Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
Fusion Proteins Containing CD47 Antibodies and Cytokines
REFERENCE TO RELATED APPLICATION
[1] This application claims priority to PCT/CN2018/114975, filed on
November 12, 2018, the
contents of which are incorporated herein by reference in their entirety.
BACKGROUND OF THE INVENTION
[2] CD47 (Cluster of Differentiation 47) was first identified as a tumor
antigen on human
ovarian cancer in the 1980s. Since then, CD47 has been found to be expressed
on multiple
human tumor types including acute myeloid leukemia (AML), chronic myeloid
leukemia, acute
lymphoblastic leukemia (ALL), non-Hodgkin's lymphoma (NHL), multiple myeloma
(MM),
bladder cancer, and other solid tumors. High levels of CD47 allow cancer cells
to avoid
phagocytosis despite having a higher level of calreticulin ¨ the dominant pro-
phagocytic signal.
[3] Also known as integrin-associated protein (IAP), ovarian cancer antigen
0A3, Rh-related
antigen and MER6, CD47 is a multi-spanning transmembrane receptor belonging to
the
immunoglobulin superfamily. Its expression and activity have been implicated
in a number of
diseases and disorders. It is a broadly expressed transmembrane glycoprotein
with a single Ig-
like domain and five membrane spanning regions, which functions as a cellular
ligand for SIRPa
with binding mediated through the NH2-terminal V-like domain of signal-
regulatory-protein a
(SIRPa). SIRPa is expressed primarily on myeloid cells, including macrophages,
granulocytes,
myeloid dendritic cells (DCs), mast cells, and their precursors, including
hematopoietic stem
cells.
[4] Macrophages clear pathogens and damaged or aged cells from the blood
stream via
phagocytosis. Cell-surface CD47 interacts with its receptor on macrophages,
SIRPa, to inhibit
phagocytosis of normal, healthy cells. SIRPa inhibits the phagocytosis of host
cells by
macrophages, where the ligation of SIRPa on macrophages by CD47 expressed on
the host
target cell generates an inhibitory signal mediated by SHP-1 that negatively
regulates
phagocytosis.
-1-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
[5] In keeping with the role of CD47 to inhibit phagocytosis of normal
cells, there is
evidence that it is transiently up-regulated on hematopoietic stem cells
(HSCs) and progenitors
just prior to and during their migratory phase, and that the level of CD47 on
these cells
determines the probability that they are engulfed in vivo.
[6] CD47 is also constitutively up-regulated on a number of cancers,
including myeloid
leukemias. Overexpression of CD47 on a myeloid leukemia line increases its
pathogenicity by
allowing it to evade phagocytosis. It has been concluded that CD47 up-
regulation is an
important mechanism for providing protection to normal HSCs during
inflammation-mediated
mobilization, and that leukemic progenitors co-opt this ability in order to
evade macrophage
killing.
[7] Certain CD47 antibodies have been shown to restore phagocytosis and
prevent
atherosclerosis. See, e.g., Kojima et al., Nature, Vol. 36, 86-90 (Aug. 4,
2016). The present
invention provides novel CD47 antibodies or immunologically active fragments
thereof that
have low immunogenicity in humans and cause low or no level of red blood cell
depletion. As
well known to a person skilled in the art, such antibodies may be
interchangeably called "anti-
CD47 antibodies."
[8] Cytokines are a broad and loose category of small proteins (-5-20 kDa)
that are
important in cell signaling. Their release has an effect on the behavior of
cells around them. It
can be said that cytokines are involved in autocrine signalling, paracrine
signalling and
endocrine signalling as immunomodulating agents. Their definite distinction
from hormones is
still part of ongoing research. Cytokines include chemokines, interferons,
interleukins,
lymphokines, and tumour necrosis factors but generally not hormones or growth
factors
(despite some overlap in the terminology). Cytokines are produced by a broad
range of cells,
including immune cells like macrophages, B lymphocytes, T lymphocytes and mast
cells, as well
as endothelial cells, fibroblasts, and various stromal cells; a given cytokine
may be produced by
more than one type of cell. They act through receptors, and are especially
important in the
immune system; cytokines modulate the balance between humoral and cell-based
immune
responses, and they regulate the maturation, growth, and responsiveness of
particular cell
populations. Some cytokines enhance or inhibit the action of other cytokines
in complex ways.
-2-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
They are important in health and disease, specifically in host responses to
infection, immune
responses, inflammation, trauma, sepsis, cancer, and reproduction.
[9] Granulocyte-macrophage colony stimulating factors (GM-CSF), a cytokine,
is a well-
known immuno-stimulator to boost the innate and adaptive immune response which
is
clinically used for myeloid reconstitution. It specifically activates
macrophage and can shift the
macrophage phenotype from M2 to Ml.
[10] No fusion proteins of CD47 antibodies and cytokines of any kind have
been reported or
even suggested to date.
SUMMARY OF THE PRESENT INVENTION
[11] In one aspect, the present invention provides isolated monoclonal
antibodies and their
immunologically active fragments that bind to human CD47. For brevity, these
CD47-binding
isolated monoclonal antibodies and their immunologically active fragments are
referred to
hereinafter as "CD47 antibodies". The CD47 antibodies of this invention are
capable of
modulating, e.g., blocking, inhibiting, reducing, antagonizing, neutralizing
or otherwise
interfering with, CD47 expression, activity and/or signaling, or the
interaction between CD47
and SIRPa. Very significantly, the CD47 antibodies of this invention do not
generally cause a
significant level of depletion or hemagglutination of human red blood cells,
and surprisingly in
many cases do not cause any depletion or hemagglutination of human red blood
cells at all.
Additionally, the CD47 antibodies of this invention have exhibited potent anti-
tumor activities.
[12] In some embodiments, the CD47 antibodies of this invention each
include (a) a variable
heavy (VH) chain sequence that is at least 90% (e.g., at least 95%) identical
to an amino acid
sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 3, SEQ
ID NO: 5, SEQ
ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, SEQ ID NO: 13, SEQ ID NO: 15, SEQ ID
NO: 17, SEQ ID NO:
19, SEQ ID NO: 21, SEQ ID NO: 23, SEQ ID NO: 25, SEQ ID NO: 27, SEQ ID NO: 29,
SEQ ID NO: 31,
SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ
ID NO: 43,
SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ
ID NO: 55,
SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ
ID NO: 67,
SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, and SEQ ID NO: 77;
and (b) a
-3-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
variable light (VL) chain sequence that is at least 90% (e.g., at least 95%)
identical to an amino
acid sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO:
4, SEQ ID NO: 6,
SEQ ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, SEQ ID NO: 14, SEQ ID NO: 16, SEQ
ID NO: 18, SEQ
ID NO: 20, SEQ ID NO: 22, SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, SEQ ID
NO: 30, SEQ ID
NO: 32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO:
42, SEQ ID NO:
44, SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54,
SEQ ID NO: 56,
SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ
ID NO: 68,
SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, and SEQ ID NO: 78.
[13] In
some other embodiments, the CD47 antibodies of this invention each include
paired
VH/VL chain sequences that are at least 90% (e.g., at least 95%, 95%, 96, 97%,
98%, 99%, or
99.5%) identical to a pair of VH and VL amino acid sequences selected from the
group
consisting of SEQ ID NO: 1 and SEQ ID NO: 2 (i.e., 1A1), SEQ ID NO: 3 and SEQ
ID NO: 4 (i.e., 1F8),
SEQ ID NO: 5 and SEQ ID NO: 6 (i.e., 2A11), SEQ ID NO: 7 and SEQ ID NO: 8
(i.e., 2C2), SEQ ID NO:
9 and SEQ ID NO: 10 (i.e., 2D7), SEQ ID NO: 11 and SEQ ID NO: 12 (i.e., 2G4),
SEQ ID NO: 13 and
SEQ ID NO: 14 (i.e., 2G11), SEQ ID NO: 15 and SEQ ID NO: 16 (i.e., 6F4), SEQ
ID NO: 17 and SEQ
ID NO: 18 (i.e., 5H1), SEQ ID NO: 19 and SEQ ID NO: 20 (i.e., 5F6), SEQ ID NO:
21 and SEQ ID NO:
22 (i.e., 1F3), SEQ ID NO: 23 and SEQ ID NO: 24 (i.e., 2A4), SEQ ID NO: 25 and
SEQ ID NO: 26 (i.e.,
2E312), SEQ ID NO: 27 and SEQ ID NO: 28 (i.e., 13A11), SEQ ID NO: 29 and SEQ
ID NO: 30 (i.e.,
15E1), SEQ ID NO: 31 and SEQ ID NO: 32 (i.e., 13H3), SEQ ID NO: 33 and SEQ ID
NO: 34 (i.e.,
14A8), SEQ ID NO: 35 and SEQ ID NO: 36 (i.e., 16H3), SEQ ID NO: 37 and SEQ ID
NO: 38 (i.e.,
1A1), SEQ ID NO: 39 and SEQ ID NO: 40 (i.e., 1A1-A), SEQ ID NO: 41 and SEQ ID
NO: 42 (i.e., 1A1-
Q), SEQ ID NO: 43 and SEQ ID NO: 44 (i.e., 1A2), SEQ ID NO: 45 and SEQ ID NO:
46 (i.e., 1A8),
SEQ ID NO: 47 and SEQ ID NO: 48 (i.e., 1B1), SEQ ID NO: 49 and SEQ ID NO: 50
(i.e., 162), SEQ ID
NO: 51 and SEQ ID NO: 52 (i.e., 1H3), SEQ ID NO: 53 and SEQ ID NO: 54 (i.e.,
1H3-Q), SEQ ID NO:
55 and SEQ ID NO: 56 (i.e., 1H3-A), SEQ ID NO: 57 and SEQ ID NO: 58 (i.e.,
2A2), SEQ ID NO: 59
and SEQ ID NO: 60 (i.e., 2A3), SEQ ID NO: 61 and SEQ ID NO: 62 (i.e., 2A6),
SEQ ID NO: 63 and
SEQ ID NO: 64 (i.e., 2A10), SEQ ID NO: 65 and SEQ ID NO: 66 (i.e., 2B1), SEQ
ID NO: 67 and SEQ
ID NO: 68 (i.e., 2C6), SEQ ID NO: 69 and SEQ ID NO: 70 (i.e., 2E7), SEQ ID NO:
71 and SEQ ID NO:
72 (i.e., 2E9), SEQ ID NO: 73 and SEQ ID NO: 74 (i.e., 2F1), SEQ ID NO: 75 and
SEQ ID NO: 76 (i.e.,
-4-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
2F3), and SEQ ID NO: 77 and SEQ ID NO: 78 (i.e., 34C5). In some instances, the
CD47 antibodies
of this invention each include a pair of VH and VL chain sequences selected
from the group
consisting of SEQ ID NO: 1 and SEQ ID NO: 2 (i.e., 1A1), SEQ ID NO: 3 and SEQ
ID NO: 4 (i.e., 1F8),
SEQ ID NO: 5 and SEQ ID NO: 6 (i.e., 2A11), SEQ ID NO: 7 and SEQ ID NO: 8
(i.e., 2C2), SEQ ID NO:
9 and SEQ ID NO: 10 (i.e., 2D7), SEQ ID NO: 11 and SEQ ID NO: 12 (i.e., 2G4),
SEQ ID NO: 13 and
SEQ ID NO: 14 (i.e., 2G11), SEQ ID NO: 15 and SEQ ID NO: 16 (i.e., 6F4), SEQ
ID NO: 17 and SEQ
ID NO: 18 (i.e., 5H1), SEQ ID NO: 19 and SEQ ID NO: 20 (i.e., 5F6), SEQ ID NO:
21 and SEQ ID NO:
22 (i.e., 1F3), SEQ ID NO: 23 and SEQ ID NO: 24 (i.e., 2A4), SEQ ID NO: 25 and
SEQ ID NO: 26 (i.e.,
2E312), SEQ ID NO: 27 and SEQ ID NO: 28 (i.e., 13A11), SEQ ID NO: 29 and SEQ
ID NO: 30 (i.e.,
15E1), SEQ ID NO: 31 and SEQ ID NO: 32 (i.e., 13H3), SEQ ID NO: 33 and SEQ ID
NO: 34 (i.e.,
14A8), SEQ ID NO: 35 and SEQ ID NO: 36 (i.e., 16H3), SEQ ID NO: 37 and SEQ ID
NO: 38 (i.e.,
1A1), SEQ ID NO: 39 and SEQ ID NO: 40 (i.e., 1A1-A), SEQ ID NO: 41 and SEQ ID
NO: 42 (i.e., 1A1-
Q), SEQ ID NO: 43 and SEQ ID NO: 44 (i.e., 1A2), SEQ ID NO: 45 and SEQ ID NO:
46 (i.e., 1A8),
SEQ ID NO: 47 and SEQ ID NO: 48 (i.e., 1B1), SEQ ID NO: 49 and SEQ ID NO: 50
(i.e., 162), SEQ ID
NO: 51 and SEQ ID NO: 52 (i.e., 1H3), SEQ ID NO: 53 and SEQ ID NO: 54 (i.e.,
1H3-Q), SEQ ID NO:
55 and SEQ ID NO: 56 (i.e., 1H3-A), SEQ ID NO: 57 and SEQ ID NO: 58 (i.e.,
2A2), SEQ ID NO: 59
and SEQ ID NO: 60 (i.e., 2A3), SEQ ID NO: 61 and SEQ ID NO: 62 (i.e., 2A6),
SEQ ID NO: 63 and
SEQ ID NO: 64 (i.e., 2A10), SEQ ID NO: 65 and SEQ ID NO: 66 (i.e., 2B1), SEQ
ID NO: 67 and SEQ
ID NO: 68 (i.e., 2C6), SEQ ID NO: 69 and SEQ ID NO: 70 (i.e., 2E7), SEQ ID NO:
71 and SEQ ID NO:
72 (i.e., 2E9), SEQ ID NO: 73 and SEQ ID NO: 74 (i.e., 2F1), SEQ ID NO: 75 and
SEQ ID NO: 76 (i.e.,
2F3), and SEQ ID NO: 77 and SEQ ID NO: 78 (i.e., 34C5).
[14] The CD47 antibodies of this invention can be chimeric or humanized.
They can prevent
or significantly reduce human CD47 from interacting with SIRPa, or promotes
macrophage-
mediated phagocytosis of a CD47-expressing cell.
[15] The CD47 antibodies of this invention do not cause a significant or
noticeable level of
hemagglutination or depletion of red blood cells, and in many cases they do
not cause
hemagglutination or depletion of red blood cells at all.
[16] In another aspect, the present invention provides isolated bispecific
monoclonal
antibodies. Each of such isolated bispecific monoclonal antibodies comprises a
first arm and a
-5-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
second arm, wherein the first arm comprises a first monoclonal antibody or
immunologically
active fragment thereof as described above which binds human CD47, and the
second arm
comprise a second monoclonal antibody that does not bind human CD47.
[17] In some embodiments, the second arm in the isolated bispecific
monoclonal antibodies
binds to a cancer cell.
[18] In some other embodiments, the bispecific monoclonal antibodies
inhibit interaction
between human CD47 and human SIRPa.
[19] Still within the scope of this invention are fusion proteins, each
comprising an isolated
monoclonal antibody or an immunologically active fragment thereof and a
cytokine, wherein
the monoclonal antibody or immunologically active fragment thereof binds to
human CD47, the
monoclonal antibody or immunologically active fragment thereof is fused to the
cytokine in the
N-terminal, with or without a linker between the monoclonal antibody or
fragment thereof and
the cytokine.
[20] In some embodiments, the isolated monoclonal antibody or
immunologically active
fragment thereof comprises:
a variable heavy (VI-I) chain sequence that is at least 95% identical to an
amino acid
sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 3, SEQ
ID NO: 5, SEQ
ID NO: 7, SEQ ID NO: 9, SEQ ID NO: 11, SEQ ID NO: 13, SEQ ID NO: 15, SEQ ID
NO: 17, SEQ ID NO:
19, SEQ ID NO: 21, SEQ ID NO: 23, SEQ ID NO: 25, SEQ ID NO: 27, SEQ ID NO: 29,
SEQ ID NO: 31,
SEQ ID NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ
ID NO: 43,
SEQ ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ
ID NO: 55,
SEQ ID NO: 57, SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ
ID NO: 67,
SEQ ID NO: 69, SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, and SEQ ID NO: 77;
and
a variable light (VL) chain sequence that is at least 95% identical to an
amino acid
sequence selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 4, SEQ
ID NO: 6, SEQ
ID NO: 8, SEQ ID NO: 10, SEQ ID NO: 12, SEQ ID NO: 14, SEQ ID NO: 16, SEQ ID
NO: 18, SEQ ID
NO: 20, SEQ ID NO: 22, SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, SEQ ID NO:
30, SEQ ID NO:
32, SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42,
SEQ ID NO: 44,
SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ
ID NO: 56,
-6-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ
ID NO: 68,
SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, and SEQ ID NO: 78.
[21] In some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof comprises a VH/VL pair, the VH/VL pair comprises VH
and VL chain
sequences at least 95% identical to a pair of VH and VL amino acid sequences
selected from the
group consisting of SEQ ID NO: 1 and SEQ ID NO: 2 (i.e., 1A1), SEQ ID NO: 3
and SEQ ID NO: 4
(i.e., 1F8), SEQ ID NO: 5 and SEQ ID NO: 6 (i.e., 2A11), SEQ ID NO: 7 and SEQ
ID NO: 8 (i.e., 2C2),
SEQ ID NO: 9 and SEQ ID NO: 10 (i.e., 2D7), SEQ ID NO: 11 and SEQ ID NO: 12
(i.e., 2G4), SEQ ID
NO: 13 and SEQ ID NO: 14 (i.e., 2G11), SEQ ID NO: 15 and SEQ ID NO: 16 (i.e.,
6F4), SEQ ID NO:
17 and SEQ ID NO: 18 (i.e., 5H1), SEQ ID NO: 19 and SEQ ID NO: 20 (i.e., 5F6),
SEQ ID NO: 21 and
SEQ ID NO: 22 (i.e., 1F3), SEQ ID NO: 23 and SEQ ID NO: 24 (i.e., 2A4), SEQ ID
NO: 25 and SEQ ID
NO: 26 (i.e., 2E312), SEQ ID NO: 27 and SEQ ID NO: 28 (i.e., 13A11), SEQ ID
NO: 29 and SEQ ID NO:
30 (i.e., 15E1), SEQ ID NO: 31 and SEQ ID NO: 32 (i.e., 13H3), SEQ ID NO: 33
and SEQ ID NO: 34
(i.e., 14A8), SEQ ID NO: 35 and SEQ ID NO: 36 (i.e., 16H3), SEQ ID NO: 37 and
SEQ ID NO: 38 (i.e.,
1A1), SEQ ID NO: 39 and SEQ ID NO: 40 (i.e., 1A1-A), SEQ ID NO: 41 and SEQ ID
NO: 42 (i.e., 1A1-
Q), SEQ ID NO: 43 and SEQ ID NO: 44 (i.e., 1A2), SEQ ID NO: 45 and SEQ ID NO:
46 (i.e., 1A8),
SEQ ID NO: 47 and SEQ ID NO: 48 (i.e., 1B1), SEQ ID NO: 49 and SEQ ID NO: 50
(i.e., 162), SEQ ID
NO: 51 and SEQ ID NO: 52 (i.e., 1H3), SEQ ID NO: 53 and SEQ ID NO: 54 (i.e.,
1H3-Q), SEQ ID NO:
55 and SEQ ID NO: 56 (i.e., 1H3-A), SEQ ID NO: 57 and SEQ ID NO: 58 (i.e.,
2A2), SEQ ID NO: 59
and SEQ ID NO: 60 (i.e., 2A3), SEQ ID NO: 61 and SEQ ID NO: 62 (i.e., 2A6),
SEQ ID NO: 63 and
SEQ ID NO: 64 (i.e., 2A10), SEQ ID NO: 65 and SEQ ID NO: 66 (i.e., 2B1), SEQ
ID NO: 67 and SEQ
ID NO: 68 (i.e., 2C6), SEQ ID NO: 69 and SEQ ID NO: 70 (i.e., 2E7), SEQ ID NO:
71 and SEQ ID NO:
72 (i.e., 2E9), SEQ ID NO: 73 and SEQ ID NO: 74 (i.e., 2F1), SEQ ID NO: 75 and
SEQ ID NO: 76 (i.e.,
2F3), and SEQ ID NO: 77 and SEQ ID NO: 78 (i.e., 34C5).
[22] In some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment comprises a VH/VL pair, wherein the VH/VL pair comprises VH
and VL chain
sequences selected from the group consisting of SEQ ID NO: 1 and SEQ ID NO: 2
(i.e., 1A1), SEQ
ID NO: 3 and SEQ ID NO: 4 (i.e., 1F8), SEQ ID NO: 5 and SEQ ID NO: 6 (i.e.,
2A11), SEQ ID NO: 7
and SEQ ID NO: 8 (i.e., 2C2), SEQ ID NO: 9 and SEQ ID NO: 10 (i.e., 2D7), SEQ
ID NO: 11 and SEQ
-7-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
ID NO: 12 (i.e., 2G4), SEQ ID NO: 13 and SEQ ID NO: 14 (i.e., 2G11), SEQ ID
NO: 15 and SEQ ID
NO: 16 (i.e., 6F4), SEQ ID NO: 17 and SEQ ID NO: 18 (i.e., 5H1), SEQ ID NO: 19
and SEQ ID NO: 20
(i.e., 5F6), SEQ ID NO: 21 and SEQ ID NO: 22 (i.e., 1F3), SEQ ID NO: 23 and
SEQ ID NO: 24 (i.e.,
2A4), SEQ ID NO: 25 and SEQ ID NO: 26 (i.e., 2E312), SEQ ID NO: 27 and SEQ ID
NO: 28 (i.e.,
13A11), SEQ ID NO: 29 and SEQ ID NO: 30 (i.e., 15E1), SEQ ID NO: 31 and SEQ ID
NO: 32 (i.e.,
13H3), SEQ ID NO: 33 and SEQ ID NO: 34 (i.e., 14A8), SEQ ID NO: 35 and SEQ ID
NO: 36 (i.e.,
16H3), SEQ ID NO: 37 and SEQ ID NO: 38 (i.e., 1A1), SEQ ID NO: 39 and SEQ ID
NO: 40 (i.e., 1A1-
A), SEQ ID NO: 41 and SEQ ID NO: 42 (i.e., 1A1-Q), SEQ ID NO: 43 and SEQ ID
NO: 44 (i.e., 1A2),
SEQ ID NO: 45 and SEQ ID NO: 46 (i.e., 1A8), SEQ ID NO: 47 and SEQ ID NO: 48
(i.e., 1B1), SEQ ID
NO: 49 and SEQ ID NO: 50 (i.e., 162), SEQ ID NO: 51 and SEQ ID NO: 52 (i.e.,
1H3), SEQ ID NO:
53 and SEQ ID NO: 54 (i.e., 1H3-Q), SEQ ID NO: 55 and SEQ ID NO: 56 (i.e., 1H3-
A), SEQ ID NO:
57 and SEQ ID NO: 58 (i.e., 2A2), SEQ ID NO: 59 and SEQ ID NO: 60 (i.e., 2A3),
SEQ ID NO: 61 and
SEQ ID NO: 62 (i.e., 2A6), SEQ ID NO: 63 and SEQ ID NO: 64 (i.e., 2A10), SEQ
ID NO: 65 and SEQ
ID NO: 66 (i.e., 2B1), SEQ ID NO: 67 and SEQ ID NO: 68 (i.e., 2C6), SEQ ID NO:
69 and SEQ ID NO:
70 (i.e., 2E7), SEQ ID NO: 71 and SEQ ID NO: 72 (i.e., 2E9), SEQ ID NO: 73 and
SEQ ID NO: 74 (i.e.,
2F1), SEQ ID NO: 75 and SEQ ID NO: 76 (i.e., 2F3), SEQ ID NO: 77 and SEQ ID
NO: 78 (i.e., 34C5),
or a combination that is at least 90% (e.g., at least 95%) identical thereto.
[23] In still some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof is chimeric or humanized.
[24] In still some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof prevents human CD47 from interacting with signal-
regulatory-protein a
(SIRPa).
[25] In still some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof does not cause a significant level of hemagglutination
or depletion of
red blood cells.
[26] In still some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof does not cause hemagglutination or depletion of red
blood cells.
-8-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[27] In still some other embodiments, the cytokine comprises a wild type or
a variant thereof,
of an immunoglobulin (Ig), a hemopoietic growth factor, an interferon, a tumor
necrosis factor,
an interleukin-17 receptor, or a monomeric glycoprotein.
[28] In still some other embodiments, the cytokine is a wild type or a
variant thereof, of the
monomeric glycoprotein. In some further embodiments, the cytokine is a wild
type or a variant
thereof, of granulocyte-macrophage colony-stimulating factor (GM-CSF).
[29] In still some other embodiments, the monoclonal antibody or
immunologically active
fragment thereof is fused to the cytokine without a linker, or with a linker
selected from the
group consisting of (G45)3, (G45)6, (GS)9, IGD(F30), IGD(F64), IGD(R30),
IGN(R64), IGD(R30-Cys),
and IGD(R64-Cys).
[30] In still some other embodiments, the fusion protein inhibits
interaction between human
CD47 and human SIRPa.
[31] In still some other embodiments of the fusion protein, the isolated
monoclonal antibody
or immunologically active fragment thereof promotes macrophage-mediated
phagocytosis of a
CD47-expressing cell.
[32] In still some other embodiments, the fusion protein further comprises
a small-molecule
therapeutic agent or a marker, and the small-molecule therapeutic agent or
marker is
conjugated with the monoclonal antibody or an immunologically active fragment
thereof or
with the cytokine. The small molecule therapeutic agent can be an anti-cancer
or anti-
inflammation agent; and the marker can be a biomarker or fluorescent marker.
[33] In still some other embodiments, the isolated monoclonal antibody or
immunologically
active fragment thereof comprises a VH/VL sequence pair that is at least 90%
(e.g., at least 95%)
identical to a pair of VH and VL amino acid sequences selected from the group
consisting of:
SEQ ID NO: 3 and SEQ ID NO: 4, and SEQ ID NO: 31 and SEQ ID NO: 32; and the
cytokine is a wild
type or a variant thereof, of granulocyte-macrophage colony-stimulating factor
(GM-CSF).
[34] In still some other embodiments, the fusion protein comprises a
variable light (VL) chain
expression vector that is at least 90% (e.g., at least 95%) identical to an
amino acid sequence
selected from the group consisting of SEQ ID NO: 108 and SEQ ID NO: 116; and a
variable heavy
(VH) chain expression vector that is at least 90% (e.g., at least 95%)
identical to an amino acid
-9-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
sequence selected from the group consisting of SEQ ID NO: 109, SEQ ID NO: 110,
SEQ ID NO:
111, SEQ ID NO: 112, SEQ ID NO: 113, SEQ ID NO: 114, SEQ ID NO: 115, SEQ ID
NO: 117, SEQ ID
NO: 118, SEQ ID NO: 119, SEQ ID NO: 120, SEQ ID NO: 121, SEQ ID NO: 122, SEQ
ID NO: 123,
SEQ ID NO: SEQ ID NO: 125, SEQ ID NO: 126, SEQ ID NO. 127, SEQ ID NO. 128, SEQ
ID NO: 129,
SEQ ID NO: 130, SEQ ID NO: 131, SEQ ID NO: 132, SEQ ID NO: 133, SEQ ID NO:
134, SEQ ID NO:
135, SEQ ID NO: 136, SEQ ID NO: 137, SEQ ID NO: 138, SEQ ID NO: 139, SEQ ID
NO: 140, SEQ ID
NO: 141, SEQ ID NO: 142, SEQ ID NO: 143, SEQ ID NO: 144, SEQ ID NO: 145, SEQ
ID NO: 146,
SEQ ID NO: 147, SEQ ID NO: 148, SEQ ID NO: 149, SEQ ID NO: 150, SEQ ID NO:
151, SEQ ID NO:
152, SEQ ID NO: 153, SEQ ID NO: 154, SEQ ID NO: 155, SEQ ID NO: 156, SEQ ID
NO: 147, and
SEQ ID NO: 158.
[35] In still some other embodiments, the fusion protein comprises a VH/VL
pair that is at
least 90% (e.g., at least 95%) identical to a pair of VH and VL amino acid
sequences selected
from the group consisting of: SEQ ID NO: 108 and SEQ ID NO: 109, SEQ ID NO:
108 and SEQ ID
NO: 110, SEQ ID NO: 108 and SEQ ID NO: 111, SEQ ID NO: 108 and SEQ ID NO: 112,
SEQ ID NO:
108 and SEQ ID NO: 113, SEQ ID NO: 108 and SEQ ID NO: 114, SEQ ID NO: 108 and
SEQ ID NO:
115, SEQ ID NO: 108 and SEQ ID NO: 117, SEQ ID NO: 108 and SEQ ID NO: 118, SEQ
ID NO: 108
and SEQ ID NO: 119, SEQ ID NO: 108 and SEQ ID NO: 120, SEQ ID NO: 108 and SEQ
ID NO: 121,
SEQ ID NO: 108 and SEQ ID NO: 122, SEQ ID NO: 108 and SEQ ID NO: 123, SEQ ID
NO: 108 and
SEQ ID NO: 124, SEQ ID NO: 108 and SEQ ID NO: 125, SEQ ID NO: 108 and SEQ ID
NO: 126, SEQ
ID NO: 108 and SEQ ID NO: 127, SEQ ID NO: 108 and SEQ ID NO: 128, SEQ ID NO:
108 and SEQ
ID NO: 129, SEQ ID NO: 108 and SEQ ID NO: 130, SEQ ID NO: 108 and SEQ ID NO:
131, SEQ ID
NO: 108 and SEQ ID NO: 132, SEQ ID NO: 108 and SEQ ID NO: 133, SEQ ID NO: 108
and SEQ ID
NO: 134, SEQ ID NO: 108 and SEQ ID NO: 135, SEQ ID NO: 108 and SEQ ID NO: 136,
SEQ ID NO:
108 and SEQ ID NO: 137, SEQ ID NO: 108 and SEQ ID NO: 138, SEQ ID NO: 108 and
SEQ ID NO:
139, SEQ ID NO: 108 and SEQ ID NO: 140, SEQ ID NO: 108 and SEQ ID NO: 141, SEQ
ID NO: 108
and SEQ ID NO: 142, SEQ ID NO: 108 and SEQ ID NO: 143, SEQ ID NO: 108 and SEQ
ID NO: 144,
SEQ ID NO: 108 and SEQ ID NO: 145, SEQ ID NO: 108 and SEQ ID NO: 146, and SEQ
ID NO: 108
and SEQ ID NO: 147; SEQ ID NO: 116 and SEQ ID NO: 109, SEQ ID NO: 116 and SEQ
ID NO: 110,
SEQ ID NO: 116 and SEQ ID NO: 111, SEQ ID NO: 116 and SEQ ID NO: 112, SEQ ID
NO: 116 and
-10-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
SEQ ID NO: 113, SEQ ID NO: 116 and SEQ ID NO: 114, SEQ ID NO: 116 and SEQ ID
NO: 115, SEQ
ID NO: 116 and SEQ ID NO: 117, SEQ ID NO: 116 and SEQ ID NO: 120, SEQ ID NO:
116 and SEQ
ID NO: 121, SEQ ID NO: 116 and SEQ ID NO: 122, SEQ ID NO: 116 and SEQ ID NO:
124, SEQ ID
NO: 116 and SEQ ID NO: 125, SEQ ID NO: 116 and SEQ ID NO: 126, SEQ ID NO: 116
and SEQ ID
NO: 127, SEQ ID NO: 116 and SEQ ID NO: 128, SEQ ID NO: 116 and SEQ ID NO: 129,
SEQ ID NO:
116 and SEQ ID NO: 130, SEQ ID NO: 116 and SEQ ID NO: 131, SEQ ID NO: 116 and
SEQ ID NO:
132, SEQ ID NO: 116 and SEQ ID NO: 133, SEQ ID NO: 116 and SEQ ID NO: 134, SEQ
ID NO: 116
and SEQ ID NO: 135, SEQ ID NO: 116 and SEQ ID NO: 136, SEQ ID NO: 116 and SEQ
ID NO: 137,
SEQ ID NO: 116 and SEQ ID NO: 138, SEQ ID NO: 116 and SEQ ID NO: 139, SEQ ID
NO: 116 and
SEQ ID NO: 140, SEQ ID NO: 116 and SEQ ID NO: 141, SEQ ID NO: 116 and SEQ ID
NO: 142, SEQ
ID NO: 116 and SEQ ID NO: 143, SEQ ID NO: 116 and SEQ ID NO: 144, SEQ ID NO:
116 and SEQ
ID NO: 145, SEQ ID NO: 116 and SEQ ID NO: 146, SEQ ID NO: 116 and SEQ ID NO:
147, SEQ ID
NO: 116 and SEQ ID NO: 148, SEQ ID NO: 116 and SEQ ID NO: 149, SEQ ID NO: 116
and SEQ ID
NO: 150, SEQ ID NO: 116 and SEQ ID NO: 151, SEQ ID NO: 116 and SEQ ID NO: 152,
SEQ ID NO:
116 and SEQ ID NO: 153, SEQ ID NO: 116 and SEQ ID NO: 154, SEQ ID NO: 116 and
SEQ ID NO:
155, and SEQ ID NO: 116 and SEQ ID NO: 156.
[36] In still another aspect, the present invention provides pharmaceutical
compositions
each containing one of the fusion proteins of this invention as described
above, and a
pharmaceutically acceptable carrier or excipient.
[37] As used herein, the term "pharmaceutically acceptable carrier or
excipient" refers to a
carrier or an excipient that is useful for preparing a pharmaceutical
composition or formulation
that is generally safe, non-toxic, and neither biologically nor otherwise
undesirable. A carrier or
excipient employed is typically one suitable for administration to human
subjects or other
mammals. In making the compositions, the active ingredient is usually mixed
with, diluted by,
or enclosed with a carrier or excipient. When the carrier or excipient serves
as a diluent, it can
be a solid, semi-solid, or liquid material, which acts as a vehicle, carrier,
or medium for the
active ingredient of the antibody.
[38] Also within the scope of the present invention is a method for
treating a disease in a
human subject in need thereof, and the method includes administering to the
subject a
-11-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
therapeutically effective amount of a fusion protein of this invention or a
pharmaceutical
composition of this invention, and the disease is a cancer, a fibrotic
disease, or any disease
related to inhibition of phagocytosis. In some instance, the cancer can be
selected from the
group consisting of ovarian cancer, colon cancer, breast cancer, lung cancer,
head and neck
cancer, bladder cancer, colorectal cancer, pancreatic cancer, non-Hodgkin's
lymphoma, acute
lymphocytic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia,
chronic
myelogenous leukemia, hairy cell leukemia (HCL), T-cell prolymphocytic
leukemia (T-PLL), large
granular lymphocytic leukemia, adult T-cell leukemia, multiple myeloma,
melanoma,
leiomyoma, leiomyosarcoma, glioma, glioblastoma, myelomas, monocytic
leukemias, B-cell
derived leukemias, T-cell derived leukemias, B-cell derived lymphomas, T-cell
derived
lymphomas, endometrial cancer, kidney cancer, melanoma, prostate cancer,
thyroid cancer,
cervical cancer, gastric cancer, liver cancer, and solid tumors; whereas the
fibrotic disease can
be selected from the group consisting of myocardial infarction, angina,
osteoarthritis,
pulmonary fibrosis, asthma, cystic fibrosis, bronchitis, and asthma. Examples
of solid tumors
include, e.g., endometrial cancer, thyroid cancer, cervical cancer, gastric
cancer, breast tumors,
ovarian tumors, lung tumors, pancreatic tumors, prostate tumors, melanoma
tumors, colorectal
tumors, lung tumors, head and neck tumors, bladder tumors, esophageal tumors,
liver tumors,
and kidney tumors, and neuroblastic-derived CNS tumors. The disease related to
inhibition of
phagocytosis can be a cardiovascular disease (e.g., atherosclerosis, stroke,
hypertensive heart
disease, rheumatic heart disease, cardiomyopathy, heart arrhythmia, congenital
heart disease,
valvular heart disease, carditis, aortic aneurysms, peripheral artery disease,
or venous
thrombosis).
[39] As
used herein, the term "effective amount" refers to that amount of a CD47
antibody
sufficient or required to effect treatment, prognosis or diagnosis of a
disease associated with
CD47 dependent signaling, as described herein, when administered to a subject.
Therapeutically effective amounts of antibodies provided herein, when used
alone or in
combination, will vary depending upon the relative activity of the antibodies
(e.g., promoting
macrophage mediated phagocytosis of cancer cells expressing CD47) and
depending upon the
subject and disease condition being treated, the weight and age of the
subject, the severity of
-12-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
the disease condition, the manner of administration and the like, which can
readily be
determined by one of ordinary skill in the art.
[40] As used herein, the term "isolated" preceding an antibody described in
this invention
(e.g., CD47 antibody) means that the antibody is substantially free of other
cellular material. In
one embodiment, an isolated antibody is substantially free of other proteins
from the same
species. In another embodiment, an isolated antibody is expressed by a cell
from a different
species and is substantially free of other proteins from the different
species. A protein may be
rendered substantially free of naturally associated components (or components
associated with
the cellular expression system used to produce the antibody) by isolation,
using protein
purification techniques well known in the art. In one embodiment, the
antibodies, or antigen
binding fragments, of the invention are isolated.
[41] As used herein, the term "biological molecules" is meant to include
synthetic antibodies
(monoclonal or bispecific), peptides, and biomimetic molecules. The term
"biomimetic
molecules" refers to molecules which are designed or developed to have
structures or
properties similar to or resembling those of naturally occurring large
compounds such as
proteins or nucleotides and which have a molecular weight of, e.g., at least
3,000, at least 5,000,
or at least 10,000.
[42] All references cited herein are incorporated by reference in their
entirety.
BRIEF DESCRIPTIONS OF THE DRAWINGS
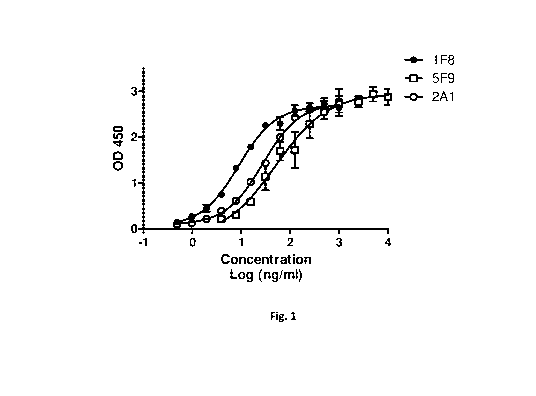
[43] Fig. 1 shows dose-dependent response of CD47 antibodies binding to
monomeric CD47-
[CD.
[44] Fig. 2a and Fig. 2b show dose-dependent response of CD47 antibodies
binding to
dimeric CD47-ECD.
[45] Fig. 3a, Fig. 3b, and Fig. 3c show dose-dependent response of CD47
antibodies blocking
the binding of CD47 to SIRPa.
[46] Fig. 4a and Fig. 4b show dose-dependent response of CD47 antibodies
binding to CD47+
Raji cells; and Fig. 4c, Fig. 4d and Fig. 4e show binding kinetics and data of
CD47 antibodies as
measured by Biacore analysis.
-13-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
[47] Fig. 5a and Fig. 5b show phagocytosis of tumor cells by human MCI)
with CD47
antibodies.
[48] Figs. 6a-6c show macrophage-mediated phagocytosis of various human
blood cancer
cell lines triggered by CD47 antibodies.
[49] Figs. 7a and 7b show red blood cells (RBC)-sparing properties in RBC
agglutination assay
with CD47 antibodies.
[50] Figs. 8a, 8b, 8c, and 8d show activities to bind RBC and induce RBC
agglutination by
CD47 antibodies at different and higher doses.
[51] Figs. 9a, 9b, 9c, and 9d show RBC-binding activities of CD47
antibodies.
[52] Fig. 10 shows results of red blood cell agglutination across multiple
human blood
samples induced by CD47 antibodies.
[53] Fig. 11 shows the human platelet binding activities of CD47 antibodies
and SIRPcc-Ig
fusion, with CD61 stained as a surface marker for platelets.
[54] Figs. 12a and 12b show the test results of cyno red blood cell
agglutination induced by
CD47 antibodies and SIRPa-Ig fusion in vitro.
[55] Fig. 13 shows the test results of phagocytosis and AML cells binding
by CD47 antibodies
and control.
[56] Fig. 14a and Fig. 14b show the efficacy of treatments with CD47
antibodies and control
on luciferase-Raji xenograft mice.
[57] Fig. 15 shows the polarization of macrophage in tumor-bearing mice
induced by CD47
antibodies and control.
[58] Fig. 16 shows the CD47 expression profiles using PDX samples of
various human cancer
types.
[59] Fig. 17 shows results of safety pharm study (hematology) in cynomolgus
monkeys.
[60] Fig. 18 shows competition in binding of CD47 between antibodies 1F8
and 5F9, and
between antibodies 1F8 and 2A1, due to their different epitopes, and
structures of the
5F9/CD47 complex and the 1F8/CD47 complex.
[61] Figs. 19a, 19b, 19c, 19d, 19e, 19f, 19g, and 19h show the effects of
the CD47 antibody
13H3 on RBC congregation, hemoglobin, platelets, and lymphocytes,
respectively.
-14-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[62] Fig. 20 shows strong binding affinity of 34C5 to recombinant CD47-ECD.
[63] Fig. 21 shows strong binding affinity of 34C5 to CD47-bearing Raji
cells.
[64] Fig. 22 shows that 34C5 was able to effectively block CD47 binding to
SIRPa, with an
ECK, of 0.30 nM.
[65] Fig. 23 shows that the antibody 34C5 promoted phagocytosis of tumor
cells by human
MCP.
[66] Fig. 24 shows the antibody 34C5 did not cause in vitro RBC
agglutination.
[67] Fig. 25 shows the antibody 34C5 decreased its binding to RBC with the
decreasing
concentration of this antibody.
[68] Fig. 26 shows that the 1F8-GMCSF fusion protein caused a larger
relative fold change of
the percentages of phagocytosed cells in CD14+ cells as compared to that of
IgG control treated
group, 1F8-treated group, and GM-CSF treated group.
[69] Fig. 27 shows that the fusion protein 1F8- GMCSF had a stronger
binding affinity to
human GMCSF receptor than the recombinant human GMCSF.
[70] Fig. 28 shows that 1F8-GMCSF had similar activities to those of GMCSF
itself in induction
of STAT5 phosphorylation.
[71] Fig. 29 shows that compared to GMCSF, the fusion protein 1F8-GMCSF
exhibited
stronger capability to stimulate TF-1 proliferation.
[72] Fig. 30(a), Fig. 30(b), Fig. 30(c) and Fig. 30(d) showed the
production of IL-6, IL-12, TNF-a,
and CD80 caused by activation of M1 macrophage in the presence of IgG, 1F8,
GMCSF or 1F8-
GMCSF fusion protein.
[73] Fig. 31 shows the 1F8-GMCSF fusion protein exhibited the best efficacy
among all the
five treatments in Raji xenograft model .
[74] Fig. 32 shows dose dependent response of the fusion protein 13H3-GMCSF
binding to
CD47+ Raji cells.
[75] Fig. 33 shows dose dependent response of the fusion protein 13H3-GMCSF
blocking the
binding of CD47 to SIRPa
[76] Fig. 34 shows the effects of 13H3-GMCSF on the phagocytosis of Raji
cells by human MCD.
-15-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
[77] Fig. 35 shows red blood cells (RBC)-sparing properties in RBC
agglutination assay with
the fusion protein 13H3-GMCSF.
[78] Fig. 36 shows dose dependent response of the fusion protein 13H3-GMCSF
binding to
GMCSF receptor.
[79] Fig. 37 shows dose dependent response of the fusion protein 13H3-GMCSF
in
stimulating STAT5 phosphorylation.
[80] Fig. 38 shows dose dependent response of the fusion protein 13H3-GMCSF
in
stimulating TF-1 proliferation.
[81] Fig. 39 shows the efficacy of treatments with the fusion protein 13H3-
GMCSF and
control on luciferase-Raji xenograft mice models.
[82] Fig. 40 shows the concentration-time curve of the serum level of 13H3-
GMCSF after a
single dose at 20 mg/kg in cynomolgus monkeys.
[83] Figs. 41a and 41b show the levels of RBC and platelet after repeat
dose of 13H3-GMCSF
or IgG at 20 mg/kg in cynomolgus monkeys.
[84] Figs. 42a, 42b and 42c show the levels of WBC, neutrophil and monocyte
after repeat
dose of 13H3-GMCSF or IgG at 20 mg/kg in cynomolgus monkeys.
[85] Fig. 43 shows dose dependent response of 13H3-GMCSF variants in
stimulating STAT5
phosphorylation.
[86] Fig. 44 shows dose dependent response of 13H3-GMCSF variants in
stimulating TF-1
proliferation.
[87] Fig. 45 shows dose dependent response of 13H3-GMCSF variants in
stimulating IL-6
production by macrophages.
[88] Fig. 46 shows the effects of the fusion protein 13H3-GMCSF variants on
the
phagocytosis of Raji cells by human MCD.
[89] Figs. 47a, 47b, and 47c show dose dependent response of 13H3-GMCSF
variants
blocking the binding of CD47 to SIRPa.
[90] Fig. 48 shows dose dependent response of 13H3-GMCSF variants binding
to red blood
cells.
-16-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[91] Fig. 49 shows red blood cells (RBC)-sparing properties in RBC
agglutination assay with
13H3-GMCSF variants.
[92] Fig. 50 shows the effects of 13H3-GMCSF with IgG1 N297A version on the
phagocytosis
of Raji cells by human MCD.
[93] Fig.51 shows the effects of 13H3-GMCSF variants with IgG1 N297A
version on the
phagocytosis of Raji cells by human MCD.
[94] Fig.52 shows dose dependent response of deglycosylated 13H3-GMCSF in
induction of
STAT5 phosphorylation.
[95] Fig.53 shows dose dependent response of deglycosylated 13H3-GMCSF in
stimulation of
TF-1 proliferation.
[96] Fig.54 shows the effects of deglycosylated 13H3-GMCSF on the
phagocytosis of Raji cells
by human MO.
[97] Fig.55 shows dose dependent response of deglycosylated 13H3-GMCSF
variants in
stimulation of TF-1 proliferation.
[98] Fig.56 shows the concentration-time curve of the serum level of the
fusion protein
13H3-GMCSF variant after a single dose at 10 mg/kg in cynomolgus monkeys.
[99] Figs.57a, 57b and 57c show the effects of the fusion protein 13H3-
GMCSF variants on
the peripheral levels of neutrophils, monocytes and leukocytes.
[100] Figs. 58a, 58b and 58c show the effects of the fusion protein 13H3-GMCSF
variants on
the peripheral levels of red blood cells, hemoglobulin and platelets.
[101] Fig.59 shows dose dependent response of 13H3-mGMCSF variants in
induction of STAT5
phosphorylation.
[102] Fig.60 shows dose dependent response of 13H3-mGMCSF variants in
stimulation of FDC-
P1 proliferation.
DETAILED DESCRIPTION OF THE INVENTION
[103] The present invention provides novel isolated monoclonal CD47 antibodies
that can
prevent human CD47 from interacting with SIRPa, or promote macrophage-mediated
phagocytosis of a CD47-expressing cell. These CD47 antibodies do not cause a
significant or
-17-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
noticeable level of hemagglutination or depletion of red blood cells, and in
many cases they do
not cause hemagglutination or depletion of red blood cells at all.
[104] As examples, a CD47 antibodies of this invention would include (a) a
variable heavy (VH)
chain sequence that is at least 90% (e.g., at least 95%) identical to an amino
acid sequence
selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 3, SEQ ID NO:
5, SEQ ID NO: 7,
SEQ ID NO: 9, SEQ ID NO: 11, SEQ ID NO: 13, SEQ ID NO: 15, SEQ ID NO: 17, SEQ
ID NO: 19, SEQ
ID NO: 21, SEQ ID NO: 23, SEQ ID NO: 25, SEQ ID NO: 27, SEQ ID NO: 29, SEQ ID
NO: 31, SEQ ID
NO: 33, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 41, SEQ ID NO:
43, SEQ ID NO:
45, SEQ ID NO: 47, SEQ ID NO: 49, SEQ ID NO: 51, SEQ ID NO: 53, SEQ ID NO: 55,
SEQ ID NO: 57,
SEQ ID NO: 59, SEQ ID NO: 61, SEQ ID NO: 63, SEQ ID NO: 65, SEQ ID NO: 67, SEQ
ID NO: 69,
SEQ ID NO: 71, SEQ ID NO: 73, SEQ ID NO: 75, and SEQ ID NO: 77; and (b) a
variable light (VL)
chain sequence that is at least 90% (e.g., at least 95%) identical to an amino
acid sequence
selected from the group consisting of SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO:
6, SEQ ID NO: 8,
SEQ ID NO: 10, SEQ ID NO: 12, SEQ ID NO: 14, SEQ ID NO: 16, SEQ ID NO: 18, SEQ
ID NO: 20,
SEQ ID NO: 22, SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, SEQ ID NO: 30, SEQ
ID NO: 32,
SEQ ID NO: 34, SEQ ID NO: 36, SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, SEQ
ID NO: 44,
SEQ ID NO: 46, SEQ ID NO: 48, SEQ ID NO: 50, SEQ ID NO: 52, SEQ ID NO: 54, SEQ
ID NO: 56,
SEQ ID NO: 58, SEQ ID NO: 60, SEQ ID NO: 62, SEQ ID NO: 64, SEQ ID NO: 66, SEQ
ID NO: 68,
SEQ ID NO: 70, SEQ ID NO: 72, SEQ ID NO: 74, SEQ ID NO: 76, and SEQ ID NO: 78.
In some
further instance, a CD47 antibodies of this invention would include a combined
VH/VL chain
sequence that is at least 90% (e.g., at least 95%) identical to an amino acid
sequence selected
from the group consisting of SEQ ID NO: 1 and SEQ ID NO: 2, SEQ ID NO: 3 and
SEQ ID NO: 4,
SEQ ID NO: 5 and SEQ ID NO: 6, SEQ ID NO: 7 and SEQ ID NO: 8, SEQ ID NO: 9 and
SEQ ID NO: 10,
SEQ ID NO: 11 and SEQ ID NO: 12, SEQ ID NO: 13 and SEQ ID NO: 14, SEQ ID NO:
15 and SEQ ID
NO: 16, SEQ ID NO: 17 and SEQ ID NO: 18, SEQ ID NO: 19 and SEQ ID NO: 20, SEQ
ID NO: 21 and
SEQ ID NO: 22, SEQ ID NO: 23 and SEQ ID NO: 24, SEQ ID NO: 25 and SEQ ID NO:
26, SEQ ID NO:
27 and SEQ ID NO: 28, SEQ ID NO: 29 and SEQ ID NO: 30, SEQ ID NO: 31 and SEQ
ID NO: 32, SEQ
ID NO: 33 and SEQ ID NO: 34, SEQ ID NO: 35 and SEQ ID NO: 36, SEQ ID NO: 37
and SEQ ID NO:
38, SEQ ID NO: 39 and SEQ ID NO: 40, SEQ ID NO: 41 and SEQ ID NO: 42, SEQ ID
NO: 43 and SEQ
-18-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
ID NO: 44, SEQ ID NO: 45 and SEQ ID NO: 46, SEQ ID NO: 47 and SEQ ID NO: 48,
SEQ ID NO: 49
and SEQ ID NO: 50, SEQ ID NO: 51 and SEQ ID NO: 52, SEQ ID NO: 53 and SEQ ID
NO: 54, SEQ ID
NO: 55 and SEQ ID NO: 56, SEQ ID NO: 57 and SEQ ID NO: 58, SEQ ID NO: 59 and
SEQ ID NO: 60,
SEQ ID NO: 61 and SEQ ID NO: 62, SEQ ID NO: 63 and SEQ ID NO: 64, SEQ ID NO:
65 and SEQ ID
NO: 66, SEQ ID NO: 67 and SEQ ID NO: 68, SEQ ID NO: 69 and SEQ ID NO: 70, SEQ
ID NO: 71 and
SEQ ID NO: 72, SEQ ID NO: 73 and SEQ ID NO: 74, SEQ ID NO: 75 and SEQ ID NO:
76, and SEQ ID
NO: 77 and SEQ ID NO: 78.
[105] As used herein, the term "antibody" is used in the broadest sense and
specifically covers
monoclonal antibodies (including full length monoclonal antibodies),
polyclonal antibodies,
multi-specific antibodies (e.g., bispecific antibodies), and antibody
fragments so long as they
exhibit the desired biological activity. "Antibodies" (or "Abs") and
"immunoglobulins" (or "Igs")
are glycoproteins having the same structural characteristics. While antibodies
exhibit binding
specificity to a specific antigen, immunoglobulins include both antibodies and
other antibody-
like molecules which lack antigen specificity. Polypeptides of the latter kind
are, for example,
produced at low levels by the lymph system and at increased levels by
myelomas.
[106] As used herein, the term "epitope" means any antigenic determinant on an
antigen to
which the paratope of an antibody binds. Epitopic determinants usually consist
of chemically
active surface groupings of molecules such as amino acids or sugar side chains
and usually have
specific three dimensional structural characteristics, as well as specific
charge characteristics.
[107] As used herein, the term "native antibodies and immunoglobulins" are
usually
heterotetrameric glycoproteins of about 150,000 daltons, composed of two
identical light (L)
chains and two identical heavy (H) chains. Each light chain is linked to a
heavy chain by one
covalent disulfide bond (also termed a "VH/VL pair"), while the number of
disulfide linkages
varies between the heavy chains of different immunoglobulin isotypes. Each
heavy and light
chain also has regularly spaced intrachain disulfide bridges. Each heavy chain
has at one end a
variable domain (VH) followed by a number of constant domains. Each light
chain has a
variable domain at one end (VL) and a constant domain at its other end; the
constant domain of
the light chain is aligned with the first constant domain of the heavy chain,
and the light chain
variable domain is aligned with the variable domain of the heavy chain.
Particular amino acid
-19-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
residues are believed to form an interface between the light- and heavy-chain
variable domains.
See, e.g., Clothia et al., J. Mol. Biol., 186:651 (1985); Novotny and Haber,
Proc. Natl. Acad. Sci.
U.S.A., 82:4592 (1985).
[108] As used herein, the term "variable" refers to the fact that certain
portions of the
variable domains differ extensively in sequence among antibodies and are used
in the binding
and specificity of each particular antibody for its particular antigen.
However, the variability is
not evenly distributed throughout the variable domains of antibodies. It is
concentrated in
three segments called complementarity-determining regions (CDRs) or
hypervariable regions
both in the light-chain and the heavy-chain variable domains. The more highly
conserved
portions of variable domains are called the framework (FR). The variable
domains of native
heavy and light chains each comprise four FR regions, largely adopting a I3-
sheet configuration,
connected by three CDRs, which form loops connecting, and in some cases
forming part of, the
I3-sheet structure. The CDRs in each chain are held together in close
proximity by the FR
regions and, with the CDRs from the other chain, contribute to the formation
of the antigen-
binding site of antibodies. See, e.g., Kabat et al., Sequences of Proteins of
Immunological
Interest, Fifth Edition, National Institute of Health, Bethesda, Md. (1991).
The constant
domains are not involved directly in binding an antibody to an antigen, but
exhibit various
effector functions, such as participation of the antibody in antibody-
dependent cellular toxicity.
Variable region sequences of interest include the provided humanized variable
region
sequences for CD47 antibodies. For instance, 1A1 includes SEQ ID NO: 1 (heavy)
and SEQ ID NO:
2 (light), 1F8 includes SEQ ID NO: 3 (heavy) and SEQ ID NO: 4 (light), and
2A11 includes SEQ ID
NO: 5 (heavy) and SEQ ID NO: 6 (light).
[109] Papain digestion of antibodies produces two identical antigen-binding
fragments, called
"Fab" fragments, each with a single antigen-binding site, and a residual "Fe"
fragment, whose
name reflects its ability to crystallize readily. Pepsin treatment yields an
F(ab')2 fragment that
has two antigen-combining sites and is still capable of cross-linking antigen.
"Fv" is the
minimum antibody fragment which contains a complete antigen-recognition and -
binding site.
In a two-chain Fv species, this region consists of a dimer of one heavy- and
one light-chain
variable domain in tight, non-covalent association. In a single-chain Fv
species (seFv), one
-20-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
heavy- and one light-chain variable domain can be covalently linked by a
flexible peptide linker
such that the light and heavy chains can associate in a "dimeric" structure
analogous to that in a
two-chain Fv species. It is in this configuration that the three CDRs of each
variable domain
interact to define an antigen-binding site on the surface of the VH-VL dimer.
Collectively, the
six CDRs confer antigen-binding specificity to the antibody. However, even a
single variable
domain (or half of an Fv comprising only three CDRs specific for an antigen)
has the ability to
recognize and bind antigen, although at a lower affinity than the entire
binding site. See, e.g.,
Pluckthun, in The Pharmacology of Monoclonal Antibodies, Vol. 113, Rosenburg
and Moore eds.,
Springer-Verlag, New York, pp. 269-315 (1994).
[110] The Fab fragment also contains the constant domain of the light chain
and the first
constant domain (CHO of the heavy chain. Fab' fragments differ from Fab
fragments by the
addition of a few residues at the carboxy terminus of the heavy chain CHi
domain including one
or more cysteines from the antibody hinge region. Fab'-SH is the designation
herein for Fab' in
which the cysteine residue(s) of the constant domains bear a free thiol group.
F(ab')2 antibody
fragments originally were produced as pairs of Fab' fragments which have hinge
cysteines
between them. Other chemical couplings of antibody fragments are also known.
[111] There are five major classes of immunoglobulins: IgA, IgD, IgE, IgG, and
IgM, and several
of these can be further divided into subclasses (isotypes), e.g., IgG1, IgG2,
IgG3, IgG4, IgA1, IgA2.
The heavy-chain constant domains that correspond to the different classes of
immunoglobulins
are called a, 5, E, y, and [4 respectively. The subunit structures and three-
dimensional
configurations of different classes of immunoglobulins are well known.
[112] As used herein, the term "antibody fragment", and all grammatical
variants thereof, are
defined as a portion of an intact antibody comprising the antigen binding site
or variable region
of the intact antibody, wherein the portion is free of the constant heavy
chain domains (i.e. CH2,
CH3, and CH4, depending on antibody isotype) of the Fc region of the intact
antibody. Examples
of antibody fragments include Fab, Fab', Fab'-SH, F(a1312, and Fv fragments;
diabodies; any
antibody fragment that is a polypeptide having a primary structure consisting
of one
uninterrupted sequence of contiguous amino acid residues (referred to herein
as a "single-
chain antibody fragment" or "single chain polypeptide"), including without
limitation (1) single-
-21-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
chain Fv (scFv) molecules, (2) single chain polypeptides containing only one
light chain variable
domain, or a fragment thereof that contains the three CDRs of the light chain
variable domain,
without an associated heavy chain moiety, and (3) single chain polypeptides
containing only
one heavy chain variable region, or a fragment thereof containing the three
CDRs of the heavy
chain variable region, without an associated light chain moiety; and multi-
specific or
multivalent structures formed from antibody fragments. In an antibody fragment
comprising
one or more heavy chains, the heavy chain(s) can contain any constant domain
sequence (e.g.
CH1 in the IgG isotype) found in a non-Fc region of an intact antibody, and/or
can contain any
hinge region sequence found in an intact antibody, and/or can contain a
leucine zipper
sequence fused to or situated in the hinge region sequence or the constant
domain sequence of
the heavy chain(s).
[113] Unless specifically indicated to the contrary, the term "conjugate" used
herein is defined
as a heterogeneous molecule formed by the covalent attachment of one or more
antibody
fragment(s) to one or more polymer molecule(s), wherein the heterogeneous
molecule is water
soluble, i.e. soluble in physiological fluids such as blood, and wherein the
heterogeneous
molecule is free of any structured aggregate. A conjugate of interest is
polyethylenglycol (PEG).
In the context of the foregoing definition, the term "structured aggregate"
refers to (1) any
aggregate of molecules in aqueous solution having a spheroid or spheroid shell
structure, such
that the heterogeneous molecule is not in a micelle or other emulsion
structure, and is not
anchored to a lipid bilayer, vesicle or liposome; and (2) any aggregate of
molecules in solid or
insolubilized form, such as a chromatography bead matrix, that does not
release the
heterogeneous molecule into solution upon contact with an aqueous phase.
Accordingly, the
term "conjugate" as defined herein encompasses the aforementioned
heterogeneous molecule
in a precipitate, sediment, bioerodible matrix or other solid capable of
releasing the
heterogeneous molecule into aqueous solution upon hydration of the solid.
[114] As used herein, the term "monoclonal antibody" (mAb) refers to an
antibody obtained
from a population of substantially homogeneous antibodies, i.e., the
individual antibodies
comprising the population are identical except for possible naturally
occurring mutations that
may be present in minor amounts. Monoclonal antibodies are highly specific,
being directed
-22-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
against a single antigenic site. Each mAb is directed against a single
determinant on the antigen.
In addition to their specificity, the monoclonal antibodies are advantageous
in that they can be
synthesized by hybridoma culture, uncontaminated by other immunoglobulins. The
modifier
"monoclonal" indicates the character of the antibody as being obtained from a
substantially
homogeneous population of antibodies, and is not to be construed as requiring
production of
the antibody by any particular method. For example, the monoclonal antibodies
to be used in
accordance with the present invention may be made in an immortalized B cell or
hybridoma
thereof, or may be made by recombinant DNA methods.
[115] The monoclonal antibodies herein include hybrid and recombinant
antibodies produced
by splicing a variable (including hypervariable) domain of an CD47 antibody
with a constant
domain (e.g. "humanized" antibodies), or a light chain with a heavy chain, or
a chain from one
species with a chain from another species, or fusions with heterologous
proteins, regardless of
species of origin or immunoglobulin class or subclass designation, as well as
antibody fragments
(e.g., Fab, F(abl, and Fv), so long as they exhibit the desired biological
activity.
[116] The monoclonal antibodies herein specifically include "chimeric"
antibodies
(immunoglobulins) in which a portion of the heavy and/or light chain is
identical with or
homologous to corresponding sequences in antibodies derived from a particular
species or
belonging to a particular antibody class or subclass, while the remainder of
the chain(s) is
identical with or homologous to corresponding sequences in antibodies derived
from another
species or belonging to another antibody class or subclass, as well as
fragments of such
antibodies, so long as they exhibit the desired biological activity.
[117] As used herein, an "isolated" antibody is one which has been identified
and separated
and/or recovered from a component of its natural environment. Contaminant
components of
its natural environment are materials which would interfere with diagnostic or
therapeutic uses
for the antibody, and may include enzymes, hormones, and other proteinaceous
or
nonproteinaceous solutes. In some embodiments, the antibody will be purified
(1) to greater
than 75% by weight of antibody as determined by the Lowry method, and most
preferably
more than 80%, 90% or 99% by weight, or (2) to homogeneity by SDS-PAGE under
reducing or
nonreducing conditions using Coomassie blue or, preferably, silver stain.
Isolated antibody
-23-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
includes the antibody in situ within recombinant cells since at least one
component of the
antibody's natural environment will not be present. Ordinarily, however,
isolated antibody will
be prepared by at least one purification step.
[118] As used herein, the term "epitope tagged" refers to a CD47 antibody
fused to an
"epitope tag". The epitope tag polypeptide has enough residues to provide an
epitope against
which an antibody can be made, yet is short enough such that it does not
interfere with activity
of the CD47 antibody. The epitope tag preferably is sufficiently unique so
that the antibody
specific for the epitope does not substantially cross-react with other
epitopes. Suitable tag
polypeptides generally have at least 6 amino acid residues and usually between
about 8-50
amino acid residues (preferably between about 9-30 residues). Examples include
the c-myc tag
and the 8F9, 3C7, 6E10, G4, B7 and 9E10 antibodies thereto (see, e.g., Evan et
al., Mol. Cell.
Biol., 5(12):3610-3616 (1985)); and the Herpes Simplex virus glycoprotein D
(gD) tag and its
antibody (see, e.g., Paborsky et al., Protein Engineering, 3(6):547-553
(1990)).
[119] As used herein, the term "label" refers to a detectable compound or
composition which
is conjugated directly or indirectly to the antibody. The label may itself be
detectable by itself
(e.g., radioisotope labels or fluorescent labels) or, in the case of an
enzymatic label, may
catalyze chemical alteration of a substrate compound or composition which is
detectable.
[120] As used herein, the term "solid phase" refers to a non-aqueous matrix to
which the
antibody of the present invention can adhere. Examples of solid phases
encompassed herein
include those formed partially or entirely of glass (e.g. controlled pore
glass), polysaccharides
(e.g., agarose), polyacrylamides, polystyrene, polyvinyl alcohol and
silicones. In certain
embodiments, depending on the context, the solid phase can comprise the well
of an assay
plate; in others it is a purification column (e.g., an affinity chromatography
column). This term
also includes a discontinuous solid phase of discrete particles. See, e.g.,
U.S. Pat. No. 4,275,149.
[121] The present invention also provides pharmaceutical compositions
containing these
CD47 antibodies and methods for treating diseases in a subject with these CD47
antibodies or
pharmaceutical compositions.
[122] As used herein, the term "treatment" or "treating" refers to both
therapeutic treatment
and prophylactic or preventative measures of a disease (such as cancer or a
fibrotic disease).
-24-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
Those in need of treatment include those already with the disease as well as
those in which the
disease is to be prevented.
[123] Examples of cancer include, but are not limited to, ovarian cancer,
colon cancer, breast
cancer, lung cancer, head and neck cancer, bladder cancer, colorectal cancer,
pancreatic cancer,
non-Hodgkin's lymphoma, acute lymphocytic leukemia, chronic lymphocytic
leukemia, acute
myeloid leukemia, chronic myelogenous leukemia, multiple myeloma, melanoma,
leiomyoma,
leiomyosarcoma, glioma, glioblastoma, myelomas, monocytic leukemias, B-cell
derived
leukemias, T-cell derived leukemias, B-cell derived lymphomas, T-cell derived
lymphomas, and
solid tumors. The fibrotic disease can be, e.g., myocardial infarction,
angina, osteoarthritis,
pulmonary fibrosis, asthma, cystic fibrosis, bronchitis, or asthma.
[124] As used herein, the term "subject" for purposes of treatment refers to
any animal
classified as a mammal, including humans, domestic and farm animals, and zoo,
sports, or pet
animals, such as dogs, horses, cats, cows, etc. Preferably, the mammal is
human.
[125] The CD47 antibodies of this invention can also be used in vitro and in
vivo to monitor
the course of CD47 disease therapy. Thus, for example, by measuring the
increase or decrease
in the number of cells expressing CD47, particularly cancer cells expressing
CD47, it can be
determined whether a particular therapeutic regimen aimed at ameliorating
disease is effective.
[126] The CD47 antibodies of this invention may be used in vitro in
immunoassays in which
they can be utilized in liquid phase or bound to a solid phase carrier. In
addition, the CD47
antibodies in these immunoassays can be detectably labeled in various ways.
Examples of
types of immunoassays which can utilize monoclonal antibodies of the invention
are flow
cytometry, e.g. FACS, MACS, immunohistochemistry, competitive and non-
competitive
immunoassays in either a direct or indirect format. Detection of the antigens
using the CD47
antibodies of this invention can be done utilizing immunoassays which are run
in either the
forward, reverse, or simultaneous modes, including immunohistochemical assays
on
physiological samples. Those of skill in the art will know, or can readily
discern, other
immunoassay formats without undue experimentation.
[127] The CD47 antibodies of the invention can be bound to many different
carriers and used
to detect the presence of CD47 expressing cells. Examples of well-known
carriers include glass,
-25-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
polystyrene, polypropylene, polyethylene, dextran, nylon, amylases, natural
and modified
celluloses, polyacrylamides, agaroses and magnetite. The nature of the carrier
can be either
soluble or insoluble for purposes of the invention. Those skilled in the art
will know of other
suitable carriers for binding monoclonal antibodies, or will be able to
ascertain such, using
routine experimentation.
[128] There are many different labels and methods of labeling known to those
of ordinary skill
in the art, which find use as tracers in therapeutic methods, for use in
diagnostic methods, and
the like. For diagnostic purposes a label may be covalently or non-covalently
attached to an
antibody of the invention or a fragment thereof, including fragments
consisting or comprising
of CDR sequences. Examples of the types of labels which can be used in the
present invention
include enzymes, radioisotopes, fluorescent compounds, colloidal metals,
chemiluminescent
compounds, and bio-luminescent compounds. Those of ordinary skill in the art
will know of
other suitable labels for binding to the monoclonal antibodies of the
invention, or will be able
to ascertain such, using routine experimentation. Furthermore, the binding of
these labels to
the monoclonal antibodies of the invention can be done using standard
techniques common to
those of ordinary skill in the art.
[129] In some embodiments, a CD47 antibody of this invention is attached to a
nanoparticle,
e.g. for use in imaging. Useful nanoparticles are those known in the art, for
example including
without limitation, Raman-silica-gold-nanoparticle (R-Si-Au-NP). The R-Si-Au-
NPs consist of a
Raman organic molecule, with a narrow-band spectral signature, adsorbed onto a
gold core.
Because the Raman organic molecule can be changed, each nanoparticles can
carry its own
signature, thereby allowing multiple nanoparticles to be independently
detected
simultaneously by multiplexing. The entire nanoparticle is encapsulated in a
silica shell to hold
the Raman organic molecule on the gold nanocore. Optional polyethylene glycol
(PEG)-ylation
of R-Si-Au-NPs increases their bioavailability and provides functional
"handles" for attaching
targeting moieties. See, e.g., Thakor et al (2011), Sci. Transl. Med.,
3(79):79ra33; Jokerst et al.
(2011) Small., 7(5):625-33; Gao et al. (2011) Biomaterials, 32(8):2141-8.
[130] For purposes of the invention, CD47 may be detected by the CD47
antibodies of this
invention when present in biological fluids and on tissues, in vivo or in
vitro. Any sample
-26-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
containing a detectable amount of CD47 can be used. A sample can be a liquid
such as urine,
saliva, cerebrospinal fluid, blood, serum and the like, or a solid or semi-
solid such as tissues,
feces, and the like, or, alternatively, a solid tissue such as those commonly
used in histological
diagnosis.
[131] Another labeling technique which may result in greater sensitivity
consists of coupling
the antibodies to low molecular weight haptens. These haptens can then be
specifically
detected by means of a second reaction. For example, it is common to use
haptens such as
biotin, which reacts with avidin, or dinitrophenol, pyridoxal, or fluorescein,
which can react with
specific anti-hapten antibodies.
[132] As a matter of convenience, a CD47 antibody of this invention can be
provided in a kit,
i.e., a packaged combination of reagents in predetermined amounts with
instructions for
performing the diagnostic assay. Where the antibody is labeled with an enzyme,
the kit will
include substrates and cofactors required by the enzyme (e.g., a substrate
precursor which
provides the detectable chromophore or fluorophore). In addition, other
additives may be
included such as stabilizers, buffers (e.g., a block buffer or lysis buffer)
and the like. The relative
amounts of the various reagents may be varied widely to provide for
concentrations in solution
of the reagents which substantially optimize the sensitivity of the assay.
Particularly, the
reagents may be provided as dry powders, usually lyophilized, including
excipients which on
dissolution will provide a reagent solution having the appropriate
concentration.
[133] Therapeutic formulations comprising one or more antibodies of the
invention are
prepared for storage by mixing the antibody having the desired degree of
purity with optional
physiologically acceptable carriers, excipients or stabilizers (see, e.g.,
Remington's
Pharmaceutical Sciences, 16th edition, Osol, A. Ed. (1980)), in the form of
lyophilized
formulations or aqueous solutions. The antibody composition will be
formulated, dosed, and
administered in a fashion consistent with good medical practice. Factors for
consideration in
this context include the particular disorder being treated, the particular
mammal being treated,
the clinical condition of the individual patient, the cause of the disorder,
the site of delivery of
the agent, the method of administration, the scheduling of administration, and
other factors
known to medical practitioners. The "therapeutically effective amount" of the
antibody to be
-27-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
administered will be governed by such considerations, and is the minimum
amount necessary
to prevent the CD47 associated disease.
[134] The therapeutic dose may be at least about 0.01 pg/kg body weight, at
least about 0.05
pg/kg body weight; at least about 0.1 pg/kg body weight, at least about 0.5
pg/kg body weight,
at least about 1 pg/kg body weight, at least about 2.5 pg/kg body weight, at
least about 5 pg/kg
body weight, and not more than about 100 pg/kg body weight. It will be
understood by one of
skill in the art that such guidelines will be adjusted for the molecular
weight of the active agent,
e.g. in the use of antibody fragments, or in the use of antibody conjugates.
The dosage may
also be varied for localized administration, e.g. intranasal, inhalation,
etc., or for systemic
administration, e.g., intraperitoneal (I.P.), intravenous (I.V.), intradermal
(I.D.), intramuscular
(I.M.), and the like.
[135] A CD47 antibody of this invention needs not be, but is optionally
formulated with one or
more agents that potentiate activity, or that otherwise increase the
therapeutic effect. These
are generally used in the same dosages and with administration routes as used
hereinbefore or
about from 1 to 99% of the heretofore employed dosages.
[136] Acceptable carriers, excipients, or stabilizers are non-toxic to
recipients at the dosages
and concentrations employed, and include buffers such as phosphate, citrate,
and other
organic acids; antioxidants including ascorbic acid and methionine;
preservatives (such as
octadecyldimethylbenzyl ammonium chloride; hexamethonium chloride;
benzalkonium
chloride, benzethonium chloride; phenol, butyl or benzyl alcohol; alkyl
parabens such as methyl
or propyl paraben; catechol; resorcinol; cyclohexanol; 3-pentanol; and m-
cresol); low molecular
weight (less than about 10 residues) polypeptides; proteins, such as serum
albumin, gelatin, or
immunoglobulins; hydrophilic polymers such as polyvinylpyrrolidone; amino
acids such as
glycine, glutamine, asparagine, histidine, arginine, or lysine;
monosaccharides, disaccharides,
and other carbohydrates including glucose, mannose, or dextrins; chelating
agents such as
EDTA; sugars such as sucrose, mannitol, trehalose or sorbitol; salt-forming
counter-ions such as
sodium; metal complexes (e.g., Zn-protein complexes); and/or non-ionic
surfactants such as
TWEENT", PLURONICSTM or polyethylene glycol (PEG). Formulations to be used for
in vivo
-28-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
administration must be sterile. This is readily accomplished by filtration
through sterile filtration
membranes.
[137] The active ingredients containing CD47 antibodies may also be entrapped
in
microcapsule prepared, e.g., by coacervation techniques or by interfacial
polymerization, for
example, hydroxymethylcellulose or gelatin-microcapsule and poly-
(methylmethacylate)
microcapsule, respectively, in colloidal drug delivery systems (for example,
liposomes, albumin
microspheres, microemulsions, nano-particles and nanocapsules) or in
macroemulsions. Such
techniques are disclosed in Remington's Pharmaceutical Sciences 16th edition,
Osol, A. Ed.
(1980).
[138] A CD47 antibody or pharmaceutical composition of this invention can be
administered
by any suitable means, including parenteral, subcutaneous, intraperitoneal,
intrapulmonary,
and intranasal. Parenteral infusions include intramuscular, intravenous,
intraarterial,
intraperitoneal, or subcutaneous administration. In addition, the anti-CD47
antibody is suitably
administered by pulse infusion, particularly with declining doses of the
antibody.
[139] For the prevention or treatment of disease, the appropriate dosage of
antibody will
depend on the type of disease to be treated, as defined above, the severity
and course of the
disease, whether the antibody is administered for preventive purposes,
previous therapy, the
patient's clinical history and response to the antibody, and the discretion of
the attending
physician. The antibody is suitably administered to the patient at one time or
over a series of
treatments.
[140] In another embodiment of the invention, an article of manufacture
containing materials
useful for the treatment of the disorders described above is provided. The
article of
manufacture comprises a container and a label. Suitable containers include,
for example,
bottles, vials, syringes, and test tubes. The containers may be formed from a
variety of
materials such as glass or plastic. The container holds a composition which is
effective for
treating the condition and may have a sterile access port (e.g., the container
may be an
intravenous solution bag or a vial having a stopper pierceable by a hypodermic
injection needle).
The active agent in the composition is the anti-CD47 antibody. The label on,
or associated with,
the container indicates that the composition is used for treating the
condition of choice. The
-29-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
article of manufacture may further comprise a second container comprising a
pharmaceutically-
acceptable buffer, such as phosphate-buffered saline, Ringer's solution and
dextrose solution. It
may further include other materials desirable from a commercial and user
standpoint, including
other buffers, diluents, filters, needles, syringes, and package inserts with
instructions for use.
[141] The following examples are put forth so as to provide those of ordinary
skill in the art
with a complete disclosure and description of how to make and use the present
invention, and
are not intended to limit the scope of what the inventors regard as their
invention nor are they
intended to represent that the experiments below are all or the only
experiments performed.
Efforts have been made to ensure accuracy with respect to numbers used (e.g.
amounts,
temperature, etc.) but some experimental errors and deviations should be
accounted for.
Unless indicated otherwise, parts are parts by weight, molecular weight is
weight average
molecular weight, temperature is in degrees Centigrade, and pressure is at or
near atmospheric.
[142] All publications and patent applications cited in this specification are
herein
incorporated by reference as if each individual publication or patent
application were
specifically and individually indicated to be incorporated by reference.
[143] The present invention has been described in terms of particular
embodiments found or
proposed by the present inventor to comprise preferred modes for the practice
of the
invention. It will be appreciated by those of skill in the art that, in light
of the present
disclosure, numerous modifications and changes can be made in the particular
embodiments
exemplified without departing from the intended scope of the invention. For
example, due to
codon redundancy, changes can be made in the underlying DNA sequence without
affecting the
protein sequence. Moreover, due to biological functional equivalency
considerations, changes
can be made in protein structure without affecting the biological action in
kind or amount. All
such modifications are intended to be included within the scope of the
appended claims.
Establishment of Phage Library
[144] CD47 is a 50 kDa membrane receptor that has extracellular N-terminal IgV
domain, five
transmembrane domains, and a short C-terminal intracellular tail. Human CD47-
IgV domain
protein conjugated with human Fc or Biotinylated human CD47-IgV domain protein
(ACRO
Biosystems) was used as antigen for phage library panning.
-30-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[145] The phage library was constructed using phagemid vectors which consisted
of the
antibody gene fragments that were amplified from spleens or bone marrows of
>50 healthy
human subjects. The antibody format is single chain variable fragment (VH +
linker +VL). The
library size was 1.1x101 and the sequence diversity was analyzed as follows.
For the 62 clones
picked up from the library and further sequenced, 16 sequences have
truncation, frameshift or
amber codon; 46 sequences have full length scFv of which all the HCDR3
sequences are unique.
In the 46 full length scFv, 13 sequences have lambda light chain and 33
sequences have kappa
light chain.
Phage Panning and Clone Selection
[146] To obtain phage clones that specifically bind to the human CD47-IgV
domain, two
methods for phage panning were used.
1. Phage library immunotube panning against human CD47-IgV
[147] In this method, the phage libraries developed as described above were
first incubated in
casein-coated immunotube for 2 hours. The human CD47-IgV-Fc fusion protein was
used for
first round of panning. Unbound phages were removed by washing with PBST for 5-
20 times.
The bound phages were eluted with freshly prepared 100 mM Triethylamine
solution and
neutralized by addition a Tris-HCI buffer, to become the first output phage
pools. This first
output phage pool was rescued through infection of E. Coli TG-1 cells for
amplification, followed
by the second round of panning using biotinylated human CD47-IgV as antigen.
The bound
phages were eluted in the same process and became the second output phage pool
which was
then rescued and then again followed by the third round of panning using human
CD47-IgV-Fc
fusion protein as antigen. The bound phages then became the third output phage
pool and
underwent the fourth round of panning using biotinylated human CD47-IgV.
2. Phage library solution panning against human CD47-IgV
[148] In this second method, the phage libraries were first incubated in
casein-blocked 100 pi
streptavdin-magnetic beads to deplete streptavdin beads binders. The
streptavidin-magnetic
beads and AG0084-hulgG1/k were used for negative depletion. The depleted
library was
rescued, which was followed by the second round of panning using biotinylated
human CD47-
1gV as antigens and further underwent negative depletion with casein blocked
streptavdin-
-31-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
magnetic beads. The unbound phages were removed by washing with PBST for 5-20
times. The
bound phages were eluted with a freshly prepared 100 mM Triethylamine
solution, neutralized
by addition of a Tris-HCI buffer, and then rescued, which was followed by the
third round of
panning using human CD47-IgV-Fc fusion protein and depleted with AG0084-
hulgG1/k. The
bound phages then become the third output phage pool and underwent the fourth
round of
panning using biotinylated human CD47-IgV and negative depletion with casein
blocked
streptavdin-magnetic beads.
[149] After this process, multiple phage clones that specifically bound to the
human CD47-IgV
domain were obtained and enriched. They were then diluted and plated to grow
at 37 C for 8
hours and captured by anti-kappa antibody-coated filter overnight.
Biotinylated human CD47-
1gV (50 nM) and NeutrAvidin-AP conjugate (1:1000 dilution) were applied to the
filter to detect
the positively bound phage clones. Positive phage plaques were picked and
eluted into 100 pi
of phage elution buffer. About 10-15 pi eluted phages were used to infect 1 mL
XL1 blue cells
to make high titer phage (HT) for Phage single point [LISA (SP[). The positive
single clones
picked from the filer lift were subjected to the binding of human CD47-IgV-Fc
fusion protein
and biotinylated human CD47-IgV domain protein. These positive single clones
were also
sequenced for their VH and VL genes. All the positive hits with unique VH and
VL genes were
cloned into expression vectors pFUSE2ss-CLIg-hk (light chain, InvivoGen, Cat
No. pfuse2ss-hclk)
and pFUSEss-CHIg-hG1 (heavy chain, InvivoGen, Cat No. pfusess-hchg1). The
antibodies were
expressed in HEK293 cells and purified by Protein A Plus Agarose.
Affinity maturation of CD-47 antibodies
[150] Binding affinity of the CD-47 antibodies of this invention can be
improved by in vitro
affinity maturation, e.g., by site-specific randomized mutation, which
resulted in mutated
sequences that are also within the scope of this invention.
[151] For example, BiaCore analysis of 1F8, a CD47 antibody of this invention,
showed a
binding affinity (KD) of 2.8 nM with a high dissociation rate of 1.04E-03 its,
which could be
improved by in vitro affinity maturation. An extensive analysis of the CDR
sequence of heavy
chain and light chain of 1F8 identified several residues in HCDR1 and LCDR1
regions that could
be randomized mutated. Therefore, the random mutagenesis libraries can be
constructed and
-32-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
introduced into the specific residues to generate a variety of new sequences.
The CDR
mutagenesis libraries are panned using biotinylated soluble CD47 [CD in
solution phase under
the equilibrium condition. After multiple rounds of panning with reduced
antigen concentration,
enriched output binders are selected for the binding ELISA test and subsequent
converted into
full IgGs which are subjected to the BiaCore analysis to specifically select
for the off-rate
improved sequence. Through this screening process, antibody molecules of this
invention can
be constructed for overall best properties for clinical applications.
Example 1. ELISA screening of phage clones binding to recombinant CD47-ECD
protein
[152] Recombinant human CD47-Fc fusion protein (Acrobiosystems) was coated at
2 ug/mL in
phosphate buffer saline (PBS) onto microtiter plates for 2 hours at the room
temperature (RT).
After coating of antigen, the wells were blocked with PBS/0.05% Tween (PBST)
with 1% BSA for
1 hour at the room temperature (RT). After washing of the wells with PBST,
purified phages
from single clones were added to the wells and incubated for 1 hour at RT. For
detection of the
binding phage clones, the HRP conjugated secondary antibodies against M13
(Jackson Immuno
Research) were added, followed by the addition of fluorogenic substrates
(Roche). Between all
incubation steps, the wells of the plate were washed with PBST three times.
Fluorescence was
measured in a TECAN Spectrafluor plate reader. The positive phage clones were
selected for
sequencing of the heavy chain and light chain genes.
[153] All of the tested CD47 antibodies of this invention showed good binding
activities for
recombinant human CD47-Fc fusion protein.
Example 2. ELISA analysis of antibodies blocking the interaction of CD47 to
SIRPa
[154] Recombinant human CD47/mouse Fc fusion protein or biotinylated CD47
protein
(Acrobiosystems) was coated at 1 ug/mL in PBS onto microtiter plates for 2
hours at RT. After
coating of antigen the wells were blocked with PBS/0.05% Tween (PBST) with 1%
BSA for 1 hour
at RT. After washing of the wells with PBST, the antibodies diluted in PBS
were added to the
wells (5 ug/mL) and incubated for 1 hour at RT. For detection of the binding
antibodies, the
HRP conjugated secondary antibodies against human Fc (Jackson Immuno Research)
were
added, followed by the addition of fluorogenic substrates (Roche). Between all
incubation
-33-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
steps, the wells of the plate were washed with PBST three times. Fluorescence
was measured
in a TECAN Spectrafluor plate reader.
[155] All of the tested CD47 antibodies of this invention showed good binding
activities for
recombinant human CD47-Fc fusion protein and biotinylated CD47 protein.
Example 3. ELISA analysis of antibodies blocking the interaction of CD47 to
SIRPa
[156] Recombinant CD47-Fc fusion protein (Acrobiosystems) was coated at 1
ug/mL in PBS
onto microtiter plates for 16 hours at 4 C. After blocking for 1 hour with 1%
BSA in PBST at RT,
1 ug/ml of SIRPa-His protein was added either in the absence or presence of
CD47 antibodies
(10 ug/mL) at RT for 1 hour. Plates were subsequently washed three times and
incubated with
an HRP-conjugated anti-His secondary antibody for 1 hour at RT. After washing,
the TMB
solution was added to each well for 30 minutes and the reaction was stopped
with 2.0 M H2504,
and OD was measured at 490 nm.
[157] All of the tested CD47 antibodies of this invention effectively blocked
the CD47 protein-
SIRPa binding.
Example 4. Dose-dependent response of CD47 antibodies binding to monomeric
CD47-ECD
[158] After direct binding and competition screening, a CD47 antibody of this
invention 1F8
was selected for this test, in comparison with two existing reference
antibodies. Biotinylated
CD47 protein (Acrobiosystems) was coated at 1 ug/mL in PBS onto microtiter
plates for 2 hours
at RT. After coating of antigen, the wells were blocked with PBS/0.05% Tween
(PBST) with 1%
BSA for 1 hour at RT. After washing of the wells with PBST, different
concentrations of CD47
antibodies were added to the well and incubated for 1 hour at RT. For
detection of the binding
antibodies, the HRP conjugated secondary antibodies against human Fc (Jackson
Immuno
Research) were added followed by the addition of fluorogenic substrates
(Roche). Between all
incubation steps, the wells of the plate were washed with PBST three times.
Fluorescence was
measured in a TECAN Spectrafluor plate reader.
[159] Reference antibodies 5F9 and 2A1 was produced according to the sequence
of Hu5F9
and CC-90002 as disclosed by researchers at Stanford University, Inhibrx LLC,
and Celgene Corp.
(see, e.g., US Pat. No. 9,017,675 B2, US Pat. No. 9,382,320, US Pat. No.
9,221,908, US Pat.
Application Pub. No. 2014/0140989 and WO 2016/109415) and used for the same
study.
-34-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[160] As shown in Fig. 1, all three antibodies (1F8, 5F9, and 2A1) showed
similar binding
activities to monomeric CD47-ECD.
Example 5. Dose-dependent response of CD47 antibodies binding to dimeric CD47-
ECD
[161] The three CD47 antibodies used in Example 4 (i.e., 1F8, 5F9, and 2A1)
were also used in
this study.
[162] CD47/mouse Fc fusion protein (Acrobiosystems) was coated at 1 ug/ml in
PBS onto
microtiter plates for 2 hours at RT. After coating of antigen the wells were
blocked with
PBS/0.05% Tween (PBST) with 1% BSA for 1 hour at RT. After washing of the
wells with PBST,
different concentrations of anti-CD47 antibodies were added to the well and
incubated for 1 at
RT. For detection of the binding antibodies, the HRP conjugated secondary
antibodies against
human Fc (Jackson Immuno Research) were added followed by the addition of
fluorogenic
substrates (Roche). Between all incubation steps, the wells of the plate were
washed with PBST
three times. Fluorescence was measured in a TECAN Spectrafluor plate reader.
[163] Likewise, as shown in Fig. 2a, among the three tested antibodies 1F8,
5F9, and 2A1, all
of them showed similar binding activities to dimeric CD47-ECD.
[164] Another binding study was conducted to compare the binding affinity of
two antibodies
of this invention, i.e., 1F8 and 13H3, to recombinant CD49-ECD. As shown in
Fig. 2b, these two
antibodies also exhibited similar binding activities in a dose-dependent
manner, with ECK, being
0.038 nM for 1F8 and 0.045 nM for 13H3.
Example 6. Dose-dependent response of CD47 antibodies blocking the binding of
CD47 to
SIRPa
[165] Three CD47 antibodies (i.e., 1F8, 5F9, and 2A1) were also used in this
study.
[166] Recombinant CD47-Fc fusion protein (Acrobiosystems) was coated at 1
ug/ml in PBS
onto microtiter plates for 16 hours at 4 C. After blocking for 1 h with 1%
BSA in PBST at RT, 1
ug/mL of SIRPa-His protein was added either in the absence or presence of
different
concentrations of anti-CD47 antibodies at RT for 1 h. Plates were subsequently
washed three
times and incubated with an HRP-conjugated anti-His secondary antibody for 1 h
at RT. After
washing, the TMB solution was added to each well for 30 min and the reaction
was stopped
with 2M H2504, and OD was measured at 490 nm.
-35-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[167] Again, as shown in Fig. 3a, all three antibodies showed similar
activities in blocking the
binding of CD47 to SIRPa.
[168] Another study was conducted to compare the ability of two CD47
antibodies of this
invention 1F8 and 13H3 to block the binding of CD47 to SIRPa. As shown in Fig.
3b and Fig. 3c,
these two antibodies also exhibited similar blocking activities in a dose-
dependent manner,
with ICso being 0.78 nM for 1F8 and 0.20 nM for 13H3.
Example 7. Dose-dependent response of CD47 antibodies binding to CD47 + Raji
cells
[169] Three CD47 antibodies (i.e., 1F8, 5F9, and 2A1) were also used in this
study.
[170] Raji cells which endogenously express human CD47 on the surface were
stained with
different concentrations of 1F8, 5F9 and 2A1 antibodies at 4 C for 30 minutes.
Then, the cells
were washed with PBS three times, followed by incubation with APC-labeled anti-
human Fc
specific antibody (Invitrogen) at 4 C for 30 minutes. Binding was measured
using a FACSCanto
(Becton-Dickinson).
[171] As shown in Fig. 4a, all three antibodies showed similar activities in
binding to CD47 + Raji
cells, following the same dose-dependent pattern.
[172] Another study was conducted to compare the ability of two CD47
antibodies of this
invention 1F8 and 13H3 to bind to CD47-bearing Raji cells. As shown in Fig.
4b, 13H3 exhibited
stronger affinity than 1F8 in binding CD47-bearing Raji cells, with ECso being
2.95 nM for 1F8
and 1.06 nM for 13H3.
[173] Fig. 4c and Fig. 4d show the binding kinetics of 1F8 and 13H3,
respectively, as measured
by Biocore analysis; and Fig. 4e shows the data.
Example 8. Study of phagocytosis of tumor cells by human macrophage (MCD)
[174] Three CD47 antibodies (i.e., 1F8, 5F9, and 2A1) were also used in this
study.
[175] PBMCs were isolated from human blood, and the monocytes were
differentiated into
macrophages for 6 days. The monocyte derived macrophages (MDMs) were scraped
and re-
plated in 24-well dishes and allowed to adhere for 24 hours. The human tumor
cell line Raji
which endogenously expressed CD47 were chosen as target cells and labeled with
1 uM CFSE
for 10 minutes, then added to MDMs at a ratio of 5:1 tumor cells per phagocyte
and CD47
antibodies was added at various doses. After incubation for 3 hours, non-
phagocytosed target
-36-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
cells were washed away with PBS and the remaining phagocytes were scraped off,
stained with
macrophage marker CD14 antibody, and analyzed by flow cytometry. Phagocytosis
was
measured by gating on CD14+ cells and then assessing the percent of CFSE
cells.
[176] As shown in Fig. 5a, all these three tested antibodies (i.e., 1F8, 5F9,
and 2A1) showed
similar activities in promoting phagocytosis of tumor cells by human MCD.
Figs. 6a, 6b, and 6c
show the macrophage-mediated phagocytosis of three different human blood
cancer cell lines,
triggered by the three CD47 antibodies.
[177] Another study was conducted to compare the ability of two CD47
antibodies of this
invention 1F8 and 13H3 to promote phagocytosis of tumor cells by human MCD. As
shown in Fig.
5b, 13H3 and 1F8 exhibited similar abilities with 13H3 slightly stronger
phagocytosis at some
concentrations.
Example 9. RBC-Sparing Property in RBC Agglutination Assay
[178] Human RBCs were diluted to 10% in PBS and incubated at 37 C for 2 hours
with a
titration of CD47 antibodies in a round bottom 96-well plate. Evidence of
hemagglutination is
demonstrated by the presence of non-settled RBCs, appearing as a haze compared
to a
punctuate red dot of non-hemagglutinated RBCs (see Figs. 7a and 8a). The
graphs in Figs. 7b
and 8b show the quantitation of the hemagglutination assay, denoted
"agglutination index"
determined by quantitating the area of the RBC pellet in the presence of the
antibody,
normalized to that of IgG control.
[179] As shown in Figs. 7a, 7b, 8a, and 8b, while CD47 antibody 5F9 already
showed significant
RBC agglutination at a concentration of or higher than 0.1 ug/uL, CD47
antibodies 1F8 and 2A1
resulted in essentially no RBC agglutination at the tested concentrations up
to 30 ug/uL (Figs. 7a
and 7b) or even up to 150 ug/mL (Figs. 8a and 8b).
[180] Likewise, Figs. 8c and 8d show that CD47 antibodies of this invention
(i.e., 1F8 and 13H3)
resulted in essentially no RBC agglutination at the tested concentrations up
to 150 ug/mL,
whereas CD47 antibody 5F9 already showed significant RBC agglutination at a
concentration of
or higher than 0.1 ug/uL.
Example 10. RBC Binding Assay
-37-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[181] Binding of CD47 antibodies against human RBCs was examined by flow
cytometry.
Human RBCs were incubated with CD47 antibodies (10 ug/mL) at 4 C for 1 hour,
followed by
the addition of APC-conjugated secondary antibody at 4 C for 30 minutes.
[182] As shown in Figs. 9a and 9b, surprisingly, CD47 antibody of this
invention 1F8 did not
bind to RBC while reference CD47 antibodies 5F9 and 2A1 did at the tested
concentrations.
[183] Likewise, Figs. 9c and 9d show that while 1F8 resulted in no RBC binding
at the tested
concentrations, 13H3 only resulted in very low RBC binding at the tested
concentrations.
Example 11. RBC Agglutination Assay
[184] RBCs were collected from six male and six female healthy individuals for
the analysis of
RBC agglutination by the addition of CD47 antibodies. Figs. 10a and 10b show
the titration
results of the hemagglutination assay, which is denoted "agglutination index"
as determined by
measuring the area of the RBC pellets in the presence of the antibody,
normalized to that of IgG
control or reference antibody.
Example 12. Platelet Binding Assay
[185] Binding of CD47 antibodies of this invention against human platelets was
examined by
flow cytometry. Human peripheral whole blood was incubated with test CD47
antibodies of
this invention (at 10 ug/mL) or SIRPa-Ig fusion and CD61 was stained as a
surface marker for
platelets. The binding of CD47 antibodies or SIRPa-Ig fusion was measured by
gating on the
CD61 positive population (platelet) and further examining the percentages of
CD47 or SIRPa-Ig
fusion binding.
[186] As shown in Fig. 11, tested CD47 antibodies of this invention did not
appreciably bind to
human platelets whereas SIRPa proteins did.
Example 13. Cyno RBC Agglutination Assay
[187] RBCs from male and female cyno monkey were diluted to 10% in PBS and
incubated at
37 C for 2 hours with the indicated concentrations of CD47 antibodies in a
round bottom 96-
well plate. Evidence of hemagglutination was demonstrated by the presence of
non-settled
RBCs, appearing as a haze compared to a punctuate red dot of non-
hemagglutinated RBCs, as
shown in Fig. 12a. Fig. 12b shows the titration results of the
hemagglutination assay, which is
-38-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
denoted "agglutination index" as determined by measuring the area of the RBC
pellets in the
presence of the antibody, normalized to that of IgG control.
[188] The data show that the tested CD47 antibodies of this invention did not
appreciably
induce cyno RBC agglutination in vitro.
Example 14. Phagocytosis of Primary Human AML Cells by CD47 Antibodies
[189] Primary PBMCs from AML patient (AML-PB003F) were labeled with 1uM CFSE
for 10
minutes, then added to MDMs at a ratio of 5:1 tumor cells per phagocyte and
the indicated
CD47 antibodies was added at various concentrations. After 3-hr incubation,
non-
phagocytosed target cells were washed away with PBS and the remaining
phagocytes were
scraped off, stained with a CD14 antibody, and analyzed by flow cytometry.
Phagocytosis was
measured by gating on CD14+ cells and then assessing the percentage of CFSE+
cells.
Phagocytosis was measured as previously mentioned.
[190] As shown in Figs. 13a-13h, the tested CD47 antibodies of this invention
all showed
significant AML binding capabilities (greater than 75%) and phagocytosis
capabilities (at least
36%), all of which are much higher than the reference CD47 antibody used in
the same essay.
Example 15. In Vivo Efficacy of 1F8 Using Luciferase-Raji Xenograft Model
(CDX)
[191] NSG mice were engrafted with Raji Luc-EGFP at a concentration of 1
million cells/mouse
via tail vein injection. They were imaged in vivo to determine the level of
engraftment five days
post engraftment. Treatment of CD47 antibodies (i.e., 1F8, 5F9, and 2A1)
started from the same
day at a dose of 10 mg/kg. All mice were injected every other day via
intraperitoneal injection.
Mice were imaged in vivo via IVIS Lumina III imaging system at the following
time points: Day 0
of antibody treatment, Day 2 of treatment, Day 6 of treatment, and Day 9 of
treatment. The
tumor growth in the mice was measured by the analysis of bioluminescent
radiance through in
vivo live imaging system.
[192] As can be seen in Fig. 14a, the analysis of bioluminescent radiance
shows that the
tumors in the mice barely grew within the first three days after the
treatments with the tested
CD47 antibody of this invention (i.e., 1F8) and the tumors reduced from day 6
after the
treatments. By comparison, the tumors in the mice treated with reference CD47
antibody
continued to grow during the same treatment period.
-39-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[193] Similarly, Fig. 14b shows that the CD47 antibody 13H3 was also effective
in vivo in Raji
xenograft model at different test concentrations.
[194] In the end of Raji-xenograft study, all the mice were euthanized by the
use of CO2 for
rodent euthanasia. The splenocytes from four groups of mice were isolated and
analyzed for
the percentage of Ml macrophages (% of CD80 positive in F4/80 positive
macrophages) and M2
macrophages (% of CD206 positive in F4/80 positive macrophages) by flow
cytometry analysis.
[195] As shown in Figs. 15a-15b, all of the tested CD47 antibodies (including
1F8) were able to
induce polarization of macrophage in tumor-bearing mice.
Example 16. CD47 expression profile using PDX samples of various human cancer
types
[196] 54 PDX samples (across 7 human cancer types) were analyzed for the
expression of
CD47 by immuno-histochemistry staining. The levels of CD47 staining in various
PDX samples
were scored by geometry and staining intensity. Figs. 16a, 16b and 16c show
the different
expression levels of CD47 after the treatments with CD47 antibodies.
Example 17. Safety Pharma Study (In vivo Cyno PK Studies)
[197] Naïve cyno monkeys were intravenously infused with vehicle (n=2), 1F8
(n=3, 15 mg/kg)
and 5F9 (n=3, 15 mg/kg). Hematology (CBC) was analyzed within 24 hours after
blood collection,
twice before the injections and at 3, 6, 10, 14 and 21 days following the
antibody administration.
CBC parameters were examined including Erythrocyte count (RBC), Hemoglobin
(HGB),
Absolute Reticulocytes and Platelet Counts. The results are depicted in Figs.
17a-17d and
showed that 1F8 treatments did not affect the hematology parameters in cyno
monkey.
[198] Similarly, Naïve cyno monkeys (n=2) were intravenously injected with
CD47 antibody
13H3 at a dose of 20 mg/kg. Their blood was collected by venipuncture into
tubes with no
anticoagulant at different time points. Serum level of the CD47 antibody 13H3
was measured
by [LISA using CD47 protein as the coating reagent, followed by detection with
an HRP-
conjugated anti-human Kappa secondary antibody. Pharmacokinetic parameters in
cyno
monkeys were analyzed by Winolin and shown in Fig. 17e and Table 1.
Table 1
T112 (h) Cmax AUCo-t AUCint CL
-40-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
(Wm!) (day*ug/m1) (day*ug/m1) (ml/hr/kg)
145.2 10.8 536.4 63.9 10692.1 1300.9 10712.5 1298.4
1.880 0.228
Safety Pharm Study (Hematology) of Antibody 13H3 in Cynomolgus Monkey
[199] Naïve cyno monkeys were intravenously infused with single dose or repeat
dose (weekly
dosing) of the antibody 13H3 (20 mg/kg). Hematology (CBC) parameters were
examined
including Erythrocyte count (RBC), Hemoglobin (HGB), Platelet Counts and
Lymphocyte Counts
at the indicated time points following the antibody administration.
[200] Figs. 19a, 19b, 19c, 19d, 19e, 19f, 19g, and 19h show the effects of the
CD47 antibody
13H3 on RBC congregation, hemoglobin, platelets, and lymphocytes.
Example 18. Structure of Antibody 1F8
[201] The epitope binning of CD47 antibodies was assessed by competition
[LISA. CD47 [CD
protein and first anti-CD47 antibody were pre-incubated and added to a
biotinylated second
anti-CD47 antibody detected by a Streptavidin-HRP antibody. If the first anti-
CD47 antibody
competed against the binding of CD47 [CD to the second antibody, both
antibodies were
placed in same or overlapping epitope bins. If not, they were placed in non-
overlapping epitope
bins. The results depicted in Figs. 18a and 18b show that CD47 antibody of
this invention 1F8
has a different epitope than those of reference antibodies 5F9 and 2A1.
[202] Fig. 18c shows the crystal structure of reference antibody 5F9 (upper
part) in complex
with human CD47-[CD (green) as reported in the literature (See, e.g., J. Clin.
Investigation, 126,
7: 2610-2620).
[203] Fig. 18d shows the crystal structure of 1F8-Fab (upper part) in complex
with human
CD47-[CD (green). The complex structure of CD47-1F8 Fab adopts straighter head
to head
orientation, unlike the complex structures of CD47-SIRPa and CD47-5F9 diabody
presenting
tilted head to head orientation. The 1F8 epitope on CD47 is discontinuous and
extensive which
includes residues L3, V25, T26, N27, M28, E29, A30, 031, T34, E35, Y37, A53,
L54, L74, K75, G76,
T99, E100, L101, T102 and R103, of which L3, N27, E29, 031, T34, E35, Y37,
A53, T99, E100,
L101, T102 and R103 are involved in the interactions with SIRPa, explaining
the antagonistic
-41-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
properties of 1F8. The complex structure also reveals VH domain of 1F8 forms 8
hydrogen
bonds and 4 salt bridges to CD47 and VL domain of 1F8 forms 8 hydrogen bonds
to CD47 as well.
[204] Unlike published CD47-IgWantibody or SIRPa complex structures, the 1F8
antibody
binds mostly different epitopes of the target although all are binding in the
similar head-to-
head orientation. The 1F8 epitope on CD47 is conformationally discontinuous
and includes a
TNMEAQ loop (residues 26-31), T34, E35, L74, and an LTR hinge (residues 101 -
103) of CD47.
Many hydrogen bond interactions are formed between side chains of antibody
residues and
CD47 main chain oxygen atoms. A salt bridge is also formed between R103 of 1F8
and E35 of
CD47. Several Van der Waals contacts are also observed which are critical to
keep appropriate
orientation. The VH domain of antibody 1F8 is primarily involved in binding to
the T34, E35 and
the LTR hinge (residues 101-103) of CD47, while the VK domain interact with
the TNMEAQ loop
(residues 26-31) and L74. These epitopes on CD47 are different from that in
5F9 antibody and
SIRPa. Structural analysis suggest that two long loops (residues 26-38 and 52-
59) of the 1F8
antibody help it bind to CD47 in a nearly vertical orientation which may lead
to the antibody to
be separated in such a way that CD47 on adjacent cells could not be bridged by
the antibody,
thereby preventing most of blood cell hemaglutination.
[205] Fig.18e shows the comparison of interaction of 5F9 and 1F8 with CD47.
[206] Superposition of reference antibody 5F9/CD47 complex structure on
complex structure
of 1F8/CD47 reveals that binding orientation of CD47 is very different between
these two
complexes. Although both antibodies have head¨to-head binding orientation,
CD47 is rotated
horizontally by about 180 degree. The structure of 1F8/CD47 complex has CD47 N-
terminal
pyroglutamate near light chain loop residues 61-64, while 5F9 has CD47 N-term
among 3 heavy
chain loops of W52, N32 and W101. In antibody 1F8, the heavy chain residues
Trp33 and
Arg103 form van der Waals contact and a salt bridge with Leu101 and Glu35 of
CD47,
respectively. At the same position, antibody 5F9's residue Tyr101 point
towards N-term of
CD47 through a van der Waals contact and Arg102 forms a hydrogen bond with
Glu104 of CD47.
Antibody 1F8's loop residues Asn31, Trp33, and hinge residues Arg53 and Asp56
form inter-
domain hydrogen bonds net, then Asn31 and Arg53 form hydrogen bonds with main
chain of
Leu101 and Thr34 in CD47. At the same interface, 5F9 does not appear to make
interaction,
-42-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
except residue Tyr 52 forms a van der Waals contact with Leu3 on CD47. The
hinge (residue 52-
56) is 3 residues shorted than that of 1F8 (residues 52-59). In light chain,
both Fab 1F8 and 5F9
have several important hydrogen bond interactions with CD47 from the loop (V29
-Y38 in 1F8
and V152-Y158 in 5F9). Residues Y97 and Y98 in 1F8 "push" the loop (residues
26-38) away,
and the latter formed 2 hydrogen bonds between 1F8 and CD47, namely between
Arg34 of 1F8
and main chain of Leu74 on CD47, and between Arg36 of 1F8 and main chain of
Thr26 on CD47.
However, 5F9's residues Gly218 and Ser219 (which correspond to Tyr97 and Tyr98
in 1F8) cause
the loop (residues 149-158) in 5F9 to form 3 hydrogen bonds with CD47 (at
Asn157-Lys39,
Tyr159-G1u104 and Lys177-Thr99,). Also like that in heavy chain, the loop
(residues 149-158) in
5F9 is about 3 residues shorter than that in 1F8 (residues 26-38). These
relative longer loops in
1F8 mainly contribute to the binding orientation of the CD47.
Example 19. CD47 Antibody 34C5
[207] To generate anti-human CD47 antibodies, different strains of 6-8-week
mice including
BALB/C, C57/BL6 or SJL mice were immunized with recombinant human CD47
extracellular
domain protein for several rounds. After immunization, mice with sufficient
titres of anti-CD47
IgG were boosted with the same antigen followed by fusion. The hybridoma
supernatants were
tested for direct binding with human CD47 [CD protein and competition of SIRPa
binding to
CD47 by [LISA screening. Through a series of screening assays, 34C5 was
selected for the
humanization and further in vitro characterization according to the assays
described above.
[208] Fig. 20 and Fig. 21 show strong binding affinity of 34C5 to recombinant
CD47-[CD (with
an ECK, of 0.27 nM) and to CD47-bearing Raji cells (with an ECK, of 0.83 nM),
respectively.
[209] Fig. 22 shows that 34C5 was able to effectively block CD47 binding to
SIRPa, with an
ECK, of 0.30 nM.
[210] Fig. 23 shows that the antibody 34C5 promoted phagocytosis of tumor
cells by human
MO.
[211] Fig. 24 shows the antibody 34C5 did not cause in vitro RBC
agglutination.
[212] Fig. 25 shows the antibody 34C5 decrease its binding to RBC with the
decreasing
concentration of this antibody.
Example 20. Preparation of Fusion Proteins
-43-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[213] Human GM-CSF cytokine was fused to the heavy chain C terminus of anti-
CD47 antibody
(1F8) via various length of linkers including (GGGGS)3, (GGGGS)6, (GGGGS)9,
IGD(F30), IGD(F64),
IGD(R30), IGN(R64), IGD(R30-Cys), and IGD(R64-Cys) or without a linker. Then,
the light chain
and heavy chain expression vectors were co-transfected into CHO cells. After
transient
transfection, the fusion proteins were purified from the medium by protein A
affinity
chromatography.
Example 21. Screening of linkers for fusion proteins
[214] Table 2 shows the agrregates, main peak, fragments, and yield of some
examples of the
fusion proteins of this invention, without a linker or with one of several
different linkers.
Table 2
Linker Aggregates Main peak Fragments Yield
(mg/L)
1F8-GMCSF 0.82% 92.13% 7.04% 2.8
1F8-(G4S)3-GMCSF I NA NA .. J ...
NA 1.9
1F8-(G4S)6-GMCSF 0.27% 93.68% .. J
6.04% 2.5
1F8-(GS)9-GMCSF 0.15% 94.83% 5.03% 2.3
1F8-IGD(F30)-GMCSF 0.21% 90.83% 8.96% 1.9
1F8-IGD(F64)-GMCSF 96.94% 3.06% 1.8
1F8-IGD(R30)-GMCSF 0.69% 92% 7.31% 1.1
1F8-IGD(R64)-GMCSF 0.75% 94.77% 4.48% 1.5
1F8-IGD(R30-Cys)-GMCSF 1 0.87% 90.79% 8.34% J
1.4
1F8-IGD(R64-Cys)-GMCSF 0.84% 91.31% 7.84% 1.4
Example 22. Binding of fusion proteins to recombinant CD47 protein
-44-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[215] Test was conducted for dose response of [LISA binding of 1F8-GMCSF, a
fusion protein
of this invention, to biotinylated human CD47-[CD protein (1 ug/m1@100 u1). In
this test,
biotinylated CD47 protein (Acrobiosystems) was coated at 1 ug/ml in PBS onto
microtiter plates
for 2 hours at the room temperature. After coating of antigen, the wells were
blocked with
PBS/0.05% Tween (PBST) with 1% BSA for 1 hour at the room temperature. After
washing of the
wells with PBST, different concentrations of 1F8-GMCSF fusion molecules were
added to the
well and incubated for 1 hour at the room temperature. For detection of the
binding
antibodies, the HRP conjugated secondary antibodies against human Fc (Jackson
Immuno
Research) were added, followed by the addition of fluorogenic substrates
(Roche). Between all
incubation steps, the wells of the plate were washed with PBST three times.
Fluorescence was
measured in a TECAN Spectrafluor plate reader. The CD47 antibody 1F8 itself
was used as
reference.
[216] The data show that CD47 antibody 1F8 and the fusion protein 1F8-GMCSF
exhibited
similar binding affinity to recombinant CD47 protein.
Example 23. Blocking of CD47-SIRPa Interaction by Fusion Proteins
[217] Blocking of CD47-SIRPa interaction was performed according to the
manufacturer's
protocol (Cisbio). Briefly, the CD47-SIRPa binding assay utilized HTRF
(Homogeneous Time-
resolved Fluorescence) technology to enable the detection of CD47-SIRPa
interaction in a high
throughput format. Antibody working solutions and Tag1-CD47/Tag-2 SIRPa
protein in the
dilution buffer were prepared. The CD47 antibodies or anti-CD47-GMCSF fusion
molecules
were added in a 384-well plate, followed by the addition of Tag1-CD47 and Tag2-
SIRPa. The
mixture was incubated at 25 C for 15 minutes, and further incubated at 25 C
for 1 hour after
conjugates pre-mixture was added. Then the plate sealer was removed and
fluorescence data
was read on a PerkinElmer Envision plate reader.
[218] The data showed that 1F8-GMCSF exhibited stronger blocking capability
than 1F8 itself,
with ICso of 1.3 nM for 1F8-GMCSF as compared to ICso of 1.6 nM for 1F8.
Example 24. RBC Sparing Properties of Fusion Proteins
[219] Human RBCs were diluted to 10% in PBS and incubated at 37 C for 2 hours
with a
titration of 1F8 or 1F8-GMCSF fusion protein in a round bottom 96-well plate.
Evidence of
-45-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
hemagglutination would be demonstrated by the presence of non-settled RBCs,
appearing as a
haze compared to a punctuate red dot of non-hemagglutinated RBCs. The result
of this study
showed that both 1F8 and 1F8-GMCSF did not induce the RBC agglutination at the
indicated
concentrations.
Example 25. Increased Phagocytosis of Tumor Cells by Fusion Proteins
[220] PBMCs were isolated from human blood, and the monocytes were
differentiated into
macrophages for 6 days. The monocyte derived macrophages (MDMs) were scraped
and re-
plated in 24-well dishes and allowed to adhere for 24 hrs. The human tumor
cell line Raji which
endogenously expressed CD47 were chosen as target cells and labeled with 1uM
CFSE for 10
minutes, then added to MDMs at a ratio of 5:1 tumor cells per phagocyte. 1F8,
GM-CSF protein,
or 1F8-GMCSF fusion protein was added at various doses. After incubation for 3
hours, non-
phagocytosed target cells were washed away with PBS and the remaining
phagocytes were
scraped off, stained with macrophage marker CD14 antibody, and analyzed by
flow cytometry.
Phagocytosis was measured by gating on CD14+ cells and then assessing the
percent of CFSE
cells.
[221] Fig. 26 shows that the 1F8-GMCSF fusion protein caused a larger relative
fold change of
the percentages of phagocytosed cells in CD14+ cells as compared to that of
IgG control treated
group, 1F8-treated group, and GM-CSF treated group.
Example 26. Binding of Fusion Protein to Human GM-CSF Receptor
[222] Test was conducted to determine dose response of [LISA binding of the
fusion protein
1F8-GMCSF to human GMCSF receptor protein (2 ug/m1@100 u1). Recombinant GMCSF
R alpha
protein (R&D Systems) was coated at 2 ug/mL in PBS onto microtiter plates for
2 hours at the
room temperature. After coating of antigen the wells were blocked with
PBS/0.05% Tween
(PBST) with 1% BSA for 1 hour at the room temperature. After washing of the
wells with PBST,
different concentrations of 1F8-GMCSF fusion protein were added to the well
and incubated for
1 hour at the room temperature. For detection of the binding antibodies, the
HRP conjugated
secondary antibodies against human Fc (Jackson Immuno Research) were added
followed by
the addition of fluorogenic substrates (Roche). Between all incubation steps,
the wells of the
-46-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
plate were washed with PBST three times. Fluorescence was measured in a TECAN
Spectrafluor
plate reader. Recombinant human GMCSF protein was used as reference.
[223] Fig. 27 shows that the fusion protein 1F8- GMCSF had a stronger binding
affinity to
human GMCSF receptor than the recombinant human GMCSF itself.
Example 27. Induction of STAT5 Activation by Fusion Protein
[224] CD14+ monocytes were purified from peripheral human blood by using CD14
positive
microbeads (Miltenyi Biotec). The purified monocytes were stimulated with the
fusion protein
1F8-GMCSF at different concentrations for 30 minutes at 37 C. After
incubation, the cells were
collected and washed with FACS buffer (1 x PBS + 2% FBS) and fixed by 2% PFA
followed by cell
permealization using ice cold methanol. Then the PE-conjugated anti-pSTAT5
antibody was
added to the cells for another incubation of 30 minutes at 4 C and analyzed by
flow cytometry.
The fold change of MFI was calculated by the MFI of test sample/MFI of IgG
control treatment.
[225] Fig. 28 shows that 1F8-GMCSF had similar induction activities to those
of GMCSF itself.
Example 28. Stimulation of TF-1 Proliferation by Fusion Proteins
[226] Prior to GMCSF stimulation, TF-1 cells were washed with RPMI1640 basal
medium and
starved for over-night. At day 2, these starved cells were collected and then
seeded at a
concentration of 3 x 105 cells/ml in 50 uL per well of a flat bottom 96-well
plate. Different
concentrations of 1F8-GMCSF fusion protein were added into the TF-1 cell
culture and
incubated for 72 hrs at 37 C. Cell proliferation was measured by CellTiter-
Glo Luminescent
Cell Viability Assay according to the manufacturer's protocol.
[227] Fig. 29 shows that compared to GMCSF, the fusion protein 1F8-GMCSF
exhibited
stronger capability to stimulate TF-1 proliferation.
Example 29. Activation of M1 Macrophage by Fusion Protein
[228] Human in vitro differentiated macrophages were co-cultured with Raji
cells at a ratio of
5:1 of tumor cells per macrophage. IgG, 1F8, GMCSF or 1F8-GMCSF fusion protein
were added
into the culture and incubated for 8 hrs. After incubation, the culture
supernatant was analyzed
for the production of IL-6, IL-12 and TNF-a by Luminex and the cells were
analyzed for the
expression of CD80 by flow cytometry. All these four parameters were the
characteristic
markers for M1 macrophage activation.
-47-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[229] Fig. 30(a), Fig. 30(b), Fig. 30(c) and Fig. 30(d) showed the production
of IL-6, IL-12, TNF-a,
and CD80 caused by activation of M1 macrophage in the presence of IgG, 1F8,
GMCSF or 1F8-
GMCSF fusion protein.
Example 30. In Vivo Efficacy of Fusion Protein in Raji Xenograft Model
[230] Raji cells were subcutaneously engrafted into the NSG mice and grown
into 100 mm3.
These mice were then treated with IgG, 1F8 alone, mouse GMCSF alone, 1F8 and
mouse GMCSF
combo, 1F8-mGMCSF fusion protein for 70 nmol per mouse twice a week. Tumor
size was
measured in two dimensions using precision calipers.
[231] Fig. 31 shows the efficacy of each of the five treatments in reducing
the tumor volume
and the 1F8-GMCSF fusion protein exhibited the best efficacy among them all.
Example 31. Fusion Protein 13H3-GMCSF
[232] To generate fusion protein 13H3-GMCSF, human GMCSF cytokine was fused to
the
heavy chain C terminus of anti-CD47 antibody (13H3) directly. Then, the light
chain and heavy
chain expression vectors were co-transfected into CHO cells. After transient
transfection, the
fusion proteins were purified from the medium by protein A affinity
chromatography.
[233] The well qualified fusion protein 13H3-GMCSF was applied to in vitro
characterization
according to the assays described above.
[234] Table 3 shows that CD47 antibody 13H3 and the fusion protein 13H3-GMCSF
exhibited
similar binding kinetics as measured by Biacore analysis.
Table 3
Molecule ka (1/Ms) kd (1/s) KD (M)
13H3 3.61E+05 2.82E-03 7.81E-09
13H3-GMCSF 7.21E+05 4.43E-03 6.14E-09
[235] Fig. 32 shows that CD47 antibody 13H3 and the fusion protein 13H3-GMCSF
exhibited
similar binding affinity to CD47-bearing Raji cells.
-48-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
[236] Fig. 33a and Fig. 33b show that 13H3-GMCSF exhibited comparable
capability with 13H3
itself in blocking CD47-SIRPa Interaction.
[237] Fig. 34 shows that the 13H3-GMCSF fusion protein exhibited potentiated
activity as
compared with 13H3 itself in promoting phagocytosis of tumor cells by human
MCD.
[238] Fig. 35 shows that 13H3-GMCSF did not cause in vitro RBC agglutination.
[239] Fig. 36 shows that 13H3-GMCSF exhibited comparable potency as the
recombinant
GMCSF protein in binding to human GMCSF receptor.
[240] Fig. 37 shows that 13H3-GMCSF exhibited comparable potency as the
recombinant
GMCSF protein in induction of STAT5 activation.
[241] Fig. 38 shows that 13H3-GMCSF exhibited comparable potency as the
recombinant GM-
CSF protein in stimulation of TF-1 proliferation.
Example 32. In Vivo Efficacy of Fusion Protein 13H3-GMCSF in Raji Xenograft
Model
[242] Raji cells were subcutaneously engrafted into the NSG mice and grown
into 100 mm3.
These mice were then treated with IgG, 13H3 alone, GMCSF alone, 13H3 and GMCSF
combo,
13H3-GMCSF fusion protein for 70 nmol per mouse twice a week. Tumor size was
measured in
two dimensions using precision calipers.
[243] Fig. 39 shows the efficacy of each of the five treatments in reducing
the tumor volume
and the 13H3-GMCSF fusion protein exhibited the best efficacy among them all.
Example 33. In vivo PK study of 13H3-GMCSF in Cynomolgus Monkey
[244] Naïve cynomolgus monkeys (n=2) were intravenously injected with the
fusion protein
13H3-GMCSF at a dose of 20 mg/kg. Their blood was collected by venipuncture
into tubes with
no anticoagulant at different time points. Serum level of the fusion protein
13H3-GMCSF was
measured by [LISA using CD47 protein as the coating reagent, followed by
detection with anti-
GMCSF secondary antibody. The concentration-time curve of the serum level of
13H3-GMCSF
after a single dose at 20 mg/kg in cynomolgus monkeys is shown in Fig. 40.
Pharmacokinetic
parameters were analyzed by Winolin and shown in Table 4.
Table 4
T112 (h) C. (1-tg/n11) AUCo_t (hr*ug/m1) MRTIast (hr)
-49-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
6.8 191 3849 11.4
Example 34. Safety Pharm Study (Hematology) of 13H3-GMCSF in Cynomolgus Monkey
[245] Naïve cynomolgus monkeys were intravenously infused with repeat dose
(weekly dosing)
of the fusion protein 13H3-GMCSF (20 mg/kg). Hematology (CBC) parameters were
examined
including the counts of erythrocyte (RBC), platelets and leukocytes (WBC),
neutrophils and
monocytes at the indicated time points following the fusion protein
administration.
[246] Figs. 41a, 41b, 42a, 42b and 42c show the effects of the fusion protein
13H3-GMCSF on
RBC, platelets, leukocytes, neutrophils and monocytes levels.
Example 35. Fusion Protein 1F8-GMCSF variants
[247] To generate fusion protein 1F8-GMCSF with attenuated GMCSF activity,
human GMCSF
part was performed site mutations. Sequences of these variants with different
site mutations
are listed in Table 5.
Table 5. Sequences of 1F8-GMCSF Wild type and Variants with Site Mutations
Molecule VL Antibody Chain VH Antibody Chain
1F8-GMCSF SEQ ID NO: 108 SEQ ID NO: 109
1F8-GMCSF, E21S SEQ ID NO: 108 SEQ ID NO: 110
1F8-GMCSF, E21A SEQ ID NO: 108 SEQ ID NO: 111
1F8-GMCSF, E21R SEQ ID NO: 108 SEQ ID NO: 112
1F8-GMCSF, E21S, D112K SEQ ID NO: 108 SEQ ID NO: 113
1F8-GMCSF, E21R, D112K SEQ ID NO: 108 SEQ ID NO: 114
1F8-GMCSF, E45K, D48K, D112K SEQ ID NO: 108 SEQ ID NO: 115
Example 36. Fusion Protein 13H3-GMCSF variants
-50-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
[248] To generate fusion protein 13H3-GMCSF with attenuated GMCSF activity,
human GM-
CSF part was performed site mutations. Sequences of these variants with
different site
mutations are listed in Table 6. Then, the light chain and heavy chain
expression vectors of
13H3-GMCSF variant were co-transfected into CHO cells. After transient
transfection, the fusion
protein variants were purified from the medium by protein A affinity
chromatography.
Table 6. Sequences of 13H3-GMCSF Wild type and Variants with Site Mutations
Molecule VL Antibody Chain VH Antibody Chain
13H3-GMCSF SEQ ID NO: 116 SEQ ID NO: 109
13H3-GMCSF, E21S SEQ ID NO: 116 SEQ ID NO: 110
13H3-GMCSF, E21A SEQ ID NO: 116 SEQ ID NO: 111
13H3-GMCSF, E21R SEQ ID NO: 116 SEQ ID NO: 112
13H3-GMCSF, E21S, D112K SEQ ID NO: 116 SEQ ID NO: 113
13H3-GMCSF, E21R, D112K SEQ ID NO: 116 SEQ ID NO: 114
13H3-GMCSF, E45K, D48K, D112K SEQ ID NO: 116 SEQ ID NO: 115
[249] The well qualified 13H3-GMCSF variants were applied to in vitro
characterization
according to the assays described above.
[250] Fig. 43 shows that 13H3-GMCSF variants exhibit attenuated potency as
compared with
the wild type fusion protein 13H3-GMCSF in induction of STAT5 activation.
[251] Fig. 44 shows that 13H3-GMCSF variants exhibit differentiated potency as
compared
with the wild type fusion protein 13H3-GMCSF in stimulation of TF-1
proliferation.
[252] Human in vitro differentiated macrophages were cultured in the presence
of 13H3-
GMCSF variants with a series of concentrations for 48 hours. The production
level of IL-6 in the
culture supernatant was analyzed by ELISA. Fig.45 shows that all the 13H3-
GMCSF variants
-51-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
exhibited lower activity to a different extent as compared with the wild type
13H3-GMCSF
fusion molecule in activation of macrophage-mediated IL-6 expression.
[253] Fig.46 shows that 13H3-GMCSF variants exhibited comparable activity with
the wildtype
13H3-GMCSF in promoting phagocytosis of tumor cells by human MCD.
[254] Table 7 shows that 13H3-GMCSF variants exhibited similar binding
kinetics with the wild
type 13H3-GMCSF as measured by Biacore analysis.
Table 7. Biacore Analysis of 13H3-GMCSF Variants
Molecule ka (1/Ms) kd (1/s) KD
(M)
13H3-GMCSF 5.40E+05 2.83E-03
5.25E-09
13H3-GMCSF E21R 4.13E+05 1.98E-03 4.80E-
09
13H3-GMCSF E21S, D112K 3.18E+05 1.97E-03 6.20E-09
[255] Figs. 47a-c show that 13H3-GMCSF variants exhibit comparable capability
with the wild
type 13H3-GMCSF in blocking CD47-SIRPa Interaction.
[256] Human RBCs were incubated with different concentrations of 13H3-GMCSF
variants
with 5F9 as reference antibody at 4 C for 30 minutes. Then, the cells were
washed with FACS
buffer twice, followed by staining with Alexa Fluor 633-conjugated anti-human
Fc specific
antibody (Invitrogen) at 4 C for 30 minutes. Binding was measured with a
FACSCelesta flow
cytometry (BD Biosciences). Fig. 48 shows that the wild type and variants of
13H3-GMCSF
exhibited very low levels of RBC binding as compared to the reference antibody
5F9.
[257] Fig. 49 shows that 13H3-GMCSF variants did not cause in vitro RBC
agglutination.
Example 37. Fusion Protein 1F8-GMCSF variants with partial silent version of
IgG1 isotype
[258] To generate fusion protein 1F8-GMCSF with attenuated Fc-mediated
effector function,
one site mutation N297A in the Fc part of human IgG1 was applied to the
wildtype and variants
-52-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
of 1F8-GMCSF. Sequences of the wild type and variants of 1F8-GMCSF with IgG1
N297A are
listed in Table 8.
Table 8. 1F8-GMCSF Fusion Proteins (Wildtype and Variants) with IgG1 N297A
Isotype
Molecule VI Antibody Chain VH Antibody Chain
1F8-GMCSF, N297A SEQ ID NO: 108 SEQ ID NO: 117
1F8-GMCSF, E21S, N297A SEQ ID NO: 108 SEQ ID NO: 118
1F8-GMCSF, E21A, N297A SEQ ID NO: 108 SEQ ID NO: 119
1F8-GMCSF, E21R, N297A SEQ ID NO: 108 SEQ ID NO: 120
1F8-GMCSF, E21S, D112K, N297A SEQ ID NO: 108 SEQ ID NO: 121
1F8-GMCSF, E21R, D112K, N297A SEQ ID NO: 108 SEQ ID NO: 122
1F8-GMCSF, E45K, D48K, D112K, SEQ ID NO: 108 SEQ ID NO: 123
N297A
Example 38. Fusion Protein 13H3-GMCSF variants with partial silent version of
IgG1 isotype
[259] To generate fusion protein 13H3-GMCSF with attenuated Fc-mediated
effector function,
one site mutation N297A in the Fc part of human IgG1 was applied to the
wildtype and variants
of 13H3-GMCSF. Sequences of the wild type and variants of 13H3-GMCSF with IgG1
N297A are
listed in Table 9. The light chain and heavy chain expression vectors of 13H3-
GMCSF variant
with IgG1 N297A were co-transfected into CHO cells. After transient
transfection, the fusion
protein variants were purified from the medium by protein A affinity
chromatography.
Table 9. 13H3-GMCSF Fusion Proteins (Wildtype and Variants) with IgG1 N297A
Isotype
Molecule VI Antibody Chain VH Antibody Chain
13H3-GMCSF, N297A SEQ ID NO: 116 SEQ ID NO: 117
13H3-GMCSF, E21R, N297A SEQ ID NO: 116 SEQ ID NO: 120
13H3-GMCSF, E215, D112K, N297A SEQ ID NO: 116 SEQ ID NO: 121
-53-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
13H3-GMCSF, E21R, D112K, N297A SEQ ID NO: 116 SEQ ID NO: 122
13H3-GMCSF, E45K, D48K, D112K, SEQ ID NO: 116 SEQ ID NO: 123
N297A
[260] Fig.50 shows that 13H3-GMCSF with IgG1 N297A isotype exhibited modest
lower
activity as compared with the wildtype 13H3-GMCSF in promoting phagocytosis of
tumor cells
by human MCD.
[261] Fig.51 shows that 13H3-GMCSF variants with IgG1 N297A isotype exhibited
modest
lower activity as compared with 13H3-GMCSF variant with IgG1 isotype in
promoting
phagocytosis of tumor cells by human MCD.
Example 39. Deglycosylated fusion protein 1F8-GMCSF
[262] Human GMCSF has two N-glycosylation sites at Asn 27 and Asn 37 and three
0-
glycosylation sites in the N-terminal region at Ser 7, Ser 9 and Thr 10. To
generate
deglycosylated fusion protein 1F8-GMCSF, sites mutations (N27Q, N370/57A, 59A,
T10A/N27Q,
N37Q, 57A, 59A, T10A) or N-terminal truncation (1-10 aa) were applied to the
GMCSF part of
the fusion protein 1F8-GMCSF with IgG1 format and IgG1 N297A format. Sequences
of the
deglycosylated fusion protein were listed in Tables 10-11.
Table 10. Sequences of deglycosylated fusion protein 1F8-GMCSF with IgG1
format
Molecule VI Antibody Chain VH Antibody Chain
1F8-GMCSF, N27Q, N37Q SEQ ID NO: 108 SEQ ID NO: 124
1F8-GMCSF, 57A, 59A, T10A SEQ ID NO: 108 SEQ ID NO: 125
1F8-GMCSF, N27Q, N37Q, 57A, 59A, SEQ ID NO: 108 SEQ ID NO: 126
T10A
1F8-GMCSF, N27Q, N37Q, 11-127 SEQ ID NO: 108 SEQ ID NO: 127
Table 11. Sequences of deglycosylated fusion protein 1F8-GMCSF with IgG1 N297A
format
Molecule VI Antibody Chain VH Antibody Chain
-54-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
1F8-GMCSF, N27Q, N37Q, N297A SEQ ID NO: 108 SEQ ID NO: 128
1F8-GMCSF, S7A, S9A, T10A, N297A SEQ ID NO: 108 SEQ ID NO: 129
1F8-GMCSF, N27Q, N37Q, S7A, S9A, SEQ ID NO: 108 SEQ ID NO: 130
T10A, N297A
1F8-GMCSF, N27Q, N37Q, N297A, SEQ ID NO: 108 SEQ ID NO: 131
11-127
Example 40. Deglycosylated fusion protein 1F8-GMCSF variants
[263] To generate deglycosylated fusion protein 1F8-GMCSF variants, site
mutations (N27Q,
N370/57A, 59A, T10A/N27Q, N37Q, 57A, 59A, T10A) or N-terminal truncation (1-10
aa) were
applied to the GMCSF part of the fusion protein 1F8-GMCSF variants with IgG1
format and IgG1
N297A format. Sequences of the deglycosylated fusion protein were listed in
Tables 12-13.
Table 12. Sequences of deglycosylated fusion protein 1F8-GMCSF variants with
IgG1 format
Molecule VI Antibody Chain VH Antibody Chain
1F8-GMCSF, E21R, N27Q, N37Q SEQ ID NO: 108 SEQ ID NO: 132
1F8-GMCSF, E21R, 57A, 59A, T10A SEQ ID NO: 108 SEQ ID NO: 133
1F8-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 108 SEQ ID NO: 134
57A, 59A, T10A
1F8-GMCSF, E21R, N27Q, N37Q, 11- SEQ ID NO: 108 SEQ ID NO: 135
127
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 136
N37Q
1F8-GMCSF, E215, D112K, 57A, 59A, SEQ ID NO: 108 SEQ ID NO: 137
T10A
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 138
N37Q, 57A, 59A, T10A
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 139
N37Q, 11-127
-55-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
Table 13. Sequences of deglycosylated 1F8-GMCSF variants with IgG1 N297A
format
Molecule VI Antibody Chain VH Antibody Chain
1F8-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 108 SEQ ID NO: 140
N297A
1F8-GMCSF, E21R, S7A, S9A, T10A, SEQ ID NO: 108 SEQ ID NO: 141
N297A
1F8-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 108 SEQ ID NO: 142
S7A, S9A, T10A, N297A
1F8-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 108 SEQ ID NO: 143
N297A, 11-127
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 144
N37Q, N297A
1F8-GMCSF, E215, D112K, 57A, 59A, SEQ ID NO: 108 SEQ ID NO: 145
T10A, N297A
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 146
N37Q, 57A, 59A, T10A, N297A
1F8-GMCSF, E215, D112K, N27Q, SEQ ID NO: 108 SEQ ID NO: 147
N37Q, N297A, 11-127
Example 41. Deglycosylated fusion protein 13H3-GMCSF
[264] To generate deglycosylated fusion protein 13H3-GMCSF, site mutations
(N27Q,
N370/57A, 59A, T10A/N27Q, N37Q, 57A, 59A, T10A) or N-terminal truncation (1-10
aa) were
applied to the GMCSF part of the fusion protein 13H3-GMCSF with IgG1 format
and IgG1 N297A
format. Then, the light chain and heavy chain expression vectors of
deglycosylated 13H3-
GMCSF were co-transfected into HEK293 cells. After transient transfection, the
fusion protein
variants were purified from the medium by protein A affinity chromatography.
Sequences of
the deglycosylated fusion protein were listed in Tables 14-15.
Table 14. Sequencecs of deglycosylated 13H3-GMCSF with IgG1 format
-56-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
Molecule VI Antibody Chain VH Antibody Chain
13H3-GMCSF, N27Q, N37Q SEQ ID NO: 116 SEQ ID NO: 124
13H3-GMCSF, S7A, S9A, T10A SEQ ID NO: 116 SEQ ID NO: 125
13H3-GMCSF, N27Q, N37Q, S7A, SEQ ID NO: 116 SEQ ID NO: 126
S9A, T10A
13H3-GMCSF, N27Q, N37Q, 11-127 SEQ ID NO: 116 SEQ ID NO: 127
Table 15. Sequences of deglycosylated 13H3-GMCSF with IgG1 N297A format
Molecule VI Antibody Chain VH Antibody Chain
13H3-GMCSF, N27Q, N37Q, N297A SEQ ID NO: 116 SEQ ID NO: 128
13H3-GMCSF, 57A, 59A, T10A, SEQ ID NO: 116 SEQ ID NO: 129
N297A
13H3-GMCSF, N27Q, N37Q, 57A, SEQ ID NO: 116 SEQ ID NO: 130
59A, T10A, N297A
13H3-GMCSF, N27Q, N37Q, N297A, SEQ ID NO: 116 SEQ ID NO: 131
11-127
[265] The well qualified deglycosylated 13H3-GMCSF with IgG1 format were
applied to in vitro
characterization according to the assays described above.
[266] Fig. 52 shows that deglycosylated 13H3-GMCSF exhibit comparable activity
with the
wildtype 13H3-GMCSF in induction of STAT5 phosphorylation.
[267] Fig. 53 shows that deglycosylated 13H3-GMCSF exhibit comparable activity
with the
wildtype 13H3-GMCSF in stimulation of TF-1 proliferation.
[268] Fig.54 shows that deglycosylated 13H3-GMCSF exhibit comparable activity
with the
wildtype 13H3-GMCSF in promoting phagocytosis of tumor cells (Raji) by human
MCD.
Example 42. Deglycosylated fusion protein 13H3-GMCSF variants
-57-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
[269] To generate deglycosylated fusion protein 13H3-GMCSF variants, site
mutations (N27Q,
N37Q/S7A, S9A, T10A/N27Q, N37Q, S7A, S9A, T10A) or N-terminal truncation (1-10
aa) were
applied to the GMCSF part of the fusion protein 13H3-GMCSF variants with IgG1
format and
IgG1 N297A format. Then, the light chain and heavy chain expression vectors of
deglycosylated
13H3-GMCSF variants were co-transfected into HEK293 cells. After transient
transfection, the
deglycosylated fusion protein variants were purified from the medium by
protein A affinity
chromatography. Sequences of the deglycosylated fusion protein were listed in
Tables 16-17.
Table 16. Sequences of deglycosylated 13H3-GMCSF variants with IgG1 format
Molecule VI Antibody Chain VH Antibody Chain
13H3-GMCSF, E21R, N27Q, N37Q SEQ ID NO: 116 SEQ ID NO: 132
13H3-GMCSF, E21R, 57A, S9A, T10A SEQ ID NO: 116 SEQ ID NO: 133
13H3-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 116 SEQ ID NO: 134
57A, 59A, T10A
13H3-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 116 SEQ ID NO: 135
11-127
13H3-GMCSF, E21S, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 136
N37Q
13H3-GMCSF, E215, D112K, 57A, SEQ ID NO: 116 SEQ ID NO: 137
S9A, T10A
13H3-GMCSF, E215, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 138
N37Q, 57A, 59A, T10A
13H3-GMCSF, E215, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 139
N37Q, 11-127
Table 17. Sequences of deglycosylated 13H3-GMCSF variants with IgG1 N297A
format
Molecule VI Antibody Chain VH Antibody Chain
13H3-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 116 SEQ ID NO: 140
N297A
-58-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
13H3-GMCSF, E21R, S7A, S9A, T10A, SEQ ID NO: 116 SEQ ID NO: 141
N297A
13H3-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 116 SEQ ID NO: 142
S7A, S9A, T10A, N297A
13H3-GMCSF, E21R, N27Q, N37Q, SEQ ID NO: 116 SEQ ID NO: 143
N297A, 11-127
13H3-GMCSF, E21S, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 144
N37Q, N297A
13H3-GMCSF, E215, D112K, 57A, SEQ ID NO: 116 SEQ ID NO: 145
59A, T10A, N297A
13H3-GMCSF, E215, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 146
N37Q, 57A, 59A, T10A, N297A
13H3-GMCSF, E215, D112K, N27Q, SEQ ID NO: 116 SEQ ID NO: 147
N37Q, N297A, 11-127
[270] The well qualified deglycosylated 13H3-GMCSF variant with IgG1 format
were applied to
in vitro characterization according to the assays described above.
[271] Fig. 55 shows that deglycosylation in GMCSF part has no effect on 13H3-
GMCSF variant-
mediated stimulation of TF-1 proliferation.
Example 43. In vivo PK study of 13H3-GMCSF variant in Cynomolgus Monkey
[272] Naïve cynomolgus monkeys (n=2) were intravenously injected with the
fusion protein
13H3-GMCSF variant (E215, D112K, IgG 1 N297A) at a dose of 10 mg/kg. Their
blood was
collected by venipuncture into tubes with no anticoagulant at different time
points. Serum
level of the fusion protein 13H3-GMCSF variant was measured by ELISA using
CD47 protein as
the coating reagent, followed by detection with anti-Fc secondary antibody.
The concentration-
time curve of the serum level of 13H3-GMCSF variant after a single dose at 10
mg/kg in
cynomolgus monkeys is shown in Fig. 56. Pharmacokinetic parameters were
analyzed by
Winolin and shown in Table 18.
Table 18. Pharmacokinetic parameters of in vivo PK study of 13H3-GMCSF
variants
-59-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
AUCall
T1/2 Cmax MRTIast
Fusion Protein. (hr* g/mL
(hr) ( g/mL) (hr)
13H3-GMCSF, E21S, D112K, N297A 22.161 450.533 1459.819 5.015
Example 44. Safety Pharm Study (Hematology) of 13H3-GMCSF variants in
Cynomolgus
Monkey
[273] Naïve cynomolgus monkeys were intravenously infused with repeated dose
at day1 and
day 4 of the fusion protein 13H3-GMCSF variants (20 mg/kg). Hematology (CBC)
parameters
were examined including the counts of erythrocyte (RBC), platelets and
leukocytes (WBC),
neutrophils and monocytes at the indicated time points following the fusion
protein
administration.
[274] Figs. 57 a-c show the effects of the fusion protein 13H3-GMCSF variants
on the
peripheral levels of neutrophils, monocytes and leukocytes.
[275] Figs. 58 a-c show the effects of the fusion protein 13H3-GMCSF variants
on the
peripheral levels of red blood cells, hemoglobulin and platelets.
Example 45. Surrogate molecule of 13H3-GMCSF
[276] To generate surrogate molecule of the fusion protein 13H3-GMCSF for
proof of concept
study, human GMCSF part was replaced by full length of murine GMCSF. Then, the
light chain
and heavy chain expression vectors of 13H3-mGMCSF were co-transfected into
HEK293 cells.
After transient transfection, the fusion protein variants were purified from
the medium by
protein A affinity chromatography. Sequences of the surrogate molecule 13H3-
mGMCSF were
listed in Table 19.
Table 19. Sequences of surrogate molecule 13H3-mGMCSF
Molecule VI Antibody Chain VH Antibody Chain
13H3-mGMCSF SEQ ID NO: 116 SEQ ID NO: 148
-60-
CA 03097443 2020-10-16
WO 2020/098232
PCT/CN2019/085096
Example 46. Surrogate molecule of 13H3-GMCSF variants
[277] To screen and generate surrogate molecule of the fusion protein 13H3-
GMCSF variants
for proof of concept study, site mutations were applied to murine GMCSF part
of the surrogate
molecule 13H3-mGMCSF to attenuate GMCSF activity. Then, the light chain and
heavy chain
expression vectors of 13H3-mGMCSF variants were co-transfected into HEK293
cells. After
transient transfection, the fusion protein variants were purified from the
medium by protein A
affinity chromatography. Sequences of the surrogate molecule 13H3-mGMCSF
variants were
listed in Table 20.
Table 16. Sequences of surrogate molecule 13H3-mGMCSF variants
Molecule VI Antibody Chain VH Antibody Chain
13H3-mGMCSF, E21S SEQ ID NO: 116 SEQ ID NO: 149
13H3-mGMCSF, E21A SEQ ID NO: 116 SEQ ID NO: 150
13H3-mGMCSF, E21R SEQ ID NO: 116 SEQ ID NO: 151
13H3-mGMCSF, K14A, H15A SEQ ID NO: 116 SEQ ID NO: 152
13H3-mGMCSF, K14A, K20A SEQ ID NO: 116 SEQ ID NO: 153
13H3-mGMCSF, K14A, E21A SEQ ID NO: 116 SEQ ID NO: 154
13H3-mGMCSF, H15A, K20A SEQ ID NO: 116 SEQ ID NO: 155
13H3-mGMCSF, H15A, E21A SEQ ID NO: 116 SEQ ID NO: 156
[278] The well qualified surrogate molecule 13H3-mGMCSF variants were applied
to in vitro
characterization according to the assays described above.
Induction of STAT5 Activation by the surrogate molecule 13H3-mGMCSF variants
[279] CD11b+ macrophages were purified from mouse spleen by using CD11b
positive
microbeads (Miltenyi Biotec). The purified macrophages were stimulated with
the surrogate
molecule 13H3-mGMCSF variants at different concentrations for 30 minutes at 37
C. After
incubation, the cells were collected and washed with FACS buffer (1 x PBS + 2%
FBS) and fixed
-61-
CA 03097443 2020-10-16
WO 2020/098232 PCT/CN2019/085096
by 2% PFA followed by cell permealization using ice cold methanol. Then the PE-
conjugated
anti-pSTAT5 antibody was added to the cells for another incubation of 30
minutes at 4 C and
analyzed by flow cytometry. The fold change of MFI was calculated by the MFI
of test
sample/MFI of IgG control treatment.
[280] Fig. 59 shows that 13H3-mGMCSF variants exhibit attenuated potency as
compared with
the wild type fusion protein 13H3-mGMCSF in induction of STAT5
phosphorylation.
Stimulation of FDC-P1 Proliferation by the surrogate molecule 13H3-mGMCSF
variants
[281] Prior to mouse GMCSF stimulation, FDC-P1 cells were collected and then
seeded at a
concentration of 2 x 105 cells/ml in 50 pi per well of a flat bottom 96-well
plate. Different
concentrations of 13H3-mGMCSF variants were added into the FDC-P1 cell culture
and
incubated for 72 hrs at 37 C. Cell proliferation was measured by CellTiter-
Glo Luminescent
Cell Viability Assay according to the manufacturer's protocol. Fig. 60 shows
that 13H3-mGMCSF
variants exhibit attenuated potency as compared with the wild type fusion
protein 13H3-
mGMCSF in stimulation of FDC-P1 proliferation.
-62-