Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
TREATING STEROID DEPENDENT LUPUS NEPHRITIS USING A COMPOSITION
THAT INHIBITS MASP-2 DEPENDENT COMPLEMENT ACTIVATION
.5 STATEMENT REGARDING SEQUENCE LISTING
The sequence listing associated with this application is provided in text
format in
lieu of a paper copy, The
name of the text tile containing the
sequence listing is
MP_i 0269_PCT Sequence Listing 20171013_ST25. The text file is 136 KB, was
created on October 10, 2017, and is being submitted via EFS-Web with the
filing of the
specification.
BACKGROUND
The complement system provides an early acting mechanism to initiate, amplify
and orchestrate the immune response to microbial infection and other acute
insults
(M.K. Liszewski and J.P. Atkinson, 1993, in Fmk/anemia' Immunology, Third
Edition,
edited by W.E. Paul, Raven Press, Ltd., New York), in humans and other
vertebrates.
While complement activation provides a valuable first-line defense against
potential
patho.4ens, the activities of complement that promote a protective immune
response can
also represent a potential threat to the host (KR. Kalli, et al., Springer
Selllill.
Ininninoputhol. 15:417-43] , 1994; B.P. Morgan, Eur. J. Clinical Investig.
24:219-228,
1004). For example, C3 and C5 proteolytic products recruit and activate
neutrophils.
While indispensable for host defense, activated neutrophils are indiscriminate
in their
release of destructive enzymes and may cause organ damage. In addition,
complement
activation rria.\ cause the deposition of lytic complement components on
nearby host cells
as well as on microbial targets, resulting- in host cell lysis.
The complement system has also been implicated in the pathogenesis of numerous
acute and chronic disease states, including: myocardial infarction, stroke,
ARDS,
reperfusion injury, septic shock, capillaiy leakage following, thermal burns,
postcardiopulmonary bypass inflammation, transplant rejection, rheumatoid
arthritis,
-1 -
Date Recue/Date Received 2021-06-21
CA 03039927 2019-04-09
WO 2018/071701 PCMJS2017/056386
multiple sclerosis, myasthenia gravis, and Alzheimer's disease. In almost all
of these
conditions, complement is not the cause but is one of several factors involved
in
pathogenesis. Nevertheless, complement activation may be a major pathological
mechanism and represents an effective point for clinical control in many of
these disease
.. states. The growing recognition of the importance of complement-mediated
tissue injury
in a variety of disease states underscores the need for effective complement
inhibitory
drugs. To date, Eculizumab (Solarist), an antibody against C5, is the only
complement-
targeting drug that has been approved for human use. Yet, C5 is one of several
effector
molecules located "downstream" in the complement system, and blockade of C5
does not
inhibit activation of the complement system. Therefore, an inhibitor of the
initiation
steps of complement activation would have significant advantages over a
"downstream"
complement inhibitor.
Currently, it is widely accepted that the complement system can be activated
through three distinct pathways: the classical pathway, the lectin pathway,
and the
alternative pathway. The classical pathway is usually triggered by a complex
composed
of host antibodies bound to a foreign particle (i.e., an antigen) and thus
requires prior
exposure to an antigen for the generation of a specific antibody response.
Since
activation of the classical pathway depends on a prior adaptive immune
response by the
host, the classical pathway is part of the acquired immune system. In
contrast, both the
lectin and alternative pathways are independent of adaptive immunity and are
part of the
innate immune system.
The activation of the complement system results in the sequential activation
of
serine protease zymogens. The first step in activation of the classical
pathway is the
binding of a specific recognition molecule, Clq, to antigen-bound IgG and
IgIVI
molecules. Clq is associated with the Clr and Cls serine protease proenzymes
as a
complex called Cl. Upon binding of Clq to an immune complex, autoproteolytic
cleavage of the Arg-Ile site of Clr is followed by Clr-mediated cleavage and
activation
of Cls, which thereby acquires the ability to cleave C4 and C2. C4 is cleaved
into two
fragments, designated C4a and C4b, and, similarly, C2 is cleaved into C2a and
C2b. C4b
fragments are able to form covalent bonds with adjacent hydroxyl or amino
groups and
generate the C3 convertase (C4b2a) through noncovalent interaction with the
C2a
fragment of activated C2. C3 convertase (C4b2a) activates C3 by proteolytic
cleavage
into C3a and C3b subcomponents leading to generation of the C5 convertase
(C4b2a3b),
-2-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
which, by cleaving C5 leads to the formation of the membrane attack complex
(C5b
combined with C6, C7, C8 and C-9, also referred to as "MAC") that can disrupt
cellular
membranes leading to cell lysis. The activated forms of C3 and C4 (C3b and
C4b) are
covalently deposited on the foreign target surfaces, which are recognized by
complement
receptors on multiple phagocytes.
Independently, the first step in activation of the complement system through
the
lectin pathway is also the binding of specific recognition molecules, which is
followed by
the activation of associated serine protease proenzymes. However, rather than
the
binding of immune complexes by Cl q, the recognition molecules in the lectin
pathway
comprise a group of carbohydrate-binding proteins (mannan-binding lectin
(MBL),
H-ficolin, M-ficolin, L-ficolin and C-type lectin CL-11), collectively
referred to as
lectins. See J. Lu et al., Blochim. Biophys. Ada 1572:387-400, (2002);
Holmskov et al.,
Annu. Rev. Immunol. 21:547-578 (2003); Teh et al., Immunology 101:225-232
(2000)).
See also J. Luet et al., Biochim Biophys Acta 1572:387-400 (2002); Holmskov et
al, Annu
Rev 1111111141101 21:547-578 (2003); Teh et al., Immunology 101:225-232
(2000); Hansen et
al, J. Immunol 185(10):6096-6104 (2010).
Ikeda et al. first demonstrated that, like Clq, MBL could activate the
complement
system upon binding to yeast mannan-coated erythrocytes in a C4-dependent
manner
(Ikeda et al., J. Biol. Chem. 262:7451-7454, (1987)). MBL, a member of the
collectin
protein family, is a calcium-dependent lectin that binds carbohydrates with 3-
and
4-hydroxy groups oriented in the equatorial plane of the pyranose ring.
Prominent
ligands for MBL are thus D-mannose and N-acetyl-D-glucosamine, while
carbohydrates
not fitting this steric requirement have undetectable affinity for MBL (Weis
et al.,
Nature 360:127-134, (1992)). The interaction between MBL and monovalent sugars
is
extremely weak, with dissociation constants typically in the single-digit
millimolar range.
MBL achieves tight, specific binding to glycan ligands by avidity, i.e., by
interacting
simultaneously with multiple monosaccharide residues located in close
proximity to each
other (Lee et al., Archly. Biochem. Biophys. 299:129-136, (1992)). MBL
recognizes the
carbohydrate patterns that commonly decorate microorganisms such as bacteria,
yeast,
parasites and certain viruses. In contrast, MBL does not recognize D-galactose
and sialic
acid, the penultimate and ultimate sugars that usually decorate "mature"
complex
glycoconjugates present on mammalian plasma and cell surface glycoproteins.
This
binding specificity is thought to promote recognition of "foreign" surfaces
and help
-3-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
protect from "self-activation." However, MBL does bind with high affinity to
clusters of
high-mannose "precursor" glycans on N-linked glycoproteins and glycolipids
sequestered
in the endoplasmic reticulum and Golgi of mammalian cells (Maynard et al., J.
Biol.
Chem. 257:3788-3794, (1982)). Therefore, damaged cells are potential targets
for lectin
pathway activation via MBL binding.
The ficolins possess a different type of lectin domain than MBL, called the
fibrinogen-like domain. Ficolins bind sugar residues in a Ca-independent
manner. In
humans, three kinds of ficolins (L-ficolin, M-ficolin and H-ficolin) have been
identified.
The two serum ficolins, L-ficolin and H-ficolin, have in common a specificity
for
N-acetyl-D-glucosamine; however, H-ficolin also binds N-acetyl-D-
galactosamine. The
difference in sugar specificity of L-ficolin, H-ficolin, CL-11, and MBL means
that the
different lectins may be complementary and target different, though
overlapping,
glycoconjugates. This concept is supported by the recent report that, of the
known lectins
in the lectin pathway, only L-ficolin binds specifically to lipoteichoic acid,
a cell wall
glycoconjugate found on all Gram-positive bacteria (Lynch et al., J. Immunol.
172:1198-1202, (2004)). The collectins (i.e., MBL) and the ficolins bear no
significant
similarity in amino acid sequence. However, the two groups of proteins have
similar
domain organizations and, like Clq, assemble into oligomeric structures, which
maximize the possibility of multi site binding.
The serum concentrations of MBL are highly variable in healthy populations and
this is genetically controlled by polymorphisms/mutations in both the promoter
and
coding regions of the MBL gene. As an acute phase protein, the expression of
MBL is
further upregulated during inflammation. L-ficolin is present in serum at
concentrations
similar to those of MBL. Therefore, the L-ficolin branch of the lectin pathway
is
potentially comparable to the MBL arm in strength. MBL and ficolins can also
function
as opsonins, which allow phagocytes to target MBL- and ficolin-decorated
surfaces (see
Jack et al., ./ Leulfoc Biol., 77(3).328-36 (2004), Matsushita and Fujita,
Iminunohiology,
205(4-5):490-7 (2002), Aoyagi et al., J linmunol, 174(1):418-25(2005). This
opsonization requires the interaction of these proteins with phagocyte
receptors (Kuhlman
et al., J. Exp. Med. /69.1733, (1989); Matsushita et al., J. Biol. Chem.
271:2448-54,
(1996)), the identity of which has not been established.
Human MBL forms a specific and high-affinity interaction through its
collagen-like domain with unique C 1r/C1s-like serine proteases, termed MBL-
associated
-4-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
serine proteases (MASPs). To date, three MASPs have been described. First, a
single
enzyme "MASP" was identified and characterized as the enzyme responsible for
the
initiation of the complement cascade (i.e., cleaving C2 and C4) (Matsushita et
al., J Exp
Med 176(6):1497-1502 (1992); Ji et al., J. Immunol. 150:571-578, (1993)). It
was
.. subsequently determined that the MASP activity was, in fact, a mixture of
two proteases:
MASP-1 and MASP-2 (Thiel et al., Nature 386:506-510, (1997)). However, it was
demonstrated that the MBL-MASP-2 complex alone is sufficient for complement
activation (Vorup-Jensen et al., I Immunol. 165:2093-2100, (2000)).
Furthermore, only
MASP-2 cleaved C2 and C4 at high rates (Ambrus et al., ./. Immunol. /70.1374-
1382,
(2003)). Therefore, MASP-2 is the protease responsible for activating C4 and
C2 to
generate the C3 convertase, C4b2a. This is a significant difference from the
Cl complex
of the classical pathway, where the coordinated action of two specific serine
proteases
(Clr and Cis) leads to the activation of the complement system. In addition, a
third
novel protease, MASP-3, has been isolated (Dahl, MR., et al., Immunity /5:127-
35,
2001). MASP-1 and MASP-3 are alternatively spliced products of the same gene.
MASPs share identical domain organizations with those of Clr and Cls, the
enzymatic components of the Cl complex (Sim et al., Biochem. Soc. Trans.
28:545,
(2000)). These domains include an N-terminal Clr/C1s/sea urchin VEGF/bone
morphogenic protein (CUB) domain, an epidermal growth factor-like domain, a
second
.. CUB domain, a tandem of complement control protein domains, and a serine
protease
domain. As in the Cl proteases, activation of MASP-2 occurs through cleavage
of an
Arg-Ile bond adjacent to the serine protease domain, which splits the enzyme
into
disulfide-linked A and B chains, the latter consisting of the serine protease
domain.
MBL can also associate with an alternatively sliced form of MASP-2, known as
.. MBL-associated protein of 19 kDa (MAp19) or small MBL-associated protein
(sMAP),
which lacks the catalytic activity of MASP-2. (Stover, I Immunol. 162:3481-90,
(1999);
Takahashi et al., Mt. Immunol. //:859-863, (1999)). MAp19 comprises the first
two
domains of MASP-2, followed by an extra sequence of four unique amino acids.
The
function of MAp19 is unclear (Degn et al., I Immunol. Methods, 2011). The MASP-
1
and MASP-2 genes are located on human chromosomes 3 and 1, respectively
(Schwaeble et al., Immunobiology 205:455-466, (2002)).
Several lines of evidence suggest that there are different MBL-MASP complexes
and a large fraction of the MASPs in serum is not complexed with MBL (Thiel,
et al., J.
-5-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Immunot /65:878-887, (2000)). Both H- and L-ficolin bind to all MASPs and
activate
the lectin complement pathway, as does MBL (Dahl et al., Immunity 15:127-35,
(2001);
Matsushita et al., I Immunol. /68:3502-3506, (2002)). Both the lectin and
classical
pathways form a common C3 convertase (C4b2a) and the two pathways converge at
this
step.
The lectin pathway is widely thought to have a major role in host defense
against
infection in the naive host. Strong evidence for the involvement of MBL in
host defense
comes from analysis of patients with decreased serum levels of functional MBL
(Kilpatri ck, Biochim. Biophys. Acta 1572:401-413, (2002)). Such patients di
splay
susceptibility to recurrent bacterial and fungal infections. These symptoms
are usually
evident early in life, during an apparent window of vulnerability as
maternally derived
antibody titer wanes, but before a full repertoire of antibody responses
develops. This
syndrome often results from mutations at several sites in the collagenous
portion of MBL,
which interfere with proper formation of MBL oligomers. However, since MBL can
.. function as an opsonin independent of complement, it is not known to what
extent the
increased susceptibility to infection is due to impaired complement
activation.
All three pathways (i.e., the classical, lectin and alternative) have been
thought to
converge at C5, which is cleaved to form products with multiple
proinflammatory effects.
The converged pathway has been referred to as the terminal complement pathway.
C5a is
.. the most potent anaphylatoxin, inducing alterations in smooth muscle and
vascular tone,
as well as vascular permeability. It is also a powerful chemotaxin and
activator of both
neutrophils and monocytes. C5a-mediated cellular activation can significantly
amplify
inflammatory responses by inducing the release of multiple additional
inflammatory
mediators, including cytokines, hydrolytic enzymes, arachi don i c acid
metabolites, and
.. reactive oxygen species. C5 cleavage leads to the formation of C5b-9, also
known as the
membrane attack complex (MAC). There is now strong evidence that sublytic MAC
deposition may play an important role in inflammation in addition to its role
as a lytic
pore-forming complex.
In addition to its essential role in immune defense, the complement system
contributes to tissue damage in many clinical conditions. Although there is
extensive
evidence implicating both the classical and alternative complement pathways in
the
pathogenesis of non-infectious human diseases, the role of the lectin pathway
is just
beginning to be evaluated. Recent studies provide evidence that activation of
the lectin
-6-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
pathway can be responsible for complement activation and related inflammation
in
ischemia/reperfusion injury. Collard et al. (2000) reported that cultured
endothelial cells
subjected to oxidative stress bind MBL and show deposition of C3 upon exposure
to
human serum (Collard et al., Am. J. Pathol. /56:1549-1556, (2000)). In
addition,
.. treatment of human sera with blocking anti-MBL monoclonal antibodies
inhibited MBL
binding and complement activation. These findings were extended to a rat model
of
myocardial ischemia-repertUsion in which rats treated with a blocking antibody
directed
against rat MBL showed significantly less myocardial damage upon occlusion of
a
coronary artery than rats treated with a control antibody (Jordan et al.,
Circulation
104:1413-1418, (2001)). The molecular mechanism of MBL binding to the vascular
endothelium after oxidative stress is unclear; a recent study suggests that
activation of the
lectin pathway after oxidative stress may be mediated by MBL binding to
vascular
endothelial cytokeratins, and not to glycoconjugates (Collard et al., Am. I
Pathol.
/59:1045-1054, (2001)). Other studies have implicated the classical and
alternative
pathways in the pathogenesis of ischemia/reperfusion injury and the role of
the lectin
pathway in this disease remains controversial (Riedermann, N.C., et al., Am. I
Pathol.
162:363-367, 2003).
Fibrosis is the formation of excessive connective tissue in an organ or
tissue,
commonly in response to damage or injury. A hallmark of fibrosis is the
production of
excessive extracellular matrix following local trauma. The normal
physiological response
to injury results in the deposition of connective tissue, but this initially
beneficial
reparative process may persist and become pathological, altering the
architecture and
function of the tissue. At the cellular level, epithelial cells and
fibroblasts proliferate and
differentiate into myofibroblasts, resulting in matrix contraction, increased
rigidity,
microvascular compression, and hypoxia. An influx of inflammatory cells,
including
macrophages and lymphocytes, results in cytokine release and amplifies the
deposition of
collagen, fibronectin and other molecular markers of fibrosis. Conventional
therapeutic
approaches have largely been targeted towards the inflammatory process of
fibrosis,
using corticosteroids and immunosuppressive drugs. Unfortunately, these anti-
inflammatory agents have had little to no clinical effect. Currently there are
no effective
treatments or therapeutics for fibrosis, but both animal studies and anecdotal
human
reports suggest that fibrotic tissue damage may be reversed (Tampe and
Zeisberg, Nat
Rev Nephrol, Vol 10:226-237, 2014).
-7-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The kidney has a limited capacity to recover from injury. Various renal
pathologies result in local inflammation that causes scarring and fibrosis of
renal tissue.
The perpetuation of inflammatory stimuli drives tubulointerstitial
inflammation and
fibrosis and progressive renal functional impairment in chronic kidney
disease. Its
progression to end-stage renal failure is associated with significant
morbidity and
mortality. Since tubulointerstitial fibrosis is the common end point of
multiple renal
pathologies, it represents a key target for therapies aimed at preventing
renal failure. Risk
factors (e.g., proteinuria) independent of the primary renal disease
contribute to the
development of renal fibrosis and loss of renal excretory function by driving
local
inflammation, which in turn enhances disease progression.
In view of the role of fibrosis in many diseases and disorders, such as, for
example, tubulointerstitial fibrosis leading to chronic kidney disease, there
is a pressing
need to develop therapeutically effective agents for treating diseases and
conditions
caused or exacerbated by fibrosis. In further view of the paucity of new and
existing
treatments targeting inflammatory pro-fibrotic pathways in renal disease,
there is a need
to develop therapeutically effective agents to treat, inhibit, prevent and/or
reverse renal
fibrosis and thereby prevent progressive chronic kidney disease.
SUMMARY
This summary is provided to introduce a selection of concepts in a simplified
form that are further described below in the Detailed Description. This
summary is not
intended to identify key features of the claimed subject matter, nor is it
intended to be
used as an aid in determining the scope of the claimed subject matter.
In one aspect, the invention provides a method for treating, inhibiting,
alleviating
.. or preventing fibrosis in a mammalian subject suffering, or at risk of
developing a disease
or disorder caused or exacerbated by fibrosis and/or inflammation, comprising
administering to the subject an amount of a MASP-2 inhibitory agent effective
to inhibit
fibrosis. In one embodiment, the MASP-2 inhibitory agent is a MASP-2 antibody
or
fragment thereof. In one embodiment, the MASP-2 inhibitory agent is a MASP-2
.. monoclonal antibody, or fragment thereof that specifically binds to a
portion of SEQ ID
NO:6. In one embodiment, the MASP-2 inhibitory agent selectively inhibits
lectin
pathway complement activation without substantially inhibiting Clq-dependent
complement activation. In one embodiment, the subject is suffering from a
disease or
-8-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
disorder caused by or exacerbated by at least one of (i) fibrosis and/or
inflammation
associated with an ischemia reperfusion injury, (ii) renal fibrosis and/or
renal
inflammation (e.g., tubulointerstitial fibrosis, chronic kidney disease,
chronic renal
failure, glomerular disease (e.g., focal segmental glomerulosclerosis), an
immune
complex disorder (e.g., IgA nephropathy, membraneous nephropathy), lupus
nephritis,
nephrotic syndrome, diabetic nephropathy, tubulointerstitial damage and
glomerulonepthritis (e.g., C3 glomerulopathy), (iii) pulmonary fibrosis and/or
inflammation (e.g., chronic obstructive pulmonary disease, cystic fibrosis,
pulmonary
fibrosis associated with scleroderma, bronchiectasis and pulmonary
hypertension), (iv)
hepatic fibrosis and/or inflammation (e.g., cirrhosis, nonalcoholic fatty
liver disease
(steatohepatitis)), liver fibrosis secondary to alcohol abuse, liver fibrosis
secondary to
acute or chronic hepatitis, biliary disease and toxic liver injury (e.g.,
hepatotoxicity due to
drug-induced liver damage induced by acetaminophen or other drug), (v) cardiac
fibrosis
and/or inflammation (e.g., cardiac fibrosis, myocardial infarction, valvular
fibrosis, atrial
fibrosis, endomyocardial fibrosis arrhythmogenic right ventricular
cardiomvopathy
(ARVC), (vi) vascular fibrosis (e.g., vascular disease, an atherosclerotic
vascular disease,
vascular stenosis, restenosis, vasculitis, phlebitis, deep vein thrombosis and
abdominal
aortic aneurysm), (vii) fibrosis of the skin (e.g., excessive wound healing,
scleroderma,
systemic sclerosis, keloids, connective tissue diseases, scarring, and
hypertrophic scars),
.. (viii) fibrosis of the joints (e.g., arthrofibrosis), (ix) fibrosis of the
central nervous system
(e.g., stroke, traumatic brain injury and spinal cord injury), (x) fibrosis of
the digestive
system (e.g., Crohn's disease, pancreatic fibrosis and ulcerative colitis),
(xi) ocular
fibrosis (e.g., anterior sub cap sul ar cataract, posterior capsule op aci fi
cati on, macular
degeneration, and retinal and vitreal retinopathy), (xii) fibrosis of
musculoskeletal soft-
tissue structures (e.g., adhesive capsulitis, Dupuytren's contracture and
myelofibrosis),
(xiii) fibrosis of the reproductive organs (e.g., endometriosis and Peyronie's
disease),
(xiv) a chronic infectious disease that causes fibrosis and/or inflammation
(e.g., alpha
virus, Hepatitis A, Hepatitis B, Hepatitis C, tuberculosis, HIV and
influenza), (xv) an
autoimmune disease that causes fibrosis and/or inflammation (e.g., scleroderma
and
systemic lupus erythematosus (SLE), (xvi) scarring associated with trauma
(e.g.,
wherein the scarring associated with trauma is selected from the group
consisting of
surgical complications (e.g., surgical adhesions wherein scar tissue can form
between
internal organs causing contracture, pain and can cause infertility),
chemotherapeutic
-9-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
drug-induced fibrosis, radiation-induced fibrosis and scarring associated with
burns), or
(xvii) organ transplant, breast fibrosis, muscle fibrosis, retroperitoneal
fibrosis, thyroid
fibrosis, lymph node fibrosis, bladder fibrosis and pleural fibrosis.
In another aspect, the present invention provides a method for treating,
inhibiting,
alleviating or preventing renal fibrosis in a mammalian subject suffering, or
at risk of
developing a disease or disorder caused or exacerbated by renal fibrosis
and/or
inflammation, comprising administering to the subject an amount of a MASP-2
inhibitory
agent effective to inhibit renal fibrosis. In one embodiment, the MASP-2
inhibitory agent
is a MASP-2 antibody or fragment thereof. In one embodiment, the MASP-2
inhibitory
agent is a MASP-2 monoclonal antibody, or fragment thereof that specifically
binds to a
portion of SEQ ID NO:6. In one embodiment, the MASP-2 antibody or fragment
thereof
specifically binds to a polypeptide comprising SEQ ID NO:6 with an affinity of
at least
10 times greater than it binds to a different antigen in the complement
system. In one
embodiment, the antibody or fragment thereof is selected from the group
consisting of a
recombinant antibody, an antibody having reduced effector function, a chimeric
antibody,
a humanized antibody and a human antibody. In one embodiment, the MASP-2
inhibitory agent selectively inhibits lectin pathway complement activation
without
substantially inhibiting Clq-dependent complement activation. In one
embodiment, the
MASP-2 inhibitory agent is administered subcutaneously, intraperitoneally,
intra-
muscularly, intra-arterially, intravenously, or as an inhalant. In one
embodiment, the
MASP-2 inhibitory agent is administered in an amount effective to inhibit
tubulointerstitial fibrosis. In one embodiment, the MASP-2 inhibitory agent
is
administered in an amount effective to reduce, delay or eliminate the need for
dialysis in
the subject. In one embodiment, the subject is suffering from a renal disease
or disorder
selected from the group consisting of chronic kidney disease, chronic renal
failure,
glomerular disease (e.g., focal segmental glomerulosclerosis), an immune
complex
disorder (e.g., IgA nephropathy, membraneous nephropathy), lupus nephritis,
nephrotic
syndrome, diabetic nephropathy, tubulointerstitial damage and
glomerulonepthritis (e.g.,
C3 glomerulopathy). In one embodiment, the subject is suffering from
proteinuria and
the MASP-2 inhibitory agent is administered in an amount effective to reduce
proteinuria
in the subject. In one embodiment, the MASP-2 inhibitory agent is administered
in an
amount and for a time effective to achieve at least a 20 percent reduction
(e.g., at least a
30 percent reduction, or at least a 40 percent reduction, or at least a 50
percent reduction)
-10-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
in 24-hour urine protein excretion as compared to baseline 24-hour urine
protein
excretion in the subject prior to treatment. In one embodiment, the subject is
suffering
from a renal disease or disorder associated with proteinuria selected from the
group
consisting of nephrotic syndrome, pre-eclampsia, eclampsia, toxic lesions of
kidneys,
amyloidosis, collagen vascular diseases (e.g., systemic lupus erythematosus),
dehydration, glomerular diseases (e.g. membranous glomerulonephritis, focal
segmental
glomerulonephritis, C3 glomerulopathy, minimal change disease, lipoid
nephrosis),
strenuous exercise, stress, benign orthostatis (postural) proteinuria, focal
segmental
glomerulosclerosis, IgA nephropathy (i.e., Berger's disease), IgM nephropathy,
membranoproliferative glomerulonephritis, membranous nephropathy, minimal
change
disease, sarcoidosis, Alport's syndrome, diabetes mellitus (diabetic
nephropathy), drug-
induced toxicity (e.g., NSAIDS, nicotine, penicillamine, lithium carbonate,
gold and
other heavy metals, ACE inhibitors, antibiotics (e.g., adriamycin) or opiates
(e.g. heroin)
or other nephrotoxins); Fabry's disease, infections (e.g., HIV, syphilis,
hepatitis A, B or
C, poststreptococcal infection, urinary schistosomiasis); aminoaciduria,
Fanconi
syndrome, hypertensive nephrosclerosis, interstitial nephritis, sickle cell
disease,
hemoglobinuria, multiple myeloma, myoglobinuria, organ rejection (e.g., kidney
transplant rejection), ebola hemorrhagic fever, Nail patella syndrome,
familial
mediterranean fever, HELLP syndrome, systemic lupus erythematosus, Wegener's
granulomatosis, Rheumatoid arthritis, Glycogen storage disease type 1,
Goodpasture's
syndrome, Henoch-Schonlein purpura, urinary tract infection which has spread
to the
kidneys, Sjogren's syndrome and post-infections glomerulonepthritis. In one
embodiment, the subject is suffering from IgA nephropathy. In one embodiment,
the
subject is suffering from membranous nephropathy.
In another aspect, the present invention provides a method of preventing or
reducing renal damage in a subject suffering from a disease or condition
associated with
proteinuria comprising administering an amount of a MASP-2 inhibitory agent
effective
to reduce or prevent proteinurea in the subject. In one embodiment, the MASP-2
inhibitory agent is a MASP-2 antibody or fragment thereof. In one embodiment,
the
MASP-2 inhibitory agent is a MASP-2 monoclonal antibody or fragment thereof
that
specifically binds to a portion of SEQ ID NO:6. In one embodiment, the MASP-2
inhibitory agent selectively inhibits lectin pathway complement activation
without
substantially inhibiting Clq-dependent complement activation. In one
embodiment, the
-11-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
disease or condition associated with proteinuria is selected from the group
consisting of
nephrotic syndrome, pre-eclampsia, ecl amp si a, toxic lesions of kidneys,
amyl oi dosi s,
collagen vascular diseases (e.g., systemic lupus erythematosus), dehydration,
glomerular
diseases (e.g. membranous glomerulonephritis, focal segmental
glomerulonephritis, C3
glomerulopathy, minimal change disease, lipoid nephrosis), strenuous exercise,
stress,
benign orthostatis (postural) proteinuria, focal segmental glomerulosclerosis,
lgA
nephropathy (i.e., Berger's disease), IgM nephropathy, membranoproliferative
glomerulonephritis, membranous nephropathy, minimal change disease,
sarcoidosis,
Alport's syndrome, diabetes mellitus (diabetic nephropathy), drug-induced
toxicity (e.g.,
NSAIDS, nicotine, penicillamine, lithium carbonate, gold and other heavy
metals, ACE
inhibitors, antibiotics (e.g., adriamycin) or opiates (e.g. heroin)); Fabry' s
disease,
infections (e.g., HIV, syphilis, hepatitis A, B or C, poststreptococcal
infection, urinary
schistosomiasis), aminoaciduria, Fanconi syndrome, hypertensive
nephrosclerosis,
interstitial nephritis, sickle cell disease, hemoglobinuria, multiple myeloma,
my ogl ob inuri a, organ rejection (e.g., kidney transplant rejection), eb ol
a hemorrhagic
fever, Nail patella syndrome, familial mediterranean fever, HELLP syndrome,
systemic
lupus erythematosus, Wegener' s granulomatosis, Rheumatoid arthritis, Glycogen
storage
disease type 1, Goodpasture' s syndrome, Henoch-Schonlein purpura, urinary
tract
infection which has spread to the kidneys, Sjogren's syndrome and post-
infections
glomerulonepthritis. In one embodiment, the MASP-2 inhibitory agent is
administered in
an amount and for a time effective to achieve at least a 20 percent reduction
(e.g., at least
a 30 percent reduction, or at least a 40 percent reduction, or at least a 50
percent
reduction) in 24-hour urine protein excretion as compared to baseline 24-hour
urine
protein excretion in the subject prior to treatment.
In another aspect, the present invention provides a method of inhibiting the
progression of chronic kidney disease, comprising administering an amount of a
MASP-2
inhibitory agent effective to reduce or prevent renal fibrosis, e.g.,
tubulointerstitial
fibrosis, in a subject in need thereof. In one embodiment, the MASP-2
inhibitory agent is
a MASP-2 antibody or fragment thereof. In one embodiment, the MASP-2
inhibitory
agent is a MASP-2 monoclonal antibody, or fragment thereof that specifically
binds to a
portion of SEQ ID NO:6. In one embodiment, the MASP-2 inhibitory agent
selectively
inhibits lectin pathway complement activation without substantially inhibiting
Clq-
dependent complement activation. In one embodiment, the subject in need
thereof
-12-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
exhibits proteinuria prior to administration of the MASP-2 inhibitory agent
and
administration of the MASP-2 inhibitory agent decreases proteinuria in the
subject. In
one embodiment, the MASP-2 inhibitory agent is administered in an amount and
for a
time effective to achieve at least a 20 percent reduction (e.g., at least a 30
percent
reduction, or at least a 40 percent reduction, or at least a 50 percent
reduction) in 24-hour
urine protein excretion as compared to baseline 24-hour urine protein
excretion in the
subject prior to treatment. In one embodiment, the MASP-2 inhibitory agent is
administered in an amount effective to reduce, delay or eliminate the need for
dialysis in
the subject.
In another aspect, the invention provides a method of protecting a kidney from
renal injury in a subject that has undergone, is undergoing, or will undergo
treatment with
one or more nephrotoxic agents, comprising administering an amount of a MASP-2
inhibitory agent effective to prevent or ameliorate drug-induced nephropathy.
In one
embodiment, the MASP-2 inhibitory agent is a MASP-2 antibody or fragment
thereof. In
one embodiment, the MASP-2 inhibitory agent is a MASP-2 monoclonal antibody or
fragment thereof that specifically binds to a portion of SEQ ID NO:6. In one
embodiment, the MASP-2 inhibitory agent selectively inhibits lectin pathway
complement activation without substantially inhibiting Clq-dependent
complement
activation.
In another aspect, the invention provides a method of treating a human subject
suffering from Immunoglobulin A Nephropathy (IgAN) comprising administering to
the
subject a composition comprising an amount of a MASP-2 inhibitory antibody, or
antigen-binding fragment thereof, effective to inhibit MASP-2-dependent
complement
activation. In one embodiment, the subject is suffering from steroid-dependent
IgAN. In
one embodiment, the MASP-2 inhibitory antibody is a monoclonal antibody, or
fragment
thereof that specifically binds to human MASP-2. In one embodiment, the
antibody or
fragment thereof is selected from the group consisting of a recombinant
antibody, an
antibody having reduced effector function, a chimeric antibody, a humanized
antibody,
and a human antibody. In one embodiment, the MASP-2 inhibitory antibody does
not
substantially inhibit the classical pathway. In one embodiment, the MASP-2
inhibitory
antibody inhibits C3b deposition in 90% human serum with an ICso of 30 nM or
less. In
one embodiment, the method further comprises identifying a human subject
having
steroid-dependent IgAN prior to the step of administering to the subject a
composition
-13-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
comprising an amount of a MASP-2 inhibitory antibody, or antigen-binding
fragment
thereof, effective to improve renal function. In one embodiment, the MASP-2
inhibitory
antibody or antigen-binding fragment thereof is administered in an amount
effective to
improve renal function. In one embodiment, the MASP-2 inhibitory antibody or
antigen-
binding fragment thereof is administered in an amount effective and for a time
sufficient
to achieve at least a 20 percent reduction in 24-hour urine protein excretion
as compared
to baseline 24-hour urine protein excretion in the subject prior to treatment.
In one
embodiment, the composition is administered in an amount sufficient to improve
renal
function and decrease the corticosteroid dosage in said subject. In one
embodiment, the
MASP-2 inhibitory antibody or antigen-binding fragment thereof comprises a
heavy
chain variable region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid
sequence set forth as SEQ ID NO.67 and a light chain variable region
comprising CDR-
Li, CDR-L2 and CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
In another aspect, the invention provides a method of treating a human subject
suffering from membranous nephropathy (MN) comprising administering to the
subject a
composition comprising an amount of a MASP-2 inhibitory antibody, or antigen-
binding
fragment thereof, effective to inhibit MASP-2-dependent complement activation.
In one
embodiment, the subject is suffering from steroid-dependent MN. In one
embodiment,
the MASP-2 inhibitory antibody is a monoclonal antibody, or fragment thereof
that
specifically binds to human MASP-2. In one embodiment, the MASP-2 inhibitory
antibody or antigen-binding fragment thereof is administered in an amount
effective to
improve renal function. In one embodiment, the MASP-2 inhibitory antibody or
antigen-
binding fragment thereof is administered in an amount effective and for a time
sufficient
to achieve at least a 20 percent reduction in 24-hour urine protein excretion
as compared
to baseline 24-hour urine protein excretion in the subject prior to treatment.
In one
embodiment, the composition is administered in an amount sufficient to improve
renal
function and decrease the corticosteroid dosage in said subject. In one
embodiment, the
MASP-2 inhibitory antibody or antigen-binding fragment thereof comprises a
heavy
chain variable region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid
sequence set forth as SEQ ID NO:67 and a light chain variable region
comprising CDR-
Li, CDR-L2 and CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
-14-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In another aspect, the invention provides a method of treating a human subject
suffering from Lupus Nephritis (LN) comprising administering to the subject a
composition comprising an amount of a MASP-2 inhibitory antibody, or antigen-
binding
fragment thereof, effective to inhibit MASP-2-dependent complement activation.
In one
embodiment, the subject is suffering from steroid-dependent LN. In one
embodiment, the
MASP-2 inhibitory antibody is a monoclonal antibody, or fragment thereof that
specifically binds to human MASP-2. In one embodiment, the MASP-2 inhibitory
antibody or antigen-binding fragment thereof is administered in an amount
effective to
improve renal function. In one embodiment, the MA SP-2 inhibitory antibody or
antigen-
binding fragment thereof is administered in an amount effective and for a time
sufficient
to achieve at least a 20 percent reduction in 24-hour urine protein excretion
as compared
to baseline 24-hour urine protein excretion in the subject prior to treatment.
In one
embodiment, the composition is administered in an amount sufficient to improve
renal
function and decrease the corticosteroid dosage in said subject. In one
embodiment, the
MASP-2 inhibitory antibody or antigen-binding fragment thereof comprises a
heavy
chain variable region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid
sequence set forth as SEQ ID NO:67 and a light chain variable region
comprising CDR-
Li, CDR-L2 and CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
In another aspect, the invention provides a method of reducing proteinuria in
a
human subject suffering from IgAN comprising administering to the subject a
MASP-2
inhibitory antibody, or antigen-binding fragment thereof, comprising a heavy
chain
variable region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid
sequence
set forth as SEQ ID NO:67 and a light chain variable region comprising CDR-L1,
CDR-
L2 and CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70 according
to a
dosage regimen as follows.
a. administering about 4 mg/kg (i.e., from 3.6 mg/kg to 4.4 mg/kg) of said
antibody to a subject suffering from IgAN once weekly intravenously for a
treatment period of at least 12 weeks; or
b. administering from about 180 mg to about 725 mg (i.e., from 162 mg to
797 mg) of said antibody to a subject suffering from IgAN once weekly
intravenously for a treatment period of at least 12 weeks,
wherein the method reduces proteinuria in said human subject.
-15-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
DESCRIPTION OF THE DRAWINGS
The foregoing aspects and many of the attendant advantages of this invention
will
become more readily appreciated as the same become better understood by
reference to
the following detailed description, when taken in conjunction with the
accompanying
drawings, wherein:
FIGURE 1 is a diagram illustrating the genomic structure of human MASP-2;
FIGURE 2A is a schematic diagram illustrating the domain structure of human
MASP-2 protein;
FIGURE 2B is a schematic diagram illustrating the domain structure of human
MAp19 protein;
FIGURE 3 is a diagram illustrating the murine MASP-2 knockout strategy;
FIGURE 4 is a diagram illustrating the human MASP-2 minigene construct,
FIGURE 5A presents results demonstrating that MASP-2-deficiency leads to the
loss of lectin-pathway-mediated C4 activation as measured by lack of C4b
deposition on
mannan, as described in Example 2;
FIGURE 5B presents results demonstrating that MASP-2-deficiency leads to the
loss of lectin-pathway-mediated C4 activation as measured by lack of C4b
deposition on
zymosan, as described in Example 2;
FIGURE 5C presents results demonstrating the relative C4 activation levels of
serum samples obtained from MASP-2+/-; MASP-2-/- and wild-type strains as
measure
by C4b deposition on mannan and on zymosan, as described in Example 2;
FIGURE 6 presents results demonstrating that the addition of murine
recombinant
MA SP-2 to MA SP-2-/- serum samples recovers lecti n-pathway-medi ated C4
activation in
a protein concentration dependent manner, as measured by C4b deposition on
mannan, as
described in Example 2;
FIGURE 7 presents results demonstrating that the classical pathway is
functional
in the MASP-2-/- strain, as described in Example 8;
FIGURE 8A presents results demonstrating that anti-MASP-2 Fab2 antibody #11
inhibits C3 convertase formation, as described in Example 10;
FIGURE 8B presents results demonstrating that anti-MASP-2 Fab2 antibody #11
binds to native rat MASP-2, as described in Example 10;
FIGURE 8C presents results demonstrating that anti-MASP-2 Fab2 antibody #41
inhibits C4 cleavage, as described in Example 10;
-16-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
FIGURE 9 presents results demonstrating that all of the anti-MASP-2 Fab2
antibodies tested that inhibited C3 convertase formation also were found to
inhibit C4
cleavage, as described in Example 10;
FIGURE 10 is a diagram illustrating the recombinant polypeptides derived from
rat MASP-2 that were used for epitope mapping of the MASP-2 blocking Fab2
antibodies, as described in Example 11;
FIGURE 11 presents results demonstrating the binding of anti-MASP-2 Fab2 #40
and #60 to rat MASP-2 polypeptides, as described in Example 11;
FIGURE 12A graphically illustrates the level of MAC deposition in the presence
or absence of human MASP-2 monoclonal antibody (0MS646) under lectin pathway-
specific assay conditions, demonstrating that 0MS646 inhibits lectin-mediated
MAC
deposition with an ICso value of approximately 1 nM, as described in Example
12;
FIGURE 12B graphically illustrates the level of MAC deposition in the presence
or absence of human MASP-2 monoclonal antibody (01\45646) under classical
pathway-
specific assay conditions, demonstrating that 0MS646 does not inhibit
classical pathway-
mediated MAC deposition, as described in Example 12;
FIGURE 12C graphically illustrates the level of MAC deposition in the presence
or absence of human MASP-2 monoclonal antibody (0MS646) under alternative
pathway-specific assay conditions, demonstrating that 0MS646 does not inhibit
alternative pathway-mediated MAC deposition, as described in Example 12;
FIGURE 13 graphically illustrates the pharmacokinetic (PK) profile of human
MASP-2 monoclonal antibody (0MS646) in mice, showing the 0MS646 concentration
(mean of n=3 animals/groups) as a function of time after administration at the
indicated
dose, as described in Example 12;
FIGURE 14A graphically illustrates the pharmacodynamic (PD) response of
human 1\4ASP-2 monoclonal antibody (0MS646), measured as a drop in systemic
lectin
pathway activity, in mice following intravenous administration, as described
in Example
12;
FIGURE 14B graphically illustrates the pharmacodynamic (PD) response of
human MASP-2 monoclonal antibody (0MS646), measured as a drop in systemic
lectin
pathway activity, in mice following subcutaneous administration, as described
in
Example 12;
-17-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
FIGURE 15 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Sirius red, wherein the tissue sections
were obtained
from wild-type and MASP-2-/- mice following 7 days of unilateral ureteric
obstruction
(UUO) and sham-operated wild-type and MASP-2-/- mice, as described in Example
14;
FIGURE 16 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with the F4/80 macrophage-specific antibody,
wherein the
tissue sections were obtained from wild-type and MASP-2-/- mice following 7
days of
unilateral ureteric obstruction (UUO) and sham-operated wild-type and MASP-2-/-
mice,
as described in Example 14
FIGURE 17 graphically illustrates the relative mRNA expression levels of
collagen-4, as measured by quantitative PCR (qPCR), in kidney tissue sections
obtained
from wild-type and MASP-2-/- mice following 7 days of unilateral ureteric
obstruction
(UUO) and sham-operated wild-type and MASP-2-/- mice, as described in Example
14.
FIGURE 18 graphically illustrates the relative mRNA expression levels of
Transforming Growth Factor Beta-1 (TGFI3-1), as measured by qPCR, in kidney
tissue
sections obtained from wild-type and MASP-2-/- mice following 7 days of
unilateral
ureteric obstruction (UUO) and sham-operated wild-type and MASP-2-/- mice, as
described in Example 14.
FIGURE 19 graphically illustrates the relative mRNA expression levels of
Interleukin-6 (IL-6), as measured by qPCR, in kidney tissue sections obtained
from wild-
type and MASP-2-/- mice following 7 days of unilateral ureteric obstruction
(UUO) and
sham-operated wild-type and MASP-2-/- mice, as described in Example 14.
FIGURE 20 graphically illustrates the relative mRNA expression levels of
Interferon-y, as measured by qPCR, in kidney tissue sections obtained from
wild-type and
MASP-2-/- mice following 7 days of unilateral ureteric obstruction (UUO) and
sham-
operated wild-type and MASP-2-/- mice, as described in Example 14.
FIGURE 21 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Siruis red, wherein the tissue sections
were obtained
following 7 days of unilateral ureteric obstruction (UUO) from wild-type mice
treated
with a MASP-2 inhibitory antibody and an isotype control antibody, as
described in
Example 15.
FIGURE 22 graphically illustrates the hydroxyl proline content from kidneys
harvested 7 days after unilateral ureteric obstruction (UUO) obtained from
wild-type
-18-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
mice treated with MASP-2 inhibitory antibody as compared with the level of
hydroxyl
proline in tissue from obstructed kidneys obtained from wild-type mice treated
with an
IgG4 isotype control, as described in Example 15.
FIGURE 23 graphically illustrates the total amount of serum proteins (mg/ml)
measured on day 15 of the protein overload study in wild-type control mice
(n=2) that
received saline only, wild-type mice that received BSA (n=6) and MASP-2-/-
mice that
received BSA (n=6), as described in Example 16
FIGURE 24 graphically illustrates the total amount of excreted protein (mg) in
urine collected over a 24 hour period on day 15 of the protein overload study
from wild-
type control mice (n=2) that received saline only, wild-type that received BSA
(n=6) and
MASP-2-/- mice that received BSA (n=6), as described in Example 16.
FIGURE 25 shows representative hematoxylin and eosin (H&E) stained renal
tissue sections from the following groups of mice on day 15 of the protein
overload study
as follows: (panel A) wild-type control mice; (panel B) MASP-2-/- control
mice, (panel
C) wild-type mice treated with BSA; and (panel D) MASP-2-/- mice treated with
bovine
serum albumin (BSA), as described in Example 16.
FIGURE 26 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with macrophage-specific antibody F4/80,
showing the
macrophage mean stained area (%), wherein the tissue sections were obtained on
day 15
of the protein overload study from wild-type control mice (n=2), wild-type
mice treated
with BSA (n=6), and MASP-2-/- mice treated with BSA (n=5), as described in
Example
16.
FIGURE 27A graphically illustrates the analysis for the presence of a
macrophage-proteinuria correlation in each wild-type mouse (n=6) treated with
BSA by
plotting the total excreted proteins measured in urine from a 24-hour sample
versus the
macrophage infiltration (mean stained area 0/0), as described in Example 16.
FIGURE 27B graphically illustrates the analysis for the presence of a
macrophage-proteinuria correlation in each MASP-2-/- mouse (n=5) treated with
BSA by
plotting the total excreted proteins in urine in a 24-hour sample versus the
macrophage
infiltration (mean stained area ?/o), as described in Example 16.
FIGURE 28 graphically illustrates the results of computer-based image analysis
of stained tissue sections with anti-TGFP antibody (measured as /0 TGFI3
antibody-
-19-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
stained area) in wild-type mice treated with BSA (n=4) and MASP-2-/- mice
treated with
BSA (n=5), as described in Example 16.
FIGURE 29 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TNFa antibody (measured as % TNFa antibody-
stained
area) in wild-type mice treated with BSA (n=4) and MASP-2-/- mice treated with
BSA
(n=5), as described in Example 16.
FIGURE 30 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-IL-6 antibody (measured as % IL-6 antibody-
stained
area) in wild-type control mice, MASP-2-/- control mice, wild-type mice
treated with
BSA (n=7) and MASP-2-/- mice treated with BSA (n=7), as described in Example
16.
FIGURE 31 graphically illustrates the frequency of TUNEL apoptotic cells
counted in serially selected 20 high power fields (HPFs) from tissue sections
from the
renal cortex in wild-type control mice (n=1), MASP-2-/- control mice (n=1),
wild-type
mice treated with BSA (n=6) and MASP-2-/- mice treated with BSA (n=7), as
described
in Example 16.
FIGURE 32 shows representative H&E stained tissue sections from the following
groups of mice at day 15 after treatment with BSA: (panel A) wild-type control
mice
treated with saline, (panel B) isotype antibody treated control mice and
(panel C) wild-
type mice treated with a MASP-2 inhibitory antibody, as described in Example
17.
FIGURE 33 graphically illustrates the frequency of TUNEL apoptotic cells
counted in serially selected 20 high power fields (HPFs) from tissue sections
from the
renal cortex in wild-type mice treated with saline control and BSA (n=8), wild-
type mice
treated with the isotype control antibody and BSA (n=8) and wild-type mice
treated with
a MASP-2 inhibitory antibody and BSA (n=7), as described in Example 17.
FIGURE 34 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TGFP antibody (measured as % TGFP antibody-
stained
area) in wild-type mice treated with BSA and saline (n=8), wild-type mice
treated with
BSA and isotype control antibody (n=7) and wild-type mice treated with BSA and
MASP-2 inhibitory antibody (n=8), as described in Example 17.
FIGURE 35 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TNFa antibody (measured as % TNFa antibody-
stained
area) in wild-type mice treated with BSA and saline (n=8), BSA and isotype
control
-20-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
antibody (n=7) and wild-type mice treated with BSA and MASP-2 inhibitory
antibody
(n=8), as described in Example 17.
FIGURE 36 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-IL-6 antibody (measured as % IL-6 antibody-
stained
.. area) in in wild-type mice treated with BSA and saline (n=8), BSA and
isotype control
antibody (n=7) and wild-type mice treated with BSA and MASP-2 inhibitory
antibody
(n=8), as described in Example 17.
FIGURE 37 shows representative H&E stained tissue sections from the following
groups of mice at day 14 after treatment with Adriamycin or saline only
(control): (panels
.. A-1, A-2, A-3) wild-type control mice treated with only saline; (panels B-
1, B-2, B-3)
wild-type mice treated with Adriamycin; and (panels C-1, C-2, C-3) MASP-2-/-
mice
treated with Adriamycin, as described in Example 18,
FIGURE 38 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with macrophage-specific antibody F4/80 showing
the
macrophage mean stained area (%) from the following groups of mice at day 14
after
treatment with Adriamycin or saline only (wild-type control): wild-type
control mice
treated with only saline; wild-type mice treated with Adriamycin; MASP-2-/-
mice
treated with saline only, and MASP-2 -/- mice treated with Adriamycin, wherein
**p=0.007, as described in Example 18;
FIGURE 39 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Sirius Red, showing the collagen
deposition stained
area (%) from the following groups of mice at day 14 after treatment with
Adriamycin or
saline only (wild-type control): wild-type control mice treated with only
saline; wild-type
mice treated with Adriamycin, MASP-2-/- mice treated with saline only, and
MASP-2 -/-
mice treated with Adriamycin, wherein **p=0.005, as described in Example 18;
FIGURE 40 graphically illustrates the urine albumin/creatinine ratio (uACR) in
two IgA patients during the course of a twelve week study with weekly
treatment with a
MASP-2 inhibitory antibody (0MS646), as described in Example 19;
FIGURE 41 graphically illustrates the uACR (mg/g) for the four IgAN patients
treated with 0MS646 over time from baseline to 120 days, as described in
Example 21;
FIGURE 42 graphically illustrates the 24-hour urine protein change from
baseline
at day 1 prior to treatment and post-treatment for the four IgAN patients
treated with
0M5646, as described in Example 21; and
-21-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
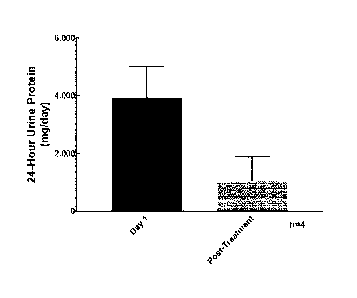
FIGURE 43 graphically illustrates the mean change from baseline to post-
treatment in 24-hour urine protein in the four IgAN patients treated with
0MS646, as
described in Example 21.
DESCRIPTION OF THE SEQUENCE LISTING
SEQ ID NO:1 human MAp19 cDNA
SEQ ID NO:2 human MAp19 protein (with leader)
SEQ ID NO:3 human MAp19 protein (mature)
SEQ ID NO:4 human MASP-2 cDNA
SEQ ID NO:5 human MASP-2 protein (with leader)
SEQ ID NO:6 human MASP-2 protein (mature)
SEQ ID NO:7 human MASP-2 gDNA (exons 1-6)
ANTIGENS: (IN REFERENCE TO THE MASP-2 MATURE PROTEIN)
SEQ ID NO:8 CUBI sequence (aa 1-121)
SEQ ID NO:9 CUBEGF sequence (aa 1-166)
SEQ ID NO:10 CUBEGFCUBII (aa 1-293)
SEQ ID NO:11 EGF region (aa 122-166)
SEQ ID NO:12 serine protease domain (aa 429 ¨ 671)
SEQ ID NO:13 serine protease domain inactive (aa 610-625 with Ser618
to Ala mutation)
SEQ ID NO:14 TPLGPKWPEPVFGRL (CUBI peptide)
SEQ ID NO:15
TAPPGYRLRLYFTHFDLELSHLCEYDFVKLSSGAKVLATLC
GQ (CUBI peptide)
SEQ ID NO:16 TFRSDYSN (MBL binding region core)
SEQ ID NO:17 FYSLGSSLDITFRSDYSNEKPFTGF (MBL binding region)
SEQ ID NO:18 IDECQVAPG (EGF PEPTIDE)
SEQ ID NO:19 ANMLCAGLESGGKDSCRGDSGGALV (serine protease
binding core)Detailed Description
PEPTIDE INHIBITORS:
SEQ ID NO:20 MBL full length cDNA
SEQ ID NO:21 MBL full length protein
SEQ ID NO:22 OGK-X-GP (consensus binding)
-22-
CA 03039927 2019-04-09
WO 2018/071701
PCT/US2017/056386
SEQ ID NO:23 OGKLG
SEQ ID NO:24 GLR GLQ GPO GKL GPO G
SEQ ID NO:25 GPO GPO GLR GLQ GPO GKL GPO GPO GPO
SEQ ID NO:26 GKDGRDGTKGEKGEPGQGLRGLQGPOGKLGPOG
SEQ ID NO:27 GAOGSOGEKGAOGPQGPOGPOGKIVIGPKGEOGDO
(human h-ficolin)
SEQ ID NO:28
GCOGLOGAOGDKGEAGTNGKRGERGPOGPOGKAGPOGPN
GAOGEO (human ficolin p35)
SEQ ID NO:29 LQRALEILPNRVTIKANRPFLVFI (C4 cleavage site)
EXPRESSION INHIBITORS:
SEQ ID NO:30 cDNA of CUBI-EGF domain (nucleotides 22-680 of SEQ
ID NO:4)
SEQ ID NO:31
5' CGGGCACACCATGAGGCTGCTGACCCTCCTGGGC 3'
Nucleotides 12-45 of SEQ ID NO:4 including the MASP-2
translation start site (sense)
SEQ ID NO:32
5'GACATTACCTTCCGCTCCGACTCCAACGAGAAG3'
Nucleotides 361-396 of SEQ ID NO:4 encoding a region
comprising the MASP-2 MBL binding site (sense)
SEQ ID NO:33
5'AGCAGCCCTGAATACCCACGGCCGTATCCCAAA3'
Nucleotides 610-642 of SEQ ID NO:4 encoding a region
comprising the CUBII domain
CLONING PRIMERS:
SEQ ID NO:34 CGGGATCCATGAGGCTGCTGACCCTC (5' PCR for
CUB)
SEQ ID NO:35 GGAATTCCTAGGCTGCATA (3' PCR FOR CUB)
SEQ ID NO:36 GGAATTCCTACAGGGCGCT (3' PCR FOR CUBIEGF)
SEQ ID NO:37 GGAATTCCTAGTAGTGGAT (3' PCR FOR
CUBIEGF CUBIT)
SEQ ID NOS:38-47 are cloning primers for humanized antibody
-23-
CA 03039927 2019-04-09
WO 2018/071701
PCT/US2017/056386
SEQ ID NO:48 is 9 aa peptide bond
EXPRESSION VECTOR:
SEQ ID NO:49 is the MASP-2 minigene insert
SEQ ID NO: 50 is the murine MASP-2 cDNA
SEQ ID NO: 51 is the murine MASP-2 protein (w/leader)
SEQ ID NO: 52 is the mature murine MASP-2 protein
SEQ ID NO: 53 the rat MASP-2 cDNA
SEQ ID NO: 54 is the rat MASP-2 protein (w/ leader)
SEQ ID NO: 55 is the mature rat MASP-2 protein
SEQ ID NO: 56-59 are the oligonucleotides for site-directed mutagenesis
of human MASP-2 used to generate human MASP-2A
SEQ ID NO: 60-63 are the oligonucleotides for site-directed mutagenesis
of murine MASP-2 used to generate murine MASP-2A
SEQ ID NO: 64-65 are the oligonucleotides for site-directed mutagenesis
of rat MASP-2 used to generate rat MASP-2A
SEQ ID NO: 66 DNA encoding 17D20_dc35VH21N11VL (0MS646)
heavy chain variable region (VH) (without signal peptide)
SEQ ID NO: 67 17D20_dc35VH21N11VL (0M5646) heavy chain
variable region (VH) polypeptide
SEQ ID NO: 68 17N16mc heavy chain variable region (VH) polypeptide
SEQ ID NO: 69 DNA encoding 17D20_dc35VH21N1IVL (0M5646)
light chain variable region (VL)
SEQ ID NO: 70 17D20_dc35VH21N11VL (0MS646) light chain variable
region (VL) polypeptide
SEQ ID NO: 71 17N16_dc17N9 light chain variable region (VL)
polypeptide
SEQ ID NO:72: SGMI-2L(full-length)
SEQ ID NO: 73: SGMI-2M (medium truncated version)
SEQ ID NO:74: SGM1-2S (short truncated version)
SEQ ID NO:75: mature polypeptide comprising the VH-1142ab6-SGIVII-2-
N and the human IgG4 constant region with hinge mutation
SEQ ID NO:76: mature polypeptide comprising the VH-M2ab6-SGMI-2-
C and the human IgG4 constant region with hinge mutation
-24-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
SEQ ID NO:77: mature polypeptide comprising the VL-M2ab6-SGMI-2-
N and the human Ig lambda constant region
SEQ ID NO:78: mature polypeptide comprising the VL-M2ab6-SGMI-2-C
and the human Ig lambda constant region
SEQ ID NO:79: peptide linker (10aa)
SEQ ID NO:80: peptide linker (6aa)
SEQ ID NO:81: peptide linker (4aa)
SEQ ID NO:82: polynucleotide encoding the polypeptide comprising the
VH-M2ab6-SGMI-2-N and the human IgG4 constant region with
hinge mutation
SEQ ID NO:83: polynucleotide encoding the polypeptide comprising the
VH-M2ab 6-SGMI-2-C and the human IgG4 constant region with
hinge mutation
SEQ ID NO:84: polynucleotide encoding the polypeptide comprising the
VL-M2ab6-SGMI-2-N and the human Ig lambda constant region
SEQ ID NO:85: polynucleotide encoding the polypeptide comprising the
VL-M2ab6-SGMI-2-C and the human Ig lambda constant region
DETAILED DESCRIPTION
The present invention is based upon the surprising discovery by the present
inventors that inhibition of mannan-binding lectin-associated serine protease-
2 (MASP-
2), the key regulator of the lectin pathway of the complement system,
significantly
reduces inflammation and fibrosis in various animal models of fibrotic disease
including
the unilateral ureteral obstruction (UUO) model, the protein overload model
and the
adriamycin-induced nephrology model of renal fibrosis. Therefore, the
inventors have
demonstrated that inhibition of MASP-2-mediated lectin pathway activation
provides an
effective therapeutic approach to ameliorate, treat or prevent renal fibrosis,
e.g.,
tubulointerstitial inflammation and fibrosis, regardless of the underlying
cause. As
.. further described herein, the use of a MASP-2 inhibitory antibody (0M5646)
is effective
to improve renal function and decrease corticosteroid needs in human subjects
suffering
from Immunoglobulin A Nephropathy (IgAN) and membranous nephropathy (MN).
-25-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
I. DEFINITIONS
Unless specifically defined herein, all terms used herein have the same
meaning
as would be understood by those of ordinary skill in the art of the present
invention. The
following definitions are provided in order to provide clarity with respect to
the terms as
they are used in the specification and claims to describe the present
invention.
As used herein, the term "MASP-2-dependent complement activation" comprises
MASP-2- dependent activation of the lectin pathway, which occurs under
physiological
conditions (i.e., in the presence of Ca') leading to the formation of the
lectin pathway C3
convertase C4b2a and upon accumulation of the C3 cleavage product C3b
subsequently
to the CS convertase C4b2a(C3b)n, which has been determined to primarily cause
op soni z ati on.
As used herein, the term "alternative pathway" refers to complement activation
that is triggered, for example, by zymosan from fungal and yeast cell walls,
lipopolysaccharide (LPS) from Gram negative outer membranes, and rabbit
erythrocytes,
as well as from many pure polysaccharides, rabbit erythrocytes, viruses,
bacteria, animal
tumor cells, parasites and damaged cells, and which has traditionally been
thought to
arise from spontaneous proteolytic generation of C3b from complement factor
C3.
As used herein, the term "lectin pathway" refers to complement activation that
occurs via the specific binding of serum and non-serum carbohydrate-binding
proteins
including mannan-binding lectin (MBL), CL-11 and the ficolins (H-ficolin, M-
ficolin, or
L-ficolin).
As used herein, the term "classical pathway" refers to complement activation
that
is triggered by antibody bound to a foreign particle and requires binding of
the
recognition molecule Clq.
As used herein, the teini "MASP-2 inhibitory agent" refers to any agent that
binds
to or directly interacts with MASP-2 and effectively inhibits MASP-2-dependent
complement activation, including anti-MASP-2 antibodies and MASP-2 binding
fragments thereof, natural and synthetic peptides, small molecules, soluble
MASP-2
receptors, expression inhibitors and isolated natural inhibitors, and also
encompasses
peptides that compete with MASP-2 for binding to another recognition molecule
(e.g.,
MBL, H-ficolin, M-ficolin, or L-ficolin) in the lectin pathway, but does not
encompass
antibodies that bind to such other recognition molecules. MASP-2 inhibitory
agents
-26-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
useful in the method of the invention may reduce MASP-2-dependent complement
activation by greater than 20%, such as greater than 50%, such as greater than
900/o. In
one embodiment, the MASP-2 inhibitory agent reduces MASP-2-dependent
complement
activation by greater than 90% (i.e., resulting in MASP-2 complement
activation of only
10% or less).
As used herein, the term -fibrosis" refers to the formation or presence of
excessive connective tissue in an organ or tissue. Fibrosis may occur as a
repair or
replacement response to a stimulus such as tissue injury or inflammation. A
hallmark of
fibrosis is the production of excessive extracellular matrix. The normal
physiological
response to injury results in the deposition of connective tissue as part of
the healing
process, but this connective tissue deposition may persist and become
pathological,
altering the architecture and function of the tissue. At the cellular level,
epithelial cells
and fibroblasts proliferate and differentiate into myofibroblasts, resulting
in matrix
contraction, increased rigidity, microvascular compression, and hypoxia.
As used herein, the term "treating fibrosis in a mammalian subject suffering
from
or at risk of developing a disease or disorder caused or exacerbated by
fibrosis and/or
inflammation" refers to reversing, alleviating, ameliorating, or inhibiting
fibrosis in said
mammalian subject.
As used herein, the term "proteinuria" refers to the presence of urinary
protein in
an abnormal amount, such as in amounts exceeding 0.3g protein in a 24-hour
urine
collection from a human subject, or in concentrations of more than 1g per
liter in a human
subject. In some embodiments, a subject suffering from proteinuria refers to
the presence
of urinary protein in amounts exceeding 1.0g protein in a 24-hour urine
collection from a
shuman subject, such as a subject suffering from immunoglobulin A (IgA)
nephropathy.
As used herein, the term "improving proteinuria" or "reducing proteinuria'
refers
to reducing the 24-hour urine protein excretion in a subject suffering from
proteinuria by
at least 20%, such as at least 30%, such as at least 40%, such at least 50% or
more in
comparison to baseline 24-hour urine protein excretion in the subject prior to
treatment
with a MASP-2 inhibitory agent. In one embodiment, treatment with a MASP-2
inhibitory agent in accordance with the methods of the invention is effective
to reduce
proteinuria in a human subject such as to achieve greater than 20 percent
reduction in 24-
hour urine protein excretion, or such as greater than 30 percent reduction in
24-hour urine
protein excretion, or such as greater than 40 percent reduction in 24-hour
urine protein
-27-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
excretion, or such as greater than 50 percent reduction in 24-hour urine
protein
excretion).
As used herein, the term "antibody" encompasses antibodies and antibody
fragments thereof, derived from any antibody-producing mammal (e.g., mouse,
rat,
.. rabbit, and primate including human), or from a hybridoma, phage selection,
recombinant
expression or transgenic animals (or other methods of producing antibodies or
antibody
fragments"), that specifically bind to a target polypeptide, such as, for
example, MASP-2,
polypeptides or portions thereof. It is not intended that the term "antibody"
limited as
regards to the source of the antibody or the manner in which it is made (e.g.,
by
hybridoma, phage selection, recombinant expression, transgenic animal, peptide
synthesis, etc). Exemplary antibodies include polyclonal, monoclonal and
recombinant
antibodies; pan-specific, multispecific antibodies (e.g., bispecific
antibodies, trispecific
antibodies); humanized antibodies; murine antibodies; chimeric, mouse-human,
mouse-primate, primate-human monoclonal antibodies; and anti-idiotype
antibodies, and
may be any intact antibody or fragment thereof. As used herein, the term
"antibody"
encompasses not only intact polyclonal or monoclonal antibodies, but also
fragments
thereof (such as dAb, Fab, Fab', F(abl)2, Fv), single chain (ScFv), synthetic
variants
thereof, naturally occurring variants, fusion proteins comprising an antibody
portion with
an antigen-binding fragment of the required specificity, humanized antibodies,
chimeric
.. antibodies, and any other modified configuration of the immunoglobulin
molecule that
comprises an antigen-binding site or fragment (epitope recognition site) of
the required
specificity.
A "monoclonal antibody" refers to a homogeneous antibody population wherein
the monoclonal antibody is comprised of amino acids (naturally occurring and
non-
naturally occurring) that are involved in the selective binding of an epitope.
Monoclonal
antibodies are highly specific for the target antigen. The term "monoclonal
antibody"
encompasses not only intact monoclonal antibodies and full-length monoclonal
antibodies, but also fragments thereof (such as Fab, Fab', F(ab')2, Fv),
single chain
(ScFv), variants thereof, fusion proteins comprising an antigen-binding
portion,
humanized monoclonal antibodies, chimeric monoclonal antibodies, and any other
modified configuration of the immunoglobulin molecule that comprises an
antigen-
binding fragment (epitope recognition site) of the required specificity and
the ability to
bind to an epitope. It is not intended to be limited as regards the source of
the antibody or
-28-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
the manner in which it is made (e.g., by hybridoma, phage selection,
recombinant
expression, transgenic animals, etc.). The term includes whole immunoglobulins
as well
as the fragments etc. described above under the definition of "antibody".
As used herein, the term "antibody fragment" refers to a portion derived from
or
related to a full-length antibody, such as, for example, an anti-MASP-2
antibody,
generally including the antigen binding or variable region thereof.
Illustrative examples
of antibody fragments include Fab, Fab', F(ab)2, F(a1:02 and Fv fragments,
scFy
fragments, diabodies, linear antibodies, single-chain antibody molecules and
multi specifi c antibodies formed from antibody fragments.
As used herein, a "single-chain Fv" or "scFv" antibody fragment comprises the
VH and VL domains of an antibody, wherein these domains are present in a
single
polypeptide chain. Generally, the Fv polypeptide further comprises a
polypeptide linker
between the VH and VL domains, which enables the scFv to form the desired
structure
for antigen binding.
As used herein, a "chimeric antibody" is a recombinant protein that contains
the
variable domains and complementarity-determining regions derived from a non-
human
species (e.g., rodent) antibody, while the remainder of the antibody molecule
is derived
from a human antibody.
As used herein, a "humanized antibody" is a chimeric antibody that comprises a
minimal sequence that conforms to specific complementarity-determining regions
derived
from non-human immunoglobulin that is transplanted into a human antibody
framework
Humanized antibodies are typically recombinant proteins in which only the
antibody
complementarity-determining regions are of non-human origin.
As used herein, the term "mannan-binding lectin" ("MBL") is equivalent to
mannan-binding protein ("MBP").
As used herein, the "membrane attack complex" ("MAC") refers to a complex of
the terminal five complement components (C5b combined with C6, C7, C8 and C-9)
that
inserts into and disrupts membranes (also referred to as C5b-9).
As used herein, "a subject" includes all mammals, including without limitation
humans, non-human primates, dogs, cats, horses, sheep, goats, cows, rabbits,
pigs and
rodents.
As used herein, the amino acid residues are abbreviated as follows: alanine
(Ala;A), asparagine (Asn;N), aspartic acid (Asp;D), arginine (Arg;R), cysteine
(Cys;C),
-29-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
glutamic acid (Glu;E), glutamine (Gin;Q), glycine (Gly;G), histidine (His;H),
isoleucine
(Ile;I), leucine (Leu;L), lysine (Lys;K), methionine (Met;M), phenylalanine
(Phe;F),
proline (Pro;P), serine (Ser;S), threonine (Thr;T), tryptophan (Trp;W),
tyrosine (Tyr;Y),
and valine (Val;V).
In the broadest sense, the naturally occurring amino acids can be divided into
groups based upon the chemical characteristic of the side chain of the
respective amino
acids. By "hydrophobic" amino acid is meant either Ile, Leu, Met, Phe, Trp,
Tyr, Val,
Ala, Cys or Pro. By "hydrophilic" amino acid is meant either Gly, Asn, Gin,
Ser, Thr,
Asp, Glu, Lys, Arg or His. This grouping of amino acids can be further
subclassed as
follows. By "uncharged hydrophilic" amino acid is meant either Ser, Thr, Asn
or Gin.
By "acidic" amino acid is meant either Glu or Asp. By "basic" amino acid is
meant either
Lys, Arg or His.
As used herein the term "conservative amino acid substitution" is illustrated
by a
substitution among amino acids within each of the following groups: (1)
glycine, alanine,
valine, leucine, and isoleucine, (2) phenylalanine, tyrosine, and tryptophan,
(3) serine and
threonine, (4) aspartate and glutamate, (5) glutamine and asparagine, and (6)
lysine,
arginine and histidine.
The term "oligonucleotide" as used herein refers to an oligomer or polymer of
ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) or mimetics thereof This
term
also covers those oligonucleobases composed of naturally-occurring
nucleotides, sugars
and covalent internucleoside (backbone) linkages as well as oligonucleotides
having
non-naturally-occurring modifications.
As used herein, an "epitope" refers to the site on a protein (e.g., a human
MASP-2
protein) that is bound by an antibody. "Overlapping epitopes" include at least
one (e.g.,
two, three, four, five, or six) common amino acid residue(s), including linear
and non-
linear epitopes.
As used herein, the terms "polypeptide," "peptide," and "protein" are used
interchangeably and mean any peptide-linked chain of amino acids, regardless
of length
or post-translational modification. The MASP-2 protein described herein can
contain or
be wild-type proteins or can be variants that have not more than 50 (e.g., not
more than
one, two, three, four, five, six, seven, eight, nine, ten, 12, 15, 20, 25, 30,
35, 40, or 50)
conservative amino acid substitutions. Conservative substitutions typically
include
substitutions within the following groups: glycine and alanine; valine,
isoleucine, and
-30-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
leucine; aspartic acid and glutamic acid; asparagine, glutamine, serine and
threonine;
lysine, histidine and arginine; and phenylalanine and tyrosine.
In some embodiments, the human MASP-2 protein can have an amino acid
sequence that is, or is greater than, 70 (e.g., 71, 72, 73, 74, 75, 76, 77,
78, 79, 80, 81, 82,
83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, or 100)
,/c. identical to the
human MASP-2 protein having the amino acid sequence set forth in SEQ ID NO: 5.
In some embodiments, peptide fragments can be at least 6 (e.g., at least 7, 8,
9, 10,
11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29,
30, 31, 32, 33, 34,
35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 55, 60, 65,
70, 75, 80, 85, 90,
95, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 250, 300, 350, 400,
450, 500,
or 600 or more) amino acid residues in length (e.g., at least 6 contiguous
amino acid
residues of SEQ ID NO: 5). In some embodiments, an antigenic peptide fragment
of a
human MASP-2 protein is fewer than 500 (e.g., fewer than 450, 400, 350, 325,
300, 275,
250, 225, 200, 190, 180, 170, 160, 150, 140, 130, 120, 110, 100, 95, 90, 85,
80, 75, 70,
65, 60, 55, 50, 49, 48, 47, 46, 45, 44, 43, 42, 41, 40, 39, 38, 37, 36, 35,
34, 33, 32, 31, 30,
29, 28, 27, 26, 25, 24, 23, 22, 21, 20, 19, 18, 17, 16, 15, 14, 13, 12, 11,
10, 9, 8, 7, or 6)
amino acid residues in length (e.g., fewer than 500 contiguous amino acid
residues in any
one of SEQ ID NOS: 5).
Percent (%) amino acid sequence identity is defined as the percentage of amino
acids in a candidate sequence that are identical to the amino acids in a
reference
sequence, after aligning the sequences and introducing gaps, if necessary, to
achieve the
maximum percent sequence identity. Alignment for purposes of determining
percent
sequence identity can be achieved in various ways that are within the skill in
the art, for
instance, using publicly available computer software such as BLAST, BLAST-2,
ALIGN,
ALIGN-2 or Megalign (DNASTAR) software. Appropriate parameters for measuring
alignment, including any algorithms needed to achieve maximal alignment over
the full-
length of the sequences being compared can be determined by known methods.
As used herein, the terms "about- or "approximately" when preceding a
numerical
value indicates the value plus or minus a range of 10%.
-31-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
II. Overview of the Invention
As described herein, the inventors have identified the central role of the
lectin
pathway in the initiation and disease progression of tubular renal pathology,
thereby
implicating a key role of the lectin pathway activation in the pathophysiology
of a diverse
range of renal diseases including IgA nephropathy, C3 glomerulopathy and other
glomerulonephritides. As further described herein, the inventors discovered
that
inhibition of mannan-binding lectin-associated serine protease-2 (MASP-2), the
key
regulator of the lectin pathway of the complement system, significantly
reduces
inflammation and fibrosis in various animal models of fibrotic disease
including the
unilateral ureteral obstruction (UUO) model, the protein overload model and
the
adriamycin-induced nephrology model of renal fibrosis. Therefore, the
inventors have
demonstrated that inhibition of MASP-2-mediated lectin pathway activation
provides an
effective therapeutic approach to ameliorate, treat or prevent renal fibrosis,
e.g.,
tubulointerstitial fibrosis, regardless of the underlying cause.
Lectins (MEL, M-ficolin, H-ficolin, L-ficolin and CL-11) are the specific
recognition molecules that trigger the innate complement system and the system
includes
the lectin initiation pathway and the associated terminal pathway
amplification loop that
amplifies lectin-initiated activation of terminal complement effector
molecules. Clq is
the specific recognition molecule that triggers the acquired complement system
and the
system includes the classical initiation pathway and associated terminal
pathway
amplification loop that amplifies Clq-initiated activation of terminal
complement effector
molecules. We refer to these two major complement activation systems as the
lectin-dependent complement system and the Cl q-dependent complement system,
respectively.
In addition to its essential role in immune defense, the complement system
contributes to tissue damage in many clinical conditions. Thus, there is a
pressing need
to develop therapeutically effective complement inhibitors to prevent these
adverse
effects. With the recognition that it is possible to inhibit the lectin
mediated MASP-2
pathway while leaving the classical pathway intact comes the realization that
it would be
highly desirable to specifically inhibit only the complement activation system
causing a
particular pathology without completely shutting down the immune defense
capabilities
of complement. For example, in disease states in which complement activation
is
-32-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
mediated predominantly by the lectin-dependent complement system, it would be
advantageous to specifically inhibit only this system. This
would leave the
C 1 q-dependent complement activation system intact to handle immune complex
processing and to aid in host defense against infection.
The preferred protein component to target in the development of therapeutic
agents to specifically inhibit the lectin-dependent complement system is MASP-
2. Of all
the known protein components of the lectin-dependent complement system (MBL,
H-ficolin, M-ficolin, L-ficolin, MASP-2, C2-C9, Factor B, Factor D, and
properdin), only
MASP-2 is both unique to the lectin-dependent complement system and required
for the
system to function. The lectins (MBL, H-ficolin, M-ficolin, L-ficolin and CL-
11) are
also unique components in the lectin-dependent complement system. However,
loss of
any one of the lectin components would not necessarily inhibit activation of
the system
due to lectin redundancy. It would be necessary to inhibit all five lectins in
order to
guarantee inhibition of the lectin-dependent complement activation system.
Furthermore,
since MBL and the ficolins are also known to have opsonic activity independent
of
complement, inhibition of lectin function would result in the loss of this
beneficial host
defense mechanism against infection. In contrast, this complement-independent
lectin
opsonic activity would remain intact if MASP-2 was the inhibitory target. An
added
benefit of MASP-2 as the therapeutic target to inhibit the lectin-dependent
complement
activation system is that the plasma concentration of MASP-2 is among the
lowest of any
complement protein 500
ng/m1); therefore, correspondingly low concentrations of
high-affinity inhibitors of MASP-2 may be sufficient to obtain full inhibition
(Moll er-Kri sten sen, M., et al., I. Iminunol Methods 282:159-167, 2003).
As described herein in Example 14, it was determined in an animal model of
fibrotic kidney disease (unilateral ureteral obstruction UUO) that mice
without the
MASP-2 gene (MASP-2-/-) exhibited significantly less kidney disease compared
to wild-
type control animals, as shown by inflammatory cell infiltrates (75%
reduction) and
histological markers of fibrosis such as collagen deposition (one third
reduction). As
further shown in Example 15, wild-type mice systemically treated with an anti-
MASP-2
monoclonal antibody that selectively blocks the lectin pathway while leaving
the classical
pathway intact, were protected from renal fibrosis, as compared to wild-type
mice treated
with an isotype control antibody. These results demonstrate that the lectin
pathway is a
key contributor to kidney disease and further demonstrate that a MASP-2
inhibitor that
-33-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
blocks the lectin pathway, such as a MASP-2 antibody, is effective as an
antifibrotic
agent. As further shown in Example 16, in the protein overload model, wild-
type mice
treated with bovine-serum albumin (BSA) developed proteinuric nephropathy,
whereas
MASP-2-/- mice treated with the same level of BSA had reduced renal injury. As
shown
in Example 17, wild-type mice systemically treated with an anti-MASP-2
monoclonal
antibody that selectively blocks the lectin pathway while leaving the
classical pathway
intact, were protected from renal injury in the protein overload model. As
described in
Example 18, MASP-2-/- mice exhibited less renal inflammation and
tubulointerstitial
injury in an Adriamycin-induced nephrology model of renal fibrosis as compared
to wild-
type mice. As described in Example 19, in an ongoing Phase 2 open-label renal
trial,
patients with IgA nephropathy that were treated with an anti-MASP-2 antibody
demonstrated a clinically meaningful and statistically significant decrease in
urine
albumin-to-creatinine ratios (uACRs) throughout the trial and reduction in 24-
hour urine
protein levels from baseline to the end of treatment. As further described in
Example 19,
in the same Phase 2 renal trial, patients with membranous nephropathy that
were treated
with an anti-MASP-2 antibody also demonstrated reductions in uACR during
treatment.
As described in Example 20, in an ongoing Phase 2 open-label renal trial, 4
out of 5
patients with Lupus Nephritis (LN) that were treated with an anti-MASP-2
antibody
demonstrated a clinically meaningful decrease in 24-hour urine protein levels
from
.. baseline to the end of treatment.
In accordance with the foregoing, the present invention relates to the use of
MASP-2 inhibitory agents, such as MASP-2 inhibitory antibodies, as
antifibrotic agents,
the use of MASP-2 inhibitory agents for the manufacture of a medicament for
the
treatment of a fibrotic condition, and methods of preventing, treating,
alleviating or
reversing a fibrotic condition in a human subject in need thereof, said method
comprising
administering to said patient an efficient amount of a MASP-2 inhibitory agent
(e.g., an
anti-MASP-2 antibody).
The methods of the invention can be used to prevent, treat, alleviate or
reverse a
fibrotic condition in a human subject suffering from any disease or disorder
caused or
exacerbated by fibrosis and/or inflammation, including diseases of the kidney
(e.g.,
chronic kidney disease, IgA nephropathy, C3 glomerulopathy and other
glomerulonephritides), lung (e.g., idiopathic pulmonary fibrosis, cystic
fibrosis,
bronchiectasis), liver (e.g., cirrhosis, nonalcoholic fatty liver disease),
heart (e.g.,
-34-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
myocardial infarction, atrial fibrosis, valvular fibrosis, endomyocardial
fibrosis), brain
(e.g., stroke), skin (e.g., excessive wound healing, scleroderma, systemic
sclerosis,
keloids), vasculature (e.g., atherosclerotic vascular disease), intestine
(e.g., Crohn's
disease), eye (e.g., anterior subcapsular cataract, posterior capsule
opacification),
musculoskeletal soft-tissue structures (e.g., adhesive capsulitis, Dupuytren's
contracture,
myelofibrosis), reproductive organs (e.g., endometriosis, Peyronie's disease),
and some
infectious diseases (e.g., alpha virus, Hepatitis C, and Hepatitis B).
III. THE ROLE OF MASP-2 IN DISEASES AND CONDITIONS CAUSED OR
EXACERBATED BY FIBROSIS
Fibrosis is the formation or presence of excessive connective tissue in an
organ or
tissue, commonly in response to damage or injury. A hallmark of fibrosis is
the
production of excessive extracellular matrix following an injury. In the
kidney, fibrosis is
characterized as a progressive detrimental connective tissue deposition on the
kidney
parenchyma which inevitably leads to a decline in renal function independently
of the
primary renal disease which causes the original kidney injury. So called
epithelial to
mesenchymal transition (EMT), a change in cellular characteristics in which
tubular
epithelial cells are transformed to mesenchymal fibroblasts, constitutes the
principal
mechanism of renal fibrosis. Fibrosis affects nearly all tissues and organ
systems and
may occur as a repair or replacement response to a stimulus such as tissue
injury or
inflammation. The normal physiological response to injury results in the
deposition of
connective tissue but, if this process becomes pathological, the replacement
of highly
differentiated cells by scarring connective tissue alters the architecture and
function of the
tissue At the cellular level, epithelial cells and fibroblasts proliferate and
differentiate
into myofibroblasts, resulting in matrix contraction, increased rigidity,
microvascular
compression, and hypoxia. Currently there are no effective treatments or
therapeutics for
fibrosis, but both animal studies and anecdotal human reports suggest that
fibrotic tissue
damage may be reversed (Tampe and Zeisberg, Nat Rev Nephrol, vol 10:226-237,
2014).
Many diseases result in fibrosis that causes progressive organ failure,
including
diseases of the kidney (e.g., chronic kidney disease, IgA nephropathy, C3
glomerulopathy
and other glomerulonephritides), lung (e.g., idiopathic pulmonary fibrosis,
cystic fibrosis,
bronchiectasis), liver (e.g., cirrhosis, nonalcoholic fatty liver disease),
heart (e.g.,
-35-
myocardial infarction, atrial fibrosis, valvular fibrosis, endomyocardial
fibrosis), brain
(e.g., stroke), skin (e.g., excessive wound healing, scleroderma, systemic
sclerosis,
keloids), vasculature (e.g., atherosclerotic vascular disease), intestine
(e.g., Crohn's
disease), eye (e.g., anterior subcapsular cataract, posterior capsule
opacification),
musculoskeletal soft-tissue structures (e.g., adhesive cap suliti s,
Dupuytren' s contracture,
myelofibrosis), reproductive organs (e.g., endometriosis, Peyronie's disease),
and some
infectious diseases (e.g., alpha virus, Hepatitis C, Hepatitis B, etc.).
While fibrosis occurs in many tissues and diseases, there are common molecular
and cellular mechanisms to its pathology. The deposition of extracellular
matrix by
fibroblasts is accompanied by immune cell infiltrates, predominately
mononuclear cells
(see Wynn T., Nat Rev buntunol 4(8).583-594, 2004).
A robust inflammatory response results in the expression of growth factors
(TGF-beta, 'VEGF, Hepatocyte Growth Factor, connective tissue growth factor),
cytokines and hormones (endothelin, IL-4, IL-6, IL-13, chemokines),
degradative
enzymes (elastase, matrix metaloproteinases, cathepsins), and extracellular
matrix
proteins (collagens, fibronectin, integrins).
In addition, the complement system becomes activated in numerous fibrotic
diseases. Complement components, including the membrane attack complex, have
been
identified in numerous fibrotic tissue specimens. For example, components of
the lectin
pathway have been found in fibrotic lesions of kidney disease (Satomura et
al., Nephron.
92(3):702-4 (2002); Sato et al., lupus 20(13):1378-86 (2011); Liu et al., Clin
Exp
binnunol, 1740:152-60 (2013)); liver disease (Rensen et al., Hepatology 50(6):
1809-17
(2009)). and lung disease (Olesen et al., Clin Innnunol 121(3):324-31 (2006)).
Overshooting complement activation has been established as a key contributor
to
immune complex-mediated as well as antibody independent glomerulonephritides.
There
is, however, a strong line of evidence demonstrating that uncontrolled
activation of
complement in situ is intrinsically involved in the pathophysiological
progression of TI
fibrosis in non-glomerular disease (Quigg R.J, J Inimunol 171:3319-3324, 2003,
Naik A.
et al., Semin Nephrol 33:575-585, 2013, Mathem D.R. et al., Chn .1 Am Soc
Nephrol
10:P1636-1650, 2015). The strong proinflammatory signals that are triggered by
local
complement activation may be initiated by complement components filtered into
the
proximal tubule and subsequently entering the interstitial space, or abnormal
synthesis of
complement components by tubular or other resident and infiltrating cells, or
by altered
-36-
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
expression of complement regulatory proteins on kidney cells, or absence or
loss or gain
for function mutations in complement regulatory components (Mathern D.R. et
al., Clin J
Am Soc Nephrol 10:P1636-1650, 2015, Sheerin N.S., et al., FASEB J 22: 1065-
1072,
2008). In mice for example, deficiency of the complement regulatory protein
CRI-
S related gene/protein y (Crry), results in tubulointerstitial (TI)
complement activation with
consequent inflammation and fibrosis typical of the injury seen in human TI
diseases
(Naik A. et al., ,S'ernin Nephrol 33:575-585, 2013, Bao L. et al.õI Am Soc
Nephrol 18:811-
822, 2007). Exposure of tubular epithelial cells to the anaphylatoxin C3a
results in
epithelial to mesenchymal transition (Tsang Z. et al., I Am Soc Nephrol 20:593-
603,
2009). Blocking C3a signaling via the C3a receptor alone has recently been
shown to
lessen renal TI fibrosis in proteinuric and non-proteinuric animals (Tsang Z.
et al., J Am
Soc Nephrol 20:593-603, 2009, Bao L. et al., Kidney Int. 80: 524-534, 2011).
As described herein, the inventors have identified the central role of the
lectin
pathway in the initiation and disease progression of tubular renal pathology,
thereby
implicating a key role of the lectin pathway activation in the pathophysiology
of a diverse
range of renal diseases including IgA nephropathy, C3 glomerulopathy and other
glomerulonephritides (Endo M. et al., Nephrol Dialysis Transplant 13: 1984-
1990, 1998;
Hisano S. et al., Am J Kidney Dis 45:295-302, 2005; Roos A. et al., J Am Soc
Nephrol 17:
1724-1734, 2006; Liu L.L. et al., Clin Exp. Immo! 174:152-160, 2013; Lhotta K.
et al.,
Nephrol Dialysis Transplant 14:881-886, 1999; Pickering et al., Kidney
International
84:1079-1089, 2013), diabetic nephropathy (Hovind P. et al., Diabetes 54:1523-
1527,
2005), ischaemic reperfusion injury (Asgari E. et al., FASEB J 28:3996-4003,
2014) and
transplant rejection (Berger S.P. et al., Am.! Transplant 5:1361-1366, 2005).
As further described herein, the inventors have demonstrated that MASP-2
.. inhibition reduces inflammation and fibrosis in mouse models of
tubulointerstitial
disease. Therefore, MASP-2 inhibitory agents are expected to be useful in the
treatment
of renal fibrosis, including tubulointerstitial inflammation and fibrosis,
proteinuria, IgA
nephropathy, C3 glomerulopathy and other glomerulonephritides and renal
ischaemia
reperfusion injury.
Kidney Diseases and Disorders
According to the National Kidney Foundation, 26 million American adults suffer
from Chronic Kidney Disease (CKD). Most patients have progressive disease
leading to
kidney failure, requiring treatment with erythropoiesis stimulating drugs,
dialysis or a
-37-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
kidney transplant for survival. There are several drugs that can treat the
main symptom
of CKD, hypertension, but currently there are no drugs that address its root
cause.
Studies have shown that progressive renal injury is caused by capillary
hypertension in substructures of the kidney known as nephrons (Whitworth J.A.,
Annals
Acad of Med, vol 34(1):2005). As nephrons (the filtration units of the kidney)
are injured
or destroyed in this process, inflammation and tissue scarring occur,
replacing nephrons
with non-functional scar tissue. As a result, the ability of the kidney to
filter blood
declines over time. This is referred to as renal fibrosis, which is the common
pathway of
progressive renal disease. Irrespective of the nature of the initial insult,
renal fibrosis is
considered to be the common final pathway by which kidney disease progresses
to end-
stage renal failure. Amelioration of renal fibrosis may be determined by one
or more of
the following: assessment of interstitial volume, collagen IV deposition,
and/or
connective tissue growth mRNA levels. The compounds and methods described
herein
are useful in the treatment of renal fibrosis.
Renal fibrosis and inflammation are prominent features of late-stage kidney
disease of virtually any etiology (see Boor et al., Boor P. et al., J of Am
Soc of
Nephrology 18:1508-1515, 2007 and Chevalier et al., Kidney International 75 :
1145-
1152, 2009). Kidney failure can be caused by a heterogeneous group of
disorders.
Progressive kidney dysfunction leads to proteinuria and renal insufficiency.
As patient
health deteriorates, dialysis may be necessary simply to forestall the damage
to the kidney
and to prevent multi-system failure. Over time, kidney failure and renal
insufficiency can
progress to end-stage renal disease (ESRD), which is total, or nearly total,
permanent loss
of kidney function. Depending on the form of kidney disease, renal function
may be lost
in a matter of days or weeks or may deteriorate slowly and gradually over the
course of
decades. Once a patient has progressed to ESRD, dialysis (hemidialysis or
peritoneal
dialysis) is required to prevent death. Patients must remain on some form of
dialysis
regimen or must obtain a kidney transplant.
Components of the lectin pathway have been found in fibrotic lesions of kidney
disease (Satomura et al., Nephron . 92(3):702-4 (2002); Sato et al., Lupus
20(13):1378-86
(2011); Liu et al., Clin Exp Immunol , 174( 1): 152-60 (2013)). In IgA
nephropathy,
patients with glomerular MBL deposition had more severe proteinuria, decreased
renal
function, lower levels of serum albumin, more severe histology, and greater
hypertension
than patients without MBL deposition (Liu et al., Clin Exp Immunol . 2013
-38-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Oct;174(1):152-60). Patients with lupus nephritis (Sato et al., Lupus,
20(13):1378-86,
2011) and chronic renal failure (Satomura et al., Nephron 92(3):702-4, 2002)
also have
increased levels of MBL and lectin pathway activity.
It has also been demonstrated that C5 deficiency led to a significant
amelioration
of major components of renal fibrosis in a nonproteinuric model of primary
tubulointerstitial damage, namely unilateral ureteral obstruction (UUO) (Boor
P. et al., J
of Am Soc of Nephrology 18:1508-1515, 2007). It has also been reported that C3
gene
expression was increased in wild-type mice following UUO, and that collagen
deposition
was significantly reduced in C3-/- mice following UUO as compared to wild-type
mice,
suggesting a role of complement activation in renal fibrosis (Fearn et al.,
Mol Innnunol
48:1666-1733, 2011: Abstract). However, prior to the discovery described
herein by the
present inventors, the complement components involved in renal fibrosis were
not well
defined. As described herein in Examples 14-17, the present inventors have
unexpectedly
determined that a deficiency of MASP-2 or blockade of MASP-2 with an
inhibitory
antibody that selectively blocks the lectin pathway, while leaving intact the
classical
pathway, clearly protects mice from renal fibrosis in various animal models of
kidney
disease.
Accordingly, in certain embodiments, the disclosure provides a method of
inhibiting renal fibrosis in a subject suffering from a kidney disease or
disorder caused or
exacerbated by fibrosis and/or inflammation comprising administering a MASP-2
inhibitory agent, such as an anti-MASP-2 antibody, to a subject in need
thereof. This
method includes administering a composition comprising an amount of a MASP-2
inhibitor effective to inhibit renal fibrosis to a subject suffering from a
kidney disease or
disorder caused or exacerbated by fibrosis and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
-39-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In certain embodiments, the MASP-2 inhibitory agents (e.g., anti-MASP-2
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying kidney disease or condition. In
certain
embodiments, the MASP-2 inhibitory agents (e.g., anti-MASP-2 antibodies) are
administered in combination with a dialysis or plasmapheresis regimen. In
certain
embodiments, the MASF'-2 inhibitory agents (e.g., anti-MASP-2 antibodies) are
used to
decrease the frequency with which dialysis or plasmapheresis is required. In
certain other
embodiments, the MASP-2 inhibitory agents (e.g., anti-MASP-2 antibodies) are
used in
combination with kidney transplantation. In certain other embodiments, the
MASP-2
inhibitory agents (e.g., anti-MASP-2 antibodies) are used to control renal
insufficiency
and prevent the further decline in renal function in patients awaiting kidney
transplantation.
By way of example, in certain embodiments, anti-MASP-2 antibodies are used to
inhibit renal fibrosis and thereby treat or ameliorate (including treating or
ameliorating
the symptoms of a disease) glomerular diseases such as focal segmental
glomerulosclerosis and nephrotic syndrome. Exemplary symptoms that can be
treated
include, but are not limited to, hypertension, proteinuria, hyperlipidemia,
hematuria, and
hypercholestermia. In some embodiments, the MASP-2 inhibitory agent inhibits
tubulointerstitial fibrosis. In certain embodiments, treating comprises
improving renal
function, decreasing proteinuria, improving hypertension, and/or decreasing
renal
fibrosis. In certain embodiments, treating comprises (i) delaying or
preventing
progression to renal insufficiency, renal failure, or ESRD; (ii) delaying,
reducing, or
preventing need for dialysis; or (iii) delaying or preventing need for kidney
transplantation.
Certain specific kidney diseases and disorders caused or exacerbated by
fibrosis
and/or inflammation are described below.
In certain embodiments, the kidney disease caused or exacerbated by fibrosis
and/or inflammation is a glomerular disease such as focal segmental
glomerulosclerosis
(FSGS). Glomerular diseases damage the glomeruli, letting protein and
sometimes red
blood cells leak into the urine. Sometimes a glomerular disease also
interferes with the
clearance of waste products by the kidney, so they begin to build up in the
blood.
Symptoms of glomerular disease include proteinuria, hematuria, reduced
glomerular
filtration rate, hypoproteinemia, and edema. A number of different diseases
can result in
-40-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
glomerular disease. It may be the direct result of an infection or a drug
toxic to the
kidneys, or it may result from a disease that affects the entire body, such as
hypertension,
diabetes or lupus. FSGS is one particular glomerular disease, but even this
particular
condition characterized by scarring in the kidney can have numerous causes.
Patients
with FSGS typically progress to end stage renal disease within 5-20 years,
although
patients with aggressive forms of the disease progress to ESRD in 2 to 3
years.
In certain embodiments, the kidney disease caused or exacerbated by fibrosis
and/or inflammation is diabetic nephropathy (DN), which is an area of
substantial unmet
medical need Diabetic nephropathy is kidney disease or damage that results as
a
complication of diabetes. The condition is exacerbated by high blood pressure,
high
blood sugar levels, and high cholesterol and lipid levels. The exact cause of
diabetic
nephropathy is unknown. However, without being bound by theory, it is believed
that
uncontrolled high blood sugar leads to the development of kidney damage, such
as
fibrosis and scarring of tissue. In humans, DN manifests as a clinical
syndrome that is
composed of albuminuria, progressively declining glomerular filtration rate
(GFR) and
increased risk for cardiovascular disease. Diabetic albuminuria is associated
with the
development of characteristic histo-pathologic features, including ticking of
the
glomerular basement membrane (GBM) and mesangial expansion. As albuminuria
progress and renal insufficiency ensues, glomerulosclerosis, arteriolar
hyalinosis and
tubulointerstitial fibrosis develop.
Accordingly, in one embodiment, the present disclosure provides methods for
treating diabetic nephropathy comprising administering an effective amount of
a MASP-2
inhibitory agent (e.g., a MASP-2 inhibitory antibody) to a subject in need
thereof. In
certain embodiments, treating comprises reducing one or more symptoms of
diabetic
nephropathy. In certain embodiments, treating comprises reducing, delaying
or
eliminating the need for dialysis. In certain embodiments, treating comprises
reducing,
delaying, or eliminating the need for kidney transplantation. In certain
embodiments,
treating comprises delaying, preventing or reversing the progression of
diabetic
nephropathy to renal failure or end stage renal disease.
In certain embodiments, the kidney disease caused or exacerbated by fibrosis
and/or inflammation is lupus nephritis. As described in more detail below,
lupus
nephritis, which is a severe complication of systemic lupus erythematosus
(SLE), is
-41-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
another example of renal fibrosis that can be treated with MASP-2 inhibitory
agents (e.g.,
anti-MA SP-2 antibodies).
Accordingly, in one embodiment, the present disclosure provides methods for
inhibiting renal fibrosis in a subject suffering from a kidney disease or
disorder caused or
exacerbated by fibrosis and/or inflammation comprising administering an
effective
amount of a MASP-2 inhibitory agent (e.g., a MASP-2 inhibitory antibody). In
some
embodiments, the kidney disease or disorder exacerbated by fibrosis and/or
inflammation
is selected from the group consisting of chronic kidney disease, chronic renal
failure,
glomerular disease (e.g., focal segmental glomerulosclerosis), an immune
complex
disorder (e.g., IgA nephropathy, membranous nephropathy), lupus nephritis,
nephrotic
syndrome, diabetic nephropathy, tubulointerstifial damage and C3
glomerulopathy or
other types of glomerulonepthritis.
Methods of Preventing or Treating Renal Injury caused by drug-induced toxicity
Another cause of renal injury includes drug-induced toxicity. For example,
nephrotoxins can cause direct toxicity on tubular epithelial cells. As
described herein,
the inventors have demonstrated that MASP-2 deficient mice are protected from
Adriamycin-induced nephropathy.
Nephrotoxins include, but are not limited to, therapeutic drugs, (e.g.,
cisplatin,
gentamicin, cephal ori dine, cyclosporin, amphotericin, Adriamycin),
radiocontrast dye,
pesticides (e.g., paraquat), and environmental contaminants (e.g.,
trichloriethylene and
dichloroacetylene). Other examples include puromycin aminonucleoside (PAN);
aminoglycosides, such as gentamicin; cephalosporins, such as cephaloridine;
calcineurin
inhibitors, such as tacrolimus or sirolimus Drug-induced nephrotoxicity may
also be
caused by non-steroidal anti-infl am matori es, anti -
retroviral s, anti-cytokin es,
immunosuppressants, oncological drugs or ACE inhibitors. The drug-induced
nephrotoxicity may further be caused by nalgesic abuse, ciprofloxacin,
clopidogrel,
cocaine, cox-2 inhibitors, diuretics, foscamet, gold, ifosfamide,
immunoglobin, Chinese
herbs, interferon, lithium, mannitol, mesalamine, mitomycin, nitrosoureas,
penicillamine,
penicillins, pentamidine, quinine, rifampin, streptozocin, sulfonamides,
ticlopidine,
triamterene, valproic acid, doxorubicin, glycerol, cidofovir, tobramycin,
neomycin
sulfate, colistimethate, vancomycin, amikacin, cefotaxime, cisplatin,
acyclovir, lithium,
interleukin-2, cyclosporin or indinavir.
-42-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Accordingly, in one embodiment, a subject at risk for developing or suffering
from renal injury may be receiving one or more therapeutic drugs that have a
nephrotoxic
effect. These subjects may be administered the MASP-2 inhibitors of the
invention prior
to or simultaneously with such therapeutic agents. Likewise, MASP-2 inhibitors
may be
administered after the therapeutic agent to treat or reduce the likelihood of
developing
nephrotoxicity.
Diseases and Conditions Associated with Proteinuria
It has been established that impaired glomerular filtration of protein results
in
proteinuria and accelerates the progressive loss of nephrons that occurs in
all chronic
renal diseases (Remuzzi and Bertani, New Eng. J Med vol 339 (20):1448-1456,
1998).
For example, in a study described in Eddy et al., Am J Pathol 135:719-33,
1989,
glomentlar filtration of albumin was consistently followed by the development
of
interstitial lesions and scarring. As further described in Eddy et al., 1989,
deposition of
complement C3 on the luminal surface of proximal tubules was observed in the
rats with
nephropathy induced by protein-overload, indicating that components of the
complement
system that are filtered by glomeruli can cause interstitial injury. It has
been
demonstrated that complement depletion or the lack of C6 ameliorated
tubulointerstitial
injury in proteinuric animal models such as mesangioproliferative
glomerulonephritis,
Adriamycin nephropathy, five-sixths nephrectomy and puromycin aminonucleoside
nephrosis (Boor et al., et al., J of Am Soc of Nephrology: JASN 18:1508-1515,
2007).
Human studies have shown that proteinuria is an independent predictor of
progression of
chronic kidney disease and that reduction in proteinuria is renal-protective
(Ruggenenti P.
et al., I Am Soc Nephrol 23 :1917-1928, 2012).
Accordingly, in one embodiment, the present disclosure provides methods for
preventing or reducing proteinurea and/or preventing or reducing renal damage
in a
subject suffering from a disease or condition associated with proteinuria
comprising
administering an amount of a MASP-2 inhibitory agent (e.g., a MASP-2
inhibitory
antibody) effective to reduce or prevent proteinurea in the subject. In
some
embodiments, the disease or condition associated with proteinuria is selected
from the
group consisting of nephrotic syndromes, pre-eclampsia, eclampsia, toxic
lesions of
kidneys, amyloidosis, collagen vascular diseases (e.g., systemic lupus
erythematosus),
dehydration, glomerular diseases (e.g. membranous glomerulonephritis, focal
segmental
glomerulonephritis, minimal change disease, lipoid nephrosis), strenuous
exercise, stress,
-43-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
benign orthostatis (postural) proteinuria, focal segmental glomerulosclerosis,
IgA
nephropathy (i.e., Berger's disease), IgM nephropathy, membranoproliferative
glomerulonephritis, membranous nephropathy, minimal change disease,
sarcoidosis,
Alport's syndrome, diabetes mellitus (diabetic nephropathy), drug-induced
toxicity (e.g.,
NSAIDS, nicotine, penicillamine, lithium carbonate, gold and other heavy
metals, ACE
inhibitors, antibiotics or opiates (e.g. heroin)); Fabry's disease, infections
(e.g., HIV,
syphilis, hepatitis A, B or C, poststreptococcal infection, urinary
schistosomiasis);
aminoaciduria, Fanconi syndrome, hypertensive nephrosclerosis, interstitial
nephritis,
sickle cell disease, hemoglobinuria, multiple myeloma, myoglobinuria, organ
rejection
(e.g., kidney transplant rejection), ebola hemorrhagic fever, Nail patella
syndrome,
familial mediterranean fever, BELLP syndrome, systemic lupus erythematosus,
Wegener's granulomatosis, Rheumatoid arthritis, Glycogen storage disease type
1,
Goodpasture's syndrome, Henoch-Schonlein purpura, urinary tract infection
which has
spread to the kidneys, Sjogren's syndrome and post-infections
glomerulonepthritis.
Liver Disease
Liver fibrosis, also called hepatic fibrosis, is caused by the accumulation of
scar
tissue in the liver and is a characteristic of most types of liver disease.
The replacement of
healthy liver tissue with scar tissue impairs the ability of the liver to
function properly. If
the condition causing the scarring is not treated, liver fibrosis may progress
to liver
cirrhosis and complete liver failure, a life-threatening condition. The major
causes of
liver fibrosis are alcohol abuse, chronic hepatitis C virus infection,
nonalcoholic
steatohepatitis and hepatotoxicity (e.g., drug-induced liver damage induced by
acetaminophen or other drug).
Components of the lectin pathway have been found in fibrotic lesions of liver
disease (Rensen et al., Hepatology 50(6): 1809-17 (2009)). For example, in
nonalcoholic
steatohepatitis (also known as fatty liver disease), there is widespread
activation of
complement system proteins, and their expression is associated with disease
severity
(Rensen et al., Hemtology 50(6): 1809-17 (2009), where in addition to C3 and
C9
deposition, MBL accumulation was found, confirming activation of the lectin
pathway.
Accordingly, in certain embodiments, the disclosure provides a method of
inhibiting hepatic fibrosis in a subject suffering from a liver disease or
disorder caused or
exacerbated by fibrosis and/or inflammation comprising administering a MASP-2
inhibitory agent, such as a MASP-2 inhibitory antibody, to a subject in need
thereof. This
-44-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
method includes administering a composition comprising an amount of a MASP-2
inhibitor effective to inhibit hepatic fibrosis to a subject suffering from a
liver disease or
disorder caused or exacerbated by fibrosis and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying liver disease or condition.
In some embodiments, the liver disease or disorder caused or exacerbated by
fibrosis and/or inflammation is selected from the group consisting of:
cirrhosis,
nonalcoholic fatty liver disease (steatohepatitis), liver fibrosis secondary
to alcohol abuse,
liver fibrosis secondary to acute or chronic hepatitis, biliary disease and
toxic liver injury
(e.g., hepatotoxicity due to drug-induced liver damage induced by
acetaminophen or
other drug).
Lung Disease
Pulmonary fibrosis is the formation or development of excess fibrous
connective
tissue in the lungs, wherein normal lung tissue is replaced with fibrotic
tissue. This
scarring leads to stiffness of the lungs and impaired lung structure and
function. In
humans, pulmonary fibrosis is thought to result from repeated injury to the
tissue within
and between the tiny air sacs (alveoli) in the lungs. In an experimental
setting, a variety
of animal models have replicated aspects of the human disease. For example, a
foreign
agent such as bleomycin, fluorescein isothiocyanate, silica, or asbestos may
be instilled
into the trachea of an animal (Gharaee-Kermani et al., Animal Models of
Pulmonary
Fibrosis. Methods Mol. Med., 2005, 117:251-259).
Accordingly, in certain embodiments, the disclosure provides a method of
inhibiting pulmonary fibrosis in a subject suffering from a lung disease or
disorder caused
or exacerbated by fibrosis and/or inflammation comprising administering a MASP-
2
-45-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
inhibitory agent, such as a MASP-2 inhibitory antibody, to a subject in need
thereof. This
method includes administering a composition comprising an amount of a MASP-2
inhibitor effective to inhibit pulmonary fibrosis, decrease lung fibrosis,
and/or improve
lung function. Improvements in symptoms of lung function include improvement
of lung
function and/or capacity, decreased fatigue, and improvement in oxygen
saturation.
In some embodiments, the disclosure provides a method of treating, inhibiting,
preventing or ameliorating pulmonary fibrosis in a subject suffering from
cystic fibrosis
comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory
antibody to a subject in need thereof.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying lung disease or condition.
Certain specific lung diseases and disorders caused or exacerbated by fibrosis
and/or inflammation are described below.
In certain embodiments, the lung disease caused or exacerbated by fibrosis
and/or
inflammation is chronic obstructive pulmonary disease (COPD). COPD is a
disease in
which airway walls are fibrotic with the accumulation of myofibroblasts and
collagen, is
a major cause of disability, and its the fourth leading cause of death in the
United States.
COPD blocks airflow and makes it increasingly difficult for a sufferer to
breathe. COPD
is caused by damage to the airways that eventually interferes with the
exchange of
oxygen and carbon dioxide in the lungs. COPD includes chronic obstructive
bronchitis
and emphysema and often both. COPD patients, whose lungs are already damaged
and
whose lung function is already compromised, are at increased risk of
complications
associated with bacterial and viral infections.
-46-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Accordingly, in one embodiment, the present disclosure provides methods for
treating chronic obstructive pulmonary disease (COPD) comprising administering
an
effective amount of a MASP-2 inhibitory agent (e.g., an anti-MASP-2 antibody)
to inhibit
and/or decrease lung fibrosis in a subject in need thereof. In certain
embodiments,
treating comprises reducing one or more symptoms of COPD. Symptoms of COPD
and/or lung fibrosis include, but are not limited to, cough with mucus,
shortness of breath
(dyspnea) that may get worse with mild activity, fatigue, frequent respiratory
infections,
wheezing, chest tightness, irregular heartbeats (arrhythmias), need for
breathing machine
and oxygen therapy, right-sided heart failure or cor pulmonale (heart swelling
and heart
failure due to chronic lung disease), pneumonia, pneumothorax, severe weight
loss and
malnutrition. Symptoms also include decrease in lung function, as evaluated
using one or
more standard tests of lung function
In certain embodiments, the lung disease caused or exacerbated by fibrosis
and/or
inflammation is pulmonary fibrosis associated with scleroderma. As described
in more
detail below, pulmonary fibrosis associated with scleroderma is another
example of
pulmonary fibrosis that can be treated with MASP-2 inhibitory agents (e.g.,
MASP-2
inhibitory antibodies).
In some embodiments, the lung disease or disorder caused or exacerbated by
fibrosis and/or inflammation is selected from the group consisting of: chronic
obstructive
pulmonary disease, cystic fibrosis, pulmonary fibrosis associated with
scleroderma,
bronchiectasis and pulmonary hypertension.
Heart and Vascular Diseases
A number of different cardiac and vascular pathologies are caused by a common
fibrotic process. Excessive deposition of fibrotic tissue in the heart results
in cardiac
pathology, in which the excess production of extracellular matrix proteins
alter the
structure, architecture, shape and affect the contractile function of the
heart (Khan and
Sheppard, Immunology 118. 10-24, 2006).
Studies indicate that fibrosis may contribute significantly to cardiac
dysfunction in
ischaemic, dilated and hypertrophic cardiomyopathy. For example, it has been
demonstrated that patients with chronic atrial fibrillation were found to have
higher levels
of myocardial interstitial fibrosis as compared to controls (Khan and
Sheppard,
Immunology 118: 10-24, 2006). As another example, it has been determined that
most
cases of arrhythmogenic right ventricular cardiomyopathy (ARVC) in the US
exhibit fat
-47-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
infiltration and scarring (fibrofatty ARVC) (Burke et al., Circulation 97:1571-
1580,
1998). In a study that examined the histopathologic characteristics of the
ventricular
myocardium in human subjects with ARVC it was determined that extensive
fibrosis was
present in biopsy specimens from pediatric patients with ARVC (Nishikawa T. et
al.,
Cardiovascular Pathology vol 8 (4):185-189, 1999).
Accordingly, in certain embodiments, the disclosure provides a method of
preventing, treating, reverting, inhibiting and/or reducing fibrosis and/or
inflammation in
a subject suffering from a cardiac or vascular disease or disorder caused or
exacerbated
by fibrosis and/or inflammation comprising administering a MASP-2 inhibitory
agent,
such as a MASP-2 inhibitory antibody, to a subject in need thereof. This
method includes
administering a composition comprising an amount of a MASP-2 inhibitor
effective to
inhibit cardiac and/or vascular fibrosis, and/or improve cardiac and/or
vascular function.
In some embodiments, the disclosure provides a method of treating, inhibiting,
preventing or ameliorating fibrosis in a subject suffering from valvular
fibrosis
comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory
antibody to a subject in need thereof.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying heart disease, or vascular disease
or condition.
In some embodiments, the cardiac or vascular disease or disorder caused or
exacerbated by fibrosis and/or inflammation is selected from the group
consisting of:
cardiac fibrosis, myocardial infarction, atrial fibrosis, endomyocardial
fibrosis
arrhythmogenic right ventricular cardiomyopathy (ARVC), vascular disease,
atherosclerotic vascular disease, vascular stenosis, restenosis, vasculitis,
phlebitis, deep
vein thrombosis and abdominal aortic aneurysm.
-48-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Chronic Infectious Diseases
Chronic infectious diseases such as Hepatitis C and Hepatitis B cause tissue
inflammation and fibrosis, and high lectin pathway activity may be
detrimental. In such
diseases, inhibitors of MASP-2 may be beneficial. For example, MBL and MASP-1
levels are found to be a significant predictor of the severity of liver
fibrosis in hepatitis C
virus (HCV) infection (Brown et al,, Chn Exp Irnmunol. 147(1):90-8, 2007;
Saadanay et
al., Arab Gastroenterol. 12(2):68-73, 2011; Saeed et al., Clin Exp Immunol.
174(2):265-
73, 2013). MASP-1 has previously been shown to be a potent activator of MASP-2
and
the lectin pathway (Megyeri et al., I Biol Chem. 29. 288(13):8922-34, 2013).
Alphaviruses such as chikungunya virus and Ross River virus induce a strong
host
inflammatory response resulting in arthritis and myositis, and this pathology
is mediated
by MBL and the lectin pathway (Gunn et al., PLoS Pathog. 8(3):e1002586, 2012).
Accordingly, in certain embodiments, the disclosure provides a method of
preventing, treating, reverting, inhibiting and/or reducing fibrosis and/or
inflammation in
a subject suffering from, or having previously suffered from, a chronic
infectious disease
that causes inflammation and/or fibrosis, comprising administering a MASP-2
inhibitory
agent, such as a MASP-2 inhibitory antibody, to a subject in need thereof.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying chronic infectious disease.
In some embodiments, the chronic infectious disease that causes inflammation
and/or fibrosis is selected from the group consisting of: alpha virus,
Hepatitis A, Hepatitis
B, Hepatitis C, tuberculosis, HIV and influenza.
Autoimmune diseases:
-49-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Scleroderma is a chronic autoimmune disease characterized by fibrosis,
vascular
alterations, and autoantibodies. There are two major forms: limited systemic
scleroderma
and diffuse systemic scleroderma. The cutaneous symptoms of limited systemic
scleroderma affect the hands, arms and face. Patients with this form of
scleroderma
frequently have one or more of the following complications: calcinosis,
Raynaud's
phenomenon, esophageal dysfunction, sclerodactyl, and telangiectasias. Diffuse
systemic
scleroderma is rapidly progressing and affects a large area of the skin and
one or more
internal organs, frequently the kidneys, esophagus, heart and/or lungs.
Scleroderma affects the small blood vessels known as arterioles, in all
organs.
First, the endothelial cells of the arteriole die off apoptotically, along
with smooth muscle
cells. These cells are replaced by collagen and other fibrous material.
Inflammatory cells,
particularly CD4+ helper T cells, infiltrate the arteriole, and cause further
damage.
The skin manifestations of scleroderma can be painful, can impair use of the
affected area (e.g., use of the hands, fingers, toes, feet, etc.) and can be
disfiguring. Skin
ulceration may occur, and such ulcers may be prone to infection or even
gangrene. The
ulcerated skin may be difficult or slow to heal. Difficulty in healing skin
ulcerations may
be particularly exacerbated in patients with impaired circulation, such as
those with
Raynaud's phenomenon. In certain embodiments, the methods of the present
disclosure
are used to treat scleroderma, for example skin symptoms of scleroderma. In
certain
embodiments, treating scleroderma comprises treating skin ulceration, such as
digital
ulcers. Administration of MASP-2 inhibitory agent such as anti-MASP-2
antibodies can
be used to reduce the fibrotic and/or inflammatory symptoms of scleroderma in
affected
tissue and/or organs.
In addition to skin symptoms/manifestations, scleroderma may also affect the
heart, kidney, lungs, joints, and digestive tract. In certain embodiments,
treating
scleroderma includes treating symptoms of the disease in any one or more of
these
tissues, such as by reducing fibrotic and/or inflammatory symptoms. Lung
problems are
amongst the most serious complications of scleroderma and are responsible for
much of
the mortality associated with the disease. The two predominant lung conditions
associated
with scleroderma are pulmonary fibrosis and pulmonary hypertension. A patient
with
lung involvement may have either or both conditions. Lung fibrosis associated
with
scleroderma is one example of pulmonary fibrosis that can be treated with MASP-
2
inhibitory agents. Scleroderma involving the lung causes scarring (pulmonary
fibrosis).
-50-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Such pulmonary fibrosis occurs in about 70% of scleroderma patients, although
its
progression is typically slow and symptoms vary widely across patients in
terms of
severity. For patients that do have symptoms associated with pulmonary
fibrosis, the
symptoms include a dry cough, shortness of breath, and reduced ability to
exercise. About
16% of patients with some level of pulmonary fibrosis develop severe pulmonary
fibrosis. Patients with severe pulmonary fibrosis experience significant
decline in lung
function and alveolitis
In certain embodiments, the methods of the present disclosure are used to
treat
scl eroderm a, for example lung fibrosis associated with scl eroderm a.
Administration of
MASP-2 inhibitory agents, such as MASP-2 inhibitory antibodies can be used to
reduce
the fibrotic symptoms of scleroderma in lung. For example, the methods can be
used to
improve lung function and/or to reduce the risk of death due to scleroderma.
Kidney involvement is also common in scleroderma patients. Renal fibrosis
associated with sclerodeima is an example of renal fibrosis that can be
treated by
administration of MASP-2 inhibitory agents, such as anti-MASP-2 antibodies. In
certain
embodiments, the methods of the present disclosure are used to treat
scleroderma, for
example kidney fibrosis associated with scleroderma. In one embodiment,
administration
of MASP-2 inhibitory antibodies can be used to reduce the fibrotic symptoms of
scleroderma in kidney. For example, the methods can be used to improve kidney
function, to reduce protein in the urine, to reduce hypertension, and/or to
reduce the risk
of renal crisis that may lead to fatal renal failure.
Systemic lupus erythematosus (SLE) is a chronic, inflammatory autoimmune
disorder characterized by spontaneous B and T cell autoreactivity and
multiorgan
immune injury and may affect the skin, joints, kidneys, and other organs.
Almost all
people with SLE have joint pain and most develop arthritis. Frequently
affected joints are
the fingers, hands, wrists, and knees. General symptoms of SLE include:
arthritis; fatigue;
general discomfort, uneasiness or ill feeling (malaise); joint pain and
swelling; muscle
aches; nausea and vomiting; and skin rash. Additionally symptoms may also
include:
abdominal pain; blood in the urine; fingers that change color upon pressure or
in the cold;
numbness and tingling; and red spots on skin. In some patients, SLE has lung
or kidney
involvement. Without being bound by theory, inflammation and/or fibrosis in
lung and
kidney damages those organs and leads to symptoms associated with lung and/or
kidney
damage. In some cases, patients with SLE develop a particular kidney condition
called
-51-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
lupus nephritis. In certain embodiments, the disclosure provides methods of
treating SLE
comprising administering an effective amount of a MASP-2 inhibitory agent such
as an
anti-MASP-2 antibody. Administering MASP-2 inhibitory antibodies can be used
to
decrease one or more symptoms of SLE. In certain embodiments, administering
anti-
MASP-2 antibodies is used to treat SLE in a patient with lupus nephritis. In
such cases,
treating SLE comprises treating lupus nephritis, such as by reducing symptoms
of lupus
nephritis In certain embodiments, treating comprises treating the skin
symptoms of SLE.
In certain embodiments, treating comprises reducing one or more symptoms of
lupus
nephritis In certain embodiments, treating comprises reducing, delaying or
eliminating
the need for dialysis. In certain embodiments, treating comprises reducing,
delaying, or
eliminating the need for kidney transplantation. In certain embodiments,
treating
comprises delaying or preventing progression of lupus nephritis to renal
failure or end
stage renal disease.
Lupus nephritis is an inflammation of the kidney, and is a severe complication
of
systemic lupus erythematosus (SLE). In the kidney, lupus nephritis can lead to
debilitating loss of function. Patients with lupus nephritis may eventually
develop kidney
failure and require dialysis or kidney transplantation. Related complications
that can also
be treated using the methods of the disclosure include interstitial nephritis
and nephrotic
syndrome. Symptoms of lupus nephritis include: blood in the urine, foamy
appearance to
.. urine, high blood pressure, protein in the urine, fluid retention, and
edema. Other
symptoms include signs and symptoms of renal fibrosis and/or kidney failure.
If left
untreated, lupus nephritis may lead to kidney failure, and even end stage
renal disease.
Accordingly, in certain embodiments, the disclosure provides a method of
preventing, treating, reverting, inhibiting and/or reducing fibrosis and/or
inflammation in
a subject suffering from an autoimmune disease that causes or exacerbates
fibrosis and/or
inflammation comprising administering a MASP-2 inhibitory agent, such as a
MASP-2
inhibitory antibody, to a subject in need thereof This method includes
administering a
composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
-52-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
.. antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying autoimmune disease.
In some embodiments, the autoimmune disease that causes or exacerbates
fibrosis
and/or inflammation is selected from the group consisting of: scleroderma and
systemic
lupus erythematosus (SLE).
Central Nervous System Diseases and Conditions:
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a disease or disorder of the central nervous system caused or exacerbated
by fibrosis
and/or inflammation comprising administering a MASP-2 inhibitory agent, such
as an
anti-MASP-2 antibody, to a subject in need thereof. This method includes
administering
a composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis
and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition during surgery or
local injection,
either directly or remotely, for example, by catheter. Alternately, the MASP-2
inhibitory
agent may be administered to the subject systemically, such as by intra-
arterial,
intravenous, intramuscular, inhalational, nasal, subcutaneous or other
parenteral
administration, or potentially by oral administration for non-peptidergic
agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying disease or disorder of the central
nervous
system.
In some embodiments, the disease or disorder of the central nervous system
caused or exacerbated by fibrosis and/or inflammation is selected from the
group
consisting of: stroke, traumatic brain injury and spinal cord injury.
Skin Diseases and Conditions
-53-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a skin disease or disorder caused or exacerbated by fibrosis and/or
inflammation
comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory
antibody, to a subject in need thereof This method includes administering a
composition
comprising an amount of a MASP-2 inhibitor effective to inhibit fibrosis
and/or
inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition to the skin, or
local application
during surgery or local injection, either directly or remotely, for example,
by catheter.
Alternately, the MASP-2 inhibitory agent may be administered to the subject
systemically, such as by intra-arterial, intravenous, intramuscular,
inhalational, nasal,
subcutaneous or other parenteral administration, by topical administration, or
potentially
by oral administration for non-peptidergic agents. Administration may be
repeated as
determined by a physician until the condition has been resolved or is
controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying skin disease or disorder.
In some embodiments, the skin disease or disorder caused or exacerbated by
fibrosis and/or inflammation is selected from the group consisting of: skin
fibrosis,
wound healing, scleroderma, systemic sclerosis, keloids, connective tissue
diseases,
scarring, and hypertrophic scars.
Musculoskeletal Bone and Soft-Tissue Disorders and Conditions
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a bone or soft-tissue disease or disorder caused or exacerbated by
fibrosis and/or
inflammation comprising administering a MASP-2 inhibitory agent, such as a
MASP-2
inhibitory antibody, to a subject in need thereof This method includes
administering a
composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis
and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application of the composition to the bone or soft-
tissue
structure, or local application during surgery or local injection, either
directly or
-54-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration, or potentially by oral administration for non-
peptidergic agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying bone or soft-tissue disease or
disorder.
In some embodiments, the bone or soft-tissue disease or disorder caused or
exacerbated by fibrosis and/or inflammation is selected from the group
consisting of:
osteoporosis and/or osteopenia associated with, for example, cystic fibrosis,
myelodysplastic conditions with increased bone fibrosis, adhesive capsulitis,
Dupuytren' s
contracture and myelofibrosis.
Joint Diseases and Conditions
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a joint disease or disorder caused or exacerbated by fibrosis and/or
inflammation
comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory
antibody, to a subject in need thereof This method includes administering a
composition
comprising an amount of a MASP-2 inhibitor effective to inhibit fibrosis
and/or
inflammation.
The MA SP-2 inhibitory composition may be administered locally to the region
of
fibrosis, such as by local application of the composition to the joint, or
local application
during surgery or local injection, either directly or remotely, for example,
by catheter.
Alternately, the MASP-2 inhibitory agent may be administered to the subject
systemically, such as by intra-arterial, intravenous, intramuscular,
inhalational, nasal,
subcutaneous or other parenteral administration, by topical administration, or
potentially
by oral administration for non-peptidergic agents. Administration may be
repeated as
determined by a physician until the condition has been resolved or is
controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying joint disease or disorder.
-55-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In some embodiments, the joint disease or disorder caused or exacerbated by
fibrosis and/or inflammation is arthrofibrosis.
Digestive Diseases and Conditions
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a digestive disease or disorder caused or exacerbated by fibrosis and/or
inflammation comprising administering a MASP-2 inhibitory agent, such as a
MASP-2
inhibitory antibody, to a subject in need thereof This method includes
administering a
composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis
and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application during surgery or local injection,
either directly or
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration, or potentially by oral administration for non-
peptidergic agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying digestive disease or disorder.
In some embodiments, the digestive disease or disorder caused or exacerbated
by
fibrosis and/or inflammation is selected from the group consisting of: Crohn's
disease,
ulcerative colitis and pancreatic fibrosis.
Ocular Diseases and Conditions
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from an ocular disease or disorder caused or exacerbated by fibrosis and/or
inflammation
comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory
antibody, to a subject in need thereof This method includes administering a
composition
comprising an amount of a MASP-2 inhibitor effective to inhibit fibrosis
and/or
inflammation.
-56-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application during surgery or local injection,
either directly or
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration to the eye (e.g., as eye drops), or potentially by oral
administration
for non-peptidergic agents. Administration may be repeated as determined by a
physician
until the condition has been resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying ocular disease or disorder.
In some embodiments, the ocular disease or disorder caused or exacerbated by
fibrosis and/or inflammation is selected from the group consisting of:
anterior
subcapsular cataract, posterior capsule opacification, macular degeneration,
and retinal
and vitreal retinopathy.
-57-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Diseases and Conditions of the Reproductive Organs
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a reproductive disease or disorder caused or exacerbated by fibrosis
and/or
inflammation comprising administering a MASP-2 inhibitory agent, such as a
MASP-2
inhibitory antibody, to a subject in need thereof This method includes
administering a
composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis
and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application during surgery or local injection,
either directly or
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration, or potentially by oral administration for non-
peptidergic agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
.. modalities appropriate for the underlying reproductive disease or disorder.
In some embodiments, the reproductive disease or disorder caused or
exacerbated
by fibrosis and/or inflammation is selected from the group consisting of:
endometriosis
and Peyronie's disease.
Scarring Associated with Trauma
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a disease or condition resulting from scarring associated with trauma
comprising
administering a MASP-2 inhibitory agent, such as a MASP-2 inhibitory antibody,
to a
subject in need thereof. This method includes administering a composition
comprising an
amount of a MASP-2 inhibitor effective to inhibit fibrosis and/or
inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application during surgery or local injection,
either directly or
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
-58-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration, or potentially by oral administration for non-
peptidergic agents.
Administration may be repeated as determined by a physician until the
condition has been
resolved or is controlled.
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying disease or disorder.
In some embodiments, the scarring associated with trauma is selected from the
group consisting of surgical complications (e.g., surgical adhesions wherein
scar tissue
can form between internal organs causing contracture, pain and can cause
infertility),
chemotherapeutic drug-induced fibrosis, radiation-induced fibrosis and
scarring
associated with burns.
Additional Diseases and Disorders caused or exacerbated by fibrosis and/or
inflammation
In certain embodiments, the disclosure provides a method of preventing,
treating,
reverting, inhibiting and/or reducing fibrosis and/or inflammation in a
subject suffering
from a disease or disorder caused or exacerbated by fibrosis and/or
inflammation selected
from the group consisting of organ transplant, breast fibrosis, muscle
fibrosis,
retroperitoneal fibrosis, thyroid fibrosis, lymph node fibrosis, bladder
fibrosis and pleural
fibrosis, comprising administering a MASP-2 inhibitory agent, such as a MASP-2
inhibitory antibody, to a subject in need thereof This method includes
administering a
composition comprising an amount of a MASP-2 inhibitor effective to inhibit
fibrosis
and/or inflammation.
The MASP-2 inhibitory composition may be administered locally to the region of
fibrosis, such as by local application during surgery or local injection,
either directly or
remotely, for example, by catheter. Alternately, the MASP-2 inhibitory agent
may be
administered to the subject systemically, such as by intra-arterial,
intravenous,
intramuscular, inhalational, nasal, subcutaneous or other parenteral
administration, by
topical administration to the eye (e.g., as eye drops), or potentially by oral
administration
for non-peptidergic agents. Administration may be repeated as determined by a
physician
until the condition has been resolved or is controlled.
-59-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In certain embodiments, the MASP-2 inhibitory agents (e.g., MASP-2 inhibitory
antibodies) are administered in combination with one or more agents or
treatment
modalities appropriate for the underlying disease or disorder.
In certain embodiments of any of the various methods and pharmaceutical
compositions described herein, the MASP-2 inhibitory antibody selectively
blocks the
lectin pathway while leaving intact the classical pathway.
IV. MASP-2 INHIBITORY AGENTS
In various aspects, the present invention provides methods of inhibiting the
adverse effects of fibrosis and/or inflammation comprising administering a
MASP-2
inhibitory agent to a subject in need thereof. MASP-2 inhibitory agents are
administered
in an amount effective to inhibit MASP-2-dependent complement activation in a
living
subject. In the practice of this aspect of the invention, representative MASP-
2 inhibitory
agents include: molecules that inhibit the biological activity of MASP-2 (such
as small
molecule inhibitors, anti-MASP-2 antibodies (e.g., MASP-2 inhibitory
antibodies) or
blocking peptides which interact with MASP-2 or interfere with a protein-
protein
interaction), and molecules that decrease the expression of MASP-2 (such as
MASP-2
antisense nucleic acid molecules, MASP-2 specific RNAi molecules and MASP-2
ribozymes), thereby preventing MASP-2 from activating the lectin complement
pathway.
The MASP-2 inhibitory agents can be used alone as a primary therapy or in
combination
with other therapeutics as an adjuvant therapy to enhance the therapeutic
benefits of other
medical treatments
The inhibition of MASP-2-dependent complement activation is characterized by
at least one of the following changes in a component of the complement system
that
occurs as a result of administration of a MASP-2 inhibitory agent in
accordance with the
methods of the invention: the inhibition of the generation or production of
MASP-2-dependent complement activation system products C4b, C3a, C5a and/or
C5b-9
(MAC) (measured, for example, as described in Example 2), the reduction of C4
cleavage
and C4b deposition (measured, for example as described in Example 2), or the
reduction
of C3 cleavage and C3b deposition (measured, for example, as described in
Example 2).
According to the present invention, MASP-2 inhibitory agents are utilized that
are
effective in inhibiting fibrosis and/or inflammation, and exhibit a detectable
antifibrotic
activity and/or induce a decrease of fibrosis. Within the context of the
invention, an anti-
-60-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
fibrotic activity may comprise at least one or more of the following: (1)
reduction in
inflammation, for example, as assessed by activation and recruitment of
macrophages and
endothelial cells; recruitment and activation of lymphocytes and/or
eosinophils via
secretion of a number of cytokines/chemokines; release of cytotoxic mediators
and
fibrogenic cytokines; (2) reduction of cell proliferation, ECM synthesis or
angiogenesis,
and/or (3) reduction in collagen deposition, as compared to the fibrotic
activity in the
absence of the MASP-2 inhibitory agent.
Assessment of an antifibrotic agent, such as a MASP-2 inhibitory agent, may be
detected using any technique known to the skilled person. For example,
assessment of an
antifibrotic agent may be assessed in a UUO model (as described in Examples 12
and 14
herein). If a detectable antifibrotic activity and/or a reduction or decrease
of fibrosis is
assessed using a MASP-2 inhibitory agent, such MASP-2 inhibitory agent is said
to be
used as a medicament for preventing, treating, reverting, and/or inhibiting
fibrosis.
The assessment of fibrosis may be carried out periodically, e.g., each week,
or
each month. The increase/decrease of fibrosis and/or presence of an
antifibrotic activity
may therefore be assessed periodically, e.g. each week, or month. This
assessment is
preferably carried out at several time points for a given subject or at one or
several time
points for a given subject and a healthy control. The assessment may be
carried out at
regular time intervals, e.g. each week, or each month. The assessment may
therefore be
assessed regularly, e.g. each week, or each month. When one assessment has led
to the
finding of a decrease of fibrosis or to the presence of an antifibrotic
activity, a MASP-2
inhibitory agent, such as a MASP-2 inhibitory antibody, is said is exhibit a
detectable
antifibrotic activity and/or inducing a reduction or decrease of fibrosis.
MASP-2 inhibitory agents useful in the practice of this aspect of the
invention
include, for example, MASP-2 antibodies and fragments thereof, MASP-2
inhibitory
peptides, small molecules, MASP-2 soluble receptors and expression inhibitors.
MASP-2
inhibitory agents may inhibit the MASP-2-dependent complement activation
system by
blocking the biological function of MASP-2. For example, an inhibitory agent
may
effectively block MASP-2 protein-to-protein interactions, interfere with MASP-
2
dimerization or assembly, block Ca2+ binding, interfere with the MASP-2 serine
protease
active site, or may reduce MASP-2 protein expression.
-61-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In some embodiments, the MASP-2 inhibitory agents selectively inhibit MASP-2
complement activation, leaving the Clq-dependent complement activation system
functionally intact.
In one embodiment, a MASP-2 inhibitory agent useful in the methods of the
.. invention is a specific MASP-2 inhibitory agent that specifically binds to
a polypeptide
comprising SEQ ID NO:6 with an affinity of at least ten times greater than to
other
antigens in the complement system. In another embodiment, a MASP-2 inhibitory
agent
specifically binds to a polypeptide comprising SEQ ID NO:6 with a binding
affinity of at
least 100 times greater than to other antigens in the complement system. In
one
embodiment, the MASP-2 inhibitory agent specifically binds to at least one of
(i) the
CCP1-CCP2 domain (aa 300-431 of SEQ ID NO:6) or the serine protease domain of
MASP-2 (aa 445-682 of SEQ ID NO:6) and inhibits MASP-2-dependent complement
activation. In one embodiment, the MASP-2 inhibitory agent is a MASP-2
monoclonal
antibody, or fragment thereof that specifically binds to MASP-2. The binding
affinity of
.. the MASP-2 inhibitory agent can be determined using a suitable binding
assay.
The MASP-2 polypeptide exhibits a molecular structure similar to MASP-1,
MASP-3, and Clr and Cis, the proteases of the Cl complement system. The cDNA
molecule set forth in SEQ ID NO:4 encodes a representative example of MASP-2
(consisting of the amino acid sequence set forth in SEQ ID NO:5) and provides
the
human MASP-2 polypeptide with a leader sequence (aa 1-15) that is cleaved
after
secretion, resulting in the mature form of human MASP-2 (SEQ ID NO:6). As
shown in
FIGURE 2, the human MASP 2 gene encompasses twelve exons. The human MASP-2
cDNA is encoded by exons B, C, D, F, G, H, I, J, K AND L. An alternative
splice results
in a 20 kDa protein termed MBL-associated protein 19 ("MAp19", also referred
to as
"sMAP") (SEQ ID NO:2), encoded by (SEQ ID NO:1) arising from exons B, C, D and
E
as shown in FIGURE 2. The cDNA molecule set forth in SEQ ID NO:50 encodes the
murine MASP-2 (consisting of the amino acid sequence set forth in SEQ ID
NO:51) and
provides the murine MASP-2 polypeptide with a leader sequence that is cleaved
after
secretion, resulting in the mature form of murine MASP-2 (SEQ ID NO:52). The
cDNA
molecule set forth in SEQ ID NO:53 encodes the rat MASP-2 (consisting of the
amino
acid sequence set forth in SEQ ID NO:54) and provides the rat MASP-2
polypeptide with
a leader sequence that is cleaved after secretion, resulting in the mature
form of rat
MASP-2 (SEQ ID NO:55).
-62-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Those skilled in the art will recognize that the sequences disclosed in SEQ ID
NO:4, SEQ ID NO:50 and SEQ ID NO:53 represent single alleles of human, murine
and
rat MASP-2 respectively, and that allelic variation and alternative splicing
are expected to
occur. Allelic variants of the nucleotide sequences shown in SEQ ID NO:4, SEQ
ID
NO:50 and SEQ ID NO:53, including those containing silent mutations and those
in
which mutations result in amino acid sequence changes, are within the scope of
the
present invention. Allelic variants of the MASP-2 sequence can be cloned by
probing
cDNA or genomic libraries from different individuals according to standard
procedures.
The domains of the human MASP-2 protein (SEQ ID NO:6) are shown in
FIGURE 1 and 2A and include an N-terminal Clr/C1s/sea urchin Vegf/bone
morphogenic protein (CUBI) domain (aa 1-121 of SEQ ID NO:6), an epidermal
growth
factor-like domain (aa 122-166), a second CUBI domain (aa 167-293), as well as
a
tandem of complement control protein domains and a serine protease domain.
Alternative splicing of the MASP 2 gene results in MAp19 shown in FIGURE 1.
MAp19
is a nonenzymatic protein containing the N-terminal CUBI-EGF region of MASP-2
with
four additional residues (EQSL) derived from exon E as shown in FIGURE 1.
Several proteins have been shown to bind to, or interact with MASP-2 through
protein-to-protein interactions. For example, MASP-2 is known to bind to, and
form
Ca2+ dependent complexes with, the lectin proteins MBL, H-ficolin and L-
ficolin. Each
MASP-2/lectin complex has been shown to activate complement through the
MASP-2-dependent cleavage of proteins C4 and C2 (Ikeda, K., et al., J. Biol.
Chem. 262:7451-7454, 1987; Matsushita, M., et al., J. Exp. Med. 176:1497-2284,
2000;
Matsushita, M., et al., J. Immunol. /68:3502-3506, 2002). Studies have shown
that the
CUBI-EGF domains of MASP-2 are essential for the association of MASP-2 with
MBL
(Thielens, N.M., et al., J. Immunol. /66:5068, 2001). It has also been shown
that the
CUBIEGFCUBII domains mediate dimerization of MASP-2, which is required for
formation of an active MBL complex (Wallis, R., et al,, .1. Biol. Chem.
275:30962-30969,
2000). Therefore, MASP-2 inhibitory agents can be identified that bind to or
interfere
with MASP-2 target regions known to be important for MASP-2-dependent
complement
activation.
ANTI-MASP-2 ANTIBODIES
In some embodiments of this aspect of the invention, the MASP-2 inhibitory
agent comprises an anti-MASP-2 antibody that inhibits the MASP-2-dependent
-63-
complement activation system. The anti-MASP-2 antibodies useful in this aspect
of the
invention include polyclonal, monoclonal or recombinant antibodies derived
from any
antibody producing mammal and may be multispecific, chimeric, humanized,
anti-idiotype, and antibody fragments. Antibody fragments include Fab, Fab',
F(ab)2,
F(ab')2, Fv fragments, scFy fragments and single-chain antibodies as further
described
herein.
MASP-2 antibodies can be screened for the ability to inhibit MASP-2-dependent
complement activation system and for antifibrotic activity and/or the ability
to inhibit
renal damage associated with proteinuria or Adriamycin-induced nephropathy
using the
assays described herein. Several MASP-2 antibodies have been described in the
literature
and some have been newly generated, some of which are listed below in TABLE 1.
For
example, as described in Examples 10 and 11 herein, anti-MASP-2 Fab2
antibodies have
been identified that block MASP-2-dependent complement activation. As
described in
Example 12, and also described in W02012/151481,
fully human MASP-2 say antibodies (e.g., 0MS646) have been identified
that block MASP-2-dependent complement activation. As described in Example 13,
and
also described in W02014/144542,
SGMT-2 peptide-bearing MASP-2 antibodies and fragments thereof with MA SP-2
inhibitory activity were generated by fusing the SGMI-2 peptide amino acid
sequence
(SEQ ID NO:72, 73 or 74) onto the amino or carboxy termini of the heavy and/or
light
chains of a human MASP-2 antibody (e.g., 0MS646-SGMI-2).
Accordingly, in one embodiment, the MASP-2 inhibitory agent for use in the
methods of the invention comprises a human antibody such as, for example
0MS646.
Accordingly, in one embodiment, a MASP-2 inhibitory agent for use in the
compositions
and methods of the claimed invention comprises a human antibody that binds a
polypeptide consisting of human MASP-2 (SEQ ID NO:6), wherein the antibody
comprises: (I) (a) a heavy-chain variable region comprising: i) a heavy-chain
CDR-H1
comprising the amino acid sequence from 31-35 of SEQ ID NO:67; and ii) a heavy-
chain
CDR-H2 comprising the amino acid sequence from 50-65 of SEQ ID NO:67; and iii)
a
heavy-chain CDR-H3 comprising the amino acid sequence froni 95-107 of SEQ ID
NO:67 and b) a light-chain variable region comprising: i) a light-chain CDR-L1
comprising the amino acid sequence from 24-34 of SEQ ID NO:70; and ii) a light-
chain
CDR-L2 comprising the amino acid sequence from 50-56 of SEQ ID NO:70; and iii)
a
-64-
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
light-chain CDR-L3 comprising the amino acid sequence from 89-97 of SEQ ID
NO:70,
or (II) a variant thereof comprising a heavy-chain variable region with at
least 90%
identity to SEQ ID NO:67 (e.g., at least 91%, at least 92%, at least 93%, at
least 94%, at
least 95%, at least 96%, at least 97%, at least 98%, at least 99% identity to
SEQ ID
NO:67) and a light-chain variable region with at least 90% identity (e.g., at
least 91%, at
least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at least
97%, at least
98%, at least 99% identity to SEQ ID NO:70.
In some embodiments, the method comprises administering to the subject a
composition comprising an amount of a MASP-2 inhibitory antibody, or antigen
binding
fragment thereof, comprising a heavy-chain variable region comprising the
amino acid
sequence set forth as SEQ ID NO:67 and a light-chain variable region
comprising the
amino acid sequence set forth as SEQ ID NO:70.
In some embodiments, the method comprises administering to the subject a
composition comprising a MASP-2 inhibitory antibody, or antigen binding
fragment
thereof, that specifically recognizes at least part of an epitope on human
MASP-2
recognized by reference antibody 0M5646 comprising a heavy-chain variable
region as
set forth in SEQ ID NO:67 and a light-chain variable region as set forth in
SEQ ID
NO:70. In one embodiment, the MASP-2 inhibitory agent for use in the methods
of the
invention comprises the human antibody 0M5646.
-65-
CA 03039927 2019-04-09
WO 2018/071701
PCT/US2017/056386
TABLE 1: EXEMPLARY MASP-2 SPECIFIC ANTIBODIES
ANTIGEN ANTIBODY TYPE REFERENCE
Recombinant Rat Polyclonal Peterson, S.V., et al., Mol.
MASP-2 Immunol. 37:803-811, 2000
Recombinant human Rat MoAb Moller-Kristensen, M., et al., J. of
CCP1/2-SP fragment (subclass IgG1) Immunol. Methods 282:159-167,
(MoAb 8B5) 2003
Recombinant human Rat MoAb Moller-Kristensen, M., et al., J. of
MAp19 (MoAb (subclass IgG1) Immunol. Methods 282:159-167,
6G12) (cross reacts 2003
with MASP-2) =
hMASP-2 Mouse MoAb (S/P) Peterson, S.V., et al., Mol.
Mouse MoAb (N-term) Immunol. 35:409, April 1998
hMASP-2 rat MoAb: Nimoab101, WO 2004/106384
(CCP1-CCP2-SP produced by hybridoma
domain cell line 03050904
(ECACC)
hMASP-2 (full murine MoAbs: WO 2004/106384
length-his tagged)
NimoAb104, produced
by hybridoma cell line
M0545YM035 (DSMZ)
NimoAb108, produced
by hybridoma cell line
M0545YM029 (DSMZ)
NimoAb109 produced
by hybridoma cell line
M0545YM046 (DSMZ)
NimoAb110 produced
by hybridoma cell line
M0545YM048 (DSMZ)
Rat MASP-2 (full- MASP-2 Fab2 antibody Example 10
length) fragments
hMASP-2 (full- Fully human scFy- clones Example 12 and W02012/151481
length)
hMASP-2 (full- SGMI-2 peptide bearing Example 13 and W02014/144542
length) MASP-2 antibodies
-66-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
ANTI-MASP-2 ANTIBODIES WITH REDUCED EFFECTOR FUNCTION
In some embodiments of this aspect of the invention, the anti-MASP-2
antibodies
have reduced effector function in order to reduce inflammation that may arise
from the
activation of the classical complement pathway. The ability of IgG molecules
to trigger
the classical complement pathway has been shown to reside within the Fc
portion of the
molecule (Duncan, A.R., et al., Nature 332:738-740 1988). IgG molecules in
which the
Fc portion of the molecule has been removed by enzymatic cleavage are devoid
of this
effector function (see Harlow, Antibodies: A Laboratory Manual, Cold Spring
Harbor
Laboratory, New York, 1988). Accordingly, antibodies with reduced effector
function
can be generated as the result of lacking the Fc portion of the molecule by
having a
genetically engineered Fc sequence that minimizes effector function, or being
of either
the human IgG2 or IgG4 isotype.
Antibodies with reduced effector function can be produced by standard
molecular
biological manipulation of the Fc portion of the IgG heavy chains as described
herein and
also described in Jolliffe et al., Intl Rev. Immunol. /0.241-250, 1993, and
Rodrigues
et al., J. Irnmunol. /5/:6954-6961, 1998. Antibodies with reduced effector
function also
include human IgG2 and IgG4 isotypes that have a reduced ability to activate
complement and/or interact with Fc receptors (Ravetch, J.V., et al., Annu.
Rev.
Immunol 9:457-492, 1991; Isaacs, J.D., et al., J. Immunol. /48:3062-3071,
1992; van de
Winkel, J.G., et al., Immunol. Today 14:215-221, 1993). Humanized or fully
human
antibodies specific to human MASP-2 comprised of IgG2 or IgG4 isotypes can be
produced by one of several methods known to one of ordinary skilled in the
art, as
described in Vaughan, T.J., et al., Nature Biotechnical 16:535-539, 1998.
PRODUCTION OF ANTI-MASP-2 ANTIBODIES
Anti-MASP-2 antibodies can be produced using MASP-2 polypeptides (e.g., full
length MASP-2) or using antigenic MASP-2 epitope-bearing peptides (e.g., a
portion of
the MASP-2 polypeptide). Immunogenic peptides may be as small as five amino
acid
residues. For example, the MASP-2 polypeptide including the entire amino acid
sequence of SEQ ID NO:6 may be used to induce anti-MASP-2 antibodies useful in
the
method of the invention. Particular MASP-2 domains known to be involved in
protein-protein interactions, such as the CUBI, and CUBIEGF domains, as well
as the
region encompassing the serine-protease active site, may be expressed as
recombinant
polypeptides as described in Example 3 and used as antigens. In addition,
peptides
-67-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
comprising a portion of at least 6 amino acids of the MASP-2 polypeptide (SEQ
ID
NO:6) are also useful to induce MASP-2 antibodies. Additional examples of MASP-
2
derived antigens useful to induce MASP-2 antibodies are provided below in
TABLE 2.
The MASP-2 peptides and polypeptides used to raise antibodies may be isolated
as
natural polypeptides, or recombinant or synthetic peptides and catalytically
inactive
recombinant polypeptides, such as MASP-2A, as further described herein. In
some
embodiments of this aspect of the invention, anti-MASP-2 antibodies are
obtained using a
transgenic mouse strain as described herein.
Antigens useful for producing anti-MASP-2 antibodies also include fusion
polypeptides, such as fusions of MASP-2 or a portion thereof with an
immunoglobulin
polypeptide or with maltose-binding protein. The polypeptide immunogen may be
a
full-length molecule or a portion thereof. If the polypeptide portion is
hapten-like, such
portion may be advantageously joined or linked to a macromolecular carrier
(such as
keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA) or tetanus toxoid)
for
immunization.
TABLE 2: MASP-2 DERIVED ANTIGENS
SEQ ID NO: Amino Acid Sequence
SEQ ID NO:6 Human MASP-2 protein
SEQ ID NO:51 Murine MASP-2 protein
SEQ ID NO:8 CUBI domain of human MASP-2
(aa 1-121 of SEQ ID NO:6)
SEQ ID NO:9 CUBIEGF domains of human MASP-2
(aa 1-166 of SEQ ID NO:6)
SEQ ID NO:10 CUBIEGFCUBlI domains of human MASP-2
(aa 1-293 of SEQ ID NO:6)
SEQ ID NO:11 EGF domain of human MASP-2
(aa 122-166 of SEQ ID NO:6)
SEQ ID NO:12 Serine-Protease domain of human MASP-2
(aa 429-671 of SEQ ID NO:6)
SEQ ID NO:13 Serine-Protease inactivated mutant form
GKDSCRGDAGGALVFL (aa 610-625 of SEQ ID NO:6 with mutated Ser 618)
SEQ ID NO:14 Human CUBI peptide
TPLGPKWPEPVFGRL
-68-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
SEQ ID NO: Amino Acid Sequence
SEQ ID NO:15: Human CUBI peptide
TAPPGYRLRLYFTHFDLEL
SHLCEYDEVKLSSGAKVL
ATLCGQ
SEQ ID NO:16: MBL binding region in human CUBI domain
TFRSDYSN
SEQ ID NO:17: MBL binding region in human CUBI domain
FYSLGSSLDITFRSDYSNEK
PFTGF
SEQ ID NO:18 EGF peptide
IDECQVAPG
SEQ ID NO:19 Peptide from serine-protease active site
ANMLCAGLESGGKDSCRG
DSGGALV
POLYCLONAL ANTIBODIES
Polyclonal antibodies against MASP-2 can be prepared by immunizing an animal
with MASP-2 polypeptide or an immunogenic portion thereof using methods well
known
to those of ordinary skill in the art. See, for example, Green et al.,
"Production of
Polyclonal Antisera," in Immunochernical Protocols (Manson, ed.), page 105.
The
immunogenicity of a MASP-2 polypeptide can be increased through the use of an
adjuvant, including mineral gels, such as aluminum hydroxide or Freund's
adjuvant
(complete or incomplete), surface active substances such as lysolecithin,
pluronic polyols,
polyanions, oil emulsions, keyhole limpet hemocyanin and dinitrophenol.
Polyclonal
antibodies are typically raised in animals such as horses, cows, dogs,
chicken, rats, mice,
rabbits, guinea pigs, goats, or sheep. Alternatively, an anti-MASP-2 antibody
useful in
the present invention may also be derived from a subhuman primate. General
techniques
for raising diagnostically and therapeutically useful antibodies in baboons
may be found,
for example, in Goldenberg et al., International Patent Publication No. WO
91/11465, and
in Losman, Mi., et al., Int. J. Cancer 46:310, 1990. Sera containing
immunologically
active antibodies are then produced from the blood of such immunized animals
using
standard procedures well known in the art.
MONOCLONAL ANTIBODIES
In some embodiments, the MASP-2 inhibitory agent is an anti-MASP-2
monoclonal antibody. Anti-MASP-2 monoclonal antibodies are highly specific,
being
-69-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
directed against a single MASP-2 epitope. As used herein, the modifier
"monoclonal"
indicates the character of the antibody as being obtained from a substantially
homogenous
population of antibodies, and is not to be construed as requiring production
of the
antibody by any particular method. Monoclonal antibodies can be obtained using
any
technique that provides for the production of antibody molecules by continuous
cell lines
in culture, such as the hybridoma method described by Kohler, G., et al.,
Nature 256:495,
1975, or they may be made by recombinant DNA methods (see, e.g., U.S. Patent
No. 4,816,567 to Cabilly). Monoclonal antibodies may also be isolated from
phage
antibody libraries using the techniques described in Clackson, T., et al.,
Nature 352:624-628, 1991, and Marks, J.D., et al.,i Mot Biol. 222:581-597,
1991. Such
antibodies can be of any immunoglobulin class including IgG, IgM, IgE, IgA,
IgD and
any subclass thereof.
For example, monoclonal antibodies can be obtained by injecting a suitable
mammal (e.g., a BALB/c mouse) with a composition comprising a MASP-2
polypeptide
or portion thereof. After a predetermined period of time, splenocytes are
removed from
the mouse and suspended in a cell culture medium. The splenocytes are then
fused with
an immortal cell line to form a hybridoma. The formed hybridomas are grown in
cell
culture and screened for their ability to produce a monoclonal antibody
against MASP-2.
Examples further describing the production of anti-MASP-2 monoclonal
antibodies are
provided herein (see also Current Protocols in Immunology, Vol. 1., John Wiley
& Sons,
pages 2.5.1-2.6.7, 1991.)
Human monoclonal antibodies may be obtained through the use of transgenic
mice that have been engineered to produce specific human antibodies in
response to
antigenic challenge. In this technique, elements of the human immunoglobulin
heavy and
light chain locus are introduced into strains of mice derived from embryonic
stem cell
lines that contain targeted disruptions of the endogenous immunoglobulin heavy
chain
and light chain loci. The transgenic mice can synthesize human antibodies
specific for
human antigens, such as the MASP-2 antigens described herein, and the mice can
be used
to produce human MASP-2 antibody-secreting hybridomas by fusing B-cells from
such
animals to suitable myeloma cell lines using conventional Kohler-Milstein
technology as
further described herein. Transgenic mice with a human immunoglobulin genome
are
commercially available (e.g., from Abgenix, Inc., Fremont, CA, and Medarex,
Inc.,
Annandale, N.J.). Methods for obtaining human antibodies from transgenic mice
are
-70-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
described, for example, by Green, L.L., etal., Nature Genet. 7:13, 1994;
Lonberg, N.,
et al., Nature 368:856, 1994; and Taylor, L.D., etal., Int. Immun. 6:579,
1994.
Monoclonal antibodies can be isolated and purified from hybridoma cultures by
a
variety of well-established techniques. Such
isolation techniques include affinity
chromatography with Protein-A Sepharose, size-exclusion chromatography, and
ion-exchange chromatography (see, for example, Coligan at pages 2.7.1-2.7.12
and
pages 2.9.1-2.9.3; Baines et al., "Purification of Immunoglobulin G (IgG)," in
Methods in
Molecular Biology, The Humana Press, Inc., Vol. 10, pages 79-104, 1992).
Once produced, polyclonal, monoclonal or phage-derived antibodies are first
tested for specific MASP-2 binding. A variety of assays known to those skilled
in the art
may be utilized to detect antibodies which specifically bind to MASP-2.
Exemplary
assays include Western blot or immunoprecipitation analysis by standard
methods (e.g.,
as described in Ausubel et al.), immunoelectrophoresis, enzyme-linked immuno-
sorbent
assays, dot blots, inhibition or competition assays and sandwich assays (as
described in
Harlow and Land, Antibodies: A Laboratory Manual, Cold Spring Harbor
Laboratory
Press, 1988). Once antibodies are identified that specifically bind to MASP-2,
the
anti-MASP-2 antibodies are tested for the ability to function as a MASP-2
inhibitory
agent in one of several assays such as, for example, a lectin-specific C4
cleavage assay
(described in Example 2), a C3b deposition assay (described in Example 2) or a
C4b
deposition assay (described in Example 2).
The affinity of anti-MASP-2 monoclonal antibodies can be readily determined by
one of ordinary skill in the art (see, e.g., Scatchard, A., NY Acad. Sci.
5/:660-672, 1949).
In one embodiment, the anti-MASP-2 monoclonal antibodies useful for the
methods of
the invention bind to MASP-2 with a binding affinity of <100 nM, preferably
<10 nM
and most preferably <2 nM.
CHIMERIC/HUMANIZED ANTIBODIES
Monoclonal antibodies useful in the method of the invention include chimeric
antibodies in which a portion of the heavy and/or light chain is identical
with or
homologous to corresponding sequences in antibodies derived from a particular
species
or belonging to a particular antibody class or subclass, while the remainder
of the chain(s)
is identical with or homologous to corresponding sequences in antibodies
derived from
another species or belonging to another antibody class or subclass, as well as
fragments
-71-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
of such antibodies (U.S. Patent No. 4,816,567, to Cabilly; and Morrison, S.L.,
et al.,
Proc. Nat7 Acad. Sci. USA 8/:6851-6855, 1984).
One form of a chimeric antibody useful in the invention is a humanized
monoclonal anti-MASP-2 antibody. Humanized forms of non-human (e.g., murine)
.. antibodies are chimeric antibodies, which contain minimal sequence derived
from
non-human immunoglobulin. Humanized monoclonal antibodies are produced by
transferring the non-human (e.g., mouse) complementarity determining regions
(CDR),
from the heavy and light variable chains of the mouse immunoglobulin into a
human
variable domain. Typically, residues of human antibodies are then substituted
in the
framework regions of the non-human counterparts. Furthermore, humanized
antibodies
may comprise residues that are not found in the recipient antibody or in the
donor
antibody. These modifications are made to further refine antibody performance.
In
general, the humanized antibody will comprise substantially all of at least
one, and
typically two variable domains, in which all or substantially all of the
hypervariable loops
.. correspond to those of a non-human immunoglobulin and all or substantially
all of the Fv
framework regions are those of a human immunoglobulin sequence. The humanized
antibody optionally also will comprise at least a portion of an immunoglobulin
constant
region (Fe), typically that of a human immunoglobulin. For further details,
see Jones,
P.T., et al., Nature 321:522-525, 1986; Reichmann, L., et al., Nature 332:323-
329, 1988;
.. and Presta, Curl-. Op. Struct. Biol. 2:593-596, 1992.
The humanized antibodies useful in the invention include human monoclonal
antibodies including at least a MASP-2 binding CDRH3 region. In addition, the
Fe
portions may be replaced so as to produce IgA or IgM as well as human IgG
antibodies.
Such humanized antibodies will have particular clinical utility because they
will
specifically recognize human MASP-2 but will not evoke an immune response in
humans
against the antibody itself. Consequently, they are better suited for in vivo
administration
in humans, especially when repeated or long-term administration is necessary.
An example of the generation of a humanized anti-MASP-2 antibody from a
murine anti-MASP-2 monoclonal antibody is provided herein in Example 6.
Techniques
for producing humanized monoclonal antibodies are also described, for example,
by
Jones, P.T., et al., Nature 321:522, 1986; Carter, P., et al., Proc. Nat'l.
Acad. Sci.
USA 89:4285, 1992; Sandhu, J.S., Cr/i. Rev. Biotech. /2:437, 1992; Singer,
et al., J.
1711172111. /50:2844, 1993; Sudhir (ed.), Antibody Engineering Protocols,
Humana Press,
-72-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Inc., 1995; Kelley, ''Engineering Therapeutic Antibodies," in Protein
Engineering:
Principles and Practice, Cleland et al. (eds.), John Wiley & Sons, Inc., pages
399-434,
1996; and by U.S. Patent No. 5,693,762, to Queen, 1997. In addition, there are
commercial entities that will synthesize humanized antibodies from specific
murine
antibody regions, such as Protein Design Labs (Mountain View, CA).
RECOMBINANT ANTIBODIES
Anti-MASP-2 antibodies can also be made using recombinant methods. For
example, human antibodies can be made using human immunoglobulin expression
libraries (available for example, from Stratagene, Corp., La Jolla, CA) to
produce
fragments of human antibodies (VH, VL, Fv, Fd, Fab or F(ab1)2). These
fragments are
then used to construct whole human antibodies using techniques similar to
those for
producing chimeric antibodies.
ANTI-IDIOTYPE ANTIBODIES
Once anti-MASP-2 antibodies are identified with the desired inhibitory
activity,
.. these_antibodies can be used to generate anti-idiotype antibodies that
resemble a portion
of MASP-2 using techniques that are well known in the art. See, e.g.,
Greenspan, N.S.,
et al., FASEB 7:437, 1993. For example, antibodies that bind to MASP-2 and
competitively inhibit a MASP-2 protein interaction required for complement
activation
can be used to generate anti-idiotypes that resemble the MBL binding site on
MASP-2
protein and therefore bind and neutralize a binding ligand of MASP-2 such as,
for
example, MBL.
IM MUNO GLOB ULIN FRAGMENT S
The MASP-2 inhibitory agents useful in the method of the invention encompass
not only intact immunoglobulin molecules but also the well known fragments
including
Fab, Fab', F(ab)2, F(ab')2 and Fv fragments, scFy fragments, diabodies, linear
antibodies,
single-chain antibody molecules and multispecific antibodies formed from
antibody
fragments.
It is well known in the art that only a small portion of an antibody molecule,
the
paratope, is involved in the binding of the antibody to its epitope (see,
e.g., Clark, W.R.,
The Experimental Foundations of Modern Immunology, Wiley & Sons, Inc., NY,
1986).
The pFc and Fc regions of the antibody are effectors of the classical
complement
pathway, but are not involved in antigen binding. An antibody from which the
pFc'
region has been enzymatically cleaved, or which has been produced without the
pFc'
-73-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
region, is designated an F(ab')2 fragment and retains both of the antigen
binding sites of
an intact antibody. An isolated F(abl)2 fragment is referred to as a bivalent
monoclonal
fragment because of its two antigen binding sites. Similarly, an antibody from
which the
Fc region has been enzymatically cleaved, or which has been produced without
the Fc
region, is designated a Fab fragment, and retains one of the antigen binding
sites of an
intact antibody molecule.
Antibody fragments can be obtained by proteolytic hydrolysis, such as by
pepsin
or papain digestion of whole antibodies by conventional methods. For example,
antibody
fragments can be produced by enzymatic cleavage of antibodies with pepsin to
provide a
5S fragment denoted F(ab')2. This fragment can be further cleaved using a
thiol reducing
agent to produce 3.5S Fab' monovalent fragments. Optionally, the cleavage
reaction can
be performed using a blocking group for the sulfhydryl groups that result from
cleavage
of disulfide linkages. As an alternative, an enzymatic cleavage using pepsin
produces
two monovalent Fab fragments and an Fc fragment directly. These methods are
described, for example, U.S. Patent No. 4,331,647 to Goldenberg; Nisonoff, A.,
et al.,
Arch. Biochem. Biophys. 89:230, 1960; Porter, R.R., Biochem. J. 73:119, 1959;
Edelman,
et al., in Methods in Enzymology /:422, Academic Press, 1967; and by Coligan
at pages
2.8.1-2.8.10 and 2.10.-2.10.4.
In some embodiments, the use of antibody fragments lacking the Fc region are
preferred to avoid activation of the classical complement pathway which is
initiated upon
binding Fc to the Fey receptor. There are several methods by which one can
produce a
MoAb that avoids Fcy receptor interactions. For example, the Fc region of a
monoclonal
antibody can be removed chemically using partial digestion by proteolytic
enzymes (such
as ficin digestion), thereby generating, for example, antigen-binding antibody
fragments
such as Fab or F(ab)2 fragments (Mariani, M., et al., Mol. Immitnol. 28:69-71,
1991).
Alternatively, the human y4 IgG isotype, which does not bind Fcy receptors,
can be used
during construction of a humanized antibody as described herein. Antibodies,
single
chain antibodies and antigen-binding domains that lack the Fc domain can also
be
engineered using recombinant techniques described herein.
SINGLE-CHAIN ANTIBODY FRAGMENTS
Alternatively, one can create single peptide chain binding molecules specific
for
MASP-2 in which the heavy and light chain Fv regions are connected. The Fy
fragments
-74-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
may be connected by a peptide linker to form a single-chain antigen binding
protein
(scFv). These single-chain antigen binding proteins are prepared by
constructing a
structural gene comprising DNA sequences encoding the VH and VL domains which
are
connected by an oligonucleotide. The structural gene is inserted into an
expression
vector, which is subsequently introduced into a host cell, such as E. coil.
The
recombinant host cells synthesize a single polypeptide chain with a linker
peptide
bridging the two V domains. Methods for producing scFvs are described for
example, by
Whitlow, et al., "Methods: A Companion to Methods in Enzymology" 2:97, 1991;
Bird,
et al., Science 242:423, 1988; U.S. Patent No. 4,946,778, to Ladner; Pack, P.,
et al.,
Bio/Technology I 1:1271, 1993.
As an illustrative example, a MASP-2 specific scFv can be obtained by exposing
lymphocytes to MASP-2 polypeptide in vitro and selecting antibody display
libraries in
phage or similar vectors (for example, through the use of immobilized or
labeled
MASP-2 protein or peptide). Genes encoding polypeptides having potential MASP-
2
polypeptide binding domains can be obtained by screening random peptide
libraries
displayed on phage or on bacteria such as E. coll. These random peptide
display libraries
can be used to screen for peptides which interact with MASP-2. Techniques for
creating
and screening such random peptide display libraries are well known in the art
(U.S.
Patent No. 5,223,409, to Lardner; U.S. Patent No. 4,946,778, to Ladner; U.S.
Patent
No. 5,403,484, to Lardner; U.S. Patent No. 5,571,698, to Lardner; and Kay et
al., Phage
Display of Peptides and Proteins Academic Press, Inc., 1996) and random
peptide
display libraries and kits for screening such libraries are available
commercially, for
instance from CLONTECH Laboratories, Inc. (Palo Alto, Calif.), Invitrogen Inc.
(San
Diego, Calif), New England Biolabs, Inc. (Ipswich, Mass.), and Pharmacia LKB
Biotechnology Inc. (Piscataway, N.J.).
Another form of an anti-MASP-2 antibody fragment useful in this aspect of the
invention is a peptide coding for a single complementarity-determining region
(CDR) that
binds to an epitope on a MASP-2 antigen and inhibits MASP-2-dependent
complement
activation. CDR peptides ("minimal recognition units") can be obtained by
constructing
genes encoding the CDR of an antibody of interest. Such genes are prepared,
for
example, by using the polymerase chain reaction to synthesize the variable
region from
RNA of antibody-producing cells (see, for example, Larrick et al., Methods: A
Companion to Methods in Enzymology 2:106, 1991; Courtenay-Luck, "Genetic
-75-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Manipulation of Monoclonal Antibodies," in Monoclonal Antibodies: Production,
Engineering and Clinical Application, Ritter et al. (eds.), page 166,
Cambridge
University Press, 1995; and Ward et al., "Genetic Manipulation and Expression
of
Antibodies," in Monoclonal Antibodies: Principles and Applications, Birch et
al. (eds.),
page 137, Wiley-Liss, Inc., 1995).
The MASP-2 antibodies described herein are administered to a subject in need
thereof to inhibit MASP-2-dependent complement activation. In some
embodiments, the
MASP-2 inhibitory agent is a high-affinity human or humanized monoclonal
anti-MA SP-2 antibody with reduced effector function.
PEP TIDE INHIBITORS
In some embodiments of this aspect of the invention, the MASP-2 inhibitory
agent comprises isolated MASP-2 peptide inhibitors, including isolated natural
peptide
inhibitors and synthetic peptide inhibitors that inhibit the MASP-2-dependent
complement activation system. As used herein, the term "isolated MASP-2
peptide
inhibitors" refers to peptides that inhibit MASP-2 dependent complement
activation by
binding to, competing with MASP-2 for binding to another recognition molecule
(e.g.,
MBL, M-ficolin, or L-ficolin) in the lectin pathway, and/or
directly interacting
with MASP-2 to inhibit MASP-2-dependent complement activation that are
substantially
pure and are essentially free of other substances with which they may be found
in nature
to an extent practical and appropriate for their intended use.
Peptide inhibitors have been used successfully in vivo to interfere with
protein-protein interactions and catalytic sites. For example, peptide
inhibitors to
adhesion molecules structurally related to LFA-1 have recently been approved
for clinical
use in coagulopathies (Ohman, EM., et al., European Heart J. /6:50-55, 1995).
Short
linear peptides (<30 amino acids) have been described that prevent or
interfere with
integrin-dependent adhesion (Murayama, 0., et al., I Biochem. 120:445-51,
1996).
Longer peptides, ranging in length from 25 to 200 amino acid residues, have
also been
used successfully to block integrin-dependent adhesion (Zhang, L., et al., I
Biol.
Chem. 271(47):29953-57, 1996). In general, longer peptide inhibitors have
higher
affinities and/or slower off-rates than short peptides and may therefore be
more potent
inhibitors. Cyclic peptide inhibitors have also been shown to be effective
inhibitors of
integrins in vivo for the treatment of human inflammatory disease (Jackson,
D.Y., et al.,
J. Med. Chem. 40:3359-68, 1997). One method of producing cyclic peptides
involves the
-76-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
synthesis of peptides in which the terminal amino acids of the peptide are
cysteines,
thereby allowing the peptide to exist in a cyclic form by disulfide bonding
between the
terminal amino acids, which has been shown to improve affinity and half-life
in vivo for
the treatment of hematopoietic neoplasms (e.g., U.S. Patent No. 6,649,592, to
Larson).
SYNTHETIC MASP-2 PEPTIDE INHIBITORS
MASP-2 inhibitory peptides useful in the methods of this aspect of the
invention
are exemplified by amino acid sequences that mimic the target regions
important for
MASP-2 function. The inhibitory peptides useful in the practice of the methods
of the
invention range in size from about 5 amino acids to about 300 amino acids.
TABLE 3
provides a list of exemplary inhibitory peptides that may be useful in the
practice of this
aspect of the present invention. A candidate MASP-2 inhibitory peptide may be
tested
for the ability to function as a MASP-2 inhibitory agent in one of several
assays
including, for example, a lectin specific C4 cleavage assay (described in
Example 2), and
a C3b deposition assay (described in Example 2).
In some embodiments, the MASP-2 inhibitory peptides are derived from MASP-2
polypeptides and are selected from the full length mature MASP-2 protein (SEQ
ID
NO:6), or from a particular domain of the MASP-2 protein such as, for example,
the
CUBI domain (SEQ ID NO:8), the CUBIEGF domain (SEQ ID NO:9), the EGF domain
(SEQ ID NO:11), and the serine protease domain (SEQ ID NO:12). As previously
described, the CUBEGFCUBII regions have been shown to be required for
dimerization
and binding with MBL (Thielens et al., supra). In particular, the peptide
sequence
TFRSDYN (SEQ ID NO:16) in the CUBI domain of MASP-2 has been shown to be
involved in binding to MBL in a study that identified a human carrying a
homozygous
mutation at Asp105 to Gly105, resulting in the loss of MASP-2 from the MBL
complex
(Stengaard-Pedersen, K., et al., New Englandi Med. 349:554-560, 2003)
In some embodiments, MASP-2 inhibitory peptides are derived from the lectin
proteins that bind to MASP-2 and are involved in the lectin complement
pathway.
Several different lectins have been identified that are involved in this
pathway, including
mannan-binding lectin (MBL), L-ficolin, M-ficolin and H-ficolin. (Ikeda, K.,
et al.,
1 Biol. Chem. 262:7451-7454, 1987; Matsushita, M., et al., I Exp. Med.
176:1497-2284,
2000; Matsushita, M., et al., I. Immunol. /68:3502-3506, 2002). These lectins
are present
in serum as oligomers of homotrimeric subunits, each having N-terminal
collagen-like
fibers with carbohydrate recognition domains. These different lectins have
been shown
-77-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
to bind to MASP-2, and the lectin/MASP-2 complex activates complement through
cleavage of proteins C4 and C2. H-ficolin has an amino-terminal region of 24
amino
acids, a collagen-like domain with 11 Gly-Xaa-Yaa repeats, a neck domain of 12
amino
acids, and a fibrinogen-like domain of 207 amino acids (Matsushita, M., et
al.,
J. ImmunoL /68:3502-3506, 2002). H-ficolin binds to GlcNAc and agglutinates
human
erythrocytes coated with LPS derived from S. typhinmrium, S. minnesota and E.
colt.
H-ficolin has been shown to be associated with MASP-2 and MAp19 and activates
the
lectin pathway. Id. L-ficolin/P35 also binds to GlcNAc and has been shown to
be
associated with MASP-2 and MAp19 in human serum and this complex has been
shown
to activate the lectin pathway (Matsushita, M., et al., I Immunol. 164:2281,
2000).
Accordingly, MASP-2 inhibitory peptides useful in the present invention may
comprise a
region of at least 5 amino acids selected from the MBL protein (SEQ ID NO:21),
the
H-ficolin protein (Genbank accession number NM 173452), the M-ficolin protein
(Genbank accession number 000602) and the L-ficolin protein (Genbank accession
number NM 015838).
More specifically, scientists have identified the MASP-2 binding site on MBL
to
be within the 12 Gly-X-Y triplets "GKD GRD GTK GEK GEP GQG LRG LQG POG
KLG POG NOG PSG SOG PKG QKG DOG KS" (SEQ ID NO:26) that lie between the
hinge and the neck in the C-terminal portion of the collagen-like domain of
MBP
(Wallis, R., et al., J. Biol. Chem. 279:14065, 2004). This MASP-2 binding site
region is
also highly conserved in human H-ficolin and human L-ficolin. A consensus
binding site
has been described that is present in all three lectin proteins comprising the
amino acid
sequence "OGK-X-GP" (SEQ ID NO:22) where the letter "0" represents
hydroxyproline
and the letter "X" is a hydrophobic residue (Wallis et al., 2004, supra).
Accordingly, in
some embodiments, MASP-2 inhibitory peptides useful in this aspect of the
invention are
at least 6 amino acids in length and comprise SEQ ID NO:22. Peptides derived
from
MBL that include the amino acid sequence "GLR GLQ GPO GKL GPO G" (SEQ ID
NO:24) have been shown to bind MASP-2 in vitro (Wallis, et al., 2004, supra).
To
enhance binding to MASP-2, peptides can be synthesized that are flanked by two
GPO
triplets at each end (''GPO GPO GLR GLQ GPO GKL GPO GGP OGP 0" SEQ ID
NO:25) to enhance the formation of triple helices as found in the native MBL
protein (as
further described in Wallis, R., et al., J. Biol. Chem. 279:14065, 2004).
-78-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2 inhibitory peptides may also be derived from human H-ficolin that
include the sequence "GAO GS0 GEK GAO GPQ GPO GPO GKM GPK GEO GDO"
(SEQ ID NO:27) from the consensus MASP-2 binding region in H-ficolin. Also
included
are peptides derived from human L-ficolin that include the sequence "GCO GLO
GAO
GDK GEA GTN GKR GER GPO GPO GKA GPO GPN GAO GEO" (SEQ ID NO:28)
from the consensus MASP-2 binding region in L-ficolin.
MASP-2 inhibitory peptides may also be derived from the C4 cleavage site such
as "LQRALEILPNRVTIKANRPFLVFI" (SEQ ID NO:29) which is the C4 cleavage site
linked to the C-terminal portion of antithrombin III (Glover, G.I., et al.,
Mol.
Irnmunot 25:1261 (1988)).
TABLE 3: EXEMPLARY MASP-2 INHIBITORY PEPTIDES
SEQ ID NO Source
SEQ ID NO:6 Human MASP-2 protein
SEQ ID NO:8 CUBI domain of MASP-2 (aa 1-121 of SEQ ID NO:6)
SEQ ID NO:9 CUBIEGF domains of MASP-2 (aa 1-166 of SEQ ID NO:6)
SEQ 1D NO:10 CUBIEGFCUBII domains of MASP-2
(aa 1-293 of SEQ ID NO:6)
SEQ ID NO:11 EGF domain of MASP-2 (aa 122-166)
SEQ ID NO:12 Serine-protease domain of MASP-2 (aa 429-671)
SEQ ID NO:16 MBL binding region in MASP-2
SEQ ID NO:3 Human MAp19
SEQ ID NO:21 Human MBL protein
SEQ ID NO:22 Synthetic peptide Consensus binding site from Human
OGK-X-GP, MBL and Human ficolins
Where "0" =
hydroxyproline and "X"
is a hydrophobic amino
acid residue
SEQ ID NO:23 Human MBL core binding site
OGKLG
-79-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
SEQ ID NO Source
SEQ ID NO:24 Human MBP Triplets 6-10- demonstrated binding to
GLR GLQ GPO GKL MASP-2
GPO G
SEQ ID NO:25 Human MBP Triplets with GPO added to enhance
GPOGPOGLRGLQGPO formation of triple helices
GKLGPOGGPOGPO
SEQ ID NO:26 Human MBP Triplets 1-17
GKDGRDGTKGEKGEP
GQGLRGLQGPOGKLG
POGNOGPSGSOGPKG
QKGDOGKS
SEQ ID NO:27 Human H-Ficolin (Hataka)
GAOGSOGEKGAOGPQ
GPOGPOGKMGPKGEO
GDO
SEQ ID NO:28 Human L-Ficolin P35
GCOGLOGAOGDKGE
AGTNGKRGERGPOGP
OGKAGPOGPNGAOGE
0
SEQ ID NO:29 Human C4 cleavage site
LQRALEILPNRVTIKA
NRPFLVFI
SEQ ID NO:72 SGMI-2L (full-length)
LEVTCEPGTTFKDKCNT
CRCGSDGKSAVCTKLW
CNQ
SEQ ID NO:73 SGM1-2M (medium truncated version)
TCEPGTTFKDKCNTCRC
GSDGKSAVCTKLWCNQ
SEQ ID NO:74 SGMI-2S (short truncated version)
TCRCGSDGKSAVCTKL
WCNQ
Note: The letter "0" represents hydroxyproline. The letter "X" is a
hydrophobic residue.
Peptides derived from the C4 cleavage site as well as other peptides that
inhibit
the MASP-2 serine protease site can be chemically modified so that they are
irreversible
protease inhibitors. For example, appropriate modifications may include, but
are not
-80-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
necessarily limited to, halomethyl ketones (Br, Cl, I, F) at the C-terminus,
Asp or Glu, or
appended to functional side chains; haloacetyl (or other a-haloacetyl) groups
on amino
groups or other functional side chains; epoxide or imine-containing groups on
the amino
or carboxy termini or on functional side chains; or imidate esters on the
amino or carboxy
termini or on functional side chains. Such modifications would afford the
advantage of
permanently inhibiting the enzyme by covalent attachment of the peptide. This
could
result in lower effective doses and/or the need for less frequent
administration of the
peptide inhibitor.
In addition to the inhibitory peptides described above, MASP-2 inhibitory
peptides useful in the method of the invention include peptides containing the
MASP-2-binding CDRH3 region of anti-MASP-2 MoAb obtained as described herein.
The sequence of the CDR regions for use in synthesizing the peptides may be
determined
by methods known in the art. The heavy chain variable region is a peptide that
generally
ranges from 100 to 150 amino acids in length. The light chain variable region
is a peptide
that generally ranges from 80 to 130 amino acids in length. The CDR sequences
within
the heavy and light chain variable regions include only approximately 3-25
amino acid
sequences that may be easily sequenced by one of ordinary skill in the art.
Those skilled in the art will recognize that substantially homologous
variations of
the MASP-2 inhibitory peptides described above will also exhibit MASP-2
inhibitory
activity. Exemplary variations include, but are not necessarily limited to,
peptides having
insertions, deletions, replacements, and/or additional amino acids on the
carboxy-terminus or amino-terminus portions of the subject peptides and
mixtures
thereof. Accordingly, those homologous peptides having MASP-2 inhibitory
activity are
considered to be useful in the methods of this invention. The peptides
described may also
include duplicating motifs and other modifications with conservative
substitutions.
Conservative variants are described elsewhere herein, and include the exchange
of an
amino acid for another of like charge, size or hydrophobicity and the like.
MASP-2 inhibitory peptides may be modified to increase solubility and/or to
maximize the positive or negative charge in order to more closely resemble the
segment
in the intact protein. The derivative may or may not have the exact primary
amino acid
structure of a peptide disclosed herein so long as the derivative functionally
retains the
desired property of MASP-2 inhibition. The modifications can include amino
acid
substitution with one of the commonly known twenty amino acids or with another
amino
-81-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
acid, with a derivatized or substituted amino acid with ancillary desirable
characteristics,
such as resistance to enzymatic degradation or with a D-amino acid or
substitution with
another molecule or compound, such as a carbohydrate, which mimics the natural
confirmation and function of the amino acid, amino acids or peptide; amino
acid deletion;
amino acid insertion with one of the commonly known twenty amino acids or with
another amino acid, with a derivatized or substituted amino acid with
ancillary desirable
characteristics, such as resistance to enzymatic degradation or with a D-amino
acid or
substitution with another molecule or compound, such as a carbohydrate, which
mimics
the natural confirmation and function of the amino acid, amino acids or
peptide; or
substitution with another molecule or compound, such as a carbohydrate or
nucleic acid
monomer, which mimics the natural conformation, charge distribution and
function of the
parent peptide. Peptides may also be modified by acetylation or amidation.
The synthesis of derivative inhibitory peptides can rely on known techniques
of
peptide biosynthesis, carbohydrate biosynthesis and the like. As a starting
point, the
artisan may rely on a suitable computer program to determine the conformation
of a
peptide of interest. Once the conformation of peptide disclosed herein is
known, then the
artisan can determine in a rational design fashion what sort of substitutions
can be made
at one or more sites to fashion a derivative that retains the basic
conformation and charge
distribution of the parent peptide but which may possess characteristics which
are not
present or are enhanced over those found in the parent peptide. Once candidate
derivative molecules are identified, the derivatives can be tested to
determine if they
function as MASP-2 inhibitory agents using the assays described herein.
SCREENING FOR MA SP-2 INHIBITORY PEPTIDES
One may also use molecular modeling and rational molecular design to generate
and screen for peptides that mimic the molecular structures of key binding
regions of
MASP-2 and inhibit the complement activities of MASP-2 The molecular
structures
used for modeling include the CDR regions of anti-MASP-2 monoclonal
antibodies, as
well as the target regions known to be important for MASP-2 function including
the
region required for dimerization, the region involved in binding to MBL, and
the serine
protease active site as previously described. Methods for identifying peptides
that bind to
a particular target are well known in the art. For example, molecular
imprinting may be
used for the de novo construction of macromolecular structures such as
peptides that bind
to a particular molecule. See, for example, Shea, K.J., "Molecular Imprinting
of
-82-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Synthetic Network Polymers: The De Novo synthesis of Macromolecular Binding
and
Catalytic Sties," TRIP 2(5) 1994.
As an illustrative example, one method of preparing mimics of MASP-2 binding
peptides is as follows. Functional monomers of a known MASP-2 binding peptide
or the
binding region of an anti-MASP-2 antibody that exhibits MASP-2 inhibition (the
template) are polymerized. The template is then removed, followed by
polymerization of
a second class of monomers in the void left by the template, to provide a new
molecule
that exhibits one or more desired properties that are similar to the template.
In addition to
preparing peptides in this manner, other MASP-2 binding molecules that are
MASP-2
inhibitory agents such as polysaccharides, nucleosides, drugs, nucleoproteins,
lipoproteins, carbohydrates, glycoproteins, steroid, lipids and other
biologically active
materials can also be prepared. This method is useful for designing a wide
variety of
biological mimics that are more stable than their natural counterparts because
they are
typically prepared by free radical polymerization of function monomers,
resulting in a
compound with a nonbiodegradable backbone.
PEP TIDE SYNTHESIS
The MASP-2 inhibitory peptides can be prepared using techniques well known in
the art, such as the solid-phase synthetic technique initially described by
Merrifield, in
Amer. Chem. Soc. 85:2149-2154, 1963. Automated synthesis may be achieved, for
example, using Applied Biosystems 431A Peptide Synthesizer (Foster City,
Calif.) in
accordance with the instructions provided by the manufacturer. Other
techniques may be
found, for example, in Bodanszky, M., et al., Peptide Synthesis, second
edition, John
Wiley & Sons, 1976, as well as in other reference works known to those skilled
in the art.
The peptides can also be prepared using standard genetic engineering
techniques
.. known to those skilled in the art. For example, the peptide can be produced
enzymatically by inserting nucleic acid encoding the peptide into an
expression vector,
expressing the DNA, and translating the DNA into the peptide in the presence
of the
required amino acids. The peptide is then purified using chromatographic or
electrophoretic techniques, or by means of a carrier protein that can be fused
to, and
subsequently cleaved from, the peptide by inserting into the expression vector
in phase
with the peptide encoding sequence a nucleic acid sequence encoding the
carrier protein.
The fusion protein-peptide may be isolated using chromatographic,
electrophoretic or
immunological techniques (such as binding to a resin via an antibody to the
carrier
-83-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
protein). The peptide can be cleaved using chemical methodology or
enzymatically, as
by, for example, hydrolases.
The MASP-2 inhibitory peptides that are useful in the method of the invention
can
also be produced in recombinant host cells following conventional techniques.
To express
a MASP-2 inhibitory peptide encoding sequence, a nucleic acid molecule
encoding the
peptide must be operably linked to regulatory sequences that control
transcriptional
expression in an expression vector and then introduced into a host cell. In
addition to
transcriptional regulatory sequences, such as promoters and enhancers,
expression vectors
can include translational regulatory sequences and a marker gene, which are
suitable for
.. selection of cells that carry the expression vector.
Nucleic acid molecules that encode a MASP-2 inhibitory peptide can be
synthesized with "gene machines" using protocols such as the phosphoramidite
method.
If chemically synthesized double-stranded DNA is required for an application
such as the
synthesis of a gene or a gene fragment, then each complementary strand is made
separately. The production of short genes (60 to 80 base pairs) is
technically
straightforward and can be accomplished by synthesizing the complementary
strands and
then annealing them. For
the production of longer genes, synthetic genes
(double-stranded) are assembled in modular form from single-stranded fragments
that are
from 20 to 100 nucleotides in length. For reviews on polynucleotide synthesis,
see, for
example, Glick and Pasternak, "Molecular Biotechnology, Principles and
Applications of
Recombinant DNA", ASM Press, 1994; Itakura, K., et al., Annu. Rev. Biochem.
53:323,
1984; and Climie, S., et al., Proc. Nat' Acad Sci. USA 87:633, 1990.
SMALL MOLECULE INHIBITORS
In some embodiments, MASP-2 inhibitory agents are small molecule inhibitors
including natural and synthetic substances that have a low molecular weight,
such as for
example, peptides, peptidomimetics and nonpeptide inhibitors (including
oligonucleotides and organic compounds). Small molecule inhibitors of MASP-2
can be
generated based on the molecular structure of the variable regions of the anti-
MASP-2
antibodies.
Small molecule inhibitors may also be designed and generated based on the
MASP-2 crystal structure using computational drug design (Kuntz ID., et al.,
Science 257:1078, 1992). The crystal structure of rat MASP-2 has been
described
(Feinberg, H., et al., Ell4B0 J. 22:2348-2359, 2003). Using the method
described by
-84-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Kuntz et al., the MASP-2 crystal structure coordinates are used as an input
for a computer
program such as DOCK, which outputs a list of small molecule structures that
are
expected to bind to MASP-2. Use of such computer programs is well known to one
of
skill in the art. For example, the crystal structure of the HIV-1 protease
inhibitor was
used to identify unique nonpeptide ligands that are HIV-1 protease inhibitors
by
evaluating the fit of compounds found in the Cambridge Crystallographic
database to the
binding site of the enzyme using the program DOCK (Kuntz, ID., et al., 1. MoL
Biol. /6/:269-288, 1982; DesJarlais, R.L., et al., PNAS 87:6644-6648, 1990).
The list of small molecule structures that are identified by a computational
method
as potential MASP-2 inhibitors are screened using a MASP-2 binding assay such
as
described in Example 10. The small molecules that are found to bind to MASP-2
are
then assayed in a functional assay such as described in Example 2 to determine
if they
inhibit MASP-2-dependent complement activation.
MASP-2 SOLUBLE RECEPTORS
Other suitable MASP-2 inhibitory agents are believed to include MASP-2 soluble
receptors, which may be produced using techniques known to those of ordinary
skill in
the art.
EXPRESSION INHIBITORS OF MASP-2
In another embodiment of this aspect of the invention, the MASP-2 inhibitory
agent is a MASP-2 expression inhibitor capable of inhibiting MASP-2-dependent
complement activation. In the practice of this aspect of the invention,
representative
MASP-2 expression inhibitors include MASP-2 antisense nucleic acid molecules
(such as
antisense mRNA, antisense DNA or antisense oligonucleotides), MASP-2 ribozymes
and
MA SP-2 RNAi molecules.
Anti-sense RNA and DNA molecules act to directly block the translation of
MASP-2 mRNA by hybridizing to MASP-2 mRNA and preventing translation of
MASP-2 protein. An antisense nucleic acid molecule may be constructed in a
number of
different ways provided that it is capable of interfering with the expression
of MASP-2.
For example, an antisense nucleic acid molecule can be constructed by
inverting the
coding region (or a portion thereof) of MASP-2 cDNA (SEQ ID NO:4) relative to
its
normal orientation for transcription to allow for the transcription of its
complement.
The antisense nucleic acid molecule is usually substantially identical to at
least a
portion of the target gene or genes. The nucleic acid, however, need not be
perfectly
-85-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
identical to inhibit expression. Generally, higher homology can be used to
compensate
for the use of a shorter antisense nucleic acid molecule. The minimal percent
identity is
typically greater than about 65%, but a higher percent identity may exert a
more effective
repression of expression of the endogenous sequence. Substantially greater
percent
identity of more than about 80% typically is preferred, though about 95% to
absolute
identity is typically most preferred.
The antisense nucleic acid molecule need not have the same intron or exon
pattern
as the target gene, and non-coding segments of the target gene may be equally
effective in
achieving antisense suppression of target gene expression as coding segments.
A DNA
sequence of at least about 8 or so nucleotides may be used as the antisense
nucleic acid
molecule, although a longer sequence is preferable. In the present invention,
a
representative example of a useful inhibitory agent of MASP-2 is an antisense
MASP-2
nucleic acid molecule which is at least ninety percent identical to the
complement of the
MASP-2 cDNA consisting of the nucleic acid sequence set forth in SEQ ID NO:4.
The
nucleic acid sequence set forth in SEQ ID NO:4 encodes the MASP-2 protein
consisting
of the amino acid sequence set forth in SEQ ID NO:5.
The targeting of antisense oligonucleotides to bind MASP-2 mRNA is another
mechanism that may be used to reduce the level of MASP-2 protein synthesis.
For
example, the synthesis of polygalacturonase and the muscarine type 2
acetylcholine
receptor is inhibited by antisense oligonucleotides directed to their
respective mRNA
sequences (U.S. Patent No. 5,739,119, to Cheng, and U.S. Patent No. 5,759,829,
to
Shewmaker). Furthermore, examples of antisense inhibition have been
demonstrated
with the nuclear protein cyclin, the multiple drug resistance gene (MDG1),
ICAM-1,
E-selectin, STK-1, striatal GABAA receptor and human EGF (see, e.g., U.S.
Patent
No. 5,801,154, to Baracchini; U.S Patent No 5,789,573, to Baker; U.S. Patent
No. 5,718,709, to Considine; and U.S. Patent No. 5,610,288, to Reubenstein).
A system has been described that allows one of ordinary skill to determine
which
oligonucleotides are useful in the invention, which involves probing for
suitable sites in
the target mRNA using RNAse H cleavage as an indicator for accessibility of
sequences
within the transcripts. Scherr, M., et al., Nucleic Acids Res. 26:5079-
5085, 1998;
Lloyd, et al., Nucleic Acids Res. 29:3665-3673, 2001. A
mixture of antisense
oligonucleotides that are complementary to certain regions of the MASP-2
transcript is
added to cell extracts expressing MASP-2, such as hepatocytes, and hybridized
in order
-86-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
to create an RNAse H vulnerable site. This method can be combined with
computer-assisted sequence selection that can predict optimal sequence
selection for
antisense compositions based upon their relative ability to form dimers,
hairpins, or other
secondary structures that would reduce or prohibit specific binding to the
target mRNA in
a host cell. These secondary structure analysis and target site selection
considerations
may be performed using the OLIGO primer analysis software (Rychlik, I., 1997)
and the
BLASTN 2Ø5 algorithm software (Altschul, S.F., et al., Nucl. Acids Res.
25:3389-3402,
1997). The antisense compounds directed towards the target sequence preferably
comprise from about 8 to about 50 nucleotides in length. Antisense
oligonucleotides
.. comprising from about 9 to about 35 or so nucleotides are particularly
preferred. The
inventors contemplate all oligonucleotide compositions in the range of 9 to 35
nucleotides
(i.e., those of 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24,
25, 26, 27, 28,
29, 30, 31, 32, 33, 34, or 35 or so bases in length) are highly preferred for
the practice of
antisense oligonucleotide-based methods of the invention. Highly preferred
target
regions of the MASP-2 mRNA are those that are at or near the AUG translation
initiation
codon, and those sequences that are substantially complementary to 5' regions
of the
mRNA, e.g., between the ¨10 and +10 regions of the MASP-2 gene nucleotide
sequence
(SEQ ID NO:4). Exemplary MASP-2 expression inhibitors are provided in TABLE 4.
-87-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
TABLE 4: EXEMPLARY EXPRESSION INHIBITORS OF MASP-2
SEQ ID NO:30 (nucleotides 22-680 of Nucleic acid sequence of MASP-2 cDNA
SEQ lID NO:4) (SEQ ID NO:4) encoding CUBIEGF
SEQ ID NO:31 Nucleotides 12-45 of SEQ ID NO:4
5'CGGGCACACCATGAGGCTGCTG including the MASP-2 translation start site
ACCCTCCTGGGC3 (sense)
SEQ ID NO:32 Nucleotides 361-396 of SEQ ID NO:4
51GACATTACCTTCCGCTCCGACTC encoding a region comprising the MASP-2
CAACGAGAAG3' MBL binding site (sense)
SEQ ID NO:33 Nucleotides 610-642 of SEQ ID NO:4
51AGCAGCCCTGAATACCCACGGCC encoding a region comprising the CUBIT
GTATCCCAAA3' domain
As noted above, the term "oligonucleotide" as used herein refers to an
oligomer or
.. polymer of ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) or
mimetics thereof.
This term also covers those oligonucleobases composed of naturally occurring
nucleotides, sugars and covalent internucleoside (backbone) linkages as well
as
oligonucleotides having non-naturally occurring modifications. These
modifications
allow one to introduce certain desirable properties that are not offered
through naturally
occurring oligonucleotides, such as reduced toxic properties, increased
stability against
nuclease degradation and enhanced cellular uptake. In illustrative
embodiments, the
antisense compounds of the invention differ from native DNA by the
modification of the
phosphodiester backbone to extend the life of the antisense oligonucleotide in
which the
phosphate substituents are replaced by phosphorothioates. Likewise, one or
both ends of
the oligonucleotide may be substituted by one or more acridine derivatives
that intercalate
between adjacent basepairs within a strand of nucleic acid.
Another alternative to antisense is the use of "RNA interference" (RNAi).
Double-stranded RNAs (dsRNAs) can provoke gene silencing in mammals in vivo.
The
natural function of RNAi and co-suppression appears to be protection of the
genome
against invasion by mobile genetic elements such as retrotransposons and
viruses that
produce aberrant RNA or dsRNA in the host cell when they become active (see,
e.g.,
Jensen, J., et al., Nat. Genet. 21:209-12, 1999). The double-stranded RNA
molecule may
be prepared by synthesizing two RNA strands capable of forming a double-
stranded RNA
-88-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
molecule, each having a length from about 19 to 25 (e.g., 19-23 nucleotides).
For
example, a dsRNA molecule useful in the methods of the invention may comprise
the
RNA corresponding to a sequence and its complement listed in TABLE 4.
Preferably, at
least one strand of RNA has a 3' overhang from 1-5 nucleotides. The
synthesized RNA
strands are combined under conditions that form a double-stranded molecule.
The RNA
sequence may comprise at least an 8 nucleotide portion of SEQ ID NO:4 with a
total
length of 25 nucleotides or less. The design of siRNA sequences for a given
target is
within the ordinary skill of one in the art. Commercial services are available
that design
siRNA sequence and guarantee at least 70% knockdown of expression (Qiagen,
Valencia,
Calif).
The dsRNA may be administered as a pharmaceutical composition and carried out
by known methods, wherein a nucleic acid is introduced into a desired target
cell.
Commonly used gene transfer methods include calcium phosphate, DEAE-dextran,
electroporation, microinjection and viral methods. Such
methods are taught in
Ausubel et al., Current Protocols in Molecular Biology, John Wiley & Sons,
Inc., 1993.
Ribozymes can also be utilized to decrease the amount and/or biological
activity
of MASP-2, such as ribozymes that target MASP-2 mRNA. Ribozymes are catalytic
RNA molecules that can cleave nucleic acid molecules having a sequence that is
completely or partially homologous to the sequence of the ribozyme. It is
possible to
design ribozyme transgenes that encode RNA ribozymes that specifically pair
with a
target RNA and cleave the phosphodiester backbone at a specific location,
thereby
functionally inactivating the target RNA. In carrying out this cleavage, the
ribozyme is
not itself altered, and is thus capable of recycling and cleaving other
molecules. The
inclusion of ribozyme sequences within anti sense RNAs confers RNA-cleaving
activity
upon them, thereby increasing the activity of the antisense constructs.
Ribozymes useful in the practice of the invention typically comprise a
hybridizing
region of at least about nine nucleotides, which is complementary in
nucleotide sequence
to at least part of the target MASP-2 mRNA, and a catalytic region that is
adapted to
cleave the target MASP-2 mRNA (see generally, EPA No. 0 321 201; W088/04300;
Haseloff, J., et al., Nature 334:585-591, 1988; Fedor, M.J., et al., Proc.
Natl. Acad. Sci.
USA 87:1668-1672, 1990; Cech, T.R., et al., Ann. Rev. Biochem. 55:599-629,
1986).
Ribozymes can either be targeted directly to cells in the form of RNA
oligonucleotides incorporating ribozyme sequences, or introduced into the cell
as an
-89-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
expression vector encoding the desired ribozymal RNA. Ribozymes may be used
and
applied in much the same way as described for antisense polynucleotides.
Anti-sense RNA and DNA, ribozymes and RNAi molecules useful in the methods
of the invention may be prepared by any method known in the art for the
synthesis of
DNA and RNA molecules. These include techniques for chemically synthesizing
oligodeoxyribonucleotides and oligoribonucleotides well known in the art, such
as for
example solid phase phosphoramidite chemical synthesis. Alternatively, RNA
molecules
may be generated by in vitro and in vivo transcription of DNA sequences
encoding the
antisense RNA molecule. Such DNA sequences may be incorporated into a wide
variety
of vectors that incorporate suitable RNA polymerase promoters such as the T7
or SP6
polymerase promoters. Alternatively, antisense cDNA constructs that
synthesize
antisense RNA constitutively or inducibly, depending on the promoter used, can
be
introduced stably into cell lines.
Various well known modifications of the DNA molecules may be introduced as a
means of increasing stability and half-life. Useful modifications include, but
are not
limited to, the addition of flanking sequences of ribonucleotides or
deoxyribonucleotides
to the 5' and/or 3' ends of the molecule or the use of phosphorothioate or 2'
0-methyl
rather than phosphodiesterase linkages within the oligodeoxyribonucleotide
backbone.
V. PHARMACEUTICAL COMPOSITIONS AND DELIVERY METHODS
DOSING
In another aspect, the invention provides compositions for inhibiting the
adverse
effects of MASP-2-dependent complement activation in a subject suffering from
a
disease or condition as disclosed herein, comprising administering to the
subject a
composition comprising a therapeutically effective amount of a MA SP-2
inhibitory agent
and a pharmaceutically acceptable carrier. The MASP-2 inhibitory agents can be
administered to a subject in need thereof, at therapeutically effective doses
to treat or
ameliorate conditions associated with MASP-2-dependent complement activation.
A
therapeutically effective dose refers to the amount of the MASP-2 inhibitory
agent
sufficient to result in amelioration of symptoms associated with the disease
or condition.
Toxicity and therapeutic efficacy of MASP-2 inhibitory agents can be
determined
by standard pharmaceutical procedures employing experimental animal models,
such as
the murine MASP-2 -/- mouse model expressing the human MASP-2 transgene
described
-90-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
in Example 1. Using such animal models, the NOAEL (no observed adverse effect
level)
and the MED (the minimally effective dose) can be determined using standard
methods.
The dose ratio between NOAEL and MED effects is the therapeutic ratio, which
is
expressed as the ratio NOAEL/MED. MASP-2 inhibitory agents that exhibit large
therapeutic ratios or indices are most preferred. The data obtained from the
cell culture
assays and animal studies can be used in formulating a range of dosages for
use in
humans. The dosage of the MASP-2 inhibitory agent preferably lies within a
range of
circulating concentrations that include the MED with little or no toxicity.
The dosage
may vary within this range depending upon the dosage form employed and the
route of
administration utilized.
In some embodiments, therapeutic efficacy of the MASP-2 inhibitory agents for
treating, inhibiting, alleviating or preventing fibrosis in a mammalian
subject suffering, or
at risk of developing a disease or disorder caused or exacerbated by fibrosis
and/or
inflammation is determined by one or more of the following: a reduction in one
of more
markers of inflammation and scarring (e.g., TGF13-1, CTFF, IL-6, apoptosis,
fibronectin,
laminin, collagens, EMT, infiltrating macrophages) in renal tissue; a
reduction in the
release of soluble markers of inflammation and fibrotic renal disease into
urine and
plasma (e.g., by the measurement of renal excretory functions).
For any compound formulation, the therapeutically effective dose can be
estimated using animal models. For example, a dose may be formulated in an
animal
model to achieve a circulating plasma concentration range that includes the
MED.
Quantitative levels of the MASP-2 inhibitory agent in plasma may also be
measured, for
example, by high performance liquid chromatography.
In addition to toxicity studies, effective dosage may also be estimated based
on
the amount of MASP-2 protein present in a living subject and the binding
affinity of the
MASP-2 inhibitory agent. It has been shown that MASP-2 levels in normal human
subjects is present in serum in low levels in the range of 500 ng/ml, and MASP-
2 levels
in a particular subject can be detelinined using a quantitative assay for MASP-
2 described
in Moller-Kristensen M., et al., I Immunol. Methods 282:159-167, 2003.
Generally, the dosage of administered compositions comprising MASP-2
inhibitory agents varies depending on such factors as the subject's age,
weight, height,
sex, general medical condition, and previous medical history. As an
illustration, MASP-2
inhibitory agents, such as anti-MASP-2 antibodies, can be administered in
dosage ranges
-91-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
from about 0.010 to 10.0 mg/kg, preferably 0.010 to 1.0 mg/kg, more preferably
0.010 to
0.1 mg/kg of the subject body weight. In some embodiments the composition
comprises
a combination of anti-MASP-2 antibodies and MASP-2 inhibitory peptides.
Therapeutic efficacy of MASP-2 inhibitory compositions and methods of the
present invention in a given subject, and appropriate dosages, can be
determined in
accordance with complement assays well known to those of skill in the art.
Complement
generates numerous specific products. During the last decade, sensitive and
specific
assays have been developed and are available commercially for most of these
activation
products, including the small activation fragments C3a, C4a, and C5a and the
large
activation fragments iC3b, C4d, Bb, and sC5b-9. Most of these assays utilize
monoclonal
antibodies that react with new antigens (neoantigens) exposed on the fragment,
but not on
the native proteins from which they are folined, making these assays very
simple and
specific. Most rely on ELISA technology, although radioimmunoassay is still
sometimes
used for C3a and C5a. These latter assays measure both the unprocessed
fragments and
.. their 'desArg' fragments, which are the major forms found in the
circulation.
Unprocessed fragments and C5adesArg are rapidly cleared by binding to cell
surface
receptors and are hence present in very low concentrations, whereas C3adesArg
does not
bind to cells and accumulates in plasma. Measurement of C3a provides a
sensitive,
pathway-independent indicator of complement activation. Alternative pathway
activation
can be assessed by measuring the Bb fragment. Detection of the fluid-phase
product of
membrane attack pathway activation, sC5b-9, provides evidence that complement
is
being activated to completion. Because both the lectin and classical pathways
generate
the same activation products, C4a and C4d, measurement of these two fragments
does not
provide any information about which of these two pathways has generated the
activation
products.
The inhibition of MASP-2-dependent complement activation is characterized by
at least one of the following changes in a component of the complement system
that
occurs as a result of administration of a MASP-2 inhibitory agent in
accordance with the
methods of the invention: the inhibition of the generation or production of
MASP-2-dependent complement activation system products C4b, C3a, C5a and/or
C5b-9
(MAC) (measured, for example, as described in measured, for example, as
described in
Example 2, the reduction of C4 cleavage and C4b deposition (measured, for
example as
-92-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
described in Example 10), or the reduction of C3 cleavage and C3b deposition
(measured,
for example, as described in Example 10).
ADDITIONAL AGENTS
In certain embodiments, methods of preventing, treating, reverting and/or
.. inhibiting fibrosis and/or inflammation include administering an MASP-2
inhibitory
agent (e.g., a MASP-2 inhibitory antibody) as part of a therapeutic regimen
along with
one or more other drugs, biologics, or therapeutic interventions appropriate
for inhibiting
fibrosis and/or inflammation. In certain embodiments, the additional drug,
biologic, or
therapeutic intervention is appropriate for particular symptoms associated
with a disease
or disorder caused or exacerbated by fibrosis and/or inflammation. By way of
example,
MASP-2 inhibitory antibodies may be administered as part of a therapeutic
regimen along
with one or more immunosuppressive agents, such as methotrexate,
cyclophosphamide,
azathioprine, and mycophenolate mofetil. By way of further example, MASP-2
inhibitory
antibodies may be administered as part of a therapeutic regimen along with one
or more
agents designed to increase blood flow (e.g., nifedipine, amlodipine,
diltiazem,
felodipine, or nicardipine). By way of further example, MASP-2 inhibitory
antibodies
may be administered as part of a therapeutic regimen along with one or more
agents
intended to decrease fibrosis, such as d-penicillamine, colchicine, PUVA,
Relaxin,
cyclosporine, TGF beta blockers and/or p38 MAPK blockers. By way of further
example, MASP-2 inhibitory antibodies may be administered as part of a
therapeutic
regimen along with steroids or broncho-dilators.
The compositions and methods comprising MASP-2 inhibitory agents (e.g.,
MA SP-2 inhibitory antibodies) may optionally comprise one or more additional
therapeutic agents, which may augment the activity of the MASP-2 inhibitory
agent or
that provide related therapeutic functions in an additive or synergistic
fashion. For
example, in the context of treating a subject suffering from a disease or
disorder caused or
exacerbated by fibrosis and/or inflammation one or more MASP-2 inhibitory
agents may
be administered in combination (including co-administration) with one or more
additional
antifibrotic agents and/or one or more anti-inflammatory and/or
immunosuppressive
.. agents.
MASP-2 inhibitory agents (e.g., MASP-2 inhibitory antibodies) can be used in
combination with other therapeutic agents such as general immunosuppressive
drugs such
as corticosteroids, immunosuppressive or cytotoxic agents, and/or antifibrotic
agents.
-93-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
PHARMACEUTICAL CARRIERS AND DELIVERY VEHICLES
In general, the MASP-2 inhibitory agent compositions of the present invention,
combined with any other selected therapeutic agents, are suitably contained in
a
pharmaceutically acceptable carrier. The carrier is non-toxic, biocompatible
and is
selected so as not to detrimentally affect the biological activity of the MASP-
2 inhibitory
agent (and any other therapeutic agents combined therewith).
Exemplary
pharmaceutically acceptable carriers for peptides are described in U.S. Patent
No. 5,211,657 to Yamada. The anti-MASP-2 antibodies and inhibitory peptides
useful in
the invention may be formulated into preparations in solid, semi-solid, gel,
liquid or
gaseous forms such as tablets, capsules, powders, granules, ointments,
solutions,
depositories, inhalants and injections allowing for oral, parenteral or
surgical
administration. The invention also contemplates local administration of the
compositions
by coating medical devices and the like.
Suitable carriers for parenteral delivery via injectable, infusion or
irrigation and
topical delivery include distilled water, physiological phosphate-buffered
saline, normal
or lactated Ringer's solutions, dextrose solution, Hank's solution, or
propanediol. In
addition, sterile, fixed oils may be employed as a solvent or suspending
medium. For this
purpose any biocompatible oil may be employed including synthetic mono- or
diglycerides. In addition, fatty acids such as oleic acid find use in the
preparation of
injectables. The carrier and agent may be compounded as a liquid, suspension,
polymerizable or non-polymerizable gel, paste or salve.
The carrier may also comprise a delivery vehicle to sustain (i.e., extend,
delay or
regulate) the delivery of the agent(s) or to enhance the delivery, uptake,
stability or
pharmacokinetics of the therapeutic agent(s). Such a delivery vehicle may
include, by
way of non-limiting example, microparticles, microspheres, nanospheres or
nanoparticles
composed of proteins, liposomes, carbohydrates, synthetic organic compounds,
inorganic
compounds, polymeric or copolymeric hydrogels and polymeric micelles. Suitable
hydrogel and micelle delivery systems include the PEO:PHB:PEO copolymers and
copolymer/cyclodextrin complexes disclosed in WO 2004/009664 A2 and the PEO
and
PEO/cyclodextrin complexes disclosed in U.S. Patent Application Publication
No. 2002/0019369 Al Such hydrogels may be injected locally at the site of
intended
action, or subcutaneously or intramuscularly to form a sustained release
depot.
-94-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
For intra-articular delivery, the MASP-2 inhibitory agent may be carried in
above-described liquid or gel carriers that are injectable, above-described
sustained-release delivery vehicles that are injectable, or a hyaluronic acid
or hyaluronic
acid derivative.
For oral administration of non-peptidergic agents, the MASP-2 inhibitory agent
may be carried in an inert filler or diluent such as sucrose, cornstarch, or
cellulose.
For topical administration, the MASP-2 inhibitory agent may be carried in
ointment, lotion, cream, gel, drop, suppository, spray, liquid or powder, or
in gel or
microcapsular delivery systems via a transdermal patch.
Various nasal and pulmonary delivery systems, including aerosols, metered-dose
inhalers, dry powder inhalers, and nebulizers, are being developed and may
suitably be
adapted for delivery of the present invention in an aerosol, inhalant, or
nebulized delivery
vehicle, respectively.
For intrathecal (IT) or intracerebroventricular (ICV) delivery, appropriately
sterile
delivery systems (e.g., liquids; gels, suspensions, etc.) can be used to
administer the
present invention.
The compositions of the present invention may also include biocompatible
excipients, such as dispersing or wetting agents, suspending agents, diluents,
buffers,
penetration enhancers, emulsifiers, binders, thickeners, flavouring agents
(for oral
administration).
PHARMACEUTICAL CARRIERS FOR ANTIBODIES AND PEPTIDES
More specifically with respect to anti-MASP-2 antibodies and inhibitory
peptides,
exemplary formulations can be parenterally administered as injectable dosages
of a
solution or suspension of the compound in a physiologically acceptable diluent
with a
pharmaceutical carrier that can be a sterile liquid such as water, oils,
saline, glycerol or
ethanol. Additionally, auxiliary substances such as wetting or emulsifying
agents,
surfactants, pH buffering substances and the like can be present in
compositions
comprising anti-MASP-2 antibodies and inhibitory peptides. Additional
components of
pharmaceutical compositions include petroleum (such as of animal, vegetable or
synthetic
origin), for example, soybean oil and mineral oil. In general, glycols such as
propylene
glycol or polyethylene glycol are preferred liquid carriers for injectable
solutions.
-95-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The anti-MASP-2 antibodies and inhibitory peptides can also be administered in
the form of a depot injection or implant preparation that can be formulated in
such a
manner as to permit a sustained or pulsatile release of the active agents.
PHARMACEUTICALLY ACCEPTABLE CARRIERS FOR EXPRESSION
INHIBITORS
More specifically with respect to expression inhibitors useful in the methods
of
the invention, compositions are provided that comprise an expression inhibitor
as
described above and a pharmaceutically acceptable carrier or diluent. The
composition
may further comprise a colloidal dispersion system.
Pharmaceutical compositions that include expression inhibitors may include,
but
are not limited to, solutions, emulsions, and liposome-containing formulations
These
compositions may be generated from a variety of components that include, but
are not
limited to, prefouned liquids, self-emulsifying solids and self-emulsifying
semisolids.
The preparation of such compositions typically involves combining the
expression
inhibitor with one or more of the following: buffers, antioxidants, low
molecular weight
polypeptides, proteins, amino acids, carbohydrates including glucose, sucrose
or dextrins,
chelating agents such as EDTA, glutathione and other stabilizers and
excipients. Neutral
buffered saline or saline mixed with non-specific serum albumin are examples
of suitable
diluents.
In some embodiments, the compositions may be prepared and formulated as
emulsions which arc typically heterogeneous systems of one liquid dispersed in
another
in the form of droplets (see, Idson, in Pharmaceutical Dosage Forms, Vol. 1,
Rieger and
Banker (eds.), Marcek Dekker, Inc., N.Y., 1988). Examples of naturally
occurring
emulsifiers used in emulsion formulations include acacia, beeswax, lanolin,
lecithin and
phosphatides
In one embodiment, compositions including nucleic acids can be formulated as
microemulsions. A microemulsion, as used herein refers to a system of water,
oil, and
amphiphile, which is a single optically isotropic and thermodynamically stable
liquid
solution (see Rosoff in Pharmaceutical Dosage Forms, Vol. 1). The method of
the
invention may also use liposomes for the transfer and delivery of antisense
oligonucleotides to the desired site.
-96-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Pharmaceutical compositions and formulations of expression inhibitors for
topical
administration may include transdermal patches, ointments, lotions, creams,
gels, drops,
suppositories, sprays, liquids and powders. Conventional pharmaceutical
carriers, as well
as aqueous, powder or oily bases and thickeners and the like may be used.
MODES OF ADMINISTRATION
The pharmaceutical compositions comprising MASF'-2 inhibitory agents may be
administered in a number of ways depending on whether a local or systemic mode
of
administration is most appropriate for the condition being treated. Further,
the
compositions of the present invention can be delivered by coating or
incorporating the
compositions on or into an implantable medical device.
SYSTEMIC DELIVERY
As used herein, the terms "systemic delivery" and "systemic administration"
are
intended to include but are not limited to oral and parenteral routes
including
intramuscular (IM), subcutaneous, intravenous (IV), intra-arterial,
inhalational,
sublingual, buccal, topical, transdermal, nasal, rectal, vaginal and other
routes of
administration that effectively result in dispersement of the delivered agent
to a single or
multiple sites of intended therapeutic action. Preferred routes of systemic
delivery for the
present compositions include intravenous, intramuscular, subcutaneous and
inhalational.
It will be appreciated that the exact systemic administration route for
selected agents
utilized in particular compositions of the present invention will be
deteimined in part to
account for the agents susceptibility to metabolic transformation pathways
associated
with a given route of administration. For example, peptidergic agents may be
most
suitably administered by routes other than oral
MASP-2 inhibitory antibodies and polypeptides can be delivered into a subject
in
need thereof by any suitable means. Methods of delivery of MASP-2 antibodies
and
polypeptides include administration by oral, pulmonary, parenteral (e.g.,
intramuscular,
intraperitoneal, intravenous (IV) or subcutaneous injection), inhalation (such
as via a fine
powder formulation), transdermal, nasal, vaginal, rectal, or sublingual routes
of
administration, and can be formulated in dosage forms appropriate for each
route of
administration.
By way of representative example, MASP-2 inhibitory antibodies and peptides
can be introduced into a living body by application to a bodily membrane
capable of
absorbing the polypeptides, for example the nasal, gastrointestinal and rectal
membranes.
-97-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The polypeptides are typically applied to the absorptive membrane in
conjunction with a
permeation enhancer. (See, e.g., Lee, V.H.L., Crit Rev. Ther. Drug Carrier
Sys. 5:69,
1988; Lee, V.H.L., I Controlled Release /3:213, 1990; Lee, V.H.L., Ed.,
Peptide and
Protein Drug Delivery, Marcel Dekker, New York (1991); DeBoer, A.G., et al.,
J. Controlled Release 13:241, 1990.) For example, STDHF is a synthetic
derivative of
fusidic acid, a steroidal surfactant that is similar in structure to the bile
salts, and has been
used as a permeation enhancer for nasal delivery. (Lee, W.A., Biopharm. 22,
Nov./Dec.
1990.)
The MASP-2 inhibitory antibodies and polypeptides may be introduced in
association with another molecule, such as a lipid, to protect the
polypeptides from
enzymatic degradation. For example, the covalent attachment of polymers,
especially
polyethylene glycol (PEG), has been used to protect certain proteins from
enzymatic
hydrolysis in the body and thus prolong half-life (Fuertges, F., et al., J.
Controlled
Release 11:139, 1990). Many polymer systems have been reported for protein
delivery
(Bae, Y.H., et al., J. Controlled Release 9:271, 1989; Hori, R., et al.,
Pharm. Res. 6:813,
1989; Yamakawa, I., et al., J. Pharm. Sci. 79:505, 1990; Yoshihiro, I., et
al., J. Controlled
Release 10:195, 1989; Asano, M., et al., J. Controlled Release 9:111, 1989;
Rosenblatt,
J., et al., J. Controlled Release 9:195, 1989; Makino, K., I Controlled
Release /2:235,
1990; Takakura, Y., et al., I Pharm. Sci. 78:117, 1989; Takakura, Y., et al.,
J. Pharm.
Sci. 78:219, 1989).
Recently, liposomes have been developed with improved serum stability and
circulation half-times (see, e.g., U.S. Patent No. 5,741,516, to Webb).
Furthermore,
various methods of liposome and liposome-like preparations as potential drug
carriers
have been reviewed (see, e.g., U.S. Patent No. 5,567,434, to Szoka; U.S.
Patent
No. 5,552,157, to Yagi; U.S. Patent No. 5,565,213, to Nakamori; U.S. Patent
No. 5,738,868, to Shinkarenko; and U.S. Patent No. 5,795,587, to Gao).
For transdermal applications, the MASP-2 inhibitory antibodies and
polypeptides
may be combined with other suitable ingredients, such as carriers and/or
adjuvants.
There are no limitations on the nature of such other ingredients, except that
they must be
pharmaceutically acceptable for their intended administration, and cannot
degrade the
activity of the active ingredients of the composition. Examples of suitable
vehicles
include ointments, creams, gels, or suspensions, with or without purified
collagen. The
-98-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2 inhibitory antibodies and polypeptides may also be impregnated into
transdermal patches, plasters, and bandages, preferably in liquid or semi-
liquid form.
The compositions of the present invention may be systemically administered on
a
periodic basis at intervals determined to maintain a desired level of
therapeutic effect.
For example, compositions may be administered, such as by subcutaneous
injection,
every two to four weeks or at less frequent intervals. The dosage regimen will
be
determined by the physician considering various factors that may influence the
action of
the combination of agents. These factors will include the extent of progress
of the
condition being treated, the patients age, sex and weight, and other clinical
factors. The
dosage for each individual agent will vary as a function of the MASP-2
inhibitory agent
that is included in the composition, as well as the presence and nature of any
drug
delivery vehicle (e.g., a sustained release delivery vehicle). In addition,
the dosage
quantity may be adjusted to account for variation in the frequency of
administration and
the pharmacokinetic behavior of the delivered agent(s).
LOCAL DELIVERY
As used herein, the term "local" encompasses application of a drug in or
around a
site of intended localized action, and may include for example topical
delivery to the skin
or other affected tissues, ophthalmic delivery, intrathecal (IT),
intracerebroventricular
(ICV), intra-articular, intracavity, intracranial or intravesicular
administration, placement
or irrigation. Local administration may be preferred to enable administration
of a lower
dose, to avoid systemic side effects, and for more accurate control of the
timing of
delivery and concentration of the active agents at the site of local delivery.
Local
administration provides a known concentration at the target site, regardless
of interpatient
variability in metabolism, blood flow, etc. Improved dosage control is also
provided by
the direct mode of delivery.
Local delivery of a MASP-2 inhibitory agent may be achieved in the context of
surgical methods for treating disease or disorder caused or exacerbated by
fibrosis and/or
inflammation such as for example during procedures such as surgery.
TREATMENT REGIMENS
In prophylactic applications, the pharmaceutical compositions comprising a
MASP-2 inhibitory agent (e.g., a MASP-2 inhibitory antibody) are administered
to a
subject susceptible to, or otherwise at risk of developing a disease or
disorder caused or
exacerbated by fibrosis and/or inflammation in an amount sufficient to inhibit
fibrosis
-99-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
and/or inflammation and thereby eliminate or reduce the risk of developing
symptoms of
the condition. In some embodiments, the pharmaceutical compositions are
administered
to a subject suspected of, or already suffering from, a disease or disorder
caused or
exacerbated by fibrosis and/or inflammation in a therapeutically effective
amount
sufficient to relieve, or at least partially reduce, the symptoms of the
condition. In both
prophylactic and therapeutic regimens, compositions comprising MASP-2
inhibitory
agents may be administered in several dosages until a sufficient therapeutic
outcome has
been achieved in the subject. Application of the MASP-2 inhibitory
compositions of the
present invention may be carried out by a single administration of the
composition, or a
limited sequence of administrations, for treatment of an acute condition
associated with
fibrosis and/or inflammation. Alternatively, the composition may be
administered at
periodic intervals over an extended period of time for treatment of chronic
conditions
associated with fibrosis and/or inflammation.
In both prophylactic and therapeutic regimens, compositions comprising MASP-2
inhibitory agents may be administered in several dosages until a sufficient
therapeutic
outcome has been achieved in the subject. In one embodiment of the invention,
the
MASP-2 inhibitory agent comprises a MASP-2 antibody, which suitably may be
administered to an adult patient (e.g., an average adult weight of 70 kg) in a
dosage of
from 0.1 mg to 10,000 mg, more suitably from 1.0 mg to 5,000 mg, more suitably
10.0
mg to 2,000 mg, more suitably 10.0 mg to 1,000 mg and still more suitably from
50.0 mg
to 500 mg. For pediatric patients, dosage can be adjusted in proportion to the
patient's
weight. Application of the MASP-2 inhibitory compositions of the present
invention may
be carried out by a single administration of the composition, or a limited
sequence of
administrations, for treatment of a subject suffering from or at risk for
developing a
disease or disorder caused or exacerbated by fibrosis and/or inflammation.
Alternatively,
the composition may be administered at periodic intervals such as daily,
biweekly,
weekly, every other week, monthly or bimonthly over an extended period of time
for
treatment of a subject suffering from or at risk for developing a disease or
disorder caused
or exacerbated by fibrosis and/or inflammation.
In both prophylactic and therapeutic regimens, compositions comprising MASP-2
inhibitory agents may be administered in several dosages until a sufficient
therapeutic
outcome has been achieved in the subject.
-100-
In some embodiments, a subject is identified to be at risk for developing a
disease
or disorder caused or exacerbated by fibrosis or inflammation by determining
that the
subject has one or more symptoms of impaired kidney function, as assessed, for
example,
by measuring serum creatinine levels, serum creatinine clearance, blood urea
nitrogen
levels, protein in the urine, and/or by measuring one or more biomarkers
associated with
a renal disease or injury.
Methods for assessing renal function are well known in the art and include,
but art
not limited to, measurements of blood systemic and glomerular capillary
pressure,
proteinuria (e.g., albuminuria), microscopic and macroscopic hematuria, serum
creatinine
level (e.g., one formula for estimating renal function in humans equates a
creatinine level
of 2.0 mg/di to 50 percent of normal kidney function and 4.0 mg/di to 25
percent),
decline in the glomerular filtration rate (e.g., rate of creatinine
clearance), and degree of
tubular damage. For example, assessment of kidney function may include
evaluating at
least one kidney function using biological and/or physiological parameters
such as serum
creatinine level, creatinine clearance rate, 24-hour urinary protein
secretion, glomerular
filtration rate, urinary albumin creatinine ratio, albumin excretion rate, and
renal biopsy
(e.g., determining the degree of renal fibrosis by measuring deposition of
collagen and/or
fibronectin).
VI. EXAMPLES
The following examples merely illustrate the best mode now contemplated for
practicing the invention, but should not be construed to limit the invention.
EXAMPLE 1
This example describes the generation of a mouse strain deficient in MASP-2
(MASP-2-/-) but sufficient of MAp19 (MAp19+/-0.
Materials and Methods: The targeting vector pKO-NTKV 1901 was designed
to disrupt the three exons coding for the C-terminal end of murine MASP-2,
including the
exon that encodes the serine protease domain, as shown in FIGURE 3.
PKO-NTKV 1901 was used to transfect the murine ES cell line E14. la (SV129
01a).
Neomycin-resistant and Thymidine Kinase-sensitive clones were selected. 600 ES
clones
were screened and, of these, four different clones were identified and
verified by southern
-101 -
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
blot to contain the expected selective targeting and recombination event as
shown in
FIGURE 3. Chimeras were generated from these four positive clones by embryo
transfer.
The chimeras were then backcrossed in the genetic background C57/BL6 to create
transgenic males. The transgenic males were crossed with females to generate F
is
with 50% of the offspring showing heterozygosity for the disrupted MASP-2
gene. The
heterozygous mice were intercrossed to generate homozygous MASP-2 deficient
offspring, resulting in heterozygous and wild-type mice in the ration of
1:2:1,
respectively.
Results and Phenotype: The resulting homozygous MASP-2-/- deficient mice
were found to be viable and fertile and were verified to be MASP-2 deficient
by southern
blot to confirm the correct targeting event, by Northern blot to confirm the
absence of
MASP-2 mRNA, and by Western blot to confii ____________________________ in the
absence of MASP-2 protein (data
not shown). The presence of MAp19 mRNA and the absence of MASP-2 mRNA were
further confirmed using time-resolved RT-PCR on a LightCycler machine. The
MASP-2-/- mice do continue to express MAp19, MASP-1, and MASP-3 mRNA and
protein as expected (data not shown). The presence and abundance of mRNA in
the
MASP-2-/- mice for Properdin, Factor B, Factor D, C4, C2, and C3 was assessed
by
LightCycler analysis and found to be identical to that of the wild-type
littermate controls
(data not shown). The plasma from homozygous MASP-2-/- mice is totally
deficient of
lectin-pathway-mediated complement activation as further described in Example
2.
Generation of a MASP-2-/- strain on a pure C57BL6 Background: The
MASP-2-/- mice were back-crossed with a pure C57BL6 line for nine generations
prior to
use of the MASP-2-/- strain as an experimental animal model.
A transgenic mouse strain that is murine MASP-2-/-, MAp19+/+ and that
expresses a human MASP-2 transgene (a murine MASP-2 knock-out and a human
MASP-2 knock-in) was also generated as follows:
Materials and Methods: A minigene encoding human MASP-2 called "mini
hMASP-2" (SEQ ID NO:49) as shown in FIGURE 4 was constructed which includes
the
promoter region of the human MASP 2 gene, including the first 3 exons (exon 1
to
exon 3) followed by the cDNA sequence that represents the coding sequence of
the
following 8 exons, thereby encoding the full-length MASP-2 protein driven by
its
endogenous promoter. The mini hMASP-2 construct was injected into fertilized
eggs of
-102-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2-/- in order to replace the deficient murine MASP 2 gene by
transgenically
expressed human MASP-2.
EXAMPLE 2
This example demonstrates that MASP-2 is required for complement activation
via the lectin pathway.
Methods and Materials:
Lectin pathway specific C4 Cleavage Assay: A C4 cleavage assay has been
described by Petersen, et al., J. Immunol. Methods 257:107 (2001) that
measures lectin
pathway activation resulting from lipoteichoic acid (LTA) from S. aureus,
which binds
L-ficolin. The assay described by Petersen et al., (2001) was adapted to
measure lectin
pathway activation via MBL by coating the plate with LPS and mannan or zymosan
prior
to adding serum from MASP-2 -/- mice as described below. The assay was also
modified
to remove the possibility of C4 cleavage due to the classical pathway. This
was achieved
by using a sample dilution buffer containing 1 M NaCl, which permits high
affinity
binding of lectin pathway recognition components to their ligands but prevents
activation
of endogenous C4, thereby excluding the participation of the classical pathway
by
dissociating the Cl complex. Briefly described, in the modified assay serum
samples
(diluted in high salt (1 M NaCl) buffer) are added to ligand-coated plates,
followed by the
addition of a constant amount of purified C4 in a buffer with a physiological
concentration of salt. Bound recognition complexes containing MASP-2 cleave
the C4,
resulting in C4b deposition.
Assay Methods:
1) Nunc
Maxisorb microtiter plates (MaxiSorb , Nunc, Cat. No. 442404,
Fisher Scientific) were coated with 1 [tg/m1 mannan (M7504 Sigma) or any other
ligand
(e.g., such as those listed below) diluted in coating buffer (15 mM Na2CO3, 35
mM
NaHCO3, pH 9.6).
The following reagents were used in the assay:
a. mannan (1 [tg/well mannan (M7504 Sigma) in 100 [11 coating
buffer):
b. zymosan (1 [tg/well zymosan (Sigma) in 100 [11 coating buffer);
c. LTA (l[t.g/well in 100 [1.1 coating buffer or 2 jig/well in 20 p1
methanol)
d. 1 [tg of the H-ficolin specific Mab 4H5 in coating buffer
e. PSA from Aerococcus viridans (2 [1g/well in 100 [il coating buffer)
-103-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
f. 100 [tl/well of formalin-fixed S. aureus DSM20233 (0D550=0.5)
in
coating buffer.
2) The plates were incubated overnight at 4 C.
3) After overnight incubation, the residual protein binding sites were
saturated by incubated the plates with 0.1% HSA-TBS blocking buffer (0.1%
(w/v) HSA
in 10 m1\4 Tris-CL, 140 mM NaCl, 1.5 m114 NaN3, pH 7.4) for 1-3 hours, then
washing
the plates 3X with TBS/tween/Ca2+ (TBS with 005% Tween 20 and 5 mM CaCl2,
1 mM MgCl2, pH 7.4).
4) Serum samples to be tested were diluted in MBL-binding buffer (1 M
NaCl) and the diluted samples were added to the plates and incubated overnight
at 4 C.
Wells receiving buffer only were used as negative controls.
5) Following incubation overnight at 4 C, the plates were washed 3X with
TBS/tween/Ca2+ Human C4 (100 1.11/well of 1 jig/m1 diluted in BBS (4 mM
barbital,
145 mM NaC1, 2 mM CaCl2, 1 mM MgCl2, pH 7.4)) was then added to the plates and
incubated for 90 minutes at 37 C. The plates were washed again 3X with
TB S/twe en/C a2+.
6) C4b deposition was detected with an alkaline phosphatase-conjugated
chicken anti-human C4c (diluted 1:1000 in TBS/tween/Ca2+), which was added to
the
plates and incubated for 90 minutes at room temperature. The plates were then
washed
again 3X with TB S/tween/Ca2 .
7) Alkaline phosphatase was detected by adding 100 jil of p-nitrophenyl
phosphate substrate solution, incubating at room temperature for 20 minutes,
and reading
the 0D405 in a microtiter plate reader.
Results: FIGURES 5A-B show the amount of C4b deposition on mannan
(FIGURE 5A) and zymosan (FIGURE 5B) in serum dilutions from MASP-2+/+
(crosses), MASP-2+/- (closed circles) and MASP-2-/- (closed triangles). FIGURE
5C
shows the relative C4 convertase activity on plates coated with zymosan (white
bars) or
mannan (shaded bars) from MASP-2-/+ mice (n=5) and MASP-2-/- mice (n=4)
relative to
wild-type mice (n=5) based on measuring the amount of C4b deposition
normalized to
wild-type serum. The error bars represent the standard deviation. As shown in
FIGURES 5A-C, plasma from MASP-2-/- mice is totally deficient in
lectin-pathway-mediated complement activation on mannan and on zymosan coated
-104-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
plates. These results clearly demonstrate that MASP-2 is an effector component
of the
lectin pathway.
Recombinant MASP-2 reconstitutes Lectin Pathway-Dependent C4
Activation in serum from the MASP-2-/- mice
In order to establish that the absence of MASP-2 was the direct cause of the
loss
of lectin pathway-dependent C4 activation in the MASP-2-/- mice, the effect of
adding
recombinant MASP-2 protein to serum samples was examined in the C4 cleavage
assay
described above. Functionally active murine MASP-2 and catalytically inactive
murine
MASP-2A (in which the active-site serine residue in the serine protease domain
was
substituted for the alanine residue) recombinant proteins were produced and
purified as
described below in Example 3. Pooled serum from 4 MASP-2 -/- mice was pre-
incubated
with increasing protein concentrations of recombinant murine MASP-2 or
inactive
recombinant murine MASP-2A and C4 convertase activity was assayed as described
above.
Results: As shown in FIGURE 6, the addition of functionally active murine
recombinant MASP-2 protein (shown as open triangles) to serum obtained from
the
MASP-2 -/- mice restored lectin pathway-dependent C4 activation in a protein
concentration dependent manner, whereas the catalytically inactive murine MASP-
2A
protein (shown as stars) did not restore C4 activation. The results shown in
FIGURE 6
are normalized to the C4 activation observed with pooled wild-type mouse serum
(shown
as a dotted line).
EXAMPLE 3
This example describes the recombinant expression and protein production of
recombinant full-length human, rat and murine MASP-2, MASP-2 derived
polypeptides,
and catalytically inactivated mutant forms of MASP-2
Expression of Full-length human, murine and rat MASP-2:
The full length cDNA sequence of human MASP-2 (SEQ ID NO: 4) was also
subcloned into the mammalian expression vector pCI-Neo (Promega), which drives
eukaryotic expression under the control of the CMV enhancer/promoter region
(described
in Kaufman R.J. et al., Nucleic Acids Research /9:4485-90, 1991; Kaufman,
Methods in
Enzymology, 185:537-66 (1991)). The full length mouse cDNA (SEQ ID NO:50) and
rat
MASP-2 cDNA (SEQ ID NO:53) were each subcloned into the pED expression vector.
-105-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The MASP-2 expression vectors were then transfected into the adherent Chinese
hamster
ovary cell line DXB1 using the standard calcium phosphate transfection
procedure
described in Maniatis et al., 1989. Cells transfected with these constructs
grew very
slowly, implying that the encoded protease is cytotoxic.
In another approach, the minigene construct (SEQ ID NO:49) containing the
human cDNA of MASP-2 driven by its endogenous promoter is transiently
transfected
into Chinese hamster ovary cells (CHO). The human MASP-2 protein is secreted
into the
culture media and isolated as described below.
Expression of Full-length catalytically inactive NIA SP-2:
Rationale: MASP-2 is activated by autocatalytic cleavage after the recognition
subcomponents MBL or ficolins (either L-ficolin, H-ficolin or M-ficolin) bind
to their
respective carbohydrate pattern. Autocatalytic cleavage resulting in
activation of
MASP-2 often occurs during the isolation procedure of MASP-2 from serum, or
during
the purification following recombinant expression. In order to obtain a more
stable
protein preparation for use as an antigen, a catalytically inactive form of
MASP-2,
designed as MASP-2A was created by replacing the serine residue that is
present in the
catalytic triad of the protease domain with an alanine residue in rat (SEQ ID
NO:55
Ser617 to Ala617); in mouse (SEQ ID NO:52 Ser617 to Ala617); or in human (SEQ
ID
NO:6 5er618 to Ala618).
In order to generate catalytically inactive human and murine MASP-2A proteins,
site-directed mutagenesis was carried out using the oligonucleotides shown in
TABLE 5.
The oligonucleotides in TABLE 5 were designed to anneal to the region of the
human
and murine cDNA encoding the enzymatically active serine and oligonucleotide
contain a
mismatch in order to change the serine codon into an alanine codon. For
example, PCR
oligonucleotides SEQ ID NOS:56-59 were used in combination with human MASP-2
cDNA (SEQ ID NO:4) to amplify the region from the start codon to the
enzymatically
active serine and from the serine to the stop codon to generate the complete
open reading
from of the mutated MASP-2A containing the Ser618 to Ala618 mutation. The PCR
products were purified after agarose gel electrophoresis and band preparation
and single
adenosine overlaps were generated using a standard tailing procedure. The
adenosine
tailed MASP-2A was then cloned into the pGEM-T easy vector, transformed into
E. coil.
A catalytically inactive rat MASP-2A protein was generated by kinasing and
annealing SEQ ID NO:64 and SEQ ID NO:65 by combining these two
oligonucleotides
-106-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
in equal molar amounts, heating at 100 C for 2 minutes and slowly cooling to
room
temperature. The resulting annealed fragment has Pstl and Xbal compatible ends
and
was inserted in place of the Pstl-Xbal fragment of the wild-type rat MASP-2
cDNA
(SEQ ID NO:53) to generate rat MASP-2A.
5 'GAGGTGACGCAGGAGGGGCATTAGTGTTT 3' (SEQ ID NO:64)
5' CTAGAAACACTAATGCCCCTCCTGCGTCACCTCTGCA 3' (SEQ ID
NO: 65)
The human, murine and rat MASP-2A were each further subcloned into either of
the mammalian expression vectors pED or pCI-Neo and transfected into the
Chinese
.. Hamster ovary cell line DX131 as described below.
In another approach, a catalytically inactive form of MASP-2 is constructed
using
the method described in Chen et al., J. Biol. Chem., 276(28):25894-25902,
2001. Briefly,
the plasmid containing the full-length human MASP-2 cDNA (described in Thiel
et al.,
Nature 386:506, 1997) is digested with Xhol and EcoR1 and the MASP-2 cDNA
.. (described herein as SEQ ID NO:4) is cloned into the corresponding
restriction sites of
the pFastBacl baculovirus transfer vector (Life Technologies, NY). The MASP-2
serine
protease active site at Ser618 is then altered to Ala618 by substituting the
double-stranded oligonucleotides encoding the peptide region amino acid 610-
625
(SEQ ID NO:13) with the native region amino acids 610 to 625 to create a MASP-
2 full
length polypeptide with an inactive protease domain.
Construction of Expression Plasmids Containing Polypeptide Regions
Derived from Human Masp-2.
The following constructs are produced using the MASP-2 signal peptide
(residues 1-15 of SEQ ID NO:5) to secrete various domains of MASP-2. A
construct
expressing the human MASP-2 CUBI domain (SEQ ID NO:8) is made by PCR
amplifying the region encoding residues 1-121 of MASP-2 (SEQ ID NO:6)
(corresponding to the N-terminal CUBI domain). A construct expressing the
human
MASP-2 CUBIEGF domain (SEQ ID NO:9) is made by PCR amplifying the region
encoding residues 1-166 of MASP-2 (SEQ ID NO:6) (corresponding to the N-
terminal
CUBIEGF domain). A construct expressing the human MASP-2 CUBIEGFCUBII
domain (SEQ ID NO:10) is made by PCR amplifying the region encoding residues 1-
293
of MASP-2 (SEQ ID NO:6) (corresponding to the N-terminal CUBIEGFCUBII domain).
The above mentioned domains are amplified by PCR using VentR polymerase and
-107-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
pBS-MASP-2 as a template, according to established PCR methods. The 5' primer
sequence of the sense primer (5'-CGGGATCCATGAGGCTGCTGACCCTC-3' SEQ ID
NO:34) introduces a BamHI restriction site (underlined) at the 5' end of the
PCR
products. Antisense primers for each of the MASP-2 domains, shown below in
TABLE 5, are designed to introduce a stop codon (boldface) followed by an
EcoRI site
(underlined) at the end of each PCR product. Once amplified, the DNA fragments
are
digested with BamHI and EcoRI and cloned into the corresponding sites of the
pFastBac I
vector. The resulting constructs are characterized by restriction mapping and
confirmed
by dsDNA sequencing.
TABLE 5: MASP-2 PCR PRIMERS
MASP-2 domain 5' PCR Primer 3' PCR Primer
SEQ ID NO:8 5'CGGGATCCATGAG 5'GGAATTCCTAGGCTGCA
CUBI (aa 1-121 of SEQ GCTGCTGACCCTC-3' TA (SEQ ID NO:35)
ID NO:6) (SEQ ID NO:34)
SEQ ID NO:9 5'CGGGATCCATGAG 5'GGAATTCCTACAGGGCG
CUBIEGF (aa 1-166 of GCTGCTGACCCTC-3' CT-3' (SEQ ID NO:36)
SEQ ID NO:6) (SEQ ID NO:34)
SEQ ID NO:10 5'CGGGATCCATGAG 51GGAATTCCTAGTAGTGG
GCTGCTGACCCTC-3' AT 3' (SEQ ID NO:37)
CUBIEGFCUBII (aa (SEQ ID NO:34)
1-293 of SEQ ID NO:6)
SEQ ID NO:4 5IATGAGGCTGCTGA 5'TTAAAATCACTAATTAT
human MASP-2 CCCTCCTGGGCCTTC GTTCTCGATC 3' (SEQ ID
3' (SEQ ID NO: 56) NO: 59) hMASP-2_reverse
hMASP-2 forward
SEQ ID NO:4 5'CAGAGGTGACGCA 5'GTGCCCCTCCTGCGTCA
human MASP-2 cDNA GGAGGGGCAC 3' CCTCTG 3' (SEQ ID NO: 57)
(SEQ ID NO: 58) hMASP-2 ala reverse
hMA SP-2 ala forward
SEQ ID NO:50 5'ATGAGGCTACTCA 5'TTAGAAATTACTTATTAT
Murine MASP-2 cDNA TCTTCCTGG3' (SEQ GTTCTCAATCC3' (SEQ ID
ID NO: 60) NO: 63) mMASP-2_reverse
mMASP-2 forward
-108-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2 domain 5' PCR Primer 3' PCR Primer
SEQ ID NO:50 5'CCCCCCCTGCGTC 5'CTGCAGAGGTGACGCAG
Murine MASP-2 cDNA ACCTCTGCAG3 (SEQ GGGGGG 3' (SEQ ID NO:
ID NO: 62) 61) mMASP-2_ala_reverse
mMASP-2 ala forward
Recombinant eukaryotic expression of MASP-2 and protein production of
enzymatically inactive mouse, rat, and human MASP-2A.
The MASP-2 and MASP-2A expression constructs described above were
transfected into DXB1 cells using the standard calcium phosphate transfection
procedure
(Maniatis et al., 1989). MASP-2A was produced in serum-free medium to ensure
that
preparations were not contaminated with other serum proteins. Media was
harvested
from confluent cells every second day (four times in total). The level of
recombinant
MASP-2A averaged approximately 1.5 mg/liter of culture medium for each of the
three
species.
MASP-2A protein purification: The MASP-2A (Ser-Ala mutant described
above) was purified by affinity chromatography on MBP-A-agarose columns. This
strategy enabled rapid purification without the use of extraneous tags. MASP-
2A
(100-200 ml of medium diluted with an equal volume of loading buffer (50 mM
Tris-C1,
pH 7.5, containing 150 mM NaCl and 25 mM CaCl2) was loaded onto an MBP-agarose
affinity column (4 ml) pre-equilibrated with 10 ml of loading buffer.
Following washing
with a further 10 ml of loading buffer, protein was eluted in 1 ml fractions
with 50 mM
Tris-C1, pH 7.5, containing 1.25 M NaCl and 10 mM EDTA. Fractions containing
the
MASP-2A were identified by SDS-polyacrylamide gel electrophoresis. Where
necessary,
MASP-2A was purified further by ion-exchange chromatography on a MonoQ column
(FIR 5/5). Protein was dialyzed with 50 mM Tris-Cl pH 7,5, containing 50 mM
NaC1 and
loaded onto the column equilibrated in the same buffer. Following washing,
bound
MASP-2A was eluted with a 0.05-1 M NaCl gradient over 10 ml.
Results: Yields of 0.25-0.5 mg of MASP-2A protein were obtained from 200 ml
of medium. The molecular mass of 77.5 kDa determined by MALDI-MS is greater
than
the calculated value of the unmodified polypeptide (73.5 kDa) due to
glycosylation.
Attachment of glycans at each of the N-glycosylation sites accounts for the
observed
mass. MASP-2A migrates as a single band on SDS-polyacrylamide gels,
demonstrating
-109-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
that it is not proteolytically processed during biosynthesis. The weight-
average molecular
mass determined by equilibrium ultracentrifugation is in agreement with the
calculated
value for homodimers of the glycosylated polypeptide.
PRODUCTION OF RECOMBINANT HUMAN MASP-2 POLYPEPTIDES
Another method for producing recombinant MASP-2 and MASP2A derived
polypeptides is described in Thielens, N.M., et al., J. Immunol. 166:5068-
5077, 2001.
Briefly, the ,õSpodoptera frupperda insect cells (Ready-Plaque Sf9 cells
obtained from
Novagen, Madison, WI) are grown and maintained in Sf90011 serum-free medium
(Life
Technologies) supplemented with 50 IU/ml penicillin and 50 mg/ml streptomycin
(Life
Technologies). The Trichoplusia ni (High Five) insect cells (provided by
Jadwiga
Chroboczek, Institut de Biologie Structurale, Grenoble, France) are maintained
in TC100
medium (Life Technologies) containing 10% FCS (Dominique Dutscher, Brumath,
France) supplemented with 50 IU/ml penicillin and 50 mg/ml streptomycin.
Recombinant baculoviruses are generated using the Bac-to-Bac system
(Life Technologies). The bacmid DNA is purified using the Qiagen midiprep
purification
system (Qiagen) and is used to transfect Sf9 insect cells using cellfectin in
Sf900 II SFM
medium (Life Technologies) as described in the manufacturer's protocol.
Recombinant
virus particles are collected 4 days later, titrated by virus plaque assay,
and amplified as
described by King and Possee, in The Baculovirus Expression System: A
Laboratory
Guide, Chapman and Hall Ltd., London, pp. 111-114, 1992.
High Five cells (1.75 x 107 cells/175-cm2 tissue culture flask) are infected
with the
recombinant viruses containing MASP-2 polypeptides at a multiplicity of
infection of 2 in
Sf900 II SFM medium at 28 C for 96 h. The supernatants are collected by
centrifugation
and diisopropyl phosphorofluoridate is added to a final concentration of 1 mM.
The MASP-2 polypeptides are secreted in the culture medium. The culture
supernatants are dialyzed against 50 mM NaC1, 1 mM CaC12, 50 mM
triethanolamine
hydrochloride, pH 8.1, and loaded at 1.5 ml/min onto a Q-Sepharose Fast Flow
column
(Amersham Pharmacia Biotech) (2.8 x 12 cm) equilibrated in the same buffer.
Elution is
conducted by applying a 1.2 liter linear gradient to 350 mM NaCl in the same
buffer.
Fractions containing the recombinant MASP-2 polypeptides are identified by
Western
blot analysis, precipitated by addition of (NH4)2504 to 60% (w/v), and left
overnight
at 4 C. The pellets are resuspended in 145 mM NaCl, 1 mM CaCl2, 50 m114
triethanolamine hydrochloride, pH 7.4, and applied onto a TSK G3000 SWG column
-110-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
(7.5 x 600 mm) (Tosohaas, Montgomeryville, PA) equilibrated in the same
buffer. The
purified polypeptides are then concentrated to 0.3 mg/ml by ultrafiltration on
Microsep
microconcentrators (m.w. cut-off= 10,000) (Filtron, Karlstein, Germany).
-111-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 4
This example describes a method of producing polyclonal antibodies against
MASP-2 polypeptides.
Materials and Methods:
MASP-2 Antigens: Polyclonal anti-human MASP-2 antiserum is produced by
immunizing rabbits with the following isolated MASP-2 polypeptides: human MASP-
2
(SEQ ID NO:6) isolated from serum; recombinant human MASP-2 (SEQ ID NO:6),
MASP-2A containing the inactive protease domain (SEQ ID NO.13), as described
in
Example 3; and recombinant CUBI (SEQ ID NO:8), CUBEGFI (SEQ ID NO:9), and
CUBEGFCUBII (SEQ ID NO:10) expressed as described above in Example 3.
Polyclonal antibodies: Six-week old Rabbits, primed with BCG (bacillus
Calmette-Guerin vaccine) are immunized by injecting 100 ug of MASP-2
polypeptide at
100 ug/m1 in sterile saline solution. Injections are done every 4 weeks, with
antibody
titer monitored by ELISA assay as described in Example 5. Culture supernatants
are
collected for antibody purification by protein A affinity chromatography.
EXAMPLE 5
This example describes a method for producing murine monoclonal antibodies
against rat or human MASP-2 polypeptides.
Materials and Methods:
Male A/J mice (Harlan, Houston, Tex.), 8-12 weeks old, are injected
subcutaneously with 100 ug human or rat rMASP-2 or rMASP-2A polypeptides (made
as
described in Example 3) in complete Freund's adjuvant (Difco Laboratories,
Detroit,
Mich.) in 200 IA of phosphate buffered saline (PBS) pH 7.4. At two-week
intervals the
mice are twice injected subcutaneously with 50 ug of human or rat rMASP-2 or
rMASP-2A polypeptide in incomplete Freund's adjuvant. On the fourth week the
mice
are injected with 50 kg of human or rat rMASP-2 or rMASP-2A polypeptide in PBS
and
are fused 4 days later.
For each fusion, single cell suspensions are prepared from the spleen of an
immunized mouse and used for fusion with Sp2/0 myeloma cells. 5x108 of the
Sp2/0 and
5x108 spleen cells are fused in a medium containing 50% polyethylene glycol
(MW. 1450) (Kodak, Rochester, N.Y.) and 5% dimethylsulfoxide (Sigma Chemical
Co.,
-112-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
St. Louis, Mo.). The cells are then adjusted to a concentration of 1.5x105
spleen cells per
200 IA of the suspension in Iscove medium (Gibco, Grand Island, N.Y.),
supplemented
with 10% fetal bovine serum, 100 units/ml of penicillin, 100 [ig/m1 of
streptomycin,
0.1 mM hypoxanthine, 0.4 !..EM aminopterin and 16 pM thymidine. Two hundred
microliters of the cell suspension are added to each well of about twenty 96-
well
microculture plates. After about ten days culture supernatants are withdrawn
for
screening for reactivity with purified factor MASP-2 in an ELISA assay.
ELISA Assay: Wells of Immulon 2 (Dynatech Laboratories, Chantilly, Va.)
microtest plates are coated by adding 50 pl of purified hMASP-2 at 50 ng/ml or
rat
rMASP-2 (or rMASP-2A) overnight at room temperature. The low concentration of
MASP-2 for coating enables the selection of high-affinity antibodies. After
the coating
solution is removed by flicking the plate, 200 [11 of BLOTTO (non-fat dry
milk) in PBS is
added to each well for one hour to block the non-specific sites. An hour
later, the wells
are then washed with a buffer PB ST (PBS containing 0.05% Tween 20). Fifty
microliters
of culture supernatants from each fusion well is collected and mixed with 50
IA of
BLOTTO and then added to the individual wells of the microtest plates. After
one hour
of incubation, the wells are washed with PBST. The bound murine antibodies are
then
detected by reaction with horseradish peroxidase (HRP) conjugated goat anti-
mouse IgG
(Fc specific) (Jackson ImmunoResearch Laboratories, West Grove, Pa.) and
diluted at
1:2,000 in BLOTTO. Peroxidase substrate solution containing 0.1% 3,3,5,5
tetramethyl
benzidine (Sigma, St. Louis, Mo.) and 0.0003% hydrogen peroxide (Sigma) is
added to
the wells for color development for 30 minutes. The reaction is terminated by
addition of
50 pl of 2M H2504 per well. The Optical Density at 450 nm of the reaction
mixture is
read with a BioTek ELISA Reader (BioTek Instruments, Winooski, Vt.).
MASP-2 Binding Assay:
Culture supernatants that test positive in the MASP-2 ELISA assay described
above can be tested in a binding assay to determine the binding affinity the
MASP-2
inhibitory agents have for MASP-2. A similar assay can also be used to
determine if the
inhibitory agents bind to other antigens in the complement system.
Polystyrene microtiter plate wells (96-well medium binding plates, Corning
Costar, Cambridge, MA) are coated with MASP-2 (20 ng/100 0/well, Advanced
Research Technology, San Diego, CA) in phosphate-buffered saline (PBS) pH 7.4
-113-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
overnight at 4 C. After aspirating the MASP-2 solution, wells are blocked with
PBS
containing 1% bovine serum albumin (BSA; Sigma Chemical) for 2 h at room
temperature. Wells without MASP-2 coating serve as the background controls.
Aliquots
of hybridoma supernatants or purified anti-MASP-2 MoAbs, at varying
concentrations in
blocking solution, are added to the wells. Following a 2 h incubation at room
temperature, the wells are extensively rinsed with PBS. MASP-2-bound anti-MASP-
2
MoAb is detected by the addition of peroxidase-conjugated goat anti-mouse IgG
(Sigma
Chemical) in blocking solution, which is allowed to incubate for lh at room
temperature.
The plate is rinsed again thoroughly with PBS, and 100 HI of 3,3',5,5'-
tetramethyl
benzidine (TMB) substrate (Kirkegaard and Perry Laboratories, Gaithersburg,
MD) is
added. The reaction of TMB is quenched by the addition of 100 IA of 1M
phosphoric
acid, and the plate is read at 450 nm in a microplate reader (SPECTRA MAX 250,
Molecular Devices, Sunnyvale, CA).
The culture supernatants from the positive wells are then tested for the
ability to
inhibit complement activation in a functional assay such as the C4 cleavage
assay as
described in Example 2. The cells in positive wells are then cloned by
limiting dilution.
The MoAbs are tested again for reactivity with hMASP-2 in an ELISA assay as
described
above. The selected hybridomas are grown in spinner flasks and the spent
culture
supernatant collected for antibody purification by protein A affinity
chromatography.
EXAMPLE 6
This example describes the generation and production of humanized murine
anti -MA SP-2 antibodies and antibody fragments.
A murine anti-MASP-2 monoclonal antibody is generated in Male A/J mice as
described in Example 5. The murine antibody is then humanized as described
below to
reduce its immunogenicity by replacing the murine constant regions with their
human
counterparts to generate a chimeric IgG and Fab fragment of the antibody,
which is useful
for inhibiting the adverse effects of MASP-2-dependent complement activation
in human
subjects in accordance with the present invention.
1. Cloning of anti-
1(ASP-2 variable region genes from murine
hybridoma cells. Total
RNA is isolated from the hybridoma cells secreting
anti-MASP-2 MoAb (obtained as described in Example 7) using RNAzol following
the
manufacturer's protocol (Biotech, Houston, Tex.). First strand cDNA is
synthesized from
-114-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
the total RNA using oligo dT as the primer. PCR is performed using the
immunoglobulin
constant C region-derived 3' primers and degenerate primer sets derived from
the leader
peptide or the first framework region of murine VH or VK genes as the 5'
primers.
Anchored PCR is carried out as described by Chen and Platsucas (Chen, P.F.,
Scand. J.
Immunot 35:539-549, 1992). For cloning the VK gene, double-stranded cDNA is
prepared using a Notl-MAK1 primer (5'-TGCGGCCGCTGTAGGTGCTGTCTTT-3'
SEQ ID NO:38). Annealed adaptors AD1 (5'-GGAATTCACTCGTTATTCTCGGA-3'
SEQ ID NO:39) and AD2 (5'-TCCGAGAATAACGAGTG-3' SEQ ID NO:40) are ligated
to both 5' and 3' termini of the double-stranded cDNA. Adaptors at the 3' ends
are
removed by Notl digestion. The digested product is then used as the template
in PCR
with the AD1 oligonucleotide as the 5' primer and MAK2
(5'-CATTGAAAGCTTTGGGGTAGAAGTTGTTC-3' SEQ ID NO:41) as the 3' primer.
DNA fragments of approximately 500 bp are cloned into pUC19. Several clones
are
selected for sequence analysis to verify that the cloned sequence encompasses
the
expected murine immunoglobulin constant region. The Notl-MAK1 and MAK2
oligonucleotides are derived from the VK region and are 182 and 84 bp,
respectively,
downstream from the first base pair of the C kappa gene. Clones are chosen
that include
the complete VK and leader peptide.
For cloning the VH gene, double-stranded cDNA is prepared using the Notl
MAGI primer (5'-CGCGGCCGCAGCTGCTCAGAGTGTAGA-3' SEQ ID NO:42).
Annealed adaptors AD1 and AD2 are ligated to both 5' and 3' termini of the
double-stranded cDNA. Adaptors at the 3' ends are removed by Notl digestion.
The
digested product are used as the template in PCR with the AD1 oligonucleotide
and
MAG2 (5'-CGGTAAGCTTCACTGGCTCAGGGAAATA-3' SEQ ID NO :43) as primers.
DNA fragments of 500 to 600 bp in length are cloned into pUC19. The Notl-MAG1
and
MAG2 oligonucleoti des are derived from the murine Cy.7.1 region, and are 180
and
93 bp, respectively, downstream from the first bp of the murine Cy. 7.1 gene.
Clones are
chosen that encompass the complete VH and leader peptide.
2.
Construction of Expression Vectors for Chimeric MASP-2 IgG and
Fab. The cloned VH and VK genes described above are used as templates in a PCR
reaction to add the Kozak consensus sequence to the 5' end and the splice
donor to the
3' end of the nucleotide sequence. After the sequences are analyzed to confirm
the
-115-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
absence of PCR errors, the VH and VK genes are inserted into expression vector
cassettes
containing human C.y1 and C. kappa respectively, to give pSV2neoVH-huCyl and
pSV2neoV-huCy. CsC1 gradient-purified plasmid DNAs of the heavy- and light-
chain
vectors are used to transfect COS cells by electroporation. After 48 hours,
the culture
supernatant is tested by ELISA to confirm the presence of approximately 200
ng/ml of
chimeric IgG. The cells are harvested and total RNA is prepared. First strand
cDNA is
synthesized from the total RNA using oligo dT as the primer. This cDNA is used
as the
template in PCR to generate the Fd and kappa DNA fragments. For the Fd gene,
PCR is
carried out using 5'-AAGAAGCTTGCCGCCACCATGGATTGGCTGIGGAACT-3'
(SEQ ID NO.44) as the 5 primer and a CH1-derived 3' primer
(5'-CGGGATCCTCAAACTTTCTTGTCCACCTTGG-3' SEQ ID NO:45). The DNA
sequence is confirmed to contain the complete VH and the CH1 domain of human
IgGl.
After digestion with the proper enzymes, the Fd DNA fragments are inserted at
the
HindIII and BamHI restriction sites of the expression vector cassette pSV2dhfr-
TUS to
give pSV2dhfrFd. The pSV2 plasmid is commercially available and consists of
DNA
segments from various sources: pBR322 DNA (thin line) contains the pBR322
origin of
DNA replication (pBR on) and the lactamase ampicillin resistance gene (Amp);
SV40
DNA, represented by wider hatching and marked, contains the SV40 origin of DNA
replication (SV40 on), early promoter (5' to the dhfr and neo genes), and
polyadenylation
signal (3' to the dhfr and neo genes). The SV40-derived polyadenylation signal
(pA) is
also placed at the 3' end of the Fd gene.
For the kappa gene, PCR is carried out using 5'-
AAGAAAGCTTGCCGCCACCATGTTCTCACTAGCTCT-3' (SEQ ID NO:46) as the 5'
primer and a CK-derived 3' primer (5'-CGGGATCCTTCTCCCTCTAACACTCT-3' SEQ
ID NO:47). DNA sequence is confirmed to contain the complete VK and human CK
regions. After digestion with proper restriction enzymes, the kappa DNA
fragments are
inserted at the HindIII and BamHI restriction sites of the expression vector
cassette
pSV2neo-TUS to give pSV2neoK. The expression of both Fd and .kappa genes are
driven by the HCMV-derived enhancer and promoter elements. Since the Fd gene
does
not include the cysteine amino acid residue involved in the inter-chain
disulfide bond, this
recombinant chimeric Fab contains non-covalently linked heavy- and light-
chains. This
chimeric Fab is designated as cFab.
-116-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
To obtain recombinant Fab with an inter-heavy and light chain disulfide bond,
the
above Fd gene may be extended to include the coding sequence for additional 9
amino
acids (EPKSCDKTH SEQ ID NO:48) from the hinge region of human IgG1 . The
BstEII-BamHI DNA segment encoding 30 amino acids at the 3' end of the Fd gene
may
be replaced with DNA segments encoding the extended Fd, resulting in
pSV2dhfrFd/9aa.
3. Expression and Purification of Chimeric Anti-MASP-2 IgG
To generate cell lines secreting chimeric anti-MASP-2 IgG, NSO cells are
transfected with purified plasmid DNAs of pSV2neoVH-huC.y1 and pSV2neoV-huC
kappa by electroporation. Transfected cells are selected in the presence of
0.7 mg/ml
G418. Cells are grown in a 250 ml spinner flask using serum-containing medium.
Culture supernatant of 100 ml spinner culture is loaded on a 10-ml PROSEP-A
column (Bioprocessing, Inc., Princeton, N.J.). The column is washed with 10
bed
volumes of PBS. The bound antibody is eluted with 50 mM citrate buffer, pH
3Ø Equal
volume of 1 M Hepes, pH 8.0 is added to the fraction containing the purified
antibody to
adjust the pH to 7Ø Residual salts are removed by buffer exchange with PBS
by
Millipore membrane ultrafiltration (MW. cut-off: 3,000). The protein
concentration of
the purified antibody is determined by the BCA method (Pierce).
4. Expression and purification of chimeric anti-MASP-2 Fab
To generate cell lines secreting chimeric anti-MASP-2 Fab, CHO cells are
transfected with purified plasmid DNAs of pSV2dhfrFd (or pSV2dhfrFd/9aa) and
pSV2neokappa, by electroporation. Transfected cells are selected in the
presence of
G418 and methotrexate. Selected cell lines are amplified in increasing
concentrations of
methotrexate Cells are single-cell subcloned by limiting dilution. High-
producing
single-cell subcloned cell lines are then grown in 100 ml spinner culture
using serum-free
medium.
Chimeric anti-MASP-2 Fab is purified by affinity chromatography using a mouse
anti-idiotypic MoAb to the MASP-2 MoAb. An anti-idiotypic MASP-2 MoAb can be
made by immunizing mice with a murine anti-MASP-2 MoAb conjugated with keyhole
limpet hemocyanin (KLH) and screening for specific MoAb binding that can be
competed with human MASP-2. For purification, 100 ml of supernatant from
spinner
cultures of CHO cells producing cFab or cFab/9aa are loaded onto the affinity
column
coupled with an anti-idiotype MASP-2 MoAb. The column is then washed
thoroughly
with PBS before the bound Fab is eluted with 50 mM diethylamine, pH 11.5.
Residual
-117-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
salts are removed by buffer exchange as described above. The protein
concentration of
the purified Fab is determined by the BCA method (Pierce).
The ability of the chimeric MASP-2 IgG, cFab, and cFAb/9aa to inhibit
MASP-2-dependent complement pathways may be determined by using the inhibitory
assays described in Example 2 or Example 7.
EXAMPLE 7
This example describes an in vitro C4 cleavage assay used as a functional
screen
to identify MASP-2 inhibitory agents capable of blocking MASP-2-dependent
complement activation via L-ficolin/P35, H-ficolin, M-ficolin or mannan.
C4 Cleavage Assay: A C4 cleavage assay has been described by Petersen,
S.V., et al., J. Imrnunol. Methods 257:107, 2001, which measures lectin
pathway
activation resulting from lipoteichoic acid (LTA) from S. aurens which binds L-
ficolin.
Reagents: Forma1in-fixed S. aureons (DSM20233) is prepared as follows:
bacteria is grown overnight at 37 C in tryptic soy blood medium, washed three
times with
PBS, then fixed for 1 h at room temperature in PBS/0.5% formalin, and washed a
further
three times with PBS, before being resuspended in coating buffer (15 mM
Na2Co3,
35 mM NaHCO3, pH 9.6).
Assay: The wells of a Nunc MaxiSorb microtiter plate (Nalgene Nunc
International, Rochester, NY) are coated with: 100 ul of formalin-fixed S.
aureus
DSM20233 (0D550 = 0.5) in coating buffer with 1 tg of L-ficolin in coating
buffer.
After overnight incubation, wells are blocked with 0.1% human serum albumin
(HSA) in
TBS (10 mM Tris-HC1, 140 mM NaC1, pH 7.4), then are washed with TBS containing
0.05% Tween 20 and 5 mM CaCl2 (wash buffer). Human serum samples are diluted
in
20 mM Tris-HC1, 1 M NaCl, 10 mM CaCl2, 0.05% Triton X-100, 0.1% HSA, pH 7.4,
which prevents activation of endogenous C4 and dissociates the CI complex
(composed
of Clq, Clr and C Is). MASP-2 inhibitory agents, including anti-MASP-2 MoAbs
and
inhibitory peptides are added to the serum samples in varying concentrations.
The diluted
samples are added to the plate and incubated overnight at 4 C. After 24 hours,
the plates
are washed thoroughly with wash buffer, then 0.1 ug of purified human C4
(obtained as
described in Dodds, A.W , Method.s Enzytnol. 223:46, 1993) in 100 ul of 4 mM
barbital,
145 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4 is added to each well. After 1.5 h
at
-118-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
37 C, the plates are washed again and C4b deposition is detected using
alkaline
phosphatase-conjugated chicken anti-human C4c (obtained from Immunsystem,
Uppsala,
Sweden) and measured using the colorimetric substrate p-nitrophenyl phosphate.
C4 Assay on mannan: The assay described above is adapted to measure lectin
.. pathway activation via MBL by coating the plate with LSP and mannan prior
to adding
serum mixed with various MASP-2 inhibitory agents.
C4 assay on H-ficolin (Hakata Ag): The assay described above is adapted to
measure lectin pathway activation via H-ficolin by coating the plate with LPS
and
H-ficolin prior to adding serum mixed with various MASP-2 inhibitory agents.
EXAMPLE 8
The following assay demonstrates the presence of classical pathway activation
in
wild-type and MASP-2-/- mice.
Methods: Immune complexes were generated in situ by coating microtiter plates
(MaxiSorb , Nunc, cat. No. 442404, Fisher Scientific) with 0.1% human serum
albumin
in 10 mM Tris, 140 m11/1 NaCl, pH 7.4 for 1 hours at room temperature followed
by
overnight incubation at 4 C with sheep anti whole serum antiserum (Scottish
Antibody
Production Unit, Carluke, Scotland) diluted 1:1000 in TBS/tween/Ca2+. Serum
samples
were obtained from wild-type and MASP-2-/- mice and added to the coated
plates.
Control samples were prepared in which Clq was depleted from wild-type and
MASP-2-/- serum samples. C 1 q-depleted mouse serum was prepared using
protein-A-coupled Dynabeads (Dynal Biotech, Oslo, Norway) coated with rabbit
anti-human Clq IgG (Dako, Glostrup, Denmark), according to the supplier's
instructions.
The plates were incubated for 90 minutes at 37 C. Bound C3b was detected with
a
polyclonal anti-human-C3c Antibody (Dako A 062) diluted in TBS/tw/ Ca ++ at
1.1000.
The secondary antibody is goat anti-rabbit IgG.
Results: FIGURE 7 shows the relative C3b deposition levels on plates coated
with IgG in wild-type serum, MASP-2-/- serum, Clq-depleted wild-type and
Clq-depleted MASP-2-/- serum. These results demonstrate that the classical
pathway is
intact in the MASP-2-i- mouse strain.
-119-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 9
The following assay is used to test whether a MASP-2 inhibitory agent blocks
the
classical pathway by analyzing the effect of a MASP-2 inhibitory agent under
conditions
in which the classical pathway is initiated by immune complexes.
Methods: To test the effect of a MASP-2 inhibitory agent on conditions of
complement activation where the classical pathway is initiated by immune
complexes,
triplicate 50 pi samples containing 90% NHS are incubated at 37 C in the
presence of
pg/ml immune complex (IC) or PBS, and parallel triplicate samples (+1-IC) are
also
included which contain 200 nM anti-properdin monoclonal antibody during the 37
C
10 incubation. After a two hour incubation at 37 C, 13 mM EDTA is added to
all samples to
stop further complement activation and the samples are immediately cooled to 5
C. The
samples are then stored at -70 C prior to being assayed for complement
activation
products (C3a and sC5b-9) using ELISA kits (Quidel, Catalog Nos. A015 and
A009)
following the manufacturer's instructions.
EXAMPLE 10
This example describes the identification of high affinity anti-MASP-2 Fab2
antibody fragments that block MASP-2 activity.
Background and rationale: MASP-2 is a complex protein with many separate
functional domains, including: binding site(s) for MBL and ficolins, a serine
protease
catalytic site, a binding site for proteolytic substrate C2, a binding site
for proteolytic
substrate C4, a MASP-2 cleavage site for autoactivation of MASP-2 zymogen, and
two
Ca binding sites. Fab2 antibody fragments were identified that bind with high
affinity
to MASP-2, and the identified Fab2 fragments were tested in a functional assay
to
determine if they were able to block MASP-2 functional activity.
To block MASP-2 functional activity, an antibody or Fab2 antibody fragment
must bind and interfere with a structural epitope on MASP-2 that is required
for MASP-2
functional activity. Therefore, many or all of the high affinity binding anti-
MASP-2
Fab2s may not inhibit MASP-2 functional activity unless they bind to
structural epitopes
on MASP-2 that are directly involved in MASP-2 functional activity.
A functional assay that measures inhibition of lectin pathway C3 convertase
formation was used to evaluate the "blocking activity" of anti-MASP-2 Fab2s.
It is
known that the primary physiological role of MASP-2 in the lectin pathway is
to generate
-120-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
the next functional component of the lectin-mediated complement pathway,
namely the
lectin pathway C3 convertase. The lectin pathway C3 convertase is a critical
enzymatic
complex (C4bC2a) that proteolytically cleaves C3 into C3a and C3b. MASP-2 is
not a
structural component of the lectin pathway C3 convertase (C4bC2a); however,
MASP-2
functional activity is required in order to generate the two protein
components (C4b, C2a)
that comprise the lectin pathway C3 convertase. Furthermore, all of the
separate
functional activities of MASP-2 listed above appear to be required in order
for MASP-2
to generate the lectin pathway C3 convertase. For these reasons, a preferred
assay to use
in evaluating the "blocking activity" of anti-MASP-2 Fab2s is believed to be a
functional
assay that measures inhibition of lectin pathway C3 convertase formation.
Generation of High Affinity Fab2s: A phage display library of human variable
light and heavy chain antibody sequences and automated antibody selection
technology
for identifying Fab2s that react with selected ligands of interest was used to
create high
affinity Fab2s to rat MASP-2 protein (SEQ ID NO:55). A known amount of rat
MASP-2
(-1 mg, >85% pure) protein was utilized for antibody screening. Three rounds
of
amplification were utilized for selection of the antibodies with the best
affinity.
Approximately 250 different hits expressing antibody fragments were picked for
ELISA
screening. High affinity hits were subsequently sequenced to determine
uniqueness of
the different antibodies.
Fifty unique anti-MASP-2 antibodies were purified and 250 jig of each purified
Fab2 antibody was used for characterization of MASP-2 binding affinity and
complement
pathway functional testing, as described in more detail below.
Assays used to Evaluate the Inhibitory (blocking) Activity of Anti-MASP-2
Fab2s
1. Assay to Measure
Inhibition of Formation of Lectin Pathway C3
Convertase:
Background. The lectin pathway C3 convertase is the enzymatic complex
(C4bC2a) that proteolytically cleaves C3 into the two potent proinflammatory
fragments,
anaphylatoxin C3a and opsonic C3b. Formation of C3 convertase appears to a key
step in
the lectin pathway in terms of mediating inflammation. MASP-2 is not a
structural
component of the lectin pathway C3 convertase (C4bC2a); therefore anti-MASP-2
antibodies (or Fab2) will not directly inhibit activity of preexisting C3
convertase.
However, MASP-2 serine protease activity is required in order to generate the
two protein
-121-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
components (C4b, C2a) that comprise the lectin pathway C3 convertase.
Therefore,
anti-MASP-2 Fab2 which inhibit MASP-2 functional activity (i.e., blocking anti-
MASP-2
Fab2) will inhibit de novo formation of lectin pathway C3 convertase. C3
contains an
unusual and highly reactive thioester group as part of its structure. Upon
cleavage of C3
by C3 convertase in this assay, the thioester group on C3b can form a covalent
bond with
hydroxyl or amino groups on macromolecules immobilized on the bottom of the
plastic
wells via ester or amide linkages, thus facilitating detection of C3b in the
ELISA assay.
Yeast mannan is a known activator of the lectin pathway. In the following
method to measure formation of C3 convertase, plastic wells coated with mannan
were
incubated for 30 min at 37 C with diluted rat serum to activate the lectin
pathway. The
wells were then washed and assayed for C3b immobilized onto the wells using
standard
ELISA methods. The amount of C3b generated in this assay is a direct
reflection of the
de novo formation of lectin pathway C3 convertase. Anti-MASP-2 Fab2s at
selected
concentrations were tested in this assay for their ability to inhibit C3
convertase
formation and consequent C3b generation.
Methods:
96-well Costar Medium Binding plates were incubated overnight at 5 C with
mannan diluted in 50 mM carbonate buffer, pH 9.5 at 1 mg/50 4/well. After
overnight
incubation, each well was washed three times with 200 4 PBS. The wells were
then
blocked with 100 4/well of 1% bovine serum albumin in PBS and incubated for
one hour at room temperature with gentle mixing. Each well was then washed
three
times with 200 4 of PBS. The anti-MASP-2 Fab2 samples were diluted to selected
concentrations in Ca ++ and Mg ++ containing GVB buffer (4.0 mM barbital, 141
mM NaC1,
1.0 mM MgCl2, 2.0 mM CaCl2, 0.1% gelatin, pH 7.4) at 5 C. A 0.5% rat serum was
added to the above samples at 5 C and 100 uL was transferred to each well.
Plates were
covered and incubated for 30 minutes in a 37 C waterbath to allow complement
activation. The reaction was stopped by transferring the plates from the 37 C
waterbath
to a container containing an ice-water mix. Each well was washed five times
with 200
4 with PBS-Tween 20 (0.05% Tween 20 in PBS), then washed two times with 200 4
PBS. A 100 4/well of 1:10,000 dilution of the primary antibody (rabbit anti-
human
C3c, DAKO A0062) was added in PBS containing 2.0 mg/ml bovine serum albumin
and
incubated 1 hr at room temperature with gentle mixing. Each well was washed 5
x 200
4 PBS. 100 4/well of 1:10,000 dilution of the secondary antibody
-122-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
(peroxidase-conjugated goat anti-rabbit IgG, American Qualex A102PU) was added
in
PBS containing 2.0 mg/ml bovine serum albumin and incubated for one hour at
room
temperature on a shaker with gentle mixing. Each well was washed five times
with 200
[IL with PBS. 100 it/well of the peroxidase substrate TMB (Kirkegaard & Perry
Laboratories) was added and incubated at room temperature for 10 min. The
peroxidase
reaction was stopped by adding 100 uL/well of 1.0 M H3PO4 and the 0D450 was
measured.
2. Assay to Measure Inhibition of A1ASP-2-dependent C4 Cleavage
Background: The serine protease activity of MASP-2 is highly specific and only
two protein substrates for MASP-2 have been identified; C2 and C4. Cleavage of
C4
generates C4a and C4b. Anti-MASP-2 Fab2 may bind to structural epitopes on
MASP-2
that are directly involved in C4 cleavage (e.g., MASP-2 binding site for C4;
MASP-2
serine protease catalytic site) and thereby inhibit the C4 cleavage functional
activity of
MASP-2.
Yeast mannan is a known activator of the lectin pathway. In the following
method to measure the C4 cleavage activity of MASP-2, plastic wells coated
with
mannan were incubated for 30 minutes at 37 C with diluted rat serum to
activate the
lectin pathway. Since the primary antibody used in this ELISA assay only
recognizes
human C4, the diluted rat serum was also supplemented with human C4 (1.0
jig/m1). The
wells were then washed and assayed for human C4b immobilized onto the wells
using
standard ELISA methods. The amount of C4b generated in this assay is a measure
of
MASP-2 dependent C4 cleavage activity. Anti-MASP-2 Fab2 at selected
concentrations
were tested in this assay for their ability to inhibit C4 cleavage.
Methods: 96-well Costar Medium Binding plates were incubated overnight at
5 C with mannan diluted in 50 mM carbonate buffer, pH 9.5 at 1.0 vg/50
uL/well. Each
well was washed 3X with 200 [IL PBS. The wells were then blocked with 100
u.L/well of
1% bovine serum albumin in PBS and incubated for one hour at room temperature
with
gentle mixing. Each well was washed 3X with 200 jiL of PBS. Anti-MASP-2 Fab2
samples were diluted to selected concentrations in Ca and Mg' containing GVB
buffer
(4.0 mM barbital, 141 mM NaCl, 1.0 mM MgCl2, 2.0 mM CaCl2, 0.1% gelatin, pH
7.4) at
5 C. 1.0 jig/m1 human C4 (Quidel) was also included in these samples. 0.5% rat
serum
was added to the above samples at 5 C and 100 [tL was transferred to each
well. The
plates were covered and incubated for 30 minutes in a 37 C waterbath to allow
-123-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
complement activation. The reaction was stopped by transferring the plates
from the
37 C waterbath to a container containing an ice-water mix. Each well was
washed
x 200 4 with PBS-Tween 20 (0.05% Tween 20 in PBS), then each well was washed
with 2X with 200 4 PBS. 100 4/well of 1:700 dilution of biotin-conjugated
chicken
5 anti-human C4c (Immunsystem AB, Uppsala, Sweden) was added in PBS containing
2.0 mg/ml bovine serum albumin (BSA) and incubated one hour at room
temperature
with gentle mixing. Each well was washed 5 x 200 4 PBS. 100 4/well of 0.1
ug/m1 of
peroxidase-conjugated streptavidin (Pierce Chemical #21126) was added in PBS
containing 2.0 mg/ml BSA and incubated for one hour at room temperature on a
shaker
with gentle mixing. Each well was washed 5 x 200 4 with PBS. 100 iL/well of
the
peroxidase substrate TMB (Kirkegaard & Perry Laboratories) was added and
incubated at
room temperature for 16 min. The peroxidase reaction was stopped by adding
100 4/well of 1.0 M H3PO4 and the OD45o was measured.
3. Binding Assay of anti-rat MASP-2 Fab2 to 'Native' rat MASP-2
Background: MASP-2 is usually present in plasma as a MASP-2 dimer complex
that also includes specific lectin molecules (mannose-binding protein (MBL)
and
ficolins). Therefore, if one is interested in studying the binding of anti-
MASP-2 Fab2 to
the physiologically relevant form of MASP-2, it is important to develop a
binding assay
in which the interaction between the Fab2 and 'native' MASP-2 in plasma is
used, rather
than purified recombinant MASP-2. In this binding assay the 'native' MASP-2-
MBL
complex from 10% rat serum was first immobilized onto mannan-coated wells. The
binding affinity of various anti-MASP-2 Fab2s to the immobilized 'native' MASP-
2 was
then studied using a standard ELIS A methodology.
Methods: 96-well Costar High Binding plates were incubated overnight at 5 C
with mannan diluted in 50 mM carbonate buffer, pH 9.5 at 1 tig/50 4/well. Each
well
was washed 3X with 200 4 PBS. The wells were blocked with 100 4/well of 0.50/0
nonfat dry milk in PBST (PBS with 0.05% Tween 20) and incubated for one hour
at room
temperature with gentle mixing. Each well was washed 3X with 200 4 of
TBS/Tween/Ca" Wash Buffer (Tris-buffered saline, 0.05% Tween 20, containing
5.0 mM CaCl2, pH 7.4. 10% rat serum in High Salt Binding Buffer (20 mM Tris,
1.0 M
NaCl, 10 mM CaCl2, 0.05% Triton-X100, 0.1% (w/v) bovine serum albumin, pH 7.4)
was prepared on ice. 100 4/well was added and incubated overnight at 5 C.
Wells were
washed 3X with 200 4 of TBS/Tween/Ca Wash Buffer. Wells were then washed 2X
-124-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
with 200 laL PBS. 100 [IL /well of selected concentration of anti-MASP-2 Fab2
diluted
in Ca +- and Mg ++ containing GVB Buffer (4.0 mM barbital, 141 mM NaCl, 1.0
m11/1
MgCl2, 2.0 mM CaCl2, 0.1% gelatin, pH 7.4) was added and incubated for one
hour at
room temperature with gentle mixing. Each well was washed 5 x 200 [IL PBS. 100
aL/well of HRP-conjugated goat anti-Fab2 (Biogenesis Cat No 0500-0099) diluted
1:5000 in 2.0 mg/ml bovine serum albumin in PBS was added and incubated for
one hour
at room temperature with gentle mixing. Each well was washed 5 x 200 L, PBS.
100
pt/well of the peroxidase substrate TMB (Kirkegaard & Perry Laboratories) was
added
and incubated at room temperature for 70 min. The peroxidase reaction was
stopped by
adding 100 tit/well of 1.0 M H3PO4 and 0D450 was measured.
RESULTS:
Approximately 250 different Fab2s that reacted with high affinity to the rat
MASP-2 protein were picked for ELISA screening. These high affinity Fab2s were
sequenced to determine the uniqueness of the different antibodies, and 50
unique
anti-MASP-2 antibodies were purified for further analysis. 250 lag of each
purified Fab2
antibody was used for characterization of MASP-2 binding affinity and
complement
pathway functional testing. The result of this analysis is shown below in
TABLE 6.
TABLE 6: ANTI-MASP-2 FAB2 THAT BLOCK LECTIN PATHWAY
COMPLEMENT ACTIVATION
Fab2 antibody # C3 Conyertase Kd C4 Cleavage
(IC50 (nM) IC50 (nM)
88 0.32 4.1 ND
41 0.35 0.30 0.81
11 0.46 0.86 <2 nM
86 0.53 1.4 ND
81 0.54 2.0 ND
66 0.92 4.5 ND
57 0.95 3.6 <2 nM
40 1.1 7.2 0.68
58 1.3 2.6 ND
60 1.6 3.1 ND
52 1.6 5.8 <2 nM
63 2.0 6.6 ND
-125-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Fab2 antibody # C3 Convertase Kd C4 Cleavage
(IC50 (nM) IC50 (nM)
49 2.8 8.5 <2 nM
89 3.0 2.5 ND
71 3.0 10.5 ND
87 6.0 2.5 ND
67 10.0 7.7 ND
As shown above in TABLE 6, of the 50 anti-MASP-2 Fab2s tested, seventeen
Fab2s were identified as MASP-2 blocking Fab2 that potently inhibit C3
convertase
formation with IC50 equal to or less than 10 nM Fab2s (a 34% positive hit
rate). Eight of
the seventeen Fab2s identified have IC50s in the subnanomolar range.
Furthermore, all
seventeen of the MASP-2 blocking Fab2s shown in TABLE 6 gave essentially
complete
inhibition of C3 convertase formation in the lectin pathway C3 convertase
assay.
FIGURE 8A graphically illustrates the results of the C3 convertase formation
assay for
Fab2 antibody #11, which is representative of the other Fab2 antibodies
tested, the results
of which are shown in TABLE 6. This is an important consideration, since it is
theoretically possible that a "blocking" Fab2 may only fractionally inhibit
MASP-2
function even when each MASP-2 molecule is bound by the Fab2.
Although mannan is a known activator of the lectin pathway, it is
theoretically
possible that the presence of anti-mannan antibodies in the rat serum might
also activate
the classical pathway and generate C3b via the classical pathway C3
convertase.
However, each of the seventeen blocking anti-MASP-2 Fab2s listed in this
example
potently inhibits C3b generation (>95 %), thus demonstrating the specificity
of this assay
for lectin pathway C3 convertase.
Binding assays were also performed with all seventeen of the blocking Fab2s in
order to calculate an apparent Kd for each. The results of the binding assays
of anti-rat
MASP-2 Fab2s to native rat MASP-2 for six of the blocking Fab2s are also shown
in
TABLE 6. FIGURE 8B graphically illustrates the results of a binding assay with
the
Fab2 antibody #11. Similar binding assays were also carried out for the other
Fab2s, the
results of which are shown in TABLE 6. In general, the apparent Kds obtained
for
binding of each of the six Fab2s to 'native MASP-2 corresponds reasonably well
with the
IC50 for the Fab2 in the C3 convertase functional assay. There is evidence
that MASP-2
-126-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
undergoes a conformational change from an 'inactive' to an 'active' form upon
activation
of its protease activity (Feinberg et al., EIVIBO J 22:2348-59 (2003); Gal et
al., J. Biol.
Chem. 280:33435-44 (2005)). In the normal rat plasma used in the C3 convertase
formation assay, MASP-2 is present primarily in the 'inactive' zymogen
conformation. In
contrast, in the binding assay, MASP-2 is present as part of a complex with
MBL bound
to immobilized mannan; therefore, the MASP-2 would be in the 'active'
conformation
(Petersen et al.õI Immunol Methods 257:107-16, 2001). Consequently, one would
not
necessarily expect an exact correspondence between the IC50 and Kd for each of
the
seventeen blocking Fab2 tested in these two functional assays since in each
assay the
Fab2 would be binding a different conformational form of MASP-2. Never-the-
less, with
the exception of Fab2 #88, there appears to be a reasonably close
correspondence
between the IC50 and apparent Kd for each of the other sixteen Fab2 tested in
the two
assays (see TABLE 6).
Several of the blocking Fab2s were evaluated for inhibition of MASP-2 mediated
cleavage of C4. FIGURE 8C graphically illustrates the results of a C4 cleavage
assay,
showing inhibition with Fab2 #41, with an IC50=0.81 nM (see TABLE 6). As shown
in
FIGURE 9, all of the Fab2s tested were found to inhibit C4 cleavage with IC50s
similar
to those obtained in the C3 convertase assay (see TABLE 6).
Although mannan is a known activator of the lectin pathway, it is
theoretically
possible that the presence of anti-mannan antibodies in the rat serum might
also activate
the classical pathway and thereby generate C4b by C Is-mediated cleavage of
C4.
However, several anti-MASP-2 Fab2s have been identified which potently inhibit
C4b
generation (>95 %), thus demonstrating the specificity of this assay for MASP-
2
mediated C4 cleavage. C4, like C3, contains an unusual and highly reactive
thioester
group as part of its structure. Upon cleavage of C4 by MASP-2 in this assay,
the
thioester group on C4b can form a covalent bond with hydroxyl or amino groups
on
macromolecules immobilized on the bottom of the plastic wells via ester or
amide
linkages, thus facilitating detection of C4b in the ELISA assay.
These studies clearly demonstrate the creation of high affinity Fab2s to rat
MASP-2 protein that functionally block both C4 and C3 convertase activity,
thereby
preventing lectin pathway activation.
-127-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 11
This Example describes the epitope mapping for several of the blocking anti-
rat
MASP-2 Fab2 antibodies that were generated as described in Example 10.
Methods:
As shown in FIGURE 10, the following proteins, all with N-terminal 6X His tags
were expressed in CHO cells using the pED4 vector:
rat MASP-2A, a full length MASP-2 protein, inactivated by altering the serine
at
the active center to alanine (S613A),
rat MASP-2K, a full-length MASP-2 protein altered to reduce autoactivation
(R424K);
CUBI-II, an N-terminal fragment of rat MASP-2 that contains the CUBI,
EGF-like and CUBIT domains only, and
CUBFEGF-like, an N-terminal fragment of rat MASP-2 that contains the CUBI
and EGF-like domains only.
These proteins were purified from culture supernatants by nickel-affinity
chromatography, as previously described (Chen et al., J. Biol. Chem. 276:25894-
02
(2001)).
A C-terminal polypeptide (CCPII-SP), containing CCPII and the serine protease
domain of rat MASP-2, was expressed in E. coil as a thioredoxin fusion protein
using
pTrxFus (Invitrogen). Protein was purified from cell lysates using Thiobond
affinity
resin. The thioredoxin fusion partner was expressed from empty pTrxFus as a
negative
control.
All recombinant proteins were dialyzed into TBS buffer and their
concentrations
determined by measuring the OD at 280 nm.
DOT BLOT ANALYSIS:
Serial dilutions of the five recombinant MASP-2 polypeptides described above
and shown in FIGURE 10 (and the thioredoxin polypeptide as a negative control
for
CCPII-serine protease polypeptide) were spotted onto a nitrocellulose
membrane. The
amount of protein spotted ranged from 100 ng to 6.4 pg, in five-fold steps. In
later
experiments, the amount of protein spotted ranged from 50 ng down to 16 pg,
again in
five-fold steps. Membranes were blocked with 5% skimmed milk powder in TBS
(blocking buffer) then incubated with 1.0 pg/m1 anti-MASP-2 Fab2s in blocking
buffer
(containing 5.0 mM Ca2+). Bound Fab2s were detected using HRP-conjugated
-128-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
anti-human Fab (AbD/Serotec; diluted 1/10,000) and an ECL detection kit
(Amersham).
One membrane was incubated with polyclonal rabbit-anti human MASP-2 Ab
(described
in Stover et al., J Immunol 163:6848-59 (1999)) as a positive control. In this
case, bound
Ab was detected using HRP-conjugated goat anti-rabbit IgG (Dako; diluted
1/2,000).
-129-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2 Binding Assay
ELISA plates were coated with 1.0 pg/well of recombinant MASP-2A or CUBI-II
polypeptide in carbonate buffer (pH 9.0) overnight at 4 C. Wells were blocked
with 1%
BSA in TBS, then serial dilutions of the anti-MASP-2 Fab2s were added in TBS
containing 5.0 mM Ca'. The plates were incubated for one hour at RT. After
washing
three times with TBS/tween/Ca", HRP-conjugated anti-human Fab (AbD/Serotec)
diluted 1/10,000 in TB S/ Ca' was added and the plates incubated for a further
one hour
at RT. Bound antibody was detected using a TMB peroxidase substrate kit
(Biorad).
RESULTS:
Results of the dot blot analysis demonstrating the reactivity of the Fab2s
with
various MASP-2 polypeptides are provided below in TABLE 7. The numerical
values
provided in TABLE 7 indicate the amount of spotted protein required to give
approximately half-maximal signal strength. As shown, all of the polypeptides
(with the
exception of the thioredoxin fusion partner alone) were recognized by the
positive control
Ab (polyclonal anti-human MASP-2 sera, raised in rabbits).
TABLE 7: REACTIVITY WITH VARIOUS RECOMBINANT RAT MASP-2
POLYPEPTIDES ON DOT BLOTS
Fab2 MASP-
2A CUBI-II CUBI/EGF-like CCPII-SP Thioredoxin
Antibody #
40 0.16 ng NR NR 0.8 ng NR
41 0.16 ng NR NR 0.8 ng NR
11 0.16 ng NR NR 0.8 ng NR
49 0.16 ng NR NR >20 ng NR
52 0.16 ng NR NR 0.8 ng NR
57 0.032 ng NR NR NR NR
58 0.4 ng NR NR 2.0 ng NR
60 0.4 ng 0.4 ng NR NR NR
63 0.4 ng NR NR 2.0 ng NR
66 0.4 ng NR NR 2.0 ng NR
67 0.4 ng NR NR 2.0 ng NR
71 0.4 ng NR NR 2.0 ng NR
81 0.4 ng NR NR 2.0 ng NR
-130-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Fab2 MA SP-2A CUBI-II CUB I/EGF ke C CPII- SP Thioredoxin
Antibody #
86 0.4 ng NR NR 10 ng NR
87 0.4 ng NR NR 2.0 ng NR
Positive <0.032 ng 0.16 ng 0.16 ng <0.032 ng NR
Control
NR = No reaction. The positive control antibody is polyclonal anti-human MASP-
2 sera,
raised in rabbits.
All of the Fab2s reacted with MASP-2A as well as MASP-2K (data not shown).
The majority of the Fab2s recognized the CCP11-SP polypeptide but not the N-
terminal
fragments. The two exceptions are Fab2 #60 and Fab2 #57 Fab2 #60 recognizes
MASP-2A and the CUBI-II fragment, but not the CUBI/EGF-like polypeptide or the
CCPII-SP polypeptide, suggesting it binds to an epitope in CUBII, or spanning
the CUBII
and the EGF-like domain. Fab2 # 57 recognizes MASP-2A but not any of the MASP-
2
fragments tested, indicating that this Fab2 recognizes an epitope in CCP1.
Fab2 #40 and
#49 bound only to complete MASP-2A. In the ELISA binding assay shown in
FIGURE 11, Fab2 #60 also bound to the CUBI-II polypeptide, albeit with a
slightly
lower apparent affinity.
These finding demonstrate the identification of unique blocking Fab2s to
multiple
regions of the MASP-2 protein.
EXAMPLE 12
This example describes the identification, using phage display, of fully human
scFv antibodies that bind to MASP-2 and inhibit lectin-mediated complement
activation
while leaving the classical (Clq-dependent) pathway component of the immune
system
intact.
Overview:
Fully human, high-affinity MASP-2 antibodies were identified by screening a
phage display library. The variable light and heavy chain fragments of the
antibodies
were isolated in both a scFv format and in a full-length IgG format. The human
MASP-2
antibodies are useful for inhibiting cellular injury associated with lectin
pathway-
mediated complement pathway activation while leaving the classical (C1 q-
dependent)
pathway component of the immune system intact. In some embodiments, the
subject
MASP-2 inhibitory antibodies have the following characteristics: (a) high
affinity for
-131-
human MASP-2 (e.g., a KD of 10 nM or less), and (b) inhibit MASP-2-dependent
complement activity in 90% human serum with an IC50 of 30 nM or less.
Methods:
Expression offull-length catalytically inactive MASP-2:
The full-length cDNA sequence of human MASP-2 (SEQ ID NO: 4), encoding
the human MASP-2 polypeptide with leader sequence (SEQ ID NO:5) was subcloned
into the mammalian expression vector pCI-Neo (Promega), which drives
eukaryotic
expression under the control of the CMV enhancer/promoter region (described in
Kaufman R.J. et al., Nucleic Acids Research /9:4485-90, 1991; Kaufman, Methods
in
Enzymology, /N5:537-66 (1991)).
In order to generate catalytically inactive human MASP-2A protein, site-
directed
mutagenesis was carried out as described in US2007/0172483.
The PCR products were purified after agarose gel electrophoresis
and band preparation and single adenosine overlaps were generated using a
standard
tailing procedure. The adenosine-tailed MASP-2A was then cloned into the pGEM-
T
easy vector and transformed into E. coli. The human MASP-2A was further
subcloned
into either of the mammalian expression vectors pED or pCI-Neo.
The MASP-2A expression construct described above was transfected into DXB1
cells using the standard calcium phosphate transfection procedure (Maniatis et
al., 1989).
MASP-2A was produced in serum-free medium to ensure that preparations were not
contaminated with other serum proteins. Media was harvested from confluent
cells every
second day (four times in total). The level of recombinant MASP-2A averaged
approximately 1.5 mg/liter of culture medium, The MASP-2A (Ser-Ala mutant
described
above) was purified by affinity chromatography on MBP-A-agarose columns
MASP-2A ELISA on .S'eFv Candidate Clones identified by panning/se&
conversion and filter screening
A phage display library of human immunoglobulin light- and heavy-chain
variable region sequences was subjected to antigen panning followed by
automated
antibody screening and selection to identify high-affinity scFv antibodies to
human
MASP-2 protein. Three rounds of panning the scFv phage library against HIS-
tagged or
biotin-tagged MASP-2A were carried out. The third round of panning was eluted
first
with MBL and then with TEA (alkaline). To monitor the specific enrichment of
phages
displaying scFv fragments against the target MASP-2A, a polyclonal phage ELISA
-132-
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
against immobilized MASP-2A was carried out. The scFv genes from panning round
3
were cloned into a pHOG expression vector and run in a small-scale filter
screening to
look for specific clones against MASP-2A.
Bacterial colonies containing plasmids encoding scFv fragments from the third
round of panning were picked, gridded onto nitrocellulose membranes and grown
overnight on non-inducing medium to produce master plates. A total of 18,000
colonies
were picked and analyzed from the third panning round, half from the
competitive elution
and half from the subsequent TEA elution. Panning of the scFv phagemid library
against
MASP-2A followed by scFv conversion and a filter screen yielded 137 positive
clones.
108/137 clones were positive in an ELISA assay for MASP-2 binding (data not
shown),
of which 45 clones were further analyzed for the ability to block MASP-2
activity in
normal human serum.
Assay to Measure Inhibition of Formation of Lectin Pathway C3 Convertase
A functional assay that measures inhibition of lectin pathway C3 convertase
formation was used to evaluate the "blocking activity" of the MASP-2 scFv
candidate
clones. MASP-2 serine protease activity is required in order to generate the
two protein
components (C4b, C2a) that comprise the lectin pathway C3 convertase.
Therefore, a
MASP-2 scFv that inhibits MASP-2 functional activity (i.e., a blocking MASP-2
scFv),
will inhibit de novo formation of lectin pathway C3 convertase. C3 contains an
unusual
and highly reactive thioester group as part of its structure. Upon cleavage of
C3 by C3
convertase in this assay, the thioester group on C3b can form a covalent bond
with
hydroxyl or amino groups on macromolecules immobilized on the bottom of the
plastic
wells via ester or amide linkages, thus facilitating detection of C3b in the
ELISA assay.
Yeast mannan is a known activator of the lectin pathway. In the following
method to measure formation of C3 convertase, plastic wells coated with mannan
were
incubated with diluted human serum to activate the lectin pathway. The wells
were then
washed and assayed for C3b immobilized onto the wells using standard ELISA
methods.
The amount of C3b generated in this assay is a direct reflection of the de
novo formation
of lectin pathway C3 convertase. MASP-2 scFv clones at selected concentrations
were
tested in this assay for their ability to inhibit C3 convertase formation and
consequent
C3b generation.
Methods:
-133-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The 45 candidate clones identified as described above were expressed, purified
and diluted to the same stock concentration, which was again diluted in Ca ++
and mg++
containing GVB buffer (4.0 mM barbital, 141 mM NaCl, 1.0 mM MgCl2, 2.0 mM
CaCl2,
0.1% gelatin, pH 7.4) to assure that all clones had the same amount of buffer.
The scFv
clones were each tested in triplicate at the concentration of 2 pg/mL. The
positive control
was OMS100 Fab2 and was tested at 0.4 pg/mL. C3c formation was monitored in
the
presence and absence of the scFv/IgG clones.
Mannan was diluted to a concentration of 20 pg/mL (1 ug/well) in 50mM
carbonate buffer (15mM Na2CO3 + 35mM NaHCO3 + 1.5 mM NaN3), pH 9.5 and coated
on an ELISA plate overnight at 4 C. The next day, the mannan-coated plates
were
washed 3 times with 200 pi PBS. 100 pi of 1% HSA blocking solution was then
added to
the wells and incubated for 1 hour at room temperature. The plates were washed
3 times
with 200[11 PBS, and stored on ice with 200 jil PBS until addition of the
samples.
Normal human serum was diluted to 0.5% in CaMgGVB buffer, and scFv clones
or the OMS100 Fab2 positive control were added in triplicates at 0.01 ug/mL; 1
us/mL
(only OMS100 control) and 10 pg/mL to this buffer and preincubated 45 minutes
on ice
before addition to the blocked ELISA plate. The reaction was initiated by
incubation for
one hour at 37 C and was stopped by transferring the plates to an ice bath.
C3b
deposition was detected with a Rabbit a-Mouse C3c antibody followed by Goat a-
Rabbit
HRP. The negative control was buffer without antibody (no antibody = maximum
C3b
deposition), and the positive control was buffer with EDTA (no C3b
deposition). The
background was determined by carrying out the same assay except that the wells
were
mannan-free. The background signal against plates without mannan was
subtracted from
the signals in the mannan-containing wells. A cut-off criterion was set at
half of the
activity of an irrelevant scFv clone (VZV) and buffer alone.
Results: Based on the cut-off criterion, a total of 13 clones were found to
block
the activity of MASP-2. All 13 clones producing > 50% pathway suppression were
selected and sequenced, yielding 10 unique clones. All ten clones were found
to have the
same light chain subclass, 2,3, but three different heavy chain subclasses:
VH2, VH3 and
VH6. In the functional assay, five out of the ten candidate scFv clones gave
IC50 nM
values less than the 25 nM target criteria using 0.5% human serum.
To identify antibodies with improved potency, the three mother scFv clones,
identified as described above, were subjected to light-chain shuffling. This
process
-134-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
involved the generation of a combinatorial library consisting of the VH of
each of the
mother clones paired up with a library of naïve, human lambda light chains
(VL) derived
from six healthy donors. This library was then screened for scFy clones with
improved
binding affinity and/or functionality.
TABLE 8: Comparison of functional potency in IC50 (nM) of the lead daughter
clones
and their respective mother clones (all in scFv format)
1% human serum 90% human serum 90% human serum
C3 assay C3 assay C4 assay
scFy clone (IC50 nM) (IC50 nM) (IC50 nM)
17D20mc 38 nd nd
17D20m d3521N11 26 >1000 140
17N16mc 68 nd nd
17N16m d17N9 48 15 230
Presented below are the heavy-chain variable region (VH) sequences for the
mother clones and daughter clones shown above in TABLE 8.
The Kabat CDRs (31-35 (H1), 50-65 (H2) and 95-107 (H3)) are bolded; and the
Chothia CDRs (26-32 (H1), 52-56 (H2) and 95-101 (H3)) are underlined.
17D20 35VH-21N11VL heavy chain variable region (VH) (SEQ ID NO:67,
encoded by SEQ ID NO:66)
QVTLKESGPVLVKPTETLTLTCTVSGF SLSRGKMGVSWIRQPPGKALEWL
AHIFSSDEKSYRTSLKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARIRRGG
IDYWGQGTLVTVSS
d17N9 heavy chain variable region (VH) (SEQ ID NO:68)
QVQL Q Q S GPGLVKP S Q TL SL TC AISGD S VS S T SAAWNWIRQ SP SRGLEWLGRTY
YRSKWYNDYAVSVKSRITINPDTSKNQF SLQLNSVTPEDTAVYYCARDPFGVPF
DIWGQGTMVTVSS
-135-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Presented below are the light-chain variable region (VL) sequences for the
mother
clones and daughter clones shown above in TABLE 8.
The Kabat CDRs (24-34 (L1); 50-56 (L2); and 89-97 (L3) are bolded; and the
Chothia CDRs (24-34 (L1); 50-56 (L2) and 89-97 (L3) are underlined. These
regions are
.. the same whether numbered by the Kabat or Chothia system.
17D20m d3521N11 light chain variable region (VL) (SEQ ID NO:70, encoded
by SEQ ID NO:69)
QPVLTQPPSL SVSPGQ TA SITC S GEKLGUKYAYWYQQKPGQ SPVLVMYQ
DKQRPSGIPERFSGSNSGNTATLTISGTQAMDEADYYCQAWDSSTAVFGGGTKL
TVL
17N16m dl7N9 light chain variable region (VL) (SEQ ID NO:71)
SYELIQPPSVSVAPGQTATITCAGDNLGKKRVHWYQQRPGQAPVLVIYD
DSDRPSGIPDRF SA SNS GNTATL TITRGEAGDEADYYCQVWDIATDHVVF GGGT
KLTVLAAAGSEQKLISE
The MASP-2 antibodies OMS100 and MoAb d3521N11VL, (comprising a heavy
chain variable region set forth as SEQ ID NO:67 and a light chain variable
region set
forth as SEQ ID NO:70, also referred to as "0M5646" and "mAb6" ), which have
both
been demonstrated to bind to human MASP-2 with high affinity and have the
ability to
block functional complement activity, were analyzed with regard to epitope
binding by
dot blot analysis. The results show that 0M5646 and OMS100 antibodies are
highly
specific for MASP-2 and do not bind to MASP-1/3. Neither antibody bound to
MAp19
nor to MASP-2 fragments that did not contain the CCP1 domain of MASP-2,
leading to
the conclusion that the binding sites encompass CCP1.
The MASP-2 antibody 0MS646 was determined to avidly bind to recombinant
MASP-2 (Kd 60-250pM) with >5000 fold selectivity when compared to Cis, Clr or
MASP-1 (see TABLE 9 below):
TABLE 9: Affinity and Specificity of 0M5646 MASP-2 antibody-MASP-2 interaction
as
assessed by solid phase ELISA studies
Antigen KID (pM)
-136-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MA SP-1 >500,000
MASP-2 62 23*
MASP-3 >500,000
Purified human Clr >500,000
Purified human Cis ¨500,000
*Mean+SD; n=12
0MS646 specifically blocks lectin-dependent activation of terminal complement
components
Methods:
The effect of 0MS646 on membrane attack complex (MAC) deposition was
analyzed using pathway-specific conditions for the lectin pathway, the
classical pathway
and the alternative pathway. For this purpose, the Wieslab Comp300 complement
screening kit (Wieslab, Lund, Sweden) was used following the manufacturer's
instructions.
Results:
FIGURE 12A graphically illustrates the level of MAC deposition in the presence
or absence of anti-MASP-2 antibody (0M5646) under lectin pathway-specific
assay
conditions. FIGURE 12B graphically illustrates the level of MAC deposition in
the
presence or absence of anti-MASP-2 antibody (0M5646) under classical pathway-
specific assay conditions. FIGURE 12C graphically illustrates the level of MAC
deposition in the presence or absence of anti-MASP-2 antibody (0M5646) under
alternative pathway-specific assay conditions.
As shown in FIGURE 12A, 0MS646 blocks lectin pathway-mediated activation
of MAC deposition with an IC50 value of approximately 1M. However, 0MS646 had
no effect on MAC deposition generated from classical pathway-mediated
activation
(FIGURE 12B) or from alternative pathway-mediated activation (FIGURE 12C)
Pharmacokinetics and Pharmacodynamics of 0M5646 following Intravenous (IV) or
Subcutaneous (SC) Administration to Mice
The pharmacokinetics (PK) and pharmacodynamics (PD) of 0MS646 were
evaluated in a 28 day single dose PK/PD study in mice. The study tested dose
levels of
-137-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
5mg/kg and 15mg/kg of 0MS646 administered subcutaneously (SC), as well as a
dose
level of 5mg/kg 0MS646 administered intravenously (IV).
With regard to the PK profile of 0MS646, FIGURE 13 graphically illustrates the
0MS646 concentration (mean of n=3 animals/groups) as a function of time after
administration of 0MS646 at the indicated dose. As shown in FIGURE 13, at
5mg/kg
SC, 0M5646 reached the maximal plasma concentration of 5-6 pg/mL approximately
1-2
days after dosing. The bioavailability of 0MS646 at 5 mg/kg SC was
approximately
60%. As further shown in FIGURE 13, at 15 mg/kg SC, 0M5646 reached a maximal
plasma concentration of 10-12 tig/mL approximately I to 2 days after dosing.
For all
groups, the 0MS646 was cleared slowly from systemic circulation with a
terminal half-
life of approximately 8-10 days. The profile of 0MS646 is typical for human
antibodies
in mice.
The PD activity of 0MS646 is graphically illustrated in FIGURES 14A and 14B.
FIGURES 14A and 14B show the PD response (drop in systemic lectin pathway
activity)
for each mouse in the 5mg/kg IV (FIGURE 14A) and 5mg/kg SC (FIGURE 14B)
groups.
The dashed line indicates the baseline of the assay (maximal inhibition; naïve
mouse
serum spiked in vitro with excess 0MS646 prior to assay). As shown in FIGURE
14A,
following IV administration of 5mg/kg of 0MS646, systemic lectin pathway
activity
immediately dropped to near undetectable levels, and lectin pathway activity
showed only
a modest recovery over the 28 day observation period. As shown in FIGURE 14B,
in
mice dosed with 5mg/kg of 0MS646 SC, time-dependent inhibition of lectin
pathway
activity was observed. Lectin pathway activity dropped to near-undetectable
levels
within 24 hours of drug administration and remained at low levels for at least
7 days.
Lectin pathway activity gradually increased with time, but did not revert to
pre-dose
levels within the 28 day observation period. The lectin pathway activity
versus time
profile observed after administration of 15mg/kg SC was similar to the 5 mg/kg
SC dose
(data not shown), indicating saturation of the PD endpoint. The data further
indicated
that weekly doses of 5mg/kg of 0MS646, administered either IV or SC, is
sufficient to
achieve continuous suppression of systemic lectin pathway activity in mice.
EXAMPLE 13
This Example describes the generation of recombinant antibodies that inhibit
MASP-2 comprising a heavy chain and/or a light chain variable region
comprising one or
more CDRs that specifically bind to MASP-2 and at least one SGMI core peptide
-138-
sequence (also referred to as an SGMI-peptide bearing MASP-2 antibody or
antigen
binding fragment thereof).
Background/Rationale:
The generation of specific inhibitors of MASP-2, termed SGMI-2, is described
in
Heja et al., J Biol Chem 287:20290 (2012) and Heja et al., PNA.S. 109:10498
(2012).
SGMI-2 is a 36 amino acid peptide
which was selected from a phage library of variants of the Schistoceint
gregaria protease
inhibitor 2 in which six of the eight positions of the protease binding loop
were fully
randomized. Subsequent in vitro evolution yielded mono-specific inhibitors
with single
.. digit nMKIvalues (Heja et al., J. Biol. Chem. 287:20290, 2012). Structural
studies
revealed that the optimized protease binding loop forms the primary binding
site that
defines the specificity of the two inhibitors. The amino acid sequences of the
extended
secondary and internal binding regions are common to the two inhibitors and
contribute
to the contact interface (Heja et al., 2012. J. Biol. Chelll. 287:20290).
Mechanistically,
SGMI-2 blocks the lectin pathway of complement activation without affecting
the
classical pathway (Heja et al., 2012. Proc. Natl. Acad Sc!. 109:10498).
The amino acid sequences of the SGMI-2 inhibitors are set forth below:
SGM1-2-full-length: LEVTCEPGTTFKDKCNTCRCGSDGKSAVCTKLWCNQ (SEQ ID
NO:72)
SGMI-2-medium:
TCEPGTTFKDKCNTCRCGSDG KSAVCTKLWCNQ (SEQ ID
NO:73)
SGMI-2-short:
....TCRCGSDGKSAVCTKLWCNQ (SEQ ID
NO :74)
As described in this Example, and also described in W02014/144542, SGMI-2
peptide-
bearing MASP-2 antibodies and fragments thereof were generated by fusing the
SGMI-2
peptide amino acid sequence (e.g., SEQ ID NO: 72, 73 or 74) onto the amino or
carboxy
termini of the heavy and/or light chains of a human MASP-2 antibody. The SGMI-
2
peptide-bearing MASP-2 antibodies and fragments have enhanced inhibitory
activity, as
compared to the naked MASP-2 scaffold antibody that does not contain the SGMI-
2
peptide sequence, when measured in a C3b or C4b deposition assay using human
serum,
as described in W02014/144542, and also have enhanced inhibitory activity as
compared
-139-
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
to the naked MASP-2 scaffold antibody when measured in a mouse model in vivo.
Methods of generating SGMI-2 peptide bearing MASP-2 antibodies are described
below.
Methods
Expression constructs were generated to encode four exemplary SGMI-2 peptide
bearing MASP-2 antibodies wherein the SGMI-2 peptide was fused either to the N-
or C-
terminus of the heavy or light chain of a representative MASP-2 inhibitory
antibody
0MS646 (generated as described in Example 12).
-140-
CA 03039927 2019-04-09
WO 2018/071701
PCT/US2017/056386
TABLE 10: MASP-2 antibody/SGMI-2 fusions
Antibody reference Peptide Location on Antibody SEQ ID
H-N H-C L-N L-C NO:
HL-M2 67 + 70
(naked MASP-2
OMS646)
H-M2-SGMI-2-N SGMI-2 75 + 70
H-M2-SGMI-2-C SGMI-2 76 +70
L-M2-SGMI-2-N SGMI-2 67+ 77
L-M2-SGMI-2-C SGMI-2 67+ 78
Abbreviations in Table 10:
"H-N"= amino terminus of heavy chain
"H-C"=carboxyl terminus of heavy chain
"L-N"=amino terminus of light chain
"L-C"=carboxyl tel ____ minus of light chain
"M2"=MASP-2 ab scaffold (representative 0MS646)
For the N-terminal fusions shown in TABLE 10, a peptide linker
('GTGGGSGSSS' SEQ ID NO: 79) was added between the SGMI-2 peptide and the
variable region.
For the C-terminal fusions shown in TABLE 10, a peptide linker (` AAGGSG'
SEQ ID NO: 80) was added between the constant region and the SGMI-2 peptide,
and a
second peptide "GSGA" (SEQ ID NO: 81) was added at the C-terminal end of the
fusion
polypeptide to protect C-terminal SGMI-2 peptides from degradation.
Amino acid sequences are provided below for the following representative
MASP-2 antibody/SGMI-2 fusions:
-141-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
H-M2ab6-SGMI-2-N (SEQ ID NO:75, encoded by SEQ ID NO:82)
LEVTCEPGTTFKDKCNTCRCGSDGKSAVCTKLWCNOGTGGGSGSSSQYTLKESG
PVLVKPTETLTLT C TV S GF SLSRGKMGVSWIRQPPGKALEWLAHIF SSDEKSYRTS
LKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARIRRGGIDWGQGTLVTVSS
ASTKGPSVFPLAPC SRST SES TAALGCLVKD YFPEP V TVS W N SGALTSGVHTFPA
VLQ SSGL Y SLS S V VT VP S S SLGTKTYTCNVDHKP SNTK VDKRVESKYGPPCPPCP
APEFLGGP S VFLFPPKPKDTLMI SRTPEVTC VVVD V S QEDPEVQF N W Y VD GVE VH
NAK TKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLP S SIEK TISKA
KGQPREPQVYTLPPSQEEMTKNQVSLTCLVKGFYP SDIAVEWESNGQPENNYKT
TPPVLD SD GSFFLY SRLTVDK SRWQEGNVF SC SVMHEALHNHYTQKSL SL SLGK
[491 aa protein, aa 1-36=5GMI-2 (underlined), aa37-46=1inker (italicized),
aa47-
164=heavy chain variable region of MASP-2 ab#6 (underlined); aa165-491=IgG4
constant region with hinge mutation.]
H-M2ab6-SGMI-2-C (SEQ ID NO:76, encoded by SEQ ID NO:83):
QVTLKE S GPVLVKPTETL TLTC TV S GF SLSRGKMGVSWIRQPPGKALEWLAHIF S
SDEKSYRTSLKSRLTISKDTSKNQVVLTMTNMDPVDTATYYCARIRRGGIDYWG
QGTLVTVS SA S TKGP S VFPLAP C SRST SE S TAALGCLVKDYFPEPVTV SWN S GAL
T SGVHTFPAVLQ S SGLYSLS SVVTVPS S SLGTKTYTCNVDEIKP SNTKVDKRVESK
YGPPCPPCPAPEFLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNW
YVDGVEVHNAKTKPREEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPS
SIEKTISK AK GQPREP QVYTLPP S QEEMTKNQV SLT CLVK GFYP SD IA VEWE SNG
QPENNYKTTPPVLD SDGSFFLYSRLTVDKSRWQEGNVF SCSVMHEALINHYTQK
SLSLSLGKAAGGSGLEVTCEPGTTFKDKCNTCRCGSDGKSAVCTKLWCNOGSGA
[491aa protein, aa1-118=heavy chain variable region of MASP-2 ab#6
(underlined); aa
119-445=IgG4 constant region with hinge mutation; aa 446-451= 1st linker
(italicized), aa
452-487=SGMI-2, aa488-491=2'llinker (italicized).]
L-M2ab6-SGMI-2-N (SEQ ID NO:77, encoded by SEQ ID NO 84):
-142-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
LEVTCEPGTTFKDKCNTCRCGSDGKSAVCTKLWCNQGTGGGSGSSSQPVLTQPPS
L SVSP GQ TA SIT C S GEKLGDKYAYWYQ QKPGQ SPVLVMYQDKQRP S GIPERF SGS
NSGNTATLTISGTQAMDEADYYCQAWDSSTAVFGGGTKLTVLGQPKAAP SVTLF
PP S SEEL QANKATLVCLI SDFYP GAVTVAWKAD S SPVKAGVETTTPSKQSNNKY
AAS SYLSLTPEQWKSHRSYSCQVMEGSTVEKTVAPTECS
[258aa protein, aa1-36=SGMI-2 (underlined); aa37-46=linker (italicized); aa47-
152=light
chain variable region of MASP-2 ab#6 (underlined); aa153-258=human Ig lambda
constant region]
L-M2ab6-SGMI-2-C (SEQ ID NO:78, encoded by SEQ ID NO:85):
QPVLT QPP SL S V SP GO TA SITC SGEKLGDKYAYWYQQKPGQSPVLVMYQDKQRP
SGIPERF SGSNSGNTATLTISGTQAMDEADYYCQAWDSSTAVFGGGTKLTVLGQP
KAAP SVTLFPP S SEEL QANKATLVCLI SDF YP GAVTVAWKAD S SPVKAGVETTTP
SKQSNNKYAASSYL SLTPEQWK SHRS Y S CQVTI-IEGS TVEKTVAP TEC SAAGGSGL
EVTCEPGTTFKDKCNTCRCGSDGKSAVCTKLWCNQGSGA
[258aa protein, aa1-106=light chain variable region of MASP-2 ab#6
(underlined); aa
107-212=human Ig lambda constant region; aa 213-218=1st linker; aa219-254=SGMI-
2;
aa255-258=2nd linker]
Functional Assays:
The four MASP-2-SGMI-2 fusion antibody constructs were transiently expressed
in Expi293F cells (Invitrogen), purified by Protein A affinity chromatography,
and tested
in 10% normal human serum for inhibition of C3b deposition in a mannan-coated
bead
assay as described below.
Testing the MASP-2-SGMI-2 fusions in the mannan-coated bead assay for C3b
deposition
The MASP-2-SGMI-2 fusion antibodies assessed for lectin pathway inhibition in
an assay of C3b deposition on mannan-coated beads. This assay, which
determines
degree of activity by flow cytometry, offers greater resolution than the
Wieslabg assay.
The lectin pathway bead assay was carried out as follows: mannan was adsorbed
to 7
[IM-diameter polystyrene beads (Bangs Laboratories; Fishers, IN, USA)
overnight at 4 C
-143-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
in carbonate-bicarbonate buffer (pH 9.6). The beads were washed in PBS and
exposed to
100/0 human serum, or 10% serum pre-incubated with antibodies or inhibitors.
The
serum-bead mixture was incubated at room temperature for one hour while
agitating.
Following the serum incubation, the beads were washed, and C3b deposition on
the beads
was measured by detection with an anti-C3c rabbit polyclonal antibody (Dako
North
America; Carpinteria, CA, USA) and a PE-Cy5 conjugated goat anti-rabbit
secondary
antibody (Southern Biotech; Birmingham, AL, USA). Following the staining
procedure,
the beads were analyzed using a FACSCalibur flow cytometer. The beads were
gated as
a uniform population using forward and side scatter, and C3b deposition was
apparent as
FL3-positive particles (FL-3, or "FL-3 channel" indicates the 3rd or red
channel on the
cytometer). The Geometric Mean Fluorescence Intensity (WI) for the population
for
each experimental condition was plotted relative to the antibody/inhibitor
concentration
to evaluate lectin pathway inhibition.
The ICso values were calculated using the GraphPad PRISM software.
Specifically, ICso values were obtained by applying a variable slope (four
parameter),
nonlinear fit to log (antibody) versus mean fluorescence intensity curves
obtained from
the cytometric assay.
The results are shown in TABLE 11.
TABLE 11: C3b deposition (mannan-coated bead assay) in 10% human serum
Construct IC50 (nM)
Naked N2 ab > 3.63 nM
(mAb#6)
H-M2- SGMI-2-N 2.11 nM
L-M2-SGMI-2-C 1.99 nM
H-M2- SGMI-2-N 2.24 nM
L-M2-SGMI-2-N 3.71 nM
Results:
The control, non-SGMI-containing MASP-2 "naked" scaffold antibody (mAb#6),
was inhibitory in this assay, with an IC50 value of > 3.63 nM, which is
consistent with
the inhibitory results observed in Example 12. Remarkably, as shown in TABLE
11, all
of the SGMI-2-MASP-2 antibody fusions that were tested improved the potency of
the
-144-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
MASP-2 scaffold antibody in this assay, suggesting that increased valency may
also be
beneficial in the inhibition of C3b deposition.
Testing the MASP-2-SGMI-2 fusions in the mannan-coated bead assay for C4b
deposition assay with 10% human serum
A C4b deposition assay was carried out with 10% human serum using the same
assay
conditions as described above for the C3b deposition assay with the following
modifications. C4b detection and flow cytometric analysis was carried out by
staining
the deposition reaction with an anti-C4b mouse monoclonal antibody (1:500,
Quidel) and
.. staining with a secondary goat anti-mouse F(ab')2 conjugated to PE Cy5
(1:200,
Southern Biotech) prior to flow cytometric analysis.
Results:
The SGMI-2-bearing MASP-2- N-teuninal antibody fusions (H-M2-SGMI-2-N:
IC50=0.34nM), L-M2-SGMI-2-N: IC50=0.41 nM)), both had increased potency as
compared to the MASP-2 scaffold antibody (HL-M2: IC50=0.78nM).
Similarly, the single SGMI-2 bearing C-terminal MASP-2 antibody fusions (H-
M2-SGMI-2-C: IC50=0.45nM and L-M2-SGMI-2C: IC50=0.47 n114) both had increased
potency as compared to the MASP-2 scaffold antibody (HL-M2: IC50=1.2 n114).
Testing the MASP-2-SGMI-2 fusions in the mannan-coated bead assay for C3b
deposition with 10% mouse serum.
A mannan-coated bead assay for C3b deposition was carried out as described
above with 10% mouse serum. Similar to the results observed in human serum, it
was
determined that the SGMI-2-bearing MASP-2 fusions had increased potency as
compared
to the MASP-2 scaffold antibody in mouse serum.
Summary of Results: The results in this Example demonstrate that all of the
SGMI-2-MASP-2 antibody fusions that were tested improved the potency of the
MASP-2
scaffold antibody.
EXAMPLE 14
This Example provides results that were generated using a Unilateral Ureteric
Obstruction (UUO) model of renal fibrosis in MASP-2 -/- deficient and MASP-2
+/+
sufficient mice to evaluate the role of the lectin pathway in renal fibrosis.
Background/Rationale:
-145-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Renal fibrosis and inflammation are prominent features of late stage kidney
disease. Renal tubulointerstitial fibrosis is progressive process involving
sustained cell
injury, aberrant healing, activation of resident and infiltrating kidney
cells, cytokine
release, inflammation and phenotypic activation of kidney cells to produce
extracellular
matrix. Renal tubulointerstitial (TI) fibrosis is the common end point of
multiple renal
pathologies and represents a key target for potential therapies aimed at
preventing
progressive renal functional impairment in chronic kidney disease (CKD). Renal
TI
injury is closely linked to declining renal function in glomerular diseases
(Risdon R.A. et
al., Lancet 1: 363-366, 1968; Schainuck L.I. et al, Hum Pathol 1: 631-640,
1970; Nath
K.A., Am J Kid Dis 20:1-17, 1992), and is characteristic of CKD where there is
an
accumulation of myofibroblasts, and the potential space between tubules and
peritubular
capillaries becomes occupied by matrix composed of collagens and other
proteoglycans.
The origin of TI myofibroblasts remains intensely controversial, but fibrosis
is generally
preceded by inflammation characterized initially by TI accumulation of T
lymphocytes
and then later by macrophages (Liu Y. et al., Nat Rev Nephrol 7:684-696, 2011;
Duffield
J. S., J Mt Invest 124:2299-2306, 2014).
The rodent model of UUO generates progressive renal fibrosis, a hallmark of
progressive renal disease of virtually any etiology (Chevalier et al., Kidney
International
75:1145-1152, 2009). It has been reported that C3 gene expression was
increased in
wild-type mice following UUO, and that collagen deposition was significantly
reduced in
C3-/- knockout mice following UUO as compared to wild-type mice, suggesting a
role of
complement activation in renal fibrosis (Fearn et al., Mol Imnntnol 48:1666-
1733, 2011).
It has also been reported that C5 deficiency led to a significant amelioration
of major
components of renal fibrosis in a model of tubulointerstitial injury (Boor P.
et al ,J of Am
Soc of Nephrology 18:1508-1515, 2007). However, prior to the study described
herein
carried out by the present inventors, the particular complement components
involved in
renal fibrosis were not well defined. Therefore, the following study was
carried out to
evaluate MASP-2 (-/-) and MASP-2 (+/+) male mice in a unilateral ureteral
obstruction
(UUO) model.
Methods:
A MASP-2-/- mouse was generated as described in Example 1 and backcrossed
for 10 generations with C57BL/6. Male wild-type (WT) C57BL/6 mice, and
homozygous MASP-2 deficient (MASP-2-/-) mice on a C57BL/6 background were kept
-146-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
under standardized conditions of 12/12 day/night cycle, fed on standard food
pellets and
given free access to food and water. Ten-week-old mice, 6 per group, were
anesthetized
with 2.5% isoflurane in 1.5 L/min oxygen. The right ureters of two groups of
ten-week-
old male C56/BL6 mice, wild-type and MASP-2-/- were surgically ligated. The
right
kidney was exposed through a lcm flank incision. The right ureter was
completely
obstructed at two points using a 6/0 polyglactin suture. Buprenorphine
analgesia was
provided perioperatively every 12 hours for up to 5 doses depending on pain
scoring.
Local bupivacaine anesthetic was given once during the surgery.
Mice were sacrificed 7 days after the surgery and kidney tissues were
collected,
fixed and embedded in paraffin blocks. Blood was collected from the mice by
cardiac
puncture under anesthesia, and mice were culled by exsanguination after
nephrectomy.
Blood was allowed to clot on ice for 2 hours and serum was separated by
centrifugation
and kept frozen as aliquots at -80 C.
Immunohistochemistry of Kidney Tissue
To measure the degree of kidney fibrosis as indicated by collagen deposition,
5
micron paraffin embedded kidney sections were stained with picrosirius red, a
collagen-
specific stain, as described in Whittaker P. et al., Basic Res Cardiol 89:397-
410, 1994.
Briefly described, kidney sections were de-paraffinized, rehydrated and
collagen stained
for 1 hour with picrosirius red aqueous solution (0.5 gm Sirius red, Sigma,
Dorset UK) in
500 mL saturated aqueous solution of picric acid. Slides were washed twice in
acidified
water (0.5% glacial acetic acid in distilled water) for 5 minutes each, then
dehydrated and
mounted
To measure the degree of inflammation as indicated by macrophage infiltration,
kidney sections were stained with macrophage-specific antibody F4/80 as
follows.
Formalin fixed, paraffin embedded, 5 micron kidney sections were
deparaffinized and
rehydrated. Antigen retrieval was performed in citrate buffer at 95 C for 20
minutes
followed by quenching of endogenous peroxidase activity by incubation in 3%
H202 for
10 minutes. Tissue sections were incubated in blocking buffer (109/0 heat
inactivated
normal goat serum with 1% bovine serum albumin in phosphate buffered saline
(PBS))
for 1 hour at room temperature followed by avidin/biotin blocking. Tissue
sections were
washed in PBS three times for 5 minutes after each step. F4/80 macrophage
primary
antibody (Santa Cruz, Dallas, TX, USA) diluted 1:100 in blocking buffer was
applied for
1 hour. A biotinylated goat anti-rat secondary antibody, diluted 1:200, was
then applied
-147-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
for 30 minutes followed by horse radish peroxidase (HRP) conjugated enzyme for
30
minutes. Staining color was developed using diaminobenzidine (DAB) substrate
(Vector
Labs, Peterborough UK) for 10 minutes and slides were washed in water,
dehydrated and
mounted without counter staining to facilitate the computer based analysis.
Image Analysis
The percentage of kidney cortical staining was determined as described in
Furness
P. N. et al., 1 Pathol 50:118-122, 1997. Briefly described, 24 bit color
images were
captured from sequential non-overlapping fields of renal cortex just beneath
the renal
capsule around the entire periphery of the section of kidney. After each image
capture
NIH Image was used to extract the red channel as an 8 bit monochrome image.
Unevenness in the background illumination was subtracted using a pre-recorded
image of
the illuminated microscope field with no section in place. The image was
subjected to a
fixed threshold to identify areas of the image corresponding to the staining
positivity.
The percentage of black pixels was then calculated, and after all the images
around the
kidney had been measured in this way the average percentage was recorded,
providing a
value corresponding to the percentage of stained area in the kidney section.
Gene Expression Analysis
Expression of several genes relevant to renal inflammation and fibrosis in
mouse
kidney were measured by quantitative PCT (qPCR) as follows. Total RNA was
isolated
from kidney cortex using Trizoll' (ThermoFisher Scientific, Paisley, UK)
according to the
manufacturer's instructions. Extracted RNA was treated with the Turbo DNA-free
kit
(ThermoFisher Scientific) to eliminate DNA contamination, and then first
strand cDNA
was synthesized using AMV Reverse Transcription System (Promega, Madison, WI,
USA). The cDNA integrity was confirmed by a single qPCR reaction using TaqMan
GAPDH Assay (Applied Biosystems, Paisley UK) followed by qPCR reaction using
Custom TaqMan Array 96-well Plates (Life Technologies, Paisley, UK)
Twelve genes were studied in this analysis:
Collagen type IV alpha 1 (col4a1; assay ID: Mm01210125 ml)
Transforming growth factor beta-1 (TGF13-1; assay ID: Mm01178820 ml);
Cadherin 1 (Cdhl; Assay ID: Mm01247357 m1);
Fibronectin 1 (Fnl; Assay ID:Mm01256744 ml);
Interleukin 6 (IL6; Assay ID Mm00446191 ml);
-148-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Interleukin 10 (IL10; Assay ID Mm00439614 ml);
Interleukin 12a (IL12a; Assay ID Mm00434165_m1);
Vimentin (Vim; Assay ID Mm01333430 ml);
Actinin alpha 1 (Actnl; Assay ID Mm01304398 ml);
Tumor necrosis factor-a (TNF-a; Assay ID Mm00443260 gl)
Complement component 3 (C3; Assay ID Mm00437838 ml);
Interferon gamma (Ifn-y; Assay ID Mm01168134)
The following housekeeping control genes were used:
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Assay ID Mm99999915_g1);
Glucuronidase beta (Gusp; Assay ID Mm00446953_m1);
Eukaryotic 18S rRNA (18S, Assay ID Hs99999901_s1);
Hypoxanthine guanine phosphoribosyl transferase (HPRT; Assay ID Mm00446968 ml)
Twenty L reactions were amplified using TaqMan Fast Universal Master Mix
(Applied
Biosystems) for 40 cycles. Real time PCR amplification data were analyzed
using
Applied Biosystems 7000 SDS v1.4 software.
RESULTS:
Following unilateral ureteric obstruction (UUO), obstructed kidneys experience
an influx of inflammatory cells, particularly macrophages, followed by the
prompt
development of fibrosis as evidenced by the accumulation of collagen alongside
tubular
dilatation and attenuation of the proximal tubular epithelium (see Chevalier
R. L. et al.,
Kidney Int 75:1145-1152, 2009).
FIGURE 15 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Sirius red, wherein the tissue sections
were obtained
from wild-type and MASP-2-/- mice following 7 days of ureteric obstruction
(UUO) or
from sham-operated control mice. As shown in FIGURE 15, kidney sections of
wild-type
mice following 7 days of ureteric obstruction showed significantly greater
collagen
deposition compared to MASP-2-/- mice (p value = 0.0096). The mean values
standard
error of means for UUO operated mice in wild-type and MASP-2-/- groups were
24.79 1.908 (n=6) and 16.58 1.3 (n=6), respectively. As further shown in
FIGURE 15,
-149-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
the tissue sections from the sham-operated control wild-type and the sham
operated
control MASP-2-/- mice showed very low levels of collagen staining, as
expected.
FIGURE 16 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with the F4/80 macrophage-specific antibody,
wherein the
tissue sections were obtained from wild-type and MASP-2-/- mice following 7
days of
ureteric obstruction or from sham-operated control mice. As shown in FIGURE
16,
compared to wild-type mice, the tissue obtained from UUO kidneys from MASP-2-/-
mice exhibited significantly less macrophage infiltration following 7 days of
ureteric
obstruction (% macrophage area stained in WT:2.23+0.4 vs MASP-2-/-: 0.53+0.06,
p=0.0035). As further shown in FIGURE 16, the tissue sections from the sham-
operated
wild-type and the sham-operated MASP-2-/- mice showed no detectable macrophage
staining.
Gene expression analysis of a variety of genes linked to renal inflammation
and
fibrosis was carried out in the kidney tissue sections obtained from wild-type
and MASP-
2-/- mice following 7 days of ureteric obstruction and sham-operated wild-type
and
MASP-2-/- mice. The data shown in FIGURES 17-20 are the Log10 of relative
quantitation to a wild-type sham operated sample and bars represent the
standard error of
means. With regard to the results of the gene expression analysis of the
fibrosis-related
genes, FIGURE 17 graphically illustrates the relative mRNA expression levels
of
collagen type IV alpha 1 (collagen-4), as measured by qPCR in kidney tissue
sections
obtained from wild-type and MASP-2-/- mice following 7 days of ureteric
obstruction
and sham-operated control mice. FIGURE 18 graphically illustrates the relative
mRNA
expression levels of Transforming Growth Factor Beta-1 (TGFP-l), as measured
by
qPCR in kidney tissue sections obtained from wild-type and MASP-2-/- mice
following 7
.. days of ureteric obstruction and sham-operated control mice As shown in
FIGURES 17
and 18, the obstructed kidneys from the wild-type mice demonstrated
significantly
increased expression of the fibrosis-related genes Collagen-type IV (FIGURE
17) and
TGF13-1 (FIGURE 18), as compared to the sham-operated kidneys in wild-type
mice,
demonstrating that these fibrosis-related genes are induced after UUO injury
in wild-type
mice, as expected. In contrast, as further shown in FIGURES 17 and 18, the
obstructed
kidneys from the MASP-2-/- subjected to the UUO injury exhibited a significant
reduction in the expression of Collagen-type IV (FIGURE 17, p=0.0388) and a
significant
-150-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
reduction in the expression of TGFI3-1 (FIGURE 18, p=0.0174), as compared to
the wild-
type mice subjected to the UU0 injury.
With regard to the results of the gene expression analysis of the inflammation-
related genes, FIGURE 19 graphically illustrates the relative mRNA expression
levels of
Interleukin-6 (IL-6), as measured by qPCR in kidney tissue sections obtained
from wild-
type and MASP-2-/- mice following 7 days of ureteric obstruction and sham-
operated
control mice. FIGURE 20 graphically illustrates the relative mRNA expression
levels of
Interferon-y, as measured by qPCR in kidney tissue sections obtained from wild-
type and
MASP-2-/- mice following 7 days of ureteric obstruction and sham-operated
control
mice. As shown in FIGURES 19 and 20, the obstructed kidneys from the wild-type
mice
demonstrated significantly increased expression of the inflammation-related
genes
Interleukin-6 (FIGURE 19) and Interferon-7 (FIGURE 20), as compared to the
sham-
operated kidneys in wild-type mice, demonstrating that these inflammation-
related genes
are induced after UUO injury in wild-type mice. In contrast, as further shown
in
FIGURES 19 and 20, the obstructed kidneys from the MASP-2-/- subjected to the
UUO
injury exhibited a significant reduction in the expression of Interleukin-6
(FIGURE 19,
p=0.0109) and Interferon-7 (FIGURE 20, p=0.0182) as compared to the wild-type
mice
subjected to the UUO injury.
It is noted that gene expression for Vim, Actn-1, TNFa, C3 and IL-10 were all
found to be significantly up-regulated in the UUO kidneys obtained from both
the wild-
type and the MASP-2-/- mice, with no significant difference in the expression
levels of
these particular genes between the wild-type and MASP-2-/- mice (data not
shown). The
gene expression levels of Cdh-1 and IL-12a did not change in obstructed
kidneys from
animals in any group (data not shown).
Discussion:
The UU0 model in rodents is recognized to induce an early, active and profound
injury in the obstructed kidney with reduced renal blood flow, interstitial
inflammation
and rapid fibrosis within one to two weeks following obstruction and has been
used
extensively to understand common mechanisms and mediators of inflammation and
fibrosis in the kidney (see e.g., Chevalier R.L., Kidney Int 75:1145-1152,
2009; Yang H.
et al., Drug Discov Today Dis Models 7:13-19, 2010).
-151-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The results described in this Example demonstrate that there is a significant
reduction in collagen deposition and macrophage infiltration in UUO operated
kidneys in
the MASP-2(-/-) mice versus the wild-type (+/+) control mice. The unexpected
results
showing a significant reduction of renal injury at both the histological and
gene
expression levels in the MASP-2-/- animals demonstrates that the lectin
pathway of
complement activation contributes significantly to the development of
inflammation and
fibrosis in the obstructed kidney. While not wishing to be bound by a
particular theory, it
is believed that the lectin pathway contributes critically to the
pathophysiology of fibrotic
disease by triggering and maintaining pro-inflammatory stimuli that perpetuate
a vicious
cycle where cellular injury drives inflammation which in turn causes further
cellular
injury, scarring and tissue loss. In view of these results, it is expected
that that inhibition
or blockade of MASP-2 with an inhibitor would have a preventive and/or
therapeutic
effect in the inhibition or prevention of renal fibrosis, and for the
inhibition or prevention
of fibrosis in general (i.e., independent of the tissue or organ).
-152-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 15
This Example describes analysis of a monoclonal MASP-2 inhibitory antibody for
efficacy in the Unilateral Ureteric Obstruction (UUO) model, a murine model of
renal
.. fibrosis.
Background/Rationale:
Amelioration of renal tubulointerstitial fibrosis, the common end point of
multiple
renal pathologies, represents a key target for therapeutic strategies aimed at
preventing
progressive renal diseases. Given the paucity of new and existing treatments
targeting
.. inflammatory pro-fibrotic pathways in renal disease, there is a pressing
need to develop
new therapies. Many patients with proteinuric renal disease exhibit
tubulointerstitial
inflammation and progressive fibrosis which closely parallels declining renal
function.
Proteinuria per se induces tubulointerstitial inflammation and the development
of
proteinuric nephropathy (Brunskill N.J. et al., J Am Soc Nephrol 15:504-505,
2004).
.. Regardless of the primary renal disease, tubulointerstitial inflammation
and fibrosis is
invariably seen in patients with progressive renal impairment and is closely
correlated
with declining excretory function (Risdon R.A. et al., Lancet 1:363-366, 1968;
Schainuck
L.I., et al., Hum Pathol 1: 631-640, 1970). Therapies with the potential to
interrupt the
key common cellular pathways leading to fibrosis hold the promise of wide
applicability
.. in renal disorders.
As described in Example 14, in the UUO model of non-proteinuric renal fibrosis
it
was determined that MASP-2-/- mice exhibited significantly less renal fibrosis
and
inflammation compared to wild-type control animals, as shown by inflammatory
cell
infiltrates (75% reduction), and histological markers of fibrosis such as
collagen (one
.. third reduction), thereby establishing a key role of the lectin pathway in
renal fibrosis.
As described in Example 13, a monoclonal MASP-2 antibody (0MS646-SGMI-2
fusion, comprising an SGMI-2 peptide fused to the C-terminus of the heavy
chain of
0MS646) was generated that specifically blocks the function of the human
lectin
pathway has also been shown to block the lectin pathway in mice. In this
example,
.. 0MS646-SGMI-2 was analyzed in the UUO mouse model of renal fibrosis in wild-
type
mice to determine if a specific inhibitor of MASP-2 is able to inhibit renal
fibrosis.
Methods:
-153-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
This study evaluated the effect of a MASP-2 inhibitory antibody (10 mg/kg
0MS646-SGMI-2), compared to a human IgG4 isotype control antibody (10 mg/kg
ET904), and a vehicle control in male WT C57BL/6 mice. The antibodies
(10mg/kg)
were administered to groups of 9 mice by intraperitoneal (ip) injection on day
7, day 4
and day 1 prior to UUO surgery and again on day 2 post-surgery. Blood samples
were
taken prior to antibody administration and at the end of the experiment to
assess lectin
pathway functional activity.
The UUO surgery, tissue collection and staining with Sirius red and macrophage-
specific antibody F4/80 were carried out using the methods described in
Example 14.
Hydroxyproline content of mouse kidneys was measured using a specific
colorimetric assay test kit (Sigma) according to manufacturer's instructions.
To assess the pharmacodynamic effect of the MASP-2 inhibitory mAb in mice,
systemic lectin pathway activity was evaluated by quantitating lectin-induced
C3
activation in minimally diluted serum samples collected at the indicated time
after
MASP-2 mAb or control mAb i.p. administration to mice. Briefly described, 7 M
diameter polystyrene microspheres (Bangs Laboratories, Fisher IN, USA) were
coated
with mannan by overnight incubation with 30 g/mL mannan (Sigma) in sodium
bicarbonate buffer (pH 9.6), then washed, blocked with 1% fetal bovine serum
in PBS
and resuspended in PBS at a final concentration of 1x108 beads/mL. Complement
deposition reactions were initiated by the addition of 2.5 !AL of mannan-
coated beads
(-250,000 beads) to 50 L of minimally diluted mouse serum samples (90% final
serum
concentration), followed by incubation for 40 minutes at 4 C. Following
termination of
the deposition reaction by the addition of 250 jiL of ice-cold flow cytometry
buffer (FB:
PBS containing 0.1% fetal bovine serum), beads were collected by
centrifugation and
washed two more times with 300 uL of ice-cold FB.
To quantify lectin-induced C3 activation, beads were incubated for 1 hour at 4
C
with 50 tiL of rabbit anti-human C3c antibody (Dako, Carpenteria, CA, USA)
diluted in
FB. Following two washes with FB to remove unbound material, the beads were
incubated for 30 minutes at 4 C with 50 !AL of goat anti-rabbit antibody
conjugated to PE-
Cy5 (Southern Biotech, Birmingham, AL, USA) diluted in FB. Following two
washes
with FB to remove unbound material, the beads were resuspended in FB and
analyzed by
a FACS Calibur cytometer. The beads were gated as a uniform population using
forward
-154-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
and side scatter, and C3b deposition in each sample was quantitated as mean
fluorescent
intensity (WI).
Results:
Assessment of Collagen Deposition:
FIGURE 21 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Siruis red, wherein the tissue sections
were obtained
following 7 days of ureteric obstruction from wild-type mice treated with
either a MASP-
2 inhibitory antibody or an isotype control antibody. As shown in FIGURE 21,
tissue
sections from kidneys harvested 7 days after obstruction (UUO) obtained from
wild-type
mice treated with MASP-2 inhibitory antibody showed a significant reduction
(p=0.0477)
in collagen deposition as compared with the amount of collagen deposition in
tissue
sections from obstructed kidneys obtained from wild-type mice treated with an
IgG4
isotype control.
Assessment of Hydroxy proline content:
Hydroxy proline was measured in kidney tissues as an indicator of collagen
content. Hydroxy proline is a parameter which is highly indicative of the
pathophysiological progression of disease induced in this model.
FIGURE 22 graphically illustrates the hydroxyl proline content from kidneys
harvested 7 days after obstruction (UUO) obtained from wild-type mice treated
with
either a MASP-2 inhibitory antibody or an isotype control antibody. As shown
in
FIGURE 22, the obstructed kidney tissues from mice treated with MASP-2
inhibitory
antibody demonstrated significantly less hydroxyl proline, an indicator of
collagen
content, than the kidneys from mice treated with the IgG4 isotype control mAb
(p=0.0439).
Assessment of inflammation:
Obstructed kidneys from wild-type, isotype control antibody-treated animals,
and
wild-type animals treated with MASP-2 inhibitory antibody demonstrated a brisk
infiltrate of macrophages. Careful quantification revealed no significant
difference in
macrophage percentage stained area between these two groups (data not shown).
However, despite equivalent numbers of infiltrating macrophages, the
obstructed kidneys
from the MASP-2 inhibitory antibody-injected animals exhibited significantly
less
fibrosis as judged by Sirius red staining, compared to obstructed kidneys from
isotype
control injected animals, which result is consistent with the results that
obstructed kidney
-155-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
tissues from mice treated with MASP-2 inhibitory antibody had significantly
less
hydroxyl proline than the kidneys treated with the IgG4 isotype control mAb.
Discussion
The results described in this Example demonstrate that the use of a MASP-2
inhibitory antibody provides protection against renal fibrosis in the UUO
model, which is
consistent with the results described in Example 14 demonstrating that MASP-2-
/- mice
have significantly reduced renal fibrosis and inflammation in the UUO model as
compared to wild-type mice. The results in this Example showing reduced
fibrosis in the
mice treated with the MASP-2 inhibitory antibody. The finding of reduced
fibrosis in the
UUO kidneys in animals with a reduction or blockade of MASP-2-dependent lectin
pathway activity is highly significant novel finding. Taken together, the
results presented
in Example 14 and in this Example demonstrate a beneficial effect of MASP-2
inhibition
on renal tubulointerstitial inflammation, tubular cell injury, profibrotic
cytokine release
and scarring. The relief of renal fibrosis remains a key goal for renal
therapeutics. The
UUO model is a severe model of accelerated renal fibrosis, and an intervention
that
reduces fibrosis in this model, such as the use of MASP-2 inhibitory
antibodies, is likely
to be used to inhibit or prevent renal fibrosis. The results from the UUO
model are likely
to be transferable to renal disease characterized by glomerular and/or
proteinuric tubular
injury.
EXAMPLE 16
This Example provides results that were generated using a protein overload
proteinurea model of renal fibrosis, inflammation and tubulointerstitial
injury in MASP-
2-/- and wild-type mice to evaluate the role of the lectin pathway in
proteinuric
nephropathy.
Background/Rationale:
Proteinuria is a risk factor for the development of renal fibrosis and loss of
renal
excretory function, regardless of the primary renal disease (Tryggvason K. et
al., J Intern
Med 254:216-224, 2003, Williams M., Am J. Nephrol 25:77-94, 2005). The concept
of
proteinuric nephropathy describes the toxic effects of excess protein entering
the
proximal tubule as a result of the impaired glomerular permselectivity
(Brunskill N.J., J
Am Soc Nephrol 15:504-505, 2004, Baines R.J., Nature Rev Nephrol 7:177-180,
2011).
This phenomenon, common to many glomerular diseases, results in a pro-
inflammatory
scarring environment in the kidney and is characterized by alterations in
proximal tubular
-156-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
cell growth, apoptosis, gene transcription and inflammatory cytokine
production as a
consequence of dysregulated signaling pathways stimulated by proteinuric
tubular
fluid. Proteinuric nephropathy is generally recognized to be a key contributor
to
progressive renal injury common to diverse primary renal pathologies.
Chronic kidney disease affects greater than 15% of the adult population in the
United States and accounts for approximately 750,000 deaths each year
worldwide
(Lozano R. et al., Lancet vol 380, Issue 9859:2095-2128, 2012). Proteinuria is
an
indicator of chronic kidney disease as well as a factor promoting disease
progression.
Many patients with proteinuric renal disease exhibit tubul ointerstiti al
inflammation and
progressive fibrosis which closely parallels declining renal function
Proteinuria per se
induces tubulointerstitial inflammation and the development of proteinuric
nephropathy
(Brunskill N.J. et al., J Am Soc Nephrol 15:504-505, 2004). In proteinuric
kidney
diseases, excessive amounts of albumin and other macromolecules are filtered
through
the glomeruli and reabsorbed by proximal tubular epithelial cells. This causes
an
inflammatory vicious cycle mediated by complement activation leading to
cytokine and
leukocyte infiltrates that cause tubule-interstitial injury and fibrosis,
thereby exacerbating
proteinuria and leading to loss of renal function and eventually progression
to end-stage
renal failure (see, e.g., Clark et al., Canadian Medical Association Journal
178:173-175,
2008). Therapies that modulate this detrimental cycle of inflammation and
proteinuria
are expected to improve outcomes in chronic kidney disease.
In view of the beneficial effects of MASP-2 inhibition in the UUO model of
tubulointerstital injury, the following experiment was carried out to
determine if MASP-2
inhibition would reduce renal injury in a protein overload model. This study
employed
protein overload to induce proteinuric kidney disease as described in Ishola
et al.,
European Renal Association 21:591-597, 2006.
Methods
A MASP-2-/- mouse was generated as described in Example 1 and backcrossed
for 10 generations with BALB/c. The current study compared the results of wild-
type
and MASP-2-/- BALB/c mice in a protein overload proteinuria model as follows.
One week prior to the experiment, mice were unilaterally nephrectomised before
protein overload challenge in order to see an optimal response. The
proteinuria inducing
agent used was a low endotoxin bovine serum albumin (BSA, Sigma) given i.p. in
normal
saline to WT (n=7) and MASP-2 -/- mice (n=7) at the following doses: one dose
each of
-157-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
2mg BSA/gm, 4mg BSA/gm, 6mg BSA/gm, 8mg BSA/gm, 10mg BSA/gm and 12mg
BSA/gm body weight, and 9 doses of 15mg BSA/gm body weight, for a total of 15
doses
administered i.p. over a period of 15 days. The control WT (n=4) and MASP-2-/-
(n=4)
mice received saline only administered i.p. After administration of the last
dose, animals
were caged separately in metabolic cages for 24 hours to collect urine. Blood
was
collected by cardiac puncture under anesthesia, blood was allowed to clot on
ice for 2
hours and serum was separated by centrifugation. Serum and urine samples were
collected at the end of the experiment on day 15, stored and frozen for
analysis.
Mice were sacrificed 24 hours after the last BSA administration on day 15 and
various tissues were collected for analysis. Kidneys were harvested and
processed for
H&E and immunostaining. Immunohistochemistry staining was carried out as
follows.
Formalin fixed, paraffin-embedded 5 micron kidney tissue sections from each
mouse
were deparaffinized and rehydrated. Antigen retrieval was performed in citrate
buffer at
95 C for 20 minutes followed by incubating tissues in 3% H202 for 10 minutes.
Tissues
were then incubated in blocking buffer (10% serum from the species the
secondary
antibody was raised in and 1% BSA in PBS) with 10% avidin solution for 1 hour
at room
temperature. Sections were washed in PBS three times, 5 minutes each, after
each step.
Primary antibody was then applied in blocking buffer with 10% biotin solution
for 1 hour
at a concentration of 1:100 for the antibodies F4/80 (Santa Cruz cat# sc-
25830), TGFI3
(Santa Cruz cat# sc-7892), IL-6 (Santa Cruz cat# sc-1265) and at 1:50 for the
TNFa
antibody (Santa Cruz cat# sc-1348). A biotinylated secondary antibody was then
applied
for 30 minutes at a concentration of 1:200 for the F4/80, TGFP and 1L-6
sections and
1:100 for the TNFa section followed by HRP conjugate enzyme for another 30
minutes.
The color was developed using diaminobenzidine (DAB) substrate kit (Vector
labs) for
10 minutes and slides were washed in water, dehydrated and mounted without
counter
staining to facilitate computer-based image analysis. Stained tissue sections
from the
renal cortex were analyzed by digital image capture followed by quantification
using
automated image analysis software.
Apoptosis was assessed in the tissue sections by staining with terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) as follows.
Apoptotic
cells in the kidney sections were stained using ApopTagg Peroxidase kit
(Millipore) as
follows. Parrafin embedded, formalin fixed kidney sections from each mouse
were
deparaffinized, rehydrated and then protein permeabilized using proteinase K
(20 ug/mL)
-158-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
which was applied to each specimen for 15 minutes at room temperature.
Specimens
were washed in PBS between steps. Endogenous peroxidase activity was quenched
by
incubating tissues in 3% H202 for 10 minutes. Tissues were then incubated in
equilibration buffer followed by incubation with TdT enzyme for 1 hour at 37
C. After
washing in stop/wash buffer for 10 minutes, anti-digoxignenin conjugate was
applied for
30 minutes at room temperature followed by washing. Color was developed in DAB
substrate kit for 4 minutes followed by washing in water. Tissues were counter
stained in
haematoxylin and mounted in DBX. The frequency of TUNEL stained (brown
colored)
apoptotic cells were manually counted in serially selected 20 high power
fields from the
cortex using Leica DBXM light microscope.
Results:
Assessment of Proteinuria
To confirm the presence of proteinuria in the mice, the total protein in serum
was
analyzed at day 15 and the total excreted proteins in urine was measured in
urine samples
collected over a 24 hour period on day 15 of the study.
FIGURE 23 graphically illustrates the total amount of serum proteins (mg/ml)
measured at day 15 in the wild-type control mice (n=2) that received saline
only, the
wild-type mice that received BSA (n=6) and the MASP-2-/- mice that received
BSA
(n=6). As shown in FIGURE 23, administration of BSA increased the serum total
protein
level in both wild-type and MASP-2-/- groups to more than double the
concentration of
the control group that received only saline, with no significant difference
between the
treated groups.
FIGURE 24 graphically illustrates the total amount of excreted protein (mg) in
urine collected over a 24 hour period on day 15 of the study from the wild-
type control
mice (n=2) that received saline only, the wild-type mice that received BSA
(n=6) and the
MASP-2-/- mice that received BSA (n=6). As shown in FIGURE 24, on day 15 of
this
study, there was an approximately six-fold increase in total excreted proteins
in urine in
the BSA treated groups as compared to the sham-treated control group that
received
saline only. The results shown in FIGURES 23 and 24 demonstrate that the
proteinuria
model was working as expected.
Assessment of Histological Changes in the Kidney
FIGURE 25 shows representative H&E stained renal tissue sections that were
harvested on day 15 of the protein overload study from the following groups of
mice:
-159-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
(panel A) wild-type control mice; (panel B) MASP-2-/- control mice; (panel C)
wild-type
mice treated with BSA; and (panel D) MASP-2-/- mice treated with BSA. As shown
in
FIGURE 25, there is a much higher degree of tissue preservation in the MASP-2-
/-
overload group (panel D) compared to the wild-type overload group (panel C) at
the same
level of protein overload challenge. For example, Bowman's capsules in the
wild-type
mice treated with BSA (overload) were observed to be greatly expanded (panel
C) as
compared to Bowman's capsules in the wild-type control group (panel A) In
contrast,
Bowman's capsules in the MASP-2-/- mice (overload) treated with the same level
of
BSA (panel D) retained morphology similar to the MASP-2-/- control mice (panel
B) and
wild-type control mice (panel A) As further shown in FIGURE 25, large protein
cast
structures have accumulated in proximal and distal tubules of the wild-type
kidney
sections (panel C), which are larger and more abundant as compared to MASP-2-/-
mice
(panel D).
It is also noted that analysis of renal sections from this study by
transmitting
electron microscope showed that the mice treated with BSA had overall damage
to the
ciliary borders of distal and proximal tubular cells, with cellular content
and nuclei
bursting into the tubule lumen. In contrast, the tissue was preserved in the
MASP-2-/-
mice treated with BSA.
Assessment of Macrophage Infiltration in the Kidney
To measure the degree of inflammation, as indicated by macrophage
infiltration,
the tissue sections of the harvested kidneys were also stained with macrophage-
specific
antibody F4/80 using methods as described in Boor et al., J of Am Soc of
Nephrology
18:1508-1515, 2007.
FIGURE 26 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with macrophage-specific antibody F4/80,
showing the
macrophage mean stained area (%), wherein the tissue sections were obtained at
day 15
of the protein overload study from wild-type control mice (n=2), wild-type
mice treated
with BSA (n=6), and MASP-2-/- mice treated with BSA (n=5). As shown in FIGURE
26, kidney tissue sections stained with F4/80 anti-macrophage antibody showed
that
while both groups treated with BSA showed a significant increase in the kidney
macrophage infiltration (measured as %F4/80 antibody-stained area) compared to
the
wild-type sham control, a significant reduction in macrophage infiltration was
observed
-160-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
in tissue sections from BSA-treated MASP-2-/- mice as compared with macrophage
infiltration in tissue sections from BSA-treated wild-type mice (p
value=0.0345).
FIGURE 27A graphically illustrates the analysis for the presence of a
macrophage-proteinuria correlation in each wild-type mouse (n=6) treated with
BSA by
plotting the total excreted proteins measured in urine from a 24 hour sample
versus the
macrophage infiltration (mean stained area %). As shown in FIGURE 27A, most of
the
samples from the wild-type kidneys showed a positive correlation between the
level of
proteinuria present and the degree of macrophage infiltration.
FIGURE 27B graphically illustrates the analysis for the presence of a
macrophage-proteinuria correlation in each MASP-2-/- mouse (n=5) treated with
BSA by
plotting the total excreted proteins in urine in a 24 hour sample versus the
macrophage
infiltration (mean stained area %). As shown in FIGURE 27B, the positive
correlation
observed in wild-type mice between the level of proteinuria and the degree of
macrophage infiltration (shown in FIGURE 27A) was not observed in MASP-2-/-
mice.
While not wishing to be bound by any particular theory, these results may
indicate the
presence of a mechanism of inflammation clearance at high levels of
proteinuria in
MASP-2-/- mice.
Assessment of Cytokine Infiltration
Interleukin 6 (IL-6), Transforming Growth Factor Beta (TGFP) and Tumor
Necrosis Factor Alpha (TNFa) are pro-inflammatory cytokines known to be up-
regulated
in proximal tubules of wild-type mice in a model of proteinuria (Abbate M. et
al., Journal
of the American Society of Nephrology: JASN, 17: 2974-2984, 2006; David S. et
al.,
Nephrol ogy, Di dalysi s, Transplantation, Official Publication of the
European Dialysis
and Transplant Association- European Renal Association 12: 51-56, 1997). The
tissue
sections of kidneys were stained with cytokine-specific antibodies as
described above.
FIGURE 28 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TGFP antibody (measured as % TGFP antibody-
stained
area) in wild-type mice treated with BSA (n=4) and MASP-2-/- mice treated with
BSA
(n=5). As shown in FIGURE 28, a significant increase in the staining of TGF13
was
observed in the wild-type BSA treated (overload) group as compared to the MASP-
2-/-
BSA treated (overload) group (p=0.026).
FIGURE 29 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TNFa antibody (measured as % TNFa antibody-
stained
-161-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
area) in wild-type mice treated with BSA (n=4) and MASP-2-/- mice treated with
BSA
(n=5). As shown in FIGURE 29, a significant increase in the staining of TNFa
was
observed in the wild-type BSA treated (overload) group as compared to the MASP-
2-/-
BSA treated (overload) group (p=0.0303).
FIGURE 30 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-IL-6 antibody (measured as % IL-6 antibody-
stained
area) in wild-type control mice, MASP-2-/- control mice, wild-type mice
treated with
BSA (n=7) and MASP-2-/- mice treated with BSA (n=7). As shown in FIGURE 30, a
highly significant increase in the staining of IL-6 was observed in the wild-
type BSA
treated group as compared to the MASP-2-/- BSA treated group (p=0.0016).
Assessment of Apoptosis
Apoptosis was assessed in the tissue sections by staining with terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and the frequency
of
TUNEL stained apoptotic cells were counted in serially selected 20 high power
fields
(HPFs) from the cortex.
FIGURE 31 graphically illustrates the frequency of TUNEL apoptotic cells
counted in serially selected 20 high power fields (HPFs) from tissue sections
from the
renal cortex in wild-type control mice (n=1), MASP-2-/- control mice (n=1),
wild-type
mice treated with BSA (n=6) and MASP-2-/- mice treated with BSA (n=7). As
shown in
FIGURE 31, a significantly higher rate of apoptosis in the cortex was observed
in kidneys
obtained from wild-type mice treated with BSA as compared to kidneys obtained
from
the MASP-2-/- mice treated with BSA (p=0.0001).
Overall Summary of Results and Conclusions:
The results in this Example demonstrate that MASP-2-/- mice have reduced renal
injury in a protein overload model. Therefore, MASP-2 inhibitory agents, such
as
MASP-2 inhibitory antibodies would be expected to inhibit or prevent the
detrimental
cycle of inflammation and proteinuria and improve outcomes in chronic kidney
disease.
-162-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 17
This Example describes analysis of a monoclonal MASP-2 inhibitory antibody for
efficacy in reducing and/or preventing renal inflammation and
tubulointerstitial injury in
a mouse protein overload proteinurea model in wild-type mice.
Background/Rationale:
As described in Example 16, in a protein overload model of proteinuria it was
determined that MASP-2-/- mice exhibited significantly better outcomes (e.g.,
less
tubulointerstitial injury and less renal inflammation) than wild-type mice,
implicating a
pathogenic role for the lectin pathway in proteinuric kidney disease.
As described in Example 13, a monoclonal MASP-2 inhibitory antibody
(0MS646-SGMI-2) was generated that specifically blocks the function of the
human
lectin pathway and has also been shown to block the lectin pathway in mice. In
this
example, the MASP-2 inhibitory antibody 0MS646-SGMI-2 was analyzed in a mouse
protein overload proteinurea model for efficacy in reducing and/or preventing
renal
inflammation and tubulointerstitial injury in wild-type mice.
Methods:
This study evaluated the effect of MASP-2 inhibitory antibody (10 mg/kg
0MS646-SGMI-2), compared to a human IgG4 isotype control antibody, ET904 (10
mg/kg), and a saline control.
Similar to the study described in Example 16, this study employed protein
overload to induce proteinuric kidney disease (Ishola et al., European Renal
Association
21:591-597, 2006). Proteinuria was induced in unilaterally nephrectomized
Balb/c mice
by daily i p. injections with escalating doses (2 g/kg to 15 g/kg) of low
endotoxin bovine
serum albumin (BSA) for a total of 15 days, as described in Example 16.
Antibody treatments were administered by biweekly i.p. injection starting 7
days
before proteinuria induction and continued throughout the study. This dosing
scheme
was selected based on previous PK/PD and pharmacoclogy studies demonstrating
sustained lectin pathway suppression (data not shown). Mice were sacrificed on
day 15
and kidneys were harvested and processed for H&E and immunostaining. Stained
tissue
sections from the renal cortex were analyzed by digital image capture followed
by
quantification using automated image analysis software.
Immunohistochemistry staining and apoptosis assessment were carried out as
described in Example 16.
-163-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Results:
Assessment of Proteinuria
To confirm the presence of proteinuria in the mice, the total excreted
proteins in
urine was measured in urine samples collected over a 24 hour period at day 15
(the end of
the experiment). It was determined that the urine samples showed a mean of
almost a
six-fold increase in total protein levels in the groups that were treated with
BSA as
compared to the control groups not treated with BSA (data not shown),
confirming the
presence of proteinuria in the mice treated with BSA. No significant
difference was
observed in the protein levels between the BSA-treated groups.
Assessment of Histological Changes
FIGURE 32 shows representative H&E stained tissue sections from the following
groups of mice at day 15 after treatment with BSA. (panel A) wild-type control
mice
treated with saline; (panel B) isotype antibody treated control mice; and
(panel C) wild-
type mice treated with MASP-2 inhibitory antibody.
As shown in FIGURE 32, there is a much higher degree of tissue preservation in
the MASP-2 inhibitory antibody-treated group (panel C) as compared to the wild-
type
group treated with saline (panel A) or isotype control (panel B) at the same
level of
protein overload challenge.
Assessment of Apoptosis
Apoptosis was assessed in the tissue sections by staining with terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and the frequency
of
TUNEL stained apoptotic cells were counted in serially selected 20 high power
fields
(HPFs) from the cortex. FIGURE 33 graphically illustrates the frequency of
TUNEL
apoptotic cells counted in serially selected 20 high power fields (HPFs) from
tissue
sections from the renal cortex in wild-type mice treated with saline control
and BSA
(n=8), wild-type mice treated with the isotype control antibody and BSA (n=8)
and wild-
type mice treated with the MASP-2 inhibitory antibody and BSA (n=7). As shown
in
FIGURE 33, a highly significantly decrease in the rate of apoptosis in the
cortex was
observed in kidneys obtained from the MASP-2 inhibitory antibody treated group
as
compared to the saline and isotype control treated group (p=0.0002 for saline
control v
MASP-2 inhibitory antibody; p=0.0052 for isotype control v. MASP-2 inhibitory
antibody).
Assessment of Cytokine Infiltration
-164-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Interleukin 6 (IL-6), Transforming Growth Factor Beta (TGFP) and Tumor
Necrosis Factor Alpha (TNFa), which are pro-inflammatory cytokines known to be
up-
regulated in proximal tubules of wild-type mice in a model of proteinuria,
were assessed
in the kidney tissue sections obtained in this study.
FIGURE 34 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TGFP antibody (measured as % TGFP antibody-
stained
area) in wild-type mice treated with BSA and saline (n=8), wild-type mice
treated with
BSA and isotype control antibody (n=7) and wild-type mice treated with BSA and
MASP-2 inhibitory antibody (n=8). As shown in FIGURE 34, quantification of the
TGFP stained areas showed a significant reduction in the levels of TGFO in the
MASP-2
inhibitory antibody-treated mice as compared to the saline and isotype control
antibody-
treated control groups (p values= 0.0324 and 0.0349, respectively).
FIGURE 35 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-TNFa antibody (measured as % TNFa antibody-
stained
area) in wild-type mice treated with BSA and saline (n=8), BSA and isotype
control
antibody (n=7) and wild-type mice treated with BSA and MASP-2 inhibitory
antibody
(n=8). As shown in FIGURE 35, analysis of stained sections showed a
significant
reduction in the level of TNFa in the MASP-2 inhibitory antibody-treated group
as
compared to the saline control group (p=0.011) as well as the isotype control
group
(p=0.0285).
FIGURE 36 graphically illustrates the results of computer-based image analysis
of
stained tissue sections with anti-IL-6 antibody (measured as % IL-6 antibody-
stained
area) in in wild-type mice treated with BSA and saline (n=8), BSA and isotype
control
antibody (n=7) and wild-type mice treated with BSA and MASP-2 inhibitory
antibody
(n=8). As shown in FIGURE 36, analysis of stained sections showed a
significant
reduction in the level of IL-6 in the MASP-2 inhibitory antibody-treated group
as
compared to the saline control group (p=0.0269) as well as to the isotype
control group
(p=0.0445).
Overall Summary of Results and Conclusions:
The results in this Example demonstrate that the use of a MASP-2 inhibitory
antibody provides protection against renal injury in a protein overload model,
which is
consistent with the results described in Example 16 demonstrating that MASP-2-
/- mice
have reduced renal injury in the proteinuria model.
-165-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
EXAMPLE 18
This Example provides results generated using an Adriamycin-induced
nephrology model of renal fibrosis, inflammation and tubulointerstitial injury
in MASP-
2-/- and wild-type mice to evaluate the role of the lectin pathway in
Adriamycin-induced
nephropathy.
Background/Rationale:
Adriamycin is an anthracycline antitumor antibiotic used in the treatment of a
wide range of cancers, including hematological malignancies, soft tissue
sarcomas and
many types of carcinomas. Adriamycin-induced nephropathy is well established
rodent
model of chronic kidney disease that has enabled a better understanding of the
progression of chronic proteinuria (Lee and Harris, Nephrology, 16:30-38,
2011). The
type of structural and functional injury in Adriamycin-induced nephropathy is
very
similar to that of chronic proteinuric renal disease in humans (Pippin et al.,
American
Journal of Renal Physiology 296:F213-29, 2009).
Adriamycin-induced nephropathy is characterized by an injury to the podocytes
followed by glomerulosclerosis, tubulointerstitial inflammation and fibrosis.
It has been
shown in many studies that Adriamycin-induced nephropathy is modulated by both
immune and non-immune derived mechanisms (Lee and Harris, Nephrology, 16:30-
38,
2011). Adriamycin-induced nephropathy has several strengths as a model of
kidney
disease. First, it is a highly reproducible and predicable model of renal
injury. This is
because it is characterized by the induction of renal injury within a few days
of drug
administration, which allows for ease of experimental design as the timing of
injury is
consistent. It is also a model in which the degree of tissue injury is severe
while
associated with acceptable mortality (<5%) and morbidity (weight loss).
Therefore, due
to the severity and timing of renal injury in Adriamycin-induced nephropathy,
it is a
model suitable for testing interventions that protect against renal injury.
As described in Examples 16 and 17, in a protein overload model of proteinuria
it
was determined that MASP-2-/- mice and mice treated with a MASP-2 inhibitory
antibody exhibited significantly better outcomes (e.g., less
tubulointerstitial injury, and
less renal inflammation) than wild-type mice, implicating a pathogenic role
for the lectin
pathway in proteinuric kidney disease.
-166-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
In this example, MASP-2-/- mice were analyzed in comparison with wild-type
mice in the Adriamycin-induced nephrology model (AN) to determine if MASP-2
deficiency reduces and/or prevents renal inflammation and tubulointerstitial
injury
induced by Adriamycin.
Methods:
1. Dosage and Time point optimization
An initial experiment was carried out to determine the dose of Adriamycin and
time
point at which BALB/c mice develop a level of renal inflammation suitable for
testing
therapeutic intervention.
Three groups of wild-type BALB/c mice (n=8) were injected with a single dose
of
Adriamycin (10.5 mg/kg) administered IV. Mice were culled at three time
points: one
week, two weeks and four weeks after Adriamycin administration. Control mice
were
injected with saline only.
Results: All mice in the three groups showed signs of glomerulosclerosis and
proteinuria, as determined by H&E staining, with incrementally increasing
degree of
tissue inflammation as measured by macrophage infiltration in the kidney (data
not
shown). The degree of tissue injury was mild in the one week group, moderate
in the two
week group and severe in the four week group (data not shown). The two week
time
point was selected for the rest of the study.
2. Analysis of Adriamycin-induced nephrology in wild-type and MASP-2-/-
mice
In order to elucidate the role of the lectin pathway of complement in the
Adriamycin-
induced nephrology, a group of MASP-2-/- mice (BALB/c) were compared to wild-
type
mice (BALB/c) at the same dose of Adriamycin. The MASP-2-/- mice were
backcrossed
with BALB/c mice for 10 generations
Wild-type (n=8) and MASP-2-/- (n=8) were injected IV with Adriamycin (10.5
mg/kg) and three mice of each strain were give saline only as a control. All
mice were
culled two weeks after the treatment and tissues were collected. The degree of
histopatholigical injury was assessed by H&E staining.
Results:
FIGURE 37 shows representative H&E stained tissue sections from the following
groups of mice at day 14 after treatment with Adriamycin or saline only
(control): (panels
A-1, A-2, A-3) wild-type control mice treated with only saline; (panels B-1, B-
2, B-3)
-167-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
wild-type mice treated with Adriamycin; and (panels C-1, C-2, C-3) MASP-2-/-
mice
treated with Adriamycin. Each photo (e.g., panel A-1, A-2, A-3) represents a
different
mouse.
As shown in FIGURE 37, there is a much higher degree of tissue preservation in
the MASP-2-/- group treated with Adriamycin as compared to the wild-type group
treated
with the same dose of Adriamycin.
FIGURE 38 graphically illustrates the results of computer-based image analysis
of kidney tissue sections stained with macrophage-specific antibody F4/80
showing the
macrophage mean stained area (%) from the following groups of mice at day 14
after
treatment with Adriamycin or saline only (wild-type control): wild-type
control mice
treated with only saline; wild-type mice treated with Adriamycin; MASP-2-/-
mice
treated with saline only, and MASP-2 -/- mice treated with Adriamycin. As
shown in
FIGURE 38, MASP-2-/- mice treated with Adriamycin have reduced macrophage
infiltration (**p=0.007) compared to wild-type mice treated with Adriamycin.
FIGURE 39 graphically illustrates the results of computer-based image analysis
of
kidney tissue sections stained with Sirius Red, showing the collagen
deposition stained
area (%) from the following groups of mice at day 14 after treatment with
Adriamycin or
saline only (wild-type control): wild-type control mice treated with only
saline; wild-type
mice treated with Adriamycin; MASP-2-/- mice treated with saline only, and
MASP-2 -/-
mice treated with Adriamycin. As shown in FIGURE 39, MASP-2-/- mice treated
with
Adriamycin have reduced collagen deposition ("p=0.005) compared to wild-type
mice
treated with Adriamycin.
Overall Summary and Conclusions:
The amelioration of renal tubulointerstitial inflammation is a key target for
the
treatment of kidney disease. The results presented herein indicate that the
lectin pathway
of complement activation contributes significantly to the development of renal
tubulointerstitial inflammation. As further demonstrated herein, a MASP-2
inhibitory
agent, such as a MASP-2 inhibitory antibody, may be used as a novel
therapeutic
approach in the treatment of proteinuric nephropathy, Adriamycin nephropathy
and
amelioration of renal tubulointerstitial inflammation.
EXAMPLE 19
-168-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
This Example describes the initial results of an ongoing Phase 2 clinical
trial to
evaluate the safety and clinical efficacy of a fully human monoclonal MASP-2
inhibitory
antibody in adults with steroid-dependent immunoglobulin A nephropathy (IgAN)
and in
adults with steroid-dependent membranous nephropathy (MN).
Background:
Chronic kidney diseases affect more than 20 million people in the United
States
(Drawz P. et al., Ann Intern Med 162(11); ITCI-16, 2015).
Glomerulonephropathies
(GNs), including IgAN and MN are kidney diseases in which the glomeruli are
damaged
and frequently lead to end-stage renal disease and dialysis. Several types of
primary GNs
exist the most common being IgAN. Many of these patients have persistent renal
inflammation and progressive deterioration. Often these patients are treated
with
corticosteroids or immunosuppressive agents, which have many serious long-term
adverse consequences. Many patients continue to deteriorate even on these
treatments.
No treatments are approved for the treatment of IgAN or MN.
IgA Nephropathy
Immunoglobulin A nephropathy (IgAN) is an autoimmune kidney disease
resulting in intrarenal inflammation and kidney injury. IgAN is the most
common form
of primary glomerulonephritis globally (Magistroni et al., Kidney Int.
88(5):974-89,
2015). With an annual incidence of approximately 2.5 per 100,000, it is
estimated that 1
in 1400 persons in the U.S. will develop IgAN. As many as 40% of patients with
IgAN
will develop end-stage renal disease (ESRD) within 20 years following
diagnosis (Coppo
R., D'Amico G., J Nephrol 18(5):503-12, 2005; Xie et al., PLoS One,
7(6):e38904
(2012)). Patients typically present with microscopic hematuri a with mild to
moderate
proteinuria and variable levels of renal insufficiency (Wyatt R.J., et al., N
Engl J Vied
368(25).2402-14, 2013). Clinical markers such as impaired kidney function,
sustained
hypertension, and heavy proteinuria (over 1 g per day) are associated with
poor prognosis
(Goto Met al., Nephrol Dial Transplant 24(10):3068-74, 2009; Berthoux F. et
al., J Am
Soc Nephrol 22(4):752-61, 2011). Proteinuria is the strongest prognostic
factor
independent of other risk factors in multiple large observational studies and
prospective
trials (Coppo R. et al., J Nephrol 18(5):503-12, 2005; Reich H. N., et al., J
Am Soc
Nephrol 18(12):3177-83, 2007). It is estimated that 15-20% of patients reach
ESRD
within 10 years of disease onset if left untreated (D'Amico G., Am J Kidney
Dis
36(2):227-37, 2000).
-169-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The diagnostic hallmark of IgAN is the predominance of IgA deposits, alone or
with IgG, IgM, or both, in the glomerular mesangium. In IgAN, renal biopsies
reveal
glomerular deposition of mannan-binding lectin (MBL), a key recognition
molecule for
activation of MASP-2, the effector enzyme of the complement system's lectin
pathway.
Glomerular MBL deposits, usually co-localized with IgA and indicating
complement
activation, and high levels of urinary MBL are associated with an unfavorable
prognosis
in IgAN, with these patients demonstrating more severe histological changes
and
mesangial proliferation than patients without MBL deposition or high levels of
urinary
MBL (Matsuda M. et al., Nephron 80(4):408-13, 1998; Liu LL et al., Clin Exp
Immunol
169(2):148-155, 2012; Roos A. et al., J Am Soc Nephrol 17(6):1724-34, 2006;
Liu LL et
al., Chn Exp1111111111101174(1).152-60, 2013). Remission rates also are
substantially lower
for patients with MBL deposition (Liu LL et al., Clin Exp Immunol 174(1):152-
60, 2013).
Current approaches to treatment of IgAN all attempt to slow, stop, or delay
deterioration of renal function. The Kidney Disease: Improving Global Outcomes
(KDIGO) clinical practice guidelines for glomerulonephritis recommend a
treatment plan
for IgAN that primarily emphasizes blood pressure control through renin-
angiotensin
system (RAS) blockade [KDIGO Work Group 2012]. For patients with persistent
daily
proteinuria of 1 g or more despite maximum tolerated doses of anti-
hypertensives and
well-controlled blood pressure, recommended treatment includes corticosteroids
and /or
other immunosuppressive agents, such as cyclophosphamide, azathioprine, or
mycofenolate mofetil. The Kidney Disease Improving Global Outcomes (KDIGO)
Guidelines for Glomerulonephritis (Int. Soc of Nephrol 2(2):139-274, 2012)
recommend
that corticosteroids should be administered to patients with proteinuria of
greater than or
equal to 1 g/day, with a usual treatment duration of 6 months. For patients
with
crescentic IgAN (defined as cresents in > 500/0 of glomeruli) and a rapid
deterioration in
renal clearance function, another immunosuppressive (e.g., cyclophosphamide)
may be
added to corticosteroids. However, even with aggressive immunosuppressive
treatment,
which is associated with serious long-term sequelae, some patients have
progressive
deterioration of renal function. There is no FDA- approved treatment for IgAN,
and even
with the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin
receptor
blockers (ARBs) to control blood pressure, increased proteinuria persists in
some
patients. None of these treatments have been shown to stop or even slow the
progression
of IgAN in patients who are at risk for rapid progression of the disease.
Alternative
-170-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
treatments that could reduce or eliminate the need for chronic corticosteroid
and/or
immunosuppressive therapies would clearly address an unmet medical need.
Membranous Nephropathy
The annual incidence of membranous nephropathy (MN) is approximately 10-12
per 1,000,000. Patients with MN can have a variable clinical course, but
approximately
25% will develop end-stage renal disease.
Membranous nephropathy is an immune-mediated glomerular disease and one of
the most common causes of the nephrotic syndrome in adults. The disease is
characterized by the formation of immune deposits, primarily IgG4, on the
outer aspect of
the glomerular basement membrane, which contain podocyte antigens and
antibodies
specific to those antigens, resulting in complement activation. Initial
manifestations of
MN are related to the nephrotic syndrome: proteinuria, hypoalbuminemia,
hyperlipidemia, and edema.
Although MN may spontaneously remit without treatment, as many as one third
of patients demonstrate progressive loss of kidney function and progress to
ESRD at a
median of 5 years after diagnosis. Often, corticosteroids are used to treat MN
and there is
a need to develop alternative therapies. Additionally, patients determined to
be at
moderate risk for progression, based on severity of proteinuria, are treated
with
prednisone in conjunction with cyclophosphamide or a calcinuerin inhibitor,
and these
two treatments together are often associated with severe systemic adverse
effects.
Methods:
Two Phase 1 clinicial trials carried out in healthy volunteers have
demonstrated
that both intravenous and subcutaneous dosing of a MASP-2 inhibitory antibody,
0MS646, resulted in sustained lectin pathway inhibition.
This Example describes interim results from an ongoing Phase 2, uncontrolled,
multicenter study of a MASP-2 inhibitory antibody, 0MS646, in subjects with
IgAN and
MN. Inclusion criteria require that all patients in this study, regardless of
renal disease
subtype, have been maintained on a stable dose of corticosteroids for at least
12 weeks
prior to study enrollment (i.e., the patients are steroid-dependent). The
study is a single-
arm pilot study with 12 weeks of treatment and a 6-week follow-up period.
Approximately four subjects are planned to be enrolled per disease. The study
is
designed to evaluate whether 0MS646 may improve renal function (e.g., improve
-171-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
proteinuria) and decrease corticosteroid needs in subjects with IgAN and MN.
To date, 2
patients with IgA nephropathy and 2 patients with membranous nephropathy have
completed treatment in the study.
At study entry each subject must have high levels of protein in the urine
despite
ongoing treatment with a stable corticosteroid dose. These criteria select for
patients who
are unlikely to spontaneously improve during the study period.
The subjects were age > 18 at screening and were only included in the study if
they had a diagnosis of one of the following: IgAN diagnosed on kidney biopsy
or
primary MN diagnosed on kidney biopsy. The enrolled patients also had to meet
all of
the following inclusion criteria.
(1) have average urine albumin/creatinine ratio > 0.6 from three samples
collected
consecutively and daily prior to each of 2 visits during the screening period,
(2) have been on > 10 mg of prednisone or equivalent dose for at least 12
weeks
prior to screening visit 1;
(3) if on immunosuppressive treatment (e.g., cyclophosphamide, mycophenolate
mofetil), have been on a stable dose for at least 2 months prior to Screening
Visit 1 with
no expected change in the dose for the study duration;
(4) have an estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73m2
calculated by the MDRD equation';
(5) are on a physician-directed, stable, optimized treatment with angiotensin
converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB)
and
have a systolic blood pressure of <150 mmHg and a diastolic blood pressure of
<90mmHg at rest;
(6) have not used belimumab, eculizumab or rituzimab within 6 months of
screening visit 1; and
(7) do not have a history of renal transplant.
1MDRD Equation: eGFR (mL/min/1.73m2) = 175 x (SCr)-1154 x (Age)-0.203
(0.742 if female) x (1.212 if African American). Note: SCr=Serum Creatinine
measurement should be mg/dL.
-172-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
The monoclonal antibody used in this study, 0MS646, is a fully human IgG4
monoclonal antibody that binds to and inhibits human MASP-2. MASP-2 is the
effector
enzyme of the lectin pathway. As demonstrated in Example 12, 0MS646 avidly
binds to
recombinant MASP-2 (apparent equilibrium dissociation constant in the range of
100
pM) and exhibits greater than 5,000-fold selectivity over the homologous
proteins Cis,
Cl r, and MASP-1. In functional assays, 0MS646 inhibits the human lectin
pathway with
nanomolar potency (concentration leading to 50% inhibition [IC50] of
approximately 3
nM) but has no significant effect on the classical pathway. 0MS646
administered either
by intravenous (IV) or subcutaneous (SC) injection to mice, non-human
primates, and
humans resulted in high plasma concentrations that were associated with
suppression of
lectin pathway activation in an ex vivo assay.
In this study, the 0MS646 drug substance was provided at a concentration of
100
mg/mL, which was further diluted for IV administration. The appropriate
calculated
volume of 0MS646 100 mg/mL injection solution was withdrawn from the vial
using a
syringe for dose preparation. The infusion bag was administered within four
hours of
preparation.
The study consists of screening (28 days), treatment (12 weeks) and follow-up
(6
weeks) periods, as shown in the Study Design Schematic below.
Study Design Schematic
(03:011
Tratemit rollow rp
=
ihm4 #1Pg
t rhstvlz
11.1is I i.4 lAtulk --------------------------- VNi :$f
1 t;tz:3;
fkSt
.;=k?1
Within the screening period and before the first 0MS646 dose, consented
subjects
provided three urine samples (collected once daily) on each of two three-
consecutive-day
periods to establish baseline values of the urine albumin-to-creatinine ratio.
Following
the screening period, eligible subjects received 0MS646 4 mg/kg IV once weekly
for 12
weeks (treatment period). There was a 6-week follow-up period after the last
dose of
OMS646.
-173-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
During the initial 4 weeks of treatment with 0MS646, subjects were maintained
on their stable pre-study dose of corticosteroids. At the end of the initial 4-
weeks of the
12-week treatment period, subjects underwent corticosteroid taper (i.e., the
corticosteroid
dose was reduced), if tolerated, over 4 weeks, followed by 4 weeks during
which the
resultant corticosteroid dose was maintained. The target was a taper to < 6 mg
prednisone (or equivalent dose) daily. Over this period, the taper was
discontinued in
subjects who had deterioration of renal function, as determined by the
investigator.
Subjects were treated with 0MS646 through the corticosteroid taper and through
the full
12 weeks of treatment. The patients were then followed for an additional 6
weeks after
their last treatment. The taper of corticosteroids and 0MS646 treatment
permitted
assessment of whether 0MS646 allowed for a decrease in the dose of
corticosteroid
required to maintain stable renal function.
The key efficacy measures in this study are the change in urine albumin-to-
creatinine ratio (uACR) and 24-hour protein levels from baseline to 12 weeks.
Measurement of urinary protein or albumin is routinely used to assess kidney
involvement and persistent high levels of urinary protein correlates with
renal disease
progression. The uACR is used clinically to assess proteinuria.
Efficacy Analyses
The analysis value for uACR is defined as the average of all the values
obtained
for a time point. The planned number of uACRs is three at each scheduled time
point.
The baseline value of the uACR is defined as the average of the analysis
values at the two
screening visits.
Results:
FIGURE 40 graphically illustrates the uACRin two IgAN patients during the
course of a twelve week study with weekly treatment with 4 mg/kg MASP-2
inhibitory
antibody (0M5646). As shown in FIGURE 40, the change from baseline is
statistically
significant at time point "a- (p=0.003); time point "b- (p=0.007) and a time
point "c"
(p=0.033) by the untransformed analysis. TABLE 12 provides the 24-hour urine-
protein
data for the two IgAN patients treated with 0MS646.
TABLE 12: 24-hour Urine Protein (mg/day) in 0MS646-treated IgAN Patients
Time of Sample Patient #1 Patient #2 Mean
-174-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
(mg/24 hours) (mg/24 hours)
Baseline 3876 2437 3156
Day 85 1783 455 1119
p= 0.017
As shown in FIGURE 40 and TABLE 12, the patients with IgAN demonstrated a
clinically and statistically significant improvement in kidney function over
the course of
the study. There were statistically significant decreases in both uACR (see
FIGURE 40)
and 24-hour urine protein concentration (see TABLE 12). As shown in the uACR
data in
FIGURE 40, the mean baseline uACR was 1264 mg/g and reached 525 mg/g at the
end of
treatment (p=0.011) decreasing to 128 mg/g at the end of the follow-up period.
As
further shown in FIGURE 40, the treatment effect was maintained throughout the
follow-
up period. Measures of 24-hour urine protein excretion tracked uACRs, with a
mean
reduction from 3156 mg/24 hours to 1119 mg/24 hours (p=0 017) Treatment
effects
across the two patients were highly consistent Both patients experienced
reductions of
approximately 2000 mg/day and both achieved a partial remission (defined as
greater
than 50 percent reduction in 24-hour urine protein excretion and/or resultant
protein
exretion less than 1000 mg/day; complete remission defined as protein
excretion less than
300 mg/day). The magnitude of the 24-hour proteinuria reductions in both IgA
nephropathy patients is associated with a significant improvement in renal
survival. Both
IgA nephropathy patients were also able to taper their steroids substantially,
each
reducing the daily dose to < 5 mg (60 mg to 0 mg; 30 mg to 5 mg).
The two MN patients also demonstrated reductions in uACR during treatment
with 0MS646. One MN patient had a decrease in uACR from 1003 mg/g to 69 mg/g
and
maintained this low level throughout the follow-up period. The other MN
patient had a
decrease in uACR from 1323 mg/g to 673 mg/g, with a variable course after
treatment.
The first MN patient showed a marked reduction in 24-hour urine protein level
(10,771
mg/24 hours at baseline to 325 mg/24 hours on Day 85), achieving partial and
nearly
complete remission, while the other remained essentially unchanged (4272 mg/24
hours
at baseline to 4502 mg/24 on Day 85). Steroids were tapered in the two MN
patients
from 30 mg to 15 mg and from 10 mg to 5 mg.
In summary, consistent improvements in renal function were observed in IgAN
and MN subjects treated with the MASP-2 inhibitory antibody 0MS646. The
effects of
-175-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
0MS646 treatment in the patients with IgAN are robust and consistent,
suggesting a
strong efficacy signal. These effects are supported by the results in MN
patients. The
time course and magnitude of the uACR changes during treatment were consistent
between all four patients with IgAN and MN. No significant safety concerns
have been
observed. Patients in this study represent a difficult-to-treat group and a
therapeutic
effect in these patients is believed to be predictive of efficacy with a MASP-
2 inhibitory
antibody, such as 0MS646, in IgAN and MN patients, such as patients suffering
from
steroid-dependent IgAN and MN (i.e., patients undergoing treatment with a
stable
corticosteroid dose prior to treatment with a MASP-2 inhibitory antibody),
including
.. those at risk for rapid progression to end-stage renal disease.
In accordance with the foregoing, in one embodiment, the invention provides a
method of treating a human subject suffering from IgAN or MN comprising
administering to the subject a composition comprising an amount of a MASP-2
inhibitory
antibody effective to inhibit MASP-2-dependent complement activation. In one
embodiment, the method comprises administering to the human subject suffering
from
IgAN or MN an amount of a MASP-2 inhibitory antibody sufficient to improve
renal
function (e.g., improve proteinuria). In one embodiment, the subject is
suffering from
steroid-dependent IgAN. In one embodiment, the subject is suffering from
steroid-
dependent MN. In one embodiment, the MASP-2 inhibitory antibody is
administered to
the subject suffering from steroid-dependent IgAN or steroid-dependent MN in
an
amount sufficient to improve renal function and/or decrease corticosteroid
dosage in said
subject.
In one embodiment, the method further comprises identifying a human subject
suffering from steroid-dependent IgAN prior to the step of administering to
the subject a
composition comprising an amount of a MASP-2 inhibitory antibody effective to
inhibit
MASP-2-dependent complement activation.
In one embodiment, the method further comprises identifying a human subject
suffering from steroid-dependent MN prior to the step of administering to the
subject a
composition comprising an amount of a MASP-2 inhibitory antibody effective to
inhibit
MASP-2-dependent complement activation.
In accordance with any of the disclosed embodiments herein, the MASP-2
inhibitory antibody exhibits at least one or more of the following
characteristics: said
antibody binds human MASP-2 with a KD of 10 nM or less, said antibody binds an
-176-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
epitope in the CCP1 domain of MASP-2, said antibody inhibits C3b deposition in
an in
vitro assay in 1% human serum at an IC50 of 10 nIVI or less, said antibody
inhibits C3b
deposition in 90% human serum with an IC50 of 30 nM or less, wherein the
antibody is
an antibody fragment selected from the group consisting of Fv, Fab, Fab',
F(ab)2 and
F(a13)2, wherein the antibody is a single-chain molecule, wherein said
antibody is an IgG2
molecule, wherein said antibody is an IgG1 molecule, wherein said antibody is
an IgG4
molecule, wherein the IgG4 molecule comprises a S228P mutation. In one
embodiment,
the antibody binds to MASP-2 and selectively inhibits the lectin pathway and
does not
substantially inhibit the classical pathway (i.e., inhibits the lectin pathway
while leaving
the classical complement pathway intact).
In one embodiment, the MASP-2 inhibitory antibody is administered in an amount
effective to improve at least one or more clinical parameters associated renal
function,
such as an improvement in proteinuria (e.g., a decrease in uACR and/or a
decrease in 24-
hour urine protein concentration, such as greater than 20 percent reduction in
24-hour
urine protein excretion, or such as greater than 30 percent reduction in 24-
hour urine
protein excretion, or such as greater than 40 percent reduction in 24-hour
urine protein
excretion, or such as greater than 50 percent reduction in 24-hour urine
protein
excretion).
In some embodiments, the method comprises administering a MASP-2 inhibitory
antibody to a subject suffering from IgAN (such as steroid-dependent IgAN),
via a
catheter (e.g., intravenously) for a first time period (e.g., at least one day
to a week or two
weeks or three weeks or four weeks or longer) followed by administering a MASP-
2
inhibitory antibody to the subject subcutaneously for a second time period
(e.g., a chronic
phase of at least two weeks or longer).
In some embodiments, the method comprises administering a MASP-2 inhibitory
agent to a subject suffering from MN (such as steroid-dependent MN), via a
catheter
(e.g., intravenously) for a first time period (e.g., at least one day to a
week or two weeks
or three weeks or four weeks or longer) followed by administering a MASP-2
inhibitory
antibody to the subject subcutaneously for a second time period (e.g., a
chronic phase of
at least two weeks or longer).
In some embodiments, the method comprises administering a MASP-2 inhibitory
antibody to a subject suffering from IgAN (such as steroid-dependent IgAN) or
MN (such
-177-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
as steroid-dependent MN) either intravenously, intramuscularly, or
subcutaneously.
Treatment may be chronic and administered daily to monthly, but preferably at
least
every two weeks, or at least once a week, such as twice a week or three times
a week.
In one embodiment, the method comprises treating a subject suffering from IgAN
(such as steroid-dependent IgAN) or MN (such as steroid-dependent MN)
comprising
administering to the subject a composition comprising an amount of a MASP-2
inhibitory
antibody, or antigen binding fragment thereof, comprising a heavy chain
variable region
comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid sequence set forth as
SEQ
ID NO:67 and a light-chain variable region comprising CDR-L1, CDR-L2 and CDR-
L3
of the amino acid sequence set forth as SEQ ID NO:70. In some embodiments, the
composition comprises a MASP-2 inhibitory antibody comprising (a) a heavy-
chain
variable region comprising: i) a heavy-chain CDR-H1 comprising the amino acid
sequence from 31-35 of SEQ ID NO:67; and ii) a heavy-chain CDR-H2 comprising
the
amino acid sequence from 50-65 of SEQ ID NO:67; and iii) a heavy-chain CDR-H3
comprising the amino acid sequence from 95-107 of SEQ ID NO:67 and b) a light-
chain
variable region comprising: i) a light-chain CDR-L1 comprising the amino acid
sequence
from 24-34 of SEQ ID NO:70; and ii) a light-chain CDR-L2 comprising the amino
acid
sequence from 50-56 of SEQ ID NO:70; and iii) a light-chain CDR-L3 comprising
the
amino acid sequence from 89-97 of SEQ ID NO:70, or (II) a variant thereof
comprising a
heavy-chain variable region with at least 90% identity to SEQ ID NO:67 (e.g.,
at least
91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at
least 97%, at
least 98%, at least 99% identity to SEQ ID NO:67) and a light-chain variable
region with
at least 90% identity (e.g., at least 91%, at least 92%, at least 93%, at
least 94%, at least
95%, at least 96%, at least 97%, at least 98%, at least 99% identity to SEQ ID
NO:70.
In some embodiments, the method comprises administering to the subject a
composition comprising an amount of a MASP-2 inhibitory antibody, or antigen
binding
fragment thereof, comprising a heavy-chain variable region comprising the
amino acid
sequence set forth as SEQ ID NO:67 and a light-chain variable region
comprising the
amino acid sequence set forth as SEQ ID NO:70.
In some embodiments, the method comprises administering to the subject a
composition comprising a MASP-2 inhibitory antibody, or antigen binding
fragment
thereof, that specifically recognizes at least part of an epitope on human
MASP-2
recognized by reference antibody 0M5646 comprising a heavy-chain variable
region as
-178-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
set forth in SEQ ID NO:67 and a light-chain variable region as set forth in
SEQ ID
NO:70.
In some embodiments, the method comprises administering to a subject suffering
from, or at risk for developing IgAN (such as steroid-dependent IgAN) or MN
(such as
steroid-dependent MN), a composition comprising a MASP-2 inhibitory antibody,
or
antigen binding fragment thereof comprising a heavy-chain variable region
comprising
the amino acid sequence set forth as SEQ ID NO:67 and a light-chain variable
region
comprising the amino acid sequence set forth as SEQ ID NO:70 in a dosage from
1
mg/kg to 10 mg/kg (i.e., 1 mg/kg, 2 mg/kg, 3 mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg,
7
mg/kg, 8 mg/kg, 9 mg/kg or 10 mg/kg) at least once weekly (such as at least
twice
weekly or at least three times weekly) for a period of at least 3 weeks, or
for at least 4
weeks, or for at least 5 weeks, or for at least 6 weeks, or for at least 7
weeks, or for at
least 8 weeks, or for at least 9 weeks, or for at least 10 weeks, or for at
least 11 weeks, or
for at least 12 weeks.
EXAMPLE 20
This Example describes the initial results of an ongoing Phase 2 clinical
trial to
evaluate the safety and clinical efficacy of a fully human monoclonal MASP-2
inhibitory
antibody in adults with steroid-dependent lupus nephritis (LN).
Background:
Chronic kidney diseases affect more than 20 million people in the United
States
(Drawz P. et al., Ann Intern Med 162(11); ITC1-16, 2015). Glom erul
onephropathi es
(GNs), including IgAN, MN and LN are kidney diseases in which the glomeruli
are
damaged and frequently lead to end-stage renal disease and dialysis. Many of
these
patients have persistent renal inflammation and progressive deterioration.
Often these
patients are treated with corticosteroids or immunosuppressive agents, which
have many
serious long-term adverse consequences. Many patients continue to deteriorate
even on
these treatments.
Lupus Nephritis
A main complication of systemic lupus erythematosus (SLE) is nephritis, also
known as lupus nephritis, which is classified as a secondary form of
glomerulonephritis.
Up to 60% of adults with SLE have some form of kidney involvement later in the
course
-179-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
of the disease (Koda-Kimble etal., Koda-Kimble and Young's Applied
Therapeutics: the
clinical use of drugs, 10th Ed, Lippincott Williams & Wilkins: pages 792-9,
2012) with a
prevalence of 20-70 per 100,000 people in the US. Lupus nephritis often
presents in
patients with other symptoms of active SLE, including fatigue, fever, rash,
arthritis,
serositis, or central nervous system disease (Pisetsky D. S. et al., Med Chn
North Am
81(1):113-28, 1997). Some patients have asymptomatic lupus nephritis; however,
during
regular follow-up, laboratory abnormalities such as elevated serum creatinine
levels, low
albumin levels, or urinary protein or sediment suggest active lupus nephritis.
Autoimmunity plays a major role in the pathogenesis of lupus nephritis. These
autoantibodies form pathogenic immune complexes intravascularly, which are
deposited
in glomeruli. Autoantibodies may also bind to antigens already located in the
glomerular
basement membrane, forming immune complexes in situ. Immune complexes promote
an
inflammatory response by activating complement and attracting inflammatory
cells
(D'Agati V.D. et al., Lupus nephritis: pathology and pathogenesis: Wallace
D.J. Hahn,
Dubois' Lupus Erythematosus, 7th Ed Philadelpha: Lippincott Williams &
Wiklins:
p1094-111, 2007). Thus, immune complex-mediated complement activation plays a
key
role in the pathogenesis of lupus nephritis. C4d deposits are present in renal
tissue and are
usually associated with immune complex deposits, Clq, and C3, invoking the
classical
pathway. In some cases C4d deposits are present without Clq, indicating
possible lectin
pathway involvement (Kim M.K., etal. Int J Chn Exp Pathol 6(10):2157-67,
2013).
In further support of an important contribution for the lectin pathway,
deposits of
MBL occur in skin lesions of SLE patients (Wallim L. R. et al., Hum Immunol
75(7):629-
32, 2014). Additionally, robust deposition of MBL and ficolins in the majority
of renal
biopsies from patients with lupus nephritis has been observed (Nisihara R M.
et al., Hum
immuno/74(8):907-10, 2013). Renal MBL deposition was most evident in patients
with
high proteinuria. Furthermore, plasma MBL levels were significantly higher in
SLE
patients than in healthy controls and MBL levels correlated with disease
activity,
suggesting that MBL levels may represent a biomarker for SLE disease activity
(Panda
A.K. et al., Arthritis Res Ther 14(5):R218, 2012). Corticosteroids are the
major
conventional treatment option for patients with mild lupus nephritis. For more
severe
cases, high-dose prednisone, methylprednisolone, mycophenolate mofetil,
cyclophosphamide, azathioprine, and cyclosporine have been used in clinical
practice.
Treatment options for SLE and lupus nephritis have high associated morbidity
and
-180-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
mortality. Side effects, particularly from long-term corticosteroid usage,
limit patient
adherence with subsequent impact on treatment efficacy. There is a need to
develop better
tolerated treatment regimens.
Methods:
As described above in Example 19, two Phase 1 clinicial trials carried out in
healthy volunteers have demonstrated that both intravenous and subcutaneous
dosing of a
MASP-2 inhibitory antibody, 0MS646, resulted in sustained lectin pathway
inhibition.
This Example describes interim results from an ongoing Phase 2, uncontrolled,
multicenter study of a MASP-2 inhibitory antibody, 0MS646, in subjects with
lupus
nephritis (LN). Inclusion criteria require that all patients in this study,
regardless of renal
disease subtype, have been maintained on a stable dose of corticosteroids for
at least 12
weeks prior to study enrollment (i.e., the patients are steroid-dependent).
The study is a
single-arm pilot study with 12 weeks of treatment and a 6-week follow-up
period.
The study is designed to evaluate whether 0MS646 may improve renal function
(e.g., improve proteinuria) and decrease corticosteroid needs in subjects with
LN. To
date, 5 patients with lupus nephritis (LN) have completed treatment in the
study.
At study entry each subject must have high levels of protein in the urine
despite
ongoing treatment with a stable corticosteroid dose. These criteria select for
patients who
are unlikely to spontaneously improve during the study period.
The subjects were age > 18 at screening and were only included in the study if
they had a diagnosis of lupus nephritis diagnosed on kidney biopsy. The
enrolled patients
also had to meet all of the following inclusion criteria:
(1) have average urine albumin/creatinine ratio > 0.6 from three samples
collected
consecutively and daily prior to each of 2 visits during the screening period;
(2) have been on > 10 mg of prednisone or equivalent dose for at least 12
weeks
prior to screening visit 1;
(3) if on immunosuppressive treatment (e.g., cyclophosphamide, mycophenolate
mofetil), have been on a stable dose for at least 2 months prior to Screening
Visit 1 with
no expected change in the dose for the study duration;
(4) have an estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73m2
calculated by the MDRD equation';
(5) are on a physician-directed, stable, optimized treatment with angiotensin
converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB)
and
-181-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
have a systolic blood pressure of <150 mmHg and a diastolic blood pressure of
<90mmHg at rest;
(6) have not used belimumab, eculizumab or rituzimab within 6 months of
screening visit 1; and
(7) do not have a history of renal transplant.
lIVIDRD Equation: eGFR (mL/min/1.73m2) = 175 x (SCr)-1154 x (Age)-0.203
(0.742 if female) x (1.212 if African American). Note: SCr=Serum Creatinine
measurement should be mg/dL.
The monoclonal antibody used in this study, 0MS646, is a fully human IgG4
monoclonal antibody that binds to and inhibits human MASP-2. MASP-2 is the
effector
enzyme of the lectin pathway. As demonstrated in Example 12, 0MS646 avidly
binds to
recombinant MASP-2 (apparent equilibrium dissociation constant in the range of
100
.. pM) and exhibits greater than 5,000-fold selectivity over the homologous
proteins Cis,
Clr, and MASP-1. In functional assays, 0MS646 inhibits the human lectin
pathway with
nanomolar potency (concentration leading to 50% inhibition [IC5o1 of
approximately 3
nM) but has no significant effect on the classical pathway. 0MS646
administered either
by intravenous (IV) or subcutaneous (SC) injection to mice, non-human
primates, and
humans resulted in high plasma concentrations that were associated with
suppression of
lectin pathway activation in an ex vivo assay.
In this study, the 0MS646 drug substance was provided at a concentration of
100
mg/mL, which was further diluted for IV administration. The appropriate
calculated
volume of 0M5646 100 mg/mL injection solution was withdrawn from the vial
using a
syringe for dose preparation. The infusion bag was administered within four
hours of
preparation.
The study consists of screening (28 days), treatment (12 weeks) and follow-up
(6
weeks) periods, as shown in the Study Design Schematic below.
-182-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Study Design Schematic
40,m:A
lip
(:030,1
TRIMACHE
i 1
i),Mn POIK
:4Cri:V3i317, rh!ittl;
f '!:q rtsttp,.:t
11 p iqf --- St9dy
. , --
µsk.cs,
Within the screening period and before the first 0MS646 dose, consented
subjects
provided three urine samples (collected once daily) on each of two three-
consecutive-day
periods to establish baseline values of the 24-hour urine protein and urine
albumin-to-
creatinine ratio. Following the screening period, eligible subjects received
0MS646 4
mg/kg IV once weekly for 12 weeks (treatment period). There was a 6-week
follow-up
period after the last dose of 0MS646.
During the initial 4 weeks of treatment with 0MS646, subjects were maintained
on their stable pre-study dose of corticosteroids. At the end of the initial 4-
weeks of the
12-week treatment period, subjects underwent corticosteroid taper (i.e., the
corticosteroid
dose was reduced), if tolerated, over 4 weeks, followed by 4 weeks during
which the
resultant corticosteroid dose was maintained. The target was a taper to < 6 mg
prednisone (or equivalent dose) daily. Over this period, the taper was
discontinued in
subjects who had deterioration of renal function, as determined by the
investigator.
Subjects were treated with 0MS646 through the corticosteroid taper and through
the full
12 weeks of treatment. The patients were then followed for an additional 6
weeks after
their last treatment. The taper of corticosteroids and 0MS646 treatment
permitted
assessment of whether 0MS646 allowed for a decrease in the dose of
corticosteroid
required to maintain stable renal function.
Efficacy Analyses
The key efficacy measure in this study is the change in 24-hour protein levels
from baseline to 12 weeks. Measurement of urinary protein or albumin is
routinely used
to assess kidney involvement and persistent high levels of urinary protein
correlates with
renal disease progression. Partial remission is defined as greater than 50
percent reduction
in 24-hour urine protein excretion.
-183-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Results:
TABLE 13 provides the 24-hour urine-protein (mg/day) for the five LN patients
treated with 0MS646.
TABLE 13: 24-hour Urine Protein (mg/day) in 0MS646-treated LN Patients
Time of Patient 41 Patient 42 Patient 43 Patient 44 Patient 45
Mean
Sample (mg/24 (mg/24 (mg/24 (mg/24 (mg/24 (patients
hours) hours) hours) hours) hours) 42-5)
Baseline 1112 7539.0 2066.9 2217.8 4067.0
15890.7
Day 85 13731 2750.9 282.0 1168.0 797.6 4998.5
Note: Patient 41 experienced a systemic disease flare during the study.
As shown in TABLE 13, the patients with LN demonstrated a clinically and
statistically significant improvement in kidney function over the course of
the study. As
shown in TABLE 13, four of five LN patients showed a substantial (mean of 69
percent)
reduction in 24-hour urine protein excretion over the treatment period. The
fifth patient
(patient 41) experienced a systemic disease flare and showed a substantial
increase. The
majority of lupus responders were able to taper their steroid doses.
In summary, significant improvements in renal function were observed in four
out
of the five LN patients treated with the MASP-2 inhibitory antibody 0M5646.
The
effects of 0M5646 treatment in the patients with LN are robust and consistent,
suggesting a strong efficacy signal. No significant safety concerns have been
observed.
Patients in this study represent a difficult-to-treat group and a therapeutic
effect in these
patients is believed to be predictive of efficacy with a MASP-2 inhibitory
antibody, such
as 0MS646, in LN patients, such as patients suffering from steroid-dependent
LN (i.e.,
patients undergoing treatment with a stable corticosteroid dose prior to
treatment with a
MASP-2 inhibitory antibody), including those at risk for rapid progression to
end-stage
renal disease.
In accordance with the foregoing, in one embodiment, the invention provides a
method of treating a human subject suffering from LN comprising administering
to the
subject a composition comprising an amount of a MASP-2 inhibitory antibody
effective
to inhibit MASP-2-dependent complement activation. In one embodiment, the
method
-184-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
comprises administering to the human subject suffering from LN an amount of a
MASP-2
inhibitory antibody sufficient to improve renal function (e.g., improve
proteinuria). In one
embodiment, the subject is suffering from steroid-dependent LN. In one
embodiment, the
MASP-2 inhibitory antibody is administered to the subject suffering from
steroid-
dependent LN in an amount sufficient to improve renal function and/or decrease
corticosteroid dosage in said subject.
In one embodiment, the method further comprises identifying a human subject
suffering from steroid-dependent LN prior to the step of administering to the
subject a
composition comprising an amount of a MASP-2 inhibitory antibody effective to
inhibit
MASP-2-dependent complement activation.
In accordance with any of the disclosed embodiments herein, the MASP-2
inhibitory antibody exhibits at least one or more of the following
characteristics: said
antibody binds human MASP-2 with a KD of 10 nIVI or less, said antibody binds
an
epitope in the CCP1 domain of MASP-2, said antibody inhibits C3b deposition in
an in
vitro assay in 1% human serum at an IC50 of 10 nM or less, said antibody
inhibits C3b
deposition in 90% human serum with an IC50 of 30 nM or less, wherein the
antibody is
an antibody fragment selected from the group consisting of Fv, Fab, Fab',
F(ab)2 and
F(abt)2, wherein the antibody is a single-chain molecule, wherein said
antibody is an IgG2
molecule, wherein said antibody is an IgG1 molecule, wherein said antibody is
an IgG4
molecule, wherein the IgG4 molecule comprises a S228P mutation. In one
embodiment,
the antibody binds to MASP-2 and selectively inhibits the lectin pathway and
does not
substantially inhibit the classical pathway (i.e., inhibits the lectin pathway
while leaving
the classical complement pathway intact).
In one embodiment, the MASP-2 inhibitory antibody is administered to a subject
suffering from LN in an amount effective to improve at least one or more
clinical
parameters associated renal function, such as an improvement in proteinuria
(e.g., a
decrease in uACR and/or a decrease in 24-hour urine protein concentration,
such as
greater than 20 percent reduction in 24-hour urine protein excretion, or such
as greater
than 30 percent reduction in 24-hour urine protein excretion, or such as
greater than 40
percent reduction in 24-hour urine protein excretion, or such as greater than
50 percent
reduction in 24-hour urine protein excretion). In some embodiments, the MASP-2
inhibitory antibody is administered to a subject suffering from LN in an
amount effective
-185-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
to result in at least a partial remission in proteinuria (i.e., greater than
50 percent
reduction in 24-hour urine protein excretion as compared to baseline).
In some embodiments, the method comprises administering a MASP-2 inhibitory
antibody to a subject suffering from LN (such as steroid-dependent LN), via a
catheter
.. (e.g., intravenously) for a first time period (e.g., at least one day to a
week or two weeks
or three weeks or four weeks or longer) followed by administering a MASP-2
inhibitory
antibody to the subject subcutaneously for a second time period (e.g., a
chronic phase of
at least two weeks or longer).
In some embodiments, the method comprises administering a MASP-2 inhibitory
.. antibody to a subject suffering from LN (such as steroid-dependent LN)
either
intravenously, intramuscularly, or subcutaneously. Treatment may be chronic
and
administered daily to monthly, but preferably at least every two weeks, or at
least once a
week, such as twice a week or three times a week.
In one embodiment, the method comprises treating a subject suffering from LN
(such as steroid-dependent LN) comprising administering to the subject a
composition
comprising an amount of a MASP-2 inhibitory antibody, or antigen binding
fragment
thereof, comprising a heavy chain variable region comprising CDR-H1, CDR-H2
and
CDR-H3 of the amino acid sequence set forth as SEQ ID NO:67 and a light-chain
variable region comprising CDR-L1, CDR-L2 and CDR-L3 of the amino acid
sequence
set forth as SEQ ID NO:70. In some embodiments, the composition comprises a
MASP-
2 inhibitory antibody comprising (a) a heavy-chain variable region comprising:
i) a
heavy-chain CDR-H1 comprising the amino acid sequence from 31-35 of SEQ ID
NO:67; and ii) a heavy-chain CDR-H2 comprising the amino acid sequence from 50-
65
of SEQ ID NO:67; and iii) a heavy-chain CDR-H3 comprising the amino acid
sequence
from 95-107 of SEQ ID NO:67 and b) a light-chain variable region comprising:
i) a light-
chain CDR-L1 comprising the amino acid sequence from 24-34 of SEQ ID NO:70;
and
ii) a light-chain CDR-L2 comprising the amino acid sequence from 50-56 of SEQ
ID
NO:70; and iii) a light-chain CDR-L3 comprising the amino acid sequence from
89-97 of
SEQ ID NO:70, or (II) a variant thereof comprising a heavy-chain variable
region with at
.. least 90% identity to SEQ ID NO:67 (e.g., at least 91%, at least 92%, at
least 93%, at
least 94%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99% identity to
SEQ ID NO:67) and a light-chain variable region with at least 90% identity
(e.g., at least
-186-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at
least 97%, at
least 98%, at least 99% identity to SEQ ID NO:70.
In some embodiments, the method comprises administering to the subject
suffering from LN (such as steroid-dependent LN) a composition comprising an
amount
.. of a MASP-2 inhibitory antibody, or antigen binding fragment thereof,
comprising a
heavy-chain variable region comprising the amino acid sequence set forth as
SEQ ID
NO:67 and a light-chain variable region comprising the amino acid sequence set
forth as
SEQ ID NO:70.
In some embodiments, the method comprises administering to the subject
suffering from LN (such as steroid-dependent LN) a composition comprising a
MASP-2
inhibitory antibody, or antigen binding fragment thereof, that specifically
recognizes at
least part of an epitope on human MASP-2 recognized by reference antibody
0MS646
comprising a heavy-chain variable region as set forth in SEQ ID NO:67 and a
light-chain
variable region as set forth in SEQ ID NO:70.
In some embodiments, the method comprises administering to a subject suffering
from, or at risk for developing LN (such as steroid-dependent LN) a
composition
comprising a MASP-2 inhibitory antibody, or antigen binding fragment thereof
comprising a heavy-chain variable region comprising the amino acid sequence
set forth as
SEQ ID NO:67 and a light-chain variable region comprising the amino acid
sequence set
forth as SEQ ID NO:70 in a dosage from 1 mg/kg to 10 mg/kg (i.e., 1 mg/kg, 2
mg/kg, 3
mg/kg, 4 mg/kg, 5 mg/kg, 6 mg/kg, 7 mg/kg, 8 mg/kg, 9 mg/kg or 10 mg/kg) at
least once
weekly (such as at least twice weekly or at least three times weekly) for a
period of at
least 3 weeks, or for at least 4 weeks, or for at least 5 weeks, or for at
least 6 weeks, or for
at least 7 weeks, or for at least 8 weeks, or for at least 9 weeks, or for at
least 10 weeks, or
for at least 11 weeks, or for at least 12 weeks.
EXAMPLE 21
This Example describes additional results obtained in the ongoing Phase 2
Clinical Trial to evaluate the safety and clinical efficacy of a fully human
monoclonal
MASP-2 inhibitory antibody, 0M5646, on reducing proteinuria in adult patients
with
glomerulopathies, including IgAN as described in Example 19.
Methods:
-187-
CA 03039927 2019-04-09
WO 2018/071701
PCT/US2017/056386
As described in Example 19, the Phase 2 trial includes patients with IgAN
receiving corticosteroids on study entry, all patients received 0MS646 in an
open-label
manner, and positive results were obtained from two IgAN patients. Dosing has
now
been completed for an additional two IgAN patients using the methods described
in
Example 19, for a total of four IgAN patients that have completed the trial.
For inclusion in this trial, patients with IgAN must have demonstrated (1)
biopsy-
diagnosed IgAN, (2) uACR>0.6 g/g, (3) eGFR>30 mL/min/1.73 m2, (4) controlled
blood
pressure on stable ACEPARB treatment, and (5) a stable steroid dose > 10 mg
prednisone
for at least 12 weeks.
All four adult patients with IgAN that were treated with 0MS646 in this trial
had
pre-existing renal impairment, with entry estimated gl omerul ar filtration
(eGFR) rates of
30 to 46 mL/mg/1.73m2 and 24-hour protein measures of 2.44 to 4.87 g/24 hours.
All
patients were receiving stable renin-angiotensin system (RAS) blockage and at
least 3
months of corticosteroid treatment at study entry.
All patients received 0MS646 IV once weekly for 12 weeks. Patients underwent
a four-week run-in period followed by 0MS646 treatment that included four
weeks on a
stable steroid dose, four weeks of steroid taper if tolerated, and four weeks
on the tapered
steroid dose. After 0MS646 treatment, patients were followed for an additional
6 more
weeks in the trial. After trial completion, patients have been followed by the
investigator.
In the trial, efficacy measures were (1) urine albumin/creatinine ratio (uACR)
measured 6 times prior to treatment (baseline) and 3 times at each efficacy
evaluation
during treatment and follow-up; and (2) 24-hour urinary protein measured once
prior to
0MS646 treatment and once 2-4 weeks after completion of 0MS646 treatment. By
protocol, corticosteroids were tapered between weeks 4 and 8 if clinically
appropriate.
Results:
Four patients with IgAN have completed the post-treatment 6 week follow-up
period. Table 14 provides the demographic and baseline characteristics of
these patients.
TABLE 14: Demographics and Baseline Characteristics
Patient 1 Patient 2 Patient 3 Patient
4
Age (years) 35 32 55 45
-188-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
Gender Male Female Female Female
Race White Asian White White
Time from 8 years 5 months 5 months 2 years
Diagnosis
uACR (mg/g) 1271 1256 1674 1628
24-hour protein 3876 2437 4872 4554
(mg)
eGFR 46 30 44 42
(mL/min/S SA)
Blood pressure 110/84 124/74 126/74 136/66
(mm Hg)
eGFR-estimated glomerular filtration rate; SSA- standard surface area (1.73
m2); uACR-urinary
albumin/creatinine ratio
All patients demonstrated marked reductions in proteinuria during 0MS646
treatment. Statistically and clinically significant improvements were observed
in both
uACR and 24-hour protein measures, as shown in FIGURES 41 and 42.
FIGURE 41 graphically illustrates the uACR (mg/g) for the four IgAN patients
treated with 0MS646 over time from baseline to 120 days. As shown in FIGURE
41,
from baseline to end of study, mean uACR decreased 1.13g/g 0.27 (77%
reduction,
p=0.026). As further shown in FIGURE 41, following 0MS646 treatment, the uACR
for
each patient was reduced by 94%, 86%, 47% and 89% (patients 1-4, respectively)
at the
last follow-up visit relative to baseline.
FIGURE 42 graphically illustrates the 24-hour urine protein change from
baseline at day 1 prior to treatment and post-treatment for the four IgAN
patients treated
with 0MS646. As shown in FIGURE 42, the 24-hour urine protein decreased 54%,
81%,
63% and 95% (patients 1-4, respectively) from baseline.
FIGURE 43 graphically illustrates the mean change from baseline to post-
treatment in 24-hour urine protein in the four IgAN patients treated with
0MS646. As
shown in FIGURE 43, the mean 24-hour urine protein decreased 2.87 1.08 g/24
hours
(73% reduction; p=0.013).
All patients were able to discontinue corticosteroids during or soon after the
study
period, demonstrating that the effect of 0MS646 on proteinuria is unlikely to
be
-189-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
corticosteroid-related. The estimated glomerular filtration rates (eGFR)
(calculated by
the Modification of Diet in Renal Disease Formula) were stable throughout the
treatment
and follow-up periods.
0MS646 was well tolerated by all patients.
In summary, in this open-label phase 2 clinical study, significant and
sustained
decreases in uACRs were observed in all patients with IgAN treated with 0MS646
for 12
weeks. The 24-hour proteinuria was significantly reduced in all patients. The
degree of
proteinuria reduction observed has been associated with substantial
improvements in
renal prognoses and clinical outcomes (Inker L. A. et al., Am Kidney /)is
68(3):392-401
(2016)). The findings of profound reduction in proteinuria allowing steroid
cessation
after treatment with 0MS646, a monoclonal antibody to MASP-2 that abrogates
the
effects of the lectin pathway of complement, support the use of 0MS646 as a
therapeutic
to improve outcomes in IgA glomerulopathy. The effects of 0MS646 in IgAN
patients
are robust and consistent, demonstrating efficacy in this population.
EXAMPLE 22
Maintenance of Remission Following Completion of 0MS646 Treatment in
Patients with IgA Nephropathy (IgAN).
Background/Rationale:
As described in Examples 19 and 21, in a phase 2 study in IgAN patients, 4
IgAN
patients were treated with 0MS646, a fully human monoclonal antibody that
inhibits
MASP-2 activity. As described in Example 19 and 21, all patients received
0MS646 IV
once weekly for 12 weeks As described in Example 21, after 0MS646 treatment,
patients were followed for 6 more weeks in the trial and all the 0MS646-
treated patients
with IgAN achieved partial remission (defined as greater than 50 percent
reduction in 24-
hour urine protein excretion and/or resultant protein excretion less than 1000
mg/day).
As described in this Example, these four patients were followed after the
trial and the
duration of remission after 0MS646 treatment was assessed.
Methods:
After completion of the phase 2 clinical trial described in Example 21, the 4
IgAN
patients treated with 0MS646 were followed by the investigator. In the trial,
endpoints
were uACR and 24-hour proteinuria. As described in Example 21, all 4 IgAN
patients
achieved partial remission at the end of the trial. In post-trial follow-up,
urinary protein-
-190-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
to-creatinine ratio (uPCR) was measured. Each uPCR value was converted to uACR
(urinary albumin/creatinine ratio) by multiplying by 0.64 (see Zhao et al.,
Chn J Am Soc
Nephrol 11:947-55, 2016).
Results:
All patients achieved partial remission following 0MS646 treatment. The mean
age of the 3 females and 1 male was 42 years, 3 are Caucasian and one is
Asian. The
mean eGFR was 41 mL/min/1.73m2 and the mean entry steroid dose was 55 mg.
Follow-
up ranged from 2 to 10 months after the last 0MS646 dose. As described in
Example 21,
during the trial the mean uACR decreased 77% (p=0.026). Three patients
maintained
partial remission during available follow-up (54%, 93% and 78% uACR decreases
at 12,
12 and 5 months, respectively). One patient had 88% of baseline uACR at 7
months.
Three patients also demonstrated improved eGFR by 7, 13 and 7 ml/min/1.73 m2
during
follow-up. The fourth patient's eGFR was stable. All patients discontinued
steroids.
0MS646 was well tolerated.
In summary, as described in Example 21, proteinuria significantly decreased in
patients with IgAN during the 12-week treatment with 0MS646 and 6-week post-
treatment period included in the trial. This reduction in proteinuria was
maintained for up
to 10 months after treatment completion. These data support the use of 0MS646
as a
therapeutic to improve outcomes in IgA glomerulopathy.
In an update from the investigator regarding the status of the four patients
described in this example at approximately one-year follow-up after the single
12-week
course of treatment with 0MS646, it was reported that three of the four
patients had
maintained reductions in proteinuria. In these three patients uACRs remained
reduced at
14 percent, 23 percent, and 24 percent of the patients' baseline values prior
to 0MS646
treatment. In addition, an improvement in estimated glomerular filtration rate
(eGFR), a
measure of renal function, was observed in 3 of the 4 patients after the
trial. The patient
with the most severe reduction in kidney function demonstrated eGFR
improvement from
mL/min/1.73 m2 to 47 mL/min/1.73 m2, an improvement of 57 percent.
In summary, the persistent reduction of proteinuria following completion of a
30 single
course of 0MS646 treatment continued to be impressive at one-year follow-up.
The improvement observed in eGFR is unexpected, especially at one-year follow-
up, as
this would be expected to take significantly longer to be evident. As
described above,
two of the four patients demonstrated a slight increase in eGFR, with one of
the patients
-191-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
showing an exciting response of 50 percent improvement. The improvements
observed
in eGFRs indicate that 0MS646 could provide further benefit to patients by
potentially
precluding or substantially extending the time to the need for dialysis and
reducing the
risk of complications associated with progression of chronic kidney disease.
In accordance with the foregoing, in one embodiment, the invention provides a
method of reducing proteinuria in a human subject suffering from IgAN
comprising
administering to the subject a MASP-2 inhibitory antibody, or antigen-binding
fragment
thereof, comprising a heavy chain variable region comprising CDR-HI, CDR-H2
and
CDR-H3 of the amino acid sequence set forth as SEQ ID NO:67 and a light chain
variable region comprising CDR-L1, CDR-L2 and CDR-L3 of the amino acid
sequence
set forth as SEQ ID NO:70 according to a dosage regimen as follows:
c. administering about 4 mg/kg (i.e., from 3.6 mg/kg to 4.4 mg/kg) of
said
antibody to a subject suffering from IgAN once weekly intravenously for a
treatment period of at least 12 weeks; or
d. administering
from about 180 mg to about 725 mg (i.e., from 162 mg to
797 mg) of said antibody to a subject suffering from IgAN once weekly
intravenously for a treatment period of at least 12 weeks,
wherein the method reduces proteinuria in said human subject.
In one embodiment, the dosage of the MASP-2 inhibitory antibody is about 4
mg/kg (i.e., from 3.6 mg/kg to 4.4 mg/kg), such as about 3.6 mg/kg, about 3.7
mg/kg,
about 3.8 mg/kg, about 3.9 m/kg, about 4.0 mg/kg, about 4.1 mg/kg, about 4.2
mg/kg,
about 4.3 mg/kg or about 4.4 mg/kg.
In one embodiment, dosage of the MASP-2 inhibitory antibody is a fixed dose
from about 180 mg to about 725 mg (i.e., from 160 mg to 800 mg, or from about
300 mg
to 500 mg, such as from about 300 mg to about 400 mg), such as about 160 mg,
about
165 mg, about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg,
about
195 mg, about 200 mg, about 205 mg, about 210 mg, about 215 mg, about 220 mg,
about
225 mg, about 230 mg, about 240 mg, about 245 mg, about 250 mg, about 255 mg,
about
260 mg, about 265 mg, about 270 mg, about 275 mg, about 280 mg, about 285 mg,
about
290 mg, about 295 mg, about 300 mg, about 305 mg, about 310 mg, about 315 mg,
about
320 mg, about 325 mg, about 330 mg, about 335 mg, about 340 mg, about 345 mg,
about
350 mg, about 355 mg, about 360 mg, about 365 mg, about 370 mg, about 375 mg,
about
380 mg, about 385 mg, about 390 mg, about 395 mg, about 400 mg, about 405 mg,
about
-192-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
410 mg, about 415 mg, about 420 mg, about 425 mg, about 430 mg, about 435 mg,
about
440 mg, about 445 mg, about 450 mg, about 455 mg, about 460 mg, about 465 mg,
about
470 mg, about 475 mg, about 480 mg, about 485 mg, about 490 mg, about 495 mg,
about
500 mg, about 505 mg, about 510 mg, about 515 mg, about 520 mg, about 525 mg,
about
530 mg, about 535 mg, about 540 mg, about 545 mg, about 550 mg, about 555 mg,
about
560 mg, about 565 mg, about 570 mg, about 575 mg, about 580 mg, about 585 mg,
about
590 mg, about 595 mg, about 600 mg, about 605 mg, about 610 mg, about 615 mg,
about
620 mg, about 625 mg, about 630 mg, about 635 mg, about 640 mg, about 645 mg,
about
650 mg, about 655 mg, about 660 mg, about 665 mg, about 670 mg, about 675 mg,
about
680 mg, about 685 mg, about 690 mg, about 695 mg, about 700 mg, about 705 mg,
about
710 mg, about 715 mg, about 720 mg, about 725 mg, about 730 mg, about 735 mg,
about
740 mg, about 745 mg, about 750 mg, about 755 mg, about 760 mg, about 765 mg,
about
770 mg, about 775 mg, about 780 mg, about 785 mg, about 790 mg, about 795 mg.
or
about 800 mg.
In one embodiment, the treatment period is 12 weeks.
In one embodiment, the treatment period is followed by a rest period (i.e., no
administration of a MASP-2 inhibitor) of at least 2 months, or a rest period
of at least 3
months, or a rest period of at least 4 months, or a rest period of at least 5
months, or a rest
period of at least 6 months or longer, such as a rest period of at least 7
months, or a rest
period of at least 8 months, or a rest period of at least 9 months, or a rest
period of at least
10 months, or a rest period of at least 11 months, or a rest period of at
least 12 months or
longer.
In some embodiments, the method further comprises periodically monitoring the
urinary protein levels in the subject during the treatment period and/or the
rest period, and
optionally resuming treatment with the MASP-2 inhibitory antibody upon a
finding of a
recurrence of proteinuria.
In some embodiments, the method is effective to reduce proteinuria in the
subject
suffering from IgAN by at least 30%, such as at least 40%, or at least 50%, or
greater
than 50% from baseline (prior to treatment) as determined at the end of the
treatment
period and/or at the end of the rest period.
In some embodiments, the method is effective to increase the estimated
glomerular filtration rate (eGFR) in the subject suffering from IgAN.
-193-
In some embodiments, the subject suffering from IgAN has proteinuria of
greater
than 1 gram protein/24 hour urine protein excretion prior to treatment and the
method is
effective to reduce proteinuria in the subject in the subject by at least 30%,
such as at
least 40%, or at least 50%, or greater than 50% from baseline (prior to
treatment) as
determined at the end of the treatment period and/or at the end of the rest
period and/or to
reduce proteinuria to less than 1 gram protein/24 hour urine protein excretion
as
determined at the end of the treatment period and/or at the end of the rest
period.
In some embodiments, the subject suffering from IgAN has proteinuria of
greater
than 1 gram protein/24 hour urine protein excretion despite maximum tolerated
doses of
antihypertensives and well-controlled blood pressure prior to treatment and
the method is
effective to reduce proteinuria in the subject in the subject by at least 30%,
such as at
least 40%, or at least 50%, or greater than 50 0 from baseline (prior to
treatment) as
determined at the end of the treatment period and/or at the end of the rest
period and/or to
reduce proteinuria to less than 1 gram protein/24 hour urine protein excretion
as
determined at the end of the treatment period and/or at the end of the rest
period.
In some embodiments, the subject suffering from IgAN has not been treated with
steroids for at least one year. In some embodiments, the subject suffering
from IgAN is
undergoing steroid treatment during at least a portion of the 12-week
treatment with
0MS646. In some embodiments, the subject suffering from IgAN is undergoing
steroid
treatment during at least a portion of the 12-week treatment with 0MS646 and
the
method is effective to reduce proteinuria and reduce or eliminate the need for
steroid
treatment by the end of the treatment period and/or at the end of the rest
period.
Other embodiments
Various modifications and variations of the described methods and compositions
of the invention will be apparent to those skilled in the art without
departing from the
scope and spirit of the invention. Although the invention has been described
in
connection with specific desired embodiments, it should be understood that the
invention
as claimed should not be unduly limited to such specific embodiments.
In accordance with the foregoing, the invention features the following
embodiments.
-194-
Date Recue/Date Received 2020-07-30
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
1A. A method for treating, inhibiting, alleviating or preventing fibrosis in a
mammalian
subject suffering, or at risk of developing a disease or disorder caused or
exacerbated by
fibrosis and/or inflammation, comprising administering to the subject an
amount of a
MASP-2 inhibitory agent effective to inhibit fibrosis.
2A. The method according to paragraph 1A, wherein the MASP-2 inhibitory agent
is a
MASP-2 antibody or fragment thereof.
3A. The method according to paragraph 2A, wherein the MASP-2 inhibitory agent
is a
MASP-2 monoclonal antibody, or fragment thereof that specifically binds to a
portion of
SEQ ID NO:6.
4A. The method according to paragraph 2A, wherein the MASP-2 antibody or
fragment
thereof specifically binds to a polypeptide comprising SEQ ID NO:6 with an
affinity of at
least 10 times greater than it binds to a different antigen in the complement
system
5A The method according to paragraph 2A, wherein the antibody or fragment
thereof is
selected from the group consisting of a recombinant antibody, an antibody
having
reduced effector function, a chimeric antibody, a humanized antibody and a
human
antibody.
6A. The method according to paragraph 1A, wherein the MASP-2 inhibitory agent
selectively inhibits lectin pathway complement activation without
substantially inhibiting
Clq-dependent complement activation.
7A. The method according to paragraph 1A, wherein the MASP-2 inhibitory agent
is
administered subcutaneously, intraperitoneally, intra-muscularly, intra-
arterially,
intravenously, or as an inhalant.
8A. The method according to any of paragraphs lA to 7A, wherein the disease or
disorder caused or exacerbated by fibrosis and/or inflammation is associated
with an
ischemia reperfusion injury.
9A. The method according to any of paragraphs lA to 7A, wherein the disease or
disorder caused or exacerbated by fibrosis and/or inflammation is not
associated with an
ischemia reperfusion injury.
-195-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
10A. The method according to any of paragraphs lA to 7A, wherein the subject
exhibits
proteinuria prior to administration of the MASP-2 inhibitory agent and
administration of
the MASP-2 inhibitory agent decreases proteinuria in the subject.
11A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by renal fibrosis
and/or
inflammation.
12A. The method according to paragraph 11A, wherein the MASP-2 inhibitory
agent is
administered in an amount effective to inhibit tubulointerstitial fibrosis.
13A. The method according to paragraph 11A, wherein the MASP-2 inhibitory
agent is
administered in an amount effective to reduce, delay or eliminate the need for
dialysis in
the subject.
14A. The method according to paragraph 11A, wherein the disease or disorder is
selected
from the group consisting of chronic kidney disease, chronic renal failure,
glom erular
disease (e.g., focal segmental glomerulosclerosis), an immune complex disorder
(e.g.,
IgA nephropathy, membraneous nephropathy), lupus nephritis, nephrotic
syndrome,
diabetic nephropathy, tubulointerstitial damage and glomerulonepthritis (e.g.,
C3
glomerulopathy).
15A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by pulmonary
fibrosis and/or
inflammation.
16A. The method according to paragraph 15A, wherein the disease or disorder is
selected
from the group consisting of chronic obstructive pulmonary disease, cystic
fibrosis,
pulmonary fibrosis associated with scleroderma, bronchiectasis and pulmonary
hypertension.
17A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by hepatic fibrosis
and/or
inflammation.
-196-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
18A. The method according to paragraph 17A, wherein the disease or disorder is
selected
from the group consisting of cirrhosis, nonalcoholic fatty liver disease
(steatohepatitis),
liver fibrosis secondary to alcohol abuse, liver fibrosis secondary to acute
or chronic
hepatitis, biliary disease and toxic liver injury (e.g., hepatotoxicity due to
drug-induced
liver damage induced by acetaminophen or other drug, such as a nephrotoxin).
19A. The method according to any of paragraphs IA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by cardiac fibrosis
and/or
inflammation.
20A. The method according to paragraph 19A, wherein the disease or condition
is
.. selected from the group consisting of cardiac fibrosis, myocardial
infarction, valvular
fibrosis, atrial fibrosis, endomyocardial fibrosis arrhythmogenic right
ventricular
cardiomyopathy (ARVC).
21A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by vascular
fibrosis.
22A. The method according to paragraph 21A, wherein the disease or disorder is
selected
from the group consisting of a vascular disease, an atherosclerotic vascular
disease,
vascular stenosis, restenosis, vasculitis, phlebitis, deep vein thrombosis and
abdominal
aortic aneurysm.
23A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
skin.
24A. The method according to paragraph 23A, wherein the disease or disorder is
selected
from the group consisting of excessive wound healing, scleroderma, systemic
sclerosis,
keloids, connective tissue diseases, scarring, and hypertrophic scars.
25A. The method according to any of paragraphs IA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
joints.
26.A The method according to paragraph 2A5, wherein the disease or disorder is
arthrofibrosis.
-197-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
27A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
central
nervous system.
28A. The method according to paragraph 27A, wherein the disease or disorder is
selected
from the group consisting of stroke, traumatic brain injury and spinal cord
injury.
29A. The method according to any of paragraphs IA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
digestive
system.
30A. The method according to paragraph 29A, wherein the disease or disorder is
selected
from the group consisting of Crohn's disease, pancreatic fibrosis and
ulcerative colitis.
31A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by ocular fibrosis.
32A. The method according to paragraph 31A, wherein the disease or disorder is
selected
from the group consisting of anterior subcapsular cataract, posterior capsule
pacification, macular degeneration, and retinal and vitreal retinopathy.
33A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
musculoskeletal bone or soft-tissue structure.
34A. The method according to paragraph 33A, wherein the disease or disorder is
selected
from the group consisting of osteoporosis and/or osteopenia associated with
cystic
fibrosis, myelodysplastic conditions with increased bone fibrosis, adhesive
capsulitis,
Dupuytren's contracture and myelofibrosis.
35A. The method according to any of paragraphs lA to 7A, wherein the subject
is
suffering from a disease or disorder caused or exacerbated by fibrosis of the
reproductive
organs.
36A. The method according to paragraph 35A, wherein the disease or disorder is
selected
from the group consisting of endometriosis and Peyronie's disease.
-198-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
37A. The method according to any of paragraphs IA to 7A, wherein the subject
is
suffering from a chronic infectious disease that causes fibrosis and/or
inflammation.
38A. The method according to paragraph 37A, wherein the infectious disease is
selected
from the group consisting of alpha virus, Hepatitis A, Hepatitis B, Hepatitis
C,
tuberculosis, HIV and influenza.
39A. The method according to any of paragraphs IA to 7A, wherein the subject
is
suffering from an autoimmune disease that causes fibrosis and/or inflammation.
40A. The method according to paragraph 39A, wherein the autoimmune disease is
selected from the group consisting of scleroderma and systemic lupus
erythematosus
(SLE).
41A. The method according to any of paragraphs 1A to 7A, wherein the subject
is
suffering from scarring associated with trauma.
42A. The method according to paragraph 41A, wherein the scarring associated
with
trauma is selected from the group consisting of surgical complications (e.g.,
surgical
adhesions wherein scar tissue can form between internal organs causing
contracture, pain
and can cause infertility), chemotherapeutic drug-induced fibrosis, radiation-
induced
fibrosis and scarring associated with burns.
43A. The method according to any of paragraphs 1A to 7A, wherein the disease
or
disorder caused or exacerbated by fibrosis and/or inflammation is selected
from the group
consisting of organ transplant, breast fibrosis, muscle fibrosis,
retroperitoneal fibrosis,
thyroid fibrosis, lymph node fibrosis, bladder fibrosis and pleural fibrosis.
1B. A method of preventing or reducing renal damage in a subject suffering
from a
disease or condition associated with proteinuria comprising administering an
amount of a
MASP-2 inhibitory agent effective to reduce or prevent proteinurea in the
subject.
2B. The method according to paragraph 1B, wherein the MASP-2 inhibitory agent
is a
MASP-2 inhibitory antibody or fragment thereof
3B. The method according to paragraph 1B or 2B, wherein the MASP-2 inhibitory
agent
is administered in an amount and for a time effective to achieve at least a 20
percent
-199-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
reduction in 24-hour urine protein excretion as compared to baseline 24-hour
urine
protein excretion prior to treatment.
4B. The method according to any of paragragphs 1B to 3B, wherein the disease
or
condition associated with proteinuria is selected from the group consisting of
nephrotic
syndrome, pre-eclampsia, eclampsia, toxic lesions of kidneys, amyloidosis,
collagen
vascular diseases (e.g., systemic lupus erythematosus), lupus nephritis,
dehydration,
glomerular diseases (e.g. membranous glomerulonephritis, focal segmental
glomerulonephritis, C3 glomerulopathy, minimal change disease, lipoid
nephrosis),
strenuous exercise, stress, benign orthostatis (postural) proteinuria, focal
segmental
glomerulosclerosis, IgA nephropathy (i.e., Berger's disease), IgM nephropathy,
membranoproliferative glomerulonephritis, membranous nephropathy, minimal
change
disease, sarcoidosis, Alport's syndrome, diabetes mellitus (diabetic
nephropathy), drug-
induced toxicity (e.g., NSAIDS, nicotine, penicillamine, lithium carbonate,
gold and
other heavy metals, ACE inhibitors, antibiotics (e.g., adriamycin) or opiates
(e.g. heroin)
or other nephrotoxins), Fabry's disease, infections (e.g., HIV, syphilis,
hepatitis A, B or
C, poststreptococcal infection, urinary schistosomiasis); aminoaciduria,
Fanconi
syndrome, hypertensive nephrosclerosis, interstitial nephritis, sickle cell
disease,
hemoglobinuria, multiple myeloma, myoglobinuria, organ rejection (e.g., kidney
transplant rejection), ebola hemorrhagic fever, Nail patella syndrome,
familial
mediterranean fever, HELLP syndrome, systemic lupus erythematosus, Wegener's
granulomatosis, Rheumatoid arthritis, Glycogen storage disease type 1,
Goodpasturc's
syndrome, Henoch-Schonlein purpura, urinary tract infection which has spread
to the
kidneys, Sj Ogren' s syndrome and post-infections glomerulonepthritis.
5B. The method according to any of paragraphs 1B to 3B, wherein the disease or
condition associated with proteinuria is IgA nephropathy (i.e., Berger's
disease).
611 The method according to any of paragraphs IB to 311, wherein the
disease or
condition associated with proteinuria is membranous nephropathy.
711. The method according to any of paragraphs I B to 3B, wherein the disease
or
condition associated with proteinuria is lupus nephritis.
-200-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
1C. A method of inhibiting the progression of chronic kidney disease,
comprising
administering an amount of a MASP-2 inhibitory agent effective to reduce or
prevent
tubulointerstitial fibrosis in a subject in need thereof.
2C. The method according to paragraph 1C, wherein the MASP-2 inhibitory agent
is a
MASP-2 inhibitory antibody, or fragment thereof.
3C. The method according to paragraph IC, wherein the subject in need thereof
exhibits
proteinuria prior to administration of the MASP-2 inhibitory agent and
administration of
the MASP-2 inhibitory agent decreases proteinuria in the subject, such that
the subject
has at least a 20 percent reduction in 24-hour urine protein excretion as
compared to
baseline 24-hour urine protein excretion in the subject prior to treatment.
4C. The method according to paragraph 1C, wherein the MASP-2 inhibitory agent
is
administered in an amount effective to reduce, delay or eliminate the need for
dialysis in
the subject.
1D. A method of protecting a kidney from renal injury in a subject that has
undergone, is
undergoing, or will undergo treatment with one or more nephrotoxic agents,
comprising
administering an amount of a MASP-2 inhibitory agent effective to prevent or
ameliorate
the incidence of drug-induced nephropathy.
2D. The method according to paragraph 1D, wherein the MASP-2 inhibitory agent
is a
MASP-2 inhibitory antibody, or fragment thereof.
3D. The method according to paragraph 1D, wherein the MASP-2 inhibitory agent
is
administered prior to said nephrotoxic agent.
4D. The method according to paragraph 1D, wherein the MASP-2 inhibitory agent
is co-
administered simultaneously with said nephrotoxic agent.
51). The method according to paragraph 1D, wherein the MASP-2 inhibitory agent
is
administered after said nephrotoxic agent to treat nephrotoxicity.
1E. A method of treating a human subject suffering from Immunoglobulin A
Nephropathy (IgAN) comprising administering to the subject a composition
comprising
-201-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
an amount of a MASP-2 inhibitory antibody, or antigen-binding fragment
thereof,
effective to inhibit MASP-2-dependent complement activation.
2E. The method according to paragraph 1E, wherein the subject is suffering
from steroid-
dependent IgAN.
3E. The method according to paragraph 1E or 2E, wherein the MASP-2 inhibitory
antibody is a monoclonal antibody, or fragment thereof that specifically binds
to human
MASP-2.
4E. The method according to any of paragraphs 1E to 3E, wherein the antibody
or
fragment thereof is selected from the group consisting of a recombinant
antibody, an
.. antibody having reduced effector function, a chimeric antibody, a humanized
antibody,
and a human antibody.
5E The method according to any of paragraphs lE to 4E, wherein the MASP-2
inhibitory
antibody does not substantially inhibit the classical pathway.
6E The method according to any of paragraphs lE to 5E, wherein the MASP-2
inhibitory antibody inhibits C3b deposition in 90% human serum with an IC5o of
30 nM
or less.
7E The method according to paragraph 2E, wherein the method further comprises
identifying a human subject having steroid-dependent IgAN prior to the step of
administering to the subject a composition comprising an amount of a MASP-2
inhibitory
antibody, or antigen-binding fragment thereof, effective to improve renal
function.
8E. The method according to any of paragraphs 1E to 7E, wherein the MASP-2
inhibitory
antibody or antigen-binding fragment thereof is administered in an amount
effective to
improve renal function.
9E. The method according to paragraph 8E, wherein the MASP-2 inhibitory
antibody or
antigen-binding fragment thereof is administered in an amount effective and
for a time
sufficient to achieve at least a 20 percent reduction in 24-hour urine protein
excretion as
compared to baseline 24-hour urine protein excretion in the subject prior to
treatment.
-202-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
10E. The method according to paragraph 1E, wherein the composition is
administered in
an amount sufficient to improve renal function and decrease the corticosteroid
dosage in
said subject.
11E. The method according to any of paragraphs lE to 10E, wherein the MASP-2
inhibitory antibody or antigen-binding fragment thereof comprises a heavy
chain variable
region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid sequence set
forth
as SEQ ID NO:67 and a light chain variable region comprising CDR-L1, CDR-L2
and
CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
1F. A method of treating a human subject suffering from membranous nephropathy
(MN) comprising administering to the subject a composition comprising an
amount of a
MA SP-2 inhibitory antibody, or antigen-binding fragment thereof, effective to
inhibit
MASP-2-dependent complement activation.
2F. The method according to paragraph 1F, wherein the subject is suffering
from steroid-
dependent MN.
3F. The method according to paragraph 1F or 2F, wherein the MASP-2 inhibitory
antibody is a monoclonal antibody, or fragment thereof that specifically binds
to human
MASP-2.
4F. The method according to any of paragraphs 1F to 3F, wherein the antibody
or
fragment thereof is selected from the group consisting of a recombinant
antibody, an
antibody having reduced effector function, a chimeric antibody, a humanized
antibody,
and a human antibody.
5F. The method according to any of paragraphs 1F to 4F, wherein the MASP-2
inhibitory antibody does not substantially inhibit the classical pathway.
6F. The method according to any of paragraphs IF to 5F, wherein the MASP-2
inhibitory antibody inhibits C3b deposition in 90% human serum with an IC5o of
30 nM
or less.
7F. The method according to paragraph IF, wherein the method further comprises
identifying a human subject having steroid-dependent MN prior to the step of
-203-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
administering to the subject a composition comprising an amount of a MASP-2
inhibitory
antibody, or antigen-binding fragment thereof, effective to improve renal
function.
8F. The method according to any of paragraphs 1F to 7F, wherein the MASP-2
inhibitory antibody or antigen-binding fragment thereof is administered in an
amount
effective to improve renal function.
9F. The method according to paragraph 8F, wherein the MASP-2 inhibitory
antibody or
antigen-binding fragment thereof is administered in an amount effective and
for a time
sufficient to achieve at least a 20 percent reduction in 24-hour urine protein
excretion as
compared to baseline 24-hour urine protein excretion in the subject prior to
treatment.
10F. The method according to paragraph 1F or 2F, wherein the composition is
administered in an amount sufficient to improve renal function and decrease
the
corticosteroid dosage in said subject.
11F. The method according to any of paragraphs IF to 10F, wherein the MA SP-2
inhibitory antibody or antigen-binding fragment thereof comprises a heavy
chain variable
region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid sequence set
forth
as SEQ ID NO:67 and a light chain variable region comprising CDR-L1, CDR-L2
and
CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
1G. A method of treating a human subject suffering from lupus nephritis
(LN)
comprising administering to the subject a composition comprising an amount of
a MASP-
2 inhibitory antibody, or antigen-binding fragment thereof, effective to
inhibit MASP-2-
dependent complement activation.
2G. The method according to paragraph 1G, wherein the subject is suffering
from
steroid-dependent LN.
3G. The method according to paragraph 1G or 2G, wherein the MASP-2 inhibitory
antibody is a monoclonal antibody, or fragment thereof that specifically binds
to human
MASP-2.
4G. The method according to any of paragraphs 1G to 3G, wherein the antibody
or
fragment thereof is selected from the group consisting of a recombinant
antibody, an
-204-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
antibody having reduced effector function, a chimeric antibody, a humanized
antibody,
and a human antibody.
5G. The method according to any of paragraphs 1G to 4G, wherein the MASP-2
inhibitory antibody does not substantially inhibit the classical pathway.
6G. The method according to any of paragraphs 1G to 5G, wherein the MASP-2
inhibitory antibody inhibits C3b deposition in 90% human serum with an ICso of
30 nM
or less.
7G. The method according to paragraph 1G, wherein the method further comprises
identifying a human subject having steroid-dependent LN prior to the step of
administering to the subject a composition comprising an amount of a MASP-2
inhibitory
antibody, or antigen-binding fragment thereof, effective to improve renal
function.
8G. The method according to any of paragraphs 1G to 7g, wherein the MA SP-2
inhibitory antibody or antigen-binding fragment thereof is administered in an
amount
effective to improve renal function.
9G. The method according to paragraph 8G, wherein the MASP-2 inhibitory
antibody or
antigen-binding fragment thereof is administered in an amount effective and
for a time
sufficient to achieve at least a 20 percent reduction in 24-hour urine protein
excretion as
compared to baseline 24-hour urine protein excretion in the subject prior to
treatment.
10G. The method according to paragraph 1G or 2G, wherein the composition is
administered in an amount sufficient to improve renal function and decrease
the
corticosteroid dosage in said subject.
11G. The method according to any of paragraphs 1G to 10G, wherein the MASP-2
inhibitory antibody or antigen-binding fragment thereof comprises a heavy
chain variable
region comprising CDR-H1, CDR-H2 and CDR-H3 of the amino acid sequence set
forth
as SEQ ID NO:67 and a light chain variable region comprising CDR-L1, CDR-L2
and
CDR-L3 of the amino acid sequence set forth as SEQ ID NO:70.
-205-
CA 03039927 2019-04-09
WO 2018/071701 PCT/US2017/056386
While illustrative embodiments have been illustrated and described, it will be
appreciated
that various changes can be made therein without departing from the spirit and
scope of
the invention
-206-