent
in EIA (with PAM4 as capture) provided positive results, indicating that the
two epitopes
may be present on the same antigenic molecule. Blocking studies (either
direction) indicated
that the epitopes bound by 45M1 and PAM4 are in fact two distinct epitopes, as
no blocking
was observed. Labeling of tissue microarrays consisting of cores from.
invasive pancreatic
carcinoma has demonstrated significant differences for expression of the 45M1
and PAM4
epitopes in individual patient specimens. Of 28 specimens, concordance was
observed in only
17 cases (61%). PAM4 was reactive with 24/28 cases (86%) while 45M] was
reactive with
13/28 (46%) cases (not shown).
103361 The results of periodate studies are consistent with glycosylation as a
factor in
MUC5AC inmmnoreactivity with the PAM4 antibody. Thus, results of studies with
apomucins may not be definitive for antigen determination.
103371 Although based on EIA capture, the PAM4 antibody appears to bind to the
same
antigenic protein as the 45M1 anti-MUC5AC MAb, it is noted that MUC5AC is not
specific
to pancreas cancer and it is found in a number of normal tissues (other than
the gastric
mucosa with which PAM4 is reactive). For example, MUC5AC is found in normal
lung,
colon and other tissues. PAM4 antibody does not bind to normal lung tissues,
except as
indicated above in few samples and to a limited or minimal amount.
103381 With respect to the effects of DTI" and periodate, it is probable that
the peptide core
disulfide bridges are identical no matter what tissue produces the protein. A
specific amino
acid sequence should fold in a specific manner, independent of the tissue
source. However,
glycosylation patterns may differ dependent upon tissue source.
Example 11. Phage Display Peptide Binding of PAM4 Antibody
103391 PAM4 antibody binding was examined with two different phage display
peptide
libraries. The first was a linear peptide library consisting of 12 amino acid
sequences and the
second was a cyclic peptide consisting of 7 amino acid sequences cyclized by a
disulfide
bridge. We panned the individual libraries alternately against hPAM4 and hLL2
(negative
selection with anti-CD22 antibody) for a combined total of 4 rounds, and then
screened the
phage displayed peptide for reactivity with both hPAM4 and mPAM4 with little
to no
reactivity against hLL2. Phage binding in a non-specific manner (i.e., binding
to epratuzumab
[hLL2]) were discarded.
105
CA 02948013 2016-11-03
WO 2016/003869 PCT/US2015/038252
103401 For the linear phage-displayed peptide, the sequence WTWNITKAYPLP (SEQ
ID
NO:7) was identified 30 times (in 35 sequenced phage), each of which were
shown to have
reactivity with PA.M.4 antibodies. A. mutational analysis was conducted in
which a library
based on this sequence and having 7.5% degeneracy at each position, was
constructed,
panned and screened as before. Variability was noted in the 19 obtained
peptide sequences
that were positive for PAM4 binding with 7 being identical to the parental
sequence, 5 having
the sequence WTWNITKEYPQP (SEQ ID NO:65) and the rest being uniquely present.
Table 11 shows the results of this mutational analysis. The upper row lists
the sequences
identified and the lower row lists the frequency with which each of the amino
acids was
identified in that position. The parent sequence is most frequent (bold) with
the next highest
variation a substitution of E for A at position 8 and a substitution of Q for
L at position 11. It
does not appear that these substitutions had any great effect upon
immunoreactivity.
Table 11. Phage Display Amino Acid Sequence (SEQ ID NO: 116) Variation with
Linear Peptide Binding to PAM4 Antibody
W I W N I T K A Y P
R R E T R Q
N R.
G F
number of 19 H H H 9 17 14 10 18 17 1 1 19
occurrences 1 2 1 5 1 2
(out of 19 2 1 1
sequences 1 1 1
analyzed) 1 1 1
1
[03411 Results with the phage displayed cyclic library were significantly
different from the
linear library (Table 12). The sequence ACPEWWGTTC (SEQ ID NO:66) was present
in 33
of 35 peptide sequences examined. Analysis of the cyclic library presented the
following
results (positions with an asterisk were invariant and not subject to
selective pressure in the
library).
106
CA 02948013 2016-11-03
WO 2016/003869 PCT/US2015/038252
Table 12. Phage Display Amino Acid Sequence (SEQ ID NO: 117) Variation with
Linear Peptide Binding to PAM4 Antibody
A
W A.
0
number of * 33 35 35 35 34 29 28
occurrences 2 1 5 4
(out of 19 1 1
sequences 1
analyzed) 1
103421 The two cysteines (at positions 2 and 10) formed a disulfide bridge.
Substitution of T
at position 9 with any amino acid greatly affected immunoreactivity. The
sequence
GITGITC (SEQ. ID NO:67) is present within the MUC5AC protein towards the amino
terminus as compared to the cyclic peptide sequence shown above, which shows
homology at
the C-terminal end of the consensus peptide sequence. However, the cyclic
peptide only
showed approximately 10% of the immunoreactivity of the linear sequence with
the PAM4
antibody. Both linear and cyclic consensus sequences are associated with a
cysteine, which
may or may not relate to the effect of DTT on IVIUC5AC immunoreactivity.
103431 The results reported herein indicate that the PAM4 epitope is dependent
upon a
specific conformation which may be produced by disulfide bridges, as well as a
specific
glycosylation pattern.
Example 12. linmunohistology of Pancreatic Cancer in a Pancreatitis Specimen
103441 Several pathologic conditions predispose patients to the development of
pancreatic
carcinoma, such as pancreatitis, diabetes, smoking and others. Within this pre-
selected group
of patients, screening measures are particularly important for the early
detection of pancreatic
neoplasia. We examined 9 specimens of chronic pancreatitis tissue from
patients having
primary diagnosis of this disease. We employed an anti-CD74 MAb, LLI, as an
indicator of
inflammatory infiltrate, and MAb-MA5 as a positive control for pancreatic
ductal and acinar
cells. Whereas the two control IvlAbs provided immunohistologic evidence
consistent with
pancreatitis, in no instance did PAM4 react with inflamed pancreatic tissue.
However, in one
case, a moderately differentiated pancreatic adenocarcinoma was also present
within the
107
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
tissue specimen. PAM4 gave an intense stain of the neoplastic cells within
this tumor. In a
second case, while the inflamed tissue was negative with PAM4, a small PanIN
precursor
lesion was identified that was labeled with PAM4. Labeling of the PanIN within
this latter
specimen is consistent with early detection of pancreatic neoplasia in a
patient diagnosed
with a non-malignant disease. These results show that detection and/or
diagnosis using the
PAM4 antibody may be performed with high sensitivity and selectivity for
pancreatic
neoplasia against a background of benign pancreatic tissues.
Example 13. Therapy of a Patient With Inoperable and Metastatic Pancreatic
Carcinoma
103451 Patient 118-001, CWG, is a 63-year-old man with Stage-IV pancreatic
adenocarcinoma with multiple liver metastases, diagnosed in November of 2007.
He agreed
to undertake combined radioimmunotherapy and gemcitabine chemotherapy as a
first
treatment strategy, and was then given a first therapy cycle of 6.5 mCi/m2
of90Y-hPAM4,
combined with 200 mg/m2 gemcitabine, whereby the gemcitabine was given once
weekly on
weeks 1-4 and 90Y-hPAM4 was given once-weekly on weeks 2-4 (3 doses). Two
months
later, the same therapy cycle was repeated, because no major toxicities were
noted after the
first cycle. Already 4 weeks after the first therapy cycle, CT evidence of a
reduction in the
diameters of the primary tumor and 2 of the 3 liver metastases surprisingly
was noted, and
this was consistent with significant decreases in the SUV values of FDG-PET
scans, with 3 of
the 4 tumors returning to normal background SUV levels at this time (FIG. 13
and FIG. 14).
The patient's pre-therapy CA-19.9 level of 1,297 dropped to a low level of 77,
further
supportive of the therapy being effective. Table 13 shows the effects of
combined
radioimmunotherapy with 90Y-hPAM4 and gemcitabine chemotherapy in this
patient. It was
surprising and unexpected that such low doses of the radionuclide conjugated
to the antibody
combined with such low, nontoxic, doses of gemcitabine showed such antitumor
activity
even after only a single course of this therapy.
Table 13. Effects of Combined Radioimmunotherapy with 9Y-hIPAM4 and
Gerncitabine
Chemotherapy in Metastatic Pancreatic Carcinoma
Tumor Location Baseline 4 wk post-Tx Baseline PET 4 wk post-Tx
Longest Longest (SUV) PET (SUV)
Diameter (cm) Diameter (cm)
Pancreatic tail 4.5 4.3 9.2 4.2
(primary)
L hepatic met 1.9 1.9 4.1 Background
108
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
R post hepatic 1.7 1.6 3.7 Background
met
R central hepatic 1.9 1.2 3.2 Background
met
Example 14. Therapy of a Patient with Inoperable Metastatic Pancreatic
Carcinoma
103461 A 56-year-old male with extensive, inoperable adenocarcinoma of the
pancreas, with
several liver metastases ranging from 1 to 4 cm in diameter, substantial
weight loss (30 lbs of
weight or more), mild jaundice, lethargy and weakness, as well as abdominal
pains requiring
medication, is given 4 weekly infusions of gemeitabine at doses of 200
mg/m2each. On the
last three gemcitabine infusions, 9 Y-DOTA-hPAM4 radiolabeled humanized
antibody is
administered at a dose of 10 mCi/m2 of 90Y and 20 mg antibody protein, in a
two-hour i.v.
infusion. Two weeks later, the patient is given a course of gemcitabine
chemotherapy
consisting of 3 weekly doses of 600 mg/m2 by i.v. infusion. The patient is
then evaluated 4
weeks later, and has a mild leukopenia (grade-2), no other major blood or
enzyme changes
over baseline, but shows an improvement in the blood CA19.9 titer from 5,700
to 1,200 and a
decrease in jaundice, with an overall subjective improvement. This follows 3
weeks later with
a repeat of the cycle of lower-dose gemeitabine (weekly x 4), with 3 doses of
90Y-DOTA-
liPAM4. Four weeks later, the patient is reevaluated, and the CT and PET scans
confirm an
approximately 40% reduction of total tumor mass (primary cancer and
metastases), with a
further reduction of the CA19.9 titer to 870. The patient regains appetite and
activity, and is
able to return to more usual daily activities without the need for pain
medication. He gains 12
lbs after beginning this experimental therapy. A repeat of the scans and blood
values
indicates that this response is maintained 6 weeks later.
Example 15. Preparation of DNUm Constructs for Pretargeting
DDD and AD Fusion Proteins
103471 The DNI.:114 technique can be used to make dimers, trimers, tetram.ers,
hexamers, etc.
comprising virtually any antibodies or fragments thereof or other effector
moieties. For
certain preferred embodiments, IgG antibodies or Fab antibody fragments may be
produced
as fusion proteins containing either a dimerization and docking domain (DDD)
or anchoring
domain (AD) sequence. Although in preferred embodiments the DDD and AD
moieties are
produced as fusion proteins, the skilled artisan will realize that other
methods of conjugation,
109
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
such as chemical cross-linking or click chemistry, may be utilized within the
scope of the
claimed methods and compositions.
103481 Bispecific antibodies may be formed by combining a Fab-DDD fusion
protein of a
first antibody with a Fab-AD fusion protein of a second antibody.
Alternatively, constructs
may be made that combine IgG-AD fusion proteins with Fab-DDD fusion proteins.
The
technique is not limiting and any protein or peptide of use may be produced as
an AD or
DDD fusion protein for incorporation into a DNI:Im construct. Where chemical
cross-linking
is utilized, the AD and DDD conjugates are not limited to proteins or peptides
and may
comprise any molecule that may be cross-linked to an AD or DDD sequence using
any cross-
linking technique known in the art. In certain exemplary embodiments, a
polyethylene glycol
(PEG) or other polymeric moiety may be incorporated into a DNLTM construct, as
described
in further detail below.
103491 For pretargeting applications, an antibody or fragment containing a
binding site for an
antigen associated with a diseased tissue, such as a tumor-associated antigen
(TAA), may be
combined with a second antibody or fragment that binds a hapten on a
targetable construct, to
which a therapeutic and/or diagnostic agent is attached. The DNUm-based
bispecific
antibody is administered to a subject, circulating antibody is allowed to
clear from the blood
and localize to target tissue, and the conjugated targetable construct is
added and binds to the
localized antibody for diagnosis or therapy.
103501 Independent transgenic cell lines may be developed for each Fab or IgG
fusion
protein. Once produced, the modules can be purified if desired or maintained
in the cell
culture supernatant fluid. Following production, any DDD-fusion protein module
can be
combined with any AD-fusion protein module to generate a bispecific DNLTM
construct. For
different types of constructs, different Al) or DDD sequences may be utilized.
Exemplary
DDD and AD sequences are provided below.
DDD1: SHIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFTRLREARA (SEQ ID
NO:68)
DDD2: CGHIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFIRLREARA (SEQ ID
NO:69)
AD!: QIEYLAKQIVDNAIQQA (SEQ ID NO:70)
AD2: CGQIEYLAKQIVDNAIQQAGC (SEQ ID NO:71)
103511 The skilled artisan will realize that DDD1 and DDD2 comprise the DDD
sequence of
the human RlIa form of protein kinase A. However, in alternative embodiments,
the DDD
110
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
and AD moieties may be based on the DDD sequence of the human Ria form of
protein
kinase A and a corresponding AKAP sequence, as exemplified in DDD3, DDD3C and
AD3
below.
DDD3
SLRECELYVQKHNIQALLKDSIVQLCTARPERPMAFLREYFERLEKEEAK (SEQ ID
NO:72)
DDD3C
MSCGGSLRECELYVQKHNTQALLKDSIVQLCTARPERPMAKREYFERLEKEEAK
(SEQ ID NO:73)
AD3
CGFEELAWKIAKM.IWSDVFQQGC (SEQ ID NO:74)
Expression Vectors
103521 The plasmid vector pdHL2 has been used to produce a number of
antibodies and
antibody-based constructs. See Gillies etal., J Immunol Methods (1989),
125:191-202;
Losman et al., Cancer (Phila.) (1997), 80:2660-6. The di-cistronic mammalian
expression
vector directs the synthesis of the heavy and light chains of IgG. The vector
sequences are
mostly identical for many different IgG-pdHL2 constructs, with the only
differences existing
in the variable domain (VH and VI) sequences. Using molecular biology tools
known to those
skilled in the art, these IgG expression vectors can be converted into Fab-DDD
or Fab-AD
expression vectors. To generate Fab-DDD expression vectors, the coding
sequences for the
hinge, CH2 and CH3 domains of the heavy chain are replaced with a sequence
encoding the
first 4 residues of the hinge, a 14 residue Gly-Ser linker and the first 44
residues of human
RlIa. (referred to as DDD I). To generate Fab-AD expression vectors, the
sequences for the
hinge, CH2 and CH3 domains of IgG are replaced with a sequence encoding the
first 4
residues of the hinge, a 15 residue Gly-Ser linker and a 17 residue synthetic
AD called
AKAP-IS (referred to as Al) 1), which was generated using bioinformatics and
peptide array
technology and shown to bind REV dimers with a very high affinity (0.4 nM).
See Alto, et al.
Proc. Natl. Acad. Sci., U.S.A (2003), 100:4445-50.
103531 Two shuttle vectors were designed to facilitate the conversion of IgG-
pd1IL2 vectors
to either Fab-DDD1 or Fab-AD1 expression vectors, as described below.
Preparation of C111
103541 The CII1 domain was amplified by PCR using the pdHL2 plasmid vector as
a
template. The left PCR primer consisted of the upstream (5') end of the C111
domain and a
111
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Sad! restriction endonuclease site, which is 5' of the CFI I coding sequence.
The right primer
consisted of the sequence coding for the first 4 residues of the hinge (PKSC
(SEQ ID NO:
118)) followed by four glycines and a serine, with the final two codons (GS)
comprising a
Barn HI restriction site. The 410 bp PCR amplimer was cloned into the PGEMT
PCR
cloning vector (PROMEGA , Inc.) and clones were screened for inserts in the T7
(5')
orientation.
103551 A duplex oligonucleotide, designated (G4S)2DDD1 C(G4S)2' disclosed as
SEQ ID NO:
119), was synthesized by Sigma GENOSYS (Haverhill, UK) to code for the amino
acid
sequence of DDD1 preceded by 11 residues of the linker peptide, with the first
two codons
comprising a Bam.HI restriction site. A stop codon and an EagI restriction
site are appended
to the 3'end. The encoded polypeptide sequence is shown below.
GSGGGGSGGGGSHIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFTRLREARA
(SEQ ID NO:75)
103561 Two oligonucleotides, designated RIIA1-44 top and RIIA1-44 bottom,
which overlap
by 30 base pairs on their 3' ends, were synthesized and combined to comprise
the central 154
base pairs of the 174 bp DDD1 sequence. The oligonucleotides were annealed and
subjected
to a primer extension reaction with Taq polymerase. Following primer
extension, the duplex
was amplified by PCR. The amplimer was cloned into PGEMT ' and screened for
inserts in
the T7 (5') orientation.
103571 A duplex oligonucleotide was synthesized to code for the amino acid
sequence of
Al)! preceded by 11 residues of the linker peptide with the first two codons
comprising a
BamIII restriction site. A stop codon and an EagI restriction site are
appended to the 3'end.
The encoded polypeptide sequence is shown below.
GSGGGGSGGGGSQIEYLAKQIVDNAIQQA (SEQ ID NO:76)
103581 Two complimentary overlapping oligonucleotides encoding the above
peptide
sequence, designated AKAP-IS Top and AKAP-IS Bottom, were synthesized and
annealed.
The duplex was amplified by PCR. The amplimer was cloned into the PGEMT
vector and
screened for inserts in the T7 (5') orientation.
Ligating DDDI with CHI
103591 A 190 bp fragment encoding the DDD1 sequence was excised from PGEMT
with
BamHI and Notl restriction enzymes and then ligated into the same sites in CH!-
PGEMT
to generate the shuttle vector CII1-DDD1-PGEMT .
Ligating ADI with CHI
112
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
103601 A 110 bp fragment containing the AD1 sequence was excised from PGEMTCR)
with
BamHI and Notl and then ligated into the same sites in CH1-PGEMT63) to
generate the
shuttle vector CHI-ADI-PGEMT .
Cloning CHI -DDD I or CHI -A.DI into pdHL2-based vectors
103611 With this modular design either CH I-DDDI or CII1-AD1 can be
incorporated into
any IgG construct in the pdHL2 vector. The entire heavy chain constant domain
is replaced
with one of the above constructs by removing the Sac11/Eagl restriction
fragment (CHI -CH3)
from pdHL2 and replacing it with the SacII/EagI fragment of CII1-DDD I or CII1-
AD1,
which is excised from the respective pGemT shuttle vector.
Consuliction of h679-F'd-ADI-pdHL2
103621 11679-Fd-AD1-pdI1L2 is an expression vector for production of h679 Fab
with AD!
coupled to the carboxyl terminal end of the CHI domain of the Fd via a
flexible Gly/Ser
peptide spacer composed of 14 amino acid residues. A pdHL2-based vector
containing the
variable domains of h679 was converted to h679-Fd-AI)I-pdHL2 by replacement of
the
SacInagl: fragment with the CII1-AD1 fragment, which was excised from the CII1-
AD1-
SV3 shuttle vector with Sac!! and Eagl.
Construction of C-DDDI-Fd-h110-14-pdf11,2
103631 C-DDDl-Fd-hMN-14-pdfiL2 is an expression vector for production of a
stable dimer
that comprises two copies of a fusion protein C-DDD1-Fab-hMN-14, in which DDD1
is
linked to hMN-14 Fab at the carboxyl terminus of CHI via a flexible peptide
spacer. The
plasmid vector hMN-14(1)-pdHL2, which has been used to produce hMN-I4 IgG, was
converted to C-DDD1-Fd-hMN-14-pdIIL2 by digestion with Sac!! and EagI
restriction
endonucleases to remove the CH1-CH3 domains and insertion of the CH1-DDD1
fragment,
which was excised from the CHI -DDD1-SV3 shuttle vector with Sac!! and Eagl.
103641 The same technique has been utilized to produce plasmids for Fab
expression of a
wide variety of known antibodies, such as hLL1, hLL2, hPAM4, hRl, hRS7, hMN-
14, hMN-
15, hA19, hA20 and many others. Generally, the antibody variable region coding
sequences
were present in a pdHL2 expression vector and the expression vector was
converted for
production of an AD- or DDD-fusion protein as described above. The AD- and DDD-
fusion
proteins comprising a Fab fragment of any of such antibodies may be combined,
in an
approximate ratio of two DDD-fusion proteins per one AD-fusion protein, to
generate a
trimeric DNUrm construct comprising two Fab fragments of a first antibody and
one Fab
fragment of a second antibody.
113
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
C-DDD2-Fd-hAIN-14-pdHL2
103651 C-DDD2-Fd-hMN-14-pdHL2 is an expression vector for production of C-DDD2-
Fab-
hMN-14, which possesses a dimerization and docking domain sequence of DDD2
appended
to the carboxyl terminus of the Fd of hMN-1 4 via a 14 amino acid residue
Cily/Ser peptide
linker. The fusion protein secreted is composed of two identical copies of hMN-
14 Fab held
together by non-covalent interaction of the DDD2 domains.
103661 The expression vector was engineered as follows. Two overlapping,
complimentary
oligonucleotides, which comprise the coding sequence for part of the linker
peptide and
residues 1-13 of DDD2, were made synthetically. The oligonucleotides were
annealed and
phosphorylated with14 PNK, resulting in overhangs on the 5' and 3' ends that
are compatible
for ligation with DNA digested with the restriction endonucleases BamIll and
PstI,
respectively.
103671 The duplex DNA was ligated with the shuttle vector CH1-DDDI-PGEMTCR.),
which
was prepared by digestion with BamHI and Pstl, to generate the shuttle vector
CHI-DDD2-
PGEMTO. A 507 bp fragment was excised from ai1-DDD2-PGEMT with Sac!! and EagI
and ligated with the 1gG expression vector hMN-14(I)-pdHL2, which was prepared
by
digestion with Sac!! and EagI. The final expression construct was designated C-
DDD2-Fd-
hMN-14-pdHL2. Similar techniques have been utilized to generated DDD2-fusion
proteins of
the Fab fragments of a number of different humanized antibodies.
h679-1-7d-AD2-pd111,2
103681 h679-Fab-AD2, was designed to pair as B to C-DDD2-Fab-hMN-14 as A. h679-
Fd-
AD2-pd11L2 is an expression vector for the production of h679-Fab-AD2, which
possesses
an anchoring domain sequence of AD2 appended to the carboxyl terminal end of
the CHI
domain via a 14 amino acid residue Gly/Ser peptide linker. AD2 has one
cysteine residue
preceding and another one following the anchor domain sequence of AD1.
103691 The expression vector was engineered as follows. Two overlapping,
complimentary
oligonucleotides (AD2 Top and AD2 Bottom), which comprise the coding sequence
for AD2
and part of the linker sequence, were made synthetically. The oligonucleotides
were annealed
and phosphorylated with 14 PNK, resulting in overhangs on the 5' and 3' ends
that are
compatible for ligation with DNA digested with the restriction endonucleases
BamIII and
Spel, respectively.
103701 The duplex DNA was ligated into the shuttle vector CII1-AD1-PGEMTO,
which was
prepared by digestion with BamIII and SpeI, to generate the shuttle vector
CII1-AD2-
114
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
PGEMT . A 429 base pair fragment containing CII1 and AD2 coding sequences was
excised from the shuttle vector with SacII and Eagl restriction enzymes and
ligated into
h679-pdHL2 vector that prepared by digestion with those same enzymes. The
final
expression vector is h679-Fd-AD2-pdHL2.
Example 16. Production of AD- and DDD-linked Fab and IgG Fusion Proteins
From Multiple Antibodies
[0371] Using the techniques described in the preceding Example, the IgG and
Fab fusion
proteins shown in Table 14 were constructed and incorporated into DNLTm
constructs. The
fusion proteins retained the antigen-binding characteristics of the parent
antibodies and the
1)NLTM constructs exhibited the antigen-binding activities of the incorporated
antibodies or
antibody fragments.
Table 14. Fusion proteins comprising IgG or Fab
Fusion Protein Binding Specificity
C-AD1-Fab-h679 HSG
C-AD2-Fab-h679 IISCi
C-(AD)2-Fab-h679 11SG
C-AD2-Fab-h734 Indium-DTPA
C-AD2-Fab-hA20 CD20
C-AD2-Fab-hA2OL CD20
C-AD2-Fab-hL243 HLA-DR
C-AD2-Fab-hLL2 C1)22
N-AD2-Fab-hLL2 CD22
C-AD2-IgG-hMN-14 CEACAM5
C-AD2-IgG-hR1 IGF -1R
C-AD2-IgG-hRS7 EGP- I
C-AD2-IgG-hPAM4 MUC
C-AD2-IgG-hLL1 CD74
C-DDD1-Fab-hMN-14 CEACAM5
C-DDD2-Fab-hIVIN-14 CEACAM5
C-DDD2-Fab-h679 HSG
C-DDD2-Fab-hA19 CD19
C-DDD2-Fab-hA20 CD20
115
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
C-DDD2-Fab-hAFP AFP
C-DDD2-Fab-hL243 HLA-DR
C-DD1)2-Fab-h1.1 I CD74
C-DDD2-Fab-hLL2 CD22
C-DDD2-Fab-hMN-3 CEACAM6
C-DDD2-Fab- hMN- 15 CEACAM6
C-1)DD2-Fab-hPA.M4 MLIC
C-DDD2-Fab-hR1 IGF -1R
C-DDD2-Fab-hRS7 EGP-1
N-DDD2-Fab-hMN-14 CEACAM5
Example 17. Sequence variants for DNLTM
103721 In certain preferred embodiments, the AD and DDD sequences incorporated
into the
DNUm construct comprise the amino acid sequences of Al)!, AD2, AD3, 1)13131,
1)1)1)2,
DDD3 or DDD3C as discussed above. However, in alternative embodiments sequence
variants of AD and/or DDD moieties may be utilized in construction of the
DNLI'm
complexes. For example, there are only four variants of human PKA. DDD
sequences,
corresponding to the DDD moieties of PKA RIa, RIIa, RIP and RII13. The Ma DDD
sequence is the basis of DDD1 and DDD2 disclosed above. The four human PKA DDD
sequences are shown below. The DDD sequence represents residues 1-44 of RIIa,
1-44 of
R1113, 12-61 of Ma and 13-66 of RIP. (Note that the sequence of DDD I is
modified slightly
from the human PKA Rila DDD moiety.)
PKA RIcE
SLRECELYVQKHNIQALLKDVSIVQLCTARPERPMAFLREYFEKLEKEEAK (SEQ ID
NO:77)
PKA RIP
SLKGCELYVQLHGIQQVLKDCIVHLCISKPERPMKFLREHFEKLEKEENRQILA (SEQ
ID NO:78)
PKA RIIa
SHIQIPPGLTELLQGYTVEVGQQPPDLVDFAVEYFTRLREARRQ (SEQ ID NO:79)
PKA RI113
SIEIPA.GLTELLQCiFTVEVLRIIQPADLLEFALQIETRLQQENER. (SEQ ID NO:80)
116
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
103731 The structure-function relationships of the AD and DDD domains have
been the
subject of investigation. (See, e.g., Burns-Hamuro et al., 2005, Protein Sci
14:2982-92; Can
et al., 2001, .1 Biol Chem 276:17332-38; Alto et al., 2003, PTOC Nati Mad Sci
USA 100:4445-
50; Hundsrucker et al., 2006, Biochem J 396:297-306; Stokka et al., 2006,
Biochem J
400:493-99; Gold et al., 2006, Mol Cell 24:383-95; Kinderman et al., 2006, Mol
Cell 24:397-
408, the entire text of each of which is incorporated herein by reference.)
103741 For example, Kinderman et al. (2006) examined the crystal structure of
the AD-DDD
binding interaction and concluded that the human DDD sequence contained a
number of
conserved amino acid residues that were important in either dimer formation or
AKAP
binding, underlined in SEQ ID NO:68 below. (See Figure 1 of Kinderman et al.,
2006,
incorporated herein by reference.) The skilled artisan will realize that in
designing sequence
variants of the DDD sequence, one would desirably avoid changing any of the
underlined
residues, while conservative amino acid substitutions might be made for
residues that are less
critical for dimerization and AKAP binding.
SITIQIPPGLTELLQGYTVEVLRQQPPDLVEFAVEYFTRLREARA. (SEQ ID NO:68)
103751 Alto et al. (2003) performed a bioinformatic analysis of the Al)
sequence of various
AKAP proteins to design an RI! selective AD sequence called AKAP-TS (SEQ ID
NO:70),
with a binding constant for DDD of 0.4 nM. The AKAP-IS sequence was designed
as a
peptide antagonist of AKAP binding to PKA. Residues in the AKAP-IS sequence
where
substitutions tended to decrease binding to DDD are underlined in SEQ ID
NO:70. The
skilled artisan will realize that in designing sequence variants of the AD
sequence, one would
desirably avoid changing any of the underlined residues, while conservative
amino acid
substitutions might be made for residues that are less critical for DDD
binding.
AKAP-IS sequence
QIEYLAKQIVDNAIQQA. (SEQ ID NO:70)
103761 Gold (2006) utilized crystallography and peptide screening to develop a
SuperAKAP-
IS sequence (SEQ ID NO:81), exhibiting a five order of magnitude higher
selectivity for the
RTI isoform of PKA compared with the RI isoform. Underlined residues indicate
the positions
of amino acid substitutions, relative to the AKAP-IS sequence, which increased
binding to
the DDD moiety of RM. In this sequence, the N-terminal Q residue is numbered
as residue
number 4 and the C-terminal A residue is residue number 20. Residues where
substitutions
could be made to affect the affinity for RIT.a were residues 8, 11, 15, 16,
18, 19 and 20 (Gold
117
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
et al., 2006). It is contemplated that in certain alternative embodiments, the
SuperAKAP-IS
sequence may be substituted for the AKAP-IS AD moiety sequence to prepare
DNLTm
constructs. Other alternative sequences that might be substituted for the AKAP-
IS Al)
sequence are shown in SEQ ID NO:82-84. Substitutions relative to the AKAP-IS
sequence
are underlined. It is anticipated that, as with the AD2 sequence shown in SEQ
ID NO:68, the
AD moiety may also include the additional N-terminal residues cysteine and
glycine and C-
terminal residues glycine and cysteine.
SuperAKAP-IS
QIEYVAKQIVDYAIHQA (SEQ ID NO:81)
Alternative A.KAP sequences
QIEYKAKQIVDHAIIIQA (SEQ ID NO:82)
QIEYHAKQIVDIIAIIIQA (SEQ ID NO:83)
QIEYVAKQIVDHAIHQA (SEQ ID NO:84)
103771 Figure 2 of Gold et al. disclosed additional DDD-binding sequences from
a variety of
AKAP proteins, shown below.
RTI-SPECIFIC AKAPS
AKAP-KL
PLEYQAGLLVQNAIQQAI (SEQ ID NO:85)
AKAP79
LLIETASSLVKNAIQLSI (SEQ ID NO:86)
AKAP-LBC
LIEEAASRIVDA.VIEQVK. (SEQ ID NO:87)
RI-SPECIFIC AKAPS
AKAPCE
ALYQFADRFSELVISEAL (SEQ ID NO:88)
RIAD
LEQVANQLADQIIKEAT (SEQ ID NO:89)
PV38
FEELAWKIAKMIWSDVF (SEQ ID NO:90)
DUAL-SPECIFICITY AKAPS
AKAP7
ELVRLSKRLVENAVLKAV (SEQ ID NO:91)
MAP2D
118
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
TAEEVSARIVQVVTAEAV (SEQ ID NO:92)
DAKAP1
QIKQAAMLISQVILEAT (SEQ ID NO:93)
DAKAP2
LAWKIAKMIVSDVMQQ (SEQ ID NO:94)
103781 Stokka et al. (2006) also developed peptide competitors of AKAP binding
to PKA,
shown in SEQ ID NO:95-97. The peptide antagonists were designated as Ht31 (SEQ
ID
NO:95), RIM) (SEQ ID NO:96) and PV-38 (SEQ ID NO:97). The Ht-31 peptide
exhibited a
greater affinity for the RII isofonn of PKA., while the RIAD and PV-38 showed
higher
affinity for RI.
Ht31
DLIEEAASRIVDAVIEQVKAAGAY (SEQ ID NO:95)
RIM)
LEQYANQLADQIIKEATE (SEQ ID NO:96)
PV-38
FEELA.WKIAKMIWSDVFQQC (SEQ ID NO:97)
103791 Hundsnicker et al. (2006) developed still other peptide competitors for
AKAP binding
to PKA., with. a binding constant as low as 0.4 nIVI to the DDD of the RH
form. of PKA.. The
sequences of various AKAP antagonistic peptides are provided in Table 1 of
Hundsrucker et
al., reproduced in Table 15 below. AKAPIS represents a synthetic RII subunit-
binding
peptide. All other peptides are derived from the Rh-binding domains of the
indicated
AKAPs.
Table 15. AKAP Peptide sequences
Peptide Sequence
AKAPT.S QT.EYLAKQIVDNATQQA (SEQ ID NO:70)
AKAPIS-P QIEYLAKQIPDNAIQQA (SEQ ID NO:98)
Ht31 KGADLIEEAASREVDAVIEQVKAAG (SEQ ID NO:99)
Ht31-P KCiA.DLIEEAASRIPDAPIEQVKAAG (SEQ ID NO:100)
AKAP7o-wt-pep PEDAELVRLSKRLVENAVLKAVQQY (SEQ ID NO:101)
AKAP7o-L304T-pep PEDAELVRTSKRLVENAVLKAVQQY (SEQ ID NO:102)
AKAP7o-L308D-pep PEDAELVRLSKRDVENAVLKAVQQY (SEQ ID NO:1.03)
AKAP7o-P-pep PEDAELVRLSKRLPENAVLKA.VQQY (SEQ ID NO:104)
119
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
AKAP7o-PP-pep PEDAELVRLSKRLPENAPLKAVQQY (SEQ ID NO:105)
AKAP7o-L314E-pep PEDAELVRLSKRLVENAVEKAVQQY (SEQ ID NO:106)
AKAP1. -pep EEGLDRNEEIKRAAFQIISQVISEA (SEQ ID NO:107)
AKAP2-pep LVDDPLEYQAGLLVQNAIQQAIAEQ (SEQ ID NO:108)
AKAP5-pep QYETLLIETASSLVKNAIQLSIEQL (SEQ ID NO:109)
AKAP9-pep LEKQYQEQLEEEVAKVIVSMSIAFA (SEQ ID NO:110)
AKAP10-pep NTDEAQEELAWKIAKMIVSDIMQQA (SEQ ID NO:1 ii)
AKAP I 1-pep VNLDKKAVLAEKIVAEAIEKAEREL (SEQ ID NO:112)
AKAP I 2-pep NGILELETKSSKLVQNIIQTAVDQF (SEQ ID NO:113)
AKAP14-pep TQDKNYEDELTQVALALVEDVINYA (SEQ ID NO:114)
Rab32-pep ETSAKDNINIEEAARFLVEKILVNII (SEQ ID NO:! 15)
103801 Residues that were highly conserved among the AD domains of different
AKAP
proteins are indicated below by underlining with reference to the AKAP IS
sequence (SEQ
ID NO:70). The residues are the same as observed by Alto et al. (2003), with
the addition of
the C-terminal alanine residue. (See FIG. 4 of Hundsrucker et al. (2006),
incorporated herein
by reference.) The sequences of peptide antagonists with particularly high
affinities for the
Rh DDD sequence were those of AKAP-TS, AKAP78-wt-pep, AKAP78-L304T-pep and
AKAP78-L308D-pep.
AKAP-IS
QIEYLAKQIVDNAIQQA (SEQ ID NO:70)
103811 Carr et al. (2001) examined the degree of sequence homology between
different
AKAP-binding DDD sequences from. human and non-human proteins and identified
residues
in the DDD sequences that appeared to be the most highly conserved among
different DDD
moieties. These are indicated below by underlining with reference to the human
PKA Rik
DDD sequence of SEQ ID NO:68. Residues that were particularly conserved are
further
indicated by italics. The residues overlap with, but are not identical to
those suggested by
Kinderman et al. (2006) to be important for binding to AKAP proteins. The
skilled artisan
will realize that in designing sequence variants of DDD, it would be most
preferred to avoid
changing the most conserved residues (italicized), and it would be preferred
to also avoid
changing the conserved residues (underlined), while conservative amino acid
substitutions
may be considered for residues that are neither underlined nor italicized..
SHIQLPPGLTELLQGYTVEVLRQQfPDLVEPAVEYFTRLREARA (SEQ ID NO:68)
120
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
103821 The skilled artisan will realize that these and other amino acid
substitutions in the
antibody moiety or linker portions of the DNLTm constructs may be utilized to
enhance the
therapeutic and/or pharmacokinetic properties of the resulting DNLTM
constructs.
Example 18. Generation of TF2 DNLTM Pretargeting Construct
103831 A trimeric DNLTM construct designated TF2 was obtained by reacting C-
DDD2-Fab-
hMN-14 with h679-Fab-AD2. A pilot batch of TF2 was generated with >90% yield
as
follows. Protein L-purified C-DDD2-Fab-hMN-14 (200 mg) was mixed with h679-Fab-
AD2
(60 mg) at a 1.4:1 molar ratio. The total protein concentration was 1.5 mg/ml
in PBS
containing 1 mM EDTA. Subsequent steps involved TCEP reduction, HIC
chromatography,
DMSO oxidation, and IMP 291 affinity chromatography. Before the addition of
TCEP, SE-
ITPLC did not show any evidence of a2b formation. Addition of 5 mM TCEP
rapidly resulted
in the formation of a2b complex consistent with a 157 kDa protein expected for
the binary
structure. TF2 was purified to near homogeneity by IMP 291 affinity
chromatography (not
shown). IMP 291 is a synthetic peptide containing the IISG hapten to which the
679 Fab
binds (Rossi et al., 2005, Clin Cancer Res 11:7122s-29s). SE-HPLC analysis of
the IMP 291
unbound fraction demonstrated the removal of a4, a, and free kappa chains from
the product
(not shown).
103841 Non-reducing SDS-PAGE analysis demonstrated that the majority of TF2
exists as a
large, covalent structure with a relative mobility near that of IgG (not
shown). The additional
bands suggest that disulfide formation is incomplete under the experimental
conditions (not
shown). Reducing SDS-PAGE shows that any additional bands apparent in the non-
reducing
gel are product-related (not shown), as only bands representing the
constituent polypeptides
of TF2 were evident (not shown). However, the relative mobilities of each of
the four
polypeptides were too close to be resolved. MALDT-TOF mass spectrometry (not
shown)
revealed a single peak of 156,434 Da, which is within 99.5% of the calculated
mass (157,319
Da) of TF2.
103851 The functionality of TF2 was determined by BIACORE assay. TF2, C-DDD1-
hMN-14+h679-ADI (used as a control sample of noncovalent a2b complex), or C-
DDD2-
hMN-14+h679-AD2 (used as a control sample of unreduced a, and b components)
were
diluted to 1 gg/m1 (total protein) and passed over a sensorchip immobilized
with HSG. The
response for TF2 was approximately two-fold that of the two control samples,
indicating that
only the h679-Fab-AD component in the control samples would bind to and remain
on the
sensorchip. Subsequent injections of WI2 IgG, an anti-idiotype antibody for
hMN-14,
121
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
demonstrated that only TF2 had a DDD-Fab-hMN-14 component that was tightly
associated
with h679-Fab-AD as indicated by an additional signal response. The additional
increase of
response units resulting from the binding of W12 to TF2 immobilized on the
sensorchip
corresponded to two fully functional binding sites, each contributed by one
subunit of C-
DDD2-Fab-hMN-14. This was confirmed by the ability of TF2 to bind two Fab
fragments of
WI2 (not shown).
Example 19. Production of TF10 Bispecific Antibody for Pretargeting
103861 A similar protocol was used to generate a trimeric TFIO DNI,Tm
construct, comprising
two copies of a C-DDD2-Fab-hPAM4 and one copy of C-AD2-Fab-679. The cancer-
targeting antibody component in TF10 was derived from hPAM4, a humanized anti-
MUCSAC MAb that has been studied in detail as a radiolabeled MAb (e.g.. Gold
et al., Clin.
Cancer Res. 13: 7380-7387, 2007). The hapten-binding component was derived
from h679, a
humanized anti-histaminyl-succinyl-glycine (HSG) MAb. The TF 10 bispecific
([11PAM4:12 x
h679) antibody was produced using the method disclosed for production of the
(anti CEA)2 x
anti IISG bsAb TF2, as described above. The TF10 construct bears two humanized
PAM4
Fabs and one humanized 679 Fab.
103871 The two fusion proteins (hPAM4-DDD and h679-AD2) were expressed
independently in stably transfected myeloma cells. The tissue culture
supernatant fluids were
combined, resulting in a two-fold molar excess of hPAM4-DDD. The reaction
mixture was
incubated at room temperature for 24 hours under mild reducing conditions
using 1 mM
reduced glutathione. Following reduction, the DNI..."4 reaction was completed
by mild
oxidation using 2 mM oxidized glutathione. IFIO was isolated by affinity
chromatography
using IMP 291-affigel resin, which binds with high specificity to the h679
Fab.
103881 A full tissue histology and blood cell binding panel has been examined
for hPAM4
TgG. and for an anti-CEA x anti-HSG bsMAb that is entering clinical trials.
hPAM4 binding
was restricted to very weak binding to the urinary bladder and stomach in 1/3
specimens (no
binding was seen in vivo), and no binding to normal tissues was attributed to
the anti-CEA x
anti-HSG bsMAb. Furthermore, in vitro studies against cell lines bearing the
HI and H2
histamine receptors showed no antagonistic or agonistic activity with the IMP
288 di-IISG
peptide, and animal studies in 2 different species showed no pharinacologic
activity of the
peptide related to the histamine component at doses 20,000 times higher than
that used for
imaging. Thus, the IISG-histamine derivative does not have pharmacologic
activity.
122
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Example 20. Imaging Studies Using Pretargeting With TF10 Bispecific Antibody
and min-Labeled Peptides
103891 The following study demonstrates the feasibility of in vivo imaging
using the
pretargeting technique with bispecific antibodies incorporating hPAM4 and
labeled peptides.
The TF 10 bispecific antibody, comprising two copies of a C-DDD2-Fab-hPAM4 and
one
copy of C-AD2-Fab-679, was prepared as described in the preceding Example.
Nude mice
bearing 0.2 to 0.3 g human pancreatic cancer xenografts were imaged, using
pretargeting with
TF 10 and "In-IMP-288 peptide. The results, shown in FIG. 15A and FIG. 15B,
demonstrate how clearly delineated tumors can be detected in animal models
using a bsMAb
pretargeting method, with min-labeled di-HSG peptide, IMP-288. The six animals
in the top
of FIG. 15A and FIG. 15B received 2 different doses of TF10 (10:1 and 20:1
mole ratio to
the moles of peptide given), and the next day they were given an "In-labeled
&I-1SG peptide
(IMP 288). The 3 other animals on the bottom of FIG. 15A and FIG. 15B received
only the
Min-IMP-288 (no pretargeting). The images shown in FIG. 15B were taken 3 h
after the
injection of the labeled peptide and show clear localization of 0.2 ¨ 0.3 g
tumors in the
pretargeted animals, with no localization in the animals given the "In-peptide
alone. Tumor
uptake averaged 20-25% ID/g with tumor/blood ratios exceeding 2000:1,
tumor/liver ratios of
170:1, and tumor/kidney ratios of 18/1.
Example 21. Production of Targeting Peptides for Use in Pretargeting and 118F
Labeling
103901 In a variety of embodiments, 18F-labeled proteins or peptides are
prepared by a novel
technique and used for diagnostic and/or imaging studies, such as PET imaging.
The novel
technique for 18F labeling involves preparation of an 18F-metal complex,
preferably an 18F-
aluminum complex, which is chelated to a chelating moiety, such as DOTA, NOTA
or NETA
or derivatives thereof. Chelating moieties may be attached to proteins,
peptides or any other
molecule using conjugation techniques well known in the art. In certain
preferred
embodiments, the 18F-Al complex is formed in solution first and then attached
to a chelating
moiety that is already conjugated to a protein, or peptide. However, in
alternative
embodiments the aluminum may be first attached to the chelating moiety and the
18F added
later.
Peptide Synthesis
103911 Peptides were synthesized by solid phase peptide synthesis using the
Fmoc strategy.
Groups were added to the side chains of diamino amino acids by using Fmoc/Aloc
protecting
123
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
groups to allow differential deprotection. The Aloe groups were removed by the
method of
Dangles et. al. (J. Org. Chem. 1987, 52:4984-4993) except that piperidine was
added in a 1:1
ratio to the acetic acid used. The unsymmetrical tetra-t-butyl DTPA was made
as described in
McBride et al. (US Patent Application Pub. No. 2005/0002945, the Examples
section of
which is incorporated herein by reference).
103921 The tri-t-butyl DOTA, symmetrical tetra-t-butyl DTPA, ITC-benzyl DTPA,
p-SCN-
Bn-NOTA. and TACN were obtained from. MACROCYCLICS (Dallas, TX). The
DiBocTACN, NODA-GA(tBu)3 and the NO2AtBu were purchased from CheMatech (Dijon,
France). The Aloc/Fmoc Lysine and Dap (diaminopropionic acid derivatives (also
Dpr)) were
obtained from CREOSALUS (Louisville, KY) or BACHEM (Torrance, CA). The
Sieber
Amide resin was obtained from NOVABIOCITEMO (San Diego, CA.). The remaining
Fmoc
amino acids were obtained from CREOSALUS , BACHEMO, PEPTECHCR) (Burlington,
MA), EMD BIOSCIENCES (San Diego, CA), CHEM IMPEX (Wood Dale, IL) or
NOVABIOCHEM . The aluminum chloride hexahydrate was purchased from SIGMA.-
ALDRICH. (Milwaukee, WI). The remaining solvents and reagents were purchased
from
FISHER SCIENTIFIC (Pittsburgh, PA) or SIGMA-ALDRICH (Milwaukee, WI). 18F was
supplied by IBA. MOLECULAR (Somerset, NJ)
18 P-= =
-Labehng of IMP 272
103931 The first peptide that was prepared and 18F-labeled was IMP 272:
DTPA-Gin-Ala-Lys(USG)-D-Tyr-Lys(HSG)-NH2 Mir 1512
103941 IMP 272 was synthesized as described (US Patent No. 7,534,431, the
Examples
section of which is incorporated herein by reference).
103951 Acetate buffer solution - Acetic acid, 1.509 g was diluted in ¨ 160 mL
water and the
pH was adjusted by the addition of 1 M NaOH then diluted to 250 mL to make a
0.1 M
solution at pII 4.03.
103961 Aluminum acetate buffer solution - A solution of aluminum was prepared
by
dissolving 0.1028 g of AlC13 hexahydrate in 42.6 mL DI water. A 4 mL aliquot
of the
aluminum solution was mixed with 16 mL of a 0.1 M Na0Ac solution at pH 4 to
provide a 2
mM Al stock solution.
103971 IMP 272 acetate buffer solution - Peptide, 0.0011 g, 7.28 x i0 moi IMP
272 was
dissolved in 364 }AL of the 0.1 M pH 4 acetate buffer solution to obtain a 2
mM stock solution
of the peptide.
124
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
103981 F-18 Labeling of IMP 272 - A 3 1.11, aliquot of the aluminum stock
solution was
placed in a REACTI-VIALTm and mixed with 501.11.. 18F (as received) and 3 pi.
of the IMP
272 solution. The solution was heated in a heating block at 110 C for 15 min
and analyzed by
reverse phase IIPLC. HPLC analysis (not shown) showed 93% free 18F and 7%
bound to the
peptide. An additional 10 p.L of the IMP 272 solution was added to the
reaction and it was
heated again and analyzed by reverse phase HPLC (not shown). The HPLC trace
showed 8%
18F at the void volume and 92% of the activity attached to the peptide. The
remainder of the
peptide solution was incubated at room temperature with 1504 PBS for ¨ 1hr and
then
examined by reverse phase HPLC. The HPLC (not shown) showed 58%18F unbound and
42% still attached to the peptide. The data indicate that 18F-Al-DTPA complex
may be
unstable when mixed with phosphate.
103991 The labeled peptide was purified by applying the labeled peptide
solution onto a 1 cc
(30 mg) WATERS HLB column (Part # 186001879) and washing with 3001.11.. water
to
remove unbound F-18. The peptide was eluted by washing the column with 2 x
1004 1:1
Et0I-VII20. The purified peptide was incubated in water at 25 C and analyzed
by reverse
phase HPLC (not shown). The HPLC analysis showed that the 18F-labeled IMP 272
was not
stable in water. After 40 min incubation in water about 17% of the 18F was
released from the
peptide, while 83% was retained (not shown).
104001 The peptide (16 ILL 2 mM IMP 272,48 lig) was labeled with 18F and
analyzed for
antibody binding by size exclusion HPLC. The size exclusion HPLC showed that
the peptide
bound hMN-14 x 679 but did not bind to the irrelevant bispecific antibody hMN-
14 x 734
(not shown).
IMP 272 18F Labeling with Other Metals
104011 A ¨3 ILL aliquot of the metal stock solution (6 x 10-9 mol) was placed
in a
polypropylene cone vial and mixed with 75 lat 18F (as received), incubated at
room
temperature for ¨2 min and then mixed with 20 1.1L of a 2 mM (4 x 108 mol) IMP
272
solution in 0.1 M Na0Ac pH 4 buffer. The solution was heated in a heating
block at 100 C
for 15 min and analyzed by reverse phase HPLC. IMP 272 was labeled with indium
(24%),
gallium (36%), zirconium (15%), lutetium (37%) and yttrium (2%) (not shown).
These
results demonstrate that the 18F metal labeling technique is not limited to an
aluminum ligand,
but can also utilize other metals as well. With different metal ligands,
different chelating
moieties may be utilized to optimize binding of an F-1.8-metal conjugate.
Production and Use of a Serum-Stable 18F-Labeled Peptide IMP 449
125
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
104021 The peptide, IMP 448 D-Ala-D-Lys(IISG)-D-Tyr-D-Lys(HSG)-N117 MIr 1009
was
made on Sieber Amide resin by adding the following amino acids to the resin in
the order
shown: Aloc-D-Lys(Fmoc)-OH, Trt-HSG-OH, the Aloc was cleaved, Fmoc-D-Tyr(But)-
OH,
Aloc-D-Lys(Fmoc)-0H, Trt-IISG-OH, the Aloc was cleaved, Fmoc-D-Ala-OH with
final
Frnoc cleavage to make the desired peptide. The peptide was then cleaved from
the resin and
purified by HPLC to produce IMP 448, which was then coupled to ITC-benzyl
NOTA. The
peptide, IMP 448, 0.0757g (7.5 x 10-5 mol) was mixed with 0.0509 g (9.09 x 10-
5 mol) ITC
benzyl NOTA and dissolved in 1 niL water. Potassium carbonate anhydrous
(0.2171 g) was
then slowly added to the stirred peptide/NOTA solution. The reaction solution
was pH 10.6
after the addition of all the carbonate. The reaction was allowed to stir at
room temperature
overnight. The reaction was carefully quenched with 1 M FICI after 14 hr and
purified by
HPLC to obtain 48 mg of IMP 449.
18F Labeling of IMP 449
104031 The peptide IMP 449 (0.002 g, 1.37 x 10-6 mol) was dissolved in 686
1.11., (2 mM
peptide solution) 0.1 M Na0Ac pH 4.02. Three microliters of a 2 mM solution of
Al in a pH
4 acetate buffer was mixed with 15 L, 1.3 mCi of 18F. The solution was then
mixed with 20
ILL of the 2 mM IMP 449 solution and heated at 105 C for 15 min. Reverse
Phase HPLC
analysis showed 35% (tR ¨ 10 min) of the activity was attached to the peptide
and 65% of the
activity was eluted at the void volume of the column (3.1 min, not shown)
indicating that the
majority of activity was not associated with the peptide. The crude labeled
mixture (5 1.11..)
was mixed with pooled human serum and incubated at 37 C. An aliquot was
removed after
15 min and analyzed by HPLC. The IIPLC showed 9.8% of the activity was still
attached to
the peptide (down from 35%). Another aliquot was removed after 1 hr and
analyzed by
HPLC. The HPLC showed 7.6% of the activity was still attached to the peptide
(down from
35%), which was essentially the same as the 15 min trace (data not shown).
High Dose 18.F Labeling
104041 Further studies with purified IMP 449 demonstrated that the 18F-labeled
peptide was
highly stable (91%, not shown) in human serum at 37 C for at least one hour
and was
partially stable (76%, not shown) in human serum at 37 C for at least four
hours. Additional
studies were performed in which the IMP 449 was prepared in the presence of
ascorbic acid
as a stabilizing agent. In those studies (not shown), the metal-18F-peptide
complex showed no
detectable decomposition in serum after 4 hr at 37 C. The m.ouse urine 30 min
after injection
of' 8F-labeled peptide was found to contain 18F bound to the peptide (not
shown). These
126
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
results demonstrate that the 18F-labeled peptides disclosed herein exhibit
sufficient stability
under approximated in vivo conditions to be used for 18F imaging studies.
104051 For studies in the absence of ascorbic acid, 18F ¨ 21 mCi in ¨ 400 IAL
of water was
mixed with 94 of 2 mM AlC13 in 0.1 M pII 4 Na0Ac. The peptide, IMP 449, 60 ttL
(0.01
M, 6 x 10 mol in 0.5 NaOH pH 4.13) was added and the solution was heated to
110 C for
15 min. The crude labeled peptide was then purified by placing the reaction
solution in the
ban-el of a I cc WATERS HLB column and eluting with water to remove unbound
18F
followed by 1:1 Et0II/1120 to elute the 18F-labeled peptide. The crude
reaction solution was
pulled through the column into a waste vial and the column was washed with 3 x
1 niL
fractions of water (18.97 mCi). The HLB column was then placed on a new vial
and eluted
with 2 x 200 ti.L 1:1 Et0II/1-120 to collect the labeled peptide (1.83 mCi).
The column
retained 0.1 mCi of activity after all of the elutions were complete. An
aliquot of the purified
18F-labeled peptide (201.11..) was mixed with 2001AL of pooled human serum and
heated at 37
C. Aliquots were analyzed by reverse phase HPLC. The results showed the
relative stability
of '8F-labeled purified IMP 449 at 37 C at time zero, one hour (91% labeled
peptide), two
hours (77% labeled peptide) and four hours (76% labeled peptide) of incubation
in human
serum (not shown). It was also observed that 18F-labeled IMP 449 was stable in
TFA
solution, which is occasionally used during reverse phase IIPLC
chromatography. There
appears to be a general correlation between stability in TFA and stability in
human serum
observed for the exemplary 18F-labeled molecules described herein. These
results
demonstrate that 18F-labeled peptide, produced according to the methods
disclosed herein,
shows sufficient stability in human serum to be successfully used for in vivo
labeling and
imaging studies, for example using PET scanning to detect labeled cells or
tissues. Finally,
since IMP 449 peptide contains a thiourea linkage, which is sensitive to
radiolysis, several
products are observed by RP-I-TPLC. IIowever, when ascorbic acid is added to
the reaction
mixture, the side products generated were markedly reduced.
Example 22. In Vivo Studies With Pretargeting TFIO DNLTM Construct and 18F-
Labeled Peptide
104061 'IT-labeled IMP 449 was prepared as follows. The 18F, 54.7 mCi in¨ 0.5
mL was
mixed with 3 tiL 2 niM Al in 0.1 M Na0Ac p114 buffer. After 3 min 101u.L of
0.05 M IMP
449 in 0.5 M pH 4 Na0Ac buffer was added and the reaction was heated in a 96
C. heating
block for 15 min. The contents of the reaction were removed with a syringe.
The crude
labeled peptide was then purified by IIPLC on a C18 column. The flow rate was
3 milmin.
127
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Buffer A was 0.1% TFA in water and Buffer B was 90% acetonitrile in water with
0.1%
TFA. The gradient went from 100% A to 75/25 A:B over 15 min. There was about 1
min
difference in retention time (tR) between the labeled peptide, which eluted
first and the
unlabeled peptide. The I-TPLC eluent was collected in 0.5 min (mL) fractions.
The labeled
peptide had a tR between 6 to 9 min depending on the column used. The I-IPLC
purified
peptide sample was further processed by diluting the fractions of interest two
fold in water
and placing the solution in the barrel of a 1 cc WATERS HLB column. The
cartridge was
eluted with 3 x 1 niL water to remove acetonitrile and TFA followed by 4001AL
1:1
Et011/H20 to elute the 18F-labeled peptide. The purified [A11811 IMP 449
eluted as a single
peak on an analytical HPLC C18 column (not shown).
104071 TA.CONICO nude mice bearing the four slow-growing sc CaPanl xenografts
were
used for in vivo studies. Three of the mice were injected with TF10 (162 gg)
followed with
[A1181-]1MP 449 18 h later. TF10 is a humanized bispecific antibody of use for
tumor
imaging studies, with divalent binding to the PAM-4 defined tumor antigen and
monovalent
binding to 11SG (see, e.g., Gold et al., 2007, J. Clin. Oncol. 25(18S):4564).
One mouse was
injected with peptide alone. All of the mice were necropsied at 1 h post
peptide injection.
Tissues were counted immediately. Comparison of mean distributions showed
substantially
higher levels of '8F-labeled peptide localized in the tumor than in any normal
tissues in the
presence of tumor-targeting bispecific antibody (data not shown).
104081 Tissue uptake was similar in animals given the [A118F] IMP 449 alone or
in a
pretargeting setting (data not shown). Uptake in the human pancreatic cancer
x.enograft,
CaPanl, at 1 h was increased 5-fold in the pretargeted animals as compared to
the peptide
alone (4.6 0.9% ID/g vs. 0.89% ID/g). Exceptional tumor/nontumor ratios were
achieved at
this time (e.g., tumor/blood and liver ratios were 23.4 2.0 and 23.5 2.8,
respectively).
104091 The results demonstrate that 18F labeled peptide used in conjunction
with a PAM4
containing antibody construct, such the TFIO DNLTM construct, provide suitable
targeting of
the 18F label to perform in vivo imaging, such as PET imaging analysis.
Example 23. Further Imaging Studies with 'TF1.0
Summary
104101 Preclinical and clinical studies have demonstrated the application of
radiolabeled
rnAb-PAM4 for nuclear imaging and radioimmunotherapy of pancreatic carcinoma.
We have
examined herein the ability of the TF10 construct to pretarget a radiolabeled
peptide for
improved imaging and therapy. Biodistribution studies and nuclear imaging of
the
128
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
radiolabeled TF10 and/or TF10-pretargeted hapten-peptide (IMP-288) were
conducted in
nude mice bearing CaPanl human pancreatic cancer xenografts.1251-TF10 cleared
rapidly
from the blood, with levels decreasing to <1% injected dose per gram (ID/g) by
16 hours.
Tumor uptake was 3.47 0.66% ID/g at this time point with no accumulation in
any normal
tissue. To show the utility of the pretargeting approach, "11n-IMP-288 was
administered 16
hours after TF10. At 3 hours postadministration of radiolabeled peptide,
imaging showed
intense uptake within the tumors and no evidence of accretion in any normal
tissue. No
targeting was observed in animals given only the 111In-peptide. Tumor uptake
of the TF10-
pretargeted "in-IMP-288 was 24.3 :la 1.7% ID/g, whereas for 111In4MP-288 alone
it was
only 0.12 0.002% ID/g at 16 hours. Tumor/blood ratios were significantly
greater for the
pretargeting group (-1,000:1 at 3 hours) compared with 11'in-PAM4-IgG (-5:1 at
24 hours; P
<0.0003). Radiation dose estimates suggested that TF10/9 Y-peptide
pretargeting would
provide a greater antitumor effect than 90Y-PAM4-igG. Thus, the results
support that TF10
pretargeting may provide improved imaging for early detection, diagnosis, and
treatment of
pancreatic cancer as compared with directly radiolabeled PAM4-IgG. (Gold et
al., Cancer Res
2008, 68(12):4819-26)
10411.1 We have identified a unique biomarker present on mucin expressedby
>85% of
invasive pancreatic adenocarcinomas, including early stage I disease and the
precursor
lesions, pancreatic intraepithelial neoplasia and intraductal papillary
mucinous neoplasia
(Gold et al., Clin Cancer Res 2007, 13:7380-87). The specific epitope, as
detected by mAb-
PAM4 (Gold etal., Int .1. Cancer 1994, 57:204-10), is absent from normal and
inflamed
pancreatic tissues, as well as most other malignant tissues. Thus, detection
of the epitope
provides a high diagnostic likelihood for the presence of pancreatic
neoplasia. Early clinical
studies using 1311- and 99mTc4abeled, murine PAM4 IgG or Fab', respectively,
showed
specific targeting in 8 of 10 patients with invasive pancreatic adenocarcinoma
(Mariani et al.,
Cancer Res 1995, 55:5911s-15s; Gold et al., Crit Rev Oncol llematol 2001,
39:147-54). Of
the two negative patients, one had a poorly differentiated pancreatic
carcinoma that did not
express the PAM4-epitope, whereas the other patient was later found to have
pancreatitis
rather than a malignant lesion.
104121 Accordingly, the high specificity of PAM4 for pancreatic cancer is of
use for the
detection and diagnosis of early disease. In addition to improved detection,
90Y-PAM4 IgG
was found to be effective in treating large human pancreatic cancer xenografts
in nude mice
(Cardillo et al., Clin Cancer Res 2001, 7:3186-92), and when combined with
gemcitabine,
129
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
further improvements in therapeutic response were observed (Gold et al., Clin
Cancer Res
2004, 10:3552-61; Gold et al., int J Cancer 2004, 109:618-26). A Phase 1
therapy trial in
patients who failed gemcitabine treatment was recently completed, finding the
maximum
tolerated dose of 90Y-humanized PAM4 IgG to be 20 mCi/m2 (Gulec et al., Proc
Amer Soc
Clin Onc, 43rd Annual Meeting, J Clin Oncol 2007, 25(18S):636s). Although all
patients
showed disease progression at or after week 8, initial shrinkage of tumor was
observed in
several cases. Clinical studies are now underway to evaluate a fractionated
dosing regimen of
90Y-hPAM4 IgG in combination with a radiosensitizing dose of gemcitabine.
104131 We report herein the development of a novel recombinant, humanized
bispecific
monoclonal antibody (mAb), TF 10, based on the targeting specificity of PAW to
pancreatic
cancer. This construct also binds to the unique synthetic hapten, histamine-
succinyl-glyeine
MSG), which has been incorporated in a number of small peptides that can be
radiolabeled
with a wide range of radionuclides suitable for single-photon emission
computed tomography
(SPEC'I) and positron emission tomography (PET) imaging, as well as for
therapeutic
purposes (Karacay et al., Clin Cancer Res 2005, 11:7879-85; Sharkey etal.,
Leukemia 2005,
19:1064-9; Rossi et al., Proc NatI Acad Sci U S A 2006, 103:6841-6; McBride et
al., J Nucl
Med 2006, 47:1678-88). These studies illustrate the potential of this new
construct to target
pancreatic adenocarcinoma for imaging or therapeutic applications.
Methods and Materials
104141 The TF2 and TF 10 bispecific DNLTM constructs and the IMP 288 targeting
peptide
were prepared as described above. Sodium iodide (1251) and indium chloride
(1111n) were
obtained from PERKIN-ELMER . IFIO was routinely labeled with 1251 by the
iodogen
method, with purification by use of size-exclusion spin columns. Radiolabeling
of DOTA-
peptide and DOTA-PAM4-igG with 111InCI was done as previously described (Rossi
et al.,
Proc Natl Acad Sci U S A 2006, 103:6841-6; McBride et al., J Nucl Med 2006,
47:1678-88).
Purity of the radiolabeled products was examined by size-exclusion high-
performance liquid
chromatography with the amount of free, unbound isotope determined by instant
TLC.
104151 For TF 10 distribution studies, female athymic nude mice -20 g (TACONIC
Farms),
bearing s.c. CaPanl human pancreatic cancer xenografts, were injected with
'251-TF 10 (10
p.Ci; 40 gg, 2.50 x 10-1 mol). At various time points, groups of mice (n =5)
were necropsied,
with tumor and nontumor tissues removed and counted in a gamma counter to
determine the
percentage of injected dose per gram of tissue (%ID/g), with these values used
to calculate
blood clearance rates and tumorinontumor ratios.
130
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
104161 For pretargeting biodistribution studies, a bispecific
rnAb/radiolabeled peptide molar
ratio of 10:1 was used. For example, a group of athymic nude mice bearing s.c.
CaPanl
human pancreatic cancer xenografts was administered TFIO (80 g, 5.07 x 100
mol),
whereas a second group was left untreated. At 16 h postinjection of TF10,
1111n-IMP-288
hapten-peptide (30 }Xi, 5.07 x 10-11 mol) was administered. Mice were
necropsied at several
time points, with tumor and nontumor tissues removed and counted in a gamma
counter to
determine the %1D/g. 'Fumor/nontumor ratios were calculated from these data.
In a separate
study, groups of mice were given I ITn-DOTA-PAM4-IgG (20 JAC, 501.1g, 3.13 x
10-40 mol)
for the purpose of comparing biodistribution, nuclear imaging, and potential
therapeutic
activity. Radiation dose estimates were calculated from the time-activity
curves with the
assumption of no activity at zero time. Student's t test was used to assess
significant
differences.
104171 To perform nuclear immunoscintigraphy, at 3 h postinjection of
radiolabeled peptide
or 24 h postinjection of radiolabeled hPAM4-IgG, tumor-bearing mice were
imaged with a
dual-head Solus gamma camera fitted with medium energy collimator for In (ADAC
Laboratories). Mice were imaged for a total of 100,000 cpm or 10 min,
whichever came first.
Results
104181 In vitro characterization of the bispecific mAb TF10. The binding of
IFIO to the
target mucin antigen was analyzed by ELISA (FIG. 16). The results showed
nearly identical
binding curves for the divalent TF10, PAM4-IgG, and PAM4-F(abi)2 (half-maximal
binding
was calculated as 1.42 0.10, 1.31 0.12, and 1.83 0.16 nmo1/1õ
respectively; P> 0.05 for
all), whereas the monovalent bsPAM4 chemical conjugate (PAM4-Fab' x anti-DTPA.-
Fab)
had a significantly lower avidity (half-maximal binding, 30.61 2.05 nmol/L;
P =0.0379,
compared with TF10), suggesting that TF10 binds in a divalent manner. The
immunoreactive
fraction of 125I-TF1.0 bound to MUC5A.0 was 87%, with 9% found as unbound TF10
and 3%
as free iodide (not shown). Ninety percent of the "In-IMP-288 bound to TF10
(not shown).
Of the total 11'In-IMP-288 bound to TF10, 92% eluted at higher molecular
weight when
excess mucin (200 p,g) was added, with only 3% eluting with the non¨mucin-
reactive TFIO
fraction. An additional 5% of the radiolabeled peptide eluted in the free
peptide volume. None
of the radiolabeled peptide bound to the mucin antigen in the absence of TF10
(not shown).
104191 Biodistribution of 125I-TF10 in CaPani tumor¨bearing nude mice. TF10
showed a
rapid clearance from the blood, starting with 21.03 1.93 %ID/g at I hour and
decreasing to
just 0.13 0.02 %ID/g at 16 hours. The biological half-life was calculated to
be 2.19 hours
131
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
[95% confidence interval (95% CI), 2.11-2.27 hours]. Tissue uptake revealed
enhanced
activity in the liver, spleen, and kidneys at 1 hour, which cleared just as
quickly by 16 hours
= 2.09 hours (95% CI, 2.08-2.10), 2.84 hours (95% Cl, 2.49-3.29), and 2.44
hours (95%
CI, 2.28-2.63) for liver, spleen, and kidney, respectively]. Activity in the
stomach most likely
reflects the accretion and excretion of radioiodine, suggesting that the
radioiodinated TF10
was actively catabolized, presumably in the liver and spleen, thereby
explaining its rapid
clearance from the blood. Nevertheless, by 16 hours, the concentration of
radioiodine
the stomach was below 1% ID/g. A group of five non-tumor-bearing nude mice
given 1251-
TF10 and necropsied at 16 hours showed similar tissue distribution, suggesting
that the tumor
had not affected the bispecific mA.b distribution and clearance from normal
tissues (data not
shown). Of course, it is possible that differences occurred before the initial
time point
examined. Tumor uptake of TF10 peaked at 6 hours postinjection (7.16 1.10
%ID/g) and
had decreased to half maximum binding (3.47 0.66 %ID/g) at 16 hours. Tumor
uptake again
decreased nearly 2-fold over the next 32 hours, but then was stable over the
following 24
hours.
104201 Biodistribution of TF10-pretargeted, 1111n-labeled peptide. Although
maximum tumor
uptake of TF 10 occurred at 6 hours, previous experience indicated that the
radiolabeled
peptide would need to be given at a time point when blood levels of TF10 had
cleared to <1%
ID/g (i.e., 16 hours). Higher levels of TF10 in the blood would lead to
unacceptably high
binding of the radiolabeled peptide within the blood (i.e., low tumor/blood
ratios), whereas
administering the peptide at a later time would mean the concentration of TF10
in the tumor
would be decreased with consequently reduced concentration of radiolabeled
peptide within
the tumor. Thus, an initial pretargeting study was done using a 16-hour
interval. With the
amount of the "In-IMP-288 held constant (30 p,Ci, 5.07 x 10-11 mol),
increasing amounts of
TF10 were given so that the administered dose off*F10 and IMP-288 expressed as
mole ratio
varied from 5:1 to 20:1 (Table 16).
Table 16. Biodistribution of 1111n-IMP-288 alone (no TF10) or pretargeted with
varying
amounts of =I'Iz I 0
:.= :.=
:.= :.=
:.=
:.=:.=
%ID/g at 3 h (mean SD)
:.= :.=
:.= :.=
:.= :.=
:.=
:.= :.=
:.=
.==
:.= :.=
:.=
:.=
:.= :.=
:.= 5:1 10:1 20:1 No TF10 :.=
:.=
.==
132
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
:.=
:.=
Tumor 19.0 3.49 24.3 1.71 28.6 0.73 0.12
0.00 :.=
:.=
:.=
:.=
:.=
:.=
:.=
Liver 0.09 0.01 0.21 0.12 0.17 0.01 0.07
0.00 :.=
:.=
:.=
:.=
:.=
:.=
:.=
Spleen 0.12 0.04 0.16
0.07 0.26 0.10 0.04 0.01
:.=
.==
:.=
:.=
:.=
Kidneys 1.59 0.11 1.72 0.24 1.53 0.14 1.71
0.22 :.=
:.=
:.=
:.=
:.=
:.=
:.=
Lungs 0.19 0.06 0.26
0.00 0.29 0.04 0.03 0.00
:.=
:.=
:.=
:.=
:.=
Blood 0.01 0.00 0.01 0.01 0.01 0.00 0.00
0.00 :.=
Stomach 0.03 0.02 0.02
0.02 0.01 0.00 0.02 0.01
=:
:.=
Small intestine 0.12 0.08 0.08 0.03 0.04
0.01 0.06 0.02
:.=
Large intestine 0.23 0.10 0.39
0.08 0.25 0.08 0.33 0.02
Pancreas 0.02 0.00 0.02
0.01 0.02 0.00 0.02 0.00
Tumor weight (g) 0.12 0.03 0.32 0.09 0.27
0.01 0.35 0.03
=
104211 At 3 hours the amount of" Iln-IMP-288 in the blood was barely
detectable (0.011)/0).
Tumor uptake increased from 19.0 3.49% TD/g to 28.55 0.73% ID/g as the
amount of
bispecific mAb administered was increased 4-fold (statistically significant
differences were
observed for comparison of each IF10/peptide ratio, one group to another; P <
0.03 or better),
but without any appreciable increase in normal tissue uptake. Tumor uptake in
the animals
given TF10 was >100-fold higher than when "In-IMP-288 was given alone.
Comparison of
"In activity in the normal tissues of the animals that either received or did
not receive prior
administration of TF1() indicated similar absolute values, which in most
instances were not
significantly different. This suggests that the bispecific mAb had cleared
sufficiently from all
normal tissues by 16 hours to avoid appreciable peptide uptake in these
tissues. Tumor/blood
ratios were >2,000:1, with other tissue ratios exceeding 100:1. Even
tumor/kidney ratios
exceeded 10:1. The highest tumor uptake of radioisotope with minimal targeting
to nontumor
tissues resulted from the 20:1 ratio; however, either of the TF10/peptide
ratios could be used
133
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
to achieve exceptional targeting to tumor, both in terms of signal intensity
and contrast ratios.
The 10:1 ratio was chosen for further study because the absolute difference in
tumor uptake of
radiolabeled peptide was not substantially different between the 10:1(24.3
1.71% ID/g) and
20:1 (28.6 0.73% ID/g) ratios.
104221 Images of the animals given TF10-pretargeted "'In-IMP-288 at a
bispecific
mAb/peptide ratio of 10:1, or the 'In-IMP-288 peptide alone, are shown in FIG.
17A, FIG.
17B and FIG. 17C. The majority of these tumors were <0.5 cm in diameter,
weighing ¨0.25
g. The images show highly intense uptake in the tumor of the TF10-pretargeted
animals (FIG.
17A). The intensity of the image background for the TF10-pretargeted animals
was increased
to match the intensity of the image taken of the animals given the min-IMP-288
alone (FIG.
17B). However, when the images were optimized for the TF10-pretargeted mice,
the signal
intensity and contrast were so high that no additional activity was observed
in the body. No
tumor localization was seen in the animals given the 111in4MP-288 alone, even
when image
intensity was enhanced (FIG. 17C).
104231 An additional experiment was done to assess the kinetics of targeting I
I lIn-hPAM4
whole-IgG compared with that of the TF10-pretargeted "lin-IMP-288 peptide.
Tumor uptake
of the min-peptide was highest at the initial time point examined, 3 hours
(15.99 4.11%
TD/g), whereas the blood concentration of radiolabeled peptide was only 0.02
0.01 % ID/g,
providing a mean tumor/blood ratio of 946.3 383Ø Over time, radiolabeled
peptide cleared
from the tumor with a biological half-life of 76.04 hours. Among nontumor
tissues, uptake
was highest in the kidneys, averaging 1.89 0.42% ID/g at 3 hours with a
steady decrease
over time (biological half-life, 33.6 hours). Liver uptake started at 0.15
0.06% ID/g and
remained essentially unchanged over time. In contrast to the TF10-pretargeted
II 'In-IMP-288,
the I I In-hPAM4-IgG had a slower clearance from the blood, albeit there was a
substantial
clearance within the first 24 hours, decreasing from 30.1% ID/g at 3 hours to
just 11.5 1.7%
ID/g at 24 hours. Variable elevated uptake in the spleen suggested that the
antibody was
likely being removed from the blood by targeting of secreted mucin that had
become
entrapped within the spleen. Tumor uptake peaked at 48 hours with 80.4 6.1%
ID/g, and
remained at an elevated level over the duration of the monitoring period. The
high tumor
uptake, paired with a more rapid than expected blood clearance for an IgG,
produced
tumor/blood ratios of 5.2 1.0 within 24 hours. FIG. 17C shows the images of
the animals at
24 hours postadministration of min-PAM4-IgG, illustrating that tumors could be
visualized
at this early time, but there was still considerable activity within the
abdomen.
134
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Tumor/nontumor ratios were mostly higher for TF10-pretargeted "In-labeled
hapten-peptide
as compared with In-hPAM4-Ig,G, except for the kidneys, where tumor/kidney
ratios with
the 1"in-IMP-288 and "In-hPAM4-IgG were similar at later times. However,
tumor/kidney
ratios for the TF10-pretargeted "In-IMP-288 were high enough (e.g., ¨7:1) at 3
hours to
easily discern tumor from normal tissue.
104241 FIG. 18A to FIG. 18D illustrates the potential therapeutic capability
of the direct and
pretargeted methods to deliver radionuclide (90Y). Although the concentration
(%ID/g) of
radioisotope within the tumor seems to be much greater when delivered by PAM4-
IgG than
by pretargeted TF10 at their respective maximum tolerated dose (0.15 mCi for
90Y-ITAM4
and 0.9 mCi for TF10-pretargeted9 Y-IMP-288) (FIG. 18A), the radiation dose to
tumor
would be similar (10,080 and 9,229 eGy for 90Y-PAM4-IgG and TF10-pretargeted 9
Y-IMP-
288, respectively) (FIG. 18C). The advantage for the pretargeting method would
be the
exceptionally low activity in blood (9 eGy), almost 200-fold less than with
the 90Y-hPAM4
IgG (1,623 Kly) (FIG. 18C). It is also important to note that the radiation
dose to liver, as
well as other nontumor organs, would be much lower with the TF10-pretargeted
90Y-IMP-288
(FIG. 18B, FIG. 18D). The exception would be the kidneys, where the radiation
dose would
be similar for both protocols at their respective maximum. dose (612 and 784
cGy for 9 Y-
PAM4-IgG and IF! 0-90Y-IMP-288, respectively) (FIG. 18B, FIG. 18D). The data
suggest
that for 90Y-PAM4-IgG, as with most other radiolabeled whole-IgG mAbs, the
dose-limiting
toxicity would be hematologic; however, for the TF10 pretargeting protocol,
the dose-limiting
toxicity would be the kidneys.
Discussion
104251 Current diagnostic modalities such as ultrasound, computerized
tomography (CT), and
magnetic resonance imaging (MRI) technologies, which provide anatomic images,
along with
PET imaging of the metabolic environment, have routinely been found to provide
high
sensitivity in the detection of pancreatic masses. However, these data are,
for the most part,
based on detection of lesions >2 cm in a population that is already presenting
clinical
symptoms. At this time in the progression of the pancreatic carcinoma, the
prognosis is rather
dismal. To improve patient outcomes, detection of small, early pancreatic
neoplasms in the
asymptomatic patient is necessary.
104261 Imaging with a mAb-targeted approach, such as is described herein with
mAb-PAM4,
may provide for the diagnosis of these small, early cancers. Of prime
importance is the
specificity of the mAb. We have presented considerable data, including
135
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
immunohistochemical studies of tissue specimens (Gold et al., Clin Cancer Res
2007;13:7380-7; Gold et al., Int J Cancer 1994;57:204-10) and immunoassay of
patient sera
(Gold et al., J Clin Oncol 2006;24:252-8), to show that mAb-PAM4 is highly
reactive with a
biomarker, the presence of which provides high diagnostic likelihood of
pancreatic neoplasia.
Furthermore, we determined that PAM4, although not reactive with normal adult
pancreatic
tissues nor active pancreatitis, is reactive with the earliest stages
ofneoplastic progression
within the pancreas (pancreatic intraepithelial neoplasia 1 and intraductal
papillary mucinous
neoplasia) and that the biomarker remains at high levels of expression
throughout the
progression to invasive pancreatic adenocarcinoma (Gold et al., Clin Cancer
Res
2007;13:7380-7). Preclinical studies with athymic nude mice bearing human
pancreatic
tumor xenografts have shown specific targeting of radiolabeled murine,
chimeric, and
humanized versions of PAM4.
104271 In the current studies, we have examined a next-generation,
recombinant, bispecific
PAM4-based construct, TF10, which is divalent for the PAM4 arm and monovalent
for the
anti-HSG hapten arm. There are several important characteristics of this
pretargeting system's
constructs, named DOCK-AND-LOCK', including its general applicability and ease
of
synthesis. However, for the present consideration, the major differences from
the previously
reported chemical construct are the valency, which provides improved binding
to tumor
antigen, and, importantly, its pharmacokinetics. TF10 clearance from nontumor
tissues is
much more rapid than was observed for the chemical conjugate. Time for blood
levels of the
bispecific constructs to reach less than 1% ID/g was 40 hours postinjection
for the chemical
construct versus 16 hours for TF10. A more rapid clearance of the pretargeting
agent has
provided a vast improvement of the tumor/blood ratio, while maintaining high
signal strength
at the tumor site (%ID/g).
104281 In addition to providing a means for early detection and diagnosis, the
results support
the use of the TF10 pretargeting system for cancer therapy. Consideration of
the effective
radiation dose to tumor and nontumor tissues favors the pretargeting method
over directly
radiolabeled PAM4-IgG. The dose estimates suggest that the two delivery
systems have
different dose-limiting toxicities: myelotoxicity for the directly
radiolabeled PAM4 versus the
kidney for the TF10 pretargeting system. This is of significance for the
future clinical
development of radiolabeled PAM4 as a therapeutic agent.
104291 Gemcitabine, the frontline drug of choice for pancreatic cancer, can
provide
significant radiosensitization of tumor cells. In previous studies, we showed
that
136
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
combinations of gemcitabine and directly radiolabeled PAM4-IgG provided
synergistic
antitumor effects compared with either arm alone (Gold et al., Clin Cancer Res
2004,
10:3552-61; Gold et al., Int i Cancer 2004, 109:618-26). The dose-limiting
factor with this
combination was overlapping hematologic toxicity. However, because the dose-
limiting
organ for TF10 pretargeting seems to be the kidney rather than hematologic
tissues,
combinations with gemcitabine should be less toxic, thus allowing increased
administration
of radioisotope with consequently greater antitumor efficacy.
104301 The superior imaging achieved with TF10 pretargeting in preclinical
models, as
compared with directly radiolabeled DOTA-PAM4-IgG, provides a compelling
rationale to
proceed to clinical trials with this imaging system. The specificity of the
tumor-targeting mA.b
for pancreatic neoplasms, coupled with the bispecific antibody platform
technology providing
the ability to conjugate various imaging compounds to the HSG-hapten-peptide
for SPECT
(It 'In), PET (68Ga), ultrasound (Au), or other contrast agents, or for that
matter 90Y or other
radionuclides for therapy, provides high potential to improve overall patient
outcomes
(Goldenberg et al., J Nucl Med 2008, 49:158-63). In particular, we believe
that a TF 10-based
ImmunoPET procedure will have major clinical value to screen individuals at
high-risk for
development of pancreatic cancer (e.g., genetic predisposition, chronic
pancreatitis, smokers,
etc.), as well as a means for follow-up of patients with suspicious abdominal
images from
conventional technologies and/or with indications due to the presence of
specific
biomarker(s) or abnormal biochemical findings. When used as part of an ongoing
medical
plan for following these patients, early detection of pancreatic cancer may be
achieved.
Finally, in combination with gemcitabine, TF10 pretargeting may provide a
better opportunity
for control of tumor growth than directly radiolabeled PAM4-IgG.
Example 24. Therapy of Pancreatic Cancer Xenografts with Gemcitabine and
"Y-Labeled Peptide Pretargeted Using TIFIO
Summwy
104311 90Y-hPAM4 IgG is currently being examined in Phase I/II trials in
combination with
gemcitabine in patients with Stage I1JJIV pancreatic cancer. We disclose a new
approach for
pretargeting radionuclides that is able to deliver a similar amount of
radioactivity to
pancreatic cancer xenografts, but with less hematological toxicity, which
would be more
amenable for combination with gemcitabine. Nude mice bearing ¨0.4 cm3 sc
CaPanl human
pancreatic cancer were administered a recombinant bsMAb, TF 10, followed 1 day
later with
a 9 Y-labeled hapten-peptide (IMP-288). Various doses and schedules of
gemcitabine were
137
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
added to this treatment, and tumor progression monitored up to 28 weeks. 0.7
mCi PT-RAIT
alone produce only a transient 60% loss in blood counts, and animals given 0.9
mCi of PT-
RATT alone and 0.7 mCi PT-RAIT +6 mg gemcitabine (human equivalent ¨1000
mg/m.2)
had no histological evidence of renal toxicity after 9 months. A single dose
of 0.25 or 0.5
mCi PT-RAIT alone can completely ablate 20% and 80% of the tumors,
respectively.
Monthly fractionated PT-RAIT (0.25 mCi/dose given at the start of each
gemcitabine cycle)
added to a standard gemcitabine regimen (6 mg wkly x 3; 1 wk oft repeat 3
times)
significantly increased the median time for tumors to reach 3.0 cm3 over PT-
RAIT alone.
Other treatment plans examining non-cytotoxic radioseasitizing dose regimens
of
gem.citabine added to PT-RATT also showed significant improvements in
treatment response
over PT-RATT alone. The results show that PT-RATT is a promising new approach
for
treating pancreatic cancer. Current data indicate combining PT-RAIT with
gemcitabine will
enhance therapeutic responses.
Methods
104321 TF10 bispecific antibody was prepared as described above. For
pretargeting, TF10
was given to nude mice bearing the human pancreatic adenocarcinoma cell line,
CaPanl.
After allowing sufficient time for TF10 to clear from the blood (16h), the
radiolabeled
divalent TISG-peptide was administered. The small molecular weight IISG-
peptide (-1.4 kD)
clears within minutes from the blood, entering the extravascular space where
it can bind to
anti-HSG arm of the pretargeted TF10 bsMAb. Within a few hours, >80% of the
radiolabeled
HSG-peptide is excreted in the urine, leaving the tumor localized peptide and
a trace amount
in the normal tissues.
Results
104331 FIG. 19 illustrates the therapeutic activity derived from a single
treatment of
established (-0.4 cm3) CaPanl tumors with 0.15 mCi of 90Y-hPAM4 IgG, or 0.25
or 0.50
mCi of TF10-pretargeted9 Y-IMP-288. Similar anti-tumor activity was observed
for the 0.5-
mCi pretargeted dose vs. 0.15-mCi dose of the directly radiolabeled IgG, but
hematological
toxicity was severe at this level of the direct conjugate (not shown), while
the pretargeted
dose was only moderately toxic (not shown). Indeed, the MTD for pretargeting
using 90Y-
IMP-288 is at least 0.9 mCi in nude mice.
104341 FIG. 20 shows that the combination of gemcitabine and PT-RAIT has a
synergistic
effect on anti-tumor therapy. IIuman equivalent doses of 1000 mg/m2 (6 mg) of
gemcitabine
(GEM) were given intraperitoneally to mice weekly for 3 weeks, then after
resting for 1
138
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
week, this regimen was repeated 2 twice. PT-RAIT (0.25 mCi of TF10-pretargeted
9 Y-IMP-
288) was given 1 day after the first GEM dose in each of the 3 cycles of
treatment. Gem
alone had no significant impact on tumor progression (survival based on time
to progress to
3.0 cm3). PT-RAIT alone improved survival compared to untreated animals, but
the
combined GEM with PT-RAIT regimen increased the median survival by nearly 10
weeks.
Because hematological toxicity is NOT dose-limiting for PT-RAIT, but it is one
of the
limitations for gemcitabine therapy, these studies suggest that PT-RAIT could
be added to a
standard GEM therapy with the potential for enhanced responses. The
significant synergistic
effect of gemcitabine plus PT-RAIT was surprising and unexpected.
104351 A further study examined the effect of the timing of administration on
the potentiation
of anti-tumor effect of gemcitabine plus PT-RAIT. A single 6-mg dose of GEM
was given
one day before or 1 day after 0.25 mCi of TF10-pretargeted 90Y-IMP-288 (not
shown). This
study confirmed what is already well known with GEM, i.e., radiosensitization
is best given
in advance of the radiation. Percent survival of treated mice showed little
difference in
survival time between PT-RATT alone and PT-RAIT with gemcitabine given 22
hours after
the radiolabeled peptide. However, administration of gemcitabine 19 hours
prior to PT-RAIT
resulted in a substantial increase in survival (not shown).
104361 Single PT-RAIT (0.25 mCi) combined with cetuximab (1 mg weekly ip; 7
weeks) or
with cetuximab + GEM (6 mg weekly x 3) in animals bearing CaPanl showed the
GEM 4-
cetuximab combination with PT-RAIT providing a better initial response (FIG.
21), but the
response associated with just cetuximab alone added to PT-RAIT was encouraging
(FIG. 21),
since it was as good or better than PT-RAIT + GEM. Because the overall
survival in this
study was excellent, with only 2 tumors in each group progressing to >2.0 cm3
after 24
weeks when the study was terminated, these results indicate a potential role
for cetuximab
when added to PT-RAIT.
Example 25. Effect of Fractionated Pretargeted Radioimmunotherapy (FT-
RAH) for Pancreatic Cancer Therapy
104371 We evaluated fractionated therapy with "Y-DOTA -di-HSG peptide (IMP-
288) and
TF10. Studies using TF10 and radiolabeled IMP-288 were performed in nude mice
bearing
s.c. CaPanl human pancreatic cancer xenografts, 0.32-0.54cm3. For therapy,
TF10-
pretargeted 90Y-IMP-288 was given [A] once (0.6mCi on wk 0) or [B]
fractionated (0.3 mCi
on wks 0 and 1), [C] (0.2 mCi on wks 0, 1 and 2) or [D] (0.2 mCi on wks 0, 1
and 4).
139
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
104381 Tumor regression (>90%) was observed in the majority of mice, 9/10,
10/10, 9/10 and
8/10 in groups [A], [B], [C] and [D], respectively. In group [A], maximum
tumor regression
in 50 % of the mice was reached at 3.7 wks, compared to 6.1, 8.1 and 7.1 wks
in [B], [C] and
[14 respectively. Some tumors showed regrowth. At week 14, the best
therapeutic response
was observed in the fractionated group (2x0.3 mCi), with 6/10 mice having no
tumors (NT)
compared to 3/10 in the 3x0.2 mCi groups and 1/10 in the lx 0.6mCi group. No
major body
weight loss was observed. Fractionated PT-RAIT provides another alternative
for treating
pancreatic cancer with minimum toxicity.
Example 26. 90Y-hPAM4 Radioimmunotherapy (RAIT) Plus Radiosensitizing
Genncitabine (GEM) Treatment in Advanced Pancreatic Cancer (PC)
104391 90Y-hPAM4, a humanized antibody highly specific for PC, showed
transient activity
in patients with advanced disease, and GEM enhanced RAIT in preclinical
studies. This study
evaluated repeated treatment cycles of9 Y-hPAM4 plus GEM in patients with
untreated,
unresectable PC. The 90Y-dose was escalated by cohort, with patients repeating
4-wk cycles
(once weekly 200 mg/m2 GEM, 90Y-hPAM4 once-weekly wks 2-4) until progression
or
unacceptable toxicity. Response assessments used CT, FDG-PET, and CA19.9 serum
levels.
104401 Of 8 patients (3F/5M, 56-72 y.o.) at the l 2 dose levels (6.5 and 9.0
mCilm290Y-
hPAM4 x 3), hematologic toxicity has been transient Grade 1-2. Two patients
responded to
initial treatment with FDG Shy and CA19.9 decreases, and lesion regression by
CT. Both
patients continue in good performance status now after 9 and 11 mo. and after
a total of 3 and
4 cycles, respectively, without additional toxicity. A 3rd patient with a
stable response by PET
and CT and decreases in CA19.9 levels after initial treatment is now
undergoing a 2nd cycle.
Four other patients had early disease progression and the remaining patient is
still being
evaluated. Dose escalation is continuing after fractionated RAIT with 90Y-
ITAM4 plus low-
dose gemeitabine demonstrated therapeutic activity at the initial 90Y-dose
levels, with
minimal hematologic toxicity, even after 4 therapy cycles.
Example 27. Early Detection of Pancreatic Carcinoma Using Mab-PAM4 and In
Vitro immunoassay
104411 Immunohistochemistry studies were performed with PAM4 antibody. Results
obtained with stained tissue sections showed no reaction of PAM4 with normal
pancreatic
ducts, ductules and acinar tissues (not shown). in contrast, use of the MA5
antibody applied
to the same tissue samples showed diffuse positive staining of normal
pancreatic ducts and
acinar tissue (not shown). In tissue sections with well differentiated or
moderately
140
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
differentiated pancreatic adenocarcinoma, PAM4 staining was positive, with
mostly
cytoplasmic staining but intensification of at the cell surface. Normal
pancreatic tissue in the
same tissue sections was unstained.
104421 Table 17 shows the results of immunohistochemical analysis with PAM4
MAb in
pancreatic adenocarcinoma samples of various stages of differentiation.
Overall, there was an
87% detection rate for all pancreatic cancer samples, with 100% detection of
well
differentiated and almost 90% detection of moderately differentiated
pancreatic cancers.
Table 17 PAM4 Labeling Pattern
Cancer n Focal Diffuse Total
Well Diff. 13 2 11 13 (100%)
Moderately Diff. 24 6 15 21 (88%)
Poorly Diff. 18 5 9 14 (78%)
Total 55 13 35 48(87%)
104431 Table 18 shows that PAM4 immunohistochemical staining also detected a
very high
percentage of precursor lesions of pancreatic cancer, including PanIn-1A to
PanIN-3, IPMN
(intraductal papillary mucinous neoplasms) and MCN (mucinous cystic
neoplasms). Overall,
PAM4 staining detected 89% of all pancreatic precursor lesions. These results
demonstrate
that PAM4 antibody-based immunodetection is capable of detecting almost 90% of
pancreatic cancers and precursor lesions by in vitro analysis. PAM4 expression
was observed
in the earliest phases of PanIN development. Intense staining was observed in
IPMN and
MCN samples (not shown). The PAM4 epitope was present at high concentrations
(intense
difflise stain) in the great majority of pancreatic adenocarcinomas. PAM4
showed diffuse,
intense reactivity with the earliest stages of pancreatic carcinoma precursor
lesions, including
PanIN-1, IPMN and MCN, yet was non-reactive with normal pancreatic tissue.
Taken
together, these results show that diagnosis and/or detection with the PAM4
antibody is
capable of detecting, with very high specificity, the earliest stages of
pancreatic cancer
development.
Table 18 PAM4 Labeling Pattern
Focal Diffuse Total
PanIn- IA 27 9 15 24 (89%)
Panin-1B 20 4 16 20 (100%)
Partin-2 11 6 4 10(91%)
PanIn-3 5 2 0 2 (40%)
141
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Total PanIn 63 21 35 56 (89%)
IPMN 36 6 25 31 (86%)
MCN 27 3 22 25 (92%)
104441 An enzyme based immunoassay for PAM4 antigen in serum samples was
developed.
FIG. 22 shows the results of differential diagnosis using PAM4 immunoassay for
pancreatic
cancer versus normal tissues and other types of cancer. The results showed a
sensitivity of
detection of pancreatic cancer of 77.4%, with a specificity of detection of
94.3%, comparing
pancreatic carcinoma (n=53) with all other specimens (n=233), including
pancreatitis and
breast, ovarian and colorectal cancer and lymphoma. The data of FIG. 22 are
presented in
tabular form in Table 19.
Table 19. PAM4-Reactive MUC5AC in Patient Sera
Mean SD Median Range # Positive
(V)
Normal 43 0.1 0.3 0.0 0-2.0 0 (0)
Pancreatifis 87 3.0 11.5 0.0 0-63.6 4 (5)
Pancreatic CA 53 171 317 31.7 0-1000 41(77)
Colorectal CA 36 3.3 7.7 0.0 0-37.8 5 (14)
Breast CA 30 3.7 10.1 0.0 0-53.5 2 (7)
Ovarian CA 15 1.8 4.3 0.0 0-16.5 1(7)
Lymphoma 19 12.3 44.2 0.0 0-194 1 (5)
104451 An ROC curve (not shown) was constructed with the data from Table 19.
Examining
a total of 283 patients, including 53 with pancreatic carcinoma, and comparing
the presence
of circulating MUC5AC in patients with pancreatic cancer to all other samples,
the ROC
curve provided an AUC of 0.88 0.03 (95% ci, 0.84-0.92) with a P value
<0.0001, a highly
significant difference for discrimination of pancreatic carcinoma from non-
pancreatic
carcinoma samples. Comparing pancreatic CA with other tumors and normal
tissue, the
PAM4 based serum assay showed a sensitivity of 77% and a specificity of 95%.
104461 A comparison was made of MUC5AC concentration in serum samples from
normal
patients, "early" (stage 1) pancreatic carcinoma and all pancreatic carcinoma
samples. The
specimens included 13 sera from healthy volunteers, 12 sera from stage-1, 13
sera from
stage-2 and 25 sera from stage-3/4 (advanced) pancreatic carcinoma. A cutoff
value of 8.8
142
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
.units/m1 (horizontal line) was used, as determined by ROC curve statistical
analysis. The
frequency distribution of PAM4 antigen concentration is shown in FIG. 23.
which shows that
92% of "early" stage-1 pancreatic carcinomas were above the cutoff line for
diagnosis of
pancreatic cancer. An ROC curve for the PAM4 based assay is shown in FIG. 24,
which
demonstrates a sensitivity of 81.6% and specificity of 84.6% for the PAM4
assay in detection
of pancreatic cancer.
104471 These results confirm that an enzyme immunoassay based on PAM4 antibody
binding
can detect and quantitate PAM4-reactive antigen in the serum of pancreatic
carcinoma
patients. The immunoassay demonstrates high specificity and sensitivity for
pancreatic
carcinoma. The majority of patients with stage 1 disease were detectable using
the PA.M.4
immunoassay.
104481 In conclusion, an immunohistology procedure employing PAM4 antibody
identified
approximately 90% of invasive pancreatic carcinoma and its precursor lesions,
Pan1N,1PMN
and MCN. A PAM.4 based enzyme immunoassay to quantitate MUC5AC in human
patient
sera showed high sensitivity and specificity for detection of early pancreatic
carcinoma. Due
to the high specificity of PAM4 for pancreatic carcinoma, the mucin biomarker
can also serve
as a target for in vivo targeting of imaging and therapeutic agents.
ImmunoPE'F imaging for
detection of "early" pancreatic carcinoma is of use for the early diagnosis of
pancreatic
cancer, when it can be more effectively treated. Use of radioimmunotherapy
with a
humanized PAM4 antibody construct, preferably in combination with a
radiosensitizing
agent, is of use for the treatment of pancreatic cancer.
Example 28. Further Studies of In Vitro Detection of PAM4 Antigen in Human
Serum
104491 In certain, embodiments, it is preferred to detect the presence of
MUC5AC and/or to
diagnose the presence of pancreatic cancer in a subject by in vitro analysis
of samples that
can be obtained by non-invasive techniques, such as blood, plasma or serum
samples. Such
ex vivo analysis may be preferred, for example, in screening procedures where
there is no a
priori reason to believe that an. individual has a pancreatic tumor in a
specific location. The
objective of the present study was to develop a reliable, accurate, serum-
based assay for
detection of pancreatic cancer at the earliest stages of the disease.
Summary
104501 A PAM4-based immunoassay was used to quantitate antigen in the serum of
healthy
volunteers (N-19), patients with known diagnosis of pancreatic adenocarcinoma
(N-68), and
143
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
patients with a primary diagnosis of chronic pancreatitis (N-29). Sensitivity
for the detection
of pancreatic adenocarcinoma was 82%, with a false-positive rate of 5% for the
healthy
controls. Patients with advanced disease had significantly higher antigen
levels than those
with early-stage disease (P<0.01), with a diagnostic sensitivity of 91%, 86%,
and 62% for
stage 3/4 advanced disease, stage-2, and stage-1, respectively. We also
evaluated chronic
pancreatitis sera, finding 38% positive for antigen. However, this observation
was discordant
with immunohistochemical findings that suggest the PAM4-antigen is not
produced by
inflamed pancreatic tissue. Furthermore, several of the serum-positive
pancreatitis patients,
for whom tissue specimens were available for pathological interpretation, had
evidence of
neoplastic precursor lesions. Immunohistochemistry of additional pancreatitis
specimens
showed 90% to be PAM4-negative with the remainder only weakly positive. This
suggested
that positive levels of PAM4-antigen within the serum are not derived from
inflamed
pancreatic tissues, but may be an early indicator of pancreatic cancer.
104511 These results show that the PAM4-serum assay may be used to detect eady-
stage
pancreatic adenocarcinoma, and that positive serum levels of PAM4-antigen are
not derived
from inflamed pancreatic tissues, but rather may provide evidence of
subclinical pancreatic
neoplasia.
Materials and Methods
104521 Human Specimens Sera (N-68) were obtained from patients with a
confirmed
diagnosis of pancreatic adenocarcinoma being treated at the Johns Hopkins
Medical Center,
Baltimore, MD, and stored frozen <5 yrs. Each of these patients underwent
surgical resection
of the pancreas, providing an opportunity for accurate diagnosis and staging.
For stage-1
disease, no neoplastic cells were observed outside of the pancreas. However,
patients with
pancreatic adenocarcinoma are likely to have undetected micrometastatic
disease at
presentation, including those patients reported with stage-1 disease. For this
reason, we
evaluated follow-up survival data. All patients described as having stage-1
disease survived
at least 1 year (time to last recorded follow-up visit), with a median
survival time of 2.70
years (25th percentile = 1.32 years) in comparison to the latest SEER data
(2002-2006), which
reports a 1.42-year median survival for patients having stage-1 disease
treated by surgical
resection.
104531 A total of 29 sera from patients with a diagnosis of chronic
pancreatitis were obtained
from the Johns Hopkins Medical Center and Zeptometrix Corp. (Franklin, MA).
Healthy
volunteers (N-19) provided blood for control specimens at the Center for
Molecular
144
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Medicine and Immunology. All specimens were de-identified, with the only
clinical data
provided to the investigators being the diagnosis, stage of disease, follow-up
survival time, and
size of the primaiy tumor.
104541 Reagents A human pancreatic mucin preparation was isolated from CaPanl,
a human
pancreatic cancer grown as xenografts in athymic nude mice. Briefly, 1 g of
tissue was
homogenized in 10 mL of 0.1 M ammonium bicarbonate containing 0.5 M sodium
chloride.
The sample was then centrifuged to obtain a supernatant that was fractionated
on a
SEPHAROSECt-4B-CL column with the void volume material chromatographed on
hydroxyapatite. The unadsorbed fraction was dialyzed extensively against
deionized water
and then lyophilized. A 1 mg/mi. solution was prepared in 0.01 M sodium.
phosphate buffer
(pH., 7.2) containing 0.15 M sodium chloride (phosphate-buffered saline
[PBS]), and used as
the stock solution for the immunoassay standards. A polyclonal, anti-mucin
antiserum was
prepared by immunization of rabbits, as described previously (Gold et al.,
Cancer Res
43:235-38, 1983). An IgG fraction was purified and assessed for purity by
sodium dodecyl
sulfate¨polyacrylamide gel electrophoresis (SDS-PAGE) and molecular-sieve high-
performance liquid chromatography. Murine MA5 antibody reactive with the MITI
protein
core was obtained from. Immunomedics, Inc. (Morris Plains, NJ). A. non-binding
isotype-
matched control antibody, Ag8, was purified from the P3X63-A.g8 murine
myeloma.
104551 Sample Preparation All assays were performed in a blinded fashion. To
prepare the
specimens for immunoassay, 3004 of serum were placed in a 2.0 mL
microcentrifuge tube
and extracted with an equal volume of 1-butanol. The tubes were vortexed
vigorously for 2
min at which time 300 L of chloroform were added and the tubes again vortexed
for 2 min;
this latter step was included in. the procedure in order to invert the aqueous
and organic
layers. The tubes were then centrifuged in a microfuge at a setting of 12,000
rpm for 5 min.
The top aqueous layer was removed to a clean tube and the sample diluted 1:2
in 2.0% (w/v)
casein-sodium salt in 0.1 M sodium phosphate buffer, pH 7.2, containing 0.15
M. sodium
chloride (PBS) for immunoassay.
104561 Enzyme immunoassay The immunoassay was performed in a 96-well polyvinyl
plate
that had been coated with 100 iL of humanized-PAM4 IgG at 20 p,,g/mL in PBS
with
incubation at 4 C overnight. The wells were then blocked by addition of 200
1.(1., of a 2.0%
(w/v) solution of casein in PBS and incubated for 1.5 h at 37 C. The blocking
solution was
removed from the wells and the plate washed 5-times with 250 1AL of PBS
containing 0.1%
(v/v) Tween-20. The standards, or unknown specimens, 1001.i.L. in triplicate,
were added to
145
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
the appropriate wells and incubated at 37 C for 1.5 h. The plate was then
washed 5-times
with PBS-Tween-20 as above.
104571 The polyclonal, rabbit anti-mucin antibody, diluted to 5 ti.g/mL in
1.00/0 (w/v) casein
in PBS containing 50 iig/mL non-specific, human IgG, was added to each well
and incubated
for 1 h at 37 C. The polyclonal antibody was then washed from the wells as
above, and
peroxidase-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch
Laboratories, West
Grove, PA), at a 1:2000 dilution in 1.0% (w/v) casein in PBS, also containing
50 gg/inL
human IgG, was added to the wells and incubated at 37 C for 1 h. After washing
the plate as
above, 100 pi of a 3,3',5,5'-tetramethylbenzidine substrate solution were
added to the wells
and incubated at room temperature for 30 min. The reaction was stopped by the
addition of
50 iL 4.0 N sulfuric acid, and the optical density read at a wavelength of 450
nm using a
SPECTRA-MAX 250 spectrophotometer (Molecular Devices, Sunnyvale, CA). Because
of
the considerable microheterogeneity of the PAM4 antigen, we chose to report
our results in
arbitrary units/mL, based on an initial reference standard purified from
xenografted CaPan-1
human pancreatic tumor.
104581 Immunohistochemisny Paraffin-embedded specimens obtained from the
Cooperative
Human Tissue Network were cut to 4 micron sections on superftost plus adhesive
slides
(Thermo Scientific, Waltham, MA). Tissue sections were then heated to 95 C for
20 min in a
pH 9.0 Tris buffer, Target Retrieval Solution (Dako, Carpinteria, CA), allowed
to cool to
room temperature, and then quenched with 3%11202 for 15 min at room
temperature.
Primary antibodies were then used at 1011g/mL with an ABC VECTASTAIN kit
(Vector
Laboratories, Burlingame, CA) for labeling the tissues. The slides were scored
independently
by two pathologists using a paradigm consistent with that reported for earlier
studies on
biomarkers in pancreatic adenocarcinoma (Gold et al., 2007, Clin Cancer Res
13:7380-87): 0-
negative, <1% of the tissue was labeled; 1-a weak, focal labeling of between
1% - 25% of the
tissue; 2-a strong, focal labeling of between 10/ - 25% of the tissue; 3-a
weak, diffuse
labeling >25% of the tissue; 4-a strong, diffuse labeling >25% of the tissue.
Only the
appropriate tissue components (e.g., adenocarcinoma cells, normal ducts, etc.)
were
considered for assessment.
104591 Statistical Analyses Standard curves were generated from the
immunoassay data,
with regression analyses performed to interpolate concentrations of the
unknown samples
(Prism 4.0 software, GraphPad, La Jolla, CA). Receiver operating
characteristic (ROC)
curves were generated by use of the Med-Calc statistical software package
(version 7.5)
146
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(Med-Calc, Mariakerke Belgium). Student's 1-test was used to compare variables
in any two
groups. The Cochran-Armitage test was used to detect a trend between detection
rates and
stage of disease.
Results
104601 Accuracy and precision of the immunoassay A set of control standards
with nominal
concentrations of 15.60, 6.20, 2.50, and 1.00 units/mL was evaluated on
several
nonconsecutive days (N=7) for determination of accuracy and precision. Curve
fitting for the
standards generally gave resultant goodness of fit values for r2 >0.990.
Accuracy was
calculated to be within 8% of the nominal value for the first three
concentrations, but fell to
approximately 22% for the 1.00 units/mL standard. Linear regression of nominal
vs.
measured units/mL in this series of controls gave a trend-line with a slope of
0.965 and y
intercept of 0.174 (r2 ¨ 0.999), where a slope of 1.00 with a y intercept of
0.00 would
constitute 100% accuracy (FIG. 25). An average absolute difference between
nominal and
recovered mass equal to 0.190 0.173 units/mL for the two lowest
concentration standards
suggested a minimum absolute error of approximately 0.2 units/mL for the EIA.
Values for
the coefficient of variation (CV) were 6.40%, 4.85%, 12.0%, and 66.4%,
respectively, for the
4 control standards. Taken together, the data suggest that the PAM4-
immunoassay provides
levels of accuracy and reproducibility that are within the guidelines
suggested for an
immunoassay measurement of an analyte; accuracy and precision were within 15%
for
concentrations above the cutoff value (2.40 units/mL), and within 20% at the
cutoff value. To
further test this, we examined 3 sera, two of which were from healthy
controls, on 3 separate
days. The two healthy controls gave average results of 0.27 :E 0.06 and 0.30
0.27 units/mL,
each of which was close to the minimum absolute error for the EIA with
consequent high CV
of 21.65 A and 88.19%, respectively. The other patient serum gave an average
of 19.45
2.51 units/mL with a CV of 12.9%.
104611 Ouantitation of antigen in patient sera In a preliminary study reported
in the Example
above, the PAM4 serum-based immunoassay had an apparent sensitivity of 77% and
a
specificity of 94% for pancreatic carcinoma. It should be noted that the
overwhelming
majority of cancer specimens of pancreatic and non-pancreatic origin had been
obtained from
patients enrolled in IRB-approved clinical trials conducted by the Garden
State Cancer Center
and stored frozen at -80 C for more than 10 yrs. However, the specimens of
pancreatitis had
been stored frozen for a considerably shorter time. We evaluated a new group
of 24 sera from
patients diagnosed with pancreatic adenocarcinoma. Only two of the sera had
levels of
147
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
PAM4-reactive antigen considered to be positive. Therefore, we considered and
evaluated
reasons why the immunoassay had not performed as expected, including the
quality of the
immunoassay reagents, the possibility that the antigen was being degraded
and/or removed
from the serum, its presence in the form of immune complexes, or being bound
by a blocking
substance. We discovered that there is a substance in fresh human serum and/or
specimens
stored frozen for short periods of time (<5 yrs) that apparently binds to the
PAM4-reactive
epitope and blocks its binding to PAM4 antibody, thus preventing detection by
immunoassay.
Percent recovery of antigen from fresh normal human serum (N-2) spiked with
PAM4-
antigen at concentrations from 5 ¨ 20 units/mL was on the order of 33% or
less.
104621 In a series of reports, Slomiany and co-workers disclosed that gastric
mucin had
covalenty bound and/or associated lipids and fatty-acids (Slomiany et al.,
1984, Arch
Biochem Biophys 229:560-67; Slomiany et al., 1986, Biochem Biophys Res Commun
141:387-93; Zalesna et al., 1989, Biochem int 18:775-84), and that these
lipids and fatty
acids had specific effects upon the physicochemical properties of the mucin.
Furthermore, it
was reported that fatty-acid synthetase levels and activity are significantly
elevated in
pancreatic adenocarcinoma, as is also the case for other forms of cancer and
other pathologic
conditions (Walter et al., 2009, Cancer Epidemiol Biomarkers Prey 19:2380-85).
Because the
blocking substance might be lipid in nature, we performed organic extraction
of sera from the
group of 24 pancreatic adenocarcinoma patients that had been stored frozen for
<5 years. As
was noted above, without prior extraction, only 2 of the 24 specimens (8.3%)
had levels of
PAM4-antigen that were considered positive, whereas after organic extraction,
22 of the 24
specimens (92%) had positive levels of the PAM4-antigen.
104631 We were also able to re-evaluate, from the study reported in the
Example above, 10
pancreatic adenocarcinoma patient sera that had been stored frozen for >15
years to confirm
the prior results. With or without extraction, all 10 specimens had levels of
antigen that were
considered to be positive . Regression analysis to compare paired results from
extracted and
non-extracted sera gave a trendline with slope of 1.10 (r2 = 0.94),
demonstrating that with or
without extraction of these long-term frozen sera, the results were similar.
It is considered
that long-term storage of the specimens resulted in degradation of the
inhibiting substance or
decreased binding to and unmasking of the epitope. All further testing of sera
was performed
with organic extraction of specimens prior to immunoassay.
104641 Specimens evaluated for PAM4-reactive antigen included 68 patients with
confirmed
pancreatic adenocarcinoma divided by stage: 21 from stage-1; 14 from stage-2;
and 33 from
148
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
stages-3 and -4 (advanced). In addition, 19 sera collected from healthy adult
volunteers and
29 patients diagnosed with chronic pancreatitis were included as control
groups. The
maximum concentration shown in the dot-plot (FIG. 26) was 80 units/mL, because
there
were insufficient volumes of sera to perform additional dilution studies.
Although a cutoff
value of 10.2 units/mL was reported in the Example above, because of the use
of an organic
extraction procedure, as well as differences in the EIA protocol (reagent
concentrations,
inclusion of human IgG in buffers), we chose to treat the current data set
independently of
prior results. A positive cutoff value of 2.4 units/mL was calculated by ROC
curve statistics
(FIG. 27) for the comparison of all pancreatic adenocarcinoma specimens versus
healthy
adults. The overall sensitivity for detection of pancreatic adenocarcinoma was
82%, with an
area under the curve of 0.92 0.03 (95% CI ¨ 0.84 ¨0.97). A.t this level of
sensitivity, a
false-positive rate of 5% was observed for the healthy control group, the
single positive case
having 3.65 units/nil, of circulating antigen, just above the cutoff value.
Insufficient volumes
of sera precluded CAI 9-9 immunoassays for comparison to the PAM4-immunoassay
results.
104651 As shown in Table 20, sensitivity for detection of early, stage-1
pancreatic
adenocarcinoma was relatively high, with 13 of 21 (62%) specimens above the
cutoff value.
As expected, this detection rate was lower than that observed for the stage-2
(86%) and
advanced stage-3 and -4 (91%) patient groups. A statistically significant
trend (P <0.01) was
noted for detection rate vs. stage of disease. We considered that this was
most likely due to
tumor size or burden. The average tumor sizes for stage-1, stage-2, and stage-
3/4 groups were
2.14 1.02 cm3, 3.36 1.18 cm3, and 3.45 1.06 cm3, respectively. While
there was no
statistically significant difference in tumor size between the stage-2 and -
3/4 groups (P
>0.41), a statistically significant difference was observed for each of these
two groups when
compared to stage-1 tumor size (P <0.004 or better). However, it should be
noted that
individual tumor size did not correlate with antigen concentration in the
serum (r2 =0.0065).
104661 Specimens reported as Stage-1 could be divided into stage-1A (N-13) and
stage-1B
(N=8) subgroups based on tumor size, with detection rates of 54% and 75%,
respectively;
however, caution is emphasized since the number of patients in each subgroup
is small. The
average tumor size for stage-1A was 1.41 0.58 cm3 (range: 0.4 cm3 ¨ 2.0 cm3)
and for
stage-1B was 3.15 I 0.44 cm3 (range: 2.5 cm3-- 4 cm3); P <0.001 for comparison
of the two
groups. While, on the whole, tumor sizes were smaller in stage-1A disease than
in stage-1B,
there was no apparent statistical correlation between individual tumor size
and concentration
of the PAM4-antigen in the blood (r2 = 0.03). Furthermore, it is important to
note that of the
149
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
13 stage-lA specimens, 4 of the 7 positive cases had PAM4-antigen levels
considerably
higher than the cutoff value, with a range of 17.65 - 32.65 units/mL.
Table 20. PAM4-reactive antigen in the sera of patients
Median T-test
True- Positive
(units/mi.) value)a
Total PC 68 9.85 81% <0.001
Stage-1 21 4.53 62% <0.002
---Stage- I A 13 3.96 54% <0.02
---Stage-1B 8 6.05 75% <0.02
Stage-2 1 10.39 86% <0.005
Stage-3/4 33 13.37 91% <0.001
Chronic Pancreatitis 29 1.28 (38% FP)
Ilealthy Volunteers 19 1.18 (5% FP)
a - All comparisons are to healthy volunteers
104671 We also evaluated a set of 29 patient sera with the primary diagnosis
of chronic
pancreatitis. At the 2.4 units/mL cutoff established by ROC evaluation of
normal and
pancreatic adenocarcinoma patients, 11 pancreatitis patients (38%) were
positive. ROC curve
analysis of pancreatitis sera compared directly to the pancreatic
adenocarcinoma specimens
gave an area under the curve of 0.77 0.05 (95% CI = 0.68 ¨ 0.85). The median
value for the
pancreatitis group was 1.28 units/mL, comparable to the healthy volunteer
group (1.18
units/mL), but considerably lower (3.5-fold) than the stage-1 pancreatic
adenocarcinoma
group (4.53 units/mL). It should be noted that our earlier results for
pancreatitis specimens
suggested a considerably lower false-positive rate, only 5%. However, those
pancreatitis
specimens were stored frozen for less than 5 years, and were not organic phase
extracted
prior to analysis.
104681 Biopsy and/or surgical specimens were available from 14 of the chronic
pancreatitis
specimens, 6 of which were from patients who were considered positive for
circulating
MUC5AC. In 3 of these 6 positive cases, precursor lesions were identified
within the tissue
sections. It was then considered whether the positive serum test was due to
pancreatitis or the
presence of neoplastic precursor lesions. We performed immunohistochemistry on
an
150
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
additional 30 biopsy specimens from patients diagnosed with pancreatitis. Of
the 30
specimens, one frank invasive pancreatic adenocarcinoma and one large PaniN-2-
3 lesion were
identified (in separate specimens) by use of PAM4 staining, while surrounding
acinar-ductal
metaplasia (ADM) and normal tissues were negative (data not shown). Of the
remaining 28
specimens, 19 had sufficient parenchyma to be evaluated, 16 of which had
evidence of ADM.
PAM4 was negative in all but two of these cases, and in each of these gave
only a very focal,
weak labeling of ADM within the specimens (data not shown).
104691 Validation studies - We have begun putting together a panel of well-
annotated serum
specimens from patients with known diagnoses. A first set of patient sera (N ¨
450) including
healthy individuals and patients having invasive pancreatic cancers
(carcinoma,
neuroendocrine, and other forms), benign disease of the pancreas (adenomatous
lesions,
pancreatitis, etc.) and non-pancreatic cancers and benign disease (biliary,
duodenal,
ampullary carcinomas, cholecystitis, gastritis, etc.) is being evaluated
(blind study) to both
confirm and extend the prior results on PAM4 specificity in a much larger
group of patients.
Table 21 presents an interim analysis based upon studies completed to date;
overall, the data
are remarkably similar to our earlier data. Employing a cutoff value
determined by ROC
analysis of PC vs Healthy Adults, the overall sensitivity for detection of
pancreatic carcinoma
was 80% at a specificity of 96%. Only 2 of 16 neuroendocrine tumors were
positive, just over
the cutoff value.
104701 To date, 14 of 53 (26%) patients with primary diagnosis of pancreatitis
have been
identified as PAM4 positive, lower than that reported in our recent
publication. We are now
attempting to correlate clinical data with results in this pancreatitis group,
as well as provide
for clinical and laboratory follow-up of these patients. Only 2 of 11 patients
with benign
adenomatous lesions (both cystadenoma) were considered positive. One other
cystadenoma
had PAM4-antigen levels greater than 200 unitsimL. The pathology report
describes the
biopsy as "very suspicious for cancer".
Table 21. PAW-reactive antigen in the sera of patients
Positive ROC-AUCb __ P
N Median' Mean SDa
(%) (95% Cl)
Pancreatic
Carcinoma 145 7.84 35.61 64.58 80 (comparisons are to PC)
Neuroendocrine 16 0.00 1.73 4.91 12.5
Healthy 27 0.40 0.54 0.53 3.7 0.90 0.02 <0.0001
151
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(0.85 ¨ 0.94)
0.85 0.28
Pancreatitis 53 0.37 1.56 3.35 26 <0.0001
(0.79 ¨ 0.90)
Pancreatic 0.90 0.03
11 0.00 0.64 0.78 18 <0.0001
Adenoma (0.84 ¨ 0.94)
a ¨ Values for Median and M.ean Si) are in Units/ml,
b ¨ Receiver Operating Characteristic Curves (ROC); Area Under the Curve
(AIJC) with
values for 95% Confidence Intervals presented.
Discussion
104711 Studies reported in the Example above that employed both
immunohistology of tissue
specimens and EIA of circulating antigen demonstrated that the PAM4-reactive
epitope is a
biomarker for invasive pancreatic adenocarcinoma and is expressed at the
earliest stages of
pancreatic neoplasia (i.e., PanIN-1). It was not detectable within normal
pancreatic tissues
(ducts, acinar and islet cells), nor the majority of non-pancreatic cancers
examined (breast,
lung, gastric, and others). Thus, an elevation of the PAM4-epitope
concentration in the serum
provided a high positive likelihood ratio of 16.8 for pancreatic
adenocarcinoma. Missing
from the prior study was clinical information regarding the stage of disease.
Consequently,
we could not evaluate the value of the immunoassay for detection of
potentially curable early
disease until now.
104721 We report herein that PAM4-based EIA using serum samples can detect
patients
having early-stage pancreatic adenocarcinoma, and can provide accurate
discrimination from
disease-free individuals. The assay's sensitivity for detection of early
pancreatic
adenocarcinoma was 62% for patients with stage-1 and 86% for patients with
stage-2 disease
and serum levels generally increased with advancing stage of disease. A high
percentage of
patients with stage-1 and -2 disease are clinically asymptomatic. We conclude
that detection
of tumor growth at these early stages using a PAM4 serum assay could provide
improved
prospects for survival.
104731 The cancer patients in this study all underwent surgical resection,
providing an
opportunity to accurately stage each patient. However, many patients with
pancreatic cancer
are suspected of having micrometastatic disease at presentation, even if they
do not have
histologically-apparent regional lymph node involvement. This highlights a
general problem
in the study of early detection, particularly with a low-incidence disease
such as pancreatic
adenocarcinoma. The accrual of specimens that are well-defined is problematic.
Further
152
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
complicating the issue is that many of these pancreatic cancers occur in the
presence of
chronic pancreatitis, cholecystitis, and neoplastic precursor lesions, amongst
other conditions.
104741 Of 29 sera with a primary diagnosis of chronic pancreatitis, 38% were
identified as
positive for PAM4-antigen. However, several of these serum-positive patients,
for whom
tissue specimens for pathological interpretation were available, had evidence
of neoplastic
precursor lesions. Furthermore, a discrepancy was observed in the comparison
of tissue
reactivity by immunohistology and serum levels of antigen by immunoassay. By
immunohistochemistry, only 10% of the evaluable specimens showed evidence of
PAM4
staining within the ADM, although this was at considerably lower intensity
than observed for
the overwhelming majority of pancreatic adenocarcinoma specimens (Gold et al.,
2007, Clin
Cancer Res 13:7380-87). Therefore, the results suggest that positive levels of
PAM4-antigen
within the serum may not be derived from inflamed pancreatic tissues, but
rather could
provide evidence of subclinical pancreatic neoplasia, such as Pan1N lesions,
and that, at the
very least, positive results provide the rationale for clinical follow-up of
these patients.
104751 Findings from genetically-engineered animal models of pancreatic
adenocarcinoma
suggest that human pancreatic neoplasia may arise before the PanIN-1 lesion
(Leach, 2004,
Cancer Cell 5:7-11). ADM was the earliest change observed in the mutant KRAS
targeted
model described by Zhu et al. (2007, Am J Pathol 171:263-73). On the other
hand, Shi et al.
(2009, Mol Cancer Res 7:230-36) reported that although KRAS gene mutations can
occur
within ADM, they occur predominantly within ADM that are associated with Pan1N
lesions.
The authors suggest this may occur by retrograde extension of the PanIN to the
surrounding
ADM. As yet, there is no conclusive evidence that ADM progress to PanIN. The
fact that
PAM4 is reactive with ADM in two patients with pancreatitis is of interest.
104761 At the present time, screening the general population for pancreatic
cancer is not
considered medically or economically worthwhile, because the disease is simply
too
infrequent. However, there is considerable interest in screening patients
predicted to have an
increased risk of developing pancreatic adenocarcinoma. Several studies have
demonstrated
that screening individuals with strong family histories of pancreatic cancer
can identify
precursor neoplasms of the pancreas that are amenable to surgical resection
(Canto et al.,
2006, Clin Gastroenterol Hepatol 4:766-81; Canto, 2005, Clin Gast-roenterol
Hepatol 3:S46-
58; Brentnall et al., 1999, Ann Intern Med 131:247-55). For example, relatives
of pancreatic
cancer patients have a significantly higher risk of developing pancreatic
cancer than the
general population (Shi et al., 2009, Arch Pathol Lab Med 133:365-74). A small
percentage
153
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
of patients with familial pancreatic cancer harbor mutations of PALB2 (partner
and localizer
of BRCA2), a susceptibility gene for pancreatic cancer (Tischkowitz et al.,
2009.
Gastroenterology 137:1183-86). Similarly, patients with long-standing chronic
pancreatitis
are at increased risk of developing pancreatic cancer, and the risk is over
30%, among
patients with early-onset (teenage) hereditary pancreatifis (Lowenfels et al.,
1993, New Eng J
Med 328:1433-37; Lowenfels et al., 1997, J Nati Cancer Inst 89:442-46). A 20-
to 34-fold
higher risk has been observed in individuals with familial atypical multiple
mole (FAMMM)
syndrome (Rutter et al., 2004, Cancer 101:2809-16). Also, several studies have
shown a
significantly increased risk of developing pancreatic cancer in diabetic
individuals who meet
certain criteria (Pannala et al., 2009, Lancet Oncol 10:88-95). Longitudinal
surveillance of
these patients by use of the PAM4-immunoassay may provide for early detection
of
neoplasia. A second potential use of the immunoassay could be as a means to
detect
recurrence of disease post-therapy, and in particular, following surgical
resection for those
patients where the tumor is supposedly confined to the pancreas.
104771 The relatively high specificity of the PAM4 antibody provides a means
to target both
imaging and therapeutic agents with high tumor uptake and high tumor/nontumor
ratios. We
have demonstrated PAM4's potential as both a directly-radiolabeled or
bispecific,
pretargeting reagent for nuclear imaging and radioimmunotherapy of pancreatic
cancer. Also,
initial results of a clinical phase lb trial to evaluate a fractionated dosing
of 90Y-PAM4 whole
IgG (clivatuzumab tetraxetan), in combination with a radiosensitizing regimen
of
gemcitabine, were reported recently (Pennington et al., 2009, J Clin Oncol
27:15s, abstract
4620). Of 22 patients with stage-3/4 disease (mostly stage-4), 68% showed
evidence of
disease control, with 23% of patients having partial responses based on RECIST
criteria.
Thus, positive results by the PAM4-based immunoassay provides a rationale to
pursue
PAM4-targeted imaging and therapy, thus providing a personalized therapy.
104781 The PAM4-based immunoassay can identify the majority of pancreatic
adenocarcinoma patients of all stages. Although a direct comparison with
CA19.9 was not
possible in the current study, a prior comparison of the two biomarkers in a
limited set of
pancreatic adenocarcinoma sera (N=41) demonstrated a statistically significant
difference
(1)-(0.01) with PAM4-antigen levels positive in 71% of patient specimens and
CA19.9-
antigen levels positive in 59% of specimens. In general, it is thought that
CA19.9 lacks the
sensitivity and specificity to provide for early detection and/or diagnosis of
pancreatic
adenocarcinoma. However, the assay does have its use for management with
continued
154
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
elevation in CA19.9 serum levels post treatment indicative of a poor
prognosis. Similarly, we
recently reported in abstract form (Pennington et al., 2009, J Clin Oncol
27:15s, abstract
4620), the use of circulating PAM4-antigen levels for prediction of anti-tumor
response.
104791 These results show that the conditions under which specimens are stored
(e.g., the
length of time they are kept frozen) can have significant effects upon
accessibility of the
epitope under study. For the PAM4-based immunoassay, a fatty acid or lipid
substance may
be able to bind the specific epitope and interfere with the immunoassay.
However, it is also
possible this material was a low-molecular weight peptide or other substance
soluble in
organic solvents. The ability to remove this substance by organic extraction
of the serum
makes the PAM4-immunoassay reproducible. In addition, the question is raised
as to the
biological significance of the circulating inhibitorMIJC5AC interaction.
However, when
using the PAM4 antibody as an in vivo targeting agent (e.g.,
radioimmunotherapy), the
presence of circulating PAM4-antigen is not a factor, since targeting of
radiolabeled-PAM4
to sites of tumor growth has been observed in the majority of patients
evaluated to date. Thus,
it appears that the PAM4-antigen within tumor is free of the blocking
substance.
Example 29. Phase IBM Study of "Y-Labeled hPAM4 Antibody and
Gemcitabine in Advanced Pancreatic Cancer
104801 A phase IBIll study of "Y-labeled hPAM4 antibody (clivatuzumab
tetraxetan) in
advanced pancreatic cancer patients was performed. A total of 100 patients
with previously
untreated Stage III or IV pancreatic cancer were enrolled into this open-label
trial to receive
gemcitabine once-weekly x 4 with "Y-clivatuznmab tetraxetan on weeks 2, 3 and
4 (therapy
cycle). The therapy cycle could be repeated until disease progression or until
the patient
displayed unacceptable toxicity. Ten patients withdrew early, while 90
patients, of whom 82
had the Stage IV (metastatic) disease, received 1 ¨ 4 therapy cycles. Tumor
responses were
assessed by CT, FDO/PET and serum CA19.9 after each cycle (initially every 4
wks).
104811 In Part I of this study, 38 patients were treated with "Y-clivattrzumab
tetraxetan at
6.5, 9, 12 or 15 mCi/m2 x 3, and a low, fixed gemcitabine dose of 200 mg/m2 x
4 for
radiosensitization. Thirteen patients were retreated with the same cycle 1 - 3
times. The
overall disease control rate, which included complete response (CR), partial
response (PR.)
and stable disease (SD), by CT-based RECIST criteria, was 58%, including 6
patients (16%)
with PR and 16 patients (42%) with SD as best response.
104821 The median overall survival (OS) for the 38 treated patients was 7.7
months, which
compares favorably with other regimens for advanced pancreatic cancer. At the
higher
155
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
therapy doses (12 and 15 mCi/m2 of 90Y-clivatuzumab tetraxetan x 3), a median
OS of 8.0
months was noted. For the 13 patients who received repeated cycles of the
combination
therapy, median OS improved to 11.8 months. Extended survival of up to 14.8
months post
therapy onset has been observed, with 8 patients achieving a survival > 6
months (3 patients
>1 yr). Anecdotal reports indicate performance status and pain level improved
with therapy.
104831 Fifty-two patients who were treated in Part II of this study received 3
weekly 90Y
doses of 12 mCi/m.2 and gemcitabine doses of 200, 600 or 1000 mg/m2x 4, with
14 patients
receiving repeated therapy cycles at the same gemcitabine dose but 9 Y doses
of 6.5, 9 or 12
mCilm2. Results were available from 47 of the 52 patients. The disease control
rate for the
200 mg/m2 group was 72%, with 19% PR and 53% SD. For the 600 and 1000 mg/m2
groups,
the disease control rates were 63% (0% PR) and 68% (18% PR), respectively.
Higher
gemcitabine doses appeared to offer no advantage in treatment response over
the lowest dose
of 200 mg/m2. At the time of reporting, survival data were not available for
this group of
patients. Treatments were well tolerated with no infusion reactions to
radiolabeled
clivatuzumab and few non-hematologic side effects. Hematologic suppression was
transient
after cycles 1 and 2.
104841 These results showed that repeated cycles of fractionated doses of
clivatuzumab
tetraxetan, labeled with yttrium-90 (90Y) and given in combination with
gemcitabine,
demonstrated therapeutic activity in patients with advanced, inoperable,
pancreatic cancer.
Therapy with repeated cycles of clivatuzumab tetraxetan plus low-dose
gemcitabine
improved overall survival over single-cycle therapy in patients with locally
advanced or
metastatic pancreatic cancer.
Example 30. Detection of Early-Stage Pancreatic Ductal Adenocarcinoma
(PDAC): Sensitivity, Specificity, and Discriminatory Properties of Serum-Based
PAM4-Immunoassay
104851 As disclosed in Example 28, a serum-based enzyme immunoassay employing
the
PAM4 antibody was able to correctly identify 81% of patients with known PDAC
and this
assay had promising sensitivity for detecting early-stage disease. These
findings have been
extended in a much larger patient population that included over 600 sera from
both malignant
and benign diseases of the pancreas and surrounding tissues. In a blinded
analysis, sera from
patients with confirmed PDAC (N=298), other cancers (N=99), benign disease of
the
pancreas (N-126), and healthy adults (N=79) were evaluated by enzyme
immunoassay for
concentration of PAM4-antigen levels.
156
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
104861 Overall sensitivity for detection of PDAC was 76%, with 64% of stage-I
patients
testing positive and a higher sensitivity (85%) for advanced disease. For the
most part, sera
from patients with neuroendocrine tumors of the pancreas or cancers of other
origin
(squamous, GIST, etc.) did not have elevated levels of the PAM4-antigen.
Approximately
half of the patients with ampullary (48%) and extrahepatic biliary (50%)
adenocarcinomas
had positive levels of circulating PAM4-antigen. Of 126 patients diagnosed
with benign
conditions of the pancreas, only 24 (19%) were positive and, in particular, 18
of 80 (23%)
patients with chronic pancreatitis (CP) were positive. ROC curve analysis
demonstrated a
statistically significant difference between the PDAC and CP groups (P
<0.0001), with an
area under the curve of 0.84 0.02 (95% CI: 0.79 ¨ 0.89). The positive- and
negative-
likelihood ratios for differentiating PDAC from benign conditions of the
pancreas were 4.00
and 0.30, respectively.
104871 In conclusion, the PAM4-immunoassay detected nearly two-thirds of stage-
1 PDAC
patients, and did so with high. discriminatory power with respect to benign
pancreatic disease.
The results provide a rationale for longitudinal surveillance of patients
considered at high-risk
for PDAC (e.g., familial pancreatic cancer, new-onset diabetes, etc.) with the
PAM4 assay.
Example 31. PAM4-Based Assay Differentiates Pancreatic Ductal
Adenocarcinoma (PDAC) From Chronic Pancreatitis and Benign Nonmucinous
Pancreatic Cysts
104881 We examined the expression of PAM4-reactive MUC5AC in chronic
pancreatitis and
benign non-mucinous cystic lesions of the pancreas. A tissue microarray of
PDAC (N=14), as
well as surgical specimens from chronic pancreatitis (N-32) and benign non-
mucinous cystic
lesions of the pancreas (N=19), were assessed by immunohistochemistry for
expression of the
PAM4-reactive MUC5AC, as well as MUC I (mA.b-MA.5), MUC4 (mAb-8G7), and
CEACAM6 (mAb-MN-15).
104891 PAM4-reactive MUC5AC, MUCI MUC4 and CEACAM6 were expressed in 79%
(11/14), 100% (14/14), 86% (12/14) and 100% (14/14) of invasive pancreatic
adenocarcinoma. PAM4 only weakly labeled 6% (1/19) of benign non-mucinous
cystic
lesions, 1 of 15 serous cystadenomas (SCAs) and 0 of 4 cysts with squamous
epithelial lining
(2 lymphoepithelial cysts, and 2 retention cysts with squamous metaplasia).
However, the
expression of MUC1, MUC4 and CEACAM6 was detected in 53% (8/15), 0% (0/15) and
13% (2/15) of SCAs, and in 4, 3 and 3 of the 4 cysts with squamous epithelial
lining,
respectively. PAM4 labeled 19% (6/32) of chronic pancreatitis specimens;
however, this
157
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
PAM4 reactivity was restricted to the PanIN precursor lesions associated with
chronic
pancreatitis. Inflamed tissue was negative. The expression of MUC1, MUC4 and
CEACAM6
was detected in 90% (27/30), 78% (25/32), and 97% (31/32) of chronic
pancreatitis. In all of
the positively-labeled specimens, the reactivity was present in non-neoplastic
inflamed
pancreatic tissue in addition to PanIN.
104901 In conclusion, the expression of PAM4 was detected in only 6% of benign
non-
mucinous cystic lesions and in the precursor lesions associated with chronic
pancreatitis.
These results suggest that PAM4, in contrast to MUC1, MUC4, and CEACAM6, may
be
useful to differentiate benign non-mucinous cystic lesions of the pancreas and
chronic
pancreatitis from PDAC.
Example 32. Combination of the PAM4 and CA19-9 Biomarkers For Improved
Detection of Pancreatic Adenocarcinoma
104911 Pancreatic ductal adenocarcinoma (PDAC) is almost universally lethal,
due mainly to
the inability to detect early-stage disease. Thus, identification of
biomarkers that can identify
patients with early-stage PDAC may improve overall survival. In a blinded
study, PAM4 and
CA19-9 immunoassays were performed on sera from 480 patients, including those
with
confirmed PDAC (N=234), other cancers (N=84), benign diseases of the pancreas
(N=89),
and healthy adults (N=50).
104921 Overall sensitivity for PDAC was similar, 74% and 77% for PAM4 and CA19-
9,
respectively. Sensitivity for detection of early, stage-1 disease (N=26),
although somewhat
higher for the PAM4-antigen, was also statistically similar, 65% and 58% for
PA.M.4 and
CA19-9, respectively (P =0.5775). However, specificity was significantly lower
for CA19-9,
particularly with respect to chronic pancreatitis (CP): 68% vs.86% for the
PAM4 assay (P =
0.014). Furthermore, CA19-9 results showed considerably higher detection rates
for non-
PDAC neoplasia, including patients with other cancers that metastasized to the
pancreas.
Thus, positive likelihood ratios (+LR) were lower for CA19-9 (+LR = 2.41) than
for the
PAM4 assay (+LR = 5.29).
104931 PAM.4 and CA19-9 antigen levels in PDAC were independent of each other
(r2 =
0.003, P=0.410); however, the positive and negative interpretations were
concordant in 68%
of the cases. Thus, a combined biomarker analysis improved the overall PDAC
detection rate
(84%), without a significant decrease in specificity (83%). Comparison of the
ROC curves
for PDAC vs. CP and PDAC vs. benign disease demonstrated a statistically
significant
158
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
improvement for the combined immunoassay, as compared to either assay alone (P
< 0.0001
in both comparisons), to detect and discriminate PDAC from benign disease.
104941 While the PAM4-immunoassay provided high sensitivity and specificity
for detection
and diagnosis of PDAC, inclusion of the CA19-9 biomarker significantly
enhanced positive
identification of PDAC patients, from 74% to 84%.
Example 33. Use of PAM4-Immunoassay as a Correlate of Tumor Response
104951 We investigated whether specific trends in PAM4-reactive MUC5AC
concentrations
(within the individual patient) can be used as an indicator of tumor response
after therapy.
Several patients from a 90Y-ITAM4 phase-lb/II clinical trial now in progress
were evaluated.
When patients were evaluated 4 weeks after treatment had ended (a treatment
cycle is 4
weeks), a decrease in serum antigen levels of >40% was suggestive of a
response. All of the
patients who had progressive disease had levels of PAM4 antigen that continued
to rise.
Trends are presented for two patients in FIG. 28A and FIG. 28B. In both cases,
trends in the
level of circulating MUC5AC were concordant with the trend in tumor volume as
determined
by CT. These results suggest that serum PAM4 levels are of use to monitor
responsiveness to
anti-cancer treatments for pancreatic cancer.
Example 34. Identification of Target Antigen for PAM4 Antibody
104961 We performed a set of blocking and capture/probe paired enzyme
immunoassays to
evaluate the relationships between the PAM4 antibody and antibodies reactive
with MUC1
(MA5, KC4, HMFG I, SM3, H23), MUC2 (G9), MUC4 (8G7) and MUC5AC (45M1). A
mucin standard derived from the CaPanl human tumor xenograft was shown to
contain the
reactive mucin species for all of these antibodies except those reactive with
09 (MUC2). Of
all MAbs examined, only 1 (45M1) reported to be reactive with MUC5AC provided
a
positive reaction in sandwich EIA. when PAM4 was used as the capture reagent.
The 45M1
antibody is reactive with a much lower percentage of pancreatic carcinomas
than PAM4 (by
IFIC on TMA) and so cannot be used as a single probe for the serum-based PAM4-
immunoassay.
104971 As described above, we performed a peptide-phage-display study by
consecutive
biopanning with the murine and humanized versions of PAM4-IgG. A consensus
sequence
(12mer - WTWNITKAYPLP (SEQ ID NO: 7)) was generated which when input into a
BLAST protein search with query coverage set at 100%, identified MUC5AC and
MUC16
with 7 of 12 and 5 of 12 identical amino acids within the 12mer sequence,
respectively.
159
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
[04981 Studies were performed using mass spectrometry to identify PAM4-
immunoprecipitated antigens from credentialed cyst fluids (these fluids were
previously
analyzed by mass spectrometry to identify specific MUCs present in the
mixtures). By PAGE
analyses of the PAM4-immunoprecipitated materials from 3 individual cyst fluid
specimens,
only two identical bands were present in each specimen (not shown). Both of
these bands
contained MUC5AC as the major mucin species.
104991 We have investigated the nature of the substance within human blood
that binds to the
PAM4-epitope, which necessitates organic extraction prior to immunoassay. As
discussed
above, Slomiany and co-workers have observed that gastric mucin had covalently
bound
and/or associated lipids and fatty-acids. Further, fatty-acid synthetase
levels and activity are
significantly elevated in pancreatic adenocarcinoma, as is also the case for
other forms of
cancer and other pathologic conditions. Speculating that the blocking
substance might be
lipid in nature, we performed an EIA (FIG. 29) in the presence and absence of
1001AM
palmitic acid and observed a statistically signficant 69% reduction in
reactivity at an 013450
equivalent of 1.0 (P<0.0001). It is noted that the normal adult serum level of
palmitic acid is
in the range of 1,480 to 3,730 ItM, considerably higher than the concentration
that was used
in this EIA experiment.
Example 35. PAM4 Differentiates Between Pancreatic Ductal Adenocarcinoma
(PDAC) and Chronic Pancreatitis (CP)
105001 Current practice guidelines suggest that patients who present with
signs and/or
symptoms suspicious of pancreatic cancer undergo a pancreatic protocol CT
imaging study
for detection of tumor mass within the pancreas. Follow-up imaging by
endoscopic
technologies (e.g., EUS, ERCP) can provide high sensitivity for detection of
disease, and
when combined with fine-needle aspiration/biopsy, can provide good diagnostic
accuracy.
However, the majority of these procedures have been performed on patients with
advanced
disease; that is, tumors greater than 2 cm. Detection of early pancreatic
cancer is still
problematic, especially when occurring in a background of pancreatitis. Thus,
the current
reality is that only 7% of all cases detected are early disease. With no
effective treatment for
advanced PC, the prognosis for these patients is dismal.
105011 Biomarkers that can reliably distinguish between cancer and benign
conditions, and/or
provide means to prioritize patients for follow-up evaluation, would be of
significant clinical
value, especially if the biomarker is capable of detecting early disease. We
have developed
monoclonal antibody PAM4 that demonstrates a high degree of specificity for
pancreatic
160
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
ductal adenocarcinoma (PDAC).
[0502] MAbs having defined reactivity with several mucin species, including
MUC1, MUC2,
MUC3, MUC4, MUC5AC, etc., were evaluated for signal response in a heterologous
PAM4-
capture sandwich EIA. The only MAbs able to provide signal response (45M1, 2-
11M1) are
known to react with specific domains of the MUC5AC mucin. Further, three
additional anti-
MUC5AC MAbs (21M1, 62M1, and 463M1) were each able to inhibit the interaction
between PAM4 and its mucin antigen. These data suggest MUC5AC as an antigen to
which
PAM4 is reactive. PAM4, unlike other anti-MUC5AC MAbs (45M1, 2-11M1, CLII2,
and
others), demonstrates greater specificity for PDAC than cancers originating
from other
organs, and may serve as a useful biomarker for PDAC, as well as a target for
antibody-
directed imaging and therapy.
Table 22. PAM4-Antigen In the Serum of Patients with Known Disease
Number of
N Median Positive Percent of
(units/mL) Cases Positive Cases
Pancreatic Cancer
atiaitAiknocaminorna 298 10,40 225 76
Neuroendoctine 20 0.08 10
Other Morphology \*7 0.51
Non-PC, Mets to the Pancreas 11 0.00
s
Ampullary Adenocarcinoma 21 1.52 0 48
Biliary Adenocarcinoma 26 4.41 13
Cholarigiocarcinoma 7 1.07 2 c)
Duodenal Adenocareinoma 7 2.80
All Biliary and Periampullary 61 1.78 48
Colon Carcinoma 32 0.15 5 16
cia.w=p.iiaoi.$mitwrisiiir- 80 0,41 18 23
Benign Cystadenoma 15 0.18 1
Benign¨Other 25 0.20 5 20
161
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
All Benign Disease 120 0.26 24 20
I leany Volunteers 79 0.27 3
Al! groups are statistically different from the pancreatic adenocarcinoma
group
with P values equal to or better than 0.0001; Mann-Whitney nonparametric test.
!Gold DV. Gaedeke I, Ghadimi BM, etal. Cancer. 2013 Feb 1;119(3):522-8.
105031 The PDAC group consisted of 40% early and 60% advanced stage patients.
Detection
rates were 64% and 85%, respectively. The sensitivity and specificity of the
PAM4 assay was
determined for PDAC vs. CP (FIG. 30A) and for PDAC vs. all benign tissue
samples (FIG.
30B). The calculated values of AUC were 0.84 and 0.85, respectively.
105041 Approximately 20% of patients with chronic pancreatitis (CP) are
positive by use of
the serum-based immunoassay. This issue is critical to the interpretation of
the results with
PAM4-positive CP patients being either false positives, or perhaps, the
discovery of occult
neoplasia. Thus, we undertook an extensive immunohistochemical evaluation of
PAM4-
reactivity in CP tissue specimens.
105051 FIG. 31 shows comparative labeling of PDAC vs. non-neoplastic prostate
tissue by
PAM4 antibody vs. antibodies against MUC1., MUC4, CEACAM6 and CA19-9. Each of
the
antibodies reacted with PDAC. PAM4 showed no reactivity with normal tissue.
The same
antibodies were compared in a sample showing a Pan.IN-2 lesion arising within
a background
of CP, with partial loss of acinar cells, some fibrosis and PanIN-associated
acinar-ductal
metapla.sia (ADM) (not shown). No labeling was observed with PAM4 in any of
the tissues
within CP, including isolated ADM (not shown). Each of the other antibodies
showed some
binding to non-neoplastic tissue (not shown). Table 23 and Table 24 show
comparative
results of labeling with PAM4 vs. antibodies against MUC1, MUC4, CEACA.M6 and
CA19-
9.
Table 23. Expression of Biornarkers in Pancreatic Ductal Adenocarcinotna
fi'AM4 MIX] Ml C4 -CEACAM6 -CA.19-9
'Number __c; 43 43 42 43
Focal Labelinga 1 (2%) tel (15%) 3 (8%) 2(5%)
114%1100:
Diffuse I 3b 42 22 35 (92%) 37 (95%)
.........
162
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(76%) (98%) (85%)
34 43 26
Total Labeled 38 (90%) 39 (91%)
(100%) (60%)
Adjacent Normal 14
O(e%) 6 (43%) 14 (100%) 14 (100%)
(N=14) (100%)
a-Focal labeling, 5% to 25% of the appropriate tissue components labeled with
the indicated
MAb; Diffuse, >25% of the appropriate tissue components labeled with the
indicated MAb;
Total, focal + diffuse. b-value provided in parenthesis is the percentage of
total N PDAC
specimens evaluated
Table 24. Expression of Bioinarkers in Chronic Panereatitis
N PAM4 M MUC4 CEACA M6 CA19-9
(himcPancreatitis 32
-Pan' Ni 5 2 1 1 5 5
- 5 4 . 4 3 5 5
-Ducts 32a 0 22 25 31 29
-Acinar cells 32a 0 '27 8 30 ¨29
-Isolated ADM 32a 0 24 0 0 26
105061 We conclude that PAM4 is not reactive with the non-neoplastic tissues
from chronic
pancreatitis (CP) patients, but rather with PDAC and its neoplastic precursor
lesions, such as
PanINs, which are known to develop within the inflamed parenchyma. Together
with results
from a prior study, we have evaluated a total of 51 specimens of CP, finding
that in no
instance was PAM4 reactive with the inflamed parenchyma. On the other hand,
each of the
other biomarkers investigated, MUC1, MUC4, CEACAM6, and CA19-9, were unable to
differentiate PDAC and benign, non-neoplastic tissues. These latter biomarkers
were
expressed to varying extents in CP-associated PanIN lesions, but also in non-
neoplastic ducts
and isolated ADM. A PAM4-based EIA to quantitate antigen in patient sera shows
high
sensitivity and specificity for detection of PDAC Approximately 2/3 of
patients with stage-1
disease are positive for circulating PAM4-antigen. We speculate that CP
patients (and
perhaps others having disease with high risk for development of PDAC), who are
found to
have positive levels of PAM4-reactive antigen in the circulation, may have
occult PDAC
and/or significant mass of precursor lesions producing the PAM4-biomarker.
Example 36. Mapping the PAM4 Epitope on MUCSAC
163
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
Summary
105071 Indirect and sandwich enzyme immunoassays (EIA) were performed to
compare and
contrast the reactivity of PAM4 with several anti-mucin antibodies having
known reactivity
to specific mucin species (e.g., MUC1, WJC4, MUC5AC, etc.). Studies designed
to block
reactivity of PAM4 with its specific antigen also were performed. We
demonstrated that
MAbs 2-11M1 and 45M1, each reactive with MUC5AC, are able to provide signal in
a
heterologous sandwich immunoassay where PAM4 is the capture antibody. Further,
we
identified MAbs 21M1, 62M1, and 463M1, each reactive with MUC5AC, as
inhibiting the
reaction of PAM4 with its specific epitope. MAbs directed to MUC I, MUC3,
MUC4,
MUC16 and CEACAM6 were not reactive with PAM4-captured antigen, nor are they
able to
block the reaction of PAM4 with its antigen. We concluded that MUC5AC is the
mucin
species to which PAM4 antibody is reactive.
Background
105081 Mucin glycoproteins are high molecular weight, heavily glycosylated,
proteins that
include at least 19 species categorized on the basis of their unique protein
cores. They can be
found as either transmembrane components of the cell or as secreted products.
Abnormal
expression of mucins is a well-known occurrence in many forms of cancer (see
Hollingsworth & Swanson, 2004, Nat Rev Cancer 4:45-60; Kufe, 2009, Nat Rev
Cancer
9:874-85; Rachagani et al., 2009, Biofactors 35:509-27), including pancreatic
ductal
adenocarcinoma (PDAC) (Ringel & Lohr, 2003, Mo lancer 2:9-13; Andrianifahanana
et al.,
2001, Clin Cancer Res 7:4033-40; Tones et al., 2012, Curr Phann Des 18:2472-
81). Neo-
expression and/or upregulation/downregulation of specific mucin species, with
and without
the generation of newly transcribed and translated splice variants (Schmid,
2003, Oncol Rep
10:1981-85), have been well-documented in the literature. Alteration of
carbohydrate
moieties through the addition of new terminal sugars (e.g., neuraminic acids),
underglycosylation, and other abnormal biochemical pathways also have been
observed
(Brockhausen, 2006, EMBO Rep 7:599-604; Yue et al., 2009, Mol Cell Proteomics
8:1697-
707; Haab et al., 2010, Ann Surg 251:937-45). These modifications may lead to
changes in.
conformational structure and/or appearance or disappearance of specific
epitopes.
Additionally, changes may be observed for the intracellular distribution of
the mucin species
under consideration, such as MUC1, which in normal tissues is a transmembrane
glycoprotein, but with neoplastic transformation is found in the cytoplasm as
well (lass et al.,
1995, J Pathol 176:143-49; Cao et al., 1997, Virchows Arch 431:159-66). These
events may
164
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
prove to be of biological and clinical significance in the process of
neoplastic development
and progression, as well as provide new biomarkers/targets for early detection
and targeted
therapy of cancer.
105091 Our laboratory initially reported the use of a polyclonal antiserum to
identify a
pancreatic ductal mucin, which at the level of sensitivity provided by
indirect
immunohistochemistry (1HC), was shown to contain an epitope relatively
specific to the
pancreas (Gold et al., 1983, Cancer Res 43:235-38), and ultimately resulted in
the
development of monoclonal antibody (MAb), PAM4 (Gold et al., 1994, Int J.
Cancer 57:204-
10), also known as clivatuzumab in its humanized form. PAM4 demonstrates high
specificity
for PDAC with little to no reactivity towards normal and benign, non-
neoplastic, pancreatic
tissues, although it does show limited reactivity (approximately 10% of all
specimens
examined) with adenocarcinomas originating in certain other organs (e.g.,
stomach, colon,
lung) (Gold et al., 1994, Int J Cancer 57:204-10; Gold et al., 2007, Clin
Cancer Res 13:7380-
87; Gold et al., 2010, Cancer Epidemiol Biomarkers Prey 19:2786-94). PAM4
identifies a
biomarker that, if present, provides a high diagnostic likelihood of the
presence of pancreatic
neoplasia (Gold et al., 2010, Cancer Epidemiol Biomarkers Prey 19:2786-94;
Gold et al.,
2006, J Clin Oncol 24:252-58; Gold et al., 2013, Cancer 119:522-28). Thus,
clinical
applications for detection of early-stage disease (Gold et al., 2010, Cancer
Epidemiol
Biomarkers Prey 19:2786-94; Gold et al., 2013, Cancer 119:522-28), and
antibody-targeted
imaging and therapy, are being pursued (Gulec et al., 2011, Clin Cancer Res
17:4091-4100;
Ocean et al., 2012, Cancer 118:5497-5506). In addition to PDAC, the PAM4-
biomarker is
expressed in the precursor lesions, pancreatic intraepithelial neoplasia
(PanIN, including the
earliest developing lesion, PanIN-1A), and intraductal papillary mucinous
neoplasia (IPMN),
suggesting that there may be oncogenic significance to its expression (Gold et
al., 2007, Clin
Cancer Res 13:7380-87). In the current study, we investigated the identity of
the mucin
species to which this clinically-relevant antibody is reactive, in order to
understand what role
this mucin may play in the development and progression of pancreatic cancers.
Methods
105101 Antigen and Antibodies - A mucin containing fraction, designated CPM1,
was
isolated, as described previously (Gold et al., 2006, J Clin Oncol 24:252-58),
from the Capan-
1 human PDAC xenograft in athymic nude mice. Briefly, this consisted of
homogenization of
the dissected tumor in 0.1M ammonium bicarbonate containing 0.5M sodium
chloride.
Following high-speed centrifugation (20,000 g x 45 min), the soluble material
was
165
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
chromatographed on a SEPHAROSE 4B-CL column, and then eluted with the
identical
ammonium bicarbonate-sodium chloride solution. The void volume material was
collected,
dialyzed against 0.01M sodium. phosphate, pH 7.2, and then passed through
hydroxyapatite to
remove nucleic acids and proteins. The non-binding, mucin-containing fraction
was again
dialyzed extensively to remove salts and used as a source of antigen.
105111 Antibodies used in the current study are listed in Table 25 with clone
and source
information. For sandwich and blocking studies, PAM4 was available in both
muiine
(mPAM4) and humanized (hPA.M4; clivatuzumab) versions provided by
Immunomedics, Inc.
(Morris Plains, NJ). All other MAbs were murine IgG. Mouse ascites fluids
containing MAbs
21M1, 45M1, 62M1 and 463M1 were kindly provided by Dr. J. Bara, INSERM, Paris,
France. PAM4 antibodies and ascites fluid containing an anti-alpha-fetoprotein
antibody,
employed as a negative control for the blocking studies (reactive with Hep-G2,
hepatoceullar
carcinoma cells) were provided by hnmunomedics, Inc. (Morris Plains, NJ). A
rabbit
polyclonal anti-CPM] (Gold et al., 1994, Int j Cancer 57:204-210; Gold etal.,
2010, Cancer
Epidemiol Biomarkers Prey 19:2786-94) IgG served as the positive control with
detection by
a horseradish peroxidase (HRP)-labeled donkey anti-rabbit IgG (Jackson
hnmunoResearch,
West Grove, PA).
Table 25. Monoclonal antibodies used
Antigen Clone name Source
MUC 1 MA5 Immunomedics
MUCI KC4 1mmunomedics
____________________________________________________________________ =
MUCI CM1 Gene Tex
MUC2 994/152 Abeam
MUC3 M3.1 Abeam
MUC3 M3A LifeSpan Bio
MUC4 8G7 Santa Cruz Biotech
MUC5AC 2-11M1 Santa Cruz Biotech
MUC5AC 45MI Santa Cruz Biotech
M1.JC5AC CLH2 Santa Cruz Biotech
= ___________________________________________________________________
MUC16 X306 Novus Bio
MUCI6 X325 Abeam
CEACAM5 MN14 1mmunomedics
166
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
CEACAM6 MN 15 Immunomedics
_
CA 19-9 CA 19-9 Santa Cruz Biotech
:
Immunomedics, inc. ¨ Morris Plains, NJ; GeneTex ¨ Irvine, CA; Abeam ¨
Cambridge, MA;
LifeSpan Biosciences, Inc. - Seattle, WA; Santa Cruz Biotechnology, Inc. -
Santa Cruz, CA;
Noy-us Biologicals ¨ Littleton, CO.
105121 Enzyme Immunoassay - Procedures have been described for both indirect
and
sandwich enzyme immunoassays (Gold et al., 1994, hit J Cancer 57:204-210; Gold
et al.,
2010, Cancer Epidemiol Biomarkers Prey 19:2786-94). For indirect immunoassays,
primary
MAbs were used at a concentration of 101.tglmL to provide high sensitivity for
signal
detection. For sandwich immunoassays, the capture MAb was coated onto the
wells at a
concentration of 10 ug/ml.õ followed by the addition of the CPM1 antigen at
various
concentrations up to 10 ug/mL. The MAb probe was then added at a high
concentration of 10
1.ig/mL for detection of response to captured antigen. Secondary IIRP-labeled
anti-species-
specific IgG (Jackson ImmunoResearch, West Grove, PA) was evaluated initially
to
determine optimum concentrations for use in the assay (usually 1:1000 or
1:2000). MAb
inhibition studies were performed by adding the inhibiting MAb to wells coated
with CPM1
antigen, starting at a high concentration of 100iug/mL of pure MAb or 1:10
dilution of ascites
fluid, and titrating to lower amounts. After incubating with the inhibiting
antibody at 37 C
for 1 h, the plates were washed, and hPAM4 added to the wells at a
concentration of 0.25
1.ig/mL. hPAM4 binding was then detected with a secondary probe, IIRP-labeled
anti-human
IgG conjugate.
105131 SDS-PAGE and Western-blotting - SDS-PAGE was performed under non-
reducing
conditions using 4-20% Tris-Glycine gels at 125V for about 2 h. Resolved
proteins were
transferred onto a nitrocellulose membrane using the Mini TRANS-BLOTS cell
system
(Bio-R.ad Laboratories, Hercules, CA) at 100 V for 1 h. To examine the
identity of
recombinant proteins, triplicate samples were run in the same gel and membrane
with
transferred samples were cut into three pieces for probing with HRP-anti-Myc,
IIRP-hPAM4,
and 45M1 plus HRP-GAM, respectively. The signals were developed with
SUPERSIGNALTm West Dura Chemiluminescent Substrate (Thermo Fisher Scientific,
Waltham, MA).
Results
105141 Several MA.bs were evaluated by indirect EIA. for reactivity with
plates coated with
CPM1 (FIG. 32), a high molecular weight mucin fraction isolated from the Capan-
1 human
167
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
pancreatic cancer xenograft. Murine PAM4 and MAbs reactive specifically with
MUC1 and
MUC5AC mucins provided elevated reactivity in this indirect immunoassay, with
minor
reactivity also observed for MA.bs directed to WO and CEACAM6. Essentially no
reaction
was seen with MAbs to MUC2, MUC4, MIJC16, and CEACAM5 glycoproteins, or the
CA19-9 carbohydrate epitope.
105151 It should be noted that a negative EIA reaction does not necessarily
indicate absence
of the mucin-antigen, because the specific epitope structure may be present,
but inaccessible
(i.e., cryptic). This is likely the case for MAb-CLII2 anti-MUC5AC generated
against a
peptide derived from the mucin's tandem repeat (Reis et al., 1997, Int j
Cancer 74:112-21),
since the other two anti-MUC5AC MAbs were highly reactive. Similarly, CM1 anti-
MUC1
was considerably less reactive than MA5 and KCA anti-MUC1 antibodies. Capan-1
cells
produce well-differentiated tumors with highly glycosylated mucins. Thus, it
is likely that
both CLH2 and CM I, reactive with the tandem repeat domains of their
respective mucins,
would not be reactive with CPM1, since the tandem repeat epitopes are
inaccessible.
105161 We then evaluated whether the anti-mucin MAbs were reactive with PAM4-
captured
mucin. Humanized PAM4 (hPAM4)-coated plates were used to capture the specific
mucin-
antigen from the CPM I fraction, which was then probed with various anti-mucin
MAbs.
Murine MAbs (mMAbs) specifically reactive with MUC1, MIJC3, MUC4, MUC16 and
CEACAM6 did not provide a signal in these heterologous sandwich immunoassays
(not
shown). On the other hand, both anti-M'UC5AC mMAbs tested, 45M1 and 2-11M1,
gave
positive reactions with the hPAM4-captured antigen (FIG. 33), with 45M I
showing
significantly greater reaction than 2-11M1 (Kd 14.32 I 1.081.ig/mL and 24.4
7.83 p.g/mL,
respectively, for MAbs 45M1 and 2-11M1; P< 0.001). However, neither of these
individual
anti-MUC5AC MA.bs provided as strong signal intensity as the rabbit anti-CPM1
polyclonal
IgG fraction. Importantly, mPAM4 did not bind to the hPAM4-captured antigen,
nor did
hPAM4 bind to mPAM4-captured antigen, suggesting that the PAM4 epitope is
present at
low density, possibly only a single site within the mucin-antigen.
105171 Follow-up studies were designed to inhibit the binding of liPAM4 to
CPM1.-coated
plates (FIG. 34A-B). Although 2-11M1 anti-MUC5AC was unable to inhibit hPAM4-
CPM1
binding, 45M1 anti-MUC5AC was able to provide a limited inhibitory effect,
with ICõ
25.5% inhibition (FIG. 34A). mF'AM4, included as a positive control, provided
ICm.=
92.4% self-inhibition at a concentration 0.1 pg/mL, while the MA.5 and KC4
anti-MUCi
antibodies provided no inhibition, even at the highest concentration evaluated
(101.igimL)
168
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
(FIG. 34A). hPAM4 was unable to completely block mPAM4 binding to the CPM1
antigen
(1Cõ,,õ---52.8%) (not shown), a not unexpected finding since the humanized
version of PAM4
is known to have a lower affinity than the murine parent. Ascites fluids
containing mMAbs
with known mapping to MUC5AC were serially diluted as inhibitory reagents,
with results
shown in FIG. 34B. mMAbs 21M1, 62M1, and 463M1 each provided inhibition
similar to
the results shown for mPAM4 self-blocking, with 45M1 ascites providing limited
inhibition,
similar to what was observed with the commercially available 45M1-IgG WIG.
34B). Ascites
fluid containing a murine anti-alpha-fetoprotein (AFP), included here as a
negative control,
provided no inhibition of the hPAM4 binding to CPM1 (FIG. 34B). Unfortunately,
insufficient volumes of ascites precluded determination of MAb concentrations,
so that
relative blocking efficiency could not be calculated.
Discussion
105181 The current Example suggests that PAM4 is reactive with the MUC5AC
mucin
glycoprotein. FIG. 35 presents a map of the MUC5AC mucin domains with reactive
epitopes
indicated for several of the anti-MUC5AC MAbs employed in our studies (Nollet
et al., 2002,
Int J Cancer 99:336-43; Nollet et al., 2004, Hybrid Hybridomics 23:93-99;
Lidell et al., 2008,
FEBS j 275:481-89). CLH2 is reactive with the peptide core of the tandem
repeat domain
(Reis et al., 1997, Int J Cancer 74:112-21), and is likely a cryptic epitope
within the Capan-I
tumor-derived MUC5AC. 2-11M1 is reactive with the N-terminus of the mucin
(Nollet et al.,
2004, Hybrid Hyridomics 23:93-99), and 45M1 at the furthest N-terminal region
of the
cysteine-rich, C-terminus (Udell et al., 2008, FEBS J 275:481-89). Both of
these MAbs were
reactive with PAM4-captured mucin, whereas MAbs to MUCs 1, 3, 4, and 16 were
not. We
observed that 45M1 provides a significantly greater signal response than 2-
11M1, suggesting
a greater density of 45M1-epitopes than 2-11M1-epitopes within CPM1. However,
this may
simply be due to a loss of 2-11M1 epitopes through proteolytic digestion of
the relatively
non-glycosylated N-terminus, and/or molecular shear of this very large
glycoprotein during
purification. In any case, the 2-11M1 antibody provided no inhibition of the
hPAM4-CPM1
interaction, suggesting the epitope is located distant to the PAM4-epitope.
105191 On the other hand, 45M1 did inhibit the hPAM4-CPM1 interaction, albeit
only
partially, suggesting that the PAM4-epitope is within the C-terminal region of
the mucin or
conthrmationally altered by interaction of this antibody with the mucin
molecule. MAbs
21M1, 62M1, and 463M1 have also been mapped to the C-terminal region of the
MUC5AC
mucin (Nollet et al., 2002 Int J Cancer 99:336-43; et al., 2004, Hybrid
Hybridomics 23:93-
169
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
99; Lidell et al., 2008, FEBS J 275:481-89), and each provided significant
inhibition of the
PAM4-mucin reaction. Taken together, our data provide direct evidence that
PAM4 is
reactive with the identical mucin (MUC5AC), and that the PAM4 epitope is
either directly-
blocked, or conformationally modified, by interaction of these MAbs with the
MUC5AC
antigen.
105201 We had initially reported that PAM4 was reactive with the MUC1 mucin
species
(Gold et al., 2007, Clin Cancer Res 13:7380-87; Gold et al., 2006, J Clin
Oncol 24:252-58).
This was based upon MUC/-gene transfection studies, whereby PAM4 was observed
to react
with the gene-transfected, MUC1+ cell line, but not the MUC1- parental cell
line or vector
control cell lines. However, other evidence acquired since then has questioned
this
interpretation, suggesting that MIJC1 transfection may have upregulated other
mucins as
well. Prior results from our laboratory lend support to the current findings.
The PAM4
epitope was found to be highly sensitive to mild reduction with dithiothreitol
(0.02M, 15 min,
20 C) or heat (100 C, 2 min), suggesting the epitope is peptide in nature, and
highly
dependent upon a specific conformation of the protein core kept intact by
disulfide bridges
(Gold et al., 1994, Int J Cancer 57:204-10). This is unlikely to be MUC1 with
all of the
cysteines located within the transmembrane domain of the mucin, but is
consistent with the
loss of reactivity shown by several anti-MUC5AC MAbs upon reduction of the
mucin
antigen. Further, employing immunohistochemical methods, we reported that
frequency of
expression and morphologic distribution of the PAM4-epitope within PDAC and
its precursor
lesions shared greater similarity to those described for MUC5AC than for MUC1
(Gold et al.,
2007, Clin Cancer Res 13:7380-87).
105211 In conclusion, antibodies that bind to the PAM4 epitope of MUC5AC are
of use for
detection and differential diagnosis of pancreatic cancer. Immunoconjugates of
such
antibodies are of use for pancreatic cancer therapy.
Example 37. DOTA Conjugates of PAM4
105221 The hPAM4 antibody was prepared as described in ExainpleThe genes of
CDR-
grafted VH and VK chains of hPAM4 were inserted into the pdHL2 plasmid vector,
a DHFR-
based amplifiable expression system. The plasmid was transfected into the
murine myeloma
cell line, Sp2/0-Ag14 (ATCC, Manassas, VA) to generate the cell clones
producing hPAM4.
The complete mature amino acid sequence is shown below.
hPAM4 Heavy Chain
170
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
105231 QVQLQQSGAEVKKPGASVKVSCEASGYTFPSYVLIIWVKQAPGQGLEWIGYI
NRYNDGTQYNEKFKGKATLTRDTSINTAYMELSRLRSDDTAVYYCARGFGGSYGFA
YWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGA
LTSGVIITFPAVLQSSGLYSLSSVVTVPSSSLGTQTYTCNVNIIKPSNTKVDKRVEPKSC
DKTITTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSITEDPEVKFNWY
VDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIE
KTISKAKGQPREPQVYTLPPSREEMIKNQVSLTCINKGFYPSDIA.VEWESNGQPENN
YKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALIINITYTQKSLSLSPG
K (SEQ ID NO:120)
hPAM4 light chain
105241 DIQLTQSPSSLSASVGDRVTMTCSASSSVSSSYLYWYQQKPGKAPKLWIYSTS
NLASGVPARFSGSGSGTDFTLTISSLQPEDSASYFCHQWNRYPYTFGGGTRLEIKRTV
AAF'SVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQD
SKDSTYSLSSTI,TLSKADYEKHKV YACEVTHQGLSSPVTKSFNRGEC (SEQ ID
NO:121)
105251 The DNA and amino acid sequences of hPAM4 Vic and VH are shown in FIG.
4A and
FIG. 4B, respectively, with the CDR.s identified in bold and underlined.
105261 The current cell clone name is hPAM4-2E3 and is produced in Sp2/0 host
cells,
DITFR expression system. The antibody is a humanized IgGh, glycoprotein. A
glycosylation
site on the heavy chain (Asn299) has a composition per mole of hPAM4-DOTA: 0.5
Fuc, 6.3
GleNAc, 6.3 Man, 0.3 Gal and 0.15 Neu5Gc; glycosylation species: GOF 70%, G IF
23%,
G2F 2%, GlFS1 4%, G2FS1 1%. There are 16 S-S bonds (32 SIT), identified and
located
exactly as theoretical prediction based on the above sequence.
105271 An hPAN44-DOTA. product was prepared from purified hPAM4 IgG that was
coupled
with the 12-membered macrocyclic chelating agent 1,4,7,10-
tetraazacyclododecane-N,
N',N",N"'-tetraacetic acid (DOTA).
105281 DOTA was conjugated via one of the carboxyl moieties to reactive sites
on the
hPAM4 antibody to generate a stable conjugate. The coupling is assumed to be
via stable
amide bond to the antibody's lysine side-chain amino group.
105291 The chemical conjugation was performed by first reacting DOTA with N-
hydroxysulth-succinimide (sulfo-NHS) in the presence of 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide (EDC) to generate activated Dom., then
incubating
activated DOTA with purified hPAM4 antibody. Conditions were optimized to
yield a
171
CA 02948013 2016-11-03
WO 2016/003869
PCT/US2015/038252
substitution ratio of 4-7 DOTA. moieties per antibody molecule, as determined
by mass
spectrometry assays.
it will be apparent to those skilled in the art that various modifications and
variations can. be
made to the products, compositions, methods and processes of this invention.
Thus, it is
intended that the present invention cover such modifications and variations,
provided they
come within the scope of the appended claims and their equivalents.
172