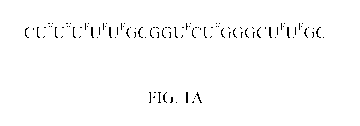Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
5-HALOURACIL-MODIFIED MICRORNAS AND THEIR USE D THE
TREATMENT OF CANCER
CROSS REFERENCE TO RELATED APPLICATIONS
100011 This application claims priority from U.S. Provisional Application No.
62/464,491,
filed February 28, 2017; U.S. Provisional Application No. 62/422,298, filed
November 15,
2016 and U.S. Provisional Application No. 62/415,740, filed November 1, 2016,
the entire
contents of which are incorporated herein by reference.
GOVERNMENT SUPPORT
[0002) The present disclosure was made with government support under grant
numbers
HL127522 and CA19709802 awarded by the National Institutes of Health. The
government
has certain rights in the disclosure.
INCORPORATION BY REFERENCE OF SEQUENCE LISTING
100031 The Sequence Listing in the ASCII text file, named as
R8859 _ PCT_ SequenceListing.txt of 7 KB, created on October 30, 2017, and
submitted to
the United States Patent and Trademark Office via EFS-Web, is incorporated
herein by
reference.
FIELD OF TH E DISCLOSURE
100041 The present disclosure is generally directed to compositions and
methods for
treating cancer, and more particularly, to methods in which modified microRNAs
alone or in
conjunction with 5-fluorouracil are used in treating cancer, particularly
colorectal, lung or
pancreatic cancer.
BACKGROUND
100051 MicroRNAs (miRNAs, miRs) are a class of highly conserved, non-coding
small
RNA molecules that mediate translation in a cell or organism by negatively
regulating the
expression of their target genes and thus causing translational arrest, mRNA
cleavage or a
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
combination thereof. See Bartel DP. Cell. (2009) 136(2):215-33. By targeting
multiple
transcripts, miRNAs regulate a wide range of biological processes, including
apoptosis,
differentiation and cell proliferation, thus aberrant microRNA function can
lead to cancer
(see Ambros V. Nature. (2004) 431(7006):350-5) and as such, miRNAs have
recently been
identified as as biomarkers, oncogenes or tumor suppressors. See, e.g., Croce,
CM, Nat Rev
Genet. (2009) 10:704-714).
[0006] Colorectal cancer (CRC) is the third most common malignancy and the
second
most common cancer-related cause of death in the United States. See, Hegde SR,
et al.,
Expert review qf gastroenterology & hepatology. (2008) 2(1):135-49. There are
many
chemotherapeutic agents used to treat cancer; however pyrimidine antagonists,
such as
fluoropyrimidine-based chemotherapeutic agents (e.g., 5-fluorouracil, S-1) are
the gold
standard for treating colorectal cancer. Pyrimidine antagonists, block the
synthesis of
pyrimidine containing nucleotides (Cytosine and Thymine in DNA; Cytosine and
Uracil in
RNA). Because pyrimidine antagonists have similar structures when compared to
endogenous nucleotides, they compete with the natural pyrimidines to inhibit
crucial
enzymatic activity involved in the replication process leading to the
prevention of DNA
and/or RNA synthesis and inhibition of cell division.
[0007] Pancreatic cancer is a deadly cancer that is very difficult to treat.
See Siegel, RL et
al. CA Cancer J. Clin. (2015) 65: 5-29. Unique aspects of pancreatic cancer
include a very
low 5 year survivial rate of less than 7% (Id.), late presentation, early
metastasis and a poor
response to chemotherapy and radiation. See Maitra A and Hruban RH, Annu Rev.
Pathol.
(2008) 3:157-188. To date gemcitabine-based chemotherapy (2', 2'-difluoro
2'deoxycytidine) is the gold standard for the treatment of pancreatic cancer,
however the
effect of therapeutic intervention is limited due to drug resistance. Oettle,
H et al. JAMA
(2013) 310: 1473-1481.
[0008] 5-fluorouracil (i.e., 5-FU, or more specifically, 5-fluoro-1H-
pyrimidine-2,4-dione)
is a well known pyrimidine antagonist that is used in many adjuvant
chemotherapeutic
medicants, such as Carac cream, Efudex , Fluoroplex , and Adrucil . It is
well
established that 5-FU targets a critical enzyme, thymidylate synthase (TYMS or
TS), which
2
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
catalyzes the methylation of deoxyuridine monophosphate (dUMP) to
deoxythymidine
monophosphate (dT1v1P) an essential step in DNA biosynthesis. Danenberg P. V.,
Biochim.
Biophys. Acta. (1977) 473(2):73-92. However, despite the steady improvement of
5-FU-
based therapy, the patient response rate to 5-FU-based chemotherapy remains
modest, due to
the development of drug resistance. Longley D. B, et al., Apoptosis, Cell
Signaling, and
Human Diseases, (2007) p. 263-78.
100091 Nevertheless, the existing cancer therapies are still in their infancy,
with many
hurdles still waiting to be improved or overcome. For example, it is well
known that,
although 5-FU is fairly efficacious in treating a variety of cancers, 5-FU
possesses
substantial toxicity and can elicit a host of adverse side effects. With
respect to miRNAs,
these compounds are known to be susceptible to enzymatic degradation when
administered,
which results in poor stabilities. Moreover, tumor cells have been known to
circumvent
apoptotic pathways by developing resistance to common therapeutic agents, such
as 5-FU
and gemcitabine. See Gottesman M. M. et al., Nature Reviews Cancer, (2002)
2(1):48-58.
Thus, there would be a significant benefit in more efficacious, stable, and
less toxic
medications for the treatment of cancer.
SUMMARY OF THE DISCLOSURE
100101 The current disclosure demonstrates that nucleic acid compositions
(i.e., a
microRNA), which incorporate a 5-halouracil base have exceptional efficacy as
anti-cancer
agents. Moreover, the data herein shows that contacting a cell with a modified
microRNA
composition of the present disclosure regulates cell cycle progression and
reduced
tumorigenesis by, for example, reducing cancer cell proliferation and
increasing the efficacy
of chemotherapeutic agents. The present disclosure is premised on the
discovery that the
incorporation of 5-halouracil bases within the nucleotide sequences of
microRNAs increases
microRNA efficacy as an anticancer therapeutic agent over the cancer
therapeutic agents
alone and/or the native microRNA.
100111 Therefore, in one aspect of the present disclosure nucleic acid
compositions that
include a modified microRNA nucleotide sequence having at least one uracil
base (U, U
3
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
bases) that has been replaced by a 5-halouracil, such as 5-fluorouracil (5-FU)
are described.
In certain embodiments, the modified microRNA has more than one, or exactly
one uracil
that has been replaced by a 5-halouracil. In some embodiments, the modified
microRNA
nucleotide sequence includes two, three, four, five, six, seven, eight or more
uracil bases that
have been replaced by a 5-halouracil. In other embodiments, all of the uracil
nucleotide
bases of the modified mRNA have been replaced by a 5-halouracil.
100121 In some embodiments, the 5-halouracil is, for example, 5-fluorouracil,
5-
chlorouracil, 5-bromouracil, or 5-iodouracil. In specific embodiments, the 5-
halouracil is 5-
fluorouracil.
100131 In certain embodiments, the modified microRNA nucleotide sequence
includes
more than one 5-halouracil whereby each of the 5-halouracils are the same. In
other
embodiments, the modified microRNA nucleotide sequence includes more than one
5-
halouracil whereby each of the 5-halouracils is different. In other
embodiments, the
modified microRNA nucleotide sequence includes more than two 5-halouracils,
whereby the
modified microRNA nucleotide sequence includes a combination of different 5-
halouracils.
100141 In an exemplary embodiment of the present disclosure, a nucleic acid
composition
that contains a miR-129 nucleotide sequence that has been modified by
replacing at least
one of the uracil nucleotide bases with a 5-halouracil is provided. More
specifically, the
nucleic acid composition contains at least the following native miR-129
nucleotide
sequence: CUUUUUGCGGUCUGGGCUUGC [SEQ ID NO. 1], wherein at least one, two,
three, four, five, six, seven, eight or all of the uracil bases in the shown
nucleic acid
sequence or that may be covalently appended to the shown sequence, are
replaced by a 5-
halouracil.
100151 In a specific embodiment of the present disclosure, the modified
microRNA has
nucleic acid sequence consisting of CUFuFuFuFuFGc GGuFcuFGGGcuFuFGC [SEQ ID
NO. 4], wherein UF is a halouracil, specifically 5-fluorouracil.
100161 In other embodiments, a seed portion of the native miR-129 nucleotide
sequence,
GUUUUUGC remains unmodified (i.e., does not include a 5-halouracil) while one
or more
4
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
(or all) of the remaining uracil nucleotide bases in the remainder of the
modified miR-129
nucleotide sequence are replaced by an equivalent number of 5-ha1ouracils. In
a specific
embodiment, the modified miR-129 microRNA of the present disclosure has
nucleic acid
sequence consisting of CUUUUUGCGGUFCUFGGGCUFUFGC [SEQ ID NO. 5], whereby
UF is a halouracil, specifically 5-fluorouracil.
[0017] In some embodiments, the 5-halouracil is, for example, 5-fluorouracil,
5-
chlorouracil, 5-bromouracil, or 5-iodouracil. In specific embodiments, the 5-
halouracil is 5-
fluorouracil.
[0018] In another embodiment of the present disclosure, nucleic acid
compositions that
contain a miR-15a nucleotide sequence that has been modified by replacing at
least one of
the uracil nucleotide bases with a 5-halouracil, such as 5-fluorouracil (5-FU)
are provided.
Specifically, the nucleic acid composition contains at least the following
native miR-15a
nucleotide sequence: UAGCAGCACAUAAUGGUUUGUG [SEQ ID NO. 2], wherein at
least one, two, three, four, five, six or all of the uracil nucleotide bases
in the shown
sequence, or that may be covalently appended to the shown sequence, are 5-
halouracils.
[0019] In a specific embodiment of the present disclosure, the modified miR-
15a
microRNA has nucleic acid sequence consisting of
UFAGCAGCACAUFAAUFGGUFUFUFGUFG [SEQ ID NO. 6], wherein UF is a halouracil,
specifically 5-fluorouracil.
[0020] In other embodiments, a seed portion of the native miR-15a nucleotide
sequence,
UAGCAGCA, remains unmodified with a 5-halouracil, while one or more (or all)
of the
remaining uracil bases in the remainder of the miR-15a nucleotide sequence
(non-seed
portion) are replaced by a 5-halouracil.
[0021] In a specific embodiment, the modified miR-129 microRNA has nucleic
acid
sequence consisting of UAGCAGCACAUF
AAuFGGuFuFuFGu
FG [SEQ ID NO. 7],
wherein UF is a halouracil, specifically 5-fluorouracil.
[0022] In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a miR-140 nucleotide sequence that has been
modified. In some
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
embodiments, the native miR-140 nucleotide sequence has been modified by
replacing at
least one of the U bases with a 5-halouracil.
100231 In one set of embodiments, precisely one of the U bases in the native
mi R-140
nucleic acid sequence sequence is a 5-halouracil. In a second set of
embodiments, precisely
or at least two U bases in the native miR-140 nucleotide sequence are replaced
by 5-
halouracils. In another set of embodiments, precisely or at least three U
bases in the miR-
140 nucleotide sequence are 5-halouracils. In other embodiments, precisely or
at least four U
bases in the native miR-140 nucleotide sequence are 5-halouracils. In some
embodiments,
precisely or at least five U bases in the miR-140 nucleotide sequence sequence
are 5-
halouracil s. In a yet other embodiments, precisely or at least six U bases in
the mi R-140
nucleotide sequence are 5-halouracils. In specific embodiments, all of the U
bases in the
mi R-140 nucleotide sequence, whether in the native and/or in an appended
portion, are 5-
halouracils.
100241 In an exemplary embodiment, the modified microRNA nucleic acid
composition of
the present disclosure has a nucleotide sequence of
CAGUFGGUUUUACCCUFAUGGUFAG [SEQ ID NO. 9], wherein UF is a halouracil,
specifically 541 El orouracil.
100251 In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a modified native miR-192 or miR-215 nucleotide
sequence that
has been modified by replacing at least one of the uracil bases with a 5-
halouracil. In some
embodiments, the modified miR-192 nucleotide sequence has been modified by
replacing at
least one of the U bases with a 5-fluorouracil.
100261 In another set of embodiments, precisely one of the U bases in the
modified miR-
192 nucleotide sequence is a 5-halouracil. In a second set of embodiments,
precisely or at
least two U bases in the modified miR-192 nucleotide sequence are 5-
halouracils. In another
set of embodiments, precisely or at least three U bases in the modified miR-
192 nucleotide
sequence are 5-halouracils. In other embodiments, precisely or at least four U
bases in the
modified miR-192 or miR-215 nucleotide sequence are 5-halouracils. In specific
6
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
embodiments, all of the U bases in the modified miR-192 or miR-215 sequence,
whether in
the native and/or in an appended portion of the nucleic acid, are 5-
halouracils.
[0027] In an exemplary embodiment, the nucleic acid composition of the present
disclosure has a modified miR-192 or modified miR-215 nucleotide sequence of
CUFGACCUFAUFGAAUFUFGACAGCC [SEQ ID NO. 11], wherein UF is a halouracil,
specifically 5-fluorouracil.
100281 In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a modified native mi R-502 nucleotide sequence that
has been
modified by replacing uracil with 5-halouracil. In some embodiments, the
modified miR-
502 nucleotide sequence has been modified by replacing at least one of the U
bases with a 5-
fluorouracil.
100291 In another set of embodiments, precisely one of the U bases in the miR-
502
nucleotide sequence is a 5-halouracil. In a second set of embodiments,
precisely or at least
two U bases in the miR-502 nucleotide sequence are 5-halouracils. In another
set of
embodiments, precisely or at least three U bases in the miR-502 nucleotide
sequence are 5-
halouracils. In other embodiments, precisely or at least four U bases in the
miR-502
nucleotide sequence are 5-halouracils. In other embodiments, precisely or at
least five U
bases in the miR-502 nucleotide sequence are 5-halouracils. In other
embodiments, precisely
or at least six U bases in the modified miR-502 nucleotide sequence are 5-
halouracils. In
other embodiments, precisely or at least seven U bases in the miR-502
nucleotide sequence
are 5-halouracils. In specific embodiments, all of the U bases in the miR-502
nucleotide
sequence, whether in the native and/or in an appended portion, are 5-
halouracils.
100301 In an exemplary embodiment, the modified miR-502 nucleic acid
composition of
the present disclosure has a modified nucleotide sequence of
AUFCCUFUFGCUAUFCUFGGGUFGCUFA [SEQ ID NO. 13], wherein UF is a halouracil,
specifically 5-fluorouracil.
100311 In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a modified miR-506 nucleotide sequence that includes
a 5-
7
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
halouracil. In some embodiments, the modified miR-506 nucleotide sequence has
been
modified by replacing at least one of the U bases with a 5-halouracil, such as
5-fluorouracil.
100321 In another set of embodiments, precisely one of the U bases in the
native mi R-506
nucleotide sequence is replaced by a 5-halouracil. In a second set of
embodiments, precisely
or at least two U bases in the modified miR-506 nucleotide sequence are 5-
ha1ouracils. In
another set of embodiments, precisely or at least three U bases in the
modified miR-506
nucleotide sequence are 5-ha1ouracils. In other embodiments, precisely or at
least four U
bases in the modified miR-506 nucleotide sequence are 5-halouracils. In other
embodiments, precisely or at least five U bases in the modified miR-506
nucleotide
sequence are 5-halouracils. In other embodiments, precisely or at least six U
bases in the
modified miR-506 nucleotide sequence are 5-halouracils. In other embodiments,
precisely
or at least seven U bases in the modified miR-506 nucleotide sequence are 5-
halouracils. In
specific embodiments, all of the U bases in the modified miR-506 nucleotide
sequence,
whether in the native and/or in an appended portion, are 5-halouracils.
100331 In an exemplary embodiment, the miR-506 nucleic acid composition of the
present
disclosure has a modified microRNA nucleotide sequence of
UFAUFUFCAGGAAGGUFGUFUFACUFUFAA [SEQ ID NO. 15], wherein UF is a
halouracil, specifically 5-fluorouracil.
100341 In some embodiments, the 5-halouracil is, for example, 5-fluorouracil,
5-
chlorouracil, 5-bromouracil, or 5-iodouracil. In specific embodiments, the 5-
halouracil is 5-
fluorouracil, or a combination thereof.
100351 The present disclosure is also directed to formulations of a modified
microRNA
composition described herein or a formulation that includes combinations
thereof. In certain
embodiments, the formulations can include pharmaceutical preparations that
comprise the
above-described nucleic acid compositions and a pharmaceutically acceptable
carrier.
100361 In another aspect, the present disclosure is directed to a method for
treating cancer
that includes administering to a subject an effective amount of one or more of
nucleic acid
compositions described herein. In certain embodiments of the present methods,
the nucleic
8
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
acid compositions include a modified miR-129, miR-15a, mi R-192, miR-215, miR-
140,
miR-502 or miR-506 nucleotide sequence wherein at least one, two, three, four,
five, six or
more of the uracil nucleotide bases in the native (unmodified) nucleotide
sequence have
been replaced by a 5-halouracil. In specific embodiments, the present methods
include
administering a nucleic acid composition of the present disclosure to a
subject having cancer
or a predisposition to cancer, whereby the nucleic acid composition is a
modified miR-129
or a modified mi R-15a nucleic acid. In a specific embodiment of the present
disclosure, the
modified microRNA administered has nucleic acid sequence selected from the
group
consisting of CUFUFUFUFUFGCGGUFCUFGGGCUFUFGC [SEQ ID NO. 4],
CUUUUUGCGGUFCUFGGGCUFUFGC [SEQ ID NO. 5],
UFAGCAGCACAUFAAUFGGUFUFUFGUFG [SEQ ID NO.6],
UAGCAGCACAUFAAUFGGUFUFUFGUFG [SEQ ID NO. 7],
CAGUFGGUUUUACCCUFAUGGUFAG [SEQ ID NO. 9],
CUFGACCUFAUFGAAUFUFGACAGCC [SEQ ID NO. 11],
AUFCCUFUFGCUAUFCUFGGGUFGCUFA [SEQ ID NO. 13], and
UFAUFUFCAGGAAGGUFGUFUFACUFUFAA [SEQ ID NO. 15].
100371 In certain embodiments, the subject is a mammal. In other embodiments,
the
subject being treated is a human, dog, horse, pig, mouse, or rat. In a
specific embodiment,
the subject is a human that has been diagnosed with cancer, or has been
identified as having
a predisposition to developing cancer. In some embodiments, the cancer being
treated can
be, for example, colorectal, stomach, esophageal, lung, ovarian, pancreatic,
or cervical
cancer. In certain embodiments, the methods of the present disclosure treat a
subject for
colorectal cancer, pancreatic cancer or breast cancer.
100381 The data provided herein surprisingly shows an increased potency of the
modified
microRNAs described herein when compared to known anticancer agents, such as 5-
FU
alone in several different cancer models, including colorectal cancer,
pancreatic cancer, and
lung cancer. For example, the present disclosure provides the unexpected
finding that the
described modified nucleic acid compositions are substantially more potent in
inhibiting
cancer progression and tumorigenesis than 5-FU, mi R-15a, mi R-129, miR-140,
miR-192,
9
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
miR-215, miR-502 or miR-506 alone, or than a combination of 5-FU and
corresponding
native microRNAs.
100391 As such, the present compositions and methods provide the additional
benefit of
permitting a lower dosing, which results in lower toxicity and fewer side
effects. A further
significant advantage exhibited by the described nucleic acid compositions is
that the instant
compositions have significantly improved efficacy compared to miR-140, miR-
192, miR-
215, miR-502 or miR-506 sequences that have not been modified with a
halouracil. Thus, at
least in view of the noted advantages, the nucleic acid compositions disclosed
herein
represent a substantial advance in the treatment of cancer.
BRIEF DESCRIPTION OF THE DRAWINGS
100401 FIGS. 1A-1H. Chemical representation of exemplary modified microRNA
nucleotide sequences of the present disclosure. (A) Chemical representation of
mi R-129
nucleotide sequence in which all U bases are replaced by a halouracil (i.e.,
UF), as set forth
in SEQ ID NO: 4. (B) Chemical representation of miR-129 in which only the non-
seed
portion of miR-129 has U bases replaced with halouracils), as set forth in SEQ
ID NO: 5.
(C) Chemical representation of miR-15a nucleotide sequence in which all U
bases are
replaced with a halouracil), as set forth in SEQ ID NO: 6. (D) Chemical
representation of
miR-15a in which only the non-seed portion of miR-15a has U bases replaced
with
halouracils), as set forth in SEQ ID NO: 7. (E) Chemical representation of the
miR-140
nucleotide sequence in which certain (3) U bases are replaced by a halouracil
as set forth in
SEQ ID NO: 9. (F) Chemical representation of the miR-192 nucleotide sequence
in which
certain (5) U bases are replaced by a halouracil as set forth in SEQ ID NO:
11. (G) Chemical
representation of the miR-502 nucleotide sequence in which certain (7) U bases
are replaced
by a halouracil as set forth in SEQ ID NO: 13. (H) Chemical representation of
the miR-506
nucleotide sequence in which all (i.e., 8) U bases are replaced by a
halouracil as set forth in
SEQ ID NO: 15.
100411 FIGS. 2A-C. Exemplary modified miR-129 nucleic acids enter cancer cells
and
effectively reduce target protein expression. (A) Graph showing target (E2F3)
specificity
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
and the ability of a modified miR-129 (with all U bases replaced with 5-FU, 5-
FU-miR-129)
compared to that of control miRNA, and an unmodified miR-129 nucleic acid. (B)
A
quantitative Real-Time PCR analysis showing that miR-129 mimics enter cancer
cells. (C)
MiR-129 mimics enter a cancer cell and break downs TS-FdUIvIP significantly
better than 5-
FU alone.
100421 FIG. 3. Graphs showing inhibition of colon cancer cell proliferation in
4 different
colon cancer cell lines (HCT116, RKO, SW480 and SW620) by an exemplary
modified
miR-129 nucleic acid (mimic) having all U bases replaced by 5-FU (- -),
as compared to
a non-specific (Negative control.) control and ectopically expressed native
miR-129 (0).
100431 FIG. 4. Combination therapy with 5-FU and modified microRNA
compositions of
the present disclosure effectively inhibit cancer cell proliferation.
Graphical comparison of
colon cancer cell proliferation for cancer cells treated with a negative
control (NC), native
mi R-129, 5-FU, an exemplary modified miR-129 nucleic acid mimic of the
present
disclosure (5-FU-miR-129), and a combination of 5-FU and the exemplary miR-129
mimic
of the present disclosure (5-FU-miR-129 + 5-FU).
100441 FIGS. 5A-B. Exemplary microRNA mimics induce apoptosis in colon cancer
cells and cause cell cycle arrest. (A) Cell death was quantified by FITC-
Annexin V
apoptosis assay to show that modified miR-129 nucleic acid compositions of the
present
disclosure induce cancer cell apoptosis at significantly higher levels than
negative controls,
or ectopically expressed native miR-129 in several different colorectal cancer
cell lines. (B)
Flow cytometiy was conducted to reveal that modified miR-129 nucleic acid
compositions
(IvIimic-1) of the present disclosure increase GI cell cycle arrest at
significantly higher
levels than negative controls, or ectopically expressed native miR-129.
100451 FIG. 6. Modified MicroRNA nucleic acid compositions of the present
disclosure
eliminate chemotherapy resistant cancer stem cells. HCT116 derived colon
cancer stem
cells were treated with increasing concentrations of exemplary modified miR-
129 nucleic
acids of the present disclosure (0) or 5-FU (0). Results show that modified
miR-129 nucleic
acids killed 5-FU resistance cancer stem cells in a dose dependent manner.
11
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
100461 FIG. 7. In vivo systemic treatment with exemplary modified miR-129
nucleic acid
compositions inhibits colon cancer metastasis without toxic side effects. A
colon cancer
metastasis mouse model was established via tail vein injection of metastatic
human colon
cancer cells. Two weeks after establishing metastasis, 40 lig of a modified
miR-129 nucleic
acid composition, as set forth in SEQ ID NO: 4 was delivered by intravenous
injection with
a treatment frequency of one injection every other day for two weeks. The
exemplary
modified mi R-129 nucleic acid (mimic) was able to inhibit colon cancer
metastasis (right
panels) while negative control miRNA (left panels) had no effect. Mice treated
with
modified miR-129 nucleic acid did not exhibit any toxicity.
100471 FIGS. 8A-B. Anti cancer activity of a second exemplary modified
microRNA of
the present disclosure. (A) Representative western blots comparing the ability
of unmodified
mi R-15a (miR-15a) and a modified mi R-15a nucleic acid composition (mimic-1)
to
modulate protein expression in colon cancer cells. Modified miR-15a, as set
forth in SEQ ID
NO: 6 (mimic-1) retains the ability to regulate miR-15a targets (YAP!, BMI-1,
DCLK1 and
ECL2) and break downs TS-FdUMP in colorectal cancer cells. (B) Modified miR-
15a
(mimic-1) showed enhanced ability to inhibit colon cancer cell proliferation
in three
different colorectal cancer cell lines (HCT116, RKO, SW620) compared to
unmodified
miR-15a (miR-15a).
100481 FIG. 9. Graph showing cell cycle control for control (Negative),
unmodified miR-
15a (miR-15a) and an exemplary modified miR-15a nucleic acid compositon as set
forth in
SEQ ID NO: 6 (mimic-1). Administration of modified miR-15a nucleic acid
induced cell
cycle arrest compared to unmodified mi R-15a as shown by an increased Gl/S
ratio exhibited
by colorectal cancer cells expressing modified miR-15a when compared to cells
ectopically
expressing native miR-15a and negative controls.
100491 FIG. 10. Modified miR-15a expression reduces the ability of cancer stem
cells to
induce cancer cell colony formation. In colon cancer stem cells, expression of
unmodified
mi R-15a (miR-15a) inhibited cancer cell colony formation when compared to the
ability of
cancer stem cells provided with a non-specific control microRNA (Negative).
Treatment
12
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
with an exemplary modified miR-15a (5-FU-miR-15a) of the present disclosure
prevented
cancer cell colony formation completely.
100501 FIG. 11. Modified miR-15a is an effective anti cancer agent in vivo. A
colon
cancer metastasis mouse model was established via tail vein injection of
metastatic human
colon cancer cells. Two weeks after establishing the metastasis, 40 lig of a
modified miR-
15a nucleic acid composition as set forth in SEQ ID NO: 6 was delivered by
intravenous
injection with treatment frequency of one injection every other day for two
weeks. The
exemplary modified miR-15a nucleic acid (mimic) was able to inhibit colon
cancer
metastasis while negative control miRNA (negative) had no effect. Mice treated
with
modified miR-15a nucleic acid did not exhibit any toxicity.
100511 FIGS. 12 A-D. Exemplary modified miR-15a and miR-129 mimics of the
present
disclosure exhibit enhanced ability to inhibit human breast cancer (A549;C,D)
and
pancreatic cancer (Panc-1(A); AsPC-1(B)) cell proliferation compared to
unmodified miR-
15a (miR-15a) or unmodified miR-129 (miR-129) or cells treated with negative
controls.
100521 FIGS. 13 A-B. Exemplary modified microRNAs of the present disclosure
exhibit
an enhanced ability to inhibit human colorectal cancer cell proliferation.
Additional
exemplary modified microRNAs were tested for their ability to inhibit
colorectal cancer cell
proliferation in HCT116 human colorectal cancer cells. (A) An exemplary
modified miR-
140 mimic as set forth in SEQ ID NO: 9 was administered to human colorectal
cancer cells
and revealed an increased ability to inhibit colorectal cancer cell
proliferation when
compared to negative control microRNAs. (B) An exemplary modified miR-192
mimic as
set forth in SEQ NO: 11 was administered to human colorectal cancer cells and
revealed
an increased ability to inhibit colorectal cancer cell proliferation when
compared to negative
control microRNAs.
100531 FIGS. 14A-D. Exemplary modified microRNAs of the present disclosure
exhibit
an enhanced ability to inhibit human pancreatic and breast cancer cell
proliferation.
Additional exemplary modified microRNAs were tested for their ability to
inhibit different
types of human cancers by examining their affects on cancer cell
proliferation. An
exemplary modified miR-502 mimic as set forth in SEQ ID NO: 13 was
administered to
1.3
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
human pancreatic cancer cells (PANC1, A) and human breast cancer (A549, C) and
revealed
an increased ability to inhibit both types of cancer cell proliferation when
compared to
negative control microRNAs. Yet another exemplary modified microRNA, a miR-506
mimic as set forth in SEQ ID NO: 15 was administered to human pancreatic
cancer cells
(PANC I, B) and human breast cancer (A549, I)) and revealed an increased
ability to inhibit
both types of cancer cell proliferation when compared to negative control
microRNAs.
DETAILED DESCRIPTION OF THE DISCLOSURE
[0054] The present disclosure provides nucleic acid compositions that
incorporate one or
more halouracil molecules. Without being bound by any one particular theory,
surprisingly,
the present disclosure reveals that the replacement of uracil nucleotides
within a microRNA
oligonucleotide sequence with a 5-halouracil increases the ability of the
microRNA to
inhibit cancer, development, progression and tumorigenesis. As such, the
present disclosure
provides various nucleic acid (e.g., microRNA) compositions having 5-
halouracil molecules
incorporated in their nucleic acid sequences and methods for using the same.
The present
disclosure further provides formulations, such as pharmaceutical compositions
comprising
the modified nucleic acid compositions, and methods for treating cancers that
include
administration of the same to a subject in need thereof.
Nucleic acid compositions.
[0055] The term "microRNA" or "miRNA" or "miR" is used interchangeably to
refer to
small non-coding ribose nucleic acid (RNA) molecules that are capable of
regulating the
expression of genes through interacting with messenger RNA molecules (mRNA),
DNA or
proteins. Typically, microRNAs are composed of nucleic acid sequences of about
19-25
nucleotides (bases) and are found in mammalian cells.
[0056] The term "modified microRNA", "modified miRNA", "modified miR" or
"mimic"
are used interchangeably herein to refer to a microRNA that differs from the
native or
endogenous microRNA (unmodified microRNA) polynucleotide. More specifically,
in the
present disclosure a modified microRNA differs from the unaltered or
unmodified
microRNA nucleic acid sequence by one or more base. In some embodiments of the
present
14
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
disclosure, a modified microRNA of the present disclosure includes at least
one uracil (U)
nucleotide base replaced by a 5-halouracil. In other embodiments a modified
microRNA
includes an additional nucleotide (i.e., adenine (A), cytosine (C), uracil
(U), and guanine
(G)) and at least one uracil base that is substituted with a 5-halouracil.
100571 In one aspect of the present disclosure, nucleic acid compositions that
include a
modified microRNA nucleotide sequence having at least one uracil base (U, U
bases) that
has been replaced with a 5-halouracil, such as 5-fluorouracil (5-FU) are
described. As
further discussed herein, the nucleic acid compositions of the present
disclosure are useful,
at least, in the treatment of cancer, particularly colorectal cancer,
pancreatic cancer and
breast cancer.
100581 In some embodiments, the nucleic acid compositions contain a nucleotide
sequence
that has been modified by derivatizing at least one of the uracil nucleobases
at the 5-position
with a group that provides a similar effect as a halogen atom. In some
embodiments, the
group providing the similar effect has a similar size in weight or spatial
dimension to a
halogen atom, e.g., a molecular weight of up to or less than 20, 30, 40, 50,
60, 70, 80, 90, or
80 g/mol. In certain embodiments, the group providing a similar effect as a
halogen atom
may be, for example, a methyl group, trihalomethyl (e.g., trifluoromethyl)
group,
pseudohalide (e.g., trifluoromethanesulfonate, cyano, or cyanate) or deuterium
(D) atom.
The group providing a similar effect as a halogen atom may be present in the
absence of or
in addition to a 5-halouracil base in the microRNA nucleotide sequence.
100591 Moreover, in other embodiments, the group providing a similar effect as
a halogen
atom may be located in the native (or seed) portion and/or in an appended
portion of the
microRNA nucleotide sequence, which will be readily identified by one of
ordinary skill in
the art. In some embodiments, one or more (or all) of the above types of
groups providing a
similar effect as a halogen atom are excluded from the modified mi RNA
nucleotide
sequence. When all such alternative groups are excluded, only one or more
halogen atoms
are present as substituents in the 5-position of one or more uracil groups in
the microRNA
nucleotide sequence.
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
[0060] In certain embodiments, the modified microRNA has more than one, or
exactly
one uracil that has been replaced with a 5-halouracil.
[0061] In some embodiments, the modified microRNA nucleotide sequence includes
three, four, five, six, seven, eight or more uracil bases that have been
replaced with a 5-
halouracil.
[0062] In other embodiments, all of the uracil nucleotide bases of the
modified mRNA
have been replaced by a 5-halouracil.
[0063] In some embodiments, the 5-halouracil is, for example, 5-fluorouracil,
5-
chlorouracil, 5-bromouracil, or 5-iodouracil. In specific embodiments, the 5-
halouracil is 5-
fluorouracil
[0064] The term "miR-129" as used herein, is meant to be synonymous with the
terms
"microRNA-129" or "miRNA-129" and refers to an oligonucleotide having the
following
nucleotide sequence: CUUUUUGCGGUCUGGGCUUGC [SEQ ID NO. 1], where it is
understood that C = cytosine, U = uracil, and G = guanine bases. The foregoing
nucleotide
sequence is herein referred to as an unmodified miR-129 (i.e., "native")
sequence unless
otherwise specified. MiR-129 may also be referred to in the field as hsa-mi R-
129 or hsa-
miR-129-5p, with accession number(s) MI0000252 and M1MAT0000242. MiR-129 is
well
known and has been studied in detail. See, e.g., J. Wu et al., Cell Cycle,
(2010) 9:9, 1809-
1818. As also well known in the art, the miR-129 sequence may be modified to
produce a
"miR-129 mimic", which has a sequence modified from the native sequence, but
that retains
the known function or activity of the native miR-129. Unless otherwise stated,
all such
modified miR-129 compositions are herein considered to be within the scope of
the term
"miR-129 mimic" as used herein.
[0065] A particular modified miR-129 nucleic acid sequence (mimic) of interest
contains
two U bases (i.e., two U-containing nucleotides) covalently appended to an end
of the miR-
129 native sequence, such as in CUUUUUGCGGUCUGGGCUUGC-UU [SEQ ID NO. 3].
In the foregoing sequence, the two terminal U bases continue or extend the miR-
129 native
sequence from 21 nucleotide bases to 23 nucleotide bases. Generally, the miR-
129 mimic
16
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
contains no more than one, two, three, four, or five additional bases (i.e.,
as additional
nucleotides) covalently appended to the miR-129 native sequence, wherein the
additional
bases are independently selected from C, U, G, and C, or the additional bases
may be
exclusively U. Typically, the miR-129 is used in single-strand form, but
double-stranded
versions are also considered herein.
100661 In one embodiment, the present disclosure is directed to nucleic acid
compositions
that contain a miR-129 nucleotide sequence that has been modified by replacing
at least one
of the uracil nucleobases (i.e., U bases) with a 5-halouracil, i.e., wherein
at least one of the
U bases in the miR-129 sequence, whether in the native and/or in an appended
portion, is a
5-halouracil. The 5-halouracil can be, for example, 5-fluorouracil, 5-
chlorouracil, 5-
bromouracil, or 5-iodouracil.
100671 In a first set of embodiments, precisely one of the U bases in the miR-
129 sequence
is a 5-halouracil. In a second set of embodiments, precisely or at least two U
bases in the
miR-129 sequence are 5-halouracils. In a third set of embodiments, precisely
or at least three
U bases in the miR-129 sequence are 5-hal ouracils. In a fourth set of
embodiments,
precisely or at least four U bases in the miR-129 sequence are 5-halouracils.
In a fifth set of
embodiments, precisely or at least five U bases in the miR-129 sequence are 5-
halouracils.
In a sixth set of embodiments, all of the U bases in the miR-129 sequence,
whether in the
native and/or in an appended portion, are 5-halouracils.
100681 In a specific embodiment, the nucleic composition of the present
disclosure has a
modified microRNA nucleotide sequence of CUFU
FuFuFuFGcG,-,¨F
CUFGGGCUFUFGC
as set forth in SEQ ID NO. 4, wherein UF is a ha1ouracil, specifically 5-
fluorouracil.
100691 The U bases that are replaced with 5-halouracils in the miR-129
sequence may be
located in an unmodified part of the miR-129 sequence, as provided above, or,
in the case of
a miR-129 mimic, may be located in one or more U bases covalently appended to
the native
miR-129, as also provided above. In other embodiments, a seed portion of the
native miR-
129 nucleotide sequence, GUUUUUGC remains unmodified with a 5-halouracil while
one
or more (or all) of the remaining U bases in the remainder of the miR-129
nucleotide
sequence are replaced with the equivalent number of 5-halouracils.
17
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
[0070] For example, in a specific embodiment, the nucleic acid composition of
the present
disclosure has a modified microRNA nucleotide sequence of
CUUUUUGCGGUFCUFGGGCUFUFGC as set forth in SEQ ID NO. 5, whereby UF is a
halouracil, specifically 5-fluorouracil.
[0071] In alternative embodiments, the nucleic acid composition contains a miR-
129
nucleotide sequence that has been modified by derivatizing at least one of the
uracil (U)
nucleobases at the 5-position with a group that provides a similar effect as a
halogen atom.
In some embodiments, the group providing the similar effect has a similar size
in weight or
spatial dimension to a halogen atom, e.g., a molecular weight of up to or less
than 20, 30, 40,
50, 60, 70, 80, 90, or 80 g/mol. The group providing a similar effect as a
halogen atom may
be, for example, a methyl group, trihalomethyl (e.g., trifluoromethyl) group,
pseudohalide
(e.g., trifluoromethanesulfonate, cyano, or cyanate) or deuterium (D) atom.
The group
providing a similar effect as a halogen atom may be present in the absence of
or in addition
to a 5-halouracil base in the miR-129 nucleotide sequence. Moreover, the group
providing a
similar effect as a halogen atom may be located in the native (or seed)
portion and/or in an
appended portion of the miR-129 nucleotide sequence. In some embodiments, one
or more
(or all) of the above types of groups providing a similar effect as a halogen
atom are
excluded from the miR-129 nucleotide sequence. When all such alternative
groups are
excluded, only one or more halogen atoms are present as substituents in the 5-
position of
one or more uracil groups in the miR-129 nucleotide sequence.
[0072] In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a mi R-15a nucleotide sequence that has been
modified. In some
embodiments, the miR-15a nucleotide sequence has been modified by replacing at
least one
of the U bases with a 5-halouracil.
[0073] The term "mi R-15a", as used herein, is meant to be synonymous with the
terms
"microRNA-15a" or "miRNA-15a" and refers to an oligonucleotide having the
following
nucleotide sequence: UAGCAGCACAUAAUGGUUUGUG [SEQ ID NO. 2], where it is
understood that A = adenine, C = cytosine, U = uracil, and G = guanine bases.
The
foregoing nucleotide sequence is herein referred to as a miR-15a unmodified
(i.e., "native")
18
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
sequence unless otherwise specified. MiR-15a may also be referred to in the
field as hsa-
miR-15a or hsa-miR-15a-5p, with accession number(s) MI0000069. MiR-15a is well
known and has been studied in detail, e.g., Xie T, et al. Clin Transl Oncol.
(2015) 17(7):504-
10; and Acunzo M, and Croce CM, Chn. Chem. (2016) 62(4):655-6. As stated above
for
miR-129 mimics, methods for creating a miR-15a mimic are known by those of
ordinary
skill in the art. Unless otherwise stated, all such modified miR-15a forms are
herein
considered to be within the scope of the term "miR-15a mimic", as used herein.
[0074] Generally, a modified miR-15a (i.e., miR-15a mimic) contains no more
than one,
two, three, four, or five additional nucleotides covalently appended to the
miR-15a native
sequence, wherein the additional bases are independently selected from C, U,
G, and C, or
the additional bases may be exclusively U. Typically, the miR-15a is used in
single-strand
form, but double-stranded versions are also considered herein.
[0075] In some embodiments, at least one of the U bases in the mi R-15a
sequence,
whether in the native and/or in an appended portion, is a 5-halouracil. The 5-
halouracil can
be, for example, 5-fluorouracil, 5-chlorouracil, 5-bromouracil, or 5-
iodouracil.
100761 In a one set of embodiments, precisely one of the U bases in the miR-
15a sequence
is a 5-halouracil. In a second set of embodiments, precisely or at least two U
bases in the
mi R-15a sequence are 5-halouracils. In another set of embodiments, precisely
or at least
three U bases in the miR-15a oligonucleotide sequence are 5-halouracils. In
other
embodiments, precisely or at least four U bases in the mi R-15a sequence are 5-
halouracils.
In some embodiments, precisely or at least five U bases in the miR-15a
sequence are 5-
halouracils. In a yet other embodiments, precisely or at least six U bases in
the miR-15a
sequence are 5-halouracils. In specific embodiments, all of the U bases in the
miR-15a
sequence, whether in the native and/or in an appended portion, are 5-
halouracils.
100771 In one embodiment, the nucleic acid composition of the present
disclosure has a
modified microRNA nucleotide sequence of UFAGCAGCACAUF
AAuFGGuFuFuFGuFG
[SEQ ID NO. 6], wherein UF is a halouracil, specifically 5-fluorouracil.
19
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
100781 The U bases that are replaced with 5-halouracils in the miR-15a
sequence may be
located in an unmodified part of the miR-15a sequence, as provided above, or,
in the case of
a miR-15a mimic, may be located in one or more uracil bases that are appended
to the native
miR-15a, as also provided above.
[0079] In other embodiments, a seed portion of the native miR-15a nucleotide
sequence,
UAGCAGCA, remains unmodified with a 5-halouracil while one or more (or all) of
the
remaining U bases in the remainder of the miR-15a nucleotide sequence (non-
seed portion)
are replaced with a 5-halouracil.
[0080] In specific embodiments, the nucleic acid composition of the present
disclosure has
a modified miR-15a nucleotide sequence of UAGCAGCACAUFAAUFGGUFUFUFGUFG
[SEQ ID NO. 71, wherein UF is a halouracil, specifically 5-fluorouracil.
[0081] In certain embodiments, the nucleic acid composition contains a miR- I
5a
nucleotide sequence that has been modified by derivatizing at least one of the
uracil (U)
nucleobases at the 5-position with a group that provides a similar effect as a
halogen atom.
In some embodiments, the group providing the similar effect has a similar size
in weight or
spatial dimension to a halogen atom, e.g., a molecular weight of up to or less
than 20, 30, 40,
50, 60, 70, 80, 90, or 80 g/mol. The group providing a similar effect as a
halogen atom may
be, for example, a methyl group, trihalomethyl (e.g., trifluoromethyl) group,
pseudohalide
(e.g., trifluoromethanesulfonate, cyano, or cyanate) or deuterium (D) atom.
The group
providing a similar effect as a halogen atom may be present in the absence of
or in addition
to a 5-halouracil base in the miR-15a nucleotide sequence. Moreover, the group
providing a
similar effect as a halogen atom may be located in the native (or seed)
portion and/or in an
appended portion of the miR-15a nucleotide sequence.
100821 In some embodiments, one or more (or all) of the above types of groups
providing
a similar effect as a halogen atom are excluded from miR-15a nucleotide
sequence. When
all such alternative groups are excluded, only one or more halogen atoms are
present as
substituents in the 5-position of one or more uracil groups in the miR-15a
nucleotide
sequence.
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
100831 In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a miR-140 nucleotide sequence that has been
modified. In some
embodiments, the miR-140 nucleotide sequence has been modified by replacing at
least one
of the U bases with a 5-halouracil.
100841 The term "miR-140", as used herein, is meant to be synonymous with the
terms
"microRNA-140" or "miRNA-140" and refers to an oligonucleotide having the
following
nucleotide sequence: CAGUGGUUUUACCCUAUGGUAG [SEQ ID NO. 8], where it is
understood that A = adenine, C = cytosine, U = uracil, and G = guanine bases.
The
foregoing nucleotide sequence is herein referred to as a miR-140 unmodified
(i.e., "native")
sequence unless otherwise specified. MiR-140 may also be referred by accession
number(s)
NT 010498 or by miRBase Accession MI0000456. MiR-140 is well known and has
been
studied in detail, e.g., Zhai, H. et al., Oncotarget. (2015) 6: 19735-46. As
stated above for
exemplary mimics miR-129 and miR-15a, methods for creating a miR-140 mimic are
known
by those of ordinary skill in the art. Unless otherwise stated, all such
modified miR-140
forms are herein considered to be within the scope of the term "miR-140
mimic", as used
herein.
100851 Generally, a modified miR-140 nucleic acid (i.e., miR-140 mimic)
contains no
more than one, two, three, four, or five additional nucleotides covalently
appended to the
miR-140 native sequence, wherein the additional bases are independently
selected from C,
U, G, and C, or the additional bases may be exclusively U. Typically, the miR-
140 mimic is
used in single-strand form, but double-stranded versions are also considered
herein.
100861 In some embodiments, at least one of the U bases in the miR-140
sequence,
whether in the native and/or in an appended portion, is a 5-halouracil. The 5-
halouracil can
be, for example, 5-fluorouracil, 5-chlorouracil, 5-bromouracil, or 5-
iodouracil.
100871 In one set of embodiments, precisely one of the U bases in the miR-140
mimic
sequence is a 5-halouracil. In a second set of embodiments, precisely or at
least two U bases
in the miR-140 sequence are 5-halouracils. In another set of embodiments,
precisely or at
least three U bases in the miR-140 oligonucleotide sequence are 5-halouracils.
In other
embodiments, precisely or at least four U bases in the miR-140 sequence are 5-
halouracils
21
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
In some embodiments, precisely or at least five U bases in the miR-140 mimic
sequence are
5-halouracils. In a yet other embodiments, precisely or at least six U bases
in the miR-140
mimic sequence are 5-halouracils. In specific embodiments, all of the U bases
in the miR-
140 sequence, whether in the native and/or in an appended portion, are 5-
halouracils.
100881 In an exemplary embodiment, the nucleic acid composition of the present
disclosure has a modified miR-140 nucleotide sequence of
CAGUFGGUUUUACCCUFAUGGUFAG [SEQ ID NO. 9], wherein UF is a halouracil.
specifically 5-fluorouracil.
[0089] The U bases that are replaced with 5-halouracils in the miR-140 mimic
sequence
may be located in an unmodified part of the miR-140 sequence, as provided
above, or may
be located in one or more uracil bases that are appended to the native miR-140
sequence, as
provided above.
[0090] In other embodiments, a seed portion of the native miR-140 nucleotide
sequence,
remains unmodified with a 5-halouracil while one or more (or all) of the
remaining U bases
in the remainder of the miR-140 nucleotide sequence (non-seed portion) are
replaced with a
5-halouracil.
[0091] In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a miR-192 nucleotide sequence that has been
modified. In some
embodiments, the miR-192 nucleotide sequence has been modified by replacing at
least one
of the U bases with a 5-halouracil.
[0092] The term "miR-192", as used herein, is meant to be synonymous with the
terms
"microRNA-192", "miRNA-192" "microRNA-215", "miR-215" or "miRNA-215" and
refers to an oligonucleotide having the following nucleotide sequence:
CUGACCUAUGAAUUGACAGCC [SEQ ID NO. 10], where it is understood that A =
adenine, C = cytosine, U = uracil, and G = guanine bases. The foregoing
nucleotide
sequence is herein referred to as a miR-192 unmodified (i.e., "native")
sequence unless
otherwise specified. MiR-192 may also be referred as hsa-mir-192, has-mir-215
or by
miRBase Accession MI0000234, or MIMAT0000222. Mi R-192 is well known and has
22
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
been studied in detail, e.g., Song, B. et al., Clin. Cancer Res. (2008), 14:
8080-8086, and
Song, B. et al., Ala Cancer. (2010), 9:96 pp. 1476-4598. As stated above for
exemplary
mimics miR-129, miR-140 and miR-15a, methods for creating a miR-192 mimics are
known
by those of ordinary skill in the art. Unless otherwise stated, all such
modified miR-192
nucleic acid forms are herein considered to be within the scope of the term
"miR-192
mimic", as used herein.
100931 Generally, a modified miR-192 (i.e., miR-192 mimic) contains no more
than one,
two, three, four, or five additional nucleotides covalently appended to the
miR-192 native
sequence, wherein the additional bases are independently selected from C, U,
G, and C, or
the additional bases may be exclusively U. Typically, the miR-192 mimic is
used in single-
strand form, but double-stranded versions are also considered herein.
100941 In some embodiments, at least one of the U bases in the miR-192 or miR-
215
sequence, whether in the native and/or in an appended portion, is a 5-
halouracil. The 5-
halouracil can be, for example, 5-fluorouracil, 5-chlorouracil, 5-bromouracil,
or 5-
iodouracil.
100951 In another set of embodiments, precisely one of the U bases in the miR-
192 mimic
sequence is a 5-halouracil. In a second set of embodiments, precisely or at
least two U bases
in the miR-192 sequence are 5-halouracils. In another set of embodiments,
precisely or at
least three U bases in the miR-I92 oligonucleotide sequence are 5-halouracils.
In other
embodiments, precisely or at least four U bases in the mi R-192 sequence are 5-
halouracils.
In specific embodiments, all of the U bases in the miR-192 sequence, whether
in the native
and/or in an appended portion, are 5-ha1ouracils.
100961 In an exemplary embodiment, the nucleic acid composition of the present
disclosure has a modified miR-192 nucleotide sequence of
CUFGACCUFAUFGAAUFUFGACAGCC [SEQ ID NO. 11], wherein UF is a halouracil,
specifically 5-fluorouraciI.
100971 The U bases that are replaced with 5-halouracils in the miR-192 mimic
sequence
may be located in an unmodified part of the miR-192 sequence, as provided
above, or may
23
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
be located in one or more uracil bases that are appended to the native miR-192
sequence, as
provided above.
[0098] In other embodiments, a seed portion of the native miR-192 nucleotide
sequence
remains unmodified with a 5-halouracil while one or more (or all) of the
remaining U bases
in the remainder of the miR-192 nucleotide sequence (non-seed portion) are
replaced with a
5-halouracil or combination thereof.
[0099] In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a mi R-502 nucleotide sequence that has been
modified. In some
embodiments, the miR-502 nucleotide sequence has been modified by replacing at
least one
of the U bases with a 5-halouracil.
[00100] The term "mi R-502", as used herein, is meant to be synonymous with
the terms
"microRNA-502" or "miRNA-502" and refers to an oligonucleotide having the
following
nucleotide sequence: AUCCUUGCUAUCUGGGUGCUA [SEQ ID NO. 12], where it is
understood that A = adenine, C = cytosine, U = uracil, and G = guanine bases.
The
foregoing nucleotide sequence is herein referred to as a miR-502 unmodified
(i.e., "native")
sequence unless otherwise specified. MiR-502 may also be referred as hsa-mir-
502 or by
miRBase Accession MI0003186, or MIMAT0002873. MiR-502 is well known and has
been studied in detail, e.g., Zhai, H, et al., Oncogene . (2013), 32:12 pp.
1570-1579. As
stated above for exemplary mimics miR-129, miR-140, miR-192 and miR-15a,
methods for
creating a miR-502 mimics are known by those of ordinary skill in the art.
Unless otherwise
stated, all such modified miR-502 nucleic acid forms are herein considered to
be within the
scope of the term "miR-502 mimic", as used herein.
[00101] Generally, a modified miR-502 (i.e., miR-502 mimic) contains no more
than one,
two, three, four, or five additional nucleotides covalently appended to the
miR-502 native
sequence, wherein the additional bases are independently selected from C, U,
G, and C, or
the additional bases may be exclusively U. Typically, the miR-502 mimic is
used in single-
strand form, but double-stranded versions are also considered herein.
24
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
1001021 In some embodiments, at least one of the U bases in the miR-502
sequence,
whether in the native and/or in an appended portion, is a 5-ha1ouracil. The 5-
halouracil can
be, for example, 5-fluorouracil, 5-chlorouracil, 5-bromouracil, or 5-
iodouracil.
[00103] In another set of embodiments, precisely one of the U bases in the miR-
502 mimic
sequence is a 5-halouracil. In a second set of embodiments, precisely or at
least two U bases
in the miR-502 sequence are 5-halouracils. In another set of embodiments,
precisely or at
least three U bases in the miR-502 oligonucleotide sequence are 5-halouracils.
In other
embodiments, precisely or at least four U bases in the miR-502 sequence are 5-
halouracils.
In other embodiments, precisely or at least five U bases in the miR-502
sequence are 5-
halouracil s. In other embodiments, precisely or at least six U bases in the
miR-502 sequence
are 5-halouracils. In other embodiments, precisely or at least seven U bases
in the miR-502
sequence are 5-halouracils. In specific embodiments, all of the U bases in the
miR-502
sequence, whether in the native and/or in an appended portion, are 5-
halouracils.
[00104] In an exemplary embodiment, the nucleic acid composition of the
present
disclosure has a modified miR-502 nucleotide sequence of
AUFCCUFUFGCUAUFCUFGGGUFGCUFA [SEQ ID NO. 13], wherein UF is a halouracil,
specifically 5-fluorouracil.
[00105] The U bases that are replaced by 5-halouracils in the miR-502 mimic
sequence
may be located in an unmodified part of the miR-502 sequence, as provided
above, or may
be located in one or more uracil bases that are appended to the native miR-502
sequence, as
provided above.
1001061 In other embodiments, a seed portion of the native miR-502 nucleotide
sequence
remains unmodified with a 5-halouracil while one or more (or all) of the
remaining U bases
in the remainder of the miR-502 nucleotide sequence (non-seed portion) are
replaced by a 5-
halouracil or combination thereof
1001071 In another exemplary embodiment, the present disclosure is directed to
nucleic acid
compositions that include a miR-506 nucleotide sequence that has been
modified. In some
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
embodiments, the miR-506 nucleotide sequence has been modified by replacing at
least one
of the U bases with a 5-halouracil.
[00108] The term "miR-506", as used herein, is meant to be synonymous with the
terins
"microRNA-506" or "miRNA-506" and refers to an oligonucleotide having the
following
nucleotide sequence: UAUUCAGGAAGGUGUUACUUAA [SEQ ID NO. 14], where it is
understood that A = adenine, C = cytosine, U = uracil, and G = guanine bases.
The
foregoing nucleotide sequence is herein referred to as a miR-506 unmodified
(i.e., "native")
sequence unless otherwise specified. MiR-506 may also be referred as hsa-mir-
506 or by
miRBase Accession M10003193, or MIMAT0022701. MiR-506 is well known and has
been studied in detail, e.g., Li, J, et al., Oncotarget. (2016), 7:38 pp.
62778-62788, and Li, J.
et al., Oncogene. (2016) 35 pp. 5501-5514. As stated above for exemplary
mimics miR-
129, miR-140, miR-502, miR-192 and miR-15a, methods for creating a miR-506
mimics are
known by those of ordinary skill in the art. Unless otherwise stated, all such
modified miR-
506 nucleic acid forms are herein considered to be within the scope of the
term "miR-506
mimic", as used herein.
[00109] Generally, a modified miR-506 (i.e., miR-506 mimic) contains no more
than one,
two, three, four, or five additional nucleotides covalently appended to the
miR-506 native
sequence, wherein the additional bases are independently selected from C, U,
G, and C, or
the additional bases may be exclusively U. Typically, the miR-506 mimic is
used in single-
strand form, but double-stranded versions are also considered herein.
[00110] In some embodiments, at least one of the U bases in the miR-506
sequence,
whether in the native and/or in an appended portion, is a 5-ha1ouracil. The 5-
halouracil can
be, for example, 5-fluorouracil, 5-chlorouracil, 5-bromouracil, or 5-
iodouracil.
[00111] In another set of embodiments, precisely one of the U bases in the miR-
506 mimic
sequence is a 5-halouracil. In a second set of embodiments, precisely or at
least two U bases
in the miR-506 sequence are 5-halouracils. In another set of embodiments,
precisely or at
least three U bases in the miR-506 oligonucleotide sequence are 5-halouracils.
In other
embodiments, precisely or at least four U bases in the miR-506 sequence are 5-
halouracils.
In other embodiments, precisely or at least five U bases in the miR-506
sequence are 5-
26
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
halouracils. In other embodiments, precisely or at least six U bases in the
miR-506 sequence
are 5-halouracils. In other embodiments, precisely or at least seven U bases
in the miR-506
sequence are 5-halouracils. In specific embodiments, all of the U bases in the
miR-506
sequence, whether in the native and/or in an appended portion, are 5-
halouracils.
1001121 In an exemplary embodiment, the nucleic acid composition of the
present
disclosure has a modified miR-506 nucleotide sequence of
UFAUFUFCAGGAAGGUFGUFUFACUFUFAA [SEQ ID NO. 15], wherein UF is a
halouracil, specifically 5-fluorouracil.
1001131 The U bases that are replaced with 5-halouracils in the miR-506 mimic
sequence
may be located in an unmodified part of the miR-506 sequence, as provided
above, or may
be located in one or more uracil bases that are appended to the native miR-506
sequence, as
provided above.
1001141 In other embodiments, a seed portion of the native miR-506 nucleotide
sequence
remains unmodified with a 5-halouracil while one or more (or all) of the
remaining U bases
in the remainder of the miR-506 nucleotide sequence (non-seed portion) are
replaced with a
5-halouracil or combination thereof.
1001151 The modified microRNA nucleic acid compositions described herein can
be
synthesized using any of the well known methods for synthesizing nucleic
acids. In
particular embodiments, the nucleic acid compositions are produced by
automated
oligonucleotide synthesis, such as any of the well-known processes using
phosphoramidite
chemistry. To introduce one or more 5-halouracil bases in a modified mi R
sequence (e.g.,
miR-15a sequence, miR-140 sequence, miR-192 sequence, miR-502 sequence, miR-
506
sequence or miR-129 sequence), a 5-halouracil nucleoside phosphoramidite can
be included
as a precursor base, along with the phosphoramidite derivatives of nucleosides
containing
natural bases (e.g., A, U, G, and C) to be included in the nucleic acid
sequence.
1001161 In some embodiments, the nucleic acid compositions of the present
disclosure may
be produced biosynthetically, such as by using in vitro RNA transcription from
plasmid,
PCR fragment, or synthetic DNA templates, or by using recombinant (in vivo)
RNA
27
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
expression methods. See, e.g., C. M. Dunham et al., Nature Methods, (2007)
4(7), pp. 547-
548. The microRNA sequence (e.g., miR-15a sequence, miR-140 sequence, miR-192
sequence, miR-502 sequence, miR-506 sequence or miR-129 sequence)may be
further
chemically modified such as by functionalizing with polyethylene glycol (PEG)
or a
hydrocarbon or a targeting agent, particularly a cancer cell targeting agent,
such as folate, by
techniques well known in the art. To include such groups, a reactive group
(e.g., amino,
aldehyde, thiol, or carboxylate group) that can be used to append a desired
functional group
may first be included in the oligonucleotide sequence. Although such reactive
or functional
groups may be incorporated onto the as-produced nucleic acid sequence,
reactive or
functional groups can be more facilely included by using an automated
oligonucleotide
synthesis in which non-nucleoside phosphoramidites containing reactive groups
or reactive
precursor groups are included.
Modjfied Nucleic Acid Formulations
[00117] In another aspect, the present disclosure is directed to formulations
of the modified
nucleic acid compositions described herein. For example, the present nucleic
acid
compositions can be formulated for pharmaceutical uses. In certain
embodiments, a
formulation is a pharmaceutical composition containing a nucleic acid
composition
described herein and a pharmaceutically acceptable carrier. In other
embodiments, a
formulation of the present disclosure comprises a modified miR-129 nucleic
acid, a
modified miR-15a nucleic acid, a modified miR-140 nucleic acid, a modified miR-
192
nucleic acid, a modified miR-502, a modified miR-506 nucleic acid or a
combination thereof
and a pharmaceutically acceptable carrier. More specifically, the modified
microRNA
nucleic acids set forth in the following nucleotide sequences can be
formulated for
pharmaceutical application and use; CUFUFUFUFUFGCGGUFCUFGGGCUFUFGC [SEQ ID
NO. 4], CUUUUUGCGGUFCUFGGGCUFUFGC [SEQ ID NO. 5],
UFAGCAGCACAUFAAUFGGUFUFUFGUFG [SEQ ID NO.6],
UAGCAGCACAUFAAUFGGUFUFUFGUFG [SEQ ID NO. 7],
CAGUFGGUUUUACCCUFAUGGUFAG [SEQ ID NO. 9],
CUFGACCUFAUFGAAUFUFGACAGCC [SEQ ID NO. 11],
28
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
AUFCCUFUFGCUAUFCUFGGGUFGCUFA [SEQ ID NO. 13], and
UFAUFUFCAGGAAGGUFGUFUFACUFUFAA [SEQ ID NO. 15].
[00118] The term "pharmaceutically acceptable carrier" is used herein as
synonymous with
a pharmaceutically acceptable diluent, vehicle, or excipient. Depending on the
type of
pharmaceutical composition and intended mode of administration, the nucleic
acid
composition may be dissolved or suspended (e.g., as an emulsion) in the
pharmaceutically
acceptable carrier. The pharmaceutically acceptable carrier can be any of
those liquid or
solid compounds, materials, compositions, and/or dosage forms which are,
within the scope
of sound medical judgment, suitable for use in contact with tissues of a
subject. The carrier
should be "acceptable" in the sense of being not injurious to the subject it
is being provided
to and is compatible with the other ingredients of the formulation, i.e., does
not alter their
biological or chemical function.
1001191 Some, non-limiting examples, of materials which can serve as
pharmaceutically
acceptable carriers include: sugars, such as lactose, glucose and sucrose;
starches, such as
corn starch and potato starch; cellulose and its derivatives, such as sodium
carboxymethyl
cellulose, ethyl cellulose and cellulose acetate; gelatin; talc; waxes; oils,
such as peanut oil,
cottonseed oil, safflower oil, sesame oil, olive oil, corn oil and soybean
oil; glycols, such as
ethylene glycol and propylene glycol; polyols, such as glycerin, sorbitol,
mannitol and
polyethylene glycol; esters, such as ethyl oleate and ethyl laurate; agar;
buffering agents;
water; isotonic saline; pH buffered solutions; and other non-toxic compatible
substances
employed in pharmaceutical formulations. The pharmaceutically acceptable
carrier may also
include a manufacturing aid (e.g., lubricant, talc magnesium, calcium or zinc
stearate, or
stearic acid), a solvent, or encapsulating material. If desired, certain
sweetening and/or
flavoring and/or coloring agents may be added. Other suitable excipients can
be found in
standard pharmaceutical texts, e.g. in "Remington's Pharmaceutical Sciences",
The Science
and Practice of Pharmacy, 19th Ed. Mack Publishing Company, Easton, Pa.,
(1995).
[00120] In some embodiments, the pharmaceutically acceptable carrier may
include
diluents that increase the bulk of a solid pharmaceutical composition and make
the
pharmaceutical dosage form easier for the patient and caregiver to handle.
Diluents for solid
29
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
compositions include, for example, microcrystalline cellulose (e.g. Avicel*),
microfine
cellulose, lactose, starch, pregelatinized starch, calcium carbonate, calcium
sulfate, sugar,
dextrates, dextrin, dextrose, dibasic calcium phosphate dihydrate, tribasic
calcium
phosphate, kaolin, magnesium carbonate, magnesi urn oxide, maltodextrin,
mannitol,
polymethacrylates (e.g. Eudragie), potassium chloride, powdered cellulose,
sodium
chloride, sorbitol and talc.
[00121] The nucleic acid compositions of the present disclosure may be
formulated into
compositions and dosage forms according to methods known in the art. In
certain
embodiments, the formulated compositions may be specially formulated for
administration
in solid or liquid form, including those adapted for the following: (1) oral
administration, for
example, tablets, capsules, powders, granules, pastes for application to the
tongue, aqueous
or non-aqueous solutions or suspensions, drenches, or syrups; (2) parenteral
administration,
for example, by subcutaneous, intramuscular or intravenous injection as, for
example, a
sterile solution or suspension; (3) topical application, for example, as a
cream, ointment or
spray applied to the skin, lungs, or mucous membranes; or (4) intravaginally
or intrarectally,
for example, as a pessary, cream or foam; (5) sublingually or buccally; (6)
ocularly; (7)
transdermally; or (8) nasally.
[00122] In some embodiments, the formulations of the present disclosure
include a solid
pharmaceutical agent that is compacted into a dosage form, such as a tablet,
may include
excipients whose functions include helping to bind the active ingredient and
other excipients
together after compression. Binders for solid pharmaceutical compositions
include acacia,
alginic acid, carbomer (e.g. carbopol), carboxymethylcellulose sodium,
dextrin, ethyl
cellulose, gelatin, guar gum, hydrogenated vegetable oil, hydroxyethyl
cellulose,
hydroxypropyl cellulose (e.g. K1uce143)), hydroxypropyl methyl cellulose (e.g.
MethoceM,
liquid glucose, magnesium aluminum silicate, maltodextrin, methylcellulose,
polymethacrylates, povidone (e.g. Kollidon , Plasdone), pregelatinized starch,
sodium
alginate and starch.
[00123] The dissolution rate of a compacted solid pharmaceutical composition
in a
subject's stomach may be increased by the addition of a disintegrant to the
composition.
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
Disintegrants include alginic acid, carboxymethylcellulose calcium,
carboxymethylcellulose
sodium (e.g. Ac-Di-Sole, Primellosee), colloidal silicon dioxide,
croscarmellose sodium,
crospovidone (e.g. Kollidoe), Polyplasdoner), guar gum, magnesium aluminum
silicate,
methyl cellulose, microcrystalline cellulose, polacrilin potassium, powdered
cellulose,
pregelatinized starch, sodium alginate, sodium starch glycolate (e.g.
Explotabe) and starch.
1001241 Therefore, in certain embodiments, glidants can be added to
formulations to
improve the flowability of a non-compacted solid agent and to improve the
accuracy of
dosing. Excipients that may function as glidants include colloidal silicon
dioxide,
magnesium trisilicate, powdered cellulose, starch, talc and tribasic calcium
phosphate.
1001251 When a dosage form such as a tablet is made by the compaction of a
powdered
composition, the composition is subjected to pressure from a punch and dye.
Some
excipients and active ingredients have a tendency to adhere to the surfaces of
the punch and
dye, which can cause the product to have pitting and other surface
irregularities. A lubricant
can be added to the composition to reduce adhesion and ease the release of the
product from
the dye. Lubricants include magnesium stearate, calcium stearate, glyceryl
monostearate,
glyceryl palmitostearate, hydrogenated castor oil, hydrogenated vegetable oil,
mineral oil,
polyethylene glycol, sodium benzoate, sodium lauryl sulfate, sodium stearyl
fumarate,
stearic acid, talc and zinc stearate.
1001261 A formulated pharmaceutical composition for tableting or capsule
filling can be
prepared by wet granulation. In wet granulation, some or all of the active
ingredients and
excipients in powder form are blended and then further mixed in the presence
of a liquid,
typically water that causes the powders to clump into granules. The granulate
is screened
and/or milled, dried and then screened and/or milled to the desired particle
size. The
granulate may then be tableted, or other excipients may be added prior to
tableting, such as a
glidant and/or a lubricant. A tableting composition may be prepared
conventionally by dry
blending. For example, the blended composition of the actives and excipients
may be
compacted into a slug or a sheet and then comminuted into compacted granules.
The
compacted granules may subsequently be compressed into a tablet.
31
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
1001271 In other embodiments, as an alternative to dry granulation, a blended
composition
may be compressed directly into a compacted dosage form using direct
compression
techniques. Direct compression produces a more uniform tablet without
granules.
Excipients that are particularly well suited for direct compression tableting
include
microcrystalline cellulose, spray dried lactose, dicalcium phosphate dihydrate
and colloidal
silica. The proper use of these and other excipients in direct compression
tableting is known
to those in the art with experience and skill in particular formulation
challenges of direct
compression tableting. A capsule filling may include any of the aforementioned
blends and
granulates that were described with reference to tableting; however, they are
not subjected to
a final tableting step
1001281 In liquid pharmaceutical compositions of the present disclosure, the
agent and any
other solid excipients are dissolved or suspended in a liquid carrier such as
water, water-for-
injection, vegetable oil, alcohol, polyethylene glycol, propylene glycol or
glycerin. Liquid
pharmaceutical compositions may contain emulsifying agents to disperse
uniformly
throughout the composition an active ingredient or other excipient that is not
soluble in the
liquid carrier. The liquid formulation may be used as an injectable, enteric,
or emollient
type of formulation. Emulsifying agents that may be useful in liquid
compositions of the
present invention include, for example, gelatin, egg yolk, casein,
cholesterol, acacia,
tragacanth, chondrus, pectin, methyl cellulose, carbomer, cetostearyl alcohol
and cetyl
alcohol.
1001291 In some embodiments, liquid pharmaceutical compositions of the present
disclosure may also contain a viscosity enhancing agent to improve the mouth-
feel of the
product and/or coat the lining of the gastrointestinal tract. Such agents
include acacia,
alginic acid bentonite, carbomer, carboxymethylcellulose calcium or sodium,
cetostearyl
alcohol, methyl cellulose, ethylcellulose, gelatin guar gum, hydroxyethyl
cellulose,
hydroxypropyl cellulose, hydroxypropyl methyl cellulose, maltodextrin,
polyvinyl alcohol,
povidone, propylene carbonate, propylene glycol alginate, sodium alginate,
sodium starch
glycolate, starch tragacanth and xanthan gum. In other embodiments, the liquid
composition
32
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
of the present disclosure may also contain a buffer, such as gluconic acid,
lactic acid, citric
acid or acetic acid, sodium gluconate, sodium lactate, sodium citrate, or
sodium acetate.
[00130] Sweetening agents, such as sorbitol, saccharin, sodium saccharin,
sucrose,
aspartame, fructose, mannitol and invert sugar, may be added to certain
formulations of the
present disclosure to improve the taste. Flavoring agents and flavor enhancers
may make
the dosage form more palatable to the patient. Common flavoring agents and
flavor
enhancers for pharmaceutical products that may be included in the composition
of the
present disclosure include maltol, vanillin, ethyl vanillin, menthol, citric
acid, fumaric acid,
ethyl maltol and tartaric acid.
[00131] Preservatives and chelating agents, such as alcohol, sodium benzoate,
butylated
hydroxy toluene, butylated hydroxyanisole and ethylenediamine tetraacetic
acid, may be
added at levels safe for ingestion to improve storage stability. Solid and
liquid compositions
may also be dyed using any pharmaceutically acceptable colorant to improve
their
appearance and/or facilitate patient identification of the product and unit
dosage level.
[00132] A dosage formulation of the present disclosure may be a capsule
containing the
composition, for example, a powdered or granulated solid composition of the
disclosure,
within either a hard or soft shell. The shell may be made from gelatin and
optionally contain
a plasticizer such as glycerin and sorbitol, and an pacifying agent or
colorant.
Methods for Treating Cancer
[00133] As stated above, the modified microRNA nucleic acid compositions of
the present
disclosure and formulations thereof show unexpected and exceptional anticancer
activity
when compared to that exhibited by a native microRNA and/or a known cancer
therapy
(chemotherapy), such as 5-FU. Therefore, another aspect of the present
disclosure provides a
method for treating cancer in a mammal by administering to the mammal an
effective
amount of one or more of the modified microRNA nucleic acid compositions of
the present
disclosure, or formulations thereof.
[00134] As shown in FIGS. 2A and 8A, exemplary modified microRNA nucleic acids
of
the present disclosure, i.e., modified miR-15a and modified MiR-129 suppress
BCI.2
33
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
expression and activity in the cancer cells of a subject, which results in an
increased amount
of available pro-apoptotic proteins which ultimately leads to increased cancer
cell death.
miR-129, for example, regulates apoptosis by directly targeting BCL2 as well
as by
impacting other critical cell death-related proteins. Further, FIG. 2A shows
that miR-129
reduces the expression, and thus activity of E2F3, a transcription factor
protein that regulates
cell cycle progression and reduces the expression or activity of thymidylate
synthase (TS)
protein levels, which results in increased cellular proliferation and
increased efficacy of
chemotherapeutic agents.
[00135] Other exemplary microRNA's such as modified miR-506, miR-140, miR-192
and
miR-502 also modulate cancer cell proliferation and cancer cell apoptosis, as
shown in
FIGS. 13A-B and 14A-D.
100136] In fact, FIGS. 7 and 11 show that intravenous treatment with two
exemplary
modified microRNA's of the present disclosure (e.g., modified miR-129 and
modified miR-
15a) effectively treat colorectal cancer by inhibiting tumor growth and
development.
[00137] Generally, the methods for treating cancer of the present disclosure
include
administering a nucleic acid composition of the present disclosure (e.g., a
modified
microRNA, such as modified miR-129 nucleic acid, a modified miR-15a nucleic
acid, a
modified mi R-140 nucleic acid, a modified miR-192 nucleic acid, a modified
miR-502, a
modified miR-506 nucleic acid or a combination thereof) to a subject. In
certain
embodiments, the nucleic acid composition can be administered as a formulation
that
includes a nucleic acid composition and a carrier. In other embodiments, the
nucleic acid
composition of the present disclosure can be administered in the absence of a
carrier (i.e.,
naked).
[00138] The term "subject" as used herein refers to any mammal. The mammal can
be any
mammal, although the methods herein are more typically directed to humans. The
phrase
'subject in need thereof' as used herein is included within the term subject
and refers to any
mammalian subject in need of a treatment, particularly cancer or has a
medically determined
elevated risk of a cancerous or pre-cancerous condition. In specific
embodiments, the
subject includes a human cancer patient. In some embodiments, the subject has
colorectal
34
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
cancer or has a medically determined elevated risk of getting colorectal
cancer. In other
embodiments, the subject has pancreatic cancer, or has a medically determined
elevated risk
of getting pancreatic cancer such as, for example, being diagnosed with
chronic pancreatitis.
In yet other embodiments, a subject of the present disclosure has lung cancer,
or has a
medically determined elevated risk of getting lung cancer.
1001391 The terms "treatment" "treat" and "treating" are synonomous with the
term "to
adminster an effective amount". These terms shall mean the medical management
of a
subject with the intent to cure, ameliorate, stabilize, reduce one or more
symptoms of or
prevent a disease, pathological condition, or disorder such as cancer. These
terms, are used
interchangeably and include the active treatment, that is, treatment directed
specifically
toward the improvement of a disease, pathological condition, or disorder, and
also include
causal treatment, that is, treatment directed toward removal of the cause of
the associated
disease, pathological condition, or disorder. In addition, treating includes
palliative
treatment, that is, treatment designed for the relief of symptoms rather than
the curing of the
disease, pathological condition, or disorder; preventative treatment, that is,
treatment
directed to minimizing or partially or completely inhibiting the development
of the
associated disease, pathological condition, or disorder; and supportive
treatment, that is,
treatment employed to supplement another specific therapy directed toward the
improvement of the associated disease, pathological condition, or disorder. It
is understood
that treatment, while intended to cure, ameliorate, stabilize, or prevent a
disease,
pathological condition, or disorder, need not actually result in the cure,
ameliorization,
stabilization or prevention. The effects of treatment can be measured or
assessed as
described herein and as known in the art as is suitable for the disease,
pathological
condition, or disorder involved. Such measurements and assessments can be made
in
qualitative and/or quarititiative terms. Thus, for example, characteristics or
features of a
disease, pathological condition, or disorder and/or symptoms of a disease,
pathological
condition, or disorder can be reduced to any effect or to any amount.
1001401 In certain embodiments, the nucleic acid compositions of the present
disclosure are
used to treat cancer, such as colorectal cancer.
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
1001411 The term "cancer", as used herein, includes any disease caused by
uncontrolled
division and growth of abnormal cells, including, for example, the malignant
and metastatic
growth of tumors. The term "cancer" also includes pre-cancerous conditions or
conditions
characterized by an elevated risk of a cancerous or pre-cancerous condition.
Thus, the
treatment of cancer is herein also considered to include a method for the
prevention of
cancer or a method for preventing a pre-cancerous condition from transforming
into a
cancerous condition or into a completely non-cancerous condition. The cancer
or pre-cancer
(neoplastic condition) can be located in any part of the body, including the
internal organs
and skin. Some examples of applicable body parts containing cancer cells
include the colon,
rectum (including anus), stomach, esophageal, digestive organs, lungs,
pancreas, and liver.
The cancer or neoplasm can also include the presence of one or more
carcinomas, sarcomas,
lymphomas, blastomas, or teratomas (germ cell tumors). In some embodiments,
the cancer
may also be a form of leukemia.
1001421 In particular embodiments, the nucleic acid compositions described
herein are used
to treat colorectal (i.e., colon or rectal), pancreatic or lung cancer in any
of its stages, as
further described below. As is well know, cancer spreads through a subject by
invading the
normal, non-cancerous tissue surrounding the tumor, via the lymph nodes and
vessels, and
by blood after the tumor invades the veins, capillaries and arteries of a
subject. When
cancer cells break away from the primary tumor ("metastasize"), secondary
tumors arise
throughout an afflicted subject forming metastatic lesions.
1001431 For example, there are four stages of colorectal cancer, which are
generally
characterized by the degree of metastasis. In Stage 0 or carcinoma in situ,
abnormal
potentially cancerous cells are found in the mucosa (innermost layer) of the
colon wall. In
Stage I, cancerous cells have formed in the mucosa of the colon wall and have
spread to the
submucosa (layer of tissue under the mucosa) and may have spread to the muscle
layer of
the colon wall. Stage II is composed of three subclasses: Stage IIA, wherein
the cancerous
tissue has spread through the muscle layer of the colon wall to the serosa
(outermost layer)
of the colon wall; Stage BB, wherein the tumor has spread through the serosa
of the colon
wall but has not spread to nearby organs; and Stage IIC, wherein the cancer
has spread
36
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
through the serosa of the colon wall and invaded nearby organs. Stage Ill is
also divided
into three subclasses: Stage IIIA, wherein the cancer may have spread through
the mucosa of
the colon wall to the submucosa and muscle layer, and has spread to one to
three nearby
lymph nodes or tissues near the lymph nodes; or the cancer has spread through
the mucosa
to the submucosa and four to six nearby lymph nodes; Stage IIIB, wherein the
tumor has
spread through the muscle layer of the colon wall to the serosa or has spread
through the
serosa but not to nearby organs and the cancer has spread to one to three
nearby lymph
nodes or to tissues near the lymph nodes; or has spread to the muscle layer or
to the serosa,
and to four to six nearby lymph nodes; or has spread through the mucosa to the
submucosa
and may have spread to the muscle layer and has spread to seven or more nearby
lymph
nodes. In Stage BIC colorectal cancer, the tumor has spread through the serosa
of the colon
wall but not to nearby organs and the cancer has spread to four to six nearby
lymph nodes;
or the cancer has spread through the muscle layer to the serosa or has spread
through the
serosa but not to nearby organs and the cancer has spread to seven or more
nearby lymph
nodes; or the cancer has spread through the serosa to nearby organs and to one
or more
nearby lymph nodes or to tissues near the lymph nodes. Finally, Stage IV colon
cancer is
divided into two subclasses: Stage IVA, wherein the cancer has spread through
the colon
wall and into nearby organs and one organ that is not near the colon or to a
distant lymph
node; and Stage IVB, wherein the cancer has spread through the colon wall and
into nearby
organs and more than one organ that is not near the colon or into the lining
of the abdominal
wall.
1001441 Yet another example of tumor staging includes the Dukes classification
system for
colorectal cancer. Here, the stages are identified as Stage A, wherein the
tumor is confined
to the intestinal wall; Stage B, wherein the tumor exhibits invasion through
the bowel but
has not invaded the lymph nodes; Stage C, wherein cancerous cells or tissue is
found within
the lymph nodes of a subject; and Stage D, wherein the tumor exhibits
widespread
metastases into several organs of the subject.
1001451 The Astler Coller classification system may alternatively be used.
Here, Stage A
colorectal cancer is identified as cancer that is only present in the mucosa
of the intestine;
37
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
Stage B1 whereby the tumor extends into muscularis propria but does not
penetrate through
it and the tumor has not metastasized into the lymph nodes, Stage B2
colorectal cancer is
denoted by a tumor that penetrates through muscularis propria and the tumor
has not
metastasized into the lymph nodes; Stage Cl is characterized by a tumor that
extends
into muscularis propria, but does not penetrate through it and the tumor has
metastasized
into the lymph nodes; Stage C2 colorectal cancer is classified as a tumor that
penetrates
through the muscularis propria where the tumor has metastasized into the lymph
nodes; and
Stage D describes a tumor that has metastasized throughout the organism or
subject.
[00146] In some embodiments, the treatment methods of the present disclosure
are more
particularly directed to cancer subjects exhibiting reduced levels of miR-129
expression,
miR-15a expression, miR-506 expression, miR-502, miR-140 or a combination
thereof. In
this respect, it is known that miR-15a is down-regulated in cancers. See, for
example, R
I Aqeilan, et al., Cell Death and Differentiation (2010) 17, pp. 215-220.
Further, it is
known that cancerous cells having reduced levels of miR-129 expression are
resistant to 5-
fluorouracil, as described, e.g., in U.S. Application Pub. No. 2016/0090636,
the contents of
which are incorporated by reference in their entirety. Additionally, it is
known that
pancreatic cancer cells exhibit reduced levels of mi R-506. See, e.g., Li, J,
et al. Oncogene.
35 pp. 5501-5514.
[00147] In yet another example, the microRNA mimics of the present disclosure
are used to
treat pancreatic cancer. Pancreatic cancer arises from precursor lesions
called pancreatic
intraepithelial neoplasia, or Pan1Ns. These lesions are typically located in
the small ducts of
the exocrine pancreas, and depending on the extent of cytologic atypia may be
classified as
low-grade dysplasia, moderate dysplasia or high-grade dysplasia lesions. Such
lesions
typically show that activating mutations in the KRAS gene present, along with
certain
inactivating mutations in CDKN2A, TP53 and SMAD4. Collectively, these genetic
mutations lead to the formation of an infiltrating cancer. Pancreatic cancer
is staged based
on size of the primary tumor and whether it has grown outside of the pancreas
into
surrounding organs; whether the tumor has spread to the nearby lymph nodes,
and whether it
has metastasized to other organs of the body (e.g., liver, lungs, abdomen).
This information
38
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
is then combined and used to provide the specific stage, i.e., 0, 1A, 1B, 2A,
2B, 3 and 4. For
stage zero (0), the pancreatic tumor is confined to the top layers of
pancreatic duct cells and
has not invaded deeper tissues. The primary tumor has not spread outside of
the pancreas
such as in pancreatic carcinoma in situ or pancreatic intraepithelial
neoplasia III. A stage
lA pancreatic tumor is typically confined to the pancreas and is 2 cm across
or smaller.
Further a stage IA pancreatic tumor has not spread to nearby lymph nodes or
distant sites. A
stage 1B pancreatic tumor confined to the pancreas and is larger than 2 cm
across. A stage
1B pancreatic tumor has not spread to nearby lymph nodes or distant sites.
Stage 2A
pancreatic tumors exhibit a tumor growing outside the pancreas but not into
major blood
vessels or nerves, but the cancer has not spread to nearby lymph nodes or
distant sites. A
subject exhibiting stage 2B pancreatic cancer presents a tumor is either
confined to the
pancreas or growing outside the pancreas but not into major blood vessels or
nerves, but has
spread to nearby lymph nodes. A subject exhibiting stage 3 pancreatic cancer
presents a
tumor that is growing outside the pancreas into major blood vessels or nerves,
but has spread
to distant sites. Stage 4 pancreatic cancer has metastasized to distant cites,
lymph nodes and
organs.
1001481 In another example, the modified microRNA nucleic acid compositions of
the
present disclosure are used to treat lung cancer. The present methods include
the treatment
of non-small cell lung cancers, such as squamous cell carcinoma,
adenocarcinoma, and large
cell carcinoma. Lung cancer often arises from malignancies in the bronchi of
the lungs and
spreads to other parts of the body, such as lymph nodes. For example, in the
case of small
cell lung cancer, a cancerous lesion is often found in once lung then spreads
to the second
lung, the fluid surrounding the lungs (pleura) or neighboring organs. Lung
cancer is staged
based on size of the primary tumor and whether it has grown outside of the
lung into lymph
nodes and whether it has metastasized to other organs of the body (e.g.,
bones, liver, breast,
brain). This information is then combined and used to provide the specific
stage, i.e., 0, 1, 2,
3 and 4. For stage zero (0), i.e., carcinoma in situ, the cancer is small in
size and has not
spread into deeper lung tissues or outside the lungs. Stage 1 lung cancer
shows cancerous
cells present in the underlying lung tissues, but the lymph nodes remain
unaffected. Stage 2
lung cancer reveals that the cancer has spread to nearby lymph nodes or into
the chest wall.
39
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
Stage 3 lung cancer is classified by a continuous spread from the lungs to the
lymph nodes
or to nearby structures and organs, such as the heart, trachea and esophagus.
Stage 4 lung
cancer exhibits metastasized cancer throughout the body, which may affect the
liver, bones
or brain.
1001491 The nucleic acid compositions according to the present disclosure may
be
administered by any of the routes commonly known in the art. This includes,
for example,
(1) oral administration; (2) parenteral administration, for example, by
subcutaneous,
intramuscular or intravenous injection; (3) topical administration; or (4)
intravaginal or
intrarectal administration; (5) sublingual or buccal administration; (6)
ocular administration;
(7) transdermal administration; (8) nasal administration; and (9)
administration directly to
the organ or cells in need thereof
1001501 The amount (dosage) of nucleic acid compositions of the present
disclosure being
administered depends on several factors, including the type and stage of the
cancer, presence
or absence of an auxiliary or adjuvant drug, and the subject's weight, age,
health, and
tolerance for the agent. Depending on these various factors, the dosage may
be, for
example, about 2 mg/kg of body weight, about 5 mg/kg of body weight, about 10
mg/kg of
body weight, about 15 mg/kg of body weight, about 20 mg/kg of body weight,
about 25
mg/kg of body weight, about 30 mg/kg of body weight, about 40 mg/kg of body
weight,
about 50 mg/kg of body weight, about 60 mg/kg of body weight, about 70 mg/kg
of body
weight, about 80 mg/kg of body weight, about 90 mg/kg of body weight, about
100 mg/kg
of body weight, about 125 mg/kg of body weight, about 150 mg/kg of body
weight, about
175 mg/kg of body weight, about 200 mg/kg of body weight, about 250 mg/kg of
body
weight, about 300 mg/kg of body weight, about 350 mg/kg of body weight, about
400 mg/kg
of body weight, about 500 mg/kg of body weight, about 600 mg/kg of body
weight, about
700 mg/kg of body weight, about 800 mg/kg of body weight, about 900 mg/kg of
body
weight, or about 1000 mg/kg of body weight, wherein the term "about" is
generally
understood to be within 10%, 5%, 2%, or 1% of the indicated value. The
dosage may also
be within a range bounded by any two of the foregoing values. Routine
experimentation
may be used to determine the appropriate dosage regimen for each patient by
monitoring the
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
compound's effect on the cancerous or pre-cancerous condition, or effect on
microRNA
expression level or activity (e.g., miR-15a, miR-129, miR-140, miR-192, miR-
502, miR-
506), or effect on BCL2 level or activity, or effect on TS level or activity,
or effect on E2F3
level or the disease pathology, all of which can be frequently and easily
monitored according
to methods known in the art. Depending on the various factors discussed above,
any of the
above exemplary doses of nucleic acid can be administered once, twice, or
multiple times
per day.
1001511 The ability of the nucleic acid compositions described herein, and
optionally, any
additional chemotherapeutic agent for use with the current methods can be
determined using
pharmacological models well know in the art, such as cytotoxic assays,
apoptosis staining
assays, xenograft assays, and binding assays.
[00152] The nucleic acid compositions described herein may or may not also be
co-
administered with one or more chemotherapeutic agents, which may be auxiliary
or adjuvant
drugs different from a nucleic composition described herein.
1001531 As used herein, "chemotherapy" or the phrase a "chemotherapeutic
agent" is an
agent useful in the treatment of cancer. Chemotherapeutic agents useful in
conjunction with
the methods described herein include any agent that modulates BCL2, E2F3 or
TS, either
directly or indirectly. Examples of chemotherapeutic agents include: anti-
metabolites such
as methotrexate and fluoropyrimidine-based pyrimidine antagonist, 5-
fluorouracil (5-FU)
(Carac cream, Efudex , Fluoroplex , Adrucile) and S-1; antifolates, including
polyglutamatable antifolate compounds; raltitrexed (Tomudexe), GW1843 and
pemetrexed
(Alimtae) and non-polyglutamatable antifolate compounds; nolatrexed
(Thymitaq0),
plevitrexed, BGC945; folic acid analogs such as denopterin, methotrexate,
pteropterin,
trimetrexate; and purine analogs such as fludarabine, 6-mercaptopurine,
thiamiprine,
thioguanine; pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine,
carmofur,
cytarabine, dideoxyuridine, doxifluridine, enocitabine, floxuridine. In a
specific embodiment
of the current disclosure, the chemotherapeutic agent is a compound capable of
inhibiting
the expression or activity of genes, or gene products involved in signaling
pathways
implicated in aberrant cell proliferation or apoptosis, such as, for example,
YAP1, BMI1,
41
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
DCLK1, BCL2, thymidylate synthase or E2F3; and pharmaceutically acceptable
salts, acids
or derivatives of any of the above.
[00154] In some embodiments, the chemotherapeutic agent is an anti-cancer
drug, or a
tissue sensitizer or other promoter for an anti-cancer drug. In some
embodiments, the co-
drug may be another nucleic acid, or another miRNA, such as a microRNA mimic
of the
present disclosure, gemctiabine or free 5-FU.
[00155] In a specific embodiment, the other nucleic acid is a short hairpin
RNA (shRNA),
siRNA, or nucleic acid complementary to a portion of the BCL2 3'UTR.
[00156] In other embodiments, the chemotherapy may be any of the following
cancer
drugs, such as one or more of methotrexate, doxorubicin, cyclophosphamide, cis-
platin,
oxaliplatin, bleomycine, vinblastine, gemcitabine, vincristine, epirubicin,
folinic acid,
paclitaxel, and docetaxel. The chemotherapeutic agent may be administered
before, during,
or after commencing therapy with the nucleic acid composition.
[00157] In some embodiments, the chemotherapeutic agent is a co-drug.
[00158] E2F transcription factor 3, E2F3 (RefSeq NG_029591.1,
N/VI_001243076.2,
NP 001230005.1) is a transcription factor that binds DNA and interacts with
effector
proteins, including but not limited to, retinoblastoma protein to regulate the
expression of
genes involved in cell cycle regulation. Therefore, any drug that inhibits the
expression of
E2F3 may be considered herein as a co-drug.
[00159] B-cell lymphoma 2 (BCL2), (RefSeq NG_009361.1, NM_000633, NP_000624)
including isoform a (NM_000633.2, NP 000624.2) and fi NM 000657.2, NP
_000648.2
thereof, are encoded by the BcI-2 gene, which is a member of the BCL2 family
of regulator
proteins that regulate mitochondria regulated cell death via the intrinsic
apoptosis pathway.
BCL2 is an integral outer mitochondrial membrane protein that blocks the
apoptotic death of
cell cells by binding BAD and BAK proteins. Non-limiting examples of BCL2
inhibitors
include antisense oligonucleotides, such as Oblimersen (Genasense; Genta
BH3
mimetic small molecule inhibitors including, ABT-737 (Abbott Laboratories,
Inc.), ABT-
42
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
199 (Abbott Laboratories, Inc.), and Obatoclax (Cephalon Inc.). Any drug that
inhibits the
expression of BCL2 may be considered herein as a co-drug.
1001601 Thymidylate synthase (RefSeq: NG_028255.1, NM_001071.2, NP_001062.1)
is a
ubiquitous enzyme, which catalyses the essential methylation of dUMP to
generate dTMP,
one of the four bases which make up DNA. The reaction requires CH Ha-folate as
a
cofactor, both as a methyl group donor, and uniquely, as a reductant. The
constant
requirement for CH H4-folate means that thymidylate synthase activity is
strongly linked to
the activity of the two enzymes responsible for replenishing the cellular
folate pool:
dihydrofolate reductase and serine transhydroxymethylase. Thymidylate synthase
is a
homodimer of 30-35kDa subunits. The active site binds both the folate cofactor
and the
dUMP substrate simultaneously, with the dUMP covalently bonded to the enzyme
via a
nucleophilic cysteine residue (See, Carreras et al, Annu. Rev. Biochem.,
(1995) 64:721-
762). The thymidylate synthase reaction is a crucial part of the pyrimidine
biosynthesis
pathway which generates dCTP and dTTP for incorporation into DNA. This
reaction is
required for DNA replication and cell growth. Thymidylate synthase activity is
therefore
required by all rapidly dividing cells such as cancer cells. Due to its
association with DNA
synthesis, and therefore, cellular replication, thymidylate synthase has been
the target for
anti-cancer drugs for many years. Non-limiting examples of thymidylate
synthase inhibitors
include folate and dUMP analogs, such as 5-fluorouracil (5-FU). Any drug that
inhibits the
expression of thymidylate synthase may be considered herein as a co-drug.
1001611 If desired, the administration of the nucleic acid composition
described herein may
be combined with one or more non-drug therapies, such as, for example,
radiotherapy,
and/or surgery. As well known in the art, radiation therapy and/or
administration of the
chemotherapeutic agent (in this case, the nucleic acid composition described
herein, and
optionally, any additional chemotherapeutic agent) may be given before surgery
to, for
example, shrink a tumor or stop the spread of the cancer before the surgery.
As also well
known in the art, radiation therapy and/or administration of the
chemotherapeutic agent may
be given after surgery to destroy any remaining cancer.
43
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
[00162] Examples have been set forth below for the purpose of illustration and
to describe
certain specific embodiments of the invention. However, the scope of this
invention is not
to be in any way limited by the examples set forth herein.
EXAMPLES
Example 1. Materials and Methods.
[00163] modyied miR-129: the 5-FU modified miR-129 molecules were synthesized
by an
automated oligonucleotide synthesis process and purified by HPLC. The two
strands were
annealed to make the mature modified 5-FU-miR-129. More specifically, a
process referred
to as "2'-ACE RNA synthesis" was used. The 2'-ACE RNA synthesis is based on a
protecting group scheme in which a silylether is employed to protect the 5'-
hydroxyl group
in combination with an acid-labile orthoester protecting group on the 2'-
hydroxy (2'-ACE).
This combination of protecting groups is then used with standard
phosphoramidite solid-
phase synthesis technology. See, for example, S.A. Scaringe, F.E. Wincott, and
M.H.
Caruthers, J. Am. Chem. Soc., 120 (45), 11820-11821(1998); International PCT
Application
WO/1996/041809; M.D. Matteucci, M.H. Caruthers, J. Am. Chem. Soc., 103, 3185-
3191
(1981); S.L. Beaucage, M.H. Caruthers, Tetrahedron Lett. 22, 1859-1862 (1981),
the entire
contents of each of which are expressly incorporated herein.
[00164] The exemplary modified miR-15a nucleic acid, modified miR-140 nucleic
acid,
modified miR-192 nucleic acid, modified miR-502, modified miR-506 nucleic acid
or any
other modified microRNAs that replace uracil with a 5-halouracil can be
synthesized in the
same manner as miR-129a.
[00165] Some exemplary structures of the protected and functionalized
ribonucleoside
phosphoramidites currently in use are shown below:
44
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
A
Ca )4;
y
s.e
: N
=====%,0-.S.;=-i),
=
rifii=
"*.*µ =======, 0:
,
rtik4, 0õõ* A
A
Nyl4
= 0:
tet,
6 \
- =-= V"(;)--.:*- :
= p \tõ..:141
:
f A *(4 0 oli6;;
,
oi cYN'Ys/
Y ........................... P
1001661 Cell culture. The human colon cancer cell lines HCT116, RKO, SW480,
SW620,
and the normal colon cell line CCD 841 CoN, pancreatic cancer cell lines ASPC-
1, Panc-1,
and lung cancer cell line A549,were obtained from the American Type Culture
Collection
(ATCC) and maintained in McCoy's 5A medium (HCT-116), DMEM (RKO, SW480,
SW620) and MEM (CCD 841 CoN) (Thermo Fischer). Media was supplemented with 10%
fetal bovine serum (Thermo Fischer).
[00167] For transfections, 1x105 cells were plated in six-well plates and
transfected with
100 nM of either mi R-15a precursor, non-specific miRNA (Thermo Fischer) or
modified
miR-15a mimics (Dharmacon) after 24 hours using Oligofectamine (Thermo
Fischer)
following the manufacturer's protocols. For reagent free transfection, cells
were plated in 6
well plates at (1x105) cells per well. Twenty-four hours later 100 pmol miRNA
(Control,
miR-15a, Mimic-1) were diluted in Optimem (Thermo Fischer) and added to the
plate.
Media was changed after 24 hours. Media was supplemented with 10% fetal bovine
serum
(Thermo Fischer). Briefly, cells were cultured in DMEM/F12 supplemented with
B27,
ng/mL bFGF, and 20 ng/mL EGF (Life Technologies) in ultra-low attachment
flasks. The
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
spheroid cells were maintained by collection through gentle centrifugation,
dissociation to
single cells and replating.
[00168] Western innnunoblot analysis: Forty-eight hours after transfection,
equal amounts
of protein (15 ps) extracted from cells lysed in RIPA buffer with protease
inhibitor (Sigma)
and were separated on 10% -12% sodium dodecyl sulfate-polyacrylamide gels
using
standard procedures. The primary antibodies used for the analysis were rabbit
anti-YAPI
monoclonal antibody (1:10000) (Cell Signaling Technologies), anti-DLCK1
(1:500)
(Abcam), anti-BCL2 (1:500) (NeoMarkers), ant-BMI-1 (1:10000) (Cell Signaling
Technologies), mouse anti-human TS antibody (1:500), anti-a-tubulin (1:50000)
(Santa
Cruz Biotech Inc.), anti-GAPDH (1:100000) (Santa Cruz Biotech Inc.), ant-E2F3
(1:500)
(Santa Cruz Biotech Inc.),. Horseradish peroxidase¨conjugated antibodies
against mouse or
rabbit (1:5000, Santa Cruz Biotech Inc.) were used as the secondary
antibodies. Protein
bands were visualized with autoradiography film using SuperSignal West Pico
Chemiluminescent Substrate (Thermo Fischer). Western blot density was
quantified using
Image J software.
[00169] Cell proliferation assay: Twenty-four hours after transfection, cells
were seeded in
96-well plates at a density of 2000 cells per well. The cell proliferation
assay was
performed on days 1 to 5 by incubating 10 I WST-1 (Roche Applied Science,
Mannheim,
Germany) in the culture medium for lh and reading the absorbance at 450 and
630nm. The
cell proliferation rate was calculated by subtracting the absorbance at 450 nm
from the
absorbance at 630 nm. Experiments for the cell proliferation assay were
performed at least
three times. The O.D. was calculated by subtracting the absorbance at 630 nm
from that at
450 nm. Proliferation experiments were performed three times.
[00170] Anchorage-independent proliferation was studied to determine cancer
cell colony
forming ability. Cancer cells were trypsinized and counted and a total of
lx105 cells per
well were transfected in 6-well plates with 25 nM modified microRNA or native
miRs or a
negative control miRNA with oligofectamine, and 6 hours after transfection,
cells were
recounted. A total of 20,000 cells in 0.35% agar (Bacto Agar; Becton
Dickinson) were
layered on top of 1 mL of a solidified 0.6% agar layer in a 35-mm dish. Growth
media with
46
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
B27, 10 ng/mL bFGF, and 20 ng/mL EGF were included in both layers. After 2
weeks of
incubation, colonies more than 50 mm in diameter were counted.
[00171] Cell cycle analysis: Twenty-four hours after transfection, cells were
harvested and
resuspended at 0.5 to 1 x 106 cells/mL in modified Krishan's buffer
supplemented with 0.02
mg/mL RNase H and 0.05 mg/mL propidium iodide. Stained cells were detected by
flow
cytometry and results were analyzed with Modfit LT'' software. The experiments
for cell
cycle analysis were performed at least three times.
[00172] Apoptosis Assay. To distinguish between early and late apoptosis, a
fluorescein
isothiocyanate (FITC)¨Annexin assay was done (Becton Dickinson). HCT116, RKO,
5W480 and 5W620 cells were plated into 6 well plates (1x105) cells per well,
after 24h,
cells were transfected with 25 nM modified miRNAs using Oligofectamine. Forty-
eight
hours after transfection, cells were harvested, stained with propidium iodide
and anti-
annexin-V antibody (Annexin V-FITC Apoptosis Detection kit, Invitrogen, CA,
USA)
following the manufacturer's protocol, and stained cells were detected by flow
cytometry.
[00173] 5-al treatment and cootoxicity assay: Twenty-four hours after
transfection,
cancer cells were plated in 96-well plates at 2 x 103 cells per well in
triplicates in 100 1.1L of
medium. After 24 hours, fresh medium containing 2 1.1M 5-FU alone, 50 nM
native
microRNA, 50 nM modified microRNA (e.g., modified miR-129), or a combination
of 2
mM 5-FU with 50 nM a modified microRNA of the present disclosure e.g.,
modified miR-
129, were added, and cells were cultured for an additional 72 hours. Cell
viability was
measured using the WST-1 assay.
[00174] Lentivirus production: Briefly, 1.5 x 106 293T cells were plated in a
10-cm dish
with 10 mL of DMEM + 10% FBS. Two days later, pEZX-MR03, a lentiviral plasmid,
expressing miR-129 or hsa-miR-15a, was transfected with Lenti-Pac HIV
expression
packaging kit following the manufacturer's protocol. Forty-eight hours later,
the virus was
harvested and concentrated with Lenti-Pac lentivirus concentration solution.
Then the titer
of the virus (approximately 1011 virus particles/ml) was determined with Lenti-
PacTm HIV
qRT-PCR titration kit. In addition, serial dilution of the virus (0.1 L, 0.5
L, 2 10 ttL,
50 pi) was used to transduce 5 x 104 HCT116 CSC to determine the transduction
efficiency.
47
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
The lowest concentration (2 pt) to achieve 100% positive expression was used
to infect the
cells for mouse in vivo treatement experiments.
[00175] Real-time qRT-PCR analysis of nucleic acid expression. The expression
levels of
microRNAs in cancer cells were quantified. Briefly, the primers specific to
the microRNA
of interest and an internal control RNU44 gene were purchased from Ambion.
cDNA
synthesis was performed by the High Capacity cDNA Synthesis Kit (Applied
Biosystems)
with miRNA-specific primers. Real-time qRT-PCR was carried out on an Applied
Biosystems 7500 Real-Time PCR machine with miRNA-specific primers by Taq/Vlan
Gene
Expression Assay (Applied Biosystems). Expression level of the exemplary miRs
of the
present disclosure was calculated by the AACT method based on the internal
control
RNU44, normalized to the control group and plotted as relative quantification.
[00176] Human cancer stern cell profiler: RNAs were extracted from cancer
cells
transfected with either exemplary microRNAs of the present disclosure or
negative mi RNA
using TRIzol reagent (Thermo Fischer) in accordance with the manufacturer's
protocol.
RNAs were transcribed to first-strand cDNA using the RT2 First Strand Kit
(Qiagen). Next,
the cDNA is mixed with RT2 SYBR Green Mastermix (Qiagen), and this mixture is
aliquoted into the wells of the Human Cancer Stem Cells RT2 Profiler PCR Array
(Qiagen).
Applied Biosystems 7500 Real-Time PCR machine was used for qRT-PCR (Applied
Biosystems), and relative expression values were determined using the AACT
method.
[00177] Mouse subcutaneous tumor implantation model: Two days before
injection,
HCT116 cancer stem cells were plated at 5 x 105/well in a 6-well ultra low
attachment plate.
20 pL of the virus or 100 pmole exemplary modified miR-129 or modified miR-15a
were
used to transduce or transfect cells. Forty-eight hours later, cells were
collected and re-
suspended at 106/m1 in DMEM/F12 knockout media with 30% matrigel. Ten-twelve
week-
old NOD/SCID mice (Jackson Laboratories, Bar Harbor, MA, USA) were used for
tumor
implantation. The mice were anesthetized by isoflurane inhalation. 1004 of
cell
suspension was injected subcutaneously into both sides of the lower back area.
The tumor
size was measured using a caliper, and tumor volume was calculated using the
formula V =
length x width2/2.
48
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
[00178] For the in vivo miRNA delivery experiments, we created colon cancer
cells that
expressed the lenti-luc reporter gene by infecting parental HCT116 cells with
a recombinant
lentivirus. Luciferase-expressing HCT116 cells (2.0x106 cells per mouse) were
suspended in
0.1 mL of PBS solution and was injected through tail vein of each mouse. Two
weeks after
injection of colon cancer cells, mice were treated via tail vein injection
with 40 i.tg of
negative control or modified miR(s) packaged with in vivo-jetPEI (Polyplus
Transfection).
Mice were treated every other day for 2 weeks (8 times). Following treatment,
mice were
screened using NIS Spectrum In vivo Imaging System (P/IS) (PerkinElmer).
[00179] RNA isolation: For mouse xenografts, sectioned tissues were
deparaffinized,
hydrated, and digested with proteinase K, respectively. Subsequently, total
RNA was
isolated using the TRizol reagent. Total RNA was also isolated from clinical
specimens by
the TRIzol -based approach.
[00180] Statistical analysis All experiments were repeated at least three
times. All
statistical analyses were performed with SigmaPlot software. The statistical
significance
between two groups was determined using Student's 1-test (paired 1-test for
clinical samples,
and unpaired 1-test for all other samples). For comparison of more than two
groups, one-way
ANOVA followed by a Bonferroni-Dunn test was used. Data were expressed as mean
standard error of the mean (SEM). The statistical significance is either
described in figure
legends, or indicated with asterisks(*). *=P <0.05; **=P<0.01; ***=P< 0.001.
Example 2: Modified microRNAs of the present disclosure have anti-cancer
activity.
[00181] As shown in FIGS. 3, 8B, 12A-B, 13A-B and 14A-D, the modified miRNAs
(modified miR: 129, 15a, 192 (215), 140, 502, and 506) are more effective in
inhibiting
colon cancer, pancreatic cancer, and lung cancer cell proliferation than non-
modified
miRNA precursor. In addition, the modified miRNAs can be delivered into cancer
cells
without the transfection reagent (data not shown). Notably, the results show
that cancer cell
proliferation across several different colorectal cancer cell lines,
pancreatic cancer cell lines,
and lung cancer cell lines, is inhibited significantly when compared to cancer
cells treated
with control microRNAs.
49
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
Example 3: Modified miR-129 nucleic acids have anti-cancer activity.
[00182] In the following experiments, 5-FU was incorporated into miR-129. In
one
experiment, all U bases in miR-129 were replaced with 5-FU, as shown in the
structure
provided in FIG. 1A, where "UF" represents 5-fluorouracil or other 5-
halouracil. In another
experiment, all U bases, except the seed region of the miR-129, were replaced
with 5-FU, as
shown in the structure provided in FIG. 1B.
[00183] Analysis of target specificity: The results of Western immunoblot
experiments in
colon cancer HCT-116 cells demonstrate that the exemplary modified miR-129
polynucleotides of the present disclosure were able to retain their target
specificity to TS,
BCL2 and E2F3 via. The results are shown in FIGS. 2A and 2B, which shows the
results
for the modified miR-129 nucleic acid having all U bases were replaced with 5-
FU, as
obtained by two separate operators as set forth in SEQ ID. NO: 4. Of further
significance,
the exemplary miR-129 mimics were found to be more potent than unmodified
(control)
miR-129 in reducing the expression levels of TS, BCL2 and E2F3.
[00184] Function enhancement of mod?fied microRNAs of the present disclosure.
The
impact of an exemplary modified miR-129 on colon cancer cell proliferation was
compared
to that of native miR-129. The results show that, at 50 nM concentration, 5-FU-
miR-129
can suppress HCT-116 tumor cell growth completely. Moreover, as shown by the
results in
FIG. 3, 5-FU-miR-129 is much more potent than the native miR-129, thereby
providing a
significantly higher inhibitory effect. Such inhibition is specific, as the
scramble control
miR has no effect on cell proliferation.
[00185] Next, the potencies of modified miR-129 and 5-FU on cell proliferation
were
compared using HCT-116 colon cancer cells. As shown by the results provided in
FIG. 4,
50 nM (40-fold less than 5-FU) of modified miR-129 is unexpectedly much more
potent
than 2 1.1M 5-FU in inhibiting tumor cell proliferation.
[00186] Exemplary modified microRNAs of the present disclosure induce
apoptosis in
colon cancer cells. With Ba2 being an important target of miR-129, the impact
of a
modified miR of the present disclosure on apoptosis was investigated.
Specifically, cell
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
death was quantified using an apoptosis assay in HCT116, RKO, SW480, and SW620
colon
cancer cells transfected with negative control miRNA, native miR-129 or an
exemplary
miR-129 mimic of SEQ ID NO: 4. Results show that the miR-129 mimic was able to
induce
apoptosis by 2 to 30-fold in all 4 colon cancer cell lines via a fluorescence-
activated cell
sorting (FACS)-based FITC¨Annexin assay compared to the native miR-129 and
negative
control miRNA (FIG. 5A).
[00187] miR-129 mimic trigger GL'S cell cycle check point control. Cell cycle
analysis was
performed using flow cytometry in HCT-116 cells treated with scramble control,
miR-129
precursor, and an exemplary miR-129 mimic. As shown in FIG. 5B, cell cycle
analysis
revealed that the miR-129 mimic impacts colon cancer cell growth by inducing
G1 arrest,
and such impact is much more potent (more than two-fold) than native miR-129.
[00188] miR-129 mimics eliminated chemotherapy resistant colon cancer stem
cells.. To
determine the impact of certain exemplary modified microRNAs of the present
disclosure
(i.e., miR-129 mimics) on 5-FU resistant colon cancer stem cells, HCT116
derived colon
cancer stem cells were treated with various concentrations of Mimic-1 or 5-FU.
The data
shown in FIG. 6 reveal that exemplary microRNA mimics of the present
disclosure are able
to eliminate 5-FU resistant colon cancer stem cells by over 80% at 100 nM
concentration,
while a lethal dose of 5-FU at 100 RM has minimal effect on tumor stem cell
viability.
[00189] Taken together, these results show that exemplary modified microRNA
polynucleotides of the present disclosure were able to inhibit cell
proliferation of HCT116
colon cancer stem cells (FIG. 6). Such inhibitory effect by modified miR-129
was much
more potent than native miR-129, as proliferation was nearly completely
blocked with 25
nM miR-129 on day 6 (FIG. 6). We also demonstrated the impact of treatment of
cells with
modified miR-129 on anchorage independent cell growth using a soft agar assay.
The
modified miR-129 treated colon cancer stem cells and formed no visible spheres
compared
to cells treated with the native miR-129 or control miRNA (similar to those
seen in FIG. 10).
1001901 miR-129 mimics inhibit colon cancer metastasis in vivo. The
therapeutic impact of
modifying miR-129 nucleic acids was evaluated using a colon cancer metastasis
model.
Two weeks after establishing metastasis, 40 lig of a miR-129 nucleic acid of
SEQ ID NO; 4
51
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
was delivered by intravenous injection with treatment frequency of one
injection every other
day for two weeks.
[00191] The results shown in FIG. 7 reveal that modified microRNA-129 inhibits
colon
cancer metastasis while negative control miRNA has no effect, while exhibiting
no toxic
side effects. .
Example 3. Modified miR-15a and anti-cancer activity thereof.
1001921 Exemplary modified miR-15a compostions have anti-cancer activity. As
shown in
FIG. 1C and FIG. 1D, exemplary modified miR-15a mimics in which all of the
uracil bases
(FIG. 1C) or only uracil bases that in the non-seed region (FIG. 1D) of the
miR-15a nucleic
acid sequence were replaced with a 5-halouracil (i.e., 5-flurouracil) were
synthesized as set
forth above.
[00193] Three days following transfection of the exemplary modified miR-15a
set forth in
FIG. 1C into HCT-116 colon cancer stem cells, protein was collected and
Western Bloting
was performed to confirm that the modified miR-15a nucleic acid compositions
of the
present disclosure maintained the ability to regulate key miR-15a targets. As
shown in FIG.
8A, miR-15a targets YAP1, BM 11, DCLK1 and BCL2 exhibited protein levels that
were
reduced upon transfection by either the unmodified miR-15a (native-miR15a) or
modified
miR-15a compositions, indicating that the 5-halouracil modification did not
inhibit the
ability of miR-15a to regulate their targets in cells.
[00194] Modified miR-15a has increased therapeutic eafficacy in vitro. In
order to
determine whether the modified miR-15a compositions of the present disclosure
demonstrated increased potency in colon cancer cell lines compared to
unmodified miR-15a,
HCT-116 colon cancer cells were transfected with a negative control (non-
specific
oligonucleotide), unmodified miR-15a or the exemplary modified miR-15a
compositions set
forth in FIG. 1C.
[00195] A WST-1 assay was used to assed cancer cell proliferation. As shown in
FIG. 8B,
six days after transfection, unmodified miR-15a had decreased cell
proliferation by 53%
compared to control. In the case of modified miR-15a, cell proliferation was
decreased by
52
CA 03042401 2019-04-30
WO 2018/085198 PCT/US2017/059011
84%. Taken together, the experimental results show that modified miR-15a is
more effective
at decreasing cancer cell proliferation compared to the unmodified miR-15a.
1001961 Modified miR-15a nucleic acids were also analyzed for their ability to
inhibit cell
cycle progression in cancer cells. FIG. 9 shows that unmodified miR-15a
induced cell cycle
arrest and lead to about 3-fold increase in the Gl/S ratio. FIG. 9 also shows
that the
exemplary modified miR-15a compositions of the present disclosure were more
effective in
stopping cell cycle progression when compared to their native counterpart. For
example, a
7-fold increase in the Gl/S ratio was exhibited by cells expressing the
exemplary modified
miR-15a nucleic acids of the present disclosure when compared to the control.
Therefore,
modified miR-15a is more effective at inducing cell cycle arrest in colon
cancer cells than
unmodified miR-15a.
[00197] The effects of the exemplary modified miR-15a compositions on colony
formation
by colon cancer stem cells in Matrigel matrix were also examined. As shown in
FIG. 10,
while many colonies were generated by cells transfected with control miRNAs
(FIG. 10,
Negative), very few colonies were generated by cells transfected with
unmodified miR-15a
(FIG. 10, miR-15a). In contrast, in the case of cells transfected with
modified miR-15a, no
colonies were observed (FIG. 10, 5-FU-miR-15a). These results indicate that
the exemplary
modified miR-15a compositions of the present disclosure are indeed more potent
inhibitors
of tumorigenesis and colorectal cancer progression.
1001981 Modified miR-1 5a inhibits cancer development and progression in vivo.
To further
our understanding of miR-15a in colon CSCs, a mouse xenograft model was
established that
included colorectal cancer cells that have been either pre transfected with
modified miR-15a
or negative control miRNA. Eight weeks after injection, tumors were measured
and
harvested. A drastic reduction in tumor size for tumors established from CSCs
expressing
modified miR-15a mimic (>25x) (n=8), as shown in FIG. 11.
1001991 The data presented here supports the viability of a novel modification
in which
halouracils (e.g., 5-FU) is incorporated into a miRNA nucleic acid sequence to
enhance the
chemotherapeutic function of the native microRNA molecule with or without the
use of
other chemotherapeutic agents.
53