Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
[ Descr i pti on]
[Title of Invention]
PHARMACEUTI CAL COMPOSI TI ON, COMPRI SI NG RECOMBI NANT
STAB! LI ZED GALECTI N 9 PROTEI N, FOR PREVENTI ON OR
TREATMENT OF RHEUMATOI D ARTHRI TI S AND BONE DI SEASE
[Techni cal Fi el d]
The present i nventi on rel at es to a recombi nant
stabilized gal ecti n 9 protein and the use thereof, and
speci f i cal I y rel at es to a pharmaceuti cal composi ti on,
compri si ng recombi nant stabilized gal ecti n 9 protei n, for
pr event i on or treatment of rheumatoi d art hritis and bone
di sease.
[ Background Art]
It has been found that there are ani mal I ecti ns that
specifically recognize sugar cqains -laving a 13-
gal act osi di c structure in living bodi es, and at least 14
genes have been i dent i f i ed so far. Gal ecti ns are
cl assi f i ed i nto prototype, chi meras, and tandem repeats
based on the structures thereof.
Gal ecti n 9, one of the tandem repeat gal ecti ns,
includes two sugar chain recogni ti on sites ( carbohydrate
recogni ti on domai ns: CRD) and a I i nk pepti de regi on
connecting the sites and has N-termi nal domain ( N -
Terminal Carbohydrate Recognition Domain; NCRD) and a C-
termi nal domain ( C- t er mi nal Carbohydrate Recogni ti on
Domai n; CCRD) connected by the I i nk pepti de regi on, and
various activities have reported so far. Regarding T
cells, gal ecti n 9 binds to Ti m-3 to induce apoptosi s of
Ti m-3- posi ti ve Th1 cells, and i nhi bits excessive Th1
responses to suppress aut oi mmune i nf I ammat i on. I n
addi ti on, gal ecti n 9 reduces Th17 cell s that are one of
CA 03157954 2022-5-10
1
causes or exacerbati on factors of van i ous i ntractabl e
di seases such as autoi mmune di seases, al I ergi es, and
cancers expressed by Tim-3.
On the other hand, gal ecti n 9 enhances i mmuni ty i n
some cases. Gal ecti n 9 bi nds to Ti m-3 on monocyt es or
dendritic cells to activate the cells and promote the
pr oduct i on of inflammatory cyt oki nes.
In addi ti on, the
i nt er act i on of gal ecti n 9 and Ti m-3 in macrophages
enhances i mmuni ty to excl ude Mycobacteri um tubercul osi s.
I n addi ti on, evi dence suggest i ng the exi stence of a
genet i c pol ymor phi sm among the gal ecti n 9 genes cl oned
from human cel I s or ti ssues is conf i rmed, and the
recombi nant gal ecti n 9 produced usi ng E. col i as a host
i s known to have an effect of i nduci ng metastasi s
i nhi bi ti on and regressi on of cancer and an effect of
i nduci ng apoptosi s of act i vat ed T cell s, part i cul ar I y
CD4- posi ti ve T cel Is which are the cause of an excessive
i mmune response, by di rect act i on on tumor cel I s
( act i vat i on to i nduce i nt ercel I ul ar adhesi on between
tumor cel I s and apoptosi s) and act i on through the i mmune
system. I n addi ti on, a t her apeut i c effect of human
i mmunodef i ci ency virus ( HI V) infection, a therapeutic
effect of at opy and asthma, and a therapeutic effect of
type 1 diabetes are known.
I n usi ng gal ecti n 9 as an actual therapeuti c agent,
in order to solve the probl ems of 1) protease
sensitivity; 2) low solubility; and 3) I ow yi el d, a study
of cleaving the linker pepti de of gal ecti n 9 to produce a
gal ect i n 9 van i ant ( G9Nul I ; Non- Patent Document 1) with
proteol yti c enzyme resistance and the like is continued,
and an effect such as KRAS mutant col on carci noma
ant it umor act i vi ty ( Non- Pat ent Document 2) i s known.
Meanwhi I e, a bone i s a dynami c ti ssue that undergoes
conti nuous removal and reconstructi on through bone
CA 03157954 2022-5-10
2
resorpti on and f ormati on. These processes are carri ed out
by two types of cel I s: osteocl asts and osteobl asts,
respectively. Speci f i cal I y, ost eobl
asts are responsi bi e
for bone f ormat i on, and osteocl asts are responsi bl e for
bone removal . I n a normal envi ronment, bone homeostasi s
is maintained by the balanced activity of the two cells.
However, homeost asi s can be di sr upt ed by abnormal
hyperactivity of these two types of cells, particularly
osteocl asts, resul ti ng i n a bone di sease such as
ar t hr i ti s, ost eopor osi s, per i pr ost het i c ost eol ysi s, and
Paget' s di sease. Therefore, accurate r egul at i on of
ost eocl ast differentiation and functions is important in
the prevent i on and treatment of a bone di sease.
For example, as a drug therapy for treating
osteoporosi s, as a drug that prevents a decrease i n bone
mass or i ncr eases bone mass by promoti ng bone f ormat i on
or suppressing bone resorption, there are bone resorption
i nhi bi tors such as cal ci um preparati ons, vi tami n D
preparati ons, bi sphosphonate preparati ons, estrogen
agoni sts/ ant agoni sts, and estrogen preparati ons, and
ost eogenesi s promoters such as ten i par at i de i nj ect i on
that i s a parathyroi d hormone preparati on.
Also, among arthritis, rheumatoid arthritis is a
typi cal chroni c autoi mmune di sease and i s a di sease
caused by i nf I ammati on i n a synovi al ti ssue that
surrounds a joint. The rheumatoid arthritis is a di sease
that can occur i n any j oi nt where the synovi al membrane
exi sts and that i nvades van i ous organs throughout the
body, and i s a chroni c i nf I ammatory di sease that destroys
articular tissues to cause severe joint disorders and
I ead to premature death. I n some severe cases, though the
cases are rare, ti ssues other than j oi nts, for exampl e,
the I ungs, the heart, the eyes, the gast roi nt est i nal
tract, the ski n, and the ki dneys may be i nvol ved.
CA 03157954 2022-5-10
3
Moreover, general 1 y, i nf I ammat ory medi at ors that are
secreted in the body in large amounts adversely affect
bone met abol i sm, thereby i ncreasi ng the r i sk of
osteoporosis and fractures. In
reality, it is conf i r med
that the i nci dence of ost eoporosi s in r heumat oi d
arthritis patients is about 15% to 20%.
Drug therapies for treating rheumatoid arthritis
i ncl ude pri mary drugs such as nonsteroi dal anti -
i nf I ammat ory drugs and st er oi d preparat i ons, whi ch are a
ki nd of hormone, and secondary drugs that suppress
rheumatoid arthritis itself by affecting the immune
system of the body, and are usual 1 y used for a long
period of time, thereby requiring attention to side
effects. In particular, steroid preparations may be
abused because good effects are exhi bi t ed i mmedi at el y,
but pr obl ems of si de effects are ser i ous. The long- t erm
use thereof may be a r i sk factor for ost eoporosi s.
Accor di ngl y, the present i nvent ors have made efforts
to devel op a new t her apeut i c agent havi ng an effective
therapeutic effect while minimizing the side effects of a
therapeutic agent for arthritis, including rheumatoid
arthritis, and/or osteoporosis. As a result, it is
conf i rmed that stabilized gal ect i n 9 protein in which
ami no aci ds of the C- t ermi nal domai n ( CCRD) of the two
sugar chain recognition sites and of link peptides of the
existing wild-type gal ect i n 9 are del et ed and substituted
have an effect of suppr essi ng Thl and Th17
differentiation and i nduci ng f i brobl ast - I i ke synovi ocyt es
( FLS) apopt osi s, thereby suppr essi ng the di f f er ent i at i on
of ost eocl ast s, and the present i nvent i on i s compl et ed on
the basis thereof.
[ Summary of I nvent i on]
[Techni cal Pr obl em]
CA 03157954 2022-5-10
4
An obj ect of the present invention is to provide a
recombi nant stabilized gal ect i n 9 prot ei n and a
pharmaceutical composition for prevention or treatment of
a bone di sease i ncl udi ng the same.
[Solution to Pr obl em]
In or der to achi eve the obj ect of the present
i nvent i on, the present i nvent i on provi des a recombi nant
stabilized gal ect i n 9 protein havi ng the ami no acid
sequence represented by SEQ ID NO: 1.
I n addi ti on, the present i nvent i on provi des a
recombi nant vector i ncl udi ng a gene that encodes the
pr ot ei n.
I n addi ti on, the present i nvent i on provi des a
t r ansf or mant i nt o whi ch the recombi nant vector i s
i nser t ed.
I n addi ti on, the present i nvent i on provi des a
pharmaceutical composition for prevention or treatment of
a bone di sease, i ncl udi ng a recombi nant st abi I i zed
gal ect i n 9 prot ei n havi ng an ami no aci d sequence
represented by SEQ I D NO: 1 or a pol ynucl eot i de that
encodes the same, as an active i ngredi ent; a met hod for
treating a bone disease, including admi ni st er i ng, to an
i ndi vi dual , a pharmaceut i cal I y effective amount of a
recombi nant stabilized gal ect i n 9 prot ei n havi ng the
ami no aci d sequence represented by SEQ I D NO: 1 or a
pol ynucl eot i de encodi ng the same; a pharmaceut i cal
composi ti on i ncl udi ng a recombi nant stabilized gal ect i n 9
pr ot ei n havi ng an ami no aci d sequence represented by SEQ
ID NO: 1 or a pol ynucl eot i de that encodes the same for
use i n the prevent i on or treatment of a bone di sease; and
the use of a recombi nant st abi I i zed gal ect i n 9 prot ei n
havi ng an ami no aci d sequence represented by SEQ I D NO: 1
or a pol ynucl eot i de that encodes the same for the
CA 03157954 2022-5-10
pr epar at i on of a phar maceut i cal composi ti on for
pr event i on or treatment of a bone di sease.
Al so, the present i nvent i on pr ovi des a health
f unct i onal food for pr event i on or i mpr ovement of a bone
di sease, i ncl udi ng a recombi nant stabilized gal ect i n 9
pr ot ei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1, as an active ingredient; a met hod for
pr event i ng or i mprovi ng a bone di sease, i ncl udi ng
admi ni st er i ng, to an individual, a pharmaceutically
effective amount of a recombinant stabilized gal ect i n 9
pr ot ei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1 or a pol ynucl eot i de that encodes the same; a
health f unct i onal food i ncl udi ng a recombi nant stabilized
gal ect i n 9 prot ei n havi ng an ami no aci d sequence
represented by SEQ I D NO: 1 or a pol ynucl eot i de that
encodes the same for use in prevention or improvement of
a bone di sease; and the use of a recombi nant st abi I i zed
gal ect i n 9 prot ei n havi ng an ami no aci d sequence
represented by SEQ I D NO: 1 or a pol ynucl eot i de that
encodes the same for the preparation of a health
f unct i onal food for pr event i ng or i mpr ovi ng a bone
di sease.
[Advantageous Effects of I nvent i on]
The recombi nant stabilized gal ect i n 9 protein of the
present i nvent i on exhi bits Th1 and Th17 cell
differentiation inhibitory effects, f i br obl ast - I i ke
synovi ocyt es ( FLS) apopt osi s effects and ost eocl ast
differentiation inhibitory effects, and thus can be
usefully used as an active ingredient in a composition
for pr event i on or treatment of a bone di sease i ncl udi ng
rheumatoid arthritis.
[Brief Descr i pt i on of Dr awi ngs]
CA 03157954 2022-5-10
6
FIG. 1 is a figure showing a binding degree of a
r ecombi nant stabilized gal ect i n 9 prot ei n ( sGal -9)
according to the present invention to Ti m- 3.
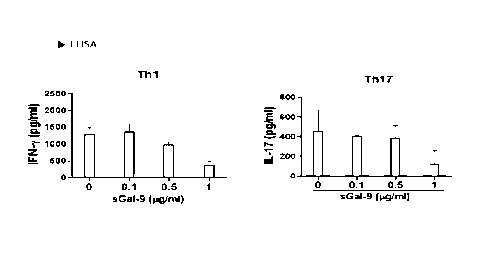
FIG. 2 is a figure showing Thl and Th17 cell
differentiation inhibitory effects of sGal -9 according to
the present i nvent i on.
FIG. 3 is a figure showing f i brobl ast- I i ke
synovi ocyt es ( FLS) apopt osi s effects of sGal - 9 according
to the present i nvent i on.
Fl G. 4 is a fi gure showi ng osteocl ast
differentiation inhibitory effects of sGal -9 according to
the present i nvent i on.
FIG. 5 is a figure showing the bone resorption
i nhi bi tory effect of sGal -9 accordi ng to the present
i nvent i on.
[ Descr i pt i on of Embodi ment s]
Her ei naf t er , embodi ments of the present i nvent i on
will be described in detail so that those of ordinary
skill in the art to which the present invention pertains
can easi I y carry out the present i nvent i on. The
embodi ment s of the present i nvent i on are pr ovi ded i n
or der to more completely explain the present invention to
those of or di nary ski II in the art to whi ch the present
i nvent i on pert ai ns. Accor di ngl y, the embodi ment of the
present invention may be modified in van i ous other forms,
and the scope of the present invention is not limited to
the embodi ment s descr i bed bel ow.
Throughout the specification of the present
invention, if it is said that any part " i ncl udes" any
component, t hi s means that other components may be
further i ncl uded, rat her than excl udi ng the other
components, unl ess otherwi se stated.
CA 03157954 2022-5-10
7
The present i nventi on provi des a recombi nant
stabilized gal ecti n 9 protein having an ami no acid
sequence represented by SEQ ID NO: 1.
I n the present i nventi on, the recombi nant stabi I i zed
gal ecti n 9 protein has a more stable molecular structure
to a protease while maintaining the sugar chain
recogni ti on activity of wild type Gal ecti n-9.
Speci f i cal I y, the recombi nant stabilized gal ecti n 9
protei n is a recombi nant protei n prepared by modi fyi ng a
I i nk r egi on connect i ng two carbohydrate recogni ti on
domains ( CRDs) of wild-type gal ecti n 9 having the
structure of NCRD- I i nker-CCRD and a C-termi nal
carbohydrate recogni ti on domai n ( CCRD) . More
speci f i cal I y, the recombi nant stabilized gal ecti n 9
protein is obtained by deleting al I peptides of the
I i nker regi on, del et i ng the ami no aci d sequence at
positions 1 to 10 ( SEQ ID NO: 3) in CCRD ( SEQ ID NO: 2),
and substituting Al a ( Al ani ne; A) at posi ti on 13 with Pro
( Prol i ne; P), may i ncl ude the ami no aci d sequence
represented by SEQ I D NO: 1, may i ncl ude an ami no aci d
sequence havi ng sequence homol ogy of 75% or more,
preferably 80% or more, more preferably 90% or more with
the ami no aci d sequence represented by SEQ I D NO: 1, and
may further i ncl ude t ar get i ng sequences, tags, I abel ed
resi dues, and an ami no aci d sequence prepared for a
specific purpose to increase half-life or peptide
stability.
I n addi ti on, the recombi nant protei n of the present
i nventi on can be obtai ned by van i ous met hods wel I known
in the art. As an example, the recombi nant protein of the
present i nventi on may be prepared usi ng pol ynucl eoti de
recombi nati on and protei n expressi on systems, may be
synthesi zed i n vitro through chemi cal synthesi s such as
pepti de synthesi s, or may be prepared by cell -free
CA 03157954 2022-5-10
8
protei n synt hesi s.
As used herein, the term "pol ynucl eoti de" refers to
a polymer to which nucleotides are bound, and serves to
transmit genetic information. For the purposes of the
present i nventi on, pol ynucl eoti de encodes the recombi nant
protei n of SEQ I D NO: 1 and may i ncl ude sequence havi ng
sequence homol ogy of 75% or more, pref erabl y 85% or more,
more pref erabl y 90% or more, and most pref erabl y 95% or
more with the pol ynucl eoti de sequence for encodi ng the
recombi nant protei n.
As used herein, the term "homology" is intended to
indicate a degree of similarity to a wild type amino acid
sequence or a pol ynucl eoti de sequence, the comparison of
such homol ogy can be performed usi ng a compari son program
well known in the art, and homol ogy between two or more
sequences can be cal cul at ed as a percentage (A).
I n addi ti on, the present i nventi on provi des a
recombi nant vector i ncl udi ng a gene that encodes the
protei n.
I n addi ti on, the present i nventi on provi des a
transf ormant i nto whi ch the recombi nant vector i s
i nsert ed.
As used herein, the term "vector" refers to a
pl asmi d, a virus or other medium well-known in the art
i nto whi eh a gene or a base sequence can be i nserted or
i ntroduced, speci f i cal I y may be I i near DNA, pl asmi d DNA,
a recombi nant non-vi ral vector, a recombi nant vi ral
vector, or an i nduci bl e gene expressi on vector system,
and the recombi nant vi ral vector may be a retrovi rus, an
adenovi r us, an adeno-associ ated vi r us, a hel per-dependent
adenovi r us, a herpes si mpl ex vi r us, a I enti vi ral vector
or a vacci ni a virus, but the vector is not limited
thereto. The base sequence accordi ng to the present
CA 03157954 2022-5-10
9
i nventi on may be operably I i nked to an expressi on control
sequence, and the operably linked base sequence may be
i ncl uded i n one expressi on vector i ncl udi ng a sel ecti on
marker and a replication origin together. Those that are
"operably I i nked" can be a gene and an expressi on control
sequence that are I i nked i n a manner that enabl es gene
expressi on when an appropri ate mol ecul e i s bound to the
expressi on control sequence. The term "expressi on control
sequence" refers to a DNA sequence that control s the
expressi on of an operabl y I i nked base sequence i n a
speci f i c host cel I . Such control sequences i ncl ude
promoters for effecting t r anscr i pt i on, any operator
sequences for regul at i ng transcri pti on, sequences for
encodi ng sui tabl e mRNA ri bosome bi ndi ng sites, and
sequences for regul at i ng the t ermi nat i on of t ranscr i pt i on
and translation.
As used her ei n, the term "transf ormati on" refers to
a change i n genet i c properti es of an organi sm by a DNA
given from the outsi de, that i s, a phenomenon i n whi ch
when a DNA, whi ch i s a type of nucl ei c aci d extracted
from a cell of a certain lineage of an organism, is
introduced into a living cell of another lineage, the DNA
enters the cel I and changes i n hereditary
char act er i sti cs. The cel I may be a prokaryoti c cel I or a
eukaryoti c cell, but is not limited thereto.
I n the present i nventi on, the gene that encodes the
recombi nant protei n can be i ntroduced i nto cel I s after
preparing a primer capable of specifically recognizing
the gene from a known sequence as descri bed above,
ampl i fyi ng the gene through a pol ymerase chai n react i on
( PCR) by using the pri mer, and introducing the amplified
gene i nto the vector as descri bed above. The i ntroducti on
methods are known, and examples thereof i ncl ude I i posome
medi at ed transf ecti on, a cal ci um phosphate method, DEAF-
CA 03157954 2022-5-10
dext ran medi at ed t r ansf ect i on, positively charged lipid
medi at ed t ransf ect i on, el ect r opor at i on, t ransduct i on
usi ng a phage system, and i nf ecti on method usi ng a vi r us.
However, the introduction met hods are not limited
thereto.
I n addi ti on, the present i nventi on provi des a
pharmaceutical composition for prevention or treatment of
a bone di sease, i ncl udi ng the recombi nant stabi I i zed
gal ecti n 9 protei n havi ng the ami no aci d sequence
represented by SEQ I D NO: 1 or a pol ynucl eoti de that
encodes the same, as an active i ngredi ent.
As used herein, the term "pr event i on" refers to any
act i on that suppresses or del ays an onset of a di sease by
admi ni strati on of a composi ti on.
As used herein, the term "treatment" refers to any
act i on i n whi ch symptoms of a di sease are i mproved or
benef i ci ally changed by admi ni strati on of the
composi ti on.
I n the present i nvent i on, the recombi nant stabi I i zed
gal ecti n 9 protein may exhibit one or more of the
f ol I owi ng properti es, thereby havi ng an effect of
pr event i ng or treati ng a bone di sease:
i ) increase in a degree of binding Ti m- 3 protein;
i i ) inhibition of Th1 and Th17 cell differentiation;
iii) inhibition of T helper cells;
iv) apoptosi s of f i brobl ast - I i ke synovi ocyt es ( FLS);
and
v) i nhi bi ti on of osteocl ast di f f erent i at i on and bone
r esor pt i on.
Speci f i cal I y, the recombi nant stabilized gal ecti n 9
protei n can i nhi bit hel per T cell s such as Th1 and Th17
through an apoptosi s i nducti on mechani sm by bi ndi ng to
Ti m-3 protei n expressed on T cel Is.
I n part i cul ar, the
CA 03157954 2022-5-10
1 1
synovi ocyt es are one of the important pat hol ogi es of
rheumatoid arthritis together with inflammatory
cytoki nes, and abnormal prol i f erat i on and act i vat i on of
synovi ocyt es becomes a problem as the di sease progresses,
and thus inflammation and destruction of joints may
conti nuousl y occur.
In addition, the recombinant stabilized gal ect i n 9
protei n can cause apoptosi s of f i brobl ast - I i ke
synovi ocyt es that destroys the matrix of articular
cartilage by secreting matrix met hal I oprotei nase ( MMP)
and cat hepsi n.
In addition, the recombinant stabilized gal ect i n 9
protein can inhibit osteocl ast differentiation and bone
resorption. In particular, the ost eocl ast s
are
multi nucl eat ed cel 1 s that destroy and absorb bone ti ssues
that are unnecessary i n bone growth, and if the bal ance
with osteobl asts that are i nvol ved i n bone gener at i on and
r egener at i on i s di sr upt ed, osteoporosi s that causes bone
loss may occur. In addition, the bone resorption is a
phenomenon in which bone is resorbed by the activity of
bone- dest royi ng cel I s, and bi sphosphonat es that are
general I y known osteoporosi s drugs i nhi bit the bone
resorption to pr event the exacerbation of osteoporosis.
I n the present i nventi on, the bone di seases may be
arthritis, osteoporosis, per i pr ost het i c ost eol ysi s,
osteol ysi s caused by f at i gue debri s of i mpl ants, Paget' s
di sease, osteomal aci a, ri ckets, osteopeni a, cal ci um
dysregul at i on, met ast at i c bone cancer, i nf I ammatory bone
I osses, secondary bone I osses by endocri ne di sease or
drugs, or per i odont al di seases accompani ed by destructi on
of al veol ar bone, but are not 1 i mi ted thereto.
In addition, the arthritis may be ost eoart hr i t i s,
degenerative arthritis, rheumatoid arthritis,
di ssoci at i ve osteochondri ti s, j oi nt I i gament damages,
CA 03157954 2022-5-10
12
meni scus damages, j oi nt mi sal i gnment, avascul ar necrosi s
or juvenile idiopathic arthritis, but is not limited
thereto.
I n a speci f i c embodi ment of the present i nvent i on,
the present i nvent ors I ysed and pun i f i ed E. col i cell s
that i nduce the expressi on of the recombi nant stabilized
gal ecti n 9 protei n havi ng the ami no aci d sequence
represented by SEQ ID NO: 1, and then prepared a
recombi nant protei n by usi ng a chromatography method. I n
addi ti on, it was conf i rmed that the recombi nant protei n
exhibited Thl and Th17 cell differentiation inhibitory
effects, f i brobl ast- I i ke- synovi ocyt es ( FLS) apopt osi s
effects and ost eocl ast di f f erent i at i on i nhi bi t or y
effects. Therefore, the recombi nant protei n and the
pol ynucl eoti de t hat encodes the same can be usef ul I y used
as an active i ngredi ent of a composi ti on for prevent i on
and treatment of a bone di sease.
Meanwhi I e, the recombi nant protei n of the present
invention or a pol ynucl eoti de that encodes the same may
be del i ver ed by phar maceut i cal I y accept abl e car r i er s such
as col I oi dal suspensi on, powder, sal i ne, I i pi ds,
I i posomes, mi crospher es, or nanospheri cal part i cl es.
These can form a complex with a vehicle or be associated
therewith and can be delivered in vivo by using a
del i very system wel I - known i n the art such as I i pi ds,
I i posomes, mi croparti cl es, gol d nanopart i cl es, pol ymers,
condensat i on reagents, pol ysacchari des, pol yami no aci ds,
dendri mers, saponi ns, adsorpti on enhanci ng substances or
fatty acids.
I n addi ti on, exampl es of pharmaceut i cal I y accept abl e
car r i er s i ncl ude I act ose, dextrose, sucrose, sor bi t ol ,
manni t ol , starch, acaci a gum, cal ci um phosphate,
al gi nate, gelatin, calcium silicate, mi crocryst al I i ne
CA 03157954 2022-5-10
13
cel I ul ose, pol yvi nyl pyrrol i done, cell ul ose, water,
syrup, methyl cell ul ose, methyl hydroxybenzoat e, pr opyl
hydroxybenzoate, t al c, magnesi urn st ear at e and mi ner al
oi I , whi ch are commonl y used i n prepar at i ons, but the
pharmaceuti cal I y accept abl e car r i ers are not I i mi ted
thereto. Further, in addition to the above components, a
I ubri cant, a wetti ng agent, a sweet eni ng agent, a
fl avori ng agent, an emul si fyi ng agent, a suspendi ng
agent, a preservati ve, and the I i ke may be addi ti onal I y
i ncl uded.
Accor di ng to a desi red method, the pharmaceuti cal
composi ti on of the present i nventi on may be admi ni stered
orally or parent eral I y (for example, intramuscularly,
i nt ravenousl y, i nt raper i t oneal I y, subcutaneously,
i ntradermal I y, or locally applied), and a dosage thereof
may vary dependi ng on states and wei ghts of pat i ents, the
severity of a disease, drug forms, routes of the
admi ni strati on and ti me, but may be appropri at el y
selected by those skilled in the art.
The pharmaceuti cal composi ti on of the present
i nventi on i s admi ni stered i n a pharmaceuti cal I y effective
amount.
As used herein, the term "pharmaceutically effective
amount" refers to an amount sufficient to treat a disease
with a reasonable benefit/risk ratio, which is applicable
to medical treatments, and the effective dose level can
be determi ned accordi ng to a pat i ent' s di sease type,
severity, drug activities, sensitivity to a drug,
admi ni strati on ti me, admi ni strati on route, excr et i on
rate, treatment durati on, factors i ncl udi ng concurrent
drugs and other factors well-known in the medical field.
The pharmaceuti cal composition according to the present
i nventi on may be admi ni stered as an i ndi vi dual
t her apeut i c agent, may be used i n combi nati on with
CA 03157954 2022-5-10
14
sur ger i es, hormone t her api es, drug t her api es and
bi ol ogi cal response modi f i ers, may be admi ni stered
si mul taneousl y, separately, or sequenti ally with the
agents, and may be admi ni stered i n a si ngl e does or
multi pl e doses. It is i mport ant to admi
ni ster an amount
capable of obtaining the maxi mum effect with a minimum
amount without si de effects i n consi der at i on of al I of
the above factors, whi ch can be easi I y determi ned by
those skilled in the art.
Specifically, the effective amount of the
pharmaceuti cal composi ti on of the present i nventi on may
vary dependi ng on a pat i ent' s age, sex, condi ti ons,
wei ght, absorpti on of the active ingredient into the
body, i nacti vat i on rate, excreti on rate, di sease type,
and drugs used i n combi nati on, and may be i ncreased or
decreased accordi ng to the admi ni strati on route, the
severity of obesity, the sex, the wei ght, the age, and
the like.
In addition, the present invention provides a health
f unct i onal food for pr event i on or i mpr ovement of a bone
di sease, i ncl udi ng the recombi nant stabi I i zed gal ecti n 9
protei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1, as an active ingredient.
In the present invention, the contents relating to
the recombi nant stabilized gal ecti n 9 protei n and bone
diseases are the same as those described above, and
specific descriptions thereof refer to the above content.
As used herein, the term "improvement" refers to any
acti ons that at I east reduces parameters rel ated to the
condi ti on bei ng treated, for exampl e, the severity of
symptoms. I n t hi s case, the health f uncti onal food
composi ti on may be used before or after the onset of the
di sease i n order to prevent or i mprove a bone di sease,
CA 03157954 2022-5-10
simultaneously with or separately from a drug for
treatment.
Meanwhi I e, i n the present i nvent i on, it was
conf i r med that the r ecombi nant stabilized gal ect i n 9
pr ot ei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1 exhibits Th1 and Th17 cell differentiation
inhibitory effects, f i br obl ast - I i ke- synovi ocyt es ( FLS)
apopt osi s effects and ost eocl ast differentiation
i nhi bi t or y effects. Therefore, the r ecombi nant pr ot ei n
can be useful I y used as an active i ngr edi ent of a health
f unct i onal food for pr event i on or i mpr ovement of a bone
di sease.
In the health functional food of the present
invention, the active ingredient may be added to food as
it is or used together with other food or food
i ngr edi ent s, and may be appr opr i at el y used accor di ng to a
met hod in the related art. The mixing amount of the
active i ngr edi ent s may be appr opr i at el y det er mi ned
dependi ng on the purpose of its use (for pr event i on or
i mpr ovement ) . In general, in the preparation of food or
beverage, the health functional food of the present
i nvent i on may be added i n an amount of pr ef er abl y 15% by
wei ght or I ess and preferably 10% by wei ght or I ess, with
respect to the raw material. However, if the purpose is
for health and hygiene or in the case of long-term intake
for the purpose of health control , the amount may be
equal to or I ess than the above range.
The health functional food of the present invention
may i ncl ude other i ngr edi ent s as essential i ngr edi ent s
without any particular limitation other than the
cont ai ni ng of the above active i ngr edi ent s. For exampl e,
as i n general beverages, van i ous fl avor i ng agents,
natural carbohydrates, or the I i ke may be i ncl uded as
CA 03157954 2022-5-10
16
addi ti onal i ngredi ents. Exampl es of the above natural
carbohydrates i ncl ude monosacchari des such as gl ucose and
fructose; di sacchari des such as maltose and sucrose; and
pal ysacchari des, for exampl e, general sugars such as
dextri n and cycl odextri n, and sugar al cohol s such as
xyl i t ol , sor bi t ol , and eryt hr i t ol .
In addi ti on to those
descr i bed above, as fl avor i ng agents, natural fl avor i ng
agents ( t haumati n, stevi a extract (for example,
rebaudi osi de A and gl ycyr r hi zi n)) and synt het ic fl avori ng
agents ( sacchari n, aspartame, and the like) may be
advantageously used. The ratio of the natural
carbohydrate may be appropri at el y determi ned by the
selection of those skilled in the art.
In addition to the above, the health functional food
of the present i nventi on may i ncl ude van i ous nut r i ents,
vitamins, minerals ( el ectrol yt es) , flavoring agents such
as synt het i c and natural fl avori ng agents, col or i ng
agents and t hi ckeners ( cheese, chocol ate, and the I i ke),
pectic acid and salts thereof, al gi ni c acid and salts
thereof, or gani c aci ds, protective col I oi dal t hi ckeners,
pH adj usters, stabi I i zers, preservati yes, gl yceri n,
al cohol s, carbonates used i n carbonated beverages, and
the I i ke. These components may be used i ndependent I y or
i n combi nati on, and the proporti on of these addi ti yes may
al so be appropri at el y sel ected by those skilled in the
art.
I n addi ti on, the present i nventi on provi des a method
for pr event i ng or t r eat i ng a bone di sease, i ncl udi ng
admi ni steri ng, to an individual, a pharmaceutically
effective amount of the recombi nant stabi I i zed gal ecti n 9
protei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1 or a pol ynucl eoti de that encodes the same.
I n addi ti on, the present i nventi on provi des a method
CA 03157954 2022-5-10
17
f or pr event i ng or i mpr ovi ng a bone disease, i ncl udi ng
admi ni st er i ng, to an individual, a pharmaceutically
effective amount of the recombi nant st abi I i zed gal ect i n 9
pr ot ei n havi ng the ami no aci d sequence represented by SEQ
ID NO: 1 or a pol ynucl eot i de that encodes the same.
The recombi nant st abi I i zed gal ect i n 9 pr ot ei n havi ng
the ami no aci d sequence represented by SEQ I D NO: 1 of
the present i nvent i on or a pol ynucl eot i de that encodes
the same exhibits Ti m-3 protein binding synergistic
effects, Thl and Th17 cell differentiation i nhi bi t or y
effects, hel per T cell inhibitory effects, f i br obl ast -
I i ke- synovi ocyt es ( FLS) apopt osi s effects, ost eocl ast
differentiation inhibitory effects and bone resorption
inhibitory effects, and thus can be usefully used to
treat a bone di sease.
I n addi ti on, the present i nvent i on provi des a
pharmaceutical composition used for prevention or
treatment of a bone di sease, i ncl udi ng the recombi nant
stabilized gal ect i n 9 protein havi ng the ami no acid
sequence represented by SEQ ID NO: 1 or a pol ynucl eot i de
that encodes the same.
In addition, the present invention provi des a health
f unct i onal food used for pr event i on or improvement of a
bone di sease, i ncl udi ng the recombi nant st abi I i zed
gal ect i n 9 prot ei n havi ng the ami no aci d sequence
represented by SEQ I D NO: 1 or a pol ynucl eot i de that
encodes the same.
I n addi ti on, the present i nvent i on provi des the use
of the recombi nant stabilized gal ect i n 9 protein havi ng
the ami no aci d sequence represented by SEQ I D NO: 1 or a
pol ynucl eot i de that encodes the same for the preparation
of a pharmaceut i cal composi ti on for pr event i on or
CA 03157954 2022-5-10
18
treatment of a bone di sease.
I n addi ti on, the present i nvent i on provi des the use
of the recombi nant stabilized gal ect i n 9 protein having
the ami no aci d sequence represented by SEQ I D NO: 1 or a
pol ynucl eot i de that encodes the same for the preparation
of a health functional food for prevention or improvement
of a bone di sease.
Her ei naf t er, the present i nvent i on i s be descr i bed
in more detail through preparation exampl es and examples.
However, the f ol I owi ng pr epar at i on exampl es and exampl es
are i nt ended to hel p the under st andi ng of the present
invention and are not intended to limit the scope of the
present i nvent i on.
<Pr epar at i on Exampl e 1> Pr epar at i on of Recombi nant
Stabilized Gal ect i n 9 Protein
An expr essi on vector i ncl udi ng a gene that encodes
the recombi nant stabilized gal ect i n 9 protein having the
ami no aci d sequence of SEQ I D NO: 1 was prepared, and the
expr essi on vector was i nt r oduced i nt o E. col i by a heat
shock method. The recombi nant pr ot ei n was expressed by
culturing E. col i in an LB medium including 50 pg/ ml of
kanamyci n and addi ng ar abi nose when the absorbance at 600
nm reached 0.7 to induce the expression of the
recombi nant prot ei n. Then, the cel I s i nduced to express
the recombi nant protein were I ysed and filtered, and the
target protei n was captured by usi ng a cat i on exchange
method, an affinity column, and the like to obtain a
highly purified recombi nant stabilized gal ect i n 9 protein
in a high yield.
<Exampl e 1> Conf i r mat i on of Ti m-3 Protein Binding Ability
of Recombi nant St abl e Gal ect i n 9 Pr ot ei n ( sGal -9)
CA 03157954 2022-5-10
19
100 pl of recombinant quman im-3 (R&D systems, Cat.
No. 10241-1I-050) at a concentration of 0.5 pg/ml was
added to each well of a 96-well ELI SA plate (Nunc, Cat.
No. 44-2404-21), the reaction was carried out at room
temperature for one night, and then the resultant was
washed three times with DPBS (Dulbecco's phosphate-
buffered saline; Wel gene, Cat. No. LB011-02) including
0.05% Tween 20 (Sigma Cat. No. P9416). After washing, 200
pl of DPBS including 1% bovine serum albumin (BSA) was
added to each well, incubated at room temperature for one
hour, and washed three times with a wash buffer. After
washing three times, the recombinant stabilized galectin
9 protein (sGal-9) obtained in <Preparation Example 1>
was diluted to various concentrations (0, 5, 10 and 20
pg/ml) and added by 100 pl to each well, incubated at
room temperature for two hours, and washed three times
with a wash buffer.
For an experimental group, Gal-9 antibody (R&D
systems, Cat. No. 1015238) was diluted to 1:1000 by using
1% BSA/DP3S and added by 100 n1 to eacq well, incubated
at room temperature for one hour, and washed three times
with a wash buffer. As a control group, an anti-mouse HRP
antibody (lnvitrogen, Cat. No. 31430) was diluted to
1:1000 by using 1% BSA/DPBS and added by 100 pl to each
well, incubated at room temperature for one hour, and
washed three times with a wash buffer.
100 pl of a 1:1 mixture of substrate reagents (R&D
systems, Cat. No. DY999) A and B was added to each washed
well and incubated at room temperature with the light
blocked for 20 minutes. After the incubation, 50 pl of a
stop solution (2 N H2SO4) was added to each well, and the
optical density (0.D) value was measured and checked at
450 nm using a microplate reader.
As a result, as shown in FIG. 1, it was confirmed
CA 03157954 2022-5-10
that the optical density increased as the concentration
of sGal -9 i ncr eased, and thus it was conf i rmed that the
binding degree of sGal - 9 and Ti m- 3 was excellent in a
concent r at i on-dependent manner ( Fl G. 1) .
<Exampl e 2> Conf i r mat i on of T Hel per Cell Differentiation
Inhibitory Effect of sGal - 9
Anti - CD3 ( eBi osci ence, Cat. No. 16-0031-82) at a
concentration of 2 pg/m1 diluted with PBS (Welgene, Cat.
No. LB 001-02) was added to a 96-well plate by 100 p1 or
to a 24-well plate by 500 u11 was reacted at 4 C for one
ni ght, was washed once with PBS, and was used for cel 1
culture. After the resultant was dispensed into 96-well
pl at es or 24- wel 1 pl at es coated with CD4, CD62L and T
cells and incubated at 37 C and 5% CO2 condi ti ons for four
days, ant i -1 L-4, ant i - I L-2 and ant i -1 L-12 cyt oki nes were
added to i nduce the di f f erent i at i on i nt o Th1 cell s and
anti-IFN-y, anti-IL-4, anti-IL-2 and anti-IL-6 cytokines
were added to i nduce di f f erent i at i on i nto Th17 cells.
After the addition of cytoki nes, sGal - 9 with a
concentration of 1 lag/m1 was added and incubated at 37 C
and 5% CO2 condi ti ons for four days.
After the culture, IFN-y cells of ml cells and IL-
17 cytoki nes of Th17 cells were measured by usi ng an
ELI SA assay kit ( Mouse DuoSet, R&D Systems) according to
the procedure of the manufacturer.
As a result, as shown in FIG. 2, it was confirmed
that the di f f erent i at i on of Th1 and Th17 cell s whi ch are
the helper T cells was inhibited as the concentration of
sGal - 9 increased, and thus it was confirmed that the
hel per T cell differentiation inhibitory effect of sGal -9
was excellent ( Fl G. 2) .
<Exampl e 3> Conf i r mat i on of Fi brobl ast- I i ke-synovi ocyt es
CA 03157954 2022-5-10
21
( FLS) Apoptosi s Effect of sGal - 9
200 pl of the FLS cells (cell passage: RA
_______________________________________________________________ FLS 2-
92#P7) were di spensed i nto each well of a 48- wel I pl ate
(SPL, Cat. No. 30048) at a cell density of 5 x 104
cells/ml, and after 24 hours, 100 pl of sGal-9 at various
concentrations (2.5, 5, and 10 pg/ml) was added to each
wel I . After 16 hours, the supernatant was col I ect ed and
stored in a deep freezer.
Cell s for fl uorescence act i vat ed cell sort i ng ( FACS)
were placed and washed in a 15 ml tube, 200 pl of FACS
buffer was added thereto, and the resultant was stored at
4 C for one hour and was stai ned with Annexi n V- Fl TC/ 7-
AAD ( Bi ol egend, Cat. No. 640922) . The stai ned cells were
filtered witq a 70 pm cell strainer (3D Falcon, Cat. No.
352350) into a tube for FACS.
As a result, as shown in FIG. 3, it was confirmed
that the f i brobl ast - I i ke- synovi ocyt es apopt osi s effect
i ncr eased as the concent r at i on of sGal -9 i ncr eased, and
thus it was conf i rmed that the synovi al cell apoptosi s
effect of sGal - 9 was excellent ( Fl G. 3) .
<Exampl e 4> Conf i r mat i on of Osteocl ast Di f f erent i at i on
Inhibitory Effect of sGal - 9
1 ml of osteocl asts was passaged i n each wel I of a
12- wel I pl ate ( Corni ng, Cat. No. 3513) at a cell densi ty
of 2.5 x 105 cel I s/ ml , and after 24 hours, sGal - 9 as an
exper i mental group at van i ous concent r at i ons (0. 1, 0. 5,
1, 2, 4, and 10 ag/m1)and PBS as a control group were put
into each well by 1 ml.
After compl et i on of di f f er ent i at i on, the stai ned
osteocl asts of the experimental group and the control
group were TRAP- stai ned by usi ng the TRAP St ai ni ng Kit
( K- ASSAY, Cat. No. KT-008) and counted by using an
opt i cal mi croscope, and then multi nucl eat ed cel I s
CA 03157954 2022-5-10
22
(TRAP( +) MNCs) i n whi ch three or more nucl ei were
observed was determined to be differentiated into
osteocl asts.
As a result, as shown in FIG. 4, it was confirmed
that the number of stai ned osteocl asts decreased as the
concent r at i on of sGal -9 i ncr eased, and thus it was
conf i rmed that the osteocl ast differentiation inhibitory
effect of sGal -9 was excellent ( Fl G. 4).
<Exampl e 5> Conf i r mat i on of Bone Resorpt i on I nhi bi tory
Effect of sGal -9
1 ml of osteocl asts was passaged at a cel I densi ty
of 2.5 x 105 cell s/ ml into each well of Osteo Assay
Surf ace multi-well plate ( Corni ng, Cat. No. 3987) coated
with i nor gani c three-di mensi onal crystal I i ne that
resembled the bone structure in vi vo, and after 24 hours,
sGal -9 as an experi mental group at van i ous concent r at i ons
(0.1, 0.5, 1, 2, /, and 10 pg/m1)and PBS as a control
group were put i nto each wel I by 1 ml . The experi mental
group ( sGal -9), the control ( PBS), and cell culture
medium ( MEM-al pha, gi bco, Cat. No. 12561-056) were
repl aced daily unt i I di f f erent i at i on was compl et ed.
After completion of differentiation, cells were
removed with 20% SOS (Bleaching solution), and the Osteo
Assay Surf ace multi -wel I pl ate was observed under a
mi croscope.
As a result, as shown in FIG. 5, it was confirmed
that the bone resorpti on inhibitory effect of sGal -9 was
excellent ( Fl G. 5).
I n concl usi on, through the results of <Exampl e 1> to
<Exampl e 5>, it was confirmed that bone diseases could be
prevented or treated by sGal -9 i n a concent rat i on-
dependent manner, through the i ncrease i n the Ti m-3
protei n bi ndi ng ability, the i ncreases i n the Thl and
CA 03157954 2022-5-10
23
Th17 cell di f f erent i at i on i nhi bi t or y effect and the
synovi al cel I apoptosi s effect, and the i ncr eases i n the
ost eocl ast di f f erent i at i on i nhi bi t or y effect and the bone
r esor pt i on i nhi bi t or y effect.
CA 03157954 2022-5-10
24