Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
1
CORRODIBLE DOWN HOLE ARTICLE
[001] This invention relates to a corrodible downhole article comprising an
aluminium alloy, a method for making such an article and the use of the
article.
[002] Background
[003] The oil and gas industries utilise a technology known as hydraulic
fracturing
or "fracking". This normally involves the pressurisation with water of a
system of
boreholes in oil and/or gas bearing rocks in order to fracture the rocks to
release the
oil and/or gas.
[004] In order to achieve this pressurisation, valves may be used to block off
or
isolate different sections of a borehole system. These valves are referred to
as
downhole valves, the word downhole being used in the context of the invention
to
refer to an article that is used in a well or borehole.
[005] One way of producing such valves involves the use of spheres (commonly
.. known as fracking balls) of multiple diameters that engage on pre-
positioned seats in
the pipe lining. Such fracking balls may be made from aluminium, magnesium,
polymers or composites. The seats are usually made of steel.
[006] An essential characteristic of the material from which the fracking ball
(ie a
corrodible downhole article) is formed is that it dissolves or corrodes under
the
conditions in the well or borehole. Such corrodible articles need to corrode
at a rate
which allows them to remain useable for the time period during which they are
required to perform their function, but that allows them to corrode or
dissolve
afterwards.
[007] As fracking ball technology has developed, valves have been designed
which
have a smaller overlap (ie contact point) between the steel seat and the
dissolvable
fracking ball. An example of such a valve is shown in Figure 1.
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
2
[008] This reduction in the surface area of the contact between the seat and
the
fracking ball results in an increase in the pressure being applied at this
contact point.
Thus, there is a need for fracking balls which are able to withstand these
increased
pressures, for example by development of materials (eg alloys) having improved
strength whilst still maintaining the desired corrosion properties (eg high
corrosion
rate, uniformity of corrosion). It is also desirable if the alloys are
processable by
extrusion. Such alloys are also sought for use in other downhole applications
where
corrodible articles are required.
[009] US 2009/0226340 Al relates to products used in oilfield exploration,
production and testing which are made from degradable aluminium alloys. The
alloys are formed by adding one or more elements to an aluminium or aluminium
alloy melt. Ga, In and Zn are described as possible additives, but the amounts
of
these elements are not mentioned.
[0010] US 2010/0209288 Al describes aged-hardenable and degradable aluminium
alloys, and their use in wellbore environments. Aluminium alloys including 0.5-
8.0
wt% Ga, 0.5-8.0 wt% Mg, less than about 2.5 wt% In and less than about 4.5 wt%
Zn
are mentioned.
[0011] WO 2014/113058 A2 relates to a degradable ball sealer for use in
hydraulic
fracturing. The degradable ball sealer includes an aluminium alloy containing
gallium, carbon particles and salt particles.
[0012] Statement of invention
[0013] This invention relates to a corrodible downhole article comprising an
aluminium alloy, wherein the aluminium alloy comprises (a) 3-15 wt% Mg, (b)
0.01-5
wt% In, (c) 0-0.25 wt% Ga, and (d) at least 60 wt% Al.
[0014] In relation to this invention, the term "alloy" is used to mean a
composition
made by mixing and fusing two or more metallic elements by melting them
together,
mixing and re-solidifying them. Thus, in the context of the inventive alloy,
any
elements mentioned are in their metallic form (and not, for example, present
as a
salt).
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
3
[0015] In particular, the aluminium alloy may comprise Mg in an amount of 3-13
wt%, more particularly 4-12 wt%, even more particularly 5-11 wt%.
[0016] More particularly, the aluminium alloy may comprise In in an amount of
0.05-
5 wt%, even more particularly 0.1-4 wt%.
[0017] In particular, the aluminium alloy may comprise In in an amount of 0.1-
1.5
wt%, more particularly 0.2-1.3 wt%, even more particularly 0.3-1.2 wt%. In
some
embodiments, the alloy may comprise these amounts of In when it comprises Sn
in
an amount of 0-0.1 wt%, more particularly 0-0.05 wt%, even more particularly
when
it is substantially free of Sn.
[0018] In particular, the aluminium alloy may comprise In in an amount of 0.5-
4.5
wt%, more particularly 0.75-4.0 wt%. In some embodiments, the alloy may
comprise
these amounts of In when it comprises Sn in an amount of 0.5-4.5 wt%, more
particularly 0.75-4.0 wt%.
[0019] More particularly, the aluminium alloy may comprise one or more
compounds
which are capable of forming an intermetallic phase. In particular, the one or
more
compounds may be selected from Ni, Fe, W, Zr and Au. More particularly, the
one
or more compounds may be Ni and/or Fe.
[0020] More particularly, the aluminium alloy may comprise Fe in an amount of
0-2.5
wt%, even more particularly 0.1-2.0 wt%, more particularly 0.5-1.8 wt%, even
more
particularly 0.5-1.50 wt%.
[0021] In particular, the aluminium alloy may comprise Ni in an amount of 0-10
wt%,
more particularly 0.1-7.5 wt%, even more particularly 0.5-6 wt%, more
particularly 1-
6 wtcYo.
[0022] More particularly, the aluminium alloy may comprise Zn in an amount of
0-15
wt%, even more particularly 0.3-14 wt%, more particularly 1-13 wt%, even more
particularly 3-10 wt%.
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
4
[0023] For example, the aluminium alloy may comprise (a) 5-11 wt% Mg, (b) 0.3-
1.2
wt% In, (c) 0-0.25 wt% Ga, (d) 0-1.8 wt% Fe, (e) 0-6 wt% Ni, and (f) 1-13 wt%
Zn. In
a further embodiment, the aluminium alloy may comprise (a) 5-11 wt% Mg, (b)
0.3-
1.2 wt% In, (c) 0-0.25 wt% Ga, (d) 0-1.5 wt% Fe, (e) 0-0.5 wt% Ni, and (f) 0.3-
10
wtc/o Zn.
[0024] In particular, the aluminium alloy may comprise Ga in an amount of 0-
0.1
wt%, more particularly 0-0.05 wt%. In some embodiments, the aluminium alloy
may
be substantially free of Ga.
[0025] More particularly, the aluminium alloy may comprise Mn in an amount of
0-
0.1 wt% more particularly 0-0.05 wt%. In some embodiments, the aluminium alloy
may be substantially free of Mn.
.. [0026] In particular, the aluminium alloy may comprise Si in an amount of 0-
0.2 wt%,
more particularly 0-0.1 wt%, even more particularly 0-0.05 wt%. In some
embodiments, the aluminium alloy may be substantially free of Si.
[0027] More particularly, the aluminium alloy may comprise Bi in an amount of
0-0.2
wt%, even more particularly 0-0.1 wt%, more particularly 0-0.05 wt%. In some
embodiments, the aluminium alloy may be substantially free of Bi.
[0028] In particular, the aluminium alloy may comprise Cu in an amount of 0-
0.2
wt%, more particularly 0-0.1 wt%, even more particularly 0-0.05 wt%. In some
embodiments, the aluminium alloy may be substantially free of Cu.
[0029] More particularly, the aluminium alloy may comprise Ca in an amount of
0-0.2
wt%, even more particularly 0-0.1 wt%, more particularly 0-0.05 wt%. In some
embodiments, the aluminium alloy may be substantially free of Ca.
[0030] In particular, the aluminium alloy may comprise carbon in an amount of
0-1
wt%, more particularly 0-0.5 wt%, even more particularly 0-0.1 wt%. In some
embodiments, the aluminium alloy may be substantially free of carbon.
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
[0031] In particular, in some embodiments the aluminium alloy may comprise an
element that is known to act as a corrosion rate modifier, e.g. a rare earth
element
other than Y, such as Ce. In the context of the invention, the rare earth
elements are
defined as the fifteen lanthanides, plus Y. The aluminium alloy may comprise
the
5 corrosion rate modifier, in an amount of 0-1 wt%, more particularly 0-0.5
wt%, even
more particularly 0-0.1 wt%. In some embodiments, the aluminium alloy may be
substantially free of the corrosion modifier.
[0032] More particularly, the aluminium alloy may comprise Ti in an amount of
0-0.5
wt%, even more particularly 00.5-0.2 wt%. In some embodiments, the aluminium
alloy may be substantially free of Ti.
[0033] In particular, the content of Al in the aluminium alloy may be at least
65wt%,
more particularly at least 70wt%. In some embodiments, the remainder of the
alloy
.. may be aluminium and incidental impurities.
[0034] In particular, the aluminium alloy may have a corrosion rate of at
least 300
mg/cm2/day, more particularly at least 500 mg/cm2/day, in some embodiments at
least 1000 mg/cm2/day, in 3 % KCI at 93 C (200 F). More particularly, the
corrosion
.. rate, in 3 % KCI at 93 C may be less than 15,000 mg/cm2/day.
[0035] In particular, the aluminium alloy may be heat treatable and/or
extrudable.
More particularly, the aluminium alloy may be heat treated and/or extruded.
[0036] In particular, the corrodible downhole article may be a downhole tool
or a
wellbore isolation device. More particularly, the wellbore isolation device
may be a
fracking ball, plug/plug component, packer or other tool assembly, even more
particularly a fracking ball. In particular, the fracking ball may be
substantially
spherical in shape.
[0037] This invention also relates to a method of making a corrodible downhole
article comprising an aluminium alloy, the method comprising the steps of:
(a) melting aluminium, Mg, In and optionally Ga, to form a
molten
aluminium alloy comprising 3-15 wt% Mg, 0.01-5 wt% In, 0-0.25 wt%
Ga, and at least 60 wt% Al,
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
6
(b) mixing the resulting molten aluminium alloy,
(c) casting the aluminium alloy or producing an aluminium alloy powder,
and
(d) forming the aluminium alloy into a corrodible downhole article.
[0038] In particular, the method may be for producing an aluminium alloy as
defined
above. Any other required components in the resulting alloy (for example,
those
listed in the preceding paragraphs describing the alloy) can be added in
melting step
(a). More particularly, the melting step may be carried out at a temperature
of 660 C
(ie the melting point of pure aluminium) or more, even more particularly less
than
2470 C (the boiling point of pure aluminium). In particular, the temperature
range
during melting and/or forming of a molten aluminium alloy may be 600 C to 850
C,
more particularly 700 C to 800 C, even more particularly about 750 C.
[0039] More particularly, in step (a) the resulting alloy may be fully molten.
In
particular, prior to melting in step (a) the alloy components may be present
in
elemental form or as one or more alloys.
[0040] In particular, in step (c) the casting may comprise pouring the molten
aluminium alloy into a mould, and then allowing it to cool and solidify. The
mould
may be a die mould, a permanent mould, a sand mould, an investment mould, a
direct chill casting (DC) mould, or other mould. More particularly, in step
(c) the
producing an aluminium alloy powder may be by casting and then grinding, or by
atomisation.
[0041] More particularly, step (d) may comprise one or more of: compacting,
additive
manufacturing, extruding, forging, rolling, and machining. In particular,
compacting
may comprise forming a Metal Matrix Composite (MMC).
[0042] In particular, after step (c) and either before or after step (d) the
method may
comprise the step of heat treating the alloy. The heat treatment may be by any
technique known in the art in relation to aluminium alloys.
[0043] In addition, this invention relates to a method of hydraulic fracturing
comprising the use of a corrodible downhole article as described above, or a
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
7
downhole tool as described above. In particular, the method may comprise
forming
an at least partial seal in a borehole with the corrodible downhole article.
The
method may then comprise removing the at least partial seal by permitting the
corrodible downhole article to corrode. This corrosion can occur at a desired
rate
with certain alloy compositions of the disclosure as discussed above. More
particularly, the corrodible downhole article may be a fracking ball, plug,
packer or
tool assembly, even more particularly a fracking ball. In particular, the
fracking ball
may be substantially spherical in shape. In some embodiments, the corrodible
downhole article, more particularly the fracking ball, may consist essentially
of the
aluminium alloy described above.
[0044] This invention will be further described by reference to the following
Figures
which are not intended to limit the scope of the invention claimed, in which:
Figure 1 shows an example of the typical geometry of a fracking ball on a
seat,
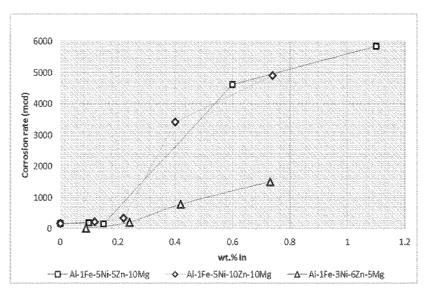
Figure 2 shows a graph of corrosion rate as a function of In content for three
alloy compositions, and
Figure 3 shows a graph of the force withstood in load with ball on seat
testing
as a function of In content for two alloy compositions.
[0045] Examples
[0046] Alloy preparation
[0047] Aluminium alloy compositions were prepared by combining the components
in the amounts listed in Table 1 below (the balance being aluminium and
incidental
impurities) and then melting them. These components were then melted by
heating
at a temperature in the range 600 C-900 C (dependent upon the alloy
components). Each melt was then cast into a billet.
[0048] Corrosion testing
[0049] In order to simulate the corrosion performance in a well, the material
was
corrosion tested by measuring weight loss in an aqueous solution of 3 wt%
potassium chloride at a constant temperature of 93 C (200 F). These results
are
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
8
shown in Table 1 below. The results demonstrate that the alloys of the
invention
achieve the desired corrosion rates.
[0050] In addition, three further alloy compositions were prepared as follows:
(i) 1 wt% Fe, 5 wt% Ni, 5 wt% Zn, 10 wt% Mg, X wt% In, remainder Al,
(ii) 1 wt% Fe, 5 wt% Ni, 10 wt% Zn, 10 wt% Mg, X wt% In, remainder Al,
and
(iii) 1 wt% Fe, 3 wt% Ni, 6 wt% Zn, 5 wt% Mg, X wt% In, remainder Al.
[0051] Various alloys were produced where the amount of In (ie the X value)
was
varied from 0-1.2 wt%. These alloys were then subjected to corrosion testing.
The
results of this testing are shown in Figure 2, which demonstrates the effect
of In
addition on corrosion behaviour.
[0052] "Ball on seat" testing
[0053] 23.5 mm diameter balls were manufactured by machining alloy billets.
The
ball on seat test is shown in Figure 1 which utilises a steel seat for the
ball test. The
seat angle was 30 and the overlap between the aluminium alloy ball and the
steel
ball seat is approximately 1.5 %, where % overlap = (1-(Diameter /Diameter ))
seat ball,/ X
100). Each ball was then forced through the steel seat using a Zwick universal
testing machine, utilising uniaxially applied compressive load, which gives a
maximum force in kN. Where a particular alloy was tested, these results are
shown
in Table 1 below. These results demonstrate that the alloys of the invention
achieve
the required force values.
[0054] In addition, two further alloy compositions were prepared as follows:
(i) 1 wt% Fe, 4 wt% Ni, 8 wt% Zn, 10 wt% Mg, X wt% In, remainder
Al,
and
(ii) 1 wt% Fe, 3 wt% Ni, 6 wt% Zn, 5 wt% Mg, X wt% In, remainder Al.
[0055] Various alloys were produced where the amount of In (ie the X value)
was
varied from 0-1.1 wt%. These alloys were then subjected to ball on seat
testing.
The results of this testing are shown in Figure 3, which demonstrates that
force can
be maintained within the desired range at varying amounts of In.
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
9
Example No. Weight % additions to aluminium
Casting Corr. Ball
base temp. Rate holding
Fe Ni Zn Mg Other ( C) (mcd)
in (kN,
additions 3% KCI, 1.5%
200 F overlap)
Comparative 1.4 2.2 7.1 6.6 740 222 28.1
Example 1
Comparative 1.4 3.9 9.3 7.6 760 240
Example 2
Comparative 1.2 4.2 9.6 8.2 1.5% Cu 760 50
Example 3
Comparative 1.1 4.4 9.3 8.3 7% Cu 760 0
Example 4
Comparative 1.5 5 7 7 1% Mn 760 67
Example 5
Comparative 3.0 10. 6.0 10.0 800 146
Example 6 0
Comparative 3.0 10. 6.0 10.0 0.1% Y 800 169
Example 7 0
Comparative 3.0 10. 6.0 10.0 0.5% Y 800 136
Example 8 0
Comparative 3.0 10. 6.0 10.0 1%Y 800 119
Example 9 0
Comparative 1.7 4.6 12.8 - 0.1% In 750 104
Example 10
Table 1
CA 03113003 2021-03-16
WO 2020/109770
PCT/GB2019/053331
Example No. Weight % additions to aluminium Casting Corr.
Ball
base temp. Rate holding
Fe Ni Zn Mg Other ( C) (mcd) in
(kN,
additions 3% KCI, 1.5%
200 F overlap)
Comparative 1.0 4.3 12.4 - 0.6% In 750 214
Example 11
Example 1 0.9 3.7 11.0 9.1 0.22% In 750 338
Example 2 0.9 3.9 11.3 9.4 0.4% In 750 3418
Example 3 1.3 4.5 10.1 8.4 0.74% In 750 4912
Example 4 1.0 3.6 6.0 9.8 0.6% In 750 .. 4615
.. 33.6
Example 5 1.0 4.5 6.3 9.8 1.1 % In 750 .. 5858
.. 31.3
Example 6 1.8 3.8 6.3 5.3 0.42% In 750 788
29.3
Example 7 1.4 3.2 6.0 5.1 0.73% In 750 .. 1511
.. 26.8
Example 8 1.0 5.0 10.0 10.0 1% In 750 1881
Example 9 1.0 5.0 10.0 10.0 1% In, 750 1804
1% Sn
Example 10 0.9 2.1 5.7 5.4 0.50 % In 700 1268 30.8
Example 11 1.4 3.2 6.0 5.1 0.73% In 700 1511 26.8
Example 12 - 3.7 0.7 7.7 1.19% In 700 .. 3709
Example 13 - 2.9 0.9 7.7 0.6 % In 700 .. 1582
Example 14 - 3.3 - 7.7 0.9% In 700 1540
Example 15 - 3.3 - 7.7 1.1 % In 700 699
Example 16 0.6 0.4 1.1 7.8 0.69% In 700 1558
Example 17 1.1 0.3 5.0 6.8 0.75% In 700 1895 26.6
Example 18 0.7 - 1.2 10.1 1.2% In 700 1761 27.7
Example 19 1.5 - 1.2 9.4 0.7% In 700 1470 28.8
Table 1 ctd