Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 2022/237720
PCT/CN2022/091679
COMBINATION THERAPIES
FIELD OF THE INVENTION
[0001] The present invention relates to novel combinations comprising a
therapeutically
effective amount of a menin-mixed-lineage leukemia 1 (menin-MLL) inhibitor of
Formula (1),
or a pharmaceutically acceptable salt or a solvate thereof; and a
therapeutically effective amount
of a B-cell lymphoma 2 (BCL-2) inhibitor; and optionally, a therapeutically
effective amount
of at least one other antineoplastic agent; as well as to methods for treating
a subject who has
been diagnosed with a hematopoietic disorder.
BACKGROUND OF THE INVENTION
[0002] Of the 10 million cancer deaths recorded by GLOBOCAN in 2020, 7.1% are
attributed
to hematopoietic disorders. Accordingly, new treatment modalities are urgently
needed for
hematopoietic disorders, including acute myeloid leukemia (AML),
myelodysplastic syndrome
(MDS) and acute lymphoblastic leukemia (ALL) as further detailed below.
[0003] AML is a common hematological malignancy whose incidence rises from
3:100,000 in
young adults to greater than 20:100,000 in older adults. For patients <60
years of age, overall
survival (OS) is 40 to 50%, but is only 5% for patients >60 years of age. The
majority of newly
diagnosed patients with AML are over the age of 60. In this patient
population, standard
induction chemotherapy is often not an option due to increased treatment-
related mortality as a
result of age and co-morbidities. Standard of care for AML patients unfit for
combination
chemotherapy is treatment with hypomethylating agents (azacitidine or
decitabine) or low dose
cytarabine. Despite these frontline treatments, median OS is only about 10
months. In all types
of AML, disease relapse is common despite an initial therapeutic response and
is the most
common reason for death. Standard chemotherapy and allogeneic stem cell
transplant (when
used) often fail to eradicate all tumor-propagating cells and select for
chemotherapy-resistant
leukemia-propagating subclones. Patients refractory to salvage therapy are
treated palliatively,
as current treatment options are extremely limited. These patients have a
median survival of 2
months. In addition, patients with newly diagnosed intermediate or higher-risk
MDS and those
who relapse after standard care have a poor prognosis and high risk of
progression to AN/It.
Therefore, there is an urgent need for new treatment modalities for
relapsed/refractory (R/R)
AML and MDS patients, newly diagnosed AML patients ineligible for induction
chemotherapy
based on age and co-morbidities, and newly diagnosed intermediate/high/very
high risk MDS
patients.
1
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0004] ALL is a hematologic malignancy propagated by impaired differentiation,
proliferation,
and accumulation of lymphoid progenitor cells in the bone marrow and/or
extramedullary sites.
ALL represents 12% of all leukemia cases and is the most common childhood
acute leukemia,
with a worldwide incidence projected to be 1 to 4.75 per 100,000 people. ALL
represents about
20% of adult leukemias. Despite high rates of complete remission (CR) (80% to
90%) with
current therapies, the majority of adult patients with ALL relapse. The 5-year
overall survival
rate is approximately 30 to 40% in adults and elderly patients. Therefore,
there is an urgent
need for new treatment modalities for relapsed/refractory ALL particularly in
adult and
especially elderly patients.
SUMMARY OF THE INVENTION
[0005] Embodiments of the present invention relate to novel combinations of a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof; and a BCL-
2 inhibitor; and optionally, at least one other antineoplastic agent.
[0006] Embodiments of the present invention relate to uses of such
combinations for treating a
subject who has been diagnosed with a hematopoietic disorder, including but
not limited to,
blood cancers, using a menin-MLL inhibitor described herein in combination
with a BCL-2
inhibitor; and optionally, at least one other antineoplastic agent.
[0007] Embodiments of the present invention relate to novel methods for
treating a subject who
has been diagnosed with a hematopoietic disorder using such combinations.
Embodiments of
the novel methods comprise administering to the subject a therapeutically
effective amount of
a menin-MLL inhibitor as described herein; and a therapeutically effective
amount of a BCL-2
inhibitor; and optionally, a therapeutically effective amount of at least one
other antineoplastic
agent; wherein the menin-MLL inhibitor is a compound of Formula (I), or a
pharmaceutically
acceptable salt or a solvate thereof.
[0008] Embodiments of the present invention relate to novel methods for
treating a subject who
has been diagnosed with a hematopoietic disorder using such combinations.
Embodiments of
the novel methods comprise administering to the subject a therapeutically
effective amount of
a menin-MLL inhibitor as described herein; and a therapeutically effective
amount of a BCL-2
inhibitor, and a therapeutically effective amount of at least one other
antineoplastic agent;
wherein the menin-MLL inhibitor is a compound of Formula (I), or a
pharmaceutically
acceptable salt or a solvate thereof
[0009] In some embodiments, the present invention is directed to methods for
treating a subject
who has been diagnosed with a hematopoietic disorder, the methods comprising
administering
2
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
to the subject a therapeutically effective amount of a menin-MLL inhibitor of
Formula (I), or a
pharmaceutically acceptable salt or a solvate thereof, and a therapeutically
effective amount of
venetoclax, or a pharmaceutically acceptable salt or solvate thereof; and a
therapeutically
effective amount of azacitidine or a pharmaceutically acceptable salt or
solvate thereof.
100101 In some embodiments, the present invention is directed to methods for
treating a subject
who has been diagnosed with a hematopoietic disorder, the methods comprising
administering
to the subject a therapeutically effective amount of a menin-MLL inhibitor of
Formula (I), or a
pharmaceutically acceptable salt or a solvate thereof; a therapeutically
effective amount of
venetoclax, or a pharmaceutically acceptable salt or solvate thereof; and a
therapeutically
effective amount of azacitidine or a pharmaceutically acceptable salt or
solvate thereof; wherein
the venetoclax, or a pharmaceutically acceptable salt or solvate thereof, is
administered to the
subject prior to, simultaneous with, or after the administration of the menin-
MLL inhibitor; and
wherein the azacitidine, or a pharmaceutically acceptable salt or solvate
thereof, is administered
to the subject prior to, simultaneous with, or after the administration of the
menin-MLL
inhibitor.
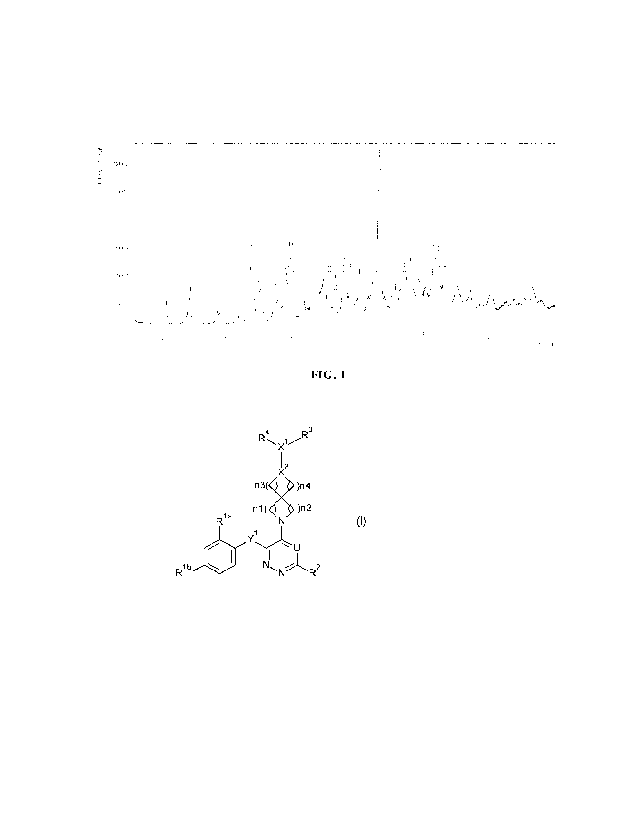
100111 In embodiments, the menin-MLL inhibitor of Formula (1) is:
4
1/R3
X
2
X
n3(X )n4
n1( )n2
Ri a
(I)
Yu
Rib 1011
and the tautomers and the stereoisomeric forms thereof, wherein
0
xa R
xb
Rla represents -C(=0)- xNR aRxb; Het; or NR
Het represents a 5- or 6-membered monocyclic aromatic ring containing one, two
or
three nitrogen atoms and optionally a carbonyl moiety;
wherein said 5- or 6-membered monocyclic aromatic ring is optionally
substituted with
one or two substituents selected from the group consisting of C3_6cycloalkyl
and C1_4alkyl;
IV and IVb are each independently selected from the group consisting of
hydrogen, Ci-
4alkyl and C3_6cycloalkyl;
3
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Rib
represents F or Cl;
Y' represents -CR5aR5b-, -0- or
R2 is selected from the group consisting of hydrogen, halo, CI-alkyl, -0-Ci-
4alkyl, and
-NR7aR7b;
U represents N or CH;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
Xl represents CH, and X2 represents N;
R4 represents isopropyl;
R5a, R5b, R5c, R7a, and km, are each independently selected from the group
consisting of
hydrogen, C1_4a1ky1 and C3_6cycloalkyl;
R3 represents -C3_6alkyl_NR8aR8b, -c _6 al kyl -C (=0)-NR9aR9b, -C _6 al kyl-
OH, or
6alkyl-NR11-C(=0)-0-Ch4alky1-0-C(=0)-Ci_4a1ky1; wherein each of the CI-alkyl
or C16alky1
moieties in the R3 definitions independently of each other may be substituted
with one, two or
three sub stituents each independently selected from the group consisting of
cyano, halo, -OH,
and -0-C14alkyl;
Rsa and leb are each independently selected from the group consisting of
hydrogen; C1-
6alkyl; -C(=0)-Ci_4alky1; -C(=0)-0-C3_4alky1; -C(=0)-NR12aRl2b; and C3_6a1kyl
substituted
with one, two or three substituents each independently selected from the group
consisting of -
OH, cyano, halo, -S (=0)2-C 3_4alkyl, -0-C 3_4a1ky1, -C(=0)-NR10aRlOb, and
_NRioc_c(=o)_c 1_
4alkyl; and
R9a, R9b, Rtoa, Riob, Rioc,
R12a, and R' are each independently selected from the
group consisting of hydrogen and C3_6alky1;
and the pharmaceutically acceptable salts and the solvates thereof.
[0012] In particular embodiments, the menin-NILL inhibitor of Formula (I) is
(R)-N-ethyl-
-fluoro-N-i sopropy1-245-(2-(64(2-methoxyethyl)(methypamino)-2-methylhexan-3 -
y1)-
2, 6-diazaspiro [3 .4] octan-6-y1)- 1, 2,4-tri azin-6 -yl)oxy)b enzami de
besylate salt
(benzenesulfonate salt):
/N \-0/
rNR-re-j besylate salt
4
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
and solvates thereof.
[0013] A skilled person will understand that the 'and solvates thereof' refer
to the besylate salt
of
(R)-N-ethyl-5 -fluoro-N-i sopropy1-2-((5 -(2-(6-((2-m ethoxy ethyl)(m
ethyl)ami no)-
2-m ethyl hexan-3 -y1)-2,6-diazaspiro[3 .4]octan-6-y1)-1 ,2,4-tri azin-6-
yl)oxy)b enzami de.
[0014] In particular embodiments, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethyl-
-fluoro-N-i sopropy1-2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-
3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide besylate salt
or hydrates
thereof.
[0015] In particular embodiments, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethyl-
5 -fluoro-N-i sop ropy1-2-((5 -(2-(6-((2-m ethoxy ethyl)(m ethyl)ami no)-2-m
ethyl hexan-3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate
salt or solvates
thereof.
[0016] In particular embodiments, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethyl-
5 -fluoro-N-i sopropy1-2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-
methylhexan-3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate
salt or hydrates
thereof.
[0017] In particular the present invention is directed to (R)-N-ethy1-5-fluoro-
N-isopropyl-
2-45 -(2-(6-((2-methoxyethyl)(m ethyl)amino)-2-m ethylhexan-3 -y1)-2,6-
diazaspiro[3 .4] octan-
6-y1)4,2,4-triazin-6-yl)oxy)benzamide bis-besylate salt 0.5-2.0 equivalents
hydrate.
[0018] In particular the present invention is directed to (R)-N-ethy1-5-fluoro-
N-isopropy1-
2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-3 -y1)-2,6-
diazaspiro[3 . octan-
6-y1)4,2,4-triazin-6-yl)oxy)benzamide bis-besylate salt 2.0 equivalents
hydrate.
[0019] In particular embodiments, the menin-MLL inhibitor of Formula (I) is a
crystalline form
A of
(R)-N-ethyl -5-fl uoro-N-i sopropy1-2-((5-(2-(6-42-m ethoxyethyl )(m
ethyl ami n o)-
2-m ethyl hexan-3 -y1)-2,6-diazaspiro[3 . 4-]octan-6-y1)-1,2,4-tri azin-6-
yl)oxy)b enzami de bis-
besylate salt hydrate.
[0020] In particular embodiments, the menin-MLL inhibitor of Formula (I) is a
crystalline form
A of
(R)-N-ethyl-5-fluoro-N-isopropy1-2-((5-(2-(6-((2-
methoxyethyl)(methyl)amino)-
2-m ethyl hexan-3 -y1)-2,6-diazaspiro[3 4]octan-6-y1)-1,2,4-tri azin-6-
yl)oxy)b enzami de bis-
besylate salt 0.5-2.0 equivalents hydrate.
[0021] More in particular the present invention is directed to a crystalline
form A of
(R)-N-ethy1-5-fluoro-N-isopropy1-245-(2-(6-((2-methoxyethyl)(methyl)amino)-
2-m ethyl hexan-3 -y1)-2,6-diazaspiro[3 .4]octan-6-y1)-1,2,4-triazin-6-
yl)oxy)benzamide bis-
besylate salt 2.0 equivalents hydrate.
5
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0022] Additional embodiments, features, and advantages of the invention will
be apparent
from the following detailed description and through practice of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] FIG. 1 is an X-ray powder diffraction (XRPD) pattern of Compound A4: a
crystalline
form A of (R)-N-ethy1-5-fluoro-N-isopropy1-2-((5-(2-(6-((2-
methoxyethyl)(methyl)amino)-
2 -m ethyl hexan-3 -y1)-2, 6 -di azaspiro [3 .4] octan-6 -y1)-1,2, 4 -tri azi
n-6 -yl)oxy)b enzami de bis-
besylate salt hydrate.
[0024] FIG. 2 depicts a comparison of tumor volumes as a function of time for
the control
group and for the treatment groups treated with a regimen comprising various
amounts of
Compound A3.
[0025] FIG. 3 depicts a comparison of tumor percent survival as a function of
time (e.g.,
Kaplan-Meier survival curves) for the control group and for the treatment
groups treated with
a regimen comprising various amounts of Compound A3.
[0026] FIG. 4A depicts a comparison of percent survival as a function of time
of mice bearing
established OCI-AML3 tumors following treatment with vehicle, monotherapy with
either
venetoclax, azacitidine or Compound Al, the doublet combination of either
venetoclax and
azacitidine, or Compound Al and venetoclax, or the triplet combination of
Compound Al,
venetoclax and azacitidine.
[0027] FIG. 4B depicts a comparison of percent survival as a function of time
of mice bearing
established MOLM-13 tumors following treatment with vehicle, monotherapy with
either
venetoclax, azacitidine or Compound Al, the doublet combination of either
venetoclax and
azacitidine, or Compound Al and venetoclax, or the triplet combination of
Compound Al,
venetoclax and azacitidine.
[0028] FIG. 5A is a contour plot for maxR which illustrates the effect of
Compound A3 in
combination with venetoclax on proliferation of MOT ,M- I 3 cells in vitro
[0029] FIG. 5B is a contour plot for maxR which illustrates the effect of
Compound A3 in
combination with azacitidine and venetoclax on proliferation of MOLM-13 cells
in vitro.
[0030] FIG. 6A is a contour plot for maxR which illustrates the effect of
Compound A4 in
combination with decitabine on proliferation of MOLM-13 cells in vitro.
[0031] FIG. 6B is a contour plot for maxR which illustrates the effect of
Compound A4 in
combination with decitabine and venetoclax on proliferation of MOLM-13 cells
in vitro.
[0032] FIG. 7A is a contour plot for maxR which illustrates the effect of
Compound A4 in
6
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
combination with decitabine on proliferation of OCI-AML3 cells in vitro.
[0033] FIG. 7B is a contour plot for maxR which illustrates the effect of
Compound A4 in
combination with decitabine and venetoclax on proliferation of OCI-AML3 cells
in vitro.
DESCRIPTION OF THE INVENTION
[0034] The term 'halo' or 'halogen' as used herein represents fluoro, chloro,
bromo and iodo.
[0035] The prefix (where x and y are integers) as used herein refers to the
number of
carbon atoms in a given group. Thus, a Ci_6alkyl group contains from 1 to 6
carbon atoms, and
so on.
[0036] The term `C1_4alkyr as used herein as a group or part of a group
represents a straight or
branched chain saturated hydrocarbon radical having from 1 to 4 carbon atoms,
such as methyl,
ethyl, n-propyl, isopropyl, n-butyl, s-butyl, t-butyl and the like.
[0037] Similar, the term 'C1_6alkyl' as used herein as a group or part of a
group represents a
straight or branched chain saturated hydrocarbon radical having from 1 to 6
carbon atoms, such
as methyl, ethyl, n-propyl, isopropyl, n-butyl, s-butyl, t-butyl, n-pentyl, n-
hexyl and the like
[0038] The term C3_6cycloalkyr as used herein as a group or part of a group
defines a saturated,
cyclic hydrocarbon radical having from 3 to 6 carbon atoms, such as
cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
[0039] It will be clear for the skilled person that S(=0)2 or SO2 represents a
sulfonyl moiety.
[0040] It will be clear for the skilled person that CO or C(=0) represents a
carbonyl moiety.
[0041] It will be clear for the skilled person that a group such as -CRR-
represents
R R
[0042] ¨C¨
. An example of such a group is -CR5aR5b-.
[0043] It will be clear for the skilled person that a group such as -NR-
represents -N-
. An
example of such a group is -N12-
10044] Non-limiting examples of monocyclic 5-or 6-membered aromatic rings
containing one,
two or three nitrogen atoms and optionally a carbonyl moiety', include, but
are not limited to
pyrazolyl, imidazolyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl,
triazinyl or 1,2-dihydro-
2-oxo-4-pyridinyl.
[0045] The skilled person will understand that a 5- or 6-membered monocyclic
aromatic ring
containing one, two or three nitrogen atoms and a carbonyl moiety includes,
but is not limited
to
7
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
0 0
0 H N H
H
,and
[0046] When any variable occurs mole than one time in any constituent, each
definition is
independent.
[0047] When any variable occurs more than one time in any formula (e.g.,
Formula (I)), each
definition is independent.
[0048] In general, whenever the term 'substituted' is used in the present
invention, it is meant,
unless otherwise indicated or clear from the context, to indicate that one or
more hydrogens, in
particular from 1 to 4 hydrogens, more in particular from 1 to 3 hydrogens,
preferably 1 or 2
hydrogens, more preferably 1 hydrogen, on the atom or radical indicated in the
expression using
'substituted' are replaced with a selection from the indicated group, provided
that the normal
valency is not exceeded, and that the substitution results in a chemically
stable compound, i.e.,
a compound that is sufficiently robust to survive isolation to a useful degree
of purity from a
reaction mixture (isolation after a reaction e g , purification by silica gel
chromatography) In a
particular embodiment, when the number of sub stituents is not explicitly
specified, the number
of substituents is one
[0049] Combinations of substituents and/or variables are permissible only if
such combinations
result in chemically stable compounds. 'Stable compound' is in this context
meant to indicate
a compound that is sufficiently robust to survive isolation to a useful degree
of purity from a
reaction mixture (isolation after a reaction e.g., purification by silica gel
chromatography).
[0050] The skilled person will understand that the term 'optionally
substituted' means that the
atom or radical indicated in the expression using 'optionally substituted' may
or may not be
substituted (this means substituted or unsubstituted respectively).
[0051] When two or more substituents are present on a moiety they may, where
possible and
unless otherwise indicated or clear from the context, replace hydrogens on the
same atom or
they may replace hydrogen atoms on different atoms in the moiety.
[0052] Within the context of this invention 'saturated' means 'fully
saturated', if not otherwise
specified.
[0053] Unless otherwise specified or clear from the context, aromatic rings
groups, can be
attached to the remainder of the molecule of Formula (1) through any available
ring carbon atom
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(C-linked) or nitrogen atom (N-linked).
[0054] Unless otherwise specified or clear from the context, aromatic rings
groups, may
optionally be substituted, where possible, on carbon and/or nitrogen atoms
according to the
embodiments.
[0055] The term "comprising" as used herein, encompasses the terms "consisting
of" and
"consisting essentially of." All embodiments described herein using the term
"comprising" are
also applicable for embodiments of the invention wherein the term "comprising"
is limited to
"consisting of." Likewise, all embodiments described herein using the term"
comprising" are
also applicable for embodiments of the invention wherein the term "comprising"
is limited to
"consisting essentially of."
[0056] The term "subject" as used herein, refers to an animal, preferably a
mammal (e.g., cat,
dog, primate or human), more preferably a human, who is or has been the object
of treatment,
observation or experiment
[0057] The term "therapeutically effective amount" as used herein, means that
amount of active
compound or pharmaceutical agent that elicits the biological or medicinal
response in a tissue
system, animal or human that is being sought by a researcher, veterinarian,
medicinal doctor or
other clinician, which includes alleviation or reversal of the symptoms of the
disease or disorder
being treated.
[0058] The term "composition" is intended to encompass a product including the
specified
ingredients in the specified amounts, as well as any product which results,
directly or indirectly,
from combinations of the specified ingredients in the specified amounts.
[0059] The terms "treatment" and "treating," as used herein, are intended to
refer to all
processes wherein there may be a slowing, interrupting, arresting or stopping
of the progression
of a disorder, or amelioration of one or more symptoms thereof, but does not
necessarily
indicate a total elimination of all symptoms
[0060] As used herein, any chemical formula with bonds shown only as solid
lines and not as
solid wedged or hashed wedged bonds, or otherwise indicated as having a
particular
configuration (e.g., R, S) around one or more atoms, contemplates each
possible stereoisomer,
or mixture of two or more stereoisomers.
[0061] Hereinbefore and hereinafter, the term "compound(s) of Formula (I)" is
meant to
include the tautomers thereof and the stereoisomeric forms thereof.
[0062] Hereinbefore and hereinafter, the term "compound(s) of Formula (Z)" is
meant to
include the tautomers thereof and the stereoisomeric forms thereof.
[0063] The terms "stereoisomers", "stereoisomeric forms" or "stereochemically
isomeric forms"
9
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
hereinbefore or hereinafter are used interchangeably.
[0064] The invention includes all stereoisomers of the compounds of the
invention either as a
pure stereoisomer or as a mixture of two or more stereoisomers.
[0065] Enantiomers are stereoisomers that are non-superimposable mirror images
of each other.
A 1:1 mixture of a pair of enantiomers is a racemate or racemic mixture.
[0066] Atropisomers (or atropoisomers) are stereoisomers which have a
particular spatial
configuration, resulting from a restricted rotation about a single bond, due
to large steric
hindrance. All atropisomeric forms of the compounds of Formula (I) are
intended to be included
within the scope of the present invention.
[0067] Diastereomers (or diastereoisomers) are stereoisomers that are not
enantiomers, i.e.,
they are not related as mirror images. If a compound contains a double bond,
the substituents
may be in the E or the Z configuration.
[0068] Substituents on bivalent cyclic saturated or partially saturated
radicals may have either
the cis- or trans-configuration; for example, if a compound contains a
disubstituted cycloalkyl
group, the substituents may be in the cis or trans configuration.
[0069] Therefore, the invention includes enantiomers, atropisomers,
diastereomers, racemates,
E isomers, Z isomers, cis isomers, trans isomers and mixtures thereof,
whenever chemically
possible.
[0070] The meaning of all those terms, i.e., enantiomers, atropisomers,
diastereomers,
racemates, E isomers, Z isomers, cis isomers, trans isomers and mixtures
thereof are known to
the skilled person.
[0071] The absolute configuration is specified according to the Cahn-Ingold-
Prelog system.
The configuration at an asymmetric atom is specified by either R or S.
Resolved stereoisomers
whose absolute configuration is not known can be designated by (+) or (-)
depending on the
direction in which they rotate plane polarized light. For instance, resolved
enantiomers whose
absolute configuration is not known can be designated by (+) or (-) depending
on the direction
in which they rotate plane polarized light.
[0072] When a specific stereoisomer is identified, this means that said
stereoisomer is
substantially free, i.e., associated with less than 50%, preferably less than
20%, more preferably
less than 10%, even more preferably less than 5%, in particular less than 2%
and most
preferably less than 1%, of the other stereoisomers. Thus, when a compound of
Formula (I) is
for instance specified as (R), this means that the compound is substantially
free of the (S) isomer;
when a compound of Formula (I) is for instance specified as E, this means that
the compound
is substantially free of the Z isomer; when a compound of Formula (I) is for
instance specified
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
as cis, this means that the compound is substantially free of the trans
isomer.
[0073] Some of the compounds according to Formula (I) may also exist in their
tautomeric
form. Such forms in so far as they may exist, although not explicitly
indicated in the above
Formula (I) are intended to be included within the scope of the present
invention. It follows that
a single compound may exist in both stereoisomeric and tautomeric form.
[0074] Pharmaceutically acceptable salts include acid addition salts and base
addition salts.
Such salts may be formed by conventional means, for example by reaction of a
free acid or a
free base form with one or more equivalents of an appropriate base or acid,
optionally in a
solvent, or in a medium in which the salt is insoluble, followed by removal of
said solvent, or
said medium, using standard techniques (e.g., in vacno, by freeze-drying or by
filtration) Salts
may also be prepared by exchanging a counter-ion of a compound of the
invention in the form
of a salt with another counter-ion, for example using a suitable ion exchange
resin.
[0075] The pharmaceutically acceptable salts as mentioned hereinabove or
hereinafter are
meant to comprise the therapeutically active non-toxic acid and base salt
forms which the
compounds of Formula (I) and solvates thereof, are able to form
[0076] Appropriate acids comprise, for example, inorganic acids such as
hydrohalic acids, e.g.,
hydrochloric or hydrobromic acid, sulfuric, nitric, phosphoric and the like
acids; or organic
acids such as, for example, acetic, propanoic, hydroxyacetic, lactic, pyruvic,
oxalic (i.e.,
ethanedioic), malonic, succinic (i.e., butanedioic acid), maleic, fumaric,
malic, tartaric, citric,
methanesulfonic, ethanesulfonic, benzenesulfonic, p-toluenesulfonic, cyclamic,
salicylic, p-
aminosalicylic, pamoic and the like acids. Conversely said salt forms can be
converted by
treatment with an appropriate base into the free base form.
[0077] The compounds of Formula (I) and solvates thereof containing an acidic
proton may
also be converted into their non-toxic metal or amine salt forms by treatment
with appropriate
organic and inorganic bases.
[0078] Appropriate base salt forms comprise, for example, the ammonium salts,
the alkali and
earth alkaline metal salts, e.g., the lithium, sodium, potassium, cesium,
magnesium, calcium
salts and the like, salts with organic bases, e.g., primary, secondary and
tertiary aliphatic and
aromatic amines such as methylamine, ethylamine, propylamine, isopropylamine,
the four
butylamine isomers, dimethylamine, diethylamine, diethanolamine,
dipropylamine,
diisopropylamine, di-n-butylamine, pyrrolidine, piperidine, morpholine,
trimethylamine,
triethylamine, tripropylamine, quinuclidine, pyridine, quinoline and
isoquinoline; the
benzathine, N-methyl-D-glucamine, hydrabamine salts, and salts with amino
acids such as, for
example, arginine, lysine and the like. Conversely the salt form can be
converted by treatment
11
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
with acid into the free acid form.
[0079] The term "prodrug" includes any compound that, following oral or
parenteral
administration, in particular oral administration, is metabolised in vivo to a
(more) active form
in an experimentally-detectable amount, and within a predetermined time (e.g.,
within a dosing
interval of between 0.5 and 24 hours, or e.g., within a dosing interval of
between 6 and 24 hours
(i.e., once to four times daily)). For the avoidance of doubt, the term
"parenteral" administration
includes all forms of administration other than oral administration, in
particular intravenous
(IV), intramuscular (IM), and subcutaneous (SC) injection.
[0080] Prodrugs may be prepared by modifying functional groups present on a
compound in
such a way that the modifications are cleaved in vivo when such prodrug is
administered to a
mammalian subject. The modifications typically are achieved by synthesizing
the parent
compound with a prodrug substituent. In general, prodrugs include compounds
wherein a
hydroxyl, amino, sulfhydryl, carboxy or carbonyl group is bonded to any group
that may be
cleaved in vivo to regenerate the free hydroxyl, amino, sulfhydryl, carboxy or
carbonyl group,
respectively.
[0081] Examples of prodrugs include, but are not limited to, esters and
carbamates of hydroxy
functional groups, esters groups of carboxyl functional groups, N-acyl
derivatives and N-
Mannich bases. General information on prodrugs may be found e.g., in
Bundegaard, H. "Design
of Prodrugs" p. 1-92, Elesevier, New York-Oxford (1985).
[0082] The term solvate comprises the solvent addition forms as well as the
salts thereof, which
the compounds of Formula (I) are able to form. Examples of such solvent
addition forms are
e.g., hydrates, alcoholates and the like.
[0083] The compounds of the invention as prepared in the processes described
below may be
synthesized in the form of mixtures of enantiomers, in particular racemic
mixtures of
enantiomers, that can be separated from one another following art-known
resolution procedures.
A manner of separating the enantiomeric forms of the compounds of Formula (I),
and
pharmaceutically acceptable salts, and solvates thereof, involves liquid
chromatography using
a chiral stationary phase. Said pure stereochemically isomeric forms may also
be derived from
the corresponding pure stereochemically isomeric forms of the appropriate
starting materials,
provided that the reaction occurs stereospecifically. Preferably if a specific
stereoisomer is
desired, said compound would be synthesized by stereospecific methods of
preparation. These
methods will advantageously employ enantiomerically pure starting materials.
[0084] The term "enantiomerically pure" as used herein means that the product
contains at least
80% by weight of one enantiomer and 20% by weight or less of the other
enantiomer. Preferably
12
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
the product contains at least 90% by weight of one enantiomer and 10% by
weight or less of
the other enantiomer. In the most preferred embodiment the term
"enantiomerically pure"
means that the composition contains at least 99% by weight of one enantiomer
and 1% or less
of the other enantiomer.
[0085] The present invention also embraces isotopically-labeled compounds
which are
identical to those recited herein, but for the fact that one or more atoms are
replaced by an atom
having an atomic mass or mass number different from the atomic mass or mass
number usually
found in nature (or the most abundant one found in nature).
[0086] All isotopes and isotopic mixtures of any particular atom or element as
specified herein
are contemplated within the scope of the invention, either naturally occurring
or synthetically
produced, either with natural abundance or in an isotopically enriched form.
Exemplary
isotopes that can be incorporated into compounds of the invention include
isotopes of hydrogen,
carbon, nitrogen, oxygen, phosphorus, sulfur, fluorine, chlorine and iodine,
such as 2H, 3H,
13c, 14c, 13N, 150, 170, 180, 32P, 33p, 35s, 18F, 36c1, 1221, 1231, 1251, 131-
,
1 75Br, 76Br, 77Br and 82Br.
Preferably, the isotope is selected from the group of 2H, 3H, 11( 13C and "F.
Preferably, the
isotope is selected from the group of 2H, 3H, 11C and 18F. More preferably,
the isotope is 2H, 3H
or 13C. More preferably, the isotope is 2H or 'C. More preferably, the isotope
is 2H. In
particular, deuterated compounds and 13C-enriched compounds are intended to be
included
within the scope of the present invention. In particular, deuterated compounds
are intended to
be included within the scope of the present invention.
[0087] Certain isotopically-labeled compounds (e.g., those labeled with 3H and
NC) may be
useful for example in substrate tissue distribution assays. Tritiated (3H) and
carbon-14 (mC)
isotopes are useful for their ease of preparation and detectability. Further,
substitution with
heavier isotopes such as deuterium (i.e., 2H) may afford certain therapeutic
advantages resulting
from greater metabolic stability (e.g., increased in vivo half-life or reduced
dosage requirements)
and hence may be preferred in some circumstances. Positron emitting isotopes
such as 150, 13N,
"C and "F are useful for positron emission tomography (PET) studies. PET
imaging in cancer
finds utility in helping locate and identify tumors, stage the disease and
determine suitable
treatment. Human cancer cells overexpress many receptors or proteins that are
potential
disease-specific molecular targets. Radiolabelled tracers that bind with high
affinity and
specificity to such receptors or proteins on tumor cells have great potential
for diagnostic
imaging and targeted radionuclide therapy (Charron, Carlie L. et al.
Tetrahedron Lett. 2016,
57(37), 4119-4127). Additionally, target-specific PET radiotracers may be used
as biomarkers
to examine and evaluate pathology, by for example, measuring target expression
and treatment
13
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
response (Austin R. etal. Cancer Letters (2016), doi: 10.1016/j
.canlet.2016.05.008).
[0088] Solid oral dosage forms such as, tablets or capsules, containing one or
more compounds
described herein may be administered in at least one dosage form at a time, as
appropriate. It
is also possible to administer the compounds in sustained release
formulations.
[0089] Additional oral forms in which the compounds described herein may be
administered
include elixirs, solutions, syrups, and suspensions; each optionally
containing flavoring agents
and coloring agents.
[0090] Alternatively, one or more compounds described herein can be
administered by
inhalation (intratracheal or intranasal) or in the form of a suppository or
pessary, or they may
be applied topically in the form of a lotion, solution, cream, ointment or
dusting powder. For
example, they can be incorporated into a cream comprising, consisting of,
and/or consisting
essentially of an aqueous emulsion of polyethylene glycols or liquid paraffin.
They can also be
incorporated, at a concentration of between about 1 % and about 10 % by weight
of the cream,
into an ointment comprising, consisting of, and/or consisting essentially of a
wax or soft
paraffin base together with any stabilizers and preservatives as may be
required. An alternative
means of administration includes transdermal administration by using a skin or
transdermal
patch.
[0091] The pharmaceutical compositions used in the methods of the present
invention (as well
as the compounds alone) can also be injected parenterally, for example,
intracavernosally,
intravenously, intramuscularly, subcutaneously, intradermally, or
intrathecally. In this case,
the compositions will also include at least one of a suitable carrier, a
suitable excipient, and a
suitable diluent.
[0092] For parenteral administration, the pharmaceutical compositions of the
present invention
are best used in the form of a sterile aqueous solution that may contain other
substances, for
example, enough salts and monosaccharides to make the solution isotonic with
blood.
[0093] For buccal or sublingual administration, the pharmaceutical
compositions of the present
invention may be administered in the form of tablets or lozenges, which can be
formulated in a
conventional manner.
[0094] By way of further example, pharmaceutical compositions containing a
compound of
Formula (I), or a pharmaceutically acceptable salt or a solvate thereof, and a
BCL-2 inhibitor
and optionally, at least one other antineoplastic agent as an active
ingredient can be prepared
by mixing the compound(s) with a pharmaceutically acceptable carrier, a
pharmaceutically
acceptable diluent, and/or a pharmaceutically acceptable excipient according
to conventional
pharmaceutical compounding techniques. The carrier, excipient, and diluent may
take a wide
14
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
variety of forms depending upon the desired route of administration (e.g.,
oral, parenteral, etc.).
Thus, for liquid oral preparations such as, suspensions, syrups, elixirs and
solutions, suitable
carriers, excipients and diluents include water, glycols, oils, alcohols,
flavoring agents,
preservatives, stabilizers, coloring agents and the like; for solid oral
preparations such as,
powders, capsules, and tablets, suitable carriers, excipients and diluents
include starches, sugars,
diluents, granulating agents, lubricants, binders, disintegrating agents and
the like. Solid oral
preparations also may be optionally coated with substances such as, sugars, or
be enterically
coated so as to modulate the major site of absorption and disintegration. For
parenteral
administration, the carrier, excipient and diluent will usually include
sterile water, and other
ingredients may be added to increase solubility and preservation of the
composition. Injectable
suspensions or solutions may also be prepared utilizing aqueous carriers along
with appropriate
additives such as, solubilizers and preservatives.
[0095] According to particular embodiments, methods using a therapeutically
effective amount
of a compound of Formula (I), or a pharmaceutically acceptable salt or a
solvate thereof, and a
BCL-2 inhibitor and optionally, at least one other antineoplastic agent, may
comprise a dose
range from about 0.1 mg to about 3000 mg, or any particular amount or range
therein, in
particular from about 1 mg to about 1000 mg, or any particular amount or range
therein, of
active ingredient in a regimen of about 1 to about (4x) per day for an average
(70 kg) human;
although, it is apparent to one skilled in the art that the therapeutically
effective amount for a
compound of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, and a BCL-
2 inhibitor and optionally, at least one other antineoplastic agent, will vary
as will the diseases,
syndromes, conditions, and disorders being treated.
[0096] An embodiment of the present invention is directed to methods of using
pharmaceutical
compositions for oral administration, comprising a compound of Formula (I), or
a
pharmaceutically acceptable salt or a solvate thereof in an amount of from
about 1 mg to about
500 mg. Advantageously, a compound of Formula (I), or a pharmaceutically
acceptable salt or
a solvate thereof may be administered in a single daily dose, or the total
daily dosage may be
administered in divided doses of two, three and (4x) daily.
[0097] Optimal dosages of a compound of Formula (I), or a pharmaceutically
acceptable salt
or a solvate thereof to be administered may be readily determined and will
vary with the
particular compound used, the mode of administration, the strength of the
preparation, and the
advancement of the hematopoietic disorder. In addition, factors associated
with the particular
subject being treated, including subject gender, age, weight, diet and time of
administration,
will result in the need to adjust the dose to achieve an appropriate
therapeutic level and desired
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
therapeutic effect. The above dosages are thus exemplary of the average case.
There can be,
of course, individual instances wherein higher or lower dosage ranges are
merited, and such are
within the scope of this invention.
[0098] Compounds of Formula (I), or a pharmaceutically acceptable salt or a
solvate thereof
may be administered in any of the foregoing compositions and dosage regimens
or by means
of those compositions and dosage regimens established in the art whenever use
of a compound
of Formula (I), or a pharmaceutically acceptable salt or a solvate thereof is
administered to a
subject in need thereof
[0099] An embodiment of the present invention is directed to methods of using
pharmaceutical
compositions for intravenous or subcutaneous administration, comprising a BCL-
2 inhibitor in
an amount of from about 1 mg to about 500 mg. Advantageously, the BCL-2
inhibitor may be
administered in a single daily dose, or the total daily dosage may be
administered in divided
doses of two, three and (4x) daily.
[0100] Optimal dosages of the BCL-2 inhibitor to be administered may be
readily determined
and will vary with the particular compound used, the mode of administration,
the strength of
the preparation, and the advancement of the disease, syndrome, condition or
disorder. In
addition, factors associated with the particular subject being treated,
including subject gender,
age, weight, diet and time of administration, will result in the need to
adjust the dose to achieve
an appropriate therapeutic level and desired therapeutic effect. The above
dosages are thus
exemplary of the average case. There can be, of course, individual instances
wherein higher or
lower dosage ranges are merited, and such are within the scope of this
invention.
[0101] The BCL-2 inhibitor may be administered in any of the foregoing
compositions and
dosage regimens or by means of those compositions and dosage regimens
established in the art
whenever use of a BCL-2 inhibitor is administered to a subject in need
thereof.
[0102] As used herein, the term "menin-MLL inhibitor" refers to an inhibitor
of the protein-
protein interaction between menin and mixed-lineage leukemia 1 (MLL1) (also
known as
histone-lysine N-methyltransferase 2A (KMT2A) protein in the scientific field
(UniProt
Accession # Q03164)) which inhibits or reduces menin-MLL 1 activity. Menin-MLL
inhibitors
described herein are disclosed in PCT/CN2020/137266 (which published as WO
2021/121327
on June 24, 2021), which is incorporated by reference herein in its entirety,
and which also
discloses corresponding synthetic schemes and analytical characterizations.
[0103] As used herein, the term "BCL-2 inhibitor" refers to an agent that
inhibits or reduces
BCL-2 activity.
[0104] As used herein, the term "antineoplastic agent" refers to any agent
that treats cancer.
16
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0105] As used herein, the term "hypomethylating agent" refers to an agent
that inhibits or
reduces DNA methylation.
[0106] As used herein, the term "kinase inhibitor" refers to an agent that
inhibits or reduce the
activity of at least one kinase (e.g., tyrosine and/or serine kinases such as
fms-like receptor
tyrosine kinase-3 (FLT3), Bruton tyrosine kinase (BTK), an Abelson tyrosine
kinase 1 (ABL),
an Aurora serine/tyrosine kinase).
[0107] As used herein, the term "FLT-3 inhibitor" refers to tyrosine kinase
inhibitors (TKI)
classified into first and next generation inhibitors based on their potency
and specificity for
fms-like receptor tyrosine kinase-3 (FLT3) and their associated downstream
targets.
[0108] As used herein, the term "CD20 inhibitor" refers to any agent that
reduces activity of
CD20.
[0109] As used herein, the term "isocitrate dehydrogenase (1DH) inhibitor"
refers to any agent
that interferes with the conversion of isocitrate to a-ketoglutarate (a-KG) in
the tricarboxylic
acid (TCA) cycle
[0110] As used herein, the term "immunomodulatory antineoplastic agent" refers
to any agent
that enhances antitumor immune cell activity.
[0111] As used herein, the term "programmed cell death protein 1 (PD-1)
inhibitor" refers to
any agent that inhibits or reduces PD-1 activity.
[0112] As used herein, the term "dihydroorotate dehydrogenase (DHODH)
inhibitor" refers to
any agent that inhibits or reduces dihydroorotate dehydrogenase activity.
[0113] As used herein, unless otherwise noted, the term "affect" or "affected"
(when referring
to a disease, disorder, or medical condition that is affected by the
inhibition or alteration of
menin-MLL activity) includes a reduction in the frequency and/or severity of
one or more
symptoms or manifestations of said hematopoietic disorder; and/or includes the
prevention of
the development of one or more symptoms or manifestations of said
hematopoietic disorder or
the development of the hematopoietic disorder.
[0114] As used herein, the term "hematopoietic disorder" refers to any
disorder associated with
the production of the cellular components of blood and blood plasma, including
but not limited
to blood cancers.
[0115] According to an embodiment, the invention provides combinations as
described herein.
[0116] According to an embodiment, the invention provides combinations as
described herein
for use as a medicament.
[0117] According to an embodiment, the invention provides combinations as
described herein
for the manufacture of a medicament.
17
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0118] According to an embodiment, the invention provides combinations as
described herein
for the manufacture of a medicament for the treatment or prevention of any one
of the disease
conditions mentioned herein.
[0119] According to an embodiment, the invention provides combinations as
described herein
for use in the prevention or treatment, in particular treatment, of diseases
as described herein.
[0120] According to an embodiment, the invention provides combinations as
described herein
for use in the prevention or treatment, in particular treatment, of a
hematopoietic disorder,
including but not limited to blood cancers, including but not limited to
lymphomas, myelomas
and leukemias.
[0121] According to an embodiment, the invention provides combinations as
described herein
for use in the prevention or treatment, in particular treatment, of a
hematopoietic disorder.
[0122] According to an embodiment, the hematopoietic disorder is selected
from, but not
limited to, lymphomas, myelomas, myelodysplasia and leukemias.
[0123] According to an embodiment, the hematopoietic disorder is a lymphoma
selected from
Hodgkin's disease lymphomas and Non-Hodgkin's lymphomas.
[0124] According to an embodiment, the lymphoma is a Non-Hodgkin's disease
that is Burkitt's
lymphoma, anaplastic large cell lymphoma, splenic marginal zone lymphoma,
hepatosplenic T-
cell lymphoma or angioimmunoblastic T-cell lymphoma (AILT).
[0125] According to an embodiment, the hematopoietic disorder is a myeloma.
According to
an embodiment, the hematopoietic disorder is a multiple myeloma, Waldenstrom
macroglobulinemia or plasmacytoma.
[0126] According to an embodiment the hematopoietic disorder is a
myelodysplasia including,
but not limited to, myelodysplastic syndrome (MDS).
[0127] According to an embodiment, the hematopoietic disorder is a leukemia.
[0128] According to an embodiment, the hematopoietic disorder is a leukemia
selected from
acute leukemias and chronic leukemias. According to an embodiment, the
leukemia is an acute
leukemia. According to an embodiment, the leukemia is chronic leukemia.
[0129] According to an embodiment, the hematopoietic disorder is a myeloid
leukemia,
myelogeneous leukemia, lymphoblastic leukemia, or lymphocytic leukemia,
According to an
embodiment, the hematopoietic disorder is a leukemia selected from, but not
limited to, acute
lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), small
lymphocytic
leukemia (SLL), acute myeloid leukemia (AML), chronic idiopathic myelofibrosis
(MF),
chronic myelogenous leukemia (CML), T-cell prolymphocytic leukemia (T-PLL), B-
cell
prolymphocytic leukemia (B-PLL), chronic neutrophilic leukemia (CNL), Hairy
cell leukemia
lg
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(HCL), T-cell large granular lymphocyte leukemia (T-LGL) and aggressive NK-
cell leukemia.
According to an embodiment, the AML is acute megakaryoblastic leukemia (AMKL).
[0130] According to an embodiment, the leukemia is MDS, CLL, SLL, ALL or AML.
According to an embodiment, the leukemia is CLL, SLL or AML. According to an
embodiment,
the leukemia is CLL or SLL. In some embodiments, the CLL or SLL is a CD20
expressing
cancer. According to an embodiment, the leukemia is ALL or AML. According to
an
embodiment, the leukemia is ALL. According to an embodiment, the leukemia is
AML.
According to an embodiment, the hematopoietic disorder is Waldenstrom
macroglobulinemia.
[0131] According to an embodiment, the hematopoietic disorder is a MILL-
rearranged leukemia,
MILL-partial tandem duplication (PTD) leukemia, MILL amplified leukemia, MILL-
positive
leukemia, or leukemia exhibiting elevated HOX/MEIS1 gene expression
signatures.
[0132] According to an embodiment, the leukemia is a MILL-rearranged leukemia
&/or a
nucleophosmin 1 (NPM1)-mutated leukemia.According to an embodiment, the
hematopoietic
disorder is a MILL-rearranged leukemia.
[0133] According to an embodiment, the hematopoietic disorder is a
nucleophosmin 1
(NPM1)-mutated leukemia (e.g., NPM1c).
[0134] According to an embodiment, the invention provides methods for
treatment of a
hematopoietic disorder that is myelodysplastic syndrome (MDS), a
myeloproliferative
neoplasm (MPN), acute lymphocytic leukemia (ALL), acute myeloid leukemia
(AML), a small
lymphocytic lymphoma (SLL) or chronic lymphocytic leukemia (CLL), comprising
administering to a subject in need thereof a therapeutically effective amount
of a compound of
Formula (I), or a pharmaceutically acceptable salt or a solvate thereof, and a
BCL-2 inhibitor,
and optionally, at least one other antineoplastic agent.
[0135] According to an embodiment, the hematopoietic disorder is
myelodysplastic syndrome
(MDS) or a myeloproliferative neoplasm (MPN).
[0136] According to an embodiment, the hematopoietic disorder is acute
lymphocytic leukemia
(ALL).
[0137] According to an embodiment, the hematopoietic disorder is acute myeloid
leukemia
(AML).
[0138] According to an embodiment, the hematopoietic disorder is a small
lymphocytic
lymphoma (SLL) or chronic lymphocytic leukemia (CLL).
[0139] According to an embodiment, the hematopoietic disorder is a SLL or CLL
where SLL
or CLL is a CD20-expressing cancer.
[0140] According to an embodiment, the hematopoietic disorder is
myelodysplastic syndrome
19
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(MDS).
[0141] According to an embodiment, the hematopoietic disorder is a my
eloproliferative
neoplasm (1VIPN).
[0142] According to an embodiment, the hematopoietic disorder is a NPM1-
mutated leukemia
with a FLT3 mutation.
[0143] According to an embodiment, the hematopoietic disorder is a FLT3-
dependent leukemia.
[0144] According to an embodiment, the hematopoietic disorder is a MEF2G-
dependent
leukemia.
[0145] According to an embodiment, the hematopoietic disorder harbours one or
more MLL1
(KMT2A) gene rearrangements or alterations (e.g., duplications or
amplification) and/or NPM1
mutations.
[0146] According to an embodiment, the hematopoietic disorder harbours (i) one
or more
MLL1 (KMT2A) gene rearrangements or alterations (e.g., duplications or
amplification) and/or
NPM1 mutations plus (ii) a FLT3 mutation.
[0147] According to an embodiment, the hematopoietic disorder is an MLL-
rearranged
leukemia.
[0148] According to an embodiment, the hematopoietic disorder is acute myeloid
leukemia
(AML).
[0149] According to an embodiment, the hematopoietic disorder is a small
lymphocytic
lymphoma (SLL).
[0150] According to an embodiment, the hematopoietic disorder is a chronic
lymphocytic
leukemia (CLL).
[0151] According to an embodiment, the hematopoietic disorder is an acute
leukemia, chronic
leukemia, myeloid leukemia, myelogeneous leukemia, lymphoblastic leukemia,
lymphocytic
leukemia, acute myelogeneous leukemia (AML), chronic myelogenous leukemia
(CIVIL), acute
lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), T cell
prolymphocytic
leukemias (T-PLL), large granular lymphocytic leukemia, Hairy cell leukemia
(HCL), MLL-
rearranged leukemia, MLL-PTD leukemia, MLL amplified leukemia, MLL-positive
leukemia,
or leukemia exhibiting elevated IIOXIMEISI gene expression signatures.
[0152] According to an embodiment, the hematopoietic disorder is AML, in
particular
nucleophosmin (NPM1)-mutated AML (i.e., NPM1" AML), more in particular
abstract
NPM I -mutated AML.
[0153] According to an embodiment, the hematopoietic disorder is a IVILL-
rearranged leukemia,
in particular MLL-rearranged AML or ALL.
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0154] According to an embodiment, the hematopoietic disorder includes a
MLL gene alteration, in particular the hematopoietic disorder is AML or ALL
with MLL gene alteration(s). In certain embodiments, the MLL gene alteration
is a duplication.
In certain embodiments the MLL gene alteration is an amplification.
[0155] According to an embodiment, the hematopoietic disorder includes a NPM1
gene
mutation and/or MILLI (also known as KiVIT2A) gene mutation.
[0156] According to an embodiment, MLL1 gene mutations include, but are not
limited to,
1VILL1 gene rearrangements, duplications or amplification.
[0157] According to an embodiment, the hematopoietic disorder is a mixed-
lineage leukemia
(MIL), MLL-related leukemia, MLL-associated leukemia, MILL-positive leukemia,
MILL-
induced leukemia, leukemia associated with a MLL, acute leukemia, chronic
leukemia,
myelodysplastic syndrome (MD S), or myeloproliferative neoplasms (MPN).
[0158] All embodiments described herein for methods for treating a
hematopoietic disorder,
are also applicable for use in treating said hematopoietic disorder.
[0159] All embodiments described herein for use in treating a hematopoietic
disorder, are also
applicable for methods for treating said hematopoietic disorder.
[0160] All embodiments described herein for methods for treating a
hematopoietic disorder,
are also applicable for use in a method for treating said hematopoietic
disorder.
[0161] All embodiments described herein for use in a method for treating a
hematopoietic
disorder, are also applicable for methods for treating said hematopoietic
disorder.
[0162] In an embodiment, the present invention relates to a novel combination
comprising:
= a therapeutically effective amount of a menin-MLL inhibitor of Formula
(I), or a
tautomer or a stereoisomeric form thereof, or a pharmaceutically acceptable
salt or a solvate
thereof;
= a therapeutically effective amount of a BCL-2 inhibitor; and
= optionally, a therapeutically effective amount of at least one other
antineoplastic agent.
[0163] According to an embodiment, compounds of Foimula (I) are menin-MLL
inhibitors
having the structure:
21
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
I 2
X
n3( ) )n4
n1( ('>)n2
1 a
(I)
Ribyu
14111
and the tautomers and the stereoisomeric forms thereof, wherein
0
xa xb
R" represents -C(=0)-
NR ax Rxb, Het, or NR R
Het represents a 5- or 6-membered monocyclic aromatic ring containing one, two
or
three nitrogen atoms and optionally a carbonyl moiety;
wherein said 5- or 6-membered monocyclic aromatic ring is optionally
substituted with
one or two substituents selected from the group consisting of C3_6cycloalky1
and C1_4alkyl;
R' and WI' are each independently selected from the group consisting of
hydrogen, Ci-
4alkyl and C3_6cycloalkyl;
Rib
represents F or Cl;
Y' represents -CR5aR5b-, -0- or
R2 is selected from the group consisting of hydrogen, halo, Ch4alkyl, -0-
Ci_4alkyl, and
-NR7aR7b;
U represents N or CH,
nl, n2, n3 and n4 are each independently selected from 1 and 2;
Xl represents CH, and X2 represents N;
R4 represents isopropyl,
Rsa, R5b, R5C, R7a, and WI', are each independently selected from the group
consisting of
hydrogen, Ci¨talkyl and C3_6eycloalkyl,
R3 represents -CI -6 alkyl-NR"R8b, -C1-6 alkyl-C (=0)-NR9aR9b, -C 1- 6 alkyl-
OH, or -C1-
6a1ky1-NR11-C(=0)-0-Ci_4a1kyl-O-C(=0)-Ci_4alkyl; wherein each of the Ci4alkyl
or Ci_6alky1
moieties in the R3 definitions independently of each other may be substituted
with one, two or
three sub stituents each independently selected from the group consisting of
cyano, halo, -OH,
and -0-Ci_4alky1;
22
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
R'a and R" are each independently selected from the group consisting of
hydrogen; Ci-
6alkyl,
-C(=0)-0-Ci_4alkyl, _c(=0)_NRI2aRi2b, and C1_6alkyl substituted
with one, two or three substituents each independently selected from the group
consisting of -
OH, cyano, halo, -s(=0)2-C3_4alkyl, -0-C3_4a1ky1, -C(=0)-NR10aRlOb, and _N-
Rioc_c(=o)_c 1_
4a1ky1; and
R9a, R9b, R10a, R1013, R10c,
R12a, and R12b are each independently selected from the
group consisting of hydrogen and C 1-6 alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0164] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
0
xa xb
Rla represents -C(=0)-NWa
Rxb; Het; or N R R
Het represents a 5- or 6-membered monocyclic aromatic ring containing one, two
or
three nitrogen atoms and optionally a carbonyl moiety;
wherein said 5- or 6-membered monocyclic aromatic ring is optionally
substituted with
one or two substituents selected from the group consisting of C3_6cycloalky1
and C1_4alkyl;
R' and Wb are each independently selected from the group consisting of
hydrogen, Ci-
4alkyl and C3_6cycloalky1;
Rib
represents F or Cl;
Y1 represents -CR5aR5b-, -0- or -NR5e-;
R2 is selected from the group consisting of hydrogen, halo, C3_4alkyl, -0-
C3_4alkyl, and
-NR7aR7b;
U represents N or CH;
n 1, n2, n3 and n4 are each independently selected from 1 and 2;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R5a, R5b, R5e, R7a, and R7b, are each independently selected from the group
consisting of
hydrogen, Ci_4alkyl and C3_6cycloalkyl;
R3 represents -Ch6alkyl-NR'R8b, -Ci_6alkyl-C(=0)-N1eaR9b, -Ci_6alkyl-OH, or -
Ci-
6alkyl-NR11-C(=0)-0 -C 1_4alkyl-O-C(=0)-C 3_4alkyl
wherein each of the Ci_4alkyl or C3_6alkyl moieties in the R3 definitions
independently
of each other may be substituted with one, two or three substituents each
independently selected
23
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
from the group consisting of cyano, halo or -0-C1_4alkyl;
R a and leb are each independently selected from the group consisting of
hydrogen, Ci-
6alkyl; -C(=0)-C1_4alky1; -C(=0)-0-C1_4alkyl; -C(=0)-NR12aRl2b; and C1_6alkyl
substituted
with one, two or three substituents each independently selected from the group
consisting of
cyano, halo, -S(=0)2-C1_4alkyl, -0-C1_4alkyl, and -C(=0)-
NRiOaRlOb; and
R9a, R9b, Rioa, R10b, R12a, and R12b are each independently
selected from the group
consisting of hydrogen and Ci_6alky1;
and the pharmaceutically acceptable salts and the solvates thereof.
[0165] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
0
xa xb
Itla represents -C(=0)-NRxa
Rxb; Het; or N R R
=
Het represents a 5- or 6-membered monocyclic aromatic ring containing one, two
or
three nitrogen atoms and optionally a carbonyl moiety;
wherein said 5- or 6-membered monocyclic aromatic ring is optionally
substituted with
one or two substituents selected from the group consisting of C3_6cycloalky1
and C1_4alkyl;
R' and Wb are each independently selected from the group consisting of
hydrogen, Ci-
-talkyl and C3_6cycloalkyl;
Rib
represents F or Cl;
Y1 represents -CR5aR5b-, -0- or -NR5e-;
R2 is selected from the group consisting of hydrogen, halo, C1_4alkyl, -0-
C1_4alkyl, and
-NR7aR7b;
U represents N or CH;
n 1, n2, n3 and n4 are each independently selected from 1 and 2;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R5a, R5b, R5e, R7a, and R7b, are each independently selected from the group
consisting of
hydrogen, Ci_4alkyl and C3_6cycloalkyl;
R3 represents -C1_6alkyl-NR'le1); wherein the C1_6alkyl moiety in the R3
definition may
be substituted with one, two or three substituents each independently selected
from the group
consisting of cyano, halo, OH, and -0-C1_4alkyl;
R' and R'b are each independently selected from the group consisting of
hydrogen; C1-
24
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
6a1ky1; and C3_6alkyl substituted with one, two or three substituents each
independently
selected from the group consisting of -OH, cyano, halo, -S(=0)2-Ci_4alkyl,
-
C(=0)- 1NR OaR1 013, and -NR"c-C(=0)-Ci_4a1kyl; and
R10a, R10b, R1 Oc are each independently selected from the group consisting of
hydrogen
and C1_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0166] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
0
xa x b
R1a represents -C(=0)-
NR ax Rxb; Het; or N R R
Het represents a 5- or 6-membered monocyclic aromatic ring containing one, two
or
three nitrogen atoms and optionally a carbonyl moiety;
wherein said 5- or 6-membered monocyclic aromatic ring is optionally
substituted with
one or two substituents selected from the group consisting of C3_6cycloalky1
and C3_4alkyl;
R' and It'th are each independently selected from the group consisting of
hydrogen, Ci-
4alkyl and C3_6cycloalkyl;
Rib
represents F or Cl;
Y1 represents -CR5aR5b-, -0- or -NR5c-;
R2 is selected from the group consisting of hydrogen, halo, C1_4alkyl, -0-
C1_4a1ky1, and
-NR7aR7b;
U represents N or CH;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R5a, R5b, R5c, R7a, and R7b, are each independently selected from the group
consisting of
hydrogen, Ci_4alkyl and C3_6cycloalkyl;
R3 represents -Ci -6alkyl-NR8aR8b; wherein the Ci_6alkyl moiety in the R3
definition may
be substituted with one, two or three substituents each independently selected
from the group
consisting of cyano, halo and -0-C1_4alkyl;
R8 and R8b are each independently selected from the group consisting of
hydrogen; Ci_
6alkyl; and C3_6alkyl substituted with one, two or three substituents each
independently selected
from the group consisting of cyano, halo, -S(=0)2-C3_4alkyl, -0-C3_4alkyl, and
-C(=0)-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
NRiOaRl0b, and
R'0a and leth are each independently selected from the group consisting of
hydrogen
and Ci_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
101671 According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rla represents -C(=0)-NR"Wb or Het;
Het represents a 6-membered monocyclic aromatic ring containing two nitrogen
atoms;
wherein said 6-membered monocyclic aromatic ring is substituted with one
C3_6cycloalkyl;
R' and Wb represent C1_4a1kyl;
Rib
represents F;
Y1 represents -0-;
R2 represents hydrogen;
U represents N or CH;
nl, n2, n3 and n4 are each independently selected from 1 and 2,
X' represents CH, and X2 represents N,
R4 represents isopropyl;
R3 represents -C1-6alkyl-NR8aR8b, -C1-6alkyl-C(=0)-NR9aR9b, -C 1- 6alkyl-OH,
or -Ci-
6alkyl-NR11-C(=0)-0-C1_4alkyl-O-C(=0)-C1_4a1 kyl ;
wherein each of the CI-alkyl or Ch6alkyl moieties in the R3 definitions
independently
of each other may be substituted with one, two or three substituents each
independently selected
from the group consisting of -OH and -0-C1_4a1kyl;
R" and R" are each independently selected from the group consisting of
hydrogen; Ci-
6alkyl; -C(=0)-Ci_4a1ky1; -C(=0)-0-C1_4a1ky1; _c (=co_NR12aR12b; and C1-6alkyl
substituted
with one, two or three substituents each independently selected from the group
consisting of -
OH, cyano, halo, -s(=0)2-C1_4alkyl,
-C(=0)-NR10aRlOb, and -NR1 c-C(=0)-C,_
4a1ky1; and
R9a, R9b, R10a, R10b, R10c, Rn, R12a, and R12b are each independently selected
from the
group consisting of hydrogen and C1_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0168] According to an embodiment, compounds of Formula q) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rla represents -C(=0)-NR"Rth or Het,
Het represents a 6-membered monocyclic aromatic ring containing two nitrogen
atoms,
26
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
wherein said 6-membered monocyclic aromatic ring is substituted with one
C3_6cycloalkyl;
R' and 1Vb represent C1_4a1kyl,
Rib
represents F;
Yl represents -0-;
R2 represents hydrogen;
U represents N or CH;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
Xl represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -Ci_6alkyl-NR8aR8b; wherein the C1_6alkyl moiety in the R3
definition may
be substituted with one, two or three substituents each independently selected
from the group
consisting of -OH and -0-Ci_4alky1;
Rsa and leb are each independently selected from the group consisting of
hydrogen; Ci
6alkyl; and Ci_6a1kyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH, cyano, halo, -S(=0)2-Ci4a1kyl,
-C(=0)-
NR1 OaR1 and -NR1cc-C(=0)-Ci_4a1ky1; and
R10a, R10b, and Rthc are each independently selected from the group consisting
of
hydrogen and Ci_6alkyl,
and the pharmaceutically acceptable salts and the solvates thereof.
101691 According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rh a represents -C(=0)- xNR aRxb;
R' and 1Vb represent C1_4a1kyl;
Rib
represents F;
Yl represents -0-;
R2 represents hydrogen;
U represents N or CH;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
X' represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -C1-6 alkyl-NR'R'; wherein the C1_6alkyl moiety in the R3
definition may
be substituted with one, two or three substituents each independently selected
from the group
consisting of -OH and -0-Ci_4alky1,
R8a and Itsb are each independently selected from the group consisting of
hydrogen, Ci
27
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
6a1ky1; and Ci_6alkyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH, cyan , halo, -S(-0)2-Ci_4alkyl, -0-
C1_4alkyl, -C(-0)-
NR10aRlOb, and _NRi0e-C(=0)-Ci4alkyl; and
R10a, R10b, and R' are each independently selected from the group consisting
of
hydrogen and Ci_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0170] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R' represents -C(=0)-NR"Wb or Het;
Het represents pyrimidinyl substituted with one C3-6cycloalkyl;
It and Itxb represent Ci_4a1kyl;
Rib
represents F;
Y1 represents -0-;
R2 represents hydrogen;
U represents N,
nl, n2, n3 and n4 are each independently selected from 1 and 2,
X1 represents CH, and X2 represents N,
R4 represents isopropyl;
R3 represents -Ci_6alkyl-NR8aR8b, wherein the C1_6alkyl moiety in the R3
definition may
be substituted with one -OH;
R8a- and Rsb are each independently selected from the group consisting of
hydrogen; CI-
6alkyl; and C1_6alkyl substituted with one or two substituents each
independently selected from
the group consisting of halo, -0-C1_4a1ky1, and -NR"-C(=0)-C1_4alkyl; and
R10a, R10b, and R1 ' are each independently selected from the group consisting
of
hydrogen and C1_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0171] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R' represents -C(=0)-
NRxaRxb or Het;
Het represents pyrimidinyl substituted with one C3_6cycloalkyl;
It' and It'd' represent C14a1kyl;
Rib represents F;
Y1 represents -0-,
R2 represents hydrogen;
28
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
U represents N;
n2 is 2,
nl, n3 and n4 are I;
X3 represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -C1_6alkyl-NleaR8b; wherein the C1_6alkyl moiety in the R3
definition may
be substituted with one -OH;
R8a- and leb are each independently selected from the group consisting of
hydrogen; Ci-
6alkyl; and Ci_6alkyl substituted with one or two substituents each
independently selected from
the group consisting of halo, -0-0 _aalkyl, and -NR1 c-C(=0)-0-talkyl; and
R10a, R10b, and R1 ' are each independently selected from the group consisting
of
hydrogen and Ci_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0172] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R1a represents -C(=0)-NR"R",
R' and R' represent Ci-talkyl,
Rib
represents F,
Y3 represents -0-;
R2 represents hydrogen;
U represents N;
n2 is 2,
nl, n3 and n4 are 1;
X3 represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -C1_6alkyl-NR8aR8b;
R8a- and R8b are each independently selected from the group consisting of
hydrogen; CI-
6alkyl; and C16alkyl substituted with one or two substituents each
independently selected from
the group consisting of halo, -0-CI_4alkyl, and -NR"c-C(=0)-CI_4alkyl; and
Riod, Riob, and Rme are each independently selected from the group consisting
of
hydrogen and C1-6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0173] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
29
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
R" represents -C(=0)-
NR ax Rxb;
R' and It represent C1_4a1kyl,
Rib represents F;
Y1 represents -0-;
R2 represents hydrogen;
U represents N;
n2 is 2,
nl, n3 and n4 are I;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -CH2-CH2-CH2-NR8aR81);
R8a- and R8b are each independently selected from the group consisting of
hydrogen; CI
-
(alkyl; and C16alkyl substituted with one or two substituents each
independently selected from
the group consisting of halo, -0-C1_4alky1, and -NR1 c-C(=0)-Ci_4alkyl; and
R10", R10', and Itme are each independently selected from the group consisting
of
hydrogen and Ci_6alkyl,
and the pharmaceutically acceptable salts and the solvates thereof.
101741 According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R' represents -C(=0)-
NR ax Rxb;
R.' and It' represent C1_4a1kyl;
Rib represents F;
Y1 represents -0-;
R2 represents hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from I and 2;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -Ci_6alkyl-NR8aR8b;
R8a and R8b are each independently selected from the group consisting of
hydrogen; C
6a1ky1; and Ch6a1kyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH, cyano, halo, -S(=0)2-C1-4alkyl, -0-Ci4alkyl,
and -C(=0)-
NR10aRlOb, and
Rioa and Rmb are each independently selected from the group consisting of
hydrogen
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
and Ci_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0175] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rla represents -C(=0)- amtx Rxb;
R' and It'd' represent Ci_4a1kyl;
Rib
represents F;
Yl represents -0-;
R2 represents hydrogen;
U represents N;
n I, n2, n3 and n4 are each independently selected from 1 and 2;
Xl represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -CH2-CH2-CH24\asaRsb;
R8a and leb are each independently selected from the group consisting of
hydrogen, Ci-
6alkyl, and Ci_6alkyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH, cyano, halo, -S(=0)2-C1-4alkyl, -0-
C1_4alkyl, and -C(=0)-
NR10aRlOb, and
RI" and Rlob are each independently selected from the group consisting of
hydrogen
and Ci_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0176] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
¨ la
tc represents -C(=0)- aNRx Rxb;
R' and Itxb represent hydrogen or Ci_4alkyl;
Rib tc represents F;
Yl represents -0-;
R2 represents hydrogen;
U represents N;
n I, n2, n3 and n4 are each independently selected from 1 and 2;
X' represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -CH2-CH2-CH2-Nlealt";
R8a and Itsb are each independently selected from the group consisting of
hydrogen, Ci-
31
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
6a1ky1, and Ci_6alkyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH, cyano, halo, -S(-0)2-Ci-4alkyl, -0-C14alkyl,
and -C(-0)-
NR10aRlOb, and
RWa and R1' are each independently selected from the group consisting of
hydrogen
and Ci_oalkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0177] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R' represents -C(=0)-NR"Wb;
R' and Wb represent hydrogen or C1_4a1ky1;
Rib
represents F;
Y3 represents -0-;
R2 represents hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from 1 and 2,
X' represents CH, and X2 represents N,
R4 represents isopropyl,
R3 represents -CH2-CH2-CH2-NR8aR"; and
R8a and R" are each independently selected from the group consisting of
hydrogen, Ci-
6alkyl; and C1_6alkyl substituted with one, two or three substituents each
independently selected
from the group consisting of -OH and -0-Ci_4alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0178] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Ria represents -C(=0)- aNRx Rxb;
R' and Wb represent Ci_aalkyl;
lb
I( represents F;
Y3 represents -0-,
R2 represents hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from 1 and 2,
X' represents CH, and X2 represents N,
R4 represents isopropyl,
R3 represents -C1_6alkyl-NR"R", and
32
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
R' and R81' are each independently selected from the group consisting of
Ci_6alkyl; and
C1_6alkyl substituted with one -0-C1_4alkyl,
and the pharmaceutically acceptable salts and the solvates thereof.
[0179] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rla represents -C(=0)- aNw wb;
R' and It'th represent Ci¨talkyl;
Rib
represents F;
Yl represents -0-;
R2 represents hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
Xl represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -CH2-CH2-CH2-NR'R"; and
RS a and le are each independently selected from the group consisting of
C1_6alkyl, and
C1_6alkyl substituted with one -0-Ci_4alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0180] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
Rla represents -C(=0)-
N-RxaRxh; or Het;
Het represents a 6-membered monocyclic aromatic ring containing two nitrogen
atoms;
wherein said 6-membered monocyclic aromatic ring is optionally substituted
with one C3-
6cycloalkyl;
Rxa- and WI' represent Ci_4alkyl;
-=-= lb
lc represents F;
Yl represents -0-;
R2 is hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
X' represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -C1-6alkyl-NleaRsb, -C1-6alkyl-C(=0)-NR9aR9b, -Ci_6alkyl-OH, or -
C1-
6alkyl-Nlel-C(=0)-0-C1_4alkyl-O-C(=0)-C1_4alkyl,
33
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
R'a and R" are each independently selected from the group consisting of
hydrogen; Ci-
6alkyl, -C(=0)-0-Ci4alkyl, _c(=0)_NRI2aRi 2h, and
C1_6a1kyl substituted
with one, two or three sub stituents each independently selected from the
group consisting of
cyano, halo, -S(=0)2-Ci_4alkyl, and -0-C1_4alkyl; and
R9a, R9b, R12a, and R' are each independently selected from the group
consisting of
hydrogen and C1_6alkyl;
and the pharmaceutically acceptable salts and the solvates thereof.
[0181] According to an embodiment, compounds of Formula (I) are as defined
herein, and the
tautomers and the stereoisomeric forms thereof, wherein
R1a represents -C(=0)- aNRx Rxb;
It and Itxb represent Ci_4alkyl;
Rib
represents F;
Y1 represents -0-;
R2 is hydrogen;
U represents N;
nl, n2, n3 and n4 are each independently selected from 1 and 2;
X1 represents CH, and X2 represents N;
R4 represents isopropyl;
R3 represents -Ci_6alkyl-NR8aR", -Ci_6alkyl-C(=0)-NR94R9b, or -Ci_6alkyl-OH;
R a and R" are each independently selected from the group consisting of
hydrogen; Ci-
6alkyl; -C(=0)-C 1_4a1ky1; -C(=0)-0-C1_4alkyl; -C(=0)-NR12aRl2b; and C 1-6
alkyl substituted
with one, two or three sub stituents each independently selected from the
group consisting of
cyano, halo, -S(=0)2-Ci_4alkyl, and -0-Ci_4alky1; and
R9a, R9b, Rua., and R12b are each independently selected from the group
consisting of
hydrogen and Ci_oalkyl;
and the pharmaceutically acceptable salts and the solvates thereof
[0182] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Rib represents F.
[0183] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R2 represents hydrogen.
[0184] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
34
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
mentioned in any of the other embodiments, wherein n1 is 1, n2 is 2, n3 is 1,
and n4 is 1.
[0185] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Yi represents -0-.
[0186] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Yi represents -0-; and U
represents N.
[0187] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Yi represents -0-; U
represents N; Rib
represents F; and R2 represents hydrogen.
[0188] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Het represents
NN
[0189] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Het represents a monocyclic
5- or 6-
membered aromatic ring containing one or two nitrogen atoms,
wherein said monocyclic 5- or 6-membered aromatic ring is substituted with one
C3_6cycloalkyl.
[0190] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Het represents a monocyclic
5- or 6-
membered aromatic ring containing one or two nitrogen atoms, wherein said
monocyclic 5- or
6-membered aromatic ring is substituted with one C3_6cycloalkyl, and Rib
represents F.
[0191] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Het represents a monocyclic
6-membered
aromatic ring containing one or two nitrogen atoms; wherein said monocyclic 6-
membered
aromatic ring is substituted with one C3_6cycloalkyl.
[0192] In an embodiment, the present invention includes compounds of Formula
(I) and the
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein Het represents a monocyclic
6-membered
aromatic ring containing one or two nitrogen atoms; and wherein said
monocyclic 6-membered
aromatic ring is substituted with one C3_6cycloalkyl; and Rib represents F.
[0193] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NleaR8b; wherein
the C1_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-
C1_4alkyl.
[0194] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C1_6alkyl-
NleaR8b; wherein
the C1_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl
[0195] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alky1-
NleaR8b.
[0196] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NR8aR8b; wherein
the C1_6alkyl moiety in the R3 definition may be substituted with one, two or
three sub stituents
each independently selected from the group consisting of cyano, halo and -0-
Ci_4alky1; R8a. and
R8b are each independently selected from the group consisting of hydrogen;
Ci_6alkyl; and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
_c(=0)_NRio _1( 10b
group consisting of -OH, cyano, halo, -S(=0)2-C1_4alkyl,
, and
-NR1 c-C(=0)-C1 _4a1ky1
[0197] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C1_6alkyl-
NR8aR8b; wherein
the Ci_6alkyl moiety in the R3 definition may be substituted with one, two or
three sub stituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl;
R8a and R81' are each independently selected from the group consisting of
hydrogen; Ci_6alkyl;
and C1_6alkyl substituted with one, two or three substituents each
independently selected from
the group consisting of -OH, cyano, halo, -S(=0)2-Ci4alkyl,
_c(=0)_NRioaRiob,
36
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
and -Nit' 'c-C(=0)-C 1_4alkyl
[0198] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NR8aR8b; wherein
the Ci_oalkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-C
i_4alkyl; lea and
Ieb are each independently selected from the group consisting of hydrogen;
Ci_6alkyl; and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of cyano, halo, -S(=0)2-Ci_4alkyl, -0-Ci_4alkyl, and -C(=0)-
ONR1 aRlOb.
[0199] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NR8aR8b; wherein
the C1_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-C
i_4alkyl; R8a and
It' are each independently selected from the group consisting of hydrogen,
C1_6alkyl, and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of cyano, halo, -S(=0)2-C1_4alkyl, -0-C 1_4alkyl,
_c(=c)_NR10aRlOb, and -
NRI c-C(=0)-C1_4alkyl.
[0200] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
INVaR8b; wherein
the C1_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl;
R8 a and R8b are each independently selected from the group consisting of
hydrogen; C1_6alkyl;
and Ci_6alkyl substituted with one, two or three substituents each
independently selected from
the group consisting of cyano, halo, -S(=0)2-Ci_4alkyl, -0-C1_4alkyl, and -
C(=0)- ONR1 aRlOb.
[0201] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NR8aR8b; wherein
the Ci_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl;
R8a. and R81' are each independently selected from the group consisting of
hydrogen; Ci_6alkyl;
and Ci_6alkyl substituted with one, two or three substituents each
independently selected from
the group consisting of cyano, halo, -S(-0)2-C1_4alkyl, -C(-0)-
ONR1 aRl0b, and -
37
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
NR1'e-C(=0)-C t-talkyl
[0202] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR5b; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-
Ci_4alkyl.
[0203] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR"; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-Ci_4alkyl.
[0204] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR"; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-
Ci_4alky1, lea and
R" are each independently selected from the group consisting of hydrogen, C
i_6alkyl, and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of -OH, cyano, halo, -S(=0)2-C1_4alkyl, -C(=0)-
NR 01 aR1013, and
_NR'
LA 0)-C
[0205] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR"; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl;
R8a and R" are each independently selected from the group consisting of
hydrogen; C1_6alkyl;
and C1_6alkyl substituted with one, two or three sub stituents each
independently selected from
the group consisting of -OH, cyano, halo, -S(=0)2-CI_4alkyl,
-C(=0)-NR10aR101),
and -NR1 c-C(=0)-C hztalkyl
[0206] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R1 represents -C2_6alkyl-
NR8aR"; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C14alkyl,
38
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
It,
and WI' are each independently selected from the group consisting of
hydrogen; C1_6a1kyl;
and C1_6alkyl substituted with one, two or three substituents each
independently selected from
the group consisting of cyano, halo, -S(=0)2-Ci_4alkyl, -0-C1_4alkyl, and -
C(=0)- ONR1 aRlOb.
[0207] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR8b; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-C
i_4alkyl; R8a and
R8b are each independently selected from the group consisting of hydrogen;
Ci_6alkyl; and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
=
group consisting of cyano, halo, -S(=0)2-C1_4alkyl, -0-C1_4alkyl, and
_c(0)_NRioaRtob.
[0208] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C1_6alky1-
NRsaR8b;
R8a and leb are each independently selected from the group consisting of
C1_6alkyl, and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of -OH, cyano, halo, -S(=0)2-Ct-4alkyl, -0-Ci4alkyl, and
_c(=0)_NRioaRtob.
[0209] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NR8aR8b; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo, -OH, and
-0-C1_4alkyl;
R8 a and R8b are each independently selected from the group consisting of
hydrogen; C1_6alkyl;
and C1_6alkyl substituted with one, two or three substituents each
independently selected from
the group consisting of cyano, halo, -S(=0)2-C1_4alkyl, -0-C1_4alkyl, -C(=0)-
ONR1 aR1013, and -
NRmc-C(=0)-C _aalkyl
[0210] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C2_6alkyl-
NleaR8b; wherein
the C2_6alkyl moiety in the R3 definition may be substituted with one, two or
three substituents
each independently selected from the group consisting of cyano, halo and -0-
C1_4alkyl; lea and
R8b are each independently selected from the group consisting of hydrogen;
Ci_6alkyl; and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of cyano, halo, -S(-0)2-C1_4alkyl, -0-C1_4alkyl,
_c(=c)_NR10aRlOb, and -
39
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
NR1'e-C(=0)-C t-talkyl
[0211] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -Ci_6alkyl-
NR8aR";
R8 a and R8b are each independently selected from the group consisting of
C1_6alkyl; and Ci-
6alkyl substituted with one, two or three substituents each independently
selected from the
group consisting of -OH, cyano, halo, -S(=0)2-Ci-4alkyl, -C(=0)-NR'a-
=-=_1( 10b
, and
-NR1 c-C(=0)-Calkyl
[0212] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -
Ci_6alkyl_NR8aR8b; Rsa
represents C1_6alkyl; and R" represents C1_6alkyl substituted with one -0-C
i_Ltalkyl.
[0213] In an embodiment, the present invention relates to methods of using the
compounds of
Formula (I) and the pharmaceutically acceptable salts, and the solvates
thereof, or any subgroup
thereof as mentioned in any of the other embodiments, wherein R3 represents -
C1_6alkyl-
NR8aR8b, -Ci_6alkyl-C(=0)-NR9aR9b, -C1_6alkyl-OH, or -Ci_6alkyl-NR11-C(=0)-0-
C1_4alkyl-O-
C(=0)-CiAalkyl, wherein each of the Ci-talkyl or Ci_6alkyl moieties in the R3
definitions
independently of each other may be substituted with one, two or three sub
stituents each
independently selected from the group consisting of cyano, halo or -0-Ci4alkyl
[0214] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C1_6alkyl-
NR8aR8b, -C1-
6alkyl-C(=0)-NR9aR9b, or -C1_6alkyl _NR i-C(=0)-0-C 1_4alkyl -0 -C(=0)-C
; wherein
each of the C1_4alkyl or Ci_6alkyl moieties in the R3 definitions
independently of each other may
be substituted with one, two or three substituents each independently selected
from the group
consisting of cyano, halo, -OH, and -0-C1_4alkyl.
[0215] In an embodiment, the present invention includes compounds of Formula
(1) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -CH2-CH2-CH2-
NR8aR8b.
[0216] In an embodiment, the present invention includes compounds of Formula
(I) and the
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein R3 represents -C1-12-0-12-
C1-12-
NR8aR8b
R a represents methyl, and R" represents -CH2-CH2-0CH3.
[0217] In an embodiment, the present invention includes compounds of Formula
(I) and the
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
pharmaceutically acceptable salts, and the solvates thereof, or any subgroup
thereof as
mentioned in any of the other embodiments, wherein C1_6alkyl in the R3
definition -Ci_6alkyl-
NR8aR8b s =
I limited to ¨CH2-CH2-CH2-.
[0218] In an embodiment, the present invention includes compounds of Formula
(I) or the
pharmaceutically acceptable salts or the solvates thereof, or any subgroup
thereof as mentioned
in any of the other embodiments, wherein the compounds of Formula (I) are
restricted to
compounds of Formula (Ia) or Formula (Ib):
R41 R3 R4 R3
x
X2 X2
n3( ) )n4 n3( ) )n4
n1( )n2 n1( )n2
R1' R1 a N
R5'
U U
N'N halo Rib halo Rib
(Ia) or (Ib),
wherein R' Rib, R3, R4, R5a, R5b,
X2, nl, n2, n3, n4 and halo are as defined for the
compounds of Formula (I) or any subgroup thereof as mentioned in any of the
other
embodiments.
[0219] In an embodiment, the compounds of Formula (1), or the pharmaceutically
acceptable
salts or the solvates thereof, or any subgroup thereof as mentioned in any of
the other
embodiments, are restricted to compounds of Formula (Ia), or the
pharmaceutically acceptable
salts or the solvates thereof. In an embodiment, the compounds of Formula (I),
or the
pharmaceutically acceptable salts or the solvates thereof, or any subgroup
thereof as mentioned
in any of the other embodiments, are restricted to compounds of Formula (Ib),
or the
pharmaceutically acceptable salts or the solvates thereof.
[0220] In an embodiment, the present invention includes compounds of Formula
(I), or the
pharmaceutically acceptable salts or the solvates thereof, or any subgroup
thereof as mentioned
in any of the other embodiments, wherein the compounds of Formula (I) are
restricted to
compounds of Formula (I-y):
cR3
,
(1-Y)
fl(1
N'N
41
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
wherein R3 is as defined for the compounds of Formula (I) or any subgroup
thereof as
mentioned in any of the other embodiments.
[0221] In Formula (I-y) n1 is 1, n2 is 2, n3 is 1, and n4 is 1.
[0222] In a particular embodiment, the compound of Formula (I) is Compound A:
0
0 \
N¨N
Compound A
or a pharmaceutically acceptable salt or solvate thereof.
[0223] In a particular embodiment, the compound of Formula (I) is Compound Al:
2 HCI
x H20
N (x : 2-3)
N¨N
Compound Al.
[0224] In a particular embodiment, the compound of Formula (I) is Compound A2:
2 HCI
2H20
0 \
N¨N
Compound A2.
[0225] In a particular embodiment, the compound of Formula (I) is Compound A3:
42
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
0 co
N- N
oxalate salt
Compound A3.
[0226] In a particular embodiment, the compound of Formula (1) is Compound A4-
a.
N is __________________________________________ / __ 0
14
\ _____________________________________________________ /
N 0
N
I
N -N besylate salt Compound A4-a
or a solvate thereof.
[0227] In a particular embodiment, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethyl-
-fluoro-N-i sopropy1-2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-
3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide besylate salt
or a hydrate
thereof.
[0228] In a particular embodiment, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethyl-
5 -fluoro-N-i sopropy1-2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-
methylhexan-3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate
salt or a solvate
thereof.
[0229] In a particular embodiment, the menin-MLL inhibitor of Formula (I) is
(R)-N-ethy1-
5-fluoro-N-i sopropy1-2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-
methylhexan-3 -y1)-
2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate
salt (Compound
A4-b) or a hydrate thereof.
[0230] In a particular embodiment, the menin-MLL inhibitor of Formula (I) is
Compound A4:
crystalline form A of (R)-N-ethy1-5 -flu
oro-N-i sopropyl-
2-((5 -(2-(6-((2-methoxyethyl)(m ethyl)amino)-2-m ethylhexan-3 -y1)-2,6-
diazaspiro[3 .4] octan-
6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate salt hydrate.
43
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0231] In a particular embodiment, the menin-MLL inhibitor of Formula (I) is a
crystalline
form A of (R)-N-ethyl-5 -fl uoro-N-i sopropy1-2-((5 -(2-(6-((2-m ethoxy
ethyl)(m ethyl)ami no)-
2-m ethyl hexan-3 -y1)-2,6-diazaspiro[3 .4]octan-6-y1)-1,2,4-triazin-6-
yl)oxy)benzamide bis-
besylate salt 0.5-2.0 equivalents hydrate.
[0232] In an embodiment, the present invention relates to a subgroup of
Formula (I) as defined
in the general reaction schemes.
[0233] In an embodiment the compound of Formula (I) is selected from the group
consisting
of any of the exemplified compounds, tautomers and stereoisomeric forms
thereof, and the free
bases, any pharmaceutically acceptable salts, and the solvates thereof.
[0234] In some embodiments, provided is a pharmaceutical composition
comprising a
pharmaceutically acceptable carrier, and as active ingredient a
therapeutically effective amount
of a combination as described in any of the other embodiments.
[0235] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and optionally, at least one other antineoplastic agent.
[0236] According to embodiments, the menin-MLL inhibitor is a compound of
Formula (1), or
a pharmaceutically acceptable salt or a solvate thereof.
[0237] According to particular embodiments, the menin-MLL inhibitor is
Compound A or a
pharmaceutically acceptable salt or solvate thereof.
[0238] According to particular embodiments, the menin-MLL inhibitor is
Compound Al.
[0239] According to particular embodiments, the menin-MLL inhibitor is
Compound A2.
[0240] According to particular embodiments, the menin-MLL inhibitor is
Compound A3.
[0241] According to particular embodiments, the menin-MLL inhibitor is
Compound A4-a or
a solvate thereof.
[0242] According to particular embodiments, the menin-MLL inhibitor is
Compound A4-b or
a hydrate thereof.
[0243] According to particular embodiments, the menin-MLL inhibitor is
Compound A4.
[0244] In particular embodiments, the menin-MLL inhibitor may have improved
metabolic
stability properties.
[0245] In particular embodiments, the menin-MLL inhibitor may have extended in
vivo half-
life (T1/2).
[0246] In particular embodiments, the menin-MLL inhibitor may have improved
oral
bioavailability.
[0247] In particular embodiments, the menin-MLL inhibitor may reduce tumor
growth e.g.,
44
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
tumors harbouring MILL (KMT2A) gene rearrangements/alterations and/or NPM1
mutations.
[0248] In particular embodiments, the menin-MLL inhibitor may have improved PD
properties
in vivo during a prolonged period of time, e.g. inhibition of target gene
expression such as
IVLEIS1 and upregulation of differentiation marker over a period of at least
16 hours.
[0249] In particular embodiments, the menin-MLL inhibitor may have an improved
safety
profile (e.g., reduced hERG inhibition; improved cardiovascular safety).
[0250] In particular embodiments, the menin-MLL inhibitor may be suitable for
Q.D. dosing
(once daily).
[0251] According to embodiments, the BCL-2 inhibitor is selected from
obatoclax, HA14-1,
navitoclax, ABT-737, TW-37, AT 1 0 1, sabutoclax, gamgogic acid and
venetoclax, or
pharmaceutically acceptable salts or solvates thereof.
[0252] According to particular embodiments, the BCL-2 inhibitor is venetoclax,
or a
pharmaceutically acceptable salt or solvate thereof.
[0253] According to embodiments, at least one other antineoplastic agent is a
hypomethylating
agent, a DNA intercalating agent, a pyrimidine analog, a purine analog, a
kinase inhibitor, a
CD20 inhibitor, an 1DH inhibitor, immunomodulatory antineoplastic agent or a
DHODH
inhibitor.
[0254] According to embodiments, at least one other antineoplastic agent is a
hypomethylating
agent, a DNA intercalating agent, a pyrimidine analog, a purine analog, a
kinase inhibitor, a
CD20 inhibitor, an isocitrate dehydrogenase (IDH) inhibitor.
[0255] According to embodiments, the hypomethylating agent includes, but is
not limited to,
azacitidine, decitabine, or pharmaceutically acceptable salts or solvates
thereof.
[0256] According to embodiments, the DNA intercalating agent includes, but is
not limited to,
an anthracycline (e.g., daunorubicin, doxorubicin, idarubicin).
[0257] According to embodiments, the DNA intercalating agent is daunorubicin.
[0258] According to embodiments, the DNA intercalating agent is doxorubicin.
[0259] According to embodiments, the DNA intercalating agent is idarubicin.
[0260] According to embodiments, the pyrimidine analog includes, but is not
limited to,
cytarabine (ARA-C).
[0261] According to embodiments, the purine analog is fiudarabine.
[0262] According to embodiments, the kinase inhibitor is a FLT-3 inhibitor, a
BTK inhibitor,
an ABL inhibitor, an Aurora inhibitor or a multi-kinase inhibitor of two or
more kinase
inhibitors thereof
[0263] According to embodiments, the kinase inhibitor is a multi-kinase
inhibitor of FLT-3
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor, ABL inhibitor, and Aurora inhibitor. According to embodiments, such
multi-kinase
inhibitor includes, but is not limited to KW-2449.
[0264] According to embodiments, the kinase inhibitor is a tyrosine kinase
inhibitor.
[0265] According to embodiments, the tyrosine kinase inhibitor is a FLT-3
inhibitor or a BTK
inhibitor.
[0266] According to embodiments, the FLT3 inhibitor includes, but is not
limited to, sorafenib,
sunitinib, midostaurin (PKC412), lestaurtinib (CEP-701), tandutinib (MLN518),
quizartinib
(AC220), gilteritinib (ASP2215), and KW-2449.
[0267] According to embodiments, the BTK inhibitor includes, but is not
limited to, ibrutinib.
[0268] According to embodiments, the CD20 inhibitor includes, but is not
limited to, an anti-
CD20 antibody (e.g., obinutuzumab (GA101)).
[0269] According to embodiments, the 1DH inhibitor includes, but is not
limited to, ivosidenib
and enasidenib.
[0270] According to embodiments, the isocitrate dehydrogenase-1 inhibitor
includes, but is not
limited to, ivosidenib.
[0271] According to embodiments, the isocitrate dehydrogenase-2 inhibitor
includes, but is not
limited to, enasidenib.
[0272] According to embodiments, the immunomodulatory antineoplastic agent
includes, but
is not limited to, PD-1 inhibitors (e.g., nivolumab, atezolizumab and
pembrolizumab),
thalidomide, lenalidomide, pomalidomide, Bacillus Calmette¨Guerin (BCG) and
levami sole.
[0273] According to embodiments, the PD-1 inhibitor includes, but is not
limited to, nivolumab,
atezolizumab and pembrolizumab.
[0274] According to embodiments, the DHODH inhibitor includes, but is not
limited to, a
compound having the structure of Formula (Z):
R.1
Y R2
XT(
R4
0
(Z), wherein
Xis CH or N;
Y is CH or N;
R1 is selected from the group consisting of: Ci_6alky1; Ci_6alkyl substituted
with OH, or
OCH3; C2_6alkenyl; Ci_6haloa1kyl; Ci_6ha1oalkyl substituted with OH, or OCH3;
C2-
46
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
6haloalkenyl; N(CH3)2; C3_6cyc1oalkyl; C3_6cycloalkyl substituted with
Ci_6alkyl; and
phenyl,
Rb
NK
N ¨Ra
R2 is 0 wherein
Rd is selected from the group consisting of: Ci_6alkyl, C1_6haloalkyl, and
C3_6cycloalkyl;
Rb is Ci_6a1kyl or Ci_6alkyl substituted with a member selected from the group
consisting of:
OH, halo, CN, OCi_6alkyl, OCi_6haloalkyl and 0C3_6cycloalkyl;
R3 is selected from the group consisting of: H, halo, CH3 and OCH3;
R4 is selected from the group consisting of:
= C1_6alkyl; Ci_6alkyl substituted with one or two OCH3; C3-6cycloalkyl; C3-
6cycloalkyl
substituted with CH3, or OCH3; CH2-C3_6cycloalkyl; and
(ReL_
= - Rd
(RG
IRc< N Re\ Rc
[1
N
I I
= Rd, N Rd NRd,N N
and and
Rc Rc
Rc¨<\I R9 ¨N
= NRd N Rd and µN---". Rd
wherein
each RC is independently selected from the group consisting of: H; halo;
C1_6alkyl; C1_6alkyl
substituted with a member selected from the group consisting of: OH, OCH3,
SCH3, and
OCF3; Ci_6haloalkyl; Ci_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; NO2; OH; 0-CH2CH2OH; and OCi_6a1kyl;
Rd is selected from the group consisting of: H; halo; Ci_6alkyl; Ci_6alkyl
substituted with a
member selected from the group consisting of: OH, OCH3, SCH3, and OCF3;
C1_6haloalkyl; C1_6haloa1kyl substituted with a member selected from the group
consisting of: OH, and OCH3; CN; and OCi_6alkyl;
47
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Rg is selected from the group consisting of: H; Ci_6alkyl; Ci_6alkyl
substituted with a
member selected from the group consisting of. OH, OCH3, SCH3, and OCF3, Ci-
6haloalkyl; and Ci_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and
n is 1, or 2;
or a pharmaceutically acceptable salt, isotope, N-oxide, solvate, or
stereoisomer thereof;
or a compound selected from
HO
N-
' - \
r
0
F-
HC
N
N. \
0
.N
02N
F HO
I N
N
ci [rya rIO
_,õ
N
or a pharmaceutically acceptable salt, N-oxide, solvate, or stereoisomer
thereof
[0275] According to embodiments, the DHODH inhibitor includes, but is not
limited to, a
compound having the structure of Formula (Z):
R1
X i R2
N
R3
Fcr
0
(Z), wherein
48
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Xis CH or N;
Y is CH or N;
R1 is selected from the group consisting of: Ci_6alkyl; Ci_6alkyl substituted
with OH, or
OCH3; C2_6alkenyl; Ci_6haloalkyl; Ci_6haloalkyl substituted with OH, or OCH3;
6haloalkenyl; N(CH3)2; C3_6cyc1oalkyl; C3_6cycloalkyl substituted with
C1_6alkyl; and
phenyl;
Rb
N
N ¨1Ra
,k \I
R2 is 0 ; wherein
Ra is selected from the group consisting of: C1_6alkyl, C1_6haloalkyl, and
C3_6cycloalky1;
Rb is C1_6alkyl or C1_6a1kyl substituted with a member selected from the group
consisting of:
OH, halo, CN, OC1_6alkyl, 0C1_6haloalkyl and 0C3_6cycloa1kyl;
R3 is selected from the group consisting of: H, halo, CH3 and OCH3;
R4 is selected from the group consisting of:
= C1_6alkyl; C1_6alky1 substituted with one or two OCH3; C3-6cycloalkyl; C3-
6cycloalkyl
substituted with CH3, or OCH3; CH2-C3_6cycloalkyl; and =
(Rc
= Rd =
(Rc
IRc< N Rcv__
Rc
N I
N
= Rd, Rd, N Rd N Rd, N N N
N
and and
Rc Rc
S
Rc¨<, IRg¨N
= A s ; - N Rd , and
N Rd
wherein
each It' is independently selected from the group consisting of. H, halo,
C1_6alkyl, C1_6alkyl
substituted with a member selected from the group consisting of: OH, OCH3,
SCH3, and
OCF3; C1_6haloalkyl; C1_6ha1oa1ky1 substituted with a member selected from the
group
consisting of: OH, and OCH3; NO2; OH; 0-CH2CH2OH; and 0C1_6a1kyl;
49
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Rd is selected from the group consisting of: H; halo; Ci_6alkyl; C1_6alkyl
substituted with a
member selected from the group consisting of. OH, OCH3, SCH3, and OCF3;
C1_6haloalkyl; C1_6haloalkyl substituted with a member selected from the group
consisting of: OH, and OCH3; CN; and OCi_6alkyl;
Rg is selected from the group consisting of: H; Ci_oalkyl; Ci_6alkyl
substituted with a
member selected from the group consisting of: OH, OCH3, SCH3, and OCF3; Ci-
6haloalkyl; and Ci_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and
n is 1, or 2;
or a pharmaceutically acceptable salt, isotope, N-oxide, solvate, or
stereoisomer thereof.
[0276] In the context of Formula (Z), the following definitions apply:
[0277] The term ''alkenyl" includes unsaturated aliphatic groups analogous in
length and
possible substitution to the alkyls described above, but that contain at least
one double bond.
For example, the term "alkenyl" includes straight-chain alkenyl groups (e.g.,
ethenyl, propenyl,
butenyl, pentenyl, hexenyl, heptenyl, octenyl, nonenyl, decenyl, etc.). The
term alkenyl further
includes alkenyl groups which include oxygen, nitrogen, sulfur or phosphorous
atoms replacing
one or more carbons of the hydrocarbon backbone. In certain embodiments, a
straight chain or
branched chain alkenyl group has 6 or fewer carbon atoms in its backbone
(e.g., C2-6 for straight
chain, C3-6 for branched chain).
[0278] The term "haloalkyl" refers to a straight- or branched-chain alkyl
group having from 1
to 6 carbon atoms in the chain optionally substituting hydrogens with
halogens. The term "CI-
6 haloalkyl" as used here refers to a straight- or branched-chain alkyl group
having from 1 to 6
carbon atoms in the chain, optionally substituting hydrogens with halogens.
The term "Ci-4
haloalkyl" as used here refers to a straight- or branched-chain alkyl group
having from 1 to 4
carbon atoms in the chain, optionally substituting hydrogens with halogens.
Examples of
"haloalkyl" groups include trifluoromethyl (CF3), difluoromethyl (CF2H),
monofluoromethyl
(CH2F), pentafluoroethyl (CF2CF3), tetrafluoroethyl (CHFCF3), monofluoroethyl
(CH2CH2F),
trifluoroethyl (CH2CF3), tetrafluorotrifluoromethylethyl (CF(CF3)2), and
groups that in light of
the ordinary skill in the art and the teachings provided herein would be
considered equivalent
to any one of the foregoing examples.
[0279] The term ''haloalkenyl" includes unsaturated aliphatic groups analogous
in length and
possible substitution to the alkyls described above, but that contain at least
one double bond
and having from 1 to 6 carbon atoms in the chain optionally substituting
hydrogens with
halogens.
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0280] The term "aryl" refers to a monocyclic, aromatic carbocycle (ring
structure having ring
atoms that are all carbon) having 6 atoms per ring. (Carbon atoms in the aryl
groups are sp2
hybridized.)
[0281] The term "heteroaryl" refers to a monocyclic or fused bicyclic
heterocycle (ring
structure having ring atoms selected from carbon atoms and up to four
heteroatoms selected
from nitrogen, oxygen, and sulfur) having from 3 to 9 ring atoms per
heterocycle. Illustrative
examples of heteroaryl groups include the following entities, in the form of
properly bonded
moieties:
,S
N
\ ¨ / N
N 0 N
I ' I I
t
N , , and
[0282] Those skilled in the art will recognize that the species listed or
illustrated above are not
exhaustive, and that additional species within the scope of these defined
terms may also be
selected.
[0283] The term "variable point of attachment" means that a group is allowed
to be attached at
more than one alternative position in a structure. The attachment will always
replace a hydrogen
atom on one of the ring atoms. In other words, all permutations of bonding are
represented by
the single diagram, as shown in the illustrations below.
N
r R N N )zz_,
represent
y- , Or
[0284] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein X is CH.
[0285] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein X is N.
[0286] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein Y is CH.
[0287] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein Y is N.
[0288] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein Rl is C1_4alkyl; C1_4alkyl substituted with OH, or OCH3;
C2_4a1keny1; Ci_4ha1oa1ky1;
C1_4haloalkyl substituted with OH, or OCH3; C2_4haloalkenyl; N(CH3)2;
cyclopropyl;
51
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
cyclopropyl substituted with Ci_zialkyl; or phenyl.
[0289] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein RI is CH3, CH2CH3,
/ 0 _______ F, \N _____________ F
, Or
F
[0290] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
F
,or -L4
(Z) wherein R1 is
[0291] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
Rb
I N¨Re
N
R2 1S 0
wherein Rb is Ci_4a1kyl substituted with OH, halo, CN, OC1_4a1kyl,
OCi4haloalkyl or
0C3_6cycloalkyl; and
Ra is Ci-talkyl, Ci-thaloalkyl, or C3_6cyc1oalky1.
[0292] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
HO
N
) N
N
(Z) wherein R2 is 0
[0293] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R3 is H.
[0294] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R3 is F.
[0295] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R3 is CH3.
[0296] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R3 is OCH3.
[0297] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R4 is
52
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
CD\sµ 0
, or
[0298] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
(Rc
R4 is Rd ;wherein
each RC is independently selected from the group consisting of: H; halo;
C1_4alkyl, CI
-
alkyl substituted with a member selected from the group consisting of: OH,
OCH3,
SCH3, and OCF3; Ci_4haloalkyl; C1_4haloalkyl substituted with a member
selected from
the group consisting of: OH, and OCH3; and NO2;
Rd is selected from the group consisting of: H; halo; Ci_zialkyl; Ci_zialkyl
substituted with
OH, OCH3, SCH3, or OCF3; Ci_4haloalkyl; Ci-ihaloalkyl substituted with OH, or
OCH3;
or OCI-4alkyl; CN; and OCI-6alkyl; and
n is 1, or 2.
[0299] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
(Rc
R4 is Rd ;
each Rc is independently selected from the group consisting of: H, halo,
Ci_4a1kyl,
Ci_4haloalkyl, NO2, 0-CH2CH2OH, and OCi4alkyl;
Rd is selected from the group consisting of: H, halo, C1_4alkyl, CN, and
OCi_6alkyl; and
f/ is 1, or 2.
[0300] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R4 is
F ' CI '
DQFO
0 '
CI
F CI , CI F
53
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
oI
oI F
0:
, CI , F ' CI ' CI , CI , CI ,
or
F 0 0 0 8
.- --- ,
HO
103011 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
CI
(Z) wherein le is or F=
103021 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
c
Rc,<Nµ, Rc .. (R
\ _ Rc N `z,
N ''--
I
U.. --- ---. --i--..
R4 is -'"---7-'Rd I.L.-C"'-'-' Rd N ',,,d
N R-d
, , ,
N N-N
II '-
N .-- ft,,,,,, .
, or
wherein
each Re is independently selected from the group consisting of: H; halo;
Ci_4alkyl; Ci_alkyl
substituted with a member selected from the group consisting of: OH, OCH3,
SCH3, and
OCF3; Ci_4haloalkyl; Ci_4haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and Rd is selected from the group consisting of:
halo;
C1_4alky1; C1-4alkyl substituted with OH, OCH3, SCH3, or OCF3; Ci_4haloalkyl;
Ci-
4haloalkyl substituted with OH, or OCH3; or 0C1_4alkyl; CN; and 0C1_6alky1.
103031 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
IR
(F2'
Rc><N...,,,µ, IR\
n N
_, I ,---`-'.---"- --
----N -1 ,õ-r21
I _, I. ..--, II --
----_------------ d N'------''Rd N''¨' Rd 1\( -- ''- N ---9- N -
- ---
R4 is ..'",---;----.."-Rd - R
, ,
,
NN
or ----%---- ; wherein
each RC is independently selected from the group consisting of: H, halo,
C1_4a1kyl,
Ci_4haloalkyl, OC1_4a1ky1, and OH;
54
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Rd is selected from the group consisting of: halo, Ci-talkyl, and CI-alkyl;
and
ri is 1, or 2.
103041 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein R4 is
I
,
-(21 CI
N[
NK NNO' NC I
I ,
oI
N , N ,N N ,N N
CI CI
HO N CN NN
0 0
I I
N, N ,
0 0
, or
HO
.
103051 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
c
NI)/ 5'
(Z) wherein R4 is \-
103061 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) wherein
Re Rc
RC I R9¨N
R4 is NRd, N Rd , orRd , wherein
Re is H; halo; Ci_4alkyl; Ci_4alkyl substituted with OH, OCH3, SCH3, or OCF3;
Cl_4haloalkyl; C1_4haloa1kyl substituted with OH, or OCH3; or CI-alkyl;
Rd is halo; CI-alkyl; Ci-talkyl substituted with OH, OCH3, SCH3, or OCF3; Ci-
thaloalkyl;
or C1_4haloalky1 substituted with OH, or OCH3; and
Rg is H; Ci_4alkyl; Ci_4alkyl substituted with OH, OCH3, SCH3, or OCF3;
Ci_4haloalkyl; or
C1_4haloalkyl substituted with OH, or OCH3.
103071 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(Z) wherein
S
F2c¨I Rg¨N
R4 is N Rd , N Rd , or snr"---'-- a =
R , wherein
Re is H or halo;
Rd is C1_4alkyl; and
Rg is H.
103081 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
CI
S
N I
HN, \S"-, , µ1\1-- , or N =
(Z) wherein R4 is N
103091 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) selected from the group consisting of:
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-(prop-1-en-2-y1)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-phenylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1, 2,4-tri azol -1-y1)-4-(prop-1-en-2-yl)i soquinol in-1(21-1)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1, 2,4-triazol-1-y1)-4-(3,3,3 -trifluoroprop-1-en-2-yl)isoquinolin-1(2H)-one,
2-(2,6-Dichloropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-4-(1-methylcy el opropyl)isoquinolin-1(2H)-one,
2-(2,6-Dichloropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-4-(prop-1-en-2-yl)isoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-4-cyclopropy1-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-
4,5-
di hydro-1H-1,2,4-tri azol-1-yl)i soquinolin- 1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-7-fluoro-44 sopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxym ethyl)-5 -oxo-4,5-di hy dro-1H- 1,2,4-triazol-1-y1)-4-
(prop- 1-en-
56
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
2-y1)-2-(2-(trifluoromethyl)phenyl)isoquinolin- 1(2H)-one;
2-(6-(4-Ethy1-3 -(hydroxymethyl)-5-oxo-4, 5 -dihydro- 1H- 1,2,4-tri azol - 1 -
y1)- 1 -oxo-4-
(prop- 1-en-2-yl)i soquinolin-2(1H)-yl)benzonitrile;
2-(2-Chloropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-4-(prop- 1-en-2-yl)i soquinolin-1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-4-
(prop- 1 -en-
2-y1)-2-(o-tolyl)i soquinolin-1(2H)-one;
2-(2-Chloropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol- 1 -y1)-4-i sopropylphthal azin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-2-
(3-
fluoropheny1)-44 sopropylphthal azin- 1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-4-i sopropylphthalazin- 1 (2H)-one;
4-Ethyl-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihy dro- 1H- 1,2,4-tri azol-
1 -y1)-2-(3 -
fluorophenyl)phthalazin-1(2H)-one;
4-Ethyl-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihy dro- 1H- 1,2,4-tri azol-
1 -y1)-2-(o-
tolyl)phthalazin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihy dro- 1H- 1,2,4-triazol- 1-y1)-7-
fl uoro-4-
sopropy1-2-(o-tolyl)i soquinolin- 1 (2H)-one,
2-(2-Chl oro-4-m ethyl pyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
1H-1,2,4-triazol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-4-(1-methyl cyclopropyl)i soquinol in- i(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-2-
(3-
fluoropheny1)-4-(2-hydroxypropan-2-ypisoquinolin- 1(2H)-one;
4-(Dimethylamino)-6-(4-ethyl -3 -(hydroxymethyl)-5-oxo-4,5-dihydro- 1H- 1,2,4-
triazol-1 -y1)-2-(o-tolypi soquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-7-fluoro-4-(prop- 1-en-2-yl)phthal azin- 1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-7-methoxy-4-(prop- 1-en-2-yl)phthal azi n- 1 (2H)-one;
2-(5 -Chl oro-3 -m ethyl - 1H-pyrazol-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-
oxo-4, 5 -
di hydro- 11/-1,2,4-triazol- 1 -y1)-7-fluoro-4-isopropyli soqui nolin- 1(2H)-
one;
2-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihy dro- 1H- 1,2,4-triazol- 1-y1)-3-
fl uoro-8-
57
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(prop- 1-en-2-y1)-6-(o-toly1)- 1, 6-naphthyri din- 5(611)-one;
2-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-
fluoro-8-
methy1-6-(o-toly1)pyri do[2, 3 -d]pyridazin-5(611)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-
fluoro-8-
isopropy1-6-(o-toly1)pyrido12,3-d]pyridazin-5(611)-one;
6-(2-Chl oro-6-fluoropheny1)-2-(4-ethy1-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro- 1H-
1,2,4-tri azol- 1-y1)-3 -fluoro-8 -(prop- 1-en-2-y1)- 1,6-naphthyri din- 5(6H)-
one;
6-(2-Chl oro-6-fluoropheny1)-2-(4-ethy1-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro- 1H-
1,2,4-tri azol- 1-y1)-3 -fluoro-8 sopropy1-1,6-naphthyridin-5(6H)-one;
(S)-2-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihydro- 111- 1,2,4-triazol- 1-
y1)-3 -fluoro-
6-(o-toly1)-8-(1, 1, 1 -trifluoropropan-2-y1)-1,6-naphthyridin-5 (611)-one;
(R)-2-(4-Ethyl-3 -(hydroxym ethyl)- 5 -oxo-4, 5-dihydro- 1H- 1,2,4-tri azol- 1-
y1)-3 -fluoro-
6-(o-toly1)-8-(1, 1, 1 -trifluoropropan-2-y1)-1,6-naphthyridin-5 (611)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(4-methylthiazol -5 -yl)i soquinolin-1(2H)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-
fluoro-8-
i sopropy1-6-(o-toly1)- 1,6-naphthyridin-5(6H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-5-methylpheny1)-4-i sopropylisoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-5-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1(2H)-one;
2-(2-Chl oro-5-m ethyl pheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4, 5-
dihydro- 1H-
1,2,4-triazol- 1-y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro- 5-methoxypheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
2-(2-Chl oropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
(prop- 1-en-2-y1)-2-(o-tolypi soquinolin- 1 (2H)-one;
2-(2-Chloro-5-methoxypheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-dihy dro-
1H-
1,2,4-triazol- 1-y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin- 1 (2H)-one;
racemi c-4-(sec-Buty1)-2-(2-chl oro-6-fluoropheny1)-6-(4-ethy1-3 -
(hydroxymethyl)-5 -
oxo-4, 5 -dihydro- 1H-1,2,4-triazol- 1-y1)-7-fluoroi soquinolin- 1(2H)-one;
2-(3 -Chloro-6-methoxypyridin-2-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
dihydro- 1H-1,2,4-triazol- 1 -y1)-7-fluoro-4-i sopropyli soquinolin- 1(2H)-
one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(2-methoxy-4-m ethylpyri din-3 -yl)i soquinolin-1(2H)-one;
2-(2-Chloro-6-fluoro-3 -methoxypheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,
5 -
di hydro- 1H-1,2,4-triazol- 1 -y1)-7-fluoro-4-(prop-1-en-2-yl)isoquinolin-
1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1,2,4-triazol- 1-y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-(o-toly1)phthalazin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-7-fluoro-4-i sopropylphthalazin-1(2H)-one;
Racemic 6-(4-Ethyl-3-(hydroxymethyl)-5-oxo-4,5 -dihydro-1H- 1,2,4-triazol-1 -
y1)-7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)phthalazin-1 (2H)-one;
(S*)-6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)phthalazin-1 (2H)-one;
(R *)-6-(4-Ethy1-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro- 1H- 1,2,4-tri azol- 1
-y1)-'7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)phthalazin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hy droxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1,2,4-tri azol- 1-y1)-7-fluoro-4-( 1-methyl cycl opropyl)i soquinolin- i(2H)-
one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
methoxy-4-methylpyridin-3-y1)-4-(prop- 1-en-2-yl)isoquinolin- 1(2H)-one;
2-(5 -Chl oro-3 -m ethyl - 1H-pyrazol-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-
oxo-4, 5 -
di hydro- 1H-1,2,4-triazol- 1 -y1)-7-fluoro-4-(prop-1-en-2-yl)isoquinolin-
1(2H)-one;
2-(3-Chloro-2-methoxy-5-methylpyridin-4-y1)-6-(4-ethy1-3 -(hydroxymethyl)- 5 -
oxo-
4, 5 -dihydro- 1H- 1,2,4-triazol- 1 -y1)-7-fluoro-4-isopropyli soquinolin-
1(2H)-one;
2-(3-Chloro-2-methoxy-5-methylpyridin-4-y1)-6-(4-ethy1-3 -(hydroxymethyl)- 5 -
oxo-
4, 5-dihydro-1H-1,2,4-triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin-
1(21-1)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro- 5-methoxypheny1)-4-i sopropylisoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(4-
fluoro-2-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one;
2-(2-Chloro-3-(2-hydroxyethoxy)pheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4,
5 -
di hydro- 1H-1,2,4-triazol- 1 -y1)-7-fluoro-4-(prop-1-en-2-yl)isoquinolin-
1(2H)-one,
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-fl
uoro-2-(2-
59
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
fluoropheny1)-4-(prop- 1 -en-2-yl)i soquinolin-1 (2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(5-
fluoro-2-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one;
2-(2, 5-Difluoropheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-i sopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-6-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one,
2-(2-Chloro-3-methoxypheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4, 5-dihy dro-
1H-
1, 2,4-triazol- 1-y1)- 7-fluoro-4-(prop- 1-en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(2-
methoxy-3, 5 -dimethylpyridin-4-y1)-4-(prop- 1 -en-2-yl)isoquinolin- 1(2H)-
one;
2-(2, 5-Difluoropheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-6-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(3 -
fluoro-2-methylpheny1)-4-i sopropylisoquinolin- 1(2H)-one;
2-(2, 5-Dimethylpheny1)-6-(4-ethyl-3 -(hy droxymethyl)-5 -oxo-4,5 -dihy dro-
1H- 1,2,4-
triazol- 1 -y1)-7-fluoro-4-i sopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(4-
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoropheny1)-44 sopropyli soquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(2-methoxy-3,5-dimethylpyridin-4-y1)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
methy1-4-
(prop- 1-en-2-y1)-2-(o-tolyl)i soquinolin- 1 (2H)-one;
2-(2-Chloro-6-fluoro-3 -methoxypheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,
5 -
di hydro- 1H-1,2,4-tri azol- 1 -y1)-7-fluoro-44 sopropyli soquinolin- 1(2H)-
one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(o-
toly1)-4-(3 ,3,3-trifluoroprop-1-en-2-yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-2-(5 -
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
methoxypheny1)-4-(prop- 1 -en-2-yl)i soquinolin- 1(2H)-one;
2-(2-Chl oro-5-m ethylpyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
1H-1,2,4-triazol- 1-y1)-7-fluoro-4-(prop-1-en-2-yl)i soqui nolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(5-
fluoro-2-methoxypyridin-4-y1)-4-(prop-1 -en-2-yl)i soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(3 -
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
2-(2, 5-Dimethylpheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soquinolin- 1(2H)-one;
Racemic-6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro- 1H- 1,2,4-tri azol- 1
-y1)-7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-2-(2-
ethylpheny1)-7-fluoro-44 sopropyli soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(2-methoxypyridin-3-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(5-
fluoro-2-methoxypyridin-4-y1)-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chl oro-5-m ethylpyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
dihy dro-
1H- 1,2,4-tri azol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one,
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(2-(methyl-d3)phenypi soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(4-methylpyrimi din-5 -yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-(2-methoxyphenypi soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(3 -
fluoro-6-methoxypyridin-2-y1)-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(3-methylpyrazin-2-yl)i soquinolin- 1(2H)-one;
2-(2-C hl oro-5-m ethyl pyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-
4,5-dihydro-
1H-1,2,4-triazol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one,
2-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-3-
flu oro-6-(2-
fluoro- 5-methylpheny1)-8-i sopropyl- 1, 6-naphthyridin- 5(611)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro 4-
61
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
isopropy1-2-(4-methylpyridazin-3-yl)isoquinolin-1(2H)-one;
(S)-6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4, 5 -dihydro- 1H- 1,2,4-triazol- 1 -
y1)-7-fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2 -yl)isoquinolin-1(2H)-one;
(R)-6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-dihydro-1H-1,2,4-triazol-1 -y1)-7-
fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2 -yl)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropyl-2-(2-(trifluoromethyl)phenyl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-(5-methylpyrimi din-4 -yl)isoquinolin-1(2H)-one;
2-(2-(Difluoromethyl)pheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -
dihydro- 1H-
1, 2,4-triazol- 1-y1)-7-fluoro-4 sopropylisoquinolin-1(2H)-one;
2-(3 -Chloro-2-methoxypyridin-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
di hydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoro-44 sopropyli soquinolin-1 (2H)-
one;
2-Cy clohexy1-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro-1H-1,2,4-tri
azol- 1-y1)-
7-fl uoro-4-isopropylisoquinolin-1(2H)-one;
2-(3 -Chloro-6-methylpyridin-2-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
1H-1,2,4-tri azol- 1-y1)-7-fluoro-4-i sopropyli soquinolin-1 (2H)-one,
2-Cy clopenty1-6-(4-ethy1-3 -(hy droxy methyl)-5 -oxo-4, 5 -dihy dro-1H-1,2,4-
triazol-1-
y1)-7-fluoro-4-i sopropylisoquinolin-1(2H)-one,
2-(3 -Chloro-4-methoxypyridin-2-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
di hydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-((1R,2 S)-2-methylcyclohexyl)isoquinolin-1(2H)-one;
2-(1,3-Dimethoxypropan-2-y1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -
dihydro-111-
1, 2,4-triazol- 1-y1)-7-fluoro-4 sopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(2-methoxy-5 -methylpyridin-4-yl)i soquinolin-1 (2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-111-1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-(( 1S,21?)-2-methylcyclohexypisoquinolin-1(2H)-one;
2-(Cyclopropylmethyl)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro-1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-i sopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-4-
sopropy1-2-(1-methoxybutan-2-yl)i soquinolin- 1(2H)-one;
2-(2-Chloropheny1)-6-(4-ethyl-3 -(hy droxymethyl)-5-oxo-4, 5 -dihy dro-111-
1,2,4-
62
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
triazol-1 -y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropyl-2-(3-methylpyridin-2-y1)i soquinolin- 1(2H)-one;
Racemic 6-(4-Ethyl-3-(hy droxymethyl)-5-oxo-4,5 -dihydro-1H- 1,2,4-triazol-1 -
y1)-7-
fluoro-4-isopropy1-2-((ci s)-3 -methoxycycl opentyl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-241R*,2R*)-2-methylcyclohexyl)i soquinolin- 1 (2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-((1 S*,2 S*)-2-methy1cyc1ohexy1)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(pentan-3 -yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(( 1R*, 2R*)-2-methy1cyc1openty1)isoquino1in- 1 (2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-241 S*,2S*)-2-methylcyclopentyl)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2((1R*,2S*)-2-methylcyclopentyl)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-((1 S*,2R*)-2-methylcyclopentyl)isoquinolin- 1(2H)-one;
Racemic 6-(4-Ethyl-3-(hy droxymethyl)-5-oxo-4,5 -dihydro-1H- 1,2,4-triazol-1 -
y1)-7-
fluoro-4-isopropy1-2-((ci s)-3 -methoxycycl ohexyl)isoquinolin- 1(2H)-one;
2-(Bicycl o[2. 2.1 ]heptan- 1-y1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -
dihy dro-/H-
1,2,4-tri azol- 1-y1)-7-fluoro-4-isopropylisoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-(2-methoxy-3 -m ethylpyri din-4-yl)isoquinolin- 1(2H)-one;
2-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-3-
fluoro-8-
i sopropy1-6-(2-methoxypheny1)- 1, 6-naphthyri din-5 (61J)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(3-methylisothiazol-4-ypisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(5-methylisothiazol-4-y1)isoquinolin-1(2H)-one;
2-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-3-
flu oro-8-
sopropy1-6-(2-(trifluoromethyl)pheny1)- 1, 6-naphthyri din-5 (61/)-one;
2-(3,6-Dimethylpyridin-2-y1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4, 5 -dihy dro-
111-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
1,2,4-triazol-1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
2-(2,5-Dimethylpyridin-4-y1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropyl-2-(4-methylpyridin-3-y1)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(3-methylpyridin-4-y1)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropyl-2-(2-methylpyridin-3-y1)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-IH-1,2,4-triazol-1-y1)-7-fluoro-
2-(2-
hydroxy-5-methylpyridin-4-y1)-4-isopropylisoquinolin-1(2H)-one;
6-(2-(Difluoromethyl)pheny1)-2-(4-ethyl-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-3-fluoro-8-isopropy1-1,6-naphthyridin-5(611)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(2-
hydroxy-3-methylpyridin-4-y1)-4-isopropylisoquinolin-1(2H)-one; and
2-(4-Ethyl-3 -(hy droxym ethyl)-5 -oxo-4, 5 -di hy dro- 1H-1,2,4-triazol-1 -
y1)-3 -fluoro-8 -
isopropy1-6-(o-D3-toly1)-1,6-naphthyridin-5(6H)-one;
and, optionally, one or more of pharmaceutically acceptable salts, isotopes, N-
oxides,
solvates, and stereoisomers thereof.
103101 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) selected from the group consisting of:
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-(prop-1-en-2-y1)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-IH-
1,2,4-triazol-1-y1)-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-phenylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-4-(prop-1-en-2-y1)isoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-IH-
1, 2,4-triazol- 1-y1)-4-(3 ,3,3 -trifluoroprop-1-en-2-yl)isoquinolin-1 (2H)-
one;
2-(2,6-Dichloropheny1)-6-(4-ethy1-3-(hy droxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-
64
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
triazol-1 -y1)-4-(1 -methylcycl opropyl)isoquinolin-1(2H)-one;
2-(2, 6-Di chl oropheny1)-6-(4-ethy1-3 -(hy droxymethyl)-5-oxo-4, 5 -dihydro-
1H- 1,2,4-
triazol-1 -y1)-4-(prop- 1-en-2-yl)i soquinolin-1 (2H)-one;
2-(2-Chloro-6-fluoropheny1)-4-cyclopropy1-6-(4-ethyl -3 -(hydroxymethyl)-5-oxo-
4, 5 -
dihydro- 1H-1,2,4-triazol- 1 -yl)i soquinolin- 1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1,2,4-tri azol- 1-y1)-7-fluoro-4-i sopropyli soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-4-
(prop- 1 -en-
2-y1)-2-(2-(trifluoromethyl)phenyl)isoquinolin- 1(2H)-one;
2-(6-(4-Ethy1-3 -(hydroxymethyl)-5-oxo-4, 5 -dihydro- 1H- 1,2,4-triazol- 1-y1)-
1 -oxo-4-
(prop- 1-en-2-yl)i soquinolin-2(1H)-yl)benzonitrile;
2-(2-Chloropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-4-(prop- soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-4-
(prop- 1 -en-
2-y1)-2-(o-tolyl)i soquinolin-1(2H)-one;
2-(2-Chloropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-4-i sopropylphthal azin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-2-(3-
fluoropheny1)-4-isopropylphthalazin- 1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-4-i sopropylphthalazin-1 (2H)-one;
4-Ethyl-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihy dro- 1H-1,2,4-tri azol-
1 -y1)-2-(3 -
fluorophenyl)phthalazin-1(2H)-one;
4-Ethyl-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihy dro- 1H-1,2,4-tri azol-
1 -y1)-2-(o-
tolyl)phthalazin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(o-tolyl)i soquinolin- 1 (2H)-one;
2-(2-Chl oro-4-m ethyl pyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
1H-1,2,4-triazol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethyl-3-(hydroxymethyl)-5-oxo-4, 5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-4-(1-methyl cyclopropyl)i soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-2-
(3 -
fluoropheny1)-4-(2-hydroxypropan-2-yl)i soquinolin- 1(2H)-one;
4-(Dimethylamino)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-dihydro- 1H-1,2,4-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
triazol-1 -y1)-2-(o-tolyl)i soquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4 -ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -dihy
dro-1H-
1, 2,4-tri azol- 1 -y1)-7-fluoro-4 -(prop-1-en-2 -yl)phthalazin-1(2H)-one;
2-(2 -Chloro-6-fluoropheny1)-6-(4 -ethy1-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro-1H-
1, 2,4-tri azol- 1 -y1)-7-methoxy-4-(prop- 1-en-2-yl)phthal azin-1 (2H)-one;
2-(5-Chloro-3 -m ethyl - 1H-pyrazol-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-
4, 5 -
di hydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoro-44 sopropyli soquinolin-1 (2H)-
one;
2-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-
fluoro-8-
(prop- 1 -en-2-y1)-6-(o-toly1)- 1, 6-naphthyri din- 5(611)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-3-
fluoro-8-
methy1-6-(o-tolyl)pyri do[2, 3 -d]pyridazin-5 (614)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-
fluoro-8-
i sopropy1-6-(o-tolyl)pyrido [2,3 -di pyridazin-5 (611)-one;
6-(2-Chloro-6-fluoropheny1)-2-(4 -ethyl-3 -(hy droxyrnethyl)-5 -oxo-4, 5 -
dihydro-1H-
1, 2,4-tri azol- 1 -y1)-3 -fluoro-8 -(prop-1-en-2 -y1)- 1,6-naphthyri din-
5(614)-one;
6-(2-Chloro-6-fluoropheny1)-2-(4 -ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro-1H-
1, 2,4-triazol-1-y1)-3-fluoro-8-i sopropy1-1,6-naphthyridin-5(6H)-one;
(S)-2-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4, 5 -dihydro-111-1,2,4-tri azol- 1-
y1)-3 -fluoro-
6-(o-toly1)-8-(1 , 1, 1 -tri fluoropropan-2 -y1)- 1,6-naphthyridin-5 (614)-
one;
(R)-2-(4-Ethyl-3 -(hydroxym ethyl)- 5 -oxo-4, 5-dihydro-111-1,2,4-tri azol-1 -
y1)-3 -fluoro-
6-(o-toly1)-8-(1, 1, 1 -trifluoropropan-2 -y1)-1,6-naphthyridin-5(614)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(4-methylthiazol -5 -yl)isoquinolin-1(2H)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-114- 1,2,4-triazol-1-y1)-3-
fluoro-8-
sopropy1-6-(o-toly1)- 1, 6-naphthyri din- 5(611)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(2-
fluoro- 5-methylpheny1)-4-i sopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-5-methylpheny1)-4-(prop-1-en-2-y1)isoquinolin-1(2H)-one;
2-(2-C hl oro-5-m ethyl pheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4, 5-
dihydro-1H-
1, 2,4-triazol-1-y1)-7-fluoro-4 -(prop-1-en-2 -yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-2-(2-
fluoro- 5-methoxypheny1)-4-(prop-1 -en-2-yl)isoquinolin-1(2H)-one;
2-(2-Chloropheny1)-6-(4-ethyl-3 -(hy droxymethyl)-5-oxo-4, 5 -dihy dro-1H-
1,2,4-
66
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
triazol-1-y1)-7-fluoro-4-(prop-1-en-2-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihy dro-1H- 1,2,4-triazol-1-y1)-7-
fl uoro-4-
(prop- 1-en-2-y1)-2-(o-tolyl)i soquinolin-1 (2H)-one,
2-(2-Chloro-5-methoxypheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4,5-dihy dro-
1H-
1, 2,4-triazol- 1-y1)-7-fluoro-4 -(prop-1-en-2 -yl)isoquinolin-1(2H)-one;
racemi c-4-(sec-Buty1)-2-(2-chl oro-6-fluoropheny1)-6-(4-ethy1-3 -
(hydroxymethyl)-5 -
oxo-4,5 -dihydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoroi soquinoli n-1 (2H)-one;
2-(3 -Chloro-6-methoxypyridin-2-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
di hydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(2-methoxy-4-m ethylpyri din-3 -yl)i soquinolin-1(2H)-one;
2-(2-Chloro-6-fluoro-3-methoxypheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4, 5
-
di hydro- 1H-1,2,4-tri azol-1-y1)-7-fluoro-4-(prop-1-en-2-y1)1 soquinolin-
1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4 -ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro-1H-
1, 2,4-triazol- 1-y1)-7-fluoro-4 -(prop-1-en-2 -yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(o-tolyl)phthalazin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4 -ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -dihy
dro-1H-
1, 2,4-tri azol- 1-y1)-7-fluoro-4 sopropylphthalazin-1(2H)-one;
Racemic 6-(4-Ethyl-3-(hy droxymethyl)-5-oxo-4,5 -dihydro-1H-1,2,4 -triazol-1 -
y1)-7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)phthalazin-1 (2H)-one;
(S*)-6-(4-Ethy1-3-(hydroxymethyl)-5 -oxo-4, 5-dihydro-1H- 1, 2,4 -triazol-1 -
y1)- 7-fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2 -yl)phthalazin-1(2H)-one;
(R *)-6-(4-Ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hydro- 1H-1,2,4-tri azol-1
-y1)-7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)phthalazin-1 (2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4 -ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -
dihydro- 1H-
1, 2,4-tri azol- 1 -y1)-7-fluoro-4 -(1-methyl cycl opropyl)i soquinolin- 1(21-
1)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
methoxy-4-methylpyridin-3 -y1)-4-(prop- 1-en-2-yl)i soquinolin-1(2H)-one;
2-(5-Chloro-3 -m ethyl - 1H-pyrazol-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-
4, 5 -
di hydro- 1H-1,2,4-tri azol- 1-y1)-7-fluoro-4-(prop-1 -en-2-yl)i soquinolin-
1(2H)-one;
2-(3 -Chloro-2-methoxy-5-methylpyridin-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)- 5 -
oxo-
4, 5 -dihydro-1H- 1,2,4-triazol-1 -y1)-7-fluoro-4-isopropyli soquinolin- 1(2H)-
one;
2-(3 -Chloro-2-methoxy-5-methylpyridin-4-y1)-6-(4-ethyl-3 -(hy droxymethyl)- 5
-oxo-
67
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
4, 5-dihydro-1H-1,2,4-triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin-
1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro- 5-methoxypheny1)-4-isopropylisoquinolin- 1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(4-
fluoro-2-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one;
2-(2-Chloro-3-(2-hydroxyethoxy)pheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,
5 -
di hydro- 1H-1,2,4-tri azol- 1 -y1)-7-fluoro-4-(prop-1-en-2-yl)i soquinolin-
1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoropheny1)-4-(prop- 1 -en-2-yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(5-
fluoro-2-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one;
2-(2, 5-Difluoropheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-i sopropyli soquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(2-
fluoro-6-methylpheny1)-44 sopropylisoquinolin- 1(2H)-one;
2-(2-Chloro-3-methoxypheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4, 5-dihydro-
1H-
1,2,4-triazol- 1-y1)-7-fluoro-4-(prop- 1-en-2-yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
methoxy-3,5-dimethylpyridin-4-y1)-4-(prop- 1 -en-2-yl)isoquinolin- 1(2H)-one;
2-(2, 5-Difluoropheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(2-
fluoro-6-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(3 -
fluoro-2-methylpheny1)-4-isopropylisoquinolin- 1(2H)-one;
2-(2, 5-Dimethylpheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(4-
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
2-(2-
fluoropheny1)-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-4-
i sopropy1-2-(2-methoxy-3 , 5-dimethylpyridin-4-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
methyl-4-
68
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(prop- 1-en-2-y1)-2-(o-tolyl)i soquinolin- 1 (2H)-one,
2-(2-Chloro-6-fluoro-3 -methoxypheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,
5 -
di hydro- 1H- 1,2,4-tri azol- 1 -y1)-7-fluoro-4-i sopropyli soquinolin- 1(2H)-
one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(o-
toly1)-4-(3 ,3,3-trifluoroprop- 1-en-2-yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(5 -
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(2-
methoxypheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1(2H)-one;
2-(2-Chl oro-5-m ethyl pyri din-3 -y1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4,
5-dihy dro-
1H- 1,2,4-tri azol- 1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soqui nolin- 1(2H)-
one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(5 -
fluoro-2-methoxypyri din-4-y1)-4-(prop- 1 -en-2-yl)i soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-7-
fluoro-2-(3 -
fluoro-2-methylpheny1)-4-(prop- 1 -en-2-yl)isoquinolin- 1 (2H)-one;
2-(2, 5-Dimethylpheny1)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-(prop- 1-en-2-yl)i soquinolin- 1(2H)-one;
Racemic-6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-dihy dro- 1 H- 1,2,4-tri azol-
1 -y1)-7-
fluoro-2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2-yl)isoquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-2-
(2-
ethylpheny1)-7-fluoro-44 sopropyli soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(2-methoxypyridin-3-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-2-(5 -
fluoro-2-methoxypyridin-4-y1)-4-i sopropyli soquinolin- 1 (2H)-one;
2-(2-Chl oro-5-m ethyl pyri din-3 -y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
1H-1,2,4-triazol- 1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(2-(methyl-d3)phenypi soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
sopropy1-2-(4-methylpyrimi din-5 -yl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-4-
sopropy1-2-(2-methoxyphenyl)i soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihy dro- 1H- 1,2,4-triazol- 1-y1)-7-
fl uoro-2-(3 -
69
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
fluoro-6-methoxypyridin-2-y1)-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(3-methylpyrazin-2-y1)isoquinolin-1(2H)-one;
2-(2 -C hl oro-5-m ethyl pyri din-3 -y1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-
4,5-dihydro-
1H-1,2,4-tri azol-1-y1)-7-fluoro-4-1 sopropyli soquinolin-1(2H)-one;
2-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-3-fluoro-
6-(2-
fluoro-5-methylpheny1)-8-i sopropyl- 1,6-naphthyridin-5(6H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro
4-
i sopropy1-2-(4-methylpyridazin-3-yl)isoquinolin-1(2H)-one;
(S)-6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4, 5 -dihydro- 1H-1,2,4-triazol- 1-y1)-
7-fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2 -yl)isoquinolin-1(2H)-one;
(R)-6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-dihydro-1H-1,2,4-tri azol-1 -y1)-
7-fluoro-
2-(o-toly1)-4-(1, 1, 1 -trifluoropropan-2 -yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(2-(trifluoromethyl)phenyl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-
fluoro-4-
i sopropy1-2-(5-methylpyrimi din-4 -yl)isoquinolin-1(2H)-one;
2-(2-(Difluoromethyl)pheny1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihy
dro-11/-
1, 2,4-triazol-1-y1)-7-fluoro-44 sopropylisoquinolin-1(2H)-one;
2-(3 -Chloro-2-methoxypyridin-4-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
di hydro- 111-1,2,4-tri azol- 1-y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-
one;
2-Cy cl ohexy1-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro-111-1,2,4-tri
azol- 1-y1)-
7-fluoro-44 sopropyli soquinolin-1(2H)-one;
2-(3 -Chl oro-6-m ethyl pyri din-2-y1)-6-(4-ethy1-3 -(hydroxymethyl)-5-oxo-4,5-
dihydro-
111-1,2,4-tri azol-1-y1)-7-fluoro-4-1 sopropyli soquinolin-1(2H)-one;
2-Cy cl openty1-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihy dro- 1H- 1,2,4-
tri azol- 1-
y1)-7-fluoro-4-i sopropylisoquinolin-1(2H)-one;
2-(3 -Chloro-4-methoxypyridin-2-y1)-6-(4-ethyl-3 -(hydroxymethyl)-5-oxo-4, 5-
di hydro- 1H-1,2,4-tri azol-1-y1)-7-fluoro-44 sopropyli soquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-((1R,2 S)-2-methylcyclohexyl)isoquinolin-1(2H)-one;
2-(1,3-Dimethoxypropan-2-y1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4,5 -dihydro-
1H-
1, 2,4-tri azol- 1-y1)-7-fluoro-4 sopropylisoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihy dro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
isopropy1-2-(2-methoxy-5-methylpyridin-4-yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-241S,2R)-2-methylcyclohexyl)isoquinolin- 1(2H)-one;
2-(Cyclopropylmethyl)-6-(4-ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro-1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
sopropy1-2-(1-methoxybutan-2-yl)i soquinolin- 1(2H)-one;
2-(2-Chl oropheny1)-6-(4-ethyl -3 -(hydroxymethyl)-5 -oxo-4, 5 -dihydro- 1H-
1,2,4-
triazol-1 -y1)-7-fluoro-4-isopropyli soquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-(3 -methylpyridin-2-yl)i soquinolin- i(2H)-one;
Racemic 6-(4-Ethyl-3-(hy droxymethyl)-5-oxo-4, 5 -dihydro-1H- 1,2,4-triazol-1 -
y1)-7-
fluoro-4-i sopropy1-2-((ci s)-3 -methoxycycl opentyl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
sopropy1-241R*,2R*)-2-methylcyclohexyl)i soquinolin- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
sopropy1-2-((1 S*,2 S*)-2-methylcyclohexyl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-fl
uoro-4-
i sopropy1-2-(pentan-3 -y1)) soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
sopropy1-241R*, 2R*)-2-methy1cyc1openty1)isoquino1in- 1 (2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-2-((1 S*,2S*)-2-methylcyclopentyl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-7-
fluoro-4-
isopropy1-241R*,2S*)-2-methylcyclopentyl)isoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
fluoro-4-
i sopropy1-2-((1 S*,2R*)-2-methylcyclopentyl)isoquinolin- 1(2H)-one;
Racemic 6-(4-Ethyl-3-(hy droxymethyl)-5-oxo-4, 5 -dihydro-1H- 1,2,4-triazol-1 -
y1)-'7-
fluoro-4-1 sopropy1-2-((ci s)-3 -methoxycycl ohexyl)isoquinolin- 1(2H)-one;
2-(Bicycl o [2. 2.1 ]heptan- 1-y1)-6-(4-ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -
dihy dro-/H-
1, 2,4-triazol- 1-y1)-7-fluoro-44 sopropylisoquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4,5-dihydro- 1H- 1,2,4-triazol- 1-y1)-7-
flu oro-4-
sopropy1-2-(2-methoxy-3 -methylpyridin-4-yl)isoquinolin-1(2H)-one;
2-(4-Ethyl-3 -(hydroxymethyl)-5-oxo-4,5-dihydro-1H- 1,2,4-triazol-1-y1)-3-fl
uoro-8-
71
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
i sopropyl- 6-(2-m ethoxypheny1)- 1, 6-naphthyri din-5 (611)-one,
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol-
1 -y1)- 7-fl u oro-4-
i sopropyl- 2 -(3 -methyl i sothi azol-4 -yl)isoquinolin-1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1,2 , 4 -tri azol- 1
-y1)- 7-flu oro-4-
sopropyl- 2 -(5-methyl i sothi azol-4 -yl)isoquinolin-1(2H)-one;
2 -(4-Ethy1-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol-
1 -y1)-3-flu oro- 8 -
sopropyl- 6-(2-(trifluorom ethy 1)pheny1)- 1, 6-n aphthyri di n-5 (611)-one;
2 -(3 , 6-Dim ethyl pyri din-2-y1)- 6 -(4-ethy1-3 -(hy droxym ethyl)- 5 -oxo-
4, 5 -di hy dro- 1H-
1, 2, 4 -tri azol- 1-y1)- 7-fluoro-4 sopropyli soquinoli n- 1(2H)-one;
2-(2, 5-Dim ethyl pyri din-4-y1)- 6 -(4-ethy1-3 -(hy droxym ethyl)- 5 -oxo-4,
5 -di hy dro- 1H-
1, 2, 4 -tri azol- 1 -y1)- 7-fluoro-4 sopropyli soquinoli n- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol- 1
-y1)- 7-flu oro-4-
i sopropyl- 2 -(4-methyl pyridin-3 -yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol- 1
-y1)- 7-flu oro-4-
i sopropyl- 2 -(3 -methyl pyridin-4-yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol- 1
-y1)- 7-flu oro-4-
sopropyl- 2 -(2-methyl pyridin-3 -yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hy droxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol-
1 -y1)- 7-fl u oro-2-(2-
hy droxy-5 -m ethyl pyri din-4-y1)-4-i sopropyli soquinolin- 1(2H)-one;
6-(2-(Difluoromethyl)pheny1)-2-(4-ethyl-3 -(hydroxymethyl)-5 -ox o-4, 5 -di hy
dro- 111-
1, 2, 4 -tri az ol- 1 -y1)-3 -fluoro-8 sopropyl- 1 ,6-nap hthyri din- 5 (611)-
one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol- 1
-y1)- 7-flu oro-2-(2-
hy droxy-3 -m ethyl pyri din-4-y1)-4-i sopropyli soquinolin- 1(2H)-one; and
2-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -triazol-1 -
y1)-3 -fluoro-8
sopropy1-6-(o-D3-toly1)- 1, 6-naphthyridin-5 (611)-one;
and, optionally, one or more of pharmaceutically acceptable salts, isotopes, N-
oxides,
solvates, and stereoisomers thereof; or
a compound selected from:
2 -(2-Chl oro- 6 -fluoroph eny1)-6-(4 -ethy1-3 -(hy droxym ethyl)-5 -oxo-4, 5 -
dihy dro- 1H-
1, 2, 4 -tri azol- 1 -yl)i soquinolin- 1(2H)-one;
6-(4-Ethyl-3 -(hydroxymethyl)-5 -oxo-4, 5 -di hy dro- 1H- 1, 2, 4 -tri azol- 1
-y1)-2-(2-fluoro-4-
nitropheny1)-4-iodoi soquinolin- 1(2H)-one;
2 -(2-Chl oro- 6 -fluoroph eny1)-7-(4 -ethy1-3 -(hy droxym ethyl)-5 -oxo-4, 5 -
dihy dro- 1H-
1, 2, 4 -tri az ol- 1 -y1)- 6-fl uoro-4 -(prop- 1 -en-2 -yl)phthal azin- 1(2H)-
one;
72
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
2-(2-Chloro-6-fluoropheny1)-7-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-6-methoxy-4-(prop-1-en-2-y1)phthalazin-1(2H)-one,
or a pharmaceutically acceptable salt, N-oxide, solvate, or stereoisomer
thereof.
103111 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) selected from the group consisting of:
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(o-toly1)isoquinolin-1(2H)-one;
2-(2-C hl oro-4-m ethyl pyri din-3 -y1)-6-(4-ethyl-3 -(hydroxy m ethyl)-5-oxo-
4, 5-di hy dro-
1H-1,2,4-triazol-1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1, 2,4-triazol-1-y1)-4-(1 -methyl cyclopropyl)i soquinolin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxyrnethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-7-fluoro-4-(prop-1-en-2-y1)phthalazin-1(2H)-one;
2-(4-Ethyl-3 -(hy droxym ethyl)-5 -oxo-4, 5 -di hy dro-1H-1,2,4-tri azol-1 -
y1)-3 -fluoro-8 -
isopropy1-6-(o-toly1)-1,6-naphthyridin-5(6H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(o-toly1)phthalazin-1(2H)-one;
2-(2-Chloro-6-fluoropheny1)-6-(4-ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-y1)-7-fluoro-4-isopropylphthalazin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropy1-2-(2-(methyl-d3)phenypisoquinolin-1(2H)-one;
(R)-6-(4-Ethyl-3 -(hydroxym ethyl)-5-oxo-4, 5-di hy dro-1H-1,2,4-tri azol -1 -
y1)-7-fluoro-
2-(o-toly1)-4-(1,1, 1 -trifluoropropan-2-yl)isoquinolin-1(2H)-one;
6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-IH-1,2,4-triazol-1-y1)-7-fluoro-
4-
isopropyl-2-(2-(trifluoromethyl)phenypisoquinolin-1(2H)-one; and
2-(2-(Difluoromethyl)pheny1)-6-(4-ethyl -3 -(hy droxym ethyl)-5 -oxo-4, 5 -di
hy dro-1H-
1,2,4-triazol-1-y1)-7-fluoro-4-isopropylisoquinolin-1(2H)-one;
and, optionally, one or more of pharmaceutically acceptable salts, isotopes, N-
oxides,
solvates, and stereoisomers thereof.
103121 In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) having the Formula (Za):
73
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
R1
Y R2
,
R4,,N
R3
0
(Za), wherein
Y is CH or N;
RI is selected from the group consisting of. C1_6alkyl; C1_6alkyl substituted
with OH, or
OCH3; C2_6alkeny1; Ch6haloalkyl; Ci_6haloa1kyl substituted with OH, or OCH3;
C2_6haloalkenyl; N(CH3)2; C3_6cycloalkyl; C3_6cyc1oalkyl substituted with
Ci_6alkyl; and
phenyl;
HO
N
N
\.N
R2 is 0
R3 is selected from the group consisting of: H, halo, CH3 and OCH3;
R4 is selected from the group consisting of:
C1_6alky1; Ci_6alky1 substituted with one or two OCH3; C3_6cyc1oalkyl;
C3_6cycloalky1
substituted with CH3, or OCH3; CH2-C3_6cycloalkyl; and
(R
Rd ;
(Rc
N Re\ Re N
N N N
Rd I I ,
Rd N Rd N Rd N
N
[I ,
and and
RG Rc
S
Rc¨<\ IRg¨N
-"-^ Rd ¨^ Rd .
N Rd , and
wherein
each It is independently selected from the group consisting of: H; halo;
Ci_6alkyl, Ci-
6alkyl substituted with a member selected from the group consisting of: OH,
OCH3,
SCH3, and OCF3; Ci_6haloa1kyl; Ci_6haloalky1 substituted with a member
selected
74
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
from the group consisting of: OH, and OCH3; NO2; OH; 0-CH2CH2OH; and OCi-
6alkyl;
Rd is selected from the group consisting of: H; halo; C1_6alkyl; Ci_6alkyl
substituted with
a member selected from the group consisting of: OH, OCH3, SCH3, and OCF3;
C1_6haloalkyl; Ci_6haloalkyl substituted with a member selected from the group
consisting of: OH, and OCH3; CN; and OCi_6alkyl;
Rg is selected from the group consisting of: H; Ci_6alkyl; Ci_6a1kyl
substituted with a
member selected from the group consisting of: OH, OCH3, SCH3, and OCF3; Ci-
6haloalkyl; and Ci_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and
n is 1, or 2;
or a pharmaceutically acceptable salt, solvate, stereoisomer, isotopic
variant, or N-oxide
thereof.
[0313] In an embodiment of the invention, the DHODH inhibitor is a compound of
Formula
(Z) having the Formula (Zb):
R1
:R2
N =*". ---
N
"I[ R3
0
(Zb), wherein
Y is CH or N:
RI is selected from the group consisting of: Ch6alkyl, C16haloalkyl and
C2_6alkenyl;
HO
N
N
R2 is 0 =
R3 is selected from the group consisting of: 1-1, halo and OCH3;
R4 is selected from the group consisting of:
(Rc RN
(Rc
Rc Rc Rc
I ,
Rd N
Rd N Ru N R'
ro " r -- µ-----",R d
Rc
Rg¨N
NRd, and Rd
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
wherein
RC is selected from the group consisting of: H, halo; C1_6alkyl, C1_6alkyl
substituted with
a member selected from the group consisting of: OH, OCH3, SCH3, and OCF3;
C 1_6haloalkyl; C 1_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and NO2;
Rd is selected from the group consisting of: H; halo; Ci_6alkyl; Ci_6alkyl
substituted with
a member selected from the group consisting of: OH, OCH3, SCH3, and OCF3;
C1_6haloalkyl; C1_6haloalkyl substituted with a member selected from the group
consisting of: OH, and OCH3; CN; and OCi_6alkyl;
Rg is selected from the group consisting of: H; Ci_6alkyl; C16alkyl
substituted with a
member selected from the group consisting of: OH, OCH3, SCH3, and OCF3; Ci-
6haloalkyl; and C1_6haloalkyl substituted with a member selected from the
group
consisting of: OH, and OCH3; and
n is 1;
or a pharmaceutically acceptable salt, solvate, stereoisomer, isotopic
variant, or N-oxide
thereof.
[0314] Exemplary compounds of Formula (Z) useful in methods of the invention
will now be
described by reference to the illustrative synthetic schemes for their general
preparation below
and examples that follow. Artisans will recognize that, to obtain the various
compounds herein,
starting materials may be suitably selected so that the ultimately desired
substituents will be
carried through the reaction scheme with or without protection as appropriate
to yield the
desired product. Alternatively, it may be necessary or desirable to employ, in
the place of the
ultimately desired substituent, a suitable group that may be carried through
the reaction scheme
and replaced as appropriate with the desired substituent. Unless otherwise
specified, the
variables are as defined above in reference to Formula (Z). Reactions may be
performed
between the melting point and the reflux temperature of the solvent, and
preferably between
0 C and the reflux temperature of the solvent. Reactions may be heated
employing
conventional heating or microwave heating. Reactions may also be conducted in
sealed pressure
vessels above the normal reflux temperature of the solvent.
[0315] All abbreviations used in the general schemes and examples for Formula
(Z) are as
defined in Table 1A. Variables are as defined in the scope or as specifically
defined in the
general Schemes.
76
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table lA ¨ Abbreviations
Abbreviation Name
A angstrom
ACN or MeCN acetonitrile
AcOH glacial acetic acid
AcOK Potassium acetate
AgBF4 Silver tetrafluoroborate
AlMe3 Trimethylaluminium
Ar Argon
aq. aqueous
AuC13 Gold(III) chloride
BC13 Boron trichloride
Bn or Bzl benzyl
Boc tert-butyloxycarbonyl
(Boc)20 Di-tert-butyl dicarbonate
Catacxium A Pd G2 AT Chi oro [(di (1 -adam
anty1)--butyl ph osphi ne)-2-(2-
aminobiphenyl)]palladium(1)
CdC12 Cadmium chloride
Celite di atom a ceous earth
conc. concentrated
CO2 Carbon dioxide
(C0C1)2 Oxalyl chloride
Cul Copper(1) iodide
Cu(OAc)2 copper(11) acetate
Cy2NMe N,N-Dicyclohexylmethylamine
Cs2CO3 Cesium carbonate
DCC N,N'-dicyclohexyl-carbodiimide
DCE dichloroethane
DCM dichloromethane
DIPEA or DIEA diisopropyl-ethyl amine
DEAD Diethyl azodicarboxylate
DEA Diethanolamine
DHP 3,4-Dihydropyran
DMA dimethylaniline
DMAP 4-dimethylaminopyridine
DME dimethoxyethane
DMf N,N-dimethylformamide
DMP
Dess¨Martin periodinane or 1,1,1-Tris(acetyloxy)-1,1-dihydro-1,2-
benziodoxo1-3-(11/)-one
DMSO dimethylsulfoxide
Dppf or DPPF 1, l'-Bis(diphenylphosphino)ferrocene
EDCI 1 -ethy1-3 -(3 -dimethylaminopropyl) carb odiimi
de
ES-API electrospray-atmospheric pressure ionization
77
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Abbreviation Name
ESI el ectrospray ionization
Et3N.3HF Triethylamine trihydrofluoride
Et0Ac or EA ethyl acetate
Et0H ethanol
Et0Na sodium ethoxide
EtMgBr Ethylmagnesium bromide
GCMS gas chromatography-mass spectrometry
h or hr(s) hour or hours
H2 Hydrogen gas
HC1 Hydrogen chloride
HPLC high performance liquid chromatography
HPA hypophosphorous acid
H2 S 04 Sulfuric acid
i-PrMgC1 Isopropylmagnesium chloride
IPA Isopropylamine
iPrOH Isopropyl alcohol or 2-propanol
isovaleraldehyde 3-methylbutanal
KT-11\41)S Potassium b i s(tri m ethyl si 1 yl) am i de
KI Potassium iodide
K20s04.2H20 Potassium osmate (VI) dihydrate
K2CO3 Potassium carbonate
K3PO4 Potassium phosphate
LCMS or LC/MS Liquid chromatography¨mass spectrometry
LiHMD S Lithium bis(trimethylsilyl)amide
Me0H methanol
MeMgBr methylmagnesium bromide
Mg(C104)2 Magnesium perchlorate
MgSO4 Magnesium sulfate
MHz megahertz
min minute or minutes
MS mass spectrometry
NaB H4 Sodium borohydride
NaHMDS Sodium bi s(trimethylsilyl)ami de
NaOH Sodium hydroxide
Na0Et Sodium ethoxide
NaHCO3 Sodium bicarbonate
Na2CO3 Sodium carbonate
NaI04 Sodium periodate
NaNO2 Sodium nitrite
Na2SO4 Sodium sulfate
N2 Nitrogen gas
78
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Abbreviation Name
NBS N-Bromosuccinimide
NC S N-chlorosuccinimide
NH2N112.H20 Hydrazine monohydrate
NIS N-iodosuccinimide
NH4C1 Ammonium chloride
NH4HCO3 Ammonium bicarbonate
NH3 H20 or NH4OH Ammonium hydroxide
NMR nuclear magnetic resonance
Allylchl oropalladi um dimer or Bi s(allyl)di chi orodipalladi um or
[Pd(ally1)C1]2 Bis(allyl)dichloropalladium or
Bis(allylchloropalladium) or
Diallyldichlorodipalladium
PdC12(PPh3)2 or
Pd(PPh3)2C12 bis(triphenylphosphine)palladium(II) dichloride
Pd(PPh3)4 tetrakis(triphenylphosphine)palladium
Pd(0Ac)2 palladium (II) acetate
P(tBu3)PdG2 or chi oroRtri -tert-butyl ph osph i n e)-2-(2-am i
n ob i ph eny1)]
tBu3PPdG2 palladium(II)
PE petrolum ether
PPh3 triphenylphosphine
ppm parts per million
Pd(dppf)C12 CH2C12 1,1! -bi s(diphenylphosphino)ferrocene -palladium(II)di chl
ori de
or Pd(dppf)C12.DCM dichloromethane complex
Pd/C Palladium on carbon
PG Protecting group
RP reverse-phase
rt or RT room temperature
Rt retention time
RhCl(PPh3)3 or
Rh(PPh3)3C1 Wilkinson's Catalyst or
Chlorotris(triphenylphosphine)rhodium(I)
Sec second or seconds
SFC supercritical fluid chromatography
5i02 silica gel
S0C12 Thionyl chloride
02 Oxygen gas
TBAB tetrabutylammonium bromide or tetra-n-
butylammonium bromide
TBDPS tert-Butyldiphenylchlorosilane
TBAF tetrabutylammonium fluoride
TBHP tert-butyl hydroperoxide
TBS tert-Butyldimethylsilyl
TES triethylsilane
TIPS triisopropylsilane
TEA or Et3N triethylamine
79
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Abbreviation Name
TFA trifluoroacetic acid
THE tetrahydrofuran
TiC14 Titanium tetrachloride
TLC thin layer chromatography
TF2NPh N-pheny lb i s(tri fluoromethan esufoni mi de)
triflate trifluoromethanesulfonyl
Tf20 Triflic anhydride or Trifluoromethanesulfonic
anhydride
TMS0iPr Is opropoxytri m ethyl silane
(PTSA or pTs0H) or
p-Toluenesulfonic acid
tosylic acid
PREPARATIVE EXAMPLES
[0316] Exemplary compounds of Formula (Z) useful in methods of the invention
will now be
described by reference to the illustrative synthetic schemes for their general
preparation below
and the specific examples to follow.
SCHEME 1
0
1.R3-NCO
0 NH2NH2+120 0
HNAN¨Ra
Bn0j-LOEt H20
N_NH2 _____________________________________________________
Et0H
2. Cyclization .. 0
(II) PG
[0317] According to SCHEME 1, a 1,2,4-triazol-5(4H)-one compound of formula
(II), where
PG is Bn, is prepared from ethyl 2-(benzyloxy)acetate in three steps. In a
first step 2-
(benzyloxy)acetohydrazide is prepared by the reaction of ethyl 2-
(benzyloxy)acetate with
hydrazine hydrate, in a suitable solvent such as Et0H, and the like; at
temperatures ranging
from 70-85 C. Reaction of the hydrazide with an isocyanate of formula Ra-NCO,
where Ra is
C1_6alkyl, in a suitable solvent such as water, and the like; provides the
corresponding
semicarbazide. Subsequent cyclization of the semicarbazide with a suitable
base such as NaOH,
in a suitable solvent such as water, provides a compound of formula (II),
where PG is Bn.
[0318] A compound of formula (II), where Ra is C1_6haloalkyl or
C3_6cycloalkyl; may be
prepared as previously described employing a suitably substituted compound of
formula Ra-
NCO, where Ra is Ci_6haloalkyl or C3_6cycloalkyl.
[0319] Protecting group exchange of a compound of formula (II), where PG is Bn
to a
compound of formula (II) where PG is TBDPS, is achieved in two steps employing
established
methodologies, such as those described in T. W. Greene and P. G. M. Wuts,
"Protective Groups
in Organic Synthesis,- 3 ed., John Wiley & Sons, 1999. In a first step,
deprotection of benzyl
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
group is achieved under hydrogenolytic conditions known to one skilled in the
art provides the
alcohol. For example, deprotection is achieved employing a palladium catalyst
such Pd/C, and
the like; under H2; in a suitable solvent such as Et0H, Me0H, Et0Ac, or a
mixture thereof,
preferably Et0H; with or without the presence HC1; for a period of 4 to 72
hrs. In a second step,
protection of the corresponding alcohol as the silyl ether, is achieved with
tert-
butyldiphenylsily1 chloride, a suitable base such as imidazole,
dimethylaminopyridine, pyridine,
and the like; in a solvent such as DMF, DCM, and the like; at temperatures
ranging from 0 C
to room temperature; affords a compound of formula (II) where PG is TBDP S.
SCHEME 2
HAL R1 Ri
Br Br Halogenation
HAL
I I Suzuki Coupling Coupling
NI I
HN 3 -B(OH)2 HN,Ir-R3 R1 HN
R R3 R4-B(OH)2 -1(
0 0 0 0
(XIV) (III) (IV)
(V)
[0320] According to SCHEME 2, a compound of formula (XIV), where R3 is H is
treated with
a halogenating reagent such as N-iodosuccinimide (NIS), and the like; in an
aprotic solvent
such as acetonitrile, and the like; under heating conditions, to afford the
halogenated compound
of formula (III), where HAL is iodide. A compound of formula R1-B(OH)2; is
reacted under
Suzuki coupling conditions known to one skilled in the art with a compound of
formula (III),
to provide a compound of formula (IV). For example, a compound of formula
(III), where HAL
is iodide, is reacted a commercially available or synthetically accessible
boronic acid (or
boronic ester) such as R1-B(OH)2, where Rl is an optionally substituted
C2_6alkenyl or aryl as
defined herein with reference to Formula (Z); a palladium catalyst such as
bi s(triphenylphosphine)pall adium(II) dichlori de, tetraki
s(triphenylphosphine)pall adium, and
the like; a suitable base such as potassium phosphate, Cs2CO3, and the like;
in a suitable solvent
such as dioxane, water, ethanol, or a mixture thereof; to provide a compound
of formula
compound (IV). A compound of formula (IV), where R3 is H, is reacted with a
compound of
formula R4-B(OH)2; under copper (11) mediated Chan-Lam coupling conditions
known to one
skilled in the art, to provide a compound of formula (V), where HAL is
bromide, X is CH and
R3 is H. For example, a compound of formula (IV) is reacted with a compound of
formula R4-
B(OH)2, where R4 is as defined herein with reference to Formula (Z); a
catalyst such as
copper(II) acetate, and the like; a base such as pyridine, NEt3, and the like;
in a suitable solvent
such as DCM, ACN, dioxane, THF, and the like; to afford a compound of formula
(V).
81
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 3
PG
0 j'Z'a
"Cd N R1 -N 0_ Ri Ri
HAL HN,N/)-----j PG
X X
NI (II) Deprotection
R3 R3 0 N
R3
R4 Coupling R4 R4
0 0
(V) (VI) (7)
[0321] According to SCHEME 3, Ullmann-type aromatic amination reaction of
compound of
formula (V), where R1 is optionally substituted C2_6alkenyl, R3 is H, R4 is
suitably substituted
phenyl as described herein with reference to Formula (Z), and HAL is Br; with
a commercially
available or synthetically accessible nucleophilic compound of formula (II),
where Ra is C1_
6a1ky1; such as suitably protected triazolones, where PG is selected from:
benzyl, 4-methoxy
benzyl, or an alkyl or aryl silane such as TBDPS, TBS, TES, or TIPS; in the
presence of
catalytic CuI and a diamine such as trans-1,2-diaminocyclohexane, and a base
such as K3PO4,
K2CO3, Cs2CO3, NaHCO3, triethylamine, and the like; in a suitable solvent such
as 1,4-dioxane,
DMSO, DMF, THF, ACN, and the like; provides a compound of formula (VI), where
X is CH
and Y is CH.
[0322] A compound of formula (VI), where PG is Bn and R1 is C2_6alkenyl, is
reacted under
Simmons-Smith cyclopropanation reaction conditions known to one skilled in the
art to provide
a compound of formula (VI) where RI is C3_6cycloalkyl substituted with
Ci_6alkyl. For example,
a compound of formula (VI), where R1 is
is reacted with diiodomethane; diethylzinc; in
a suitable solvent such as toluene, and the like; at temperatures ranging from
0 C to room
temperature; for a period of 3 to 26 h; to provide a compound of formula (VI),
where Rl is
cyclopropyl substituted with CH3.
[0323] Subsequent deprotection employing established methodologies, such as
those described
in T. W. Greene and P. G M. Wuts, "Protective Groups in Organic Synthesis," 3
ed., John
Wiley & Sons, 1999), provides a compound of Formula (Z), where X and Y are CH.
For
example, compound of formula (VI), where R3 is H, and PG is TBDPS, is
deprotected
employing conditions known to one skilled in the art, preferably with TBAF in
a suitable
solvent such as THF, and the like. In a preferred method, PG is TBDPS, and Ra
is C1_6a1kyl.
Alternately, removal of a TBDPS protecting group is achieved employing
triethylamine
trihydrogen fluoride (Et3N=31-IF).
[0324] Removal of the Bn protecting group is achieved in the presence of
hydrogen gas, in the
presence of a catalyst such as Palladium on carbon (Pd/C). Removal of the
protecting group
82
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Bn is also achieved employing TFA, at a temperature of about 80 C.
[0325] A compound of Formula (Z), where X is CH; Y is CH; R2, R3, R4 is each
defined as
described herein with reference to Formula (Z); and R4 is C2_6alkenyl, is
reduced
employing hydrogenation conditions known to one skilled in the art, for
example, reaction with
Pd/C or Wilkinson's Catalyst [RhCl(PPh3).3] under H2; in a suitable solvent
such as Me0H, THF,
Et0Ac, and the like; provides a compound of Formula (Z) where RI is C2_6alkyl.
SCHEME 4
HAL
R4
R3
00a R5
0
Reductive
Br ') Br (V)
Amination Cyclization
NH
R4¨N H2 R4' DMAP/NEt3 R4' N
R1
DCM 0
HAL
(VII) (VIII) (IX) 31(
R4 R3
0
(Va)
[0326] According to SCHEME 4, reductive amination of a compound of formula
(VII), with
oc, I3-unsaturated aldehyde such as 3-methyl-2-butenal, 3-methylpent-2-enal,
and the like;
employing TiC14; and a base such as triethylamine; in an aprotic solvent such
as
dichloromethane (DCM), and the like; provides an enamine intermediate which is
subsequently
reduced employing a reducing agent such as Nal3ILI, and the like; to afford a
compound of
formula (VIII) where R5 is C1_4alkyl, R4 is as defined herein with reference
to Formula (Z). A
compound of formula (VIII) is coupled with commercially available or
synthetically accessible
4-bromo-2-iodobenzoyl chloride employing a base such as triethylamine and 4-
dimethylaminopyridine (DMAP); in an anhydrous aprotic solvent such as
dichloromethane
(DCM), and the like; to afford a compound of formula (IX). Treatment of a
compound of
formula (IX), with palladium (II) acetate, tetrabutylammonium bromide, and
potassium acetate
under heating Heck reaction conditions, affords the intramolecular cyclized
compounds of
formula (V), wherein RI- is optionally substituted C2_6alkyl, R3 is H, X is
CH, and HAL is Br;
and (Va) wherein R1 is optionally substituted C2_6alkenyl, R3 is H, X is CH2,
and HAL is Br.
SCHEME 5
PG PG
PG
0 r
/¨
N3A N
ps
ty N¨R3 N¨R.
,O. (j Hydrolysis I Ai
Chlorination
I =
N
R5 if R3'o - HO IV 'Rs CI
R3
base, heating
(X) (YI) (X1a)
(XII)
83
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0327] According to SCHEME 5, the reaction of a commercially available or
synthetically
accessible compound of formula (X), where HAL is F, R3 is F, and R5 is H or
C1_4alkyl, with a
commercially available or synthetically accessible nucleophilic compound of
formula (II),
where IV is C1_6alkyl, such as suitably protected triazolones, where PG is
selected from: benzyl,
4-methoxy benzyl, or an alkyl or aryl silane such as TBDPS, TBS, TES, or TIPS;
in the presence
of a base such as K3PO4, K2CO3, Cs2CO3, NaHCO3, triethylamine, and the like;
in a suitable
solvent such as DMSO, DMF, THF, ACN, and the like; affords a compound of
formula (XI).
In a preferred method, PG is Bn, and IV is Ci_6alkyl. The ester of formula
(XI), when R5 is C1_
4a1ky1, is hydrolyzed to its corresponding acid, under acidic or basic
conditions. For example,
the treatment of tert-butyl ester (R5 is tert-Bu) with TFA; or alternately,
hydrolysis with a base
like NaOH, in an aqueous solvent, affords a compound of formula (XIa), where
R5 is H. A
compound of formula (Xla) is chlorinated, employing conditions known to one
skilled in the
art, to provide the acyl chloride of formula (XII). For example, a compound of
formula (XIa)
is heated in S0C12; or treated with oxalyl chloride in DCM
SCHEME 6
PG PG
PG
R5
/¨O
N
f N_Ra ,NH I N¨Ra
R4
(VIII) 1
Cyclization X
Cl 0 0
0
R3
Base R4 R3
0 0 0
(XII) (XIII)
(VI)
[0328] According to SCHEME 6, a compound of formula (XII), where R3 is H or F,
PG is Bn,
and Ra is C1-6alkyl; is reacted with a compound of formula (VIII), where R5 is
Ci_4alkyl,
employing a base such as a mixture of triethylamine (TEA) and 4-
dimethylaminopyridine
(DMAP); in an anhydrous aprotic solvent such as dichloromethane (DCM), and the
like; to
afford a compound of formula (XIII). A compound of formula (VI), where X is CH
and Y is
CH, is obtained by treatment of a compound of formula (XIII), where RI is
optionally
substituted C1_6alkyl as described herein with reference to Formula (Z); with
palladium (II)
acetate, tetrabutylammonium bromide, and potassium acetate under heating Heck
reaction
conditions, that affords a mixture of intramolecular cyclized compounds, which
is then
separated to isolate an intermediate compound where R1 is C2_6alkyl, and R3 is
H or F.
84
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 7
PG
PG
0 ,Ra _TO
PG N R8 HAL
1\1
FIN,/ NN¨
B
Halogenation
[ I
Coupling 0
__________________________________ HN
IR3 HNI,--,1
R, 0
HN,r--,R3
0 0 0
(XIV) (XV) (XVI)
[0329] According to SCHEME 7, Ullmann-type aromatic arnination reaction of a
compound of
formula (XIV), where R3 is H or F, with a compound of formula (II); such as
suitably protected
triazolones, where PG is selected from: benzyl, 4-methoxy benzyl, or an alkyl
or aryl silane
such as TBDPS, TBS, TES, or TIPS; according to methods previously described;
affords a
compound of formula (XV). In a preferred method, PG is Bn, and IV is
Ci_6alkyl. A compound
of formula (XV) is treated with a halogenating reagent such as N-
iodosuccinimide (NIS), and
the like; in an aprotic solvent such as acetonitrile, and the like; under
heating conditions; affords
a halogenated compound of formula (XVI), where Y is CH and HAL is iodide.
SCHEME 8
0
R5'0
R'
PG
0 0 =
R1 0,
R1 PG FzI N
0
1 Br (XVIla)
1 Br NH2NH2 H20 X
Br
(II) )1(1;111
P3 Coupling HN
R,
0 0"" I -k,= 0
(V) 0
(XVIII)
R5
0
(XVIIb)
[0330] According to SCHEME 8, compounds of formula (XVIIa) and (XVIIb) are
prepared
from 5-bromoi sob enzofuran- 1 , 3 -di one in two steps. 5 -B rom oi sob
enzofuran- 1,3 -di one is
reacted with a commercially available or synthetically accessible suitably
substituted alkyl
Grignard reagent such as i-PrMgC1, EtMgBr, and the like; in the presence of
CdC12; in aprotic
solvent like THF, and the like; followed by subsequent treatment with an
alkylating agent of
formula R5-I, where R5 is C1_4alkyl (such as iodomethane or iodoethane); in
the presence of
base like K2CO3, Cs2CO3, and the like; in a aprotic solvent such as DMF, DMSO,
and the like;
affords a mixture of regio-isomeric esters of formula (XVIIa) and (XVIIb),
where Rl is an
optionally substituted Ci_6alkyl. In a similar fashion, aryl Grignard reagents
may be used to
provide compounds of formula (XVIIa) and (VXIIb), where R1 is a suitably
substituted phenyl.
The regio-isomers of formula (XVIIa) and (XVIIb) are not separated but are
used directly and
converted into the corresponding phthalazinone (mixture). For example, a
mixture of formula
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(XVIIa) and formula (XVIIb) are treated with excess hydrazine; in a suitable
solvent such as
ethanol or methanol, at temperatures ranging from room temperature to 90 C,
for a period of
6 to 20 hours. The desired phthalazinone compound of formula (V) can be
readily separated
from the other regio-isomer by precipitation, crystallization, or purified by
flash
chromatography. Ullmann-type aromatic amination reaction of a compound of
formula (V),
with a suitably protected triazolone of formula (II), where Ra is Ci_6a1ky1,
and PG is selected
from: benzyl, 4-methoxy benzyl, or an alkyl or aryl silane such as TBDPS, TBS,
TES, or TIPS;
in the presence of catalytic CuI and a diamine such as trans-1,2-
diaminocyclohexane, and a
base such as K3PO4, K2CO3, Cs2CO3, NaHCO3, triethylamine, and the like; in a
suitable solvent
such as I,4-dioxane, DMSO, DMF, THE, ACN, and the like; affords a compound of
formula
(XVIII), where X is N.
[0331] A compound of formula (XVIII), where RI is C2_6a1keny1, is reacted
under Simmons-
Smith cyclopropanation reaction conditions known to one skilled in the art, to
provide a
compound of formula (XVIII) where RI is C3_6cyc10a1ky1 substituted with
Ci_6alkyl. For
example, a compound of formula (XVIII), where R1 is C2_6alkenyl, is reacted
with
diiodomethane; diethylzinc; in a suitable solvent such as toluene, and the
like; at temperatures
ranging from 0 C to room temperature; for a period of 24 to 26 h; to provide
a compound of
formula (XVIII), where RI is cyclopropyl substituted with CH3.
SCHEME 9
R PG
N
0 HN_N----j PG R1 R1 Ra 121
I HAL HAL (ii)
0 HAL sl\IHNH2> X I
n I I
rz, 0
o Couphng
Rs-' R3 Coupling Rs- y 12' 11 R3
R4
0 0 0
(X) (XIX) (V)
(VI)
[0332] According to SCHEME 9, a compound of formula (X), where HAL is Br, R3
is H, and
R5 is CH3, is coupled in a palladium catalyzed carbonylation reaction with a
commercially
available or synthetically accessible aldehyde of formula RI-CHO, where RI is
C1_6alkyl; to
afford the corresponding ketone compound of formula (XIX), (similar
transformation has been
reported by Suchand et a1õ/. Org. Chem. 2016, 81, 6409-6423). For example,
reaction of
methyl 4-bromo-2-iodobenzoate with isobutylaldehye; in the presence of a
palladium catalyst
such as Pd(OAc)2; Ag2O; and an oxidizing agent such as aqueous solution of
tert-butyl
hydroperoxide (TBHP); at a temperature of about 120 C; for a period of 10-14
h; provided
methyl 4-bromo-2-i sobutyrylbenzoate. A ketone compound of formula (XIX) is
reacted with
hydrazine le-NHNH2, where R4 is suitably substituted aryl such as 2-chloro-6-
86
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
fluorophenylhydrazine; to afford a compound of formula (V), where X is N.
Ullmann-type
aromatic amination reaction of a compound of formula (V) with a suitably
protected triazolone
(II) as previously described, affords a compound of formula (VI), where Y is
CH, and R1 is
selected from C1-6a1kyl.
[0333] A compound of formula (VI), where Y is CH, and R1 is phenyl and X is N,
may be
prepared in a similar fashion, employing methods previously describe by
coupling methyl 4-
bromo-2-iodobenzoate with a commercially available or synthetically accessible
aldehyde of
formula RI--CHO, where Rl is phenyl.
SCHEME 10
PG Suzuki Coupling
PG
r
/¨(5PG
R1-B(OH)2
R1 R1
HAL N¨Ra
N¨R'
1. Suzuki (R1= alkenyl) Coupling
I I 0 2. Oxidation I
.R3 0 R4-B(CH)2
0
HN, R4 R3
11 3. Grignard 0 0
(xviii) (VI)
(xvi) HH(cH3)2. H20
Heat
[0334] According to SCHEME 10, a compound of formula R'-B(OH)2; is reacted
under Suzuki
coupling conditions known to one skilled in the art, with a compound of
formula (XVI), to
provide a compound of formula (XVIII), where Xis CH. For example, a compound
of formula
(XVI), where Y is CH and HAL is iodide, is reacted a commercially available or
synthetically
accessible boronic acid (or boronic ester) such as R1-B(OH)2, where Rl is an
optionally
substituted C2_6alkenyl, C3_6cycloalkyl or aryl as defined herein with
reference to Formula (Z);
a palladium catalyst such as bis(triphenylphosphine)palladium(II) dichloride,
and the like; a
suitable base such a potassium phosphate, Cs2CO3, and the like; in a suitable
solvent such as
dioxane, water, ethanol, or a mixture thereof; to provide a compound of
formula compound
(XVIII), where X is CH. It has been noticed that during the coupling reaction
as described
above, loss of the iodide during the reaction conditions afforded a compound
of formula
compound (XVIII), where X is CH, and RI- is H. A compound of formula (XVIII),
where X is
CH or N, is reacted with a compound of formula R4-B(OH)2; under copper (II)
mediated Chan-
Lam coupling conditions known to one skilled in the art, or as previously
described, to provide
a compound of formula (VI), where X is CH or N, 11.1 is optionally substituted
C2_6alkeny1, R3
is H or F, and R4 is a suitably substituted phenyl as described herein with
reference to Formula
(Z).
[0335] A compound of formula (XVIII), where R1 is N(CH3)2 is prepared from a
compound of
87
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
formula (XVI), where HAL is Br and PG is Bn. Reaction of a compound of formula
(XVI) with
an amine bs such as NH(CH3)2 in water, at a temperature of about 110 C, for a
period of 96
hours h; affords a compound of formula (XVIII) where R1 is N(CH3)2, and IV is
Ci_6alkyl. A
compound of Formula (Z), where R1 is N(CH3)2 is prepared according to methods
described
above.
[0336] A compound of formula (XVIII), where R1 is Ci_6alkyl substituted with
OH, is prepared
from a compound of formula (XVIII), where R1 is C2_6alkeny1, and PG is Bn in
two steps. In a
first step, reaction of a compound of formula (XVIII), where R1 is
, under oxidizing
conditions such as NaI04, and K20s04.2H20 or 0s04; in a suitable solvent such
as THF/H20;
at temperatures ranging from 0 C to room temperature; for a period of 48 to
72 hours; affords
a ketone intermediate compound. In a second step, reaction of the ketone
intermediate
compound with a Grignard reagent such as methylmagnesium bromide; in a
suitable solvent
such as diethyl ether; at temperatures ranging from 0 C to room temperature;
for a period of 3
to 30 hours; affords a compound of formula (XVIII), where le is C1_6alkyl
substituted with OH.
SCHEME 11
0-1f
,F
R4
NH2NH ¨R4 1-IN¨N
Base Hy Tf2
N
R3
R4 R3 Base R4
R3 0 0 0 0
(XX) (XXI)
(XXII)
[0337] According to SCHEME 11, 4,5-difluorophthalic anhydride is reacted with
a hydrazine
compound of formula R4-NHNH2, where R4 is a suitably substituted phenyl or
heteroaryl such
as (2-chloro-6-fluorophenyl)hydrazine hydrochloride; in acetic acid; at a
temperature of about
125 C; for a period of about 1.5 h to afford a compound of formula (XX),
where R3 is F.
Rearrangement of a compound of formula (XX) affords a ring expansion compound
of formula
(XXI), under basic conditions such as sodium ethoxide or sodium methoxide; in
a protic solvent
such as ethanol, methanol, and the like; at room temperature; for a period of
about 1.5 h.
Derivation of a compound of formula (XXI), with a sulfonate-based leaving
group such as
trifluoromethanesulfonyl (triflate), is achieved by is by reaction with a
triflating agent such as
trifluoromethanesulfonic anhydride (Tf20), a base such as triethylamine (TEA),
pyridine, and
the like, in a suitable solvent such as DCM and the like, to provide a
compound of formula
(XXII). Milder triflating agents such as N-
phenylbis(trifluoromethanesufonimide) (TF2NPh),
a base such as TEA, DIEA, and the like, in a suitable solvent such as DCM, and
the like; may
be used.
gg
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 12
PG
N"-Cd
N "a
)r,1
0
N N 3
D
PG R4 '`
OTf Ri
HN,
0
(II)
Suzuki Coupling x HAL
(VI) +
R3
R3 R1-B(OH)2
R4 R4 Base
0 0 R1
R2
(XXII) (V) X
,PG
0
R4 N I
0 .R'
0
(Via)
[0338] According to SCHEME 12, a compound of formula R1-B(OH)2; is reacted
under Suzuki
coupling conditions previously described, with a compound of formula (XXII),
to provide a
compound of formula (V), where X is N. For example, a compound of formula
(XXII), is
reacted a commercially available or synthetically accessible boronic acid (or
boronic ester) such
as R'-B(OH)2, where R" is C2_6alkenyl or C2_6haloalkenyl as defined herein
with reference to
Formula (Z); a palladium catalyst such as 1, l'-b i s(di phenyl ph osphi n
o)ferrocene-
palladium(II)dichloride or bis(triphenylphosphine)palladium(II) dichloride,
and the like; a
suitable base such a potassium phosphate, Cs2CO3, K2CO3, and the like; in a
suitable solvent
such as dioxane, water, ethanol, or a mixture thereof; to provide a compound
of formula
compound (V). A compound of formula (V), where RI- is C2_6alkyl or
C2_6haloa1kyl, is readily
prepared by selective hydrogenation of a compound of formula (V), where It' is
C2_6alkenyl or
C2_6haloalkenyl. For example, reaction of a compound of formula (V), where It'
is
F
F , under hydrogenation conditions employing a catalyst such as Pd/C and the
like,
in a suitable solvent such as Et0Ac, and the like; under an atmosphere of
hydrogen gas (20-45
psi) at room temperature; for a period of 4 to 24 hours; affords a compound of
formula (V),
, or
F
where R1 is F F. The reaction of a compound of formula (V),
with a suitably
protected triazolone of formula (II), employing conditions previously
described, affords a
mixture of compounds of formula (VI) and (VIa) which can be separated before
or after
deprotection of the protecting group.
89
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 13
Rd Rd PG
PG
PG '
/¨ci
R1 N--=-Cci
F NO2 RI N=-A/¨C)
N-R" RI
i
N¨Rd
(XXIII) Rd X"' All N.--1(
Reduction Rd X
N -_.,.
irp, 0\ip
o
(IP o R3
HN --' R3
Y- Fe base I
0
0 H2N
02N.- IR' (XXV)
(XVIII) Re (XXIV)
PG
RI '
N1,----00
isii N-Rd
Diazotization Rd
R3
Reduction l'
0
Re (XXVI)
[0339] According to SCHEME 13, N-arylation of a compound of formula (XVIII) is
achieved
by reaction of suitably substituted commercially available or synthetically
accessible fluoro
compound of formula (XXIII), where R' and Rd are as defined herein with
reference to Formula
(Z). A compound of formula (XVIII), where R1 is H, C2_6alkenyl , C2_6hal oal
kenyl, C3-
6cycl oalkyl, C3-6cycl alkyl substituted with C1-6a1ky1, and X is CH or N, is
reacted under
nucleophilic displacement reaction conditions, with a commercially available
or synthetically
accessible fluoro compound of formula (XXIII); in the presence of a base like
K2CO3, Cs2CO3,
and the like; in aprotic solvent such as DMF, DMSO, and the like; at
temperatures ranging from
65 to 100 "V; to afford a compound of formula (XXIV).
[0340] Reduction of compound of formula (XXIV) is achieved employing zinc or
iron and
NH4C1; in a mixed solvent of methanol and water; to provide an amino compound
of formula
(XXV).
[0341] Di azotizati on of a compound in formula (XXV) with NaNO2; in an acidic
aqueous
solution or other nitrite reagents; in an organic solvent, such as Et0H, and
the like; at a
temperature of 0 C; and subsequent the reduction of diazo group with zinc at
temperatures
ranging from 0 to 85 C; or by treatment with H3P02; affords a compound of
formula (XXVI),
where R and Rd are as defined as described herein with reference to Formula
(Z).
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 14
Rib Rla
HN
N-Ra PG
RR' RtRIa
N=Cd
Br 0 a
I HAL (XXV F N-R
I)
.HAL (II) 1"0 " Gyclization
PG
Base 0 R3 0
4111111VIP 123
R1
N
N-Re
0 (XXVIII) (XXIX)
"
(X) PG
R3 0
¨rd 0
0
(XXX)
H0
R
Cy2N Me
0
(X la) Getecxiurn A Pd
G2
[0342] According to SCHEME 14, a compound of formula (X), where HAL is F, R5
is H and
R3 is F, is reacted with a commercially available or synthetically accessible
compound of
formula (XXVII), where Ria and Rib are each independently H or Ci_4alky1, such
as 1-bromo-
3-methy1-2-butene; in the presence of a base such as K2CO3, Cs2CO3, and the
like; in a suitable
solvent such as DMSO, DIvIIF, THF, ACN, and the like; to afford an ester
compound of formula
(XXVIII), where R3 is F, and HAL is F. A compound of formula (XXVIII), where
R1a and Rib
are each independently selected from Ci_4haloalkyl or C3_6cycloalkyl may be
made in a similar
fashion. The reaction of an ester of formula (XXVIII) with a suitably
protected triazolone
compound of formula (11); in the presence of a base such as K3PO4, K2CO3,
Cs2CO3, NaHCO3,
triethylamine, and the like; in a suitable solvent such as 1,4-dioxane, DMSO,
DMF, THF, ACN,
and the like; affords a compound of formula (XXIX). In a preferred method, PG
is Bn, and Ra
is Ci_6alkyl. A compound of formula (XXIX), where R3 is H or F, undergoes
intramolecular
cyclization under Heck reaction conditions, such as employing at catalyst such
as chloroRtri-
tert-butylphosphine)-2-(2-aminobi phenyl)] palladium (II) (P(tBu3)PdG2), N-
cycl oh exyl -N-
methyl-cyclohexanamine, in a suitable solvent such as toluene, and the like;
at a temperature of
about 15 to 80 C; for a period of about 18 to 36 hours; to provide an
isocoumarin compound of
formula (XXX), where Y is CH and R1 is isopropyl, R3 is H or F, Ra and PG are
defined as
described above.
[0343] An isocoumarin compound of formula (XXX), where Ri is ¨ is prepared
from a
compound of formula (XIa) and methylbuta-1,2-dien-l-y1 acetate. Methylbuta-1,2-
dien-l-y1
acetate is commercially available or prepared in two steps from 2-methyl-3-
butyn-2-ol. Acetic
anhydride is reacted with 2-methyl-3-butyn-2-ol, in the presence of a catalyst
such as
Mg(C104)2; in a suitable solvent such as DCM, and the like; to afford 2-
methylbut-3-yn-2-y1
acetate. 2-Methylbut-3-yn-2-y1 acetate is reacted with a catalytic amount of a
Lewis acid such
91
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
as AgBF4, AgC104, PtC12, and the like; to provide 3-methylbuta-1,2-dien-1-y1
acetate. 3-
Methylbuta-1,2-dien-1-y1 acetate is coupled with a compound of formula (XIa),
where IV is H,
employing intermolecular cyclization under Heck reaction conditions as
previously described,
such as employing at catalyst such as Catacxium A Pd G2, and Cy2NMe palladium
(II) acetate,
phase transfer reagent like tetrabutylammonium bromide, and a base like
potassium acetate, in
a suitable solvent such as DMF, and the like; at a temperature of 70 to 90 C;
for a period of 10
to 16 hours; to provide the isocoumarin compound of formula (XXX), where Y is
CH and le
is
[0344] A compound of formula (XXX), where Y is CH and is -- is selectively
reduced
under hydrogenation conditions employing at catalyst such as Wilkinson's
Catalyst
[RhCl(PPh3)3] and the like, in a suitable solvent such as THF, and the like;
at room temperature,
provide an isocoumarin compound of formula (XXX), where It1 is isopropyl.
SCHEME 15
0 HO
0\
OEt
Wittig Reaction Reduction Oxidation
0
0
[0345] According to SCHEME 15, 2-butanone is converted to ethyl 3-methylpent-2-
enoate
employing Wittig reaction conditions known to one skilled in the art. For
example, 2-butanone
is reacted with a triphenyl phosphonium ylide such as (carbethoxymethylene)
triphenylphosphorane, with or without an additive such as benzoic acid, LiC1,
and sodium
dodecyl sulfate (SDS), and the like, in a suitable solvent such as toluene, at
temperatures
ranging from rt to the reflux temperature of the solvent, for a period of 12-
24 h. Ethyl 3-
methylpent-2-enoate is reduced to 3-methylpent-2-en-1-ol employing a suitable
reducing agent
such as DIBAL-H, in a suitable solvent such as toluene, and the like, at
temperatures ranging
from -78 C to room temperature. 3 -Methylpent-2-en-l-ol is oxidized to 3-
methylpent-2-enal
employing oxidation conditions known to one skilled in the art, for example,
DMP (Dess-
Martin periodinane), S03-pyridine, Swern conditions RCOC1)2, DMSO, Et3IN],
PCC, and the
like, in a solvent such as Et0Ac, DMSO, DCM, and the like, at temperatures
ranging from
about -78 C to room temperature (about 23 C). In a preferred method, 3-
methylpent-2-en-1-
ol is oxidized to 3-methylpent-2-enal with Dess-Martin periodinane, in DCM, at
25 C for a
period of 1-4 h.
92
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 16
PG
PG 0
d
R1 N%----C
Ri N ------ 1 N¨Ra
1 N¨Ra R4¨N H2
I , I , (VII)
__________________________________________________ ..-
Oy----.õ-_,----- R3 0 Lewis acid R4-N-TIR 03
0
0
(XXX) (XXXI)
103461 According to SCHEME 16, an isocoumarin of compound of formula (XXX),
where Y
is CH, is reacted with a commercially available or synthetically accessible
amine compound of
formula R4-NH2, where R4 is as defined herein with reference to Formula (Z); a
Lewis acid
such as like AlMe3, A1C13, and the like; in a suitable aprotic solvent such as
DCM, toluene, and
the like; to provide a compound of formula (XXXI), where Y is CH, and le, R3,
R4 and IV are
defined as described herein with reference to Formula (Z).
SCHEME 17
PG
1. sea, j/ ________ N 0_
A
N---=1\
.- i F HN,N----/ PG \ i N¨Ra
R
R5
'ClYR3 X 0 3 __________________
R3
base, heating
0 HO = 0
(X) 0
(XXXII!)
(XXXIV)
PG PG
0 0'
4
N --,----C R1 N N¨Ra
1 iK,_,
Lewis acid NI N-,\_c
Cycliz ...e¨Raation i ..- õ 0 0
Rearrangement `-' R3
0 0
(XXXV) (XXX)
103471 An isocoumarin compound of formula (XXX) may be prepared according to
SCHEME
17. 4,5-Difluoro-2-iodobenzoyl chloride is prepared from a compound of formula
(X), where
HAL is F, R5 is H and R3 is F, employing conditions known to one skilled in
the art such as
oxalyl chloride or thionyl chloride, in the presence of a catalytic amount of
DMF, in a suitable
solvent such as an aprotic non-polar solvent such as dichloromethane (DCM),
tetrahydrofuran
(THF), acetonitrile (ACN), toluene, and the like, at a temperatures ranging
from 0 C to room
temperature to form 4,5-difluoro-2-iodobenzoyl chloride. 4,5-Difluoro-2-
iodobenzoyl chloride
may be reacted with commercially available or synthetically accessible 2-
methylbut-3-yn-2-ol,
93
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
in the presence of a base such as triethylamine and DMAP; in a suitable
solvent such as DCM,
and the like, to afford an ester compound of formula (XXXIII). A compound of
formula
(XXXIII) may be reacted with a compound of formula (II), employing methods as
previously
described to afford a compound of formula (XXXIV). Treatment of a compound of
formula
(XXXIV) with a catalytic amount of a Lewis acid such as AgC104, PtC12, and the
like; may
afford the rearranged compound of formula (XXXV). A compound of formula
(XXXV), where
R3 is H or F, may undergo an intramolecular cyclization under Heck reaction
conditions, such
as employing a catalyst such as palladium (II) acetate, a phase transfer
reagent like
tetrabutylammonium bromide, and a base like potassium acetate, in a suitable
solvent such as
DMF, and the like; at a temperature of 70 to 90 C; for a period of 1 to 3
hours; to provide an
isocoumarin compound of formula (XXX), where Y is CH.
SCHEME 18
PG
o N=C6
ci y rj N--F PG
HN PG
,N HALNJ 0
IN=4,,
R- Coupling
\(j
0 R5 (XXXIX) EtO15-0 0
(Xa) Suzuki R5
R3
Coupling
Or PG (XL)
N-IR'
N-Ac
0
R3
R5
(XI)
103481 According to SCHEME 18, a compound of formula (Xa), where HAL is Cl, R5
is
CH(CH3)2, and R3 is F, is commercially available or synthetically accessible
according to
methods as described in Chen, et al, US Patent Publication No. US2016-0176869.
Reaction of
a compound of formula (Xa) with a commercially available or synthetically
accessible
nucleophilic compound of formula (II), where PG is benzyl, and Re is
C1_6alkyl; in the presence
of a base such as K2CO3, Cs2CO3, NaHCO3, triethyl amine, and the like; in a
suitable solvent
such as dimethylsulfoxide (DMSO), DMF, THE', ACN, and the like; affords a
compound of
formula (XXXIX), where Y is N. A compound of formula (XXXIX) or formula (XI),
where R3
is F, and R5 is Ci_4alkyl; is reacted a commercially available 1-ethoxyethene-
2-boronic acid
pinacol ester, a palladium catalyst such as
bis(triphenylphosphine)palladium(II) dichloride,
1,11-bis(diphenylphosphino)ferrocene-palladium(II)dichloride and the like; a
suitable base such
as Cs2CO3, and the like; in a suitable solvent such as dioxane, water,
ethanol, or a mixture
thereof; employing conventional or microwave heating; to provide a compound of
formula (XL),
94
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
where Y is N or CH.
SCHEME 19
PG ci PG PG
] N-R
R4-NH2 Et0, Y=--
/-0
AcOH N=id
.
1
Et0,,,,-1/.., ,N-_,\ (VII) Y L1N-Ra
I 0 Al(CH3)3 ,
H ,,,Y.,..N.INI-Ra or TFA
1 r 1
zØ.1.R3 0 Heating N --,_õ-, 0
R`I-NR3
R5 DCM R4- - R3
0 0 0
(XL) (XLI) (XLII)
PG PG
ci d
HAL N=-/
R1 N=-
/¨
I NR' \1 1 1-s
" X Th--1
Halogenation l', N ...., \, R1-B(OH)2 N1 R
-
1 ,-- -1 ---. ' -1
N, -. 0 Suzuki Coupling rj , I 0
R4- R3 ---,-..,----.. 3
R4- Tr R
o o
(xvi) (vi)
[0349] According to SCHEME 19, a compound of formula R4-NH2, where R4 is as
defined
herein with reference to Formula (7); is reacted with trimethyl aluminum; in a
suitable solvent
such as dichloromethane, toluene, or a mixture thereof; the resulting solution
is combined with
a compound of formula (XL), where Y is CH or N; to provide a compound of
formula (XLI).
A compound of formula (XLI), where Y is CH or N, is treated with acetic acid
or trifluoroacetic
acid under heating conditions between 50 C to 90 C, to provide a compound of
formula (XLII).
A compound of formula (XLII) is halogenated employing N-bromosuccinimide in
anhydrous
dimethylformamide at room temperature, to provide a compound of formula (XVI),
where HAL
is Br. A compound of formula R1-B(OH)2; is reacted under Suzuki coupling
conditions known
to one skilled in the art, or as previously described with a compound of
formula (XVI), to
provide a compound of formula (VI), where R1 is an optionally substituted
C2_6alkenyl, C2-
6ha1oa1keny1, or aryl as defined herein with reference to Formula (Z). A
compound of formula
(VI), where R1 is an optionally substituted C2_6alkenyl or C2_6haloalkenyl is
reacted under
hydrogenation conditions using Wilkinson catalyst ((PPh3)3RhC1) to provide a
compound of
formula (VI), where R1 is C2_6alkyl or C2_6haloalkyl.
SCHEME 20
PG
PG PG
R1
N=Cd
1. R4-NH2 i
N-Ro
N=--Cd RI N=id
I N-Ra ________ 1 y ij N¨Ra mi ,_ 1
1 R4
CI -)1-_, N -1
0
2. AcOH -- 1r --'----1R3 [Pd(ally1)C1]2, DPPF
- r'i"
0
R3 0
0 0 (VI)
R5
(xxxix, (xxx)
[0350] According to SCHEME 20, 3-methylbutanal is reacted with a compound of
formula
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
(XXXIX), where Y is N and R5 is CH(CH3)2, with a palladium catalyst such as
allylpalladium(II) chloride dimer, and the like; a ligand such as 1,1'-
bis(diphenylphosphino)ferrocene (dppf), and the like; a suitable base such as
Cs2CO3, and the
like; in the presence of water scavenger such as molecular sieve (4A); in a
suitable solvent such
as dioxane thereof; to provide a compound of formula compound (X)0C), where Rl
is isopropyl.
A compound of formula R4-NH2, where R4 is as defined herein with reference to
Formula (Z);
is reacted with trimethyl aluminum; in a suitable solvent such as
dichloromethane, toluene, or
a mixture thereof; the resulting solution is combined with a compound of
formula (XXX),
followed by subsequent treatment with acetic acid under heating temperature of
80-100 C for
a period of time ranging from 5 to 24 hours; to provide a compound of formula
(VI); where X
is CH, Y is N, R1 is isopropyl, R3 is F.
SCHEME 21
PG
N----f 6
NI -Re
I õ,,r.N -....,\(\
/-01:'G
,¨ CPG
N=--- N ----X
R5 II (i)-5<-
o (XI)
\I
PG "-----õ,z, B-0 \ ...:7..--õ,_,,õYõ,,,,N --_, K20s04. 0)'12 -
I 4
or R5 N,--id
Suzuki -R o Naio
3 R5 ,fly-^-
..õ---R3
Coupling
1 N-Re 0 0
CI _,,
(XLIII)
(XLIV)
I 0
R50
(XXXIX)
po PG
R1 N\ R1 R1 N=CO
1. 121-MgBr .. 0 1 --' A\ o NI-I2NH-R4 o --- 3
2. Dess-Martin Fzcz' 1.1"-----------R 0
0 0
(XLV) (VI)
I1. Stille Coupling
2. Hydrolysis
PG
N=C6
I N-
CI ...,y,õN,..4
0 `.... 0
F
0
R5
(XXXIX)
103511 According to SCHEME 21, A compound of formula (XXXIX) or formula (XI),
where
R3 is F, and R5 is CI-alkyl; is reacted a commercially available vinylboronic
acid pinacol ester;
a palladium catalyst such as bis(triphenylphosphine)palladium(II) dichloride,
or 1,1-
96
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
bis(diphenylphosphino)ferrocene-palladium(II)dichloride dichloromethane
complex, and the
like, a suitable base such as Cs2CO3, and the like, in a suitable solvent such
as dioxane, water,
ethanol, or a mixture thereof; to provide a compound of formula compound
(XLIII), where Y
is N or CH. The vinyl group in a compound of formula (XLIII) is selectively
converted into an
aldehyde group of formula (XLIV) employing potassium osmate (VI) dihydrate/
sodium
periodate, or ozonolysis, and the like. A compound of formula (XLIV) is
reacted with a
commercially available or synthetically accessible suitably substituted alkyl
Grignard reagent
such as i-PrMgC1, and the like; in aprotic solvent like THF, and the like;
followed by subsequent
treatment with an oxidizing reagent such as Dess-Martin reagent, or Swern
oxidation conditions,
and the like; to afford a ketone compound of formula (XLV).
[0352] A compound of formula (XLV) is prepared from a compound of formula
(XXXIX) in
two steps. A compound of formula (XXXIX), where R3 is F, and R5 is Ch4alkyl;
is reacted a
commercially available tributy1(1-ethoxyvinyl)tin; a palladium catalyst such
as
bis(triphenylphosphine)pall adium(II) di chl oride, 1, 1Lb i
s(diphenylphosphino)ferrocene-
palladium(II)dichl oride and the like; in a suitable solvent such as dioxane,
water, ethanol, or a
mixture thereof Subsequent acidic hydrolysis employing conditions such as
treatment with
aqueous HC1 solution at room temperature affords a compound of formula (XLV),
where X is
N, Y is N or CH, RI is methyl.
[0353] A commercially or synthetically available hydrazine R4-NHNH2, where R4
is as defined
herein with reference to Formula (Z), such as 2-chloro-6-
fluorophenylhydrazine, o-
tolylhydrazine; is condensed with a compound of formula (XLV); in the presence
of a base such
as potassium carbonate, and the like; under the heating conditions such as 70-
120 C; in a
suitable solvent such as toluene, or a mixture thereof; afford a compound of
formula (VI), where
X is N, Y is CH or N, and R4 is as defined herein with reference to Formula
(Z).
SCHEME 22
PG
GC)
HN N Re 0 ,R 0, tr
N N 0_
PG ,0 PG R4-NH2
Br,
(II) PG Br HO T.J.r,
11
, F
[Pd(elly1)CIL DPPF CLIf AcOH -
R3
8- Base No molecular 0 0
(XLVI) Sieve (XLVII) (VI)
[0354] According to SCHEME 22, the reaction of methyl 2-bromo-4,5-
difluorobenzoate with
a suitably protected triazolone compound of formula (II); in the presence of a
base such as
K3PO4, K2CO3, Cs2CO3, NaHCO3, triethylamine, and the like; in a suitable
solvent such as 1,4-
dioxane, DMSO, DMF, TifF, ACN, and the like; affords a compound of formula
(XLVI). In a
preferred method, PG is Bn, and R" is C1_6alkyl (as previously described in
Scheme 14). 3-
97
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Methylbutanal is reacted with a compound of formula (XLVI), with a palladium
catalyst such
as allylpalladium(II) chloride dimer, and the like, a ligand such as 1,1'-
bis(diphenylphosphino)ferrocene (dppf), and the like; a suitable base such as
Cs2CO3, and the
like; in the absence of water scavenger such as molecular sieve (4A); in a
suitable solvent such
as dioxane thereof; to provide a compound of formula compound (XLVII). A
compound of
formula le-NH2, where R4 is as defined herein with reference to Formula (Z);
is reacted with
trimethyl aluminum; in a suitable solvent such as dichloromethane,
dichloroethane, toluene, or
a mixture thereof; the resulting solution is combined with a compound of
formula (XLVII),
followed by subsequent treatment with acetic acid under heating temperature of
80-100 C for
a period of time ranging from 5 to 24 hours; to provide a compound of formula
(VI); where X
is CH, Y is CH, R1 is isopropyl, R3 is F. In certain cases, a compound of
formula R4-NH2 such
as o-toluidine and the like; is directly condensed with a compound of formula
compound
(XLVII) in acetic acid under heating temperature of 80-100 'V for a period of
time ranging
from 10 to 24 hours; to provide a compound of formula (VI); where X, Y,
R3 are defined
above.
SCHEME 23
PG PG
PG
N=C N==c
N=id
C I N N¨Ra N¨Ra
N ¨Ra
1.Sonogoshira AuCll
0 0
2. TBAF MeCN 0
0
R5 R5
(XXXIX) (XLVI I I) (XLI X)
PG
/¨
N=Cd
N
1. R PG 4-NH2 N¨Ra R1
(VII)
X 1 . Halogenation
X
2. AcOH (j2. Coupling
NI
0
R4
0 0
(L) (VI)
[0355] According to SCHEME 23, a compound of formula (XXXIX), where R5 is
Ci_4alkyl, Y
is N, and R3 is F, is subjected to a Sonogashira coupling reaction with a
silyl protected alkyne,
such as trimethyl silyl acetyl ene, a palladium
catalyst such as
palladium(11)bis(triphenylphosphine) dichloride and the like; a copper
catalyst such as copper
iodide and the like; with a suitable base, such as triethylamine; in a
suitable solvent such as
ACN, toluene, and the like. Deprotection reaction employing TBAF in a suitable
solvent such
as THF, and the like; at room temperature affords a compound of formula
(XLVIII). A
98
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
compound of formula (XLIX) is obtained using a gold catalyst, preferably AuC13
in a suitable
solvent mixture, such as MeCN. Similar transformation by AuC13-catalyzed
cyclization has
been described by Marchal, E. et al in Tetrahedron 2007, 63, 9979-9990. A
compound of
formula R4-NH2, where R4 is as defined herein with reference to Formula (Z);
is reacted with
trimethylaluminum; in a suitable solvent such as dichloromethane,
dichloroethane, toluene, or
a mixture thereof; the resulting solution is combined with a compound of
formula (XLIX),
followed by subsequent treatment with acetic acid under heating temperature of
80-100 C for
a period of time ranging from 5 to 24 hours; to provide a compound of formula
(L). Employing
NBS in a suitable solvent, preferably DMF, at room temperature followed by
cross coupling
using conditions known to one skilled in the art, preferably a palladium
catalyst such as
palladium(II)bis(triphenylphosphine) dichloride, a base such as Cs2CO3,
Na2CO3, and the like;
in a solvent mixture composed of 1,4-dioxane and water; at a temperature of
100 C provides a
compound of formula (VI), where X is CH, Y is N, R3 is F, and 111, Ra and PG
are defined as
previously described.
SCHEME 24
PG
Ri N ¨id Ri
_y N¨Ra Deprotection
, R2
x
0
rj I
R3 R3
0 0
(VI) (z)
103561 According to SCHEME 24, a compound of formula (VI), where PG is Bn, is
deprotected
employing conditions known to one skilled in the art, preferably in neat TFA
in a sealed tube,
at a temperature of about 60 to 90 C; or employing BC13, at a temperature of
about -78 C, in a
suitable solvent such as in DCM; or treatment with hydrogen gas, in the
presence of a catalyst
such as Palladium on carbon (Pd/C), affords a compound of Formula (Z).
103571 In a similar fashion, N-arylation and in-situ TBDPS deprotection of a
compound of
formula (XVIII), where R1 is 1 and PG is TBDPS, and Xis N; is achieved
employing conditions
known to one skilled in the art or as previously described, to afford a
compound of Formula
(Z).
103581 A compound of Formula (Z), where R3 is F is reacted in a nucleophilic
aromatic
substitution reaction to provide a compound of Formula (Z), where R3 is OCH3.
For example,
reaction of a compound of Formula (Z), where R3 is F, with a suitable base
such as NaOH, and
99
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
the like; in a suitable solvent such as Me0H, and the like; to provide a
compound of Formula
(Z) where Y is CH and R3 is OCH3.
[0359] Compounds of Formula (Z) may be converted to their corresponding salts
using
methods known to one of ordinary skill in the art. For example, an amine of
Formula (Z) is
treated with trifluoroacetic acid, HC1, or citric acid in a solvent such as
Et20, CH2C12, THF,
Me0H, chloroform, or isopropanol to provide the corresponding salt form.
Alternately,
trifluoroacetic acid or formic acid salts are obtained as a result of reverse
phase HPLC
purification conditions. Crystalline forms of pharmaceutically acceptable
salts of compounds
of Formula (Z) may be obtained in crystalline form by recrystallization from
polar solvents
(including mixtures of polar solvents and aqueous mixtures of polar solvents)
or from non-polar
solvents (including mixtures of non-polar solvents).
[0360] Where the compounds according to this invention have at least one
chiral center, they
may accordingly exist as enantiomers. Where the compounds possess two or more
chiral centers,
they may additionally exist as diastereomers. It is to be understood that all
such isomers and
mixtures thereof are encompassed within the scope of the present invention.
[0361] Compounds prepared according to the schemes described above may be
obtained as
single forms, such as single enantiomers, by form-specific synthesis, or by
resolution.
Compounds prepared according to the schemes above may alternately be obtained
as mixtures
of various forms, such as racemic (1:1) or non-racemic (not 1:1) mixtures.
Where racemic and
non-racemic mixtures of enantiomers are obtained, single enantiomers may be
isolated using
conventional separation methods known to one of ordinary skill in the art,
such as chiral
chromatography, recrystallization, diastereomeric salt formation,
derivatization into
diastereomeric adducts, biotransformation, or enzymatic transformation. Where
regioisomeric
or diastereomeric mixtures are obtained, as applicable, single isomers may be
separated using
conventional methods such as chromatography or crystallization.
[0362] The following specific examples are provided to further illustrate
compounds of
Formula (Z) and various preferred embodiments.
EXAMPLES
[0363] In obtaining the compounds of Formula (Z) described in the examples
below and the
corresponding analytical data, the following experimental and analytical
protocols were
followed unless otherwise indicated.
[0364] Unless otherwise stated, reaction mixtures were magnetically stirred at
room
temperature (rt) under a nitrogen atmosphere. Where solutions were "dried,"
they were
100
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
generally dried over a drying agent such as Na2SO4 or MgSO4. Where mixtures,
solutions, and
extracts were "concentrated", they were typically concentrated on a rotary
evaporator under
reduced pressure.
[0365] Normal-phase silica gel chromatography (FCC) was performed on silica
gel (SiO2)
using prepacked cartridges.
[0366] Preparative reverse-phase high performance liquid chromatography (RP
HPLC) was
performed on either:
METHOD A. A Gilson GX-281 semi-prep-HPLC with Phenomenex Synergi C18(10 ttm,
150 x 25mm), or Boston Green ODS C18 (51.tm, 150 x 30mm), and mobile phase of
5-99%
ACN in water (with 0.225%FA) over 10 min and then hold at 100% ACN for 2 min,
at a flow
rate of 25 mL/min; or
METHOD B. A Gilson GX-281 semi-prep-HPLC with Phenomenex Synergi C18(10p.m,
150 x 25mm) , or Boston Green ODS C18 (4tm, 150 x 30mm), and mobile phase of 5-
99%
ACN in water(0.1%TFA) over 10 min and then hold at 100% ACN for 2 min, at a
flow rate of
25 mL/min; or
METHOD C. A Gilson GX-281 semi-prep-IIPLC with Phenomenex Synergi C18(10ttm,
150 x 25mm), or Boston Green ODS C18 (51.tm, 150 x 30mm), and mobile phase of
5-99%
ACN in water(0.05%HC1) over 10 min and then hold at 100% ACN for 2 min, at a
flow rate of
25 mL/min; or
METHOD D. a Gilson GX-281 semi-prep-HPLC with Phenomenex Gemini C18 (10[Im,
150 x 25mm), AD(10m, 250mm x 30mm), or Waters XBridge C18 column (51.tm, 150 x
30mm), mobile phase of 0-99% ACN in water (with 0.05% ammonia hydroxide v/v)
over 10
min and then hold at 100% ACN for 2 min, at a flow rate of 25 mL/min; or
METHOD E. a Gilson GX-281 semi-prep-HPLC with Phenomenex Gemini C18 (lOttm,
150 x 25mm), or Waters )(Bridge C18 column (51,tm, 150 x 30mm), mobile phase
of 5-99%
ACN in water(lOmM NH4HCO3) over 10 min and then hold at 100% ACN for 2 min, at
a flow
rate of 25 mL/min.
[0367] Preparative supercritical fluid high performance liquid chromatography
(SFC) was
performed either on a Thar 80 Prep-SFC system, or Waters 80Q Prep-SFC system
from Waters.
The ABPR was set to 100bar to keep the CO2 in SF conditions, and the flow rate
may verify
according to the compound characteristics, with a flow rate ranging from
50g/min to 70g/min.
The column temperature was ambient temperature.
[0368] Mass spectra (MS) were obtained on a SHIMADZU LCMS-2020 MSD or Agilent
101
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
1200\G6110A MSD using electrospray ionization (ESI) in positive mode unless
otherwise
indicated. Calculated (calcd.) mass corresponds to the exact mass.
[0369] Nuclear magnetic resonance (NMR) spectra were obtained on Bruker model
AVIII 400
spectrometers. Definitions for multiplicity are as follows: s = singlet, d =
doublet, t= triplet, q
= quartet, dd = doublet of doublets, ddd = doublet of doublet of doublets, td
= triplet of doublets,
dt = doublet of triplets, spt = septet, quin = quintet, m = multiplet, br =
broad. It will be
understood that for compounds comprising an exchangeable proton, said proton
may or may
not be visible on an NMR spectrum depending on the choice of solvent used for
running the
NMR spectrum and the concentration of the compound in the solution.
[0370] Chemical names were generated using ChemDraw Ultra 12.0, ChemDraw Ultra
14.0
(CambridgeSoft Corp., Cambridge, MA) or ACD/Name Version 10.01 (Advanced
Chemistry).
Compounds designated as R* or S* are enantiopure compounds where the absolute
configuration was not determined.
Intermediate 1: 3 -((B enzy loxy)methyl)-4-ethyl- 1H-1,2,4-tri azol-5(4H)-one.
Bn0
N
N
HN-_1
0
[0371] Step A. 2-(Benzyloxy)acetohydrazide.
103721 To a solution of ethyl 2-(benzyloxy)acetate (55 g, 283.17 mmol) in Et0H
(500 mL) was
added NH2NH2-1-120 (28.3 g, 566 mmol, 27.5 mL). The reaction mixture was
heated at 78 C
for 6 h. The reaction mixture was concentrated under reduced pressure to
afford the title product
(52 g, crude) as a colorless oil, which was used directly in the next step
without further
purification.
[0373] Step B. 3 -((B enzyl oxy)m ethyl)-4-ethy1-1H-1, 2,4-triazol -5(4H)-one.
[0374] To a solution of 2-(benzyloxy)acetohydrazide (52 g, 288 mmol) in H20
(500 mL) was
added dropwise isocyanatoethane (25.1 g, 346 mmol, 27.9 mL) at 0 C. After the
addition was
complete, the mixture was stirred at 25 C for 12 hr. To the mixture was added
H20 (20 mL),
and an aqueous solution (120 mL) of NaOH (57.7 g, 1.44 mol). The mixture was
stirred at
95 C for 12 hr. The reaction mixture was cooled to rt, then quenched with HC1
(12 M) at 0 C
and adjusted to "pH" 6. The solid was filtered and dried under reduced
pressure to afford the
title compound as a white solid (61 g, 91% yield). 1-1-1NIMR (400 MHz, CDC13)
ö ¨9.23 - 9.09
(m, 1H),-7.41 - 7.31 (m, 5H),-4.58 -4.53 (m, 2H),-4.45 - 4.42 (m, 2H),-3.82 -
3.75 (m, 2H),-
102
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
1.33 - 1.29 (m, 3H) ppm.
Intermediate 2: 5-(((tert-Butyldiphenylsilypoxy)methyl)-4-ethyl-2,4-dihydro-3H-
1,2,4-triazol-
3 -one.
TBDPSO
N
I N
HN
0
103751 Step A. 4-Ethyl-5-(hydroxymethyl)-2,4-dihydro-3H-1,2,4-triazol-3-one.
103761 To a solution of 5-[(benzyloxy)methyl]-4-methyl-2,4-dihydro-3H-1,2,4-
triazol-3-one
(8 g, 34.3 mmol, 1.0 eq.) in methanol (200 mL) was added Pd/C (2 g). The
resulting mixture
was maintained under hydrogen and stirred at rt for 6 h. Then the resulting
mixture was filtered
and the filtrate was concentrated to afford the crude product 4-ethy1-5-
(hydroxym ethyl)-2,4-
dihydro-3H-1,2,4-triazol-3-one as a white solid (4.3 g, 88 % yield). 1H NMR
(400 MHz,
DMSO-d6) 6 11.52 (s, 1H), 5.55 (t, J= 5.50 Hz, 1H), 4.32 (d, J = 5.50 Hz, 2H),
3.64 (q, J =
6.97 Hz, 2H), 1.18 (t, J= 6.97 Hz, 3H) ppm.
103771 Step B. 5-(((tert-Butyldiphenylsilypoxy)methyl)-4-ethyl-2,4-dihydro-3H-
1,2,4-triazol-
3 -one .
103781 To a solution of 4-ethyl-5-(hydroxymethyl)-2,4-dihydro-3H-1,2,4-triazol-
3-one (3 g, 21
mmol, 1.0 eq.) in DCM (30 mL) was added tert-butylchlorodiphenylsilane (6.5
mL, 25 mmol,
1.2 eq.) and pyridine (1.86 mL, 23 mmol, 1.1 eq.). The resulting mixture was
stirred at rt
overnight. The reaction mixture was quenched with water (100 mL). The
resulting mixture was
extracted with DCM (3 x 100 mL). The organic layers were combined, dried over
anhydrous
sodium sulfate, filtered and concentrated. The residue was purified by silica
gel
chromatography (SiO2, 50-80% ethyl acetate / petroleum ether) to afford 5-
(((tert-
butyldiphenylsilyl)oxy)methyl)-4-ethyl-2,4-dihydro-3H-1,2,4-triazol-3-one as a
white solid
(4.9 g, 61 % yield). LCMS (ES-API): mass calcd. for C211-127N302Si, 381.2; m/z
found, 382.2
[M-41]'. 1H NMR (400 MHz, CDC13) 6 9.98 (s, 1H), 7.61-7.72 (m, 4H), 7.32-7.54
(m, 6H),
4.54 (s, 2H), 3.84 (q, J = 7.34 Hz, 2H), 1.33 (t, J= 7.34 Hz, 311), 1.07 (s,
9H) ppm.
Intermediate 3: 5-((B enzyl oxy)m ethyl)-4-ethy1-2-(7-fluoro- 1- oxo-
4-(prop-l-cn-2-y1)- 1H-
i sochromen-6-y1)-2,4-dihydro-3H-1.2,4-tri azol-3- one.
103
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
Bn0
N
N
0, o
if
0
[0379] Step A. tert-Butyl 4,5-difluoro-2-iodobenzoate.
[0380] 4,5-Difluoro-2-iodobenzoic acid (3 g, 11 mmol) was dissolved in TI-IF
(30 mL), then
di-tert-butyl dicarbonate (4.6 g, 21 mmol) was added followed by DMAP (645 mg,
5.3 mmol).
The reaction mixture was stirred under nitrogen at 50 C overnight, then
cooled down to room
temperature. The solvent was evaporated under reduced pressure. The residue
was diluted with
Et0Ac then washed with brine. The organic layer was separated, dried with
Na2SO4, filtered,
and concentrated. The residue was purified by silica column chromatography
(gradient elution:
0 - 5% Et0Ac in petroleum ether) to give the title compound as a yellow oil
(2.9 g, yield: 79%).
1H NMR (400 MHz, CDC13) 6 7.77 (dd, J= 10.2, 7.9 Hz, 1 H), 7.63 (dd, J= 10.2,
7.9 Hz, 1
H), 1.62 (s, 911) ppm; 19F NMR (376 'VLF-1z, CDC13) 6 -131.55 - -131.13 (m, 1
F), -136.97 - -
136.65 (m, 1 F) ppm.
[0381] Step B. tert-Butyl 4-(3 -((b enzyl oxy)m ethyl)-4-ethy1-5 -ox o-4,5 -di
hy dro-11-1-1,2, 4-
tri azol-1-y1)-5 -fluoro-2-i odob enzoate.
[0382] A mixture of tert-butyl 4,5-difluoro-2-iodobenzoate (3.2 g, 9.4 mmol),
3-
((b enzyl oxy)m ethyl)-4-ethy1-1H-1 ,2,4-tri azol-5(4H)-one (Intermediate 1,
2.6 g, 11.2 mmol)
and Cs2CO3 (6.1 g, 18.7 mmol) in anhydrous DMF (30 mL) was stirred under
nitrogen at 75 C
for 1 h, then cooled to room temperature. The mixture was filtered through a
pad of Celite ,
and the pad was washed with Et0Ac. The filtrate was combined, washed with
brine, and
concentrated. The residue was purified by silica column chromatography
(gradient elution: 0 -
40% Et0Ac in petroleum ether) to give the title compound as a colorless
amorphous solid (5 g,
yield: 96%). ESI-MS: mass calcd. for C23H25FIN304, 553.1; m/z found, 554.1
[M+Hr 1H
NMR (400 MHz, CDC13) 6 8.16 (d, J= 7.1 Hz, 1 H), 7.62 (d, J= 10.8 Hz, 1 H),
7.29 - 7.45
(m, 5 H), 4.61 (s, 2 H), 4.50 (s, 2 H), 3.84 (q, J= 7.2 Hz, 2 H), 1.63 (s, 9
H), 1.35 (t, J= 7.2 Hz,
3 H) ppm; 19F NMR (376 MHz, CDC13) 6 -119.09 (dd, J= 10.6, 7.0 Hz, 1 F) ppm.
[0383] Step C. 4-(3-((B enzyloxy)methyl)-4-ethy1-5 -oxo-4,5 -dihydro-1H-1, 2,4-
triazol-1 -y1)-5-
fluoro-2-iodobenzoic acid.
[0384] To a solution of tert-butyl 4-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-
dihydro-1H-
1,2,4-triazol-1-y1)-5-fluoro-2-iodobenzoate (5 g, 9 mmol) in DCM (50 mL) was
slowly added
104
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
TFA (10 mL). The reaction mixture was stirred at room temperature overnight.
The reaction
mixture was concentrated under vacuum. The obtained residue was triturated
with petroleum
ether at room temperature for 30 min. The mixture was filtered and the solid
was rinsed with
petroleum ether. The precipitate was collected and dried in yam() to give the
title compound
as a white solid (4.1 g, yield: 91%). ESI-MS: mass calcd. for C19H17FIN304,
497.0; m/z
found, 498.0 [M-h_El] .1H NMR (400 MHz, DMSO-d6) 6 8.16 (d, J= 7.3 Hz, 1 H),
7.78 (d, J
= 11.0 Hz, 1 H), 7.28 - 7.43 (m, 5 H), 4.60 (s, 2 H), 4.57 (s, 2 H), 3.74 (q,
.1 = 7.2 Hz, 2 H),
1.23 (t, J= 7.2 Hz, 3 H) ppm; 19F NMR (376 MHz, DMSO-d6) 6 -119.91 (s, 1 F)
ppm.
[0385] Step D. 5-((Benzyl oxy)m ethyl )-4-ethy1-2-(7-fluoro-1-oxo-4-
(prop-1 -en -2-y1)-1H-
isochromen-6-y1)-2,4-dihydro-3H-1,2,4-triazol-3-one.
[0386] To a mixture of 3-methylbuta-1,2-dien-1-y1 acetate (Intermediate 12,
280 mg, 2.2
mmol), 4-(3 -((b enzyl oxy)methyl)-4-ethy1-5 -oxo-4,5-dihydro-1H-1, 2,4-tri
azol-1 -y1)-5-fluoro-
2-i odobenzoi c acid (1.1 g, 2.2 mmol) and Cy2N1Me (867 mg, 4.4 mmol) in DMF
(7 mL) was
added Catacxium A Pd G2 (74.2 mg, 0.11 mmol) under nitrogen. The reaction
mixture was
stirred under nitrogen at 90 'V for overnight. The mixture was then cooled to
room temperature,
diluted with Et0Ac and washed with brine. The organic layer was separated and
the aqueous
layer was combined and extracted with Et0Ac. The combined organic layer was
dried over
Na2SO4, filtered and concentrated. The residue was purified by silica column
chromatography
(gradient elution: 0 - 80% Et0Ac in petroleum ether) to give the title
compound as yellow solid
(240 mg, yield: 25%). ESI-MS: mass calcd. for C24H22FN304, 435.2; m/z 436.2 [M-
41]+.1H
NMR (400 MHz, CDC13) 6 8.15 (d, J = 10.5 Hz, 1 H), 7.86 (d, J = 6.8 Hz, 1 H),
7.30 - 7.45
(m, 5 H), 7.19 (s, 1 H), 5.37 -5.39 (m, 1 H), 5.18 (s, 1 H), 4.62 (s, 2 H),
4.53 (s, 2 H), 3.87 (q,
J = 7.1 Hz, 2H), 2.11 (s, 3 H), 1.37 (t, J= 7.2 Hz, 3 H) ppm.
Intermediate 4: 5-((Benzyloxy)methyl)-4-ethy1-2-(7-fluoro-4-isopropyl-1-oxo-1H-
isochromen-6-y1)-2,4-dihydro-3H-1,2,4-triazol-3-one.
Bn0
N
I N
N,Af
0 0
0
Method I:
[0387] Step A. 3-Methylbut-2-en- 1-yl 4,5-difluoro-2-iodobenzoate.
105
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0388] To the mixture of 4,5-difluoro-2-iodobenzoic acid (1.4 g, 4.9 mmol) and
Cs2CO3 (4.8 g,
14.8 mmol) in anhydrous DMF (20 mL) was added 1-bromo-3-methyl-2-butene (1.5
g, 9.9
mmol). The reaction mixture was stirred at room temperature for 18 h. The
mixture was diluted
with water, and the mixture was extracted with DCM and Et0Ac. The combined
organic extract
was dried over Na2SO4, filtered and concentrated. The residue was purified by
flash
chromatography (SiO2, gradient elution: 10-20% Et0Ac in heptane) to give the
desired product
as a colorless oil (1.6 g, yield: 92%). II-INMR (400 MHz, CDC13) 6 7.80 (dd, J
=7 .58, 9.54 Hz,
1H), 7.73 (dd, .1 = 7.83, 10.76 Hz, 1H), 5.42-5.52 (m, 1H), 4.82 (d, .1 = 7.34
Hz, 2H), 1.80 (s,
3H), 1.78(s, 3H) ppm.
[0389] Step B. 3 -Methylbut-2 -en-1 -y1 4-(3 -((b enzyloxy)m ethyl)-4-ethy1-5 -
oxo-4, 5-di hy dro-
1H-1,2,4-triazol-1-y1)-5-fluoro-24 odobenzoate.
[0390] To a mixture of 3-methylbut-2-en- 1 -y1 4,5-difluoro-2-iodobenzoate
(1.6 g, 4.5 mmol),
3-((benzyloxy)methyl)-4-ethyl-1H-1,2,4-triazol-5(4H)-one (Intermediate 1, 2.1
g, 9.1 mmol) in
anhydrous DMF (25 mL) was added Cs2CO3 (2.9 g, 9.1 mmol). The reaction mixture
was
heated under nitrogen at 85 C for 1 h, then cooled to room temperature. The
mixture was
diluted with water, and the mixture was extracted with DCM and Et0Ac. The
combined organic
extract was dried over Na2SO4, filtered and concentrated. The residue was
purified by flash
chromatography (SiO2, gradient elution: 20-50% Et0Ac in heptane) to give the
title compound
as a white solid (2.4 g, yield: 93%). LCMS (ES-API): mass calcd. for
C24H25FIN304, 565.1;
m/z found, 566.2 [M-41]". NMR (400 MHz, CDC13) 6 8.21 (d, J = 6.85 Hz,
1H), 7.73 (d, J
= 11.25 Hz, 1H), 7.29-7.44 (m, 5H), 5.41-5.53 (m, 1H), 4.84 (d, J= 7.34 Hz,
2H), 4.60 (s, 2H),
4.50 (s, 2H), 3.84 (q, J = 7.22 Hz, 2H), 1.80 (s, 3H), 1.78 (d, J= 0.98 Hz,
3H), 1.34 (t, J = 7.22
Hz, 3H) ppm.
[0391] Step C. 5-((Benzyloxy)methyl)-4-ethy1-2-(7-fluoro-44 sopropy1-1-oxo-1H-
i sochrom en-
6-y1)-2,4-di hy dro-3H-1,2,4-tri azol-3 -one.
[0392] To a mixture of 3 -methylbut-2-en-l-y1 4-(3 -((b enzyloxy)methyl)-4-
ethy1-5 -oxo-4, 5 -
dihydro-1H-1,2,4-triazol-1-y1)-5-fluoro-2-iodobenzoate (4 g, 6.86 mmol, 1 eq)
in toluene (200
mL) was added (tBu3P)PdG2 (351 mg, 0.69 mmol, 0.1 eq), N-cyclohexyl-N-methyl-
cyclohexanamine (1.60 mL, 7.54 mmol, 1.1 eq) respectively. The reaction
mixture was
degassed with nitrogen for three times, and then heated under nitrogen
atmosphere at 80 C for
18 h. LCMS analysis showed -18% of starting material remained. The mixture was
cooled to
15 C, and additional N-cyclohexyl-N-methyl- cyclohexanamine (0.72 mL, 3.43
mmol, 0.5 eq)
and tBu3PPdG2 (176 mg, 0.34 mmol, 0.05 eq) were added. The reaction mixture
was degassed
106
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
with nitrogen, and then heated under nitrogen atmosphere at 80 C for 16 h. The
mixture was
concentrated under reduced pressure, then diluted with H20 (200 mL), and
extracted with
Et0Ac (150 mL x 3). The combined organic layers were washed with brine (100 mL
x 2), dried
over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The
residue was
purified by flash chromatography (SiO2, Petroleum ether/Ethyl acetate=5/1 to
3/1) to give the
title compound as a yellow oil (1.1 g, yield: 35%). ESI-MS: mass calcd. for
C24H24FN304, 437.2;
m/z found, 438.5 [M+H]. 1H NMR (400 MHz, CDC13) 6 8.16(d, J= 6.6 Hz, 1H), 7.96
(d, J
= 6.6 Hz, 1H), 7.42 -7.34 (m, 5H), 7.13 (s, 1H), 4.63 (s, 2H), 4.54 (s, 2H),
3.88 (dd, I= 7.2,
14.4 Hz, 2H), 3.13- 3.06(m, 1H), 1.38 ( tõI= 7.2 Hz, 3H), 1.32 (dõI = 6.8 Hz,
6H) ppm.
Method II:
103931 To a mixture of 5-((benzyloxy)methyl)-4-ethy1-2-(7-fluoro-1-oxo-4-(prop-
1-en-2-y1)-
1H-isochromen-6-y1)-2,4-dihydro-3H-1,2,4-triazol-3-one (Intermediate 3, 5.9 g,
13.5 mmol) in
THF (100 mL) at room temperature was added Wilkinson's Catalyst [RhCl(PP103]
(3.8 g, 4.1
mmol). The mixture was degassed and purged with hydrogen gas. The reaction
mixture was
stirred under an atmosphere of hydrogen (15 Psi) at room temperature for 12 h.
The mixture
was concentrated. The residue was purified by silica column chromatography
(elution: 0 -25%
Et0Ac in petroleum ether) to give the title compound as a yellow solid (1.5 g,
yield: 77%). ESI-
MS: mass calcd. for C24H24FN304, 437.2; m/z found 438.2 [M-FH]+. 1H NMR (400
MHz,
DMSO-d6) 6 8.10 (d, J= 10.5 Hz, 1 H), 8.01 (d, J= 7.0 Hz, 1 H), 7.46 (s, 1 H),
7.26 - 7.42 (m,
H), 4.61 (s, 2 H), 4.59 (s, 2 H), 3.77 (q, J= 7.3 Hz, 2 1-1), 3.08 (dt, J=
13.4, 6.8 Hz, 1 H), 1.22
- 1.28 (m, 911) ppm; 19F NMR (376 MHz, DMSO-d6) 6 -118.29 (br s, 1 F) ppm.
Intermediate 5: 4-(3-((Benzyloxy)methyl)-4-ethy1-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-
5-fluoro-2-iodobenzoyl chloride.
Bn0
IN
CI 0
0
[0394] Step A. tert-Butyl 4,5-difluoro-2-iodobenzoate.
[0395] 4,5-Difluoro-2-iodobenzoic acid (3 g, 11 mmol) was dissolved in THE (30
mL), then
di-tert-butyl dicarbonate (4.6 g, 21 mmol) was added followed by DMAP (645 mg,
5.3 mmol).
The reaction mixture was stirred under nitrogen at 50 C overnight, then
cooled down to room
temperature. The solvent was evaporated under reduced pressure. The residue
was diluted with
107
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Et0Ac then washed with brine. The organic layer was separated, dried with
Na2SO4, filtered,
and concentrated. The residue was purified by silica column chromatography
(gradient elution:
0 - 5% Et0Ac in petroleum ether) to give the title compound as a yellow oil
(2.9 g, yield: 79%).
1H NMR (400 MHz, CDC13) 6 7.77 (dd, J = 10.2, 7.9 Hz, 1 H), 7.63 (dd, J =
10.2, 7.9 Hz, 1
H), 1.62 (s, 911) ppm; 19F NMIR (376 MHz, CDC13) 6 -131.55 - -131.13 (m, 1 F),
-136.97 - -
136.65 (m, 1 F) ppm.
[0396] Step B. tell-Butyl 4-(3 -((b enzyloxy )m ethyl)-4-ethy1-5 -ox o-4,5 -di
hy dro-11-1-1,2, 4-
tri azol-1-y1)-5 -fluoro-2-iodob enzoate.
[0397] A mixture of tert-butyl 4,5-di fluoro-2-i odobenzoate (3.2 g, 9.4
mmol), 3-
((benzyloxy)methyl)-4-ethy1-1H-1,2,4-triazol-5(4H)-one (Intermediate 1, 2.6 g,
11.2 mmol)
and Cs2CO3 (6.1 g, 18.7 mmol) in anhydrous DMF (30 mL) was stirred under
nitrogen at 75 C
for 1 h, then cooled to room temperature. The mixture was filtered through a
pad of Celite ,
and the pad was washed with Et0Ac. The filtrate was combined, washed with
brine, and
concentrated. The residue was purified by silica column chromatography
(gradient elution: 0 -
40% Et0Ac in petroleum ether) to give the title compound as a colorless
amorphous solid (5 g,
yield: 96%). ESI-MS: mass calcd. for C23H25FIN304, 553.1; m/z found, 554.1
[M+H]t 1H
NMR (400 MHz, CDC13) 6 8.16 (d, J = 7.1 Hz, 1 H), 7.62 (d, J = 10.8 Hz, 1 H),
7.29 - 7.45
(m, 5 H), 4.61 (s, 2 H), 4.50 (s, 2 H), 3.84 (q, J= 7.2 Hz, 211), 1.63 (s,
9H), 1.35 (t, J = 7.2 Hz,
3 H) ppm; 19F NMR (376 MHz, CDC13) 6 -119.09 (dd, J= 10.6, 7.0 Hz, 1 F) ppm.
[0398] Step C. 4-(34(Benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-5-
fluoro-2-iodobenzoic acid.
[0399] To a solution of tert-butyl 4-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-
dihydro-111-
1,2,4-triazol-1-y1)-5-fluoro-2-iodobenzoate (5 g, 9 mmol) in DCM (50 mL) was
slowly added
TFA (10 mL). The reaction mixture was stirred at room temperature overnight.
The reaction
mixture was concentrated under vacuum. The obtained residue was triturated
with petroleum
ether at room temperature for 30 min. The mixture was filtered and the solid
was rinsed with
petroleum ether. The precipitate was collected and dried in vacuo to give the
title compound as
a white solid (4.1 g, yield: 91%). ESI-MS: mass calcd. for C191117FIN304,
497.0; m/z found,
498.0 [M Hr.1H NMR (400 MHz, DMSO-d6) 6 8.16 (d, J= 7.3 Hz, 111), 7.78 (d, J =
11.0
Hz, 1 H), 7.28 - 7.43 (m, 5 H), 4.60 (s, 2 H), 4.57 (s, 2 H), 3.74 (q, J= 7.2
Hz, 2 H), 1.23 (t, J
= 7.2 Hz, 3 H) ppm; 19F NMR (376 MHz, DMSO-d6) 6 -119.91 (s, 1 F) ppm.
[0400] Step D. 4-(34(Benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1.,2,4-
triazol-1-y1)-5-
fluoro-2-iodobenzoyl chloride.
108
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0401] A solution of 4-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-1-
y1)-5-fluoro-2-iodobenzoic acid (3.5 g, 7 mmol) in SOC12 (14 mL) was heated at
reflux for 15
min. The reaction mixture was cooled to room temperature and concentrated. To
the residue
was added anhydrous toluene, then the mixture was evaporated to give the crude
product as a
yellow gum (3.6 g), which was directly used for the next step without further
purification.
Intermediate 6: 2-Chloro-6-fluoro-N-(3-methylpent-2-en-1-yl)aniline.
CI
[0402] Step A. Ethyl 3-methylpent-2-enoate.
[0403] To a solution of 2-butanone (52 g, 717.6 mmol) and
(carbethoxymethylene)
triphenylphosphorane (50 g, 143.5 mmol) in toluene (65 mL) was added benzoic
acid (3.5 g,
28.7 mmol). The reaction mixture was heated at reflux for 16 h. The mixture
was diluted with
petroleum ether and filtered through a short pad of silica gel. The silica gel
was washed with
hexane. The filtrate was concentrated under reduced pressure at 0-2 C. The
residue was
purified by silica column chromatography (elution: 0 - 10% Et0Ac in petroleum
ether) to give
the title compound as a colorless liquid (23.3 g crude). 1-fl NMR (400 MHz,
CDC13) 6 5.58 -
5.69 (m, 1 H), 4.13 (qd, J= 7.1, 4.9 Hz, 2 H), 2.62 (q, J= 7.5 Hz, 1 H), 2.09 -
2.20 (m, 3 H),
1.86 (d, J= 1.2 Hz, 1 H), 1.24- 1.28 (m, 3 H), 1.01 - 1.09 (m, 3 H) ppm.
[0404] Step B. 3-Methylpent-2-en-1 -ol.
[0405] To a toluene solution (1 M) of DIBAL-H (118 mL, 118 mmol) at -78 C was
added a
toluene solution (40 mL) of ethyl 3-methylpent-2-enoate (20 g crude) dropwise
under nitrogen.
The reaction mixture was stirred at -78 'V for 2 h. The mixture was warmed to
room
temperature and slowly poured into saturated aqueous potassium sodium tartrate
solution at
0 C. The mixture was stirred for 2 h and filtered through a short pad of
Celite . The pad was
washed with DC1V1/Et0Ac (v/v, 3/1), and the filtrate was extracted with DCM.
The organic
extract was separated, dried over Na2SO4, filtered and concentrated. The
residue was purified
by silica column chromatography (elution: 0 - 100% DCM in petroleum ether,
then 0 - 30%
Et0Ac in DCM) to give the title compound as a colorless liquid (7 g, yield of
two steps: 57%).
1I-1 NM:ft (400 MiElz, CDC13) 6 5.35- 5.46(m, 1 H), 4.11 - 4.21 (m, 2 H), 2.02
- 2.13 (m, 2 H),
1.67- 1.76 (m, 311), 0.98 - 1.06 (m, 3 H) ppm.
[0406] Step C. 3-Methylpent-2-enal.
109
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0407] To a solution of 3-methylpent-2-en-1-ol (2 g, 20.0 mmol) in DCM (20 mL)
was added
Dess-martin periodinane (10 g, 24.0 mmol). The reaction mixture was stirred at
room
temperature for 1 h. The mixture was filtered through a short pad of Centel.
The pad was
washed with DCM. The combined filtrate was washed with saturated aqueous
NaHCO3 solution.
The organic layer was separated, dried over Na2SO4, filtered and concentrated
under reduced
pressure at 0 - 2 'C. The crude was purified by silica column chromatography
(elution: DCM)
to give the title compound as a colorless liquid (1.5 g, yield: 77%). 1H NMR
(400 MHz, CDC13)
6 9.91 - 10.04 (m, 1 H), 5.78 - 5.90 (m, 1 H), 2.58 (q, J= 7.6 Hz, 1 H), 2.23
(d, J= 7.3 Hz, 1
H), 2.16 (s, 2 H), 1.96 (dõI = 1.1 Hz, 1 H), 1.16 (tõI = 7.6 Hz, 1 H), 1.09
(tõI = 7.4 Hz, 2 H)
PPm -
[0408] Step D. N-(2-Chloro-6-fluoropheny1)-3-methylpent-2-en-1-imine.
[0409] To a mixture of 2-chloro-6-fluoroaniline (1.2 g, 8.2 mmol) and 3-
methylpent-2-enal
(0.97 g, 9.9 mmol) in DCM (18 mL) under nitrogen at 0 C was added
triethylamine (4.6 mL,
33 mmol), followed by the addition of a DCM solution (1 M) of TiC14 (5 mL, 5
mmol) dropwise.
The resulting mixture was stirred at 0 C for 1 h, then warmed to room
temperature and stirred
for 4 h. The mixture was poured into saturated aqueous NH4C1 solution. The
mixture became
cloudy and filtered through a pad of Celite . The pad was washed with Et0Ac.
The combined
filtrate was diluted with DCM and water. The organic layer was separated, and
aqueous layer
was extracted with DCM. The combined organic layers were washed with brine,
dried over
Na2SO4, filtered and concentrated. The residue product was purified by silica
gel column
chromatography (gradient elution: 0 - 5% DCM in petroleum ether) to give the
title compound
as a pale yellow oil (1.3 g, yield: 70%).
[0410] Step E. 2-Chl oro-6-fluoro-N -(3 -methylp ent-2 -en-1 -yl)aniline.
[0411] To a solution of N-(2-chloro-6-fluoropheny1)-3 -methyl pent-2-en-1 -
imine (1.3 g, 5.76
mmol) in Me0H (20 mL) was added NaBH4 (218 mg, 5.8 mmol), and after 1 h,
another batch
of NaBH4 (218 mg, 5.8 mmol) was added. A total of NaBH4 (1.1 g, 29 mmol) was
added. The
reaction mixture was stirred at room temperature overnight. The mixture was
concentrated, and
then diluted with water and extracted with Et0Ac. The organic layer was
separated, washed
with brine, dried over Na2SO4, filtered and concentrated. The residue was
purified by combi
flash column chromatography over silica gel (eluent: 0 - 5% DCM in petroleum
ether) to give
the title compound as a yellow oil (430 mg, yield: 33%). 1H NM:1Z (400 MHz,
CDC13) 6 6.95 -
7.02 (m, 1 H), 6.84 (ddd, J = 12.2, 8.3, 1.3 Hz, 1 H), 6.52 - 6.63 (m, 1 H),
5.17 -5.28 (m, 1 H),
3.85 (d, J= 5.6 Hz, 2 H), 3.73 (s, 1 H), 1.91 -2.07 (m, 2 H), 1.58 - 1.68 (m,
3 H), 0.89 - 0.96
110
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(m, 3 H).
Intermediate 7: 5-Chloro-3-methy1-1-(tetrahydro-2H-pyran-2-y1)-1H-pyrazol-4-
amine.
NH2
THP
[0412] Step A. 5-Chloro-3-methy1-4-nitro-1-(tetrahydro-2H-pyran-2-y1)-1H-
pyrazole.
[0413] To a solution of 3-methyl-4-nitropyrazole (2 g, 15.7 mmol) in Et0Ac (20
mL) was
added DHP (2 g, 23.6 mmol) and Ts0H.H20 (150 mg, 0.79 mmol) at room
temperature. The
mixture was stirred at room temperature for overnight. Et3N (0.4 mL) was added
and the
mixture was washed with brine. Then the organic layer was separated, dried
over anhydrous
Na2SO4, filtered and concentrated. The residue was dissolved in THE (45 mL)
and the
temperature was lowered to -78 C. A THF (1 M) solution of LiHMDS (10.6 mL,
13.8 mmol)
was added to the mixture under nitrogen. After 45 minutes at -78 C, the
solution of
hexachloroethane (8.9 g, 37.8 mmol) in THE (20 mL) was added dropwise. The
reaction
mixture was warmed to room temperature and stirred for overnight. The mixture
was poured
into saturated aqueous NTI4C1 solution and extracted with Et0Ac. The organic
phase was
separated, washed with brine, dried over anhydrous Na2SO4, filtered and
concentrated. The
residue was purified by silica column chromatography (gradient elution: 0 -
40% Et0Ac in
petroleum ether) to give the title compound as white solid (1.8 g, yield:
58%). 1H NAAR (400
MHz, CDC13) 6 5.52 (dd, J = 10.0, 2.7 Hz, 1 H), 4.07 - 4.15 (m, 1 H), 3.70
(td, J = 11.3, 2.8
Hz, 1 H), 2.57 (s, 3 H), 2.37 - 2.47 (m, 1 H), 2.11 -2.19 (m, 1 H), 1.86- 1.90
(m, 1 H), 1.72 -
1.75 (m, 1 H), 1.64 (d, J= 2.0 Hz, 1 H), 1.53 (d, J= 6.6 Hz, 1 H) ppm.
[0414] Step B. 5 -C hl oro-3 -methyl-1 -(tetrahy dro-2H-pyran-2-y1)-1H-pyrazol-
4-ami ne.
[0415] To a mixture of 5 -chl oro-3 -methyl -4-nitro-1 -(tetrahy dro-2H-pyran-
2-y1)-1H-pyrazol e
(100 mg, 0.4 mmol) in Me0H/THF/H20 (v/v/v, 1/1/1, 3 mL) was added iron powder
(114 mg,
2.0 mmol) and NH4C1 (109 mg, 2.0 mmol). The mixture was stirred at 70 "V for
1.5 h. The
mixture was cooled to room temperature and filtered through a pad of Celite.
The pad was
washed with Et0Ac. The combined filtrate was washed with saturated aqueous
NaHCO3
solution. The organic layer was separated, and the aqueous layer was extracted
with Et0Ac.
The combined organic extract was washed with brine, dried over anhydrous
Na2SO4, filtered
and concentrated. The residue was purified by silica column chromatography
(gradient elution:
0 - 50% Et0Ac in petroleum ether) to give the title compound as a yellow oil
(70 mg, yield:
111
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
79%). ESI-MS: mass calcd. for C9H14C1N30, 215.1; m/z found, 216.1 [M+H].
Intermediate 8: 3 -(2-((tert-Butyl diphenyl silyl)oxy)ethoxy)-2-chloroaniline.
NH2
Sc'
TBDPS
[0416] Step A. tert-Butyl (2-(2-chl oro-3 -nitrophenoxy) eth oxy)diphenyl
silane .
[0417] To a mixture of 2-chloro-3-nitrophenol (200 mg, 1.2 mmol), 2-((tert-
butyldiphenylsilyl)oxy)ethan-1 -ol (554 mg, 1.8 mmol) and PPh3 (453 mg, 1.7
mmol) in TT-IF
(10 mL) was added DEAD (281 mg, 161 mmol) at 0 C under nitrogen. The mixture
was
warmed to room temperature and stirred at room temperature for 12 h. Saturated
aqueous
NH4C1 solution was added, and the mixture was extracted with Et0Ac. The
organic was
separated, washed with brine, dried over anhydrous Na2SO4, filtered and
concentrated. The
residue was purified by silica column chromatography (gradient elution: 0 -
10% Et0Ac in
petroleum ether) to give the title compound as a yellow oil (240 mg, yield:
46%). 1H NAIR (400
MHz, CDC13) 6 7.72 (dd, J = 7.8, 1.5 Hz, 4 H), 7.35 - 7.49 (m, 7 H), 7.30 (t,
J = 8.2 Hz, 1 H),
7.12 (dd, J= 8.3, 1.2 Hz, 1 H), 4.20 - 4.25 (m, 2 H), 4.06 (t, J= 4.9 Hz, 2
H), 1.06 (s, 9 H) ppm.
[0418] Step B. 3-(2-((tert-Butyldiphenylsilyl)oxy)ethoxy)-2-chloroaniline.
[0419] To a mixture of tert-buty1(2-(2-chloro-3-
nitrophenoxy)ethoxy)diphenylsilane (220 mg,
0.5 mmol), NH4C1 (258 mg, 4.8 mmol) in THE (3 mL), Me0H (3 mL) and H20 (3 mL)
was
added iron powder (269 mg, 4.8 mmol). The reaction mixture was stirred at 70
C for 2 h. The
mixture was cooled to room temperature, diluted with Et0Ac, and filtered
through a pad of
Celite . The Celite was washed with Et0Ac. The combined filtrate was washed
with brine,
dried over Na2SO4, filtered and concentrated. The residue was purified by
silica column
chromatography (gradient elution: 0 - 11% Et0Ac in petroleum ether) to give
the title
compound as a yellow solid (192 mg, yield: 92%). ESI-MS: mass calcd. for
C24H28C1NO2Si,
425.2; m/z found, 426.1[M+H]t 1H NMR (400 MHz, CDC13) 6 7.75 (dd, J= 7.9, 1.6
Hz, 4H),
7.35 - 7.48 (m, 6H), 6.97 (t, J= 8.1 Hz, 1 H), 6.42 (dd, J = 8.2, 1.1 Hz, 1
H), 6.33 (dd, J = 8.2,
1.1 Hz, 1 H), 4.13 - 4.17 (m, 2H), 4.07 - 4.13 (m, 2 H), 4.01 -4.06 (m, 2 H),
1.06 (s, 9 H) ppm.
Intermediate 9: 5-((B enzyloxy)m ethyl)-4-ethy1-2-(7-m ethyl -1 -oxo-4-(prop-1-
en-2-y1)-1H-
i sochromen-6-y1)-2,4-dihydro-3H-1,2,4-tri azol -3- one.
112
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Bn0
0,1f
0
[0420] Step A. tert-Butyl 2-bromo-4-fluoro-5-methylbenzoate.
[0421] To a solution of 2-bromo-4-fluoro-5-methylbenzoic acid (1 g, 4.3 mmol)
in TI-IF (10
mL) was added (Boc)20 (1.9 g, 8.6 mmol), followed by the addition of DMAP (262
mg, 2.1
mmol). The reaction mixture turned orange and was stirred under nitrogen at 50
C for
overnight. The mixture was cooled to room temperature, diluted with Et0Ac, and
then washed
with brine. The organic layer was separated, dried over Na2SO4, filtered and
concentrated. The
residue was purified by silica column chromatography (elution: 0 - 3% Et0Ac in
petroleum
ether) to give the title compound as colorless oil (900 mg, yield: 72%). 1H
NMIt (400 MHz,
CDC13) 6 7.57 (d, J= 8.1 Hz, 1 H), 7.24 (d, J= 4.6 Hz, 1 H), 2.22 (d, J= 1.5
Hz, 3 H), 1.58 (s,
9 H) ppm.
[0422] Step B. tert-Butyl 4-(3 -((b en zyl oxy)m ethyl)-4-ethyl-5 -ox o-4,5 -
di hydro-1H-1,2, 4-
tri azol-1-y1)-2 -bromo-5-methylbenzoate.
[0423] A mixture of tert-butyl 2-bromo-4-fluoro-5-methylbenzoate. (750 mg, 2.6
mmol), 5-
((benzyloxy)methyl)-4-ethy1-2,4-dihydro-3H-1,2,4-triazol-3-one (800 mg, 3.4
mmol) and
Cs2CO3 (1.7 g, 5.2 mmol) in DMF (8 mL) was stirred at 90 C for 16 h. The
reaction was
quenched by the addition of aqueous saturated N1H4C1 solution. The mixture was
extracted with
Et0Ac. The organic layer was separated, washed with brine, dried over Na2SO4,
filtered and
concentrated. The residue was purified by combi-flash chromatography (SiO2,
eluent: 0 - 22%
Et0Ac in petroleum ether) to give the title compound as colorless gum (1 g,
yield: 71%). ESI-
MS: mass calcd. for C24H28BrN304, 501.1; m/z found, 502.1 [M+H]t 1H NAIR (400
MHz,
CDC13) 6 7.64 (d, J= 6.1 Hz, 2 H), 7.33 - 7.43 (m, 5 H), 4.61 (s, 2 H), 4.50
(s, 2 H), 3.85 (qõI
= 7.3 Hz, 2 H), 2.31 (s, 3 H), 1.62 (s, 9 H), 1.36 (t, J= 7.2 Hz, 3 H) ppm.
[0424] Step C. 4-(34(Benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-2-
bromo-5-methylbenzoic acid.
[0425] To a mixture of tert-butyl 4-(3 -((b enzyl oxy)methyl)-4-ethy1-5 -oxo-
4, 5 -di hy dro-1H-
1,2,4-triazol-1-y1)-2-bromo-5-methylbenzoate (500 mg, 0.90 mmol) in DCM (5 mL)
was added
TFA (1 mL). The mixture was stirred at room temperature for 12 h. The mixture
was
concentrated. The residue was dissolved with DCM, and petroleum ether was
added slowly.
The mixture was stirred at room temperature for 30 min. The mixture was
filtered, and the
113
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
precipitate was rinsed with petroleum ether. The solid was collected and dried
in vacuo to give
the title compound as a white solid (360 mg, yield: 86%). ESI-MS: mass calcd.
for
C201-120BrN304, 445.1; m/z found, 446.0 [M+H] . 1H NMR (400 MHz, DMSO-d6) 6
7.77 (s, 1
H), 7.70 (s, 1 H), 7.29 -7.41 (m, 5 H), 4.59 (s, 2 H), 4.56 (s, 2 H), 3.74 (q,
J= 7.0 Hz, 2 H),
2.24 (s, 3 H), 1.23 (t, J= 7.2 Hz, 3 H) ppm.
[0426] Step D. 5 -((B enzyl oxy)methyl)-4-ethy1-2 -(7-methyl- 1 -oxo-4-(prop-1
-en-2-y1)-1H-
isochromen-6-y1)-2,4-dihydro-3H-1,2,4-triazol-3-one.
[0427] To a mixture of 4-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-dihydro-1H-
1,2,4-triazol-
-y1)-2-bromo-5-methylbenzoi c acid (560 mg, 1.26 mmol), 3-methyl buta-1,2-di
en-l-yl acetate
(Intermediate 12, 1.58 g, 12.5 mmol), AcOK (369 mg, 3.76 mmol) and TBAB (809
mg, 2.51
mmol) in DMF (3.9 mL) under nitrogen was added Pd(OAc)2 (141 mg, 0.63 mmol).
The
reaction mixture was stirred under nitrogen at 90 C for overnight. The
mixture was cooled to
room temperature, diluted with Et0Ac, and washed with brine. The organic layer
was separated,
and the aqueous layer was extracted with Et0Ac. The combined organic layers
were combined,
dried over Na2SO4, filtered, and concentrated. The residue was purified by
silica column
chromatography (gradient elution: 0 - 70% Et0Ac in petroleum ether) to give
the title
compound as a yellow solid (410 mg, yield: 73%). ESI-MS: mass calcd. for
C25H25N304, 431.2;
m/z found, 432.1 [M+H] . 1H NIVIR (400 MHz, CDC13) 6 8.28 (s, 1 H), 7.59 (s, 1
H), 7.31 -
7.46 (m, 5 H), 7.17 (s, 1 H), 5.30 - 5.37 (m, 1 H), 5.15 (s, 1 H), 4.63 (s, 2
H), 4.52 (s, 2 H), 3.87
(q, 1=7.1 Hz, 2 H), 2.46 (s, 3 H), 2.10 (s, 3 IT), 1.38 (t, = 7.2 Hz, 31-1)
ppm.
Intermediate 10: Isopropyl 6-(3 -((benzyloxy)methyl)-4-ethyl-5-oxo-4, 5-dihy
dro-1H-1, 2,4-
tri azol-1-y1)-2-chl oro-5 -fl uoroni coti nate.
/--OBn
CI N
reA
\r0
0
0
[0428] Step A. 2,6-Dichloro-5-fluoronicotinoyl chloride.
[0429] To a solution of 2,6-dichloro-5-fluoronicotinic acid (20 g, 95 mmol) in
THF (200 mL)
was added (C0C1)2 (12.7 g, 10.0 mmol) and DMF (69.6 mg, 0.952 mmol) at 0 C
dropwise.
The mixture was stirred at 0 C for 30 min, then warmed to 25 C, and stirred
for 1 h. The
reaction mixture was concentrated under reduced pressure to afford desired
product (21.7 g,
crude) as a colorless oil, which was used without further purification.
114
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0430] Step B. Isopropyl 2,6-dichloro-5-fluoronicotinate.
[0431] To a mixture of propan-2-ol (8.56 g, 142 mmol, 10.9 mL) and pyridine
(9.02 g, 114
mmol) in THF (200 mL) was added a solution of 2,6-dichloro-5-fluoronicotinoyl
chloride (21.7
g, 96.0 mmol) in THF (50 mL) at 0 C. The mixture was stirred at 25 C for 1
h. The mixture
was poured into water (300 mL). The aqueous phase was extracted with ethyl
acetate (300 mL).
The combined organic phase was dried with anhydrous Na2SO4, filtered and
concentrated. The
residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl
acetate=1/1 to
10:1) to afford the title compound (21 g, 86.82% yield). MS (ESI): mass calcd.
for
C9H8C12FN02, 250.1; m/z found, 252.0 [M+H]+. 1H NMR (400 MHz, CDC13) 6 7.97 -
7.95 (d,
J= 7.2 Hz, 1H), 5.32 - 5.25 (m, 1H), 1.58 - 1.39 (in, 6H) ppm.
[0432] Step C. Isopropyl 6-(3-((benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-
1,2,4-
tri azol-1-y0-2 -chloro-5 -fl uoroni cotinate.
[0433] To a mixture of isopropyl 2,6-dichloro-5-fluoronicotinate (4 g, 15.87
mmol) in DMSO
(40 mL) was added 3-((benzyloxy)methyl)-4-ethyl-1H-1,2,4- triazol-5(4H)-one
(3.89 g, 16.66
mmol) and K2CO3 (3.29 g, 23.80 mmol). The mixture was stirred at 80 C for 3
hr. LCMS
showed the starting material was consumed and desired mass was detected. The
mixture was
diluted with H20 (30 mL) and extracted with Et0Ac (50 mL x 3). The combined
organic layers
were washed with brine (100 mL), dried over Na2SO4, filtered and concentrated
under reduced
pressure. The residue was purified by column chromatography (SiO2, Petroleum
ether/Ethyl
acetate=1/0 to 1:1) to afford the title compound (5.7g, 79.86% yield). MS
(ESI): mass calcd.
for C21H22C1FN404, 448.1; m/z found, 449.2 [M+H]+. 1H NMR (400 MHz, CDC13) 6
8.10 (d,
J= 8.8 Hz, 1H), 7.43 - 7.31 (m, 5H), 5.30 (td, J= 6.3, 12.5 Hz, 1H), 4.61 (s,
2H), 4.54 (s, 2H),
3.85 (q, J= 7.2 Hz, 2H), 1.41 (d, J= 6.2 Hz, 6H), 1.37 - 1.31 (m, 3H) ppm.
Intermediate 11: 5-((B enzyl oxy)m ethyl)-4-ethy1-2-(7-fluoro-3 -hy droxy-4-i
sopropy1-1-
oxoi sochrom an-6-y1)-2,4- di hy dro-3H-1,2,4-tri azol-3 -one.
0
Fy
N N OH
Bn0
[0434] Step A. Methyl 4-(3-((benzyl oxy)methyl)-4-ethyl -5-oxo-4, 5-di hydro-
1H-1,2,4-tri azol -
1-y1)-2-bromo-5-fluorobenzoate.
115
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0435] To a flask charged with methyl 2-bromo-4,5-difluorobenzoate (100.0 g,
398 mmol), 5-
((b enzyl oxy)m ethyl)-4-ethy1-2,4-dihy dro-3H-1,2,4-tri azol-3 -one
(Intermediate 1, 113.5 g, 508
mmol) and K2CO3 (100.0 g, 724 mmol) was added anhydrous DNIF (1000 mL). The
reaction
mixture was heated under nitrogen at 50 C for 16 h, and then additional 5-
((benzyloxy)methyl)-4-ethy1-2,4-dihydro-3H-1,2,4-triazol-3-one (11 g, 51 mmol)
was added.
The reaction mixture continued to be stirred at 50 C. The mixture was cooled
to room
temperature and stirred for 10 min. Water (1000 mL) was added dropwise, and
the mixture was
stirred at room temperature for 2 h. The precipitate was collected by
filtration and dried to give
the crude product (190 g). The product was stirred in DMF (500 mL) for 30 min,
then water
(500 mL) was added. The mixture was stirred for 2 h. The precipitate was
collected by filtration
and dried to give the title compound (180 g, yield: 90%). 1H NMR (300 MHz,
CDC13) 6 7.95
(d, J= 6.7 Hz, 1H), 7.73 (d, J= 10.7 Hz, 1H), 7.44-7.27 (m, 5 H), 4.60 (s, 2
H), 4.50 (s, 2 H),
3.95 (s, 3H), 3.84 (q, .1 = 7.2 Hz, 2 H), 1.34 (t, .1 = 7.2 Hz, 3 H) ppm.
[0436] Step B. 5 -((B enzyl oxy)methyl)-4-ethy1-2-(7-fluoro-3 -
hydroxy -4-i sopropy1-1-
oxoi sochrom an-6 -y1)-2,4-di hy dro-3H-1, 2,4-tri azol-3 -one.
[0437] To a mixture of methyl 4-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-
dihydro-1H-1,2,4-
triazol-1-y1)-2-bromo-5-fluorobenzoate (30 g, 65.9 mmol), Xantphos (4.02 g,
6.6 mmol),
[Pd(ally1)C1]2 (1.38 g, 3.77 mmol), Cs2CO3 (42.6 g, 130 mmol) in
dimethylacetamide (300 mL)
was added 3-methylbutanal (41.4 mL, 386 mmol) slowly. The reaction mixture was
heated
under nitrogen at 80 C for 22 h. The mixture was filtered and quenched with
aqueous NU1IC1
solution until "pH" turned 7-8. The mixture was extracted with ethyl acetate
(2000 mL x2). The
combined organic extract was concentrated. The residue was purified by column
chromatography (SiO2, gradient elution: 1-33% ethyl acetate in petroleum
ether) to give the
title compound as an oil (61.7 g, yield: 56%). 111 NMIR (300 1V111z, CDC13) 6
7.93 (d, J = 10.4
Hz, 1H), 7.55 (d, J= 6.7 Hz, 1H), 7.44-7.28 (m, 5H), 5.92 (s, 1H), 4.61 (s,
2H), 4.51 (s, 2H),
4.37 (br s, 1H), 3.85 (q, J = 7.2 Hz, 2H), 2.80 (d, J= 7.1 Hz, 1H), 1.92 (spt,
J= 6.8 Hz, 1H),
1.35 (t, J= 7.2 Hz, 3H), 1.05 (d, J= 6.8 Hz, 3H), 0.93 (d, 1= 6.8 Hz, 3H) ppm.
Intermediate 12: 3 -Methylbuta-1,2-di en-1 -y1 acetate.
0
- 0
[0438] Step A. 2-Methylbut-3-yn-2-y1 acetate.
[0439] To a mixture of Mg(C104)2 (796 mg, 3.6 mmol) in acetic anhydride (38 g,
371 mmol)
116
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
at 0 C was added 2-methyl-3-butyn-2-ol (30 g, 357 mmol) dropwise. The
reaction mixture was
stirred at 0 'V for 10 min, then warmed to room temperature and stirred for
overnight. The
reaction mixture was diluted with DCM, then washed with aqueous saturated
NaHCO3 solution
and aqueous saturated Na2CO3 solution. The organic layer was separated, dried
over Na2SO4,
filtered and concentrated at 0 C. The residue was purified by silica column
chromatography
(elution: DCM) to give the title compound as pale yellow oil (35.8 g, yield:
80%). 1H NMR
(400 MHz, CDC13) 5 2.54 (s, 1 H), 2.03 (s, 3 H), 1.67 (s, 6 H) ppm.
[0440] Step B. 3-Methylbuta-1,2-dien-1 -y1 acetate.
[0441] To a solution of 2-methylbut-3-yn-2-y1 acetate (2.5 g, 20 mmol) in DCM
(20 mL) was
added AgBF4 (117 mg, 0.6 mmol) under nitrogen. The resulting colorless
solution was stirred
under nitrogen at 35 C for 2 h until the mixture turned into a black
solution. The mixture was
washed with aqueous ammonia (10%). The organic layer was separated, and the
aqueous layer
was extracted with DCM. The combined organic layer was dried over Na2SO4,
filtered and
concentrated. The residue was purified by silica column chromatography
(gradient elution: 0 ¨
3% Et0Ac in petroleum ether) to give the title compound as yellow oil (650 mg,
yield: 26%).
1H NMR (400 MHz, CDC13) 5 7.20 (dt, J= 4.1, 2.0 Hz, 1 H), 2.11 (s, 3 H), 1.81
(d, J= 2.0 Hz,
6 H) ppm.
Example 22 of PCT/IB2020/053601 (which published as WO 2020/212897 on October
22,
2020) discloses 6-(4-Ethyl-3-(hydroxym ethyl)-5-oxo-4,5-di hydro- 1H-1,2,4-tri
azol -1-y1)-7-
fluoro-4-isopropy1-2-(o-tolyl)isoquinolin-1(2H)-one (Compound 22).
HO
1tII
Ns-2
N
0
[0442] Step A. 6-(3-((Benzyloxy)methyl)-4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-
triazol-1-y1)-7-
fluoro-4-isopropyl-2-(o-tolypisoquinolin-1(2H)-one.
[0443] To a mixture of 5-((benzyloxy)methyl)-4-ethy1-2-(7-fluoro-3-hydroxy-4-
isopropyl-1-
oxoisochroman-6-y1)-2,4-dihydro-3H-1,2,4-triazol-3-one (Intermediate 11, 56 g,
123 mmol) in
AcOH (160 mL) was added o-toluidine (14.8 g, 138 mmol). The reaction mixture
was heated
at 80 C for 16 h. The mixture was concentrated, and then the -pH- was
adjusted to 7-8 with
aqueous NaHCO3 solution. The mixture was extracted with ethyl acetate (160 mL
x2). The
117
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
combined organic extract was concentrated. The residue was purified by flash
chromatography
(SiO2, 0-20% Ethyl acetate in DCM) to give the title compound as an oil (32.5
g, yield: 50%).
MS (ESI): mass calcd. for C311-131FN403, 526.2; m/z found, 527.4 [M-F1-1] .
NMR (300 MHz,
CDC13) 6 8.33 (d, J = 10.9 Hz, 1H), 8.07 (d, J = 6.8 Hz, 1H), 7.44-7.27 (m,
9H), 6.84 (s, 1H),
4.64 (s, 2H), 4.55 (s, 2H), 3.89 (q, J= 7.2 Hz, 2H), 3.23 (spt, J= 6.8 Hz,
1H), 2.16 (s, 3H), 1.39
(t, J= 7.2 Hz, 3H), 1.31 (dd, J= 6.8, 2.1 Hz, 6H) ppm.
[0444] Step B. 6-(4-Ethy1-3-(hydroxymethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-
1-y1)-7-
fluoro-4-isopropy1-2-(o-toly1)isoquinolin-1(2H)-one.
[0445] To a stirred solution of 6-(3-((benzyloxy)methyl)-4-ethy1-5-oxo-4,5-
dihydro-1H-1,2,4-
tri azol
sopropy1-2-(o-tol yl)i soquinolin-1(2H)-one (26. 5 g, 50.3 mmol) in
DCM (230 mL) at -78 C was added a DCM solution (1 M) of BC13 (290 mL, 290 mL)
under
nitrogen. The reaction mixture was stirred at 15 C for 0.5 h. The reaction
was quenched by
Me0H (100 mL) at -78 C to -20 C. The mixture was partitioned between water and
DCM. The
organic layer was separated, and the aqueous layer was extracted with DCM (110
mL x 2). The
combined organic extract was washed with brine (30 mLx2), dried with anhydrous
Na2SO4,
filtered, and concentrated to give a crude product (27.5 g). The product was
triturated with
methyl ethyl ketone (82 mL) and heptane (290 mL) to give a pure product (17.5
g), which was
re-crystallized in ethanol and water to give the title compound as a white
solid (16 g, yield:
73%). MS (ESI): mass caled. for C24H25FN403, 436.2; m/z found, 437.2 [M+Hr 11-
INMR (400
MHz, CDC13) 6 8.33 (d, J = 11.2, 1 H), 8.08 (d, 1= 6.8, 1
7.39-7.33 (m, 3 H), 7.28 (s, 1 H),
6.85 (s, 1 H), 4.69 (br s, 2 H), 3.94 (q, J= 7.11 Hz, 2 H), 3.27 (td, J=
13.66, 6.82 Hz, 1 H),
2.32 (br s, 1 H), 2.17 (s, 3 H), 1.45 (t, J = 7.11 Hz, 3 H), 1.32 (dd, J =
6.82, 1.83 Hz, 6 H) ppm.
[0446] It is noted that compounds of Formula (Z) described herein are
described in
PCT/IB2020/053601 (which published as WO 2020/212897 on October 22, 2020),
which is
incorporated by reference herein, in its entirety for all purposes.
[0447] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent.
[0448] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a hypomethylating agent.
[0449] In some embodiments, provided is a combination therapy comprising a
menin-MLL
118
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a hypomethylating
agent.
[0450] According to embodiments, the hypomethylating agent is azacitidine,
decitabine, or
pharmaceutically acceptable salts or solvates thereof.
[0451] According to particular embodiments, the hypomethylating agent is
azacitidine, or a
pharmaceutically acceptable salt or solvate thereof.
[0452] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof and a BCL-
2 inhibitor.
[0453] In some embodiments, provided is a combination therapy consisting of a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof and a BCL-
2 inhibitor.
[0454] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof and
venetoclax, or a pharmaceutically acceptable salt or solvate thereof.
[0455] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof and venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof.
[0456] In some embodiments, provided is a combination therapy comprising
Compound Al
and venetoclax, or a pharmaceutically acceptable salt or solvate thereof.
[0457] In some embodiments, provided is a combination therapy comprising
Compound A2
and venetoclax, or a pharmaceutically acceptable salt or solvate thereof.
[0458] In some embodiments, provided is a combination therapy comprising
Compound A3
and venetoclax, or a pharmaceutically acceptable salt or solvate thereof.
[0459] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof and venetoclax, or a pharmaceutically acceptable salt or
solvate thereof.
[0460] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof and venetoclax, or a pharmaceutically acceptable salt or
solvate thereof.
[0461] In some embodiments, provided is a combination therapy comprising
Compound A4
and venetoclax, or a pharmaceutically acceptable salt or solvate thereof.
[0462] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent is azacitidine, or a
pharmaceutically
119
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
acceptable salt or solvate thereof.
[0463] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof, and at least one
other antineoplastic
agent is azacitidine, or a pharmaceutically acceptable salt or solvate
thereof.
[0464] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is at least one other
antineoplastic agent is azacitidine, or a pharmaceutically acceptable salt or
solvate thereof
[0465] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is azacitidine, or a pharmaceutically acceptable salt or
solvate thereof
[0466] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is azacitidine, or a pharmaceutically acceptable salt or
solvate thereof.
[0467] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is azacitidine, or a pharmaceutically acceptable salt or
solvate thereof.
[0468] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is azacitidine, or a pharmaceutically
acceptable salt or
solvate thereof.
[0469] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is azacitidine, or a pharmaceutically
acceptable salt or
solvate thereof.
[0470] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is azacitidine, or a pharmaceutically acceptable salt or
solvate thereof.
[0471] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent is azacitidine, or a
pharmaceutically
acceptable salt or solvate thereof.
[0472] In some embodiments, provided is a combination therapy comprising a
menin-MILL
120
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof, and azacitidine, or
a pharmaceutically
acceptable salt or solvate thereof.
[0473] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and azacitidine, or a pharmaceutically
acceptable salt or
solvate thereof.
[0474] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and
azacitidine, or a
pharmaceutically acceptable salt or solvate thereof.
[0475] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and
azacitidine, or a
pharmaceutically acceptable salt or solvate thereof.
[0476] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and
azacitidine, or a
pharmaceutically acceptable salt or solvate thereof.
[0477] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and
azacitidine, or a pharmaceutically acceptable salt or solvate thereof.
[0478] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and
azacitidine, or a pharmaceutically acceptable salt or solvate thereof.
[0479] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and
azacitidine, or a
pharmaceutically acceptable salt or solvate thereof.
[0480] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent is decitabine, or a
pharmaceutically
acceptable salt or solvate thereof.
[0481] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent is decitabine, or a pharmaceutically acceptable salt or solvate thereof.
[0482] In some embodiments, provided is a combination therapy comprising
Compound A or
121
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is decitabine, or a
pharmaceutically acceptable salt or solvate thereof.
[0483] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is decitabine, or a pharmaceutically acceptable salt or
solvate thereof
[0484] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is decitabine, or a pharmaceutically acceptable salt or
solvate thereof
[0485] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is decitabine, or a pharmaceutically acceptable salt or
solvate thereof.
[0486] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is decitabine, or a pharmaceutically
acceptable salt or
solvate thereof.
[0487] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is decitabine, or a pharmaceutically
acceptable salt or
solvate thereof.
[0488] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is decitabine, or a pharmaceutically acceptable salt or
solvate thereof.
[0489] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a DNA intercalating agent.
[0490] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a DNA intercalating
agent
[0491] According to embodiments, the DNA intercalating agent is an
anthracycline.
[0492] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
122
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is an anthracycline.
[0493] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is an anthracycline.
[0494] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is an anthracycline.
[0495] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is an anthracycline.
[0496] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is an anthracycline.
[0497] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is an anthracycline.
[0498] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is an anthracycline.
[0499] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is an anthracycline.
[0500] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is an anthracycline.
[0501] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a pyrimidine analog
[0502] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
123
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
agent, wherein at least one other antineoplastic agent is a pyrimidine analog.
[0503] According to embodiments, the pyrimidine analog is cytarabine.
[0504] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is cytarabine.
[0505] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is cytarabine
[0506] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is cytarabine.
[0507] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is cytarabine.
[0508] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is cytarabine.
[0509] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is cytarabine.
[0510] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is cytarabine.
[0511] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is cytarabine.
[0512] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is cytarabine
[0513] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
124
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
agent is a purine analog.
[0514] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a purine analog.
[0515] According to embodiments, the purine analog is fludarabine.
[0516] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is fludarabine
[0517] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is fludarabine
[0518] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is fludarabine.
[0519] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is fludarabine.
[0520] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is fludarabine.
[0521] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is fludarabine.
[0522] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is fludarabine.
[0523] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is fludarabine.
[0524] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
125
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
antineoplastic agent is fludarabine.
[0525] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is an IDH inhibitor.
[0526] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is an isocitrate
dehydrogenase-1 inhibitor
(e.g., ivosidenib)
[0527] According to embodiments, the IDH inhibitor is ivosidenib.
[0528] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is ivosidenib
[0529] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is ivosidenib.
[0530] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is ivosidenib.
[0531] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ivosidenib.
[0532] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ivosidenib.
[0533] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ivosidenib
[0534] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ivosidenib.
126
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0535] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ivosidenib.
[0536] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ivosidenib.
[0537] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is an isocitrate
dehydrogenase-2 inhibitor
(e.g., enasidenib).
[0538] According to embodiments, the 1DH inhibitor is enasidenib.
[0539] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is enasidenib.
[0540] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is enasidenib.
[0541] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is enasidenib
[0542] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is enasidenib.
[0543] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is enasidenib.
[0544] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is enasidenib.
[0545] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
127
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
least one other antineoplastic agent is enasidenib
[0546] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is enasidenib.
[0547] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is enasidenib.
[0548] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is an immunomodulatory antineoplastic agent.
[0549] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is an immunomodulatory antineoplastic agent which is a PD-1 inhibitor.
[0550] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is an immunomodulatory
antineoplastic
agent.
[0551] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is an immunomodulatory
antineoplastic
agent which is a PD-1 inhibitor.
[0552] According to embodiments, the immunomodulatory antineoplastic agent is
nivolumab,
atezolizumab, pembrolizumab, thalidomide, lenalidomide, pomalidomide, Bacillus
Calmette¨
Guerin (BCG) or levamisole.
[0553] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is nivolumab.
[0554] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
128
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is nivolumab.
[0555] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is nivolumab.
[0556] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is nivolumab.
[0557] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is nivolumab.
[0558] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is nivolumab
[0559] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is nivolumab.
[0560] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is nivolumab.
[0561] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is nivolumab
[0562] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is atezolizumab.
[0563] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is atezolizumab
[0564] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is atezolizumab.
129
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0565] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is atezolizumab.
[0566] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is atezolizumab.
[0567] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is atezolizumab.
[0568] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is atezolizumab.
[0569] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is atezolizumab
[0570] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is atezolizumab.
[0571] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is pembrolizumab.
[0572] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is pembrolizumab.
[0573] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is pembrolizumab.
[0574] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pembrolizumab.
[0575] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
130
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
antineoplastic agent is pembrolizumab.
[0576] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pembrolizumab.
[0577] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is pembrolizumab.
[0578] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is pembrolizumab
[0579] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pembrolizumab.
[0580] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is thalidomide.
[0581] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is thalidomide.
[0582] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is thalidomide
[0583] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is thalidomide.
[0584] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is thalidomide.
[0585] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is thalidomide.
[0586] In some embodiments, provided is a combination therapy comprising
Compound A4-a
131
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is thalidomide.
[0587] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is thalidomide.
[0588] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is thalidomide.
[0589] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is lenalidomide.
[0590] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is lenalidomide.
[0591] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is lenalidomide.
[0592] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lenalidomide.
[0593] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lenalidomide.
[0594] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lenalidomide.
[0595] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is lenalidomide.
[0596] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is lenalidomide.
132
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0597] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lenalidomide.
[0598] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is pomalidomide.
[0599] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is pomalidomide.
[0600] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is pomalidomide
[0601] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pomalidomide.
[0602] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pomalidomide.
[0603] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pomalidomide
[0604] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is pomalidomide.
[0605] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is pomalidomide.
[0606] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is pomalidomide.
[0607] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
133
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is BCG.
[0608] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is BCG.
[0609] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is BCG
[0610] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is BCG.
[0611] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is BCG
[0612] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is BCG.
[0613] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is BCG.
[0614] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is BCG.
[0615] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is BCG.
[0616] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is 1 evami sole
[0617] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
134
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
agent, wherein at least one other antineoplastic agent is levamisole.
[0618] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is levamisole.
[0619] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is levamisole.
[0620] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is levamisole
[0621] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is levamisole.
[0622] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is levamisole.
[0623] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is levamisole.
[0624] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is levamisole.
[0625] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a DHODH inhibitor.
[0626] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a DHODH inhibitor.
[0627] According to embodiments, the DHODH inhibitor is a compound of Formula
(Z), or a
pharmaceutically acceptable salt, isotope, N-oxide, solvate, or stereoisomer
thereof.
[0628] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
135
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a DHODH inhibitor of Formula (Z), or a pharmaceutically acceptable
salt, isotope, N-
oxide, solvate, or stereoisomer thereof.
[0629] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a DHODH inhibitor of
Formula (Z), or
a pharmaceutically acceptable salt, isotope, N-oxide, solvate, or stereoisomer
thereof.
[0630] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is a DHODH
inhibitor of Formula (Z), or a pharmaceutically acceptable salt, isotope, N-
oxide, solvate, or
stereoisomer thereof.
[0631] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically acceptable
salt, isotope, N-oxide, solvate, or stereoisomer thereof.
[0632] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically acceptable
salt, isotope, N-oxide, solvate, or stereoisomer thereof.
[0633] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically acceptable
salt, isotope, N-oxide, solvate, or stereoisomer thereof.
[0634] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically
acceptable salt, isotope, N-oxide, solvate, or stereoisomer thereof.
[0635] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically
acceptable salt, isotope, N-oxide, solvate, or stereoisomer thereof
[0636] In some embodiments, provided is a combination therapy comprising
Compound A4,
136
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a DHODH inhibitor of Formula (Z), or a
pharmaceutically acceptable
salt, isotope, N-oxide, solvate, or stereoisomer thereof.
[0637] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a kinase inhibitor.
[0638] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a kinase inhibitor.
[0639] According to embodiments, the kinase inhibitor is a serine and/or
tyrosine kinase
inhibitor.
[0640] According to embodiments, the kinase inhibitor is an inhibitor of FLT3
and/or BTK
[0641] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a FLT3 inhibitor.
[0642] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a FLT3 inhibitor.
[0643] According to embodiments, the FLT3 inhibitor is sorafenib, sunitinib,
midostaurin
(PKC412), lestaurtinib (CEP-701), tandutinib (MLN518), quizartinib (AC220),
gilteritinib
(ASP2215), or KW-2449.
[0644] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is sorafenib
[0645] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is sorafenib.
[0646] In some embodiments, provided is a combination therapy comprising
Compound A or
137
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is sorafenib.
[0647] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sorafenib.
[0648] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sorafenib.
[0649] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sorafenib.
[0650] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is sorafenib.
[0651] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is sorafenib.
[0652] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sorafenib.
[0653] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is sunitinib.
[0654] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is sunitinib.
[0655] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is sunitinib.
[0656] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sunitinib.
138
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0657] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sunitinib.
[0658] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sunitinib.
[0659] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is sunitinib.
[0660] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is sunitinib.
[0661] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is sunitinib
[0662] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is midostaurin.
[0663] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is midostaurin
[0664] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is midostaurin
[0665] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is midostaurin.
[0666] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is midostaurin.
[0667] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
139
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
antineoplastic agent is midostaurin.
[0668] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is midostaurin.
[0669] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is midostaurin.
[0670] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is midostaurin
[0671] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is lestaurtinib
[0672] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is lestaurtinib.
[0673] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is lestaurtinib.
[0674] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lestaurtinib.
[0675] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lestaurtinib.
[0676] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lestaurtinib.
[0677] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is lestaurtinib.
[0678] In some embodiments, provided is a combination therapy comprising
Compound A4-b
140
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is lestaurtinib.
[0679] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is lestaurtinib.
[0680] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is tandutinib.
[0681] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is tandutinib
[0682] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is tandutinib.
[0683] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is tandutinib.
[0684] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is tandutinib.
[0685] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is tandutinib.
[0686] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is tandutinib.
[0687] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is tandutinib.
[0688] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is tandutinib.
141
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0689] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is AC220.
[0690] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is AC220.
[0691] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is AC220.
[0692] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is AC220
[0693] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is AC220.
[0694] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is AC220.
[0695] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is AC220
[0696] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is AC220
[0697] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is AC220.
[0698] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is ASP2215.
[0699] In some embodiments, provided is a combination therapy comprising a
menin-MILL
142
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is ASP2215.
[0700] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is ASP2215.
[0701] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ASP2215.
[0702] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ASP2215.
[0703] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ASP2215.
[0704] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ASP2215.
[0705] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ASP2215.
[0706] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ASP2215.
[0707] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is KW-2449.
[0708] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is KW-2449.
[0709] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
143
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
acceptable salt or solvate thereof and at least one other antineoplastic agent
is KW-2449.
[0710] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is KW-2449.
[0711] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is KW-2449.
[0712] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is KW-2449
[0713] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is KW-2449.
[0714] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is KW-2449.
[0715] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is KW-2449.
[0716] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a BTK inhibitor.
[0717] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a BTK inhibitor.
[0718] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is a BTK inhibitor.
[0719] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a BTK inhibitor.
[0720] In some embodiments, provided is a combination therapy comprising
Compound A2,
144
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a BTK inhibitor.
[0721] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a BTK inhibitor.
[0722] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is a BTK inhibitor.
[0723] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is a BTK inhibitor.
[0724] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a BTK inhibitor.
[0725] According to embodiments, the BTK inhibitor is ibrutinib
[0726] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is ibrutinib.
[0727] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is ibrutinib
[0728] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is ibrutinib.
[0729] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ibrutinib
[0730] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ibrutinib
[0731] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
145
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
antineoplastic agent is ibrutinib
[0732] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ibrutinib.
[0733] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is ibrutinib.
[0734] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is ibrutinib
[0735] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is a CD20 inhibitor.
[0736] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (1), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is a CD20 inhibitor.
[0737] According to embodiments, the CD20 inhibitor is an anti-CD20 antibody,
in particular
obinutuzumab (GA101).
[0738] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is a CD20 inhibitor.
[0739] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a CD20 inhibitor.
[0740] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a CD20 inhibitor.
[0741] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a CD20 inhibitor.
[0742] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
146
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
least one other antineoplastic agent is a CD20 inhibitor.
[0743] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is a CD20 inhibitor.
[0744] In some embodiments, provided is a combination therapy comprising
Compound A4,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is a CD20 inhibitor.
[0745] In some embodiments, provided is a combination therapy comprising a
menin-MILL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, a BCL-2
inhibitor and at least one other antineoplastic agent, wherein at least one
other antineoplastic
agent is GA101.
[0746] In some embodiments, provided is a combination therapy comprising a
menin-MLL
inhibitor of Formula (I), or a pharmaceutically acceptable salt or a solvate
thereof, venetoclax,
or a pharmaceutically acceptable salt or solvate thereof and at least one
other antineoplastic
agent, wherein at least one other antineoplastic agent is GA101
[0747] In some embodiments, provided is a combination therapy comprising
Compound A or
a pharmaceutically acceptable salt or solvate thereof, venetoclax, or a
pharmaceutically
acceptable salt or solvate thereof and at least one other antineoplastic agent
is GA101.
[0748] In some embodiments, provided is a combination therapy comprising
Compound Al,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is GA101.
[0749] In some embodiments, provided is a combination therapy comprising
Compound A2,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is GA101.
[0750] In some embodiments, provided is a combination therapy comprising
Compound A3,
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is GA101.
[0751] In some embodiments, provided is a combination therapy comprising
Compound A4-a
or a solvate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is GA101.
[0752] In some embodiments, provided is a combination therapy comprising
Compound A4-b
or a hydrate thereof, venetoclax, or a pharmaceutically acceptable salt or
solvate thereof and at
least one other antineoplastic agent is GA101.
[0753] In some embodiments, provided is a combination therapy comprising
Compound A4,
147
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
venetoclax, or a pharmaceutically acceptable salt or solvate thereof and at
least one other
antineoplastic agent is GA101.
[0754] All possible combinations of the above indicated embodiments are
considered to be
embraced within the scope of the invention
[0755] In some embodiments, provided are methods for treating a subject who
has been
diagnosed with a hematopoietic disorder. The present invention relates, for
example, to novel
methods that comprise administering to the subject a therapeutically effective
amount of a
menin-MLL inhibitor as described herein; and a therapeutically effective
amount of a BCL-2
inhibitor; and optionally, a therapeutically effective amount of at least one
other antineoplastic
agent.
[0756] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (1), or a pharmaceutically acceptable salt or
a solvate thereof
is administered orally to a subject.
[0757] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (I), or a pharmaceutically acceptable salt or
a solvate thereof
is administered in a dose of from about 1 mg/kg to about 50 mg/kg to the
subject.
[0758] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (I), or a pharmaceutically acceptable salt or
a solvate thereof
is administered in a dose of from about 2.5 mg/kg to about 25 mg/kg to the
subject.
[0759] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (I), or a pharmaceutically acceptable salt or
a solvate thereof
is administered in a dose of from about 7.5 mg/kg to about 12.5 mg/kg to the
subject.
[0760] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (I), or a pharmaceutically acceptable salt or
a solvate thereof
is administered in a dose of from about 8 mg/kg to about 10 mg/kg to the
subject.
[0761] An additional embodiment of the invention relates to methods as
described herein
wherein the compound of Formula (1), or a pharmaceutically acceptable salt or
a solvate thereof
is administered in a dose of from about 0.1 mg to about 5 mg to the subject.
[0762] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered orally to a subject.
[0763] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered in a dose of from about 1 mg/kg to
about 50 mg/kg
to the subject.
[0764] An additional embodiment of the invention relates to methods as
described herein
148
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
wherein the BCL-2 inhibitor is administered in a dose of from about 2.5 mg/kg
to about 25
mg/kg to the subject.
[0765] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered in a dose of from about 7.5 mg/kg
to about 12.5
mg/kg to the subject.
[0766] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered in a dose of from about 8 mg/kg to
about 10 mg/kg
to the subject.
[0767] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered in a dose of from about 0,1 mg to
about 5 mg to
the subject.
[0768] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered to the subject
intravenously or
subcutaneously.
[0769] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered intravenously
or subcutaneously
in a dose of from about 10 mg/m2 to about 250 mg/m2 to the subject.
[0770] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered intravenously
or subcutaneously
in a dose of from about 50 mg/m2 to about 150 mg/m2 to the subject.
[0771] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered intravenously
or subcutaneously
in a dose of from about 60 mg/m2 to about 100 mg/m2 to the subject.
[0772] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered intravenously
or subcutaneously
in a dose of about 75 mg/m2 to the subject.
[0773] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered to the subject
orally in a dose of
from about 1 mg to about 500 mg to the subject.
[0774] An additional embodiment of the invention relates to methods for
treating a subject who
has been diagnosed with cancer (e.g., wherein the cancer is a hematopoietic
disorder, such as
myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), acute
lymphoblastic
leukemia (ALL), a small lymphocytic lymphoma (SLL) or chronic lymphocytic
leukemia
(CLL)), wherein the method comprises administering to the subject:
149
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
a therapeutically effective amount of a menin-MLL inhibitor of Formula (I), or
a
tautomer or a stereoisomeric form thereof, or a pharmaceutically acceptable
salt or a solvate
thereof; and
a therapeutically effective amount of a DHODH inhibitor of Formula (Z), or a
pharmaceutically acceptable salt, isotope, N-oxide, solvate, or stereoisomer
thereof;
for example, wherein the menin-MLL inhibitor and the DHODH inhibitor are
administered in accordance with their dosing schedules for a time period,
e.g., (i)
simultaneously or sequentially in either order on the same day(s) within a
time period (e.g., a
21-day period, or a 28-day period, or a 3-month period, or a 6-month period,
or a one-year
period, etc.), and/or (ii) on different days within a time period.
[0775] An additional embodiment of the invention relates to methods for
treating a subject who
has been diagnosed with cancer (e.g., wherein the cancer is a hematopoietic
disorder, such as
myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), acute
lymphoblastic
leukemia (ALL), a small lymphocytic lymphoma (SLL) or chronic lymphocytic
leukemia
(CLL)), wherein the method comprises administering to the subject:
a therapeutically effective amount of a menin-MLL inhibitor that is Compound A
or a
pharmaceutically acceptable salt or solvate thereof (e.g., Compound Al, or
Compound A2, or
Compound A3, or Compound A4-a or a solvate thereof, or Compound A4-b or a
hydrate thereof,
or Compound A4); and
a therapeutically effective amount of a DHODH inhibitor that is Compound 22 or
a
pharmaceutically acceptable salt, solvate, stereoisomer, isotopic variant, or
N-oxide thereof
(e.g., 6-(4-Ethyl -3 -(hy droxym ethyl)-5-oxo-4,5-di hy dro-1H-
1,2,4-tri azol -1 -y1)-7-fluoro-4-
i sopropy1-2-(o-tolypi soquinolin-1(2H)-one);
for example, wherein the menin-MLL inhibitor and the DHODH inhibitor are
administered in accordance with their dosing schedules for a time period,
e.g., (i)
simultaneously or sequentially in either order on the same day(s) within a
time period (e.g., a
21-day period, or a 28-day period, or a 3-month period, or a 6-month period,
or a one-year
period, etc.), and/or (ii) on different days within a time period.
[0776] An additional embodiment of the invention relates to methods as
described herein
wherein compound of Formula (I), or a pharmaceutically acceptable salt or a
solvate thereof is
administered to the subject daily.
[0777] An additional embodiment of the invention relates to methods as
described herein
wherein compound of Formula (I), or a pharmaceutically acceptable salt or a
solvate thereof is
administered to the subject daily for at least 7 days.
150
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0778] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered to the subject daily.
[0779] An additional embodiment of the invention relates to methods as
described herein
wherein the BCL-2 inhibitor is administered to the subject daily for at least
7 days.
[0780] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered to the subject
daily.
[0781] An additional embodiment of the invention relates to methods as
described herein
wherein at least one other antineoplastic agent is administered to the subject
daily for at least
21 days.
[0782] Optimal dosages of any of the therapeutic compounds described herein to
be
administered may be readily determined and will vary with the particular
compound used, the
mode of administration, the strength of the preparation, and the advancement
of the disease,
syndrome, condition or disorder. In addition, factors associated with the
particular subject
being treated, including subject gender, age, weight, diet and time of
administration, will result
in the need to adjust the dose to achieve an appropriate therapeutic level and
desired therapeutic
effect.
[0783] The above dosages are thus exemplary of the average case. There can be,
of course,
individual instances wherein higher or lower dosage ranges are merited, and
such are within the
scope of this invention.
[0784] The therapeutic compounds described herein may be administered in any
of the
foregoing compositions and dosage regimens or by means of those compositions
and dosage
regimens established in the art whenever use of the therapeutic compounds
described herein is
administered to a subject in need thereof
[0785] The therapeutic compounds described herein may be administered to the
subject
simultaneously or sequentially.
[0786] When administered sequentially, the menin-lVILL inhibitor of Formula
(I) may be
administered first. When administration is simultaneous, the combination may
be administered
either in the same or a different pharmaceutical composition. For instance,
the menin-MLL
inhibitor of Formula (I) may be administered prior to, simultaneous with, or
after the
administration of the BCL-2 inhibitor. For instance, the BCL-2 inhibitor may
be administered
prior to, simultaneous with, or after the administration of the optional other
antineoplastic agent.
Adjunctive therapy, i.e., where one or two agent(s) are used as the primary
treatment and the
other agent is used to assist that primary treatment, is also an embodiment of
the present
invention.
151
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0787] An embodiment of the invention relates to a therapeutically effective
amount of a
menin-MLL inhibitor including the compounds of Formula (I) and the
pharmaceutically
acceptable salts, and the solvates thereof, or any subgroup thereof as
mentioned in any of the
embodiments, for use in combination with a therapeutically effective amount of
a BCL-2
inhibitor; and optionally, a therapeutically effective amount of at least one
other antineoplastic
agent. In certain embodiments, the aforementioned therapeutically effective
amounts are
administered in separate dosage forms for use in treating a subject who has
been diagnosed with
a hematopoietic disorder.
[0788] An embodiment of the invention relates to a pharmaceutical product
including a menin-
MILL inhibitor of Formula (I) and the pharmaceutically acceptable salts, and
the solvates thereof,
or any subgroup thereof as mentioned in any of the embodiments, and a BCL-2
inhibitor; and
optionally, at least one other antineoplastic agent as a combined preparation
for simultaneous,
separate or sequential use in treating a subject who has been diagnosed with a
hematopoietic
disorder.
[0789] The following Examples are provided to illustrate some of the concepts
described within
this disclosure. While the Example is considered to provide an embodiment, it
should not be
considered to limit the more general embodiments described herein.
EXAMPLES
General Synthetic Schemes
Venetoclax
[0790] Venetoclax is available commercially.
Azacitidine
[0791] Azacitidine is available commercially.
Compounds of Formula I
[0792] In this section, as in all other sections unless the context indicates
otherwise,
references to Formula (I) also include all other sub-groups and examples
thereof as defined
herein.
[0793] The general preparation of some typical examples of the compounds of
Formula (I) is
described hereunder and in the specific examples, and are generally prepared
from starting
materials which are either commercially available or prepared by standard
synthetic processes
commonly used by those skilled in the art of organic chemistry. The following
schemes are only
152
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
meant to represent examples of the invention and are in no way meant to be a
limit of the
invention.
[0794] Alternatively, compounds of Formula (I) may also be prepared by
analogous reaction
protocols as described in the general schemes below, combined with standard
synthetic
processes commonly used by those skilled in the art.
[0795] The skilled person will realize that in the reactions described in the
Schemes, although
this is not always explicitly shown, it may be necessary to protect reactive
functional groups
(for example hydroxy, amino, or carboxy groups) where these are desired in the
final product,
to avoid their unwanted participation in the reactions. In general,
conventional protecting
groups (PG) can be used in accordance with standard practice. The protecting
groups may be
removed at a convenient subsequent stage using methods known from the art.
[0796] The skilled person will realize that in the reactions described in the
Schemes, it may be
advisable or necessary to perform the reaction under an inert atmosphere, such
as for example
under N2-gas atmosphere.
[0797] It will be apparent for the skilled person that it may be necessary to
cool the reaction
mixture before reaction work-up (refers to the series of manipulations
required to isolate and
purify the product(s) of a chemical reaction such as for example quenching,
column
chromatography, extraction).
[0798] The skilled person will realize that heating the reaction mixture under
stirring may
enhance the reaction outcome. In some reactions microwave heating may be used
instead of
conventional heating to shorten the overall reaction time.
[0799] The skilled person will realize that another sequence of the chemical
reactions shown
in the Schemes below, may also result in the desired compound of Formula (I).
[0800] The skilled person will realize that intermediates and final compounds
shown in the
Schemes below may be further functionalized according to methods well-known by
the person
skilled in the art. The intermediates and compounds described herein can be
isolated in free
form or as a salt, or a solvate thereof. The intermediates and compounds
described herein may
be synthesized in the form of mixtures of tautomers and stereoisomeric forms
that can be
separated from one another following art-known resolution procedures.
[0801] All abbreviations used in the general schemes for Formula (I) are as
defined in Table
1B below in the part Examples. Variables are as defined in the scope or as
specifically defined
in the general Schemes.
153
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Part A) Schemes la, lb, lc, 2a, 2b and 3
Rn = C1_6alkyl-NR8aPG or C1_6alkyl-OPG or C1_6alkyl-C(=0)0R9', PG = protecting
group
Scheme la 0
k ,PG halo Step 1 R4_,
,Ci.6alkyl-NHPG
+ I. If
, R4 -Mg 0
Ci_6alkyl'
HO Rn ________________________
Step 2 H Rn + R4 _ivighalo Step 3
R4, ,Rn Step 2 R4, ,Rn
).- y _________________________________________________ > T
11
---- __________________________________________________________________ >
o OH
0
0
_ PG step 4 HO Rn Step 5 N Rn halo Step 6
R4 Rn
,'- N-, _______________________________ 43- y + R4-Mg or
R4 Li 1- ---Tr-
,
0
. 0
C1 .6alkyl'
Scheme lb HO, _OH
0
4:11 + e
gab OH Step 7
__________________________________________________________ ).- 0 OH }
halo
Riblitillij
R1'
Rib OH
Rxb Rxb
HO 0
, 4 0 , r:, 0
Rib
Rxa Rxb Step 5 Rxa Step 8 Rxa
II1T
b
,+ -N-
H
Ri
R16el Rib
Scheme lc PG
PG N
N halo n3(X)n4
r13% + )n4 hal '
J,õ Step 9
n1( )n2
U
I _________________________________________________ J.-- N
n1( )n2 N,-51,
N half,i)
N R2
H I
N N../..,
R2
[0802] In Scheme la, lb and lc the following reaction conditions apply.
[0803] Step 1: at a suitable temperature such as for example -70 C, in the
presence of a suitable
base such as for example TMEDA and a suitable organometallic reagent such as
for example
isopropylmagnesium bromide, in a suitable solvent such as for example THF;
[0804] Step 2: at a suitable temperature such as for example from 0 C to RT,
in the presence
of a suitable oxidative reagent such as for example DMP, in a suitable solvent
such as for
example DCM;
[0805] Step 3: at a suitable temperature such as for example from -20 C to RT,
in the presence
of a suitable organometallic reagent such as for example isopropylmagnesium
bromide, in a
suitable solvent such as for example THF;
[0806] Step 4: at a suitable temperature such as for example 80 C, in the
presence of a suitable
base such as for example NaOH, in suitable solvents such as for example THY
and H20;
154
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0807] Step 5: at a suitable temperature such as for example RT, in the
presence of suitable
amide condensation reagents such as for example EDCI and HOBt, in the presence
of a suitable
base such as for example NiVEVI, in a suitable solvent such as for example
DCM;
[0808] Step 6: at a suitable temperature such as for example -70 C, in the
presence of a suitable
organometallic reagent such as for example isopropyllithium, in a suitable
solvent such as for
example THE;
[0809] Step 7: at a suitable temperature such as for example 90 C, in the
presence of a suitable
organometallic catalyst such as for example Pd(dppf)C12, in the presence of a
suitable base such
as for example Na2CO3, in suitable solvents such as for example 1,4-dioxane
and H20;
[0810] Step 8: at a suitable temperature such as for example from 0 C to RT,
in the presence
of a suitable Lewis acid such as for example BBr3, in a suitable solvent such
as for example
DCM;
[0811] Step 9: at a suitable temperature such as for example from -78 C to 40
'V, in particular
from 0 C to RT, in the presence of a suitable base such as for example TEA,
DBU or K2CO3,
in a suitable solvent such as for example DCM, TETT or DIVIT;
Scheme 2a
PG PG
PG
I N
N
N
Rla n3(X)n4 n3()n2 )n4 n3%)n4
OH
Step 9), n1( Step 10
n1( )n2
n1(
)n2
N Rla
Rib + . ''.- hale,
U o)-1N R2 = halo
1j1
I
R1' IV-I%1
N-N-,,
-,--, R2
N-N/I,R2 R1 bill.
Step 11 1
R2 = H
R4Rn
H
N
N n3(X)n4
n3()n4
R4 ,, Rn
n1(X )n2 11õ n1( )n2
0 Wa
N
Ri a N
-.4 ______________________________________________________________
Rib 0-11)'-= Step 12 u
I
1
N-N.)
0 NI,N Rib.
155
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Scheme 2b
n3( )X
n1( )n2 0
PG
Step 12
R4 Rn R4,y, Rn
Step 11 N R4
Rn
n3()n4 n3( )n4
n1( )n2 n1( )n2
n3(X)n4
PG Step 14
n1( )n2
R1 a
0 OH
R1 a R1a OH
Step 13OyN IV
R
oN Rib
N NN
Rib
ib
Step 41
Rla -C1_4alkyl
ON
R1b NN%
1 Step 9
Ria 0 0
'Ci_4alkyl
OH + CI 1N
R" NNJ
[0812] In Scheme 2a and 2b, the following reaction conditions apply:
[0813] Step 9: See Step 9 in Scheme 1;
[0814] Step 10: at a suitable temperature such as for example RT, in the
presence of a suitable
catalyst such as for example Pd/C, in the presence of a suitable reductive
reagent such as for
example H2, optionally in the presence of a suitable base such as for example
TEA, in a suitable
solvent such as for example THF; Alternatively, at a suitable temperature such
as RT, in the
presence of a suitable catalyst such as for example Pd(dpp0C12-13CM complex, a
suitable
reducing agent such NaBH4, a suitable base such as for example TMEDA, in a
suitable solvent
such as for example THF.
[0815] Step 11: for N deprotection, at a suitable temperature such as for
example RT, in the
presence of a suitable acid as for example TFA, in a suitable solvent such as
for example DCM;
for 0 deprotection, at a suitable temperature such as for example RT, in the
presence of a
156
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
suitable acid as for example 4-methylbenzenesulfonic acid, in a suitable
solvent such as for
example Me0H,
[0816] Step 12: at a suitable temperature such as for example 80 C,
optionally in the presence
of a suitable Lewis acid such as for example ZnC12, in the presence of a
suitable reductive
reagent such as for example NaBH3CN, in a suitable solvent such as for example
Me0H;
[0817] Step 13: at a suitable temperature such as for example RT, in the
presence of a suitable
organometallic catalyst such as for example Ag(Phen)20Tf, in the presence of a
suitable
brominating reagent such as for example 1,3-dibromo-1,3,5-triazinane-2,4,6-
trione, in a
suitable solvent such as for example DCE;
[0818] Step 14: at a suitable temperature such as for example RT, in the
presence of a suitable
chlorinating reagent such as for example oxalyl chloride, in the presence of
DMF, in a suitable
solvent such as for example DCM.
Scheme 3
y.
When Rn = C1_salkyl-NR8'PG R4 cioikyi-
NR8aReb
N
n3(X )n4
n1( )n2
R4yC1.Ealkyl-NR8aPG R4 C16alkyl-NHR8e 0 R1a N
N N abi rk,N
n1(
n3(X)n4 n3(X)n4 <05/
isl, N
S,,,/ RibilliW )n2 Step 11
n1( )n2
R1' N _____ y
N (31-)--u it
CJ
,),,,, Febglam
Rib 'N
124 yCi.oalkyl-NRii -C(=0)-0-C1_4alkyl-O-C(=0)-C1.4alkyl
N
n3(X)n4
n1( )n2
Rla grah 0Nu
Rib FtlIF N
When Rn = Ci_Galkyl-OPG R4i Ci _6alkyl-OPG R4,f, Ci_6alkyl-OH
N N
"3K ,)n4 n3(X)n4
Step 11
n1( )n2 ____ v) ___ n1( )n2
R1a N R1 t a N
iiiii Oyk,, u a (3?-'1_1
' 1,1
Ribl NN Rib
IPI .11P1' 'NJ
When Rn = C1_olkyl-C(=0)0R9 R4õCi.6alkyl-C(.0)0R9a Fl4, ,Ci.5alkyl-
C(.0)-NRIaR91'
T T
N N
n3(X)n4 n3(X)n4
Step 16
n1( )n2 _____ 7.-- n1( )n2
R1' N 121' N
gli lb
NI, NI,
121 b.11'111IPP. N Rib"-LIPIP N
157
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
[0819] In Scheme 3, the following reaction conditions apply:
[0820] Step 11-12: See Step 11-12 in Scheme 2;
[0821] Step 15: at a suitable temperature such as for example 80 C, in the
presence of a suitable
base such as for example Cs2CO3, in suitable solvent such as for example DMF;
[0822] Step 16: at a suitable temperature such as for example 40 C, in the
presence of a suitable
base such as for example ammonia, in suitable solvent such as for example 1,4-
dioxane.
Part B) Schemes 4, 5, 6, 7, 8, 9, 10, 11 and 12
Scheme 4 4 H0_13,0H
1)
0 + OH Step 1
_______________________________________________________ > / OH }
halo 1
R1a
Rib -,,,
Rib ..j.,,. OH
o i xb Rxb
1
HO 0
' ,N a Rib
X
O
+ Fe; .. Rxb Step 2
.,
___________________________________ ).- R..,N 0 Step 3 Rxa
_______________________________________________________ ).-- OH
1 H 0-
,.._,,,,
Rib
Rib Rib
Ri a 0-,......,.õ.0,R9a Ri a 0,.,,,,.0, R9a Ria
OOH
OH OH Jitep5
+ Cl- - N Step 4 S
7
- -,
N, -,J
N'N-,-- N,
--õ,---
Rib Rib N Rib
N
R1a O'-:,--- OH
Ria OH R1a OCF3
0, Step 6
N 7
NI
N'N 0 --i----I Step
N, .,,,,,/,- N,ie
Rib Rib N Rib
[0823] In Scheme 4, the following reaction conditions apply:
[0824] Step 1: at a suitable temperature such as for example 90 C, in the
presence of a suitable
organometallic catalyst such as for example Pd(dppf)C12, in the presence of a
suitable base such
as for example Na2CO3, in suitable solvents such as for example 1,4-dioxane
and H20;
[0825] Step 2: at a suitable temperature such as for example RT, in the
presence of suitable
amide condensation reagent such as for example HATU, in the presence of a
suitable base such
as for example DIEA, in a suitable solvent such as for example DCM;
[0826] Step 3: at a suitable temperature such as for example from -78 C to
RT, in the presence
of a suitable Lewis acid such as for example BBr3, in a suitable solvent such
as for example
DCM;
[0827] Step 4: at a suitable temperature such as for example from -78 C to 40
C, in particular
from 0 C to RT, in the presence of a suitable base such as for example TEA,
DBU or K2CO3,
158
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
in a suitable solvent such as for example DCM, THY or DMF;
[0828] Step 5: at a suitable temperature such as for example RT, in the
presence of a suitable
base such as for example LiORH20, in suitable solvents such as for example THF
and H20;
[0829] Step 6: at a suitable temperature such as for example RT, in the
presence of a suitable
organometallic catalyst such as for example Ag(Phen)20Tf, in the presence of a
suitable
brominating reagent such as for example 1,3-dibromo-1,3,5-triazinane-2,4,6-
trione, in a
suitable solvent such as for example DCE;
[0830] Step 7: at a suitable temperature such as for example RT, in the
presence of a suitable
brominating reagent such as 1,3-dibromo-1,3,5-tri azinane-2,4,6-tri one, in
the presence of 2,2,2-
trifluoroethan-1-ol as solvent.
Scheme 5 PG
PG
ll
N
PG
N halo n3( ) )n4 R1a
n3(X)n4
n3)n4 + hal,ort, Step 8 n1( )n2 Step +
R1 St 9 n1( )n2
'' U _____________________________ 1.- ,-.)./OH ___ )....-
N
n1( )n2
halo I,
ii ' II R1b
,,,-,--
(3-1----LU
H
N. -R2
I
N,N ,i-,R
N
R1b
R2 = hay
2 Step 11
Step 10
H PG
H
N N
N
n3(X)n4 n3(X)n4 n3%)n4
n1( )n2 Step 11 n1( )n2
Ria N Ri'
N
NI' N.J l=
el I , N I
Kill
Rib Rlb Rib -
R4 R3
H
Rt_ ,R3 Rty,R3 I
N
N T halo
n3(X)n4
n3( ) )n4 + RR3 Step 123._ n3(XN )n4 Step 11 N
halo) Step 8
,,
n1( )n2
X
0 n1( )n2 > n3( ) )n4 +
N
--.halo
N n1( )n2 N.N
PIG N N
hahrz,)
PG H
I --- N
N.N.J,, halo
[0831] In Scheme 5, the following reaction conditions apply:
[0832] Step 8: at a suitable temperature such as for example from -78 C to 40
'V, in particular
from 0 C to RT, in the presence of a suitable base such as for example TEA,
DBU or K2CO3,
in a suitable solvent such as for example DCM, THE or DMF;
[0833] Step 9: at a suitable temperature such as for example from -78 C to 40
C, in particular
from 0 C to RT, in the presence of a suitable base such as for example TEA,
DBU or K2CO3,
in a suitable solvent such as for example DCM, TELF or DMF;
159
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0834] Step 10: at a suitable temperature such as for example RT, in the
presence of a suitable
organometallic catalyst as for example Pd/C and a suitable base as for example
TEA, in a
suitable solvent such as for example Me0H under H2 atmosphere;
[0835] Step 11: When PG is Boc, at a suitable temperature such as for example
RT, in the
presence of a suitable acid as for example TFA, in a suitable solvent such as
for example DCM.
Scheme 6
(Rw)In m = 1, 2, 3
R4 C,,,alkyl-OPS Rw = H, CN, halo,
OH, 0-C1alkyl
(R.). N
Step 12 n3(X)n4
RAõCi.fialkyl-OPG _______ ).
if n1( )n2
0 Ria N
60,___õ,..
, ii
H Feb 'N
N (R). (R,,)õ,
n3(g)n4
R4,(04.6alkyl-OH R4.õ Ci,salkyl-LG
n1( )n2 T
We N N N
Step 12 n3(X)n4 Step 13 n3(
_X)n4
0 ) _____________________ )
n1( )n2 ,....
n1( Au
Rib 'N R1' N R1' N
(3 S '
--"I'-' 0
.0
124yC1.6alkyl-NRa'Rsb
a ILI 7
n3(%
Rib R1e-
Y
R4 Ci_Balkyl-OH HNRR'RRb (R).,,0 t''67v (R.).
0
...""9" '
R' n1( )n2
0 N
R4 Ci_5alkyl¨< ] R4 Ci_51kyl¨/( cnN.0`
Y o --r- H
R4 C15alkyl¨( 1
[1 0 n1( )n2
..,,,
, o: Step 12
,,3(X)4 N
Step 15 n3r4 Rib -N--
.õ,, _____________________ )
' ___________________________________________ ).-
n1( )n2
0 Rta N Rla N
0 O
Rib N Rib N
[0836] In Scheme 6, the following reaction conditions apply:
[0837] Step 12: reductive amination condition, at a suitable temperature such
as for example
from RT to 80 C, in the presence or absence of a suitable Lewis acid such as
for example ZnC12
or an acid for example AcOH, in the presence of a suitable reducing agent such
as for example
NaBH3CN, in a suitable solvent such as for example Me0H;
[0838] Step 13: at a suitable temperature such as for example 0 C, in the
presence of a suitable
electrophile as for example MsCl, in the presence of a suitable base such as
for example TEA,
in a suitable solvent such as for example DCM;
[0839] Step 14: at a suitable temperature such as for example from 0 C to RT,
in the presence
of a suitable oxidizing agent as for example DAV, in a suitable solvent such
as for example
DCM,
[0840] Step 15: at a suitable temperature such as for example 50 C, in the
presence of a suitable
acid as for example HC1, in a suitable solvent such as for example ACN;
[0841] Step 16: at a suitable temperature such as for example RT, in the
presence or absence
160
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
of a suitable base as for example TEA, in a suitable solvent such as for
example TEIF.
Scheme 7
m = 1, 2, 3
Rw = H, ON, halo, OH, 0-C1.4alkyl
(Rw)m
H R4 Ci.Galkyl-NRgaRgb
N
n3 ,)n4 N
(Rw). Step 12 Ri n3(X)n4
nif )n2 11
R1a N + R4. Ci_calkyl-NR8aRth ... -
n1( )n2
N
R,
NM + I I ri
IRIE. N --N_,L,' R2
(R.A.
R4 Ci Balkyl-N123 PG Step 12
_
/Step12 or Step 17
0
(Fly.). (Rw).
(Rw)nl
R4 Ci_0a1ky1-NR8ePG R4 01_6alkyl-NHR8a R
Ci_olkyl-NR"
Y Y 4
N N N 0
0
n3()n4 Step 11 n3(X)n4 Step 17 n3(X )n4
1
Cr
n1( )n2 n1( )n2 02N n1( )n2
1
R1a N 12 iF
1e N Rla N
0-'''''Ci,alkyl
u
el ii ___________________________________________________ 0
--,--0 --T)--,u 0}0"---'0)1'Ci_4alkyl _i----..- ,,,--
I I 1 Y ul
1216,-----:õ._,, - N'N---)-õR2 --,_ ,--- N N --,:-1, R2
Rib ---,,,,-- N,N!---,R,
Rib
H
N
n3%)n4 (Rw)m (Rw)m
n1( )n2 R4yCi.salky1-0(.0)0R9' R4 , C1.6alkyl-
C(.0)-NRg'R9b
R1' N -r--
N N
n3(X)n4 n3(X)n4 II ill Step 12 Step 18
+ FINR9'reb -)'-
N,...",,R, n1( )n2 n1( )n2
Rib -I- R1' N R1a N
0,
------i
R4yCi.olkyl-C(=0)0R9
R16 'N" R2 121b
.----,..,..- N N,,,- ,R2
0
[0842] In Scheme 7, the following reaction conditions apply:
[0843] Step 11: When PG is Boc, at a suitable temperature such as for example
RT, in the
presence of a suitable acid as for example TFA, in a suitable solvent such as
for example DCM;
[0844] Step 12: reductive amination condition, at a suitable temperature such
as for example
from RT to 80 C, in the presence or absence of a suitable Lewis acid such as
for example ZnC12
or an acid for example AcOH, in the presence of a suitable reducing agent such
as for example
NaBH3CN, in a suitable solvent such as for example Me0H;
[0845] Step 17: at a suitable temperature such as for example from RT to 80
C, in the presence
of a suitable base such as for example DIEA or Cs2CO3, in suitable solvent
such as for example
DCM or DMF;
[0846] Step 18: at a suitable temperature such as for example 40 'V, in the
presence of a suitable
base such as for example ammonia, in suitable solvent such as for 1,4-dioxane.
161
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
Scheme 8
Rta OH
, _.J-, 0 _ RtT,R3
R4,T,R3 Rib j f 1-õIN N
Ir , µ94
r.3%).4
n3(X= )n4 + n1( )n2
R1' N
n1( )n2 ,0S
N Ria 171--C F3 .µ . j1----:,
H j II
AO Or
,o,J,N
Rib -.N'-- N-N
N--
Rib 1 Step 10
R'1R3 RR3 R4r.R3
T
N N
N
RI n3(X)n4 n3(X)n4 n3(X)n4
j__ _OH Step 9 Step 21
--- -,--- + ________ n1( )n2 ).- __ n1( )n2 S¨ n1(
)n2
N Ria N Rla
N
Rtb
hal.-1-- - 0 0
' N
I
N,=
----(L-I N
'---(L-I N
N halo Rib N -N-1, halo Rib =
-N 0C1.4alkyl
Step 20 ly Step 22
R4 R3
RR3
N
N n3( )
On4
n3(X)n4
n1(
)n2
n1( )n2
Rla N
Ria N
o.--1---k N
1 f 1
NI
R1b N NR7aR7b
Rtb
[0847] In Scheme 8, the following reaction conditions apply:
[0848] Step 9: at a suitable temperature such as for example from -78 C to 40
C, in particular
from 0 C to RT, in the presence of a suitable base such as for example TEA,
DBU or K2CO3,
in a suitable solvent such as for example DCM, Tlif or DMF;
[0849] Step 10: at a suitable temperature such as for example RT, in the
presence of a suitable
organometallic catalyst as for example Pd/C, optionally in the presence of a
suitable base as for
example TEA, in a suitable solvent such as for example Me0H under H2
atmosphere;
[0850] Step 19: at a suitable temperature such as for example RT, in the
presence of a suitable
chlorinating reagent such as for example oxalyl chloride, in the presence of
DMF, in a suitable
solvent such as for example DCM;
[0851] Step 20: at a suitable temperature such as for example 90 C, in the
presence of a suitable
nucleophilic amine, in a suitable solvent such as for example Et0H;
[0852] Step 21: at a suitable temperature such as for example RT, in the
presence of a suitable
162
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
acid such as for example HC1 in dioxane, in a suitable solvent such as for
example Me0H;
[0853] Step 22: at a suitable temperature such as for example 110 "V, in the
presence of a
suitable boron reagent such as for example trimethylboroxine, in the presence
of a suitable
organometallic catalyst such as for example
tetrakis(triphenylphosphine)palladium(0), in the
presence of a suitable base such as for example K2CO3, in a suitable solvent
such as for example
1,4-dioxane;
Scheme 9
CI CI
CO2C1_4alkyl
Step 23 CO2C1_4alkyl
Step 24 ¨0
n1(4)n2 + 1.----",ci
).-- n1( )n2 ¨)."- n1( )n2
N N N
PG PG PG
0 0
0H
0 'N Step
28
R4i,11,0H LC) Step 25 ll'ir-I-LO Step 260.. _________
,_...---- Step 27 . R -4'--- Boco,
R4 N __ I.- +
HN , NH .:,-.3.., ,--1\ Boc
-Boc 0 0"-C\ Boc 0 0 HNIRsiRsb
R4 R4
R8b
R4 -,,,r,--, N - R8 b CI Step 29 N,R8b Step
30 NH , ) \ Step 31
8'
HN kBa __ ). \ ______ ..- N
12 N-Rsb
NH2 R83
'Boc n1( )112
R8''
+ N n1( )n2
CI PG N
¨0 PG
n1( )n2
'N
PG
FeN_Rsb
R4
N 118' 1- -Isl
N 128a
R4
R1'
) n2 + OH N 12811 n1( )n2
Step 33 0
N ___________________________________________ > 1.11{X
Step 34
N _____________________________ Raa __________________________________________
H 4
Step 32 N
_________________________ > halo
halcLri R1bõN
rr1(X)n2 halo
N 1 NNK
R2
PG -;:d..,
- N R2 "
1221 N,R8b R(--..õ...,-,N_R8b
--r-
N 148a N 1482
n1( )n2 _________ Step 35 ).- n1(X)n2
R1 N R2= halo R1' N
0 oI'N
N*
- - 3
RR1"-N R2 Rib N -N
[0854] In Scheme 9, the following reaction conditions apply:
[0855] Step 23: at a suitable temperature such as for example from -78 C to -
25 C, in the
presence of suitable bases such as for example DMA and n-BuLi, in a suitable
solvent such as
for example THF;
[0856] Step 24: at a suitable temperature such as for example between -65 C
and ¨ 55 C, in
163
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
the presence of suitable reducing agent such as for example DIBAL-H, in a
suitable solvent
such as for example toluene, preferably conducted in a suitable flow chemistry
system,
[0857] Step 25: first at a suitable temperature such as for example from -10
C to 10 C, in the
presence of a suitable base such as for example DMAP, in the presence of a
suitable
condensation agent such as for example DCC, in a suitable solvent such as for
example DCM;
then at a suitable temperature such as for example from -10 C to 0 C, in the
presence of a
suitable acid such as for example AcOH, in the presence of a suitable reducing
agent such as
for example NaBH4, in a suitable solvent such as for example DCM;
[0858] Step 26: in a suitable solvent such as for example toluene and heated
to reflux;
[0859] Step 27: at a suitable temperature such as for example from -5 C to 5
C, in the presence
of suitable reducing agent such as for example LiBH4, in a suitable solvent
such as for example
2-methyltetrahydrofuran;
[0860] Step 28: at a suitable temperature such as for example from 15 'V to 25
'V, in the
presence of a suitable reducing agent such as for example NaBH(OAc)3, in a
suitable solvent
such as for example DCM;
[0861] Step 29: at a suitable temperature such as for example from 15 C to 25
'V, in the
presence of a suitable acid such as for HC1, in a suitable solvent such as for
example IPA;
[0862] Step 30: at a suitable temperature such as for example from 5 C to 30
C, in the presence
of a suitable base such as for example TEA, in the presence of suitable
reducing agent such as
for example NaBH(OAc)3, in a suitable solvent such as for example toluene;
[0863] Step 31: at a suitable temperature such as for example from 50 C to 55
C, in the
presence of a suitable base such as for example K2HPO4, in a suitable solvent
such as for
example H20;
[0864] Step 32: When PG is Bn at a suitable temperature such as for example
from -5 C to
45 'V, under a hydrogen atmosphere within a suitable pressure range such as
for example from
0.27 to 0.40 MPa, in the presence of a suitable catalyst such as for example
palladium hydroxide
on carbon, in the presence of a suitable acid as for example MSA in a suitable
solvent such as
Et0H;
[0865] Step 33: at a suitable temperature such as for example from -50 C to -
40 C, in the
presence of suitable base such as for example TEA, in a suitable solvent such
as 2-
methyltetrahydrofuran;
[0866] Step 34: at a suitable temperature such as for example from 20 C to 30
C, in the
presence of suitable base such as for example TMG, in a suitable solvent such
as 2-
m ethy ltetrahy drofuran ;
164
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0867] Step 35: at a suitable temperature such as for example from 20 C to 30
C, under a
hydrogen atmosphere within a suitable pressure range such as for example from
0.20 to 0.30
Mpa, in the presence of a suitable catalyst such as for example palladium on
carbon, in a suitable
solvent such as Me0H;
[0868] alternatively, at a suitable temperature such as room temperature, in
the presence of a
suitable catalyst such as for example I, F-Bis(diphenylphosphino)ferrocene-
palladium(H)
dichloride dichloromethane complex, a suitable reducing agent such sodium
borohydride, a
suitable base such as for example N,N,N',N'-tetramethylethylenediamine, in a
suitable solvent
such as for example tetrahydrofuran.
SCHEME 10
[0869] In general, compounds of Formula (1) wherein Y1 is limited to -CH2-,
and R2 is limited
to W1, hereby named compounds of Formula (Ia), can be prepared according to
the following
reaction Scheme 10. In Scheme 10, W1 represents chloro, bromo or iodo; all
other variables are
defined according to the scope of the present invention.
Scheme 10
R4i R3 R4Xi
R3
'X '
X2 X2
n3(X)n4 Ria n3( )n4
CH2ZnBr Step 36
n1( )n2 n1(
)n2
Ria
R1b
I U
N'Nhalo R1
NhaIo
(la)
[0870] In Scheme 10, the following reaction conditions apply:
[0871] Step 36: at a suitable temperature ranged from 60 C to 100 C, in
presence of a suitable
catalyst such as palladium acetate (Pd(OAc)2) or
tris(dibenzylideneacetone)dipalladium(0)
(Pd2(dba)3) or tetrakis(triphenylphosphine)palladium(0), in a suitable solvent
such as for
example tetrahydrofuran or dioxane.
[0872] The skilled person will realize that starting from compound (Ia),
analogous chemistry
as reported in step 10 in scheme 5 and in steps 20, 21 and 22 in scheme 8
could be performed.
165
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
SCHEME 11
[0873] In general, compounds of Formula (I) wherein Y' is limited to -CR5aR5b-
and R2 is
limited to Wl, hereby named compounds of Formula (Ib), can be prepared
according to the
following reaction Scheme 11. In Scheme 11 at least one of R5a and R5b is
other than hydrogen.
All other variables are defined according to the scope of the present
invention.
Scheme 11
R4 R3 R4 R3
'Xi
X2 X2
n3(/) )n4 R1a R5a n3( )n4
R5b Step 37
niV n2 n1( )n2
RiaR5a R5b N
Rib
w(LU U
N halo R1 halo
(Lb)
[0874] In Scheme 11, the following reaction condition apply:
[0875] Step 37: at a suitable temperature ranged from 80 C to 200 C, in
presence of a suitable
catalyst such as palladium acetate (Pd(OAc)2), in the presence of a suitable
ligand such as for
example triphenylphosphine or tricyclohexylphosphine, in a suitable solvent
such as for
example dioxane, preferably in sealed conditions, optionally under microwave
irradiation.
[0876] The skilled person will realize that starting from compound (Ib),
analogous chemistry
as reported in step 10 in scheme 5 and in steps 20, 21 and 22 in scheme 8
could be performed.
SCHEME 12
Scheme 12
PG PG
Ri a n3(< )n4 n3( )
)n4
NHR5c n1( )n2 Step 38 n1( )n2
R1a R5c N
Rib halo
R2 N
RibXIII5 'N R2
[0877] In Scheme 12, the following reaction condition apply:
[0878] Step 38: at a suitable temperature such as for example from RT to 80
C, in the presence
of a suitable base such as for example DIEA, Cs2CO3 or DBU, in suitable
solvent such as for
example DCM, THE or DMF;
166
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0879] Alternatively, at a suitable temperature such as for example RT to 100
C, in the presence
of a suitable catalyst such as for example Pd2dba3, in the presence of a
suitable ligand such as
for example Xantphos, in the presence of a suitable base such as Cs2CO3 or
Na2CO3, in a
suitable solvent such dioxane or a mixture of dioxane and water.
[0880] The skilled person will realize that starting from intermediate Z,
analogous chemistry
as reported in case Yl represents 0 can be performed.
[0881] It will be appreciated that where appropriate functional groups exist,
compounds of
various formulae or any intermediates used in their preparation may be further
derivatized by
one or more standard synthetic methods employing condensation, substitution,
oxidation,
reduction, or cleavage reactions. Particular substitution approaches include
conventional
alkylation, arylation, heteroarylation, acylation, sulfonylation,
halogenation, nitration,
formylation and coupling procedures.
[0882] The compounds of Formula (I) may be synthesized in the form of racemic
mixtures of
enantiomers which can be separated from one another following art-known
resolution
procedures. The racemic compounds of Formula (I) containing a basic nitrogen
atom may be
converted into the corresponding diastereomeric salt forms by reaction with a
suitable chiral
acid. Said diastereomeric salt forms are subsequently separated, for example,
by selective or
fractional crystallization and the enantiomers are liberated therefrom by
alkali. An alternative
manner of separating the enantiomeric forms of the compounds of Formula (I)
involves liquid
chromatography using a chiral stationary phase. Said pure stereochemically
isomeric forms
may also be derived from the corresponding pure stereochemically isomeric
forms of the
appropriate starting materials, provided that the reaction occurs
stereospecifically.
[0883] In the preparation of compounds of Formula (I), protection of remote
functionality (e.g.,
primary or secondary amine) of intermediates may be necessary. The need for
such protection
will vary depending on the nature of the remote functionality and the
conditions of the
preparation methods. Suitable amino-protecting groups (NH-Pg) include acetyl,
trifluoroacetyl,
t-butoxycarbonyl (Boc), benzyloxycarbonyl (CBz) and 9-fluorenyl-
methyleneoxycarbonyl
(Fmoc). The need for such protection is readily determined by one skilled in
the art. For a
general description of protecting groups and their use, see T. W. Greene and
P. G. M. Wuts,
Protective Groups in Organic Synthesis, 4th ed., Wiley, Hoboken, New Jersey,
2007.
Several methods for preparing the compounds of Formula (I) are illustrated in
the following
examples. Unless otherwise noted, all starting materials were obtained from
commercial
suppliers and used without further purification, or alternatively can be
synthesized by a skilled
person by using well-known methods.
167
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 1B - Abbreviations
Abbreviation Meaning
Ag(Phen)20Tf silver triflate¨bis(1,10-phenanthroline) complex
2-MeTHF 2-methyltetrahydrofuran
ACN acetonitrile
AcC1 acetyl chloride
AcOH acetic acid
Ac20 acetic anhydride
aq. aqueous
Ar argon
BBr3 tribromoborane
bn benzyl
Boc tert-butyloxycarbonyl
Boc20 di-tert-butyl dicarbonate
n-BuLi n-butyllithium
Cbz benzyloxycarbonyl
CD3OD Methanol-d4
CHC13 chloroform
Cs2CO3 cesium carbonate
conc. concentrated
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
DCC dicyclohexylcarbodiimide
DCE dichloroethane
DCM dichloromethane
DDQ 4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-
dicarbonitrile
DEA diethylamine
DIBAL-H diisobutylaluminum hydride
DIEA or DIPEA N,N-diisopropylethylamine
DMAP N,N-dimethylpyridin-4-amine
DMF N,N-dimethylformamide
DMP Dess-Martin periodinane
DMSO dimethyl sulfoxide
dppf 1,1'-ferrocenediyl-bis(diphenylphosphine)
EDCI N-(3-Dimethylaminopropy1)-N-ethylcarbodiimide
hydrochloride
EA or Et0Ac ethyl acetate
Et0H ethanol
eq. equivalent(s)
FA formic acid
FCC flash column chromatography
hour(s)
112 hydrogen
HAT U 1 -[bi s(dimethylamino)methyl ene] -1H-1,2,3 -
triazol o [4, 5-b]
pyridinium 3-oxid hexafluorophosphate
1120 water
HC1 hydrochloric acid
HOBt 1-Hydroxybenzotriazole
HPLC high performance liquid chromatography
ICH2C1 chloroiodomethane
168
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Abbreviation Meaning
IPA isopropyl alcohol
IPAc isopropyl acetate
K2CO3 potassium carbonate
KI potassium iodide
K2 HP 04 dipotassium phosphate
K3P 04 tripotassium phosphate
LiAlD4 lithium aluminum deuteride
LAH lithium aluminum hydride
LiBH4 lithium borohydride
LDA lithium diisopropylamide
Lid lithium chloride
LG leaving group
Me methyl
Me0H methanol
2-MeTHF 2-methyltetrahydrofuran
min minute(s)
mL milliliters
mmol millimoles
mg milligram
MgSO4 magnesium sulfate
MSA methanesulfonic acid
MsC1 methanesulfonyl chloride
MS molecular sieve
MTBE methyl tert-butyl ether
N2 nitrogen
NA not available
NaBH3CN sodium cyanoborohydride
NaBH(OAc)3 sodium triacetoxyborohydride
NaBD3CN sodium cyanoborodeuteride
Na2CO3 sodium carbonate
NaH sodium hydride
NaHCO3 sodium bicarbonate
Na! sodium iodide
Na0Ac sodium acetate
NaOH sodium hydroxide
Na2S03 sodium sulfite
Na2SO4 sodium sulfate
NH4C1 ammonium chloride
NMM 1-4-Methylmorpholine
Pd2dba3 tris(dibenzylideneacetone)dipalladium(0)
[1,1 1-bi s(diphenylphosphino)ferrocene] di chloropall adium(II),
Pd(dppf)C12.DCM
complex with di chloromethane
Pd(PPh3)4 tetrakis(triphenylphosphine)palladium(0)
PE petroleum ether
PG protecting group
Phen phenanthroline
psi pound per square inch
169
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Abbreviation Meaning
p-Ts0H p-toluenesulfonic acid
p-Ts011.1120 p-toluenesulfonic acid monohydrate
Rt retention time
Rochelle's salt potassium sodium tartrate tetrahydrate
RT room temperature
sat. saturated
SFC supercritical fluid chromatography
TBAF tetrabutylammonium fluoride
TBDMS tert-butyldimethylsilyl
TBDPS tert-butyldiphenylsilyl
t-BuOK potassium tert-butoxide
TEA triethylamine
Tf trifluoromethanesulfonyl
TFA trifluoroacetic acid
THF tetrahydrofuran
Ti(0iPr).1 titanium(IV) isopropoxide
TLC thin layer chromatography
TMEDA N,N,N1,AP-tetramethylethylenediamine
TMG 1,1,3,3-tetramethylguanidine
TMSI iodotrimethylsilane
Ts p-toluenesulfonyl
TsC1 p-toluenesulfonyl chloride
v/v volume per volume
vol. volume(s)
wt weight
Xantphos 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene
[0884] As understood by a person skilled in the art, compounds synthesized
using the protocols
as indicated may exist as a solvate e.g., hydrate, and/or contain residual
solvent or minor
impurities. Compounds or intermediates isolated as a salt form, may be integer
stoichiometric
i.e., mono- or di-salts, or of intermediate stoichiometry. When an
intermediate or compound in
the experimental part below is indicated as `HC1 salt' without indication of
the number of
equivalents of HC1, this means that the number of equivalents of HC1 was not
determined. The
same principle will also apply to all other salt forms referred to in the
experimental part, such
0
OH
as e.g., 'oxalate salt', 'formate salt' or' 0 salt
[0885] A skilled person will realize that, even where not mentioned explicitly
in the
experimental protocols below, typically after a column chromatography
purification, the
desired fractions were collected and the solvent was evaporated.
[0886] In case no stereochemistry is indicated, this means it is a mixture of
stereoisomers,
170
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
unless otherwise is indicated or is clear from the context.
[0887] When a stereocenter is indicated with 'RS' this means that a racemic
mixture was
obtained at the indicated center, unless otherwise indicated.
Example 1 ¨ Synthesis of (R)-N-ethyl-5-fluoro-N-isopropyl-24(5-(2-(6-((2-
methoxyethyl)
(methyl)amino)-2-methylhexan-3-y1)-2,6-diazaspiro [3.4] octan-6-yl)-1,2,4-
triazin-6-yl)oxy)
benzamide (Compound A) ¨ Preparation Method A
Preparation of intermediate I - tert-butyl (5-methyl-4-orohexAcarbamate
0
Boc > Mg TMEDA, THF
Br
[0888] To a solution of tert-butyl 2-oxopyrrolidine-1-carboxylate (5.0 g, 27
mmol) and
TMEDA (5.0 mL, 33 mmol) in THF (60 mL) cooled at -70 C was slowly added
isopropylmagnesium bromide solution (19 mL, 55 mmol, 2.9 M in 2-
methyltetrahydrofuran),
the resulting mixture was slowly warmed to RT and stirred for 12 h. The
mixture was poured
into sat. aq. NH4C1 (50 mL) solution and extracted with Et0Ac (50 mL x 3). The
combined
organic layers were dried over anhydrous Na2SO4, filtered, and concentrated
under reduced
pressure to give the crude product, which was further purified by FCC
(PE/Et0Ac = 1:0 to
100:1) to afford the title intermediate (3.7 g, 60% yield) as a yellow oil.
Preparation of intermediate 13 - tert-hutyl 6-(3,6-dichloro-1,2,4-triazin-5-
y1)-2,6-diazaspiro
[3.4Joctane-2-carboxylate
Boc
Boc
CI
N TEA, DCM
N
-N CI N Cl.'1)I N
N-NCI
[0889] To the solution of 3,5,6-trichloro-1,2,4-triazine (10.0 g, 54.2 mmol)
and TEA (15.2 mL,
109 mmol) in DCM (100 mL) cooled at 0 C was added tert-butyl 2,6-
diazaspiro[3.4]octane-
2-carboxylate (9.21 g, 43.4 mmol), the mixture was warmed to RT and stirred
for 1 h. The
mixture was diluted with water (20 mL) and extracted with DCM (30 mL x 3). The
combined
organic layers were washed with brine, dried over Na2SO4, filtered and
concentrated under
reduced pressure to give the crude product which was purified by FCC on silica
gel (PE/Et0Ac
171
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
= 1:0 to 3:1) to afford the title intermediate (12.0 g, 58% yield) as a yellow
solid.
Preparation of intermediate 27- N-ethyl-5-fluoro-N-isopropyl-2-
methoxybenzamide
HO 0
HATU, DIEA, DCM 0
+
0
[0890] To the mixture of 5-fluoro-2-methoxybenzoic acid (8.00 g, 47.0 mmol)
and N-
ethylpropan-2-amine (8.19 g, 94.0 mmol) in dry DCM (150 m_L) cooled at 0 C,
were slowly
added HATU (21.5 g, 56.5 mmol) and DMA (9.10 g, 70.4 mmol) in portions. The
resulting
mixture was slowly warmed to RT and stirred for 8 h. The organic layer was
washed with water
(20 mL x 3) and dried over anhydrous Na2SO4. After filtration, the solvent was
removed under
reduced pressure and the crude product was purified by FCC (Et0Ac/PE = 0% to
20%) to afford
the title intermediate (12.0 g, 96% yield) as a white solid.
Preparation of intermediate 28 - N-ethy1-5-fluoro-2-hydroxy-N-
isopropyThenzarnide
N 0 N 0
BBr3, DCM
0 OH
[0891] To the solution of N-ethyl-5-fluoro-N-isopropy1-2-methoxybenzamide
(intermediate 27)
(12.0 g, 50.1 mmol) in dry DCM (100 mL) cooled at -78 C was slowly added BBr3
(14.4 mL,
152 mmol), the resulting mixture was slowly warmed to RT and stirred for 8 h.
The mixture
was cooled to -78 C again and Me0H (5 mL) was added dropwise to quench the
reaction. The
resulting mixture was slowly warmed to RT and the pH value was adjusted to
about 8 by adding
sat. aq. NaHCO3 solution. The aqueous layer was extracted by DCM (50 mL x 3)
and the
combined organic layers were dried over anhydrous Na2SO4, filtered and
concentrated under
reduced pressure to give the crude product which was purified by FCC (Et0Ac/PE
= 0% to
20%) to afford the title intermediate (9.0 g, 78% yield) as a white solid.
172
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Alternative preparation of intermediate 28
HO 0
N 0
OH 1,1-Carbonyl diimidazole (1.2 eq.)
THF,15-25 C OH
[0892] A mixture of 5-fluoro-2-hydroxy-benzoic acid (14.0 kg, 89.68mo1, 1.0
equiv.) in THF
(168 L, 12 volumes) was adjusted to between 15-25 C, and 1,1-
carbonyldiimidazole, (17.45kg,
107.62 mol, 1.2 equiv.) was added over a period of 1 hour. After addition, the
mixture was
stirred for 18 hours at 15-25 'C. After this time N-ethylpropan-2-amine
(14.85kg, 170.39mo1,
1.9 equiv.) was added to the mixture at 15-25 C over a period of 2 hours. The
resulting mixture
was further aged for between 18-24 hours at 15-25 C. The pH was the adjusted
to between
pH4-5 with aq. 10% H2SO4(140kg, 10 volumes) and the layers were separated. The
organic
phase was concentrated to between 42-56L maintaining a temperature below 40 C,
and then n-
heptane (43kg, 4.5 volumes) was added to the mixture at 15-25 C over a period
of 3 hours. The
mixture was then cooled to 0-10 C and stirred for an additional 6 hours. The
resulting slurry
was filtered and the cake was washed with a tert-butyl methyl ether (MTBE):n-
heptane mixture
(25 kg of a 2:3 volume/volume mixture of MTBE:n-heptane, 2.5 volumes). The
cake wash was
repeated a further two times and the resulting solid was dried in-vacuo at 50
C to afford
intermediate 28 (16.5 kg, purity: 99.1%, yield: 80.4%).
Preparation of intermediate 14 - tert-butyl 6-(3-chloro-6-(2-
(ethyl(isopropyl)earbamoyl)-4-
fluorophenoxy)-1,2,4-triazin-5-y1)-2,6-diazaspiro[3.41octane-2-carboxylate
poc
Boc
N
DB U, THF N 0
OH
CI
N-NCI NCI
[0893] The mixture of tert-butyl 6-(3,6-dichloro-1,2,4-triazin-5-y1)-2,6-
diazaspiro[3.4]octane-
2-carboxyl ate (intermediate 13) (12.0 g, 33.3 mmol), AT-ethy1-5-fluoro-2-
hydroxy-AT-
isopropylbenzamide (intermediate 28) (7.5 g, 33.3 mmol) and DBU (6.1 g, 40.1
mmol) in THY
(120 mL) was stirred at 25 C for 8 h. The mixture was diluted with water (30
mL) and extracted
with DCM (30 mL x 3). The combined organic layers were washed with brine,
dried over
173
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Na2SO4, filtered and concentrated under reduced pressure to give the crude
product which was
purified by FCC (PE/Et0Ac = 1:0 to 3:1) to afford the title intermediate (14.0
g, 73% yield) as
green solid.
Preparation of intermediate 2 - tert-butyl 6-(6-(2-(ethyl(isopropyl)carbamoyI)-
47fhtoro-
phenoxy)-1,2,4-triazin-5-y1)-2,6-d1azasp1ro[3. 4Joctane-2-carboxylate
Synthesis method A for intermediate 2:
,Boc
,Boc
NaBH4, TMEDA
0N 0
Pd(dppf)C12=DCM, THF
I
NCI F
NN
108941 To the mixture of tert-butyl 6-(3-chloro-6-(2-
(ethyl(isopropyl)carbamoy1)-4-
fluorophenoxy)-1,2,4-triazin-5-y1)-2,6-diazaspiro[3.4]octane-2-carboxylate
(intermediate 14)
(20 g, 36.4 mmol), NaBH4 (2.48 g, 65.7 mmol) and TMEDA (8.54 g, 73.5 mmol) in
THF (500
mL) was added Pd(dppf)C12=DCM (1.70 g, 2.08 mmol) under N2 atmosphere. After
addition,
the reaction mixture was stirred at 25 C for 14 h. The reaction mixture was
filtered and the
filtrate was concentrated, the residue was purified by FCC on silica gel
(Et0Ac) to afford the
title intermediate (15 g, 93% purity, 74% yield) as brown solid.
Synthesis method B fbr intermediate 2:
,Boc
,Boc
Pd/C, H2
TEA, Me0H
OyJN OyJN
N-NCI
108951 To the solution of tert-butyl 6-(3-chloro-6-(2-
(ethyl(isopropyl)carbamoy1)-4-
fluorophenoxy)-1,2,4-tri azin-5-y1)-2,6-di azaspiro[3 .4]octane-2-carboxyl ate
(intermediate 14)
(22.0 g, 40.1 mmol), TEA (15 mL) in Me0H (100 mL) was added Pd/C (wet, 5.0 g,
10%) The
resulting mixture was stirred under H2 atmosphere (30 psi) at 25 C for 8 h.
The reaction mixture
was filtered through a celite pad and the filtrate was concentrated in men to
afford the title
intermediate (25.0 g, crude), which was used directly in next step without
further purification.
174
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Preparation of intermediate 3 - 2-((5-(2,6-diazaspiro[3.4Joclan-6-y1)-1,2,4-
triazin-6-y1)oxy)-
N-ethyl-5-fluoro-N-isopropylbenzamide
,Boc
NH
r' TFA, DCM 0
0
-N
I
N I
N
F N -
[0896] To the solution of tert-butyl 6-(6-(2-(ethyl(isopropyl)carbamoy1)-4-
fluorophenoxy)-
1,2,4-triazin-5-y1)-2,6-diazaspiro[3.4]octane-2-carboxylate (intermediate 2)
(300 mg, 0.583
mmol) in DCM (5 mL) was added TFA (0.5 mL, 6.4 mmol), the resulting mixture
was stirred
at RT for 3 h. Then 10% NaOH (5 mL) solution was slowly added into the mixture
to adjust
the pH value to about 12, the resulting mixture was extracted with DCM (10 mL
x 3). The
combined organic layers were dried over anhydrous Na2SO4, filtered, and
concentrated in vacuo
to afford the title intermediate (220 mg, 90% yield) as a white solid.
Preparation of Compound 61 - tell-butyl (4-(6-(6-(2-
(ethyl(isopropyl)carbamoy1)-4-
.fluorophenoxy)-1,2,4-triazin-5-y1)-2,6-diazaspiro[3.4loctan-2-y1)-5-
inethylhexyl)carbamate
NH
r--
ZnCl2, NaBH3CN
HN¨Boc
0,
HN¨Boc Me0H, 80'C NO N
NN T NJ
öOLN
[0897] The mixture 2-45-(2,6-diazaspiro[3 .4]octan-6-y1)-1,2,4-triazin-6-
yl)oxy)-N-ethy1-5-
fluoro-N-isopropylbenzamide (intermediate 3) (1.0 g, 2.4 mmol), tert-butyl (5 -
methy1-4-
oxohexyl)carbamate (intermediate 1) (830 mg, 3.62 mmol) and ZnC12 (660 mg,
4.84 mmol) in
Me0H (15 mL) was stirred at 80 C for 0.5 h. Then NaBH3CN (310 mg, 4.93 mmol)
was added
and the resulting mixture was stirred at 80 C for 6 h. After cooled to RT,
the mixture was
concentrated under reduced pressure to give the crude product, which was
further purified by
preparative HPLC using a Waters Xbridge Prep OBD (column: C18 150x40 mm 10 um;
eluent:
ACN/H20 (0.05% ammonia) from 45% to 75% v/v) to afford the title compound (700
mg, 46%
yield) as colorless oil.
175
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Preparation of Compounds 62 and 63 - le rt-butyl (R)-(4-(6-(6-(2-
(ethyl(isopropyl) carbainoy1)-
47fluorophenoxy)-1,2,4-triazin-5-y1)-2,6-diazaspiro[3.4Joctan-2-y1)-5-
methylhexyl)carbamate
and ten-butyl (S)-(4-(6-(6-(2-(ethyl(isopropyl)cctrbamoyh-47fluorophenoxy)-
1,2,4-triazin-5-
y1)-2,6-diazaspiro[3.4loctan-2-y1)-5-methylhexyl)carbamate
N R __________________________________________________________
HN¨Boc HN¨Boc
SFC
0 C3 0 N./
0 0
N
N-NJ
Compound 61 Compound 62
N\
HN¨Boc
0
yL N
Compound 63
[0898] tert-butyl (4-(6-(6-(2-(ethyl(i sopropyl)carb amoy1)-4-fluorophenoxy)-
1,2,4-tri azin-5 -
y1)-2,6-diazaspirot3 .4] octan-2-y1)-5-methylhexyl)carbamate (compound 61)
(200 mg, 0.319
mmol) was purified by SFC over DAICEL CHlRALPAK IG (column: 250x30 mm 10 um;
isocratic elution: Et0H (containing 0.1% of 25% ammonia): supercritical CO2,
40%: 60% (v/v))
to afford the title compounds (compound 62) (85 mg, 42% yield) and (compound
63) (80 mg,
40% yield) both as light yellow oil.
Compound 64 - (R)-2-((5-(2-(6-amino-2-methylhexan-3-y1)-2,6-
diazaspiro[3.4Joctan-6-y1)-
1,2,4-triazin-6-yl)oxy)-N-ethyl-5-fluoro-N-isopropylbenzamide
\ \
N R __________________________________________________________________ R __
r- HN¨Boc TFA, DCM
N.2
0 0 Q
IN
176
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
[0899] To the solution of tert-butyl (R)-(4-(6-(6-(2-
(ethyl(isopropyl)carbamoy1)-4-
fluorophenoxy)-1,2,4-triazin-5-y1)-2,6-diazaspiro[3.4]octan-2-y1)-5-
methylhexyl)carbamate
(compound 62) (550 mg, 0.876 mmol) in DCM (4 mL) was slowly added TFA (4 mL),
and the
resulting mixture was stirred at 25 C for 1 h. The reaction mixture was
concentrated under
reduced pressure to give a residue. The residue was diluted in DCM (40 mL) and
the pH value
was adjusted to around 12 by aq. NaOH (2 M, 16 mL) solution. The aqueous layer
was extracted
with DCM (10 mL x 2). The combined organic layers were dried over anhydrous
Na2SO4,
filtered and concentrated in vacuo to afford the title compound (460 mg,
crude) as yellow solid,
which was used directly in next step without further purification.
Compound 11 - (R)-N-ethy1-5-fluoro-N-isopropy1-245-(2-(6-((2-
methoxyethyl)amino)-2-
methylhexan-3-y1)-2,6-diazaspiro[3.4Joctan-6-y1)-1,2,4-triazin-6-
y1)oxy)benzamide
0\
NR ________________________ NH2
N R _____________________________________________________________________
FI/N
+ , Cs2CO3,N. Nal
)._
0 isC5-3 Br 0 N
DMF, 80 C MW
0 1, 0 1,
)N
N, N,
Ni
[0900] The mixture of (R)-24(5-(2-(6-amino-2-methylhexan-3-y1)-2,6-
diazaspiro[3.4]octan-6-
y1)-1,2,4-triazin-6-yl)oxy)-N-ethy1-5-fluoro-N-isopropylbenzamide (compound
64) (120 mg,
crude), 1-bromo-2-methoxyethane (32 mg, 0.23 mmol), Cs2CO3 (222 mg, 0.681
mmol), NaI
(102 mg, 0.680 mmol) in DMF (1 mL) was stirred at 80 C via microwave
irradiation for 1 h.
After cooling to RT, the mixture was diluted with H20 (10 mL) and extracted
with Et0Ac (3 x
mL). The combined organic layers were washed with H20 (10 mL), dried over
Na2SO4,
filtered and concentrated under reduced pressure to afford the crude product
which was further
purified by HPLC over a Phenomenex Gemini-NX (column: 150x30 mm 5 um; eluent:
ACN/H20 (10mM NH4HCO3) from 51% to 71% (v/v)) and further purified by SFC over
DAICEL CHIRALCEL OD-H (column: 250x30 mm 5 urn; eluent: supercritical CO2 in
Et0H
(0.1% v/v ammonia) 25/25, v/v) to afford the title compound (5.13 mg, 96%
purity) as yellow
solid.
[0901] LC-MS (ESI) (Method 1): Rt = 2.997 min, m/z found 586.3 [M+E-1] .
Compound A (R)-N-ethyl-5-iluoro-N-isopropyl-245-(2-(642-methoxyethyl)
(methyl)amino)-2-methylhexan-3-y1)-2,6-diazaspiro13.4Joctan-6-y1)- I ,2,4-
triazin-6-yl)oxy)
177
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
benzamide
N
H/N¨\\_
R __________________________________ 0
N R _____________________________________________________________________ /
0 4,N HCOH, NaBH3CN 0
N AcOH, Me0H
Compound A
N-N I I
Compound 11
[0902] The mixture of (R)-N-ethyl-5-fluoro-N-i sopropy1-
2-((5-(2-(6-((2-
methoxyethyl)amino)-2-methylhexan-3 -y1)-2, 6-di azaspiro [3.4] octan-6-y1)-
1,2, 4-triazin-6-
yl)oxy)benzami de (compound 11) (40.0 mg, 0.068 mmol), formaldehyde (55.4 mg,
0.683 mol,
37% in water) and AcOH (8.2 mg, 0.137 mmol) in anhydrous Me0H (2 mL) was
stirred at
45 C for 1 h. Then, NaBH3CN (8.6 mg, 0.137 mmol) was added to the mixture and
the resulting
mixture was stirred at 45 C for another 1 h. After cooling to RT, the
reaction mixture was
treated with sat. aq. NaHCO3 (40 mL) to adjust the pH value to about 8 and
further extracted
with DCM (20 mL x 3). The combined organic layers were dried over anhydrous
Na2SO4,
filtered and concentrated under reduced pressure to give the crude which was
purified by
preparative 1-1PLC over Boston Prime (column: C18 150x30mm 5um, Mobile Phase
A: H20
(0.04% ammonia+10mM NH4HCO3), Mobile Phase B: ACN, Flow rate: 25 mL/min,
gradient
condition B/A from 50% to 80% (50%B to 80% B)) to afford the title compound
(9.62 mg,
99.10% purity, 23.3% yield) as yellow oil.
Example 2 ¨ Synthesis of (R)-N-ethyl-5-fluoro-N-isopropyl-2-45-(2-(6-02-
methoxyethyl)
(methyl)amino)-2-methylhexan-3-y1)-2,6-diazaspiro [3.4] oetan-6-yl)-1,2,4-
triazin-6-yl)oxy)
benzamide (Compound A) ¨ Preparation Method B
Preparation of intermediate 7 - 4-((tert-butoxycarbonyl)(methyl)amino)butanoic
acid
(130c)20 TEA, Me0H
0 0
[0903] To a solution of 4-(methylamino)butanoic acid hydrochloride (3.0 g,
19.5 mmol) and
TEA (7.78 mL, 58.6 mmol) in Me0H (30 mL) was added Boc20 (4.69 g, 21.5 mmol)
dropwise.
The mixture was stirred at RT for 2 h. The mixture was concentrated under
reduced pressure
and the residue was diluted with Et0Ac (100 mL), washed with cooled 0.1 N HC1
(70 mL x 2),
178
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
H20 (50 mL x 2) and brine (50 mL), dried over Na2SO4, filtered and
concentrated to afford the
title intermediate (1.80 g, crude) as colorless oil.
Preparation of intermediate 8 - tert-butyl (4-(methoxy(methyl)amino)-4-
oxobutyl)(methyl)
carbamate
HO, Boc o EDCI, HOBt, NMM, CHCI3
N
IV"-B G
0
[0904] To a solution of 4-((tert-butoxycarbonyl)(methyl)amino)butanoic acid
(intermediate 7)
(1.80 g, crude) in CHC13 (30 mL) was added N,O-dimethylhydroxylamine
hydrochloride (960
mg, 9.84 mmol), H013t (1.24 g, 9.18 mmol) and NMM (2.80 mL, 25.1 mmol). And,
then EDO-
(2.23 g, 11.6 mmol) was added and the reaction mixture was stirred at RT for 4
h. The reaction
mixture was diluted with DCM (100 mL), washed with 1N HC1 (30 mL x 3), sat.
aq. NaHCO3
(30 mL x 3) and brine (30 mL), dried over Na2SO4, filtered and concentrated
under in lactic) to
afford the title intermediate (1.70 g, crude) as colorless oil.
Preparation of intermediate 9 - tert-butyl methyl(5-methyl-4-
orohexyl)carbamate
THF, -70 C
Boc
,,F.Boc
0 ) __ Li
0 0
[0905] To a solution of tert-butyl (4-(methoxy(methyl)amino)-4-
oxobutyl)(methyl)carbamate
(intermediate 8) (200 mg, crude) in TUT' (5 mL) cooled at -70 C under N2
atmosphere was
added dropwise isopropyllithium (3.2 mL, 2.24 mmol, 0.7M in pentane). The
resulting mixture
was stirred at -70 C for 2 h. The mixture was quenched with sat. aq. NH4C1
(15 mL), extracted
with Et0Ac (30 mL x 2). The combined organic layers were washed with brine (30
mL), dried
over Na2SO4, filtered and concentrated under reduced pressure to give a crude
product. The
crude product was further purified by FCC (PE/Et0Ac = 10:1) to afford the
title intermediate
(60 mg) as colorless oil.
Preparation of Compound 60 - tert-butyl 64-(6-(6-(2-
(ethyl(isopropyl)carbamoyl)-1-
fluor ophenoxy)- I , 2 ,,l-triazin-5-yl)-2,6-diazaspiro [3 . octan-2-y1)-5-
methylhexyl) (methyl)
carbatnate
179
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
NH
\N-Boc
õT. N
-N + Boc ZnC12, NaBH3CN
,N ,0
_0
0 Me0H, 80 C
ii i Oyj'N
F
[0906] To a solution of 24(5-(2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-
yl)oxy)-N-ethyl-
5-fluoro-N-isopropylbenzamide (intermediate 3) (600 mg, 1.45 mmol) and tert-
butyl methyl(5-
methy1-4-oxohexyl)carbamate (intermediate 9) (330 mg, 1.37 mmol) in Me0H (50
mL) was
added ZnC12 (789 mg, 5.79 mmol). The resulting mixture was stirred at 80 C
for 2 h. Then
NaBH3CN (729 mg, 11.6 mmol) was added and the reaction mixture was stirred at
80 C
overnight. After cooling to RI, the mixture was concentrated under reduced
pressure to give a
crude residue, which was diluted with DCM (50 mL), quenched with sat. aq.
NH4C1 (50 mL)
and extracted with DCM (50 rriL x 3). The combined organic layers were washed
with brine
(50 mL), dried over Na2SO4, filtered and the filtrate was concentrated under
reduced pressure
to give a crude product which was further purified by FCC (DCM/Me0H = 10:1) to
afford the
title compound (400 mg, 42% yield) as white solid.
Compound 67- N-ethyl-5-fluoro-N-isopropyl-245-(2-(2-methyl-6-
(methylamino)hexan-3-yl)-
2,6-diazaspiro[3.4loctan-6-yl)-1,2,4-triazin-6-yhoxj)benzamide hydrochloride
\ Boc \ /NH
HCl/1,4-dioxane
ciN
0 DCM
0N
I
N I
HCl salt
[0907] To a solution of tert-butyl (4-(6-(6-(2-(ethyl(isopropyl)carbamoy1)-4-
fluorophenoxy)-
1,2,4-triazin-5-y1)-2,6-diaza spiro[3 4] octan-2-y1)-5-
methylhexyl)(methyl)carbamate
(compound 60) (1 g, 1.56 mmol) in DCM (10 mL) was added 4M HC1 in dioxane (5
mL, 20
mmol), the resulting mixture was stirred at RT for 1 h. The reaction mixture
was concentrated
in vacuo to afford the title compound (960 mg, crude, HC1 salt) which was used
directly in next
step without further purification.
Compound A - (R)-N-ethyl-57fluoro-N-isopropyl-2-((5-(2-(6-((2-methoxyethyl)
(methyl)
amino)-2 -methylhexan- 3-yl)-2, 6-diazaspiro [3 . octan-6-yh- I, 2 ,z1-triazin-
6-yl)oxy) benzamide
180
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
\ F17¨
N 0
K2CO3, Nal
____________________________________________________ -.1=1 0
y-k-N Br
I
N DMF, 50 C
oY14
HC I salt F
N 1 Compound
68
Compound 67
SFC
5-1\s __________________________________________________________________ \_./
0
0yJN Compound A
[0908] To the mixture of AT-ethyl-5-fluoro-AT-i sopropy1-245-(2-(2-m ethyl -6-
(m ethyl amino)
hexan-3 -y1)-2,6-diazaspiro[3 .4] octan-6-y1)-1,2,4-tri azin-6-yl)oxy)b
enzamide hydrochloride
(compound 67) (480 mg, crude), K2CO3 (700 mg, 5.07 mmol) and NaI (400 mg, 2.67
mmol) in
D1VIF (5 mL) was added 1-bromo-2-methoxyethane (230 mg, 1.65 mmol). The
resulting
mixture was stirred at 50 C overnight. After cooled to RT, the reaction
mixture was quenched
with H20 (30 mL) and extracted with DCM (30 mL x 3). The combined organic
layers were
washed with brine (30 mL x 3), dried over Na2SO4, filtered and concentrated to
give a crude
residue. The residue was purified by FCC (DCM/Me0H = 10:1) to afford N-ethy1-5-
fluoro-N-
i sopropy1-2-45-(2-(6-((2-m ethoxy ethyl)(m ethyl)ami no)-2-m ethylhexan-3 -
y1)-2,6-
diazaspiro[3 .4]octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide (compound 68)
(250 mg, 48%
yield) as yellow oil.
[0909] The N-ethyl-5-fluoro-N-i sopropy1-24(5-(2-(64(2-
methoxyethyl)(methypamino)-2-
methylhexan-3-y1)-2,6-diazaspiro[3 . 4] octan-6-y1)-1,2,4-triazin-6-yl)oxy)b
enzamide
(compound 68) (960 mg, combined from several batches obtained by Method B) was
first
separated by SFC using DA10EL CH1RALPAK 1G (column: 250x30mm 10um; Mobile
phase:
A: Supercritical CO2, B: Et0H (0.1% ammonia), A:B=40:60 at 60 mL/min) and
further purified
by preparative ITPLC using Boston Prime (column: 150x30mm 5um, Mobile Phase A:
1420
(10mM NH4HCO3), Mobile Phase B: ACN, Flow rate: 25 mL/min, gradient condition
B/A from
55% to 85%) to afford the title compound (270 mg) as colorless oil.
181
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0910] 1H NMR (400 MHz, Methanol-d4): 6 = 8.40 (s, 1H), 7.47-7.32 (m, 1H),
7.30-7.10 (m,
2H), 4.24-4.01 (m, 2H), 3.89-3.60 (m, 3H), 3.48 (br s, 3H), 2.63-2.51 (m, 2H),
2.43-2.32 (m,
2H), 2.29-2.07 (m, 6H), 1.86-1.72 (m, 1H), 1.62-1.44 (m, 2H), 1.39-1.02 (m,
10H), 0.99-0.66
(m, 9H). Some protons were hidden by the solvent peak and are not reported.
[0911] LCMS (ES!) (Method 2): Rt = 1.965 min, m/z found 600.3 [M-P111+.
[0912] SFC (Method 11): Rt = 4.904 min.
Example 3 ¨ Synthesis of (R)-N-ethyl-5-fluoro-N-isopropyl-2-45-(2-(6-02-
methoxyethyl)
(methyl)amino)-2-methylhexan-3-y1)-2,6-diazaspiro [3.4] octan-6-yl)-1,2,4-
triazin-6-yl)oxy)
benzamide (Compound A) ¨ Preparation Method C
Preparation of intermediate 227- tert-butyl (R)-(1-(2,2-dimethy1-4,6-dioxo-1,3-
dioxan-5-y1)-3-
methylbutan-2-Acarbaniate
0
1. 0 0
0 0
DCC, DMAP, DCM
0
HN'Boc 2. NaBF14, AcOH
Boc,NH00
[0913] Boc-L-valine (44.9 kg), 2,2-dimethy1-1,3-dioxane-4,6-dione (32.9 kg)
and DMAP (35.5
kg) in DCM (607 kg) pre-cooled at -10 to 0 C were added to a solution of DCC
(55.5 kg) in
DCM (613 kg) over 3 h and aged for 16 h at -10 to 0 C. 10% citric acid aqueous
solution (449
kg) was added whilst maintaining a temperature below 10 C. The resulting
slurry was aged for
2 h at 0 to 10 C then filtered. The filter cake was washed with DCM (91 kg).
The filtrate was
separated and the organic layer was washed with 10% citric acid aqueous
solution (two times
450 kg) and 10% NaCl aqueous solution (449 kg). To organic phase (1200 kg),
was added
acetic acid (75.0 kg) whilst maintaining a temperature between -10 to 0 C.
Sodium Borohydride
(18.0 kg) was added in portions over 5 h whilst maintaining a temperature in
the range -10 to
0 C and then resulting mixture was aged at -10 to 0 C for an additional 16 h.
The mixture was
warmed to 15 to 25 C, and aged for 2 h. The mixture was then washed with 14%
NaCl aqueous
solution (450 kg) followed by a second wash with 14% NaCl aqueous solution
(432 kg) and a
final water wash (444 kg). The organic phase was concentrated under reduced
pressure to 2-4
vol. !so-propanol (143 kg) was added to the residue and concentrated to 4-5
vol. under reduced
pressure. After cooling to -10 to 0 C and aging for 8 h, the resulting slurry
was filtered, washed
182
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
with IPA (38 kg) and dried to afford the title intermediate (46.7 kg, 69%
yield) as a white solid.
Preparation of intermediate 228 - tert-butyl (R)-2-isopropy1-5-aropyrrolidine-
I-carboxy1ate
0
Reflux
Boc",NH00 Toluene -7i-- Boo
[0914] tert-butyl (R)-(1-(2,2-di m ethy1-4, 6-di oxo-1,3 -di oxan-
5 -y1)-3 -methylbutan-2-
yl)carbamate (intermediate 227) (46.7 kg) in toluene (333 kg) was heated to
reflux and aged for
4 h. The mixture was cooled to ambient temperature, filtered and washed with
toluene (20 kg).
The combined filtrates were concentrated to dryness at reduced pressure to
afford the desired
compound (31.05 kg, 96% yield) as an oil which was used directly without
further purification.
Preparation of intermediate 229 - tert-butyl (5R)-2-hydroxy-5-
isopropylpyrrolidine-1-
carboxylate
0 LiBH4 OH
Boo 2-MeTHF
[0915] tert-butyl (R)-2-i sopropy1-5 -oxopyrrol i di ne-l-carb oxyl ate
(intermediate 228) (30.9 kg)
in 2-MeTHF (26.7 kg) was cooled to -5 to 5 C. A solution of LiBH4in 2-MeTHF
(1M, 45.2 kg,
54.4 mol) was added over 3 h and the mixture was aged for 4 h. A cold aqueous
solution of 5%
NaHCO3 (163 kg) was added at -5 to 5 C over 3h and aged for an additional 2
h. The mixture
was warmed to ambient temperature and aged for a further 2 h. The aqueous
layer was separated
and the organic layer was washed with 10% NaCl aqueous solution (170 kg) and
water (155
kg). During the water wash, an emulsion formed and solid NaCl (3.1 kg) was
added to affect
the separation. After removal of the aqueous layer, the organic layer was
concentrated under
reduced pressure to dryness to afford the desired compound (28.5 kg, 91%
yield) as an oil,
which was used directly without further purification.
Preparation of intermediate 230 - tert-butyl (R)-(64(2-
methoxyethyl)(methyl)amino)-2-
methylhexan-3-Acarbamate
183
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
MeOCH2CH2NHMe
NaBH(OAc)3
OH DCM
BocHN'Boc
[0916] tert-butyl (5R)-2-hydroxy-5 sopropylpyrrol i dine-1 -carboxyl ate
(intermediate 229)
(28.55 kg) in DCM (344 kg), at 15 to 25 C was treated with 2-methoxy-N-
methylethan-1 -amine
(12.3 kg, 138.0 mol) and the resulting mixture was aged for 1 h. Sodium
triacetoxyborohydride
(40.12 kg) was added in portions over 5h whilst maintaining a temperature
between 15 to 25 C
and the resulting mixture was aged for 48 h. The reaction mixture was quenched
by the addition
of 8% NaOH aqueous solution (184 kg) over 2 h whilst maintaining a temperature
between 15
to 25 C and the mixture was aged for a further 2 h. The water layer was
separated, and the
organic layer was washed with water (169 kg). The organic layer was then
concentrated under
reduced pressure to dryness to afford the title intermediate (33.26 kg, 88%
yield) as an oil which
was used directly without further purification.
Preparation of intermediate 231 - (R)-N1-(2-methoxyethyl)-N1,5-dimethylhexane-
1,4-diamine,
dihydrochloride
HCI / IPA
HN,
Boc NH2 2 HCI
[0917] To 4 molar solution of HC1 in iso-propanol (84.80 kg) at ambient
temperature was added
a solution of tert-butyl (R)-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-
3-
yl)carbamate (intermediate 230) (32.38 kg) in iso-propanol (25.6 kg) over 3 h
and the mixture
was aged at ambient temperature for an additional 19 h. Methyl tert-butyl
ether (95.25 kg) was
then added over 1 h and the mixture was aged for 2.5 h. The resulting slurry
was filtered and
washed with MTBE (53 kg). The filter cake was dried to afford the title
compound (23.92 kg,
81% yield) as a white solid.
Preparation of intermediate 232 - ethyl 1-benzy1-3-(chloromethyl)pyrrolicline-
3-carboxylate
CO2Et DIPEA (1.1 eq.) CI
'(n-BuLi (1.0 eq.) 47\5CO2Et N.0 THF
ICH2CI (1.2 eq.)
Bn -78 to -60 C
[0918] To a solution of D1PEA (952 g, 1.1 eq.) in THY (6 L) which was cooled
to -35 to -25 C
184
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
was added n-BuLi (2.33 kg, 2.5 M in hexane, 1.0 eq.) whilst maintaining a
temperature below
-25 C. The resulting mixture was aged at -35 to -25 C for an additional 30 min
then cooled to
between -78 to -60 C. A solution of ethyl 1-benzylpyrrolidine-3-carboxylate (2
kg, 1.0 eq.) in
THF (2 L) at -78 to -60 C was added and stirred for an addition 30 min.
Chloroiodomethane
(1.81 kg, 1.2 eq.) was then charged at -78 to -60 C. The reaction mixture was
aged at -60 to -
40 C for 2 h. To the reaction mixture was added to citric acid aqueous
solution (660 g in 6 L
H20) at a temperature between 0 to 10 C and the resulting mixture was aged at
20 to 30 C for
an additional 20 min. After separating the layers, the aqueous layer was
extracted with Et0Ac
(6 L) and the combined organic layers washed with brine (6 L) then warmed to
50 to 60 C.
Oxalic acid (2.22 kg) was charged at 50 to 60 C. The resulting mixture was
stirred at 50 to 60 C
for 3 h then cooled to 20 to 30 C and aged overnight. The resulting solid was
filtered and the
cake was washed with ethyl acetate (2 L). The wet cake was added to toluene (4
L), H20 (8 L)
and K3PO4 (1.5 eq.) and the resulting mixture was aged at 20 to 30 C for 20
min. After
separating the layers, the aqueous layer was extracted with toluene (2 L). The
organic layers
were combined and washed twice with water (2 L). The organic phase was
concentrated under
reduced pressure to afford 4.2 kg of the desired compound as a toluene
solution (46 wt % by
assay, giving an assay yield of 80%).
Preparation of intermediate 233 - 1-benzy1-3-(ch1oromethyOpyrrolidine-3-
carbaldehyde
CI Flow
CO2Et 10)* CI '0
DIBAL-H (2.0 eq.) Ns
Bn toluene Bn
-65 to -55 C
[0919] Reaction conducted in a flow chemistry system: A solution of ethyl 1-
benzy1-3-
(chloromethyl)pyrrolidine-3-carboxylate (intermediate 232) (4.4 kg) in toluene
(26 L) was
pumped at 26.7 mL/min and cooled to -60 C. After cooling, it was then mixed
with a cooled
solution of DIBAL-H (28.1 mol) in toluene at -60 C (28 L) with a pumping rate
of 32.1 mL/min.
The mixture was passed through a Perfluoroalkoxy (PFA) coil tube reactor at
¨60 C (total flow
rate of 58.8 mL/min with a residence time of 5 seconds). The resulting mixture
was mixed with
cooled Me0H (-60 C) which was pumped at the rate of 15.2 mL/min. This mixed
solution was
pumped to another PFA coil tube reactor at ¨60 C (total flow rate of 74 mL/min
with a residence
time of 5 seconds). The resulting mixture was collected into a receiver which
contained 20 wt %
aq. solution Rochelle' s salt (20 V). The layers were separated, and the
organic phase was twice
185
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
washed with water (2 x 44 L). The organic phase was combined with another 3.0
kg batch
prepared in an analogous manner and concentrated under reduced pressure to
afford 20.8 kg of
a toluene solution of the desired compound (25.5 wt % assay by HPLC, giving an
assay yield
of 85%) which was used directly without further purification.
[0920] 111 NMR (300 MHz, Chloroform-d): 6 9.62 (s, 1H), 7.39 - 7.20 (m, 5H),
3.83 - 3.57
(m, 4H), 2.96 (d, J = 10.2 Hz, 1H), 2.80 - 2.55 (m, 3H), 2.17 (ddd, J = 13.9,
7.9, 6.1 Hz, 1H),
1.83 (ddd, J = 13.4, 7.8, 5.5 Hz, 1H).
Preparation of intermediate 234 - (R)-4-(6-benzy1-2,6-diazaspiro[3.4loctan-2-
y1)-N-(2-
methoxy ethyl)-N,5-dimethylhexan-l-amine
OMe
<
N- Cl2cCo K2HPO4 0.0 eq.)`
NH2 2 HCI ci FIN A H20 __
R
\-14, __________________________ k N-\ N-
Bn Et3N (2.0 eq.) \-0Me 50-55 C
\-0Me
NaBH(OAc)3 (3.2 eq.) N2 N
toluene
Bn
- Bn
15-25 C
[0921] To a solution of 1-benzy1-3-(chloromethyl)pyrrolidine-3-carbaldehyde
(intermediate
233) in toluene (3.0 kg, 10 wt %) diluted with toluene (30 L) and (R)-N1-(2-
methoxyethyl)-
M,5-dimethylhexane-1,4-diamine, dihydrochloride (intermediate 231) (3.47 kg)
was added
triethylamine (2.55 kg, 25.2 mol) at 20 to 30 C. The resulting mixture was
aged for 2 h at 20 to
30 C. Then sodium triacetoxyborohydride (9.0 kg) was charged at 20 to 30 C and
the mixture
was aged for 12 h. The reaction mixture was cooled to 5 to 15 C and 25 wt %
NaOH aqueous
solution (25 L, -16.75 eq.) was added maintaining a temperature below 35 C.
The resulting
mixture was aged at 20 to 30 C for 25 mins and the layers were separated. The
organic layer
was washed with 15 wt % aq. NaCl (10 L) and the layers were again separated
and water (18
L) was charged to the organic phase. The pH of the aqueous phase was adjusted
to 6-7 with
4M aq. HC1 whilst maintaining an internal temperature below 35 C. The organic
phase was
then discarded and the aqueous phase was separated and basified to pH 8-9 with
K2HPO4.
[0922] The resulting mixture was warmed to 50 to 55 C and aged for 3 h. The
reaction mixture
was then cooled to ambient temperature and combined with other two batches
(2.4 kg + 3.0 kg).
The combined streams were washed with methyl tert-butyl ether three times (3 x
40 L). To the
resulting aqueous layer was added additional methyl tert-butyl ether (83 L)
and the aqueous
phase was basified to pH 9-10 using 8 wt % aq. NaOH whilst maintaining a
temperature
between 15 to 35 C. The aqueous layer was separated, and the organic layer was
washed with
three times water (3 x 30 L). The organic layer was then concentrated under
reduced pressure
186
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
to approximately 3 volumes and then flushed with methanol three times (3 x 30
L) and
concentrated to dryness to afford the desired intermediate (12.4 kg, 90%
isolated yield) as light-
yellow oil, which was used directly without further purification.
Preparation of intermediate 234a (citric acid salt of intermediate 234)
citric acid salt
(equivalents not determined)
N
'B
[0923] Et0H (80 ml) and intermediate 234 (20 g) were added in a round bottom
flask. Next, a
0.5 M solution of citric acid in Et0H (100 ml; 1 equivalent) was added to the
mixture in the
round bottom flask at room temperature. Subsequently, the mixture was
evaporated till dryness
(Rotavap, 40 C). Acetonitrile (200 ml) was added to the residue and the
mixture was
evaporated till dryness (Rotavap, 40 C). Acetonitrile (100 ml) was added to
the residue and
stirred overnight on a magnetic heating plate at room temperature. Finally,
intermediate 234a
was filtered off and dried at room temperature.
Preparation of crystalline form of citric acid salt of intermediate 234
(intermediate 2346)
CO2H
. HO2C4\CO2H
OH
Bn
crystalline
ratio intermediate/citric acid 3/2
[0924] Intermediate 234a (3.72 g) was added to acetonitrile (20 ml) at room
temperature and
the mixture was stirred. The mixture was heated to 60 C until the reaction
mixture became
homogeneous (about 10 minutes). Next, the mixture was cooled to 50 C at a
rate of 0.5 C/min.
Next, seeds were added (19 mg of intermediate 234a; 0.5 w/w %) and the mixture
was aged
while stirring during 3 hours and 30 minutes. Next, the mixture was cooled non-
linear to 20 C
over 8 hours with an exponent of 2,3. The obtained mixture was stirred
overnight and the
product was filtered off and dried (overnight at room temperature in hood).
After isolation,
187
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
intermediate 234b was obtained (2.75 g; yield 73.9%) as the crystalline form
of the citric acid
salt of intermediate 234. The obtained ratio of the intermediate/citric acid
is 3/2 (NMR).
The non-linear cooling referred to above was done according to the formula
below:
A new linear ramp is started every 30 seconds during the defined duration of
the cooling. The
ramp is calculated according to the following equation:
t
T action 30s I
set T T
startvalue [(Tstartvalue endvalue) * (
Duration
Tset: Set value for each new ramp
Tstart value: Measured mixture temperature at the start of the cooling
trajectory
Tend value: Defined end value of cooling trajectory
taction: Actual time from the start of the cooling
Duration: Defined cooling duration
n: Exponent
1-1-1 NMR (400 MHz, Me0H-d4) 6 ppm 0.91 (3 H, d, J=6.88 Hz) 0.98 (3 H, d,
J=6.88 Hz) 1.46
- 1.57(2 H, m) 1.67- 1.87(2 H, m) 1.94 - 2.03 (1 H, m) 2.20 - 2.29 (2 H, m)
2.62 - 2.69 (2 H,
m) 2.72 -2.77 (4 H, m) 2.77 -2.82 (2 H, m) 2.90 (2 H, t, J=7.32 Hz) 2.95 -
3.02 (2 H, m) 3.07
- 3.16 (2 H, m) 3.16 - 3.22 (2 H, m) 3.37(3 H, s) 3.68 -3.72 (2 H, m) 3.83 -
3.89 (2 H, m) 3.90
- 3.92 (2 H, m) 3.94 -4.06 (2 H, m) 7.32 - 7.43 (5 H, m).
Preparation of intermediate 224 - (R)-N-(2-methoxyethyl)-N,5-dimethy1-4-(2,6-
diazaspiro
/3. 41 octan-2-yl)hexan- 1 -amine
\ \
NR _______________________________ Pd(OH)2/C, MSA
N R _______________________________________________________________
H2
\-0Me
OMe
Bn
109251 To palladium hydroxide on carbon (1.2kg) in Et0H (1.47 kg) cooled to -5
to 5 C were
added methanesulfoni c acid (MS A) (11kg), (R)-4-(6-benzy1-2,6-di azaspiro[3
.4]octan -2-y1 - N-
(2-methoxyethyl)-N,5-dimethylhexan-1-amine (intermediate 234) (10kg) and Et0H
(250L).
The mixture was warmed to 35-45 C and stirred under a hydrogen atmosphere
(0.27 to 0.40
1\SPa) for 16-20h. The mixture was filtered over diatomite (20kg) and the pad
was washed with
Et0H (24L). The filtrate was concentrated under reduced pressure (<40 C) to 2-
3 vol. and then
flushed twice with 2-MeTHF (73kg and 47kg) to give a 2-3 vol. solution. After
dilution with
188
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
2-MeTHF (65kg), 10% aq. sodium sulfate (30kg) was added and the mixture was
cooled to 0
to 10 C, followed by the addition of 16% aq. NaOH (50kg) to adjust the pH to
13-14. The
temperature was adjusted to 15 to 25 C and stirred for 30 to 60 min. The
aqueous layer was
separated and extracted twice with 2-MeTHF (47kg x 2). The combined organic
layers were
concentrated under reduced pressure (<40 C) to 3-4 vol. and 2-MeTHE (950g) was
added.
After concentration under reduced pressure (<40 C) to 3-4 vol., the resulting
solution was
diluted with 2-MeTHF (30kg), dried by passing through 4A molecular sieves
(25kg) and
washed with 2-MeTHF (30kg). The final solution was concentrated to afford the
desired
compound (6.7kg) as an oil with 90.1% assay purity in a 79% corrected yield.
Preparation of intermediate 225 - (R)-4-(6-(3,6-dichloro-1,2,4-triazin-5-y1)-
2,6-diazaspiro
[3.4]octan-2-y1)-N-(2-methoxyethyl)-N,5-dimethylhexan-1-amine
1. TEA(1.0 eq.), 2-MeTHF
R \¨\
N R ______________________________ 2. CI N CI
/ \-0
I \¨ -pi0 a
CI N-
N
-`N
NI
(0.95-1.0 eq)
c
109261 To (R)-N-(2-methoxyethyl)-N, 5 -dimethy1-4 -(2, 6-diazaspiro[3 .4]
octan-2-yl)hexan-1 -
ami ne (intermediate 224) (100 g) was added 2-MeTHF (430 g) and TEA (68 g) and
the mixture
was cooled to -50 to -40 C. 3,5,6-trichloro-1,2,4-triazine (62 g) in 2-MeTHF
(172 g) was added
and the mixture was stirred for 1 to 3 h. The resulting mixture was warmed to -
20 to -10 C and
a 7% NaHCO3 aqueous solution was added, the mixture was warmed to 20 to 30 C
and stirred
for 30 to 60 min. The aqueous layer was removed and the organic layer was
washed with 10%
Na2SO4 (500 g). The organic layer was dried by passing through 4A molecular
sieves (220 g)
and washed with 2-MeTHF (180 g). The title intermediate was afforded in 90%
assay yield as
a solution 14.8 wt% in 2-MeTHF.
Compound 393 - (R)-2-((3-chloro-5-(2-(64(2-methoxyethyl)(methypamino)-2-
methylhexan-3-
y1)-2,6-diazaspiro[3.4]octan-6-y1)-1,2,4-triazin-6-y1)oxy)-N-ethyl-5-fluoro-N-
isopropyl-
benzamide
189
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Synthesis method A for Compound 393:
N
OH
.71¨\ DBU, THF
\-0 ______________________________________________
N 0 )
N
NCI N ci
[0927] The mixture of N-ethyl-5-fluoro-2-hydroxy-N-isopropylbenzamide
(intermediate 28)
(1.10 g, 4.88 mmol), (R)-4-(6-(3,6-dichloro-1,2,4-triazin-5-y1)-2,6-
diazaspiro[3.4]octan-2-y1)-
N-(2-methoxyethyl)-N,5-dimethylhexan-1-amine (intermediate 225) (1.70 g, 3.82
mmol) and
DBU (750 mg, 4.93 mmol) in anhydrous THF (15 mL) was stirred at 40 C for 8 h.
After cooled
to RT, the mixture was concentrated under reduced pressure, the resulting
residue was diluted
with DCM (60 mL) and washed with H20 (20 mL x 3). The organic layer was dried
over
anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the
crude product
which was purified FCC (Me01-I/DCM = 0% to 10%) to afford a yellow oil (1.40
g), which was
further separated by SFC over DAICEL CHIRALPAK AD (column: 250x50 mm,10 um;
Mobile phase: A: Supercritical CO2, B: Et0H (0.1% ammonia), A:B = 50:50 at 70
mL/min;
Column Temp: 38 C; Nozzle Pressure: 100Bar; Nozzle Temp: 60 C; Evaporator
Temp: 20 C;
Trimmer Temp: 25 C; Wavelength: 220nm) to afford the title compound (1.0 g).
Synthesis method A for Compound 393:
NI/ R ________________________________ N
N R _________________________________________________________________
õ,e0
0 _________________________________________ 2-MeTH F
TMG
OH \¨ N az\
I
CI N 0 1,
N
I N 1
N
N CI NCI
[0928] To a 2-MeTHF solution
of (R)-4-(6-(3,6-dichloro-1,2,4-triazin-5-y1)-2, 6-
di azospiro [3 .4] octan-2-y1)-N-(2-m ethoxy ethyl)-N,5 -dim ethylhexan-1 -
amine (intermediate 225)
(676g of a 14.8 wt% solution in 2-MeTLIF, 100g corrected of intermediate 225)
and N-ethy1-5-
fluoro-2-hydroxy-N-isopropylbenzamide (intermediate 28) (50.6 g) in 2-MeTHF
(40 g) at 20
to 30 C was added tetramethylguanidine (31 g) and the mixture was stirred for
40 to 48 h. A
7% NaHCO3 aqueous solution (500g) was added and the mixture was stirred for 30
to 60 min.
The aqueous layer was removed and the organic layer was washed with twice with
4% NaOH
aqueous solution (2 x 500 g) and once with 10% Na2SO4 aqueous solution (500
g). The organic
layer was concentrated under reduced pressure (<40 C) to 2.2-3.0 vol. and
flushed three times
190
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
with Me0H (1 x 790g and 2 x 395g) until both 2-MeTHF and water content were
both <1.0%
to afford the desired compound in 86% assay yield as a 60.1 wt% solution in
methanol.
Compound A - (R)-N-ethyl-5-fluoro-N-isopropyl-2-((5-(2-(6-((2-methoxyethyl)
(methyl)
cunino)-2-methylhexan-3-yl)-2,6-diazaspiro[3.4J0c1an-6-yl)- I ,2,4-trictzin-6-
yl)oxy) benzcunide
\NI_/-13
R ___________________________________ 1. Wet Pd/C, H2 R __
2. MeOH, 20-30 C.
3. Filter.
N 0 7 \
_ 0
Compound 393
Compound A
N
TI
NNCI
[0929] A methanol solution of (R)-2-((3-chloro-5-(2-(6-((2-
methoxyethyl)(methyl)amino)-2-
methylhexan-3-y1)-2,6-diazaspiro[3 4] octan-6-y1)-1,2,4-tri azin-6-yl)oxy-N-
ethy1-5-fluoro-N-
isopropylbenzamide (compound 393) (163.93g of a 60.1 wt % solution in Me0H,
100g
corrected of compound 393), palladium on carbon (10 g) and Me0H (316 g) was
stirred at 20
to 30 C under a hydrogen atmosphere (0.20 to 0.30 Mpa) for 18 h. The mixture
was filtered
over diatomite (75 g) and the cake was washed with Me0H (158 g). The filtrate
was
concentrated under reduced pressure (<40 C) to ¨3 vol., then flushed with
isopropyl acetate
(IPAc, 870 g) concentrating to ¨3 vol. The mixture was then diluted with IPAc
(696 g) and a
20% Na2CO3 aqueous solution was added (500 g). The mixture was stirred for 30
to 60 min.
The aqueous layer was removed. The organic layer was washed with water (500 g)
then
concentrated under reduced pressure <45 C to ¨3 vol. The title intermediate
was afforded in
approximately 90% assay yield as a 48.1 wt% solution in IPAc.
Example 4 ¨ Synthesis of (R)-N-ethyl-5-fluoro-N-isopropyl-24(5-(2-(6-((2-
methoxyethyl)
(methyl)am ino)-2-methylhexan-3-y1)-2,6-diazaspiro 13.41octan-6-yl)-1,2,4-
triazin-6-yl)oxy)
benzamide oxalate (Compound A3)
N ___________________________ \ \
o
e
N
I
'lei oxalate salt
191
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Compound A3
[0930] To a solution of (R)-N-ethyl-5-fluoro-N-isopropy1-2-05-(2-(6-((2-
methoxyethyl)
(methyl)amino)-2-methylhexan-3 -y1)-2,6-diazaspiro[3 4] octan-6-y1)-1,2,4-tri
azin-6-yl)oxy)
benzamide (Compound A) (270 mg, 0.450 mmol) in 20 mL of ACN (20 mL) was added
oxalic
acid (81.0 mg, 0.900 mmol). After addition, the reaction mixture was stirred
at RT for 1 h. Then
the reaction mixture was concentrated, the residue was re-dissolved in ACN and
deionized
water, and lyophilized to afford the title compound (350 mg) as white solid.
[0931] 111 NMR (400 MHz, Methanol-4): 6 = 8.48 (s, 1H), 7.52-7.11 (m, 3H),
4.54-3.64 (m,
12H), 3.40-3.34(m, 5H), 3.23-3.13 (m, 2H), 2.90(s, 3H), 2.54-2.27 (m, 2H),
2.19-2.03 (m, 1H),
1.97-1.77 (m, 2H), 1.75-1.50 (m, 2H), 1.35-065 (m, 17H).
[0932] 111 NMR (400 MHz, DMSO-d6): = 8.51 (s, 1H), 7.51-7.29 (m, 3H), 4.29-
3.34 (m,
12H), 3.23-2.84 (m, 7H), 2.70 (s, 3H), 2.35-2.09 (m, 2H), 2.05-1.85 (m, 1H),
1.81-1.58 (m, 2H),
1.56-1.33 (m, 2H), 1.18-0.60 (m, 1711).
[0933] LCMS (ES!) (Method 2): It, = 1.969 min, m/z found 600.4 [M+H]
Example 5¨ Synthesis of Compound Al
N
1. Conc. HCI(1.9 eq), Et0H
2, IPAC, seed( 2%). 2
HCI
3. IPAc N
x H20
4. Filter.
0
2-3)
N¨N N¨N
Compound A Compound
Al
[0934] To a solution of Compound A (207.90 g of a 48 wt% solution in IPAc,
100g of active
Compound A) in IPAc (360 g) was added Et0H (63 g) at 20 to 25 C. The solution
was then
treated with conc. HCl (32.9 g) in Et0H (49.5 g) over ¨15 min. The mixture was
seeded with
crystalline Compound Al seed (2 g, 2% seed load) then aged for 18 h. IPAc (870
g) was added
slowly over 4 h at between 20 to 25 C and the slurry was stirred for an
additional 18 h. After
cooling to ¨5 C, the product was filtered, washed with IPAc (522 g) and dried
under vac at 20-
30 C to afford the weakly crystalline Compound Al as a white solid (91.0%
yield, 115.4 g).
(Note: A small amount of seed material used in the reaction was obtained via
an analogous
reaction protocol on small-scale.)
[0935] Recrystallisation: A solution of weakly crystalline Compound Al (100
g), Et0H (166
192
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
g), purified water (21.5 g) and IPAc (178 g) was stirred at 20 to 30 C for 0.5-
2 h to get a clear
solution. Extra IPAc (522 g) was added dropwise over 1-2 h, and then the
mixture was seeded
with crystalline Compound Al seed (2 g, 2% seed load). Then the mixture was
aged for 18 ¨20
h, IPAc (348 g) was added slowly over 12 h at between 20 to 30 C, and the
slurry was stirred
for an additional 55-60 h. The product was filtered, washed with IPAc (158 g)
and dried in
vacuo at 20-30 C to afford Compound Al as a white solid (85% yield, 85.0 g,
net).
[0936] 114NMR (DMSO-d6, 400MHz): 6 = 11.60 (1H, brs), 10.8 (1H, brs), 8.52
(1H, s), 7.36
(3H, m), 3.97-4.20 (7H, m), 3.64-3.71 (4H, m), 3.47 (7H, m), 3.25 (2H, m),
3.05 (3H, m), 2.73
(3H, s), 2.10-2.45 (1H, m), 1.99 (1H, m), 1.78 (2H, m), 1.55 (2H, m), 0.83-
1.12 (12H, m), 0.70
(2H, m).
[0937] LCMS (Method 7): Rt = 0.669 min, m/z found 600.5 [M-41]t
Example 6 ¨ Synthesis of crystalline form A of (R)-N-ethyl-5-fluoro-N-
isopropyl-
2-054246-02-m ethoxyethyl)(methyl)amino)-2-methylhexan-3-y1)-
2,6-diazaspiro [3.4]octan-6-yl)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate
salt hydrate
(Compound A4) (equivalent water not determined)
R _____________________________
_______________________________ \No
\
NR \
\ /
___________________________________________ = N 0
N 0
FNN
Compound A
I
N,N
I
bis-besylate salt hydrate
Crystalline form A
Compound A4
[0938] 43.06 g benzenesulfonic acid (2 equivalents with respect to the free
base Compound A)
was added to 840 ml of an acetone/water 95/5 v/v mixture and dissolved. 192.8
g of a solution
of Compound A (containing 80 g API) in IPAc was added. The material was
dissolved, resulting
in a clear solution. A further 80 ml of IPAc is added and the temperature was
adjusted to 25 C.
2% of seeds were added and the mixture is stirred for an hour at 25 C. Then
28.8 V (2312 ml)
of IPAc was added over a period of 8 hours. Afterwards the suspension was
stirred for 18 hours
at 25 C. The suspension was filtered and washed with 320 ml of a mixture of
acetone/water/IPAc 23.75/1.75/75 v/v/v. 122.91 g of crystalline form A bis-
besylate hydrate
(equivalent water not determined) was obtained.
193
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[0939] A skilled person will understand that a small amount of initial seed
material used in the
reaction above can be obtained via an analogous reaction protocol on small-
scale without
addition of seeds and wait for spontaneous
nucleation.
Initial seeds of the besylate salt were also obtained during salt screening
experiments. In these
experiments 100 mg of the free base was weighed into 2mL vials, and then 200pL
of ethyl
acetate or acetone was added to dissolve the free base. 1 eq counter-ions
(benzenesulfonic acid)
were added to the samples, and the samples were stirred at 25 C for 3 days.
The suspension
obtained was centrifuged and yielded initial seeds.
[0940] An appropriate amount of crystalline form A of (R)-N-ethy1-5-fluoro-N-
isopropy1-
2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-3 -y1)-2,6-
diazaspiro[3 .4] octan-
6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate salt hydrate was dissolved
in deuterated
DMSO and the 1D NMR spectrum was recorded.
[0941] A Bruker AVANCE NEO-600 MHz NMR spectrometer equipped with a Bruker 5
mm
PA BBO 600S3 BB-H-D-05 Z-GRD high resolution probe and running TOPSPIN 4.0
software,
was used to collect a 1-dimensional proton experiment at 300K on the sample in
deuterated
DMSO.
[0942]
NMR (600 MHz, DMSO-d6) 6 ppm 0.69 (br s, 2 H) 0.82 - 0.98 (m, 9 H) 1.07
(br s,
4 H) 1.31 - 1.46 (m, 1 H) 1.51 (br d, J=2.91 Hz, 1 H) 1.69 (br d, J=3.45 Hz, 2
H) 1.98 (br s, 1
H) 2.06 - 2.45 (m, 2 H) 2.77 (br s, 3 H) 2.87 - 3.19 (m, 3 H) 3.24 (br s, 1 H)
3.31 (s, 6 H) 3.64
(br s, 4 H) 3.71 -4.59 (m, 7 H) 7.24 - 7.54 (m, 9 H) 7.61 (br d, J=7.27 Hz, 4
H) 8.45 - 8.60 (m,
1 H) 9.24 (br s, 1 H) 9.44 -9.82 (m, 1 H).
Example 7 - Alternative synthesis of crystalline form A of (R)-N-ethy1-5-
fluoro-
N-isopropy1-2-05-(2-(6-02-m ethoxyethyl)(m ethyl)am in o)-2-m ethyl h ex an-3-
y1)-
2,6- diazas piro [3.4] octan-6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-
besylate salt hydrate
(Compound A4) (equivalent water not determined)
[0943] A mixture of isopropanol/water 95/5 (24 ml) was charged in a flask and
heated to 40 C.
Benzenesulfonic acid (4.31 g; 98%) was added. Subsequently, 19.3 g of a
solution of
Compound A (containing 8 g of Compound A) in lPAc was added. Another 16 ml of
IPAc was
added. 2% of seeds were added and the mixture was stirred for 1 hour at 40 C.
Then IPAc was
added (115.2 ml) dropwisc over a period of 8 hours. Next, the mixture was
cooled to 0 C for
15 hours. The suspension was filtered and the wet cake was washed with
(IPA/H20 95/5)/1PAc
1/6 (32 ml) The wet cake was dried at 25 C for 16 hours to obtain 11.44 g of
crystalline form
A bi s-besyl ate hydrate (equivalent water not determined).
194
CA 03215313 2023- 10- 12
WO 2022/237720 PCT/CN2022/091679
Crystalline form A
[0944] Crystalline form A of (R)-N-
ethy1-5-fluoro-N-isopropy1-
2-((5-(2-(6-((2-methoxyethyl)(methyl)amino)-2-methylhexan-3 -y1)-2,6-
diazaspiro[3 .4] octan-
6-y1)-1,2,4-triazin-6-yl)oxy)benzamide bis-besylate salt hydrate may be
characterised by an X-
ray powder diffraction pattern.
[0945] X-ray powder diffraction (XRPD) analysis was carried out on a
PANalytical Empyrean
diffractometer. The instrument is equipped with a Cu-Ka X-ray tube using iCore
and dCore
tunable optics for the incident and the diffracted beam, respectively. The
compound was loaded
into the cavity of a 16mm sample holder using the back loading technique.
[0946] Samples were run on XRPD using the method below:
Tube: Cu: K-Alpha (2=1.541874A)
Generator: Voltage: 45 kV; Current: 40 mA
Geometry: Bragg-Brentano
Scan mode: Continuous Scan
Scan Range: 3 to 35 deg.
Step size: 0.0131 deg.
Counting time: 30s
Spinner revolution time: 1 sec
Incident beam path (iCore)
Program. divergence slit: automatic
Irradiated length: 10 mm
Soller slit: 0.03 rad
Mask 1: 14 mm
Mask 2: 6 mm
Width: 7.7 mm
Diffracted beam path (dCore)
Anti scatter slit: automatic
Irradiated length: 10 mm
Soller slit: 0.04 rad
Detector: PIXcel3D- Medipix3 lx1
[0947] One skilled in the art will recognize that diffraction patterns and
peak positions are
195
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
typically substantially independent of the diffractometer used and whether a
specific calibration
method is utilized. Typically, the peak positions may differ by about 0.2
two theta, or less.
The intensities (and relative intensities) of each specific diffraction peak
may also vary as a
function of various factors, including, but not limited to particle size,
orientation, sample purity,
etc.
[0948] The X-ray powder diffraction pattern comprises peaks at 5.4, 7.2, 11.1,
11.9 and 21.7
degrees two theta 0.2 degrees two theta. The X-ray powder diffraction
pattern may further
comprise at least one peak selected from 13.7, 14.5, 14.7, 15.0, 16.5, 17.8,
19.0, 19.4, 20.1
degrees two theta 0.2 degrees two theta.
[0949] Form A may further be characterized by an X-ray powder diffraction
pattern having
four, five, six, seven, eight, nine or more peaks selected from those peaks
identified in Table 2.
[0950] Form A may further be characterized by an X-ray powder diffraction
pattern comprising
those peaks identified in Table 2, wherein the relative intensity of the peaks
is greater than about
2%, preferably greater than about 5%, more preferably greater than about 10%,
more preferably
greater than about 15%. However, a skilled person will realize that the
relative intensity of the
peaks may vary between different samples and different measurements on the
same sample.
[0951] Form A may further be characterized by an X-ray powder diffraction
pattern
substantially as depicted in FIG. 1.
[0952] Table 2 provides peak listings and relative intensity for the XPRD of
Crystalline form
A of
(R)-N-ethy1-5-fluoro-N-isopropy1-2-((5-(2-(6-((2-
methoxyethyl)(methyl)amino)-
2-m ethyl hexan-3 -y1)-2,6-di azaspiro[3 .4]octan-6-y1)-1,2,4-triazin-6-
yl)oxy)benzamide bis-
besylate salt hydrate BSA salt (FIG. 1).
Table 2 - Peak Listings and Relative Intensity for XRPD of Form A
Pos. [ 2Th.] Rel. Int. [%]
5.3965 16.30
7.1906 23.69
9.2513 8.14
9.4433 7.39
11.0719 11.34
11.9144 73.29
12.3921 29.17
12.5717 22.93
12.8791 8.93
13.6790 26.58
13.8694 15.67
14.4793 38.19
14.7398 55.26
14.9599 56.99
196
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
15.8715 20.66
16.4606 22.37
17.0459 20.43
17.4421 34.59
17.8203 46.78
18.2871 30.73
18.9573 43.91
19.4485 41.00
20.1190 35.53
20.7356 18.05
21.0535 30.09
21.6801 100.00
22.0236 18.06
22.7925 29.92
23.5044 41.92
23.9959 43.96
24.5555 31.47
25.1401 25.01
25.7588 59.24
26.0910 53.05
26.6137 39.47
27.5409 24.89
28.5493 22.44
29.1699 13.97
30.1441 21.01
31.2560 14.66
31.8783 16.47
32.7054 17.11
33.2797 24.40
33.9762 15.63
Compound A4 described herein is disclosed in PCT/CN2021/100466 (filed June 17,
2021),
which is incorporated by reference herein in its entirety, for all purposes.
ANALYTICAL METHODS
[0953] The analytical information in the compounds above or in the Tables
below, was
generated by using the analytical methods described below.
M1R-Methods
[0954] Some NAIR experiments were carried out using a Bruker Avance III 400
spectrometer
at ambient temperature (298.6 K), using internal deuterium lock and equipped
with BBO
400MHz Si 5 mm probe head with z gradients and operating at 400 MI-lz for the
proton and
197
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
100MHz for carbon. Chemical shifts (6) are reported in parts per million
(ppm). J values are
expressed in Hz.
[0955] Some NMR experiments were carried out using a Varian 400-MR
spectrometer at
ambient temperature (298.6 K), using internal deuterium lock and equipped with
Varian 400
4NUC PFG probe head with z gradients and operating at 400 MHz for the proton
and 100MHz
for carbon_ Chemical shifts (6) are reported in parts per million (ppm). J
values are expressed
in Hz.
[0956] Some NMR experiments were carried out using a Varian 400-VNMRS
spectrometer at
ambient temperature (298.6 K), using internal deuterium lock and equipped with
Varian 400
ASW PFG probe head with z gradients and operating at 400 MHz for the proton
and 100MHz
for carbon. Chemical shifts (6) are reported in parts per million (ppm). J
values are expressed
in Hz.
[0957] Some NMR experiments were carried out using a Bruker AVANCE III ETD 300
spectrometer at ambient temperature (298.6 K), using internal deuterium lock
and equipped
with PA BBO 300S1 BRF-1-1-D-05 7 5 mm probe head with z gradients and
operating at 300
MHz for the proton and 75 MHz for carbon. Chemical shifts (d) are reported in
parts per million
(ppm). J values are expressed in Hz.
LCMS (Liquid chromatography/Mass õspectrometry)
General procedure
109581 The High Performance Liquid Chromatography (HPLC) measurement was
performed
using a LC pump, a diode-array (DAD) or a UV detector and a column as
specified in the
respective methods. HPLC details are provided below in Table 3. If necessary,
additional
detectors were included (see Tables 3 and 4 below).
[0959] Flow from the column was brought to the Mass Spectrometer (MS) which
was
configured with an atmospheric pressure ion source. It is within the knowledge
of the skilled
person to set the tune parameters (e.g., scanning range, dwell time...) in
order to obtain ions
allowing the identification of the compound's nominal monoisotopic molecular
weight (MW).
Data acquisition was performed with appropriate software.
[0960] Compounds are described by their experimental retention times (Rt) and
ions. If not
specified differently in the table of data, the reported molecular ion
corresponds to the [M+H]+
(protonated molecule) and/or [M-H]- (deprotonated molecule). In case the
compound was not
directly ionizable the type of adduct is specified (i.e., [M+NH4] , [M+HC00]-,
etc...). For
198
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
molecules with multiple isotopic patterns (Br, Cl..), the reported value is
the one obtained for
the lowest isotope mass. All results were obtained with experimental
uncertainties that are
commonly associated with the method used.
[0961] Hereinafter, "SQD" means Single Quadrupole Detector, "RT- room
temperature, "BEET'
bridged ethylsiloxane/silica hybrid, "HS S" High Strength Silica, "DAD" Diode
Array Detector.
Table 3 - LCMS Method Codes
Flow
Method
Run
Instrument Column Mobile phase Gradient
code Column
time
100%A was held for 1 min, A
Waters Mobile phase A: gradient from 100%A to 40%
0.8
XBridge H20 with A is applied in 4 min, and
C18 0.04 % TFA; 40%A down to 15%A in 2.5
1 Agilent
10
(2.0x50 mobile phase B: min. And then return to
mm, 5 ACN with 100%A in 2 min and held for
u M) 0.02 % TFA 0.5 min. The post time is 0.5
min.
First, 90% A was held for 0.8
Waters Mobile phase A:
min. Then a gradient was
XBridge H20 with
C18 0.04 % TFA;
applied to 20% A and 80% B 0.8
2 Agilent in 3.7 min and held for 3
min. ---- 10
(2.0x50 mobile phase B:
And then return to 90%A in 2 50
mm, 5 ACN with
min and held for 0.5 min. The
urn) 0.02 % TFA
post time is 0.5 min.
Time (min) A% 13%
Mobile phase A
XBridge 0.05% 95 5 1.5
0.05% TFA in
Agilent LC C18, 4.6 H20 11.0 65 35
7 1260 with x150 13.0 5 95
20
Mobile phase B
MS6120 mm, 15.0 5 95
0.05 % TFA in
3.5 iõtrn 16.0 95 5 45
ACN
20.0 95 5
Flow expressed in mL/min; column temperature (T) in C; run time in minutes
Analytical SFC
General procedure for SFC methods
[0962] The SFC measurement was performed using an Analytical Supercritical
fluid
chromatography (SFC) system composed by a binary pump for delivering carbon
dioxide (CO2)
and modifier, an autosampler, a column oven, a diode array detector equipped
with a high-
pressure flow cell standing up to 400 bars. Analytical SFC details are
provided below in Table
4. If configured with a Mass Spectrometer (MS) the flow from the column was
brought to the
(MS). It is within the knowledge of the skilled person to set the tune
parameters (e.g., scanning
range, dwell time...) in order to obtain ions allowing the identification of
the compound's
nominal monoisotopic molecular weight (MW). Data acquisition was performed
with
199
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
appropriate software.
Table 4 - Analytical SFC Details
Run
Method Flowtime
column mobile phase gradient
code
Col T
RPR
Waters UPCC from 5% to 40% of
2-8 8
with PDA A: Supercritical B in 4 min and
11 (Chiralpak IG-3 CO2 B: Et0II hold 40% for 2.5
100x4.6 mm (0.05% DEA) min, then 5% of B 35
1500 psi.
ID., 3 urn) for 1.5 min
Flow expressed in mL/min; column temperature (T) in C; run time in minutes,
Backpressure
(BPR) in bars or pound-force per square inch (psi). "ACN- means acetonitrile;
"Me0H" means
methanol; "Et0H" means ethanol; "DEA" means diethylamine. All other
abbreviations used in
Table 4 above are as defined before.
PHARMACOLOGICAL PART
1) Menin/1V1LL Homogenous Time-Resolved Fluorescence (HTRF) Assay
[0963] To an untreated, white 384-well microtiter plate was added 40 nL 200X
test compound
in DMSO and 4 itL 2X terbium chelate-labeled menin (vide infra for
preparation) in assay
buffer (40 mM Tris=HC1, pH 7.5, 50 mM NaC1, 1 mM DTT (dithiothreitol) and
0.05% Pluronic
F-127). After incubation of test compound and terbium chelate-labeled menin
for 30 min at
ambient temperature, 4 uL 2X FITC-1VIBM1 peptide (FITC-I3-alanine-SARWRFPARPGT-
NH2) ("FITC" means fluorescein isothiocyanate) in assay buffer was added, the
microtiter plate
centrifuged at 1000 rpm for 1 min and the assay mixtures incubated for 15 min
at ambient
temperature. The relative amount of meninTITC-MBM1 complex present in an assay
mixture
is determined by measuring the homogenous time-resolved fluorescence (HTRF) of
the
terbium/FITC donor /acceptor fluorophore pair using an EnVision microplate
reader (ex. 337
nm/terbium em. 490 nm/FITC em. 520 nm) at ambient temperature. The degree of
fluorescence
resonance energy transfer (the HTRF value) is expressed as the ratio of the
fluorescence
emission intensities of the FITC and terbium fluorophores (Pm 520 nm/Fm 490
nm). The final
concentrations of reagents in the binding assay are 200 pM terbium chelate-
labeled menin, 75
nM FITC-1VIBM1 peptide and 0.5% DMSO in assay buffer. Dose-response titrations
of test
compounds are conducted using an 11 point, four-fold serial dilution scheme,
starting typically
at 10 itM.
[0964] Compound potencies were determined by first calculating % inhibition at
each
200
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
compound concentration according to equation 1:
% inhibition = ((HC - LC) - (HTRFcompound LC)) / (HC - LC)) *100 (Eqn 1)
where LC and HC are the HTRF values of the assay in the presence or absence of
a saturating
concentration of a compound that competes with FITC-MBM1 for binding to menin,
and
HTRFenmP"nd is the measured HTRF value in the presence of the test compound.
HC and LC
HTRF values represent an average of at least 10 replicates per plate. For each
test compound, %
inhibition values were plotted vs. the logarithm of the test compound
concentration, and the
1C'50 value derived from fitting these data to equation 2:
% inhibition = Bottom + (Top -B ottom)/(1+10^((log/C50-log [cm pd]) * h)) (Eqn
2)
where Bottom and Top are the lower and upper asymptotes of the dose-response
curve,
respectively, /C50 is the concentration of compound that yields 50% inhibition
of signal and h
is the Hill coefficient.
[0965] Preparation of Terbium cryptate labeling of Menin: Menin (a.a 1-610-
6xhis tag, 2.3
mg/mL in 20mM Hepes (2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethane sulfonic
acid), 80 mM
NaCl, 5mM DTT (Dithiothreitol), pH 7.5) was labeled with terbium cryptate as
follows. 200
ug of Menin was buffer exchanged into lx Hepes buffer. 6.67 uM Menin was
incubated with
8-fold molar excess NHS (N-hydroxysuccinimide)-terbium cryptate for 40 minutes
at room
temperature. Half of the labeled protein was purified away from free label by
running the
reaction over a NAPS column with elution buffer (0.1M Hepes, pH 7 + 0.1% BSA
(bovine
serum albumin)). The other half was eluted with 0.1M phosphate buffered saline
(PBS), pH7.
400 ul of eluent was collected for each, aliquoted and frozen at -80 C. The
final concentration
of terbium-labeled Menin protein was 115 ug/mL in Hepes buffer and 85 itg/mL
in PBS buffer,
respectively.
MENIN Protein Sequence (SEO ID NO: I):
[0966] MGLKAAQKTLFPLRSIDDVVRLFAAELGREEPDLVLLSLVLGFVEHFLAVNRV
1P TN VPELTF QP SPAPDPP GGL T YFP VADL SIIAAL Y ARF TAQ1RGAVDL SL Y PRE GGV S
SRELVKKV SD V1WN SLSRSYFKDRAHIQSLF SF1TGTKLD S SGVAFAVVGACQALGLR
DVHLALSEDHAWVVF GPNGEQ TAEVTWHGKGNEDRRGQTVNAGVAERSWLYLKG
SYMRCDRKMEVAFMVCAINPSIDLHTDSLELLQLQQKLLWLLYDLGHLERYPMALG
NLADLEELEP TP GRPDPL TLYHKGIA S AK T YYRDEHIYP YMYLAGYH CRNRNVREAL
QAWAD TATVIQDYNYCREDEEIYKEFFEVANDVIPNLLKEAA SLLEAGEERP GEQ SQ
GT Q S Q GS ALQDPECF AHLLRF YDGICKWEEGSP TPVLHVGWATFLVQ SLGRFEGQVR
QKVRIVSREAEAAEAEEPWGEEAREGRRRGPRRESKPEEPPPPKKPALDKGLGTGQG
201
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
AVS GPPRKPP GT VAGTARGPEGGS TAQVPAPAA SPPPEGPVLTF Q SEKMKGMKELLV
ATKINS S AIKLQL TAQ S Q VQMKKQKVS TP SDYTL SFLKRQRKGLE11-11111HH
2a) Proliferation Assay
[0967] The anti-proliferative effect of menin/MLL protein/protein interaction
inhibitor test
compounds was assessed in human leukemia cell lines. The cell line MOLM-14
harbors a MILL
translocation and expresses the WILL fusion protein MILL-AF9, respectively, as
well as the
wildtype protein from the second allele. OCI-AMIL3 cells that carry the NPM1c
gene mutation
were also tested. MILL rearranged cell lines (e.g., MOLM-14) and NPM1c mutated
cell lines
exhibit stem cell-like elevated HOXA/MEIS1 gene expression signatures. KO-52
was used as
a control cell line containing two MLL (KIVIT2A) wildtype alleles in order to
exclude compounds
that display general cytotoxic effects.
[0968] MOLM-14 cells were cultured in RPM1-1640 (Sigma Aldrich) supplemented
with 10%
heat-inactivated fetal bovine serum (HyClone), 2 mM L-glutamine (Sigma
Aldrich) and
50 g/m1 gentamycin (Gibco). KO-52 and OCI-AML3 cell lines were propagated in
alpha-
MEM (Sigma Aldrich) supplemented with 20% heat-inactivated fetal bovine serum
(HyClone),
2 mM L-glutamine (Sigma Aldrich) and 50 j.i.g/m1 gentamycin (Gibco). Cells
were kept at 0.3
¨ 2.5 million cells per ml during culturing and passage numbers did not exceed
20.
[0969] In order to assess the anti-proliferative effects, 200 MOLM-14 cells,
200 OCI-A1VIL3
cells or 300 KO-52 cells were seeded in 200 p1 media per well in 96-well round
bottom, ultra-
low attachment plates (Costar, catalogue number 7007). Cell seeding numbers
were chosen
based on growth curves to ensure linear growth throughout the experiment. Test
compounds
were added at different concentrations and the DMSO content was normalized to
0.3%. Cells
were incubated for 8 days at 37 C and 5% CO2. Spheroid like growth was
measured in real-
time by live-cell imaging (IncuCyteZOOM, Essenbio, 4x objective) acquiring
images at day 8.
Confluence (%) as a measure of spheroid size was determined using an
integrated analysis tool.
[0970] In order to determine the effect of the test compounds over time, the
confluence in each
well as a measure of spheroid size, was calculated. Confluence of the highest
dose of a reference
compound was used as baseline for the LC (Low control) and the confluence of
DMSO treated
cells was used as 0% cytotoxicity (High Control, HC).
[0971] Absolute IC50 values were calculated as percent change in confluence as
follows:
LC = Low Control: cells treated with e.g., I i_tM of the cytotoxic agent
staurosporin, or e.g.,
cells treated with a high concentration of an alternative reference compound;
HC = High Control: Mean confluence (%) (DMSO treated cells);
202
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
% Effect = 100 - (100*(Sample-LC)/(HC-LC)); and
GraphPad Prism (version 7.00) was used to calculate the IC50. Dose-response
equation was used
for the plot of % Effect vs Log10 compound concentration with a variable slope
and fixing the
maximum to 100% and the minimum to 0%.
2b) MEISI niRNA Expression Assay
[0972] MEIS1 mRNA expression upon treatment of compound was examined by
Quantigene
Singleplex assay (Thermo Fisher Scientific). This technology allows for direct
quantification
of mRNA targets using probes hybridizing to defined target sequences of
interest and the signal
is detected using a Multimode plate reader Envision (PerkinElmer). The MOLM-14
cell line
was used for this experiment. Cells were plated in 96-well plates at 3,750
cells/well in the
presence of increasing concentrations of compounds. After incubation of 48
hours with
compounds, cells were lysed in lysis buffer and incubated for 45 minutes at 55
C. Cell lysates
were mixed with human ATEIS1 specific capture probe or human RPL28 (Ribosomal
Protein
L28) specific probe as a normalization control, as well as blocking probes.
Cell lysates were
then transferred to the custom assay hybridization plate (Thermo Fisher
Scientific) and
incubated for 18 to 22 hours at 55 C. Subsequently, plates were washed to
remove unbound
materials followed by sequential addition of preamplifiers, amplifiers, and
label probe. Signals
(= gene counts) were measured with a Multimode plate reader Envision. IC5os
were calculated
by dose-response modelling using appropriate software. For all non-housekeeper
genes
response equal counts corrected for background and relative expression. For
each sample, each
test gene signal (background subtracted) was divided by the normalization gene
signal (RPL28:
background subtracted). Fold changes were calculated by dividing the
normalized values for
the treated samples by the normalized values for the DMSO treated sample. Fold
changes of
each target gene were used for the calculation of IC5(is.
109731 The results are summarized below in Table 5.
Table 5 - Biological data ¨ HTRF, Proliferation and MEIS1 mRNA Expression
Assays
HTRF- MEIS1 spheroid OCI- spheroid
Compound 30min ICso assay_OneTime AML3 assay_OneTime
Number incubation (pM) MOLM-14 ICso ICso KO-52
ICso
IC50 (nM) (PM) (p1M) (PM)
11 0.11 0.018 0.021 0.21 8.38
A 0.09 0.02 0.021 0.091 6.85
A3 0.098 0.017 0.017 0.12 7.75
Al 0.18 0.017 0.011 0.08
203
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
3) Mouse PK (In vivo T y? and oral bioavailahility)
[0974] In vivo pharmacokinetics (PK) were assessed in fasted male CD-1 mice
(age 6-8 weeks)
following a single intravenous (IV, 0.5 or 1.0 mg/kg administered at 2.5
ml/kg) or oral (PO, 5
mg/kg administered at 10 ml solution/kg) dose of test article formulated in a
20% (w:vol) HP-
I3-CD solution or in Pyrogen free water.
[0975] Plasma and/or whole blood samples were collected from the dorsal
metatarsal vein at
desired timepoints via serial capillary microsampling (approx. 0.03 mL) using
EDTA as an
anticoagulant. Concentrations of compound in the plasma and blood samples were
analyzed
using a qualified LC-MS/MS method. In silico analysis of main pharmacokinetic
parameters
was performed using WinNonlin (PhoenixTM, version 6.1) or similar software.)
4) Metabolic Stability in Human/Mouse Liver Microsomes
Experimental Procedure
[0976] The objective of this study is to measure in vitro metabolic stability
of test compound(s)
in human and mouse liver microsomes and provide quantitative information on
the rate of
metabolic turnover (i e , determination of the apparent intrinsic clearance of
test).
[0977] Test items were prepared at a stock concentration of 10 mM in DMSO. For
determination of metabolic turnover, a final working solution was prepared by
adding 2 pL of
mM DMSO stock solution for test compound or positive control compounds to 198
pL, of
acetonitrile (100 p..M final concentration).
[0978] Incubations were performed as follows: First, liver microsomes were
thawed on ice and
a master solution containing liver microsomes in 100 mM PBS (phosphate-
buffered saline) at
pH 7.4 is prepared. Next, the liver microsomes solution was added to the
incubation plates and
10 mM NADPH (Nicotinamide-adenine dinucleotide phosphate) was added (MW: 833.4
g/mol;
Roche Diagnostics GmbH, Germany. Dissolved in phosphate buffer (100 mmol/L, pH
7.4)).
The mixture was mixed for 10 seconds and pre-warmed in the incubation plate at
37 C for 10
minutes. The metabolic reaction was initiated with the addition of 5 pL of the
100 WV working
solution for test compound or positive control compounds to incubation plate
(final test item
concentration = 1 p.M). The reaction final mixture should contain 1 mM NADPH,
0.5 mg/mL
microsomes protein and 1 [TM test compound or positive control compound in 100
mM PBS at
pH 7.4. The percentage of organic solvent in incubation mixture is 1% with
DMSO 0.02%.
[0979] The reaction was quenched by transferring 50 ML of the incubated
mixture at selected
204
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
time points into the quenching plate containing 200 p.1_, of cold methanol.
After sampling of all
the timepoints the quenching plate was centrifuged at 4000 rpm for 40 minutes
to precipitate
protein. A total of 90 [IL of the supernatant was transferred to an analysis
plate and ultra-pure
H20 water is added into each well for LC/MS/MS analysis. All incubations and
analysis were
performed in duplicate.
Data analysis
[0980] All calculations were carried out using Microsoft Excel. The slope
value, k, was
determined by linear regression of the natural logarithm of the remaining
percentage of the
parent drug vs. incubation time curve. The results are summarized below in
Table 6.
[0981] The in vitro half-life (in vitro ti/2) was determined from the slope
value:
in vitro t1i2= - (0.693 / k)
Conversion of the in vitro t1/2 (in min) into the in vitro intrinsic clearance
(in vitro CLint, in
L/min/mg proteins) was done using the following equation:
0.693 volume of incubation (4)
in vitro Clint_ = ( ______________________ ) * _____________________
ti amount of proteins (mg)
Table 6 - Mouse PK and Metabolic Stability
In vivo
Bio- Human LM Mouse LM
Example Formulating T1/2
availability Clint
Clint
number agent (IV)
(PO) (%) i/mg) ( 1/m
in/m g)
(h)
A HP-13-CD 6.7 17 19
<7.5
Pyrogen free
A3 9.0 34 19 <7.5
water
11 HP-f3-CD NA NA 14
<7.5
"NA" means not analyzed
5) Protocol JOT Pharmacodynamics (PI)) Activity in Subcutaneous
(sc or SC) Xenografts
of1VIOL11/1-14 or OCI-Alt/IL3 Cells
Test Agents and Controls
[0982] Compound A3 was formulated in 20% hydroxypropyl-beta-cyclodextrin (HP-
I3-CD)
and prepared to reach a total volume of 0.2 mL (10 mL/kg) per dose for a 20 g
animal. Doses
were adjusted by individual body weight each day. Working stocks of Compound
A3 were
205
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
prepared once per week for each study and stored at room temperature. Compound
A3 was
administered orally (PO), daily.
Assay
109831 The in vivo pharmacodynamics (PD) activity of compounds was evaluated
in
subcutaneous (SC) xenografts of MOLM-14 cells or OCI-AML3 cells. Nude NMIRI
mice
(Crl:NMIRI-Foxn lnu/-) harboring MOLM-14 or OCI-AML3 tumors were treated with
3 daily
doses of vehicle or compounds. Plasma samples were collected at 23 hours after
day 2 dose,
0.5 hours post final dose, and 16 hours post final dose and tumor samples were
collected 16
hours post final dose. To examine the effects of compounds on the expression
of multiple
Menin-KMT2A target genes (e.g., MEISI, MEF2C, FLT3) QuantiGene Plex technology
(Thermo Fisher Scientific) was used. Frozen tumors were homogenized and
transferred to
individual lysing matrix tubes in lysis buffer and incubated for 30 minutes at
55 C. Cell lysates
were mixed with target-specific capture probes, Luminex beads, and blocking
probes,
transferred to the custom assay hybridization plate (Thermo Fisher Scientific)
and incubated
for 18 to 22 hours at 54 C. Subsequently, plates were transferred to a
magnetic separation plate
and washed to remove unbound materials from beads followed by sequential
hybridization of
preamplifiers, amplifiers, and label probe and subsequent streptavidin
phycoerythrin binding.
Signals from the beads were measured with a Luminex FlexMap three-dimensional
instrument.
For all non-housekeeper genes response equal counts corrected for background
and relative
expression. For each sample, each test gene signal (background subtracted) was
divided by the
normalization gene signal (RPL19, RPL28, ATP6V1A: background subtracted). Fold
changes
were calculated by dividing the normalized values for the treated samples by
the normalized
values for the DMSO treated sample. The results are summarized below in Tables
7 and 8.
Table 7 - Expression Level (% relative to vehicle) of Selected Genes from
MOLM-14 SC Model (mean values and standard deviations)
Compound A3 (mg/kg) MEIS1 FLT3 MEF2C
0 101.30 + 15.06 104.80 + 10.07 103.50 +
11.02
3 83.49 + 25.48 78.67 + 20.74 85.50 +
22.77
62.84 + 4.06 74.91 + 8.97 68.04 + 14.43
30 23.16 + 2.75 52.61 + 4.51 27.83
2.17
50 14.40 + 3.39 36.14 + 3.50 18.75 +
2.38
100 10.97 + 3.21 35.82 + 1.10 14.18 +
1.56
206
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 8 - Expression Level (% relative to vehicle) of Selected Genes
from OCI-AML3 SC Model (mean values and standard deviations).
Compound A3 (mg/kg) MEIS1
0 100.30 8.53
3 87.90 + 39.75
48.81 15.30
30 32.66 + 3.71
50 23.83 1.34
100 16.76 1.92
[0984] Tables 7a and 8a show median values based on repeated experiments in
optimized
conditions with fresh tumor samples.
Table 7a - Expression level ("A relative to vehicle) of selected genes from
MOLM-14 SC
model (Median values and Standard Deviations).
Compound A3 (mg/kg) MEIS1 FLT3 MEF2C
0 100.0 + 13.5 100.0 + 10.1
100.0 + 11.0
3 83.7 22.8 89.2 20.7
87.7 + 22.8
10 49.3 + 5.9 79.8 + 9.0
64.6 + 14.4
30 14.7 3.9 54.5 4.5
28.8 22
50 4.7 1.1 37.6 3.5
18.8 2.4
100 3.3 + 1.4 35.4 + 1.1
13.6 + 1.6
Table 8a - Expression level ("/0 relative to vehicle) of selected gene from
OCI-AML3 SC
model (Median values and Standard Deviations).
Compound A3 (mg/kg) MEIS1
0 100.0 11.2
3 71.2 15.1
10 26.5 + 4.3
30 25.1 11.2
50 8.5 2.2
100 9.4 1.2
6) Efficacy Study in MOLM-121 Subcutaneous Model
Test Agents and Controls
[0985] Compound A3 was formulated in 20% hydroxypropyl-beta-cyclodextrin (HIP-
13-CD)
and prepared to reach a total volume of 0.2 mL (10 mL/kg) per dose for a 20 g
animal. Doses
were adjusted by individual body weight each day. Working stocks of Compound
A3 were
prepared once per week for each study and stored at 25 C.
207
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Animals
[0986] Female MARI Nude mice (MOLM-14 SC) were used when they were
approximately 6
to 8 weeks of age and weighed approximately 25 g. All animals could acclimate
and recover
from any shipping-related stress for a minimum of 7 days prior to experimental
use. Autoclaved
water and irradiated food were provided ad libitum, and the animals were
maintained on a 12
hour light and dark cycle. Cages, bedding, and water bottles were autoclaved
before use and
changed weekly. Further details are provided below in Table 9.
Table 9 - Tissue Culture and Cell Injection Reagents
DPBS (Dulbecco's phosphate-buffered saline)
Heat-inactivated fetal bovine serum
RPMI 1640 medium
L-glutamine
Gentamycin
T175 Culture Flask
Roller Bottle
Tumor Model and Cell Culture Method
[0987] Human AML MOLM-14 cells were cultured at 37 C, 5% CO2 in the indicated
complete
culture media (RPMI 1640 + 10% HI-FBS + 2mM L-glutamine + 50ug/m1 Gentamycin
). Cells
were harvested while in logarithmic growth and resuspended in cold (4 C)
Roswell Park
Memorial Institute (RPMI) 1640 in serum-free medium.
[0988] Each mouse received 5 x106 MOLM-14 cells in 50% Matrigel in the right
flank, in a
total volume of 0.2 mL using a lcc syringe and a 27-gauge needle.
Study Designs
[0989] Compound A3 was administered orally (PO), daily.
[0990] Day 0 is the day of tumor cell implantation and study initiation.
[0991] Mice bearing SC MOLM-14 tumors were randomized on Day 16 post-tumor
implantation and assigned to treatment groups according to tumor volume (mean
of ¨130 mm3;
n-10/group). Treatment with vehicle or Compound A3 (at 30 and 100 mg/kg) was
initiated on
the same day, with daily oral dosing for 21 days. Plasma was collected at 1,
2, 4, 8, and 23 hours
after the last dose (n=4-5/group/time point) for PK (pharmacokinetics)
analysis.
Animal Monitoring
[0992] SC tumor volume was measured for each animal 2 to 3 times per week or
more
208
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
throughout the study.
Calculations
[0993] Tumor volume was calculated using the formula:
Tumor volume (mm3) = (Dxd2/2); where 'ID' represents the larger diameter and
d' the smaller
diameter of the tumor as determined by caliper measurements. Tumor volume data
was graphed
as the mean tumor volume SEM.
[0994] The % ATGI was defined as the difference between mean tumor burden of
the treatment
and control groups, calculated as % ATGI = ([(TVeTVco)(TVITVio)]/(TVc TVco))x
100 where
`I'Ve' is the mean tumor burden of a given control group, TVco' is the mean
initial tumor
burden of a given control group, 'TV' is the mean tumor burden of the
treatment group, and
`TVto' is the mean initial tumor burden of the treatment group. % TGI was
defined as the
difference between.
[0995] Mean tumor volumes of the treated and control groups, calculated as:
% TGI = ((TV:IVO/TN/) x100 where 'TV: is the mean tumor volume of the control
group and
TVt' is the mean tumor volume of the treatment group. As defined by National
Cancer Institute
criteria, >60% TGI is considered biologically significant.
[0996] The % Tumor Regression (TR), quantified to reflect the treatment-
related reduction of
tumor volume as compared to baseline independent of the control group, was
calculated
as %TR= (1-mean (TVti/TVtoi)) x 100 where `I'Vti' is the tumor burden of
individual animals
in a treatment group, and TVtoi' is the initial tumor burden of the animal.
Data Analysis
[0997] Tumor volumes were graphed using Prism software (GraphPad version 7 or
8).
Statistical significance for most studies was evaluated for Compound A3-
treated groups
compared with HPI3CD vehicle-treated controls on the last day of the study
when 2/3 or more
mice remained in each group. Differences between groups were considered
significant when
p<0.05.
[0998] Statistical significance for animal tumor volume was calculated using
the linear mixed-
effects (LME) analysis in R software version 3.4.2 (using Janssen' s
internally developed Shiny
application version 4.0), with treatment and time as fixed effects and animal
as random effect.
Logarithmic transformation was performed if individual longitudinal response
trajectories were
not linear.
[0999] The information derived from this model was used to make pairwise
treatment
209
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
comparisons of tumor volumes to that of the control group or between all the
treatment groups.
The results are shown in FIG. 2.
7) Cardio-Electrophysiological Effects of the Testing Compounds in
Synchronously
Beating Human Pluripotent Stem Cell-Derived Cardionlyocytes (hSC-CMs) Using a
Ca2+ -
Fluorescence Assay (CTCM human)
Protocol
[1000] Compounds were tested in the 96-well plates.
[1001] Compounds were tested at 0.1 tiM, 0.2 tiM, 0.5 laM, 1 laM, 2.5 laM and
5 laM (n = 4 per
dose) on Cor.4U 0-Cardiomyocytes or on iCell Cardiomyocytes2.
[1002] Alternatively, compounds were tested at 0.1 M, 0.3 M; 1 jiM, 3 p.M,10
p.M and 30
[tM (n = 4 per dose) mostly on iCell Cardiomyocytes2.
[1003] Positive and Negative controls
Dofetilide at 3 nM
lsoproterenol at 100 nM
Nimodipine at 100-300 nM
Cetirizine at 3 1.1.M.
Vehicle control: Dimethylsulfoxide (DMSO). The solutions of the compound in
DMSO or its
solvent (final concentration of 0.1% DMSO; n = 8).
Preparation of Test Article and Controls
[1004] Tested compounds were dissolved in DMSO at 1000-fold the intended
concentrations.
A compound "mother-plate" was made, containing the test compounds and positive
and
negative controls at 1000-fold the final concentrations. At the experiment
day, these stock
solutions were diluted with Tyrode (Sigma), supplemented with 10 mM HEPES
(Gibco), to 2-
fold the intended concentration (in round bottom compound plates). Final DMSO
concentration
in test solutions and vehicle control was 0.1%.
Cells
[1005] hSC-CMs (Cor.4U Cardiomyocytes) were obtained from CDI (Ncardia,
Germany).
Cells are pre-plated and seeded in fibronectin-coated 96-well plates at a
density suited to form
a monolayer and maintained in culture in a stage incubator (37 C, 5% CO2),
according to the
instructions of the cell provider.
210
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[1006] Second line hSC derived cardiomyocyte called iCelle Cardiomyocytes2
were
purchased from FUJIFILM Cellular Dynamics (USA). The experiments with test
drugs are
carried out 5 to 7 days after plating the cells onto the plate to have a
living, beating monolayer
of hiPSC-derived cardiomyocytes. The beating monolayer in 96-well-plates are
normally taken
from 2 Vials of frozen iCelle Cardiomyocytes2 (--z5 million cells/vial), which
will be plated
onto three 96-well plates (50K/well).
Before Start of Experiment
[1007] At least one hour before the start of the experiments the normal cell
medium was
replaced with Tyrode solution with Calcium dye (see below).
[1008] Cal 520 dye (AAT Bioquest) was dissolved in 11 ml of Tyrode
supplemented with 10
mM HEPES and warmed up to 37 C before adding to the cells.
[1009] 35 ul cell culture medium was removed from each well and replaced with
35 [11 of pre-
warmed Cal 520 dye solution and cell plate was incubated for 45 min at 37 C /
5% CO2. Cells
were incubated for 5 min at 37 C.
Experiment
[1010] Spontaneous electrical activity is recorded, using Cal520TM (AAT
Bioquest) calcium
fluorescence-dye signalling. This dye integrates the total intracellular
calcium activity over the
whole well. A bottle of Ca1520 dye (50 g, MW: 1103/mol) is dissolved with 50
ul DMSO as a
stock solution of 0.9 mM. 50 ML of the stock solution of the dye was added to
10 ml Tryodes
solution to have dye concentration of 4.5 uM. Subsequently, 35 Ml of this dye
solution was
added into each well, to have a final dye concentration of 1.58 MM. The
current dye protocol
on this CTCM human assay was established recently (Ivan Kopljar et al, Journal
of
Pharmacological and toxicological methods 2018. 91: 80-86; Lu et al., Tox Sci
2019. 170 (2):
345-356).
[1011] Fluorescent signals (Ca2 transient morphology) were measured using the
Functional
Drug Screen System (FDSS/pCell; Hamamatsu, Japan) and the recordings were
subsequently
analyzed off-line, using appropriate software e.g., Notocord.
[1012] The cell plate was loaded into the FDSS/pCell for a test run: Ca2+
transients were
measured for 4 minutes to check for synchronous beating of the cardiomyocytes
in each well.
All 96 wells were measured simultaneously (sampling interval: 0.06 s, short
exposure time: 10
ms; excitation wavelength 480 nm; emission wavelength 540 nm; FDSS/uCell
warmed to 37 C).
When all showed synchronous beating, the 96-well plate was measured repeatedly
for 3 times
211
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(to verify synchronous beating in all 96-well at baseline, wells that did not
meet the preset
criteria were excluded from the study and not treated with compound):
T = 0: control period (-5 to -1 min) + compound addition, followed for 3 min.
T ¨ 30: measured from 29 to 34 min after compound addition
[1013] During the compound addition step, 100 jul of the respective double-
concentrated test
solutions was pipetted into each well simultaneously.
[1014] Data were analyzed off-line using appropriate software e.g., Notocord-
Hem (version
4.3).
[1015] The following parameters of the Ca2+ transient morphology were
measured:
- beat rate (BR)
- amplitude of the Ca" transient (Amp),
- CTD90: Ca2+ transient duration at 90% (time to 90% of the initial base
value)
[1016] The presence of various 'arrhythmia-like' activities were also noted
during the
experimental periods. These included:
= 'early afterdepolarization-like' (EAD-like) events (defined as "an extra
small peak
of the transient waveform following the initial peak of the transient"),
= 'ventricular tachycardia-like' (VT-like) events (defined as a very fast
beating rate)
or
= 'ventricular fibrillation-like' (VF -like) events (defined as "small
amplitude, fast-
rate Ca' waveforms with irregularities and non-measurable transient
potentials)
= 'cessation of beating' of the cells (no Ca2+ transients observed).
If compound-induced changes on the calcium transient signal could not be
analyzed by the
software, then these signals were identified as BQL (below quality analyses
level).
Data Analysis
[1017] Data, measured from the FDSS- Cell, were copied for off-line analysis
and were
analyzed and uploaded in SPEC-II (our operational management system) for
further analysis.
The values of the variables before and after administration of the compound
were collected and
transferred into an Excel workbook.
[1018] All values (actual units and percentage changes from the baseline
values) are expressed
as median (minimum and maximum). Changes versus the corresponding baseline
values (in
actual units) observed in the compound group were compared with those in the
solvent control
group using the Wilcoxon-Mann-Whitney Test. Two-tailed tests with Bonferroni
correction for
multiplicity adjustment were conducted. Since there are 10 treatment groups
each compared to
212
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
the solvent group, alpha level of 0.05/10 (0.005) was considered to reflect a
statistically
significant difference from the solvent group. All statistical analysis was
performed using
appropriate software e.g., R software version 3.5.2.
[1019] Quality Control of the hiPSC-CMs in the plate:
Plates were rejected if they did not meet following criteria:
- Stable regular beating
- Amplitude > 500 relative units
- Beat rate between 25 and 80 beats per minute
- CTD90 between 300 and 800 ms.
[1020] In the present study, the hiPSC-CMs in the plates met the above
criteria.
[1021] These parameters combined with incidence of arrhythmia or cessation of
beating were
used to calculate the potential hazard level using a weighted scoring method
(based on Kopljar
et al., Stem Cell Reports 2018. 11, 1365-1377). This hazard score is
calculated per
concentration by adding weighted points based on the Tolerance Intervals (TI)
on the changes
of CTD90, the beat rate and amplitude (AA%) and incidence of beating stop and
early
afterdepolarization (EAD). Consequently, for each concentration one of four
different hazard
levels will be generated. This will be done after 30-min of incubated with
compound. The
hazard levels are:
No hazard: within the vehicle effect levels or small non-relevant changes.
Low hazard: relevant effect but potentially low risk for cardiac liabilities.
High hazard: relative high risk for cardiac liabilities.
Very high hazard: very high risk due to arrhythmic like events (EAD' s).
[1022] The 'Hazard Score' results provide an identification for potential
acute cardiac drug-
induced effects at free drug equivalent (as no plasma proteins are added to
the wells).
Evaluation of hazard identification is conducted using a 'scoring reference
book' called
CTCM Scoring version 1 (Kopljar et al., Stem Cell Reports 2018. 11: 1365-
1377), and levels
are indicated according to the following color scheme of Table 10.
Table 10 ¨ Color Schemes of Hazardous Identification Legend
Green No concern
Yellow Low concern
Red High concern
Black Very high concern due to
arrhythmic events
Ranking of a testing compound according to hazard score severity on the Ca'
transient assay
213
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
measured in HiPSc-CMs as listed above in different colors and in the
associated table.
RESULTS
Using iCell Cardiomyocytes2 as cell line
[1023] Positive and negative controls: The positive and negative controls all
had expected
pharmacological effects in this assay. The results are summarized below in
Tables 11 and 12.
Table 11 ¨ Hazard Scoring for Compound A3
rnpoun Co d Color @ Color @ Color @ Color @ Color @ Color @
0.1 uM 0.21.tM 0.51.tM luM 2.5 M
luM
A3 Green Green Green Green Green
Green
Table 12 ¨ Hazard Scoring for Compounds Al and 11
Compound Color @ Color @ Color .0/ Color @ Color @ Color @
0.1 uM 0.3 M luM 3 pM 10 MM
30pM
Al Green Green Green Green Green
yellow
11 Green Green Green Green Green
Green
[1024] For Compound Al: with an efficacious dose in mouse xenograft models of
30 mg/kg,
CTCM human concentration vs free Cmax would be estimated as follows:
Margin CTCM human 10 MM vs free Cmax >16 (mouse, human)
Margin CTCM human 30 MM vs free Cmax >45 (mouse, human).
8)
Effect on the Membrane Potassium Current 'Kr in hERG Transfected Cell Lines
[1025] Protocol 1:
Abbreviations
CHO Chinese hamster ovary cell line
DMSO Dimethylsulfoxide
hERG human ether-a-go-go-related gene
rapidly activating delayed-rectifier I( current
Methods
[1026] Experiments were performed using CHO cells stably expressing the hERG
potassium
channel. Cells were grown at 37 C and 5% CO2 in culture flasks in Ham's F12
Medium
supplemented with 10% heat-inactivated fetal calf serum, hygromycin B (100
p.g/m1) and
geneticin (100 pg/m1). For use in the automated patch-clamp system QPatch
(Sophion) cells
were harvested to obtain cell suspension of single cells.
[1027] Solutions: The bath solution contained (in mM) 145 NaCl, 4 KC1, 10
glucose, 10
214
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
HEPES ((4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 2 CaCl2 and 1
MgCl2 (pH 7.4
with NaOH). The pipette solution contained (in mM) 120 KC1, 10 EGTA (Ethylene
glycol-
bis(2-aminoethylether)-N,N,N,N-tetraacetic acid), 10 HEPES, 5.374 CaC12 and
1.75 MgCl2
(pH 7.2 with KOH).
[1028] Patch-clamp experiments were performed in the voltage-clamp mode and
whole-cell
currents were recorded with an automated patch-clamp assay utilizing the
QPatch system
(Sophion). Current signals were amplified and digitized, stored and analyzed
by using the
QPatch assay software.
[1029] The holding potential was -80 mV. The hERG current (Ktselective outward
current)
was determined as the maximal tail current at -40 mV after a 2 second
depolarization to +60
mV. Pulse cycling rate was 15 s. A short pulse (90 ms) to -40 mV served as a
baseline step to
calculate the tail current amplitude. After establishing whole-cell
configuration and a stability
period, the solvent control (0.3% DMSO) was applied for 5 minutes followed by
the test
substance by four increasing concentrations of 3 x 10-7 M, 3 x 10' M, 10-5 M
and 3 x 10-5 M.
Each concentration of the test substance was applied twice. The effect of each
concentration
was determined after 5 min as an average current of 3 sequential voltage
pulses. To determine
the extent of block the residual current was compared with vehicle pre-
treatment.
[1030] Concentration/response relations were calculated by non-linear least-
squares fits to the
individual data points. The half-maximal inhibiting concentration (IC50) was
calculated by the
fitting routine.
[1031] Protocol 2:
Cells
[1032] The compound, vehicle control and positive control were tested on hERG-
transfected
EfEK293 cells. A human embryonic kidney cell line (FIEK293) with a stable
transfection of
hERG (Zhou Z et al. Biophysical Journal 1998. 74, 230-241; McDonald T.V.et al,
Nature 1997.
388, 289-292) was used (University of Wisconsin, Madison, USA). The cells were
kept in
culture inlMEM (Minimum Essential Medium, Gibco) which was supplemented with
(amounts
indicated added to 500 ml 1MEM): 5 ml L-Glutamine-Penicillin-Streptomycin
(Sigma), 50 ml
Fetal Bovine serum (Bio-Whittaker), 5 ml Non-essential Amino Acids 100x
(Gibco), 5 ml
sodium pyruvate 100 mM (Gibco) and 4 ml geneticin 50 mg/ml (Gibco) using T175
flasks. The
cells were incubated at 37 C in 5% CO2 atmosphere (in air).
215
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Cell Harvesting for Assay
[1033] Cells were harvested as described below using accumaxTM (Sigma) as the
dissociating
reagent. Cells were then resuspended in a mixture of 33% DMEM/F12 (Dulbecco's
Modified
Eagle Medium/Nutrient Mixture F-12 - Sigma) media/67% extracellular
physiological solution.
[1034] The flasks were washed twice carefully with ¨5-10 ml phosphate buffered
saline (PBS)
(GibcoTM) containing 2mM EDTA (Ethylenediaminetetraacetic acid) (Sigma). The
cells were
dissociated using ¨3 ml of accumaxTM (cell detachment solution) and incubated
for ¨5 to 10
min. at 37 C. Cold external physiological solution (2-5 ml) was added and the
flasks are
incubated at ¨4 C for 5-10 min. Then, the cell suspension in each flask was
gently dissociated
with a 5m1 pipette. The cell suspension was transferred to a low binding petri-
dish (-10 mm
diameter). Each flask was washed with ¨ additional 5 ml cold external
physiological solution
and this solution was also added to the petri-dish. The petri-dish was then
incubated for another
to 10 minutes at ¨4 C. After another gentle dissociation of the cell
suspension in the petri
dish, the cells were transferred to a reservoir kept on an orbital shaker at
200 rpm at 16 C.
Before experiments were performed, the cells recovered for ¨20 min.
Compounds
[1035] A 10 mM solution of the compound was used and plated in a 384 well
plate. Aliquots
of the stock solutions are diluted with the recording solution using automated
liquid handling
(Biomek FXP; final DMSO concentration: 0.03 to 0.3 %). A standard range of
screening
concentrations was used ranging from 1 uM to 30 uM.
[1036] A positive control (E-4031) was included within each run to evaluate
the sensitivity of
the assay. Table 13 summarizes the external and intracellular solutions used
in the experiments.
In Table 13, the composition of the intracellular and external buffer
solutions is shown in [mM]
and "NIVIDG" means N-methyl-D-glucamine.
216
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 13 - External and Intracellular Solutions
Extracellular
Intracellular Physiological Seal Enhancer
Recording Solution
Solution Solution / Chip Fill Solution
Solution
KC1 10 NaCl 140 NMDG 60 NMDG 60
KF 110 KCl 4 NaCl 80 NaCl 80
NaCl 10 Glucose 5 KC1 4 KC1 4
HEPES 10 HEPE S 10 CaCl2 10 CaCl2 2
EGTA 10 CaCl2 2 MgCl2 1 MgCl2 1
MgCl2 1 Glucose 5 Glucose 5
HEPE S 10 HEPE S
10
pH 7.2 (KOH) pH 7.4 NaOH)( pH 7.4 (HCL) pH 7.4 (HCL)
Study Design
[1037] The whole cell patch clamp technique on transfected cells allows the
study of ion-
channels with no - or limited interference from other ion-channels. The
effects of the
compounds on the hERG current were studied with an automated planar patch
clamp system,
SyncroPatch 384PE (Obergrussberger et al, Journal of Laboratory Automation
2016. 21 (6),
779-793). All cells were recorded in the whole cell mode of the patch clamp
technique. The
module is incorporated in a liquid handling pipetting robot system, Biomek
FXP, for application
of cells and compounds, vehicle control and positive control.
[1038] The different concentrations of the compounds were applied in two
cumulatively
increasing concentrations for the compounds (1 M and 10 M, and 3 MM and 30
MM,
respectively). The hERG current was determined as the maximal tail current at -
30 mV and
percent inhibition upon compound or vehicle and positive control addition was
reported.
[1039] After cells are caught onto the individual holes of the recording chips
using the chip fill
solution, the seal is increased with the seal enhancer solution (increased
[Ca2]; then the cells
were washed twice with recording solution before using a pressure protocol to
go into the whole
cell mode.
[1040] After the whole cell mode was achieved, test pulses were given for ¨10
minutes to
quantify the hERG current in control conditions. During this control period
vehicle control
solution (recording solution containing 0.03% DMSO) was added three times into
the
individual wells. While continuing the pulse protocol, cumulatively increasing
concentrations
of the vehicle control, compound or positive control was added. The effect of
the vehicle,
compound and positive control was measured after 5 minutes of drug
application. Two
217
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
concentrations of the compound were tested per cell.
[1041] The use of the internal and recording solutions will result in ¨10 mV
liquid junction
potential and the command voltage step will take this into account.
[1042] Electrophysiological measurements: The membrane current of the cells
was measured
at distinct membrane potentials with the patch clamp technique by means of an
automated patch
clamp system The holding potential is -70 mV The hERG current (K-h-sel ective
outward
current) was determined as the maximal tail current at -30 mV after a 2 second
depolarization
to +70 mV (refs. 1, 4). Pulse cycling rate was 15 s.
Data analysis
[1043] The leak corrected hERG current (K -selective outward current) was
determined as the
maximal tail current at -30 mV after a 2-second of depolarization to +70 mV
measured between
2336.3 ms and 3083.6 ms. The median of three current amplitudes was taken at
the end of the
control period and at the end of each addition of compound, vehicle and
positive control to
calculate the percent inhibition.
[1044] QC parameters were set in the SyncroPatch 384PE PatchContro1384
software to
automatically exclude wells from the analysis if values fall outside the
range. The QC criteria
are dependent on the type of recording plate (chip). Typically, a 4xChip
(medium size hole)
was used to record from hERG-transfected 1-1EK293 cells. QC criteria 4-6 were
set before the
first addition of the compound, QC criteria 4 and 5 were also set at the end
of each compound
addition.
[1045] QC Criteria and acceptable ranges:
1. Board Check: -500pA - 500pA
2. Contact seal resistance: -100kOhm - 10MOhm
3. Junction potential offset: 0 - 100mV
4. Rseal > 100 MOhm
5. Rseries: between 1 - 25 MOhm
6. hERG tail current? 0.2 nA before compound addition
[1046] Each compound was replicated on the same plate in at least 5 wells.
Percent inhibition
of at least 2-3 replicates per concentration will be reported as median. The
results are
summarized below in Tables 14 and 15 for Protocol 1 and Protocol 2,
respectively.
218
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 14 - hERG IC50 (pM) from Protocol 1
Compound Number hERG- ICso (M)
A >30.2
Table 15 - hERG IC50 ( M) from Protocol 2
Example Number hERG ICso p.M
11 >30.2
9) Efficacy Study in Disseminated OCI-AML3 Model
Test Agents and Controls
[1047] Compound A3 was formulated in 20% hydroxypropyl-beta-cyclodextrin (EIP-
13-CD)
and prepared to reach a total volume of 0.2 mL (10 mL/kg) per dose for a 20 g
animal. Doses
were adjusted by individual body weight each day. Working stocks of Compound
A3 were
prepared once per week for each study and stored at 25 C.
Animals
[1048] Female SCID beige mice (CB17 Cg-PrkdcscidLystbg-J/Cr1/-) were used when
they
were approximately 6 to 8 weeks of age and weighed approximately 25 g. All
animals could
acclimate and recover from any shipping-related stress for a minimum of 7 days
prior to
experimental use. Autoclaved water and irradiated food were provided ad
libitum, and the
animals were maintained on a 12 hour light and dark cycle. Cages, bedding, and
water bottles
were autoclaved before use and changed weekly. The tissue culture and cell
injection reagents
are summarized below in Table 16.
Table 16 - Tissue Culture and Cell Injection Reagents
DPBS (Dulbecco's phosphate-buffered saline)
Heat-inactivated fetal bovine serum
MiEM Alpha medium
L-glutamine
Gentamycin
T175 Culture Flask
Roller Bottle
Tumor Model and Cell Culture Method
[1049] Human AML cell line OCI-AML3 was cultured at 37 C, 5% CO2 in the
indicated
complete culture media (MiEM Alpha + 20% HI-FBS (Heat-Inactivated Fetal Bovine
Serum) +
219
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
2mM L-glutamine + 50ug/m1 Gentamycin). Cells were harvested while in
logarithmic growth
and resuspended in cold (4 C) MEM ((Minimum Essential Medium) Alpha in serum-
free
medium.
[1050] For the disseminated OCI-AML3 model, each mouse received 5x105 cells
via IV
injection in a total volume of 0.2 mL using a 26-gauge needle.
Study Designs
[1051] Compound A3 was administered orally (PO), daily.
[1052] Day 0 is the day of tumor cell implantation and study initiation.
[1053] In the efficacy study, mice bearing IV OCI-AML3 xenograft tumors were
randomly
assigned to treatment groups 3 days post-tumor cell engraftment. Treatment
with vehicle or
Compound A3 (at 30, 50, 100 mg/kg) was initiated on the same day, with daily
dosing for 28
days.
Animal Monitoring
[1054] Animals were monitored daily for clinical signs related to either
compound toxicity or
tumor burden (i.e., hind limb paralysis, lethargy, etc.).
Calculations
[1055] For survival assessment, results were plotted as the percentage
survival against days
post tumor implant. Negative clinical signs and/or >20% body weight loss was
used as a
surrogate endpoint for death. Median survival was determined utilizing Kaplan-
Meier survival
analysis. The percent increased life span (ILS) was calculated as: ((median
survival day of
treated group - median survival day of control group) / median survival day of
control group)
100. Animals failing to reach the surrogate endpoint due to adverse clinical
signs (such as
ulcerated tumors, body weight loss, etc.) or death unrelated to treatment were
censored for the
survival assessment. As defined by NCI criteria, >25% lLS is considered
biologically
significant. (Johnson JI et al. Br J Cancer. 2001. 84(10), 1424-1431).
Data Analysis
[1056] Survival and body weight data were graphically represented utilizing
Prism (Version 7).
Statistical significance for body weights was evaluated as described above.
Statistical
significance was evaluated for Kaplan-Meier survival plots comparing
therapeutic treatment
group vs. appropriate vehicle-treated control using log-rank (Mantel-Cox) test
in R software
version 3.4.2. Differences between groups were considered significant when the
p value was
220
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
<0.05.
Survival
[1057] The Kaplan-Meier survival curve is shown in FIG. 3. Mice bearing
established OCI-
AML3 tumors were orally dosed daily with Compound A3 at 30, 50, 100 mg/kg in
20% HP-13-
CD formulation for a total of 28 days (n=9-10/group). For Compound A3 treated
groups, the
median days of survival were reached at the following days for 30 mg/kg at day
75.5, for 50
mg/kg at day 58.5 and for 100 mg/kg at day 75 this compared to a median
survival of 38.5 days
for the vehicle-treated control group. Compound A3 treatment resulted in
statistically
significant increased lifespan of OCI-AML3 tumor-bearing mice by 96.1%, 51.9%
and 94.8%
(at the 30, 50 and 100 mg/kg dose levels) as compared to that of control mice,
(p<0.001). This
was a biologically significant ILS as per NCI criteria threshold of >25% ILS
(Johnson JI et al.
Br. J Cancer. 2001. 84(10), 1424-1431).
10) Efficacy of Menin-MLL Inhibitor in Combination with Venetoclax, and
Optionally,
Azacitidine, in OCI-AML3 (NPM1c) IV Model in NSG Mice
110581 The disseminated OCI-AML3 model described above was also used to
examine the
efficacy of menin-MLL-inhibitor (formulated as described above) in combination
with
venetoclax (formulated in polyethylene glycol [PEG]-400/Phosa150/dimethyl
sulfoxide
[DMSO]), and optionally azacitidine (formulated in 10% DMSO), with the
following
differences: (i) instead of female SC1D beige, N SG (non-obese diabetic [NOD]
scid gamma or
NOD.Cg-Prkdcse'd Il2rgtml wil/SzJ) were utilized, (ii) instead of 5x105 cells,
each mouse received
an injection of 1x106 cells, (iii) instead of 3 days, treatment with
compound(s) was initiated 5
days after cell injection, and (iv) instead of a total volume of 0.2 mL (10
ml/kg) per dose,
compound(s) prepared to reach a volume of 8 ml/kg Compound Al was given in the
morning,
and venetoclax or azacitidine was given in the afternoon, approximately 6-8 hr
apart. The
treatment of each group using compound(s) in this efficacy study is summarized
in Table 17.
221
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 17 ¨ Treatment of OCI-AML3 tumors in mice
GROUP TREATMENT (n=10) DOSE
ROUTE SCHEDULE
1 Vehicles 8 mL/kg ip/po/po QD x 7/QD x
28/QD x 28
2 Azacitidine 2 rag/kg ip QD x 7
3 Venetoclax 100 mg/kg po QD x 28
4 Compound Al 30 mg/kg po QD x 28
Azacitidine 2 mg/kg ip/po QD x 7/QD x 28
+Venetoclax 100 mg/kg
6 Venetoclax 100 mg/kg po/po/po QD x 28/QD
x 28
+Compound Al 30 mg/kg
7 Azacitidinc 2 mg/kg ip/po/po QD x 7/QD x
28/QD x 28
+Venetoclax 100 mg/kg
+Compound Al 30 mg/kg
[1059] The Kaplan-Meier survival curve is shown in FIG. 4A. The impact on life
span of
treating mice bearing established OCI-AML3 tumors for 4 weeks is summarized in
Table 18.
Table 18 Impact on Life Span following Treatment of OCI-AML3-tumors in mice
TREATMENT %ILS
Azacitidine 18
Venetoclax 0 (ns)
Compound Al 33
Azacitidine 14
+Venetoclax
Venetoclax 33
+Compound Al
Azacitidine 35*
+Venetoclax
+Compound Al
p<0.05 as compared with vehicle-treated control mice except where noted as not
significant
(ns). *p<0.05 versus Aza and Ven monotherapies, and Aza+Ven combination
doublet.
110601 As reflected in Table 18, treatment of mice with Compound Al in
combination with
either venetoclax or venetoclax plus azacitidine resulted in statistically
significant increased
lifespan (1LS) of OC1-AML3 tumor-bearing mice as compared to that of vehicle
control treated
mice or with either azacitidine or venetoclax alone or the doublet combination
of ventetoclax
plus azacitidine. Notably, there is no antagonism with the triplet combination
(Compound Al
plus venetoclax and azacitidine) or doublets (venetoclax plus azacitidine or
Compound Al),
222
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
and efficacy seems solely driven by Compound Al.
11) Efficacy of Menin-MLT Inhibitor in Combination with Venetoclax
and Optionally,
Azacitidine, in MOLM-13 (KMT2A-r) IV Model in NSG Mice
[1061] A similar approach to the disseminated OCI-AML3 model described herein
for
examining the efficacy of menin-MLL-inhibitor in combination with venetoclax,
and optionally
azacitidine, was adopted with the MOLM-13 cells being used instead of OCI-AML3
cells.
Tumor Model and Cell Culture Method
[1062] Human AML cell line MOLM-13 was cultured at 37 C, 5% CO2 in the
indicated
complete culture media (RPMI, Roswell Park Memorial Institute [RPMI] 1640 10%
HI FBS
2mM L-glutamine 50 jig/m1 Gentamycin). Cells were harvested while in
logarithmic
growth and resuspended in cold (4 C) serum-free RPMI 1640 medium Each mouse
received
an injection of 1x105 MOLM-13 cells, and all other parameters were conducted
as described
for experiment (10).
[1063] The treatment of each group using compound(s) in this efficacy study is
summarized in
Table 19.
Table 19 ¨ Treatment of MOLM-13-tumors in mice
GROUP TREATMENT (n=10) DOSE ROUTE SCHEDULE
1 Vehicles 8 mL/kg ip/po/po QD x 7/QD x
28/QD x 28
2 Azacitidine 2 mg/kg ip QD x 7
3 Venetoclax 100 mg/kg po QD x 28
4 Compound All 10 mg/kg po QD x 28
Azacitidine 2 mg/kg ip/po QD x 7/QD x 28
+Vene toclax 100 mg/kg
6 Venetoclax 100 mg/kg po/po +QD x 28/QD
x 28
+Compound Al 10 mg/kg
7 Azacitidine 2 mg/kg ip/po/po QD x 7/QD x
28/QD x 28
+Venetoclax 100 mg/kg
+Compound Al 10 mg/kg
[1064] The Kaplan-Meier survival curve is shown in FIG. 4B. The impact on life
span of
treating mice bearing established MOLM-13 tumors for 4 weeks is summarized in
Table 20.
223
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 20 - Impact on Life Span following Treatment of MOLM-13 tumors in Mice
TREATMENT %ILS
Azacitidine 35
Venetoclax 6
Compound Al 35*
Azacitidine 53*
+Venetoclax
Venetoclax 256**
+Co mpound Al
Azacitidine 277**
+Venetoclax
+Compound Al
p<0.05 as compared with vehicle-treated control mice except where noted as not
significant
(ns). *p<0.05 versus Aza and Yen monotherapies. **p<0.05 versus Compound Al,
Aza, and
Yen monotherapies, and Aza+Ven combination doublet.
[1065] As reflected in Table 20, treatment of mice with Compound Al in
combination with
either venetoclax or venetoclax plus azacitidine resulted in statistically
significant increased
lifespan (TLS) of MOT,M-13 tumor-bearing mice as compared to that of mice
treated with
venetoclax alone or the doublet combination of ventetoclax plus azacitidine.
(12) In Vitro Proliferation Protocol
(A) Menin-MLL Inhibitor in Combination with Venetoclax and Optionally,
Azacitidine
[1066] The effect of Compound A3 in combination with venetoclax, or in
combination with
venetoclax and azacitidine, was determined in proliferation assays using the
MOLM-13
(KiVIT2A-r) cell line. MOLM-13 cells were seeded at 500 cells/well in 96-well
plates and
exposed to the indicated drug alone or in combination at the specified
concentration detailed in
Table 21A and Table 21B. In particular, for the combination of Compound A3
with venetoclax
(without azacitidine) a concentration range of Compound A3 (a 7-point, 4-fold
serial dilution
starting at an initial concentration of 1 i_tM) was combined venetoclax (a 6-
point, 5-fold serial
dilution starting at an initial concentration of 1 [tM). For the combination
of Compound A3
with venetoclax and azacitidine a concentration range of Compound A3 (a 6-
point, 4-fold serial
dilution starting at an initial concentration of 1 1.,IM) was combined with
the combination of
azacitidine (a 7-point, 5-fold serial dilution starting at an initial
concentration of 25 M) and
venetoclax (a 7-point, 5-fold serial dilution starting at an initial
concentration of 1 MM) in a
224
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
25:1 ratio. Compound A3 and azacitidine (when added) were treated at day 0 and
venetoclax
was added at day 4. MOLM-13 cells were incubated at 37 C, 5% CO2 with the
indicated drug
combination concentration for 8 days (Compound A3 and azacytidine) and for 4
days
(venetoclax) in quadruplicate.
110671 Spheroid-like growth was monitored in real-time by non-invasive live-
cell imaging
using the 4x objective of the Incucyte ZOOM live cell imaging system (Essen
BioScience), and
acquiring images on Day 8. The percent confluence, as a measure of spheroid
size, was
determined using an integrated analysis tool that is the part of the Incucyte
ZOOM software
"IncuCyte ZOOM 2016B" (Essen BioScience). The DMSO content was normalized to
0.3%
and the percent confluence from the DMSO well was used as baseline response.
[1068] Potential combination effects were evaluated at day 8 and analyzed for
synergy using
an R-based Biochemically Intuitive Generalized Loewe (BIGL) model implemented
with a
highest single agent (HSA) null model (Van der Borght 2017). Data from 2
independent
experiments were pooled and analyzed.
Results
[1069] The doublet combination of Compound A3 with venetoclax led to
significantly
increased inhibition of cell proliferation compared to Compound A3 or
venetoclax
monotherapy (see FIG. 5A). Strong synergistic effects were observed across a
range of
concentrations in MOLM-13 cells as reflected in the contour plot (FIG. 5A) and
detailed below
in Table 21A where effect size is represented as the difference between the
observed outcome
and the expected outcome of combination treatments with 95% confidence
interval shown in
parentheses.
[1070] The triplet combination of Compound A3 with venetoclax and azacitidine
led to
significantly increased inhibition of cell proliferation compared to Compound
A3 monotherapy
or the doublet combination of venetoclax and azacitidine (see FIG. 5B) Strong
synergistic
effects were observed across a range of concentrations in MOLM-13 cells as
reflected in the
contour plot (FIG. 5B) and detailed below in Table 21B where effect size is
represented as the
difference between the observed outcome and the expected outcome of
combination treatments
with 95% confidence interval shown in parentheses.
225
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 21A - Effect of Compound A3 in Combination with Venetoclax on MOLM-13
Cell
Proliferation.
,
_______________________________________________________________________________
__
0.03 -0.01 -0.00 -0.03 -0.00
0.00
1 (-0.02, 0.07) (-0.05, 0.04) (-0.04, (:.O4) (-0.08, 0.01)
(-0.05, 0.04) (-0.04, 0.04)
0.25 0.05 0.02 -0.00 -0.02 0.00 I -0.01
O..% (0.01, 0.09) ( 0.02, 0.07) ( 0.04,
0.04' ( 0.06, 0.03) (0.05, 0.05) 1 ( 0.06, 0.03)
M 1 __
0 0625 0.06* -0.03 -0.09 -0.22" -0.22" -0.26"
../.... .
(0.01,011) (-0.07,0.02) (-0.14,-0.04) (-
0.26,Ø17) (-0.26, -0.17) (-0.31,-0.22)
en . ______________________________________________________
< -
"r0 0.0156 0.02 -0.01 0.00 -0.09 -0.17" -
0.12"
C (-0.02, 0 07) (-0.05Ø03) (-0.04,0.05) (-
0.13, -0.05) (-0.22-0.13) (-0.17, -0.07)
=
O 0.004 0.04 -0.06 0.01 0.02
-0.01 0.03
11.
(0.01, 0.08) (-0.09, -0.021 (-003,0.05) (-
0.03, 0.06) (-0.03, 0.04) (-0.01,0.08)
E
o 0.01 0.02 0.01 0.03
0.01 -0.00
µ11 0.001 (-0.01, 0.05) (-0.01, 0.06) (-n_oz. oil-, (-
0.m, (Lim) (41.01, 0.06) (-0.04, 0.07)
0.02 0.09* 0.02 -0.01 0.04
0.02
0.0003 (-0.02, 0.05) (0.05, 0.13) (-0_02, 0.06) (-0.05, 0.04)
(4...00, 0.09) (-0.03,0.06)
Venetoclax ( M} 0.0003 0.0016 0.008 0.04 0.2
I.
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by" * "
and synergy is indicated by" ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
Table 21B - Effect of Compound A3 in Combination with Venetoclax and
Azacytidine on
MOLM-13 Cell Proliferation.
0.01 4.01 -0.00 -0.05 -0.12" 0.00 0.00
i. (-0.05, 0.07) (-0.08, 0.05) (-0.06, ).06) (-0.11,
0.02) (-0.18. -0.06) (-0.05Ø06) (-0.05Ø06)
.........
0.00 0.03 -0.02 -0.02 -0.11" -0.00 0.00
I. 0.25
(-106, 0.06) (-0.03,0.09) (-(L08, 0.04) (-
0.08, 0.04) (-0.17, -0.05) (-0.06,0.05) (-0.05,0.06)
......... _________________________________________________________ 7
rn 0.0625 um -0.00 0.05 -0.13" -0.41" 0.01 0.00
< (-0.06,0.09) (4.08,0.08) (-0.03, 0.13) 1-
0.20, -0.06) (-0.48, -0.34) (-0.05,0.06) (-0.05,0.06)
C 0.04 -0.40 -0.01 0.01 -0.15" 0.00 0.00
= 0.0156
O (-0.03, 0.10) (-0.07, 0.07) (-
0.08,0.05) (-0.06, 0.08) (-0.23, -0.08) (-0.05,0.06) (-0.05Ø06)
CI.
E 0.004 0.06 0.04 -0.05 -0.02 0.07* 0.00 0.01
O (0.00, 0.12) (4.02,0.10) (-
(L11, 0.00) (-0.08, 0.05) (-0.01, 0.15) (-(L05, 0.06) j-0.05,
0.06)
I.)
0.04 0.02 0.01 0.02 0.09' 0.00 0.01
0.001
(-0 12, 0.10) f. -0.03, 0.08) (-0.04, ,.07) (-
0.05, 0.08) '0.01, 0.17) (-0.05, 0.06) (-0.04,0.07)
Ven et oc Tax (WO) GE-05 0.0003 0.0016 0.008 0.04
0.2 1
Azacitidine (p.M) 0.002 0.u08 0.04 0... 1 5
25
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by" * "
and synergy is indicated by" ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
226
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
(B) Menin-MLL Inhibitor in Combination with Decitabine and Venetoclax
The effect of Compound A4 in combination with decitabine as compared to in
combination
with decitabine and venetoclax was determined in proliferation assays using
the MOLM-13
(KIVIT2A-r) and OCI-A1V11L3 (NPM1c) cell lines.
Cell lines
AML cell lines MOLM-13 and OCI-AML3 were purchased from DSMZ. MOLM-13 were
grown in RPMI medium supplemented with 10% Fetal Bovine Serum (FBS) and 1%
penicillin/streptomycin. OCI-AML3 cells were cultured in 80-90% alpha-MEM
(with ribo-
and deoxyribonucleosides) + 10-20% FB S. All cell lines were cultured at 37 C
in 5% CO2
atmosphere.
Cell Titer Glo assays
110711 A1V1L cell lines, MOLM-13 and 0C1-AML3, (5 x 103 cells/well) were
seeded in 96-well
plates and grown for 6 days in serum (10%)-containing medium in the presence
or absence of
inhibitors at the indicated concentrations detailed below in Table 22A and
Table 22B.
Proliferation was analyzed by means of a Cell Titer Glo assay using the
CellTiter 96 Aqueous
One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), according
to the
manufacturer's instructions. Data are mean with standard deviation from two to
four
independent experiments in technical triplicates. Compound A4 and decitabine
were added at
day 0 and venetoclax (when added) was added at day 4.
Synergy calculations
[1072] R-based Biochemically Intuitive Generalized Loewe (BIGL) model
implemented with
a highest single agent (HSA) null model. Specifically, the B1GL methodology
was applied to
calculate drug-drug interactions (Van der Borght, K., Tourny, A., Bagdziunas,
R. et al. B1GL:
Biochemically Intuitive Generalized Loewe null model for prediction of the
expected combined
effect compatible with partial agonism and antagonism. Sci Rep 7, 17935
(2017); Thas, 0.,
Tourny, A., Verbist, B., Hawinkel, S., Nazarov, M., Mutambanengwe, K., &
Bijnens, L.
Statistical detection of synergy: New methods and a comparative study.
Pharmaceutical
Statistics (2021)).
227
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
[1073] Synergy matrix results of the BIGL analysis of the cell line data with
HSA as mean
model calculated on the basis of the cellular metabolic activity using Cell
Titer-Glo assay.
Bootstrap confidence intervals are indicated. Effect sizes and their
confidence intervals are
shown. Notably, each data point based on the p-value and sign of the
respective maxR statistic
with the size of the dots reflecting the degree of synergy or antagonism
corresponding to graded
scale. No significant average effect if zero is included in the interval.
Results
A pairwise matrix combination of decitabine and Compound A4 in the absence or
presence of
venetoclax was evaluated in MOLM-13 (KMT2A-AF9; FLT3-ITD) and OCI-AML3 (NPM1c
AML) cells using a 6-day CellTiter-Glo assay format. Notably, the combination
of decitabine
and Compound A4, with or without venetoclax, was synergistically cytotoxic in
MOLM-13
(KMT2A-AF9; FLT3-ITD) cells as reflected in the contour plots (FIG. 6A and
FIG. 6B) and
detailed below in Tables 22A and 22B, respectively.
Table 22A - Effect of Compound A4 in Combination with Decitabine on MOLM-13
Cell
Proliferation.
MOLM-13
-0.0237 -0.0274 -0.0185 -0.0029 -
0.0014
Et 0577,11.015- p..n1 031 (-0_11436,11.11066)
(-EL01 57,0_1111LN 0.01 3,14,0.
2 c -0.0156 -0.0637** -0.0463 -00109
41.0059
5- 10566,0.025d ).1007,41.0267) (-0.0706;0.022)
(41.0228,0.0009) (41.0179,0.0062)
-0.0232 -0.1574" -0.16 4" -0.0192 -
0.0101
0.0569,0.0104 11879,-0.1268) (-0.1846,-
(L1442) (-0.0305,-0.008) (-0.0219,0.0017)
-0.0058 4/1435" -0.1783" -0.0211 -
0.0107
0.1
0 0363,0024/ 11703,-0.1166) (-0.198,-0.1587)
(-0.0323,-0.01) (-0.0224,0.001)
Ø0041 -0.1694" -0.1895" -0.022 -
0.0114
0
4.) 00328,0.0245 11932,-01456) (-02087,-0.1703)
(-0.033,-0.0109) (-0.0231,0.0003)
0.0387 0.2" 0.1958" 0.0214
0.0116
0.0665,-0.010 -0.223,-0.177) (-02148,-01768)
(-0.0325,-0.0102) (-0.0233,0.0001)
Dedtablne (ph4) 0.0015 0.015 0.15 1.5 3
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by * "
and synergy is indicated by ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
228
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Table 22B - Effect of Compound A4 in Combination with Venetoclax and
Decitabine on
MOLM-13 Cell Proliferation.
MOLM-13
0 001 -9.043 -0.0028 -0.0228 -0.0034 0.0004
.
(-0.139,0.053) (-0.0897,0.084) (4.0725,0.0269)
(4.0297,0.0228) (-0.0272,0.028)
2 0.01 -0.0293 -0.0208 -0.0376 -0.0034 0.0904
=. (-0.1306,0.072) (-0.1067,0.0651) (-0.086,0.0107)
(4.0296,0.0229) (-0.0273,0.028)
Mt MO5 4.0059 -0.2397** -0.057 4.0037 -0.0001
<
(-0.0895,0.0778) (-0.3118,-0.1675) (-0.1035,-0.0104) (-
0.0299,0.0224) (-0.0276,0.0275)
*0
C 0.1 -0.0258 4.2477** -0.0607* -0.0036 -0.0003
0
0 (-0.0979,0.0462) (-0.3043,-0.1. .1) (41068,-
0.0145) (4.0298,0.0226) (-0.0278,0.0272)
_
E -0.0128 426** 4.0654** -0.0041 -0.0002
3 1
(-0.0835,0.0579 (-0.3178,-0 7077) (-0.1111,4)0197)
(4.0307,0.07)) (41.0778,(10773)
10 -0.0363 -0.2876** -0.0676" -0.0039 -0.0003
(-0.1062,0.0336) (-0.339,4.2363) (41131,-0.0221) (-
0.03,0.0223) (-0.0278,0.0273)
Decitabine (pm) moan 0.015 0.15 1.5 3
Venetodax µ1.tM) 0.6..002 0.0002 0.002 0.02 0.2
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by" * "
and synergy is indicated by" ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
Antagonistic effects in OCT-AML3 (NP1\41c AML) cells were observed at low
doses of
decitabine (15 n1V1), with or without venetoclax, as reflected in the contour
plots (FIG. 7A and
FIG. 7B) and detailed below in Tables 23A and 23B, respectively.
Table 23A - Effect of Compound A4 in Combination with Decitabine on OCI-AML3
Cell
Proliferation.
OCI-ANIL3
r
-0.0483 -0.0238 -0.0156 -0.024 -0.0127
0.001
(-0.1014,0.0047) (-0.0813,0.033: -0.065,0.0338)
(4.0524,0.0043) (-0.0404,0.0151)
2 0.01 -0.0234 -0.1175** -0.038 40267 -0.0128
=_ (-0.0831,0.0364) (-0.1731,-0.06:
0.0869,0.0109) (-0.055,0.0015) (-0.0405,0.0149)
.....
er 0.0136 0.0588 -0.0571 4.0258 -0.0136
< 0.05
(-0.0364,0.0636) (0.0078,0.109", ).1055,-0.0086) (-
0.0541,0.0025) (-0.0413,0.0141)
"0
= 0.0139 0.1279* -0.0603 -0.0228 -0.0136
= 0.1
0 (-0.0299,0.0576) (0.0814,0.174! ).1086,-0.0119)
(-0.0512,0.0056) (-0.0413,0.0141)
CL
E 0.0141 0.1688* -0.0504 -0.0246 -0.013
0 1
. (-0.0288;0.057) (0.122,0.2156 0.094,-0.0067) (-0.053,0.0037)
(-0.04.07,0.0147)
(...) __________________________________________________________ . _
0.0288 0.1406* -0.0324 -0.018 -0.0114
(-0.0156,0.0734 (0.0934,0.1877) , 0.0767,0.012) (-
0.0466,0_0106) (-0.0392,0.0163)
- Dedtabine (niVT) 0.0015 0.015 0.15 1.5 3
229
CA 03215313 2023- 10- 12
WO 2022/237720
PCT/CN2022/091679
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by " * "
and synergy is indicated by" ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
Table 23B - Effect of Compound A4 in Combination with Venetoclax and
Decitabine on
OCT-AML3 Cell Proliferation.
OCI-AML 3
t
-0.0424 -0.0091 0.0094 -0.0239 -0.0114
0.001
10831,-0.0017) _ _ _ (-0.0527,0.0345) (-0.0278,0.0467) (-
0.0485,0.0007) 1 (-0.0356,0.0128)
+-
-
2 c ! 0.0237 -0.0788 -0.0187 -0.023 -
0.0127
8.0209,0.0683) (-0.1204,-0.0372) (-0.0555,0.0181) (-0.0476,0.0016) i (-
0.0369,0.0115)
V 0.0187 0.1041* -0.0351 -0.0224 -
0.0118
04 (
8.0182,0.0557) (0.0658,111424) (-0.0716,0.0014) (-0.047,0.0022)
(41036,0.8124)
12
C 0.0242 0.15' -0.0295 -0.0237 -
0.0117
7
17 0.1
0. i-0.01,0_0584) (0.1137,0.1864) (-0.0662,0.0071) (-
0.0483,0.0009) (-0.0359,0.0125)
_
E 1 0.0362 0.2025* -0.0159 0.0222 -
0.0109
a
3.0036,0.0689) (0.1668,0.2381) (-0.0476,0.0159) (-0.0468,0.0024) (-
0.0352,0.0133)
0.06 0.1657* 0.005 4.0167 -0.0099
3.0257,0.0942) (0.1297,0.2018) (-0.0282,0.0382) (-0.0414,0.0081) (-
0.0341,0.0144)
i
Decitabine (jiM) 0.0015 0.015 0.15 1.5 3
Venetaclax ( M) 0.00002 0.0002 0.002 0.02 0.2
Note that absence of any asterisk indicates an additive effect, antagonism is
indicated by" * "
and synergy is indicated by " ** ". Antagonistic and synergistic effects are
derived from the
significant calls based on the maxR test and numbers associated with same
interpreted as
antagonistic or synergistic, respectively.
230
CA 03215313 2023- 10- 12