Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03200150 2023-04-28
Description
ANTIBODY AGAINST NOVEL CORONAVIRUS, AND TEST KIT FOR
NOVEL CORONAVIRUS DETECTION
Cross-Reference to Related Application
The present disclosure claims the priority of Chinese Patent Application with
the application No.
202011179251.2, filed to the China National Intellectual Property
Administration on October 29,
2020 and entitled "Antibody against Novel Coronavirus, and Kit for Novel
Coronavirus Detection",
the entire content of which is incorporated in this application by reference.
Technical Field
The present disclosure relates to the technical field of antibodies, and
specifically, to an
antibody against novel coronavirus, and a kit for novel coronavirus detection.
Background
A structural protein of 2019 novel coronavirus (2019-nCoV) is classified into
spike glycoprotein
(S protein), envelope glycoprotein (E protein), membrane glycoprotein (M
protein) and nucleocapsid
protein (N protein), and these proteins include a plurality of antigen
epitopes. The N protein
intertwines with viral genome RNA to form a viral nucleocapsid, which plays an
important role in the
synthesis of virus RNA. In addition, the N protein is relatively conserved and
makes up the largest
proportion of the structural proteins of the virus; and high levels of
antibodies against the N protein
can be produced in the body early in the infection. Finally, the N protein is
an important marker
protein of the novel coronavirus. Using the principle of specific binding of
antigens and antibodies,
the presence of the antigens may be detected by an N protein monoclonal
antibody, so as to directly
prove the presence of the novel coronavirus in a sample and achieving the
detection of the novel
coronavirus.
Antibodies to be detected are mainly classified into two categories, which are
IgM and IgG.
Currently, there is a lack of systematic studies on the production and
duration of these two types of
antibodies to the novel coronavirus. Generally, IgM antibodies are produced
early, and once
infected, the antibodies are produced quickly, maintained for a short period
of time, and disappear
quickly. A positive test in the blood may reflect that the body is in an acute
state of infection, which
may be used as an indicator of early infection. Compared with a nucleic acid
testing method,
samples of an antibody test are serum or plasma, and are less affected by
sample sampling,
1/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
thereby facilitating early diagnosis and exclusion of suspicious cases; and in
addition, the test is
rapid, convenient and suitable for large-scale screening.
Since the outbreak of the novel coronavirus 2019-nCoV pneumonia, it has spread
globally,
posing a serious threat to human life safety and health. Respiratory droplets
and close contact
transmission are the main transmission routes of the novel coronavirus
pneumonia, and the
potential for aerosol transmission exists in relatively closed environments
with prolonged exposure
to high aerosol concentration. The 2019-nCoV is highly infectious, most
patients develop clinical
symptoms after infection, and some patients are asymptomatic infections.
Common signs in people
infected with coronavirus include respiratory symptoms, fever, cough,
shortness of breath, and
difficult breathing. In more severe cases, the infection may lead to
pneumonia, severe acute
respiratory syndrome, renal failure, and even death. Although there is no
specific treatment for the
disease caused by the novel coronavirus, treatment of mild or asymptomatic
patients may greatly
improve the cure rate and shorten treatment time. Therefore, the detection or
identification of the
patients becomes particularly important.
At present, nucleic acid testing and viral gene sequencing are mainly used as
confirmatory
evidence of pathogenesis, and nucleic acid testing is subject to false
negative results due to various
factors such as sampling, operation and reagents. The detection rate of
positive viral nucleic acid in
patients with highly suspected 2019-nCoV infection is only 30%-50%. In
addition, nucleic acid
testing requires high requirements for instruments and equipment, testing
sites and environmental
conditions, and has disadvantages of long testing time and low throughput, not
facilitating
large-scale testing of the population under the current epidemic. Therefore,
there is an urgent need
to develop a rapid and convenient detection kit for clinical detection, to
isolate the infected
population as soon as possible, so as to stop the spread of the virus.
Therefore, antibody detection
kits have become more important.
There are few 2019-nCoV N protein monoclonal antibody products with varied
performance.
Summary
The present disclosure provides an antibody or a functional fragment thereof
against novel
coronavirus or an N protein thereof. The antibody or the functional fragment
thereof includes the
following complementarity determinant regions:
CDR-VH1: G-X1-T-F-T-X2-Y-X3-M-N, wherein X1 is Y or F, X2 is D or N, and X3 is
A or G;
CDR-VH2: W-X1-N-T-Y-X2-G-E-P-T-Y-A-X3-X4-F, wherein X1 is L or I, X2 is T or
S, X3 is D or
E, and X4 is D or E;
2/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
CDR-VH3: A-R-X1-A-X2-X3-R-S-Y, wherein X1 is S or T, X2 is I or L, and X3 is I
or L;
CDR-VL1: K-A-S-X1-D-X2-S-T-A-X3-A, wherein X1 is Q or E, X2 is I, V or L, and
X3 is I, V or L;
CDR-VL2: W-X1-S-T-R-H-X2, wherein X1 is A or G, and X2 is T or S; and
CDR-VL3: Q-Q-H-X1-S-T-P-X2, wherein X1 is Y or W, and X2 is L, V or I.
In some implementations,
in CDR-VH1, X1 is Y;
in CDR-VH2, X1 is I;
in CDR-VH3, X1 is S;
in CDR-VL1, X1 is Q; and
in CDR-VL2, X1 is A.
In some implementations, in CDR-VH1, X2 is D.
In some implementations, in CDR-VH1, X2 is N.
In some implementations, in CDR-VH1, X3 is A.
In some implementations, in CDR-VH1, X3 is G.
In some implementations, in CDR-VH2, X2 is T.
In some implementations, in CDR-VH2, X2 is S.
In some implementations, in CDR-VH2, X3 is D.
In some implementations, in CDR-VH2, X3 is E.
In some implementations, in CDR-VH2, X4 is D.
In some implementations, in CDR-VH2, X4 is E.
In some implementations, in CDR-VH3, X2 is I.
In some implementations, in CDR-VH3, X2 is L.
In some implementations, in CDR-VH3, X3 is I.
In some implementations, in CDR-VH3, X3 is L.
3/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
In some implementations, in CDR-VL1, X2 is I.
In some implementations, in CDR-VL1, X2 is V.
In some implementations, in CDR-VL1, X2 is L.
In some implementations, in CDR-VL1, X3 is I.
In some implementations, in CDR-VL1, X3 is V.
In some implementations, in CDR-VL1, X3 is L.
In some implementations, in CDR-VL2, X2 is T.
In some implementations, in CDR-VL2, X2 is S.
In some implementations, in CDR-VL3, X1 is Y.
In some implementations, in CDR-VL3, X1 is W.
In some implementations, in CDR-VL3, X2 is L.
In some implementations, in CDR-VL3, X2 is V.
In some implementations, in CDR-VL3, X2 is I.
In some implementations, each complementarity determinant region of the
antibody or the
functional fragment thereof is any one selected from the following mutation
combinations 1-42:
CDR-VH1 CDR-VH2 CDR-VH3 CDR-VL1 CDR-VL2 CDR-VL3
X2/X3 X2/X3/X4 X2/X3 X2/X3 X2 X1/X2
Mutation combination 1 D/A S/E/D I/L L/L S W/L
Mutation combination 2 N/A S/E/E I/1 V/V T Y/L
Mutation combination 3 D/G S/D/E L/L I/1 T W/V
Mutation combination 4 N/G S/D/D I/1 I/L S YN
Mutation combination 5 D/A T/E/D I/L IN T W/I
Mutation combination 6 D/G T/E/E L/L I/1 T Y/I
Mutation combination 7 N/A T/D/E L/L V/L T Y/L
Mutation combination 8 N/G T/D/D I/L V/V S W/V
Mutation combination 9 D/A T/E/D L/I V/I S Y/I
4/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation combination 10 N/A S/E/D L/L L/L S W/L
Mutation combination 11 D/G T/E/E I/1 L/V T Y/V
Mutation combination 12 N/G S/E/E I/1 L/I T W/I
Mutation combination 13 N/A T/D/E L/L V/I S Y/V
Mutation combination 14 D/G S/D/E I/1 L/I T W/L
Mutation combination 15 D/A T/D/D L/I I/L S Y/I
Mutation combination 16 N/G S/D/D I/L V/L T Y/L
Mutation combination 17 D/G T/E/E L/L L/V T W/V
Mutation combination 18 N/A S/D/D I/L I/1 T W/I
Mutation combination 19 N/G T/D/D L/L V/V T Y/L
Mutation combination 20 D/A S/D/E I/L L/L S W/I
Mutation combination 21 D/A T/D/E I/1 IN T Y/V
Mutation combination 22 D/G S/E/E I/1 V/I S W/L
Mutation combination 23 N/A T/E/D I/L L/I S Y/I
Mutation combination 24 N/G S/E/D L/I I/1 T W/V
Mutation combination 25 N/A T/E/D I/1 V/V T W/L
Mutation combination 26 D/G S/E/E L/I L/V S Y/L
Mutation combination 27 D/A T/D/E L/L IN T W/V
Mutation combination 28 N/G S/D/D L/I V/L T Y/V
Mutation combination 29 D/G S/E/D L/L L/L T W/I
Mutation combination 30 N/A T/E/E I/1 I/L T Y/I
Mutation combination 31 N/G S/D/E I/L L/L T Y/L
Mutation combination 32 D/A T/D/D L/I V/L T W/V
Mutation combination 33 D/A S/D/D I/L I/L S Y/I
Mutation combination 34 N/A T/D/E L/L L/V S W/L
Mutation combination 35 D/G S/D/E L/L V/V S Y/V
Mutation combination 36 N/G T/E/E L/L IN T W/I
Mutation combination 37 D/A S/E/E I/L L/I S Y/L
5/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation combination 38 DIG T/E/D III VII T W/V
Mutation combination 39 N/A S/E/D I/L III T W/I
Mutation combination 40 N/G T/D/D I/L V/V S Y/L
Mutation combination 41 DIG T/E/D L/I L/L S W/I
Mutation combination 42 N/A S/E/D I/L III S Y/V
In some implementations, the antibody or the functional fragment thereof binds
to the N protein
of novel coronavirus with an affinity of KID8x10-9mol/L. In some
implementations, KID4x10-19mol/L.
In some implementations,
in CDR-VH1, X1 is F;
in CDR-VH2, X1 is L;
in CDR-VH3, X1 is T;
in CDR-VL1, X1 is E; and
in CDR-VL2, X1 is G.
In some implementations, each complementarity determinant region of the
antibody or the
functional fragment thereof is any one selected from the following mutation
combinations 43-48:
CDR-VH1 CDR-VH2 CDR-VH3 CDR-VL1 CDR-VL2 CDR-VL3
X2/X3 X2/X3/X4 X2/X3 X2/X3 X2 X1/X2
Mutation combination 43 D/A S/E/D I/L L/L S W/L
Mutation combination 44 N/G T/D/E L/I L/I T W/L
Mutation combination 45 N/G T/E/E L/I L/I S Y/V
Mutation combination 46 N/A T/D/E L/I V/V T Y/L
Mutation combination 47 N/A S/D/D I/L I/1 S Y/I
Mutation combination 48 N/G T/D/E L/I L/V T Y/V
In some implementations, the antibody includes light chain framework regions
FR1-L, FR2-L,
FR3-L and FR4-L, of which sequences are successively shown in SEQ ID NO:1-4,
and/or heavy
chain framework regions FR1-H, FR2-H, FR3-H and FR4-H, of which sequences are
successively
shown in SEQ ID NO:5-8.
6/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
In some implementations, the antibody further includes a constant region.
In some implementations, the constant region is any one selected from a
constant region of
IgG1, IgG2, IgG3, IgG4, IgA, IgM, IgE and IgD.
In some implementations, the constant region is derived from species of
cattle, horse, dairy
cow, pig, sheep, goat, rat, mouse, dog, cat, rabbit, camel, donkey, deer,
mink, chicken, duck, goose,
turkey, gamecock or human.
In some implementations, the constant region is derived from the mouse.
In some implementations, the sequence of a light chain constant region of the
constant region
is shown in SEQ ID NO:9, and the sequence of a heavy chain constant region of
the constant region
is shown in SEQ ID NO:10.
In some implementations, the functional fragment is any one selected from VHH,
F(ab')2, Fab',
Fab, Fv or scFv of the antibody.
In some implementations, the antibody includes light chain framework regions
FR1-L, FR2-L,
FR3-L and FR4-L respectively successively having at least 80% identity with
sequences SEQ ID
NO:1, 2, 3 and 4, and/or heavy chain framework regions FR1-H, FR2-H, FR3-H and
FR4-H
respectively successively having at least 80% identity with sequences 5, 6, 7
and 8.
The present disclosure provides a reagent or a kit for detecting novel
coronavirus or an N
protein thereof. The reagent or the kit includes any one of the above
antibodies or the functional
fragments thereof.
In some implementations, antibody or the functional fragment thereof is
labeled with a
detectable marker.
In some implementations, the detectable marker is selected from a fluorescent
dye, an enzyme
that catalyzes color development of substrates, a radio isotope, a
chemiluminescence reagent or a
nanoparticle marker.
In some implementations, the fluorescent dye is selected from fluorescein dyes
and derivatives
thereof, rhodamine dyes and derivatives thereof, Cy series dyes and
derivatives thereof, Alexa
series dyes and derivatives thereof, and protein dyes or derivatives thereof.
In some implementations, the enzyme that catalyzes color development of
substrates is
selected from horseradish peroxidase, alkaline phosphatase, 8-galactosidase,
glucose oxidase,
carbonic anhydrase, acetylcholinesterase or glucose 6-phosphate deoxygenase.
7/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
In some implementations, the radio isotope is selected from 212Bi, 1311,
111in, 90y, 186Re, 211At, 1251,
188Re, 153sm, 213Bi, 32R, 94mTb, 99mTb, 203pb, 67Ga, 68Ga, 43sb, 47sb, 0min,
97Ru, 62cu, 64cu, 67cu,
68cu, 86y, 88y, 121sn, 161Tb, 166H0, 105Rh, 177Lu, 172Lu or 18F.
In some implementations, the chemiluminescence reagent is selected from
luminol and
derivatives thereof, lucigenin, crustacean fluorescein and derivatives
thereof, bipyridine ruthenium
and derivatives thereof, acridinium ester and derivatives thereof,
dioxycyclohexane and derivatives
thereof, lophine and derivatives thereof, and peroxyoxalate or derivatives
thereof.
In some implementations, the nanoparticle marker is selected from
nanoparticles, colloids,
organic nanoparticles, magnetic nanoparticles, quantum dot nanoparticles or
rare earth complex
nanoparticles.
In some implementations, the colloids are selected from colloidal metals,
disperse dyes, dye
labeled microspheres, or latexes.
In some implementations, the colloidal metals are selected from colloidal
gold, colloidal silver
or colloidal selenium.
The present disclosure provides a nucleic acid molecule. The nucleic acid
molecule encodes
any one of the above antibodies or the functional fragments thereof.
The present disclosure provides a vector. The vector includes a nucleic acid
fragment encoding
any one of the above antibodies or the functional fragments thereof.
The present disclosure provides a recombinant cell. The recombinant cell
includes the vector.
The present disclosure provides use of the antibody or the functional fragment
thereof or the
reagent or kit in detection of novel coronavirus.
The present disclosure provides use of the antibody or the functional fragment
thereof or the
reagent or kit for detecting novel coronavirus.
The present disclosure provides a method for detecting novel coronavirus. The
method
includes the following operations.
A) Under conditions sufficient for a binding reaction to occur, any one of the
above antibodies
or the functional fragments thereof is contacted with a sample, so as to
perform a binding reaction.
B) An immune complex produced by the binding reaction is detected.
The present disclosure provides a method for diagnosing a subject in a novel
coronavirus
8/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
infection or a disease associated with the novel coronavirus infection. The
method includes the
following operations.
A) Under conditions sufficient for a binding reaction to occur, any one of the
above antibodies
or the functional fragments thereof is contacted with a sample from the
subject, so as to perform a
binding reaction.
B) An immune complex produced by the binding reaction is detected.
In some implementations, the disease associated with the novel coronavirus
infection includes
respiratory symptoms, fever, cough, shortness of breath, difficult breathing,
pneumonia, severe
acute respiratory syndrome, or renal failure.
In some implementations, the subject in the novel coronavirus infection
includes an
asymptomatic patient, an infected patient without obvious symptoms, or a
symptomatic infected
patient.
The present disclosure provides a method for preparing any one of the above
antibodies or the
functional fragments thereof. The method includes: culturing the recombinant
cell, and obtaining the
antibody or the functional fragment from a culture product by means of
separation and purification.
Brief Description of the Drawings
In order to illustrate the technical solutions in the embodiments of the
present disclosure more
clearly, the drawings used in the embodiments will be briefly introduced
below. It is to be understood
that the following drawings only illustrate some embodiments of the present
disclosure, which
therefore should not be regarded as limitations to the scope. For those of
ordinary skill in the art,
other related drawings may also be obtained in accordance with these drawings
without creative
efforts.
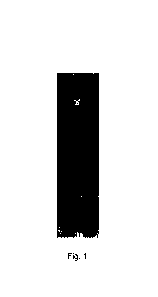
Fig. 1 shows reductive SDS-PAGE results of an antibody against novel
coronavirus according
to Embodiment 1.
Detailed Description of the Embodiments
In order to make the purposes, technical solutions and advantages of the
embodiments of the
present disclosure clearer, the technical solutions in the embodiments of the
present disclosure will
be clearly and completely described below. If specific conditions are not
indicated in the
implementations and embodiments, the implementations are carried out in
accordance with the
conventional conditions or the conditions recommended by manufacturers.
Reagents or
9/ 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
instruments used are conventional products that may be purchased commercially
if the
manufacturers are not specified.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by those of ordinary skill in the art to which
the disclosure
belongs. Although any methods and materials similar or equivalent to those
described herein may
be used in the practice or testing of preparations or unit doses herein, some
methods and materials
are now described. Unless otherwise stated, the technologies used or
considered herein are
standard methods. The materials, methods, and examples are illustrative only
and not limiting.
Unless otherwise indicated, the present disclosure will be practiced by using
conventional
technologies of cell biology, molecular biology (including recombinant
technologies), microbiology,
biochemistry and immunology, and the conventional technologies are within the
competence of
those skilled in the art. The technologies are well explained in the
literature, such as Molecular
Cloning:A Laboratory Manual (2nd edition, Sambrook et. al., 1989);
Oligonucleotide Synthesis
(M.J.Gait, 1984); Animal Cell Culture (R.I.Freshney, 1987); Methods in
Enzymology (Academic
Press, Inc.); Handbook of Experimental Immunology (D.M.Weir and
C.C.Blackwell); Gene Transfer
Vectors for Mammalian Cells (J.M.Miller and M.PCalos, 1987); Current Protocols
in Molecular
Biology (F.M.Ausubel et. aL, 1987); PCR:The Polymerase Chain Reaction (Mullis
et. al., 1994); and
Current Protocols in Immunology (J.E.Coligan et. aL, 1991). Each of the
literature is explicitly
incorporated herein by reference.
Term definition
As used herein, the term "complementarity determinant region" means that an
intact or
complete antibody includes two heavy chains and two light chains. Each heavy
chain includes a
variable region (VH) and a constant region (CH); and each light chain includes
a variable region (VL)
and a constant region (CL). The antibody is in a "Y" shape, and the stem of Y
consists of the second
and third constant regions of two heavy chains bound together by means of
disulfide bonds. Each
arm of Y includes the variable region and the first constant region of the
single heavy chain in
combination with the variable region and the constant region of the single
light chain. The variable
region of the light chain and the variable region of the heavy chain are
responsible for antigen
binding. The variable regions in two chains generally include three highly
variable regions, called
complementarity determinant regions.
As used herein, when being used to represent an antibody, the term "functional
fragment"
refers to a portion of the antibody including a heavy or light chain
polypeptide, and the polypeptide
retains some or all of the binding activity of the antibody from which the
fragment originated. These
10/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
functional fragments may include (for example) Fd, Fv, Fab, F(ab'), F(ab)2,
F(a13)2, single-chain Fv
(scFv), a double-chain antibody (diabody), a triple-chain antibody (triabody),
a four-chain antibody
(tetrabody), and a microbody (minibody). Other functional fragments may
include (for example)
heavy or light chain polypeptides, variable region polypeptides or CDR
polypeptides or portions
thereof, as long as these functional fragments retain the binding activity.
As used herein, the term "constant region" refers to the region of an antibody
molecule near a
C-terminal amino acid sequence that is relatively stable in both the light and
heavy chains.
As used herein, the term "variable region" refers to the region of the
antibody molecule near an
N-terminal amino acid sequence that is highly variable in the light and heavy
chains.
As used herein, the term "naked antibody stability" refers to an unlabeled
antibody or a
functional fragment thereof, such as an antibody or a functional fragment
thereof that is not labeled
with a detectable marker.
Some implementations of the present disclosure provide an antibody against
novel coronavirus,
and a kit for detecting novel coronavirus. The antibody may specifically bind
to an N protein of the
novel coronavirus, has high affinity for the N protein, and has good
sensitivity and specificity for
detecting the novel coronavirus. According to the present disclosure, a wider
range of antibody
options are provided for detection of the novel coronavirus.
Some implementations of the present disclosure provide an antibody or a
functional fragment
thereof against novel coronavirus or an N protein thereof. The antibody or the
functional fragment
thereof includes the following complementarity determinant regions:
CDR-VH1: G-X1-T-F-T-X2-Y-X3-M-N, wherein X1 is Y or F, X2 is D or N, and X3 is
A or G;
CDR-VH2: W-X1-N-T-Y-X2-G-E-P-T-Y-A-X3-X4-F, wherein X1 is L or I, X2 is T or
S, X3 is D or
E, and X4 is D or E;
CDR-VH3: A-R-X1-A-X2-X3-R-S-Y, wherein X1 is S or T, X2 is I or L, and X3 is I
or L;
CDR-VL1: K-A-S-X1-D-X2-S-T-A-X3-A, wherein X1 is Q or E, X2 is I, V or L, and
X3 is I, V or L;
CDR-VL2: W-X1-S-T-R-H-X2, wherein X1 is A or G, and X2 is T or S; and
CDR-VL3: Q-Q-H-X1-S-T-P-X2, wherein X1 is Y or W, and X2 is L, V or I.
The antibody or the functional fragment thereof against novel coronavirus
provided in the
present disclosure includes the above complementarity determinant region
structures. The above
complementarity determinant region structures may make the antibody or the
functional fragment
11/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
thereof specifically bind to the N protein of the novel coronavirus, such that
the antibody or the
functional fragment thereof has high affinity for the N protein, and has good
sensitivity and
specificity for detecting the novel coronavirus. According to the present
disclosure, a wider range of
antibody options are provided for detection of the novel coronavirus.
In an optional implementation, in CDR-VH1, X1 is Y; in CDR-VH2, X1 is I; in
CDR-VH3, X1 is S;
in CDR-VL1, X1 is Q; and in CDR-VL2, X1 is A.
Experimental results in this embodiment show that the antibody shows higher
affinity for the N
protein of the novel coronavirus when the above mutation sites in each
complementarity
determinant region are the above amino acid residues.
In an optional implementation, in CDR-VH1, X2 is D.
In an optional implementation, in CDR-VH1, X2 is N.
In an optional implementation, in CDR-VH1 , X3 is A.
In an optional implementation, in CDR-VH1, X3 is G.
In an optional implementation, in CDR-VH2, X2 is T.
In an optional implementation, in CDR-VH2, X2 is S.
In an optional implementation, in CDR-VH2, X3 is D.
In an optional implementation, in CDR-VH2, X3 is E.
In an optional implementation, in CDR-VH2, X4 is D.
In an optional implementation, in CDR-VH2, X4 is E.
In an optional implementation, in CDR-VH3, X2 is I.
In an optional implementation, in CDR-VH3, X2 is L.
In an optional implementation, in CDR-VH3, X3 is I.
In an optional implementation, in CDR-VH3, X3 is L.
In an optional implementation, in CDR-VL1, X2 is I.
In an optional implementation, in CDR-VL1, X2 is V.
In an optional implementation, in CDR-VL1, X2 is L.
12/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
In an optional implementation, in CDR-VL1, X3 is I.
In an optional implementation, in CDR-VL1, X3 is V.
In an optional implementation, in CDR-VL1, X3 is L.
In an optional implementation, in CDR-VL2, X2 is T.
In an optional implementation, in CDR-VL2, X2 is S.
In an optional implementation, in CDR-VL3, X1 is Y.
In an optional implementation, in CDR-VL3, X1 is W.
In an optional implementation, in CDR-VL3, X2 is L.
In an optional implementation, in CDR-VL3, X2 is V.
In an optional implementation, in CDR-VL3, X2 is I.
In an optional implementation, each complementarity determinant region of the
antibody or the
functional fragment thereof is any one selected from the following mutation
combinations 1-42:
CDR-VH1 CDR-VH2 CDR-VH3 CDR-VL1 CDR-VL2 CDR-VL3
X2/X3 X2/X3/X4 X2/X3 X2/X3 X2 X1/X2
Mutation combination 1 D/A S/E/D I/L L/L S W/L
Mutation combination 2 N/A S/E/E I/1 V/V T Y/L
Mutation combination 3 D/G S/D/E L/L I/1 T W/V
Mutation combination 4 N/G S/D/D I/1 I/L S YN
Mutation combination 5 D/A T/E/D I/L IN T W/I
Mutation combination 6 D/G T/E/E L/L I/1 T Y/I
Mutation combination 7 N/A T/D/E L/L V/L T Y/L
Mutation combination 8 N/G T/D/D I/L V/V S W/V
Mutation combination 9 D/A T/E/D L/I V/I S Y/I
Mutation combination 10 N/A S/E/D L/L L/L S W/L
Mutation combination 11 D/G T/E/E I/1 L/V T Y/V
Mutation combination 12 N/G S/E/E I/1 L/I T W/I
Mutation combination 13 N/A T/D/E L/L V/I S Y/V
13/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation combination 14 DIG S/D/E III L/I T W/L
Mutation combination 15 D/A T/D/D L/I I/L S Y/I
Mutation combination 16 N/G S/D/D I/L V/L T Y/L
Mutation combination 17 DIG T/E/E L/L L/V T W/V
Mutation combination 18 N/A S/D/D I/L I/1 T W/I
Mutation combination 19 N/G T/D/D L/L V/V T Y/L
Mutation combination 20 D/A S/D/E I/L L/L S W/I
Mutation combination 21 D/A T/D/E I/1 IN T Y/V
Mutation combination 22 DIG S/E/E I/1 VII S W/L
Mutation combination 23 N/A T/E/D I/L L/I S Y/I
Mutation combination 24 N/G S/E/D L/I I/1 T W/V
Mutation combination 25 N/A T/E/D I/1 V/V T W/L
Mutation combination 26 DIG S/E/E L/I L/V S Y/L
Mutation combination 27 D/A T/D/E L/L IN T W/V
Mutation combination 28 N/G S/D/D L/I V/L T Y/V
Mutation combination 29 DIG S/E/D L/L L/L T W/I
Mutation combination 30 N/A T/E/E I/1 I/L T Y/I
Mutation combination 31 N/G S/D/E I/L L/L T Y/L
Mutation combination 32 D/A T/D/D L/I V/L T W/V
Mutation combination 33 D/A S/D/D I/L I/L S Y/I
Mutation combination 34 N/A T/D/E L/L L/V S W/L
Mutation combination 35 DIG S/D/E L/L V/V S Y/V
Mutation combination 36 N/G T/E/E L/L IN T W/I
Mutation combination 37 D/A S/E/E I/L L/I S Y/L
Mutation combination 38 DIG T/E/D I/1 V/I T W/V
Mutation combination 39 N/A S/E/D I/L I/1 T W/I
Mutation combination 40 N/G T/D/D I/L V/V S Y/L
Mutation combination 41 DIG T/E/D L/I L/L S W/I
14 / 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation combination 42 N/A S/E/D I/L I/1 S Y/V
In an optional implementation, the antibody or the functional fragment thereof
binds to an N
protein of novel coronavirus with an affinity of KID8x10-9m01/L.
In an optional implementation, KID4x10-19m01/L.
In an optional implementation, KID7x10-9m01/L, KID6x10-9mol/L, KID5x10-9m01/L,
KID4x10-9mol/L, KID3x10-9m01/L, KID2x10-9m01/L,
KID1 x1 0-9m01/L, KID9x10-19m01/L,
KID8x 1O-10m01/L, KID7x 1 0-19mol/L, KID6x 1 0-19m01/L,
KID5x 1 0-19m01/L, KID4x1 0-19m01/L,
KID3x10-19m01/L, KID2x10-19m01/L, KID1 x10-19m01/L, KID9x10-11m01/L, KID8x10-
11mol/L or
KID7x 1 0-11mol/L.
In an optional implementation, 7.54x10-11mol/LKID3.28x10-19mol/L.
KD is detected by referring to the method in the embodiments of the present
disclosure.
In an optional implementation, in CDR-VH1, X1 is F; in CDR-VH2, X1 is L; in
CDR-VH3, X1 is T;
in CDR-VL1, X1 is E; and in CDR-VL2, X1 is G.
In an optional implementation, each complementarity determinant region of the
antibody or the
functional fragment thereof is any one selected from the following mutation
combinations 43-48:
CDR-VH1 CDR-VH2 CDR-VH3 CDR-VL1 CDR-VL2 CDR-VL3
X2/X3 X2/X3/X4 X2/X3 X2/X3 X2 X1/X2
Mutation combination 43 D/A S/E/D I/L L/L S W/L
Mutation combination 44 N/G T/D/E L/I L/I T W/L
Mutation combination 45 N/G T/E/E L/I L/I S Y/V
Mutation combination 46 N/A T/D/E L/I V/V T Y/L
Mutation combination 47 N/A S/D/D I/L I/1 S Y/I
Mutation combination 48 N/G T/D/E L/I L/V T Y/V
In an optional implementation, the antibody includes light chain framework
regions FR1-L,
FR2-L, FR3-L and FR4-L, of which sequences are successively shown in SEQ ID
NO:1-4, and/or
heavy chain framework regions FR1-H, FR2-H, FR3-H and FR4-H, of which
sequences are
successively shown in SEQ ID NO:5-8.
Generally, a variable region (VH) of the heavy chain and a variable region
(VL) of the light chain
may be obtained by connecting the following numbered CDRs and FRs in the
following combination
15/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
arrangement: FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4.
In some implementations, the antibody includes light chain framework regions
FR1-L, FR2-L,
FR3-L and FR4-L respectively successively having at least 80% homology to
sequences SEQ ID
NO:1, 2, 3 and 4, and/or heavy chain framework regions FR1-H, FR2-H, FR3-H and
FR4-H
respectively successively having at least 80% homology to sequences SEQ ID NO:
5, 6, 7 and 8.
In some implementations, the antibody includes light chain framework regions
FR1-L, FR2-L,
FR3-L and FR4-L respectively successively having at least 80% identity with
sequences SEQ ID
NO:1, 2, 3 and 4, and/or heavy chain framework regions FR1-H, FR2-H, FR3-H and
FR4-H
respectively successively having at least 80% identity with sequences SEQ ID
NO: 5, 6, 7 and 8.
It is to be noted that, in other implementations and embodiments, the amino
acid sequence of
each framework region of the antibody or the functional fragment thereof
provided in the present
disclosure may have at least 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%,
90%, 91%,
92%, 93%, 94%, 95%, 96%, 97%, 98% or 99% identity with that of the above
corresponding
framework region (SEQ ID NO:1, 2, 3, 4, 5, 6, 7 or 8).
In an optional implementation, the antibody further includes a constant
region.
In an optional implementation, the constant region is any one selected from a
constant region
of IgG1, IgG2, IgG3, IgG4, IgA, IgM, IgE and IgD.
In an optional implementation, the constant region is derived from the species
of mammals or
poultry animals. In an optional implementation, the mammals include cattle,
horse, dairy cow, pig,
sheep, goat, rat, mouse, dog, cat, rabbit, camel, donkey, deer, mink, or
human. In an optional
implementation, the poultry animals include chicken, duck, goose, turkey, or
gamecock.
In an optional implementation, the constant region is derived from species of
cattle, horse, dairy
cow, pig, sheep, goat, rat, mouse, dog, cat, rabbit, camel, donkey, deer,
mink, chicken, duck, goose,
turkey, gamecock or human.
In an optional implementation, the constant region is derived from the mouse.
In an optional implementation, the sequence of a light chain constant region
of the constant
region is shown in SEQ ID NO:9, and the sequence of a heavy chain constant
region of the constant
region is shown in SEQ ID NO:10.
In an optional implementation, the functional fragment is any one selected
from VHH, F(ab')2,
Fab', Fab, Fv or scFv of the antibody.
16/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
The functional fragment of the above antibody usually has the same binding
specificity as its
source antibody. It would have been readily understood by those skilled in the
art according to
content recorded in the present disclosure, the functional fragment of the
above antibody may be
obtained by means of methods including, for example, but not limited to,
enzymatic digestion
(including but not limited to pepsin or papain) and/or by means of a method of
splitting disulfide
bonds through chemical reduction. On the basis of an intact antibody structure
provided in the
present disclosure, the functional fragment would have been readily obtained
by those skilled in the
art.
The functional fragment of the antibody may also be obtained by means of
recombinant genetic
technologies also known to those skilled in the art or by means of synthesis,
for example, by
automated peptide synthesizers, such as those sold by, but not limited to,
Applied BioSystems, etc.
An implementation of the present disclosure provides a reagent or a kit for
detecting novel
coronavirus or an N protein thereof. The reagent or the kit includes any one
of the above antibodies
or the functional fragments thereof.
In an optional implementation, the antibody or the functional fragment thereof
in the reagent or
kit is labeled with a detectable marker.
As used herein, the "detectable marker" refers to a class of substances having
properties such
as luminescence, color development, radioactivity, etc., that can be directly
observed by naked
eyes or detected or detected by an instrument, and by means of the properties,
qualitative or
quantitative detection of a corresponding target may be achieved.
In an optional implementation, the detectable marker includes, but is not
limited to, a
fluorescent dye, an enzyme that catalyzes color development of substrates, a
radio isotope, a
chemiluminescence reagent and a nanoparticle marker.
In an actual use process, those skilled in the art may choose the appropriate
marker according
to detection conditions or practical needs, regardless of the use of any
marker, it is within the scope
of protection of the present disclosure.
In an optional implementation, the fluorescent dye includes, but is not
limited to, fluorescein
dyes and derivatives thereof (for example, including, but not limited to,
Fluorescein Isothiocyanate
(FITC), hydroxyfluorescein (FAM) and Tetrachloro Fluorescein (TET) or
analogues thereof),
rhodamine dyes and derivatives thereof (for example, including, but not
limited to, red rhodamine
(RBITC), tetramethyl rhodamine (TAMRA) and rhodamine B (TRITC) or analogues
thereof), Cy
series dyes and derivatives thereof (for example, including, but not limited
to, Cy2, Cy3, Cy3B,
17/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Cy3.5, Cy5, Cy5.5 and Cy3 or analogues thereof), Alexa series dyes and
derivatives thereof (for
example, including, but not limited to, AlexaFluor350, 405, 430, 488, 532,
546, 555, 568, 594, 610,
33, 647, 680, 700 and 750 or analogues thereof), and protein dyes and
derivatives thereof (for
example, including, but not limited to, Phycoerythrin (PE), Phycocyanin (PC),
Allophycocyanin
(APC), polymethionin-Chlorophyll Protein (preCP)).
In an optional implementation, the enzyme that catalyzes color development of
substrates
includes, but is not limited to, horseradish peroxidase, alkaline phosphatase,
8-galactosidase,
glucose oxidase, carbonic anhydrase, acetylcholinesterase and glucose 6-
phosphate
deoxygenase.
In an optional implementation, the radio isotope includes, but is not limited
to, 212Bi, 1311, 111in,
90y, 186Re, 211At, 1251, 188Re, 153sm, 213Bi, 32p, 94mTb, 99mTb, 203pb, 67Ga,
68Ga, 43sb, 47sb, 0min, 97RU,
62cu, 64cu, 67cu, 68cu, 86y, 88y, 121sn, 161Tb, 166H0, 105Rb, 177Lu, 172Lu and
18F.
In an optional implementation, the chemiluminescence reagent includes, but is
not limited to,
luminol and derivatives thereof, lucigenin, crustacean fluorescein and
derivatives thereof, bipyridine
ruthenium and derivatives thereof, acridinium ester and derivatives thereof,
dioxycyclohexane and
derivatives thereof, lophine and derivatives thereof, and peroxyoxalate and
derivatives thereof.
In an optional implementation, the nanoparticle marker includes, but is not
limited to,
nanoparticles, colloids, organic nanoparticles, magnetic nanoparticles,
quantum dot nanoparticles
and rare earth complex nanoparticles.
In an optional implementation, the colloids include, but are not limited to,
colloidal metals,
disperse dyes, dye labeled microspheres, and latexes.
In an optional implementation, the colloidal metals include, but are not
limited to, colloidal gold,
colloidal silver and colloidal selenium.
An implementation of the present disclosure provides a nucleic acid molecule,
which encodes
the above antibody or the functional fragment thereof. An implementation of
the present disclosure
provides a vector including a nucleic acid molecule. An implementation of the
present disclosure
provides a recombinant cell including the vector. Some implementations of the
present disclosure
provide use of the antibody or the functional fragment thereof or the reagent
or the kit in detection of
novel coronavirus.
Some implementations of the present disclosure provide use of the antibody or
the functional
fragment thereof or the reagent or kit for detecting novel coronavirus.
18/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Some implementations of the present disclosure provide a method for detecting
novel
coronavirus. The method includes the following operations.
A) Under conditions sufficient for a binding reaction to occur, the antibody
or the functional
fragment thereof is contacted with a sample, so as to perform a binding
reaction.
B) An immune complex produced by the binding reaction is detected.
A method for diagnosing a subject in a novel coronavirus infection or a
disease associated with
the novel coronavirus infection includes the following operations.
A) Under conditions sufficient for a binding reaction to occur, the antibody
or the functional
fragment thereof is contacted with a sample from the subject, so as to perform
a binding reaction.
B) An immune complex produced by the binding reaction is detected.
In some implementations, the disease associated with the novel coronavirus
infection includes
respiratory symptoms, fever, cough, shortness of breath, difficult breathing,
pneumonia, severe
acute respiratory syndrome, and renal failure.
In some implementations, the subject in the novel coronavirus infection
includes an
asymptomatic patient, an infected patient without obvious symptoms, and a
symptomatic infected
patient.
An implementation of the present disclosure provides a method for preparing
the antibody or
the functional fragment thereof. The method includes: culturing the
recombinant cell, and obtaining
the antibody or the functional fragment from a culture product by means of
separation and
purification.
On the basis of the amino acid sequence of the antibody or the functional
fragment thereof
provided in the present disclosure, it would have been readily conceivable to
those skilled in the art
to prepare the antibody or the functional fragment thereof with a genetic
engineering technology or
other technologies (chemical synthesis and hybridoma cells). For example, the
antibody or the
functional fragment thereof is isolated and purified from a culture product of
a recombinant cell
capable of recombining and expressing any one of the above antibodies or the
functional fragments
thereof. It would have been readily achievable for those skilled in the art.
Based on this, the
antibody or the functional fragment thereof of the present disclosure is
within the scope of protection
of the present disclosure regardless of used preparation technologies.
Embodiments
19/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
The characteristics and performance of the present disclosure are further
described below in
detail with reference to embodiments.
Embodiment 1
In this embodiment, restriction endonuclease and Prime Star DNA polymerase
were purchased
from Takara. A MagExtractor-RNA extraction kit was purchased from TOYOBO. A BD
SMARTTm
RACE cDNA Amplification Kit was purchased from Takara. A pMD-18T vector was
purchased from
Takara. A plasmid extraction kit was purchased from Tengen Corporation. Primer
synthesis and
gene sequencing were performed by Invitrogen.
1 Construction of recombinant plasmid
(1) Antibody gene preparation
mRNA was extracted from a hybridoma cell line secreting an antibody against an
N protein of
novel coronavirus; a DNA product was obtained by means of RT-PCR; the product
was inserted into
a pMD-18T vector after rTaq DNA polymerase was used to perform an A tailing
reaction, and was
transformed into DH5a competent cells; and after colonies were grown, heavy
chain and light chain
gene clones were taken, and four clones each were sent to a gene sequencing
company for
sequencing.
(2) Sequence analysis of antibody variable region genes
The gene sequences obtained from the above sequencing were placed in the IMGT
antibody
database (which was derived from http://www.imgt.org) for analysis, and VNTI
11.5 software was
used for analysis, so as to determine that both heavy and light chain primers
were correct for
amplified genes. In the gene fragment amplified by the light chain primer
pair, a VL gene sequence
is 324bp and belonged to a Vkll gene family, there is a 57 bp leader peptide
sequence in front of it;
and in the gene fragment amplified by the heavy chain primer pair, a VH gene
sequence is 357bp
and belonged to a VH1 gene family, there is a 57 bp leader peptide sequence in
front of it.
(3) Construction of recombinant antibody expression plasmid
pcDNATM 3.4 TOPO vector was the constructed recombinant antibody eukaryotic
expression
vector. The polyclonal restriction sites such as HindIII, BamHI and EcoRI have
been introduced into
the expression vector, and is named as pcDNA3.4A expression vector, and then
referred to as 3.4A
expression vector. Based on the above sequencing results of antibody variable
region genes in
pMD-18T, specific primers for VL and VH genes of -the antibody were designed
with HindlIl and
EcoRI restriction sites and protection base at both ends, respectively, and a
0.73 kb light chain gene
20/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
fragment and a 1.42 kb heavy chain gene fragment were amplified by means of
PCR amplification.
The heavy chain and light chain gene fragments were double digested with
HindIII/EcoRl; the
3.4A vector was double digested by the HindIII/EcoRl; the heavy chain gene and
light chain gene
were ligated into the 3.4A expression vector after the fragments and the
vectors were purified and
recycled, so as to obtain the recombinant expression plasmids for the heavy
chain and the light
chain.
2 Stable cell line screening
(1) Transient transfection of CHO cells with recombinant antibody expression
plasmids to
determine the activity of expression plasmid
The plasmid obtained from steps 1-(3) was diluted with ultrapure water to
400ng/ml, CHO cells
were adjusted to 1.43x 107cells/m1 in a centrifuge tube, 100pL plasmid and
700pL cells were mixed
and transferred into an electroporation cup, electroporating, sampling and
counting were performed
on days 3, 5 and 7, and samples were collected for detection on day 7.
A 2019-nCoV N protein antigen was diluted with coating liquid (with a main
component
NaHCO3) to 1pg/ml, with 100pL per well, and stayed overnight at 4 C; on the
next day, it was
washed twice with a washing solution (with a main component being
Na2HPO4+NaCI), and patted
dry; blocking solution (20%BSA+80%PBS) was added, 120pL per well, and
incubated at 37 C for
lh and patted dry; the diluted CHO cell supernatant was added, 100pL/well, and
incubated at 37 C
for 30min (partial supernatant, 1h); it was washed with the washing solution
for 5 times, and
patted dry; goat anti-mouse IgG-HRP (wherein, HRP is labeled by horseradish
peroxidase) was
added, 100pL per well, 37 C, 30min; it was washed with the washing solution
for 5 times, and
patted dry; a color developing solution A (50pL/well, containing citric
acid+sodium
acetate+acetanilide+urea peroxide) was added, and then a color developing
solution B (50pL/well,
containing citric acid+EDTA 2Na+TMB+concentrated HCL) was added, 10min; a stop
solution
(50pL/well, containing EDTA-2Na + concentrated H2504) was added; and an OD
value was read at
450nm (refer to 630 nm) on a microplate reader. Results showed that the
reaction OD was still
greater than 1.0 after 1000-fold dilution of cell supernatant, and the
reaction OD of the wells without
cell supernatant was less than 0.1, indicating that the antibody produced
after plasmid transient
transfer was active against the 2019-nCoV N protein antigen.
(2) Linearization of recombinant antibody expression plasmid
Preparation of the following reagents: 50pL of buffer, 100pg/tube of DNA, 10pL
of a Puv
21 / 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
enzyme, sterile water added up to 500pL, enzyme digestion was performed
overnight in a 37 C
water bath; extraction with equal volumes of phenol/chloroform/isoamyl alcohol
(lower layer)
25:24:1 was performed firstly, followed with chloroform (aqueous phase) in
sequence; and
precipitation was performed with 0.1 times the volume (aqueous phase) of 3M
sodium acetate and
2 times the volume of ethanol on ice, rinsing and precipitation was performed
with 70% ethanol,
organic solvents were removed, after the ethanol was completely evaporated, re-
melting was
performed with proper amount of sterilized water, and finally the
concentration was determined.
(3) Stable transfection of recombinant antibody expression plasmid, and
pressurized screening
of stable cell lines
The plasmid obtained from steps 2-(2) was diluted to 400ng/mlwith ultrapure
water, CHO cells
were adjusted to 1.43x 107cells/m1 in a centrifuge tube, 100pL plasmid and
700pL cells were mixed
and transferred into an electroporation cup, electroporating, counting was
performed on the next
day; and pressurized culture was performed for about 25 days, 25um01/L MSX 96
well.
Cloned wells with cells were observed and labeled under a microscope, and
confluence was
recorded; culture supernatant was taken, and samples are sent for detection;
cell lines with
antibody concentration and high relative concentration were selected to be
transferred to 24 wells,
and transferred to 6 wells in about 3 days; after 3 days of seeding and batch
culture, cell density
was adjusted to 0.5x 106 cells/ml, 2.2m1 was taken for batch culture, and cell
density was adjusted to
0.3x 106 cells/ml, 2m1 was taken for seeding; 7-day 6-well batch culture
supernatant was send for
detection, and the cell lines with small antibody concentration and cell
diameter were selected to be
transferred in TPP for seed preservation and passage.
3 Recombinant antibody production
(1) Cell expansion culture
The cells obtained by means of passaging in steps 2-(3) were resuscitated and
then cultured in
a 125 ml shake flask, with an inoculum volume of 30 ml, and a culture medium
was a 100%
Dynamis culture medium, and was placed in a shaker at 120 r/min, 37 C, and 8%
CO2. Culture was
performed for 72h, inoculation expansion culture was performed with the
inoculation density of
500,000 cells/ml, the volume of expansion culture was calculated according to
production
requirements, and the culture medium was the 100% Dynamis culture medium.
Then, expansion
culture was performed every 72h. When a cell volume met the production
requirements, the
inoculation density was strictly controlled to about 500,000 cells/ml for
production.
(2) Shake flask production and purification
22/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Shake flask parameters: 120r/min, 37 C and 8% CO2. Fed-batch cultivation:
material
replenishment was started when cultured in the shake flask up to 72 h, HyClone
Cell Boost Feed 7a
was fed daily with 3% of an initial culture volume, and Feed 7b was fed daily
with one thousandth of
the initial culture volume until day 12 (material replenishment at day 12).
Glucose was replenished
with 3g/L at day 6. Samples were collected at day 13. Affinity purification
was performed with protein
A affinity chromatography columns. 4 pg of the purified antibody was taken for
reductive SDS-PAGE,
and 4 pg of a foreign control antibody was used as a control,
electrophoretogram was shown in Fig.
1 below and showed two bands after reductive SDS-PAGE, and one Mr was 50 KD
(heavy chain,
SEQ ID NO:14) and the other Mr was 28 KD (light chain, SEQ ID NO:13).
Embodiment 2
Performance detection of antibody
(1) Activity detection of the antibody and mutants thereof in Embodiment 1
The sequence of the antibody (WT) in Embodiment 1 was analyzed, and the heavy
chain
variable region was shown in SEQ ID NO:12. Herein the amino acid sequence of
each
complementarity determinant region in the heavy chain variable region was as
follows.
CDR-VH1: G-F(X1)-T-F-T-D(X2)-Y-A(X3)-M-N;
CDR-VH2: W-L(X1)-N-T-Y-S(X2)-G-E-P-T-Y-A-E(X3)-D(X4)-F;
CDR-VH3: A-R-T(X1)-A-I(X2)-L(X3)-R-S-Y;
The light chain variable region was shown in SEQ ID NO:11. The amino acid
sequence of each
complementarity determinant region in the light chain variable region was as
follows.
CDR1-VL: K-A-S-E(X1)-D-L(X2)-S-T-A-L(X3)-A;
CDR-VL2: W-G(X1)-S-T-R-H-S(X2);
CDR-VL3: Q-Q-H-W(X1)-S-T-P-L(X2).
On the basis of the antibody (WT) against novel coronavirus in Embodiment 1,
mutations were
made in the complementarity determinant regions for sites related to the
activity of the antibody,
herein X1, X2, X3, and X4 were mutation sites, as shown in Table 1 below.
Table 1 Mutation site related to the activity of the antibody
CDR-VH 1 CDR- CDR- CDR- CDR-
23/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
WT F L T E G
Mutation 1 Y 1 S Q A
Mutation 2 Y L T E A
Mutation 3 F 1 S E A
Mutation 4 Y 1 S Q G
Mutation 5 Y H S Q A
Mutation 6 Y L T F G
Detection on the binding activity of the antibody in Table 1:
A 2019-nCoV N protein antigen was diluted to 1pg/m1 with the coating liquid
(with a main
component NaHCO3) for microporous plate coating, with 100p1 per well,
overnight at 4 C; on the
next day, it was washed twice with a washing solution, and patted dry;
blocking solution (20%
BSA+80%PBS) was added, 120pL per well, and incubated at 37 C for 1h, and
patted dry; diluted
monoclonal antibody in Table 1 was added, 100pL/well, and incubated at 37 C
for 30min-60min; it
was washed with a washing solution for 5 times, and patted dry; goat anti-
mouse IgG-HRP was
added , 100pL/well, 37 C, and 30min; it was washed with a washing solution
(PBS) for 5 times, and
patted dry; a color developing solution A (50p1/well, containing 2.1g/L of
citric acid, 12.25g/L of citric
acid, 0.07g/L of acetanilide, and 0.5g/L of urea peroxide) was added, and then
a color developing
solution B (50p1/well, containing 1.05g/L of citric acid, 0.186g/L of EDTA
2Na, 0.45g/L of TMB, and
0.2mI/L of concentrated HCI), was added for 10min; a stop solution (50p1/well,
containing 0.75g of
EDTA-2Na and 10.2mI/L of concentrated H2504) was added; and an OD value was
read at 450nm
(referring to 630nm) on a microplate reader. Results were shown in Table 2
below.
Table 2 Activity data of WT antibody and mutants thereof
Antibody concentration (ng/ml) 5000 1000 500 250 125 0.00
WT 0.712 0.731 0.351 0.152 0.52 0.090
Mutation 1 0.952 0.825 0.625 0.364 0.204 0.025
Mutation 2 0.912 0.831 0.631 0.402 0.214 0.028
Mutation 3 0.825 0.841 0.594 0.387 0.198 0.022
Mutation 4 0.847 0.918 0.542 0.362 0.148 0.023
Mutation 5 0.168 0.021 - - - -
24 / 38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation 6 0.146 0.02 - - - -
The results in Table 2 showed that WT, the antibodies of mutation 1 to
mutation 4 have higher
activity against the N protein antigen, and mutation 5 and mutation 6 have
poor activity; and
mutation 1 has the highest activity.
(2) Affinity detection of the antibody and mutants thereof
(a) On the basis of mutation 1, other sites were mutated, and the sequence of
each mutation
was shown in Table 3.
Table 3 Mutation site related to the affinity of an antibody
CDR- CDR-VH2 CDR-VH3 CDR- CDR- CDR-
Mutation 1 D/A S/E/D I/L L/L S W/L
Mutation 1-1 N/A S/E/E I/1 V/V T Y/L
Mutation 1-2 D/G S/D/E L/L I/1 T W/V
Mutation 1-3 N/G S/D/D I/1 I/L S YN
Mutation 1-4 D/A T/E/D I/L IN T W/I
Mutation 1-5 D/G T/E/E L/L I/1 T Y/I
Mutation 1-6 N/A T/D/E L/L V/L T Y/L
Mutation 1-7 N/G T/D/D I/L V/V S W/V
Mutation 1-8 D/A T/E/D L/I V/I S Y/I
Mutation 1-9 N/A S/E/D L/L L/L S W/L
Mutation 1-10 D/G T/E/E I/1 LN T YN
Mutation 1-11 N/G S/E/E I/1 L/I T W/I
Mutation 1-12 N/A T/D/E L/L V/I S YN
Mutation 1-13 D/G S/D/E I/1 L/I T W/L
Mutation 1-14 D/A T/D/D L/I I/L S Y/I
Mutation 1-15 N/G S/D/D I/L V/L T Y/L
Mutation 1-16 D/G T/E/E L/L LN T W/V
Mutation 1-17 N/A S/D/D I/L I/1 T W/I
Mutation 1-18 N/G T/D/D L/L V/V T Y/L
25/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation 1-19 D/A S/D/E I/L L/L S W/I
Mutation 1-20 D/A T/D/E I/1 IN T YN
Mutation 1-21 DIG S/E/E I/1 V/I S W/L
Mutation 1-22 N/A T/E/D I/L L/I S Y/I
Mutation 1-23 N/G S/E/D L/I I/1 T W/V
Mutation 1-24 N/A T/E/D I/1 V/V T W/L
Mutation 1-25 DIG S/E/E L/I L/V S Y/L
Mutation 1-26 D/A T/D/E L/L IN T W/V
Mutation 1-27 N/G S/D/D L/I V/L T YN
Mutation 1-28 DIG S/E/D L/L L/L T W/I
Mutation 1-29 N/A T/E/E I/1 I/L T Y/I
Mutation 1-30 N/G S/D/E I/L L/L T Y/L
Mutation 1-31 D/A T/D/D L/I V/L T W/V
Mutation 1-32 D/A S/D/D I/L I/L S Y/I
Mutation 1-33 N/A T/D/E L/L L/V S W/L
Mutation 1-34 DIG S/D/E L/L V/V S YN
Mutation 1-35 N/G T/E/E L/L IN T W/I
Mutation 1-36 D/A S/E/E I/L L/I S Y/L
Mutation 1-37 DIG T/E/D I/1 V/I T W/V
Mutation 1-38 N/A S/E/D I/L I/1 T W/I
Mutation 1-39 N/G T/D/D I/L V/V S Y/L
Mutation 1-40 DIG T/E/D L/I L/L S W/I
Mutation 1-41 N/A S/E/D I/L I/1 S YN
Affinity analysis
An AMC sensor was used, the purified antibody was diluted to 10 pg/mL with
phosphate tween
buffer (PBST, with main components being Na2HPO4+NaCI+TW-20), and the 2019-
nCoV N protein
antigen was diluted with the PBST in a gradients: 1.41pg/mL, 0.70pg/mL,
0.35pg/mL, 0.18pg/mL,
0.09pg/mL, and 0.04pg/mL.
26/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Operational process: equilibrium was performed in buffer 1 (PBST) for 60s, the
antibody was
cured in an antibody solution for 300s, incubation was performed in buffer 2
(PBST) for 180s,
binding was performed in an antigen solution for 420s, dissociation was
performed in buffer 2 for
1200s, sensor regeneration was performed with a 10mM pH 1.69 GLY solution and
buffer 3, and
data were outputted. KD represents an equilibrium dissociation constant, that
was, the affinity; kon
represents the binding rate; and kdis represents the dissociation rate.
Results were shown in Table
4 below.
Table 4 Affinity detection data
KD(M) kon(1/Ms) kdis(1/s)
Mutation 1 1.21E-10 3.51E+06 4.24E-04
Mutation 1-1 1.03E-10 4.21E+06 4.35E-04
Mutation 1-2 1.29E-10 2.42E+06 3.12E-04
Mutation 1-3 1.49E-10 3.19E+06 4.76E-04
Mutation 1-4 1.22E-10 2.58E+06 3.14E-04
Mutation 1-5 3.28E-10 2.07E+06 6.78E-04
Mutation 1-6 2.47E-10 3.03E+06 7.48E-04
Mutation 1-7 1.67E-10 3.78E+06 6.32E-04
Mutation 1-8 3.15E-10 2.26E+06 7.12E-04
Mutation 1-9 1.13E-10 4.68E+06 5.31E-04
Mutation 1-10 1.43E-10 4.18E+06 5.98E-04
Mutation 1-11 1.07E-10 4.98E+06 5.35E-04
Mutation 1-12 1.46E-10 4.72E+06 6.89E-04
Mutation 1-13 1.59E-10 4.68E+06 7.43E-04
Mutation 1-14 3.17E-10 2.21E+06 7.01E-04
Mutation 1-15 7.54E-11 5.00E+06 3.77E-04
Mutation 1-16 1.78E-10 2.06E+06 3.67E-04
Mutation 1-17 1.27E-10 4.88E+06 6.21E-04
Mutation 1-18 1.93E-10 3.41E+06 6.59E-04
Mutation 1-19 9.97E-11 3.93E+06 3.92E-04
27/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Mutation 1-20 8.94E-11 5.00E+06 4.47E-04
Mutation 1-21 1.55E-10 4.50E+06 6.96E-04
Mutation 1-22 1.42E-10 2.76E+06 3.91E-04
Mutation 1-23 8.79E-11 4.78E+06 4.20E-04
Mutation 1-24 1.50E-10 3.65E+06 5.48E-04
Mutation 1-25 1.79E-10 3.12E+06 5.59E-04
Mutation 1-26 1.01E-10 4.75E+06 4.79E-04
Mutation 1-27 1.64E-10 2.38E+06 3.91E-04
Mutation 1-28 1.66E-10 2.66E+06 4.42E-04
Mutation 1-29 2.24E-10 3.48E+06 7.80E-04
Mutation 1-30 1.74E-10 4.57E+06 7.97E-04
Mutation 1-31 1.30E-10 4.31E+06 5.59E-04
Mutation 1-32 1.41E-10 4.17E+06 5.90E-04
Mutation 1-33 3.03E-10 2.32E+06 7.04E-04
Mutation 1-34 1.61E-10 2.81E+06 4.53E-04
Mutation 1-35 1.35E-10 2.42E+06 3.27E-04
Mutation 1-36 1.41E-10 2.22E+06 3.12E-04
Mutation 1-37 8.89E-11 4.95E+06 4.40E-04
Mutation 1-38 1.05E-10 3.38E+06 3.55E-04
Mutation 1-39 1.26E-10 4.31E+06 5.42E-04
Mutation 1-40 1.09E-10 4.36E+06 4.75E-04
Mutation 1-41 1.31E-10 3.10E+06 4.06E-04
The data in Table 4 showed that both mutation 1 and series mutants thereof
have higher affinity
for the N protein antigen, indicating that all antibodies obtained by means of
mutation on the basis
of mutation 1 and according to the manner in Table 3 have higher affinity.
(b) On the basis of WT, and other sites were mutated; the affinity of the
mutants were detected
by using the affinity determination method of 2(a). The sequences of mutations
were shown in Table
below, and corresponding affinity data were shown in Table 6.
28/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Table 5 Mutation performed using WT as a framework
CDR-VH1 CDR-VH2 CDR-VH3 CDR-VL1 CDR-VL2 CDR-VL3
X2/X3 X2/X3/X4 X2/X3 X2/X3 X2 X1/X2
WT D/A S/E/D I/L L/L S W/L
\NT1 N/G T/D/E L/I L/I T W/L
\NT2 N/G T/E/E L/I L/I S Y/V
\NT3 N/A T/D/E L/I V/V T Y/L
\NT4 N/A S/D/D I/L I/1 S Y/I
\NT5 N/G T/D/E L/I L/V T Y/V
Table 6 Affinity detection results of the WT antibody and mutants thereof
KD(M) kon(1/Ms) kdis(1/s)
WT 7.25E-09 1.80E+05 1.31E-03
WT 1 2.65E-09 2.28E+05 6.05E-04
WT 2 2.48E-09 3.10E+05 7.69E-04
WT 3 7.08E-09 1.36E+05 9.63E-04
\NT 4 4.62E-09 1.15E+05 5.31E-04
WT 5 2.20E-09 3.56E+05 7.82E-04
The data in Table 6 showed that the WT and the mutants thereof have higher
affinity for the N
protein antigen, indicating that, on the basis of the WT, all antibodies
obtained by means of mutation
on the basis of WT and according to the manner in Table 5 have higher
affinity.
(3) Naked antibody stability assessment
The above antibodies were placed at 4 C (refrigerator), -80 C (refrigerator)
and 37 C
(incubator) for 21 days; samples at day 7, day 14 and day 21 were taken for
state observation, and
the samples at day 21 were detected for activity. The results show that the
antibodies do not show
any obvious protein state change under three assessment conditions for 21
days, and the activity
does not show a decreasing trend with the rising of an assessment temperature,
indicating that the
above antibodies were stable. Table 7 below shows OD results of the enzyme
immunoassay activity
assay for the mutation 1 antibody with 21 days of assessment.
Table 7
29/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Sample concentration (ng/ml) 500 250 0
4 C, samples at day 21 0.708 0.414 0.034
-80 C, samples at day 21 0.752 0.521 0.032
37 C, samples at day 21 0.731 0.551 0.037
The above are only the preferred embodiments of the disclosure and are not
intended to limit
the disclosure. For those skilled in the art, the disclosure may have various
modifications and
variations. Any modifications, equivalent replacements, improvements and the
like made within the
spirit and principle of the disclosure shall fall within the scope of
protection of the disclosure.
Industrial Applicability
The present disclosure provides an antibody against novel coronavirus, and a
kit for detecting
the novel coronavirus. The antibody may specifically bind to an N protein of
the novel coronavirus,
has high affinity for the N protein, and has good sensitivity and specificity
for detecting the novel
coronavirus. In addition, the detection kit provided in the present disclosure
also has the same
technical effect as the antibody, and has a wide application prospect and high
market value.
30/38
PN208315FPSW
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
SEQUENCE LISTING
<110> FAPON BIOTECH INC.
<120> Antibody against Novel Coronavirus, and Kit for Novel Coronavirus
Detection
<130> 55213348-135CA
<140> n/a
<141> 2020-10-29
<150> 202011179251.2
<151> 2020-10-29
<160> 14
<170> PatentIn version 3.5
<210> 1
<211> 23
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 1
Asp Ile Val Met Thr Gln Ser His Lys Phe Met Ser Thr Ser Val Gly
1 5 10 15
Asp Arg Val Ser Ile Thr Cys
<210> 2
<211> 15
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 2
Trp Cys Gln Gln Lys Pro Gly Gln Ser Pro Lys Leu Leu Ile Tyr
1 5 10 15
<210> 3
<211> 32
Date recue/Date received 2023-04-28
<212> PRT CA 03200150 2023-04-28
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 3
Gly Val Pro Asp Arg Phe Thr Gly Ile Arg Ser Gly Thr Asp Tyr Thr
1 5 10 15
Leu Thr Ile Ser Ser Val Gin Ala Glu Asp Leu Ala Leu Tyr Tyr Cys
20 25 30
<210> 4
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 4
Thr Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys Arg
1 5 10
<210> 5
<211> 25
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 5
Gin Ile Gin Leu Val Gin Ser Gly Pro Glu Leu Lys Lys Pro Gly Glu
1 5 10 15
Thr Val Lys Ile Ser Cys Lys Ala Ser
20 25
<210> 6
<211> 14
<212> PRT
<213> Artificial Sequence
<220>
Date recue/Date received 2023-04-28
<223> Synthesized CA 03200150 2023-04-28
<400> 6
Trp Val Lys Gin Ala Pro Gly Lys Gly Leu Lys Trp Met Gly
1 5 10
<210> 7
<211> 32
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 7
Lys Gly Arg Phe Ala Phe Ser Leu Glu Thr Ser Ala Ser Thr Ala Tyr
1 5 10 15
Leu Gin Ile Asn Asn Leu Lys Asn Glu Asp Thr Ala Thr Tyr Phe Cys
20 25 30
<210> 8
<211> 14
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 8
Phe Asp Tyr Trp Gly Gin Gly Thr Thr Leu Thr Val Ser Ser
1 5 10
<210> 9
<211> 106
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 9
Ala Asp Ala Ala Pro Thr Val Ser Ile Phe Pro Pro Ser Ser Glu Gin
1 5 10 15
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Leu Thr Ser Gly Gly Ala Ser Val Val Cys Phe Leu Asn Asn Phe Tyr
20 25 30
Pro Lys Asp Ile Asn Val Lys Trp Lys Ile Asp Gly Ser Glu Arg Gln
35 40 45
Asn Gly Val Leu Asn Ser Trp Thr Asp Gln Asp Ser Lys Asp Ser Thr
50 55 60
Tyr Ser Met Ser Ser Thr Leu Thr Leu Thr Lys Asp Glu Tyr Glu Arg
65 70 75 80
His Asn Ser Tyr Thr Cys Glu Ala Thr His Lys Thr Ser Thr Ser Pro
85 90 95
Ile Val Lys Ser Phe Asn Arg Asn Glu Cys
100 105
<210> 10
<211> 324
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 10
Ala Lys Thr Thr Pro Pro Ser Val Tyr Pro Leu Ala Pro Gly Ser Ala
1 5 10 15
Ala Gln Thr Asn Ser Met Val Thr Leu Gly Cys Leu Val Lys Gly Tyr
20 25 30
Phe Pro Glu Pro Val Thr Val Thr Trp Asn Ser Gly Ser Leu Ser Ser
35 40 45
Gly Val His Thr Phe Pro Ala Val Leu Gln Ser Asp Leu Tyr Thr Leu
50 55 60
Ser Ser Ser Val Thr Val Pro Ser Ser Thr Trp Pro Ser Glu Thr Val
65 70 75 80
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Thr Cys Asn Val Ala His Pro Ala Ser Ser Thr Lys Val Asp Lys Lys
85 90 95
Ile Val Pro Arg Asp Cys Gly Cys Lys Pro Cys Ile Cys Thr Val Pro
100 105 110
Glu Val Ser Ser Val Phe Ile Phe Pro Pro Lys Pro Lys Asp Val Leu
115 120 125
Thr Ile Thr Leu Thr Pro Lys Val Thr Cys Val Val Val Asp Ile Ser
130 135 140
Lys Asp Asp Pro Glu Val Gln Phe Ser Trp Phe Val Asp Asp Val Glu
145 150 155 160
Val His Thr Ala Gln Thr Gln Pro Arg Glu Glu Gln Phe Asn Ser Thr
165 170 175
Phe Arg Ser Val Ser Glu Leu Pro Ile Met His Gln Asp Trp Leu Asn
180 185 190
Gly Lys Glu Phe Lys Cys Arg Val Asn Ser Ala Ala Phe Pro Ala Pro
195 200 205
Ile Glu Lys Thr Ile Ser Lys Thr Lys Gly Arg Pro Lys Ala Pro Gln
210 215 220
Val Tyr Thr Ile Pro Pro Pro Lys Glu Gln Met Ala Lys Asp Lys Val
225 230 235 240
Ser Leu Thr Cys Met Ile Thr Asp Phe Phe Pro Glu Asp Ile Thr Val
245 250 255
Glu Trp Gln Trp Asn Gly Gln Pro Ala Glu Asn Tyr Lys Asn Thr Gln
260 265 270
Pro Ile Met Asp Thr Asp Gly Ser Tyr Phe Val Tyr Ser Lys Leu Asn
275 280 285
Val Gln Lys Ser Asn Trp Glu Ala Gly Asn Thr Phe Thr Cys Ser Val
290 295 300
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Leu His Glu Gly Leu His Asn His His Thr Glu Lys Ser Leu Ser His
305 310 315 320
Ser Pro Gly Lys
<210> 11
<211> 108
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 11
Asp Ile Val Met Thr Gin Ser His Lys Phe Met Ser Thr Ser Val Gly
1 5 10 15
Asp Arg Val Ser Ile Thr Cys Lys Ala Ser Glu Asp Leu Ser Thr Ala
20 25 30
Leu Ala Trp Cys Gin Gin Lys Pro Gly Gin Ser Pro Lys Leu Leu Ile
35 40 45
Tyr Trp Gly Ser Thr Arg His Ser Gly Val Pro Asp Arg Phe Thr Gly
50 55 60
Ile Arg Ser Gly Thr Asp Tyr Thr Leu Thr Ile Ser Ser Val Gin Ala
65 70 75 80
Glu Asp Leu Ala Leu Tyr Tyr Cys Gin Gin His Trp Ser Thr Pro Leu
85 90 95
Thr Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys Arg
100 105
<210> 12
<211> 119
<212> PRT
<213> Artificial Sequence
<220>
Date recue/Date received 2023-04-28
<223> Synthesized CA 03200150 2023-04-28
<400> 12
Gin Ile Gin Leu Val Gin Ser Gly Pro Glu Leu Lys Lys Pro Gly Glu
1 5 10 15
Thr Val Lys Ile Ser Cys Lys Ala Ser Gly Phe Thr Phe Thr Asp Tyr
20 25 30
Ala Met Asn Trp Val Lys Gin Ala Pro Gly Lys Gly Leu Lys Trp Met
35 40 45
Gly Trp Leu Asn Thr Tyr Ser Gly Glu Pro Thr Tyr Ala Glu Asp Phe
50 55 60
Lys Gly Arg Phe Ala Phe Ser Leu Glu Thr Ser Ala Ser Thr Ala Tyr
65 70 75 80
Leu Gin Ile Asn Asn Leu Lys Asn Glu Asp Thr Ala Thr Tyr Phe Cys
85 90 95
Ala Arg Thr Ala Ile Leu Arg Ser Tyr Phe Asp Tyr Trp Gly Gin Gly
100 105 110
Thr Thr Leu Thr Val Ser Ser
115
<210> 13
<211> 214
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthesized
<400> 13
Asp Ile Val Met Thr Gin Ser His Lys Phe Met Ser Thr Ser Val Gly
1 5 10 15
Asp Arg Val Ser Ile Thr Cys Lys Ala Ser Glu Asp Leu Ser Thr Ala
20 25 30
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Leu Ala Trp Cys Gln Gln Lys Pro Gly Gln Ser Pro Lys Leu Leu Ile
35 40 45
Tyr Trp Gly Ser Thr Arg His Ser Gly Val Pro Asp Arg Phe Thr Gly
50 55 60
Ile Arg Ser Gly Thr Asp Tyr Thr Leu Thr Ile Ser Ser Val Gln Ala
65 70 75 80
Glu Asp Leu Ala Leu Tyr Tyr Cys Gln Gln His Trp Ser Thr Pro Leu
85 90 95
Thr Phe Gly Ala Gly Thr Lys Leu Glu Leu Lys Arg Ala Asp Ala Ala
100 105 110
Pro Thr Val Ser Ile Phe Pro Pro Ser Ser Glu Gln Leu Thr Ser Gly
115 120 125
Gly Ala Ser Val Val Cys Phe Leu Asn Asn Phe Tyr Pro Lys Asp Ile
130 135 140
Asn Val Lys Trp Lys Ile Asp Gly Ser Glu Arg Gln Asn Gly Val Leu
145 150 155 160
Asn Ser Trp Thr Asp Gln Asp Ser Lys Asp Ser Thr Tyr Ser Met Ser
165 170 175
Ser Thr Leu Thr Leu Thr Lys Asp Glu Tyr Glu Arg His Asn Ser Tyr
180 185 190
Thr Cys Glu Ala Thr His Lys Thr Ser Thr Ser Pro Ile Val Lys Ser
195 200 205
Phe Asn Arg Asn Glu Cys
210
<210> 14
<211> 443
<212> PRT
<213> Artificial Sequence
<220>
Date recue/Date received 2023-04-28
<223> Synthesized CA 03200150 2023-04-28
<400> 14
Gin Ile Gin Leu Val Gin Ser Gly Pro Glu Leu Lys Lys Pro Gly Glu
1 5 10 15
Thr Val Lys Ile Ser Cys Lys Ala Ser Gly Phe Thr Phe Thr Asp Tyr
20 25 30
Ala Met Asn Trp Val Lys Gin Ala Pro Gly Lys Gly Leu Lys Trp Met
35 40 45
Gly Trp Leu Asn Thr Tyr Ser Gly Glu Pro Thr Tyr Ala Glu Asp Phe
50 55 60
Lys Gly Arg Phe Ala Phe Ser Leu Glu Thr Ser Ala Ser Thr Ala Tyr
65 70 75 80
Leu Gin Ile Asn Asn Leu Lys Asn Glu Asp Thr Ala Thr Tyr Phe Cys
85 90 95
Ala Arg Thr Ala Ile Leu Arg Ser Tyr Phe Asp Tyr Trp Gly Gin Gly
100 105 110
Thr Thr Leu Thr Val Ser Ser Ala Lys Thr Thr Pro Pro Ser Val Tyr
115 120 125
Pro Leu Ala Pro Gly Ser Ala Ala Gin Thr Asn Ser Met Val Thr Leu
130 135 140
Gly Cys Leu Val Lys Gly Tyr Phe Pro Glu Pro Val Thr Val Thr Trp
145 150 155 160
Asn Ser Gly Ser Leu Ser Ser Gly Val His Thr Phe Pro Ala Val Leu
165 170 175
Gin Ser Asp Leu Tyr Thr Leu Ser Ser Ser Val Thr Val Pro Ser Ser
180 185 190
Thr Trp Pro Ser Glu Thr Val Thr Cys Asn Val Ala His Pro Ala Ser
195 200 205
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Ser Thr Lys Val Asp Lys Lys Ile Val Pro Arg Asp Cys Gly Cys Lys
210 215 220
Pro Cys Ile Cys Thr Val Pro Glu Val Ser Ser Val Phe Ile Phe Pro
225 230 235 240
Pro Lys Pro Lys Asp Val Leu Thr Ile Thr Leu Thr Pro Lys Val Thr
245 250 255
Cys Val Val Val Asp Ile Ser Lys Asp Asp Pro Glu Val Gin Phe Ser
260 265 270
Trp Phe Val Asp Asp Val Glu Val His Thr Ala Gin Thr Gin Pro Arg
275 280 285
Glu Glu Gin Phe Asn Ser Thr Phe Arg Ser Val Ser Glu Leu Pro Ile
290 295 300
Met His Gin Asp Trp Leu Asn Gly Lys Glu Phe Lys Cys Arg Val Asn
305 310 315 320
Ser Ala Ala Phe Pro Ala Pro Ile Glu Lys Thr Ile Ser Lys Thr Lys
325 330 335
Gly Arg Pro Lys Ala Pro Gin Val Tyr Thr Ile Pro Pro Pro Lys Glu
340 345 350
Gin Met Ala Lys Asp Lys Val Ser Leu Thr Cys Met Ile Thr Asp Phe
355 360 365
Phe Pro Glu Asp Ile Thr Val Glu Trp Gin Trp Asn Gly Gin Pro Ala
370 375 380
Glu Asn Tyr Lys Asn Thr Gin Pro Ile Met Asp Thr Asp Gly Ser Tyr
385 390 395 400
Phe Val Tyr Ser Lys Leu Asn Val Gin Lys Ser Asn Trp Glu Ala Gly
405 410 415
Date recue/Date received 2023-04-28
CA 03200150 2023-04-28
Asn Thr Phe Thr Cys Ser Val Leu His Glu Gly Leu His Asn His His
420 425 430
Thr Glu Lys Ser Leu Ser His Ser Pro Gly Lys
435 440
Date recue/Date received 2023-04-28