Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
METHODS, COMPOSITIONS, AND DEVICES FOR INFORMATION STORAGE
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims priority to U.S. Provisional Patent Application
Nos. 62/301,538,
filed February 29, 2016, and 62/415,430, filed October 31, 2016, the contents
of each of which
are hereby incorporated by reference in their entireties.
FIELD
[002] The invention relates to novel methods, compositions and devices useful
for information
storage and retrieval, using nanopore devices to synthesize and sequence
polymers, e.g., nucleic
acids.
BACKGROUND
[003] There is a continuing demand to store ever more data on or in physical
media, with
storage devices getting ever smaller as their capacity gets bigger. The amount
of data stored is
reportedly doubling in size every two years, and according to one study, by
2020 the amount of
data we create and copy annually will reach 44 zetabytes, or 44 trillion
gigabytes. Moreover,
existing data storage media such as hard drives, optical media, and magnetic
tapes, are relatively
unstable and become corrupted after prolonged storage.
[004] There is an urgent need for alternative approaches to storing large
volumes of data for
extended periods, e.g. decades or centuries.
[005] Some have proposed using DNA to store data. DNA is extremely stable and
could in
theory encode vast amounts of data and store the data for very long periods.
See, for example,
Bancroft, C., et al., Long-Term Storage of Information in DNA, Science (2001)
293: 1763-1765.
Additionally, DNA as a storage medium is not susceptible to the security risks
of traditional
digital storage media. But there has been no practical approach to
implementing this idea.
[006] WO 2014/014991, for example, describes a method of storing data on DNA
oligonucleotides, wherein information is encoded in binary format, one bit per
nucleotide, with a
96 bit (96 nucleotide) data block, a 19 nucleotide address sequence, and
flanking sequences for
amplification and sequencing. The code is then read by amplifying the
sequences using PCR and
sequencing using a high speed sequencer like the Illumina HiSeq machine. The
data block
sequences are then arranged in the correct order using the address tags, the
address and flanking
1
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
sequences are filtered out, and the sequence data is translated into binary
code. Such an approach
has significant limitations. For example, the 96 bit data block could encode
only 12 letters
(using the conventional one byte or 8 bits per letter or space). The ratio of
useful information
stored relative to "housekeeping" information is low ¨ approximately 40% of
the sequence
information is taken up with the address and the flanking DNA. The
specification describes
encoding a book using 54,898 oligonucleotides. The ink-jet printed, high-
fidelity DNA
microchips used to synthesize the oligonucleotides limited the size of the
oligos (159-mers
described were at the upper limit). Furthermore, reading the oligonucleotides
requires
amplification and isolation, which introduces additional potential for error.
See also, WO
2004/088585A2; WO 03/025123 A2; C. BANCROFT: "Long-Term Storage of Information
in
DNA", Science (2001) 293 (5536): 1763c-1765; COX J P L: "Long-term data
storage in DNA",
Trends in Biotechnology (2001)19(7): 247-250.
[007] While the potential information density and stability of DNA make it an
attractive vehicle
for data storage, as has been recognized for over twenty-five years, there is
still no practical
approach to writing and reading large amounts of data in this form.
BRIEF SUMMARY
[008] We have developed a new approach to nucleic acid storage, using
nanofluidic systems to
synthesize the nucleic acid sequences and nanopore readers to read the
sequences. Our approach
allows for the synthesis, storage and reading of DNA strands which are
hundreds, thousands or
even millions of bases long. Because the sequences are long, only a relatively
small proportion
of the sequence is taken up with identifying information, so that the
information density is much
higher than in the approach described above. Moreover, in some embodiments,
the nucleic acid
as synthesized will have a specific location on a nanochip, so the sequence
can be identified even
without identifying information. The sequencing carried out in nanochambers is
very rapid, and
reading the sequence through a nanopore can be extremely rapid, on the order
of up to one
million bases per second. Since only two base types are required, the
sequencing can be faster
and more accurate than sequencing procedures that must distinguish among four
nucleotide base
types (adenine, thymine, cytosine, guanine). In particular embodiments, the
two bases will not
pair with one another and form secondary structures and will also be of
different sizes. For
2
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
example, adenine and cytosine would be better for this purpose than adenine
and thymine, which
tend to hybridize, or adenine and guanine, which are of similar size.
[009] In some embodiments, this system can be used to synthesize long polymers
encoding
data, which can be amplified and/or released, and then sequenced on a
different sequencer. In
other embodiments, the system can be used to provide custom DNA sequences. In
still other
embodiments, the system can be used to read DNA sequences.
[010] The nanochips used in one embodiment contain at least two separate
reaction
compartments connected by at least one nanopore, which prevents at least some
of the
components from mixing, but allows as few as a single molecule of DNA, or
other charged
polymers, e.g., RNA or peptide nucleic acid (PNA), to cross from one reaction
compartment into
another in a controllable manner. The transfer of the polymer (or at least the
end of the polymer
to which monomers are added) from one compartment to another permits
sequential
manipulations/ reactions to the polymer, such as addition of bases, using
enzymes which are
prevented from crossing through the nanopore, for example because they are too
large or because
they are tethered to a substrate or bulky portion. Nanopore sensors report
back on the movement
or location of the polymer and its state, for example its sequence and whether
the attempted
reaction was successful. This allows data to be written, stored, and read, for
example wherein the
base sequence corresponds to a machine readable code, for example a binary
code, with each
base or group of bases corresponding to a 1 or 0.
[011] Accordingly, the invention includes, inter alia, the following
embodiments,
= A nanochip for synthesis of an electrically charged polymer, e.g.,
DNA, comprising at least two distinct monomers, the nanochip comprising two or
more reaction chambers separated by one or more nanopores, wherein each
reaction chamber comprises an electrolytic fluid, one or more electrodes to
draw
the electrically charged polymer into the chamber and one or more reagents to
facilitate addition of monomers or oligomers to the polymer. The nanochip may
optionally be configured with functional elements to guide, channel and/or
control
the DNA, it may optionally be coated or made with materials selected to allow
smooth flow of DNA or to attach the DNA, and it may comprise nanocircuit
elements to provide and control electrodes proximate to the nanopores. For
example, the one or more nanopores may optionally each be associated with
3
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
electrodes which can control the movement of the polymer though the nanopore
and/or detect changes in electric potential, current, resistance or
capacitance at the
interface of the nanopore and the polymer, thereby detecting the sequence of
the
polymer as it passes through the one or more nanopores. In particular
embodiments, the oligomers are synthesized using polymerases or site specific
recombinases. In some embodiments, the polymer is sequenced during the course
of synthesis, to allow for the detection and optionally correction of
mistakes. In
some embodiments, the polymer thus obtained is stored on the nanochip and can
be sequenced when it is desired to access the information encoded in the
polymer
sequence.
= A method of synthesizing a polymer, e.g., DNA, using a nanochip as
described.
= A single stranded DNA molecule wherein the sequence consists essentially
of
only nonhybridizing nucleotides, for example adenine and cytosine nucleotides
(As and Cs), which are arranged in sequence to correspond to a binary code,
e.g.,
for use in a method of data storage.
= A double stranded DNA comprising a series of nucleotide sequences
corresponding to a binary code, wherein the double stranded DNA further
comprises
= A method of reading binary code encoded in DNA, comprising using a
nanopore
sequencer.
= A method of data storage and devices therefor, using the above nanochip
to make
an electrically charged polymer, e.g., DNA, comprising at least two distinct
monomers, wherein the monomers are arranged in sequence to correspond to a
binary code.
[012] Further aspects and areas of applicability of the present invention will
become apparent
from the detailed description provided hereinafter. It should be understood
that the detailed
description and specific examples, while indicating preferred embodiments of
the invention, are
intended for purposes of illustration only and are not intended to limit the
scope of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
4
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[013] The present invention will become more fully understood from the
detailed description
and the accompanying drawings, wherein:
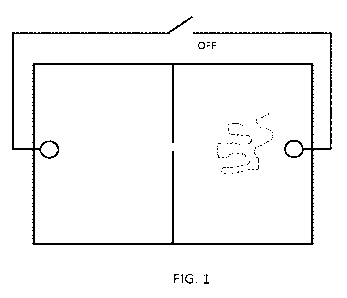
[014] Figure 1 shows a diagram of a simple two-chamber nanochip design, with a
dividing
membrane perforated by a nanopore, and electrodes on either side of the
membrane.
[015] Figures 2 and 3 show how the charged polymer, e.g. DNA, is drawn towards
the anode.
[016] Figures 4 and 5 show that the polymer can be moved back by reversing the
polarity of the
electrodes.
[017] Figure 6 shows a two chamber nanochip design for DNA synthesis, in which
a
polymerase enzyme is located in one chamber, a de-blocking enzyme is in the
other chamber,
and neither can pass through the nanopore.
[018] Figure 7 shows addition of an adenine nucleotide when a 3'-blocked dATP
(A) flows
through left chamber, and the current is set 'forward' to bring the DNA into
the chamber.
[019] Figure 8 shows deprotection of the oligonucleotide so an additional
nucleotide can be
added. For example, deprotection occurs after moving the DNA into the chamber
by setting the
current to 'reverse'.
[020] Figure 9 shows addition of a 3'-blocked dCTP (C). In certain
embodiments, fluid flow is
used to exchange the contents of this chamber, e.g., as depicted, previously
there was 'A' in this
chamber.
[021] Figure 10 shows how multiple separate retaining chambers can be provided
while the
flow chamber becomes a single lane to provide reagents.
[022] Figure 11 shows an approach to keeping the DNA associated with its
chamber, by
attaching to the chamber (upper DNA fragment in figure) or by coupling to a
bulky group that
cannot get through the nanopore (lower DNA fragment in figure). In this
system, the end of the
DNA can still move into the flow chamber and receive additional nucleotides,
but the other end
remains in the retaining chamber.
[023] Figure 12 shows a configuration where the DNA is attached to the wall of
the chamber
and controlled by multiple electrodes.
[024] Figure 13 shows how the DNA can be retained in the chamber when desired,
simply by
controlling the polarity of the electrodes.
[025] Figure 14 shows an array with free-flowing reagents through both sides,
with the DNA
bound to the surface of a chamber.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[026] Figure 15 shows an alternate design with the electrodes on the sides
adjacent to the
dividing membrane, which allows for less expensive manufacture.
[027] Figure 16 shows a three-compartment arrangement, where the DNA can be
moved from
compartment to compartment by the electrodes. This system does not require
significant flow of
reagents during synthesis.
[028] Figure 17 shows an example of how reagents could be configured in a
three compartment
arrangement.
[029] Figure 18 depicts an oligonucleotide tethered adjacent to a nanopore,
where the nanopore
has electrode elements on either side of the membrane.
[030] Figure 19 depicts a series of DNA molecules attached along a membrane
comprising
nanopores and each under control of electrodes adjacent to a nanopore, with a
flow lane on either
side of the membrane. For example, as depicted, the left flow lane provides a
flow of buffer
wash / 3'-blocked dATP (A) / buffer wash / 3'-blocked dCTP (C) / buffer wash,
wherein the
DNA molecules are brought into the flow chamber only when the desired
nucleotide is present.
The right lane provides deblocking agent(s) to deprotect the 3' end of the
nucleotide and allow
for addition of another nucleotide. In one embodiment, the deblocking agent(s)
flow when the
left lane is being washed with buffer. In another embodiments, the
deprotecting agent(s) are too
bulky to cross to the left lane via the nanopores.
[031] Figures 20-22 depict the schematically the proof of concept experiments
wherein the bits
used to encode the data are short oligomers attached using topoisomerase.
[032] Figure 23 depicts a format for a nanopore sequencer wherein the polymer
sequence is
read using capacitive variance. In this capacitive readout scheme, electrodes
form the top and
bottom plates of a capacitor, separated by a membrane comprising a nanopore.
The capacitor is
embedded in a resonant circuit, wherein a pulsating direct current can draw
the charged polymer
through the nanopore. The change in capacitance is measured as the polymer,
e.g. DNA, passes
through the nanopore, using high frequency impedance spectroscopy. A major
advantage of this
approach, particularly with DNA, is that the measurement frequency can be very
high
(effectively a measurement for every cycle, so a 100MHz frequency corresponds
to 100 million
measurements per second), and much greater than the rate of transfer of
monomers through the
nanopore (DNA, for example, unless somehow constrained, will pass through the
nanopore in
response to electrical current at a speed on the order of 1 million
nucleotides per second).
6
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[033] Figure 24 depicts a dual addition chamber layout, suitable for adding
two different types
of monomers or oligomers, e.g., for 2-bit or binary encoding. The upper part
of the figure shows
a top view. The lower part shows a side view cross-section. The full device in
this embodiment
can be assembled from up to 3 independently fabricated layers and joined by
wafer bonding, or
may be formed by etching a single substrate. The chip comprises an electrical
control layer (1), a
fluidics layer (2) which contains the two addition chambers atop a reserve
chamber, with the
charged polymer (e.g., DNA) anchored between nanopore entrances to the first
and second
addition chambers, and an electrical ground layer (3).
[034] Figure 25 depicts the operation of the dual addition chamber layout of
Figure 24. It will
be observed that at the base of each addition chamber, there is a nanopore
(4). The nanopore is
made, for example, by drilling with FIB, TEM, wet or dry etching, or via
dielectric breakdown.
The membrane (5) comprising the nanopores is, e.g.,from 1 atomic layer to 10's
of nm thick. It is
made from, e.g., SiN, BN, SiOx, Graphene, transition metal dichalcogenides
e.g. WS2 or MoS2.
Underneath the nanopore membrane (5) there is a reserve or deblocker chamber
(6), which
contains reagents for deprotection of the polymer following addition of a
monomer or oligomer
in one of addition chambers (it will be recalled that the monomers or
oligomers are added in end-
protected form, so that only a single monomer or oligimer is added at a time).
The polymer (7)
can be drawn into or out of the addition chambers by changing the polarity of
the electrodes in
the electrical control layer (1).
[035] Figure 26 depicts a top view of similar layout to figures 24 and 25, but
here there are four
addition chambers which share a common reserve or deblocker chamber and the
polymer is
tethered at a position (9) with access to each of the four chambers. The cross
section of this
layout would be as depicted in Figures 24 and 25, and the charged polymer can
be moved into
each of the four addition chambers by operation of the electrodes in the
electrical control layer (1
in Figure 24).
[036] Figure 27 depicts a top view of a nanopore chip having multiple sets of
dual addition
chambers as depicted in Figure 24 and 25, allowing multiple polymers to be
synthesized in
parallel. The monomers are (here dATP and dGTP nucleotides represented as A
and G) are
loaded into each chamber via serial flow paths. One or more common deblocker
flow cells
allows for the polymers to be deprotected after addition of a monomer or
oligomer in one of the
addition chambers. This also allow the polymers to be detatched on demand (for
example using
7
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
a restriction enzyme in the case of DNA, or a chemical detachment from the
surface adjacent to
the nanopore, and collected externally. In this particular embodiment, the
deblocker flow cells
are perpendicular to the fluidics loading channels used to fill the addition
chambers.
[037] Figure 28 depicts further details of the wiring for the dual addition
chamber layouts. The
electrical control layer (1) includes wiring made from metal or polysilicon.
The wiring density is
increased by 3D stacking, with electrical isolation provided by dielectric
deposition (e.g., via
PECVD, sputtering, ALD etc). The contact (11) to the top electrode by in the
addition chamber
in in one embodiment is made using Through Silicon Via (TSV) by Deep Reactive
Ion Etch
(DRIE) (cryo or BOSCH process). Individual voltage control (12) allows for
each addition
chamber to be addressed individually, allowing fine control of the sequence of
multiple polymers
in parallel. The right side of the figure depicts a top view illustrating
wiring to multiple addition
cells. The electrical ground layer (3) may be common (as shown) or split to
reduce cross
coupling between the cells.
[038] Figure 29 depicts an alternative configuration where the control
electrodes (13) for the
addition chambers may be deposited on the side of the chamber in a wrap around
fashion instead
of at the top of the chamber.
[039] Figure 30 depicts a SDS-PAGE gel confirming that topoisomerase addition
protocol as
described in Example 3 works, with bands corresponding to the expected A5 and
B5 products
being clearly visible. .
[040] Figure 31 depicts an agarose gel confirming that the PCR product of
Example 5 is the
correct size. Lane 0 is a 25 base pair ladder; lane 1 is product of
experiment, line corresponding
to expected molecular weight; lane 2 is negative control #1; lane 3 is
negative control #2; lane
4is negative control #4.
[041] Figure 32 depicts an agarose gel confirming that the restriction enzyme
as described in
Example 5 produces the expected product. The ladder on the left is a 100 base
pair ladder. Lane
1 is undigested NAT1/NAT9c, Lane 2 is digested NAT1/NAT9c. Lane 3 is
undigested
NAT1/NAT9cI, Lane 4 is digested NAT1/NAT9cI.
[042] Figure 33 depicts Immobilization of DNA near nanopore. Panel (1) shows
DNA with an
origami structure on one end in the left chamber (in the actual nanochip,
there initially are many
such origami structures in the left chamber). Panel (2) illustrates the system
with anode on the
right, which drives the DNA to the nanopore. While the DNA strand is able to
transit the
8
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
nanopore, the origami structure is too large to pass through, so the DNA is
'stuck'. Turning the
current off (panel 3) allows the DNA to diffuse. With suitable chemistry, the
end of the DNA
strand is able to bind when it comes in contact with the surface near the
nanopore. In panel (4) a
restriction enzyme is added, which cuts the origami structure from the DNA.
The chamber is
washed to remove enzyme and residual DNA. The final result is a single DNA
molecule attached
near a nanopore, able to be moved back and forth through the nanopore.
[043] Figure 34 depicts a basic functioning nanopore. In each panel, the y-
axis is current (nA)
and the x-axis is time (s). The left panel "Screening of RF Noise" illustrates
the utility of the
Faraday cage. A chip with no nanopore is placed in the flow cell and 300mV
applied. When the
lid of the Faraday cage is closed (first arrow) the noise reduction can be
seen. A small spike
occurs when the latch is closed (second arrow). Notice the current is ¨0 nA.
After pore
manufacture (middle panel), application of 300mV (arrow) results in a current
of ¨3.5 nA. When
DNA is applied to the ground chamber and +300mV is applied DNA translocations
(right panel)
can be observed as transient decreases in the current. (Note, in this case the
TS buffer is used:
50mM Tris, pH 8, 1M NaCl). Lambda DNA is used for this DNA translocation
experiment.
[044] Figure 35 depicts a simplified picture illustrating the main features of
the DNA origami
structure: a large single stranded region, the cubic origami structure, and
the presence of 2
restriction sites (SwaI and AlwN1) near the origami structure.
[045] Figure 36 depicts an electron microscope image of the manufactured DNA
origami
structure, and demonstrates the expected topology. Origami is made in 5mM Tris
base, 1 mM
EDTA, 5 mM NaCl, 5mM MgCl2. In order to maintain the origami structure, it is
preferable to
have Mg concentrations of ¨5mM or Na /K+ concentrations around 1M. The origami
structure
is stored at 4 C at 500nM.
[046] Figure 37 depicts a restriction digestion of the DNA origami to confirm
correct assembly
and function. The lane on the far left provides MW standards. The restriction
sites are tested by
digesting the origami with AlwN1 and Swal. The four test lanes contain
reagents as follows
(units are microliters):
9
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1
................................. \\µµ = :=k
5weli
NE13. 3.1.10x: .3
2
[047] Test lane (1) is a negative control; (2) is digestion with Swal; (3) is
digestion with
AlwN1; (4) is double digestion with Swal/AlwNl. Digestion is performed at room
temperature
for 60 minutes, followed by 37 C for 90 minutes. Agarose gel 1/2x TBE-Mg
(1/2x TBE with
5mM MgCl2), visualized with ethidium bromide staining. Individual digestion
with either
enzyme shows no mobility effect in a gel, but digestion with both enzymes
together (lane 4)
results in two fragments of different lengths, as expected.
[048] Figure 38 depicts binding of biotin-labeled oligonucleotides to
streptavadin-coated beads
vs. binding to control BSA coated beads. The y-axis is fluorescence units,
'pre-binding' is oligo
fluorescence from test solution prior to binding beads, (-) controls are
fluorescence seen after
binding to two different batches of BSA-conjugated beads, SA-1 and SA-2 are
fluorescence seen
after binding to 2 different batches of streptavidin-conjugated beads. A small
apparent amount of
binding is observed with BSA-conjugated beads, but much larger binding is seen
with the
streptavidin-conjugated beads.
[049] Figure 39 depicts binding of biotin-labeled oligonucleotides to
streptavadin-coated beads
vs. binding to control BSA coated beads in different buffer systems, MPBS and
HK buffer. The
left bar 'Neg Ctrl' is the oligo fluorescence from test solution prior to
binding the beads. Middle
column shows fluorescence of 'BSA beads' and right column of `SA beads' after
binding to
BSA or streptavidin beads respectively. In both buffer systems, the
fluoresence is reduced by the
streptavidin beads relative to controls, indicating that the biotin-labeled
oligonucleotides are
binding well to streptavadin-coated beads in different buffer systems.
[050] Figure 40 depicts a functioning conjugated SiO2 nanopore, wherein the
surface is
strepavidin coated on one side and BSA coated on the other. The x-axis is time
and the y-axis is
current. The dot shows the point where the current is reversed. There is a
brief overshoot when
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
the current is reversed, then the current settles to approximately the same
absolute value. The
nanopore shows a current of ¨ 3nA at 200mV and --3nA at -200mV.
[051] Figure 41 shows a representation of an origami DNA structure inserted
into a nanopore.
[052] Figure 42 shows a representation of attachment of the single stranded
DNA to the
streptavidin-coated surface adjacent to the nanopore.
[053] Figure 43 shows experimental results of an origami DNA attached to the
surface near a
nanopore. Current is + or - ¨2.5nA in both directions, which is less than the
original current of
+/- ¨3nA, reflecting partial obstruction by the origami structure. The x-axis
is time (s), y-axis is
current (nA), circles represent voltage switch points.
[054] Figure 44 shows the insertion of origami DNA, resulting in a slight drop
in current. The
origami immediately exits the nanopore when the current is released. The x-
axis is time (s), y-
axis is current (nA), circles represent voltage switch points.
[055] Figure 45 shows a representation of controlled movement of a DNA strand
back and forth
through a nanopore by application of current. On the left side the DNA is in
the pore, so the
observed current will be lower than if there was no DNA in the pore. When the
current is
reversed (right side) the is no DNA in the pore so the current will be
unchanged.
[056] Figure 46 shows experimental results confirming this representation.
When a positive
voltage is applied the current is ¨3nA, comparable to the current typically
observed when the
pore is open. When the voltage is reversed the current is --2.5nA. This is
lower than the current
typically seen when the pore is open, and corresponds to the current typically
observed when the
pore is blocked by a strand of DNA. Several sequential voltage switches show
consistent results,
suggesting that the DNA is alternating in configuration as depicted in Figure
45.
[057] Figure 47 shows different conjugation chemistries to link the DNA to the
surface
adjacent to the naopore.
DETAILED DESCRIPTION
[058] The following description of the preferred embodiment(s) is merely
exemplary in nature
and is in no way intended to limit the invention, its application, or uses.
[059] As used throughout, ranges are used as shorthand for describing each and
every value
that is within the range. Any value within the range can be selected as the
terminus of the range.
In addition, all references cited herein are hereby incorporated by referenced
in their entireties.
11
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
In the event of a conflict in a definition in the present disclosure and that
of a cited reference, the
present disclosure controls.
[060] Unless otherwise specified, all percentages and amounts expressed herein
and elsewhere
in the specification should be understood to refer to percentages by weight.
The amounts given
are based on the active weight of the material.
[061] "Nanochip" as used herein refers to a nanofluidic device, comprising
multiple chambers
containing fluid and optionally channels allowing for fluid flow, wherein the
critical dimensions
of the features of the nanochip, for example the width of the elements
dividing the chambers
from one another, are from one atom to 10 microns in thickness, e.g., smaller
than one micron,
e.g. 0.01-1 micron. The flow of materials in the nanochip may be regulated by
electrodes. For
example, as DNA and RNA are negatively charged, they will be drawn to a
positively charged
electrode. See, e.g., Gershow, M, et al., Recapturing and Trapping Single
Molecules with a
Solid State Nanopore, Nat Nanotechnol. (2007) 2(12): 775-779, incorporated
herein by
reference. The flow of fluids may in some cases also be regulated by gate
elements, and by
flushing, injecting, and/or suctioning fluids into or out of the nanochip. The
system is capable of
precise multiplexed analysis of nucleic acids (DNA/RNA). In certain
embodiments, the nanochip
can be made of a silicon material, for example silicon dioxide or silicon
nitride. Silicon nitride
(e.g., Si3N4) is especially desirable for this purpose because it is
chemically relatively inert and
provides an effective barrier against diffusion of water and ions even when
only a few nm thick.
Silicon dioxide (as used in the examples herein) is also useful, because it is
a good surface to
chemically modify. Alternatively, in certain embodiments, the nanochip, may be
made in whole
or in part out of materials which can form sheets as thin as a single molecule
(sometimes referred
to as single layered materials), for example graphene, e.g., as described in
Heerema, SJ, et al,
Graphene nanodevices for DNA sequencing, Nature Nanotechnology (2016) 11: 127-
136; Garaj
S et al., Graphene as a subnanometre trans-electrode membrane, Nature (2010)
467 (7312),
190-193, the contents of each of which are incorporated herein by reference,
or a transition
metal dichalcogenide, e.g., molybendum disulfide (MoS2) as described in Feng,
et al.,
Identification of single nucleotides in MoS2 nanopores, Nat Nanotechnol.
(2015) 10(12):1070-
1076, the contents of which are incorporated herein by reference, or boron
nitride, as described
in Gilbert, et al. Fabrication of Atomically Precise Nanopores in Hexagonal
Boron Nitride, eprint
arXiv:1702.01220 (2017).
12
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[062] In some embodiments, the nanochip comprises such a single layered
material which is
relatively stiff and inert, e.g., at least as inert and stiff as graphene,
such as MoS2. Single layered
materials may, for example be used as all or part of the membrane comprising
the nanopore. The
nanochip may be lined in parts with metal, for example the walls may be
layered (e.g. metal ¨
silicon nitride ¨ metal), and the metal can then be configured to provide a
controllable pair of
electrodes near the nanopore, so that the nucleic acid can be moved back and
forth through the
nanopore by electromotive force, and also can be sequenced by measuring the
change in electric
potential as the nucleic acid passes through the nanopore.
[063] Nanochip nanofluidic devices for sequencing DNA are generally known, for
example as
described in Li, J., et al, Solid-state nanopore for detecting individual
biopolymers, Methods Mol
Biol. (2009)544:81-93; Smeets RM, et al. Noise in solid-state nanopores, PNAS
(2008)105(2):417-21; Venta K, et al., Differentiation of short, single-
stranded DNA
homopolymers in solid-state nanopores, ACS Nano. (2013)7(5):4629-36; Briggs K,
et al.
Automated fabrication of 2-nm solid-state nanopores for nucleic acid analysis,
Small
(2014)10(10):2077-86; and Chen Z, DNA translocation through an array of kinked
nanopores,
Nat Mater. (2010)9(8):667-75; the entire contents of each of which are
incorporated herein by
reference, e.g. for their teachings on the design and manufacture of nanochips
comprising
nanopores.
[064] "Nanopore" as used herein is pore having a diameter of less than 1
micron, e.g., 2-20 nm
diameter, for example on the order of 2-5 nm. Single stranded DNA can pass
through a 2 nm
nanopore; single or double stranded DNA can pass through a 4 nm nanopore.
Having a very
small nanopore, e.g., 2-5 nm, allows the DNA to pass through, but not the
larger protein
enzymes, thereby allowing for controlled synthesis of the DNA (or other
charged polymer).
Where larger nanopores (or smaller protein enzymes) are used, the protein
enzyme may be
conjugated to a substrate that will prevent it from passing though the
nanopore, e.g. to a larger
molecule, such as a larger protein, to a bead, or to a surface in the chamber.
Different types of
nanopores are known. For example, biological nanopores are formed by assembly
of a pore-
forming protein in a membrane such as a lipid bilayer. For example, a-
hemolysin and similar
protein pores are found naturally in cell membranes, where they act as
channels for ions or
molecules to be transported in and out of cells, and such proteins can be
repurposed as
nanochannels. Solid-state nanopores are formed in synthetic materials such as
silicon nitride or
13
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
graphene e.g., by configuring holes in the synthetic membrane, e.g. using
feedback controlled
low energy ion beam sculpting (IBS) or high energy electron beam illumination.
Hybrid
nanopores can be made by embedding a pore-forming protein in synthetic
material. Where there
is a metal surface or electrode at either end or either side of the nanopore,
a current flow across
the nanopore may be established through the nanopore via an electrolyte media.
Electrodes may
be made of any conductive material, for example silver, gold, platinum,
copper, titanium dioxide,
for example silver coated with silver chloride.
[065] Methods for configuring a nanopore in a solid state, e.g., silicon
nitride, membrane, are
known. In one approach, a silicon substrate is coated with the membrane
material, e.g., silicon
nitride, and the overall configuration of the membrane is created using
photolithography and wet
chemical etching, to provide silicon nitride membranes of the desired size for
incorporation into
a nanochip, e.g., about 25x25 microns. Initial 0.1 micron diameter holes or
cavities are punched
in the silicon nitride membrane using a focused ion beam (FIB). Ion beam
sculpting can
configure the nanopore either by shrinking a larger pore, e.g., by ion beam
induced lateral mass
transport on the membrane surface, or by removing membrane material by ion
beam sputtering
layer by layer from the flat side of the membrane containing a cavity from
opposing sides, so that
when the cavity is ultimately reached, there is a sharp-edged nanopore. The
ion beam exposure is
extinguished then the ion current transmitted through the pore is appropriate
for the desired pore
size. See, e.g.. Li, J., et al., Solid-state nanopore for detecting individual
biopolymers, Methods
Mol Biol. (2009)544:81-93. Alternatively, the nanopores can be configured
using high energy
(200-300 keV) electron beam illumination in a TEM. Using semiconductor
processing
techniques, e-beam lithography, reactive-ion etching of 5i02 mask layers, and
anisotropic KOH
etching of Si, pyramidal 20 x 20 nm and larger pores are made in a 40 nm thick
membrane. The
electron beam in a TEM is used to shrink the larger 20 nm pores to smaller
ones. The TEM
allows the shrinking process to be observed in real-time. Using a thinner
membrane (e.g., < 10
nm thick) nanopores can be drilled with a high energy focused electron beam in
a TEM. See,
generally, Storm AJ, et al. Fabrication of solid-state nanopores with single-
nanometre precision.
Nature Materials (2003) 2:537-540; Storm AJ, et al. Translocation of double-
stranded DNA
through a silicon oxide nanopore. Phys. Rev. E (2005)71:051903; Heng JB, et
al. Sizing DNA
Using a Nanometer-Diameter Pore. Biophys. J (2004) 87(4):2905-11; the contents
of each of
which are incorporated herein by reference.
14
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[066] In other embodiments, the nanopores are made using dielectric breakdown,
using a
relatively high voltage potential across the membrane, wherein the voltage is
raised until current
is detected, e.g., as described in Kwok, et al., "Nanopore Fabrication by
Controlled Dielectric
Breakdown," PLOS ONE (2014) 9(3): e92880, the contents of which are
incorporated herein by
reference.
[067] Using these techniques, and depending of course on the exact technique
used and the
thickness and exact composition of the membrane, the overall shape of the
nanopore in a solid
material such a silicon nitride may roughly resemble two funnels with their
apexes coming
together at the narrowest point, i.e., the actual nanopore. Such a double cone
shape is conducive
to steering the polymer through the nanopore and back. Imaging techniques, for
example atomic
force microscopy (AFM) or transmission electron microscopy (TEM), particularly
TEM, can be
used to verify and measure the size, location and configuration of the
nanomembranes, the FIB
holes or cavities, and the final nanopores.
[068] In some embodiments, one end of the polymer, e.g., DNA, is tethered near
the nanopore
or on the inner wall of the funnel leading to the nanopore. Since the polymer
approaches the
nanopore initially by diffusion, then is driven by the electrical gradient,
the gradient-driven
motion is maximized and the diffusive motion minimized, and speed and
efficiency thereby
enhanced, if one end of the polymer is tethered close to the nanopore. See,
e.g. Wanunu M,
Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt
gradient, Nat
Nanotechnol. (2010) 5(2):160-5; Gershow M., Recapturing and trapping single
molecules with a
solid-state nanopore. Nat Nanotechnol. (2007) 2(12):775-9; Gershow, M.,
Recapturing and
Trapping Single Molecules with a Solid State Nanopore. Nat Nanotechnol. (2007)
2(12): 775-
779.
[069] In one embodiment, one end of the polymer, e.g., DNA, is attached to a
bead and the
polymer is driven through the pore. Attachment to the bead will stop the
polymer from moving
all the way through the nanopore on the opposite side of the dividing membrane
in an adjacent
chamber. The current is then turned off, and the polymer, e.g., DNA, attaches
to the surface
adjacent to the nanopore in a chamber on the other side of the dividing
membrane. For example,
in one embodiment, one end of ssDNA is covalently attached to a 50nm bead, and
the other end
is biotinylated. Streptavidin is bound to the area at the desired point of
attachment in the
chamber on the other side of the dividing membrane. The DNA is pulled through
the nanopore
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
by an electrical potential, and the biotin attaches to the streptavidin. The
attachments to the bead
and/or the surface adjacent to the nanopore can be either covalent bonds or
strong noncovalent
bonds (like the biotin-streptavidin bond). The bead is then cut off with an
enzyme and flushed
away. In some embodiments, the single stranded DNA is cleaved with a
restriction enzyme
which cleaves single stranded DNA, e.g., as described in K. Nishigaki, Type II
restriction
endonucleases cleave single-stranded DNAs in general. Nucleic Acids Res.
(1985) 13(16):
5747-5760, incorporated herein by reference. In other embodiments, a
complementary
oligonuceotide is provided to make a double-stranded restriction site, which
can then be cleaved
with the corresponding restriction enzyme.
[070] As the polymer passes through the nanopore, the change in electric
potential or current
across the nanopore caused by the partial blockage of the nanopore as the
polymer passes
through can be detected and used to identify the sequence of monomers in the
polymer, as the
different monomers can be distinguished by their different sizes and
electrostatic potentials.
[071] The use of nanochips comprising nanopores in a method of DNA
fabrication, as
described herein, is not disclosed in the art, but such chips are well known
and commercially
available for rapid sequencing of DNA. For example, the MinION (Oxford
Nanopore
Technologies, Oxford, UK) is small and can be attached to a laptop computer.
As a single strand
of DNA passes through a protein nanopore at 30 bases per second, the MinION
measures the
electrical current. The DNA strands in the pore disrupts the ionic flow,
resulting in changes in
current corresponding to the nucleotides in the sequence. Mikheyev, AS, et al.
A first look at the
Oxford Nanopore MinION sequencer, Mol. Ecol. Resour. (2014)14,1097-1102. While
the
accuracy of the MinION is poor, requiring repeated resequencing, the speed and
accuracy of the
sequencing using the nanochips of the present invention can be greatly
improved if the DNA
being read contains only two easily distinguishable bases, e.g. A and C.
[072] The membrane comprising the nanopores may, in some embodiments, have a
trilayer
configuration, with a metal surface on either side of an insulating core
material, e.g.. a silicon
nitride membrane. In this embodiment, the metal surfaces are configured, e.g.,
by lithographic
means, to provide a microcircuit with paired electrodes, one at each end of
each nanopore, e.g.,
such that a current flows across the nanopore may be established between the
electrodes and
through the nanopore via an electrolyte media, which current can draw the
polymer through the
nanopore and by reversing the polarity, can draw it back. As the polymer
passes through the
16
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
nanopore, the electrodes can measure the change in electric potential across
the nanopore so as to
identify the sequence of monomers in the polymer.
[073] In some embodiments, the sequence of the polymer is designed to store
data. In some
embodiments, the data is stored in a binary code (1's and O's). In some
embodiments each base
corresponded to a 1 or 0. In other embodiments, an easily recognized sequence
of two or more
bases corresponds to a 1 and another easily recognized sequence of two or more
bases
corresponds to a 0. In other embodiments, the data is can be stored in a
ternary, quaternary or
other code. In a particular embodiment, the polymer is DNA, for example single
stranded DNA,
wherein the DNA contains only two base types and does not contain any bases
capable of self-
hybridizing, e.g., wherein the DNA comprises adenines and guanines, adenines
and cytosines,
thymidines and guanines, or thymidines and cytosines. In some embodiments, the
two bases
may be interspersed with one or more additional bases, for example A and C may
contain a T to
"punctuate" the sequence, e.g., by indicating a break in a coding sequence, at
a frequency that
does not result in significant self-hybridization. In other embodiments, e.g.,
where the nucleic
acid is double stranded, some or all available bases may be employed.
[074] The nucleotide bases may be natural or may in some embodiments consist
of or include
nonnatural bases, e.g. as described in Malyshev, D. et al. "A semi-synthetic
organism with an
expanded genetic alphabet", Nature (2014) 509: 385-388, incorporated herein by
reference.
[075] In one embodiment, the data is stored by addition of single monomers,
e.g., single
nucleotides in the case of DNA, to the polymer. In one embodiment, the polymer
is DNA and the
monomers are adenine (A) and cytosine (C) residues. A and C residues have an
advantage
because (i) A and C have a large size difference, so differentiation through
the nanopore should
be facilitated, (ii) A and C do not pair with one another so do not form
significant secondary
structure which could complicate interpretation of the nanopore signal, and
(iii) for the same
reason, G's are less preferred as they are know to form guanine tetrads.
Nucleotides are added by
terminal transferase (or polynucleotide phosphorylase), but the nucleotides
are 3'-blocked so that
only a single nucleotide is added at a time. The block is removed prior to
addition of the next
nucleotide.
[076] In some embodiments, the DNA is left in the nanochip. In other
embodiments, it is
removed, and optionally converted to double stranded DNA and/or optionally
converted to
crystalline form, e.g. to enhance long term stability. In still other
embodiments, DNA can be
17
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
amplified and the amplified DNA removed for long term storage, while the
original template
DNA, for example DNA bound to the wall of a chamber in the nanochip, can be
left in the
nanochip, where it can be read and/or used as a template to make additional
DNA.
[077] In some embodiments, the DNA or other polymer is anchored to a surface
proximate to
the nanopore during synthesis. For example, in one embodiment, single stranded
DNA
molecules are each attached at the 5' end to a surface proximate to a
nanopore, wherein the
current at each nanopore can be independently regulated by electrodes for that
nanopore, so that
the 3'end of the DNA molecule can be pulled forward through the nanopore from
a retaining
chamber into a flow chamber containing a flow of 3'-protected dNTPs together
with a
polymerase or terminal transferase enzyme to add a 3'-protected dNTPs, or
retained in the
retaining chamber where the nanopore excludes the enzyme, so that the dNTP is
not added. See,
e.g., depictions at Figures 12-16 and also Figures 18 and 19. In other
embodients, single
stranded DNA is built by addition to the 5' end (with the 3' end attached),
using topisomerase, as
described more fully below. By controlling whether or not each DNA molecule
participates in
each cycle, the sequence of each DNA molecule can be precisely controlled,
e.g., as follows:
Step Flow Nanopore 1 Nanopore 2
Chamber
0 Retain Retain
1 flow 'A'
2 Forward into flow retain
chamber
'A' gets added
3 Reverse back into retain
resting chamber;
oligo is deprotected
4 Flush with retain retain
buffer
flow 'C' forward forward
'C' gets added 'C' gets added
18
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
6 reverse reverse
oligo is deprotected oligo is deprotected
7 retain forward
'C' gets added
8 retain reverse
oligo is deprotected
9 Flush with retain retain
buffer
Flow A = 3'-protected dATP
Flow C = 3'-protected dCTP
Nanopore 1 and Nanopore 2 in this schematic are associated with different DNA
strands and the
positions of which (in or out of the flow chambers) are separately
controllable. The DNA can be
deprotected either by a specific enzyme in the retaining chamber, or by
changing the flow in the
flow chamber to provide deprotection by enzymatic, chemical, light-catalyzed
or other means. In
one embodiment, the deblocking agent(s) flow between cycles of Flow A and Flow
C, e.g., when
the flow chamber is being washed with buffer, so that the deblocking agent
does not deprotect
the nucleotide building blocks. In other embodiments, the deprotecting
agent(s) are too bulky to
cross to the flow chamber via the nanopores.
[078] The end result in the foregoing example would be that an A and a C were
added to the
DNA at Nanopore 1, and a C and a C were added to the DNA at Nanopore 2.
[079] In another embodiment, the chamber configuration is similar, but with
double stranded
DNA anchored to the surface proximate to a nanopore, and oligonucleotide
fragments, for
example of two or more types, each corresponding to a binary code, are added
sequentially, e.g.,
using site-specific recombinases, i.e., enzymes that spontaneously recognize
and cleave at least
one strand of a double strand of nucleic acids within a sequence segment known
as the site-
specific recombination sequence, for example using topoisomerase-charged
oligonucleotides as
described below.
[080] In certain embodiments, it may be desirable to keep the electrically
charged polymer,
e.g., DNA, in a condensed state subsequent to synthesis. There are several
reasons for this:
19
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
= the polymer should be more stable in this form,
= condensing the polymer will keep down crowding and allow use of longer
polymers in
small volumes,
= orderly condensation can reduce potential that the polymer will form
knots or tangles,
= if any of the chambers are interconnected it will help keep the polymer
from getting so
long that it goes through a different pore than it is supposed to when current
is applied,
= condensation will help keep polymer away from the electrodes, where
electrochemistry
could damage the polymer.
A human cell is about 10 microns but contains 8 billion base pairs of DNA.
Stretched out it
would be over a meter long. The DNA fits into the cell because it is wound
around histone
proteins. In certain embodiments, histones or similar proteins provide a
similar function in the
nanochips of the invention. In some embodiments, the interior surfaces of the
nanochips are
slightly positively charged so that electrically charged polymer, e.g.,DNA
tends to stick weakly
to them.
[081] In certain embodiments, the charged polymer, e.g., single or double
stranded DNA,
bound to a surface proximate to a nanopore. This can be accomplished in
various ways.
Generally, the polymer is localized to the nanopore by attaching the polymer
to a relatively bulky
structure (e.g. a bead, a protein, or a DNA origami structure (described
below), having a
diameter too large to fit through the nanopore, e.g., >10nm, e.g., about 20-
50nm), pulling the
charged polymer through the nanopore using current, anchoring the end of the
polymer distal to
the bulky structure to the surface adjacent to the nanopore, and cleaving off
the bulky structure.
[082] The step of anchoring the end of the polymer distal to the bulky
structure to the surface
adjacent to the nanopore, can be accomplished in various ways. In one
embodiment, the polymer
is a single stranded DNA, and there are pre-attached DNA strands (about 50bp)
which are
complementary to part of the single stranded DNA, so that the single stranded
DNA and the pre-
attached DNA strands can join via base pairing. If the pairing is strong
enough, it will be
sufficient to keep the DNA anchored even while being manipulated. An advantage
of this
method of attachment is that it allows the DNA to be removed from the nanopore
chip if desired
for long term storage of the DNA. Alternatively, the strand is attached to the
surface covalently,
either using conjugation chemistry, e.g., streptavidin-biotin conjugation as
described in Example
1 below, or 'click' chemistry (see Kolb, et al. Angew. Chem. Int. Ed.
(2001)40: 2004-2021,
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
incorporated herein by reference, and/or using enzymatic attachment, for
example by pre-
attaching oligos covalently to the distal surface, and then using DNA ligase
to connect them.
[083] Once the distal end of the strand is attached to the surface adjacent to
the nanopore, the
bulky structure is cleaved off, e.g., using an endonuclease which cleaves at a
restriction site near
the bulky structure.
[084] The bulky structure may be a bead, a bulky molecule, e.g., a protein
which is reversibly
bound to a DNA strand, or a DNA origami structure. DNA origami involves the
use of base
pairing to create three dimensional DNA structures. DNA origami techniques are
generally
described in Bell, et al, Nano Lett. (2012)12: 512-517, incorporated herein by
reference. For
example, in the current invention, DNA origami can be used to attach the
single DNA molecule
to a surface adjacent to the nanopore. In one embodiment, the structure is a
'honey comb cube',
e.g., about 20nm on each side. This prevents this part of the DNA from going
through the
nanopore (just like in the attached paper). There is a long strand of DNA
(single or double
stranded) attached to the origami structure. The DNA strand goes through
through the nanopore,
until the origami cube meets the nanopore and blocks further progress. The
current is then turned
off and the strand is attached to the surface adjacent to the nanopore.
[085] In another embodiment, the electrically charged polymer, e.g., DNA, with
the origami
structure is in the middle chamber of a three chamber configuration. The
origami will keep the
DNA from completely entering the other 2 chambers (or other one chamber in the
2 chamber
example). Thus, in this example the polymer doesn't need to be anchored to the
surface. This
reduces the risk that the polymer will knot up and avoids the need for the
step of binding one end
of the polymer to the surface and cleaving off the bulky portion at the other
end. The volume of
the chamber with the origami should be kept as small as practical so that the
polymer stays
relatively close to the pore, which will help ensure that it translocates
quickly when current is
applied. It should be noted that while the middle chamber containing the
origami portion of the
polymer can't be interconnected with other middle chambers (or else the
different polymers will
get mixed up), the other chambers (or sets of chambers in the 3 chamber
example) can be
interconnected. These other chambers can have larger volumes if desired, as
the polymer will
necessarily be close to the pore (some of it will be in the pore in fact) when
the DNA is moved to
that chamber.
21
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[086] In some embodiments, the device comprises three in-line chambers,
wherein the addition
chambers are contiguous to allow for flow, and have common electrodes, while
the `deprotece
chambers are fluidically isolated except for the flow through the nanopore and
have unique
electrodes.
[087] In other embodiments, the DNA or other charged polymer is not anchored
but can move
between synthesis chamber(s) and deprotection chamber(s), under control of
electrodes in the
chambers, while the polymerase and the deprotecting agents are restricted from
movement
between chambers because they are too bulky to pass through the nanopores
connecting the
chambers and/or are anchored to a surface in a chamber. See, e.g., Figures 1-9
and 16-17.
[088] The current needed to move the charged polymer through the nanopore
depends on, e.g.,
the nature of the polymer, the size of the nanopore, the material of the
membrane containing the
nanopore, and the salt concentrations, and so will be optimized to the
particular system as
required. In the case of DNA as used in the examples herein, examples of
voltage and current
would be, e.g., 50-500mV, typically 100-200mV, and 1-10nA, e.g., about 4nA,
with salt
concentrations on the order of 100mM to 1M.
[089] The movement of charged polymer, e.g., DNA, through the nanopore is
normally very
rapid, e.g., 1 to 51.ts per base, so on the order of one million bases per
second (1 MHz, if we adopt
the nomenclature of frequency), which presents challenges for getting an
accurate reading
distinct from the noise in the system. Using current methods, either (i) a
nucleotide needed to be
repeated in a sequence, e.g., ca. 100 times successively, in order to produce
a measurable
characteristic change, or (ii) using protein pores, such as Alpha hemolysin
(aHL) or
Mycobacterium smegmatis porin A (MspA), which provide a relatively long pore
with potential
for multiple reads as the base moves through the polymer, and in some cases,
can be adapted to
provide a controlled feed of DNA through the pore one base at a time, in some
cases using an
exonuclease to cleave each base as it passes through. Various approaches are
possible, e.g.,
= slowing down the speed of the polymer, from ca. 1MHz to ca. 100-200Hz,
for example
using a medium comprising an electrorheological fluid in which becomes more
viscous
when a voltage is applied, thereby slowing down the speed of the polymer
through the
nanopore, or a plasmonic fluid system, wherein the viscosity of the medium can
be
controlled by light; or a molecular motor or ratchet;
22
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
= providing a sequence in the polymer, e.g., in single stranded DNA, which
will form a
bulky secondary structure, e.g., a "hairpin", "hammerhead", or "dumbbell"
configuration,
which will have to be linearized in order to fit through the nanopore, thereby
making the
information less dense and providing a signal having a longer duration;
= providing many reads of the same sequence, e.g., by using rapidly
alternating current,
allowing for many reads of the same sequence frame, and combined with brief
bursts of
direct current to pull the molecule to the next sequence frame, by reading the
entire
sequence multiple times, or by reading multiple identical sequences in
parallel, in each
case collating the reads to provide a consensus read that amplifies the signal
;
= measuring an impedance change in a high frequency signal induced by a
change in
capacitance as monomers (e.g., nucleotides) pass through the nanopore, rather
than
measuring changes in current flow or resistance directly;
= enhancing the differences in current, resistance or capacitance between
different bases,
e.g., by using non-natural bases which have a greater difference in size or
are otherwise
modified to give different signals, or by forming larger secondary structures
within the
DNA, such as a "hairpin", "hammerhead", or "dumbbell" configuration, which
provide
an enhanced signal because of their larger size;
= using an optical reading system, for example using an integrated optical
antenna adjacent
to the nanopore, which acts as an optical transducer (or optical signal
enhancer) to
complement or replace standard ion current measurements, e.g., as described in
Nam, et
al., "Graphene Nanopore with a Self-Integrated Optical Antenna", Nano Lett.
(2014)14:
5584-5589, the contents of which are incorporated herein by reference. In some
embodiments, the monomers , e.g. DNA nucleotides, are labeled with fluorescent
dyes so
that each different monomer fluoresces at a signature intensity as it passes
through the
junction of the nanopore and its optical antenna. In some embodiments, a solid-
state
nanopore strips off fluorescent labels, leading to a series of detectable
photon bursts, as
the polymer passes through the nanopore at high speed, e.g. as described in
McNally et
al., "Optical recognition of converted DNA nucleotides for single molecule DNA
sequencing using nanopore arrays", Nano Lett. (2010)10(6): 2237-2244, and
Meller A.,
"Towards Optical DNA Sequencing Using Nanopore Arrays", I. Biomol Tech. (2011)
22(Suppl): S8¨S9, the contents of each of which are incorporated herein by
reference.
23
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[090] In one embodiment, the charged polymer is a nucleic acid, e.g., single
stranded DNA,
wherein the sequences provide a secondary structure. Bell, et al., Nat
Nanotechnol.(2016)11(7):645-51, incorporated herein by reference, describes
using a relatively
short sequence of dumbbell configurations detectible in a solid state nanopore
format, to label
antigens in an immunoassay. The nanopores used in Bell, et al. were relatively
large, so the
entire dumbbell structure could pass through the pore, but using nanopores
smaller than the
diameter of the dumbbell configuration, the DNA will "unzip" and become
linearized. More
complex configurations can be used, e.g. wherein each bit corresponds to a
sequence similar to a
tRNA (see, e.g., Henley, et al. Nano Lett. (2016)16: 138-144, incorporated
herein by reference).
Thus the invention provides charged polymers, e.g. single stranded DNA, having
at least two
types secondary structure, wherein the secondary structure encode data (e.g.
binary data, wherein
one secondary structure type is a 1 and a second is a 0). In other
embodiments, secondary
structures are used to slow down the passage of the DNA through the nanopore
or to provide
breaks in the sequence, to facilitate reading of the sequence.
[091] In another embodiment, the invention utilizes a DNA molecule comprising
a series of at
least two different DNA motifs, wherein each motif specifically binds to a
particular ligand, for
example a gene regulatory protein for double stranded DNA or a tRNA for single
stranded DNA,
wherein the at least two different DNA motifs encode information, e.g. in a
binary code, wherein
one motif is a 1 and a second is a 0, e.g., wherein the ligand enhances the
signal difference (e.g.
change in current or capacitance) across the nanopore as the DNA passes
through the nanopore.
[092] As discussed above, when different monomers pass through the nanopore,
they affect the
current flow through the nanopore, primarily by physically blocking the
nanopore and changing
the conductance across the nanopore. In existing nanopore systems, this change
in current is
measured directly. The problem with current reading systems is that there is
considerable noise
in the system, and in the case of DNA, for example, when measuring current
fluctuations as
different nucleotide units pass through the nanopore, a relatively long
integration time, on the
order of one hundredth of a second, is needed to accurately detect differences
between different
monomers, e.g., between different bases. Recently, it has been shown that
changes in impedance
and capacitance can be useful to study cells and biological systems, despite
the potential for
complex interactions with salts and biological molecules. For example,
Laborde, et al. Nat
24
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
Nano. (2015)10(9):791-5 (incorporated herein by reference), demonstrates that
high-frequency
impedance spectroscopy can be used to detect small changes in capacitance
under physiological
salt conditions and image microparticles and living cells beyond the Debye
limit.
[093] In one embodiment of the invention, therefore, we measure capacitive
variance rather
than measuring current variance directly, for example, wherein the sequence of
the charged
polymer is identified by measuring the phase change in a radiofrequency signal
induced by
change in capacitance as the monomers (e.g., nucleotides) pass through the
nanopore.
[094] Simply stated, capacitance exists in any circuit where there is a gap
between one
electrical conductor and another. While current varies directly with
capacitance, it does not vary
simultaneously with capacitance. For example, if we were to plot the current
and voltage over
time in a capacitive circuit with an alternating electrical current, we would
see that while both
current and voltage each form a sine wave, the waves are out of phase. When
there is a change in
current, there is a change in capacitance, which is reflected in a change in
the phase of the signal.
A radiofrequency alternating current provides a signal with fixed frequency
and amplitude, while
the phase of the signal will vary with the capacitance of the circuit. In our
system, we use a
pulsating direct current rather than an alternating current (i.e., the voltage
alternates between two
values, but the voltage does not cross the "zero" line, such that polarity is
maintained and one
electrode remains positive and the other negative), so that the charged
polymer can be drawn
through the nanopore (towards the positive electrode in the case of DNA). When
there is
nothing in the nanopore, the capacitance has one value, which changes as the
different monomers
of the polymer pass through the nanopore. Suitable frequency ranges are in the
radiofrequency
range, e.g. 1 MHz to 1GHz, e.g. 50-200MHz, for example about 100MHz, e.g.
below higher
microwave frequencies that could cause significant dielectric heating of the
medium. To reduce
the potential for interference, different frequencies can be applied at
different nanopores so that
multiple nanopores can be measured simultaneously with a single radiofrequency
input line.
[095] Measuring impedance changes (due to, e.g. changes in capacitance) at
high frequencies
increases the signal to noise available within a certain time span, as it
reduces the effects of 1/f
noise, or 'pink' noise that is inherent in electronic measurement circuits.
Using a high frequency
signal enhances the signal-to-noise ratio, as many measurements are made
within a given time
span, providing a more stable signal which is readily distinguished from
impedance changes due
to environmental or device variation and fluctuation.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[096] Applying these principles to the instant invention, the invention
provides in one
embodiment, a method of measuring an impedance change in a high frequency
signal induced by
a change in capacitance as monomers (e.g., nucleotides) pass through a
nanopore, for example, a
method of reading a monomer sequence of a charged polymer comprising at least
two different
types of monomers, for example a DNA molecule, comprising applying a
radiofrequency
pulsating direct current, e.g. at a frequency of 1 MHz to 1GHz, e.g. 50-
200MHz, for example
about 100MHz, across a nanopore, wherein the pulsating direct current draws
the charged
polymer through the nanopore and the monomer sequence is read by measuring the
capacitive
variance across the nanopore as the charged polymer goes through the nanopore.
[097] In some embodiments, the invention provides a nanochip for sequencing an
electrically
charged polymer, e.g., DNA, comprising at least two distinct monomers, the
nanochip
comprising at least a first and second reaction chambers, each comprising
electrolytic medium,
and separated by a membrane comprising one or more nanopores, wherein a pair
of electrodes
(for example in the form of opposing plates), connected in circuit, is
disposed on either side of
the membrane comprising one or more nanopores, the electrodes being separated
by a distance of
1-30 microns, e.g., about 10 microns, such that the gap between the electrodes
has a capacitance
when a radiofrequency pulsating direct current, e.g. 1 MHz to 1GHz, is applied
to the electrodes
so as to draw the electrically charged polymer through the nanopore, e.g.,
from one chamber to
the next, and such that the phase of the pulsating direct radiofrequency
current changes with
changes in capacitance as the electrically charged polymer passes through the
nanopore, thereby
allowing detection of the monomer sequence of the electrically charged
polymer. In certain
embodiments, the nanochip comprises multiple sets of reaction chambers wherein
the reaction
chambers within a set are separated by membrane having one or more nanopores,
and the sets of
reaction chambers are separated by a screening layer to minimize electrical
interference between
the sets of reaction chambers and/or to separate multiple linear polymers and
allow them to be
sequenced in parallel.
[098] For example, in one embodiment the electrodes form the top and bottom
plates of a
capacitor embedded in a resonant circuit, and the change in capacitance is
measured as the DNA
passes through the pore between the plates.
[099] In certain embodiments, the nanochip further comprises reagents for
synthesizing the
polymer, e.g. DNA, e.g., according to any of Nanochip 1, et seq., below.
26
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0100] In one embodiment, therefore the invention provides a method (Method 1)
for
synthesizing a charged polymer [e.g., a nucleic acid (e.g., DNA or RNA)]
comprising at least
two distinct monomers in a nanochip, the nanochip comprising
one or more addition chambers containing reagents for addition of one or more
monomers [e.g. nucleotides] or oligomers [e.g., oligonucleotides] to the
charged polymer
in a buffer solution in terminal protected form, such that only a single
monomer or
oligomer can be added in one reaction cycle; and
one or more reserve chambers containing buffer solution but not all reagents
necessary
for addition of the one or more monomers or oligomers,
wherein the chambers are separated by one or more membranes comprising one or
more
nanopores and
wherein the charged polymer can pass through the nanopore but the least one of
the
reagents for addition of one or more monomers or oligomers cannot,
the method comprising
a) moving the first end of a charged polymer having a first end and a second
end, by
electrical attraction into an addition chamber, whereby monomers or oligomers
are added
to said first end in blocked form,
b) moving the first end of the charged polymer with the added monomer or
oligomer in
blocked form into a reserve chamber,
c) deblocking the added monomer or oligomer, and
d) repeating steps a-c, wherein the monomers or oligomers added in step a) are
the same
or different, until the desired polymer sequence is obtained.
[0101] For example, the invention provides
1.1. Method 1, wherein the polymer is nucleic acid, e.g., wherein the
polymer is DNA
or RNA, e.g., wherein it is DNA, e.g dsDNA or ssDNA.
1.2. Any foregoing method wherein the second end of the polymer, e.g. the
nucleic
acid, is either protected or bound to a substrate adjacent to the nanopore.
1.3. Any foregoing method wherein the electrical attraction is provided by
applying an
electric potential between the electrodes in each chamber, wherein the
polarity and
27
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
current flow between the electrodes can be controlled, e.g., such that the
nucleic acid is
attracted to a positive electrode.
1.4. Any foregoing method wherein the polymer is a nucleic acid and
(i) the said first end of the nucleic acid is the 3'-end, the addition of
nucleotides is in the 5' to 3' direction and is catalyzed by a polymerase,
e.g.,
wherein the polymerase is hindered (e.g. due to its size or due to being
tethered to
a substrate in the first chamber) from passing through the nanopore, the
nucleotides are 3'-protected when added, and following addition of the 3'-
protected nucleotide to the 3'-end of the nucleic acid, the 3'-protecting
group on
the nucleic acid is removed, e.g., in the reserve chamber; or
(ii) the said first end of the nucleic acid is the 5' end, the addition of
nucleotides is in the 3' to 5' direction, the nucleotides are 5'-protected
when
added, and following addition of the 5'-protected nucleotide to the 5'-end of
the
nucleic acid, the 5'protecting group is removed, e.g., in the second chamber;
(for
example wherein the phosphate on the 5'-protected nucleotide is a nucleoside
phosphoramidite coupled via the 5'-protecting group to a bulky group which
cannot pass through the nanopore, so that following coupling to the nucleic
acid,
the unreacted nucleotides are flushed away, the bulky 5'-protecting group is
cleaved from the nucleic acid, and flushed away, and the 5'-end of the nucleic
acid can be moved into the reserve chamber);
wherein the addition of nucleotides to the nucleic acid is controlled by
movement of the first end of the nucleic acid into and out of the one or more
addition chambers, and the cycle is continued until the desired sequence is
obtained.
1.5. Any foregoing method wherein the sequence of monomers or oligomers in the
polymer [e.g., the sequence of nucleotides in the nucleic acid] thus
synthesized
corresponds to a binary code.
1.6. Any foregoing method wherein the polymer thus synthesized is single
stranded
DNA.
1.7. Any foregoing method wherein the sequence of the polymer [e.g. the
nucleic acid]
is checked during the process or synthesis by sequencing the monomers or
oligomers
28
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[e.g., nucleotide bases] as they pass through the nanopore to identify errors
in
sequencing.
1.8. Any foregoing method wherein the polymer thus synthesized is single
stranded
DNA, wherein at least 95%, e.g at least 99%, e.g., substantially all of the
bases in the
sequence are selected from two bases that do not hybridize with other bases in
the strand,
e.g. bases selected from adenine and cytosine.
1.9. Any foregoing method wherein a multiplicity of polymers [e.g.
oligonucleotides]
are synthesized independently in parallel, such that polymers
[oligonucleotides] having
different sequences are obtained by separately controlling whether they are
present in one
or more addition chambers or one or more reserve chambers.
1.10. Any foregoing method wherein there are at least two addition chambers
that
contain reagents suitable for adding different monomers or oligmers, e.g.
different
nucleotides, e.g., wherein there are one or more addition chambers containing
reagents
suitable for adding a first monomer or oligomer and one or more addition
chambers
containing reagents suitable for adding a second different monomer or
oligomer, for
example wherein there are one or more addition chambers containing reagents
suitable
for adding adenine nucleotides and one or more addition chambers containing
reagents
suitable for adding cytosine nucleotides.
1.11. Any foregoing method wherein at least one addition chamber is a flow
chamber,
providing a flow cycle comprising (i) providing to the flow chamber reagents
suitable for
adding a first monomer or oligomer, (ii) flushing, (iii) providing to the flow
chamber
reagents suitable for adding a second different monomer or oligomer, and (iv)
flushing,
and repeating the cycle, until the synthesis is complete, wherein the sequence
of
monomers or oligomers in the polymer is controlled by introducing or excluding
the first
end of the polymer from the flow chamber during step (i) or (iii) in each
cycle;
1.12. Any foregoing method wherein the polymer is DNA and at least one
addition
chamber is a flow chamber, providing a flow cycle comprising (i) providing to
the flow
chamber reagents suitable for adding a first type of nucleotide, (ii)
flushing, (iii)
providing to the flow chamber reagents suitable for adding a second type of
nucleotide,
and (iv) flushing, and repeating the cycle until the synthesis is complete,
wherein the
29
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
sequence is controlled by controlling the presence or absence of the first end
of the DNA
(e.g. the 3'-end) in the flow chamber.
1.13. Any foregoing method wherein the polymer is DNA and at least one
addition
chamber is a flow chamber, providing a flow cycle comprising (i) providing to
the flow
chamber reagents suitable for adding a first type of nucleotide, (ii)
flushing, (iii)
providing to the flow chamber reagents suitable for adding a second type of
nucleotide,
and (iv) flushing, (i) providing to the flow chamber reagents suitable for
adding a third
type of nucleotide, (ii) flushing, (iii) providing to the flow chamber
reagents suitable for
adding a fourth type of nucleotide, and (iv) flushing, and repeating the cycle
until the
synthesis is complete, wherein the sequence is controlled by controlling the
presence or
absence of the first end of the DNA (e.g. the 3'-end) in the flow chamber when
reagents
suitable for adding the different types of nucleotides are present.
1.14. Any foregoing method wherein the polymer is DNA and the nanochip
comprises
two addition chambers which are flow chambers, (a) the first flow chamber
providing a
flow cycle comprising (i) providing to the first flow chamber reagents
suitable for adding
a first type of nucleotide, (ii) flushing, (iii) providing to the first flow
chamber reagents
suitable for adding a second different type of nucleotide, and (iv) flushing,
and repeating
the cycle until the synthesis is complete, and (b) the second flow chamber
providing a
flow cycle comprising (i) providing to the second flow chamber reagents
suitable for
adding a third type of nucleotide, (ii) flushing, (iii) providing to the
second flow chamber
reagents suitable for adding a fourth different type of nucleotide, and (iv)
flushing, and
repeating the cycle until the synthesis is complete, wherein the nucleotides
are selected
from dATP, dTTP, dCTP, and dGTP and wherein the sequence is controlled by
directing
the first end of the DNA (e.g. the 3'-end) into the flow chamber where the
next desired
nucleotide is provided.
1.15. Any foregoing method wherein the polymer is DNA and the nanopore chip
comprises one or more addition chambers for adding dATP, one or more addition
chambers for adding dTTP, one or more addition chambers for adding dCTP, and
one or
more addition chambers for adding dGTP.
1.16. Any foregoing method wherein the polymers [e.g. nucleic acids]
synthesized are
each bound via their second end to a surface proximate to a nanopore.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1.17. Any foregoing method wherein the sequence of the polymer [e.g. nucleic
acid] is
determined following each cycle by detecting the change in electric potential,
current,
resistance, capacitance, and/or impedance as the polymer passes through the
nanopore.
1.18. Any foregoing method wherein the polymer is a nucleic acid and synthesis
of the
nucleic acid takes place in a buffer solution, e.g., a solution comprising a
buffer for pH 7-
8.5, e.g. ca. pH 8, e,g, a buffer comprising tris(hydroxymethyl)aminomethane
(Tris), a
suitable acid, and optionally a chelator, e.g., ethylenediaminetetraacetic
acid (EDTA), for
example TAE buffer containing a mixture of Tris base, acetic acid and EDTA or
TBE
buffer comprising a mixture of Tris base, boric acid and EDTA; for example a
solution
comprising 10mM Tris pH 8, 1 mM EDTA, 150 mM KC1, or for example, 50 mM
Potassium Acetate, 20 mM Tris-acetate, 10 mM Magnesium Acetate, pH 7.9 @ 25 C.
1.19. Any foregoing method wherein the polymer is single stranded DNA further
comprising converting the synthesized single stranded DNA into double stranded
DNA.
1.20. Any foregoing method further comprising removing the polymer [e.g. the
nucleic
acid] from the nanochip after the polymer synthesis is complete.
1.21. Any foregoing method wherein the polymer is a nucleic acid, further
comprising
amplifying and retrieving copies of synthesized nucleic acid using an
appropriate primer
and a polymerase (e.g. Phi29).
1.22. Any foregoing method wherein the polymer is a nucleic acid, further
comprising
cleaving the synthesized nucleic acid with a restriction enzyme and removing
the the
nucleic acid from the nanochip.
1.23. Any foregoing method wherein the polymer is a nucleic acid, further
comprising
amplifying the nucleic acid thus synthesized.
1.24. Any foregoing method further comprising removing the polymer [e.g., the
nucleic
acid] from the nanochip and crystallizing the polymer.
1.25. Any foregoing method wherein the polymer is a nucleic acid, further
comprising
stabilizing the nucleic acid, e.g., by drying a solution comprising the
nucleic acid together
with one or more of a buffer (e.g., a borate buffer), an antioxidant, a
humectant, e.g. a
polyol, and optionally a chelator, for example as described in US 8283165 B2,
incorporated herein by reference; or by forming a matrix between the nucleic
acid and a
polymer, such as poly(ethylene glycol)¨poly(1-lysine) (PEG¨PLL) AB type block
31
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
copolymer; or by addition of a complementary nucleic acid strand or a protein
that binds
the DNA.
1.26. Any foregoing method comprising:
(i) reacting a nucleic acid with a 3'-protected nucleotide in an addition
chamber,
in the presence of a polymerase which catalyzes the addition of the 3'-
protected
nucleotide to the 3' end of the nucleic acid,;
(ii) drawing at least the 3' end of the 3'-protected nucleic acid thus
obtained out
of the addition chamber, through the at least one nanopore, into a reserve
chamber, wherein the polymerase is hindered (e.g. due to its size or due to
being
tethered to a substrate in the first chamber) from passing through the
nanopore;
(iii) deprotecting the 3'-protected nucleic acid, e.g., chemically or
enzymatically;
and
(iv) if it is desired that an additional 3'-protected dNTP be added to the
oligonucleotide, drawing the 3' end of the oligonucleotide into the same or
different addition chamber, so that steps (i) ¨ (iii) are repeated, or if it
is not so
desired, allowing the 3' end of the nucleic acid to remain in the reserve
chamber
until a further cycle wherein the desired 3'-protected dNTP is provided to the
addition chamber; and
(v) repeating the cycle of steps (i) ¨ (iv) until the desired nucleic acid
sequence is
obtained.
1.27. Any foregoing method wherein the polymer is nucleic acid single-stranded
DNA
(ssDNA) and the one or more nanopores have a diameter allowing ssDNA to pass
but not
double stranded DNA (dsDNA), e.g., a diameter of about 2nm.
1.28. Any foregoing method wherein the monomer is a 3'-protected nucleotide,
e.g.,
deoxynucleotide triphosphate (dNTP), e.g. selected from deoxyadenosine
triphosphate
(dATP), deoxyguanosine triphosphate (dGTP), deoxycytidine triphosphate (dCTP),
deoxythymidine triphosphate (dTTP), for example dATP or dCTP.
1.29. Any foregoing method wherein the polymer is a nucleic acid and the
addition of
the nucleotide to the nucleic acid is catalyzed by a polymerase, e.g., a
template
independent polymerase, e.g., terminal deoxynucleotidyl transferase (TdT), or
32
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
polynucleotide phosphorylase, e.g., wherein the polymerase catalyzes the
incorporation
of a deoxynucleotide at the 3'-hydroxyl terminus of DNA.
1.30. Any foregoing method wherein the membrane contains a multiplicity of
nanopores and a multiplicity of polymers each bound to a surface proximate to
a
nanopore, e.g., a multiplicity of nucleic acids each bound via their 5' end to
a surface
proximate to a nanopore.
1.31. Any foregoing method wherein a multiplicity of polymers each bound to a
surface
proximate to a nanopore, e.g., a multiplicity of nucleic acids each bound at
the 5' end to a
surface proximate to a nanopore, are synthesized independently, wherein each
nanopore
has an associated pair of electrodes, wherein one electrode in the pair is
located
proximate to one end of the nanopore and the other electrode located proximate
to the
other end of the nanopore, such that each polymer can be independently moved
between
the first and second chamber by current provided by the pair of electrodes.
1.32. Any foregoing method wherein the polymer is a 3'-protected nucleic acid
bound
at the 5' end to a surface proximate to a nanopore and the 3' end of the 3'-
protected
nucleic acid is drawn through the nanopore by using an electrical force, e.g.,
by using an
electrical force applied from an electrode in an adjacent chamber.
1.33. Method 1.20 wherein the new 3'-protected dNTP is the same or different
from the
first 3'-protected dNTP.
1.34. Method 1.20 wherein the 3'-protected dNTP used in step (i) of the cycle
alternates
with each cycle between 3'-protected dATP and 3'-protected dCTP.
1.35. Any foregoing method wherein the polymer is a nuceic acid and
deprotection of
the nucleic acid is carried out by an enzyme that removes a 3'-protecting
group on
ssDNA but not on a 3'protected dNTP.
[0101] For example, the invention provides a method for synthesizing a nucleic
acid in a
nanochip, comprising at least a first chamber and a second chamber separated
by a membrane
comprising at least one nanopore, the synthesis being carried out in a buffer
solution by a cycle
of nucleotide addition to a first end of a nucleic acid having a first end and
a second end, wherein
the first end of the nucleic acid is moved by electrical attraction between
one or more addition
chambers (which contains reagents capable of adding nucleotides) and one or
more reserve
chambers (which do not contain reagents necessary to add nucleotides), the
chambers being
33
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
separated by one or more membranes each comprising one or more nanopores,
wherein the
nanopore is large enough to permit passage of the nucleic acid but is too
small to permit passage
of at least one reagent essential for adding a nucleotide, e.g, wherein the
method corresponds to
any of Method 1, et seq.
[0102] In certain embodiments, the sequence of the polymer corresponds to a
binary code, for
example where the polymer is a nucleic acid and the sequence corresponds to a
binary code,
where each bit (0 or 1) is represented by a base, e.g. A or C.
[0103] In certain embodiments, the polymer is DNA
[0104] In certain other embodiments, each bit is represented by a short
sequence of monomers
rather than by a single monomer. For example, in one such embodiment, blocks
of DNA are
synthesized, where each block generates a unique signal via the nanopore and
corresponds to a
zero or a one. This embodiment has certain advantages in that single
nucleotides are more
difficult to detect in nanopores, especially solid-state nanopores, so using
blocks is less prone to
reading errors, although the information density in the polymer is
correspondingly reduced.
[0105] For example, blocks of (double stranded) nucleotides can be added,
using site-specific
recombinases, i.e., enzymes that spontaneously recognize and cleave at least
one strand of a
double strand of nucleic acids within a sequence segment known as the site-
specific
recombination sequence. In one such embodiment, the site specific recombinase
is a
topoisomerase used to ligate a topo-conjugated dsDNA oligonucleotide block to
the sequence.
These oligonucleotides themselves will not have a structure compatible with
further ligation until
they are cleaved with a restriction enzyme. Vaccinia virus topoisomerase I
specifically
recognises DNA sequence 5'-(C/T)CCTT-3'. The topoisomerase binds to double-
stranded DNA
and cleaves it at the 5'-(C/T)CCTT-3' cleavage site. Note that the cleavage is
not complete, as
the topoisomerase only cleaves the DNA on one strand (although having a nearby
nick on the
other strand does cause a double-strand break of sorts), and when it cleaves,
the topoisomerase
attaches covalently to the 3' phosphate of the 3' nucleotide. The enzyme then
remains covalently
bound to the 3' end of the DNA, and can either religate the covalently held
strand at the same
bond as originally cleaved (as occurs during DNA relaxation), or it can
religate to a heterologous
acceptor DNA having compatible overhangs, creating a recombinant molecule. In
this
embodiment, we create dsDNA donor oligonucleotides (e.g., comprising one of at
least two
different sequences, one for '0' and the other for '1') flanked by a
topoisomerase recombination
34
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
site and a restriction site that generates a topoisomerase ligation site. The
cassettes are Topo-
charged; that is, they are covalently bound to a topoisomerase, which will
bind them to a
topoisomerase ligation site on the receiver oligonucleotide. When the growing
DNA chain of the
receiver is cleaved with a restriction enzyme it becomes capable of ligation
to a Topo-charged
cassette. So, one just needs to cycle the growing DNA from restriction enzyme
to Topo-charged
cassette successively, with each cycle adding another donor oligonucleotide. A
related approach
has been described for cloning, see, e.g., Shuman S., Novel approach to
molecular cloning and
polynucleotide synthesis using vaccinia DNA topoisomerase. J Biol Chem.
(1994);
269(51):32678-84, the contents of which are incorporated by reference.
[0106] Single bases can be added using a similar strategy. In the presence of
a suitable single
stranded `deprotected"acceptor' DNA, the topo-charged DNA is enzymatically and
covalently
ligated ('added') to the acceptor by the topoisomerase, which in the process
becomes removed
from the DNA. A type ITS restriction enzyme can then cleave all of the added
DNA with the
exception of a single base (the base which is being 'added'). This process of
deprotect-add can
be repeated to add additional bases (bits). As demonstrated in the examples
herein, it is feasible
to use a Topo / TypeIIS restriction enzyme combination to add a single
nucleotide to the 5' end
of a target single stranded DNA. The use of a TypeIIS restriction enzyme
enables cleavage of
DNA in a location different from that of the recognition sequence (other
TypeIIS restriction
enzymes can be found at https://www.neb.com/tools-and-
resources/selectioncharts/type-iis-
restriction-enzymes). The use of inosines (which act as 'universal bases' and
pair with any other
base) in this system allows this reaction to occur without any specific
sequence requirements in
the target DNA. The identity of the nucleotide added to the single strand
target DNA is the 3'
nucleotide to which vaccinia topoisomerase conjugates via the 3' phosphate.
Since the
recognition sequence of vaccinia topoisomerase is (C/T)CCTT, we have used this
system to add
a 'T' to the target DNA. There is a related topoisomerase, SVF, that can use
the recognition
sequence CCCTG (https://www.ncbi.nlm.nih.gov/pubmed/8661446). Thus SVF can be
used to
add a `G' instead of a 'T'. Paired with vaccinia topo, binary data can be
encoded in T's and G's.
[0107] In another approach to single base addition, a 5'phosphate provides a
blocking group to
provide single base addition in the 3' to 5' direction. The charging reaction
charges the
topoisomerase with a single T (or G, or other nucleotide as desired), having a
5' phosphate
group. When the charged topoisomerase 'sees' a free 5' unblocked
(unphosphorylated) single
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
stranded DNA chain it will add the T to that chain, providing a DNA with a T
added to the 5'.
This addition is facilitated by the presence of an adapter DNA having
sequences to which the
topoisomerase and the single stranded acceptor DNA can bind. (Note that the
adapter DNA is
catalytic ¨ it can be reused as a template in repeated reactions.) The added
nucleotide has a 5'
phosphate on it, so it won't be a substrate for further addition until it is
exposed to a phosphatase,
which removes the 5' phosphate. The process is repeated, using vaccinia
topoisomerase to add a
single "T" to the 5' end of a target single stranded DNA and SVF topoisomerase
to add a single
'G', thus allowing construction of a sequence encoding binary information with
T and G. Other
topoisomerases can be used to add A's or C's, although this reaction is less
efficient.
[0108] One advantage of using a topoisomerase-mediated strategy is that the
monomer is
covalently attached to the topoisomerase, and therefore cannot "escape" to
interfere with other
reactions. When polymerase is used, the monomers can diffuse so the
polymerases and/or the
deblocking agents shoudl be specific (e.g. selective for A vs C, for example)
or alternatively, the
monomers are provided by a flow so they don't have a chance to mix.
[0109] In one aspect, the invention provides a topoisomerase charged with a
single nucleotide,
i.e., a topoisomerase conjugated to a single nucleotide, e.g., wherein the
topoisomerase is
conjugated via the 3'-phosphate of the nucleotide, and the nucleotide is
protected, e.g.,
phosphorylated, at the 5'-position.
[0110] In another aspect the invention provides a method (Method A) of
synthesizing a DNA
molecule using topoisomerase-mediated ligation, by adding single nucleotides
or oligomers to a
DNA strand in the 3' to 5' direction, comprising (i) reacting a DNA molecule
with a
topoisomerase charged with the desired nucleotide or oligomer wherein the
nucleotide or
oligomer is blocked from further addition at the 5' end, then (ii) deblocking
the 5' end of the
DNA thus formed, and repeating steps (i) and (ii) until the desired nucleotide
sequence is
obtained, e.g.,
A1.1. Method A which is a method of synthesizing a DNA molecule by
adding single
nucleotides in the 3' to 5' direction comprising (i) reacting a DNA molecule
with a
topoisomerase charged with the desired nucleotide in 5'protected form, e.g.,
5'phosphorylated form, such that the desired nucleotide in 5'protected form is
added to
the 5' end of the DNA, then (ii) deprotecting the 5' end of the DNA thus
formed through
36
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
the use of a phosphatase enzyme, and repeating steps (i) and (ii) until the
desired
nucleotide sequence is obtained; or
A1.2. Method A which is a method of synthesizing a DNA molecule by adding
oligomers in the 3' to 5' direction comprising (i) reacting a DNA molecule
with a
topoisomerase charged with the desired oligomer, thereby ligating the oligomer
to the
DNA molecule, then (ii) using a restriction enzyme to provide a 5' site for a
topoisomerase-mediated ligation for another oligomer, and repeating steps (i)
and (ii)
until the desired oligimer sequence is obtained.
A1.3. Any foregoing method comprising providing ligase and ATP to seal
nicks in the
DNA [NB: the topoisomerase ligation only ligates one strand].
A1.4. Any foregoing method wherein the topoisomerase-charged donor
oligonucleotide
comprises a 5' overhang on the strand complementary to the strand bearing the
topoisomerase, comprising a polyinosine sequence [NB: inosines act as
'universal bases'
and pair with any other base].
A1.5. Any foregoing method wherein the restriction enzyme is a type ITS
restriction
enzyme which can cleave all of the added DNA with the exception of a single
base (the
base which is being 'added').
A1.6. Any foregoing method wherein the toposiomerase is selected from
vaccinia
topoisomerase and SVF topoisomerase I.
A1.7. Any foregoing method wherein vaccinia topoisomerase (which recognizes
(C/T)CCTT) is used to add dTTP nucleotides and SVF topoisomerase I (which
recognizes CCCTG) is used to add dGTP nucleotides, e.g., to provide binary
code
A1.8. Any foregoing method wherein the DNA is double stranded and the
reserve
chamber further comprises a ligase and ATP, to repair the DNA strand not
joined by the
topoisomerase.
A1.9. Any foregoing method comprising use of a topoisomerase inhibitor to
suppress
binding and activity of free topoisomerase to the DNA oligomer, e.g., wherein
the
inhibitors is selected from novobiocin and coumermycin.
A1.10. Any foregoing method wherein the DNA strand thus provided has a
sequence
comprising thymidine (T) nucleosides and deoxyguanisine (G) nucleosides.
37
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
A1.11. Any foregoing method wherein the topoisomerase adds a single base,
but the
restriction enzyme cleaves at a position which is one nucleotide in the 5'
direction from
the base added by topoisomerase.
A1.12. Any foregoing method wherein the DNA strand thus provided has a
sequence
comprising a sequence of 'TT' and `TG' dinucleotides.
A1.13. Any foregoing method wherein the DNA is single stranded,
A1.14. Any foregoing method wherein the DNA double stranded.
A1.15. Any foregoing method wherein the DNA is on a substrate or magnetic
bead,
where it can be selectively exposed to or removed from the reagents as
required to
provide the desired sequence.
A1.16. Any foregoing method wherein some or all of the reagents for adding
or
deblocking the DNA are supplied by flow and removed by flushing.
A1.17. Any foregoing method wherein the attachment of the single
nucleotides or
oligomers to a single-stranded DNA is facilitated by the presence of an
adapter DNA
having sequences to which the topoisomerase and the single stranded acceptor
DNA can
bind.
A1.18. Any foregoing method carried out in a system where a nanopore
separates a
chamber comprising the topoisomerase from a chamber comprising the phosphatase
or
restriction enzyme, wherein the nanopore allows movement of the DNA by
electrical
attraction, but not the enzymes, e.g. as described in any of Method 2, et seq.
[0111] One possible concern is poly-G sequences may form G-quartet secondary
structures. By
moving the restriction enzyme back one base (to the 5' of the topo sequence)
and following a
similar Topo/IIS strategy a 'TT' or `TG' can be added, each of which can
represent a different
bit. While this would require 2 bases to encode a bit, it has the advantage of
avoiding poly-G
sequences. In other embodiments, other bases in the 3' end of the topo
recognition sequence -
although less efficient than (C/T)CCTT, can allow conjugation using poxvirus
topoisomerase
with (C/T)CCTA, (C/T)CCTC and (C/T)CCTG
(https://www.ncbi.nlm.nih.gov/pubmed/17462694). Protein engineering/selection
techniques can
be used to improve the efficiency of these reactions as well, and similar
approaches can be used
to add non-canonical bases.
38
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0112] In certain embodiments, the method of synthesizing DNA by this method
includes
treating the DNA with a ligase and ATP. The topoisomerase only joins together
one side of the
DNA (the other is essentially nicked). The ligase would repair the nick and
ensure that the
topoisomerase itself doesn't recut the reaction product and cleave it.
[0113] In certain embodiments, the method comprises using a topoisomerase
inhibitor to
suppress binding and activity of free topoisomerase to the DNA oligomer.
Suitable inhibitors
include novobiocin and coumermycin. Note that complete inhibition is not
desirable, as a low
level of topoisomerase activity can help 'relax' coiled DNA, which is useful
especially when
synthesizing long DNA chains.
[0114] Thus, in another embodiment, the disclosure provides a method (Method
2) for
synthesizing DNA in a nanochip, comprising one or more addition chambers
containing a
topoisomerase-charged oligonucleotide (i.e.. oligonucleotide bound at the 3'
end to a
topoisomerase), and one or more reserve chambers comprising a restriction
enzyme or deblocker,
e.g., phosphatase, said chambers also containing compatible buffer solution
and being separated
by a membrane comprising at least one nanopore, wherein the topoisomerase and
the restriction
enzyme are prevented from passing through the nanopore (e.g. because they are
too large and/or
because they are tethered to a substrate in the first and second chambers
respectively), the
synthesis being carried out by a cycle of adding single nucleotides or short
oligonucleotide
blocks to a first end of a nucleic acid having a first end and a second end,
wherein the first end of
the nucleic acid is moved by electrical attraction between an addition
chambers and a reserve
chamber, for example in one embodiment as follows:
(i) moving the 5' end of a receiver DNA (e.g., a double-stranded DNA) into a
first
addition chamber, by means of an electrical force,
(ii) providing in the first addition chamber a topoisomerase-charged donor
oligonucleotide, wherein the donor oligonucleotide comprises a topoisomerase
binding
site, an informational sequence (e.g., selected from at least two different
nucleotides or
sequences, e.g., wherein one sequence corresponds to '0' and the other to '1'
in a binary
code), and a restriction site which when cleaved by a restriction enzyme will
yield a
topoisomerase ligation site;
(iii) allowing sufficient time for the donor olignucleotide to ligate to and
thereby extend
the receiver DNA;
39
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
(iv) moving the 5' end of the receiver DNA thus extended into the reserve
chamber, by
means of an electrical force, e.g., so that the restriction enzyme cleaves the
receiver DNA
to provide a topoisomerase ligation site, or in the case of single nucleotide
addition, the
deblocker, e.g., phosphatase, generates a 5' unblocked nucleotide on the
single stranded
DNA; and
(v) repeating the cycle of steps (i) ¨ (iv), adding oligonucleotides having
the same or
different informational sequence, until the desired DNA sequence or sequences
are
obtained.
[0115] For example, the invention provides
2.1. Method 2 wherein the 3' end of the receiver DNA is attached
proximate to a
nanopore and the 5'end of the receiver oligonucleotide comprises a
topoisomerase
ligation site, and comprising a step after step (iv) of adding an additional
oligonucleotide
to the 5' end of the receiver DNA by flushing the first addition chamber and
providing
new topoisomerase-charged donor oligonucleotide to the first addition chamber,
wherein
the new donor oligonucleotide has a different informational sequence from the
previous
donor oligonucleotide; and if desired that the new donor oligonucleotide be
added to the
receiver DNA, drawing the 5' end of the receiver nucleic acid back into the
first chamber,
and repeating steps (i) ¨ (iii), or if not so desired, allowing the receiver
DNA to remain in
the second chamber until the desired donor oligonucleotide is provided to the
first
chamber.
2.2. Any foregoing method wherein a multiplicity of receiver DNA molecules are
synthesized independently in parallel, such that DNA molecules having
different
sequences are obtained by separately controlling whether they are present in
the first
chamber.
2.3. Any foregoing method wherein a multiplicity of receiver DNA molecules
each
bound at the 3' end to a surface proximate to a nanopore are synthesized
independently,
wherein each nanopore has an associated pair of electrodes, wherein one
electrode in the
pair is located proximate to one end of the nanopore and the other electrode
located
proximate to the other end of the nanopore, such that each receiver DNA
molecule can be
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
independently moved between the first and second chamber by current provided
by the
pair of electrodes.
2.4. Any foregoing method wherein the donor oligonucleotides used in step
(i) of the
cycle alternate with each cycle between donor oligonucleotides comprising a
first
informational sequence and donor oligonucleotides comprising a second
informational
sequence.
2.5. Method 2 comprising the step of adding an additional oligonucleotide
to the 5'
end of the receiver DNA by returning the 5' end of the receiver DNA to the
first addition
chamber to add an oligonucleotide having the same informational sequence or
moving
the 5' end of the receiver DNA to a second addition chamber to having a donor
oligonucleotide bound at the 3' end to a topoisomerase, wherein the donor
oligonucleotide in the second addition chamber has a different informational
sequence
from the donor oligonucleotide in the first addition chamber.
2.6. Any foregoing method wherein the donor oligonucleotide comprises a
structure as
follows:
5' CGAAGGG <Informational sequence A or B> GTCGACNNNNN
3' GCTTCCC < ------------------- Complement ------------ > CAGCTGNNNNN
wherein N refers to any nucleotide and the restriction enzyme is Accl, which
can cut the
DNA (e.g. GTCGAC in the above sequence) so as to provide an appropriate
overhang.
2.7. Any foregoing method wherein the donor oligonucleotide has a hairpin
structure,
e.g., 2.6 wherein the NNNNN groups on the top and bottom strands are joined.
2.8. Any foregoing method wherein at least one of the topoisomerase charged
oligonucleotides has a structure as follows:
5' CGAAGGG <Informational sequence A or B> GTCGACNNNNN
3' *TTCCC < -------------------- Complement ------------ > CAGCTGNNNNN
(* = topoisomerase)
2.9. Any foregoing method wherein at least one of the topoisomerase charged
oligonucleotides has a structure as follows:
5' pCACGTCAGGCGTATCCATCCCTT*
3' GTGCAGTCCGCATAGGTAGGGAAGCGC
41
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
2.10. The preceding method wherein the topoisomerase charged oligonucleotide
2.11. Any foregoing method wherein the sequence of DNA synthesized is
determined
following each cycle by detecting the change in electric potential, current,
resistance,
capacitance and/or impedance as the oligonucleotide passes through the
nanopore.
2.12. Any foregoing method wherein the synthesis of the DNA takes place in a
buffer
solution, e.g., a solution comprising a buffer for pH 7-8.5, e.g. ca. pH 8,
e,g, a buffer
comprising tris(hydroxymethyl)aminomethane (Tris), a suitable acid, and
optionally a
chelater, e.g., ethylenediaminetetraacetic acid (EDTA), for example TAE buffer
containing a mixture of Tris base, acetic acid and EDTA or TBE buffer
comprising a
mixture of Tris base, boric acid and EDTA; for example a solution comprising
10mM
Tris pH 8, 1 mM EDTA, 150 mM KC1, or for example, 50 mM Potassium Acetate, 20
mM Tris-acetate, 10 mM Magnesium Acetate, pH 7.9 @ 25 C.
2.13. Any foregoing method further comprising removing the DNA from the
nanochip.
2.14. Any foregoing method further comprising amplifying the DNA thus
synthesized.
2.15. Any foregoing method further comprising removing the DNA from the
nanochip
and crystallizing the DNA.
2.16. Any foregoing method further comprising stabilizing the DNA, e.g., by
drying a
solution comprising the DNA together with one or more of a buffer (e.g., a
borate buffer),
an antioxidant, a humectant, e.g. a polyol, and optionally a chelator, for
example as
described in US 8283165 B2, incorporated herein by reference, or by forming a
matrix
between the nucleic acid and a polymer, such as poly(ethylene glycol)¨poly(1-
lysine)
(PEG¨PLL) AB type block copolymer.
2.17. Any foregoing method comprising providing ligase and ATP to seal nicks
in the
DNA [NB: the topoisomerase ligation only ligates one strand].
2.18. Any foregoing method wherein the topoisomerase-charged donor
oligonucleotide
comprises a 5' overhang on the strand complementary to the strand bearing the
topoisomerase, comprising a polyinosine sequence [NB: inosines act as
'universal bases'
and pair with any other base].
2.19. Any foregoing method wherein the restriction enzyme is a type ITS
restriction
enzyme which can cleave all of the added DNA with the exception of a single
base (the
base which is being 'added').
42
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
2.20. Any foregoing method wherein the toposiomerase is selected from vaccinia
topoisomerase and SVF topoisomerase I.
2.21. Any foregoing method wherein vaccinia topoisomerase (which recognizes
(C/T)CCTT) is used to add dTTP nucleotides and SVF topoisomerase I (which
recognizes CCCTG) is used to add dGTP nucleotides, e.g., to provide binary
code
information.
2.22. Any foregoing method wherein the reserve chamber further comprises a
ligase
and ATP, to repair the DNA strand not joined by the topoisomerase.
2.23. Any foregoing method comprising use of a topoisomerase inhibitor to
suppress
binding and activity of free topoisomerase to the DNA oligomer, e.g., wherein
the
inhibitors is selected from novobiocin and coumermycin.
2.24. Any foregoing method wherein the DNA strand thus provided has a sequence
comprising thymidine (T) nucleosides and deoxyguanisine (G) nucleosides.
2.25. Any foregoing method wherein the topoisomerase adds a single base, but
the
restriction enzyme cleaves at a position which is one nucleotide in the 5'
direction from
the base added by topoisomerase.
2.26. Any foregoing method wherein the DNA strand thus provided has a sequence
comprising a sequence of 'TT' and `TG' dinucleotides.
2.27. Any foregoing method which is a method of synthesizing a DNA molecule by
adding single nucleotides in the 3' to 5' direction comprising (i) reacting a
DNA
molecule with a topoisomerase charged with the desired nucleotide in
5'protected form,
e.g., 5'phosphorylated form, such that the desired nucleotide in 5'protected
form is added
to the 5' end of the DNA, then (ii) deprotecting the 5' end of the DNA thus
formed
through the use of a phosphatase enzyme, and repeating steps (i) and (ii)
until the desired
nucleotide sequence is obtained.
2.28. Any foregoing method which is a method of synthesizing a DNA molecule by
adding oligomers in the 3' to 5' direction comprising (i) reacting a DNA
molecule with a
topoisomerase charged with the desired oligomer, thereby ligating the oligomer
to the
DNA molecule, then (ii) using a restriction enzyme to provide a 5' site for a
topoisomerase-mediated ligation for another oligomer, and repeating steps (i)
and (ii)
until the desired nucleotide sequence is obtained.
43
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
2.29. Any foregoing method which is a method in accordance with any of Method
A, et
seq.
[0116] The product of the synthesis reactions can be detected, reviewed for
quality control
purposes, and read to extract the data encoded on the polymer. For example the
DNA may be
amplified and sequenced by conventional means to confirm that the nanopore
sequencing is
robust.
[0117] In another embodiment, the invention provides an oligonulceotide
comprising a
topoisomerase binding site, an informational sequence (e.g., selected from at
least two different
sequences, e.g., wherein one sequence corresponds to '0' and the other to '1'
in a binary code),
and a restriction site which when cleaved by a restriction enzyme will yield a
topoisomerase
ligation site, e.g., comprising the following sequence:
5' CGAAGGG <Informational sequence A or B> GTCGAC
3' GCTTCCC < ----------------------- Complement --------------------- > CAGCTG
wherein the Informational Sequence A or B is a sequence of 3-12, e.g., about 8
nucleotides.
[0118] In another embodiment, the invention provides a topoisomerase charged
oligonucleotide
wherein the oligonucleotide comprises a topoisomerase binding site, an
informational sequence
(e.g., selected from at least two different sequences, e.g., wherein one
sequence corresponds to
'0' and the other to '1' in a binary code), and a restriction site which when
cleaved by a
restriction enzyme will yield a topoisomerase ligation site; for example a
topoisomerase charged
oligonucleotide having a structure as follows:
5' CGAAGGG <Informational sequence A or B> GTCGACNNNNN
3' *TTCCC < ------------------ Complement --------------------------- >
CAGCTGNNNNN
wherein the Informational Sequence A or B is a sequence of 3-12, e.g., about 8
nucleotides and *
is topoisomerase covalently bound to the oligonucleotide; e.g., wherein the
topisomerase is
Vaccinia virus topoisomerase I.
[0119] In another embodiment, the invention provides a single or double
stranded DNA
molecule as described above, wherein the single strand or the coding sequence
consists
essentially of nonhybridizing bases, for example adenines and cytosines (As
and Cs), which are
arranged in sequence to correspond to a binary code, e.g., for use in a method
of data storage.
44
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
For example, the invention provides DNA (DNA 1), wherein the DNA is single or
double
stranded, at least 1000 nucleotides long, e.g., 1000 ¨ 1,000,000 nucleotides
or, for example,
5,000 to 20,000 nucleotides long, wherein the sequence of the nucleotides
corresponds to a
binary code; e.g.,
1.1. DNA 1 wherein the DNA is single stranded.
1.2. DNA 1 wherein the DNA is double stranded.
1.3. Any foregoing DNA wherein the nucleotides in a single strand or in the
coding
strand are selected from adenine, thymine and cytosine nucleotides, e.g. are
selected from
adenine and cytosine nucleotides or thymine and cytosine nucleotides
1.4. Any foregoing DNA consisting primarily of nonhybridizing nucleotides,
so that it
will not form significant secondary structures when in the form of a single
strand.
1.5. Any foregoing DNA wherein the nucleotides are at least 95%, e.g. 99%,
e.g.,
100% adenine and cytosine nucleotides.
1.6. Any foregoing DNA comprising a nucleotide or sequence of nucleotides
added to
separate or punctuate the nucleotides comprising a binary code, e.g., to
separate the l's
and 0's or groups of l's and 0's, so that consecutive l's or O's can be more
easily read.
1.7. Any foregoing DNA wherein (a) each bit in the binary code corresponds
to a
single nucleotide, e.g. each of 1 and 0 correspond to A or C; or (b) each bit
in the binary
code corresponds to a series of more than 1 nucleotides, e.g. 2, 3 or 4
nucleotides, e.g.,
AAA or CCC.
1.8. Any foregoing DNA which is crystallized.
1.9. Any foregoing DNA which is provided in a dry form together with one or
more of
a buffer salt (e.g., a borate buffer), an antioxidant, a humectant, e.g. a
polyol, and
optionally a chelator, for example as described in US 8283165 B2, incorporated
herein by
reference; and/or in a matrix between the nucleic acid and a polymer, such as
poly(ethylene glycol)¨poly(1-lysine) (PEG¨PLL) AB type block copolymer; ;
and/or
together with a complementary nucleic acid strand or a protein that binds the
DNA.
1.10. Any foregoing DNA made by any of Method 1 et seq. or Method 2 et seq. or
Method A, et seq..
[0120] The nanochips can be fabricated for example as depicted in figures 23-
29. For example,
in one format, each polymer strand is associated with two or four addition
chambers, wherein the
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
two addition chamber format is useful for encoding binary code in the polymer,
and the four
addition chamber format is particularly useful for making custom DNA
sequences. Each
addition chamber contains a separately controllable electrode. The addition
chambers contain
reagents to add monomers to the polymer in buffer. The addition chambers are
separated by a
membrane comprising one or more nanopores from a reserve chamber, which may be
common
to multiple addition chambers, and which contains deprotection reagents and
buffer, to deprotect
the protected monomers or oligomers added in the addition chambers. The
nanochips comprise a
multiplicity of addition chamber sets, to allow parallel synthesis of many
polymers.
[0121] High-bandwidth and low-noise nanopore sensor and detection electronics
are important
to achieving single-DNA base resolution. In certain embodiments, the nanochip
is electrically
linked to a Complementary Metal-Oxide Semiconductor (CMOS) chip. Solid-state
nanopores
can be integrated within a CMOS platform, in close proximity to the biasing
electrodes and
custom-designed amplifier electronics, e.g., as described in Uddin, et al.,
"Integration of solid-
state nanopores in a 0.5 [tm cmos foundry process", Nanotechnology (2013)
24(15): 155501, the
contents of which are incorporated herein by reference.
[0122] In another embodiment, the disclosure provides a nanochip (Nanochip 1)
for synthesis of
and/or sequencing an electrically charged polymer, e.g., DNA, comprising at
least two distinct
monomers, the nanochip comprising at least a first and second reaction
chambers, separated by a
membrane comprising one or more nanopores, wherein each reaction chamber
comprises one or
more electrodes to draw the electrically charged polymer into the chamber and
further comprises
an electrolytic media and optionally reagents for addition of monomers to the
polymer, for
example,
1.1. Nanochip 1 wherein the nanopore has a diameter of 2-20 nm, e.g. 2-10
nm, for
example 2-5 nm.
1.2. Any foregoing nanochip wherein the some or all of the walls of the
reaction
chambers of the nanochip comprise a silicon material, e.g., silicon, silicon
dioxide,
silicon nitride, or combinations thereof, for example silicon nitride.
1.3. Any foregoing nanochip wherein the some or all of the walls of the
reaction
chambers of the nanochip comprise a silicon material, e.g., silicon, silicon
dioxide,
silicon nitride, or combinations thereof, for example silicon nitride, and
some or all of the
nanopores are made by ion bombardment.
46
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1.4. Any foregoing nanochip wherein some or all of the nanopores are comprised
of a
pore-forming protein, a-hemolysin, in a membrane, e.g. a lipid bilayer.
1.5. Any foregoing nanochip wherein some or all of the walls of the
reaction chambers
are coated to minimize interactions with the reagents, e.g., coated with a
polymer such as
polyethylene glycol, or with a protein, such a bovine serum albumin.
1.6. Any foregoing nanochip comprising an electrolyte media.
1.7. Any foregoing nanochip comprising an electrolyte media comprising a
buffer,
e.g., a buffer for pH 7-8.5, e.g. ca. pH 8, e,g, a buffer comprising
tris(hydroxymethyl)aminomethane (Tris), a suitable acid, and optionally a
chelater, e.g.,
ethylenediaminetetraacetic acid (EDTA), for example TAE buffer containing a
mixture
of Tris base, acetic acid and EDTA or TBE buffer comprising a mixture of Tris
base,
boric acid and EDTA; for example a solution comprising 10mM Tris pH 8, 1 mM
EDTA,
150 mM KC1, or for example, 50 mM Potassium Acetate, 20 mM Tris-acetate, 10 mM
Magnesium Acetate, pH 7.9 @ 25 C.
1.8. .. Any foregoing nanochip comprising reagents for addition of monomers to
the
polymer.
1.9. Any foregoing nanochip capable of both synthesizing ("writing", e.g.,
by adding
monomers or groups of monomers sequentially to the polymer) and sequencing
("reading", e.g., by measuring changes in current and/or inductance as the
monomers
pass through the nanopore) the polymer.
1.10. Any foregoing nanochip wherein the membrane comprising one or more
nanopores comprises a metal surface on both sides, the metal surface being
separated by
an insulator, e.g. a silicon nitride membrane, the metal surfaces being
configured, e.g., by
lithographic means, to provide electrodes at either end of each nanopore,
e.g., such that a
current flow across the nanopore may be established through the nanopore via
an
electrolyte media, e.g., such that the currant can draw the polymer through
the nanopore
and as the polymer passes through the nanopore, the change in electric
potential across
the nanopore can be measured and used to identify the sequence of monomers in
the
polymer.
1.11. Any foregoing nanochip comprising an electrically charged polymer which
is
DNA.
47
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1.12. Any foregoing nanochip comprising an electrically charged polymer which
is
single stranded DNA (ssDNA).
1.13. Any foregoing nanochip comprising an electrically charged polymer which
is
DNA comprising a predetermined restriction site.
1.14. Any foregoing nanochip comprising an electrically charged polymer which
is
DNA wherein the DNA is a DNA as described in any of DNA 1, et seq., above.
1.15. Any foregoing nanochip comprising an electrically charged polymer which
is
DNA, wherein the DNA comprises at least 95%, e.g. 99%, e.g., 100% adenines and
cytosines.
1.16. Any foregoing nanochip comprising an electrically charged polymer which
is
DNA, wherein the DNA comprises only adenines and cytosines.
1.17. Any foregoing nanochip comprising one or more ports to permit
introduction of
and flushing out of buffer and reagents.
1.18. Any foregoing nanochip comprising a buffer solution, e.g., a solution
comprising
a buffer for pH 7-8.5, e.g. ca. pH 8, e,g, a buffer comprising
tris(hydroxymethyl)aminomethane (Tris), a suitable acid, and optionally a
chelater, e.g.,
ethylenediaminetetraacetic acid (EDTA), for example TAE buffer containing a
mixture
of Tris base, acetic acid and EDTA or TBE buffer comprising a mixture of Tris
base,
boric acid and EDTA; for example a solution comprising 10mM Tris pH 8, 1 mM
EDTA,
150 mM KC1, or for example, 50 mM Potassium Acetate, 20 mM Tris-acetate, 10 mM
Magnesium Acetate, pH 7.9 @ 25 C..
1.19. Any foregoing nanochip which is or is capable of being lyophilized for
storage
and subsequently rehydrated, e.g., wherein the structure of the nanochip
comprises a
hydratable or water permeable polymer.
1.20. Any foregoing nanochip which is synthesized in a dry form, e.g., wherein
the
structure of the nanochip comprises a hydratable or water permeable polymer,
followed
by hydration prior to use, optionally followed by lyophilization for long term
storage
once the write process is complete.
1.21. Any foregoing nanochip wherein the electrically charged polymer, e.g.,
DNA, is
stabilized with histone.
1.22. Any foregoing nanochip wherein the interior surface is positively
charged.
48
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1.23. Any foregoing nanochip wherein the electrodes are operably connected in
a
capacitive circuit capable of providing a radiofrequency pulsating direct
current, e.g. at a
frequency of 1 MHz to 1GHz, e.g. 50-200MHz, for example about 100MHz, across
the
nanopore, e.g., wherein the pulsating direct current can draw the charged
polymer
through the nanopore and the monomer sequence can be determined by measuring
the
capacitive variance across the nanopore as the charged polymer goes through
the
nanopore.
1.24. Any foregoing nanochip comprising a reserve or deblocker chamber, which
contains reagents for deprotection of the polymer following addition of a
monomer or
oligomer in one of addition chambers.
1.25. Any foregoing nanochip comprising a multiplicity of pairs of addition
chambers.
1.26. Any foregoing nanochip comprising an electrical control layer, a
fluidics layer
and an electrical ground layer, e.g., as depicted in figure 24, joined by
wafer bonding.
1.27. Any foregoing nanochip wherein the nanopore is made by drilling with
FIB,
TEM, wet or dry etching.
1.28. Any foregoing nanochip wherein the membrane comprising the nanopores is
from
1 atomic layer to 30 nm thick.
1.29. Any foregoing nanochip wherein the membrane comprising the nanopores is
made of SiN, BN, SiOx, Graphene, transition metal dichalcogenides e.g. W52 or
MoS2..
1.30. Any foregoing nanochip comprising wiring made from metal or polysilicon.
1.31. Any foregoing nanochip wherein the wiring density is increased by 3D
stacking,
with electrical isolation provided by dielectric deposition (e.g., via PECVD,
sputtering,
ALD etc).
1.32. Any foregoing nanochip wherein the contact to the electrode in the
addition
chamber is made using Through Silicon Via (TSV) by Deep Reactive Ion Etch
(DRIE),
e.g. using cryo or BOSCH process, or via wet silicon etching.
1.33. Any foregoing nanochip wherein individual voltage control for the
electrode in
each addition chamber allows the electrode in each addition chamber to be
controlled and
monitored individually.
1.34. Any foregoing nanochip wherein each polymer is associated with a first
addition
chamber, a second addition chamber, and a deblocking chamber.
49
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
1.35. Any foregoing nanochip wherein one or more chambers have fluid flow.
1.36. Any foregoing nanochip wherein one or more chambers are fluidically
isolated.
1.37. Any foregoing nanochip wherein the deblocking chamber has fluid flow..
1.38. Any foregoing nanochip wherein addition chambers have common fluid flow.
1.39. Any foregoing nanochip wherein wiring between chambers is common among
chambers of similar type (e.g. among first addition chambers, among second
addition
chambers, and among deblocking chambers.)
1.40. Any foregoing nanochip wherein the addition chambers have individual
voltage
control and the deblocking chambers have a common electrical ground.
1.41. Any foregoing nanochip wherein the deblocking chambers have individual
voltage control, the first addition chambers have a common electrical ground
and the
second addition chambers have a common electrical ground.
1.42. Any foregoing nanochip wherein the nanochip is fabricated by wafer
bonding,
and the chambers are prefilled with desired reagents prior to bonding.
1.43. Any foregoing nanochip wherein one or more internal surfaces are
silanized.
1.44. Any foregoing nanochip which has one or more ports for introduction or
removal
of fluid.
1.45. Any foregoing nanochip wherein the electrodes in the chambers are
restricted
from direct contact with the charged polymer, e.g.,wherein the electrode is
placed too far
from the nanopore to be reached by a charged polymer bound to a surface
adjacent to the
nanopore, or wherein the electrode is protected by a material which will
permit the
passage of water and single atom ions (e.g., Na+, K+ and Cl- ions) but not the
passage of
the polymer or monomer or oligomer reagents to be joined to the polymer.
1.46. Any foregoing nanochip which is electrically linked to a Complementary
Metal-
Oxide Semiconductor (CMOS) chip.
[0123] For example, in one embodiment, the invention provides a nanochip,
e.g., according to
any of Nanochip 1, et seq., for sequencing an electrically charged polymer,
e.g.,
DNA, comprising at least two distinct monomers, the nanochip comprising at
least a first and
second reaction chambers comprising an electrolyte media and separated by a
membrane
comprising one or more nanopores, wherein each reaction chamber comprises at
least one pair of
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
electrodes disposed on opposite sides of the membrane, wherein the electrodes
are operably
connected in a capacitive circuit capable of providing a radiofrequency
pulsating direct current,
e.g. at a frequency of 1 MHz to 1GHz, e.g. 50-200MHz, for example about
100MHz, across the
nanopore, e.g., wherein the pulsating direct current can draw the charged
polymer through the
nanopore and the monomer sequence can be determined by measuring the
capacitive variance
across the nanopore as the charged polymer goes through the nanopore.
[0124] In another embodiment, the invention provides a method of reading a
monomer sequence
of a charged polymer comprising at least two different types of monomers, for
example a DNA
molecule, comprising applying a radiofrequency pulsating direct current, e.g.
at a frequency of 1
MHz to 1GHz, e.g. 50-200MHz, for example about 100MHz, across a nanopore,
wherein the
pulsating direct current draws the charged polymer through the nanopore and
the monomer
sequence is read by measuring the capacitive variance across the nanopore as
the charged
polymer goes through the nanopore.
[0125] In another embodiment, the invention provides the use of any of DNA 1,
et seq. in a
method for storing information.
[0126] In another embodiment, the invention provides the use of a single
stranded DNA in a
method for storing information, e.g., wherein the sequence is substantially
non-self-hybridizing.
[0127] In another embodiment, the invention provides a method of data storage
and device,
using a nanochip, e.g.,any of Nanochip 1 et seq. to make an electrically
charged polymer, e.g.,
DNA, comprising at least two distinct monomers or oligomers, wherein the
monomers or
oligomers are arranged in sequence to correspond to a binary code, e.g., in
accordance with any
of the foregoing Methods 1 and/or 2 et seq.
[0128] For example, in one embodiment, the nanochip comprising the polymer
thus synthesized
provides a data storage device, as the nanochip can be activated and the
sequence of the polymer
detected by passing it through a nonopore at any time. In other embodiments
the polymer is
removed from the nanochip, or amplified and the amplified polymer removed from
the nanochip,
stored until required, and then read using a conventional sequencer, e.g., a
conventional
nanopore sequencing device,
51
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0129] In another embodiment, the invention provides a method of storing
information
comprising synthesizing any of DNA 1, et seq., e.g., in accordance with any of
Methods 1, et
seq. or Methods 2, et seq.
[0130] In another embodiment, the invention provides a method of reading a
binary code, e.g., as
encoded on any of DNA 1, et seq., using a nanopore sequencer, for example
using Nanochip 1 et
seq., as described herein.
[0131] Any foregoing method wherein the nanochip is erased using an enzyme
which lyses the
charged polymer, e.g., a deoxyribonuclease (DNase) to hydrolyze DNA.
EXAMPLES
Example I ¨ Immobilizing one end of DNA adjacent to nanopore and controlled
back and
forth movement of DNA via electrical current
[0132] Experimental procedures are developed to demonstrate that DNA is moved
back and
forth between two chambers separated by a nanopore, via an electrical current,
under conditions
that a relevant protein does not move between chambers.
[0133] A nanochip comprising two chambers is fabricated from silicon nitride.
Nanopores of <4
nm (for dsDNA or ssDNA) and 2 nm (for ssDNA only) are prepared as described in
Briggs K, et
al. Automated fabrication of 2-nm solid-state nanopores for nucleic acid
analysis, Small
(2014)10(10):2077-86. The two chambers are referred to as a 'near' and 'far'
chamber, the far
chamber being the chamber where 3' end of DNA is conjugated.
[0134] It is shown that ssDNA (2nm pore) and ssDNA+dsDNA (4nm pore) but not
protein pass
through the nanopore. Passing through the nanopores is detected by electrical
current disruption.
[0135] Conjugation of DNA to pore surface: Attach 5' amino modified DNA to
carboxy-coated
polystyrene beads (Fluoresbrite BB Carboxylate Microspheres 0.05m, from
Polysciences,
Inc.) via carbodiimide mediated attachment. 3' of DNA is labeled with biotin.
DNA is of a pre-
specified length.
[0136] Strepatividin conjugation: Conjugation was performed on the 'far' side
of a silicon
nitride nanopore conjugate streptavidin to the surface, as described in
Arafat, A. Covalent
Biofunctionalization of Silicon Nitride Surfaces. Langmuir (2007) 23 (11):
6233-6244.
[0137] Immobilization of DNA near the nanopore: DNA conjugated polystyrene
beads in buffer
is added to 'near' chamber and buffer is added to 'far' chamber (standard
buffer: 10mM Tris pH
52
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
8, 1 mM EDTA, 150 mM KC1). Voltage (-100 mV) is applied until current
disruption is
observed (use an Axon Nanopatch200B patch-clamp amplifier). 50 nm beads cannot
pass
through the nanopore, so when a DNA strand has gone through and a bead is
pressed against an
end of the nanopore the current is highly disrupted. Current is maintained 1-2
mins until DNA is
irreversibly bound to immobilized streptavidin on the far side via binding of
biotin. To confirm
that the DNA has been immobilized, the current is reversed. Different currents
are observed if
DNA is in or out of the pore. If it appears that DNA is not immobilized, then
the process is
repeated.
[0138] Release the bead via endonuclease: Restriction enzyme in restriction
enzyme buffer is
added to the chamber where the DNA is attached. In one embodiment, the DNA is
single
stranded and contains a restriction site cleavable by an enzyme that will
cleave single stranded
DNA. See, e.g., Nishigaki, K., Type II restriction endonucleases cleave single-
stranded DNAs in
general. Nucleic Acids Res. (1985) 13(16): 5747-5760. In an alternative
embodiment, a
complementary oligonucleotide is added to the chamber where the DNA is
attached and allowed
to hybridize for 30 minutes to create dsDNA, then the restriction enzyme is
added. Once the bead
is released, it is washed away. Current is switched between forward and
reverse to confirm that
the DNA goes into/through and out of the pore.
[0139] Demonstrate controlled back and forth movement: Using standard buffer,
current is
applied in forward direction until signal disruption is observed and then
reverted to 'normal'
after the DNA passes. Reverse current is applied until signal disruption is
observed. It is
observed that the signal does not go back to normal as the DNA remains in the
pore. Application
of current in forward and reverse direction is repeated for several cycles to
confirm that DNA
moves back and forth through the nanopore.
Example la: Immobilizing DNA strand adjacent to nanopore in a silicon dioxide
chip
[0140] A nanochip interior wall is fabricated from silicon dioxide. Both sides
are silanized, but
the oligonucleotide is conjugated to just one side of the chip wall, then a
nanopore is created.
[0141] Silanization: The surface of the chip wall is cleaned with piranha
solution (various brands
commercially available, generally comprising a mixture of sulfuric acid
(H2504) and hydrogen
peroxide (H202), which remove organic residues from the surface) at 30 C, and
washed with
double-distilled water. A stock solution of (3-aminopropyl)triethoxysilane
(APTES) is prepared,
53
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
with 50% methanol (Me0H), 47.5% APTES, 2.5% nanopure H20, and aged > lhour at
4 C.
The APTES stock is then diluted 1:500 in Me0H and applied to and incubated
with the chip wall
at room temperature. The chip wall is then rinsed with Me0H and dried at 110 C
for 30
minutes.
[0142] Conjugation: The chip wall is then incubated for 5 hours at room
temperature in a 0.5%
w/v solution of 1õ4-phenylene diisothiocyanate (PDC) in dimethyl sulfoxide
(DMSO). It is
washed briefly twice with DMSO and the briefly twice with double distilled
water. The chip wall
is then incubated with 100 nM amine-modified single stranded DNA oligomers
(ca. 50-mers) in
double distilled water (pH 8) overnight at 37 C. Then the chip wall is washed
twice with 28%
ammonia solution to deactivate any unreacted material, and washed twice with
double distilled
water. One or more nanopores are then created in the wall.
[0143] Once the fabrication of the nanochip is complete, the interior wall is
coated with DNA
oligomers ca. 50bp long. This permits a single stranded DNA having an end-
terminal sequence
complementary to the surface bound DNA to be localized to a nanopore by
attaching the ssDNA
to a relatively bulky structure (e.g. a bead, a protein, or a DNA origami
structure having a
diameter too large to fit through the nanopore,), wherein the sequence
complementary to the
surface-bound DNA is distal to the bulky structure, pulling the charged
polymer through the
nanopore using current, allowing the ssDNA to bind to a complementary surface
bound DNA
oligomer adjacent to the nanopore, and cleaving off the bulky structure.
Example 2: DNA synthesis ¨ single nucleotide addition
[0144] DNA is moved to 'reserve' chamber by applying appropriate current and
detecting DNA
movement.
[0145] Terminal transferase enzyme (TdT, New England Biolabs) in appropriate
buffer (50 mM
Potassium Acetate, 20 mM Tris-acetate, 10 mM Magnesium Acetate, pH 7.9 @ 25
C), plus
reversibly blocked-dATP* is added to the 'addition' chamber. The buffer is
also added to the
'reserve' chamber.
[0146] dNTPs that have reversible blocks on the 3' ¨OH are used to add
nucleotides to the DNA.
When added to the DNA chain, the next dNTP cannot be added until the blocked
dNTP is
unblocked.
[0147] Deblocking can be chemical or enzymatic. Different approaches are
utilized:
54
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0148] a. 3' 0-allyl: Allyl is removed by Pd-catalyzed deallylation in
aqueous buffer
solution as described in Ju J, Four-color DNA sequencing by synthesis using
cleavable
fluorescent nucleotide reversible terminators. Proc Natl Acad Sci USA.
(2006);103(52):19635-
40; or by using using iodine (10 mol%) in polyethylene glycol-400, as
described in Shankaraiah
G., et al., Rapid and selective deallylation of allyl ethers and esters using
iodine in polyethylene
glycol-400. Green Chem. (2011)13: 2354-2358
[0149] b. 3' 0-NH2: Amine is removed in buffered NaNO2, as described in US
8034923.
[0150] c. 3'-phosphate. Phosphate is hydrolyzed with Endonuclease IV (New
England
Biolabs). Other possible 3' modifications which can also be removed with
Endonuclease IV
include phosphoglycoaldehyde and deoxyribose-5-phosphate.
[0151] d. 3'-0-Ac: Acetate is removed by enzymatic hydrolysis as described
in Ud-Dean, A
theoretical model for template-free synthesis of long DNA sequence. Syst Synth
Biol (2008)
2:67-73,
[0152] The DNA is then moved to the 'far' chamber by applying appropriate
current and
detecting DNA movement. DNA is deprotected by switching out buffers and adding
deblocking
buffer/solution as described in a. ¨ d. above.
[0153] Process is repeated as desired to make sequence of interest.
Example 3: DNA synthesis: block oligonucleotide addition
[0154] The 3' end of double stranded DNA is attached adjacent to a nanopore
with 4nm
aperture. The 5' end of the DNA has an overhang of CG (reading from 5' to 3').
[0155] Oligo cassettes A and B are made as follows:
5' CGAAGGG <CODEA OR B> GTCGACNNNNN
3' GCTTCCC <COMPLEMENT> CAGCTGNNNNN
[0156] CodeA and CodeB each represent an informational sequence. Ns refer to
any nucleotide.
The 5' sequence comprises a topoisomerase recognition site and the 3' sequence
comprises an
Accl restriction site. The oligo is exposed to topoisomerase and the
toposisomerase binds to 3'
thymidine:
5' CGAAGGG <CODEA OR B> GTCGACNNNNN
3' *TTCCC <COMPLEMENT> CAGCTGNNNNN (* = topoisomerase)
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0157] DNA is moved to 'near' chamber by applying appropriate current and
detecting DNA
movement. The topoisomerase-charged `codeA' oligo is provided in the
'addition' chamber.
The DNA is moved into the addition chamber by applying appropriate current and
detecting
DNA movement, whereupon the code A oligo is bound to the DNA, Accl is added to
the
'reserve' chamber, where it cleaves at the restriction site to provide a
topoisimerase ligation site.
[0158] The process is repeated until the desired sequence is reached, adding
other 'code A' or
'code B.' Note that it is not required to continually add new Accl to the
'reserve' chamber; it is
just needed to flush out codeA or codeB oligos in 'addition' chamber when
switching from
codeA or codeB.
[0159] For sequencing a pore that only allows ssDNA to pass, some
modifications to the
protocol above are required. It is already known that when dsDNA encounters a
small pore
(2nm) only ssDNA will go through and the complement will be 'stripped' off.
Thus, if doing this
synthesis with a 2nm pore one must ensure that the proper dsDNA is able to
'reform' on the
other side. To do this one would add "CGAAGGG <CODEA OR B> GTCGACNNNNN" to the
near chamber (to ensure a restriction site is created) and "CGAAGGG <CODEA OR
B> GT" to
the far chamber (to ensure a topo-compatible site is generated).
[0160] Elaborating on the foregoing method, we demonstrate the sequential
'addition' of DNA-
encoded information into a growing DNA chain with >2 sequential additions
(representing 2 bits
of data), each of which comprise an 'add' and a `deprotece step. Initial
experiments for
optimization and proof of concept are performed in microtubes.
[0161] In the approach described in this example, one bit of information is
encoded in a string of
nucleotides. The DNA bit to be 'added' is a short dsDNA sequence conjugated to
vaccinia
topoisomerase I (topo). In the presence of a suitable `deprotected"acceptor'
DNA, the topo-
charged DNA 'bit' is enzymatically and covalently linked ('added') to the
acceptor by the
topoisomerase, which in the process becomes removed from the DNA. A
restriction enzyme can
then cleave the added bit to `deprotece it and create of suitable 'acceptor'
sequence for addition
of the next bit.
[0162] Topo Charging: A generic charging scheme is as follows, depicted
schematically in
figure 22 and below, where N indicates any nucleotide, and A, T, G, and C
represent nucleotides
with adenine, thymine, guanine and cytosine bases respectively. N's on top of
one another are
56
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
complementary. While this example uses the restriction enzyme HpyCH4III, the
basic strategy
will work with other restriction enzymes, e.g., as demonstrated in Example 4.
N-N-N-N-N-N-N-N-N A-G-G-G-N-N-N-N-N-N-N-N-N-N
N-N-N-N-N-N-N-N-N-T-T-C-C-C-N-N-N-N-N-N-N-N-N-N
+
Topoisomerase *
N-N-N-N-N-N-N-N-N
N-N-N-N-N-N-N-N-N
+
A-G-G-G-N-N-N-N-N-N-N-N-N-N
*-T-T-C-C-C-N-N-N-N-N-N-N-N-N-N
(topo charged)
(N's on top of one another are complementary)
Addition
Generic 'add' reaction:
N-N-N-N-N-N-N-N-N-N-A
N-N-N-N-N-N-N-N-N-N..
(acceptor)
+
A-G-G-G-N-N-N-N-N-N-N-N-N-N
*-T-T-C-C-C-N-N-N-N-N-N-N-N-N-N
(topo charged)
N-N-N-N-N-N-N-N-N-N-A A-G-G-G-N-N-N-N-N-N-N-N-N-N
N-N-N-N-N-N-N-N-N-N-T-T-C-C-C-N-N-N-N-N-N-N-N-N-N
+
*
(free topo)
Deprotection
Generic 'deprotection' reaction:
57
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
...N-N-A-C-A-G-T-N-N-N-N-N-N-N-N-N-N
...N-N-T-G-T-C-A-N-N-N-N-N-N-N-N-N-N
+
HpyCH4III
(restriction enzyme)
...N-N-A-C-A
...N-N-T-G..
(deprotected)
+
..G-T-N-N-N-N-N-N-N-N-N-N
T-C-A-N-N-N-N-N-N-N-N-N-N
(side product)
[0163] The following oligonucleotides are ordered from Integrated DNA
Technologies (IDT).
The "b" at the end of some of the oligonucleotides indicates biotin):
BAB:
CGATAGTCTAGGCACTGTTTGCTGCGCCCTTGTCCGTGTCGCCCTTATCTACTT
AAGAGATCATACAGCATTGCGAGTACG
Bl: b-CACGTACTCGCAATGCTGTATGATCTCTTAAGTAGATA
B2: ATCTACTTAAGAGATCATACAGCATTGCGAGTACG
TAl: b-CACACTCATGCCGCTGTAGTCACTATCGGAAT
TA2: AGGGCGACACGGACAGTTTGAATCATACCG
TA3b:
AACTTAGTATGACGGTATGATTCAAACTGTCCGTGTCGCCCTTATTCCG
ATAGTGACTACAGCGGCATGAG
TB1: b-CACACTCATGCCGCTGTAGTCACTATCGGAAT
TB2: AGGGCGCAGCAAACAGTGCCTAGACTATCG
TB3b:
AACTTAGTATGACGATAGTCTAGGCACTGTTTGCTGCGCCCTTATTCCGATAG
TGACTACAGCGGCATGAG
FP1: CACGTACTCGCAATGCT
58
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
FP2: CGGTATGATTCAAACTGTCCG
FP3: GCCCTTGTCCGTGTC
[0164] Oligonucleotides are solubilized to 100uM in TE buffer and stored at -
20C.
[0165] Hybridized oligonucleotides are made by mixing oligonucleotides as
described below,
heating to 95 C for 5 minutes, and then dropping the temperature by 5 C every
3 minutes until
the temperature reaches 20 C. Hybridized oligonucleotides are stored at 4 C or
-20 C. The
combinations of oligonucleotide are as follows:
B1/2
48 uLB1
48 uL B2
4 uL 5M NaCl
AS
20 uL TA1
20 uL TA2
uL TA3b
4 uL 5M NaCl
51 uL TE
B5
20 uL TB1
20 uL TB2
5 uL TB3b
4 uL 5M NaCl
51 uL TE
[0166] The following buffers and enzymes are used in this example:
59
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
TE: 10M Tris pH 8.0, 1mM EDTA, pH 8.0
WB: 1M NaC1, 10mM Tris pH8.0, 1mM EDTA pH8.0
lx Topo: 20mM Tris pH7.5, 100mM NaC1, 2mM DTT, 5mM MgCl2
lx RE: 50mM K-acetate, 20mM Tris-acetate, 10mM Mg-acetate, 100ug/m1 BSA pH 7.9
@ 25C.
Vaccinia DNA Topoisomerase I (topo) is purchased from Monserate Biotech
(10,000
U/mL)
HypCH4III is purchased from NEB
Streptavidin-coated magnetic beads (s-MagBeads) are purchased from
ThermoFisher.
[0167] Acceptor is prepared as follows: 5uL of s-magbeads are washed one time
in 200uL WB
(binding time 1 minute). 5uL B1/2 + 195uL WB is added to beads and incubated
15 minutes at
room temperature, then washed one time with 200uL WB, then washed one time
with 200uL lx
Topo, and resuspended in 150uL of lx Topo
[0168] Topo-charged A5 (see figure 20) is prepared as follows: 4uL 10x topo
buffer + 23 uL
water + 8uL A5 + 5 uL topo are incubated at 37 C for 30 minutes, added to to
5uL s-magbeads
(washed lx with 200uL WB, lx with 200uL lx Topo, resuspended in 150uL lx
topo), and
allowed to bind for 15 minutes at room temperature.
[0169] 'Add' charged AS to Acceptor: s-magbeads are removed from from Topo-
charged-A5,
added to Acceptor, and incubated at 37 C for 60 minutes. The aliquot is
removed, diluted 1/200
in TE, and stored at -20C
[0170] Deprotection: The material is washed one time with 200uL of WB, when
washed one
time with 200uL of lx RE, and resuspended in 15uL 10x RE and 120uL water).
15uL
HypCH4III is added. The mixture is incubated at 37 C for 60 minutes, then
washed one time
with 200uL WB, washed one time with 200uL lx topo, to produce a product which
we term
'Acceptor-A5'.
[0171] Topo charged B5 (see figure 21) is prepared as follows: 4uL 10x topo
buffer. 23 uL
water and 8uL B5 + 5 uL topo are combined and incubated at 37 C for 30 min.
the product is
added to 5uL s-magbeads (washed one time with 200uL WB, one time with 200uL lx
Topo, and
resuspended in 150uL lx topo) and allowed to bind for 15 minutes at room
temperature.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0172] 'Add' charged B5 to Acceptor-A5: s-magbeads are removed from Topo-
charged-B5,
added to Acceptor-A5 and incubated at 37 C for 60 minutes. The aliquot is then
removed, diluted
1/200 in TE, and stored at -20 C
[0173] Deprotection: The material is washed one time with 200uL of WB, then
washed one time
with 200uL of lx RE, and resuspended in 15uL 10x RE and 120uL water. 15uL
HypCH4III is
added, and the mixture is ncubated at 37 C for 60 minutes.
[0174] Confirmation that the above reactions worked is provided by PCR
amplification of
aliquots from A5 (Acceptor with A5 added: step iii, `A5 Added' in schematic)
and B5
(Acceptor-A5 with B5 added: step vi, 135 Added' in schematic). 'No template'
is used as
negative control for A5, A5 is used as negative control for B5, oligo BAB is
used as positive
control for B5. The expected product size for A5 PCR is 68bp, the expected
product size for B5
PCR is 57bp. (B1/2 is also run on the gel, expected size is ¨47bp, but this
may be approximate as
there are overhangs and it is biotinylated). PCR reactions (30 cycles of
95/55/68 (1 minutes each)
are carried out as follows:
A5 (-) ctrl A5 B5 (-) ctrl B5
Template 1 uL 1 uL 1 uL
FP1 1 uL 1 uL 1 uL 1 uL
FP2 1 uL 1 uL
FP3 1 uL 1 uL
Water 8 uL 7 uL 7 uL 7 uL
Maxima MM 10 uL 10 uL 10 uL 10 uL
[0175] SDS-PAGE using 4-20% Tris-glycine gels is used to confirm that expected
size
oligonucleotides are produced. Charging is performed as described above, but
directly after
charging (37 C incubation step), loading buffer is mixed in and samples are
heated to 70 C for 2
minutes and allowed to cool prior to running the gel. Gel is stained with
Coomassie. For the
negative control, water is added to the reaction instead of topo. Figure 30
depicts the results,
clearly showing bands corresponding to the expected product sizes for AS PCR
and for B5 PCR.
[0176] DNA bit addition via topoisomerase-charged DNA cassettes and
deprotection performed
via restriction enzyme are thus shown to be feasible. In these proof of
concept experiments the
61
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
DNA is immobilized via streptavidin-conjugated magnetic beads, and moved
sequentially into
different reaction mixes, but in the nanopore chip format, we create separate
reaction chambers
and use electrical current to move the DNA into those different reaction
chambers.
[0177] Finally, PCR demonstrates that the expected DNA sequences are created
when
performing sequential additions of DNA sequences corresponding to 'bits' of
information. These
reactions have worked as designed, even with minimal optimization.
[0178] DNA made as described in examples 2 and 3 is recovered and sequenced,
using a
commercial nanopore sequencer (MinION from Oxford Nanopore), confirming that
the desired
sequence is obtained.
Example 4¨ DNA synthesis: block oligonucleotide addition, using a different
restriction
enzyme
[0179] The following synthesis is carried analogously to Example 3, but using
the restriction
enzyme Mini, which cuts at `ACGCGI" to form:
...NNNA. CGCGTNNN...
...NNNTGCGC ANNN_
In this example TOPO is charged to form a complex with sequence
complementarity that will
enable the charged TOPO to transfer DN.A to DNA cut with MiuT
5' pCACGTCAGGCGTATCCATCCOTTCGCGTTCACGTACTCGCAATGCTGTAG
3' GTGCAGTCCGCATAGGTAGGGAAGCGC AGTGCATGAGCGTTACGAGATCb
-4-
TOPO
5' pCACGTCAGGCGTATCCATCCCTT*
3 GTGCAGTCCGCATAGGTAGGGAAGCGC
("' indicates TOPO bound at 3' phosphate)
62
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
CGCGTTCACGTACTCGCAATGCTGTAG
AGTGCATGAGCGTTACGAGATCb
(b,biotin. This can be removed with streptavidin)
[0180] By a process analogous to the preceding example, the charged TOPO is
then used to add
the oligomer to the 5' end of strand being synthesized, having a complementary
acceptor
sequence, thereby releasing the TOPO, and the strand is then "deprotected"
using the Mini, and
the cycle repeated until the desired sequence of oli.gomers is obtained.
Example 5¨ Addition of single base using topoisomerase strategy
[0181] We have found that the topoisomerase system can also be designed to add
single bases to
a single stranded DNA chain (in comparison to Example 3, which describes
adding 'cassettes').
The DNA bit to be 'added' is contained in a short DNA sequence conjugated to
vaccinia
topoisomerase I (topo). In the presence of a suitable single stranded
`deprotected"acceptor'
DNA, the topo-charged DNA is enzymatically and covalently ligated ('added') to
the acceptor
by the topoisomerase, which in the process becomes removed from the DNA. A
type ITS
restriction enzyme can then cleave all of the added DNA with the exception of
a single base (the
base which is being 'added'). This process of deprotect-add is repeated to add
additional bases
(bits).
[0182] Topo Charging: A generic charging protocol is as follows, similar to
Example 3:
...N-N-N-N-N-N-N-N-N-C-C-C-T-T-N-N-N-N-N-N-N-N-N-N-N-N-N_
...N-N-N-N-N-N-N-N-N-N-N-N-N-N-I-I-I-I-I N-N-N-N-N-N-N_biotin
Topoisomerase (*)
N-N-N-N-N-N-N-N-N-N-N-N-N-N-N
(by-proct)
...N-N-N-N-N-N-N-N-N-C-C-C-T-T*
...N-N-N-N-N-N-N-N-N-N-N-N-N-N-I-I-I-I-I
(topo charged)
As in Example 3, the N's on top of one another are complementary. I is
inosine. The biotin is
63
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
used to remove unreacted product and byproduct. Addition of a single base is
carried out as
follows
N-N-N-N-N-N-N-N-N-N_
(acceptor sequence nucleotides indicated in italics)
...N-N-N-N-N-N-N-N-N-C-C-C-T-T*
...N-N-N-N-N-N-N-N-N-N-N-N-N-N-I-I-I-I-I
(topo charged)
...N-N-N-N-N-N-N-N-N-C-C-C-T-T-N-N-N-N-N-N-N-N-N-N_
...N-N-N-N-N-N-N-N-N-N-N-N-N-N-I-I-I-I-I
(free topo)
Deprotection is illustrated as follows, using BciVI restriction enzyme (site
in bold):
...N-G-T-A-T-C-C-N-N-C-C-C-T-T-N-N-N-N-N-N-N-N-N-N_
...N-N-N-N-N-N-N-N-N-N-N-N-N-N-I-I-I-I-I
BciVI
(restriction enzyme)
T-N-N-N-N-N-N-N-N-N-N_
(note a 'T' has been added to the 5' of the acceptI
N-N-I-I-I-I-I
(dissociated*)
C-T-A-T-C-C-N-N-C-C-C-T
T-N-N-N-N-N-N-N-N-N-N_
N-N-I-I-I-I-I
64
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
T-N-N-N-N-N-N-N-N-N-N... + N-N-I-I-I-I- I
(NNIIIII dissociates from the single strand with added base)
[0183] The following oligonucleotides are synthesized commercially (B=biotin,
P-phosphate, I =
inosine):
NAT1 CACGTCAGGCGTATCCATCCCTTCACGTACTCGCAATGCTGTATGGCGAT
NAT1b P-CACGTCAGGCGTATCCATCCCTTCACGTACTCGCAATGCTGTATGGCGAT-B
NAT9cI P-IIIIIAAGGGATGGATACGCCTGACGTG
NAT9x P-ATCGCCATACAGCATTGCGAG
NAT9 ACGTGAAGGGATGGATACGCCTGACGTG
Nat9Acc CACGTAGCAGCAAACAGTGCCTAGACTATCG
Nat1P CACGTCAGGCGTATCCATCC
FP4 CGATAGTCTAGGCACTGTTTG
[0184] The oligonucleotides are solubilized to 10011M in TE buffer and stored
at -20 C.
[0185] Hybridization: The following hybridized oligonucleotides are made by
mixing the
oligonucleotides as described, heating to 95 C for 5 minutes, and then
dropping the temperature
by 5 C every 3' until the temperature reaches 20 C. Hybridized
oligonucleotides are stored at 4
C or -20 C.
NAT1b/NAT9cI/NAT9x
8 1.iL NAT1B
[IL NAT9cI
10 [IL NAT9x
48 1.iL TE
4 [IL 5M NaCl
NAT1/NAT9cI
10 [IL NATI
10 [IL NAT9cI
80 uL PBS
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
NAT1/NAT9
[IL NATI
10 !IL NAT9
80 uL PBS
[0186] Buffers & Enzymes: The following buffers are used:
TE: 10M Tris pH 8.0, 1mM EDTA, pH 8.0
PBS: phosphate buffered saline (137mM NaCl, 2.7mM KC1, 10mM Na2HPO4, 1.8mM
KH2PO4)(pH 7.4)
10x Cutsmart: 500 mM KAc, 200 mM Tris-Ac, 100 mM Mg-Ac, 1 mg/mL BSA pH 7.9
BciVI is purchased from NEB and Streptavidin-coated magnetic beads (s-
MagBeads) are
purchased from ThermoFisher
[0187] The addition reaction is carried out as follows.
[0188] 1. Topo charge: The reagents are assembled as per table:
Experiment (-) control #1 (-) control #2
10x topo buffer 3 3 3
Water 17 21 23
NAT1B/NAT9c1/NAT9x 6 6 -
Topo 4 - 4
The reagents are then incubated at 37 C for 30 minutes. The byproducts are
removed using
streptavidin magnetic beads (5uL) in lx topo buffer after 10 minutes at room
temperature to
allow binding.
[0189] 2. Reaction: The reagents are assembled as per table:
Experiment (-) control #1 (-) control #2
From A.1.c 1 1 1
66
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
NAT9Acc 1 1 1
10x topo buffer 1 1 1
water 7 7 7
The reagents are then incubated at 37 C for 30 minutes. The addition reaction
is expected to
proceed as follows:
NAT1B 5 p-CACGTCAGGCGTATCCATCCCTTOACGTACTCGCAATGCTGTATGGCGAT-B
NAT 9cI 3 ' GTGC.AGTC.CGCATAGGTAGGGAAI L III GAGCGTTACGACATACCGCTA-p NAT9x
TOPO
CACGTCAGGCGTATCCATCCCTT*
3 GTGCAGTCCGCATAGGTAGGGAAII I 3: I]=.; =
[0190] The asterisk (*) represents topoisomerase. Note that NAT9cI is
phosphorylated, but this
isn't shown for illustration purposes.
[0191] When the charged topo is in the presence of an acceptor sequence, it
undergoes the
following reaction:
5' p-CACGTCAGGCGTATCCATCCCTT*
GTGCAGTCCGCATAGGTAGGGAAIIIII
CACGTAGCAGCAAACAGTGCCTAGACTATCG
5' p-CACGTCAGGCGTATCCATCCCTTCACGTAGCAGCAAACAGTGCCTAGACTATCG
GTGCAGTCCGCATAGGTAGGGAAIIIII
[0192] PCR amplification and measurement of the molecular weights of the
product on agarose
gel confirms the expected product is produced. See Figure 30, depicting
correct sized band in
lane 1 (experiment), no bands in negative controls.
67
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0193] B. Deprotection Reaction: The reagents are assembled as per table:
1 2 3
4
NAT1/NAT9 1 1 -
_
NAT 1/NAT9cI - - 1
1
10x cutsmart 2 2 2
2
water 17 16 17
16
0
BciVI 0 1 1
The reagents are incubated at 37 C for 90 minutes. For the deprotection
reaction, a
representative product of an addition reaction is created using purchased
oligonucleotides, and
tested for digestion with the BciVI restriction enzyme:
NAT1 5' CACGTCAGGCGTATCCATCCCTTCACGTACTCGCAATGCTGTATGGCGAT
NAT9cI 3' GTGCAGTCCGCATAGGTAGGGAAIIIII
+
BciVI
NAT1 5' CACGTCAGGCGTATCCATCCCT TCACGTACTCGCAATGCTGTATGGCGAT
NAT9cI 3' GTGCAGTCCGCATAGGTAGGG AAIIIII
It was not known whether the the restriction enzyme would cut the DNA as
intended, given that
3' of the cut site are a series of inosines as opposed to 'regular' bases. As
a positive control, the
'appropriately' base-paired equivalent of NAT1/NAT9cI is made (NAT1/NAT9c):
NAT1 5' CACGTCAGGCGTATCCATCCCTTCACGTACTCGCAATGCTGTATGGCGAT
NAT9c 3' GTGCAGTCCGCATAGGTAGGGAAGTGCA
PCR amplification of the product followed by measurement of molecular weight
on agarose gels
68
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
(Figure 31) shows that the enzyme works as intended. For the positive control,
a larger band is
observed when undigested (lane 1), but a smaller band/s are observed with
digestion. The same
pattern is observed with NAT1/NAT9cI, showing that the presence of inosines
does not negate
or interfere with digestion. A small amount of undigested product seems to
remain with
NAT1/NAT9cI, suggesting that the cleavage is not as effective, at least under
these conditions,
as with NAT1/NAT9c. Cleavage efficiency may be improved by altering buffer
conditions
and/or addition of more inosines at the 5' end of NAT9cI.
[0194] The foregoing example demonstrates that it is feasible to use a Topo /
TypeIIS restriction
enzyme combination to add a single nucleotide to the 5' end of a target single
stranded DNA. A
related topoisomerase, SVF, that recognizes the sequence CCCTG
(https://ww w.nebi.nimmih.govipiEbnial/8661446) is used to add a `G' instead
of a 'T', using an
analogous process, thus allowing construction of a sequence encoding binary
information with T
and G.
[0195] As noted above, where dsDNA is generated using topoisomerase
strategies, nicks in
DNA on the opposing strand can be repaired using a ligase together with ATP.
But when doing
the single nucleotide addition, as in this example, we are building a single
stranded DNA, so
there are no nicks that need to be repaired and no need to use ligase.
Example 6¨ Addition of single base using topoisomerase strategy couple with 5'
phosphate
coupling
[0196] In another approach to single base addition, we use a 5'phosphate as a
blocking group to
provide single base pair addition in the 3' to 5' direction. The charging
reaction charges the
topoisomerase with a single T (or G, or other nucleotide as desired), having a
5' phosphate
group. When the charged topoisomerase 'sees' a free 5' unblocked
(unphosphorylated) single
stranded DNA chain it will add the T to that chain, providing a DNA with a T
added to the 5'.
This addition is facilitated by the presence of an adapter DNA having
sequences to which the
topoisomerase and the single stranded acceptor DNA can bind. (Note that the
adapter DNA is
catalytic ¨ it can be reused as a template in repeated reactions.) The added
nucleotide has a 5'
phosphate on it, so it won't be a substrate for further addition until it is
exposed to a phosphatase,
which removes the 5' phosphate. The process is repeated, using Topo to add a
single "T" to the
5' end of a target single stranded DNA and SVF topoisomerase to add a single
`G', thus allowing
69
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
construction of a sequence encoding binary information with T and G. The
process is depicted
schematically as follows:
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
GEN ER1CALLY:
CHARGING:
N-N-N-N-N-N-N-N-C-C-C-TT-N-N-N-N-N-N-N-N (T is 5 phosphorylated)
TOPO
N-N-N-N-N-N-N-N-C-C-C-T N-N-N-N-N-N-N-N
T-TOPO (T is 5' phosphorylated)
TRANSFER:
T-TOPO (T is 5' phosphorylated)
N-N-N-N-N-N-N-N-N-N-N-N (5' N has 5' OH)
=
T-N-N-N-N-N-N-N-N-N-N-N-N (T is 5' phosphorylated)
TOPO
DEB LOCKING:
T-N-N-N-N-N-N-N-N-N-N-N-N (T is 5' phosphorylated)
Phosphatase
T-N-N-N-N-N-N-N-N-N-N-N-N (T is 5' dephosphoryiated, now has 5' OH)
71
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
*****ALTERNATE TRANSFER MECHANISM************
T-TOPO (T is 5 phosphorylated)
N-N-N-N-N-N-N-N-N-N-N-N (acceptor) (5' N has 5' OH)
N-N-N-N-N-N-N-C-C-C-T
N-N-N-N-N-N-N-N-N-N-N-A-I-I-I-I-I
(adapter)
TOPO
T-N-N-N-N-N-N-N-N-N-N-N-N
N-N-N-N-N-N-N-N-N-N-N-A-
this transient intermediate that breaks down to ¨>
T-N-N-N-N-N-N-N-N-N-N-N-N (T is 5' phosbhorylated)
N-N-N-N-N-N-N-C-C-C-T
N-N-N-N-N-N-N-N-N-N-N-A-I-I-I-I-I
Example 7- Using DNA origami to aid in attaching DNA adjacent to nanopore
[0197] A DNA strand with a large origami structure on one end is captured in a
nanopore, and
immobilized to surface-conjugated streptavidin through a terminal biotin
moiety on the DNA.
After restriction enzyme cleavage of the origami structure, the immobilized
DNA can be moved
back and forth through the pore, as confirmed by current disruption. The
immobilization enables
a controlled movement of a single DNA molecule through the pore, which in turn
enables both
the 'reading' and 'writing' of information to DNA.
[0198] As depicted in Figure 35, a bulky double-stranded DNA unit is formed,
which is too large
to fit through the nanopore, with a single stranded region, linked to the
bulky portion by two
short double stranded regions having which serves to anchor the DNA to be
added to in the
72
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
synthesis. The single stranded region can then be detached and anchored to the
surface adjacent
to the nanopore, and the origami structure released. See Figure 33.
[0199] Nanopores are formed in 3mm chips with 20nm 5i02, with 50 * 50 pm
windows. Chip
are provided by Nanopore Solutions. Nanopore cassette holders and flow cells
are provided by
Nanopore Solutions. The amplifier is a Tecella Pico 2 amplifier. This is a usb-
powered amplifier
that uses a usb-computer interface for control. Tecella supplies (Windows)
software to control
the amplifier. The multimeter is a FLUKE 17B+ Digital Multimeter, capable of
detecting current
as low as 0.1 uA. For screening of radiofrequency noise we use a Concentric
Technology
Solutions TC-5916A Shield Box (Faraday Cage) with USB interface.
Oligonucleotides are
obtained from IDT.com. "PS" is Proparyl Silane - 0-(PROPARGYL)-N-
(TRIETHOXYSILYLPROPYL) CARBAMATE fromhttp:llwww.gelest.corn/productio-
proparg,371-n-triethoxysilylpropylearbatnate-90/.
[0200] The origami structure is based on single-stranded m13 with a
'honeycomb' cube origami
structure which is ¨ 20nm on one side. There are double stranded regions
adjacent to the
honeycomb each containing a unique restriction site. One of those sites is
used to attach modified
DNA to enable attachment near the nanopore, the other is used for cleaving off
the origami
structure once the DNA is attached.
[0201] Nanopore Formation: Nanopores are formed in the chips using dielectric
breakdown, as
follows:
1. Chips are carefully mounted in the cassettes
2. Wetting: 100% ethanol is carefully pipetted on the chip. Bubbles must be
removed.
However, direct pipetting of solution on the chip should be avoided or the
chip can crack
(5i02 is only 20nm).
3. Surface treatment: ethanol is removed, and freshly prepared Piranah
solution (75%
sulfuric acid, 25% hydrogen peroxide (30%)) is pipetted onto the chip. (let
piranha
solution come to room temperature). Leave on for 30 minutes.
4. Rinse 4 times with distilled water.
5. Rinse 2 times with HK buffer (10mM HEPES pH 8, 1M KC1)
6. Assemble cassette into flow cell.
7. Add 700 [IL HK buffer to each chamber of the flow cell.
8. Insert silver electrodes attached to the amplifier and close the Faraday
cage.
73
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
9. Test resistance with 300 mV. No current should be detected. If it is
detected, the chip
is likely cracked and one must start again.
10. Connect electrodes to a DC current of 6 V and test the current with a
multimeter.
Current should be low and should not change. Increase voltage by 1.5 V and
hold the
voltage until the resistance increases. If resistance does not increase after
5-10
minutes, increase the voltage another 1.5 V and try again. Repeat until
resistance
increases, at which point the applied voltage should be stopped immediately.
(with
sufficient voltage, dielectric breakdown occurs and a 'hole' is created in the
SiO2
membrane. When initially created the hole is small, but will increase in size
as the
voltage is maintained.)
11. Test the pore using the amplifier. At 300 mV one should see current of a
few to
several nA. The more current, the larger the pore.
[0202] Figure 34 depicts a basic functioning nanopore. In each panel, the y-
axis is current (nA)
and the x-axis is time (s). The left panel "Screening of RF Noise" illustrates
the utility of the
Faraday cage. A chip with no nanopore is placed in the flow cell and 300mV
applied. When the
lid of the Faraday cage is closed (first arrow) the noise reduction can be
seen. A small spike
occurs when the latch is closed (second arrow). Notice the current is ¨0 nA.
After pore
manufacture (middle panel), application of 300mV (arrow) results in a current
of ¨3.5 nA. When
DNA is applied to the ground chamber and +300mV is applied DNA translocations
(right panel)
can be observed as transient decreases in the current. (Note, in this case the
TS buffer is used:
50mM Tris, pH 8, 1M NaCl). Lambda DNA is used for this DNA translocation
experiment.
[0203] Silver Chloride Electrodes:
1. Silver wire is soldered to insulated copper wire.
2. Copper wire is grounded, and silver is dipped into fresh 30% sodium
hypochlorite for
30 minutes.
3. Silver should acquire a dark gray coating (silver chloride).
4. Silver wire is rinsed extensively in distilled water and dried.
5. It is now ready for use.
[0204] Silanization of Beads: The silanization method is initially
developed/tested on 5i02
coated magnetic beads (GBioscience). The following protocol is adopted:
1. Pretreat beads in fresh pirannah solution for 30 minutes.
74
CA 03016037 2018-08-28
WO 2017/151680
PCT/US2017/020044
2. Wash 3x with distilled water.
3. Wash 2x in methanol.
4. Dilute APTES stock 1:500 in methanol.
5. Add diluted APTES to beads, incubate at RT for 45 minutes.
6. Rinse with methanol.
7. 100 C for 30 minutes.
8. Store under vacuum.
[0205] Silanization of silicon chip
1. Mount chip with nanopore in a cassette.
2. Rinse with methanol, carefully removing any air bubbles.
3. Add fresh pirannah solution (equilibrated to room temperature) and incubate
for 30
minutes.
4. Wash 4x with distilled water.
5. Wash 3x with methanol.
6. Dilute APTES stock 1:500 in methanol and use to wash chip 2x. Incubate at
RT for
45 minutes.
7. Rinse 2x with methanol.
8. Dry under and air stream.
9. Store under vacuum overnight.
[0206] Streptavidin conjugation of beads: The streptavidin conjugation is
initially
developed/tested on the silanized beads prepared above.
1. Wash silanized beads with Modified Phosphate-Buffered Saline (MPBS)
2. Make a fresh solution of 1.25% glutaraldehyde in MPBS (using 50%
glutaraldehyde
stock, stored frozen).
3. Add 1.25% glutaraldehyde to beads and let stand for 60' with gentle up-down
pipetting every 15 minutes.
4. Wash 2x with MPBS.
5. Wash 2x with water.
6. Let dry under vacuum.
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
7. Add streptavidin (500 1.tg/mL in MPBS) to beads and incubate 60 minutes.
(For
negative control beads, use bovine serum albumin (BSA) (2mg/mL in MPBS) in
place of
streptavidin).
8. Remove streptavidin and add BSA (2mg/mL in MPBS). Incubate 60 minutes.
9. Wash 2x in MPBS.
10. Store at 4 C.
[0207] Streptavidin conjugation of silicon chip
1. Rinse silanized chip with ethanol 2x
2. Rinse silanized chip with MPBS 2x
3. Make a fresh solution of 1.25% glutaraldehyde in MPBS (using 50%
glutaraldehyde
stock, stored frozen).
4. Rinse chip with 1.25% glutaraldehyde 2x, let stand for 60' with gentle up-
down
pipetting every 15 minutes
5. Wash 2x with MPBS
6. Wash 2x with water
7. Let dry under air stream
8. To one half of the chip add BSA (2 mg/mL in MPBS), and to the other add
streptavidin (500 ttg/mL in MPBS). Incubate 60 minutes. Make a marking on the
cassette
to indicate which half of the chip is streptavidin modified.
9. Rinse both halfs of the chip with BSA (2 mg/mL in MPBS). Incubate 60
minutes.
10. Wash in MPBS.
[0208] The buffers used herein are made as follows:
MPBS: 8 g/L NaCl, 0.2 g/L KC1, 1.44 g/L disodium-phosphate, 0.240 g/L
potassium
phosphate, 0.2 g/L polysorbate-20 (pH 7.2)
PBS: 8 g/L NaCl, 0.2 g/L KC1, 1.44 g/L disodium-phosphate, 0.240 g/L potassium
phosphate
TS: 50 mM Tris pH 8.0, 1M NaCl
HK: 10 mM HEPES pH 8.0, 1M KC1
TE: 10 mM Tris, 1 mM EDTA, pH 8.0
Pirannah Solution: 75% hydrogen peroxide (30%) + 25% sulfuric acid
APTES stock: 50% methanol, 47.5% APTES, 2.5% nanopure water. Age at 4 C for at
76
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
least 1 hour. Store at 4 C.
PDC stock: 0.5% w/v 1,4-phenylene diisothiocyanate in DMSO
[0209] Oligonucleotides (5' TO 3') are ordered:
o1 CTGGAACGGTAAATTCAGAGACTGCGCTTTCCATTCTGGCTTTAATG
03 GGAAAGCGCAGTCTCTGAATTTAC
Ni CTTACTGGAACGGCTATCGATATCGCAGCAGGACAGA
BN1 Biotin-CTTACTGGAACGGCTATCGATATCGCAGCAGGACAGA
N2 GTCCTGCTGCGATATCGATAGCCGTTCCAGTAAG
[0210] Oligonucleotide pair hybridization is carried out as follows:
1. Make stock solutions of oligo's at 100uM concentration in TE buffer
2. Dilute oligos to 10 ttM in PBS
3. Heat to 85 C for 5' in a thermal cycler
4. Ramp heat down by 5 C every 3' until 25 C
5. Store at 4 C or -20 C
[0211] Streptavidin Conjugation: Streptavidin conjugation to 5i02 is developed
and tested using
5i02 coated magnetic beads, and the protocols were then adapted for 5i02
chips. Binding of
biotinylated oligos to both streptavidin and BSA conjugated beads are tested.
As expected,
negligible binding is observed with BSA-conjugated beads, while strong binding
is observed
with streptavidin conjugated beads. See Figure 38. Since it would be more
convenient to perform
the conjugation in high salt (DNA movement is performed in high salt), the
ability of the beads
to bind in HK buffer is also tested. Binding in HK buffer is comparable to
binding in MPBS
buffer (Figure 39).
[0212] Origami constructs are made and confirmed operable as described above
in Figures 35-
37. Biotinylation of the origami structure is tested using oligonucleotides.
We already know
from the `Orgami' results described above for Figure 37 that the AlwNI site is
active. An
oligonucleotide pair that recreates a segment of the exact sequence in the
origami DNA is used
below (ol/o3). The origami molecule is depicted as follows:
77
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
saa.,õuõ:Laai Ilk
....... rs T C
=Vi`,.eLt
' t" :it: a a v. s$
z
......... 4 ...............
[0213] The oligo pair o1/o3 is
CTGGAACGGTAAATTCAGAGACTGCGCTTTCCATTCTGGCTTTAATG ol
CATTTAAGTCTCTGACGCGAAAGG 03
[0214] The DNA is digested with AlwNI in the presence of T4 DNA ligase, and a
biotinylated
oligo that is complementary to the overhang on the 3' side of the origami
sequence (which itself
is attached to a long ssDNA sequence which itself is attached to the other
side of the origami),
according to the following reaction:
78
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
CTGGAACGGTAAATTCAGAGACTGCGCTTTCCATTCTGGC=AATG o1
OATTTAAGTCTCTGACGCGAAGG 3
A wN
C T GGAAC GGTAAAT TCAGAGA CTGCGCTTTCcArrcrEGGCTrIAATG
CAT TTAAGTC TCTGACGCGAAAGG
B-crrAcTGGAAccIscTATc:GATATCGCAGCAG:GACACA BN 1
G.AAT CAC:1:1"r GCCGATAGC 'r TAGC Gr.1' C TCC T G 1µ12
+ q
B-CTTACTGGIaCGGCTATCGATATCSCAGCAGG'ACAGACTGCGCTTTCCATTCTGGCTTTAATG
GAATGAC:CT ' T A GC G T t:-.:.T(:C'E'CTCTGACGCGAAAGG
In this strategy, AlwN1 cleaves the target DNA. When the ligase is added it is
possible for this
DNA to be religated, but the restriction enzyme will cut it again. However,
if/when the (right)
fragment (of ol/o3) binds to BN1/N2, the restriction site is NOT recreated,
thus this product will
not be cut. Specific attachment is confirmed, by testing with and without the
restriction enzyme:
\it\
AVAita
1.0x lig
water IS 14.5
All reagents except ligase are added and solution is incubated at 37 C for 60
minutes. Ligase is
added and solution incubated overnight at 16 C. 10x hg buff refers to NEB 10x
T4 DNA ligase
buffer. Ligase in NEB T4 DNA ligase. ol/o3 and nl/n2 refer to annealed oligo
pairs, as depicted
above. Units are microliters. Agarose gel analysis confirms that in the
presence of the AlwNI, a
larger product is formed, corresponding to the biotinylated oligonucleotide
attached to the long
ssDNA arm attached to the origami structure. A similar strategy is used for 3'
biotinylation,
where desired.
79
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0215] Above we demonstrate the ability to form and use a nanopore to detect
voltage induced
transit of DNA across the pore, the creation of an origami molecule with a
long ss region
attached at its' far end to a biotin, and the conjugation of streptavidin to
silicon dioxide, and to
use that to capture biotinylated DNA. These tools are used to attach and
control the movement of
a single DNA molecule next to a nanopore.
[0216] The first step is to conjugate streptavidin to one surface of an SiO2
nanopore (and BSA to
the other side). This is accomplished according to the protocol above. The
resulting pores tend to
have a lower current than they initially have. After some brief 6v pulses, the
currents return to be
near their original current. A functioning nanopore at this point is shown in
Figure 40.
[0217] Next, the origami DNA is inserted. When the origami DNA is added to the
appropriate
chamber and the current turned on, the origami will insert into the chamber. A
representation of
this is shown in Figure 41. Experimental results when the origami is
introduced at a final
concentration of 50 pM confirm that the DNA with the origami inserts into the
pore relatively
soon (typically in seconds), which is detectable by the resulting reduction of
current flow across
the nanopore (e.g., in these experiments, current before origami insertion is
¨3nA, and ¨2.5nA
after insertion). If the current is allowed to run for longer times, double
insertions can be
observed. If higher concentrations are used, insertion occurs too quickly to
be observed.
[0218] Binding of inserted DNA to chip. After the origami is inserted into the
nanopore, 15
minutes are allowed to elapse before voltage is applied again. The end of the
ssDNA region of
the origami contains a biotin, and streptavidin is conjugated to the surface
of the nanopore.
Streptavidin binds to avidin with an affinity constant that approaches that of
a covalent bond.
The 15 minute time allows the DNA to diffuse and for the biotin end to find
and bind to the
streptavidin. If the DNA has in fact become attached to the surface, when the
voltage is reversed
the observed current should be slightly less than what was seen previously.
Also, switching the
current back and forth should result in currents in both directions that are
lower than that seen
with a free pore.. In the example shown here, a free pore shows a current of
¨3nA. Figure 42
shows a representation of attached DNA, and Figure 43 shows experimental
results of voltage
switching the attached origami DNA. Note that the currents seen in both
directions are ¨+/-2.5
nA, which is lower than the ¨+/-3nA observed with a free pore. If the DNA
hasn't bound to the
surface, the original current will be recovered when the voltage is switched
(Figure 44).
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
[0219] In order to remove the origami structure, the buffer in the flow cell
chamber containing
the origami structure was removed and replaced with lx Swal buffer with luL
Swal/20 L. The
buffer in the other flow cell chamber is replaced with lx Swal buffer without
Swal. This is
incubated at room temperature for 60 minutes, then washed with HK buffer, and
voltage applied.
Movement of the DNA back and forth as represented in Figure 45 is confirmed by
the
experimental data in Figure 46, showing controlled movement of immobilized DNA
through a
SiO2 nanopore.
Example 8 ¨ Alternative means to attach the polymer to the surface adjacent to
the nanopore
[0220] The foregoing examples describe attachment of DNA to the surface
adjacent to the
nanopore by biotinylating the DNA and coating the attachment surface with
streptavidin. Some
alternative means of attaching the polymer are depicted in Figure 47.
[0221] a) DNA Hybridizaation: In one method, the DNA which is extended in the
methods of
the invention is hybridized to a short oligonucleotide which is attached near
the nanopore. Once
the synthesis is complete, the synthesized DNA can be easily removed without a
need for
restriction enzymes, or alternatively the double strand formed by the bound
oligonucleotide and
the synthesized DNA can provide a substrate for a restriction enzyme. In this
example, the short
oligomers are conjugated to the surface using biotin-strepavidin, or ligated
using 1,4-phenylene
diisothiocyanate as follows:
Conjugation of biotinylated DNA to 5i02:
A. SILANIZE:
1. Pre-treatment: nha solution for 30 minutes, wash with double distilled H20
(ddH20)
2. Prepare APTES stock: 50% Me0H, 47.5% APTES, 2.5% nanopure H20: age >lhr 4 C
3. dilute APTES stock 1:500 in Me0H
4. incubate chips at room temperature
5. rinse Me0H
6. dry
7. heat at 110 C for 30 minutes
CONJUGATE:
1. Treat chip with PDC stock 5h (room temperature) (PDC stock: 0.5% w/v 1,4-
phenylene diisothiocyanate in DMSO)
81
CA 03016037 2018-08-28
WO 2017/151680 PCT/US2017/020044
2. 2 washes in DMSO (brief!)
3. 2 washes in ddH20 (brief!)
4. 100 nM amino-modified DNA in ddH20 (pH 8) 0/N 37 C
5. 2 washes 28% ammonia solution (deactivate)
6. 2 washes ddH20
[0222] Single stranded DNA having a terminal sequence complementary to the
attached
oligonucleotides is introduced as described above and allowed to hybridize
with the attached
oligonucleotides.
[0223] b) Click chemistry: Click chemistry is a general term for reactions
that are simple and
thermodynamically efficient, do not create toxic or highly reactive
byproducts, and operate in
water or biocompatible solvents, and are often used to join substrates of
choice with specific
biomolecules. The click conjugation in this case uses similar chemistry as
used in a) to attach the
oligonucleotides, only it is here used to attach the polymer which is extended
in the course of
synthesis in the methods of the invention. While in this example, DNA is the
polymer, this
chemistry would work to attach other polymers which have been functionalized
by addition of a
compatible azide group.
SILANIZE:
1. Pre-treatment: piranha solution for 30', wash ddH20
2. Prepare PS (propargyl silane) stock: 50% Me0H, 47.5% PS, 2.5% nanopure H20:
age >lhr 4C
3. Dilute APTES stock 1:500 in Me0H
4. Incubate chips at room temperature
5. Rinse Me0H
6. Dry
7. Heat at 110 C for 30 minutes
[0224] DNA which is terminated in an azide functional group will covalently
bind to this surface
(as shown in Figure 47). Azide terminated oligos are ordered, and attached to
the longer origami
DNA, as described for the biotin addition to the DNA previously.
82